Abstract
Since Isaac's and Lindenmann's seminal experiments over 50 years ago demonstrating a soluble factor generated from heat killed virus-stimulated chicken embryos could inhibit live influenza virus replication, the term interferon has been synonymous with inhibition of virus replication. While the antiviral properties of type 1 interferon (IFN-I) are undeniable, recent studies have reported expanding and somewhat unexpected roles of IFN-I signaling during both acute and persistent viral infections. IFN-I signaling can promote morbidity and mortality through induction of aberrant inflammatory responses and recruitment of inflammatory innate immune cell populations during acute respiratory viral infections. During persistent viral infection, IFN-I signaling promotes containment of early viral replication/dissemination, however, also initiates and maintains immune suppression, lymphoid tissue disorganization, and CD4 T cell dysfunction through modulation of multiple immune cell populations. Finally, new data are emerging illuminating how specific IFN-I species regulate immune pathology and suppression during acute and persistent viral infections, respectively. Systematic characterization of the cellular populations that produce IFN-I, how the timing of IFN-I induction and intricacies of subtype specific IFN-I signaling promote pathology or immune suppression during acute and persistent viral infections should inform the development of treatments and modalities to control viral associated pathologies.
Keywords: Immune pathology, Acute virus, Persistent virus, Interferon, Immune suppression, Antiviral, T cells
1. Type 1 Interferon Signaling During Acute Viral Infection
1.1. Suppressing Viral Replication/Dissemination
Many viruses harbor viral proteins with specific functions geared toward preventing IFN-I production and/or signaling, highlighting the evolutionary selective pressure exerted by IFN-I during viral replication (Devasthanam, 2014). The absence of IFN-I signaling during acute virus infection in vivo increases virus replication, dissemination, and lethality during multiple viral infections in animal models. Global deletion of IFNAR1 results in enhanced mortality during vesicular stomatitis virus (VSV), vaccinia virus (VV), West Nile virus (WNV), and lymphocytic choriomeningitis virus (LCMV) infections (Muller et al., 1994). Moreover, infection of IFNAR1 KO mice with acute LCMV Armstrong (Arm) (Nakayama et al., 2010, Zhou et al., 2012) and treatment of Arm-infected mice with an IFNAR1 neutralizing antibody elevated viral loads and promoted virus persistence (Teijaro et al., 2013, Wilson et al., 2013). Dendritic cell-specific deletion of IFNAR1 results in elevated virus replication and systemic persistence of the CW3 strain of murine Norovirus (MNoV) despite increased cell-mediated and humoral adaptive immune responses (Nice et al., 2016). IFN-I signaling has been shown to be essential for controlling WNV infection and restricting viral pathogenesis (Sheehan, Lazear, Diamond, & Schreiber, 2015). Mice deficient in IFNAR1 signaling display increased susceptibility to WNV infection (Pinto et al., 2014, Samuel and Diamond, 2005). During infection with the Coronavirus, mouse hepatitis virus (MHV-A59), the magnitude of the IFN-I and -II responses directly correlated with viral loads (Raaben, Koerkamp, Rottier, & de Haan, 2009). Moreover, IFN-I produced by plasmacytoid dendritic cells (pDCs) was essential to control virus replication and prevent mortality following MHV-A59 infection in mice (Cervantes-Barragan et al., 2007). During experimental infection of mice and nonhuman primates with the Lassa hemorrhagic fever virus, delayed or reduced induction of IFN-I and downstream gene signatures correlated with high viral loads and fatal outcome (Baize et al., 2009, Yun et al., 2012).
Deletion of IFN-I related signaling pathways during respiratory virus infections in animal models results in diverse effects depending on the virus strain and genetic background (Durbin et al., 2000, Garcia-Sastre, Durbin, et al., 1998, Price et al., 2000). In the context of respiratory viral infection, genetic deletion of STAT1 reduced virus control, enhanced pathology, and mortality during SARS-CoV and influenza virus infection (Durbin et al., 2000, Frieman et al., 2010). Interestingly, STAT1-deficient animals were highly susceptible to influenza virus infection, displaying elevated viral titers and increased pathology compared to STAT1-sufficient mice. Studies in mouse models of influenza virus have revealed conflicting evidence for the role of IFNAR1 in controlling influenza virus replication, morbidity, and mortality. Infection of IFNAR1−/− mice with the PR8 strain of influenza virus resulted in altered recruitment of Ly6Chi vs Ly6Cint monocytes in the lung, translating into increased production of the neutrophil chemoattractant, KC (CXCL8), elevated numbers of neutrophils in the lung and increased morbidity and mortality (Seo et al., 2011). Therefore, modulation of type 1 interferon signaling and production needs to be balanced to have enough to control virus infection but not promote excessive inflammation. The discrepancy between influenza pathogenicity in IFNAR1 and STAT1-deficient mice was later clarified when animals lacking both IFNAR1/IFN-λ were unable to control influenza virus replication. This is further supported in humans where null mutations in the human Interferon regulatory factor-7 gene results in reduced IFN-I and -III production from myeloid DCs and pDCs and life-threatening seasonal influenza virus infection (Ciancanelli et al., 2015). Exposure of bone marrow cells to IFN-I prior to their recruitment to lung endows these cells with an antiviral program that protects from virus infection after entry into the infected lung (Hermesh, Moltedo, Moran, & Lopez, 2010). Deletion of the IFN-β or IFNAR1 genes in mice with a functional Mx1 gene increased virus replication and reduced the LD50 20-fold (Koerner, Kochs, Kalinke, Weiss, & Staeheli, 2007). Infection of IFNAR1-deficient mice with low dose mouse adapted H1N1 influenza viruses resulted in mortality, elevated viral loads, exacerbated lung pathology, and reduced numbers of IL-10-producing cells as compared to IFNAR1-sufficient controls (Arimori et al., 2013). Moreover, exogenous administration of IL-10 to IFNAR1-deficient animals following influenza virus infection partially restored survival and ameliorated lung pathology. Thus, IFN-I can be protective during influenza virus infection either through suppressing virus spread or prompting induction of immune-suppressive cytokines to reign in excessive inflammation.
1.2. Promote Antiviral Immune Responses
In addition to directly inhibiting virus propagation, IFN-I also has potent immune stimulatory functions which support the resolution of virus infection. IFN-I promotes upregulation of MHC-I expression in multiple cell lineages (Lindahl et al., 1976a, Lindahl et al., 1976b), which is required for optimal T cell stimulation, differentiation, expansion, and killing of virus-infected cells. Autocrine signaling of IFN-I on dendritic cells promotes their activation and T cell stimulatory capacity (Montoya et al., 2002). IFN-I signaling during virus infection promotes conversion of pDCs into myeloid derived DCs and impairs hematopoietic differentiation of bone marrow progenitors into DCs (Sevilla et al., 2004, Zuniga et al., 2004). Following exposure to IFN-I, metallophilic macrophages induce expression of the Usp18 protein which prevents Jak1 phosphorylation and inhibits IFN-I signaling in these cells. In turn, repression of IFN-I signaling allows for restricted virus replication in these macrophages, promoting the production of viral antigens which are recognized by B cells, the final result is the facilitation of antiviral antibody generation and enhanced virus control (Honke et al., 2012).
IFN-I also exerts potent costimulatory effects directly on CD8 T cells, enhancing CD8 T cell proliferation upon IFNAR1 signaling (Curtsinger et al., 2005, Kolumam et al., 2005). The timing of CD8 T cell exposure to IFN-I significantly influences the differentiation and magnitude of the response (Welsh, Bahl, Marshall, & Urban, 2012). Exposure of naïve CD8 T cells to APC and IFN-I prior to antigenic stimulation promotes the maintenance of a naïve phenotype with reduced proliferation despite production of effector cytokines. Direct IFN-I signaling on naïve and memory T cells promotes rapid apoptosis, inhibits proliferation, and promotes early effector differentiation of memory cells upon exposure. Blockade of IFN-I signaling during WNV infection has significant effects on T cell expansion, cytokine production, and differentiation when administered during the maturation phase of the T cell response, however, had no effect when given prior to infection (Pinto et al., 2011). Moreover, low dose priming with the VV Ankara strain had little effect on effector or memory T cell recall in IFNAR1−/− mice (Volz, Langenmayer, Jany, Kalinke, & Sutter, 2014). In addition to T cells, IFN-I signaling is known to be important for NK cell function. IFN-I signaling promotes NK cell cytolytic capacity and survival during acute viral infection (Hwang et al., 2012, Martinez et al., 2008, Nguyen et al., 2002) and was recently reported to protect antiviral CD8 T cells from NK cell lytic effects (Crouse et al., 2014, Xu et al., 2014). Reconstitution of IFNAR1−/− mice with IFNAR1+/+ NK cells restored early control of VV infection in vivo (Martinez et al., 2008), suggesting that NK cell intrinsic IFNAR1 signaling is important for early control of VV replication. Moreover, direct IFN-I signaling on NK cells was required to induce NK cell IFN-γ production during acute LCMV infection. Early IFN-γR signaling was required for promoting initial virus control in the peritoneum (Mack, Kallal, Demers, & Biron, 2011), suggesting that IFN-I signaling directly on NK cells promotes virus control during acute LCMV infection. IFN-I signaling during viral infection can also signal to regulatory T cells and subsequently alter their suppressive functions. It was recently demonstrated that IFNAR1 signaling on FoxP3+ Tregs limits their suppressive function during acute LCMV infection, thus promoting virus control (Srivastava, Koch, Pepper, & Campbell, 2014). Deletion of IFNAR1 on FoxP3+ cells blunted virus-specific T cell responses and elevated virus loads. Thus, IFN-I signaling on suppressive T cell populations temporarily suspends suppressive function and allows for optimal antiviral T cell responses during an ongoing viral infection.
Similar to effects on T cells, IFN-I signaling has both positive (Le Bon et al., 2001) and negative effects on antiviral B cell responses. The survival and maturation of immature B cells can be inhibited by IFN-I signaling (Lin, Dong, & Cooper, 1998). In contrast to immature B cells, IFN-I signaling promotes B cell activation, antibody production, and isotype switch following influenza, VSV, and WNV infection (Coro et al., 2006, Fink et al., 2006, Purtha et al., 2008, Rau et al., 2009). However, it was also reported that influenza virus-specific antibody levels were elevated at later time points following influenza virus challenge in IFNAR1-deficient mice compared to IFNAR1-sufficient controls (Price et al., 2000). During acute LCMV infection, blockade of IFN-I signaling in both wild-type and STAT3-deficient mice enhanced T follicular helper cell (TFH), germinal center B cell differentiation, and anti-LCMV antibody responses (Ray et al., 2014). Elevated antibody responses during acute viral infections following IFNAR1 blockade suggest that, in certain circumstances, IFN-I signaling can restrain optimal antiviral antibody responses.
1.3. Augment Pathological Immune Responses
The correlation of an aggressive immune response and severe disease following influenza virus infection in humans and animal models has been discussed previously (La Gruta, Kedzierska, Stambas, & Doherty, 2007). An aggressive innate response, with elevated recruitment of inflammatory leukocytes to lung, likely contributed to the morbidity of the 1918 influenza infection (Ahmed et al., 2007, Kobasa et al., 2007). In fact, lung injury during infection of macaques with the 1918 H1N1 influenza virus strain directly correlated with early dysregulated inflammatory gene expression, including elevated IFN-I signatures (Cilloniz et al., 2009, Kobasa et al., 2007). More recently, clinical studies on avian H5N1-infected humans documented a significant association between excessive early cytokine responses and immune cell recruitment as predictive of poor outcome (de Jong et al., 2006). An aberrant cytokine/chemokine response was observed in patients with severe disease during the most recent H1N1 pandemic in 2009 (Arankalle et al., 2010). Type I interferon signaling is well known to inhibit influenza virus replication and spread (Garcia-Sastre & Biron, 2006). The production of the NS1 protein, one of 11 viral proteins, acts to inhibit type 1 interferon production and signaling (Hale, Randall, Ortin, & Jackson, 2008), suggesting that IFN-I signaling exerts substantial selection pressure on virus fitness. Deletion or mutation of the NS1 gene results in significant increases in the levels of type 1 interferon in infected cells and significantly lower virus titers both in vitro and in vivo (Garcia-Sastre, Egorov, et al., 1998, Jiao et al., 2008, Kochs et al., 2007). Despite strong evidence demonstrating extensive antiviral properties of IFN-I, several studies also suggest pathogenic roles for IFN-α during influenza virus infection. The production of several proinflammatory cytokines and chemokines is known to be amplified by IFN-I receptor signaling. In addition to protective effects of IFN-I signaling, pathogenic roles for IFN-I have been reported during influenza virus infection (Fig. 1A). Appearance of IFN-α in lavage fluid directly coincides with symptom onset during human experimental influenza virus infection (Hayden et al., 1998), suggesting that IFN-I signaling and pathological responses in humans temporally coincide. Recently, it was paradoxically reported that deletion of IFNAR1 or depletion of pDCs in SvEv129 mice inhibited pulmonary pathology and improved survival following lethal influenza virus challenge (Davidson, Crotta, McCabe, & Wack, 2014). Reduced immune pathology and enhanced survival in mice deficient in IFN-I signaling transpired without significant increases in viral loads or impediment of eventual viral clearance (Fig. 1B). In contrast to deletion of IFN-I signaling, treatment of influenza virus-infected mice with IFN-α resulted in enhanced morbidity and mortality; thus, IFN-I can promote pathological consequences during acute influenza virus infection.
Fig. 1.

IFN-I signaling enhances cytokine/chemokine amplification, innate immune cell recruitment, and immune pathology during respiratory viral infections. (A) Viral infection in the lung with Influenza or SARS-CoV promotes the induction of delayed IFN-I production which enhances cytokine/chemokine production, recruitment of NK cells, and neutrophils and inflammatory macrophage/monocytes all which contribute to lung immune-mediated pathology. (B) Blockade or genetic deletion of IFNAR1 blunts cytokine/chemokine amplification, inhibits recruitment of NK cells, neutrophils, and inflammatory macrophages/monocytes resulting in reduced immunopathology, and improved survival. Treatment of mice with S1P1R agonists early during influenza virus infection suppresses IFN-I amplification from plasmacytoid dendritic cells which lowers IFN-I levels. The end result is blunting of cytokine/chemokine amplification, inhibition of NK cell, neutrophil, and inflammatory macrophage/monocyte recruitment into the lung, reduced immunopathology, and improved survival.
Over the past 5 years, we identified that therapeutic administration of sphingosine 1 phosphate (S1P) analogs early during influenza virus infection in mice resulted in reduced morbidity and mortality (Walsh et al., 2011). S1P is a lipid metabolite converted from ceramide precursors to sphingosine. The subsequent phosphorylation by sphingosine kinase 1 and 2 produces bioactive S1P in vivo where it acts on S1P-specific G-protein couples receptors (GPCRs) (Chalfant & Spiegel, 2005). The levels of bioactive S1P are regulated through the actions of S1P phosphatases and lyases which dephosphorylate and degrade S1P, respectively. Highest levels of S1P are found in the blood and lymph with significantly lower levels maintained in peripheral tissues (Cyster, 2005). S1P binds and signals through five GPCRs denoted as S1PR1-5 which couple to various G-protein signaling effectors. The expression of S1P receptors is heterogeneous, being found on both hematopoietic and nonhematopoietic lineages (Im, 2010). The functional coupling to multiple heterotrimeric G-proteins promote the diverse cellular functions associated with S1P receptor signaling. Signaling through these five receptors is known to modulate multiple cellular processes including: cell adhesion, migration, survival, proliferation, endocytosis, barrier function, and cytokine production (Rivera, Proia, & Olivera, 2008).
Recently, we identified a novel regulatory function of S1PR1 signaling in blunting early cytokine amplification and innate immune cell recruitment following influenza virus infection (Fig. 1B). Early administration of a promiscuous S1PR agonist, AAL-R, or an S1P1R-selective agonist (CYM-5442) significantly blunted production of multiple pro-inflammatory cytokines and chemokines following infection with either WSN or human pandemic H1N1 2009 influenza virus (Teijaro et al., 2011, Walsh et al., 2011). Further, both AAL-R- and CYM-5442-mediated reduction of early innate immune cell recruitment and cytokine/chemokine production correlated directly with reduced lung pathology and improved survival during H1N1 2009 influenza virus infection. While these S1PR agonists clearly inhibited innate immune responses, significant inhibition of activated T cell recruitment into the lung at various times post infection occurred in mouse adapted (Marsolais et al., 2009) and human pathogenic strains of influenza virus (Walsh et al., 2011). The above findings were extended using genetic and chemical tools to probe functions of the S1P1 receptor (S1P1 GFP knockin transgenic mice, S1P1 receptor agonists and antagonists), revealing that pulmonary endothelial cells modulate innate immune cell recruitment and cytokine/chemokine responses early following influenza virus infection (Teijaro et al., 2011). Importantly, S1P1R agonist treatment blunted cytokine/chemokine production and innate immune cell recruitment in the lung independently of endosomal and cytosolic innate sensing pathways (Teijaro, Walsh, Rice, Rosen, & Oldstone, 2014). Further, S1P1R signaling suppression of cytokine amplification was independent of multiple innate signaling adaptor pathways but required the MyD88 adaptor for cytokine amplification following influenza virus challenge. Immune cell infiltration and cytokine production were found to be distinct events, both orchestrated by signaling through the S1P1R. Suppression of early innate immune responses through S1P1R signaling also reduced mortality during infection with human pathogenic strains (H1N1/2009 swine) of influenza virus in a ferret model, demonstrating that S1PR1-mediated blunting of influenza virus pathogenesis in mice could be extended to a model more closely resembling human disease.
The link between S1PR1 and IFN-α amplification following influenza virus infection was striking. In fact, the absence of IFNAR1 abolished cytokine amplification and the capacity of S1P1R agonists to further blunt cytokine/chemokine responses (Teijaro et al., 2016, Teijaro et al., 2011). To understand how S1PR1 signaling regulates IFN-α and cytokine amplification, we assessed the pulmonary cell subsets that produce IFN-α and cytokines/chemokines following influenza virus challenge. Expression of S1P1R was quickly observed in purified pDCs; moreover, S1P1R agonists suppressed IFN-I induction/amplification from both mouse and human pDCs following influenza virus simulation (Teijaro et al., 2016). Further mechanistic studies revealed that S1P1R agonist-mediated suppression was independent of Gi/o signaling and required signaling through the S1P1R C-terminus. Biochemically, S1P1R agonists accelerated the turnover of IFNAR1 and promoted trafficking to lysosomes for degradation, abrogating STAT1 phosphorylation, blunting the IFN-I autoamplification loop. The fact that IFN-I production/signaling can down modulate S1PR1 expression/activity indirectly through upregulation of CD69 which promotes internalization of S1PR1 in T cells is significant (Shiow, 2006) and suggests that S1P1R and IFN-I signaling are closely linked and capable of counter regulating one another. An additional study also reported IFN-I modulation in pDCs via other S1PRs (Dillmann et al., 2016), suggesting that this phenomenon could be more promiscuous than originally thought.
Similar to influenza virus infection, aberrant innate cytokine/chemokine responses and immune cell recruitment into lungs correlate with disease severity in human patients (Huang et al., 2005). IFN-I signaling during murine SARS-CoV infection appears to be dispensable for virus control while also potentiating immune pathology. However, the role IFN-I signaling plays in this pathology has only recently been systematically addressed. Deletion of IFNAR1 in mice does not mirror the enhanced viral loads or pathological consequences observed in STAT1−/− mice in SARS-CoV infection, suggesting an IFNAR1-independent STAT-1-dependent pathway is necessary for controlling SARS-CoV (Frieman et al., 2010). This study provocatively suggests that IFN-I signaling is dispensable for controlling SARS-CoV replication in vivo. Recently, an important study was published where the authors further highlighted the importance of IFN-I signaling in respiratory virus pathology by reporting that delayed IFN-I induction and signaling during SARS-CoV infection in mice promoted the development and infiltration of inflammatory monocyte–macrophages into the lung, resulting in exacerbated lung pathology and lethal pneumonia (Channappanavar et al., 2016). Attenuation of IFN-I signaling either through genetic deletion or through antibody neutralization of IFNAR1 prevented inflammatory monocyte–macrophage infiltration into the lung, abrogated lung immune pathology, and resulted in mild clinical disease. Importantly, genetic deletion or blockade of IFN-I signaling resulted in control of viral loads similar to control animals, reinforcing that IFN-I signaling is dispensable for control of SARS-CoV infection in vivo. One possibility is that in the absence of IFN-I signaling, induction of an IFN-III (IFN-λ) antiviral program may effectively limit viral replication. The results found in this study were strikingly similar to those found in influenza virus-infected SvEv129 mice and suggest that strategic modulation of IFN-I signaling could ameliorate pathologies associated with severe respiratory virus infection.
Collectively, the studies above suggest that IFN-I signaling is essential to cytokine and chemokine amplification and innate immune cell recruitment and can promote excessive immunopathology during acute respiratory viral infections (Fig. 1). Importantly, that IFN-I production and signaling can be blunted without enhancing virus propagation following acute respiratory viral infection suggests that this pathway can be modulated without compromising host antiviral responses. The correlation between blunting IFN-I signaling, lessened immune pathology, and improved survival during multiple respiratory viral infections highlight the need to mechanistically dissect how IFN-I promotes immune pathology during these infections.
2. Type I Interferon Signaling and Persistent/Chronic Viral Infection
2.1. Controlling Virus Replication/Dissemination
The role of IFN-I signaling in restraining chronic/persistent viral infection is well documented. Inhibition of IFN-I signaling by antibody blockade of IFNAR1 results in elevated virus replication early following LCMV Cl13 infection and treatment of mice with IFN-I during the early stages of persistent LCMV infection promotes rapid virus control (Wang et al., 2012). Mechanistically, IFN-I therapy increased expansion of virus-specific CD8 T cells and prevented T cell exhaustion; however, whether this was due to IFN-I-mediated immune stimulatory effects, lowering of antigen levels, or both was not systematically addressed. An additional study reported that deletion of the 2′–5′ oligoadenylate synthetase-like 1 gene prior to LCMV Cl13 infection facilitated sustained IFN-I production/signaling, promoted T cell expansion, reduced T cell exhaustion, and promoted rapid virus control (Lee, Park, Jeong, Kim, & Ha, 2013). Similar to persistent LCMV infection, IFN-I administration can exert protective effects through slowing SIV replication and disease progression if administered early following infection (Sandler et al., 2014) and has shown some efficacy in patients with persistent HIV infection (Asmuth et al., 2010, Azzoni et al., 2013). Moreover, treatment with pegylated IFN-α in conjunction with the antiviral drug Ribavirin was the standard of care for treating patients with chronic hepatitis C virus (HCV) infection until recently (Heim, 2013, Moreno-Otero, 2005). However, despite success in HCV therapy, the modest efficacy observed following IFN-α administration requires Ribavirin and, even in combination, only a slim majority of patients respond. Moreover, patients who fail to control HCV following IFN-I therapy were reported to express a higher IFN-I gene signature prior to treatment (Sarasin-Filipowicz et al., 2008). Similar trends were observed following IFN-I administration during HIV and SIV infections, where IFN-I administration had only the modest effects if given during established persistent infection (Asmuth et al., 2008, Hubbard et al., 2012). The reasons for the discrepancies observed in human persistent viral infections, where IFN-I therapy can promote control (50–60% of HCV patients) while in others (during established HIV infection) minimal benefit is observed, remain unknown. One could imagine a scenario where in some persistently infected HCV patients, elevated IFN-I signatures persist, and addition of pegylated IFN-α provides minimal benefit while patients with lower IFN-I signatures respond to the therapy. Whether treatment with pegylated IFN-α earlier during infection (prior to sustained IFN-I signatures) would be beneficial would be interesting to discern. A similar profile appears to exist in persistent SIV infection, where early administration of IFN-I promotes control of viral loads and pathogenesis, while later administration has modest effects on viral titers and disease outcome. During infection with a model Gamma Herpesvirus, MHV68, the lack of IFN-I signaling exacerbated virus replication, increased reactivation from latency, and resulted in enhanced morbidity and mortality (Barton et al., 2005, Dutia et al., 1999). Taken together, IFN-I therapy may be beneficial during the early stages of persistent, latent chronic viral infection, or infections with lower IFN-I signatures; however, blocking IFN-I signaling either alone or in conjunction with antiviral or immune checkpoint therapies may prove more effective once virus persistence and elevated IFN-I signatures are established. However, the ultimate outcome will likely depend on the persistent virus studied, genetic susceptibilities of individuals, and subtype and timing of IFN-I species produced; all which require further investigation. Moreover, given the undesirable side effects of IFN-I administration, IFN therapy can do as much harm as good during viral infection, highlighting the need for developing alternative approaches to treat persistent viral infections.
2.2. Shaping the Immune Suppressive Environment
During persistent viral infections, chronic immune activation, negative immune regulator expression, an elevated interferon signature, and lymphoid tissue destruction correlate with disease progression. Elevated IFN-I signatures have been observed during LCMV infection in mice (Hahm, Trifilo, Zuniga, & Oldstone, 2005) and HIV and HCV infections in humans and nonhuman primates (Bosinger et al., 2009, Jacquelin et al., 2009, Wieland et al., 2014). Chronic immune activation following HIV infection has been reported, and suppression of this hyperactivated state has been proposed as a potential strategy to alleviate HIV-associated pathologies (Boasso et al., 2008, Boasso and Shearer, 2008, d'Ettorre et al., 2011). Disease following experimental SIV infection in rhesus macaques correlates with elevated IFN-I production and inflammatory signatures (Jacquelin et al., 2009, Manches and Bhardwaj, 2009). In contrast, SIV infection in sooty mangabeys and African green monkeys, which develop modest pathology despite equivalent viral loads as macaques, correlate with reduced IFN-I and inflammatory gene signatures (Bosinger et al., 2009). Similar correlations with respect to reduced immune activation exist in HIV-infected elite controllers, although whether reduced immune activation follows virus control is uncertain (Deeks and Walker, 2007, Saez-Cirion et al., 2007). Blockade of PD-1 signaling during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques and lowers IFN-I signatures in the blood and colon (Dyavar Shetty et al., 2012). Moreover, an elevated interferon signature is observed in HCV-infected patients despite limited control of virus replication and development of liver pathology (Guidotti and Chisari, 2006, Su et al., 2002, Wieland et al., 2014). In fact, HCV infection in culture blocks ISG protein expression through activation of RNA-dependent protein kinase (Garaigorta & Chisari, 2009), creating a paradoxical IFN-I-dependent viral advantage. Thus, IFN-I signaling pathways have the potential to aid viral fitness and promote pathology during persistent viral infection. These studies further highlight the viability of the IFN-I signaling system as a target to promote control of persistent viral infection.
While the literature suggests a causative role for IFN-I in contributing to pathogenesis of persistent virus infections, definitive studies assessing how IFN-I neutralization affects the outcome of virus persistence were lacking until recently. Two laboratories assessed the role IFN-I signaling plays during persistent infection using the LCMV Clone-13 (Cl13) strain of virus. During their investigation, they found that blockade of IFN-I signaling using an IFNAR1 neutralizing antibody reduced immune system activation, decreased expression of negative immune regulatory molecules IL-10 and PD-L1 and restored lymphoid architecture in mice persistently infected with LCMV (Fig. 2 ). Importantly, blockade of IFNAR1 both prior to and following established persistent LCMV infection promoted faster virus clearance and required an intact CD4 T cell compartment (Teijaro et al., 2013, Wilson et al., 2013). Blockade of IFN-I signaling significantly enhanced CD4 T cell differentiation into Th1 effectors as well as increased TFH cell differentiation (Osokine et al., 2014). The above studies demonstrate for the first time a direct causal link between IFN-I signaling, immune activation, negative immune regulator expression, lymphoid tissue disorganization, and long-term virus persistence. More recently, it was reported that during Cl13 infection, both type I and II interferon promoted the induction and suppressive capacity of CD95+CD39+ immune regulatory DCs (iregDCs), respectively (Cunningham et al., 2016). While IFN-γ promoted the differentiation of iregDCs from monocytes, IFN-I promoted the suppressive functions of iregDCs. Genetic deletion of IFNAR1 prevented the expression of PD-L1 and production of IL-10 from iregDCs, relieving their suppressive capabilities. In addition to modulating the suppressive capacity of iregDCs, IFN-I signaling also limited their generation/expansion. During MNoV infection, selective genetic deletion of IFNAR1 in DCs increased expression of the cellular activation markers CD80, CD86, and MHCII, suggesting that direct IFN-I signaling on DCs may be responsible for restraining DC function in vivo (Nice et al., 2016). Generation of elevated numbers of iregDCs was also observed during HIV and mycobacterium tuberculosis infections as well as cancer, suggesting that iregDC generation is common in immunosuppressive environments. The IFN-I-driven immune-suppressive state during persistent LCMV infection also inhibits macrophage function. A recent study found that mice infected with the persistent docile strain of LCMV have impaired humoral immune responses to a superinfecting VSV infection (Honke et al., 2016). The absence of virus replication in CD169+ macrophages was not due to antiviral CD8 T cell-mediated killing of CD169+ macrophages but instead the result of sustained IFN-I responses and an elevated IFN-I antiviral gene program. In turn, reduction in VSV replication and antigen production in CD169+ macrophages reduced antigen production in these cells which was essential for antiviral antibody generation.
Fig. 2.
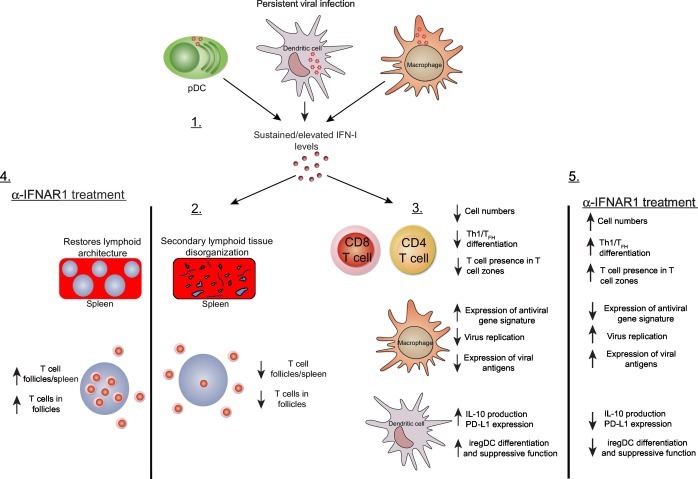
Elevated IFN-I signatures during persistent viral infection support a global immunosuppressive program. 1. Infection with persistent viruses results in elevated IFN-I production and downstream gene signatures, which are maintained throughout the infection even without detectable IFN-I protein levels. 2. At the organ level, elevated IFN-I signatures prevent proper organization of secondary lymphoid architecture, with fewer T cell zones and less recruitment of T cells into T cell zones. 3. Elevated IFN-I signatures promote T cell exhaustion reducing T cells numbers and preventing Th1/TFH differentiation. Upregulation of antiviral gene expression in marginal zone macrophages results in inhibition of virus replication in these cells and suboptimal levels of viral antigen required for triggering antiviral humoral immune responses. IFN-I signaling on dendritic cells promotes sustained expression of the negative immune regulatory molecules IL-10 and PD-L1, promotes the generation of iregDCs, and maintains T cell immune suppression. 4. Blockade of IFN-I signaling during persistent viral infection using an anti-IFNAR1 monoclonal antibody restores lymphoid architecture and promotes T cell migration/residence in T cell zones. 5. Restores T cell function, increases expression of viral antigens in CD169+ macrophages, blunts production of the negative immune regulatory molecules IL-10 and PD-L1, and inhibits differentiation and suppressive function of iregDCs. The above restoration of immune cell functions following IFN-I blockade ultimately results in hastened clearance of the persistent viral infection.
The existence of multiple IFN-I subspecies (14 IFN-α species in mice and 13 in humans in addition to IFN-β) suggests that either the IFN-I system requires redundancy to be effective or that individual IFN-I species evolved to execute specific functions. Certainly, different IFN-α species and β display varying degrees of affinity for the IFNAR1/2 receptor complex (Ng et al., 2016, Thomas et al., 2011), with IFN-β displaying the highest binding affinity. LCMV persistence was influenced more by IFN-β than IFN-α signaling as treatment of mice infected with LCMV Cl13 with an IFN-β neutralizing antibody displayed accelerated virus clearance compared to a polyclonal IFN-α antibody which had minimal effects on virus control (Ng et al., 2015). IFN-β neutralization did not exacerbate early virus replication, improved lymphoid architecture, and enhanced virus-specific CD4 and CD8 T cell responses. However, while IFN-β neutralization clearly promoted faster virus clearance as compared to neutralization with a polyclonal IFN-α antibody, the contribution of IFN-α species not neutralized by the polyclonal antibody used was not investigated. Nevertheless, neutralizing IFN-β may promote adaptive immune control of virus without significantly affecting virus replication and thus may represent a safer approach to promoting control of persistent virus infection in vivo. The dichotomy between IFN-α and β was further highlighted upon infection of New Zealand black (NZB) mice with LCMV Cl13. Infection of NZB mice with Cl13 resulted in early lethality that was found to be due to CD8 T cell-dependent thrombocytopenia and pulmonary endothelial cells loss (Baccala et al., 2014). Interestingly, despite upregulation of PD-1/PD-L1 expression and IL-10 production, T cell function remained intact. Moreover, this enhanced pathology correlated with elevated IFN-I protein levels and gene signatures; however, unlike infection in C57BL/6J mice, the pathology required IFN-α signaling and was IFN-β independent. It was recently reported that IFN-β signaling required binding to IFNAR1 but was independent of IFNAR2. Deletion of IFNAR1 ameliorated LPS-induced sepsis induction, while IFNAR2−/− mice were unaffected (de Weerd et al., 2013); thus, it would be interesting to test how IFNAR2−/− NZB mice respond to Cl13 infection. The above studies demonstrate that IFN-α and -β species can differentially modulate immune responses in various viral infections, highlighting the importance of future investigation into how different IFN-I subtypes modulate viral control and disease pathogenesis.
3. Perspective
Several important questions still remain that provide exciting avenues for investigating the roles of IFN-I signaling during viral infection in the future. Although IFN-I signaling can trigger various downstream effector pathways, how signaling via select IFN-I species dictate specific outcomes following viral infections remain incompletely understood. Specifically, there is a great need to understand the roles individual IFN-I-α and -β subsets play in restraining viral replication or promoting immune inflammatory/suppressive programs in vivo. Further, how IFN-I signaling in specific cellular subsets in vivo regulates immune pathological and immune-suppressive responses will be interesting to dissect. The IFNAR1-floxed mouse strain which was generated recently will be instrumental in future studies to investigate this question. Illuminating what cell types require IFN-I signaling in vivo should pave the way for generating a detailed understanding of the cellular and molecular mechanisms by which IFN-I signaling acts to promote immune pathology and suppression in acute and persistent viral infections.
The capacity of IFN-I signaling to promote immune pathology during acute respiratory viral infection appears in animal models of both influenza and SARS-CoV infection. The necessity of IFN-I signaling to restrain viral spread during acute viral infection suggest that targeting the IFN-I signaling pathway may be ill advised. However, one wonders whether targeting specific IFN-I species to suppress detrimental inflammation can be achieved without compromising virus clearance during acute respiratory viral infections. Moreover, the production of IFN-λ during respiratory viral infection may be sufficient to control viral loads while IFN-I signaling is inhibited. Recent results in mouse models suggest this may be possible; however, further studies are needed. Moreover, whether the effects observed in mice will translate to human respiratory viral infections is unknown and should be investigated with caution.
In the context of the immune-suppressive programs elicited by IFN-I signaling during persistent virus infection, the recent demonstration that blockade of IFN-β enhanced virus control by inducing improved lymphoid architecture and enhanced virus-specific CD4 and CD8 T cell responses, suggest that targeting selective IFN-I species can redirect immune responses sufficiently to promote immune-mediated virus control. Importantly, relief of the immune-suppressive environment in this case was not accompanied by elevated viral loads following treatment with IFN-β-neutralizing antibody, suggesting that more selective modulation of specific IFN-I species can allow for preservation of some antiviral functions.
The mechanisms by which the different IFN-I species interact with the IFNAR1 and IFNAR2 receptors to induce differential downstream signaling suggests this pathway could be manipulated pharmacologically. It is interesting to postulate whether small molecules or biologics could be developed to block binding/signaling of specific IFN-I species (i.e., IFN-β or specific α-species). For example, could IFN-β signaling be selectively inhibited without altering IFN-α species engagement with the IFNAR1/2 receptor complex during ongoing viral infection using a small molecule or antibody therapeutic? Could a small molecule be designed to reverse aspects of the immune-suppressive environment and promote virus control without compromising virus replication? On the contrary, could selective IFN-I agonists be developed to increase IFN-I signaling in a productive way to lower viral loads and bring persistent/chronic viral infection under control? A similar question could be posited during acute viral infections where IFN-I signaling promotes aberrant inflammation and immune pathology. Moreover, it would be interesting to investigate whether selective biological or pharmacological modulation of IFN-I signaling may translate to treat autoimmune disease states associated with elevated and sustained IFN-I signaling. However, any therapy that enhances or blocks IFN-I signaling will need to be approached carefully, given the delicate balancing act required for controlling virus replication while safely modulating immune responses.
References
- Ahmed R., Oldstone M.B., Palese P. Protective immunity and susceptibility to infectious diseases: Lessons from the 1918 influenza pandemic. Nature Immunology. 2007;8:1188–1193. doi: 10.1038/ni1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arankalle V.A., Lole K.S., Arya R.P., Tripathy A.S., Ramdasi A.Y., Chadha M.S. Role of host immune response and viral load in the differential outcome of pandemic H1N1 (2009) influenza virus infection in Indian patients. PloS One. 2010;5:e13099. doi: 10.1371/journal.pone.0013099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimori Y., Nakamura R., Yamada H., Shibata K., Maeda N., Kase T. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral Research. 2013;99:230–237. doi: 10.1016/j.antiviral.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Asmuth D.M., Abel K., George M.D., Dandekar S., Pollard R.B., Miller C.J. Pegylated interferon-alpha 2a treatment of chronic SIV-infected macaques. Journal of Medical Primatology. 2008;37:26–30. doi: 10.1111/j.1600-0684.2007.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmuth D.M., Murphy R.L., Rosenkranz S.L., Lertora J.J., Kottilil S., Cramer Y. Safety, tolerability, and mechanisms of antiretroviral activity of pegylated interferon Alfa-2a in HIV-1-monoinfected participants: A phase II clinical trial. The Journal of Infectious Diseases. 2010;201:1686–1696. doi: 10.1086/652420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzoni L., Foulkes A.S., Papasavvas E., Mexas A.M., Lynn K.M., Mounzer K. Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. The Journal of Infectious Diseases. 2013;207:213–222. doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccala R., Welch M.J., Gonzalez-Quintial R., Walsh K.B., Teijaro J.R., Nguyen A. Type I interferon is a therapeutic target for virus-induced lethal vascular damage. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8925–8930. doi: 10.1073/pnas.1408148111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baize S., Marianneau P., Loth P., Reynard S., Journeaux A., Chevallier M. Early and strong immune responses are associated with control of viral replication and recovery in lassa virus-infected cynomolgus monkeys. Journal of Virology. 2009;83:5890–5903. doi: 10.1128/JVI.01948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton E.S., Lutzke M.L., Rochford R., Virgin H.W. Alpha/beta interferons regulate murine gammaherpesvirus latent gene expression and reactivation from latency. Journal of Virology. 2005;79:14149–14160. doi: 10.1128/JVI.79.22.14149-14160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boasso A., Hardy A.W., Anderson S.A., Dolan M.J., Shearer G.M. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PloS One. 2008;3:e2961. doi: 10.1371/journal.pone.0002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boasso A., Shearer G.M. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clinical Immunology. 2008;126:235–242. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosinger S.E., Li Q., Gordon S.N., Klatt N.R., Duan L., Xu L. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. The Journal of Clinical Investigation. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Barragan L., Zust R., Weber F., Spiegel M., Lang K.S., Akira S. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfant C.E., Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: Expanding roles in cell signaling. Journal of Cell Science. 2005;118:4605–4612. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host & Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciancanelli M.J., Huang S.X., Luthra P., Garner H., Itan Y., Volpi S. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348:448–453. doi: 10.1126/science.aaa1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilloniz C., Shinya K., Peng X., Korth M.J., Proll S.C., Aicher L.D. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathogens. 2009;5:e1000604. doi: 10.1371/journal.ppat.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coro E.S., Chang W.L., Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. Journal of Immunology. 2006;176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- Crouse J., Bedenikovic G., Wiesel M., Ibberson M., Xenarios I., Von Laer D. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity. 2014;40:961–973. doi: 10.1016/j.immuni.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Cunningham C.R., Champhekar A., Tullius M.V., Dillon B.J., Zhen A., de la Fuente J.R. Type I and type II interferon coordinately regulate suppressive dendritic cell fate and function during viral persistence. PLoS Pathogens. 2016;12:e1005356. doi: 10.1371/journal.ppat.1005356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger J.M., Valenzuela J.O., Agarwal P., Lins D., Mescher M.F. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. Journal of Immunology. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- Cyster J.G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annual Review of Immunology. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- Davidson S., Crotta S., McCabe T.M., Wack A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nature Communications. 2014;5:3864. doi: 10.1038/ncomms4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong M.D., Simmons C.P., Thanh T.T., Hien V.M., Smith G.J., Chau T.N. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nature Medicine. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerd N.A., Vivian J.P., Nguyen T.K., Mangan N.E., Gould J.A., Braniff S.J. Structural basis of a unique interferon-beta signaling axis mediated via the receptor IFNAR1. Nature Immunology. 2013;14:901–907. doi: 10.1038/ni.2667. [DOI] [PubMed] [Google Scholar]
- Deeks S.G., Walker B.D. Human immunodeficiency virus controllers: Mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- d'Ettorre G., Paiardini M., Ceccarelli G., Silvestri G., Vullo V. HIV-associated immune activation: From bench to bedside. AIDS Research and Human Retroviruses. 2011;27:355–364. doi: 10.1089/aid.2010.0342. [DOI] [PubMed] [Google Scholar]
- Devasthanam A.S. Mechanisms underlying the inhibition of interferon signaling by viruses. Virulence. 2014;5:270–277. doi: 10.4161/viru.27902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillmann C., Ringel C., Ringleb J., Mora J., Olesch C., Fink A.F. S1PR4 signaling attenuates ILT 7 internalization to limit IFN-alpha production by human plasmacytoid dendritic cells. Journal of Immunology. 2016;196:1579–1590. doi: 10.4049/jimmunol.1403168. [DOI] [PubMed] [Google Scholar]
- Durbin J.E., Fernandez-Sesma A., Lee C.K., Rao T.D., Frey A.B., Moran T.M. Type I IFN modulates innate and specific antiviral immunity. Journal of Immunology. 2000;164:4220–4228. doi: 10.4049/jimmunol.164.8.4220. [DOI] [PubMed] [Google Scholar]
- Dutia B.M., Allen D.J., Dyson H., Nash A.A. Type I interferons and IRF-1 play a critical role in the control of a gammaherpesvirus infection. Virology. 1999;261:173–179. doi: 10.1006/viro.1999.9834. [DOI] [PubMed] [Google Scholar]
- Dyavar Shetty R., Velu V., Titanji K., Bosinger S.E., Freeman G.J., Silvestri G. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. The Journal of Clinical Investigation. 2012;122:1712–1716. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K., Lang K.S., Manjarrez-Orduno N., Junt T., Senn B.M., Holdener M. Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. European Journal of Immunology. 2006;36:2094–2105. doi: 10.1002/eji.200635993. [DOI] [PubMed] [Google Scholar]
- Frieman M.B., Chen J., Morrison T.E., Whitmore A., Funkhouser W., Ward J.M. SARS-CoV pathogenesis is regulated by a STAT1 dependent but a type I, II and III interferon receptor independent mechanism. PLoS Pathogens. 2010;6:e1000849. doi: 10.1371/journal.ppat.1000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaigorta U., Chisari F.V. Hepatitis C virus blocks interferon effector function by inducing protein kinase R phosphorylation. Cell Host & Microbe. 2009;6:513–522. doi: 10.1016/j.chom.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A., Biron C.A. Type 1 interferons and the virus-host relationship: A lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A., Durbin R.K., Zheng H., Palese P., Gertner R., Levy D.E. The role of interferon in influenza virus tissue tropism. Journal of Virology. 1998;72:8550–8558. doi: 10.1128/jvi.72.11.8550-8558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A., Egorov A., Matassov D., Brandt S., Levy D.E., Durbin J.E. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- Guidotti L.G., Chisari F.V. Immunobiology and pathogenesis of viral hepatitis. Annual Review of Pathology. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- Hahm B., Trifilo M.J., Zuniga E.I., Oldstone M.B. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity. 2005;22:247–257. doi: 10.1016/j.immuni.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Hale B.G., Randall R.E., Ortin J., Jackson D. The multifunctional NS1 protein of influenza A viruses. The Journal of General Virology. 2008;89:2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- Hayden F.G., Fritz R., Lobo M.C., Alvord W., Strober W., Straus S.E. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. The Journal of clinical investigation. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim M.H. 25 years of interferon-based treatment of chronic hepatitis C: An epoch coming to an end. Nature Reviews. Immunology. 2013;13:535–542. doi: 10.1038/nri3463. [DOI] [PubMed] [Google Scholar]
- Hermesh T., Moltedo B., Moran T.M., Lopez C.B. Antiviral instruction of bone marrow leukocytes during respiratory viral infections. Cell Host & Microbe. 2010;7:343–353. doi: 10.1016/j.chom.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honke N., Shaabani N., Cadeddu G., Sorg U.R., Zhang D.E., Trilling M. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nature Immunology. 2012;13:51–57. doi: 10.1038/ni.2169. [DOI] [PubMed] [Google Scholar]
- Honke N., Shaabani N., Merches K., Gassa A., Kraft A., Ehrhardt K. Immunoactivation induced by chronic viral infection inhibits viral replication and drives immunosuppression through sustained IFN-I responses. European Journal of Immunology. 2016;46:372–380. doi: 10.1002/eji.201545765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C. An interferon-gamma-related cytokine storm in SARS patients. Journal of Medical Virology. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J.J., Greenwell-Wild T., Barrett L., Yang J., Lempicki R.A., Wahl S.M. Host gene expression changes correlating with anti-HIV-1 effects in human subjects after treatment with peginterferon Alfa-2a. The Journal of Infectious Diseases. 2012;205:1443–1447. doi: 10.1093/infdis/jis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I., Scott J.M., Kakarla T., Duriancik D.M., Choi S., Cho C. Activation mechanisms of natural killer cells during influenza virus infection. PloS One. 2012;7:e51858. doi: 10.1371/journal.pone.0051858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im D.S. Pharmacological tools for lysophospholipid GPCRs: Development of agonists and antagonists for LPA and S1P receptors. Acta Pharmacologica Sinica. 2010;31:1213–1222. doi: 10.1038/aps.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquelin B., Mayau V., Targat B., Liovat A.S., Kunkel D., Petitjean G. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. The Journal of Clinical Investigation. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao P., Tian G., Li Y., Deng G., Jiang Y., Liu C. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. Journal of Virology. 2008;82:1146–1154. doi: 10.1128/JVI.01698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D., Jones S.M., Shinya K., Kash J.C., Copps J., Ebihara H. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Kochs G., Garcia-Sastre A., Martinez-Sobrido L. Multiple anti-interferon actions of the influenza A virus NS1 protein. Journal of Virology. 2007;81:7011–7021. doi: 10.1128/JVI.02581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner I., Kochs G., Kalinke U., Weiss S., Staeheli P. Protective role of beta interferon in host defense against influenza A virus. Journal of Virology. 2007;81:2025–2030. doi: 10.1128/JVI.01718-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam G.A., Thomas S., Thompson L.J., Sprent J., Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. The Journal of Experimental Medicine. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gruta N.L., Kedzierska K., Stambas J., Doherty P.C. A question of self-preservation: Immunopathology in influenza virus infection. Immunology and Cell Biology. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- Le Bon A., Schiavoni G., D'Agostino G., Gresser I., Belardelli F., Tough D.F. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Park C.H., Jeong Y.H., Kim Y.J., Ha S.J. Negative regulation of type I IFN expression by OASL1 permits chronic viral infection and CD8(+) T-cell exhaustion. PLoS Pathogens. 2013;9:e1003478. doi: 10.1371/journal.ppat.1003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Dong C., Cooper M.D. Impairment of T and B cell development by treatment with a type I interferon. The Journal of Experimental Medicine. 1998;187:79–87. doi: 10.1084/jem.187.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P., Gresser I., Leary P., Tovey M. Enhanced expression of histocompatibility antigens of lymphoid cells in mice treated with interferon. The Journal of Infectious Diseases. 1976;133(Suppl.):A66–A68. doi: 10.1093/infdis/133.supplement_2.a66. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Gresser I., Leary P., Tovey M. Interferon treatment of mice: Enhanced expression of histocompatibility antigens on lymphoid cells. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:1284–1287. doi: 10.1073/pnas.73.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack E.A., Kallal L.E., Demers D.A., Biron C.A. Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection. mBio. 2011;2:1–10. doi: 10.1128/mBio.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manches O., Bhardwaj N. Resolution of immune activation defines nonpathogenic SIV infection. The Journal of Clinical Investigation. 2009;119:3512–3515. doi: 10.1172/JCI41509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D., Hahm B., Walsh K.B., Edelmann K.H., McGavern D., Hatta Y. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Huang X., Yang Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. Journal of Immunology. 2008;180:1592–1597. doi: 10.4049/jimmunol.180.3.1592. [DOI] [PubMed] [Google Scholar]
- Montoya M., Schiavoni G., Mattei F., Gresser I., Belardelli F., Borrow P. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- Moreno-Otero R. Therapeutic modalities in hepatitis C: Challenges and development. Journal of Viral Hepatitis. 2005;12:10–19. doi: 10.1111/j.1365-2893.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Muller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Nakayama Y., Plisch E.H., Sullivan J., Thomas C., Czuprynski C.J., Williams B.R. Role of PKR and Type I IFNs in viral control during primary and secondary infection. PLoS Pathogens. 2010;6:e1000966. doi: 10.1371/journal.ppat.1000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C.T., Mendoza J.L., Garcia K.C., Oldstone M.B. Alpha and beta type 1 interferon signaling: Passage for diverse biologic outcomes. Cell. 2016;164:349–352. doi: 10.1016/j.cell.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C.T., Sullivan B.M., Teijaro J.R., Lee A.M., Welch M., Rice S. Blockade of interferon Beta, but not interferon alpha, signaling controls persistent viral infection. Cell Host & Microbe. 2015;17:653–661. doi: 10.1016/j.chom.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K.B., Salazar-Mather T.P., Dalod M.Y., Van Deusen J.B., Wei X.Q., Liew F.Y. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. Journal of Immunology. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- Nice T.J., Osborne L.C., Tomov V.T., Artis D., Wherry E.J., Virgin H.W. Type I interferon receptor deficiency in dendritic cells facilitates systemic murine norovirus persistence despite enhanced adaptive immunity. PLoS Pathogens. 2016;12:e1005684. doi: 10.1371/journal.ppat.1005684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osokine I., Snell L.M., Cunningham C.R., Yamada D.H., Wilson E.B., Elsaesser H.J. Type I interferon suppresses de novo virus-specific CD4 Th1 immunity during an established persistent viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7409–7414. doi: 10.1073/pnas.1401662111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A.K., Daffis S., Brien J.D., Gainey M.D., Yokoyama W.M., Sheehan K.C. A temporal role of type I interferon signaling in CD8 + T cell maturation during acute West Nile virus infection. PLoS Pathogens. 2011;7:e1002407. doi: 10.1371/journal.ppat.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A.K., Ramos H.J., Wu X., Aggarwal S., Shrestha B., Gorman M. Deficient IFN signaling by myeloid cells leads to MAVS-dependent virus-induced sepsis. PLoS Pathogens. 2014;10:e1004086. doi: 10.1371/journal.ppat.1004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G.E., Gaszewska-Mastarlarz A., Moskophidis D. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. Journal of Virology. 2000;74:3996–4003. doi: 10.1128/jvi.74.9.3996-4003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtha W.E., Chachu K.A., Virgin H.W., Diamond M.S. Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling. Journal of Virology. 2008;82:10964–10974. doi: 10.1128/JVI.01646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaben M., Koerkamp M.J.A.G., Rottier P.J.M., de Haan C.A.M. Type I interferon receptor-independent and -dependent host transcriptional responses to mouse hepatitis coronavirus infection in vivo. BMC Genomics. 2009;10:350. doi: 10.1186/1471-2164-10-350. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau F.C., Dieter J., Luo Z., Priest S.O., Baumgarth N. B7-1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. Journal of Immunology. 2009;183:7661–7671. doi: 10.4049/jimmunol.0803783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J.P., Marshall H.D., Laidlaw B.J., Staron M.M., Kaech S.M., Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J., Proia R.L., Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nature Reviews. Immunology. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Cirion A., Pancino G., Sinet M., Venet A., Lambotte O., ANRS EP36 HIV Controllers Study Group HIV controllers: How do they tame the virus? Trends in Immunology. 2007;28:532–540. doi: 10.1016/j.it.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Samuel M.A., Diamond M.S. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. Journal of Virology. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler N.G., Bosinger S.E., Estes J.D., Zhu R.T., Tharp G.K., Boritz E. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin-Filipowicz M., Oakeley E.J., Duong F.H., Christen V., Terracciano L., Filipowicz W. Interferon signaling and treatment outcome in chronic hepatitis C. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7034–7039. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.U., Kwon H.J., Ko H.J., Byun Y.H., Seong B.L., Uematsu S. Type I interferon signaling regulates Ly6C(hi) monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathogens. 2011;7:e1001304. doi: 10.1371/journal.ppat.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla N., McGavern D.B., Teng C., Kunz S., Oldstone M.B. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. The Journal of Clinical Investigation. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan K.C., Lazear H.M., Diamond M.S., Schreiber R.D. Selective blockade of interferon-alpha and -beta reveals their non-redundant functions in a mouse model of West Nile virus infection. PloS One. 2015;10:e0128636. doi: 10.1371/journal.pone.0128636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow L.R. CD69 acts downstream of interferon-[alpha]/[beta] to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Koch M.A., Pepper M., Campbell D.J. Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. The Journal of Experimental Medicine. 2014;211:961–974. doi: 10.1084/jem.20131556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su A.I., Pezacki J.P., Wodicka L., Brideau A.D., Supekova L., Thimme R. Genomic analysis of the host response to hepatitis C virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Ng C., Lee A.M., Sullivan B.M., Sheehan K.C.F., Welch M. Persistent LCMV infection is controlled by blockade of type 1 interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Studer S., Leaf N., Kiosses W.B., Nguyen N., Matsuki K. S1PR1-mediated IFNAR1 degradation modulates plasmacytoid dendritic cell interferon-alpha autoamplification. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:1351–1356. doi: 10.1073/pnas.1525356113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Walsh K.B., Cahalan S., Fremgen D.M., Roberts E., Scott F. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Walsh K.B., Rice S., Rosen H., Oldstone M.B. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3799–3804. doi: 10.1073/pnas.1400593111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Moraga I., Levin D., Krutzik P.O., Podoplelova Y., Trejo A. Structural linkage between ligand discrimination and receptor activation by type I interferons. Cell. 2011;146:621–632. doi: 10.1016/j.cell.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz A., Langenmayer M., Jany S., Kalinke U., Sutter G. Rapid expansion of CD8 + T cells in wild-type and type I interferon receptor-deficient mice correlates with protection after low-dose emergency immunization with modified vaccinia virus Ankara. Journal of Virology. 2014;88:10946–10957. doi: 10.1128/JVI.00945-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K.B., Teijaro J.R., Wilker P.R., Jatzek A., Fremgen D.M., Das S.C. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12018–12023. doi: 10.1073/pnas.1107024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Swiecki M., Cella M., Alber G., Schreiber R.D., Gilfillan S. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host & Microbe. 2012;11:631–642. doi: 10.1016/j.chom.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R.M., Bahl K., Marshall H.D., Urban S.L. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathogens. 2012;8:e1002352. doi: 10.1371/journal.ppat.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S., Makowska Z., Campana B., Calabrese D., Dill M.T., Chung J. Simultaneous detection of hepatitis C virus and interferon stimulated gene expression in infected human liver. Hepatology. 2014;59:2121–2130. doi: 10.1002/hep.26770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E.B., Elsaesser D.H., Herskovitz J., Deng J., Cheng G., Aronow B. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.C., Grusdat M., Pandyra A.A., Polz R., Huang J., Sharma P. Type I interferon protects antiviral CD8 + T cells from NK cell cytotoxicity. Immunity. 2014;40:949–960. doi: 10.1016/j.immuni.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Yun N.E., Poussard A.L., Seregin A.V., Walker A.G., Smith J.K., Aronson J.F. Functional interferon system is required for clearance of lassa virus. Journal of Virology. 2012;86:3389–3392. doi: 10.1128/JVI.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Cerny A.M., Fitzgerald K.A., Kurt-Jones E.A., Finberg R.W. Role of interferon regulatory factor 7 in T cell responses during acute lymphocytic choriomeningitis virus infection. Journal of Virology. 2012;86:11254–11265. doi: 10.1128/JVI.00576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga E.I., McGavern D.B., Pruneda-Paz J.L., Teng C., Oldstone M.B. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nature Immunology. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]


