Abstract
Feline coronavirus (FCoV) is an etiological agent that causes a benign enteric illness and the fatal systemic disease feline infectious peritonitis (FIP). The FCoV spike (S) protein is considered the viral regulator for binding and entry to the cell. This protein is also involved in FCoV tropism and virulence, as well as in the switch from enteric disease to FIP. This regulation is carried out by spike's major functions: receptor binding and virus-cell membrane fusion. In this review, we address important aspects in FCoV genetics, replication and pathogenesis, focusing on the role of S. To better understand this, FCoV S protein models were constructed, based on the human coronavirus NL63 (HCoV-NL63) S structure. We describe the specific structural characteristics of the FCoV S, in comparison with other coronavirus spikes. We also revise the biochemical events needed for FCoV S activation and its relation to the structural features of the protein.
Keywords: Coronavirus, Feline coronavirus, Feline infectious peritonitis, Spike protein, Cleavage activation, Tropism, Pathogenesis, Serotype, Spike structure
1. Introduction and historical aspects
Feline coronavirus (FCoV) is the etiological agent of severe disease in domestic and wild felids, known as feline infectious peritonitis (FIP). The disease was first reported in the early 1960s by Holzworth, who included it in the manuscript: “Some important disorders of cats” (Holzworth, 1963). However the viral etiology was only suggested until 1966, when experimental infections of healthy animals were performed using tissues from infected cats (Wolfe and Griesemer, 1966). Viral etiology was finally confirmed in 1968 (Ward et al., 1968, Zook et al., 1968). Two different clinical forms, biotypes or pathotypes have been described for the clinical forms of FCoV (Kipar and Meli, 2014). The first, feline enteric coronavirus (FECV), is a characterized by a mild infection of the enteric tract and is considered ubiquitous in most healthy cats (Pedersen, 2009). The second, feline infectious peritonitis virus (FIPV), is considered the virulent pathotype of FCoV and, in contrast to FECV, the disease caused by FIPV is almost always lethal. However only a relatively small percentage of cats will develop FIP (Brown et al., 2009, Foley et al., 1997).
The virus was first isolated in 1976 using autochthonous peritoneal cells (Pedersen, 1976), then propagated in cell culture using Felis catus kidney cells (CRFK). This first isolated strain was named “TN-406” and later known as “FIPV I Black” (Black, 1980, Pedersen et al., 1981a). However, isolation and growing of FCoV has always been difficult, resulting in only few cell culture adapted strains available. Within those strains, WSU 79-1146 and WSU 79-1683 (later known as FECV II 79-1683 and FIPV II 79-1146, respectively) were reported in 1987 and since their isolation they have served as models for the study of FCoV in vitro (McKeirnan et al., 1987). Several studies have been carried out in an attempt to understand the evolution and emergence of FCoV (Chang et al., 2011, Pedersen et al., 1978, Ward, 1970). The first evidence that FCoV was related to other coronaviruses (CoVs) was reported by Ward in 1970. Using electron microscopy, he observed viral particles in tissues from animals infected with an FIP virus and described similarities between these particles and the previously reported human coronavirus 229E and mouse hepatitis virus (Ward, 1970). Following this finding, the relationship between FCoV and other animal coronaviruses (e.g. dogs and pigs) was reported (Pedersen et al., 1978), which served as a starting point for subsequent studies that addressed the close relation between some FCoV and canine coronavirus (CCoV) (Herrewegh et al., 1998). Nevertheless, emergence of FCoV during the last decades is not only related to the FCoV evolution alongside other CoVs, but also to the specific behavioral characteristics of the virus (i.e. switching from FECV to FIPV), and external factors as the increasing popularity of cats as pets, deriving from new and sometimes more intensive breeding methods that favor viral circulation in cat populations (Pedersen, 2009).
2. Taxonomy, morphology and genetics
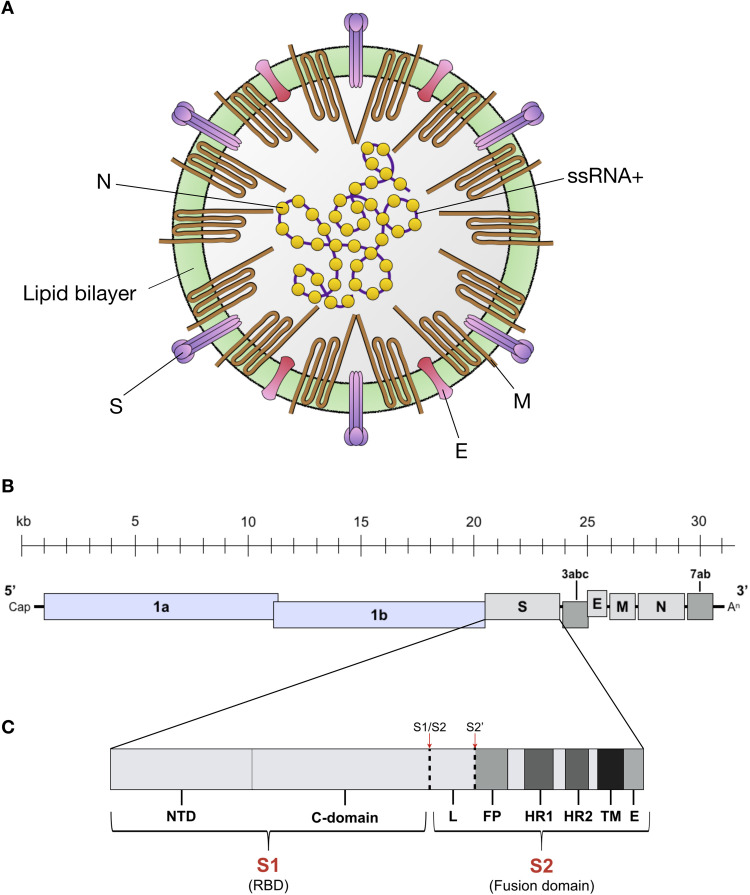
FCoV (known as Alphacoronavirus 1), belongs to the Alphacoronavirus genus (Order: Nidovirales, Subfamily: Coronavirinae, Family: Coronaviridae).1 Members of the Coronaviridae family are grouped in four genera, where Alphacoronavirus and Betacoronavirus include viruses that principally infect mammals, and are derived from the bat gene pool; whereas Gammacoronavirus and Deltacoronavirus group viruses that infect birds and mammals and are derived from the avian and pig gene pool ( Table 1) (Woo et al., 2012). The viral structure is composed by the nucleocapsid (containing the viral genome) and the outer envelope ( Fig. 1A). These two elements stabilize and protect the RNA genome of the virus (Masters and Perlman, 2013). FCoV virions are usually spherical with a moderate level of pleomorphism, with a size range between 80 and 120 nm and club-like surface projections or spikes about 12–24 nm that give the virus its crown-like appearance from where the coronavirus name is derived (Fig. 1A) (Barcena et al., 2009, Fehr and Perlman, 2015). Inside the outer envelope, a helically symmetrical nucleocapsid is found protecting the viral genome, a 5’ capped and 3’ poly-A tailed single-stranded positive sense RNA (ssRNA+) approximately ~ 29 kilobases (kb) in length (Kipar and Meli, 2014, Masters and Perlman, 2013). The FCoV genome has 11 open reading frames (ORFs) encoding four structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N), and seven non-structural proteins: the accessory proteins 3a, 3b, 3c, 7a and 7b, and the replicases 1a and 1b (Fig. 1B) (Dye and Siddell, 2005, Pedersen, 2009).
Table 1.
Genus and species from Coronavirinae subfamily.
| Genus | Species | Viruses |
|---|---|---|
| Alphacoronavirus | Alphacoronavirus 1 | Transmissible gastroenteritis virus (TEGV) |
| Feline coronavirus (FCoV) | ||
| Porcine epidemic diarrhea virus (PEDV) | ||
| Human coronavirus NL63 (HCoV-NL63) | ||
| Human coronavirus 229E (HCoV-229E) | ||
| Betacoronavirus | Betacoronavirus 1 | Bovine coronavirus (BCoV) |
| Human coronavirus OC43 (HCoV-OC43) | ||
| Middle East respiratory syndrome-related coronavirus (MERS-CoV) | ||
| Murine coronavirus | Mouse hepatitis virus (MHV) | |
| Severe acute respiratory syndrome-related coronavirus (SARS-CoV) | ||
| Human coronavirus HKU1 (HCoV-HKU1) | ||
| Gammacoronavirus | Avian coronavirus | Infectious bronchitis virus (IBV) |
| Turkey coronavirus | ||
| Beluga whale coronavirus SW1 | ||
| Deltacoronavirus | Coronavirus HKU15 (also known as porcine coronavirus PCoV-HKU15) | |
Most representative species of Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus.
Fig. 1.
FCoV structure and genome. (A) FCoV structure and proteins. Spike (S), matrix (M), envelope (E) and nucleocapsid (N). Adapted from: Kipar and Meli, 2013. (B) FCoV ssRNA+ is about ~ 29 kilobases (kb) long and has 11 ORF encoding 7 non-structural proteins (Replicase proteins 1a and 1b, and accessory proteins 3a, 3b, 3c, 7a and 7b); and 4 structural proteins (S, M, E, and N). Adapted from: Masters and Pearlman, 2013. (C) S gene diagram. FCoV S is composed by two subunits: S1 (receptor binding domain – RBD) and S2 (fusion domain). The S1 subunit is divide in two functional domains: N-terminal domain (NTD) and C-terminal domain (C-domain or CTD). The S2 subunit is composed by the fusion peptide (FP), two heptad repeats (HR1 and HR2), a transmembrane domain (TM) and an endodomain (E). The two S activation sites S1/S2 and S2’ are indicated (red arrows and dashed lines), as well as the linker (L) region between S1/S2 and S2’. Adapted from: Millet and Whittaker (2015).
The viral helical nucleocapsid is composed of multiple copies of the RNA binding protein N, a 50 kDa protein which is composed of two domains (NTD and CTD) both with the same affinity to bind RNA, but through different mechanisms. However, both domains are necessary for N binding to the viral RNA (Chang et al., 2006, Hurst et al., 2009). In fact, binding to the viral RNA instead of non-viral RNA is suggested to be enhanced by phosphorylation of the N protein, which induces the specific structural conformation to accomplish this function (Fehr and Perlman, 2015). The most important function of N is to bind and protect the viral genomic RNA (Fig. 1A); however, unlike other viral nucleocapsid proteins (e.g. rhabdoviruses and paramyxoviruses), FCoV (and most coronaviruses) N protein is inefficient at protecting the RNA from ribonuclease activity (Masters and Perlman, 2013, Olsen, 1993). In addition, N protein has been shown to induce cell-mediated immunity, which suggests a possible role in vaccine response studies (Hohdatsu et al., 2003). The mechanism through N can induce immunity is not known, but evidence of interaction between N and M proteins (as well as between N and the nsp3 component of the replicase complex) has been described, and could be related to this feature (Hurst et al., 2013, Sturman et al., 1980).
The FCoV envelope is composed of four main elements: a lipid bilayer derived from the host cell endoplasmic reticulum – Golgi intermediate compartment (ERGIC), and viral proteins E, M and S (Fig. 1A). M is the most abundant structural protein in the virus (Masters and Perlman, 2013). This medium-sized (about ~ 25 to 30 kDa) N-linked glycosylated protein is randomly distributed along the viral envelope, anchored through three transmembrane domains (Armstrong et al., 1984). M has an extensive C-terminal endodomain; and a small ectodomain (about 10% of the N-terminal portion) which makes it less antigenic despite its high abundance (Neuman et al., 2011, Olsen, 1993). The M protein is translated and inserted into endoplasmic reticulum (ER) for viral assembly, but no specific signaling for ER retention has been found in the protein (Fehr and Perlman, 2015). Nevertheless, the M protein interaction with the nucleocapsid, as well as its function in viral membrane remodeling during CoV assembly at the ERGIC, has been described (Neuman et al., 2011).
The small E protein (~ 8 to 12 kDa) is a type III membrane protein and is also inserted in the viral envelope but is present at a much lesser extent than M or S (Masters and Perlman, 2013). Both E protein C- terminal endodomain and N-terminal ectodomain have been described to have ion channel activity (Fehr and Perlman, 2015, Pervushin et al., 2009). While the function of CoVs E protein (including FCoV) has been associated with assembly at the ERGIC compartment (Corse and Machamer, 2002, Kipar and Meli, 2014), several studies have shown that unlike other CoV structural proteins, viruses with deletions or inactivation of the E protein are less virulent, raising the question of the importance of this protein in viral fitness (Pervushin et al., 2009). However, this specific feature is virus type dependent (DeDiego et al., 2007). The role of the E protein in FCoV replication and pathogenesis has not been studied yet. However, in a study performed in the CoV severe acute respiratory syndrome (SARS-CoV), the authors demonstrated that E ion exchange function in the viral membrane is relevant for viral pathogenesis, as mice infected with mutated or knocked down E protein presented less clinical signs and recovered after infection, while the ones infected with wild type virus did not survive. Nevertheless, the defective E protein did not affect the viral replication (Nieto-Torres et al., 2014).
Among the viral structural proteins, S could be considered the most important in terms of FCoV pathogenesis. The coronavirus S protein is a class I viral fusion protein and is considered the major viral regulator in host cell entry (Bosch et al., 2003, White et al., 2008). Viral fusion proteins are grouped in three different classes according to their structure and biochemical activation processes, where class I proteins are characterized by predominant α-helical secondary structures and a trimeric organization of their pre-fusion and post-fusion state (Harrison, 2013, White et al., 2008). One interesting aspect about class I fusion proteins is the differences in activation of their fusion mechanisms, despite their conserved structure (Millet and Whittaker, 2015, White et al., 2008). However, all fusion proteins in this class undergo major structural changes that allow the viral fusion peptide to contact and anchor into the target cell membrane, and the formation of the “trimer of hairpins” structure followed by the fusion of the outer membranes (hemifusion) and the formation of the fusion pore (White et al., 2008). To successfully induce fusion, a proteolytic activation of the viral protein subunits is often necessary, and this can vary significantly between different fusion proteins. Specific aspects about FCoV S function and structure will be addressed in detail later in this review.
FCoV non-structural proteins are encoded by the ORFs 1ab, 3abc and 7ab. The replicase complex (encoded by the ORF1ab) is translated through a ribosome frameshifting mechanism, resulting in the generation of polyproteins that are later processed by virally-encoded proteases into 16 active subunits (Brierley et al., 1989, Enjuanes et al., 2006, Kipar and Meli, 2014). Together, the 16 subunits of the replicase complex, carry out the viral genome replication as well as generate the template for the transcription of the structural and accessory proteins (Enjuanes et al., 2006, Masters and Perlman, 2013). The specific functions for the 3abc accessory proteins are not completely understood. Nevertheless, the 3c proteins appears to be necessary for viral replication and several studies have related it to changes in FCoV virulence and tropism (Chang et al., 2010, Pedersen et al., 2009). Finally, the 7ab accessory proteins function is not fully understood, but they are reported to play a role in the immune response to FCoV infection (Dedeurwaerder et al., 2013). The 7a protein is a type I interferon (INF) antagonist and is suggested to protect the virus interfering with the INF response (Dedeurwaerder et al., 2014). On the other hand, the 7b glycoprotein has been reported to induce antibody responses in naturally infected cats (Kennedy et al., 2008).
3. Serotype and biotype
Antigenically, FCoV viruses can be classified in two groups or serotypes: I and II. This antigenic classification is based on differences of the FCoV S protein amino acid sequence and antibody neutralization (Kipar and Meli, 2014, Lewis et al., 2015, Shiba et al., 2007). The S protein antigenic differences are derived from the genetic origin of each serotype. The serotype I is the original and predominant type, with an S protein entirely derived from FCoV. Viral strains from this serotype are clinically more common and subsequently display higher epidemiological importance (Benetka et al., 2004, Pedersen, 2009). However, a prevalence of 30% of FCoV serotype II was reported in Japan in the early 1990s, and also coinfections FCoV I-II have been reported (An et al., 2011, Hohdatsu et al., 1992). Isolation and cell culture adaptation of serotype I strains, is considered to be difficult (Lewis et al., 2015). In fact, most of the available cell culture adapted strains correspond to the serotype II, which complicates the in vitro study of the clinically important serotype I. The cell culture adaptation of FCoV is suggested to be entirely related with the S protein, as recombinant FCoV I viruses carrying a FCoV II S protein displays faster growing in cell culture, as well as an expansion in the receptor usage (Tekes et al., 2010).
Serotype II viruses are less common and in contrast to serotype I viruses, the genetic origin is derived from recombination events between at least two different viruses, which is an event that has been reported in other CoVs both in vitro in cell culture and in vivo under normal field conditions (Herrewegh et al., 1998). Specifically, FCoV serotype II viruses resulted from a double recombination between FCoV and CCoV, and while there is still some debate about the where in the viral genome the recombination events happened, bioinformatics analyses along with antibody neutralization assays, have shown a higher homology between FCoV II and CCoV S proteins (Kipar and Meli, 2014). Amino acid sequence homology between FCoV II S protein and CCoV or also transmissible gastroenteritis virus (TEGV) S proteins is significantly higher (about 91% between the FCoV II strain 79-1146 and CCoV, or 81% with TEGV), compared with the homology between FCoV II and FCoV I S proteins (about 46% between FCoV II strain 79-1146 and FCoV I strain TN406) (Herrewegh et al., 1998, Wesseling et al., 1994).
As well as showing distinct serological differences, serotype I and serotype II FCoVs have recently been proposed to have quite distinct taxonomical features, in line with their distinct biological properties. Within the Alphacoronavirus 1 species, two clades have been proposed as an improved way to classify the Alphacoronavirus genus; clade A viruses encompassing serotype I FCoV and CCoV and clade B viruses including serotype II FCoV and CCoV and TGEV-like viruses (Whittaker et al., 2018).
Both FCoV serotypes (I and II), can occur in two antigenically and morphologically undistinguishable biotypes (or pathotypes): FECV and FIPV. The ubiquitous and usually asymptomatic FECV is considered to be globally distributed and its prevalence in feral cats (as well as in cats from breeding facilities) can reach 90% (Hohdatsu et al., 1992, Pedersen et al., 1984, Pedersen et al., 2004, Shiba et al., 2007). The FECV biotype was previously described to only replicate in the intestinal epithelium, considering it an “enteric exclusive” virus and only differentiating from the “systemic” (monocyte/macrophage targeting) FIPV biotype in this aspect (Kipar and Meli, 2014, Porter et al., 2014). However, recent evidence has demonstrated that FECV can also replicate in monocytes, challenging the previous concepts about FECV exclusive tropism (Kipar et al., 2010). In fact, current studies performed from our laboratory, have shown the presence of viral RNA in blood samples from both FIP symptomatic cats and healthy housemates; in fact, viral RNA can also be detected in fecal samples from viremic animals with or without clinical signs (data not published). These results have been also reported by other groups, supporting the hypothesis that FECV is a precursor form of FIPV (Gunn-Moore et al., 1998, Meli et al., 2004).
Contrary to the asymptomatic FECV, the FIPV biotype is considered highly virulent and leads to the severe and always-lethal disease FIP (Licitra et al., 2013, Pedersen et al., 1984). While there is no definitive evidence of what makes FIPV virulent compared to FECV, there are several hypotheses about this topic, and most commonly accepted is related to the specific FIPV tropism change to monocytes and macrophages (Pedersen, 2009, Pedersen et al., 1984, Pedersen et al., 1981b). The switch in the tropism from localized cells (enteric epithelium for the FECV biotype) to systemically distributed cells (monocytes and macrophages), is considered the major reason behind FIPV virulence. However, the finding of FCoV RNA in blood samples from healthy animals, argues against the tropism switch as the solely cause of FIPV virulence (Kipar et al., 2001, Meli et al., 2004). Changes in the viral tropism are the consequence of interactions between the virus and the host cells, where the presence or absence of receptors, co-receptors, biochemical conditions (i.e. proteases, ions, pH) or even access to the susceptible cell can be determinants for these changes. However, despite several years of investigation trying to understand the virulence switch between FECV and FIPV, the specific mechanisms behind it remain to be uncovered.
It is generally accepted by the scientific community that FIPV occurrence is the consequence of mutations in the virus in currently FECV infected cats, and that transmission of FIPV between animals appears to be unlikely. However, FIP “outbreaks” have been documented (Pedersen et al., 1981b). The mutation theory suggests that asymptomatic FECV infected cats will be exposed to repeated cycles of infection-recovery-reinfection with the virus, and mutations of the FECV inside the animal will allow the virus to expand its virulence and tropism, becoming an FIPV, but losing its transmissibility (Pedersen, 2009, Pedersen et al., 1981b, Poland et al., 1996). Several viral genes have been proposed to carry those mutations (e.g. S, 3abc and 7ab genes) (Chang et al., 2010, Chang et al., 2012, Chang et al., 2011, Herrewegh et al., 1995, Kennedy et al., 2001, Licitra et al., 2013, Pedersen et al., 2012, Vennema et al., 1998). The S protein is considered the major regulator of FCoV infection to the host cell. This protein possesses the receptor binding domain (RBD) to attach the cellular receptor, but also the fusion peptide (FP) to induce fusion between virus and cellular membranes during viral entry (Belouzard et al., 2012, Regan et al., 2008).
Mutations in the FCoV S gene have been related to the FECV-FIPV switch, specifically in the region encoding the so called S1/S2 cleavage site (which is only present in FCoV serotype I viruses). In a study by Licitra et al., the authors found that FECVs possess a S1/S2 sequence that is likely to be cleaved by furin-like proteases, while FIPVs possess a mutated S1/S2 sequence that is suggested to be activated by other proteases, suggesting a relation between FCoV S protease activation requirements and biotype switch (Licitra et al., 2013).
A second S cleavage site, S2’ (present in both FCoV serotypes), has been also suggested to participate in FCoV pathogenesis, but its relation with its virulence and tropism is still unclear (Millet and Whittaker, 2015). Nevertheless, a study in 2008 with FCoV serotype II viruses (which only possesses the S2’ cleavage site), showed differences in S proteolytic requirements between FECV and FIPV. In this study, the FCoV serotype II strains FECV II 79-1683 and FIPV II 79-1146 were unable to grow in presence of an inhibitor of the cysteine protease cathepsin B, while only FECV II 1683 was unable to grow in presence of a cathepsin L inhibitor. Later the authors showed that both virus S proteins were cleaved by cathepsin B, but only FECV II 79-1683 S protein was cleaved by cathepsin L, suggesting that specific FCoV S activation requirements are related with changes in biotype (Regan et al., 2008). Previous and current studies in our laboratory have found specific mutations in S2’ cleavage site in clinical samples from healthy and FIP cats (Licitra et al., 2014). Our recent results suggest these mutations to be likely related with FECV-FIPV switch in both FCoV serotype I and II (data not published). Similar to FCoV, other CoVs have been reported to have specific sequences at the S2' cleavage site, and mutations on it can lead to changes in protease activation requirements, and possibly tropism (Belouzard et al., 2009, de Haan et al., 2008, Millet and Whittaker, 2014). For instance, mutations in infectious bronchitis virus (IBV) S2' site have been suggested to play a role in the cell culture adaptation and virulence attenuation of the strain Beaudette; which unlike other IBV strains possesses a unique S2' site that is likely to be cleaved by furin-like proteases (Millet and Whittaker, 2015).
In addition to the mutations at S1/S2 and S2’ cleavage site regions, other mutations located close to the FP region have been also reported to be specific to FECVs or FIPVs. In a study from Chang et al., the authors analyzed FECV and FIPV sequences from healthy and clinically sick cats from the Netherlands, and were able to identify a single amino acid mutation Met-Leu mapped to position 1058 within S2, that allow them to almost perfectly differentiate (> 95%) between FECV and FIPV sequences (Chang et al., 2012). Evidence of the functional consequences of this mutation and the specific relation of this mutation with the FECV-FIPV switch were not explored by the authors. However, a later study re-interpreted these results and found that the mutation Met-Leu was consistent with an enteric or systemic FCoV infection, respectively (Porter et al., 2014). According to the authors, a Met in the amino acid position 1058 of the FCoV spike, was detected in fecal samples from FIP cats (77%) and non-FIP cats (100%). In contrast, FCoV detected in tissue samples from FIP cats (91%) and non-FIP cats (89%), present a Leu in this amino acid position. Nevertheless, recent studies have suggested that mutation analysis of the FCoV S protein are not completely reliable for the differentiation of FIP from FCoV positive samples, so further studies are needed in this topic (Barker et al., 2017).
Mutations in the 3abc and 7ab ORFs have been also related with FCoV biotype switch, and from those, one of the genes that has been most studied is the 3c gene. Several authors have suggested that truncations of the 3c gene are determinants for the FECV-FIPV switch (Chang et al., 2010, Chang et al., 2011, Pedersen et al., 2009, Pedersen et al., 2012, Vennema et al., 1998). However, the results are not completely consistent and sometimes contradictory. Initial sequencing studies found that truncation or deletion of the 3c gene frequently correlated with the FIPV biotype, while an intact (non truncated) 3c gene correlated with FECVs (Vennema et al., 1998). A following study showed that FCoVs detected in tissues from sick cats always had a mutated (truncated or deleted) 3c gene, suspecting them to be FIPVs, while the FCoVs from healthy animals showed an intact 3c protein, supporting the previous results (Pedersen et al., 2009). However, more recent studies showed that while most of the FECVs (from healthy animals) carried an intact 3c gene and most of the FIPVs (from infected tissues) carried a mutated 3c, some tissue-associated viruses carried an intact 3c gene, suggesting that 3c mutations are not always characteristic of FIPVs (Chang et al., 2010, Pedersen et al., 2012). The relation between 7ab proteins and FCoV biotype was also suggested in the past. Early studies indicated that deletions of the 7ab genes were characteristic of FIPVs (Kennedy et al., 2001, Vennema et al., 1998). Nonetheless, FIPVs that have lost the 7a, 7b or 7ab genes showed to keep their virulence in cell culture, but displayed reduced virulence in experimental infections (Dedeurwaerder et al., 2013, Haijema et al., 2004).
4. Replication
FCoV replication starts with the attachment of the virion to the host cell membrane ( Fig. 2) (Cham et al., 2017). The interaction between the virus and the cell is first accomplished by binding the viral S protein to the receptor in the cellular membrane. This interaction is key to the determination of viral tropism and host range (Balint et al., 2012, Cham et al., 2017, Lai, 1990). Receptor usage by CoVs has been studied for several years and protein and glycan cellular receptors have been reported for different CoVs ( Table 2). Several Alphacoronaviruses have been reported to use the aminopeptidase N (APN, also known as CD13), as their cellular receptor (Table 2). APN is a cell surface glycoprotein (about 150 kDa), expressed by granulocyte-monocyte lineage progenitors, as well as by fibroblasts, neurons, renal, respiratory and digestive epithelial cells, among others (Hohdatsu et al., 1998, Look et al., 1989). APN functions are variable and usually related with the type of cell where is being expressed, but an enzymatic metalloprotease activity is commonly observed (Tresnan et al., 1996). For instance, APN proteins in the intestine are suggested to participate in the maturation of small proteins, as well as helping the peptide absorption at the enterocyte membrane (Hohdatsu et al., 1998). As with other Alphacoronavirus, it has been demonstrated that FCoV S protein from serotype II but not serotype I viruses, binds APN (specifically the feline form - fAPN) to use it as a receptor for entry in vitro (Dye et al., 2007, Hohdatsu et al., 1998, Van Hamme et al., 2011). In fact, recombinant FCoV serotype I viruses encoding FCoV II S protein, have been shown to infect cells constitutively or engineered expressing fAPN receptors, while wild type FCoV I viruses failed to infect these cells (Rossen et al., 2001, Tekes et al., 2010). To date, the role of fAPN for FCoV II cell entry in live animals, as well as the specific cellular receptor for FCoV serotype I, remain unclear.
Fig. 2.
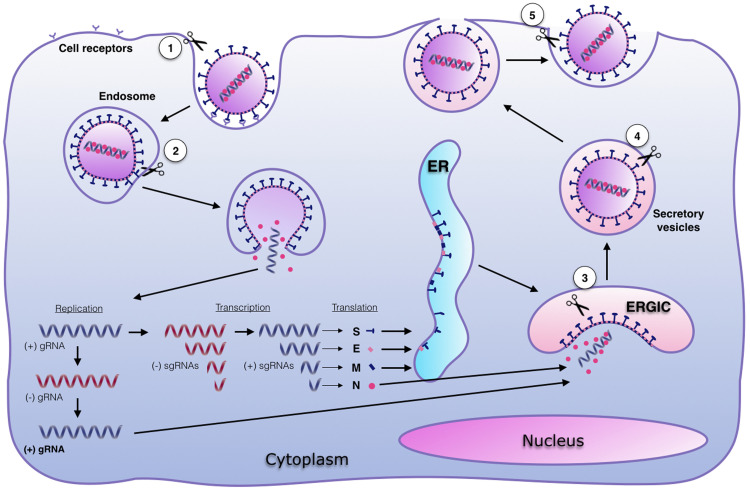
FCoV replication cycle and sites for S activation. Replication cycle starts with viral binding to the cellular receptor. The virus is endocyted and viral-cell fusion allows the delivery of the (+) genomic RNA (gRNA) to the cytoplasm, to initiate the genome replication and the protein synthesis. Sub-genomic RNAs (sgRNAs) are transcribed and translated. Structural proteins S, E and M are folded and post-translationally modified at the ER. Viral assembly takes place in the ERGIC and viruses are released through exocytosis. Activation of S proteins (scissors) can take place at: 1. The cell membrane during viral attachment (S1/S2 site); 2. The endosome to induce viral-cell fusion (S2’ site); 3. The trans-Golgi network during viral assembly (S1/S2 site); 4. The secretory vesicle during viral egress (S1/S2 site); and 5. The cell membrane during viral release (S1/S2 site). Adapted from: Millet and Whittaker (2015).
Table 2.
Cellular receptors for CoV.
| Genus | Virus | Receptor |
|---|---|---|
| Alphacoronavirus | TGEV | pAPN |
| FCoV II | fAPN | |
| PEDV | pAPN | |
| HCoV-NL63 | ACE2 | |
| HCoV-229E | hAPN | |
| Betacoronavirus | BCoV | 9-O-acetylated neuramininc acid |
| HCoV-OC43 | 9-O-acetylated neuramininc acid | |
| MERS-CoV | DPP4 | |
| MHV | mCEACAM1 | |
| SARS-CoV | ACE2 | |
| Gammacoronavirus | IBV | α-2,3-linked sialic acid |
| IBV Beaudette | HS |
Viruses: Transmissible gastroenteritis virus (TEGV), porcine endemic diarrhea virus (PEDV), feline coronavirus serotype II (FCoV II), human coronavirus NL63 (HCoV-NL63), human coronavirus 229E (HCoV-229E), severe acute respiratory syndrome-coronavirus (SARS-CoV), bovine coronavirus (BCoV), Middle East respiratory syndrome coronavirus (MERS-CoV), mouse hepatitis virus (MHV), infectious bronchitis virus (IBV). Receptors: Porcine aminopeptidase N (pAPN), feline aminopeptidase N (fAPN), human aminopeptidase N (hAPN), angiotensin-converting enzyme (ACE2), dipeptidyl peptidase 4 (DPP4), murine carcinoembryonic antigen-related adhesion molecule 1 (mCEACAM1), heparan sulfate (HS). Revised in Belouzard et al. (2012)).
In addition to fAPN, the role of C-type lectins as mediators for CoVs entry into the cell has been reported. It is known that several viruses including: human immunodeficiency virus type 1 (HIV-1) and CoVs (i.e. SARS-CoV, HCoV-229E, HCoV-NL63), utilize C-type lectins as entry factors, in addition to the specific receptor (Regan and Whittaker, 2008). Indeed, FCoVs can also use C-type lectins as co-factors for cell entry, and specifically, the dendritic cell (DC)-specific intercellular adhesion molecule (ICAM) grabbing non-integrin (DC-SIGN) is reported to facilitate the FCoV II infection. However, DC-SIGN enhances FCoV II replication only in fAPN expressing cells, suggesting that its presence is not sufficient to completely mediate the viral entry (Regan and Whittaker, 2008, Van Hamme et al., 2011). The specific mechanisms by which FCoV interacts with DC-SIGN, and if the S protein participates in those interactions, is still unknown. Some CoVs possess membrane proteins in addition to S, that participate as collaborators for virus-cell binding. The hemagglutinin esterase (HE) is a structural protein found in the viral envelope of a subset of Betacoronaviruses, with two specific functions: bind to sialic acid moieties found on cell surface glycoproteins and glycolipids (hemagglutinin function), and acetylesterase activity over 9-O- or 4-O-acetylated sialic acids (Wurzer et al., 2002). These characteristics are considered to assist the viral binding to the host cell, as a cofactor for S protein in cell host entry (Vlasak et al., 1988). However, no accessory FCoV proteins are known to facilitate viral entry into the cell.
A receptor-independent mechanism called antibody-dependent enhancement (ADE) is well recognized for FCoV (specifically FIPV biotype) entry into the cell. This mechanism takes advantage of the host immune response to allow the virus to infect monocytes and macrophages (Corapi et al., 1992). In brief, ADE occurs when FIPVs bound to anti-FIPV-S antibodies infect monocytes/macrophages using the Fc-binding portion of Fc receptors in the cell as receptors for viral entry. This mechanism has been reported to promote FIPV II entry in absence of a fAPN receptor in vitro, but also for FIPV I in vivo (Takano et al., 2008a, Takano et al., 2008b).
Once FCoV is bound to the cellular receptor, the virus needs to access the cellular cytoplasm to continue its replication cycle. FCoV entry into the host cell occurs when the receptor-bound virus is endocytosed by the cell and fusion between viral and cellular membrane is induced (Fig. 2) (Burkard et al., 2014). Viral-cell membrane fusion is mediated by FCoV S protein, through the fusion peptide located in the second domain (S2) of the protein. In addition to receptor binding, two biochemical events are thought to be necessary to successfully induce membrane fusion at the endocytic vesicle: a protease activation or cleavage of FCoV S, and a drop of the endosomal pH (Fehr and Perlman, 2015, Millet and Whittaker, 2015, Regan et al., 2008). Protease activation of S is a complex process and it is suggested that each CoV has its own proteolytic requirements. Furin-like proteases are a group of enzymes that have been reported to activate several CoVs proteins, including: SARS-CoV, Middle East respiratory syndrome-CoV (MERS-CoV), and IBV strain Beaudette, among others. Along with furin, other proteases (i.e. cathepsins, trypsin, transmembrane protease/serine (TMPRSS), elastase, plasmin) have been shown to participate in CoV S activation (Millet and Whittaker, 2015).
FCoV S requires proteolytic activation at least at one specific site in the protein, to induce the membrane fusion. The necessity of more than one proteolytic activation event in FCoV S is directly related to the serotype of the virus; FCoV I viruses are reported to have two specific activation sites: S1/S2 and S2’, while FCoV II viruses are reported to have only the S2’ site (Fig. 1C) (Licitra et al., 2013, Millet and Whittaker, 2015). Nonetheless, not all the proteolytic activation of FCoV S occurs at the endosome (Fig. 2). In fact, the first event at the S1/S2 cleavage site is suggested to happen before the viral attachment to the cell, likely during S maturation during FCoV assembly and release (Millet and Whittaker, 2015). As mentioned previously, furin-like proteases are suggested to activate FCoV S at the S1/S2 site, while cathepsins activate the S2’ for the endosomal route of entry (Licitra et al., 2013, Regan et al., 2008). However, other proteases can also be playing a role in this process and this is currently an active area of research. The second necessary event to induce the FCoV-cell membrane fusion is a drop in the endosomal pH. This pH change is reported to be necessary for protease activity, but may also collaborate with the unfolding of S after activation (Regan et al., 2008, White and Whittaker, 2016). However, not all CoVs are equally dependent on a pH drop to induce fusion, as was demonstrated in FCoV, where FIPV II 79-1146 fusion showed to be consistently less dependent on low pH, when compared with FECV II 79-1683 in vitro (Regan et al., 2008). Combined, the two biochemical events (activation and pH drop) aim for the exposing of the S fusion peptide, which is the official regulator of the fusion process. It was recently shown for SARS-CoV that additional ionic factors (ie, Ca2+ ions) are also driving forces in S-mediated fusion, but a role of Ca2+ ions for FCoV is currently unknown. A more detailed explanation of the virus-cell membrane fusion is presented in the next section of this review.
As a result of the membrane fusion, the FCoV nucleocapsid releases the viral genome (viral uncoating) into the cytoplasm where the viral genome replication and the protein synthesis is carried out (Fig. 2). Following the release of the genome, FCoV needs to synthetize the replicase proteins to initiate the genome replication (Masters and Perlman, 2013). The replicase genes are encoded by the ORFs rep1a and rep1b, which are translated immediately after the uncoating through a ribosome frameshifting. Two polyproteins pp1a and pp1ab are synthetized and then cleaved to 11 and 16 nonstructural proteins, respectively. Once processed, the nonstructural proteins assemble what is called the replicase-transcriptase complex, creating the conditions for the RNA replication and subsequent transcription of the structural proteins (Fehr and Perlman, 2015). The replication machinery in FCoV, including a RNA-dependent RNA polymerase (RdRp), is also encoded by ORFs 1a and 1ab (Masters and Perlman, 2013). After synthesis and processing of replicase proteins, the viral ssRNA+ genome is copied into a ssRNA- intermediate molecule, from which the remaining structural and accessory genes are transcribed in sub-genomic RNAs (sgRNAs) (Fig. 2) (Enjuanes et al., 2006, Masters and Perlman, 2013).
Maturation of the FCoV S, M and E structural proteins occurs at the ER in the cytoplasm. There, the proteins are inserted and maturation processes as glycosylation occurs during the transport of the viral proteins through the secretory pathway into the ERGIC (Krijnse-Locker et al., 1994). It has been reported that activation events in the S protein can also occur during the viral steps at the ERGIC, as well as during viral release. In fact, furin-like proteins are usually associated with this cellular compartment, making possible the S1/S2 cleavage at this stage in the viral replication (Belouzard et al., 2012, Millet and Whittaker, 2015). In a parallel process at the same compartment, N proteins bind to the recently synthetized genomic ssRNA+ molecules, to be enclosed into the mature virions. Assembly of the new viral particles is a process that is mainly regulated by the M protein, through the interaction with N at the ERGIC. These interactions, promote the completion of the virion assemble (Fehr and Perlman, 2015).
Finally, once the virions are completely assembled, the new viruses are transported to the cell surface through secretory vesicles and released in an endosome-cell membrane fusion process that is not regulated by the virus (Masters and Perlman, 2013). FCoV is reported to be released to the basolateral portion of the cell membrane (Rossen et al., 2001, Takano et al., 2008a). As a consequence of this process, fusion between cellular membranes occurs, inducing the so-called syncytia, a particular characteristic of the FCoV infection (Pedersen, 2009).
5. S protein structure, function and modeling analysis
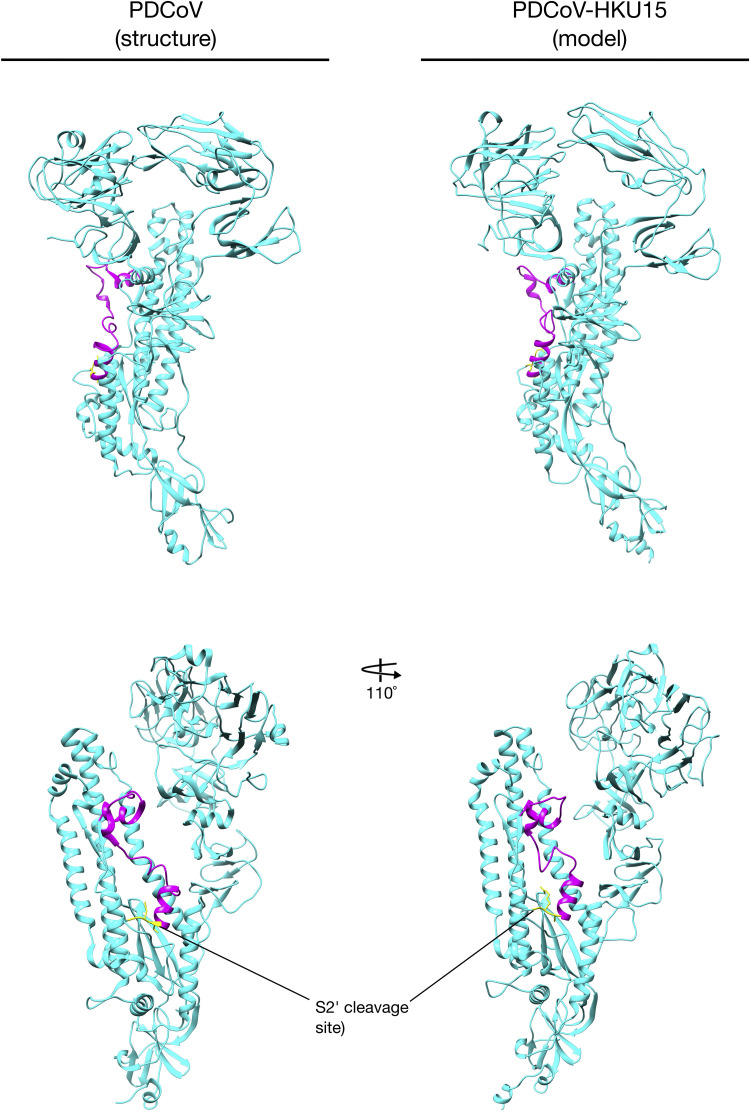
As mentioned before, FCoV S is the major viral mediator for entry into the host cell. Its function can be divided in two processes: 1. Binding to the cellular receptor, and 2. Inducing the fusion between the viral and the cellular membranes (Belouzard et al., 2012). The FCoV spike is a type I transmembrane protein (180–220 kDa, ∼ 1460 amino acids) and is encoded by the S gene (Fig. 1C). It is highly glycosylated containing ~ 35 N-glycosylation sites, and assembles as trimers on the surface of the virion with a club-shaped conformation (Belouzard et al., 2012, Millet and Whittaker, 2015). The almost complete cryo-electron microscopy (cryo-EM) structure of several CoV S proteins has been recently reported, including: Mouse hepatitis virus (MHV), human coronavirus HKU1 (HCoV-HKU1), human coronavirus NL63 (HCoV-NL63), SARS-CoV, MERS-CoV, porcine deltacoronavirus (PDCoV) (Gui et al., 2017, Kirchdoerfer et al., 2016, Shang et al., 2017, Walls et al., 2016a, Walls et al., 2016b, Xiong et al., 2017, Yuan et al., 2017). Unfortunately, no FCoV S structure has been solved yet, limiting the understanding of the specific structural aspects of this protein. However, protein modeling can be very useful to better study the FCoV S protein. Taking advantage of this tool, we constructed S protein models for the Alphacoronavirus: FCoV, strains: FIPV I Black (Susan Baker, Loyola University Chicago, personal communication), FECV II 79-1683 (GenBank # AFH58021.1) and FIPV II 79-1146 (GenBank # AAY32596.1) and Gammacoronavirus: IBV strain Beaudette (GenBank # AJ311362.1) using Chimera software (UCSF Chimera v. 1.11.2, University of California) and PyMOL 2.0 (PyMOL Molecular Graphics System v.2.0.3, Schrodinger LLC), based on the only Alphacoronavirus S protein structure available in the literature: HCoV-NL63, deposited in the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) accession number 5SZS (RCSB PDB # 5SZS) ( Fig. 3, Fig. 4) (alignments available in the Supplementary material) (Walls et al., 2016b). These models were used for further comparison and analysis of specific CoV S protein features. Additionally, in order to be able to cover all the CoVs, we also used the solved structure of the Betacoronavirus: MHV S protein (RCSB PDB # 3JCL) and the Deltacoronavirus: PDCoV (RCSB PDB # 6B7N) in our analyses (Shang et al., 2017, Walls et al., 2016a).
Fig. 3.
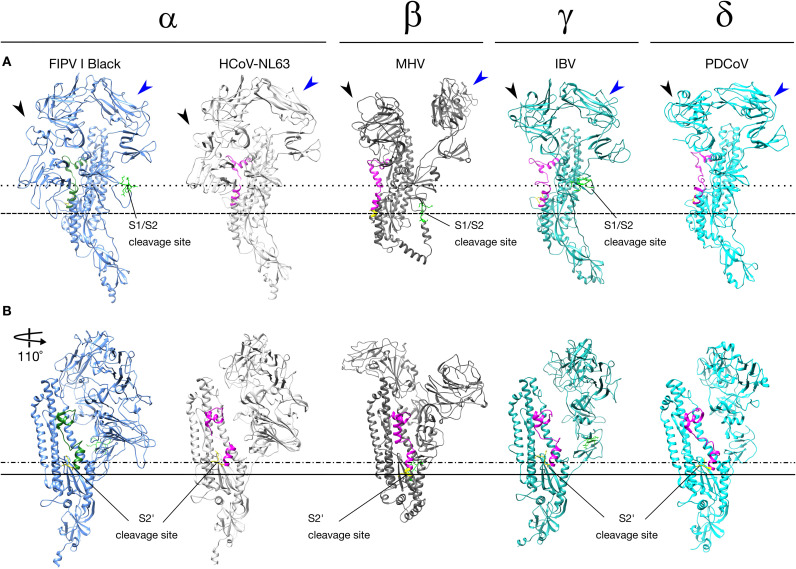
Alphacoronavirus (α), Betacoronavirus (β), Gammacoronavirus (γ) and Deltacoronavirus (δ) spike protein. Comparison between S protein monomer structures from HCoV-NL63 (RCSB PDB # 5SZS), MHV (RCSB PDB # 3JCL) and PDCoV (RCSV PDB # 6B7N), and the S protein monomer models from FIPV I Black and IBV strain Beaudette. The NTD (black arrow heads) and C-domain (blue arrow heads) are signaled. (A) S1/S2 (bright green) cleavage site is present in FCoV, MHV and IBV. FCoV and IBV S1/S2 cleavage site are located at the same relative position (dotted line), while MHV S1/S2 cleavage site is located lower compared with FCoV and IBV dashed line). (B) S2’ (yellow) cleavage site is present in all CoVs spike. All CoV S2’ cleavage sites are located at the same relative position (dashed and dotted line). MHV S2’ is located lower compared with other CoV (solid line). The fusion peptide located at the same relative position in all spikes (FCoV = green, other CoV = magenta).
Fig. 4.
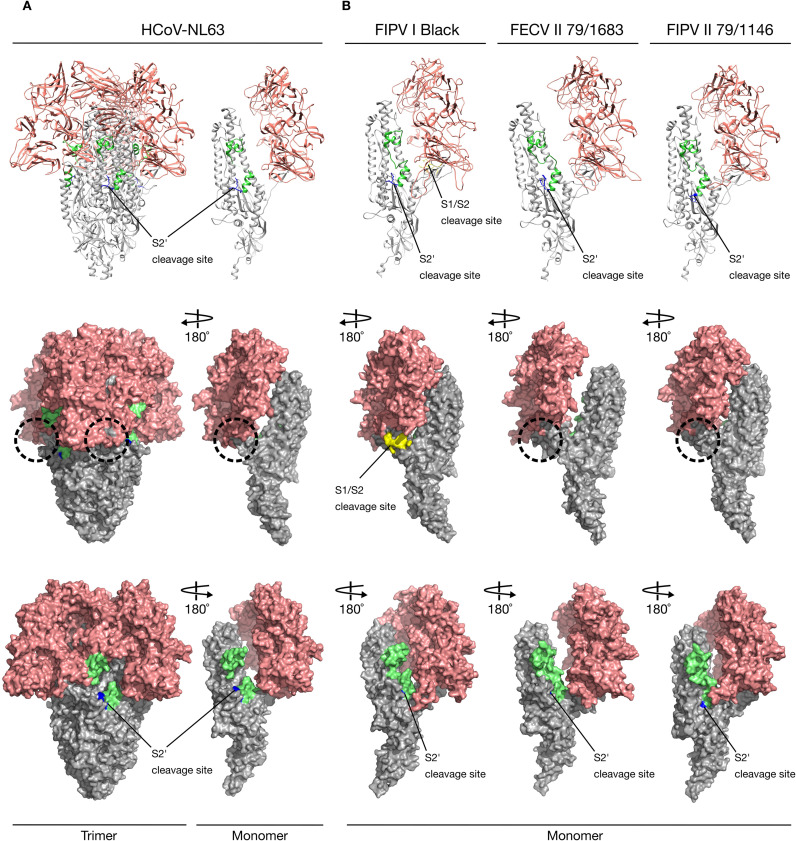
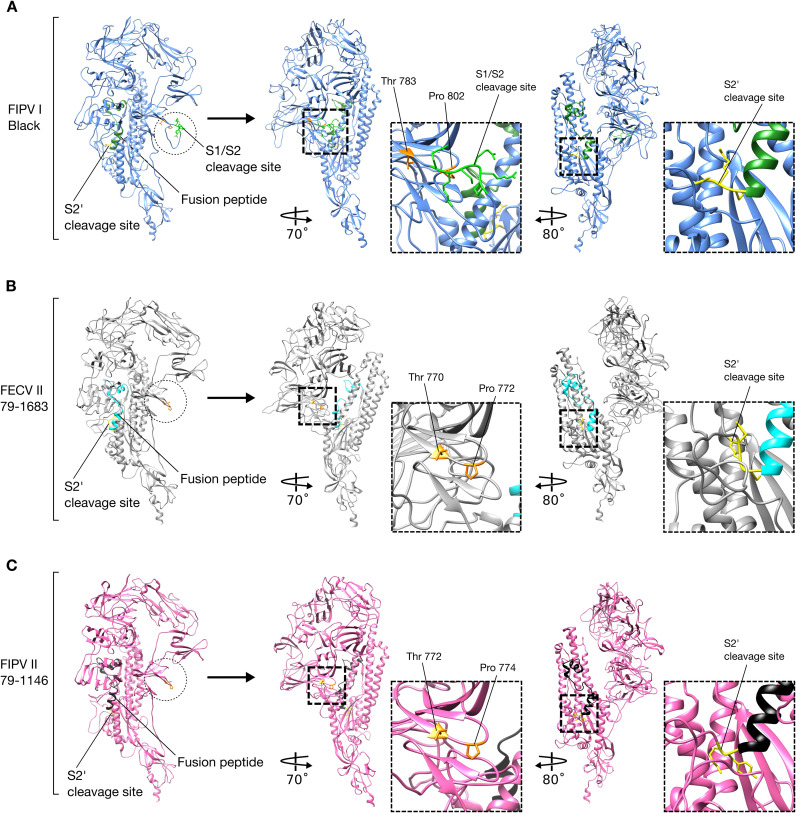
Alphacoronavirus S proteins. Ribbon and surface images of the structure (HCoV-NL63) and models (FCoV) of S proteins and their cleavage sites. S1 domain (salmon), S2 domain (gray), fusion peptide (lime green). (A) HCoV-NL63 S protein structures (RCSB PDB # 5SZS) with its unique S2’ cleavage site (blue) and the missing S1/S2 cleavage site expected location (dashed circle). (B) FIPV I Black, FECV II 78/1683 and FIPV II 79-1146S proteins models based on HCoV-NL63 structure. Notice that only FIPV I Black possesses two cleavage sites: S1/S2 (yellow) and S2’ (blue) while FCoV II viruses possess the S2’ cleavage site (blue) but no S1/S2 (dashed circles).
The S protein is composed of three segments: an ectodomain (which contains most of the functional elements of the protein), a transmembrane domain, and a small endodomain (containing the short intracellular terminal tail) (Li, 2016). As expected, the molecular structures and the predicted models show the S ectodomain to be composed of two functional subunits: S1 and S2 (Fig. 1C) (Belouzard et al., 2012). The S1 subunit contains the receptor binding domain (RBD) and the S2 subunit (or fusion domain) contains all the key features of a class I fusion protein: a fusion peptide (FP) and two heptad repeats (HR1 and HR2, located right before the transmembrane domain (TM) and the short cytoplasmic tail or endodomain (E) (Fig. 1C). Two subdomains have been described in the S1 subunit: N-terminal domain (NTD), known for being able to bind carbohydrates (with the only exception of MHV), such as 9-O-acetylated neuramininc acid in bovine coronavirus (BCoV) and human coronavirus HCoV-OC43; and C-terminal domain (C-domain or CTD), which binds to protein receptors in the cell (Li, 2016, Millet and Whittaker, 2015).
Using the previously reported structures of HCoV-NL63, MHV and PDCoV S proteins and the constructed models for FCoV I and II, and IBV S proteins, we performed a structure analysis of the topology and organization of this proteins. To do this, we first compared the structure of S proteins from each genus, focusing on the overall structure of the proteins. Similarities in the organization and final shape of the S protein were predicted in all the compared spikes from each genus (Fig. 4). All of the proteins were club shaped, with a stalk portion containing the activation sites and the FP, and a globular portion where the RBD is located. However, because the models were based on the HCoV-NL63 spike structure, this raised questions about their accuracy. In order to address this concern, we also constructed models of FCoV and IBV spikes, based on the MHV S reported structure (Walls et al., 2016a). Our models did not predict any major differences in the S protein organization of the models based on MHV, compared with the models based on HCoV-NL63 (data not shown). Additionally, we constructed a PDCoV (GenBank # YP_005352831.1) model based on the HCoV-NL63 structure, and compared it to the recently published PDCoV structure. We found an overall similarity between the predicted PDCoV model and the actual PDCoV structure, in the major organization and location of the regions of interest for our study (i.e. S2’ sites and FP) (Suplementary Fig. 1) (Shang et al., 2017). The major structural differences were found in the Betacoronavirus MHV, compared with the HCoV-NL63 structure and the other CoV spike models. In MHV spike, the S1 subunit NTD domain (Fig. 3A black arrowheads) is specifically separated from the C-domain (Fig. 3A blue arrowheads), which gives it a wider conformation of the globular portion of the protein compared with the other spikes (Fig. 4A). MHV is the only known coronavirus which spike binds the protein receptor murine carcinoembryonic antigen-related adhesion molecule 1 (mCEACAM1) through its NTD, instead of its C-domain (Kubo et al., 1994). While there are no studies that have addressed the relationship between the particular conformation of MHV S1 subunit and the binding to its cellular receptor, we cannot dismiss that this conformation is the reason of the special ability of the MHV NTD to bind proteins instead of carbohydrates. In addition to this analysis, we also compared the overall organization of the three FCoV modeled S proteins from FIPV I Black, FECV II 79-1683 and FIPV II 79-1146. No major differences in the shape and organization between the three spikes were found, not even when compared with the HCoV-NL63 structure, suggesting a conserved organization for Alphacoronavirus spikes (Fig. 4). These findings agree with a previous study of the S1 subunit, where despite the major differences in the amino acid sequence of different CoVs, the S1 subunit adopts common structural topologies, mostly referring to connectivity between secondary structures. However, the author also mentions that despite the common evolutionary origin of the CoVs spikes, the S1 subunits differs in their primary, secondary and tertiary structures as a result of a divergent evolutionary process (Li, 2012). A further analysis focused in the topology of three functional elements: S1/S2 and S2’ cleavage sites, and the FP among the CoVs structures, was also performed and will be described later in this section.
Binding to a receptor is a specific process known to be the principal determinant for viral tropism. Having a carbohydrate receptor for host cell entry is not an uncommon feature. In CoV S proteins, the binding to the receptor is mainly regulated by the S1 subunit. Structural differences and similitudes in the S1 subunit among the four CoVs, were studied by Li (2012). In his study, he found major differences in the amino acid sequence (about only 10% of similarity) between the Alphacoronavirus: HCoV-NL63 and TEGV, the Betacoronavirus: MHV, BCoV and SARS-CoV, and the Gammacoronavirus: IBV. Equally, he found major differences in the primary, secondary and tertiary S1 structures of HCoV-NL63, MHV and SARS-CoV. However, despite these differences there is a remarkably similarity in their structural topologies, sharing common active spots to interact with cellular receptors and suggesting a convergent evolution of the CoV S1 subunit (Li, 2012).
The two functional domains NTD and C-domain (also known as CTD) possess a receptor binding function, and binding to at least one of them is enough for viral attachment to the cell (Li, 2016). As mentioned before, several cellular receptors have been reported to be used by CoVs attachment and entry (Table 2), but the use of those receptors seems to be virus-specific (Belouzard et al., 2012). For FCoV serotype I, no protein or carbohydrate receptor has been reported yet, and it is also not known which domain of the S1 subunit (NTD or C-domain) is carrying out the viral binding function. On the other hand, FCoV II viruses are known to bind the protein fAPN receptor, but as with the previous serotype, the specifics of the S protein interaction with the receptor are still unknown. However, the possibility of FCoV binding a carbohydrate receptor cannot be ruled out. It is known that the Gammacoronavirus IBV NTD of the S1 subunit, can bind to carbohydrate residues like: α-2,3-linked sialic acid and heparan sulfate (HS) (Madu et al., 2007, Winter et al., 2006). The IBV S1 subunit is highly variable and shares low homology among IBV strains, showing amino acid differences between 2 and 50% (Jackwood, 2012, Wickramasinghe et al., 2014). This high variability agrees with the broad tropism of IBV, but contrasts with the limited cell culture adaptation of IBV strains (a characteristic that IBV shares with FCoV), suggesting that the virus might require additional or more specific receptors. In fact, studies with IBV strains Beaudette and Mass41, have shown that the S1 NTD is determinant for the tropism differences between these two strains (Promkuntod et al., 2014). However, the IBV infection of non-susceptible cells expressing DC-SIGN has been reported, suggesting that other elements (including receptors and co-receptors) could be participating in IBV cell entry (Zhang et al., 2012). Betacoronaviruses such as BCoV and HCoV-OC43 also binds to carbohydrate residues; however, additional proteins in the virus (e.g. HE protein) seem to play a major role in carbohydrate binding (Masters and Perlman, 2013). Finally, it has been shown that MERS-CoV is able to bind both protein and carbohydrate receptors in the cell. According with a recent study, a dual-receptor binding (protein and glycan) was observed in MERS-CoV replication (Li et al., 2017). In the study, the authors demonstrated that in addition to the previously known MERS-CoV protein receptor dipeptidyl peptidase 4 (DPP4), the virus can also bind to a sialic acid residues through its NTD domain of the S protein. These results, open the possibility that FCoV could use one or more receptors for viral attachment to the host cell.
As described previously, right after the viral attachment to the cell, an endocytic process may occur, followed by a membrane fusion event. Fusion of the viral and the cellular membrane is a complex process that is mainly mediated by the S2 subunit of the S protein (Belouzard et al., 2012). To induce membrane fusion, the S2 subunit is subjected to a series of biochemical events (e.g. protease activation and pH dropping), resulting in modification of the S2 structure and the exposition of the functional FP. (White et al., 2008). Proteolytic activation of the FCoV spike, occurs at two specific regions in the protein for FCoV I viruses: S1/S2 and S2’; or at one specific region for FCoV II viruses: S2’ (for FCoV II viruses) (Millet and Whittaker, 2015). Having a S1/S2 cleavage site, is a common characteristic for most viruses from the Betacoronavirus and Gammacoronavirus, but in the Alphacoronavirus genus only FCoV I and CCoV have been reported to have a S1/S2 cleavage site (Fig. 4). When comparing the topology of the S1/S2 cleavage site of MHV (Betacoronavirus) and IBV (Gammacoronavirus), relative to FCoV (Alphacoronavirus) S1/S2 site, our models predicted a similar localization of the cleavage site for both FCoV and IBV, but not for MHV (Fig. 3A). In fact, MHV S1/S2 site appears to be located not only in a lower location in the stalk of the protein, but in a different conformational loop (Fig. 3A, compare dotted and dashed lines). These differences positioned MHV spike as the most structurally distant protein of the analyzed CoVs.
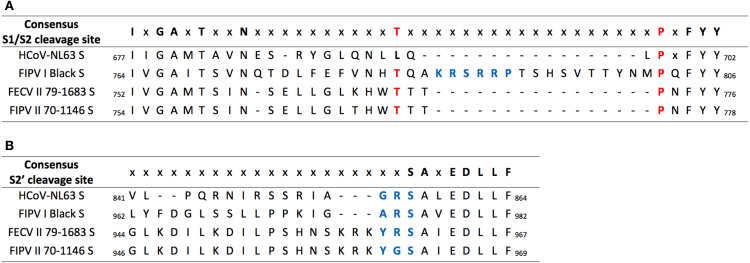
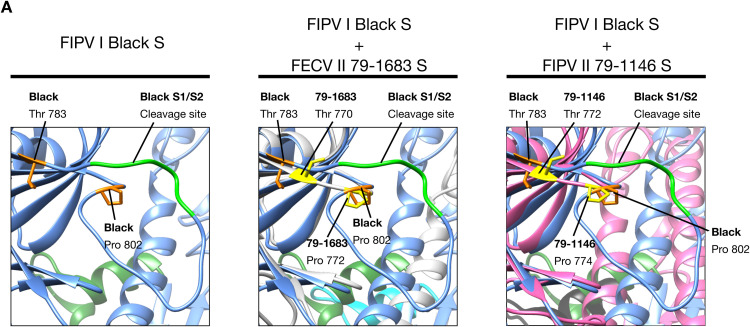
One of the major differences between FCoV serotypes I and II spikes, is the presence or not of the S1/S2 cleavage site. The fact that FCoV I but not FCoV II viruses possess a S1/S2 cleavage site, was previously addressed in this review. We compared the models of three FCoV S proteins (FIPV I Black, FECV II 79-1683 and FIPV II 79-1146), to better understand the implications of the presence or absence of the S1/S2 site (Fig. 3, Fig. 5). As predicted, in a preliminary analysis of the amino acid sequence alignment of the studied FCoV S proteins, the absence of the S1/S2 cleavage site in the two FCoV serotype II viruses was observed and then corroborated in the modeled spikes, while the FCoV I virus possessed the site. Surprisingly, in addition to the absence of the S1/S2 site in the FCoV II viruses, a protruding loop next to the S1/S2 cleavage site (S1/S2 loop) was observed in the FIPV I Black S, but not in the FECV II 79-1683 and FIPV II 79-1146 ( Fig. 5ABC, first panel dotted circles). This loop was also not observed in the Betacoronavirus and Gammacoronavirus S1/S2 sites (Fig. 3A). Taking into account these findings, we went back to the sequence alignment and performed a more detailed analysis of the S1/S2 region, where we found a 16 amino acid gap (including the 6 amino acids of the S1/S2 site) in both FCoV serotype II, as well as in HCoV-NL63 S sequences ( Fig. 6A). Two residues flanking the S1/S2 loop in FIPV I Black S: A threonine residue in position 783 (Thr 783) and a proline residue in position 802 (Pro 802) (Fig. 5A second panel and 6), were identified to be conserved in both FCoV II viruses at positions: Thr 770 and Pro 772 in FECV II 79-1683, and Thr 772 and Pro 774 in FIPV II 1146 (Fig. 5BC second panel and 6). Interestingly, when mapped in the FCoV II spike models, the position of the residues matched the exact position of the residues in FIPV I Black, despite the presence of the loop ( Fig. 7). In fact, a perfect co-localization of the residues can be observed when the FIPV I Black S model is overlaid to each FCoV II models (Fig. 7). The impact of the S1/S2 loop on FIPV I Black spike function is unknown, as well as its absence in both FCoV II viruses. However, the exposed nature of this loop in the S structure, places the S1/S2 cleavage site in an easily accessible position for protease activation, suggesting that this cleavage event plays a major role in FIPV I Black spike function.
Fig. 5.
FCoV I and II spike protein. Comparison between FCoV I and II S protein monomer models. (A) FIPV I Black S protein model and cleavage sites. S1/S2 cleavage site is protruding from the protein forming a 19 amino acids loop (dotted line circle). Location and magnification of FIPV I Black S1/S2 cleavage site (bright green) and flanking amino acids (orange): Thr 783 and Pro 802. Location and magnification of FIPV I Black S2’ cleavage site (yellow). (B) FECV II 79-1683S protein model and cleavage sites. Notice the absence of the S1/S2 cleavage site and loop (dotted line circle). Expected location of FECV II 79–1683 S1/S2 loop and flanking amino acids (yellow): Thr 770 and Pro 772. Location and magnification of FECV II 79–1683 S2’ cleavage site (yellow). (C) FIPV II 79-1146S protein model and cleavage sites. Notice the absence of the S1/S2 cleavage site and loop (dotted line circle). Expected location of FIPV II 79-1146 S1/S2 loop and flanking amino acids (yellow): Thr 772 and Pro 774. Location and magnification of FIPV II 79-1146 S2’ cleavage site (yellow).
Fig. 6.
HCoV-NL63, FCoV I and II S1/S2 and S2’ amino acid sequence alignment. Amino acid alignment of HCoV-NL63, FIPV I Black, FECV II 1683 and FIPV II 1146 S1/S2 and S2’ region. (A) A 16 amino acids gap corresponding to the S1/S2 cleavage site and loop is missing in HCoV-NL63 and in both FCoV II S sequences. FIPV I Black S1/S2 cleavage site is highlighted in blue. Corresponding S1/S2 flanking amino acids (Thr and Pro) for all FCoVs are highlighted in red. (B) A three amino acids gap immediately before the S2’ cleavage site is observed in HCoV-NL63 and FIPV I Black S. The S2’ cleavage sites are highlighted in blue.
Fig. 7.
FCoV S1/S2 cleavage site. (A) FIPV I Black S1/S2 cleavage site (bright green) and flanking amino acids (orange): Thr 783 and Pro 802. (B) Superposition of FECV II 79-1683 (light gray) and FIPV I Black (light blue) S1/S2 regions. FECV II 79-1683 amino acids Thr 770 and Pro 772 (yellow), are located in the same position than FIPV I Black S1/S2 flanking amino acids (orange). (C) Superposition of FIPV II 79-1146 (pink) and FIPV I Black (light blue) S1/S2 regions. FIPV II 79-1146 amino acids Thr 772 and Pro 774 (yellow), are located in the same position than FIPV I Black S1/S2 flanking amino acids (orange). Notice that in both FCoV II S proteins the complete S1/S2 loop (including the S1/S2 cleavage site) is missing.
A CoV common S2’ cleavage site is located immediately before the FP, and proteolytic processing at this site is believed to activate the fusion function of the S2 subunit (Burkard et al., 2014). Similar to the S1/S2 site, the topology of the S2’ cleavage site of alpha-, gamma- and delta-CoV is practically the same (Fig. 3B dotted and dashed line); and again, MHV S2’ cleavage site appears to be located in a different position, relative to FIPV I Black model (Fig. 3B solid line). Interestingly, the topology of the both S1/S2 and S2’ cleavage sites, appears to not alter the topology of the FP itself. We also compared the position of the S2’ cleavage site in the three studied FCoV (Fig. 3 and 5ABC third panel). No variation in topology or structure was found at the S2’ site in the studied FCoV S (Fig. 5ABC third panel). This denotes the high conservation of this region among CoVs and suggests a determinant role of this site in S function.
Along with the S2’ activation, pH is a key factor for triggering the FCoV virus-cell membrane fusion (Regan et al., 2008). Once the virus is inside the endosome, pH variation can participate in two different steps of the membrane fusion: first, providing the specific conditions for protease cleavage of the S2’ site; and second, inducing a set of conformational changes on S repositioning the FP, to make it able to anchor into the cell membrane (Regan et al., 2008, White et al., 2008, White and Whittaker, 2016). Previous studies from our group described the role of the S2’ site in the exposition and activation of the FP in SARS-CoV. In this study, a motif with the characteristics of a viral FP located immediately after the S2’ site was described to be highly conserved across CoVs, suggesting the key role of S2’ activation in the FP function (Madu et al., 2009). Nevertheless, recent studies also from our group have re-defined the FP pre-fusion structure, and have found indications of additional requirements (e.g. ions) for inducing the fusion function of the FP (Lai et al., 2017). In this study, two α-helical subdomains (called FP1 and FP2) bound by a flexible loop region were described in the SARS-CoV's FP, based on a modeling approach. Interestingly, comparing these findings with reported CoVs spike protein structures, a similar organization of the FP can be observed. We compared the topology of the FP in the studied CoV spikes. Interestingly, the topology of the FP is conserved between all the studied spikes, and is not affected by the position of the S1/S2 or S2’ cleavage sites (Figs. 3 and 4B). This remarkable conservation of the FP, highlights the evolutionary and functional relevance of this protein for FCoV replication. Nonetheless, FCoV S2’ cleavage site appears to be less exposed compared with HCoV-NL63, as was observed in the surface models (Fig. 4), suggesting that previous unfolding steps could be necessary to expose the S2’ site for protease cleavage. In fact, a recent study showed that unfolding is essential for cleaving within α-helical structures (as CoVs FP), which supports the necessity of structural changes in S to expose S2’ prior to activation (Robertson et al., 2016). While the specific reasons behind the hidden location of the S2’ site are not known, we hypothesize that preventing S activation at the S2’ site before receptor binding, is important for the stability of the spike protein. We also compared the S2’ amino acid sequence for the studied FCoVs. A three amino acid gap was found in FIPV I Black and HCoV-NL63, but no structural implications of this gap were found (Fig. 6B).
The insertion of the FP in the cell membrane, leads to the subsequent conformational change in the spike protein, where the two HR regions located downstream the FP initiates a fold-back process between the viral and cell membranes (White et al., 2008). The HR are amino acid chains of seven residues with a conserved pattern HPPHCPC (where H are hydrophobic residues, P are polar or hydrophilic residues, and C are other charged residues), that allows the formation of compact alpha helical structures of one HR wrapping over the other, known as six-helix bundle (6HB) (Chambers et al., 1990, Millet and Whittaker, 2015, White et al., 2008). Finally, as a result of the 6HB formation both viral and cell membranes come into a close proximity, allowing the membrane fusion and the subsequent fusion pore formation, necessary for delivering FCoV genome to the cytoplasm to continue the replication cycle (White and Whittaker, 2016).
6. Conclusions
Several decades of FCoV research have led to the understanding of some of the most important clinical and epidemiological aspects of FIP. However, despite those research efforts, many aspects of FCoV pathogenesis, especially those related with the infection of the host cell, as well as the mechanisms through the virus expands its tropism and cause disease, are still not well understood, making FIP a current and major threat to feline health. FCoV spike is considered a key regulator in FCoV pathogenesis. Indeed, CoV spike proteins have been studied and its role in the viral entry into the host cell has been well described (Belouzard et al., 2012, White et al., 2008, White and Whittaker, 2016). In this review, we addressed the major elements of FCoV infection and we explored the role of the S protein as a regulator in FCoV pathogenesis. Using a modeling approach based on the previously reported HCoV-NL63 S protein, we were able to identify specific characteristics and differences of the spike protein of several CoVs.
The overall structure of the modeled S proteins, resembles almost identically the HCoV-NL63 spike. Major structural differences were only seen when compared with MHV spike (Fig. 3). It is known, that MHV spike is unique between other CoVs, due its ability to bind protein receptors through its NTD domain (Kubo et al., 1994). While is not possible to affirm that this specific characteristic is the reason behind the particular structure of MHV S, it is important to mention that opposite to MHV, most of CoV spikes binds protein receptors through the C-domain of the S protein (Li, 2016).
Activation of the S protein is necessary for the virus to infect the host cell, and the differences in the activation mechanisms of the protein are suggested to be determinant in the viral pathogenesis (Millet and Whittaker, 2015). Comparing the FCoV S structure of the two serotypes, a major structural and functional difference is the presence of the S1/S2 cleavage site in the serotype I virus FIPV I Black (Fig. 4, Fig. 5). Previous studies have shown that this additional cleavage site is present in FCoV I but not in FCoV II viruses (Licitra et al., 2014, Millet and Whittaker, 2015). In fact, most Alphacoronaviruses do not possess this cleavage site, making FCoV I viruses special in terms of proteolytic activation of their spike protein. Little is known about the implications of having the S1/S2 cleavage site in the pathogenesis of FIPV I viruses, but previous studies have suggested a relation between the S1/S2 proteolytic activation requirements and changes in virulence and tropism (de Haan et al., 2008, Licitra et al., 2013). In these studies, it was proposed that mutations in the spike cleavage sites might result in a change in the FCoV biotype from FECV to FIPV (Licitra et al., 2013, Licitra et al., 2014). Interestingly, we observed in our predicted models that FIPV I Black possesses a 16 amino acids total insertion in the S1/S2 region, that forms an extra loop in the spike structure, making the cleavage site more accessible for proteolytic processing (Fig. 4, Fig. 5, Fig. 7).
The S2’ cleavage site appears to be conserved across CoVs. When comparing the CoV models and structures, no differences in topology were predicted in the studied spikes (Fig. 3). This aspect highlights the common evolutionary origin of CoV spikes and suggests a major role in S function. The S2’ topology, is also conserved among the FCoV spikes and despite the amino acid sequence between the studied FCoVs, no other major changes were detected in this region (Fig. 4, Fig. 5).
In conclusion, we addressed the most relevant aspects of FCoV pathogenesis and revised the role of the S protein in the virulence and tropism of the virus. We predicted that the S protein structure is conserved across CoVs, including FCoV. However, differences in the activation requirements between serotype I and serotype II viruses are present, but their role in S function is not well understood. While there is no doubt about the importance of FCoV S in the viral pathogenesis, future studies to uncover the specific mechanisms through FCoV S participate in the virulence and tropism switch are still necessary.
Acknowledgements
This work was supported by the Morris Animal Foundation (Grant no. D15FE-028, 2015); the Winn Feline Foundation (Grant no. W15-026); and the Feline Health Center, Cornell University (Grant FY16-17).
Footnotes
Revised on: International Committee on Taxonomy of Viruses (ICTV). Virus Taxonomy: The Classification and Nomenclature of Viruses The Online (10th) Report of the ICTV. Updated: August 2016. https://talk.ictvonline.org/ictv-reports/ictv_online_report/.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.virol.2017.12.027.
Appendix A. Supplementary material
Fig. S1.
PDCoV spike structure and predicted model. PDCoV spike structure (RCSV PDB # 6B7N) and predicted PDCoV-HKU15 strain MI6148 spike model (GenBank # AIA99518.1). Supplementary material 1: HCoV-NL63 S and FIPV I Black S amino acid alignment. HCoV-NL63 (RCSV PDB # 5SZS) and FIPV I Black S (Susan Baker, Loyola University Chicago, personal communication). Supplementary material 2: HCoV-NL63 S and FECV II 79-1683 S amino acid alignment. HCoV-NL63 S (RCSV PDB # 5SZS) and FECV II 79-1683 S (GenBank # AFH58021.1). Supplementary material 3: HCoV-NL63 S and FIPV II 79-1146 S amino acid alignment. HCoV-NL63 S (RCSV PDB # 5SZS) and FIPV II 79-1146 S (GenBank # AAY32596.1). Supplementary material 4: HCoV-NL63 S and IBV strain Beaudette S amino acid alignment. HCoV-NL63 S (RCSV PDB # 5SZS) and IBV strain Beaudette S (GenBank # AJ311362.1).
References
- An D.J., Jeoung H.Y., Jeong W., Park J.Y., Lee M.H., Park B.K. Prevalence of Korean cats with natural feline coronavirus infections. Virol. J. 2011;8:455. doi: 10.1186/1743-422X-8-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J., Niemann H., Smeekens S., Rottier P., Warren G. Sequence and topology of a model intracellular membrane protein, E1 glycoprotein, from a coronavirus. Nature. 1984;308:751–752. doi: 10.1038/308751a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint A., Farsang A., Zadori Z., Hornyak A., Dencso L., Almazan F., Enjuanes L., Belak S. Molecular characterization of feline infectious peritonitis virus strain DF-2 and studies of the role of ORF3abc in viral cell tropism. J. Virol. 2012;86:6258–6267. doi: 10.1128/JVI.00189-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcena M., Oostergetel G.T., Bartelink W., Faas F.G., Verkleij A., Rottier P.J., Koster A.J., Bosch B.J. Cryo-electron tomography of mouse hepatitis virus: insights into the structure of the coronavirion. Proc. Natl. Acad. Sci. USA. 2009;106:582–587. doi: 10.1073/pnas.0805270106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker E.N., Stranieri A., Helps C.R., Porter E.L., Davidson A.D., Day M.J., Knowles T., Kipar A., Tasker S. Limitations of using feline coronavirus spike protein gene mutations to diagnose feline infectious peritonitis. Vet. Res. 2017;48:60. doi: 10.1186/s13567-017-0467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetka V., Kubber-Heiss A., Kolodziejek J., Nowotny N., Hofmann-Parisot M., Mostl K. Prevalence of feline coronavirus types I and II in cats with histopathologically verified feline infectious peritonitis. Vet. Microbiol. 2004;99:31–42. doi: 10.1016/j.vetmic.2003.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J.W. Recovery and in vitro cultivation of a coronavirus from laboratory-induced cases of feline infectious peritonitis (FIP) Vet. Med. Small Anim. Clin.: VM SAC. 1980;75:811–814. [PubMed] [Google Scholar]
- Bosch B.J., van der Zee R., de Haan C.A.M., Rottier P.J.M. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley I., Digard P., Inglis S.C. Characterization of an efficient coronavirus ribosomal frameshifting signal: requirement for an RNA pseudoknot. Cell. 1989;57:537–547. doi: 10.1016/0092-8674(89)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.A., Troyer J.L., Pecon-Slattery J., Roelke M.E., O’Brien S.J. Genetics and pathogenesis of feline infectious peritonitis virus. Emerg. Infect. Dis. 2009;15:1445–1452. doi: 10.3201/eid1509.081573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., Pelkmans L., Rottier P.J., Bosch B.J., de Haan C.A. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10:e1004502. doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham T.-C., Chang Y.-C., Tsai P.-S., Wu C.-H., Chen H.-W., Jeng C.-R., Pang V.F., Chang H.-W. Determination of the cell tropism of serotype 1 feline infectious peritonitis virus using the spike affinity histochemistry in paraffin-embedded tissues. Microbiol. Immunol. 2017;61:318–327. doi: 10.1111/1348-0421.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers P., Pringle C.R., Easton A.J. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J. Gen. Virol. 1990;71(Pt 12):3075–3080. doi: 10.1099/0022-1317-71-12-3075. [DOI] [PubMed] [Google Scholar]
- Chang C.K., Sue S.C., Yu T.H., Hsieh C.M., Tsai C.K., Chiang Y.C., Lee S.J., Hsiao H.H., Wu W.J., Chang W.L., Lin C.H., Huang T.H. Modular organization of SARS coronavirus nucleocapsid protein. J. Biomed. Sci. 2006;13:59–72. doi: 10.1007/s11373-005-9035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.W., de Groot R.J., Egberink H.F., Rottier P.J. Feline infectious peritonitis: insights into feline coronavirus pathobiogenesis and epidemiology based on genetic analysis of the viral 3c gene. J. Gen. Virol. 2010;91:415–420. doi: 10.1099/vir.0.016485-0. [DOI] [PubMed] [Google Scholar]
- Chang H.W., Egberink H.F., Halpin R., Spiro D.J., Rottier P.J. Spike protein fusion peptide and feline coronavirus virulence. Emerg. Infect. Dis. 2012;18:1089–1095. doi: 10.3201/eid1807.120143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.W., Egberink H.F., Rottier P.J. Sequence analysis of feline coronaviruses and the circulating virulent/avirulent theory. Emerg. Infect. Dis. 2011;17:744–746. doi: 10.3201/eid1704.102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corapi W.V., Olsen C.W., Scott F.W. Monoclonal antibody analysis of neutralization and antibody-dependent enhancement of feline infectious peritonitis virus. J. Virol. 1992;66:6695–6705. doi: 10.1128/jvi.66.11.6695-6705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corse E., Machamer C.E. The cytoplasmic tail of infectious Bronchitis Virus E protein directs golgi targeting. J. Virol. 2002;76:1273–1284. doi: 10.1128/JVI.76.3.1273-1284.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan C.A., Haijema B.J., Schellen P., Wichgers Schreur P., te Lintelo E., Vennema H., Rottier P.J. Cleavage of group 1 coronavirus spike proteins: how furin cleavage is traded off against heparan sulfate binding upon cell culture adaptation. J. Virol. 2008;82:6078–6083. doi: 10.1128/JVI.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeurwaerder A., Desmarets L.M., Olyslaegers D.A., Vermeulen B.L., Dewerchin H.L., Nauwynck H.J. The role of accessory proteins in the replication of feline infectious peritonitis virus in peripheral blood monocytes. Vet. Microbiol. 2013;162:447–455. doi: 10.1016/j.vetmic.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeurwaerder A., Olyslaegers D.A., Desmarets L.M., Roukaerts I.D., Theuns S., Nauwynck H.J. ORF7-encoded accessory protein 7a of feline infectious peritonitis virus as a counteragent against IFN-alpha-induced antiviral response. J. Gen. Virol. 2014;95:393–402. doi: 10.1099/vir.0.058743-0. [DOI] [PubMed] [Google Scholar]
- DeDiego M.L., Alvarez E., Almazan F., Rejas M.T., Lamirande E., Roberts A., Shieh W.J., Zaki S.R., Subbarao K., Enjuanes L. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. J. Virol. 2007;81:1701–1713. doi: 10.1128/JVI.01467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C., Siddell S.G. Genomic RNA sequence of Feline coronavirus strain FIPV WSU-79/1146. J. Gen. Virol. 2005;86:2249–2253. doi: 10.1099/vir.0.80985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C., Temperton N., Siddell S.G. Type I feline coronavirus spike glycoprotein fails to recognize aminopeptidase N as a functional receptor on feline cell lines. J. Gen. Virol. 2007;88:1753–1760. doi: 10.1099/vir.0.82666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Almazan F., Sola I., Zuniga S. Biochemical aspects of coronavirus replication and virus-host interaction. Annu. Rev. Microbiol. 2006;60:211–230. doi: 10.1146/annurev.micro.60.080805.142157. [DOI] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J.E., Poland A., Carlson J., Pedersen N.C. Risk factors for feline infectious peritonitis among cats in multiple-cat environments with endemic feline enteric coronavirus. J. Am. Vet. Med. Assoc. 1997;210:1313–1318. [PubMed] [Google Scholar]
- Gui M., Song W., Zhou H., Xu J., Chen S., Xiang Y., Wang X. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 2017;27:119–129. doi: 10.1038/cr.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn-Moore D.A., Gruffydd-Jones T.J., Harbour D.A. Detection of feline coronaviruses by culture and reverse transcriptase-polymerase chain reaction of blood samples from healthy cats and cats with clinical feline infectious peritonitis. Vet. Microbiol. 1998;62:193–205. doi: 10.1016/S0378-1135(98)00210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijema B.J., Volders H., Rottier P.J. Live, attenuated coronavirus vaccines through the directed deletion of group-specific genes provide protection against feline infectious peritonitis. J. Virol. 2004;78:3863–3871. doi: 10.1128/JVI.78.8.3863-3871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.C. Principles of virus structure. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Lippincot Williams & Wilkins; Philadelphia, PA: 2013. pp. 52–86. [Google Scholar]
- Herrewegh A.A., Vennema H., Horzinek M.C., Rottier P.J., de Groot R.J. The molecular genetics of feline coronaviruses: comparative sequence analysis of the ORF7a/7b transcription unit of different biotypes. Virology. 1995;212:622–631. doi: 10.1006/viro.1995.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh A.A.P.M., Smeenk I., Horzinek M.C., Rottier P.J.M., de Groot R.J. feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J. Virol. 1998;72:4508–4514. doi: 10.1128/jvi.72.5.4508-4514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Izumiya Y., Yokoyama Y., Kida K., Koyama H. Differences in virus receptor for type I and type II feline infectious peritonitis virus. Arch. Virol. 1998;143:839–850. doi: 10.1007/s007050050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Okada S., Ishizuka Y., Yamada H., Koyama H. The prevalence of types I and II feline coronavirus infections in cats. J. Vet. Med. Sci. 1992;54:557–562. doi: 10.1292/jvms.54.557. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T., Yamato H., Ohkawa T., Kaneko M., Motokawa K., Kusuhara H., Kaneshima T., Arai S., Koyama H. Vaccine efficacy of a cell lysate with recombinant baculovirus-expressed feline infectious peritonitis (FIP) virus nucleocapsid protein against progression of FIP. Vet. Microbiol. 2003;97:31–44. doi: 10.1016/j.vetmic.2003.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzworth J. Some important disorders of cats. Cornell Vet. 1963;53:157–160. [PubMed] [Google Scholar]
- Hurst K.R., Koetzner C.A., Masters P.S. Identification of in vivo-interacting domains of the murine coronavirus nucleocapsid protein. J. Virol. 2009;83:7221–7234. doi: 10.1128/JVI.00440-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst K.R., Koetzner C.A., Masters P.S. Characterization of a critical interaction between the coronavirus nucleocapsid protein and nonstructural protein 3 of the viral replicase-transcriptase complex. J. Virol. 2013;87:9159–9172. doi: 10.1128/JVI.01275-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56:634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- Kennedy M., Boedeker N., Gibbs P., Kania S. Deletions in the 7a ORF of feline coronavirus associated with an epidemic of feline infectious peritonitis. Vet. Microbiol. 2001;81:227–234. doi: 10.1016/S0378-1135(01)00354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M.A., Abd-Eldaim M., Zika S.E., Mankin J.M., Kania S.A. Evaluation of antibodies against feline coronavirus 7b protein for diagnosis of feline infectious peritonitis in cats. Am. J. Vet. Res. 2008;69:1179–1182. doi: 10.2460/ajvr.69.9.1179. [DOI] [PubMed] [Google Scholar]
- Kipar A., Kohler K., Leukert W., Reinacher M. A comparison of lymphatic tissues from cats with spontaneous feline infectious peritonitis (FIP), cats with FIP virus infection but no FIP, and cats with no infection. J. Comp. Pathol. 2001;125:182–191. doi: 10.1053/jcpa.2001.0501. [DOI] [PubMed] [Google Scholar]
- Kipar A., Meli M.L. Feline infectious peritonitis: still an enigma? Vet. Pathol. 2014;51:505–526. doi: 10.1177/0300985814522077. [DOI] [PubMed] [Google Scholar]
- Kipar A., Meli M.L., Baptiste K.E., Bowker L.J., Lutz H. Sites of feline coronavirus persistence in healthy cats. J. Gen. Virol. 2010;91:1698–1707. doi: 10.1099/vir.0.020214-0. [DOI] [PubMed] [Google Scholar]
- Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., Corbett K.S., Graham B.S., McLellan J.S., Ward A.B. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnse-Locker J., Ericsson M., Rottier P.J., Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J. Cell Biol. 1994;124:55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo H., Yamada Y.K., Taguchi F. Localization of neutralizing epitopes and the receptor-binding site within the amino-terminal 330 amino acids of the murine coronavirus spike protein. J. Virol. 1994;68:5403–5410. doi: 10.1128/jvi.68.9.5403-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A.L., Millet J.K., Daniel S., Freed J.H., Whittaker G.R. The SARS-CoV fusion peptide forms an extended bipartite fusion platform that perturbs membrane order in a calcium-dependent manner. J. Mol. Biol. 2017 doi: 10.1016/j.jmb.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M. Coronavirus: organization, replication and expression of genome. Annu. Rev. Microbiol. 1990;44:303–333. doi: 10.1146/annurev.mi.44.100190.001511. [DOI] [PubMed] [Google Scholar]
- Lewis C.S., Porter E., Matthews D., Kipar A., Tasker S., Helps C.R., Siddell S.G. Genotyping coronaviruses associated with feline infectious peritonitis. J. Gen. Virol. 2015;96:1358–1368. doi: 10.1099/vir.0.000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Evidence for a common evolutionary origin of coronavirus spike protein receptor-binding subunits. J. Virol. 2012;86:2856–2858. doi: 10.1128/JVI.06882-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Hulswit R.J.G., Widjaja I., Raj V.S., McBride R., Peng W., Widagdo W., Tortorici M.A., van Dieren B., Lang Y., van Lent J.W.M., Paulson J.C., de Haan C.A.M., de Groot R.J., van Kuppeveld F.J.M., Haagmans B.L., Bosch B.J. Identification of sialic acid-binding function for the middle east respiratory syndrome coronavirus spike glycoprotein. Proc. Natl. Acad. Sci. USA. 2017 doi: 10.1073/pnas.1712592114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licitra B.N., Millet J.K., Regan A.D., Hamilton B.S., Rinaldi V.D., Duhamel G.E., Whittaker G.R. Mutation in spike protein cleavage site and pathogenesis of feline coronavirus. Emerg. Infect. Dis. 2013;19:1066–1073. doi: 10.3201/eid1907.121094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licitra, B.N., Sams, K.L., Lee, D.W., Whittaker, G.R., 2014. Feline Coronaviruses Associated with Feline Infectious Peritonitis Have Modifications to Spike Protein Activation Sites at Two Discrete Positions, arXiv. Cornell University Library, p. 5.
- Look A.T., Ashmun R.A., Shapiro L.H., Peiper S.C. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J. Clin. Investig. 1989;83:1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madu I.G., Chu V.C., Lee H., Regan A.D., Bauman B.E., Whittaker G.R. Heparan sulfate is a selective attachment factor for the avian coronavirus infectious bronchitis virus beaudette. Avian Dis. 2007;51:45–51. doi: 10.1637/0005-2086(2007)051[0045:HSIASA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Madu I.G., Roth S.L., Belouzard S., Whittaker G.R. Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide. J. Virol. 2009;83:7411–7421. doi: 10.1128/JVI.00079-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P.S., Perlman S. Coronaviridae. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Lippincot Williams & Wilkins; Philadelphia, PA: 2013. pp. 825–858. [Google Scholar]
- McKeirnan A.J., Evermann J.F., Davis E.V., Ott R.L. Comparative properties of feline coronaviruses in vitro. Can. J. Vet. Res. = Rev. Can. De. Rech. Vet. 1987;51:212–216. [PMC free article] [PubMed] [Google Scholar]
- Meli M., Kipar A., Muller C., Jenal K., Gonczi E., Borel N., Gunn-Moore D., Chalmers S., Lin F., Reinacher M., Lutz H. High viral loads despite absence of clinical and pathological findings in cats experimentally infected with feline coronavirus (FCoV) type I and in naturally FCoV-infected cats. J. Feline Med. Surg. 2004;6:69–81. doi: 10.1016/j.jfms.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. USA. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S., Droese B., Klaus J.P., Makino S., Sawicki S.G., Siddell S.G., Stamou D.G., Wilson I.A., Kuhn P., Buchmeier M.J. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011;174:11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Torres J.L., DeDiego M.L., Verdia-Baguena C., Jimenez-Guardeno J.M., Regla-Nava J.A., Fernandez-Delgado R., Castano-Rodriguez C., Alcaraz A., Torres J., Aguilella V.M., Enjuanes L. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10:e1004077. doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C.W. A review of feline infectious peritonitis virus: molecular biology, immunopathogenesis, clinical aspects, and vaccination. Vet. Microbiol. 1993;36:1–37. doi: 10.1016/0378-1135(93)90126-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. Morphologic and physical characteristics of feline infectious peritonitis virus and its growth in autochthonous peritoneal cell cultures. Am. J. Vet. Res. 1976;37:567–572. [PubMed] [Google Scholar]
- Pedersen N.C. A review of feline infectious peritonitis virus infection: 1963–2008. J. Feline Med. Surg. 2009;11:225–258. doi: 10.1016/j.jfms.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Black J.W., Boyle J.F., Evermann J.F., McKeirnan A.J., Ott R.L. Pathogenic differences between various feline coronavirus isolates. Adv. Exp. Med. Biol. 1984;173:365–380. doi: 10.1007/978-1-4615-9373-7_36. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Boyle J.F., Floyd K. Infection studies in kittens, using feline infectious peritonitis virus propagated in cell culture. Am. J. Vet. Res. 1981;42:363–367. [PubMed] [Google Scholar]
- Pedersen N.C., Boyle J.F., Floyd K., Fudge A., Barker J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis. Am. J. Vet. Res. 1981;42:368–377. [PubMed] [Google Scholar]
- Pedersen N.C., Liu H., Dodd K.A., Pesavento P.A. Significance of coronavirus mutants in feces and diseased tissues of cats suffering from feline infectious peritonitis. Viruses. 2009;1:166–184. doi: 10.3390/v1020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Liu H., Scarlett J., Leutenegger C.M., Golovko L., Kennedy H., Kamal F.M. Feline infectious peritonitis: role of the feline coronavirus 3c gene in intestinal tropism and pathogenicity based upon isolates from resident and adopted shelter cats. Virus Res. 2012;165:17–28. doi: 10.1016/j.virusres.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Sato R., Foley J.E., Poland A.M. Common virus infections in cats, before and after being placed in shelters, with emphasis on feline enteric coronavirus. J. Feline Med. Surg. 2004;6:83–88. doi: 10.1016/j.jfms.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Ward J., Mengeling W.L. Antigenic relationship of the feline infectious peritonitis virus to coronaviruses of other species. Arch. Virol. 1978;58:45–53. doi: 10.1007/BF01315534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervushin K., Tan E., Parthasarathy K., Lin X., Jiang F.L., Yu D., Vararattanavech A., Soong T.W., Liu D.X., Torres J. Structure and inhibition of the SARS coronavirus envelope protein ion channel. PLoS Pathog. 2009;5:e1000511. doi: 10.1371/journal.ppat.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A.M., Vennema H., Foley J.E., Pedersen N.C. Two related strains of feline infectious peritonitis virus isolated from immunocompromised cats infected with a feline enteric coronavirus. J. Clin. Microbiol. 1996;34:3180–3184. doi: 10.1128/jcm.34.12.3180-3184.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter E., Tasker S., Day M.J., Harley R., Kipar A., Siddell S.G., Helps C.R. Amino acid changes in the spike protein of feline coronavirus correlate with systemic spread of virus from the intestine and not with feline infectious peritonitis. Vet. Res. 2014;45:49. doi: 10.1186/1297-9716-45-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promkuntod N., van Eijndhoven R.E., de Vrieze G., Grone A., Verheije M.H. Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology. 2014;448:26–32. doi: 10.1016/j.virol.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan A.D., Shraybman R., Cohen R.D., Whittaker G.R. Differential role for low pH and cathepsin-mediated cleavage of the viral spike protein during entry of serotype II feline coronaviruses. Vet. Microbiol. 2008;132:235–248. doi: 10.1016/j.vetmic.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan A.D., Whittaker G.R. Utilization of DC-SIGN for entry of feline coronaviruses into host cells. J. Virol. 2008;82:11992–11996. doi: 10.1128/JVI.01094-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A.L., Headey S.J., Ng N.M., Wijeyewickrema L.C., Scanlon M.J., Pike R.N., Bottomley S.P. Protein unfolding is essential for cleavage within the alpha-helix of a model protein substrate by the serine protease, thrombin. Biochimie. 2016;122:227–234. doi: 10.1016/j.biochi.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Rossen J.W., Kouame J., Goedheer A.J., Vennema H., Rottier P.J. Feline and canine coronaviruses are released from the basolateral side of polarized epithelial LLC-PK1 cells expressing the recombinant feline aminopeptidase-N cDNA. Arch. Virol. 2001;146:791–799. doi: 10.1007/s007050170147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Zheng Y., Yang Y., Liu C., Geng Q., Tai W., Du L., Zhou Y., Zhang W., Li F. Cryo-EM structure of porcine delta coronavirus spike protein in the pre-fusion state. J. Virol. 2017 doi: 10.1128/JVI.01556-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba N., Maeda K., Kato H., Mochizuki M., Iwata H. Differentiation of feline coronavirus type I and II infections by virus neutralization test. Vet. Microbiol. 2007;124:348–352. doi: 10.1016/j.vetmic.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L.S., Holmes K.V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J. Virol. 1980;33:449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Katada Y., Moritoh S., Ogasawara M., Satoh K., Satoh R., Tanabe M., Hohdatsu T. Analysis of the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection: aminopeptidase N is not important and a process of acidification of the endosome is necessary. J. Gen. Virol. 2008;89:1025–1029. doi: 10.1099/vir.0.83558-0. [DOI] [PubMed] [Google Scholar]
- Takano T., Kawakami C., Yamada S., Satoh R., Hohdatsu T. Antibody-dependent enhancement occurs upon re-infection with the identical serotype virus in feline infectious peritonitis virus infection. J. Vet. Med. Sci. 2008;70:1315–1321. doi: 10.1292/jvms.70.1315. [DOI] [PubMed] [Google Scholar]
- Tekes G., Hofmann-Lehmann R., Bank-Wolf B., Maier R., Thiel H.J., Thiel V. Chimeric feline coronaviruses that encode type II spike protein on type I genetic background display accelerated viral growth and altered receptor usage. J. Virol. 2010;84:1326–1333. doi: 10.1128/JVI.01568-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresnan D.B., Levis R., Holmes K.V. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J. Virol. 1996;70:8669–8674. doi: 10.1128/jvi.70.12.8669-8674.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hamme E., Desmarets L., Dewerchin H.L., Nauwynck H.J. Intriguing interplay between feline infectious peritonitis virus and its receptors during entry in primary feline monocytes. Virus Res. 2011;160:32–39. doi: 10.1016/j.virusres.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., Poland A., Foley J., Pedersen N.C. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150–157. doi: 10.1006/viro.1998.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Spaan W., Palese P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc. Natl. Acad. Sci. USA. 1988;85:4526–4529. doi: 10.1073/pnas.85.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Tortorici M.A., Bosch B.J., Frenz B., Rottier P.J., DiMaio F., Rey F.A., Veesler D. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531:114–117. doi: 10.1038/nature16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Tortorici M.A., Frenz B., Snijder J., Li W., Rey F.A., DiMaio F., Bosch B.J., Veesler D. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat. Struct. Mol. Biol. 2016;23:899–905. doi: 10.1038/nsmb.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J.M. Morphogenesis of a virus in cats with experimental feline infectious peritonitis. Virology. 1970;41:191–194. doi: 10.1016/0042-6822(70)90070-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J.M., Munn R.J., Gribble D.H., Dungworth D.L. An observation of feline infectious peritonitis. Vet. Rec. 1968;83:416–417. doi: 10.1136/vr.83.16.416. [DOI] [PubMed] [Google Scholar]
- Wesseling J.G., Vennema H., Godeke G.J., Horzinek M.C., Rottier P.J. Nucleotide sequence and expression of the spike (S) gene of canine coronavirus and comparison with the S proteins of feline and porcine coronaviruses. J. Gen. Virol. 1994;75(Pt 7):1789–1794. doi: 10.1099/0022-1317-75-7-1789. [DOI] [PubMed] [Google Scholar]
- White J.M., Delos S.E., Brecher M., Schornberg K. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 2008;43:189–219. doi: 10.1080/10409230802058320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.M., Whittaker G.R. Fusion of enveloped viruses in endosomes. Traffic. 2016;17:593–614. doi: 10.1111/tra.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker G.R., André N., Millet J.K. Improving virus taxonomy by re-contextualizing sequence-based classification with biologically-relevant data: the case of the Alphacoronavirus 1 species. mSphere. 2018;3:e00463–00417. doi: 10.1128/mSphereDirect.00463-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe I.N., van Beurden S.J., Weerts E.A., Verheije M.H. The avian coronavirus spike protein. Virus Res. 2014;194:37–48. doi: 10.1016/j.virusres.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C., Schwegmann-Wessels C., Cavanagh D., Neumann U., Herrler G. Sialic acid is a receptor determinant for infection of cells by avian Infectious bronchitis virus. J. Gen. Virol. 2006;87:1209–1216. doi: 10.1099/vir.0.81651-0. [DOI] [PubMed] [Google Scholar]
- Wolfe L.G., Griesemer R.A. Feline infectious peritonitis. Pathol. Vet. 1966;3:255–270. doi: 10.1177/030098586600300309. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M., Zheng B.J., Chan K.H., Yuen K.Y. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzer W.J., Obojes K., Vlasak R. The sialate-4-O-acetylesterases of coronaviruses related to mouse hepatitis virus: a proposal to reorganize group 2 Coronaviridae. J. Gen. Virol. 2002;83:395–402. doi: 10.1099/0022-1317-83-2-395. [DOI] [PubMed] [Google Scholar]
- Xiong X., Tortorici M.A., Snijder J., Yoshioka C., Walls A.C., Li W., McGuire A.T., Rey F.A., Bosch B.J., Veesler D. Glycan shield and fusion activation of a deltacoronavirus spike glycoprotein fine-tuned for enteric infections. J. Virol. 2017 doi: 10.1128/JVI.01628-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Cao D., Zhang Y., Ma J., Qi J., Wang Q., Lu G., Wu Y., Yan J., Shi Y., Zhang X., Gao G.F. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat. Commun. 2017;8:15092. doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Buckles E., Whittaker G.R. Expression of the C-type lectins DC-SIGN or L-SIGN alters host cell susceptibility for the avian coronavirus, infectious bronchitis virus. Vet. Microbiol. 2012;157:285–293. doi: 10.1016/j.vetmic.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook B.C., King N.W., Robison R.L., McCombs H.L. Ultrastructural evidence for the viral etiology of feline infectious peritonitis. Pathol. Vet. 1968;5:91–95. [Google Scholar]