Abstract
Steroids that contain a 3-hydroxyl group (3-OH steroids) are widely distributed in nature. During analysis with ESI-MS, they easily become dehydrated while in the protonated form, resulting in the production of several precursor ions and leading to low sensitivity of detection. To address this analytical challenge, here, we developed a method for the quantitation of 3-OH steroids by LC-MS/MS coupled with post-column addition of lithium (Li) ions to the mobile phase. The Li ion has a high affinity for the keto group of steroids, stabilizing their structures during ionization and permitting detection of analytes exclusively as the lithiated form. This not only improved the intensities of the precursor ions, but also promoted the formation of typical lithiated fragment ions. This improvement made the quantitation by multiple reaction monitoring more sensitive and reliable, as evidenced by 1.53–188 times enhanced detection sensitivity of 13 steroids that contained at least one keto and two hydroxyl groups or one keto and one 5-olefinic double bond, among 16 different 3-OH steroids. We deployed our newly developed method for profiling steroids in mouse brain tissue and identified six steroids in one tissue sample. Among these, 16-hydroxyestrone, tetrahydrocorticosterone, and 17α-hydroxypregnenolone were detected for the first time in the mouse brain. In summary, the method described here enables the detection of lithiated steroids by LC-MS/MS, including three 3-OH steroids not previously reported in the mouse brain. We anticipate that this new method may allow the determination of 3-OH steroids in different brain regions.
Keywords: mass spectrometry, hormones/steroid, brain lipids, corticosteroids, estrogen, androgens, 3-hydroxyl steroid hormone, mouse brain, liquid chromatography, ultra-performance liquid chromatography-tandem mass spectrometry
Steroids influence brain development, behavior, cognition, neuroplasticity, and neuroinflammation, and can be synthesized within the brain starting from a cholesterol backbone (a planar tetracyclic ring) (1–3). Steroid hormones can be enzymatically transformed into different classes of steroids, including estrogens (female reproductive steroids), androgens (male reproductive steroids), progestogens (pregnancy steroids), and corticosteroids (stress steroids) (4).
The three position of a steroid skeleton contains either a keto group or a hydroxyl group. Progesterone, cortisol, testosterone, and some other steroids that are produced via the main biosynthesis routes contain a 3-keto group, while pregnenolone (P5), dehydroepiandrosterone (DHEA), and some other steroids contain a 3-OH group (3-OH steroids). The 3-OH steroids play important roles in the brain. Tetrahydrocortisol (TH-COL), tetrahydrocortisone (TH-COR), 3β,5α-tetrahydrodeoxycorticosterone (TH-DOC), tetrahydrodeoxycortisol (THS), and tetrahydrocorticosterone (THB) are tetra-hydrocorticosteroids that are associated with stress (5, 6). P5, DHEA, androsterone (An), and their hydroxylated derivatives, such as 17α-hydroxypregnenolone (17-OH-P5), 7α-hydroxydehydroepiandrosterone (7-OH-DHEA), and 11-β-hydroxyandrosterone (11-OH-An), are classified as neurosteroids because of their relevance as modulators of various receptors, such as the GABA receptor (3, 7–9).
Because of its high sensitivity, selectivity, and multi-analyte capacity, LC-MS/MS has become a popular method for the analysis of steroids in biosamples (10–13). Compared with steroids containing a 3-keto group, the structures of which are maintained during ionization, 3-OH steroids are easily dehydrated when measurements are made in the positive-ion mode (14). In this case, the precursor ions of 3-OH steroids for the multiple reaction monitoring (MRM) mode are [M+H-H2O]+ or [M+H-2H2O]+ rather than [M+H]+ (11, 12, 15–18). However, the formation of multiple dehydration products in the ion source ultimately decreases the sensitivity of detection of the precursor ion to be subjected to MRM. The lack of product ions derived from the backbone might also result in an ambiguous structural characterization. Sodium ions, which have an affinity similar to protons and are often observed as the adduct ion, also have an effect on the stability of quantitation in MRM. This dehydration phenomenon can be essentially eliminated when 3-OH steroids are deprotonated or ammoniated. In the former case, estradiol and estriol (E3) were measured with [M-H]− as the precursor ion (16). However, not all 3-OH steroids are deprotonated in the negative mode. [M+NH4]+ was used for the detection of P5 and DHEA when the ESI was operated at room temperature (19). Silver ions have also been used to form adducts of 11-OH-An and DHEA (20). However, because Ag is made up of two stable isotopes, 107Ag (51.839%) and 109Ag (48.161%), all possible silverated adducts are observed at approximately half intensity. Moreover, [M+Ag-2H2O]+ is also produced to some extent and has a high affinity for certain types of sulfur compounds, which is, in general, not desirable for the analysis of biosamples. Chemical derivatization is another strategy for avoiding dehydration when the ketone or phenolic group is blocked: The Girard-P reagent or hydroxylamine has been used for the derivatization of the keto-steroids; dansyl chloride is a well-known estrogen labeling reagent (12, 13, 21, 22). However, chemical derivatization, in general, requires additional incubation, purification, or extraction steps (23).
Lithium (Li) ions are able to form cationic adducts with a variety of compounds (24, 25). Adams and Gross (26, 27) used the Li ion to improve the fragmentation of lipids and the determination of the double bond location in 1980s. A post-column modification method using 0.25 mM Li chloride (LiCl) was used to analyze Li adducts of ouabain in human serum (28, 29). Collision-induced dissociation (CID) mass spectra of Li-adducted pseudoprotodioscin and methyl protodioscin were obtained using ESI-MSn (30). Having a structure similar to a steroid, 1α,25-dihydroxyvitamin D was determined in lithiated form by adding Li acetate (LiOAc) to the mobile phase (31, 32). Using a similar strategy, steroid glycoside conjugates were quantified in porcine plasma samples (33). These cases prompted Keski-Rahkonen et al. (34) to develop a similar method for the analysis of estradiol in human serum and tissue. However, in this case, a Li adduct was not efficiently formed, which could be explained by the fact that estradiol contains only two hydroxyl groups, thus lowering the affinity of this compound for Li ions, compared with 3-OH steroids with multiple hydroxyl and/or keto groups. In 2013, Bao, Wang, and Tang (35) used 25 mM LiOAc in the mobile phase to form the Li adduct of 20(S)-protopanaxadiol. Because 20(S)-protopanaxadiol, which does not contain a keto group, showed a moderate affinity for Li, a relatively higher concentration (25 mM) of LiOAc was used. Moreover, the setting of the MRM transition ([M+Li]+ → [M+Li-H2O]+) seemed unreliable because many natural metabolites also produce dehydrated ions upon CID. All of these compounds, described above, have a steroid skeleton, and all contain a 3-OH or a 3-OR group. The free steroid hormones, as reported above, have never been quantified in the form of their Li adducts by LC-MS/MS.
In this work, a method for the quantitation of steroids containing a 3-hydroxyl group by LC-MS/MS coupled with the post-column introduction of Li ions in the mobile phase was developed. Because Li ions had a higher affinity for carbonyl groups, compared with protons and ammonium and sodium ions, it was possible to detect 3-OH steroids that contain keto groups solely as the lithiated forms, leading to an enhancement in their ion intensities. In addition, 3-OH steroids that contain one keto and two hydroxyl groups and give typical lithiated fragment ions derived from the lithiated precursors made quantitation by MRM more sensitive and stable. The method was applied to the analysis of mouse brain tissue and allowed six steroids to be identified from one mouse brain tissue sample, among which 16-hydroxyestrone (16-OH-E1), THB, and 17-OH-P5 were, for the first time, identified in the mouse brain.
MATERIALS AND METHODS
Chemicals
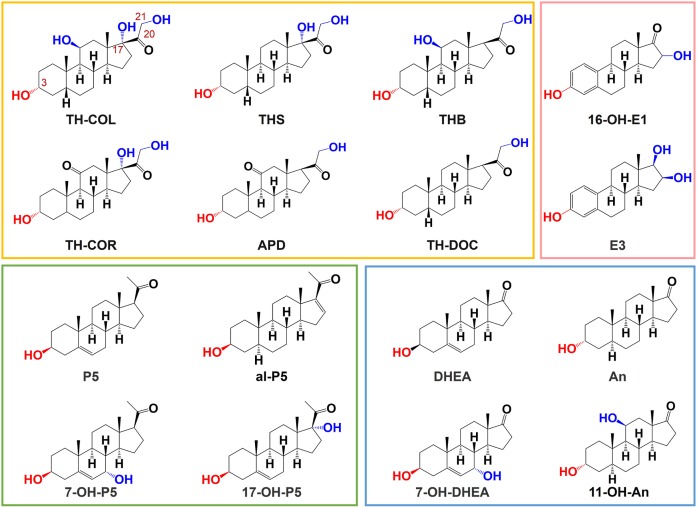
HPLC grade methanol (MeOH), acetonitrile (ACN), and ethyl acetate, 99.998% trace metals basis LiCl, 99.95% trace metals basis LiOAc, analytical reagent-grade acetate acid, sodium chloride (NaCl), P5, 17-OH-P5, DHEA, E3, dehydroepiandrosterone-2,2,3,4,4-d5 (DHEA-d5), and P5-20,21-13C2-16,16-d2 (P5-13C2,d2) were purchased from Sigma-Aldrich (Tokyo, Japan). Alphadolone (APD), allopregnenolone (al-P5), TH-COL, TH-COR, 11-OH-An, THB, TH-DOC, THS, 16-OH-E1, 16-keto-17β-estradiol-2,4,6,6,9-d5 (16-keto-E2-d5), and TH-COR-2,2,4,4,21,21-d6 (TH-COR-d6) were supplied by Toronto Research Chemicals (North York, Canada). An was purchased from Tokyo Chemical Industry (Tokyo, Japan). 7-OH-DHEA and 7α-hydroxypregnenolone (7-OH-P5) were supplied by Nacalai Tesque (Kyoto, Japan). HPLC grade formic acid was obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Ultrapure water was prepared using a Puric ω (Organo, Tokyo, Japan). The HF Bond Elute C18 (1 ml, 60 mg) was purchased from Agilent Technologies, Inc. Santa Clara, CA. The structures of the 3-OH steroids in this analysis are shown in Fig. 1.
Fig. 1.
Structures of the 3-OH steroids used in this analysis. The boxes shown in yellow, pink, green, and blue represent different classes of steroid hormones, i.e., corticosteroids, estrogens, progestogens, and androgens, respectively.
Animals
All animal experiments were performed in compliance with the Animal Experimental Committee of the Institute for Protein Research at Osaka University. C57BL/6NJcl mice were obtained from CLEA Japan, Inc. (Tokyo, Japan). Mice were maintained in a quiet environment with the temperature controlled at 24 ± 1°C. Mice were on a 12 h light/dark cycle with free access to standard chow and water at all times. Mice, at the age of 7 weeks, were deeply anesthetized with isoflurane, and blood samples were obtained via cardiac puncture. The mice were subsequently euthanized by decapitation and whole brains were quickly removed from the skull. The removed whole brains were immediately frozen in liquid nitrogen and stored in a −80°C freezer until further use.
Sample preparation
Samples were thawed on ice. A 7.5 ml aliquot of 0.25 M acetate acid was added per gram of sample. Brains were homogenized in a Nippi (Tokyo, Japan) Biomasher SP disposable pestle with a power masher electronic attachment. After thawing, the brains were placed in the pestle and homogenized while buffer was added incrementally. After the entire amount of buffer was added and the mixture homogenized thoroughly, the homogenate was sonicated with a TAITEC (Tokyo, Japan) VP-050 (settings PWM 30%, on:off cycle 4:1 s) for 120 s. Samples were frozen until further use.
After homogenization, the homogenate was divided into three parallel samples and transferred to 10 ml glass tubes for liquid-liquid extraction. Internal standards (6 μl of a mixture of 200 pg μl−1 TH-COR-d6 and P5-13C2,d2 and 400 pg μl−1 of 16-keto-E2-d5, and DHEA-d5 in 40% MeOH) were added to each brain sample. A 3,000 μl portion of ethyl acetate and NaCl was added until saturation was reached. The mixture was then vortexed for 10 min and centrifuged for 10 min at 700 g. The organic layer was collected, and the remaining matrix was reextracted using the above procedure two times. The three combined organic layers were dried under a stream of nitrogen at room temperature. The subsequent residue was redissolved in 400 μl of 50% ACN and diluted with 1,600 μl of ultrapure water. The supernatant was loaded on a Bond Elute, which was pretreated with 2 ml 80% ACN and 2 ml 10% ACN, followed by washing three times with 1,000 μl of 10% ACN and eluted with 1,000 μl of 80% ACN. The eluate was evaporated to dryness with a speed-vac and the resulting solid redissolved in 12 μl of 40% MeOH. The above extraction procedures were optimized as described in the supplemental data.
LC-MS/MS
The UPLC-MS/MS analysis was performed using an Agilent 1290 Infinity II and a 6470 triple quadrupole mass spectrometer equipped with an ESI ion source (Agilent Technologies, Inc.). Chromatographic separation was achieved with an Agilent Eclipse Plus C18 RRHD 2.1 × 100 mm, 1.8 μm column, which was maintained at 40°C. The mobile phase consisted of solvent A (0.1% formic acid in deionized water) and solvent B (0.1% formic acid in MeOH). The elution gradient was from 40% to 80% B from 0 to 8 min, maintained at 80% B from 8 to 10 min, 80–40% B from 10.0 to 10.10 min, and held at 40% B from 10.1 to 13.1 min. The injection volume was 10 μl. The MRM mode was applied for the detection and quantitation of all steroids with two transitions optimized for each targeted compound. The ESI source parameters were set as follows: the capillary voltage was −4,500 V in the positive ion mode, the nebulizer (N2) pressure was 55 psi, the drying gas (N2) temperature and flow rate were 210°C and 13 liters min−1, respectively, and sheath gas temperature was 275°C. The post-column addition of 0.2 mM LiCl in water was carried out with an Agilent 1100 binary pump as the auxiliary pump. As shown in supplemental Fig. S1, the column effluent (0.4 ml min−1) and auxiliary solution (0.4 ml min−1) were mixed at the T-connector and passed through two in-line filters (Agilent 1290 inline filter, 0.3 μm), which were connected in tandem, prior to reaching to the ion source. The MRM parameters for 3-OH steroids are summarized in supplemental Table S1. Note that because Li ion salts, such as LiCl and LiOAc, are nonvolatile, the amount of LiCl introduced to the ion source was reduced to 0.2 mM, which was sufficient to form complete Li adducts of the 3-OH steroids (see Fig. 2), and the absolute amount of LiCl introduced into MS was reduced to 0.48 μmol (20.3 μg) per analysis by controlling the two-way switching valve to either MS (6 min for the analysis) or waste (7 min for the column equilibration) by an Agilent MassHunter Acquisition system (see supplemental Fig. S1). A Li adduct of 20(S)-protopanaxadiol was observed with 25 mM LiOAc in a previous report (35), which might cause ionization to be suppressed or plug the capillary inlet.
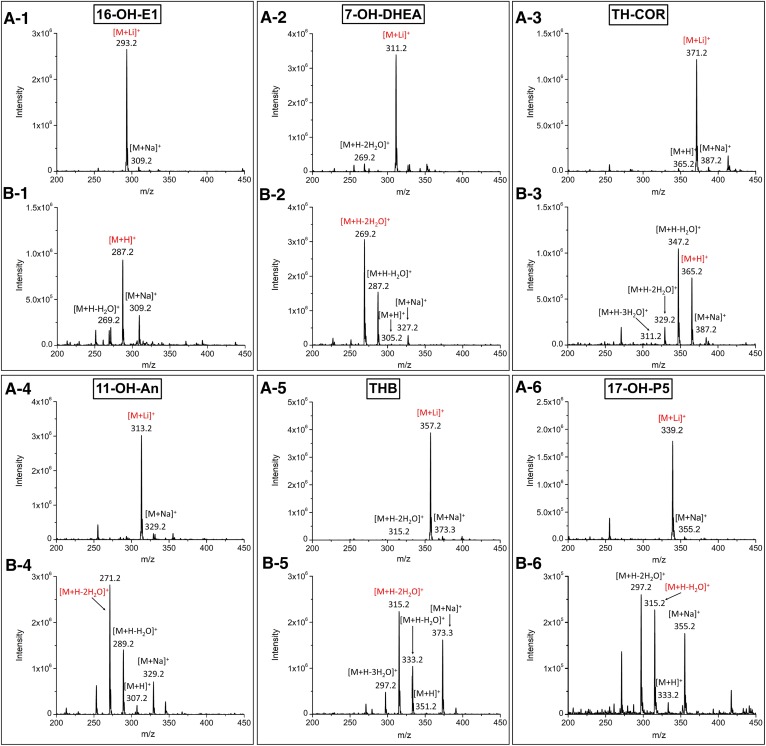
Fig. 2.
ESI-MS spectra of the 3-OH steroids. The mobile phase consisted of solvent A [0.2 mM LiCl in deionized water (A) and 0.1% FA in deionized water (B)] and solvent B (MeOH). 1, 16-OH-E1; 2, 7-OH-DHEA; 3, TH-COR; 4, 11-OH-An; 5, THB; 6, 17-OH-P5. Two hundred nanograms of each steroid on column, in 20% of solvent B, were introduced directly to the mass spectrometer. The red ions represent the precursor ions used for the MRM analyses.
To compare the Li adduct method (Li method) with the previous protonated method (H method), ESI source parameters were set similarly in both methods, which were slightly different from the above: the nebulizer (N2) pressure was set at 30 psi and the sheath gas temperature was 375°C to enhance the dehydration of steroids. The auxiliary pump was off when conducting the H method. The MRM parameters for the H method are summarized in supplemental Table S2.
Data processing
Data were acquired using an Agilent MassHunter Acquisition system and processed using Agilent MassHunter Quantitive Analysis, Microsoft Excel, and OriginLab 2018 (Academic). Method validation is described in the supplemental data.
RESULTS AND DISCUSSION
MS
The mass spectrometric behavior and tandem mass spectrometric behavior of the lithiated steroids were examined in the positive-ion ESI mode by direct infusion. As shown in Fig. 2, when the mobile phase was aqueous 0.1% FA and MeOH, numerous signals for dehydrated ions and sodium adducts were observed in the mass spectra. Because TH-COR contains three hydroxyl groups, multi-dehydrated ions were observed, as shown in . This phenomenon was also observed for other steroids, such as 7-OH-DHEA (Fig. 2B-2), 11-OH-An (Fig. 2B-4), THB (Fig. 2B-5), and 17-OH-P5 (Fig. 2B-6), all of which contain two hydroxyl groups. The sodium adduct peaks were very strong for 16-OH-E1 (Fig. 2B-1), 11-OH-An, THB, and 17-OH-P5. When the mobile phase was aqueous 0.2 mM LiCl and MeOH, the Li adducts became the dominant peaks in the spectra (Fig. 2A). Li+, which acts as a Lewis acid, has a stronger affinity for the lone pair electrons of the hydroxyl or carbonyl groups on the steroids compared with other metal ions.
MS/MS fragmentation
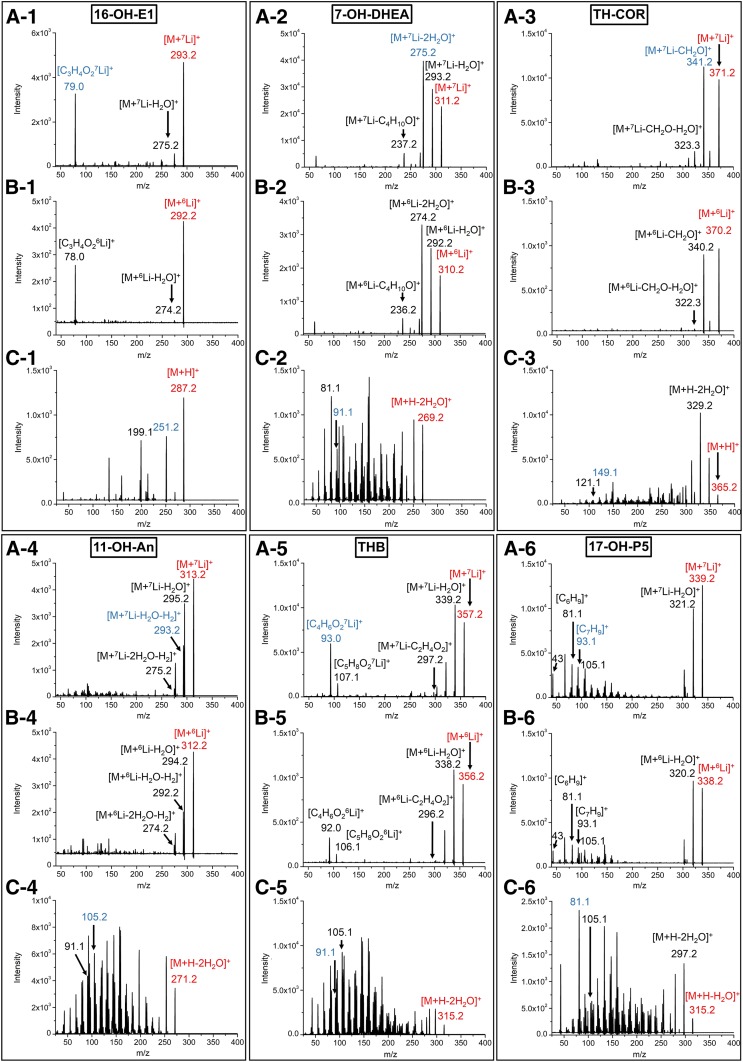
Product ions for lithiated steroids were mainly derived from dehydration that occurred at the hydroxyl groups or by ring-cleavage (Fig. 3) and were clearly observed with a relatively high collision energy (more than 20 eV) (supplemental Table S1). Because the Li ions were preferably attached to keto groups, the fragmentation behavior was simple, and some specific fragments were produced (Fig. 3A). Meanwhile, protonated steroids (dehydrated forms) showed complex fragmentation patterns upon CID (Fig. 3C). Those fragments could be originated from the tetracyclic rings and were produced with a higher collision energy (supplemental Table S2). In addition, because the proton was delocalized in the molecules, the complex fragmentation, which was mainly observed at a 14 Da interval, can be attributed to the cleavage in each carbon-carbon bond on the skeleton. The satellite peaks with ±2 Da to the main peaks could be accounted for by the unsaturated double bonds in the structure. Owing to the complexity and dispersion of the fragment ions, it becomes difficult to define the MRM transition and to obtain sufficient intensities for the MRM transitions.
Fig. 3.
Product ion spectra of the 3-OH steroids. The precursor ions are [M+7Li]+ (A) and [M+6Li]+ (B). The mobile phase consisted of solvent A [0.2 mM LiCl in deionized water (A, B) and 0.1% FA in deionized water (C)] and solvent B (MeOH). 1, 16-OH-E1; 2, 7-OH-DHEA; 3, TH-COR; 4, 11-OH-An; 5, THB; 6, 17-OH-P5. The amount of steroid injected was: 16-OH-E1 and 7-OH-DHEA, 1 ng; TH-COR and THB, 1 ng for A and B and 20 ng for C; 11-OH-An and 17-OH-P5, 20 ng. The analytes were introduced through a two-way valve directly to the mass spectrometer in 20% of solvent B. The m/z of the product ions in each spectrum reflected the ion forms assigned to the structures. The red ions represent the precursor ions for the product ion scan. The blue ions represent the product ions used for the quantitative analysis by MRM.
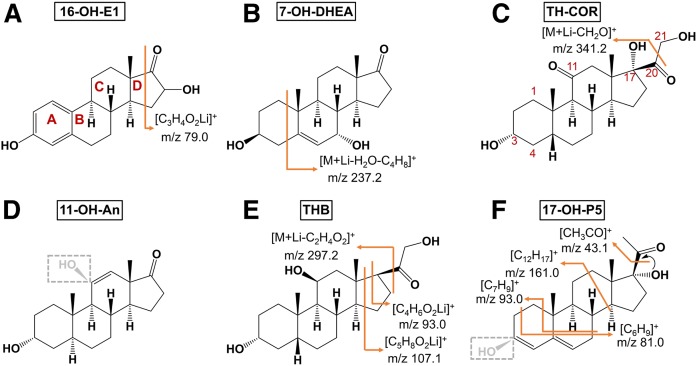
Prominent fragment peaks were observed for 16-OH-E1 at m/z 79.0 (Fig. 4A), which were determined to be lithiated by comparison with the MS/MS from the 6Li-adduct (Fig. 3A-1, B-1), and were produced by the cleavage of the D ring. In the case of 7-OH-DHEA and 7-OH-P5, the loss of [H2O+C4H8] from the A ring was found (Fig. 4B, supplemental Table S1), which were confirmed by MS/MS of the 6Li adduct (Fig. 3A-2, B-2). [M+Li-CH2O]+ was the most intense peak for TH-COL, TH-COR, and THS (Fig. 3A-3). These steroids contain an OH group at the 17 position, which promotes the loss of CH2O at position 20 (Fig. 4C). Dehydrogenation was observed only for 11-OH-An following dehydration (Fig. 3A-4), and gave a [M+Li-H2O-H2]+ ion (Fig. 4D). THB, APD, and TH-DOC showed prominent peaks at m/z 93 and m/z 107 (Fig. 3A-5), which could be assigned, respectively, to [C4H6O2Li]+ and [C5H8O2Li]+ and were derived from the D ring (Fig. 4E). The m/z 297.2 peak was produced by the loss of C2H4O2 at the 17 position. P5 and 17-OH-P5 gave fragment ions at m/z 93.0 (Fig. 4F), but this fragment was obviously different from that of corticosteroids because it was not shifted in the MS/MS from the 6Li-adduct precursor, suggesting that this fragment did not contain Li and originated from the cleavage of the A and B rings (Fig. 3A-6, B-6).
Fig. 4.
Fragmentation behavior of lithiated 16-OH-E1 (A), 7-OH-DHEA (B), TH-COR (C), 11-OH-An (D), THB (E), and 17-OH-P5 (F).
Chromatography
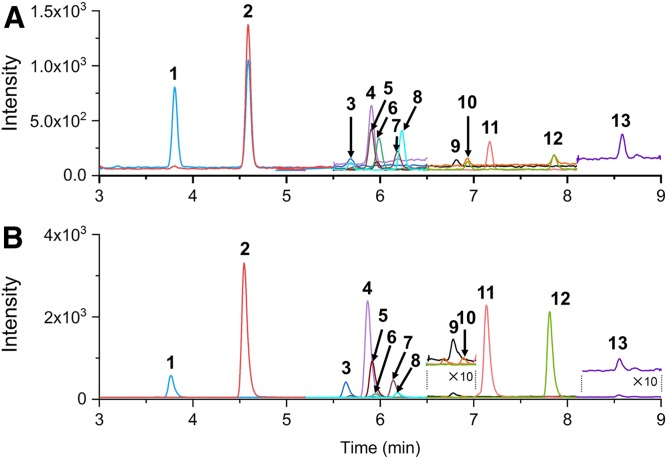
MeOH was used as the mobile phase for separating the lithiated 3-OH steroids and gave good separation and higher intensity for 3-OH steroids, over ACN (Fig. 5, supplemental Fig. S2). This is partly due to the splitting of the precursor ion into the unique adduct, [M+ACN+Li]+, during the ionization (supplemental Fig. S3). Such an adduct ion was not observed at all in cases where MeOH was used as the solvent, and the degree of formation of adduct ions varied, depending on the specific steroid. The thirteen steroids examined in this study could be analyzed within 9 min (Fig. 5) by the solvent system, 0.1% FA in water (mobile phase A)/0.1% FA in MeOH (mobile phase B). When 0.1 mM LiCl in water as mobile phase A and MeOH as mobile phase B were used, the retention time for each steroid was retarded, indicating that the acidic solvent system results in species that are relatively more hydrophilic than those produced in the neutral solvent system (supplemental Fig. S4). It is noteworthy that the use of a lower percentage of organic solvent resulted in an improved ionization efficiency for all of the steroids examined in this study (supplemental Fig. S2). Thus, in order to prevent the steroids from being eluted with higher concentrations of mobile phase B, the aqueous Li ion-containing solution was mixed into the effluent after the column via an auxiliary pump. In view of the increased sensitivity of the steroids, post-column mixing of an equivalent volume of aqueous solution to that of the separation pump decreased the percentage of organic solvent by half (see the Materials and Methods section), and thereby increased overall detection sensitivity (supplemental Fig. S2). The mixing of the solvents from the main and axial pumps could be efficiently achieved by using two in-line filters (Agilent 1290 inline filter, 0.3 μm), connected in tandem, after the T-piece that connects the two flows. In addition, several concentrations of Li, ranging from 0.05 to 1 mM of LiCl, were examined in order to produce lithiated forms more efficiently by using the same flow rate as that of the main pump. As a result, nearly all of the Li adduct ions of steroids could be observed as the predominant species with no less than 0.2 mM LiCl (supplemental Fig. S5, Fig. 2). This suggests that the 0.1 mM LiCl in the spraying droplets is sufficient to allow all of the 3-OH steroids to stably form Li adducts during ionization. Note that Li ions could also be supplied with LiOAc; however, soon after mixing with the FA (0.1%) solvent, the counter anion of AcO− was readily replaced with HCOO− to form LiOOCH, which had a propensity to be associated with steroids to form [M+(LiOOCH)+Li]+ (supplemental Fig. S6), the degree of which varied depending on the specific steroid. This adduct ion was not observed at all in cases when LiCl was used, thus allowing 3-OH steroids to be observed with a higher intensity (see supplemental Fig. S7).
Fig. 5.
Chromatograms for the separation of steroids with 100 pg being injected: without LiCl (H method) (A); with LiCl (Li method) (B) (see the Materials and Methods). 1, 16-OH-E1; 2, 7-OH-DHEA; 3, TH-COL; 4, 7-OH-P5; 5, TH-COR; 6, 11-OH-An; 7, THB; 8: APD; 9, DHEA; 10, 17-OH-P5; 11, TH-DOC; 12, THS; 13, P5. The MRM transitions used in this analysis were shown in supplemental Tables S1 and S2. The peak traces depicted in colors correspond to those obtained by the transitions indicated in bold, which were used for the quantification.
Features of high sensitivity
The LOD (signal to noise ratio = 3) and limit of quantitation (LOQ) (signal to noise ratio = 10) of the standard solution was determined with (Li method) and without (H method) the use of a LiCl solution. The detection sensitivity enhancement (fold) was defined as the ratio of the LOD obtained by the H method over that by the Li method. The sensitivity for 16-OH-E1, 7-OH-DHEA, TH-COL, 7-OH-P5, TH-COR, 11-OH-An, APD, THB, 17-OH-P5, TH-DOC, and THS were enhanced by 1.78–188 times by the Li method (supplemental Table S3). These steroids contain at least two hydroxyl groups and one carbonyl group (Fig. 1); whereas, the sensitivities for E3, An, and al-P5 were decreased by about one tenth, which can be attributed to the poor formation of fragment ions. The structure of 16-OH-E1 is similar to that of E3, but its sensitivity was increased by 3.14 times (see Fig. 1). This can be attributed to the fact that 16-OH-E1 contains a carbonyl group at the 17 position, which has a relatively higher affinity for Li+ than a hydroxyl group and, therefore, produced an intense fragment ion at m/z 79.0 (see Fig. 4). In addition, compared with 11-OH-An, An itself contains only a single hydroxyl group, thus resulting in a lower abundance of the dehydrated ion, [M+Li-H2O]+, upon CID (Fig. 1). The al-P5 molecule, which lacks an olefinic double bond at the five position, gave a lower intensity of [M+Li-H2O]+, compared with P5, because al-P5 is unable to form conjugated double bonds after dehydration (Fig. 1). The results for P5 and DHEA, both of which contain a single hydroxyl group but could form conjugated double bonds upon dehydration, showed slight enhancement by 1.53- and 2.69-fold over those with H method, respectively. Based on these findings, we conclude that 3-OH steroids that contain at least one carbonyl group and two hydroxyl groups or one carbonyl group and a 5-olefinic double bond (13 steroids) would give better results when the Li method is used (supplemental Table S3). The thirteen steroids could be detected with a lower background by the Li method within 9 min (Fig. 5).
Optimization of sample preparation
The liquid-liquid extraction coupled with the solid phase extraction preparation procedure was optimized. Details can be found in the supplemental data.
Blank matrix
For method validation, a blank mouse brain matrix was prepared. Because neurosteroids play important roles in mouse brain development, it was impossible to find a mouse species for which the brains contained no steroids. Charcoal extraction was used for the blank matrix preparation procedure (36). However, charcoal might strip out the interfering compounds, which might be the weakness of using a blank matrix prepared in this way.
Method validation
The method was validated in terms of the LOD, LOQ, linearity range, precision, accuracy, matrix effect, and recovery. The LOQs for the 13 steroids in mouse brain tissue blank matrix were determined to be 0.024–1.540 pg mg−1. Details can be found in the supplemental data.
Biosample analysis
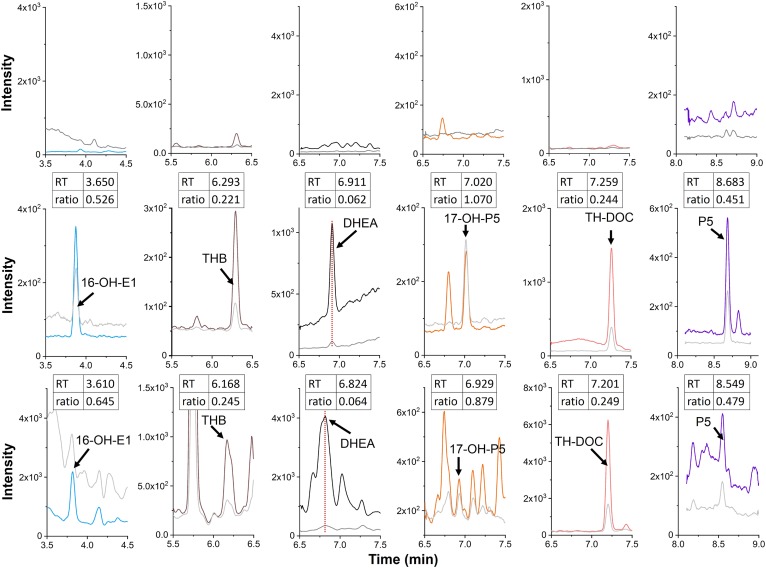
The new method was applied for the analysis of steroids in brain tissue from five healthy male mice. Six steroids were identified and quantified in whole brain samples. The steroids were identified by comparing the retention time and the peak intensity ratio of two MRM transitions of the samples with that for a spiked blank matrix sample. The criteria for the identification was as follows: the relative difference in retention time should be less than 2%; the relative difference in the ratio of the peak intensity of two MRM transitions should be less than 20%. The method allowed six steroids to be identified from one mouse brain tissue sample (Fig. 6). Note that there are shoulders to the THB peak on the right side and to the DHEA peak on the left side (lower panel of Fig. 6), which were, most probably, endogenous metabolites in the brain extract that were slightly observed for the blank matrix sample (upper panel of Fig. 6). As shown in Table 1, the amount of steroids in the tissue varied from sample to sample, ranging from 0.117 to 27.01 pg mg−1. In earlier studies, DHEA, TH-DOC, and P5 were quantified in whole rat brain at concentrations of 0.05–7 pg mg−1 (37–40). The levels of TH-DOC and P5 in the mouse brain are similar to those in the rat brain. However, in our analysis, the level of DHEA was found to be much higher in the mouse brain tissue. The previous work by Sjövall and colleagues indicated that the cholesterol autoxidation took place mainly during chemical derivatization for GC-MS, where the high temperature and the existence of catalysts for the derivatization promoted the formation of DHEA and P5 from cholesterol (41). In our experiment, the sample collection and homogenization were conducted on ice and the extraction was performed at room temperature (see the Materials and Methods). Meanwhile, Jäntti et al. found DHEA in the mouse brain, however the concentration was lower than LOQ (2.88 pg/mg) (19). We might speculate that the relatively high level of DHEA obtained in the present study could be ascribed to the species of mice used. THB was detected in the brains of rats that had been subjected to immobilization but not in brains from unstressed rats (42). This is the first report of the presence of 16-OH-E1, THB, and 17-OH-P5 in an unstressed control mouse brain.
Fig. 6.
MRM chromatograms of steroids from a charcoal-treated blank matrix sample (upper), a charcoal-treated spiked sample (middle), and a whole mouse brain (lower). Each steroid was identified using two MRM transitions (supplemental Table S1). The colored traces in each chromatogram are depicted with the values (= transitions) in bold in supplemental Table S1, which were used for the quantification (16-OH-E1, m/z 293.2→79.1; THB, m/z 357.2→93; DHEA, m/z 295.2→277.2; 17-OH-P5, m/z 339.2→93.1; TH-DOC, m/z 341.2→93; and P5, m/z 323.2→43.1). The gray traces represent the transitions used for the qualification in supplemental Table S1 (16-OH-E1, m/z 293.2→275.2; THB, m/z 357.2→107.1; DHEA, m/z 295.2→105.1; 17-OH-P5, m/z 339.2→81.1; TH-DOC, m/z 341.2→107; and P5, m/z 323.2→105.1). The amounts of steroids spiked to the charcoal-treated sample were 50 pg for 16-OH-E1, THB, and TH-DOC, and 500 pg for DHEA, 17-OH-P5, and P5. The ratios indicated in the insets were calculated on the basis of the peak height ratios of the two transitions (normal over bold values, obtained for each steroid, in supplemental Table S1).
TABLE 1.
Quantitative results for the steroids of whole mouse brain tissue and a comparison with values reported in previous studies
| Steroid | Concentration in Whole Brain (pg mg−1), n = 5a | Literature Concentration in Male Rat Brain (pg mg−1) | Literature Concentration in Male Rat Cortex (pg mg−1) |
| 16-OH-E1 | 0.97–2.38 | — | — |
| THB | 0.117–0.462 | 1.38 ± 0.54 (stressed) (42) | — |
| DHEA | 15.59–27.01 | 0.05–0.11 (37), 0.2–0.6 (39) | 46–79 (43) |
| 17-OH-P5 | <LOQ | — | — |
| TH-DOC | 0.129–0.535 | 1 (40) | — |
| P5 | 1.64–1.98 | 0.60–1.2 (37), 7 (38) | 12–15 (44) |
The concentration in each individual brain is shown in supplemental Table S7.
In conclusion, the objective of this study was to develop a method for the sensitive and universal quantitation of multi-class 3-OH steroids in a mouse brain sample. By the post-column addition of Li ions into the mobile phase, the sensitivity and selectivity could be significantly enhanced by 1.53–188 times for 13 different types of 3-OH steroids. To the authors’ knowledge, this represents the first published method describing the analysis of lithiated steroids by LC-MS/MS and the first report of the detection of 16-OH-E1, THB, and 17-OH-P5 in a control mouse brain; although the actual role of these steroids in the brain will require further investigation. The method has the potential for allowing the determination of various 3-OH steroids in separate regions of the brain, such as the cerebrum, cerebellum, hippocampus, and hypothalamus. It is noteworthy that the H method had a higher sensitivity for An, E3, and 3-keto steroids. In addition, Because the analytical settings for the H method and the Li method are quite similar, they could be simultaneously operated in conjunction with ordinary LC-MS/MS, which would allow for a more comprehensive analysis of multi-class steroids in bio-samples.
Supplementary Material
Acknowledgments
The authors appreciate the assistance of Mr. Yoshiyuki Nishioka from the Kinryo Electric Co. Ltd. and Dr. Di Chen for technical support and Miss Huibin Zhu who helped to prepare appropriately sized figures.
Footnotes
Abbreviations:
- ACN
- acetonitrile
- al-P5
- allopregnenolone
- An
- androsterone
- APD
- alphadolone
- CID
- collision-induced dissociation
- DHEA
- dehydroepiandrosterone
- DHEA-d5
- dehydroepiandrosterone-2,2,3,4,4-d5
- E3
- estriol
- 16-keto-E2-d5
- 16-keto-17β-estradiol-2,4,6,6,9-d5
- Li
- lithium
- LiCl
- lithium chloride
- LiOAc
- lithium acetate
- LOQ
- limit of quantitation
- MeOH
- methanol
- MRM
- multiple reaction monitoring
- NaCl
- sodium chloride
- 3-OH steroids
- steroids that contain a 3-hydroxyl group
- 11-OH-An
- 11-β-hydroxyandrosterone
- 7-OH-DHEA
- 7α-hydroxydehydroepiandrosterone
- 16-OH-E1
- 16-hydroxyestrone
- 7-OH-P5
- 7α-hydroxypregnenolone
- 17-OH-P5
- 17α-hydroxypregnenolone
- P5
- pregnenolone
- P5-13C2
- d2, pregnenolone-20,21-13C2-16,16-d2
- THB
- tetrahydrocorticosterone
- TH-COL
- tetrahydrocortisol
- TH-COR
- tetrahydrocortisone
- TH-COR-d6
- tetrahydrocortisone-2,2,4,4,21,21-d6
- TH-DOC
- 3β,5α-tetrahydrodeoxycorticosterone
- THS
- tetrahydrodeoxycortisol
This study was supported by Japan Society for the Promotion of Science Grant-in-Aid for Specially Promoted Research 17H06096 (to Y.F.). The authors declare that they have no conflicts of interest with the contents of this article.
The online version of this article (available at https://www.jlr.org) contains a supplement.
REFERENCES
- 1.Taves M. D., Ma C., Heimovics S. A., Saldanha C. J., and Soma K. K.. 2011. Measurement of steroid concentrations in brain tissue: methodological considerations. Front. Endocrinol. (Lausanne). 2: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanukoglu I. 1992. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J. Steroid Biochem. Mol. Biol. 43: 779–804. [DOI] [PubMed] [Google Scholar]
- 3.Baulieu E. E. 1998. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 23: 963–987. [DOI] [PubMed] [Google Scholar]
- 4.Falkenstein E., Tillmann H-C., Christ M., Feuring M., and Wehling M.. 2000. Multiple actions of steroid hormones—a focus on rapid, nongenomic effects. Pharmacol. Rev. 52: 513–556. [PubMed] [Google Scholar]
- 5.Erickson K., Drevets W., and Schulkin J.. 2003. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci. Biobehav. Rev. 27: 233–246. [DOI] [PubMed] [Google Scholar]
- 6.Groeneweg F. L., Karst H., and Joëls M.. 2011. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 209: 153–167. [DOI] [PubMed] [Google Scholar]
- 7.Mellon S. H., and Griffin L. D.. 2002. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol. Metab. 13: 35–43. [DOI] [PubMed] [Google Scholar]
- 8.Stoffel-Wagner B. 2003. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann. N. Y. Acad. Sci. 1007: 64–78. [DOI] [PubMed] [Google Scholar]
- 9.Belelli D., and Lambert J. J.. 2005. Neurosteroids: endogenous regulators of the GABA A receptor. Nat. Rev. Neurosci. 6: 565–575. [DOI] [PubMed] [Google Scholar]
- 10.Wozniak B., Matraszek-Zuchowska I., and Zmudzki J.. 2012. LC-MS/MS fast analysis of androgenic steroids in urine. Anal. Bioanal. Chem. 403: 2965–2972. [DOI] [PubMed] [Google Scholar]
- 11.Galuska C. E., Hartmann M. F., Sanchez-Guijo A., Bakhaus K., Geyer J., Schuler G., Zimmer K. P., and Wudy S. A.. 2013. Profiling intact steroid sulfates and unconjugated steroids in biological fluids by liquid chromatography-tandem mass spectrometry (LC-MS-MS). Analyst. 138: 3792–3801. [DOI] [PubMed] [Google Scholar]
- 12.Boggs A. S., Bowden J. A., Galligan T. M., Guillette L. J. Jr., and Kucklick J. R.. 2016. Development of a multi-class steroid hormone screening method using liquid chromatography/tandem mass spectrometry (LC-MS/MS). Anal. Bioanal. Chem. 408: 4179–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Z., Wang C., Zhang J., and Wang Z.. 2017. A UHPLC-MS/MS method for profiling multifunctional steroids in human hair. Anal. Bioanal. Chem. 409: 4751–4769. [DOI] [PubMed] [Google Scholar]
- 14.Pozo O. J., Van Eenoo P., Deventer K., and Delbeke F. T.. 2007. Ionization of anabolic steroids by adduct formation in liquid chromatography electrospray mass spectrometry. J. Mass Spectrom. 42: 497–516. [DOI] [PubMed] [Google Scholar]
- 15.Caruso D., Scurati S., Maschi O., De Angelis L., Roglio I., Giatti S., Garcia-Segura L. M., and Melcangi R. C.. 2008. Evaluation of neuroactive steroid levels by liquid chromatography-tandem mass spectrometry in central and peripheral nervous system: effect of diabetes. Neurochem. Int. 52: 560–568. [DOI] [PubMed] [Google Scholar]
- 16.Ceglarek U., Kortz L., Leichtle A., Fiedler G. M., Kratzsch J., and Thiery J.. 2009. Rapid quantification of steroid patterns in human serum by on-line solid phase extraction combined with liquid chromatography-triple quadrupole linear ion trap mass spectrometry. Clin. Chim. Acta. 401: 114–118. [DOI] [PubMed] [Google Scholar]
- 17.Surowiec I., Koc M., Antti H., Wikstrom P., and Moritz T.. 2011. LC-MS/MS profiling for detection of endogenous steroids and prostaglandins in tissue samples. J. Sep. Sci. 34: 2650–2658. [DOI] [PubMed] [Google Scholar]
- 18.Koal T., Schmiederer D., Pham-Tuan H., Rohring C., and Rauh M.. 2012. Standardized LC-MS/MS based steroid hormone profile-analysis. J. Steroid Biochem. Mol. Biol. 129: 129–138. [DOI] [PubMed] [Google Scholar]
- 19.Jäntti S. E., Tammimäki A., Raattamaa H., Piepponen P., Kostiainen R., and Ketola R. A.. 2010. Determination of steroids and their intact glucuronide conjugates in mouse brain by capillary liquid chromatography-tandem mass spectrometry. Anal. Chem. 82: 3168–3175. [DOI] [PubMed] [Google Scholar]
- 20.Kim S. H., Cha E. J., Lee K. M., Kim H. J., Kwon O. S., and Lee J.. 2014. Simultaneous ionization and analysis of 84 anabolic androgenic steroids in human urine using liquid chromatography-silver ion coordination ionspray/triple-quadrupole mass spectrometry. Drug Test. Anal. 6: 1174–1185. [DOI] [PubMed] [Google Scholar]
- 21.Wang C., Wu C., Zhang L., and Zhang J.. 2016. Ultraperformance liquid chromatography–tandem mass spectrometry method for profiling ketolic and phenolic sex steroids using an automated injection program combined with diverter valve switch and step analysis. Anal. Chem. 88: 7878–7884. [DOI] [PubMed] [Google Scholar]
- 22.Keski-Rahkonen P., Huhtinen K., Poutanen M., and Auriola S.. 2011. Fast and sensitive liquid chromatography–mass spectrometry assay for seven androgenic and progestagenic steroids in human serum. J. Steroid Biochem. Mol. Biol. 127: 396–404. [DOI] [PubMed] [Google Scholar]
- 23.Soldin S. J., and Soldin O. P.. 2009. Steroid hormone analysis by tandem mass spectrometry. Clin. Chem. 55: 1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostiainen R., and Kauppila T. J.. 2009. Effect of eluent on the ionization process in liquid chromatography-mass spectrometry. J. Chromatogr. A. 1216: 685–699. [DOI] [PubMed] [Google Scholar]
- 25.Bayer E., Gfrörer P., and Rentel C.. 1999. Coordination-ionspray-MS (CIS-MS), a universal detection and characterization method for direct coupling with separation techniques. Angew. Chem. Int. Ed. Engl. 38: 992–995. [DOI] [PubMed] [Google Scholar]
- 26.Adams J., and Gross M. L.. 1986. Energy requirement for remote charge site ion decompositions and structural information from collisional activation of alkali metal cationized fatty alcohols. J. Am. Chem. Soc. 108: 6915–6921. [Google Scholar]
- 27.Adams J., and Gross M. L.. 1987. Tandem mass spectrometry for collisional activation of alkali metal-cationized fatty acids: a method for determining double bond location. Anal. Chem. 59: 1576–1582. [Google Scholar]
- 28.Pitzalis M. V., Hamlyn J. M., Messaggio E., Iacoviello M., Forleo C., Romito R., de Tommasi E., Rizzon P., Bianchi G., and Manunta P.. 2006. Independent and incremental prognostic value of endogenous ouabain in idiopathic dilated cardiomyopathy. Eur. J. Heart Fail. 8: 179–186. [DOI] [PubMed] [Google Scholar]
- 29.Baecher S., Kroiss M., Fassnacht M., and Vogeser M.. 2014. No endogenous ouabain is detectable in human plasma by ultra-sensitive UPLC-MS/MS. Clin. Chim. Acta. 431: 87–92. [DOI] [PubMed] [Google Scholar]
- 30.Lin S., Wang D., Yang D., Yao J., Tong Y., and Chen J.. 2007. Characterization of steroidal saponins in crude extract from Dioscorea nipponica Makino by liquid chromatography tandem multi-stage mass spectrometry. Anal. Chim. Acta. 599: 98–106. [DOI] [PubMed] [Google Scholar]
- 31.Yuan C., Kosewick J., He X., Kozak M., and Wang S.. 2011. Sensitive measurement of serum 1alpha,25-dihydroxyvitamin D by liquid chromatography/tandem mass spectrometry after removing interference with immunoaffinity extraction. Rapid Commun. Mass Spectrom. 25: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 32.Casetta B., Jans I., Billen J., Vanderschueren D., and Bouillon R.. 2010. Development of a method for the quantification of 1alpha,25(OH)2-vitamin D3 in serum by liquid chromatography tandem mass spectrometry without derivatization. Eur. J. Mass Spectrom. (Chichester). 16: 81–89. [DOI] [PubMed] [Google Scholar]
- 33.van Platerink C. J., Janssen H. G., Graf B., Abrahamse L., and Haverkamp J.. 2011. Quantification of steroid glycosides from Hoodia gordonii in porcine plasma using high performance liquid chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879: 819–825. [DOI] [PubMed] [Google Scholar]
- 34.Keski-Rahkonen P., Huhtinen K., Desai R., Tim Harwood D., Handelsman D. J., Poutanen M., and Auriola S.. 2013. LC-MS analysis of estradiol in human serum and endometrial tissue: Comparison of electrospray ionization, atmospheric pressure chemical ionization and atmospheric pressure photoionization. J. Mass Spectrom. 48: 1050–1058. [DOI] [PubMed] [Google Scholar]
- 35.Bao Y., Wang Q., and Tang P.. 2013. Lithium adduct as precursor ion for sensitive and rapid quantification of 20 (S)-protopanaxadiol in rat plasma by liquid chromatography/quadrupole linear ion trap mass spectrometry and application to rat pharmacokinetic study. J. Mass Spectrom. 48: 399–405. [DOI] [PubMed] [Google Scholar]
- 36.Konkle A. T., and McCarthy M. M.. 2011. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 152: 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S., Sjövall J., and Griffiths W. J.. 2003. Neurosteroids in rat brain: extraction, isolation, and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal. Chem. 75: 5835–5846. [DOI] [PubMed] [Google Scholar]
- 38.Higashi T., Takido N., and Shimada K.. 2005. Studies on neurosteroids XVII.: Analysis of stress-induced changes in neurosteroid levels in rat brains using liquid chromatography–electron capture atmospheric pressure chemical ionization-mass spectrometry. Steroids. 70: 1–11. [DOI] [PubMed] [Google Scholar]
- 39.Pesaresi M., Maschi O., Giatti S., Garcia-Segura L. M., Caruso D., and Melcangi R. C.. 2010. Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm. Behav. 57: 46–55. [DOI] [PubMed] [Google Scholar]
- 40.Park M. H., Rehman S. U., Kim I. S., Choi M. S., and Yoo H. H.. 2017. Stress-induced changes of neurosteroid profiles in rat brain and plasma under immobilized condition. J. Pharm. Biomed. Anal. 138: 92–99. [DOI] [PubMed] [Google Scholar]
- 41.Liere P., Pianos A., Eychenne B., Cambourg A., Bodin K., Griffiths W., Schumacher M., Baulieu E. E., and Sjovall J.. 2009. Analysis of pregnenolone and dehydroepiandrosterone in rodent brain: cholesterol autoxidation is the key. J. Lipid Res. 50: 2430–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higashi T., Yokoi H., Maekubo H., Honda A., and Shimada K.. 2007. Studies on neurosteroids XXIII. Analysis of tetrahydrocorticosterone isomers in the brain of rats exposed to immobilization using LC-MS. Steroids. 72: 865–874. [DOI] [PubMed] [Google Scholar]
- 43.Rustichelli C., Pinetti D., Lucchi C., Ravazzini F., and Puia G.. 2013. Simultaneous determination of pregnenolone sulphate, dehydroepiandrosterone and allopregnanolone in rat brain areas by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 930: 62–69. [DOI] [PubMed] [Google Scholar]
- 44.Giatti S., D’Intino G., Maschi O., Pesaresi M., Garcia-Segura L. M., Calza L., Caruso D., and Melcangi R. C.. 2010. Acute experimental autoimmune encephalomyelitis induces sex dimorphic changes in neuroactive steroid levels. Neurochem. Int. 56: 118–127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.