Abstract
Objectives
In 2018, Brazilian guidelines changed to recommend tuberculosis (TB) preventive therapy for all people with HIV and a CD4≤350 cells/μL, but only for those with a positive tuberculin skin test (TST) if CD4>350 cells/μL. We determined the potential effectiveness of CD4-based guidelines for TB testing and preventive therapy.
Design
Secondary analysis of the stepped-wedge, cluster-randomized THRio trial for isoniazid preventive therapy (IPT).
Methods
We analyzed data from 4,114 newly-registered patients with HIV in 29 clinics followed until TB diagnosis, death, or administrative censoring. We compared incidence rates of TB and TB/death between CD4, TST, IPT, and antiretroviral therapy (ART) categories.
Results
Initial CD4 count was ≤350 in 2,138 (52%) and >350 in 1,976 (48%) patients. TST was performed for 2,922 (71%), of whom 657 (16%) were TST-positive (278 [13%] CD4≤350 vs. 379 [19%] CD4>350). A total of 619 (15%) received IPT and 2,806 (68%) received ART. For patients with CD4≤350 who did not receive IPT, the incidence rate of TB was 1.79/100 person-years (pys) and TB/death was 3.89/100pys. For patients with CD4>350 who did not receive IPT, the incidence rates of TB and TB/death were 0.57/100pys and 1.49/100pys for TST-negatives, and 1.05/100pys and 1.64/100pys for TST-unknowns.
Conclusions
TB incidence was high among all patients who did not receive IPT, including those with CD4>350 and negative or unknown TST results. TB preventive therapy should be provided to all PLWH in medium burden settings, regardless of CD4 count and TST status.
Keywords: tuberculosis, HIV, LTBI, tuberculin skin testing, TB preventive therapy
BACKGROUND
Tuberculosis (TB) preventive therapy is highly effective for reducing TB incidence and mortality among people living with HIV (PLWH) [1–7]. While prior studies showed the effectiveness of TB preventive therapy to be greatest for patients with a positive tuberculin skin test (TST) or interferon gamma release assay (IGRA) [1], more recent studies conducted among patients receiving antiretroviral therapy (ART) show a benefit for patients with a negative TST or IGRA [3,6], and international guidelines state that a positive test is not a requirement for initiating preventive therapy in PLWH [8]. Nevertheless, TB preventive therapy is vastly underutilized globally [9], and is not widely prescribed for those without a positive TST or IGRA.
In 2018, Brazilian guidelines for TB preventive therapy for PLWH were updated to incorporate CD4 count stratification, with TB preventive therapy recommended for 1) all patients with CD4 counts ≤350 cells/μL, regardless of TST/IGRA status and 2) only for patients with a positive TST or IGRA if CD4 count is >350 cells/μL [10]. CD4 count stratification was previously incorporated in guidelines for TB preventive therapy for pregnant women in South Africa [11] and for patients from low- and medium-burden countries residing in the United Kingdom [12], however there is little evidence of implementation. While it is known that the sensitivity of TST and IGRA is diminished for HIV-infected individuals and suspected that the prevalence of anergy increases with decreasing CD4 count [13,14], CD4 count stratification has not been evaluated as a strategy to guide testing and treatment of latent TB infection (LTBI) for PLWH.
To determine the potential effectiveness of Brazil’s CD4 count-based guidelines for LTBI evaluation and TB preventive therapy, we evaluated TB incidence among patients with known or unknown TST results by baseline CD4 count in an individual patient analysis of participants enrolled in the TB/HIV in Rio (THRio) study, a cluster-randomized, phased implementation trial of isoniazid preventive therapy (IPT) for PLWH in Rio de Janeiro, Brazil.
METHODS
Study design and participants
Methods [15] and results [2,4] from the THRio study have been previously described. Briefly, THRio was a cluster-randomized trial conducted in 29 HIV clinics in Rio de Janeiro, Brazil that evaluated the impact of an intervention to increase use of TST and IPT on incidence of TB and mortality among PLWH. For our analysis of CD4 count stratification, we included adults (≥18 years) newly registered at THRio study clinics from 2005–2009, excluding those who were already registered at the clinics at the start of the study. We excluded patients with active TB diagnosed within 30 days of clinic registration, as these were likely prevalent TB cases. We used data collected through medical record review during the THRio data collection period (September 1, 2005-August 31, 2010), including patient demographic information; CD4 counts; and dates of TST results, IPT initiation, ART initiation, TB diagnosis, and death. TB and death dates were also obtained by linkage with the Rio de Janeiro mortality and TB registries through October 31, 2012.
TB testing and treatment
Nurses were trained to perform TST for all patients who had not previously been diagnosed with TB, prescribed IPT, or had a history of a positive TST. TST was performed with purified protein derivative RT23 (Statens Serum Institut, Copenhagen, Denmark), with results read in the clinic within 2–4 days. Nurses were trained to rule out active TB for patients with a positive TST using a clinical history and chest radiography, and to prescribe isoniazid 300 mg with pyridoxine 25 mg per day for 6 months, with refills at 30- or 90-day intervals. Active TB was diagnosed according to Brazilian national guidelines, which required ≥1 positive culture for Mycobacterium tuberculosis, positive acid-fast bacilli smear, or clinical and radiographic presentation consistent with TB and response to anti-TB treatment [16]. Brazilian national guidelines recommended ART for patients with a CD4 count <200 cells/μL through 2007, <350 cells/μL from 2008–2009, and <500 cells/μL starting in 2010.
Statistical analysis
For the primary analyses, we excluded patients with an unknown baseline CD4 count (see Supplemental Tables 1 and 2 for characteristics of patients with unknown baseline CD4 counts). We used Chi-square or Wilcoxon rank-sum tests to compare characteristics of patients with baseline CD4 counts ≤350 cells/μL vs. >350 cells/μL, with baseline CD4 count defined as the CD4 count closest to the time of clinic registration. We followed patients for up to 7 years, from THRio enrollment to incident TB, death, or administrative censoring on October 31, 2012.
Our primary outcomes were 1) incident TB and 2) incident TB or death, the endpoints of the THRio trial. To determine the potential effectiveness of CD4 count stratification to guide TST and TB preventive therapy, we calculated incidence rates per 100 person-years with 95% confidence intervals (CIs) based on the Poisson distribution, and incidence rate ratios comparing incidence rates among patients receiving 1) IPT vs. no IPT and 2) ART vs. no ART, stratified by baseline CD4 count and TST status. In addition, we calculated the cumulative hazard of our outcomes using the Nelson–Aalen approach and compared hazards using the log-rank test.
TST status, IPT, and ART were treated as time-dependent variables. Time from enrollment to TST was considered “TST-unknown,” and changed to “TST-negative” or “TST-positive” on the date the TST result was reported. Patients who had multiple TSTs and converted from TST-negative to -positive were considered “TST-unknown” prior to the first TST result, “TST-negative” starting the date the first negative TST result was reported, and “TST-positive” starting the date the first positive TST result was reported. Time until start of IPT was considered “no IPT” and changed to “IPT” on the date treatment was initiated; time until start of ART was considered “no ART” and changed to “ART” on the date treatment was initiated. All analyses were adjusted for age at enrollment and sex.
Ethics approval
The THRio study was approved by the institutional review boards of the Johns Hopkins Medical Institutions and the Municipal Health Secretariat of Rio de Janeiro. The requirement for informed consent to participate was waived, as the intervention was training staff to better implement practices already recommended in national guidelines.
RESULTS
Patient characteristics
Of 4,607 newly-enrolled THRio participants, we excluded 46 with a prior positive TST, 1 with active TB diagnosed within 30 days of clinic registration, and 492 with missing baseline CD4 counts (Supplemental Figure 1). Of the remaining 4,114 newly registered patients, 2,138 (52%) had a baseline CD4≤350 and 1,976 (48%) had a baseline CD4>350. Median time from enrollment to CD4 count measurement was 22 days (interquartile range [IQR] 6–72 days). A total of 1,553 (38%) patients were female and median age was 35 years (IQR 28–43, Table 1). 382 (9%) were on ART at the time of clinic registration, including 180 (8%) with CD4≤350 and 202 (10%) with CD4>350 (p=0.05). Total follow-up time was 19,814 person-years, with patients with CD4≤350 contributing 10,048 person-years and patients with CD4>350 contributing 9,766 person-years.
Table 1.
Patient characteristics and TST results
| Total N=4,114 | CD4≤350 N=2,138 | CD4>350 N=1,976 | p-value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Female sex | 1,553 (38%) | 758 (35%) | 795 (40%) | 0.002 |
| Median age, years (IQR) | 35 (28–43) | 37 (30–45) | 33 (27–41) | <0.001 |
| Prior ART | 382 (9%) | 180 (8%) | 202 (10%) | 0.05 |
| Median days on ART (IQR) | 690 (162–1,689) | 332 (78–1,237) | 973 (281–2,017) | <0.001 |
| TST results | ||||
| TST-negative | 2,265 (55%) | 1,202 (56%) | 1,063 (54%) | <0.001 |
| TST-positive* | 657 (16%) | 278 (13%) | 379 (19%) | |
| TST-unknown** | 1,192 (29%) | 658 (31%) | 534 (27%) | |
| Median days to TST (IQR) | 46 (10–180) | 45 (11–210) | 47 (9–165) | 0.17 |
| ART at TST | 1,181 (40%) | 772 (52%) | 409 (28%) | <0.001 |
Abbreviations: IQR, interquartile range; ART, antiretroviral therapy; TST, tuberculin skin test
Includes 155 patients who converted from TST-negative to -positive over follow-up
TST not placed and/or read
TST
2,922 (71%) patients had a TST placed and read, including 1,480 (69%) with CD4≤350 and 1,442 (73%) with CD4>350 (p=0.01, Table 1). Median time to first TST was 46 days (IQR 10–180) and did not differ by baseline CD4 count (p=0.17). Among those with a TST, 1,181 (40%) were receiving ART at the time of TST, including 772 (52%) with CD4≤350 and 409 (28%) with CD4>350 (p<0.001). The prevalence of a positive first TST was 17% overall (502/2,922), with 189 (13%) TST-positive patients with CD4≤350 and 313 (22%) with CD4>350 (p<0.001). Repeat tests were performed in 1,044/2,922 (36%) patients, of whom 155 (15%) converted from TST-negative to -positive (89/583 [15%] with CD4≤350 vs. 66/461 [14%] with CD4>350, p=0.18). The proportion of patients with a positive TST result increased with increasing baseline CD4 count (Supplementary Figure 2).
IPT and ART
A total of 619 (15%) patients received IPT during follow-up, including 260 (12%) with CD4≤350 and 359 (18%) with CD4>350 (p<0.001, Table 2). Overall, 538 (82%) patients with positive TSTs received IPT, compared with 65 (3%) TST-negatives and 16 (1%) TST-unknowns (p<0.001). The proportion of TST-positive patients receiving IPT was similar by CD4 count (79% CD4≤350 vs. 84% CD4>350, p=0.24). ART was received by 2,806 (68%) patients (1,993 [93%] CD4≤350 vs. 813 [41%] CD4>350, p<0.001) and 387 (9%) received both IPT and ART (251 [12%] CD4≤350 vs. 136 [7%] CD4>350, p<0.001).
Table 2.
IPT and ART initiation, and patient outcomes
| CD4≤350 | CD4>350 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n=2,138) | TST-negative (n=1,202) | TST-positive* (n=278) | TST-unknown (n=658) | p-value | Total (n=1,976) | TST-negative (n=1,063) | TST-positive* (n=379) | TST-unknown (n=534) | p-value | |
| IPT and ART | ||||||||||
| IPT | 260 (12%) | 33 (3%) | 219 (79%) | 8 (1%) | <0.001 | 359 (18%) | 32 (3%) | 319 (84%) | 8 (2%) | <0.001 |
| ART** | 1,993 (93%) | 1,152 (96%) | 263 (95%) | 578 (88%) | <0.001 | 813 (41%) | 473 (45%) | 142 (37%) | 198 (37%) | 0.01 |
| IPT and ART | 251 (12%) | 15 (1%) | 212 (76%) | 8 (1%) | <0.001 | 136 (7%) | 15 (1%) | 118 (31%) | 3 (1%) | <0.001 |
| Outcomes | ||||||||||
| TB | 170 (8%) | 63 (5%) | 34 (12%) | 73 (11%) | <0.001 | 92 (5%) | 28 (3%) | 32 (8%) | 32 (6%) | <0.001 |
| Died*** | 254 (12%) | 122 (10%) | 24 (9%) | 108 (16%) | <0.001 | 115 (6%) | 56 (5%) | 20 (5%) | 39 (7%) | 0.23 |
Abbreviations: TST, tuberculin skin test; IPT, isoniazid preventive therapy; ART, antiretroviral therapy; TB, tuberculosis
155 patients converted from TST-negative to -positive over follow-up
382 (9%) patients were on ART at baseline (180 [8%] CD4≤350, 202 [10%] CD4>350) and 37 (1%) initiated ART after a TB diagnosis (22 [1%] CD4≤350, 15 [1%] CD4>350)
73 (2%) died after a TB diagnosis (53 [2%] CD4≤350 vs. 20 [1%] CD4>350, p<0.001)
TB and mortality
There were 262 patients diagnosed with TB and 369 deaths during follow-up, for a TB incidence rate of 1.32 per 100 person-years (95% CI 1.17–1.49) and a TB or death incidence rate of 2.82 per 100 person-years (95% CI 2.59–3.06). Among patients with CD4≤350, there were 170 patients diagnosed with TB and 254 deaths, for a TB incidence rate of 1.69 per 100 person-years (95% CI 1.46–1.97) and a TB or death rate of 3.69 per 100 person-years (95% CI 3.34–4.09). Among patients with CD4>350, there were 92 patients diagnosed with TB and 115 deaths, for a TB incidence rate of 0.94 per 100 person-years (95% CI 0.77–1.16) and a TB or death rate of 1.91 per 100 person-years (95% CI 1.66–2.21). The 7-year cumulative hazard of TB was 9% for patients with CD4≤350 and 6% for patients with CD4>350 (log-rank p<0.001, Supplementary Figure 3).
For patients with CD4≤350 who initiated IPT, the adjusted incidence rate ratio was 0.54 (95% CI 0.29–1.01) for TB and 0.55 (95% CI 0.36–0.83) for TB or death compared to those not receiving IPT; and 0.98 (95% CI 0.67–1.45) for TB and 1.23 (95% CI 0.93–1.64) for TB or death for patients who received ART compared to those who did not (Supplemental Table 3). For patients with CD4>350, the adjusted incidence rate ratio was 0.75 (95% CI 0.40–1.40) for TB and 0.91 (95% CI 0.61–1.36) for TB or death for patients who initiated IPT compared with those who did not; and 0.83 (95% CI 0.53–1.29) for TB and 1.04 (95% CI 0.77–1.39) for TB or death for patients who received ART compared with those who did not.
For patients with CD4≤350, there were 63 TB cases and 122 deaths among 1,202 TST-negatives and 73 TB cases and 108 deaths among 658 TST-unknowns, compared with 34 TB cases and 24 deaths among 278 TST-positives (Table 2). The adjusted incidence rate ratios for patients who initiated IPT compared with those who did not were 1.49 (95% CI 0.37–6.06) for TST-negatives and 0.07 (95% CI 0.03–0.16) for TST-positives for TB, with no TB cases among TST-unknowns who received IPT (Table 3); and 0.76 (95% CI 0.24–2.39) for TST-negatives and 0.15 (95% CI 0.08–0.26) for TST-positives for TB or death, with no TB cases or deaths among TST-unknowns who received IPT (Table 4). The adjusted incidence rate ratios for patients who received ART compared to those who did not were 0.70 (95% CI 0.28–1.77) for TST-negatives, 1.18 (95% CI 0.08–0.37) for TST-positives, and 1.98 (95% CI 1.16–3.39) for TST-unknowns for TB (Table 3) and 0.70 (95% CI 0.38–1.30) for TST-negatives, 0.22 (95% CI 0.11–0.46) for TST-positives, and 2.31 (95% CI 1.59–3.36) for TST-unknowns for TB or death (Table 4).
Table 3.
Impact of a) IPT and b) ART on TB incidence
| CD4≤350 N=2,138 | CD4>350 N=1,976 | |||||||
|---|---|---|---|---|---|---|---|---|
| TB cases | Person-years | IR per 100 pys (95% CI) | IRR (95% CI) | TB cases | Person-years | IR per 100 pys (95% CI) | IRR (95% CI) | |
| a) IPT | ||||||||
| TST-negative / no IPT | 61 | 5,410 | 1.13 (0.88–1.45) | REF | 28 | 4,894 | 0.57 (0.39–0.83) | REF |
| TST-negative / IPT | 2 | 137 | 1.46 (0.36–5.83) | 1.49 (0.37–6.06) | 0 | 127 | 0 | — |
| TST-positive / no IPT | 26 | 183 | 14.25 (9.70–20.92) | REF | 21 | 268 | 7.83 (5.10–12.00) | REF |
| TST-positive / IPT | 8 | 923 | 0.87 (0.43–1.73) | 0.07 (0.03–0.16) | 11 | 1,385 | 0.79 (0.44–1.43) | 0.11 (0.06–0.21) |
| TST-unknown / no IPT | 73 | 3,361 | 2.17 (1.72–2.73) | REF | 32 | 3,054 | 1.05 (0.74–1.48) | REF |
| TST-unknown / IPT | 0 | 34 | 0 | — | 0 | 37 | 0 | — |
| b) ART | ||||||||
| TST-negative / no ART | 5 | 264 | 1.89 (0.79–4.54) | REF | 21 | 2,758 | 0.76 (0.50–1.17) | REF |
| TST-negative / ART | 58 | 4,890 | 1.19 (0.92–1.53) | 0.70 (0.28–1.77) | 7 | 1,892 | 0.37 (0.18–0.78) | 0.49 (0.21–1.13) |
| TST-positive / no ART | 10 | 53 | 18.95 (10.19–35.21) | REF | 24 | 1,053 | 2.28 (1.52–3.40) | REF |
| TST-positive / ART | 24 | 1,005 | 2.39 (1.60–3.56) | 0.18 (0.08–0.37) | 8 | 510 | 1.57 (0.79–3.14) | 0.69 (0.30–1.62) |
| TST-unknown / no ART | 17 | 1,445 | 1.18 (0.73–1.89) | REF | 19 | 2,619 | 0.73 (0.46–1.14) | REF |
| TST-unknown / ART | 56 | 2,391 | 2.34 (1.80–3.04) | 1.98 (1.16–3.39) | 13 | 934 | 1.39 (0.81–2.40) | 1.76 (0.88–3.52) |
Abbreviations: IPT, isoniazid preventive therapy; ART, antiretroviral therapy; TB, tuberculosis; IR, incidence rate; CI, confidence interval; IRR, incidence rate ratio; TST, tuberculin skin test
TST, IPT, and ART time-dependent; IRRs adjusted for sex and age at enrollment
Table 4.
Impact of a) IPT and b) ART on TB or death
| CD4≤350 N=2,138 | CD4>350 N=1,976 | |||||||
|---|---|---|---|---|---|---|---|---|
| TB or death | Person-years | IR per 100 pys (95% CI) | IRR (95% CI) | TB or death | Person-years | IR per 100 pys (95% CI) | IRR (95% CI) | |
| a) IPT | ||||||||
| TST-negative / no IPT | 163 | 5,410 | 3.01 (2.58–3.51) | REF | 73 | 4,894 | 1.49 (1.19–1.88) | REF |
| TST-negative / IPT | 3 | 137 | 2.19 (0.71–6.78) | 0.76 (0.24–2.39) | 2 | 127 | 1.57 (0.39–6.29) | 1.22 (0.30–4.98) |
| TST-positive / no IPT | 29 | 183 | 15.89 (11.04–22.87) | REF | 23 | 268 | 8.57 (5.70–12.90) | REF |
| TST-positive / IPT | 20 | 923 | 2.17 (1.40–3.36) | 0.15 (0.08–0.26) | 24 | 1,385 | 1.73 (1.16–2.59) | 0.21 (0.13–0.37) |
| TST-unknown / no IPT | 156 | 3,361 | 4.64 (3.97–5.43) | REF | 64 | 3,054 | 2.10 (1.64–2.68) | REF |
| TST-unknown / IPT | 0 | 34 | 0 | — | 1 | 37 | 2.71 (0.38–19.27) | 1.46 (0.21–10.10) |
| b) ART | ||||||||
| TST-negative / no ART | 11 | 264 | 4.16 (2.30–7.51) | REF | 47 | 2,758 | 1.70 (1.28–2.27) | REF |
| TST-negative / ART | 146 | 4,890 | 2.99 (2.54–3.51) | 0.70 (0.38–1.30) | 28 | 1,892 | 1.48 (1.02–2.14) | 0.77 (0.49–1.22) |
| TST-positive / no ART | 10 | 53 | 18.95 (10.19–35.21) | REF | 33 | 1,053 | 3.13 (2.23–4.41) | REF |
| TST-positive / ART | 39 | 1,005 | 3.88 (2.83–5.31) | 0.22 (0.11–0.46) | 14 | 510 | 2.75 (1.63–4.64) | 0.80 (0.41–1.57) |
| TST-unknown / no ART | 34 | 1,445 | 2.35 (1.68–3.29) | REF | 38 | 2,619 | 1.45 (1.06–1.99) | REF |
| TST-unknown / ART | 131 | 2,391 | 5.48 (4.62–6.50) | 2.31 (1.59–3.36) | 27 | 934 | 2.89 (1.98–4.22) | 1.84 (1.14–2.97) |
Abbreviations: IPT, isoniazid preventive therapy; ART, antiretroviral therapy; TB, tuberculosis; IR, incidence rate; CI, confidence interval; IRR, incidence rate ratio; TST, tuberculin skin test
TST, IPT, and ART time-dependent; IRRs adjusted for sex and age at enrollment
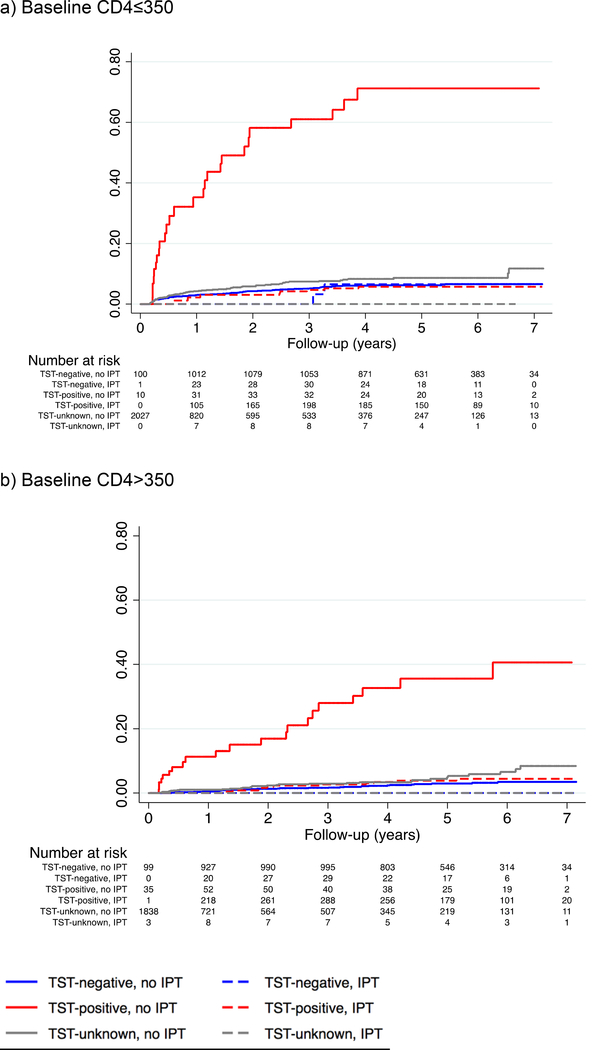
For patients with CD4>350, there were 28 TB cases and 56 deaths among 1,063 TST-negatives and 32 TB cases and 39 deaths among 534 TST-unknowns, compared with 32 TB cases and 20 deaths among 379 TST-positives (Table 2). The adjusted incidence rate ratio for patients who initiated IPT compared with those who did not was 0.11 (95% CI 0.06–0.21) for TST-positives for TB, with no TB cases among TST-negatives or -unknowns who received IPT (Table 3); and 1.22 (95% CI 0.30–4.98) for TST-negatives, 0.21 (95% CI 0.13–0.37) for TST-positives, and 1.46 (95% CI 0.21–10.10) for TST-unknowns for TB or death (Table 4). The adjusted incidence rate ratios for patients who received ART compared with those who did not were 0.49 (95% CI 0.21–1.13) for TST-negatives, 0.69 (95% CI 0.30–1.62) for TST-positives, and 1.76 (95% CI 0.88–3.52) for TST-unknowns for TB (Table 3) and 0.77 (95% CI 0.49–1.22) for TST-negatives, 0.80 (95% CI 0.41–1.57) for TST-positives, and 1.84 (95% CI 1.14–2.97) for TST-unknowns for TB or death (Table 4). Cumulative hazards for TB by CD4, TST, and IPT status are presented in Figure 1 and cumulative hazards for TB or death are presented in Supplemental Figure 5.
Figure 1.
Cumulative hazard of TB by IPT and TST status
Abbreviations: TB, tuberculosis; IPT, isoniazid preventive therapy; TST, tuberculin skin test
a) For patients with baseline CD4≤350, the 7-year cumulative hazard of TB was: 7% for TST-negatives who did not receive IPT; 7% for TST-negatives who received IPT; 71% for TST-positives who did not receive IPT; 6% for TST-positives who received IPT; 12% for TST-unknowns who did not receive IPT; and 0% for TST-unknowns who received IPT (log-rank p<0.001). b) For patients with baseline CD4>350, the 7-year cumulative hazard of TB was: 3% for TST-negatives who did not receive IPT; 0% for TST-negatives who received IPT; 41% for TST-positives who did not receive IPT; 4% for TST-positives who received IPT; 8% for TST-unknowns who did not receive IPT; and 0% for TST-unknowns who received IPT (log-rank p<0.001).
DISCUSSION
Brazil has recently recommended TB preventive therapy for all PLWH with CD4 counts ≤350 cells/μL, but for individuals with a CD4>350 cells/μL a positive TST is needed before initiating TB preventive therapy. Our results show that TB incidence is high among all patients who did not receive IPT, including those with high baseline CD4 counts and negative or unknown TST results, suggesting that these new guidelines will lead to missed opportunities to prevent TB in PLWH who are at risk of TB or death.
While at least two countries have previously incorporated CD4 count stratification in guidelines for LTBI testing and treatment [11,12], this strategy has not been evaluated and is not supported by WHO guidelines, which explicitly state that TST/IGRA is not required to initiate TB preventive therapy for PLWH [8]. Guidelines recommending CD4 count stratification to guide TST and TB preventive therapy are likely based on several assumptions: first, that the risk of TB is substantially higher in individuals with lower CD4 cell counts [2], who also have an increased prevalence of anergy; and second, that the benefits of TB preventive therapy in those with higher CD4 cell counts are limited to those with a positive TST or IGRA. In addition, logistical and economic challenges have historically resulted in delays in performing TSTs, and many patients develop TB without being tested [2,17]. Thus, providing TB preventive treatment to those patients at highest risk, while continuing to test patients more likely to mount an immune response, is a positive step towards increasing use of TB preventive therapy, and simultaneously helps to address the problem of global tuberculin shortages. This framework, however, has several flaws. First, PLWH have significantly higher rates of TB than HIV-negative persons, regardless of TST/IGRA status. Our results confirm that PLWH with negative TSTs are at high risk of active TB: in our study population, the TB incidence rate was 0.86 per 100 person-years among TST-negative individuals, over 20-fold higher than that of HIV-negative persons in Brazil [9]. Importantly, this risk was not limited to those with low CD4 counts; individuals with negative TST results and high CD4 counts had a TB incidence rate 15-fold higher than HIV-negative persons in Brazil. Second, while earlier trials (from the pre-ART era) suggested that only those with positive tests for LTBI benefit from TB preventive therapy [1], recent studies conducted among patients receiving ART clearly show the effectiveness of TB preventive therapy for PLWH with negative TST/IGRA results [3,6]. Of particular relevance is the Temprano trial, which found that IPT reduced the hazard of death similarly for patients with positive and negative IGRA results and high baseline CD4 counts (median CD4 count 465 cells/μL) [6]. A widely-cited earlier study in anergic people in the United States with advanced HIV, though considerably underpowered, showed that IPT reduced TB incidence by over 50%, with a TB incidence rate in the control group that was over 100-fold higher than the general population [18]. These trials, along with the study conducted by Rangaka and colleagues in South African patients with advanced HIV disease, which also demonstrated efficacy of IPT in TST- and IGRA-negative people [3], confirm that LTBI testing is unnecessary for initiating preventive therapy in PLWH, regardless of CD4 count. While false-negative results are more likely to occur among individuals with lower CD4 counts [13,14], there is no established CD4 count threshold to clearly distinguish reactors from non-reactors. For these reasons, expanding TB preventive therapy recommendations to include all PLWH without active TB, regardless of CD4 count or TST/IGRA status, is likely to reduce TB incidence in Brazil.
Implementation of TB preventive therapy has been limited in part due to a belief that ART is sufficient for preventing TB among PLWH, and that the benefits of TB preventive therapy will therefore be minimal in Treat All era. However, the independent effects of ART and IPT are well established [4–6] and our results suggest that patients receiving ART remain at high risk of TB: in our study population, the overall incidence rate of TB was 1.43 per 100 person-years among patients receiving ART compared with 1.17 per 100 person-years among those not receiving ART. Patients with high baseline CD4 counts and negative TSTs receiving ART were also at risk, with an incidence rate of 0.37 per 100 person-years, demonstrating that ART was insufficient for eliminating the risk of TB.
Additional concerns surrounding TB preventive therapy for PLWH without LTBI testing include potential overtreatment of individuals believed to be at low risk for progression to active TB who may experience unnecessary adverse drug effects, and costs to health systems in resource limited settings. Identifying and targeting treatment to at-risk individuals only would be the ideal preventive strategy. However, until highly sensitive and specific biomarkers predicting TB risk are developed, we are limited to using epidemiological factors to target at-risk groups. Given the high TB incidence rates in our study population across all CD4 and TST statuses, CD4 count-based risk stratification does not appear to be adequate to identify all PLWH at risk of TB, even in a medium-burden setting. Isoniazid has previously been shown to be well-tolerated in this setting. In the overall THRio study population, 1.5% of 1,472 patients who initiated IPT experienced adverse events leading to therapy interruption, and liver toxicity occurred in only three patients [17]. Finally, concerns of potential subclinical TB among severely immunosuppressed HIV patients may also contribute to poor uptake of TB preventive therapy. However, the REMEMBER trial found that even among HIV patients with very low CD4 counts (<50 cells/μL), empiric active TB treatment did not reduce mortality compared to IPT [19].
Importantly, 11% of patients did not have any CD4 count recorded, and for patients who did have CD4 counts the median time from clinic registration to a CD4 count measurement was 22 days, underscoring a key challenge in implementing treatment guidelines that rely on measurement of this clinical marker. With CD4 counts no longer tied to ART initiation in the Treat All era, clinician reliance on CD4 monitoring may decline, thereby limiting opportunities for CD4 count risk stratification to guide TST and TB preventive therapy. Furthermore, for those who do undergo CD4 testing, the delay between clinic registration and receiving results creates an avoidable risk that patients will disengage from care prior to initiating preventive therapy.
Patients without TST results appear to be a particularly high-risk group. In our study, 40% of TB cases occurred among patients without TSTs, and the 7-year cumulative hazard of TB for patients with unknown TSTs was high (10% for TST-unknowns vs. 16% for TST-positives, Supplemental Figure 3b). In addition, 40% of all deaths occurred among patients without TSTs, and death was more common for TST-unknowns than both TST-positives and -negatives. We could not quantify clinic attendance in our study, however patients without TST results were much more likely to also be missing a CD4 count than those with a TST result (86% vs. 29% missing, p<0.001), indicating that patients without TST results may represent a population less engaged in HIV care with a higher risk of TB infection and poor clinical outcomes. This highlights the need to emphasize retention in care and promote comprehensive TB evaluation at the point of HIV diagnosis.
Our study has several limitations. First, we had insufficient power to evaluate the impact of IPT on the rates of TB among study participants with negative or unknown TST results. Very few individuals with negative or unknown results received IPT, reflecting national guidelines at the time of the study, which recommended IPT only for patients who were TST-positive or contacts of known TB cases. However, as previously noted, TB preventive therapy has been proven to be highly protective for patients with negative TST/IGRA results with both low [3] and high [6] CD4 counts, albeit in high TB burden settings. Second, we did not stratify our analyses of IPT by ART status due to the relatively small number of events in each TST/CD4 group, though previous analyses demonstrated that IPT and ART independently reduced TB risk in the overall THRio study population [2,4].
In conclusion, TB preventive therapy remains an extremely underutilized strategy for global TB control, despite its clear effectiveness. The Treat All era offers an important opportunity to scale-up the use of TB preventive therapy for PLWH, as patients are initiated on ART earlier and have more frequent contact with health care providers. Removing remaining barriers to TB preventive therapy is key to improving uptake. Eliminating the need for CD4 count risk stratification will streamline TB evaluation for PLWH in Brazil, thereby increasing use of preventive therapy and reducing TB incidence and mortality.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients, clinicians, and staff who participated in the THRio study. In addition, we thank Larry Moulton and Aletta Nonyane of the Johns Hopkins Bloomberg School of Public Health Department of Biostatistics for their statistical guidance. This study was supported by the Bill & Melinda Gates Foundation (19790.01 to the Consortium to Respond Effectively to the AIDS-Tuberculosis Epidemic); the Johns Hopkins Center for AIDS Research (P30 AI094189); and the Johns Hopkins HIV Epidemiology and Prevention Sciences Training Program (T32 AI102623 to LHC). Additional support was received from the Johns Hopkins Bloomberg School of Public Health Department of Epidemiology (to LHC). The funding organizations had no role in the design, collection, analysis, or interpretation of data. An earlier version of this analysis was presented in poster exhibitions at the 10th International AIDS Society Conference on HIV Science in Mexico City, Mexico on July 20 and 23, 2019.
Conflicts of Interest and Source of Funding: This study was supported by the Bill & Melinda Gates Foundation (19790.01 to the Consortium to Respond Effectively to the AIDS-Tuberculosis Epidemic); the Johns Hopkins Center for AIDS Research (P30 AI094189); and the Johns Hopkins HIV Epidemiology and Prevention Sciences Training Program (T32 AI102623 to LHC). Additional support was received from the Johns Hopkins Bloomberg School of Public Health Department of Epidemiology (to LHC). The authors declare no conflicts of interest.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010; :CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durovni B, Saraceni V, Moulton LH, Pacheco AG, Cavalcante SC, King BS, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster-randomised trial. Lancet Infect Dis 2013; 13:852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangaka MX, Wilkinson RJ, Boulle A, Glynn JR, Fielding K, van Cutsem G, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet 2014; 384:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golub JE, Cohn S, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, et al. Long-term protection from isoniazid preventive therapy for tuberculosis in HIV-infected patients in a medium-burden tuberculosis setting: the TB/HIV in Rio (THRio) study. Clin Infect Dis 2015; 60:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med 2015; 373:808–822. [DOI] [PubMed] [Google Scholar]
- 6.Badje A, Moh R, Gabillard D, Guehi C, Kabran M, Ntakpe J-B, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health 2017; 5:e1080–e1089. [DOI] [PubMed] [Google Scholar]
- 7.Swindells S, Ramchandani R, Gupta A, Benson CA, Leon-Cruz J, Mwelase N, et al. One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis. N Engl J Med 2019; 380:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO | Latent TB Infection : Updated and consolidated guidelines for programmatic management. WHO. http://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/ (accessed 16 Mar2019). [PubMed]
- 9.WHO | Global tuberculosis report 2018. https://www.who.int/tb/publications/global_report/en/ (accessed 25 Jun 2019).
- 10.Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis: Brasília. Protocolo de vigilância da infecção latente pelo Mycobacterium tuberculosis no Brasil.; 2018.
- 11.National Department of Health, Republic of South Africa. National Tuberculosis Management Guidelines 2014. Pretoria, South Africa:; 2014. [Google Scholar]
- 12.Pozniak AL, Coyne KM, Miller RF, Lipman MCI, Freedman AR, Ormerod LP, et al. British HIV Association guidelines for the treatment of TB/HIV coinfection 2011. HIV Med 2011; 12:517–524. [DOI] [PubMed] [Google Scholar]
- 13.Rangaka MX, Wilkinson KA, Seldon R, Van Cutsem G, Meintjes GA, Morroni C, et al. Effect of HIV-1 infection on T-Cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med 2007; 175:514–520. [DOI] [PubMed] [Google Scholar]
- 14.Santin M, Casas S, Saumoy M, Andreu A, Moure R, Alcaide F, et al. Detection of latent tuberculosis by the tuberculin skin test and a whole-blood interferon-gamma release assay, and the development of active tuberculosis in HIV-seropositive persons. Diagn Microbiol Infect Dis 2011; 69:59–65. [DOI] [PubMed] [Google Scholar]
- 15.Moulton LH, Golub JE, Durovni B, Cavalcante SC, Pacheco AG, Saraceni V, et al. Statistical design of THRio: a phased implementation clinic-randomized study of a tuberculosis preventive therapy intervention. Clin Trials 2007; 4:190–199. [DOI] [PubMed] [Google Scholar]
- 16.Conde MB, Melo FAF de, Marques AMC, Cardoso NC, Pinheiro VGF, Dalcin P de TR, et al. III Brazilian Thoracic Association Guidelines on tuberculosis. J Bras Pneumol 2009; 35:1018–1048. [DOI] [PubMed] [Google Scholar]
- 17.Durovni B, Cavalcante SC, Saraceni V, Vellozo V, Israel G, King BS, et al. The implementation of isoniazid preventive therapy in HIV clinics: the experience from the TB/HIV in Rio (THRio) study. AIDS 2010; 24 Suppl 5:S49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordin FM, Matts JP, Miller C, Brown LS, Hafner R, John SL, et al. A controlled trial of isoniazid in persons with anergy and human immunodeficiency virus infection who are at high risk for tuberculosis. Terry Beirn Community Programs for Clinical Research on AIDS. N Engl J Med 1997; 337:315–320. [DOI] [PubMed] [Google Scholar]
- 19.Hosseinipour MC, Bisson GP, Miyahara S, Sun X, Moses A, Riviere C, et al. Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet 2016; 387:1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.