Abstract
Introduction:
Checkpoint inhibitors have improved outcomes in metastatic melanoma, with 4-year overall survival (OS) of 46% for anti-PD-1 alone or 53% in combination with anti-CTLA-4. However, the median progression free survival is 6.9 and 11.5 months, respectively. Many who progress have gone on to alternative treatments, including surgery, yet the outcome of patients selected for surgery after checkpoint blockade remains unclear.
Methods:
Patients who were treated with checkpoint blockade from 2003 to 2017, followed by metastasectomy, were identified from a prospectively maintained institutional melanoma database. Response to immunotherapy was assessed at the time of surgery. Patients were categorized as having responding, isolated progressing, or multiple progressing lesions.
Results:
Of the 237 total patients identified, 208 (88%) had stage IV disease and 29 (12%) had unresectable stage III disease at the start of immunotherapy. Median OS following first resection was 21 months. Median follow-up among survivors was 23 months. Complete resection at the first operation (n=87, 37%), was associated with improved survival compared with patients with incomplete resection (n=150, 63%) (median OS not reached (NR) versus 10.8 months, respectively; 95% CI: 7.3,14.8; p<0.0001). Patients resected for an isolated progressing or responding tumor had a longer median survival compared to those with multiple progressing lesions (NR versus 7.8 months, 95% CI: 6.2,11.2; p<0.0001).
Conclusion:
Patients selected for surgical resection following checkpoint blockade have a relatively favorable survival, especially if they had a response to immunotherapy and undergo complete resection of isolated progressing or responding disease.
Keywords: Stage IV, Melanoma, Immunotherapy, Metastases, Metastatic Melanoma, Metastasectomy, Immunotherapy, Checkpoint Blockade
INTRODUCTION
Prior to the introduction of effective systemic therapies, surgical resection of metastatic melanoma in selected patients has been associated with long term survival.1-10 For example, the SWOG 9430 trial reported a 4-year overall survival (OS) of 31% in patients with completely resected disease.11 In addition, follow-up data from the Multicenter Selective Lymphadenectomy Trial (MSLT-I) demonstrated an improved 4-year OS in patients chosen for surgery as a component of treatment (21% vs 7% for systemic treatment alone).12
The rationale for surgical resection in patients with stage IV melanoma is also supported by a phase III randomized trial evaluating the efficacy of an allogenic melanoma vaccine, Canvaxin plus BCG, compared to BCG alone, following complete resection of all metastatic sites.13 While the trial did not demonstrate a survival difference between Canvaxin and BCG, there was a notable 43% 5-year OS in both treatment arms following complete surgical resection of up to five sites of metastatic disease. These data also suggest the possibility of improved survival in the context of appropriate surgical selection and possibly an immunotherapy agent.1-9,11-13
The emergence of effective systemic therapies in the form of both targeted therapy and checkpoint blockade, has revolutionized the treatment of patients with advanced Stage III and Stage IV melanoma.14-19 Recently patients with advanced melanoma treated with the combination of nivolumab and ipilimumab had a 4-year OS of 53%, compared to 46% in those treated with nivolumab alone or 30% in those treated with ipilimumab alone.20 However, the median progression free survival (PFS), even in the combination anti-CTLA-4 and anti-PD-1 group, was 11.5 months; meaning that approximately 50% of patients will recur by one year. At present there is no standardized approach to address the role of surgery in stage IV melanoma. Therefore, clinicians are faced with the challenge of deciding between additional systemic treatment or surgical resection, which is still largely individualized. The aim of this study is to describe the outcomes of patients who underwent surgery after checkpoint blockade, to provide a framework for more informed patient selection for metastasectomy in the future.
METHODS
This study was approved by the institutional review board at Memorial Sloan Kettering Cancer Center (MSKCC). A total of 1615 patients with cutaneous, ocular, mucosal, acral and unknown primary melanoma who underwent treatment with checkpoint inhibitor therapy (CPI) from 2003 to 2017 were retrospectively identified from a prospectively maintained database. CPI was defined as any single agent or combination use of CTLA-4, PD-1 and PD-L1 blockade. There was no standardized number of doses nor duration of CPI treatment for these patients. Database query for “surgical intervention” after CPI identified 596 patients who had surgical intervention after at least one dose of CPI. Of these, 355 patients were excluded for diagnostic and/or non-therapeutic procedures, such as mediport placement, endoscopy, biopsy, and tube thoracoscopy. Two additional patients were excluded with advanced Stage II mucosal melanoma. The final cohort of patients included in this study were 237 patients with advanced Stage III or Stage IV melanoma who underwent treatment with checkpoint inhibitor therapy followed by metastasectomy. Unresectable or advanced stage III melanoma was defined at the time of treatment, by review of the multidisciplinary melanoma disease management team, as patients with large lymph nodes, and/or numerous in-transit lesions.
Clinical and pathologic factors examined included age, sex, location of primary melanoma, preoperative neutrophil to lymphocyte ratio (NLR), site of metastatic disease, type of CPI, response to CPI, and site of surgical resections. Age and NLR were analyzed as continuous variables. NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. All patients had a NLR recorded within six weeks prior to surgery; NLR closest to first resection was used as the preoperative NLR measurement.
Overall survival (OS) was defined as the time from the date of first surgical resection after CPI to the date of death or last follow-up. OS was evaluated using Kaplan Meier method stratified by response to CPI and compared using the log-rank test. Univariate Cox regression model was used to examine the association of age at first resection, sex, preoperative NLR, resection to NED, and response group categorization. Characteristics significant on univariate analysis with a p value of ≤0.10 were entered into a multivariate Cox proportional hazards model. A p-value of <0.05 was considered significant in this study. Statistical analysis was performed using SAS (version 9.2, Cary, NC, USA).
Distant disease-free interval (DFI) was evaluated using Kaplan Meier method and calculated from date of initial melanoma diagnosis to date of stage IV diagnosis. Patients with stage III disease (n=29) and those with stage IV disease at diagnosis, or no history of primary melanoma (n=31), were excluded from the distant DFI analysis, which left a cohort of 177 patients. Patients with a history of more than one primary melanoma had their most advanced lesion used for the calculation of DFI.
All pathologic specimens were evaluated by dermatopathologists at our institution. Pre-operative biopsy confirming metastatic melanoma was not performed prior to surgical resection for all patients. Pathologic complete response (pCR) was defined as no residual tumor in resected tumor specimen.
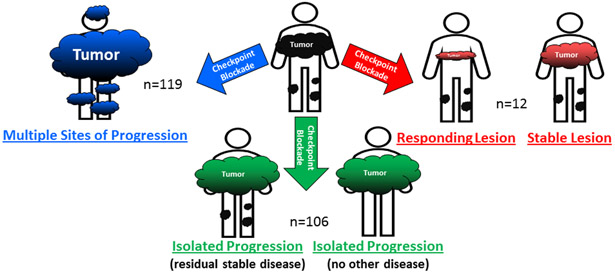
Many patients lacked formal RECIST assessment, modified RECIST21 or immune related response criteria (irRC)22 assessment which was not introduced until 2009. Therefore, to assess response for the entire cohort retrospectively, patients were categorized into three groups based upon systemic response to immunotherapy. Response to CPI was determined by chart review of radiology assessment within 30 days prior to surgery and categorized into three groups. Group 1, responding category, represented a patient with an overall response to immunotherapy, but with a single residual lesion that was radiologically smaller or stable (n=12). Group 2, isolated progressor category, represented a patient with a single site of progressing disease, while the other systemic disease had responded or was stable, (n=106). Lastly, group 3, the multiple progressor category included patients (n=119) with multifocal progression with most of these patients undergoing surgery for palliation (Figure 1).
Figure 1:
Patients Classified by Radiologically Assessed Response to Immunotherapy
RESULTS
Patient Characteristics
Patient characteristics are described in Table 1 for the entire cohort (n=237) and by response group to CPI. Overall, the median age at the start of immunotherapy was 61 years (range 19–90) and 63 years at the time surgical resection (range 19–92). Sixty-two percent of patients were male. Eighty eight percent of patients had Stage IV disease, 12% had unresectable stage III disease at the start of immunotherapy. Most commonly patients had a cutaneous primary melanoma (68.5%) followed by melanoma of unknown primary (16.5%). Median pre-operative NLR for the entire cohort was 4 (range 0.3-36). Age at initiation of CPI (p = 0.77), age at time of surgical resection (p=0.72), and gender (p=0.31) did not differ significantly between response groups. Amongst the three groups, there was a lower NLR in the responding (group 1) and isolated progressor group (group 2) compared to the multiple progressor group (group 3) (p=0.009). Additionally, a higher percentage of patients were resected to NED in groups 1 and 2 compared to group 3.
Table 1.
Demographic and surgical characteristics of the study population.
| Characteristics | All Patients N=237 (%) |
Group 1 (N=12) |
Group 2 (N=106) |
Group 3 (n=119) |
|---|---|---|---|---|
| Age at Start of Checkpoint Inhibitor, | ||||
| Median years (range) | 61(19-90) | 65 (33-78) | 61 (19-88) | 61 (19-90) |
| Age at Metastasectomy, | ||||
| Median years (range) | 63 (19-92) | 66 (33-79) | 63 (19-89) | 62 (19-92) |
| Gender | ||||
| Female | 91 (38) | 5 (42) | 47 (44) | 39 (33) |
| Male | 146 (62) | 7 (58) | 59 (56) | 80 (67) |
| Stage III | 29 (12) | 3 (25) | 17 (16) | 9 (8) |
| Stage III (Unknown Primary) | 2 (1) | 0 (0) | 1 (1) | 1 (1) |
| Stage IIIB | 6 (3) | 0 (0) | 5 (5) | 1 (1) |
| Stage IIIC | 21 (9) | 3 (25) | 11(10) | 7 (6) |
| Stage IV | 208 (88) | 9 (75) | 89(84) | 110 (92) |
| Site of Primary Melanoma | ||||
| Cutaneous | 162 (68.5) | 7 (58) | 79 (74.5) | 76 (64) |
| Ocular/Uveal | 3 (1) | 0 (0) | 2 (2) | 1 (1) |
| Mucosal | 16 (7) | 1 (8) | 4 (3.5) | 11 (9) |
| Acral | 17 (7) | 2 (17) | 3 (3) | 12 (10) |
| Unknown Primary | 39 (16.5) | 2 (17) | 18 (17) | 19 (16) |
| Pre-Operative Neutrophil to Lymphocyte Ratio (NLR) | ||||
| Mean | 5.4 | 3.8 | 4.5 | 6.3 |
| Median (range) | 4 (0.3-36) | 2.6 (1.5-9.3) | 3.6 (0.3-20.2) | 4.1 (0.3-36.0) |
| Total Number of Resections, % | 349 | 17 (5) | 163 (47) | 169 (48) |
| Total Resections per Patient (N=237) | ||||
| 1 | 162 (69) | 8 (67) | 70 (66) | 84 (71) |
| 2 | 52 (21) | 3 (25) | 25 (24) | 24 (20) |
| 3 | 12 (5) | 1 (8) | 4 (4) | 7 (6) |
| 4 | 9 (4) | 0 (0) | 5 (5) | 4 (3) |
| 5 | 1 (<1) | 0 (0) | 1 (<1) | 0 (0) |
| 6 | 1 (<1) | 0 (0) | 1 (<1) | 0 (0) |
| Resected to No Evidence of Disease (NED) Following 1st Surgery | ||||
| No | 150 (63) | 0 (0) | 35 (33) | 115 (97) |
| Yes | 87 (37) | 12 (100) | 71 (67) | 4 (3) |
| Pre-operative Checkpoint Inhibitor | ||||
| Anti-CTLA4 | 148 (62) | 5 (42) | 65 (61) | 78 (65) |
| Anti-PD1 | 18 (8) | 1 (8) | 10 (10) | 78 (65) |
| Combination anti-CTLA4 and anti-PD1 | 69 (29) | 6 (50) | 30 (28) | 33 (28) |
| Other (anti-CTLA4 and anti-PDL1) | 2 (1) | 0 (0) | 1 (1) | 1 (1) |
| Post-operative Therapy | ||||
| No Further Therapy | 109(46) | 5 (42) | 44 (42) | 60 (50) |
| Anti-CTLA4 | 17 (7) | 1 (8) | 10 (10) | 6 (5) |
| Anti-PD1 | 44 (19) | 1 (8) | 18 (17) | 25 (21) |
| Combination anti-CTLA4 and anti-PD1 | 19 (8) | 2 (17) | 12 (11) | 5 (4) |
| Other (anti-PDL1) | 2 (1) | 0 (0) | 0 (0) | 2 (2) |
| Targeted Therapy | 25 (10) | 2 (17) | 11(10) | 12 (10) |
| Chemotherapy | 21 (9) | 1 (8) | 11(10) | 9 (8) |
Checkpoint Inhibitor (CPI) Preceding Surgery
Prior to surgical resection, 62% of patients were treated with anti-CTLA-4 alone, 8% received anti-PD-1 alone, 29% received therapy with anti–CTLA-4 and anti–PD1, either concomitantly or sequentially (Table 1; Supplemental Figure 1A).
Surgical Resection
Most patients (n=162, 69%) had only 1 operation following CPI. The remaining 75 patients (31%) had 2 or more operations, for a total of 349 surgeries (Table 1). The median time from initiation of immunotherapy to initial resection was 6.9 months (range <1 month to 101 months). As shown in Table 2, the most common site of initial resection was skin and/or soft-tissue metastases (30%), followed by brain or central nervous system lesions (CNS) (26.5%). Also shown is the response to immunotherapy at the time of surgery by site of resection. Most patients in groups 1 and 2 underwent resection for lymph node (42% and 28%, respectively) and skin and/or soft tissue metastases (25% and 27%, respectively). In group 3, 45% of patients underwent resection for CNS or brain metastases and 55% underwent resection for extra-CNS disease.
Table 2.
Site of Surgical Resection by Response to Checkpoint Inhibitor Therapy at Time of Initial Operation (N=237)
| Site of Initial Operation (N=237) |
N (%) | Group 1 (N=12) |
Group 2 (N=106) |
Group 3 (N=119) |
|---|---|---|---|---|
| All Patients | 237 (100) | 12 (5) | 106 (45) | 119 (50) |
| Skin/Soft Tissue | 72 (30) | 3 (25) | 29 (27) | 40 (34) |
| Lymph Nodes | 42 (18) | 5 (42) | 30 (28) | 7 (6) |
| Lung | 11 (5) | 2 (17) | 9 (8) | 0 |
| Liver | 3 (1) | 0 (0) | 3 (3) | 0 |
| Other Abdominal Disease | 42 (18) | 1 (8) | 25 (24) | 16 (13) |
| Bone | 4 (1.5) | 0 (0) | 2 (2) | 2 (2) |
| Brain/CNS | 63 (26.5) | 1 (8) | 8 (8) | 54 (45) |
All disease was resected, i.e. resection to NED, in 87 (37%) patients, which included all the patients in group 1 (single site of remaining disease, n=12, 14%), 71 (82%) patients in group 2 (isolated progressor), and 4 (4%) patients in group 3 (multifocal progressor). The remaining 63% of patients (n=150) underwent resection of a metastatic lesion while disease at other sites was not removed; these patients were from group 2 (n=35, 23%) and group 3 (n=115, 77%). Most patients resected to NED underwent surgery for lymph node metastases (33%), skin or soft tissue metastases (25%), and abdominal visceral metastases (24%) (Supplemental Table 1). In the multiple progressor category there were four patients who had two separate progressing lesions resected and were rendered NED by surgery. These patients, due to more than one site of progression, were categorized as being in the multiple progressor (group 3) but were ultimately able to be resected to NED. These patients were classified by their most advanced site of metastatic disease (e.g. simultaneous resection of a brain metastasis and a femur metastasis and is classified by his CNS/brain site of resection).
Following surgical resection 46% (n=109) of patients received no further treatment. Forty-four patients (19%) received anti–PD-1 therapy alone and 10% received combination targeted therapy with a BRAF and MEK inhibitor following resection. The remainder went on to receive chemotherapy (9%), combination checkpoint blockade (8%), anti-CTLA4 (7%), and other (1%) therapies as part of a clinical trial (Table 1, Supplemental Figure 1B). Of the 109 patients who did not receive further systemic therapy following surgery, 43 (39%) were resected to NED. Of the remaining 66 patients with residual disease, 58 were from multiple progressing group (group 3) and 8 were from the isolated progressing group (group 2). Most of these patients had multi-focal progression and were referred for palliative or supportive care, including hospice.
Survival Outcomes
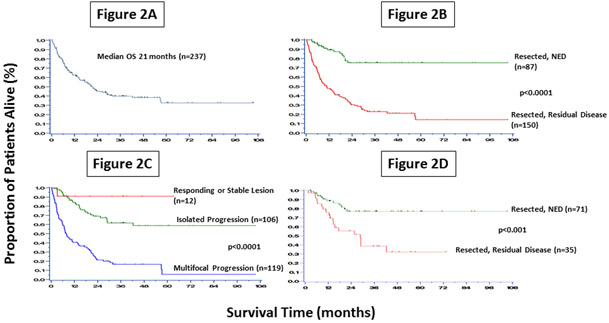
Median OS from the time of initial resection for the entire cohort was 21 months, with a median follow-up among survivors of 23 months (Figure 2A). Median OS was not reached in the 87 patients rendered NED by surgical resection compared to a median OS of 10.8 months in the 150 patients with residual disease following surgery (p<0.0001); this translates to an estimated 5-year OS rate of 75% compared to 15% in the group not resected to NED (p<0.0001) (Figure 2B).
Figure 2:
Overall Survival for all 237 patients (2A), OS Startified by Resection Status (2B), OS Startified by Response to Checkpoint Blockade (2C), OS in Isolated Progressing Lesions Startified by Resection Status (2D).
When patients were stratified by response to CPI at the time of surgery, there was a dramatic separation in survival. Group 1, responding or stable disease (n=12), had an estimated 90% 5-year survival. Patients in group 2, with one isolated progressing lesion, (n=106) had a 60% 5-year OS. The median OS was not reached in either of these groups. Patients in group 3, who underwent surgical resection in the context of multifocal progressive disease (n=119) had a median OS of 7.8 months (p<0.0001) (Figure 2C) and an estimated 5-year OS of 6%. Non-cutaneous melanoma subtypes (ocular, mucosal and acral) were included in the analysis and as shown in Table 1 were distributed across all three subgroups.
Patients in group 2 were further analyzed by disease status following resection. As expected, the patients who underwent complete resection of their sole site of progressive disease (n=71) had improved survival, with an estimated 5-year OS of 75%, while patients undergoing resection of an enlarging lesion, and disease at other sites not removed, (n=35) had a 5-year OS of 30% (p<0.001) (Figure 2D).
Factors associated with worse OS on multivariate analysis were patients who had residual disease following resection (i.e. those not rendered NED with surgery) (HR 2.75, 95% CI 1.44– 5.25, p=0.002), a higher preoperative NLR (HR 1.04, 95% CI: 1.01-1.08, p=0.024), and multifocal progression on CPI (Table 3). Patients with multiple sites of progressing disease had a higher risk of death than those with stable or responding disease (HR 7.57, 95% CI: 1.02–56.01, p<0.001). Systemic response to immunotherapy at time of surgery was strongly associated with outcome; however, a traditional marker of good outcome in metastasectomy, time to development of metastatic disease or DFI (≤12 or >12 months), was not significant (p=0.09) (Supplemental Figure 2).
Table 3.
Multivariate Analysis of Factors Associated with Overall Survival in the 237 Patients With Advanced Melanoma.
| Clinical Factors | Hazard Ratio |
95% Confidence Interval |
p-value |
|---|---|---|---|
| Complete Resection to NED (No vs Yes) | 2.75 | 1.44-5.25 | .002 |
| Pre-Operative Neutrophil to Lymphocyte Ratio (NLR) (continuous) | 1.04 | 1.01-1.08 | .024 |
| Response to Checkpoint Blockade | |||
| Multiple Progressors vs Responding/Stable Disease | 7.57 | 1.02-56.01 | <.001 |
| Isolated Progressor vs Responding/Stable Disease | 3.05 | 0.42-22.36 |
NED, no evidence of disease.
Pathologic Complete Response
A pathologic complete response (pCR) was noted in only 14 out of 349 (4%) specimens. Among the 12 patients in group 1, responding to immunotherapy, who underwent resection of a residual tumor, 6 (or 50% of group 1) were found to have no viable tumor. The remaining 8 pCR specimens showed growth on radiologic assessment prior to surgery, and therefore came from the isolated or multiple progressing groups. Pre-operative CPI treatment of these 14 patients consisted of anti-CTLA-4 alone in 8 patients (57%), anti-PD1 alone in 1 patient (7%), and combination anti-CTLA-4 and anti-PD-1 in 5 patients (36%). Of the 8 patients with a pCR from groups 2 and 3, 4 patients were resected to NED while 4 had other sites of progressing disease.
DISCUSSION
With the increased efficacy of modern systemic treatments, the use of surgery for metastatic disease has increased across cancer types, including melanoma.23 To date, the majority of studies have focused on the role of metastasectomy in the pre-immunotherapy era, with a 5-year OS of 20-30%, and as high as 43% in a clinical trial evaluating cancer vaccination.1-13 This study is the largest analysis of outcomes after surgery in patients previously treated with CPI therapy.
Recently, retrospective analyses have suggested that resection after targeted or immunotherapy can be associated with prolonged OS. He et al., reported 18 patients with advanced stage III and IV melanoma treated with vemurafenib, BRAF inhibitor therapy, within 30 days of surgery.24 The study showed elective resections were associated with improved survival compared to patients requiring more urgent surgery, and there was durable postoperative disease control in patients undergoing resection of oligometastatic disease.
Another study by Prabhakaran et al. examined 54 patients who underwent surgery for GI melanoma metastases from 2007 to 2013, 15 (28%) of whom received preoperative immunotherapy.25 When divided based on curative or palliative surgical resection, median OS was not reached in the curative (n=13) versus 9.5 months in the palliative (n=41) group. On multivariate analysis, resection to NED (p = 0.012) and the presence of single metastases (p = 0.031) were associated with improved OS. However, given the small number of patients that received preoperative immunotherapy it is unclear if response to therapy influenced survival.
A larger series from the John Wayne Cancer Institute analyzed survival in patients with resectable abdominal melanoma metastasis from a more recent era of 2004-2014 (n=320), and prior to 2004 (n=1303), with the assumption that the modern era received more effective systemic therapy.26 Patients had improved survival in the more recent era, with a median survival of 11 months vs 8 months (p=0.003). However, the study failed to demonstrate any survival advantage in the subset of patients undergoing surgery and immunotherapy over surgery alone, even in the best cohort of patients with gastrointestinal tract disease (OS of 23 months for the combination versus 21 months for surgery alone).While results may be confounded by the smaller sample size and the variability in that some patients received immunotherapy pre-operatively and some received it post-operatively, the study highlights the difficultly in retrospectively ascertaining the individual contribution of two effective therapies in metastatic melanoma: surgery and checkpoint blockade.
The factors that go into patient selection for melanoma metastasectomy are difficult to quantify. Although no level I evidence exists, retrospective data suggest that predictors of improved survival following metastasectomy include low disease burden, ability to remove all disease, and a longer DFI.7,11,12,27 Interestingly, DFI was not a significant factor associated with survival in this cohort, suggesting that an effective immunotherapy may be able to overcome some cancers with aggressive biology. A higher neutrophil to lymphocyte ratio (NLR) was associated with worse OS in this study. Elevated NLR has previously been associated with poor outcomes in patients treated with CPI alone.28 Additionally, a higher preoperative NLR has been linked to worse OS in other types of malignancies such as non-metastatic renal cell carcinoma,29 pancreatic neuroendocrine tumors,30 pancreatic ductal adenocarcinoma,31 and colorectal adenocarcinoma.32 Elevated preoperative NLR has also been correlated with both worse OS and higher rates of recurrence in metastatic cancer, specifically in patients who have been diagnosed with resectable synchronous colorectal liver metastases. 33 Together these findings suggest that NLR may be an important factor to consider prior to surgical intervention, and also to include in future cohorts of patients undergoing surgery.
Historically, patients with melanoma CNS metastases, even those undergoing resection, have had poor survival outcomes and were excluded from studies.34-36 However, there have been durable CNS responses to checkpoint blockade in asymptomatic patients, and therefore this group was included in this study.37 While many of the palliative resections in group 3 were for CNS disease (45%), there was also a large contribution from other disease sites such as skin/soft tissue (34%). In addition, some patients with CNS disease had an isolated site of progression and were in group 2. Therefore, patients with CNS disease were included to represent a real-world population.
Response to systemic therapy has also been shown in retrospective series to be a predictor of improved survival following surgery.24,38,39 In this study systemic response to CPI at the time of surgery categorized patients into groups with dramatically different survival. Patients in group 1, with a single responding lesion that had not completely resolved after CPI, have an excellent survival (90% estimated 5-year survival). Most interesting is the survival of patients in group 2, the isolated progressor group. Clinically, these patients have an overall response to CPI, but have a single site of progression, or an escape lesion. Research has suggested that immunoediting occurs, particularly in the setting of an active immune response, and can result in escape tumors that have decreased MHC expression, loss of B2M, alterations in IFN gamma signaling, or other mechanisms which make them less responsive to immunotherapy.40,41 While additional systemic therapies maybe an effective choice for patients with escape lesions, this study suggests that surgical resection is also a good option, particularly if surgery is able to render patients NED, as this group had a 75% estimated 5-year overall survival. In addition, 46% of patients, primarily from group 1 and group 2, did not receive systemic therapy postoperatively, suggesting that surgery can have a durable effect on removal of escape lesions. Patients in group 3 with multifocal progression, however, represent a group with a poor outcome and surgery is unlikely to alter the disease course in this cohort.
CONCLUSION
In the era of checkpoint inhibition, patients who underwent surgery with a systemic overall response to CPI had a 5-year OS of 90%. Patients able to be resected to NED following CPI had a 5-year OS of 75%. These outcomes are better than previously reported for surgery alone in patients with metastatic melanoma. While further follow-up is needed to assess the durability of these results, this study supports that strong consideration should be given to surgery as a component of systemic treatment in metastatic melanoma, particularly in escape lesions that can be removed completely.
Supplementary Material
Synopsis: The survival of patients with melanoma selected for metastasectomy after checkpoint therapy was evaluated. Surgery for patients with a solitary progressing lesion or responding disease had a more favorable outcome then those with multiple sites of progression.
ACKNOWLEDGEMENTS
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748, Ludwig Collaborative and Swim Across America Laboratory, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA. Parker Institute for Cancer Immunotherapy, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA. Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA, Weill Cornell Medicine, New York, NY 10065, USA
Footnotes
Disclosures: This study was presented in plenary podium format at the 71st Annual Meeting of the Society of Surgical Oncology, March 21-24, 2018, Chicago, IL. Drs Hollmann, Coit, Momtaz and Brady have no disclosures. Dr Bello has worked as a consultant for survivornet. Dr. Panageas has stock ownership in Johnson and Johnson, Pfizer, Viking Therapeutics, and Catalyst Biotech. Dr. Shoushtari serves on the advisory board for Bristol-Myers Squibb (BMS), Castle Biosciences, and Immunocore. He receives institutional research support from BMS, Immunocore, AstraZeneca, and Xcovery. Dr. Chapman receives consulting, advisory, or speaking compensation from Immunocore, Merck, Cell Medica, Takeda Millenium, and AstraZeneca. He holds stock in Rgenix. He receives research support from Pfizer. Dr. Postow has worked as a consultant for: BMS, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, Aduro. He has received honoraria from: BMS and Merck. He receives institutional research support from: RGenix, Infinity, BMS, Merck, Array BioPharma, Novartis, and AstraZeneca. Dr. Callahan reports grants from and employment of a family member by BMS; personal fees for advisory/consulting role from AstraZeneca/MedImmune, Incyte, Moderna and Merck. Dr. Wolchok is a consultant for: Adaptive Biotech, Advaxis, Amgen, Apricity, Array BioPharma, Ascentage Pharma, Astellas, Bayer, Beigene, BMS, Celgene, Chugai, Elucida, Eli Lilly, F Star, Genentech, Imvaq, Janssen, Kleo Pharma, Linneaus, MedImmune, Merck, Neon Therapeutics, Ono Pharmaceuticals, Polaris Pharma, Polynoma, Psioxus, Puretech, Recepta, Sellas Life Sciences, Serametrix, Surface Oncology, Syndax. He receives research support from: BMS, Medimmune, Genentech. He has equity in: Potenza Therapeutics, Tizona Pharmaceuticals, Adaptive Biotechnologies, Elucida, Imvaq, Beigene, Trieza, Serametrix, Linneaus. He receives honorarium from: Esanex. Dr. Ariyan served on an advisory board for BMS.
REFERENCES
- 1.Karakousis CP, Velez A, Driscoll DL, Takita H. Metastasectomy in malignant melanoma. Surgery 1994;115:295–302. [PubMed] [Google Scholar]
- 2.Ollila DW, Essner R, Wanek LA, Morton DL. Surgical resection for melanoma metastatic to the gastrointestinal tract. Arch Surg 1996;131:975–9; 9-80. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher WS, Pommier RF, Lum S, Wilmarth TJ. Surgical treatment of metastatic melanoma. Am J Surg 1998;175:413–7. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal S, Yao TJ, Coit DG. Surgery for melanoma metastatic to the gastrointestinal tract. Ann Surg Oncol 1999;6:336–44. [DOI] [PubMed] [Google Scholar]
- 5.Ollila DW, Hsueh EC, Stern SL, Morton DL. Metastasectomy for recurrent stage IV melanoma. J Surg Oncol 1999;71:209–13. [DOI] [PubMed] [Google Scholar]
- 6.Wood TF, DiFronzo LA, Rose DM, et al. Does complete resection of melanoma metastatic to solid intra-abdominal organs improve survival? Ann Surg Oncol 2001;8:658–62. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RP, Hanish SI, Haney JC, et al. Improved survival with pulmonary metastasectomy: an analysis of 1720 patients with pulmonary metastatic melanoma. J Thorac Cardiovasc Surg 2007;133:104–10. [DOI] [PubMed] [Google Scholar]
- 8.Collinson FJ, Lam TK, Bruijn WM, et al. Long-term survival and occasional regression of distant melanoma metastases after adrenal metastasectomy. Ann Surg Oncol 2008;15:1741–9. [DOI] [PubMed] [Google Scholar]
- 9.Mittendorf EA, Lim SJ, Schacherer CW, et al. Melanoma adrenal metastasis: natural history and surgical management. Am J Surg 2008;195:363–8; discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 10.Neuman HB, Patel A, Hanlon C, Wolchok JD, Houghton AN, Coit DG. Stage-IV melanoma and pulmonary metastases: factors predictive of survival. Ann Surg Oncol 2007;14:2847–53. [DOI] [PubMed] [Google Scholar]
- 11.Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430. Cancer 2011;117:4740–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard JH, Thompson JF, Mozzillo N, et al. Metastasectomy for distant metastatic melanoma: analysis of data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I). Ann Surg Oncol 2012;19:2547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faries MB, Mozzillo N, Kashani-Sabet M, et al. Long-Term Survival after Complete Surgical Resection and Adjuvant Immunotherapy for Distant Melanoma Metastases. Ann Surg Oncol 2017;24:3991–4000. [DOI] [PubMed] [Google Scholar]
- 14.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:1270–1. [DOI] [PubMed] [Google Scholar]
- 19.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1480–92. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 22.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412–20. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett EK, Simmons KD, Wachtel H, et al. The rise in metastasectomy across cancer types over the past decade. Cancer 2015;121:747–57. [DOI] [PubMed] [Google Scholar]
- 24.He M, Lovell J, Ng BL, et al. Post-operative survival following metastasectomy for patients receiving BRAF inhibitor therapy is associated with duration of pre-operative treatment and elective indication. J Surg Oncol 2015;111:980–4. [DOI] [PubMed] [Google Scholar]
- 25.Prabhakaran S, Fulp WJ, Gonzalez RJ, et al. Resection of Gastrointestinal Metastases in Stage IV Melanoma: Correlation with Outcomes. Am Surg 2016;82:1109–16. [PubMed] [Google Scholar]
- 26.Deutsch GB, Flaherty DC, Kirchoff DD, et al. Association of Surgical Treatment, Systemic Therapy, and Survival in Patients With Abdominal Visceral Melanoma Metastases, 1965-2014: Relevance of Surgical Cure in the Era of Modern Systemic Therapy. JAMA Surg 2017;152:672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer T, Merkel S, Goehl J, Hohenberger W. Surgical therapy for distant metastases of malignant melanoma. Cancer 2000;89:1983–91. [DOI] [PubMed] [Google Scholar]
- 28.Cassidy MR, Wolchok RE, Zheng J, et al. Neutrophil to Lymphocyte Ratio is Associated With Outcome During Ipilimumab Treatment. EBioMedicine 2017;18:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimes N, Hannan C, Tyson M, Thwaini A. The role of neutrophil-lymphocyte ratio as a prognostic indicator in patients undergoing nephrectomy for renal cell carcinoma. Can Urol Assoc J 2018;12:E345–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou B, Zhan C, Wu J, Liu J, Zhou J, Zheng S. Prognostic Significance of Preoperative Neutrophil-to-Lymphocyte Ratio in Surgically Resectable Pancreatic Neuroendocrine Tumors. Med Sci Monit 2017;23:5574–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abe T, Amano H, Kobayashi T, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognosticator in early stage pancreatic ductal adenocarcinoma. Eur J Surg Oncol 2018;44:1573–9. [DOI] [PubMed] [Google Scholar]
- 32.Kubo H, Murayama Y, Arita T, Kuriu Y, Nakanishi M, Otsuji E. The Prognostic Value of Preoperative Neutrophil-to-Lymphocyte Ratio in Colorectal Cancer. World J Surg 2016;40:2796–802. [DOI] [PubMed] [Google Scholar]
- 33.Kim H, Jung HI, Kwon SH, et al. Preoperative neutrophil-lymphocyte ratio and CEA is associated with poor prognosis in patients with synchronous colorectal cancer liver metastasis. Ann Surg Treat Res 2019;96:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wronski M, Arbit E. Surgical treatment of brain metastases from melanoma: a retrospective study of 91 patients. J Neurosurg 2000;93:9–18. [DOI] [PubMed] [Google Scholar]
- 35.Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol 2004;22:1293–300. [DOI] [PubMed] [Google Scholar]
- 36.Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011;117:1687–96. [DOI] [PubMed] [Google Scholar]
- 37.Kluger HM, Chiang V, Mahajan A, et al. Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. J Clin Oncol 2019;37:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang JC, Abad J, Sherry R. Treatment of oligometastases after successful immunotherapy. Semin Radiat Oncol 2006;16:131–5. [DOI] [PubMed] [Google Scholar]
- 39.Faries MB, Leung A, Morton DL, et al. A 20-year experience of hepatic resection for melanoma: is there an expanding role? J Am Coll Surg 2014;219:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gyorki DE, Yuan J, Mu Z, et al. Immunological insights from patients undergoing surgery on ipilimumab for metastatic melanoma. Ann Surg Oncol 2013;20:3106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 2016;375:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.