Abstract
Context:
A large body of experimental and observational data has implicated vitamin D deficiency in the development of cardiovascular disease. However, evidence to support routine vitamin D supplementation to prevent or treat cardiovascular disease is lacking.
Evidence Acquisition:
A comprehensive literature review was performed using Pubmed and other literature search engines.
Evidence Synthesis:
Mounting epidemiological evidence and data from Mendelian randomization studies support a link between vitamin D deficiency and adverse cardiovascular health outcomes, but randomized trial evidence to support vitamin D supplementation is sparse. Current public health guidelines restrict vitamin D intake recommendations to the maintenance of bone health and prevention of fractures. Two recently published large trials (VITAL and ViDA) that assessed the role of moderate-to-high dose vitamin D supplementation as primary prevention for cardiovascular outcomes in the general population had null results, and previous randomized trials have also been generally negative. These findings from general population cohorts that are largely replete in vitamin D may not be applicable to chronic kidney disease (CKD) populations, in which the use of active (1α-hydroxylated) vitamin D compounds is prevalent, or to other high-risk populations. Additionally, recent trials in the CKD population, and trials using vitamin D analogues have been limited.
Conclusions:
Current randomized trials of vitamin D supplementation do not support benefits for cardiovascular health, but the evidence remains inconclusive. Additional randomized trials assessing larger numbers of participants with low baseline vitamin D levels, having longer follow-up periods, and testing higher vitamin D dosages, are needed to guide clinical practice.
Keywords: Vitamin D, atherosclerosis, cardiovascular disease, chronic kidney disease, clinical trials
INTRODUCTION
Cardiovascular disease (CVD) is one of the leading non-communicable causes of mortality(1), causing one third of deaths worldwide. Global health studies have revealed an epidemiologic transition from infectious to non-communicable diseases such as CVD as the predominant cause of death in industrialized countries.(2) This trend is also becoming apparent in low and middle-income countries, commonly affecting individuals at premature ages or from underserved ethnic groups or regions.(3,4) Atherosclerotic CVD (ASCVD), including coronary heart disease and stroke, tends to be a chronic inflammatory and thrombo-occlusive condition affecting medium-sized and large arteries. (5) ASCVD in regions such as Sub-Saharan Africa is now an established major cause of death, albeit largely secondary to non-atherosclerotic conditions such as rheumatic heart disease, HIV-related disorders and hypertensive heart disease.(6) In industrialized countries and regions where population aging has evolved such as in South America, unhealthy lifestyles have become increasingly prevalent accounting for higher rates of ASCVD.(4,7) It is therefore not surprising that CVD represents a significant healthcare economic burden worldwide.
CVD burden has been attributed to certain behaviors such sedentary lifestyle, unhealthy dietary patterns, smoking and excessive alcohol consumption.(3) Growing evidence supports significant cause-effect relationships of most of these factors with CVD.(8,9) Other environmental factors such as air pollution and conditions such as inflammatory arthropathies, HIV, diabetes and chronic kidney disease (CKD) are now known to be important risk factors for CVD. A considerable body of evidence has also implicated hypovitaminosis D as an important risk for CVD.
Low blood levels of vitamin D have been linked to a number of adverse health outcomes including all-cause mortality, cardiovascular disease as well as reduced bone density and fracture risk, metabolic syndrome, malignancy, autoimmune conditions and infection.(10) Overt vitamin D deficiency results in the striking phenotype of rickets in children, and osteomalacia in adults and children. It has therefore been intensively studied for almost a century. Animal models have shown that vitamin D receptor (VDR) knockout mice develop overt CVD, including upregulation of the renin-angiotensin-aldosterone (RAAS) system, hypertension, left ventricular hypertrophy and heart failure.(11,12) In humans, a large meta-analysis of observational studies (n=849 412) found that low concentrations of 25-hydroxyvitamin D (25(OH)D) were associated with an increased risk of cardiovascular as well as cancer, and non-cardiovascular non-cancer deaths.(13) Each 10 nmol/L reduction in 25(OH)D was associated with a 16% increase in all-cause mortality; the pooled relative risk (RR) was 1.35 (95% CI 1.13 to 1.61) for death from cardiovascular disease, 1.14 (1.01 to 1.29) for death from cancer, 1.30 (1.07 to 1.59) for non-vascular, non-cancer death, and 1.35 (1.22 to 1.49) for all-cause mortality.(13) These data were supported by findings from a Scandinavian Mendelian randomization study (n=95,766) where a genetically determined difference in circulating 25-hydroxyvitamin D (25(OH)D) concentrations of 20 nmol/L was associated with a significantly increased risk of all-cause, cardiovascular and cancer mortality (HR 1.19, 1.18 and 1.12 respectively),(14) although other genomic studies have been inconsistent. Given the considerable proportion of the general population at risk for vitamin D inadequacy, establishing the implications for cardiovascular health is of high importance: the National Health and Nutrition Examination Survey (NHANES) for 2007 through 2010 revealed that 13% of Americans aged 1 to 70 years of age are at risk for vitamin D inadequacy.(15) Additionally, NHANES identified a marked decrease from 45% (43–47%) to 23% (20–26%) in serum 25(OH)D levels of 30 ng/ml or more between NHANES III (1988–1994) and NHANES IV (2001–2004).(16) However, some of these changes may be attributed to fluctuations in the DiaSorin RIA assay kit used.(17)
Serum 25(OH)D is commonly measured, and low levels are often treated in clinical practice with over-the-counter supplements.(10) National and international guidelines set thresholds for deficiency, insufficiency and repletion (based mostly on skeletal outcomes), and recommend daily intakes for the general population.(18) In kidney disease, vitamin D has been used for more than 6 decades to treat renal bone disease,(19) though mostly by using synthetic vitamin D compounds rather than naturally occurring ones. Despite this, there is a lack of data on the effect of such practices on meaningful clinical outcomes over and above biochemical parameters such as parathyroid hormone (PTH) and calcium.(20) The 2011 dietary reference (DRI) intake report by the Institute of Medicine (IOM) concluded that bone health was the only outcome whereby causality was established by available evidence.(21) Using a risk assessment framework, the IOM committee determined that for CVD, cancer, diabetes, falls, physical performance, autoimmune disorders and other extraskeletal chronic disease outcomes, the evidence was deemed to be inconsistent, inconclusive as to causality and insufficient to serve as a basis for DRI development.
This review will consider contemporary data on vitamin D and their application to clinical practice for the management of ASCVD. We conducted a comprehensive literature review considering the biological mechanisms and current experimental, clinical and randomized trial data available to guide vitamin D therapy in cardiovascular disease prevention and/or treatment. PubMed and other literature search engines were utilized.
Vitamin D nomenclature and physiology
Vitamin D is a generic term that refers to a group of compounds derived from 7-dehydrocholesterol (7DHC) (Figure 1). In this review however, this term is exclusively applied to cholecalciferol (vitamin D3), formed through dermal photoconversion of 7DHC. Cholecalciferol is biologically inert but is converted to compounds that bind to the nuclear vitamin D receptor. From skin, cholecalciferol enters the circulation, where it has a half-life of ~24h due to substrate-dependent hepatic conversion to 25-hydroxyvitamin D (25(OH)D). 25(OH)D is the dominant circulating vitamin D metabolite, has a long half-life of ~3 weeks, and is used to determine vitamin D status given that its concentrations reflect supply, metabolism and catabolism over time and low concentrations are ubiquitous in vitamin D deficiency rickets or osteomalacia.(22) 25(OH)D is converted to 1,25-dihydroxyvitamin D (1,25(OH)2D, calcitriol) by the 1α-hydroxylase (CYP27B1). 1,25(OH)2D is a calcitropic steroid hormone with a short half-life of only 4–6 hours and a high affinity for the nuclear vitamin D receptor (VDR).
Figure 1: Vitamin D compounds and synthesis:
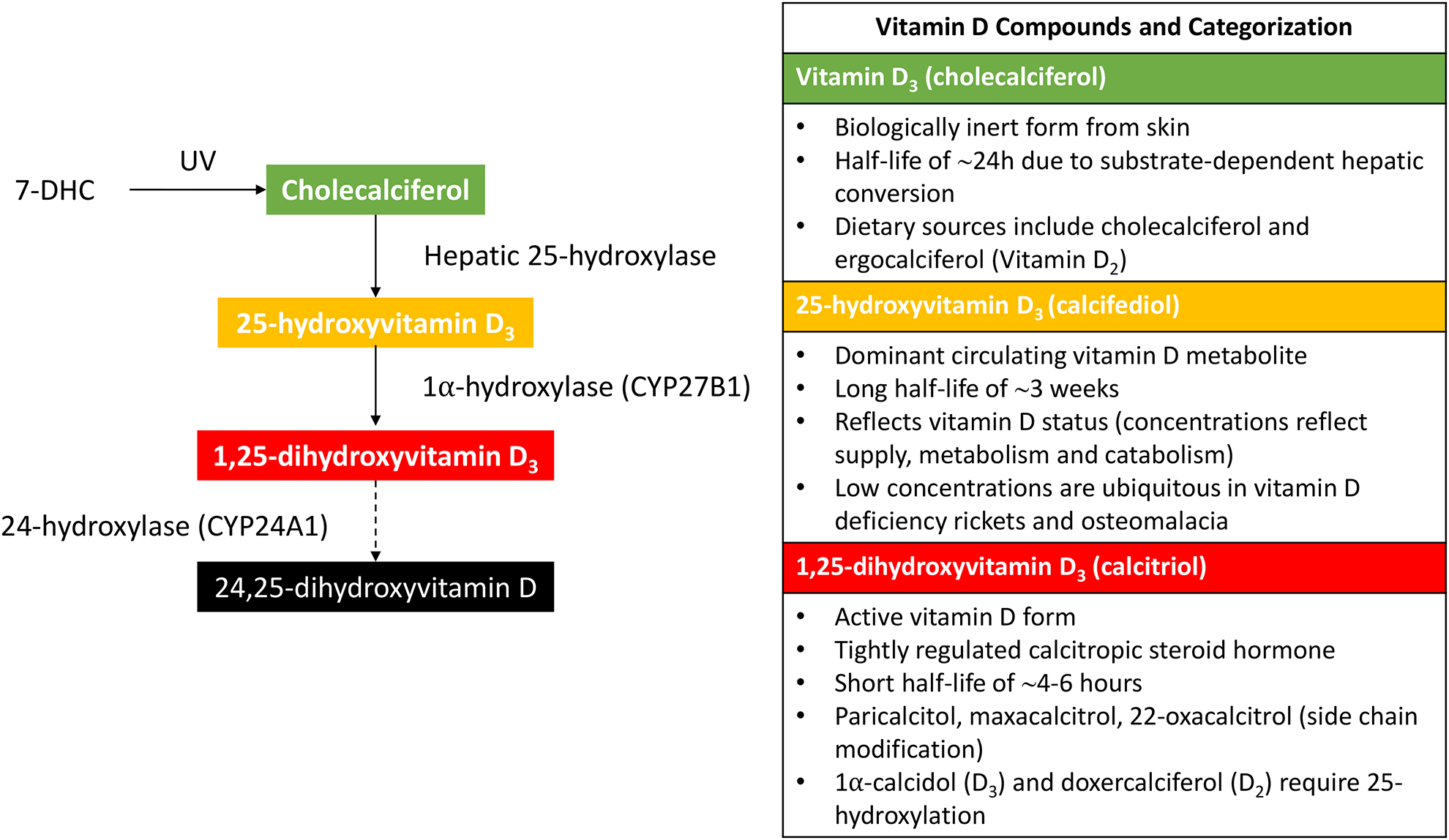
Choleclaciferol is formed through dermal photoconversion of 7-dehydrocholesterol (7DHC). Cholecalciferol is biologically inert, but is converted by various enzymes to compounds that bind the nuclear vitamin D receptor (VDR). 1,25-dihydroxyvitamin D (1,25(OH)2D, calcitriol) is the active form of vitamin D that is synthesized from 25(OH) 2D by 1α-hydroxylase (CYP27B1), now known to be expressed in almost all human tissues. 1,25(OH) 2D has a high affinity for the VDR.
1α-hydroxylase was initially identified in proximal tubular cells of the kidney, but is now known to be expressed throughout the nephron including the distal tube and collecting duct.(23) Renal 1α-hydroxylase appears to account for the majority of circulating 1,25(OH)2D that mediates endocrine (or classical) effects. Outside of the kidney, 1α-hydroxylase has been found to be expressed in almost all human tissue types, including the skin (basal keratinocytes, hair follicles), lymph nodes (granulomata), colon (epithelial cells and parasympathetic ganglia), brain (cerebellum and cerebral cortex), prostate, breast, testes, and placenta (decidual and trophoblastic cells), pancreas (islets), adrenal medulla, and notably in myocardium and vascular cells (endothelial and vascular smooth muscle cells).(24) Extra-renal synthesis of vitamin D mediates autocrine/paracrine or “non-classical” functions of vitamin D. It is unclear how much extra-renal 1α-hydroxylase activity contributes to circulating vitamin D levels. However, anephric individuals who were administered inactive vitamin D3 have been shown to have detectable serum 1,25(OH)2D levels suggesting significant extrarenal 1α-hydroxylase activity.(25)
Similar to 1α-hydroxylase, the VDR is ubiquitously expressed and this explains the multi-system effects observed during hypovitaminosis D.(26) Vitamin D deficiency results in notable mineral-bone abnormalities, including reduced intestinal calcium and phosphate absorption, increased parathyroid hormone (PTH) concentrations and reduced bone mineral density,(10,26) features recapitulated in VDR−/− mice.(27) This is not surprising given that 1,25(OH)2D levels are tightly regulated by PTH, serum calcium, phosphorus and the phosphatonin, fibroblast growth factor (FGF)-23. Dietary calcium can exert direct regulatory effects on the 1α-hydroxylase enzyme through modulations in serum calcium levels. Both dietary calcium and phosphate indirectly regulate the 1α-hydroxylase enzyme by modulating PTH production at the parathyroid gland. Low calcium and phosphate concentrations lead to the stimulation of 1α-hydroxylase to produce active 1,25(OH)2D.
FGF-23 is a bone-derived hormone that directly suppresses 1α-hydroxylase activity at the kidney and its function is dependent upon expression of a specialized functional receptor at the kidney, composed of fibroblast growth factor receptor (FGFR)-1 and the transmembrane α-Klotho protein.(28,29) High FGF-23 levels lead to suppressed of 1α-hydroxylase activity and consequently, reduced 1,25(OH)2D production. Given that α-Klotho is widely expressed in extrarenal tissues in humans including arterial, epithelial, endocrine, reproductive and neuronal tissues,(30) it is possible that FGF-23 can regulate extrarenal 1,25(OH)2D synthesis in α-Klotho producing tissues. High 1,25(OH)2D concentrations reduces 1α-hydroxylase enzyme activity via inhibition of PTH production, forming a negative feedback loop to prevent the development of hypervitaminosis D. Additionally, upregulation of the 24-hydroxylase enzyme by high 1,25(OH)2D stimulates the catabolic pathway resulting in reduced 1,25(OH)2D concentrations.
MOLECULAR EFFECTS ON THE ATHEROGENIC PROCESS
Robust experimental studies have shown strong links between vitamin D deficiency and pathogenic processes underlying the development of cardiac failure, such as endothelial dysfunction, reduced coronary flow, and subclinical atherosclerosis in patients with normal, or near-normal coronary arteries.(11,31) Signalling components of the vitamin D hormonal system are widely expressed across various cell types of the cardiovascular system, including myocardial cells, endothelial cells, vascular smooth muscle cells (VSMCs), fibroblasts and pericytes. This reflects the critical role of vitamin D in the regulation of cardiovascular health, particularly considering the role that these cell types, in addition to immune cells, play in the development of atherosclerosis. The presence of vitamin D signalling components and a functional 1α-hydroxylase enzyme in multiple cardiovascular cell types reflect an interplay between local production to exert autocrine/paracrine effects and response to endocrine effects in the regulation of normal cardiovascular physiology.(32)
VDR knockout mice exhibit prominent cardiovascular dysfunction, including upregulation of the renin-angiotensin-aldosterone (RAAS) system, hypertension, left ventricular hypertrophy and heart failure in addition to changes resembling premature aging such as alopecia, abnormal blood mineral levels, hyperparathyroidism, and defective T cell and macrophage function.(33,34) Conversely, repletion of vitamin D has been shown to reverse these changes in mice.(35) Significantly, VDR knockout mice develop multi-organ thrombus formation after administration of lipopolysaccharide.(36) These mice were found to have enhanced platelet aggregation, reduced nitric oxide (NO) synthase expression, and reduced expression of anti-thrombin in the liver, and thrombomodulin in the aorta, liver and kidney, thereby promoting the atherogenic process. Vitamin D exerts pleiotropic anti-atherogenic effects, such as regulation of vascular cell growth, migration and differentiation, immune response modulation, and regulation of cytokine production, inflammation and fibrotic pathways all of which play a critical role in atherosclerotic plaque formation and rupture.(37) Potential mechanisms by which VDR activation can modulate the development of cardiovascular disease is summarized in figure 2.
Figure 2: Vitamin D receptor (VDR) activation and the atherogenic process:
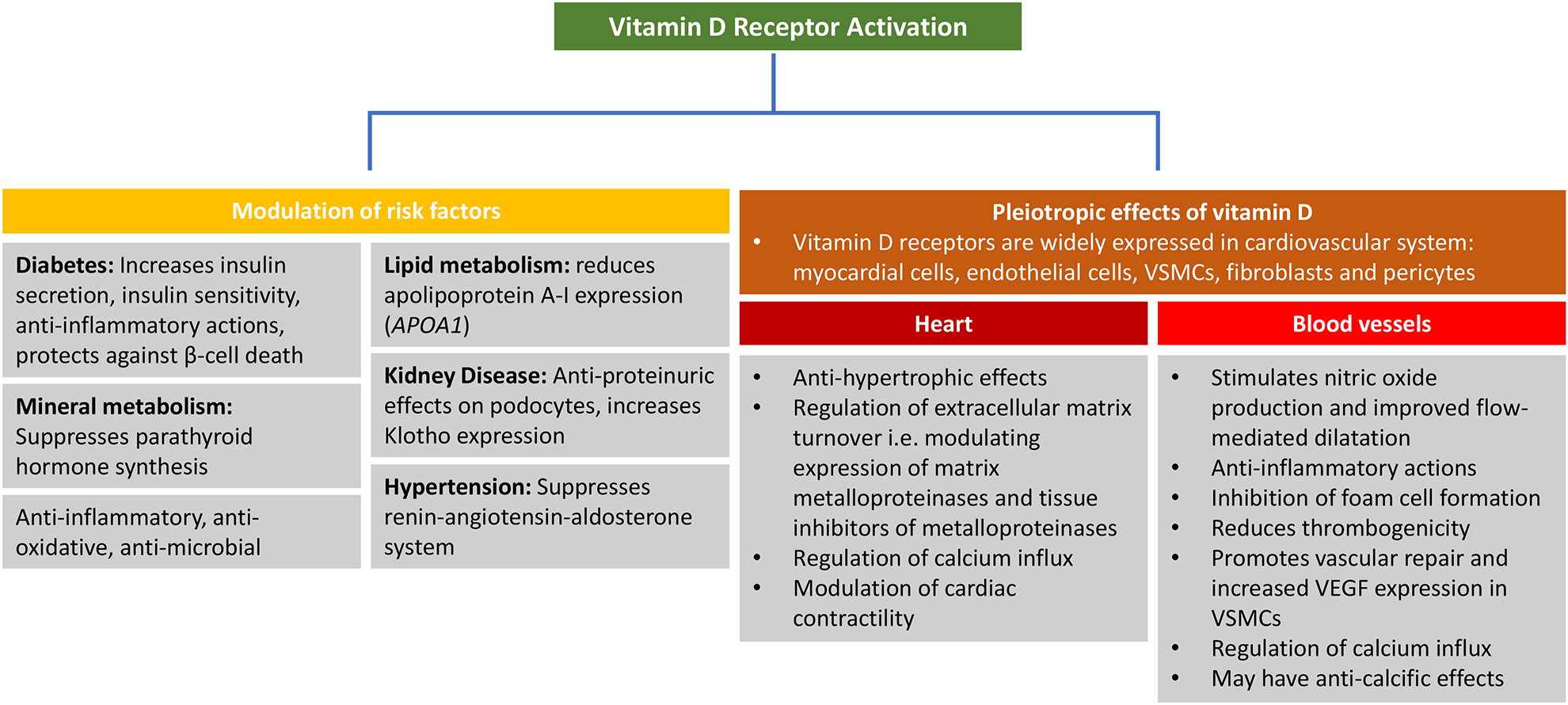
VDR activation can exert pleiotropic cardiovasculo-protective effects through multiple mechanisms. VSMCs (Vascular smooth muscle cells); VEGF (vascular endothelial growth factor).
Effects on vascular cells
VDR activation in endothelial cells (ECs) can modulate vascular tone through various mechanisms: Vitamin D stimulates nitric oxide (NO) production by activating NO synthase,(38,39) regulates the release of vasoconstrictor metabolites of arachidonic acid(38,40) and downregulates expression of cyclooxygenase-1, the major source of endothelium-derived contracting factors(41) By reducing calcium influx into ECs, VDR activation can acutely modulate vascular tone.(42) Vitamin D exerts anti-oxidative effects in ECs, in vitro by suppressing NADPH oxidase, leading to reduced superoxide synthesis(43) and blocking the extrinsic caspase cascade.(44) Importantly, vitamin D can inhibit intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in ECs in vitro, factors that are involved in subendothelial migration of monocytes and subsequent transformation into foam cells.(45,46)
Vascular smooth muscle cells (VSMCs) form the medial layer of the arterial wall and play a pivotal role in pathogenesis of atherosclerosis through proliferation and migration from the media to the intima, morphological changes and secretion of inflammatory factors.(47) Vitamin D has been shown to stimulate antiproliferative effects of VSMCs via inhibition of epidermal growth factor and endothelin(48,49), and regulate acute influx of Ca2+ into the cell, in vitro.(50) High doses of 1,25(OH)2D have been shown to induce VSMC migration while physiological doses of 25(OH)D and 1,25(OH)2D inhibits migration and proliferation.(51–53) Activation of VDRs increases elastogenesis and stabilization of the VSMC layer by stimulating production of myosin, collagen type 1, matrix metalloproteinase-9 and elastin.(54) By stimulating prostacyclin production via the cyclooxygenase pathway in VSMCs in vitro, 1,25(OH)2D may inhibit aggravation of atherosclerosis.(55) Activated vitamin D analogs have been shown to suppress plasminogen activator inhibitor-1, a pro-thrombotic and pro-atherogenic factor in coronary artery smooth muscle cells, in vitro.(56)
Vascular calcification occurs in healthy aging, in conjunction with atherosclerosis (intimal calcification) and at an accelerated rate in CKD (intimal and medial calcification), where it is strongly associated with cardiovascular events and mortality. Vitamin D deficiency has been associated with increased arterial calcification across a number of studies,(57–60) and with the development of coronary artery calcification.(61) Vitamin D can promote the production of vitamin K-dependent proteins and exert synergistic effects. This interaction appears critical given that vitamin K deficiency is associated with vascular calcification.(62) Experimental studies suggest that the role of VDRAs in the regulation of vascular calcification is dependent on the specific analogue. In animal models, administration of pharmacological doses of 1,25(OH)2D resulted in increased aortic calcification, however this was not seen in animals treated with the synthetic 1,25(OH)2D analogue 19-nor-1,25-dihydroxyvitamin D2 (paricalcitol).(63) In one model, both 1,25(OH)2D and paricalcitol were protective against vascular calcification at dosages sufficient to correct secondary hyperparathyroidism.(64)
Administration of active vitamin D sterols result in a reduction of plasma vitamin D concentrations, due to potent induction of the vitamin D catabolic pathway, in vivo.(58) These findings are consistent with evidence that polymorphisms in CYP24A1 that result in lower 25(OH)D concentrations are associated with increased coronary artery calcification.(65) Ensuring vitamin D repletion may therefore allow the autocrine and paracrine synthesis of 1,25(OH)2D in cardiovascular tissues, activating VDR-dependent calcification inhibitory pathways. 1,25(OH)2D and its analogues, administered systemically may overwhelm the autoregulatory system by potently inducing 24-hydroxylase expression and potentially result in local vitamin D deficiency at the tissue level.
Regulation of immune cells
The atherogenic process involves both the innate and adaptive immune system. Vitamin D is carried by low-density lipoprotein particles and is internalized by various cells, such as monocytes that contain a functional 1α-hydroxylase enzyme. Monocytes migrate trans-endothelially and are transformed into foam cells. Local synthesis of 1,25(OH)2D can decrease expression of monocyte adhesion expression and alter macrophaqe gene expression, facilitate cholesterol egress, lower acetylated and oxidized LDL uptake, and reduce foam cell production(66,67) and influence the atherosclerotic process by regulating endothelial cells and VSMCs (e.g. type I collagen, VEGF production, matrix metalloproteinases). VDR activation results in inhibition of interferon-ϒ and upregulation of IL-10, vitro.(68,69) Additionally, VDRs inhibit IL-1β and IL-6 production preventing macrophage activation and plaque instability.(70) Stimulation of IL-4 synthesis by VDR activation promote anti-atherogenic properties of Th2 cells in vitro, which secrete anti-atherogenic cytokines such as IL-5, IL-10 and IL-13.(71) These changes, seem likely to underlie some of the vasculo-protective properties of VDR activators.
Indirect effects of vitamin D on atheroma formation
VDR activators can protect against atherogenic formation indirectly by regulating other systemic processes. Vitamin D has been shown to regulate the renin angiotensin aldosterone (RAAS), a key regulator of blood pressure. Both angiotensin II and aldosterone are also involved in the atherogenic process(72) and this is promoted by vitamin D deficiency leading to activation of the RAAS system.(26) Conversely, VDR activators can suppress renin and angiotensinogen gene expression by blocking the NF-kB pathway.(11,73) There is also growing evidence that vitamin D can regulate β-cell function and insulin sensitivity(74) and that deficiency is associated with dyslipidemia,(75) both of which can promote atheroma formation. However, a detailed discussion of this is beyond the scope of this article.
EPIDEMIOLOGICAL AND INTERVENTIONAL DATA IN THE GENERAL POPULATION
Many studies have demonstrated efficacy of cholecalciferol, alone or with calcium supplements, in reducing osteoporotic fracture risk, and calcium and vitamin D have been integral to the treatment of osteoporosis for several decades, as remains the case today.(76) A considerable body of clinical and epidemiologic data suggests a role for vitamin D in reducing cardiovascular disease and mortality. Observational studies have shown that vitamin D deficiency is associated with hypertension, LVH, overt coronary heart disease and myocardial infarction, and is prognostic for major post-infarction adverse events including, heart failure hospitalizations, recurrent acute myocardial infarction and death(77–80). In a meta-analysis of observational studies including 65 994 participants, Wang et al showed that vitamin D deficiency was associated with an increased risk of cardiovascular disease, cardiovascular mortality, myocardial infarction and stroke.(12) Similarly, Chowdhury et al demonstrated a significant association of low vitamin D concentrations with cardiovascular death in a meta-analysis of observational studies including 849 412 participants comparing the lower to the upper vitamin D tertile (pooled relative risk 1.35, 95%CI 1.13 to 1.61).(13) In another meta-analysis that investigated the relationship between vitamin D and carotid atherosclerosis, vitamin D concentrations were inversely associated with carotid atherosclerosis.(81)
Findings from Mendelian Randomization (MR) studies have been inconsistent. Aspelund et al reported a HR of of 1.20 (95% CI 1.15–1.25) for all-cause mortality of a 20nmol/L difference in 25(OH)D in 10,501 patients.(82) In a large study of 95,766 Danish patients, the same reduction in 25(OH)D was associated with increased all-cause and cancer mortality, but not with increased cardiovascular mortality.(14) This lack of association with cardiovascular mortality is consistent with a recent report by Brondum-Jacobsen et al, where genetic determinants of 25(OH)D were not significantly associated with atherosclerotic ischemic heart disease (HR 0.98, 95% CI 0.76–1.26) or myocardial infarction (HR 1.15 (95% CI 0.83–1.59) in 92,416 Danish participants.(83) Other smaller MR studies have failed to show an association of genetic determinants of circulating 25(OH)D concentrations with all-cause, cardiovascular or noncardiovascular deaths(84) or diabetic microvascular complications (DMI).(85) A small but statistically significant reduction in systolic BP of 0.37mmHg was found to be associated with a genetically determined increment of 10 nmol/l in circulating 1,25(OH)2D in a large mendelian randomisation study (n=49,363).(86) However, the Women’s Genome Health Study (WGHS) that included 23,294 women of European ancestry did not find any association between 5 selected vitamin D-related single nucleotide polymorphisms (SNPs) with systolic or diastolic blood pressure.(87) It is noteworthy that there is some evidence that the association of vitamin D deficiency with health outcomes may in turn vary with common vitamin D receptor polymorphisms, and this may complicate interpretation of MR studies.(88) Further, polymorphisms investigated in MR studies vary, and may not include all genetic variants driving circulating 25(OH)D concentration.(89)
Risk factors for ASCVD such as dyslipidemia, hypertension and obesity, features that capitulate the metabolic syndrome significantly increase the risk of ASCVD. Several cross-sectional studies have shown that high serum 25(OH)D levels are associated with a favourable serum lipid profile.(90–92) However, clinical trials have showed conflicting results as discussed in further detail below.
Treatment with vitamin D in the general population
A number of meta-analyses have examined vitamin D replacement therapy with all-cause mortality and cardiovascular outcomes. A meta-analysis of randomized trials including 13,637 participants randomized to cholecalciferol or placebo reported that supplementation with cholecalciferol in subjects without kidney failure reduced all-cause mortality (HR 0.89, 95% CI 0.80 – 0.99).(13) Interestingly, there was no benefit with ergocalciferol. This is consistent with reports indicating that greater increments in total and free 25(OH)D are achieved with equivalent doses of cholecalciferol compared to ergocalciferol.(93,94) The benefit associated with cholecalciferol may be mediated by its effects on a variety of established cardiovascular risk factors. Mirhosseini et al reported a meta-analysis including data from 81 randomised trials, demonstrating that randomization to vitamin D resulted in significant reductions in systolic blood pressure, diastolic blood pressure, serum PTH, hs-CRP, total cholesterol, LDL, triglycerides and a significant increase in HDL.(95) In contrast to the reported effect on blood pressure, the Women’s Health Initiative trial failed to identify any reduction in SBP (0.22 mmHg, 95% CI −0.05 to 0.49) or DBP (0.11 mmHg, 95% CI −0.04 to 0.27) in 36,282 post-menopausal women randomised to elemental calcium and cholecalciferol or placebo with a median follow-up of 7 years.(96) The investigators also found no reduction in coronary or cerebrovascular risk in the treatment arm.(97) However, this trial used a very low daily dose of cholecalciferol (400IU) in combination with 1000 mg of elemental calcium. Additionally, in a meta-analysis by Elamin, et al that examined 51 trials, vitamin D supplementation was not associated with changes in diastolic or systolic blood pressure.(98) Others have reported similar findings in this highlight the inconsistencies in present trial data.
Although not directly comparable, it is noteworthy that a significant reduction in blood pressure was observed in 281 patients with diabetes and albuminuria after randomisation to the synthetic calcitriol analogue paricalcitol in the Selective Vitamin D Receptor Activator for Albuminuria Lowering Study.(99) The vast majority of these patients were hypertensive at entry.(99). Increased arterial stiffness as determined by pulse wave velocity has been associated with low 25(OH)D concentrations.(100) In a recent placebo controlled RCT in 120 patients with CKD3/4 and vitamin D insufficiency (25(OH)D < 50nmol/L), cholecalciferol 300,000 IU at baseline and 8 weeks significantly decreased pulse wave velocity (1.24 (2.16 – 0.74) m/s, p<0.001) compared with placebo after 16 weeks (secondary outcome). Similarly, Levin et al noted that in a 3-arm double blind RCT of 119 patients with CKD3b/4, calcidiol (25(OH)D) 5000IU 3x/week and calcitriol 0.5ug 3x/week both decreased PWV when compared to placebo.(101) Given the strong association of PWV with cardiovascular events and mortality, and also as a surrogate for arterial calcification, these data are of considerable importance.
Carotid intima-media thickness (CMT) is a marker of atherosclerosis and has been linked to vitamin D deficiency.(81) Unfortunately, few randomized trials have assessed the effects of vitamin D supplementation on CMT in the general population. In one trial that included 80 patients randomized to receive either 50,000 IU vitamin D/week or placebo for 16 weeks, the investigators found no significant different in CMT. However, pro-atherogenic inflammatory markers such as IL-6 levels were reduced.(67) Further, cholecalciferol may also have an impact on endothelial function. Kumar et al showed a significant increase in brachial arterial flow-mediated dilatation (FMD) with cholecalciferol but not placebo.(102) This is consistent with findings from the Paricalcitol and Endothelial Function in Chronic Kidney Disease (PENNY) trial, where paricalcitol 2ug/day increased FMD when compared to placebo.(103) In contrast, other RCTs have shown conflicting results. Kendrick et al reported no effect of cholecalciferol 2000IU per day on FMD over 6 months, possibly due to the lower dose of cholecalciferol used.(104) Chandler, et al. showed that vitamin D supplementation (at 1000, 2000 or 4000 IU/d orally compared to placebo for 3 months) did not change inflammatory markers in African Americans.(105)
The effect of Vitamin D supplementation on lipid profile and dyslipidemia is unclear, with conflicting results from several randomized trials. In one small randomized trial that included 8 patients with metabolic syndrome receiving 50,000 IU vitamin D or placebo weekly for 16 weeks, only triglyceride levels were significantly reduced.(106) However, in a large retrospective cohort study by Ponda et al., raising vitamin D levels from < 20 to ≥30 ng/ml (n=6260) compared with remaining at < 20 ng/ml (n=2332) was associated with an increase in total cholesterol (0.77 mg/dL, 95% CI 0.18–1.36) and high-density lipoprotein (0.42 mg/dL, CI 0.08–0.76) but no significant changes in low-density lipoprotein or triglycerides. In a meta-analysis that assessed the effects of vitamin D supplementation on blood lipids that included a total of 1346 participants, no statistically significant changes were observed for cholesterol, high-density lipoprotein, or triglyceride levels.(107)
These and other data have led to two recent large randomised placebo-controlled trials in the general population. The ViDA (Vitamin D Assessment) trial enrolled 5,108 participants in New Zealand aged 50–84 years.(108) In the treatment arm, participants received an initial dose of 200,000 IU followed a month later by monthly 100,000 IU or placebo for a median of 3.3 years. The investigators found no significant reduction in incident CVD and death in the treatment group compared to placebo (adjusted hazard ratio 1.02; 95% CI, 0.87–1.20). The VITAL trial examined the benefits and risks of cholecalciferol at a dose of 2000 IU per day and marine n-3 fatty acids at a dose of 1g per day (in a 2 × 2 factorial design) for the primary prevention of cardiovascular disease or cancer with median follow-up of 5.3 years.(109) VITAL included a total of 25,871 participants (men aged 50 years of age or older and women aged 55 years of age or older) in a randomized, double-blind, placebo-controlled design. Vitamin D did not lower the incidence of major cardiovascular events (396 in vitamin D group, 409 in placebo group; hazard ratio, 0.97; 95% CI, 0.85–1.12, P=0.69). Results were not modified by baseline serum 25(OH)D levels, with similar results for those above and below a baseline 25(OH)D level of 20 ng/mL. However, it is noteworthy that in both trial populations were mostly vitamin D replete prior to treatment (ViDA 66.25 ± 22.5 nmol/L; VITAL 77 ± 25 nmol/L). While ViDA and VITAL indicate that significant doses of cholecalciferol do not reduce cardiovascular risk in a generally replete population, they did not assess the effect of treating to sufficiency a vitamin D deficient population. Further, ViDA had a median follow-up time of 3.3 years and VITAL 5.3 years. Latency effects cannot be confidently excluded. Other limitations include the monthly dosing schedule in ViDA which is associated with nonphysiological fluctuations in blood levels of vitamin D.(110) There is therefore still a need for trials in vitamin D insufficient populations with a longer follow-up duration.
A summary of recent major randomized trials is provided in table 1.
Table 1:
Recent major RCTs of vitamin D and CVD events or CVD risk factors
| Trial name | Population | Intervention | Follow-up | Outcomes and main findings | Year | Ref |
|---|---|---|---|---|---|---|
| The VITAL (Vitamin D and Omega-3) trial | 25 871 men 50 years or older and women 55 years or older | Vitamin D3 (cholecalciferol) 2000 IU per day and omega-3 1g per day or placebo | Median 5.3 years | Vitamin D did not lower incidence of major cardiovascular events or incidence of invasive cancer compared to placebo | 2018 | 109 |
| The ViDA (Vitamin D Assessment) trial | 5110 community-resident adults, aged 50 to 84 years | Vitamin D3 (200 000IU), followed a month later by monthly 100 000 IU or placebo | Median 3.3 years | Monthly high-dose vitamin D supplementation did not prevent incident CVD and death compared to placebo | 2017 | 108 |
| The effect of vitamin D statues on endothelial function trial | 114 post-menopausal women with 25D concentrations between 10 and 60ng/mL | Vitamin D3 2500 IU or placebo | 4 months | No significant differences between groups in changes in brachial artery flow-mediated vasodilation (FMD), pulse wave velocity (PWV), augmentation index (AI) or CRP. | 2012 | 67 |
| The effect of vitamin D repletion on small low density lipoprotein (LDL) particle number in subjects at elevated cardiovascular risk trial | 151 adults with 25D concentrations < 20 ng/mL | Vitamin D3 50 000 IU weekly or placebo | 8 weeks | Vitamin D repletion did not change small low-density lipoprotein (LDL) levels, total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) or triglyceride levels compared to placebo | 2012 | 144 |
| Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative (WHI) trial | 754 women aged 50 to 59 | Calcium carbonate (1000mg elemental calcium) + vitamin D3 400 IU daily or placebo nested within WHI trial of estrogen among women who underwent hysterectomy | 7 years | Treatment with vitamin D3 and calcium did not alter coronary artery calcification (CAC) measurements as assessed by cardiac CT compared to placebo | 2010 | 145 |
| Vitamin D Effects in Overweight Patients (SMART) trial | Healthy overweight subjects (n=200) with mean 25D concentration of 30 nmol/L | Vitamin D preparation (Vigantol oil, Merck) 83μg per day or placebo | 12 months | Weight loss was not affected by vitamin D supplementation. Vitamin D group had lower PTH, triglyceride and TNF-α levels compared to placebo. | 2009 | 147 |
| Calcium/vitamin D supplementation and cardiovascular events in the Women’s Health Initiative (WHI) trial | 36,282 women aged 50 to 79 | Calcium carbonate (1000mg elemental calcium) + vitamin D3 400 IU daily or placebo | 7 years | Vitamin D3 and calcium supplementation did not increase or decrease coronary or cerebrovascular risk in healthy postmenopausal women | 2007 | 97 |
| Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure | 93 patients with congestive heart failure (CHF), New York Heart Association class ≥ 2 | Vitamin D3 50μg daily + 500mg calcium or placebo + 500mg calcium | 9 months | Pro-inflammatory cytokine TNF-α increased in the placebo group but remained constant in the vitamin D3 treatment group. There was no change in survival rate between the two groups. | 2006 | 146 |
| Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community | 2686 adults aged 65–85 years | Vitamin D3 100 000 IU or placebo every four months | 5 years | There was no significant difference in all-cause mortality or cardiovascular disease between the two groups. | 2003 | 148 |
| Vitamin D and the health of blood vessels in kidney disease trial | 119 patients eGFR 15–45 ml/min/1.73m2 | Calcifediol 5000 IU, calcitriol 0.5μg or placebo thrice weekly | 6 months | PWV decreased in the calcifediol group, remained unchanged in the calcitriol group and increased in the placebo group. | 2017 | 101 |
| The OPERA trial (Effect of Paricalcitol on Left Ventricular Mass and Function in CKD) trial | 60 patients with stage 3–5 CKD | Paricalcitol 1μg per day or placebo | 52 weeks | Paricalcitol significantly reduced PTH, alkaline phosphatase levels, and cardiovascular-related hospitalizations compared to placebo. There was no change in LV mass index as determined by cardiac MRI | 2014 | 134 |
| The PRIMO (Paricalcitol Capsule Benefits in Renal Failure– Induced Cardiac Morbidity) study | 227 CKD patients with mild to moderate LVH and preserved EF | Paricalcitol 2μg per day or placebo | 48 weeks | Paricalcitol reduced PTH levels within 4 weeks. At 48 weeks, there was no difference in left ventricular mass index compared to placebo as determined by cardiac MRI | 2012 | 133 |
| Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes study | 281 type-2 diabetic patients receiving RAAS inhibitor | Paricalcitol 1μg or 2μg per day, or placebo | 24 weeks | Addition of 2μg per day to RAAS inhibition lowered residual albuminuria in patients with diabetic nephropathy | 2010 | 99 |
LESSONS FROM KIDNEY DISEASE
CVD remains the leading cause of death among patients with CKD. End-stage renal disease (ESRD) patients on dialysis have a 10- to 30-fold higher CVD mortality compared with the general population.(111) CKD patients experience both accelerated atherosclerotic and non-atherosclerotic CVD, involving arterial calcification and left ventricular (LV) hypertrophy with the consequence of increased incidence of arrhythmia, congestive heart failure and sudden cardiac death.(112) CKD aggravates pre-existing traditional risk factors for atherosclerosis, such as hypertension and dyslipidemia. However, non-traditional or novel risk factors such as uremia, pro-inflammatory cytokines, mineral dysregulation and volume shifts on dialysis can all contribute to the development of accelerated atherosclerosis.(113,114) Perturbed vitamin D metabolism is a hallmark of kidney failure and CKD therefore represents a valuable model for vitamin D deficiency. Untreated kidney failure is associated with the classical skeletal features of vitamin D deficiency.
Plasma 25(OH)D concentrations begin to decline when the glomerular filtration rate (GFR) falls below 50 ml/min/1.73m2.(115) There are multiple contributing factors to the vitamin D deficiency observed in CKD, including urinary losses in patients with proteinuric kidney disease,(116) impaired hepatic 25-hydroxylation in the context of uremia,(117) reduced dietary intake, and increased catabolism through induction of the catabolic 24-hydroxylase(118) in addition to the known risk factors for deficiency. Further, elevated concentrations of FGF-23 induce the vitamin D catabolic pathway in the kidney and simultaneously inhibit 1α-hydroxylation. Once patients reach ESRD, only a minority are vitamin D sufficient. In a cohort of 6,518 German dialysis patients, 76% were vitamin D deficient,(119) with smaller studies reporting deficiency in 69 – 87%.(120–124)
Approximately 80% of all vitamin D metabolites are carried in blood bound to vitamin D binding protein (VDBP). A 58kDa protein, VDBP is a negative acute phase reactant with a short half-life (1–2 days). In acute illness, VDBP concentrations (and consequently total vitamin D concentrations) reduce. In patients with proteinuric kidney disease, urinary losses of VDBP and its cargo of vitamin D metabolites occur.(116) It is also readily lost through plasma exchange,(124) and these factors may contribute to its deficiency in patients with CKD. Given these variations in VDBP (and their impact on measured total vitamin D), some have proposed determining ‘free’ or bioavailable vitamin D. Indeed, bioavailable vitamin D appears to correlate more closely with markers of mineral metabolism in CKD than does total 25(OH)D.(125) The discovery of genetic polymorphisms in VDBPs and racial differences have added further dimensions of complexity to this field(126).
Challenges with conventional use of 1α-hydroxylated compounds
Given the high prevalence of vitamin D deficiency in CKD populations and the association with adverse health outcomes of particular relevance to CKD, existing CKD treatment guidelines recommend measuring 25(OH)D and replenishing vitamin D with cholecalciferol, aiming for a target threshold of 75 nmol/L (30 ng/mL) or above.(20) However, this is not consistently done since supportive evidence is limited (The Kidney Disease Improving Global Outcomes - KDIGO treatment guidelines grades this as a 2C recommendation), vitamin D measurement is costly, and it is unclear whether the associations observed with deficiency apply in the context of concomitant treatment with 1α-hydroxylated vitamin D; osteomalacia and rickets are both effectively treated with 1,25(OH)2D,(127) and it is not currently known whether the same applies to other sequelae of vitamin D deficiency in patients with CKD.
Although PTH suppression is readily achieved through administration of 1α-hydroxylated vitamin D, the effect this might have on tissue concentrations of 1,25(OH)2D is unknown. The existing treatment paradigm of reliance on 1α-hydroxylated vitamin D compounds presents several concerns: 1) systemic administration increases plasma calcium and phosphate; 2) dose is limited by hypercalcemia; 3) it may potentiate vascular calcification and 4) potent induction of the vitamin D catabolic pathway occurs, paradoxically potentiating vitamin D deficiency by catabolism of 1,25(OH)2D and 25(OH)D at tissue level. In contrast, the administration of inactive cholecalciferol could circumvent all of these limitations. The systemic administration of cholecalciferol results in high tissue concentrations of 1,25(OH)2D without the same risk of causing hypercalcemia seen with 1α-hydroxylated VDRAs.(128,129) Furthermore, cholecalciferol use results in significant reductions in PTH, and either reduced or unchanged plasma calcium and phosphate concentrations.(122,130) Cholecalciferol also results in reductions in bone alkaline phosphatase (ALP), and improves the achievement of treatment targets for calcium, phosphate and PTH concentrations.(122)
The fact that the VDR and CYP27B1 are ubiquitously expressed mandates a reappraisal of the current practice of administering mostly 1α-hydroxylated compounds to patients with ESRD, since this strategy was predicated on the now disproven belief that 1α-hydroxylation was exclusive to the kidney. The ubiquitous use of 1α-hydroxylated compounds (alfacalcidol, calcitriol, paricalcitol etc.) in CKD presents the greatest challenge to interpretation of evidence from studies in the general population to the CKD setting.
Therapeutic targeting of CVD in CKD with vitamin D
In a retrospective observational study, Lishmanov et al compared patients with CKD3/4 and vitamin D < 75 nmol/L that were prescribed ergocalciferol replacement (n=90) to those that received no replacement (n=30). Patients receiving ergocalciferol had a significantly lower risk of cardiovascular events over a mean follow-up of 27.2 months (HR 0.37, 95% CI 0.14 – 1.0).(131) While observational studies such as this support therapeutic supplementation of vitamin D in CKD patients for cardiovascular benefit, unfortunately interventional studies have demonstrated conflicting results. It should be noted that randomized controlled trials that specifically examine atherosclerosis as the primary prespecified outcome in CKD patients are severely lacking.
In the recent J-DAVID (Japan Dialysis Active Vitamin D) trial, 976 hemodialysis patients with serum PTH levels less than or equal to 180pg/ml were recruited.(132) The patients were randomized to receive either 0.5μg oral alfacalcidiol per day or no vitamin D receptor activator with median follow-up of 4 years. The investigators found no significant difference in composite outcome of cardiovascular events in the intervention group (21.1%) compared to the control group (17.9%; absolute difference 3.25% [95% CI, −1.75% to 8.24%; HR 1.25 [95% CI, 0.94–1.67], p=0.13).
The PRIMO (Paricalcitol Capsule Benefits in Renal Failure – Induced Cardiac Morbdity) study recruited 227 patients in a multinational double-blinded placebo-controlled trial designed to study the effects of paricalcitol on left ventricular mass index (LVMI) using cardiac magnetic resonance (CMR) imaging (133). G3 and G4 CKD patients with mild-to-moderate left ventricular hypertrophy (LVH) and preserved LV ejection fraction at baseline were included and followed over a period of 48 weeks. The study found that paricalcitol did not alter LVMI (0.34 g/m2.7, 95% CI −0.14–0.83 g/m2) compared to placebo (−0.07, g/m2.7, 95% CI −0.55–0.42 g/m2). In the paricalcitol arm, PTH levels were reduced to 30% of baseline, markedly increased concentrations of serum calcium and phosphate and a lower number of cardiovascular hospitalizations. There was no change in pre-specified measures of diastolic function, or SBP. The OPERA trial (Effect of Paricalcitol on Left Ventricular Mass and Function in CKD) was a prospective, double-blinded, randomized, placebo-controlled trial to determine whether oral activated vitamin D reduced LV mass as examined by echocardiography (134). Patients were randomly assigned to receive either oral paricalcitol (n=30) or matching placebo (n=30) for 52 weeks. Similar to the PRIMO study, the study did not find a difference in LV mass between the two arms.
Despite several animal models of LVH that have shown promising therapeutic effects of vitamin D, including the spontaneously hypertensive rat model(135), the Dahl salt sensitive rat(136) and the pressure overload rat(137), clinical trials have not shown benefit. A number of possibilities exists that could explain these findings, such as treatment duration that may have been too short to detect a difference, the degree of LVH was too far advanced, or high FGF-23 levels that can directly induce LVH development(138) and mask beneficial effects of vitamin D. One should also entertain the possibility that the primary hypothesis that vitamin D can reduce cardiovascular disease in CKD is in fact null in humans.
There is evidence linking vitamin D deficiency with SCD in hemodialysis patients.(139) Vitamin D treatment of hemodialysis patients has been associated with a reduced QTc interval, prolongation of which is a significant risk factor for SCD(140). In one large cross-sectional study that recruited 3299 Caucasian patients who were routinely referred to coronary angiography, low 25(OH)D and 1,25(OH)2D concentrations were associated with SCD(77).
HOW MUCH VITAMIN D IS ENOUGH?
Vitamin D status is determined by measuring 25(OH)D concentrations. The 2011 report by the IOM suggests that 25(OH)D levels of 16 ng/ml (40nmol/liter) meet the needs of approximately half the population (median population requirement) and levels of 20ng/ml (50 nmol/liter) meet the needs of at least 97.5% of the population. These findings consider the observation that lower concentrations were associated with rickets and osteomalacia (21) and that thresholds are reached for intestinal absorption of calcium and for bone mineral density at these 25(OH)D concentrations, There remains controversy as to the definitions of vitamin D deficiency and varying definitions have caused confusion. For example, the UK Department of Health and Scientific Advisory Committee on Nutrition (SACN) define vitamin D deficiency as < 25 nmol/l (10ng/ml). Others have argued that the deficiency threshold should be significantly higher, at 50 nmol/L (20 ng/dL).(10) There is additional controversy regarding the upper limit of normal or tolerable upper intake levels; different cut-offs have been proposed ranging from 50 ng/ml to 150ng/ml. Excess vitamin D, usually at 25(OH)D concentrations > 150ng/ml can cause vitamin D toxicity that results in hypercalcemia, renal failure, neuropsychiatric and gastrointestinal symptoms as well as cardiovascular manifestations such as hypertension, arrhythmia and heart block. In extreme cases, vitamin D toxicity can cause coma and death.(141)
Although the thresholds for defining sufficiency have historically been based on skeletal health, there is increasing recognition that non-skeletal disease outcomes should also be taken into account. For example, while levels > 10 ng/ml are optimal to prevent rickets and osteomalacia, levels > 30 ng/ml may be needed to prevent both secondary hyperparathyroidism and osteoporosis. However, the utility of PTH concentrations for identifying the optimal level of vitamin D remains controversial and the relationship between 25(OH)D and PTH is inconsistent.(15) Additionally, recommendations should take into account the fact that vitamin D concentrations are influenced by sunlight exposure, increasing latitude, skin pigmentation, adiposity, low physical activity, poor diet, diabetes and CKD.(142)
The Recommended Dietary Allowance (RDA) provided by the IOM for vitamin D is 600 IU per day for individuals 1 to 70 years of age and 800 IU/d for those above 70 years of age. These recommendations are pooled from 32 study averages of serum 25(OH)D concentrations. Per the IOM, this RDA is expected to achieve a 25(OH)D level of 50 nmol/L or more in 97.5% of healthy individuals.(18) A common misconception is that the RDA defines a threshold or “cut point” of which virtually the entire population must have a serum 25(OH)D concentration above 20ng/ml to achieve adequate bone health.(15) The reality is that most of the population (about 97.5%) has a requirement of 20ng/ml or less and approximately half of the population has a requirement of 16ng/ml or less. (15)
A number of current trials are seeking to reduce the uncertainty over the benefits of vitamin D supplementation. VITAL will soon report on additional health outcomes, including heart failure, cognition, diabetes, depression, and autoimmune diseases. The D-Health trial is assessing the effect of 60,000IU cholecalciferol monthly on all cause and cancer mortality in 21,315 Australian adults.(143) The Finnish Vitamin D Trial (FIND) is assessing the effects of up to 3,600IU daily of cholecalciferol in reducing cancer and cardiovascular risk in 2495 participants in men aged 60 years or older and women 65 years or older (NCT01463813). DO-HEALTH is testing vitamin D3 2000 IU daily for bone health, fall reduction, physical performance, and other outcomes among >2000 European adults. One large randomised trial of cholecalciferol versus standard care in dialysis is currently underway in the United Kingdom (The SIMPLIFIED Trial, EudraCT 2015–005003-88). These and other trials (Table 2) should generate evidence for the general and CKD population of the efficacy and effectiveness of vitamin D in reducing cardiovascular and cancer risk.
Table 2:
Ongoing Vitamin D clinical trials
| Trial name | Population | Intervention | Main outcomes | Results expected | Country |
|---|---|---|---|---|---|
| VIDAL (Vitamin D and Longevity trial) | 20,000 adults, 65–85 years old | 100,000 IU D3 monthly, placebo or open control | Mortality, morbidity (infections, doctor’s visits, cancer) and vitamin D levels | 2020 | United Kingdom |
| SIMPLIFIED (Survival Improvement with Cholecalciferol in Patients on Dialysis) | 4,200 adults, aged > 18 years | Cholecalciferol 60,000 IU fortnightly | Mortality, health-related quality of life, hospital admission, cardiovascular events, cancer incidence | 2023 | United Kingdom |
| TARGET-D (Trial Evaluating Vitamin D Normalization on Major Adverse Cardiovascular-Related Events Among Myocardial Infarction Patients) | 890 adults, aged > 18 years | Target 25D levels > 40ng/mL with cholecalciferol or standard of care (no intervention) | Mortality, myocardial infarction, heart failure hospitalization and cerebral vascular accident | 2021 | United States |
| FIND (Finnish Vitamin D Trial) | 2495 adults, men > 60 years and women > 65 years | 3200 D3 daily, 1600 D3 daily or placebo | Cardiovascular disease, cancer and diabetes mellitus | 2019 | Finland |
| Effect of Vitamin D Supplementation in Patients with Heart Failure and Vitamin D Deficiency | 60 adults, aged 45 to 85 years | 5 000IU vitamin D D vs placebo | Myocardial structure and function by MRI, myocardial infarction | 2019 | Mexico |
| Effect of Vitamin D on Ventricular Remodeling in Patients with Acute Myocardial Infarction (VITDAMI) | 144 adults, aged 40 to 85 years | 15,690 IU Calcifediol vs placebo | Change in cardiac remodeling by MRI, echo parameters | 2019 | Spain |
| Magnesium and Vitamin D Supplementation and Cardiometabolic Outcomes | 123 adults, aged 30 to 70 years | 1000U vitamin D and 360 magnesium vs 1000U vitamin D vs placebo | PTH levels, inflammatory cytokines, lipid profile, blood pressure, serum osteocalcin | 2020 | United States |
| Vitamin D Prophylaxis in the Prevention of Hypertensive Disorders of Pregnancy | 412 adults, aged > 18 years | 3000 IU vitamin D daily or 4000 IU daily with and without prenatal vitamins | Blood pressure, new onset proteinuria, thrombocytopenia, impaired liver function testing, renal insufficiency | 2019 | United States |
| DO-HEALTH (Vitamin D3-Omega3-Home Exercise – Healthy Ageing and Longevity Trial) | 2157 adults, aged 70 years and older | 1) 2000 IU vitamin D compared to placebo; 2) 1g omega-3 fatty acid compared to placebo; 3) home exercise of 30 minutes 3 times a week compared to control exercise program 30 minutes 3 times a week | Incident non-vertebral fractures, functional decline (measured by short physical performance test battery), blood pressure, cognitive decline (by montreal cognitive assessment), immunity (by rate of any infections) | 2019 | Switzerland |
Conclusion
Clear scientific evidence supports a key role for vitamin D in skeletal health. However, there is inconclusive evidence to support benefits of vitamin D supplementation for extraskeletal health outcomes. Furthermore, existing evidence suggests that most of the population is meeting their requirements, at suggested RDAs by IOM and at the 25(OH)D level of at least 20ng/ml (50 nmol/liter) even under conditions of low sun exposure.(21) Additional trials assessing whether vitamin D supplementation in populations with lower serum 25(OH)D levels, with treatment and follow-up over longer durations, and testing higher dosages of vitamin D, will confer CVD benefits are needed. Also, additional data on cardiovascular outcomes such as heart failure will be of great importance.
In high risk groups such as the CKD population, however, vitamin D deficiency is prevalent. The alternative strategy of administering 1α-hydroxylated compounds for treatment of secondary hyperparathyroidism and renal osteodystrophy has prevailed over recent decades and continues to do so in this population, but the rationale for the preferential use of these drugs is not strong, nor supported by robust evidence from adequately powered randomized trials. For patients not currently enrolled in trials, treating established vitamin D deficiency with cholecalciferol appears to be a rational and reasonable way forward while further evidence accumulates.
Disclosures
T.F.H receives funding from the National Institute for Health Research NIHR HTA 14/49/127, 16/167/120 and 17/83/06. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care, UK. K.L. received an NIH (K23 DK115683-01) award. R.T. is a consultant to Fresenius Medical Care North America and has filed patents in the area of bioavailable vitamin D. JEM receives funding from the National Institutes of Health to conduct the VITamin D and OmegA-3 TriaL (VITAL). The National Cancer Institute, National Heart Lung and Blood Institute, and other NIH institutes and agencies provide funding for the trial. Study pills are donated by Pharmavite LLC of Northridge, Calif (vitamin D) and Pronova BioPharma, Norway and BASF (omega-3s).
References
Table 1 citations: (108,109) (67) (144) (145) (147) (97) (146) (148) (101) (134) (133) (99)
- 1.Nascimento BR, Brant LC, Moraes DN, Ribeiro AL. Global health and cardiovascular disease. Heart. 2014;100(22):1743–1749. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. [DOI] [PubMed] [Google Scholar]
- 3.O’Flaherty M, Buchan I, Capewell S. Contributions of treatment and lifestyle to declining CVD mortality: why have CVD mortality rates declined so much since the 1960s? Heart. 2013;99(3):159–162. [DOI] [PubMed] [Google Scholar]
- 4.Baena CP, Chowdhury R, Schio NA, Sabbag AE Jr., Guarita-Souza LC, Olandoski M, Franco OH, Faria-Neto JR. Ischaemic heart disease deaths in Brazil: current trends, regional disparities and future projections. Heart. 2013;99(18):1359–1364. [DOI] [PubMed] [Google Scholar]
- 5.Insull W Jr. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med. 2009;122(1 Suppl):S3–S14. [DOI] [PubMed] [Google Scholar]
- 6.Ntsekhe M, Damasceno A. Recent advances in the epidemiology, outcome, and prevention of myocardial infarction and stroke in sub-Saharan Africa. Heart. 2013;99(17):1230–1235. [DOI] [PubMed] [Google Scholar]
- 7.de Fatima Marinho de Souza M, Gawryszewski VP, Ordunez P, Sanhueza A, Espinal MA. Cardiovascular disease mortality in the Americas: current trends and disparities. Heart. 2012;98(16):1207–1212. [DOI] [PubMed] [Google Scholar]
- 8.Sekikawa A, Miura K, Lee S, Fujiyoshi A, Edmundowicz D, Kadowaki T, Evans RW, Kadowaki S, Sutton-Tyrrell K, Okamura T, Bertolet M, Masaki KH, Nakamura Y, Barinas-Mitchell EJ, Willcox BJ, Kadota A, Seto TB, Maegawa H, Kuller LH, Ueshima H, Group EJS. Long chain n-3 polyunsaturated fatty acids and incidence rate of coronary artery calcification in Japanese men in Japan and white men in the USA: population based prospective cohort study. Heart. 2014;100(7):569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackshaw A, Morris JK, Boniface S, Tang JL, Milenkovic D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018;360:j5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 11.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, Eaton CB, May HT, Anderson JL, Sesso HD. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5(6):819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB, Feldman BS, Pan A, Johnson L, Crowe F, Hu FB, Franco OH. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. 2014;349:g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manson JE, Brannon PM, Rosen CJ, Taylor CL. Vitamin D Deficiency - Is There Really a Pandemic? N Engl J Med. 2016;375(19):1817–1820. [DOI] [PubMed] [Google Scholar]
- 16.Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, Eckfeldt JH, Fleet JC, Howard G, Hoofnagle AN, Hui SL, Lensmeyer GL, Massaro J, Peacock M, Rosner B, Wiebe D, Bailey RL, Coates PM, Looker AC, Sempos C, Johnson CL, Picciano MF, Vitamin DRotNMoSDAC, Options for Resolving T. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr. 2010;140(11):2030S–2045S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Nutrition Board IoM. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC, USA: The National Academic Press. [Google Scholar]
- 19.Kleeman CR, Bernstein D. Chronic renal failure. Its effect on calcium, phosphorus and osseous metabolism unified approach. Calif Med. 1961;94:335–338. [PMC free article] [PubMed] [Google Scholar]
- 20.Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, Gutierrez OM, Bansal V, Rosas SE, Nigwekar S, Yee J, Kramer H. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis. 2017;70(6):737–751. [DOI] [PubMed] [Google Scholar]
- 21.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr. 2008;88(2):500S–506S. [DOI] [PubMed] [Google Scholar]
- 23.Zehnder D, Quinkler M, Eardley KS, Bland R, Lepenies J, Hughes SV, Raymond NT, Howie AJ, Cockwell P, Stewart PM, Hewison M. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008;74(10):1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888–894. [DOI] [PubMed] [Google Scholar]
- 25.Jongen MJ, van der Vijgh WJ, Lips P, Netelenbos JC. Measurement of vitamin D metabolites in anephric subjects. Nephron. 1984;36(4):230–234. [DOI] [PubMed] [Google Scholar]
- 26.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8–28. [DOI] [PubMed] [Google Scholar]
- 27.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94(18):9831–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. [DOI] [PubMed] [Google Scholar]
- 29.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. [DOI] [PubMed] [Google Scholar]
- 30.Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, James S, Wilkinson IB, Ting S, Hsiao LL, Hiemstra TF, Zehnder D. alpha-Klotho Expression in Human Tissues. J Clin Endocrinol Metab. 2015;100(10):E1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oz F, Cizgici AY, Oflaz H, Elitok A, Karaayvaz EB, Mercanoglu F, Bugra Z, Omer B, Adalet K, Oncul A. Impact of vitamin D insufficiency on the epicardial coronary flow velocity and endothelial function. Coron Artery Dis. 2013;24(5):392–397. [DOI] [PubMed] [Google Scholar]
- 32.Hewison M, Zehnder D, Bland R, Stewart PM. 1alpha-Hydroxylase and the action of vitamin D. J Mol Endocrinol. 2000;25(2):141–148. [DOI] [PubMed] [Google Scholar]
- 33.Weishaar RE, Simpson RU. Vitamin D3 and cardiovascular function in rats. J Clin Invest. 1987;79(6):1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol. 1990;258(1 Pt 1):E134–142. [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124(17):1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aihara K, Azuma H, Akaike M, Ikeda Y, Yamashita M, Sudo T, Hayashi H, Yamada Y, Endoh F, Fujimura M, Yoshida T, Yamaguchi H, Hashizume S, Kato M, Yoshimura K, Yamamoto Y, Kato S, Matsumoto T. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem. 2004;279(34):35798–35802. [DOI] [PubMed] [Google Scholar]
- 37.Lim K, Hamano T, Thadhani R. Vitamin D and Calcimimetics in Cardiovascular Disease. Semin Nephrol. 2018;38(3):251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molinari C, Uberti F, Grossini E, Vacca G, Carda S, Invernizzi M, Cisari C. 1alpha,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell Physiol Biochem. 2011;27(6):661–668. [DOI] [PubMed] [Google Scholar]
- 39.Queen LR, Ji Y, Xu B, Young L, Yao K, Wyatt AW, Rowlands DJ, Siow RC, Mann GE, Ferro A. Mechanisms underlying beta2-adrenoceptor-mediated nitric oxide generation by human umbilical vein endothelial cells. J Physiol. 2006;576(Pt 2):585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanhoutte PM. Endothelium-dependent contractions in hypertension: when prostacyclin becomes ugly. Hypertension. 2011;57(3):526–531. [DOI] [PubMed] [Google Scholar]
- 41.Wong MS, Man RY, Vanhoutte PM. Calcium-independent phospholipase A(2) plays a key role in the endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2010;298(4):H1260–1266. [DOI] [PubMed] [Google Scholar]
- 42.Wong MS, Delansorne R, Man RY, Vanhoutte PM. Vitamin D derivatives acutely reduce endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2008;295(1):H289–296. [DOI] [PubMed] [Google Scholar]
- 43.Hirata M, Serizawa K, Aizawa K, Yogo K, Tashiro Y, Takeda S, Moriguchi Y, Endo K, Fukagawa M. 22-Oxacalcitriol prevents progression of endothelial dysfunction through antioxidative effects in rats with type 2 diabetes and early-stage nephropathy. Nephrol Dial Transplant. 2013;28(5):1166–1174. [DOI] [PubMed] [Google Scholar]
- 44.Polidoro L, Properzi G, Marampon F, Gravina GL, Festuccia C, Di Cesare E, Scarsella L, Ciccarelli C, Zani BM, Ferri C. Vitamin D protects human endothelial cells from H(2)O(2) oxidant injury through the Mek/Erk-Sirt1 axis activation. J Cardiovasc Transl Res. 2013;6(2):221–231. [DOI] [PubMed] [Google Scholar]
- 45.Martinesi M, Bruni S, Stio M, Treves C. 1,25-Dihydroxyvitamin D3 inhibits tumor necrosis factor-alpha-induced adhesion molecule expression in endothelial cells. Cell Biol Int. 2006;30(4):365–375. [DOI] [PubMed] [Google Scholar]
- 46.Stach K, Kalsch AI, Nguyen XD, Elmas E, Kralev S, Lang S, Weiss C, Borggrefe M, Kalsch T. 1alpha,25-dihydroxyvitamin D3 attenuates platelet activation and the expression of VCAM-1 and MT1-MMP in human endothelial cells. Cardiology. 2011;118(2):107–115. [DOI] [PubMed] [Google Scholar]
- 47.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28(5):812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13(6 Pt 2):954–959. [DOI] [PubMed] [Google Scholar]
- 49.Chen S, Law CS, Gardner DG. Vitamin D-dependent suppression of endothelin-induced vascular smooth muscle cell proliferation through inhibition of CDK2 activity. J Steroid Biochem Mol Biol. 2010;118(3):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies MR, Hruska KA. Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int. 2001;60(2):472–479. [DOI] [PubMed] [Google Scholar]
- 51.Rebsamen MC, Sun J, Norman AW, Liao JK. 1alpha,25-dihydroxyvitamin D3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circ Res. 2002;91(1):17–24. [DOI] [PubMed] [Google Scholar]
- 52.Tukaj C, Trzonkowski P, Pikula M, Hallmann A, Tukaj S. Increased migratory properties of aortal smooth muscle cells exposed to calcitriol in culture. J Steroid Biochem Mol Biol. 2010;121(1–2):208–211. [DOI] [PubMed] [Google Scholar]
- 53.Raymond MA, Desormeaux A, Labelle A, Soulez M, Soulez G, Langelier Y, Pshezhetsky AV, Hebert MJ. Endothelial stress induces the release of vitamin D-binding protein, a novel growth factor. Biochem Biophys Res Commun. 2005;338(3):1374–1382. [DOI] [PubMed] [Google Scholar]
- 54.Norman PE, Powell JT. Vitamin D, shedding light on the development of disease in peripheral arteries. Arterioscler Thromb Vasc Biol. 2005;25(1):39–46. [DOI] [PubMed] [Google Scholar]
- 55.Wakasugi M, Noguchi T, Inoue M, Kazama Y, Tawata M, Kanemaru Y, Onaya T. Vitamin D3 stimulates the production of prostacyclin by vascular smooth muscle cells. Prostaglandins. 1991;42(2):127–136. [DOI] [PubMed] [Google Scholar]
- 56.Wu-Wong JR, Nakane M, Ma J. Effects of vitamin D analogs on the expression of plasminogen activator inhibitor-1 in human vascular cells. Thromb Res. 2006;118(6):709–714. [DOI] [PubMed] [Google Scholar]
- 57.Lai S, Fishman EK, Gerstenblith G, Brinker J, Tai H, Chen S, Li J, Tong W, Detrick B, Lai H. Vitamin D deficiency is associated with coronary artery calcification in cardiovascularly asymptomatic African Americans with HIV infection. Vasc Health Risk Manag. 2013;9:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ellam T, Hameed A, ul Haque R, Muthana M, Wilkie M, Francis SE, Chico TJ. Vitamin D deficiency and exogenous vitamin D excess similarly increase diffuse atherosclerotic calcification in apolipoprotein E knockout mice. PLoS One. 2014;9(2):e88767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt N, Brandsch C, Kuhne H, Thiele A, Hirche F, Stangl GI. Vitamin D receptor deficiency and low vitamin D diet stimulate aortic calcification and osteogenic key factor expression in mice. PLoS One. 2012;7(4):e35316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young KA, Snell-Bergeon JK, Naik RG, Hokanson JE, Tarullo D, Gottlieb PA, Garg SK, Rewers M. Vitamin D deficiency and coronary artery calcification in subjects with type 1 diabetes. Diabetes Care. 2011;34(2):454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20(8):1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Ballegooijen AJ, Pilz S, Tomaschitz A, Grubler MR, Verheyen N. The Synergistic Interplay between Vitamins D and K for Bone and Cardiovascular Health: A Narrative Review. Int J Endocrinol. 2017;2017:7454376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mizobuchi M, Finch JL, Martin DR, Slatopolsky E. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72(6):709–715. [DOI] [PubMed] [Google Scholar]
- 64.Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol. 2008;19(8):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen H, Bielak LF, Ferguson JF, Streeten EA, Yerges-Armstrong LM, Liu J, Post W, O’Connell JR, Hixson JE, Kardia SL, Sun YV, Jhun MA, Wang X, Mehta NN, Li M, Koller DL, Hakonarson H, Keating BJ, Rader DJ, Shuldiner AR, Peyser PA, Reilly MP, Mitchell BD. Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arterioscler Thromb Vasc Biol. 2010;30(12):2648–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riek AE, Oh J, Sprague JE, Timpson A, de las Fuentes L, Bernal-Mizrachi L, Schechtman KB, Bernal-Mizrachi C. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J Biol Chem. 2012;287(46):38482–38494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riek AE, Oh J, Bernal-Mizrachi C. 1,25(OH)2 vitamin D suppresses macrophage migration and reverses atherogenic cholesterol metabolism in type 2 diabetic patients. J Steroid Biochem Mol Biol. 2013;136:309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168(3):1181–1189. [DOI] [PubMed] [Google Scholar]
- 69.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195(5):603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panichi V, De Pietro S, Andreini B, Bianchi AM, Migliori M, Taccola D, Giovannini L, Tetta C, Palla R. Calcitriol modulates in vivo and in vitro cytokine production: a role for intracellular calcium. Kidney Int. 1998;54(5):1463–1469. [DOI] [PubMed] [Google Scholar]
- 71.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–4980. [DOI] [PubMed] [Google Scholar]
- 72.Werner C, Poss J, Bohm M. Optimal antagonism of the Renin-Angiotensin-aldosterone system: do we need dual or triple therapy? Drugs. 2010;70(10):1215–1230. [DOI] [PubMed] [Google Scholar]
- 73.Deb DK, Chen Y, Zhang Z, Zhang Y, Szeto FL, Wong KE, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-{kappa}B pathway. Am J Physiol Renal Physiol. 2009;296(5):F1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–825. [DOI] [PubMed] [Google Scholar]
- 75.Querfeld U, Hoffmann MM, Klaus G, Eifinger F, Ackerschott M, Michalk D, Kern PA. Antagonistic effects of vitamin D and parathyroid hormone on lipoprotein lipase in cultured adipocytes. J Am Soc Nephrol. 1999;10(10):2158–2164. [DOI] [PubMed] [Google Scholar]
- 76.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, Hope S, Kanis JA, McCloskey EV, Poole KES, Reid DM, Selby P, Thompson F, Thurston A, Vine N, National Osteoporosis Guideline G. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pilz S, Marz W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, Boehm BO, Dobnig H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93(10):3927–3935. [DOI] [PubMed] [Google Scholar]
- 78.Ng LL, Sandhu JK, Squire IB, Davies JE, Jones DJ. Vitamin D and prognosis in acute myocardial infarction. Int J Cardiol. 2013;168(3):2341–2346. [DOI] [PubMed] [Google Scholar]
- 79.Correia LC, Sodre F, Garcia G, Sabino M, Brito M, Kalil F, Barreto B, Lima JC, Noya-Rabelo MM. Relation of severe deficiency of vitamin D to cardiovascular mortality during acute coronary syndromes. The American journal of cardiology. 2013;111(3):324–327. [DOI] [PubMed] [Google Scholar]
- 80.Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29(4):636–645. [DOI] [PubMed] [Google Scholar]
- 81.Chen FH, Liu T, Xu L, Zhang L, Zhou XB. Association of Serum Vitamin D Level and Carotid Atherosclerosis: A Systematic Review and Meta-analysis. J Ultrasound Med. 2018;37(6):1293–1303. [DOI] [PubMed] [Google Scholar]
- 82.Aspelund T, Grubler MR, Smith AV, Gudmundsson EF, Keppel M, Cotch MF, Harris TB, Jorde R, Grimnes G, Joakimsen R, Schirmer H, Wilsgaard T, Mathiesen EB, Njolstad I, Lochen ML, Marz W, Kleber ME, Tomaschitz A, Grove-Laugesen D, Rejnmark L, Swart KMA, Brouwer IA, Lips P, van Schoor NM, Sempos CT, Durazo-Arvizu RA, Skrabakova Z, Dowling KG, Cashman KD, Kiely M, Pilz S, Gudnason V, Eiriksdottir G. Effect of Genetically Low 25-Hydroxyvitamin D on Mortality Risk: Mendelian Randomization Analysis in 3 Large European Cohorts. Nutrients. 2019;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brondum-Jacobsen P, Benn M, Afzal S, Nordestgaard BG. No evidence that genetically reduced 25-hydroxyvitamin D is associated with increased risk of ischaemic heart disease or myocardial infarction: a Mendelian randomization study. Int J Epidemiol. 2015;44(2):651–661. [DOI] [PubMed] [Google Scholar]
- 84.Trummer O, Pilz S, Hoffmann MM, Winkelmann BR, Boehm BO, Marz W, Pieber TR, Obermayer-Pietsch B, Renner W. Vitamin D and mortality: a Mendelian randomization study. Clin Chem. 2013;59(5):793–797. [DOI] [PubMed] [Google Scholar]
- 85.Liu Z, Liu L, Chen X, He W, Yu X. Associations study of vitamin D receptor gene polymorphisms with diabetic microvascular complications: a meta-analysis. Gene. 2014;546(1):6–10. [DOI] [PubMed] [Google Scholar]
- 86.Vimaleswaran KS, Cavadino A, Berry DJ, LifeLines Cohort Study i, Jorde R, Dieffenbach AK, Lu C, Alves AC, Heerspink HJ, Tikkanen E, Eriksson J, Wong A, Mangino M, Jablonski KA, Nolte IM, Houston DK, Ahluwalia TS, van der Most PJ, Pasko D, Zgaga L, Thiering E, Vitart V, Fraser RM, Huffman JE, de Boer RA, Schottker B, Saum KU, McCarthy MI, Dupuis J, Herzig KH, Sebert S, Pouta A, Laitinen J, Kleber ME, Navis G, Lorentzon M, Jameson K, Arden N, Cooper JA, Acharya J, Hardy R, Raitakari O, Ripatti S, Billings LK, Lahti J, Osmond C, Penninx BW, Rejnmark L, Lohman KK, Paternoster L, Stolk RP, Hernandez DG, Byberg L, Hagstrom E, Melhus H, Ingelsson E, Mellstrom D, Ljunggren O, Tzoulaki I, McLachlan S, Theodoratou E, Tiesler CM, Jula A, Navarro P, Wright AF, Polasek O, International Consortium for Blood P, Cohorts for H, Aging Research in Genomic Epidemiology c, Global Blood Pressure Genetics c, Caroline H, Wilson JF, Rudan I, Salomaa V, Heinrich J, Campbell H, Price JF, Karlsson M, Lind L, Michaelsson K, Bandinelli S, Frayling TM, Hartman CA, Sorensen TI, Kritchevsky SB, Langdahl BL, Eriksson JG, Florez JC, Spector TD, Lehtimaki T, Kuh D, Humphries SE, Cooper C, Ohlsson C, Marz W, de Borst MH, Kumari M, Kivimaki M, Wang TJ, Power C, Brenner H, Grimnes G, van der Harst P, Snieder H, Hingorani AD, Pilz S, Whittaker JC, Jarvelin MR, Hypponen E. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L, Chu A, Buring JE, Ridker PM, Chasman DI, Sesso HD. Common genetic variations in the vitamin D pathway in relation to blood pressure. Am J Hypertens. 2014;27(11):1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levin GP, Robinson-Cohen C, de Boer IH, Houston DK, Lohman K, Liu Y, Kritchevsky SB, Cauley JA, Tanaka T, Ferrucci L, Bandinelli S, Patel KV, Hagstrom E, Michaelsson K, Melhus H, Wang T, Wolf M, Psaty BM, Siscovick D, Kestenbaum B. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA. 2012;308(18):1898–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pilz S, Verheyen N, Grubler MR, Tomaschitz A, Marz W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. 2016;13(7):404–417. [DOI] [PubMed] [Google Scholar]
- 90.Jorde R, Figenschau Y, Hutchinson M, Emaus N, Grimnes G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur J Clin Nutr. 2010;64(12):1457–1464. [DOI] [PubMed] [Google Scholar]
- 91.Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res. 2011;50(4):303–312. [DOI] [PubMed] [Google Scholar]
- 92.Zittermann A, Gummert JF, Borgermann J. The role of vitamin D in dyslipidemia and cardiovascular disease. Curr Pharm Des. 2011;17(9):933–942. [DOI] [PubMed] [Google Scholar]
- 93.Shieh A, Chun RF, Ma C, Witzel S, Meyer B, Rafison B, Swinkels L, Huijs T, Pepkowitz S, Holmquist B, Hewison M, Adams JS. Effects of High-Dose Vitamin D2 Versus D3 on Total and Free 25-Hydroxyvitamin D and Markers of Calcium Balance. J Clin Endocrinol Metab. 2016;101(8):3070–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones G. Extrarenal vitamin D activation and interactions between vitamin D(2), vitamin D(3), and vitamin D analogs. Annu Rev Nutr. 2013;33:23–44. [DOI] [PubMed] [Google Scholar]
- 95.Mirhosseini N, Rainsbury J, Kimball SM. Vitamin D Supplementation, Serum 25(OH)D Concentrations and Cardiovascular Disease Risk Factors: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2018;5:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Margolis KL, Ray RM, Van Horn L, Manson JE, Allison MA, Black HR, Beresford SA, Connelly SA, Curb JD, Grimm RH Jr., Kotchen TA, Kuller LH, Wassertheil-Smoller S, Thomson CA, Torner JC, Women’s Health Initiative I. Effect of calcium and vitamin D supplementation on blood pressure: the Women’s Health Initiative Randomized Trial. Hypertension. 2008;52(5):847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M, Women’s Health Initiative I. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846–854. [DOI] [PubMed] [Google Scholar]
- 98.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, Erwin PJ, Hensrud DD, Murad MH, Montori VM. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(7):1931–1942. [DOI] [PubMed] [Google Scholar]
- 99.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–1551. [DOI] [PubMed] [Google Scholar]
- 100.Luo Q, Wang LL, Gao YH. Association between serum 25-hydroxyvitamin D and arterial stiffness in non-dialysis-dependent CKD. Eur J Clin Nutr. 2016;70(2):274–276. [DOI] [PubMed] [Google Scholar]
- 101.Levin A, Tang M, Perry T, Zalunardo N, Beaulieu M, Dubland JA, Zerr K, Djurdjev O. Randomized Controlled Trial for the Effect of Vitamin D Supplementation on Vascular Stiffness in CKD. Clin J Am Soc Nephrol. 2017;12(9):1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kumar V, Yadav AK, Lal A, Kumar V, Singhal M, Billot L, Gupta KL, Banerjee D, Jha V. A Randomized Trial of Vitamin D Supplementation on Vascular Function in CKD. J Am Soc Nephrol. 2017;28(10):3100–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zoccali C, Curatola G, Panuccio V, Tripepi R, Pizzini P, Versace M, Bolignano D, Cutrupi S, Politi R, Tripepi G, Ghiadoni L, Thadhani R, Mallamaci F. Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension. 2014;64(5):1005–1011. [DOI] [PubMed] [Google Scholar]
- 104.Kendrick J, Andrews E, You Z, Moreau K, Nowak KL, Farmer-Bailey H, Seals DR, Chonchol M. Cholecalciferol, Calcitriol, and Vascular Function in CKD: A Randomized, Double-Blind Trial. Clin J Am Soc Nephrol. 2017;12(9):1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chandler PD, Scott JB, Drake BF, Ng K, Manson JE, Rifai N, Chan AT, Bennett GG, Hollis BW, Giovannucci EL, Emmons KM, Fuchs CS. Impact of vitamin D supplementation on inflammatory markers in African Americans: results of a four-arm, randomized, placebo-controlled trial. Cancer Prev Res (Phila). 2014;7(2):218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salekzamani S, Mehralizadeh H, Ghezel A, Salekzamani Y, Jafarabadi MA, Bavil AS, Gargari BP. Effect of high-dose vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: a randomized controlled double-blind clinical trial. J Endocrinol Invest. 2016;39(11):1303–1313. [DOI] [PubMed] [Google Scholar]
- 107.Wang H, Xia N, Yang Y, Peng DQ. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2012;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, Murphy J, Khaw KT, Camargo CA Jr. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study : A Randomized Clinical Trial. JAMA Cardiol. 2017;2(6):608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE, Group VR. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med. 2018. [DOI] [PubMed] [Google Scholar]
- 110.Hollis BW, Wagner CL. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98(12):4619–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease HBPRCC, Epidemiology, Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–2169. [DOI] [PubMed] [Google Scholar]
- 112.USRDS. US Renal Data System (USRDS). Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage renal Disease in the United States. National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2013. [Google Scholar]
- 113.Chade AR, Lerman A, Lerman LO. Kidney in early atherosclerosis. Hypertension. 2005;45(6):1042–1049. [DOI] [PubMed] [Google Scholar]
- 114.Reiss AB, Miyawaki N, Moon J, Kasselman LJ, Voloshyna I, D’Avino R Jr., De Leon J. CKD, arterial calcification, atherosclerosis and bone health: Inter-relationships and controversies. Atherosclerosis. 2018;278:49–59. [DOI] [PubMed] [Google Scholar]
- 115.Oh YJ, Kim M, Lee H, Lee JP, Kim H, Kim S, Oh KH, Joo KW, Lim CS, Kim S, Kim YS, Kim DK. A threshold value of estimated glomerular filtration rate that predicts changes in serum 25-hydroxyvitamin D levels: 4th Korean National Health and Nutritional Examination Survey 2008. Nephrol Dial Transplant. 2012;27(6):2396–2403. [DOI] [PubMed] [Google Scholar]
- 116.Barragry JM, France MW, Carter ND, Auton JA, Beer M, Boucher BJ, Cohen RD. Vitamin-D metabolism in nephrotic syndrome. Lancet. 1977;2(8039):629–632. [DOI] [PubMed] [Google Scholar]
- 117.Michaud J, Naud J, Ouimet D, Demers C, Petit JL, Leblond FA, Bonnardeaux A, Gascon-Barre M, Pichette V. Reduced hepatic synthesis of calcidiol in uremia. J Am Soc Nephrol. 2010;21(9):1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ye JJ, Zhou TB, Zhang YF, Wang Q, Su YY, Tang JM, Li HY. Levels of vitamin D receptor and CYP24A1 in patients with end-stage renal disease. Afr Health Sci. 2016;16(2):462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krause R, Schober-Halstenberg HJ, Edenharter G, Haas K, Roth HJ, Frei U. Vitamin D status and mortality of German hemodialysis patients. Anticancer Res. 2012;32(1):391–395. [PubMed] [Google Scholar]
- 120.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA Jr., Tonelli M, Thadhani R. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004–1013. [DOI] [PubMed] [Google Scholar]
- 121.Shaffi K, Tighiouart H, Scott T, Lou K, Drew D, Weiner D, Sarnak M. Low 25-hydroxyvitamin D levels and cognitive impairment in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(6):979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jean G, Souberbielle JC, Chazot C. Monthly cholecalciferol administration in haemodialysis patients: a simple and efficient strategy for vitamin D supplementation. Nephrol Dial Transplant. 2009;24(12):3799–3805. [DOI] [PubMed] [Google Scholar]
- 123.Drechsler C, Verduijn M, Pilz S, Dekker FW, Krediet RT, Ritz E, Wanner C, Boeschoten EW, Brandenburg V, Group NS. Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant. 2011;26(3):1024–1032. [DOI] [PubMed] [Google Scholar]
- 124.Hiemstra TF, Casian A, Boraks P, Jayne DR, Schoenmakers I. Plasma exchange induces vitamin D deficiency. QJM. 2014;107(2):123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82(1):84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trachtman H, Gauthier B. Parenteral calcitriol for treatment of severe renal osteodystrophy in children with chronic renal insufficiency. J Pediatr. 1987;110(6):966–970. [DOI] [PubMed] [Google Scholar]
- 128.Wagner D, Trudel D, Van der Kwast T, Nonn L, Giangreco AA, Li D, Dias A, Cardoza M, Laszlo S, Hersey K, Klotz L, Finelli A, Fleshner N, Vieth R. Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and ki67 labeling in prostate cancer patients. J Clin Endocrinol Metab. 2013;98(4):1498–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA, Feldman D. Dietary vitamin D(3) and 1,25-dihydroxyvitamin D(3) (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology. 2012;153(6):2576–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010;5(5):905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lishmanov A, Dorairajan S, Pak Y, Chaudhary K, Chockalingam A. Treatment of 25-OH vitamin D deficiency in older men with chronic kidney disease stages 3 and 4 is associated with reduction in cardiovascular events. Am J Ther. 2013;20(5):480–486. [DOI] [PubMed] [Google Scholar]
- 132.Investigators JD, Shoji T, Inaba M, Fukagawa M, Ando R, Emoto M, Fujii H, Fujimori A, Fukui M, Hase H, Hashimoto T, Hirakata H, Honda H, Hosoya T, Ikari Y, Inaguma D, Inoue T, Isaka Y, Iseki K, Ishimura E, Itami N, Ito C, Kakuta T, Kawai T, Kawanishi H, Kobayashi S, Kumagai J, Maekawa K, Masakane I, Minakuchi J, Mitsuiki K, Mizuguchi T, Morimoto S, Murohara T, Nakatani T, Negi S, Nishi S, Nishikawa M, Ogawa T, Ohta K, Ohtake T, Okamura M, Okuno S, Shigematsu T, Sugimoto T, Suzuki M, Tahara H, Takemoto Y, Tanaka K, Tominaga Y, Tsubakihara Y, Tsujimoto Y, Tsuruya K, Ueda S, Watanabe Y, Yamagata K, Yamakawa T, Yano S, Yokoyama K, Yorioka N, Yoshiyama M, Nishizawa Y. Effect of Oral Alfacalcidol on Clinical Outcomes in Patients Without Secondary Hyperparathyroidism Receiving Maintenance Hemodialysis: The J-DAVID Randomized Clinical Trial. JAMA. 2018;320(22):2325–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. Jama. 2012;307(7):674–684. [DOI] [PubMed] [Google Scholar]
- 134.Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, Lo G, Lai KN, Lo WK, Lam CW, Yu CM. Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J Am Soc Nephrol. 2014;25(1):175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Przybylski R, McCune S, Hollis B, Simpson RU. Vitamin D deficiency in the spontaneously hypertensive heart failure [SHHF] prone rat. Nutr Metab Cardiovasc Dis. 2010;20(9):641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Choi JH, Ke Q, Bae S, Lee JY, Kim YJ, Kim UK, Arbeeny C, Thadhani R, Kang PM. Doxercalciferol, a pro-hormone of vitamin D, prevents the development of cardiac hypertrophy in rats. J Card Fail. 2011;17(12):1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meems LM, Cannon MV, Mahmud H, Voors AA, van Gilst WH, Sillje HH, Ruifrok WP, de Boer RA. The vitamin D receptor activator paricalcitol prevents fibrosis and diastolic dysfunction in a murine model of pressure overload. J Steroid Biochem Mol Biol. 2012;132(3–5):282–289. [DOI] [PubMed] [Google Scholar]
- 138.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, Espe K, Dekker F, Brandenburg V, Marz W, Ritz E, Wanner C. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31(18):2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim HW, Park CW, Shin YS, Kim YS, Shin SJ, Kim YS, Choi EJ, Chang YS, Bang BK. Calcitriol regresses cardiac hypertrophy and QT dispersion in secondary hyperparathyroidism on hemodialysis. Nephron Clin Pract. 2006;102(1):c21–29. [DOI] [PubMed] [Google Scholar]
- 141.Marcinowska-Suchowierska E, Kupisz-Urbanska M, Lukaszkiewicz J, Pludowski P, Jones G. Vitamin D Toxicity-A Clinical Perspective. Front Endocrinol (Lausanne). 2018;9:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Palaniswamy S, Hypponen E, Williams DM, Jokelainen J, Lowry E, Keinanen-Kiukaanniemi S, Herzig KH, Jarvelin MR, Sebert S. Potential determinants of vitamin D in Finnish adults: a cross-sectional study from the Northern Finland birth cohort 1966. BMJ Open. 2017;7(3):e013161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neale RE, Armstrong BK, Baxter C, Duarte Romero B, Ebeling P, English DR, Kimlin MG, McLeod DS, RL OC, van der Pols JC, Venn AJ, Webb PM, Whiteman DC, Wockner L. The D-Health Trial: A randomized trial of vitamin D for prevention of mortality and cancer. Contemp Clin Trials. 2016;48:83–90. [DOI] [PubMed] [Google Scholar]
- 144.Ponda MP, Dowd K, Finkielstein D, Holt PR, Breslow JL. The short-term effects of vitamin D repletion on cholesterol: a randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2012;32(10):2510–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Manson JE, Allison MA, Carr JJ, Langer RD, Cochrane BB, Hendrix SL, Hsia J, Hunt JR, Lewis CE, Margolis KL, Robinson JG, Rodabough RJ, Thomas AM, Women’s Health I, Women’s Health Initiative-Coronary Artery Calcium Study I. Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative. Menopause. 2010;17(4):683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–759. [DOI] [PubMed] [Google Scholar]
- 147.Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koerfer R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89(5):1321–1327. [DOI] [PubMed] [Google Scholar]
- 148.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469. [DOI] [PMC free article] [PubMed] [Google Scholar]


