Abstract
Parasitic infections are a major source of human suffering, mortality, and economic loss, but drug development for these diseases has been stymied by the significant expense involved in bringing a drug though clinical trials and to market. Identification of single compounds active against multiple parasitic pathogens could improve the economic incentives for drug development as well as simplifying treatment regimens. We recently performed a screen of repurposed compounds against the protozoan parasite Entamoeba histolytica, causative agent of amebic dysentery, and identified four compounds (anisomycin, prodigiosin, obatoclax and nithiamide) with low micromolar potency and drug-like properties. Here, we extend our investigation of these drugs. We assayed the speed of killing of E. histolytica trophozoites and found that all four have more rapid action than the current drug of choice, metronidazole. We further established a multi-institute collaboration to determine whether these compounds may have efficacy against other parasites and opportunistic pathogens. We found that anisomycin, prodigiosin and obatoclax all have broad-spectrum antiparasitic activity in vitro, including activity against schistosomes, T. brucei, and apicomplexan parasites. In several cases, the drugs were found to have significant improvements over existing drugs. For instance, both obatoclax and prodigiosin were more efficacious at inhibiting the juvenile form of Schistosoma than the current standard of care, praziquantel. Additionally, low micromolar potencies were observed against pathogenic free-living amebae (Naegleria fowleri, Balamuthia mandrillaris and Acanthamoeba castellanii), which cause CNS infection and for which there are currently no reliable treatments. These results, combined with the previous human use of three of these drugs (obatoclax, anisomycin and nithiamide), support the idea that these compounds could serve as the basis for the development of broad-spectrum anti-parasitic drugs.
Author summary
Parasitic diseases are a major cause of human morbidity and mortality worldwide, as well as a significant economic drain in developing countries. Many parasites have limited treatment options with low efficacy and significant side effects, however research into new therapeutics suffers from a lack of investment. In this study, we characterize four potential anti-parasitic drugs: anisomycin, nithiamide, prodigiosin and obatoclax. These drugs were previously shown to effectively inhibit Entamoeba histolytica, the parasite that causes amebic dysentery. Here, we demonstrate that these drugs have activity against a wide variety of parasites from different taxonomic groups. Additionally, we assessed the speed of killing of these compounds against E. histolytica and the brain pathogen Balamuthia mandrillaris, and show that several are faster acting than current drugs. Two of these drugs (prodigiosin and obatoclax) had broad-spectrum activity, including against life stages not treated by current drugs such as juvenile schistosome worms, and three (obatoclax, nithiamide and anisomycin) have been used previously in humans. Although more study will be needed to adapt these drugs to the varying requirements for treatment of each parasitic disease, this work is a promising beginning towards identifying drugs against multiple parasites that are human pathogens.
Introduction
Parasitic diseases cause a significant public health burden, especially in the developing world. A 2013 survey of the causes of mortality worldwide estimated ~1 million deaths due to parasitic diseases, with parasitic protists such as Plasmodium being the most common [1]. In addition to this loss of life, significant morbidities such as cognitive impairment and growth stunting often result from parasitic infections [2]. Despite this large impact on human health, drug discovery efforts to develop new treatments for parasitic diseases have significant underinvestment. Given the high cost of bringing a drug to market [3], economic considerations are challenging for developing therapies for diseases mostly prevalent in low resource environments. At the same time, biological barriers also exist which hinder successful drug development. These include the tendency of many parasites to become resistant to treatment as well as drug toxicity, which can be severe with drugs for eukaryotic pathogens due to conservation between parasite and host pathways [4]. In addition, many parasites have multiple life stages, which may have differing drug susceptibilities. Due to these issues, the idea of repurposing drugs originally developed for other diseases to treat parasitic infections has been growing in popularity. This approach can significantly lower the cost of bringing drugs to market by reducing the need for extensive pre-clinical testing and clinical trials [5].
We recently performed a screen of repurposing libraries, totaling ~4000 compounds, to identify compounds targeting the protozoan parasite Entamoeba histolytica [6]. From this work we identified four compounds: nithiamide (a nitroimidazole agent), anisomycin (an antibiotic isolated from Streptomyces), prodigiosin (a natural pigment isolated from a bacterium), and obatoclax (a synthetic analog of prodigiosin thought to inhibit BCL-2). Three of these, prodigiosin, obatoclax and anisomycin, had activity against both the trophozoite and cyst stages of E. histolytica and, importantly, were also active against metronidazole-resistant parasites. Of these compounds, anisomycin, nithiamide and obatoclax all have been used in humans, either in clinical trials or historically [7–9]. This finding validates our approach of screening with repurposed drug libraries, as drugs that have previously been used safely in humans should have a faster and cheaper regulatory route.
We next asked whether these four compounds are active against a broad-range of parasitic pathogens including (i) another anaerobic enteric parasite (Giardia lamblia), (ii) free-living amebae (Naegleria fowleri, Acanthamoeba castellanii, and Balamuthia mandrillaris), (iii) apicomplexan parasites (Plasmodium falciparum and Cryptosporidium parvum), (iv) trypanosomatids (Trypanosoma brucei), and (v) multi-cellular worm (Schistosoma mansoni). Giardia lamblia is an anaerobic protozoan parasite that inhabits the small intestine of humans and other animals. It is the most common intestinal parasite [10], causing severe diarrhea in over 100 million people per year [11]. The free-living amebae Naegleria fowleri, Acanthamoeba castellanii and Balamuthia mandrillaris are opportunistic pathogens that can cause rare but dangerous infections of the central nervous system (CNS); Acanthamoeba can cause ocular disease and both Balamuthia and Acanthamoeba can cause systemic disease. Current drug regimens for CNS infections with these amebae are sorely inadequate, and even with treatment fatality rates are >90% [12]. Parasites of the phylum Apicomplexa are responsible for many of the most common parasitic diseases, including malaria. P. falciparum is responsible for ~80% of malaria cases worldwide [13], causing severe fever due to the lysis of infected red blood cells, and leading to an estimated 620,000 deaths per year [1]. Cryptosporidium parvum has been recognized as a serious cause of childhood diarrhea in the developing world [14]. Human African Trypanosomiasis (HAT), which is caused by two subspecies of T. brucei, is lethal in humans if not treated appropriately. There is no vaccine and treatment relies on a small number of mostly old drugs that must be administered parenterally, have a range of often serious side-effects and for which resistance has been a problem [15–17]. Schistosomiasis, caused by trematode worms of the genus Schistosoma, affects an estimated 250 million people in at least 76 countries worldwide [18]. Symptoms include fever and bloody diarrhea, as well as hepatic and renal pathology, caused by the large numbers of eggs laid by female worms [18].
We now further characterize anisomycin, prodigiosin, obatoclax, and nithiamide for activity against a broad range of parasites and show that all compounds have activity against at least one other parasite group. Prodigiosin and its related compound obatoclax had the broadest-spectrum activity, showing the ability to kill at least one parasite from each of the groups listed above. Importantly, both prodigiosin and obatoclax effectively kill juvenile schistosome worms with high efficacy. We performed a preliminary investigation of in vivo activity using a mouse model of Cryptosporidium infection but found no significant improvement in parasite burden. Despite this negative result, important information about the tolerability and dosage of oral prodigiosin was gained. Anisomycin was also a promising lead, due to its activity against Naegleria, apicomplexan parasites, and T. brucei, along with its previous history of established human use. This study provides exciting new leads for drug development efforts for single compounds to target multiple parasites that cause serious human disease.
Results
E. histolytica speed of killing
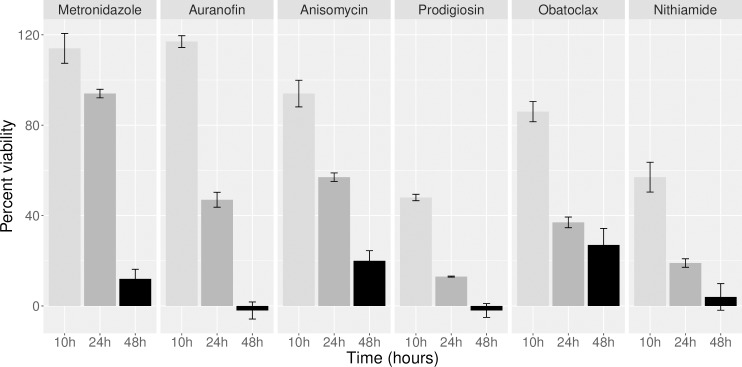
Rapid action of antiparasitic drugs is important for improving efficacy and reducing treatment duration. Additionally, better understanding of the kinetics of activity can offer insight into the mechanism of action of lead compounds [19]. In order to determine speed of killing of E. histolytica trophozoites, we performed a time course experiment in which parasite growth was assayed at 10, 24 and 48h post treatment. The four compounds were assayed at 2 times the previously established EC50 [6] and compared to metronidazole and auranofin [20], also at 2x the EC50. Fluorescence after incubation with FDA was compared to parasites treated with 0.6% DMSO from the same time point and three independent biological replicates were performed. All four compounds inhibited trophozoite growth more rapidly than metronidazole (Fig 1). Prodigiosin and nithiamide were the fastest acting, with ~50% inhibition by as early as 10h post treatment. The result with nithiamide was especially notable, considering that it is chemically similar to metronidazole and they are presumed to have the same mechanism of action. Anisomycin and obatoclax were slower acting, but both had strong activity by 24h, compared to metronidazole which did not have significant inhibition until 48h of treatment. It is important to note that control parasites did not exhibit significant growth over this short incubation period (compared to parasite number at time zero), indicating that there was likely an actual reduction in parasite viability, and not simply an inhibition of growth.
Fig 1. Speed of killing for Entamoeba histolytica.
Results from experiment to assess kinetics of drug action against E. histolytica trophozoites. Drugs were assayed at the following concentrations (2x the previously measured EC50 [6]): metronidazole, 17μM; auranofin 0.5μM; anisomycin, 1.4μM; prodigiosin, 1.4μM; obatoclax, 1μM; nithiamide, 10μM. Graph shows FDA signal as a percent of DMSO control. Killing was assayed at 10, 24 and 48 hours, and three biological replicates were performed for each data point.
Parasite drug susceptibility testing
In order to determine if the compounds with activity against Entamoeba are also active against other parasitic diseases, we tested their activity against (i) other anaerobic enteric parasites (Giardia lamblia), (ii) free-living amebae (Naegleria fowleri, Acanthamoeba castellanii, and Balamuthia mandrillaris), (iii) apicomplexan parasites (Plasmodium falciparum and Cryptosporidium parvum), (iv) trypanosomatid (Trypanosoma brucei), and (v) multi-cellular worm (Schistosoma mansoni). The EC50s for all drugs and parasites tested are shown in Table 1. A survey of publicly available toxicity and pharmicokinetic data is shown in Table 2.
Table 1. Potency of candidate compounds in various parasite systems.
| Parasite | Anisomycin | Prodigiosin | Obatoclax | Nithiamide | Control |
|---|---|---|---|---|---|
| Entamoeba | 0.7 | 0.7 | 0.5 | 5 | Metronidazole (8.9μM) |
| Giardia | 18.5 | 3.8 | 0.9 | 2.6 | Metronidazole (6.4μM) |
| Naegleria | 4.7 | 6.4 | 3.6 | no activity | Amphotericin (0.2μM) |
| Acanthamoeba | no activity | 2.2 | 0.5 | no activity | PHMB (9.8μM) |
| Balamuthia (trophozoites) | no activity | 4 | 1.5 | no activity | Nitroxoline (2.8μM) |
| Balamuthia (cysts) | no activity | 3.8 | 1.7 | no activity | Nitroxoline (15.5 μM) |
| Plasmodium | 0.1 | nt* | nt* | no activity | Atovaquone (23nM) |
| Cryptosporidium | 0.08 | 0.09 | 2.1 | no activity | Nitazoxanide (2.07μM) |
| Schistosoma (juvenile) | no activity | 1 | 0.6 | no activity | Praziquantel (4.7μM) |
| T. brucei | 0.10 | 0.03 | 0.04 | 3.6 | Pentamidine (0.11μM) |
The EC50 values for anisomycin, prodigiosin, obatoclax and nithiamide for each parasite tested are shown. All concentrations are micromolar. Results for control compounds are from the same experiment except as indicated: metronidazole (Entamoeba) [6], nitroxoline (Balamuthia) [21], Atovaquone (Plasmodium) [22]; praziquantel (juvenile Schistosoma) [23]. * indicates that compound was not tested as it was incompatible with the assay.
Table 2. Pharmacokinetic and cytotoxicity properties.
| Drug | Anisomycin | Prodigiosin | Obatoclax | Nithiamide |
|---|---|---|---|---|
| in vivo data | ||||
| Animal | rat | mouse | mouse | mouse |
| Toxicity | LD50: 72mg/kg (oral) 1 | LD50: 18mg/kg (ip) 2 | >5mg/kg (IV) 3 | LD50: 300mg/kg (ip) 4 |
| Cmax in μM | 0.01 (150mg/kg subQ) 5 | na | 0.24 (4.8mg/kg IV) 6 | na |
| Cytotox (CC50) | ||||
| HepG2 | 0.39μM | 0.21μM | 0.21μM | no activity |
| HEK293 | 0.08μM | 0.13μM | 0.8μM | no activity |
| MDCK | na | no activity 7 | 1.4μM 8 | na |
| PBMCs | na | 241μM 9 | >4μM 3 | na |
| Vero | 45μM 10 | na | 6.6μM 11 | na |
| CHO | na | na | na | no activity 12 |
| Huh-7 | 2.4μM 13 | na | 0.38μM 14 | na |
| A549 | no activity 15 | na | 9.9μM 16 | na |
| hFF-1 | >25μM | 0.14μM | 0.11μM | no activity |
LD50 and Cmax for published animal studies, and a selection of publicly available cytotoxicity data for each compound are shown. Route of administration and dosage in parenthesis. 'na' indicates that no data was available. Results for HepG2 and HEK293 cytotoxicity from Calibr (reframedb.org). Cytotoxicity against human foreskin fibroblast cells (hFF-1) was determined as mentioned in methods. Other data are as follows: 1. [24]; 2. [25]; 3. [26] Truedel 2007; 4. [27]; 5. [28]; 6. [29]; 7. [30]; 8. [31]; 9. [32]; 10. [33]; 11. [34]; 12. [35]; 13. [36] (pubchem AID:449705); 14. [37] (pubchem AID:742238); 15. [38] (pubchem AID:720523); 16. [39]
Giardia lamblia
Current treatment of Giardia is based on nitroimidazole agents (tinidazole and metronidazole) and nitazoxanide, a thiazolide-family compound. However, treatment failure is noted in up to 20% of isolates and re-treatment or combination therapies are needed [40]. In our testing, we found that Giardia was susceptible to nithiamide, as previously reported [35], with an EC50 of 2.6μM. This compound belongs to the nitroimidazole class, similar to metronidazole, although it was slightly more efficacious (metronidazole EC50 = 6.4μM). Additionally, we found that both prodigiosin and obatoclax inhibited Giardia with good potency (EC50s 3.8 and 0.9μM respectively) (Table 1). As these compounds are chemically unrelated to metronidazole, and were effective against metronidazole resistant Entamoeba [6], they may make good candidates for treatment of resistant strains of Giardia [41]. Some anti-Giardia activity was also seen with anisomycin, but efficacy was poor (EC50 of 18.5μM) compared to the other compounds tested and to current treatments.
Free-living amebae Naegleria, Acanthamoeba and Balamuthia
Current treatment of CNS disease caused by the free-living ameba is based on a multi drug regimen often including antibiotics such as azithromycin and pentamidine as well as amphotericin B and miltefosine. However, these protocols have poor efficacy and mortality rates remain very high [42]. We found that all three amebae were inhibited by prodigiosin and obatoclax, with EC50s in the low micromolar range (Table 1). Additionally, anisomycin was active against Naegleria. These results compare favorably to current treatments; for instance, miltefosine killing of Naegleria has an EC50 of ~48μM [43]. However, it is important to note that in vitro EC50s do not necessarily translate to in vivo success. For instance, amphotericin has sub-micromolar EC50 in vitro but is not effective in patients. An additional aspect in the treatment of these parasites is that they can cause infection in multiple different tissues (CNS, eye, skin), requiring distinct properties such as CNS penetrance. Alternate formulations (such as for ocular topical application) of medicinal chemistry efforts to enhance CNS availability may be important for developing these compounds into useful drugs. Significantly, both compounds also were active against Balamuthia cysts, which are typically less sensitive to many drugs than are Balamuthia trophozoites [21].
Balamuthia phenotypic assays
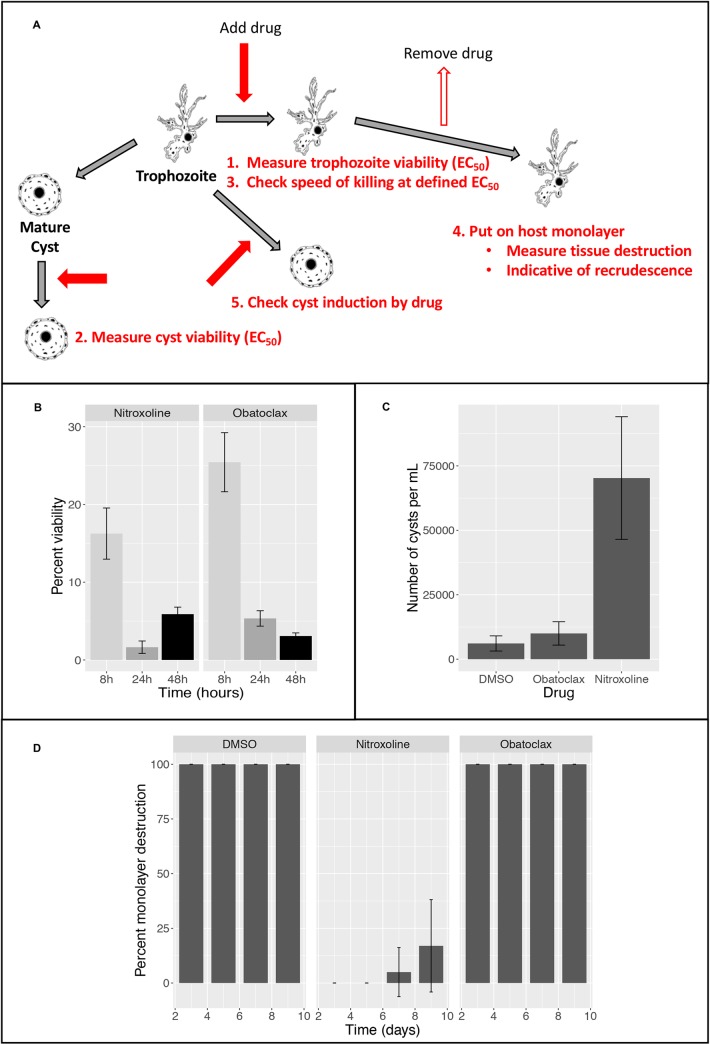
Given the potency of obatoclax against both life stages of Balamuthia, as well as evidence that it can penetrate the CNS [29], we wanted to further characterize the activity of this drug with a variety of phenotypic assays (Fig 2A). To determine the kinetics of the killing of Balamuthia trophozoites, we performed a similar assay to that used for Entamoeba. The compound nitroxoline, which has previously been shown to have rapid in vitro activity [21] was used as a control. Both compounds showed significant reduction of signal compared to the DMSO control by 8 hours and had >90% inhibition by 24 hours after treatment (Fig 2B), indicating rapid action that could be critical in the treatment of this fatal disease.
Fig 2. Phenotypic assays for Balamuthia mandrillaris.
(A) Assays performed: Schematic representing the workflow for each of the assays performed for Balamuthia. Red arrows represent addition of drug and outline arrows represent drug removal. (B) Speed of killing: Results from experiment to asses kinetics of drug action against Balamuthia trophozoites. Drugs were assayed at 2x the EC50 as previously assessed [21, 44]: nitroxoline at 15.6μM and obatoclax at 3.2μM. Graph shows FDA signal as a percent of 0.5% DMSO control. Killing was assayed at 8, 24 and 48 hours, and three biological replicates were performed for each data point. (C) Cyst induction: Trophozoites were treated for 72h with obatoclax (3.2μM), nitroxoline (15.6μM) or DMSO (0.5%), in 24-well plates, then the numbers of trophozoites and cysts were counted. Nitroxoline induces substantial cyst formation, as previously observed [21], but obatoclax is similar to control. (D) Parasite recrudescence: Trophozoites were treated for 72h with obatoclax (3.2μM), nitroxoline (15.6μM) or DMSO (0.5%), in flasks, then drug was removed, and Balamuthia were seeded on a monolayer of mammalian cells. The monolayer was rapidly destroyed by trophozoites after obatoclax treatment, indicating that this concentration and/or duration of treatment was insufficient to prevent recrudescence.
It has previously been found that some drugs can induce encystation in B. mandrillaris [21]. This property could slow disease progression, as cysts are non-proliferative, though it could also make clearance of the infection more difficult. To determine whether obatoclax has this property, we treated Balamuthia trophozoites with obatoclax and nitroxoline, both at 2x the EC50 concentration for 72h. We determined that, in contrast to nitroxoline, obatoclax did not significantly enhance encystation, compared to the DMSO control (Fig 2C).
Finally, we explored the potential of obatoclax to delay Balamuthia recrudescence after treatment. For this assay, trophozoites were treated with drug at 2x the EC50 for 72h, then washed and seeded on a monolayer of hFF cells in 24-well plates. Cells were monitored at 3, 5, 7 and 9 days, and the percent of intact monolayer was recorded. Since untreated or DMSO treated Balamuthia trophozoites quickly destroy host cells, this assay gives a clear measure of viability after drug removal. We found that the obatoclax treated trophozoites were able to completely destroy the hFF monolayer in 3 days, showing that 72h treatment was insufficient to prevent recrudescence (Fig 2D). This finding may indicate that more extended treatment with obatoclax would be required in a clinical setting.
Schistosoma mansoni
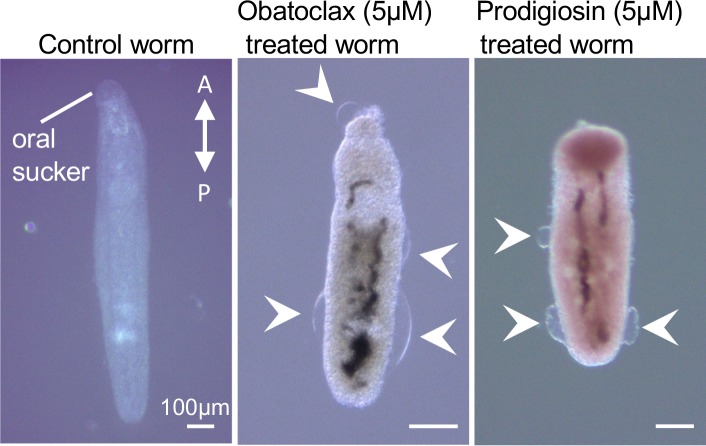
The current drug of choice, praziquantel, is cheap and effective against adult schistosomes, but has reduced activity in killing juveniles [45]. Additionally, resistance to the drug has been seen both in vitro and in the clinic [46]. The recovery of juvenile worms after praziquantel treatment may contribute to drug resistance in human populations; for these reasons, attempts to identify drugs that are effective at all life stages is a vital area of research [46, 47]. We tested our four lead compounds in a viability assay against juvenile forms of Schistosoma mansoni. Of the compounds we tested, only prodigiosin and obatoclax were effective, with EC50 values of 1μM and 0.6μM respectively (Table 1). To determine whether the worms could recover from the drugs, we transferred the worms after 72h 5μM drug treatment to fresh media and monitored activity. For both prodigiosin and obatoclax, worm motility was not restored even after 72 hours recovery, contrasting significantly with results seen after praziquantel treatment where juvenile worms were found to have significantly better recovery after drug treatment than adult worms [48, 49]. Imaging of prodigiosin and obatoclax treated worms revealed that both drugs resulted in significant damage to the tegument, with numerous areas of blebbing and separation from the worm body (Fig 3), a phenotype previously seen in with other anti-schistosomal drugs, and associated with increased ion flow across the tegument, exposure to host immune system, and eventual loss of viability [50, 51].
Fig 3. S. mansoni phenotypes after treatment with obatoclax and prodigiosin.
Imaging of juvenile Schistosoma after 72h treatment with DMSO control (panel 1) 5μM obatoclax (panel 2) or 5μM prodigiosin (panel 3). Note changes in gross morphology as well as tegument blebbing (arrows). Position of oral sucker and anterior-posterior axis are indicated.
Trypanosoma brucei
Treatment for Human African Trypanosomiasis, caused by the parasite T. brucei, varies based on subspecies and disease stage but includes intravenous dosing of drugs such as melarsoprol which can have significant side effects [16]. Fexinidazole has been recently approved for treatment of gambiense HAT [52] and clinical trials are ongoing for its utility against rhodesiense HAT. All four compounds were active against T. brucei with EC50 values ranging from 3.6 μM (nithiamide) to 0.03μM (prodigiosin) (Table 1). Except for nithiamide, all were more potent than the control compound, pentamidine, in this assay.
Apicomplexan parasites
The first line treatment for uncomplicated P. falciparum malaria involves treating malaria with artemisinin-based combination therapy [13], involving combining treatments such as such as artemether + lumefantrine or artesunate + amodiaquine. These treatments can be highly effective in most cases; however drug resistance is a major problem, already affecting even advanced treatments [53]. Hence, new drugs for this parasite are constantly needed. Due to the fluorescence-based nature of the assay used we were unable determine efficacy of either prodigiosin or obatoclax, both of which had interfering fluorescence. However, there are previously published reports of anti-malarial activity with this compound class [54]. We did find potent activity with anisomycin (EC50 0.1μM, Table 1). This is consistent with previously published results [55], indicating a potential for this drug to be added to the anti-malarial arsenal. Nithiamide had no activity in this parasite.
C. parvum is an intestinal parasite that causes diarrheal symptoms and can result in malnutrition and impaired growth in children [56]. The most important current treatment is nitazoxanide [57], which can reduce diarrhea symptoms in adults but has reduced efficacy in children and immunocompromised patients. Of our compounds, anisomycin, prodigiosin and obatoclax all had potent activity (EC50s: 0.08μM, 0.09μM and 2.1μM respectively) (Table 1). The efficacy of anisomycin against both apicomplexan parasites was intriguing, opening the possibility that it could have wider use against other members of this group such as Toxoplasma and Cyclospora. Although we could not assay prodigiosin or obatoclax killing of Plasmodium, their strong inhibition of C. parvum as well as many of the other parasites tested point to it as a potential lead compound for the development of a broad-spectrum anti-parasitic agent.
Cryptosporidium in vivo model for testing prodigiosin efficacy
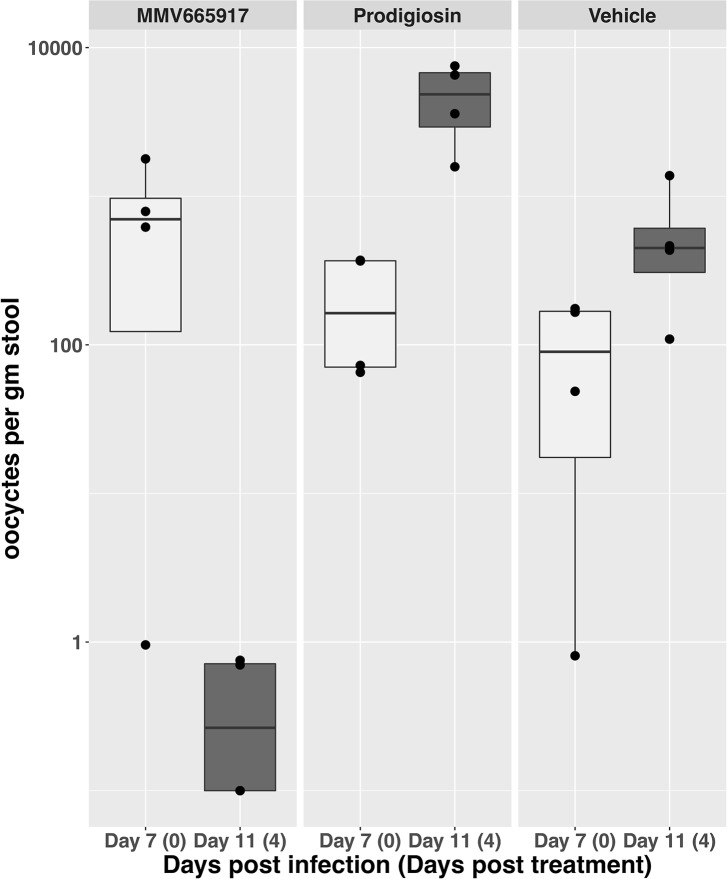
Based on these promising results, we decided to test prodigiosin in an in vivo mouse model of Cryptosporidium infection. NOD SCID gamma mice were infected with C. parvum oocysts and the infection was allowed to progress for seven days. On day seven, mice were treated orally with either vehicle (5% DMSO in 1% HPMC), prodigiosin (25mg/kg), or MMV665917 (60mg/kg), a recently discovered lead compound with in vivo activity [58], as a positive control. Both compounds were dosed twice daily for four days, and oocyst shedding was monitored daily. Comparison of the number of oocysts shed on day 7 (before start of treatment) and day 11 showed that while MMV665917 significantly reduced the number of oocysts, oocyst shedding increased during this time period for mice treated with either prodigiosin or the vehicle control (Fig 4). In addition, some weight loss was observed in prodigiosin mice, although this effect reversed upon cessation of treatment. Reasons for this lack of in vivo efficacy despite in vitro potency include potential pharmacokinetic issues or insufficient dosing. Unfortunately, considering the side effects noted with prodigiosin at the dose used, increasing dosage is likely to be unsuccessful. Obatoclax, which has a record of safe human use [7] and also had in vitro activity against C. parvum (Table 1) is a potential candidate for further in vivo studies.
Fig 4. Effect of prodigiosin on C. parvum infection in an in vivo mouse model.
Mice infected with C. parvum oocysts were treated beginning on day 7 post infection with Vehicle (DMSO), positive control (MMV665917 at 60 mg/kg twice daily) or prodigiosin at 25mg/kg twice daily. Number of oocysts in the stool were counted at day 7 (day one of treatment) and day 11 (day four of treatment). Four mice per condition were used. Results for each individual animal are shown by a black dot (•).
Potential for BCL2-like inhibitors targeting parasitic pathogens
Obatoclax, which is active against all parasites assayed in our study, is a potent inhibitor of the apoptosis regulator BCL-2 [59]. However, except for Schistosoma all the organisms are single-celled protists which do not have apparent BCL-2 homologs. In order to gain a better understanding of the mechanism of action of obatoclax, we obtained a number of BCL-2 inhibitors (Venetoclax, Navitoclax, A-1331852, A-1210477 and S63845) and tested them in our assays against a subset of the parasites. Surprisingly, no effect was seen on viability of juvenile schistosome worms, at concentrations up to 20μM (S1 Table), indicating that obatoclax may have another mechanism of action in schistosomes. This may be due to sequence divergence, as the S. mansoni BCL-2 (XP_018654288.1) is only ~20% identical and ~60% similar to the human protein based on clustal omega alignment. Of the protists, some weak activity was seen against Naegleria with A-1331852 (EC50 ~30μM). Intriguingly, Navitoclax, A-1331852, A-1210477 and S63845 all had activities against Cryptosporidium in the 3–20μM range. No effect was seen on any of the other protists tested (E. histolytica, B. mandrillaris, T. brucei). A blastP search of the C. parvum genome indicates that the protein with the closest homology to human BCL-2 is an uncharacterized protein cgd4_3520 (e-value 0.026). Given the absence of an obvious Cryptosporidium BCL-2 homologue, obatoclax likely effects C. parvum growth either by inhibiting host cell BCL-2 or via a non-BCL-2 dependent mechanism. Overall, these results indicate that obatoclax most likely kills the parasites tested though a non-BCL-2 dependent mechanism.
Discussion
Development of novel therapeutics against parasitic pathogens would benefit from identification of drugs that can work against multiple organisms as a means to reduce drug development and regulatory costs. We previously identified four compounds with potent activity against the protozoan parasite E. histolytica, and we now expand our exploration of these compounds looking at their potential as broad spectrum anti-parasitic compounds. We found that two of the compounds, prodigiosin and obatoclax, have broad spectrum anti-parasitic activity, including against T. brucei and the juvenile form of Schistosoma mansoni [47]. Of these, obatoclax is particularly interesting, due to its lower cost and safe record of human use. Both compounds have demonstrated ability to kill tumor-derived cell lines, but lower toxicity towards non-malignant cells such as PBMCs and MDCK cells (Table 2 and [30]). Anisomycin had efficacy in fewer systems, but its good potency against both apicomplexan parasites tested, and history of safe human use [8, 60] make it a potential candidate for further development.
In considering one drug for multiple pathogens, we have to also consider the Target Product Profile (TPP) for each disease. Entamoeba, Giardia, and Cryptosporidium all cause gastrointestinal disease. For these parasites, oral formulations that can kill the parasites in the intestinal lumen are ideal. In addition, treatments that are cheap and have a good safety profile are necessary, as they would likely be used widely in resource-poor endemic regions. Anisomycin has good efficacy against Entamoeba and Cryptosporidium, and significant animal toxicity and pharmacokinetic data including oral and IV treatment in rodents (Table 2 and [24, 28]). Chronic treatment in female macaques was tolerated in doses up to 64mg/kg daily, for 30 weeks [24]. These data, along with its past use as an oral antibiotic [8, 60], supports the idea that anisomycin could, with further in vivo testing and clinical trials, become a useful addition to current treatment options. Prodigiosin was able to inhibit all three parasites in vitro. However, the toxicities noted during in vivo treatment may indicate potential difficulties in developing a safe drug for human use. Obatoclax had similar EC50s and has a more favorable safety profile, making it a better candidate for further in vivo testing and development. In clinical trials for leukemia, IV treatment of obatoclax resulted in mostly transient neurological side effects (euphoria, dizziness, somnolence) [7], with a maximum tolerated dose of 28mg/m2 [61]. However, it would need to be tested in oral formulation for use against these parasites. It is possible that the failure of prodigiosin in our in vivo Cryptosporidium trial was in part due to pharmacokinetic issues leading to low drug concentrations in the colon. Development of obatoclax or any drug for this use would have to take these properties into account; absorption rate in the small intestine and interactions with the colonic epithelium would have to be monitored in any new formulation.
Like the GI parasites, an effective treatment for schistosomes ideally should be cheap and have few side effects. However, ability of a compound to achieve significant plasma concentrations is important, as worms are found in the bloodstream. The current standard of care, praziquantel, is inexpensive and safe enough to be given prophylactically as a single oral dose in at-risk areas [62]. However, for treatment of infected individuals, the ineffectiveness against juvenile stages may require repeated dosing to achieve a cure. Thus, a compound with activity against the juvenile stages would be of interest. Obatoclax, which had potent activity against juvenile worms, could be used either as an IV treatment for severe cases or reformulated into an oral medication. Malaria has a similar TPP to schistosomiasis, requiring a safe, low cost drug capable of achieving high levels in the blood stream. From our candidate drugs, anisomycin had good potency, and a promising pharmacokinetic profile [28].
In contrast to these parasites, the free-living amebae, Naegleria, Balamuthia and Acanthamoeba, rarely cause disease in humans, but when they infect the CNS are almost universally fatal with no good current therapies. Two different TPPs would need to be considered: one for CNS disease (with any of the three amebae) where patients are urgently ill and a second for skin or systemic symptoms (with Acanthamoeba or Balamuthia) where patients are chronically ill. Additionally, Acanthamoeba causes keratitis, a serious eye infection found most prevalently in contact lens wearers. Amebic keratitis is treatable, but current regiments are prolonged and have significant side-effects, largely due to the need to eliminate the resistant cyst form of Acanthamoeba [63]. To determine if prodigiosin or obatoclax could be a useful treatment for keratitis would require testing for effective killing of cysts, as well as re-formulation for ocular use. It should be noted that although activity against Acanthamoeba cysts was not tested, we did see efficacy of obatoclax against cysts of Balamuthia, a related parasite [64]. For the CNS diseases, since patients are generally hospitalized throughout treatment, higher drug toxicity levels and costs may be acceptable, and IV dosing would be preferred. A key factor in effectiveness for a drug for this indication is penetration of the blood-brain barrier, as all three parasites infect the CNS. Obatoclax has previously been shown to have high CNS levels [29], and has been in phase II clinical trials in IV formulation. If sufficient CNS levels are not reached, intrathecal delivery, which has proven effective for some antibiotics [65] could also be considered. It should be noted that due to the extreme rarity of CNS and systemic diseases caused by the free-living ameba, clinical trials would not be feasible. Demonstration of efficacy in animal models and an established record of safety in previous human trials can lead to approval for clinical use in these cases. For ocular disease, multiple animal models exist, which could be used to test safety and efficacy [66].
Current drug treatment for Trypanosoma brucei is complicated, often requiring repeated parenteral administration of drugs or drug combinations under clinical supervision to treat either Stage 1 (hemolymphatic) or Stage 2 (CNS-infiltrated) disease. Fortunately, one new drug, fexinidazole, was approved in 2018 as the first all-oral treatment of both stage 1 and 2 West African (gambiense) trypanosomiasis, and clinical trials are ongoing regarding its potential to treat East African (rhodesiense) disease [52]. Although, this is excellent progress, the goal should be to generate a portfolio of drugs in the case (as has happened for trypanocidal drugs in the past) of resistance emerging. Of the four drugs tested here against T. brucei, all except nithiamide yielded EC50 values less than the current Stage 1 treatment option, pentamidine (EC50 = 0.11 μM), including, encouragingly, the brain-penetrant obatoclax (EC50 = 0.044 μM). Further investigations, including with other strains of T. brucei, are ongoing.
In this study we have identified compounds efficacious against a diverse collection of parasites. Such broad-spectrum drugs could be of great utility in situations where diagnosis is uncertain (for instance, in the case of the free-living ameba which can all cause encephalitis) or when a patient is infected with multiple pathogens. Additionally, there could be economic advantages to a "one drug multiple-bug" approach. Currently, development of drugs for neglected tropical diseases is stymied by the high cost and low economic incentive to provide drugs for developing countries [67]. A single drug with broad spectrum activity would have a larger potential market, altering the cost benefit analysis for pharmaceutical companies. With further characterization of in vivo efficacy and toxicity, the compounds identified in this study, in particular obatoclax and anisomycin, have the potential to become useful tools in the treatment of multiple parasitic disease.
Methods
Culture and strains used
Entamoeba histolytica strain HM-1:IMSS trophozoites were grown and maintained at 37°C in TYI media under standard conditions [68].
Giardia lamblia strain WB was grown and maintained at 37°C in TYI media.
Acanthamoeba castellanii strain Ma trophozoites (T4 genotype) were cultured at 28°C in PYG medium according to a modified technique [69].
Naegleria fowleri strain KUL trophozoites were axenically cultured in Nelson's medium supplemented with 10% FBS at 37°C [70, 71].
Balamuthia mandrillaris ATCC strain PRA-291 were grown in axenic modified Cerva’s medium [72] at 37°C and 5% CO2. Encystation was induced by incubation in medium with galactose (12% final concentration) as previously described [21, 73].
Cryptosporidium parvum strain Iowa oocysts were obtained from Bunch Grass Farm (Deary, ID) [58].
Plasmodium falciparum strain Dd2attB (MRA-843) were obtained from MR4. Parasites were grown in human erythrocytes (2% hematocrit) in RPMI 1640 media supplemented with 0.25% Albumax II (GIBCO Life Technologies), 2 g/L sodium bicarbonate, 0.1mM hypoxanthine, 25mM HEPES (pH 7.4), and 50μg/L gentamycin, at 37°C, 5% O2, and 5% CO2 [74].
Juvenile Schistosoma mansoni from the Biomedical Research Institute were obtained from infected mice ~3 weeks post-infection by hepatic portal vein perfusion using 37°C DMEM with 5% heat inactivated FBS. Worms were rinsed to remove mouse blood and cultured at 37°C/5% CO2 in Basch Medium 169 supplemented with 1X antibiotic-antimycotic [75].
Bloodstream forms of T. b. brucei Lister 427 were cultured in vitro in T25 vented flasks (Thermo Fisher Scientific) in a humidified atmosphere of 5% CO2 at 37°C, using a modified Iscove’s medium (HMI-11) with 20% heat-inactivated fetal bovine serum (FBS; Gibco, Carlsbad, CA) [76]. Parasites are maintained in log-phase growth (between 1×105 and 1×106 parasites/mL) and passaged every 48 h.
hFF-1 cells (gift from John Boothroyd lab, Stanford University) were cultured in DMEM with 1g/L glucose, Sodium Pyruvate and L-glutamine (Gibco) completed with 10% FBS, and 100 U/mL penicillin/streptomycin at 37°C with 5% CO2.
hFF-1 cytotoxicity experiments
hFF-1 cells were plated in 96-well plates in 50μl DMEM at a density of 2000 cells/well, and incubated at 37°C for ~6h to allow adherence. Drug was then added in 50μl volume to give final concentrations from 60–0.03μM, and plates were returned to the incubator. After 72h, viability was assed using CellTiter-Glo Luminescent Cell Viability Assay (Promega). Results are from two independent experiments and EC50s were calculated using the GraphPad prism.
Speed of killing experiments
E. histolytica
To determine the kinetics of our compounds activity against E. histolytica, we adapted the viability assay we developed in our previous studies [6] and performed a time course to measure changes in the number of live trophozoites over 48 hours. E. histolytica trophozoites (10,000 parasites in 150μl media) were seeded into 96-well plates and allowed to grow overnight in an anaerobic chamber. The next day, a single plate was removed for analysis, and drug was added to remaining plates at 2x the previously calculated EC50 concentration. Parasite viability was assayed at 10, 24 and 48h using the live cell marker fluorescein diacetate (Sigma) as previously described [6]. Three independent biological replicates were performed.
B. mandrillaris
For the speed of killing assay, trophozoites of B. mandrillaris (10,000 parasites in 100 μL) were treated with the compounds in black, clear bottom 96-well plates for 8, 24, and 48h. Viability was determined after incubation with fluorescein diacetate for 30 minutes, fixing the trophozoites with 4% PFA and measuring fluorescence using a Tecan Infinite M1000 pro fluorometer following incubation. The percentage viability of trophozoites treated with different compounds at different time points was calculated. 0.5% DMSO was used as a negative control and 15.5μM nitroxoline (2x the published EC50 [21]) was used as a positive control. Three independent biological replicates were performed.
Balamuthia encystment induction
Balamuthia trophozoites (50,000 in 500μL media per well) were plated in in 24 well plates and incubated at 37°C for 72h with drug (15.6 μM nitroxoline and 3.2μM obatoclax) or DMSO (0.5%). Trophozoites were fixed by adding 8% paraformaldehyde to a final concentration of 4%, and cysts were counted using a hemocytometer. Trophozoites and cysts were distinguished by visual inspection under light microscopy. Results are expressed as number of cysts per mL of media.
Balamuthia Recrudescence
Recrudescence assays to asses recovery from drug treatment were adapted from a previous study [21]. B. mandrillaris trophozoites (105 cells in 4mL of media) were exposed to obatoclax at a concentration of 3.2μM for 72h at 37°C. Treated B. mandrillaris parasites were then centrifuged, washed once with PBS and resuspended in 4mL of complete DMEM media. Resuspended trophozoites (0.5mL) were transferred in duplicate to each well of a 24-well plate containing hFF-1 cells that had been seeded at 5x104 per well and incubated for 72h at 37°C. Plates were fixed with 4% PFA after 3, 5, 7 and 9 days, and the percent of the HFF monolayer lysed was observed using light microscopy and recorded for each timepoint. 0.5% DMSO was used as a negative control and 15.6 μM nitroxoline [21] was used as a positive control. Three independent biological replicates were performed.
Parasite drug susceptibility testing
Giardia lamblia
The compounds were screened against G. lamblia trophozoites following an ATP-bioluminescence based assay for cell growth and survival [77]. For primary screen, parasites were seeded into a 96-well plate at 5,000 trophozoites per well with a final concentration of test compound of 50μM in 100μl media. Negative controls in the screen plates contained 0.5% DMSO and positive controls contained 50μM of metronidazole. For 16-point dose response study, stock compounds were serially diluted with DMSO to yield a final 16-point concentration range spanning 0.0015μM-50μM. Assay plates were incubated for 48h at 37°C in the GasPak EZ gas-generating anaerobe pouch system (VWR), and at the end of incubation 50μL of CellTiter-Glo Luminescent Cell Viability Assay (Promega) was added in each well of the 96-well plates to induce cell lysis. The resulting ATP-bioluminescence of the trophozoites was measured at room temperature using an Envision plate reader from PerkinElmer. Percent inhibition was calculated, and the relative dose response data in triplicate were exported to GraphPad Prism software 8.0 for EC50 calculations and statistical analysis.
Acanthamoeba castellanii and Naegleria fowleri
Trophozoites were screened using the same protocol as Giardia, with the following modifications: no GasPak was used; for N. fowleri, 10,000 trophozoites were seeded per well and 50μM amphotericin B was used as a positive control; for A. castellanii 5,000 trophozoites were seeded per well and 50μM of chlorhexidine was used as a positive control [70].
Balamuthia mandrillaris
Trophozoites and cysts were screened using protocols adapted from [21]. Briefly, 6000 trophozoites or 4000 cysts were seeded into opaque 96-well plates and incubated with drug (50–0.4μM) or DMSO (0.5%) in 100μl media for 72h. Viability was assessed using CellTiter-Glo. EC50 values were determined using the GraphPad Prism 4-parametric sigmoidal curve-fitting model, with the bottom and top constraints set to 0 and 1, respectively.
Plasmodium falciparum
Assays were performed as in [78]. Cultures were grown in 125μL volume in 96-well plates containing serial dilution of drugs in triplicate. Growth was initiated with ring-stage parasites at 1% parasitemia and 1% hematocrit. Drug (100–0.1μM) or vehicle (DMSO) was added and plates were incubated for 72h. Growth was terminated by fixation with 1% formaldehyde and parasitized cells were stained with 50nM YOYO-1 (Invitrogen). Parasitemia was determined by flow cytometry. Data were analyzed by BD C6 Accuri C-Sampler software, and EC50 curves plotted by GraphPad Prism.
Cryptosporidium parvum
Growth inhibition was determined by an Immunofluorescence assay adapted from [79]. Oocysts were excysted by treatment with 10mM hydrochloric acid (10 min at 37°C), followed by exposure to 2mM sodium taurocholate (Sigma-Aldrich) in PBS for 10 min at 16°C. Excysted oocysts were then added to >95% confluent HCT-8 cell monolayers in 384-well clear-bottom plates at a concentration of 5,500 Iowa isolate oocysts per well. Compounds were added just before or 3h after infection, and assay plates were incubated for 48h post-infection at 37°C under 5% CO2. Wells were then washed three times with PBS containing 111mM D-galactose, fixed with 4% formaldehyde in PBS for 15 min at room temperature, permeabilized with 0.25% Triton X-100 for 10 min at 37°C, washed three times with PBS with 0.1% Tween 20, and blocked with 4% bovine serum albumin (BSA) in PBS for 2 h at 37°C or 4°C overnight. Parasitophorous vacuoles were stained with 1.33 micrograms/ml of fluorescein-labeled Vicia villosa lectin (Vector Laboratories) diluted in 1% BSA in PBS with 0.1% Tween 20 for 1h at 37°C, followed by the addition of Hoechst 33258 (AnaSpec) at a final concentration of 0.09mM for another 15 min at 37°C. Wells were then washed five times with PBS containing 0.1% Tween 20. A Nikon Eclipse TE2000 epifluorescence microscope with an automated stage was programmed using NIS-Elements Advanced Research software (Nikon, USA) to focus on the center of each well and take a 3-by-3 composite image using an EXi Blue digital camera (QImaging, Canada) with a 20x objective (numerical aperture, 0.45). Nucleus and parasite images were analyzed using macros developed on the ImageJ platform (National Institutes of Health). The only modification from the published macro used to count parasites was that the lower size threshold for parasites was decreased from 16.5 to 4 pixels (1 pixel = 0.65 micrometers). Graphs were plotted and EC50 values calculated using GraphPad Prism software, version 6.01.
Schistosoma mansoni
Juvenile worms were randomly distributed to 24-well plate. To each well was added 2mL drug, diluted to the desired concentration (0.05–5μM) or fresh medium. After 72-hour drug treatment, the mobility of worms was examined. Worms that were not mobile within the 2-minute observation time period were counted as dead worms. After 72h, drug-containing media was removed worms were washed once in medium and returned to fresh-drug free media. Worms were observed for 3 days to monitor recovery from drug treatment. For EC50 calculations, two independent biological experiments were performed. For imaging, drug treated worms were imaged on a Zeiss Stemi 508 microscope at 5x magnification. Untreated control worms were washed briefly in 0.6M MgCl2 and then in PBS + Triton X-100 (0.3%) prior to imaging to reduce motility. To avoid morphological changes of worms, fixation step was omitted for both drug-treated and control worms.
Trypanosoma brucei
The SYBR Green assay ([80] as modified by Monti et al. [81]) was employed to measure the effect of test compounds on cell viability. Compounds in 100% DMSO stocks were diluted in 100μL HMI-10 modified medium in 96-well plates (Corning 3903) such that the final DMSO concentration was 0.5%. Eight-point dose response assays (ranging from final concentrations of compound from 4μM to 0.8nM) were set up in duplicate. Bloodstream-form trypanosomes in log-phase growth were then diluted to 2x105 cells/mL in HMI-10 medium and dispensed into the previously prepared 96-well plates at 100μL per well. After 72h, trypanosomes were lysed by the addition of 50μL/well of lysis buffer containing 1 × SYBR Green. Plates were read on the 2104 EnVision multilabel plate reader (PerkinElmer) with excitation at 485 nm/emission at 535 nm. The activity of test compounds was normalized against controls from the same plate according to the following formula: Activity (%) = [1 − (FCpd − blank) / (FNeg − blank)] × 100, where FCpd corresponds to the emitted fluorescent signal expressed in arbitrary fluorescence units for the test compound; and FNeg and blank correspond to the mean fluorescent signal of the negative control wells and the background signal, respectively. EC50 values, i.e., the concentration of drug required to inhibit trypanosome growth by 50%, were calculated using GraphPad Prism software, version 6.00 for Apple Macintosh. Each assay was performed as three experimental replicates and means ±SD values are shown.
Animal studies with Cryptosporidium and drug susceptibility
In vivo mouse experiments were performed following a protocol based on [58]. All NOD SCID gamma mouse studies were performed in compliance with animal care guidelines and were approved by the University of Vermont Institutional Animal Care and Use Committee. NOD SCID gamma mice with normal flora (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and were housed for at least a week for acclimatization. At the age of 4 to 5 weeks, mice were infected with 105 C. parvum Iowa isolate oocysts. Fecal oocyst shedding is detected 6 days after infection using a quantitative PCR (qPCR assay), so treatment was started on day 7 after infection. Mice (4 per experimental group) were treated orally with prodigiosin at 25mg/kg twice daily, with MMV665917 at 60mg/kg twice daily as a positive control [58], or with vehicle alone. A final concentration of 5% DMSO was used in 100μl of 1% HPMC per dose. Mice were treated on days 7, 8, 9, and 10 post-infection. Oocyst shedding in feces was monitored by qPCR just prior to treatment and 1 day following completion of treatment.
Compound sources
Compounds were obtained from the following vendors: Prodigiosin: BOC Sciences (Catalog number 82-89-3); Obatoclax: Apex Bio (Catalog number A419); Anisomycin: Sigma (Catalog number A9789); Nithiamide: Sigma (Catalog number 33978); Venetoclax: Selleckchem (Catalog Number S8048); Navitoclax: Selleckchem (Catalog Number S1001); A-1331852: Selleckchem (Catalog Number S7801); A-1210477: Selleckchem (Catalog Number S7790); S63845: Selleckchem (Catalog Number S8383).
Supporting information
The EC50s for BCL-2 inhibitors for each parasite tested are shown. All concentrations are micromolar.
(XLSX)
Acknowledgments
We would like the thank the Stanford HTBC for technical help and members of the Singh lab for valuable discussion. Important consultation was provided by SPARK advisors, especially Toni Kline, Steve Schow, Marcus Parrish, Jeewon Kim, and Kevin Grimes.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Funding for this project was provided by SPARK Translational Research Program at Stanford University; funding from the Stanford Department of Medicine Translational Research and applied Medicine Center; The Child Health Research Institute, Lucile Packard Foundation for Children’s Health; and through the NIH (R21-AI123594 to US). Giardia, Naegleria and Acanthamoeba work was supported by 1KL2TR001444, R21AI133394 and R21AI141210 to AD. Cryptosporidium work was supported by grants from The Bill and Melinda Gates Foundation (OPP1132796) and the NIH (R21-AI130807) to CDH. PL and BW were supported by a Beckman Young Investigator Award. Screening of T. brucei was supported by NIH-NIAID R21AI133394 and R21AI141210. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. The Lancet. 2002;359(9306):564–71. 10.1016/S0140-6736(02)07744-9 [DOI] [PubMed] [Google Scholar]
- 3.DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ. 2016;47:20–33. 10.1016/j.jhealeco.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 4.Fairlamb AH, Gow NA, Matthews KR, Waters AP. Drug resistance in eukaryotic microorganisms. Nat Microbiol. 2016;1(7):16092 10.1038/nmicrobiol.2016.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janes J, Young ME, Chen E, Rogers NH, Burgstaller-Muehlbacher S, Hughes LD, et al. The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. Proc Natl Acad Sci U S A. 2018;115(42):10750–5. 10.1073/pnas.1810137115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrenkaufer GM, Suresh S, Solow-Cordero D, Singh U. High-Throughput Screening of Entamoeba Identifies Compounds Which Target Both Life Cycle Stages and Which Are Effective Against Metronidazole Resistant Parasites. Front Cell Infect Microbiol. 2018;8:276 10.3389/fcimb.2018.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schimmer AD, Raza A, Carter TH, Claxton D, Erba H, DeAngelo DJ, et al. A multicenter phase I/II study of obatoclax mesylate administered as a 3- or 24-hour infusion in older patients with previously untreated acute myeloid leukemia. PLoS One. 2014;9(10):e108694 10.1371/journal.pone.0108694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez Constandse R. [Anisomycin in intestinal amebiasis; study of 30 clinical cases]. Prensa Med Mex. 1956;21(7–10):114–5. [PubMed] [Google Scholar]
- 9.Willcox RR. Treatment of vaginal trichomoniasis with 2-acetylamino-5-nitrothiazole (aminitrozole) given orally. Br J Vener Dis. 1957;33(2):115–7. 10.1136/sti.33.2.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogitsh BJ, Carter CE, Oeltmann TN. Chapter 5—Visceral Protistans II: Flagellates In: Bogitsh BJ, Carter CE, Oeltmann TN, editors. Human Parasitology (Fifth Edition): Academic Press; 2019. p. 71–82. [Google Scholar]
- 11.Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015;12(12):e1001920 10.1371/journal.pmed.1001920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trabelsi H, Dendana F, Sellami A, Sellami H, Cheikhrouhou F, Neji S, et al. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol Biol (Paris). 2012;60(6):399–405. 10.1016/j.patbio.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 13.Organization WH. World malaria report 2016. World Health Organization; 2016. [Google Scholar]
- 14.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–22. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 15.Krishna S, Stich A. African trypanosomiasis: Clinical manifestations, diagnosis, and treatment 2019. Available from: https://www.uptodate.com/contents/african-trypanosomiasis-clinical-manifestations-diagnosis-and-treatment#H2359284. [Google Scholar]
- 16.Pepin J, Milord F. The treatment of human African trypanosomiasis. Adv Parasitol. 1994;33:1–47. 10.1016/s0065-308x(08)60410-8 [DOI] [PubMed] [Google Scholar]
- 17.Baker CH, Welburn SC. The Long Wait for a New Drug for Human African Trypanosomiasis. Trends Parasitol. 2018;34(10):818–27. 10.1016/j.pt.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 18.Bogitsh BJ, Carter CE, Oeltmann TN. Chapter 11—Blood Flukes In: Bogitsh BJ, Carter CE, Oeltmann TN, editors. Human Parasitology (Fifth Edition): Academic Press; 2019. p. 193–209. [Google Scholar]
- 19.Sanz LM, Crespo B, De-Cozar C, Ding XC, Llergo JL, Burrows JN, et al. P. falciparum in vitro killing rates allow to discriminate between different antimalarial mode-of-action. PLoS One. 2012;7(2):e30949 10.1371/journal.pone.0030949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, et al. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med. 2012;18(6):956–60. Epub 2012/05/23. nm.2758 [pii] 10.1038/nm.2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurie MT, White CV, Retallack H, Wu W, Moser MS, Sakanari JA, et al. Functional Assessment of 2,177 U.S. and International Drugs Identifies the Quinoline Nitroxoline as a Potent Amoebicidal Agent against the Pathogen Balamuthia mandrillaris. MBio. 2018;9(5). 10.1128/mBio.02051-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Thapar MM, Wernsdorfer WH, Bjorkman A. In vitro interactions of artemisinin with atovaquone, quinine, and mefloquine against Plasmodium falciparum. Antimicrob Agents Chemother. 2002;46(5):1510–5. 10.1128/AAC.46.5.1510-1515.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansour NR, Paveley R, Gardner JM, Bell AS, Parkinson T, Bickle Q. High Throughput Screening Identifies Novel Lead Compounds with Activity against Larval, Juvenile and Adult Schistosoma mansoni. PLoS Negl Trop Dis. 2016;10(4):e0004659 10.1371/journal.pntd.0004659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardocki JF, Timmens EK, Wilson LB, Sodergren JO, Hettinger BR, P'An SY. Acute and chronic toxicity of anisomycin in experimental animals. Antibiot Chemother (Northfield). 1955;5(9):490–5. [PubMed] [Google Scholar]
- 25.Health NIfOSa. Registry of Toxic Effects of Chemical Substances1987.
- 26.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109(12):5430–8. 10.1182/blood-2006-10-047951 [DOI] [PubMed] [Google Scholar]
- 27.Cuckler AC, Kupferberg AB, Millman N. Chemotherapeutic and tolerance studies on amino-nitro-thiazoles. Antibiot Chemother (Northfield). 1955;5(10):540–50. [PubMed] [Google Scholar]
- 28.Tolić L, Grujić S, Mojović M, Jovanović M, Lubec G, Bačić G, et al. Determination of anisomycin in tissues and serum by LC-MS/MS: application to pharmacokinetic and distribution studies in rats. RSC Advances. 2016;6(95):92479–89. 10.1039/C6RA16083B [DOI] [Google Scholar]
- 29.Barrett JS, Zhang AY, Urtishak KA, Danet-Desnoyers G, Carroll AJ, Hunger SP, et al. Predicting Clinical Dose-Exposure and Exposure-Response Relationships of Pan-Antiapoptotic BCL-2 Family Inhibitor Obatoclax in MLL Rearranged Infant Leukemias From Preclinical Disease Models and Adult Experience. Blood. 2011;118:2580–. [Google Scholar]
- 30.Montaner B, Navarro S, Pique M, Vilaseca M, Martinell M, Giralt E, et al. Prodigiosin from the supernatant of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. Br J Pharmacol. 2000;131(3):585–93. 10.1038/sj.bjp.0703614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denisova OV, Kakkola L, Feng L, Stenman J, Nagaraj A, Lampe J, et al. Obatoclax, saliphenylhalamide, and gemcitabine inhibit influenza a virus infection. The Journal of biological chemistry. 2012;287(42):35324–32. 10.1074/jbc.M112.392142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahul S, Chandrashekhar P, Hemant B, Bipinchandra S, Mouray E, Grellier P, et al. In vitro antiparasitic activity of microbial pigments and their combination with phytosynthesized metal nanoparticles. Parasitol Int. 2015;64(5):353–6. 10.1016/j.parint.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 33.Torres NI, Castilla V, Bruttomesso AC, Eiras J, Galagovsky LR, Wachsman MB. In vitro antiviral activity of dehydroepiandrosterone, 17 synthetic analogs and ERK modulators against herpes simplex virus type 1. Antiviral Res. 2012;95(1):37–48. 10.1016/j.antiviral.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 34.Varghese FS, Rausalu K, Hakanen M, Saul S, Kummerer BM, Susi P, et al. Obatoclax Inhibits Alphavirus Membrane Fusion by Neutralizing the Acidic Environment of Endocytic Compartments. Antimicrob Agents Chemother. 2017;61(3). 10.1128/AAC.02227-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen CZ, Kulakova L, Southall N, Marugan JJ, Galkin A, Austin CP, et al. High-throughput Giardia lamblia viability assay using bioluminescent ATP content measurements. Antimicrob Agents Chemother. 2011;55(2):667–75. 10.1128/AAC.00618-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, et al. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc Natl Acad Sci U S A. 2008;105(26):9059–64. 10.1073/pnas.0802982105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang W, Soares J, Greninger P, Edelman EJ, Lightfoot H, Forbes S, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Research. 2012;41(D1):D955–D61. 10.1093/nar/gks1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh A, Venkannagari S, Oh KH, Zhang YQ, Rohde JM, Liu L, et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem Biol. 2016;11(11):3214–25. 10.1021/acschembio.6b00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YJ, Cubitt B, Chen E, Hull MV, Chatterjee AK, Cai Y, et al. The ReFRAME library as a comprehensive drug repurposing library to identify mammarenavirus inhibitors. Antiviral Res. 2019;169:104558 10.1016/j.antiviral.2019.104558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tejman-Yarden N, Eckmann L. New approaches to the treatment of giardiasis. Current Opinion in Infectious Diseases. 2011;24(5):451–6. 10.1097/QCO.0b013e32834ad401 [DOI] [PubMed] [Google Scholar]
- 41.Leitsch D. Drug Resistance in the Microaerophilic Parasite Giardia lamblia. Curr Trop Med Rep. 2015;2(3):128–35. 10.1007/s40475-015-0051-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS immunology and medical microbiology. 2007;50(1):1–26. Epub 2007/04/13. 10.1111/j.1574-695X.2007.00232.x [DOI] [PubMed] [Google Scholar]
- 43.Cope JR, Conrad DA, Cohen N, Cotilla M, DaSilva A, Jackson J, et al. Use of the Novel Therapeutic Agent Miltefosine for the Treatment of Primary Amebic Meningoencephalitis: Report of 1 Fatal and 1 Surviving Case. Clin Infect Dis. 2016;62(6):774–6. 10.1093/cid/civ1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kangussu-Marcolino M, Ehrenkaufer G, Chen E, Debnath A, Singh U. Identification of plicamycin, TG02, panobinostat, lestaurtinib, and GDC-0084 as promising compounds for the treatment of central nervous system infections caused by the free-living amebae Naegleria, Acanthamoeba and Balamuthia. International Journal for Parasitology: Drugs and Drug Resistance. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vale N, Gouveia MJ, Rinaldi G, Brindley PJ, Gartner F, Correia da Costa JM. Praziquantel for Schistosomiasis: Single-Drug Metabolism Revisited, Mode of Action, and Resistance. Antimicrob Agents Chemother. 2017;61(5). 10.1128/AAC.02582-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, Wang L, Liang YS. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol Res. 2012;111(5):1871–7. 10.1007/s00436-012-3151-z [DOI] [PubMed] [Google Scholar]
- 47.Pica-Mattoccia L, Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int J Parasitol. 2004;34(4):527–33. 10.1016/j.ijpara.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 48.Kasinathan RS, Sharma LK, Cunningham C, Webb TR, Greenberg RM. Inhibition or knockdown of ABC transporters enhances susceptibility of adult and juvenile schistosomes to Praziquantel. PLoS Negl Trop Dis. 2014;8(10):e3265 10.1371/journal.pntd.0003265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao SH, Catto BA, Webster LT Jr. Effects of praziquantel on different developmental stages of Schistosoma mansoni in vitro and in vivo. J Infect Dis. 1985;151(6):1130–7. 10.1093/infdis/151.6.1130 [DOI] [PubMed] [Google Scholar]
- 50.Buchter V, Hess J, Gasser G, Keiser J. Assessment of tegumental damage to Schistosoma mansoni and S. haematobium after in vitro exposure to ferrocenyl, ruthenocenyl and benzyl derivatives of oxamniquine using scanning electron microscopy. Parasit Vectors. 2018;11(1):580 10.1186/s13071-018-3132-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.William S, Botros S, Ismail M, Farghally A, Day TA, Bennett JL. Praziquantel-induced tegumental damage in vitro is diminished in schistosomes derived from praziquantel-resistant infections. Parasitology. 2001;122 Pt 1:63–6. [DOI] [PubMed] [Google Scholar]
- 52.Deeks ED. Fexinidazole: First Global Approval. Drugs. 2019;79(2):215–20. 10.1007/s40265-019-1051-6 [DOI] [PubMed] [Google Scholar]
- 53.Tse EG, Korsik M, Todd MH. The past, present and future of anti-malarial medicines. Malar J. 2019;18(1):93 10.1186/s12936-019-2724-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papireddy K, Smilkstein M, Kelly JX, Shweta, Salem SM, Alhamadsheh M, et al. Antimalarial activity of natural and synthetic prodiginines. J Med Chem. 2011;54(15):5296–306. 10.1021/jm200543y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ekong RM, Kirby GC, Patel G, Phillipson JD, Warhurst DC. Comparison of the in vitro activities of quassinoids with activity against Plasmodium falciparum, anisomycin and some other inhibitors of eukaryotic protein synthesis. Biochem Pharmacol. 1990;40(2):297–301. 10.1016/0006-2952(90)90691-d [DOI] [PubMed] [Google Scholar]
- 56.Moore SR, Lima NL, Soares AM, Oria RB, Pinkerton RC, Barrett LJ, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010;139(4):1156–64. 10.1053/j.gastro.2010.05.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of Nitazoxanide. J Infect Dis. 2001;184(1):103–6. 10.1086/321008 [DOI] [PubMed] [Google Scholar]
- 58.Jumani RS, Bessoff K, Love MS, Miller P, Stebbins EE, Teixeira JE, et al. A Novel Piperazine-Based Drug Lead for Cryptosporidiosis from the Medicines for Malaria Venture Open-Access Malaria Box. Antimicrob Agents Chemother. 2018;62(4). 10.1128/AAC.01505-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oki Y, Copeland A, Hagemeister F, Fayad LE, Fanale M, Romaguera J, et al. Experience with obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma. Blood. 2012;119(9):2171–2. 10.1182/blood-2011-11-391037 [DOI] [PubMed] [Google Scholar]
- 60.Martin Abreu L. [Action of anisomycin in amebiasis and giardiasis]. Rev Med Hosp Gen (Mex). 1962;25:103–8. [PubMed] [Google Scholar]
- 61.O'Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113(2):299–305. 10.1182/blood-2008-02-137943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.WHO. 2019. Available from: https://www.who.int/schistosomiasis/strategy/en/.
- 63.Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS microbiology reviews. 2006;30(4):564–95. Epub 2006/06/16. 10.1111/j.1574-6976.2006.00023.x [DOI] [PubMed] [Google Scholar]
- 64.Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA. Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am J Trop Med Hyg. 2003;68(1):65–9. [PubMed] [Google Scholar]
- 65.Falagas ME, Bliziotis IA, Tam VH. Intraventricular or intrathecal use of polymyxins in patients with Gram-negative meningitis: a systematic review of the available evidence. Int J Antimicrob Agents. 2007;29(1):9–25. 10.1016/j.ijantimicag.2006.08.024 [DOI] [PubMed] [Google Scholar]
- 66.Ren M, Wu X. Evaluation of three different methods to establish animal models of Acanthamoeba keratitis. Yonsei Med J. 2010;51(1):121–7. 10.3349/ymj.2010.51.1.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Renslo AR, McKerrow JH. Drug discovery and development for neglected parasitic diseases. Nat Chem Biol. 2006;2(12):701–10. 10.1038/nchembio837 [DOI] [PubMed] [Google Scholar]
- 68.Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–2. 10.1016/0035-9203(78)90144-x [DOI] [PubMed] [Google Scholar]
- 69.Debnath A, Tunac JB, Silva-Olivares A, Galindo-Gomez S, Shibayama M, McKerrow JH. In vitro efficacy of corifungin against Acanthamoeba castellanii trophozoites and cysts. Antimicrob Agents Chemother. 2014;58(3):1523–8. 10.1128/AAC.02254-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Debnath A, Calvet CM, Jennings G, Zhou W, Aksenov A, Luth MR, et al. CYP51 is an essential drug target for the treatment of primary amoebic meningoencephalitis (PAM). PLoS Negl Trop Dis. 2017;11(12):e0006104 10.1371/journal.pntd.0006104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Debnath A, Nelson AT, Silva-Olivares A, Shibayama M, Siegel D, McKerrow JH. In Vitro Efficacy of Ebselen and BAY 11–7082 Against Naegleria fowleri. Front Microbiol. 2018;9:414 10.3389/fmicb.2018.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lares-Jimenez LF, Gamez-Gutierrez RA, Lares-Villa F. Novel culture medium for the axenic growth of Balamuthia mandrillaris. Diagn Microbiol Infect Dis. 2015;82(4):286–8. 10.1016/j.diagmicrobio.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 73.Siddiqui R, Jarroll EL, Khan NA. Balamuthia mandrillaris: role of galactose in encystment and identification of potential inhibitory targets. Exp Parasitol. 2010;126(1):22–7. 10.1016/j.exppara.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 74.Gisselberg JE, Zhang L, Elias JE, Yeh E. The Prenylated Proteome of Plasmodium falciparum Reveals Pathogen-specific Prenylation Activity and Drug Mechanism-of-action. Mol Cell Proteomics. 2017;16(4 suppl 1):S54–S64. Epub 12/31. 10.1074/mcp.M116.064550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Basch PF. Cultivation of Schistosoma mansoni in vitro. I. Establishment of cultures from cercariae and development until pairing. J Parasitol. 1981;67(2):179–85. [PubMed] [Google Scholar]
- 76.Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75(6):985–9. [PubMed] [Google Scholar]
- 77.Debnath A, Shahinas D, Bryant C, Hirata K, Miyamoto Y, Hwang G, et al. Hsp90 inhibitors as new leads to target parasitic diarrheal diseases. Antimicrob Agents Chemother. 2014;58(7):4138–44. 10.1128/AAC.02576-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu W, Herrera Z, Ebert D, Baska K, Cho SH, DeRisi JL, et al. A chemical rescue screen identifies a Plasmodium falciparum apicoplast inhibitor targeting MEP isoprenoid precursor biosynthesis. Antimicrobial agents and chemotherapy. 2015;59(1):356–64. Epub 11/03. 10.1128/AAC.03342-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bessoff K, Sateriale A, Lee KK, Huston CD. Drug repurposing screen reveals FDA-approved inhibitors of human HMG-CoA reductase and isoprenoid synthesis that block Cryptosporidium parvum growth. Antimicrob Agents Chemother. 2013;57(4):1804–14. 10.1128/AAC.02460-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faria J, Moraes CB, Song R, Pascoalino BS, Lee N, Siqueira-Neto JL, et al. Drug discovery for human African trypanosomiasis: identification of novel scaffolds by the newly developed HTS SYBR Green assay for Trypanosoma brucei. J Biomol Screen. 2015;20(1):70–81. 10.1177/1087057114556236 [DOI] [PubMed] [Google Scholar]
- 81.Monti L, Wang SC, Oukoloff K, Smith AB 3rd, Brunden KR, Caffrey CR, et al. Brain-Penetrant Triazolopyrimidine and Phenylpyrimidine Microtubule Stabilizers as Potential Leads to Treat Human African Trypanosomiasis. ChemMedChem. 2018;13(17):1751–4. 10.1002/cmdc.201800404 [DOI] [PMC free article] [PubMed] [Google Scholar]