Abstract
Campylobacter jejuni is one of the most prevalent causes of bacterial gastroenteritis worldwide, and it is largely associated with consumption of contaminated poultry. Current Campylobacter control measures at the poultry production level remain insufficient, and hence there is the need for alternative control strategies. We evaluated the potential of the monoterpene (-)-α-pinene for control of C. jejuni in poultry. The antibacterial and resistance-modulatory activities of (-)-α-pinene were also determined against 57 C. jejuni strains. In addition, the anti-quorum-sensing activity of (-)-α-pinene against C. jejuni NCTC 11168 was determined for three subinhibitory concentrations (125, 62.5, 31.25 mg/L) over three incubation times using an autoinducer-2 bioassay based on Vibrio harveyi BB170 bioluminescence measurements. The effects of a subinhibitory concentration of (-)-α-pinene (250 mg/L) on survival of C. jejuni, and in combination with enrofloxacin on fluoroquinolone resistance development in C. jejuni, were determined in a broiler chicken model, by addition of (-)-α-pinene to the broiler water supply. The reduction of C. jejuni numbers by (-)-α-pinene was further determined in broiler chickens that were colonized with either fluoroquinolone-susceptible or -resistant strains, by direct gavage treatment. We observed weak in vitro antimicrobial activity for (-)-α-pinene alone (MIC >500 mg/L), but strong potentiating effects on antibiotics erythromycin and ciprofloxacin against different Campylobacter strains (>512 fold change). After 24 h of treatment of C. jejuni with (-)-α-pinene, its quorum-sensing signaling was reduced by >80% compared to the untreated control. When given in the drinking water, (-)-α-pinene did not show any significant inhibitory effects on the level of C. jejuni in the colonized chickens, and did not reduce fluoroquinolone resistance development in combination with enrofloxacin. Conversely, when (-)-α-pinene was administered by direct gavage, it significantly reduced the number of fluoroquinolone susceptible C. jejuni in the colonized broiler chickens. These results demonstrate that (-)-α-pinene modulates quorum-sensing in Campylobacter, potentiates antibiotics against different Campylobacter strains, and reduces Campylobacter colonization in broiler chickens.
Introduction
Campylobacter jejuni represents a food safety hazard worldwide. It can cause campylobacteriosis, which is one of the most widespread bacterial foodborne zoonoses reported for the European Union and the United States [1–3]. Campylobacteriosis is commonly associated with ingestion of contaminated poultry, water, or milk, and manifests as acute watery/bloody diarrhea, fever, and cramps. This can also lead to post-infection development of the severe neurological condition known as Guillain-Barre syndrome [1]. An additional risk is the increasing antimicrobial resistance in Campylobacter. In particular, Campylobacter resistance to fluoroquinolones and macrolides compromises effectiveness of antibiotic therapies and poses a heightened food safety concern in the food chain [2,4].
Currently, there are no fully effective and practical measures for the control of poultry contamination with the avian commensal C. jejuni. Control of Campylobacter includes pre-harvest measures on poultry farms and post-harvest approaches in processing plants. Pre-harvest biosecurity and hygiene measures can be used to prevent entrance of Campylobacter onto a farm and to limit its spread between flocks, whereas post-harvest measures focus on decontamination of carcasses [5–7].
To mitigate transmission of Campylobacter from food animals to humans through the food supply chain, effective pathogen control measures are needed. These must be designed to reduce the Campylobacter load at the farm and/or slaughterhouse level, with emphasis on poultry production, where Campylobacter resides as a commensal [2,4,8]. Even a relatively small reduction in C. jejuni numbers in the chicken cecum by 1 log10 CFU can reduce the public health risk by more than 50% [8].
A number of natural products have been shown to have anti-Campylobacter activities and have been studied as feed additives, such as essential oils and their components [9,10]. The majority of studies have been focused on the bactericidal aspects of the antimicrobial actions of natural compounds, while their potential for reduction of pathogen virulence through inhibition of efflux pumps, quorum sensing or other factors contributing to colonization of a host, remains largely unexplored [11,12].
In C. jejuni, quorum sensing is mediated by the furanosyl borate diester autoinducer-2 (AI-2) signal that is produced as a result of the action of the S-ribosylhomociateinase LuxS, encoded by the luxS gene [13]. The C. jejuni mutant lacking the luxS gene shows impaired biofilm formation, motility, resistance against oxidative stress, invasion of Caco-2 cells, virulence in the host, and colonization of the chicken intestine [13–18]. This suggests that inhibition of C. jejuni quorum sensing in the host might result in reduction of C. jejuni in the feces, and thus control C. jejuni spread in the environment.
Only a few plant extracts have been reported to show anti-quorum-sensing effects in C. jejuni (e.g., citrus extracts, Evodia ruticarpa extracts) to date [19,20].
In a previous study, we also demonstrated efflux-inhibitory and resistance-modulatory activities of the monoterpene (-)-α-pinene in Campylobacter [21]. These findings suggest that plant extracts, such as (-)-α-pinene, modulate multiple physiological functions in C. jejuni. However, the effects of these plant extracts have not been examined using an in vivo system, which would allow for determination of their potential use in food-animal production. In this study we further investigated (-)-α-pinene bioactivities, including: (i) inhibition of C. jejuni quorum sensing in vitro; (ii) modulation of C. jejuni resistance to fluoroquinolones in broiler chickens; and (iii) reduction of C. jejuni colonization in broiler chickens.
Materials and methods
Bacterial strains and growth conditions
The Campylobacter jejuni strains shown in S1 and S2 Tables were isolated and characterized by Luangtongkum et al. [22], and were stored at -80°C in 80% Mueller Hinton broth (MHB: Oxoid, UK) with 20% glycerol. They were then grown on Mueller-Hinton agar (MHA; Oxoid, UK) at 42°C under microaerobic conditions (5% O2, 10% CO2, 85% N2) for 24 h. The second passage from each culture was used in the experiments. When necessary, MHA was supplemented with selective medium (SR01176; Oxoid, UK) and growth medium (SR0232E; Oxoid, UK) (MHA-SS), 30 mg/L kanamycin (Merck, Germany), or 4 mg/L ciprofloxacin (Merck, Germany). The Vibrio harveyi BB170 reporter strain [19,23] was grown on autoinducer bioassay (AB) medium at 30°C, which contained 17 g/L NaCl (Merck, Germany), 12.3 g/L MgSO4 (Merck, Germany), 2 g/L casamino acids (BD Bacto; Fisher Scientific), 1 mM K2HPO4 (Kemika, Croatia), 0.1 mM L-arginine (Sigma Aldrich, Germany), and 1% (v/v) glycerol (Kemika, Croatia).
Antimicrobial and resistance-modulatory activities of (-)-α-pinene in vitro
The minimal inhibitory concentrations (MICs) of (-)-α-pinene (Sigma Aldrich, Germany) were determined against all of the 57 C. jejuni strains that were sourced according to S1 Table, using the broth microdilution method, as described previously [21]. The reported MIC50 and MIC90 values represent the MICs that inhibited at least 50% and 90%, respectively, of the tested strains. The resistance-modulatory activity of (-)-α-pinene was determined in combination with the clinically relevant antibiotics ciprofloxacin and erythromycin (Fluka Chemie, Germany), using the broth microdilution method [21]. (-)-α-Pinene was added to these antibiotics at the subinhibitory concentration of 125 mg/L. The MICs were determined, along with the fold-changes (FC) between the MICs of the antibiotics alone and their MICs with the addition of (-)-α-pinene. These were calculated according to Eq (1):
| (1) |
where MICAb is the MIC of the antibiotic alone, and MICAbAp is the MIC of the antibiotic in the presence of 125 mg/L (-)-α-pinene. FC ≥2 was considered as indicative of biologically significant resistance modulation.
Quorum-sensing inhibition in vitro
To determine the influence of (-)-α-pinene on C. jejuni quorum sensing, autoinducer-2 bioassays were performed. C. jejuni NCTC 11168 and C. jejuni 11168ΔluxS (negative control; [18]) cultures in MHB were adjusted to OD600 0.1. The (-)-α-pinene stock solutions were prepared in 100% dimethylsulfoxide (DMSO) at 6.25 g/L, 12 g/L, and 25 g/L. Fifty microliters of each stock was added to 10 mL of each culture for the final (-)-α-pinene concentrations of 31.25 mg/L, 62.5 mg/L, and 125 mg/L. Untreated cultures were used as controls. The cultures were incubated under microaerobic conditions at 42°C for 24 h. Samples of 3 mL were taken after 4 h, 8 h, and 24 h, and filter sterilized using 0.2-μm syringe filters (Sartorius, Germany), for the cell-free supernatants.
The autoinducer-2 bioassay was performed as previously described [19], with some modifications. The quorum-sensing inhibition bioassays were carried out using a V. harveyi BB170 reporter strain [23]that was grown for 16 h at 30°C and 150 rpm, and used at the final concentration of 5 ×104 CFU/mL in AB medium. Filter sterilized C. jejuni cell-free supernatants were added to the suspensions of the reporter strain to a final concentration of 10% (v/v) (i.e., 20 μL cell-free supernatant added to 180 μL reporter strain suspension). Sterile medium was used as the blank (10% [v/v] MHB, 90% [v/v] AB medium). Kinetic measurements were carried out for the bioluminescence signals of V. harveyi BB170 produced as a result of the presence of the quorum-sensing signal that originated from the C. jejuni cell-free supernatants. The relative luminescence signals were measured at 15-min intervals over 20 h at 30°C, in white microtiter plates (Nunc, Thermo Scientific) incubated in a microplate reader (Varioskan Lux; Thermo Scientific).
Vibrio harveyi produces a background luminescence signal that increases with the concentration of the culture. To define the most stable point of signal production, V. harveyi growth and signal production was measured in AB supplemented with MHB (180 μL:20 μL) at 30°C. The signal stabilized when V. harveyi entered the stationary phase (S1 Fig). The time point when V. harveyi enters the stationary phase (after 9 h incubation) was used in the calculation of the quorum-sensing signals attributed to C. jejuni.
The relative luminescence signals were interpreted as the quorum-sensing signal in the C. jejuni cell-free supernatants (i.e., a higher signal indicated a higher concentration of quorum-sensing signaling molecules produced by C. jejuni), and are shown in S2 Fig.
Cell-free supernatants from C. jejuni 11168ΔluxS, a mutant that cannot produce the quorum-sensing signal (AI-2), were used as the negative control, and fresh MHB as the blank. To determine the inhibition rates of the quorum sensing by (-)-α-pinene, the blank values were subtracted from all of the test sample values. These corrected test values were used to calculate the reduction in quorum sensing using Eq (2):
| (2) |
The experiments were performed as three independent biological replicates and three technical replicates.
Broiler chicken colonization with C. jejuni
Broiler chicks (Cornish Rock strain, unspecified sex) were obtained from the Welp Hatchery in Iowa (USA) on the day of hatching, and were divided into four groups of 10 broilers each. The broiler chickens were kept in sanitized wire-floored cages (each group, n = 10/cage), and provided with feed and water ad libitum. Cloacal swabs were taken from each broiler chicken prior to the experiment and plated onto MHA-SS to confirm that they showed no Campylobacter colonization prior to inoculation. No Campylobacter was detected in any of the broiler chickens tested. At the age of day 5, each bird was inoculated with 3.6 ×106 CFU C. jejuni NCTC 11168 by oral gavage. To confirm colonization, cloacal swabs were collected 3 days after the inoculation.
At the age of day 8, medicated water was given to birds for 5 consecutive days to evaluate the synergistic effects of (-)-α-pinene and enrofloxacin on Campylobacter fluoroquinolone resistance development. Since enrofloxacin and (-)-α-pinene were dissolved in DMSO, the medicated water contained 0.5% DMSO for all groups, with the following additions for each group: (1) none (DMSO; control group); (2) 250 mg/L (-)-α-pinene (AP); (3) 50 mg/L enrofloxacin (ENRO) (Sigma Aldrich); and (4) 250 mg/L (-)-α-pinene and 50 mg/L enrofloxacin (ENRO+AP). Cloacal swabs were collected every other day, and 3 days after (day 16 of age) the final day of the treatment for Campylobacter culture.
As the culture results of the cloacal swabs showed, all of the birds in all of the groups were colonized by C. jejuni. In addition, enrofloxacin treatment resulted in development of fluoroquinolone resistance in C. jejuni in the treated groups (FQ-R; groups 3 (ENRO) and 4 (ENRO+AP) above), while the groups that were not treated with enrofloxacin remained colonized by fluoroquinolone sensitive C. jejuni (FQ-S; groups 1 (DMSO) and 2 (AP) above). To further determine the effects of (-)-α-pinene on susceptible and resistant C. jejuni in vivo, one group of each category (groups 2 and 4) were given an additional 250 mg/L (-)-α-pinene directly by oral gavage (i.e., the FQ-S treated and FQ-R treated groups) while the other two (groups 1 and 3) did not receive any (-)-α-pinene (i.e., the FQ-S untreated and FQ-R untreated groups). The gavage water (0.4 mL/bird/day) was started at the age of 18 days for 3 consecutive days, and it contained 0.5% DMSO for all four groups. Direct gavage treatment was used to minimize the variability of the dosing between the broiler chickens. All of the broiler chickens were sacrificed at 21 days of age, at which time cecum contents were collected for Campylobacter culture.
For determination of Campylobacter numbers, all of the fecal swabs and the cecum contents collected were suspended in MHB (1 mL MHB/swab with 100 mg feces), serially diluted, plated onto MHA-SS (for total C. jejuni numbers) and onto MHA-SS supplemented with 4 mg/L ciprofloxacin (for fluoroquinolone-resistant C. jejuni), and incubated at 42°C under microaerobic conditions for 48 h. The detection limit of the culture method for C. jejuni was 100 CFU/g feces. To further confirm the emergence of fluoroquinolone-resistant C. jejuni mutants, colonies from MHA-SS were also collected for each group at every sampling, and antimicrobial sensitivity testing was performed using E-test strips (0.002–32 mg/L ciprofloxacin; AB Biodisk, Sweden).
Ethics statement
All of the animal protocols and procedures used in this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Iowa State University (Ames, Iowa, USA) before the start of the experiments. The approved protocol identification number is: 2-07-6304-G. The animal care and use protocol used in this study adhered to regulations and guidelines provided in the “Guide for the Care and Use of Laboratory Animals”, 8th edition, and the “Guide for the Care and Use of Agricultural Animals in Research and Teaching”, 3rd edition.
Statistical analyses
All of the data were tested for normality with Kolmogorov-Smirnov and Shapiro-Wilk tests. The statistical significances of the quorum-sensing inhibition and antimicrobial and resistance-modulatory activities were calculated using one-way ANOVA with Tukey’s post-hoc tests. The associations between antibiotic resistance and resistance modulation were calculated using Chi-squared tests with Cramer’s V strength tests. Differences in colonization between the treated and untreated broiler chickens were analyzed using Student’s t-tests. Emergence of fluoroquinolone-resistant mutants in groups was compared using Student’s t-tests. All of the analyses were performed using the SPSS software, version 21 (IBM Corp., Armonk, NY, USA).
Results
(-)-α-Pinene shows weak antimicrobial activity but strong resistance-modulatory activity against C. jejuni in vitro
To evaluate the clinical relevance of previously reported antimicrobial and resistance-modulatory activities of (-)-α-pinene [21], these activities were tested across 57 broiler, turkey, and human C. jejuni strains, in addition to the reference strain (NCTC 11168), which were sourced as listed in S1 Table. The following criteria were defined for the antimicrobial activities of (-)-α-pinene alone: high: MIC ≤31.25 mg/L; intermediate: MIC from 62.5 mg/L to 1000 mg/L; low: MIC at 2000 mg/L; none: MIC >2000 mg/L. Based on these criteria, and collectively considering these 57 C. jejuni strains, (-)-α-pinene alone showed low antimicrobial activity, with the overall MIC50 of 2000 mg/L (concentration of (-)-α-pinene that inhibited at least 50% of the strains; Table 1). Considering the strains individually, the majority of these strains showed low antimicrobial activities of (-)-α-pinene (n = 39; 68%), with no effects seen against 12% (n = 7) (Table 1). These data thus demonstrate the relatively weak antimicrobial activity of (-)-α-pinene alone against C. jejuni.
Table 1. Antimicrobial and resistance modulatory activity of (-)-α-pinene with antibiotics ciprofloxacin and erythromycin in 57 Campylobacter jejuni strains from chicken meat (strain code, CB), turkey meat (strain code, CT), human feces (strain codes F, X) and the reference strain NCTC 11168.
| Strain | (-)-α-Pinene | Ciprofloxacin | Erythromycin | ||||
|---|---|---|---|---|---|---|---|
| code | MIC (mg/L) | MIC (mg/L) | Fold | MIC (mg/L) | Fold | ||
| Alone | Alone | Plus (-)-α-pinenea | changeb | Alone | Plus (-)-α-pinenea | changeb | |
| CB1:6 | 1000 | 16 | <0.125 | >128 | 0.5 | <0.002 | >256 |
| CB1:14 | 1000 | 16 | <0.125 | >128 | 0.5 | <0.002 | >256 |
| CB1:18 | 1000 | 16 | <0.125 | >128 | 0.5 | 0.125 | 4 |
| CB2:6 | 2000 | 64 | 16 | 4 | 0.5 | 0.06 | 8 |
| CB2:8 | 2000 | 64 | 32 | 2 | 0.5 | 0.25 | 2 |
| CB2:11 | 1000 | 8 | 4 | 2 | 0.5 | 0.25 | 2 |
| CB3:1 | 2000 | 0.125 | 0.06 | 2 | 0.25 | 0.06 | 4 |
| CB3:5 | 2000 | 0.25 | 0.06 | 4 | 0.5 | 0.25 | 2 |
| CB 4:21 | 1000 | 0.06 | 0.03 | 2 | 0.06 | <0.002 | >32 |
| CB 4:22 | 2000 | 0.125 | 0.001 | 128 | 0.06 | 0.002 | 32 |
| CB 6:8 | 2000 | 0.06 | 0.008 | 8 | 0.125 | <0.002 | >64 |
| CB 6:9 | 2000 | 0.06 | 0.03 | 2 | 0.125 | 0.06 | 2 |
| CB 6:26 | 2000 | 0.06 | 0.03 | 2 | 0.25 | 0.125 | 2 |
| CB 7:15 | 1000 | 8 | <0.06 | >128 | 0.25 | <0.002 | >128 |
| CB 7:21 | 2000 | 8 | 1 | 8 | 0.125 | <0.002 | >64 |
| CB 8:14 | 2000 | 0.06 | 0.001 | 64 | 0.125 | 0.002 | 64 |
| CB 8:15 | 1000 | 0.06 | 0.001 | 64 | 0.5 | <0.002 | >256 |
| CT 1:1 | 2000 | 16 | 1 | 16 | 0.5 | 0.03 | 16 |
| CT 1:9 | 1000 | 16 | 2 | 8 | 0.5 | 0.06 | 8 |
| CT 2:2 | 2000 | 16 | <0.06 | >256 | 256 | <1 | >256 |
| CT 3:5 | 2000 | 0.06 | <0.001 | >64 | 0.03 | <0.002 | 16 |
| CT3:11 | 500 | 4 | <0.06 | >64 | - | - | - |
| CT3:19 | 2000 | 16 | <0.06 | >256 | 512 | <1 | >512 |
| CT4:4 | 2000 | 16 | 8 | 2 | 128 | 64 | 2 |
| CT4:14 | 2000 | 8 | 8 | 1 | 128 | 64 | 2 |
| CT5:2 | 2000 | 16 | 4 | 4 | 256 | 256 | 1 |
| CT5:8 | 2000 | 8 | 2 | 4 | 256 | 16 | 16 |
| CT5:10 | 2000 | 16 | <0.06 | >256 | 256 | <1 | >256 |
| CT5:12 | 2000 | 16 | 4 | 4 | 256 | 32 | 8 |
| CT5:18 | 2000 | 16 | 4 | 4 | 256 | 64 | 4 |
| CT 6:18 | 2000 | 8 | 0.5 | 16 | 128 | <1 | >128 |
| CT 6:8 | 2000 | 16 | 2 | 8 | 256 | <1 | >256 |
| CT 6:16 | 2000 | 0.03 | <0.001 | >32 | 128 | <1 | >128 |
| CT 7:2 | 2000 | 0.03 | <0.001 | >32 | 0.06 | 0.002 | 32 |
| CT 8: 28 | 2000 | 8 | 1 | 8 | 0.25 | <0.008 | >32 |
| CT 8:29 | >2000 | 4 | <0.06 | >64 | 64 | 8 | 8 |
| CT 8:22 | 2000 | 4 | 0.5 | 8 | 0.5 | 0.5 | 1 |
| CT 9:14 | 2000 | 0.25 | <0.002 | >128 | 2 | <0.008 | >256 |
| CT 10:18 | 1000 | 0.06 | <0.002 | >32 | 0.25 | <0.008 | >32 |
| CT 9:21 | 1000 | 0.125 | <0.002 | >64 | 2 | <0.008 | >256 |
| F6501 | >2000 | 0.125 | 0.06 | 2 | 0.25 | 0.125 | 2 |
| H2958 | 2000 | 0.125 | 0.03 | 4 | 0.5 | 0.25 | 2 |
| M63885 | 2000 | 0.25 | <0.002 | >128 | 0.5 | <0.008 | >64 |
| T59822 | 2000 | 0.125 | 0.016 | 8 | 0.25 | 0.06 | 4 |
| W14861 | >2000 | 0.25 | 0.25 | 1 | 2 | 2 | 1 |
| X60179 | 2000 | 8 | <0.06 | >128 | 0.25 | <0.002 | >128 |
| F15871 | >2000 | 0.125 | 0.06 | 2 | 1 | 0.5 | 2 |
| W11805 | 2000 | 0.06 | 0.031 | 2 | 0.25 | 0.06 | 4 |
| M402 | >2000 | 0.06 | 0.031 | 2 | 1 | 0.5 | 2 |
| W28752 | 2000 | 0.06 | 0.008 | 8 | 0.5 | <0.002 | >256 |
| M33323 | 2000 | 0.06 | 0.06 | 1 | 0.25 | 0.25 | 1 |
| W64861 | 2000 | 0.125 | 0.06 | 2 | 0.25 | 0.25 | 1 |
| M76297 | 2000 | 0.06 | 0.03 | 2 | 0.25 | 0.125 | 2 |
| E46972 | >2000 | 1 | <0.002 | >512 | 0.25 | <0.008 | >32 |
| M36292 | 2000 | 0.06 | <0.002 | >32 | 0.25 | <0.008 | >32 |
| X7199 | >2000 | 16 | 16 | 1 | 0.25 | 0.125 | >32 |
| NCTC 11168 | 2000 | 0.06 | 0.03 | 2 | 0.25 | 0.06 | 4 |
| MIC90c | 2000 | 16 | 4 | 4 | 256 | 16 | 16 |
| MIC50d | 2000 | 0.25 | 0.06 | 4 | 0.5 | 0.06 | 8 |
The resistance-modulatory activity of (-)-α-pinene in C. jejuni with two clinically important antibiotics (i.e., ciprofloxacin, erythromycin) was tested at the subinhibitory concentration of 125 mg/L (-)-α-pinene. These data are reported as fold-changes (FC) in terms of the decrease in the MICs of the antibiotics when combined with (-)-α-pinene (Table 1). The following criteria were set for the resistance-modulatory activities in terms of the fold-changes: high: ≥32; intermediate: <32 to ≥8; low: <8 to ≥2; and no activity, 1.
The FC differed among the strains, from 1 (i.e., no activity) to >512 (i.e., high activity). When combined with ciprofloxacin, (-)-α-pinene showed strong and intermediate resistance-modulatory activities in 39% (n = 22) and 18% (n = 10) of the strains, respectively. The susceptibility to ciprofloxacin was affected marginally by (-)-α-pinene (i.e., low activity) in 37% (n = 21) of the strains, and not affected at all in 7% (n = 4) of the strains. The antimicrobial activity of erythromycin was enhanced by (-)-α-pinene in the majority of the tested strains. In 46% (n = 26), (-)-α-pinene showed high resistance-modulatory activity; in 13% (n = 7), intermediate, and in 32% (n = 18), low activity. (-)-α-Pinene did not increase the susceptibility to erythromycin in 9% (n = 5) of the tested strains. It was interesting to note that only strains M33323 and W14861 did not show any changes in susceptibility to both ciprofloxacin and erythromycin when combined with (-)-α-pinene.
We then compared the resistance-modulatory activity of (-)-α-pinene for ciprofloxacin and erythromycin with the available antibiotic susceptibility data for a range of antibiotics (i.e., ampicillin, tetracycline, kanamycin, gentamicin, erythromycin, clindamycin, ciprofloxacin, nalidixic acid, norfloxacin), using 37 of the broiler and turkey strains (S2 Table). Here, no significant associations were seen between the susceptibilities to any specific antibiotic and the resistance-modulatory activities of (-)-α-pinene. Thus, these resistance-modulating activities of (-)-α-pinene did not depend on the susceptibility to any of the antibiotics tested.
Campylobacter jejuni quorum sensing is inhibited by (-)-α-pinene in vitro
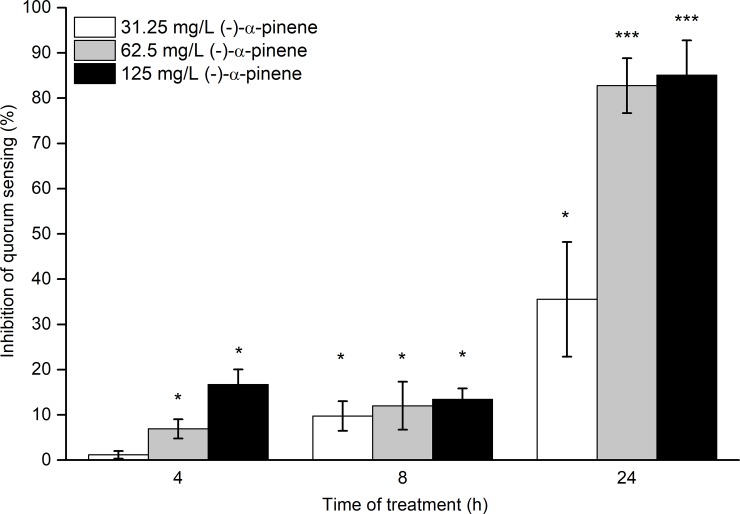
To determine the potential of (-)-α-pinene for inhibition of quorum sensing, C. jejuni NCTC 11168 was treated with three subinhibitory concentrations of (-)-α-pinene (i.e., 31.25, 62.5, 125 mg/L) for 4 h, 8 h, and 24 h. The reductions in the quorum-sensing signaling molecules produced in the treated cultures were calculated and compared to that for the untreated cultures. Inhibition of C. jejuni quorum sensing was seen for all of these samples treated with (-)-α-pinene, regardless of the concentration added, and at all time-points (Fig 1). After 8 h treatment with (-)-α-pinene, the quorum-sensing inhibition was in the same range for all of the (-)-α-pinene concentrations used (10%-13% inhibition; p >0.05). After 4 h and 8 h of treatments, the highest quorum-sensing inhibition by (-)-α-pinene did not exceed 20%. After 24 h of treatment, there was higher quorum-sensing inhibition in all of the samples treated with (-)-α-pinene, compared to the shorter incubation times (p <0.01). The 24-h treatment with 31.25 mg/L (-)-α-pinene resulted in 36% inhibition of quorum sensing, while the higher treatments with 62.5 mg/L and 125 mg/L (-)-α-pinene showed 83% (p = 0.001) and 85% (p <0.001) inhibition, respectively, of quorum sensing compared to the untreated control. These data thus showed concentration-dependent quorum-sensing inhibitory activities of (-)-α-pinene, which was emphasized by the prolonged treatment times.
Fig 1. Time-course of (-)-α-pinene inhibition of quorum sensing in C. jejuni NCTC11168.
Data are means ±standard deviation of relative reduction of quorum sensing signal (as luminescence of V. harveyi BB170) in the treated C. jejuni cell free supernatants (CFS) versus the untreated C. jejuni CFS, calculated from three replicates. * p <0.05, *** p ≤0.001.
(-)-α-Pinene does not reduce fluoroquinolone resistance development when added to enrofloxacin in broiler chickens
Although the use of enrofloxacin is prohibited in poultry production in the USA due to its rapid induction of fluoroquinolone resistance in C. jejuni [25], it is still used in veterinary medicine in the European Union [26]. Based on the obvious activity of (-)-α-pinene in modulating fluoroquinolone resistance (Table 1), we investigated whether a subinhibitory concentration of (-)-α-pinene can delay development of fluoroquinolone resistance of C. jejuni in the chicken model, with the addition of (-)-α-pinene and enrofloxacin into the broilers water supply. Here, the (-)-α-pinene concentration used was doubled (but still subinhibitory) compared to the in vitro studies, to ensure sufficient concentration in the intestinal tract in chickens.
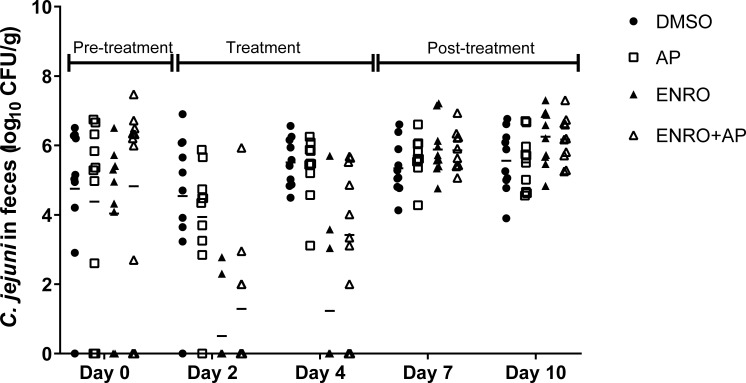
As treatments with enrofloxacin are known to induce fluoroquinolone resistance in Campylobacter [25], these analyses included enumeration of both the total numbers of Campylobacter and the proportion of ciprofloxacin-resistant Campylobacter in each broiler chicken fecal sample. The results showed that all of the Campylobacter isolates (100%), in all of the colonized birds treated with enrofloxacin developed fluoroquinolone resistance at all of the sampling times after the treatment had begun, regardless of inclusion of (-)-α-pinene in the drinking water. In contrast, Campylobacter isolates from the broiler chicken groups treated with DMSO and (-)-α-pinene alone (i.e., no enrofloxacin treatment) did not develop any resistance (0%) to fluoroquinolones at any point during the experiment. During these treatments, the mean Campylobacter numbers for (-)-α-pinene alone (4.37 log10 CFU/g) tended to be lower than that of the DMSO control (5.05 log10 CFU/g); however, there was wide variability within each of these treatment groups, so these data did not reach statistical significance (Fig 2).
Fig 2. Time-courses of the effects of (-)-α-pinene in the water supply of broiler chickens inoculated with C. jejuni NCTC 11168 3 days before (-)-α-pinene treatment (started day 0).
Data are C. jejuni counts (log10 CFU/g feces) in cloacal swabs from individual broiler chickens in the treatment groups: DMSO, no treatment control; AP, 250 mg/L (-)-α-pinene; ENRO, 50 mg/L enrofloxacin; ENRO+AP, combination of 50 mg/L enrofloxacin and 250 mg/L (-)-α-pinene. The detection limit of the culture method was approximately 2 log10 CFU/g feces, and the means are indicated by the horizontal lines.
These data showed that (-)-α-pinene did not have any resistance-modulatory activity in vivo, nor did it reduce the rapid development of fluoroquinolone resistance of C. jejuni after exposure to enrofloxacin. However, addition of (-)-α-pinene alone to the broiler chicken water supply reduced the average numbers of Campylobacter in the already colonized broiler chickens, although this did not reach statistical significance. Of note, with the (-)-α-pinene here added to the water supply, the amount of (-)-α-pinene ingested by each broiler chicken could not be controlled, which is likely to explain the large variations in these data.
Reduction of Campylobacter in broiler chicken cecum after direct gavage with (-)-α-pinene
To better evaluate whether (-)-α-pinene can modulate C. jejuni colonization in the same broiler chicken experiment described above, chickens colonized by FQ-S Campylobacter and FQ-R Campylobacter were treated with (-)-α-pinene by direct oral gavage. With this treatment, the amount of (-)-α-pinene consumed by each animal was better controlled.
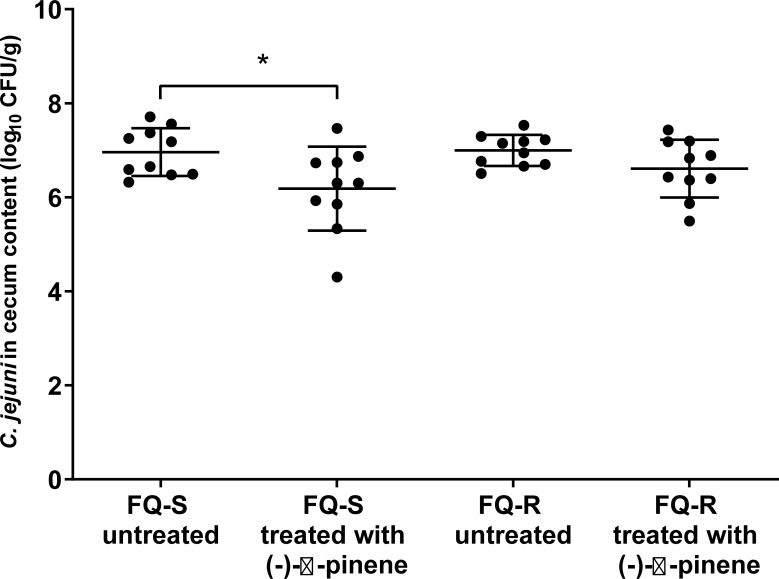
These data indicated that there were significantly lower C. jejuni counts in the broiler chickens colonized with FQ-S C. jejuni when treated with (-)-α-pinene (FQ-S treated), with a reduction of 0.8 log10 CFU/g unit (p = 0.028; Fig 3) compared to the untreated group (FQ-S untreated). No significant differences were seen between the nontreated and (-)-α-pinene–treated groups that were colonized with FQ-R C. jejuni (FQ-R untreated, FQ-R treated), although a slight mean reduction from 7.0 to 6.6 log10 CFU/g cecum content (p = 0.095; Fig 3) was observed in the treated group compared to untreated. These data show that (-)-α-pinene reduced colonization of the FQ-S C. jejuni in broiler chickens when administered by direct gavage, but it had no significant effect on FQ-R C. jejuni.
Fig 3. Campylobacter counts (log10 CFU/g) in the cecum content of broiler chickens following treatments without and with a subinhibitory (-)-α-pinene concentration via direct gavage for 3 consecutive days (0.4 mL, 250 mg/L, daily).
The samples were collected 24 h after the last treatment, and cultured for Campylobacter. Data are means ±standard deviation from individual broiler chickens that were colonized with either FQ-S or FQ-R C. jejuni prior to the treatment. FQ-S/FQ-R untreated, fluoroquinolone-sensitive/resistant C. jejuni (controls); FQ-S/FQ-R treated, fluoroquinolone-sensitive/resistant C. jejuni plus treatment with (-)-α-pinene. *p <0.05.
Discussion
The effects of (-)-α-pinene have been seen to be versatile, from antioxidative to cell protective [27], and to anti-cancer [28], with only weak antimicrobial activities reported previously [29]. In the present study, we showed that the concentrations of (-)-α-pinene needed for antimicrobial effects against C. jejuni were high, and were similar for all of the strains (n = 57) tested regardless of host origin (e.g., chicken, turkey, human) and susceptibility profiles to a range of antibiotics. These data confirm the observations of Kovač et al. [21], where (-)-α-pinene also showed weak antimicrobial activity against nine C. jejuni strains tested. We also confirmed earlier indications of the strong in-vitro resistance-modulatory activity of (-)-α-pinene for clinically important antibiotics (i.e., ciprofloxacin and erythromycin) against this large and diverse collection of C. jejuni strains [21]. These data suggest that (-)-α-pinene may have potential as an adjunctive therapy, in various hosts, to increase the efficacy of macrolides and fluoroquinolones against C. jejuni resistant to these antimicrobials.
Subinhibitory concentrations of (-)-α-pinene have been shown to evoke diverse transcriptional responses in C. jejuni, although the main mechanisms of its resistance-modulatory activity appear to be inhibition of the CmeABC efflux pump and induction of membrane damage [21]. Similarly, Oh and Jeon [30] reported that different monoterpenes can show synergistic effects when combined with ciprofloxacin or erythromycin, due to the modulation of antibiotic influx and efflux in C. jejuni.
Correct functioning of the efflux pumps, such as the CmeABC multidrug efflux pump (the major efflux pump in C. jejuni), is needed not only to enhance bacterial resistance to antibiotics, but to also increase bacterial resistance to bile salts, and thus to facilitate the colonization of the gastrointestinal tract in animals and humans by C. jejuni [31]. This suggests that after exposure to an efflux pump inhibitor (e.g., pinene), C. jejuni sensitivity to antimicrobials can increase, its virulence can decrease, and its colonization can become impaired [32]. We therefore tested the potential of (-)-α-pinene to modulate C. jejuni resistance to fluoroquinolones, to attenuate the development of resistance to fluoroquinolones and impair C. jejuni colonization in vivo in a broiler chicken model.
In the broiler chicken model, (-)-α-pinene did not act as a modulator of C. jejuni resistance when administered together with enrofloxacin, nor did it change the development of resistance in C. jejuni to fluoroquinolones when NCTC 11168 was used as the model strain (Fig 2). Due to the ever-growing antibiotic resistance of C. jejuni [33], the concept of a natural compound that can hinder resistance development or have a synergistic activity with antibiotics would open new and attractive opportunities for combating antibiotic resistance. However, the reality is often more complex, as compounds that demonstrate activity in vitro do not always maintain the same activity in vivo, as additional factors, that cannot be controlled, are introduced.
It has been suggested that a reduction of Campylobacter in the chicken intestine by 1 log10 CFU can reduce the public health risk by 50% to 90%, and a 2 log10 CFU reduction can reduce the risk by >90% [8,34,35]. This can be achieved with natural compounds [10]. Supplementation of poultry feed and water with natural compounds has been shown to reduce Campylobacters in poultry, and in some cases, this has improved animal health and yield as well. For example, feed supplementation with carvacrol and thymol at inhibitory concentrations has shown significant reduction of Campylobacter and Salmonella colonization [36] and growth enhancement in broiler chickens [37]. Grilli et al. [9] lowered Campylobacter counts in the broiler chicken cecum by 1 log10 CFU/g with feed additives of essential oils at 5000 mg/L, which represented an antimicrobial concentration. In the present study, a 0.8 log10 CFU reduction in Campylobacter counts in the broiler chicken cecum was obtained using a lower, and subinhibitory, concentration of (-)-α-pinene (250 mg/L) (Fig 3). This suggests that even lower concentrations of natural compounds, where bioactivity is still observed, can contribute to Campylobacter control in broilers, thus reducing the amount of treatment needed. The C. jejuni colonization reduction by (-)-α-pinene in a subinhibitory concentration can be explained by its efflux pump inhibitory [21] and quorum sensing inhibitory (Fig 1) activities exhibited at sub-inhibitory concentrations in vitro. In C. jejuni, both intact efflux pump activity [31] and quorum sensing [17] are important for colonization of the host, thus can the inhibition of these systems, by an external source such as (-)-α-pinene, contribute to C. jejuni control.
When a substance is introduced into an animal host to promote the reduction of pathogens, it is important to consider both the host response [38] and the response of the pathogen in question to the substance. An important factor for C. jejuni host colonization is cell-to-cell communication, or quorum sensing [13,39]. Disruption of the quorum-sensing system of C. jejuni interferes with its motility and autoagglutination, its production of cytolethal distending toxin, and its host colonization [17,18].
Essential oils and their constituents, such as cinnamaldehyde and cinnamon bark essential oil, can inhibit quorum sensing [40]. Furthermore, in C. jejuni, quorum-sensing inhibitors such as epigallocatechin gallate and extracts of Euodia ruticarpa can reduce motility and biofilm formation [19,20], although the potential in-vivo effects of these on C. jejuni colonization are not known. Brackman et al. [40] demonstrated 65% inhibition of quorum sensing by cinnamaldehyde in Vibrio spp. This was lower compared to that of (-)-α-pinene against C. jejuni in the present study, where the inhibition was >80% when treated with subinhibitory (-)-α-pinene (Fig 1). The quorum-sensing inhibition in Vibrio spp. resulted in down-regulation of virulence factors and weaker cytotoxicity toward Caenorhabditis elegans [40], which indicated that cinnamaldehyde can modulate the pathogen–host interactions. In the present study, we observed changes in pathogen–host interactions in terms of C. jejuni colonization in broiler chicken cecum content after treatment with (-)-α-pinene by direct gavage (Fig 3).
Although the anti-Campylobacter activity of (-)-α-pinene under in vitro conditions was similar against both ciprofloxacin-susceptible and -resistant C. jejuni strains (Table 1), the reduction of FQ-R Campylobacters in the broiler chickens treated with (-)-α-pinene did not reach significance. These findings can be explained by the observations of Luo et al. [25], who showed that FQ-R C. jejuni strains have better fitness in vivo compared to FQ-S strains, and are thus more tolerant to stressors, such as (-)-α-pinene treatment.
The present study stresses the importance of improving the control of Campylobacter in poultry production so as to reduce the public health risk. It also exposes the problem of development of antibiotic resistance in poultry production and the difficulties in the management of human foodborne infections by antibiotic-resistant C. jejuni. Further investigations into the mechanisms of action of natural compounds that might be used for manipulation of pathogen–host interactions and reduction of host colonization in vivo is highly warranted. Furthermore, it is important to consider the effects and mechanisms of action of such compounds at subinhibitory concentrations. This can allow improved prediction of their activity in live systems (i.e., in the animal), where it can be difficult to control the exact amounts that are ingested. For example, for Pseudomonas aeruginosa, inhibitors of quorum sensing have been shown to mitigate infections even without showing strong antibacterial effects [41,42]. This makes the inhibitory effects on quorum sensing an important aspect when searching for new anti-infectious compounds.
For (-)-α-pinene, in C. jejuni it was shown previously to evoke stress and heat-shock responses, to inhibit the multidrug efflux pumps, and to increase membrane permeability [21]. The present study further indicates that it can inhibit quorum sensing in C. jejuni. It is likely that all of these in vitro activities of (-)-α-pinene might have contributed to its in vivo effects observed in the current study on colonization by fluoroquinolone-susceptible C. jejuni in chickens.
Conclusions
The findings from this study indicate that despite showing poor antimicrobial activity against Campylobacter, even at high concentrations, (-)-α-pinene can modulate C. jejuni quorum sensing and colonization of broiler hosts when administered at subinhibitory concentrations. Further in vivo studies are warranted to better evaluate the effects of (-)-α-pinene on colonization by Campylobacter, including different species and strains with different antimicrobial resistance profiles (e.g., erythromycin resistance), as well as various treatment regimens (e.g., therapeutic vs. preventive).
Supporting information
Data are means ±standard deviation. Framed time points (i.e. 9 h) are those best suited for further evaluation of quorum sensing inhibition.
(DOCX)
Data are means ±standard deviation for luminescent signal (relative luminescent units, RLU). Framed time point is that used in the calculation of quorum sensing inhibition.
(DOCX)
(DOCX)
(DOCX)
Data Availability
"All data for the manuscript is available in Figshare, specifically: Fig 1. 10.6084/m9.figshare.8234153https://figshare.com/s/23ec3f570d93069ae7fbFig 2. 10.6084/m9.figshare.8234192https://figshare.com/s/aa309738e3cdbe99717eFig 3. 10.6084/m9.figshare.8234189https://figshare.com/s/55589de9346a17749cd5S1 and S2 Figs 10.6084/m9.figshare.8234156https://figshare.com/s/2559324ea098365d3e8cS1 and S2 Tables. 10.6084/m9.figshare.8234159https://figshare.com/s/9a452dc1b939e7d9de30".
Funding Statement
This study was financed by the Slovenian-American bilateral projects BI-SLO-USA 2014/2015 and 2018/19 and P4-0116 funded by the Slovenian Research Agency (ARRS) awarded to SSM. http://www.arrs.si/en/index.asp JK was supported by the USDA National Institute of Food and Hatch Appropriations under Project #PEN04646 and Accession #1015787. https://nifa.usda.gov/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin Microbiol Rev. 2015; 28(3): 687–720. 10.1128/CMR.00006-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EFSA and ECDC. European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018;16(12): e05500 10.2903/j.efsa.2018.5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellyn P. Marder MPH. Preliminary incidence and trends of infections with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. Sites, 2006–2017. MMWR Morb Mortal Wkly Rep. 2018;67 10.15585/mmwr.mm6711a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovač J, Stessl B, Čadež N, Gruntar I, Cimerman M, Stingl K, et al. Population structure and attribution of human clinical Campylobacter jejuni isolates from central Europe to livestock and environmental sources. Zoonoses Public Health. 2018;65(1):51–8. 10.1111/zph.12366 [DOI] [PubMed] [Google Scholar]
- 5.Sahin O, Kassem II, Shen Z, Lin J, Rajashekara G, Zhang Q. Campylobacter in Poultry: ecology and potential interventions. Avian Dis. 2015;59(2):185–200. 10.1637/11072-032315-Review [DOI] [PubMed] [Google Scholar]
- 6.Dittoe DK, Ricke SC, Kiess AS. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front Vet Sci. 2018;5 10.3389/fvets.2018.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riedel CT, Brøndsted L, Rosenquist H, Haxgart SN, Christensen BB. Chemical decontamination of Campylobacter jejuni on chicken skin and meat. J Food Prot. 2009. June;72(6): 1173–80. 10.4315/0362-028x-72.6.1173 [DOI] [PubMed] [Google Scholar]
- 8.BIOHAZ. EFSA Panel on Biological Hazards. Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011;9(4). 10.2903/j.efsa.2011.2105 [DOI] [Google Scholar]
- 9.Grilli E, Vitari F, Domeneghini C, Palmonari A, Tosi G, Fantinati P, et al. Development of a feed additive to reduce caecal Campylobacter jejuni in broilers at slaughter age: from in vitro to in vivo, a proof of concept. J Appl Microbiol. 2013;114(2): 308–17. 10.1111/jam.12053 [DOI] [PubMed] [Google Scholar]
- 10.Kelly C, Gundogdu O, Pircalabioru G, Cean A, Scates P, Linton M, et al. The in vitro and in vivo effect of carvacrol in preventing Campylobacter infection, colonization and in improving productivity of chicken broilers. Foodborne Pathog Dis. 2017;14(6): 341–9. 10.1089/fpd.2016.2265 [DOI] [PubMed] [Google Scholar]
- 11.LaSarre B, Federle MJ. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev. 2013;77(1): 73–111. 10.1128/MMBR.00046-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reuter K, Steinbach A, Helms V. Interfering with bacterial quorum sensing. Perspect Medicin Chem. 2016; 8, 1 10.4137/PMC.S13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elvers KT, Park SF. Quorum sensing in Campylobacter jejuni: detection of a luxS encoded signalling molecule. Microbiology. 2002. May;148(Pt 5): 1475–81. 10.1099/00221287-148-5-1475 [DOI] [PubMed] [Google Scholar]
- 14.Golden NJ, Acheson DWK. Identification of motility and autoagglutination Campylobacter jejuni mutants by random transposon mutagenesis. Infect Immun. 2002; 70 (4): 1761–71. 10.1128/IAI.70.4.1761-1771.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon B, Itoh K, Misawa N, Ryu S. Effects of quorum sensing on flaA transcription and autoagglutination in Campylobacter jejuni. Microbiol Immunol. 2003;47(11): 833–9. 10.1111/j.1348-0421.2003.tb03449.x [DOI] [PubMed] [Google Scholar]
- 16.Holmes K, Tavender TJ, Winzer K, Wells JM, Hardie KR. AI-2 does not function as a quorum sensing molecule in Campylobacter jejuni during exponential growth in vitro. BMC Microbiol. 2009; 9 (1): 214 10.1186/1471-2180-9-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quiñones B, Miller WG, Bates AH, Mandrell RE. Autoinducer-2 Production in Campylobacter jejuni contributes to chicken colonization. Appl Environ Microbiol. 2009;75(1): 281–5. 10.1128/AEM.01803-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plummer P, Sahin O, Burrough E, Sippy R, Mou K, Rabenold J, et al. Critical role of LuxS in the virulence of Campylobacter jejuni in a guinea pig model of abortion. Infect Immun. 2012. February;80(2): 585–93. 10.1128/IAI.05766-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezek K, Kurinčič M, Knauder E, Klančnik A, Raspor P, Bucar F, et al. Attenuation of adhesion, biofilm formation and quorum sensing of Campylobacter jejuni by Euodia ruticarpa. Phytother Res. 2016. September;30(9): 1527–32. 10.1002/ptr.5658 [DOI] [PubMed] [Google Scholar]
- 20.Castillo S, Heredia N, García S. 2(5H)-Furanone, epigallocatechin gallate, and a citric-based disinfectant disturb quorum-sensing activity and reduce motility and biofilm formation of Campylobacter jejuni. Folia Microbiol. 2015. January 1;60(1): 89–95. [DOI] [PubMed] [Google Scholar]
- 21.Kovač J, Šimunović K, Wu Z, Klančnik A, Bucar F, Zhang Q, et al. Antibiotic resistance modulation and modes of action of (-)-α-pinene in Campylobacter jejuni. PLOS ONE. 2015. April 1;10(4): e0122871 10.1371/journal.pone.0122871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luangtongkum T, Morishita TY, Ison AJ, Huang S, McDermott PF, Zhang Q. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Appl Environ Microbiol. 2006. May 1;72(5): 3600–7. 10.1128/AEM.72.5.3600-3607.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassler BL, Greenberg EP, Stevens AM. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol. 1997. June;179(12): 4043–5. 10.1128/jb.179.12.4043-4045.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz S, Silley P, Simjee S, Woodford N, van Duijkeren E, Johnson AP, et al. Editorial: Assessing the antimicrobial susceptibility of bacteria obtained from animals. J Antimicrob Chemother. 2010. April 1;65(4):601–4. 10.1093/jac/dkq037 [DOI] [PubMed] [Google Scholar]
- 25.Luo N, Pereira S, Sahin O, Lin J, Huang S, Michel L, et al. Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. PNAS. 2005. January 18;102(3): 541–6. 10.1073/pnas.0408966102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Medicines Agency (EMA). Questions and answers on use of enrofloxacin-containing veterinary medicines administered via drinking water to chickens and turkeys. Follow-up assessment after the referral under Article 35 of Directive 2001/82/EC (EMEA/V/A/089). EMA/184512/2018. 2018. URL: https://www.ema.europa.eu/en/documents/referral/enrofloxacin-article-35-referral-questions-answers-use-enrofloxacin-containing-veterinary-medicines_en.pdf
- 27.Bouzenna H, Hfaiedh N, Giroux-Metges M-A, Elfeki A, Talarmin H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed Pharmacother. 2017. September 1;93: 961–8. 10.1016/j.biopha.2017.06.031 [DOI] [PubMed] [Google Scholar]
- 28.Aydin E, Türkez H, Geyikoğlu F. Antioxidative, anticancer and genotoxic properties of α-pinene on N2a neuroblastoma cells. Biologia. 2013;68(5): 1004–1009. [Google Scholar]
- 29.Silva ACR da Lopes PM, Azevedo MMB de Costa DCM, Alviano CS Alviano DS. Biological activities of a-pinene and β-pinene enantiomers. Molecules. 2012;17(6): 6305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh E, Jeon B. Synergistic anti-Campylobacter jejuni activity of fluoroquinolone and macrolide antibiotics with phenolic compounds. Front Microbiol. 2015;6 10.3389/fmicb.2015.01129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Sahin O, Michel LO, Zhang Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect Immun. 2003. August 1;71(8): 4250–9. 10.1128/IAI.71.8.4250-4259.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinn T, Bolla J-M, Pagès J-M, Fanning S. Antibiotic-resistant Campylobacter: could efflux pump inhibitors control infection? J Antimicrob Chemother. 2007. June 1;59(6):1230–6. 10.1093/jac/dkl470 [DOI] [PubMed] [Google Scholar]
- 33.EFSA and ECDC. European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA J. 2018; 16 10.2903/j.efsa.2018.5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robyn J, Rasschaert G, Pasmans F, Heyndrickx M. Thermotolerant Campylobacter during broiler rearing: risk factors and intervention. Compr Rev Food Sci Food Saf. 2015; 14, 81–105. 10.1111/1541-4337.12124 [DOI] [PubMed] [Google Scholar]
- 35.Meunier M, Guyard‐Nicodème M, Dory D, Chemaly M. Control strategies against Campylobacter at the poultry production level: biosecurity measures, feed additives and vaccination. J Appl Microbiol. 2016. 120: 1139–1173. [DOI] [PubMed] [Google Scholar]
- 36.Alali WQ, Hofacre CL, Mathis GF, Faltys G. Effect of essential oil compound on shedding and colonization of Salmonella enterica serovar Heidelberg in broilers. Poult Sci. 2013. March;92(3): 836–41. 10.3382/ps.2012-02783 [DOI] [PubMed] [Google Scholar]
- 37.Tiihonen K, Kettunen H, Bento MHL, Saarinen M, Lahtinen S, Ouwehand AC, et al. The effect of feeding essential oils on broiler performance and gut microbiota. Br Poult Sci. 2010. June 1;51(3): 381–92. 10.1080/00071668.2010.496446 [DOI] [PubMed] [Google Scholar]
- 38.Reyer H, Zentek J, Männer K, Youssef IMI, Aumiller T, Weghuber J, et al. Possible molecular mechanisms by which an essential oil blend from star anise, rosemary, thyme, and oregano and saponins increase the performance and ileal protein digestibility of growing broilers. J Agric Food Chem. 2017. August 16;65(32): 6821–30. 10.1021/acs.jafc.7b01925 [DOI] [PubMed] [Google Scholar]
- 39.Plummer PJ. LuxS and quorum-sensing in Campylobacter. Front Cell Infect Microbiol. 2012;2: 22 10.3389/fcimb.2012.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brackman G, Celen S, Hillaert U, Calenbergh SV, Cos P, Maes L, et al. Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp. PLOS ONE. 2011. January 13;6(1): e16084 10.1371/journal.pone.0016084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann N, Lee B, Hentzer M, Rasmussen TB, Song Z, Johansen HK, et al. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(-/-) mice. Antimicrob Agents Chemother. 2007. October;51(10): 3677–87. 10.1128/AAC.01011-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harjai K, Kumar R, Singh S. Garlic blocks quorum sensing and attenuates the virulence of Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2010. March 1;58(2): 161–8. 10.1111/j.1574-695X.2009.00614.x [DOI] [PubMed] [Google Scholar]