Abstract
Background
Lower respiratory tract infections in the first years of life are associated with later asthma, and this observation has led to a focus on the potential causal role of specific respiratory viruses, such as rhinoviruses and respiratory syncytial virus, in asthma development. However, many respiratory viruses and bacteria trigger similar respiratory symptoms and it is possible that the important risk factors for asthma are the underlying susceptibility to infection and the exaggerated reaction to such triggers rather than the particular triggering agent.
Objective
We sought to study the association between specific infections in early life and development of asthma later in childhood.
Methods
Three hundred thirteen children were followed prospectively in the Copenhagen Prospective Studies of Asthma in Childhood2000 high-risk birth cohort. Nine respiratory virus types (respiratory syncytial virus, rhinoviruses, other picornaviruses, coronaviruses 229E and OC43, parainfluenza viruses 1-3, influenza viruses AH1, AH3, and B, human metapneumovirus, adenoviruses, and bocavirus) and 3 pathogenic airway bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis) were identified in airway secretions sampled during episodes of troublesome lung symptoms in the first 3 years of life. Asthma was determined by age 7 years.
Results
In unadjusted analyses, all viruses and pathogenic bacteria identified during episodes of troublesome lung symptoms were associated with increased risk of asthma by age 7 years with similar odds ratios for all viruses and pathogenic bacteria. After adjustment for the frequency of respiratory episodes, the particular triggers were no longer associated with asthma.
Conclusion
The number of respiratory episodes in the first years of life, but not the particular viral trigger, was associated with later asthma development. This suggests that future research should focus on the susceptibility and exaggerated response to lower respiratory tract infections in general rather than on the specific triggering agent.
Key words: Child, asthma, virus, bacteria, respiratory tract infection
Abbreviations used: COPSAC, Copenhagen Prospective Studies on Asthma in Childhood; OR, Odds ratio; RSV, Respiratory syncytial virus
It is a solid clinical observation that asthmatic children experience exacerbations in relation to the common cold, and viruses are more commonly found during asthma exacerbations than in the asymptomatic state.1 In addition, viral respiratory tract infections in the first years of life have been suspected to initiate a chronic disease trajectory leading to recurrent wheeze and asthma later in childhood, and this putative causal role in asthma development has stimulated a strong focus on the role and pathogenic mechanisms of specific viral agents.2, 3, 4, 5, 6, 7 With respect to long-term consequences on asthma risk, there has previously been a particular focus on respiratory syncytial virus (RSV) infection as a cause of instigating abnormal pulmonary function, wheezing, and asthma in childhood,2, 3, 4, 5, 6 although recently there has been a focus on the role of human rhinovirus infection in asthma development.7
However, several viral and bacterial agents can elicit similar asthmatic symptoms in young children8 and trigger asthma exacerbations in school-aged children.1 Furthermore, it is clear that individual susceptibility plays an important role in the response to respiratory tract infections. Asthmatic patients have an altered epithelial immune response to rhinoviruses9 and they are more susceptible to lower respiratory tract infections in relation to these.10 Also, hyperreactive airways are a hallmark of asthmatic children and we and others have shown that children who later have asthma display increased bronchial responsiveness before any lower respiratory tract illness.11, 12
Therefore, it is possible that the important risk factors for asthma are the pre-existing susceptibility and inflammatory response to respiratory tract infections in general rather than the specific triggering agent. This is a key question for directing future research: if specific viruses confer a specific risk, then virus-specific mechanisms should be the focus. Alternatively, future research should focus on the underlying susceptibility and hyperreactivity to infections in general.
We analyzed the association between specific viruses and pathogenic bacteria detected during episodes of troublesome lung symptoms in early life and asthma status at age 7 years in the Copenhagen Prospective Studies on Asthma in Childhood2000 (COPSAC2000) birth cohort.
Methods
Study subjects
COPSAC2000 is a prospective clinical birth cohort study conducted in accordance with the Declaration of Helsinki and approved by the Copenhagen and Frederiksberg Ethics Committee (KF 01-289/96 and KF 11-107/02) and the Danish Data Protection Agency (2008-41-1754). From 1998 to 2001, the study enrolled 411 neonates at 1 month of age who were born to mothers with a history of asthma, excluding children born before 36 weeks of gestation and anyone suspected of chronic diseases or lung symptoms before inclusion, as previously described in detail.13, 14
Respiratory tract infections in early life
Significant troublesome lung symptoms were recorded in daily diaries from 1 month of age until age 3 years, as recently described and analyzed in detail.15 Parents were taught to record their child's symptoms with emphasis on the lower airways. Troublesome lung symptoms were translated to the parents as any symptom significantly affecting the child's breathing, such as noisy breathing (wheeze or whistling sounds), shortness of breath, or persistent troublesome cough affecting sleep or the activity of the child. Daily symptoms were recorded as composite dichotomized scores (yes/no) each day (ie, the parents were taught to make a global assessment). The complexity of symptoms was detailed in a book that was given to the parents (www.copsac.com/content/literature-parents). The diary cards were collected and reviewed by physicians at the half-yearly clinic visits.
Parents were encouraged to bring the children to the research clinic for an acute visit after each 3-day episode of troublesome lung symptoms recorded in the diary. The research physicians examined the children at each acute visit in accordance with predefined standard procedures. The children received a standardized physical examination, including lung auscultation. Physicians performed nasopharyngeal and hypopharyngeal aspirates under aseptic conditions, with 2 separate soft suction catheters passed into the upper rhinopharynx and hypopharynx for analysis of viruses and bacteria, respectively.8
A respiratory episode defined as 3 consecutive days of troublesome lung symptoms with available viral and/or bacterial aspirates was the predefined base unit of symptom burden and the number of such episodes was summarized as an indicator reflecting respiratory symptom burden.15
Viruses were detected by using PCR analysis of nasopharyngeal aspirate samples for RSV; rhinoviruses; other picornaviruses; coronaviruses 229E and OC43; parainfluenza viruses 1 to 3, influenza viruses AH1, AH3, and B; human metapneumovirus; adenoviruses; and bocavirus, as previously described.8
Pathogenic airway bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis) were identified according to standard procedures, as previously described,8 and analyzed as the dichotomized measure of at least 1 bacterium detected.
Sensitization
Sensitization was determined based on skin prick test responses, specific IgE levels, or both, to any allergens at 6 years of age. Specific IgE levels were measured by means of ImmunoCAP (Thermo Fischer Scientific Inc, Waltham, Mass)16 against cat, dog, horse, birch, timothy grass, Dermatophagoides pteronyssinus, Dermatophagoides farinae, mugwort, molds (Paspalum notatum, Cladosporium herbarium, Aspergillus fumigatus, and Alternaria alternata), egg, milk, peanut, cod, wheat, and soya bean allergens. Skin prick tests were performed with cat, dog, horse, birch, timothy grass, D pteronyssinus, D farinae, mugwort, A alternata, C herbarium, egg, milk, peanut, cod, wheat, rye, beef, pork, and soya bean allergen extracts (ALK, Hørsholm, Denmark), as well as raw egg and milk.17 Sensitization was defined as having any positive skin prick test response (>3 mm) or any specific IgE level of greater than 3.5 kU/L.
Infant lung function
Infant lung function was measured at 1 month of age based on the rapid raised-volume thoracoabdominal compression technique, as previously described.11 Forced expiratory flow at 50% of functional vital capacity was used in this study because this was the measure previously shown to be most closely associated with later asthma.11
Asthma at age 7 years
Asthma at age 7 years was diagnosed by the physicians at the research unit in accordance with international guidelines. The burden of recurrent symptoms was quantified from an algorithm of 5 episodes of troublesome lung symptoms, as defined above, within 6 months and need for short-acting β2-agonists, as previously described in detail.13, 15 The quality of symptoms was judged by the study clinician to be typical of asthma including exercise-induced symptoms, prolonged nocturnal cough, persistent cough outside common cold, and symptoms causing awakening at night. Furthermore, the diagnosis required symptom improvement during a 3-month trial of inhaled corticosteroids and relapse when this medication was stopped.
Confounders
Possible confounding effects were evaluated for selected risk factors with suspected relevance to risk of childhood asthma: 17q21 locus (ORMDL3) 18 and filaggrin genotypes,19 mother's level of education, nicotine in hair by 12 months, and father's asthma.
Statistical analyses
Distribution of risk factors in the study population and in children excluded from the analysis was compared by using the χ2 test for categorical variables and the t test for continuous variables. Nicotine in hair was log-transformed to obtain normal distribution.
Children with a positive identification of a particular virus or bacteria during an infection were compared with children who never had a positive identification of this specific pathogen, either because their aspirates were never positive for that particular pathogen or because they were never ill enough to have nasopharyngeal suction performed in the first 3 years of life. Associations between each specific virus or bacteria and asthma at age 7 years were investigated with logistic regression. Subsequently, we adjusted for the child's total number of respiratory episodes with an available sample for virus or bacteria to obtain the specific trigger effect adjusted for the total burden of respiratory episodes.
Possible interaction with allergic sensitization and infant lung function was investigated by including the interaction term in the regression analysis.
A significance level of .05 was used in all types of analyses, and all analyses were conducted with SAS, version 9.3, software for Windows (SAS Institute, Cary, NC).
Results
Study base
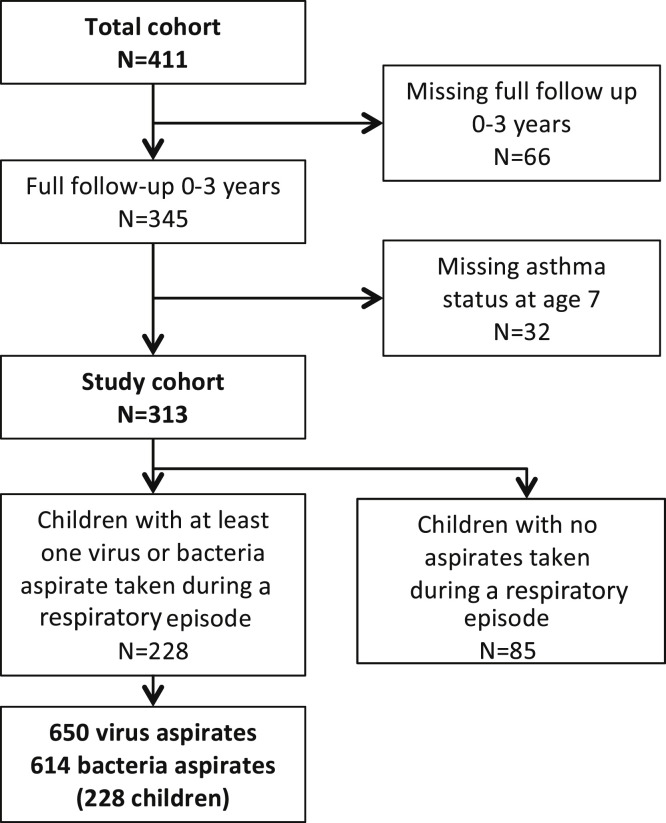
The COPSAC2000 birth cohort included 411 newborns. This study analyzed those 313 children with complete follow-up in the first 3 years of life (66 children missing) and known asthma status at 7 years of age (32 children missing, Fig 1 ).
Fig 1.
Flow chart for the study population.
Children in the study group and excluded children did not differ with respect to 17q21 locus and filaggrin genotypes, mother's level of education or nicotine in hair by 12 months, but father's asthma was more prevalent in the study group than the excluded group (20% vs 7%, P = .0022).
Symptom burden
The majority of children had between 1 and 5 respiratory episodes; 10% never reported troublesome lung symptoms in their diary cards, and 30% had episodes of troublesome lung symptoms in the diary card but were not seen at the clinic and hence had no virus or bacteria aspirate taken. These children were assumed not to have had any clinically significant episodes of troublesome lung symptoms. In total, 228 of the 313 children had at least 1 respiratory episode fulfilling the definition of 3 consecutive days of troublesome lung symptoms with a virus or bacteria aspirate taken.
Prevalence of viruses and bacteria
A total of 650 viral aspirates were collected. Viruses were identified in 423 (65%) of 650 samples. Rhinoviruses were the most frequent and found in 147 (23%) samples, followed by RSV in 124 (19%) samples and coronaviruses in 79 (12%) samples. One hundred eighty-four children had at least 1 identifiable virus, and 106 had several viruses, some on several occasions.
A total of 614 bacterial aspirates were collected. Bacteria were identified in 533 (87%) of these. S pneumoniae was found in 295 (48%) samples, H influenzae was found in 275 (45%) samples, and M catarrhalis was found in 304 (50%) samples. Two hundred six children had at least 1 identifiable pathogenic bacteria, and 132 children had several identifiable pathogenic bacteria.
Asthma prevalence
Asthma prevalence by age 7 years was 15% (46/313). Thirty percent of the children with asthma and 17% of the children without asthma were sensitized to 1 or more allergens at 6 years of age (P = .052).
Risk of asthma in relation to respiratory tract infections
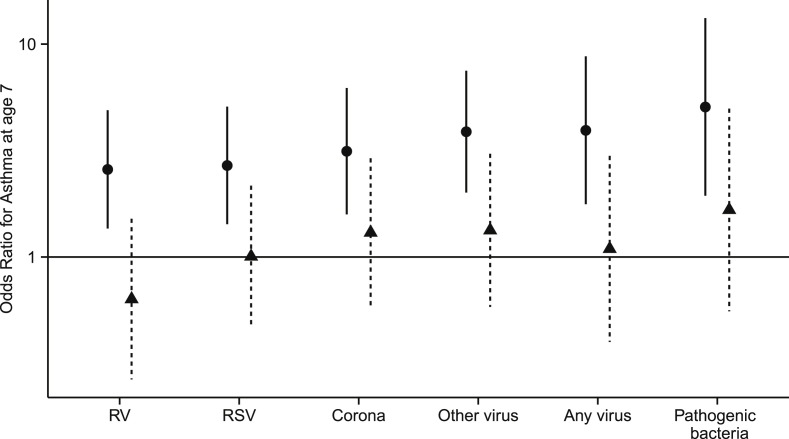
Simple logistic regression analyses showed that children with rhinovirus infections were at significantly greater risk of asthma at age 7 years compared with subjects never having rhinovirus infections (odds ratio [OR], 2.58 [95% CI, 1.36-4.89]; P = .0037). The risk of asthma was similarly increased in children with RSV (OR, 2.69 [95% CI, 1.43-5.09]; P = .0023); coronavirus (OR, 3.14 [95% CI, 1.59-6.22]; P = .0010), other viruses (OR, 3.88 [95% CI, 2.01-7.49]; P < .0001), or pathogenic bacteria present (OR, 5.07 [95% CI, 1.94-13.25]; P < .0001) (Table I ). Having a respiratory episode without any virus detected was also a significant risk factor for asthma at age 7 years (OR, 3.62 [95% CI, 1.82-7.20]; P =.0002; data not shown). Also, the simple number of respiratory episodes per child was significantly associated with asthma (OR, 1.43 [95% CI, 1.26-1.61]; P < .0001; Table I).
Table I.
Risk of asthma by age 7 years in relation to lower respiratory tract infections within the first 3 years of life
| No. of children with virus/no. of children with no virus | Unadjusted OR (95% CI); P value | Adjusted for the total no. of respiratory episodes, OR (95% CI); P value | |
|---|---|---|---|
| Rhinovirus | 92/221 | 2.58 (1.36-4.89); .004 | 0.63 (0.27-1.51); .30 |
| RSV | 101/212 | 2.69 (1.42-5.09); .002 | 1.01 (0.47-2.16); .99 |
| Coronavirus | 59/254 | 3.14 (1.59-6.22); .001 | 1.30 (0.58-2.91); .52 |
| Other virus | 117/196 | 3.88 (2.01-7.49); <.001 | 1.33 (0.58-3.05); .49 |
| Any virus | 184/129 | 3.94 (1.77-8.76); <.001 | 1.09 (0.40-2.99); .86 |
| Any pathogenic bacteria | 206/107 | 5.07 (1.94-13.25); <.001 | 1.06 (0.12-9.17); .96 |
| No. of children with and without any episodes (clinic visit) | Unadjusted OR (95% CI); P value | Adjusted for any viral/bacterial infection OR [95% CI]; P value | |
| Respiratory episode (any) | 228/85 | 1.43 (1.26-1.61); <.001 | 1.39 (1.21-1.59); <.001 |
Values in boldface indicate statistical significance.
There was no significant risk from any of the viruses or bacteria when the simple association analyses were adjusted for the child's total number of respiratory episodes (Fig 2 and Table I), whereas the association between the number of respiratory episodes and asthma remained significant after adjustment for any positive viral or bacterial aspirate (OR, 1.39 [95% CI, 1.21-1.59]; P < .0001; Table I).
Fig 2.
Risk of asthma at age 7 years in relation to viral or bacterial respiratory tract infection in the first 3 years of life. Circles, Crude ORs with 95% CIs; triangles, adjusted ORs (adjusted for the child's total number of respiratory episodes where an aspirate was taken). RV, Rhinovirus.
Analyzing the number of episodes with specific virus types and bacteria produced similar results (Table II ). The effect estimate was similar for all of the different triggers in the unadjusted analyses. In the multivariable model, including both numbers of the specific infection and the total number of respiratory episodes, only the total number of respiratory episodes remained significantly associated with asthma.
Table II.
Risk of asthma by age 7 years in relation to the number of lower respiratory tract infections within the first 3 years of life
| Effect of no. of respiratory episodes with a specific agent, unadjusted, OR (95% CI); P value | Effect of no. of respiratory episodes with a specific agent, adjusted for the total no. of respiratory episodes, OR (95% CI); P value | Effect of the total no. of respiratory episodes, adjusted for no. of respiratory episodes with each agent, OR (95% CI); P value | |
|---|---|---|---|
| Rhinovirus | 1.94 (1.43-2.62); <.001 | 0.89 (0.56-1.4); .60 | 1.47 (1.24-1.74); <.001 |
| RSV | 1.98 (1.31-3.01); .002 | 0.94 (0.55-1.59); .81 | 1.44 (1.25-1.65); <.001 |
| Coronavirus | 2.14 (1.40-3.27); <.001 | 1.19 (0.73-1.94); .49 | 1.40 (1.22-1.60); <.001 |
| Other virus | 1.81 (1.35-2.43); <.001 | 0.97 (0.65-1.42); .86 | 1.44 (1.23-1.68); <.001 |
| Any virus | 1.63 (1.36-1.95); <.001 | 1.09 (0.75-1.58); .65 | 1.36 (1.06-1.74); .016 |
| Any pathogenic bacteria | 1.57 (1.34-1.84); <.001 | 1.15 (0.83-1.61); .39 | 1.30 (1.01-1.66); .040 |
Values in boldface indicate statistical significance.
Limiting the analyses to events in the first year of life (see Table E1 and the Results section in this article's Online Repository at www.jacionline.org) or to children having at least 1 respiratory episode in the first 3 years of life (see Table E2 and the Results section in this article's Online Repository at www.jacionline.org) did not change the conclusions.
The associations between specific respiratory tract infections and asthma were largely unchanged after adjustment for sensitization at age 6 years or infant lung function (data not shown). We further tested whether sensitization or infant lung function modified the association between specific infections and later asthma. There were some trends toward stronger association between specific viral infections and asthma in some strata, including a stronger association for RSV in children with lower infant lung function and an opposite tendency for RSV and coronavirus infection. However, overall, there was no statistical evidence of effect modification (all interaction P values were >.12; see Table E3 and the Results section in this article's Online Repository at www.jacionline.org).
Discussion
Simple association analyses showed association between common respiratory viral infections and later development of asthma but with no differences in risk between the most common virus types (rhinovirus, RSV, and coronavirus). There was no significant risk of school-age asthma from any virus or pathogenic bacteria when adjusting for the total number of respiratory episodes; that is, for a given burden of acute respiratory episodes, it did not matter whether any of these or how many of these were related to a particular trigger. This suggests that the most important risk factor for later development of asthma is the number of acute respiratory episodes rather that the specific viral trigger.
Strengths of the study
The major strength of COPSAC is the meticulous prospective clinical monitoring, diagnosis, and treatment of lung symptoms based on standard operating procedures. The birth cohort was followed prospectively with diary cards and 6-month routine visits (3-month visits for children with a diagnosis of asthma). In addition, the children attended the clinic for acute respiratory episodes, which was part of the nested randomized controlled trial of inhaled corticosteroids, showing no clinical effect compared with placebo.20 At each visit, the child was seen by the research doctor at the dedicated research clinic, where full clinical work-up was performed, including objective assessment of the respiratory status of the child and aspirates for microbiology.
The base unit of symptom burden was a “respiratory episode,” defined as a 3-day episode of lung symptoms significantly affecting the well-being of the child, excluding croup, and having information on the microbial trigger from airway aspirates. We avoided the use of specific terms, such as wheeze, which we have demonstrated has a very low sensitivity to asthma.15, 21, 22 This base unit was defined objectively from diary cards, which we have previously found to be a more sensitive measure of asthma propensity, with a closer association with known risk factors of asthma, such as 17q21 locus genotypes, than the traditional temporal categories.15, 23
Asthma diagnosis followed a standardized algorithm based on the recorded symptom severity and included a request for improvement upon treatment and relapse after treatment, as well as a clinical history compatible with asthma, as judged by the study physician.
When investigating any specific virus, we chose to include all other clinically assessed respiratory episodes in the comparison group; the nonexposed group included children with exacerbations with triggers other than the case trigger. This was a conservative approach. If we had used the group of children with no respiratory tract infections ever, this would have boosted the unadjusted ORs but would not affect the result of the adjustment for respiratory episodes.
Limitations of the study
Similar to previous studies on this subject,7 this is an observational study and we cannot draw conclusions about potential causal effects of viral respiratory tract infections. For example, children with a lower respiratory tract infection triggered by rhinovirus had a higher risk of later asthma than children not experiencing such an episode, however, this might also be due to underlying asthma constitution or the lower respiratory tract illness itself, irrespective if the trigger. We tried to investigate this further by including multivariable analyses adjusting for the total number of respiratory episodes and thereby estimating the effect of a specific triggering agent for a given burden of episodes.
We presumed that children active in the cohort but without clinic visits for respiratory episodes in the first 3 life years did not have clinically significant respiratory episodes. This introduces a possible bias against the null hypothesis; that is, if children in the control group had similar symptomatic episodes, this would reduce the contrast between 2 groups and reduce the risk estimates of asthma. However, we found similar results when we limited the analyses to the 228 children presenting to the clinic with respiratory episodes.
Because of the size of the study, we had limited statistical power to detect effect modification by allergic sensitization or infant lung function.
The external validity of this study is limited by the high-risk nature of the cohort. Still, this is unlikely to affect the distribution of virus types or the risk of asthma between them.
Interpretation
Our study suggests that the specific microbial trigger is not important for the risk of later asthma development. This directs future research toward studies of individual susceptibility and inflammatory consequences of lower respiratory tract infections in general.
Our findings contrast previous reports from another birth cohort study finding that acute respiratory episodes with detection of rhinovirus were more strongly associated with later asthma than episodes with detection of RSV.7 However, those data are from a relatively small study, and the very high risk estimates observed for rhinovirus are associated with considerable statistical uncertainty, as evidenced by the wide CIs. In line with our findings, another birth cohort study reported that respiratory tract infections with RSV and rhinovirus in the first year of life were associated with wheeze and asthma by age 5 years, with no statistically significant difference between the 2 viruses.24 We have previously shown that pathogenic bacteria are associated with wheezing episodes in young children.8 Similar to our finding for viruses, it did not seem to determine the long-term course of asthma if the episodes were triggered by bacteria. None of the previous studies of viral infections analyzed the presence of pathogenic bacteria.
Our study relates to the ongoing debate on early viral infections being causative for asthma, merely a marker of the underlying asthma constitution, or perhaps both. There is a significant body of work published on the association between particularly RSV2, 3, 4, 5, 6 and more recently rhinovirus7 and later development of asthma. On the other hand, predisposition to asthma was associated with increased risk of lower respiratory tract infection and RSV bronchiolitis, the intermediary phenotype associated with asthma.25 Furthermore, early wheezy symptoms were found to be a strong risk factor for subsequent RSV-related hospitalization.26 Recently, we demonstrated that children with acute severe virus-induced bronchiolitis were born with increased bronchial responsiveness compared with children without bronchiolitis27 and that allergic sensitization was a risk factor for rhinovirus-induced wheezing.28 The present study does not support causal effects of specific viruses: for a given number of respiratory episodes it did not influence the risk of asthma whether any of these, or how many of these, were triggered by a specific virus. A differential risk from different virus types during acute episodes could be interpreted in support of a causal effect from certain viruses. However, our study did not support this observation. Similar to other observational studies, we cannot draw any conclusions on causality of early respiratory tract infections on later asthma from this study. It has been reported that timing of birth in relation to the yearly peak in viral respiratory tract infections was associated with later asthma in support of a causal role of early respiratory infections.29 A causal role can only be proved in interventional studies showing that removing the virus or preventing infection changes the risk of subsequent asthma. A recent randomized trial of mAbs against RSV demonstrated a protective effect against wheeze in the first year of life and also after the end of treatment, and further follow-up will provide information on the causal role of early RSV infection on asthma.30 Our results showing a nondifferential response to different viral infections would suggest that a potential causal effect on asthma would not be restricted to RSV infections.
It is plausible that early respiratory tract infections interact with genetic susceptibility to asthma and initiate a trajectory toward asthma. An interaction between early respiratory tract infections and the asthma locus at chromosome 17q21 was recently reported from the COPSAC and COAST (Childhood Origins of ASThma) birth cohorts.31 This locus seemed primarily to be associated with increased susceptibility to respiratory episodes triggered by rhinovirus infection. Future studies must show whether genetics can improve our understanding on the relationship between early infections and asthma.
Conclusion
Our study suggests that the number of early respiratory episodes, but not the specific viral trigger, is associated with development of school-age asthma. In particular, we saw no difference in asthma risk associated with rhinovirus or RSV. This suggests that the susceptibility and response to early respiratory tract infections in general, rather than the specific triggering agent, should be the target of future research.
Key messages.
-
•
Early respiratory infections are associated with later asthma, and this has led to a focus on a potential causal role of specific viral triggers, particularly rhinoviruses and RSV.
-
•
In this study, the number of early respiratory episodes was associated with asthma, irrespective of virus type.
-
•
This suggests that future research should focus on the susceptibility and response to lower respiratory tract infections in general rather than the specific triggering agent.
Acknowledgments
We thank the children and families of the COPSAC2000 cohort study for their support and commitment. We also acknowledge and appreciate the unique efforts of the COPSAC research team and T. Kebadze and J. Aniscenko for virologic analyses and thank Thermo Fischer Scientific Inc for sponsoring the IgE analyses.
Footnotes
Data in this manuscript were published as an abstract at the European Academy of Allergy and Clinical Immunology Congress 2014 but not in any other format.
Disclosure of potential conflict of interest: Copenhagen Prospective Studies on Asthma in Childhood (COPSAC) is funded by private and public research funds all listed on www.copsac.com. The Lundbeck Foundation (grant no. R16-A1694); the Ministry of Health (grant no. 903516); the Danish Council for Strategic Research (grant no. 0603-00280B); the Danish Council for Independent Research and the Capital Region Research Foundation have provided core support for COPSAC. Thermo Fischer Scientific Inc sponsored the IgE analyses. No pharmaceutical company was involved in the study. The funding agencies did not have any role in design and conduct of the study; collection, management, and interpretation of the data; or preparation, review, or approval of the manuscript. S. L. Johnston is supported by ERC FP7 Advanced grant 233015, a Chair from Asthma UK (CH11SJ), MRC Centre Grant G1000758, and Predicta FP7 Collaborative Project grant 260895. H. Bisgaard is employed by the Capital Region of Denmark, which has received funding from the Lundbeck Foundation and has received or has grants pending from the European Research Council, National Institutes of Health, and Novo Nordisk and has received compensation for providing expert testimony from the European Medicines Agency. The rest of the authors declare that they have no relevant conflicts of interest.
Results
Analysis restricted to infections in the first year of life
Table E1 presents the analysis of viral respiratory tract infections restricted to the first year of life. The study base was slightly increased to 329 children with full follow-up for the first year of life and asthma status at age 7 years. There were fewer cases, but the results were overall similar to the main analysis of viral respiratory tract infections in the first 3 years of life (Table I).
Analysis restricted to children with respiratory episodes and viral/bacteria sampling
Table E2 presents the analysis of a study population reduced to the 228 children having at least 1 clinical visit with viral or bacterial sampling during the first 3 years of life.
Interaction with allergic sensitization at age 6 years and infant lung function
Two hundred ninety (93%) of the 313 children were tested for sensitization at age 6 years by using either skin prick tests or specific IgE measurements. Fifty-eight (19%) children were sensitized to 1 or more common allergens. Three hundred eight of the 313 children had an assessment of infant lung function (ie, forced expiratory flow at 50% of functional vital capacity). One hundred fifty-three of these had a value less than the study mean, and 155 had a value greater than the mean.
Table E3 presents the potential effect modification of viral respiratory tract infections by sensitization and infant lung function (stratified according to mean population level). There was no significant effect modification by sensitization or infant lung function on the effect of any specific respiratory tract infection.
Table E1.
Risk of asthma by age 7 years in relation to lower respiratory tract infections within the first year of life
| Explanatory variable | No. of cases/no. of control subjects | Unadjusted OR (95% CI); P value | Adjusted for the total no. of respiratory episodes, OR (95% CI); P value |
|---|---|---|---|
| Rhinovirus | 56/273 | 2.86 (1.42-5.75); .003 | 0.89 (0.35-2.27); .81 |
| RSV | 52/277 | 2.82 (1.38-5.77); .005 | 1.26 (0.54-2.91); .59 |
| Coronavirus | 26/303 | 1.97 (0.75-5.21); .17 | 0.70 (0.23-2.17); .54 |
| Other virus | 57/272 | 3.15 (1.58-6.28); .001 | 1.20 (0.50-2.86); .68 |
| Any virus | 117/212 | 2.74 (1.45-5.17); .002 | 0.89 (0.36-2.18); .79 |
| Any pathogenic bacteria | 128/201 | 2.85 (1.50-5.40); .001 | 1.30 (0.60-2.82); .51 |
| No. of children with and without any episodes (clinic visit) | Adjusted for any virus or bacterial infection, OR (95% CI); P value | ||
| Respiratory episode (any) | 163/166 | 1.63 (1.34-1.99); <.001 | 1.63 (1.25-2.12); .0003 |
Values in boldface indicate statistical significance.
Table E2.
Risk of asthma by age 7 years in relation to lower respiratory tract infections within the first 3 years of life among children with at least 1 respiratory episode
| Explanatory variable | No. of cases/no. of control subjects | Unadjusted OR (95% CI); P value | Adjusted for the total no. of respiratory episodes, OR (95% CI); P value |
|---|---|---|---|
| Rhinovirus | 92/136 | 1.82 (0.93-3.58); .081 | 0.63 (0.27-1.49); .29 |
| RSV | 101/127 | 1.89 (0.96-3.72); .066 | 0.98 (0.45-2.11); .95 |
| Coronavirus | 59/169 | 2.33 (1.15-4.72); .0187 | 1.28 (0.58-2.86); .54 |
| Other virus | 117/111 | 2.84 (1.37-5.90); .0049 | 1.30 (0.56-3.02); .54 |
| Any virus | 184/44 | 2.60 (0.88-7.73); .0849 | 0.95 (0.29-3.15); .93 |
| Any pathogenic bacteria | 206/220 | 5.22 (0.68-39.93); .11 | 2.52 (0.32-20.04); .38 |
Values in boldface indicate statistical significance.
Table E3.
Possible effect modification by infant lung function (infant FEF50) and allergic sensitization on association between lower respiratory tract infections and risk of asthma at age 7 years
| No. | Variable (exposure) | OR for asthma, unadjusted | OR for asthma, adjusted for the total no. of respiratory episodes | Interaction term, P value | |
|---|---|---|---|---|---|
| Strata by infant FEF50 | |||||
| Low | 153 | Rhinovirus | 2.00 (0.85-4.69); .11 | 0.33 (0.09-1.17); .086 | .29 |
| High | 155 | Rhinovirus | 3.15 (1.16-8.60); .025 | 1.11 (0.31-3.92); .88 | |
| Low | 153 | RSV | 3.79 (1.59-9.03); .002 | 1.51 (0.54-4.22); .43 | .14 |
| High | 155 | RSV | 1.30 (0.46-3.72); .62 | 0.37 (0.10-1.40); .14 | |
| Low | 153 | Coronavirus | 1.99 (0.77-5.12); .1536 | 0.82 (0.26-2.63); .74 | .24 |
| High | 155 | Coronavirus | 5.29 (1.84-15.17); .002 | 2.50 (0.75-8.33); .14 | |
| Low | 153 | Other virus | 3.48 (1.44-8.37); .0054 | 1.42 (0.51-3.96); .50 | .80 |
| High | 155 | Other virus | 3.95 (1.43-10.93); .008 | 1.18 (0.28-5.08); .82 | |
| Low | 153 | Any virus | 3.54 (1.15-10.85); .027 | 0.89 (0.22-3.51); .87 | .86 |
| High | 155 | Any virus | 3.76 (1.18-12.01); .025 | 1.16 (0.25-5.37); .85 | |
| Low | 153 | Any pathogenic bacteria | 7.69 (1.74-33.94); .007 | 2.56 (0.50-13.14); .26 | .45 |
| High | 155 | Any pathogenic bacteria | 3.06 (0.84-11.07); .089 | 1.00 (0.22-4.64); .99 | |
| Strata by current sensitization (6 y) | |||||
| No | 166 | Rhinovirus | 1.81 (0.77- 4.23); .171 | 0.41 (0.13-1.35); .143 | .12 |
| Yes | 105 | Rhinovirus | 3.84 (1.30-11.35); .015 | 1.10 (0.28-4.36); .892 | |
| No | 166 | RSV | 1.98 (0.85-4.61); .11 | 0.78 (0.28-2.14); .62 | .39 |
| Yes | 105 | RSV | 3.35 (1.17-9.58); .024 | 1.00 (0.26-3.8); .99 | |
| No | 166 | Coronavirus | 4.86 (1.98-11.94); <.001 | 2.10 (0.74-6.02); .16 | .48 |
| Yes | 105 | Coronavirus | 1.25 (0.31-4.98); .75 | 0.87 (0.2-3.79); .85 | |
| No | 166 | Other virus | 3.01 (1.25-7.23); .014 | 0.89 (0.28-2.80); .84 | .28 |
| Yes | 105 | Other virus | 3.90 (1.36-11.2); .012 | 1.50 (0.43-5.24); .52 | |
| No | 166 | Any virus | 2.56 (0.91-7.19); .075 | 0.64 (0.17-2.39); .51 | .18 |
| Yes | 105 | Any virus | 5.87 (1.59-21.76); .008 | 1.64 (0.30-9.03); .57 | |
| No | 166 | Any pathogenic bacteria | 3.75 (1.07-13.14); .039 | 1.16 (0.28-4.92); .84 | .29 |
| Yes | 105 | Any pathogenic bacteria | 7.47 (1.62-34.44); .01 | 2.65 (0.46-15.09); .27 | |
FEF50, Forced expiratory flow at 50% of functional vital capacity.
Values in boldface indicate statistical significance.
References
- 1.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein R.T., Sherrill D., Morgan W.J., Holberg C.J., Halonen M., Taussig L.M. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 3.Sigurs N., Gustafsson P.M., Bjarnason R., Lundberg F., Schmidt S., Sigurbergsson F. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 4.Kuehni C.E., Spycher B.D., Silverman M. Causal links between RSV infection and asthma: no clear answers to an old question. Am J Respir Crit Care Med. 2009;179:1079–1080. doi: 10.1164/rccm.200904-0567ED. [DOI] [PubMed] [Google Scholar]
- 5.Pullan C.R., Hey E.N. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed) 1982;284:1665–1669. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McBride J.T. Pulmonary function changes in children after respiratory syncytial virus infection in infancy. J Pediatr. 1999;135:28–32. [PubMed] [Google Scholar]
- 7.Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisgaard H., Hermansen M.N., Bønnelykke K., Stokholm J., Baty F., Skytt N.L. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards M.R., Regamey N., Vareille M., Kieninger E., Gupta A., Shoemark A. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013;6:797–806. doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corne J.M., Marshall C., Smith S., Schreiber J., Sanderson G., Holgate S.T. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 11.Bisgaard H., Jensen S.M., Bønnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185:1183–1189. doi: 10.1164/rccm.201110-1922OC. [DOI] [PubMed] [Google Scholar]
- 12.Martinez F.D., Morgan W.J., Wright A.L., Holberg C.J., Taussig L.M. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 13.Bisgaard H., Hermansen M.N., Buchvald F., Loland L., Halkjaer L.B., Bønnelykke K. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 14.Bisgaard H. The Copenhagen Prospective Study on Asthma in Childhood (COPSAC): design, rationale, and baseline data from a longitudinal birth cohort study. Ann Allergy Asthma Immunol. 2004;93:381–389. doi: 10.1016/S1081-1206(10)61398-1. [DOI] [PubMed] [Google Scholar]
- 15.Bisgaard H., Pipper C.B., Bønnelykke K. Endotyping early childhood asthma by quantitative symptom assessment. J Allergy Clin Immunol. 2011;127:1155–1164.e2. doi: 10.1016/j.jaci.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Paganelli R., Ansotegui I.J., Sastre J., Lange C.E., Roovers M.H., de Groot H. Specific IgE antibodies in the diagnosis of atopic disease. Clinical evaluation of a new in vitro test system, UniCAP, in six European allergy clinics. Allergy. 1998;53:763–768. [PubMed] [Google Scholar]
- 17.Bisgaard H., Li N., Bonnelykke K., Chawes B.L., Skov T., Paludan-Müller G. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128:646–652.e5. doi: 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 18.Moffatt M.F., Kabesch M., Liang L., Dixon A.L., Strachan D., Heath S. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 19.Bønnelykke K., Pipper C.B., Tavendale R., Palmer C.N., Bisgaard H. Filaggrin gene variants and atopic diseases in early childhood assessed longitudinally from birth. Pediatr Allergy Immunol. 2010;21:954–961. doi: 10.1111/j.1399-3038.2010.01073.x. [DOI] [PubMed] [Google Scholar]
- 20.Bisgaard H., Hermansen M.N., Loland L., Halkjaer L.B., Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354:1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 21.Skytt N., Bønnelykke K., Bisgaard H. “To wheeze or not to wheeze”: that is not the question. J Allergy Clin Immunol. 2012;130:403–407.e5. doi: 10.1016/j.jaci.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 22.Bisgaard H., Swern A.S., Knorr B. “To wheeze or not to wheeze”: that is not the question—the sequel. J Allergy Clin Immunol. 2012;130:531–532. doi: 10.1016/j.jaci.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 23.Bisgaard H., Bønnelykke K., Sleiman P.M.A., Brasholt M., Chawes B., Kreiner-Møller E. Chromosome 17q21 gene variants are associated with asthma and exacerbations but not atopy in early childhood. Am J Respir Crit Care Med. 2009;179:179–185. doi: 10.1164/rccm.200809-1436OC. [DOI] [PubMed] [Google Scholar]
- 24.Kusel M.M.H., de Klerk N.H., Kebadze T., Vohma V., Holt P.G., Johnston S.L. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goetghebuer T., Kwiatkowski D., Thomson A., Hull J. Familial susceptibility to severe respiratory infection in early life. Pediatr Pulmonol. 2004;38:321–328. doi: 10.1002/ppul.20069. [DOI] [PubMed] [Google Scholar]
- 26.Stensballe L.G., Kristensen K., Simoes E.A., Jensen H., Nielsen J., Benn C.S. Atopic disposition, wheezing, and subsequent respiratory syncytial virus hospitalization in Danish children younger than 18 months: a nested case-control study. Pediatrics. 2006;118:e1360–e1368. doi: 10.1542/peds.2006-0907. [DOI] [PubMed] [Google Scholar]
- 27.Chawes B.L.K., Poorisrisak P., Johnston S.L., Bisgaard H. Neonatal bronchial hyperresponsiveness precedes acute severe viral bronchiolitis in infants. J Allergy Clin Immunol. 2012;130:354–361.e3. doi: 10.1016/j.jaci.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson D.J., Evans M.D., Gangnon R.E., Tisler C.J., Pappas T.E., Lee W.M. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–285. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu P., Dupont W.D., Griffin M.R., Carroll K.N., Mitchel E.F., Gebretsadik T. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanken M.O., Rovers M.M., Molenaar J.M., Winkler-Seinstra P.L., Meijer A., Kimpen J.L. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 31.Calışkan M., Bochkov Y.A., Kreiner-Møller E., Bønnelykke K., Stein M.M., Du G. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]