Abstract
Detailed information about the replication cycle of viruses and their interactions with host organisms is required to develop strategies to stop them. Cell biology studies, live-cell imaging, and systems biology have started to illuminate the multiple and subtly different pathways that animal viruses use to enter host cells. These insights are revolutionizing our understanding of endocytosis and the movement of vesicles within cells. In addition, such insights reveal new targets for attacking viruses before they can usurp the host-cell machinery for replication.
Main Text
Introduction
Viruses are obligatory intracellular parasites; therefore, their replication (and the pathogenic consequences of infection) depends critically on the ability to transmit their genomes from infected to noninfected host organisms and from infected to uninfected cells. The small size, structural simplicity, and lack of any metabolic or motile activities severely limit the types of processes that virus particles can themselves undertake to promote the transfer process. As passive, inert particles, they have evolved to exploit the behavior and the physiology of their hosts.
At the cell level, this is manifested in the activation of endogenous cellular responses that provide assistance to viruses so that they can cross membranes and other barriers and deliver their genes into the cytosol or the nucleus. Recent studies indicate that cells offer a variety of endocytosis, trafficking, and sorting mechanisms that animal viruses can take. Moreover, viruses have become valuable tools to study some of these processes. Here, we review the general concepts in virus entry and discuss some of the emerging issues.
Virus Particles as Devices for Targeted Gene Transfer
A viral particle is composed of nucleic acids (RNA or DNA), protein, and, in the case of enveloped viruses, membrane lipids. The proteins include structural components, such as capsid proteins; matrix proteins; membrane glycoproteins; and, in many cases, accessory proteins such as reverse transcriptases, RNA polymerases, kinases, and proteases. One or more shells of protein protect the RNA or DNA genome in the so-called capsid, which is often helical or icosahedral. Enveloped viruses have, in addition, a lipid bilayer membrane that serves to protect the capsid and the genome and operates as a “transport vesicle” during cell-to-cell transmission.
Transmission involves three main stages: the assembly of virus particles in infected cells, their release to the extracellular space, and entry into a new cell. Nonenveloped virus particles and the capsids of enveloped viruses are assembled in the cytosol or the nucleus of the infected cell. The lipid bilayer membrane (envelope) of enveloped viruses is acquired during a budding process through a cellular membrane. With few exceptions, the key proteins in the capsid and the envelope are encoded by the viral genome.
Given that the components of the virus particle are often synthesized in different parts of the cell, the assembly of a particle is a remarkable example of coordinated molecular sorting. The assembly processes involve the establishment of a complex network of specific interactions that bring the relevant viral components together into a particle with precise stoichiometry and geometry and with the exclusion of most cellular components. That all this is possible with the limited genetic information contained in viral genomes is impressive. It serves as a testament to the viruses' ingenious structural designs and the generous assistance of the cell.
Enveloped particles leave the infected cell inconspicuously by budding and secretion. Nonenveloped viruses are usually thought to undergo release through cell lysis, but some may escape by secretory mechanisms after budding into membrane bound compartments and then losing their membrane (Altenburg et al., 1980). Others may subvert cellular autophagy pathways to gain access to exocytic organelles (Jackson et al., 2005).
The particles released from cells are stable structures crosslinked by networks of intermolecular interactions. They are resistant to the stresses encountered in the extracellular space during transmission from cell to cell and host to host. However, at the same time, the assembly and maturation program has made sure that the particles can fall apart at a moment's notice during entry into a new cell. The reversibility of assembly is important to liberate the genome in infectious form and release the accessory proteins. Many of the stabilizing interactions in the particle must be undone: the envelope must be shed, the capsid opened, and the nucleic acids decondensed. To make this possible, the entire virus particle or specific proteins in it are locked in metastable conformations and poised to undergo major conformational changes when triggered by appropriate cues during entry (Steven et al., 2005). The uncoating program is thus built into the virion during assembly, allowing major transformations in the particle without the need for external energy.
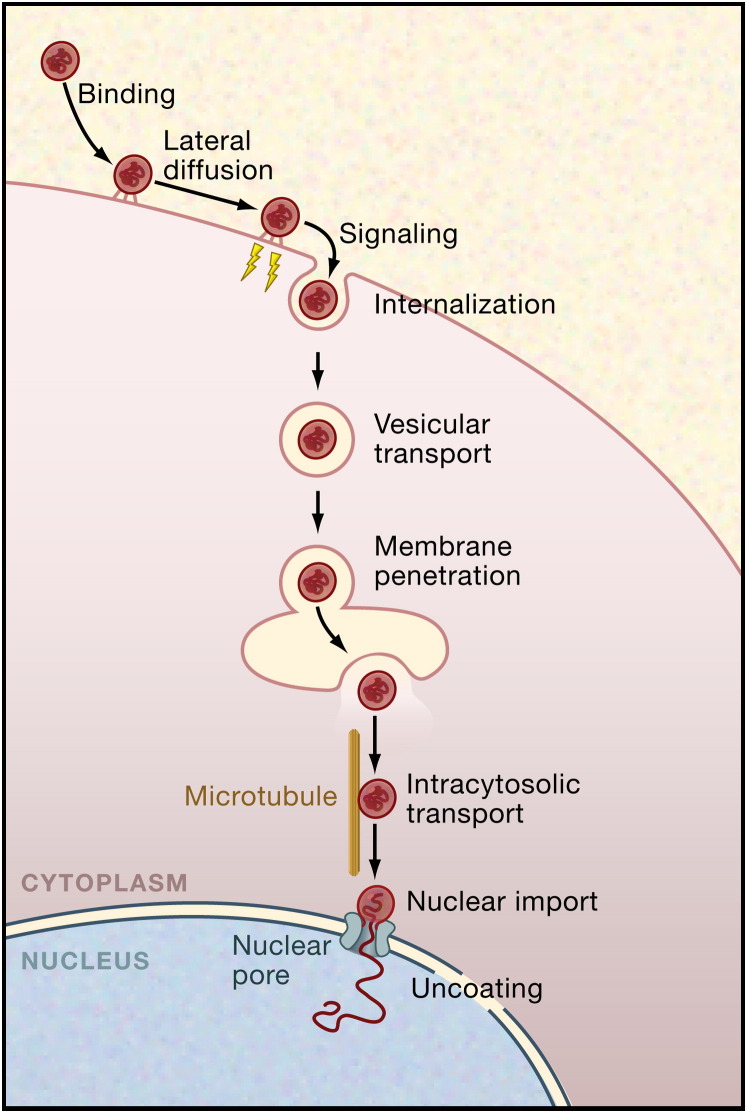
Like assembly, entry and uncoating typically occur in several tightly controlled, consecutive steps. These steps, shown in Figure 1 for a virus that enters via endocytosis and moves to the nucleus (e.g., an adeno- or polyomavirus), start with events at the cell surface and end with the decondensation of the genome at the site of replication. As the virus progresses in its entry program, it undergoes changes that lead to events such as penetration, capsid destabilization, and uncoating of the genome. Many of these changes result from conformational alterations in metastable viral structures. They are triggered by receptor binding, exposure to low pH, reentry into a reducing environment, enzyme-induced covalent modifications, and other cellular cues (Earp et al., 2005, Harrison, 2005, Hogle, 2002, Smith and Helenius, 2004). Viruses exploit such signals not only to induce changes in the particle and dissociation of protein subunits but also to coordinate movement from one compartment and location in the cell to another, ensuring that each step in the uncoating program occurs at the right point in the sequence, at the right time, and in the right place.
Figure 1.
Steps in the Endocytic Entry Program of a Typical Animal Virus
Whether enveloped or nonenveloped, many viruses depend on the host cell's endocytic pathways for entry. They follow a multistep entry and uncoating program that allows them to move from the cell periphery to the perinuclear space. In this example, the virus proceeds to deliver its uncoated genome into the nucleoplasm. The interaction between the virus and the host cell starts with virus binding to attachment factors and receptors on the cell surface, followed by lateral movement of the virus-receptor complexes and the induction of signals that result in the endocytic internalization of the virus particle. After vesicular trafficking and delivery into the lumen of endosomes, caveosomes, or the ER, a change in the virus conformation is induced by cellular cues. This alteration results in the penetration of the virus or its capsids through the vacuole membrane into the cytosolic compartment. Enveloped viruses use membrane fusion for penetration, whereas nonenveloped viruses induce lysis or pore formation. After targeting and transport along microtubules, the virus or the capsid binds, as in this example, to the nuclear pore complex, undergoes a final conversion, and releases the viral genome into the nucleus. The details in the entry program vary for different viruses and cell types, but many of the key steps shown here are general.
Although the incoming viruses depend on cues from the cell, the cell also responds to signals induced by the virus. Simply by binding to surface receptors, and perhaps by clustering them, many viruses “knock at the door” of the cell by activating cellular signaling cascades. This is often essential because it results in the local activation of ligand-triggered processes that viruses require for entry, such as caveolar/raft endocytosis, clathrin-coat assembly, and actin-cortex dissociation. This aspect of virus entry is complex but increasingly central for understanding the infection process.
Virus Receptors
In order to infect, viruses must first bind to the cell surface. The surface structures that they bind to are of two general types depending on the functional consequences of the interaction. Attachment factors serve to bind the particles and thus help to concentrate viruses on the cell surface. Such interactions can be relatively nonspecific. Often they involve interactions with heparan sulfate or other carbohydrate structures on the cell surface (Ugolini et al., 1999, Vlasak et al., 2005, Young, 2001).
Unlike attachment factors, virus receptors actively promote entry. They can do so by initiating conformational changes in the virus particle, by activating signaling pathways, and by promoting endocytic internalization. Often the receptors accompany the virus into the cell during endocytic uptake and may then play a role intracellularly in the penetration reaction.
Given that the interactions are usually highly specific, the presence of receptors determines to a large degree which cell types and species can be infected. These interactions and the molecules involved are of crucial importance for understanding not only the mechanisms of entry but also the biology of infection and pathogenesis. In recent years, hundreds of attachment factors and receptors for different viruses have been identified, resulting in a large body of valuable information (Young, 2001). The crystal structures of several viruses and viral proteins bound to receptors have also been solved (Kwong et al., 1998, Rossmann et al., 2002, Skehel and Wiley, 2000, Stewart et al., 2003). Within the scope of this review, it is not possible to discuss the attachment factors and receptors in depth; however, it is important to emphasize that the list encompasses a wide variety of different proteins, lipids, and carbohydrates. Included are ion transporters, adhesion factors, signaling proteins, and a variety of other cell-surface receptors. Proteoglycans and glycolipids are commonly used as virus receptors or attachment factors (Young, 2001). Moreover, influenza viruses and some paramyxoviruses have spike glycoproteins with lectin domains that bind to sialic acid, and the major coat protein of polyomaviruses, VP1, binds to the glycan moiety of specific gangliosides (Skehel and Wiley, 2000, Stehle and Harrison, 1997, Tsai et al., 2003).
Although individual interactions between viruses and their receptors are specific, they are often of low affinity. However, the avidity increase resulting from multiple receptor binding sites on virus particles often guarantees nearly irreversible binding. Moreover, multisite binding is likely to cluster receptor proteins, which in turn may activate signaling pathways and/or recruitment to endocytic structures (see below).
Numerous viruses are known to use more than one type of receptor, either in parallel or in series. The consecutive use of CD4 and a chemokine receptor by the human immunodeficiency virus (HIV type 1) is a well-studied example of the latter. In this case, the two receptors are needed to induce major conformational changes in the HIV envelope protein that initiate membrane fusion (Berger et al., 1999). Interestingly, HIV-1 can also bind to glycosylceramides and heparan sulfate, interactions that may facilitate the initial recruitment of virus to susceptible cells (Long et al., 1994, Ugolini et al., 1999). Moreover, the presence of specific glycosphingolipids in the target cell membrane can enhance CD4/coreceptor-dependent fusion (Puri et al., 1998). Viruses, in particular those that undergo rapid mutation, are also known to be able to switch receptors (Byrnes and Griffin, 2000, Klimstra et al., 1998) or adapt to use alternative receptors when the primary receptor is absent (Vlasak et al., 2005). This is one of the risks posed by the current avian influenza epidemic: the avian hemagglutinin glycoprotein may, through mutations, evolve to interact more potently with glycoconjugates on human cells.
The entry of coxsackie B virus provides an interesting example of how viruses can exploit the different properties of cell-surface receptors to bring about stepwise entry. In this case, the host cells are epithelial cells that grow in tight monolayers. Coxsackie B viruses are human picornaviruses that cause meningitis and myocarditis. They are simple, nonenveloped RNA viruses that replicate in the cytosol and do not need low pH for penetration. They share an essential receptor molecule with adenoviruses, a glycoprotein called CAR (the coxsackie and adenovirus receptor) (Zhang and Bergelson, 2005). In epithelial cells, CAR is a component of tight junctions and is therefore not accessible to incoming viruses from the apical side. A recent study addressed the question of how the virus gains access to its receptor, i.e., how it breaches the epithelial barrier (Coyne and Bergelson, 2006).
The starting point for the study was the observation that many coxsackie B strains interact with a coreceptor present on the apical surface of epithelial cells, the decay-accelerating factor (DAF, a GPI-anchored protein) (Shieh and Bergelson, 2002). Virus binding leads to crosslinking of DAF molecules, inclusion into lipid rafts, and activation of the tyrosine kinase c-Abl (Coyne and Bergelson, 2006). Once activated, c-Abl was found to activate the Rho-family small GTPase Rac, which in turn induced a reorganization of the actin cytoskeleton and promoted transfer of bound viruses from the apical surface to the tight-junction region of the cell. This made it possible for the virus to associate with CAR and for CAR to induce a conformational change in the virus particle, an obligatory step in picornavirus uncoating and entry (Hogle, 2002). To release its RNA into the cytoplasm, the modified particles were then internalized by caveolar endocytosis activated by phosphorylation of Tyr14 in caveolin-1 by Fyn, a member of the Src family of nonreceptor tyrosine kinases. The detailed route taken by the virus inside the cell remains unclear at this point, but the final release of the viral RNA into the cytosol is likely to occur in the endoplasmic reticulum (ER).
This study elegantly demonstrates how a virus exploits multiple cellular receptors and functions to overcome the obstacles it encounters during entry into cells. In this particular cellular context, the coreceptor is needed to overcome the inaccessibility of the main receptor. As discussed below, the signaling cascades are often important in both entry steps and downstream activation of the cytoskeleton and endocytic machinery.
Virus-Induced Signaling
It is apparent that many viruses make use of the cell's signaling pathways during entry. This was first recognized for adenoviruses, which use CAR as a primary receptor and integrins as coreceptors (Li et al., 1998, Nemerow and Stewart, 1999). Nemerow and coworkers demonstrated that the interaction between adenovirus pentons (protein complexes which together with the hexons make up the adenovirus capsid) and integrins activates phosphatidylinositol 3-kinase (PI(3)K), which in turn activates Rac and Cdc42, resulting in the polymerization of actin- and clathrin-mediated endocytosis of the virus. Activation of many different signaling pathways has since been described with the involvement of a variety of factors, including serine/threonine, tyrosine, and PI kinases; phosphatases; and a variety of small GTPases (including Arf, Rab, and Rho family members) (Greber, 2002, Pelkmans et al., 2005).
Viruses use signaling activities to induce changes in the cell that promote viral entry and early cytoplasmic events, as well as to optimize later processes in the replication cycle. Initially, the viruses need to make their presence on the cell surface known so that the cell can launch an endocytic response to bring them in. For example, the internalization of SV40 by caveolar/raft endocytosis is regulated by at least five different kinases (Pelkmans et al., 2005). Inhibition of tyrosine kinases in particular blocks internalization and dramatically reduces infection (Chen and Norkin, 1999, Pelkmans et al., 2002). In other cases, a virus may need to induce lateral movement along the membrane. This can be seen very dramatically when viruses such as murine leukemia virus and HIV-1 bind to filopodia and proceed to “surf” on the outside of these structures toward the cell body (Lehmann et al., 2005). As discussed above, lateral movement is also needed for coxsackie B viruses to reach the CAR receptor in epithelial cells, and this movement requires activation of c-Abl following virus-induced crosslinking of DAF (Coyne and Bergelson, 2006).
The signals can be generated in several ways. The viruses may activate cellular signaling molecules directly by using them as receptors. This may be why so many viruses bind integrins. Viruses may also induce signaling by clustering specific cell-surface proteins or lipids. Interestingly, a number of viruses use GPI-anchored proteins and gangliosides, which are only associated with the outer leaflet of the plasma membrane, as their receptors. That this often leads to activation of tyrosine kinases on the cytosolic side may be related to the fact that the GPI-anchored proteins and gangliosides become lipid-raft associated when clustered (Coyne and Bergelson, 2006, Parton, 1994, Parton and Richards, 2003, Pelkmans et al., 2002, Sharma et al., 2004). Being dually acylated, some Src-family kinases (though not Src itself) are also enriched in lipid-raft microdomains, but on the cytoplasmic surface of the membrane. Accordingly, there is increasing evidence that many viruses associate with lipid rafts to initiate intracellular signaling (Coyne and Bergelson, 2006, Damm et al., 2005, Ono and Freed, 2005).
Virus Penetration: The Cell Surface versus Intracellular Membranes
The transfer of the genome and accessory proteins through the barrier of a cellular membrane into the cytosol is called penetration. For enveloped viruses, penetration involves membrane fusion, and, for nonenveloped viruses, it involves pore formation or membrane lysis. The molecular mechanisms of viral membrane fusion are beginning to be understood in increasingly fine detail but will not be considered here (Earp et al., 2005, Harrison, 2005, Kielian and Rey, 2006). For the most part, the penetration mechanisms of nonenveloped viruses are less well understood. However, recent single-particle cryo-EM analysis of polioviruses bound to receptor-containing membranes has started to provide detailed information on the structural changes involved in picornavirus penetration and the organization of poliovirus-induced membrane pores through which the viral RNA is believed to enter the cytoplasm (Bubeck et al., 2005).
The cellular membrane penetrated during virus entry is either the plasma membrane or the limiting membrane of an intracellular organelle, the lumen of which viruses reach after endocytosis. In all known cases, the viruses or their capsids penetrate first into the cytosol. Most RNA viruses (though not all) replicate in the cytosol, often in contact with specific organelles (Salonen et al., 2005). With the exception of poxviruses and iridoviruses, DNA viruses and their capsids are subsequently transported to the nucleus for replication.
It has been known for a long time that certain enveloped viruses such as herpes simplex virus 1 (HSV-1); Sendai virus; and many retroviruses, including HIV, have pH-independent fusion proteins and can therefore penetrate into cells by fusing directly with the plasma membrane. It is generally assumed that fusion events at the plasma membrane lead to productive infection, although this is difficult to prove because virus particles are also continuously endocytosed. Among nonenveloped viruses, several families, including some picornaviruses (such as polio) and polyomaviruses, do not require low pH for penetration. While many of these are known to require endocytosis for penetration, it has been argued that some may penetrate directly through the plasma membrane, though this remains contentious (Hogle, 2002).
However, if we view the whole spectrum of viruses, the majority do need endocytic internalization for penetration and productive infection, most likely because endocytosis offers real advantages. Viruses that are ferried into cells inside endocytic vesicles can move deep into the cytoplasm, bypassing many of the barriers associated with the membrane cortex and cytosolic crowding (Marsh and Bron, 1997) and exploiting the molecular motors that are normally recruited to endocytic vesicles (Dohner and Sodeik, 2005). A dependence on low pH for penetration allows viruses to use the decreasing pH of endocytic organelles as a cue to activate the penetration reactions and allows viral escape to the cytoplasm at specific locations or before the virus is delivered to the hydrolytic lysosomes (Helenius et al., 1980). In addition, no viral components remain on the cell surface after penetration for detection by the host's immune defenses.
Virus Penetration through Clathrin-Mediated Endocytosis
Of the endocytic pathways taken by viruses, the most commonly used is the clathrin-mediated endocytic route ( Figure 2C and Figures 3A and 3B). It transports incoming viruses together with their receptors into early and late endosomes. Clathrin-mediated endocytosis is a continuous process, and, for virus entry, it is usually rapid and efficient (Marsh and Helenius, 1989). The incoming viruses are often exposed to the acidic milieu of endosomes within minutes after internalization, and many respond to the pH drop by undergoing changes that lead to penetration. Depending on the pH threshold, the site of penetration is either the early (pH 6.5 to 6.0) or the late endosome (pH 6.0 to 5.5). In some cases, such as for Ebola virus, SARS coronavirus, and the nonenveloped mammalian reoviruses, acidic pH alone is not sufficient to induce fusion, and proteolytic cleavages in viral proteins by acid-dependent endosomal proteases, in particular cathepsins L and B, are needed to trigger the change to the penetration-competent state (Chandran et al., 2005, Ebert et al., 2002, Simmons et al., 2005). For avian leukosis virus, both interaction of the viral envelope protein with a specific receptor (Damico et al., 1998, Hernandez et al., 1997) and low pH (Mothes et al., 2000) are required for fusion.
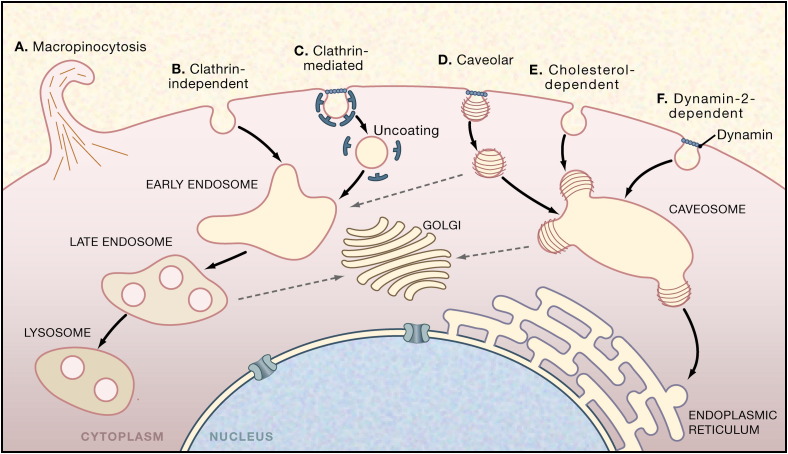
Figure 2.
Endocytic Pathways Used by Viruses
In mammalian cells, many different mechanisms are available for the endocytic internalization of virus particles. Some of these mechanisms, such as clathrin-mediated endocytosis, are ongoing, whereas others, such as caveolae, are ligand and cargo induced. Currently, there is evidence for six pathways.
(A) Macropinocytosis is involved in the entry of adenoviruses.
(B) A clathrin-independent pathway from the plasma membrane has been shown to exist for influenza virus and arenaviruses.
(C) The clathrin-mediated pathway is the most commonly observed uptake pathway for viruses. The viruses are transported via early endosomes to late endosomes and eventually to lysosomes.
(D) The caveolar pathway is one of several closely related, cholesterol-dependent pathways that bring viruses including SV40, coxsackie B, mouse polyoma, and Echo 1 to caveosomes, from which many of them continue, by a second vesicle transport step, to the ER.
(E) A cholesterol-dependent endocytic pathway devoid of clathrin and caveolin-1, used by polyomavirus and SV40.
(F) A pathway similar to (D) except dependent on dynamin-2. It is used by Echo virus 1.
Depending on the virus and cell type, penetration reactions occur in five locations: the plasma membrane, early and late endosomes, caveosomes, and the ER. Note that the additional endocytic mechanism of phagocytosis also operates in many cells but has not as yet been linked to virus entry and is not included here.
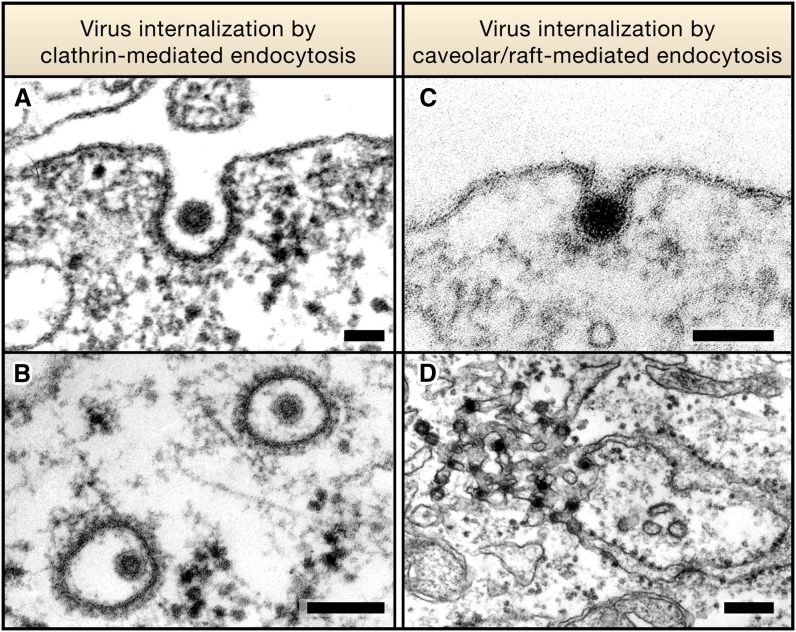
Figure 3.
Electron Micrographs Showing Virus Internalization by Clathrin- or Caveolar/Raft-Mediated Endocytosis
(A and B) Semliki Forest virus, a 70 nm diameter enveloped alphavirus, is internalized by clathrin-coated pits (A) and vesicles (B) for endocytosis and infection. Here, the virus is interacting with a BHK-21 cell.
(C and D) Simian virus 40, a small 50 nm diameter nonenveloped DNA virus, binds to gangliosides in the plasma membrane of CV-1 cells and enters via caveolae (C) and tight-fitting small vesicles. The viruses are transported through caveosomes to the ER, where many accumulate in smooth membrane domains (D). Scale bar in (A) and (C) = 100 nm, (B) = 200 nm, and (D) = 250 nm. (A), (C), and (D) are courtesy of J. Kartenbeck and A.H. (B) is reproduced from Helenius et al. (1980), The Journal of Cell Biology, 1980, volume 84, pp. 404–420 by copyright permission of The Rockefeller University Press.
Single-particle tracking of influenza virus in cultured simian kidney epithelial cells shows that, although 60% of the particles enter via clathrin-coated pits, 40% use a clathrin-independent pathway (Rust et al., 2004) (Figure 2B). Surprisingly, of those that use the clathrin-dependent pathway, only 5% associate with preexisting clathrin-coated domains in the membrane; the majority enter via coated pits that assemble underneath the surface bound viral particles. This implies induction of transmembrane signaling by the receptor bound virus particles. A similar induction of coated-pit formation has also been observed for reovirus and Semliki Forest virus (SFV) (Ehrlich et al., 2004; A. Vonderheit and A.H., unpublished data); whether this represents recruitment of clathrin-coat components to the clustered cytoplasmic domains of viral receptor proteins or a more complex signaling cascade leading to clathrin recruitment is unclear.
Endocytic clathrin-coated vesicles deliver their contents to early endosomes (Figure 2C). These organelles, though morphologically complex, have usually been considered to be a homogeneous population. However, there is increasing evidence that viruses and other endocytosed cargo are selectively targeted to specific populations of endosomes (Kirkham et al., 2005). For example, the early endosomes that receive the incoming influenza viruses belong to a population that rapidly relocates the viruses, by microtubule-mediated transport, to the perinuclear region, where penetration occurs by fusion (Lakadamyali et al., 2003). SFV fuses soon after delivery to Rab5 and EEA1-positive early endosomes, but the fused virus is then transported in Rab7-positive carriers to late endosomes (Vonderheit and Helenius, 2005). There is also a recent observation that vesicular stomatitis virus (VSV) may fuse with the internal membrane vesicles of multivesicular endosomes and that these internal membranes subsequently fuse with the limiting membrane of late endosomes to release the viral RNA to the cytoplasm (Le Blanc et al., 2005). In this case, penetration would involve two membrane fusion events, the first mediated by the viral envelope protein and the second by an as yet uncharacterized cellular mechanism. Interestingly, the endocytic uptake and penetration of anthrax toxin also appears to involve a two-step process, the first involving interaction of the toxin with the internal membranes of a multivesicular endosome and the second a back fusion of these membranes with the endosome-limiting membrane (Abrami et al., 2004).
The complexity of the clathrin-mediated endocytic pathway is illustrated by the increasing number of alternative cofactors, adaptors, and tethering proteins that are involved in the formation of clathrin-coated pits and vesicles (Robinson, 2004). Using small interfering RNA (siRNA) silencing screens, more than 90 different kinases have recently been shown to regulate the clathrin-mediated internalization and early steps of infection of HeLa cells by VSV (Pelkmans et al., 2005). These kinases belong to many different classes, including regulators of the cytoskeleton, cell cycle, cell growth, and membrane trafficking. They either increase or decrease the efficiency of VSV entry and early steps in the infection cycle. Many of the kinases were shown to have a direct effect on the clathrin-mediated endocytosis of other ligands.
Virus Penetration Following Clathrin-Independent Endocytosis
That some viruses use clathrin-independent pathways for endocytosis and infection is now widely accepted. The best studied of these are the caveolar/raft pathways first observed for SV40, a simple nonenveloped DNA virus (Anderson et al., 1996, Kartenbeck et al., 1989, Stang et al., 1997). The caveolar/raft pathways, of which there are at least three related variants (Figures 2D–2F), seem to specialize in the internalization of lipids including cholesterol, GPI-anchored proteins, and components of cholesterol-rich microdomains (lipid rafts). Caveolae are also involved in the transcytosis of serum components in endothelial cells, and many cargo molecules of these pathways seem to have a role in signaling.
The capsids of SV40 and the related polyomavirus are composed of 72 homopentameric VP1 protein units that resemble cholera toxin B chain pentamers (Stehle et al., 1996, Stehle and Harrison, 1997). Like cholera toxin, these viruses bind to the sugar moiety of gangliosides and enter cells via caveolar/raft pathways that are dependent on cholesterol (Figures 2D and 2E) and the activation of tyrosine-kinase signaling cascades (Anderson et al., 1996, Pelkmans et al., 2001, Smith et al., 2003a, Stang et al., 1997, Tsai et al., 2003). Activation involves tyrosine kinases, PI kinases, and raft lipids. Dynamin 2, actin, caveolin-1, and Rho GTPases are also involved, depending on the virus and the cell type (Pelkmans et al., 2002). Internalization occurs via caveolae that are activated for long-distance transport in the cytosol or via small vesicles that lack caveolin-1 (Damm et al., 2005, Tagawa et al., 2005) (Figures 3C and 3D).
The caveolar/raft pathway takes the majority of internalized viruses first to pH-neutral organelles in the cytoplasm called caveosomes (Figure 2D). Following a second activation step, the virions are then transported by caveolin-free, microtubule-dependent vesicles trafficking to the ER, where penetration occurs (Pelkmans et al., 2001). A recent study suggests that a lumenal ER protein, ERp29, a thioredoxin homolog without thiol oxidoreductase activity, facilitates penetration of polyomavirus by exposing the hydrophobic, C-terminal arm of VP1 (Magnuson et al., 2005). In the case of SV40, we have found that the redox conditions in the ER, as well as two thiol oxidoreductases, PDI and ERp57, are important for SV40 uncoating and infection (M. Schelhaas, L. Pelkmans, and A.H., unpublished data). Since siRNA silencing of derlin 1, a protein linked to the reverse translocation of substrates for ER-associated protein degradation (ERAD), inhibits infection, it is likely that components of the ERAD pathway play a central role in SV40 penetration from the ER. Interestingly, a similar mechanism may explain how the cholera toxin A chains penetrate into the cytosol (Tsai et al., 2002).
RNAi silencing screens with the entire human kinome have demonstrated that, in HeLa cells, the caveolar/raft pathways that mediate SV40 entry depend on some 80 different kinases. The set of kinases show only partial overlap with those that regulate the clathrin-mediated entry of VSV (Pelkmans et al., 2005). Interestingly, many of the kinases involved in SV40 entry are known to have functions in integrin signaling and actin regulation.
Other viruses that use caveolar/raft endocytosis include Echo 1, a picornavirus that binds to integrins, and coxsackie B in human Caco-2 epithelial cells (Coyne and Bergelson, 2006, Pietiainen et al., 2005). For Echo 1 virus, caveolar/raft uptake and entry require signaling that involves protein kinase C, and penetration seems to occur in caveosomes without the involvement of the ER (Pietiainen et al., 2004, Upla et al., 2004).
In addition to these pathways, there is increasing evidence for clathrin-independent pathways of virus entry into the endosomal system (Figure 2B). Such a pathway has been observed for the arenavirus lymphocytic choriomeningitis virus (LCMV) and, as already mentioned, for a fraction of incoming influenza viruses (Borrow and Oldstone, 1994, Rust et al., 2004, Sieczkarski and Whittaker, 2002). The molecular mechanisms involved in the pathway (or pathways) are not understood. By contrast, following binding to CAR and αv integrin coreceptors, adenovirus type 2 (Ad2) activates an actin-dependent process classified as macropinocytosis (Figure 2A), but virus endocytosis occurs via clathrin-coated pits. Inhibition of macropinocytosis inhibits virus entry, but how exactly this endocytic process influences virus penetration is unclear (Meier et al., 2002). Although the formation of primary vesicles displays great heterogeneity, the subsequent steps inside the cell involve either endosomes or caveosomes and require cholesterol (Imelli et al., 2004). That these two organelle classes are not entirely independent of each other is shown by their regulation through a small subset of common kinases and by the observation that cargo can be transferred between them (Pelkmans et al., 2004).
Together with bacterial toxins such as cholera, anthrax, and Shiga toxin, viruses are emerging as valuable ligands for charting the various ligand-inducible pathways of endocytosis. The spectrum of pathways is often redundant and cell-type specific. Differences in sensitivity to inhibitors, expression of dominant-negative mutants, and live-cell microscopy show that the pathways differ in their kinetics and their dependence on host-cell kinases; dynamins; Rac-, Rab-, and Arf-family GTPases; actin and tubulin; and cholesterol. The pathways are also likely to be physiologically regulated (e.g., the upregulation of compensatory pathways when clathrin-mediated endocytosis is inhibited; Damke et al., 1995). This may be one reason why viruses such as SV40 and influenza can use several different pathways, enabling them to infect a wide range of cells under various conditions. The question arises as to whether the pathways that have been identified in tissue culture systems, in some cases under unusual experimental conditions, occur in the relevant cells in vivo or whether there are additional endocytic entry mechanisms still to be discovered.
It will take some effort to define how many different endocytic pathways operate in cells and their normal functional activities. Further analysis using siRNA screens, with larger siRNA libraries and other viruses, may allow the identification of unique sets of proteins that are required for the entry of viruses that use a specific endocytic mechanism, thus providing a genetic fingerprint for each pathway. Such analyses should demonstrate whether these different pathways involve distinct molecular mechanisms or whether overlapping sets of proteins mediate several variants of essentially a single endocytic mechanism.
Virus Transmission: Cell-free versus Cell-Cell Infection
The existence of pH-independent fusion proteins has an important consequence for some viruses. When these proteins are expressed on the cell surface, they can allow fusion of the infected cell with neighboring noninfected cells expressing appropriate receptors. This leads to formation of heterokaryons, or syncytia, and potentially allows for the dissemination of the virus without the formation of mature virus particles. Nevertheless, in the majority of cases, the transfer of viral genomes from cell to cell appears to occur through the formation of virus particles that are released from infected cells and use the mechanisms described above to enter new uninfected hosts. Interestingly, the herpes virus varicella-zoster (VZV) appears to use cell-free viruses to spread from human to human but direct cell-to-cell transfer, without formation of infectious virions, to spread within an infected host (Chen et al., 2004). Most studies of viral entry are, by necessity, conducted in tissue culture systems using cell lines that differ from the cells that are the normal targets for infection in vivo. Although these systems have contributed greatly to our understanding of virus entry, they do not fully replicate the in vivo scenario, and, as a consequence, crucial aspects of entry mechanisms may be missed.
In experimental systems, the entry stage of infection is usually studied by adding cell-free virus particles to cells. Often the efficiency of entry is low, and experimenters frequently enhance virus adsorption by including charged polymers, such as polybrene, in the medium or by gently centrifuging virus particles onto cells (O'Doherty et al., 2000). Viruses that have adapted to growing in tissue culture cells can show an enhanced tendency to use proteoglycans for initial recruitment to cell surfaces (de Haan et al., 2005, Vlasak et al., 2005). Indeed, HIV-1 strains adapted to grow in T cell lines appear to bind to cells via proteoglycans before engaging CD4 and coreceptors (Ugolini et al., 1999). Together this suggests that the events involved in virus adsorption and entry in tissue culture may be different from those that occur in vivo. A number of recent studies suggest why this is the case.
Transmission of viruses from cell to cell in epithelial cells, for example, may occur through the basolateral domains, where a virus released from one cell can be effectively deposited on adjacent cells, minimizing the events associated with virus recruitment to the cell surface. Vaccinia virus (VV) provides an extreme example. VV is produced in several forms, of which the intracellular mature virus (IMV) and extracellular enveloped virus (EEV) are capable of infecting target cells. The EEV is initially assembled with three membranes and is released to the cell surface when the outer membrane fuses with the plasma membrane to produce an extracellular double-membraned particle. The EEV remains bound to the surface of the producer cell and induces the polymerization of cytoplasmic actin comets that push the virus around on the surface of the cell, often at the tips of filopodium-like projections (Smith et al., 2003b, Wolffe et al., 1998) (see Figure 4 and Greber and Way, 2006 [this issue of Cell]). In confluent cultures, and presumably in vivo as well, these projections push viruses into neighboring cells and enhance cell-to-cell transfer. Deletion of viral proteins involved in transmembrane signaling and actin nucleation reduces the viral plaque size in tissue culture and pathogenesis in vivo (Smith et al., 2003b, Wolffe et al., 1998).
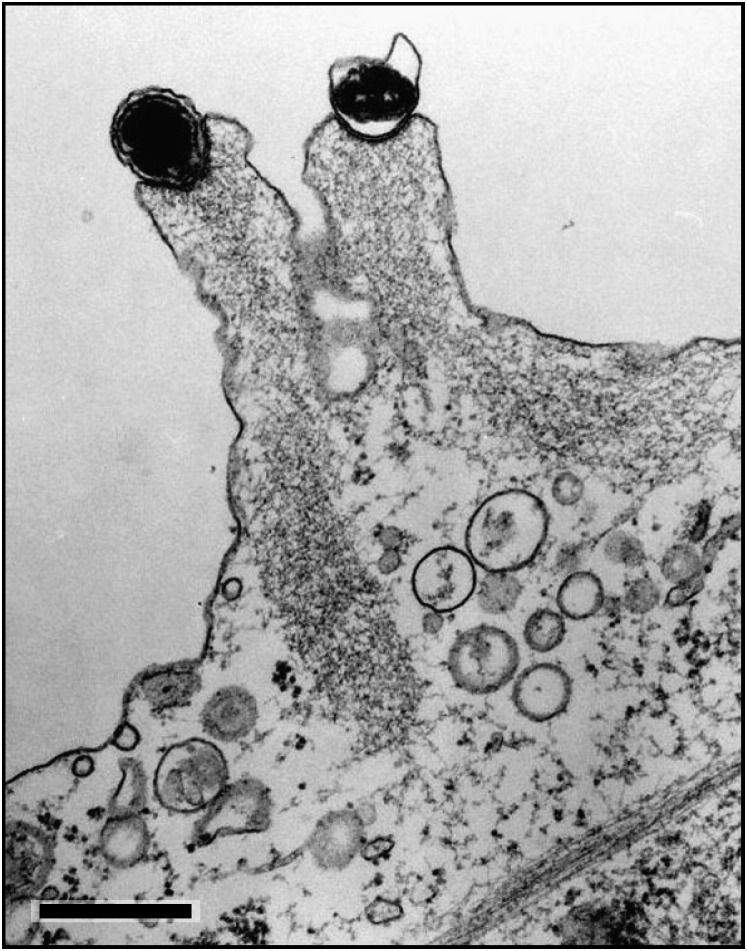
Figure 4.
Swine Pox Viruses at the Tip of Projections in the Plasma Membrane of the Infected Cell
After release from the cell, the viruses remain associated with the plasma membrane, where they induce assembly of actin filaments. The projections that are formed are motile and push the viruses into contact with neighboring cells. In this way, they promote infection within tissues. Note the actin filaments emanating from the area of virus cell contact. Courtesy of J. Kartenbeck. Scale bar = 500 nm.
Recently, structures termed infectious or virological synapses have been increasingly implicated in cell-to-cell virus transfer. Initially described for the transfer of the human T cell leukemia virus type 1 (HTLV-1) from infected to uninfected T cells, these areas of intimate contact between the infected and uninfected cells were recognized to have features in common with immunological synapses (Igakura et al., 2003). In contrast to T cell killing or antigen presentation, virological-synapse formation between T cells does not require major histocompatibility antigens (Jolly and Sattentau, 2004). The synapse provides a domain where virus assembly can be focused to release particles efficiently to a target cell. Similar means of transfer have been described for herpes viruses (Johnson and Huber, 2002) and for the T cell to T cell transfer of HIV (Jolly and Sattentau, 2004).
A particularly intriguing example of infectious synapse function is that described for dendritic cells. For many years it has been recognized that a highly efficient way of infecting T cells with HIV in culture is to first present the virus to dendritic cells (Cameron et al., 1992). HIV infection of dendritic cells can occur but is generally regarded as being inefficient. Recent studies have suggested an alternative mode of interaction whereby viruses may be captured by C type lectins (Geijtenbeek et al., 2000, Turville et al., 2002), and perhaps other receptors involved in antigen acquisition, and internalized into endocytic vesicles without infecting the dendritic cell. This virus can be regurgitated when dendritic cells interact with CD4-positive T cells and is able to infect the T cell in trans through infectious or virological synapses—a process that mimics the normal course of antigen presentation (McDonald et al., 2003). Viruses can be harbored in endocytic vesicles for hours, if not days, without losing infectivity, potentially allowing the dendritic cells in vivo to undergo maturation and relocation from peripheral tissue, where they would initially encounter viruses, to lymphoid organs. The endocytic compartment in which endocytosed viruses are sequestered is morphologically complex and bears some similarity to the compartments in macrophages where HIV assembles (Garcia et al., 2005). Although trans-infection has been documented in tissue culture, it remains unclear whether it operates in the same manner in vivo where, for HIV at least, infection of dendritic cells and the production of viruses in infected dendritic cells may be more relevant (Turville et al., 2004). Regardless, the transfer of viruses through infectious synapses is likely to operate in both scenarios.
Similar dendritic-cell-mediated trans-infection routes have been proposed for a number of other viruses, including herpes, filo-, and flaviviruses (van Kooyk and Geijtenbeek, 2003). Thus, these viruses have adapted to exploit a loophole in the defense strategies ranged against them by effectively using the cell's endocytosis and/or secretory mechanisms to allow their release to be temporally and spatially coordinated to focus infectious viruses on target cells. It is likely that similar strategies are used in various situations by different viruses; in particular, following reactivation of latent viruses, the transfer of HSV and varicella-zoster virus (chicken pox) from infected neurones to epithelial cells is likely to exploit specialized cell junctions (Johnson and Huber, 2002).
Thus, although the full life cycle of a virus requires assembly, dissemination by release from cells, and entry into new targets, the time during which viruses are genuinely cell free may be minimal. These mechanisms may limit the extent to which a virus is exposed to the humoral immune system (Johnson and Huber, 2002) and may also explain, for example, why, in HIV patients, infected T cells harbor multiple proviral genomes, suggestive of cells having undergone multiple, reasonably synchronous infection events (Jung et al., 2002).
Viral Restriction
Many viruses only infect a restricted range of cells, limiting their species tropism and cell tropism. Frequently, the restriction on infection is due to the absence of appropriate receptors. With retroviruses, however, a number of other mechanisms to limit viral replication have been discovered, and many of these operate at the level of entry (Towers and Goff, 2003). For HIV in particular, several mechanisms have now been described through which virus replication can be limited independently of receptor expression. Cellular RNA-editing enzymes can be packaged into virus particles and can introduce mutations in the viral genome during the reverse-transcription event that occurs shortly after virus entry. HIV-1 uses its Vif protein to overcome this restriction by preventing packaging of the editing enzymes into virus particles (Sheehy et al., 2002). In another example, the so-called Lv1 (or Ref1) restriction that prevents HIV-1 replication in Old World monkeys is mediated by a homo-oligomeric protein called Trim 5α (Stremlau et al., 2004, Towers, 2005). Trim 5α appears to bind to an exposed loop on the capsid protein that becomes available when the capsid is released to the cytoplasm after fusion (Ylinen et al., 2005). Trim 5α appears to prevent uncoating of the incoming particles by crosslinking the capsid protein subunits, though in some cases it may operate after reverse transcription (Ylinen et al., 2005). The Fv1 restriction seen with murine leukemia viruses operates after reverse transcription and prevents nuclear entry (Towers et al., 2000). Interestingly, the Fv1 restriction maps to an endogenous retroviral gag gene (Best et al., 1996), suggesting that interaction between incoming capsids and an endogenous Gag protein prevents infection.
In another form of restriction, now termed Lv2, some strains of HIV-1 and HIV-2 fail to replicate efficiently in HeLa cells. The phenotype maps to specific amino acids in both Env and capsids. For the Env-mediated restriction, at least, the failure to efficiently infect cells appears to be related to virus delivery to a raft-mediated endocytic pathway, and experimental manipulations that disrupt raft-mediated endocytosis overcome the restriction, as does diverting the virus to a clathrin-mediated pathway by pseudotyping the virus with the VSV-G protein (Marchant et al., 2005, Schmitz et al., 2004). Thus, not only must viruses find their way to the correct pathway for infection, but diversion to an alternative pathway, or blind alley, can limit the potential for subsequent infection and replication. Other mechanisms through which the mode of penetration determines the outcome of subsequent events in virus entry appear to operate for other related viruses (Kim et al., 2001).
Perspectives
Among the many challenges for the future is the need to identify exactly how many endocytic mechanisms contribute to virus entry and to determine which viruses use these pathways. RNAi screens similar to those described by Pelkmans et al. (2005) will allow a systematic approach to these questions with the possibility that specific routes can be defined by the requirement for a unique set of genes and their encoded proteins. Thus, each endocytic mechanism might be given a unique genetic fingerprint. Understanding these processes may allow specific cellular pathways or molecular machines to be targeted pharmacologically to inhibit the entry of any virus that uses the route for infection. Such an approach may offer the advantage that it will be more difficult for a virus to find a way around the block by mutation. It will also be important to show that the pathways identified in tissue culture experiments operate in vivo during a normal infection. Such experiments will be difficult, especially with human viruses, but inhibitors identified through tissue culture experiments may make it possible to address these issues.
At the level of cells and virus particles, developments in morphological techniques from high-end light microscopy to electron microscopy are allowing the events involved in virus entry to be analyzed with increased spatial and temporal resolution. Single-particle tracking of virus particles containing components labeled with different fluorochromes or fluorescent proteins is allowing specific events to be followed in remarkable detail. For some viruses with high infectious-unit-to-particle ratios, such as SFV, such studies provide direct information on entry. More careful attention to the preparation and storage of other viruses may allow similar morphological experiments to be interpreted with the confidence that the events observed represent the behavior of infectious particles. The ability to use microscopes in high-throughput screens for inhibitors of virus entry or to determine key components in the entry pathway will have a far-reaching impact on our understanding of virus entry. Together, these studies should provide detailed insights to the cellular mechanisms of endocytosis. The identification of natural ligands will also be key in establishing the biological relevance of these pathways. If we revisit this topic in 5 years' time, we will have a very much more detailed picture of virus entry. Viruses will continue to hold crucial cards in our attempts to understand cells and their pathogens.
Acknowledgments
M.M. is supported by the UK Medical Research Council. A.H. is supported by the Swiss National Research Foundation, the European Union Fifth Framework (EUROGENEDRUG), and the ETH Zurich. We thank Annegret Pelchen-Matthews, Stefan Moese, Peter Rottier, Varpu Marjomäki, Urs Greber, and Thomas Kershaw for critical comments on the manuscript, and we apologize to colleagues whose work has not been directly cited due to space limitations.
References
- Abrami L., Lindsay M., Parton R.G., Leppla S.H., van der Goot F.G. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J. Cell Biol. 2004;166:645–651. doi: 10.1083/jcb.200312072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenburg B.C., Graham D.Y., Estes M.K. Ultrastructural study of rotavirus replication in cultured cells. J. Gen. Virol. 1980;46:75–85. doi: 10.1099/0022-1317-46-1-75. [DOI] [PubMed] [Google Scholar]
- Anderson H.A., Chen Y., Norkin L.C. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E.A., Murphy P.M., Farber J.M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Best S., Le Tissier P., Towers G., Stoye J.P. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature. 1996;382:826–829. doi: 10.1038/382826a0. [DOI] [PubMed] [Google Scholar]
- Borrow P., Oldstone M.B. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology. 1994;198:1–9. doi: 10.1006/viro.1994.1001. [DOI] [PubMed] [Google Scholar]
- Bubeck D., Filman D.J., Cheng N., Steven A.C., Hogle J.M., Belnap D.M. The structure of the poliovirus 135S cell entry intermediate at 10-angstrom resolution reveals the location of an externalized polypeptide that binds to membranes. J. Virol. 2005;79:7745–7755. doi: 10.1128/JVI.79.12.7745-7755.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes A.P., Griffin D.E. Large-plaque mutants of Sindbis virus show reduced binding to heparan sulfate, heightened viremia, and slower clearance from the circulation. J. Virol. 2000;74:644–651. doi: 10.1128/jvi.74.2.644-651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P.U., Freudenthal P.S., Barker J.M., Gezelter S., Inaba K., Steinman R.M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- Chandran K., Sullivan N.J., Felbor U., Whelan S.P., Cunningham J.M. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308:1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.J., Zhu Z., Gershon A.A., Gershon M.D. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell. 2004;119:915–926. doi: 10.1016/j.cell.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Chen Y., Norkin L.C. Extracellular simian virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp. Cell Res. 1999;246:83–90. doi: 10.1006/excr.1998.4301. [DOI] [PubMed] [Google Scholar]
- Coyne C.B., Bergelson J.M. Virus-induced Abl and Fyn kinase signals permit coxsakievirus entry through epithelial tight junctions. Cell. 2006 doi: 10.1016/j.cell.2005.10.035. in press. [DOI] [PubMed] [Google Scholar]
- Damico R.L., Crane J., Bates P. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc. Natl. Acad. Sci. USA. 1998;95:2580–2585. doi: 10.1073/pnas.95.5.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H., Baba T., van der Bliek A.M., Schmid S.L. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J. Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm E.M., Pelkmans L., Kartenbeck J., Mezzacasa A., Kurzchalia T., Helenius A. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 2005;168:477–488. doi: 10.1083/jcb.200407113. Published online January 24, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan C.A., Li Z., te Lintelo E., Bosch B.J., Haijema B.J., Rottier P.J. Murine coronavirus with an extended host range uses heparan sulfate as an entry receptor. J. Virol. 2005;79:14451–14456. doi: 10.1128/JVI.79.22.14451-14456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohner K., Sodeik B. The role of the cytoskeleton during viral infection. Curr. Top. Microbiol. Immunol. 2005;285:67–108. doi: 10.1007/3-540-26764-6_3. [DOI] [PubMed] [Google Scholar]
- Earp L.J., Delos S.E., Park H.E., White J.M. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 2005;285:25–66. doi: 10.1007/3-540-26764-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D.H., Deussing J., Peters C., Dermody T.S. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 2002;277:24609–24617. doi: 10.1074/jbc.M201107200. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Boll W., Van Oijen A., Hariharan R., Chandran K., Nibert M.L., Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Garcia E., Pion M., Pelchen-Matthews A., Collinson L., Arrighi J.F., Blot G., Leuba F., Escola J.M., Demaurex N., Marsh M., Piguet V. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic. 2005;6:488–501. doi: 10.1111/j.1600-0854.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Kwon D.S., Torensma R., van Vliet S.J., van Duijnhoven G.C., Middel J., Cornelissen I.L., Nottet H.S., KewalRamani V.N., Littman D.R. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Greber U.F. Signalling in viral entry. Cell. Mol. Life Sci. 2002;59:608–626. doi: 10.1007/s00018-002-8453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U.F., Way M. A superhighway to virus infection. Cell. 2006;124:741–754. doi: 10.1016/j.cell.2006.02.018. this issue. [DOI] [PubMed] [Google Scholar]
- Harrison S.C. Mechanism of membrane fusion by viral envelope proteins. Adv. Virus Res. 2005;64:231–261. doi: 10.1016/S0065-3527(05)64007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J. Cell Biol. 1980;84:404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L.D., Peters R.J., Delos S.E., Young J.A., Agard D.A., White J.M. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J. Cell Biol. 1997;139:1455–1464. doi: 10.1083/jcb.139.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle J.M. Poliovirus cell entry: common structural themes in viral cell entry pathways. Annu. Rev. Microbiol. 2002;56:677–702. doi: 10.1146/annurev.micro.56.012302.160757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igakura T., Stinchcombe J.C., Goon P.K., Taylor G.P., Weber J.N., Griffiths G.M., Tanaka Y., Osame M., Bangham C.R. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- Imelli N., Meier O., Boucke K., Hemmi S., Greber U.F. Cholesterol is required for endocytosis and endosomal escape of adenovirus type 2. J. Virol. 2004;78:3089–3098. doi: 10.1128/JVI.78.6.3089-3098.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson W.T., Giddings T.H., Jr., Taylor M.P., Mulinyawe S., Rabinovitch M., Kopito R.R., Kirkegaard K. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. Published online April 26, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.C., Huber M.T. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 2002;76:1–8. doi: 10.1128/JVI.76.1.1-8.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C., Sattentau Q.J. Retroviral spread by induction of virological synapses. Traffic. 2004;5:643–650. doi: 10.1111/j.1600-0854.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- Jung A., Maier R., Vartanian J.P., Bocharov G., Jung V., Fischer U., Meese E., Wain-Hobson S., Meyerhans A. Multiply infected spleen cells in HIV patients. Nature. 2002;418:144. doi: 10.1038/418144a. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J., Stukenbrok H., Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J. Cell Biol. 1989;109:2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M., Rey F.A. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat. Rev. Microbiol. 2006;4:67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.S., You X.J., Harmon M.E., Overbaugh J., Fan H. Use of helper-free replication-defective simian immunodeficiency virus-based vectors to study macrophage and T tropism: evidence for distinct levels of restriction in primary macrophages and a T-cell line. J. Virol. 2001;75:2288–2300. doi: 10.1128/JVI.75.5.2288-2300.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham M., Fujita A., Chadda R., Nixon S.J., Kurzchalia T.V., Sharma D.K., Pagano R.E., Hancock J.F., Mayor S., Parton R.G. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J. Cell Biol. 2005;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimstra W.B., Ryman K.D., Johnston R.E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 1998;72:7357–7366. doi: 10.1128/jvi.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong P.D., Wyatt R., Robinson J., Sweet R.W., Sodroski J., Hendrickson W.A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakadamyali M., Rust M.J., Babcock H.P., Zhuang X. Visualizing infection of individual influenza viruses. Proc. Natl. Acad. Sci. USA. 2003;100:9280–9285. doi: 10.1073/pnas.0832269100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc I., Luyet P.P., Pons V., Ferguson C., Emans N., Petiot A., Mayran N., Demaurex N., Faure J., Sadoul R. Endosome-to-cytosol transport of viral nucleocapsids. Nat. Cell Biol. 2005;7:653–664. doi: 10.1038/ncb1269. Published online June 1, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M.J., Sherer N.M., Marks C.B., Pypaert M., Mothes W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J. Cell Biol. 2005;170:317–325. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Stupack D., Bokoch G.M., Nemerow G.R. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J. Virol. 1998;72:8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D., Berson J.F., Cook D.G., Doms R.W. Characterization of human immunodeficiency virus type 1 gp120 binding to liposomes containing galactosylceramide. J. Virol. 1994;68:5890–5898. doi: 10.1128/jvi.68.9.5890-5898.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson B., Rainey E.K., Benjamin T., Baryshev M., Mkrtchian S., Tsai B. ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol. Cell. 2005;20:289–300. doi: 10.1016/j.molcel.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Marchant D., Neil S.J., Aubin K., Schmitz C., McKnight A. An envelope-determined, pH-independent endocytic route of viral entry determines the susceptibility of human immunodeficiency virus type 1 (HIV-1) and HIV-2 to Lv2 restriction. J. Virol. 2005;79:9410–9418. doi: 10.1128/JVI.79.15.9410-9418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Bron R. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J. Cell Sci. 1997;110:95–103. doi: 10.1242/jcs.110.1.95. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv. Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D., Wu L., Bohks S.M., KewalRamani V.N., Unutmaz D., Hope T.J. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- Meier O., Boucke K., Hammer S.V., Keller S., Stidwill R.P., Hemmi S., Greber U.F. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 2002;158:1119–1131. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes W., Boerger A.L., Narayan S., Cunningham J.M., Young J.A. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell. 2000;103:679–689. doi: 10.1016/s0092-8674(00)00170-7. [DOI] [PubMed] [Google Scholar]
- Nemerow G.R., Stewart P.L. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 1999;63:725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U., Swiggard W.J., Malim M.H. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 2000;74:10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono A., Freed E. Role of lipid rafts in virus replication. Adv. Virus Res. 2005;64:311–357. doi: 10.1016/S0065-3527(05)64010-9. [DOI] [PubMed] [Google Scholar]
- Parton R.G. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J. Histochem. Cytochem. 1994;42:155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- Parton R.G., Richards A.A. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Kartenbeck J., Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Puntener D., Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Bürli T., Zerial M., Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Fava E., Grabner H., Hannus M., Habermann B., Krausz E., Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- Pietiainen V., Marjomaki V., Upla P., Pelkmans L., Helenius A., Hyypia T. Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin II, and signaling events. Mol. Biol. Cell. 2004;15:4911–4925. doi: 10.1091/mbc.E04-01-0070. Published online September 8, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietiainen V.M., Marjomaki V., Heino J., Hyypia T. Viral entry, lipid rafts and caveosomes. Ann. Med. 2005;37:394–403. doi: 10.1080/07853890510011976. [DOI] [PubMed] [Google Scholar]
- Puri A., Hug P., Jernigan K., Barchi J., Kim H.Y., Hamilton J., Wiels J., Murray G.J., Brady R.O., Blumenthal R. The neutral glycosphingolipid globotriaosylceramide promotes fusion mediated by a CD4-dependent CXCR4-utilizing HIV type 1 envelope glycoprotein. Proc. Natl. Acad. Sci. USA. 1998;95:14435–14440. doi: 10.1073/pnas.95.24.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Rossmann M.G., He Y., Kuhn R.J. Picornavirus-receptor interactions. Trends Microbiol. 2002;10:324–331. doi: 10.1016/s0966-842x(02)02383-1. [DOI] [PubMed] [Google Scholar]
- Rust M.J., Lakadamyali M., Zhang F., Zhuang X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 2004;11:567–573. doi: 10.1038/nsmb769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen A., Ahola T., Kaariainen L. Viral RNA replication in association with cellular membranes. Curr. Top. Microbiol. Immunol. 2005;285:139–173. doi: 10.1007/3-540-26764-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C., Marchant D., Neil S.J., Aubin K., Reuter S., Dittmar M.T., McKnight A. Lv2, a novel postentry restriction, is mediated by both capsid and envelope. J. Virol. 2004;78:2006–2016. doi: 10.1128/JVI.78.4.2006-2016.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D.K., Brown J.C., Choudhury A., Peterson T.E., Holicky E., Marks D.L., Simari R., Parton R.G., Pagano R.E. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol. Biol. Cell. 2004;15:3114–3122. doi: 10.1091/mbc.E04-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy A.M., Gaddis N.C., Choi J.D., Malim M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- Shieh J.T., Bergelson J.M. Interaction with decay-accelerating factor facilitates coxsackievirus B infection of polarized epithelial cells. J. Virol. 2002;76:9474–9480. doi: 10.1128/JVI.76.18.9474-9480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieczkarski S.B., Whittaker G.R. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J. Virol. 2002;76:10455–10464. doi: 10.1128/JVI.76.20.10455-10464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J., Wiley D. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- Smith A.E., Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- Smith A.E., Lilie H., Helenius A. Ganglioside-dependent cell attachment and endocytosis of murine polyomavirus-like particles. FEBS Lett. 2003;555:199–203. doi: 10.1016/s0014-5793(03)01220-1. [DOI] [PubMed] [Google Scholar]
- Smith G.L., Murphy B.J., Law M. Vaccinia virus motility. Annu. Rev. Microbiol. 2003;57:323–342. doi: 10.1146/annurev.micro.57.030502.091037. [DOI] [PubMed] [Google Scholar]
- Stang E., Kartenbeck J., Parton R.G. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell. 1997;8:47–57. doi: 10.1091/mbc.8.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle T., Harrison S.C. High-resolution structure of a polyomavirus VP1-oligosaccharide complex: implications for assembly and receptor binding. EMBO J. 1997;16:5139–5148. doi: 10.1093/emboj/16.16.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle T., Gamblin S.J., Yan Y., Harrison S.C. The structure of simian virus 40 refined at 3.1 A resolution. Structure. 1996;4:165–182. doi: 10.1016/s0969-2126(96)00020-2. [DOI] [PubMed] [Google Scholar]
- Steven A.C., Heymann J.B., Cheng N., Trus B.L., Conway J.F. Virus maturation: dynamics and mechanism of a stabilizing structural transition that leads to infectivity. Curr. Opin. Struct. Biol. 2005;15:227–236. doi: 10.1016/j.sbi.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P.L., Dermody T.S., Nemerow G.R. Structural basis of nonenveloped virus cell entry. Adv. Protein Chem. 2003;64:455–491. doi: 10.1016/s0065-3233(03)01013-1. [DOI] [PubMed] [Google Scholar]
- Stremlau M., Owens C.M., Perron M.J., Kiessling M., Autissier P., Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Tagawa A., Mezzacasa A., Hayer A., Longatti A., Pelkmans L., Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J. Cell Biol. 2005;170:769–779. doi: 10.1083/jcb.200506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers G., Bock M., Martin S., Takeuchi Y., Stoye J.P., Danos O. A conserved mechanism of retrovirus restriction in mammals. Proc. Natl. Acad. Sci. USA. 2000;97:12295–12299. doi: 10.1073/pnas.200286297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers G.J. Control of viral infectivity by tripartite motif proteins. Hum. Gene Ther. 2005;16:1125–1132. doi: 10.1089/hum.2005.16.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers G.J., Goff S.P. Post-entry restriction of retroviral infections. AIDS Rev. 2003;5:156–164. [PubMed] [Google Scholar]
- Tsai B., Ye Y., Rapoport T.A. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat. Rev. Mol. Cell Biol. 2002;3:246–255. doi: 10.1038/nrm780. [DOI] [PubMed] [Google Scholar]
- Tsai B., Gilbert J.M., Stehle T., Lencer W., Benjamin T.L., Rapoport T.A. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 2003;22:4346–4355. doi: 10.1093/emboj/cdg439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turville S.G., Cameron P.U., Handley A., Lin G., Pohlmann S., Doms R.W., Cunningham A.L. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 2002;3:975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- Turville S.G., Santos J.J., Frank I., Cameron P.U., Wilkinson J., Miranda-Saksena M., Dable J., Stossel H., Romani N., Piatak M., Jr. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- Ugolini S., Mondor I., Sattentau Q.J. HIV-1 attachment: another look. Trends Microbiol. 1999;7:144–149. doi: 10.1016/s0966-842x(99)01474-2. [DOI] [PubMed] [Google Scholar]
- Upla P., Marjomaki V., Kankaanpaa P., Ivaska J., Hyypia T., Van Der Goot F.G., Heino J. Clustering induces a lateral redistribution of alpha 2 beta 1 integrin from membrane rafts to caveolae and subsequent protein kinase C-dependent internalization. Mol. Biol. Cell. 2004;15:625–636. doi: 10.1091/mbc.E03-08-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y., Geijtenbeek T.B. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- Vlasak M., Goesler I., Blaas D. Human rhinovirus type 89 variants use heparan sulfate proteoglycan for cell attachment. J. Virol. 2005;79:5963–5970. doi: 10.1128/JVI.79.10.5963-5970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheit A., Helenius A. Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol. 2005;3:e233. doi: 10.1371/journal.pbio.0030233. Published online June 21, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe E.J., Weisberg A.S., Moss B. Role for the vaccinia virus A36R outer envelope protein in the formation of virus-tipped actin-containing microvilli and cell-to-cell virus spread. Virology. 1998;244:20–26. doi: 10.1006/viro.1998.9103. [DOI] [PubMed] [Google Scholar]
- Ylinen L.M., Keckesova Z., Wilson S.J., Ranasinghe S., Towers G.J. Differential restriction of human immunodeficiency virus type 2 and simian immunodeficiency virus SIVmac by TRIM5alpha alleles. J. Virol. 2005;79:11580–11587. doi: 10.1128/JVI.79.18.11580-11587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.T.Y. Virus entry and uncoating. In: Knipe D.M., Howley P.M., editors. Fields Virology. Fourth Edition. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 87–103. [Google Scholar]
- Zhang Y., Bergelson J.M. Adenovirus receptors. J. Virol. 2005;79:12125–12131. doi: 10.1128/JVI.79.19.12125-12131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]