Abstract
The ADAMs (a disintegrin and metalloproteinase) are a fascinating family of transmembrane and secreted proteins with important roles in regulating cell phenotype via their effects on cell adhesion, migration, proteolysis and signalling. Though all ADAMs contain metalloproteinase domains, in humans only 13 of the 21 genes in the family encode functional proteases, indicating that at least for the other eight members, protein–protein interactions are critical aspects of their biological functions. The functional ADAM metalloproteinases are involved in “ectodomain shedding” of diverse growth factors, cytokines, receptors and adhesion molecules. The archetypal activity is shown by ADAM-17 (tumour necrosis factor-α convertase, TACE), which is the principal protease involved in the activation of pro-TNF-α, but whose sheddase functions cover a broad range of cell surface molecules. In particular, ADAM-17 is required for generation of the active forms of Epidermal Growth Factor Receptor (EGFR) ligands, and its function is essential for the development of epithelial tissues. Several other ADAMs have important sheddase functions in particular tissue contexts. Another major family member, ADAM-10, is a principal player in signalling via the Notch and Eph/ephrin pathways. For a growing number of substrates, foremost among them being Notch, cleavage by ADAM sheddases is essential for their subsequent “regulated intramembrane proteolysis” (RIP), which generates cleaved intracellular domains that translocate to the nucleus and regulate gene transcription. Several ADAMs play roles in spermatogenesis and sperm function, potentially by effecting maturation of sperm and their adhesion and migration in the uterus. Other non-catalytic ADAMs function in the CNS via effects on guidance mechanisms. The ADAM family are thus fundamental to many control processes in development and homeostasis, and unsurprisingly they are also linked to pathological states when their functions are dysregulated, including cancer, cardiovascular disease, asthma, Alzheimer’s disease. This review will provide an overview of current knowledge of the human ADAMs, discussing their structure, function, regulation and disease involvement.
Keywords: ADAM, Metalloproteinase, Disintegrin, Sheddase
1. Introduction
The ADAMs (a disintegrin and metalloproteinase) are a family of transmembrane and secreted proteins of approximately 750 amino acids in length, with functions in cell adhesion and proteolytic processing of the ectodomains of diverse cell surface receptors and signalling molecules. Their name derives from their modular construction and makes an entertaining allusion to the initial characterization of members of the family in snake venom and as sperm proteins associated with fertility (Wolfsberg et al., 1995). They have been identified in many species, from the nematode Caenorhabditis elegans through to the expanded family found in vertebrates (Huxley-Jones et al., 2007). ADAM-like sequences have been found in the fission yeast Schizosaccharomyces pombe but are absent from Saccharomyces cerevisiae and plants. The biological processes to which ADAMs have been linked functionally include sperm–egg interactions, cell fate determination in the nervous system, cell migration, axon guidance, muscle development and diverse aspects of immunity. In humans, ADAM-17 (otherwise known as tumour necrosis factor-α converting enzyme, TACE) orchestrates immune and inflammatory responses via activation of pro-TNF-α, but is also essential during development for activation of membrane-associated ligands of the epidermal growth factor receptor (EGFR) (Blobel, 2005). Ectodomain shedding of several substrates is coupled to their subsequent cleavage by “regulated intramembrane proteolysis” (RIP) generating nucleus-targeted signals, foremost among these being Notch (Saftig and Hartmann, 2005). ADAM-10 is the principal sheddase involved in Notch signalling and it has also emerged as a key player in fate specification and guidance mechanisms via Eph tyrosine kinases and their ligands, ephrins (Janes et al., 2005). The ADAMs play a potentially protective role in the pathogenesis of Alzheimer’s disease by participating in non-amyloidogenic processing of amyloid-β precursor protein (APP). However, in cancer several ADAMs enhance the malignant aspects of tumour behaviour by stimulating cell proliferation via EGFR Receptor activation (Borrell-Pages et al., 2003, Kenny and Bissell, 2007) and by induction of epithelial–mesenchymal transition via cleavage of E-cadherin (Maretzky et al., 2005a). Thus, particular ADAMs are attractive targets for drug development (Moss et al., 2008a), though it is clearly essential to understand the complex repertoire of physiological roles of these important and multifaceted molecules in order to avoid the disappointments that attended clinical use of broad spectrum matrix metalloproteinase inhibitors (Coussens et al., 2002).
This chapter reviews current knowledge of the ADAM metalloproteinases, with a major focus on the human ADAMs and their pathophysiological roles. Attention will be concentrated on the ADAMs proper, and we exclude from this survey detailed discussion of the related and fascinating ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family, since there have been several excellent recent reviews of this group, whose roles are fundamentally distinct from those of the ADAMs (Apte, 2004, Jones and Riley, 2005, Porter et al., 2005).
The reader is directed to a number of other valuable sources of information on the ADAMs, including the book “The ADAM Family of Proteases”, edited by Hooper and Lendeckel (2005, Springer, The Netherlands) (White et al., 2005), and important recent reviews (Arribas et al., 2006, Blobel, 2005, Garton et al., 2006, Mochizuki and Okada, 2007, Rocks et al., 2008, Seals and Courtneidge, 2003, Tousseyn et al., 2006, White, 2003, Murphy et al., 2008).
1.1. Overview of the ADAM family: modular proteins with proteolytic and non-proteolytic functions
The ADAMs belong to the M12B adamalysin protease subfamily in the MEROPS classification (http://merops.sanger.ac.uk/; Rawlings et al., 2008), which includes the closely related class PIII snake venom reprolysins and the ADAMTSs. They have also in the past been referred to as the MDC (metalloproteinase disintegrin cysteine-rich) family (Blobel, 1997), but the ADAM nomenclature is now established in common use, though their MDC alternative names are also provided for the examples listed in Table 1 . The typical modular layout of the ADAMs is shown in Fig. 1 , which also contrasts their structure with those of their closest relatives.
Table 1.
The human ADAMs
| Gene | Other aliases | Accession number | Location | Proteolytic activity? | Present in Mus musculus | Inhibition by TIMPs | Major sites of expression in human tissues |
|---|---|---|---|---|---|---|---|
| ADAM1 | Fertilin alpha, FTNAP, Ftna, PH-30a | NG_001216 | 12q24.13 | Pseudogene | Yes (NM_172126, NM_172125) | – | – |
| ADAM2 | Fertilin beta, CRYN1, CRYN2, FTNB, PH-30b, PH30 | NM_001464 | 8p11.2 | No | Yes (NM_009618) | No study | Testis |
| ADAM3 | CYRN1, tMDCI | NR_001569 | 8p21-p12 | Pseudogene | Yes (NM_009619) | – | – |
| ADAM5 | TMDCII | NR_001448 | 8p11.23 | Pseudogene | Yes (NM_007401) | – | – |
| ADAM6 | t MDCIV; C14orf96 | NR_002224 | 14q32.33 | Probably pseudogene | Yes (NM_174885) | – | – |
| ADAM7 | EAPI, GP-83 | NM_003817 | 8p21.2 | No | Yes (NM_007402) | – | Testis, erythrocytes |
| ADAM8 | CD156, MGC134985, MS2 | NM_001109 | 10q26.3 | Yes | Yes (NM_007403) | None | Bone marrow lymphoid/ myeloid cells, lymphatic system, haematopoietic stem cells, peripheral blood lymphoid/myeloid cells |
| ADAM9 | KIAA0021, MCMP, MDC9, Mltng, meltrin gamma | v1 NM_003816 | 8p11.23 | Yes | Yes (NM_007404) | None | Mesenchymal stem cells, placenta, pancreas, adult stem cells, adipose tissue |
| v2 NM_001005845 | |||||||
| ADAM10 | CD156c, HsT18717, MADM, kuz | NM_001110 | 15q22 | Yes | Yes (NM_007399) | TIMP-1 TIMP-3 | Mesenchymal stem cells, placenta, blood myeloid cells, bladder, bone marrow myeloid cells |
| ADAM11 | MDC | NM_002390 | 17q21.3 | No | Yes (NM_009613, NM_001110778) | – | Erythrocytes, central and peripheral nervous systems, liver and biliary system, salivary gland |
| ADAM12 | RP11-295J3.5, MCMP, MCMPMltna, MLTN, MLTNA, Meltrin alpha | v1 NM_003474 | 10q26.3 | Yes | Yes (NM_007400) | TIMP-2 | Placenta, mesenchymal stem cells, adult stem cells |
| v2 NM_021641 | TIMP-3 | ||||||
| ADAM15 | MDC15, Metargidin | v1 NM_207191 | 1q21.3 | Yes | Yes (NM_001037722, NM_009614) | No study | Widespread (highest in mesenchymal stem cells and urogenital system) |
| v2 NM_003815 | |||||||
| v3 NM_207194 | |||||||
| v4 NM_207195 | |||||||
| v5 NM_207196 | |||||||
| v6 NM_207197 | |||||||
| v6a AB209157 | |||||||
| v6b AY560599 | |||||||
| v7a AY560600 | |||||||
| v7b AY560601 | |||||||
| v8 AY576417 | |||||||
| ADAM17 | CD156b, MGC71942, TACE, cSVP | NM_003183 | 2p25 | Yes | Yes (NM_009615) | TIMP-3 | Widespread (highest in lymphatic) |
| ADAM18 | ADAM27, MGC41836, MGC88272, tMDCIII | NM_014237 | 8p11.22 | No | Yes (NM_010084) | – | Testis, erythrocytes, bone marrow, pancreas) |
| ADAM19 | FKSG34, MADDAM, MLTNB | v1 NM_023038 v2 NM_033274 | 5q32–q33 | Yes | Yes (NM_009616) | None | Widespread (highest in placenta, mesenchymal stem cells, lymphatic system, heart) |
| ADAM20 | NM_003814 | 14q24.1 | Yes | No | No study | Testis, erythrocytes, bone marrow | |
| ADAM21 | ADAM31, MGC125389 | NM_003813 | 14q24.1 | Yes | Yes (NM_020330) | No study | Testis, erythrocytes, central and peripheral nervous systems |
| ADAM 22 | MDC2, MGC149832 | v1 NM_021723 | 7q21 | No | Yes (NM_001007220, NM_001007221, NM_001098225) | – | Peripheral and central nervous systems |
| v2 NM_021722 | |||||||
| v3 NM_016351 | |||||||
| v4 NM_004194 | |||||||
| v5 NM_021721 | |||||||
| ADAM23 | MDC3 | NM_003812 | 2q33 | No | Yes (NM_011780) | Peripheral and central nervous systems, heart | |
| ADAM28 | ADAM23, MDC-Lm, MDC-Ls, MDCL, eMDC | v1 NM_014265 | 8p21.2 | Yes | Yes (NM_010082, NM_183366) | TIMP-3 | Haematopoietic stem cells, pancreas, gastrointestinal system, bone marrow myeloid cells, lymphatic system, respiratory system, bladder |
| v3 NM_021777 | TIMP-4 | ||||||
| ADAM29 | svph1 | NM_014269 | 4q34 | No | Yes (NM_175939) | – | Testis |
| ADAM30 | svph4 | NM_021794 | 1p13-p11 | Yes | Yes (NM_027665) | No study | Testis |
| ADAM32 | FLJ26299, FLJ29004 | NM_145004 | 8p11.23 | No | Yes (NM_153397) | – | Blood lymphoid cells |
| ADAM33 | RP5-964F7.2, DJ964F7.1, DKFZp434K0521, FLJ35308, FLJ36751, MGC149823, MGC71889 | v1 NM_025220 | 20p13 | Yes | Yes (NM_033615) | TIMP-3 | Uterus, other urogenital system, respiratory system, gastrointestinal system, tongue, endocrine system |
| v2 NM_153202 | TIMP-4 | ||||||
| ADAMDEC1 (ADAM-like, decysin 1) | M12.219 | NM_014479 | 8p21.2 | Yes | Yes (NM_021475) | No study | Lymphatic system, bladder, gastrointestinal system |
Records for ADAM4 and ADAM25 have been discontinued by NCBI.
Also present in Mus musculus Adam4 (NM_009620), Adam4b (EG214321 – predicted gene), Adam24 (NM_010086), Adam25 (NM_011781), Adam26 (NM_010085), Adam31 (AF251559), Adam34 (NM_145745), Adam36 (BN000114), Adam37 (BN000115), Adam38 (NM_001009548), Adam39 (NM_001025380).
Fig. 1.
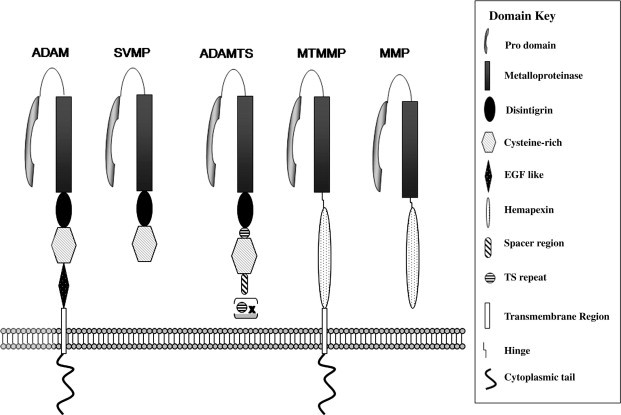
Comparison of the domain structures of the ADAM, MMP, SVMP and ADAMTS metalloproteinases. The domain organization of the proteases is compared, and their membrane or secreted location is indicated. Domains are not drawn to scale.
The ADAMs, matrix metalloproteinases (MMPs), ADAMTSs and snake venom metalloproteinases (SVMPs) all possess signal sequences at their N-termini, which direct the proteins to the secretory pathway. This is followed in all by a pro-domain, which acts as an intramolecular chaperone to ensure correct protein folding (Roghani et al., 1999) and functions via a cysteine-switch mechanism to maintain enzyme latency. The pro-domain is generally removed intracellularly during transit through the Golgi system by pro-protein convertases (Lum et al., 1998), though for ADAM-8 and -28 there is evidence that this can involve an autocatalytic mechanism (Howard et al., 2000, Schlomann et al., 2002). The metalloproteinase (MP) domain is immediately C-terminal to the pro-domain, and at this point the structural organization begins to diverge dramatically in different MP families. In the ADAMs, ADAMTSs and SVMPs, the MP domain is followed by a disintegrin-like domain, so-called for the ability of small proteins found in hemorrhagic snake venoms to bind platelet integrin αIIbβIIIa thereby blocking platelet aggregation (Niewiarowski et al., 1994). The disintegrin domain contains a 14-amino acid stretch known as the “disintegrin loop” that is implicated in interactions between ADAMs and integrins (White, 2003). [An exception to this is ADAMDEC-1, which is unusual in being truncated part way through the disintegrin domain through the introduction of a stop codon (Bates et al., 2002)]. The disintegrin domain is followed in the ADAMs by the cysteine-rich domain, which is also seen in the SVMPs and ADAMTSs, though in the latter the first of a series of C-terminal thrombospondin-like repeats is interposed (Porter et al., 2005). Most ADAMs then have an EGF-like domain [though this is absent in ADAM-10 and -17 (Janes et al., 2005)], adjacent to a membrane-spanning region, which is followed by a cytoplasmic tail that varies extensively in length and sequence between different ADAM family members. ADAM cytoplasmic domains have been shown to interact with proteins involved in intracellular signalling, trafficking and cytoarchitecture, and several display proline-rich motifs that can act as ligands for SH3 (Src homology region-3)-domain containing proteins, which will be discussed more extensively in Section 2.5.
Several ADAM genes display alternatively spliced transcripts that give rise to variant proteins. In particular, ADAM12 encodes both a long form (ADAM-12L) that has the typical transmembrane modular structure, and also ADAM-12S which is a secreted form that lacks the transmembrane and cytoplasmic domains (Wewer et al., 2006). A similar situation occurs for ADAM9 (Mazzocca et al., 2005), ADAM11 (Katagiri et al., 1995) and ADAM28 (Fourie et al., 2003, Roberts et al., 1999), while ADAM15 gives rise to as many as 13 different splice variants, all of which affect exons encoding the cytoplasmic domain (Kleino et al., 2007). The pattern of splice variants of ADAM15 is conserved between mouse and humans (Kleino et al., 2007), and this also applies for ADAM22, where again the alternative splicing affects only the cytoplasmic domain (Sagane et al., 2005). Alternative splicing of ADAM19 gives rise to a short form that lacks the pro-, metalloproteinase and disintegrin domains, and is found exclusively intracellularly (Kurisaki et al., 2002).
Thirty-eight members of the ADAM family have been catalogued from various species (http://people.virginia.edu/~jw7g/Table_of_the_ADAMs.html; http://www.uniovi.es/degradome/tables/table_met.htm). There is considerable variation in the ADAM genes between rodents and humans (Puente and Lopez-Otin, 2004, Puente et al., 2003). The human genome contains 25 ADAM genes of which 4 appear to be pseudogenes (see Table 1). In contrast, mouse and rat have 37 and 34 Adam genes, many of which are specifically expressed in testis (Puente and Lopez-Otin, 2004). Of the 21 presumed functional human ADAMs, 13 encode proteins that possess the characteristic reprolysin-type active site (HEXGHXXGXXHD) in the metalloproteinase domain followed downstream by the “Met turn” that is the signature of the Metzincin enzymes (Bode et al., 1993), indicating functional proteolytic capability. Although it is not included in other surveys of the ADAMs, we have chosen to include ADAMDEC-1 (also known as decysin), since we feel it has a legitimate claim for ADAM status. ADAMDEC-1 has the third of the histidines in the Zn-binding site replaced by an aspartate residue (HELGHVLGMPDV), a feature that is also seen in M6 and M7 family metzincins that are active metalloproteinases, and thus ADAMDEC-1 is likely also to be an active endopeptidase (Bates et al., 2002). The catalytically active human ADAM proteases are thus: ADAMDEC-1, ADAM-8, -9, 10, -12, -15, -17, -19, -20, -21, -28, -30 and -33. In contrast, ADAM-2, -7, -11, -18, -22, -23, -29 and -32 lack one or more critical features in the Zn-binding active site, suggesting that the metalloproteinase domain in these proteins may play roles in protein folding and protein–protein interactions rather than catalysis. However, based on studies with gene knockout mice which will be discussed in Section 1.3, it is clear that there are important developmental roles for individual ADAMs of both the active and proteolytically inactive groupings. Table 2 provides an overview of the substrates that have been identified for the catalytically active ADAMs, many of which will be considered in more detail when ADAM functions are explored in Section 3.
Table 2.
ADAM substrates
In humans and other vertebrates, ADAM2, 7, 18, 20, 21, 29 and 30 are expressed primarily in the testis, consistent with their involvement in spermatogenesis and sperm function (Fig. 2 ). Of the remaining genes, ADAM9, 10, 12, 15, 17 and 19 are expressed quite broadly in somatic tissues, while ADAM28 and 33 show a more restricted tissue range and ADAM8 is primarily active in hematopoietic cell types. Table 1 highlights the profound differences in tissue expression of the family members, based on In Silico Transcriptomic Datamining studies (K. Iljin and O. Kallioniemi, personal communication). Noteworthy is the expression of ADAM11, ADAM22 and ADAM23 predominantly in the central and peripheral nervous systems, and ADAM12 in placenta and mesenchymal or adult stem cell populations.
Fig. 2.
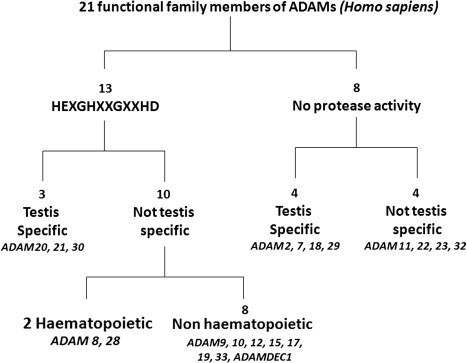
The 21 members of the human ADAM family, in relation to their metalloproteinase activity and sites of expression.
For the MMPs, a major level of control occurs via their interaction with specific tissue inhibitors of metalloproteinases (TIMPs), of which there are four in mammals (Baker et al., 2002). TIMPs inhibit MMPs by forming 1:1 tight, non-covalent complexes with active enzymes. With a few notable exceptions, TIMPs are broadly interchangeable in their ability to inhibit the MMPs, an exception being TIMP-1, which is inactive against MT1-MMP/MMP-14 and certain other MMPs (Baker et al., 2002). However, the TIMPs show much greater specificity towards the ADAM proteinases (see Table 1). In general, TIMP-3 is a functional inhibitor of the majority of proteolytically active ADAMs, and individual ADAMs display characteristic susceptibilities to the other TIMPs. The catalytic domain of ADAM-10 is inhibited by both TIMP-1 and TIMP-3 (Amour et al., 2000), whereas ADAM-8, -9 and -19 defy any pattern and are insensitive to TIMP inhibition (Amour et al., 2002, Chesneau et al., 2003). In contrast, ADAM-12S has recently been shown to be inhibited by TIMP-3 and TIMP-2 in preference to TIMP-1 (Jacobsen et al., 2008). ADAM-33 is moderately inhibited by TIMP-3 and TIMP-4, but weakly by TIMP-2 and is insensitive to TIMP-1 (Zou et al., 2004). These highly divergent characteristics reflect the importance of not only the MP domain in binding of TIMPs to active metalloproteinases, and this aspect will be discussed further in a following section. In addition to TIMPs, REversion-inducing Cysteine-rich protein with Kazal motifs (RECK), an unrelated protein that also displays MMP inhibitory capability (Baker et al., 2002) has been shown to be a physiological inhibitor of ADAM-10 in development of the nervous system (Muraguchi et al., 2007).
ADAM activity is also regulated by diverse stimuli. We will discuss this in more depth in Section 3.2 on ectodomain shedding, but suffice here to point out that ADAM-mediated shedding is both constitutive and inducible by agents such as G-protein coupled receptor (GPCR) activators, PKC activators, Ca ionophores, and other experimental or natural stimuli. The mechanisms are poorly understood, though involvement of the cytoplasmic domain is an obvious possibility. Phosphorylation or other modifications of the cytoplasmic domain may influence the interactions of ADAMs with adapter or trafficking proteins that direct substrate interactions or location in membrane sub-domains or other specific cellular locations. However, the cytoplasmic domain may be necessary only for particular functions, since the membrane localization and stimulus-induced shedding elicited by ADAM-17 and -19 has been shown, at least in some model systems, to occur in the absence of their cytoplasmic domains, and is dependent only on a membrane-spanning region (Reddy et al., 2000, Wakatsuki et al., 2004). Following stimulation, ADAM-17 rapidly disappears from the cell surface via endocytosis (Doedens and Black, 2000) which represents another potential control point for regulation of ADAM function.
1.2. Evolution of the ADAM genes
Insights into the evolutionary origins of human ADAM genes is emerging from comparison with other sequenced genomes, including rodents (Puente and Lopez-Otin, 2004), the primitive chordate Ciona intestinalis and the zebrafish Danio rerio (Huxley-Jones et al., 2007), and sea urchin (Angerer et al., 2006). The C. intestinalis genome is particularly insightful because Ciona is one of the closest invertebrate relatives of vertebrates. Its genome contains 4 ADAM genes: ADAMa and ADAMb are orthologous to ADAM17 and ADAM10, respectively, and define an A sub-group, while ADAMc1 and c2 genes define a separate B sub-group that corresponds to human ADAM2, 7–9, 11, 12, 15, 18, 19, 22, 23, 28, 32, and 33. The remaining human ADAMs are specific to the vertebrate lineage, though as the authors point out, it is possible that invertebrate orthologs of these genes may have been lost during evolution. Like Ciona, Drosophila melanogaster contains orthologs of ADAM17 and ADAM10. Indeed there are two Drosophila genes that are highly similar to ADAM10 – kuzbanian (kuz) and kul (Huxley-Jones et al., 2007). Drosophila also has two genes corresponding to the B subgroup seen in Ciona. The sea urchin Strongylocentrus purpuratus contains only 2 ADAM genes, and does not seem to have an ortholog of ADAM10, though an ortholog exists in the nematode Caenorhabditis elegans (Angerer et al., 2006). The two sea urchin genes appear to be most closely related to ADAM17 and one of the B subgroup Ciona ADAMs (described as ADAM12/15/19/33-like) (Angerer et al., 2006). The absence of an ADAM10 ortholog in sea urchin is perhaps surprising, given the central role of this protease in signalling via the Notch pathway.
Based on the sequence of the metalloproteinase domain, the human ADAMs can be arranged in the family tree shown in Fig. 3 , which emphasizes the separation of ADAM10 and 17 from the other ADAMs, and draws attention to particular sub-groups such as a proteolytically active cluster encompassing ADAMDEC1, ADAM8, 12, 15, 19, 28 and 33. Some of these relationships are borne out by chromosomal locations: for instance, ADAMDEC1, ADAM7 and ADAM28 are clustered together on chromosome 8p12, suggesting a common origin from an ancestral gene by duplication (Bates et al., 2002). Other sub-groups of non-proteolytically active ADAMs are also apparent, such as the ADAM11, 22, 23 set and ADAM2, 18 and 32. A broadly similar subgroup analysis has been reported recently based on comparison of the full protein coding sequences of the ADAMs, and also on gene exon–intron organization (Kleino et al., 2007).
Fig. 3.
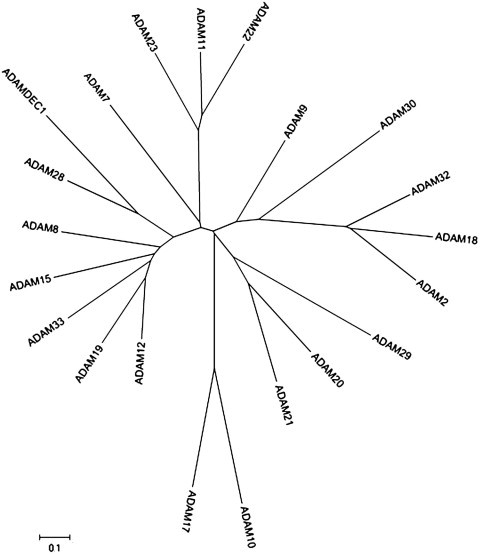
Phylogenetic tree of human ADAMs based on the sequences of their metalloproteinase domains. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 4 (Tamura et al., 2007). The scale bar represents amino acid substitutions per site, such that 0.1 represents 10% of sites having a substitution.
Further discussion on the evolution of ADAMs can be found in Section 3.1 on the role of ADAMs in fertility. Comparative analysis of the sequences of mammalian ADAM genes has revealed that the testis-specific genes are evolving at the fastest rate, with positive selection (as opposed to purifying selection) of codons in the disintegrin/cysteine-rich domains, which likely play a role in sperm adhesion to the uterus and oocyte (Glassey and Civetta, 2004). This was not true for the more broadly expressed ADAMs, which are subject to purifying selection.
1.3. Insights from gene knockout mice
Table 3 shows a summary of the phenotypes of the Adam-null mice that have been generated to date. More details will be provided on particular genetic knockouts (e.g. Adam1a, Adam1b, Adam2, Adam10, Adam17) in subsequent sections. We note below a few central observations for particular genes:
Table 3.
Phenotypes of Adam knockout mice
| ADAM knockout | Phenotype | References |
|---|---|---|
| Adam1a | Male infertility, defects in sperm migration | Nishimura et al. (2004) |
| Adam2 | Male infertility, defects in sperm migration and adhesion | Cho et al., 1998, Nishimura et al., 2001, Nishimura et al., 2007 |
| Defects in migration of neuroblasts to olfactory bulb | Murase et al. (2008) | |
| Adam3 | Male infertility, defects in sperm migration and adhesion | Cho et al., 1998, Kim et al., 2006, Nishimura et al., 2001, Stein et al., 2005 |
| Adam8 | Viable, fertile, no pathology (reduced CHL-1 shedding in brain) | Kelly et al. (2005) |
| Adam9 | Viable, fertile, no pathology | Sahin et al. (2004) |
| Adam10 | Early lethality E9.5dpc, defective CNS and heart development, somite formation and vasculogenesis. Phenocopies Notch deficiency (more severe than presenilin 1 and 2 double knockouts. | Hartmann et al. (2002) |
| Adam11 | Viable, fertile, impaired hippocampal-dependent spatial learning and altered nociception responses | Takahashi et al., 2006b, Takahashi et al., 2006a |
| Adam12 | Viable, fertile, 30% embryonic lethality, brown adipose abnormalities, no muscle defect | Kurisaki et al., 2003, Masaki et al., 2005, Sahin et al., 2004 |
| Adam15 | Viable, fertile, tumour neovascularization reduced, age onset osteoarthritis | Bohm et al., 2005, Horiuchi et al., 2003, Sahin et al., 2004 |
| Adam17 | Perinatal lethality, probably due to heart defects; pulmonary hypoplasia; problems with epithelial tissue maturation, phenocopies defects seen in EGFR, TGFα, HB-EGF and amphiregulin knockout mice | Jackson et al., 2003, Peschon et al., 1998, Shi et al., 2003, Zhao et al., 2001 |
| Adam19 | 80% postnatal lethality, multiple cardiovascular defects | Horiuchi et al., 2005, Kurohara et al., 2004, Sahin et al., 2004 |
| Adam22 | Postnatal lethality, ataxia and peripheral nerve hypomyelination | Sagane et al. (2005) |
| Adam33 | Viable, fertile, no pathology | Chen et al. (2006) |
Adam8. ADAM8 is primarily expressed in hematopoetic cell types (Table 1) and roles for ADAM-8 in leukocyte infiltration in neurodegenerative diseases have been suggested (Horiuchi et al., 2005). Shedding of the adhesion molecule CHL-1 by ADAM-8 has been linked to neurite extension and promotion of neuronal survival, and though Adam8−/− mice have no overt phenotype (Kelly et al., 2005), they show much reduced CHL-1 shedding in the brain (Naus et al., 2004). Elevated expression of Adam8 has been seen in Wobbler mice, along with increased CHL-1 shedding, suggesting possible involvement of ADAM-8 in the pathology of neurodegeneration (Naus et al., 2004). ADAM-8 has also been linked to asthma since allergens strongly induced Adam8 expression in peribronchial and perivascular inflammatory cells in an experimental asthma model (King et al., 2004).
Adam10. ADAM-10 is the principal ADAM sheddase involved in Regulated Intramembrane Proteolysis (RIP), with critical actions on Notch/Delta signalling and APP processing (Saftig and Hartmann, 2005; Fig. 4 ). Notch is a transmembrane protein that undergoes three proteolytic cleavages during its functional lifecycle. The first is cleavage by a pro-protein convertase in the secretary apparatus, so that the protein appears on the surface as a heterodimeric protein (Artavanis-Tsakonas et al., 1999). The second (S2) cleavage occurs upon activation by binding of one of several ligands (e.g. Delta and Jagged), followed by a third cleavage in the plane of the membrane by γ-secretase, which is composed of the aspartate proteases, presenilins 1 and 2 (Selkoe, 2001). This final cleavage releases the cytoplasmic domain of Notch, which then binds to a member of the CSL (CBF1, Su[H], Lag-1) family of transcription factors, which activate downstream genes (Schlondorff and Blobel, 1999). Evidence is now very strong that ADAM-10 is the main protease responsible for the S2 cleavage. Loss of ADAM-10 leads to early embryonic lethality around 9.5 days post coitum, with multiple defects in the developing central nervous and cardiovascular systems and somites, which closely resemble those seen in mice deficient in Notch/Delta signalling (Hartmann et al., 2002). This observation concurs with the findings from Drosophila, which indicated that the Kuzbanian (Kuz) gene was essential for controlling neural cell fate decisions in the developing nervous system via proteolytic cleavage of the transmembrane receptor Notch (Pan and Rubin, 1997, Rooke et al., 1996). Likewise the C. elegans orthologue (sup-17) was identified as a suppressor of the phenotype caused by constitutively active Notch/LIN-12 (Saftig and Hartmann, 2005). This therefore represents a fundamental biological signalling mechanism that is conserved from worms to mammals (Horiuchi et al., 2005).
Fig. 4.

Regulated intramembrane proteolysis of Notch and Amyloid Precursor Protein (APP).
Controversially, ADAM-17 was also reported to participate in Notch cleavage in vitro and in cultured mammalian cells (Brou et al., 2000). However, Adam17−/− mice do not show any defects related to Notch signalling (see below). Similar to Notch, APP is a transmembrane protein subject to RIPping via metalloproteinase and γ-secretase action (Saftig and Hartmann, 2005). Ectodomain shedding can involve either α- or β-secretases, which cleave differentially, the latter giving rise to Aβ peptides that result in the deposition of amyloid plaque, whereas the former produces a soluble extracellular fragment (APPsα) that is non-amyloidogenic (Schlondorff and Blobel, 1999). It is clear that both ADAM-10 and -17 can act as α-secretases (Allinson et al., 2003), and fibroblasts from Adam10−/− mice showed no deficiency in α-secretase activity, probably due to compensation by ADAM-17 (Hartmann et al., 2002). However, overexpression of functional ADAM-10 in neurons in transgenic mice that also overexpress human APP led to increased production of APPsα and reduced amyloid plaque formation and overall cognitive deficits (Postina et al., 2004). In contrast an inactive ADAM-10 promoted Alzheimer-like pathology. These findings strongly implicate ADAM-10 as a physiologically relevant α-secretase. But in addition to Notch/Delta, ADAM-10 has many other biologically relevant targets, including ephrin-A5 and E-cadherin ((Janes et al., 2005, Maretzky et al., 2005a); Table 1). It is likely that the early lethality of Adam10−/− mice due to the Notch signalling defect masks detection of other critical developmental processes in which ADAM-10 participates.
Adam11. Though Adam11−/− mice are apparently normal, these mice show defects in learning in the water maze task and motor coordination in the rotating rod task (Takahashi et al., 2006a). This is consistent with the expression of ADAM11 in the hippocampus and cerebellum. These mice also show reduced pain perception responses (Takahashi et al., 2006b).
Adam12. Several Adam knockouts result in viable mice with no obvious pathologies, including Adam9−/−, Adam12−/− and Adam15−/− mice, though 30% mortality of Adam12−/− pups in the first week after birth has been seen in one study (Kurisaki et al., 2003). However, in another study triple knockout Adam9/12/15−/− are also viable and fertile and are generated in the correct Mendelian ratios (Sahin et al., 2004), suggesting that these differences may in part be attributable to the genetic background (Horiuchi et al., 2005). As meltrin-α, ADAM-12 had originally been implicated in muscle formation, where its expression in myoblasts along with ADAM-19 (Meltrin-β) and ADAM-9 (Meltrin-γ) was linked to myoblast fusion to generate multinucleated myotubes (Yagami-Hiromasa et al., 1995). [Parenthetically, the notion that ADAMs had roles in cell fusion arose from their first identification as Fertilins, with a suggested role in sperm–egg fusion via a fusion peptide sequence in the Cys-rich sequence of fertilin-α (Blobel et al., 1992) but to date experimental proof of a role for any ADAM in cell fusion is lacking (Tousseyn et al., 2006)]. No defect in muscle formation has been observed in Adam12−/−, or in Adam9−/− animals. Overexpression of ADAM-12 on a muscle-specific promoter was shown to alleviate the muscular dystrophy pathology in mdx mice in the short term (Moghadaszadeh et al., 2003), but in aged mdx mice this situation was reversed, with accentuation of the dystrophic phenotype and suppression of muscle regeneration following freeze-thaw injury (Jorgensen et al., 2007). Expression of ADAM-12 has been linked to the formation of quiescent reserve cells, otherwise known as satellite cells, that have the properties of self-renewing stem cells and are stimulated to proliferate and contribute to muscle formation following injury or repair (Cao et al., 2003). Conceivably forced overexpression of ADAM-12 may interfere with subsequent recruitment of these cells. Thus the role of ADAM-12 in muscle formation is unclear, particularly as Adam9/12/15/19-quadruple-null mice show no apparent muscle defect, arguing against possible compensation by other Meltrins (Horiuchi et al., 2005). However, Adam12-null animals show minor defects in brown adipose tissue formation (Kurisaki et al., 2003), and transgenic over-expression enhanced adipogenesis (Kawaguchi et al., 2002). Furthermore, Adam12−/− mice showed reduced weight gain and numbers of adipocytes when fed a high fat diet, suggesting a role in adipocyte proliferation and differentiation (Masaki et al., 2005).
Adam15. Mice deficient in ADAM-15 show no overt phenotype, though tumour neovascularisation and growth was reduced (Horiuchi et al., 2003). Aged male Adam15−/− mice displayed accelerated development of osteoarthritis, which may be associated with the ability of ADAM-15 to promote adhesion of chondrocytes to cartilage collagens types II and VI (Bohm et al., 2005).
Adam17. The characterization of ADAM-17 as the TNF-α converting enzyme (TACE) has unlocked an unexpected wealth of information about protein ectodomain shedding that has had repercussions throughout cell and developmental biology. In particular, since mice lacking TNF-α are overtly normal, a key finding from knockout mice in which Adam17 was disrupted (by deletion of the Zn-binding site in the MP domain) was that ADAM-17 has roles in addition to pro-TNF-α activation, and is in fact required for EGF receptor (EGFR) signalling during normal development (Peschon et al., 1998). The Adam17−/− mice died close to birth (at around 17.5 days post coitum), with open eye lids, hair and skin abnormalities and defects in epithelial maturation of multiple organs, all of which resemble defects seen in mice lacking EGFR or its ligands heparin-binding-EGF (HB-EGF), amphiregulin and transforming growth factor-α (TGF-α) (Blobel, 2005, Horiuchi et al., 2005). In particular, HB-EGF−/− mice die before weaning with defects in valvulogenesis in the heart and hypoplastic, poorly differentiated lungs that parallel those seen in Adam17−/− mice (Jackson et al., 2003, Shi et al., 2003, Zhao et al., 2001). It appears that both ADAM-9 and -19 also contribute to heart development, since double knockouts with Adam17 led to exacerbated heart phenotypes, suggesting redundant or compensatory roles for these ADAMs, which are only unmasked in the absence of the major HB-EGF sheddase, ADAM-17 (Horiuchi et al., 2005).
Adam22. Adam22−/− mice are born in the appropriate Mendelian ratio but die before weaning, which has been linked to hypomyelination of peripheral nerves, leading to ataxia (Sagane et al., 2005). ADAM-22 has been shown to be the receptor for a secreted neuronal protein LGI1, mutations in which cause a genetically determined form of epilepsy [autosomal dominant lateral temporal epilepsy, ADLTE; (Fukata et al., 2006)]. The LGI1 is expressed from the presynaptic neuron and binds to ADAM-22 on the surface of post-synaptic neurons. A model has been presented in which the cytoplasmic domain of ADAM-22 interacts with a scaffold protein PSD-95, which in turn can interact with the AMPA receptor responsible for glutaminergic signalling (Snyder, 2006). Defects in this pathway may lead to the aberrant excitation associated with epilepsy. However no mutations were found in ADLTE patients previously identified as negative for LGI1 mutations (Chabrol et al., 2007), so the role of ADAM-22 in neuronal function requires further exploration.
2. ADAM structure and regulation
2.1. Overall topology
There is detailed knowledge on the structure of the metalloproteinase domain of ADAM proteins drawn from X-ray crystal data, though for the most part this has been derived from studies of the SVMPs (reviewed in Gomis-Ruth (2003)). However, most of this information has come from study of the isolated catalytic domain, and only recently has the overall molecular architecture of the complete metalloproteinase/disintegrin/cysteine-rich (MDC) structure begun to emerge (Igarashi et al., 2007, Takeda et al., 2006). These new structures have again come from the SVMPs, namely, vascular apoptosis-inducing factor-1 (VAP-1) and VAP-2B, but as the residues identified in these studies as important for stabilizing the multidomain architecture appear to be strictly conserved throughout all the ADAMs, they no doubt shed light on general mechanisms of substrate recognition and cleavage by mammalian ADAMs. The key message from the recent findings is that the overall MDC domain structure results in a C-shaped molecule, whereby part of the Cys-rich domain faces towards the catalytic site in the M domain (Takeda et al., 2006). This part of the C-domain is comprises a hyper-variable region (HVR) that differs markedly between ADAM family members and it is proposed that this plays an important role in substrate binding. This “C-shape” structure also has implications for the potential of the disintegrin domain to participate in adhesive interactions, which will be discussed further.
The metalloproteinase catalytic domain in the ADAMs is typical of the metzincin clan, which is a globular structure divided into two subdomains, with the active site cleft running between the two (Gomis-Ruth, 2003). The upper, N-terminal subdomain consists of a highly twisted, 5-stranded β-pleated sheet, the strands of which lie parallel to each other and to the substrate, which binds in an extended fashion, essentially running horizontally, left to right along the groove in the schematic of the SVMP Adamalysin II shown in Fig. 5 A. Multiple α-helices also create structural features in the upper subdomain, with the lower αB helix of the Adamalysin II structure containing the HEXXH of the catalytic Zn-binding site. The clan-defining feature of the Metzincins is an absolutely conserved methionine residue creating a 1,4-β-turn (Met-turn) (Bode et al., 1993) that then correctly positions the lower subdomain to create the catalytic cleft. This lower subdomain has relatively few features apart from an α-helix towards the C-terminus. The catalytic Zn atom sits at the bottom of the groove between the subdomains, with subsites in the groove () determining specificity for particular amino acid sequences () in the substrate, where cleavage occurs between the and residues (Fig. 5B). The topology of this groove, and in particular the depth and hydrophobicity of the specificity pocket, is critical for building selectivity into the design of synthetic metalloproteinase inhibitors. Three disulfide bonds are also involved in stabilizing the ADAM metalloproteinase domain structure, along with a structural Ca2+ in many, including ADAM-33 (Orth et al., 2004), but not in ADAM-17 (Maskos et al., 1998). Within this overall consensus structure, the presence of different numbers of α-helices and insertion loops between them create unique surface features that would be expected to affect substrate recognition. Thus although ADAM-17 and ADAM-33 (which to date are the only members of the mammalian ADAM family proper for which the structures have been solved) possess the same layout of secondary structure elements, their catalytic domains share only 26% identity (Orth et al., 2004).
Fig. 5.
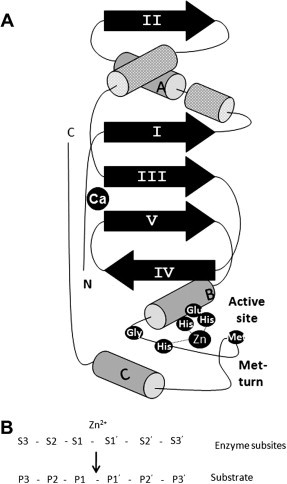
Structure of the ADAM metalloproteinase domain. (A) The schematic depicts structural features determined by X-ray crystallographic analysis of the SVMP Adamalysin II (Gomis-Ruth, 2003). (B) Nomenclature for the amino acid sequence in the cleavage site of substrates and the corresponding sub-sites in the active site of the protease.
The disintegrin (D) domain that follows the metalloproteinase domain has been seen in the VAP1 and VAP2B structures to be subdivided again into 2 sub-domains, described as the “shoulder” (Ds) and “arm” (Da) domains. The N-terminus region of the Cys-rich (C) domain that follows is termed a “wrist” (Cw) domain, and together the Ds, Da and Cw domains shape the whole molecule into the form of a C (Takeda et al., 2006). There are few secondary structure elements in the resulting C-shaped arm, with disulfide bonds and two Ca2+-binding sites holding the structure together. Most importantly in this structure, the disintegrin loop that is widely considered to be responsible for the interactions between ADAMs and integrins (which will be discussed in detail in Section 2.3) is packed against the Cw-domain and is thus inaccessible for binding (Takeda et al., 2006). The “wrist” Cw domain is followed by a “hand” Ch segment, which contains at its distal portion the hypervariable region (HVR), which is proposed by Takeda and co-workers to be a key exosite involved in target recognition (Igarashi et al., 2007). The crystal structure of ADAMTS-1 has also recently been solved, and here again the Cys-rich domain appears to stack against the active site (Gerhardt et al., 2007).
These main structural features are summarised in the schematic shown in Fig. 6 .
Fig. 6.
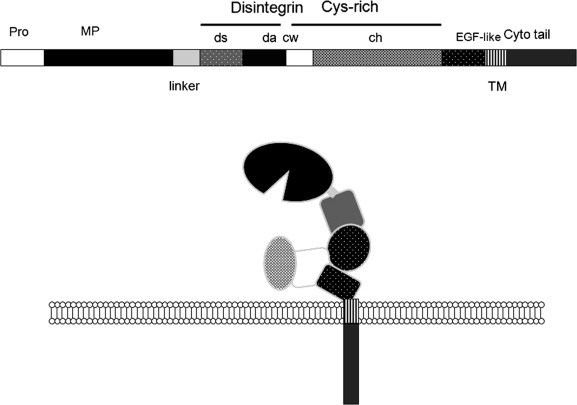
The disposition of the modular domains of ADAMs. The schematic shows a representation of the likely arrangement of ADAM domains based on the crystallographic data of Takeda et al., 2006, Igarashi et al., 2007.
The overall C-shaped MDC structure has flexibility via a hinge between the metalloproteinase and disintegrin domains. In many SVMPs and also in sperm ADAMs such as ADAM-2 (fertilin-β) there is cleavage of the molecules in this region. In contrast ADAM1 (fertilin-α) is cleaved at Ca-binding site-III. So in addition to regulating the proteolytic aspect of ADAM function, such modifications would profoundly affect protein–protein interaction capabilities. Whether this might unmask the disintegrin loop for association with integrins remains to be established.
2.2. Activation of ADAM zymogens, maturation and trafficking
For the majority of the ADAMs, pro-domain removal likely involves the action of pro-protein convertases (PCs) such as furin and takes place intracellularly in the secretory pathway (Endres et al., 2003, Seals and Courtneidge, 2003). These enzymes cleave at a consensus RX(R/K)R motif. The pro-domain functions via a Cysteine switch mechanism, as in the MMPs (Loechel et al., 1999, Roghani et al., 1999). Further support for this view comes from mutational studies of the putative PC site, which is shown to block the activation of ADAM-10 (Anders et al., 2001). However, since the autocatalytic processing of PCs is itself regulated, in some cases involving retention of the cleaved pro-domain in complex with the PC, there is potential for regulated activation of their ADAM zymogens at the cell surface (Mayer et al., 2008). Moreover for at least two ADAMs (ADAM-8 and -28) there is evidence from analysis of inactive E-A mutations in the Zn-binding site for autocatalytic removal of the pro-domain (Howard et al., 2000, Schlomann et al., 2002).
As discussed above, following activation by pro-domain removal, further proteolytic processing of downstream ADAM domains may significantly affect protein conformation and function. In addition to the processing of sperm ADAMs, this has been documented for ADAM-8, which has been observed as a cleaved, cell-associated form lacking the metalloproteinase domain (Schlomann et al., 2002). ADAM-19 also undergoes autolytic processing in its Cys-rich domain, but the N-terminal metalloproteinase-disintegrin modules remain associated with the C-terminal fragment via disulfide bonding (Kang et al., 2004).
The isolated pro-domains of ADAMs can act as potent, selective inhibitors of the active mature forms of the enzymes, as has been documented for ADAM-10 and ADAM-17 (Gonzales et al., 2004, Moss et al., 2007b). Interestingly, at least for ADAM-17 the inhibition of active forms via isolated pro-domains does not seem to involve the Cys-switch mechanism involved in maintaining latency (Gonzales et al., 2004), and indeed it has been suggested that the main functions of the pro-domain are to act as a chaperone and to protect the enzymes from degradation in the secretory system (Anders et al., 2001, Milla et al., 2006). This is suggested by the ability of the pro-domain of ADAM-10 to rescue in trans an otherwise inactive ADAM-10 mutant lacking the pro-domain (Anders et al., 2001). For ADAM-12 the excised pro-domain has been observed to remain non-covalently associated with the mature form of the molecule extracellularly, with the whole molecule adopting a “four-leaf clover” structure where one of the four globular domains is the pro-domain (Wewer et al., 2006). Thus the pro-domains of ADAMs have multiple functions in maintaining latency and stability, ensuring proper folding and entry into the secretory pathway, and perhaps also functional interactions outside the cell.
Other potential control points for ADAM activity include the trafficking of enzymes to particular sites within the cell, the activation of enzyme activity following cell stimulation, and the subsequent removal of active enzyme by internalization or other mechanisms. There are striking examples for all of these types of controls acting for particular ADAMs. Recent data indicate that ADAM-19 and mature, active ADAM-17 are sequestered within cholesterol-rich membrane microdomains, otherwise know as lipid rafts or detergent-resistant microdomains (DRMs) (Tellier et al., 2006, Wakatsuki et al., 2004). Numerous substrates of ADAM-17 and -19 are also localized in these rafts, along with furin. Depletion of cholesterol from the lipid rafts by cyclodextrin or high-density lipoprotein treatment increased the shedding of ADAM-17 substrates without increasing ADAM-17 activity (Tellier et al., 2008, Tellier et al., 2006).
2.3. The disintegrin–integrin connection
Integrins are α and β chain heterodimeric adhesion receptors that are encoded by 18 α and 8 β chain genes in mammals, to give 28 different αβ combinations, each of which has distinctive ligand binding characteristics (reviewed in Hynes (2002)). “True” snake venom disintegrins are small 50–90 amino acid proteins that competitively inhibit integrin-mediated adhesion of platelets to RGD sequences in fibrinogen, by virtue of an RGD or related sequence that is presented at the end of an extended loop, termed the disintegrin loop. In the SVMPs, ADAMs and ADAMTSs, the disintegrin domain should more accurately be called “disintegrin-like”, because with the exception of human ADAM-15 they lack the RGD sequence in their disintegrin loops and they also have additional Cys residues missing from “true” venom disintegrins (Evans, 2001). The initial identification of disintegrin-like domains when the first mammalian ADAMs were identified led to the hypothesis that these regions would interact with integrins, like the related sequences in snake venoms (Wolfsberg et al., 1995). There is now considerable evidence that extracellular domains of ADAMs do indeed interact with integrins, and studies of recombinant disintegrin domains have identified a consensus motif present in their disintegrin loops, namely CRXXXXXCDXXEXC (White et al., 2005). In many cases these interactions have been shown to influence cell adhesion and cell–cell interactions. Table 4 summarizes the known interactions of ADAMs with specific integrins, including α2β1, α4β1, α4β7, α5β1, α6β1, α6β4, α9β1, α2β1, αVβ3, αVβ5 (Arribas et al., 2006, Eto et al., 2002, Tomczuk et al., 2003, White et al., 2005).
Table 4.
ADAM-Integrin associations
| α2β1 | α4β1 | A4 β7 | α5β1 | α6β1 | α6β4 | α9β1 | αVβ3 | αVβ5 | |
|---|---|---|---|---|---|---|---|---|---|
| ADAM1 | • | ||||||||
| ADAM2 | • | • | • | ||||||
| ADAM3 | • | • | • | ||||||
| ADAM7 | • | • | • | ||||||
| ADAM9 | • | • | • | • | • | ||||
| ADAM12 | • | • | |||||||
| ADAM15 | • | • | • | ||||||
| ADAM17 | • | ||||||||
| ADAM19 | • | • | |||||||
| ADAM23 | • | ||||||||
| ADAM28 | • | • | • | ||||||
| ADAM33 | • | • | • |
• indicates an association has been reported.
ADAM–Integrin interactions, adapted from Arribas et al. (2006).
Human ADAM-15 provided interesting insights due to the unique RGD motif in its disintegrin loop, which allowed Eto et al. (2002) to dissect the requirements for RGD-dependent binding to integrin αVβ3 versus the RGD-independent association with α9β1. This mapped the α9β1-interaction site to a motif RXXXXXXDLPEF (residues 481–492 in human ADAM-15, in which the RGD motif occurs at residues 484–486) that is conserved in all ADAMs except ADAM-10 and -17. Indeed, they then demonstrated that the disintegrin domains of several ADAMs associated with α9β1, but not those of ADAM-10 and -17 (Eto et al., 2002).
A potential issue concerning many of these studies is that they have been conducted with recombinant disintegrin domains, removed from the context of the native proteins, and as we have seen earlier, the current structural view of the ADAMs is that the disintegrin-loop region is buried internally in the full-length molecule, unavailable for interaction. However a few studies have looked at the effects of ADAMs on adhesion and migration of cells by studying the intact molecule. If we consider ADAM-12, whose disintegrin domain has previously been shown to interact with integrin α4β1, expression of full-length ADAM-12 in 3T3-L1 preadipocytes revealed an ADAM-12/β1 association, which impaired integrin-mediated adhesion and spreading (Kawaguchi et al., 2003). When expressed in CHO cells that carried defined integrins (α4β1, α5β1 or both), ADAM12 inhibited migration of α4β1-expressing cells but not those expressing α5β1, i.e. the pattern mirrored that of the recombinant disintegrin domain (Huang et al., 2005). Likewise ADAM-19 and ADAM-33 inhibited migration consistent with the binding preferences of their isolated disintegrin domains. Bax et al. (2004) have also presented evidence for interactions between the full extracellular portion of ADAM-17 and α5β1 both in cis and in trans, and then narrowed the association down to the distintegrin-cysteine-rich region.
It is clear that full length ADAMs can influence adhesion in either a positive or negative manner, independently of their metalloproteinase functions, and likely through their associations with integrins (for further review see Arribas et al. (2006)). These observations raise interesting mechanistic questions, which might be resolved by detailed crystal structure information of intact cellular ADAMs. Also, a key aspect may relate to the involvement of intracellular interaction partners of the ADAMs via their cytoplasmic domains. We have recently observed that two splice variants of ADAM-15 that differ only in their cytoplasmic domains by the inclusion of an additional proline-rich sequence in the longer of the two, have opposing effects on adhesion and migration (Zhong et al., 2008). These different cytoplasmic domain have common (Grb2, FISH) and distinct (Src, Brk) interaction partners, which may lead to isoform-specific effects on intracellular signalling or protein localization or trafficking.
2.4. The Cys-rich domain
There is strong support for a role for the Cys-rich domain in regulating ADAM function. For ADAM-12, the Cys-rich domain mediates adhesive interactions with syndecans, leading subsequently to engagement with β1-containing integrins (Iba et al., 2000, Thodeti et al., 2003). In Xenopus laevis the metalloproteinase activity of ADAM-13 is required for cranial neural crest cell migration (Alfandari et al., 2001), and when ADAM-13 is over-expressed in embryos it leads to hyperplasia of the cement gland (Smith et al., 2002). Though the catalytic domain of ADAM-10 can be substituted for that of ADAM-13, the cement gland hyperplasia phenotype critically requires the Cys-rich domain, which is consistent with involvement of the Cys-rich domain in substrate interactions. This is further supported by demonstration of the association of the ADAM-13 disintegrin/Cys-rich domains with fibronectin (Gaultier et al., 2002). Also, domain deletion analysis showed that the Cys-rich domain of TACE is required for the shedding of the IL-1 Receptor-II, but not for its ability to shed TNF-α or the p75TNF receptor (Reddy et al., 2000).
An exciting recent insight into role of the Cys-rich domain has come fro m the study of the role of ADAM-10 in ephrin-Eph signalling (Janes et al., 2005, Mancia and Shapiro, 2005). Ephrins are cell-associated ligands (either GPI-anchored in the case of the ephrin-A ligands or transmembrane for the ephrin-B class) for Eph tyrosine kinase receptors which generate bidirectional signalling in adjacent cells upon ephrin-Eph engagement (Pasquale, 2008). Fundamentally, ephrin-Eph signalling involves cell repulsion following engagement, which is important for neuronal guidance and also for establishment of the arterial and venous vascular networks. This repulsion involves cleavage of the ephrin ligand following binding to its cognate Eph (Hattori et al., 2000). Janes et al. (2005) have shown that ADAM-10 constitutively associates with the ephrin binding domain of EphA3, and that formation of the EphA3/ephrin-A5 complex creates a high affinity binding site for the Cys-rich domain of ADAM-10, which binds to neither component alone. This in turn results in a conformational switch such that the ADAM-10 metalloproteinase domain is positioned to be able to cleave the ephrin-A5 in trans. The key feature is an acidic pocket in the C-terminus of the Cys-rich domain, likely the hypervariable region observed by Takeda et al. (2006). This model may be a paradigm for a much wider range of ADAM actions.
2.5. The cytoplasmic domain
The transmembrane ADAMs have cytoplasmic domains that vary widely in length and sequence, ranging from the 11 residues of ADAM-11 to 231 residues for ADAM-19 (Seals and Courtneidge, 2003). Several contain one or more PXXP motifs that can act as binding sites for SH3-domain containing proteins, and Ser, Thr and tyrosine residues that are potential sites for phosphorylation by diverse kinases. This led to speculation that the cytoplasmic domains may play important roles in regulating protease function in response to intracellular signalling events (i.e. inside–out signalling), or also that they could be involved in outside–in signalling following engagement of their ectodomains with substrates or adhesion partners, leading to recruitment of signalling molecules and adapters. A large number of interaction partners have now been identified using yeast 2-hybrid, pull-down assay and co-immunoprecipitation, and Table 5 summarises current knowledge of these interactions. The ADAMs that contain potential SH3 binding sites are: ADAM-7, -8, -9, 10, -12, -15, -17, -19, -22, -29 and -33. ADAM-12 and -15 contain the most extensive repertoires of Pro-rich motifs (9 and 13, respectively, depending on the splice variant for ADAM15), and have been most extensively studied with regard to their interaction partners.
Table 5.
ADAM cytoplasmic domain interaction partners
The significance of the cytoplasmic domains for ADAM functions rests on several observations. First, although the overall sequence identity between ADAM orthologues is less in the cytoplasmic tail regions than in sequences encoding the ectodomains, the Pro-rich motifs are generally well conserved. For example, comparison of mammalian and zebrafish ADAM12 shows that 5 of the PxxP are conserved with good identity in surrounding sequence blocks and several others may be substituted functionally since the Danio rerio gene has 12 copies of the motif overall in its cytoplasmic domain. Second, there are now clear examples where the cytoplasmic domain plays a key role in ADAM function, either by regulating catalytic activity or by trafficking the protein to the correct cellular location (Cao et al., 2002). ADAM-12 is stored intracellularly as a mature, active protein but appears on the surface in response to PMA stimulation (Hougaard et al., 2000), and this regulated translocation requires the cytoplasmic domain and is dependent on PKCε (Sundberg et al., 2004). The SH3-domain-containing protein PACSIN3, which is involved in endocytosis and interacts with proteins such as dynamin and N-WASP, interacts with the cytoplasmic domains of several ADAMs including ADAM-9, -10, -12, 15 and -19, but not that of ADAM-17 (Mori et al., 2003). The ADAM-12/PACSIN3 association was shown to be functionally important for ADAM-12 mediated shedding of HB-EGF in response to PMA or the GPCR agonist angiotensin II. Likewise another ADAM-12 interactor, Eve-1, which is an SH3-containing adapter protein that also binds with several ADAM cytoplasmic domains including ADAM-17 has been shown by siRNA knockdown studies to be important for stimulus-activated shedding by ADAM-12 (Tanaka et al., 2004). Yet another ADAM-12 interacting protein is the scaffolding protein Tks5/Fish, which is a Src substrate that contains 5 SH3 domains and a Phox homology domain that binds phosphatidyl inositols (Abram et al., 2003). Tks5/Fish co-localizes with ADAM-12 in actin-rich podosomes, sometimes known as invadopodia, suggesting that it may be important for spatially organizing ADAM-12 activity. This suggests an interesting interplay of factors regulating signalling at the cell surface, since ADAM-12 itself binds Src and has been reported to activate Src activity in C2C12 cells (Kang et al., 2000), whilst also recruiting and activating phosphatidylinositol 3-kinase (PI3K) via SH3 domain/pro-motif interactions (Kang et al., 2001).
A proline-rich stretch in the cytoplasmic domain of ADAM-10 has recently been shown to be important for the correct basolateral location of ADAM-10 in polarized epithelial cells, endowing the enzyme with the ability to cleave E-cadherin and promote cell migration when it is correctly localized (Wild-Bode et al., 2006). It is unclear at present what intracellular protein mediates this trafficking, though Synapse Associated Protein-97 (SAP97) is an SH3-domain containing protein that interacts with this region of ADAM-10 (Marcello et al., 2007) and is responsible for correct localization of ADAM-10 in synaptic membranes, thereby promoting its α-secretase activity. Interestingly SAP97 also interacts with the extreme C-terminal region of ADAM-17, but in this case it involves the third PDZ domain of SAP97 (Peiretti et al., 2003). This suggests a common control point for integration of the α-secretase activities of ADAM-10 and -17.
Despite the intense activity on the study of ADAM-17 and its involvement in stimulus-induced ectodomain shedding, there are still conflicting views as to the role of its cytoplasmic domain. Its association with the PDZ-domain containing protein tyrosine phosphatise-1H (PTP1H) inhibits PMA-stimulated pro-TNF-α activation (Zheng et al., 2002). ADAM-17 is able to associate with Erk MAP kinase and is phosphorylated by it on Thr 735 leading to enhanced cleavage of the TrkA neurotrophin receptor in CHO cells (Diaz-Rodriguez et al., 2002). Moreover, Soond et al. (2005) have reported that trafficking of ADAM-17 from the endoplasmic reticulum to the cell surface was dependent on Erk phosphorylation at Thr 735. Meanwhile Fan et al. (2003) observed PMA-induction of phosphorylation at Ser819, but neither mutation of this site or deletion of the entire cytoplasmic domain affected serum-induced pro-TGF-α cleavage, which is consistent with the studies from Black and co-workers (Doedens et al., 2003, Reddy et al., 2000), where full PMA-inducible ADAM-17 activity occurred with inclusion of just the membrane spanning region for correct insertion in the membrane (Reddy et al., 2000, Doedens et al., 2003). Thus the mechanism responsible for PMA activation of ADAM-17 is still unclear. This could involve PMA-induced release of an inhibitor, such as the pro-domain of ADAM-17 itself, or TIMP-3, though the experiments of Doedens et al. (2003) argue against these proposals. An alternative may be that PMA activation may modify another protein leading to an interaction with ADAM-17, potentially altering its engagement with substrates within the lipid-rich domains where it is primarily localized (see Section 2.2).
On balance, however, it seems likely that the cytoplasmic domain does indeed play a key role in coupling ADAM-17 and other ADAMs to specific signalling events such as GPCR activated ADAM-mediated EGFR ligand release, termed “triple membrane-passing signal” (TMPS), which will be discussed further in Section 3.3. Recent data have helped unravel the GPCR-EGFR crosstalk elicited by gastrin-releasing peptide, which involves phosphorylation of ADAM-17 by PDK1, as a result of Src and PI3K activation by the GRP receptor (Zhang et al., 2006). This leads to amphiregulin release and EGFR signalling. Thus the cytoplasmic domains of ADAMs likely couple ADAM activity to signalling pathways in a cell- and stimulus-specific fashion.
Finally, further support for the notion of functional relevance for the cytoplasmic domain comes from analysis of ADAM-15 cytoplasmic domain variants, whose expression in human breast cancer shows isoform-specific associations with clinical outcome and also with effects on cell adhesion and migration (Zhong et al., 2008). Recent studies indicate differential abilities of two of the main cytoplasmic variants of ADAM-15 to cleave fibroblast growth factor receptor-2 IIIb (FGFR2-IIIb), with the Src-associating ADAM15B isoform displaying enhanced activity compared to the ADAM15A variant that lacks the ability to associate with Src (Maretzky et al., submitted for publication).
2.6. ADAM inhibitors
The ability of natural TIMPs to inhibit ADAMs is summarised in Table 1. In general, TIMPs display much greater selectivity towards the ADAMs than MMPs, and several ADAMs are inhibited exclusively by TIMP-3 (Baker et al., 2002). Indeed this latter characteristic has often been used to characterize the nature of the metalloprotease involvement in a shedding event, though as we learn more about the interplay between TIMPs and ADAMs more detailed evidence should be presented if ADAM involvement is suggested. TIMPs are two-domain molecules, with a larger N-terminal domain that is responsible for MMP inhibition, and a smaller C-terminal domain that provides specificity for additional protein–protein interactions including the pro-forms of particular MMPs. The mechanism of TIMP-MMP inhibition was solved by the crystal structure of the MMP-3/TIMP-1 complex (Gomis-Ruth et al., 1997), which revealed an elongated wedge-shaped ridge of TIMP-1 that docks with the active site cleft. This mechanism critically depends upon the sequence of the N-terminus of the mature TIMP molecule, since the first four residues lie in the active site in a fashion equivalent to the P1–P1′–P2′–P3′ residues of a peptide substrate. The N-terminal Cys1 residue is responsible for Zn-chelation via its α-amino and carbonyl groups. Extension of TIMPs by a single residue at the N-terminus renders them non-functional against MMPs, however a N-terminal domain TIMP-3 mutant extended by an Ala residue at its N-terminus retained activity against ADAM-17, indicating that the inhibitory mechanism is dissimilar (Wei et al., 2005). Moreover, a Thr2Gly replacement at position 2, which reduces the affinity of TIMP-1 for MMPs by three orders of magnitude, retained potency against ADAM-17. Further protein engineering studies of TIMPs have dissected the basis of TIMP-MMP and TIMP–ADAM interactions, and it is clear that sites distal to those involved in interactions in the active site cleft have profound influences. Indeed, although the N-terminal domain and full-length form of TIMP-3 are equally potent against the isolated catalytic domain of ADAM-17 (Lee et al., 2001), the extra-catalytic domains of ADAM-17, and in particular the Cys-rich region, substantially weakened the inhibitory effects of N-TIMP3 (Lee et al., 2003). The TIMP-3 forms studied by Wei et al. (2005) show positive co-operativity in binding to the whole ectodomain of ADAM-17, implying multiple interactions. Recent studies using stopped-flow X-ray spectroscopy show dynamic charge transitions at the active site Zn of ADAM-17 prior to substrate engagement with the ion, which implies communication between distal sites of the molecule and the catalytic core (Solomon et al., 2007). Further support for the importance of distal protein interactions between TIMPs and exosites away from the catalytic cleft come from the observations that the N-terminal domains of TIMP-1 and TIMP-3 are ineffective as inhibitors of ADAM-10 compared to the full-length TIMPs (Rapti et al., 2008).
There has been considerable interest in the development of synthetic inhibitors of ADAMs as therapeutic agents (Moss and Bartsch, 2004). Given the central importance of ADAM-17 in inflammatory processes, and the success of anti-TNF-α therapies in the treatment of rheumatoid arthritis, there is a strong case for the design of selective TACE inhibitors (Moss et al., 2008a). Likewise, ADAM-10 involvement in the shedding of HER2 from the cell surface (Liu et al., 2006) makes it a promising target for anti-cancer therapy (Moss et al., 2008b). As we have seen, the metalloproteinase catalytic domain has overall structural similarity with other metzincins (Section 2.1), but there are distinctive features that have allowed the development of a number of selective compounds. In particular the deep, curved hydrophobic S1′ pocket of ADAM-17, which merges below the surface with the deep S3′ pocket (Maskos et al., 1998) has been exploited to develop a range of compounds showing good selectivity and potency, with K i’s in the lower nM range (Condon et al., 2007, Huang et al., 2007, Niu et al., 2006, Wasserman et al., 2003). Four selective ADAM-17 inhibitors are in clinical trials for treatment of rheumatoid arthritis: BMS-561392 and TMI-005 (apratastat) progressed to phase II trials, and GI5402 and R618 are in phase I (Moss et al., 2008a). However further development of some of these compounds has already halted, including those in phase II trials, due to mechanism-based liver toxicity. It is possible that despite their toxicity profiles some of these potent, selective compounds might find clinical utility in other diseases such as cancer. In this regard, INCB3619 is a potent inhibitor of both ADAM-10 and -17 which reduced the processing of multiple EGFR ligands in vitro and in vivo in tumour-bearing mice, and reduced tumour growth appreciably (Fridman et al., 2007).
Among inhibitors that have proved to be selective for other ADAMs, G1254023X has 100× higher inhibitory activity against ADAM-10 compared to ADAM17 (Ludwig et al., 2005). This compound was used to provide evidence that ADAM-10 is involved in constitutive shedding of IL6R from cells, whereas PMA-stimulated shedding was inhibited by GW280264X, a mixed ADAM10/ADAM-17 inhibitor (Hundhausen et al., 2003; Ludwig et al., 2005). CGS27023 is a very efficient inhibitor of ADAM-9, with a K i of 1 nM (Mochizuki and Okada, 2007). KB-R7785 is a potent ADAM-12 inhibitor that was identified by virtue of its ability to block HB-EGF shedding in cardiomyocytes (Asakura et al., 2002). This inhibitor blocked HB-EGF shedding and attenuated hypertrophy in mice treated with GPCR agonists.
Further insights into ADAM regulation will no doubt come from the demonstration that RECK (reversion-inducing cysteine-rich protein with Kazal motifs) inhibits ADAM-10-mediated Notch signalling in neural precursor cells (Muraguchi et al., 2007).
3. The biological functions of the ADAMs
In the sections that follow we review information on the actions of particular ADAMs in fertility, ectodomain shedding and cell signalling.
3.1. Fertility
The first mammalian ADAMs identified were the two subunits of the heterodimeric sperm protein fertilin, ADAM1 (fertilin-α) and ADAM2 (fertilin-β; Blobel et al., 1992), whose functions have been studied extensively in rodents (Primakoff and Myles, 2000, Primakoff and Myles, 2002). These proteins were originally proposed to be important for sperm–egg binding and fusion, since an anti-fertilin-β monoclonal antibody blocked guinea pig sperm–egg fusion. Fertilin-α and -β are proteolytically processed at different stages of sperm maturation by cleavage after the MP domain, leaving the disintegrin as the N-terminus of the sperm-associated proteins and thus potentially free to engage with egg integrins (Primakoff and Myles, 2000). In support of this, fertilin-β was shown to bind to α6β1 integrin (Almeida et al., 1995), and a peptide based on the disintegrin domain of fertilin-β blocked sperm–egg binding (Primakoff and Myles, 2000). Fertilin-β-null mice are infertile, but fertilin-β−/− sperm fuse with eggs in vitro at approximately 50% of the level of wild-type sperm, so fertilin-β is not essential for fusion (Primakoff and Myles, 2002). Sperm from these animals in fact show multiple defects in sperm–egg adhesion and fusion, in migration from the uterus, and in binding to the zona pellucida surrounding the egg (Cho et al., 1998). It transpires that there are complex relationships between ADAMs that are expressed in spermatogenic cells and sperm, such that deficiencies in one ADAM lead to reductions in the levels of several others that appear on the sperm surface (Kim et al., 2006). Adam3 (Cyritestin)-null male mice are also infertile (Primakoff and Myles, 2002) and ADAM-7 and ADAM-5 are reduced on the surface of sperm from fertilin-β- and Adam3-null mice (Kim et al., 2006) Moreover, eggs from integrin α6-null mice bind and fuse normally with sperm (Primakoff and Myles, 2002). Thus the precise contributions of ADAMs in sperm function are still not clear. Some may function at early stages in spermatogenesis and in sperm migration in the uterus/oviduct and in zona pellucida binding, while some may function as chaperones to allow the correct assembly of ADAMs or other proteins on the sperm surface. How the situation in rodents relates to spermatogenesis and fertility in humans is also unclear, since many of the testis-specific Adam genes of rodents are represented only as pseudogenes in humans, including ADAM1 and ADAM3 (Puente and Lopez-Otin, 2004).
These testis-specific ADAMs are in an interesting state of evolutionary flux: ADAMs 1, 4, 6, 20, 21, 24, 25, 26, 29, 30 and 34 – where present in a particular mammalian species – lack introns in their coding sequences, suggestive of pseudogenization (e.g. ADAM20 is human-specific; ADAM25 is a pseudogene in humans; Adam26 is missing from humans, and a pseudogene in rat). Adam24 encodes Testase-1, an apparently functional metalloprotease that is activated on the sperm plasma membrane (rather than in the Golgi apparatus) as sperm mature during transit in the epididymis (Zhu et al., 2001), implicating it in sperm function; again, however, the gene is missing in humans. Perhaps these testis- and sperm-specific ADAMs are indeed drivers of speciation events, whose substrates or adhesion partners on the egg or other female reproductive structures may mirror their rates of evolutionary change?
3.2. Ectodomain shedding The ADAM-17/TACE paradigm
The identification of the metalloproteinase responsible for the activation of the membrane-associated precursor form of tumour necrosis factor-α (TNF-α) was a landmark event in the protease field (Black et al., 1997, Moss et al., 1997). The involvement of the cytokine TNF-α in a variety of diseases associated with inflammation, including rheumatoid arthritis, cancer and Crohn’s disease, made the mechanism of its activation of great clinical and pharmaceutical relevance. However, as discussed in Section 1.3, ADAM-17 has emerged as a sheddase with an extremely broad substrate range, with fundamental links to EGFR signalling. Since EGF and related ligands (HB-EGF, TGF-α, epiregulin, amphiregulin and betacellulin) are all produced initially as membrane-associated molecules that require proteolytic processing, this identifies ADAM-17 (and related sheddases) as upstream regulators of one of the most important signalling pathways in vertebrates.
Shedding of a membrane-anchored cytokine or growth factor by an ADAM proteinase could be relevant to various modes of signal transduction, including paracrine, autocrine and juxtacrine (Fig. 7 ). On the one hand, shedding may be required for the ligand to diffuse to engage with a receptor on the same cell (autocrine), or on neighbouring cells to act in a paracrine fashion; in addition proteolytic processing may activate a ligand that remains membrane associated, which can activate cells in a juxtacrine or autocrine fashion. Shedding may remove signalling ligands to down-modulate or terminate signalling. Likewise, cleavage and release of signalling receptors may down-modulate signalling capability on the cell surface, or generate soluble receptors that act as decoys by sequestering ligands.
Fig. 7.
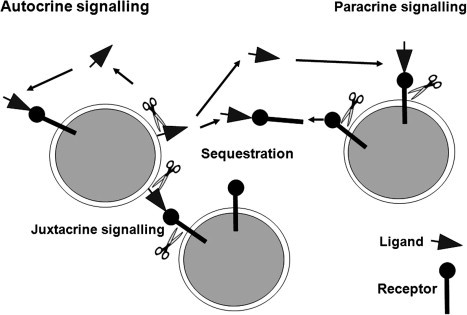
ADAM metalloproteinases can influence autocrine, paracrine and juxtacrine signalling. The schematic depicts the potential actions of ADAMs (scissors) on receptor–ligand pairs, acting to release ligand that acts upon receptors on the cell of origin (autocrine), or on distant cells (paracrine). Juxtacrine signalling involving adjacent cells may involve ADAMs for correct presentation of the ligand, or for cleavage of the ligand following receptor engagement, or for receptor activation. Shed receptors may act as decoys, or cleavage of the receptor may itself activate or modulate signalling.
In the years since its discovery, the repertoire of membrane-associated proteins that are susceptible to shedding/processing by ADAM-17 has grown immensely, as shown by the detailed listing in Table 2. However, other proteolytically active ADAMs can also act as sheddases, and two of the key concepts that have emerged are (1) that constitutive shedding may involve different enzymes from those that participate in signal-activated ectodomain cleavage and (2) different members of a family of substrates may be acted upon by different ADAM proteases in the same cell. The availability of cells from gene knockout mice has revolutionized the analysis of ADAM involvement in ectodomain shedding. For instance, study of cells from cells derived from mice deficient in either ADAM-9, -10, 12, -15, 17 or -19 has shown that within the EGFR ligand family, ADAM-10 is the main sheddase for EGF and betacellulin, whilst ADAM-17 is largely responsible for cleavage of TGF-α, epiregulin, amphiregulin and HB-EGF (Sahin et al., 2004).
In many studies ADAM-17 has been shown to be responsible for induced shedding following activation of protein kinase-C (PKC) by phorbol esters such as PMA, whereas constitutive, PKC-independent shedding involves a distinct activity (Horiuchi et al., 2007b). For instance, using Adam-null cell lines and transfecting in functional ADAM proteins, Zheng et al. (2004) demonstrated involvement of ADAM-17 in phorbol-stimulated TNF-α shedding, but ADAM-19 was able to cause constitutive release following transfection of Adam17−/− cells, whereas ADAM-9, 10, and -19 could not. A typical pattern is shown by the L1 adhesion molecule, for which ADAM-10 mediates constitutive shedding while ADAM-17 carries out cleavage after phorbol stimulation or cholesterol depletion (Maretzky et al., 2005b). The calcium ionophore ionomycin activates ADAM-10 mediated shedding of CD30 (Eichenauer et al., 2007) and the chemokines CX3CL1 and CXCL16 (Hundhausen et al., 2007). It is important to understand the full physiological repertoire of the shedding events controlled by ADAMs such as ADAM-17 and the redundancy and compensation by multiple ADAM family members if these enzymes are to be targeted by pharmacological inhibitors.
3.3. GPCR-EGFR crosstalk: “Triple Membrane-Passing Signalling”
There is now a wealth of evidence that the activation of G-protein coupled receptors (GPCRs) leads to downstream activation of EGFR signalling via activation of ADAM-mediated EGFR ligand release (Blobel, 2005, Fischer et al., 2003). Table 6 lists several of these studies and further information can be found in the review by Ohtsu et al. (2006). The first clear demonstration that this GPCR-EGFR crosstalk involved cleavage of an EGFR ligand came from the work of Prenzel et al. (1999), who highlighted an absolute requirement for metalloproteinase activity by using batimastat, a broad spectrum synthetic inhibitor of MMPs and ADAMs, and further showed that that HB-EGF was involved from use of a mutant diphtheria toxin (Crm 7) that blocks HB-EGF function. This study explained the inability to identify free ligand in conditioned medium, since shed HB-EGF was retained in the pericellular ECM, and activation of EGFR signalling was critically dependent on high cell density. The transmembrane form of pro-TGFα is inactive in stimulating the EGFR on adjacent cells, such that ADAM-17 is essential for juxtacrine signalling (Borrell-Pages et al., 2003). In part this may involve ligand solubilisation to allow diffusion and action in a paracrine fashion, but by analogy with the ephrin-A5/EphA3 signalling scenario discussed earlier (Janes et al., 2005) it is possible that ADAM-17-mediated cleavage of the TGFα/EGFR complex may be necessary to elicit EGFR activation.
Table 6.
ADAM involvement in GPCR-EGFR Crosstalk
| Agonist | ADAM | EGFR ligand | References |
|---|---|---|---|
| Angiotensin II | ADAM17 | HB-EGF TGFα | Lautrette et al., 2005, Mifune et al., 2005, Schafer et al., 2004a |
| ADAM12 | HB-EGF | Asakura et al. (2002) | |
| Lysophosphatidic acid (LPA) | ADAM15 | ? | Schafer et al. (2004b) |
| ADAM17 | HB-EGF | Hart et al. (2005) | |
| Thrombin | ADAM17 | HB-EGF | Hart et al. (2005) |
| ADAM15 | HB-EGF | Hart et al. (2005) | |
| Phenylephrine | ADAM12 | HB-EGF | Asakura et al. (2002) |
| Bombesin | ADAM10 | HB-EGF | Yan et al. (2002) |
| Carbachol | ADAM17 | Amphiregulin | Gschwind et al. (2003) |
| Serotonin | ADAM-17 | HB-EGF | Gooz et al. (2006) |
Depending on the cell and the stimulus involved the mechanisms involved in this cross-talk may differ, though the basic strategy of the “Triple Membrane-Passing Signal” (TMPS; Fischer et al., 2003) remains the same. This concept is encapsulated in Fig. 8 . The intracellular linkages between GPCR and ADAM activation are still opaque, but participation of the cytoplasmic domain of the ADAM seems both logical and efficient. This aspect has been addressed by Grandis and colleagues, who have studied activation of EGFR signalling following stimulation of cells with gastrin-releasing peptide. They have demonstrated that Src is essential downstream of GPCR signalling to facilitate metalloproteinase-mediated amphiregulin release and EGFR activation (Zhang et al., 2004). Src was required for the GPCR-mediated activation of phosphatidyl inositol-3 kinase (PI-3K), which in turn led to activation of phosphatidyl inositol-dependent kinase-1 (PDK1), which directly phosphorylated ADAM-17 (Zhang et al., 2006). It is possible that in some circumstances TMPS may not involve the ADAM cytoplasmic domain since as we saw previously, phorbol ester activation of ADAM-17 can occur – at least in transfected cells – in the absence of the cytoplasmic domain (Reddy et al., 2000). However this type of mechanism is appealing since it provides a way to build specificity into the GPCR-ADAM crosstalk. This also could provide insights into the functional significance of the cytoplasmic domain splice variants seen, for example, for ADAM15 (Zhong et al., 2008) and ADAM22 (Godde et al., 2006, Sagane et al., 2005).
Fig. 8.
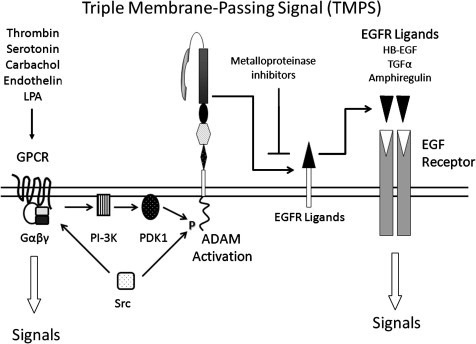
Triple membrane passing signal: involvement of ADAMs in the crosstalk between GPCR and EGFR signalling. The model proposed is based on studies reported in Zhang et al. (2006).
3.4. Regulated intramembrane proteolysis (RIP)
Regulated intramembrane proteolysis (RIP) can be defined as a sequential proteolytic cascade that involves an initial ectodomain sheddase action on a transmembrane protein, followed by a subsequent cleavage within the membrane itself, which in many cases generates a signal-transducing intracellular domain fragment. The classical examples of regulated intramembrane proteolysis are Notch signalling and APP processing, which we have discussed in Section 1.3 in relation to the results from knockout mouse studies. These two processes are illustrated in Fig. 4, and further details can be found in Saftig and Hartmann, 2005, Tousseyn et al., 2006, Murphy et al., 2008. The number of proteins that are now known to be subject to RIPping is growing. Many of these are adhesion molecules and substrates of ADAM-10, though RIPping is likely to be a widespread phenomenon involving many ADAM sheddases. RIPping substrates include CD44 (Nagano et al., 2004), N-cadherin (Reiss et al., 2005), E-cadherin (Maretzky et al., 2005a) and L1 (Maretzky et al., 2005b). In each of these cases, as with the Notch paradigm, cleavage by the extracellular ADAM creates a truncated transmembrane stub that becomes accessible for cleavage by an intramembrane-cleaving protease (I-CLiP), of which four types are known: the presenilins (which are the proteolytic component of γ-secretase), the signal peptide peptidases, S2P, and the rhomboids (Spasic and Annaert, 2008). In many instances these cleavages generate an intracellular domain (ICD) that moves off to the nucleus and influences gene transcription, as is the case for Notch (Fig. 4). For both N- and E-cadherin, cleavage leads to disruption of cell adhesion mediated by the extracellular domains, and a redistribution of β-catenin from the cell surface to the nucleus to activate target genes, leading to epithelial–mesenchymal transition in the case of E-cadherin (Maretzky et al., 2005a, Reiss et al., 2005). In addition the N-cadherin ICD is reported to act as a signal transducer by binding to the transcriptional coactivator CBP and inducing its proteasomal degradation (Marambaud et al., 2003).
In addition to Notch, the Notch ligand Delta 1 is also subject to RIPping (Six et al., 2003). Cleavage of Delta by ADAM-10 had originally been considered to allow the ligand to diffuse to distant Notch receptors (Qi et al., 1999) but subsequently it was recognized that the released ligand was inactive, leading to the suggestion that this was a mechanism to allow spatial control of Notch signalling (Mishra-Gorur et al., 2002). However it was subsequently realized that the ADAM-10-mediated cleavage of Delta1 led to γ-secretase-mediated processing of the transmembrane stub generating an ICD that translocated to the nucleus (Six et al., 2003). Thus this could represent the same bidirectional signalling that has been seen for the proteolysis of the ephrin-A5/EphA3 complex by ADAM-10 (Janes et al., 2005). The ICDs generated by RIPping may have multiple roles in cell signalling in addition to effects on transcription in the nucleus: for instance, the ICD generated by γ-secretase action on ephrinB2 causes activation of Src by binding and inhibiting Csk, a kinase that phosphorylates and inhibits Src. Src in turn phosphorylates ephrinB2, preventing its cleavage by γ-secretase and establishing a feedback loop (Georgakopoulos et al., 2006).
Although much of the current data focus on ADAM-10 as the major “RIPping sheddase”, we should not exclude other ADAMs from consideration in this role. There are reports that ADAM-17 cleaves the p75 neurotrophin receptor (p75NTR), followed by nuclear translocation of the ICD and induction of apoptosis (Srinivasan et al., 2007). RIPping is an important and much-employed biological mechanism in which many ADAMs likely participate.
4. ADAM involvement in human disease
The fundamental nature of the biological processes controlled by ADAM proteinases mandates that dysregulation of these enzymes will contribute to pathogenetic mechanisms of human disease. ADAMs have been implicated in cancer, neurological and cardiovascular diseases, asthma, infection and inflammation. Table 7 provides information on some of the known disease associations and further information can be found in two excellent recent reviews (Mochizuki and Okada, 2007, Tousseyn et al., 2006). Here we will highlight some of the key mechanistic principles.
Table 7.
Associations of ADAMs with human diseases
| ADAM | Pathology association | Observation | References |
|---|---|---|---|
| ADAM8 | Cancer | Overexpression in pancreatic cancer associated with reduced patient survival | Valkovskaya et al. (2007) |
| Upregulated in gliomas and associated with local invasion | Wildeboer et al. (2006) | ||
| Rheumatoid arthritis | Levels of shed ADAM-8 in synovial fluid correlate with joint inflammation | Gomez-Gaviro et al. (2007) | |
| ADAM9 | Cancer | Upregulated in breast cancer | O’Shea et al. (2003) |
| Promotes invasion of colon cancer cells by binding to α6β4 and α2β1 integrins | Mazzocca et al. (2005) | ||
| Upregulation in prostate, breast and intestinal cancers. Mouse knockout suppressed malignancy in a model of prostate cancer | Peduto et al. (2005) | ||
| High ADAM9 levels predict higher efficacy of tamoxifen therapy in ER+ breast cancers | Sieuwerts et al., 2005 | ||
| Overexpression in non-small cell lung cancer linked to brain metastasis | Shintani et al. (2004) | ||
| Cytoplasmic ADAM9 overexpression associated with poorer survival in pancreatic ductal adenocarcinoma | Grutzmann et al. (2004) | ||
| ADAM10 | Inflammation | Cleavage of VE-cadherin and increased endothelial permeability | (Schulz et al., 2008 |
| Cancer | Upregulated in prostate cancer | McCulloch et al. (2000) | |
| Nuclear localization associated with Gleason score in prostate cancer | Arima et al. (2007) | ||
| Shedding of CD30 from lymphoma cells linked to failure of antibody-based immunotherapy | Eichenauer et al., 2007 | ||
| Ageing | Shedding of anti-ageing Klotho protein | Chen et al. (2007) | |
| Allergic responses Alzheimer’s | Shedding of soluble CD23 promotes IgE production | Weskamp et al., 2006, Lemieux et al., 2007 | |
| disease | Reduced immunostaining in neurons in AD brains versus controls | Bernstein et al. (2003) | |
| ADAM12 | Cancer | Increased levels in urine of breast cancer patients | Roy et al. (2004) |
| Upregulated in breast cancer and associated with decreased tumour cell apoptosis | Kveiborg et al. (2005) | ||
| Increased mutation frequency detected in breast cancer genome sequencing | Sjoblom et al. (2006) | ||
| Increased in bladder cancer | Fröhlich et al. (2006) | ||
| Pre-eclampsia | Reduced maternal serum ADAM-12 levels | Laigaard et al. (2005) | |
| Down syndrome | Reduced maternal serum ADAM-12 levels | Laigaard et al. (2003) | |
| ADAM15 | Cancer | Increased expression in aggressive breast and prostate cancer | Kuefer et al. (2006) |
| Differential association of splice variants with prognosis in breast cancer | Zhong et al. (2008) | ||
| Increased expression in lung cancer cells at invasion front | Schutz et al. (2005) | ||
| Increased copy number in breast cancer cell lines | Ortiz et al. (2004) | ||
| ADAM17 | Cancer | Over-expressed in breast cancer at protein level and required for activation of pro-TGFα | Borrell-Pages et al. (2003) |
| Increased protein expression in breast cancer | Santiago-Josefat et al. (2007) | ||
| Over-expressed in breast cancer and associated with tumour progression and metastasis | McGowan et al. (2007) | ||
| Correlation with TGFα expression in breast cancer and predictive of outcome | Kenny and Bissell (2007) | ||
| Predicts adverse outcome in breast cancer | McGowan et al. (2008) | ||
| Rheumatoid arthritis | Upregulation in synovial cells in RA but not OA, and associated with HIF-1 expression | Charbonneau et al. (2007) | |
| Multiple sclerosis | Increased expression in blood vessels, macrophages and astrocytes in active lesions | Plumb et al. (2006) | |
| Diabetes | Increased expression in monocytes in response to LDL from diabetic patients versus controls | Worley et al. (2007) | |
| ADAM19 | Cancer | Upregulated in gliomas and associated with local invasion | Wildeboer et al. (2006) |
| ADAM22 | Cancer | Decreased expression in gliomas | D’Abaco et al. (2006) |
| ADAM29 | Cancer | Increased mutation frequency detected in colorectal cancer genome sequencing | Sjoblom et al. (2006) |
| ADAM33 | Asthma | SNPs associated with asthma susceptibility | Van Eerdewegh et al. (2002) |
| Psoriasis | SNPs associated with psoriasis | Lesueur et al. (2007) | |
| ADAMDEC1 | Atherosclerosis | Upregulated in unstable versus stable atherosclerotic plaque | Papaspyridonos et al. (2006) |
As active metalloproteinases, ADAMs may have a role in promotion of tumour invasion and metastasis via cleavage of extracellular matrix proteins. Certainly ECM targets are included among lists of known substrates (Table 2), but in general this does not seem to be the major connection of these enzymes to cancer progression. That said, there are exceptions, as is the case for ADAM-9 which can cleave laminin and promote invasion (Mazzocca et al., 2005). ADAMs may directly modulate tumour cell adhesion in by interactions with integrins and proteoglycans as discussed in Sections 2.3, 2.4 (and see also (Arribas et al., 2006)). Isolated domains of ADAMs can have potent effects on these aspects of tumourigenesis, as evidenced by the antiangiogenic and anti-metastatic actions of the disintegrin domain of ADAM-15 (Trochon-Joseph et al., 2004); whether these effects are relevant to naturally occurring forms of these molecules is still unclear. Several studies have however linked ADAM-10 to cleavage of the adhesion molecules N-, E- and VE-cadherin (Maretzky et al., 2005a, Reiss et al., 2005, Schulz et al., 2008). These will have profound effects on tumour cell phenotype, resulting in altered patterns of adhesion and migration, and influence on downstream gene expression via activation of β-catenin signalling or direct effects of the RIPped intracellular domain. Likewise, the shedding of adhesion molecules such as Selectins, ICAM-1, VCAM-1, PECAM-1 by ADAMs will affect tumour cell adhesion to the vasculature. By ectodomain shedding ADAMs may mobilize growth factors, chemokines and other soluble mediators or influence signalling via cognate receptors as we have discussed in Section 3.2. This is particularly pertinent for ADAM-17 and EGFR signalling, where direct relationships between ADAM-17 and TGFα have been clearly documented in human breast cancer (Borrell-Pages et al., 2003, Kenny and Bissell, 2007).
An important area of ADAM participation in normal and pathogenetic processes is their influence on inflammatory responses (reviewed by Garton et al., 2006, Ponnuchamy and Khalil, 2008, Murphy et al., 2008). Leukocyte recruitment in response to inflammatory stimuli is a multistep process that involves (1) rolling and tethering on the vascular endothelium, which is mediated by Selectins and their ligands; (2) activation and firm adhesion, which depends on chemokine activation of leukocyte integrins and their associations with vascular adhesion moledules such as ICAM-1 and VCAM-1; (3) locomotion to endothelial cell junctions, again dependent on leukocyte integrins and adhesion partners; and (4) diapediesis across the endothelial layer and basement membrane, which involves PECAM-1 and CD99 (Garton et al., 2006, Ponnuchamy and Khalil, 2008). L-selectin shedding by ADAMs affects the rolling of leukocytes across the endothelium such that blockade of shedding promotes leukocyte adhesion and activation (Hafezi-Moghadam and Ley, 1999). ADAM-mediated L-selectin shedding is necessary also to allow transendothelial migration (Faveeuw et al., 2001). Evidence points to involvement of ADAM-17 in shedding of L-selectin, ICAM-1 and V-CAM-1 (Garton et al., 2006) and ADAM-10 in cleavage of VE-cadherin, thereby enhancing vascular permeability and transmigration of leukocytes (Schulz et al., 2008). Moreover, both ADAM-10 and -17 process chemokines (see Table 2) which drive the activation process and facilitate chemoattraction. ADAM-17 controls TNF-α-mediated inflammation by regulating pro-TNF-α activation and the shedding of TNFRs. The importance of TNFR shedding is highlighted by the discovery that the dominantly inherited chronic inflammatory disorder TNFR-associated periodic syndrome (TRAP) is caused by mutations that impair shedding of TNF-R1 (McDermott et al., 1999). Inflammation is essential for the body to cope with infectious agents, but excessive inflammation contributes to disease pathogenesis in arthritis, cancer, cardiovascular disease, and a host of autoimmune conditions. Thus many of the disease connections of ADAMs may actually be underpinned by fundamental roles in inflammation.
Extending the immunological connection of ADAMs in human pathologies, we come to the association of ADAM33 with asthma. Linkage studies identified ADAM33 as a candidate asthma-susceptibility locus, and numerous polymorphisms were identified in both coding and non-coding regions, the latter suggestive of effects on splicing or transcriptional control (Van Eerdewegh et al., 2002). The function of ADAM-33 in asthma is currently unknown. It is expressed by smooth muscle cells and fibroblasts of the airway rather than inflammatory cells, and many splice variants have been reported, some of which omit the metalloproteinase domain (Powell et al., 2004). Thus its role may be in adhesion or via critical protein–protein interactions. Notably ADAM8¸ which is predominantly expressed by hematopoietic cells, is upregulated in inflammatory cells in an experimental allergen-induced model (King et al., 2004). Expression of both ADAM33 and ADAM8 increases in line with disease severity in bronchial samples from patients with asthma, consistent with roles in promoting disease progression (Foley et al., 2007).
Finally with regard to Alzheimer’s disease, it is now clear that ADAM-9, -10, and -17 can act as α-secretases, possibly acting as a team (Allinson et al., 2003), and thus potentially alter the balance of metabolism of APP towards the non-amyloidogenic pathway. The reduced expression of ADAM-10 in neurons in AD brains compared to controls is consistent with a primary role for this enzyme (Bernstein et al., 2003). Conceivably, therapies could be envisaged that increase α-secretase levels and there is evidence to support positive effects of steroid hormones, non-steroidal anti-inflammatory drugs, metal ions and cholesterol lowering drugs (Allinson et al., 2003). However, the caveat is that we need to understand much more about the multifaceted roles of ADAMs in tissue homeostasis and their positive links with disease pathogenesis that are summarised in Table 7.
5. Concluding remarks
Our knowledge of the remarkable mammalian ADAM family has come a long way since the discovery of the first members as sperm-associated proteins in the early 1990s (Blobel, 1997). It is now clear that these proteases regulate cell phenotype and behaviour by ectodomain shedding and RIPping, and that separately from their proteolytic functions, many influence cell-cell communication via adhesive interactions. There are growing links with human diseases, many reflecting involvement in inflammatory processes, and ADAMs are thus attractive targets for novel therapies. There are several priority areas for future investigations. Firstly, we need more information on ADAM structure, and in particular the structure of full-length molecules. The recent crystallographic analysis of SVMPs have provided radical new insights into the topology of the molecules and how domains may act together to execute substrate docking and catalysis. In these new structures the disintegrin loop is buried and inaccessible, which raises questions about its role in protein–protein interactions. Thus more knowledge of the post-translational processing of the ADAMs and the structures of these proteolytically cleaved or otherwise modified forms will unlock a wealth of mechanistic information. This information will also be valuable for the design of more selective inhibitors that might interfere with function via interactions with exosites involved in substrate recognition or protein–protein interactions, rather than catalysis per se. A second and related issue is the need to understand the significance of variant form of the ADAMs that are produced by alternative splicing. As we have seen, many of these affect the cytoplasmic domain, and we need to look more closely at the how these domains may be involved in particular aspects of ADAM function such as regulated trafficking, interaction partner selection and coupling to signal transduction pathways. Thirdly, given the critical roles of many ADAMs in key developmental processes, use of conditional and inducible mouse knockouts in conjunction with disease models is essential. Finally, this review has not dealt extensively with the control of ADAM expression since very little is known at present. Though it is clear that several ADAMs are subject to transcriptional regulation (Worley et al., 2003), it is likely that control at this level may not be as significant as that of their relatives the MMPs (Clark et al., 2008) since there are already strong clues that post-transcriptional regulation is key, at least for ADAM17 (Santiago-Josefat et al., 2007). Thus the study of the roles of microRNAs in ADAM control may prove very revealing (Dalmay and Edwards, 2006).
Acknowledgements
This work was supported by grants from the Wellcome Trust, the Biotechnology and Biological Sciences Research Council, the Big C Appeal, Cancer Research-UK and the EU Framework Programme 6 Cancerdegradome Project LSHC-CT-2003-503297.
References
- Abram C.L., Seals D.F., Pass I., Salinsky D., Maurer L., Roth T.M., Courtneidge S.A. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J. Biol. Chem. 2003;278(19):16844–16851. doi: 10.1074/jbc.M300267200. [DOI] [PubMed] [Google Scholar]
- Alfa Cisse M., Sunyach C., Slack B.E., Fisher A., Vincent B., Checler F. M1 and M3 muscarinic receptors control physiological processing of cellular prion by modulating ADAM17 phosphorylation and activity. J. Neurosci. 2007;27(15):4083–4092. doi: 10.1523/JNEUROSCI.5293-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfandari D., Cousin H., Gaultier A., Smith K., White J.M., Darribere T., DeSimone D.W. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest-cell migration. Curr. Biol. 2001;11(12):918–930. doi: 10.1016/s0960-9822(01)00263-9. [DOI] [PubMed] [Google Scholar]
- Allinson T.M., Parkin E.T., Turner A.J., Hooper N.M. ADAMs family members as amyloid precursor protein alpha-secretases. J. Neurosci. Res. 2003;74(3):342–352. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- Almeida E.A., Huovila A.P., Sutherland A.E., Stephens L.E., Calarco P.G., Shaw L.M., Mercurio A.M., Sonnenberg A., Primakoff P., Myles D.G., White J.M. Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell. 1995;81(7):1095–1104. doi: 10.1016/s0092-8674(05)80014-5. [DOI] [PubMed] [Google Scholar]
- Althoff K., Mullberg J., Aasland D., Voltz N., Kallen K., Grotzinger J., Rose-John S. Recognition sequences and structural elements contribute to shedding susceptibility of membrane proteins. Biochem. J. 2001;353(Pt 3):663–672. doi: 10.1042/0264-6021:3530663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amour A., Knight C.G., English W.R., Webster A., Slocombe P.M., Knauper V., Docherty A.J., Becherer J.D., Blobel C.P., Murphy G. The enzymatic activity of ADAM8 and ADAM9 is not regulated by TIMPs. FEBS Lett. 2002;524(1-3):154–158. doi: 10.1016/s0014-5793(02)03047-8. [DOI] [PubMed] [Google Scholar]
- Amour A., Knight C.G., Webster A., Slocombe P.M., Stephens P.E., Knauper V., Docherty A.J., Murphy G. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 2000;473(3):275–279. doi: 10.1016/s0014-5793(00)01528-3. [DOI] [PubMed] [Google Scholar]
- Anders A., Gilbert S., Garten W., Postina R., Fahrenholz F. Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 2001;15(10):1837–1839. doi: 10.1096/fj.01-0007fje. [DOI] [PubMed] [Google Scholar]
- Angerer L., Hussain S., Wei Z., Livingston B.T. Sea urchin metalloproteases: a genomic survey of the BMP-1/tolloid-like, MMP and ADAM families. Dev. Biol. 2006;300(1):267–281. doi: 10.1016/j.ydbio.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Apte S.S. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int. J. Biochem. Cell Biol. 2004;36(6):981–985. doi: 10.1016/j.biocel.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Arima T., Enokida H., Kubo H., Kagara I., Matsuda R., Toki K., Nishimura H., Chiyomaru T., Tatarano S., Idesako T., Nishiyama K., Nakagawa M. Nuclear translocation of ADAM-10 contributes to the pathogenesis and progression of human prostate cancer. Cancer Sci. 2007;98(11):1720–1726. doi: 10.1111/j.1349-7006.2007.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas J., Bech-Serra J.J., Santiago-Josefat B. ADAMs, cell migration and cancer. Cancer Metastasis Rev. 2006;25(1):57–68. doi: 10.1007/s10555-006-7889-6. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Asakura M., Kitakaze M., Takashima S., Liao Y., Ishikura F., Yoshinaka T., Ohmoto H., Node K., Yoshino K., Ishiguro H., Asanuma H., Sanada S., Matsumura Y., Takeda H., Beppu S., Tada M., Hori M., Higashiyama S. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 2002;8(1):35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- Baker A.H., Edwards D.R., Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J. Cell Sci. 2002;115(Pt 19):3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- Bates E.E., Fridman W.H., Mueller C.G. The ADAMDEC1 (decysin) gene structure: evolution by duplication in a metalloprotease gene cluster on chromosome 8p12. Immunogenetics. 2002;54(2):96–105. doi: 10.1007/s00251-002-0430-3. [DOI] [PubMed] [Google Scholar]
- Bax D.V., Messent A.J., Tart J., van Hoang M., Kott J., Maciewicz R.A., Humphries M.J. Integrin alpha5beta1 and ADAM-17 interact in vitro and co-localize in migrating HeLa cells. J. Biol. Chem. 2004;279(21):22377–22386. doi: 10.1074/jbc.M400180200. [DOI] [PubMed] [Google Scholar]
- Bech-Serra J.J., Santiago-Josefat B., Esselens C., Saftig P., Baselga J., Arribas J., Canals F. Proteomic identification of desmoglein 2 and activated leukocyte cell adhesion molecule as substrates of ADAM17 and ADAM10 by difference gel electrophoresis. Mol. Cell Biol. 2006;26(13):5086–5095. doi: 10.1128/MCB.02380-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H.G., Bukowska A., Krell D., Bogerts B., Ansorge S., Lendeckel U. Comparative localization of ADAMs 10 and 15 in human cerebral cortex normal aging, Alzheimer disease and Down syndrome. J. Neurocytol. 2003;32(2):153–160. doi: 10.1023/b:neur.0000005600.61844.a6. [DOI] [PubMed] [Google Scholar]
- Black R.A., Rauch C.T., Kozlosky C.J., Peschon J.J., Slack J.L., Wolfson M.F., Castner B.J., Stocking K.L., Reddy P., Srinivasan S., Nelson N., Boiani N., Schooley K.A., Gerhart M., Davis R., Fitzner J.N., Johnson R.S., Paxton R.J., March C.J., Cerretti D.P. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385(6618):729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Blobel C.P. Metalloprotease-disintegrins: links to cell adhesion and cleavage of TNF alpha and Notch. Cell. 1997;90(4):589–592. doi: 10.1016/s0092-8674(00)80519-x. [DOI] [PubMed] [Google Scholar]
- Blobel C.P. ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005;6(1):32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Blobel C.P., Wolfsberg T.G., Turck C.W., Myles D.G., Primakoff P., White J.M. A potential fusion peptide and an integrin ligand domain in a protein active in sperm–egg fusion. Nature. 1992;356(6366):248–252. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- Bode W., Gomis-Ruth F.X., Stockler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins’. FEBS Lett. 1993;331(1–2):134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- Bohm B.B., Aigner T., Roy B., Brodie T.A., Blobel C.P., Burkhardt H. Homeostatic effects of the metalloproteinase disintegrin ADAM15 in degenerative cartilage remodeling. Arthritis Rheum. 2005;52(4):1100–1109. doi: 10.1002/art.20974. [DOI] [PubMed] [Google Scholar]
- Borrell-Pages M., Rojo F., Albanell J., Baselga J., Arribas J. TACE is required for the activation of the EGFR by TGF-alpha in tumors. EMBO J. 2003;22(5):1114–1124. doi: 10.1093/emboj/cdg111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brou C., Logeat F., Gupta N., Bessia C., LeBail O., Doedens J.R., Cumano A., Roux P., Black R.A., Israel A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell. 2000;5(2):207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- Budagian V., Bulanova E., Orinska Z., Duitman E., Brandt K., Ludwig A., Hartmann D., Lemke G., Saftig P., Bulfone-Paus S. Soluble Axl is generated by ADAM10-dependent cleavage and associates with Gas6 in mouse serum. Mol. Cell Biol. 2005;25(21):9324–9339. doi: 10.1128/MCB.25.21.9324-9339.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Buxbaum J.D., Liu K.N., Luo Y., Slack J.L., Stocking K.L., Peschon J.J., Johnson R.S., Castner B.J., Cerretti D.P., Black R.A. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem. 1998;273(43):27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- Canault M., Tellier E., Bonardo B., Mas E., Aumailley M., Juhan-Vague I., Nalbone G., Peiretti F. FHL2 interacts with both ADAM-17 and the cytoskeleton and regulates ADAM-17 localization and activity. J. Cell Physiol. 2006;208(2):363–372. doi: 10.1002/jcp.20671. [DOI] [PubMed] [Google Scholar]
- Cao Y., Kang Q., Zhao Z., Zolkiewska A. Intracellular processing of metalloprotease disintegrin ADAM12. J. Biol. Chem. 2002;277(29):26403–26411. doi: 10.1074/jbc.M110814200. [DOI] [PubMed] [Google Scholar]
- Cao Y., Kang Q., Zolkiewska A. Metalloprotease-disintegrin ADAM 12 interacts with alpha-actinin-1. Biochem. J. 2001;357(Pt 2):353–361. doi: 10.1042/0264-6021:3570353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Zhao Z., Gruszczynska-Biegala J., Zolkiewska A. Role of metalloprotease disintegrin ADAM12 in determination of quiescent reserve cells during myogenic differentiation in vitro. Mol. Cell Biol. 2003;23(19):6725–6738. doi: 10.1128/MCB.23.19.6725-6738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrol E., Gourfinkel-An I., Scheffer I.E., Picard F., Couarch P., Berkovic S.F., McMahon J.M., Bajaj N., Mota-Vieira L., Mota R., Trouillard O., Depienne C., Baulac M., LeGuern E., Baulac S. Absence of mutations in the LGI1 receptor ADAM22 gene in autosomal dominant lateral temporal epilepsy. Epilepsy Res. 2007;76(1):41–48. doi: 10.1016/j.eplepsyres.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Chalaris A., Rabe B., Paliga K., Lange H., Laskay T., Fielding C.A., Jones S.A., Rose-John S., Scheller J. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood. 2007;110(6):1748–1755. doi: 10.1182/blood-2007-01-067918. [DOI] [PubMed] [Google Scholar]
- Charbonneau M., Harper K., Grondin F., Pelmus M., McDonald P.P., Dubois C.M. Hypoxia-inducible factor mediates hypoxic and tumor necrosis factor alpha-induced increases in tumor necrosis factor-alpha converting enzyme/ADAM17 expression by synovial cells. J. Biol. Chem. 2007;282(46):33714–33724. doi: 10.1074/jbc.M704041200. [DOI] [PubMed] [Google Scholar]
- Chen C., Huang X., Sheppard D. ADAM33 is not essential for growth and development and does not modulate allergic asthma in mice. Mol. Cell Biol. 2006;26(18):6950–6956. doi: 10.1128/MCB.00646-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.D., Podvin S., Gillespie E., Leeman S.E., Abraham C.R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl. Acad. Sci. USA. 2007;104(50):19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesneau V., Becherer J.D., Zheng Y., Erdjument-Bromage H., Tempst P., Blobel C.P. Catalytic properties of ADAM19. J. Biol. Chem. 2003;278(25):22331–22340. doi: 10.1074/jbc.M302781200. [DOI] [PubMed] [Google Scholar]
- Cho C., Bunch D.O., Faure J.E., Goulding E.H., Eddy E.M., Primakoff P., Myles D.G. Fertilization defects in sperm from mice lacking fertilin beta. Science. 1998;281(5384):1857–1859. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- Cho R.W., Park J.M., Wolff S.B., Xu D., Hopf C., Kim J.A., Reddy R.C., Petralia R.S., Perin M.S., Linden D.J., Worley P.F. mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron. 2008;57(6):858–871. doi: 10.1016/j.neuron.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse M.A., Sunyach C., Lefranc-Jullien S., Postina R., Vincent B., Checler F. The disintegrin ADAM9 indirectly contributes to the physiological processing of cellular prion by modulating ADAM10 activity. J. Biol. Chem. 2005;280(49):40624–40631. doi: 10.1074/jbc.M506069200. [DOI] [PubMed] [Google Scholar]
- Clark I.M., Swingler T., Sampieri C.L., Edwards D.R. The regulation of matrix metalloproteinases and their inhibitors. Int J. Biochem. Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Condon J.S., Joseph-McCarthy D., Levin J.I., Lombart H.G., Lovering F.E., Sun L., Wang W., Xu W., Zhang Y. Identification of potent and selective TACE inhibitors via the S1 pocket. Bioorg Med Chem Lett. 2007;17(1):34–39. doi: 10.1016/j.bmcl.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Contin C., Pitard V., Itai T., Nagata S., Moreau J.F., Dechanet-Merville J. Membrane-anchored CD40 is processed by the tumor necrosis factor-alpha-converting enzyme. Implications for CD40 signaling. J. Biol. Chem. 2003;278(35):32801–32809. doi: 10.1074/jbc.M209993200. [DOI] [PubMed] [Google Scholar]
- Cousin H., Gaultier A., Bleux C., Darribere T., Alfandari D. PACSIN2 is a regulator of the metalloprotease/disintegrin ADAM13. Dev. Biol. 2000;227(1):197–210. doi: 10.1006/dbio.2000.9871. [DOI] [PubMed] [Google Scholar]
- Coussens L.M., Fingleton B., Matrisian L.M. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- D’Abaco G.M., Ng K., Paradiso L., Godde N.J., Kaye A., Novak U. ADAM22, expressed in normal brain but not in high-grade gliomas, inhibits cellular proliferation via the disintegrin domain. Neurosurgery. 2006;58(1):179–186. doi: 10.1227/01.neu.0000192363.84287.8b. (discussion 179–186) [DOI] [PubMed] [Google Scholar]
- Dalmay T., Edwards D.R. MicroRNAs and the Hallmarks of Cancer. Oncogene. 2006;25:6170–6175. doi: 10.1038/sj.onc.1209911. [DOI] [PubMed] [Google Scholar]
- Diaz-Rodriguez E., Montero J.C., Esparis-Ogando A., Yuste L., Pandiella A. Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735: a potential role in regulated shedding. Mol. Biol. Cell. 2002;13(6):2031–2044. doi: 10.1091/mbc.01-11-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens J.R., Black R.A. Stimulation-induced down-regulation of tumor necrosis factor-alpha converting enzyme. J. Biol. Chem. 2000;275(19):14598–14607. doi: 10.1074/jbc.275.19.14598. [DOI] [PubMed] [Google Scholar]
- Doedens J.R., Mahimkar R.M., Black R.A. TACE/ADAM-17 enzymatic activity is increased in response to cellular stimulation. Biochem. Biophys. Res. Commun. 2003;308(2):331–338. doi: 10.1016/s0006-291x(03)01381-0. [DOI] [PubMed] [Google Scholar]
- Dyczynska E., Sun D., Yi H., Sehara-Fujisawa A., Blobel C.P., Zolkiewska A. Proteolytic processing of delta-like 1 by ADAM proteases. J. Biol. Chem. 2007;282(1):436–444. doi: 10.1074/jbc.M605451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenauer D.A., Simhadri V.L., von Strandmann E.P., Ludwig A., Matthews V., Reiners K.S., von Tresckow B., Saftig P., Rose-John S., Engert A., Hansen H.P. ADAM10 inhibition of human CD30 shedding increases specificity of targeted immunotherapy in vitro. Cancer Res. 2007;67(1):332–338. doi: 10.1158/0008-5472.CAN-06-2470. [DOI] [PubMed] [Google Scholar]
- Endres K., Anders A., Kojro E., Gilbert S., Fahrenholz F., Postina R. Tumor necrosis factor-alpha converting enzyme is processed by proprotein-convertases to its mature form which is degraded upon phorbol ester stimulation. Eur. J. Biochem. 2003;270(11):2386–2393. doi: 10.1046/j.1432-1033.2003.03606.x. [DOI] [PubMed] [Google Scholar]
- Eto K., Huet C., Tarui T., Kupriyanov S., Liu H.Z., Puzon-McLaughlin W., Zhang X.P., Sheppard D., Engvall E., Takada Y. Functional classification of ADAMs based on a conserved motif for binding to integrin alpha 9beta 1: implications for sperm–egg binding and other cell interactions. J. Biol. Chem. 2002;277(20):17804–17810. doi: 10.1074/jbc.M200086200. [DOI] [PubMed] [Google Scholar]
- Evans J.P. Fertilin beta and other ADAMs as integrin ligands: insights into cell adhesion and fertilization. Bioessays. 2001;23(7):628–639. doi: 10.1002/bies.1088. [DOI] [PubMed] [Google Scholar]
- Fabre-Lafay S., Garrido-Urbani S., Reymond N., Goncalves A., Dubreuil P., Lopez M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J. Biol. Chem. 2005;280(20):19543–19550. doi: 10.1074/jbc.M410943200. [DOI] [PubMed] [Google Scholar]
- Fan H., Turck C.W., Derynck R. Characterization of growth factor-induced serine phosphorylation of tumor necrosis factor-alpha converting enzyme and of an alternatively translated polypeptide. J. Biol. Chem. 2003;278(20):18617–18627. doi: 10.1074/jbc.M300331200. [DOI] [PubMed] [Google Scholar]
- Faveeuw C., Preece G., Ager A. Transendothelial migration of lymphocytes across high endothelial venules into lymph nodes is affected by metalloproteinases. Blood. 2001;98(3):688–695. doi: 10.1182/blood.v98.3.688. [DOI] [PubMed] [Google Scholar]
- Fischer O.M., Hart S., Gschwind A., Ullrich A. EGFR signal transactivation in cancer cells. Biochem. Soc. Trans. 2003;31(Pt 6):1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- Foley S.C., Mogas A.K., Olivenstein R., Fiset P.O., Chakir J., Bourbeau J., Ernst P., Lemiere C., Martin J.G., Hamid Q. Increased expression of ADAM33 and ADAM8 with disease progression in asthma. J. Allergy Clin. Immunol. 2007;119(4):863–871. doi: 10.1016/j.jaci.2006.12.665. [DOI] [PubMed] [Google Scholar]
- Fourie A.M., Coles F., Moreno V., Karlsson L. Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J. Biol. Chem. 2003;278(33):30469–30477. doi: 10.1074/jbc.M213157200. [DOI] [PubMed] [Google Scholar]
- Franzke C.W., Tasanen K., Borradori L., Huotari V., Bruckner-Tuderman L. Shedding of collagen XVII/BP180: structural motifs influence cleavage from cell surface. J. Biol. Chem. 2004;279(23):24521–24529. doi: 10.1074/jbc.M308835200. [DOI] [PubMed] [Google Scholar]
- Fridman J.S., Caulder E., Hansbury M., Liu X., Yang G., Wang Q., Lo Y., Zhou B.B., Pan M., Thomas S.M., Grandis J.R., Zhuo J., Yao W., Newton R.C., Friedman S.M., Scherle P.A., Vaddi K. Selective inhibition of ADAM metalloproteases as a novel approach for modulating ErbB pathways in cancer. Clin. Cancer Res. 2007;13(6):1892–1902. doi: 10.1158/1078-0432.CCR-06-2116. [DOI] [PubMed] [Google Scholar]
- Fröhlich C., Albrectsen R., Dyrskjøt L., Rudkjaer L., Ørntoft T.F., Wewer U.M. Molecular profiling of ADAM12 in human bladder cancer. Clin. Cancer Res. 2006;12(24):7359–7368. doi: 10.1158/1078-0432.CCR-06-1066. [DOI] [PubMed] [Google Scholar]
- Fukata Y., Adesnik H., Iwanaga T., Bredt D.S., Nicoll R.A., Fukata M. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science. 2006;313(5794):1792–1795. doi: 10.1126/science.1129947. [DOI] [PubMed] [Google Scholar]
- Galliano M.F., Huet C., Frygelius J., Polgren A., Wewer U.M., Engvall E. Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha -actinin-2, is required for myoblast fusion. J. Biol. Chem. 2000;275(18):13933–13939. doi: 10.1074/jbc.275.18.13933. [DOI] [PubMed] [Google Scholar]
- Garton K.J., Gough P.J., Philalay J., Wille P.T., Blobel C.P., Whitehead R.H., Dempsey P.J., Raines E.W. Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1) is mediated by tumor necrosis factor-alpha-converting enzyme (ADAM 17) J. Biol. Chem. 2003;278(39):37459–37464. doi: 10.1074/jbc.M305877200. [DOI] [PubMed] [Google Scholar]
- Garton K.J., Gough P.J., Raines E.W. Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J. Leukoc. Biol. 2006;79(6):1105–1106. doi: 10.1189/jlb.0106038. [DOI] [PubMed] [Google Scholar]
- Gaultier A., Cousin H., Darribere T., Alfandari D. ADAM13 disintegrin and cysteine-rich domains bind to the second heparin-binding domain of fibronectin. J. Biol. Chem. 2002;277(26):23336–23344. doi: 10.1074/jbc.M201792200. [DOI] [PubMed] [Google Scholar]
- Gavert N., Sheffer M., Raveh S., Spaderna S., Shtutman M., Brabletz T., Barany F., Paty P., Notterman D., Domany E., Ben-Ze’ev A. Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res. 2007;67(16):7703–7712. doi: 10.1158/0008-5472.CAN-07-0991. [DOI] [PubMed] [Google Scholar]
- Georgakopoulos A., Litterst C., Ghersi E., Baki L., Xu C., Serban G., Robakis N.K. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25(6):1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt S., Hassall G., Hawtin P., McCall E., Flavell L., Minshull C., Hargreaves D., Ting A., Pauptit R.A., Parker A.E., Abbott W.M. Crystal structures of human ADAMTS-1 reveal a conserved catalytic domain and a disintegrin-like domain with a fold homologous to cysteine-rich domains. J. Mol. Biol. 2007;373(4):891–902. doi: 10.1016/j.jmb.2007.07.047. [DOI] [PubMed] [Google Scholar]
- Glassey B., Civetta A. Positive selection at reproductive ADAM genes with potential intercellular binding activity. Mol. Biol. Evol. 2004;21(5):851–859. doi: 10.1093/molbev/msh080. [DOI] [PubMed] [Google Scholar]
- Godde N.J., D’Abaco G.M., Paradiso L., Novak U. Efficient ADAM22 surface expression is mediated by phosphorylation-dependent interaction with 14-3-3 protein family members. J. Cell Sci. 2006;119(Pt 16):3296–3305. doi: 10.1242/jcs.03065. [DOI] [PubMed] [Google Scholar]
- Gomez-Gaviro M., Dominguez-Luis M., Canchado J., Calafat J., Janssen H., Lara-Pezzi E., Fourie A., Tugores A., Valenzuela-Fernandez A., Mollinedo F., Sanchez-Madrid F., Diaz-Gonzalez F. Expression and regulation of the metalloproteinase ADAM-8 during human neutrophil pathophysiological activation and its catalytic activity on L-selectin shedding. J. Immunol. 2007;178(12):8053–8063. doi: 10.4049/jimmunol.178.12.8053. [DOI] [PubMed] [Google Scholar]
- Gomis-Ruth F.X. Structural aspects of the metzincin clan of metalloendopeptidases. Mol. Biotechnol. 2003;24(2):157–202. doi: 10.1385/MB:24:2:157. [DOI] [PubMed] [Google Scholar]
- Gomis-Ruth F.X., Maskos K., Betz M., Bergner A., Huber R., Suzuki K., Yoshida N., Nagase H., Brew K., Bourenkov G.P., Bartunik H., Bode W. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature. 1997;389(6646):77–81. doi: 10.1038/37995. [DOI] [PubMed] [Google Scholar]
- Gonzales P.E., Solomon A., Miller A.B., Leesnitzer M.A., Sagi I., Milla M.E. Inhibition of the tumor necrosis factor-alpha-converting enzyme by its pro domain. J. Biol. Chem. 2004;279(30):31638–31645. doi: 10.1074/jbc.M401311200. [DOI] [PubMed] [Google Scholar]
- Gooz M., Gooz P., Luttrell L.M., Raymond J.R. 5-HT2A receptor induces ERK phosphorylation and proliferation through ADAM-17 tumor necrosis factor-alpha-converting enzyme (TACE) activation and heparin-bound epidermal growth factor-like growth factor (HB-EGF) shedding in mesangial cells. J. Biol. Chem. 2006;281(30):21004–21012. doi: 10.1074/jbc.M512096200. [DOI] [PubMed] [Google Scholar]
- Grutzmann R., Luttges J., Sipos B., Ammerpohl O., Dobrowolski F., Alldinger I., Kersting S., Ockert D., Koch R., Kalthoff H., Schackert H.K., Saeger H.D., Kloppel G., Pilarsky C. ADAM9 expression in pancreatic cancer is associated with tumour type and is a prognostic factor in ductal adenocarcinoma. Br. J. Cancer. 2004;90(5):1053–1058. doi: 10.1038/sj.bjc.6601645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwind A., Hart S., Fischer O.M., Ullrich A. TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO J. 2003;22(10):2411–2421. doi: 10.1093/emboj/cdg231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi-Moghadam A., Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J. Exp. Med. 1999;189(6):939–948. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. USA. 2008;105(22):7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H.P., Recke A., Reineke U., Von Tresckow B., Borchmann P., Von Strandmann E.P., Lange H., Lemke H., Engert A. The ectodomain shedding of CD30 is specifically regulated by peptide motifs in its cysteine-rich domains 2 and 5. FASEB J. 2004;18(7):893–895. doi: 10.1096/fj.03-0901fje. [DOI] [PubMed] [Google Scholar]
- Hart S., Fischer O.M., Prenzel N., Zwick-Wallasch E., Schneider M., Hennighausen L., Ullrich A. GPCR-induced migration of breast carcinoma cells depends on both EGFR signal transactivation and EGFR-independent pathways. Biol. Chem. 2005;386(9):845–855. doi: 10.1515/BC.2005.099. [DOI] [PubMed] [Google Scholar]
- Hartmann D., de Strooper B., Serneels L., Craessaerts K., Herreman A., Annaert W., Umans L., Lubke T., Lena Illert A., von Figura K., Saftig P. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum. Mol. Genet. 2002;11(21):2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- Hattori M., Osterfield M., Flanagan J.G. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289(5483):1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- Hermey G., Sjogaard S.S., Petersen C.M., Nykjaer A., Gliemann J. Tumour necrosis factor alpha-converting enzyme mediates ectodomain shedding of Vps10p-domain receptor family members. Biochem. J. 2006;395(2):285–293. doi: 10.1042/BJ20051364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiipakka M., Saksela K. Versatile retargeting of SH3 domain binding by modification of non-conserved loop residues. FEBS Lett. 2007;581(9):1735–1741. doi: 10.1016/j.febslet.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Hikita A., Yana I., Wakeyama H., Nakamura M., Kadono Y., Oshima Y., Nakamura K., Seiki M., Tanaka S. Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-kappaB ligand. J. Biol. Chem. 2006;281(48):36846–36855. doi: 10.1074/jbc.M606656200. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Le Gall S., Schulte M., Yamaguchi T., Reiss K., Murphy G., Toyama Y., Hartmann D., Saftig P., Blobel C.P. Substrate selectivity of epidermal growth factor-receptor ligand sheddases and their regulation by phorbol esters and calcium influx. Mol. Biol. Cell. 2007;18(1):176–188. doi: 10.1091/mbc.E06-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K., Miyamoto T., Takaishi H., Hakozaki A., Kosaki N., Miyauchi Y., Furukawa M., Takito J., Kaneko H., Matsuzaki K., Morioka H., Blobel C.P., Toyama Y. Cell surface colony-stimulating factor 1 can be cleaved by TNF-alpha converting enzyme or endocytosed in a clathrin-dependent manner. J. Immunol. 2007;179(10):6715–6724. doi: 10.4049/jimmunol.179.10.6715. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Weskamp G., Lum L., Hammes H.P., Cai H., Brodie T.A., Ludwig T., Chiusaroli R., Baron R., Preissner K.T., Manova K., Blobel C.P. Potential role for ADAM15 in pathological neovascularization in mice. Mol. Cell Biol. 2003;23(16):5614–5624. doi: 10.1128/MCB.23.16.5614-5624.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K., Zhou H.M., Kelly K., Manova K., Blobel C.P. Evaluation of the contributions of ADAMs 9, 12, 15, 17, and 19 to heart development and ectodomain shedding of neuregulins beta1 and beta2. Dev. Biol. 2005;283(2):459–471. doi: 10.1016/j.ydbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hougaard S., Loechel F., Xu X., Tajima R., Albrechtsen R., Wewer U.M. Trafficking of human ADAM 12-L: retention in the trans-Golgi network. Biochem. Biophys. Res. Commun. 2000;275(2):261–267. doi: 10.1006/bbrc.2000.3295. [DOI] [PubMed] [Google Scholar]
- Howard L., Maciewicz R.A., Blobel C.P. Cloning and characterization of ADAM28: evidence for autocatalytic pro-domain removal and for cell surface localization of mature ADAM28. Biochem. J. 2000;348(Pt 1):21–27. [PMC free article] [PubMed] [Google Scholar]
- Howard L., Nelson K.K., Maciewicz R.A., Blobel C.P. Interaction of the metalloprotease disintegrins MDC9 and MDC15 with two SH3 domain-containing proteins, endophilin I and SH3PX1. J. Biol. Chem. 1999;274(44):31693–31699. doi: 10.1074/jbc.274.44.31693. [DOI] [PubMed] [Google Scholar]
- Huang A., Joseph-McCarthy D., Lovering F., Sun L., Wang W., Xu W., Zhu Y., Cui J., Zhang Y., Levin J.I. Structure-based design of TACE selective inhibitors: manipulations in the S1’-S3’ pocket. Bioorg. Med. Chem. 2007;15(18):6170–6181. doi: 10.1016/j.bmc.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Huang J., Bridges L.C., White J.M. Selective modulation of integrin-mediated cell migration by distinct ADAM family members. Mol. Biol. Cell. 2005;16(10):4982–4991. doi: 10.1091/mbc.E05-03-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Feng L., Yang L., Zhou W., Zhao S., Li C. Screen and identification of proteins interacting with ADAM19 cytoplasmic tail. Mol. Biol. Rep. 2002;29(3):317–323. doi: 10.1023/a:1020409217215. [DOI] [PubMed] [Google Scholar]
- Hundhausen C., Misztela D., Berkhout T.A., Broadway N., Saftig P., Reiss K., Hartmann D., Fahrenholz F., Postina R., Matthews V., Kallen K.J., Rose-John S., Ludwig A. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102(4):1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- Hundhausen C., Schulte A., Schulz B., Andrzejewski M.G., Schwarz N., von Hundelshausen P., Winter U., Paliga K., Reiss K., Saftig P., Weber C., Ludwig A. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J. Immunol. 2007;178(12):8064–8072. doi: 10.4049/jimmunol.178.12.8064. [DOI] [PubMed] [Google Scholar]
- Huxley-Jones J., Clarke T.K., Beck C., Toubaris G., Robertson D.L., Boot-Handford R.P. The evolution of the vertebrate metzincins; insights from Ciona intestinalis and Danio rerio. BMC Evol. Biol. 2007;7:63. doi: 10.1186/1471-2148-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Iba K., Albrechtsen R., Gilpin B., Frohlich C., Loechel F., Zolkiewska A., Ishiguro K., Kojima T., Liu W., Langford J.K., Sanderson R.D., Brakebusch C., Fassler R., Wewer U.M. The cysteine-rich domain of human ADAM 12 supports cell adhesion through syndecans and triggers signaling events that lead to beta1 integrin-dependent cell spreading. J. Cell Biol. 2000;149(5):1143–1156. doi: 10.1083/jcb.149.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T., Araki S., Mori H., Takeda S. Crystal structures of catrocollastatin/VAP2B reveal a dynamic, modular architecture of ADAM/adamalysin/reprolysin family proteins. FEBS Lett. 2007;581(13):2416–2422. doi: 10.1016/j.febslet.2007.04.057. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Hirata M., Hasuwa H., Iwamoto R., Umata T., Miyado K., Tamai Y., Kurisaki T., Sehara-Fujisawa A., Ohno S., Mekada E. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 1998;17(24):7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.F., Qiu T.H., Sunnarborg S.W., Chang A., Zhang C., Patterson C., Lee D.C. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22(11):2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J., Visse R., Sorensen H.P., Enghild J.J., Brew K., Wewer U.M., Nagase H. Catalytic properties of ADAM12 and its domain deletion mutants. Biochemistry. 2008;47(2):537–547. doi: 10.1021/bi701629c. [DOI] [PubMed] [Google Scholar]
- Janes P.W., Saha N., Barton W.A., Kolev M.V., Wimmer-Kleikamp S.H., Nievergall E., Blobel C.P., Himanen J.P., Lackmann M., Nikolov D.B. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123(2):291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Jones G.C., Riley G.P. ADAMTS proteinases: a multi-domain, multi-functional family with roles in extracellular matrix turnover and arthritis. Arthritis Res. Ther. 2005;7(4):160–169. doi: 10.1186/ar1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen L.H., Jensen C.H., Wewer U.M., Schroder H.D. Transgenic overexpression of ADAM12 suppresses muscle regeneration and aggravates dystrophy in aged mdx mice. Am. J. Pathol. 2007;171(5):1599–1607. doi: 10.2353/ajpath.2007.070435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczur V., Puskas L.G., Nagy Z.U., Miled N., Rebai A., Juhasz F., Kupihar Z., Zvara A., Hackler L., Jr., Farid N.R. Cleavage of the human thyrotropin receptor by ADAM10 is regulated by thyrotropin. J. Mol. Recognit. 2007;20(5):392–404. doi: 10.1002/jmr.851. [DOI] [PubMed] [Google Scholar]
- Kalus I., Bormann U., Mzoughi M., Schachner M., Kleene R. Proteolytic cleavage of the neural cell adhesion molecule by ADAM17/TACE is involved in neurite outgrowth. J. Neurochem. 2006;98(1):78–88. doi: 10.1111/j.1471-4159.2006.03847.x. [DOI] [PubMed] [Google Scholar]
- Kang Q., Cao Y., Zolkiewska A. Metalloprotease-disintegrin ADAM 12 binds to the SH3 domain of Src and activates Src tyrosine kinase in C2C12 cells. Biochem. J. 2000;352(Pt 3):883–892. [PMC free article] [PubMed] [Google Scholar]
- Kang Q., Cao Y., Zolkiewska A. Direct interaction between the cytoplasmic tail of ADAM 12 and the Src homology 3 domain of p85alpha activates phosphatidylinositol 3-kinase in C2C12 cells. J. Biol. Chem. 2001;276(27):24466–24472. doi: 10.1074/jbc.M101162200. [DOI] [PubMed] [Google Scholar]
- Kang T., Park H.I., Suh Y., Zhao Y.G., Tschesche H., Sang Q.X. Autolytic processing at Glu586-Ser587 within the cysteine-rich domain of human adamalysin 19/disintegrin-metalloproteinase 19 is necessary for its proteolytic activity. J. Biol. Chem. 2002;277(50):48514–48522. doi: 10.1074/jbc.M208961200. [DOI] [PubMed] [Google Scholar]
- Kang T., Tschesche H., Amy Sang Q.X. Evidence for disulfide involvement in the regulation of intramolecular autolytic processing by human adamalysin19/ADAM19. Exp. Cell Res. 2004;298(1):285–295. doi: 10.1016/j.yexcr.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Karkkainen S., Hiipakka M., Wang J.H., Kleino I., Vaha-Jaakkola M., Renkema G.H., Liss M., Wagner R., Saksela K. Identification of preferred protein interactions by phage-display of the human Src homology-3 proteome. EMBO Rep. 2006;7(2):186–191. doi: 10.1038/sj.embor.7400596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T., Harada Y., Emi M., Nakamura Y. Human metalloprotease/disintegrin-like (MDC) gene: exon-intron organization and alternative splicing. Cytogenet. Cell Genet. 1995;68(1-2):39–44. doi: 10.1159/000133884. [DOI] [PubMed] [Google Scholar]
- Kawaguchi N., Horiuchi K., Becherer J.D., Toyama Y., Besmer P., Blobel C.P. Different ADAMs have distinct influences on Kit ligand processing: phorbol-ester-stimulated ectodomain shedding of Kitl1 by ADAM17 is reduced by ADAM19. J. Cell Sci. 2007;120(Pt 6):943–952. doi: 10.1242/jcs.03403. [DOI] [PubMed] [Google Scholar]
- Kawaguchi N., Sundberg C., Kveiborg M., Moghadaszadeh B., Asmar M., Dietrich N., Thodeti C.K., Nielsen F.C., Moller P., Mercurio A.M., Albrechtsen R., Wewer U.M. ADAM12 induces actin cytoskeleton and extracellular matrix reorganization during early adipocyte differentiation by regulating beta1 integrin function. J. Cell Sci. 2003;116(Pt 19):3893–3904. doi: 10.1242/jcs.00699. [DOI] [PubMed] [Google Scholar]
- Kawaguchi N., Xu X., Tajima R., Kronqvist P., Sundberg C., Loechel F., Albrechtsen R., Wewer U.M. ADAM 12 protease induces adipogenesis in transgenic mice. Am. J. Pathol. 2002;160(5):1895–1903. doi: 10.1016/S0002-9440(10)61136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Hutchinson G., Nebenius-Oosthuizen D., Smith A.J., Bartsch J.W., Horiuchi K., Rittger A., Manova K., Docherty A.J., Blobel C.P. Metalloprotease-disintegrin ADAM8: expression analysis and targeted deletion in mice. Dev. Dyn. 2005;232(1):221–231. doi: 10.1002/dvdy.20221. [DOI] [PubMed] [Google Scholar]
- Kenny P.A., Bissell M.J. Targeting TACE-dependent EGFR ligand shedding in breast cancer. J. Clin. Invest. 2007;117(2):337–345. doi: 10.1172/JCI29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Oh J., Woo J.M., Choi E., Im S.H., Yoo Y.J., Kim D.H., Nishimura H., Cho C. Expression and relationship of male reproductive ADAMs in mouse. Biol. Reprod. 2006;74(4):744–750. doi: 10.1095/biolreprod.105.048892. [DOI] [PubMed] [Google Scholar]
- King N.E., Zimmermann N., Pope S.M., Fulkerson P.C., Nikolaidis N.M., Mishra A., Witte D.P., Rothenberg M.E. Expression and regulation of a disintegrin and metalloproteinase (ADAM) 8 in experimental asthma. Am. J. Respir. Cell Mol. Biol. 2004;31(3):257–265. doi: 10.1165/rcmb.2004-0026OC. [DOI] [PubMed] [Google Scholar]
- Kirkin V., Cahuzac N., Guardiola-Serrano F., Huault S., Luckerath K., Friedmann E., Novac N., Wels W.S., Martoglio B., Hueber A.O., Zornig M. The Fas ligand intracellular domain is released by ADAM10 and SPPL2a cleavage in T-cells. Cell Death Differ. 2007;14(9):1678–1687. doi: 10.1038/sj.cdd.4402175. [DOI] [PubMed] [Google Scholar]
- Kleino I., Ortiz R.M., Huovila A.P. ADAM15 gene structure and differential alternative exon use in human tissues. BMC Mol. Biol. 2007;8:90. doi: 10.1186/1471-2199-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H., Tomioka S., Sorimachi H., Saido T.C., Maruyama K., Okuyama A., Fujisawa-Sehara A., Ohno S., Suzuki K., Ishiura S. Membrane-anchored metalloprotease MDC9 has an alpha-secretase activity responsible for processing the amyloid precursor protein. Biochem. J. 1999;343(Pt 2):371–375. [PMC free article] [PubMed] [Google Scholar]
- Kopitz C., Gerg M., Bandapalli O.R., Ister D., Pennington C.J., Hauser S., Flechsig C., Krell H.W., Antolovic D., Brew K., Nagase H., Stangl M., von Weyhern C.W., Brucher B.L., Brand K., Coussens L.M., Edwards D.R., Kruger A. Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res. 2007;67(18):8615–8623. doi: 10.1158/0008-5472.CAN-07-0232. [DOI] [PubMed] [Google Scholar]
- Kuefer R., Day K.C., Kleer C.G., Sabel M.S., Hofer M.D., Varambally S., Zorn C.S., Chinnaiyan A.M., Rubin M.A., Day M.L. ADAM15 disintegrin is associated with aggressive prostate and breast cancer disease. Neoplasia. 2006;8(4):319–329. doi: 10.1593/neo.05682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisaki T., Masuda A., Sudo K., Sakagami J., Higashiyama S., Matsuda Y., Nagabukuro A., Tsuji A., Nabeshima Y., Asano M., Iwakura Y., Sehara-Fujisawa A. Phenotypic analysis of Meltrin alpha (ADAM12)-deficient mice: involvement of Meltrin alpha in adipogenesis and myogenesis. Mol. Cell Biol. 2003;23(1):55–61. doi: 10.1128/MCB.23.1.55-61.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisaki T., Wakatsuki S., Sehara-Fujisawa A. Meltrin beta mini, a new ADAM19 isoform lacking metalloprotease and disintegrin domains, induces morphological changes in neuronal cells. FEBS Lett. 2002;532(3):419–422. doi: 10.1016/s0014-5793(02)03732-8. [DOI] [PubMed] [Google Scholar]
- Kurohara K., Komatsu K., Kurisaki T., Masuda A., Irie N., Asano M., Sudo K., Nabeshima Y., Iwakura Y., Sehara-Fujisawa A. Essential roles of Meltrin beta (ADAM19) in heart development. Dev. Biol. 2004;267(1):14–28. doi: 10.1016/j.ydbio.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Kveiborg M., Frohlich C., Albrechtsen R., Tischler V., Dietrich N., Holck P., Kronqvist P., Rank F., Mercurio A.M., Wewer U.M. A role for ADAM12 in breast tumor progression and stromal cell apoptosis. Cancer Res. 2005;65(11):4754–4761. doi: 10.1158/0008-5472.CAN-05-0262. [DOI] [PubMed] [Google Scholar]
- Laigaard J., Sorensen T., Frohlich C., Pedersen B.N., Christiansen M., Schiott K., Uldbjerg N., Albrechtsen R., Clausen H.V., Ottesen B., Wewer U.M. ADAM12: a novel first-trimester maternal serum marker for Down syndrome. Prenat. Diagn. 2003;23(13):1086–1091. doi: 10.1002/pd.762. [DOI] [PubMed] [Google Scholar]
- Laigaard J., Sorensen T., Placing S., Holck P., Frohlich C., Wojdemann K.R., Sundberg K., Shalmi A.C., Tabor A., Norgaard-Pedersen B., Ottesen B., Christiansen M., Wewer U.M. Reduction of the disintegrin and metalloprotease ADAM12 in preeclampsia. Obstet. Gynecol. 2005;106(1):144–149. doi: 10.1097/01.AOG.0000165829.65319.65. [DOI] [PubMed] [Google Scholar]
- Lambert D.W., Clarke N.E., Hooper N.M., Turner A.J. Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett. 2008;582(2):385–390. doi: 10.1016/j.febslet.2007.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280(34):30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautrette A., Li S., Alili R., Sunnarborg S.W., Burtin M., Lee D.C., Friedlander G., Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat. Med. 2005;11(8):867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- Lee M.H., Dodds P., Verma V., Maskos K., Knauper V., Murphy G. Tailoring tissue inhibitor of metalloproteinases-3 to overcome the weakening effects of the cysteine-rich domains of tumour necrosis factor-alpha converting enzyme. Biochem. J. 2003;371(Pt 2):369–376. doi: 10.1042/BJ20021538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.H., Knauper V., Becherer J.D., Murphy G. Full-length and N-TIMP-3 display equal inhibitory activities toward TNF-alpha convertase. Biochem. Biophys. Res. Commun. 2001;280(3):945–950. doi: 10.1006/bbrc.2000.4192. [DOI] [PubMed] [Google Scholar]
- Lemieux G.A., Blumenkron F., Yeung N., Zhou P., Williams J., Grammer A.C., Petrovich R., Lipsky P.E., Moss M.L., Werb Z. The low affinity IgE receptor (CD23) is cleaved by the metalloproteinase ADAM10. J. Biol. Chem. 2007;282(20):14836–14844. doi: 10.1074/jbc.M608414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesueur F., Oudot T., Heath S., Foglio M., Lathrop M., Prud’homme J.F., Fischer J. ADAM33, a new candidate for psoriasis susceptibility. PLoS ONE. 2007;2(9):e906. doi: 10.1371/journal.pone.0000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Wang Y., Forbes K., Vignali K.M., Heale B.S., Saftig P., Hartmann D., Black R.A., Rossi J.J., Blobel C.P., Dempsey P.J., Workman C.J., Vignali D.A. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007;26(2):494–504. doi: 10.1038/sj.emboj.7601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.C., Liu X., Li Y., Covington M., Wynn R., Huber R., Hillman M., Yang G., Ellis D., Marando C., Katiyar K., Bradley J., Abremski K., Stow M., Rupar M., Zhuo J., Li Y.L., Lin Q., Burns D., Xu M., Zhang C., Qian D.Q., He C., Sharief V., Weng L., Agrios C., Shi E., Metcalf B., Newton R., Friedman S., Yao W., Scherle P., Hollis G., Burn T.C. Identification of ADAM10 as a major source of HER2 ectodomain sheddase activity in HER2 overexpressing breast cancer cells. Cancer Biol. Ther. 2006;5(6):657–664. doi: 10.4161/cbt.5.6.2708. [DOI] [PubMed] [Google Scholar]
- Loechel F., Fox J.W., Murphy G., Albrechtsen R., Wewer U.M. ADAM 12-S cleaves IGFBP-3 and IGFBP-5 and is inhibited by TIMP-3. Biochem. Biophys. Res. Commun. 2000;278(3):511–515. doi: 10.1006/bbrc.2000.3835. [DOI] [PubMed] [Google Scholar]
- Loechel F., Overgaard M.T., Oxvig C., Albrechtsen R., Wewer U.M. Regulation of human ADAM 12 protease by the prodomain. Evidence for a functional cysteine switch. J. Biol. Chem. 1999;274(19):13427–13433. doi: 10.1074/jbc.274.19.13427. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Hundhausen C., Lambert M.H., Broadway N., Andrews R.C., Bickett D.M., Leesnitzer M.A., Becherer J.D. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb. Chem. High Throughput Screen. 2005;8(2):161–171. doi: 10.2174/1386207053258488. [DOI] [PubMed] [Google Scholar]
- Lum L., Reid M.S., Blobel C.P. Intracellular maturation of the mouse metalloprotease disintegrin MDC15. J. Biol. Chem. 1998;273(40):26236–26247. doi: 10.1074/jbc.273.40.26236. [DOI] [PubMed] [Google Scholar]
- Lum L., Wong B.R., Josien R., Becherer J.D., Erdjument-Bromage H., Schlondorff J., Tempst P., Choi Y., Blobel C.P. Evidence for a role of a tumor necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J. Biol. Chem. 1999;274(19):13613–13618. doi: 10.1074/jbc.274.19.13613. [DOI] [PubMed] [Google Scholar]
- Maatta J.A., Sundvall M., Junttila T.T., Peri L., Laine V.J., Isola J., Egeblad M., Elenius K. Proteolytic cleavage and phosphorylation of a tumor-associated ErbB4 isoform promote ligand-independent survival and cancer cell growth. Mol. Biol. Cell. 2006;17(1):67–79. doi: 10.1091/mbc.E05-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia F., Shapiro L. ADAM and Eph: how Ephrin-signaling cells become detached. Cell. 2005;123(2):185–187. doi: 10.1016/j.cell.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Marambaud P., Wen P.H., Dutt A., Shioi J., Takashima A., Siman R., Robakis N.K. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114(5):635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Marcello E., Gardoni F., Mauceri D., Romorini S., Jeromin A., Epis R., Borroni B., Cattabeni F., Sala C., Padovani A., Di Luca M. Synapse-associated protein-97 mediates alpha-secretase ADAM10 trafficking and promotes its activity. J. Neurosci. 2007;27(7):1682–1691. doi: 10.1523/JNEUROSCI.3439-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzky T., Reiss K., Ludwig A., Buchholz J., Scholz F., Proksch E., de Strooper B., Hartmann D., Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc. Natl. Acad. Sci. USA. 2005;102(26):9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzky T., Schulte M., Ludwig A., Rose-John S., Blobel C., Hartmann D., Altevogt P., Saftig P., Reiss K. L1 is sequentially processed by two differently activated metalloproteases and presenilin/gamma-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol. Cell Biol. 2005;25(20):9040–9053. doi: 10.1128/MCB.25.20.9040-9053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki M., Kurisaki T., Shirakawa K., Sehara-Fujisawa A. Role of meltrin {alpha} (ADAM12) in obesity induced by high-fat diet. Endocrinology. 2005;146(4):1752–1763. doi: 10.1210/en.2004-1082. [DOI] [PubMed] [Google Scholar]
- Maskos K., Fernandez-Catalan C., Huber R., Bourenkov G.P., Bartunik H., Ellestad G.A., Reddy P., Wolfson M.F., Rauch C.T., Castner B.J., Davis R., Clarke H.R., Petersen M., Fitzner J.N., Cerretti D.P., March C.J., Paxton R.J., Black R.A., Bode W. Crystal structure of the catalytic domain of human tumor necrosis factor-alpha-converting enzyme. Proc. Natl. Acad. Sci. USA. 1998;95(7):3408–3412. doi: 10.1073/pnas.95.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer G., Hamelin J., Asselin M.C., Pasquato A., Marcinkiewicz E., Tang M., Tabibzadeh S., Seidah N.G. The regulated cell surface zymogen activation of the proprotein convertase PC5A directs the processing of its secretory substrates. J. Biol. Chem. 2008;283(4):2373–2384. doi: 10.1074/jbc.M708763200. [DOI] [PubMed] [Google Scholar]
- Mazzocca A., Coppari R., De Franco R., Cho J.Y., Libermann T.A., Pinzani M., Toker A. A secreted form of ADAM9 promotes carcinoma invasion through tumor-stromal interactions. Cancer Res. 2005;65(11):4728–4738. doi: 10.1158/0008-5472.CAN-04-4449. [DOI] [PubMed] [Google Scholar]
- McCulloch D.R., Harvey M., Herington A.C. The expression of the ADAMs proteases in prostate cancer cell lines and their regulation by dihydrotestosterone. Mol. Cell Endocrinol. 2000;167(1-2):11–21. doi: 10.1016/s0303-7207(00)00305-1. [DOI] [PubMed] [Google Scholar]
- McDermott M.F., Aksentijevich I., Galon J., McDermott E.M., Ogunkolade B.W., Centola M., Mansfield E., Gadina M., Karenko L., Pettersson T., McCarthy J., Frucht D.M., Aringer M., Torosyan Y., Teppo A.M., Wilson M., Karaarslan H.M., Wan Y., Todd I., Wood G., Schlimgen R., Kumarajeewa T.R., Cooper S.M., Vella J.P., Amos C.I., Mulley J., Quane K.A., Molloy M.G., Ranki A., Powell R.J., Hitman G.A., O’Shea J.J., Kastner D.L. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97(1):133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- McGowan P.M., McKiernan E., Bolster F., Ryan B.M., Hill A.D., McDermott E.W., Evoy D., O’Higgins N., Crown J., Duffy M.J. ADAM-17 predicts adverse outcome in patients with breast cancer. Ann. Oncol. 2008;19(6):1075–1081. doi: 10.1093/annonc/mdm609. [DOI] [PubMed] [Google Scholar]
- McGowan P.M., Ryan B.M., Hill A.D., McDermott E., O’Higgins N., Duffy M.J. ADAM-17 expression in breast cancer correlates with variables of tumor progression. Clin. Cancer Res. 2007;13(8):2335–2343. doi: 10.1158/1078-0432.CCR-06-2092. [DOI] [PubMed] [Google Scholar]
- Mechtersheimer S., Gutwein P., Agmon-Levin N., Stoeck A., Oleszewski M., Riedle S., Postina R., Fahrenholz F., Fogel M., Lemmon V., Altevogt P. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J. Cell Biol. 2001;155(4):661–673. doi: 10.1083/jcb.200101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J.F., McFall C., Rosenwasser L.J. Polymorphism R62W results in resistance of CD23 to enzymatic cleavage in cultured cells. Genes Immun. 2007;8(3):215–223. doi: 10.1038/sj.gene.6364376. [DOI] [PubMed] [Google Scholar]
- Mifune M., Ohtsu H., Suzuki H., Nakashima H., Brailoiu E., Dun N.J., Frank G.D., Inagami T., Higashiyama S., Thomas W.G., Eckhart A.D., Dempsey P.J., Eguchi S. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J. Biol. Chem. 2005;280(28):26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- Milla M.E., Gonzales P.E., Leonard J.D. The TACE zymogen: re-examining the role of the cysteine switch. Cell Biochem. Biophys. 2006;44(3):342–348. doi: 10.1385/CBB:44:3:342. [DOI] [PubMed] [Google Scholar]
- Mishra-Gorur K., Rand M.D., Perez-Villamil B., Artavanis-Tsakonas S. Down-regulation of Delta by proteolytic processing. J. Cell Biol. 2002;159(2):313–324. doi: 10.1083/jcb.200203117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui Y., Mochizuki S., Kodama T., Shimoda M., Ohtsuka T., Shiomi T., Chijiiwa M., Ikeda T., Kitajima M., Okada Y. ADAM28 is overexpressed in human breast carcinomas: implications for carcinoma cell proliferation through cleavage of insulin-like growth factor binding protein-3. Cancer Res. 2006;66(20):9913–9920. doi: 10.1158/0008-5472.CAN-06-0377. [DOI] [PubMed] [Google Scholar]
- Mochizuki S., Okada Y. ADAMs in cancer cell proliferation and progression. Cancer Sci. 2007;98(5):621–628. doi: 10.1111/j.1349-7006.2007.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki S., Shimoda M., Shiomi T., Fujii Y., Okada Y. ADAM28 is activated by MMP-7 (matrilysin-1) and cleaves insulin-like growth factor binding protein-3. Biochem. Biophys. Res. Commun. 2004;315(1):79–84. doi: 10.1016/j.bbrc.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Moghadaszadeh B., Albrechtsen R., Guo L.T., Zaik M., Kawaguchi N., Borup R.H., Kronqvist P., Schroder H.D., Davies K.E., Voit T., Nielsen F.C., Engvall E., Wewer U.M. Compensation for dystrophin-deficiency: ADAM12 overexpression in skeletal muscle results in increased alpha 7 integrin, utrophin and associated glycoproteins. Hum. Mol. Genet. 2003;12(19):2467–2479. doi: 10.1093/hmg/ddg264. [DOI] [PubMed] [Google Scholar]
- Mohan S., Thompson G.R., Amaar Y.G., Hathaway G., Tschesche H., Baylink D.J. ADAM-9 is an insulin-like growth factor binding protein-5 protease produced and secreted by human osteoblasts. Biochemistry. 2002;41(51):15394–15403. doi: 10.1021/bi026458q. [DOI] [PubMed] [Google Scholar]
- Mori S., Tanaka M., Nanba D., Nishiwaki E., Ishiguro H., Higashiyama S., Matsuura N. PACSIN3 binds ADAM12/meltrin alpha and up-regulates ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. J. Biol. Chem. 2003;278(46):46029–46034. doi: 10.1074/jbc.M306393200. [DOI] [PubMed] [Google Scholar]
- Moss M.L., Bartsch J.W. Therapeutic benefits from targeting of ADAM family members. Biochemistry. 2004;43(23):7227–7235. doi: 10.1021/bi049677f. [DOI] [PubMed] [Google Scholar]
- Moss M.L., Bomar M., Liu Q., Sage H., Dempsey P., Lenhart P.M., Gillispie P.A., Stoeck A., Wildeboer D., Bartsch J.W., Palmisano R., Zhou P. The ADAM10 prodomain is a specific inhibitor of ADAM10 proteolytic activity and inhibits cellular shedding events. J. Biol. Chem. 2007;282(49):35712–35721. doi: 10.1074/jbc.M703231200. [DOI] [PubMed] [Google Scholar]
- Moss M.L., Jin S.L., Milla M.E., Bickett D.M., Burkhart W., Carter H.L., Chen W.J., Clay W.C., Didsbury J.R., Hassler D., Hoffman C.R., Kost T.A., Lambert M.H., Leesnitzer M.A., McCauley P., McGeehan G., Mitchell J., Moyer M., Pahel G., Rocque W., Overton L.K., Schoenen F., Seaton T., Su J.L., Becherer J.D. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385(6618):733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- Moss M.L., Rasmussen F.H. Fluorescent substrates for the proteinases ADAM17, ADAM10, ADAM8, and ADAM12 useful for high-throughput inhibitor screening. Anal. Biochem. 2007;366(2):144–148. doi: 10.1016/j.ab.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Moss M.L., Sklair-Tavron L., Nudelman R. Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2008;4(6):300–309. doi: 10.1038/ncprheum0797. [DOI] [PubMed] [Google Scholar]
- Moss M.L., Stoeck A., Yan W., Dempsey P.J. ADAM10 as a target for anti-cancer therapy. Curr. Pharm. Biotechnol. 2008;9(1):2–8. doi: 10.2174/138920108783497613. [DOI] [PubMed] [Google Scholar]
- Muraguchi T., Takegami Y., Ohtsuka T., Kitajima S., Chandana E.P., Omura A., Miki T., Takahashi R., Matsumoto N., Ludwig A., Noda M., Takahashi C. RECK modulates Notch signaling during cortical neurogenesis by regulating ADAM10 activity. Nat. Neurosci. 2007;10(7):838–845. doi: 10.1038/nn1922. [DOI] [PubMed] [Google Scholar]
- Murase S., Cho C., White J.M., Horwitz A.F. ADAM2 promotes migration of neuroblasts in the rostral migratory stream to the olfactory bulb. Eur. J. Neurosci. 2008;27(7):1585–1595. doi: 10.1111/j.1460-9568.2008.06119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Murthy A., Khokha R. Clipping, shedding and RIPping keep immunity on cue. Trends Immunol. 2008;29(2):75–82. doi: 10.1016/j.it.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Nagano O., Murakami D., Hartmann D., De Strooper B., Saftig P., Iwatsubo T., Nakajima M., Shinohara M., Saya H. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J. Cell Biol. 2004;165(6):893–902. doi: 10.1083/jcb.200310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najy A.J., Day K.C., Day M.L. The Ectodomain Shedding of E-cadherin by ADAM15 Supports ErbB Receptor Activation. J. Biol. Chem. 2008;283(26):18393–18401. doi: 10.1074/jbc.M801329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naus S., Richter M., Wildeboer D., Moss M., Schachner M., Bartsch J.W. Ectodomain shedding of the neural recognition molecule CHL1 by the metalloprotease-disintegrin ADAM8 promotes neurite outgrowth and suppresses neuronal cell death. J. Biol. Chem. 2004;279(16):16083–16090. doi: 10.1074/jbc.M400560200. [DOI] [PubMed] [Google Scholar]
- Nelson K.K., Schlondorff J., Blobel C.P. Evidence for an interaction of the metalloprotease-disintegrin tumour necrosis factor alpha convertase (TACE) with mitotic arrest deficient 2 (MAD2), and of the metalloprotease-disintegrin MDC9 with a novel MAD2-related protein, MAD2beta. Biochem. J. 1999;343(Pt 3):673–680. [PMC free article] [PubMed] [Google Scholar]
- Niewiarowski S., McLane M.A., Kloczewiak M., Stewart G.J. Disintegrins and other naturally occurring antagonists of platelet fibrinogen receptors. Semin. Hematol. 1994;31(4):289–300. [PubMed] [Google Scholar]
- Nishimura H., Cho C., Branciforte D.R., Myles D.G., Primakoff P. Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin beta. Dev. Biol. 2001;233(1):204–213. doi: 10.1006/dbio.2001.0166. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Kim E., Nakanishi T., Baba T. Possible function of the ADAM1a/ADAM2 Fertilin complex in the appearance of ADAM3 on the sperm surface. J. Biol. Chem. 2004;279(33):34957–34962. doi: 10.1074/jbc.M314249200. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Myles D.G., Primakoff P. Identification of an ADAM2-ADAM3 complex on the surface of mouse testicular germ cells and cauda epididymal sperm. J. Biol. Chem. 2007;282(24):17900–17907. doi: 10.1074/jbc.M702268200. [DOI] [PubMed] [Google Scholar]
- Niu X., Umland S., Ingram R., Beyer B.M., Liu Y.H., Sun J., Lundell D., Orth P. IK682, a tight binding inhibitor of TACE. Arch. Biochem. Biophys. 2006;451(1):43–50. doi: 10.1016/j.abb.2006.03.034. [DOI] [PubMed] [Google Scholar]
- O’Shea C., McKie N., Buggy Y., Duggan C., Hill A.D., McDermott E., O’Higgins N., Duffy M.J. Expression of ADAM-9 mRNA and protein in human breast cancer. Int. J. Cancer. 2003;105(6):754–761. doi: 10.1002/ijc.11161. [DOI] [PubMed] [Google Scholar]
- Ohtsu H., Dempsey P.J., Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am. J. Physiol. Cell Physiol. 2006;291(1):C1–10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- Okada A., Mochizuki S., Yatabe T., Kimura T., Shiomi T., Fujita Y., Matsumoto H., Sehara-Fujisawa A., Iwamoto Y., Okada Y. ADAM-12 (meltrin alpha) is involved in chondrocyte proliferation via cleavage of insulin-like growth factor binding protein 5 in osteoarthritic cartilage. Arthritis Rheum. 2008;58(3):778–789. doi: 10.1002/art.23262. [DOI] [PubMed] [Google Scholar]
- Orth P., Reichert P., Wang W., Prosise W.W., Yarosh-Tomaine T., Hammond G., Ingram R.N., Xiao L., Mirza U.A., Zou J., Strickland C., Taremi S.S., Le H.V., Madison V. Crystal structure of the catalytic domain of human ADAM33. J. Mol. Biol. 2004;335(1):129–137. doi: 10.1016/j.jmb.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Ortiz R.M., Karkkainen I., Huovila A.P. Aberrant alternative exon use and increased copy number of human metalloprotease-disintegrin ADAM15 gene in breast cancer cells. Genes Chromosomes Cancer. 2004;41(4):366–378. doi: 10.1002/gcc.20102. [DOI] [PubMed] [Google Scholar]
- Pan D., Rubin G.M. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90(2):271–280. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- Papaspyridonos M., Smith A., Burnand K.G., Taylor P., Padayachee S., Suckling K.E., James C.H., Greaves D.R., Patel L. Novel candidate genes in unstable areas of human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2006;26(8):1837–1844. doi: 10.1161/01.ATV.0000229695.68416.76. [DOI] [PubMed] [Google Scholar]
- Pasquale E.B. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133(1):38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Peduto L., Reuter V.E., Shaffer D.R., Scher H.I., Blobel C.P. Critical function for ADAM9 in mouse prostate cancer. Cancer Res. 2005;65(20):9312–9319. doi: 10.1158/0008-5472.CAN-05-1063. [DOI] [PubMed] [Google Scholar]
- Peiretti F., Deprez-Beauclair P., Bonardo B., Aubert H., Juhan-Vague I., Nalbone G. Identification of SAP97 as an intracellular binding partner of TACE. J. Cell Sci. 2003;116(Pt 10):1949–1957. doi: 10.1242/jcs.00415. [DOI] [PubMed] [Google Scholar]
- Peschon J.J., Slack J.L., Reddy P., Stocking K.L., Sunnarborg S.W., Lee D.C., Russell W.E., Castner B.J., Johnson R.S., Fitzner J.N., Boyce R.W., Nelson N., Kozlosky C.J., Wolfson M.F., Rauch C.T., Cerretti D.P., Paxton R.J., March C.J., Black R.A. An essential role for ectodomain shedding in mammalian development. Science. 1998;282(5392):1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Plumb J., McQuaid S., Cross A.K., Surr J., Haddock G., Bunning R.A., Woodroofe M.N. Upregulation of ADAM-17 expression in active lesions in multiple sclerosis. Mult. Scler. 2006;12(4):375–385. doi: 10.1191/135248506ms1276oa. [DOI] [PubMed] [Google Scholar]
- Poghosyan Z., Robbins S.M., Houslay M.D., Webster A., Murphy G., Edwards D.R. Phosphorylation-dependent interactions between ADAM15 cytoplasmic domain and Src family protein-tyrosine kinases. J. Biol. Chem. 2002;277(7):4999–5007. doi: 10.1074/jbc.M107430200. [DOI] [PubMed] [Google Scholar]
- Ponnuchamy B., Khalil R.A. Role of ADAMs in endothelial cell permeability: cadherin shedding and leukocyte rolling. Circ. Res. 2008;102(10):1139–1142. doi: 10.1161/CIRCRESAHA.108.177394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S., Clark I.M., Kevorkian L., Edwards D.R. The ADAMTS metalloproteinases. Biochem. J. 2005;386(Pt 1):15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postina R., Schroeder A., Dewachter I., Bohl J., Schmitt U., Kojro E., Prinzen C., Endres K., Hiemke C., Blessing M., Flamez P., Dequenne A., Godaux E., van Leuven F., Fahrenholz F. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J. Clin. Invest. 2004;113(10):1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell R.M., Wicks J., Holloway J.W., Holgate S.T., Davies D.E. The splicing and fate of ADAM33 transcripts in primary human airways fibroblasts. Am. J. Respir. Cell Mol. Biol. 2004;31(1):13–21. doi: 10.1165/rcmb.2003-0330OC. [DOI] [PubMed] [Google Scholar]
- Prenzel N., Zwick E., Daub H., Leserer M., Abraham R., Wallasch C., Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Myles D.G. The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet. 2000;16(2):83–87. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Myles D.G. Penetration, adhesion, and fusion in mammalian sperm–egg interaction. Science. 2002;296(5576):2183–2185. doi: 10.1126/science.1072029. [DOI] [PubMed] [Google Scholar]
- Puente X.S., Lopez-Otin C. A genomic analysis of rat proteases and protease inhibitors. Genome Res. 2004;14(4):609–622. doi: 10.1101/gr.1946304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente X.S., Sanchez L.M., Overall C.M., Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nat. Rev. Genet. 2003;4(7):544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- Qi H., Rand M.D., Wu X., Sestan N., Wang W., Rakic P., Xu T., Artavanis-Tsakonas S. Processing of the notch ligand delta by the metalloprotease Kuzbanian. Science. 1999;283(5398):91–94. doi: 10.1126/science.283.5398.91. [DOI] [PubMed] [Google Scholar]
- Rapti M., Atkinson S.J., Lee M.H., Trim A., Moss M., Murphy G. The isolated N-terminal domains of TIMP-1 and TIMP-3 are insufficient for ADAM10 inhibition. Biochem. J. 2008;411(2):433–439. doi: 10.1042/BJ20071430. [DOI] [PubMed] [Google Scholar]
- Rawlings N.D., Morton F.R., Kok C.Y., Kong J., Barrett A.J. MEROPS: the peptidase database. Nucleic Acids Res. 2008;36(Database issue):D320–325. doi: 10.1093/nar/gkm954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P., Slack J.L., Davis R., Cerretti D.P., Kozlosky C.J., Blanton R.A., Shows D., Peschon J.J., Black R.A. Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J. Biol. Chem. 2000;275(19):14608–14614. doi: 10.1074/jbc.275.19.14608. [DOI] [PubMed] [Google Scholar]
- Reiss K., Maretzky T., Haas I.G., Schulte M., Ludwig A., Frank M., Saftig P. Regulated ADAM10-dependent ectodomain shedding of gamma-protocadherin C3 modulates cell-cell adhesion. J. Biol. Chem. 2006;281(31):21735–21744. doi: 10.1074/jbc.M602663200. [DOI] [PubMed] [Google Scholar]
- Reiss K., Maretzky T., Ludwig A., Tousseyn T., de Strooper B., Hartmann D., Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell–cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005;24(4):742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio C., Buxbaum J.D., Peschon J.J., Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. J. Biol. Chem. 2000;275(14):10379–10387. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- Roberts C.M., Tani P.H., Bridges L.C., Laszik Z., Bowditch R.D. MDC-L, a novel metalloprotease disintegrin cysteine-rich protein family member expressed by human lymphocytes. J. Biol. Chem. 1999;274(41):29251–29259. doi: 10.1074/jbc.274.41.29251. [DOI] [PubMed] [Google Scholar]
- Rocks N., Paulissen G., El Hour M., Quesada F., Crahay C., Gueders M., Foidart J.M., Noel A., Cataldo D. Emerging roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie. 2008;90(2):369–379. doi: 10.1016/j.biochi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Roghani M., Becherer J.D., Moss M.L., Atherton R.E., Erdjument-Bromage H., Arribas J., Blackburn R.K., Weskamp G., Tempst P., Blobel C.P. Metalloprotease-disintegrin MDC9: intracellular maturation and catalytic activity. J. Biol. Chem. 1999;274(6):3531–3540. doi: 10.1074/jbc.274.6.3531. [DOI] [PubMed] [Google Scholar]
- Rooke J., Pan D., Xu T., Rubin G.M. KUZ, a conserved metalloprotease-disintegrin protein with two roles in Drosophila neurogenesis. Science. 1996;273(5279):1227–1231. doi: 10.1126/science.273.5279.1227. [DOI] [PubMed] [Google Scholar]
- Roy R., Wewer U.M., Zurakowski D., Pories S.E., Moses M.A. ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J. Biol. Chem. 2004;279(49):51323–51330. doi: 10.1074/jbc.M409565200. [DOI] [PubMed] [Google Scholar]
- Ruhe J.E., Streit S., Hart S., Ullrich A. EGFR signaling leads to downregulation of PTP-LAR via TACE-mediated proteolytic processing. Cell Signal. 2006;18(9):1515–1527. doi: 10.1016/j.cellsig.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Saftig P., Hartmann D. ADAM10 A major membrane protein ectodomain sheddase involved in regulated intramembrane proteolysis. In: Hooper N.M., Lendeckel U., editors. The Adam Family of Proteases. Springer; 2005. pp. 85–121. [Google Scholar]
- Sagane K., Hayakawa K., Kai J., Hirohashi T., Takahashi E., Miyamoto N., Ino M., Oki T., Yamazaki K., Nagasu T. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 2005;6(1):33. doi: 10.1186/1471-2202-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U., Blobel C.P. Ectodomain shedding of the EGF-receptor ligand epigen is mediated by ADAM17. FEBS Lett. 2007;581(1):41–44. doi: 10.1016/j.febslet.2006.11.074. [DOI] [PubMed] [Google Scholar]
- Sahin U., Weskamp G., Kelly K., Zhou H.M., Higashiyama S., Peschon J., Hartmann D., Saftig P., Blobel C.P. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 2004;164(5):769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Josefat B., Esselens C., Bech-Serra J.J., Arribas J. Post-transcriptional up-regulation of ADAM17 upon epidermal growth factor receptor activation and in breast tumors. J. Biol. Chem. 2007;282(11):8325–8331. doi: 10.1074/jbc.M608826200. [DOI] [PubMed] [Google Scholar]
- Schafer B., Gschwind A., Ullrich A. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene. 2004;23(4):991–999. doi: 10.1038/sj.onc.1207278. [DOI] [PubMed] [Google Scholar]
- Schafer B., Marg B., Gschwind A., Ullrich A. Distinct ADAM metalloproteinases regulate G protein-coupled receptor-induced cell proliferation and survival. J. Biol. Chem. 2004;279(46):47929–47938. doi: 10.1074/jbc.M400129200. [DOI] [PubMed] [Google Scholar]
- Schantl J.A., Roza M., Van Kerkhof P., Strous G.J. The growth hormone receptor interacts with its sheddase, the tumour necrosis factor-alpha-converting enzyme (TACE) Biochem. J. 2004;377(Pt 2):379–384. doi: 10.1042/BJ20031321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlomann U., Wildeboer D., Webster A., Antropova O., Zeuschner D., Knight C.G., Docherty A.J., Lambert M., Skelton L., Jockusch H., Bartsch J.W. The metalloprotease disintegrin ADAM8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. J. Biol. Chem. 2002;277(50):48210–48219. doi: 10.1074/jbc.M203355200. [DOI] [PubMed] [Google Scholar]
- Schlondorff J., Blobel C.P. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J. Cell Sci. 1999;112(Pt 21):3603–3617. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- Scholz F., Schulte A., Adamski F., Hundhausen C., Mittag J., Schwarz A., Kruse M.L., Proksch E., Ludwig A. Constitutive expression and regulated release of the transmembrane chemokine CXCL16 in human and murine skin. J. Invest. Dermatol. 2007;127(6):1444–1455. doi: 10.1038/sj.jid.5700751. [DOI] [PubMed] [Google Scholar]
- Schulte A., Schulz B., Andrzejewski M.G., Hundhausen C., Mletzko S., Achilles J., Reiss K., Paliga K., Weber C., John S.R., Ludwig A. Sequential processing of the transmembrane chemokines CX3CL1 and CXCL16 by alpha- and gamma-secretases. Biochem. Biophys. Res. Commun. 2007;358(1):233–240. doi: 10.1016/j.bbrc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- Schulte M., Reiss K., Lettau M., Maretzky T., Ludwig A., Hartmann D., de Strooper B., Janssen O., Saftig P. ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death. Cell Death Differ. 2007;14(5):1040–1049. doi: 10.1038/sj.cdd.4402101. [DOI] [PubMed] [Google Scholar]
- Schulz B., Pruessmeyer J., Maretzky T., Ludwig A., Blobel C.P., Saftig P., Reiss K. ADAM10 regulates endothelial permeability and T-Cell transmigration by proteolysis of vascular endothelial cadherin. Circ. Res. 2008;102(10):1192–1201. doi: 10.1161/CIRCRESAHA.107.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz A., Hartig W., Wobus M., Grosche J., Wittekind C., Aust G. Expression of ADAM15 in lung carcinomas. Virchows Arch. 2005;446(4):421–429. doi: 10.1007/s00428-004-1193-z. [DOI] [PubMed] [Google Scholar]
- Seals D.F., Courtneidge S.A. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17(1):7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- Selkoe D.J. Presenilin, Notch, and the genesis and treatment of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2001;98(20):11039–11041. doi: 10.1073/pnas.211352598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Chen H., Sun J., Buckley S., Zhao J., Anderson K.D., Williams R.G., Warburton D. TACE is required for fetal murine cardiac development and modeling. Dev. Biol. 2003;261(2):371–380. doi: 10.1016/s0012-1606(03)00315-4. [DOI] [PubMed] [Google Scholar]
- Shintani Y., Higashiyama S., Ohta M., Hirabayashi H., Yamamoto S., Yoshimasu T., Matsuda H., Matsuura N. Overexpression of ADAM9 in non-small cell lung cancer correlates with brain metastasis. Cancer Res. 2004;64(12):4190–4196. doi: 10.1158/0008-5472.CAN-03-3235. [DOI] [PubMed] [Google Scholar]
- Sieuwerts A.M., Meijer-van Gelder M.E., Timmermans M., Trapman A.M., Garcia R.R., Arnold M., Goedheer A.J., Portengen H., Klijn J.G., Foekens J.A. How ADAM-9 and ADAM-11 differentially from estrogen receptor predict response to tamoxifen treatment in patients with recurrent breast cancer: a retrospective study. Clin. Cancer Res. 2005;11(20):7311–7321. doi: 10.1158/1078-0432.CCR-05-0560. [DOI] [PubMed] [Google Scholar]
- Singh R.J., Mason J.C., Lidington E.A., Edwards D.R., Nuttall R.K., Khokha R., Knauper V., Murphy G., Gavrilovic J. Cytokine stimulated vascular cell adhesion molecule-1 (VCAM-1) ectodomain release is regulated by TIMP-3. Cardiovasc. Res. 2005;67(1):39–49. doi: 10.1016/j.cardiores.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Six E., Ndiaye D., Laabi Y., Brou C., Gupta-Rossi N., Israel A., Logeat F. The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase. Proc. Natl. Acad. Sci. USA. 2003;100(13):7638–7643. doi: 10.1073/pnas.1230693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoblom T., Jones S., Wood L.D., Parsons D.W., Lin J., Barber T.D., Mandelker D., Leary R.J., Ptak J., Silliman N., Szabo S., Buckhaults P., Farrell C., Meeh P., Markowitz S.D., Willis J., Dawson D., Willson J.K., Gazdar A.F., Hartigan J., Wu L., Liu C., Parmigiani G., Park B.H., Bachman K.E., Papadopoulos N., Vogelstein B., Kinzler K.W., Velculescu V.E. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314(5797):268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- Slack B.E., Ma L.K., Seah C.C. Constitutive shedding of the amyloid precursor protein ectodomain is up-regulated by tumour necrosis factor-alpha converting enzyme. Biochem. J. 2001;357(Pt 3):787–794. doi: 10.1042/0264-6021:3570787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.M., Gaultier A., Cousin H., Alfandari D., White J.M., DeSimone D.W. The cysteine-rich domain regulates ADAM protease function in vivo. J. Cell Biol. 2002;159(5):893–902. doi: 10.1083/jcb.200206023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S.H. Neuroscience. Adam finds an exciting mate. Science. 2006;313(5794):1744–1745. doi: 10.1126/science.1133501. [DOI] [PubMed] [Google Scholar]
- Solomon A., Akabayov B., Frenkel A., Milla M.E., Sagi I. Key feature of the catalytic cycle of TNF-alpha converting enzyme involves communication between distal protein sites and the enzyme catalytic core. Proc. Natl. Acad. Sci. USA. 2007;104(12):4931–4936. doi: 10.1073/pnas.0700066104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soond S.M., Everson B., Riches D.W., Murphy G. ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. J. Cell Sci. 2005;118(Pt 11):2371–2380. doi: 10.1242/jcs.02357. [DOI] [PubMed] [Google Scholar]
- Spasic D., Annaert W. Building gamma-secretase: the bits and pieces. J. Cell Sci. 2008;121(Pt 4):413–420. doi: 10.1242/jcs.015255. [DOI] [PubMed] [Google Scholar]
- Srinivasan B., Wang Z., Brun-Zinkernagel A.M., Collier R.J., Black R.A., Frank S.J., Barker P.A., Roque R.S. Photic injury promotes cleavage of p75NTR by TACE and nuclear trafficking of the p75 intracellular domain. Mol. Cell Neurosci. 2007;36(4):449–461. doi: 10.1016/j.mcn.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Stein K.K., Go J.C., Primakoff P., Myles D.G. Defects in secretory pathway trafficking during sperm development in Adam2 knockout mice. Biol. Reprod. 2005;73(5):1032–1038. doi: 10.1095/biolreprod.105.040972. [DOI] [PubMed] [Google Scholar]
- Sundberg C., Thodeti C.K., Kveiborg M., Larsson C., Parker P., Albrechtsen R., Wewer U.M. Regulation of ADAM12 cell-surface expression by protein kinase C epsilon. J. Biol. Chem. 2004;279(49):51601–51611. doi: 10.1074/jbc.M403753200. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Kadota N., Hara T., Nakagami Y., Izumi T., Takenawa T., Sabe H., Endo T. Meltrin alpha cytoplasmic domain interacts with SH3 domains of Src and Grb2 and is phosphorylated by v-Src. Oncogene. 2000;19(51):5842–5850. doi: 10.1038/sj.onc.1203986. [DOI] [PubMed] [Google Scholar]
- Takahashi E., Sagane K., Nagasu T., Kuromitsu J. Altered nociceptive response in ADAM11-deficient mice. Brain Res. 2006;1097(1):39–42. doi: 10.1016/j.brainres.2006.04.043. [DOI] [PubMed] [Google Scholar]
- Takahashi E., Sagane K., Oki T., Yamazaki K., Nagasu T., Kuromitsu J. Deficits in spatial learning and motor coordination in ADAM11-deficient mice. BMC Neurosci. 2006;7:19. doi: 10.1186/1471-2202-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Igarashi T., Mori H., Araki S. Crystal structures of VAP1 reveal ADAMs’ MDC domain architecture and its unique C-shaped scaffold. EMBO J. 2006;25(11):2388–2396. doi: 10.1038/sj.emboj.7601131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Nanba D., Mori S., Shiba F., Ishiguro H., Yoshino K., Matsuura N., Higashiyama S. ADAM binding protein Eve-1 is required for ectodomain shedding of epidermal growth factor receptor ligands. J. Biol. Chem. 2004;279(40):41950–41959. doi: 10.1074/jbc.M400086200. [DOI] [PubMed] [Google Scholar]
- Tellier E., Canault M., Poggi M., Bonardo B., Nicolay A., Alessi M.C., Nalbone G., Peiretti F. HDLs activate ADAM17-dependent shedding. J. Cell Physiol. 2008;214(3):687–693. doi: 10.1002/jcp.21265. [DOI] [PubMed] [Google Scholar]
- Tellier E., Canault M., Rebsomen L., Bonardo B., Juhan-Vague I., Nalbone G., Peiretti F. The shedding activity of ADAM17 is sequestered in lipid rafts. Exp. Cell Res. 2006;312(20):3969–3980. doi: 10.1016/j.yexcr.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Thodeti C.K., Albrechtsen R., Grauslund M., Asmar M., Larsson C., Takada Y., Mercurio A.M., Couchman J.R., Wewer U.M. ADAM12/syndecan-4 signaling promotes beta 1 integrin-dependent cell spreading through protein kinase Calpha and RhoA. J. Biol. Chem. 2003;278(11):9576–9584. doi: 10.1074/jbc.M208937200. [DOI] [PubMed] [Google Scholar]
- Tomczuk M., Takahashi Y., Huang J., Murase S., Mistretta M., Klaffky E., Sutherland A., Bolling L., Coonrod S., Marcinkiewicz C., Sheppard D., Stepp M.A., White J.M. Role of multiple beta1 integrins in cell adhesion to the disintegrin domains of ADAMs 2 and 3. Exp. Cell Res. 2003;290(1):68–81. doi: 10.1016/s0014-4827(03)00307-0. [DOI] [PubMed] [Google Scholar]
- Tousseyn T., Jorissen E., Reiss K., Hartmann D. (Make) stick and cut loose—disintegrin metalloproteases in development and disease. Birth Defects Res. C Embryo Today. 2006;78(1):24–46. doi: 10.1002/bdrc.20066. [DOI] [PubMed] [Google Scholar]
- Tran T., Hoffmann S., Wiesehan K., Jonas E., Luge C., Aladag A., Willbold D. Insights into human Lck SH3 domain binding specificity: different binding modes of artificial and native ligands. Biochemistry. 2005;44(45):15042–15052. doi: 10.1021/bi051403k. [DOI] [PubMed] [Google Scholar]
- Trochon-Joseph V., Martel-Renoir D., Mir L.M., Thomaidis A., Opolon P., Connault E., Li H., Grenet C., Fauvel-Lafeve F., Soria J., Legrand C., Soria C., Perricaudet M., Lu H. Evidence of antiangiogenic and antimetastatic activities of the recombinant disintegrin domain of metargidin. Cancer Res. 2004;64(6):2062–2069. doi: 10.1158/0008-5472.can-03-3272. [DOI] [PubMed] [Google Scholar]
- Valkovskaya N., Kayed H., Felix K., Hartmann D., Giese N.A., Osinsky S.P., Friess H., Kleeff J. ADAM8 expression is associated with increased invasiveness and reduced patient survival in pancreatic cancer. J. Cell Mol. Med. 2007;11(5):1162–1174. doi: 10.1111/j.1582-4934.2007.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eerdewegh P., Little R.D., Dupuis J., Del Mastro R.G., Falls K., Simon J., Torrey D., Pandit S., McKenny J., Braunschweiger K., Walsh A., Liu Z., Hayward B., Folz C., Manning S.P., Bawa A., Saracino L., Thackston M., Benchekroun Y., Capparell N., Wang M., Adair R., Feng Y., Dubois J., FitzGerald M.G., Huang H., Gibson R., Allen K.M., Pedan A., Danzig M.R., Umland S.P., Egan R.W., Cuss F.M., Rorke S., Clough J.B., Holloway J.W., Holgate S.T., Keith T.P. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418(6896):426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- Wakatsuki S., Kurisaki T., Sehara-Fujisawa A. Lipid rafts identified as locations of ectodomain shedding mediated by Meltrin beta/ADAM19. J. Neurochem. 2004;89(1):119–123. doi: 10.1046/j.1471-4159.2003.02303.x. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sul H.S. Ectodomain shedding of preadipocyte factor 1 (Pref-1) by tumor necrosis factor alpha converting enzyme (TACE) and inhibition of adipocyte differentiation. Mol. Cell Biol. 2006;26(14):5421–5435. doi: 10.1128/MCB.02437-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman Z.R., Duan J.J., Voss M.E., Xue C.B., Cherney R.J., Nelson D.J., Hardman K.D., Decicco C.P. Identification of a selectivity determinant for inhibition of tumor necrosis factor-alpha converting enzyme by comparative modeling. Chem. Biol. 2003;10(3):215–223. doi: 10.1016/s1074-5521(03)00044-9. [DOI] [PubMed] [Google Scholar]
- Wei S., Kashiwagi M., Kota S., Xie Z., Nagase H., Brew K. Reactive site mutations in tissue inhibitor of metalloproteinase-3 disrupt inhibition of matrix metalloproteinases but not tumor necrosis factor-alpha-converting enzyme. J. Biol. Chem. 2005;280(38):32877–32882. doi: 10.1074/jbc.C500220200. [DOI] [PubMed] [Google Scholar]
- Weskamp G., Ford J.W., Sturgill J., Martin S., Docherty A.J., Swendeman S., Broadway N., Hartmann D., Saftig P., Umland S., Sehara-Fujisawa A., Black R.A., Ludwig A., Becherer J.D., Conrad D.H., Blobel C.P. ADAM10 is a principal ‘sheddase’ of the low-affinity immunoglobulin E receptor CD23. Nat. Immunol. 2006;7(12):1293–1298. doi: 10.1038/ni1399. [DOI] [PubMed] [Google Scholar]
- Weskamp G., Kratzschmar J., Reid M.S., Blobel C.P. MDC9, a widely expressed cellular disintegrin containing cytoplasmic SH3 ligand domains. J. Cell Biol. 1996;132(4):717–726. doi: 10.1083/jcb.132.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp G., Schlondorff J., Lum L., Becherer J.D., Kim T.W., Saftig P., Hartmann D., Murphy G., Blobel C.P. Evidence for a critical role of the tumor necrosis factor alpha convertase (TACE) in ectodomain shedding of the p75 neurotrophin receptor (p75NTR) J. Biol. Chem. 2004;279(6):4241–4249. doi: 10.1074/jbc.M307974200. [DOI] [PubMed] [Google Scholar]
- Wewer U.M., Morgelin M., Holck P., Jacobsen J., Lydolph M.C., Johnsen A.H., Kveiborg M., Albrechtsen R. ADAM12 is a four-leafed clover: the excised prodomain remains bound to the mature enzyme. J. Biol. Chem. 2006;281(14):9418–9422. doi: 10.1074/jbc.M513580200. [DOI] [PubMed] [Google Scholar]
- White J.M. ADAMs: modulators of cell–cell and cell–matrix interactions. Curr. Opin. Cell Biol. 2003;15(5):598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- White J.M., Bridges L., DeSimone D., Tomczuk M., Wolfsberg T. Introduction to the ADAM Family. In: Hooper N.M., Lendeckel U., editors. The ADAM Family of Proteases. Springer; 2005. pp. 1–28. [Google Scholar]
- Wild-Bode C., Fellerer K., Kugler J., Haass C., Capell A. A basolateral sorting signal directs ADAM10 to adherens junctions and is required for its function in cell migration. J. Biol. Chem. 2006;281(33):23824–23829. doi: 10.1074/jbc.M601542200. [DOI] [PubMed] [Google Scholar]
- Wildeboer D., Naus S., Amy Sang Q.X., Bartsch J.W., Pagenstecher A. Metalloproteinase disintegrins ADAM8 and ADAM19 are highly regulated in human primary brain tumors and their expression levels and activities are associated with invasiveness. J. Neuropathol. Exp. Neurol. 2006;65(5):516–527. doi: 10.1097/01.jnen.0000229240.51490.d3. [DOI] [PubMed] [Google Scholar]
- Wolfsberg T.G., Straight P.D., Gerena R.L., Huovila A.P., Primakoff P., Myles D.G., White J.M. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain. Dev. Biol. 1995;169(1):378–383. doi: 10.1006/dbio.1995.1152. [DOI] [PubMed] [Google Scholar]
- Worley J.R., Baugh M.D., Hughes D.A., Edwards D.R., Hogan A., Sampson M.J., Gavrilovic J. Metalloproteinase expression in PMA stimulated THP-1 cells: effects of PPARgamma agonists and 9-cis-retinoic acid. J. Biol. Chem. 2003;278(51):51340–51346. doi: 10.1074/jbc.M310865200. [DOI] [PubMed] [Google Scholar]
- Worley J.R., Hughes D.A., Dozio N., Gavrilovic J., Sampson M.J. Low density lipoprotein from patients with Type 2 diabetes increases expression of monocyte matrix metalloproteinase and ADAM metalloproteinase genes. Cardiovasc. Diabetol. 2007;6:21. doi: 10.1186/1475-2840-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagami-Hiromasa T., Sato T., Kurisaki T., Kamijo K., Nabeshima Y., Fujisawa-Sehara A. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995;377(6550):652–656. doi: 10.1038/377652a0. [DOI] [PubMed] [Google Scholar]
- Yan Y., Shirakabe K., Werb Z. The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J. Cell Biol. 2002;158(2):221–226. doi: 10.1083/jcb.200112026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokozeki T., Wakatsuki S., Hatsuzawa K., Black R.A., Wada I., Sehara-Fujisawa A. Meltrin beta (ADAM19) mediates ectodomain shedding of Neuregulin beta1 in the Golgi apparatus: fluorescence correlation spectroscopic observation of the dynamics of ectodomain shedding in living cells. Genes Cells. 2007;12(3):329–343. doi: 10.1111/j.1365-2443.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Thomas S.M., Lui V.W., Xi S., Siegfried J.M., Fan H., Smithgall T.E., Mills G.B., Grandis J.R. Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation. Proc. Natl. Acad. Sci. USA. 2006;103(18):6901–6906. doi: 10.1073/pnas.0509719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Thomas S.M., Xi S., Smithgall T.E., Siegfried J.M., Kamens J., Gooding W.E., Grandis J.R. SRC family kinases mediate epidermal growth factor receptor ligand cleavage, proliferation, and invasion of head and neck cancer cells. Cancer Res. 2004;64(17):6166–6173. doi: 10.1158/0008-5472.CAN-04-0504. [DOI] [PubMed] [Google Scholar]
- Zhao J., Chen H., Peschon J.J., Shi W., Zhang Y., Frank S.J., Warburton D. Pulmonary hypoplasia in mice lacking tumor necrosis factor-alpha converting enzyme indicates an indispensable role for cell surface protein shedding during embryonic lung branching morphogenesis. Dev. Biol. 2001;232(1):204–218. doi: 10.1006/dbio.2001.0176. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Saftig P., Hartmann D., Blobel C. Evaluation of the contribution of different ADAMs to tumor necrosis factor alpha (TNFalpha) shedding and of the function of the TNFalpha ectodomain in ensuring selective stimulated shedding by the TNFalpha convertase (TACE/ADAM17) J. Biol. Chem. 2004;279(41):42898–42906. doi: 10.1074/jbc.M403193200. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Schlondorff J., Blobel C.P. Evidence for regulation of the tumor necrosis factor alpha-convertase (TACE) by protein-tyrosine phosphatase PTPH1. J. Biol. Chem. 2002;277(45):42463–42470. doi: 10.1074/jbc.M207459200. [DOI] [PubMed] [Google Scholar]
- Zhong J.L., Poghosyan Z., Pennington C.J., Scott X., Handsley M.M., Warn A., Gavrilovic J., Honert K., Kruger A., Span P.N., Sweep F.C., Edwards D.R. Distinct functions of natural ADAM-15 cytoplasmic domain variants in human mammary carcinoma. Mol. Cancer Res. 2008;6(3):383–394. doi: 10.1158/1541-7786.MCR-07-2028. [DOI] [PubMed] [Google Scholar]
- Zhu G.Z., Myles D.G., Primakoff P. Testase 1 (ADAM 24) a plasma membrane-anchored sperm protease implicated in sperm function during epididymal maturation or fertilization. J. Cell Sci. 2001;114(Pt 9):1787–1794. doi: 10.1242/jcs.114.9.1787. [DOI] [PubMed] [Google Scholar]
- Zhu L., Bergmeier W., Wu J., Jiang H., Stalker T.J., Cieslak M., Fan R., Boumsell L., Kumanogoh A., Kikutani H., Tamagnone L., Wagner D.D., Milla M.E., Brass L.F. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc. Natl. Acad. Sci. USA. 2007;104(5):1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P., Sun Y., Xu R., Sang Y., Zhao J., Liu G., Cai L., Li C., Zhao S. The interaction between ADAM 22 and 14-3-3zeta: regulation of cell adhesion and spreading. Biochem. Biophys. Res. Commun. 2003;301(4):991–999. doi: 10.1016/s0006-291x(03)00056-1. [DOI] [PubMed] [Google Scholar]
- Zou J., Zhu F., Liu J., Wang W., Zhang R., Garlisi C.G., Liu Y.H., Wang S., Shah H., Wan Y., Umland S.P. Catalytic activity of human ADAM33. J. Biol. Chem. 2004;279(11):9818–9830. doi: 10.1074/jbc.M309696200. [DOI] [PubMed] [Google Scholar]


