Abstract
Baculoviruses play an important ecological role regulating the size of insect populations. For many years, baculoviruses have been applied as targeted biocontrol agents against forestry and agriculture pests. Baculovirus insecticides are effective against insect pests such as velvetbean caterpillar (Anticarsia gemmatalis), cotton bollworm (Helicoverpa zea), and gypsy moth (Lymantria dispar). Baculoviruses are transmitted to insects by the oral route mediated by the occlusion-derived virus (ODV). The ODV is also specialized to exploit the insect midgut that is one of the most extreme biological environments where the viruses are subject to caustic pH and digestive proteases. The molecular biology of the ODV reveals new frontiers in protein chemistry. Finally, ODVs establishes infection in insect gut tissues that are virtually nonsupportive to virus replication and which are continuously sloughed away. ODVs carry with them a battery of proteins that enable them to rapidly exploit and harness these unstable cells for virus replication.
I. Introduction
A. Baculoviruses
Baculoviruses are a family of arthropod‐specific viruses found ubiquitously in the environment and have been isolated from more than 600 host insect species including the orders Lepidoptera, Hymenoptera, Diptera, Orthoptera, Coleoptera, Neuroptera, Thysanera, and Trichoptera (Adams 1991, Herniou 2003, Larsson 1984, Martignoni 1986, Murphy 1995, Tinsley 1985). Most baculovirus species have been isolated from Lepidoptera and the majority of nonlepidopteran isolates have not been well characterized. They are DNA viruses with closed, circular, double‐stranded DNA genomes ranging from 80 to 180 kbp in size. The genomes are packaged in bacillus‐shaped nucleocapsids, and the name “baculovirus” is in reference to the nucleocapsid shape. Presently, the genomes from 29 baculovirus species have been sequenced providing a database of more than 4000 genes (Hiscock and Upton, 2000).
Baculoviruses play an important ecological role regulating the size of insect populations (Evans 1986, Odindo 1983). For many decades, baculoviruses have been applied as targeted biocontrol agents against forestry and agriculture pests. Baculovirus insecticides have been effective against insect pests such as velvetbean caterpillar (Anticarsia gemmatalis) (Moscardi, 1999), cotton bollworm (Helicoverpa zea) (Zhang, 1994), and gypsy moth (Lymantria dispar) (Cook et al., 2003). Baculovirus‐based biocontrol applications have been restricted to lepidopteran and hymenopteran (sawflies) pests. Mosquito‐specific baculoviruses have been characterized (Andreadis 2003, Moser 2001) with the potential to develop them for biocontrol of mosquitoes.
Baculoviruses are transmitted to insects by the oral route mediated by the occlusion‐derived virus (ODV). This is reference to the occlusion of orally infectious baculoviruses in protein crystals called occlusion bodies (OBs). It is important to study the structure and function of ODVs and OBs because it expands the horizon for the application of baculovirus as insecticides. The ODV is also specialized to exploit the insect midgut which is one of the most extreme biological environments where the viruses are subject to caustic pH and digestive proteases. Understanding the molecular biology of the ODV should reveal new frontiers in protein chemistry. Finally, ODVs establish infection in insect gut tissues that are virtually nonsupportive to virus replication and which are continuously sloughed away. ODVs carry with them a battery of proteins that enable them to rapidly exploit and harness these unstable cells for virus replication. Learning about these proteins will have implications in biocontrol and biotechnology.
In the following sections, we will overview the basic biology of ODVs and OBs and will thoroughly examine the proteins within the ODV. Reviews on the ODV structure, function, and molecular biology have been lacking and, therefore, the goal of this chapter is to provide a more comprehensive picture and to stimulate future research.
An extensive analysis of the protein composition of the AcMNPV ODV virion was done in 2003 (Braunagel et al., 2003). That study produced a list of 44 ODV‐associated viral proteins. We use this landmark study as the framework for much of this chapter. Among these 44 AcMNPV‐specific ODV proteins, 21 are conserved among all baculovirus genomes (Table I ).
Table I.
Summary of Occlusion‐Derived Virion Proteins
 |
The table summarizes the 44 ODV‐associated proteins identified by Braunagel et al., (2003), and also includes PIF‐1, ‐2, ‐3, and 11K proteins. The sequences were analyzed for baculovirus homologues on the Viral Bioinformatics Resources Center (Hiscock and Upton, 2000). These resources searched baculovirus genomes of 7 group I NPVs, 12 group II NPVs, 2 hymenopteran NPVs, 1 dipteran NPV, and 7 GVs. The completely conserved proteins are shaded.
B. Origin of Baculoviruses
Fossil records tell us that terrestrial organisms originated from the sea and one of the earliest groups to venture out on to land was the arthropods, the predecessors of today's insects. Insects have been evolving and diversifying for the past 400 million years (Labanderia and Sepkoski, 1993) and so must have their viruses. Insect viruses of today may be related to the viruses brought onto land by ancient arthropod ancestors. The Baculoviridae is a family of insect viruses with complexity in form and function would suggest a long evolutionary lineage. Baculoviruses are genetically and morphologically distinct from other virus families and have an unusually stringent specificity for arthropod hosts. It has been suggested that baculovirus coevolved with Lepidoptera (Zanotto et al., 1993) given that most baculoviruses are isolated from this order and that there is high species specificity among lepidopteran baculovirus isolates. Baculoviruses isolated from other orders such as Diptera and Hymenoptera are also comparably less complex than the lepidopteran baculoviruses (Afonso 2001, Garcia‐Maruniak 2004, Herniou 2003, Lauzon 2004). The Lepidoptera were one of the last insect orders to experience significant species divergence, which occurred in the later half of the Cenozoic age (65 million years ago) (Labanderia and Sepkoski, 1993). If baculoviruses are as old as Lepidoptera, their survival as a virus family today can be attributed to the evolution of the ability of baculoviruses to occlude virions in protein crystals called OBs.
C. Baculovirus Occlusion
The baculovirus OB is a distinctive structure in virology. The OBs of baculoviruses are large enough to be observed by light microscopy and range from 500 to 2000 nm in diameter (Adams 1991, Bilimoria 1991, Boucias 1998) depending on the virus. This feature led to baculoviruses being the earliest described virus particles. Occlusion is an adaptation to permit baculoviruses to remain in a dormant but viable state in the environment for decades and perhaps even centuries (Bergold, 1963b). Baculovirus virions have lipid bilayer envelopes which normally make viruses susceptible to desiccation and loss of viability outside the host (Cox, 1989). The survival strategy of many enveloped viruses is to persist in living hosts as latent viruses or to infect a reservoir host species. For example, herpesviruses remain latent for decades in human hosts (Efstathiou and Preston, 2005) and the SARS Coronavirus was shown to persist in bats and civets (Li et al., 2005a). Insect viruses cannot rely on latency or reservoir strategies for several reasons. Insects are short lived compared to other animal classes thus limiting the chances for transmission of latent viruses between insect hosts. The dramatic physiological changes that occur in insects as they go from egg to adult also create complications at a cellular level that are not conducive to virus persistence. Probably the most important factor is that insect populations are seasonal and cyclic. There are long periods of time when insect species simply are not around in significant densities to transmit viruses. Insect viruses must persist for long periods in the environment waiting for population surges of their hosts.
To persist in the environment, baculoviruses evolved to surround their enveloped virions in the protective protein layers. In essence, they have acquired the hardier characteristics of nonenveloped viruses without significantly altering the biology of virion entry and attachment to host cells. Occluded viruses do not infect any species outside of invertebrates suggesting that persistence in the environment is critical for insect viruses. The occlusion strategy has evolved in parallel with some other insect viruses such as entomopoxviruses (Poxviridae) and cytoplasmic polyhedroviruses (Reoviridae) (Adams 1991, Rohrmann 1986). However, baculoviruses have evolved occlusion to several orders of complexity above that of other occluded viruses. First, baculovirus OBs may contain multiple numbers of virions and second, the virions may contain multiple nucleocapsids. A single OB of some baculoviruses species may deliver dozens of virions to a tissue and infect a cell with multiple copies of the viral genome. In the next few sections, we will discuss the advantages of this strategy in baculovirus biology.
II. The Baculovirus Life Cycle
A. Virus Entry into the Midgut
The transmission and replication of baculoviruses occur exclusively in the larval stages of insects. Transmission occurs by the oral or “per os” route when insects inadvertently consume OB‐contaminated food. OBs and food particles travel through the foregut and enter the midgut (Fig. 1 ). Lepidopterans have alkaline midguts (pH 10–11) (Terra and Ferreira, 1994) and baculoviruses have evolved to tolerate and exploit this extreme microenvironment. The alkalinity of the insect midgut triggers the dissolution of OBs and the release of occluded virions into the midgut lumen. These released virus particles are, therefore, precisely defined as ODVs. Occluded ODVs are released from OBs within 12 min post entry into the insect midgut (Adams and McClintock, 1991) and once released into the midgut, ODVs must breach the peritrophic membrane (PM).
Fig 1.
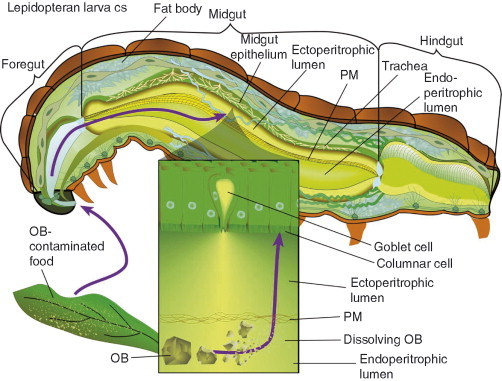
Per os infection by baculoviruses. A cross‐sectional representation of the anatomy of an insect larva is depicted. A baculovirus OB enters by the per os route in contaminated food. OBs pass through the foregut and enter the midgut where they dissolve in the alkaline midgut lumen and release ODVs. The inset figure depicts the translocation of released ODVs past the peritrophic membrane (PM) to midgut columnar epithelial cells. The midgut region surrounded by the PM has been referred to as the endoperitrophic lumen and the region outside the PM has been referred to as the ectoperitrophic lumen.
The PM is a net or lattice of chitin and protein that is produced along the length of the midgut such that it forms a hollow tube protecting the delicate midgut epithelium from direct contact with food particles. The PM is the insect equivalent of mucous, providing lubrication for food particle passage. The PM lattice has pore sizes ranging from 21 to 36 nm in diameter (Barbehenn and Martin, 1995). Small particles, such as digestive enzymes, can pass freely through the lattice into the endoperitrophic lumen to liberate peptides, sugars, and nutrients. The PM provides an immune defense by restricting the passage of larger particles such as bacteria, fungi, and viruses. To gain access to midgut tissues, ODVs must breach the PM through chance encounter of lesions or by releasing the PM‐compromising viral protease called enhancins (Greenspan 1991, Hashimoto 1991, Hotchkin 1981, Lepore 1996, Ohba 1983, Wang 1997b, Wang 1994). Enhancins are co‐occluded with ODVs in the OB matrix (Greenspan 1991, Hashimoto 1991, Hotchkin 1981, Lepore 1996, Ohba 1983, Wang 1994) or are present on ODV surfaces (Slavicek and Popham, 2005). Enhancins are metalloproteases, which cleave mucin‐like proteins bridging chitin strands in the PM lattice (Wang 1997a, Wang 1997b). Even baculoviruses that do not encode an enhancin degrade the PM by other viral factors (Derksen and Granados, 1988). Enhancins are an example of specialized adaptation of baculoviruses to their insect hosts. The study of baculovirus enhancins is of great importance not only for viral biology but also for the fact that these proteins expose vulnerabilities in the insect immune system.
B. The Structure of OBs
Early on in the study of the Baculoviridae family, it was recognized that, based on OB morphology, there were two major divisions or genera; the nucleopolyhedroviruses (NPVs) and the granuloviruses (GVs). The OB morphologies are illustrated in Fig. 2 . The virions of both of these baculovirus groups were occluded in proteinaceous OBs that could be seen under compound light microscope. The OBs of NPVs are most easily seen due to their larger size and their light refractory polyhedral (multisided crystal) structure. The average diameter of NPVs is within the range of 800–2000 nm (Bilimoria, 1991). The OBs of GVs appear as dark granules and are comparatively more difficult to resolve on the light microscope. They are ovoid shaped and about 500‐nm long and 200‐nm wide (Boucias and Pendland, 1998). The OBs of NPVs are called polyhedra and the OBs of GVs are called granula. Following this nomenclature, the major protein component of polyhedra is polyhedrin and the major protein component granula is granulin. Polyhedrins and granulins are ∼30 kDa in molecular weight. Both polyhedrin and granulin form a crystalline lattice that occludes virions. Dozens of virions are occluded in a polyhedron, while only a single virion is occluded in a granulum (Fig. 2). Although crystals are the natural state of polyhedrin and granulin, the atomic structures of neither of these proteins have been determined. The major reason for this is that polyhedra and granules form only inside the nuclei of insect cells. Abundant amounts of both of these proteins can be easily acquired but they have not been successfully solubilized and recrystallized into forms suitable for NMR or X‐ray crystallography. X‐ray diffraction has been done on OBs themselves (Bergold 1963a, Harrap 1972a) and these data along with electron microscopy (EM) studies suggest polyhedrin proteins contact each other at six points as nodular spheres. Despite the uniformity of the polyhedrin crystal, ODVs are randomly oriented within OBs. In addition, the interface between the ODV virion lipid bilayer envelope and the polyhedrin matrix contains a fibrous network of which the composition is unknown (Adams and McClintock, 1991). It has been suggested that virion envelopes themselves catalyze polyhedrin polymerization (Wood, 1980). The biology behind the occlusion of ODV virions in polyhedrin or granulin is not well understood but there are likely unique interactions among virion envelope proteins and the protein matrix of the OB.
Fig 2.
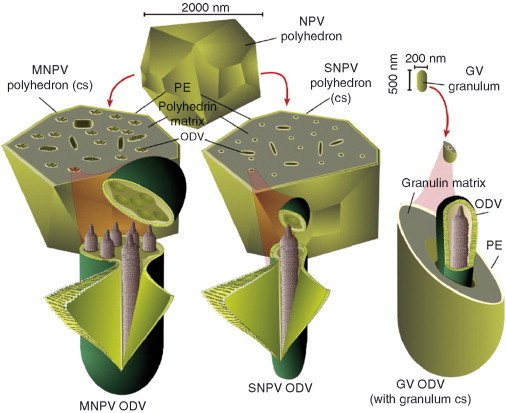
Major occlusion‐derived virion forms. Three major OB phenotypes are illustrated in the background. The nucleopolyhedrovirus (NPV) OBs are larger than the Granulovirus (GV) OBs due to the fact that they contain multiple numbers of ODV virions. The OBs of GVs are capsule shaped and contain only single virions. The OBs of NPVs are multisided crystals or polyhedra. Some species of NPVs produce cuboidal OBs. The NPVs are further divided into the multiple nucleopolyhedroviruses (MNPVs) and single nucleopolyhedroviruses (SNPVs). The multiple (M) and single (S) designations are in reference to the number of nucleocapsids that are found in each virion. The ODVs of MNPVs, SNPVs, and GVs are depicted in the foreground. The ODVs are illustrated in dissected views and the GV ODV is illustrated as partially encapsulated.
Surrounding the OBs of NPVs and GVs is a glycoprotein multilayered lattice termed the calyx (Carstens 1992, Whitt 1988), or the polyhedron membrane (Adams and McClintock, 1991) or the polyhedral envelope (PE) (Gross 1993, Gross 1994, Russell 1990). The PE is porous and does not resemble a conventional lipid bilayer virus envelope (Adams 1991, Harrap 1972b, Robertson 1974). The PE is a protein/carbohydrate matrix that forms a lattice or net with hexagonal pores ranging from 6 to 15 nm in diameter (Adams 1991, Harrap 1972b). The primary PE carbohydrate sugars are hexoses (60%), pentoses (29%), uronic acids, and hexosamines (Minion et al., 1979). The primary PE protein is a phosphoprotein called polyhedral envelope protein (PEP) or PP34. PEP is covalently linked to carbohydrates by disulfide bonds (Gombart 1989, Whitt 1988). Early PE structures form in the nucleus as concentric rings (Adams 1977, Goldberg 2002). The PEs surround OBs during their formation and they are the last structure that physically separates the OB from its environment and, therefore, serves to protect the integrity of OBs during and after their release to the environment. The OBs of many baculoviruses are released in a bath of a viral protease. One of the more interesting modes of baculovirus transmission is through predators that consume virus‐infected prey (Abbas and Boucias, 1984). The OBs survive predator digestive proteases and glycosidases such that viable OBs are dispersed in predator's feces. Presumably, the PE helps the OB to resist digestion from viral and nonhost enzymes. The PE is an elegantly designed structure for ensuring the protection and integrity of OBs until they are consumed by an insect host. The PE lattice is fine enough to restrict access of large enzymes to the OB matrix but would permit rapid permeation by anions from the alkaline host midgut. This would presumably lead to rapid decrystallization of polyhedrin or granulin, rupturing of the PE, and the release of ODV into the midgut. The complete release of ODVs from OB proteins may be further enhanced by OB‐associated alkaline proteases that have been reported to be present in OBs from larvae (Eppstein 1975, Eppstein 1975, Langridge 1981, Maeda 1983).
C. Infection of the Midgut Epithelium
On entering the ectoperitrophic space, ODVs diffuse to the midgut epithelium. The lepidopteran midgut epithelium is primarily composed of goblet and columnar cells. Goblet cells are involved in potassium ion transport from the hemolymph into the midgut and are not infected by baculoviruses (Adams and McClintock, 1991). ODVs primarily infect the predominant columnar cells, which are involved in secreting digestive enzymes and absorbing nutrients. On the luminal side, columnar cell surfaces are covered in brush border microvilli. ODVs have the specificity to bind to the apical ends of microvilli (Adams and McClintock, 1991). ODVs have lipid bilayer envelopes that fuse directly with midgut cell membranes (Haas‐Stapleton 2004, Horton 1993) resulting in the release of nucleocapsids into the cytosol (Fig. 3 ). These nucleocapsids migrate to the nucleus where they are unpackaged and the viral DNA genome is released. Virus genes are expressed, products of structural genes are synthesized, DNA replication ensues, and new progeny viruses are assembled and released. In the primitive hymenopteran baculoviruses, new progeny virions are occluded and released after cell lysis back into the midgut lumen (Ackermann 1983, Garcia‐Maruniak 2004, Lauzon 2004, Podgwaite 1984).
Fig 3.
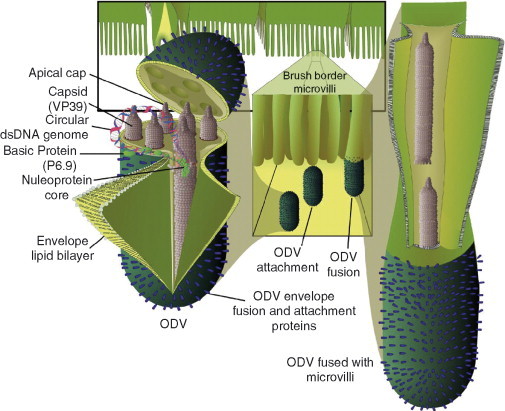
The ODV phenotype. The illustration on the left side represents a dissected view of the structure of the ODV. The DNA genome is shown expanding from the nucleocapsid to emphasize the presence of one viral genome in each nucleocapsid. This illustration is done in the context of an MNPV ODV. In the background and expanded into the center are representations of the insect midgut epithelium. The ODV‐specific processes of attachment and viral envelope fusion with membranes of the brush border microvilli are shown. Expanded on the right hand side is resulting the release of ODV‐nucleocapsids which are translocated up the microvillus into the midgut cell.
The insect midgut is a poor tissue for viruses to infect. Midgut cells are sloughed off regularly (Engelhard and Volkman, 1995) and are prone to apoptosis (Uwo et al., 2002) and, it becomes a race against time for the baculoviruses to establish infection in these cells. In addition, midgut cells are in a cell cycle arrest that is not conducive for virus DNA replication. Tissues beyond the midgut columnar epithelium have actively dividing cells that are more suitable for virus replication. However, the ODV is narrowly specialized to infect midgut epithelial cells in an extreme alkaline environment and has been rarely observed to infect other cell types. Because insect viruses rely on chance encounter infection, maximal progenies are needed to ensure survival. It is a significant advantage for baculoviruses to infect tissues beyond the midgut as many more viral progeny can be produced in a given host. This advantage has driven baculoviruses to evolve a second, radically different, budded virus (BV) phenotype (Fig. 4 ). BVs are broadly specialized to infect many internal tissues of the host including tracheoblasts, hemocytes, and fat body. BV entry occurs by receptor‐mediated, absorptive endocytosis (Volkman and Goldsmith, 1985), and acid‐triggered fusion of BV envelopes with endosomal membranes (Blissard 1992, Leikina 1992). The existence of two virion phenotypes in one virus is a major distinction of the Baculoviridae family. In later sections, we will describe these virion phenotypes in more detail.
Fig 4.
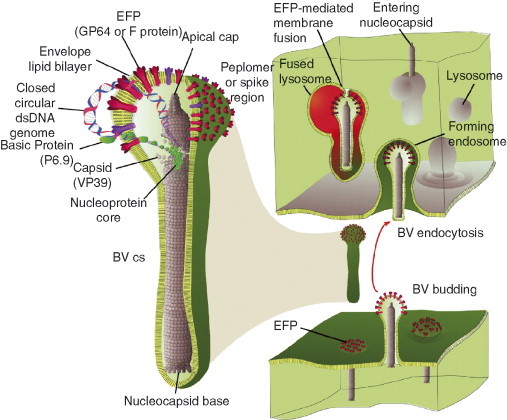
The BV phenotype. The illustration on the left side represents a dissected view of the structure of the BV. The DNA genome is shown expanding from the nucleocapsid in order to emphasize its presence in the nucleocapsid. The major BV envelope fusion proteins (EFPs), GP64 and F protein are shown at the upper peplomer end of the virion. This illustration is in the context of a group I NPV baculovirus. The right hand side illustrates the processes of BV egress from an infected cell (lower right) and BV infection of a new cell (upper right). Nucleocapsids bud out of the infected cell membrane where viral EFPs have concentrated. In budding, the virion acquires EFPs and the cell membrane as its virion envelope. The BV diffuses across to a new cell where it is taken into the cell by receptor‐mediated endocytosis. The BV‐containing endosome fuses with an acidifying lysosome. This pH shift triggers EFP‐mediated envelope fusion with the endosomal membrane and release the BV nucleocapsid into the cytosol. The nucleocapsid then translocates to the nucleus.
D. Infection of Tissues Beyond the Midgut
After ODVs infect midgut cells, BVs that are produced which bud from the basement membrane, penetrate the basal lamina and infect tissues of the hemocoel. Penetration of the basal lamina by BV is not well understood. Often EM images of this process show a clearing zone around BV as they traverse the basal lamina. The basal lamina is composed of protein, and it is possible that proteases produced by columnar epithelial cells are being directed by the virus to be released at the basal lamina. In addition, a viral‐encoded cathepsin (V‐CATH) has been copurified with BV (Lanier and Volkman, 1998). In cancer metastasis, malignant tumor cells become invasive to tissues by producing cathepsins on their surfaces that degrade the extracellular matrix (Nomura 2005, Yamaguchi 1990). V‐CATH may have an analogous function enabling BV to be projected through the protein matrix of the basal lamina.
For most baculoviruses, the midgut epithelium does not serve as a major tissue of viral replication but those infected cells serve to produce the initial supply of BV needed to infect other susceptible cells and tissues in larvae (Fig. 5 ). Often baculoviruses completely bypass replication in midgut cells in a process which will be discussed in later sections. As a defense mechanism against viruses, the infected midgut cell is sloughed off and new cells are generated to aid in gut recovery. Permitting the midgut epithelium to recover allows the host to continue to eat and grow and, in turn, this allows for the virus to replicate and maximize production of progeny.
Fig 5.
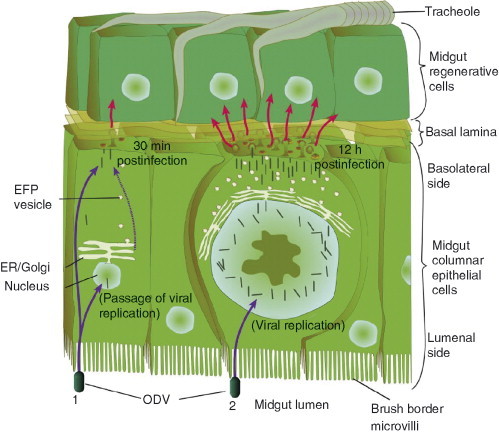
Two modes of infection in the midgut. Most baculoviruses use the midgut only to produce the first generation of BV progeny that bud out from the basal lateral side of the midgut cell to infect other tissues. ODVs that enter midgut cells may (1) bypass virus replication in the midgut cell or (2) initiate virus replication. When MNPVs bypass replication, most nucleocapsids translocate to the basolateral side of the midgut cell. One or a few nucleocapsids enter the nucleus, unpackaged, and expresses envelope fusion protein (efp) genes. EFP proteins are translocated via the endoplasmic reticulum (ER) and Golgi to the basal lateral side of the cell where they meet up with nucleocapsids which then bud out. This is the passage effect. When MNPVs replicate in the midgut cell significantly greater numbers of BV are produced. However, this mode of infection requires 8–12 h vs 30 min when the virus bypasses viral replication.
The major conduit for virus spread from the midgut is the tracheal system (Engelhard 1994, Kirkpatrick 1994, Washburn 1995). The tracheal system is the respiratory system of insects which begins on the outside as openings called spiracles along the lateral sides of insects. Tracheal tubes branch extensively as they lead from spiracles into the hemocoel. These branches transition abruptly into a network of very small tubes called tracheoles. Tracheoles are lined by tracheolar cells which can be 200–400 nm in length (Wigglesworth, 1984). Tracheoles impregnate nearly every tissue including the midgut epithelium. A midgut cell‐derived BV infecting a tracheolar cell can bypass the basal lamina and its BV progeny will have access deep into the tissues of the hemocoel. To a lesser degree, midgut regenerative cells that lie basolateral to the midgut epithelium also have been shown to be sites of midgut cell‐derived BV virus infection (Flipsen et al., 1995). Once in the hemocoel, BV is further dispersed by infecting hemocytes. These cells circulate throughout the open circulatory system. Hemocytes are mostly involved in immune response and infection of these cells has the added effect of reducing the ability of the host to combat the virus infection.
An important tissue of baculovirus replication is the fat body that acts as the insect liver (Dean et al., 1985) and is responsible for the storage and metabolism of lipids and sugars. It also produces vitellogenin, the primary egg protein. In the lepidopteran larva, the fat body is an amorphous and protuberant organ running throughout the insect. The tissue is highly accessible to BV and the energy‐rich cells of the fat body are ideal for producing abundant virus progeny. The fat body often becomes engorged with OBs such that the insect takes on an opalescent white, puffy appearance prior to death.
Exploiting the inner tissues of the insect host gives the virus the advantage of being able to produce enormous numbers of progeny. However, this virus is trapped inside the host, which is not necessarily a problem given that predators will often target slow moving, sick larvae and disperse OBs after feeding (Abbas and Boucias, 1984). Cannibalism among some insect species may also contribute to horizontal transmission. These modes of transmission are not efficient and most baculoviruses release OBs from the host by virus‐induced tissue liquefaction and the cuticle rupture after death. These processes are facilitated by the synergistic interaction of the viral protease, V‐CATH (Ohkawa 1994, Slack 1995), and the viral chitinase, ChiA (Hawtin et al., 1997). As the liquefied remains ooze out from the dead host, OBs are broadly dispersed along food surfaces that are eaten by a new host.
III. Cytopathology and Virion Phenotypes
A. The Early Phases
With the exception of some cell types, the baculovirus replication cycle includes a nonlytic phase of BV production, followed by ODV production and ending with the lytic release of OBs. These phases have been illustrated in Fig. 6 . The baculovirus infection cycle stages occur at predictable time intervals. The length of those time intervals is virus species specific and many baculoviruses require longer replication times than the model AcMNPV virus. The baculovirus replication cycle begins almost immediately after the nucleocapsid delivers the viral genome into host cell nucleus. Viral immediate early genes are expressed within 30 minutes postinfection (Chisholm and Henner, 1988) and their protein products along with virion‐associated proteins begin to manipulate the host cell to become competent for DNA replication. The structure of the nucleus is modified resulting in its expansion or nuclear hypertrophy. For AcMNPV, this process can be observed in the first 6 h. An electron dense, irregular‐shaped, granular region begins to form in the center of the nucleus (Harrap 1972a, Young 1993). This region is called the virogenic stroma and it is the site of viral RNA transcription, DNA replication, and nucleocapsid assembly. Concomitant with the appearance of the virogenic stroma is the movement of cellular heterochromatin to the edges of the nucleus along the inner nuclear membrane (INM) (Williams and Faulkner, 1997). Heterochromatin is highly condensed, histone‐associated host genomic DNA. The rearrangement of the nucleus has been shown to be mediated by viral interaction with tubulin (Volkman and Zaal, 1990). The virus infection causes the nucleus to partition itself into two major regions, the virogenic stroma and the peristromal space or nuclear ring zone. The heterochromatin on the outer edges of the nuclear ring zone gradually disappears over time (Williams and Faulkner, 1997). As the virogenic stroma expands, cavities or lacunae form inside it and nucleocapsids are assembled along the edges inside these cavities (Young et al., 1993). The stroma is composed mostly of RNA with DNA and a protein scaffolding that supports RNA and DNA complexes (Young et al., 1993). At about 12 hours postinfection (hpi), the virogenic stroma expands to fill most of the nucleus. Between 12 and 20 hpi, the BV virion phenotype is produced. The earliest made nucleocapsids in the baculovirus infection migrate out of the virogenic stroma, across the ring zone and to the nuclear membrane. Nucleocapsids are transported through the nuclear membrane and migrate across the cytosol to the cell membrane where they bud out. The process involved in translocation of nucleocapsids is not well understood although there is growing evidence of cellular actin involvement (Beniya 1998, Braunagel 2001, Lanier 1998, Lanier 1996, Lu 2004, Lu 2002). In the process of budding, nucleocapsid are enveloped in the host membrane and acquire virus‐encoded proteins in their envelopes. In midgut columnar epithelial cells, BV nucleocapsids show basal polarity (Keddie et al., 1989).
Fig 6.
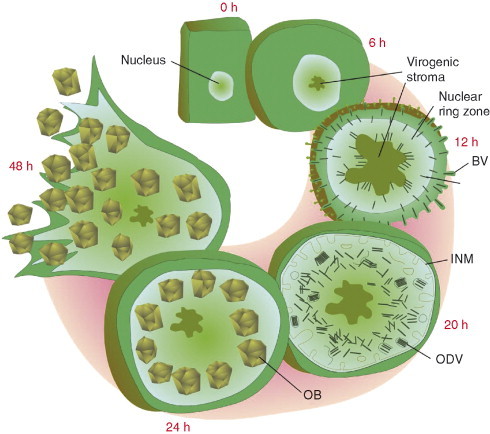
Baculovirus infection cycle. Several phases of virus replication are illustrated beginning with the rounding of newly infected cells and finishing with the lytic release of OBs. Indicated times are relative to the infection cycle of AcMNPV. The purpose of the figure is to illustrate the progression of phases from BV production to ODV production. Nucleocapsids are initially translocated to the cell membrane for BV production and later become retained in the nuclear ring zone for ODV production. INM is in reference to the inner nuclear membrane (INM) which provides the ODV envelope.
B. The Budded Virus
BV virions only contain single nucleocapsids and their envelopes appear loose such that cytoplasmic proteins may be present in the space between the nucleocapsid and the envelope. A computer illustrated structure of BV is presented in Fig. 4. The BV envelope contains the major viral glycoproteins, GP64/GP67 (Whitford et al., 1989), and fusion protein or F protein (Lung 2002, Pearson 2000, Pearson 2001, Westenberg 2002, Westenberg 2004, WF 2000). These proteins have also been called ENV proteins as they mediate budding, attachment, and entry of BVs (Blissard 1992, Hefferon 1999, Oomens 1999). In transmission EM preparations, BV envelopes are bulbous at one end and the surface is serrated (Adams et al., 1977). The serrations or notches are called peplomers and are thought to contain GP64 and F protein given that antibodies specific for BV envelope proteins localize in the peplomer region (Volkman et al., 1984). BV envelope proteins are N‐glycosylated and are sorted in the endoplasmic reticulum (ER) in order to be transported to the cell membrane. The GP64 protein is only present among group I NPVs and the F protein of these viruses has apparently lost its ENV functions (Whitford et al., 1989). The group II NPVs and GVs all encode for F proteins (Bulach 1999, Hashimoto 2000, Hayakawa 1999, Herniou 2001, Lange 2003, Luque 2001, Slack 2004, Wormleaton 2003, Zanotto 1993). F protein differs from GP64 in that it requires proteolytic cleavage before it can be functional (Westenberg et al., 2002). The only baculoviruses that do not have F protein homologues are the hymenopteran baculoviruses NeleNPV (Lauzon et al., 2004) and NeseNPV (Garcia‐Maruniak et al., 2004). These baculoviruses likely do not to produce BVs.
C. The Occlusion‐Derived Virus
Beginning at 20 hpi by AcMNPV, the virogenic stroma recedes and the infection shifts to favor the production of ODVs. A computer illustrated ODV structure is presented in Fig. 3. The virogenic stroma becomes more condensed and the nuclear ring zone expands to accommodate accumulating nucleocapsids. Nucleocapsids in the nuclear ring zone become ODVs as they acquire lipid bilayer envelopes. The retention and formation of enveloped virions in the nucleus is a unique biological phenomenon. The ODV envelope is a lipid bilayer that resembles, but is not identical to, the INM in composition (Braunagel and Summers, 1994). The ODV envelope is more rigid than the BV envelope due to the presence of more saturated fatty acid phospholipids. The ODV envelope also contains phosphatidylcholine instead of phosphatidylserine which is found in BV envelopes.
There has been some controversy as to whether the ODV envelope is synthesized de novo or whether it is acquired from the INM. The time of ODV morphogenesis coincides with the appearance of membranous structures which are believed to be the precursors of ODV envelopes (Adams 1991, Fraser 1986, Hong 1994). Considerable evidence has been generated to favor an INM source which invaginates as microvesicles into the ring zone late in infection (Braunagel 1996a, Hong 1994). It should be pointed out that even by this mechanism, the virus would have to induce “de novo” INM synthesis to ensure enough material for nucleocapsid envelopment. The ODV envelope contains a number of integral and associated viral proteins. These proteins are more diverse than BV envelope proteins. They are mostly nonglycosylated and they are transported to the ODV envelope by unique mechanisms. The ODV envelope proteins will be described more in detail in later sections of this chapter. Unlike BV nucleocapsids, ODV nucleocapsids are also packaged in a proteinaceous virus derived tegument prior to envelopment. After occlusion, this tegument becomes more condensed around nucleocapsids (Knudsen and Harrap, 1976).
Soon after ODVs begin to appear in the ring zone, occlusion begins to occur and by 24 hpi, fully formed OBs are present. By 48 hpi, OBs are liberated from the nuclei of infected cells by lytic release. The viral protein V‐CATH that has been previously mentioned to be important for liquefaction of the host also plays a role in releasing OBs from infected cells. The OBs of V‐CATH deletion mutant AcMNPV viruses are not released from infected cells in cell culture. V‐CATH has been linked with appearance of free OBs in the hemolymph of BmNPV‐infected insects (Suzuki et al., 1997). P10, which forms fibrillar bodies in the nucleus and cytoplasm, has been shown to interact with host cell microtubules (Patmanidi et al., 2003) and also to be involved in cell lysis (Williams et al., 1989).
D. Multiple Occlusion and Bypass of Midgut Cell Replication
The NPVs are further divided into single and multiple occlusion “subphenotypes” (SNPV and MNPV). The virions of SNPV contain single nucleocapsids and the virions of MNPV contain multiple nucleocapsids. MNPV virions can contain more than 40 nucleocapsids (Kawamoto and Asayama, 1975). The MNPVs have, however, only been observed in lepidopteran baculovirus isolates (Rohrmann, 1986). It could be that there are not enough examples of nonlepidopteran baculoviruses. While it has been somehow debatable whether to retain the “S” and “M” designations in baculovirus nomenclature, single and multiple capsid subphenotypes have been suggested to be anomalous and with no genetic basis or biological importance (Herniou et al., 2003). The more compelling argument for the distinction of SNPV and MNPV are biological studies that demonstrate the advantage of the MNPV over the SNPV in oral infectivity (Washburn 1999, Washburn 2003b). These studies are difficult to do as the genes for multiple and single occlusion are not yet known and comparisons must be made between related but not identical MNPV and SNPV baculoviruses.
One of the earliest proposed theories for multiple occlusions was that it was a way for baculoviruses to bypass replication in the midgut and to deliver virions to the hemocoel via the tracheoles or other cell types (Keddie et al., 1989). This has been termed the “passage effect” (Granados and Lawler, 1981) (Fig. 5). It was demonstrated that when MNPV ODVs infect midgut cells, some nucleocapsids bypass the nucleus, migrate to the basal lamina side (basement membrane) of the midgut cell, and bud through to infect other cell types (Adams 1977, Granados 1981). This can occur because other nucleocapsids from the MNPV ODV virion enter the nucleus, uncoat, and express early genes including the BV envelope fusion protein gene, gp64. The gp64 gene has a bipartite early and late promoter and the GP64 protein is produced by 6 hpi by AcMNPV (Blissard and Rohrmann, 1989). The GP64 protein accumulates on the basement membrane of midgut cells (Keddie et al., 1989) and enables ODV‐derived nucleocapsids to bud as infectious BV. This theory has been supported by the demonstration that the elimination of the gp64 early promoter reduces the oral infectivity of AcMNPV (Washburn et al., 2003a). F proteins such as Ld130 of LdMNPV are produced at late times postinfection (Pearson et al., 2001). However, the promoter structures of the f protein genes of LdMNPV and Spodoptera exigua multiple nucleopolyhedrovirus (SeMNPV) contain early and late virus transcriptional motifs (IJkel 1999, Kuzio 1999).
Group I MNPVs such as AcMNPV retain F protein homologues (Ac23) that are unable to perform any fusion function in the BV (Monsma et al., 1996) but which have been shown to enhance baculovirus oral infectivity (Lung et al., 2003). This is intriguing given that the AcMNPV F protein present in ODV envelopes (Braunagel et al., 2003). The presence of F protein in the ODV and its enhancement of oral infectivity points to F protein being an important ODV protein. ODVs enter midgut cells by direct fusion of the virion envelope with the midgut cell membrane (Horton and Burand, 1993) and the simplest explanation is that F protein is involved in the fusion of ODV envelopes with the midgut cell membrane. Presumably the F protein is C‐terminally anchored in the ODV envelope with its N‐terminus exposed.
Another possible role of F protein may involve the passage effect. After fusion of the ODV with the midgut cell membrane, there would be opportunity for F protein transfer into midgut cell membranes. F protein could be absorbed by pinocytosis and subsequently translocated along side nucleocapsids to the basement membrane. This would be a rapid mechanism for ODV virions to pass through the midgut cell cytoplasm and to form BV at the basement membrane. GVs such as EpapGV appear only to infect midgut cells but completely evade midgut replication and infect fat body, epidermis, and tracheolar tissues (Goldberg et al., 2002). GVs have a single nucleocapsid in each OB and the early ENV protein expression strategy would not work. F protein membrane transfer and translocation may offer these viruses a mechanism of passage through the midgut. The F proteins of group II NPVs and GVs have not yet been identified in the ODV. It has been noted that the nonfunctional AcMNPV F protein homologue has low identity to the functional F protein homologues of group II NPVs and GVs (Lung et al., 2002). However, F protein homologues of group I NPVs, group II NPVs, and GVs have common structural features (Lung 2002, Lung 2003) and thus would be expected to be also localized in the ODV envelope.
It has been postulated that gp64 was acquired during baculovirus evolution (Pearson et al., 2000) and it is established that GP64 is not present in ODV envelopes (Hohmann 1983, Keddie 1989, Wang 1985). The gp64 gene has a common origin with tick‐transmitted mammalian arboviruses (Morse et al., 1992) while the f protein gene has an insect origin (Lung and Blissard, 2005). The different origins of these proteins would influence how they function in the virus.
There are other explanations of the evolution of multiple occlusions. For example, ODV nucleocapsids may be delivering associated proteins that augment virus replication. Multiple occluded nucleocapsids would effectively deliver more of these factors. In addition, delivering several copies of viral genomes decreases the chances that mutation will compromise infection. After waiting for long periods of time in the environment, mutations that occur would be random and multiple genome copies could complement each other.
IV. ODV Nucleocapsid Proteins
A. The Nucleocapsid
The nucleocapsids of the ODV and BV have many similarities as they both contain complete viral genomes and have major proteins in common. The ODV nucleocapsid is illustrated in Fig. 7 . Baculovirus nucleocapsids are 40–70 nm in diameter and 250–400 nm in length (Boucias and Pendland, 1998). The size of the viral genome determines the length of the nucleocapsid. Nucleocapsids are polar, with a claw or base on one end and a nipple or apical cap on the other end (Federici 1986, Fraser 1986). The apical cap is oriented toward the virogenic stroma during nucleocapsid assembly (Fraser, 1986). It is also found associated with the forming envelopes of the ODV and is oriented on the peplomer end of BV (Adams 1991, Fraser 1986). The nucleocapsid is composed of an outer protein capsid surrounding a nucleoprotein core (Arif, 1986). The most abundant structural protein of the nucleocapsid is VP39 (Blissard 1989, Guarino 1990, Pearson 1988, Thiem 1989b) with monomers arranged in stacked rings around the nucleoprotein core (Federici, 1986).
Fig 7.
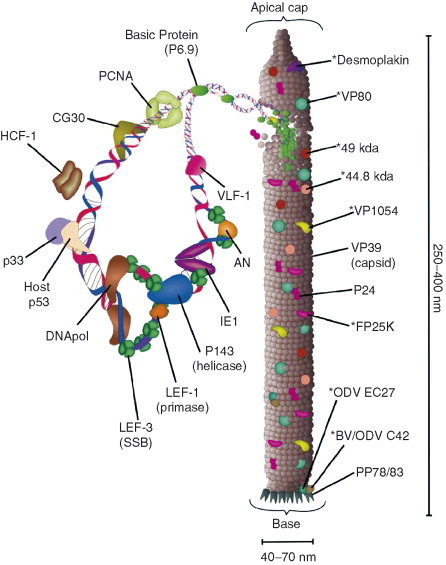
Proteins of the ODV nucleocapsid. Some of the proteins found associated with the ODV nucleocapsid are illustrated. The figure is in the context of AcMNPV. The “*” indicates that the proteins for which the specific location on the nucleocapsids has not been confirmed. We have also placed nucleocapsid‐associated proteins which have DNA‐binding activities or potential transcriptional activation activities on the viral genome. The intent of the figure is to reveal the nucleocapsid as a complex structure of multiple protein types instead of simply protein sheath surrounding Basic Protein and the viral genome.
In addition to the major VP39 capsid protein, there are number of other minor but important capsid‐associated proteins. The PP78/83 (ORF1629) protein is a phosphoprotein that was first identified in viral fractionation studies as a BV/ODV envelope protein and/or ODV tegument protein (Pham et al., 1993). EM studies later revealed that PP78/83 is associated with the nucleocapsid base (Russell 1997, Vialard 1993). Discovery that PP78/83 nucleates actin polymerization has lead to the suggestion that this protein is involved in nucleocapsid translocation into the nucleus after infection (Lanier and Volkman, 1998). Only 19 PP78/83 homologues are found among 29 baculovirus genomes. PP78/83 appears to play a critical and a multifunctional role (Kitts and Possee, 1993). The study of a number of baculovirus proteins often reveals multifunctional roles. The name PP78/83 is in reference to the unphosphorylated and phosphorylated state of this protein (Vialard and Richardson, 1993). PP78/83 has also been shown to be a component of the virus‐encoded RNA polymerase complex (Iorio et al., 1998). It is difficult to find a linkage between transcription and virion translocation; however, PP78/83 would be translocated to the nucleus to participate in the transcriptome.
BV/ODV‐C42 is a capsid‐associated protein that was shown by yeast two‐hybrid analysis to interact directly with PP78/83 (Braunagel et al., 2001). Homologues to the bv/odv‐c42 gene are present in all sequenced baculoviruses except CuniNPV. BV/ODV‐C42 has a conserved nuclear localization signal and localizes along with PP78/83 in the DNA‐rich virogenic stroma. BV/ODV‐C42 has not been specifically localized to the capsid base. However, we consider this likely given its association with PP78/83. BV/ODV‐C42 interacts directly with another highly conserved capsid‐associated viral protein called ODV‐EC27 (Braunagel et al., 2001). ODV‐EC27, which is also associated with the ODV envelope, is involved in regulating the cell cycle and will be covered in detail in following sections. It is possible that BV/ODV‐C42, ODV‐EC27, and PP68/83 are found associated together as complex at the nucleocapsid base.
The VP80 protein is a capsid‐associated structural protein that was first identified as P87 in OpMNPV (Muller et al., 1990). Other homologues were characterized in AcMNPV (VP80) (Lu and Carstens, 1992) and CfMNPV (P82) (Li et al., 1997). Gene homologues to the vp80 gene are only found in NPV genomes. The vp80 gene is transcribed late in infection and the protein localizes in the nucleocapsids of BV and ODV (Li 1997, Muller 1990). The VP80 homologue of CfMNPV has 72 and 82 kDa molecular weight protein forms (Li et al., 1997) and only the 82‐kDa protein is associated with ODV nucleocapsids. The 82‐kDa form is the result of posttranslational modification given that this protein has a predicted mass of 71.2 kDa. N‐Glycosylation was eliminated as a possibility (Li et al., 1997) and phosphorylation is an alternative explanation given that this protein has 52 potential phosphorylation sites. Phosphorylation also is prevalent with other capsid‐associated proteins such as PP78/83.
The capsid‐associated protein P24 is conserved among some but not all NPVs and GVs. The p24 gene is an auxiliary gene as it is absent from variants of LdMNPV (Slavicek and Hayes‐Plazolles, 2003). It was confirmed by EM and virus fractionation immunoanalysis that P24 was a component of the nucleocapsids of BV and ODV (Wolgamot et al., 1993). The P24 homologue of SpltNPV is present only in the ODV nucleocapsids and migrates in SDS‐PAGE at significantly higher molecular weight than expected (79 kDa instead of 36 kDa) (Li et al., 2005c). It was suggested that this protein is a homodimer in ODV given that the 36‐kDa monomeric form was detected in infected cell lysates.
B. Nucleoprotein Core
Within the nucleocapsid is a nucleoprotein core composed of supercoiled viral DNA and viral protein. The predominant protein in the nucleoprotein core is a small peptide with a pI value of 12 that is aptly named, Basic Protein. It is has been suggested that the positively charged arginine residues of Basic Protein interact with the negatively charged DNA backbone to enable its condensation in the nucleocapsid (Kelly et al., 1983). Basic Protein is protamine‐like (Tweeten et al., 1980) and has been called P6.9 or VP12 depending on the baculovirus (Wilson 1988, Wilson 1987). Basic Protein is also a phosphoprotein and its phosphorylation is inhibited by the presence of Zn2+ (Funk 1992, Funk 1993, Wilson 1985). When nucleocapsids are unpackaged in a new cell, cellular chelators remove Zn2+ which promotes Basic Protein to be phosphorylated by capsid‐associated kinases (Funk 1993, Miller 1983, Wilson 1985). The resulting change in charge dissociates Basic Protein from viral DNA which then unpackages from the nucleoprotein core (Funk 1993, Wilson 1985). This unpackaging may occur at nuclear pores or in the nucleus. Ultimately, the viral genome is delivered into the nucleus where it is bound up by host histones (Wilson and Miller, 1986).
On the basis of pH and salt‐dissociation properties, it was suggested that there is only one major viral protein that is associated with packaged viral DNA (Young et al., 1993). However, a significant number of ODV‐associated proteins have DNA‐binding activity and are thus likely minor constituents of the packaged nucleosome.
The AcMNPV ORF59 gene is predicted to encode for an 8.2‐kDa unnamed protein that is associated with ODV nucleocapsids (Braunagel et al., 2003). This protein bears strong homology to the Escherichia coli protein, ChaB, and we suggest naming this protein, V‐ChaB. There are 20 V‐ChaB homologues among the group I and group II NPVs. The structure of the E. coli ChaB has been solved and it has been suggested that ChaB is involved in the regulation of divalent cation transport (Osborne et al., 2004). The most important divalent cation in the nucleocapsid is Zn2+ (Funk 1992, Funk 1993, Wilson 1985) and perhaps V‐ChaB protein is involved in the transport of this cation during the condensation of the viral genome. The observed condensation of the tegument after ODV occlusion (Knudsen and Harrap, 1976) may be facilitated by the removal of ions from the ODV.
C. Nucleocapsid Proteins Involved in DNA Packaging
There is evidence that some capsid‐associated proteins are involved in nucleocapsid assembly and DNA packaging. VP1054 is an essential 42‐kDa baculovirus protein that is required for capsid assembly and the arrangement of VP39 monomers (Olszewski and Miller, 1997a). Temperature sensitive (Ts) mutants of the VP1054 protein produce spherical capsids in the virogenic stroma instead of tubular capsids (Olszewski and Miller, 1997a). Association of this protein with nucleocapsids (Braunagel et al., 2003) may suggest interaction with VP39 during the formation or stacking of VP39 oligomer rings. In one model of nucleocapsid assembly, the capsid base and main body are preassembled in specific locations in the virogenic stroma (Fraser, 1986). The nucleoprotein passes through the apical cap and is supercoiled as it is packaged into empty capsid tubes. The supercoiling of viral DNA into the capsid has not been characterized. The nucleocapsid‐associated protein, desmoplakin (AcMNPV ORF66), has some homology to type I topoisomerases and could be involved in mediating the supercoiling of DNA. There are 55 homologues of baculovirus desmoplakins shared among 29 baculovirus genomes. Type I topoisomerases are found associated with the nucleocapsids of Vaccinia virus and it has been shown that these proteins accelerate the expression of early viral genes (Da Fonseca and Moss, 2003).
Several nucleocapsid‐associated proteins have been characterized to have activities suggestive of involvement in viral DNA packaging. Alkaline nuclease (AN or Alk Exo) is a highly conserved and essential nucleocapsid‐associated protein that may be involved in the processing of the viral genome into nucleocapsids by removing aberrant DNA branched structures from condensing DNA (Li 2000, Okano 2004).
Very late factor‐1 (VLF‐1) is a protein that binds to burst elements on delayed late gene promoters and is associated with enhancement of delayed late gene expression (McLachlin 1994, Mistretta 2005, Yang 1998b). VLF‐1 is essential for virus replication (Li 2005b, Vanarsdall 2004, Yang 1998a) and it is associated with BV and ODV nucleocapsids (Yang and Miller, 1998b). The VLF‐1 protein binds to cruciform DNA structures and may have a role in processing of the viral genome during nucleocapsid assembly (Mikhailov and Rohrmann, 2002b). On the basis of the DNA‐binding activities, VLF‐1 and AN would be expected to be associated with the viral genome in the nucleoprotein core region. The persistence of VLF‐1 and AN with ODV nucleocapsids may point to additional functions for these proteins. Perhaps VLF‐1 binding to burst elements blocks early transcription of very late promoters so that essential early promoters are favored.
D. DNA Replication Proteins in the Nucleocapsid
As has been discussed earlier, baculoviruses have evolved to minimize or bypass viral replication in columnar epithelial cells. One of the reasons for this is that columnar epithelial cells are in an arrested state. Many of the host DNA replication factors are not present in arrested cells and this is a challenge for replication of a large genome DNA virus. The nucleocapsids of ODVs have consequentially evolved to carry with them viral DNA replication factors along with the viral genome. The six core baculovirus DNA replication proteins are immediate early 1 (IE‐1), DNA polymerase (DNApol), P143 (helicase), LEF‐1 (primase), LEF‐2 (primase cofactor), and LEF‐3 (single‐stranded DNA‐binding protein or SSB) (Kool 1994, Lu 1995b). ODV nucleocapsids contain all of these proteins but for LEF‐2 (Braunagel et al., 2003). The absence of LEF‐2 is surprising given its oligomerization with LEF‐1 (Evans 1997, Hefferon 2002) and its stabilization of LEF‐1 binding to DNA (Mikhailov and Rohrmann, 2002a). BV nucleocapsids do not contain IE‐1 or P143 (Braunagel et al., 2003).
All of the core DNA replication factors bind to DNA and the binding by DNApol and P143 is inferred by their functions in DNA synthesis and in strand separation, respectively. IE‐1 binds to replication origin DNA sequences called homologous repeats (hrs) (Kovacs 1992, Pearson 1992). LEF‐3 binds to single‐stranded DNA (Hang et al., 1995) and binds to P143 (Wu and Carstens, 1998) and to IE‐1 (Hefferon and Miller, 2002). The baculovirus exonuclease AN has been shown to bind to DNA and to complex with LEF‐3 (Mikhailov et al., 2003). The location of DNA replication factors in the ODV nucleocapsid has not been determined. However, their DNA‐binding activities and cointeractions would lead to the conclusion that these factors are bound to the viral genome in the nucleoprotein core of the nucleocapsid.
Other ODV capsid‐associated proteins with potential involvement in DNA replication include host cell factor HCF‐1 (Braunagel et al., 2003) and viral proliferating cell nuclear antigen (PCNA) (O'Reilly et al., 1989). HCF‐1 is required for AcMNPV DNA replication in Trichoplusia ni cell lines (Lu and Miller, 1995a) and it augments the ability of this virus to orally infect T. ni larvae (Lu and Miller, 1996).
PCNA, which functions as a DNA replication factor has strong DNA‐binding tendencies and is probably part of the nucleosome. Eukaryotic PCNAs have been correlated with DNA synthesis, DNA repair, and cell cycle regulation (Bravo 1986, Kurki 1986, Madsen 1985, Solomon 2004). The viral PCNA is localized in viral DNA replication complexes (Iwahori et al., 2002) and is not essential (Iwahori et al., 2004) or stimulatory (Kool et al., 1994) for viral DNA replication in insect cell lines. In cell culture, the host PCNA appears to compensate for absence of the viral PCNA (Iwahori et al., 2004). Supplementing the ODV virion with viral PCNA may be advantageous to the virus as insect midgut cells produce low levels of cellular PCNA (Zudaire et al., 2004).
E. DNA Repair Proteins of the Nucleocapsid
One of the major antagonists to the viability of baculoviruses is exposure to light and when absorbed by the virions, it changes the energetic state of proteins and DNA. This leads to uncontrolled covalent bond breakages and functional inactivation. In one study examining the effects of time, temperature and light, the latter was the major factor influencing viability (Jarvis and Garcia, 1994). Ultraviolet (UV) light only represents 5% of sunlight energy but is the form of energy that most damages baculovirus viability (Shapiro et al., 2002). Baculovirus OBs persist in the environment for long periods of time and are frequently exposed to UV light. Exposure of OBs to UV light results in dramatic decrease of oral infectivity (Shapiro et al., 2002). Natural reservoirs of viable baculovirus OBs are most prevalent on plant stems and nonlight exposed surfaces (Raymond et al., 2005).
When insect cells are exposed to UV radiation and subsequently are infected with UV‐inactivated baculoviruses, these cells are able to recover viral viability (Witt, 1984). It was hypothesized that UV induces synthesis of host enzymes that enable repair of UV‐damaged viral genomes. Some baculoviruses may have acquired the host DNA repair genes such as viral‐three prime repair exonuclease (V‐TREX) and photolyase. Homologues to the v‐trex gene have only been identified in AgMNPV (Slack 2004, Slack 2004), CfMNPV (Yang et al., 2004), and CfDEFNPV (Lauzon et al., 2005). The v‐trex gene may have been acquired from the host given its homology to insect genes (Slack et al., 2004). Although there is no direct evidence of virion localization, V‐TREX overproduction leads to significantly increased levels of associated 3′ to 5′ exonuclease activity in BV (Slack and Shapiro, 2004). The astounding success of AgMNPV as viral insecticide may be partially attributed to the resistance of this virus to UV damage in the field. Another example of a candidate baculovirus DNA repair enzyme is a photolyase that has been identified in the genomes of TnSNPV (Willis et al., 2005) and ChchNPV (van Oers et al., 2005). The baculovirus photolyase and V‐TREX proteins point to a selective pressure for baculoviruses to acquire DNA repair enzyme genes.
It would be advantageous for ODV virions to carry DNA repair proteins so that lethal mutations can be repaired immediately after the genome is released into a new host cell. ODV virions carry with them a number of proteins that could be involved in DNA repair. For example, damaged DNA strands could be removed by the 5′ to 3′ activities of the exonuclease AN. The DNApol complex proteins could also play a role in the resynthesis of damaged DNA.
It is possible that the viral PCNA is involved in DNA repair for viral genomes after infection. In some systems, PCNA has been shown to be specifically involved in the repair of UV‐damaged DNA (Aboussekhra 1995, Li 1996).
The ODV‐associated peptide product of AcMNPV, ORF79, has 20 other baculovirus homologues and is very similar to a family of UvrC intron‐encoded nucleases (URI) (Aravind et al., 1999). This family of enzymes is involved in bacterial DNA repair defenses after UV damage (Aravind et al., 1999). The URI nucleases enable repair of damaged DNA by making strand incisions on both sides of the DNA lesion. In bacterial systems, they function as part of a complex with ABC‐type UvrA ATPases and UvrB helicases (Lin 1992, Lin 1992). It is likely that the AcMNPV ORF79 gene was acquired from bacteria or another insect virus. The entomopathogenic nematode bacteria, Photorhabdus luminescens and the insect iridovirus type 6, both encode ORF79 homologues.
Some researchers have engineered DNA repair genes into baculoviruses such as the DNA glycosylase, cv‐PDG (Petrik et al., 2003). This resulted in increased tolerance of the BV form of baculoviruses to UV‐C radiation but had only negligible effects on the ability of ODVs to tolerate UV light. Failure of this strategy may have been due to poor engineering of the protein to be a component of the ODV nucleocapsid. This kind of approach attempting to engineer baculovirus tolerance to UV light will likely become more important in the future. Given the complexity of the ODV, it may be simplest to evaluate some of less common baculovirus auxiliary UV protection gene candidates such as v‐trex or photolyase before venturing into other systems.
F. Cell Cycle Regulator Proteins in the Nucleocapsid
As has been mentioned previously, midgut cells are in a state that metabolically will not support viral DNA replication. In addition to DNA replication factors, baculovirus ODV virions carry proteins that promote midgut cell rejuvenation. Differentiated midgut cells are in a nondividing, Gap 0 (G0) cell cycle phase (Garcia 2001, Loeb 2003). Dividing cells have four possible phases; G1 phase (gap 1), S phase (DNA synthesis), G2 phase (gap 2), and M phase (mitosis). ODV nucleocapsids carry with them proteins that appear designed to shift the cell cycle out of the G0 phase and into the S phase or DNA synthesis phase.
ODV‐EC27, which is conserved among all baculovirus genomes, is the first ODV protein to be described with involvement in the cell cycle (Belyavskyi et al., 1998). This protein has the “EC” designation due to localization in the envelopes and nucleocapsids of ODVs (Braunagel et al., 1996b). When isolated from ODVs, this protein migrates at 27 kDa in reducing SDS‐PAGE gels which is less than the expected size of 33 kDa (Braunagel et al., 1996b). Other larger sizes of ODV‐EC27 were also detected in ODVs and BVs, which may be homodimers and homotrimers (Belyavskyi 1998, Braunagel 1996b).
One of the more important avenues of investigation of ODV‐EC27 centered around a cyclin box motif that was identified in residues 80–110 of AcMNPV ODV‐EC27 (Belyavskyi et al., 1998). Cyclins are proteins that mediate shifting of the cell cycle through interaction with cyclin‐dependent kinases (CDKs). ODV‐EC27 was shown to bear significant similarity to Bombyx mori cyclin B (Baluchamy and Gopinathan, 2005). Notably, the authors of this study refer to the BmNPV ODV‐EC27 homologue as “viral cyclin” or V‐CYC.
Immunoprecipitation experiments demonstrated that the ODV‐EC27 coprecipitates with CDKs and that these complexes will phosphorylate the known CDK substrates, histone H1 and retinoblastoma (pRB) (Belyavskyi et al., 1998). Immunoprecipitation experiments ODV‐EC27 binding specificity to cell division cycle kinase 2 (Cdc2) (Belyavskyi et al., 1998). Cdc2 forms complexes with cyclin B to trigger cells to enter mitosis and divide (Nurse, 1990). In other words, cyclin B/Cdc2 complexes induce the G2/M phase transition. At late times in baculovirus infection, cyclin B is rapidly degraded and cells enter G2/M phase transition arrest (Braunagel et al., 1998). This loss of cyclin B coincides with formation of ODV‐EC27/Cdc2 complexes (Belyavskyi et al., 1998). ODV‐EC27 lacks a cyclin‐like destruction box motif thus providing mechanism to maintain ODV‐EC27/Cdc2 complexes and G2/M phase arrest. It has been suggested that maintaining G2/M phase arrest at late times in infection is very important from the prospective of the ODV assembly (Belyavskyi et al., 1998). During M phase, the nuclear envelope breaks down to permit chromosomal segregation and cell division. Arresting the cells before the onset of M phase may be required for ODV assembly given that they acquire their envelopes from the INM, which would be lost if cells were permitted to proceed through M phase. The inner nuclear envelope may also have physical characteristics at the end of the G2 phase which may make it more easily acquired to form ODV envelopes. It should be noted however, that the nuclear membrane completely disintegrates prior to the assembly of GV ODVs (Goldberg et al., 2002).
Why is ODV‐EC27 a structural protein of the ODV given its involvement in G2/M phase arrest at late times in infection? ODV‐EC27 also coimmunoprecipitates with cyclin‐dependent kinase 6 (Cdk6) (Belyavskyi et al., 1998). Cdk6 normally binds to cyclin D to promote cells to proceed from quiescent G0 and G1 phases to the DNA synthesis S phase (for review see Ewen, 2000). DNA viruses require cells to enter the S phase in order that nucleotides and other cellular resources are made available for virus DNA replication. Emphasis on S phase transition early in baculovirus infection is supported by studies of the baculovirus immediate early gene, IE‐2, which independently causes cells to enter S phase arrest (Prikhod'ko et al., 1999a). Curiously, IE‐2 has not been detected in the ODV.
ODV‐EC27 complexes with cellular PCNA and possibly viral PCNA in nucleocapsids of BV and ODV (Belyavskyi et al., 1998). In addition to being involved in DNA synthesis and repair, PCNA has also been shown in mammalian models (Xiong 1993, Zhang 1993) and insect models (Kisielewska et al., 2005) to form complexes with cyclin D and CDKs to promote cell cycle shifts into S phase (for review see Maga and Hubscher, 2003). The viral PCNA is nonessential for baculovirus replication but appears to enhance infectivity (Iwahori 2002, Iwahori 2004). Providing the PCNA cell cycle factors may provide an advantage for ODVs as cellular PCNA is present in low abundance in insect midgut cells (Zudaire et al., 2004).
Yeast two‐hybrid experiments revealed that ODV‐EC27 binds directly with nucleocapsid proteins BV/ODV‐C42 and PP78/83 (Braunagel et al., 2001). As mentioned earlier, PP78/83 is a nucleocapsid base‐associated structural protein. BV/ODV‐C42 was identified to contain the canonical pRB‐binding motif (LXCXE) (Braunagel 2001, Forng 1999). This may be significant for association of ODV‐EC27 with pRB (Belyavskyi et al., 1998). The LXCXE motif is however only conserved among three BV/ODV‐C42 homologues (AcMNPV, BmNPV, and RoMNPV).
There are likely more ODV‐associated protein involved in shifting the cell cycle that have not been characterized. The 13.3‐kDa predicted product of AcMNPV ORF102 is may be one of those proteins. A BLAST search of this protein's sequence identified some similarity to NIMA kinases. NIMA is an acronym for Never In Mitosis gene A which is a type of protein involved in regulation of the cell cycle (Noguchi 2002, Noguchi 2004).
G. Apoptosis Inhibitor Proteins in the Nucleocapsid
One of the primary strategies that cells use to evade virus infection is a self‐destructive response called programmed cell death or apoptosis. This altruistic behavior sacrifices the virus‐infected cell to mitigate virus replication and spread throughout the organism. Insect midgut epithelial cells are easily triggered to undergo apoptosis not only from response to viral infections but also from their routine turnover in the midgut.
Baculoviruses produce two classes of proteins that can prevent cells from undergoing apoptosis, P35 (Clem et al., 1991) and inhibitors of apoptosis (IAP) type (Thiem and Chejanovsky, 2004) (for review see Clem, 1995). Baculoviruses frequently encode both classes of p35 and iap genes and even multiple copies. The P35 and IAP proteins are synthesized abundantly at the onset of infection due to strong early promoters. Given the obvious emphasis baculoviruses place on apoptosis inhibitors, it is surprising that P35 and IAP have not been localized in ODV or BV. This may have something to do with localization of P35 and IAP in the cytoplasm and assembly of nucleocapsids in the nucleus.
The nucleocapsid‐associated baculovirus protein P33 may be involved in apoptosis given that this protein binds to murine or human p53 (Prikhod'ko et al., 1999b). The cellular p53 protein is involved in apoptosis and cell cycle regulation (for review see Bai 2005, Collot‐Teixeira 2004). Cosynthesis of P33 with the cellular protein p53 in insect cells leads to apoptosis (Prikhod'ko et al., 1999b). The P33 protein appears essential for baculovirus infection (Prikhod'ko et al., 1999b), and existence of an insect homologue to p53 (Jin et al., 2000) leads to the possibility that P33 is involved in mechanisms which target p53 in order to inhibit apoptosis or permit cell cycle progression. P53 is a DNA‐binding protein and it is possible that P33 associates with host P53 proteins on the viral genome in nucleocapsids. The apoptosis observed with the coexpression of p33 and p53 would be inhibitory to virus infection and thus the virus likely retains p33 to interact with additional proteins.
It was hypothesized that baculovirus ring finger proteins like CG30, IE‐2, PE38, BmNPV ORF35 (AcMNPV ORF44), IAP1, and IAP2 were ubiquitin ligases that may catalyze the ubiquitination and destruction of cellular p53 (Imai et al., 2003). Herpesviruses and adenoviruses shift the cell cycle for DNA replication by employing virus‐encoded ring finger/ubiquitin ligases to targeting cellular P53 for ubiquitination by viral (Boutell 2003, Weger 2002). Only IAP2, IE‐2, and PE38 had ubiquitin ligase activities and such activities were inconclusive for CG30 (Imai et al., 2003). CG30 is the only protein among these that have been localized to ODVs.
H. Nucleocapsid‐Associated Proteins Involved in Transcriptional Activation
The IE‐1 protein is not only involved in DNA replication but is also a highly conserved transcriptional activator of viral genes (Choi 1995a, Choi 1995b, Choi 1995c, Kremer 1998, Passarelli 1993) and is also a required component of viral DNA replication complex (Kool 1994, Lu 1995b). IE‐1 predominantly localizes in the virogenic stroma where DNA replication occurs (Kawasaki et al., 2004). IE‐1 also colocalizes with VP39 in the virogenic stroma (Kawasaki et al., 2004) and thus is present in regions of capsid assembly. The ie‐1 gene is strongly expressed early in infection and its transcripts can be detected within 20 minutes postinfection (Kovacs et al., 1991). One the potential roles of nucleocapsid‐associated IE‐1 may be to transcriptionally activate the expression of early viral genes immediately after the genome has been unpackaged in the nucleus of a new host cell.
The nucleocapsid‐associated protein PNK/PNL (polynucleotide kinase/polynucleotide ligase) is an 80‐kDa protein that was first identified as a unique gene in the genome of AcMNPV (Ayres et al., 1994). The pnk/pnl gene is a nonconserved auxiliary gene and is not present in many baculoviruses including variants of AcMNPV such as AcMNPV 1.2, GmMNPV, and BmNPV (Yanase et al., 2000). The pnk/pnl gene is expressed early in infection and is nonessential for virus replication (Durantel et al., 1998). RoMNPV is the only other baculovirus known to encode for this 80‐kDa peptide. It was shown that PNK/PNL has three functionally active domains with kinase, phosphatase, and RNA ligase activities, respectively (Martins and Shuman, 2004). Speculation was made that PKN/PNL enabled the virus to evade an RNA damage‐based host defense.
PNK/PNL could be involved in the RNA splicing and repair activities that occur with the expression of the immediate early transcription factor gene ie0. The ie0 gene is the only known early baculovirus gene to be spliced (Kovacs 1991, Pearson 1997, Theilmann 2001) and it plays an essential role establishing baculovirus infection (Pearson 1997, Stewart 2005). The ie0 gene results from the splicing of a small exon onto the 5′ end of the ie‐1 gene (Chisholm and Henner, 1988). Expression of ie0 occurs very rapidly in the first few hours of infection and then diminishes while ie‐1 expression continues late into the virus replication cycle (Kovacs et al., 1991). At the beginning of infection, there is likely an immediate need for robust RNA‐splicing activities to ensure translation of IE0. The capsid‐associated PNK/PNL protein may be present to augment these cellular splicing activities. This may be necessary in some cell types that the virus infects. The ratios of IE0 and IE‐1 also affect baculovirus host specificity (Lu 1996, Pearson 1997, Stewart 2005) and thus PNK/PNL may be playing a role in specificity through RNA splicing.
The CG30 protein is a nucleocapsid‐associated protein with a very distinctive C‐terminal C3HC4 zinc ring finger motif, an N‐terminal leucine zipper motif (Thiem and Miller, 1989a). The leucine zipper and zinc finger are considered DNA‐binding motifs; it is likely that CG30 is binding to viral genomic DNA within nucleocapsids.
The CG30 protein is nonessential for virus replication (Passarelli and Miller, 1994) and was suggested to be involved in transcriptional activation due to the presence of a central acidic domain (Passarelli 1994, Thiem 1989a). Oddly, the deletion mutant of CG30 was slightly more orally infectious and produced more BV (Passarelli and Miller, 1994). Given its immediate cost on the virus, cg30 must be an auxiliary gene with long‐term evolutionary benefits such as host range. Homologues to the cg30 gene are present only in NPVs and in all cases, the cg30 gene is downstream and in the same orientation as the major capsid protein encoding gene vp39. The cg30 gene is transcribed from an early promoter and late vp39 transcripts are bicistronic such that they include the cg30 ORF in addition to the vp39 ORF (Blissard 1989, Lu 1996, Thiem 1989a). There is no direct evidence that CG30 is translated from this late transcript; however, only 2 bp separate the end of the vp39 ORF from the beginning of the cg30 ORF and from what is known of baculovirus bicistronic translation (Chang and Blissard, 1997), CG30 is likely synthesized in lesser amounts but at the same time as VP39. This timing of production would provide CG30 proteins for inclusion with nucleocapsids.
V. ODV Envelope and Tegument Proteins
A. ODV Proteins Interacting with the Tegument
Tegument proteins are defined by viral fractionation techniques involving several detergents. In these techniques, ODVs are treated with detergents such as Nonidet P‐40 (NP‐40) and the soluble proteins are defined as envelope proteins and the remaining insoluble proteins are assumed to be nucleocapsid proteins. Tegument proteins are solublized by NP‐40 due to loss of viral envelopes retaining them within the virions. Discriminating ODV integral envelope proteins from tegument proteins can be done using phase separation and the detergent Triton X‐114 (TX‐114) (Bordier, 1981). The tegument protein GP41 is released from ODVs by NP‐40 detergent (Pan 2005, Whitford 1992), and some have suggested GP41 is an integral ODV envelope protein (Pan et al., 2005). However, GP41 partitions entirely into the aqueous phase in TX‐114 detergent separation experiments (Whitford and Faulkner, 1992). Hidden Markov analysis (Krogh et al., 2001) of all GP41 homologues found no predicted transmembrane domains.
The GP41 protein is also an O‐glycosylated protein (Jain 2004, Pan 2005, Whitford 1992) and to our knowledge is the only O‐glycosylated baculovirus protein. The significance of this to the function of GP41 is unknown. The only functional studies of GP41 suggest this ODV structural protein affects BV production. A Ts mutant of GP41 blocked nucleocapsid exit from the nucleus at Ts conditions (33°C) and prevented formation of BV (Olszewski and Miller, 1997b). It was suggested that presence of GP41 in the ODV tegument was “adventitious” and was the result of the proteins presence in the nucleoplasm. This may be a premature interpretation of the role of GP41. This protein is conserved among all baculovirus genomes sequenced so far. The hymenopteran NeseNPV and NeleNPV baculoviruses contain gp41 gene homologues (Garcia‐Maruniak 2004, Lauzon 2004) and these viruses appear to produce only ODV. Earlier observations could have been the result of a Ts mutant GP41 inappropriately interacting with nucleocapsids exiting the nucleus. ODVs are not occluded in wt AcMNPV at 33°C (Lee and Miller, 1979) and thus the role of GP41 in the ODV could not be examined for GP41 Ts mutants.
ODV‐EC43 was first characterized in the baculovirus HaSNPV (Fang et al., 2003) and was found to be localized both in the ODV nucleocapsid and in the envelope. Some homologues of this protein contain transmembrane domain motifs, although the majority do not. ODV‐EC43 is conserved among all baculovirus genomes and is the predicted product of AcMNPV ORF109. ODV‐EC43 was also detected in the ODVs of AcMNPV (Braunagel et al., 2003).
P91 is a highly conserved baculovirus protein that may be in the ODV tegument cross linking the virion envelope with nucleocapsids (Russell and Rohrmann, 1997). P91 is present in the OpMNPV ODV as 91‐ and 102‐kDa molecular weight forms. P91 is not specific to the ODV and is also found in the BV as single 91‐kDa form. The homologues to this protein consistently contain highly hydrophobic N‐terminal transmembrane motifs similar to ones present in ODV other envelope proteins (Hong 1997, Slack 2001). The localization pattern of P91 is in the nuclear ring zone (Kawasaki 2004, Russell 1997) similar to what is observed for ODV envelope proteins such as ODV‐E66 (Hong et al., 1994) and P74 (Slack et al., 2001). Immuno‐EM images showed association of P91 with envelopes and with nucleocapsids (Russell and Rohrmann, 1997). P91 could not be dissociated completely from ODV virions by NP‐40 detergents or by disulfide reducing agents. This is thought to be due to unknown covalent bonds between P91 and nucleocapsid proteins (Russell and Rohrmann, 1997).
B. ODV Envelope Proteins
ODV envelope proteins are more diverse in composition compared to those of BV envelopes. ODV envelope proteins are playing biological roles in ODV occlusion and interaction with the midgut. ODV envelope proteins include a group of proteins called per os infectivity factors (PIFs). The core conserved ODV envelope proteins are ODV‐E18, ODV‐E56, P74, PIF‐1, PIF‐2, Ac115 (PIF‐3), and Ac150 (PIF‐4). These proteins are shown as components of the ODV envelope in Fig. 8 . ODV‐E18 is listed as not to be present in the mosquito baculovirus CuniNPV (Afonso et al., 2001). However, CuniNPV ORF31 appears to be a distant homologue to odv‐e18. It is located upstream of odv‐ec27, and its protein product is predicted to have a hydrophobicity profile similar to ODV‐E18. In addition to the universally conserved ODV envelope proteins, ODV envelope proteins ODV‐E66 and ODV‐E25 are in all lepidopteran baculoviruses.
Fig 8.
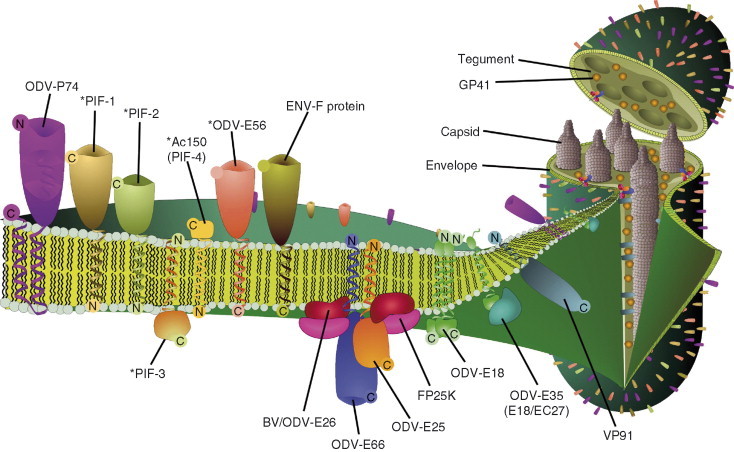
Proteins of the ODV envelope and tegument. The illustration represents a dissected view of the structure of the ODV with emphasis on the envelope. The figure is in the context of AcMNPV. The major ODV‐associated proteins with predicted transmembrane domains are shown in the ODV envelope. The “*” indicates that there is no data to suggest the orientation of these proteins in the ODV envelope. FP25K, which is capsid associated, also appears on the ODV envelope due to its apparent interaction with the transmembrane domains of ODV‐E66 and ODV‐E25. Tegument proteins are also illustrated in the main virion on the right side.
C. ODV‐E18, ODV‐E35, and ODV‐EC27
As covered earlier, ODV‐EC27 is a nucleocapsid‐associated protein involved in altering the host cell replication cycle. The AcMNPV homologue of ODV‐EC27 lacks a transmembrane domain motif and we are reluctant to classify ODV‐EC27 as an envelope protein. In the NPVs, the odv‐e18 gene is found immediately upstream and in the same orientation as the odv‐ec27 gene. ODV‐E18 is an integral ODV envelope protein and all homologues have N‐terminal transmembrane motif regions. ODV‐E18 and ODV‐EC27 were first characterized as products from AcMNPV (strain E2) (Braunagel et al., 1996b). For this virus, the odv‐e18 gene is 269 bp long and encodes for a predicted 9.5‐kDa protein product. However, the ODV‐E18 protein migrates at an estimated size of 18 kDa in reducing SDS‐PAGE gels (Braunagel et al., 1996b). Post secondary modification has been ruled out and it has been proposed that ODV‐E18 is a strongly associated homodimer (Braunagel et al., 1996b). ODV‐EC27 is translated from the 872‐bp ORF, 15 bp downstream of the ODV‐E18 ORF. The ODV‐EC27 gene product has a predicted mass of 33 kDa but migrates at 27 kDa in SDS‐PAGE.
Immuno‐based experiments suggest that ODV‐E18 and ODV‐EC27 produce a chimeric fusion protein or a heterodimer called ODV‐E35 (Braunagel et al., 1996b). In AcMNPV, ODV‐E18, and ODV‐EC27, ORFs are only separated by 15 bp and they are transcribed as a bicistron mRNA. The ODV‐E18/ODV‐EC27 bicistron is conserved among the following baculoviruses (the distance between ORFs is indicated in brackets); AcMNPV (15 bp), AdhoNPV (31 bp), BmNPV (14 bp), CfDEFNPV (14 bp), HearSNPV (14 bp), HzSNPV (14 bp), EppoNPV (14 bp), NeleNPV (14 bp), NeseNPV (25 bp), OpMNPV (26 bp), LdMNPV (17 bp), CfMNPV (28 bp), ChchNPV (36 bp), MacoNPV‐A (38 bp), MacoNPV‐B (38 bp), RaouNPV (16 bp), SeMNPV (54 bp), SpltNPV (31 bp), and TnSNPV (40 bp).
The genetic linkage of ODV‐E18 and ODV‐EC27 is not present in GV baculoviruses. In these viruses, the genes are separated by great distances: AgseGV (NC_005839) (77,419 bp), AdorGV (NC_005038) (57,419 bp), CyleGV (65,249 bp), CypoGV (69,143 bp), PlxyGV (54,987 bp), PhopGV (69,183 bp), and XecnGV (96,723 bp). We noted that the ODV‐EC27 homologues of these viruses all contain strong transmembrane motifs in their C‐terminal regions that are not present in the NPV homologues. It is possible that ODV‐EC27 localizes exclusively in the ODV envelopes of GVs.
Braunagel 1996a, Braunagel 1996b suggested that ODV‐E35 is produced from translational frameshifting between ODV‐E18 and ODV‐EC27. Translational frame shifting in the context of baculovirus infection has been observed before (Williams et al., 1989). The combined molecular weights of 9‐kDa monomeric E18 and 27‐kDa EC27 would produce a protein migrating at 36 kDa.
ODV‐E35 is an integral ODV envelope protein and we speculate that ODV‐E35 may be employed by the virus to localize ODV‐EC27 peptide domains on the tegument side of the envelope. As mentioned earlier, ODV‐EC27 binds to a number of nucleocapsid‐associated proteins including BV/ODV‐C42 and PP78/83 (Braunagel et al., 2001). ODV‐E35 may be stabilizing the ODV virion structure by cross‐linking the envelope with nucleocapsid bound proteins.
D. ODV‐E66 and ODV‐E25
ODV‐E25 is an integral ODV envelope protein that is N‐terminally anchored in the envelope (Hong 1997, Russell 1993). The single N‐terminal transmembrane motif of ODV‐E25 is conserved among homologues. ODV‐E25 is also found in BV but is comparatively more abundant in ODV (Russell and Rohrmann, 1993).
ODV‐E66 is an integral ODV envelope protein that like ODV‐E25 is N‐terminally anchored in envelope (Hong 1997, Russell 1993). ODV‐E66 is however exclusively found in the envelope (Hong et al., 1994). In addition to a conserved N‐terminal transmembrane motif, some ODV‐E66 homologues have internal transmembrane motifs. However, they are not conserved in position and do not have as high transmembrane probability scores. Yeast two‐hybrid and immunoprecipitation experiments showed that the exposed C‐terminal regions of ODV‐E66 and ODV‐E25 interact (Braunagel et al., 1999). This would argue that ODV‐E66 and ODV‐E25 have common orientation in the ODV envelope. Experiments with membrane‐trafficking proteins also show that ODV‐E25 and ODV‐E66 have common orientation in the ER membrane (Saksena et al., 2004).
Data has been published addressing the orientation of ODV‐E66 and ODV‐E25 in the ODV envelope. In a study where the transmembrane region of ODV‐E66 was fused to the N‐terminus of GFP, the resulting protein chimera was exposed on microvesicle surfaces (Braunagel et al., 2004). This data was extrapolated to suggest that ODV‐E66 is exposed on the cytoplasmic side of the ER and the nucleoplasmic side of the INM. Several indirect lines of evidence point to ODV‐E66 and ODV‐E25 being oriented on the inner side of the ODV envelope. First, ODV‐E25 is not completely released from ODV by NP‐40 detergent and remains residually associated with the nucleocapsid (Russell and Rohrmann, 1997). Second, yeast two‐hybrid and immunoprecipitation show that ODV‐E66 binds to the major capsid protein VP39 (Braunagel et al., 1999). From this we would suggest that ODV‐E66 and ODV‐E25 are involved in ODV capsid envelopment. A caveat to our prediction of ODV‐E66 orientation is that the ODV‐E66 of AcMNPV may be cleaved by trypsins in ODV preparations. This would indicate surface exposure of ODV‐E66. When this 66‐kDa protein was purified from OBs, a smaller 60‐kDa molecular weight form appeared (Hong et al., 1994). N‐terminal sequencing suggested that this was a tryptic digest product of ODV‐E66 (Hong et al., 1994). The trypsin cleavage site is not conserved among ODV‐E66 homologues and the authors of this study did not speculate on the origin of the trypsin activity present in ODV preparations.
Deletion mutants of ODV‐E66 demonstrate that this protein is not required for BV production (Russell and Rohrmann, 1997). Insect oral bioassay data has not been published on these viruses so it is not known if ODV‐E66 is an essential PIF. Deletion mutants have not been made for ODV‐E25. If we assume orientation inside the virion, ODV‐E66 and ODV‐E25 are not likely to be involved in ODV attachment or fusion to the midgut.
E. Trafficking of ODV Envelope Proteins, FP25K and BV/ODV‐E26
Studying ODV envelope proteins is made more difficult by the complexity of the translocation of viral proteins into the ODV envelope. The ER, outer nuclear membrane (ONM), INM, and nuclear pore complex are a continuous network of membranes exploited by baculoviruses to deliver proteins to the ODV envelope (Braunagel et al., 2004). The INM is presumed to be the source of ODV envelopes (Hong 1994, Hong 1997). ODV envelope proteins which begin in the ER are targeted to the INM which blebs off as ODV envelope precursor microvesicles in the nuclear ring zone (Hong 1994, Hong 1997).
The major ODV envelope proteins do not have N‐terminal membrane insertion signal peptides and ODV envelope proteins are almost never N‐glycosylated (Braunagel et al., 2004). One exception is the Spodoptera litura MNPV ORF137 ODV envelope protein that has been shown to be N‐glycosylated (Yin et al., 2003). There are few other viral homologues to this protein. F protein is an N‐glycosylated protein with an N‐terminal signal peptide and although it is associated in the ODV, F protein is considered specific for the BV. The primary ODV envelope proteins have prominent hydrophobic transmembrane motifs that are involved in membrane insertion, anchoring, and localization in the ODV envelope (Hong 1994, Hong 1997, Slack 2001). ODV envelope proteins exhibit the ability to spontaneously insert themselves into membranes (Hong 1997, Slack 2001, Yao 2004). These hydrophobic transmembrane motifs have been termed INM sorting motifs (Braunagel et al., 2004).
The proteins FP25K and BV/ODV‐E26 (Beniya 1998, Braunagel 1999) may play an important role in the trafficking of ODV envelope proteins. FP25K associates with BV and ODV nucleocapsids (Braunagel et al., 1999) and BV/ODV‐E26 associates with BV and ODV envelopes (Beniya et al., 1998). BV/ODV‐E26 has been localized on the plasma membrane, BV envelopes, INM microvesicles, and ODV envelopes (Beniya et al., 1998). BV/ODV‐E26 lacks transmembrane domain motifs and is released from ODVs by NP‐40 detergent treatment (Beniya et al., 1998). Like the tegument protein GP41 (Whitford and Faulkner, 1992), BV/ODV‐E26 fractionates into the aqueous phase of TX‐114 detergent phase partition assays (Beniya et al., 1998). BV/ODV‐E26 is thus a peripheral membrane protein and interaction with FP25K would orient BV/ODV‐E26 to inner side of the ODV envelope. A small proportion of BV/ODV‐E26 protein remains with purified BV and ODV nucleocapsids (Beniya et al., 1998). Presumably this is due to association with FP25K.
FP25K studies began with a few polyhedra (FP) mutant baculovirus phenotypes that occurred during high titer, serial passage in cell culture (Fraser 1982, Fraser 1983, Stairs 1981). Many FP mutant baculovirus phenotypes resulted from the disruption of the fp25k gene (Beames and Summers, 1989), thus the nomenclature “few polyhedra 25K” or FP25K. The lack of polyhedra in FP25K mutants was first correlated with the inappropriate localization of polyhedrin (Jarvis et al., 1992) and decreased polyhedrin gene transcription (Harrison et al., 1996). FP25K mutants failed to translocate ODV‐E66 to INM vesicles (Rosas‐Acosta et al., 2001) and ODV‐E66 peptide became significantly less abundant while the mRNA transcripts of odv‐e66 were unchanged (Braunagel 1999, Rosas‐Acosta 2001). Immunoprecipitation and yeast two‐hybrid analysis revealed interaction of FP25K with ODV envelope proteins, ODV‐E25 in addition to ODV‐E66 (Braunagel 1999, Katsuma 1999).
Despite being localized in BV nucleocapsids, FP25K is not required for BV production. Absence of FP25K results in increased abundance of GP64 (Braunagel et al., 1999) and increased production of BV (Harrison 1995, Wu 2005). BV budding is driven by GP64 accumulation on the cell membrane (Oomens and Blissard, 1999). Coinciding with GP64 accumulation, FP25K deletion mutants also produce more BV/ODV‐E26 (Braunagel et al., 1999). Homologues to BV/ODV‐E26 are exclusive to group I NPVs that use GP64 as their primary ENV protein. However, BV/ODV‐E26 has not been shown to interact with GP64 and paradoxically, FP25K coimmunoprecipitates GP64 (Braunagel et al., 1999).
It is interesting that FP25K deletion mutants also affect the transport of the viral protein V‐CATH (Katsuma et al., 1999). V‐CATH is cathepsin‐like proteinase involved in the lytic release of OBs from virus‐infected cells and from host tissues (Ohkawa 1994, Slack 1995, Suzuki 1997). Both V‐CATH and GP64 are N‐glycoproteins with membrane insertion signal peptides and are likely sharing common pathways in the ER. Normally, V‐CATH copurifies with BVs (Lanier et al., 1996) but is apparently not being secreted in FP25K mutants.
It has been suggested that FP25K mutant effects may be “stoichiometric” in nature (Braunagel et al., 1999) and not directly caused by FP25K mutation. FP25K is definitively directly involved in the transport of membrane proteins. Covalent cross‐linking experiments revealed that FP25K and also BV/ODV‐E26 bind to the transmembrane region sorting motifs of ODV‐E66 and ODV‐E25 (Braunagel 2004, Saksena 2004). Interaction between BV/ODV‐E26 and FP25K may determine the trafficking of BV bound envelope proteins (GP64) and ODV bound envelope proteins (ODV‐E25, ODV‐E66). The mechanism by which FP25K and BV/ODV‐E26 traffic proteins may involve actin or cystoskeleton filaments. FP25K has been identified to contain three myosin‐like regions and one actin‐binding motif (Beniya 1998, Braunagel 1999). BV/ODV‐E26 also has a tropomysosin motif. The nucleocapsids of FP25K deletion mutants were not efficiently enveloped (Katsuma et al., 1999). Given the role FP25K plays in ODV‐E66 translocation, this indirectly supports the premise that ODV‐E66 is involved in ODV capsid envelopment.
The mosquito baculovirus CuniNPV (Afonso et al., 2001) and the sawfly baculoviruses NeleNPV (Lauzon et al., 2004) and NeseNPV (Garcia‐Maruniak et al., 2004) lack ODV‐E25 and ODV‐E66 and yet the major ODV envelope proteins ODV‐E56, PIF‐1, PIF‐2, and P74 are present in these viruses. This would imply that FP25K is specifically involved in translocation of ODV‐E66 and ODV‐E25 and not required for transport of the other ODV envelope peptides.
F. ODV‐E56
ODV‐E56 is another protein that is exclusively associated with the envelope (Braunagel 1996a, Theilmann 1996). The OpMNPV ODV‐E56 homologue migrated in SDS‐PAGE as 46‐ and 43‐kDa proteins (Theilmann et al., 1996) and the AcMNPV ODV‐E56 homologue migrated as a 67‐ and 56‐kDa proteins (Braunagel et al., 1996a). Both homologues are posttranslationally modified as the OpMNPV and AcMNPV homologues should migrate at 40 to 41 kDa, respectively. N‐Gycosylation was ruled out (Braunagel et al., 1996a) and proteolytic cleavage has proposed to the potential source of multiple forms (Theilmann et al., 1996).
ODV‐E56 has a strong transmembrane motif on its C‐terminus that is required for translocation into the nucleus and localization into ODV envelopes (Braunagel et al., 1996a). It is not required for BV production, ODV assembly, or occlusion (Braunagel et al., 1996a). Bioassay data have not been presented on ODV‐E56 deletion mutant viruses. The transmembrane domain prediction profile of ODV‐E56 is similar to P74. ODV‐E56 also has a central region that contains a membrane insertion signal peptide motif and a single C‐terminal transmembrane domain. It is not established if ODV‐E56 is exposed on the ODV surface. In our model, we placed it to be exposed on the surface of the ODV and have suggested that only the C‐terminal transmembrane domain is utilized by this protein for envelope insertion.
G. P74 (PIF‐0)
P74 has been shown to be an ODV envelope protein by viral fractionation (Faulkner et al., 1997) and by EM (Rashidan et al., 2005). P74 was the first ODV envelope protein to be demonstrated essential for oral infection (Kuzio 1989, Yao 2004, Zhou 2005). Perhaps P74 should be renamed PIF‐0 or P74‐PIF‐0 as it was the first PIF to be discovered. The P74 protein is C‐terminally anchored in the ODV envelope (Slack et al., 2001) and is N‐terminally exposed on the ODV surface (Faulkner et al., 1997). P74 has an internal transmembrane domain and a C‐terminal double transmembrane domain. The internal transmembrane domain has characteristics of an internal transmembrane insertion signal peptide with positively charged amino acids on one side and negatively charged amino acids on other side (Alberts et al., 1994). Experimental data suggest that the central transmembrane motif is not inserted into membranes and remains buried in the protein. A chimeric P74‐GFP protein lacking only the P74 C‐terminal double transmembrane domain is soluble and not associated with membranes (Slack et al., 2001). The double transmembrane domain motif on the C‐terminus of P74 is odd because of the closeness of these domains and suggests a “hairpin” or folded transmembrane domain that may spontaneously insert into membranes. It was demonstrated that bacterially expressed and purified P74 proteins could spontaneously insert into ODVs and rescue oral infectivity of a P74 null virus (Yao et al., 2004). This work also indicates that P74 does not require post secondary modification to be functional.
Evidence from several studies suggests P74 is an ODV attachment protein that binds virions to midgut cells (Haas‐Stapleton 2004, Yao 2004). It has been known for over a decade that ODVs bind to midgut protein receptors (Horton and Burand, 1993) and data have shown that P74 binds to a 30‐kDa insect midgut receptor protein (Yao et al., 2004).
It was shown that P74 was proteolytically cleaved on its N‐terminus by insect midgut trypsins (Slack and Lawrence, 2005). Earlier studies did not observe this phenomenon because the antisera were specific for the N‐terminal cleaved region of P74 (Faulkner 1997, Slack 2005). Among all P74 homologues including the “pseudobaculovirus” Hz‐1, there is one conserved trypsin cleavage site corresponding to R156 in AcMNPV. The position of this site correlates with the size of the P74 cleavage product produced after incubation in the midgut trypsins. The P74 is the most complex of the ODV envelope proteins. We speculate that that the central transmembrane domain region may be involved in membrane fusion and is perhaps exposed by protease cleavage.
H. Proteases, P74, and Oral Infectivity
Data suggesting that ODV envelope peptide P74 may be cleaved by insect midgut trypsins leads to questions about the role of trypsins in oral infection. The role that insect midgut proteases play in baculovirus infection is not been well investigated. The lepidopteran midgut is rich in trypsin and chymotrypsin (Johnston 1991, Terra 1994) and it is likely that baculoviruses have evolved to tolerate and exploit these proteases just as they have for midgut alkalinity. Our work suggests that supplementation of baculoviruses with trypsin enhances oral infectivity and addition of soybean trypsin inhibitors decreases oral infectivity. Tryptic cleavage activation is documented for other viruses including Coronavirus (Frana et al., 1985), Rotavirus (Vonderfecht et al., 1988), Sendai virus (Muramatsu and Homma, 1980), and Vaccinia virus (Ichihashi and Oie, 1982). Tryptic activation occurs in other entomopathogens as Bacillus thuringiensis (Bt). The midgut binding Bt toxin protein requires trypsin cleavage to be activated (Miranda et al., 2001).
Occlusion is a complex viral evolutionary development and it can be assumed that baculovirus ancestors were not occluded. These nonoccluded ancestors may have required alkaline proteolytic activation to infect midgut tissues. Midgut alkalinity is the primary trigger for the release of ODVs from OBs. Why might baculoviruses retain the need for trypsin activation? One possible reason is that viral attachment protein receptor‐binding domains may need to be shielded from the OB matrix proteins. Another possibility is that hydrophobic membrane fusion elements must be exposed during ODV assembly. When N‐terminally truncated forms of the ODV envelope protein P74 are synthesized in insect cells, the protein becomes trapped nonspecifically in regions of the cell (Slack et al., 2001).
Baculovirus OB‐associated alkaline serine proteases (Eppstein 1975, Eppstein 1975, Langridge 1981, Maeda 1983) may be playing a synergistic role in infection by ensuring activation of released ODVs. The OB‐associated proteases are of host origin and are not present in tissue culture derived OBs (McCarthy and Dicapua, 1979). It is notable that the hymenopteran baculoviruses NeleNPV (Lauzon et al., 2004) and NeseNPV (Garcia‐Maruniak et al., 2004) encode for serine protease genes. These “ancestral” baculoviruses do not appear to have the BV phenotype and their replication is restricted to the midgut. As the infection ensues, the midgut physiology is likely affected and protease production may be compromised. Perhaps these viruses are supplementing insect midgut trypsins with viral trypsin in order to infect more tissues of the midgut in secondary rounds of infection.
I. PIF‐1 and PIF‐2
In the past, ODV‐associated proteins were discovered by chance as the viral genes were methodically characterized. A per os bioassay functional genomic approach has been used to identify PIF proteins that are essential for oral infection (Kikhno 2002, Pijlman 2003). The first PIFs to be identified this way were PIF‐1 (originally PIF) and PIF‐2 (Kikhno 2002, Pijlman 2003). PIF‐1 was characterized as the ORF7 gene product of the Spodoptera littoralis nucleopolyhedrovirus (SpliNPV) (Kikhno et al., 2002). PIF‐2 was characterized as the ORF35 gene product of the SeMNPV (Pijlman et al., 2003). The pif‐1 and pif‐2 gene homologues are ORF119 and ORF22 of AcMNPV, respectively. The 60‐kDa PIF‐1 protein and the 44‐kDa PIF‐2 protein contain prominent N‐terminal transmembrane motifs that is likely to serve as transmembrane anchors. When we analyzed all homologues of these two proteins for transmembrane motifs (Krogh et al., 2001), we identified only a single N‐terminal transmembrane motif for both of these proteins. These hydrophobic N‐terminal transmembrane motifs are similar to the INM sorting motifs present on ODV‐E66 and ODV‐E25 (Braunagel 2004, Hong 1997).
PIF‐1 was confirmed to be an integral membrane protein of the ODV envelope (Kikhno et al., 2002) but the exposure of the C‐terminal portion of PIF‐1 on the ODV surface has not been shown. There are presently no data published to show that SeMNPV PIF‐2 is a component of the ODV; however, the AcMNPV homologue was detected in ODVs (Braunagel et al., 2003). Interestingly, the AcMNPV ORF119 PIF‐1 protein product was not identified in this study as an ODV component. PIF‐1 is produced in low abundance in SpliNPV (Gutierrez et al., 2004) and may not be easily detected in AcMNPV ODV preparations. PIF‐1 and PIF‐2 have been shown to be involved in ODV binding to midgut cells (Ohkawa et al., 2005). This would suggest that these proteins are exposed on the surface of the ODV along with P74.
J. PIF‐3 and 11K Proteins
As has been emphasized throughout this review that the ODV is a highly complex virus phenotype. The number of PIF protein types associated with the ODV continues to grow. PIF‐3 is a 23‐kDa protein encoded by AcMNPV ORF Ac115 (Ohkawa et al., 2005). The protein is conserved among all baculoviruses and is essential for oral infection. It has a good N‐terminal transmembrane domain and could be an integral ODV envelope protein. However, PIF‐3 does not affect ODV binding or envelope fusion with midgut cells (Ohkawa et al., 2005). It was noted by Ohkawa et al. (2005) that ODV infection involves processes beyond attachment and fusion and that PIF‐3 may play a role in the translocation of ODV capsids along the microvillus. Perhaps PIF‐3 is exposed to the inside of the ODV envelope where it can interact with cytoskeletal elements.
Another group of proteins that play an enhancing role in oral infection are the 11K proteins (Lapointe 2004, Zhang 2005). Like the PIFs, these proteins are conserved among many baculovirus genomes and in addition, homologues are found in entomopoxviruses (Dall et al., 2001). The 11K protein homologues of AcMNPV are encoded by ORFs Ac145 and Ac150 (Lapointe et al., 2004). Their distinguishing feature is not so much amino acid sequence identity but the presence of C6 chitin‐binding motifs or peritrophin‐C domains that are common among mucins, peritrophins, and chitinases (Tellam et al., 1999). This pointed to the possibility that 11K proteins interact with and compromise the PM similar to enhancins (Lapointe et al., 2004). The experimental evidence suggests that the 11K protein enhancement of oral infection does not involve the PM (Zhang et al., 2005). In some circumstances, the Ac145 protein acts synergistically with the Ac150 protein (Lapointe et al., 2004) while in other cases Ac150 alone acts as an enhancer of oral infection (Zhang et al., 2005). Ac150 contains a prominent N‐terminal transmembrane domain and it is associated with BV and OBs (Lapointe et al., 2004). Immuno‐EM data showed that Ac150 could be an ODV envelope protein (Lapointe et al., 2004) and possibly a PIF‐4 given that it has been classified as a PIF (Zhang et al., 2005). However, the oral infectivity enhancement effects of Ac150 only occur with OBs and not with alkali liberated ODVs (Zhang et al., 2005). Perhaps Ac150 is exposed on the ODV surface and is involved in the timely release of ODVs from the OB protein matrix.
As in the case of PIF‐1, the proteins of PIF‐3 and Ac150 were not identified as components of the ODV when ODVs were analyzed directly for protein composition (Braunagel et al., 2003), which provides a baseline for studying the ODV, but it appears that functional genomics is a critical tool for discovering the proteins that are needed for oral infection.
K. Summary of ODV Envelope Proteins
Of the 44 proteins identified as components of the AcMNPV ODV (Braunagel et al., 2003), 8 have transmembrane domain motifs. Included among these are VP91, P74, ODV‐E56, ODV‐E66, ODV‐E25, ODV‐E18, PIF‐2, and F protein. PIF‐1, PIF‐3, and Ac150 (PIF‐4) would bring the number of potential ODV “integral” envelope proteins to 11. Deletion mutants or gene fusion disruption mutants have been generated for P74 (Kuzio et al., 1989), PIF‐1 (Kikhno et al., 2002), PIF‐2 (Pijlman et al., 2003), PIF‐3 (Ohkawa et al., 2005), Ac150 (Lapointe 2004, Zhang 2005), ODV‐E66 (Hong et al., 1997), and ODV‐E56 (Braunagel et al., 1996a). Relevant biological data relating to the requirement of ODV‐E66 and ODV‐E56 for oral infection have not been generated, but it may be predicted that these proteins could also be PIFs. P74 is the only ODV envelope protein that has been experimentally demonstrated to be exposed on the ODV surface (Faulkner et al., 1997). Our illustration of ODV envelope proteins (Fig. 8) is thus speculative and much work remains to be done to determine the orientation of the other ODV envelope proteins.
Considerable progress has been made using ODV envelope proteins to understand the morphogenesis of the ODV envelope from the INM (Pijlman et al., 2003). However, a model for how INM vesicles form the ODV envelope after entry into the nuclear ring zone has not been presented. We suggest that this process may be similar to the process that occurs with BV formation (Fig. 9 ). The lumen of the ER is continuous with the lumen between the ONM and INM. For BV formation, ER secretory vesicles containing GP64 and F protein are translocated (through the Golgi complex) to the cell membrane where they fuse. In these ER‐originating vesicles, GP64 and F protein are oriented toward the lumen to result in their orientation on the outside of the cell membrane. BV nucleocapsids then bud out acquiring these proteins as BV envelope proteins. For ODV formation, INM vesicles containing ODV envelope proteins are released into the nucleus. ODV nucleocapsids attach to these INM vesicles just as BV nucleocapsids attach to the cell membrane. It has been suggested that ODV‐E66 is exposed on the surfaces of INM vesicles (Pijlman et al., 2003). We propose that ODV‐E66 interacts directly with the nucleocapsids as they bind to INM vesicles. The ODV nucleocapsids essentially bud into INM microvesicles and are enveloped. Membrane proteins oriented on the luminal side of INM vesicles are consequently exposed on the surfaces of ODVs. Thus, F protein and P74 would be expected to be present on the luminal side INM vesicles prior to their becoming ODV envelope proteins. This is presently only a hypothesis and much work is still needed to understand the morphogenesis of the ODV.
Fig 9.
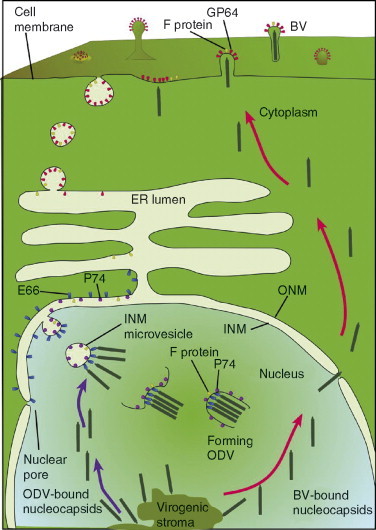
A simple model for baculovirus virion envelopment and envelope protein translocation. The BV and ODV forms in distinctly different locations in the cell. BV nucleocapsid must translocate through nuclear pores out of the nucleus, across the cytosol, and to the cell membrane. ODV nucleocapsids are retained within the nuclear ring zone. The formation of enveloped virions in the nucleus is unusual for viruses. However, there may be a common thread between the ODV and the BV morphogenesis. Cell surface proteins such as BV integral envelope fusion proteins (EFPs) are synthesized in the ER such that they are exposed in the ER lumen. EFPs are translocated to the cell surface in ER vesicles, which after sorting in the Golgi complex, fuse with the host cell membrane. This permits EFPs to be presented on the outside of BVs as they bud out of the cell. ODV virions may acquire their envelopes by “budding” into INM vesicles. The double nuclear membrane is continuous with the ER. In this model, ODV envelope proteins such as P74 and F protein would be displayed on the luminal side of the ER and nuclear double membrane. Envelope proteins such as E66 that may interact with nucleocapsids would be displayed on the nucleoplasmic side of INM vesicles.
VI. Conclusions
Most baculovirus research has been directed at studying the BV, which has been the direct path for several reasons. Experiments involving BV function can be done in cell culture while ODV studies are best when they include feeding bioassays with insects. For most laboratories, rearing insects is messy and the quality of animals can vary from even the best equipped facilities. Consequently, many ODV studies have been lacking due to the omission of an animal model. The media environment of cell culture is well defined while the animal model and particularly the midgut are infinitely more complex and have an extreme microenvironment for which the biology is still not fully understood. Finally, the ODV is a far more complex structure than the BV. Multiple capsids per virion, occlusion, more diverse viral protein composition, and the tegument all contribute to the complexity of ODV virions. The ODV‐associated proteins with functions involving viral DNA packaging, capsid translocation, cell cycle disruption, DNA replication, and DNA repair have been the easiest proteins to study given that their activities can be evaluated in insect cell culture.
ODV proteins that interact with the OB matrix are yet to be described. Occlusion is a unique biological phenomenon. Understanding the mechanism behind this could have important applications beyond baculovirus biology. It may be possible to engineer baculoviruses to occlude vaccines or biopharmaceutical products. The OB would be an ideal package of preservation as contained particles can be rapidly released with just a change in pH. Preliminary studies hint that it may be possible to alter or control the shape of OBs (Lin et al., 2000). This has the potential to lead to applications in nanobiotechnology.
ODV proteins that interact with the insect midgut have been more difficult to investigate. Simple experiments such as protease digest determination of the surface exposure of ODV envelope proteins have not been done despite the fact that many of these proteins have single transmembrane anchor regions. Significant progress in this area is anticipated. The availability of insect genomes and midgut cDNA/EST libraries will expedite the discovery of ODV receptors on the midgut. Indeed, may be through these studies that the ODV attachment protein(s) become characterized. No doubt, baculoviruses have evolved to target highly conserved midgut receptor proteins. Identifying these receptors will have implications in baculovirus biology, insect biology, and will potentially lead to new biocontrol technologies.
Acknowledgments
Part of this research was supported by a grant from Genome Canada through the Ontario Genomics Institute, the Biotechnology Strategy Fund of Canada, and the Biocontrol Network of Canada.
The authors would like to thank Hilary Lauzon for helpful discussions and Chris Upton for introducing the Viral Bioinformatics Resource Center, University of Victoria.
References
- Abbas M., Boucias D.G. Interaction between nuclear polyhedrosis virus‐infected Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae and predator Podisus maculiventris (Say) (Hemiptera: Pentatomidae) Environ. Entomol. 1984;13(2):599–602. [Google Scholar]
- Aboussekhra A., Wood R.D. Detection of nucleotide excision repair incisions in human fibroblasts by immunostaining for PCNA. Exp. Cell Res. 1995;221(2):326–332. doi: 10.1006/excr.1995.1382. [DOI] [PubMed] [Google Scholar]
- Ackermann H.W., Smirnoff W.A. A morphological investigation of 23 baculoviruses. J. Invertebr. Pathol. 1983;41(3):269–280. [Google Scholar]
- Adams J.R., Bonami J.R. Atlas of invertebrate viruses. In: Adams J.R., Bonami J.R., editors. “Atlas of InvertebrateViruses”. CRC Press; Boca Raton: 1991. [Google Scholar]
- Adams J.R., McClintock J.T. Baculoviridae, nuclear polyhedrosis viruses Part 1. Nuclear polyhedrosis viruses of insects. In: Adams J.R., Bonami J.R., editors. “Atlas of Invertebrate Viruses”. CRC Press; Boca Raton: 1991. pp. 87–180. Chapter 6. [Google Scholar]
- Adams J.R., Goodwin R.H., Wilcox T.A. Electron microscopic investigations on invasion and replication of insect baculoviruses in vivo and in vitro. Biologie Cellulaire. 1977;28(3):261–268. [Google Scholar]
- Afonso C.L., Tulman E.R., Lu Z., Balinsky C.A., Moser B.A., Becnel J.J., Rock D.L., Kutish G.F. Genome sequence of a baculovirus pathogenic for Culex nigripalpus. J. Virol. 2001;75:11157–11165. doi: 10.1128/JVI.75.22.11157-11165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B., Bray D., Lewis J., Raff M., Roberts K., Watson J.D. Intracellular compartments and protein sorting. In “Molecular Biology of the Cell”. In: Robertson M., Adams R., editors. 3rd Ed. Garland Publishing, Inc.; New York: 1994. pp. 551–594. Chapter 12. [Google Scholar]
- Andreadis T., Becnel J., White S. Infectivity and pathogencity of a novel baculovirus, CuniNPV from Culex nigripalpus (Diptera: Culicidae) for thirteen species and four genera of mosquitoes. Encyclopedia Entomol. 2003;40:512–517. doi: 10.1603/0022-2585-40.4.512. [DOI] [PubMed] [Google Scholar]
- Aravind L., Walker D.R., Koonin E.V. Conserved domains in DNA repair proteins and evolution of repair systems. Nucleic Acids Res. 1999;27(5):1223–1242. doi: 10.1093/nar/27.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif B. Structure of the viral genome. In: Doerler W., Bohm P., editors. “Molecular Biology of Baculoviruses”. Springer; Berlin: 1986. pp. 31–50. [Google Scholar]
- Ayres M.D., Howard S.C., Kuzio J., Lopez‐Ferber M., Possee R.D. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology. 1994;202:586–605. doi: 10.1006/viro.1994.1380. [DOI] [PubMed] [Google Scholar]
- Bai M., Papoudou‐Bai A., Kitsoulis P., Horianopoulos N., Kamina S., Agnantis N.J., Kanavaros P. Cell cycle and apoptosis deregulation in classical Hodgkin lymphomas. In Vivo. 2005;19(2):439–453. [PubMed] [Google Scholar]
- Baluchamy S., Gopinathan K.P. Characterization of a cyclin homolog from Bombyx mori nucleopolyhedrovirus. Virus Res. 2005;108(1–2):69–81. doi: 10.1016/j.virusres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Barbehenn R.V., Martin M.M. Peritrophic envelope permeability in herbivorous insects. J. Insect. Physiol. 1995;41:301–311. doi: 10.1016/s0022-1910(96)00098-4. [DOI] [PubMed] [Google Scholar]
- Beames B., Summers M.D. Location and nucleotide sequence of the 25k protein missing from baculovirus few polyhedra FP mutants. Virology. 1989;168(2):344–353. doi: 10.1016/0042-6822(89)90275-4. [DOI] [PubMed] [Google Scholar]
- Belyavskyi M., Braunagel S.C., Summers M.D. The structural protein ODV‐EC27 of Autographa californica nucleopolyhedrovirus is a multifunctional viral cyclin. Proc. Natl. Acad. Sci. USA. 1998;95(19):11205–11210. doi: 10.1073/pnas.95.19.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beniya H., Braunagel S.C., Summers M.D. Autographa californica nuclear polyhedrosis virus: Subcellular localization and protein trafficking of BV/ODV‐E26 to intranuclear membranes and viral envelopes. Virology. 1998;240(1):64–75. doi: 10.1006/viro.1997.8903. [DOI] [PubMed] [Google Scholar]
- Bergold G.H. The molecular structure of some insect virus inclusion bodies. J. Ultrastruct. Res. 1963;8:360–378. doi: 10.1016/s0022-5320(63)90013-3. [DOI] [PubMed] [Google Scholar]
- Bergold G.H. The nature of nuclear polyhedrosis viruses. In: Steinhaus E.A., editor. “Insect Pathology: An Advance Treatise”. Academic Press; New York: 1963. pp. 413–455. [Google Scholar]
- Bilimoria S.L. The biology of nuclear polyhedrosis viruses. In: Kurstak E., editor. “Viruses of Invertebrates”. Marcel Dekker Inc.; New York: 1991. pp. 1–60. [Google Scholar]
- Blissard G.W., Rohrmann G.F. Location, sequence, transcriptional mapping, and temporal expression of the gp64 envelope glycoprotein gene of the Orgyia pseudotsugata multicapsid nuclear polyhedrosis virus. Virology. 1989;170(2):537–555. doi: 10.1016/0042-6822(89)90445-5. [DOI] [PubMed] [Google Scholar]
- Blissard G.W., Wenz J.R. Baculovirus GP64 envelope glycoprotein is sufficient to mediate pH dependent membrane fusion. J. Virol. 1992;66:6829–6835. doi: 10.1128/jvi.66.11.6829-6835.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blissard G.W., Quant‐Russell R.L., Rohrmann G.F., Beaudreau G.S. Nucleotide sequence, transcriptional mapping, and temporal expression of the gene encoding P39, a major structural protein of the multicapsid nuclear polyhedrosis virus of Orgyia pseudotsugata. Virology. 1989;168(2):354–362. doi: 10.1016/0042-6822(89)90276-6. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X‐114. J. Biol. Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- Boucias D.G., Pendland J.C. “Principles of Insect Pathology,”. Kluwer Academic Publishers; Norwell: 1998. Baculoviruses; pp. 111–146. [Google Scholar]
- Boutell C., Everett R.D. The herpes simplex virus type 1 (HSV‐1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 2003;278(38):36596–36602. doi: 10.1074/jbc.M300776200. [DOI] [PubMed] [Google Scholar]
- Braunagel S.C., Summers M.D. Autographa californica nuclear polyhedrosis virus, PDV, and ECV viral envelopes and nucleocapsids: Structural proteins, antigens, lipid and fatty acid profiles. Virology. 1994;202(1):315–328. doi: 10.1006/viro.1994.1348. [DOI] [PubMed] [Google Scholar]
- Braunagel S.C., Elton D.M., Ma H., Summers M.D. Identification and analysis of an Autographa californica nuclear polyhedrosis virus structural protein of the occlusion‐derived virus envelope: ODV‐E56. Virology. 1996;217(1):97–110. doi: 10.1006/viro.1996.0097. [DOI] [PubMed] [Google Scholar]
- Braunagel S.C., He H., Ramamurthy P., Summers M.D. Transcription, translation, and cellular localization of three Autographa californica nuclear polyhedrosis virus structural proteins: ODV‐E18, ODV‐E35, and ODV‐EC27. Virology. 1996;222(1):100–114. doi: 10.1006/viro.1996.0401. [DOI] [PubMed] [Google Scholar]
- Braunagel S.C., Parr R., Belyavskyi M., Summers M.D. Autographa californica nucleopolyhedrovirus infection results in Sf9 cell cycle arrest at G2/M phase. Virology. 1998;244(1):195–211. doi: 10.1006/viro.1998.9097. [DOI] [PubMed] [Google Scholar]
- Braunagel S.C., Burks J.K., Rosas‐Acosta G., Harrison R.L., Ma H., Summers M.D. Mutations within the Autographa californica nucleopolyhedrovirus FP25K gene decrease the accumulation of ODV‐E66 and alter its intranuclear transport. J. Virol. 1999;73(10):8559–8570. doi: 10.1128/jvi.73.10.8559-8570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunagel S.C., Guidry P.A., Rosas‐Acosta G., Engelking L., Summers M.D. Identification of BV/ODV‐C42, an Autographa californica nucleopolyhedrovirus orf101‐encoded structural protein detected in infected‐cell complexes with ODV‐EC27 and p78/83. J. Virol. 2001;75(24):12331–12338. doi: 10.1128/JVI.75.24.12331-12338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunagel S.C., Russell W.K., Rosas‐Acosta G., Russell D.H., Summers M.D. Determination of the protein composition of the occlusion‐derived virus of Autographa californica nucleopolyhedrovirus. Proc. Natl. Acad. Sci. USA. 2003;100(17):9797–9802. doi: 10.1073/pnas.1733972100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunagel S.C., Williamson S.T., Saksena S., Zhong Z., Russell W.K., Russell D.H., Summers M.D. Trafficking of ODV‐E66 is mediated via a sorting motif and other viral proteins: Facilitated trafficking to the inner nuclear membrane. Proc. Natl. Acad. Sci. USA. 2004;101(22):8372–8377. doi: 10.1073/pnas.0402727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R. Synthesis of the nuclear protein cyclin (PCNA) and its relationship with DNA replication. Exp. Cell Res. 1986;163(2):287–293. doi: 10.1016/0014-4827(86)90059-5. [DOI] [PubMed] [Google Scholar]
- Bulach D.M., Kumar C.A., Zaia A., Liang B., Tribe D.E. Group II nucleopolyhedrovirus subgroups revealed by phylogenetic analysis of polyhedrin and DNA polymerase gene sequences. J. Invertebr. Pathol. 1999;73(1):59–73. doi: 10.1006/jipa.1998.4797. [DOI] [PubMed] [Google Scholar]
- Carstens E.B., Williams G.V., Faulkner P., Partington S. Analysis of polyhedra morphology mutants of Autographa‐californica nuclear polyhedrosis virus molecular and ultrastructural features. J. Gen. Virol. 1992;73(6):1471–1479. doi: 10.1099/0022-1317-73-6-1471. [DOI] [PubMed] [Google Scholar]
- Chang M.‐J., Blissard G.W. Baculovirus gp64 gene expression: Negative regulation by a minicistron. J. Virol. 1997;71:7448–7460. doi: 10.1128/jvi.71.10.7448-7460.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm G.E., Henner D.J. Multiple early transcripts and splicing of the Autographa californica nuclear polyhedrosis virus IE‐1 gene. J. Virol. 1988;62(9):3193–3200. doi: 10.1128/jvi.62.9.3193-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Guarino L.A. The baculovirus transactivator IE‐l binds to viral enhancer elements in the absence of insect cell factors. J. Virol. 1995;69:4548–4551. doi: 10.1128/jvi.69.7.4548-4551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Guarino L.A. Expression of the IE1 transactivator of Autographa californica nuclear polyhedrosis virus during viral infection. Virology. 1995;209:99–107. doi: 10.1006/viro.1995.1234. [DOI] [PubMed] [Google Scholar]
- Choi J., Guarino L.A. A temperature‐sensitive IE1 protein of Autographa californica nuclear polyhedrosis virus has altered transactivation and DNA binding activities. Virology. 1995;209:90–98. doi: 10.1006/viro.1995.1233. [DOI] [PubMed] [Google Scholar]
- Clem R.J. The role of apoptosis in defense against baculovirus infection in insects. Curr. Top. Microbiol. Immunol. 1995;289:113–129. doi: 10.1007/3-540-27320-4_5. [DOI] [PubMed] [Google Scholar]
- Clem R.J., Fechheimer M., Miller L.K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254(5036):1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- Collot‐Teixeira S., Bass J., Denis F., Ranger‐Rogez S. Human tumor suppressor p53 and DNA viruses. Rev. Med. Virol. 2004;14(5):301–319. doi: 10.1002/rmv.431. [DOI] [PubMed] [Google Scholar]
- Cook S.P., Webb R.E., Podgwaite J.D., Reardon R.C. Increased mortality of gypsy moth Lymantria dispar (L.) (Lepidoptera: Lymantriidae) exposed to gypsy moth nuclear polyhedrosis virus in combination with the phenolic gycoside salicin. J. Econ. Entomol. 2003;96(6):1662–1667. doi: 10.1603/0022-0493-96.6.1662. [DOI] [PubMed] [Google Scholar]
- Cox C.S. Airborne bacteria and viruses. Sci. Prog. 1989;73(292 Pt 4):469–499. [PubMed] [Google Scholar]
- Da Fonseca F., Moss B. Poxvirus DNA topoisomerase knockout mutant exhibits decreased infectivity associated with reduced early transcription. Proc. Natl. Acad. Sci. USA. 2003;100(20):11291–11296. doi: 10.1073/pnas.1534874100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall D., Luque T., O'Reilly D. Insect‐virus relationships: Sifting by informatics. Bioessays. 2001;23(2):184–193. doi: 10.1002/1521-1878(200102)23:2<184::AID-BIES1026>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Dean R.L., Locke M., Collins J.V. Structure of the fat body. In: Kerkut G.A., Gilbert L.I., editors. Vol.9. Pergamont Press; London: 1985. pp. 155–210. (“Comprehensa Insect Physiology, Biochemistry and Pharmacology”). [Google Scholar]
- Derksen A.C.G., Granados R.R. Alteration of a lepidopteran peritrophic membrane by baculoviruses and enhancement of viral infectivity. Virology. 1988;167:242–250. doi: 10.1016/0042-6822(88)90074-8. [DOI] [PubMed] [Google Scholar]
- Durantel D., Croizier L., Ayres M.D., Croizier G., Possee R.D., Lopez‐Ferber M. The pnk/pnl gene (ORF 86) of Autographa californica nucleopolyhedrovirus is a non‐essential, immediate early gene. J. Gen. Virol. 1998;79(Pt. 3):629–637. doi: 10.1099/0022-1317-79-3-629. [DOI] [PubMed] [Google Scholar]
- Efstathiou S., Preston C.M. Towards an understanding of the molecular basis of herpes simplex virus latency. Virus Res. 2005;111(2):108–119. doi: 10.1016/j.virusres.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Engelhard E.K., Volkman L.E. Developmental resistance in fourth instar Trichoplusia ni orally inoculated with Autographa californica M nuclear polyhedrosis virus. Virology. 1995;209(2):384–389. doi: 10.1006/viro.1995.1270. [DOI] [PubMed] [Google Scholar]
- Engelhard E.K., Kam‐Morgan L.N.W., Washburn J.O., Volkman L.E. The insect tracheal system: A conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc. Natl. Acad. Sci. USA. 1994;91(8):3224–3227. doi: 10.1073/pnas.91.8.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppstein D.A., Thoma J.A. Alkaline protease associated with the matrix protein of a virus infecting the cabbage looper. Biochem. Biophys. Res. Commun. 1975;62:478–484. doi: 10.1016/s0006-291x(75)80163-x. [DOI] [PubMed] [Google Scholar]
- Eppstein D.A., Thoma J.A., Scott H.A., Young S.Y. Degradation of matrix protein from the nuclear‐polyhedrosis virus of Trichoplusia ni by an endogenous protease. Virology. 1975;67:591–594. doi: 10.1016/0042-6822(75)90459-6. [DOI] [PubMed] [Google Scholar]
- Evans H.F. Ecology and epizootiology of baculoviruses. In: Granados R.R., Federici B.A., editors. Vol. 2. CRC Press; Boca Raton: 1986. pp. 89–100. (“The biology of baculoviruses”). 2 vols. [Google Scholar]
- Evans J.T., Leisy D.J., Rohrmann G.F. Characterization of the interaction between the baculovirus replication factors LEF‐1 and LEF‐2. J. Virol. 1997;71(4):3114–3119. doi: 10.1128/jvi.71.4.3114-3119.1997. 2 vols. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M.E. Where the cell cycle and histones meet. Genes Dev. 2000;14(18):2265–2270. doi: 10.1101/gad.842100. [DOI] [PubMed] [Google Scholar]
- Fang M., Wang H., Yuan L., Chen X., Vlak J.M., Hu Z. Open reading frame 94 of Helicoverpa armigera single nucleocapsid nucleopolyhedrovirus encodes a novel conserved occlusion‐derived virion protein, ODV‐EC43. J. Gen. Virol. 2003;84(Pt. 11):3021–3027. doi: 10.1099/vir.0.19291-0. [DOI] [PubMed] [Google Scholar]
- Faulkner P., Kuzio J., Williams G.V., Wilson J.A. Analysis of p74, a PDV envelope protein of Autographa californica nucleopolyhedrovirus required for occlusion body infectivity in vivo. J. Gen. Virol. 1997;78(Pt. 12):3091–3100. doi: 10.1099/0022-1317-78-12-3091. [DOI] [PubMed] [Google Scholar]
- Federici B.A. Ultrastructure of baculoviruses. In: Granados R.R., Federici B.A., editors. Vol. I. CRC Press; Boca Raton, Florida: 1986. pp. 61–88. (“The Biology of Baculoviruses”). 2 vols. [Google Scholar]
- Flipsen J.T., Martens J.W., van Oers M.M., Vlak J.M., van Lent J.W. Passage of Autographa californica nuclear polyhedrosis virus through the midgut epithelium of Spodoptera exigua larvae. Virology. 1995;208(1):328–335. doi: 10.1006/viro.1995.1156. [DOI] [PubMed] [Google Scholar]
- Forng R.‐Y., Atreya C.D. Mutations in the retinoblastoma protein‐binding LXCXE motif of rubella virus putative replicase affect virus replication. J. Gen. Virol. 1999;80:327–332. doi: 10.1099/0022-1317-80-2-327. [DOI] [PubMed] [Google Scholar]
- Frana M.F., Behnke J.N., Sturman L.S., Holmes K.V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: Host‐dependent differences in proteolytic cleavage and cell fusion. J. Virol. 1985;56(3):912–920. doi: 10.1128/jvi.56.3.912-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M.J. Ultrastructural observations of virion maturation in Autographa californica nuclear polyhedrosis virus infected Spodoptera frugiperda cell cultures. J. Ultrastruct. Mol. Struct. Res. 1986;95(1–3):189–195. [Google Scholar]
- Fraser M.J., Hink W.F. The isolation and characterization of the MP and FP plaque variants of Galleria mellonella nuclear polyhedrosis virus. Virology. 1982;117(2):366–378. doi: 10.1016/0042-6822(82)90476-7. [DOI] [PubMed] [Google Scholar]
- Fraser M.J., Smith G.E., Summers M.D. Aquisition of host cell DNA sequences by baculoviruses; Relationships between host DNA insertions and FP mutants of Autographa californica and Galleria mellonella nuclear polyhedrosis virus. J. Virol. 1983;47:287–300. doi: 10.1128/jvi.47.2.287-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C.J., Consigli R.A. Evidence for zinc binding by two structural proteins of Plodia interpunctella granulosis virus. J. Virol. 1992;66(5):3168–3171. doi: 10.1128/jvi.66.5.3168-3171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C.J., Consigli R.A. Phosphate cycling on the basic protein of Plodia interpunctella granulosis virus. Virology. 1993;193(1):396–402. doi: 10.1006/viro.1993.1136. [DOI] [PubMed] [Google Scholar]
- Garcia J.J., Li G., Wang P., Zhong J., Granados R.R. Primary and continuous midgut cell cultures from Pseudaletia unipuncta (Lepidoptera: Noctuidae) In Vitro Cell. Dev. Biol. Anim. 2001;37(6):353–359. doi: 10.1007/BF02577570. [DOI] [PubMed] [Google Scholar]
- Garcia‐Maruniak A., Maruniak J.E., Zanotto P.M., Doumbouya A.E., Liu J.C., Merritt T.M., Lanoie J.S. Sequence analysis of the genome of the Neodiprion sertifer nucleopolyhedrovirus. J. Virol. 2004;78(13):7036–7051. doi: 10.1128/JVI.78.13.7036-7051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A.V., Romanowski V., Federici B.A., Sciocco de Cap A. Effects of the Epap granulovirus on its host, Epinotia aporema (Lepidoptera: Tortricidae) J. Invertebr. Pathol. 2002;80(3):148–159. doi: 10.1016/s0022-2011(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Gombart A.F., Pearson M.N., Rohrmann G.F., Beaudreau G.S. A baculovirus polyhedral envelope‐associated protein: Genetic location, nucleotide sequence, and immunocytochemical characterization. Virology. 1989;169(1):182–193. doi: 10.1016/0042-6822(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Granados R.R., Lawler K.A. In vivo pathway of Autographa californica baculovirus invasion and infection. Virology. 1981;l08:297–308. doi: 10.1016/0042-6822(81)90438-4. [DOI] [PubMed] [Google Scholar]
- Greenspan G.L., Corsaro B.G., Hughes P.R., Granados R.R. In‐vivo enhancement of baculovirus infection by the viral enhancing factor of a granulosis virus of the cabbage looper trichoplusia‐ni lepidoptera noctuidae. J. Invertebr. Pathol. 1991;58(2):203–210. [Google Scholar]
- Gross C., Rohrmann G.F. Analysis of the role of 5′ promoter elements and 3′ flanking sequences on the expression of a baculovirus polyhedron envelope protein gene. Virology. 1993;192(1):273–281. doi: 10.1006/viro.1993.1030. [DOI] [PubMed] [Google Scholar]
- Gross C.H., Russell R.L.Q., Rohrmann G.F. Orgyia pseudotsugata baculovirus p10 and polyhedron envelope protein genes: Analysis of their relative expression levels and role in polyhedron structure. J. Gen. Virol. 1994;75(5):1115–1123. doi: 10.1099/0022-1317-75-5-1115. [DOI] [PubMed] [Google Scholar]
- Guarino L.A., Smith M.W. Nucleotide sequence and characterization of the 39k gene region of Autographa californica nuclear polyhedrosis virus. Virology. 1990;179(1):1–8. doi: 10.1016/0042-6822(90)90266-t. [DOI] [PubMed] [Google Scholar]
- Gutierrez S., Kikhno I., Ferber M.L. Transcription and promoter analysis of pif, an essential but low‐expressed baculovirus gene. J. Gen. Virol. 2004;85:331–341. doi: 10.1099/vir.0.19623-0. [DOI] [PubMed] [Google Scholar]
- Haas‐Stapleton E.J., Washburn J.O., Volkman L.E. P74 mediates specific binding of Autographa californica M nucleopolyhedrovirus occlusion‐derived virus to primary cellular targets in the midgut epithelia of Heliothis virescens Larvae. J. Virol. 2004;78:6786–6791. doi: 10.1128/JVI.78.13.6786-6791.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang X., Dong W., Guarino L.A. The lef‐3 gene of Autographa calfonica nuclear polyhedrosis virus encodes a single‐stranded DNA‐binding protein. J. Virology. 1995;69(6):3924–3928. doi: 10.1128/jvi.69.6.3924-3928.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrap K.A. The structure of nuclear polyhedrosis viruses. Virology. 1972;50:114–123. doi: 10.1016/0042-6822(72)90351-0. [DOI] [PubMed] [Google Scholar]
- Harrap K.A. The structure of nuclear polyhedrosis viruses. I. The inclusion body. Virology. 1972;50(1):114–123. doi: 10.1016/0042-6822(72)90351-0. [DOI] [PubMed] [Google Scholar]
- Harrison R.L., Summers M.D. Mutations in the Autographa californica multinucleocapsid nuclear polyhedrosis virus 25 kDa protein gene result in reduced virion occlusion, altered intranuclear envelopment and enhanced virus production. J. Gen. Virol. 1995;76:1451–1459. doi: 10.1099/0022-1317-76-6-1451. [DOI] [PubMed] [Google Scholar]
- Harrison R.L., Jarvis D.L., Summers M.D. The role of AcMNPV 25K gene, “FP25”, in baculovirus polh and p10 expression. Virology. 1996;226:34–46. doi: 10.1006/viro.1996.0625. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Corsaro B.G., Granados R.R. Location and nucleotide sequence of the gene encoding the viral enhancing factor of the Trichoplusia ni granulosis virus. J. Gen. Virol. 1991;72(11):2645–2652. doi: 10.1099/0022-1317-72-11-2645. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Hayakawa T., Ueno Y., Fujita T., Sano Y., Matsumoto T. Sequence analysis of the Plutella xylostella granulovirus genome. Virology. 2000;275:358–372. doi: 10.1006/viro.2000.0530. [DOI] [PubMed] [Google Scholar]
- Hawtin R.E., Zarkowska T., Arnold K., Thomas C.J., Gooday G.W., King L.A., Kuzio J.A., Possee R.D. Liquefaction of Autographa californica nucleopolyhedrovirus‐infected insects is dependent on the integrity of virus‐encoded chitinase and cathepsin genes. Virology. 1997;238(2):243–253. doi: 10.1006/viro.1997.8816. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Ko R., Okano K., Seong S.‐I., Goto C., Maeda S. Sequence analysis of the Xestia c‐nigrum granulovirus genome. Virology. 1999;262:277–297. doi: 10.1006/viro.1999.9894. [DOI] [PubMed] [Google Scholar]
- Hefferon K.L., Miller L.K. Reconstructing the replication complex of AcMNPV. Eur. J. Biochem. 2002;269(24):6233–6240. doi: 10.1046/j.1432-1033.2002.03342.x. [DOI] [PubMed] [Google Scholar]
- Hefferon K.L., Oomens A.G., Monsma S.A., Finnerty C.M., Blissard G.W. Host cell receptor binding by baculovirus GP64 and kinetics of virion entry. Virology. 1999;258(2):455–468. doi: 10.1006/viro.1999.9758. [DOI] [PubMed] [Google Scholar]
- Herniou E.A., Luque T., Chen X., Vlak J.M., Winstanley D., Cory J.S., O'Reilly D.R. Use of whole genome sequence data to infer baculovirus phylogeny. J. Virol. 2001;75(17):81117–81126. doi: 10.1128/JVI.75.17.8117-8126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herniou E.A., Olszewski J.A., Cory J.S., O'Reilly D.R. The genome sequence and evolution of baculoviruses. Ann. Rev. Entomol. 2003;48:211–234. doi: 10.1146/annurev.ento.48.091801.112756. [DOI] [PubMed] [Google Scholar]
- Hiscock D., Upton C. Viral genome database: A tool for storing and analyzing genes and proteins from complete viral genomes. Bioinformatics. 2000;16:484–485. doi: 10.1093/bioinformatics/16.5.484. [DOI] [PubMed] [Google Scholar]
- Hohmann A.W., Faulkner P. Monoclonal antibodies to baculovirus structural proteins: Determination of specificities by Western blot analysis. Virology. 1983;125(2):432–444. doi: 10.1016/0042-6822(83)90214-3. [DOI] [PubMed] [Google Scholar]
- Hong T., Braunagel S.C., Summers M.D. Transcription, translation, and cellular localization of PDV‐E66: A structural protein of the PDV envelope of Autographa californica nuclear polyhedrosis virus. Virology. 1994;204(1):210–222. doi: 10.1006/viro.1994.1525. [DOI] [PubMed] [Google Scholar]
- Hong T., Summers M.D., Braunagel S.C. N‐terminal sequences from Autographa californica nuclear polyhedrosis virus envelope proteins ODV‐E66 and ODV‐E25 are sufficient to direct reporter proteins to the nuclear envelope, intranuclear microvesicles and the envelope of occlusion derived virus. Proc. Natl. Acad. Sci. USA. 1997;94(8):4050–4055. doi: 10.1073/pnas.94.8.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton H.M., Burand J.P. Saturable attachment sites for polyhedron‐derived baculovirus on insect cells and evidence for entry via direct membrane fusion. J. Virol. 1993;67(4):1860–1868. doi: 10.1128/jvi.67.4.1860-1868.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkin P.G. Comparison of virion proteins and granulin from a granulosis virus produced in two host species. J. Invertebr. Pathol. 1981;38(2):303–304. [Google Scholar]
- Ichihashi Y., Oie M. Proteolytic activation of vaccinia virus for the penetration phase of infection. Virology. 1982;116(1):297–305. doi: 10.1016/0042-6822(82)90421-4. [DOI] [PubMed] [Google Scholar]
- IJkel W., van Strien E.A., Heldens J.G., Broer R., Zuidema D., Goldbach R.W., Vlak J.M. Sequence and organization of the Spodoptera exigua multicapsid nucleopolyhedrosis virus genome. J. Gen. Virol. 1999;80:3289–3304. doi: 10.1099/0022-1317-80-12-3289. [DOI] [PubMed] [Google Scholar]
- Imai N., Matsuda N., Tanaka K., Nakano A., Matsumoto S., Kang W. Ubiquitin ligase activities of Bombyx mori nucleopolyhedrovirus RING finger proteins. J. Virol. 2003;77(2):923–930. doi: 10.1128/JVI.77.2.923-930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio C., Vialard J.E., McCracken S., Lagace M., Richardson C.D. The late expression factors 8 and 9 and possibly the phosphoprotein p78/83 of Autographa californica multicapsid nucleopolyhedrovirus are components of the virus‐induced RNA polymerase. Intervirology. 1998;41(1):35–46. doi: 10.1159/000024913. [DOI] [PubMed] [Google Scholar]
- Iwahori S., Ikeda M., Kobayashi M. Comparative characterization of pcna genes from Hyphantria cunea nucleopolyhedrovirus and two cell lines from the fall armyworm, Spodoptera frugiperda, and the fall webworm, Hyphantria cunea. J. Insect. Biotechnol. Sericol. 2002;71:25–34. [Google Scholar]
- Iwahori S., Ikeda M., Kobayashi M. Association of Sf9 cell proliferating cell nuclear antigen with the DNA replication site of Autographa californica multicapsid nucleopolyhedrovirus. J. Gen. Virol. 2004;85(Pt. 10):2857–2862. doi: 10.1099/vir.0.80114-0. [DOI] [PubMed] [Google Scholar]
- Jain M., Das R.H. Nucleotide sequence and molecular characterization of the structural glycoprotein gp41 gene homologue of Spodoptera litura nucleopolyhedrosis virus (spltNPV‐I) Mol. Biol. Rep. 2004;31(4):231–239. doi: 10.1007/s11033-005-1405-x. [DOI] [PubMed] [Google Scholar]
- Jarvis D.L., Garcia A., Jr. Long‐term stability of baculoviruses stored under various conditions. Biotechniques. 1994;16(3):508–513. [PubMed] [Google Scholar]
- Jarvis D.L., Bohlmeyer D.A., Garcia A.J. Enhancement of polyhedrin nuclear localization during baculovirus infection. J. Virol. 1992;66(12):6903–6911. doi: 10.1128/jvi.66.12.6903-6911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Martinek S., Joo W.S., Wortman J.R., Mirkovic N., Sali A., Yandell M.D., Pavletich N.P., Young M.W., Levine A.J. Identification and characterization of a p53 homologue in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2000;97(13):7301–7306. doi: 10.1073/pnas.97.13.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K.A., Lee M.J., Gatehouse J.A., Anstee J.H. The partial purification and characterization of serine protease activity in Helicoverpa armigera. Biochemistry. 1991;21:389–397. [Google Scholar]
- Katsuma S., Noguchi Y., Zhou C.L., Kobayashi M., Maeda S. Characterization of the 25K FP gene of the baculovirus Bombyx mori nucleopolyhedrovirus: Implications for post‐mortem host degradation [In Process Citation] J. Gen. Virol. 1999;80(Pt. 3):783–791. doi: 10.1099/0022-1317-80-3-783. [DOI] [PubMed] [Google Scholar]
- Kawamoto F., Asayama T. Studies on the arrangement patterns of nucleocapsids within envelopes of nuclear‐polyhedrosis virus in the fat‐body cells of the brown tail moth, Euproctis similis. J. Invertebr. Pathol. 1975;26(1):47–55. doi: 10.1016/0022-2011(75)90168-8. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y., Matsumoto S., Nagamine T. Analysis of baculovirus IE1 in living cells: Dynamics and spatial relationships to viral structural proteins. J. Gen. Virol. 2004;85(Pt. 12):3575–3583. doi: 10.1099/vir.0.80418-0. [DOI] [PubMed] [Google Scholar]
- Keddie B.A., Aponte G.W., Volkman L.E. The pathway of infection of Autographa californica nuclear polyhedrosis virus in an insect host. Science. 1989;243(4899):1728–1730. doi: 10.1126/science.2648574. [DOI] [PubMed] [Google Scholar]
- Kelly D.C., Brown D.A., Ayres M.D., Allen C.J., Walker I.O. Properties of the major nucleocapsid protein of Heliothis zea singly enveloped nuclear polyhedrosis virus. J. Gen. Virol. 1983;64(2):399–408. [Google Scholar]
- Kikhno I., Gutierrez S., Croizier L., Croizier G., Ferber M.L. Characterization of pif, a gene required for the per os infectivity of Spodoptera littoralis nucleopolyhedrovirus. J. Gen. Virol. 2002;83:3013–3022. doi: 10.1099/0022-1317-83-12-3013. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B.A., Washburn J.O., Engelhard E.K., Volkman L.E. Primary infection of insect tracheae by Autographa californica M nuclear polyhedrosis virus. Virology. 1994;203(1):184–186. doi: 10.1006/viro.1994.1472. [DOI] [PubMed] [Google Scholar]
- Kisielewska J., Lu P., Whitaker M. GFP‐PCNA as an S‐phase marker in embryos during the first and subsequent cell cycles. Biol. Cell. 2005;97(3):221–229. doi: 10.1042/BC20040093. [DOI] [PubMed] [Google Scholar]
- Kitts P.A., Possee R.D. A method for producing recombinant baculovirus expression vectors at high frequency. Biotechniques. 1993;14(5):810–817. [PubMed] [Google Scholar]
- Knudsen D.L., Harrap K.A. Replication of a nuclear polyhedrosis virus in a continuous cell culture of Spodoptera frugiperda: Microscopy study of the sequence of events of virus infection. J. Virol. 1976;17:254–268. doi: 10.1128/jvi.17.1.254-268.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M., Ahrens C.H., Goldbach R.W., Rohrmann G.F., Vlak J.M. Identification of genes involved in DNA replication of the Autographa californica baculovirus. Proc. Natl. Acad. Sci. USA. 1994;91(23):11212–11216. doi: 10.1073/pnas.91.23.11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G.R., Guarino L.A., Summers M.D. Novel regulatory properties of the IE1 and IE0 transactivators encoded by the baculovirus Autographa californica multicapsid nuclear polyhedrosis virus. J. Virol. 1991;65(10):5281–5288. doi: 10.1128/jvi.65.10.5281-5288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G.R., Choi J., Guarino L.A., Summers M.D. Functional dissection of the Autographa californica nuclear polyhedrosis virus immediate‐early 1 transcriptional regulatory protein. J. Virol. 1992;66(12):7429–7437. doi: 10.1128/jvi.66.12.7429-7437.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer A., Knebel‐Morsdorf D. The early baculovirus he65 promoter: On the mechanism of transcriptional activation by IE1. Virology. 1998;249:336–351. doi: 10.1006/viro.1998.9288. [DOI] [PubMed] [Google Scholar]
- Krogh A., Larsson B., von Heijne G., Sonnhammer E.L.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001;305(3):567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Kurki P., Vanderlaan M., Dolbeare F., Gray J., Tan E.M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp. Cell Res. 1986;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- Kuzio J., Jaques R., Faulkner P. Identification of p74 a gene essential for virulence of baculovirus occlusion bodies. Virology. 1989;173(2):759–763. doi: 10.1016/0042-6822(89)90593-x. [DOI] [PubMed] [Google Scholar]
- Kuzio J.M., Pearson M.N., Harwood S.H., Funk C.J., Evans J.T., Slavicek J.M., Rohrmann G.F. Sequence analysis of the genome of a baculovirus pathogenic for Lymantria dispar. Virology. 1999;253:17–34. doi: 10.1006/viro.1998.9469. [DOI] [PubMed] [Google Scholar]
- Labanderia C.C., Sepkoski J.J. Insect diversity in the fossil record. Science. 1993;261(July 16):310–314. doi: 10.1126/science.11536548. [DOI] [PubMed] [Google Scholar]
- Lange M., Jehle J.A. The genome of the Cryptophlebia leucotreta granulovirus. Virology. 2003;317(2):220–236. doi: 10.1016/s0042-6822(03)00515-4. [DOI] [PubMed] [Google Scholar]
- Langridge W.H.R., Balter K. Protease activity associated with the capsule protein of Estigmene acrea granulovirus virus. Virology. 1981;114:595–600. doi: 10.1016/0042-6822(81)90243-9. [DOI] [PubMed] [Google Scholar]
- Lanier L.M., Volkman L.E. Actin binding and nucleation by Autographa california M nucleopolyhedrovirus. Virology. 1998;243(1):167–177. doi: 10.1006/viro.1998.9065. [DOI] [PubMed] [Google Scholar]
- Lanier L.M., Slack J.M., Volkman L.E. Actin binding and proteolysis by the baculovirus AcMNPV: The role of virion‐associated V‐CATH. Virology. 1996;216(2):380–388. doi: 10.1006/viro.1996.0073. [DOI] [PubMed] [Google Scholar]
- Lapointe R., Popham H.J., Straschil U., Goulding D., O'Reilly D.R., Olszewski J.A. Characterization of two Autographa californica nucleopolyhedrovirus proteins, Ac145 and Ac150, which affect oral infectivity in a host‐dependent manner. J. Virol. 2004;78(12):6439–6448. doi: 10.1128/JVI.78.12.6439-6448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson R. Insect pathological investigations on Swedish Thysanura: A nuclear polyhedrosis virus of the bristletail Dilta hibernica. J. Invertebr. Pathol. 1984;44(2):172–177. [Google Scholar]
- Lauzon H.A., Lucarotti C.J., Krell P.J., Feng Q., Retnakaran A., Arif B.M. Sequence and organization of the Neodiprion lecontei nucleopolyhedrovirus genome. J. Virol. 2004;78(13):7023–7035. doi: 10.1128/JVI.78.13.7023-7035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon H.A., Jamieson P.B., Krell P.J., Arif B.M. Gene organization and sequencing of the Choristoneura fumiferana defective nucleopolyhedrovirus genome. J. Gen. Virol. 2005;86(Pt. 4):945–961. doi: 10.1099/vir.0.80489-0. [DOI] [PubMed] [Google Scholar]
- Lee H.H., Miller L.K. Isolation, complementation, and initial characterization of temperature‐sensitive mutants of the baculovirus Autographa californica nuclear polyhedrosis virus. J. Virol. 1979;31:240–252. doi: 10.1128/jvi.31.1.240-252.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E., Onaran H.O., Zimmerberg J. Acidic pH induces fusion of cells infected with baculovirus to form syncytia. FEBS Lett. 1992;304(2–3):221–224. doi: 10.1016/0014-5793(92)80623-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore L.S., Roelvink P.R., Granados R.R. Enhancin, the granulosis virus protein that facilitates nucleopolyhedrovirus (NPV) infections, is a metalloprotease. J. Invertebr. Pathol. 1996;68(2):131–140. doi: 10.1006/jipa.1996.0070. [DOI] [PubMed] [Google Scholar]
- Li L., Rohrmann G.F. Characterization of a baculovirus alkaline nuclease. J. Virol. 2000;74(14):6401–6407. doi: 10.1128/jvi.74.14.6401-6407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Hannon G.J., Beach D., Stillman B. Subcellular distribution of p21 and PCNA in normal and repair‐deficient cells following DNA damage. Curr. Biol. 1996;6(2):189–199. doi: 10.1016/s0960-9822(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H., Zhang J., McEachern J. Bats are natural reservoirs of SARS‐like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Li X., Pang A., Lauzon H.A., Sohi S.S., Arif B.M. The gene encoding the capsid protein P82 of the Choristoneura fumiferana multicapsid nucleopolyhedrovirus: Sequencing, transcription and characterization by immunoblot analysis. J. Gen. Virol. 1997;78(Pt. 10):2665–2673. doi: 10.1099/0022-1317-78-10-2665. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang J., Deng R., Zhang Q., Yang K., Wang X. vlf‐1 deletion brought AcMNPV to defect in nucleocapsid formation. Virus Genes. 2005;31(3):275–284. doi: 10.1007/s11262-005-3242-3. [DOI] [PubMed] [Google Scholar]
- Li Z., Li C., Pan L., Yu M., Yang K., Pang Y. Characterization of p24 gene of Spodoptera litura multicapsid nucleopolyhedrovirus. Virus Genes. 2005;30(3):349–356. doi: 10.1007/s11262-004-6778-8. [DOI] [PubMed] [Google Scholar]
- Lin G.Y., Zhong J., Wang X. Abnormal formation of polyhedra resulting from a single mutation in the polyhedrin gene of Autographa californica multicapsid nucleopolyhedrovirus. J. Invertebr. Pathol. 2000;76:13–19. doi: 10.1006/jipa.2000.4934. [DOI] [PubMed] [Google Scholar]
- Lin J.J., Sancar A. Active site of (A)BC excinuclease. I. Evidence for 5′ incision by UvrC through a catalytic site involving Asp399, Asp438, Asp466, and His538 residues. J. Biol. Chem. 1992;267(25):17688–17692. [PubMed] [Google Scholar]
- Lin J.J., Phillips A.M., Hearst J.E., Sancar A. Active site of (A)BC excinuclease. II. Binding, bending, and catalysis mutants of UvrB reveal a direct role in 3′ and an indirect role in 5′ incision. J. Biol. Chem. 1992;267(25):17693–17700. [PubMed] [Google Scholar]
- Loeb M.J., Clark E.A., Blackburn M., Hakim R.S., Elsen K., Smagghe G. Stem cells from midguts of Lepidopteran larvae: Clues to the regulation of stem cell fate. Arch. Insect. Biochem. Physiol. 2003;53(4):186–198. doi: 10.1002/arch.10098. [DOI] [PubMed] [Google Scholar]
- Lu A., Carstens E.B. Nucleotide sequence and transcriptional analysis of the p80 gene of Autographa californica nuclear polyhedrosis virus: A homologue of the Orgyia pseudotsugata nuclear polyhedrosis virus capsid‐associated gene. Virology. 1992;190(1):201–209. doi: 10.1016/0042-6822(92)91206-a. [DOI] [PubMed] [Google Scholar]
- Lu A., Miller L.K. Differential requirements for baculovirus late expression factor genes in two cell lines. J. Virol. 1995;69(10):6265–6272. doi: 10.1128/jvi.69.10.6265-6272.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A., Miller L.K. The roles of eighteen baculovirus late expression factor genes in transcription and DNA replication. J. Virol. 1995;69(2):975–982. doi: 10.1128/jvi.69.2.975-982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A., Miller L.K. Species‐specific effects of the hcf‐1 gene on baculovirus virulence. J. Virol. 1996;70(8):5123–5130. doi: 10.1128/jvi.70.8.5123-5130.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Iatrou K. The genes encoding the P39 and CG30 proteins of Bombyx mori nuclear polyhedrosis virus. J. Gen. Virol. 1996;77(Pt. 12):3135–3143. doi: 10.1099/0022-1317-77-12-3135. [DOI] [PubMed] [Google Scholar]
- Lu S., Ge G., Qi Y. Ha‐VP39 binding to actin and the influence of F‐actin on assembly of progeny virions. Arch. Virol. 2004;149(11):2187–2198. doi: 10.1007/s00705-004-0361-4. [DOI] [PubMed] [Google Scholar]
- Lu S.Y., Qi Y.P., Ge G.Q. Interaction of Heliothis armigera nuclear polyhedrosis viral capsid protein with its host actin. J. Biochem. Mol. Biol. 2002;35(6):562–567. doi: 10.5483/bmbrep.2002.35.6.562. [DOI] [PubMed] [Google Scholar]
- Lung O., Blissard G.W. A cellular Drosophila melanogaster protein with similarity to baculovirus F envelope fusion proteins. J. Virol. 2005;79(13):7979–7989. doi: 10.1128/JVI.79.13.7979-7989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung O., Westenberg M., Vlak J.M., Zuidema D., Blissard G.W. Pseudotyping Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV): F proteins from group II NPVs are functionally analogous to AcMNPV GP64. J. Gen. Virol. 2002;76:5729–5736. doi: 10.1128/JVI.76.11.5729-5736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung O.Y., Cruz‐Alvarez M., Blissard G.W. Ac23, an envelope fusion protein homolog in the baculovirus Autographa californica multicapsid nucleopolyhedrovirus, is a viral pathogenicity factor. J. Virol. 2003;77(1):328–339. doi: 10.1128/JVI.77.1.328-339.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque T., Finch R., Crook N., O'Reilly D.R., Winstanley D. The complete sequence of the Cydia pomonella granulovirus genome. J. Gen. Virol. 2001;82:2531–2547. doi: 10.1099/0022-1317-82-10-2531. [DOI] [PubMed] [Google Scholar]
- Madsen P., Celis J.E. S‐phase patterns of cyclin (PCNA) antigen staining resemble topographical patterns of DNA synthesis. A role for cyclin in DNA replication? FEBS Lett. 1985;193(1):5–11. doi: 10.1016/0014-5793(85)80068-5. [DOI] [PubMed] [Google Scholar]
- Maeda S., Nagata M., Tanada Y. Ionic conditions affecting the release and absorption of an alkaline protease associated with occlusion bodies of insect baculoviruses. J. Invertebr. Pathol. 1983;42:376–383. [Google Scholar]
- Maga G., Hubscher U. Proliferating cell nuclear antigen (PCNA): A dancer with many partners. J. Cell Sci. 2003;116(Pt. 15):3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- Martignoni M.E., Iwai P.J. USDA Forest Service PNW‐195. 4th ed. 1986. A catalog of viral diseases of insects, mites, and ticks; p. 51. [Google Scholar]
- Martins A., Shuman S. Characterization of a baculovirus enzyme with RNA ligase, polynucleotide 5′‐kinase, and polynucleotide 3′‐phosphatase activities. J. Biol. Chem. 2004;279(18):18220–18231. doi: 10.1074/jbc.M313386200. [DOI] [PubMed] [Google Scholar]
- McCarthy W.J., Dicapua R.A. Characterization of solubilized proteins from tissue culture‐ and host‐derived nuclear polyhedra of Lymantria dispar and Autographa californica. Intervirology. 1979;11:174–181. doi: 10.1159/000149030. [DOI] [PubMed] [Google Scholar]
- McLachlin J.R., Miller L.K. Identification and characterization of vlf‐1, a baculovirus gene involved in very late gene expression. J. Virol. 1994;68(12):7746–7756. doi: 10.1128/jvi.68.12.7746-7756.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov M.V., Rohrmann G.F. Baculovirus replication factor LEF‐1 is a DNA primase. J. Virol. 2002;76(5):2287–2297. doi: 10.1128/jvi.76.5.2287-2297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov M.V., Rohrmann G.F. Binding of the baculovirus very late expression factor I (VLF‐1) to different DNA structures. BMC Mol. Biol. 2002;3(14):1–11. doi: 10.1186/1471-2199-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov V.S., Okano K., Rohrmann G.F. Baculovirus alkaline nuclease possesses a 5′ –> 3′ exonuclease activity and associates with the DNA‐binding protein LEF‐3. J. Virol. 2003;77(4):2436–2444. doi: 10.1128/JVI.77.4.2436-2444.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.K., Adang M.J., Browne D. Protein kinase activity associated with the extracellular and occluded forms of the baculovirus Autographa californica nuclear polyhedrosis virus. J. Virol. 1983;46(1):275–278. doi: 10.1128/jvi.46.1.275-278.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minion F.C., Coons L.B., Broome J.R. Characterization of the polyhedral envelope of the nuclear polyhedrosis virus of Heliothis virescens. J. Invertebr. Pathol. 1979;34:303–307. [Google Scholar]
- Miranda R., Zamudio F.Z., Bravo A. Processing of Cry1Ab delta‐endotoxin from Bacillus thuringiensis by Manduca sexta and Spodoptera frugiperda midgut proteases: Role in protoxin activation and toxin inactivation. Insect. Biochem. Mol. Biol. 2001;31:1155–1163. doi: 10.1016/s0965-1748(01)00061-3. [DOI] [PubMed] [Google Scholar]
- Mistretta T.A., Guarino L.A. Transcriptional activity of baculovirus very late factor 1. J. Virol. 2005;79(3):1958–1960. doi: 10.1128/JVI.79.3.1958-1960.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma S.A., Oomens A.G.P., Blissard G.W. The GP64 envelope fusion protein is an essential baculovirus protein required for cell to cell transmission of infection. J. Virol. 1996;70:4607–4616. doi: 10.1128/jvi.70.7.4607-4616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M.A., Marriott A.C., Nuttall P.A. The glycoprotein of thogoto virus (a tick‐borne orthomyxo‐like virus) is related to the baculovirus glycoprotein gp64. Virology. 1992;186:640–646. doi: 10.1016/0042-6822(92)90030-s. [DOI] [PubMed] [Google Scholar]
- Moscardi F. Assessment of the application of baculoviruses for control of Lepidoptera. Annu. Rev. Entomol. 1999;44:257–289. doi: 10.1146/annurev.ento.44.1.257. [DOI] [PubMed] [Google Scholar]
- Moser B., Becnel J., White S., Afonso C., Kutish G., Shanker S., Almira E. Morphological and molecular evidence that Culex nigripalpus baculovirus is an unusual member of the family Baculoviridae. J. Gen. Virol. 2001;82(Pt. 2):283–297. doi: 10.1099/0022-1317-82-2-283. [DOI] [PubMed] [Google Scholar]
- Muller R., Pearson M.N., Russell R.L., Rohrmann G.F. A capsid‐associated protein of the multicapsid nuclear polyhedrosis virus of Orgyia pseudotsugata: Genetic location, sequence, transcriptional mapping, and immunocytochemical characterization. Virology. 1990;176(1):133–144. doi: 10.1016/0042-6822(90)90238-m. [DOI] [PubMed] [Google Scholar]
- Muramatsu M., Homma M. Trypsin action on the growth of Sendai virus in tissue culture cells. V. An activating enzyme for Sendai virus in the chorioallantoic fluid of the embryonated chicken egg. Microbiol. Immunol. 1980;24(2):113–122. doi: 10.1111/j.1348-0421.1980.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Murphy F.A., Fauquet C.M., Bishop D.H.L., Ghabrial S.A., Jarvis A.W., Martelli G.P., Mayo M.P., Summers M.D. Classification and nomenclature of viruses: Sixth report of the international committee for the taxonomy of viruses. Arch. Virol. Suppl. 1995;10:1–586. [PubMed] [Google Scholar]
- Noguchi K., Fukazawa H., Murakami Y., Uehara Y. Nek11, a new member of the NIMA family of kinases, involved in DNA replication and genotoxic stress responses. J. Biol. Chem. 2002;277(42):39655–39665. doi: 10.1074/jbc.M204599200. [DOI] [PubMed] [Google Scholar]
- Noguchi K., Fukazawa H., Murakami Y., Uehara Y. Nucleolar Nek11 is a novel target of Nek2A in G1/S‐arrested cells. J. Biol. Chem. 2004;279(31):32716–32727. doi: 10.1074/jbc.M404104200. [DOI] [PubMed] [Google Scholar]
- Nomura T., Katunuma N. Involvement of cathepsins in the invasion, metastasis and proliferation of cancer cells. J. Med. Invest. 2005;52(1–2):1–9. doi: 10.2152/jmi.52.1. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating the onset of M‐phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Odindo M.O. Epizootiological observations on a nuclear polyhedrosis of the African armyworm Spodoptera exempta (Walk.) Insect. Sci. Appl. 1983;4(3):291–298. [Google Scholar]
- Ohba M., Tanada Y. A synergistic factor enhances the in vitro infection of an insect baculovirus. Naturwissenschaften. 1983;70(12):613–614. [Google Scholar]
- Ohkawa T., Majima K., Maeda S. A cysteine protease encoded by the baculovirus Bombyx mori nuclear polyhedrosis virus. J. Virol. 1994;68(10):6619–6625. doi: 10.1128/jvi.68.10.6619-6625.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa T., Washburn J.O., Sitapara R., Sid E., Volkman L.E. Specific binding of Autographa californica M nucleopolyhedrovirus occlusion‐derived virus to midgut cells of Heliothis virescens larvae is mediated by products of pif genes Ac119 and Ac022 but not Ac115. J. Virol. 2005;79(24):15258–15264. doi: 10.1128/JVI.79.24.15258-15264.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano K., Vanarsdall A.L., Rohrmann G.F. Characterization of a baculovirus lacking the alkaline nuclease gene. J. Virol. 2004;78(19):10650–10656. doi: 10.1128/JVI.78.19.10650-10656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski J., Miller L.K. Identification and characterization of a baculovirus structural protein, VP1054, required for nucleocapsid formation. J. Virol. 1997;71(7):5040–5050. doi: 10.1128/jvi.71.7.5040-5050.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski J., Miller L.K. A role of baculovirus GP41 in budded virus production. Virology. 1997;233(2):292–301. doi: 10.1006/viro.1997.8612. [DOI] [PubMed] [Google Scholar]
- Oomens A.G., Blissard G.W. Requirement for GP64 to drive efficient budding of Autographa californica multicapsid nucleopolyhedrovirus. Virology. 1999;254(2):297–314. doi: 10.1006/viro.1998.9523. [DOI] [PubMed] [Google Scholar]
- O'Reilly D.R., Crawford A.M., Miller L.K. Viral proliferating cell nuclear antigen. Nature. 1989;337(6208):606. doi: 10.1038/337606a0. [DOI] [PubMed] [Google Scholar]
- Osborne M.J., Siddiqui N., Iannuzzi P., Gehring K. The solution structure of ChaB, a putative membrane ion antiporter regulator from Escherichia coli. BMC Struct. Biol. 2004;4(1):9. doi: 10.1186/1472-6807-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L., Li Z., Gong Y., Yu M., Yang K., Pang Y. Characterization of gp41 gene of Spodoptera litura multicapsid nucleopolyhedrovirus. Virus Res. 2005;110(1–2):73–79. doi: 10.1016/j.virusres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Passarelli A.L., Miller L.K. Three baculovirus genes involved in late and very late gene expression: Ie‐1, ie‐n, and lef‐2. J. Virol. 1993;67(4):2149–2158. doi: 10.1128/jvi.67.4.2149-2158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarelli A.L., Miller L.K. In vivo and in vitro analyses of recombinant baculoviruses lacking a functional cg30 gene. J. Virol. 1994;68(2):1186–1190. doi: 10.1128/jvi.68.2.1186-1190.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patmanidi A.L., Possee R.D., King L.A. Formation of P10 tubular structures during AcMNPV infection depends on the integrity of host‐cell microtubules. Virology. 2003;317(2):308–320. doi: 10.1016/j.virol.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Pearson M., Bjornson R., Pearson G., Rohrmann G. The Autographa californica baculovirus genome: Evidence for multiple replication origins. Science (Washington DC) 1992;257(5075):1382–1384. doi: 10.1126/science.1529337. [DOI] [PubMed] [Google Scholar]
- Pearson M.N., Rohrmann G.F. Splicing is required for transactivation by the immediate early gene 1 of the Lymantria dispar multinucleocapsid nuclear polyhedrosis virus. Virology. 1997;235(1):153–165. doi: 10.1006/viro.1997.8687. [DOI] [PubMed] [Google Scholar]
- Pearson M.N., Quant‐Russell R.L., Rohrmann G.F., Beaudreau G.S. P39, a major baculovirus structural protein: Immunocytochemical characterization and genetic location. Virology. 1988;167:407–413. [PubMed] [Google Scholar]
- Pearson M.N., Groten C., Rohrmann G.F. Identification of the Lymantria dispar nucleopolyhedrovirus envelope fusion protein provides evidence for a phylogenetic division of the Baculoviridae. J. Virol. 2000;74(13):6126–6131. doi: 10.1128/jvi.74.13.6126-6131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M.N., Russell R.L., Rohrmann G.F. Characterization of a baculovirus‐encoded protein that is associated with infected‐cell membranes and budded virions. Virology. 2001;291(1):22–31. doi: 10.1006/viro.2001.1191. [DOI] [PubMed] [Google Scholar]
- Petrik D.T., Iseli A., Montelone B.A., Van Etten J.L., Clem R.J. Improving baculovirus esistance to UV inactivation: Increased virulence resulting from expression of a DNA repair enzyme. J. Invertebr. Pathol. 2003;82(1):50–56. doi: 10.1016/s0022-2011(02)00197-0. [DOI] [PubMed] [Google Scholar]
- Pham D.Q., Hice R.H., Sivasubramanian N., Federici B.A. The 1629‐bp open reading frame of the Autographa californica multinucleocapsid nuclear polyhedrosis virus encodes a virion structural protein. Gene. 1993;137(2):275–280. doi: 10.1016/0378-1119(93)90020-4. [DOI] [PubMed] [Google Scholar]
- Pijlman G.P., Pruijssers A.J., Vlak J.M. Identification of pif‐2, a third conserved baculovirus gene required for per os infection of insects. J. Gen. Virol. 2003;84:2041–2049. doi: 10.1099/vir.0.19133-0. [DOI] [PubMed] [Google Scholar]
- Podgwaite J.D., Rush P., Hall D., Walton G.S. Efficacy of the Neodiprion sertifer (Hymenoptera: Diprionidae) nucleopolyhedrosis virus (Baculovirus) product, Neochek‐S. J. Econ. Entomol. 1984;77(2):525–528. [Google Scholar]
- Prikhod'ko E.A., Lu A., Wilson J.A., Miller L.K. In vivo and in vitro analysis of baculovirus ie‐2 mutants. J. Virol. 1999;73(3):2460–2468. doi: 10.1128/jvi.73.3.2460-2468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prikhod'ko G.G., Wang Y., Freulich E., Prives C., Miller L.K. Baculovirus p33 binds human p53 and enhances p53‐mediated apoptosis. J. Virol. 1999;73(2):1227–1234. doi: 10.1128/jvi.73.2.1227-1234.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidan K.K., Nassoury N., Giannopoulos P.N., Guertin C. Transcription, translation, and immunolocalization of ODVP‐6E/ODV‐E56 and p74 proteins: Two highly conserved ODV‐associated envelope proteins of Choristoneura fumiferana Granulovirus. J. Biochem. Mol. Biol. 2005;38(1):65–70. doi: 10.5483/bmbrep.2005.38.1.065. [DOI] [PubMed] [Google Scholar]
- Raymond B., Hartley S.E., Cory J.S., Hails R.S. The role of food plant and pathogen‐induced behaviour in the persistence of a nucleopolyhedrovirus. J. Invertebr. Pathol. 2005;88(1):49–57. doi: 10.1016/j.jip.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Robertson J.S., Harrap K.A., Longworth J.F. Baculovirus morphogenesis: The acquisition of the virus envelope. J. Invertebr. Pathol. 1974;23(2):248–251. doi: 10.1016/0022-2011(74)90193-1. [DOI] [PubMed] [Google Scholar]
- Rohrmann G.F. Evolution of occluded baculoviruses. In: Granados R.R., Federici B.A., editors. Vol. 1. CRC Press; Boca Raton, FL: 1986. pp. 203–315. (“The Biology of Baculoviruses”). 2 vols. [Google Scholar]
- Rosas‐Acosta G., Braunagel S.C., Summers M.D. Effects of deletion and overexpression of the Autographa californica nuclear polyhedrosis virus FP25K gene on synthesis of two occlusion‐derived virus envelope proteins and their transport into virus‐induced intranuclear membranes. J. Virol. 2001;75(22):10829–10842. doi: 10.1128/JVI.75.22.10829-10842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R.L., Rohrmann G.F. Characterization of P91, a protein associated with virions of an Orgyia pseudotsugata baculovirus. Virology. 1997;233(1):210–223. doi: 10.1006/viro.1997.8599. [DOI] [PubMed] [Google Scholar]
- Russell R.L.Q., Rohrmann G.F. A baculovirus polyhedron envelope protein immunogold localization in infected cells and mature polyhedra. Virology. 1990;174(1):177–184. doi: 10.1016/0042-6822(90)90066-z. [DOI] [PubMed] [Google Scholar]
- Russell R.L.Q., Rohrmann G.F. A 25‐kDa protein is associated with the envelopes of occluded baculovirus virions. Virology. 1993;195(2):532–540. doi: 10.1006/viro.1993.1404. [DOI] [PubMed] [Google Scholar]
- Russell R.L.Q., Funk C.J., Rohrmann G.F. Association of a baculovirus‐encoded protein with the capsid basal region. Virology. 1997;227(1):142–152. doi: 10.1006/viro.1996.8304. [DOI] [PubMed] [Google Scholar]
- Saksena S., Shao Y., Braunagel S.C., Summers M.D., Johnson A.E. Cotranslational integration and initial sorting at the endoplasmic reticulum translocon of proteins destined for the inner nuclear membrane. Proc. Natl. Acad. Sci. USA. 2004;101(34):12537–12542. doi: 10.1073/pnas.0404934101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M., Farrar R.R., Jr., Domek J., Javaid I. Effects of virus concentration and ultraviolet irradiation on the activity of corn earworm and beet armyworm (Lepidoptera: Noctuidae) nucleopolyhedroviruses. J. Econ. Entomol. 2002;95(2):243–249. doi: 10.1603/0022-0493-95.2.243. [DOI] [PubMed] [Google Scholar]
- Slack J.M., Lawrence S.D. Evidence for proteolytic cleavage of the baculovirus occlusion‐derived virion envelope protein P74. J. Gen. Virol. 2005;86(Pt. 6):1637–1643. doi: 10.1099/vir.0.80832-0. [DOI] [PubMed] [Google Scholar]
- Slack J.M., Shapiro M. Anticarsia gemmatalis multicapsid nucleopolyhedrovirus v‐trex gene encodes a functional 3′ to 5′ exonuclease. J. Gen. Virol. 2004;85(Pt. 10):2863–2871. doi: 10.1099/vir.0.80109-0. [DOI] [PubMed] [Google Scholar]
- Slack J.M., Kuzio J., Faulkner P. Characterization of v‐cath, a cathepsin L‐like proteinase expressed by the baculovirus Autographa californica multiple necular polyhedrosis virus. J. Gen. Virol. 1995;76:1091–1098. doi: 10.1099/0022-1317-76-5-1091. [DOI] [PubMed] [Google Scholar]
- Slack J.M., Dougherty E.M., Lawrence S.D. A study of the Autographa californica multiple nucleopolyhedrovirus ODV envelope protein p74 using a GFP tag. J. Gen. Virol. 2001;82(Pt. 9):2279–2287. doi: 10.1099/0022-1317-82-9-2279. [DOI] [PubMed] [Google Scholar]
- Slack J.M., Ribeiro B.M., Lobo de Souza M. The gp64 locus of the Anticarsia gemmatalis multicapsid nucleopolyhedrovirus contains a three prime repair exonuclease homologue and lacks v‐cath and ChiA genes. J. Gen. Virol. 2004;85:211–219. doi: 10.1099/vir.0.19617-0. [DOI] [PubMed] [Google Scholar]
- Slavicek J.M., Hayes‐Plazolles N. The Lymantria dispar nucleopolyhedrovirus contains the capsid‐associated p24 protein gene. Virus Genes. 2003;26(1):15–18. doi: 10.1023/a:1022317819378. [DOI] [PubMed] [Google Scholar]
- Slavicek J.M., Popham H.J. The Lymantria dispar nucleopolyhedrovirus enhancins are components of occlusion‐derived virus. J. Virol. 2005;79(16):10578–10588. doi: 10.1128/JVI.79.16.10578-10588.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon D.A., Cardoso M.C., Knudsen E.S. Dynamic targeting of the replication machinery to sites of DNA damage. J. Cell Biol. 2004;166(4):455–463. doi: 10.1083/jcb.200312048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs G.R., Fraser T., Fraser M. Changes in growth and virulence of a nuclear polyhedrosis virus from choristoneura fumiferana after passage in Trichoplusia ni and galleria mellonella. J. Invertebr. Pathol. 1981;38(2):230–235. [Google Scholar]
- Stewart T.M., Huijskens I., Willis L.G., Theilmann D.A. The Autographa californica multiple nucleopolyhedrovirus ie0‐ie1 gene complex is essential for wild‐type virus replication, but either IE0 or IE1 can support virus growth. J. Virol. 2005;79(8):4619–4629. doi: 10.1128/JVI.79.8.4619-4629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Kanaya T., Okazaki H., Ogawa K., Usami A., Watanabe H., Kadono‐Okuda K., Yamakawa M., Sato H., Mori H., Takahashi S., Oda K. Efficient protein production using a Bombyx mori nuclear polyhedrosis virus lacking the cysteine proteinase gene. J. Gen. Virol. 1997;78(Pt. 12):3073–3080. doi: 10.1099/0022-1317-78-12-3073. [DOI] [PubMed] [Google Scholar]
- Tellam R.L., Wijffels G., Willadsen P. Peritrophic matrix proteins. Insect. Biochem. Mol. Biol. 1999;29(2):87–101. doi: 10.1016/s0965-1748(98)00123-4. [DOI] [PubMed] [Google Scholar]
- Terra W.R., Ferreira C. Insect digestive enzymes: Properties, compartmentalization and function. Com. Biochem. Physiol. 1994;109B:1–62. [Google Scholar]
- Theilmann D.A., Chantler J.K., Stweart S., Flipsen H.T., Vlak J.M., Crook N.E. Characterization of a highly conserved baculovirus structural protein that is specific for occlusion‐derived virions. Virology. 1996;218(1):148–158. doi: 10.1006/viro.1996.0175. [DOI] [PubMed] [Google Scholar]
- Theilmann D.A., Willis L.G., Bosch B.J., Forsythe I.J., Li Q. The baculovirus transcriptional transactivator ie0 produces multiple products by internal initiation of translation. Virology. 2001;290(2):211–223. doi: 10.1006/viro.2001.1165. [DOI] [PubMed] [Google Scholar]
- Thiem S.M., Chejanovsky N. The role of baculovirus apoptotic suppressors in AcMNPV‐mediated translation arrest in Ld652Y cells. Virology. 2004;319(2):292–305. doi: 10.1016/j.virol.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Thiem S.M., Miller L.K. A baculovirus gene with a novel transcription pattern encodes a polypeptide with a zinc finger and a leucine zipper. J. Virol. 1989;63(11):4489–4497. doi: 10.1128/jvi.63.11.4489-4497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiem S.M., Miller L.K. Identification, sequence, and transcriptional mapping of the major capsid protein gene of the baculovirus Autographa californica nuclear polyhedrosis virus. J. Virol. 1989;63(5):2008–2018. doi: 10.1128/jvi.63.5.2008-2018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley T.W., Kelly D.C. Taxonomy and nomenclature of insect pathogenic viruses. In: Maramorosch K., Sherman K.E., editors. Vol. 3. Academic Press; Orlando, FL: 1985. (“Insecticides for Biological Control”). [Google Scholar]
- Tweeten K.A., Bulla L.A., Consigli R.A. Characterization of an extremely basic protein derived from granulosis virus nucleocapsid. J. Virol. 1980;33:866–876. doi: 10.1128/jvi.33.2.866-876.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwo M.F., Ui‐Tei K., Park P., Takeda M. Replacement of midgut epithelium in the greater wax moth, Galleria mellonela, during larval‐pupal moult. Cell Tissue Res. 2002;308(2):319–331. doi: 10.1007/s00441-002-0515-1. [DOI] [PubMed] [Google Scholar]
- van Oers M.M., Abma‐Henkens M.H., Herniou E.A., de Groot J.C., Peters S., Vlak J.M. Genome sequence of Chrysodeixis chalcites nucleopolyhedrovirus, a baculovirus with two DNA phytolyase genes. J. Gen. Virol. 2005;86(7):2069–2080. doi: 10.1099/vir.0.80964-0. [DOI] [PubMed] [Google Scholar]
- Vanarsdall A.L., Okano K., Rohrmann G.F. Characterization of a baculovirus with a deletion of vlf‐1. Virology. 2004;326(1):191–201. doi: 10.1016/j.virol.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Vialard J.E., Richardson C.D. The 1629‐nucleotide open reading frame located downstream of the Autographa californica nuclear polyhedrosis virus polyhedrin gene encodes a nucleocapsid‐associated phosphoprotein. J. Virol. 1993;67(10):5859–5866. doi: 10.1128/jvi.67.10.5859-5866.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman L.E., Goldsmith P.A. Mechanism of neutralization of budded Autographa californica nuclear polyhedrosis virus by a monoclonal antibody: Inhibition of entry by adsorptive endocytosis. Virology. 1985;143(1):185–195. doi: 10.1016/0042-6822(85)90107-2. [DOI] [PubMed] [Google Scholar]
- Volkman L.E., Zaal K.J.M. Autographa‐californica m nuclear polyhedrosis virus microtubules and replication. Virology. 1990;175(1):292–302. doi: 10.1016/0042-6822(90)90211-9. [DOI] [PubMed] [Google Scholar]
- Volkman L.E., Goldsmith P.A., Hess R.T., Faulkner P. Neutralization of budded Autographa californica NPV by a monoclonal antibody: Identification of the target antigen. Virology. 1984;133(2):354–362. doi: 10.1016/0042-6822(84)90401-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderfecht S.L., Miskuff R.L., Wee S.B., Sato S., Tidwell R.R., Geratz J.D., Yolken R.H. Protease inhibitors suppress the in vitro and in vivo replication of rotavirus. J. Clin. Invest. 1988;82(6):2011–2016. doi: 10.1172/JCI113821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Granados R.R. An intestinal mucin is the target substrate for a baculovirus enhancin. Proc. Natl. Acad. Sci. USA. 1997;94(13):6977–6982. doi: 10.1073/pnas.94.13.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Granados R.R. Molecular cloning and sequencing of a novel invertebrate intestinal mucin cDNA. J. Biol. Chem. 1997;272(26):16663–16669. doi: 10.1074/jbc.272.26.16663. [DOI] [PubMed] [Google Scholar]
- Wang P., Hammer D.A., Granados R.R. Interaction of Trichoplusia ni granulosis virus‐encoded enhancin with the midgut epithelium and peritrophic membrane of four lepidopteran insects. J. Gen. Virol. 1994;75(8):1961–1967. doi: 10.1099/0022-1317-75-8-1961. [DOI] [PubMed] [Google Scholar]
- Wang X., Kelly C.C. Neutralisation of Trichoplusia ni nuclear polyhedrosis virus with antisera to two forms of the virus. Microbiologica (Bologna) 1985;8(2):141–149. [PubMed] [Google Scholar]
- Washburn J.O., Kirkpatrick B.A., Volkman L.E. Comparative pathogenesis of Autographa californica M nuclear polyhedrosis virus in larvae of Trichoplusia ni and Heliothis virescens. Virology. 1995;209:561–568. doi: 10.1006/viro.1995.1288. [DOI] [PubMed] [Google Scholar]
- Washburn J.O., Lyons E.H., Haas‐Stapleton E.J., Volkman L.E. Multiple nucleocapsid packaging of Autographa californica nucleopolyhedrovirus accelerates the onset of systemic infection in Trichoplusia ni. J. Virol. 1999;73(1):411–416. doi: 10.1128/jvi.73.1.411-416.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn J.O., Chan E.Y., Volkman L.E., Aumiller J.J., Jarvis D.L. Early synthesis of budded virus envelope fusion protein GP64 enhances Autographa californica multicapsid nucleopolyhedrovirus virulence in orally infected Heliothis virescens. J. Virol. 2003;77(1):280–290. doi: 10.1128/JVI.77.1.280-290.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn J.O., Trudeau D., Wong J.F., Volkman L.E. Early pathogenesis of Autographa californica multiple nucleopolyhedrovirus and Helicoverpa zea single nucleopolyhedrovirus in Heliothis virescens: A comparison of the ‘M’ and ‘S’ strategies for establishing fatal infection. J. Gen. Virol. 2003;84(Pt. 2):343–351. doi: 10.1099/vir.0.18701-0. [DOI] [PubMed] [Google Scholar]
- Weger S., Hammer E., Heilbronn R. Topors, a p53 and topoisomerase I binding protein, interacts with the adeno‐associated virus (AAV‐2) Rep78/68 proteins and enhances AAV‐2 gene expression. J. Gen. Virol. 2002;83(Pt. 3):511–516. doi: 10.1099/0022-1317-83-3-511. [DOI] [PubMed] [Google Scholar]
- Westenberg M., Wang H., WF I.J., Goldbach R.W., Vlak J.M., Zuidema D. Furin is involved in baculovirus envelope fusion protein activation. J. Virol. 2002;76(1):178–184. doi: 10.1128/JVI.76.1.178-184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenberg M., Veenman F., Roode E.C., Vlak J.M., Zuidema D. Functional analysis of the putative fusion domain of the baculovirus envelope fusion protein F. J. Virol. 2004;78:6946–6954. doi: 10.1128/JVI.78.13.6946-6954.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WF I.J., Westenberg M., Goldbach R.W., Blissard G.W., Vlak J.M., Zuidema D. A novel baculovirus envelope fusion protein with a proprotein convertase cleavage site. Virology. 2000;275(1):30–41. doi: 10.1006/viro.2000.0483. [DOI] [PubMed] [Google Scholar]
- Whitford M., Faulkner P. A structural polypeptide of the baculovirus Autographa californica nuclear polyhedrosis virus contains O‐linked N‐acetylglucosamine. J. Virol. 1992;66(6):3324–3329. doi: 10.1128/jvi.66.6.3324-3329.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford M., Stewart S., Kuzio J., Faulkner P. Identification and sequence analysis of a gene encoding gp67, an abundant envelope glycoprotein of the baculovirus Autographa californica nuclear polyhedrosis virus. J. Virol. 1989;63(3):1393–1399. doi: 10.1128/jvi.63.3.1393-1399.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt M.A., Manning J.S. A phosphorylated 34‐kDa protein and a subpopulation of polyhedrin are thiol linked to the carbohydrate layer surrounding a baculovirus occlusion body. Virology. 1988;163(1):33–42. doi: 10.1016/0042-6822(88)90231-0. [DOI] [PubMed] [Google Scholar]
- Wigglesworth V.B. “Insect Physiology,”. 8th Ed. Chapman and Hall; New York: 1984. Respiration; pp. 18–21. [Google Scholar]
- Williams G.V., Faulkner P. Cytological changes and viral morphogenesis during Baculovirus infection. In: Miller L.K., editor. “The Baculoviruses”. Plenum Press; New York: 1997. pp. 61–108. [Google Scholar]
- Williams G.V., Rohel D.Z., Kuzio J., Faulkner P. A cytopathological investigation of Autographa californica nuclear polyhedrosis virus p10 gene function using insertion‐deletion mutants. J. Gen. Virol. 1989;70(1):187–202. doi: 10.1099/0022-1317-70-1-187. [DOI] [PubMed] [Google Scholar]
- Willis L.G., Siepp R., Stewart T.M., Erlandson M.A., Theilmann D.A. Sequence analysis of the complete genome of Trichoplusia ni single nucleopolyhedrovirus and the identification of a baculoviral photolyase gene. Virology. 2005;338(2):209–226. doi: 10.1016/j.virol.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Wilson M.E., Consigli R.A. Functions of a protein kinase activity associated with purified capsids of the granulosis virus infecting Plodia interpunctella. Virology. 1985;143:526–535. doi: 10.1016/0042-6822(85)90391-5. [DOI] [PubMed] [Google Scholar]
- Wilson M.E., Miller L.K. Changes in the nucleoprotein complexes of a baculovirus DNA during infection. Virology. 1986;151:315–328. doi: 10.1016/0042-6822(86)90052-8. [DOI] [PubMed] [Google Scholar]
- Wilson M.E., Price K.H. Association of Autographa californica nuclear polyhedrosis virus (AcMNPV) with the nuclear matrix. Virology. 1988;167(1):233–241. doi: 10.1016/0042-6822(88)90073-6. [DOI] [PubMed] [Google Scholar]
- Wilson M.E., Mainprize T.H., Friesen P.D., Miller L.K. Location, transcription, and sequence of a baculovirus gene encoding a small arginine‐rich polypeptide. J. Virol. 1987;61(3):661–666. doi: 10.1128/jvi.61.3.661-666.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt D.J. Photoreactivation and ultraviolet‐enhanced reactivation of ultraviolet‐irradiated nuclear polyhedrosis virus by insect cells. Arch. Virol. 1984;79(1–2):95–107. doi: 10.1007/BF01314307. [DOI] [PubMed] [Google Scholar]
- Wolgamot G.M., Gross C.H., Russell R.L., Rohrmann G.F. Immunocytochemical characterization of p24, a baculovirus capsid‐associated protein. J. Gen. Virol. 1993;74(Pt. 1):103–107. doi: 10.1099/0022-1317-74-1-103. [DOI] [PubMed] [Google Scholar]
- Wood H.A. Isolation and replication of an occlusion body mutant of the Autographa californica nuclear polyhedrosis virus. Virology. 1980;105:338–344. doi: 10.1016/0042-6822(80)90035-5. [DOI] [PubMed] [Google Scholar]
- Wormleaton S., Kuzio J., Winstanley D. The complete sequence of the Adoxophyes orana granulovirus genome. Virology. 2003;311(2):350–365. doi: 10.1016/s0042-6822(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Wu D., Deng F., Sun X., Wang H., Yuan L., Vlak J.M., Hu Z. Functional analysis of FP25K of Helicoverpa armigera single nucleocapsid nucleopolyhedrovirus. J. Gen. Virol. 2005;86(Pt. 9):2439–2444. doi: 10.1099/vir.0.81110-0. [DOI] [PubMed] [Google Scholar]
- Wu Y., Carstens E.B. A baculovirus single‐stranded DNA binding protein, LEF‐3, mediates the nuclear localization of the putative helicase P143. Virology. 1998;247(1):32–40. doi: 10.1006/viro.1998.9235. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. Subunit rearrangement of the cyclin‐dependent kinases is associated with cellular transformation. Genes Dev. 1993;7(8):1572–1583. doi: 10.1101/gad.7.8.1572. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N., Chung S.M., Shiroeda O., Koyama K., Imanishi J. Characterization of a cathepsin L‐like enzyme screted from human pancreatic cancer cell line HPC‐YP. Cancer Res. 1990;50(3):658–663. [PubMed] [Google Scholar]
- Yanase T., Hashimoto Y., Kawarabata T. Identification of insertion and deletion genes in Autographa californica nucleopolyhedrovirus variants isolated from Galleria mellonella, Spodoptera exigua, Spodoptera litura and Xestia c‐nigrum. Virus Genes. 2000;21(3):167–177. doi: 10.1023/a:1008183329145. [DOI] [PubMed] [Google Scholar]
- Yang D.H., de Jong J.G., Makhmoudova A., Arif B.M., Krell P.J. Choristoneura fumiferana nucleopolyhedrovirus encodes a functional 3′‐5′ exonuclease. J. Gen. Virol. 2004;85(Pt. 12):3569–3573. doi: 10.1099/vir.0.80592-0. [DOI] [PubMed] [Google Scholar]
- Yang S., Miller L.K. Control of baculovirus polyhedrin gene expression by very late factor 1. Virology. 1998;248(1):131–138. doi: 10.1006/viro.1998.9272. [DOI] [PubMed] [Google Scholar]
- Yang S., Miller L.K. Expression and mutational analysis of the baculovirus very late factor 1 (vlf‐1) gene. Virology. 1998;245(1):99–109. doi: 10.1006/viro.1998.9152. [DOI] [PubMed] [Google Scholar]
- Yao L., Zhou W., Xu H., Zheng Y., Qi Y. The Heliothis armigera single nucleocapsid nucleopolyhedrovirus envelope protein P74 is required for infection of the host midgut. Virus Res. 2004;104(2):111–121. doi: 10.1016/j.virusres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Yin C., Yu J., Wang L., Li Z., Zhang P., Pang Y. Identification of a novel protein associated with envelope of occlusion‐derived virus in Spodoptera litura multicapsid nucleopolyhedrovirus. Virus Genes. 2003;26(1):5–13. doi: 10.1023/a:1022310202540. [DOI] [PubMed] [Google Scholar]
- Young J.C., Mackinnon E.A., Faulkner P. The architecture of the virogenic stroma in isolated nuclei of Spodoptera frugiperda cells in vitro infected by Autographa californica nuclear polyhedrosis virus. J. Struct. Biol. 1993;110(2):141–153. [Google Scholar]
- Zanotto P.M., Kessing B.D., Maruniak J.E. Phylogenetic interrelationships among baculoviruses: Evolutionary rates and host associations. J. Invertebr. Pathol. 1993;62(2):147–164. doi: 10.1006/jipa.1993.1090. [DOI] [PubMed] [Google Scholar]
- Zhang G. Research, development and application of Heliothis viral pesticide in China. Resour. Environ. Yangtze Valley. 1994;3:1–6. [Google Scholar]
- Zhang H., Xiong Y., Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol. Biol. Cell. 1993;4(9):897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.H., Ohkawa T., Washburn J.O., Volkman L.E. Effects of Ac150 on virulence and pathogenesis of Autographa californica multiple nucleopolyhedrovirus in noctuid hosts. J. Gen. Virol. 2005;86(Pt. 6):1619–1627. doi: 10.1099/vir.0.80930-0. [DOI] [PubMed] [Google Scholar]
- Zhou W., Yao L., Xu H., Yan F., Qi Y. The function of envelope protein P74 from Autographa californica multiple nucleopolyhedrovirus in primary infection to host. Virus Genes. 2005;30(2):139–150. doi: 10.1007/s11262-004-5623-4. [DOI] [PubMed] [Google Scholar]
- Zudaire E., Simpson S.J., Illa I., Montuenga L.M. Dietary influences over proliferating cell nuclear antigen expression in the locust midgut. J. Exp. Biol. 2004;207(Pt. 13):2255–2265. doi: 10.1242/jeb.01004. [DOI] [PubMed] [Google Scholar]


