Abstract
Regulation of protein synthesis by viruses occurs at all levels of translation. Even prior to protein synthesis itself, the accessibility of the various open reading frames contained in the viral genome is precisely controlled. Eukaryotic viruses resort to a vast array of strategies to divert the translation machinery in their favor, in particular, at initiation of translation. These strategies are not only designed to circumvent strategies common to cell protein synthesis in eukaryotes, but as revealed more recently, they also aim at modifying or damaging cell factors, the virus having the capacity to multiply in the absence of these factors. In addition to unraveling mechanisms that may constitute new targets in view of controlling virus diseases, viruses constitute incomparably useful tools to gain in-depth knowledge on a multitude of cell pathways.
Abbreviations of virus names
- AAV-2
Adeno-associated virus type 2
- AMCV
Artichoke mottled crinkle virus
- APV
Achritosiphon pisum virus
- ASLV
Avian sarcoma leukemia virus
- BDV
Borna disease virus
- BLV
Bovine leukemia virus
- BNYVV
Beet necrotic yellow vein virus
- BSBV
Beet soil-borne virus
- BSMV
Barley stripe mosaic virus
- BVQ
Beet virus Q
- BWYV
Beet western yellows virus
- BYDV
Barley yellow dwarf virus
- BYV
Beet yellows virus
- CaMV
Cauliflower mosaic virus
- CarMV
Carnation mottle virus
- CCFV
Cardamine chlorotic fleck virus
- CCSV
Cucumber chlorotic spot virus
- CoMV
Cocksfoot mottle virus
- CNV
Cucumber necrosis virus
- CPMV
Cowpea mosaic virus
- CrPV
Cricket paralysis virus
- CRSV
Carnation ringspot virus
- CTV
Citrus tristeza virus
- CVB
Coxsackie virus B
- CyRSV
Cymbidium ringspot virus
- DmeGypV
Drosophila melanogaster gypsy virus
- EAV
Equine arterivirus
- EBV
Epstein–Barr virus
- EIAV
Equine infectious anemia virus
- EMCV
Encephalomyocarditis virus
- EqTV
Equine torovirus
- FCV
Feline calicivirus
- FMDV
Foot-and-mouth disease virus
- HAstV
Human astrovirus
- HAV
Hepatitis A virus
- HBV
Hepatitis B virus
- HCMV
Human cytomegalovirus
- HCV
Hepatitis C virus
- HDV
Hepatitis delta virus
- HIV-1
Human immunodeficiency virus 1
- HPIV-1
Human parainfluenza virus 1
- HPV
Human papillomavirus
- HRV
Human rhinovirus
- HSV-1
Herpes simplex virus 1
- HTLV-1
Human T-lymphotropic virus 1
- IBV
Infectious bronchitis virus
- LIYV
Lettuce infectious yellows virus
- LRV1-1
Leishmania RNA virus 1-1
- MCMV
Maize chlorotic mottle virus
- MHV
Murine hepatitis virus
- MLV
Murine leukemia virus
- MMTV
Mouse mammary tumor virus
- MNSV
Melon necrotic spot virus
- MoMLV
Moloney murine leukaemia virus
- NV
Norwalk virus
- OCSV
Oat chlorotic stunt virus
- PCMV
Peach chlorotic mottle virus
- PCV
Peanut clump virus
- PEBV
Pea early-browning virus
- PEMV
Pea enation mosaic virus
- PLRV
Potato leafroll virus
- PPV
Plum pox virus
- PSIV
Plautia stali intestine virus
- PVM
Potato virus M
- RCNMV
Red clover necrotic mottle virus
- RhPV
Rhodopalosiphum padi virus
- RTBV
Rice tungro bacilliform virus
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- SbDV
Soybean dwarf virus
- SBWMV
Soil-borne wheat mosaic virus
- SceTy1V
Saccharomyces cerevisiae Ty1 virus
- SceTy3V
Saccharomyces cerevisiae Ty3 virus
- SCNMV
Sweet clover necrotic mottle virus
- ScV-L-A
Saccharomyces cerevisiae virus L-A
- SFV
Semliki Forest virus
- SINV
Sindbis virus
- STNV
Satellite tobacco necrosis virus
- SV40
Simian virus 40
- TBSV
Tomato bushy stunt virus
- TCV
Turnip crinkle virus
- TEV
Tobacco etch virus
- TMEV
Theiler's murine encephalomyelitis virus
- TMV
Tobacco mosaic virus
- TNV
Tobacco necrosis virus
- TRV
Tobacco rattle virus
- TuMV
Turnip mosaic virus
- VSV
Vesicular stomatitis virus
- WDSV
Walleye dermal sarcoma virus
- Other abbreviations
- aa
amino acid
- CAT
chloramphenicol acetyltransferase
- 3'-CITE
3'-cap-independent translation element
- CP
coat protein
- eEF
eukaryotic elongation factor
- eIF
eukaryotic initiation factor
- eRF
eukaryotic release factor
- 4E-BP
eIF4E-binding protein
- GCN2
general control nonderepressible-2
- GP
glycoprotein
- IGR
intergenic region
- IRES
internal ribosome entry site
- ITAF
IRES trans-acting factor
- nt
nucleotide
- ORF
open reading frame
- P
phosphoprotein
- PABP
poly(A) binding protein
- Paip1
PABP-interacting protein 1
- PCBP
poly(rC) binding protein
- PERK
PKR-like endoplasmic reticulum kinase
- PKR
protein kinase RNA
- PTB
pyrimidine tract binding protein
- RdRp
RNA-dependent RNA polymerase
- sORF
short ORF
- sg
subgenomic
- TAV
transactivator
- TC
ternary complex (eIF2-GTP-Met-tRNAiMet)
- TE
translation enhancer
- TLS
tRNA-like structure
- unr
upstream of N-ras
- uORF2
upstream ORF2
- UTR
untranslated region
- VPg
viral protein genome linked
I. Introduction
Because of the small size of their genomes and hence of their limited coding capacity, viruses have evolved a cohort of strategies to synthesize a few—and borrow from their host many—of the numerous elements required for their multiplication. The sophistication of the strategies elaborated by viruses is unsurpassed, and many of these strategies are common among viruses, but are rare or even nonexistent in uninfected cells. Many were first demonstrated in viral systems before being described in cell systems (reviewed in Bernardi and Haenni, 1998). The genome of viruses is compact and used to its limits: overlapping open reading frames (ORFs) are frequent, intergenic regions (IGRs) are usually short, and noncoding as well as coding regions are often involved in regulation of replication, transcription, and/or translation.
This chapter presents an overview of the strategies used by viruses of eukaryotes to regulate the expression of their viral genomes, ranging from the production of the RNA templates to translation of the encoded proteins. Emphasis is placed on RNA viruses, in which most of the strategies were originally described; moreover, only a few examples are taken from retroviruses, since the strategies used by these viruses have been discussed at length in several recent review articles (Balvay et al., 2007, Brierley and Dos Ramos, 2006, Goff, 2004, Yilmaz et al., 2006). For further information dealing with certain aspects of translation regulation mechanisms used by viruses, the reader may wish to turn to other reviews (Bushell and Sarnow, 2002, Gale et al., 2000, Mohr et al., 2007, Ryabova et al., 2002).
II. Regulation Prior to Translation
Viruses use several regulation strategies prior to translation to obtain maximum protein diversity from their small genomes. Prior to initiation of translation, the viral RNA, due to serve as template for protein synthesis, can be modified so as to favor synthesis of certain viral proteins, sometimes to the detriment of cell proteins. This can be achieved by various mechanisms such as editing, splicing, and the production of subgenomic (sg) RNAs including cap-snatching. The importance of regulation at this level has, moreover, been highlighted in recent publications showing that viral translation and transcription are coupled (Barr, 2007, Katsafanas and Moss, 2007, Sanz et al., 2007).
A. Editing
Editing is a mechanism in which an RNA-encoded nucleotide (nt) is modified, or one, two, or more pseudotemplated nts are inserted at the editing site; various forms of editing have been described (Weissmann et al., 1990). Viruses resort to editing by nt modification in the case of Hepatitis delta virus (HDV; genus Deltavirus), and by the addition of one or more nts in paramyxoviruses.
1. Editing by nucleotide modification
HDV is a highly pathogenic subviral particle totally dependent on the DNA virus Hepatitis B virus (HBV; family Hepadnaviridae) for its propagation (reviewed in Taylor, 2006); it requires the HBV envelope proteins to assemble into HDV particles. The genome of HDV is a (−) sense, closed circular, and highly structured single-stranded RNA (of ∼ 1680 nts in HDV genotype III) referred to as the genomic RNA (Fig. 1 ); it is devoid of coding capacity (i.e., devoid of ORF). However, the complementary antigenomic RNA contains a unique ORF for the short surface antigen HDAg-S of 195 amino acids (aa); HDAg-S is produced from an 800‐nt long linear sg mRNA that is both capped and polyadenylated (Gudima et al., 2000). The protein is produced throughout infection and is required for HDV replication. At late times in infection, editing of the antigenomic RNA occurs by deamination of the A residue (position 1012) of the UAG codon that ends the HDAg-S ORF; editing does not occur on the HDAg-S mRNA. Hence the antigenome, which is the template for editing, must be replicated to yield the edited genomic RNA prior to being transcribed to produce the edited sg mRNA that is also capped and polyadenylated. Editing also requires previous refolding of the antigenome, from a rod-like to a branched double-hairpin structure in HDV genotype III, or to a highly conserved base-paired structure in HDV genotype I (Casey, 2002, Cheng et al., 2003).
Figure 1.
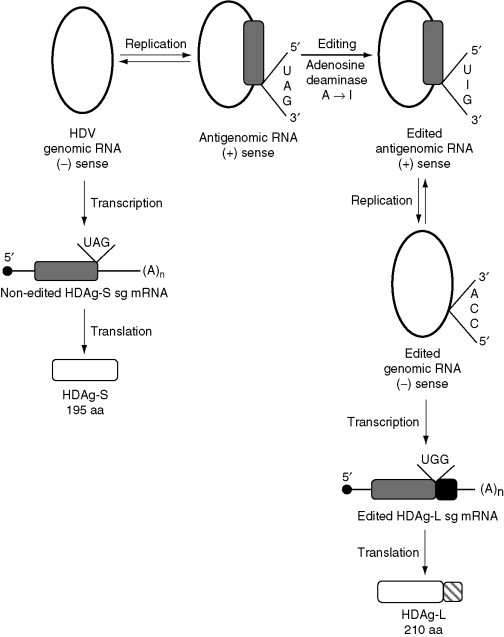
RNA editing of the antigenomic RNA during HDV replication. Editing allows the virus to express two proteins HDAg-S and HDAg-L from one coding sequence. The black circle represents the 5′-capped terminus of the mRNA. (A)n represents a poly(A) tail. Gray rectangles represent ORFs, the black rectangle represents the edited ORF region, the white rectangles represent the HDAg protein, and the gray slashed rectangle represents the extended C-terminal region in the edited protein.
Deamination of the A residue in UAG leading to an I (inosine) residue and producing the triplet UIG (Fig. 1) is triggered by a host adenosine deaminase that acts on RNA substrates. Upon replication of the edited antigenome, the I residue recognized as G leads to ACC in the edited genomic RNA that is then transcribed as UGG coding for tryptophane in the edited mRNA. As a consequence, the edited sg mRNA presents an extended ORF and produces HDAg-L of 210 aa. The two viral proteins share the same N-terminal region, the longer protein bearing an extended C-terminal region; they are responsible for two distinct functions in the HDV-infected cell. The longer protein inhibits replication and editing and is necessary for virus assembly, whereas the shorter protein is required for replication (Cheng et al., 2003). Editing is, therefore, a vital process for HDV propagation, and an exquisite balance between the nonedited and edited mRNAs, and between replication and virus production is a major factor in maintaining optimum virus production. How this equilibrium is reached remains largely speculative, although editing is known to involve specific structural elements that depend on the HDV genotype considered (Casey, 2002, Cheng et al., 2003).
2. Editing by nucleotide addition
In a coding RNA, the introduction of nontemplated nts leads to the production of a new edited mRNA. In such an mRNA, a change in reading frame at the point of editing has occurred, resulting in the synthesis of a new protein. The new “edited” protein is identical to the “original” protein resulting from the nonedited mRNA, from the 5′ terminus to the editing site, but different thereafter. The protein resulting from editing is usually endowed with properties and/or activities that are absent from the original protein.
Paramyxoviruses are animal viruses that belong to the order Mononegavirales. They possess a nonsegmented (also known as monopartite) (−) strand RNA genome of 15–16 kb (reviewed in Nagai, 1999). Their genome encodes a minimum of six structural proteins that are produced from six capped and polyadenylated mRNAs. In the complementary antigenomic RNA, the ORFs are separated by conserved sequences that dictate initiation and termination of the six transcripts. Except for the phosphoprotein (P) mRNA, each mRNA expresses a single protein from a single ORF. The P gene is more complex. In most members of the subfamily Paramyxovirinae (family Paramyxoviridae), editing of the P mRNA results in the insertion of 1–5 nontemplated G residues within a run of Gs at the level of a conserved AnGn editing sequence (Cattaneo et al., 1989, Mahapatra et al., 2003, Steward et al., 1993; reviewed in Strauss and Strauss, 1991), presumably as a result of a stuttering process (Vidal et al., 1990). This causes a shift within the P ORF and may lead to the synthesis of up to six nonstructural proteins from edited and nonedited mRNAs depending on the virus. In Sendai virus, two mRNAs can be produced by editing of the P/C (also known as P) mRNA, the V mRNA (insertion of 1 G), and the W mRNA (insertion of 2 or 5 Gs) (Fig. 2A ) (Curran et al., 1991). The V protein, a cysteine-rich protein, binds Zn+ 2, a characteristic related to virus pathogenicity in mice. Indeed, mutation of the cysteine residues in the corresponding V protein in the Sendai virus genome reduces Zn+ 2 binding and pathogenicity (Fukuhara et al., 2002).
Figure 2.
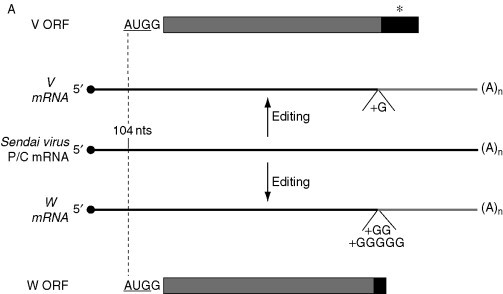
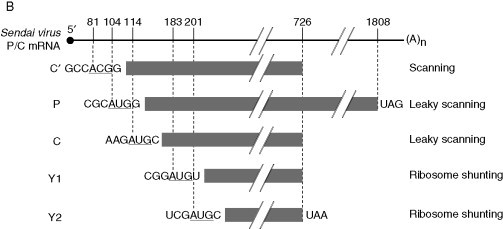
Schematic representation of the Sendai virus P/C mRNA expression. (A) Regulation prior to translation: editing of P/C mRNA. *, Cysteine-rich coding region. Initiation codon is underlined. (B) Regulation at initiation of translation of P/C mRNA. Initiation codons of each ORF are underlined and the sequence contexts are presented; termination codons are shown. Other indications are as in legend of Fig. 1.
Editing is also observed for the synthesis of the structural glycoproteins (GPs) of Ebola virus (family Filoviridae, order Mononegavirales), whose monopartite (−) strand RNA genome contains seven genes. Two GPs are produced from the GP gene, a short and a long form that make up 80% and 20% of the total GP protein synthesized, respectively; they differ in their C-terminal region. The short form of GP is produced by the unedited transcript, whereas the long form results from an edited transcript that has acquired an additional nontemplated A residue within a stretch of seven conserved A residues in the GP ORF. The long form possesses a transmembrane anchor sequence absent from the short form (Sanchez et al., 1996, Volchkov et al., 1995).
B. Splicing
Splicing is a strategy used by DNA viruses such as those of the family Adenoviridae and Polyomaviridae (reviewed in Ziff, 1980, Ziff, 1985), the Caulimoviridae (reviewed in Ryabova et al., 2006), the Baculoviridae (Chisholm and Henner, 1988, Kovacs et al., 1991), and of the genus Mastrevirus, family Geminiviridae (Schalk et al., 1989). It is less frequently encountered among RNA viruses, although it is observed in certain RNA viruses that replicate in the nucleus. This is the case of retroviruses whose mRNAs undergo a complicated cascade of splicing and alternative splicing events. The splicing mechanisms used by these viruses will not be developed here, having received considerable attention in several review articles (Cullen, 1998, Stoltzfus and Madsen, 2006). Examples of nonretroviruses whose RNA genomes multiply in the nucleus and employ splicing are briefly presented here; they are Borna disease virus (BDV) and Influenza virus.
BDV (family Bornaviridae) belongs to the order Mononegavirales. However, it differs from the other members of this order by several unique features (reviewed in de la Torre, 2002, Tomonaga et al., 2002). As opposed to the other members of this order whose life cycle occurs entirely in the cytoplasm, BDV is replicated and transcribed in the nucleus of the infected cell and employs the cellular RNA splicing machinery. The two splice donor and three splice acceptor sites follow the general mammalian splice site consensus (Fig. 3 ). The six ORFs contained in the antigenome are not separated by conserved IGRs as in other mononegavirales. Rather, the six proteins of BDV are translated from capped and polyadenylated transcripts that are initiated at only three sites (S1–S3) and terminate at five possible sites (T1–T4, t6). The nucleoprotein (known as N) is produced from a transcript initiated at S1, and the X protein and P from a transcript initiated at S2. The matrix (M), glycoprotein (G), and polymerase (L) are all produced from transcripts initiated at S3, and resort to alternative splicing for the production of the transcripts required. Splicing of intron I that overlaps the M ORF abolishes synthesis of the corresponding protein and produces protein G, while splicing of introns I and II (the latter corresponds to most of the G ORF) leads to the synthesis of the L protein. Additionally, splicing of intron III that uses the same 5′ splice donor site as intron II but another 3′ splice acceptor site also eliminates most of the G ORF as well as the 5′ region of the L ORF. This could lead to the production of yet another BDV protein; this putative protein has so far not been identified (reviewed in Jordan and Lipkin, 2001). Although translation of M is prevented by splicing of intron I, this leaves a minicistron corresponding to the N-terminal region of M which enhances translation of G, presumably by promoting ribosomal reinitiation. However, mutation experiments using the unspliced transcript, suggest that leaky scanning is also a mechanism that could lead to the synthesis of G from the unspliced transcript (Schneider et al., 1997).
Figure 3.
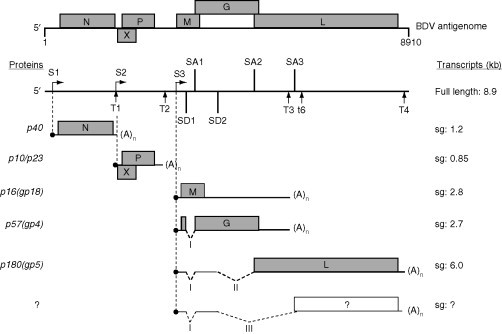
Regulation prior to translation by splicing in BDV. S1–S3, transcription initiation sites; T1–T4 and t6, polyadenylation/termination sites; SA1–SA3, splice acceptor sites; SD1 and SD2, splice donor sites. I, II, and III are introns. N, nucleoprotein; P, phosphoprotein; M, matrix; G, glycoprotein; and L, polymerase. The open rectangle corresponds to the ORF of a putative protein. Other indications are as in legend of Fig. 1.
Influenza viruses (family Orthomyxoviridae) are enveloped viruses with a segmented (−) strand RNA genome; they are replicated and transcribed in the nucleus by the viral RNA-dependent RNA polymerase (RdRp) complex composed of PB1, PB2, and PA. In the nucleus of infected cells, transcription of the viral RNAs into mRNAs by the RdRp requires cooperation with ongoing transcription by the cellular RNA polymerase II, since the RdRp initiates synthesis of viral mRNAs via cap-snatching using capped cellular mRNAs (see below; reviewed in Lamb and Krug, 2001, Rao et al., 2003). Influenza A virus and Influenza B virus are composed of eight RNA segments. Alternative splicing leads to the synthesis of two proteins from segments seven and eight of Influenza A virus. Regulation of the choice of the 5′ or 3′ splice sites is finely controlled. Although alternative splicing occurs in many viruses, only in a few cases have viral proteins been shown to be involved in this mechanism. In segment seven of Influenza A virus, two alternative 5′ splice sites control the production of the shorter (mRNA3: 111 nts) and the longer (M2 mRNA: 151 nts) spliced mRNAs from the pre-mRNA known as M1 mRNA. Both spliced RNAs use the same 3′ splice site. At early times after infection, the more favorable upstream 5′ splice site is used, leading to the synthesis of mRNA3 that potentially codes for a 9-aa peptide (as yet undetected). At later times after infection, the RdRp complex now produced in sufficient amounts binds to and blocks the upstream 5′ splice site, forcing the cell splicing machinery to switch to the less favorable downstream 5′ splice site. As a consequence, M2 mRNA is synthesized as is also its encoded M2 ion channel protein of 97 aa (Shih et al., 1995).
C. Subgenomic RNA synthesis
Contrary to mRNAs of eukaryotic cells that are largely monocistronic, the RNA genomes of many eukaryotic viruses contain multiple ORFs of which generally only the 5′-proximal ORF is accessible for translation. Thus, viruses have evolved several strategies to synthesize the proteins corresponding to 5′-distal ORFs (reviewed in Miller and Koev, 2000, White, 2002). One of the most common mechanisms is the production of 3′-coterminal sgRNAs. In such templates, the internally positioned and the 3′-proximal ORFs in the genome of (+) strand RNA viruses are accessed by sgRNAs in which these ORFs become 5′-proximal and serve as mRNAs. sgRNAs are generally synthesized by internal initiation of RNA synthesis on the complementary (−) RNA strand. They are 5′-truncated versions of the genomic RNA and therefore perfect copies of the region of the genome from which they derive.
A particular mechanism of sgRNA production is used by RNA viruses whose genome segments are ambisense or of (−) polarity and resort to cap-snatching. This mechanism was first described for the synthesis of the mRNAs of Influenza virus (Bouloy et al., 1978, Krug et al., 1979). The endonuclease activity of the viral RdRp cleaves nuclear cellular capped RNAs to generate capped primers of up to about 20 nts in length for viral mRNA synthesis. As a result, the viral mRNAs contain capped nonviral oligonucleotides at their 5′ end. Several plant (members of the family Bunyaviridae and of the genus Tenuivirus) and animal (members of the family Bunyaviridae) viruses with (−) strand or ambisense RNA genomes also use this transcription initiation mechanism. Since these viruses multiply in the cytoplasm, they use cytoplasmic rather than nuclear cellular capped RNAs as primers (Garcin and Kolakofsky, 1990, Garcin et al., 1995, Huiet et al., 1993, Raju et al., 1990, Ramírez et al., 1995, Vialat and Bouloy, 1992).
III. Initiation of Translation
A. Cap-dependent initiation
The most common strategy of translation initiation encountered among eukaryotes is cap-dependent translation (reviewed in Jackson and Kaminski, 1995, Pestova et al., 2007). This occurs in capped, generally monocistronic mRNAs, whose initiation codon lies close to the 5′ cap structure, and whose leader sequence also called 5′ untranslated region (UTR) possesses varying degrees of secondary structure. A number of complex steps lead to binding of the small 40S ribosomal subunit to the mRNA. The assembly of the eukaryotic initiation factor (eIF) 2, GTP, and Met-tRNAiMet forms the ternary complex (TC). Interaction of the TC with the 40S ribosomal subunit, facilitated by eIF1, eIF1A, and eIF3, leads to the formation of the 43S preinitiation complex. eIF3 is composed of 13 subunits (eIF3a–eIF3m) (Hinnebusch, 2006). The cap structure is recognized by the heterotrimer eIF4F composed of eIF4G (multivalent scaffolding protein), eIF4E (cap-binding protein), and eIF4A (ATP-dependent helicase). The 43S preinitiation complex binds to the 5′ end of the mRNA with the help of eIF4F in the presence of eIF4B, and the complex scans the mRNA leader sequence until it reaches the initiation codon to form the 48S initiation complex (Kozak and Shatkin, 1978). The initiation codon is usually the first AUG codon encountered; it is recognized by base-pairing with the anticodon of Met-tRNAiMet and the efficiency of recognition depends on the sequence context surrounding the initiation codon. The most favorable context in mammals is RCCAUGG with purine (R) at position − 3 (Kozak, 1986, Kozak, 1991), and in plants it is ACAAUGG (Fütterer and Hohn, 1996). At this step the 48S initiation complex is joined by the large 60S ribosomal subunit to form the 80S ribosome. Joining requires two additional factors: eIF5 and eIF5B. Hydrolysis of eIF2-bound GTP induced by eIF5 leads to reduction in the affinity of eIF2 for Met-tRNAiMet. In turn, the essential ribosome-dependent GTPase activity of eIF5B leads to displacement of the eIF2-bound GDP and other initiation factors from the 40S subunit (reviewed in Pestova et al., 2007). The assembled 80S ribosome contains the initiator Met-tRNAiMet in the ribosomal P (peptidyl) site and another aa-tRNA in the ribosomal A (aminoacyl) site. The delivery of the aa-tRNA is mediated by the eukaryotic elongation factor (eEF) 1A–GTP complex. After peptide bond formation (triggered by the peptidyl transferase in the ribosome) eEF2 binding and subsequent GTP hydrolysis catalyze ribosomal translocation, and the elongation cycle begins (Frank et al., 2007). This general strategy is also adopted by a large number of eukaryotic viruses. Yet the RNA genome of certain viruses lacks a cap structure; the 5′ end of such RNA genomes can carry a covalently bound viral protein designated viral protein genome-linked (VPg), or begin with a di- (or a mono-) phosphate. In other cases, the 5′ UTR of the viral RNA contains an internal ribosome entry site (IRES) responsible for initiation of translation.
B. Closed-loop model or circularization
In most eukaryotic mRNAs, the 5′ cap structure and the 3′ poly(A) tail appear to work together leading to efficient translation initiation. This is believed to occur when the 5′ and 3′ ends are brought in close proximity, referred to as the mRNA circularization or closed-loop model. The existence of cellular polyribosomes arranged in a circle was visualized using electron microscopy (Christensen et al., 1987). Circularization is brought about by binding of the initiation factor eIF4E to the 5′ cap and to eIF4G. In turn, eIF4G binds to the poly(A)-binding protein (PABP) bound to the 3′ poly(A) tail (Fig. 4A ). PABP contains four conserved RNA recognition motifs in its N-terminal domain that are involved in RNA and eIF4G interactions, and a C-terminal domain that binds to several proteins, including eIF4B, the eukaryotic release factor (eRF) 3 and the PABP-interacting protein 1 (Paip1). Therefore, PABP promotes the formation of the closed-loop complex by binding directly to eIF4G (Gallie, 1998, Imataka et al., 1998, Gale et al., 2000; reviewed in Dreher and Miller, 2006) or through Paip1 binding to eIF4A (Craig et al., 1998) or by PABP interaction with eIF4B (Bushell et al., 2001, Le et al., 1997) (Fig. 4A). Circularization thus appears to be mediated by RNA–protein and protein–protein interactions. Increasing evidence has been provided for the involvement of both 5′ and 3′ UTRs of eukaryotic mRNAs and viral mRNAs in initiation of translation (reviewed in Edgil and Harris, 2006, Hentze et al., 2007, Komarova et al., 2006, Mazumder et al., 2003, Wilkie et al., 2003). In addition to contributing to mRNA stabilization, circularization probably facilitates ribosome recruiting from the 3′ end of the mRNA after a terminated round of translation, to the 5′ region for initiation of a second round. This could be achieved via interaction of PABP with eRF3 that by interacting with eRF1, would result in the formation of a closed loop by way of the 5′ cap—eIF4E–eIF4G–PABP–eRF3–eRF1—termination codon (Fig. 4A) (Uchida et al., 2002).
Figure 4.
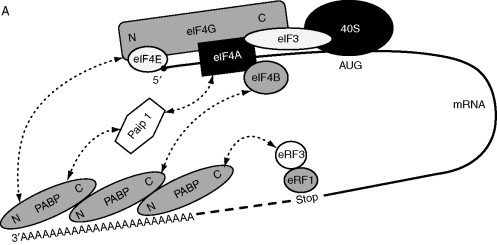
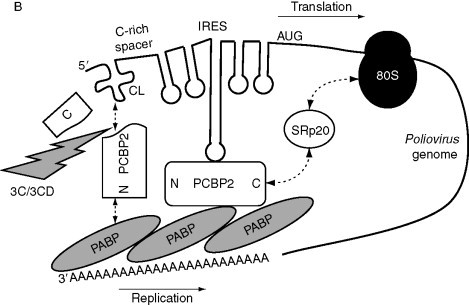
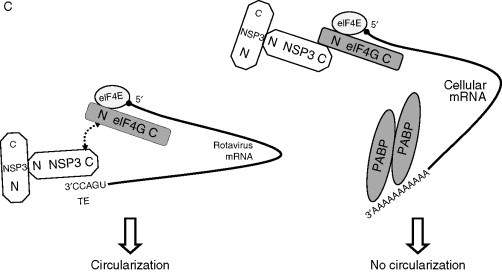
Possible models of circularization. (A) Closed-loop model or circularization of cellular mRNAs. eIF4E+eIF4G+eIF4A, eIF4F complex; Stop, termination codon. (B) Models of circularization of Poliovirus genome. CL, cloverleaf structure; 3C and 3CD, viral proteases. (C) Role of rotavirus NSP3 in mRNA circularization. NSP3 mediates viral mRNA circularization (left) and inhibits cellular mRNA circularization (right). N and C, N- and C-terminal regions of proteins; TE, translation enhancer. Dashed arrows indicate interactions between proteins. Other indications are as in legend of Fig. 1.
Certain viral mRNAs are devoid of 5′ cap structure or VPg, and some of them are devoid of 3′ poly(A) tail. Nevertheless, such mRNAs are highly efficient, and circularization presumably required for efficient translation is achieved via various mechanisms. In the case of viral RNAs with a poly(A) tail, circularization has been investigated by looking for viral and/or host proteins that participate in circularization. For instance, in Poliovirus (family Picornaviridae) RNA whose VPg is removed soon after entry of the virus into the cell, the long 5′ UTR of its genomic RNA contains an IRES preceded by a cloverleaf structure (Fig. 4B). PABP interacts with the poly(A) tail of the viral RNA. It also binds to the poly(rC)-binding protein 2 (PCBP2) that binds to the IRES structure to circularize the viral mRNA for translation (Blyn et al., 1997, Silvera et al., 1999, Walter et al., 2002). Binding of PCBP2 to PABP leads to circularization of the viral RNA with the formation of an RNA–protein–protein–RNA bridge. In addition, the cellular protein SRp20 that is involved in cellular mRNA splicing and nucleocytoplasmic trafficking and also cofractionates with ribosomal subunits, interacts with PCBP2, and promotes Poliovirus IRES-driven translation (Bedard et al., 2007).
Circularization can also be achieved by direct base pairing between a region in the 5′ UTR and a region in the 3′ UTR of an mRNA; it is used in particular by viral mRNAs that possess neither cap (or VPg) nor poly(A) tail. In several instances, specific interactions have been detected and their functional significance investigated by phylogenetic studies of conserved regions within the ends of viral RNAs, and by mutation analyses of the base-paired regions presumably involved (reviewed in Miller and White, 2006). Among plant RNA viruses, the region of the 3′ UTR required for translation is frequently referred to as 3′-cap-independent translation element (3′-CITE). Several classes of 3′-CITEs have been described (reviewed in Miller et al., 2007). They presumably operate by long-distance base pairing between the 3′ UTR and a complementary region in the 5′ UTR, leading to interactions known as kissing stem–loop interactions. Some of the well-studied cases are those of Tobacco necrosis virus (TNV; family Tombusviridae; Meulewaeter et al., 2004, Shen and Miller, 2004), Satellite tobacco necrosis virus (STNV; Guo et al., 2001, Meulewaeter et al., 1998) Tomato bushy stunt virus (TBSV; family Tombusviridae; Fabian and White, 2004, Fabian and White, 2006), Barley yellow dwarf virus (BYDV; family Luteoviridae; Guo et al., 2000, Guo et al., 2001), and Maize necrotic streak virus (family Tombusviridae; Scheets and Redinbaugh, 2006). An interesting outcome of these studies has been the observation that in certain cases, part of the 3′ UTR can function by recruiting initiation factors required for initiation of translation. This seems to be the case of STNV in which a 3′ translation enhancer (TE) mimics a 5′ cap structure (Gazo et al., 2004) by binding to eIF4E and this binding is enhanced by eIF4G (or eIFiso4E and eIFiso4G of eIFiso4F, isoforms only found in plants). Moreover, the 3′ UTR of STNV RNA contains a region that has been reported to be complementary of the 3′ end of the ribosomal 18S RNA (Danthinne et al., 1993). Thus, long-distance RNA–RNA interaction between the 5′ and 3′ UTRs might bring eIF4F and the 40S subunit positioned on 3′ UTR close to the initiation codon, favoring initiation of translation. Phylogenetic studies suggest that similar mechanisms may be involved in stimulating translation of other viral mRNAs devoid of cap and poly(A) tail (Gazo et al., 2004, Miller et al., 2007, Shen and Miller, 2004, Treder et al., 2008).
Rotaviruses (family Reoviridae) contain 11 double-stranded generally monocistronic RNAs; they are capped, and most of them contain a short conserved sequence (UGACC) at their 3′ end. This sequence serves as 3′ TE. Enhanced gene expression by the 3′ TE requires the viral nonstructural protein NSP3 (Fig. 4C). This protein binds not only to the 3′ TE but also to eIF4G, suggesting that it behaves as a functional homolog of PABP, leading to circularization of the mRNA (Piron et al., 1998). Moreover, upstream of the common UGACC sequence in its 3′ UTR, the mRNA of gene 6 coding for the structural protein VP6 possesses a unique gene-specific TE that does not require NSP3 for activity (Yang et al., 2004).
Finally, in the tripartite (+) strand RNA virus Alfalfa mosaic virus (family Bromoviridae), whose RNAs are capped but lack a poly(A) tail, a few molecules of coat (also known as capsid) protein (CP) appear to replace PABP in promoting translation: the CP binds strongly to specific regions in the 3′ UTR and also to eIF4G (or eIFiso4G; Krab et al., 2005; reviewed in Bol, 2005). When an artificial poly(A) tail is tagged to the 3′ UTR, CP molecules are no longer required for translation (Neeleman et al., 2001).
C. VPg and initiation
The presence of a VPg linked covalently to the 5′ end of an RNA is characteristic of members of various virus families such as the Birnaviridae, Caliciviridae, Picornaviridae, Potyviridae, Comoviridae, and Luteoviridae (reviewed in Sadowy et al., 2001). The size of the VPg varies from 3 kDa (members of the Picornaviridae family) to 90 kDa (members of the Birnaviridae family). Binding of VPg to eIF3 and eIF4E suggests that an initiation complex is formed and recruited to the viral mRNA, a complex in which VPg would behave as a cap substitute. VPg may therefore interfere with translation by interacting with initiation factors that are required for initiation of both cap-dependent and IRES-containing mRNA translation.
In Poliovirus, whose genome contains a VPg and an IRES in its 5′ UTR, the VPg is removed from the genomic RNA early in infection and the viral mRNA lacks a VPg. Therefore, VPg does not regulate initiation of translation in this virus and probably in other members of the Picornaviridae family.
The genome of Turnip mosaic virus (TuMV, family Potyviridae) is devoid of IRES and cap structure. Its VPg in the precursor form 6K-VPg-Pro appears to favor translation of viral proteins by interacting with eIFiso4E (Leonard et al., 2004, Wittmann et al., 1997). TuMV and Tobacco etch virus (TEV; family, Potyviridae) can interfere in vitro with the formation of a translation initiation complex on host plant cellular mRNA by sequestering eIFiso4E, since the binding affinity of VPg for eIFiso4E is stronger than that of capped RNA. VPg enhances uncapped viral mRNA translation and inhibits capped mRNA translation. Moreover, it appears to function as an alternative cap-like structure by forming a complex with eIFiso4E and eIFiso4G (Khan et al., 2008, Miyoshi et al., 2006).
Furthermore, for viruses such as those of the family Caliciviridae that are also devoid of cap or IRES, evidence for the involvement of the VPg in translation initiation has been documented: in addition to binding to the eIF3 complex (in particular to its eIF3d subunit), the Norwalk virus (NV) VPg inhibits translation of cap-dependent and of IRES-containing reporter mRNAs in vitro (Daughenbaugh et al., 2003). In Feline calicivirus (FCV), the VPg directly interacts with eIF4E in vitro (Goodfellow et al., 2005) and removal of the VPg from the FCV RNA results in dramatic reduction of viral protein synthesis (Herbert et al., 1997).
D. IRES-directed initiation
Initiation of translation of the genome of numerous RNA viruses does not comply with the general cap-dependent scanning mechanism of eukaryotic protein synthesis (reviewed in Doudna and Sarnow, 2007, Kneller et al., 2006). Rather, initiation can occur downstream of a (usually) long GC-rich 5′ UTR known as IRES that in contrast to classical cap-dependent initiation of translation, plays an active role in 40S ribosomal subunit recruitment. These viral 5′ UTRs are generally highly structured, thereby hindering movement of the scanning ribosomes. Animal viruses that resort to this strategy are picornaviruses (reviewed in Belsham and Jackson, 2000, Martínez-Salas and Fernández-Miragall, 2004, Martínez-Salas et al., 2001, Pestova et al., 2001) and pestiviruses (Pisarev et al., 2005).
Ribosomal entry directly to an internal AUG initiation codon on an mRNA devoid of cap structure was demonstrated by placing an IRES between two mRNA cistrons in a dicistronic construct. The presence of the IRES allowed the expression of the downstream cistron independently of the upstream cistron (Jang et al., 1988, Pelletier and Sonenberg, 1988). Hence, cis-acting elements in the IRES appear to cap independently recruit ribosomes to the initiation codon, and the 5′ UTR can be considered an IRES if it drives initiation of translation of the downstream cistron.
Considerable work has been directed toward deciphering the sequence elements involved in IRES-mediated initiation, and the protein factors participating in this step of translation. Translation by an IRES obviates the need of certain host eIFs (that differ for different groups of IRESs), and often requires additional host proteins, the IRES trans-acting factors (ITAFs). These are mRNA-binding proteins such as the pyrimidine tract-binding protein (PTB), ITAF45, PCBP2, the cellular cytoplasmic RNA-binding protein designated upstream of N-ras (unr), and the La autoantigen. The IRESs involved in initiation are part of the 5′ UTR or of the IGR, and their integrity is required for full activity. They sometimes include 5′ nts of the ORF following the IRES (Rijnbrand et al., 2001). Based on their sequence and structure, the IRESs of members of the Picornaviridae family can be divided into three major groups; (1) Enterovirus (Poliovirus) and Rhinovirus (Human rhinovirus, HRV), (2) Cardiovirus (Encephalomyocarditis virus, EMCV and Theiler's murine encephalomyelitis virus, TMEV) and Aphthovirus (Foot-and-mouth disease virus, FMDV), and (3) Hepatovirus (Hepatitis A virus, HAV) (reviewed in Belsham and Jackson, 2000, Kean et al., 2001, Martínez-Salas and Fernández-Miragall, 2004).
Studies in vitro showed that the EMCV IRES-mediated initiation of translation is ATP dependent and requires eIF2, eIF3, eIF4A, and eIF4B as well as the central region of eIF4G to which eIF4A binds. eIF4E is not required, and therefore cleavage of eIF4G as well as the absence of eIF1 which is important for 40S ribosomal subunit scanning do not abolish EMCV IRES function. The same applies to the FMDV IRES-mediated translation from the first initiator AUG (reviewed in Pestova et al., 2001). PTB, an auxiliary cellular 57‐kDa protein with four RNA recognition motifs, strongly stimulates initiation of translation of all group 1 and 2 IRESs (Andreev et al., 2007, Borovjagin et al., 1994, Gosert et al., 2000, Hunt and Jackson, 1999, Pilipenko et al., 2000). ITAF45 is additionally required for FMDV IRES-mediated translation, and PCBP2 as well as unr for Poliovirus and Rhinovirus IRESs (Andreev et al., 2007, Blyn et al., 1997, Boussadia et al., 2003, Pilipenko et al., 2000). The La autoantigen stimulates PV IRES-mediated translation (Costa-Mattioli et al., 2004).
The 5′ UTR of hepatovirus RNAs such as Hepatitis C virus (HCV; family Flaviviridae) are 342–385 nts long. Initiation at their IRES differs from initiation in picornavirus IRESs in vitro: binding of the 40S ribosomal subunit to the HCV IRES occurs directly, without requirement for the translation initiation factors eIF4F and eIF4B (reviewed in Pestova et al., 2001). Thus, the IRES functionally replaces eIF4F on the 40S ribosomal subunit (Siridechadilok et al., 2005). Lack of eIF4F is compensated by conformational modifications in the 40S ribosomal subunit (Spahn et al., 2001). Moreover, the activity of HCV-like IRESs is also affected by the coding sequence immediately downstream of the initiation codon. Not only flaviviruses but also RNA genomes of some picornaviruses such as Porcine Teschovirus carry HCV-like IRES elements within their 5′ UTR (Pisarev et al., 2004).
An interesting variant of IRESs exists in viruses of the Dicistroviridae family such as Cricket paralysis virus (CrPV), viruses originally believed to be the insect counterpart of mammalian picornaviruses (reviewed in Doudna and Sarnow, 2007, Jan, 2006). The monopartite RNA genome of CrPV harbors a 5′ VPg and a 3′ poly(A) tail. It contains two nonoverlapping ORFs separated by an IGR (as opposed to picornaviruses that have only one ORF); the expression of the two ORFs is triggered by two distinct IRESs, one in the 5′ UTR and the other in the IGR (Jan et al., 2003, Sasaki and Nakashima, 1999, Sasaki and Nakashima, 2000, Wilson et al., 2000b). As shown using the long (580 nts) 5′ IRES contained in the Rhopalosiphum padi virus (RhPV) RNA, the 5′ UTR initiates translation of a nonstuctural polyprotein at the expected AUG codon. No specific boundaries of this IRES can be defined, suggesting that the IRES contains multiple domains capable of recruiting ribosomes for translation (Terenin et al., 2005). The IGR of 175–533 nts separating the two ORFs contains the second IRES (∼ 180 nts) that initiates synthesis of a structural polyprotein on a non-AUG codon and requires neither initiation factors nor Met-tRNAiMet but a small domain (domain 3) downstream of the IGR IRES that docks into the 40S ribosomal P site mimicking the tRNA anticodon-loop structure during translation initiation (Costantino et al., 2008, Jan and Sarnow, 2002, Wilson et al., 2000a). In most cases, initiation from the second IRES begins at a GCU, GCA, or GCC triplet coding for alanine or at a CAA triplet coding for glutamine (reviewed in Pisarev et al., 2005). In the model proposed for the initiation of the second IRES, domain 3 within the IGR occupies the ribosomal P site, the ribosomal A site remaining accessible for the Ala-tRNA or the Gln-tRNA, and translocation occurs on the ribosome without peptide bond formation (designated as the elongation-competent assembly of ribosome). As in the case of the HCV IRES, binding of the CrPV IGR to the 40S ribosomal subunit induces conformational changes on the ribosome (Costantino et al., 2008, Pfingsten et al., 2006, Spahn et al., 2004).
The rates of cap- and IRES-dependent initiation pathways in vitro are different: using FMDV RNA as template it was shown that cap-dependent assembly of the 48S ribosomal complex occurs faster than IRES-mediated assembly (Andreev et al., 2007). Moreover, some viruses have evolved sequences that prevent their IRESs from functioning. For example, the HCV IRES possesses a conserved stem–loop structure containing the initiation codon and this structure has been shown to decrease IRES efficiency (Honda et al., 1996). One possible explanation is that for successful viral infection, IRESs should work at a very specific level of efficiency, which does not necessarily correspond to maximum efficiency.
As opposed to animal virus IRESs, plant virus IRES elements are shorter and less structured. Moreover, such elements are not confined to the 5′ UTRs on the genome of plant RNA viruses, and they are then at times referred to as TEs. Depending on the plant viral genome, the IRES is located (1) in the 5′ UTR such as in the picorna-like virus Potato virus Y (family Potyviridae; Levis and Astier-Manifacier, 1993), (2) within or between ORFs such as in a crucifer-infecting Tobacco mosaic virus (TMV, genus Tobamovirus; Jaag et al., 2003, Skulachev et al., 1999, Zvereva et al., 2004) and in Potato leafroll virus (PLRV, family Luteoviridae; Jaag et al., 2003), or (3) in the 3′ UTRs of viruses such as in BYDV (Guo et al., 2001, Wang et al., 1997). In the Hibiscus chlorotic ringspot virus (family Tombusviridae) genome, the activity of the 5′-located IRES is enhanced by the presence of a TE (also known as CITE) located in the 3′ UTR (Koh et al., 2002, Koh et al., 2003). The possible mechanisms of action of 3′ TEs has in recent years revealed the immense variety of strategies used in translation initiation by plant RNA viruses (reviewed in Miller and White, 2006).
E. Non-AUG initiation codons
In some cases in eukaryotes as also in prokaryotes, initiation of translation of cellular and viral mRNAs occurs on a non-AUG codon. Table I summarizes the situation for viruses that initiate some of their proteins on non-AUG codons.
Table I.
Viruses shown or postulated to use non-AUG codons as initiators of protein synthesis
| Family/genus | RNAa | Initiation codon | Protein | References |
|---|---|---|---|---|
| Plant viruses | ||||
| Caulimoviridae | ||||
| Tungrovirus | ||||
| RTBV | 1 | AUU | ORF1 | Fütterer et al. (1996) |
| Furovirus | ||||
| SBWMV | 2 | CUG | 28Kb | Shirako (1998) |
| (Flexiviridae) | ||||
| (Foveavirus) | ||||
| PCMV | 1 | AUC | ORF1 | James et al. (2007) |
| 1 | AUA | ORF 5b | James et al. (2007) | |
| Animal viruses | ||||
| Parvoviridae | ||||
| Dependovirus | ||||
| AAV-2 | 1 | ACG | B | Becerra et al. (1985) |
| Retroviridae | ||||
| Lentivirus | ||||
| EIAV | 1 | CUG | Tat | Carroll and Derse (1993) |
| Gammaretrovirus | ||||
| MoMLV | 1 | CUG | Pr75gag | Prats et al. (1989) |
| Deltaretrovirus | ||||
| HTLV-1 | 1 | GUG | Rex | Corcelette et al. (2000) |
| CUG | Tax | Corcelette et al. (2000) | ||
| Paramyxoviridae | ||||
| Respirovirus | ||||
| Sendai virus | 1 | ACG | C′ | Boeck and Kolakofsky, 1994, Curran and Kolakofsky, 1988, Gupta and Patwardhan, 1988 |
| HPIV-1 | 1 | GUG | C′ | Boeck et al. (1992) |
| Picornaviridae | ||||
| Cardiovirus | ||||
| TMEV | 1 | AUG/ACGc | L* | van Eyll and Michiels (2002) |
| Flaviviridae | ||||
| Flavivirus | ||||
| HCV | 1 | GUG/GCGc | F | Baril and Brakier-Gingras (2005) |
| Dicistroviridae | ||||
| Cripavirus | ||||
| CrPV | 1 | GCUd | ORF 2 | Wilson et al. (2000a) |
| PSIV | 1 | CAAe | ORF 2 | Sasaki and Nakashima, 2000, Yamamoto et al., 2007 |
| RhPV | 1 | GCAd | ORF 2 | Domier et al. (2000) |
For each virus, the RNA segment whose protein is initiated at a non-AUG codon is indicated as also the initiation codon used, and the designation of the resulting protein. The brackets surrounding Flexiviridae and Foveavirus indicate that PCMV is presumed to belong to this family and genus.
RTBV contains a double-stranded DNA; AAV-2 contains a single-stranded DNA.
CP ORF.
Depending on the variant.
Ala as initiator.
Gln as initiator.
In addition to containing the P ORF, the Sendai virus P mRNA harbors the C ORF in another reading frame that leads to the synthesis of a nested set of C-coterminal proteins (proteins C′, C, Y1, and Y2) known jointly as the C proteins (Fig. 2B). Except for C′ that is initiated upstream of the P protein on the mRNA, the other C proteins are entirely contained within the P ORF. C′ is initiated on an ACG codon in an optimum sequence context, and nts + 5 and + 6 also appear to be important for initiation at such non-AUG codons. The other C proteins (C, Y1, and Y2) are initiated on downstream-located AUG codons in suboptimal contexts and are presumably synthesized by leaky scanning or ribosome shunting (Curran and Kolakofsky, 1988, Gupta and Patwardhan, 1988, Kato et al., 2004). Use of ACG as initiation codon has been described in the neurovirulent strains of TMEV (van Eyll and Michiels, 2002).
The initiation codon in Human parainfluenza virus 1 (HPIV-1, family Paramyxoviridae) for the synthesis of C′ is a GUG codon. In vivo, GUG appears nearly as efficient as AUG in initiating C′ expression in the same context (Boeck et al., 1992).
The Moloney murine leukemia virus (MoMLV, family Retroviridae) genomic RNA codes for two in-phase precursor proteins Pr65gag and Pr75gag. It uses an upstream CUG as translation initiation codon for the synthesis of Pr75gag that migrates to the cell surface and is involved in virus spread (Prats et al., 1989).
Among plant viruses, a non-AUG initiation codon exists in the polycistronic mRNA of the pararetrovirus Rice tungro bacilliform virus (RTBV; family Caulimoviridae). ORF I of the mRNA (harboring ORFs I–III) is accessed by reinitiation after translation of a short ORF (sORF). Following a long 5′ leader sequence harboring several sORFs that are bypassed by ribosome shunting, synthesis is initiated on an AUU codon at ORF I (Fütterer and Hohn, 1996). Only 10% of the ribosomes initiate at ORF I; the remaining 90% reach ORFs II and III and initiate on an AUG codon (reviewed in Ryabova et al., 2006). A similar situation occurs in other pararetroviruses. RNA 2 of the bipartite (+) sense single-stranded RNA genome of Soil-borne wheat mosaic virus (SBWMV, genus Furovirus) codes for two proteins; the shorter (19K) CP is produced via conventional AUG initiation, whereas the N-terminally extended 28K protein is initiated at a CUG codon upstream of the CP ORF (Shirako, 1998). Under certain conditions, AUU codons located in the 5′ UTR of the TMV RNA can serve as initiation codons (Schmitz et al., 1996).
In the cases presented earlier, the non-AUG codons allow initiation with a methionine residue. There is however an interesting situation of methionine-independent translation initiation (reviewed in Pisarev et al., 2005, Touriol et al., 2003). This is the case of the IRES-dependent initiation of translation of members of the Dicistroviridae family whose structural protein encoded by ORF 2 lacks an AUG initiation codon and translation initiation occurs at a CAA (coding for Gln) or GCU or GCA (coding for Ala) codon, depending on the virus (Table I).
F. Multiple reading frames
Whereas eukaryotic cell mRNAs are usually monocistronic, the mRNAs of eukaryotic viruses frequently contain several ORFs, the AUG positioned close to the 5′ end of the RNA generally constituting the initiation codon. To reach downstream initiation codons that correspond to internal ORFs on polycistronic RNAs lacking an IRES, viruses resort to either leaky scanning, reinitiation, or shunting.
1. Leaky scanning
A mechanism commonly used by viruses to express polycistronic RNAs is leaky scanning (reviewed in Ryabova et al., 2006), in which when the initiation codon lies within less than 10 nts from the cap structure, or when it is embedded in a poor context for initiation, some of the scanning ribosomes bypass this first initiation codon and start translation on a downstream-located initiation codon whose context is more appropriate for initiation (Fig. 2B). Leaky scanning also occurs when initiation is at a non-AUG codon in an optimal context followed by an AUG codon. Two possible situations can arise: in-frame initiation or overlapping ORFs.
a. In-frame initiation
This occurs when an ORF harbors more than one potential in-frame initiation codon; it is codon context-dependent. The outcome of in-frame initiation is the production of two proteins that are identical over the total length of the shorter protein. Table II lists the cases of in-frame initiation reported. In FMDV and Plum pox potyvirus (PPV, family Potyviridae), in-frame initiation is cap independent (Andreev et al., 2007, Simon-Buela et al., 1997).
Table II.
In-frame initiation
| Family/genus | Genome segment | Protein | References |
|---|---|---|---|
| Comoviridae | |||
| Comovirus | |||
| CPMV | RNA M | Movement protein | Verver et al. (1991) |
| Hordevirus | |||
| BSMV | RNA β | Movement protein | Petty and Jackson (1990) |
| Furovirus | |||
| SBWMV | RNA 2 | Coat protein | Shirako (1998) |
| Potyviridae | |||
| Potyvirus | |||
| PPV | RNA | Polyprotein | Simon-Buela et al. (1997) |
| Bornaviridae | |||
| Bornavirus | |||
| BDV | RNA P | 24‐ and 16‐kDa phosphoproteins | Kobayashi et al. (2000) |
| Picornaviridae | |||
| Aphthovirus | |||
| FMDV | RNA | Polyprotein | Andreev et al. (2007) |
For each virus the genome segment that undergoes in-frame initiation is indicated.
b. Overlapping ORFs
This strategy is extremely common among viruses and is generally also codon context-dependent. The result of this strategy is the synthesis of two different proteins. A situation common to plant viruses belonging to several genera such as the carlaviruses and potexviruses (family Flexiviridae), and the viruses of the genera Furovirus and Hordeivirus is the presence within their (+) sense single-stranded RNA genome of a group of three ORFs known as the triple gene block whose expression leads to three proteins involved in movement of the virus within the plant. Synthesis of these proteins requires the production of two sgRNAs. The 5′-proximal ORF is translated from a functionally monocistronic sgRNA, whereas the two subsequent ORFs are translated from the second sgRNA. Expression of the third ORF, which overlaps the second ORF, occurs by leaky scanning and is codon context-dependent (Verchot et al., 1998, Zhou and Jackson, 1996).
Peanut clump virus (PCV, genus Pecluvirus) contains a bipartite (+) sense single-stranded strand RNA genome. In RNA2, the first of two ORFs that codes for the virus CP terminates with a UGA codon that overlaps the AUG codon initiating the second ORF: AUGA. About one-third of the ribosomes fail to initiate translation of the CP and scan the template initiating translation of the second ORF, more than 100 residues downstream of the first ORF (Herzog et al., 1995). RTBV contains a closed-circular double-stranded DNA genome that is transcribed yielding two mRNAs. The longer polycistronic mRNA (known as pregenomic RNA) encodes three ORFs (I, II, and III) that are linked by AUGA, the termination codon of the upstream ORF overlapping the initiation codon of the downstream ORF (Fütterer et al., 1997). ORF I is initiated at an AUU codon, preceded by a long 5′ UTR with several sORFs that are bypassed by ribosome shunting. On the other hand, ORFs II and III initiate at a conventional AUG codon. However, the AUG initiating ORF II is in a poor context, and the majority of the ribosomes bypass this AUG to reach the downstream more favorable AUG of ORF III. Leaky scanning therefore accounts for initiation of translation of ORFs II and III.
Turnip yellow mosaic virus (family Tymoviridae) is a monopartite (+) sense single-stranded RNA virus that bears a cap structure, and harbors a tRNA-like structure (TLS) at its 3′end that can be valylated in vitro and in vivo. Its first two 5′-proximal and largely overlapping ORFs code for the movement protein (ORF1), and the replicase polyprotein (ORF2) in a different reading frame. It has been reported that the valylated viral RNA serves as bait for ribosomes directing them to initiate synthesis of ORF2, and donating its valine residue for the N-terminus of the polyprotein in a cap- and initiator-independent manner (Barends et al., 2003); interaction between the 3′ TLS and the initiation codon of ORF2 would lead to circularization of the RNA. However, recent studies suggest that initiation of translation of the polyprotein is cap and context dependent, the TLS having only a positive effect on translation of ORF2 without being indispensable (Matsuda and Dreher, 2007). This mechanism allows dicistronic expression from initiation codons that are closely spaced.
2. Reinitiation
Another possibility for initiation at an internal start codon in a polycistronic mRNA is reinitiation of translation of downstream ORFs following expression of a 5′-proximal ORF (of 30 codons or less; reviewed in Ryabova et al., 2006). Reinitiation requires that the 40S ribosomal subunit remain on the mRNA after terminating synthesis of the 5′-proximal ORF. Efficiency of reinitiation decreases with increasing length of the IGR between the 5′-proximal and the next ORF.
Among eukaryotic viruses, polycistronic mRNAs have been the most thoroughly examined in viruses of the family Caulimoviridae, in particular in the double-strand DNA virus Cauliflower mosaic virus (CaMV). The large 35S mRNA of CaMV and related viruses contains up to seven ORFs (Fig. 5 ), and for some of them recurrent translation depends on reinitiation activated by the transactivator (TAV). The TAV protein is encoded by ORF VI contained in the pregenomic (or polycistronic) 35S mRNA; it is expressed by the 19S sgRNA in which it is the only ORF (Pooggin et al., 2001). In dicistronic constructs harboring the CaMV ORF VII followed by ORF I (or by ORFs II, III, IV, V, or an artificial ORF) fused to the chloramphenicol acetyltransferase (CAT) gene, very low levels of CAT activity were obtained in plant protoplasts; however, when the product of ORF VI was included, considerably higher levels of CAT activity were observed (Bonneville et al., 1989, Fütterer and Hohn, 1991). The second ORF of the dicistronic construct is synthesized by reinitiation and not by an IRES, since a stem structure positioned at various sites upstream of this ORF hinders its translation (Fütterer and Hohn, 1991). TAV-stimulated initiation of the second ORF does not depend on the distance separating the two ORFs, since the distance can be abolished as in a quadruplet AUGA, or the ORFs can be separated by as many as 700 nts, and even limited overlap between the ORFs is possible. TAV directly binds to the eIF3g subunit of eIF3 and associates with the L18 and L24 proteins of the 60S ribosomal subunit (Leh et al., 2000, Park et al., 2001). These interactions result in TAV–eIF3 complex association with the translocating ribosome during translation, favoring reinitiation of downstream ORFs. On the other hand, eIF4B can compete with TAV for binding to eIF3g, since the binding sites of these two proteins on eIF3g overlap. Overexpression of eIF4B inhibits TAV-mediated reinitiation of a second ORF, probably by inhibiting TAV–eIF3g-40S complex formation (Park et al., 2004).
Figure 5.
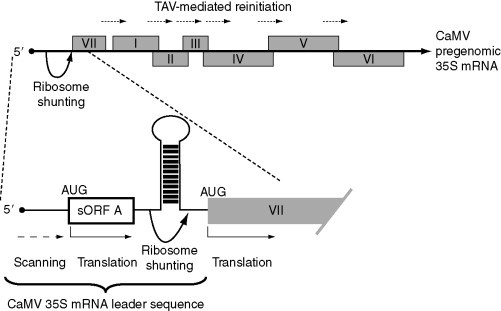
Schematic representation of the organization of the CaMV pregenomic 35S mRNA and strategies of translation initiation. I–VII are ORFs. TAV, transactivator. Arrows show migration of ribosomes by reinitiation (dotted), scanning (dashed), and shunting (curved). Translation is represented by a bent arrow. Other indications are as in legend of Fig. 1.
The members of the Calicivirus family contain a (+) sense single-stranded RNA carrying a VPg. The sgRNAs of these viruses that also contain a VPg represent widely studied examples of reinitiation by mammalian ribosomes after translation of a long ORF. The Rabbit hemorrhagic disease virus genomic RNA codes for a large polyprotein ORF1 that is subsequently processed producing the viral nonstructural proteins and the 3′ terminally located major CP VP60, as well as a small 3′ terminally located ORF2 in another reading frame. The 3′-terminal part of ORF1 overlaps the 5′ region of ORF2. Expression of ORF2 yields the minor CP VP10 and is produced from a sgRNA that also contains the region of ORF1 expressing VP60. Thus, the sgRNA codes for the major VP60 encoded by the 3′-terminal part of ORF1, and for the minor VP10 produced by ORF2. The two ORFs overlap by AUGUCUGA such that the termination codon (UGA) of ORF1 lies downstream of the initiation codon (AUG) of ORF2. Synthesis of VP10 occurs from the genomic as well as from the sgRNA and involves an unusual translation termination/reinitiation process. Indeed, synthesis of VP10 depends strictly on the presence of the termination codon ending ORF1 preceded by a sequence element of about 80 nts (Meyers, 2003). The sequence element contains two motifs that are essential for expression of ORF2, one of which is conserved among caliciviruses and is complementary to a sequence in the 18S ribosomal RNA. In FCV, sgORF1 and sgORF2 overlap by 4 nts (AUGA) and translation in a reticulocyte lysate of the FCV sgRNAs showed that ORF1/ORF2 termination/reinitiation does not require the eIF4F complex and that the 3′-terminal RNA sequence of ORF1 binds to the 40S ribosomal subunit and to IF3 (Luttermann and Meyers, 2007, Meyers, 2007, Pöyry et al., 2007). Thus, the termination/reinitiation process requires sequence elements that could prevent dissociation of postterminating ribosomes via RNA–RNA, RNA–protein, and/or protein–protein interactions.
3. Shunting
A ribosome shunting mechanism has been proposed to explain how initiation of translation occurs in viral polycistronic mRNAs that have a long leader sequence with generally several sORFs, a long low-energy hairpin structure and a probable packaging signal within the 5′ UTR (reviewed in Ryabova et al., 2006). This is the case of CaMV (Fig. 5). The ribosomes having entered at the level of the cap structure on the 35S mRNA would scan a few nts, then skip from a “take-off site” over part of the leader sequence containing a structural element and sORFs, to reach a “landing site,” and finally scan to the downstream ORF. It has been suggested that formation of a leader hairpin between the two sites would bring these sites in close proximity, favoring shunting. It is generally assumed that shunting is more easily achieved if the upstream ORF is short, such that the initiation factors that allowed initiation of translation of the sORF may have at least partly remained on the ribosome during translation (reviewed in Jackson, 2005). In addition to the size of the sORF, the time required for scanning seems also to be important (Pöyry et al., 2004), the eIF4F initiation complex remaining on the ribosome for a few seconds without interruption of sORF translation. The leader sequence of the CaMV 35S mRNA is replete with sORFs. Of these, the 5′-proximal sORF, sORF A, is indispensable for ribosome shunting and infectivity; its aa sequence is generally not important but it must be translated and should be between 2 and 10 codons long for efficient shunting. Another important cis-acting element for shunting includes the distance between the termination codon of sORF A and the base of the leader hairpin (reviewed in Ryabova et al., 2006). Finally, it has been reported that TAV promotes expression of ORF VII (Pooggin et al., 2001).
Shunting may explain translation of polycistronic mRNAs in other viruses, generally by examining the effect on translation of inserting a strong hairpin structure near the 5′ end or in the middle region of the leader sequence, or by inserting AUG codons within the leader, as done for CaMV. Shunting occurs in the case of the 200 nt-long leader, the tripartite leader, in the Adenovirus late mRNAs from the major late promoter. This highly conserved leader contains a 25–44 nt-long unstructured 5′ region, followed by highly structured hairpins devoid of sORFs. Shunting has been reported to be enhanced by complementarity between the tripartite leader and the 3′ hairpin of the 18S ribosomal RNA (Xi et al., 2004, Yueh and Schneider, 2000).
In the polycistronic P/C mRNA of Sendai virus (Fig. 2B), proteins P and C are presumably initiated by leaky scanning, whereas proteins Y1 and Y2 most possibly arise by shunting. This was suggested because changing the ACG codon of C′ to AUG dramatically reduced the synthesis of the P and C proteins, but had virtually no effect on the synthesis of Y1 and Y2 (Latorre et al., 1998). Yet to date, no specific sites have been detected in the mRNA to account for shunting.
G. Modification of cell factors involved in initiation
Shutoff of host protein synthesis is the process in which cell protein synthesis is inhibited during viral infection due to the use by the virus of the host metabolism (reviewed in Gale et al., 2000, Randall and Goodbourn, 2008). Host shutoff reflects the competition between viral and host mRNAs for the translation machinery, and results in selective translation of viral mRNAs over endogenous host mRNAs. Early translational switch is accompanied by disaggregation of polysomes containing capped cellular mRNAs, followed by reformation of polysomes containing exclusively viral mRNAs (reviewed in Lloyd, 2006).
It is at first sight rather surprising that in plants, no infection by a plant virus has so far been conclusively demonstrated to hinder host translation in planta so as to favor synthesis of viral proteins. Host translational shutoff by plant viruses has been reported only in in vitro translation studies of the potyviruses TuMV and TEV (Cotton et al., 2006, Khan et al., 2008, Miyoshi et al., 2006). The authors reported different causes for the inhibition of cellular mRNA translation. On one hand inhibition would be the result of competition between cellular-capped mRNAs and VPg for eIFiso4E, the binding affinity of VPg for eIFiso4E being stronger that of the capped mRNA (Khan et al., 2008, Miyoshi et al., 2006). On the other hand, inhibition of cell mRNA translation by TuMV would not be mediated by the interaction of VPg-Pro (precursor of VPg) with eIFiso4E but by VP-Pro-induced degradation of RNAs (Cotton et al., 2006).
It has been established for several plants that variation in eIF4E and eIFiso4E is involved in natural recessive resistance against potyviruses (reviewed in Kang et al., 2005, Robaglia and Caranta, 2006). Resistance and complementation assays provide evidence for coevolution between pepper eIF4E and potyviral VPg (Charron et al., 2008). Some recessive plant virus resistance genes code for eIF4E with the aa substitution Gly107Arg, and this substitution was shown to abolish the ability of eIF4E to bind TEV VPg and the cap, providing resistance against TEV infection (Yeam et al., 2007). Recently, a functional map of lettuce eIF4E was obtained, and the results using mutated eIF4E suggest that the function of eIF4E in the potyvirus cycle might be distinct from its physiological function of binding the cap structure at the 5′ ends of mRNAs to initiate translation; thus eIF4E may be required for virus RNA replication or other processes of the virus cycle (German-Retana et al., 2008).
1. Phosphorylation of eIF2α
The function of eIF2 in protein synthesis is the formation of the TC and its delivery to the 40S ribosomal subunit. eIF2 is a complex composed of the three subunits α, β, and γ (Fig. 6 ). Phosphorylation of eIF2α inhibits the exchange of GDP for GTP catalyzed by the exchange factor eIF2B, and leads to the sequestration of eIF2B in a complex with eIF2 resulting in general inhibition of protein synthesis (Sudhakar et al., 2000; reviewed in Hinnebusch, 2005). The amount of eIF2B in cells is limiting as compared to eIF2. Thus, even small changes in the phosphorylation status of eIF2α have a drastic effect on translation due to eIF2B sequestration (Balachandran and Barber, 2004, Krishnamoorthy et al., 2001, Sudhakar et al., 2000, Yang and Hinnebusch, 1996). For several mRNAs the eIF2 complex is replaced by a single polypeptide designated eIF2A that directs codon-dependent and GTP-independent Met-tRNAiMet binding to the 40S ribosomal subunit and may act by favoring expression of specific proteins (Adams et al., 1975, Merrick and Anderson, 1975, Zoll et al., 2002).
Figure 6.
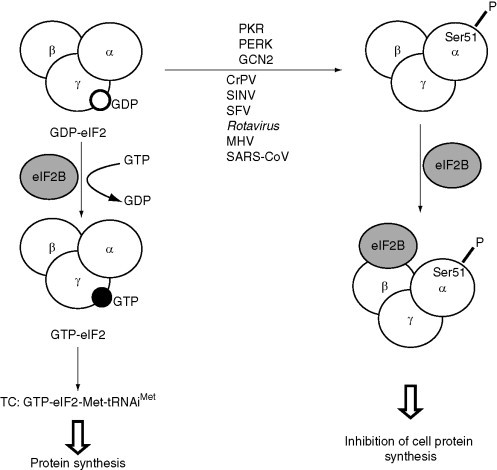
Phosphorylation of eIF2α in virus-infected cells. TC, ternary complex; P, phosphorylation. α, β, and γ are the subunits of eIF2.
Four cellular eIF2α kinases are known to phosphorylate the eIF2α subunit at residue Ser51. Three of the kinases—the protein kinase RNA (PKR), the PKR-like endoplasmic reticulum kinase (PERK), and the general control nonderepressible-2 (GCN2) kinase—play a prominent role in virus-infected cells (Fig. 6). PKR binds to and is activated by double-strand RNAs that are generated during replication and transcription of viral genomes. Accumulation of unfolded proteins in the endoplasmic reticulum during viral infection induces a signaling cascade from the cytoplasmic kinase domain of PERK, leading to induction of eIF2α phosphorylation. Finally, GCN2 kinase is reported to be activated upon Sindbis virus (SINV, family Togaviridae) infection (Berlanga et al., 2006).
Many viruses evolved diverse strategies to prevent PKR or PERK activation in infected cells; these strategies have been discussed in detail in recent reviews (Dever et al., 2007, Garcia et al., 2007, Mohr, 2006, Mohr et al., 2007). However, there are several examples in which viruses use eIF2α phosphorylation to switch off cell translation and direct the cell machinery to synthesize their own proteins (Fig. 6). A classical illustration of how eIF2 modification fosters translation of viral mRNAs is initiation of translation on the CrPV IRES. The IRES contained in the IGR promotes initiation of protein synthesis without the assistance of any initiation factors, including eIF2 (reviewed in Doudna and Sarnow, 2007, Pisarev et al., 2005). Moreover, CrPV stimulates eIF2α phosphorylation; this inactivates host mRNA translation by decreasing the amount of preinitiation 43S ribosomal complexes formed and facilitates translation initiation on the CrPV IRES. Indeed, lowering the amounts of TC and 43S ribosomal complexes increases the efficiency of initiation on the CrPV IRES (Pestova et al., 2004, Thompson et al., 2001). HCV encodes proteins known to inactivate PKR (or PKR + PERK) function(s) (Garcia et al., 2007). However, HCV IRES-driven translation initiation can also be maintained in the presence of activated PKR and reduced TCs (Robert et al., 2006). A new pathway of eIF2- and eIF5-independent initiation of translation on the HCV IRES has been proposed recently in which assembly of the 80S complex requires only two initiation factors, eIF5B and eIF3 (Terenin et al., 2008).
Infection by viruses of the genus Alphavirus (family Togaviridae) such as SINV or Semliki forest virus (SFV) activates PKR, which results in almost complete phosphorylation of eIF2α at late times postinfection. Translation of the viral sg 26S mRNA takes place efficiently during this time, whereas translation of genomic mRNA is impaired by eIF2α phosphorylation (Molina et al., 2007, Ventoso et al., 2006). It was shown that a hairpin loop structure within the 26S mRNA-coding region, located downstream of the AUG initiation codon, promotes eIF2-independent translation with the help of eIF2A (Ventoso et al., 2006). However, the fact that translation of the 26S mRNA must be coupled to transcription to be efficient in infected cells suggests that additional viral or cellular factors are involved in translation initiation on the 26S mRNA (Sanz et al., 2007).
Early in the infection process rotaviruses take over the host translation machinery, and this is achieved via interaction of the viral NSP3 with eIF4G and phosphorylation of eIF2α (Figure 4, Figure 6; Montero et al., 2008, Piron et al., 1998). These two mechanisms may explain the severe shutoff of cell protein synthesis observed during rotavirus infection, although it is not clear how capped viral mRNAs are efficiently translated in such eIF2α-sequestered conditions.
Murine hepatitis virus (MHV) as well as Severe acute respiratory syndrome coronavirus (SARS-CoV), both of the family Coronaviridae, induce host translational shutoff. This is achieved via different mechanisms: degradation of cell mRNAs including mRNAs encoding translation-related factors (Leong et al., 2005, Raaben et al., 2007), increase in eIF2α phosphorylation presumably via PERK, and formation of stress granules and processing bodies that are thus sites of mRNA stalling and degradation, respectively (Chan et al., 2006, Raaben et al., 2007, Versteeg et al., 2006). Expression of the SARS-CoV NSP1 is involved in degradation of several host mRNAs and in host translation shutoff (Kamitani et al., 2006). Surprisingly, despite eIF2α phosphorylation the SARS-CoV proteins are still efficiently synthesized even though coronaviral mRNAs are structurally equivalent to host mRNAs (Hilton et al., 1986, Siddell et al., 1981).
It is interesting to observe that despite considerable work performed in recent years, phosphorylation of eIF2α still represents one of the most intriguing problems in translational control during viral infection, since it is still not clear why the phosphorylation of eIF2 affects cellular protein synthesis without impairing translation initiation of many viral RNAs.
2. Modification of eIF4E and 4E-BP
eIF4E is believed to be the least abundant of all initiation factors and, therefore, to be a perfect target for regulation of protein synthesis. It interacts with the cap structure of mRNAs, with the scaffold protein eIF4G and with repressor proteins known as eIF4E-binding proteins (4E-BPs). eIF4E undergoes regulated phosphorylation on Ser209 mediated by the eIF4G-associated MAPK signal-integrating kinases, Mnk1 and Mnk2 (Fig. 7 ) (Pyronnet et al., 1999, Raught and Gingras, 2007). Uninfected cells growing exponentially typically possess roughly equal amounts of phosphorylated and nonphosphorylated forms of eIF4E (Feigenblum and Schneider, 1993) and the ratio shifts toward the phosphorylated form of eIF4E following treatment of the cells with growth factors, hormones, and mitogens (Flynn and Proud, 1995, Joshi et al., 1995, Makkinje et al., 1995). However, the functional role of eIF4E phosphorylation remains elusive. Indeed, there is no direct link between eIF4E phosphorylation and the enhanced translation observed as a result of these stimuli, since recent studies showed that phosphorylation of eIF4E decreases the affinity of eIF4E for capped mRNA. Thus, the working hypothesis is that the nonphosphorylated form of eIF4E within the eIF4F complex (eIF4E, eIF4G, and eIF4A) binds to the cap structure on the mRNA, and that eIF4E phosphorylation accompanies initiation complex transition to elongation (reviewed in Scheper and Proud, 2002). In addition, phosphorylation could dissociate eIF4E from the cap and enable the eIF4F complex to move along the 5′ UTR and unwind the secondary structure.
Figure 7.
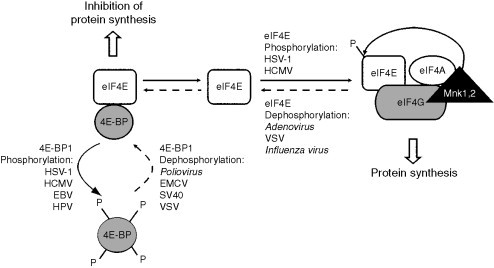
Phosphorylation and dephosphorylation of eIF4E and 4E-BP1. eIF4E+eIF4G+eIF4A form eIF4F. Other indications are as in legends of Figure 1, Figure 6.
4E-BP constitutes a family of translation repressors that prevent eIF4F assembly and act as negative growth regulators (Raught and Gingras, 2007). 4E-BPs are phosphoproteins, 4E-BP1 being the best studied of the three 4E-BPs known in mammals. It undergoes phosphorylation at multiple sites leading to its dissociation from eIF4E, leaving eIF4E free to bind eIF4G and to form the eIF4F complex (Fig. 7) (Lin et al., 1994, Pause et al., 1994). The mechanism proposed is that eIF4E possesses an eIF4G-binding site which overlaps with 4E-BP motifs; thus, 4E-BP and eIF4G binding to eIF4E would be mutually exclusive (Haghighat et al., 1995, Marcotrigiano et al., 1999).
a. Dephosphorylation of eIF4E and of 4E-BP1
Adenovirus (family Adenoviridae), Vesicular stomatitis virus (VSV; family Rhabdoviridae), and Influenza virus infections lead to accumulation of nonphosphorylated eIF4E and subsequent inhibition of host protein synthesis. Adenovirus mediates the quantitative dephosphorylation of eIF4E (up to 95% of the total eIF4E) leading to suppression of cellular protein synthesis (Fig. 7) (Feigenblum and Schneider, 1993). The Adenovirus late protein designated 100K is synthesized at high levels at the onset of the late phase of infection (Bablanian and Russell, 1974, Oosterom-Dragon and Ginsberg, 1980). It interacts with the C-terminus of eIF4G (Cuesta et al., 2000) and with the tripartite leader sequence of viral late mRNAs (Xi et al., 2004). Binding of the 100K protein to eIF4G evicts Mnk1 from the eIF4F complex, thus impairing eIF4E phosphorylation in the initiation complex and inhibiting translation of host mRNAs (Cuesta et al., 2000). On the other hand, adenoviral late mRNAs are translated efficiently via ribosome shunting (Xi et al., 2004, Xi et al., 2005). VSV infection causes dephosphorylation of eIF4E and 4E-BP1 thus hampering host protein synthesis (Fig. 7). The resulting changes in eIF4F do not inhibit translation of viral mRNAs, although the detailed mechanism of how VSV mRNAs that are capped and possess poly(A) tails overcome the obstacle created has not been elucidated (Connor and Lyles, 2002). Influenza virus infection results in partial (up to 70%) dephosphorylation of eIF4E and concomitant loss of eIF4F activity (Fig. 7). Thus, Influenza virus mRNAs that are capped via cap-snatching and polyadenylated (Herz et al., 1981, Krug et al., 1979, Luo et al., 1991) are translated efficiently under conditions of partial inactivation of eIF4F (Feigenblum and Schneider, 1993) when host protein synthesis is blocked (Katze and Krug, 1990). Several studies have shown that the NS1 viral protein selectively promotes translation of viral mRNAs by increasing their rate of initiation (de la Luna et al., 1995, Enami et al., 1994, Katze et al., 1986, Park and Katze, 1995) and interacts with PABP and eIF4GI (one of the two isoforms of eIF4G in animals) in viral mRNA translation initiation complexes (Aragón et al., 2000, Burgui et al., 2003). Moreover, a recent report has provided evidence that the Influenza virus RdRp substitutes for eIF4E in viral mRNA translation and binds to the translation preinitiation complex (Burgui et al., 2007). One can speculate that the combination of dephosphorylation of eIF4E, hyperphosphorylation of eIF4G, and binding of RdRp to the preinitiation complex and of NS1 to eIF4GI creates an eIF4F factor more specific for Influenza virus mRNA translation.
4E-BP1 is dephosphorylated following infection with Poliovirus or EMCV (Fig. 7). This is a well-established example of viral switch from cap-dependent to IRES-mediated initiation of translation in picornavirus-infected cells (Gingras et al., 1996, Svitkin et al., 2005). Simian virus 40 (SV40; family Polyomaviridae) is a recent example of a virus that causes significant decrease in phosphorylation of 4E-BP1 late in lytic infection. This process is specifically mediated by the SV40 small t antigen. As in the case of Poliovirus and EMCV, dephosphorylation of 4E-BP1 and its subsequent binding to eIF4E displaces eIF4E from the eIF4F complex. This mechanism functions as a switch in translation initiation mechanisms favoring IRES-mediated translation (Yu et al., 2005). Indeed, recent studies have shown that the SV40 late 19S mRNA possesses an IRES (Yu and Alwine, 2006).
b. Phosphorylation of eIF4E and 4E-BP1
Members of the Herpesviridae family of the Alphaherpesvirinae subfamily, such as Herpes Simplex Virus 1 (HSV-1), and of the Betaherpesvirinae subfamily, such as Human cytomegalovirus (HCMV), can stimulate the assembly of eIF4F complexes in primary human cells; this is partly achieved by phosphorylation of eIF4E and 4E-BP1 early in the productive viral growth cycle (Fig. 7) (Kudchodkar et al., 2006, Walsh and Mohr, 2004, Walsh et al., 2005). At the same time HSV-1 infection dramatically impairs host protein synthesis (Elgadi et al., 1999, Everly et al., 2002, Sciabica et al., 2003) whereas with HCMV the effect on host protein synthesis is weak (Stinski, 1977). Interestingly, the ratio of eIF4F over 4E-BP1 increases in cells infected with either HSV-1 or HCMV, promoting assembly of eIF4F complexes. For HSV-1 this is achieved exclusively through proteasome-mediated degradation of 4E-BP1 (Walsh and Mohr, 2004), whereas for HCMV, replication induces an increase in the overall abundance of the eIF4F components eIF4E and eIF4G, and also of PABP relative to the translational repressor 4E-BP1 (Walsh et al., 2005). However, liberation of eIF4E from 4E-BP1 in the case of HSV-1 is insufficient to accelerate eIF4E incorporation into the eIF4F complex. A recent study showed that the HSV-1 ICP6 gene product binds to eIF4G promoting association of eIF4E with the N-terminus of eIF4G and facilitating eIF4E phosphorylation. This suggests a chaperone role for ICP6 in eIF4F assembly (Walsh and Mohr, 2006).
4E-BP1 is hyperphosphorylated (Fig. 7) following infection by Epstein–Barr Virus (EBV; family Herpesviridae, subfamily Gammaherpesvirinae) (Moody et al., 2005) or Human papillomavirus (HPV; family Papillomaviridae) (Moody et al., 2005, Munger et al., 2004, Oh et al., 2006).
3. Modification of eIF4G
a. Cleavage of eIF4G
The large modular protein eIF4G serves as a docking site for initiator factors and other proteins involved in initiation of RNA translation. Due to the central role of eIF4G in translation initiation, many viruses belonging to the families Picornaviridae, Retroviridae, and Caliciviridae have evolved mechanisms to modify the function of eIF4G so as to prevent cell protein synthesis. These viruses induce cleavage of eIF4G, separating the N-terminal eIF4E-binding domain from the C-terminal eIF4A- and eIF3-binding domains (Fig. 8 ). As a consequence, the capacity of eIF4G to connect capped mRNAs to the 40S ribosome is abolished by the virus, inducing host translation shutoff.
Figure 8.
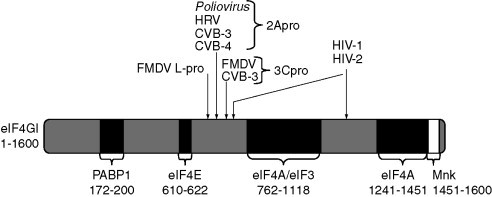
eIF4GI protein-binding sites and cleavage sites by viral proteases. Black or white rectangles represent regions of eIF4G interacting with other proteins. Numbers correspond to aa. Arrows correspond to sites of cleavage.
Host shutoff during infection by picornaviruses such as Poliovirus, HRV and human Coxsackie virus B (CVB)-3 and CVB-4 results in part from cleavage of eIF4GI by the viral 2A protease (2Apro) at aa 681/682 (Baxter et al., 2006, Lamphear et al., 1993, Sommergruber et al., 1994, Sousa et al., 2006). The Poliovirus and HRV 2Apro also cleave eIF4GII (at aa 699/670) but more slowly than cleavage of eIF4GI (Gradi et al., 1998, Svitkin et al., 1999). FMDV has evolved an alternate papain-like protease, L-pro in place of 2Apro to cleave both isoforms of eIF4G (Gradi et al., 2004); it cleaves eIF4GI 7 aa upstream of 2Apro (Fig. 8), and eIF4GII 1 aa downstream of the 2Apro cleavage site (reviewed in Lloyd, 2006). Poliovirus infection also activates two cell proteases that cleave eIF4GI close to the 2Apro cleavage site (Zamora et al., 2002). The 3Cpro of FMDV and the 2Apro and 3Cpro of CVB-3 also cleave eIF4GI (Fig. 8) (Chau et al., 2007, Strong and Belsham, 2004). Degradation of eIF4GI has been observed in CD4+ cells infected with Human immunodeficiency virus 1 (HIV-1; family Retroviridae) (Ventoso et al., 2001). The HIV-1 protease efficiently cleaves eIF4GI at multiple sites, but not eIF4GII (Ohlmann et al., 2002). Proteases of HIV-2 and of members of the family Retroviridae (Human T-lymphotropic virus 1 (HTLV-1), Simian immunodeficiency virus, and Mouse mammary tumor virus (MMTV)) also cleave eIF4GI (Alvarez et al., 2003; reviewed in Lloyd, 2006). Finally, infection of cells with FCV leads to cleavage of eIF4GI and eIF4GII and host translation shutoff (Willcocks et al., 2004); the identity of the protease responsible for cleavage of eIF4G is unknown, but it could be a cellular protease activated by the infection.
b. Phosphorylation of eIF4G
eIF4G is 10-fold more phosphorylated in Influenza virus-infected than in noninfected cells and phosphorylated eIF4G still interacts with eIF4A and eIF4E. Cleavage of eIF4G by the Poliovirus 2Apro inhibits translation of the Influenza virus mRNAs (Feigenblum and Schneider, 1993, Garfinkel and Katze, 1992). Phosphorylation of eIF4G in HCMV-infected cells is one of the mechanisms that enhances eIF4F activity during the viral replication cycle (Kudchodkar et al., 2004, Walsh et al., 2005). eIF4G phosphorylation is induced throughout infection with SV40 (Yu et al., 2005).
4. Cleavage of PABP
Certain viruses cleave the C-terminal domain of PABP thereby destroying its interactions with eIF4B, eRF3, or Paip1 (Fig. 4A). PABP is targeted for cleavage by the 2Apro and 3Cpro of Poliovirus and CVB-3 (Joachims et al., 1999, Kerekatte et al., 1999, Kuyumcu-Martinez et al., 2002, Kuyumcu-Martinez et al., 2004b), by L-pro of FMDV (Rodríguez Pulido et al., 2007), and by 3Cpro of HAV (Zhang et al., 2007). PABP is proteolytically processed by the calicivirus 3C-like protease (Kuyumcu-Martinez et al., 2004a), and HIV-1 and HIV-2 proteases are also able to cleave PABP in the absence of other viral proteins (Alvarez et al., 2006). The contribution of PABP cleavage versus eIF4G cleavage in shutoff of host or viral protein synthesis has not been compared directly. Poliovirus cleavage of PABP appears to be promoted by the interaction of PABP with translation initiation factors, ribosomes or poly(A)-containing RNAs (Kuyumcu-Martinez et al., 2002, Rivera and Lloyd, 2008). Processing of PABP could either occur through one of the components that provides shutoff of host translation or could favor the switch from translation to replication of viral genomes as for example PABP cleavage by 3Cpro in Poliovirus- and HAV-infected cells (Bonderoff et al., 2008, Zhang et al., 2007).
5. Substitution of PABP
Severe inhibition of host mRNA translation due to competition between the viral protein NSP3 and PABP for eIF4G was shown in cells infected with rotaviruses. The viral NSP3 protein binds to the conserved motif UGACC located at the 3′ end of the viral mRNA, and circularizes the mRNA via interaction with eIF4G (Fig. 4C). Since NSP3 has a higher affinity for eIF4G than does PABP, it replaces PABP and disrupts host mRNA circularization (Michel et al., 2000, Vende et al., 2000). NSP3–eIF4G interaction results in reduced efficiency of host mRNA translation. NSP3-mediated circularization has been reported to enhance Rotavirus mRNA translation (Vende et al., 2000) and to be dispensable for translation of the viral mRNAs (Montero et al., 2006). X-ray structure and biophysical studies have shown that NSP3 forms an asymmetric homodimer around the conserved motif at the 3′ end of Rotavirus mRNAs (Deo et al., 2002).
6. Cleavage of PBCP2
During the mid-to-late phase of Poliovirus infection, PCBP2 is cleaved by the viral proteases 3C and 3CD (Fig. 4B); the cleaved protein is no longer able to bind to the IRES and initiate translation, but it binds to the 5′-terminal cloverleaf structure or simultaneously to the cloverleaf structure and to the adjacent C-rich spacer circularizing the viral genome for replication. Hence, the formation of two different closed loop structures could favor the switch from translation to replication of the Poliovirus genome (Gamarnik and Andino, 1998, Herold and Andino, 2001, Perera et al., 2007, Toyoda et al., 2007).
IV. Elongation of Translation
A. Frameshift
This is the mechanism whereby during the course of peptide chain elongation, certain ribosomes shift from the original ORF (0 frame) on the mRNA by one nt, either in the 5′ direction (− 1 frame) or in the 3′ direction (+ 1 frame), and continue protein synthesis in the new frame. This results in the synthesis of two proteins, the “stopped” and “transframe” proteins; they are identical from the N-terminus to the frameshift site but differ thereafter, and the stopped protein is always the more abundant of the two proteins (reviewed in Farabaugh, 2000). The occurrence of − 1 frameshift is more frequent and has been more extensively studied than + 1 frameshift. − 1 Frameshift is common among (+) strand RNA viruses; it has been found in most retroviruses, in coronaviruses, L-A viruses of yeast and in several plant viruses belonging to diverse groups (Table III ). Frameshift is observed during translation of RNA genomes exhibiting overlapping gene arrangements. It usually allows the expression of the viral replicase, the transframe protein in most cases harboring the polymerase or the reverse transcriptase. It has recently been reported (Chung et al., 2008) that TuMV, in addition to synthesizing a large polyprotein that undergoes cleavage, also harbors a frameshift protein embedded in the P3 region of the polyprotein: frameshift leads to the expression of a protein designated P3-PIPO. PIPO is essential for infectivity of the virus, although its precise role has not been established.
Table III.
Viruses of eukaryotes shown or postulated to regulate elongation of translation by frameshifting
| Family/genus | RNA | Type of FS | Proteins | References |
|---|---|---|---|---|
| Plant virusesa | ||||
| Carlavirus | ||||
| PVM | 1 | − 1 | CP/12K | Gramstat et al. (1994) |
| Sobemovirus | ||||
| BWYV | 1 | − 1 | 66K/67K | Veidt et al., 1988, Veidt et al., 1992 |
| CoMV | 1 | − 1 | 64K/56K | Mäkinen et al. (1995) |
| Closteroviridae | ||||
| Closterovirus | ||||
| BYV | 1 | + 1 | 295K/48K | Agranovsky et al. (1994) |
| CCSV | 1 | + 1 | ORF1a/b | ten Dam et al. (1990) |
| CTV | 1 | + 1 | 349K/57K | Karasev et al. (1995) |
| Crinivirus | ||||
| LIYV | 1 | + 1 | 217K/55K | Klaassen et al. (1995) |
| Luteoviridae | ||||
| Enamovirus | ||||
| PEMV | 1 | − 1 | 84K/67K | Demler and de Zoeten (1991) |
| 2 | − 1 | 33K/65K | Demler et al. (1993) | |
| Luteovirus | ||||
| BYDV-PAV | 1 | − 1 | 39K/60K | Di et al. (1993) |
| Polerovirus | ||||
| PLRV | 1 | − 1 | 70K/67K | Prüfer et al. (1992) |
| Tombusviridae | ||||
| Dianthovirus | ||||
| CRSV | 1 | − 1 | 27K/54K | Kujawa et al., 1993, Ryabov et al., 1994 |
| RCNMV | 1 | − 1 | 27K/57K | Kim and Lommel, 1994, Xiong et al., 1993 |
| SCNMV | 1 | − 1 | 27K/57K | Ge et al. (1993) |
| Animal viruses | ||||
| Pseudoviridaeb | ||||
| Pseudovirus | ||||
| SceTy1V | 1 | + 1 | Gag/Pol | Belcourt and Farabaugh, 1990, Clare et al., 1988 |
| Metaviridaeb | ||||
| Metavirus | ||||
| SceTy3V | 1 | + 1 | Gag/Pol | Hansen et al. (1992) |
| Errantivirus | ||||
| DmeGypV | 1 | − 1 | Gag/Pol | Bucheton (1995) |
| Retroviridaeb | ||||
| Alpharetrovirus | ||||
| ASLV | 1 | − 1 | Pro/Pol | Arad et al. (1995) |
| Betaretrovirus | ||||
| MMTV | 1 | − 1 | Gag/Pro/Pol | Jacks et al. (1987) |
| Deltaretrovirus | ||||
| HTLV-1 | 1 | − 1 | Gag/Pro/Pol | Nam et al. (1993) |
| Lentivirus | ||||
| HIV-1 | 1 | − 1 | Gag/Pro | Parkin et al. (1992) |
| Totiviridaec | ||||
| Totivirus | ||||
| ScV-L-A | 1 | − 1 | Gag/Pol | Dinman et al. (1991) |
| Leishmaniavirus | ||||
| LRV1-1 | 1 | + 1 | CP/RdRp | Stuart et al. (1992) |
| Astroviridaea | ||||
| Astrovirus | ||||
| HAstV | 1 | − 1 | ORF1a/ORF1b | Lewis and Matsui (1996) |
| Coronaviridaea | ||||
| Coronavirus | ||||
| IBV | 1 | − 1 | Pol1a/Pol1b | Brierley et al., 1987, Brierley et al., 1989 |
| SARS-CoV | Plant and Dinman (2006) | |||
| Torovirus | ||||
| EqTV | 1 | − 1 | ORF1a/ORF1b | Lai and Cavanagh (1997) |
| Arteriviridaea | ||||
| Arterivirus | ||||
| EAV | 1 | − 1 | ORF1a/ORF1b | den Boon et al., 1991, Napthine et al., 2003 |
| Unassigned virusa | ||||
| APV | 1 | − 1 | ORF1/ORF2 | van der Wilk et al. (1997) |
| Measles virus | 1 | − 1 | P/R | Liston and Briedis (1995) |
| BLV | Rice et al. (1985) |
For each virus the genome segments that undergo frameshifting (FS), the type of FS, and the “stopped” and “transframe” proteins involved are indicated.
(+) Sense single-stranded RNA viruses.
Reverse-transcribing RNA viruses.
dsRNA viruses.
Three RNA signals are important in − 1 frameshifting, a slippery heptanucleotide sequence where frameshift occurs, a downstream hairpin that in many instances can additionally form a pseudoknot, and a spacer element between the slippery sequence and the hairpin structure; the length of the spacer varies between 4 and 9 nts, depending on the viral genome. The viral sequences appear to be optimized for a suitable level of stopped and transframe proteins required for viral replication rather than for maximum frameshift (Kim et al., 2001).
B. Modification of elongation factors
eEF-1 is composed of eEF1A (formerly called eEF-1α) the transporter of aa-tRNAs to the A site on the ribosomes during elongation in conjunction with GTP hydrolysis, and a trimeric complex known as eEF1B (formerly called eEF-1βγδ) responsible for the regeneration of GTP from GDP on eEF-1A (Slobin and Moller, 1978). eEF-2 promotes translocation of the aa- or peptidyl-tRNA from the A to the P site on the ribosome in a GTP-dependent reaction.
Given the fact that strong evidence for deviations from the norm during elongation of protein synthesis does not seem to exist, it is not surprising that the cases of modification of elongation factors caused by viral infection appear to be virtually nonexistent. Indeed, such modifications would most likely equally affect cellular and viral protein synthesis. Nevertheless, a case of elongation factor modification has been documented during infection by viruses of the Herpesviridae family.
The mammalian eEF-1δ subunit of eEF1B is phosphorylated in vitro in the same position by the cell kinase cdc2, and hyperphosphorylated by a viral kinase conserved in all the subfamilies of the Herpesviridae family, such as the HSV-1 UL13 kinase, the EBV BGLF4 kinase, and the HCMV UL97 kinase (Kato et al., 2001, Kawaguchi et al., 1999, Kawaguchi et al., 2003). How phosphorylation of eEF-1δ by the viral kinases affects translation elongation remains obscure.
V. Termination of Translation
Termination of translation occurs when the ribosome encounters one of the three termination codons that defines the 3′ boundary of the ORF on the mRNA: UAG, UGA, or UAA. It involves termination codon recognition at the ribosomal A site, peptidyl-tRNA hydrolysis, and release of ribosomes from the mRNA. The participation of two proteins, the eukaryotic release factors eRF1 and eRF3, in termination codon recognition has been demonstrated (Drugeon et al., 1997, Janzen et al., 2002, Karamysheva et al., 2003). The three termination codons are decoded by eRF1 that catalyzes ester bond hydrolysis in peptidyl-tRNA at the ribosomal peptidyl-transferase center. eRF1 functions cooperatively with the GTPase eRF3 whose activity is ribosome and eRF1 dependent (Kononenko et al., 2008, Pisareva et al., 2006). Final events leading to complete disassembly of the posttermination 80S ribosome require eIF1, eIF1A, and eIF3 (Pisarev et al., 2007). Efficiency of termination appears to be determined by competition between eRF binding to the ribosome and alternative translational events that allow ribosomes to continue decoding. The processes that can circumvent termination codons include: ribosomal frameshift, readthrough or suppression of termination by natural cellular tRNAs, and binding of release factors.
A. Readthrough
In readthrough, a cellular aa-tRNA, called a natural suppressor, decodes the termination codon and translation continues in the same frame up to the next in-frame termination codon. Readthrough is commonly encountered in plant single-stranded RNA viruses and in some animal viruses. Table IV presents the families, genera, and viruses whose genomes have been shown or postulated to resort to readthrough. Readthrough usually allows the synthesis of the RdRp, the reverse transcriptase or of a CP-fusion protein, depending on the virus. The CP-fusion protein is present in the virus particles and is needed for encapsidation and/or for vector transmission.
Table IV.
Viruses shown or postulated to regulate termination of translation by readthrough
| Family/genus | RNA | Termination codon | Proteins | References |
|---|---|---|---|---|
| Plant virusesa | ||||
| Benyvirus | ||||
| BNYVV | 2 | UAG | CP/75K | Niesbach-Klosgen et al., 1990, Schmitt et al., 1992 |
| Furovirus | ||||
| SBWMV | 1 | UGA | 150K/209K | Shirako and Wilson (1993) |
| 2 | UGA | CP/84K | Yamamiya and Shirako (2000) | |
| Peclovirus | ||||
| PCV | 1 | UGA | 103K/191K | Herzog et al. (1994) |
| Pomovirus | ||||
| BVQ | 1 | UAA | 149K/207K | Koenig et al. (1998) |
| 2 | UAG | CP/54K | Koenig et al. (1998) | |
| BSBV | 1 | UAA | 145K/204K | Koenig and Loss (1997) |
| 2 | UAG | CP/104K | Koenig et al. (1997) | |
| Tobamovirus | ||||
| TMV | 1 | UAG | 126K/183K | Ishikawa et al., 1986, Pelham, 1978, Skuzeski et al., 1991 |
| Tobravirus | ||||
| PEBV | 1 | UGA | 141K/201K | MacFarlane et al. (1989) |
| TRV | 1 | UGA | 134K/194K | Hamilton et al. (1987) |
| Tombusviridae | ||||
| Avenavirus | ||||
| OCSV | 1 | UAG | 23K/84K | Boonham et al. (1995) |
| Carmovirus | ||||
| CarMV | 1 | UAG,UAG | 27K/86K/98K | Guilley et al. (1985) |
| CCFV | 1 | UAG | 28K/87K | Skotnicki et al. (1993) |
| MNSV | 1 | UAG | 29K/89K | Riviere and Rochon (1990) |
| UAG | 7K/14K | Riviere and Rochon (1990) | ||
| TCV | 1 | UAG | 28K/88K | White et al. (1995) |
| Machlomovirus | ||||
| MCMV | 1 | UAG | 50K/111K | Nutter et al. (1989) |
| UGA | 9K/33K | Nutter et al. (1989) | ||
| Necrovirus | ||||
| TNV | 1 | UAG | 23K/82K | Meulewaeter et al. (1990) |
| Tombusvirus | ||||
| AMCV | 1 | UAG | 33K/92K | Tavazza et al. (1994) |
| CNV | 1 | UAG | 33K/92K | Rochon and Tremaine (1989) |
| CyRSV | 1 | UAG | 33K/92K | Grieco et al. (1989) |
| TBSV | 1 | UAG | 33K/92K | Hearne et al. (1990) |
| Luteoviridae | ||||
| Enamovirus | ||||
| PEMV | 1 | UGA | CP/55K | Demler and de Zoeten (1991) |
| SbDV | 1 | UAG | CP/80K | Rathjen et al. (1994) |
| Luteovirus | ||||
| BYDV-PAV | 1 | UAG | CP/72K | Dinesh-Kumar et al., 1992, Filichkin et al., 1994, Miller et al., 1988, Wang et al., 1995 |
| Polerovirus | ||||
| BWYV | 1 | UAG | CP/74K | Brault et al., 1995, Veidt et al., 1988, Veidt et al., 1992 |
| PLRV | 1 | UAG | CP/80K | Bahner et al., 1990, Rohde et al., 1994 |
| Animal viruses | ||||
| Retroviridaeb | ||||
| Gammaretrovirus | ||||
| MLV | 1 | UAG | Gag/Pol | Etzerodt et al., 1984, Herr, 1984 |
| Epsilonretrovirus | ||||
| WDSV | 1 | UAG | Gag/Pro | Holzschu et al. (1995) |
| Togaviridaea | ||||
| Alphavirus | ||||
| SINV | 1 | UGA | P123/nsP4 | Strauss and Strauss (1994) |
For each virus, the RNA segment whose protein undergoes readthrough, the nature of the suppressible termination codon, and the designation of the stopped (indicated as CP or by its size if not the CP) and readthrough proteins (indicated by the total size of the resulting protein) are indicated. Other members of the Alphavirus genus (O'nyong-nyong virus and SFV) have CGA (Arg); one SINV strain has UGU (Cys); in all cases, the importance of a C residue 3′ of UGA, CGA, or UGU has been emphasized.
(+) Sense single-stranded RNA viruses.
Reverse-transcribing RNA viruses.
Readthrough of termination codons requires the positioning of a suppressor aa-tRNA in the ribosomal A site where it competes with eRF1 for the termination codon. Two proteins are produced in the presence of a suppressor aa-tRNA that recognizes the termination codon at the 3′ end of an ORF: the expected “stopped” protein that terminates at the termination codon of the ORF, and the longer “readthrough” protein that extends to the next in-frame termination codon. The two proteins are identical over the total length of the stopped protein. Synthesis of the stopped protein is always more abundant than that of the readthrough protein.
A well-known example of readthrough occurs in the TMV (+) single-stranded RNA genome. The 5′-proximal ORF codes for the 126K protein that contains a putative methyltransferase and a helicase domain. Readthrough of its UAG termination codon leads to the synthesis of the 183K readthrough product that harbors the highly conserved GDD (Gly-Asp-Asp) motif, responsible for replicase activity (reviewed in Beier and Grimm, 2001, Maia et al., 1996).
Many members of the genus Alphavirus harbor a suppressible UGA codon separating the regions coding for the NSP3 and NSP4 proteins. The NSP4 protein shares homologous aa sequences with the RdRp of Poliovirus and plant RNA viruses.
In addition to the termination codon, other cis elements on the mRNA are required for efficient readthrough. These elements are either the sequence surrounding the termination codon preferentially on the 3′ side and/or a hairpin or pseudoknot structure also located downstream of the suppressible termination codon. In the case of TMV RNA, the nature of the two codons following the suppressible UAG codon affects the level of readthrough (Valle et al., 1992). The requirements in BYDV are very different: two elements are mandatory for readthrough of the UAG codon in vitro and in vivo: a proximal and a distal element located, respectively, 6–15 nts and about 700 nts downstream of the suppressible UAG codon (Brown et al., 1996). The distal element is conserved among luteoviruses and in Pea enation mosaic enamovirus (PEMV, family Luteoviridae), suggesting that it might also participate in readthrough in these viruses.
Readthrough was clearly demonstrated in mouse cells infected with Murine leukemia virus (MLV; family Retroviridae). Here, most ribosomes terminate synthesis at the UAG codon to produce the Gag protein, but when termination is suppressed, a glutamine residue from Gln-tRNAGln is incorporated at the level of the UAG codon and elongation continues to form the Gag–Pol product. This latter protein is then cleaved to yield Gag, a protease (whose corresponding gene segment harbors the suppressed UAG codon) and the reverse transcriptase (Yoshinaka et al., 1985). In retroviruses, suppression of termination is controlled by structures within the RNA itself: it requires a few specific nts immediately downstream of the termination codon, followed by a spacer region of a few nts and a hairpin that in some cases forms a pseudoknot. In MLV, suppression of the gag UAG codon depends on specific downstream sequences and on a pseudoknot structure (reviewed in Gale et al., 2000).
B. Suppressor tRNAs
Misreading of termination codons is achieved by a variety of naturally occurring suppressor tRNAs that normally recognize a cognate codon, but at times recognize one of the termination codons by “improper” base pairing (reviewed in Beier and Grimm, 2001).
1. Suppressors of UAG/UAA codons
The first natural UAG suppressor tRNA identified was the cytoplasmic tRNATyr bearing a GΨA anticodon purified from tobacco leaves and Drosophila melanogaster (Beier et al., 1984, Bienz and Kubli, 1981). Pseudouiridine (Ψ) can form a classical base pair with adenosine. The Ψ modification at the second anticodon position is necessary to read the UAG codon; it enhances the unconventional G:G interaction at the first anticodon position. Mutating the suppressible TMV UAG codon to UAA leads to virion formation in plants, implying that a tRNA recognizing the UAA codon is present in the host. It was shown that the UAA codon, if placed in the TMV context, was also recognized in vitro by the suppressor tobacco tRNATyr. A second UAG/UAA suppressor is the cytoplasmic tRNAGln with CUG or UmUG (Um is 2′-O-methyluridine) anticodons. tRNAGln is present in almost all prokaryotes and eukaryotes. Interaction of the two tRNAGln isoacceptors with UAG or UAA requires an unconventional G:U base pair at the third anticodon position. Probably an unmodified A in the tRNA immediately 3′ of the anticodon facilitates noncanonical base pairing. Other UAG suppressors are the cytoplasmic tRNALeu with a CAA or a CAG anticodon. Here, recognition of the UAG codon requires an unusual A:A pair in the second position of both the CAA and the CAG anticodons and also a G:U pair in the third position of the CAG anticodon.
2. Suppressors of UGA codons
Two UGA suppressors, a chloroplast and a cytoplasmic tRNATrp with the anticodon CmCA (Cm is 2′-O-methylcytidine) were isolated from tobacco plants and shown to suppress the Tobacco rattle virus (TRV; genus, Tobravirus) RNA1 UGA codon. Several reports indicate that a tRNATrp with UGA suppressor activity is also present in vertebrates (Cordell et al., 1980, Geller and Rich, 1980). Recognition of the UGA codon by tRNATrp requires an unusual Cm:A pair in the first position of the CmCA anticodon. A tRNACys with a GCA anticodon was isolated from tobacco plants and shown to suppress the UGA in TRV RNA1. Misreading of UGA by tRNACys involves a G:A pair at the first GCA anticodon position. The two tRNAArg with an U*CG (U* is 5-methoxy-carbonylmethyluridine) or ICG anticodon stimulate UGA readthrough in the context of TRV RNA1. Interaction of tRNAArg with the UGA codon requires a G:U base pair at the third U*CG anticodon position.
C. Binding of release factors
The reverse transcriptase of MLV interacts with eRF1. This interaction displaces eRF3 from the release factor complex and increases synthesis of the readthrough protein. This function of the reverse transcriptase is required for appropriate levels of the readthrough and stopped proteins (Orlova et al., 2003; reviewed in Goff, 2004).
Interaction between the nascent peptidyl-tRNA during translation of the 22-codon upstream ORF2 (uORF2) and eRF1 of HCMV inhibits expression of the downstream UL4 gene. The peptide product of uORF2 inhibits its own translation termination by forming a stable peptidyl-prolyl-tRNA-ribosome complex that prevents peptide release and stalls the elongating ribosome at the uORF2 termination codon (Janzen et al., 2002).
VI. Conclusions
The study of the regulation of gene expression has known various phases over the decades, ever since some of its major players, such as messenger RNAs and ribosomes had been identified. It first led to examining the initiation, elongation, and termination steps of protein biosynthesis using bacterial extracts and artificial RNAs or bacteriophage RNA genomes as mRNAs, and defining the proteins involved in each step. Thereafter, the availability of cell mRNAs greatly facilitated the study of protein biosynthesis in extracts of eukaryotic cells. This revealed the vast number of protein factors involved in particular at the initiation step of protein synthesis, and the mechanism of action of these factors. In recent years, the sequencing of an ever increasing number of viral RNA genomes shown to function as mRNAs has brought a wealth of new information regarding the fundamental role played by the modulation of the structure of mRNAs in regulating gene expression. It has, for instance, led to numerous studies that consider circularization of mRNAs an important step in promoting protein synthesis. In addition, it has also highlighted the variety of strategies developed by viruses to perturb host protein synthesis so as to favor synthesis of viral proteins. Such evasion of host protein synthesis is now leading to a variety of fascinating studies showing that this involves a complex yet balanced interplay between the host cell translation machinery, the viral mRNA, and the viral proteins resulting from expression of the viral genome. Further experiments will undoubtedly unveil other new venues in this intriguing and multifaceted aspect of cell development.
Acknowledgments
We are very grateful to Hans Gross for suggesting that we write a review on the present topic, and for his encouragements. We are most thankful to Ivan N. Shatsky and Nahum Sonenberg for critical reading of the review and for their valuable and constructive suggestions. We are most grateful to Katherine M. Kean for her support during the writing of this article.
This work was supported by DARRI (Direction des Applications de la Recherche et des Relations Industrielles de l'Institut Pasteur) to A. V. K. and by grants from SIDACTION and the ANRS (Association Nationale de Recherche contre le SIDA) to B. C. R.
A. V. K. thanks her father, Vasiliy B. Poltaraus, for supporting and educating her. The authors thank the members of their laboratories for their help and constant support.
References
- Adams S.L., Safer B., Anderson W.F., Merrick W.C. Eukaryotic initiation complex formation. Evidence for two distinct pathways. J. Biol. Chem. 1975;250:9083–9089. [PubMed] [Google Scholar]
- Agranovsky A.A., Koonin E.V., Boyko V.P., Maiss E., Frotschl R., Lunina N.A., Atabekov J.G. Beet yellows closterovirus: Complete genome structure and identification of a leader papain-like thiol protease. Virology. 1994;198:311–324. doi: 10.1006/viro.1994.1034. [DOI] [PubMed] [Google Scholar]
- Alvarez E., Castello A., Menendez-Arias L., Carrasco L. HIV protease cleaves poly(A)-binding protein. Biochem. J. 2006;396:219–226. doi: 10.1042/BJ20060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez E., Menendez-Arias L., Carrasco L. The eukaryotic translation initiation factor 4GI is cleaved by different retroviral proteases. J. Virol. 2003;77:12392–12400. doi: 10.1128/JVI.77.23.12392-12400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev D.E., Fernandez-Miragall O., Ramajo J., Dmitriev S.E., Terenin I.M., Martínez-Salas E., Shatsky I.N. Differential factor requirement to assemble translation initiation complexes at the alternative start codons of foot-and-mouth disease virus RNA. RNA. 2007;13:1366–1374. doi: 10.1261/rna.469707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arad G., Bar-Meir R., Kotler M. Ribosomal frameshifting at the Gag-Pol junction in avian leukemia sarcoma virus forms a novel cleavage site. FEBS Lett. 1995;364:1–4. doi: 10.1016/0014-5793(95)00302-p. [DOI] [PubMed] [Google Scholar]
- Aragón T., de la Luna S., Novoa I., Carrasco L., Ortín J., Nieto A. Eukaryotic translation initiation factor 4GI is a cellular target for NS1 protein, a translational activator of influenza virus. Mol. Cell. Biol. 2000;20:6259–6268. doi: 10.1128/mcb.20.17.6259-6268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bablanian R., Russell W.C. Adenovirus polypeptide synthesis in the presence of non-replicating poliovirus. J. Gen. Virol. 1974;24:261–279. doi: 10.1099/0022-1317-24-2-261. [DOI] [PubMed] [Google Scholar]
- Bahner I., Lamb J., Mayo M.A., Hay R.T. Expression of the genome of potato leafroll virus: Readthrough of the coat protein termination codon in vivo. J. Gen. Virol. 1990;71:2251–2256. doi: 10.1099/0022-1317-71-10-2251. [DOI] [PubMed] [Google Scholar]
- Balachandran S., Barber G.N. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5:51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Balvay L., Lopez Lastra M., Sargueil B., Darlix J.L., Ohlmann T. Translational control of retroviruses. Nat. Rev. Microbiol. 2007;5:128–140. doi: 10.1038/nrmicro1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barends S., Bink H.H., van den Worm S.H., Pleij C.W., Kraal B. Entrapping ribosomes for viral translation: tRNA mimicry as a molecular Trojan horse. Cell. 2003;112:123–129. doi: 10.1016/s0092-8674(02)01256-4. [DOI] [PubMed] [Google Scholar]
- Baril M., Brakier-Gingras L. Translation of the F protein of hepatitis C virus is initiated at a non-AUG codon in a + 1 reading frame relative to the polyprotein. Nucleic Acids Res. 2005;33:1474–1486. doi: 10.1093/nar/gki292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr J.N. Bunyavirus mRNA synthesis is coupled to translation to prevent premature transcription termination. RNA. 2007;13:731–736. doi: 10.1261/rna.436607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter N.J., Roetzer A., Liebig H.D., Sedelnikova S.E., Hounslow A.M., Skern T., Waltho J.P. Structure and dynamics of coxsackievirus B4 2A proteinase, an enyzme involved in the etiology of heart disease. J. Virol. 2006;80:1451–1462. doi: 10.1128/JVI.80.3.1451-1462.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra S.P., Rose J.A., Hardy M., Baroudy B.M., Anderson C.W. Direct mapping of adeno-associated virus capsid proteins B and C: A possible ACG initiation codon. Proc. Natl. Acad. Sci. USA. 1985;82:7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K.M., Daijogo S., Semler B.L. A nucleo-cytoplasmic SR protein functions in viral IRES-mediated translation initiation. EMBO J. 2007;26:459–467. doi: 10.1038/sj.emboj.7601494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Barciszewska M., Krupp G., Mitnacht R., Gross H.J. UAG readthrough during TMV RNA translation: Isolation and sequence of two tRNAs with suppressor activity from tobacco plants. EMBO J. 1984;3:351–356. doi: 10.1002/j.1460-2075.1984.tb01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Grimm M. Misreading of termination codons in eukaryotes by natural nonsense suppressor tRNAs. Nucleic Acids Res. 2001;29:4767–4782. doi: 10.1093/nar/29.23.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcourt M.F., Farabaugh P.J. Ribosomal frameshifting in the yeast retrotransposon Ty: tRNAs induce slippage on a 7 nucleotide minimal site. Cell. 1990;62:339–352. doi: 10.1016/0092-8674(90)90371-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham G.J., Jackson R.J. Translational initiation on picornavirus RNA. In: Sonenberg N., Hershey J.W.B., Mathews M.B., editors. Translational Control of Gene Expression. CSHL Press; Cold Spring Harbor, NY: 2000. pp. 869–900. [Google Scholar]
- Berlanga J.J., Ventoso I., Harding H.P., Deng J., Ron D., Sonenberg N., Carrasco L., de Haro C. Antiviral effect of the mammalian translation initiation factor 2alpha kinase GCN2 against RNA viruses. EMBO J. 2006;25:1730–1740. doi: 10.1038/sj.emboj.7601073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi F., Haenni A.L. Viruses: Exquisite models for cell strategies. Biochimie. 1998;80:1035–1041. doi: 10.1016/s0300-9084(99)80009-1. [DOI] [PubMed] [Google Scholar]
- Bienz M., Kubli E. Wild-type tRNATyrG reads the TMV RNA stop codon, but Q base-modified tRNATyrQ does not. Nature. 1981;294:188–190. doi: 10.1038/294188a0. [DOI] [PubMed] [Google Scholar]
- Blyn L.B., Towner J.S., Semler B.L., Ehrenfeld E. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J. Virol. 1997;71:6243–6246. doi: 10.1128/jvi.71.8.6243-6246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeck R., Curran J., Matsuoka Y., Compans R., Kolakofsky D. The parainfluenza virus type 1 P/C gene uses a very efficient GUG codon to start its C′ protein. J. Virol. 1992;66:1765–1768. doi: 10.1128/jvi.66.3.1765-1768.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeck R., Kolakofsky D. Positions + 5 and + 6 can be major determinants of the efficiency of non-AUG initiation codons for protein synthesis. EMBO J. 1994;13:3608–3617. doi: 10.1002/j.1460-2075.1994.tb06668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol J.F. Replication of alfamo- and ilarviruses: Role of the coat protein. Annu. Rev. Phytopathol. 2005;43:39–62. doi: 10.1146/annurev.phyto.43.101804.120505. [DOI] [PubMed] [Google Scholar]
- Bonderoff J.M., Larey J.L., Lloyd R.E. Cleavage of Poly(A)‐binding protein by poliovirus 3C proteinase inhibits viral internal ribosome entry site-mediated translation. J. Virol. 2008;82:9389–9399. doi: 10.1128/JVI.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville J.M., Sanfacon H., Fütterer J., Hohn T. Posttranscriptional trans-activation in cauliflower mosaic virus. Cell. 1989;59:1135–1143. doi: 10.1016/0092-8674(89)90769-1. [DOI] [PubMed] [Google Scholar]
- Boonham N., Henry C.M., Wood K.R. The nucleotide sequence and proposed genome organization of oat chlorotic stunt virus, a new soil-borne virus of cereals. J. Gen. Virol. 1995;76:2025–2034. doi: 10.1099/0022-1317-76-8-2025. [DOI] [PubMed] [Google Scholar]
- Borovjagin A., Pestova T., Shatsky I. Pyrimidine tract binding protein strongly stimulates in vitro encephalomyocarditis virus RNA translation at the level of preinitiation complex formation. FEBS Lett. 1994;351:299–302. doi: 10.1016/0014-5793(94)00848-5. [DOI] [PubMed] [Google Scholar]
- Bouloy M., Plotch S.J., Krug R.M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc. Natl. Acad. Sci. USA. 1978;75:4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussadia O., Niepmann M., Creancier L., Prats A.C., Dautry F., Jacquemin-Sablon H. Unr is required in vivo for efficient initiation of translation from the internal ribosome entry sites of both rhinovirus and poliovirus. J. Virol. 2003;77:3353–3359. doi: 10.1128/JVI.77.6.3353-3359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V., van den Heuvel J.F., Verbeek M., Ziegler-Graff V., Reutenauer A., Herrbach E., Garaud J.C., Guilley H., Richards K., Jonard G. Aphid transmission of beet western yellows luteovirus requires the minor capsid read-through protein P74. EMBO J. 1995;14:650–659. doi: 10.1002/j.1460-2075.1995.tb07043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley I., Boursnell M.E., Binns M.M., Bilimoria B., Blok V.C., Brown T.D., Inglis S.C. An efficient ribosomal frame-shifting signal in the polymerase-encoding region of the coronavirus IBV. EMBO J. 1987;6:3779–3785. doi: 10.1002/j.1460-2075.1987.tb02713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley I., Digard P., Inglis S.C. Characterization of an efficient coronavirus ribosomal frameshifting signal: Requirement for an RNA pseudoknot. Cell. 1989;57:537–547. doi: 10.1016/0092-8674(89)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley I., Dos Ramos F.J. Programmed ribosomal frameshifting in HIV-1 and the SARS-CoV. Virus Res. 2006;119:29–42. doi: 10.1016/j.virusres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.M., Dinesh-Kumar S.P., Miller W.A. Local and distant sequences are required for efficient readthrough of the barley yellow dwarf virus PAV coat protein gene stop codon. J. Virol. 1996;70:5884–5892. doi: 10.1128/jvi.70.9.5884-5892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheton A. The relationship between the flamenco gene and gypsy in Drosophila: How to tame a retrovirus. Trends Genet. 1995;11:349–353. doi: 10.1016/s0168-9525(00)89105-2. [DOI] [PubMed] [Google Scholar]
- Burgui I., Aragón T., Ortín J., Nieto A. PABP1 and eIF4GI associate with influenza virus NS1 protein in viral mRNA translation initiation complexes. J. Gen. Virol. 2003;84:3263–3274. doi: 10.1099/vir.0.19487-0. [DOI] [PubMed] [Google Scholar]
- Burgui I., Yanguez E., Sonenberg N., Nieto A. Influenza virus mRNA translation revisited: Is the eIF4E cap-binding factor required for viral mRNA translation? J. Virol. 2007;81:12427–12438. doi: 10.1128/JVI.01105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell M., Sarnow P. Hijacking the translation apparatus by RNA viruses. J. Cell Biol. 2002;158:395–399. doi: 10.1083/jcb.200205044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell M., Wood W., Carpenter G., Pain V.M., Morley S.J., Clemens M.J. Disruption of the interaction of mammalian protein synthesis eukaryotic initiation factor 4B with the poly(A)-binding protein by caspase- and viral protease-mediated cleavages. J. Biol. Chem. 2001;276:23922–23928. doi: 10.1074/jbc.M100384200. [DOI] [PubMed] [Google Scholar]
- Carroll R., Derse D. Translation of equine infectious anemia virus bicistronic tat-rev mRNA requires leaky ribosome scanning of the tat CTG initiation codon. J. Virol. 1993;67:1433–1440. doi: 10.1128/jvi.67.3.1433-1440.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J.L. RNA editing in hepatitis delta virus genotype III requires a branched double-hairpin RNA structure. J. Virol. 2002;76:7385–7397. doi: 10.1128/JVI.76.15.7385-7397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Kaelin K., Baczko K., Billeter M.A. Measles virus editing provides an additional cysteine-rich protein. Cell. 1989;56:759–764. doi: 10.1016/0092-8674(89)90679-x. [DOI] [PubMed] [Google Scholar]
- Chan C.P., Siu K.L., Chin K.T., Yuen K.Y., Zheng B., Jin D.Y. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2006;80:9279–9287. doi: 10.1128/JVI.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron C., Nicolai M., Gallois J.L., Robaglia C., Moury B., Palloix A., Caranta C. Natural variation and functional analyses provide evidence for co-evolution between plant eIF4E and potyviral VPg. Plant J. 2008;54:56–68. doi: 10.1111/j.1365-313X.2008.03407.x. [DOI] [PubMed] [Google Scholar]
- Chau D.H., Yuan J., Zhang H., Cheung P., Lim T., Liu Z., Sall A., Yang D. Coxsackievirus B3 proteases 2A and 3C induce apoptotic cell death through mitochondrial injury and cleavage of eIF4GI but not DAP5/p97/NAT1. Apoptosis. 2007;12:513–524. doi: 10.1007/s10495-006-0013-0. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Jayan G.C., Casey J.L. Differential inhibition of RNA editing in hepatitis delta virus genotype III by the short and long forms of hepatitis delta antigen. J. Virol. 2003;77:7786–7795. doi: 10.1128/JVI.77.14.7786-7795.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm G.E., Henner D.J. Multiple early transcripts and splicing of the Autographa californica nuclear polyhedrosis virus IE-1 gene. J. Virol. 1988;62:3193–3200. doi: 10.1128/jvi.62.9.3193-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A.K., Kahn L.E., Bourne C.M. Circular polysomes predominate on the rough endoplasmic reticulum of somatotropes and mammotropes in the rat anterior pituitary. Am. J. Anat. 1987;178:1–10. doi: 10.1002/aja.1001780102. [DOI] [PubMed] [Google Scholar]
- Chung B.Y., Miller W.A., Atkins J.F., Firth A.E. An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. USA. 2008;105:5897–5902. doi: 10.1073/pnas.0800468105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare J.J., Belcourt M., Farabaugh P.J. Efficient translational frameshifting occurs within a conserved sequence of the overlap between the two genes of a yeast Ty1 transposon. Proc. Natl. Acad. Sci. USA. 1988;85:6816–6820. doi: 10.1073/pnas.85.18.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J.H., Lyles D.S. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J. Virol. 2002;76:10177–10187. doi: 10.1128/JVI.76.20.10177-10187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcelette S., Masse T., Madjar J.J. Initiation of translation by non-AUG codons in human T-cell lymphotropic virus type I mRNA encoding both Rex and Tax regulatory proteins. Nucleic Acids Res. 2000;28:1625–1634. doi: 10.1093/nar/28.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., DeNoto F.M., Atkins J.F., Gesteland R.F., Bishop J.M., Goodman H.M. The forms of tRNATrp found in avian sarcoma virus and uninfected chicken cells have structural identity but functional distinctions. J. Biol. Chem. 1980;255:9358–9368. [PubMed] [Google Scholar]
- Costa-Mattioli M., Svitkin Y., Sonenberg N. La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Mol. Cell. Biol. 2004;24:6861–6870. doi: 10.1128/MCB.24.15.6861-6870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino D.A., Pfingsten J.S., Rambo R.P., Kieft J.S. tRNA-mRNA mimicry drives translation initiation from a viral IRES. Nat. Struct. Mol. Biol. 2008;15:57–64. doi: 10.1038/nsmb1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton S., Dufresne P.J., Thivierge K., Ide C., Fortin M.G. The VPgPro protein of Turnip mosaic virus: In vitro inhibition of translation from a ribonuclease activity. Virology. 2006;351:92–100. doi: 10.1016/j.virol.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.W., Haghighat A., Yu A.T., Sonenberg N. Interaction of polyadenylate-binding protein with the eIF4G homologue PAIP enhances translation. Nature. 1998;392:520–523. doi: 10.1038/33198. [DOI] [PubMed] [Google Scholar]
- Cuesta R., Xi Q., Schneider R.J. Adenovirus-specific translation by displacement of kinase Mnk1 from cap-initiation complex eIF4F. EMBO J. 2000;19:3465–3474. doi: 10.1093/emboj/19.13.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B.R. Retroviruses as model systems for the study of nuclear RNA export pathways. Virology. 1998;249:203–210. doi: 10.1006/viro.1998.9331. [DOI] [PubMed] [Google Scholar]
- Curran J., Boeck R., Kolakofsky D. The Sendai virus P gene expresses both an essential protein and an inhibitor of RNA synthesis by shuffling modules via mRNA editing. EMBO J. 1991;10:3079–3085. doi: 10.1002/j.1460-2075.1991.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J., Kolakofsky D. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. EMBO J. 1988;7:245–251. doi: 10.1002/j.1460-2075.1988.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danthinne X., Seurinck J., Meulewaeter F., Van Montagu M., Cornelissen M. The 3′ untranslated region of satellite tobacco necrosis virus RNA stimulates translation in vitro. Mol. Cell. Biol. 1993;13:3340–3349. doi: 10.1128/mcb.13.6.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughenbaugh K.F., Fraser C.S., Hershey J.W., Hardy M.E. The genome-linked protein VPg of the Norwalk virus binds eIF3, suggesting its role in translation initiation complex recruitment. EMBO J. 2003;22:2852–2859. doi: 10.1093/emboj/cdg251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Luna S., Fortes P., Beloso A., Ortín J. Influenza virus NS1 protein enhances the rate of translation initiation of viral mRNAs. J. Virol. 1995;69:2427–2433. doi: 10.1128/jvi.69.4.2427-2433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J.C. Molecular biology of Borna disease virus and persistence. Front. Biosci. 2002;7:D569–D579. doi: 10.2741/torre. [DOI] [PubMed] [Google Scholar]
- Demler S.A., de Zoeten G.A. The nucleotide sequence and luteovirus-like nature of RNA 1 of an aphid non-transmissible strain of pea enation mosaic virus. J. Gen. Virol. 1991;72:1819–1834. doi: 10.1099/0022-1317-72-8-1819. [DOI] [PubMed] [Google Scholar]
- Demler S.A., Rucker D.G., de Zoeten G.A. The chimeric nature of the genome of pea enation mosaic virus: The independent replication of RNA 2. J. Gen. Virol. 1993;74:1–14. doi: 10.1099/0022-1317-74-1-1. [DOI] [PubMed] [Google Scholar]
- den Boon J.A., Snijder E.J., Chirnside E.D., de Vries A.A., Horzinek M.C., Spaan W.J. Equine arteritis virus is not a togavirus but belongs to the coronaviruslike superfamily. J. Virol. 1991;65:2910–2920. doi: 10.1128/jvi.65.6.2910-2920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo R.C., Groft C.M., Rajashankar K.R., Burley S.K. Recognition of the rotavirus mRNA 3′ consensus by an asymmetric NSP3 homodimer. Cell. 2002;108:71–81. doi: 10.1016/s0092-8674(01)00632-8. [DOI] [PubMed] [Google Scholar]
- Dever T.E., Dar A.C., Sicheri F. The eIF2α Kinases. In: Mathews M.B., Sonenberg N., Hershey J.W.B., editors. Translational Control in Biology and Medicine. CSHL Press; Cold Spring Harbor, NY: 2007. pp. 319–344. [Google Scholar]
- Di R., Dinesh-Kumar S.P., Miller W.A. Translational frameshifting by barley yellow dwarf virus RNA (PAV serotype) in Escherichia coli and in eukaryotic cell-free extracts. Mol. Plant Microbe Interact. 1993;6:444–452. doi: 10.1094/mpmi-6-444. [DOI] [PubMed] [Google Scholar]
- Dinesh-Kumar S.P., Brault V., Miller W.A. Precise mapping and in vitro translation of a trifunctional subgenomic RNA of barley yellow dwarf virus. Virology. 1992;187:711–722. doi: 10.1016/0042-6822(92)90474-4. [DOI] [PubMed] [Google Scholar]
- Dinman J.D., Icho T., Wickner R.B. A − 1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc. Natl. Acad. Sci. USA. 1991;88:174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domier L.L., McCoppin N.K., D′Arcy C.J. Sequence requirements for translation initiation of Rhopalosiphum padi virus ORF2. Virology. 2000;268:264–271. doi: 10.1006/viro.2000.0189. [DOI] [PubMed] [Google Scholar]
- Doudna J.A., Sarnow P. Translational initiation by viral internal ribosome entry sites. In: Mathews M.B., Sonenberg N., Hershey J.W.B., editors. Translational Control in Biology and Medicine. CSHL Press; Cold Spring Harbor, NY: 2007. pp. 129–153. [Google Scholar]
- Dreher T.W., Miller W.A. Translational control in positive strand RNA plant viruses. Virology. 2006;344:185–197. doi: 10.1016/j.virol.2005.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugeon G., Jean-Jean O., Frolova L., Le Goff X., Philippe M., Kisselev L., Haenni A.L. Eukaryotic release factor 1 (eRF1) abolishes readthrough and competes with suppressor tRNAs at all three termination codons in messenger RNA. Nucleic Acids Res. 1997;25:2254–2258. doi: 10.1093/nar/25.12.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgil D., Harris E. End-to-end communication in the modulation of translation by mammalian RNA viruses. Virus Res. 2006;119:43–51. doi: 10.1016/j.virusres.2005.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgadi M.M., Hayes C.E., Smiley J.R. The herpes simplex virus vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J. Virol. 1999;73:7153–7164. doi: 10.1128/jvi.73.9.7153-7164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami K., Sato T.A., Nakada S., Enami M. Influenza virus NS1 protein stimulates translation of the M1 protein. J. Virol. 1994;68:1432–1437. doi: 10.1128/jvi.68.3.1432-1437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzerodt M., Mikkelsen T., Pedersen F.S., Kjeldgaard N.O., Jorgensen P. The nucleotide sequence of the Akv murine leukemia virus genome. Virology. 1984;134:196–207. doi: 10.1016/0042-6822(84)90285-x. [DOI] [PubMed] [Google Scholar]
- Everly D.N., Jr., Feng P., Mian I.S., Read G.S. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: Genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 2002;76:8560–8571. doi: 10.1128/JVI.76.17.8560-8571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian M.R., White K.A. 5′-3′ RNA-RNA interaction facilitates cap- and poly(A) tail-independent translation of tomato bushy stunt virus mRNA: A potential common mechanism for tombusviridae. J. Biol. Chem. 2004;279:28862–28872. doi: 10.1074/jbc.M401272200. [DOI] [PubMed] [Google Scholar]
- Fabian M.R., White K.A. Analysis of a 3′-translation enhancer in a tombusvirus: A dynamic model for RNA–RNA interactions of mRNA termini. RNA. 2006;12:1304–1314. doi: 10.1261/rna.69506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P.J. Translational frameshifting: Implications for the mechanism of translational frame maintenance. Prog. Nucleic Acid Res. Mol. Biol. 2000;64:131–170. doi: 10.1016/s0079-6603(00)64004-7. [DOI] [PubMed] [Google Scholar]
- Feigenblum D., Schneider R.J. Modification of eukaryotic initiation factor 4F during infection by influenza virus. J. Virol. 1993;67:3027–3035. doi: 10.1128/jvi.67.6.3027-3035.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filichkin S.A., Lister R.M., McGrath P.F., Young M.J. In vivo expression and mutational analysis of the barley yellow dwarf virus readthrough gene. Virology. 1994;205:290–299. doi: 10.1006/viro.1994.1645. [DOI] [PubMed] [Google Scholar]
- Flynn A., Proud C.G. Serine 209, not serine 53, is the major site of phosphorylation in initiation factor eIF-4E in serum-treated Chinese hamster ovary cells. J. Biol. Chem. 1995;270:21684–21688. doi: 10.1074/jbc.270.37.21684. [DOI] [PubMed] [Google Scholar]
- Frank J., Gao H., Sengupta J., Gao N., Taylor D.J. The process of mRNA-tRNA translocation. Proc. Natl. Acad. Sci. USA. 2007;104:19671–19678. doi: 10.1073/pnas.0708517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara N., Huang C., Kiyotani K., Yoshida T., Sakaguchi T. Mutational analysis of the Sendai virus V protein: Importance of the conserved residues for Zn binding, virus pathogenesis, and efficient RNA editing. Virology. 2002;299:172–181. doi: 10.1006/viro.2002.1516. [DOI] [PubMed] [Google Scholar]
- Fütterer J., Hohn T. Translation of a polycistronic mRNA in the presence of the cauliflower mosaic virus transactivator protein. EMBO J. 1991;10:3887–3896. doi: 10.1002/j.1460-2075.1991.tb04958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Hohn T. Translation in plants—Rules and exceptions. Plant Mol. Biol. 1996;32:159–189. doi: 10.1007/BF00039382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Potrykus I., Bao Y., Li L., Burns T.M., Hull R., Hohn T. Position-dependent ATT initiation during plant pararetrovirus rice tungro bacilliform virus translation. J. Virol. 1996;70:2999–3010. doi: 10.1128/jvi.70.5.2999-3010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Rothnie H.M., Hohn T., Potrykus I. Rice tungro bacilliform virus open reading frames II and III are translated from polycistronic pregenomic RNA by leaky scanning. J. Virol. 1997;71:7984–7989. doi: 10.1128/jvi.71.10.7984-7989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M., Jr., Tan S.L., Katze M.G. Translational control of viral gene expression in eukaryotes. Microbiol. Mol. Biol. Rev. 2000;64:239–280. doi: 10.1128/mmbr.64.2.239-280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D.R. A tale of two termini: A functional interaction between the termini of an mRNA is a prerequisite for efficient translation initiation. Gene. 1998;216:1–11. doi: 10.1016/s0378-1119(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Gamarnik A.V., Andino R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 1998;12:2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M.A., Meurs E.F., Esteban M. The dsRNA protein kinase PKR: Virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Garcin D., Kolakofsky D. A novel mechanism for the initiation of tacaribe arenavirus genome replication. J. Virol. 1990;64:6196–6203. doi: 10.1128/jvi.64.12.6196-6203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin D., Lezzi M., Dobbs M., Elliott R.M., Schmaljohn C., Kang C.Y., Kolakofsky D. The 5′ ends of Hantaan virus (Bunyaviridae) RNAs suggest a prime-and-realign mechanism for the initiation of RNA synthesis. J. Virol. 1995;69:5754–5762. doi: 10.1128/jvi.69.9.5754-5762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel M.S., Katze M.G. Translational control by influenza virus. Selective and cap-dependent translation of viral mRNAs in infected cells. J. Biol. Chem. 1992;267:9383–9390. [PubMed] [Google Scholar]
- Gazo B.M., Murphy P., Gatchel J.R., Browning K.S. A novel interaction of Cap-binding protein complexes eukaryotic initiation factor (eIF) 4F and eIF(iso)4F with a region in the 3′-untranslated region of satellite tobacco necrosis virus. J. Biol. Chem. 2004;279:13584–13592. doi: 10.1074/jbc.M311361200. [DOI] [PubMed] [Google Scholar]
- Ge Z., Hiruki C., Roy K.L. Nucleotide sequence of sweet clover necrotic mosaic dianthovirus RNA-1. Virus Res. 1993;28:113–124. doi: 10.1016/0168-1702(93)90130-f. [DOI] [PubMed] [Google Scholar]
- Geller A.I., Rich A. A UGA termination suppression tRNATrp active in rabbit reticulocytes. Nature. 1980;283:41–46. doi: 10.1038/283041a0. [DOI] [PubMed] [Google Scholar]
- German-Retana S., Walter J., Doublet B., Roudet-Tavert G., Nicaise V., Lecampion C., Houvenaghel M.C., Robaglia C., Michon T., Le Gall O. Mutational analysis of a plant cap-binding protein eIF4E reveals key amino-acids involved in biochemical functions and potyvirus infection. J. Virol. 2008;92:7601–7612. doi: 10.1128/JVI.00209-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A.C., Svitkin Y., Belsham G.J., Pause A., Sonenberg N. Activation of the translational suppressor 4E-BP1 following infection with encephalomyocarditis virus and poliovirus. Proc. Natl. Acad. Sci. USA. 1996;93:5578–5583. doi: 10.1073/pnas.93.11.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S.P. Genetic reprogramming by retroviruses: Enhanced suppression of translational termination. Cell Cycle. 2004;3:123–125. [PubMed] [Google Scholar]
- Goodfellow I., Chaudhry Y., Gioldasi I., Gerondopoulos A., Natoni A., Labrie L., Laliberte J.F., Roberts L. Calicivirus translation initiation requires an interaction between VPg and eIF4E. EMBO Rep. 2005;6:968–972. doi: 10.1038/sj.embor.7400510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosert R., Chang K.H., Rijnbrand R., Yi M., Sangar D.V., Lemon S.M. Transient expression of cellular polypyrimidine-tract binding protein stimulates cap-independent translation directed by both picornaviral and flaviviral internal ribosome entry sites in vivo. Mol. Cell. Biol. 2000;20:1583–1595. doi: 10.1128/mcb.20.5.1583-1595.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradi A., Foeger N., Strong R., Svitkin Y.V., Sonenberg N., Skern T., Belsham G.J. Cleavage of eukaryotic translation initiation factor 4GII within foot-and-mouth disease virus-infected cells: Identification of the L-protease cleavage site in vitro. J. Virol. 2004;78:3271–3278. doi: 10.1128/JVI.78.7.3271-3278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradi A., Svitkin Y.V., Imataka H., Sonenberg N. Proteolysis of human eukaryotic translation initiation factor eIF4GII, but not eIF4GI, coincides with the shutoff of host protein synthesis after poliovirus infection. Proc. Natl. Acad. Sci. USA. 1998;95:11089–11094. doi: 10.1073/pnas.95.19.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramstat A., Prüfer D., Rohde W. The nucleic acid-binding zinc finger protein of potato virus M is translated by internal initiation as well as by ribosomal frameshifting involving a shifty stop codon and a novel mechanism of P-site slippage. Nucleic Acids Res. 1994;22:3911–3917. doi: 10.1093/nar/22.19.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco F., Burgyan J., Russo M. The nucleotide sequence of cymbidium ringspot virus RNA. Nucleic Acids Res. 1989;17:6383. doi: 10.1093/nar/17.15.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudima S., Wu S.Y., Chiang C.M., Moraleda G., Taylor J. Origin of hepatitis delta virus mRNA. J. Virol. 2000;74:7204–7210. doi: 10.1128/jvi.74.16.7204-7210.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Carrington J.C., Balazs E., Jonard G., Richards K., Morris T.J. Nucleotide sequence and genome organization of carnation mottle virus RNA. Nucleic Acids Res. 1985;13:6663–6677. doi: 10.1093/nar/13.18.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Allen E., Miller W.A. Structure and function of a cap-independent translation element that functions in either the 3′ or the 5′ untranslated region. RNA. 2000;6:1808–1820. doi: 10.1017/s1355838200001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Allen E.M., Miller W.A. Base-pairing between untranslated regions facilitates translation of uncapped, nonpolyadenylated viral RNA. Mol. Cell. 2001;7:1103–1109. doi: 10.1016/s1097-2765(01)00252-0. [DOI] [PubMed] [Google Scholar]
- Gupta K.C., Patwardhan S. ACG, the initiator codon for a Sendai virus protein. J. Biol. Chem. 1988;263:8553–8556. [PubMed] [Google Scholar]
- Haghighat A., Mader S., Pause A., Sonenberg N. Repression of cap-dependent translation by 4E-binding protein 1: Competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 1995;14:5701–5709. doi: 10.1002/j.1460-2075.1995.tb00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W.D., Boccara M., Robinson D.J., Baulcombe D.C. The complete nucleotide sequence of tobacco rattle virus RNA-1. J. Gen. Virol. 1987;68:2563–2575. doi: 10.1099/0022-1317-68-10-2563. [DOI] [PubMed] [Google Scholar]
- Hansen L.J., Chalker D.L., Orlinsky K.J., Sandmeyer S.B. Ty3 GAG3 and POL3 genes encode the components of intracellular particles. J. Virol. 1992;66:1414–1424. doi: 10.1128/jvi.66.3.1414-1424.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearne P.Q., Knorr D.A., Hillman B.I., Morris T.J. The complete genome structure and synthesis of infectious RNA from clones of tomato bushy stunt virus. Virology. 1990;177:141–151. doi: 10.1016/0042-6822(90)90468-7. [DOI] [PubMed] [Google Scholar]
- Hentze M.W., Gebauer F., Preiss T. cis-Regulatory sequences and trans-acting factors in translational control. In: Mathews M.B., Sonenberg N., Hershey J.W.B., editors. Translational Control in Biology and Medicine. CSHL Press; Cold Spring Harbor, NY: 2007. pp. 269–295. [Google Scholar]
- Herbert T.P., Brierley I., Brown T.D. Identification of a protein linked to the genomic and subgenomic mRNAs of feline calicivirus and its role in translation. J. Gen. Virol. 1997;78:1033–1040. doi: 10.1099/0022-1317-78-5-1033. [DOI] [PubMed] [Google Scholar]
- Herold J., Andino R. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol. Cell. 2001;7:581–591. doi: 10.1016/S1097-2765(01)00205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J. Virol. 1984;49:471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz C., Stavnezer E., Krug R., Gurney T., Jr. Influenza virus, an RNA virus, synthesizes its messenger RNA in the nucleus of infected cells. Cell. 1981;26:391–400. doi: 10.1016/0092-8674(81)90208-7. [DOI] [PubMed] [Google Scholar]
- Herzog E., Guilley H., Fritsch C. Translation of the second gene of peanut clump virus RNA 2 occurs by leaky scanning in vitro. Virology. 1995;208:215–225. doi: 10.1006/viro.1995.1145. [DOI] [PubMed] [Google Scholar]
- Herzog E., Guilley H., Manohar S.K., Dollet M., Richards K., Fritsch C., Jonard G. Complete nucleotide sequence of peanut clump virus RNA 1 and relationships with other fungus-transmitted rod-shaped viruses. J. Gen. Virol. 1994;75:3147–3155. doi: 10.1099/0022-1317-75-11-3147. [DOI] [PubMed] [Google Scholar]
- Hilton A., Mizzen L., MacIntyre G., Cheley S., Anderson R. Translational control in murine hepatitis virus infection. J. Gen. Virol. 1986;67:923–932. doi: 10.1099/0022-1317-67-5-923. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A.G. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A.G. eIF3: A versatile scaffold for translation initiation complexes. Trends Biochem. Sci. 2006;31:553–562. doi: 10.1016/j.tibs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Holzschu D.L., Martineau D., Fodor S.K., Vogt V.M., Bowser P.R., Casey J.W. Nucleotide sequence and protein analysis of a complex piscine retrovirus, walleye dermal sarcoma virus. J. Virol. 1995;69:5320–5331. doi: 10.1128/jvi.69.9.5320-5331.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M., Brown E.A., Lemon S.M. Stability of a stem loop involving the initiator AUG controls the efficiency of internal initiation of translation on hepatitis C virus RNA. RNA. 1996;2:955–968. [PMC free article] [PubMed] [Google Scholar]
- Huiet L., Feldstein P.A., Tsai J.H., Falk B.W. The maize stripe virus major noncapsid protein messenger RNA transcripts contain heterogeneous leader sequences at their 5′ termini. Virology. 1993;197:808–812. doi: 10.1006/viro.1993.1662. [DOI] [PubMed] [Google Scholar]
- Hunt S.L., Jackson R.J. Polypyrimidine-tract binding protein (PTB) is necessary, but not sufficient, for efficient internal initiation of translation of human rhinovirus-2 RNA. RNA. 1999;5:344–359. doi: 10.1017/s1355838299981414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imataka H., Gradi A., Sonenberg N. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 1998;17:7480–7489. doi: 10.1093/emboj/17.24.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Motoyoshi F., Takamatsu N., Okada Y. In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus. Nucleic Acids Res. 1986;14:8291–8305. doi: 10.1093/nar/14.21.8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaag H.M., Kawchuk L., Rohde W., Fischer R., Emans N., Prüfer D. An unusual internal ribosomal entry site of inverted symmetry directs expression of a potato leafroll polerovirus replication-associated protein. Proc. Natl. Acad. Sci. USA. 2003;100:8939–8944. doi: 10.1073/pnas.1332697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Townsley K., Varmus H.E., Majors J. Two efficient ribosomal frameshifting events are required for synthesis of mouse mammary tumor virus gag-related polyproteins. Proc. Natl. Acad. Sci. USA. 1987;84:4298–4302. doi: 10.1073/pnas.84.12.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R.J. Alternative mechanisms of initiating translation of mammalian mRNAs. Biochem. Soc. Trans. 2005;33:1231–1241. doi: 10.1042/BST0331231. [DOI] [PubMed] [Google Scholar]
- Jackson R.J., Kaminski A. Internal initiation of translation in eukaryotes: The picornavirus paradigm and beyond. RNA. 1995;1:985–1000. [PMC free article] [PubMed] [Google Scholar]
- James D., Varga A., Croft H. Analysis of the complete genome of peach chlorotic mottle virus: Identification of non-AUG start codons, in vitro coat protein expression, and elucidation of serological cross-reactions. Arch. Virol. 2007;152:2207–2215. doi: 10.1007/s00705-007-1050-x. [DOI] [PubMed] [Google Scholar]
- Jan E. Divergent IRES elements in invertebrates. Virus Res. 2006;119:16–28. doi: 10.1016/j.virusres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Jan E., Kinzy T.G., Sarnow P. Divergent tRNA-like element supports initiation, elongation, and termination of protein biosynthesis. Proc. Natl. Acad. Sci. USA. 2003;100:15410–15415. doi: 10.1073/pnas.2535183100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan E., Sarnow P. Factorless ribosome assembly on the internal ribosome entry site of cricket paralysis virus. J. Mol. Biol. 2002;324:889–902. doi: 10.1016/s0022-2836(02)01099-9. [DOI] [PubMed] [Google Scholar]
- Jang S.K., Krausslich H.G., Nicklin M.J., Duke G.M., Palmenberg A.C., Wimmer E. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol. 1988;62:2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen D.M., Frolova L., Geballe A.P. Inhibition of translation termination mediated by an interaction of eukaryotic release factor 1 with a nascent peptidyl-tRNA. Mol. Cell. Biol. 2002;22:8562–8570. doi: 10.1128/MCB.22.24.8562-8570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachims M., Van Breugel P.C., Lloyd R.E. Cleavage of poly(A)-binding protein by enterovirus proteases concurrent with inhibition of translation in vitro. J. Virol. 1999;73:718–727. doi: 10.1128/jvi.73.1.718-727.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan I., Lipkin W.I. Borna disease virus. Rev. Med. Virol. 2001;11:37–57. doi: 10.1002/rmv.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi B., Cai A.L., Keiper B.D., Minich W.B., Mendez R., Beach C.M., Stepinski J., Stolarski R., Darzynkiewicz E., Rhoads R.E. Phosphorylation of eukaryotic protein synthesis initiation factor 4E at Ser-209. J. Biol. Chem. 1995;270:14597–145603. doi: 10.1074/jbc.270.24.14597. [DOI] [PubMed] [Google Scholar]
- Kamitani W., Narayanan K., Huang C., Lokugamage K., Ikegami T., Ito N., Kubo H., Makino S. Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mRNA degradation. Proc. Natl. Acad. Sci. USA. 2006;103:12885–12890. doi: 10.1073/pnas.0603144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B.C., Yeam I., Jahn M.M. Genetics of plant virus resistance. Annu. Rev. Phytopathol. 2005;43:581–621. doi: 10.1146/annurev.phyto.43.011205.141140. [DOI] [PubMed] [Google Scholar]
- Karamysheva Z.N., Karamyshev A.L., Ito K., Yokogawa T., Nishikawa K., Nakamura Y., Matsufuji S. Antizyme frameshifting as a functional probe of eukaryotic translational termination. Nucleic Acids Res. 2003;31:5949–5956. doi: 10.1093/nar/gkg789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasev A.V., Boyko V.P., Gowda S., Nikolaeva O.V., Hilf M.E., Koonin E.V., Niblett C.L., Cline K., Gumpf D.J., Lee R.F., Garnsey S.M., Lewandowski D.J. Complete sequence of the citrus tristeza virus RNA genome. Virology. 1995;208:511–520. doi: 10.1006/viro.1995.1182. [DOI] [PubMed] [Google Scholar]
- Kato A., Cortese-Grogan C., Moyer S.A., Sugahara F., Sakaguchi T., Kubota T., Otsuki N., Kohase M., Tashiro M., Nagai Y. Characterization of the amino acid residues of sendai virus C protein that are critically involved in its interferon antagonism and RNA synthesis down-regulation. J. Virol. 2004;78:7443–7454. doi: 10.1128/JVI.78.14.7443-7454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Kawaguchi Y., Tanaka M., Igarashi M., Yokoyama A., Matsuda G., Kanamori M., Nakajima K., Nishimura Y., Shimojima M., Phung H.T., Takahashi E. Epstein–Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1delta (EF-1delta): EF-1delta is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 2001;82:1457–1463. doi: 10.1099/0022-1317-82-6-1457. [DOI] [PubMed] [Google Scholar]
- Katsafanas G.C., Moss B. Colocalization of transcription and translation within cytoplasmic poxvirus factories coordinates viral expression and subjugates host functions. Cell Host Microbe. 2007;2:221–228. doi: 10.1016/j.chom.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M.G., DeCorato D., Krug R.M. Cellular mRNA translation is blocked at both initiation and elongation after infection by influenza virus or adenovirus. J. Virol. 1986;60:1027–1039. doi: 10.1128/jvi.60.3.1027-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M.G., Krug R.M. Translational control in influenza virus-infected cells. Enzyme. 1990;44:265–277. doi: 10.1159/000468764. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Kato K., Tanaka M., Kanamori M., Nishiyama Y., Yamanashi Y. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1delta. J. Virol. 2003;77:2359–2368. doi: 10.1128/JVI.77.4.2359-2368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Matsumura T., Roizman B., Hirai K. Cellular elongation factor 1delta is modified in cells infected with representative alpha-, beta-, or gammaherpesviruses. J. Virol. 1999;73:4456–4460. doi: 10.1128/jvi.73.5.4456-4460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean K.M., Michel Y.M., Malnou C.E., Paulous S., Borman A.M. Roles and mechanisms of mRNA 5′–3′ end cross-talk in translation initiation on animal virus RNAs. Rec. Res. Dev. Virol. 2001;3:165–176. [Google Scholar]
- Kerekatte V., Keiper B.D., Badorff C., Cai A., Knowlton K.U., Rhoads R.E. Cleavage of Poly(A)-binding protein by coxsackievirus 2A protease in vitro and in vivo: Another mechanism for host protein synthesis shutoff? J. Virol. 1999;73:709–717. doi: 10.1128/jvi.73.1.709-717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A., Miyoshi H., Gallie D.R., Goss D.J. Potyvirus genome-linked protein, VPg, directly affects wheat germ in vitro translation: Interactions with translation initiation factors eIF4F and eIFiso4F. J. Biol. Chem. 2008;283:1340–1349. doi: 10.1074/jbc.M703356200. [DOI] [PubMed] [Google Scholar]
- Kim K.H., Lommel S.A. Identification and analysis of the site of − 1 ribosomal frameshifting in red clover necrotic mosaic virus. Virology. 1994;200:574–582. doi: 10.1006/viro.1994.1220. [DOI] [PubMed] [Google Scholar]
- Kim Y.G., Maas S., Rich A. Comparative mutational analysis of cis-acting RNA signals for translational frameshifting in HIV-1 and HTLV-2. Nucleic Acids Res. 2001;29:1125–1131. doi: 10.1093/nar/29.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen V.A., Boeshore M.L., Koonin E.V., Tian T., Falk B.W. Genome structure and phylogenetic analysis of lettuce infectious yellows virus, a whitefly-transmitted, bipartite closterovirus. Virology. 1995;208:99–110. doi: 10.1006/viro.1995.1133. [DOI] [PubMed] [Google Scholar]
- Kneller E.L., Rakotondrafara A.M., Miller W.A. Cap-independent translation of plant viral RNAs. Virus Res. 2006;119:63–75. doi: 10.1016/j.virusres.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Watanabe M., Kamitani W., Tomonaga K., Ikuta K. Translation initiation of a bicistronic mRNA of Borna disease virus: A 16-kDa phosphoprotein is initiated at an internal start codon. Virology. 2000;277:296–305. doi: 10.1006/viro.2000.0592. [DOI] [PubMed] [Google Scholar]
- Koenig R., Commandeur U., Loss S., Beier C., Kaufmann A., Lesemann D.E. Beet soil-borne virus RNA 2: Similarities and dissimilarities to the coat protein gene-carrying RNAs of other furoviruses. J. Gen. Virol. 1997;78:469–477. doi: 10.1099/0022-1317-78-2-469. [DOI] [PubMed] [Google Scholar]
- Koenig R., Loss S. Beet soil-borne virus RNA 1: Genetic analysis enabled by a starting sequence generated with primers to highly conserved helicase-encoding domains. J. Gen. Virol. 1997;78:3161–3165. doi: 10.1099/0022-1317-78-12-3161. [DOI] [PubMed] [Google Scholar]
- Koenig R., Pleij C.W., Beier C., Commandeur U. Genome properties of beet virus Q, a new furo-like virus from sugarbeet, determined from unpurified virus. J. Gen. Virol. 1998;79:2027–2036. doi: 10.1099/0022-1317-79-8-2027. [DOI] [PubMed] [Google Scholar]
- Koh D.C., Liu D.X., Wong S.M. A six-nucleotide segment within the 3′ untranslated region of hibiscus chlorotic ringspot virus plays an essential role in translational enhancement. J. Virol. 2002;76:1144–1153. doi: 10.1128/JVI.76.3.1144-1153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D.C., Wong S.M., Liu D.X. Synergism of the 3′-untranslated region and an internal ribosome entry site differentially enhances the translation of a plant virus coat protein. J. Biol. Chem. 2003;278:20565–20573. doi: 10.1074/jbc.M210212200. [DOI] [PubMed] [Google Scholar]
- Komarova A.V., Brocard M., Kean K.M. The case for mRNA 5′ and 3′ end cross talk during translation in a eukaryotic cell. Prog. Nucleic Acid Res. Mol. Biol. 2006;81:331–367. doi: 10.1016/S0079-6603(06)81009-3. [DOI] [PubMed] [Google Scholar]
- Kononenko A.V., Mitkevich V.A., Dubovaya V.I., Kolosov P.M., Makarov A.A., Kisselev L.L. Role of the individual domains of translation termination factor eRF1 in GTP binding to eRF3. Proteins. 2008;70:388–393. doi: 10.1002/prot.21544. [DOI] [PubMed] [Google Scholar]
- Kovacs G.R., Guarino L.A., Graham B.L., Summers M.D. Identification of spliced baculovirus RNAs expressed late in infection. Virology. 1991;185:633–643. doi: 10.1016/0042-6822(91)90534-i. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J. Biol. Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- Kozak M., Shatkin A.J. Migration of 40 S ribosomal subunits on messenger RNA in the presence of edeine. J. Biol. Chem. 1978;253:6568–6577. [PubMed] [Google Scholar]
- Krab I.M., Caldwell C., Gallie D.R., Bol J.F. Coat protein enhances translational efficiency of Alfalfa mosaic virus RNAs and interacts with the eIF4G component of initiation factor eIF4F. J. Gen. Virol. 2005;86:1841–1849. doi: 10.1099/vir.0.80796-0. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy T., Pavitt G.D., Zhang F., Dever T.E., Hinnebusch A.G. Tight binding of the phosphorylated alpha subunit of initiation factor 2 (eIF2alpha) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol. Cell. Biol. 2001;21:5018–5030. doi: 10.1128/MCB.21.15.5018-5030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R.M., Broni B.A., Bouloy M. Are the 5′ ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell. 1979;18:329–334. doi: 10.1016/0092-8674(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Kudchodkar S.B., Yu Y., Maguire T.G., Alwine J.C. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J. Virol. 2004;78:11030–11039. doi: 10.1128/JVI.78.20.11030-11039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudchodkar S.B., Yu Y., Maguire T.G., Alwine J.C. Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of raptor- and rictor-containing complexes. Proc. Natl. Acad. Sci. USA. 2006;103:14182–14187. doi: 10.1073/pnas.0605825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A.B., Drugeon G., Hulanicka D., Haenni A.L. Structural requirements for efficient translational frameshifting in the synthesis of the putative viral RNA-dependent RNA polymerase of potato leafroll virus. Nucleic Acids Res. 1993;21:2165–2171. doi: 10.1093/nar/21.9.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyumcu-Martinez M., Belliot G., Sosnovtsev S.V., Chang K.O., Green K.Y., Lloyd R.E. Calicivirus 3C-like proteinase inhibits cellular translation by cleavage of poly(A)-binding protein. J. Virol. 2004;78:8172–8182. doi: 10.1128/JVI.78.15.8172-8182.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyumcu-Martinez N.M., Joachims M., Lloyd R.E. Efficient cleavage of ribosome-associated poly(A)-binding protein by enterovirus 3C protease. J. Virol. 2002;76:2062–2074. doi: 10.1128/jvi.76.5.2062-2074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyumcu-Martinez N.M., Van Eden M.E., Younan P., Lloyd R.E. Cleavage of poly(A)-binding protein by poliovirus 3C protease inhibits host cell translation: A novel mechanism for host translation shutoff. Mol. Cell. Biol. 2004;24:1779–1790. doi: 10.1128/MCB.24.4.1779-1790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M., Cavanagh D. The molecular biology of coronaviruses. Adv. Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R.A., Krug R.M. Orthomyxoviridae: The viruses and their replication. In: Knipe D.M., Howley P.W., editors. Fields Virology. Lippincott Williams & Wilkens; Philadelphia, PA: 2001. pp. 1487–1531. [Google Scholar]
- Lamphear B.J., Yan R., Yang F., Waters D., Liebig H.D., Klump H., Kuechler E., Skern T., Rhoads R.E. Mapping the cleavage site in protein synthesis initiation factor eIF-4 gamma of the 2A proteases from human Coxsackievirus and rhinovirus. J. Biol. Chem. 1993;268:19200–19203. [PubMed] [Google Scholar]
- Latorre P., Kolakofsky D., Curran J. Sendai virus Y proteins are initiated by a ribosomal shunt. Mol. Cell. Biol. 1998;18:5021–5031. doi: 10.1128/mcb.18.9.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le H., Tanguay R.L., Balasta M.L., Wei C.C., Browning K.S., Metz A.M., Goss D.J., Gallie D.R. Translation initiation factors eIF-iso4G and eIF-4B interact with the poly(A)-binding protein and increase its RNA binding activity. J. Biol. Chem. 1997;272:16247–16255. doi: 10.1074/jbc.272.26.16247. [DOI] [PubMed] [Google Scholar]
- Leh V., Yot P., Keller M. The cauliflower mosaic virus translational transactivator interacts with the 60S ribosomal subunit protein L18 of Arabidopsis thaliana. Virology. 2000;266:1–7. doi: 10.1006/viro.1999.0073. [DOI] [PubMed] [Google Scholar]
- Leonard S., Viel C., Beauchemin C., Daigneault N., Fortin M.G., Laliberté J.F. Interaction of VPg-Pro of turnip mosaic virus with the translation initiation factor 4E and the poly(A)-binding protein in planta. J. Gen. Virol. 2004;85:1055–1063. doi: 10.1099/vir.0.19706-0. [DOI] [PubMed] [Google Scholar]
- Leong W.F., Tan H.C., Ooi E.E., Koh D.R., Chow V.T. Microarray and real-time RT-PCR analyses of differential human gene expression patterns induced by severe acute respiratory syndrome (SARS) coronavirus infection of Vero cells. Microbes Infect. 2005;7:248–259. doi: 10.1016/j.micinf.2004.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis C., Astier-Manifacier S. The 5′ untranslated region of PVY RNA, even located in an internal position, enables initiation of translation. Virus Genes. 1993;7:367–379. doi: 10.1007/BF01703392. [DOI] [PubMed] [Google Scholar]
- Lewis T.L., Matsui S.M. Astrovirus ribosomal frameshifting in an infection-transfection transient expression system. J. Virol. 1996;70:2869–2875. doi: 10.1128/jvi.70.5.2869-2875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.A., Kong X., Haystead T.A., Pause A., Belsham G., Sonenberg N., Lawrence J.C., Jr. PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science. 1994;266:653–656. doi: 10.1126/science.7939721. [DOI] [PubMed] [Google Scholar]
- Liston P., Briedis D.J. Ribosomal frameshifting during translation of measles virus P protein mRNA is capable of directing synthesis of a unique protein. J. Virol. 1995;69:6742–6750. doi: 10.1128/jvi.69.11.6742-6750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R.E. Translational control by viral proteinases. Virus Res. 2006;119:76–88. doi: 10.1016/j.virusres.2005.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G.X., Luytjes W., Enami M., Palese P. The polyadenylation signal of influenza virus RNA involves a stretch of uridines followed by the RNA duplex of the panhandle structure. J. Virol. 1991;65:2861–2867. doi: 10.1128/jvi.65.6.2861-2867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttermann C., Meyers G. A bipartite sequence motif induces translation reinitiation in feline calicivirus RNA. J. Biol. Chem. 2007;282:7056–7065. doi: 10.1074/jbc.M608948200. [DOI] [PubMed] [Google Scholar]
- MacFarlane S.A., Taylor S.C., King D.I., Hughes G., Davies J.W. Pea early browning virus RNA1 encodes four polypeptides including a putative zinc-finger protein. Nucleic Acids Res. 1989;17:2245–2260. doi: 10.1093/nar/17.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra M., Parida S., Egziabher B.G., Diallo A., Barrett T. Sequence analysis of the phosphoprotein gene of peste des petits ruminants (PPR) virus: Editing of the gene transcript. Virus Res. 2003;96:85–98. doi: 10.1016/s0168-1702(03)00176-x. [DOI] [PubMed] [Google Scholar]
- Maia I.G., Séron K., Haenni A.L., Bernardi F. Gene expression from viral RNA genomes. Plant Mol. Biol. 1996;32:367–391. doi: 10.1007/BF00039391. [DOI] [PubMed] [Google Scholar]
- Mäkinen K., Naess V., Tamm T., Truve E., Aaspollu A., Saarma M. The putative replicase of the cocksfoot mottle sobemovirus is translated as a part of the polyprotein by − 1 ribosomal frameshift. Virology. 1995;207:566–571. doi: 10.1006/viro.1995.1118. [DOI] [PubMed] [Google Scholar]
- Makkinje A., Xiong H., Li M., Damuni Z. Phosphorylation of eukaryotic protein synthesis initiation factor 4E by insulin-stimulated protamine kinase. J. Biol. Chem. 1995;270:14824–14828. doi: 10.1074/jbc.270.24.14824. [DOI] [PubMed] [Google Scholar]
- Marcotrigiano J., Gingras A.C., Sonenberg N., Burley S.K. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol. Cell. 1999;3:707–716. doi: 10.1016/s1097-2765(01)80003-4. [DOI] [PubMed] [Google Scholar]
- Martínez-Salas E., Fernández-Miragall O. Picornavirus IRES: Structure function relationship. Curr. Pharm. Des. 2004;10:3757–3767. doi: 10.2174/1381612043382657. [DOI] [PubMed] [Google Scholar]
- Martínez-Salas E., Ramos R., Lafuente E., López de Quinto S. Functional interactions in internal translation initiation directed by viral and cellular IRES elements. J. Gen. Virol. 2001;82:973–984. doi: 10.1099/0022-1317-82-5-973. [DOI] [PubMed] [Google Scholar]
- Matsuda D., Dreher T.W. Cap- and initiator tRNA-dependent initiation of TYMV polyprotein synthesis by ribosomes: Evaluation of the Trojan horse model for TYMV RNA translation. RNA. 2007;13:129–137. doi: 10.1261/rna.244407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder B., Seshadri V., Fox P.L. Translational control by the 3′-UTR: The ends specify the means. Trends Biochem. Sci. 2003;28:91–98. doi: 10.1016/S0968-0004(03)00002-1. [DOI] [PubMed] [Google Scholar]
- Merrick W.C., Anderson W.F. Purification and characterization of homogeneous protein synthesis initiation factor M1 from rabbit reticulocytes. J. Biol. Chem. 1975;250:1197–1206. [PubMed] [Google Scholar]
- Meulewaeter F., Danthinne X., Van Montagu M., Cornelissen M. 5′- and 3′-sequences of satellite tobacco necrosis virus RNA promoting translation in tobacco. Plant J. 1998;14:169–176. doi: 10.1046/j.1365-313x.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- Meulewaeter F., Seurinck J., Van Emmelo J. Genome structure of tobacco necrosis virus strain A. Virology. 1990;177:699–709. doi: 10.1016/0042-6822(90)90536-z. [DOI] [PubMed] [Google Scholar]
- Meulewaeter F., van Lipzig R., Gultyaev A.P., Pleij C.W., Van Damme D., Cornelissen M., van Eldik G. Conservation of RNA structures enables TNV and BYDV 5′ and 3′ elements to cooperate synergistically in cap-independent translation. Nucleic Acids Res. 2004;32:1721–1730. doi: 10.1093/nar/gkh338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers G. Translation of the minor capsid protein of a calicivirus is initiated by a novel termination-dependent reinitiation mechanism. J. Biol. Chem. 2003;278:34051–34060. doi: 10.1074/jbc.M304874200. [DOI] [PubMed] [Google Scholar]
- Meyers G. Characterization of the sequence element directing translation reinitiation in RNA of the calicivirus rabbit hemorrhagic disease virus. J. Virol. 2007;81:9623–9632. doi: 10.1128/JVI.00771-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel Y.M., Poncet D., Piron M., Kean K.M., Borman A.M. Cap-poly(A) synergy in mammalian cell-free extracts. Investigation of the requirements for poly(A)-mediated stimulation of translation initiation. J. Biol. Chem. 2000;275:32268–32276. doi: 10.1074/jbc.M004304200. [DOI] [PubMed] [Google Scholar]
- Miller W.A., Koev G. Synthesis of subgenomic RNAs by positive-strand RNA viruses. Virology. 2000;273:1–8. doi: 10.1006/viro.2000.0421. [DOI] [PubMed] [Google Scholar]
- Miller W.A., Wang Z., Treder K. The amazing diversity of cap-independent translation elements in the 3′-untranslated regions of plant viral RNAs. Biochem. Soc. Trans. 2007;35:1629–1633. doi: 10.1042/BST0351629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.A., Waterhouse P.M., Gerlach W.L. Sequence and organization of barley yellow dwarf virus genomic RNA. Nucleic Acids Res. 1988;16:6097–6111. doi: 10.1093/nar/16.13.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.A., White K.A. Long-distance RNA-RNA interactions in plant virus gene expression and replication. Annu. Rev. Phytopathol. 2006;44:447–467. doi: 10.1146/annurev.phyto.44.070505.143353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Suehiro N., Tomoo K., Muto S., Takahashi T., Tsukamoto T., Ohmori T., Natsuaki T. Binding analyses for the interaction between plant virus genome-linked protein (VPg) and plant translational initiation factors. Biochimie. 2006;88:329–340. doi: 10.1016/j.biochi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Mohr I. Phosphorylation and dephosphorylation events that regulate viral mRNA translation. Virus Res. 2006;119:89–99. doi: 10.1016/j.virusres.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Mohr I.J., Pe'ery T., Mathews M.B. Protein synthesis and translational control during viral infection. In: Mathews M.B., Sonenberg N., Hershey J.W.B., editors. Translational Control in Biology and Medicine. CSHL Press; Cold Spring Harbor, NY: 2007. pp. 545–599. [Google Scholar]
- Molina S., Sanz M.A., Madan V., Ventoso I., Castello A., Carrasco L. Differential inhibition of cellular and Sindbis virus translation by brefeldin A. Virology. 2007;363:430–436. doi: 10.1016/j.virol.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Montero H., Arias C.F., López S. Rotavirus nonstructural protein NSP3 is not required for viral protein synthesis. J. Virol. 2006;80:9031–9038. doi: 10.1128/JVI.00437-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero H., Rojas M., Arias C.F., López S. Rotavirus infection induces the phosphorylation of eIF2{alpha} but prevents the formation of stress granules. J. Virol. 2008;82:1496–1504. doi: 10.1128/JVI.01779-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody C.A., Scott R.S., Amirghahari N., Nathan C.A., Young L.S., Dawson C.W., Sixbey J.W. Modulation of the cell growth regulator mTOR by Epstein–Barr virus-encoded LMP2A. J. Virol. 2005;79:5499–5506. doi: 10.1128/JVI.79.9.5499-5506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K., Baldwin A., Edwards K.M., Hayakawa H., Nguyen C.L., Owens M., Grace M., Huh K. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y. Paramyxovirus replication and pathogenesis. Reverse genetics transforms understanding. Rev. Med. Virol. 1999;9:83–99. doi: 10.1002/(sici)1099-1654(199904/06)9:2<83::aid-rmv244>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Nam S.H., Copeland T.D., Hatanaka M., Oroszlan S. Characterization of ribosomal frameshifting for expression of pol gene products of human T-cell leukemia virus type I. J. Virol. 1993;67:196–203. doi: 10.1128/jvi.67.1.196-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napthine S., Vidakovic M., Girnary R., Namy O., Brierley I. Prokaryotic-style frameshifting in a plant translation system: Conservation of an unusual single-tRNA slippage event. EMBO J. 2003;22:3941–3950. doi: 10.1093/emboj/cdg365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeleman L., Olsthoorn R.C., Linthorst H.J., Bol J.F. Translation of a nonpolyadenylated viral RNA is enhanced by binding of viral coat protein or polyadenylation of the RNA. Proc. Natl. Acad. Sci. USA. 2001;98:14286–14291. doi: 10.1073/pnas.251542798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesbach-Klosgen U., Guilley H., Jonard G., Richards K. Immunodetection in vivo of beet necrotic yellow vein virus-encoded proteins. Virology. 1990;178:52–61. doi: 10.1016/0042-6822(90)90378-5. [DOI] [PubMed] [Google Scholar]
- Nutter R.C., Scheets K., Panganiban L.C., Lommel S.A. The complete nucleotide sequence of the maize chlorotic mottle virus genome. Nucleic Acids Res. 1989;17:3163–3177. doi: 10.1093/nar/17.8.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh K.J., Kalinina A., Park N.H., Bagchi S. Deregulation of eIF4E: 4E-BP1 in differentiated human papillomavirus-containing cells leads to high levels of expression of the E7 oncoprotein. J. Virol. 2006;80:7079–7088. doi: 10.1128/JVI.02380-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmann T., Prevot D., Decimo D., Roux F., Garin J., Morley S.J., Darlix J.L. In vitro cleavage of eIF4GI but not eIF4GII by HIV-1 protease and its effects on translation in the rabbit reticulocyte lysate system. J. Mol. Biol. 2002;318:9–20. doi: 10.1016/S0022-2836(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Oosterom-Dragon E.A., Ginsberg H.S. Purification and preliminary immunological characterization of the type 5 adenovirus, nonstructural 100, 000-dalton protein. J. Virol. 1980;33:1203–1207. doi: 10.1128/jvi.33.3.1203-1207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova M., Yueh A., Leung J., Goff S.P. Reverse transcriptase of Moloney murine leukemia virus binds to eukaryotic release factor 1 to modulate suppression of translational termination. Cell. 2003;115:319–331. doi: 10.1016/s0092-8674(03)00805-5. [DOI] [PubMed] [Google Scholar]
- Park H.S., Browning K.S., Hohn T., Ryabova L.A. Eucaryotic initiation factor 4B controls eIF3-mediated ribosomal entry of viral reinitiation factor. EMBO J. 2004;23:1381–1391. doi: 10.1038/sj.emboj.7600140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.S., Himmelbach A., Browning K.S., Hohn T., Ryabova L.A. A plant viral “reinitiation” factor interacts with the host translational machinery. Cell. 2001;106:723–733. doi: 10.1016/s0092-8674(01)00487-1. [DOI] [PubMed] [Google Scholar]
- Park Y.W., Katze M.G. Translational control by influenza virus. Identification of cis-acting sequences and trans-acting factors which may regulate selective viral mRNA translation. J. Biol. Chem. 1995;270:28433–28439. doi: 10.1074/jbc.270.47.28433. [DOI] [PubMed] [Google Scholar]
- Parkin N.T., Chamorro M., Varmus H.E. Human immunodeficiency virus type 1 gag-pol frameshifting is dependent on downstream mRNA secondary structure: Demonstration by expression in vivo. J. Virol. 1992;66:5147–5151. doi: 10.1128/jvi.66.8.5147-5151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A., Belsham G.J., Gingras A.C., Donze O., Lin T.A., Lawrence J.C., Jr., Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Pelham H.R. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature. 1978;272:469–471. doi: 10.1038/272469a0. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Perera R., Daijogo S., Walter B.L., Nguyen J.H., Semler B.L. Cellular protein modification by poliovirus: The two faces of poly(rC)-binding protein. J. Virol. 2007;81:8919–8932. doi: 10.1128/JVI.01013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T.V., Kolupaeva V.G., Lomakin I.B., Pilipenko E.V., Shatsky I.N., Agol V.I., Hellen C.U. Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. USA. 2001;98:7029–7036. doi: 10.1073/pnas.111145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T.V., Lomakin I.B., Hellen C.U. Position of the CrPV IRES on the 40S subunit and factor dependence of IRES/80S ribosome assembly. EMBO Rep. 2004;5:906–913. doi: 10.1038/sj.embor.7400240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T.V., Lorsch J.R., Hellen C.U. The mechanism of translation initiation in eukaryotes. In: Mathews M.B., Sonenberg N., Hershey J.W.B., editors. Translational Control in Biology and Medicine. CSHL Press; Cold Spring Harbor, NY: 2007. pp. 87–128. [Google Scholar]
- Petty I.T., Jackson A.O. Two forms of the major barley stripe mosaic virus nonstructural protein are synthesized in vivo from alternative initiation codons. Virology. 1990;177:829–832. doi: 10.1016/0042-6822(90)90559-a. [DOI] [PubMed] [Google Scholar]
- Pfingsten J.S., Costantino D.A., Kieft J.S. Structural basis for ribosome recruitment and manipulation by a viral IRES RNA. Science. 2006;314:1450–1454. doi: 10.1126/science.1133281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko E.V., Pestova T.V., Kolupaeva V.G., Khitrina E.V., Poperechnaya A.N., Agol V.I., Hellen C.U. A cell cycle-dependent protein serves as a template-specific translation initiation factor. Genes Dev. 2000;14:2028–2045. [PMC free article] [PubMed] [Google Scholar]
- Piron M., Vende P., Cohen J., Poncet D. Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J. 1998;17:5811–5821. doi: 10.1093/emboj/17.19.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarev A.V., Chard L.S., Kaku Y., Johns H.L., Shatsky I.N., Belsham G.J. Functional and structural similarities between the internal ribosome entry sites of hepatitis C virus and porcine teschovirus, a picornavirus. J. Virol. 2004;78:4487–4497. doi: 10.1128/JVI.78.9.4487-4497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarev A.V., Hellen C.U., Pestova T.V. Recycling of eukaryotic posttermination ribosomal complexes. Cell. 2007;131:286–299. doi: 10.1016/j.cell.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarev A.V., Shirokikh N.E., Hellen C.U. Translation initiation by factor-independent binding of eukaryotic ribosomes to internal ribosomal entry sites. C. R. Biol. 2005;328:589–605. doi: 10.1016/j.crvi.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Pisareva V.P., Pisarev A.V., Hellen C.U., Rodnina M.V., Pestova T.V. Kinetic analysis of interaction of eukaryotic release factor 3 with guanine nucleotides. J. Biol. Chem. 2006;281:40224–40235. doi: 10.1074/jbc.M607461200. [DOI] [PubMed] [Google Scholar]
- Plant E.P., Dinman J.D. Comparative study of the effects of heptameric slippery site composition on − 1 frameshifting among different eukaryotic systems. RNA. 2006;12:666–673. doi: 10.1261/rna.2225206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooggin M.M., Fütterer J., Skryabin K.G., Hohn T. Ribosome shunt is essential for infectivity of cauliflower mosaic virus. Proc. Natl. Acad. Sci. USA. 2001;98:886–891. doi: 10.1073/pnas.98.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöyry T.A., Kaminski A., Connell E.J., Fraser C.S., Jackson R.J. The mechanism of an exceptional case of reinitiation after translation of a long ORF reveals why such events do not generally occur in mammalian mRNA translation. Genes Dev. 2007;21:3149–3162. doi: 10.1101/gad.439507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöyry T.A., Kaminski A., Jackson R.J. What determines whether mammalian ribosomes resume scanning after translation of a short upstream open reading frame? Genes Dev. 2004;18:62–75. doi: 10.1101/gad.276504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats A.C., De Billy G., Wang P., Darlix J.L. CUG initiation codon used for the synthesis of a cell surface antigen coded by the murine leukemia virus. J. Mol. Biol. 1989;205:363–372. doi: 10.1016/0022-2836(89)90347-1. [DOI] [PubMed] [Google Scholar]
- Prüfer D., Tacke E., Schmitz J., Kull B., Kaufmann A., Rohde W. Ribosomal frameshifting in plants: A novel signal directs the − 1 frameshift in the synthesis of the putative viral replicase of potato leafroll luteovirus. EMBO J. 1992;11:1111–1117. doi: 10.1002/j.1460-2075.1992.tb05151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyronnet S., Imataka H., Gingras A.C., Fukunaga R., Hunter T., Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 1999;18:270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaben M., Groot Koerkamp M.J., Rottier P.J., de Haan C.A. Mouse hepatitis coronavirus replication induces host translational shutoff and mRNA decay, with concomitant formation of stress granules and processing bodies. Cell Microbiol. 2007;9:2218–2229. doi: 10.1111/j.1462-5822.2007.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Raju L., Hacker D., Garcin D., Compans R., Kolakofsky D. Nontemplated bases at the 5′ ends of Tacaribe virus mRNAs. Virology. 1990;174:53–59. doi: 10.1016/0042-6822(90)90053-t. [DOI] [PubMed] [Google Scholar]
- Ramírez B.C., Garcin D., Calvert L.A., Kolakofsky D., Haenni A.L. Capped nonviral sequences at the 5′ end of the mRNAs of Rice hoja blanca virus RNA4. J. Virol. 1995;69:1951–1954. doi: 10.1128/jvi.69.3.1951-1954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall R.E., Goodbourn S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Rao P., Yuan W., Krug R.M. Crucial role of CA cleavage sites in the cap-snatching mechanism for initiating viral mRNA synthesis. EMBO J. 2003;22:1188–1198. doi: 10.1093/emboj/cdg109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen J.P., Karageorgos L.E., Habili N., Waterhouse P.M., Symons R.H. Soybean dwarf luteovirus contains the third variant genome type in the luteovirus group. Virology. 1994;198:671–679. doi: 10.1006/viro.1994.1079. [DOI] [PubMed] [Google Scholar]
- Raught B., Gingras A.-C. Signaling to translation initiation. In: Mathews M.B., Sonenberg N., Hershey J.W.B., editors. Translational Control in Biology and Medicine. CSHL Press; Cold Spring Harbor, NY: 2007. pp. 369–400. [Google Scholar]
- Rice N.R., Stephens R.M., Burny A., Gilden R.V. The gag and pol genes of bovine leukemia virus: Nucleotide sequence and analysis. Virology. 1985;142:357–377. doi: 10.1016/0042-6822(85)90344-7. [DOI] [PubMed] [Google Scholar]
- Rijnbrand R., Bredenbeek P.J., Haasnoot P.C., Kieft J.S., Spaan W.J., Lemon S.M. The influence of downstream protein-coding sequence on internal ribosome entry on hepatitis C virus and other flavivirus RNAs. RNA. 2001;7:585–597. doi: 10.1017/s1355838201000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C.I., Lloyd R.E. Modulation of enteroviral proteinase cleavage of poly(A)-binding protein (PABP) by conformation and PABP-associated factors. Virology. 2008;375:59–72. doi: 10.1016/j.virol.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere C.J., Rochon D.M. Nucleotide sequence and genomic organization of melon necrotic spot virus. J. Gen. Virol. 1990;71:1887–1896. doi: 10.1099/0022-1317-71-9-1887. [DOI] [PubMed] [Google Scholar]
- Robaglia C., Caranta C. Translation initiation factors: A weak link in plant RNA virus infection. Trends Plant Sci. 2006;11:40–45. doi: 10.1016/j.tplants.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Robert F., Kapp L.D., Khan S.N., Acker M.G., Kolitz S., Kazemi S., Kaufman R.J., Merrick W.C., Koromilas A.E., Lorsch J.R., Pelletier J. Initiation of protein synthesis by hepatitis C virus is refractory to reduced eIF2⋅GTP⋅Met-tRNA(i)(Met) ternary complex availability. Mol. Biol. Cell. 2006;17:4632–4644. doi: 10.1091/mbc.E06-06-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon D.M., Tremaine J.H. Complete nucleotide sequence of the cucumber necrosis virus genome. Virology. 1989;169:251–259. doi: 10.1016/0042-6822(89)90150-5. [DOI] [PubMed] [Google Scholar]
- Rodríguez Pulido M., Serrano P., Sáiz M., Martínez-Salas E. Foot-and-mouth disease virus infection induces proteolytic cleavage of PTB, eIF3a, b, and PABP RNA-binding proteins. Virology. 2007;364:466–474. doi: 10.1016/j.virol.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Rohde W., Gramstat A., Schmitz J., Tacke E., Prüfer D. Plant viruses as model systems for the study of non-canonical translation mechanisms in higher plants. J. Gen. Virol. 1994;75:2141–2149. doi: 10.1099/0022-1317-75-9-2141. [DOI] [PubMed] [Google Scholar]
- Ryabov E.V., Generozov E.V., Kendall T.L., Lommel S.A., Zavriev S.K. Nucleotide sequence of carnation ringspot dianthovirus RNA-1. J. Gen. Virol. 1994;75:243–247. doi: 10.1099/0022-1317-75-1-243. [DOI] [PubMed] [Google Scholar]
- Ryabova L.A., Pooggin M.M., Hohn T. Viral strategies of translation initiation: Ribosomal shunt and reinitiation. Prog. Nucleic Acid Res. Mol. Biol. 2002;72:1–39. doi: 10.1016/S0079-6603(02)72066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabova L.A., Pooggin M.M., Hohn T. Translation reinitiation and leaky scanning in plant viruses. Virus Res. 2006;119:52–62. doi: 10.1016/j.virusres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Sadowy E., Milner M., Haenni A.L. Proteins attached to viral genomes are multifunctional. Adv. Virus Res. 2001;57:185–262. doi: 10.1016/s0065-3527(01)57004-9. [DOI] [PubMed] [Google Scholar]
- Sanchez A., Trappier S.G., Mahy B.W., Peters C.J., Nichol S.T. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc. Natl. Acad. Sci. USA. 1996;93:3602–3607. doi: 10.1073/pnas.93.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz M.A., Castello A., Carrasco L. Viral translation is coupled to transcription in Sindbis virus-infected cells. J. Virol. 2007;81:7061–7068. doi: 10.1128/JVI.02529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki J., Nakashima N. Translation initiation at the CUU codon is mediated by the internal ribosome entry site of an insect picorna-like virus in vitro. J. Virol. 1999;73:1219–1226. doi: 10.1128/jvi.73.2.1219-1226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki J., Nakashima N. Methionine-independent initiation of translation in the capsid protein of an insect RNA virus. Proc. Natl. Acad. Sci. USA. 2000;97:1512–1515. doi: 10.1073/pnas.010426997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk H.J., Matzeit V., Schiller B., Schell J., Gronenborn B. Wheat dwarf virus, a geminivirus of graminaceous plants needs splicing for replication. EMBO J. 1989;8:359–364. doi: 10.1002/j.1460-2075.1989.tb03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheets K., Redinbaugh M.G. Infectious cDNA transcripts of Maize necrotic streak virus: Infectivity and translational characteristics. Virology. 2006;350:171–183. doi: 10.1016/j.virol.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Scheper G.C., Proud C.G. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur. J. Biochem. 2002;269:5350–5359. doi: 10.1046/j.1432-1033.2002.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C., Balmori E., Jonard G., Richards K.E., Guilley H. In vitro mutagenesis of biologically active transcripts of beet necrotic yellow vein virus RNA 2: Evidence that a domain of the 75-kDa readthrough protein is important for efficient virus assembly. Proc. Natl. Acad. Sci. USA. 1992;89:5715–5719. doi: 10.1073/pnas.89.13.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Prüfer D., Rohde W., Tacke E. Non-canonical translation mechanisms in plants: Efficient in vitro and in planta initiation at AUU codons of the tobacco mosaic virus enhancer sequence. Nucleic Acids Res. 1996;24:257–263. doi: 10.1093/nar/24.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P.A., Kim R., Lipkin W.I. Evidence for translation of the Borna disease virus G protein by leaky ribosomal scanning and ribosomal reinitiation. J. Virol. 1997;71:5614–5619. doi: 10.1128/jvi.71.7.5614-5619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciabica K.S., Dai Q.J., Sandri-Goldin R.M. ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation. EMBO J. 2003;22:1608–1619. doi: 10.1093/emboj/cdg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R., Miller W.A. The 3′ untranslated region of tobacco necrosis virus RNA contains a barley yellow dwarf virus-like cap-independent translation element. J. Virol. 2004;78:4655–4664. doi: 10.1128/JVI.78.9.4655-4664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih S.R., Nemeroff M.E., Krug R.M. The choice of alternative 5′ splice sites in influenza virus M1 mRNA is regulated by the viral polymerase complex. Proc. Natl. Acad. Sci. USA. 1995;92:6324–6328. doi: 10.1073/pnas.92.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirako Y. Non-AUG translation initiation in a plant RNA virus: A forty-amino-acid extension is added to the N terminus of the soil-borne wheat mosaic virus capsid protein. J. Virol. 1998;72:1677–1682. doi: 10.1128/jvi.72.2.1677-1682.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirako Y., Wilson T.M. Complete nucleotide sequence and organization of the bipartite RNA genome of soil-borne wheat mosaic virus. Virology. 1993;195:16–32. doi: 10.1006/viro.1993.1342. [DOI] [PubMed] [Google Scholar]
- Siddell S., Wege H., Barthel A., ter Meulen V. Intracellular protein synthesis and the in vitro translation of coronavirus JHM mRNA. Adv. Exp. Med. Biol. 1981;142:193–207. doi: 10.1007/978-1-4757-0456-3_17. [DOI] [PubMed] [Google Scholar]
- Silvera D., Gamarnik A.V., Andino R. The N-terminal K homology domain of the poly(rC)-binding protein is a major determinant for binding to the poliovirus 5′-untranslated region and acts as an inhibitor of viral translation. J. Biol. Chem. 1999;274:38163–38170. doi: 10.1074/jbc.274.53.38163. [DOI] [PubMed] [Google Scholar]
- Simon-Buela L., Guo H.S., Garcia J.A. Cap-independent leaky scanning as the mechanism of translation initiation of a plant viral genomic RNA. J. Gen. Virol. 1997;78:2691–2699. doi: 10.1099/0022-1317-78-10-2691. [DOI] [PubMed] [Google Scholar]
- Siridechadilok B., Fraser C.S., Hall R.J., Doudna J.A., Nogales E. Structural roles for human translation factor eIF3 in initiation of protein synthesis. Science. 2005;310:1513–1515. doi: 10.1126/science.1118977. [DOI] [PubMed] [Google Scholar]
- Skotnicki M.L., Mackenzie A.M., Torronen M., Gibbs A.J. The genomic sequence of cardamine chlorotic fleck carmovirus. J. Gen. Virol. 1993;74:1933–1937. doi: 10.1099/0022-1317-74-9-1933. [DOI] [PubMed] [Google Scholar]
- Skulachev M.V., Ivanov P.A., Karpova O.V., Korpela T., Rodionova N.P., Dorokhov Y.L., Atabekov J.G. Internal initiation of translation directed by the 5′-untranslated region of the tobamovirus subgenomic RNA I(2) Virology. 1999;263:139–154. doi: 10.1006/viro.1999.9928. [DOI] [PubMed] [Google Scholar]
- Skuzeski J.M., Nichols L.M., Gesteland R.F., Atkins J.F. The signal for a leaky UAG stop codon in several plant viruses includes the two downstream codons. J. Mol. Biol. 1991;218:365–373. doi: 10.1016/0022-2836(91)90718-l. [DOI] [PubMed] [Google Scholar]
- Slobin L.I., Moller W. Purification and properties of an elongation factor functionally analogous to bacterial elongation factor Ts from embryos of Artemia salina. Eur. J. Biochem. 1978;84:69–77. doi: 10.1111/j.1432-1033.1978.tb12142.x. [DOI] [PubMed] [Google Scholar]
- Sommergruber W., Ahorn H., Klump H., Seipelt J., Zoephel A., Fessl F., Krystek E., Blaas D., Kuechler E., Liebig H.D., Skern T. 2A proteinases of coxsackie- and rhinovirus cleave peptides derived from eIF-4γ via a common recognition motif. Virology. 1994;198:741–745. doi: 10.1006/viro.1994.1089. [DOI] [PubMed] [Google Scholar]
- Sousa C., Schmid E.M., Skern T. Defining residues involved in human rhinovirus 2A proteinase substrate recognition. FEBS Lett. 2006;580:5713–5717. doi: 10.1016/j.febslet.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Spahn C.M., Jan E., Mulder A., Grassucci R.A., Sarnow P., Frank J. Cryo-EM visualization of a viral internal ribosome entry site bound to human ribosomes: The IRES functions as an RNA-based translation factor. Cell. 2004;118:465–475. doi: 10.1016/j.cell.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Spahn C.M., Kieft J.S., Grassucci R.A., Penczek P.A., Zhou K., Doudna J.A., Frank J. Hepatitis C virus IRES RNA-induced changes in the conformation of the 40s ribosomal subunit. Science. 2001;291:1959–1962. doi: 10.1126/science.1058409. [DOI] [PubMed] [Google Scholar]
- Steward M., Vipond I.B., Millar N.S., Emmerson P.T. RNA editing in Newcastle disease virus. J. Gen. Virol. 1993;74:2539–2547. doi: 10.1099/0022-1317-74-12-2539. [DOI] [PubMed] [Google Scholar]
- Stinski M.F. Synthesis of proteins and glycoproteins in cells infected with human cytomegalovirus. J. Virol. 1977;23:751–767. doi: 10.1128/jvi.23.3.751-767.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C.M., Madsen J.M. Role of viral splicing elements and cellular RNA binding proteins in regulation of HIV-1 alternative RNA splicing. Curr. HIV Res. 2006;4:43–55. doi: 10.2174/157016206775197655. [DOI] [PubMed] [Google Scholar]
- Strauss E.G., Strauss J.H. RNA viruses: Genome structure and evolution. Curr. Opin. Genet. Dev. 1991;1:485–493. doi: 10.1016/S0959-437X(05)80196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J.H., Strauss E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R., Belsham G.J. Sequential modification of translation initiation factor eIF4GI by two different foot-and-mouth disease virus proteases within infected baby hamster kidney cells: Identification of the 3Cpro cleavage site. J. Gen. Virol. 2004;85:2953–2962. doi: 10.1099/vir.0.80254-0. [DOI] [PubMed] [Google Scholar]
- Stuart K.D., Weeks R., Guilbride L., Myler P.J. Molecular organization of Leishmania RNA virus 1. Proc. Natl. Acad. Sci. USA. 1992;89:8596–8600. doi: 10.1073/pnas.89.18.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakar A., Ramachandran A., Ghosh S., Hasnain S.E., Kaufman R.J., Ramaiah K.V. Phosphorylation of serine 51 in initiation factor 2 alpha (eIF2 alpha) promotes complex formation between eIF2 alpha(P) and eIF2B and causes inhibition in the guanine nucleotide exchange activity of eIF2B. Biochemistry. 2000;39:12929–12938. doi: 10.1021/bi0008682. [DOI] [PubMed] [Google Scholar]
- Svitkin Y.V., Gradi A., Imataka H., Morino S., Sonenberg N. Eukaryotic initiation factor 4GII (eIF4GII), but not eIF4GI, cleavage correlates with inhibition of host cell protein synthesis after human rhinovirus infection. J. Virol. 1999;73:3467–3472. doi: 10.1128/jvi.73.4.3467-3472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkin Y.V., Herdy B., Costa-Mattioli M., Gingras A.C., Raught B., Sonenberg N. Eukaryotic translation initiation factor 4E availability controls the switch between cap-dependent and internal ribosomal entry site-mediated translation. Mol. Cell. Biol. 2005;25:10556–10565. doi: 10.1128/MCB.25.23.10556-10565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazza M., Lucioli A., Calogero A., Pay A., Tavazza R. Nucleotide sequence, genomic organization and synthesis of infectious transcripts from a full-length clone of artichoke mottle crinkle virus. J. Gen. Virol. 1994;75:1515–1524. doi: 10.1099/0022-1317-75-7-1515. [DOI] [PubMed] [Google Scholar]
- Taylor J.M. Hepatitis delta virus. Virology. 2006;344:71–76. doi: 10.1016/j.virol.2005.09.033. [DOI] [PubMed] [Google Scholar]
- ten Dam E.B., Pleij C.W., Bosch L. RNA pseudoknots: Translational frameshifting and readthrough on viral RNAs. Virus Genes. 1990;4:121–136. doi: 10.1007/BF00678404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenin I.M., Dmitriev S.E., Andreev D.E., Royall E., Belsham G.J., Roberts L.O., Shatsky I.N. A cross-kingdom internal ribosome entry site reveals a simplified mode of internal ribosome entry. Mol. Cell. Biol. 2005;25:7879–7888. doi: 10.1128/MCB.25.17.7879-7888.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenin I.M., Dmitriev S.E., Andreev D.E., Shatsky I.N. Eukaryotic translation initiation machinery can operate in a bacterial-like mode without eIF2. Nat. Struct. Mol. Biol. 2008;15:836–841. doi: 10.1038/nsmb.1445. [DOI] [PubMed] [Google Scholar]
- Thompson S.R., Gulyas K.D., Sarnow P. Internal initiation in Saccharomyces cerevisiae mediated by an initiator tRNA/eIF2-independent internal ribosome entry site element. Proc. Natl. Acad. Sci. USA. 2001;98:12972–12977. doi: 10.1073/pnas.241286698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga K., Kobayashi T., Ikuta K. Molecular and cellular biology of Borna disease virus infection. Microbes Infect. 2002;4:491–500. doi: 10.1016/s1286-4579(02)01564-2. [DOI] [PubMed] [Google Scholar]
- Touriol C., Bornes S., Bonnal S., Audigier S., Prats H., Prats A.C., Vagner S. Generation of protein isoform diversity by alternative initiation of translation at non-AUG codons. Biol. Cell. 2003;95:169–178. doi: 10.1016/s0248-4900(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Franco D., Fujita K., Paul A.V., Wimmer E. Replication of poliovirus requires binding of the poly(rC) binding protein to the cloverleaf as well as to the adjacent C-rich spacer sequence between the cloverleaf and the internal ribosomal entry site. J. Virol. 2007;81:10017–10028. doi: 10.1128/JVI.00516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treder K., Kneller E.L., Allen E.M., Wang Z., Browning K.S., Miller W.A. The 3′ cap-independent translation element of Barley yellow dwarf virus binds eIF4F via the eIF4G subunit to initiate translation. RNA. 2008;14:134–147. doi: 10.1261/rna.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N., Hoshino S., Imataka H., Sonenberg N., Katada T. A novel role of the mammalian GSPT/eRF3 associating with poly(A)-binding protein in Cap/Poly(A)-dependent translation. J. Biol. Chem. 2002;277:50286–50292. doi: 10.1074/jbc.M203029200. [DOI] [PubMed] [Google Scholar]
- Valle R.P., Drugeon G., Devignes-Morch M.D., Legocki A.B., Haenni A.L. Codon context effect in virus translational readthrough. A study in vitro of the determinants of TMV and Mo-MuLV amber suppression. FEBS Lett. 1992;306:133–139. doi: 10.1016/0014-5793(92)80984-o. [DOI] [PubMed] [Google Scholar]
- van der Wilk F., Dullemans A.M., Verbeek M., Van den Heuvel J.F. Nucleotide sequence and genomic organization of Acyrthosiphon pisum virus. Virology. 1997;238:353–362. doi: 10.1006/viro.1997.8835. [DOI] [PubMed] [Google Scholar]
- van Eyll O., Michiels T. Non-AUG-initiated internal translation of the L* protein of Theiler's virus and importance of this protein for viral persistence. J. Virol. 2002;76:10665–10673. doi: 10.1128/JVI.76.21.10665-10673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veidt I., Bouzoubaa S.E., Leiser R.M., Ziegler-Graff V., Guilley H., Richards K., Jonard G. Synthesis of full-length transcripts of beet western yellows virus RNA: Messenger properties and biological activity in protoplasts. Virology. 1992;186:192–200. doi: 10.1016/0042-6822(92)90073-x. [DOI] [PubMed] [Google Scholar]
- Veidt I., Lot H., Leiser M., Scheidecker D., Guilley H., Richards K., Jonard G. Nucleotide sequence of beet western yellows virus RNA. Nucleic Acids Res. 1988;16:9917–9932. doi: 10.1093/nar/16.21.9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vende P., Piron M., Castagne N., Poncet D. Efficient translation of rotavirus mRNA requires simultaneous interaction of NSP3 with the eukaryotic translation initiation factor eIF4G and the mRNA 3′ end. J. Virol. 2000;74:7064–7071. doi: 10.1128/jvi.74.15.7064-7071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventoso I., Blanco R., Perales C., Carrasco L. HIV-1 protease cleaves eukaryotic initiation factor 4G and inhibits cap-dependent translation. Proc. Natl. Acad. Sci. USA. 2001;98:12966–12971. doi: 10.1073/pnas.231343498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventoso I., Sanz M.A., Molina S., Berlanga J.J., Carrasco L., Esteban M. Translational resistance of late alphavirus mRNA to eIF2alpha phosphorylation: A strategy to overcome the antiviral effect of protein kinase PKR. Genes Dev. 2006;20:87–100. doi: 10.1101/gad.357006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verchot J., Angell S.M., Baulcombe D.C. In vivo translation of the triple gene block of potato virus X requires two subgenomic mRNAs. J. Virol. 1998;72:8316–8320. doi: 10.1128/jvi.72.10.8316-8320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg G.A., Slobodskaya O., Spaan W.J. Transcriptional profiling of acute cytopathic murine hepatitis virus infection in fibroblast-like cells. J. Gen. Virol. 2006;87:1961–1975. doi: 10.1099/vir.0.81756-0. [DOI] [PubMed] [Google Scholar]
- Verver J., Le Gall O., van Kammen A., Wellink J. The sequence between nucleotides 161 and 512 of cowpea mosaic virus M RNA is able to support internal initiation of translation in vitro. J. Gen. Virol. 1991;72:2339–2345. doi: 10.1099/0022-1317-72-10-2339. [DOI] [PubMed] [Google Scholar]
- Vialat P., Bouloy M. Germiston virus transcriptase requires active 40S ribosomal subunits and utilizes capped cellular RNAs. J. Virol. 1992;66:685–693. doi: 10.1128/jvi.66.2.685-693.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Curran J., Kolakofsky D. A stuttering model for paramyxovirus P mRNA editing. EMBO J. 1990;9:2017–2022. doi: 10.1002/j.1460-2075.1990.tb08330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchkov V.E., Becker S., Volchkova V.A., Ternovoj V.A., Kotov A.N., Netesov S.V., Klenk H.D. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology. 1995;214:421–430. doi: 10.1006/viro.1995.0052. [DOI] [PubMed] [Google Scholar]
- Walsh D., Mohr I. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 2004;18:660–672. doi: 10.1101/gad.1185304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D., Mohr I. Assembly of an active translation initiation factor complex by a viral protein. Genes Dev. 2006;20:461–472. doi: 10.1101/gad.1375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D., Perez C., Notary J., Mohr I. Regulation of the translation initiation factor eIF4F by multiple mechanisms in human cytomegalovirus-infected cells. J. Virol. 2005;79:8057–8064. doi: 10.1128/JVI.79.13.8057-8064.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter B.L., Parsley T.B., Ehrenfeld E., Semler B.L. Distinct poly(rC) binding protein KH domain determinants for poliovirus translation initiation and viral RNA replication. J. Virol. 2002;76:12008–12022. doi: 10.1128/JVI.76.23.12008-12022.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.Y., Chay C., Gildow F.E., Gray S.M. Readthrough protein associated with virions of barley yellow dwarf luteovirus and its potential role in regulating the efficiency of aphid transmission. Virology. 1995;206:954–962. doi: 10.1006/viro.1995.1018. [DOI] [PubMed] [Google Scholar]
- Wang S., Browning K.S., Miller W.A. A viral sequence in the 3′-untranslated region mimics a 5′ cap in facilitating translation of uncapped mRNA. EMBO J. 1997;16:4107–4116. doi: 10.1093/emboj/16.13.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C., Cattaneo R., Billeter M.A. RNA editing. Sometimes an editor makes sense. Nature. 1990;343:697–699. doi: 10.1038/343697a0. [DOI] [PubMed] [Google Scholar]
- White K.A. The premature termination model: A possible third mechanism for subgenomic mRNA transcription in (+)-strand RNA viruses. Virology. 2002;304:147–154. doi: 10.1006/viro.2002.1732. [DOI] [PubMed] [Google Scholar]
- White K.A., Skuzeski J.M., Li W., Wei N., Morris T.J. Immunodetection, expression strategy and complementation of turnip crinkle virus p28 and p88 replication components. Virology. 1995;211:525–534. doi: 10.1006/viro.1995.1434. [DOI] [PubMed] [Google Scholar]
- Wilkie G.S., Dickson K.S., Gray N.K. Regulation of mRNA translation by 5′- and 3′-UTR-binding factors. Trends Biochem. Sci. 2003;28:182–188. doi: 10.1016/S0968-0004(03)00051-3. [DOI] [PubMed] [Google Scholar]
- Willcocks M.M., Carter M.J., Roberts L.O. Cleavage of eukaryotic initiation factor eIF4G and inhibition of host-cell protein synthesis during feline calicivirus infection. J. Gen. Virol. 2004;85:1125–1130. doi: 10.1099/vir.0.19564-0. [DOI] [PubMed] [Google Scholar]
- Wilson J.E., Pestova T.V., Hellen C.U., Sarnow P. Initiation of protein synthesis from the A site of the ribosome. Cell. 2000;102:511–520. doi: 10.1016/s0092-8674(00)00055-6. [DOI] [PubMed] [Google Scholar]
- Wilson J.E., Powell M.J., Hoover S.E., Sarnow P. Naturally occurring dicistronic cricket paralysis virus RNA is regulated by two internal ribosome entry sites. Mol. Cell. Biol. 2000;20:4990–4999. doi: 10.1128/mcb.20.14.4990-4999.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann S., Chatel H., Fortin M.G., Laliberté J.F. Interaction of the viral protein genome linked of turnip mosaic potyvirus with the translational eukaryotic initiation factor (iso) 4E of Arabidopsis thaliana using the yeast two-hybrid system. Virology. 1997;234:84–92. doi: 10.1006/viro.1997.8634. [DOI] [PubMed] [Google Scholar]
- Xi Q., Cuesta R., Schneider R.J. Tethering of eIF4G to adenoviral mRNAs by viral 100k protein drives ribosome shunting. Genes Dev. 2004;18:1997–2009. doi: 10.1101/gad.1212504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q., Cuesta R., Schneider R.J. Regulation of translation by ribosome shunting through phosphotyrosine-dependent coupling of adenovirus protein 100k to viral mRNAs. J. Virol. 2005;79:5676–5683. doi: 10.1128/JVI.79.9.5676-5683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z., Kim K.H., Kendall T.L., Lommel S.A. Synthesis of the putative red clover necrotic mosaic virus RNA polymerase by ribosomal frameshifting in vitro. Virology. 1993;193:213–221. doi: 10.1006/viro.1993.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamiya A., Shirako Y. Construction of full-length cDNA clones to soil-borne wheat mosaic virus RNA1 and RNA2, from which infectious RNAs are transcribed in vitro: Virion formation and systemic infection without expression of the N-terminal and C-terminal extensions to the capsid protein. Virology. 2000;277:66–75. doi: 10.1006/viro.2000.0587. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Nakashima N., Ikeda Y., Uchiumi T. Binding mode of the first aminoacyl-tRNA in translation initiation mediated by Plautia stali intestine virus internal ribosome entry site. J. Biol. Chem. 2007;282:7770–7776. doi: 10.1074/jbc.M610887200. [DOI] [PubMed] [Google Scholar]
- Yang A.D., Barro M., Gorziglia M.I., Patton J.T. Translation enhancer in the 3′-untranslated region of rotavirus gene 6 mRNA promotes expression of the major capsid protein VP6. Arch. Virol. 2004;149:303–321. doi: 10.1007/s00705-003-0211-9. [DOI] [PubMed] [Google Scholar]
- Yang W., Hinnebusch A.G. Identification of a regulatory subcomplex in the guanine nucleotide exchange factor eIF2B that mediates inhibition by phosphorylated eIF2. Mol. Cell. Biol. 1996;16:6603–6616. doi: 10.1128/mcb.16.11.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeam I., Cavatorta J.R., Ripoll D.R., Kang B.C., Jahn M.M. Functional dissection of naturally occurring amino acid substitutions in eIF4E that confers recessive potyvirus resistance in plants. Plant Cell. 2007;19:2913–2928. doi: 10.1105/tpc.107.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A., Bolinger C., Boris-Lawrie K. Retrovirus translation initiation: Issues and hypotheses derived from study of HIV-1. Curr. HIV Res. 2006;4:131–139. doi: 10.2174/157016206776055039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T.D., Oroszlan S. Murine leukemia virus protease is encoded by the gag-pol gene and is synthesized through suppression of an amber termination codon. Proc. Natl. Acad. Sci. USA. 1985;82:1618–1622. doi: 10.1073/pnas.82.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Alwine J.C. 19S late mRNAs of simian virus 40 have an internal ribosome entry site upstream of the virion structural protein 3 coding sequence. J. Virol. 2006;80:6553–6558. doi: 10.1128/JVI.00517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Kudchodkar S.B., Alwine J.C. Effects of simian virus 40 large and small tumor antigens on mammalian target of rapamycin signaling: Small tumor antigen mediates hypophosphorylation of eIF4E-binding protein 1 late in infection. J. Virol. 2005;79:6882–6889. doi: 10.1128/JVI.79.11.6882-6889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh A., Schneider R.J. Translation by ribosome shunting on adenovirus and hsp70 mRNAs facilitated by complementarity to 18S rRNA. Genes Dev. 2000;14:414–421. [PMC free article] [PubMed] [Google Scholar]
- Zamora M., Marissen W.E., Lloyd R.E. Multiple eIF4GI-specific protease activities present in uninfected and poliovirus-infected cells. J. Virol. 2002;76:165–177. doi: 10.1128/JVI.76.1.165-177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Morace G., Gauss-Muller V., Kusov Y. Poly(A) binding protein, C-terminally truncated by the hepatitis A virus proteinase 3C, inhibits viral translation. Nucleic Acids Res. 2007;35:5975–5984. doi: 10.1093/nar/gkm645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Jackson A.O. Expression of the barley stripe mosaic virus RNA beta “triple gene block”. Virology. 1996;216:367–379. doi: 10.1006/viro.1996.0072. [DOI] [PubMed] [Google Scholar]
- Ziff E.B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980;287:491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]
- Ziff E.B. Splicing in adenovirus and other animal viruses. Int. Rev. Cytol. 1985;93:327–358. doi: 10.1016/s0074-7696(08)61377-7. [DOI] [PubMed] [Google Scholar]
- Zoll W.L., Horton L.E., Komar A.A., Hensold J.O., Merrick W.C. Characterization of mammalian eIF2A and identification of the yeast homolog. J. Biol. Chem. 2002;277:37079–37087. doi: 10.1074/jbc.M207109200. [DOI] [PubMed] [Google Scholar]
- Zvereva S.D., Ivanov P.A., Skulachev M.V., Klyushin A.G., Dorokhov Y.L., Atabekov J.G. Evidence for contribution of an internal ribosome entry site to intercellular transport of a tobamovirus. J. Gen. Virol. 2004;85:1739–1744. doi: 10.1099/vir.0.79792-0. [DOI] [PubMed] [Google Scholar]


