Abstract
Circular RNAs are generated at low levels from many protein-coding genes. Liu et al. now reveal that many of these transcripts bind and inhibit the double-stranded RNA (dsRNA)-dependent kinase PKR. Upon viral infection, circular RNAs are globally degraded to release PKR, which becomes activated to aid in the immune response.
Circular RNAs are generated at low levels from many protein-coding genes. Liu et al. now reveal that many of these transcripts bind and inhibit the double-stranded RNA (dsRNA)-dependent kinase PKR. Upon viral infection, circular RNAs are globally degraded to release PKR, which becomes activated to aid in the immune response.
Main Text
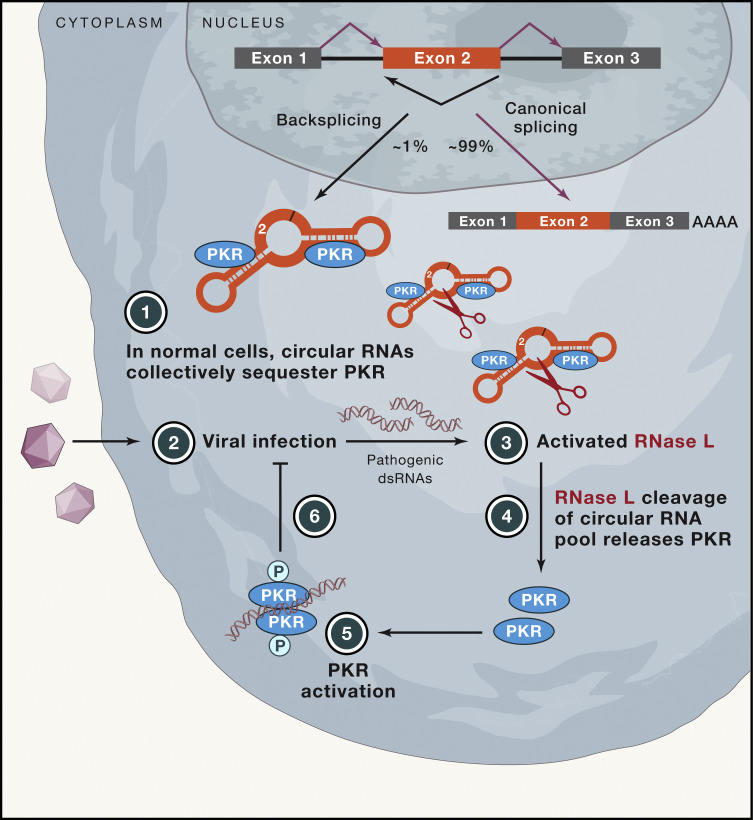
Most eukaryotic genes are interrupted by intronic sequences that must be removed from pre-messenger RNAs by the splicing machinery. These introns are typically spliced out in a sequential order, resulting in the production of a linear mRNA. However, the splicing machinery can also “backsplice” and join a splice donor to an upstream splice acceptor (e.g., join the end of exon 2 to the beginning of exon 2), thereby generating a circular RNA with covalently linked ends (Figure 1 ). Thousands of genes can generate circular RNAs that accumulate in the cytoplasm, but most rarely do so because backsplicing is far less efficient (∼1%) than canonical splicing (reviewed in Wilusz, 2018). Nevertheless, some circular RNAs accumulate to high levels and sequester microRNAs or RNA binding proteins or, alternatively, serve as templates for translation. Most other individual circular RNAs are expressed at exceedingly low levels, so it has remained unclear what biological function (if any) they exert. In this issue of Cell, Liu et al. (2019) reveal that circular RNAs can collectively bind and suppress activation of the kinase PKR, thereby controlling innate immune responses.
Figure 1.
Circular RNAs Can Collectively Modulate Innate Immune Responses
A pre-mRNA can be spliced to generate a linear mRNA or a circular RNA. In normal uninfected cells, many circular RNAs act as a group to bind and inhibit activity of the PKR kinase (1). Upon viral infection (2), pathogenic double-stranded RNAs (dsRNAs) can be produced that lead to activation of RNase L (3), which endonucleolytically cleaves circular RNAs. This releases PKR (4) which can then bind the pathogenic dsRNAs and become activated (5) to inhibit the viral infection (6).
The innate immune system is the first line of defense against invading pathogens and involves a set of receptors that recognize pathogen structures (reviewed in Mogensen, 2009). Among these receptors, PKR recognizes long (>33 bp) dsRNAs in the cytoplasm and then inhibits protein synthesis. PKR thus needs to be readily activatable yet maintained in an inactive state in uninfected cells to prevent inappropriate reactions and autoimmunity. Previous work has shown that PKR activation can be blocked upon binding the adenovirus small noncoding VAI RNA (Kitajewski et al., 1986) or short (16–33 bp) dsRNAs (Zheng and Bevilacqua, 2004), and Liu et al. (2019) now find that many endogenous circular RNAs are able to bind PKR. Interestingly, when the binding profiles of linear and circular RNAs of the same sequence were compared, circular RNAs bound much more strongly to PKR. This suggested that circular RNAs have distinct structures from linear RNAs. Indeed, structural mapping revealed that most circular RNAs in cells form stable secondary structures with short (16–26 bp) imperfect duplexes, while linear RNAs folded into more dynamic, unstable structures (Liu et al., 2019).
The short dsRNA regions within circular RNAs enable binding to PKR, but for what purpose? Liu et al. (2019) found that high levels of individual circular RNAs are sufficient to suppress PKR activity in vitro, but most circular RNAs are expressed at only a handful of copies per cell. It is thus highly unlikely that any individual circular RNA can function as an efficient PKR inhibitor in vivo. Nevertheless, if one considers all circular RNAs as a group, there are ∼9,000–10,000 copies of circular RNAs in each HeLa cell, and most form 1–4 dsRNA regions. This translates to no less than 10,000 dsRNA regions present within circular RNAs that could potentially bind and inhibit PKR. To test this stoichiometry-based model, Liu et al. (2019) used plasmids to express individual circular RNAs to very high levels (5,000–6,000 copies per cell), thereby increasing the overall circular RNA pool. They then examined PKR activation kinetics after these HeLa cells were stimulated with the dsRNA mimic poly(I:C) or infected with an RNA virus, encephalomyocarditis virus (EMCV). Compared to wild-type cells, PKR activity was greatly reduced by circular RNA overexpression. In contrast, overexpression of a linear RNA of the same sequence had no effect on PKR, nor did overexpression of a circular RNA that lacked dsRNA regions. Collectively, these results indicate that endogenous circular RNAs can bind PKR to shield its dsRNA-mediated activation even in the presence of pathogenic dsRNAs.
If circular RNAs naturally bind and inhibit PKR, the question then becomes how PKR is activated when needed. Circular RNAs have been considered to be highly stable transcripts as their covalently closed structures make them resistant to exonucleases. Remarkably, Liu et al. (2019) show that the vast majority (80%–90%) of circular RNAs are degraded within 1-2 hr of cells being stimulated with poly(I:C) or infected with EMCV. This is due to activation of oligoadenylate synthetase (OAS) and the cytoplasmic endonuclease RNase L, which catalyzes cleavage of viral and cellular RNAs after UN dinucleotides (where N = A, C, G, or U) (Wreschner et al., 1981). While 10%–30% of linear mRNAs were degraded after poly(I:C) stimulation or EMCV infection, the depletion of mature circular RNAs was much more drastic (Liu et al., 2019). This is largely because circular RNAs are rarely generated, and the amount of nascent circular RNAs being produced cannot compensate for the rapid degradation. The authors thus propose that this rapid fall in mature circular RNA levels enables PKR to be released and then activated upon recognizing pathogenic dsRNAs. When circular RNA levels remained high (e.g., due to the circular RNA overexpression plasmids), Liu et al. (2019) found that PKR failed to be efficiently activated. This suggests that RNase L may act upstream of PKR, but there are previous studies that have found no requirement for RNase L in PKR activation, e.g., during infection with murine coronavirus (Kindler et al., 2017). In fact, extended PKR activation was observed in RNase L knockout mouse embryonic fibroblasts after stimulation with poly(I:C) or EMCV infection (Khabar et al., 2003). The underlying reason(s) for these conflicting results are currently unclear.
Liu et al. (2019) further found a global reduction in circular RNAs in peripheral blood mononuclear cells (PBMCs) from patients with the autoimmune disease systemic lupus erythematosus (SLE) compared to normal controls. This was coupled to increased RNase L activity (perhaps due to more dsRNA being present in patient-derived cells) and enhanced PKR activation. Nevertheless, overexpression of circular RNAs was able to reverse these phenotypes and cause reduced expression of IFNβ and type I IFN-induced genes. This suggests the exciting potential of modulating circular RNA levels as a therapeutic strategy for SLE. For example, exogenously produced circular RNAs could be introduced, although the dosages would need to be well controlled, especially since overexpression of circular and lariat RNAs can facilitate some viral infections (Zhang et al., 2018, Liu et al., 2019). In addition, there are conflicting reports on whether such exogenously produced circular RNAs themselves trigger immune responses (Wesselhoeft et al., 2019).
Considering that little long dsRNA is thought to be present in the cytoplasm of uninfected normal cells, it will be very important in the future to clarify why PKR would need to be subjected to such active suppression by circular RNAs. Interestingly, circular RNA levels were not modulated by many other immune stimulatory treatments examined by Liu et al. (2019), including lipopolysaccharide or interferon-β. This indicates context specificity and further work is now needed to determine if circular RNAs play any role in the activation of other innate immune receptors, especially in well-characterized disease and animal models.
Acknowledgments
I thank Susan R. Weiss and Robert H. Silverman for helpful convesations. Work in J.E.W.’s laboratory is supported by NIH grants R35-GM119735 and R01-NS099371 and by the Rita Allen Foundation.
Declaration of Interests
J.E.W. is a consultant for VL50, Inc.
References
- Khabar K.S., Siddiqui Y.M., al-Zoghaibi F., al-Haj L., Dhalla M., Zhou A., Dong B., Whitmore M., Paranjape J., Al-Ahdal M.N. RNase L mediates transient control of the interferon response through modulation of the double-stranded RNA-dependent protein kinase PKR. J. Biol. Chem. 2003;278:20124–20132. doi: 10.1074/jbc.M208766200. [DOI] [PubMed] [Google Scholar]
- Kindler E., Gil-Cruz C., Spanier J., Li Y., Wilhelm J., Rabouw H.H., Züst R., Hwang M., V’kovski P., Stalder H. Early endonuclease-mediated evasion of RNA sensing ensures efficient coronavirus replication. PLoS Pathog. 2017;13:e1006195. doi: 10.1371/journal.ppat.1006195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajewski J., Schneider R.J., Safer B., Munemitsu S.M., Samuel C.E., Thimmappaya B., Shenk T. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986;45:195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Liu C.-X., Li X., Nan F., Jiang S., Gao X., Guo S.-K., Xue W., Cui Y., Dong K., Ding H. Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell. 2019;177:865–880. doi: 10.1016/j.cell.2019.03.046. this issue. [DOI] [PubMed] [Google Scholar]
- Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselhoeft R.A., Kowalski P.S., Parker-Hale F.C., Huang Y., Bisaria N., Anderson D.G. RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Mol. Cell. 2019 doi: 10.1016/j.molcel.2019.02.015. S1097-2765(19)30105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J.E. A 360° view of circular RNAs: From biogenesis to functions. Wiley Interdiscip. Rev. RNA. 2018;9:e1478. doi: 10.1002/wrna.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreschner D.H., McCauley J.W., Skehel J.J., Kerr I.M. Interferon action--sequence specificity of the ppp(A2'p)nA-dependent ribonuclease. Nature. 1981;289:414–417. doi: 10.1038/289414a0. [DOI] [PubMed] [Google Scholar]
- Zhang S.Y., Clark N.E., Freije C.A., Pauwels E., Taggart A.J., Okada S., Mandel H., Garcia P., Ciancanelli M.J., Biran A. Inborn Errors of RNA Lariat Metabolism in Humans with Brainstem Viral Infection. Cell. 2018;172:952–965. doi: 10.1016/j.cell.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Bevilacqua P.C. Activation of the protein kinase PKR by short double-stranded RNAs with single-stranded tails. RNA. 2004;10:1934–1945. doi: 10.1261/rna.7150804. [DOI] [PMC free article] [PubMed] [Google Scholar]