Abstract
Background
The occurrence of respiratory tract viral infections in patients with primary hypogammaglobulinemia has not been studied.
Objective
We conducted a prospective 12-month follow-up study of respiratory tract infections in 12 adult patients with primary hypogammaglobulinemia.
Methods
Nasal swab samples and induced sputum samples were taken at the onset of acute respiratory tract infection and every 3 months thereafter. Samples were tested for bacteria and viruses. PCR tests were performed for 15 respiratory tract viruses. In case the results for rhinovirus were positive, follow-up nasal swab samples were taken every 2 weeks until rhinoviral PCR results became negative. Patients completed symptom diaries, which were collected every month. The spouses of the patients served as healthy control subjects.
Results
During the 12-month period, the 12 patients had 65 episodes of acute respiratory tract infections, and the 11 spouses had 12 acute episodes (P < .001). Respiratory tract viruses were found in sputum in 54% of the infections. Rhinovirus was the most common virus. In more than half of our patients, rhinoviral PCR results stayed positive for more than 2 months. The most long-acting persistence with the same rhinovirus was 4 months.
Conclusions
Despite adequate immunoglobulin replacement therapy, patients with primary hypogammaglobulinemia have increased susceptibility to respiratory tract viral infections. Rhinoviral infections are frequent and prolonged.
Key words: Primary immunodeficiency, hypogammaglobulinemia, X-linked agammaglobulinemia, common variable immunodeficiency, rhinovirus, respiratory virus, respiratory tract infection, immunoglobulin replacement therapy
Abbreviations used: Btk, Bruton tyrosine kinase; CVID, Common variable immunodeficiency; RSV, Respiratory syncytial virus; TLR, Toll-like receptor; XLA, X-linked agammaglobulinemia
Patients with primary hypogammaglobulinemia typically experience recurrent bacterial infections.1 It is generally considered that patients with hypogammaglobulinemia are not more prone to viral infections than immunocompetent subjects. Enteroviruses, however, are an exception. Systemic enterovirus infections (echoviruses, coxsackieviruses, and vaccine-related polioviruses) have caused severe morbidity and high mortality rates in patients with hypogammaglobulinemia.2, 3 Fatal enterovirus-induced meningoenchephalitis has been described in case reports in patients with primary X-linked agammaglobulinemia (XLA). However, chronic enterovirus-induced meningitis has become less common in recent years since the use of higher immunoglobulin doses.4 The occurrence of other picornaviruses, such as rhinoviral infections in patients with primary hypogammaglobulinemia, is not known. Thus far, respiratory tract viral infections have not been studied in patients with primary hypogammaglobulinemia. Using modern diagnostic techniques, we wanted to study the occurrence of respiratory tract infections, especially viral infections, in patients with primary hypogammaglobulinemia who were receiving regular immunoglobulin replacement therapy.
Methods
Patients
Twelve adult patients with primary hypogammaglobulinemia participated in the study (Table I ). Two patients had XLA, and 10 had common variable immunodeficiency (CVID). Their ages ranged from 32 to 79 years (median, 47.5 years). CVID was characterized by decreased serum immunoglobulin levels (>2 SDs less than the age-adjusted mean), defective in vitro antibody formation, and exclusion of other known causes of humoral immune defects. The diagnosis of XLA was based on early-onset, very low serum immunoglobulin concentrations; male sex; and a lack of circulating mature B lymphocytes in the peripheral blood and mutation analysis of the Bruton tyrosine kinase (Btk) gene.5 All patients were receiving regular immunoglobulin replacement therapy (dose, 400-600 mg/kg/mo). The trough serum IgG concentration was greater than 5 g/L in all patients; 9 patients had serum IgG levels of 6.0 g/L or greater. No patients were receiving prophylactic antibiotic treatment at the beginning of the study. All patients were nonsmokers.
Table I.
Patient demographics
| Patient no. | Sex | Age (y) | Diagnosis | Age at diagnosis (y) | Serum IgG/IgA/IgM level at diagnosis (g/L) | Through serum IgG level (g/L) | Replacement therapy | Pulmonary complications |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 79 | CVID | 59 | 2.5/<0.1/0.1 | 5.5 | IVIG | Bronchiectasis, fibrosis |
| 2 | M | 32 | XLA | 1 | 1/<0.1/0.1 | 9 | IVIG | Bronchiectasis |
| 3 | M | 36 | CVID | 32 | 3.2/>0.2/<0.2 | 5.5 | IVIG | Bronchiectasis, noduli |
| 4 | F | 45 | CVID | 33 | 2.5/<0.2/0.3 | 5.4 | IVIG | Bronchiectasis |
| 5 | M | 54 | CVID | 29 | 1.5/<0.2/<0.2 | 6.5 | IVIG | Fibrosis, mild bronchiectasis |
| 6 | M | 39 | CVID | 24 | 4.0/<0.2/0.4 | 6.0 | IVIG | Bronchiectasis, noduli |
| 7 | M | 42 | XLA | 1 | 1/<0.2/0.2 | 7.6 | IVIG/SCIG | Bronchiectasis |
| 8 | M | 57 | CVID | 22 | 2.5/<0.2/<0.2 | 7.0 | IVIG | Bronchiectasis |
| 9 | F | 49 | CVID | 33 | 2.3/<0.2/<0.2 | 6.0 | IVIG | Bronchiectasis |
| 10 | M | 51 | CVID | 48 | <0.5/<0.2/<0.2 | 6.2 | IVIG | Bronchiectasis, noduli |
| 11 | M | 46 | CVID | 18 | 2.2/<0.2/<0.2 | 6.5 | IVIG | Bronchiectasis |
| 12 | M | 68 | CVID | 24 | 1.7/<0.2/<0.2 | 6.0 | IVIG | Bronchiectasis |
F, Female; IVIG, intravenous immunoglobulin; M, male; SCIG, subcutaneous immunoglobulin.
Eleven spouses of the 12 patients served as healthy control subjects. In 3 families there were school-aged children. One of the patients was a widow and lived alone.
Study design
This was a prospective, 12-month longitudinal study from February 2006 through March 2007 designed to capture all acute symptomatic episodes of respiratory tract infections. The study was carried out at Turku University Hospital, Finland. Informed consent was obtained from the patients and healthy control subjects, and the study was approved by the Joint Commission on Ethics of the Turku University Hospital and the University of Turku.
The primary outcome variable was an acute episode of respiratory tract infection (the common cold) defined on the basis of new symptoms (ie, excessive sneezing, sore throat, nasal discharge, nasal obstruction, and cough). The secondary outcome variable was viral cause of this acute respiratory episode. We searched for 15 viruses: adenovirus; coronaviruses OC43, 229E, HKU1, and NL63; enteroviruses; human bocavirus; human metapneumovirus; influenza A and B viruses, parainfluenza type 1, 2, and 3 viruses; rhinovirus and respiratory syncytial virus (RSV).
At the first visit and follow-up visits every 3 months, patients were examined (L.K.). Four nasal swabs were taken, and the patients provided induced sputum samples.
At the onset of respiratory tract infection, the patients were asked to contact the investigator (L.K.) if they presented with new symptoms of respiratory tract infection. The patient was examined (L.K.) within 2 days. Three nasal swabs were taken, and the patients provided an induced sputum sample.
Enteroviral and rhinoviral PCR, as well as viral and bacterial cultivation, were performed from the swab and sputum specimens. If rhinoviral PCR results were positive in the nasal swab samples, sputum samples, or both, new nasal swabs were taken every 2 weeks until rhinoviral RNA results became negative. The research nurse taught the patient to take nasal swabs at home, and swabs were sent in vials by post to the laboratory.6
If the bacterial culture of the sputum sample was positive, the patient received antibiotic treatment. A new induced sputum sample was taken after the antibiotic treatment. Antibiotic treatment was also given in some patients with bacterial culture–negative acute infections.
Samples from healthy control subjects
If the spouse of the patient had acute symptoms of respiratory tract infection, she or he took nasal swabs at home according to the instructions of the research nurse and sent the vials by post. In cases in which the rhinoviral RNA results were positive, new nasal swabs were taken every 2 weeks until they became negative.
Symptom diary
Patients and spouses completed a common symptom diary, recording fever, nasal discharge, cough, sputum amount and color, absenteeism from work, and antibiotic treatments. The diaries were returned monthly and checked by the research nurse. If questions arose, the research nurse called the patient or the spouse.
Microbiological samples
Nasal swab
Nasal swabs were obtained from the nostrils at a depth of 2 to 3 cm by using a sterile cotton swab.6 The nasal swab for respiratory tract viral PCR was inserted into a dry vial. The nasal swab for viral culture was inserted into a vial containing 2.5 mL of viral transport medium (5% tryptose phosphate broth, 0.5% BSA, and antibiotics in PBS). The nasal swab for the bacterial culture was inserted into a culture medium vial.
Induced sputum sample
Sputum production was induced by means of inhalation of 5% hypertonic saline solution according to the method of Zar et al.7 The quality of the sputum sample was assessed by means of light microscopy. The samples were classified in 6 categories, of which classes 4 to 6 (ie, those with >25 leukocytes and <10 squamous cells per field) were further processed. The samples were cultured on standard media, and potential respiratory tract pathogens were identified and tested for antimicrobial susceptibility.
Viral analysis
Nasal swab samples were analyzed immediately for viral cultivation and PCR analysis for enteroviruses and rhinovirus.6 Sputum samples for viral cultivation were stored at −70°C until analysis. Viral cultivation from nasal swabs and induced sputum specimens was performed according to standard roller tube methods in LLC-Mk2, HeLa Ohio, and A549 cells and in human foreskin fibroblasts.
Nasal swabs were eluted by vortexing with 1 mL of PBS. Nucleic acids were extracted from 220-μL aliquots of the specimens with the QIAamp MinElute Virus Spin kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions, and from 550-μL induced sputum specimens with the NucliSens EasyMag automated extractor (BioMerieux, Boxtel, The Netherlands) with an elution volume of up to 55 μL.
Extracts from nasal swabs and sputum specimens were analyzed for enteroviruses, rhinovirus, and RSV according to standard PCR protocols used in the Virus Diagnostic Laboratory of the Department of Virology, Turku University. Extracts from the sputum specimens were also analyzed for adenovirus and human bocavirus by using PCR assays in accordance with standard protocols of the Department of Virology, Turku University, and for human metapneumovirus by using the Amplitect Quantification Kit for Metapneumovirus Genomes (AME Biosciences, Toroed, Norway). A multiplex PCR assay (The Seeplex Respiratory Virus Detection assay; Seegene, Inc, Seoul, Korea) was used for detection of 13 respiratory tract viruses (adenovirus, influenza A and B viruses, coronaviruses OC43/HKU1 and 229E/NL63, rhinovirus, human metapneumovirus, RSV A and B, and parainfluenza type 1, 2, and 3 viruses).
Rhinovirus-positive cDNAs were amplified for sequence analysis, as described earlier.6 The primers from the 5′ noncoding region generate 397-bp-long amplicons, which were sequenced in the DNA Sequence Service Laboratory of the Turku Center for Biotechnology. The partial sequences were aligned with ClustalW software. Viruses with greater than 98% sequence homology were considered the same type.6
The phylogenetic tree was computed by using the Jukes-Cantor algorithm and the neighbor-joining method. Phylogenetic analyses were conducted by using the MEGA4 program and the bootstrap consensus tree inferred from 1000 replicates.
Statistical methods
Statistical analysis were performed with SPSS for Windows software (version 16.0; SPSS, Inc, Chicago, Ill). A paired-samples t test was conducted to compare respiratory tract infection episodes in patients and spouses and to compare respiratory tract viral infections and rhinoviral infections, respectively.
Results
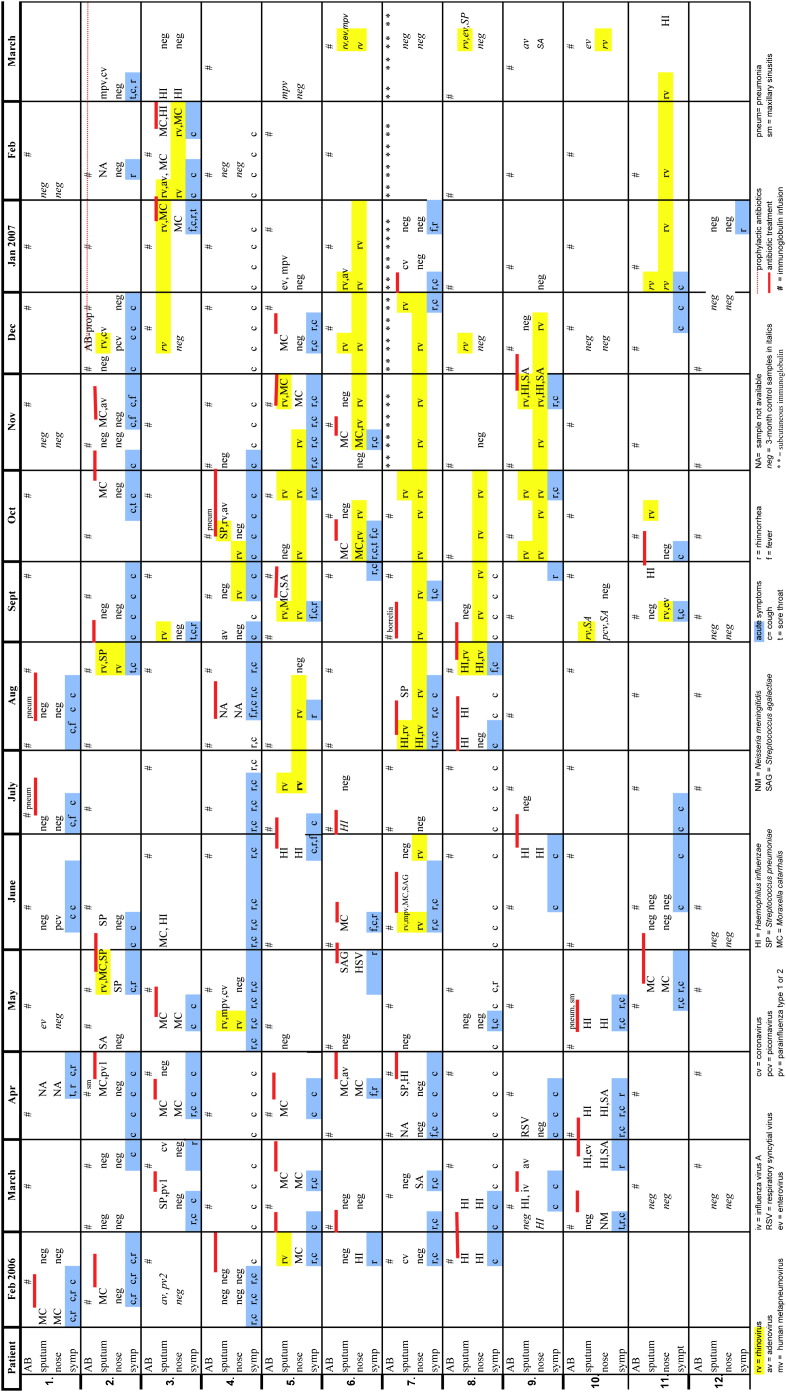
During the 12-month follow-up, 12 patients had 65 episodes of acute respiratory tract infections. In sputum samples at acute infection, a possible causative agent was found in 57 (88%) of 65 episodes. Bacteria were found in sputum samples in 39 (60%) of 65 episodes and in 17 episodes were found together with a virus/viruses. Respiratory tract viruses were found in sputum samples in 35 (54%) of 65 episodes: 1 virus in 12 episodes, 2 to 3 viruses in 6 episodes, and 1 to 2 viruses together with bacteria in 17 episodes. In nasal swab samples at acute infection, bacteria were found in 24 (37%) of 65 episodes, and viruses were found in 19 (29%) of 65 episodes. Both viral cultivation and PCR tests were done for 105 mucus samples. Viral cultivation results were positive in 13% of the samples, and PCR results were positive in 30% of the samples. Bacterial and viral findings are shown in Table II , and symptoms and infections of the patients are shown in Fig 1 .
Table II.
Bacteria and viruses in sputum and nasal swab samples at the time of acute infection and at control or follow-up visits
| Sputum at acute infection | Nasal swab at acute infection | Sputum at control | Nasal swab at control | |
|---|---|---|---|---|
| Single bacteria | ||||
| Moraxella catarrhalis | 10 | 8 | 0 | 0 |
| Haemophilus influenzae | 7 | 3 | 1 | 0 |
| Staphylococcus aureus | 2 | 6 | 0 | 0 |
| Streptococcus agalactiae | 1 | 0 | 0 | 0 |
| Streptococcus pneumoniae | 1 | 0 | 0 | 0 |
| Two bacteria | ||||
| M catarrhalis + H influenzae | 1 | 0 | 1 | 0 |
| H influenzae + S aureus | 0 | 2 | 0 | 0 |
| Bacterial and viral coinfection | ||||
| S pneumoniae + rhinovirus | 2 | 1 | 0 | 0 |
| S pneumoniae + parainfluenza type 1 virus | 1 | 0 | 0 | 0 |
| S pneumoniae + H influenzae + enterovirus | 1 | 0 | 0 | 0 |
| M catarrhalis + rhinovirus | 3 | 1 | 0 | 0 |
| M catarrhalis + adenovirus | 1 | 0 | 0 | 0 |
| M catarrhalis + parainfluenza type 1 virus | 1 | 0 | 0 | 0 |
| M catarrhalis + S pneumoniae + rhinovirus | 1 | 0 | 0 | 0 |
| M catarrhalis + rhinovirus + adenovirus | 1 | 0 | 0 | 0 |
| M catarrhalis + S agalactiae + rhinovirus + metapneumovirus | 1 | 0 | 0 | 0 |
| H influenzae + rhinovirus | 3 | 3 | 0 | 0 |
| H influenzae + enterovirus | 1 | 0 | 0 | 0 |
| H influenzae + influenza | 1 | 0 | 0 | 0 |
| Single virus | ||||
| Rhinovirus | 6 | 14 | 7 | 24 |
| Coronavirus | 4 | NS | 1 | NS |
| Adenovirus | 1 | 0 | 2 | 0 |
| RSV | 1 | 0 | 0 | 0 |
| Enterovirus | 0 | 0 | 2 | 0 |
| Two or 3 viruses | ||||
| Rhinovirus + adenovirus | 2 | 0 | 1 | 0 |
| Rhinovirus + enterovirus + metapneumovirus | 1 | 0 | 1 | 0 |
| Rhinovirus + metapneumovirus + coronavirus | 1 | 0 | 0 | 0 |
| Enterovirus + metapneumovirus | 1 | 0 | 1 | 0 |
| Metapneumovirus + coronavirus | 1 | 0 | 0 | 0 |
| Adenovirus + parainfluenza type 2 virus | 0 | 0 | 1 | 0 |
NS, Not studied.
Fig 1.
Symptoms, infections, microbiological findings, antibiotic treatments, and immunoglobulin infusions over 12 months in 12 patients.
The most common bacteria in induced sputum samples during acute infections were Moraxella catarrhalis (19/65 [29%]) and nontypeable Haemophilus influenzae (14/65 [22%]).
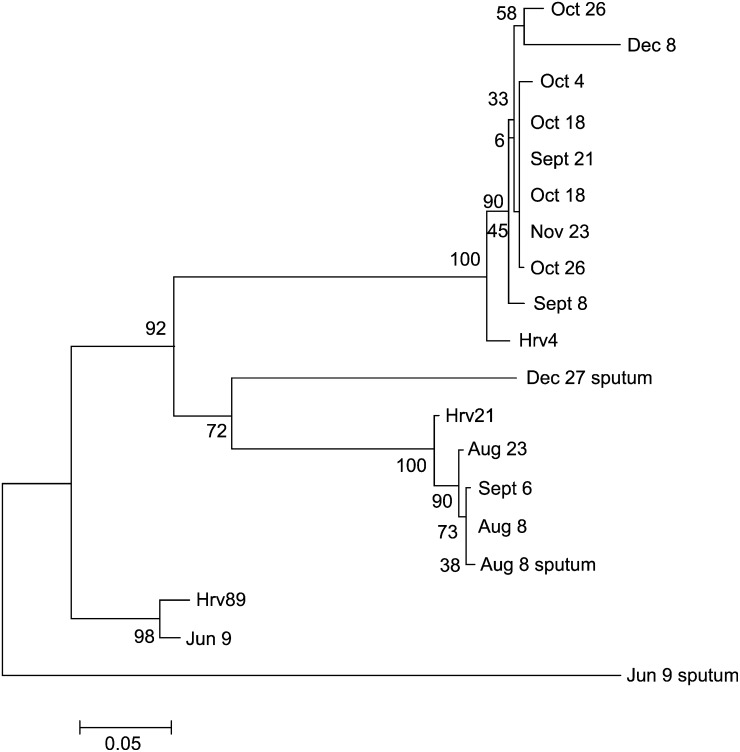
Rhinovirus was the most common respiratory tract virus. It was identified in 10 of 12 patients on several occasions during the study period. At the onset of acute respiratory tract symptoms, it was found in sputum in 21 (32%) of 65 episodes either as a single virus (6/65 [9%]), together with bacteria (11/65 [17%]), or together with other viruses (4/65 [6%]). Rhinovirus was found concomitantly in sputum samples and nasal swabs in 11 acute episodes (Table II). In 7 patients the rhinoviral PCR results were positive for 8 or more weeks in nasal swabs taken every 2 weeks. In a patient with XLA rhinovirus, PCR results stayed positive for 5 months. The same rhinoviral type, with more than 98% partial 5′ nucleotide sequence homology, was found for 4 months (Fig 2 ). In a patient with CVID, the same rhinoviral type persisted for 6 weeks (score of 98% similarity). The majority of rhinoviral infections occurred during the fall months (Fig 1).
Fig 2.
Phylogenetic tree of rhinovirus partial 5′ NCR sequences of nasal and sputum specimens of a patient with XLA for a 5-month period and rhinovirus prototypes HRV4, HRV21, and HRV89 (GenBank accession numbers DQ473490, FJ445121, and FJ445184). Scale bars indicate nucleotide substitutions per position.
Adenovirus was found in the sputum samples of 5 patients 9 times. In 1 patient adenovirus was found 3 times during 8 months; however, it was not found in subsequent samples. Results for human metapneumovirus were positive on 6 occasions. Coronavirus was found on 6 occasions, and on 3 occasions, it was the only pathogen found at the time the patient had symptoms. Results for enterovirus were positive in sputum samples on 6 occasions (4 acute episodes), always together with bacteria, other viruses, or both. Parainfluenza type 1 virus was found to be positive twice, and parainfluenza type 2 virus, influenza A virus, and RSV were found to be positive once. Human bocavirus was not found.
At control or follow-up visits, when patients did not have signs of acute respiratory tract infection, viruses where found in sputum samples altogether 16 times. Rhinovirus was the most common virus (Table II).
The mean number of respiratory tract infections during the 12-month period per patient was 5 (range, 1-8), and the mean number of received antibiotic treatments was 4 (range, 1-8). The most common clinical signs and symptoms of acute respiratory tract infections were cough and rhinitis and increased sputum volume in patients with chronic cough. Fever (>37.0°C) was present in 14 of 65 episodes. There were no differences in the symptoms in patients with viral infection or viral-bacterial coinfection (data not shown). The mean duration of symptoms was 11 days. Two patients required hospitalization because of pneumonia. Results for rhinovirus and adenovirus were positive in sputum before pneumonia in 1 patient. Bacteria were not found in the blood or sputum of the patients with pneumonia.
Six of the 11 spouses had 12 acute respiratory tract infections during the 12-month period. Five had no infections. Rhinovirus was found in only 1 (8%) of 12 episodes, and results were negative in a control sample 2 weeks later. Parainfluenza viruses were detected in 3 episodes. In 1 family both the patient and the spouse had parainfluenza type 1 viral infections at the same time.
There was a significant difference in the number of respiratory tract infection episodes in patients and spouses: patients had 4.4 episodes more than the spouses (95% CI, 2.6-6.1; P < .001). Patients had also significantly more viral infections than the spouses (mean difference, 2.7; 95% CI, 1.3-4.2; P = .002) and more rhinoviral infections (mean difference, 1.8; 95% CI, 0.9-2.8; P = .002), respectively.
Discussion
Our prospective 12-month study has 2 new observations. First, despite adequate immunoglobulin replacement therapy, most patients with primary hypogammaglobulinemia had increased susceptibility to respiratory tract viral infections. Second, many patients specifically had recurrent and persistent rhinoviral infections.
In this study patients with hypogammaglobulinemia had a median of 5 episodes of respiratory tract infection in a year, which was significantly more than seen in their spouses. It is well established that otherwise healthy adults have annually 1 to 2 episodes of respiratory tract infection.8 Half of the 65 respiratory episodes in our patients were associated with respiratory tract viruses, showing the increased susceptibility to respiratory tract viral infections. There are no previous studies focused on respiratory tract viruses in acute or chronic respiratory tract infections in patients with hypogammaglobulinemia. We have earlier searched for respiratory tract viruses in bronchoalveolar lavage fluid and maxillary sinus lavage samples at the time when patients with hypogammaglobulinemia were free from acute infection.9, 10
An interesting observation in this study was that patients with primary hypogammaglobulinemia had recurrent and persistent rhinoviral infections. Rhinovirus was the most common pathogen in respiratory tract infections, and results for it were positive in one third of the infections. Rhinovirus was found both as a sole pathogen and also together with bacteria. Rhinoviruses have been shown to induce proinflammatory response in the lower airways.11, 12, 13 Chronic inflammation might predispose patients to a progression of pulmonary changes, even if the immunoglobulin replacement therapy is adequate.14 Previously, chronic rhinoviral infection with the same virus in the lower respiratory tract has been described in immunocompromised lung transplant recipients.15 In immunocompetent adults rhinoviral clearance is usually rapid, on average 1 to 2 weeks.7, 16 In immunocompetent children rhinoviral shedding has been shown to persist for up to 3 weeks, but prolonged shedding for months has not been documented in immunocompetent subjects.17 Consequently, continuous viral RNA detection for months suggests active replication and persistent infection. In more than half of our patients, rhinoviral PCR results were positive for more than 2 months. Frequent rhinoviral infections raise the question of the possible role of the absence of secretory IgA in the defense against rhinoviruses.
Our patients also had adenoviral, metapneumoviral, and coronaviral infections. It was of interest that 5 patients had a positive adenoviral PCR result in sputum samples, and in 3 patients it was found more than once. Four of the patients with recurrent adenovirus had prominent bronchiectasis. There are several studies showing that persistent or latent adenovirus might contribute to chronic respiratory diseases.18, 19 Furthermore, in 4 episodes adenovirus was found concomitantly with other viruses or bacteria.
In the present study enteroviruses were found on 6 occasions in the respiratory tract, although the initial presumption was that the patients would have recurrent enteroviral infections because it is known that patients with primary immunodeficiency are unusually susceptible to enteroviral infections. Chronic enteroviral encephalitis is frequently a fatal complication in patients with agammaglobulinemia.20, 21 In a review of 90 cases of chronic enteroviral infections in patients with primary immunodeficiency, enteroviruses were usually isolated from the cerebrospinal fluid, and the major signs and symptoms were neurological. Echoviruses were the most common enteroviruses. In only 5 patients were enteroviruses documented in the respiratory tract.3 A more recent report of 201 patients with XLA also showed that enteroviruses were the most common causes in central nervous system infections.22 Several vaccine- and non–vaccine-associated poliomyelitis case reports have been published, and patients with hypogammaglobulinemia have been reported to chronically excrete poliovirus.23, 24, 25
Patients with primary hypogammaglobulinemia appear to have a more severe clinical course of certain viral infections, such as hepatitis C virus, herpes simplex virus, and varicella zoster virus, than immunocompetent subjects.26, 27, 28 Wheat et al29 studied the prevalence of human herpesvirus 8 infection in patients with CVID and found that patients with granulomatous or lymphocytic interstitial lung disease had a markedly higher prevalence of human herpesvirus 8 than patients without granulomatous/lymphocytic interstitial lung disease. These data further suggest that antiviral competence is not intact in a group of patients with primary hypogammaglobulinemia.
Acute respiratory tract infections were common even if patients had a trough serum IgG concentration of more than 6.5 g/L. Repeated antibiotic treatments were considered necessary in many patients. This is in agreement with the observations of a recent follow-up study in Italy that showed that patients with primary hypogammaglobulinemia tend to have recurrent bacterial bronchitis despite replacement therapy.30
Mixed viral-bacterial coinfection was detected in one fourth of the patients at the onset of acute infection. Viral-bacterial infection might induce more severe inflammation in the lower respiratory tract than sole viral or bacterial infection.31 It has recently been shown that rhinovirus induces impairment of the antibacterial host defense and thereby predisposes the patient to coinfections with bacteria and probably with other viruses and furthermore to development of pulmonary changes.32
The mechanisms of increased susceptibility to respiratory tract viral infections in hypogammaglobulinemic patients are unclear. In patients with XLA, Btk is defective. Btk is a cytoplasmic nonreceptor tyrosine kinase and a member of the Tec family. Btk is important in Toll-like receptor (TLR) 8 and TLR9 signalling. Both TLR8 and TLR9 are important in the activation of host defense against viral and bacterial infections. Defective triggering of TLRs leads to impaired production of proinflammatory cytokines, such as TNF-α and IL-6. TLR8 recognizes single-stranded RNA. TLR9 recognizes unmethylated CpG motifs that exist in both viral and bacterial DNA. Patients with XLA have impaired IL-6, which might explain their susceptibility to viral infections.33 Patients with CVID also presented with prolonged persistence of rhinovirus. Impaired IL-6 and IL-10 production, as well as TLR9 activation defects, have been demonstrated in patients with CVID.34 This might partly explain the persistence of rhinovirus.
Our study has some limitations that should be taken into account in interpreting the results. The number of patients was small. The spouses did not visit the study clinic. Nasal swabs were done based on self-assessment, and their quality might not be comparable with those taken by the research nurse. In addition, we used cotton swabs, and flocked swabs might have provided a better yield. Rhinoviral shedding in healthy spouses could not be properly studied because only 1 spouse had rhinoviral infection. Furthermore, association of rhinoviral PCR positivity with clinical illness is not yet fully understood.
In conclusion, our study shows that in addition to bacterial lower respiratory tract infections, patients with primary hypogammaglobulinemia are prone to recurrent respiratory tract viral infections. Especially rhinoviral infections were frequent and prolonged. Recurrent and persistent respiratory tract viral infections, often in association with bacteria, most probably predispose patients to chronic pulmonary changes. Adequate immunoglobulin replacement therapy is not effective in preventing respiratory tract viral infection, and other kinds of treatments are needed. Unfortunately, the availability of antivirals is still very limited. However, our observations suggest that the possibility of respiratory viruses should be investigated by means of PCR more often in patients with hypogammaglobulinemia because viral infection often explains the respiratory signs and symptoms of the patient.
Clinical implications.
Respiratory tract viruses should be investigated by means of PCR more often in patients with primary hypogammaglobulinemia. Bacterial and viral coinfections might predispose patients to chronic pulmonary changes.
Acknowledgments
We thank Aino Ruohola, MD, for helpful discussions and Kaisu Kaistinen, RN, for excellent assistance in data collection.
Footnotes
Supported by grants from the government to Turku University Hospital and the Research Foundation of Orion Corporation.
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
References
- 1.Oksenhendler E., Gérard L., Fieschi C., Malphettes M., Mouillot G., Jaussaud R. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis. 2008;46:1547–1554. doi: 10.1086/587669. [DOI] [PubMed] [Google Scholar]
- 2.MacLennan C., Dunn G., Huissoon A.P., Kumararatne D.S., Martin J., O'Leary P. Failure to clear persistent vaccine-derived neurovirulent poliovirus infection in an immunodeficient man. Lancet. 2004;8:1509–1513. doi: 10.1016/S0140-6736(04)16150-3. [DOI] [PubMed] [Google Scholar]
- 3.Rudge P., Webster A.D., Revesz T., Warner T., Espanol T., Cunningham-Rundles C. Encephalomyelitis in primary hypogammaglobulinemia. Brain. 1996;119:1–15. doi: 10.1093/brain/119.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Halliday E., Winkelstein J., Webster A.D. Enteroviral infections in primary immunodeficiency (PID): a survey of morbidity and mortality. J Infect. 2003;46:1–8. doi: 10.1053/jinf.2002.1066. [DOI] [PubMed] [Google Scholar]
- 5.International Union of Immunological Societies Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. Clin Exp Immunol. 1999;118(suppl 1):1–28. doi: 10.1046/j.1365-2249.1999.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peltola V., Waris M., Österback R., Susi P., Ruuskanen O., Hyypiä T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infection. J Infect Dis. 2008;197:382–389. doi: 10.1086/525542. [DOI] [PubMed] [Google Scholar]
- 7.Zar H.J., Tannenbaum E., Hanslo D., Hussey G. Sputum induction as a diagnostic tool for community-acquired pneumonia in infants and young children from a high HIV prevalence area. Pediatr Pulmonol. 2003;36:58–62. doi: 10.1002/ppul.10302. [DOI] [PubMed] [Google Scholar]
- 8.Monto A.S. Epidemiology of viral respiratory infections. Am J Med. 2002;112(suppl):4S–12S. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 9.Kainulainen L., Suonpää J., Nikoskelainen J., Svedström E., Vuorinen T., Meurman O. Bacteria and viruses in maxillary sinuses of patients with primary hypogammaglobulinemia. Arch Otolaryngol Head Neck Surg. 2007;133:597–602. doi: 10.1001/archotol.133.6.597. [DOI] [PubMed] [Google Scholar]
- 10.Kainulainen L., Nikoskelainen J., Vuorinen T., Tevola K., Liippo K., Ruuskanen O. Viruses and bacteria in bronchial samples from patients with primary hypogammaglobulinemia. Am J Respir Crit Care Med. 1999;159:1199–1204. doi: 10.1164/ajrccm.159.4.9807067. [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulos N.G., Bates P.J., Bardin P.G., Papi A., Leir S.H., Fraenkel D.J. Rhinovirus infects the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 12.Gern J.E., Vrtis R., Grindle K.A., Swenson C., Busse W.W. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 13.Wos M., Sanak M., Soja J., Olechnowicz H., Busse W.W., Szczeklik A. The presence of rhinovirus in lower airways of patients with bronchial asthma. Am J Respir Crit Care Med. 2008;177:1082–1089. doi: 10.1164/rccm.200607-973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kainulainen L., Varpula M., Liippo K., Svedström E., Nikoskelainen J., Ruuskanen O. Pulmonary abnormalities in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 1999;104:1031–1036. doi: 10.1016/s0091-6749(99)70085-0. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser L., Aubert J.D., Pache J.C., Deffernez C., Rochat T., Garbino J. Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med. 2006;174:1392–1399. doi: 10.1164/rccm.200604-489OC. [DOI] [PubMed] [Google Scholar]
- 16.van Elden L.J., Sachs A.P., van Loon A.M., Haarman M., van de Vijver D.A., Kimman T.G. Enhanced severity of virus associated lower respiratory tract disease in asthma patients may not be associated with delayed viral clearance and increase viral load in the respiratory tract. J Clin Virol. 2008;41:116–121. doi: 10.1016/j.jcv.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winther B., Hayden F.G., Hendley J.O. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J Med Virol. 2006;78:644–650. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 18.Matsuse T., Hayashi S., Kuwano K., Keunecke H., Jeffries W.A., Hogg J. Latent adenoviral infection in the pathogenesis of chronic airway obstruction. Am Rev Respir Dis. 1992;146:177–184. doi: 10.1164/ajrccm/146.1.177. [DOI] [PubMed] [Google Scholar]
- 19.Retamales I., Elliot W.M., Meshi B., Coxson H.O., Pare P.D., Sciurba F.C. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164:469–473. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- 20.Wilfert C.M., Buckley R.H., Mohanakumar T., Griffith J.F., Katz S.L., Whisnant J.K. Persistent and fatal central-nervous-system ECHO virus infections in patients with agammaglobulinemia. N Engl J Med. 1977;296:1485–1489. doi: 10.1056/NEJM197706302962601. [DOI] [PubMed] [Google Scholar]
- 21.McKinney R.E., Jr., Katz S.L., Wilfert C.M. Chronic enteroviral meningoenchephalitis in agammaglobulinemic patients. Rev Infect Dis. 1987;9:334–356. doi: 10.1093/clinids/9.2.334. [DOI] [PubMed] [Google Scholar]
- 22.Winkelstein J.A., Marino M.C., Lederman H.M., Jones S.M., Sullivan K., Burks A.W. X-Linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine. 2006;85:193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 23.Hara M., Saito Y., Komatsu T., Kodama H., Abo W., Chiba S. Antigenic analysis of polioviruses isolated from a child with agammaglobulinemia and paralytic poliomyelitis after Sabin vaccine administration. Microbiol Immunol. 1981;25:905–913. doi: 10.1111/j.1348-0421.1981.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 24.Abo W., Chiba S., Yamanaka T., Nakao T., Hara M., Tagaya I. Paralytic poliomyelitis in a child with agammaglobulinemia. Eur J Pediatr. 1979;132:11–16. doi: 10.1007/BF00443199. [DOI] [PubMed] [Google Scholar]
- 25.MacLennan C., Dunn G., Huissoon A.P., Kumararatne D.S., Martin J., O'Leary P. Failure to clear persistent vaccine-derived neurovirulent poliovirus infection in an immunodeficient man. Lancet. 2004;363:1509–1513. doi: 10.1016/S0140-6736(04)16150-3. [DOI] [PubMed] [Google Scholar]
- 26.Lederman H.A., Winkelstein J.A. X-linked agammaglobulinemia: an analysis of 96 patients. Medicine. 1985;64:145–156. [PubMed] [Google Scholar]
- 27.Bjøro K., Skaug K., Haaland T., Frøland S.S. Long-term outcome of chronic hepatitis C virus infection in primary hypogammaglobulinaemia. QJM. 1999;92:433–441. doi: 10.1093/qjmed/92.8.433. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham-Rundles C., Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 29.Wheat W.H., Cool C.D., Norimoto Y., Rai P.R., Kirkpatrick C.H., Lindenbaum B.A. Possible role of human herpes virus 8 in the lymphoproliferative disorders in common variable immunodeficiency. J Exp Med. 2005;202:479–484. doi: 10.1084/jem.20050381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinti I., Soresina A., Spadaro G., Martino S., Donnanno S., Agostini C. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. Clin Immunol. 2007;27:308–316. doi: 10.1007/s10875-007-9075-1. [DOI] [PubMed] [Google Scholar]
- 31.Jennings L.C., Anderson T.P., Beynon K.A., Chua A., Laing R.T., Werno A.M. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 32.Oliver B.G., Lim S., Wark P., Laza-Stanca V., King N., Black J.L. Rhinovirus exposure impairs immune responses to bacterial products in alveolar macrophages. Thorax. 2008;63:519–525. doi: 10.1136/thx.2007.081752. [DOI] [PubMed] [Google Scholar]
- 33.Doyle S.L., Jefferies C.A., Feighery C., O'Neill L.A. Signalling by Toll-like receptors 8 and 9 requires Bruton's tyrosine kinase. J Biol Chem. 2007;282:36953–36960. doi: 10.1074/jbc.M707682200. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham-Rundles C., Radigan L., Knight A.K., Bauer L., Nakazawa A. TLR9 activation is defective in common variable immunodeficiency. J Immunol. 2006;176:1978–1987. doi: 10.4049/jimmunol.176.3.1978. [DOI] [PubMed] [Google Scholar]