Research highlights
▶ 1.28 mM potassium is favourable to the growth of Houttuynia cordata Thunb. ▶ High photosynthetic rate is due to increased chlorophyll and stable chloroplast. ▶ Stomatal conductance is limited by potassium supplies. ▶ Potassium starvation and high potassium reduce water content and root growth. ▶ Potassium starvation and high potassium cause oxidative stress.
Abbreviations: CAT, catalase; GAP, Good Agricultural Practices; H2O2, hydrogen peroxide; K, potassium; •O2–, superoxide radicals; POD, peroxidase; ROS, reactive oxygen species; SOD, superoxide dismutase
Keywords: Houttuynia cordata Thunb., Potassium, Growth, Photosynthesis, Transpiration, Oxidative stress
Abstract
Houttuynia cordata Thunb. is an edible herb with a variety of pharmacological activities, but only limited information is available about its response towards potassium supplementation. Sterile plantlets were cultured in media with different potassium levels, and parameters related to growth, foliar potassium, water and chlorophyll contents, photosynthesis, transpiration, H2O2 contents and antioxidative enzyme activities were determined after a month. Results showed that 1.28 mM potassium was the optimum for H. cordata as highest values of dry weight, shoot height, root length and number were obtained at this concentration. The optimum potassium concentration resulted in the maximum net photosynthetic rate which could be associated with the highest chlorophyll content rather than limited stomatal conductance. The supply of surplus potassium resulted in higher content of foliar potassium, but negatively correlated with the biomass. Both potassium starvation (0 mM) and high potassium (>1.28 mM) could lead to water loss through high transpiration rate and low water absorption, respectively, and resulted in H2O2 accumulation and increased activities of catalase and peroxidase, which suggested induction of oxidative stress. Moreover, H. cordata showed the minimum of H2O2 content and the maximum of superoxide dismutase activity on 1.28 mM potassium, implying its role in inducing tolerance against oxidative stress.
1. Introduction
Potassium (K) as quality element is one of the major nutrients for plant growth and development (Besford and Maw, 1975). It is the most abundant cation in plant cells and plays important roles in metabolisms like enzyme activities, water and assimilate transport, and protein synthesis (Yin and Vyn, 2002, Yin and Vyn, 2003, Véry and Sentenac, 2003, Pettigrew, 2008). Different potassium supplement levels significantly affect physiological and biochemical characters of plants (Chartzoulakis et al., 2006). Appropriate potassium concentrations effectively improve plant productivity (Yurtseven et al., 2005), while its deficiency leads to a decrease in chlorophyll content and photosynthetic rate (Zhao et al., 2001, Gerardeaux et al., 2010), and inhibits root and shoot elongation (Drew, 1975, Bednarz et al., 1998, Shin et al., 2005, Kanai et al., 2007). Inappropriate potassium levels may also induce stress responses, and many stresses can result in the accumulation of reactive oxygen species (ROS), such as superoxide radicals (•O2 –) and hydrogen peroxide (H2O2) (Shin and Schachtman, 2004). Plants have evolved antioxidant enzyme system including superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) to prevent damage from ROS (Bowler et al., 1992, Nayyar and Chander, 2004). SOD scavenges •O2 –, resulting in H2O2 and O2 formation (Amor et al., 2006). CAT and POD are the main enzymes to eliminate H2O2 (León et al., 2002, Passardi et al., 2005).
Houttuynia cordata Thunb. is a pungent, heart-like leafed perennial herb and constitutes a single species of the genus Houttuynia in the ancient Saururaceae and in Chinese is known as ‘Yuxingcao’, which means ‘producing unique fishy smell’. It belongs to thermophilic and hygrophilous species, native to Eastern Asia, especially distributed in middle, southeastern and southwestern provinces and regions in China. It can often be found in ravines, streamsides, forests, wet meadows, slopes, thicket and field margins, trailsides, roadsides or ditch banks in these regions (Wu et al., 2005a).
H. cordata has been identified as one of the most potential medical and edible wild plant resources (Wu et al., 2005a). Its young plants are popularly used as wild vegetable, while its mature plants are commonly used as a traditional medical herb in some Asian countries, such as China (Wu et al., 2005b), Korea (Kim et al., 2001), India (Chakraborti et al., 2006), Vietnam (Ogle et al., 2003) and Thailand (Nuengchamnong et al., 2009). H. cordata contains six major effective chemical components, namely essential oils, flavonoids, alkaloids, fatty acids, sterols and polyphenolic acids, and these compounds exhibit a variety of pharmacological activities like anti-cancer, anti-oxidative, anti-hypertension, anti-inflammatory, anti-mutagenic, anti-bacterial, and anti-purulent. It is effective in treating pneumonia, severe acute respiratory syndrome, human immunodeficiency virus and influenza virus and refractory hemoptysis (Lu et al., 2006, Lau et al., 2008, Nuengchamnong et al., 2009).
Due to these advantages the requirement of H. cordata has been increasing in the last several years. However, the wild resources in China cannot meet such huge demands. In recent years farmers are trying to increase its production by artificial cultivation. Being different from field crop plants such as cotton, wheat, maize and rice (Koch and Estes, 1975, Bednarz et al., 1998, Weng et al., 2007, Pettigrew, 2008, Gerardeaux et al., 2010), only limited information is available about its response towards fertilization formulae. Therefore, it is necessary to establish an efficient fertilization system for its commercial cultivation. However, the effects of different potassium supplement levels on its growth and physiology are largely unknown. Therefore, the main objective of the present study was to filter out the optimal supplement level of potassium for H. cordata growth by investigating its effects on growth, plant K concentrations, water contents, chlorophyll contents, photosynthesis, transpiration, H2O2 contents and antioxidative enzyme activities.
2. Materials and methods
2.1. Plant materials and growth conditions
H. cordata new line w01-100 with desirable traits like disease resistance, high-quality and yield, was selected out from a collection of more than one hundred H. cordata accessions present in China. The line belongs to chemotype myrcene (Chen et al., 2008) and has chromosome number 90 (Wu et al., 2003). It has been planted for commercial purposes for years in Good Agricultural Practices (GAP) base of 999 Pharmaceutical Group (China).
Sterile plantlets with three leaves were used in this experiment. The uniform plantlets were selected and cultured on MS (Murashige and Skoog, 1962) media, having five different potassium levels (0, 1.28, 2.56, 5.31 and 10.26 mM). The treatment with 0 mM K was negative control. Each treatment consisted of three replicates with at least 30 plants. The control culture conditions were maintained at 24 ± 2 °C under 12 h photoperiod with light intensity of 30 μmol m−2 s−1 provided by cool-white fluorescent lamps (Philips, China). Plants were harvested after a month to determine water contents, growth parameters, foliar K concentrations, chlorophyll contents, photosynthesis parameters, H2O2 contents and antioxidative enzyme activities.
2.2. Growth parameters and water contents
Water content and growth parameters including dry weight, shoot height, root length and number were measured. Shoot height and root length were determined using a vernier caliper. The shoot height indicates the value between the top of plantlet and stem base. The length of taproot of individual plantlet represents the root length.
2.3. K concentrations
The second fully expanded leaves were used for determination of K concentrations. Samples were dried as following: firstly, fresh leaves were fixed quickly at 105 °C for 30 min, and then oven-dried at 70 °C for 48 h. The dried material was milled to pass a 0.5 mm sieve. The powder (0.01 g) soaked in 1 mM hydrochloric acid for 5 h, vibrated for 30 min, and filtered, and then settled to the constant volume of 10 mL using 1 mM hydrochloric acid. The extraction solution was used to determine K concentration by atomic emission spectrophotometry. Foliar K content was calculated in terms of U g−1 dry weight (DW).
2.4. Chlorophyll contents
Chlorophyll contents were estimated by a portable chlorophyll meter (SPAD-502, Konica Minolta, Tokyo, Japan) from the second fully expanded leaves from the top of individual plants. SPAD value was an indicator of chlorophyll content. The average SPAD value of three points per leaf at upper, middle and lower positions was used.
2.5. Photosynthesis and transpiration parameters
The second fully expanded leaves were used for determination of net photosynthetic rate (Pn), stomatal conductance (Cond), transpiration rate (Tr) and intercellular CO2 concentration (Ci). These parameters were measured by a portable photosynthesis system (LI-6400, Li-Cor, Lincoln, NE, USA) with the air temperature, relative humidity, CO2 concentration and light intensity inside the leaf chamber controlled at 25 °C, 55%, 450 μmol CO2 mol−1, 1000 μmol m−2 s−1, respectively.
2.6. Antioxidative enzyme assays
The second fully expanded leaves were used for determination of antioxidative enzyme assays. Leaves (0.5 g) were homogenized with mortar and pestle in 10 mL 50 mM sodium phosphate buffer (pH 7.8 for SOD, pH 6.0 for POD and 7.0 for CAT) containing 1% polyvinylpyrrolidone (w/v). The homogenate was centrifuged at 15,000 × G for 10 min and the supernatant as enzyme extract was used for antioxidative enzyme assays. The whole extraction procedure was carried out at 4 °C.
SOD activity was determined by monitoring its ability to inhibit photochemical reduction of nitroblue tetrazolium (NBT) at 560 nm (Beauchamp and Fridovich, 1971). Absorbance values were recorded on an ultraviolet and visible (UV-Vis) spectrophotometer (UV-2450, Shimazu Co., Kyoto, Japan). One unit (U) of SOD was defined as the amount of enzyme necessary to inhibit the reduction of NBT by 50%. Enzyme activity was calculated in terms of U g−1 fresh weight (FW). The reaction mixture contained of 50 mM sodium phosphate buffer (pH 7.8), 13 mM l-methionine, 75 μM NBT, 10 μM ethylene diamine tetraacetic acid (EDTA) -Na2, 2 μM riboflavin, 0.1 mL enzyme extract.
POD activity was determined based on guaiacol oxidation (Hassan et al., 2005). Increase in the absorbance due to guaiacol oxidation was measured at 470 nm. Absorbance values were recorded on the UV-Vis spectrophotometer. One unit of activity was determined by the variety of 0.01 min−1. Enzyme activity was expressed as U g−1 FW. The reaction mixture contained of 50 mM sodium phosphate buffer (pH 6.0), 5 mM guaiacol, 10 mM H2O2, and 0.05 mL enzyme extract.
CAT activity was determined by monitoring the destruction of H2O2 (Rout and Shaw, 2001). Decrease in the absorbance due to decomposition of H2O2 was measured at 240 nm. Absorbance values were recorded on the UV-Vis spectrophotometer. One unit of activity was determined by the variety of 0.01 min−1. Enzyme activity was calculated in terms of U g−1 FW. The reaction mixture consisted of 200 mM sodium phosphate buffer (pH 7.0), 10 mM H2O2, 0.1 mL enzyme extract.
2.7. H2O2 contents
H2O2 content was determined as titanium complex (Brennan and Frenkel, 1977). Leaf samples (0.5 g) were homogenized in 10 mL cold (4 °C) acetone. The homogenate was centrifuged (15,000 × G) at 4 °C for 5 min. Subsequently, the supernatant (1 mL) was mixed with 0.1 mL titanium reagent (20% titanic tetrachloride in concentrated hydrochloric acid, v/v), followed by the addition of 0.2 mL concentrated ammonia to precipitate the peroxide–titanium complex. The mixture was then centrifuged at 15,000 × G for 5 min. The precipitate was washed with acetone repeatedly and then solubilized in 5 mL of 2 M sulphuric acid. The intensity of yellow color of supernatant was measured at 415 nm by UV-Vis spectrophotometer. The concentration of H2O2 in the supernatant was calculated by comparing its absorbance to a standard calibration curve representing H2O2–titanium complex from 0 to 1 mM and expressed as μmol g−1 FW.
2.8. Statistical analysis
Values were presented as means ± standard errors (SE) from three independent treatments. These data were subjected to analysis of variance, correlation and Duncan's multiple range test (P < 0.05) using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Growth parameters
H. cordata growth was significantly influenced by potassium concentrations (Fig. 1 ). The four growth parameters including dry weight, shoot height, root length and number showed significant differences with treatments (Table 1 ). Treatment of 1.28 mM K represented the highest values of these four growth parameters, while potassium starvation (0 mM) severely inhibited the growth of H. cordata. Similarly high potassium concentrations (>1.28 mM) also caused severe reduction in these parameters. The shortest root and least root number were both found in the treatment of the highest potassium level (10.26 mM).
Fig. 1.

H. cordata plantlets cultured in MS medium with different potassium supplies for a month. (a)–(e) represent 0, 1.28, 2.56, 5.31 and 10.26 mM potassium, respectively.
Table 1.
Effects of different potassium supplies on foliar K, water and chlorophyll contents and growth parameters of H. cordata.
| K1 | K2 | K3 | K4 | K5 | |
|---|---|---|---|---|---|
| Dry weight (mg plant−1) | 4.87 ± 0.39 (c) | 7.45 ± 0.50 (a) | 6.13 ± 0.11 (b) | 5.73 ± 0.19 (bc) | 5.56 ± 0.44 (bc) |
| Shoot height (cm) | 0.50 ± 0.03 (d) | 0.95 ± 0.01 (a) | 0.75 ± 0.04 (b) | 0.64 ± 0.07 (c) | 0.67 ± 0.09 (bc) |
| Root length (cm) | 0.51 ± 0.01 (c) | 1.16 ± 0.11 (a) | 0.70 ± 0.07 (b) | 0.53 ± 0.08 (c) | 0.19 ± 0.01 (d) |
| Root number | 2.13 ± 0.13 (c) | 4.13 ± 0.30 (a) | 2.82 ± 0.07 (b) | 2.95 ± 0.25 (b) | 1.72 ± 0.25 (d) |
| Foliar K (mg g−1 DW) | 13.50 ± 0.71 (d) | 20.00 ± 1.00 (c) | 34.00 ± 3.46 (a) | 26.33 ± 0.58 (b) | 22.00 ± 1.73 (bc) |
| Water content (g 100 g−1) | 92.08 ± 0.18 (c) | 94.09 ± 0.16 (a) | 93.46 ± 0.25 (b) | 93.42 ± 0.15 (b) | 92.52 ± 0.31 (c) |
| Chlorophyll content | 23.80 ± 0.60 (e) | 33.01 ± 0.78 (a) | 25.13 ± 0.35 (d) | 27.25 ± 0.84 (c) | 31.10 ± 0.70 (b) |
Note: Each value is mean ± SE (n = 3). Values followed by the different letter in the same lines are significantly different according to Duncan's multiple range test (P < 0.05). K1, K2, K3, K4 and K5 indicate 0, 1.28, 2.56, 5.13 and 10.26 mM K, respectively. Chlorophyll contents are expressed as SPAD value.
3.2. Leaf K, water and chlorophyll contents
Foliar K contents were significantly affected by the supplemental levels of potassium (Table 1). The maximum of leaf K concentrations was recorded in the treatment of 2.56 mM K, while higher potassium supplementation led to a decrease of potassium absorption as compared to 2.56 mM K. However, the maximum of potassium absorption did not represent the maximum of dry matter (Table 1).
Plant water contents were also significantly different between treatments (Table 1). The water content was maximum on 1.28 mM K while both no potassium and high potassium resulted in water loss in plant. Coarse small shrinkage of blade was observed in the treatments of potassium starvation and high potassium (Fig. 1).
Maximum chlorophyll content was recorded on 1.28 mM K (Table 1), and represented the darker hue in green leaves (Fig. 1), while the least was observed on no potassium treatment. Surprisingly the chlorophyll content dropped to the next minimum at 2.56 mM and then again showed a slight increase with higher potassium levels (>2.56 mM).
3.3. Photosynthesis and transpiration parameters
The net photosynthetic rate was maximum on 1.28 mM K, while it dropped dramatically on all other treatments, which were almost at par with each other (Table 2 ). The plants on this treatment had the minimum intercellular CO2 concentration (Table 2), thereby representing the maximum of carbon assimilation too, which could directly contribute to dry matter production. However, the maximum of net photosynthetic rate seemed to be not correlated with limited stomatal conductance (Table 2). But there was a positive correlation between the net photosynthetic rates and chlorophyll contents, and the Pearson correlation coefficient was 0.75.
Table 2.
Effects of different potassium supplies on net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), transpiration rate (Tr) and stomatal conductance (Cond) in the leaves of H. cordata.
| K1 | K2 | K3 | K4 | K5 | |
|---|---|---|---|---|---|
| Pn (μmol CO2 m−2 s−1) | 1.92 ± 0.14 (b) | 9.52 ± 0.72 (a) | 0.37 ± 0.06 (c) | 1.45 ± 0.39 (b) | 2.17 ± 0.40 (b) |
| Ci (μmol CO2 mol−1) | 433.98 ± 2.05 (a) | 383.87 ± 2.83 (b) | 438.74 ± 2.54 (a) | 428.73 ± 5.82 (a) | 429.20 ± 4.38 (a) |
| Tr (mmol H2O m−2 s−1) | 6.33 ± 1.06 (a) | 2.42 ± 0.26 (b) | 2.68 ± 0.35 (b) | 2.30 ± 0.11 (b) | 3.37 ± 0.68 (b) |
| Cond (mol H2O m−2 s−1) | 0.57 ± 0.03 (a) | 0.36 ± 0.04 (b) | 0.39 ± 0.03 (b) | 0.36 ± 0.10 (b) | 0.42 ± 0.08 (b) |
Note: Each value is means ± SE (n = 3). Values followed by the different letter in the same lines are significantly different according to Duncan's multiple range test (P < 0.05). K1, K2, K3, K4 and K5 indicate 0, 1.28, 2.56, 5.13 and 10.26 mM K, respectively.
Transpiration rates were significantly different between potassium starvation and the other four treatments with potassium, which were found at par with each other (Table 2). Similar trend was observed for stomatal conductance, which is usually associated with transpiration (Table 2). This suggested that potassium starvation strongly stimulated stomatal opening and then transpiration, and stomatal conductance was limited in the treatments with potassium.
3.4. H2O2 contents and antioxidative enzyme activities
H2O2 productions were significantly affected by potassium treatments (Fig. 2a). The minimum of H2O2 contents was recorded in the treatment of 1.28 mM K. H2O2 contents showed a continuous increment in the treatments of high potassium levels (>1.28 mM). It suggested that the absence of potassium and high potassium stimulated H2O2 accumulation, leading to oxidative stress.
Fig. 2.
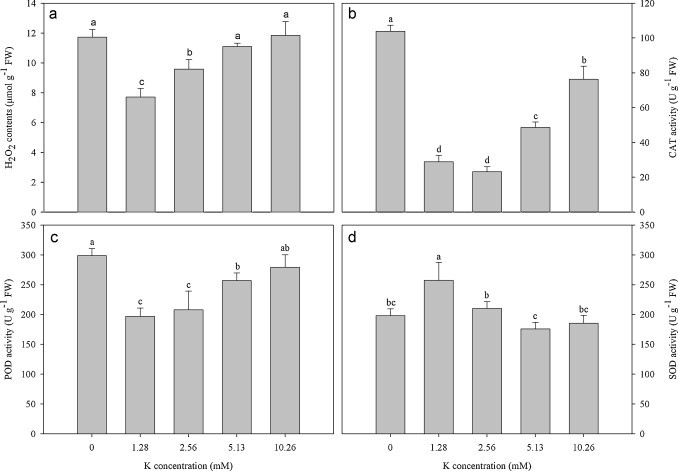
Effects of different potassium supplies on H2O2 contents and antioxidative enzyme activities in H. cordata. (a) H2O2, (b) CAT, (c) POD and (d) SOD. Values are means ± SE (n = 3). Vertical line on top of the bars means SE. Bars carrying different letters are significantly different at P < 0.05.
The activities of antioxidative enzymes got significantly affected by potassium concentrations. Both CAT and POD activities were low on 1.28 mM K, and both absence of potassium as well as high potassium stimulated their activities (Fig. 2b and c). The increases in CAT and POD activities might play a H2O2-scavenging role against induced oxidative stress. Interestingly, SOD activity reached maximum on 1.28 mM K, while it showed significantly reduced activity on potassium starvation as well as potassium surplus conditions (Fig. 2d).
4. Discussion
Potassium, at optimum concentration, is essential for H. cordata growth. Many studies have demonstrated an obvious increment in plant stature and yield with proper supply of potassium (Mullins et al., 1994, Heckman and Kamprath, 1995, Pettigrew and Meredith, 1997, Buah et al., 2000, Vyn and Janovicek, 2001). In the present study, H. cordata plants acquired highest values of dry weight, shoot height, root length and number on 1.28 mM K, and as such represented the most favourable treatment for its growth and development. Potassium starvation, as well as its surplus, caused severe reduction in its growth and development. The length and surface area of root affected the range of nutrient absorption, having direct negative consequences on plant productivity (Cakmak, 2005, Hermans et al., 2006, Pettigrew, 2008). Therefore, the decreases in dry weight and shoot height of H. cordata were partly due to similar changes of root length and number. Moreover, although the supply of surplus potassium resulted in higher contents of foliar potassium as compared to 1.28 mM K, the higher leaf K did not improve the biomass. This might contribute to a hypothesis that plant probably needs critical cytoplasmic concentration of K in a certain range (Leigh and Wyn Jones, 1984).
Photosynthesis was significantly affected by potassium concentrations, determining yield. It is well known that photosynthesis is related to carbon assimilation and dry matter production (Gifford and Evans, 1981, Kramer, 1981, Zelitch, 1982) and that chlorophyll content, chloroplast ultrastructure and stomatal conductance are the major factors in photosynthetic rate (Farquhar and Sharkey, 1982, Zhao et al., 2001), However, the optimum concentration of potassium in the present study did not show an increase in the stomatal conductance, and wrinkled leaves as observed in the treatments of potassium starvation and high potassium might be due to water loss and therefore represent poor chloroplast ultrastructure (not measured in this study). Many studies have shown that appropriate potassium concentration can enhance chlorophyll content and photophosphorylase activity and maintain chloroplast inner membrane and proton gradient of thylakoid membranes, promoting photosynthetic phosphorylation (Véry and Sentenac, 2003, Yurtseven et al., 2005, Chartzoulakis et al., 2006, Hermans et al., 2006). Therefore the maximum of net photosynthetic rate on 1.28 mM K may be mainly associated with high chlorophyll content and stable chloroplast ultrastructure, rather than limited stomata conductance.
Appropriate potassium concentration could maintain a critical water content in H. cordata. It is well known that potassium plays an important role in controlling stomatal aperture because concentration gradient of potassium between inside and outside of stomatal guard cells affects solute potential (Fischer, 1968, Fischer and Hsiao, 1968). In the present study the optimum concentration of potassium showed lower stomatal conductance and transpiration rate than potassium starvation, resulting in higher water content, which is important for cell functions. However, although the stomata conductance and transpiration rate of the four treatments with potassium were found at par with each other, high potassium resulted in lower water content as compared to 1.28 mM K. This suggested that high potassium reduced water absorption.
Oxidative stress could be induced by potassium starvation and high potassium according to the increase in H2O2 content (Fig. 2a). This result is well in accordance with some other studies (Cakmak, 1994, Cakmak, 2005, Shin and Schachtman, 2004). Excess H2O2 is harmful primarily due to reaction with lipids, proteins, and nucleic acids thus resulting in lipid peroxidation, membrane leakage, enzyme inactivation, and DNA breaks or mutations (Romero-Puertas et al., 2007), chlorophyll destruction (Cakmak, 1994) and growth reduction (Molassiotis et al., 2006). The increased activity of CAT and POD as observed in the present study might be to protect biomolecules from being attacked by H2O2. However, SOD activity did not show an increase with potassium starvation and high potassium. Previous studies have demonstrated that oxygen-dependence photosynthesis may be “leaking” energy to molecular oxygen, forming ROS such as •O2 – (Wise and Naylor, 1987, Ort and Baker, 2002). It therefore was suggested that accumulation of •O2 – might be partially suppressed by the low photosynthesis rates in these treatments. All of these implied that potassium starvation and high potassium might not result in an increase of •O2 – and excess H2O2 should have accumulated from other sources rather than from the conversion of •O2 – to H2O2. Moreover, the minimum of H2O2 content and the maximum of SOD activity at 1.28 mM K suggested that the optimal level of potassium resulted in lower oxidative stress and endowed plants with the resistance against oxidative stress, respectively.
Acknowledgments
This work was financial supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholar in China. We thanks Ruru Yao and Degang Wu for helping in tissue culture, and Shuaishuai Lv, Yi Yu and Yezhu Cao for assistance with enzyme assays and chlorophyll content determination.
References
- Amor N.B., Jiménez A., Megdiche W., Lundqvist M., Sevilla F., Abdelly C. Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol. Plant. 2006;126:446–457. [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bednarz C.W., Oosterhuis D.M., Evans R.D. Leaf photosynthesis and carbon isotope discrimination of cotton in response to potassium deficiency. Environ. Exp. Bot. 1998;39:131–139. [Google Scholar]
- Besford R., Maw G. Effect of potassium nutrition on tomato plant growth and fruit development. Plant Soil. 1975;42:395–412. [Google Scholar]
- Bowler C., Montagu M.V., Inze D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Phys. 1992;43:83–116. [Google Scholar]
- Brennan T., Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977;59:411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buah S., Polito T.A., Killorn R. No-tillage corn response to placement of fertilizer nitrogen, phosphorus, and potassium. Commun. Soil Sci. Plan. 2000;31:3121–3133. [Google Scholar]
- Cakmak I. Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced in magnesium- and potassium-deficient leaves, but not in phosphorus-deficient leaves. Environ. Exp. Bot. 1994;45:1259–1266. [Google Scholar]
- Cakmak I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005;168:521–530. [Google Scholar]
- Chakraborti S., Sinha S., Sinha R. High-frequency induction of multiple shoots and clonal propagation from rhizomatous nodal segments of Houttuynia cordata Thunb. – an ethnomedicinal herb of India. In Vitro Cell Dev: Pl. 2006;42:394–398. [Google Scholar]
- Chartzoulakis K., Psarras G., Vemmos S., Loupassaki M., Bertaki M. Response of two olive cultivars to salt stress and potassium supplement. J. Plant Nutr. 2006;29:2063–2078. [Google Scholar]
- Chen L., Wu W., Huang C.Y., Yang Y.X., Zheng Y.L. Composition and variability of the essential oil of Houttuynia of China. Chem. Nat. Compd. 2008;44:778–783. [Google Scholar]
- Drew M.C. Comparison of the effects of a localized supply of phosphate, nitrate, ammonium, and potassium on the growth of the seminal root system and the shoot in barley. New Phytol. 1975;75:479–490. [Google Scholar]
- Farquhar G.D., Sharkey T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982;33:317–345. [Google Scholar]
- Fischer R.A. Stomatal opening: role of potassium uptake by guard cells. Science. 1968;160:784–785. doi: 10.1126/science.160.3829.784. [DOI] [PubMed] [Google Scholar]
- Fischer R.A., Hsiao T.C. Stomatal opening in isolated epidermal strips of Vicia faba. II. Responses to KCl concentration and the role of potassium absorption. Plant Physiol. 1968;43:1953–1958. doi: 10.1104/pp.43.12.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardeaux E., Jordan-Meille L., Constantin J., Pellerin S., Dingkuhn M. Changes in plant morphology and dry matter partitioning caused by potassium deficiency in Gossypium hirsutum (L.) Environ. Exp. Bot. 2010;67:451–459. [Google Scholar]
- Gifford R.M., Evans L.T. Photosynthesis, carbon partitioning, and yield. Annu. Rev. Plant Physiol. 1981;32:485–509. [Google Scholar]
- Hassan M.J., Shao G., Zhang G. Influence of cadmium toxicity on growth and antioxidant enzyme activity in rice cultivars with different grain cadmium accumulation. J. Plant Nutr. 2005;28:1259–1270. [Google Scholar]
- Heckman J.R., Kamprath E.J. Potassium accumulation and soybean yield related to potassium fertilizer rate and placement. Commun. Soil Sci. Plan. 1995;26:123–143. [Google Scholar]
- Hermans C., Hammond J.P., White P.J., Verbruggen N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006;11:610–617. doi: 10.1016/j.tplants.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Kanai S., Ohkura K., Adu-Gyamfi J.J., Mohapatra P.K., Nguyen N.T., Saneoka H., Fujita K. Depression of sink activity precedes the inhibition of biomass production in tomato plants subjected to potassium deficiency stress. J. Exp. Bot. 2007;58:2917–2928. doi: 10.1093/jxb/erm149. [DOI] [PubMed] [Google Scholar]
- Kim S.K., Ryu S., No J., Choi S., Kim Y. Cytotoxic alkaloids from Houttuynia cordate. Arch. Pharm. Res. 2001;24:518–521. doi: 10.1007/BF02975156. [DOI] [PubMed] [Google Scholar]
- Koch D.W., Estes G.O. Influence of potassium stress on growth, stomatal behavior, and CO2 assimilation in corn. Crop Sci. 1975;15:697–699. [Google Scholar]
- Kramer P.J. Carbon dioxide concentration, photosynthesis, and dry matter production. Bioscience. 1981;31:29–33. [Google Scholar]
- Lau K.M., Lee K.M., Koon C.M., Cheung C.S.F., Lau C.P., Ho H.M., Lee M.Y.H., Au S.W.N., Cheng C.H.K., Lau C.B.S., Tsui S.K.W., Wan D.C.C., Waye M.M.Y., Wong K.B., Wong C.K., Lam C.W.K., Leung P.C., Fung K.P. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008;118:79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León A.M., Palma J.M., Corpas F.J., Gómez M., Romero-Puertas M.C., Chatterjee D., Mateos R.M., del Río L.A., Sandalio L.M. Antioxidative enzymes in cultivars of pepper plants with different sensitivity to cadmium. Plant Physiol. Biochem. 2002;40:813–820. [Google Scholar]
- Leigh R.A., Wyn Jones R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984;97:1–13. [Google Scholar]
- Lu H.M., Liang Y.Z., Chen S. Identification and quality assessment of Houttuynia cordata injection using GC–MS fingerprint: a standardization approach. J. Ethnopharmacol. 2006;105:436–440. doi: 10.1016/j.jep.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Molassiotis A., Sotiropoulos T., Tanou G., Diamantidis G., Therios I. Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM 9 (Malus domestica Borkh) Environ. Exp. Bot. 2006;56:54–62. [Google Scholar]
- Mullins G., Reeves D., Burmester C., Bryant H. In-row subsoiling and potassium placement effects on root growth and potassium content of cotton. Agron. J. 1994;86:136–1136. [Google Scholar]
- Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- Nayyar H., Chander S. Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J. Agron. Crop Sci. 2004;190:355–365. [Google Scholar]
- Nuengchamnong N., Krittasilp K., Ingkaninan K. Rapid screening and identification of antioxidants in aqueous extracts of Houttuynia cordata using LC-ESI-MS coupled with DPPH assay. Food Chem. 2009;117:750–756. [Google Scholar]
- Ogle B., Tuyet H., Duyet H., Xuan Dung N. Food, feed or medicine: the multiple functions of edible wild plants in Vietnam. Econ. Bot. 2003;57:103–117. [Google Scholar]
- Ort D.R., Baker N.R. A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr. Opin. Plant Biol. 2002;5:193–198. doi: 10.1016/s1369-5266(02)00259-5. [DOI] [PubMed] [Google Scholar]
- Passardi F., Cosio C., Penel C., Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005;24:255–265. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- Pettigrew W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 2008;133:670–681. doi: 10.1111/j.1399-3054.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- Pettigrew W.T., Meredith W.R. Dry matter production, nutrient uptake, and growth of cotton as affected by potassium fertilization. J. Plant Nutr. 1997;20:531–548. [Google Scholar]
- Romero-Puertas M.C., Corpas F.J., Rodríguez-Serrano M., Gómez M., del Río L.A., Sandalio L.M. Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. J. Plant Physiol. 2007;164:1346–1357. doi: 10.1016/j.jplph.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Rout N.P., Shaw B.P. Salt tolerance in aquatic macrophytes: possible involvement of the antioxidative enzymes. Plant Sci. 2001;160:415–423. doi: 10.1016/s0168-9452(00)00406-4. [DOI] [PubMed] [Google Scholar]
- Shin R., Berg R.H., Schachtman D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005;46:1350–1357. doi: 10.1093/pcp/pci145. [DOI] [PubMed] [Google Scholar]
- Shin R., Schachtman D.P. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8827–8832. doi: 10.1073/pnas.0401707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry A.A., Sentenac H. Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol. 2003;54:575–603. doi: 10.1146/annurev.arplant.54.031902.134831. [DOI] [PubMed] [Google Scholar]
- Vyn T.J., Janovicek K.J. Potassium placement and tillage system effects on corn response following long-term no till. Agron. J. 2001;93:487–495. [Google Scholar]
- Weng X.-Y., Zheng C.-J., Xu H.-X., Sun J.-Y. Characteristics of photosynthesis and functions of the water–water cycle in rice (Oryza sativa) leaves in response to potassium deficiency. Physiol. Plant. 2007;131:614–621. doi: 10.1111/j.1399-3054.2007.00978.x. [DOI] [PubMed] [Google Scholar]
- Wise R.R., Naylor A.W. Chilling-enhanced photooxidation: evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol. 1987;83:278–282. doi: 10.1104/pp.83.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Zheng Y.L., Chen L., Wei Y.M., Yan Z.H. Genetic diversity among the germplasm resources of the genus Houttuynia Thunb. in China based on RAMP markers. Genet. Resour. Crop Evol. 2005;52:473–482. [Google Scholar]
- Wu W., Zheng Y.L., Chen L., Wei Y.M., Yang R.W., Yan Z.H. Evaluation of genetic relationships in the genus Houttuynia Thunb. in China based on RAPD and ISSR markers. Biochem. Syst. Ecol. 2005;33:1141–1157. [Google Scholar]
- Wu W., Zheng Y.L., Yang R.W., Chen L., Wei Y.M. Variation of chromosome number and cytomixis of Houttuynia cordata Thunb. from China. Acta Phytotaxon. Sin. 2003;41:245–257. [Google Scholar]
- Yin X., Vyn T.J. Soybean responses to potassium placement and tillage alternatives following no-till. Agron. J. 2002;94:1367–1374. [Google Scholar]
- Yin X., Vyn T.J. Potassium placement effects on yield and seed composition of no-till soybean seeded in alternate row widths. Agron. J. 2003;95:126–132. [Google Scholar]
- Yurtseven E., Kesmez G.D., Ünlükara A. The effects of water salinity and potassium levels on yield, fruit quality and water consumption of a native central anatolian tomato species (Lycopersicon esculantum) Agric. Water Manage. 2005;78:128–135. [Google Scholar]
- Zelitch I. The close relationship between net photosynthesis and crop yield. Bioscience. 1982;32:796–802. [Google Scholar]
- Zhao D., Oosterhuis D.M., Bednarz C.W. Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica. 2001;39:103–109. [Google Scholar]


