Abstract
This review discusses in vivo airway aspects of plasma exudation in relation to current views on epithelial permeability and epithelial regeneration in health and disease. Microvascular-epithelial exudation of bulk plasma proteins characteristically occurs in asthmatic patients, being especially pronounced in those with severe and exacerbating asthma. Healthy human and guinea pig airways challenged by noninjurious histamine-leukotriene–type autacoids also respond through prompt mucosal exudation of nonsieved plasma macromolecules. Contrary to current beliefs, epithelial permeability in the opposite direction (ie, absorption of inhaled molecules) has not been increased in patients with asthma and allergic rhinitis or in acutely exuding healthy airways. A slightly increased subepithelial hydrostatic pressure produces such unidirectional outward perviousness to macromolecules. Lack of increased absorption permeability in asthmatic patients can further be reconciled with occurrence of epithelial shedding, leaving small patches of denuded basement membrane. Counteracting escalating barrier breaks, plasma exudation promptly covers the denuded patches. Here it creates and sustains a biologically active barrier involving a neutrophil-rich, fibrin-fibronectin net. Furthermore, in the plasma-derived milieu, all epithelial cell types bordering the denuded patch dedifferentiate and migrate from all sides to cover the denuded basement membrane. However, this speedy epithelial regeneration can come at a cost. Guinea pig in vivo studies demonstrate that patches of epithelial denudation regeneration are exudation hot spots evoking asthma-like features, including recruitment/activation of granulocytes, proliferation of fibrocytes/smooth muscle cells, and basement membrane thickening. In conclusion, nonsieved plasma macromolecules can operate on the intact airway mucosa as potent components of first-line innate immunity responses. Exuded plasma also takes center stage in epithelial regeneration. When exaggerated, epithelial regeneration can contribute to the inception and development of asthma.
Key words: Plasma proteins, innate immunity, airway epithelium, airway microcirculation, epithelial permeability, epithelial regeneration, asthma pathogenesis
Abbreviations used: AMP, Antimicrobial peptide; BAL, Bronchoalveolar lavage; CC16, Club cell protein; PAF, Platelet activating factor
Epithelial barrier dysfunction allowing exaggerated absorption of inhaled molecules is considered a major pathogenic feature of asthmatic and allergic airways. This paradigm is currently supported by observations on epithelial cells in culture.1 By contrast, careful noninvasive in vivo studies have demonstrated a lack of increased bronchial permeability to inhaled molecules in asthmatic patients.2, 3, 4, 5, 6 Similarly, nasal absorption permeability has not been increased in patients with seasonal or perennial rhinitis.7, 8, 9, 10 This in vitro–in vivo dichotomy ought to have some bearing on several areas of research. However, the iconoclastic in vivo data demonstrating maintained epithelial barrier function in patients with asthma or allergic rhinitis are not extensively noted in the current literature.
Known features of asthma can contribute to the belief that there must be increased tissue penetration of inhaled molecules in patients with this disease. A prime example is increased shedding of epithelial cells. Clearly, an abnormal loss of barrier cells in patients with asthma11, 12 and rhinitis13, 14 would lead to increased penetration of molecules deposited on the airway surface. To make matters worse, molecular biology data produced in vitro are now interpreted to indicate impaired epithelial repair in asthmatic patients.15, 16
How then can we interpret the patient data on maintained barrier function? Is there a possibility that epithelial barrier restitution somehow is highly efficient in vivo in patients with this desquamative disease? And, if so, is exaggerated regeneration, rather than the reputed permeability hypothesis, a pathogenic epithelial aspect of asthma?
Most conspicuous with regard to epithelial permeability might be the observation of increased levels of plasma macromolecules on the airway surface. This feature is now widely considered to reflect a generally increased epithelial perviousness. The inference is that airway mucosal absorption permeability must also be increased in airways in which plasma exudation occurs.1, 17 Based on evidence cumulating over a century or more, microvascular-epithelial exudation of plasma macromolecules is an established feature of asthma and allergic rhinitis.18, 19, 20 However, the understanding of epithelial transmission of plasma is limited, as is understanding of the potential role of exuded plasma in epithelial regeneration.
Discussing innate immune defense in patients with obstructive lung disease, Curtis et al21 maintain that plasma protein exudation reflects “microscopic bleedings.” By contrast, several authors have mentioned in passing that plasma exudation can be part of the first line of defense in the airway mucosa.22, 23 Is it then possible that epithelial transmission of extravasated plasma is so swift that subepithelial edema does not develop? Furthermore, is there an asymmetry in airway epithelial lining cells such that it can let through bulk plasma macromolecules without allowing increased penetration of inhaled molecules? What mechanism then might allow increased plasma exudation in airways in which permeability in the opposite direction is not increased?
This article discusses epithelial barrier function in vivo, with a focus on opportunities for plasma macromolecules to be involved in first-line defense of the intact airway mucosa, epithelial regeneration, and asthma in association with inflammation and exaggerated epithelial regeneration.
Plasma exudation contributing to first-line defense of the intact airway mucosa
Prompt, reversible, and reproducible plasma exudation
Using challenges with noninjurious histamine-leukotriene–type autacoids, animal and human in vivo experiments have demonstrated how swift plasma exudation responses are evoked in tracheobronchial and nasal airways. Importantly, many autacoid “inflammatory” stimuli increase permeability in airway subepithelial microvessels but are without this effect in the pulmonary circulation.20 Within a few minutes after exposure to histamine-type mediators (including leukotrienes, prostaglandins, platelet activating factor [PAF]-acether, and bradykinin), plasma protein indices are significantly increased in sampled airway surface liquids in guinea pig and human airways.24, 25, 26, 27, 28, 29 This response is dose dependent, reversible, and reproducible. In accordance with this, the exudative response to histamine has been used to assess the onset and duration of action of antihistamine drugs in human nasal airways.30
Nonsieved and noninjurious passage of plasma macromolecules from the subepithelial microcirculation to the epithelial surface
Passage of plasma proteins from the subepithelial microcirculation through mucosal tissue, including basement membranes, and then paracellularly across intact pseudostratified epithelial lining cells (Fig 1 )20, 31, 32, 33, 34 involves little sieving. An early demonstration of prompt challenge-induced microvascular-epithelial exudation in guinea pig tracheobronchial airways suggested that plasma macromolecules appeared on the mucosal surface independent of their charge and size.29 The indiscriminate microvascular-epithelial passage of macromolecules explains why the process is called exudation rather than transudation, the latter name emphasizing fluid movement.35, 36, 37 Thus different-sized plasma proteins have appeared on challenged but intact guinea pig and human airway mucosal surfaces in nearly the same concentration ratios as occur in circulating blood.24, 29, 31, 38 Similar data have been reported in asthmatic patients by Out et al.32
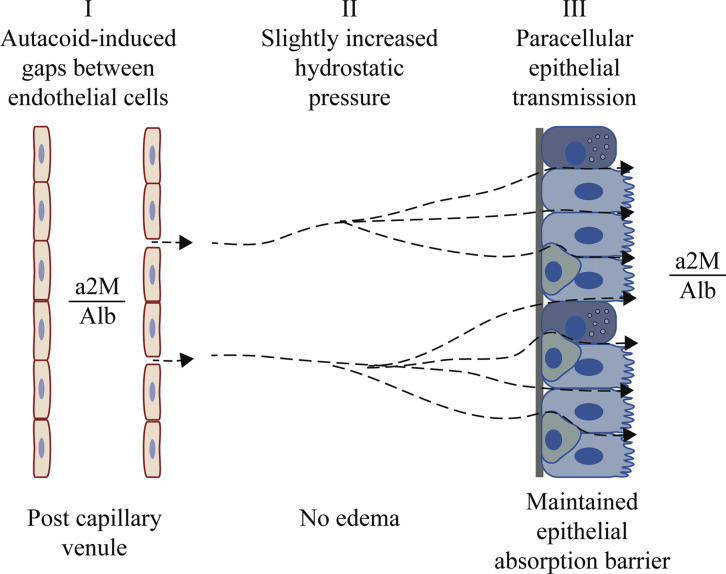
Fig 1.
The 3 major steps involved in airway microvascular-epithelial exudation of nonsieved plasma proteins. After local mucosal challenge, autacoids induce high-permeability gaps between endothelial cells in postcapillary venules (I). Venules belong to a profuse subepithelial microcirculation distinct from the pulmonary circulation.20 Through these gaps, plasma macromolecules enter interstitial spaces, where an increased hydrostatic pressure (II) will affect basolateral aspects of epithelial cells. A pressure increase of only 5 cm of H2O is sufficient for inducing paraepithelial transmission of plasma macromolecules (III). Exudation occurs without prior edema formation, without sieving, and without compromising the epithelial barrier. In accord with this, histamine-challenged human airway mucosa in vivo exudes albumin (Alb) and α2-macroglobulin (a2M) at a concentration ratio (a2M/Alb) that is similar to that in circulating plasma.31, 32 Furthermore, absorption of molecules deposited on the mucosal surface is not increased. Plasma extravasation (I) is an active endothelial cell event under physiologic and pharmacologic control.33, 34 The ensuing epithelial passage (III) appears to be a hydrostatic pressure–driven hydraulic mechanism driven by the extravasated plasma itself (II). Microvascular-epithelial exudation of nonsieved plasma macromolecules is a prompt, reversible, repeatable, and usually short-lasting innate immune response.
Accordingly, the large plasma protein α2-macroglobulin has emerged as a well-validated index of plasma exudation in nasal and bronchial airways in numerous human in vivo studies.31, 32, 39, 40, 41, 42, 43, 44 Further reflecting the nonsieved nature of plasma exudation responses, a tangled and sticky macromolecule, such as fibrinogen, is also used as an index of plasma exudation.38, 45 Yet the ultrastructure of the epithelial lining has not been affected by transmission of bulk plasma.46, 47, 48, 49
Plasma exudation without mucosal edema
Studies in guinea pigs and rats indicate that extravasated plasma is promptly transmitted to the mucosal surface between and around epithelial cells48 in the challenged tracheobronchial area.24, 27, 47, 48, 50 Studies in mice are of limited value in this field because this species lacks a bronchial circulation.51 There is no known type of mucosal challenge that results in selective extravasation without the further transmission of plasma across the epithelial lining. Furthermore, the plasma exudation response to autacoids has not been associated with an increase in lymphatic transport of plasma tracers.27 Hence, in agreement with a lack of high-permeability edema, increased draining of proteins from the mucosal tissues has not occurred. The wet/dry weight ratio of the airway wall has not increased29 nor has the subepithelial tissue been appreciably expanded to indicate presence of actual edema.47, 52 A morphometric study in allergic rats also demonstrated that the airway-narrowing effect of allergen challenge reflected smooth muscle constriction without edema.53 Prolonged stimulation of rat airways with allergens, such as house dust mite, has increased the number of subepithelial microvessels, although with little signs of mucosal edema.51
Also, in human asthma there is some increased vascularity51 but little evidence for bronchial mucosal edema. Even at sites at which the bronchial lumen is filled with mucus and exudate, the adjacent subepithelial mucosal tissue has exhibited no signs of edema.54 The bronchoscopic in vivo view is known to give the impression of mucosal edema. However, it is unclear whether the appearance of a thickened mucosa represents edema or is a result of circular smooth muscle impacting on the mucosal tissue at different degrees of bronchoconstriction.
Clearly, plasma exudation involves some deposition of plasma macromolecules in mucosal tissues, most commonly recorded as distribution of Evan's blue dye bound to albumin.50 Importantly, such observations reflect the capacity of extravasated plasma proteins to contribute to an active mucosal tissue milieu (edema is surplus liquid in the tissue and therefore might not be equated with merely the occurrence of Evan's blue–colored tissue).
The basic nature of plasma exudation can be compared with mucus secretion
The above observations indicate that the basic nature of the plasma exudation process is more physiologic than pathophysiologic. A noninjurious, reversible, and reproducible response of the airway mucosa, plasma exudation is in several respects comparable with the mucosal secretory response,55 and yet they are distinctly regulated. Admittedly, histamine-leukotriene–type mediators causing plasma exudation can also be active secretagogues,55 suggesting ample opportunities for concomitant occurrence of mucus and plasma proteins on the airway surface. However, the archetypal cholinergic agents causing mucosal secretion are without plasma exudation effects.28, 56, 57 Furthermore, the reputed neurogenic plasma exudation response, which is typically evoked by exposure to capsaicin, seems confined to rodents.58 In human airways capsaicin consistently induces coughing59 but no plasma exudation response.28, 43 Hence it is the nonneurogenic plasma exudation responses in rats and guinea pigs that translate well to human subjects.
Plasma exudation occurs without increased absorption of mucosal surface molecules
Supporting the physiologic aspect, the epithelial barrier against the environment has not been compromised by exudation of nonsieved plasma. Both in animal and human airways, challenge with histamine-type mediators causes prompt plasma exudation without being associated with any increase in absorption of tracers applied on the mucosal surface.24, 52, 60, 61
This feature is important in several respects. One consequence is that the mere increase in plasma protein levels on the airway epithelial surface cannot be interpreted as a sign of general perviousness of that epithelial lining. There is also little support for an old belief that plasma exudation can induce shedding when forcing itself into the lumen.18 Hence any inward permeability change has to be validated by specific data on absorption rates.
Also, in the intestine challenge-induced plasma exudation responses have been demonstrated to occur without being associated with increased absorption permeability.62, 63, 64 Therefore plasma exudation and increased penetration of mucosal surface solutes should be considered distinct features.
Both plasma and leukocytes can traverse the airway epithelial lining without compromising its integrity as a barrier
Notations of plasma and leukocyte extravasation as components of tissue response date from the latter half of the 19th century. Among the central issues discussed even then, Julius Cohnheim36 emphasized that in mucosally lined hollow organs, extravasated plasma and leukocytes had access to convenient elimination through epithelial transmission. However, in recent decades, the role of transepithelial exit in elimination of mucosal leukocytes has largely been ignored and severely eclipsed by a focus on the biology of cell apoptosis.
Cell death through apoptosis followed by engulfment has rapidly gained a hegemonic position as a mechanism responsible for cell elimination. Apoptosis has been particularly hailed as a mechanism causing resolution of cell-driven airway inflammation. This largely in vitro data–based view seems unfortunate, especially concerning a major granulocyte in asthmatic patients: the eosinophil. With apologies to apoptosis proponents, it needs underscoring that apoptotic eosinophils have not been demonstrated in human living blood–perfused airway tissues, not even at resolution phases.65, 66 Also, the belief that successful treatment with anti–IL-5 biological agents reflects eosinophil apoptosis is not supported by compelling data.67, 68 Yet eosinophils do die in mucosal tissue of asthmatic patients. However, their death in vivo is almost exclusively through primary cytolysis, which might cause rather than resolve inflammatory disease conditions.66, 69
Concomitant with the increasing focus on molecular mechanisms of apoptosis, clinical and experimental in vivo data have continued to be publicized, indicating that elimination of these cells in asthmatic patients occurs through swift migration across the airway epithelium. Thus epithelial transmission has emerged as a noninjurious cell exit pathway for resolving mucosal tissue eosinophilia in asthmatic patients.65 Also, other inflammatory cells appear to use this pathway at resolution of inflammation.70
The plasticity of mucosal epithelial cell junctions obviously operates well at both paracellular migration of leukocytes and plasma exudation. The ability of epithelial cells to transiently yield and still maintain tightness is involved in both. However, modes of epithelial transmission differ and so do modes of extravasation. Leukocyte migration is driven by chemotactic signals separately regulating extravasation, tissue dwelling, and transepithelial passage.71, 72, 73 By contrast, the fate of extravasated plasma seems less to dwell in the tissue than to move promptly to the mucosal surface. It is also of note that the endothelial gaps through which plasma macromolecules escape the circulation are not commonly used by leukocytes.74, 75
Specificity of plasma exudation as a first-line innate immune response
A kind of specificity lies in the strict location and brief duration of the plasma exudation response. There is a profuse subepithelial microcirculation carrying systemic blood in human nasal airways, as well as in the entire human tracheobronchial airway.20 At any challenge site, postcapillary venules of this circulation can react through prompt formation of high-permeability endothelial gaps. This noninjurious response reflects an active process in endothelial cells that is under physiologic and pharmacologic control.33, 34, 36 , Gaps might open preferentially in the venular wall facing the epithelium.74, 76 At a given challenge burden, the gaps will be open for only a limited time. For example, at a given concentration of histamine, the duration of plasma extravasation might not last more than about 10 to 20 minutes, even in the continued presence of this type of agent.33, 36 , It is only if the challenge burden increases that the extravasation will be longer lasting. Challenge with allergen or PAF-acether can cause both an early- and a late-phase plasma extravasation response.27, 50, 77 Reflecting the time course of extravasation, plasma proteins then emerge early and late on the mucosal surface at the site of challenge (Fig 1).
Despite its basic physiologic nature, plasma exudation is a powerful innate immunity response. It also allows major combat against threatening noxious factors to take place on the tissue surface rather than inside the tissue, where the risk of harmful results is so much greater. Plasma exudation contributes to a complex scene of biological activity in vivo. Its indiscriminate nature means that all the potent protein systems of circulating plasma will appear on the mucosal surface, with ample opportunities for activation and action. Whether there is selective activation of individual plasma protein systems depending on the type of exudative challenge agent/insult is not known.
Mediators of plasma exudation in healthy airways
What factors are causally involved in plasma exudation responses in healthy airways? Because neurogenic plasma exudation might not occur in human airways, a major physiologic mechanism has been ruled out. In airways challenged by simple “irritants” that evoke neurogenic activity, responses can thus be confined to increased mucus secretion and increased blood flow. Plasma exudation steps up the airway response to inhalant insults.
There is a wide range of cell-derived mediators with a known capacity to produce plasma exudation responses in the airways. Histamine-type autacoids, including leukotrienes, kinins, and PAF-acether, produce prompt plasma exudation effects without causing epithelial injury, with leukotriene C4 and D4 being by far the most potent.29 Plasma exudation might depend on release of such mediators from tissue-dwelling mast cells, granulocytes, and other cells variably occurring in the normal airway mucosa. Plasma exudation induced by microbial exposure can involve both neutrophil activation15 and kinins.78 Environmental challenges that evoke plasma exudation responses can involve epithelial factors operating in local recruitment and activation of inflammatory cells with the capacity to release vascular permeability agents.79 Epithelial factors can also contribute negatively by tonically suppressing airway plasma exudation.43, 80 It is not known whether epithelial cells release vasoactive mediators in vivo that directly cause plasma exudation as a first-line airway defense response.
Antimicrobial defense at the surface of an intact airway mucosa
Based on the present mechanism (Fig 1), it can be inferred that exuded plasma, with its content of immunoglobulins, complement factors, coagulation proteins, and more, can combat microbes deposited on the surface of uninjured mucosal lining. Such a versatile content of multiple profoundly active protein systems might be needed to counteract evasion strategies of pathogens.81, 82
However, the possibility of a role of airway plasma exudation does not seem to be mentioned in the huge literature that has developed in the field of mucosal surface antimicrobial defense. Instead, the focus is on antimicrobial peptides (AMPs; some 10,000 PubMed articles on AMPs have appeared in the last 2 decades). The biology of epithelially derived AMPs has been explored in detail in cell cultures. Still, their actual role in vivo has not been fully assessed, in part because the AMPs are rapidly catabolized.83, 84 It also seems unlikely that they would survive in a protease-active plasma exudate milieu. Hence plasma exudation and AMPs might not act together but would be separate antimicrobial mechanisms operating on the intact mucosal surface. This possibility is now speculative. Not enough can be known about the in vivo efficacy of AMPs, and the antimicrobial efficacy of airways exudation of plasma has not yet been subject to an in-depth investigation.
Plasma exudation is a first-line defense mechanism. However, it also occurs as a sign of tissue engagement at established microbial infections. Thus mucosal exudation of plasma is a general inflammatory feature of virally infected human nasal and bronchial airways.45, 85 The observation of exaggerated sputum fibrinogen concentrations in asthmatic patients with the common cold compared with a control group with the common cold45 further agrees with the notion that virus-induced exacerbations of this disease in part reflect a poor tolerance to viral infection.86 It has been suggested that both viral and bacterial pathogens increase microvascular permeability in part through activation of the “contact system,” causing release of the vasoactive autacoid bradykinin.87 Mechanisms involving endothelial Toll-like receptors 2, 4, and 9 have also been reported to be involved in the increased vascular permeability caused by bacterial infection, but these mechanisms concern pathophysiology of sepsis with no known relevance to mucosal defense.88
Innovative in vivo approaches seem required to explore the possibility that disease-inducing microbes deposited on the intact mucosal surface can evoke highly localized plasma exudation responses. A critical aspect concerns the early events before any established infection. Is there a window of opportunity for plasma exudation to assist in averting a threatening infection of airway mucosal tissues?
Mechanism of epithelial transmission of extravasated macromolecules
Transmission of bulk plasma outward across the airway epithelial lining should reflect a mechanism that can accommodate a number of in vivo features (Box 1 ).
Box 1. Features that define the basic physiology of plasma exudation.
- Direction and injury aspects of plasma exudation:
-
•The outward paracellular epithelial transmission of macromolecules should be a unidirectional perviousness, allowing extravasated plasma proteins to appear locally on the challenged airway mucosal surface without being associated with any increase in inward permeability.
-
•Although plasma exudation is an important response to epithelial denudation, its basic nature involves transmission of macromolecules across pseudostratified airway epithelial lining without compromising the ultrastructure or functional tightness of the epithelial lining cells as an absorption barrier.
-
•
- Epithelial transmission of bulk plasma preventing formation of subepithelial edema:
-
•The epithelial exudation mechanism transmits mucosal challenge–induced, extravasated plasma macromolecules so swiftly that the increased vascular high permeability will not result in edema in the subepithelial area of interest.
-
•Autacoids and drugs known to cause and inhibit, respectively, vascular permeability should not affect the epithelial transmission of extravasated plasma. Indeed, reducing the ability of the epithelial transmission of plasma would promote development of subepithelial edema.
-
•
- Time aspects and reproducibility:
-
•Epithelial transmission of plasma macromolecules should occur promptly within a few minutes after their extravasation. With a short delay, it represents the time course of extravasation from the profuse network of subepithelial postcapillary venules.
-
•Thus the epithelial component of the exudation mechanism sees to it that the mucosal challenges, which increase subepithelial venular leakage of plasma macromolecules, will automatically be followed by appearance of extravasated plasma proteins on the mucosal surface at the very site of challenge.
-
•The plasma exudation response is fully reversible and repeatable.
-
•
A hydrostatic pressure hypothesis and an approach to testing it
Considering the aspects listed in Box 1, the idea arises that somehow the extravasated plasma itself brings about its own further passage across the epithelial lining. Is it possible that the liberated plasma could increase the local hydrostatic pressure to affect the basolateral aspects of epithelial cells in such a manner so as to create paracellular pathways to the mucosal surface? Little was known about the effects on airway transepithelial macromolecular flux in response to small nondistorting increases in hydrostatic pressure.
Intact segments of guinea pig trachea were dissected to test the hypothesis of hydrostatic pressure–driven epithelial transmission of macromolecules. Segments were mounted in organ baths and attached at both ends to liquid-filled tubing by using extreme care to avoid edge injuries. By using this set-up, serosal and mucosal hydrostatic pressures could be regulated separately and affect epithelial transmission of macromolecular tracers in either direction.89, 90 The guinea pig trachea was considered suited for these experiments because of similarities to human airways, including a pseudostratified epithelium and a profuse subepithelial microcirculation. The guinea pig trachea also exhibited similar responses to human airways with regard to plasma exudation evoked by allergen and nonneural mediator challenges.91 Additionally, the tracheal cartilage should be an asset because it would counteract confounding distortions of the tissue when pressure is increased.
In vitro support for the hydrostatic pressure hypothesis
Increasing serosal pressure in the isolated tubal segment of guinea pig trachea by only 5 cm of H2O, markedly less than the microvascular pressure,33, 49, 92 caused prompt transepithelial passage of tracer macromolecules from serosal to mucosal surface liquids. This transmission continued at the same increased rate as long as the pressure was increased. Importantly, the transepithelial movement of serosal macromolecules was not associated with increased passage of macromolecular tracers in the opposite direction. Thus during and after 30 minutes of a serosal pressure challenge, mucosal absorption permeability was not increased.90 In agreement with this, there was no sign of any damage to or structural change of the epithelium.89 Furthermore, the hydrostatic pressure–induced movement of serosal macromolecules to the mucosal surface was well repeatable and reproducible.
Finally, autacoids and drugs, which are known to either induce or inhibit plasma exudation in vivo, had no influence on the hydrostatic pressure–induced epithelial transmission of macromolecules in vitro.89 This observation supports the notion that the epithelial passage of extravasated plasma reflects the effect of hydrostatic pressure alone without any need for other effects on the epithelium to facilitate the passage.
In summary, there was tantalizing agreement between experimental hydrostatic pressure–induced transmission of macromolecules in the isolated tracheal tubes and the in vivo plasma exudation response to histamine-type mediators in animal and human airways. Taken together, these observations support the idea that the extravasated plasma itself, by slightly increasing epithelial basolateral hydrostatic pressures, could create paracellular epithelial macromolecular exit paths. It was proposed that such a mechanism operated at plasma exudation responses in vivo.
The hydrostatic pressure hypothesis confirmed in vivo
The hydrostatic pressure hypothesis received specific in vivo support. In a segment of rat trachea, Serikov et al49 demonstrated that an increase in hydrostatic pressure of 4.5 cm of H2O in the airway lumen was sufficient to prevent a plasma exudation response in vivo. As with the in vitro experiments, use of the trachea was probably crucial for the success of the in vivo experiments: the applied pressure would not affect the airway tissue layers because of the stabilizing effect of cartilage rings. Using scanning electron microscopy, Serikov et al49 further confirmed that plasma exudation occurred without affecting epithelial structural integrity.48
Hence both in vitro and in vivo data support the notion that a slightly increased hydrostatic pressure is the gross operational mechanism involved in the basic plasma exudation response to noninjurious mucosal challenges. Tokuda et al93 have reported that an increased hydrostatic pressure gradient from the basolateral to apical sides of epithelial cells caused reversible changes in tight junction proteins. However, few studies have determined molecular mechanisms associated with hydrostatic pressure–evoked paraepithelial flux of macromolecular solutes in the airways.
Airway plasma exudation and absorption in patients with asthma and allergic rhinitis
Exudative diseases without increased mucosal absorption permeability
Direct noninvasive measurements of airway permeability seem to have clearly disproved the hypothesis of increased bronchial absorption of inhaled molecules as a basic defect in asthmatic patients. Thus work by leading pulmonary research groups demonstrated that absorption of inhaled technetium 99m–labeled diethylene-triamine-pentaacetate (DTPA) is unrelated to bronchial hyperresponsiveness and that it is not increased in asthmatic patients.2, 3, 4, 94 Lack of increased permeability has been confirmed in studies involving more asthmatic patient groups and in which absorption from different lung zones has been examined.5 Furthermore, in patients with perennial allergic rhinitis, in whom symptoms were induced by stopping treatment, absorption permeability was not increased.8
Using dual-isotope techniques, determining concomitant mucociliary clearance and absorption of inhaled DTPA, Halpin et al7, 10 reported consistently reduced bronchial absorption in asthmatic patients compared with that in healthy subjects. Similarly, already in 1975, indications of reduced nasal airway absorption in patients with allergic rhinitis had been demonstrated.9 In accord with this, significantly reduced absorption permeability was observed in patients with seasonal rhinitis along with plasma exudation and allergic symptoms. In these studies absorption tracer concentration, influence of mucociliary clearance, exposed airway surface area, and exposure time were controlled.7, 10
A transient phase of increased absorption permeability can occur in patients with acute asthma.94 It is possible that extensive denudation is involved as a causative factor in such cases (see below). Challenge with house dust mite, an allergen that stands out as particularly pathogenic, has also caused transiently increased airway absorption in vivo in mice.95 These data might need confirmation in other species because mice lack a bronchial microcirculation.51 Taken together, in vivo data suggest that increased permeability of inhaled molecules is not a basic feature of asthma and allergic rhinitis, although plasma exudation does occur in these patients.
The paradigm of increased epithelial permeability in asthmatic patients might be unduly strengthened by misrepresentation of in vivo data
The studies disproving the permeability paradigm are rarely cited in current literature. Instead, quite unspecific data now serve to support the established notion that asthma is a disease with abnormal penetration of inhaled molecules across the bronchial mucosa. In both human and animal studies, observations of increased airway surface levels of plasma proteins are commonly reported as a compelling sign of compromised epithelial integrity.17 In view of the known features of plasma exudation, this interpretation seems unwarranted.
Proteins, such as club cell protein (CC16) and surfactant protein D, are secreted constituents of airway surface liquids. Reduced levels of these proteins in airway samples or increased levels in blood and urine have been reported to support the hypothesis of increased absorption permeability in asthmatic patients. However, it has been pointed out that data are inconsistent.1, 96 Blood and urine data on CC16 also vary greatly. A recent study additionally demonstrated a puzzling dichotomy: CC16 levels were increased in plasma but reduced in urine after allergen challenge in asthmatic patients.97 Bronchial surface levels of surfactant protein D in patients with severe asthma can be both increased and decreased,98, 99 questioning its value as a permeability probe. Unsurprisingly, replacement of these indirect indices with direct noninvasive markers of airway absorption permeability has been strongly recommended.1 As discussed above, absorption of inhaled permeability tracers has not been increased in asthmatic patients.
Airway surface proteins in asthmatic patients: Produced locally or emanating from exuded plasma?
Given the versatility of culture conditions, airway structural cells and leukocytes can exhibit an intriguing ability in vitro to produce proteins that also occur in plasma. Therefore an uncertainty might concern the source of proteins in the airways, whether produced locally or as a component of exuded plasma. There is now a predominance of cell biology studies over explorative and independent in vivo research. Hence the notion of local airway cellular production of plasma proteins is favored perhaps more than justified with regard to medical importance.
From its nonsieved nature, it is predictable100, 101 that coagulation and complement proteins will appear on the human airway surface at plasma exudation events. Yet even in established plasma exudative conditions, such as after airway allergen challenge in atopic subjects,100, 102 local production of complement proteins has been forwarded as the preferred source.103, 104 As demonstrated in sensitized guinea pigs and in patients with allergic rhinitis and allergic asthma, allergen challenge has caused both early- and late-phase plasma exudation responses.27, 105, 106 Yet in studies in which appearance of complement and coagulation products would reflect this biphasic feature, the role of plasma exudation has not been considered.107
It is of interest that plasma proteins, such as complement and coagulation proteins,108, 109 are considered central in asthma pathogenesis. However, it is surprising that local occurrence of leukocytes with merely a presumed capacity to produce the protein in question has sufficed to indicate local production; lack of systemic inflammation has similarly been used as an argument to favor a local source.103, 104 This kind of evidence/reasoning might not compellingly support a local cellular source. Rather, such widely disseminated arguments likely reflect lack of topical discussions of plasma exudation mechanisms.
Meerschaert et al110 made commendable attempts at identifying the source of increased bronchoalveolar lavage (BAL) fluid fibronectin after segmental allergen challenge in asthmatic patients. In a small subset of their patients, they analyzed BAL fluid by using an antibody directed at a splice variant of fibronectin considered to be of cellular rather than plasma origin. Possibly reflecting the small number of patients or methodological issues, the spread in values was large, and statistical significance of their intriguing data was not reached. Yet the authors' message was that the BAL fluid fibronectin was largely derived from cells.
To strengthen their argument, the authors also focused on significant correlations between fibronectin concentrations and leukocyte numbers in all BAL fluid, finding r values of between 0.6 and 0.8. However, they might have missed an opportunity to demonstrate superior correlation between fibronectin and albumin levels, both of which had increased by a factor of 6 on day 2 after antigen (mean BAL fluid values compared with antigen levels on day 0).
Local production of fibronectin at allergen exposure obviously needed to be confirmed, but when the same laboratory later reported BAL fluid fibronectin data after allergen challenge in asthmatic patients, the source of this protein was not considered.111, 112 The latter reports included a study in which both thrombin activity and fibronectin levels were determined in BAL fluid obtained 2 days after segmental allergen challenge in asthmatic patients.112 Thrombin activity correlated nicely with both total protein and fibronectin levels (r > 0.8), supporting the possibility that both thrombin and fibronectin were components of exuded plasma that would have been produced, accumulated, and remained to a significant extent in airway surface liquids until BAL was carried out 48 hours after allergen challenge.
Albumin or other plasma protein as biomarkers of airway plasma exudation?
Endobronchial allergen challenge in patients with allergic asthma produced significant BAL fluid levels of fibrinogen, even when the increased levels of albumin did not reach significance. However, there was correlation between albumin and fibrinogen levels (r = 0.9) at the bronchial allergic reaction.100 Varying baseline levels make the relatively small and diffusible albumin molecule a less specific plasma exudation tracer compared with higher-weight proteins. In animal tracheobronchial experiments, as well as in human nasal studies, cumulated mucosal surface albumin can be washed away, creating a suitable baseline condition.52, 113 Then albumin is a consistent plasma exudation tracer along with larger molecules, including fibrinogen and α2-macroglobulin.
In patients with chronic severe eosinophilic asthma, increased sputum levels of α2-macroglobulin indicated significant plasma exudation; however, fibrinogen levels were not increased. Instead, levels of fibrin degradation products were increased.42 Under chronic disease conditions, fibrinogen can be trapped as fibrin. When fibrin is deposited and tethered to the airway surface, it might not become a component of sputum as readily as its degradation products. These data support the use of α2-macroglobulin as a biomarker of plasma exudation in chronic disease conditions. Local production can be considered concerning proteins that are increased to a greater degree than the plasma tracer.42
Esnault et al114 compared the ratio of their designated BAL fluid protein to albumin levels and showed that it deviated from the ratio in plasma. Their conclusion was that contribution from plasma leak was unlikely. However, relatively larger concentrations of the protein were observed in plasma. In such cases work might be required to elucidate potentially confounding factors, such as binding, breakdown, and other modes through which exuded proteins can escape detection.
A further confounding factor concerns the possibility that the binding capacity of plasma proteins can bring mucosal tissue-dwelling cell-derived molecules to the surface at plasma exudation. Thus in patients with seasonal rhinitis, levels of eosinophil-derived proteins were increased in sputum only after histamine inhalations had produced acute bronchial plasma exudation.115 Effects of plasma exudation on the distribution of mucosal cell-derived molecules might need consideration in studies of airway samples.
Plasma exudation and its potentially pathogenic actions in patients with severe asthma
Although plasma exudation might be a common feature of asthma in general,42, 116 the best evidence for occurrence of plasma exudation in asthmatic patients might emanate from studies of patients with severe eosinophilic42, 117 or neutrophilic118 asthma and from studies in which asthmatic patients are challenged by insults/interventions known to worsen asthma: the common cold,45 allergen exposure,102 or a stepwise reduction in anti-inflammatory treatment.117, 119 Plasma exudation indices might even be a dominating inflammatory index of worsening asthma.120
Exudative hyperresponsiveness, which was determined based on exaggerated plasma exudation in response to histamine-type challenges, has been demonstrated in airways subjected to allergic disease and viral infection.26, 121, 122, 123 Thus it has occurred in human nasal airways in adults and children with seasonal allergic rhinitis122, 123 and in patients with coronavirus-induced common cold.26 A common cause of asthma exacerbations, viral infections cause plasma exudation in human nasal and bronchial airways.45, 85 Possibly as a reflection of exudative hyperresponsiveness, particularly large sputum concentrations of fibrinogen were observed in asthmatic patients in association with the natural common cold.45
Leukotrienes C4 and D4 are several orders of magnitude more potent than other common autacoids as inducers of plasma exudation in guinea pig tracheobronchial airways.29 Eosinophil granule proteins have caused plasma extravasation in the hamster cheek pouch.124 TNF-α has induced a late plasma exudation response in human nasal airways.125 However, little is known regarding which factors are actually involved in increased plasma exudation in asthmatic patients.
Although plasma exudation might not cause edema,27, 47 physical encroachment of the airway lumen can still result and aggravate the effects of bronchoconstriction.126 Exposure to plasma proteins can increase mucus production. Exuded plasma can also interact with mucus to increase viscosity and immobilize airway surface material.127, 128 This effect would be particularly unwanted in the small airways, with a risk of bronchiolar closure.129 It has been suggested that mucus plugs in asthmatic airways might be mucus-plasma plugs, but the extent to which plasma contributes to airway plugs remains to be determined in detail.
The exuded plasma proteins offer a Pandora's box, with many intact proteins and many of their breakdown molecules offering biological actions of interest. There is established interest in pathogenic roles of complement proteins, coagulation proteins, and kinins in asthmatic patients.108, 109, 130 Several plasma proteins can have crucial roles in epithelial regeneration (discussed below). However, specific roles of individual plasma proteins in patients with disease await the critical tests that trials with selectively targeted interventions will provide.
A maintained airway barrier against environmental challenges at shedding of columnar epithelial cells: A role in innate airway defense?
Although a versatile epithelial barrier is a topical theme, barrier restitution events after different degrees of shedding might not be widely discussed. The most common mode of epithelial shedding is loss of columnar cells. There is interest in apoptosis of epithelial cells, but it is not known whether exiting columnar epithelial cells are at any stage of apoptosis. Single columnar cells can leave without a trace because neighboring cells move in, squeezing the leaving cell. Such shed single cells can be found in airspace samples with a tapered-off tail. Also sheets of columnar cells are commonly shed in asthmatic patients (connected ciliated cells with still beating cilia have been found in asthmatic sputa).19, 131, 132 Columnar epithelial cells have some contact with the basement membrane, but it is not known whether their removal will cause any plasma exudation. Indeed, a plasma exudation response might not be needed because the remaining basal cells have remarkable transformative capacities.
After selective gentle removal of columnar cells from human bronchi or guinea pig tracheas, the exposed, normally loose, and cobblestone-like basal cells produce a barrier structure within a few minutes. They flatten to cover the basement membrane and create interdigitating cell contacts all around.133 Hence limited shedding exclusively of columnar cells might be a normal event serving to rid the airway of cells that are somehow defective. Alternatively, the cells might need to be sacrificed as part of airway defense operations. Shedding of columnar epithelial cells might not necessarily result in increased absorption permeability or might evoke plasma exudation.
Exaggerated plasma exudation reflects in part exaggerated epithelial regeneration in asthmatic patients
Nonsanguineous epithelial denudation in asthma guides in vivo experiments
Both plasma exudation and epithelial shedding are increased in asthmatic patients, especially in those with severe disease and during exacerbations.11, 18, 19 There are major aspects to consider in experimental approaches aimed at producing disease-like epithelial denudation in vivo. Extensive shedding of epithelial cells in asthmatic patients might not involve any bleeding. Thus when both columnar and basal cells are shed, no bleeding should occur. Shedding in asthmatic patients also leaves an apparently intact basement membrane. The discussion below concerns in vivo studies involving guinea pig airways in which the above-mentioned features have been accommodated.
Ingrid Erjefält's skillful in vivo interventions without any surgery and involving physiologic baselines134 have been essential both for studies of challenge-induced plasma exudation and for studies of shedding-like denudation in guinea pig tracheobronchial airways. Some important in vivo research was not cited, mostly because of, for example, more mucosal damage than involved in asthma-like epithelial shedding, involvement of toxic gases, and use of a species that lacks a bronchial microcirculation.
Epithelial denudation causes plasma exudation that creates a provisional barrier
In experiments involving gentle nonsanguineous mechanical denudation in vivo of a patch of dorsal trachea, it has been demonstrated that the remaining intact epithelial basement membrane is not left naked. Locally exuded plasma promptly spreads over the surface of the entire denuded basement membrane but not much further afield. A plasma-derived gel is thus created to cover the area of interest.135, 136 Such gels are not seen in common graphs because they are washed away during preparations for histologic examinations.
Mechanisms specifically involved in the plasma exudation response to denudation have not been determined but might in part reflect removal of tonic suppression of microvascular permeability by epithelial factors.80 The exudation is of much longer duration than the response to histamine-type autacoids.135 It sustains the cover gel with fresh plasma until the gel is not needed.135, 136 The gel is also dislodged as soon as the previously denuded area is fully covered by rapidly migrating epithelial regeneration (discussed below).
The plasma exudation response to disease-like denudation has several connotations. Based on its physical appearance, its capacity to trap molecules and particles, and its biological defense capacity, the gel is already a coarse barrier. Even just the fibrinogen component and associated mechanisms have major immune defense capacities.137 Consistent with the abundant occurrence of neutrophils in the epithelial regeneration area,74 fibrin is known to bind neutrophils and other leukocytes, as well as a range of cell- and plasma-derived molecules.138 Through its integrins, eosinophils exhibit intriguing interactions with fibrin and fibronectin.139 The continuous supply by exuded plasma provides a highly dynamic in vivo milieu suited for both barrier activities and regeneration of an epithelial cell barrier (Fig 2 ).
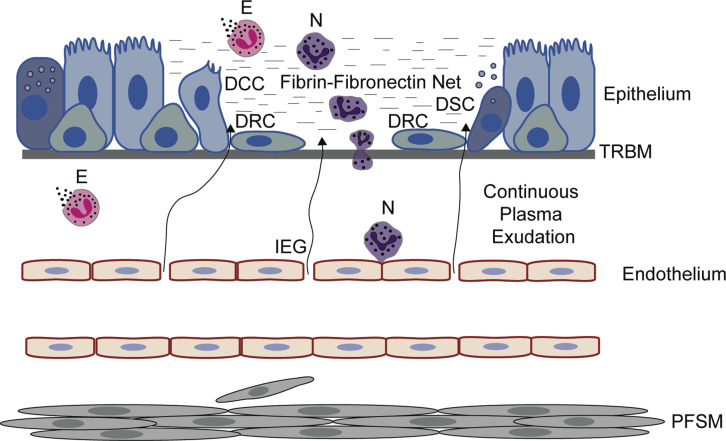
Fig 2.
Events occurring in vivo after epithelial denudation, leaving an uninjured basement membrane. No bleeding occurs, but the basement membrane is not left naked. Plasma exudation produces and continuously supplies a gel-like cover involving a fibrin-fibronectin net. Also, leukocytes, especially neutrophils, are recruited and activated. Eosinophils already present in the airway are activated by primary cytolysis. The gel serves as a highly active provisional barrier and milieu for speedy epithelial regeneration. All epithelial cell types bordering the denuded patch, notably including both secretory and ciliated cells, promptly dedifferentiate into flattened regeneration cells. As in micrographs of bronchi of asthmatic patients, basal cells might not be readily distinguished from regeneration cells. Loosely linked, the latter migrate from all sides over the denuded patch at a speed of several micrometers per minute. When a new cell cover is complete, plasma exudation stops, the plasma-derived gel dislodges, and proliferation plus redifferentiation toward a normal epithelium begins in the previously denuded area. Epithelial regeneration in vivo alone induces recruitment and activation of granulocytes, hyperexudation, hypersecretion, proliferation not only of epithelial cells but also of fibrocyte/smooth muscle cells, and thickening of the epithelial reticular basement membrane. In patients with desquamating asthma, multiple patches of epithelial regeneration would contribute to known pathogenic inflammatory and remodeling effects. DCC, Dedifferentiating ciliated cell internalizing or shedding its cilia, flattening, and migrating; DRC, dedifferentiated mesenchymal-like regeneration cells migrating speedily; DSC, dedifferentiating secretory cell releasing its secretory granules, flattening, and migrating; E, eosinophils undergoing cytolysis and liberating its protein-rich granules; IEG, venular interendothelial gaps through which nonsieved plasma proteins extravasate; N, neutrophils; PFSM, proliferating fibrocytes/smooth muscle cells; TRBM, thickened reticular basement membrane.
Epithelial regeneration occurs in an active soup of plasma proteins
An exclusive in vivo phenomenon, microvascular-epithelial exudation of plasma might not be a well-recognized mechanism in today's topical discussions, which are largely based on data in which the dynamic influence of plasma extravasation is absent. For example, it is not widely appreciated that after disease-like epithelial denudation in guinea pig tracheas, regeneration proteins, such as fibronectin and fibrinogen, emanate entirely from extravasated plasma.135 At epithelial injury sites, a novel cell sheet consisting of flattened repair epithelium is thus created in a dynamically active, plasma-derived gel milieu. This is also an active defense milieu. For example, basal cell–like epithelial repair cells might need this protection to compensate for their sensitivity to microbial exposure.140, 141 The in vivo exudation milieu seems more important than reflected in the current literature.142
Epithelial denudation in vivo appears to be exceedingly patchy
A helicopter view of wholemount airway preparations has revealed numerous tiny denudation spots after in vivo allergen challenge in guinea pigs. Each denudation patch is a hotspot with intense exudation and repair events.143, 144 Notably, the area of these spots has been much less than the denudation area revealed by sectioning of such a challenged airway wall.144 Hence although large areas of denudation in histologic preparations can reflect epithelial fragility, they might in part be sectioning artifacts.144, 145
It is not known in detail what decides the patchiness of denudation. Strikingly, this phenomenon is conspicuous, even in airways uniformly exposed to noxious agents, including allergens and dry air.143, 146 Davis and Freed147 thus carried out segmental dry air challenges in dogs to produce patchy bronchial denudation. Using a wedged bronchoscope, these authors further noted local accumulation of “mucus” and suggested that such material (actual components were not determined) could serve as a surrogate barrier.147 Mosser et al148 demonstrated that only a small fraction of epithelial cells became infected when exposed to rhinovirus. This observation is consistent with a patchy distribution of virus-induced epithelial injury. Patchy distribution of epithelial derangement, including early signs of epithelial regeneration, might be common in publicized histologic graphs of diseased airways.149 Some information on patchy epithelial damage and repair can also be found in studies exploring bronchial mucosal distribution of molecules of interest.150, 151 However, a systematic analysis seems to be lacking.
Speedy restitution of the epithelial cell barrier at patchy exudation sites of denudation
Small-sized areas of epithelial denudation are essential for speedy repair. At the loss of neighboring epithelium, all epithelial cell types bordering a denuded patch will promptly dedifferentiate into poorly differentiated flattened cells that cause epithelial regeneration (here called regeneration or regenerative cells). Already during dedifferentiation, cells that previously were fully differentiated into secretory and ciliated cells start to migrate. From all sides, such local regenerative cells move speedily in a tethered fashion over the denuded basement membrane (Fig 2).136 Thus a novel cell sheet consisting of flattened regenerated epithelium is created. The new epithelial cell barrier has reduced paracellular stretches available for passage of molecules, possibly explaining part of the reduced absorption demonstrated in patients with asthma and allergic rhinitis (see above). The speedy epithelial restitution requires an intact basement membrane. From its inception, it occurs in a dynamic, plasma-derived, gel-like milieu.135
A maintained airway mucosal barrier against environmental challenges at epithelial denudation avoids ever-escalating disease conditions
When shedding is intense and involves denudation, the situation is potentially the start of a spiral of extreme pathogenic consequences. If the barrier against hostile airspace factors is lost and not restored speedily, ever-increasing inflammation and harm to the airways would be expected to occur. The initial denudation challenge would make it so much easier for additional and more severe challenge-induced effects. In turn, more areas would be denuded and more airway tissues would be directly exposed to noxious mucosal surface material. Hence it is possible that plasma exudation and speedy barrier restitution mechanisms, such as those discussed above, have evolved to avoid a vicious circle.
A maintained airway barrier in conditions of denudation comes at a cost
The sustained plasma exudation response is associated with a range of intriguing effects
The first barrier created after epithelial denudation in vivo is the provisional gel cover produced and maintained by sustained plasma exudation. Especially in patients with severe asthma with extensive shedding, epithelial injury repair processes can contribute to the increased plasma exudation recorded in this condition. As discussed above, exaggerated plasma exudation alone might be of pathogenic importance in asthmatic patients because of its physical and biological effects. However, plasma exudation is not the only in vivo response to denudation (Fig 2). The epithelial regeneration process in vivo has evoked a range of additional effects that disseminate into the neighborhood of hot spot denudation patches. Such effects include accumulation and activation of granulocytes, increased mucus secretion, and increased proliferation of epithelial and fibroblast/smooth muscle cells.74, 136, 152, 153
Little support for involvement of epithelial-mesenchymal units
Dedifferentiated and migrating regeneration cells can be considered mesenchymal-like cells. However, they have not been seen to migrate across the plasma-covered basement membrane. Hence the idea of dissemination of epithelial-mesenchymal units into the lamina propria154 is not supported. Instead, the dedifferentiated cells have proliferated and eventually undergone redifferentiation to a normal epithelium.136 Pain et al154 have also underscored that evidence for occurrence of epithelial-mesenchymal units in asthmatic patients is lacking. Thus the increased numbers of fibrocytes in airways of asthmatic patients155, 156 might simply reflect proliferation of these cells occurring in response to signals emanating from inflammatory processes,154 including signals from sites of epithelial denudation regeneration sites.136
Exaggerated epithelial regeneration can contribute to the inflammatory and remodeling aspects of asthma
Recruitment and activation of neutrophils are prominent in the regeneration exudation area. Repeated denudation regeneration processes further result in reticular basement membrane thickening.152, 153 Eosinophil-derived proteins have been suggested to cause part of the epithelial shedding in asthmatic patients.157 Free eosinophil granules, which are known to be produced by eosinophil cytolysis and release of toxic proteins,69 are also intriguingly associated with epithelial cell loss in asthmatic patients.158 In patients with severe asthma, eosinophil cytolysis–derived free granules in sputum are a prominent indicator of the disease state.117 In guinea pig airways subjected to denudation, eosinophils already present in the area of interest are activated through primary cytolysis to release clusters of free eosinophil granules.74 Hence epithelial regeneration–induced eosinophil cytolysis might be part of a pathogenic circle in asthmatic patients.158
Exaggerated epithelial regeneration at both inception and severe stages of asthma
Notably, the asthma-like effects evoked, along with epithelial regeneration, have been produced in vivo also in airways in which no inflammation existed before epithelial denudation.136 Thus it is of interest that Creola bodies (clusters of desquamated epithelial cells) in aspirated sputum, independent of other factors, were reported to predict asthma development in wheezing infants.159 Creola bodies in sputum have also been significantly associated with asthma exacerbations in adults and children.11, 160 The occurrence of very large numbers of Creola bodies (up to 400 clusters in just 2 smears of sputum11) agrees with a pronounced patchiness of epithelial damage in asthma (see above).
The multiple effects of epithelial denudation regeneration alone (Fig 2) support the idea of its operational role both in early initiation of asthma and in patients with severe asthma with exacerbations. The possibility of a pathogenic role of this epithelial process has also been entertained in discussions of exercise-induced asthma.161
Furthermore, as demonstrated in guinea pigs in vivo, plasma exudation and epithelial regeneration responses to airway epithelial denudation have been refractory to steroid treatment.162 This observation is of interest because steroid treatment is not known to prevent asthma development, and in patients with severe asthma, plasma exudation,117 along with central disease features, can also be refractory in part to steroid treatment effects.163, 164
Therapeutic implications
Airway plasma exudation not only reflects an active inflammatory process but also is a significant feature of epithelial regeneration, and it has a role in first-line innate defense. Thus increased bronchial exudation of plasma in asthmatic patients might reflect a mixture of different types of mucosal events. This diversity has therapeutic implications.
The discovery that β-agonist bronchodilators have additional microvascular antipermeability actions substantiates the view that venular endothelial permeability is under physiologic and pharmacologic control.165 Furthermore, β-agonists simultaneously increased mucosal blood flow,165 which supports the view that mucosal blood flow is not a critical component of airway plasma exudation. A limited vascular antipermeability effect of inhaled β-agonists has been demonstrated in human healthy bronchi subjected to histamine challenge.166 However, whether these drugs reduce plasma exudation to any significant extent in asthmatic patients is not known.
Effective glucocorticoid treatment of asthma reduces plasma exudation117 and can reduce an exudate hyperresponsiveness.123 Reversibly, when the antiasthma treatment dose of steroids is tapered, plasma exudation is increased, and asthma is worsened.119, 120 The antiexudative actions of glucocorticoids seem to reflect a general anti-inflammatory efficacy of these drugs rather than direct effects on microvascular permeability. Supporting this view, a study in human nasal airways demonstrated that topical glucocorticoid treatment did not reduce histamine challenge–induced plasma exudation.167 These data suggest that glucocorticoid-treated airway mucosa remains vigilant when defense calls for a plasma exudation response. Also, topical glucocorticoid treatment inhibited neither the plasma exudation response nor the speedy epithelial regeneration seen after epithelial denudation in vivo in guinea pig trachea.162 Together, these in vivo observations tally with glucocorticoids sparing several innate immunity mechanisms, as reported by Schleimer.168 Desired features of an anti-inflammatory, antiasthma drug would include efficacy against pathogenic plasma exudation without inhibiting the involvement of this microvascular response when it is expressed as a component of mucosal defense and epithelial regeneration, respectively.
The idea that exaggerated epithelial regeneration qualifies as a causative component of asthma pathogenesis (Box 2 ) has therapeutic implications. As discussed above, there is a risk of ever-escalating disease conditions if epithelial regeneration is not a prompt and speedy process. A desired feature of future treatments would be to find means by which epithelial cell loss can be reduced rather than interfering with mechanisms associated with epithelial regeneration. This goal supports the importance of targeting airway epithelial cells in pharmacologic research on epithelial cytoprotection.
Box 2. Changing concepts of epithelial permeability and regeneration.
- The hitherto acknowledged permeability concept (largely based on observations in cell cultures and misrepresentation of in vivo data):
-
•Asthma is characterized by increased permeability to inhaled molecules.
-
•
- The current permeability concept (based on actual observations in vivo in patients)
-
•Asthma is characterized by unchanged or even decreased permeability to inhaled molecules.
-
•
Collateral evidence regarding plasma exudation and epithelial regeneration supporting and expanding “the present permeability concept”
- Epithelial features in vivo, as summarized below (I-IV), agree with a well-maintained barrier function in the airways that nevertheless exhibit epithelial shedding and/or plasma exudation.
-
I.Reflecting a pronounced asymmetry of the intact pseudostratified epithelium, increased airways exudation of nonsieved plasma macromolecules is not associated with increased permeability in the opposite inward direction. (Specific data were obtained in guinea pig and human airways.)
-
II.At asthma-like nonsanguineous denudation, a well-sustained, plasma exudation–derived active barrier involving a leukocyte-rich fibrin-fibronectin net promptly covers the naked basement membrane. (Specific data were obtained in guinea pig airways).
-
III.In the dynamic exudate gel cover, all types of epithelial cells bordering the denuded area promptly dedifferentiate into flattened epithelial regeneration cells that migrate speedily in a tethered fashion over the basement membrane. (Specific data were obtained in guinea pig airways.)
-
IV.At exposure to inhaled insults, which cause epithelial shedding in asthmatic patients, the ensuing denudation areas can be exceedingly patchy. This limitation is important because epithelial regeneration cells are produced all around individual denudation patches. Thus dedifferentiated epithelial cells will rapidly replace provisional covers of plasma exudate–derived gels in small-sized denuded spots. (Specific data were obtained in guinea pigs and with support from observations on patchy denudation in canine airways and in the setting of asthma).
-
I.
A novel concept of asthma pathogenesis (based on observations in vivo in asthmatic patients indicating that epithelial shedding is not associated with increased absorption permeability and on observations in vivo in guinea pigs indicating that epithelial regeneration processes alone produce neutrophil recruitment and activation, lytic activation of eosinophils, plasma exudation, increased mucus secretion, proliferation of fibrocyte/smooth muscle cells, and reticular basement membrane thickening).
Exaggerated epithelial regeneration at multiple denudation patches, rather than increased epithelial permeability, is pathogenic in asthmatic patients.
Limitations
Exploratory in vivo research often produces unexpected data. If not in accord with accepted notions, the observations are not always pursued vigorously; however, they can still be significant. This overview is partly based on in vivo data that disagree with popular research paradigms. Accordingly, the present attempt at understanding airway mucosal features has been little helped by the advanced cell biology research that supports currently leading notions. The ensuing limitation of the present discourse is underscored by its focus on events involving plasma exudation, which is almost exclusively an in vivo phenomenon. As indicated in this article, there is a need for further work. This need is also implicit based on the limited number of research groups involved in generation of some of the data that underpin the views presented here.
Conclusion
Independent observations in vivo in patients and guinea pigs underpin the notions discussed in this review. The notions are novel in the sense that they, more or less explicitly, challenge several current paradigms in airway and asthma research. Inferentially, changing concepts are introduced, including supplementary idea regarding the pathogenesis of asthma (Box 2).
Not long ago, increased “capillary permeability” and plasma extravasation were considered to reflect vascular injury. This view no longer prevails. Numerous elegant studies have revealed mechanisms of reversible formation of venular endothelial gaps leading to transient extravasation of nonsieved plasma macromolecules. This mode of extravasation occurs in the profuse airway subepithelial microcirculation in response to allergens and other significant inhalant challenges.
Here I have discussed the possibility that the further passage of extravasated nonsieved plasma across airway epithelial linings is also, in its basic shape, a noninjurious physiologic event. Without causing appreciable edema, extravasated plasma macromolecules move between yielding epithelial cells to appear locally on the mucosal surface at the very site of challenge. A slightly increased hydrostatic pressure affecting the basolateral aspects of epithelial cells seems causally involved in the paracellular epithelial transmission of extravasated plasma. Unless the challenge grows, this prompt local exudative response is of brief duration.
Despite the nonsieved nature of the microvascular-epithelial plasma exudation process, absorption of molecules deposited on the mucosal surface is not increased. The epithelial lining also remains structurally intact. These are important features of a first-line defense response. They can also explain in part why a disease such as asthma, which exhibits airway plasma exudation, might not display an abnormal inward perviousness.
At the time of epithelial shedding, diverse mechanisms are operating to maintain tightness and prevent undue penetration of mucosal surface material. Even when both columnar and basal epithelial cells are shed (denudation), the naked basement membrane will promptly receive a provisional barrier of exuded plasma. The activated plasma forms a gel-like structure involving a leukocyte-rich fibrin-fibronectin net covering the basement membrane. The gel remains and is continuously supplied by fresh plasma until a new cover of regenerated epithelial cells is established. In a dynamic plasma-derived molecular milieu, ciliated and secretory epithelial cells surrounding denuded spots dedifferentiate into flattened and rapidly migrating regeneration cells. Thus airways with patchy denudation sites are not without barriers or might only transiently exhibit a reduced absorption barrier. Barrier restoration is potentially prompt, even in patients with severe conditions with extensive but patchy denudation of the epithelium.
Prompt and speedy epithelial regeneration in patients with severe asthma counteracts the risk of severely escalating barrier breaks. However, epithelial regeneration processes also evoke disease-like effects. In experimental in vivo studies the mere process of denudation/regeneration on top of an uninjured basement membrane has caused plasma exudation, granulocyte recruitment/activation, mucus secretion, proliferation of fibroblast/smooth muscle cells, and thickening of the reticular basement membrane. Hence exaggerated epithelial regeneration occurring in plasma exudation hot spots seems worthy of consideration as a factor contributing not only to a speedy epithelial regeneration and a maintained absorption barrier but also to inception and worsening of asthma.
What is currently known?
-
•
The bronchial mucosa in vivo in asthmatic patients exhibits exudation of plasma and epithelial shedding, yet there is no increased absorption of inhaled molecules.
-
•The above 3 features can be reconciled thanks to additional in vivo observations:
-
—The asymmetry and plasticity of the epithelial lining allows outward passage of macromolecules without altering its tightness as a barrier toward molecules deposited on the mucosal surface. The epithelial transmission of nonsieved plasma macromolecules appears to be driven by a small subedematous increase in hydrostatic pressure transiently and noninjuriously affecting the basolateral aspect of the pseudostratified epithelial lining cells.
-
—Epithelial regeneration mechanisms after shedding operate promptly to restitute barrier functions in vivo. Even at denudation, the naked but intact basement membrane will be promptly covered, first by a plasma-derived fibrin-fibronectin net and with continuously by fresh plasma and leukocytes. This cover is a coarse physical and highly potent biological barrier. Furthermore, it provides a milieu in which all types of epithelial cells bordering the denuded area dedifferentiate into speedily migrating regeneration cells, which are tethered together. In the likely event of quite patchy denudation in vivo, a cellular barrier will thus speedily replace the provisional plasma-derived barrier, leaving little opportunity for increased absorption of inhaled molecules. However, the regeneration sites are also inflammatory and remodeling hotspots, suggesting the possibility that exaggerated epithelial regeneration at multiple denudation sites contributes to development and worsening of asthma.
-
—
What more do we need to know?
-
•
The basic features of airway plasma exudation indicate that it has roles in first-line defense on the surface of the intact mucosa in health and disease. Hence we need to know further details of the involved mechanisms and how their in vivo functions fare in comparison with other defense mechanisms operating at the mucosal surface, including mucus secretion and AMP production.
-
•
We need to define in detail several additional features of plasma exudation in asthma, as summed up by the following questions: To what extent does plasma exudation actually occur? Is there an exudative hyperresponsiveness to challenge with microvascular permeability agents? To what extent does exuded plasma contribute to airway plugs? What is the relative contribution of cell-derived versus plasma-derived proteins in asthmatic patients, including coagulation and complement molecules? On that note, is plasma exudation an “all-in” defense/disease mechanism or are there conditions when individual plasma protein systems are selectively activated?
-
•
Plasma exudation in response to asthma-like epithelial denudation is distinguished by means of sustained occurrence, providing both physical and biological protection. We need to know what plasma-derived molecules are essential for epithelial regeneration and whether any cell-derived molecule is also essential in this process in vivo. We need to know the extent of patchiness of epithelial denudation and how we can define such hot spots in asthmatic patients. How do inflammatory and remodeling responses evoked by epithelial regeneration events fare compared with other such mechanisms in vivo at asthma inception, asthma development, and asthma exacerbation?
Footnotes
Disclosure of potential conflict of interest: C. Persson declares that he has no relevant conflicts of interest.
References
- 1.Georas S.N., Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134:509–520. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elwood R.K., Kennedy S., Belzberg A., Hogg J.C., Pare P.D. Respiratory mucosal permeability in asthma. Am Rev Respir Dis. 1983;128:523–527. doi: 10.1164/arrd.1983.128.3.523. [DOI] [PubMed] [Google Scholar]
- 3.O'Byrne P.M., Dolovich M., Dirks R., Roberts R.S., Newhouse M.T. Lung epithelial permeability: relation to nonspecific airway responsiveness. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:77–84. doi: 10.1152/jappl.1984.57.1.77. [DOI] [PubMed] [Google Scholar]
- 4.Chan T.B. Pulmonary epithelial permeability in normal individuals and asthmatic patients. Ann Acad Med Singapore. 1985;14:462–464. [PubMed] [Google Scholar]
- 5.Del Donno M., Chetta A., Foresi A., Gavaruzzi G., Ugolotti G., Olivieri D. Lung epithelial permeability and bronchial responsiveness in subjects with stable asthma. Chest. 1997;111:1255–1260. doi: 10.1378/chest.111.5.1255. [DOI] [PubMed] [Google Scholar]
- 6.Persson C., Uller L. Epithelial perviousness in allergic airways disease. J Allergy Clin Immunol. 2017;140:1211. doi: 10.1016/j.jaci.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 7.Greiff L., Andersson M., Svensson C., Lundin S., Wollmer P., Persson C.G. Reduced airway absorption in seasonal allergic rhinitis. Am J Respir Crit Care Med. 1997;156:783–786. doi: 10.1164/ajrccm.156.3.9607013. [DOI] [PubMed] [Google Scholar]
- 8.Greiff L., Andersson M., Svensson J., Wollmer P., Lundin S., Persson C.G. Absorption across the nasal airway mucosa in house dust mite perennial allergic rhinitis. Clin Physiol Funct Imaging. 2002;22:55–57. [PubMed] [Google Scholar]
- 9.Kontou-Karakitsos K., Salvaggio J.E., Mathews K.P. Comparative nasal absorption of allergens in atopic and nonatopic subjects. J Allergy Clin Immunol. 1975;55:241–248. doi: 10.1016/0091-6749(75)90143-8. [DOI] [PubMed] [Google Scholar]
- 10.Greiff L., Wollmer P., Svensson C., Andersson M., Persson C.G. Effect of seasonal allergic rhinitis on airway mucosal absorption of chromium-51 labelled EDTA. Thorax. 1993;48:648–650. doi: 10.1136/thx.48.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naylor B. The shedding of the mucosa of the bronchial tree in asthma. Thorax. 1962;17:69–72. doi: 10.1136/thx.17.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montefort S., Djukanovic R., Holgate S.T., Roche W.R. Ciliated cell damage in the bronchial epithelium of asthmatics and non-asthmatics. Clin Exp Allergy. 1993;23:185–189. doi: 10.1111/j.1365-2222.1993.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 13.Cruz A.A., Naclerio R.M., Proud D., Togias A. Epithelial shedding is associated with nasal reactions to cold, dry air. J Allergy Clin Immunol. 2006;117:1351–1358. doi: 10.1016/j.jaci.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 14.Berger G., Bernheim J., Ophir D. Epithelial shedding of the inferior turbinate in perennial allergic and nonallergic rhinitis: a riddle to solve. Arch Otolaryngol Head Neck Surg. 2007;133:78–82. doi: 10.1001/archotol.133.1.78. [DOI] [PubMed] [Google Scholar]
- 15.Kicic A., Stevens P.T., Sutanto E.N., Kicic-Starcevich E., Ling K.M., Looi K. Impaired airway epithelial cell responses from children with asthma to rhinoviral infection. Clin Exp Allergy. 2016;46:1441–1455. doi: 10.1111/cea.12767. [DOI] [PubMed] [Google Scholar]
- 16.Sweerus K., Lachowicz-Scroggins M., Gordon E., LaFemina M., Huang X., Parikh M. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017;139:72–81.e1. doi: 10.1016/j.jaci.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loser S., Lloyd C.M., Gregory L.G. Reply. J Allergy Clin Immunol. 2017;140:1211–1212. doi: 10.1016/j.jaci.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 18.Dunnill M.S. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persson C.G. Centennial notions of asthma as an eosinophilic, desquamative, exudative, and steroid-sensitive disease. Lancet. 1997;350:1021–1024. doi: 10.1016/s0140-6736(96)02335-5. [DOI] [PubMed] [Google Scholar]
- 20.Persson C.G., Svensson C., Greiff L., Anderson M., Wollmer P., Alkner U. The use of the nose to study the inflammatory response of the respiratory tract. Thorax. 1992;47:993–1000. doi: 10.1136/thx.47.12.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis J.L., Freeman C.M., Hogg J.C. The immunopathogenesis of chronic obstructive pulmonary disease: insights from recent research. Proc Am Thorac Soc. 2007;4:512–521. doi: 10.1513/pats.200701-002FM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schleimer R.P., Berdnikovs S. Etiology of epithelial barrier dysfunction in patients with type 2 inflammatory diseases. J Allergy Clin Immunol. 2017;139:1752–1761. doi: 10.1016/j.jaci.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomazic P.V., Birner-Gruenberger R., Leitner A., Obrist B., Spoerk S., Lang-Loidolt D. Nasal mucus proteomic changes reflect altered immune responses and epithelial permeability in patients with allergic rhinitis. J Allergy Clin Immunol. 2014;133:741–750. doi: 10.1016/j.jaci.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 24.Erjefalt I., Persson C.G. Inflammatory passage of plasma macromolecules into airway wall and lumen. Pulm Pharmacol. 1989;2:93–102. doi: 10.1016/0952-0600(89)90030-6. [DOI] [PubMed] [Google Scholar]
- 25.Svensson C., Baumgarten C.R., Pipkorn U., Alkner U., Persson C.G. Reversibility and reproducibility of histamine induced plasma leakage in nasal airways. Thorax. 1989;44:13–18. doi: 10.1136/thx.44.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greiff L., Andersson M., Akerlund A., Wollmer P., Svensson C., Alkner U. Microvascular exudative hyperresponsiveness in human coronavirus-induced common cold. Thorax. 1994;49:121–127. doi: 10.1136/thx.49.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erjefalt I., Greiff L., Alkner U., Persson C.G. Allergen-induced biphasic plasma exudation responses in guinea pig large airways. Am Rev Respir Dis. 1993;148:695–701. doi: 10.1164/ajrccm/148.3.695. [DOI] [PubMed] [Google Scholar]
- 28.Halldorsdottir H., Greiff L., Wollmer P., Andersson M., Svensson C., Alkner U. Effects of inhaled histamine, methacholine and capsaicin on sputum levels of alpha 2-macroglobulin. Thorax. 1997;52:964–968. doi: 10.1136/thx.52.11.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persson C.G., Erjefalt I., Andersson P. Leakage of macromolecules from guinea-pig tracheobronchial microcirculation. Effects of allergen, leukotrienes, tachykinins, and anti-asthma drugs. Acta Physiol Scand. 1986;127:95–105. doi: 10.1111/j.1748-1716.1986.tb07880.x. [DOI] [PubMed] [Google Scholar]
- 30.Greiff L., Andersson M., Svensson C., Persson C.G. Topical azelastine has a 12-hour duration of action as assessed by histamine challenge-induced exudation of alpha 2-macroglobulin into human nasal airways. Clin Exp Allergy. 1997;27:438–444. [PubMed] [Google Scholar]
- 31.Greiff L., Andersson M., Erjefalt J.S., Svensson C., Persson C.G. Loss of size-selectivity at histamine-induced exudation of plasma proteins in atopic nasal airways. Clin Physiol Funct Imaging. 2002;22:28–31. doi: 10.1046/j.1475-097x.2002.00390.x. [DOI] [PubMed] [Google Scholar]
- 32.Out T.A., van de Graaf E.A., Jansen H.M. Permeability or local production of immunoglobulins and other inflammatory proteins in asthma. Eur Respir J Suppl. 1991;13:148s–155s. [PubMed] [Google Scholar]
- 33.Grega G.J., Adamski S.W. The role of venular endothelial cells in the regulation of macromolecular permeability. Microcirc Endothelium Lymphatics. 1988;4:143–167. [PubMed] [Google Scholar]
- 34.Svensjo E., Arfors K.E., Raymond R.M., Grega G.J. Morphological and physiological correlation of bradykinin-induced macromolecular efflux. Am J Physiol Heart Circ Physiol. 1979;236:H600–H606. doi: 10.1152/ajpheart.1979.236.4.H600. [DOI] [PubMed] [Google Scholar]
- 35.Persson C.G. Role of plasma exudation in asthmatic airways. Lancet. 1986;2:1126–1129. doi: 10.1016/s0140-6736(86)90533-7. [DOI] [PubMed] [Google Scholar]
- 36.Persson C.G. Plasma exudation and asthma. Lung. 1988;166:1–23. doi: 10.1007/BF02714025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung K.F., Rogers D.F., Barnes P.J., Evans T.W. The role of increased airway microvascular permeability and plasma exudation in asthma. Eur Respir J. 1990;3:329–337. [PubMed] [Google Scholar]
- 38.Svensson C., Alkner U., Pipkorn U., Persson C.G. Histamine-induced airway mucosal exudation of bulk plasma and plasma-derived mediators is not inhibited by intravenous bronchodilators. Eur J Clin Pharmacol. 1994;46:59–65. doi: 10.1007/BF00195917. [DOI] [PubMed] [Google Scholar]
- 39.Nocker R.E., Out T.A., Weller F.R., de Riemer M.J., Jansen H.M., van der Zee J.S. Induced sputum and bronchoalveolar lavage as tools for evaluating the effects of inhaled corticosteroids in patients with asthma. J Lab Clin Med. 2000;136:39–49. doi: 10.1067/mlc.2000.107305. [DOI] [PubMed] [Google Scholar]
- 40.Van Rensen E.L., Hiemstra P.S., Rabe K.F., Sterk P.J. Assessment of microvascular leakage via sputum induction: the role of substance P and neurokinin A in patients with asthma. Am J Respir Crit Care Med. 2002;165:1275–1279. doi: 10.1164/rccm.2110092. [DOI] [PubMed] [Google Scholar]
- 41.Greiff L., Andersson M., Svensson C., Akerlund A., Alkner U., Persson C.G. Effects of two weeks of topical budesonide treatment on microvascular exudative responsiveness in healthy human nasal airways. Eur Respir J. 1997;10:841–845. [PubMed] [Google Scholar]
- 42.Brims F.J., Chauhan A.J., Higgins B., Shute J.K. Coagulation factors in the airways in moderate and severe asthma and the effect of inhaled steroids. Thorax. 2009;64:1037–1043. doi: 10.1136/thx.2009.114439. [DOI] [PubMed] [Google Scholar]
- 43.Greiff L., Andersson M., Svensson C., Nilsson M., Erjefalt I., Erjefalt J.S. Topical nitroprusside may reduce histamine-induced plasma exudation in human nasal airways. Allergy. 1995;50:593–597. doi: 10.1111/j.1398-9995.1995.tb01205.x. [DOI] [PubMed] [Google Scholar]
- 44.Van de Graaf E.A., Out T.A., Roos C.M., Jansen H.M. Respiratory membrane permeability and bronchial hyperreactivity in patients with stable asthma. Effects of therapy with inhaled steroids. Am Rev Respir Dis. 1991;143:362–368. doi: 10.1164/ajrccm/143.2.362. [DOI] [PubMed] [Google Scholar]
- 45.Pizzichini M.M., Pizzichini E., Efthimiadis A., Chauhan A.J., Johnston S.L., Hussack P. Asthma and natural colds. Inflammatory indices in induced sputum: a feasibility study. Am J Respir Crit Care Med. 1998;158:1178–1184. doi: 10.1164/ajrccm.158.4.9712082. [DOI] [PubMed] [Google Scholar]
- 46.Luts A., Sundler F., Erjefalt I., Persson C.G. The airway epithelial lining in guinea pigs is intact promptly after the mucosal crossing of a large amount of plasma exudate. Int Arch Allergy Appl Immunol. 1990;91:385–388. doi: 10.1159/000235146. [DOI] [PubMed] [Google Scholar]
- 47.Rogers D.F., Alton E.W., Aursudkij B., Boschetto P., Dewar A., Barnes P.J. Effect of platelet activating factor on formation and composition of airway fluid in the guinea-pig trachea. J Physiol. 1990;431:643–658. doi: 10.1113/jphysiol.1990.sp018352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erjefalt J.S., Erjefalt I., Sundler F., Persson C.G. Epithelial pathways for luminal entry of bulk plasma. Clin Exp Allergy. 1995;25:187–195. doi: 10.1111/j.1365-2222.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 49.Serikov V.B., Jang Y.J., Widdicombe J.H. Estimate of the subepithelial hydrostatic pressure that drives inflammatory transudate into the airway lumen. J Appl Physiol (1985) 2002;92:1702–1708. doi: 10.1152/japplphysiol.00645.2001. [DOI] [PubMed] [Google Scholar]
- 50.Olivenstein R., Du T., Xu L.J., Martin J.G. Microvascular leakage in the airway wall and lumen during allergen induced early and late responses in rats. Pulm Pharmacol Ther. 1997;10:223–230. doi: 10.1006/pupt.1997.0095. [DOI] [PubMed] [Google Scholar]
- 51.Wagner E.M., Jenkins J., Schmieder A., Eldridge L., Zhang Q., Moldobaeva A. Angiogenesis and airway reactivity in asthmatic Brown Norway rats. Angiogenesis. 2015;18:1–11. doi: 10.1007/s10456-014-9441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erjefalt I., Luts A., Persson C.G. Appearance of airway absorption and exudation tracers in guinea pig tracheobronchial lymph nodes. J Appl Physiol (1985) 1993;74:817–824. doi: 10.1152/jappl.1993.74.2.817. [DOI] [PubMed] [Google Scholar]
- 53.Du T., Xu L.J., Lei M., Wang N.S., Eidelman D.H., Ghezzo H. Morphometric changes during the early airway response to allergen challenge in the rat. Am Rev Respir Dis. 1992;146:1037–1041. doi: 10.1164/ajrccm/146.4.1037. [DOI] [PubMed] [Google Scholar]
- 54.Malmstrom K., Lohi J., Sajantila A., Jahnsen F.L., Kajosaari M., Sarna S. Immunohistology and remodeling in fatal pediatric and adolescent asthma. Respir Res. 2017;18:94. doi: 10.1186/s12931-017-0575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers D.F. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol. 2004;4:241–250. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Greiff L., Svensson C., Andersson M., Persson C.G. Effects of topical capsaicin in seasonal allergic rhinitis. Thorax. 1995;50:225–229. doi: 10.1136/thx.50.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaulbach H.C., White M.V., Igarashi Y., Hahn B.K., Kaliner M.A. Estimation of nasal epithelial lining fluid using urea as a marker. J Allergy Clin Immunol. 1993;92:457–465. doi: 10.1016/0091-6749(93)90125-y. [DOI] [PubMed] [Google Scholar]
- 58.Germonpre P.R., Joos G.F., Pauwels R.A. Characterization of the neurogenic plasma extravasation in the airways. Arch Int Pharmacodyn Ther. 1995;329:185–203. [PubMed] [Google Scholar]
- 59.Midgren B., Hansson L., Karlsson J.A., Simonsson B.G., Persson C.G. Capsaicin-induced cough in humans. Am Rev Respir Dis. 1992;146:347–351. doi: 10.1164/ajrccm/146.2.347. [DOI] [PubMed] [Google Scholar]
- 60.Erjefalt I., Persson C.G. Allergen, bradykinin, and capsaicin increase outward but not inward macromolecular permeability of guinea-pig tracheobronchial mucosa. Clin Exp Allergy. 1991;21:217–224. doi: 10.1111/j.1365-2222.1991.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 61.Greiff L., Wollmer P., Pipkorn U., Persson C.G. Absorption of 51Cr EDTA across the human nasal airway barriers in the presence of topical histamine. Thorax. 1991;46:630–632. doi: 10.1136/thx.46.9.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gustafsson B., Persson C.G. Allergen-induced mucosal exudation of plasma into rat ileum and its inhibition by budesonide. Scand J Gastroenterol. 1992;27:587–593. doi: 10.3109/00365529209000123. [DOI] [PubMed] [Google Scholar]
- 63.Persson C.G., Gustafsson B., Erjefalt J.S., Sundler F. Mucosal exudation of plasma is a noninjurious intestinal defense mechanism. Allergy. 1993;48:581–586. doi: 10.1111/j.1398-9995.1993.tb00752.x. [DOI] [PubMed] [Google Scholar]
- 64.McHugh K.J., Svensjo E., Persson C.G. Exudative and absorptive permeability in different phases of an experimental colitis condition. Scand J Gastroenterol. 1996;31:900–905. doi: 10.3109/00365529609051999. [DOI] [PubMed] [Google Scholar]
- 65.Persson C., Uller L. Transepithelial exit of leucocytes: inflicting, reflecting or resolving airway inflammation? Thorax. 2010;65:1111–1115. doi: 10.1136/thx.2009.133363. [DOI] [PubMed] [Google Scholar]
- 66.Persson C., Ueki S. Lytic eosinophils produce extracellular DNA traps as well as free eosinophil granules. J Allergy Clin Immunol. 2017;141:1164. doi: 10.1016/j.jaci.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 67.Persson C. Eosinophil apoptosis-inducing drugs risk worsening rather than resolving asthma. J Allergy Clin Immunol. 2015;135:1662. doi: 10.1016/j.jaci.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 68.Bochner B.S., Kiwamoto T., Katoh T., Zhu Z., Tiemeyer M. Reply. J Allergy Clin Immunol. 2015;135:1662–1663. doi: 10.1016/j.jaci.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 69.Persson C., Uller L. Theirs but to die and do: primary lysis of eosinophils and free eosinophil granules in asthma. Am J Respir Crit Care Med. 2014;189:628–633. doi: 10.1164/rccm.201311-2069OE. [DOI] [PubMed] [Google Scholar]
- 70.Persson C., Uller L. Resolution of leucocyte-mediated mucosal diseases. A novel in vivo paradigm for drug development. Br J Pharmacol. 2012;165:2100–2109. doi: 10.1111/j.1476-5381.2011.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu L., Mul F.P., Kuijpers T.W., Lutter R., Roos D., Knol E.F. Neutrophil transmigration across monolayers of endothelial cells and airway epithelial cells is regulated by different mechanisms. Ann N Y Acad Sci. 1996;796:21–29. doi: 10.1111/j.1749-6632.1996.tb32563.x. [DOI] [PubMed] [Google Scholar]
- 72.Adcock I.M., Caramori G. Chemokine receptor inhibitors as a novel option in treatment of asthma. Curr Drug Targets Inflamm Allergy. 2004;3:257–261. doi: 10.2174/1568010043343660. [DOI] [PubMed] [Google Scholar]
- 73.Nourshargh S., Hordijk P.L., Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 74.Erjefalt J.S., Sundler F., Persson C.G. Eosinophils, neutrophils, and venular gaps in the airway mucosa at epithelial removal-restitution. Am J Respir Crit Care Med. 1996;153:1666–1674. doi: 10.1164/ajrccm.153.5.8630618. [DOI] [PubMed] [Google Scholar]
- 75.Baluk P., Bolton P., Hirata A., Thurston G., McDonald D.M. Endothelial gaps and adherent leukocytes in allergen-induced early- and late-phase plasma leakage in rat airways. Am J Pathol. 1998;152:1463–1476. [PMC free article] [PubMed] [Google Scholar]
- 76.McDonald D.M. Endothelial gaps and permeability of venules in rat tracheas exposed to inflammatory stimuli. Am J Physiol. 1994;266:L61–L83. doi: 10.1152/ajplung.1994.266.1.L61. [DOI] [PubMed] [Google Scholar]
- 77.O'Donnell S.R., Erjefalt I., Persson C.G. Early and late tracheobronchial plasma exudation by platelet-activating factor administered to the airway mucosal surface in guinea pigs: effects of WEB 2086 and enprofylline. J Pharmacol Exp Ther. 1990;254:65–70. [PubMed] [Google Scholar]
- 78.Imamura T., Tanase S., Szmyd G., Kozik A., Travis J., Potempa J. Induction of vascular leakage through release of bradykinin and a novel kinin by cysteine proteinases from Staphylococcus aureus. J Exp Med. 2005;201:1669–1676. doi: 10.1084/jem.20042041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cromwell O., Hamid Q., Corrigan C.J., Barkans J., Meng Q., Collins P.D. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology. 1992;77:330–337. [PMC free article] [PubMed] [Google Scholar]
- 80.Erjefalt J.S., Erjefalt I., Sundler F., Persson C.G. Mucosal nitric oxide may tonically suppress airways plasma exudation. Am J Respir Crit Care Med. 1994;150:227–232. doi: 10.1164/ajrccm.150.1.8025753. [DOI] [PubMed] [Google Scholar]
- 81.Blom A.M., Hallstrom T., Riesbeck K. Complement evasion strategies of pathogens-acquisition of inhibitors and beyond. Mol Immunol. 2009;46:2808–2817. doi: 10.1016/j.molimm.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 82.Egesten A., Frick I.M., Morgelin M., Olin A.I., Bjorck L. Binding of albumin promotes bacterial survival at the epithelial surface. J Biol Chem. 2011;286:2469–2476. doi: 10.1074/jbc.M110.148171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Wetering S., Sterk P.J., Rabe K.F., Hiemstra P.S. Defensins: key players or bystanders in infection, injury, and repair in the lung? J Allergy Clin Immunol. 1999;104:1131–1138. doi: 10.1016/s0091-6749(99)70004-7. [DOI] [PubMed] [Google Scholar]
- 84.Lecaille F., Lalmanach G., Andrault P.M. Antimicrobial proteins and peptides in human lung diseases: a friend and foe partnership with host proteases. Biochimie. 2016;122:151–168. doi: 10.1016/j.biochi.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 85.Akerlund A., Greiff L., Andersson M., Bende M., Alkner U., Persson C.G. Mucosal exudation of fibrinogen in coronavirus-induced common colds. Acta Otolaryngol. 1993;113:642–648. doi: 10.3109/00016489309135878. [DOI] [PubMed] [Google Scholar]
- 86.Uller L., Persson C. Viral induced overproduction of epithelial TSLP: role in exacerbations of asthma and COPD? J Allergy Clin Immunol. 2018;142:712. doi: 10.1016/j.jaci.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 87.Oehmcke-Hecht S., Kohler J. Interaction of the human contact system with pathogens—an update. Front Immunol. 2018;9:312. doi: 10.3389/fimmu.2018.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khakpour S., Wilhelmsen K., Hellman J. Vascular endothelial cell Toll-like receptor pathways in sepsis. Innate Immun. 2015;21:827–846. doi: 10.1177/1753425915606525. [DOI] [PubMed] [Google Scholar]
- 89.Persson C.G., Erjefalt I., Gustafsson B., Luts A. Subepithelial hydrostatic pressure may regulate plasma exudation across the mucosa. Int Arch Allergy Appl Immunol. 1990;92:148–153. doi: 10.1159/000235206. [DOI] [PubMed] [Google Scholar]
- 90.Gustafsson B.G., Persson C.G. Asymmetrical effects of increases in hydrostatic pressure on macromolecular movement across the airway mucosa. A study in guinea-pig tracheal tube preparations. Clin Exp Allergy. 1991;21:121–126. doi: 10.1111/j.1365-2222.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 91.Persson C.G., Erjefalt J.S., Greiff L., Andersson M., Erjefalt I., Godfrey R.W. Plasma-derived proteins in airway defence, disease and repair of epithelial injury. Eur Respir J. 1998;11:958–970. doi: 10.1183/09031936.98.11040958. [DOI] [PubMed] [Google Scholar]
- 92.Intaglietta M. Microvascular pressure measurements by cannulation: independency of concentration gradients and deviations in micro-servonulling. Microvasc Res. 1971;3:396–399. doi: 10.1016/0026-2862(71)90041-0. [DOI] [PubMed] [Google Scholar]
- 93.Tokuda S., Miyazaki H., Nakajima K., Yamada T., Marunaka Y. Hydrostatic pressure regulates tight junctions, actin cytoskeleton and transcellular ion transport. Biochem Biophys Res Commun. 2009;390:1315–1321. doi: 10.1016/j.bbrc.2009.10.144. [DOI] [PubMed] [Google Scholar]
- 94.Lemarchand P., Chinet T., Collignon M.A., Urzua G., Barritault L., Huchon G.J. Bronchial clearance of DTPA is increased in acute asthma but not in chronic asthma. Am Rev Respir Dis. 1992;145:147–152. doi: 10.1164/ajrccm/145.1.147. [DOI] [PubMed] [Google Scholar]
- 95.Steelant B., Farre R., Wawrzyniak P., Belmans J., Dekimpe E., Vanheel H. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. 2016;137:1043–1053.e5. doi: 10.1016/j.jaci.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 96.Lakind J.S., Holgate S.T., Ownby D.R., Mansur A.H., Helms P.J., Pyatt D. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers. 2007;12:445–467. doi: 10.1080/13547500701359327. [DOI] [PubMed] [Google Scholar]
- 97.Stenberg H., Wadelius E., Moitra S., Aberg I., Ankerst J., Diamant Z. Club cell protein (CC16) in plasma, bronchial brushes, BAL and urine following an inhaled allergen challenge in allergic asthmatics. Biomarkers. 2018;23:51–60. doi: 10.1080/1354750X.2017.1375559. [DOI] [PubMed] [Google Scholar]
- 98.Emmanouil P., Loukides S., Kostikas K., Papatheodorou G., Papaporfyriou A., Hillas G. Sputum and BAL Clara cell secretory protein and surfactant protein D levels in asthma. Allergy. 2015;70:711–714. doi: 10.1111/all.12603. [DOI] [PubMed] [Google Scholar]
- 99.Mackay R.M., Grainge C.L., Lau L.C., Barber C., Clark H.W., Howarth P.H. Airway surfactant protein D deficiency in adults with severe asthma. Chest. 2016;149:1165–1172. doi: 10.1016/j.chest.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salomonsson P., Gronneberg R., Gilljam H., Andersson O., Billing B., Enander I. Bronchial exudation of bulk plasma at allergen challenge in allergic asthma. Am Rev Respir Dis. 1992;146:1535–1542. doi: 10.1164/ajrccm/146.6.1535. [DOI] [PubMed] [Google Scholar]
- 101.Andersson M., Michel L., Llull J.B., Pipkorn U. Complement activation on the nasal mucosal surface—a feature of the immediate allergic reaction in the nose. Allergy. 1994;49:242–245. doi: 10.1111/j.1398-9995.1994.tb02656.x. [DOI] [PubMed] [Google Scholar]
- 102.Svensson C., Gronneberg R., Andersson M., Alkner U., Andersson O., Billing B. Allergen challenge-induced entry of alpha 2-macroglobulin and tryptase into human nasal and bronchial airways. J Allergy Clin Immunol. 1995;96:239–246. doi: 10.1016/s0091-6749(95)70013-7. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y., Miwa T., Ducka-Kokalari B., Redai I.G., Sato S., Gullipalli D. Properdin contributes to allergic airway inflammation through local C3a generation. J Immunol. 2015;195:1171–1181. doi: 10.4049/jimmunol.1401819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krug N., Tschernig T., Erpenbeck V.J., Hohlfeld J.M., Kohl J. Complement factors C3a and C5a are increased in bronchoalveolar lavage fluid after segmental allergen provocation in subjects with asthma. Am J Respir Crit Care Med. 2001;164:1841–1843. doi: 10.1164/ajrccm.164.10.2010096. [DOI] [PubMed] [Google Scholar]
- 105.Lopuhaa C.E., de Riemer M.J., Out T.A., Sjamsoedin D.H., Aalberse R.C., Jansen H.M. Comparison of allergen-induced late inflammatory reactions in the nose and in the skin in house dust mite-allergic patients with or without asthma. Int Arch Allergy Immunol. 2003;130:266–274. doi: 10.1159/000070213. [DOI] [PubMed] [Google Scholar]
- 106.Elovsson S., Smailagic A., Erjefalt I., Zackrisson C., Eriksson C., Wang X. Evaluation of nasal barrier dysfunction at acute- and late-phase reactions in a guinea pig model of allergic rhinitis. Vascul Pharmacol. 2005;43:267–276. doi: 10.1016/j.vph.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 107.Thwaites R.S., Gunawardana N.C., Broich V., Mann E.H., Ahnstrom J., Campbell G.A. Biphasic activation of complement and fibrinolysis during the human nasal allergic response. J Allergy Clin Immunol. 2018;141:1892–1895.e6. doi: 10.1016/j.jaci.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wills-Karp M. Complement activation pathways: a bridge between innate and adaptive immune responses in asthma. Proc Am Thorac Soc. 2007;4:247–251. doi: 10.1513/pats.200704-046AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Boer J.D., Majoor C.J., van 't Veer C., Bel E.H., van der Poll T. Asthma and coagulation. Blood. 2012;119:3236–3244. doi: 10.1182/blood-2011-11-391532. [DOI] [PubMed] [Google Scholar]
- 110.Meerschaert J., Kelly E.A., Mosher D.F., Busse W.W., Jarjour N.N. Segmental antigen challenge increases fibronectin in bronchoalveolar lavage fluid. Am J Respir Crit Care Med. 1999;159:619–625. doi: 10.1164/ajrccm.159.2.9806053. [DOI] [PubMed] [Google Scholar]
- 111.Becky Kelly E.A., Busse W.W., Jarjour N.N. A comparison of the airway response to segmental antigen bronchoprovocation in atopic asthma and allergic rhinitis. J Allergy Clin Immunol. 2003;111:79–86. doi: 10.1067/mai.2003.28. [DOI] [PubMed] [Google Scholar]
- 112.Terada M., Kelly E.A., Jarjour N.N. Increased thrombin activity after allergen challenge: a potential link to airway remodeling? Am J Respir Crit Care Med. 2004;169:373–377. doi: 10.1164/rccm.200308-1156OC. [DOI] [PubMed] [Google Scholar]
- 113.Greiff L., Pipkorn U., Alkner U., Persson C.G. The 'nasal pool' device applies controlled concentrations of solutes on human nasal airway mucosa and samples its surface exudations/secretions. Clin Exp Allergy. 1990;20:253–259. doi: 10.1111/j.1365-2222.1990.tb02680.x. [DOI] [PubMed] [Google Scholar]
- 114.Esnault S., Kelly E.A., Sorkness R.L., Evans M.D., Busse W.W., Jarjour N.N. Airway factor XIII associates with type 2 inflammation and airway obstruction in asthmatic patients. J Allergy Clin Immunol. 2016;137:767–773.e6. doi: 10.1016/j.jaci.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Greiff L., Andersson M., Svensson C., Linden M., Wollmer P., Persson C.G. Demonstration of bronchial eosinophil activity in seasonal allergic rhinitis by induced plasma exudation combined with induced sputum. Thorax. 1999;54:33–36. doi: 10.1136/thx.54.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schoonbrood D.F., Lutter R., Habets F.J., Roos C.M., Jansen H.M., Out T.A. Analysis of plasma-protein leakage and local secretion in sputum from patients with asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150:1519–1527. doi: 10.1164/ajrccm.150.6.7952610. [DOI] [PubMed] [Google Scholar]
- 117.Pizzichini M.M., Pizzichini E., Clelland L., Efthimiadis A., Mahony J., Dolovich J. Sputum in severe exacerbations of asthma: kinetics of inflammatory indices after prednisone treatment. Am J Respir Crit Care Med. 1997;155:1501–1508. doi: 10.1164/ajrccm.155.5.9154849. [DOI] [PubMed] [Google Scholar]
- 118.Desai D., Gupta S., Siddiqui S., Singapuri A., Monteiro W., Entwisle J. Sputum mediator profiling and relationship to airway wall geometry imaging in severe asthma. Respir Res. 2013;14:17. doi: 10.1186/1465-9921-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Persson C., Uller L. Roles of plasma exudation in asthma and COPD. Clin Exp Allergy. 2009;39:1626–1629. doi: 10.1111/j.1365-2222.2009.03373.x. [DOI] [PubMed] [Google Scholar]
- 120.Khor Y.H., Teoh A.K., Lam S.M., Mo D.C., Weston S., Reid D.W. Increased vascular permeability precedes cellular inflammation as asthma control deteriorates. Clin Exp Allergy. 2009;39:1659–1667. doi: 10.1111/j.1365-2222.2009.03349.x. [DOI] [PubMed] [Google Scholar]
- 121.Erjefalt I., Persson C.G. Increased sensitivity to toluene diisocyanate (TDI) in airways previously exposed to low doses of TDI. Clin Exp Allergy. 1992;22:854–862. doi: 10.1111/j.1365-2222.1992.tb02831.x. [DOI] [PubMed] [Google Scholar]
- 122.Svensson C., Andersson M., Greiff L., Alkner U., Persson C.G. Exudative hyperresponsiveness of the airway microcirculation in seasonal allergic rhinitis. Clin Exp Allergy. 1995;25:942–950. doi: 10.1111/j.1365-2222.1995.tb00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meyer P., Andersson M., Persson C.G., Greiff L. Steroid-sensitive indices of airway inflammation in children with seasonal allergic rhinitis. Pediatr Allergy Immunol. 2003;14:60–65. doi: 10.1034/j.1399-3038.2003.02102.x. [DOI] [PubMed] [Google Scholar]
- 124.Minnicozzi M., Duran W.N., Gleich G.J., Egan R.W. Eosinophil granule proteins increase microvascular macromolecular transport in the hamster cheek pouch. J Immunol. 1994;153:2664–2670. [PubMed] [Google Scholar]
- 125.Widegren H., Korsgren M., Andersson M., Greiff L. Effects of TNFalpha on the human nasal mucosa in vivo. Respir Med. 2007;101:1982–1987. doi: 10.1016/j.rmed.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 126.Hutt G., Wick H. Contraction of circular muscle, thickness of inner lining and their mutual interference in the constriction of tubular transverse sectionsPflugers Arch Gesamte Physiol Menschen Tiere. 1954;258:311–314. doi: 10.1007/BF00363639. [DOI] [PubMed] [Google Scholar]
- 127.Aitken M.L., Verdugo P. Donnan mechanism of mucin release and conditioning in goblet cells: the role of polyions. Symp Soc Exp Biol. 1989;43:73–80. [PubMed] [Google Scholar]
- 128.Innes A.L., Carrington S.D., Thornton D.J., Kirkham S., Rousseau K., Dougherty R.H. Ex vivo sputum analysis reveals impairment of protease-dependent mucus degradation by plasma proteins in acute asthma. Am J Respir Crit Care Med. 2009;180:203–210. doi: 10.1164/rccm.200807-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sturton G., Persson C., Barnes P.J. Small airways: an important but neglected target in the treatment of obstructive airway diseases. Trends Pharmacol Sci. 2008;29:340–345. doi: 10.1016/j.tips.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 130.Proud D. The kinin system in rhinitis and asthma. Clin Rev Allergy Immunol. 1998;16:351–364. doi: 10.1007/BF02737656. [DOI] [PubMed] [Google Scholar]
- 131.Naylor B., Railey C. A pitfall in the cytodiagnosis of sputum of asthmatics. J Clin Pathol. 1964;17:84–89. doi: 10.1136/jcp.17.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Montefort S., Roberts J.A., Beasley R., Holgate S.T., Roche W.R. The site of disruption of the bronchial epithelium in asthmatic and non-asthmatic subjects. Thorax. 1992;47:499–503. doi: 10.1136/thx.47.7.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Erjefalt J.S., Sundler F., Persson C.G. Epithelial barrier formation by airway basal cells. Thorax. 1997;52:213–217. doi: 10.1136/thx.52.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Erjefalt I.A., Wagner Z.G., Strand S.E., Persson C.G. A method for studies of tracheobronchial microvascular permeability to macromolecules. J Pharmacol Methods. 1985;14:275–283. doi: 10.1016/0160-5402(85)90003-8. [DOI] [PubMed] [Google Scholar]
- 135.Erjefalt J.S., Erjefalt I., Sundler F., Persson C.G. Microcirculation-derived factors in airway epithelial repair in vivo. Microvasc Res. 1994;48:161–178. doi: 10.1006/mvre.1994.1047. [DOI] [PubMed] [Google Scholar]
- 136.Erjefalt J.S., Erjefalt I., Sundler F., Persson C.G. In vivo restitution of airway epithelium. Cell Tissue Res. 1995;281:305–316. doi: 10.1007/BF00583399. [DOI] [PubMed] [Google Scholar]
- 137.Delvaeye M., Conway E.M. Coagulation and innate immune responses: can we view them separately? Blood. 2009;114:2367–2374. doi: 10.1182/blood-2009-05-199208. [DOI] [PubMed] [Google Scholar]
- 138.Mosesson M.W. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 139.Johansson M.W., Annis D.S., Mosher D.F. alpha(M)beta(2) integrin-mediated adhesion and motility of IL-5-stimulated eosinophils on periostin. Am J Respir Cell Mol Biol. 2013;48:503–510. doi: 10.1165/rcmb.2012-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Plotkowski M.C., Zahm J.M., Tournier J.M., Puchelle E. Pseudomonas aeruginosa adhesion to normal and injured respiratory mucosa. Mem Inst Oswaldo Cruz. 1992;87(suppl 5):61–68. doi: 10.1590/s0074-02761992000900008. [DOI] [PubMed] [Google Scholar]
- 141.Jakiela B., Brockman-Schneider R., Amineva S., Lee W.M., Gern J.E. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cyranoski D. Surgeon commits misconduct. Nature. 2015;521:406–407. doi: 10.1038/nature.2015.17605. [DOI] [PubMed] [Google Scholar]
- 143.Erjefalt J.S., Korsgren M., Nilsson M.C., Sundler F., Persson C.G. Association between inflammation and epithelial damage-restitution processes in allergic airways in vivo. Clin Exp Allergy. 1997;27:1344–1355. [PubMed] [Google Scholar]
- 144.Erjefalt J.S., Korsgren M., Nilsson M.C., Sundler F., Persson C.G. Prompt epithelial damage and restitution processes in allergen challenged guinea-pig trachea in vivo. Clin Exp Allergy. 1997;27:1458–1470. doi: 10.1046/j.1365-2222.1997.1200932.x. [DOI] [PubMed] [Google Scholar]
- 145.Ordonez C., Ferrando R., Hyde D.M., Wong H.H., Fahy J.V. Epithelial desquamation in asthma: artifact or pathology? Am J Respir Crit Care Med. 2000;162:2324–2329. doi: 10.1164/ajrccm.162.6.2001041. [DOI] [PubMed] [Google Scholar]
- 146.Davis M.S., Schofield B., Freed A.N. Repeated peripheral airway hyperpnea causes inflammation and remodeling in dogs. Med Sci Sports Exerc. 2003;35:608–616. doi: 10.1249/01.MSS.0000058660.88987.A0. [DOI] [PubMed] [Google Scholar]
- 147.Davis M.S., Freed A.N. Repeated hyperventilation causes peripheral airways inflammation, hyperreactivity, and impaired bronchodilation in dogs. Am J Respir Crit Care Med. 2001;164:785–789. doi: 10.1164/ajrccm.164.5.2003081. [DOI] [PubMed] [Google Scholar]
- 148.Mosser A.G., Brockman-Schneider R., Amineva S., Burchell L., Sedgwick J.B., Busse W.W. Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J Infect Dis. 2002;185:734–743. doi: 10.1086/339339. [DOI] [PubMed] [Google Scholar]
- 149.Laitinen L.A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985;131:599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- 150.Lackie P.M., Baker J.E., Gunthert U., Holgate S.T. Expression of CD44 isoforms is increased in the airway epithelium of asthmatic subjects. Am J Respir Cell Mol Biol. 1997;16:14–22. doi: 10.1165/ajrcmb.16.1.8998074. [DOI] [PubMed] [Google Scholar]
- 151.Spina D., Rigby P.J., Paterson J.W., Goldie R.G. Autoradiographic localization of beta-adrenoceptors in asthmatic human lung. Am Rev Respir Dis. 1989;140:1410–1415. doi: 10.1164/ajrccm/140.5.1410. [DOI] [PubMed] [Google Scholar]
- 152.Persson C.G., Erjefalt J.S., Erjefalt I., Korsgren M.C., Nilsson M.C., Sundler F. Epithelial shedding—restitution as a causative process in airway inflammation. Clin Exp Allergy. 1996;26:746–755. [PubMed] [Google Scholar]
- 153.Erjefalt J.S., Persson C.G. Airway epithelial repair: breathtakingly quick and multipotentially pathogenic. Thorax. 1997;52:1010–1012. doi: 10.1136/thx.52.11.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pain M., Bermudez O., Lacoste P., Royer P.J., Botturi K., Tissot A. Tissue remodelling in chronic bronchial diseases: from the epithelial to mesenchymal phenotype. Eur Respir Rev. 2014;23:118–130. doi: 10.1183/09059180.00004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nihlberg K., Larsen K., Hultgardh-Nilsson A., Malmstrom A., Bjermer L., Westergren-Thorsson G. Tissue fibrocytes in patients with mild asthma: a possible link to thickness of reticular basement membrane? Respir Res. 2006;7:50. doi: 10.1186/1465-9921-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gizycki M.J., Adelroth E., Rogers A.V., O'Byrne P.M., Jeffery P.K. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol. 1997;16:664–673. doi: 10.1165/ajrcmb.16.6.9191468. [DOI] [PubMed] [Google Scholar]
- 157.Motojima S., Frigas E., Loegering D.A., Gleich G.J. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am Rev Respir Dis. 1989;139:801–805. doi: 10.1164/ajrccm/139.3.801. [DOI] [PubMed] [Google Scholar]
- 158.Persson C. Primary lysis of eosinophils in severe desquamative asthma. Clin Exp Allergy. 2014;44:173–183. doi: 10.1111/cea.12255. [DOI] [PubMed] [Google Scholar]
- 159.Yamada Y., Yoshihara S., Arisaka O. Creola bodies in wheezing infants predict the development of asthma. Pediatr Allergy Immunol. 2004;15:159–162. doi: 10.1111/j.1399-3038.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 160.Ogata H., Motojima S., Fukuda T., Makino S. Creola bodies and the eosinophil cationic protein in sputum in acute asthmatic attacks with respect to their clinical significanceArerugi. 1990;39:1567–1575. [PubMed] [Google Scholar]
- 161.Anderson S.D., Kippelen P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol. 2008;122:225–237. doi: 10.1016/j.jaci.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 162.Erjefalt J.S., Erjefalt I., Sundler F., Persson C.G. Effects of topical budesonide on epithelial restitution in vivo in guinea pig trachea. Thorax. 1995;50:785–792. doi: 10.1136/thx.50.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wenzel S.E., Szefler S.J., Leung D.Y., Sloan S.I., Rex M.D., Martin R.J. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156:737–743. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 164.Louis R., Schleich F., Barnes P.J. Corticosteroids: still at the frontline in asthma treatment? Clin Chest Med. 2012;33:531–541. doi: 10.1016/j.ccm.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 165.Erjefalt I., Persson C.G. Pharmacologic control of plasma exudation into tracheobronchial airways. Am Rev Respir Dis. 1991;143:1008–1014. doi: 10.1164/ajrccm/143.5_Pt_1.1008. [DOI] [PubMed] [Google Scholar]
- 166.Greiff L., Wollmer P., Andersson M., Svensson C., Persson C.G. Effects of formoterol on histamine induced plasma exudation in induced sputum from normal subjects. Thorax. 1998;53:1010–1013. doi: 10.1136/thx.53.12.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Greiff L., Andersson M., Svensson C., Alkner U., Persson C.G. Glucocorticoids may not inhibit plasma exudation by direct vascular antipermeability effects in human airways. Eur Respir J. 1994;7:1120–1124. [PubMed] [Google Scholar]
- 168.Schleimer R.P. Glucocorticoids suppress inflammation but spare innate immune responses in airway epithelium. Proc Am Thorac Soc. 2004;1:222–230. doi: 10.1513/pats.200402-018MS. [DOI] [PubMed] [Google Scholar]