Abstract
Background
Simple and safe strategies for the prevention of viral respiratory tract infections (RTIs) are needed.
Objective
We hypothesized that early prebiotic or probiotic supplementation would reduce the risk of virus-associated RTIs during the first year of life in a cohort of preterm infants.
Methods
In this randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov no. NCT00167700), 94 preterm infants (gestational age, ≥32 + 0 and ≤36 + 6 weeks; birth weight, >1500 g) treated at Turku University Hospital, Turku, Finland, were allocated to receive oral prebiotics (galacto-oligosaccharide and polydextrose mixture, 1:1), a probiotic (Lactobacillus rhamnosus GG, ATCC 53103), or placebo (microcrystalline cellulose) between days 3 and 60 of life. The primary outcome was the incidence of clinically defined virus-associated RTI episodes confirmed from nasal swabs by using nucleic acid testing. Secondary outcomes were the severity and duration of RTIs.
Results
A significantly lower incidence of RTIs was detected in infants receiving prebiotics (rate ratio [RR], 0.24; 95% CI, 0.12-0.49; P < .001) or probiotics (RR, 0.50; 95% CI, 0.28-0.90; P = .022) compared with those receiving placebo. Also, the incidence of rhinovirus-induced episodes, which comprised 80% of all RTI episodes, was found to be significantly lower in the prebiotic (RR, 0.31; 95% CI, 0.14-0.66; P = .003) and probiotic (RR, 0.49; 95% CI, 0.24-1.00; P = .051) groups compared with the placebo group. No differences emerged among the study groups in rhinovirus RNA load during infections, duration of rhinovirus RNA shedding, duration or severity of rhinovirus infections, or occurrence of rhinovirus RNA in asymptomatic infants.
Conclusions
Gut microbiota modification with specific prebiotics and probiotics might offer a novel and cost-effective means to reduce the risk of rhinovirus infections.
Key words: Galacto-oligosaccharide, gut microbiota, Lactobacillus rhamnosus GG, polydextrose, prebiotic, preterm infant, probiotic, respiratory tract infections, rhinovirus
Abbreviations used: RR, Rate ratio; RSV, Respiratory syncytial virus; RTI, Respiratory tract infection
Prebiotics and probiotics modulate the gut microbiota and interact with innate and adaptive immunity.1, 2 The association between early contact with microbes and the subsequent development of infectious diseases has prompted interest in the modulation of early host-microbe interaction. The number and severity of respiratory tract infections (RTIs), the most common cause of pathogen-related infant morbidity in developed and developing countries, has been shown in some studies to be reduced by probiotic or prebiotic intervention, although the effects are not well established.3, 4
Premature infants lack initial maturation signals for their immature immune system, and their stepwise compositional development of the gut microbiota is disturbed. This is due to delayed introduction of enteral feeding, lack of fresh breast milk, frequent antibiotic use, and the neonatal intensive care unit environment.5 Consequently, these infants have a heightened susceptibility to infections.
Identifying simple, cost-effective, and safe strategies for the prevention of RTIs is essential. With this in mind, we hypothesized that early modification of the gut microbial and immunologic environment with specific prebiotics or probiotics would reduce the risk of viral RTIs in preterm infants during the first year of life.
Methods
Study design and population
This randomized, double-blind, placebo-controlled study was conducted with 94 preterm infants treated at Turku University Hospital, Turku, Finland, between June 2008 and May 2012 (http://www.clinicaltrials.gov/ct/gui/show/NCT00167700). The infants were recruited between the first and third days of life. The inclusion criteria were as follows: gestational age between 32 + 0 and 36 + 6 weeks, birth weight of greater than 1500 g, and absence of any congenital defects preventing enteral nutrition. The standard of care in our unit is to give either maternal or pasteurized donated breast milk to all preterm infants. The study complied with the Declaration of Helsinki, as revised in 2000. Written informed consent was obtained from the parents of the infants, and the study protocol was approved by the Ethics Committee of the Hospital District of South-West Finland. The major challenge in this trial was the recruitment of patients. In Finland the use of commercially available probiotic products, even among newborns, is highly widespread, and most of the parents recruited did not want to participate in the study because they wanted to give their infant probiotics.
Randomization and masking
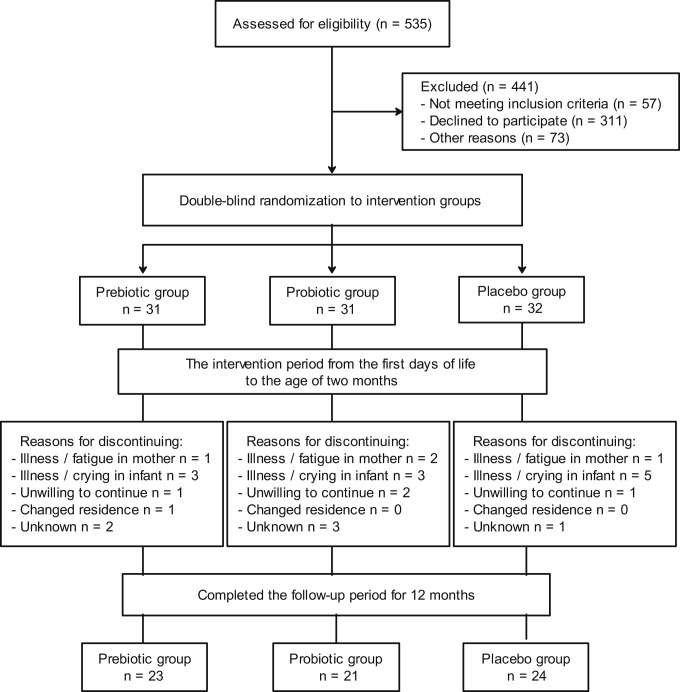
At baseline, subjects were randomly assigned to one of 3 study groups (Fig 1 ) according to computer-generated block randomization of 6 infants: the prebiotic, probiotic, or placebo groups. The randomization list was generated by a statistician who was not involved in the recruitment of study subjects. Families and study and hospital personnel were all blinded to the randomization code. Sealed envelopes contained subject numbers corresponding to numbered prebiotic, probiotic, and placebo containers, which were coded according to the randomization list by a member of the research group not involved with the conduct or reporting of the study. Research nurses and researchers ensured that preparations with corresponding numbers were given to the subjects. The trial data were collected on printed case record forms, and the members of the research group performed data entry. All data were kept confidential.
Fig 1.
Trial flow of patients.
Randomization to receive prebiotics (a mixture of polydextrose [Danisco Sweeteners, Surrey, United Kingdom] and galacto-oligosaccharides [Friesland Foods Domo, Zwolle, The Netherlands]) in a 1:1 ratio at 1 × 600 mg/day for 1 to 30 days and 2 × 600 mg/day for 31 to 60 days, a probiotic (Lactobacillus rhamnosus GG, ATCC 53103; Mead Johnson & Co, Evansville, Ind) at a dose of 1 × 109 colony-forming units/day for 1 to 30 days and 2 × 109 colony-forming units/day for 31 to 60 days, or placebo (microcrystalline cellulose and dextrose anhydrate [Chr. Hansen, Hørsholm, Denmark]) took place in a double-blind manner (Fig 1). All study products were prepared in the Turku University Hospital Pharmacy. Prebiotic, probiotic, and placebo preparations looked, smelled, and tasted identical. All containers were stored at +5°C, and the viability of the probiotic was confirmed by means of regular analysis by a blinded laboratory in the Functional Foods Forum at Turku University. Compliance in consumption of study containers was assessed by means of interview. The parents were taught by the study nurse to mix the products immediately before administration to the infant, with approximately 10 mL of breast milk or formula administered by spoon or bottle.
Study conduct and outcomes
After the enrollment day, follow-up visits were scheduled at the ages of 1, 2, 4, 6, and 12 months with the same study nurse. In addition to the scheduled study visits in the study clinic, there was an additional telephone call to the parents at infant's age of 9 months. Weight, height, and head circumference were measured, and growth charts were sketched at each visit. During all study visits, parents reported the infant's behavior patterns, including sleeping patterns, fussing, crying, irritability, feeding, vomits, stool consistency (normal, loose, firm, hard, and atypical) and frequency (1/wk, 2-3/wk, daily, 1-2/d, 2-3/d, and >3/d), infection and other diseases, and medication use. Additionally, adverse events were queried from the parents during all visits. These data will be published in detail elsewhere.6 Furthermore, parents kept a structured diary on the child's physician's office visits and pharmacologic treatments during the follow-up period. The information was collected from the diaries, as well as from the parents, during the study visits. The infants were clinically examined by a physician at the age of 12 months and when deemed necessary, such as on signs and symptoms of acute infection.
The primary outcome in the present study was the incidence of defined viral RTIs confirmed from nasal swabs by using nucleic acid testing. Secondary outcomes were the effect of intervention on the severity and duration of these defined viral RTIs. The symptomatic RTI episode was defined when the child had at least 1 of the following symptoms: fever (temperature >38.0°C), rhinitis, or cough. Infectious disease symptoms were documented by parents using a structured (same questions to all parents) daily diary. In addition, the following symptoms were recorded: nasal congestion, excessive crying/restlessness/irritability, poor appetite, vomiting (throwing up partially digested foods and drinks), or diarrhea (≥4 loose stools daily). For the occurrence and duration of fever, a temperature of greater than 38.0°C was considered significant. The severity of the symptoms individually for fever, rhinitis, cough, nasal congestion, excessive crying/restlessness/irritability, poor appetite, vomiting (throwing up partially digested foods and drinks), and diarrhea (≥4 loose stools daily) was scored by the parents daily as mild (1), moderate (2), or severe (3). The mean of these symptom scores was regarded as the severity score of an episode. The duration of symptoms was measured in days.
Sample collection
When having symptoms of an RTI, nasal swabs (flocked nylon nasal swabs [no. 553C]; Copan, Brescia, Italy) from a depth of 2 to 3 cm were taken at home on days 1, 5, 10, and 15 of the illness for detection of respiratory tract viruses by using nucleic acid testing. In addition, nasal swabs were taken during the study visits at the ages of 2, 4, 6, and 12 months if the infant was asymptomatic. If the infant had RTI symptoms at the time of the study visit, the asymptomatic sample was taken 2 weeks after the symptoms had disappeared. In a subgroup of children (n = 5) swabs were collected on days 5, 10, and 15 after a verified subclinical rhinovirus infection. A research nurse taught the parents the technique for obtaining a nasal swab sample. The swabs were mailed to the laboratory in dry sterile tubes and stored at −70°C until analysis.7
Virus detection
The swab specimen was suspended in 0.8 mL of PBS. Total nucleic acids were extracted from 200 μL of the suspension by using a MagNA Pure 96 extractor (Roche, Mannheim, Germany) with an elution volume of 50 μL. Human bocavirus DNA was detected by using quantitative PCR from 5 μL of the extract.8 Reverse transcription was performed in 20-μL reactions by using 8 μL of the extract, random hexamer primers, and RevertAid H-cDNA synthesis reagents (Fermentas, St Leon-Rot, Germany). Rhinovirus, enteroviruses, and respiratory syncytial virus (RSV) RNA were detected from 5 μL of the cDNA by using quantitative PCR.9 In addition, adenovirus, coronaviruses 229E/NL63 and OC43/HKU1, influenza A and B viruses, human metapneumovirus, parainfluenza virus types 1 to 3, RSV groups A and B, and rhinovirus were detected by using a Seeplex RV12 multiplex PCR assay (Seegene, Seoul, Korea). Altogether, 659 PCR tests were carried out.
Statistics
The overall aim of this study was to balance the gut microbiota composition of preterm infants with prebiotic and probiotic supplementation and consequently improve the infant's well-being and reduce the disease risk. The study had several outcome variables. The present article describes the effect of prebiotic and probiotic intervention on the incidence of RTIs. Data were analyzed based on complete case analysis. Categorical variables were analyzed by using the χ2 test and the Fisher exact test for dichotomous variables. Univariate associations between continuous variables were analyzed by using ANOVA or the Kruskall-Wallis test. Negative binomial regression was used to compare the incidence of RTIs or rhinovirus infections among the study groups. The results are given as rate ratios (RRs) with 95% CIs. In addition, this association was studied by using antenatal steroids, delivery method, sex, postnatal antibiotics, and older siblings at home as covariates. Because the same child could have multiple viral episodes, the following analyses were made with repeated-measures methods. Viral copy numbers were compared between the study groups by using nonparametric repeated-measures analysis. Comparisons between groups were made by using generalized estimating equations for the duration and intensity of clinical symptoms. Associations between the area under the curve of viral copy numbers and duration and severity of symptoms were studied by using mixed models.
Results
Subjects' characteristics
The mean gestational age of the infants was 35 + 0 weeks (range, 32 + 0 to 36 + 6 weeks), and the mean birth weight was 2393 g (range, 1550-3965 g). The 12-month study period was completed by 68 (72.3%) of 94 of the infants (Fig 1). Twenty-five of 26 of the infants discontinuing the study dropped out before the age of 1 month, and no RTIs were reported before that age point. The baseline clinical characteristics of these infants, the pregnancies, and the mothers of the infants (Table I ) were found to be representative of the original population recruited. Of the total participating infants, 98% (69/71) at the age of 1 month and 94% (64/68) at the age of 2 months consumed the capsules regularly daily, without a significant difference among the study groups (P = .566 and .619, respectively). In this study population no adverse effects were recorded, and an adverse event was not the reason for noncompliance in any case. No infant had a severe infection during the follow-up period.
Table I.
Clinical characteristics of mothers, pregnancies, and infants in the 3 study groups
| Group | Prebiotic (n = 23) | Probiotic (n = 21) | Placebo (n = 24) | P value |
|---|---|---|---|---|
| Clinical characteristics of mothers and pregnancies | ||||
| Primipara | 17 (73.9%) | 14 (66.7%) | 13 (54.2%) | .358 |
| Gestational diabetes mellitus | 4 (17.4%) | 2 (9.5%) | 1 (4.2%) | .326 |
| Twins | 10 (43.5%) | 4 (19.0%) | 10 (41.7%) | .171 |
| Antenatal corticosteroids (yes) | 18 (78.3%) | 9 (42.9%) | 9 (37.5%) | .011 |
| Maternal antibiotics during delivery (yes) | 4 (17.4%) | 9 (42.9%) | 7 (29.2%) | .142 |
| Duration of gestation (wk) | 33.9 (1.3) | 34.6 (0.9) | 34.9 (1.1) | .008 |
| Cesarean delivery (yes) | 9 (39.1%) | 4 (19.0%) | 10 (41.7%) | .223 |
| Clinical characteristics of infants | ||||
| Sex (male) | 11 (47.8%) | 14 (66.7%) | 19 (79.2%) | .078 |
| Birth weight (g) | 2123 (390) | 2511 (401) | 2412 (484) | .010 |
| Birth weight <10th percentile (yes) | 4 (17.4%) | 2 (9.5%) | 5 (20.8%) | .579 |
| Birth length (cm) | 45.5 (2.2) | 47.0 (2.0) | 46.3 (3.0) | .136 |
| Head circumference at birth (cm) | 31.4 (1.2) | 32.4 (1.3) | 32.5 (1.3) | .009 |
| 5-min Apgar score | 8 (1) | 8 (1) | 8 (2) | .925 |
| Need of NICU care | 19 (82.6%) | 17 (81.0%) | 17 (70.8%) | .575 |
| No. of days treated in NICU | 14 (10) | 8 (4) | 13 (8) | .034 |
| Need of mechanical ventilation (yes) | 2 (8.7%) | 4 (19.0%) | 2 (8.3%) | .460 |
| Surfactant treatment (yes) | 1 (4.3%) | 2 (9.5%) | 1 (4.2%) | .695 |
| Need of postnatal antibiotic treatment | 14 (60.9%) | 15 (71.4%) | 15 (62.5%) | .735 |
| No. of postnatal days with antibiotics | 2.4 (2.7) | 2.3 (2.2) | 1.7 (1.9) | .492 |
| Hyperbilirubinemia | 13 (56.5%) | 9 (42.9%) | 12 (50%) | .664 |
| Older siblings at home (yes) | 6 (26.1%) | 7 (33.3%) | 11 (45.8%) | .358 |
| Exclusively breast-fed (mo) | 1.1 (1.7) | 1.7 (2.2) | 2.0 (2.2) | .335 |
| Total duration of breast-feeding (mo) | 5.4 (3.4) | 7.2 (4.4) | 5.7 (4.5) | .321 |
| Day care at age 12 mo | 3 (13.0%) | 2 (9.5%) | 2 (8.3%) | .742 |
| Weight (g) at age of 12 mo | 9374 (2342) | 9717 (1415) | 10107 (1633) | .415 |
| Length (cm) at age of 12 mo | 75.4 (2.3) | 75.4 (3.3) | 75.9 (2.7) | .773 |
| Head circumference (cm) at age of 12 mo | 46.4 (1.3) | 46.4 (1.6) | 47.1 (1.7) | .237 |
| No. of antibiotic courses prescribed during the first 12 mo∗ | 0.3 (0.8) | 0.1 (0.5) | 0.8 (1.7) | .241 |
Results are given as numbers (percentages) of subjects or as means (SDs). The variables were analyzed by using the χ2 test and Fisher exact test for dichotomous variables and ANOVA or the Kruskall-Wallis test for continuous variables.
NICU, Neonatal intensive care unit.
Data from parents.
Episodes of acute viral RTIs
The total number of clinical RTI episodes in the whole study population during the 12-month follow-up period was 102. The number of RTI episodes was 14 in the prebiotic group, 26 in the probiotic group, and 62 in the placebo group. In 96% of cases, a putative causative virus was detected. Rhinovirus was the most common virus and was found in 80% of the episodes. Two viruses were detected in 20 cases, 3 in 3 cases, and 4 in 1 case (Table II ). The rate of findings of multiple viruses was 2 in the prebiotic group, 6 in the probiotic group, and 19 in the placebo group.
Table II.
Detection of respiratory tract viruses in acute RTI episodes in the 3 study groups during the 12-month study period
| Group | Prebiotic (n = 23) | Probiotic (n = 21) | Placebo (n = 24) | P value∗ | |
|---|---|---|---|---|---|
| Virus | Rate of multiple viruses | ||||
| Adenovirus | 0 | 2 | 1 | 3 (100%) | NS |
| Coronavirus type 229E/NL63 | 0 | 2 | 3 | 2 (40%) | NS |
| Coronavirus type OC43/HKU1 | 0 | 1 | 3 | 2 (50%) | NS |
| Influenza A virus | 1 | 1 | 1 | 1 (33%) | NS |
| Influenza B virus | 0 | 0 | 1 | – | NS |
| Human metapneumovirus | 0 | 0 | 1 | 1 (100%) | NS |
| Parainfluenza virus type 1 | 0 | 0 | 0 | – | – |
| Parainfluenza virus type 2 | 0 | 0 | 0 | – | – |
| Parainfluenza virus type 3 | 2 | 2 | 7 | 8 (73%) | NS |
| RSV group A | 0 | 3 | 3 | 4 (67%) | NS |
| RSV group B | 1 | 0 | 0 | 1 (100%) | NS |
| Rhinovirus | 13 | 19 | 50 | 24 (29%) | .015 |
| Human enterovirus | 0 | 2 | 2 | 3 (75%) | NS |
| Human bocavirus | 0 | 0 | 5 | 5 (100%) | NS |
NS, Not significant.
Kruskall-Wallis test.
A significant difference was detected among the groups in the incidence of RTIs, with the mean being 0.6 (SD, 0.8) in the prebiotic group, 1.2 (SD, 1.6) in the probiotic group, and 2.5 (SD, 2.0) in the placebo group (P = .001, Table III ). This difference was seen throughout the 12-month study period. Taking rhinovirus as the sole source of infections, the incidence was significantly lower in the prebiotic group (RR, 0.31; 95% CI, 0.14-0.66; P = .003) and the probiotic group (RR, 0.49; 95% CI, 0.24-1.00; P = .051) when compared with the placebo group, whereas the difference between the 2 intervention groups did not reach statistical significance (RR, 0.6; 95% CI, 0.3-1.5; P = .280).
Table III.
Number of acute viral RTI episodes during the 12-month study period
| Group | Prebiotic (n = 23) | Probiotic (n = 21) | Placebo (n = 24) | P value∗ |
|---|---|---|---|---|
| No. of episodes | ||||
| 0 | 14 (60.9%) | 10 (47.6%) | 4 (16.7%) | .005 |
| 1-3 | 9 (39.1%) | 9 (42.9%) | 12 (50.0%) | .652 |
| >3 | 0 (0%) | 2 (9.5%) | 8 (33.3%) | .005 |
Fisher exact test.
As a sensitivity analysis, we reanalyzed our main results (incidence of RTIs and rhinovirus infections) assuming that all missing subjects had either a very good outcome (10th percentile) or a very bad outcome (90th percentile). Assuming that missing subjects had a very good outcome (10th percentile), the results remained alike: a significantly lower incidence of RTIs was detected in infants receiving prebiotics (RR, 0.23; 95% CI, 0.11-0.51; P < .001) or probiotics (RR, 0.43; 95% CI, 0.21-0.87; P = .023) compared with those receiving placebo. Also, the incidence of rhinovirus-induced episodes was found to be significantly lower in the prebiotic (RR, 0.29; 95% CI, 0.12-0.70; P = .010) and probiotic (RR, 0.43; 95% CI, 0.19-0.96; P = .041) groups compared with the placebo group. Assuming that missing subjects would have had a very bad outcome (90th percentile), the result was as follows: a significantly lower incidence of RTIs was detected in infants receiving prebiotics (RR, 0.53; 95% CI, 0.33-0.84; P =.012) but not probiotics (RR, 0.76; 95% CI, 0.49-1.17; P = .210) compared with those receiving placebo. The incidence of rhinovirus-induced episodes tended to be lower in the prebiotic (RR, 0.63; 95% CI, 0.37-1.07; P = .091) but not in the probiotic (RR, 0.82; 95% CI 0.9-1.38; P = .462) group compared with the placebo group.
Similarly, when the incidence of RTIs or rhinovirus infections was adjusted to use of antenatal corticosteroids or postnatal antibiotics, mode of delivery, sex, or presence of older siblings at home, a significant difference emerged among the study groups (P = .001). Five cases of human bocavirus infections in 3 infants were detected, all in the placebo group and all concomitant with rhinovirus.
Rhinovirus RNA copy numbers and duration of shedding
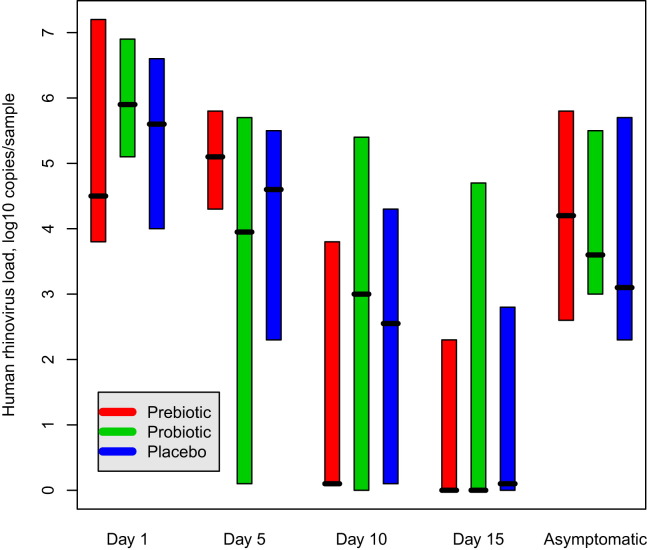
The mean duration of symptoms in rhinovirus episodes was 9.9 (SE, 1.1) days in the prebiotic group, 9.7 (SE, 0.6) days in the probiotic group, and 10.7 (SE, 0.7) days in the placebo group (P = .597). No significant differences were detected among the groups in the combined severity score of clinical symptoms in rhinovirus episodes, with the median of the median scores being 3.0 in the prebiotic group, 2.0 in the probiotic group, and 2.0 in the placebo group (P = .262). On symptomatic rhinovirus episodes (day 1), the median copy number of rhinovirus RNA was 4.5 (interquartile range, 3.8-7.2) log10 copies/sample in the prebiotic group, 5.9 (interquartile range, 5.1-6.9) log10 copies/sample in the probiotic group, and 5.6 (interquartile range, 4.0-6.6) log10 copies/sample in the placebo group (P = .650). The rhinovirus RNA copy numbers on days 1, 5, 10, and 15 of the episodes are presented in Fig 2 . The relative rhinovirus copy numbers correlated positively with the duration (P = .021) but not with the severity of symptoms (data not shown), with the association being similar in all groups. Furthermore, the time (in days) needed for virus eradication during symptomatic rhinovirus episodes did not differ among the groups (P = .838): the median eradication time was between 10 and 15 days in the prebiotic and probiotic groups and greater than 15 days in the placebo group.
Fig 2.
Median (range) rhinovirus load (log10 copies per sample) obtained when respiratory symptoms were present on days 1, 5, 10, and 15 of the episodes and in asymptomatic infants in the 3 study groups. No significant differences were detected among the study groups at any time point (P = .650 on day 1, P = .605 on day 5, P = .856 on day 10, P = .121 on day 15, and P = .990 in asymptomatic infants). The difference between symptomatic infants on day 1 and asymptomatic infants was significant (P < .001).
Respiratory tract viruses in asymptomatic subjects
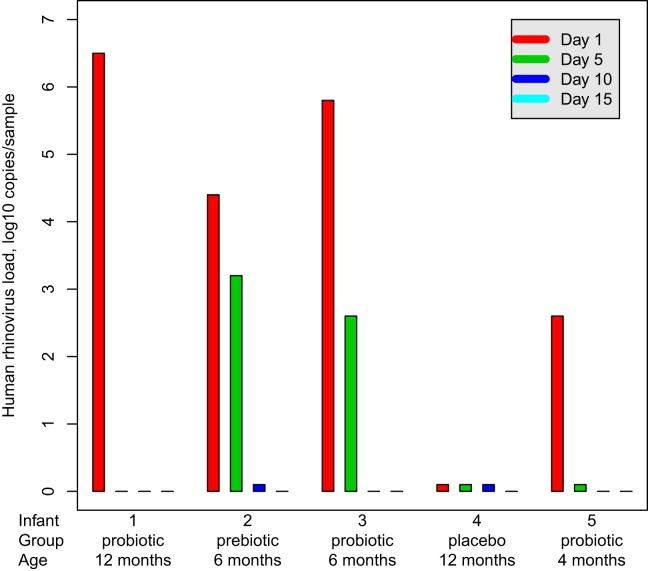
Rhinovirus was the most common virus detected in asymptomatic infants (62/86 virus-positive findings, Table IV ). In 10 (12%) cases 2 viruses and in 1 case 3 viruses were found concomitantly. The rate of findings of multiple viruses in asymptomatic infants was 3 in the prebiotic group, 4 in the probiotic group, and 4 in the placebo group. No significant differences were found among the study groups in the occurrence of rhinovirus RNA in asymptomatic infants. The median copy number of rhinovirus RNA in nasal swabs was 3.4 (interquartile range, 2.6-4.8) log10 copies/sample in the whole population, with no significant difference among the study groups (P = .990, Fig 2). The copy number of rhinovirus RNA in the nasal swabs from symptomatic infants on day 1 was significantly higher than in the asymptomatic subjects (P < .001, Fig 2). The duration of rhinovirus RNA shedding in asymptomatic infants (n = 5) is presented in Fig 3 , all being RNA negative on day 15 and the difference from symptomatic infants being significant in this respect (P = .040).
Table IV.
The detection rates of respiratory viruses in asymptomatic infants in the 3 study groups
| Group | Prebiotics (n = 23) | Probiotic (n = 21) | Placebo (n = 24) | P value∗ | |
|---|---|---|---|---|---|
| Virus | Rate of multiple viruses | ||||
| Adenovirus | 0 | 3 | 0 | 1 (33%) | NS |
| Coronavirus type 229E/NL63 | 1 | 1 | 1 | 1 (33%) | NS |
| Coronavirus type OC43/HKU1 | 2 | 3 | 1 | 1 (17%) | NS |
| Influenza A virus | 0 | 0 | 0 | – | – |
| Influenza B virus | 0 | 0 | 0 | – | – |
| Human metapneumovirus | 0 | 0 | 0 | – | – |
| Parainfluenza virus type 1 | 0 | 0 | 0 | – | – |
| Parainfluenza virus type 2 | 0 | 0 | 0 | – | – |
| Parainfluenza virus type 3 | 1 | 3 | 2 | 3 (50%) | NS |
| RSV group A | 0 | 2 | 0 | 1 (50%) | NS |
| RSV group B | 1 | 0 | 1 | 1 (50%) | NS |
| Rhinovirus | 21 | 20 | 21 | 8 (13%) | NS |
| Human enterovirus | 2 | 2 | 3 | 2 (29%) | NS |
| Human bocavirus | 4 | 1 | 6 | 5 (45%) | NS |
NS, Not significant.
Kruskall-Wallis test.
Fig 3.
Rhinovirus load (log10 copies per sample) obtained in the 5 infants with asymptomatic rhinovirus-positive findings. Rhinovirus quantitative RT-PCR was performed on days 5, 10, and 15 after a verified asymptomatic rhinovirus RNA finding (day 1) to determine the duration of viral shedding.
Discussion
In this study a significant reduction in the incidence of RTIs and especially of rhinovirus infections during the first year of life in a preterm population was achieved with specific prebiotics and probiotics, namely galacto-oligosaccharide:polydextrose and Lactobacillus rhamnosus GG. Our results support previously published randomized, double-blind prebiotic and/or probiotic trials conducted in healthy term infants during the first year of life, with the incidence of clinical RTIs representing outcome (Table V ),10, 11, 12, 13, 14, 15, 16, 17 and extend these to an objective characterization of the viral cause. In contrast to earlier studies, this study was conducted in premature infants, and supplementation commenced within the first week of life, which might have increased the protective efficacy against RTIs. Moreover, the effect exerted by prebiotics tended to outweigh that of the probiotic. One putative explanation for this finding might lie in the pre-existence of bifidobacteria-dominated gut microbiota in our breast-fed preterm population,6 which strengthens the effect of prebiotics in selective stimulation of the growth of beneficial intestinal microorganisms.2
Table V.
Previously published randomized, double-blind, placebo-controlled prebiotic interventions, probiotic interventions, or both conducted in infants aged less than 1 year with incidence of RTIs as outcome
| First author, publication year, country | Age group/duration of intervention | Duration of follow-up | No. of infants included | Type of intervention | Outcomes | Result regarding the incidence of RTIs (as reported in original articles) |
|---|---|---|---|---|---|---|
| Weizman et al,10 2005, Israel | 4-10 mo/12 wk | 12 wk | 201; 73 + 8 + 60 | Formula supplemented with Bifidobacterium lactis Bb12, Lactobacillus reuteri, or placebo | No. of days or episodes with fever, diarrhea, or respiratory illness | No difference in rate or duration of RTIs |
| Arslanoglu et al,11, 12 2007 and 2008, Italy | <2 wk, 6 mo | 2 y | 259; 129 + 130 | Formula supplemented with galacto-oligosaccharide and fructo-oligosaccharide or placebo | Incidence of allergic manifestations, number of infectious episodes | Significantly fewer of all types of infections (P = .01), upper RTIs (P < .01), fever episodes (P < .0001), and fewer antibiotic prescriptions (P < .05) |
| Kukkonen et al,13 2008, Finland | (4 wk before delivery), 6 mo | 2 y | 1018; 506 + 512 | Lactobacillus rhamnosus GG, LC705, Bifidobacterium breve Bb99, and Propionibacterium shermanii + postpartum galacto-oligosaccharide or placebo (capsules) | Incidence of allergic manifestations, safety, number of RTIs and intestinal infections | Significantly fewer RTIs (ratio, 0.87; P = .009) |
| Rautava et al,14 2009, Finland | <2 mo to age of 12 mo | 12 mo | 81; 38 + 43 | Formula supplemented with Lactobacillus rhamnosus GG + Bifidobacterium lactis Bb12 or placebo | Incidence of respiratory and gastrointestinal recurrent (≥3) infections before the age of 7 mo | Significantly fewer acute otitis media (RR, 0.44; P = .014) need for antibiotics (RR, 0.52; P = .015) and recurrent RTIs (RR, 0.51; P = .022); no difference in rate of RTIs per se |
| Taipale et al,15 2011, Finland | 1 mo to age of 8 mo | 8 mo | 109; 55 + 54 | Bifidobacterium lactis Bb12 or placebo tablets in pacifier | Incidence of RTI and gastrointestinal infections | Significantly fewer RTIs (RR, 0.69; P = .014) |
| Maldonado et al,16 2012, Spain | 6 mo to age of 12 mo | 6 mo | 215; 117 + 98 | Formula supplemented with Lactobacillus fermentum or placebo | Incidence of all kinds of infections | Significantly fewer respiratory (IR, 0.729; P = .026) and total number of infections (IR, 0.70; P = .003) |
| Gil-Campos et al,17 2012, Spain | 1 mo to age of 6 mo | 5 mo | 137; 71 + 66 | Formula supplemented with Lactobacillus fermentum or placebo | Weight gain, other anthropometric data, and incidence of all kinds of infections | No difference in incidence of RTIs |
IR, Incidence ratio; RR, risk ratio.
In this preterm population the protective effect of prebiotics and probiotics achieved against RTIs and rhinovirus infections was shown to extend throughout the 12-month follow-up period. The immunomodulatory effects accomplished during the time when the child's immunologic phenotype is consolidated can thus confer clinical benefits beyond the intervention period, as we have previously documented in the risk reduction of eczema at the age of 7 years with a perinatal probiotic intervention.18 Our observation is in agreement with the programming theory, whereby early-life exposures can carry effects into later life, with this now being extended to microbial contact.19, 20 In fact, this is in accordance with a previously published clinical study in which early colonizers were shown to have potential to exert their immunologic effects years later.21 Moreover, prevention of early rhinovirus infections might be of great clinical importance in that early rhinovirus infections have recently been recognized as a major predisposing factor in the development of asthma.22
The rationale of probiotic use in the prevention of RTIs is based on their ability to reduce pathogen colonization in the respiratory epithelium and to regulate not only mucosal immunity through activation of inflammasomes but also systemic immune responses.23, 24 The health benefits of probiotic bacteria in viral diseases, previously best demonstrated in gastrointestinal infections, are related to maintenance of epithelial barrier integrity, production of antimicrobial factors, control of the balance between proinflammatory and anti-inflammatory cytokines, and reinforced production of antigen-specific secretory IgA.25 Most importantly, probiotics have been shown to regulate the expression of genes related to innate immune-mediated cytokine responses in the intestinal26 and also the respiratory mucosa, creating an anti-inflammatory milieu and thus modulating beneficially respiratory mucosal antibacterial and antiviral immunity.27, 28 Recently, in mouse model studies lactobacilli induced changes in expression of interferon-stimulated genes and increases in IFN-γ, IL-6, and IL-10 levels, with beneficial modulation of the respiratory mucosal antiviral immunity.27 These mechanisms are analogous to the shielding effect of breast-feeding against gastrointestinal infections and RTIs, being mediated by passive immune protection by IgA and oligosaccharides and by colonizing bacteria and their growth factors and molecules regulating microbial recognition.29
Rhinovirus is the most commonly detected respiratory tract virus, with a wide range of clinical presentations.30, 31 In this study up to 6 rhinovirus infections were recorded in a year in our preterm population, although the overall rate of RTIs was low. There are few putative explanations for this: the majority of the infants (65%) were firstborn, all of them were breast-fed, and only 10% were in day care at the age of 12 months. Interestingly, prebiotics and probiotics prevented symptomatic infection but not the occurrence of rhinovirus RNA in asymptomatic infants. In agreement with the findings of Jansen et al,32 the rhinovirus RNA copy number was found to be lower in asymptomatic infants. There was a significant correlation between rhinovirus RNA load and the duration of clinical symptoms. Although both prebiotic and probiotic supplementation prevented rhinovirus infections, the intervention had no effect on the duration or severity of symptomatic rhinovirus infections. Furthermore, neither of the interventions influenced the duration of viral shedding. A new observation was that the period of rhinovirus RNA shedding was significantly shorter among the asymptomatic infants.
We focused here on premature infants being physiologically immunodeficient because of an immature innate immune system. In this study 83% of the infants in the placebo group had at least 1 RTI during the first year of life. Preterm infants have significantly reduced proinflammatory cytokine (IL-1β, IL-6, and TNF-α) responses, and they produce decreased amounts of antiviral IFN-α.5 The mechanisms involved lie in reduced pattern recognition receptor expression. On the other hand, they tend to mount aberrant responses to antigen challenges with an overexpression of proinflammatory cytokines, which leads to an overwhelming hyperreactive state. In consequence, this can lead to increased susceptibility to viral infections. It is also well established that prematurity is the major risk factor for severe RSV infections,33, 34 and many recent studies have shown that premature infants also have severe rhinovirus infections.35, 36
There are important limitations to our study. We studied preterm infants, which could question the generalizability of our results to full-term and older infants. Furthermore, a substantial number of premature infants who underwent randomization could not be evaluated for the 12-month study period. Additionally, it must be acknowledged that not all prebiotics and probiotics are the same, and drawing conclusions on the relationship between such interventions and the effects of any other prebiotic supplementation or probiotic species is not possible. On the other hand, our study is the first to report the effect of prebiotics and probiotics on the prevention of defined viral RTIs in a preterm cohort. The results enhance the placebo-controlled evidence noted in several reviews (also the Cochrane review),3, 4, 37 especially data for infancy. Our study also provides novel clinical evidence as to rhinovirus RNA shedding and its relation to the clinical picture in symptomatic and asymptomatic infants. Furthermore, given the fact that probiotics are live microorganisms and that both probiotics and prebiotics possess numerous immunologic properties, uncertainty still prevails regarding the long-term safety of their use among preterm infants during the period when the child's immunologic phenotype is consolidated. The absence of adverse effects in this study cohort represents safety documentation for the use of these prebiotics and probiotics in this sensitive infant population.
Worthy of note is that 11% of all infants are born premature, and this population thus represents some 12.9 million infants per year worldwide.5 Preterm infants carry a heightened risk of infectious ailments of both bacterial and viral cause and undernourishment, aggravating this susceptibility. RTIs are a major cause of mortality and morbidity worldwide, particularly during the first years of life, with rhinovirus being the main pathogen responsible for this socioeconomic burden. Although strict hygiene measures have been shown to reduce viral transmission and thus diminish the incidence of rhinovirus infections, no definitive preventive measures have thus far been discovered for the effective control of this entity. On the basis of our results, gut microbiota modulation with specific prebiotics, probiotics, or both could offer a cost-effective tool in the fight against RTIs, hopefully also in the developing world.
In conclusion, we demonstrated that early manipulation of the microbiota in preterm infants by dietary means might induce long-lasting effects and could reduce the risk of viral RTIs.
Clinical implications.
Manipulation of complex interaction between the colonizing microbes and the developing immune system of the preterm neonatal intestine with prebiotics and probiotics can have a beneficial effect on the subsequent risk of RTIs.
Acknowledgments
We thank the families participating in our study; Turku University Hospital Pharmacy for study product preparation; Anna Pärtty, MD, Anne Yrjänä, RN, and Ulla-Maija Eriksson, RN, for their assistance in the study clinic; Tiina Ylinen, laboratory engineer, in the virus laboratory; Jaakko Matomäki, MSc, for help with statistical analyses; and Robert MacGilleon, MA, for language review of the manuscript.
Footnotes
The Mead Johnson Nutrition Company USA covered the costs of the prebiotic and probiotic products (prepared by Turku University Hospital Pharmacy) and part of the salary for the first author. Financial support for the study was provided by the Juho Vainio Foundation, the Päivikki and Sakari Sohlberg Foundation, the Jenny and Antti Wihuri Foundation, and EVO Funding of Turku University Hospital and Satakunta Central Hospital.
Disclosure of potential conflict of interest: R. Luoto has received grants from the Juho Vainio Foundation and the EVO Funding of Satakunta Central Hospital. M. Waris has received grants from the Jenny and Antti Wihuri Foundation. E. Isolauri has received grants from the Päivikki and Sakari Sohlberg Foundation and the EVO Funding of Turku University Hospital. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Food and Agriculture Organization of the United Nations, World Health Organization. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Available at: www.who.int/entity/foodsafety/publications/fs_management/en/probiotics.pdf. Accessed November 2009.
- 2.Pineiro M., Asp N.G., Reid G., Macfarlane S., Morelli L., Brunser O. FAO Technical meeting on prebiotics. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S156–S159. doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- 3.Vouloumanou E.K., Makris G.C., Karageorgopoulos D.E., Falagas M.E. Probiotics for the prevention of respiratory tract infections: a systematic review. Int J Antimicrob Agents. 2009;34:197. doi: 10.1016/j.ijantimicag.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Weichert S., Schroten H., Adam R. The role of prebiotics and probiotics in prevention and treatment of childhood infectious diseases. Pediatr Infect Dis J. 2012;31:859–862. doi: 10.1097/INF.0b013e3182620e52. [DOI] [PubMed] [Google Scholar]
- 5.Sharma A.A., Jen R., Butler A., Lavoie P.M. The developing human preterm neonatal immune system: a case for more research in this area. Clin Immunol. 2012;145:61–68. doi: 10.1016/j.clim.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pärtty A., Luoto R., Kalliomäki M., Salminen S., Isolauri E. Effects of early prebiotic and probiotic supplementation on development of gut microbiota and fussing and crying in preterm infants: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2013 Jul 31 doi: 10.1016/j.jpeds.2013.05.035. doi:10.1016/j.jpeds.2013.05.035 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Peltola V., Waris M., Osterback R., Susi P., Ruuskanen O., Hyypiä T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197:382–389. doi: 10.1086/525542. [DOI] [PubMed] [Google Scholar]
- 8.Koskenvuo M., Möttönen M., Waris M., Allander T., Salmi T.T., Ruuskanen O. Human bocavirus in children with acute lymphoblastic leukemia. Eur J Pediatr. 2008;167:1011–1015. doi: 10.1007/s00431-007-0631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLeish N.J., Witteveldt J., Clasper L., McIntyre C., McWilliam Leitch EC. Development and assay of RNA transcripts of enterovirus species A to D, rhinovirus species A to C, and human parechovirus: assessment of assay sensitivity and specificity of real-time screening and typing methods. J Clin Microbiol. 2012;50:2910–2917. doi: 10.1128/JCM.01172-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weizman Z., Asli G., Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005;115:5–9. doi: 10.1542/peds.2004-1815. [DOI] [PubMed] [Google Scholar]
- 11.Arslanoglu S., Moro G.E., Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. 2007;137:2420–2424. doi: 10.1093/jn/137.11.2420. [DOI] [PubMed] [Google Scholar]
- 12.Arslanoglu S., Moro G.E., Schmitt J., Tandoi L., Rizzardi S., Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138:1091–1095. doi: 10.1093/jn/138.6.1091. [DOI] [PubMed] [Google Scholar]
- 13.Kukkonen K., Savilahti E., Haahtela T., Juntunen-Backman K., Korpela R., Poussa T. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: randomized, double-blind, placebo-controlled trial. Pediatrics. 2008;122:8–12. doi: 10.1542/peds.2007-1192. [DOI] [PubMed] [Google Scholar]
- 14.Rautava S., Salminen S., Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy−a randomised, double-blind, placebo-controlled study. Br J Nutr. 2009;101:1722–1726. doi: 10.1017/S0007114508116282. [DOI] [PubMed] [Google Scholar]
- 15.Taipale T., Pienihäkkinen K., Isolauri E., Larsen C., Brockmann E., Alanen P. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in infancy. Br J Nutr. 2011;105:409–416. doi: 10.1017/S0007114510003685. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado J., Cañabate F., Sempere L., Vela F., Sánchez A.R., Narbona E. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J Pediatr Gastroenterol Nutr. 2012;54:55–61. doi: 10.1097/MPG.0b013e3182333f18. [DOI] [PubMed] [Google Scholar]
- 17.Gil-Campos M., López MÁ, Rodriguez-Benítez M.V., Romero J., Roncero I., Linares M.D. Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1-6 months of age: a randomized controlled trial. Pharmacol Res. 2012;65:231–238. doi: 10.1016/j.phrs.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Kalliomäki M., Salminen S., Poussa T., Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1019–1021. doi: 10.1016/j.jaci.2006.12.608. [DOI] [PubMed] [Google Scholar]
- 19.Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olszak T., An D., Zeissig S., Vera M.P., Richter J., Franke A. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faith J.J., Guruge J.L., Charbonneau M., Subramanian S., Seedorf H., Goodman A.L. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jartti T., Korppi M. Rhinovirus-induced bronchiolitis and asthma development. Pediatr Allergy Immunol. 2011;22:350–355. doi: 10.1111/j.1399-3038.2011.01170.x. [DOI] [PubMed] [Google Scholar]
- 23.Glück U., Gebbers J.O. Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and beta-hemolytic streptococci) Am J Clin Nutr. 2003;77:517–520. doi: 10.1093/ajcn/77.2.517. [DOI] [PubMed] [Google Scholar]
- 24.Ichinohe T., Pang I.K., Kumamoto Y., Peaper D.R., Ho J.H., Murray T.S. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gareau M.G., Sherman P.M., Walker W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7:503–514. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganguli K., Meng D., Rautava S., Lu L., Walker W.A., Nanthakumar N. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am J Physiol Gastrointest Liver Physiol. 2013;304:G132–G141. doi: 10.1152/ajpgi.00142.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villena J., Chiba E., Tomosada Salva S., Marranzino G., Kitazawa H. Orally administered Lactobacillus rhamnosus modulates the respiratory immune response triggered by the viral pathogen-associated molecular pattern poly(I: C) BMC Immunol. 2012;13:53. doi: 10.1186/1471-2172-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Archambaud C., Nahori M.A., Soubigou Bécavin C., Laval L., Lechat P. Impact of lactobacilli on orally acquired listeriosis. Proc Natl Acad Sci U S A. 2012;109:16684–16689. doi: 10.1073/pnas.1212809109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr. 2010;156(suppl):S8–S15. doi: 10.1016/j.jpeds.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs S.E., Lamson D.M., St George K., Walsh T.J. Human rhinoviruses. Clin Microbiol Rev. 2012;26:135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruuskanen O., Waris M., Ramilo O. New aspects on human rhinovirus infections. Pediatr Infect Dis J. 2013;32:553–555. doi: 10.1097/INF.0b013e3182833c90. [DOI] [PubMed] [Google Scholar]
- 32.Jansen R.R., Wieringa J., Koekkoek S.M., Visser C.E., Pajkrt D., Molenkamp R. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49:2631–2636. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krilov L.R., Weiner L.B., Yogev R., Fergie J., Katz B.Z., Henrickson K.J. The 2009 COID recommendations for RSV prophylaxis: issues of efficacy, cost, and evidence-based medicine. Pediatrics. 2009;124:1682–1684. doi: 10.1542/peds.2009-2681. [DOI] [PubMed] [Google Scholar]
- 34.Blanken M.O., Rovers M.M., Molenaar J.M., Winkler-Seinstra P.L., Meijer A., Kimpen J.L. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 35.van Piggelen R.O., van Loon A.M., Krediet T.G., Verboon-Maciolek M.A. Human rhinovirus causes severe infection in preterm infants. Pediatr Infect Dis J. 2010;29:364–365. doi: 10.1097/INF.0b013e3181c6e60f. [DOI] [PubMed] [Google Scholar]
- 36.Miller E.K., Bugna J., Libster R., Shepherd B.E., Scalzo P.M., Acosta P.L. Human rhinoviruses in severe respiratory disease in very low birth weight infants. Pediatrics. 2012;129:e60–e67. doi: 10.1542/peds.2011-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hao Q., Lu Z., Dong B.R., Huang C.Q., Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2011;9:CD006895. doi: 10.1002/14651858.CD006895.pub2. [DOI] [PubMed] [Google Scholar]