Abstract
Understanding the molecular and cellular mechanisms underlying membrane traffic pathways is crucial to the treatment and cure of human disease. Various human diseases caused by changes in cellular homeostasis arise through a single gene mutation(s) resulting in compromised membrane trafficking. Many pathogenic agents such as viruses, bacteria, or parasites have evolved mechanisms to subvert the host cell response to infection, or have hijacked cellular mechanisms to proliferate and ensure pathogen survival. Understanding the consequence of genetic mutations or pathogenic infection on membrane traffic has also enabled greater understanding of the interactions between organisms and the surrounding environment. This review focuses on human genetic defects and molecular mechanisms that underlie eukaryote exocytosis and endocytosis and current and future prospects for alleviation of a variety of human diseases.
Key words: Membrane traffic, Secretory pathway, Endocytosis, Exocytosis, Secretion, Genetic disease
I. Introduction
The human cell is a complex network of membranes and protein enclosed in a membrane lipid bilayer. The interactions within and associated with such biomembrane bilayers have profound consequences for the organism as a whole; a single defect in just 1 of the potential 30–40,000 gene products made by each cell can cause devastating, if not fatal, effects for the whole organism. In addition to this, humans pass genetic information onto their offspring and, with it, any genetic mutations or polymorphisms. It is believed that at least 1 in 10 people have, or will eventually develop, a disease caused by mutation or variation at the gene level. Understanding how genetic mutations increase risk for human disease is critical in our understanding and treatment of the majority of human ailments that are caused by interactions between the organism and the environment.
This review focuses on the research undertaken in the past 30 years relating to the molecular mechanisms that underlie membrane trafficking within eukaryotic cells. We address mechanisms and factors that control protein progression through the secretory and internalization pathways and highlight key human diseases that illuminate mechanisms of membrane trafficking. In addition, current and future strategies for therapeutic intervention in such genetic disorders are considered.
II. Principles of Membrane Traffic in Eukaryotic Cells
Common to all eukaryotic cells is the presence of multiple biomembrane lipid bilayer compartments, or organelles, which are maintained by specific protein–protein and protein–lipid interactions. Such interactions are maintained within each compartment in spite of continuous trafficking of membrane‐bound and soluble components to different intracellular locations, and for secretion from the cell. In the majority of cases, this transfer of material occurs through vesicular movement: fission, docking, and fusion of membrane bilayer‐enclosed intermediates occurs between donor and acceptor compartments (Palade, 1975). Proteins, including membrane‐bound receptors, secreted enzymes, and antibodies, begin their journey by entering the early secretory pathway at the endoplasmic reticulum (ER). From here they are transported through the Golgi apparatus and finally distributed to their final destination such as other intracellular organelles, the plasma membrane, or the extracellular environment.
But how does a specific protein “know” how to reach a specific cellular destination when hundreds of newly synthesized, different molecules require specific transport and targeting? Many of these transport intermediates or vesicles, whether derived from the ER, other internal organelles, or the plasma membrane, are “coated” with unique protein complexes, tethering factors, and regulatory factors that ensure correct targeting to an acceptor compartment. Vesicle coat proteins, such as the clathrin or coat protein (COP) complexes, are relatively well studied. Such complexes are assembled onto the cytoplasmic face of donor compartments to facilitate the fission of transport intermediates. Allied with these coat proteins are different molecules that mediate recognition of cytoplasmic motifs in cargo proteins either directly (e.g., transmembrane proteins) or indirectly (e.g., soluble secreted enzymes).
The SNARE hypothesis is central to our understanding of vesicular targeting to intracellular compartments (Rothman 1994, Sollner 1993). Initially uncovered in a screen for intra‐Golgi transport docking and fusion regulators, the SNARE (soluble N‐ethylmaleimide‐sensitive fusion attachment protein receptor) proteins have been found to regulate different membrane interactions in all eukaryotes via a highly conserved mechanism for membrane trafficking based on accessory docking and fusion regulators. SNARE proteins are present on both the vesicle (vesicular or v‐SNARE) and the acceptor (target or t‐SNARE) and comprise coiled‐coil domains that assemble to facilitate vesicle docking and membrane fusion (Bennett 1995, Pelham 2001).
In conjunction with SNARE proteins, small Ras‐related Rab GTPases are implicated in further ensuring the fidelity of vesicle docking and fusion (Olkkonen and Stenmark, 1997). These 20‐ to 25‐kDa proteins are GTP‐hydrolyzing enzymes that act to recruit different proteins or effectors to membranes in a GTP/GDP‐regulated manner (Collins, 2003). Rab GTPase activity and protein conformation are regulated by interaction with soluble and membrane‐bound proteins; such regulators can also tether vesicles to acceptor membranes and mediate intracellular signaling.
III. Secretory Pathways
A. Early Secretory Pathway
1. ER Quality Control
The endoplasmic reticulum (ER) is the first stage of quality control along the secretory pathway. Proteins destined for secretion (e.g., hormones), the plasma membrane (e.g., membrane‐bound receptors), or other intracellular membrane compartments such as the lysosome (e.g., lysosomal proteases) are cotranslationally inserted into the ER lumen through a protein complex referred to as the Sec61 translocon (Swanton and Bulleid, 2003). Here they are folded, glycosylated, and, in some cases, assembled into oligomeric complexes before passage along the secretory pathway to the Golgi apparatus. Proteins in transit through the Golgi apparatus can be subject to the action of proteases and glycosylating enzymes, resulting in modifications characteristic of passage through a specific subcompartment. Secreted proteins and lipids are finally sorted at the trans‐Golgi network (TGN) to their final destination. The ER therefore plays a rate‐determining role as the first compartment along this route by ensuring proteins are assembled and folded correctly before ER export. The ER thus contains a variety of resident enzymes, lectins, and chaperones that perform the quality control steps involved in protein assembly and export. A protein that does not pass this initial quality assessment, perhaps because of a mutation that does not allow correct folding or oligomerization, will be retained within the ER and subsequently degraded (Section III.A.2). In severe cases, where the ER cannot remove such a misfolded protein, an ER stress response is initiated that results in apoptosis, or cell suicide, in an attempt to preserve the functionality of the tissue or organ (Kaufman, 1999).
The effectiveness of the ER as a quality control checkpoint along the secretory pathway is reflected by the large variety of genetic mutations in proteins that cause aberrant ER retention, accumulation, or activation of the ER stress response (see Table I ). An important human disease that highlights this phenomenon is cystic fibrosis (CF): nearly 70% of CF patients have a 3‐bp deletion in the gene encoding the chloride channel transmembrane regulator (CFTRΔF508) (Bertrand and Frizzell, 2003), which causes defective chloride transport across the apical epithelial membrane and enhanced sodium absorption through various basolateral membrane Na+/K+‐ATPases. These changes lead to a net increase in water absorption and a characteristic thickening of lung mucus in CF patients. Whereas both wild‐type CFTR and CFTRΔF508 interact with ER chaperones, mutant CFTR shows prolonged interaction with ER chaperones Hsp70/Hdj‐1 and calnexin (Amaral 2004, Pind 1994).
Table I.
Human Diseases and Associated Membrane Trafficking Defects
| Human disease | Protein | Membrane trafficking defect | Clinical features | References | OMIMa |
|---|---|---|---|---|---|
| α1‐Antitrypsin deficiency | α1‐Antitrypsin | Inhibited export from the ER of this secreted protein. Lung and liver damage by proteases | Emphysema and liver cirrhosis | (Perlmutter, 2004) | 107400 |
| Acute myeloid leukemia | Endophillin II | Clathrin–coated pit formation | Leukemia | (Dreyling 1996, Jones 2001, Narita 1999, Tebar 1999) | 604465 |
| Alzheimer's disease | Presenilin 1 | Presenilin 1–involved in cleavage and trafficking of amyloid precursor protein to plasma membrane | Neurodegenerative disorder | (Uemura et al., 2004) | 104300 |
| Tau | Tau – microtubular stability through formation of aggregates | ||||
| Autosomal dominant polycystic kidney disease (ADPKD) | Polycystin‐1 or 2 | Causes a defect in E‐cadherin assembly and basolateral trafficking | Renal cysts in kidney and other tissues leading to end‐ stage renal failure | (Charron et al., 2000) | 173900 |
| Autosomal dominant retinitis pigmentosa | Rhodopsin | Inhibited interaction of rhodopsin and ARF4, leading to inhibited post‐Golgi delivery to rod outer segment | Narrowing of visual fields, night blindness | (Deretic et al., 2005) | 180380 |
| Autosomal dominant ventricular tachycardia | Ryanodine receptor | Mutations in lumenal and transmembrane domains | Cardiac arrhythmia, hyperthermia | (Yano et al., 2005) | 604722 |
| Autosomal recessive primary hyperoxaluria | Alanine‐glyoxylate aminotransferase | Mistargeting of peroxisomal proteins to mitochondria | Kidney disease | (Danpure, 1998) | 259900 |
| Aβ‐lipoproteinaemia | MTP | ER retention thus preventing ApoB secretion | Vascular disease | (Sharp et al., 1993) | 200100 |
| Batten's disease | CLN1‐CLN8 | Group of gene products implicated in regulating the processing and targeting of lysosomal and synaptic proteins | Neurological disease | (Pearce, 2000) | 204200 |
| Breast cancer | Caveolin‐1 | Deletion or dominant negative mutation of caveolin‐1 promotes tumor progression | Breast cancer | (Bouras et al., 2004; Williams and Lisanti, 2005) | 601047 |
| Brugada syndrome | SCN5A, α subunit of cardiac sodium channel | ER retention of sodium channel subunits and defective cell surface sodium transport | Cardiac disease | (Baroudi et al., 2004) | 601144 |
| Charcot‐Marie‐Tooth disease, demyelinating, type 1B | Myelin protein zero gene, MPZ | ER retention of integral membrane protein | Neurological and degenerative muscle disease | (Hayasaka 1993, Matsuyama 2002) | 118200 |
| Charcot‐Marie‐Tooth disease, axonal, type 2A1 | KIF1B | Microtubular transport of synaptic vesicles | Neurological and degenerative muscle disease | (Zhao et al., 2001) | 118210 |
| Chediak‐Higashi syndrome (CHS) | CHS1/Lyst | Lyst involved in regulation of protein secretion from lysosomes – enlarged lysosomes | Partial albinism, recurrent bacterial infections, impaired chemotaxis and abnormal natural killer cell function | (Shiflett 2002, Ward 2003) | 214500 |
| Choroideremia (CHM) | Rab Escort Protein 1 (REP1) | RAB27a remains cytosolic due to defective geranylgeranyl modification in CHM lymphoblasts | X‐linked form of retinal degeneration | (Seabra et al., 2002) | 303100 |
| Combined factors V and VIII deficiency | ERGIC‐53/p58 C‐type lectin | ER retention and defective secretion of factors V and VIII | Blood disease | (Nichols et al., 1998) | 227300 |
| Congenital Finnish nephritic syndrome | Nephrin (NPHS1), podocin (NPHS2) | ER retention | Kidney inflammation | (Kestila 1998, Kramer‐Zucker 2005) | 256300 600995 |
| Congenital hyperinsulinism | Pancreatic ATP‐sensitive potassium channel (K‐ATP) | ER or Golgi retention of K‐ATP due to mutations in its sulfonylurea‐1 (SUR1) subunit | Excess insulin leading to hypoglycaemia | (Dunne 2004, Yan 2004) | 602485 |
| Congenital hypothyroid goiter | Thyroglobulin | ER storage disease. Thyroglobulin is misfolded and accumulates in ER | Constipation, large tongue, swelling around the eyes, failure to suckle, mental retardation | (Hishinuma 1998, Kim 1998) | 188450 |
| Congenital sucrase‐isomaltase deficiency | Sucrase‐isomaltase | ER retention instead of brush border membrane localization | Gastrointestinal disease | (Naim et al., 1998) | 222900 |
| Cystic fibrosis | Cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel | Trafficking of the chloride channel to the plasma membrane is defective | Multi‐organ disease, most commonly lungs and pancreas | (Heda et al., 2001) | 219700 |
| Demyelinating polyneuropathy | EEA1 | Autoantibody against EEA1 | Limb weakness | (Selak et al., 2003) | 605070 |
| Dent's disease | CLC‐5 voltage‐gated chloride channel | Inhibited post‐Golgi transport to cell surface | Progressive renal failure | (Carr 2003, Ludwig 2005) | 300009 |
| Diabetes insipidus (nephrogenic) | Vasopressin V2 receptor | ER storage disease leading to retention of receptor in the ER | Excessive water secretion through kidneys (diabetes insipidus) | (Kim 1998, Morello 2000) | 304800 |
| Diabetes mellitus (Type 2) | Insulin receptor | Functional defects or ER retention | Diabetes mellitus; polyuria, polydipsia, tiredness, increased appetite | (Kadowaki et al., 1991) | 125853 |
| Dubin‐Johnson syndrome | MRP2 | ER retention | Liver disease | (Mor‐Cohen et al., 2001) | 237500 |
| Fabry's disease | α‐Galactosidase A | In this fat storage disorder, lysosomal α‐galactosidase is retained in the ER, preventing degradation of glycosphingolipids | Cloudiness of eyes, burning sensation in hands and feet, skin blemishes, renal failure, myocardial infarction | (Fan 1999, Garman 2002) | 301500 |
| Familial hemophagocytic lymphoschistiocytosis (FHL) | Perforin | Perforin – defective CTL (cytotoxic T lymphocytes) mediated killing | Immunodeficiency | (Feldmann 2003, Stepp 1999) | 603553 |
| Munc 13–4 | Munc 13–4 – inhibited release of secretory lysosomes from CTLs | ||||
| Familial hypercholesterolemia | Low density lipoprotein receptor (LDLR) | ER retention and degradation of LDLR | Increased blood cholesterol, atherosclerosis, heart disease | (Defesche, 2004) | 143890 |
| Familial intrahepatic cholestasis | MDR3 | ABC transporter of phosphatidylcholine out of cell | Liver disease | (de Vree et al., 1998) | 602347 |
| Griscelli syndrome | Myosin Va or Rab27A | Inhibited transport of melanosomes to plasma membrane in melanocytes | Albinism, silvery hair, neurological defects, immunodeficiency | (Menasche et al., 2000) | 214450 607624 |
| Hereditary myeloperoxidase | MPO | ER retention and degradation | Cancer, immunodeficiency | (DeLeo et al., 1998) | 606989 |
| Hereditary hemochromatosis | Hemochromatosis (HFE) | Mutant HFE fails to bind transferrin receptor at cell surface, resulting in iron overload | Liver cirrhosis, diabetes mellitus, cardiomyopathy | (Miyajima, 2002) | 235200 |
| Hereditary spherocytosis | Chloride/bicarbonate anion exchanger | Misfolding and accumulation in the ER without rapid degradation or severe aggregation | Blood disease | (Quilty and Reithmeier, 2000) | 182900 |
| Hermansky‐Pudlak syndrome | β subunit of AP3 | AP3 – compromised lysosomal trafficking | Partial albinism, bleeding, ceroid accumulates in lysosomal structures | (Detter 2000, Huizing 2002) | 203300 |
| RabGGT‐α subunit | RabGGT‐α – inhibited Rab prenylation and membrane association | ||||
| Human neutropenia | Neutrophil elastase Occasionally other genes | Cyclic neutropenia. Excessive routing of NE to granules | Alternate 21 day cycling of neutrophils and monocytes | (Benson 2003, Berliner 2004, Horwitz 2004) | 162800 |
| Severe congenital neutropenia. Impaired association with AP3; NE redirected from lysosome to plasma membrane | Promyelocytic arrest in bone marrow | 202700 | |||
| Huntington's disease | Huntingtin (htt) | Microtubular transport of BDNF | Neurodegeneration | (Gauthier et al., 2004) | 143100 |
| I‐cell disease | NAGT1 phosphotransferase | Defect in mannose‐6‐ phosphate addition to lysosomal enzymes resulting in aberrant targeting | Neurological disease | (Ben‐Yoseph et al., 1987) | 252500 |
| Laron syndrome | Growth hormone receptor | Low levels of cell surface protein caused by ER retention | Dwarfism | (Wojcik et al., 1998) | 245590 |
| Leukocyte adhesion deficiency type I | CD18 | Leukocyte rolling and adhesion during immune reaction | Recurrent bacterial and fungal infections, poor wound healing | (Hogg 1999, Mathew 2000) | 116920 |
| Limb girdle muscular dystrophy 1C, rippling muscle disease, distal myopathy | Caveolin‐3 | Abnormal caveolin‐3 traps normal caveolin‐3 in the Golgi of skeletal muscle cells | Muscle diseases | (Woodman et al., 2004) | 601253 |
| Lissencephaly | LIS1 or doublecortin | Microtubular motor function or stabilization of microtubules | Retardation, epilepsy | (Reiner et al., 1993) | 607432 |
| Listeria monocytogenes infection | Hepatocyte growth factor receptor (HGFR) | HGFR on the host cell internalizes bacteria via binding to the surface protein internalin B | Symptoms of food poisoning | (Li, et al., 2005b) | 164860 |
| Long QT‐2 syndrome | Human ether‐a‐go‐go (HERG) potassium channel | ER retention of HERG preventing trafficking to cell surface | Abnormal electrical cardiac impulses and ventricular tachycardia | (Kupershmidt et al., 2002) | 192500 |
| Menkes' disease | ATP7A (Menkes disease protein) | Mislocalization and/or degradation of this copper transporter leads to copper deficiency | Mental retardation, skeletal abnormalities, kinky hair. Usually lethal before the age of 3 | (Lutsenko and Petris, 2003) | 309400 |
| Multiple exostoses syndrome | Golgi‐localized EXT1 and EXT2 complex | Mutations reducing glucoronyltransferase and N‐acetyl‐D‐ glucosaminotransferase activity inhibit post‐ER transport of EXT1/EXT2 | Skeletal dysplasia, connective tissue disorder | (McCormick et al., 2000) | 133700 |
| Nephrogenic diabetes insipidus | Water channel aquaporin‐2 | ER retention; some AQP2 mutants can leave ER using chemical chaperones | Excessive water secretion through kidneys (dilute urine and excess water loss) | (Tamarappoo et al., 1999) | 125800 |
| Niemann‐Pick disease type C | NPC1 | Lysosomal accumulation of LDL‐derived cholesterol | Neurodegenerative disease | (Liscum, 2000) | 257220 |
| Occipital horn syndrome | ATP7A (Menkes disease protein) | ER retention of ATP7A results in a milder form of Menkes disease | Mental retardation, skeletal abnormalities | (Kaler 1998, Qi 1998) | 304150 |
| Ocular and oculocutaneous albinism | Tyrosinase, GPCR‐like OA1 gene product | Unstable or mislocalized proteins | Eye pigmentation defects | (D'Addio et al., 2000) | 300500 |
| Oculocerebrorenal syndrome of Lowe | OCRL1 | Perturbed endosome‐to‐ TGN trafficking | Cataracts, mental retardation, renal failure | (Lowe, 2005) | 309000 |
| Osteogenesis imperfecta | Type I collagen | Defective trafficking or ER retention of collagen | Brittle bones and teeth, hearing loss | (Pochampally et al., 2005) | 166200 |
| Paraneoplastic stiff‐person syndrome | Amphiphysin I | Clathrin‐coated vesicle formation compromised | Autoimmune disease | (De Camilli et al., 1993) | 184850 |
| Pelizaeus‐Merzbacher disease | Proteolipid protein (PLP) gene | ER retention leading to ER stress signaling and apoptosis | Neurological disease | (Gow et al., 1998) | 312080 |
| Pendred syndrome | Pendrin (anion transporter) | ER retention of a cell surface iodide transporter | Hypothyroidism, deafness | (Taylor et al., 2002) | 274600 |
| Persistent hyperinsulinemic hypoglycemia of infancy (PHHI) | Potassium channel (Kir6.2) and sulfonylurea receptor (SUR1) subunits | ER or TGN retention of a cell surface potassium channel involved in insulin secretion | Hyperinsulinism, hypoglycemia | (Taschenberger et al., 2002) | 601820 |
| Prion diseases | Prion protein (PrP) | Abnormal PrP accumulate in the ER; perturbed trafficking to cell surface | Neurodegenerative disorders | (Harris, 2003) | 176640 |
| Rhizomelic chondrodysplasia puncta | Pex7 | Defective import of peroxisomal matrix proteins | Skeletal defect, neurological disease | (Terlecky and Fransen, 2000) | 215100 |
| Stargardt‐like macular dystrophy | ABCA4 gene; vitamin A transport | Defective localization | Blindness | (Edwards 2001, Sun 1999) | 248200 |
| Usher's syndrome | Myosin VIIA | Melanosome transport | Blindness, deafness | (Liu et al., 1998) | 276903 |
| Wilson's disease | ATP7B (Wilson's disease protein) | ER retention and inhibited secretion of copper‐containing enzymes from liver | Neurological disease, liver cirrhosis | (Cox and Gitlin, 2003; Moore, 2002) | 277900 |
| Wiskott‐Aldrich syndrome | Wiskott‐Aldrich syndrome protein (WASP) | WASP regulation of actin cytoskeleton | Immunodeficiency, autoimmune disease, hematologic malignancy | (Burns et al., 2004) | 301000 |
Online Mendelian Inheritance in Man reference at http://www.ncbi.nlm.nih.gov/omim/.
Another key example of misfolded proteins being retained in the ER is Menkes disease, a rare and severe X‐linked recessive disorder characterized by abnormal hair, neurodegeneration, and early childhood fatality. The disease is due to copper deficiency along the secretory pathway caused by the malfunctioning of the Menkes disease protein (ATP7A). This gene product is a multiple transmembrane domain protein and copper transporter of the P‐type ATPase family responsible for translocating copper ions across intracellular membranes. Fibroblasts from patients who carry a genetic mutation resulting in the G1019D amino acid substitution in ATP7A show ER retention of this P‐type ATPase (Kim et al., 2002).
Three forms of Menkes disease can arise from different mutations in the ATP7A gene: premature stop codons, deletions, or splicing defects. These can prevent ATP7A function and/or trafficking. Classical Menkes disease is the most common and fatality usually results by the age of 3 years. In two other nonfatal forms of Menkes, mild and occipital horn syndrome, ATP7A maintains the ability to transport copper ions across intracellular membranes, although trafficking to the plasma membrane can be compromised (La Fontaine et al., 1999).
ATP7A is ubiquitously expressed and is the major copper transporter in cells of the intestine, kidney, and brain. In the liver, however, the major copper transporter is the Wilson's disease protein, ATP7B (Bull et al., 1993). This second P‐type ATPase shares strong similarity with ATP7A and also translocates copper ions across membranes. Although these gene products share functional similarities, mutations in ATP7B result in copper accumulation in the liver and brain.
Familial hypercholesterolemia is an autosomal dominantly inherited disease caused by mutations in the low‐density lipoprotein receptor (LDLR), leading to premature atherosclerosis and coronary heart disease. In healthy individuals, the LDLR is expressed on the surface of cells, where it binds circulating LDL particles and promotes uptake and cellular metabolism of its constituents, which includes cholesterol. In these patients, LDLR alleles display amino acid substitutions (Cassanelli 1998, Jensen 1997), truncations (Lehrman et al., 1987), or missense mutations (Leitersdorf et al., 1993), which can result in ER retention and degradation.
The point mutation at residue 209 of the insulin receptor compromises the ability of the receptor to dimerize correctly within the ER, therefore leading to ER retention. Decreased plasma membrane levels of insulin receptor cause inhibited insulin binding after stimulus by a meal, and subsequent elevations in plasma glucose levels. This then leads to type II diabetes mellitus (Kadowaki et al., 1991).
A number of human diseases can induce the ER stress response. Here, the mutant protein is retained within the ER, resulting in either dilation of the organelle, such as in congenital hyperthyroidism (Medeiros‐Neto et al., 1996) and hypofibrinogenemia (Callea et al., 1992), or chronic ER stress as is the case for hereditary emphysema (Perlmutter, 2003). In Pelizaeus‐Merzbacher disease, an X‐linked leukodystrophy disease, ER accumulation of proteolipid protein (PLP) results in oligodendrocyte apoptosis (Gow et al., 1998) and the subsequent disruption of white matter formation in the brain observed in humans and mouse models. PLP is a central nervous system protein that is the major component of myelin and, when expressed in cultured fibroblasts, is localized to the plasma membrane (Gow et al., 1994). The link between PLP and the ER stress response provides a tool for elucidating the cellular response to misfolded protein accumulation (Swanton et al., 2003).
2. ER‐Associated Degradation
Accumulation of proteins within the ER, leading to blockage of protein secretion, is an unwanted cellular property and mechanisms have evolved to overcome such events. This disposal of unwanted proteins is termed ER‐associated degradation (ERAD) (Fig. 1 ). As recently as the early 1990s, it was still believed that aberrant proteins were degraded within the ER (Fra and Sitia, 1993); however, current models suggest that aberrant ER‐retained proteins actually undergo retrotranslocation and subsequent degradation in the cytoplasm. Retrotranslocation has been proposed to occur through the same “pore” used to translocate nascent proteins into the ER lumen during translation, namely the Sec61 translocon (Biederer 1996, Romisch 1999).
Fig. 1.
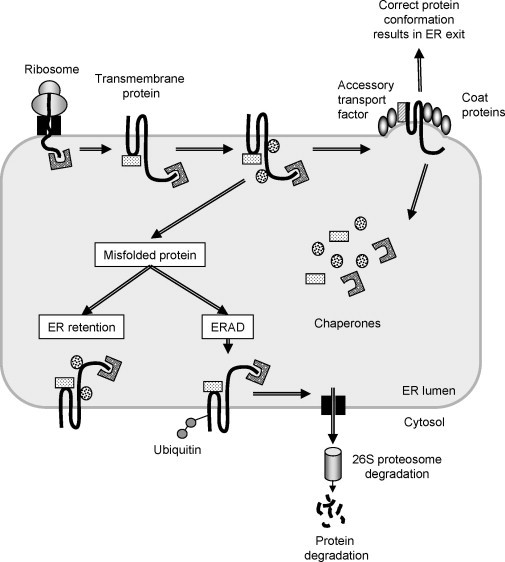
Quality control of protein assembly within the endoplasmic reticulum. Proteins destined for the secretory pathway (this example shows a transmembrane protein) are cotranslationally translocated from the ribosome into the lumen of the endoplasmic reticulum (ER) through a portal referred to as the Sec61 translocon. As the newly synthesized protein enters the ER, quality control mechanisms in the form of protein chaperones bind to it and fold it to its correct conformation. Further processing occurs through interactions with other chaperones before the successfully folded protein is loaded into COPII‐coated vesicles and shuttled from the ER to the Golgi apparatus. However, if the protein carries a mutation that causes it to take on an aberrant conformation the ER chaperones will trigger a misfolded protein response. This has two outcomes: either the chaperones will remain bound to the misfolded protein, preventing its escape from the organelle (ER retention), or the protein will be ubiquitinated and retrotranslocated through the Sec61 complex for proteasomal degradation in the cell cytoplasm. A number of human genetic diseases are a result of key proteins failing to traffic through the secretory pathway and as a consequence are retained or degraded in this manner.
Various yeast and mammalian proteins have been shown to be retrotranslocated from the ER and degraded within the cytoplasm in a proteasome‐dependent manner, including the budding yeast proteins carboxypeptidase Y, and a mutant pro‐α‐factor. When a mammalian protein such as CFTR is expressed in budding yeast, it matures relatively slowly within the yeast ER, leading to retrotranslocation to the cytoplasm and degradation (Ward et al., 1995). A further example is that of α1‐antitrypsin deficiency. α1‐Antitrypsin is responsible for inactivating the enzyme elastase produced by lung neutrophils. In this inherited disease, a mutated form of α1‐antitrypsin is retrotranslocated and degraded in proteasomes, leading to retention of active elastase in lung tissues and thus is a cause of lung emphysema (Rutishauser and Spiess, 2002).
However, retrotranslocation and proteasomal degradation may not be functionally coupled processes. Pharmacological inhibitors that cause proteasome inactivation lead to egress of molecules such as MHC class I (Wiertz 1996a, Wiertz 1996b), ribophorin (de Virgilio et al., 1998), and carboxypeptidase Y (Biederer et al., 1997) from the ER to the cell cytoplasm. In contrast, inhibition of protein ubiquitination results in the retention of such molecules within the ER. Schmitz et al. (2004) suggest that two distinct proteasome‐regulated pathways mediate degradation of retrotranslocated β‐amyloid precursor protein.
Interestingly, endocytosed toxins that target key cytosolic factors appear to use the ERAD pathway to move out of the ER and into the cytosol (Deeks 2002, Hazes 1997). Cholera and ricin toxins are routed from the cell surface through the Golgi apparatus and to the ER before being retrotranslocated into the cell cytosol. It is believed that the unusually low lysine content of these protein toxins prevents subsequent ER‐associated ubiquitination for degradation by the cytosolic proteasome.
3. Protein Traffic between the ER and Golgi Apparatus
Protein cargo is shuttled between the ER and Golgi within vesicular intermediates or 50‐nm‐diameter spherical vesicles containing coat protein complexes such as COPI or COPII. Initially discovered in mammals and yeast (Kaiser 1990, Malhotra 1989, Novick 1980, Rothman 1996), COP complexes are required for the formation of vesicles at the ER, ER–Golgi intermediate compartment (ERGIC), and Golgi apparatus. COP recruitment to membranes facilitates the specific capture, packaging, transport, and delivery of membrane‐bound and soluble protein cargo to an acceptor compartment.
COPII recruitment to sites on the smooth ER initiates the formation of anterograde (forward) transport vesicles. These COPII vesicles move from the ER to the ERGIC, or vesicular tubular clusters (VTCs). From here, COPI‐coated vesicles are thought to mediate the continued anterograde movement from the ERGIC to the cis face of the Golgi apparatus (Scales et al., 1997).
The Sar1p GTPase regulates COPII vesicle formation via interaction with the Sec12p guanine exchange factor (GEF). Sec12p‐mediated activation of Sar1p to a GTP‐bound form leads to recruitment of the Sec23p–Sec24p heterodimer to membranes; this also initiates protein cargo selection within the ER and recruitment of v‐SNAREs such as Bet1p and Bos1p. Binding of Sec23p–Sec24p mediates further recruitment of the Sec13p–Sec31p complex. This COPII complex then acts as a protein scaffold that causes deformation of the membrane, resulting in vesicular fission, with anterograde movement of protein cargo‐containing COPII vesicles to the ERGIC. COPII docking at an acceptor compartment is thought to trigger Sec23p function, causing a conformational change in Sar1p and GTP hydrolysis and dissociation or uncoating of the COPII complex. Thus COPII vesicle docking and fusion with an acceptor compartment are mediated by cognate v‐SNARE/t‐SNARE interactions (Kirchhausen 2000, Kuehn 1998, Matsuoka 1998, Tang 2005).
A severe hereditary bleeding disorder called combined deficiency of factor V factor VIII (F5F8D) highlights the functional importance of trafficking between the ER and ERGIC. Some F5F8D patients are deficient in the ERGIC‐localized ERGIC‐53 (LMAN1) protein and display defective secretion of the factor V and VIII clotting factors. ERGIC‐53 is a mannose‐binding lectin that acts as a “cargo receptor” and recycles between the ER and ERGIC (Neerman‐Arbez 1999, Nichols 1998). However, ∼30% of F5F8D patients show normal levels of ERGIC‐53/LMAN1, but are deficient in an associated protein, MCFD2, another ERGIC resident that interacts with ERGIC‐53/LMAN1 in a calcium‐dependent manner (Zhang et al., 2003).
Small intestinal cells called enterocytes absorb fats and fat‐soluble vitamins from food in the form of fatty acids and monoglycerides. The fats enter the lumenal surface of absorptive enterocytes by free diffusion across their membranes, and emerge from the basolateral surface as particulate structures referred to as chylomicrons. Formation of chylomicrons occurs within the ER and Golgi apparatus by vesicular transport before being trafficked from the Golgi to the plasma membrane. Chylomicron retention disease (CMRD), Anderson disease, and a neuromuscular disorder, CMRD associated with Marinesco‐Sjögren syndrome (CMRD‐MSS), are examples of inherited diseases that result in compromised fat absorption, low blood cholesterol, and severely depleted blood chylomicron levels. Jones et al. (2003) identified eight mutations in the Sar1p gene product and COPII component associated with these lipid absorption diseases, thus strongly implicating a role for the COPII vesicular transport system in the movement of dietary fats from the intestine to the circulating bloodstream.
COPII mediates anterograde traffic from the ER to the Golgi apparatus; however, COPI vesicles appear to function primarily in the retrograde (backward) transfer of proteins from the Golgi and ERGIC back to the ER. This retrograde traffic is necessary for recovering escaped ER resident proteins, coat and SNARE proteins that have arrived at the ERGIC and Golgi from COPII vesicles, or glycosylation enzymes that have been incorrectly modified (Duden 2003, Lee 2004). The Golgi‐associated COPI coatomer is a complex of seven polypeptides: α‐, β‐, β′‐, γ‐, δ‐, ε‐, and ζ‐COP gene products, which interact with the donor membrane to form COPI vesicles. Vesicle formation is triggered by the GTPase ADP‐ribosylation factor 1 (ARF1), which recruits COPI coatomer to the donor membrane. Transmembrane proteins containing cytoplasmic lysine‐based motifs such as KKXX or KXKXX, or soluble proteins containing the C‐terminal KDEL motif, are recycled by COPI‐coated vesicles from the Golgi apparatus back to the ER. The KDEL motif, present in soluble ER chaperones such as BiP and protein disulfide isomerase, is recognized by the membrane‐bound KDEL receptor (Majoul et al., 2001). In both cases, cytoplasmic motifs in these transmembrane proteins are recognized and bound by COPI coatomer, promoting inclusion into vesicles destined for the ER. Actin microfilaments are also involved in this retrograde transport step (Valderrama et al., 2001). This Golgi–ER step is regulated by the GTPase Cdc42 and N‐WASP protein (Luna et al., 2002), factors previously implicated in actin‐linked processes at the plasma membrane.
Live imaging of cells expressing an engineered fluorescent and temperature‐sensitive vesicular stomatitis virus G‐glycoprotein (ts045VSVG) demonstrated sequential action of COPII‐ and COPI‐coated vesicles (Scales et al., 1997). VSVG accumulated in structures close to the ER that contained intermediate compartment resident proteins. These structures then matured into vesicles that contained COPI proteins. Stephens et al. (2000) showed that this “segregation” between COPII and COPI vesicles occurred at a location in close proximity to exit sites on ER membranes.
A COP‐independent mechanism has also been implicated in retrograde traffic between the Golgi apparatus and the ER. The Rab6 GTPase is implicated in regulating the movement of bacterial Shiga toxin B fragment (STB) via a retrograde step from the Golgi apparatus to the ER. Expression of a dominant‐negative GDP‐bound form of Rab6 inhibited STB retrograde movement, whereas COPI transport was unaffected (White et al., 1999).
4. Intra‐Golgi Transport
The Golgi apparatus is composed of flattened cisternae and membrane compartments that are closely juxtaposed in a stack‐like appearance. In mammalian cells these stacks are positioned end‐to‐end, forming a ribbon‐like structure near the nucleus (Barr and Warren, 1996). The Golgi apparatus is a highly dynamic organelle sited at the hub of the secretory pathway with key processing and sorting functions. The Golgi is a polarized structure with proteins and lipids from the ER received at the cis side, followed by the medial and trans subcompartments, where further glycosylation modifications occur; the trans‐Golgi network (TGN) is the final subcompartment where sorting and packaging events take place. The Golgi apparatus also sorts proteins and lipids bound on a retrograde pathway from the cis‐Golgi back to the ER. In addition, proteins can also return to the TGN from the endomembrane/lysosomal system (Fig. 2 ).
Fig. 2.
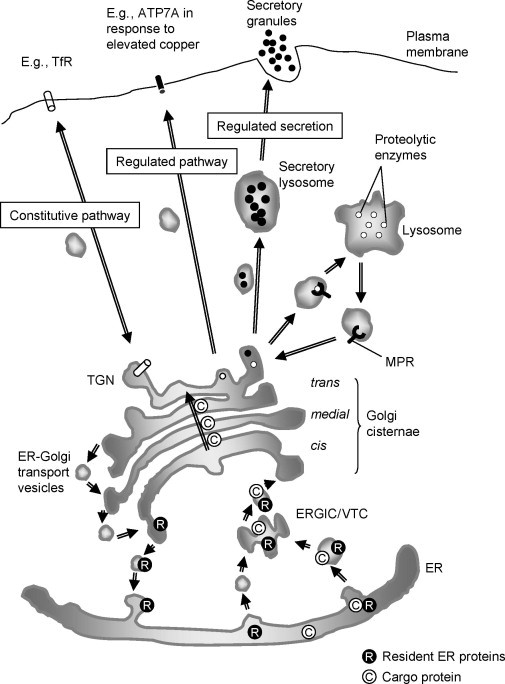
The secretory pathway and vesicular trafficking. Protein enters the secretory pathway at the endoplasmic reticulum (ER) and is trafficked in COPII‐coated vesicular structures to the intermediate compartment (ERGIC/VTC), from which COPI‐coated vesicles carry it to the cis face of the Golgi. Cargo protein (C) continues along the secretory pathway through the Golgi apparatus to the trans‐Golgi network (TGN). Retention signals in ER resident proteins (R) ensure they undergo retrograde trafficking from the Golgi in COPI vesicles. Retrograde transport of Golgi enzymes that may have escaped their resident cisternae also occurs in COPI‐coated vesicles. At the distal face of the Golgi the TGN ensures the correct targeting of proteins, either constitutively or regulated to the plasma membrane, or to intracellular membrane compartments such as proteolytic or secretory lysosomes.
Controversy exists regarding the mechanism for anterograde movement of cargo proteins within the Golgi apparatus. The Golgi apparatus contains secretory proteins that can vary in physical size, from relatively small polypeptides to large, bulky multisubunit complexes; all need to reach the TGN for final sorting into transport intermediates. There are also resident glycosylation enzymes that have spatially restricted functions within the Golgi, that is, enzymes that function within specific subcompartments to ensure the correct addition or trimming of N‐ and O‐linked sugars on secreted proteins as they progress through the pathway. This raises a key question: how do protein and lipid cargo move through the Golgi apparatus while resident enzymes retain their localization? We know that many Golgi enzymes contain transmembrane Golgi localization signals that mediate targeting to a specific compartment (Munro, 1998). Two models have been proposed: the cisternal maturation model and the vesicular transport model (Elsner 2003, Storrie 2000). Briefly, the cisternal maturation model suggests that large proteins or aggregates remain within a single Golgi cisterna, which matures through the retrograde transfer of resident enzymes via COPI vesicles. In contrast, the vesicular transport model proposes that newly synthesized protein is trafficked from cisterna to cisterna via COPI‐coated vesicles that sequentially bud off membranes and fuse with the next subcompartment. In either case, COPI‐coated vesicles play a central role in intra‐Golgi transport. A number of SNARE proteins, such as membrin, rbet1, GS27, and syntaxin‐5, have also been localized to the Golgi apparatus and are required for intra‐Golgi transport and homeostasis (Nichols and Pelham, 1998).
Golgi‐tethering molecules called golgins and Golgi reassembly stacking proteins (GRASPs) belong to a family of regulatory factors involved in Golgi maintenance and vesicular transport. The reader is pointed to an in‐depth review that covers golgins in more detail (Short et al., 2005). In brief, the golgins can be anchored to Golgi membranes through various mechanisms and contain characteristic coiled‐coil domains that extend from the membranes as a rod‐like structure (Burkhard et al., 2001). Golgins such as giantin and golgin‐84 are securely anchored to the membrane via a transmembrane domain near their C terminus. Electrostatic or ionic interactions mediate the attachment of other golgins to membranes. For example, proteins of the GRASP family (GRASP65 and GRASP55) bind to GM130 and golgin‐45 to recruit these factors to the cis and medial Golgi membranes, respectively. Moreover, a large number of golgins are recruited to membranes via interactions with the Rab, ARF, and ARL (ARF‐like) GTPases. Vesicular and cis‐Golgi membrane recruitment of golgin p115 is regulated by Rab1, whereas membrane attachment of yeast golgin Rud3p is regulated by ARF1p. Golgin‐97 binds to membranes by interaction with ARL1p, a member of a new class of ARF‐like GTPases termed ARLs (Short et al., 2005). Interestingly, autoantibodies directed against giantin, golgin‐245, golgin‐160, GM130, and golgin‐97 golgins and GRASPs are present in patients with autoimmune conditions such as Sjögren's syndrome and systemic lupus erythematosus.
In Sjögren's syndrome, moisture‐producing glands are targeted by the autoimmune response, resulting in dry eyes and mouth (Lichtenfeld et al., 1976). Systemic lupus erythematosus is a chronic rheumatic condition that affects joints and muscles, causing skin rash and kidney problems. Sjögren's syndrome patients can also simultaneously display both rheumatoid arthritis and systemic lupus erythematosus.
Golgi biogenesis requires golgin function at different stages during cell division. Mammalian p115 is crucial for maintenance of the stacked nature of the Golgi cisternae (Puthenveedu and Linstedt, 2004). During mitosis, the Golgi stack disperses into clustered vesicles. These vesicles then fuse in the daughter cells to form new cisternae, alignment and stacking of which result in the formation of a fully functional organelle. GRASP65 tethers have been proposed to hold cisternae in close proximity through interactions with p115 and GM130 (Shorter and Warren, 1999). The golgin p115 is also involved in tethering COPI vesicles to Golgi membranes (Sonnichsen et al., 1998) and may be needed for SNARE complex assembly (Shorter et al., 2002). The budding yeast p115 homolog (Uso1p) tethers COPII‐coated vesicles to Golgi membranes during anterograde transport from ER exit sites to the cis‐Golgi (Barlowe 1997, Cao 1998, Sapperstein 1996). Mammalian p115 is also essential for the tethering of transport vesicles to the cis‐Golgi (Alvarez et al., 2001) and during intra‐Golgi transport (Seemann 2000, Waters 1992).
Golgins such as golgin‐84 are implicated in the regulation of Golgi structure and the formation of the Golgi ribbon (Diao et al., 2003). Golgin‐97 may function as a tethering molecule in retrograde traffic from the endosome to the TGN (Lu et al., 2004). Moreover golgins are also implicated as tethering components between the cytoskeleton and the Golgi apparatus (Short et al., 2005).
B. Late Secretory Pathway: Post‐TGN Trafficking
The trans‐Golgi network (TGN) is the final Golgi subcompartment where secreted proteins are sorted, packaged, and directed to their final destination. Trafficking from the TGN can occur in either a constitutive or regulated manner. Constitutive transport is the continuous release of protein from the trans‐Golgi network. Regulated secretion occurs in response to extracellular stimuli such as secretagogues, metal ions, hormones, or growth factors, which trigger the docking and fusion of secretory granules or vesicles with the plasma membrane.
Various mechanisms control the trafficking of proteins from the TGN by the formation and delivery of membrane‐derived transport vesicles to the plasma membrane, endosomes, or lysosomal structures (Ponnambalam and Baldwin, 2003). The expression of inactive (dominant‐negative) protein kinase D isoforms in tumor lines (Liljedahl et al., 2001), polarized canine kidney cells (Yeaman et al., 2004), and mouse fibroblasts (Prigozhina and Waterman‐Storer, 2004) has been shown to inhibit vesicle fission (release) from the TGN. Vesicle release is modulated by this family of kinases in response to cellular diacylglycerol (Baron and Malhotra, 2002) and binding to an as yet unknown effector protein on the cytoplasmic face of the TGN (Van Lint et al., 2002). In addition, the Cdc42 GTPase is linked to actin remodeling and has been shown to inhibit the exit of basolateral targeted proteins in polarized cells (Kroschewski 1999, Musch 2001) and copper‐regulated protein transport (Cobbold et al., 2002).
Copper is an essential element and cofactor required for functionality of many secreted enzymes (cuproenzymes). At steady state, ATP7A (Menkes disease copper transporter; Section III.A.1) resides in the TGN, where it provides newly synthesized cuproenzymes such as lysyl oxidase with copper ions as they traverse the secretory pathway. When intracellular copper ion levels rise, ATP7A responds to this environmental danger by redistributing to the plasma membrane in a Cdc42‐regulated manner (Cobbold et al., 2002). Here, ATP7A acts as a copper efflux pump to remove copper ions from the cytoplasm to maintain homeostatic function and prevent toxicity. When copper levels are reduced, ATP7A recycles back to the TGN. This endocytic internalization and sorting event is independent of both clathrin and caveolae (Cobbold et al., 2003), although relying on a cytoplasmic dileucine motif present in the ATP7A C–terminus (Francis 1999, Petris 1999).
Dent's disease, an X‐linked kidney disorder that presents with hypercalciuria, nephrocalcinosis (kidney stone formation), and progressive renal failure, is caused by missense, nonsense, and deletion mutations within the endosomal CLC‐5 voltage‐gated chloride channel. CLC‐5 is a member of a large family of voltage‐gated chloride channels that have a diverse array of cellular functions including membrane excitability, transepithelial ion transport, and cell volume regulation (Thakker, 1997). When expressed in Xenopus oocytes, a number of missense mutations in the CLC‐5 gene localized the channel to the Golgi apparatus and showed reduced conductance and significantly reduced plasma membrane (PM) localization (Ludwig et al., 2005). Similarly, expression of mutant CLC‐5 alleles in cultured cells revealed an approximate 5‐fold increase in Golgi retention (Carr et al., 2003).
IV. Internalization and Recycling
A. Receptor‐Mediated Endocytosis
1. Clathrin‐Coated Vesicles
Clathrin‐coated vesicles (CCVs) are a route for protein internalization conserved from yeast to humans. Roth and Porter (1964) first observed this process in mosquito oocytes and these vesicles have subsequently become one of the best characterized membrane transport steps in eukaryotes. Clathrin is one of the principal proteins involved in this transport step and, in combination with more than 25 clathrin‐associated factors, this unique structural component forms transport vesicles on the cytoplasmic face of the TGN, endosomes, and the plasma membrane. Clathrin‐coated vesicles bud from their donor membranes and are directed to target membranes by associated proteins and factors.
This highly conserved 600‐kDa clathrin complex comprises heavy (180 kDa) and light (25 kDa) chain proteins that are assembled into a three‐legged structure called a triskelion. Triskelions can be polymerized by accessory factors into striking lattice‐like “cages” comprising pentagons and hexagons, resembling a soccer ball structure or buckminsterfullerene. Clathrin cages are ∼70–120 nm in diameter; significantly larger than COPI or COPII vesicles (CCVs).
Clathrin‐coated vesicles are believed to assemble through a sequence of events that can be designated as activation, cargo capture, coat assembly, scission, movement, and vesicle uncoating (Kirchhausen, 2000). Members of a class of clathrin‐associated factor termed adaptor protein (AP) complexes are recruited to donor membranes through interactions with a docking complex, which then further interacts with motifs within the cytoplasmic tail of cargo proteins, resulting in “cargo capture.” This leads to clathrin cage assembly and the concomitant polymerization of the clathrin triskelion and resultant deformation of the donor membrane. Scission, or vesicle release from the plasma membrane, is believed to occur through the action of the GTPase dynamin and other accessory proteins, such as amphiphysin (Wigge et al., 1997). In the fruit fly Drosophila melanogaster, a dynamin gene mutation (shibire) causes temperature‐sensitive paralysis. This is likely due to a block in the endocytic uptake of synaptic vesicle proteins at the plasma membrane, leading to a block in recycling and reformation of competent synaptic vesicles at nerve terminals (Chen 1991, Koenig 1989, Kosaka 1983, van der Bliek 1991). The expression of a dominant‐negative GDP‐bound dynamin mutant, K44A, results in compromised CCV formation (Herskovits 1993, van der Bliek 1993) and inhibition of clathrin‐mediated internalization of the glucose transporter GLUT4 (Al‐Hasani et al., 1998), human immunodeficiency virus (HIV) (Daecke et al., 2005), and influenza virus (Roy et al., 2000). The scission function of dynamin is assisted by specific lipid‐modifying enzymes such as endophilin, synaptojanin, and phospholipase D (Bi 1997, Haffner 1997, Ringstad 1999, Schmidt 1999, Woscholski 1997). Finally, CCV uncoating at the target membrane occurs through the actions of the heat shock protein Hsc70 (Schlossman et al., 1984) and auxilin (Ungewickell et al., 1995).
Sorting of proteins from donor to target membranes involves the recognition of cytoplasmic sequences in membrane proteins by clathrin‐associated AP complexes. Four adaptor protein complexes (AP1–AP4), each comprising four different subunits, have been identified (Robinson, 2004). The AP1 complex is involved in clathrin‐coated vesicle formation at the TGN for transport to late endosomes; evidence has also implicated a role for this complex in a TGN‐to‐plasma membrane step (Folsch et al., 2003). AP2 is the best‐studied of the four complexes and mediates internalization of transmembrane receptors at the plasma membrane via clathrin‐coated vesicles. The AP3 complex is involved in trafficking from early endosomes to either late endosomes or lysosome‐related organelles such as melanosomes, platelet‐dense bodies, and antigen‐processing compartments. Finally, the AP4 complex was the last to be cloned (Hirst 1999, Dell'Angelica 1999a). In contrast to AP1–AP3, AP4 does not possess the β “ear” domain (see below), which allows interaction with clathrin and other cytosolic factors such as Eps15 and auxilin 2 (Lundmark and Carlsson, 2002). By electron microscopy, AP4 has been localized to vesicles at the TGN, plasma membrane, and early endosomes, although there is debate as to whether these vesicles are clathrin‐coated (Hirst 1999, Barois 2005). Interestingly, AP3 and AP4 may function independently of clathrin (Hirst 1999, Vowels 1998), suggesting the existence of another, as yet unidentified, coat protein that is analogous to clathrin.
All four AP complexes comprise two large 100‐kDa subunits: a β subunit (β1–β4) plus a γ (AP1), α (AP2), δ (AP3), or ε (AP4) subunit. In addition, each AP complex contains a 50‐kDa subunit (μ1–μ4) and a small 20‐kDa subunit (σ1–σ4). AP1, ‐2, and ‐3 contain two carboxyl “ear” domains connected to the head of each large 100‐kDa subunit by a flexible hinge of approximately 20–30 residues. Importantly, the ear domain of the β subunit and the hinge domains of the γ and α subunits have been shown to bind clathrin (Goodman 1995, Morgan 2000, Owen 2000), and consensus sequences in the hinge domains of β1 and β2 have clathrin‐binding properties (Dell'Angelica et al., 1998).
The β and μ subunits of the AP complex interact with motifs present in the cytoplasmic domains of transmembrane proteins to mediate cargo recruitment into clathrin‐coated vesicles. Such motifs include NPXY, YXXØ, and dileucine‐based sequences (Ø represents a bulky hydrophobic amino acid). One such motif, NPXY, is present in key cellular receptors such as low density lipoprotein receptor (LDLR), epidermal growth factor receptor (EGFR or Erb1), and insulin receptor, and mediates endocytosis and sorting. Importantly, the JD mutation (Y807C) in LDLR lies within this key motif and causes familial hypercholesterolemia (Knoblauch et al., 2000). The second tyrosine‐based motif, YXXØ, mediates plasma membrane internalization, lysosomal targeting, and basolateral targeting of cargo. This motif is found in lysosomal residents such as LAMP‐1 and ‐2, CD63, the recycling transferrin receptor (TfR), and TGN‐associated recycling membrane proteins, furin and TGN38. Di‐leucine motifs present on transmembrane transporters such as GLUT4 (glucose transporter), ATP7A, and mannose‐6‐phosphate receptors (M6PR) can fall into two categories: [DE]XXX[LI] and DXXLL related motifs. The [DE]XXX[LI] motif is associated with proteins internalized from the plasma membrane and targeted to lysosomes, while DXXLL motif is found in transmembrane proteins that shuttle between the TGN and endosomal system (Bonifacino and Traub, 2003).
Another class of clathrin‐associated factor is the Golgi‐localized, γ‐ear‐containing, ARF‐binding proteins (GGAs) found on the TGN and postulated to interact with AP1 to mediate transport of M6PR (Section V.B) to endosomes (Doray et al., 2002). GGAs can act as multifunctional adaptors that link transmembrane proteins, ARF GTPases, clathrin and accessory proteins at sites of CCV formation (Robinson and Bonifacino, 2001).
The disease oculocerebrorenal syndrome of Lowe (OCRL) is an X‐linked disorder caused by mutations in the OCRL1 gene (Lowe, 2005). The gene product is an inositol 5′‐phosphatase that catalyzes the removal of the phosphate from this position on the inositol moiety. The preferred OCRL1 substrate is PI(4,5)P2, a phosphoinositide shown to be important in endocytosis because of its central role in recruiting accessory proteins to CCVs (Padron et al., 2003). OCRL1 has been localized to clathrin‐coated vesicles associated with endosomal and TGN membranes (Choudhury et al., 2005). This is not surprising as OCRL1 interacts with clathrin and promotes its assembly into clathrin lattices and cages (Choudhury 2005, Ungewickell 2004). OCRL1 also interacts with the Rac1 GTPase that regulates actin dynamics, possibly via a GTPase activation domain to accelerate GTP hydrolysis (Faucherre et al., 2003). Although the exact function of OCRL1 is still unclear, the disease phenotype hints to OCRL1 function in membrane trafficking. OCRL1 mutations can cause loss of protein expression and phosphatase activity. RNAi‐mediated inhibition of OCRL1 expression in cultured human cells results in partial redistribution of a cation‐independent mannose‐6‐phosphate receptor and a TGN recycling protein (TGN46) to early endosomes (Choudhury et al., 2005). This suggests that loss of OCRL1 perturbs endosome‐to‐TGN vesicle transport, suggesting a functional requirement for this membrane traffic step. It is possible that OCRL1 plays a role in anterograde trafficking from the TGN‐to‐endosomes as well, since OCRL1 is abundantly present on TGN‐associated clathrin buds destined for the endocytic pathway.
OCRL disease symptoms include congenital cataracts, mental retardation, and renal tubular dysfunction (Lowe et al., 1952). Renal failure in OCRL patients is probably partly caused by defects in solute and protein re‐adsorption in kidney proximal tubules. This is likely due to missorting of megalin and cubilin, cell surface receptors involved in kidney solute uptake. In OCRL1 patients plasma membrane shedding of these receptors is reduced (Norden et al., 2002), indicating OCRL1 regulation of either receptor trafficking from the TGN‐to‐plasma membrane or recycling from plasma membrane‐to‐TGN.
Paraneoplastic stiff‐person syndrome (SPS) is a neurological autoimmune disease characterized by severe muscle stiffness and spasms, and often has secondary symptoms including diabetes, epilepsy, and breast cancer. Autoantibodies are produced against the clathrin‐associated regulator, amphiphysin I (De Camilli et al., 1993), a protein shown to bind dynamin in nerve terminals (David et al., 1996) and which is implicated in regulating the endocytosis of neuronal synaptic vesicle components (Burns, 2005). In support of this hypothesis, Sommer et al. (2005) showed that SPS‐like symptoms could be triggered in rats injected with anti‐amphiphysin antibodies from a human SPS patient.
Genetic translocations leading to the formation of hybrid clathrin‐accessory proteins can lead to other forms of acute myeloid leukemia, lymphoblastic leukemia and acute megakaryoblastic leukemia (Dreyling 1996, Jones 2001, Narita 1999, Tebar 1999). In these diseases, an aberrant hybrid protein consisting of the putative transcription factor AF10 and the clathrin accessory protein CALM (clathrin assembly lymphoid myeloid leukemia protein) is formed because of a partial inversion of the AF10 gene on chromosome 11 (Salmon‐Nguyen et al., 2000).
Finally, in Hermansky‐Pudlak syndrome (HPS) type 2, a condition that results in partial albinism and prolonged bleeding, mutations have been found in the β3A gene that encodes a subunit of the AP3 adaptor complex (Dell'Angelica et al., 1999b). HPS is discussed in more detail in Section V.C.
B. Alternative Internalization Routes
1. Caveolae
Originally identified more than 50 years ago (Palade 1953, Yamada 1955), caveolae are flask‐shaped invaginations of approximately 50–100 nm in diameter at the plasma membrane. These plasma membrane profiles are related to lipid rafts and contain unique mixtures of GPI‐anchored proteins, transmembrane proteins, signaling factors and lipids, such as cholesterol. Caveolae are believed to mediate the uptake of small solutes, regulate protein trafficking (Hommelgaard 2005, Tagawa 2005), transcytosis (transport across endothelial cells) (Simionescu et al., 2002), signal transduction (Insel 2005, Lisanti 1994, Ostrom 2004) and cholesterol homeostasis (Fielding and Fielding, 2001). However, their exact role in the internalization of membrane proteins and soluble protein ligands is controversial.
Caveolin‐1, also known as VIP21, is a structural component essential for the formation and stability of caveolae (Kurzchalia 1992, Rothberg 1992). Of the three members of the caveolin gene family (caveolin‐1, ‐2, and ‐3) (Scherer 1996, Tang 1996), caveolin‐1 and ‐2 are abundant in a wide variety of cell types including endothelial cells, adipocytes, alveolar type I pneumocytes, and smooth muscle cells (Williams and Lisanti, 2004), whereas caveolin‐3 is a muscle‐specific isoform expressed in striated muscle cells such as cardiac and skeletal myocytes (Cohen 2004, Tang 1996). Caveolin‐1 and ‐3 are both able to induce formation of caveolae at the plasma membrane (Galbiati 2001, Li 1996). However, caveolin‐2 requires the presence of caveolin‐1 for expression, membrane localization, and formation of caveolae (Razani et al., 2002).
Caveolae are absent from cells that lack caveolin‐1 but can be induced by ectopic expression of the gene (Fra et al., 1995). Caveolins adopt a hairpin‐like structure that inserts into the membrane such that the N and C termini are cytoplasmic. Caveolins can polymerize to form a striated coat surrounding an invagination site (Pelkmans, 2005). Caveolin‐1 can bind cholesterol (Murata et al., 1995), which is enriched within both caveolae and lipid rafts (Sargiacomo et al., 1993); this may explain why caveolae have been considered a subset of lipid rafts. However, caveolae and lipid rafts are considered to be independent entities as some proteins can be found in one but not the other (Liu et al., 1997). Certain ligands can internalize via a lipid raft‐dependent but clathrin‐independent mechanism in cells that lack caveolae (Lamaze et al., 2001).
A large pool of the plasma membrane caveolar vesicles cluster into dense grape‐like structures where individual caveolae appear stacked on top of each another (Thomsen et al., 2002). These structures are intimately associated with the actin cytoskeleton (Stahlhut and van Deurs, 2000); caveola‐associated proteins are also implicated in regulating plasma membrane dynamics and cellular movement. A small pool of “transport‐competent” caveolar vesicles may undergo short‐range constitutive fusion and budding cycles just under the plasma membrane (Pelkmans, 2005). Caveolae and caveolins can also be detected at the TGN (Dupree 1993, Kurzchalia 1992) and may form stable “platforms” for the movement of proteins and lipids from the TGN to the plasma membrane (Tagawa et al., 2005).
The caveolar pathway can be hijacked and used by pathogens or toxins to gain entry into the cell. Viruses such as polyomavirus, echovirus 1, and simian virus 40 (SV40) use caveolae to internalize viral particles. These viruses cluster lipid rafts and sequester them into caveolae through interactions with raft components such as integrins and glycosphingolipids (Pelkmans, 2005); in the case of SV40, the virus binds to the raft component ganglioside GM1 (Tsai et al., 2003). Tagawa et al. (2005) have shown that SV40 can trigger the long‐range movement of transport‐competent caveolar vesicles. Moreover, cell infection with SV40 more than doubles the number of caveolae capable of undergoing viral internalization and long‐range trafficking.
Caveolae contain much of the molecular machinery required for “classical” vesicle fission, docking, and fusion, for example, SNARE proteins, monomeric and trimeric GTPases, annexins II and VI, N‐ethylmaleimide (NEM)‐sensitive fusion protein (NSF), and ATPases (Schnitzer et al., 1995). Caveolae also contain the dynamin GTPases, which can be transiently recruited to SV40‐loaded caveolae and implicated in membrane scission (Henley 1998, Oh 1998, Pelkmans 2002). Internalized caveola‐derived vesicles move to an endocytic compartment termed the “caveosome” and eventually arrive at the early endosome. After fusion with the target compartment, caveolae do not disassemble but maintain their integrity in the membrane, preserving their compartmentalization and retaining their lipid and protein components (Pelkmans et al., 2004). The fate of internalized SV40 viruses after reaching the caveosome eventually results in arrival at the smooth ER (Pelkmans et al., 2001).
Interestingly, mutations in caveolin have been implicated in muscular dystrophy and cardiovascular disease, and mutations causing the downregulation of caveolin have been linked to the progression of various human carcinomas; it is therefore possible that caveolins may have a tumor suppressor role. The caveolin‐1 and caveolin‐2 genes are located on human 7q31.1 near the microsatellite repeat marker D7S522. This region is commonly deleted in various cancers (Engelman et al., 1998), hinting that caveolin gene deletion may be advantageous for tumor progression. In one report, the caveolin‐1 P132L mutation was present in 16% of breast cancer patients studied (Hayashi et al., 2001). The P132L mutation was also linked to the metastatic potential of tumors and disease prognosis. The caveolin‐1 P132L mutation also conferred increased cell migration and altered morphology. Caveolin‐1 protein levels can be reduced or absent from a number of human breast cancer cell lines compared with normal mammary cells (Lee et al., 1998). Similarly, silent and missense mutations in caveolin‐1 have also been associated with oral carcinomas (Han et al., 2004). Caveolin‐1, and to a lesser extent caveolin‐2, gene expression is downregulated in some cases of thyroid carcinoma (Aldred et al., 2003).
Although it remains unclear as to why the loss of caveolin causes cell proliferation diseases such as cancer, one can speculate on the role of caveolin in regulating signaling pathways. In endothelial cells, which have a high abundance of caveolin, the key vascular endothelial growth factor receptor 2 (VEGFR2) has been shown to be inactive when localized to caveolae (Labrecque et al., 2003). This receptor tyrosine kinase modulates the endothelial response to the key VEGF‐A cytokine and controls angiogenesis and new blood vessel formation, thus regulating neovascularization and tumor growth (Neufeld et al., 1999). Similarly, platelet‐derived growth factor (PDGF) receptor tyrosine kinase activity is reduced when associated with caveolae (Yamamoto et al., 1999). In addition to VEGFR2 and PDGFR, a number of G protein‐coupled receptors (GPCRs) have been shown to interact with caveola‐associated factors (Insel et al., 2005). GPCRs are a large family of transmembrane receptors involved in a variety of signal transduction events. These receptors are activated by a range of ligands, including hormones and peptides, and have been linked to a number of cancers such as thyroid, lung, and gastric. The presence of a number of GPCRs in caveolae suggests that these plasma membrane structures may interact with GPCRs and modulate their signaling potential. Lisanti and others (Li et al., 1995a) have shown that caveolin‐1 interacts solely with inactive forms of G‐protein α subunits, lending credence to the negative regulation hypothesis caused by the association of caveolae with transmembrane signaling receptors.
A number of mutations in muscle‐specific caveolin‐3 have been associated with four distinct but related autosomal dominant muscle disease phenotypes (Woodman et al., 2004): limb girdle muscular dystrophy type 1c (Minetti et al., 1998), rippling muscle disease, hyperCKemia (persistently elevated levels of serum creatine kinase), and distal myopathy. Some mutations cause aberrant retention of caveolin‐3 in the Golgi and subsequent degradation; other mutations may cause mutant caveolin‐3 to act in a dominant‐negative manner by forming unstable aggregates with wild‐type caveolin‐3 (Galbiati 1999, Sotgia 2003a, Sotgia 2003b). Hypertrophic cardiomyopathy (HCM) patients have a caveolin‐3 T63S mutation that reduces plasma membrane levels (Hayashi et al., 2004).
Caveolin gene knockout mice are providing insights into protein function in different human diseases. For example, lack of caveolins can cause diabetes, atherosclerosis, and cardiomyopathies in mouse models (Cohen 2004, Williams 2004). However, such phenotypes have yet to be linked to caveolin dysfunction in humans.
2. Phagocytosis
Phagocytosis is a process used by white blood cells such as macrophages, neutrophils, and dendrites to ingest large particulate material into specialized vesicles called phagosomes. These professional phagocytes are paramount in the defense against infection as they engulf and ingest whole microorganisms such as bacteria. They also use this route for “mopping up” apoptotic debris or senescent cells from tissues. In contrast to constitutive pinocytic transport, phagocytosis is regulated by cell surface‐localized Fc receptor (FcR) contact or interaction with complement‐ or antibody‐coated particles which results in clustering of FcR on the cell surface, a step important for subsequent intracellular signaling and cellular activation (Daeron, 1997).
Polymorphisms in leukocyte‐specific Fcγ receptors may contribute to autoimmune diseases such as Guillain‐Barré syndrome or rheumatoid arthritis, and enhanced susceptibility to infection (van Sorge et al., 2003). Fc‐mediated binding can trigger a complex signaling response involving extrusion of fine plasma membrane projections (pseudopodia) from the macrophage to surround and engulf the pathogen, forming a phagosome. The signaling response is reviewed in greater detail elsewhere (Bokoch 2005, Chimini 2000, Niedergang 2005). In brief, the activation of tyrosine kinases and Rho GTPases is triggered through FcR signaling. The Rac and Cdc42 GTPases, in conjunction with the downstream effector WASP, mediate remodeling of the actin cytoskeleton, leading to pseudopodium formation and phagosome closure (Castellano 2001, Chimini 2000). In contrast, the complement mediated‐uptake of opsonized particles differs such that they appear to “fall” into the cell in a process that requires Rho, but not Rac or Cdc42 (Bokoch, 2005).
Phagocytosis, although designed to destroy pathogens, can paradoxically be used as a route of entry by pathogens such as Mycobacterium (M. leprae and M. tuberculosis) or Leishmania (Nguyen 2005, Scott 2003). Normally, internalized pathogens are destroyed successfully through phagosome maturation into lysosomes and subsequent degradation. Mycobacterium can evade host degradation by secreting a soluble serine/threonine protein kinase G molecule into the phagosome. This molecule initiates a signaling response that interferes with phagosome–lysosome fusion, and promotes intracellular pathogen survival (Walburger et al., 2004). Furthermore, phagosome maturation is compromised by a pathogen induced block of p38 MAP (mitogen‐activated protein) kinase recruitment to the tethering molecule early endosome antigen 1 (Fratti et al., 2003).
The Leishmania protozoan parasite, which is transmitted to humans by sand flies, produces a membrane molecule called a lipophosphoglycan, which is inserted into the lipid bilayer of the phagosome in infected macrophages. This lipophosphoglycan is thought to modulate intracellular signaling pathways, resulting in a less fusogenic phagosome and preventing maturation; this would facilitate pathogen replication and disease progression (Lodge and Descoteaux, 2005).
V. Protein Trafficking Through the Endosomal–Lysosomal System
A. Endosomal Sorting and Recycling
Molecules internalized from the cell surface by receptor‐mediated endocytosis and clathrin‐coated vesicles are delivered to the early endosome for sorting. Molecules such as low‐density lipoprotein receptor (LDLR) and transferrin receptor (TfR) are efficiently recycled between the early endosome and the plasma membrane. However, after ligand‐mediated activation (Fig. 3 ), receptor tyrosine kinases such as epidermal growth factor receptor (EGFR) are sorted along the endocytic pathway for degradation.
Fig. 3.
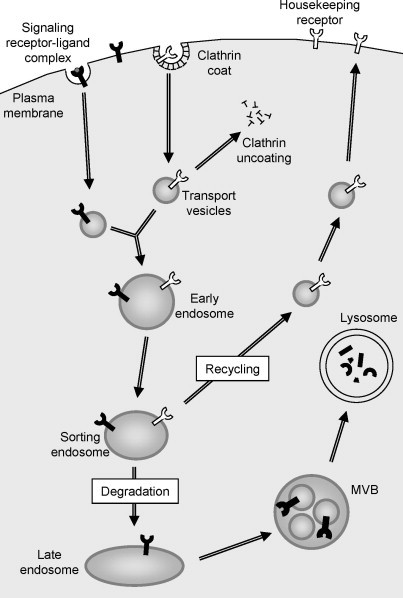
Protein trafficking through the endosomal–lysosomal system. Cell surface receptors are internalized through clathrin‐coated vesicles (CCVs) at the plasma membrane. In the cell cytoplasm, CCVs shed their coat components and fuse to produce endosomes. Internalized receptors are either recycled from sorting endosomes (housekeeping receptors, e.g., transferrin receptor) or targeted for degradation within the lysosome (signaling receptors, e.g., growth factor receptors) after movement through the late endosome and multivesicular body (MVB) compartments.
Early endosomes are thought to be formed through the fusion of internalized vesicles and recruitment of specific proteins and lipids. One key endosomal regulator is the ubiquitously expressed Rab5a GTPase. Rab5a is present on the cytosolic face of the plasma membrane, vesicles, and tubular endosomal profiles (Chavrier et al., 1990). A number of Rab5a‐associated effector proteins regulate endosomal fusion and mediate protein cargo movement and endosomal sorting (Zerial and McBride, 2001). Such effector proteins, including Rabaptin‐5, Rabex‐5, and Rabenosyn‐5, are clustered on the cytosolic face of the early endosome and stabilize the GTP‐bound Rab5a in an activated state (Horiuchi et al., 1997). GTP‐bound Rab5a directly binds to early endosome antigen EEA1 to regulate vesicular and endosomal tethering. EEA1 contains a C‐terminal Rab5a‐binding domain, and a phosphatidylinositol 3‐phosphate‐binding zinc finger domain referred to as an FYVE (conserved in Fab1, YOTB, Vac1, and EEA1) domain (Gaullier 1998, Stenmark 1996). Overexpression of wild‐type Rab5a, or a constitutively active Rab5a mutant, causes endosome enlargement and defective trafficking through this compartment, whereas expression of a constitutively inactive Rab5a mutant leads to formation of small endosomes and decreased endocytosis (Bucci et al., 1992). A family of effector proteins that accelerate GTPase hydrolysis (RabGAPs) have been identified: RabGAP‐5 binds to Rab5a and regulates trafficking through the endocytic pathway (Haas et al., 2005).
The importance of Rab5a activity is further illustrated in the genetic disorder tuberous sclerosis (TS), a disease that causes tumors in the brain, eyes, heart, kidneys, lungs, and skin. TS arises when the tumor suppressor gene, tuberous sclerosis complex (TSC), is absent; introduction of the wild‐type TSC2 gene into an animal model or cultured cells results in tumor suppression and reduced cellular proliferation (Kobayashi 1995, Yeung 1994). Interestingly, the TSC2 gene product (tuberin) is implicated in regulating GTP/GDP exchange on Rab5a, thus regulating trafficking through this endosome system (Xiao et al., 1997). In chronic myelomonocytic leukemia (CMML) a genetic translocation causes fusion of Rab5a effector Rabaptin‐5 and the PDGFβR (Magnusson et al., 2001). This chromosomal translocation results in enhanced cellular proliferation by compromising endosomal fusion and trafficking, and thus regulation of growth factor degradation. It is likely that this aberrant gene product is not degraded and triggers sustained intracellular signaling, leading to cell proliferation and tumor progression in a subset of lymphoid cells.
Recycling from endosomes back to the cell surface is often used by receptors that internalize nutrients such as lipoproteins and ions. Receptor recycling rather than degradation conserves receptor functionality and nutrient uptake and reduces energy expenditure in the synthesis of new receptors (Mukherjee et al., 1997). Genetic screens in the nematode Caenorhabditis elegans identified RME‐1 and delineated a new family of conserved class of Eps15 homology (EH) domain proteins (Grant et al., 2001). Both the worm and mouse homologs of RME‐1 are associated with the endosomal compartment: a dominant‐negative RME‐1 G429R mutant had little effect on receptor‐mediated endocytosis but had a substantial effect on endosomal recycling, suggesting a functional role in this step (Lin et al., 2001).
Although information is currently limited, a number of neurological diseases are associated with dysfunction of early endosomal proteins. In some cases of demyelinating polyneuropathy, characterized by progressive weakening and sensory dysfunction of the legs and arms, EEA1 autoantibodies have been detected (Selak et al., 1999). A number of disorders, from muscular dystrophy to rheumatoid arthritis, reveal the presence of circulating anti‐EEA1 antibodies. Interestingly, EEA1 epitopes recognized by such autoantibodies varied from patient to patient (Selak et al., 2003). Autoantibodies against EEA1 have also been detected in cases of subacute cutaneous systemic lupus erythematosus (SCLE), characterized by the appearance of an unsightly red rash, often occurring after sun exposure (Mu et al., 1995).
B. Lysosomal Sorting and Protein Degradation
Lysosomes are terminal, membrane‐enclosed degradative compartments that interact with other organelles through vesicular transport originating from the secretory, endocytic, and autophagic pathways. This organelle stores various proteases, lipases, hydrolases, and degradative enzymes within an acidic environment that maximizes enzymatic activity and degradation. Resident lysosomal membrane proteins, integral proteins, and glycoproteins are targeted to the organelle via the endosome. Lysosomal proteases such as cathepsin D are processed in the Golgi apparatus to add a mannose 6‐phosphate (M6P) moiety to N‐linked sugars. The M6P moiety is recognized by plasma membrane or TGN‐resident mannose 6‐phosphate receptors (M6PRs) and sorted to the late endosome and eventually the lysosome. Here, the acidic pH (pH < 5.5) results in receptor–ligand disassociation and recycling of the M6PR to the TGN. Fusion between the endosome and preexisting primary lysosomes allows the delivery of lysosomal resident proteins.
The importance of M6P‐mediated targeting of lysosomal proteins is highlighted in the human neurological disorder, I‐cell disease (mucolipidosis II), where lysosomal enzymes are secreted from cells rather than targeted to the lysosome. The defect in I‐cell disease involves lack of M6P moiety addition as a result of mutations to the N‐acetylglucosamine‐1‐phosphotransferase enzyme usually present within the Golgi apparatus (Ben‐Yoseph et al., 1987).
How lysosomes are formed is still unclear (Luzio et al., 2003). Three mechanisms have been proposed to explain lysosomal biogenesis: vesicular transport between late endosomes and preformed primary lysosomes (Griffiths and Gruenberg, 1991), early endosomal “maturation” to lysosomes (Murphy, 1991), or the current favored model of “kiss‐and‐run,” in which transient interactions between endosomes and lysosomes transfer endosomal contents to the latter compartment (Duclos 2003, Storrie 1996). Late endosome and lysosome interactions in the kiss‐and‐run model are thought to be regulated by the Rab7 GTPase, which is present on late endosomes; a Vps complex, homologous to budding yeast vacuole fusion regulators, is also implicated in sorting and delivery to lysosomes (Seals et al., 2000). The mammalian form of the Vps complex interacts with syntaxin‐7, a t‐SNARE that is concerned in regulating membrane dynamics along this route (Kim et al., 2001).
Danon disease is caused by point mutations in, or complete absence of lysosome‐associated membrane protein 2 (LAMP2) or complete absence of this protein: changes which result in cardiomyopathy, myopathy, and mental retardation. In Danon disease patients and LAMP2‐deficient mice, autophagic vacuoles accumulate within the cytoplasm; these vacuoles arise via intracellular engulfment of old membranes to form an autophagosome, thus sequestering membranes and proteins for eventual degradation (Shintani and Klionsky, 2004). Autophagosomes fuse with lysosomes, leading to degradation for provision of molecules for cellular homeostasis. The accumulation of autophagic vacuoles in LAMP2‐deficient cells suggests that LAMP2 mediates interactions between autophagosomes and lysosomes. This pathway is commonly activated during conditions of cellular stress such as starvation or pathogenic infection (Kirkegaard et al., 2004).
Lysosomal storage diseases are caused through insufficient degradation of targeted components within lysosomes, leading to substrate accumulation and lysosome enlargement. More than 40 lysosomal storage diseases have been documented and generally manifest themselves as neurological disorders; disease severity correlates with the levels of lysosomal enzyme activity. Niemann‐Pick disease is a neurodegenerative condition caused by sphingomyelin accumulation in reticuloendothelial cells and ganglion neurons, leading to cell death. It is classified into five types (A–E), each distinguished by either clinical severity or age‐related disease phenotype. Niemann‐Pick type A (NPA) is most common, with death occurring before 3 years of age. NPA patients have point mutations in the SMPD1 gene that encodes a lysosomal sphingomyelinase (Levran 1991, Takahashi 1992). Interestingly, in NPC patients, endocytosed LDL particles are not fully degraded in lysosomes, leading to defects in cholesterol metabolism (Li et al., 2005a). The NPC disease is caused by mutations in the NPC1 gene, which encodes a lysosomal resident protein with similarity to sterol‐sensing enzymes and proteins (Scott and Ioannou, 2004).
Fabry disease is an X‐linked condition caused by changes in lysosomal α‐galactosidase activity resulting in glycosphingolipid accumulation within vascular endothelial lysosomes. This leads to angiokeratomas (a wart‐like thickening of the skin), progressive renal impairment, cardiomyopathy, and cerebrovascular disease. Mutations in the α‐galactosidase A gene can also show reduced enzymatic activity of the encoded protein and retention within the endoplasmic reticulum (Yasuda et al., 2003).
Receptor tyrosine kinases such as epidermal growth factor receptor (EGFR) are degraded by lysosomes after ligand binding and receptor activation. EGFR lysosomal targeting is dependent on ligand‐stimulated ubiquitination of the cytoplasmic domain. Binding of EGF to EGFR causes downstream signaling, clathrin‐mediated internalization, and trafficking to endosomes. Internalized receptor–ligand complexes are sorted to the late endosome or multivesicular bodies (MVBs), which eventually deliver their contents to the lysosome (Katzmann et al., 2002). Whereas other receptors such as TfR are recycled to the plasma membrane, EGFR is moved through the endosome–lysosome system by a ubiquitin‐dependent sorting and recognition system. These include the Hrs/STAM heterodimer and TSG101 (Bilodeau et al., 2002) present on endosomal membranes. The TSG101 tumor suppressor gene is mutated in nearly 50% of breast cancer patients and encodes a membrane‐associated protein (Lee and Feinberg, 1997). This factor participates in the sorting of ubiquitinated proteins on the endosome, but its exact function is not clear.
C. Secretory Lysosomes
In some specialized cells, such as cytotoxic T lymphocytes (CTLs), platelets, and melanocytes, regulated secretion can be routed through compartments other than the TGN. Such cells have evolved mechanisms whereby modified or secretory lysosomes release their contents at the plasma membrane in response to extracellular stimuli. These secretory lysosomes (SLs) share lysosomal characteristics such as acid pH and LAMP (lysosome‐associated membrane proteins) residents but also contain unique markers such as tyrosinase, present in melanosomes. The CTL secretory lysosomes contain unique components such as perforin and granzymes required for triggering apoptosis in target cells. On CTL contact with a target cell, SLs traffic toward the immunological synapse formed between the CTL and target cell. A signal then causes SL fusion with the CTL plasma membrane (Stinchcombe et al., 2001), and release of SL contents and subsequent target cell death.
A number of autosomal genetic diseases causing immunodeficiency and albinism involve defects in regulated lysosomal secretion (Stinchcombe et al., 2004). In the rare, fatal disease familial hemophagocytic lymphohistiocytosis (FHL) SLs congregate at the plasma membrane in CTLs, where they can dock but cannot fuse with the membrane. In one group of FHL patients, this disease is due to a mutation in the gene encoding Munc13‐4; this is closely related to the neuronal Munc13‐1 gene product that is involved in SNARE complex formation in neuronal cells (Feldmann et al., 2003). Assembly of this neuronal syntaxin‐1, SNAP‐25, and synaptobrevin complex is regulated by Munc18‐1, which binds and locks syntaxin‐1 (t‐SNARE) in a closed, inactive conformation, thus preventing it from interacting with SNAP‐25 (Yang et al., 2000). However, Munc13‐1 (Sassa et al., 1999) and RIM (Rab3a‐interacting protein effector) (Koushika et al., 2001) can compete with Munc18‐1 and displace it from syntaxin‐1. This reinforces the syntaxin‐1 open conformation and allows SNARE complex formation to occur. Munc13‐1 may act as a conformational switch to promote t‐SNARE into an “open” state, thus allowing formation of the SNARE complex that mediates synaptic vesicle docking and fusion. From observations of FHL patients, one speculation is that Munc13‐4 has a role similar to that of Munc13‐1 in regulating SNARE complex formation for SL docking and fusion in CTLs (Yang et al., 2000).
Chediak‐Higashi syndrome (CHS) is a key example of a disease affecting SL function in CTLs, with patients displaying hypopigmentation (Stinchcombe et al., 2004). CHS patients have genetic mutations in the Lyst or CHS1 gene (Barbosa 1996, Perou 1996) and produce CTLs containing strikingly enlarged SLs that are able to polarize to the immunological synapse but are unable to fuse with the PM. This suggests a role for the CHS1 gene product in regulating membrane docking and fusion (Clark 2003, Clark 2003). Overexpression of CHS1 leads to the presence of small lysosomes, indicating increased lysosomal fission (Ward et al., 2003). In addition, CHS1 interacts with SNARE proteins, further indicating a role in SL fusion (Tchernev et al., 2002).
Griscelli syndrome patients also display defects in SL dynamics within CTLs and exhibit hypopigmentation and silvery hair. In melanocytes, cells responsible for pigment storage and production, Rab27a is required to recruit melanophilin to pigment granules called melanosomes (SLs). Melanophilin binds the myosin motor protein myosin Va and regulates melanosome movement along actin cables to the plasma membrane (Hume 2002, Strom 2002, Wu 2002). In type 1 Griscelli syndrome patients, Rab27a GTPase is missing or defective, whereas in type 2 Griscelli syndrome patients the myosin Va motor protein is absent. These defects are also evident in mouse models such as ashen (Rab27a defective), dilate (myosin Va defective), and leaden (melanophilin defective). In both the human Griscelli syndromes and the mouse models, melanosomes are clustered in a perinuclear location, a defect attributed to Rab27a dysfunction (Wilson 2000, Wu 2001). Interestingly, CTLs isolated from type 1 Griscelli syndrome patients and ashen mice (Rab27a deficient) are unable to kill target cells, whereas type 2 Griscelli syndrome patient and dilate mouse CTLs are functional. This suggests that Rab27a interacts with different effectors to induce SL fusion with the plasma membrane in different cell types (Haddad et al., 2001).
Hermansky‐Pudlak syndrome (HPS) is a fourth example of SL dysfunction and is characterized by oculocutaneous albinism, ceroid deposition, and excessive prolonged bleeding (Hermansky 1959, Swank 2000). However, HPS cannot be viewed as a single disease but a group of at least seven autosomal genetic disorders. Each of the seven subgroups (HPS1–7) is due to mutations in individual genes, most of which encode components of multisubunit protein complexes involved in vesicle trafficking (Li et al., 2003), whereas the function of others remains unclear. Three of these complexes, termed BLOCs (biogenesis of lysosome‐related organelle complexes), play a role in regulating trafficking involved in platelet and melanosome secretion, but their exact functions are unclear (Di Pietro and Dell'Angelica, 2005). In HPS2 patients a nonsense mutation in the gene encoding the β3A subunit of the AP3 adaptor protein prevents expression of this subunit (Huizing et al., 2002). As previously mentioned, AP3 is involved in the recruitment of transmembrane proteins into vesicles at the early endosome for delivery to the lysosome (Peden et al., 2004). In melanocytes derived from HPS2 patients, the tyrosinase that catalyzes the formation of melanin is not transported to maturing melanosomes (Huizing et al., 2001). This leads to the characteristic pattern of albinism seen in patients with this condition.
Furthermore, patients with HPS2 display an impaired CTL response and immune response. CTLs from HPS2 patients have lytic granules that cannot move in an oriented fashion toward the microtubule‐organizing center; therefore, when CTLs are stimulated by contact with target cells, the lytic granules are not targeted to the immunological synapse for cell killing (Clark et al., 2003).
Studies on the cell biology of HIV infection have suggested the existence of a viral secretory compartment. Work by Marsh and others (Garcia 2005, Kramer 2005, Pelchen‐Matthews 2003) has localized viral envelope (gp120) and matrix proteins (p17) to tetraspanin‐positive endosome‐related organelles in infected macrophages and dendritic cells. These viral secretory compartments move from an intracellular localization to an infectious synapse when infected macrophages or dendritic cells form an immunological synapse with activated T cells. This may be one mechanism for subsequent viral infection of CD4‐positive T cells, thus causing the impaired immune response seen in patients with acquired immunodeficiency syndrome (AIDS).
VI. The Cytoskeleton and Disease
Cells require a highly organized framework or cytoskeleton to station and move membrane organelles within three‐dimensional space. Components of the cytoskeleton can guide organelles or vesicles to specific destinations within the cell. The microtubule cytoskeleton is commonly associated with the directional movement of intracellular transport vesicles or intermediates. In contrast, actin has been envisioned to have a structural role in determining cell shape, plasma membrane dynamics, and cell locomotion. However, evidence points to a role for actin in regulated trafficking from the TGN (Allan 2002, Badizadegan 2004, Cobbold 2004) and endocytosis (Ascough 2004, Engqvist‐Goldstein 2003).
The cytoskeleton is a dynamic structure likened to a collapsible scaffold that can be rapidly disassembled and reconstituted depending on cellular requirements. Actin or tubulin polymerization (elongation) and depolymerization (breakdown) rely on the controlled addition or removal of monomers in a polarized and energy‐dependent manner. Protofilaments in either structure are both polarized, with the plus end growing at a faster rate. Actin cables are each composed of two parallel protofilaments that twist around each other, whereas microtubules are composed of a hollow cylindrical structure comprising 13 parallel protofilaments.
Actin nucleation is an initial step required for elongation involving formation of a stable trimer subunit base for protofilament elongation. A heptameric complex termed ARP2/3 (actin‐related protein) binds to the ends and sides of actin filaments to nucleate and further accelerate the growth of the actin network (Millard et al., 2004). The function of the ARP2/3 complex can be regulated by membrane‐associated Rho GTPases. These regulators, which include Cdc42 and various Rac isoforms, act as molecular switches that cycle between an active GTP‐bound state and an inactive GDP‐bound state. Cdc42 regulates ARP2/3 indirectly through its downstream target Wiskott‐Aldrich syndrome protein (WASP), which binds directly to the ARP2/3 complex (Jaffe and Hall, 2005). Patients with X‐linked Wiskott‐Aldrich syndrome display mutations in the WASP gene and have thrombocytopenia (reduced platelet count), eczema, recurrent infections, hematologic malignancy, and autoimmune disorders (Lemahieu et al., 1999). Approximately 300 disease mutations in WASP have been reported, which lead to defective control of WASP in actin polymerization and severe disease phenotypes (Burns et al., 2004). WASP expression is restricted to hematopoietic cells, although the ubiquitously expressed N‐WASP is present in various cells and tissues (Burns et al., 2004).
The actin network is important for the formation of immunological synapses between cytotoxic T lymphocytes (CTLs) and their targets, as well as T lymphocytes and antigen‐presenting cells such as macrophages. The formation of the immunological synapse in CTLs is essential for the transport, docking, and fusion of SLs and subsequent destruction of the target cell as described above. Defective WASP inhibits the formation of the immunological synapse and T cell activation (Notarangelo and Ochs, 2003), probably causing the immunological deficiencies observed in Wiskott‐Aldrich syndrome patients. WASP deficiency in T lymphocytes also affects the regulation and composition of lipid rafts (Dupre et al., 2002), indicating that the formation of the immunological synapse is dependent on lipid rafts, WASP, and actin dynamics.
Motor proteins provide the physical force to move membrane vesicles along the polymerized cytoskeletal filaments via ATP‐dependent hydrolysis. Actin‐based motor proteins belong to the myosin superfamily. The myosin Va gene is mutated in a small number of patients with Griscelli syndrome (Bahadoran 2003, Pastural 2000) (other trafficking mutations contributing to Griscelli syndrome are discussed in Section V.C). Mutations in the myosin VIIa gene can cause Usher's syndrome, resulting in blindness and deafness. Intracellular transport is probably compromised in Usher syndrome patients; the mouse shaker model has a mutant myosin VIIa gene, displaying defective melanosome transport in retinal pigment epithelial cells (Liu et al., 1998) and altered distribution in photoreceptor cells (Richardson et al., 1997).
Microtubule motor proteins, which actively move vesicles along the microtubules, and microtubule‐associated proteins (MAPs), serve as docking molecules to bind cargo to motor proteins (Gerdes and Katsanis, 2005). Microtubule motor proteins belong to either the kinesin or dynein families. With the exception of the C‐terminal kinesins, kinesin‐based motors generally transport cargo toward the plus end of the microtubule, whereas the dyneins are minus end‐directed motors.
Long‐range vesicular transport is particularly important in neurons, where axons can reach up to 1 m in length. Newly synthesized lipids, and secreted or membrane‐associated proteins, are made in the cell body; long‐range and directional transport is crucial for replenishing the constituents of the presynaptic cleft (at the terminal end of the axon) with synaptic vesicles and plasma membrane receptors (Holzbaur, 2004).
A number of human neurological diseases are linked to mutations in microtubule motors and associated proteins. Lissencephaly, a Greek term meaning “smooth brain,” causes severe brain malformation resulting in mental retardation and epilepsy. One of the genes mutated in the disease is LIS1 (originally identified in Miller‐Dieker syndrome patients with lissencephaly) (Reiner et al., 1993). The LIS1 protein regulates microtubule motor function by binding dynein1 and p150Glued, a component of the dynactin complex that binds to and activates dynein (Smith et al., 2000). It is proposed that LIS1 regulates retrograde axonal transport. Another gene mutated in some patients with lissencephaly is doublecortin, a microtubule‐associated protein that binds tubulin and stabilizes microtubules (Horesh 1999, Moores 2004).
The KIF1B kinesin regulates transport of synaptic vesicle precursors along the neuronal axon. Patients with Charcot‐Marie‐Tooth disease type 2A display neuronal axonal degeneration due to a loss‐of‐function mutation in the motor domain of KIF1B (Zhao et al., 2001). In Alzheimer's disease, a classical sign is hyperphosphorylated aggregates of the microtubule‐associated protein, tau, in neuronal cells and tissues. Tau protein can influence vesicular transport (Ebneth et al., 1998) by regulating the attachment of motors to microtubules (Trinczek et al., 1999). One theory is that the tau protein can interfere with kinesin‐dependent transport by blocking motor access to microtubules, thus slowing or preventing vesicle movement along axons (Mandelkow et al., 2003). Moreover, an early sign of Alzheimer's disease is the loss of synapses and retrograde degeneration of neurons, complemented by a breakdown in intracellular transport.
The disruption of microtubule‐mediated vesicular trafficking may also be a causative factor of the neurodegenerative phenotype of Huntington's disease. This disease is caused by expansion of polyglutamine repeats occurring in the brain‐enriched protein Huntingtin (Htt). It has been demonstrated that Htt enhances vesicular transport of brain‐derived neurotrophic factor (BDNF) along microtubules (Gauthier et al., 2004). Htt is localized in the cytoplasm and is associated with vesicular and microtubule‐based traffic through its ability to bind Huntingtin‐associated protein 1 (HAP1) (Li et al., 1995b), a protein that has affinity for the dynactin p150Glued subunit (Engelender et al., 1997). Dysfunctional polyQ‐Htt associated with the disease state may disrupt the transport of BDNF by binding and blocking the HAP1/dynactin‐mediated delivery of BDNF vesicles along microtubules (Gauthier et al., 2004). This is further supported by the finding that BDNF levels are decreased in brains of Huntington's disease patients (Ferrer et al., 2000).
VII. Therapeutic Strategies
A. Current Treatments
The current treatment of genetic disorders involves addressing the symptoms rather than the cause. To that end, many mild forms of disorders such as Niemann‐Pick disease, and familial hypercholesterolemia, can be controlled by diet regimens and lifestyle changes. In contrast, a life‐threatening disease such as cystic fibrosis requires extensive physiotherapy and pulmonary exercise to loosen and prevent mucus accumulation within the lungs.
New antidementia drugs are increasingly successful in treating neurological disorders such as Alzheimer's disease. Drugs such as galantamine, donepezil, rivastigmine, and memantine target the posttranslational processing of βAPP to reduce amyloid deposits (Prasher, 2004). In familial hypercholesterolemia, statin treatment is a common strategy for reducing plasma LDL and cholesterol levels by targeting HMG‐CoA reductase, the rate‐limiting enzyme in cellular cholesterol biosynthesis. Furthermore, less commonly used LDL‐lowering drugs such as probucol have shown some success in lowering circulating lipoprotein and cholesterol levels (Buckley et al., 1989). The administration of adrenalin receptor antagonists (β‐blockers) to patients with the cardiac condition long‐QT syndrome reduces arrhythmia risk. Enzyme replacement therapy (ERT) has been carried out in patients with Fabry's disease, a lysosomal storage disease. Patients are given recombinant lysosomal α‐galactosidase (Mignani and Cagnoli, 2004) to reduce the risks of strokes and kidney failure associated with the condition. Finally, organ transplantation is occasionally carried out for some disease states: for example, bone marrow transplants for Chediak‐Higashi syndrome patients (Liang et al., 2000) and Rab27a‐defective patients with Griscelli syndrome (Schuster et al., 2001) and Wiskott‐Aldrich syndrome (Filipovich et al., 2001). However, although transplant operations can be successful in alleviating the immunological issues associated with these diseases, it does not address problems associated with the nervous system or pigmentation.
B. Gene Therapy: The Next Generation of Medical Treatment?
Completion of the human genome sequencing project has given science the ability to track gene(s) responsible for potentially any genetic disorder and, as a consequence, to allow these genes to be corrected in patients. This is the goal of gene therapy research. Of course, gene therapy has a fundamental limitation: it is only really suitable for single‐gene defect diseases, and multigenic or chromosomal defects will be beyond the ability of the technique because of the complex nature of the disease. However, there are more than 2500 single gene defects that cause human disease, so there are many diseases requiring such approaches. The history of gene therapy is discussed in more detail by Russell 1997, Scollay 2001.
1. Gene Delivery
Much effort has been made in developing techniques that allow successful replacement or augmentation of defective genes. Gene therapy is performed by introducing a gene vehicle directly into the patient (in vivo) or by removing cells from a patient, introducing the gene into these cells in culture, and replacing the cells back in the patient (ex vivo). Most studies have focused on the use of viral vectors as delivery vehicles.
Retroviruses are potentially the best gene delivery system (Kurian et al., 2000). These RNA viruses are able to infect a great many cell types and replicate by inserting their viral genes into the genome of the host. The host cellular machinery is then modulated to produce and assemble viral particles. In gene therapy, retroviruses could be used to express the target gene to be replaced but be modified to prevent viral disease (HIV, which is the causative agent of AIDS, is also a retrovirus). The principal drawback of a retrovirus vector is the possibility that genomic integration could elevate oncogene expression, thus causing cancer. Therefore, the majority of clinical trials using retrovirus vectors have been performed ex vivo. A “successful” gene therapy experiment was exemplified in the case of a 4‐year‐old female patient lacking adenosine deaminase (ADA), which results in severely compromised immunodeficiency (ADA‐SCID) and dysfunctional T cells (Blaese et al., 1995). In this case, a retroviral vector was used to deliver the coding sequence for ADA into cells, resulting in successful expression of this enzyme in hitherto defective cells. Although successful, it is uncertain whether enzyme replacement treatment (recombinant ADA injections) also influenced the patient outcome.
Adenovirus (AdV) (McConnell and Imperiale, 2004) is a DNA virus and key gene therapy vehicle that maintains the viral genome as a separate transmissible episome within the nuclei of infected host cells. The use of attenuated or inactivated AdV for human gene delivery has attracted much interest. The advantages of AdV gene transfer are that its genome can easily be manipulated and recombinant virus can be grown to high titers in vitro with efficient transduction of target cells in vitro or in vivo. As AdV can effectively infect nondividing cells such as lung pulmonary tissues it is a popular vector of choice for gene therapy to treat cystic fibrosis (CF) patients. Although there are promising studies (Zabner et al., 1993), failures have also been noted (Knowles et al., 1995).
A major disadvantage of an AdV‐based approach is the triggering of a strong host immune response to the virus, which becomes a serious problem in subsequent long‐term delivery of recombinant virus for disease alleviation. One approach to circumventing such an issue is to use a viral delivery system that produces a low host immune response such as the adeno‐associated virus (AAV) (Flotte, 2005). AAV is a nonpathogenic virus that requires coinfection with a helper virus to replicate. However, AAV has broad host cell specificity and is difficult to grow in large quantities, probably because of its reliance on a helper virus.
Finally, nonviral methods are increasingly available for the delivery of DNA constructs directly into cells and tissues. These are often lipid‐based reagents (e.g., liposomes) that bind to the plasmid DNA and fuse with the plasma membrane, thus enabling cytosolic delivery of the gene. The plasmid DNA would then be transported into the host nucleus by endogenous cellular machinery. This type of gene delivery can only be performed ex vivo and can be limited by the poor DNA transfection efficiency of primary cells or tissues. This type of method, however, is a potentially useful method for delivering genes into progenitors or precursors (e.g., stem cells) before cellular differentiation and tissue formation within a particular microenvironment in the body (Mendell et al., 1995).
C. Other Potential Therapeutic Methods
1. Chemical and Pharmacological Chaperones
Numerous disease states are caused by protein misfolding within the ER, leading to degradation (Table I; 1, 2). One strategy would be to promote the correct protein conformation in a mutant gene product either chemically or pharmacologically. A number of membrane‐diffusible chemical and pharmacological “chaperones” have been identified that could affect protein folding in cells. Chemical chaperones such as glycerol and trimethylamine N‐oxide (TMAO) can restore the wild‐type trafficking and activity of CFTRΔF508 in cultured epithelial cells (Brown et al., 1996), and porcine kidney epithelial cells expressing CFTRΔF508 and treated with dimethyl sulfoxide (DMSO) increased plasma membrane levels of the channel protein (Bebok et al., 1998). Loo et al. (2005) have demonstrated that a novel quinazoline derivative specific for CFTR will rescue the defective trafficking of CFTRΔF508 in cultured cells. Cell surface levels of a water channel, aquaporin‐2, can be enhanced with DMSO (Tamarappoo and Verkman, 1998). Defects in this gene can result in X‐linked nephrogenic diabetes insipidus, a condition in which patients are unable to concentrate their urine because of an inability to reabsorb water from the kidneys into the blood.
Although chemical chaperones are somewhat nonspecific in their action (the protein folding of the whole cell is affected and not just the target protein), pharmacological chaperones can be tailored to individual proteins. For example, the compound SR121463A is a nonpeptide vasopressin V2 receptor antagonist (Morello 2000, Robert 2005). Patients with a mutant vasopressin V2 receptor can also display nephrogenic diabetes insipidus. On treatment, the cell‐permeant SR121463A compound would act as a chaperone and accompany the mutant V2 receptor to the cell surface to rescue correct functionality.
Geldanamycin, a naturally occurring antifungal agent, has potential as an anticancer drug (Beliakoff 2004, Miyata 2005). Geldanamycin interacts with and inhibits activity of the heat shock protein and chaperone Hsp90, a cytosolic cellular stress protein that supports the correct folding, stability, and function of “client” proteins. Many Hsp90 client proteins are implicated in cell cycle progression, proliferation, and angiogenesis (Whitesell and Lindquist, 2005). The ErbB2 tyrosine kinase complex is implicated in regulation and development of epithelial breast tumors and is an Hsp90 client. Inhibition of Hsp90 action by geldanamycin results in degradation of both ErbB2 and downstream signaling effectors, resulting in reduced cellular growth and tumor formation (Citri et al., 2004).
2. Cell‐Penetrating Peptides
A number of cell‐permeable peptide sequences found in viruses and host proteins have been discovered that mediate the delivery of cargo (proteins, drugs, plasmid DNA, oligonucleotides) directly into cells (Brooks 2005, Gupta 2005, Schwartz 2000). Such peptide sequences could be fused or attached to recombinant or engineered proteins and administered to patients to complement defects of a particular gene product. For example, the Drosophila melanogaster antennapedia homeodomain (Antp) transcription factor contains a short 16‐residue sequence that mediates protein translocation across biological membrane bilayers in an energy‐independent manner (Derossi 1994, Joliot 1991).
Other sources of cell membrane‐permeable proteins have been uncovered in viruses. The HIV‐1 replication protein Tat contains a basic, arginine‐ and lysine‐rich peptide sequence (residues 47–57) that modulates the translocation of exogenous Tat across the plasma membrane in a number of cell types, and is able to activate intracellular genes controlled by an HIV promoter (Frankel 1988, Mann 1991). This basic 10‐residue sequence can internalize conjugated β‐galactosidase and horseradish peroxidase (Fawell et al., 1994) as well as a Fab antibody fragment (Anderson et al., 1993). The major structural protein of herpesvirus (HSV‐1), VP22, can traffic between cells (Elliott and O'Hare, 1997), whereas the PreS‐2 domain of hepatitis B virus surface antigen acts as a shuttle for peptides and functional proteins (such as EGFP) in hepatocytes and other cells (Oess and Hildt, 2000), suggesting further the existence of naturally occurring peptide sequences that may act as drug delivery vectors. Finally, a “synthetic” amphipathic peptide, FLUOS‐KLALKLALKALKAALKLA‐NH2, has been shown to be internalized in mast and endothelial cells (Oehlke et al., 1998).
3. Small Molecule Therapeutics
The employment of small molecular inhibitors as a method of treating human disease has moved at exponential pace. A number of compounds have been synthesized or isolated from nonhuman organisms that directly affect cellular function and have been used in research on a variety of diseases. Plant‐ and microorganism‐derived polyhydroxylated alkaloids referred to as iminosugars have been used in the treatment of patients with Gaucher disease (Cox et al., 2000). Gaucher disease type I and type II is a lysosomal storage disorder caused by a mutation in the gene encoding the acid β‐glucosidase (GBA) enzyme and results in the accumulation of toxic glucosylceramide in a patient's spleen, liver, and bones; manifesting itself in enlargement of these organs, as well as heart and lung disease. Iminosugars act on glycosylating enzymes present within the ER and Golgi and inhibit their ability to transfer sugar moieties onto proteins. One member of the iminosugar family, N‐butyldeoxynojirimycin (NB‐DNJ; also called miglustat or Zavesca) inhibits the enzyme important in the maturation of the GBA substrate glucocerebroside, namely ceramide glucosyltransferase (CGT) (Butters et al., 2003). Inhibition of CGT has resulted in significantly reduced levels of glucocerebroside in the liver and spleen of patient in clinical trials (Cox et al., 2000). However, nearly 80% of patients in the trials displayed osmotic diarrhea as a side effect of the treatment. In a mouse model for human Tay‐Sachs disease, which is caused by a mutation in the gene encoding hexosamidase A, levels of the harmful glycophospholipid GM2 were significantly reduced on treatment with NB‐DNJ (Platt et al., 1997). In addition to the treatment of lysosomal storage diseases, an NB‐DNJ derivative called miglitol has been used in the treatment of diabetes mellitus, resulting in reduced activity of the sucrose–isomaltase enzyme complex and reduction of carbohydrate digestion (Mitrakou et al., 1998).
A major aspect of human disease is the production and subsequent degradation of misfolded proteins, either by the proteasome or within the lysosome. Lysosomotropic agents such as chloroquine cause an increase in the intralumenal pH of endosomes and lysosomes, reducing lysosomal protease activities and the trafficking of proteins through the endosome–lysosome system. A number of proteasome inhibitors such as MG132, lactacystin, and ALLN can specifically inhibit the activity of a range of serine and cysteine proteases and chymotrypsin‐like enzymes (Kisselev and Goldberg, 2001). Proteasome inhibition has been linked with a number of aspects of human disease. Treatment of endothelial cells with proteasome inhibitors resulted in apoptosis of proliferating cells (Drexler et al., 2000) and inhibition of plasminogen activator levels; this factor promotes angiogenesis and new blood vessel sprouting (Oikawa et al., 1998). However, inhibiting proteasome function has broad cytotoxic and apoptotic effects in cells and tissues.
Chemotherapeutic agents targeting signaling pathways are currently of much interest in relation to cancer therapeutics. Cellular proliferation can be regulated by growth factor binding to a cell surface receptor and signaling through either the mitogen‐activated protein kinase (MAPK) or phosphoinositide‐3‐kinase (PI3K) cascades. Activation of these pathways induces the expression of oncogenes such as c‐jun and c‐fos and inhibits apoptosis through a sequence of protein phosphorylation events. Shelton et al. (2003) demonstrated that inhibition of the MAPK pathway with small molecule inhibitors specific for Raf‐1 or MEK reduced cell proliferation and induced apoptosis in conditionally transformed hematopoietic cells. However, such pathways also regulate other cellular functions besides proliferation or apoptosis and there are likely to be consequences for cellular homeostasis.
Structural studies are important in the development of new small molecule inhibitors that target specific enzymes and regulators. c‐Akt (PKB) is a serine/threonine protein kinase required for survival and proliferation in many human cancers and its structure has been elucidated (Kumar 2005, Yang 2002; and references therein). Chemotherapeutic agents have been consequently designed that inhibit c‐Akt activity; molecules such as H‐89 target the ATP‐binding pocket in c‐Akt (Kumar and Madison, 2005). Compounds that bind specifically to c‐Akt isoforms or target specific domains within the kinase have been reported (Barnett et al., 2005), but there are no reports of clinical trials with such compounds (Kumar and Madison, 2005).
Finally, small molecule inhibitors are being developed to target the posttranslational processing of proteins or peptides implicated in human disease. The enzyme that catalyzes the initial steps in β‐amyloid synthesis, γ‐secretase, is an attractive target for prevention of amyloid deposits in Alzheimer's disease patients (Churcher and Beher, 2005). Such small molecule inhibitors could also be used to treat pathogenic infections such as those caused by severe acute respiratory syndrome (SARS), influenza, HIV, or hepatitis C viruses. Attractive targets are virus‐encoded or host proteases required for processing of viral proteins to generate infectious virus particles from the host cell. In the case of the SARS virus, a viral chymotrypsin‐like cysteine protease is responsible for processing SARS viral proteins required for viral replication. Inhibition of this protease would effectively inhibit viral replication. A molecule referred to as CS11 was found to inhibit the replication of human SARS with no toxic effect on normal cells (Dooley et al., 2006). Much work is also being carried out in targeting host proteases required for the processing of HIV envelope glycoproteins by the biosynthetic secretory pathway to generate viral gp120 and gp41 polypeptides.
VIII. Concluding Remarks
The completion of the human genome sequencing project has led to the prediction that a large number of diseases will be identified and understood at the gene level (Collins et al., 2003). As noted in this review, a number of examples exist in which a single gene mutation can have devastating effects on human function. At present, the symptoms of some mild forms of genetic diseases can be modulated through diet or drug regimens, and some success has been achieved with organ transplantation. Gene therapy has attracted much attention but has suffered setbacks due to viral toxicity issues. An alternative strategy is the use of small molecule therapeutics, which may override specific defects or target specific pathways to compensate for gene defect(s). In addition, our understanding of how we respond at a genetic level to pathological infection will enable us to design effective drug strategies to presently chronic infections. In essence, understanding the cell biological basis for human diseases will enable us to design effective methods to deliver therapeutic strategies to patients.
References
- Aldred M.A., Ginn‐Pease M.E., Morrison C.D., Popkie A.P., Gimm O., Hoang‐Vu C., Krause U., Dralle H., Jhiang S.M., Plass C., Eng C. Caveolin‐1 and caveolin‐2,together with three bone morphogenetic protein‐related genes, may encode novel tumor suppressors down‐regulated in sporadic follicular thyroid carcinogenesis. Cancer Res. 2003;63:2864–2871. [PubMed] [Google Scholar]
- Al‐Hasani H., Hinck C.S., Cushman S.W. Endocytosis of the glucose transporter GLUT4 is mediated by the GTPase dynamin. J. Biol. Chem. 1998;273:17504–17510. doi: 10.1074/jbc.273.28.17504. [DOI] [PubMed] [Google Scholar]
- Allan V.J., Thompson H.M., McNiven M.A. Motoring around the Golgi. Nat. Cell Biol. 2002;4:E236. doi: 10.1038/ncb1002-e236. [DOI] [PubMed] [Google Scholar]
- Alvarez C., Garcia‐Mata R., Hauri H.‐P., Sztul E. The p115‐interactive proteins GM130 and giantin participate in endoplasmic reticulum‐Golgi traffic. J. Biol. Chem. 2001;276:2693–2700. doi: 10.1074/jbc.M007957200. [DOI] [PubMed] [Google Scholar]
- Amaral M.D. CFTR and chaperones: Processing and degradation. J. Mol. Neurosci. 2004;23:41–48. doi: 10.1385/JMN:23:1-2:041. [DOI] [PubMed] [Google Scholar]
- Anderson D.C., Nichols E., Manger R., Woodle D., Barry M., Fritzberg A.R. Tumor cell retention of antibody Fab fragments is enhanced by an attached HIV TAT protein‐derived peptide. Biochem. Biophys. Res. Commun. 1993;194:876–884. doi: 10.1006/bbrc.1993.1903. [DOI] [PubMed] [Google Scholar]
- Ascough K.R. Endocytosis: Actin in the driving seat. Curr. Biol. 2004;14:R124–R126. [PubMed] [Google Scholar]
- Badizadegan K., Wheeler H.E., Fujinaga Y., Lencer W.I. Trafficking of cholera toxin‐ganglioside GM1 complex into Golgi and induction of toxicity depend on actin cytoskeleton. Am. J. Physiol. Cell Physiol. 2004;287:C1453–C1462. doi: 10.1152/ajpcell.00189.2004. [DOI] [PubMed] [Google Scholar]
- Bahadoran P., Ortonne J.P., Ballotti R., de Saint‐Basile G. Comment on Elejalde syndrome and relationship with Griscelli syndrome. Am. J. Med. Genet. 2003;116A:408–409. doi: 10.1002/ajmg.a.10065. [DOI] [PubMed] [Google Scholar]
- Barbosa M.D., Nguyen Q.A., Tchernev V.T., Ashley J.A., Detter J.C., Blaydes S.M., Brandt S.J., Chotai D., Hodgman C., Solari R.C., Lovett M., Kingsmore S.F. Identification of the homologous beige and Chediak‐Higashi syndrome genes. Nature. 1996;382:262–265. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J. Cell Biol. 1997;139:1097–1108. doi: 10.1083/jcb.139.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett S.F., Bilodeau M.T., Lindsley C.W. The Akt/PKB family of protein kinases: A review of small molecule inhibitors and progress towards target validation. Curr. Top. Med. Chem. 2005;5:109–125. doi: 10.2174/1568026053507714. [DOI] [PubMed] [Google Scholar]
- Barois N., Bakke O. The adaptor protein AP‐4 as a component of the clathrin coat machinery: A morphological study. Biochem. J. 2005;385:503–510. doi: 10.1042/BJ20041010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C.L., Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- Baroudi G., Napolitano C., Priori S.G., Del Bufalo A., Chahine M. Loss of function associated with novel mutations of the SCN5A gene in patients with Brugada syndrome. Can. J. Cardiol. 2004;20:425–430. [PubMed] [Google Scholar]
- Barr F.A., Warren G. Disassembly and reassembly of the Golgi apparatus. Semin. Cell Dev. Biol. 1996;7:505–510. [Google Scholar]
- Bebok Z., Venglarik C.J., Panczel Z., Jilling T., Kirk K.L., Sorscher E.J. Activation of DeltaF508 CFTR in an epithelial monolayer. Am. J. Physiol. Cell Physiol. 1998;275:C599–C607. doi: 10.1152/ajpcell.1998.275.2.C599. [DOI] [PubMed] [Google Scholar]
- Beliakoff J., Whitesell L. Hsp90: An emerging target for breast cancer therapy. Anticancer Drugs. 2004;15:651–662. doi: 10.1097/01.cad.0000136876.11928.be. [DOI] [PubMed] [Google Scholar]
- Bennett M.K. SNAREs and the specificity of transport vesicle targeting. Curr. Opin. Cell Biol. 1995;7:581–586. doi: 10.1016/0955-0674(95)80016-6. [DOI] [PubMed] [Google Scholar]
- Benson K.F., Li F.‐Q., Person R.E., Albani D., Duan Z., Wechsler J., Meade‐White K., Williams K., Acland G.M., Niemeyer G., Lothrop C.D., Horwitz M. Mutations associated with neutropenia in dogs and humans disrupt intracellular transport of neutrophil elastase. Nat. Genet. 2003;35:90. doi: 10.1038/ng1224. [DOI] [PubMed] [Google Scholar]
- Ben‐Yoseph Y., Potier M., Mitchell D.A., Pack B.A., Melancon S.B., Nadler H.L. Altered molecular size of N‐acetylglucosamine 1‐phosphotransferase in I‐cell disease and pseudo‐Hurler polydystrophy. Biochem. J. 1987;248:697–701. doi: 10.1042/bj2480697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner N., Horwitz M., Loughran T.P., Jr. Congenital and acquired neutropenia. Hematology. 2004;2004:63–79. doi: 10.1182/asheducation-2004.1.63. [DOI] [PubMed] [Google Scholar]
- Bertrand C.A., Frizzell R.A. The role of regulated CFTR trafficking in epithelial secretion. Am. J. Physiol. Cell Physiol. 2003;285:C1–C18. doi: 10.1152/ajpcell.00554.2002. [DOI] [PubMed] [Google Scholar]
- Bi K., Roth M.G., Ktistakis N.T. Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr. Biol. 1997;7:301–307. doi: 10.1016/s0960-9822(06)00153-9. [DOI] [PubMed] [Google Scholar]
- Biederer T., Volkwein C., Sommer T. Degradation of subunits of the Sec61p complex, an integral component of the ER membrane, by the ubiquitin‐proteasome pathway. EMBO J. 1996;15:2069–2076. [PMC free article] [PubMed] [Google Scholar]
- Biederer T., Volkwein C., Sommer T. Role of Cue1p in ubiquitination and degradation at the ER surface. Science. 1997;278:1806–1809. doi: 10.1126/science.278.5344.1806. [DOI] [PubMed] [Google Scholar]
- Bilodeau P.S., Urbanowski J.L., Winistorfer S.C., Piper R.C. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 2002;4:534–539. doi: 10.1038/ncb815. [DOI] [PubMed] [Google Scholar]
- Blaese R.M., Culver K.W., Miller A.D., Carter C.S., Fleisher T., Clerici M., Shearer G., Chang L., Chiang Y., Tolstoshev P., Greenblatt J.J., Rosenberg S.A., Klein H., Berger M., Mullen C.A., Ramsey W.J., Muul L., Morgan R.A., Anderson W.F. T lymphocyte‐directed gene therapy for ADA‐SCID: Initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- Bokoch G.M. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Bonifacino J.S., Traub L.M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Bouras T., Lisanti M.P., Pestell R.G. Caveolin‐1 in breast cancer. Cancer Biol. Ther. 2004;3:931–941. doi: 10.4161/cbt.3.10.1147. [DOI] [PubMed] [Google Scholar]
- Brooks H., Lebleu B., Vives E. Tat peptide‐mediated cellular delivery: Back to basics. Adv. Drug Deliv. Rev. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Brown C.R., Hong‐Brown L.Q., Biwersi J., Verkman A.S., Welch W.J. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones. 1996;1:117–125. doi: 10.1379/1466-1268(1996)001<0117:ccctmp>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C., Parton R.G., Mather I.H., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Buckley M.M., Goa K.L., Price A.H., Brogden R.N. Probucol. A reappraisal of its pharmacological properties and therapeutic use in hypercholesterolaemia. Drugs. 1989;37:761–800. doi: 10.2165/00003495-198937060-00002. [DOI] [PubMed] [Google Scholar]
- Bull P.C., Thomas G.R., Rommens J.M., Forbes J.R., Cox D.W. The Wilson disease gene is a putative copper transporting P‐type ATPase similar to the Menkes gene. Nat. Genet. 1993;5:327. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- Burkhard P., Stetefeld J., Strelkov S. Coiled coils: A highly versatile protein folding motif. Trends Cell Biol. 2001;11:82. doi: 10.1016/s0962-8924(00)01898-5. [DOI] [PubMed] [Google Scholar]
- Burns S., Cory G.O., Vainchenker W., Thrasher A.J. Mechanisms of WASP‐mediated hematologic and immunologic disease. Blood. 2004;104:3454–3462. doi: 10.1182/blood-2004-04-1678. [DOI] [PubMed] [Google Scholar]
- Burns T.M. A step forward for stiff‐person syndrome. Lancet. 2005;365:1365–1367. doi: 10.1016/S0140-6736(05)66349-0. [DOI] [PubMed] [Google Scholar]
- Butters T.D., Mellor H.R., Narita K., Dwek R.A., Platt F.M. Small‐molecule therapeutics for the treatment of glycolipid lysosomal storage disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:927–945. doi: 10.1098/rstb.2003.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callea F., Brisigotti M., Fabbretti G., Bonino F., Desmet V.J. Hepatic endoplasmic reticulum storage diseases. Liver. 1992;12:357–362. doi: 10.1111/j.1600-0676.1992.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Cao X., Ballew N., Barlowe C. Initial docking of ER‐derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr G., Simmons N., Sayer J. A role for CBS domain 2 in trafficking of chloride channel CLC‐5. Biochem. Biophys. Res. Commun. 2003;310:600. doi: 10.1016/j.bbrc.2003.09.057. [DOI] [PubMed] [Google Scholar]
- Cassanelli S., Bertolini S., Rolleri M., De Stefano F., Casarino L., Elicio N., Naselli A., Calandra S. A “de novo” point mutation of the low‐density lipoprotein receptor gene in an Italian subject with primary hypercholesterolemia. Clin. Genet. 1998;53:391–395. doi: 10.1111/j.1399-0004.1998.tb02752.x. [DOI] [PubMed] [Google Scholar]
- Castellano F., Chavrier P., Caron E. Actin dynamics during phagocytosis. Semin. Immunol. 2001;13:347. doi: 10.1006/smim.2001.0331. [DOI] [PubMed] [Google Scholar]
- Charron A.J., Bacallao R.L., Wandinger‐Ness A. ADPKD: A human disease altering Golgi function and basolateral exocytosis in renal epithelia. Traffic. 2000;1:675–686. doi: 10.1034/j.1600-0854.2000.010811.x. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R.G., Hauri H.P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chen M.S., Obar R.A., Schroeder C.C., Austin T.W., Poodry C.A., Wadsworth S.C., Vallee R.B. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Chimini G., Chavrier P. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat. Cell Biol. 2000;2:E191. doi: 10.1038/35036454. [DOI] [PubMed] [Google Scholar]
- Choudhury R., Diao A., Zhang F., Eisenberg E., Saint‐Pol A., Williams C., Konstantakopoulos A., Lucocq J., Johannes L., Rabouille C., Greene L.E., Lowe M. Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans‐Golgi network. Mol. Biol. Cell. 2005;16:3467–3479. doi: 10.1091/mbc.E05-02-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churcher I., Beher D. Gamma‐secretase as a therapeutic target for the treatment of Alzheimer's disease. Curr. Pharm. Des. 2005;11:3363–3382. doi: 10.2174/138161205774370771. [DOI] [PubMed] [Google Scholar]
- Citri A., Kochupurakkal B.S., Yarden Y. The achilles heel of ErbB‐2/HER2: Regulation by the Hsp90 chaperone machine and potential for pharmacological intervention. Cell Cycle. 2004;3:51–60. [PubMed] [Google Scholar]
- Clark R., Griffiths G.M. Lytic granules, secretory lysosomes and disease. Curr. Opin. Immunol. 2003;15:516. doi: 10.1016/s0952-7915(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Clark R.H., Stinchcombe J.C., Day A., Blott E., Booth S., Bossi G., Hamblin T., Davies E.G., Griffiths G.M. Adaptor protein 3‐dependent microtubule‐mediated movement of lytic granules to the immunological synapse. Nat. Immunol. 2003;4:1111. doi: 10.1038/ni1000. [DOI] [PubMed] [Google Scholar]
- Cobbold C., Ponnambalam S., Francis M.J., Monaco A.P. Novel membrane traffic steps regulate the exocytosis of the Menkes disease ATPase. Hum. Mol. Genet. 2002;11:2855–2866. doi: 10.1093/hmg/11.23.2855. [DOI] [PubMed] [Google Scholar]
- Cobbold C., Coventry J., Ponnambalam S., Monaco A.P. The Menkes disease ATPase (ATP7A) is internalized via a Rac1‐regulated, clathrin‐ and caveolae‐independent pathway. Hum. Mol. Genet. 2003;12:1523–1533. doi: 10.1093/hmg/ddg166. [DOI] [PubMed] [Google Scholar]
- Cobbold C., Coventry J., Ponnambalam S., Monaco A.P. Actin and microtubule regulation of trans‐Golgi network architecture, and copper‐dependent protein transport to the cell surface. Mol. Membr. Biol. 2004;21:59–66. doi: 10.1080/096870310001607350. [DOI] [PubMed] [Google Scholar]
- Cohen A.W., Hnasko R., Schubert W., Lisanti M.P. Role of caveolae and caveolins in health and disease. Physiol. Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Collins F.S., Morgan M., Patrinos A. The Human Genome Project: Lessons from large‐scale biology. Science. 2003;300:286–290. doi: 10.1126/science.1084564. [DOI] [PubMed] [Google Scholar]
- Collins R.N. Rab and ARF GTPase regulation of exocytosis. Mol. Membr. Biol. 2003;20:105–115. doi: 10.1080/0968768031000085892. [DOI] [PubMed] [Google Scholar]
- Cox T., Lachmann R., Hollak C., Aerts J., van Weely S., Hrebicek M., Platt F., Butters T., Dwek R., Moyses C., Gow I., Elstein D., Zimran A. Novel oral treatment of Gaucher's disease with N‐butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 2000;355:1481–1485. doi: 10.1016/S0140-6736(00)02161-9. [DOI] [PubMed] [Google Scholar]
- D'Addio M., Pizzigoni A., Bassi M.T., Baschirotto C., Valetti C., Incerti B., Clementi M., De Luca M., Ballabio A., Schiaffino M.V. Defective intracellular transport and processing of OA1 is a major cause of ocular albinism type 1. Hum. Mol. Genet. 2000;9:3011–3018. doi: 10.1093/hmg/9.20.3011. [DOI] [PubMed] [Google Scholar]
- Daecke J., Fackler O.T., Dittmar M.T., Krausslich H.G. Involvement of clathrin‐mediated endocytosis in human immunodeficiency virus type 1 entry. J. Virol. 2005;79:1581–1594. doi: 10.1128/JVI.79.3.1581-1594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeron M. Fc receptor biology. Annu. Rev. Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- Danpure C.J. The molecular basis of alanine: Glyoxylate aminotransferase mistargeting: The most common single cause of primary hyperoxaluria type 1. J. Nephrol. 1998;11(Suppl. 1):8–12. [PubMed] [Google Scholar]
- David C., McPherson P.S., Mundigl O., de Camilli P. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc. Natl. Acad. Sci. USA. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Thomas A., Cofiell R., Folli F., Lichte B., Piccolo G., Meinck H.M., Austoni M., Fassetta G., Bottazzo G. The synaptic vesicle‐associated protein amphiphysin is the 128‐kD autoantigen of stiff‐man syndrome with breast cancer. J. Exp. Med. 1993;178:2219–2223. doi: 10.1084/jem.178.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo F.R., Goedken M., McCormick S.J., Nauseef W.M. A novel form of hereditary myeloperoxidase deficiency linked to endoplasmic reticulum/proteasome degradation. J. Clin. Invest. 1998;101:2900–2909. doi: 10.1172/JCI2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks E.D., Cook J.P., Day P.J., Smith D.C., Roberts L.M., Lord J.M. The low lysine content of ricin A chain reduces the risk of proteolytic degradation after translocation from the endoplasmic reticulum to the cytosol. Biochemistry. 2002;41:3405–3413. doi: 10.1021/bi011580v. [DOI] [PubMed] [Google Scholar]
- Defesche J.C. Low‐density lipoprotein receptor—its structure, function, and mutations. Semin. Vasc. Med. 2004;4:5–11. doi: 10.1055/s-2004-822993. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E.C., Klumperman J., Stoorvogel W., Bonifacino J.S. Association of the AP‐3 adaptor complex with clathrin. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E.C., Mullins C., Bonifacino J.S. AP‐4, a novel protein complex related to clathrin adaptors. J. Biol. Chem. 1999;274:7278–7285. doi: 10.1074/jbc.274.11.7278. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E.C., Shotelersuk V., Aguilar R.C., Gahl W.A., Bonifacino J.S. Altered trafficking of lysosomal proteins in Hermansky‐Pudlak syndrome due to mutations in the beta 3A subunit of the AP‐3 adaptor. Mol. Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- Deretic D., Williams A.H., Ransom N., Morel V., Hargrave P.A., Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP‐ribosylation factor 4 (ARF4) Proc. Natl. Acad. Sci. USA. 2005;102:3301–3306. doi: 10.1073/pnas.0500095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derossi D., Joliot A.H., Chassaing G., Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- Detter J.C., Zhang Q., Mules E.H., Novak E.K., Mishra V.S., Li W., McMurtrie E.B., Tchernev V.T., Wallace M.R., Seabra M.C., Swank R.T., Kingsmore S.F. Rab geranylgeranyl transferase alpha mutation in the gunmetal mouse reduces Rab prenylation and platelet synthesis. Proc. Natl. Acad. Sci. USA. 2000;97:4144–4149. doi: 10.1073/pnas.080517697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Virgilio M., Weninger H., Ivessa N.E. Ubiquitination is required for the retro‐translocation of a short‐lived luminal endoplasmic reticulum glycoprotein to the cytosol for degradation by the proteasome. J. Biol. Chem. 1998;273:9734–9743. doi: 10.1074/jbc.273.16.9734. [DOI] [PubMed] [Google Scholar]
- de Vree J.M.L., Jacquemin E., Sturm E., Cresteil D., Bosma P.J., Aten J., Deleuze J.‐F., Desrochers M., Burdelski M., Bernard O., Elferink R.P.J.O., Hadchouel M. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc. Natl. Acad. Sci. USA. 1998;95:282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao A., Rahman D., Pappin D.J., Lucocq J., Lowe M. The coiled‐coil membrane protein golgin‐84 is a novel rab effector required for Golgi ribbon formation. J. Cell Biol. 2003;160:201–212. doi: 10.1083/jcb.200207045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro S.M., Dell'Angelica E.C. The cell biology of Hermansky‐Pudlak syndrome: Recent advances. Traffic. 2005;6:525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Dooley A.J., Shindo N., Taggart B., Park J.G., Pang Y.P. From genome to drug lead: Identification of a small‐molecule inhibitor of the SARS virus. Bioorg. Med. Chem. Lett. 2006;16:830–833. doi: 10.1016/j.bmcl.2005.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray B., Ghosh P., Griffith J., Geuze H.J., Kornfeld S. Cooperation of GGAs and AP‐1 in packaging MPRs at the trans‐Golgi network. Science. 2002;297:1700–1703. doi: 10.1126/science.1075327. [DOI] [PubMed] [Google Scholar]
- Drexler H.C., Risau W., Konerding M.A. Inhibition of proteasome function induces programmed cell death in proliferating endothelial cells. FASEB J. 2000;14:65–77. doi: 10.1096/fasebj.14.1.65. [DOI] [PubMed] [Google Scholar]
- Dreyling M.H., Martinez‐Climent J.A., Zheng M., Mao J., Rowley J.D., Bohlander S.K. The t(10;11)(p13;q14) in the U937 cell line results in the fusion of the AF10 gene and CALM, encoding a new member of the AP‐3 clathrin assembly protein family. Proc. Natl. Acad. Sci. USA. 1996;93:4804–4809. doi: 10.1073/pnas.93.10.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos S., Corsini R., Desjardins M. Remodeling of endosomes during lysosome biogenesis involves “kiss and run” fusion events regulated by rab5. J. Cell Sci. 2003;116:907–918. doi: 10.1242/jcs.00259. [DOI] [PubMed] [Google Scholar]
- Duden R. ER‐to‐Golgi transport: COP I and COP II function (Review) Mol. Membr. Biol. 2003;20:197–207. doi: 10.1080/0968768031000122548. [DOI] [PubMed] [Google Scholar]
- Dunne M.J., Cosgrove K.E., Shepherd R.M., Aynsley‐Green A., Lindley K.J. Hyperinsulinism in infancy: From basic science to clinical disease. Physiol. Rev. 2004;84:239–275. doi: 10.1152/physrev.00022.2003. [DOI] [PubMed] [Google Scholar]
- Dupre L., Aiuti A., Trifari S., Martino S., Saracco P., Bordignon C., Roncarolo M.‐G. Wiskott‐Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity. 2002;17:157. doi: 10.1016/s1074-7613(02)00360-6. [DOI] [PubMed] [Google Scholar]
- Dupree P., Parton R.G., Raposo G., Kurzchalia T.V., Simons K. Caveolae and sorting in the trans‐Golgi network of epithelial cells. EMBO J. 1993;12:1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebneth A., Godemann R., Stamer K., Illenberger S., Trinczek B., Mandelkow E. Overexpression of tau protein inhibits kinesin‐dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: Implications for Alzheimer's disease. J. Cell Biol. 1998;143:777. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A.O., Donoso L.A., Ritter R., 3rd. A novel gene for autosomal dominant Stargardt‐like macular dystrophy with homology to the SUR4 protein family. Invest. Ophthalmol. Vis. Sci. 2001;42:2652–2663. [PubMed] [Google Scholar]
- Elliott G., O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- Elsner M., Hashimoto H., Nilsson T. Cisternal maturation and vesicle transport: Join the band wagon! (Review) Mol. Membr. Biol. 2003;20:221–229. doi: 10.1080/0968768031000114024. [DOI] [PubMed] [Google Scholar]
- Engelender S., Sharp A.H., Colomer V., Tokito M.K., Lanahan A., Worley P., Holzbaur E.L., Ross C.A. Huntingtin‐associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum. Mol. Genet. 1997;6:2205–2212. doi: 10.1093/hmg/6.13.2205. [DOI] [PubMed] [Google Scholar]
- Engelman J.A., Zhang X.L., Lisanti M.P. Genes encoding human caveolin‐1 and ‐2 are co‐localized to the D7S522 locus (7q31.1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett. 1998;436:403. doi: 10.1016/s0014-5793(98)01134-x. [DOI] [PubMed] [Google Scholar]
- Engqvist‐Goldstein A.E., Drubin D.G. Actin assembly and endocytosis: From yeast to mammals. Annu. Rev. Cell Dev. Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- Fan J.Q., Ishii S., Asano N., Suzuki Y. Accelerated transport and maturation of lysosomal alpha‐galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat. Med. 1999;5:112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- Faucherre A., Desbois P., Satre V., Lunardi J., Dorseuil O., Gacon G. Lowe syndrome protein OCRL1 interacts with Rac GTPase in the trans‐Golgi network. Hum. Mol. Genet. 2003;12:2449–2456. doi: 10.1093/hmg/ddg250. [DOI] [PubMed] [Google Scholar]
- Fawell S., Seery J., Daikh Y., Moore C., Chen L.L., Pepinsky B., Barsoum J. Tat‐mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann J., Callebaut I., Raposo G., Certain S., Bacq D., Dumont C., Lambert N., Ouachee‐Chardin M., Chedeville G., Tamary H. Munc13‐4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Goutan E., Marin C., Rey M.J., Ribalta T. Brain‐derived neurotrophic factor in Huntington disease. Brain Res. 2000;866:257–261. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- Fielding C.J., Fielding P.E. Caveolae and intracellular trafficking of cholesterol. Adv. Drug Deliv. Rev. 2001;49:251. doi: 10.1016/s0169-409x(01)00140-5. [DOI] [PubMed] [Google Scholar]
- Filipovich A.H., Stone J.V., Tomany S.C., Ireland M., Kollman C., Pelz C.J., Casper J.T., Cowan M.J., Edwards J.R., Fasth A., Gale R.P., Junker A., Kamani N.R., Loechelt B.J., Pietryga D.W., Ringden O., Vowels M., Hegland J., Williams A.V., Klein J.P., Sobocinski K.A., Rowlings P.A., Horowitz M.M. Impact of donor type on outcome of bone marrow transplantation for Wiskott‐Aldrich syndrome: Collaborative study of the International Bone Marrow Transplant Registry and the National Marrow Donor Program. Blood. 2001;97:1598–1603. doi: 10.1182/blood.v97.6.1598. [DOI] [PubMed] [Google Scholar]
- Flotte T.R. Adeno‐associated virus‐mediated gene transfer for lung diseases. Hum. Gene Ther. 2005;16:643–648. doi: 10.1089/hum.2005.16.643. [DOI] [PubMed] [Google Scholar]
- Folsch H., Pypaert M., Maday S., Pelletier L., Mellman I. The AP‐1A and AP‐1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 2003;163:351–362. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fra A., Sitia R. The endoplasmic reticulum as a site of protein degradation. Subcell. Biochem. 1993;21:143–168. doi: 10.1007/978-1-4615-2912-5_7. [DOI] [PubMed] [Google Scholar]
- Fra A.M., Williamson E., Simons K., Parton R.G. De novo formation of caveolae in lymphocytes by expression of VIP21‐caveolin. Proc. Natl. Acad. Sci. USA. 1995;92:8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M.J., Jones E.E., Levy E.R., Martin R.L., Ponnambalam S., Monaco A.P. Identification of a di‐leucine motif within the C terminus domain of the Menkes disease protein that mediates endocytosis from the plasma membrane. J. Cell Sci. 1999;112:1721–1732. doi: 10.1242/jcs.112.11.1721. [DOI] [PubMed] [Google Scholar]
- Frankel A.D., Pabo C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Fratti R.A., Chua J., Deretic V. Induction of p38 mitogen‐activated protein kinase reduces early endosome autoantigen 1 (EEA1) recruitment to phagosomal membranes. J. Biol. Chem. 2003;278:46961–46967. doi: 10.1074/jbc.M305225200. [DOI] [PubMed] [Google Scholar]
- Galbiati F., Engelman J.A., Volonte D., Zhang X.L., Minetti C., Li M., Hou H., Jr., Kneitz B., Edelmann W., Lisanti M.P. Caveolin‐3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin‐glycoprotein complex, and T‐tubule abnormalities. J. Biol. Chem. 2001;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- Galbiati F., Volonte D., Minetti C., Chu J.B., Lisanti M.P. Phenotypic behavior of caveolin‐3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD‐1C). Retention of LGMD‐1C caveolin‐3 mutants within the Golgi complex. J. Biol. Chem. 1999;274:25632–25641. doi: 10.1074/jbc.274.36.25632. [DOI] [PubMed] [Google Scholar]
- Garcia E., Pion M., Pelchen‐Matthews A., Collinson L., Arrighi J.F., Blot G., Leuba F., Escola J.M., Demaurex N., Marsh M., Piguet V. HIV‐1 trafficking to the dendritic cell‐T‐cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic. 2005;6:488–501. doi: 10.1111/j.1600-0854.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- Garman S.C., Garboczi D.N. Structural basis of Fabry disease. Mol. Genet. Metab. 2002;77:3–11. doi: 10.1016/s1096-7192(02)00151-8. [DOI] [PubMed] [Google Scholar]
- Gaullier J.M., Simonsen A., D'Arrigo A., Bremnes B., Stenmark H., Aasland R. FYVE fingers bind PtdIns(3)P. Nature. 1998;394:432–433. doi: 10.1038/28767. [DOI] [PubMed] [Google Scholar]
- Gauthier L.R., Charrin B.C., Borrell‐Pages M., Dompierre J.P., Rangone H., Cordelieres F.P., De Mey J., MacDonald M.E., Lessmann V., Humbert S., Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Gerdes J.M., Katsanis N. Microtubule transport defects in neurological and ciliary disease. Cell. Mol. Life Sci. 2005;62:1556. doi: 10.1007/s00018-005-5007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman O.B., Jr., Keen J.H. The alpha chain of the AP‐2 adaptor is a clathrin binding subunit. J. Biol. Chem. 1995;270:23768–23773. doi: 10.1074/jbc.270.40.23768. [DOI] [PubMed] [Google Scholar]
- Gow A., Friedrich V.L., Jr., Lazzarini R.A. Intracellular transport and sorting of the oligodendrocyte transmembrane proteolipid protein. J. Neurosci. Res. 1994;37:563–573. doi: 10.1002/jnr.490370503. [DOI] [PubMed] [Google Scholar]
- Gow A., Southwood C.M., Lazzarini R.A. Disrupted proteolipid protein trafficking results in oligodendrocyte apoptosis in an animal model of Pelizaeus‐Merzbacher disease. J. Cell Biol. 1998;140:925–934. doi: 10.1083/jcb.140.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B., Zhang Y., Paupard M.C., Lin S.X., Hall D.H., Hirsh D. Evidence that RME‐1, a conserved C. elegans EH‐domain protein, functions in endocytic recycling. Nat. Cell Biol. 2001;3:573–579. doi: 10.1038/35078549. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Gruenberg J. The arguments for pre‐existing early and late endosomes. Trends Cell Biol. 1991;1:5–9. doi: 10.1016/0962-8924(91)90047-d. [DOI] [PubMed] [Google Scholar]
- Gupta B., Levchenko T.S., Torchilin V.P. Intracellular delivery of large molecules and small particles by cell‐penetrating proteins and peptides. Adv. Drug Deliv. Rev. 2005;57:637–651. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Haas A.K., Fuchs E., Kopajtich R., Barr F.A. A GTPase‐activating protein controls Rab5 function in endocytic trafficking. Nat. Cell Biol. 2005;7:887–893. doi: 10.1038/ncb1290. [DOI] [PubMed] [Google Scholar]
- Haddad E.K., Wu X., Hammer J.A., 3rd, Henkart P.A. Defective granule exocytosis in Rab27a‐deficient lymphocytes from Ashen mice. J. Cell Biol. 2001;152:835–842. doi: 10.1083/jcb.152.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner C., Takei K., Chen H., Ringstad N., Hudson A., Butler M.H., Salcini A.E., Di Fiore P.P., De Camilli P. Synaptojanin 1: Localization on coated endocytic intermediates in nerve terminals and interaction of its 170 kDa isoform with Eps15. FEBS Lett. 1997;419:175–180. doi: 10.1016/s0014-5793(97)01451-8. [DOI] [PubMed] [Google Scholar]
- Han S.E., Park K.H., Lee G., Huh Y.J., Min B.M. Mutation and aberrant expression of caveolin‐1 in human oral squamous cell carcinomas and oral cancer cell lines. Int. J. Oncol. 2004;24:435–440. [PubMed] [Google Scholar]
- Harris D.A. Trafficking, turnover and membrane topology of PrP. Br. Med. Bull. 2003;66:71–85. doi: 10.1093/bmb/66.1.71. [DOI] [PubMed] [Google Scholar]
- Hayasaka K., Himoro M., Sato W., Takada G., Uyemura K., Shimizu N., Bird T.D., Conneally P.M., Chance P.F. Charcot‐Marie‐Tooth neuropathy type 1B is associated with mutations of the myelin P0 gene. Nat. Genet. 1993;5:31–34. doi: 10.1038/ng0993-31. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Matsuda S., Machida K., Yamamoto T., Fukuda Y., Nimura Y., Hayakawa T., Hamaguchi M. Invasion activating caveolin‐1 mutation in human scirrhous breast cancers. Cancer Res. 2001;61:2361–2364. [PubMed] [Google Scholar]
- Hayashi T., Arimura T., Ueda K., Shibata H., Hohda S., Takahashi M., Hori H., Koga Y., Oka N., Imaizumi T. Identification and functional analysis of a caveolin‐3 mutation associated with familial hypertrophic cardiomyopathy. Biochem. Biophys. Res. Commun. 2004;313:178. doi: 10.1016/j.bbrc.2003.11.101. [DOI] [PubMed] [Google Scholar]
- Hazes B., Read R.J. Accumulating evidence suggests that several AB‐toxins subvert the endoplasmic reticulum‐associated protein degradation pathway to enter target cells. Biochemistry. 1997;36:11051–11054. doi: 10.1021/bi971383p. [DOI] [PubMed] [Google Scholar]
- Heda G.D., Tanwani M., Marino C.R. The delta F508 mutation shortens the biochemical half‐life of plasma membrane CFTR in polarized epithelial cells. Am. J. Physiol. Cell Physiol. 2001;280:C166–C174. doi: 10.1152/ajpcell.2001.280.1.C166. [DOI] [PubMed] [Google Scholar]
- Henley J.R., Krueger E.W.A., Oswald B.J., McNiven M.A. Dynamin‐mediated internalization of caveolae. J. Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansky F., Pudlak P. Albinism associated with hemorrhagic diathesis and unusual pigmented reticular cells in the bone marrow: Report of two cases with histochemical studies. Blood. 1959;14:162–169. [PubMed] [Google Scholar]
- Herskovits J.S., Burgess C.C., Obar R.A., Vallee R.B. Effects of mutant rat dynamin on endocytosis. J. Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Bright N.A., Rous B., Robinson M.S. Characterization of a fourth adaptor‐related protein complex. Mol. Biol. Cell. 1999;10:2787–2802. doi: 10.1091/mbc.10.8.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishinuma A., Kasai K., Masawa N., Kanno Y., Arimura M., Shimoda S.I., Ieiri T. Missense mutation (C1263R) in the thyroglobulin gene causes congenital goiter with mild hypothyroidism by impaired intracellular transport. Endocr. J. 1998;45:315–327. doi: 10.1507/endocrj.45.315. [DOI] [PubMed] [Google Scholar]
- Hogg N., Stewart M.P., Scarth S.L., Newton R., Shaw J.M., Law S.K., Klein N. A novel leukocyte adhesion deficiency caused by expressed but nonfunctional beta2 integrins Mac‐1 and LFA‐1. J. Clin. Invest. 1999;103:97–106. doi: 10.1172/JCI3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur E.L.F. Motor neurons rely on motor proteins. Trends Cell Biol. 2004;14:233. doi: 10.1016/j.tcb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Hommelgaard A.M., Roepstorff K., Vilhardt F., Torgersen M.L., Sandvig K., van Deurs B. Caveolae: Stable membrane domains with a potential for internalization. Traffic. 2005;6:720–724. doi: 10.1111/j.1600-0854.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- Horesh D., Sapir T., Francis F., Grayer Wolf S., Caspi M., Elbaum M., Chelly J., Reiner O. Doublecortin, a stabilizer of microtubules. Hum. Mol. Genet. 1999;8:1599. doi: 10.1093/hmg/8.9.1599. [DOI] [PubMed] [Google Scholar]
- Horiuchi H., Lippe R., McBride H.M., Rubino M., Woodman P., Stenmark H., Rybin V., Wilm M., Ashman K., Mann M., Zerial M. A novel Rab5 GDP/GTP exchange factor complexed to rabaptin‐5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Horwitz M., Benson K.F., Duan Z., Li F.‐Q., Person R.E. Hereditary neutropenia: Dogs explain human neutrophil elastase mutations. Trends Mol. Med. 2004;10:163. doi: 10.1016/j.molmed.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Huizing M., Sarangarajan R., Strovel E., Zhao Y., Gahl W.A., Boissy R.E. AP‐3 mediates tyrosinase but not TRP‐1 trafficking in human melanocytes. Mol. Biol. Cell. 2001;12:2075–2085. doi: 10.1091/mbc.12.7.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing M., Scher C.D., Strovel E., Fitzpatrick D.L., Hartnell L.M., Anikster Y., Gahl W.A. Nonsense mutations in ADTB3A cause complete deficiency of the {beta}3A subunit of adaptor complex‐3 and severe Hermansky‐Pudlak syndrome type 2. Pediatr. Res. 2002;51:150–158. doi: 10.1203/00006450-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Hume A.N., Collinson L.M., Hopkins C.R., Strom M., Barral D.C., Bossi G., Griffiths G.M., Seabra M.C. The leaden gene product is required with Rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic. 2002;3:193–202. doi: 10.1034/j.1600-0854.2002.030305.x. [DOI] [PubMed] [Google Scholar]
- Insel P.A., Head B.P., Ostrom R.S., Patel H.H., Swaney J.S., Tang C.‐M., Roth D.M. Caveolae and lipid rafts: G protein‐coupled receptor signaling microdomains in cardiac myocytes. Ann. NY Acad. Sci. 2005;1047:166–172. doi: 10.1196/annals.1341.015. [DOI] [PubMed] [Google Scholar]
- Jaffe A.B., Hall A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jensen H.K., Holst H., Jensen L.G., Jorgensen M.M., Andreasen P.H., Jensen T.G., Andresen B.S., Heath F., Hansen P.S., Neve S., Kristiansen K., Faergeman O., Kolvraa S., Bolund L., Gregersen N. A common W556S mutation in the LDL receptor gene of Danish patients with familial hypercholesterolemia encodes a transport‐defective protein. Atherosclerosis. 1997;131:67–72. doi: 10.1016/s0021-9150(96)06059-5. [DOI] [PubMed] [Google Scholar]
- Joliot A., Pernelle C., Deagostini‐Bazin H., Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA. 1991;88:1864–1868. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.K., Chaplin T., Shankar A., Neat M., Patel N., Samuel D.P., Hill A.S., Debernardi S., Bassini A., Young B.D., Saha V. Identification and molecular characterisation of a CALM‐AF10 fusion in acute megakaryoblastic leukaemia. Leukemia. 2001;15:910–914. doi: 10.1038/sj.leu.2402140. [DOI] [PubMed] [Google Scholar]
- Jones B., Jones E.L., Bonney S.A., Patel H.N., Mensenkamp A.R., Eichenbaum‐Voline S., Rudling M., Myrdal U., Annesi G., Naik S., Meadows N., Quattrone A., Islam S.A., Naoumova R.P., Angelin B., Infante R., Levy E., Roy C.C., Freemont P.S., Scott J., Shoulders C.C. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat. Genet. 2003;34:29–31. doi: 10.1038/ng1145. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Kadowaki H., Accili D., Yazaki Y., Taylor S.I. Substitution of arginine for histidine at position 209 in the alpha‐subunit of the human insulin receptor. A mutation that impairs receptor dimerization and transport of receptors to the cell surface. J. Biol. Chem. 1991;266:21224–21231. [PubMed] [Google Scholar]
- Kaiser C.A., Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Kaler S.G. Metabolic and molecular bases of Menkes disease and occipital horn syndrome. Pediatr. Dev. Pathol. 1998;1:85–98. doi: 10.1007/s100249900011. [DOI] [PubMed] [Google Scholar]
- Katzmann D.J., Odorizzi G., Emr S.D. Receptor downregulation and multivesicular‐body sorting. Nat. Rev. Mol. Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Kaufman R.J. Stress signaling from the lumen of the endoplasmic reticulum: Coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Kestila M., Lenkkeri U., Mannikko M., Lamerdin J., McCready P., Putaala H., Ruotsalainen V., Morita T., Nissinen M., Herva R., Kashtan C.E., Peltonen L., Holmberg C., Olsen A., Tryggvason K. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Kim P.S., Arvan P. Endocrinopathies in the family of endoplasmic reticulum (ER) storage diseases: Disorders of protein trafficking and the role of ER molecular chaperones. Endocr. Rev. 1998;19:173–202. doi: 10.1210/edrv.19.2.0327. [DOI] [PubMed] [Google Scholar]
- Kim B.E., Smith K., Meagher C.K., Petris M.J. A conditional mutation affecting localization of the Menkes disease copper ATPase. Suppression by copper supplementation. J. Biol. Chem. 2002;277:44079. doi: 10.1074/jbc.M208737200. 4408. [DOI] [PubMed] [Google Scholar]
- Kim B.Y., Kramer H., Yamamoto A., Kominami E., Kohsaka S., Akazawa C. Molecular characterization of mammalian homologues of class C Vps proteins that interact with syntaxin‐7. J. Biol. Chem. 2001;276:29393–29402. doi: 10.1074/jbc.M101778200. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Three ways to make a vesicle. Nat. Rev. Mol. Cell Biol. 2000;1:187–198. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K., Taylor M.P., Jackson W.T. Cellular autophagy: Surrender, avoidance and subversion by microorganisms. Nat. Rev. Microbiol. 2004;2:301–314. doi: 10.1038/nrmicro865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev A.F., Goldberg A.L. Proteasome inhibitors: From research tools to drug candidates. Chem. Biol. 2001;8:739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- Knoblauch H., Muller‐Myhsok B., Busjahn A., Ben Avi L., Bahring S., Baron H., Heath S.C., Uhlmann R., Faulhaber H.D., Shpitzen S., Aydin A., Reshef A., Rosenthal M., Eliav O., Muhl A., Lowe A., Schurr D., Harats D., Jeschke E., Friedlander Y., Schuster H., Luft F.C., Leitersdorf E. A cholesterol‐lowering gene maps to chromosome 13q. Am. J. Hum. Genet. 2000;66:157–166. doi: 10.1086/302704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M.R., Hohneker K.W., Zhou Z., Olsen J.C., Noah T.L., Hu P.C., Leigh M.W., Engelhardt J.F., Edwards L.J., Jones K.R. A controlled study of adenoviral‐vector‐mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N. Engl. J. Med. 1995;333:823–831. doi: 10.1056/NEJM199509283331302. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Hirayama Y., Kobayashi E., Kubo Y., Hino O. A germline insertion in the tuberous sclerosis (Tsc2) gene gives rise to the Eker rat model of dominantly inherited cancer. Nat. Genet. 1995;9:70–74. doi: 10.1038/ng0195-70. [DOI] [PubMed] [Google Scholar]
- Koenig J.H., Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J. Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T., Ikeda K. Possible temperature‐dependent blockage of synaptic vesicle recycling induced by a single gene mutation in Drosophila. J. Neurobiol. 1983;14:207–225. doi: 10.1002/neu.480140305. [DOI] [PubMed] [Google Scholar]
- Koushika S.P., Richmond J.E., Hadwiger G., Weimer R.M., Jorgensen E.M., Nonet M.L. A post‐docking role for active zone protein Rim. Nat. Neurosci. 2001;4:997. doi: 10.1038/nn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer B., Pelchen‐Matthews A., Deneka M., Garcia E., Piguet V., Marsh M. HIV interaction with endosomes in macrophages and dendritic cells. Blood Cells Mol. Dis. 2005;35:136–142. doi: 10.1016/j.bcmd.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Kramer‐Zucker A.G., Wiessner S., Jensen A.M., Drummond I.A. Organization of the pronephric filtration apparatus in zebrafish requires nephrin, podocin and the FERM domain protein mosaic eyes. Dev. Biol. 2005;285:316–329. doi: 10.1016/j.ydbio.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski R., Hall A., Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat. Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- Kuehn M.J., Herrmann J.M., Schekman R. COPII‐cargo interactions direct protein sorting into ER‐derived transport vesicles. Nature. 1998;391:187–190. doi: 10.1038/34438. [DOI] [PubMed] [Google Scholar]
- Kumar C.C., Madison V. AKT crystal structure and AKT‐specific inhibitors. Oncogene. 2005;24:7493–7501. doi: 10.1038/sj.onc.1209087. [DOI] [PubMed] [Google Scholar]
- Kupershmidt S., Yang T., Chanthaphaychith S., Wang Z., Towbin J.A., Roden D.M. Defective human ether‐a‐go‐go‐related gene trafficking linked to an endoplasmic reticulum retention signal in the C terminus. J. Biol. Chem. 2002;277:27442–27448. doi: 10.1074/jbc.M112375200. [DOI] [PubMed] [Google Scholar]
- Kurian K.M., Watson C.J., Wyllie A.H. Retroviral vectors. Mol. Pathol. 2000;53:173–176. doi: 10.1136/mp.53.4.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T.V., Dupree P., Parton R.G., Kellner R., Virta H., Lehnert M., Simons K. VIP21, a 21‐kD membrane protein is an integral component of trans‐Golgi‐network‐derived transport vesicles. J. Cell Biol. 1992;118:1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque L., Royal I., Surprenant D.S., Patterson C., Gingras D., Beliveau R. Regulation of vascular endothelial growth factor receptor‐2 activity by caveolin‐1 and plasma membrane cholesterol. Mol. Biol. Cell. 2003;14:334–347. doi: 10.1091/mbc.E02-07-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fontaine S., Firth S.D., Lockhart P.J., Brooks H., Camakaris J., Mercer J.F. Intracellular localization and loss of copper responsiveness of Mnk, the murine homologue of the Menkes protein, in cells from blotchy (Mo blo) and brindled (Mo br) mouse mutants. Hum. Mol. Genet. 1999;8:1069–1075. doi: 10.1093/hmg/8.6.1069. [DOI] [PubMed] [Google Scholar]
- Lamaze C., Dujeancourt A., Baba T., Lo C.G., Benmerah A., Dautry‐Varsat A. Interleukin 2 receptors and detergent‐resistant membrane domains define a clathrin‐independent endocytic pathway. Mol. Cell. 2001;7:661. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- Lee M.P., Feinberg A.P. Aberrant splicing but not mutations of TSG101 in human breast cancer. Cancer Res. 1997;57:3131–3134. [PubMed] [Google Scholar]
- Lee S.W., Reimer C.L., Oh P., Campbell D.B., Schnitzer J.E. Tumor cell growth inhibition by caveolin re‐expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- Lee M.C., Miller E.A., Goldberg J., Orci L., Schekman R. Bi‐directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Leitersdorf E., Reshef A., Meiner V., Dann E.J., Beigel Y., van Roggen F.G., van der Westhuyzen D.R., Coetzee G.A. A missense mutation in the low density lipoprotein receptor gene causes familial hypercholesterolemia in Sephardic Jews. Hum. Genet. 1993;91:141–147. doi: 10.1007/BF00222714. [DOI] [PubMed] [Google Scholar]
- Lemahieu V., Gastier J.M., Francke U. Novel mutations in the Wiskott‐Aldrich syndrome protein gene and their effects on transcriptional, translational, and clinical phenotypes. Hum. Mutat. 1999;14:54–66. doi: 10.1002/(SICI)1098-1004(1999)14:1<54::AID-HUMU7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Levran O., Desnick R.J., Schuchman E.H. Niemann‐Pick disease: A frequent missense mutation in the acid sphingomyelinase gene of Ashkenazi Jewish type A and B patients. Proc. Natl. Acad. Sci. USA. 1991;88:3748–3752. doi: 10.1073/pnas.88.9.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Okamoto T., Chun M., Sargiacomo M., Casanova J.E., Hansen S.H., Nishimoto I., Lisanti M.P. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J. Biol. Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- Li X.‐J., Li S.‐H., Sharp A.H., Nucifora F.C., Schilling G., Lanahan A., Worley P., Snyder S.H., Ross C.A. A huntingtin‐associated protein enriched in brain with implications for pathology. Nature. 1995;378:398. doi: 10.1038/378398a0. [DOI] [PubMed] [Google Scholar]
- Li S., Song K.S., Lisanti M.P. Expression and characterization of recombinant caveolin. J. Biol. Chem. 1996;271:568–573. [PubMed] [Google Scholar]
- Li W., Zhang Q., Oiso N., Novak E.K., Gautam R., O'Brien E.P., Tinsley C.L., Blake D.J., Spritz R.A., Copeland N.G., Jenkins N.A., Amato D., Roe B.A., Starcevic M., Dell'Angelica E.C., Elliott R.W., Mishra V., Kingsmore S.F., Paylor R.E., Swank R.T. Hermansky‐Pudlak syndrome type 7 (HPS‐7) results from mutant dysbindin, a member of the biogenesis of lysosome‐related organelles complex 1 (BLOC‐1) Nat. Genet. 2003;35:84. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Repa J.J., Valasek M.A., Beltroy E.P., Turley S.D., German D.C., Dietschy J.M. Molecular, anatomical, and biochemical events associated with neurodegeneration in mice with Niemann‐Pick type C disease. J. Neuropathol. Exp. Neurol. 2005;64:323–333. doi: 10.1093/jnen/64.4.323. [DOI] [PubMed] [Google Scholar]
- Li N., Xiang G.S., Dokainish H., Ireton K., Elferink L.A. The listeria protein internalin B mimics hepatocyte growth factor‐induced receptor trafficking. Traffic. 2005;6:459–473. doi: 10.1111/j.1600-0854.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Liang J.S., Lu M.Y., Tsai M.J., Lin D.T., Lin K.H. Bone marrow transplantation from an HLA‐matched unrelated donor for treatment of Chediak‐Higashi syndrome. J. Formos. Med. Assoc. 2000;99:499–502. [PubMed] [Google Scholar]
- Lichtenfeld J.L., Kirschner R.H., Wiernik P.H. Familial Sjogren's syndrome with associated primary salivary gland lymphoma. Am. J. Med. 1976;60:286–292. doi: 10.1016/0002-9343(76)90439-3. [DOI] [PubMed] [Google Scholar]
- Liljedahl M., Maeda Y., Colanzi A., Ayala I., Van Lint J., Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans‐Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- Lin S.X., Grant B., Hirsh D., Maxfield F.R. Rme‐1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat. Cell Biol. 2001;3:567–572. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- Lisanti M.P., Scherer P.E., Tang Z., Sargiacomo M. Caveolae, caveolin and caveolin‐rich membrane domains: A signalling hypothesis. Trends Cell Biol. 1994;4:231. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Liu J., Oh P., Horner T., Rogers R.A., Schnitzer J.E. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol‐anchored protein microdomains. J. Biol. Chem. 1997;272:7211–7222. doi: 10.1074/jbc.272.11.7211. [DOI] [PubMed] [Google Scholar]
- Liu X., Ondek B., Williams D.S. Mutant myosin VIIa causes defective melanosome distribution in the RPE of shaker‐1 mice. Nat. Genet. 1998;19:117. doi: 10.1038/470. [DOI] [PubMed] [Google Scholar]
- Lodge R., Descoteaux A. Modulation of phagolysosome biogenesis by the lipophosphoglycan of Leishmania. Clin. Immunol. 2005;114:256–265. doi: 10.1016/j.clim.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Loo T.W., Bartlett M.C., Clarke D.M. Rescue of deltaF508 and other misprocessed CFTR mutants by a novel quinazoline compound. Mol. Pharm. 2005;2:407–413. doi: 10.1021/mp0500521. [DOI] [PubMed] [Google Scholar]
- Lowe C.U., Terrey M., Mac L.E. Organic‐aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am. J. Dis. Child. 1952;83:164–184. doi: 10.1001/archpedi.1952.02040060030004. [DOI] [PubMed] [Google Scholar]
- Lowe M. Structure and function of the Lowe syndrome protein OCRL1. Traffic. 2005;6:711–719. doi: 10.1111/j.1600-0854.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Lu L., Tai G., Hong W. Autoantigen golgin‐97, an effector of Arl1 GTPase, participates in traffic from the endosome to the trans‐Golgi network. Mol. Biol. Cell. 2004;15:4426–4443. doi: 10.1091/mbc.E03-12-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M., Doroszewicz J., Seyberth H.W., Bokenkamp A., Balluch B., Nuutinen M., Utsch B., Waldegger S. Functional evaluation of Dent's disease‐causing mutations: Implications for ClC‐5 channel trafficking and internalization. Hum. Genet. 2005;117:228–237. doi: 10.1007/s00439-005-1303-2. [DOI] [PubMed] [Google Scholar]
- Luna A., Matas O.B., Martinez‐Menarguez J.A., Mato E., Duran J.M., Ballesta J., Way M., Egea G. Regulation of protein transport from the Golgi complex to the endoplasmic reticulum by CDC42 and N‐WASP. Mol. Biol. Cell. 2002;13:866–879. doi: 10.1091/mbc.01-12-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark R., Carlsson S.R. The beta‐appendages of the four adaptor‐protein (AP) complexes: Structure and binding properties, and identification of sorting nexin 9 as an accessory protein to AP‐2. Biochem. J. 2002;362:597–607. doi: 10.1042/0264-6021:3620597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsenko S., Petris M.J. Function and regulation of the mammalian copper‐transporting ATPases: Insights from biochemical and cell biological approaches. J. Membr. Biol. 2003;191:1–12. doi: 10.1007/s00232-002-1040-6. [DOI] [PubMed] [Google Scholar]
- Luzio J.P., Poupon V., Lindsay M.R., Mullock B.M., Piper R.C., Pryor P.R. Membrane dynamics and the biogenesis of lysosomes. Mol. Membr. Biol. 2003;20:141–154. doi: 10.1080/0968768031000089546. [DOI] [PubMed] [Google Scholar]
- Magnusson M.K., Meade K.E., Brown K.E., Arthur D.C., Krueger L.A., Barrett A.J., Dunbar C.E. Rabaptin‐5 is a novel fusion partner to platelet‐derived growth factor beta receptor in chronic myelomonocytic leukemia. Blood. 2001;98:2518–2525. doi: 10.1182/blood.v98.8.2518. [DOI] [PubMed] [Google Scholar]
- Majoul I., Straub M., Hell S.W., Duden R., Soling H.D. KDEL‐cargo regulates interactions between proteins involved in COPI vesicle traffic: Measurements in living cells using FRET. Dev. Cell. 2001;1:139–153. doi: 10.1016/s1534-5807(01)00004-1. [DOI] [PubMed] [Google Scholar]
- Malhotra V., Serafini T., Orci L., Shepherd J.C., Rothman J.E. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- Mandelkow E.M., Stamer K., Vogel R., Thies E., Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol. Aging. 2003;24:1079. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Mann D.A., Frankel A.D. Endocytosis and targeting of exogenous HIV‐1 Tat protein. EMBO J. 1991;10:1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew E.C., Shaw J.M., Bonilla F.A., Law S.K., Wright D.A. A novel point mutation in CD18 causing the expression of dysfunctional CD11/CD18 leucocyte integrins in a patient with leucocyte adhesion deficiency (LAD) Clin. Exp. Immunol. 2000;121:133–138. doi: 10.1046/j.1365-2249.2000.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Orci L., Amherdt M., Bednarek S.Y., Hamamoto S., Schekman R., Yeung T. COPII‐coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- Matsuyama W., Nakagawa M., Takashima H., Osame M. Altered trafficking and adhesion function of MPZ mutations and phenotypes of Charcot‐Marie‐Tooth disease 1B. Acta Neuropathol. (Berl.) 2002;103:501–508. doi: 10.1007/s00401-001-0497-1. [DOI] [PubMed] [Google Scholar]
- McConnell M.J., Imperiale M.J. Biology of adenovirus and its use as a vector for gene therapy. Hum. Gene Ther. 2004;15:1022–1033. doi: 10.1089/hum.2004.15.1022. [DOI] [PubMed] [Google Scholar]
- McCormick C., Duncan G., Goutsos K.T., Tufaro F. The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc. Natl. Acad. Sci. USA. 2000;97:668–673. doi: 10.1073/pnas.97.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros‐Neto G., Kim P.S., Yoo S.E., Vono J., Targovnik H.M., Camargo R., Hossain S.A., Arvan P. Congenital hypothyroid goiter with deficient thyroglobulin. Identification of an endoplasmic reticulum storage disease with induction of molecular chaperones. J. Clin. Invest. 1996;98:2838–2844. doi: 10.1172/JCI119112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche G., Pastural E., Feldmann J., Certain S., Ersoy F., Dupuis S., Wulffraat N., Bianchi D., Fischer A., Le Deist F., de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- Mendell J.R., Kissel J.T., Amato A.A., King W., Signore L., Prior T.W., Sahenk Z., Benson S., McAndrew P.E., Rice R. Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N. Engl. J. Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- Mignani R., Cagnoli L. Enzyme replacement therapy in Fabry's disease: Recent advances and clinical applications. J. Nephrol. 2004;17:354–363. [PubMed] [Google Scholar]
- Millard T.H., Sharp S.J., Machesky L.M. Signalling to actin assembly via the WASP (Wiskott‐Aldrich syndrome protein)‐family proteins and the Arp2/3 complex. Biochem. J. 2004;380:1–17. doi: 10.1042/BJ20040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti C., Sotgia F., Bruno C., Scartezzini P., Broda P., Bado M., Masetti E., Mazzocco M., Egeo A., Donati M.A., Volonte D., Galbiati F., Cordone G., Bricarelli F.D., Lisanti M.P., Zara F. Mutations in the caveolin‐3 gene cause autosomal dominant limb‐girdle muscular dystrophy. Nat. Genet. 1998;18:365. doi: 10.1038/ng0498-365. [DOI] [PubMed] [Google Scholar]
- Mitrakou A., Tountas N., Raptis A.E., Bauer R.J., Schulz H., Raptis S.A. Long‐term effectiveness of a new alpha‐glucosidase inhibitor (BAY m1099‐miglitol) in insulin‐treated type 2 diabetes mellitus. Diabet. Med. 1998;15:657–660. doi: 10.1002/(SICI)1096-9136(199808)15:8<657::AID-DIA652>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Miyajima H. Genetic disorders affecting proteins of iron and copper metabolism: Clinical implications. Intern. Med. 2002;41:762–769. doi: 10.2169/internalmedicine.41.762. [DOI] [PubMed] [Google Scholar]
- Miyata Y. Hsp90 inhibitor geldanamycin and its derivatives as novel cancer chemotherapeutic agents. Curr. Pharm. Des. 2005;11:1131–1138. doi: 10.2174/1381612053507585. [DOI] [PubMed] [Google Scholar]
- Moores C.A., Perderiset M., Francis F., Chelly J., Houdusse A., Milligan R.A. Mechanism of microtubule stabilization by doublecortin. Mol. Cell. 2004;14:833. doi: 10.1016/j.molcel.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Mor‐Cohen R., Zivelin A., Rosenberg N., Shani M., Muallem S., Seligsohn U. Identification and functional analysis of two novel mutations in the multidrug resistance protein 2 gene in Israeli patients with Dubin‐Johnson syndrome. J. Biol. Chem. 2001;276:36923–36930. doi: 10.1074/jbc.M105047200. [DOI] [PubMed] [Google Scholar]
- Morello J.P., Petaja‐Repo U.E., Bichet D.G., Bouvier M. Pharmacological chaperones: A new twist on receptor folding. Trends Pharmacol. Sci. 2000;21:466–469. doi: 10.1016/s0165-6147(00)01575-3. [DOI] [PubMed] [Google Scholar]
- Morgan J.R., Prasad K., Hao W., Augustine G.J., Lafer E.M. A conserved clathrin assembly motif essential for synaptic vesicle endocytosis. J. Neurosci. 2000;20:8667–8676. doi: 10.1523/JNEUROSCI.20-23-08667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu F.T., Callaghan J.M., Steele‐Mortimer O., Stenmark H., Parton R.G., Campbell P.L., McCluskey J., Yeo J.P., Tock E.P., Toh B.H. EEA1, an early endosome‐associated protein. EEA1 is a conserved alpha‐helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin‐binding IQ motif. J. Biol. Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Ghosh R.N., Maxfield F.R. Endocytosis. Physiol. Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Munro S. Localization of proteins to the Golgi apparatus. Trends Cell Biol. 1998;8:11–15. doi: 10.1016/S0962-8924(97)01197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M., Peranen J., Schreiner R., Wieland F., Kurzchalia T.V., Simons K. VIP21/caveolin is a cholesterol‐binding protein. Proc. Natl. Acad. Sci. USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R.F. Maturation models for endosome and lysosome biogenesis. Trends Cell Biol. 1991;1:77–82. doi: 10.1016/0962-8924(91)90022-2. [DOI] [PubMed] [Google Scholar]
- Musch A., Cohen D., Kreitzer G., Rodriguez‐Boulan E. cdc42 regulates the exit of apical and basolateral proteins from the trans‐Golgi network. EMBO J. 2001;20:2171–2179. doi: 10.1093/emboj/20.9.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M., Shimizu K., Hayashi Y., Taki T., Taniwaki M., Hosoda F., Kobayashi H., Nakamura H., Sadamori N., Ohnishi H., Bessho F., Yanagisawa M., Ohki M. Consistent detection of CALM‐AF10 chimaeric transcripts in haematological malignancies with t(10;11)(p13;q14) and identification of novel transcripts. Br. J. Haematol. 1999;105:928–937. doi: 10.1046/j.1365-2141.1999.01433.x. [DOI] [PubMed] [Google Scholar]
- Neerman‐Arbez M., Johnson K.M., Morris M.A., McVey J.H., Peyvandi F., Nichols W.C., Ginsburg D., Rossier C., Antonarakis S.E., Tuddenham E.G. Molecular analysis of the ERGIC‐53 gene in 35 families with combined factor V‐factor VIII deficiency. Blood. 1999;93:2253–2260. [PubMed] [Google Scholar]
- Neufeld G., Cohen T., Gengrinovitch S., Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Nguyen L., Pieters J. The Trojan horse: Survival tactics of pathogenic mycobacteria in macrophages. Trends Cell Biol. 2005;15:269–276. doi: 10.1016/j.tcb.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Nichols B.J., Pelham H.R. SNAREs and membrane fusion in the Golgi apparatus. Biochim. Biophys. Acta. 1998;1404:9–31. doi: 10.1016/s0167-4889(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Nichols W.C., Seligsohn U., Zivelin A., Terry V.H., Hertel C.E., Wheatley M.A., Moussalli M.J., Hauri H.P., Ciavarella N., Kaufman R.J., Ginsburg D. Mutations in the ER‐Golgi intermediate compartment protein ERGIC‐53 cause combined deficiency of coagulation factors V and VIII. Cell. 1998;93:61–70. doi: 10.1016/s0092-8674(00)81146-0. [DOI] [PubMed] [Google Scholar]
- Niedergang F., Chavrier P. Regulation of phagocytosis by Rho GTPases. Curr. Top. Microbiol. Immunol. 2005;291:43–60. doi: 10.1007/3-540-27511-8_4. [DOI] [PubMed] [Google Scholar]
- Norden A.G.W., Lapsley M., Igarashi T., Kelleher C.L., Lee P.J., Matsuyama T., Scheinman S.J., Shiraga H., Sundin D.P., Thakker R.V., Unwin R.J., Verroust P., Moestrup S.K. Urinary Megalin deficiency implicates abnormal tubular endocytic function in Fanconi syndrome. J. Am. Soc. Nephrol. 2002;13:125–133. doi: 10.1681/ASN.V131125. [DOI] [PubMed] [Google Scholar]
- Notarangelo L.D., Ochs H.D. Wiskott–Aldrich syndrome: A model for defective actin reorganization, cell trafficking and synapse formation. Curr. Opin. Immunol. 2003;15:585. doi: 10.1016/s0952-7915(03)00112-2. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post‐translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Oehlke J., Scheller A., Wiesner B., Krause E., Beyermann M., Klauschenz E., Melzig M., Bienert M. Cellular uptake of an alpha‐helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non‐endocytically. Biochim. Biophys. Acta. 1998;1414:127–139. doi: 10.1016/s0005-2736(98)00161-8. [DOI] [PubMed] [Google Scholar]
- Oess S., Hildt E. Novel cell permeable motif derived from the PreS2‐domain of hepatitis‐B virus surface antigens. Gene Ther. 2000;7:750–758. doi: 10.1038/sj.gt.3301154. [DOI] [PubMed] [Google Scholar]
- Oh P., McIntosh D.P., Schnitzer J.E. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP‐driven fission from the plasma membrane of endothelium. J. Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T., Sasaki T., Nakamura M., Shimamura M., Tanahashi N., Omura S., Tanaka K. The proteasome is involved in angiogenesis. Biochem. Biophys. Res. Commun. 1998;246:243–248. doi: 10.1006/bbrc.1998.8604. [DOI] [PubMed] [Google Scholar]
- Olkkonen V.M., Stenmark H. Role of Rab GTPases in membrane traffic. Int. Rev. Cytol. 1997;176:1–85. doi: 10.1016/s0074-7696(08)61608-3. [DOI] [PubMed] [Google Scholar]
- Ostrom R.S., Insel P.A. The evolving role of lipid rafts and caveolae in G protein‐coupled receptor signaling: Implications for molecular pharmacology. Br. J. Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D.J., Vallis Y., Pearse B.M., McMahon H.T., Evans P.R. The structure and function of the beta 2‐adaptin appendage domain. EMBO J. 2000;19:4216–4227. doi: 10.1093/emboj/19.16.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padron D., Wang Y.J., Yamamoto M., Yin H., Roth M.G. Phosphatidylinositol phosphate 5‐kinase I{beta} recruits AP‐2 to the plasma membrane and regulates rates of constitutive endocytosis. J. Cell Biol. 2003;162:693–701. doi: 10.1083/jcb.200302051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G.E. Fine structure of blood capillaries. J. Appl. Phys. 1953;24:1424. [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pastural E., Ersoy F., Yalman N., Wulffraat N., Grillo E., Ozkinay F., Tezcan I., Gedikoglu G., Philippe N., Fischer A., de Saint Basile G. Two genes are responsible for Griscelli syndrome at the same 15q21 locus. Genomics. 2000;63:299. doi: 10.1006/geno.1999.6081. [DOI] [PubMed] [Google Scholar]
- Pearce D.A. Localization and processing of CLN3, the protein associated to Batten disease: Where is it and what does it do? J. Neurosci. Res. 2000;59:19–23. [PubMed] [Google Scholar]
- Peden A.A., Oorschot V., Hesser B.A., Austin C.D., Scheller R.H., Klumperman J. Localization of the AP‐3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 2004;164:1065–1076. doi: 10.1083/jcb.200311064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen‐Matthews A., Kramer B., Marsh M. Infectious HIV‐1 assembles in late endosomes in primary macrophages. J. Cell Biol. 2003;162:443–455. doi: 10.1083/jcb.200304008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H.R. SNAREs and the specificity of transport vesicle targeting. Trends Cell Biol. 2001;11:99–101. doi: 10.1016/s0962-8924(01)01929-8. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Kartenbeck J., Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two‐step vesicular‐transport pathway to the ER. Nat. Cell Biol. 2001;3:473. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Burli T., Zerial M., Helenius A. Caveolin‐stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Pelkmans L. Secrets of caveolae‐ and lipid raft‐mediated endocytosis revealed by mammalian viruses. Biochim. Biophys. Acta. 2005;1746:295–304. doi: 10.1016/j.bbamcr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Perlmutter D.H. Alpha1‐antitrypsin deficiency: Liver disease associated with retention of a mutant secretory glycoprotein in the endoplasmic reticulum. Methods Mol. Biol. 2003;232:39–56. doi: 10.1385/1-59259-394-1:39. [DOI] [PubMed] [Google Scholar]
- Perlmutter D.H. Alpha‐1‐antitrypsin deficiency: Diagnosis and treatment. Clin. Liver Dis. 2004;8:839–859. doi: 10.1016/j.cld.2004.06.001. , viii–ix. [DOI] [PubMed] [Google Scholar]
- Perou C.M., Moore K.J., Nagle D.L., Misumi D.J., Woolf E.A., McGrail S.H., Holmgren L., Brody T.H., Dussault B.J., Monroe C.A., Duyk G.M., Pryor R.J., Li L., Justice M.J., Kaplan J. Identification of the murine beige gene by YAC complementation and positional cloning. Nat. Genet. 1996;13:303. doi: 10.1038/ng0796-303. [DOI] [PubMed] [Google Scholar]
- Petris M.J., Mercer J.F. The Menkes protein (ATP7A; MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C‐terminal di‐leucine endocytic signal. Hum. Mol. Genet. 1999;8:2107–2115. doi: 10.1093/hmg/8.11.2107. [DOI] [PubMed] [Google Scholar]
- Pind S., Riordan J.R., Williams D.B. Participation of the endoplasmic reticulum chaperone calnexin (p88, IP90) in the biogenesis of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 1994;269:12784–12788. [PubMed] [Google Scholar]
- Platt F.M., Neises G.R., Reinkensmeier G., Townsend M.J., Perry V.H., Proia R.L., Winchester B., Dwek R.A., Butters T.D. Prevention of lysosomal storage in Tay‐Sachs mice treated with N‐butyldeoxynojirimycin. Science. 1997;276:428–431. doi: 10.1126/science.276.5311.428. [DOI] [PubMed] [Google Scholar]
- Pochampally R.R., Horwitz E.M., Digirolamo C.M., Stokes D.S., Prockop D.J. Correction of a mineralization defect by overexpression of a wild‐type cDNA for COL1A1 in marrow stromal cells (MSCs) from a patient with osteogenesis imperfecta: A strategy for rescuing mutations that produce dominant‐negative protein defects. Gene Ther. 2005;14:1119–1125. doi: 10.1038/sj.gt.3302514. [DOI] [PubMed] [Google Scholar]
- Ponnambalam S., Baldwin S.A. Constitutive protein secretion from the trans‐Golgi network to the plasma membrane. Mol. Membr. Biol. 2003;20:129–139. doi: 10.1080/0968768031000084172. [DOI] [PubMed] [Google Scholar]
- Prasher V.P. Review of donepezil, rivastigmine, galantamine and memantine for the treatment of dementia in Alzheimer's disease in adults with Down syndrome: Implications for the intellectual disability population. Int. J. Geriatr. Psychiatry. 2004;19:509–515. doi: 10.1002/gps.1077. [DOI] [PubMed] [Google Scholar]
- Prigozhina N.L., Waterman‐Storer C.M. Protein kinase D‐mediated anterograde membrane trafficking is required for fibroblast motility. Curr. Biol. 2004;14:88–98. doi: 10.1016/j.cub.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Puthenveedu M.A., Linstedt A.D. Gene replacement reveals that p115/SNARE interactions are essential for Golgi biogenesis. Proc. Natl. Acad. Sci. USA. 2004;101:1253–1256. doi: 10.1073/pnas.0306373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M., Byers P.H. Constitutive skipping of alternatively spliced exon 10 in the ATP7A gene abolishes Golgi localization of the Menkes protein and produces the occipital horn syndrome. Hum. Mol. Genet. 1998;7:465–469. doi: 10.1093/hmg/7.3.465. [DOI] [PubMed] [Google Scholar]
- Quilty J.A., Reithmeier R.A. Trafficking and folding defects in hereditary spherocytosis mutants of the human red cell anion exchanger. Traffic. 2000;1:987–998. doi: 10.1034/j.1600-0854.2000.011208.x. [DOI] [PubMed] [Google Scholar]
- Razani B., Wang X.B., Engelman J.A., Battista M., Lagaud G., Zhang X.L., Kneitz B., Hou H., Jr., Christ G.J., Edelmann W., Lisanti M.P. Caveolin‐2‐deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol. Cell Biol. 2002;22:2329–2344. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O., Carrozzo R., Shen Y., Wehnert M., Faustinella F., Dobyns W.B., Caskey C.T., Ledbetter D.H. Isolation of a Miller‐Dicker lissencephaly gene containing G protein [beta]‐subunit‐like repeats. Nature. 1993;364:717. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Richardson G.P., Forge A., Kros C.J., Fleming J., Brown S.D.A.M., Steel K.P. Myosin VIIA is required for aminoglycoside accumulation in cochlear hair cells. J. Neurosci. 1997;17:9506–9519. doi: 10.1523/JNEUROSCI.17-24-09506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad N., Gad H., Low P., Di Paolo G., Brodin L., Shupliakov O., De Camilli P. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin‐mediated synaptic vesicle endocytosis. Neuron. 1999;24:143–154. doi: 10.1016/s0896-6273(00)80828-4. [DOI] [PubMed] [Google Scholar]
- Robert J., Auzan C., Ventura M.A., Clauser E. Mechanisms of cell‐surface rerouting of an endoplasmic reticulum‐retained mutant of the vasopressin V1b/V3 receptor by a pharmacological chaperone. J. Biol. Chem. 2005;280:42198–42206. doi: 10.1074/jbc.M510180200. [DOI] [PubMed] [Google Scholar]
- Robinson M.S., Bonifacino J.S. Adaptor‐related proteins. Curr. Opin. Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- Robinson M.S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Romisch K. Surfing the Sec61 channel: Bidirectional protein translocation across the ER membrane. J. Cell Sci. 1999;112:4185–4191. doi: 10.1242/jcs.112.23.4185. [DOI] [PubMed] [Google Scholar]
- Roth T.F., Porter K.R. Yolk protein uptake in the oocyte of the mosquito Aedes aegypti. l. J. Cell Biol. 1964;20:313–332. doi: 10.1083/jcb.20.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K.G., Heuser J.E., Donzell W.C., Ying Y.S., Glenney J.R., Anderson R.G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rothman J.E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman J.E., Wieland F.T. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Roy A.‐M.M., Parker J.S., Parrish C.R., Whittaker G.R. Early stages of influenza virus entry into Mv‐1 lung cells: Involvement of dynamin. Virology. 2000;267:17–28. doi: 10.1006/viro.1999.0109. [DOI] [PubMed] [Google Scholar]
- Russell S.J. Science medicine, and the future. Gene therapy. Br. Med. J. 1997;315:1289–1292. doi: 10.1136/bmj.315.7118.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser J., Spiess M. Endoplasmic reticulum storage diseases. Swiss Med. Wkly. 2002;132:211–222. doi: 10.4414/smw.2002.09861. [DOI] [PubMed] [Google Scholar]
- Salmon‐Nguyen F., Busson M., Daniel M., Leblanc T., Bernard O.A., Berger R. CALM‐AF10 fusion gene in leukemias: Simple and inversion‐associated translocation (10;11) Cancer Genet. Cytogenet. 2000;122:137–140. doi: 10.1016/s0165-4608(00)00277-6. [DOI] [PubMed] [Google Scholar]
- Sapperstein S.K., Lupashin V.V., Schmitt H.D., Waters M.G. Assembly of the ER to Golgi SNARE complex requires Uso1p. J. Cell Biol. 1996;132:755–767. doi: 10.1083/jcb.132.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M.P. Signal transducing molecules and glycosyl‐phosphatidylinositol‐linked proteins form a caveolin‐rich insoluble complex in MDCK cells. J. Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa T., Harada S.‐I., Ogawa H., Rand J.B., Maruyama I.N., Hosono R. Regulation of the UNC‐18‐Caenorhabditis elegans syntaxin complex by UNC‐13. J. Neurosci. 1999;19:4772–4777. doi: 10.1523/JNEUROSCI.19-12-04772.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales S.J., Pepperkok R., Kreis T.E. Visualization of ER‐to‐Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Scherer P.E., Okamoto T., Chun M., Nishimoto I., Lodish H.F., Lisanti M.P. Identification, sequence, and expression of caveolin‐2 defines a caveolin gene family. Proc. Natl. Acad. Sci. USA. 1996;93:131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman D.M., Schmid S.L., Braell W.A., Rothman J.E. An enzyme that removes clathrin coats: Purification of an uncoating ATPase. J. Cell Biol. 1984;99:723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Wolde M., Thiele C., Fest W., Kratzin H., Podtelejnikov A.V., Witke W., Huttner W.B., Soling H.D. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature. 1999;401:133–141. doi: 10.1038/43613. [DOI] [PubMed] [Google Scholar]
- Schmitz A., Schneider A., Kummer M.P., Herzog V. Endoplasmic reticulum‐localized amyloid beta‐peptide is degraded in the cytosol by two distinct degradation pathways. Traffic. 2004;5:89–101. doi: 10.1111/j.1600-0854.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- Schnitzer J.E., Liu J., Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J. Biol. Chem. 1995;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- Schuster F., Stachel D.K., Schmid I., Baumeister F.A., Graubner U.B., Weiss M., Haas R.J., Belohradsky B.H. Griscelli syndrome: Report of the first peripheral blood stem cell transplant and the role of mutations in the RAB27A gene as an indication for BMT. Bone Marrow Transpl. 2001;28:409–412. doi: 10.1038/sj.bmt.1703114. [DOI] [PubMed] [Google Scholar]
- Schwartz J.J., Zhang S. Peptide‐mediated cellular delivery. Curr. Opin. Mol. Ther. 2000;2:162–167. [PubMed] [Google Scholar]
- Scollay R. Gene therapy: A brief overview of the past, present, and future. Ann. NY Acad. Sci. 2001;953:26–30. doi: 10.1111/j.1749-6632.2001.tb11357.x. [DOI] [PubMed] [Google Scholar]
- Scott C.C., Botelho R.J., Grinstein S. Phagosome maturation: A few bugs in the system. J. Membr. Biol. 2003;193:137. doi: 10.1007/s00232-002-2008-2. [DOI] [PubMed] [Google Scholar]
- Scott C., Ioannou Y.A. The NPC1 protein: Structure implies function. Biochim. Biophys. Acta. 2004;1685:8–13. doi: 10.1016/j.bbalip.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Seabra M.C., Mules E.H., Hume A.N. Rab GTPases, intracellular traffic and disease. Trends Mol. Med. 2002;8:23–30. doi: 10.1016/s1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- Seals D.F., Eitzen G., Margolis N., Wickner W.T., Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl. Acad. Sci. USA. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J., Jokitalo E.J., Warren G. The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol. Biol. Cell. 2000;11:635–645. doi: 10.1091/mbc.11.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak S., Chan E.K., Schoenroth L., Senecal J.L., Fritzler M.J. Early endosome antigen. 1: An autoantigen associated with neurological diseases. J. Investig. Med. 1999;47:311–318. [PubMed] [Google Scholar]
- Selak S., Mahler M., Miyachi K., Fritzler M.L., Fritzler M.J. Identification of the B‐cell epitopes of the early endosome antigen 1 (EEA1) Clin. Immunol. 2003;109:154–164. doi: 10.1016/s1521-6616(03)00169-4. [DOI] [PubMed] [Google Scholar]
- Sharp D., Blinderman L., Combs K.A., Kienzle B., Ricci B., Wager‐Smith K., Gil C.M., Turck C.W., Bouma M.E., Rader D.J. Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinaemia. Nature. 1993;365:65–69. doi: 10.1038/365065a0. [DOI] [PubMed] [Google Scholar]
- Shelton J.G., Moye P.W., Steelman L.S., Blalock W.L., Lee J.T., Franklin R.A., McMahon M., McCubrey J.A. Differential effects of kinase cascade inhibitors on neoplastic and cytokine‐mediated cell proliferation. Leukemia. 2003;17:1765–1782. doi: 10.1038/sj.leu.2403052. [DOI] [PubMed] [Google Scholar]
- Shiflett S.L., Kaplan J., Ward D.M. Chediak‐Higashi syndrome: A rare disorder of lysosomes and lysosome related organelles. Pigment Cell Res. 2002;15:251–257. doi: 10.1034/j.1600-0749.2002.02038.x. [DOI] [PubMed] [Google Scholar]
- Shintani T., Klionsky D.J. Autophagy in health and disease: A double‐edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short B., Haas A., Barr F. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim. Biophys. Acta Mol. Cell Res. 2005;1744:383–395. doi: 10.1016/j.bbamcr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Shorter J., Warren G. A role for the vesicle tethering protein, p115, in the post‐mitotic stacking of reassembling Golgi cisternae in a cell‐free system. J. Cell Biol. 1999;146:57–70. doi: 10.1083/jcb.146.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J., Beard M.B., Seemann J., Dirac‐Svejstrup A.B., Warren G. Sequential tethering of golgins and catalysis of SNAREpin assembly by the vesicle‐tethering protein p115. J. Cell Biol. 2002;157:45–62. doi: 10.1083/jcb.200112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu M., Gafencu A., Antohe F. Transcytosis of plasma macromolecules in endothelial cells: A cell biological survey. Microsc. Res. Tech. 2002;57:269–288. doi: 10.1002/jemt.10086. [DOI] [PubMed] [Google Scholar]
- Smith D.S., Niethammer M., Ayala R., Zhou Y., Gambello M.J., Wynshaw‐Boris A., Tsai L.‐H. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat. Cell Biol. 2000;2:767. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- Sollner T., Whiteheart S.W., Brunner M., Erdjument‐Bromage H., Geromanos S., Tempst P., Rothman J.E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Sommer C., Weishaupt A., Brinkhoff J., Biko L., Wessig C., Gold R., Toyka K.V. Paraneoplastic stiff‐person syndrome: Passive transfer to rats by means of IgG antibodies to amphiphysin. Lancet. 2005;365:1406–1411. doi: 10.1016/S0140-6736(05)66376-3. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B., Lowe M., Levine T., Jamsa E., Dirac‐Svejstrup B., Warren G. A role for Giantin in docking COPI vesicles to Golgi membranes. J. Cell Biol. 1998;140:1013–1021. doi: 10.1083/jcb.140.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgia F., Woodman S.E., Bonuccelli G., Capozza F., Minetti C., Scherer P.E., Lisanti M.P. Phenotypic behavior of caveolin‐3 R26Q, a mutant associated with hyperCKemia, distal myopathy, and rippling muscle disease. Am. J. Physiol. Cell Physiol. 2003;285:C1150–C1160. doi: 10.1152/ajpcell.00166.2003. [DOI] [PubMed] [Google Scholar]
- Sotgia F., Bonuccelli G., Minetti C., Woodman S.E., Capozza F., Kemp R.G., Scherer P.E., Lisanti M.P. Phosphofructokinase muscle‐specific isoform requires caveolin‐3 expression for plasma membrane recruitment and caveolar targeting: Implications for the pathogenesis of caveolin‐related muscle diseases. Am. J. Pathol. 2003;163:2619–2634. doi: 10.1016/S0002-9440(10)63616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut M., van Deurs B. Identification of filamin as a novel ligand for caveolin‐1: Evidence for the organization of caveolin‐1‐associated membrane domains by the actin cytoskeleton. Mol. Biol. Cell. 2000;11:325. doi: 10.1091/mbc.11.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Aasland R., Toh B.H., D'Arrigo A. Endosomal localization of the autoantigen EEA1 is mediated by a zinc‐binding FYVE finger. J. Biol. Chem. 1996;271:24048–24054. doi: 10.1074/jbc.271.39.24048. [DOI] [PubMed] [Google Scholar]
- Stepp S.E., Dufourcq‐Lagelouse R., Le Deist F., Bhawan S., Certain S., Mathew P.A., Henter J.‐I., Bennett M., Fischer A., de Saint Basile G., Kumar V. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- Stephens D.J., Lin‐Marq N., Pagano A., Pepperkok R., Paccaud J.P. COPI‐coated ER‐to‐Golgi transport complexes segregate from COPII in close proximity to ER exit sites. J. Cell Sci. 2000;113(Pt. 12):2177–2185. doi: 10.1242/jcs.113.12.2177. [DOI] [PubMed] [Google Scholar]
- Stinchcombe J.C., Bossi G., Booth S., Griffiths G.M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- Stinchcombe J., Bossi G., Griffiths G.M. Linking albinism and immunity: The secrets of secretory lysosomes. Science. 2004;305:55–59. doi: 10.1126/science.1095291. [DOI] [PubMed] [Google Scholar]
- Storrie B., Desjardins M. The biogenesis of lysosomes: Is it a kiss and run, continuous fusion and fission process? BioEssays. 1996;18:895–903. doi: 10.1002/bies.950181108. [DOI] [PubMed] [Google Scholar]
- Storrie B., Pepperkok R., Nilsson T. Breaking the COPI monopoly on Golgi recycling. Trends Cell Biol. 2000;10:385–391. doi: 10.1016/s0962-8924(00)01818-3. [DOI] [PubMed] [Google Scholar]
- Strom M., Hume A.N., Tarafder A.K., Barkagianni E., Seabra M.C. A family of Rab27‐binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 2002;277:25423–25430. doi: 10.1074/jbc.M202574200. [DOI] [PubMed] [Google Scholar]
- Sun H., Molday R.S., Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor‐specific ATP‐binding cassette transporter responsible for Stargardt disease. J. Biol. Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- Swank R.T., Novak E.K., McGarry M.P., Zhang Y., Li W., Zhang Q., Feng L. Abnormal vesicular trafficking in mouse models of Hermansky‐Pudlak syndrome. Pigment Cell Res. 2000;13(Suppl. 8):59–67. doi: 10.1034/j.1600-0749.13.s8.12.x. [DOI] [PubMed] [Google Scholar]
- Swanton E., Bulleid N.J. Protein folding and translocation across the endoplasmic reticulum membrane. Mol. Membr. Biol. 2003;20:99–104. doi: 10.1080/0968768031000069241. [DOI] [PubMed] [Google Scholar]
- Swanton E., High S., Woodman P. Role of calnexin in the glycan‐independent quality control of proteolipid protein. EMBO J. 2003;22:2948–2958. doi: 10.1093/emboj/cdg300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa A., Mezzacasa A., Hayer A., Longatti A., Pelkmans L., Helenius A. Assembly and trafficking of caveolar domains in the cell: Caveolae as stable, cargo‐triggered, vesicular transporters. J. Cell Biol. 2005;170:769–779. doi: 10.1083/jcb.200506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Suchi M., Desnick R.J., Takada G., Schuchman E.H. Identification and expression of five mutations in the human acid sphingomyelinase gene causing types A and B Niemann‐Pick disease. Molecular evidence for genetic heterogeneity in the neuronopathic and non‐neuronopathic forms. J. Biol. Chem. 1992;267:12552–12558. [PubMed] [Google Scholar]
- Tamarappoo B.K., Verkman A.S. Defective aquaporin‐2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J. Clin. Invest. 1998;101:2257–2267. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarappoo B.K., Yang B., Verkman A.S. Misfolding of mutant aquaporin‐2 water channels in nephrogenic diabetes insipidus. J. Biol. Chem. 1999;274:34825–34831. doi: 10.1074/jbc.274.49.34825. [DOI] [PubMed] [Google Scholar]
- Tang Z., Scherer P.E., Okamoto T., Song K., Chu C., Kohtz D.S., Nishimoto I., Lodish H.F., Lisanti M.P. Molecular cloning of caveolin‐3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- Tang B.L., Wang Y., Ong Y.S., Hong W. COPII and exit from the endoplasmic reticulum. Biochim. Biophys. Acta. 2005;1744:293–303. doi: 10.1016/j.bbamcr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Taschenberger G., Mougey A., Shen S., Lester L.B., LaFranchi S., Shyng S.L. Identification of a familial hyperinsulinism‐causing mutation in the sulfonylurea receptor 1 that prevents normal trafficking and function of KATP channels. J. Biol. Chem. 2002;277:17139–17146. doi: 10.1074/jbc.M200363200. [DOI] [PubMed] [Google Scholar]
- Taylor J.P., Metcalfe R.A., Watson P.F., Weetman A.P., Trembath R.C. Mutations of the PDS gene, encoding pendrin, are associated with protein mislocalization and loss of iodide efflux: Implications for thyroid dysfunction in Pendred syndrome. J. Clin. Endocrinol. Metab. 2002;87:1778–1784. doi: 10.1210/jcem.87.4.8435. [DOI] [PubMed] [Google Scholar]
- Tchernev V.T., Mansfield T.A., Giot L., Kumar A.M., Nandabalan K., Li Y., Mishra V.S., Detter J.C., Rothberg J.M., Wallace M.R., Southwick F.S., Kingsmore S.F. The Chediak‐Higashi protein interacts with SNARE complex and signal transduction proteins. Mol. Med. 2002;8:56–64. [PMC free article] [PubMed] [Google Scholar]
- Tebar F., Bohlander S.K., Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: Localization in endocytic‐coated pits, interactions with clathrin, and the impact of overexpression on clathrin‐mediated traffic. Mol. Biol. Cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlecky S.R., Fransen M. How peroxisomes arise. Traffic. 2000;1:465–473. doi: 10.1034/j.1600-0854.2000.010604.x. [DOI] [PubMed] [Google Scholar]
- Thakker R.V. Chloride channels cough up. Nat. Genet. 1997;17:125–127. doi: 10.1038/ng1097-125. [DOI] [PubMed] [Google Scholar]
- Thomsen P., Roepstorff K., Stahlhut M., van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol. Biol. Cell. 2002;13:238–250. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinczek B., Ebneth A., Mandelkow E.M., Mandelkow E. Tau regulates the attachment/detachment but not the speed of motors in microtubule‐dependent transport of single vesicles and organelles. J. Cell Sci. 1999;112:2355. doi: 10.1242/jcs.112.14.2355. [DOI] [PubMed] [Google Scholar]
- Tsai B., Gilbert J.M., Stehle T., Lencer W., Benjamin T.L., Rapoport T.A. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 2003;22:4346–4355. doi: 10.1093/emboj/cdg439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K., Kuzuya A., Shimohama S. Protein trafficking and Alzheimer's disease. Curr. Alzheimer Res. 2004;1:1–10. doi: 10.2174/1567205043480528. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Ungewickell H., Holstein S.E., Lindner R., Prasad K., Barouch W., Martin B., Greene L.E., Eisenberg E. Role of auxilin in uncoating clathrin‐coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- Ungewickell A., Ward M.E., Ungewickell E., Majerus P.W. The inositol polyphosphate 5‐phosphatase Ocrl associates with endosomes that are partially coated with clathrin. Proc. Natl. Acad. Sci. USA. 2004;101:13501–13506. doi: 10.1073/pnas.0405664101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valderrama F., Duran J.M., Babia T., Barth H., Renau‐Piqueras J., Egea G. Actin microfilaments facilitate the retrograde transport from the Golgi complex to the endoplasmic reticulum in mammalian cells. Traffic. 2001;2:717–726. doi: 10.1034/j.1600-0854.2001.21006.x. [DOI] [PubMed] [Google Scholar]
- van der Bliek A.M., Meyerowitz E.M. Dynamin‐like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- van der Bliek A.M., Redelmeier T.E., Damke H., Tisdale E.J., Meyerowitz E.M., Schmid S.L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint J., Rykx A., Maeda Y., Vantus T., Sturany S., Malhotra V., Vandenheede J.R., Seufferlein T. Protein kinase D: An intracellular traffic regulator on the move. Trends Cell Biol. 2002;12:193–200. doi: 10.1016/s0962-8924(02)02262-6. [DOI] [PubMed] [Google Scholar]
- van Sorge N.M., van der Pol W.L., van de Winkel J.G.J. FcgammaR polymorphisms: Implications for function, disease susceptibility and immunotherapy. Tissue Antigens. 2003;61:189–202. doi: 10.1034/j.1399-0039.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Vowels J.J., Payne G.S. A dileucine‐like sorting signal directs transport into an AP‐3‐dependent, clathrin‐independent pathway to the yeast vacuole. EMBO J. 1998;17:2482–2493. doi: 10.1093/emboj/17.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walburger A., Koul A., Ferrari G., Nguyen L., Prescianotto‐Baschong C., Huygen K., Klebl B., Thompson C., Bacher G., Pieters J. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science. 2004;304:1800–1804. doi: 10.1126/science.1099384. [DOI] [PubMed] [Google Scholar]
- Ward C.L., Omura S., Kopito R.R. Degradation of CFTR by the ubiquitin‐proteasome pathway. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- Ward D.M., Shiflett S.L., Huynh D., Vaughn M.B., Prestwich G., Kaplan J. Use of expression constructs to dissect the functional domains of the CHS/Beige protein: Identification of multiple phenotypes. Traffic. 2003;4:403–415. doi: 10.1034/j.1600-0854.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- Waters M.G., Clary D.O., Rothman J.E. A novel 115‐kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J. Cell Biol. 1992;118:1015–1026. doi: 10.1083/jcb.118.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Johannes L., Mallard F., Girod A., Grill S., Reinsch S., Keller P., Tzschaschel B., Echard A., Goud B., Stelzer E.H. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol. 1999;147:743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell L., Lindquist S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- Wiertz E.J., Tortorella D., Bogyo M., Yu J., Mothes W., Jones T.R., Rapoport T.A., Ploegh H.L. Sec61‐mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- Wiertz E.J., Jones T.R., Sun L., Bogyo M., Geuze H.J., Ploegh H.L. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- Wigge P., Kohler K., Vallis Y., Doyle C.A., Owen D., Hunt S.P., McMahon H.T. Amphiphysin heterodimers: Potential role in clathrin‐mediated endocytosis. Mol. Biol. Cell. 1997;8:2003–2015. doi: 10.1091/mbc.8.10.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T.M., Lisanti M.P. The caveolin genes: From cell biology to medicine. Ann. Med. 2004;36:584–595. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- Wilson S.M., Yip R., Swing D.A., O'Sullivan T.N., Zhang Y., Novak E.K., Swank R.T., Russell L.B., Copeland N.G., Jenkins N.A. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc. Natl. Acad. Sci. USA. 2000;97:7933–7938. doi: 10.1073/pnas.140212797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman S.E., Sotgia F., Galbiati F., Minetti C., Lisanti M.P. Caveolinopathies: Mutations in caveolin‐3 cause four distinct autosomal dominant muscle diseases. Neurology. 2004;62:538–543. doi: 10.1212/wnl.62.4.538. [DOI] [PubMed] [Google Scholar]
- Wojcik J., Berg M.A., Esposito N., Geffner M.E., Sakati N., Reiter E.O., Dower S., Francke U., Postel‐Vinay M.C., Finidori J. Four contiguous amino acid substitutions, identified in patients with Laron syndrome, differently affect the binding affinity and intracellular trafficking of the growth hormone receptor. J. Clin. Endocrinol. Metab. 1998;83:4481–4489. doi: 10.1210/jcem.83.12.5357. [DOI] [PubMed] [Google Scholar]
- Woscholski R., Finan P.M., Radley E., Totty N.F., Sterling A.E., Hsuan J.J., Waterfield M.D., Parker P.J. Synaptojanin is the major constitutively active phosphatidylinositol‐3,4,5‐trisphosphate 5‐phosphatase in rodent brain. J. Biol. Chem. 1997;272:9625–9628. doi: 10.1074/jbc.272.15.9625. [DOI] [PubMed] [Google Scholar]
- Wu X., Rao K., Bowers M.B., Copeland N.G., Jenkins N.A., Hammer J.A., 3rd. Rab27a enables myosin Va‐dependent melanosome capture by recruiting the myosin to the organelle. J. Cell Sci. 2001;114:1091–1100. doi: 10.1242/jcs.114.6.1091. [DOI] [PubMed] [Google Scholar]
- Wu X.S., Rao K., Zhang H., Wang F., Sellers J.R., Matesic L.E., Copeland N.G., Jenkins N.A., Hammer J.A., 3rd. Identification of an organelle receptor for myosin‐Va. Nat. Cell Biol. 2002;4:271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- Xiao G.H., Shoarinejad F., Jin F., Golemis E.A., Yeung R.S. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J. Biol. Chem. 1997;272:6097–6100. doi: 10.1074/jbc.272.10.6097. [DOI] [PubMed] [Google Scholar]
- Yamada E. The fine structure of the gall bladder epithelium of the mouse. J. Cell Biol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Toya Y., Jensen R.A., Ishikawa Y. Caveolin is an inhibitor of platelet‐derived growth factor receptor signaling. Exp. Cell Res. 1999;247:380–388. doi: 10.1006/excr.1998.4379. [DOI] [PubMed] [Google Scholar]
- Yan F., Lin C.‐W., Weisiger E., Cartier E.A., Taschenberger G., Shyng S.‐L. Sulfonylureas correct trafficking defects of ATP‐sensitive potassium channels caused by mutations in the sulfonylurea receptor. J. Biol. Chem. 2004;279:11096–11105. doi: 10.1074/jbc.M312810200. [DOI] [PubMed] [Google Scholar]
- Yang B., Steegmaier M., Gonzalez L.C., Jr., Scheller R.H. nSec1 binds a closed conformation of syntaxin1A. J. Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M., Yamamoto T., Ikemoto N., Matsuzaki M. Abnormal ryanodine receptor function in heart failure. Pharmacol. Ther. 2005;107:377–391. doi: 10.1016/j.pharmthera.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Yasuda M., Shabbeer J., Benson S.D., Maire I., Burnett R.M., Desnick R.J. Fabry disease: Characterization of alpha‐galactosidase A double mutations and the D313Y plasma enzyme pseudodeficiency allele. Hum. Mutat. 2003;22:486–492. doi: 10.1002/humu.10275. [DOI] [PubMed] [Google Scholar]
- Yeaman C., Ayala M.I., Wright J.R., Bard F., Bossard C., Ang A., Maeda Y., Seufferlein T., Mellman I., Nelson W.J., Malhotra V. Protein kinase D regulates basolateral membrane protein exit from trans‐Golgi network. Nat. Cell Biol. 2004;6:106–112. doi: 10.1038/ncb1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung R.S., Xiao G.H., Jin F., Lee W.C., Testa J.R., Knudson A.G. Predisposition to renal carcinoma in the Eker rat is determined by germ‐line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc. Natl. Acad. Sci. USA. 1994;91:11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J., Couture L.A., Gregory R.J., Graham S.M., Smith A.E., Welsh M.J. Adenovirus‐mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]
- Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang B., Cunningham M.A., Nichols W.C., Bernat J.A., Seligsohn U., Pipe S.W., McVey J.H., Schulte‐Overberg U., de Bosch N.B., Ruiz‐Saez A., White G.C., Tuddenham E.G., Kaufman R.J., Ginsburg D. Bleeding due to disruption of a cargo‐specific ER‐to‐Golgi transport complex. Nat. Genet. 2003;34:220–225. doi: 10.1038/ng1153. [DOI] [PubMed] [Google Scholar]
- Zhao C., Takita J., Tanaka Y., Setou M., Nakagawa T., Takeda S., Yang H.W., Terada S., Nakata T., Takei Y. Charcot‐Marie‐Tooth disease type 2A caused by mutation in a microtubule motor KIF1B[beta] Cell. 2001;105:587–597. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]
- Lehrman M.A., Schneider W.J., Brown M.S., Davis C.G., Elhammer A., Russell D.W., Goldstein J.L. The Lebanese allele at the low density lipoprotein receptor locus. Nonsense mutation produces truncated receptor that is retained in endoplasmic reticulum. J. Biol. Chem. 1987;262:401–410. [PubMed] [Google Scholar]
- Yang J., Cron P., Good V.M., Thompson V., Hemmings B.A., Barford D. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3‐peptide and AMP‐PNP. Nat. Struct. Biol. 2002;9:940–944. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
Further Reading
- Cox D.W., Moore S.D. Copper transporting P‐type ATPases and human disease. J. Bioenerg. Biomembr. 2002;34:333–338. doi: 10.1023/a:1021293818125. [DOI] [PubMed] [Google Scholar]
- Gitlin J.D. Wilson disease. Gastroenterology. 2003;125:1868–1877. doi: 10.1053/j.gastro.2003.05.010. [DOI] [PubMed] [Google Scholar]
- Naim H.Y., Roth J., Sterchi E.E., Lentze M., Milla P., Schmitz J., Hauri H.P. Sucrase‐isomaltase deficiency in humans. Different mutations disrupt intracellular transport, processing, and function of an intestinal brush border enzyme. J. Clin. Invest. 1988;82:667–679. doi: 10.1172/JCI113646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.S., Römisch K. Surfing the Sec61 channel: Bidirectional protein translocation across the ER membrane. J. Cell Sci. 1999;112(Pt. 23):4185–4191. doi: 10.1242/jcs.112.23.4185. [DOI] [PubMed] [Google Scholar]


