Abstract
Since the discovery that baculoviruses can efficiently transduce mammalian cells, baculoviruses have been extensively studied as potential vectors for both in vitro and in vivo gene therapy. This chapter reviews the history of this research area, cells permissive to baculovirus transduction, factors influencing transduction and transgene expression, efforts to improve transduction, mechanisms of virus entry and intracellular trafficking, applications for in vivo and ex vivo gene therapy, as well as advantages, limitations, and safety issues concerning use of baculoviruses as gene therapy vectors. Recent progress and efforts directed toward overcoming existing bottlenecks are emphasized.
I. Baculovirus Transduction of Mammalian Cells
A. Historical Overview
Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is one of the most well‐studied baculoviruses. In 1983, baculoviruses were first explored as vectors for the expression of human interferon β (IFN‐β) in insect cells (Smith et al., 1983). Since then, the potential of the baculovirus–insect cell expression system has been fully exploited for the production of numerous recombinant proteins (reviewed in Beljelarskaya 2002, Luckow 1988, Patterson 1995) and baculovirus research advanced insect cell culture as a force in the field of biotechnology. One of the reasons for the increasing popularity of the baculovirus–insect cell expression system is safety, because baculoviruses are regarded as nonpathogenic to humans, and the baculovirus host range is restricted to insects and invertebrates.
In 1983, however, Tjia et al. (1983) first found that baculoviruses can be internalized by mammalian cells and at least some of the viral DNA reached the nucleus. The nuclear DNA, however, did not persist and there was no evidence that baculovirus DNA was transcribed in mammalian cells (Tjia et al., 1983). Later, Volkman and Goldsmith (1983) demonstrated that baculoviruses can be internalized by nontarget vertebrate cells such as human lung carcinoma cell line A427. Carbonell et al. (1985) further confirmed that baculoviruses entered mammalian cells and mediated very low‐level expression of Escherichia coli chloramphenicol acetyltransferase (CAT) under the control of polyhedrin and Rous sarcoma virus (RSV) promoters. However, the significance of these findings was not widely noted until a decade later.
In the mid‐1990s, two pioneer groups reported that recombinant baculoviruses harboring a cytomegalovirus (CMV) promoter‐luciferase gene cassette (Hofmann et al., 1995) or an RSV long terminal repeat (LTR) promoter‐β‐galactosidase (β‐gal) gene cassette (Boyce and Bucher, 1996) efficiently expressed the reporter genes in mammalian cells. The data suggested a strong preference of baculovirus vectors to transduce hepatocytes of different origins (e.g., human and rabbit), because efficient transduction and high‐level expression were only observed in primary hepatocytes and hepatoma cells. Significantly, lower reporter gene expression was observed in several other cell lines (e.g., COS‐1 and 293) and little to no expression was observed in other cell types including A549, CHO, NIH‐3T3, and CV‐1 cells. The authors suggested that the block to expression in less susceptible cells might be subsequent to viral entry, rather than the ability to be internalized by the target cells, because high‐ and low‐expressing cell lines internalized similar amounts of virus (Boyce and Bucher, 1996). One factor accounting for the low apparent transduction efficiency in certain cell types is promoter strength; Shoji et al. (1997) showed that cells that were not transduced by a baculovirus‐expressing β‐gal under the control of the CMV promoter could be efficiently transduced by a baculovirus expressing the same reporter protein under the transcriptional control of the stronger CAG promoter. Furthermore, Shoji et al. (1997) compared the gene expression by baculovirus and adenovirus vectors using the same expression unit and observed the same level of expression in HepG2, HeLa, and COS‐7 cells by both vectors. They even demonstrated efficient expression and proper processing of hepatitis C virus (HCV) protein mediated by a baculovirus vector. These pioneering studies paved the way for use of baculoviral vectors as tools for gene delivery into mammalian cells.
B. Cells Permissive to Baculovirus Transduction
The list of cells permissive to baculovirus transduction has rapidly expanded. These cells include cell lines originating from cells of human (e.g., HeLa, Huh‐7, HepG2, keratinocytes, bone marrow fibroblasts), rodent (e.g., CHO, BHK), porcine (e.g., CPK, PK15), bovine (e.g., BT), and even fish (e.g., EPC, CHH‐1) origin (Table I ). In addition, baculoviruses are capable of transducing nondividing cells, such as PK1 cells arrested in S phase (van Loo et al., 2001). Transduction of primary cells, such as human neural cells (Sarkis et al., 2000), pancreatic islet cells (Ma et al., 2000), and rat articular chondrocytes (Ho et al., 2004), has also been observed. In addition, Wagle and Jesuthasan (2003) showed that baculoviruses successfully transduced the embryos of zebrafish. EphrinB2a is normally expressed in the posterior region of developing somites and baculovirus‐mediated misexpression by injection of the baculovirus expressing ephrinB2a into specific tissues, caused abnormal somite boundary formation (Wagle and Jesuthasan, 2003). Moreover, we demonstrated that baculoviruses are capable of transducing mesenchymal stem cells (MSC) derived from human umbilical cord blood and bone marrow (Ho et al., 2005), as well as MSC‐derived adipogenic, osteogenic, and chondrogenic progenitor cells (Ho et al., 2006). Despite the rapidly growing list of cells permissive to baculovirus transduction, however, baculovirus transduction of cell lines of hematopoietic origin, such as U937, K562, Raw264.7 (Condreay et al., 1999), LCL‐cm, and Raji (Cheng et al., 2004), is inefficient. As the spectrum of cell types permissive to baculovirus transduction expands, the potential applications of baculovirus vectors are receiving increasing attention. Table II lists some of the applications of baculoviruses that have been explored (see also chapter by Condreay et al., pp. 255–286; van Oers, pp. 193–253; and Mäkelä and Oker‐Blom, pp. 91–112, this volume).
Table I.
Some Cell Types that Are Permissive to Baculovirus Transduction
Table II.
Applications of the Baculovirus/Mammalian Cell System
| Application | References |
|---|---|
| In vitro and in vivo gene therapy | Airenne 2000, Boyce 1996, Hofmann 1995, Pieroni 2001, Sarkis 2000, Tani 2003 |
| Cell‐based assays | Ames 2004, Jenkinson 2003, Katso 2005 |
| Studies of gene function | Clay 2003, Pfohl 2002 |
| Studies of virology | Delaney 1998, Dwarakanath 2001, Lopez 2002, McCormick 2002, Zhou 2000 |
| Protein production | Chen 2005, Ojala 2004, Ramos 2002 |
| Virus vector production | Cheshenko 2001, McCormick 2002, Poomputsa 2003, Sollerbrant 2001 |
| Surface display | Ernst 1998, Grabherr 2001 |
| Vaccine candidates | Abe 2003, Aoki 1999, Facciabene 2004, Tami 2000, Yoshida 2003 |
C. Mechanisms of Baculovirus Entry into Mammalian Cells
1. Importance of the Envelope Glycoprotein gp64
The baculovirus gp64 glycoprotein is a major component of the budded virus envelope and is essential for virus entry into insect cells by receptor‐mediated endocytosis (Wickham et al., 1990). Following virus entry, gp64 further mediates the acid‐induced endosomal escape, thus allowing for nucleocapsid transport into the cytoplasm and nucleus (Blissard and Wenz, 1992). Similarly, gp64 is essential for virus attachment and endosomal escape in mammalian cells (Hofmann et al., 1998). In support of the importance of gp64 is the finding that a monoclonal antibody specific for gp64 abolishes the capability of baculovirus vectors to transduce mammalian cells (Gronowski et al., 1999). A baculovirus overexpressing gp64 from an additional gp64 gene can incorporate ∼1.5 times the normal amount of gp64 on the virion surface and exhibit 10‐ to 100‐fold more reporter gene expression in a variety of mammalian cells when compared to the control baculovirus (Tani et al., 2001). The importance of gp64 for virus transduction is further substantiated as a mutant virus lacking gp64 on the viral envelope failed to transduce mammalian cells (Abe et al., 2005). Furthermore, gp64 of AcMNPV was shown to rescue transduction of mammalian cells by HaSNPV (Helicoverpa armigera single nucleopolyhedrovirus), a virus that does not transduce mammalian cells. The range of mammalian cell types transduced by HaSNPV expressing the gp64 of AcMNPV was consistent with those transduced by AcMNPV (Lang et al., 2005).
2. Surface Molecules for Virus Docking
Although the importance of gp64 for virus entry has been documented, the nature of the cell surface molecule that interacts with the virus is unclear in both insect and mammalian cells (Kukkonen et al., 2003). Initially, it was suggested that baculovirus transduction was liver specific and that asialoglycoprotein could be involved in virus binding (Boyce 1996, Hofmann 1995). However, van Loo et al. (2001) showed that Pk1 cells, which do not express asialoglycoprotein receptors, can be successfully transduced, and hence asialoglycoprotein is not a key determinant. It was also shown that electrostatic interactions may be necessary for baculovirus binding to the mammalian cell surface because preincubation of 293 cells with polybrene, a cationic compound that neutralizes negatively charged epitopes on the cell membrane, resulted in a rapid decrease in virus binding (Duisit et al., 1999). The same group also suggested that heparan sulfate may act as an important docking motif for baculovirus binding because removal of heparan sulfate from the cell surface by heparanase I or III prior to transduction reduced transgene (LacZ) expression by ≈50% (Duisit et al., 1999). Aside from heparan sulfate, phospholipids on the cell surface were suggested to serve as an important docking point for gp64, thus facilitating viral entry into mammalian cells (Tani et al., 2001).
On the other hand, by transient depletion of calcium using EGTA pretreatment, Bilello et al. (2003) demonstrated that paracellular junction complexes are important barriers for baculoviral entry into primary hepatocytes. In contrast, we found that EGTA treatment of Huh‐7 cells and chondrocytes does not significantly enhance transduction efficiencies although disruption of cell junctions was apparent (unpublished data). Despite the discrepancies in identification of the surface receptors, multiple lines of evidence suggest that baculoviruses are internalized by endocytosis (Condreay 1999, van 2001). By electron and confocal microscopy, Matilainen et al. confirmed that baculoviruses enter HepG2 cells via clathrin‐mediated endocytosis. However, baculovirus attachment to clathrin‐coated pits seemed to be a relatively rare phenomenon, and therefore other internalization mechanisms (possibly via macropinocytosis) may also exist (Matilainen et al., 2005). Virus attachment does not appear to be limiting, because baculoviruses can efficiently bind to NIH‐3T3, a cell line less susceptible to baculovirus transduction, even at 4°C (Stanbridge et al., 2003).
3. Endosomal Escape and Intracellular Trafficking
Having entered the mammalian cell, budded virus is transported to the endosome, followed by acid‐induced endosomal escape of the nucleocapsid mediated by gp64. Endosomal escape was first uncovered by treating baculovirus‐transduced mammalian cells with a lysosomotropic agent (e.g., chloroquine), which inhibits endosomal maturation and subsequent baculovirus‐mediated gene expression (Boyce 1996, Hofmann 1995). The importance of endosomal escape was further confirmed by treating HepG2 cells with monensin, which blocked early endosome acidification and trapped the nucleocapsids in the endosome (Kukkonen et al., 2003). Therefore, it is generally assumed that escape from the endosomes blocks baculovirus transduction of some mammalian cells (Barsoum 1997, Boyce 1996). However, Kukkonen et al. (2003) suggested that the block may lie not in escape from the endosome, but rather in cytoplasmic trafficking or nuclear import of the nucleocapsids. In cells nonpermissive to baculovirus‐mediated transduction (e.g., EAHY, MG63, and NHO cells), virus is internalized and routed to the endosome 30 min posttransduction, and escapes from the endosome by 4 h posttransduction, but the nucleocapsid does not enter the nucleus efficiently. Accordingly, no detectable transgene expression is observed even with a very high virus load. In contrast, baculoviruses are capable of entering HepG2 cells (which are highly permissive to baculovirus transduction), escaping from the endosome and entering the nucleus 4 h after transduction. Consistent with this notion is that direct injection of nucleocapsids into the cytoplasm does not affect the translocation of nucleocapsids into the nucleus, demonstrating that endosomal escape is not necessarily a critical step (Salminen et al., 2005). Nucleocapsids are transported into different subcellular compartments in different cells (Abe et al., 2005). In 293T cells, the nucleocapsids reached the nucleus where the transgene was efficiently transcribed following uncoating. However, in the nonpermissive macrophage RAW264.7 cells, the nucleocapsids appeared to be trapped by the phagocytic pathway, and degraded viral DNA was then transported into toll‐like receptor 9 (TLR9)‐expressing intracellular compartments (Abe et al., 2005).
In the cytoplasm, the nucleocapsids seem to induce the formation of actin filaments which probably facilitate the transport of nucleocapsids into the nucleus. Cytochalasin D, an agent causing reversible depolymerization of actin filaments, strongly inhibits reporter gene expression, but does not prevent the uptake of enveloped virions inside cytoplasmic vesicles, or prevent their escape into the cytoplasm (van Loo et al., 2001). More recently, it was shown that disintegration of microtubules by microtubule‐depolymerizing agents (e.g., nocodazole and vinblastine) significantly enhanced the nuclear transport of virus and subsequent transgene expression in HepG2 cells (Salminen et al., 2005), suggesting that intact microtubules constituted a barrier to baculovirus transport toward the nucleus. The viral genome, major capsid protein, and electron‐dense capsids were also found inside the nucleus, suggesting that the nucleocapsid was transported through the nuclear pore (van Loo et al., 2001). All of these studies highlight the importance of intracellular trafficking for transgene expression. The proposed route of baculovirus entry and intracellular trafficking is illustrated in Fig. 1 .
Fig 1.
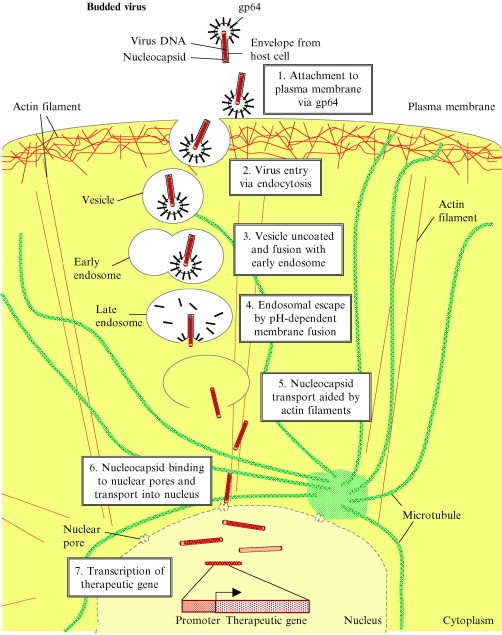
Proposed entry and intracellular trafficking of a baculovirus vector for expression of a therapeutic gene in a mammalian cell.
D. Transduction Efficiency and Level of Transgene Expression
1. Dependence on Cell Types
Baculovirus transduction efficiencies vary considerably according to the cell type and can range from 95% for BHK cells (Wang et al., 2005) to lower than 10% for NIH‐3T3 cells (Cheng et al., 2004). Baculovirus transduction of hepatocytes (e.g., HepG2, Huh‐7) is particularly efficient, with efficiencies of up to 80% (Wang et al., 2005). Because plasmid transfection of hepatocytes, a common approach for gene delivery into liver cells, is notoriously difficult (with a typical delivery efficiency of 5–30%), the highly efficient baculovirus‐mediated gene delivery has been exploited to study hepatitis B virus replication in HepG2 cells (Delaney and Isom, 1998) and to produce hepatitis delta virus‐like particles (HDV VLP) in hepatocytes (Wang et al., 2005).
The transduction efficiency may also be dependent on cellular differentiation state because transduction efficiency is only ≈30% for undifferentiated human neural progenitor cells, but can be up to ≈55% for differentiated neural cells at a multiplicity of infection (MOI) of 25 (Sarkis et al., 2000). Likewise, transduction efficiency (21–90%), transgene expression level and duration (7–41 days) vary widely with the differentiation state and lineage of the adipogenic, osteogenic, and chondrogenic progenitors originating from human MSCs (Ho et al., 2006). The transduction efficiency is very high for adipogenic and osteogenic progenitors, but is relatively low for chondrogenic progenitors (Fig. 2 ).
Fig 2.
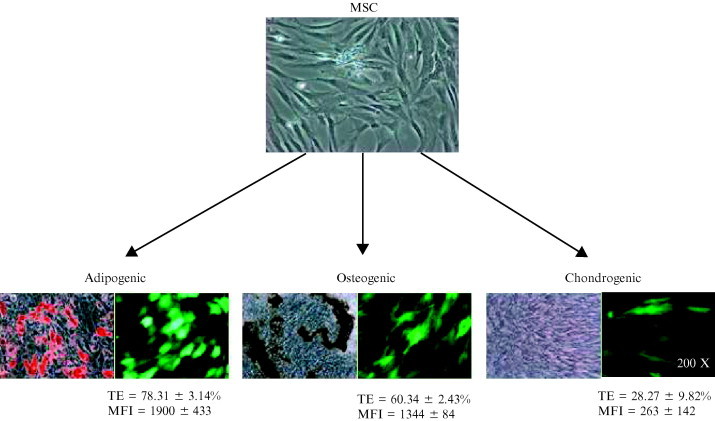
Baculovirus transduction of adipogenic, osteogenic, and chondrogenic progenitors. Human mesenchymal stem cells (MSC) were induced to differentiate into adipogenic, osteogenic, and chondrogenic pathways. The adipogenic, osteogenic, and chondrogenic progenitors were revealed by staining with oil red‐O, von Kossa, and safranin‐O staining, respectively, two weeks postinduction (left panels of each lineage pathway). The progenitors were transduced with baculoviruses expressing enhanced green fluorescent protein (EGFP) 2 weeks postinduction and exhibited different degrees of EGFP expression (right panel of each lineage pathway). The transduction efficiency (TE) and mean fluorescence intensity (MFI) were measured by flow cytometry.
2. Effects of Promoter
The transduction efficiency is also promoter‐dependent because Shoji et al. (1997) demonstrated that baculovirus‐mediated luciferase expression driven by the CAG promoter was tenfold higher than that driven by the CMV promoter. Thus it is of interest to examine the efficiency of different promoters of viral and cellular origins in baculovirus vectors in mammalian cells. Although various promoters have been cloned into baculovirus vectors to drive gene transcription (Table III ), only recently have Spenger et al. (2004) systematically compared the transgene expression driven by Simian virus 40 (SV40), CMV, RSV, and a cellular promoter (human ubiquitin C) in CHO, COS‐1, and HEK293 cells. The CMV and RSV promoters were the most active in all cell lines tested, followed by the ubiquitin C promoter. SV40 promoter was the weakest among these four promoters.
Table III.
Mammalian Promoters Inserted into Baculovirus Vectors
| Promoter | References |
|---|---|
| Rous sarcoma virus long terminal repeat (RSV‐LTR) promoter | Boyce and Bucher, 1996 |
| Cytomegalovirus immediate early promoter CMV‐IE | Hofmann 1995, Sollerbrant 2001 |
| Simian virus 40 (SV40) promoter | Spenger et al., 2004 |
| Hybrid chicken β‐actin promoter (CAG) | Shoji 1997, Stanbridge 2003 |
| Hepatitis B virus (HBV) promoter/enhancer | Delaney and Isom, 1998 |
| Human α‐fetoprotein promoter/enhancer | Park et al., 2001 |
| Human ubiquitin C promoter | Spenger et al., 2004 |
| Hybrid neuronal promoter | Li 2004, Wang 2005 |
| Drosophila heat shock protein (hsp70) | Viswanathan et al., 2003 |
3. Effects of Drugs
The transduction efficiency can be markedly enhanced by the addition of sodium butyrate, trichostatin A (Condreay et al., 1999), or valproic acid (Hu et al., 2003a). These compounds are histone deacetylase inhibitors that induce histone hyperacetylation and lead to a relaxed chromatin structure (Kramer et al., 2001). The use of these drugs enhances baculovirus‐mediated gene transcription, thereby highlighting the importance of the chromatin state of the baculovirus genome in the transduced cells for transgene expression. Note, however, that cytotoxicity is often associated with the use of these drugs (Hu et al., 2003a) and the extent to which gene expression is upregulated is dependent on the promoter and the particular cell line (Spenger et al., 2004).
4. Effects of Baculovirus Genomic Enhancer
Another factor influencing the transduction efficiency and expression level in certain cell lines is the activation of mammalian promoters (e.g., the CMV promoter and the heat shock promoter) by a homologous region (hr) in the baculovirus (AcMNPV) genome (Viswanathan et al., 2003). One of the hr regions, hr1, enhances transcription from the polyhedrin and the Drosophila heat shock protein (hsp70) promoters in insect cells (e.g., Sf9) in trans (Venkaiah et al., 2004). Yet hr1 also functions in mammalian cells (e.g., Vero and HepG2) as an enhancer when present in cis and in trans (Viswanathan et al., 2003). The upregulation of gene expression by hr1 probably stems from binding of hr1 with high affinity and specificity to nuclear factors in mammalian cells, thereby stimulating transcription (Viswanathan et al., 2003). The insertion of an additional copy of the hr1 region into the AcMNPV genome thus represents an attractive approach for overexpression of foreign proteins in mammalian cells (Venkaiah et al., 2004). The additional hr1 also improves the genetic stability of the bacmid‐derived baculovirus and consequently prolongs expression of the heterologous protein, because spontaneous deletion of the heterologous gene(s) in the foreign bacterial artificial chromosome sequences readily occurs (Pijlman 2001, Pijlman 2004).
5. Transduction Protocols
Another approach to enhancing the efficiency of baculovirus transduction of mammalian cells is to alter the transduction protocol. For transduction, typically the baculovirus is concentrated by ultracentrifugation and resuspended in phosphate‐buffered saline (PBS). The cells are then incubated with the virus for 1 h at 37°C in growth medium (e.g., DMEM) (Boyce 1996, Shoji 1997, Tani 2003). We developed a protocol by which incubation of unconcentrated virus (i.e., virus supernatant harvested from infected cell culture) with cells at lower temperature (e.g., 25°C or 27°C) for 4–8 h in PBS resulted in gene transfer into HeLa, chondrocytes (Ho 2004, Hsu 2004), and human MSC (Ho et al., 2005) with efficiencies comparable or superior to those using traditional protocols. Specifically, the transduction efficiencies of human MSC derived from umbilical cord blood can be elevated from 42% (using the conventional protocol) to 73% (using the modified protocol). This protocol eliminates the need for virus ultracentrifugation, and hence not only represents a simpler approach but also reduces the chance for virus loss or inactivation during ultracentrifugation.
A key determinant for the improved transduction efficiency is the incubation medium. We found that PBS is superior to DMEM or TNM‐FH (the medium for baculovirus production) in terms of transduction efficiency and transgene expression (Ho 2005, Hsu 2004). Comparison between the major components in PBS and medium revealed that NaHCO3 present in DMEM or TNM‐FH significantly reduced transduction efficiency (unpublished data), but the reason for this is unknown.
6. Modifications of the Baculovirus Vector for Improved Gene Delivery
The tropism and transduction efficiency of baculoviruses has been manipulated by modifying the envelope protein. Modification can be performed by fusing a heterologous gene in frame at the 5′ end of the gp64 gene under the control of the polyhedrin or p10 promoter. The fusion protein, after expression as an additional copy, is translocated to the plasma membrane and incorporated into the viral envelope on virus budding. Use of this approach was first demonstrated by fusion of human immunodeficiency virus‐1 (HIV‐1) envelope proteins to gp64 and the modified budded virus bound to the CD4 receptor on T cells (Boublik et al., 1995). A similar strategy was applied to construct avidin‐displaying baculoviruses, which showed a 5‐fold increase in transduction efficiency in rat malignant glioma cells and a 26‐fold increase in transduction efficiency in rabbit aortic smooth muscle cells compared to the wild‐type baculovirus (Raty et al., 2004). Baculoviruses displaying heterologous envelope proteins, such as vesicular stomatitis virus G protein (VSVG), have also been constructed. These vectors transduce human hepatoma and rat neuronal cells at efficiencies roughly 10‐ to 100‐fold greater than baculoviruses lacking VSVG (Barsoum et al., 1997). This pseudotyped virus also transduced cell lines that are transduced at very low levels by the unmodified baculovirus, thus broadening the tropism. The enhanced transduction efficiency and wider tropism are attributed to the increased transport of baculovirus DNA into nuclei rather than to the increased binding or virus uptake (Barsoum et al., 1997).
In contrast, specific targeting of baculoviruses to mammalian cells by displaying a single‐chain antibody fragment specific for the carcinoembryonic antigen (CEA) or synthetic IgG‐binding domains was also demonstrated (Mottershead 2000, Ojala 2001). Such viral targeting could reduce the virus dose required for in vivo gene therapy regimes, if baculoviruses can be engineered to bind efficiently to specific cell types.
II. Baculovirus Vectors for Gene Therapy
A. In Vivo Gene Therapy
Given the highly efficient gene delivery into many cell types, baculoviruses have captured increasing interest as vectors for in vivo gene delivery. Tissues that have been targeted include rabbit carotid artery (Airenne et al., 2000), rat liver (Huser et al., 2001), rat brain (Lehtolainen 2002, Sarkis 2000, Wang 2005), mouse brain (Sarkis et al., 2000), mouse skeletal muscle (Pieroni et al., 2001), mouse cerebral cortex and testis (Tani et al., 2003), and mouse liver (Hoare et al., 2005). For baculovirus‐mediated in vivo gene therapy, however, the complement system appears to be a significant barrier because systemic or intraportal application as well as direct injection into the liver parenchyma fail to result in detectable gene expression (Sandig et al., 1996). This failure stems from inactivation of the baculovirus vector in the presence of native serum, because baculoviruses activate the classical pathway of the complement system (Hofmann et al., 1998). Hoare et al. (2005) further showed that both classical and alternative pathways are involved in the inactivation and suggested that naturally occurring IgM antibodies with high affinity for baculoviruses may be partially responsible for the inactivation.
Various strategies have been employed to avoid complement inactivation. Hofmann and Strauss (1998) demonstrated that the survival of a baculovirus vector in human serum can be enhanced through treatment with a functional antibody‐blocking complement component 5 (C5). Meanwhile, the complement inhibitor sCR1 (soluble complement receptor type 1) protects baculoviruses from serum inactivation in vitro and coadministration of sCR1 along with intraportal administration of the baculovirus vector leads to hepatic expression in mice (Hoare et al., 2005). Inactivation of baculoviruses in human plasma and whole blood can be prevented by treatment with cobra venom factor (CVF), an inhibitor of the complement system (Hofmann and Strauss, 1998). By injecting CVF into mice one day prior to baculovirus administration, Sarkis et al. (2000) demonstrated that the baculovirus was not inactivated by the complement system and could transduce neural cells (mainly astrocytes) in vivo when directly injected into the brain of rodents. Surprisingly, they observed the same level of expression in animals without treatment of CVF, suggesting that the baculovirus was not inactivated by the complement system. These contradictory observations were probably caused by the particular immunological characteristics of the brain (Sarkis et al., 2000) or differences in stereotaxic coordinates and injection pressure, or speed (Lehtolainen et al., 2002).
The complement inactivation problem can also be minimized by avoiding contact of the baculovirus vectors with blood components. By using a silastic collar, transduction of adventitial cells in rabbit carotid arteries was achieved and the efficiencies were comparable to those obtained with adenoviral vectors (Airenne et al., 2000). Gene expression was transient remaining high level for 1 week but disappearing by day 14, and the arterial structure and endothelium remained intact after baculovirus transduction. Baculovirus vectors have also been injected into the rodent brain where complement proteins may be absent because of the blood–brain barrier, or the complement level in the brain may be insufficient to affect gene transfer (Lehtolainen et al., 2002). After in vivo injection into the brain, baculoviruses specifically transduced the epithelium of the choroids plexus in ventricles and the transduction efficiency was as high as 76 ± 14%. In contrast, adenovirus vectors showed preference to corpus callosum glial cells and ventricular ependymal lining. Hence, baculovirus vectors are especially useful for targeting of choroids plexus cells (Lehtolainen et al., 2002).
A more cutting‐edge approach to alleviate complement inactivation is the generation of complement‐resistant baculoviruses by display on the viral envelope of decay‐accelerating factor (DAF), a regulator that blocks complement at the central step of both the classical and alternative pathways (Huser et al., 2001). Such complement‐resistant baculovirus vectors allow for a substantial improvement of gene transfer into neonatal rats in vivo after local injection into the liver parenchyma. Expression of the transgene (human coagulation factor IX, hFIX) was transient probably as a result of the generation of antibodies directed against the transgene product hFIX, which might lead to clearance of either expressed hFIX protein and/or positively transduced cells. Alternatively, baculoviruses can be pseudotyped by displaying VSVG on the envelope. The VSVG‐modified virus enhanced gene transfer efficiencies into mouse skeletal muscle in vivo and the transgene expression lasted 178 days in DBA/2J mice and 35 days in BALB/c and C57BL/6 mice (Pieroni et al., 2001). The VSVG‐modified baculovirus also exhibited greater resistance to inactivation by animal sera and could transduce cerebral cortex and testis of mice by direct inoculation in vivo (Tani et al., 2003).
In addition to expressing therapeutic proteins, it has been shown that transduction of Saos‐2, HepG2, Huh‐7, and primary human hepatic stellate cells with a baculovirus expressing shRNAs (short‐hairpin RNAs) targeting lamin A/C effectively knocked down expression of the corresponding mRNA and protein (Nicholson et al., 2005). More recently, baculoviruses have been used to mediate RNA interference (RNAi) using a novel hybrid promoter consisting of the CMV enhancer and polymerase III H1 promoter. The recombinant baculovirus was capable of suppressing expression of the target gene by 95% in cultured cells and by 82%in vivo in rat brain (Ong et al., 2005). These data suggest that baculoviruses may be used as delivery vectors for triggering RNA interference for in vivo gene therapy.
B. Ex Vivo Gene Therapy
To date, most gene therapy studies using baculovirus vectors have focused on in vivo applications, yet relatively little is known about the potential of baculoviruses for ex vivo therapy. One relevant study was performed by establishing an ex vivo perfusion model for human liver segments (Sandig et al., 1996). The recombinant baculovirus was perfused through the liver segments for 15 min and reasonable transduction rates were achieved in all perfused parts of the liver tissue. This study verified for the first time that baculovirus‐mediated gene transfer is possible in liver tissue and is encouraging for future studies including in situ perfusion of intact livers with baculovirus vectors in animal models.
In addition, we have demonstrated highly efficient baculovirus‐mediated gene transfer into articular chondrocytes (Ho et al., 2004), human MSCs (Ho et al., 2005), and MSC‐derived progenitors (Ho et al., 2006), all being candidate cell sources for the treatment of disorders in connective tissues, particularly cartilage and bone. Importantly, differentiation states of chondrocytes, MSC, and MSC‐derived progenitor cells were not affected after baculovirus transduction. Further, the transduction of primary rabbit articular chondrocytes with baculoviruses expressing BMP‐2 significantly improved the secretion of extracellular matrix (ECM) and promoted the expression of chondrocyte‐specific genes (unpublished data), thus implicating the potential use of baculoviruses for delivery of genes encoding growth factors for cartilage and bone tissue engineering.
The ECM represents a barrier to baculovirus entry into chondrocytes because treatment of rat articular chondrocytes that had been cultured for 10 days with enzymes (hyaluronadase and heparanase) effectively removed the ECM and enhanced virus uptake and gene expression (unpublished data). Unfortunately, articular chondrocytes and osteoblasts are embedded in the ECM in vivo. Therefore, the dense ECM surrounding the target cells constitutes a formidable barrier for in vivo baculovirus‐mediated gene therapy for tissue engineering. As such, ex vivo gene therapy may be a more appropriate choice in the context of bone and cartilage tissue engineering. For instance, differentiation of MSCs or progenitors toward a specific lineage pathway (e.g., osteogenic) may be modulated by ex vivo transduction with recombinant baculoviruses expressing appropriate growth factors (e.g., BMP‐2). Given the efficient transduction of the partially differentiated progenitors, these cells may be transduced again with baculoviruses expressing identical (or different) factors with high efficiency, followed by seeding into scaffolds and implantation into animal models. The transduced cells may continue to express the appropriate factors in vivo, thereby stimulating cell differentiation and tissue (e.g., bone) regeneration in an autocrine or paracrine fashion.
III. Advantages and Limitations of Baculoviruses as Gene Therapy Vectors
A. Advantages
To date, vectors used for gene therapy are divided into two categories: nonviral and viral. Nonviral vectors comprise polymers (or liposomes) conjugated with polycations or other targeting molecules. However, the application of nonviral vectors is often restricted by the poor efficiency of delivery and transgene expression (Verma and Somia, 1997). In contrast, viral vectors, such as retroviral, lentiviral, adenoviral, and adeno‐associated viral (AAV) vectors are in common use due to the more efficient cellular uptake and transgene expression. Despite this, each vector has intrinsic advantages and disadvantages (Table IV ). For example, retroviruses can mediate integration of viral DNA into the host chromosome for permanent genetic modification; however, transcriptional silencing often occurs and results in transient expression. More critically, the random integration could lead to activation of oncogenes or inactivation of tumor suppressor genes and has resulted in unfortunate leukemia‐like diseases in two X‐linked SCID patients treated with retroviral vectors (Check, 2002). Lentiviral vectors, derived from Human or Simian immunodeficiency virus (HIV or SIV), are emerging vectors capable of long‐term expression in dividing and nondividing cells. However, the pathogenic nature of HIV or SIV raises serious concerns about the safety of these vectors, and the production of high‐titer virus stock is inefficient (Lundstrom, 2003). Adenoviruses can effectively infect dividing and nondividing cells and mediate high‐level transgene expression, but the transgene expression is often transient due to the elicitation of strong humoral and cellular immunity, which has also resulted in the death of a patient (Marshall, 1999). AAV vectors can mediate sustained expression, but the packaging capacity is restricted and large‐scale vector production is difficult. Furthermore, the preexisting immunity to human AAV vectors is comparable to that of adenoviral vectors (Thomas et al., 2003).
Table IV.
Comparison of Baculovirus and Other Viral Vectors
| Features | Retroviral | Lentiviral | Adenoviral | AAV | Baculoviral |
|---|---|---|---|---|---|
| Ease of preparation | No | No | Yes | No | Yes |
| Packaging capacity | 7–7.5 kb | 7–7.5 kb | Up to 30 kb | 3.5–4 kb | >38 kb |
| Route of administration | Ex vivo | Ex/in vivo | Ex/in vivo | Ex/in vivo | Ex/in vivo |
| Vector genome forms | Integrated | Integrated | Episomal | Episomala | Episomal |
| Gene expression duration | Short | Long | Short | Long | Short |
| Tropism | Dividing cell | Broad | Broad | Broad | Broad |
| Immune response | Low | Low | High | Unknown | Unknown |
| Preexisting immunity | Unlikely | Unlikely | Yes | Yes | Unlikely |
| Safety | Integration may induce oncogenesis | Integration may induce oncogenesis | Inflammatory response, toxicity | Inflammatory response, toxicity | Highb |
AAV can mediate site‐specific integration into human chromosome 19, but common AAV vectors do not contain the rep gene and thus cannot mediate integration. However, random integration may occur.
Baculoviruses are considered safe, but more studies are required to confirm the safety in in vivo and ex vivo applications.
In comparison with these common viral vectors, baculoviruses possess a number of advantages:
1. Lack of Toxicity and Replication
Baculovirus transduction is nontoxic to mammalian cells and does not hinder cell growth even at high MOI (Gao 2002, Hofmann 1995). Our studies again confirmed this notion because transduction with a wild‐type baculovirus did not cause any observable adverse effects to chondrocytes or human MSC (Ho et al., 2004, 2005). Cell proliferation, however, may be slightly retarded by transgene products, such as EGFP (Ho et al., 2004), which could be toxic and might even induce apoptosis in some cells (Detrait 2002, Liu 1999). Fortunately, the cell growth rate was restored after several passages as EGFP expression attenuates (Ho et al., 2005). Moreover, baculoviruses do not replicate in transduced mammalian cells (Hofmann 1995, Kost 2002, Sandig 1996, Shoji 1997).
The nonreplicative and nontoxic attributes of baculoviruses are particularly important because retroviruses, lentiviruses, and adenoviruses are human pathogens, and hence emergence of replication‐competent viruses (RCV) raises serious safety concerns. In contrast, baculoviruses are not pathogenic to humans, and hence the emergence of RCV is not an issue for baculovirus‐meditaed gene therapy.
2. Large Cloning Capacity
The baculovirus (AcMNPV) genome is large (≈130 kb) and the maximum cloning capacity is at least 38 kb because the adenovirus genome has been cloned into a baculovirus vector (Cheshenko et al., 2001). Such a large cloning capacity provides flexibility for multiple genes or large inserts. This flexibility is particularly advantageous in comparison with retroviral and AAV vectors whose cloning capacities are limited to 7–7.5 kb and 3.5–4 kb, respectively, and prohibit the cloning of regulatory sequences or large gene fragments (e.g., dystrophin).
3. Ease of Production
The production of retroviral, lentiviral, and AAV vectors requires transfection of plasmids encoding essential genes into packaging cells (Thomas et al., 2003). The transfection process, however, is cumbersome, costly, and difficult to scale up. In sharp contrast, baculovirus propagation can easily be achieved by infecting insect cells in suspension culture (e.g., in spinner flasks or bioreactors) and harvesting the supernatant 3–4 days postinfection. Scale‐up of the production process is straightforward because large‐scale insect cell culture processes are well‐established. The production phase is initiated simply by adding virus solution to cultured cells. Furthermore, the construction, propagation, and handling of baculoviruses can be performed readily in Biosafety Level 1 laboratories without the need for specialized equipment.
4. Lack of Preexisting Immunity
One of the problems associated with adenoviral and AAV vectors is that most people are exposed to these viruses and develop corresponding neutralizing antibodies. Circulating virus‐neutralizing antibodies can preclude efficient transduction with the viral vector. In contrast, it is unlikely that people develop such preexisting immunity against baculoviruses. The use of baculovirus vectors in gene therapy, therefore, may avoid the problem of preexisting immunity.
B. Limitations
Despite the promising aspects, baculoviruses have a number of disadvantages as gene therapy vectors.
1. Transient Expression
In vitro, baculovirus‐mediated expression usually lasted from 7 to 14 days for common cell lines such as CHO, HeLa, and BHK cells (Hu et al., 2003a), although expression continued for 41 days in adipogenic progenitor cells (Ho et al., 2006). In vivo, transgene expression typically declines by day 7 and disappears by day 14 (Airenne 2000, Lehtolainen 2002). The duration of in vitro transgene expression can be enhanced by prolonging the transduction period (e.g., upto 8 h) (Hsu et al., 2004) or by supertransduction (Hu et al., 2003a). Nonetheless, the extent to which expression can be prolonged is limited because expression is generally restricted to less than 1 month, which is significantly shorter than expression (in the range of months) mediated by retroviral, lentiviral, and AAV vectors.
One key difference between baculoviral and other viral vectors is that the genes carried by other vectors can persist in the host nucleus, either in an integrated or episomal form, for a longer period. However, Tjia et al. (1983) demonstrated that baculoviral DNA persists in the nuclei of transduced mammalian cells for only 24–48 h, as determined by Southern blot (Tjia et al., 1983). We also found that the total transgene (egfp) copy number within baculovirus‐transduced chondrocytes declined 11‐fold (as determined by quantitative real‐time PCR) while cell number increases 3.5‐fold in 11 days, indicating that baculoviral DNA degrades over time (Ho et al., 2004). The declining egfp copy number was concomitant with the decrease in mRNA transcription level (unpublished data) as well as fluorescence intensity.
To prolong transgene expression, Palombo et al. (1998) designed hybrid baculovirus–AAV vectors which contained a transgene cassette composed of the β‐gal reporter gene and hygromycin resistance gene (Hygr) flanked by the AAV‐inverted terminal repeats (ITR), which are necessary for AAV replication and integration into the host genome (Palombo et al., 1998). Hybrid baculovirus–AAV vectors were derived with or without the AAV rep gene (whose gene products are essential for viral DNA replication and integration) cloned in different positions with respect to the baculovirus polyhedrin promoter. Transduction of 293 cells with the hybrid vector expressing the rep gene resulted in specific integration of ITR‐flanked DNA into the AAVS1 site of chromosome 19 (Palombo et al., 1998). A similar baculovirus–AAV hybrid vector incorporating an ITR‐flanked luciferase gene under a neuron‐specific promoter was also employed for in vivo studies (Wang and Wang, 2005). Even without the help of rep gene expression, the viral vector was able to provide transgene expression for at least 90 days when tested in rat brains. These studies demonstrate an effective methodology for engineering of baculoviral vectors for sustained transgene expression.
2. Inactivation by Serum Complement
As described earlier, contact between baculoviruses and serum complement results in rapid inactivation. Despite various attempts to minimize complement inactivation, to date the number of successful baculovirus‐mediated in vivo gene therapy experiments in complement‐competent animals is limited (Hoare et al., 2005). However, the complement system is also a potent barrier to in vivo administration of other gene delivery systems such as liposomes (Marjan et al., 1994), murine retrovirus (Takeuchi et al., 1996), and various synthetic DNA complexes (Plank et al., 1996).
3. Inhibition of Transduction by Intercellular Junctions
Intercellular junctions may be an additional hurdle to baculovirus‐mediated gene therapy because transient disruption of these junctions by EGTA treatment prior to transduction improved gene delivery efficiency into long‐term cultures of primary hepatocytes (Bilello et al., 2001). Bilello et al. (2003) also suggested the importance of the basolateral surface for virus entry at least for some cell types. In our laboratory, however, transient disruption of cell junctions failed to effectively enhance baculovirus‐mediated gene transfer into chondrocytes and HepG2 cells that were cultured to overconfluence (unpublished data), implying that other factors in addition to the paracellular junction complexes might be involved in transduction of these cells.
4. Fragility of Budded Virus
Another drawback associated with baculoviruses as gene delivery vectors is that the nucleocapsid is enveloped with lipids derived from the host cell membrane. The envelope structure is essential for virus infectivity due to the anchored gp64 (Blissard and Wenz, 1992), but it also renders virus vulnerable to mechanical force and results in relatively low virus stability, a common problem also observed for other enveloped viruses such as retrovirus (Wu et al., 2000). Typically, baculoviral vectors are concentrated by ultracentrifugation after harvesting from cell culture and resuspended in PBS prior to use. However, ultracentrifugation often leads to significant loss of infectivity probably due to damage to viral envelopes. Ultracentrifugation also tends to result in virus aggregation (Barsoum, 1999). To alleviate these problems, we constructed a recombinant baculovirus with a hexahistidine (His6) tag displayed on the viral envelope, which enables virus purification by a simple immobilized metal affinity chromatography (IMAC) (Hu et al., 2003b). The IMAC methodology results in high purity (87%) and obviates the need for successive ultracentrifugation steps. However, the recovery yield in terms of infectious titer is lower than expected (<10%), probably because of damage to the viral envelope during the binding, washing, and elution steps. One possibility to alleviate virus loss during chromatographic purification steps is to display VSVG protein on the baculoviral envelope. Display of VSVG on the retrovirus envelope has been shown to enhance virus stability and the same strategy may be applied to enhancing baculovirus stability.
Besides sensitivity to mechanical force, the half‐life of baculoviruses is drastically decreased from 173 h at 27°C to 7–8 h at 37°C (Hsu et al., 2004). Such labile thermal stability, in conjunction with the tendency to be inactivated by serum complement, may further restrict the in vivo application of baculovirus gene delivery vectors.
IV. Safety Issues Concerning the Use of Baculoviruses for Gene Therapy
A. DNA Integration and Viral Gene Expression
As mentioned earlier, baculoviruses are nonpathogenic to humans and are nonreplicative in mammalian cells. Also, baculovirus DNA tends to be degraded in mammalian cells. However, Condreay et al. (1999) demonstrated that a recombinant baculovirus containing two expression cassettes (one harboring GFP under the control of the CMV promoter and the other harboring neomycin phosphotransferase under the control of the SV40 promoter) is capable of mediating stable expression. When transduced cells were selected with the antibiotic G418, cell lines that stably maintain the foreign expression cassettes can be obtained at high frequency and exhibit stable, high‐level expression of the reporter gene for at least 25 passages. The frequency ranged from one clone in 39 transduced cells to one clone in 109 transduced cells, indicating that stable transduction is an efficient event. Stably transduced derivatives have been selected from a substantial number of cell types (e.g., CHO, Huh‐7, HeLa, K562), suggesting that stable cell lines can be derived from any cell type that exhibits transient expression (Condreay et al., 1999).
Such a stably expressing derivative (CHO cell) was later confirmed to stem from the integration of baculovirus DNA into the host cell genome as small, discrete single‐copy fragments (Merrihew et al., 2001). These fragments, ranging in size from 5 to 18 kb, had randomly distributed breakpoints outside the selected region, suggesting an illegitimate mode of integration (little or no homology between recombining DNA molecules). Such integration resulted in at least two clones that expressed GFP for up to 5 months.
Since leukemia‐like conditions developed in two of the 11 SCID patients treated by retrovirus‐mediated gene therapy, safety issues regarding whether and/or how vector DNA integrates into the genomic DNA are under scrutiny. Although these stably expressing cell clones are obtained under antibiotic selection, and the integration occurs in a way different from that of retroviruses (which encode a viral integrase directing nearly full‐length, single‐copy integration events), the possibility that baculoviruses mediate spontaneous integration into genomic DNA cannot be excluded. To date, there is no direct evidence showing that spontaneous integration of baculoviral DNA occurs in the absence of an antibiotic resistance gene and selective pressure, but extensive studies examining the state and fate of introduced viral DNA are necessary to further prove the safety of baculovirus gene therapy vectors.
Another concern regarding the use of baculoviruses is whether baculovirus endogenous genes are expressed. As long ago as 1983, Tjia et al. (1983) showed that baculovirus endogenous gene transcription is absent in transduced HeLa cells. Using RT‐PCR, Stanbridge et al. (2003) assessed the expression of a number of baculovirus genes after transduction of human cells, and found no baculovirus gene transcripts in human cells. However, a study demonstrated that the baculoviral genomic early‐to‐late (ETL) promoter is active and able to drive reporter gene expression in mammalian cells (Liu et al., 2006). Although gene expression does not equate to virus replication, the possibility that other baculoviral promoters are also active in the transduced mammalian cells cannot be excluded. Whether and how expression of baculoviral proteins at basal levels in the mammalian cells induces immune responses and how this may influence cellular gene expression and physiological state requires further investigation.
B. Immune Response and Potential as a Vaccine Vector
The “Achilles heel” of gene therapy is that immune responses used to tackle wild‐type infections are activated against the vectors and/or the new transgene products (Thomas et al., 2003). Although baculoviruses were found capable of entering mammalian cells as early as in 1983 (Tjia 1983, Volkman 1983), the host response to baculovirus uptake, either in vitro or in vivo, was not evaluated until 1999 when Gronowski et al. (1999) reported that administration of baculoviruses in vitro induced the production of IFN‐α and IFN‐β from human and murine cell lines. The IFN‐stimulating activity of baculoviruses required live virus and was not due to the presence of viral RNA or DNA. Furthermore, administration of baculoviruses induced in vivo protection of mice from encephalomyocarditis virus infection (Gronowski et al., 1999). A subsequent study discovered that baculovirus transduction of cultured rat hepatocytes disrupted phenobarbital (PB) gene induction, a potent transcriptional activation event characteristic of highly differentiated hepatocytes, and repressed expression of the albumin gene (Beck et al., 2000). But neither cAMP nor PKA activities were affected by the virus. Baculovirus transduction also induced the expression of cytokines, such as TNF‐α, IL‐1α, and IL‐1β, in primary rat hepatocytes, however, TNF‐β, IL‐2, IL‐3, IL‐4, IL‐5, IL‐6, and IFN‐γ were not detected in any of the baculovirus‐exposed hepatocytes (Beck et al., 2000). Airenne et al. (2000) found that in vivo administration of baculovirus to rabbit carotid artery resulted in signs of inflammation. More recently, Abe et al. (2003) demonstrated that inoculation of baculovirus induced the secretion of inflammatory cytokines, such as TNF‐α and IL‐6, in a murine macrophage cell line, RAW264.7. In the same study, they also demonstrated that intranasal inoculation with a wild‐type baculovirus elicited a strong innate immune response that protected mice from a lethal challenge of influenza virus (Abe et al., 2003). This protective immune response was induced via the TLR9/MyD88‐dependent signaling pathway (Abe et al., 2005). The production of inflammatory cytokines was severely reduced in peritoneal macrophages (PECs) and splenic CD11c+ dendritic cells (DCs) derived from mice deficient in MyD88 or TLR9 after stimulation with baculovirus. In contrast, a significant amount of IFN‐α was still detectable in the PECs and DCs of these mice after stimulation with baculovirus, suggesting that a TLR9/MyD88‐independent signaling pathway may also participate in the production of IFN‐α (Abe et al., 2005). The induction of cytokines required gp64, however, gp64 itself did not directly participate in the TLR‐mediated immune response. Instead, the authors concluded that internalization of viral DNA via gp64‐mediated membrane fusion and endosomal maturation which released the viral genome into TLR9‐expressing cellular compartments were necessary for the induction of innate responses (Abe et al., 2005). As mentioned earlier, membrane fusion and endosomal escape via gp64 are essential for nucleocapsid transport into the nucleus. Hence it appears that transgene expression may be coincident with induction of the TLR9/MyD88‐signaling pathway.
Taken together, these findings suggest that baculoviruses may induce various immune responses in vitro and in vivo as for other viruses (e.g., adenovirus), thus raising questions as to whether this will compromise the use of baculovirus vectors for in vivo human gene therapy. Which cytokines are induced by baculoviruses, and how cytokines modulate cellular and humoral immunities in vivo are not completely understood. The question of whether baculovirus‐mediated ex vivo gene therapy elicits the immune response is also of interest. All of these questions need to be answered with more in‐depth investigations to ensure the safe application of baculoviral gene therapy vectors.
The immune response induced by baculoviruses makes it a promising candidate as a novel vaccine vehicle against infectious diseases (see chapter by van Oers, this volume, pp. 193–253). The ability of baculoviruses to induce immune responses was first exploited by Aoki et al. (1999), who found that a recombinant baculovirus‐expressing glycoprotein gB of pseudorabies virus induced antibodies against gB protein in mice, suggesting that this recombinant baculovirus could serve as a vaccine candidate for pseudorabies. The feasibility of using baculoviruses as vaccine carriers was also demonstrated by Abe et al. (2003), who found that intranasal inoculation with a recombinant baculovirus expressing hemagglutinin (HA) of the influenza virus under the control of the CAG promoter elicited the innate immune response and provided mice with a high level of protection from a lethal challenge of influenza virus. The level of protection is dependent on the route of administration, and intranasal administration is considerably superior to intramuscular administration although the latter induces significantly higher anti‐HA IgG levels. More recently, Facciabene et al. (2004) demonstrated that intramuscular injection of a baculovirus expressing carcinoembryonic antigen (CEA) induced a measurable anti‐CEA–specific CD4+ T cell response. The immunogenic properties of baculoviruses are not restricted to CEA because intramuscular injection of another baculovirus (Bac‐E2) expressing the E2 glycoprotein of HCV induced an anti‐E2 CD8+ T cell response as well as the innate immune response such as natural killer (NK) cell cytolytic activity (Facciabene et al., 2004). Interestingly, when Bac‐E2 is pseudotyped to display VSVG on the envelope, the minimal dose required to elicit a measurable T cell response was tenfold less, indicating that the VSVG‐pseudotyped Bac‐E2 was a more potent vaccine carrier than the unmodified virus. This finding agrees with the previous statement that baculoviruses displaying VSVG provide for more efficient immunogen expression in transduced cells.
Baculoviruses can also provoke an immune response against an antigen when it is displayed on the viral surface. For instance, immunization with adjuvant‐free baculovirus displaying rodent malaria Plasmodium berghei circumsporozoite protein (PbCSP) on the envelope induced high levels of antibodies and IFN‐γ–secreting cells against PbCSP, and protected 60% of mice against sporozoite challenge (Yoshida et al., 2003). A more recent study further showed that baculovirus displaying severe acute respiratory syndrome‐coronavirus (SARS‐CoV) spike protein on the envelope induced the release of IL‐8 in lung cells (Chang et al., 2004). These studies substantiate the potential of baculoviruses displaying immunogens as vaccine candidates.
V. Conclusions and Prospects
The broad range of mammalian cells permissive to baculovirus transduction, the nontoxic and nonreplicative nature, large packaging capacity, and ease of production make baculoviruses promising tools for gene therapy. Despite these advantages, baculoviruses are inactivated by serum complement, which restricts the application of baculovirus vectors for in vivo gene therapy. Additionally, the duration of transgene expression is generally short, thus baculoviruses may not be suited for long‐term gene therapy unless a hybrid vector capable of integrating the expression cassette into the host genome (e.g., baculovirus–AAV) is employed. Nonetheless, baculoviruses, in conjunction with other viral vectors (e.g., adenoviral or lentiviral), may be administered sequentially to escape either preexisting or therapy‐induced antiviral immunity. Additionally, baculoviruses may serve as delivery vectors for triggering RNA interference. Baculoviruses carrying tumor‐suppressor or suicide genes may also be used in combination with other treatments for cancer therapy (Song 2001, Stanbridge 2003). Given the highly efficient gene transfer to chondrocytes and MSCs, baculoviruses expressing appropriate growth factors may be used for ex vivo genetic modification of cells prior to transplantation into animals. The growth factors, acting in either an autocrine or a paracrine fashion, potentially accelerate tissue regeneration in vivo. Unlike the treatment of chronic disease, it is neither necessary nor desirable for transgene expression to persist beyond the few weeks or months needed to achieve healing (Huard 2003, Lieberman 2002). As a result, long‐term transgene expression is not critical in tissue engineering. Hence, the combination of baculovirus‐mediated gene therapy and tissue engineering may hold great promise. Of course, to address the safety issues of employing baculoviruses in gene therapy, the DNA integration, expression of baculovirus endogenous genes, and baculovirus‐induced immune responses should be investigated.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Health Research Institute (grant NHRI‐EX94–9412EI) and Ministry of Economic Affairs (MOEA 94A0317P2), Taiwan.
References
- Abe T., Takahashi H., Hamazaki H., Miyano‐Kurosaki N., Matsuura Y., Takaku H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 2003;171:1133–1139. doi: 10.4049/jimmunol.171.3.1133. [DOI] [PubMed] [Google Scholar]
- Abe T., Hemmi H., Miyamoto H., Moriishi K., Tamura S., Takaku H., Akira S., Matsuura Y. Involvement of the toll‐like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J. Virol. 2005;79:2847–2858. doi: 10.1128/JVI.79.5.2847-2858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airenne K.J., Hiltunen M.O., Turunen M.P., Turunen A.M., Laitinen O.H., Kulomaa M.S., Yla‐Herttuala S. Baculovirus‐mediated periadventitial gene transfer to rabbit carotid artery. Gene Ther. 2000;7:1499–1504. doi: 10.1038/sj.gt.3301269. [DOI] [PubMed] [Google Scholar]
- Ames R.S., Fornwald J.A., Nuthulaganti P., Trill J.J., Foley J.J., Buckley P.T., Kost T.A., Wu Z.N., Romanos M.A. BacMam recombinant baculoviruses in G protein‐coupled receptor drug discovery. Receptors Channels. 2004;10:99–107. doi: 10.1080/10606820490514969. [DOI] [PubMed] [Google Scholar]
- Aoki H., Sakoda Y., Jukuroki K., Takada A., Kida H., Fukusho A. Induction of antibodies in mice by a recombinant baculovirus expressing pseudorabies virus glycoprotein B in mammalian cells. Vet. Microbiol. 1999;68:197–207. doi: 10.1016/s0378-1135(99)00110-8. [DOI] [PubMed] [Google Scholar]
- Barsoum J. Concentration of recombinant baculovirus by cation‐exchange chromatography. Biotechniques. 1999;26:834–840. doi: 10.2144/99265bm07. [DOI] [PubMed] [Google Scholar]
- Barsoum J., Brown R., McKee M., Boyce F.M. Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum. Gene. Ther. 1997;8:2011–2018. doi: 10.1089/hum.1997.8.17-2011. [DOI] [PubMed] [Google Scholar]
- Beck N.B., Sidhu J.S., Omiecinski C.J. Baculovirus vectors repress phenobarbital‐mediated gene induction and stimulate cytokine expression in primary cultures of rat hepatocytes. Gene. Ther. 2000;7:1274–1283. doi: 10.1038/sj.gt.3301246. [DOI] [PubMed] [Google Scholar]
- Beljelarskaya S.N. A baculovirus expression system for insect cells. Mol. Biol. 2002;36:281–292. [Google Scholar]
- Bilello J.P., Delaney W.E., Boyce F.M., Isom H.C. Baculovirus entry into nondividing hepatocytes is enhanced by transient disruption of intercellular junctions. Hepatology. 2001;34:834. doi: 10.1128/JVI.75.20.9857-9871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilello J.P., Cable E.E., Myers R.L., Isom H.C. Role of paracellular junction complexes in baculovirus‐mediated gene transfer to nondividing rat hepatocytes. Gene Ther. 2003;10:733–749. doi: 10.1038/sj.gt.3301937. [DOI] [PubMed] [Google Scholar]
- Blissard G., Wenz J.R. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH‐dependent membrane fusion. J. Virol. 1992;66:6829–6835. doi: 10.1128/jvi.66.11.6829-6835.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boublik Y., Di Bonito P., Jones I.M. Eukaryotic virus display: Engineering the major surface glycoprotein of the Autographa californica nuclear polyhedrosis virus (AcNPV) for the presentation of foreign proteins on the virus surface. Biotechnology. 1995;13:1079–1084. doi: 10.1038/nbt1095-1079. [DOI] [PubMed] [Google Scholar]
- Boyce F.M., Bucher N.L.R. Baculovirus‐mediated gene transfer into mammalian cells. Proc. Natl. Acad. Sci. USA. 1996;93:2348–2352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell L.F., Klowden M.J., Miller L.K. Baculovirus‐mediated expression of bacterial genes in dipteran and mammalian cells. J. Virol. 1985;56:153–160. doi: 10.1128/jvi.56.1.153-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.J., Liu C.Y.Y., Chiang B.L., Chao Y.C., Chen C.C. Induction of IL‐8 release in lung cells via activator protein‐1 by recombinant baculovirus displaying severe acute respiratory syndrome‐coronavirus spike proteins: Identification of two functional regions. J. Immunol. 2004;173:7602–7614. doi: 10.4049/jimmunol.173.12.7602. [DOI] [PubMed] [Google Scholar]
- Check E. Gene therapy: A tragic setback. Nature. 2002;420:116–118. doi: 10.1038/420116a. [DOI] [PubMed] [Google Scholar]
- Chen Y.‐H., Wu J.‐C., Wang K.‐C., Chiang Y.‐W., Lai C.‐W., Chung Y.‐C., Hu Y.‐C. Baculovirus‐mediated production of HDV‐like particles in BHK cells using a novel oscillating bioreactor. J. Biotechnol. 2005;118:135–147. doi: 10.1016/j.jbiotec.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Cheng T., Xu C.‐Y., Wang Y.‐B., Chen M., Wu T., Zhang J., Xia N.‐S. A rapid and efficient method to express target genes in mammalian cell by baculovirus. World J. Gastroenterol. 2004;10:1612–1618. doi: 10.3748/wjg.v10.i11.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N., Krougliak N., Eisensmith R.C., Krougliak V.A. A novel system for the production of fully deleted adenovirus vectors that does not require helper adenovirus. Gene Ther. 2001;8:846–854. doi: 10.1038/sj.gt.3301459. [DOI] [PubMed] [Google Scholar]
- Clay W.C., Condreay J.P., Moore L.B., Weaver S.L., Watson M.A., Kost T.A., Lorenz J.J. Recombinant baculoviruses used to study estrogen receptor function in human osteosarcoma cells. Assay Drug Dev. Technol. 2003;1:801–810. doi: 10.1089/154065803772613435. [DOI] [PubMed] [Google Scholar]
- Condreay J.P., Witherspoon S.M., Clay W.C., Kost T.A. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc. Natl. Acad. Sci. USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney W.E., Isom H.C. Hepatitis B virus replication in human HepG2 cells mediated by hepatitis B virus recombinant baculovirus. Hepatology. 1998;28:1134–1146. doi: 10.1002/hep.510280432. [DOI] [PubMed] [Google Scholar]
- Detrait E.R., Bowers W.J., Halterman M.W., Giuliano R.E., Bennice L., Federoff H.J., Richfield E.K. Reporter gene transfer induces apoptosis in primary cortical neurons. Mol. Ther. 2002;5:723–730. doi: 10.1006/mthe.2002.0609. [DOI] [PubMed] [Google Scholar]
- Duisit G., Saleun S., Douthe S., Barsoum J., Chadeuf G., Moullier P. Baculovirus vector requires electrostatic interactions including heparan sulfate for efficient gene transfer in mammalian cells. J. Gene Med. 1999;1:93–102. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<93::AID-JGM19>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Dwarakanath R.S., Clark C.L., McElroy A.K., Spector D.H. The use of recombinant baculoviruses for sustained expression of human cytomegalovirus immediate early proteins in fibroblasts. Virology. 2001;284:297–307. doi: 10.1006/viro.2001.0924. [DOI] [PubMed] [Google Scholar]
- Ernst W., Grabherr R., Wegner D., Borth N., Grassauer A., Katinger H. Baculovirus surface display: Construction and screening of a eukaryotic epitope library. Nucleic Acids Res. 1998;26:1718–1723. doi: 10.1093/nar/26.7.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciabene A., Aurisicchio L., La Monica N. Baculovirus vectors elicit antigen‐specific immune responses in mice. J. Virol. 2004;78:8663–8672. doi: 10.1128/JVI.78.16.8663-8672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., McCormick C.J., Arthur M.J.P., Ruddle R., Oakley F., Smart D.E., Murphy F.R., Harris M.P.G., Mann D.A. High efficiency gene transfer into cultured primary rat and human hepatic stellate cells using baculovirus vectors. Liver. 2002;22:15–22. doi: 10.1046/j.0106-9543.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- Grabherr R., Ernst W. The Baculovirus expression system as a tool for generating diversity by viral surface display. Comb. Chem. High Throughput Screen. 2001;4:185–192. doi: 10.2174/1386207013331165. [DOI] [PubMed] [Google Scholar]
- Gronowski A.M., Hilbert D.M., Sheehan K.C.F., Garotta G., Schreiber R.D. Baculovirus stimulates antiviral effects in mammalian cells. J. Virol. 1999;73:9944–9951. doi: 10.1128/jvi.73.12.9944-9951.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y.‐C., Chen H.‐C., Wang K.‐C., Hu Y.‐C. Highly efficient baculovirus‐mediated gene transfer into rat chondrocytes. Biotechnol. Bioeng. 2004;88:643–651. doi: 10.1002/bit.20239. [DOI] [PubMed] [Google Scholar]
- Ho Y.‐C., Chung Y.‐C., Hwang S.‐M., Wang K.‐C., Hu Y.‐C. Transgene expression and differentiation of baculovirus‐transduced human mesenchymal stem cells. J. Gene Med. 2005;7:860–868. doi: 10.1002/jgm.729. [DOI] [PubMed] [Google Scholar]
- Ho Y.‐C., Lee H.‐P., Hwang S.‐M., Lo W.‐H., Chen H.‐C., Chung C.‐K., Hu Y.‐C. Gene Ther. 2006. Baculovirus transduction of human mesenchymal stem cell‐derived progenitor cells: Variation of transgene expression with cellular differentiation states. (in press) [DOI] [PubMed] [Google Scholar]
- Hoare J., Waddington S., Thomas H.C., Coutelle C., McGarvey M.J. Complement inhibition rescued mice allowing observation of transgene expression following intraportal delivery of baculovirus in mice. J. Gene Med. 2005;7:325–333. doi: 10.1002/jgm.671. [DOI] [PubMed] [Google Scholar]
- Hofmann C., Strauss M. Baculovirus‐mediated gene transfer in the presence of human serum or blood facilitated by inhibition of the complement system. Gene Ther. 1998;5:531–536. doi: 10.1038/sj.gt.3300607. [DOI] [PubMed] [Google Scholar]
- Hofmann C., Sandig V., Jennings G., Rudolph M., Schlag P., Strauss M. Efficient gene‐transfer into human hepatocytes by baculovirus vectors. Proc. Natl. Acad. Sci. USA. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C., Lehnet W., Strauss M. The baculovirus system for gene delivery into hepatocytes. Gene Ther. Mol. Biol. 1998;1:231–239. [Google Scholar]
- Hsu C.‐S., Ho Y.‐C., Wang K.‐C., Hu Y.‐C. Investigation of optimal transduction conditions for baculovirus‐mediated gene delivery into mammalian cells. Biotechnol. Bioeng. 2004;88:42–51. doi: 10.1002/bit.20213. [DOI] [PubMed] [Google Scholar]
- Hu Y.‐C., Tsai C.‐T., Chang Y.‐J., Huang J.‐H. Enhancement and prolongation of baculovirus‐mediated expression in mammalian cells: Focuses on strategic infection and feeding. Biotechnol. Prog. 2003;19:373–379. doi: 10.1021/bp025609d. [DOI] [PubMed] [Google Scholar]
- Hu Y.‐C., Tsai C.‐T., Chung Y.‐C., Lu J.‐T., Hsu J.T.‐A. Generation of chimeric baculovirus with histidine‐tags displayed on the envelope and its purification using immobilized metal affinity chromatography. Enzyme Microb. Technol. 2003;33:445–452. [Google Scholar]
- Huard J., Li Y., Peng H.R., Fu F.H. Gene therapy and tissue engineering for sports medicine. J. Gene Med. 2003;5:93–108. doi: 10.1002/jgm.344. [DOI] [PubMed] [Google Scholar]
- Huser A., Rudolph M., Hofmann C. Incorporation of decay‐accelerating factor into the baculovirus envelope generates complement‐resistant gene transfer vectors. Nat. Biotechnol. 2001;19:451–455. doi: 10.1038/88122. [DOI] [PubMed] [Google Scholar]
- Jenkinson S., McCoy D., Kerner S., Fox T., Ferris R., Lawrence W., Condreay P., Clay W., Smith C. Development of a novel surrogate assay to measure HIV envelope/CCR5/CD4‐mediated viral/cell fusion using BacMam baculovirus technology. FASEB J. 2003;17:A665. doi: 10.1177/1087057103255747. A665. [DOI] [PubMed] [Google Scholar]
- Katso R.M., Parham J.H., Caivano M., Clay W.C., Condreay J.P., Gray D.W., Lindley K.M., Mason S.J., Rieger J., Wakes N.C., Cairns W.J., Merrhiew R.V. Evaluation of cell‐based assays for steroid nuclear receptors delivered by recombinant baculoviruses. J. Biomol. Screen. 2005;10:715–724. doi: 10.1177/1087057105278873. [DOI] [PubMed] [Google Scholar]
- Kost T.A., Condreay J.P. Recombinant baculoviruses as mammalian cell gene delivery vectors. Trends Biotechnol. 2002;20:173–180. doi: 10.1016/s0167-7799(01)01911-4. [DOI] [PubMed] [Google Scholar]
- Kramer O.H., Gottlicher M., Heinzel T. Histone deacetylase as a therapeutic target. Trends Endocrinol. Metab. 2001;12:294–300. doi: 10.1016/s1043-2760(01)00438-6. [DOI] [PubMed] [Google Scholar]
- Kronschnabl M., Marschall M., Stamminger T. Efficient and tightly regulated expression systems for the human cytomegalovirus major transactivator protein IE2p86 in permissive cells. Virus Res. 2002;83:89–102. doi: 10.1016/s0168-1702(01)00422-1. [DOI] [PubMed] [Google Scholar]
- Kukkonen S.P., Airenne K.J., Marjomaki V., Laitinen O.H., Lehtolainen P., Kankaanpaa P., Mahonen A.J., Raty J.K., Nordlund H.R., Oker‐Blom C., Kulomaa M.S., Yla‐Herttuala S. Baculovirus capsid display: A novel tool for transduction imaging. Mol. Ther. 2003;8:853–862. doi: 10.1016/j.ymthe.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Lang C.Y., Song J.H., Chen X.W. The GP64 protein of Autographa californica multiple nucleopolyhedrovirus rescues Helicoverpa armigera nucleopolyhedrovirus transduction in mammalian cells. J. Gen. Virol. 2005;86:1629–1635. doi: 10.1099/vir.0.80857-0. [DOI] [PubMed] [Google Scholar]
- Lehtolainen P., Tyynela K., Kannasto J., Airenne K.J., Yla‐Herttuala S. Baculoviruses exhibit restricted cell type specificity in rat brain: A comparison of baculovirus‐ and adenovirus‐mediated intracerebral gene transfer in vivo. Gene Ther. 2002;9:1693–1699. doi: 10.1038/sj.gt.3301854. [DOI] [PubMed] [Google Scholar]
- Leisy D.J., Lewis T.D., Leong J.A.C., Rohrmann G.F. Transduction of cultured fish cells with recombinant baculoviruses. J. Gen. Virol. 2003;84:1173–1178. doi: 10.1099/vir.0.18861-0. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang X., Guo H., Wang S. Axonal transport of recombinant baculovirus vectors. Mol. Ther. 2004;10:1121–1129. doi: 10.1016/j.ymthe.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Lieberman J.R., Ghivizzani S.C., Evans C.H. Gene transfer approaches to the healing of bone and cartilage. Mol. Ther. 2002;6:141–147. doi: 10.1006/mthe.2000.0663. [DOI] [PubMed] [Google Scholar]
- Liu H.S., Jan M.S., Chou C.K., Chen P.H., Ke N.J. Is green fluorescent protein toxic to the living cells? Biochem. Biophys. Res. Commun. 1999;260:712–717. doi: 10.1006/bbrc.1999.0954. [DOI] [PubMed] [Google Scholar]
- Liu Y.K., Chu C.C., Wu T.Y. Baculovirus ETL promoter acts as a shuttle promoter between insect cells and mammalian cells. Acta. Pharmacol. Sin. 2006;27:321–327. doi: 10.1111/j.1745-7254.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- Lopez P., Jacob R.J., Roizman B. Overexpression of promyelocytic leukemia protein precludes the dispersal of ND10 structures and has no effect on accumulation of infectious herpes simplex virus 1 or its proteins. J. Virol. 2002;76:9355–9367. doi: 10.1128/JVI.76.18.9355-9367.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow V.A., Summers M.D. Trends in the development of baculovirus expression vectors. Bio/Technology. 1988;6:47–55. [Google Scholar]
- Lundstrom K. Latest development in viral vectors for gene therapy. Trends Biotechnol. 2003;21:117–122. doi: 10.1016/S0167-7799(02)00042-2. [DOI] [PubMed] [Google Scholar]
- Ma L., Tamarina N., Wang Y., Kuznetsov A., Patel N., Kending C., Hering B.J., Philipson L.H. Baculovirus‐mediated gene transfer into pancreatic islet cells. Diabetes. 2000;49:1986–1991. doi: 10.2337/diabetes.49.12.1986. [DOI] [PubMed] [Google Scholar]
- Marjan J., Xie Z.C., Devine D.V. Liposome‐induced activation of the classical complement pathway does not require immunoglobulin. BBA‐Biomembranes. 1994;1192:35–44. doi: 10.1016/0005-2736(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- Matilainen H., Rinne J., Gilbert L., Marjomaki V., Reunanen H., Oker‐Blom C. Baculovirus entry into human hepatoma cells. J. Virol. 2005;79:15452–15459. doi: 10.1128/JVI.79.24.15452-15459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C.J., Rowlands D.J., Harris M. Efficient delivery and regulable expression of hepatitis C virus full‐length and minigenome constructs in hepatocyte‐derived cell lines using baculovirus vectors. J. Gen. Virol. 2002;83:383–394. doi: 10.1099/0022-1317-83-2-383. [DOI] [PubMed] [Google Scholar]
- Merrihew R.V., Clay W.C., Condreay J.P., Witherspoon S.M., Dallas W.S., Kost T.A. Chromosomal integration of transduced recombinant baculovirus DNA in mammalian cells. J. Virol. 2001;75:903–909. doi: 10.1128/JVI.75.2.903-909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottershead D.G., Alfthan K., Ojala K., Takkinen K., Oker‐Blom C. Baculoviral display of functional scFv and synthetic IgG‐binding domains. Biochem. Biophys. Res. Commun. 2000;275:84–90. doi: 10.1006/bbrc.2000.3264. [DOI] [PubMed] [Google Scholar]
- Nakamichi K., Matsumoto Y., Tohya Y., Otsuka H. Induction of apoptosis in rabbit kidney cell under high‐level expression of bovine herpesvirus 1 U(s)ORF8 product. Intervirology. 2002;45:85–93. doi: 10.1159/000063236. [DOI] [PubMed] [Google Scholar]
- Nicholson L.J., Philippe M., Paine A.J., Mann D.A., Dolphin C.T. RNA interference mediated in human primary cells via recombinant baculoviral vectors. Mol. Ther. 2005;11:638–644. doi: 10.1016/j.ymthe.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Ojala K., Mottershead D.G., Suokko A., Oker‐Blom C. Specific binding of baculoviruses displaying gp64 fusion proteins to mammalian cells. Biochem. Biophys. Res. Commun. 2001;284:777–784. doi: 10.1006/bbrc.2001.5048. [DOI] [PubMed] [Google Scholar]
- Ojala K., Tikka P.J., Kautto L., Kapyla P., Marjomaki V., Oker‐Blom C. Expression and trafficking of fluorescent viral membrane proteins in baculovirus‐transduced BHK cells. J. Biotechnol. 2004;114:165–175. doi: 10.1016/j.jbiotec.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Ong S.T., Li F., Du J., Tan Y.W., Wang S. Hybrid cytomegalovirus enhancer H‐1 promoter‐based plasmid and baculovirus vectors mediate effective RNA interference. Hum. Gene. Ther. 2005;16:1404–1412. doi: 10.1089/hum.2005.16.1404. [DOI] [PubMed] [Google Scholar]
- Palombo F., Monciotti A., Recchia A., Cortese R., Ciliberto G., La Monica N. Site‐specific integration in mammalian cells mediated by a new hybrid baculovirus‐adeno‐associated virus vector. J. Virol. 1998;72:5025–5034. doi: 10.1128/jvi.72.6.5025-5034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.W., Lee H.K., Kim T.G., Yoon S.K., Paik S.Y. Hepatocyte‐specific gene expression by baculovirus pseudotyped with vesicular stomatitis virus envelope glycoprotein. Biochem. Biophys. Res. Commun. 2001;289:444–450. doi: 10.1006/bbrc.2001.6009. [DOI] [PubMed] [Google Scholar]
- Patterson R.M., Selkirk J.K., Merrick B.A. Baculovirus and insect cell gene expression‐review of baculovirus biotechnology. Environ. Health Perspect. 1995;103:756–759. doi: 10.1289/ehp.95103756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfohl J.L., Worley J.F., Condreay J.P., An G., Apolito C.J., Kost T.A, Truax J.F. Titration of K‐ATP channel expression in mammalian cells utilizing recombinant baculovirus transduction. Receptors Channels. 2002;8:99–111. [PubMed] [Google Scholar]
- Pieroni L., Maione D., La Monica N. In vivo gene transfer in mouse skeletal muscle mediated by baculovirus vectors. Hum. Gene. Ther. 2001;12:871–881. doi: 10.1089/104303401750195845. [DOI] [PubMed] [Google Scholar]
- Pijlman G.P., van den Born E., Martens D.E., Vlak J.M. Autographa californica baculoviruses with large genomic deletions are rapidly generated in infected insect cells. Virology. 2001;283:132–138. doi: 10.1006/viro.2001.0854. [DOI] [PubMed] [Google Scholar]
- Pijlman G.P., de Vrij J., van den End F.J., Vlak J.M., Martens D.E. Evaluation of baculovirus expression vectors with enhanced stability in continuous cascaded insect‐cell bioreactors. Biotechnol. Bioeng. 2004;87:743–753. doi: 10.1002/bit.20178. [DOI] [PubMed] [Google Scholar]
- Plank C., Mechtler K., Szoka F.C., Wagner E. Activation of the complement system by synthetic DNA complexes: A potential barrier for intravenous gene delivery. Hum. Gene. Ther. 1996;7:1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- Poomputsa K., Kittel C., Egorov A., Ernst W., Grabherr R. Generation of recombinant influenza virus using baculovirus delivery vector. J. Virol. Methods. 2003;110:111–114. doi: 10.1016/s0166-0934(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Ramos L., Kopec L.A., Sweitzer S.M., Fornwald J.A., Zhao H.Z., McAllister P., McNulty D.E., Trill J.J., Kane J.F. Rapid expression of recombinant proteins in modified CHO cells using the baculovirus system. Cytotechnology. 2002;38:37–41. doi: 10.1023/A:1021189628274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raty J.K., Airenne K.J., Marttila A.T., Marjomaki V., Hytonen V.P., Lehtolainen P., Laitinen O.H., Mahonen A.J., Kulomaa M.S., Yla‐Herttuala S. Enhanced gene delivery by avidin‐displaying baculovirus. Mol. Ther. 2004;9:282–291. doi: 10.1016/j.ymthe.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Salminen M., Airenne K.J., Rinnankoski R., Reimari J., Valilehto O., Rinne J., Suikkanen S., Kukkonen S., Yla‐Herttuala S., Kulomaa M.S., Vihinen‐Ranta M. Improvement in nuclear entry and transgene expression of baculoviruses by disintegration of microtubules in human hepatocytes. J. Virol. 2005;79:2720–2728. doi: 10.1128/JVI.79.5.2720-2728.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandig V., Hofmann C., Steinert S., Jennings G., Schlag P., Strauss M. Gene transfer into hepatocytes and human liver tissue by baculovirus vectors. Hum. Gene. Ther. 1996;7:1937–1945. doi: 10.1089/hum.1996.7.16-1937. [DOI] [PubMed] [Google Scholar]
- Sarkis C., Serguera C., Petres S., Buchet D., Ridet J.L., Edelman L., Mallet J. Efficient transduction of neural cells in vitro and in vivo by a baculovirus‐derived vector. Proc. Natl. Acad. Sci. USA. 2000;97:14638–14643. doi: 10.1073/pnas.260472897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji I., Aizaki H., Tani H., Ishii K., Chiba T., Saito I., Miyamura T., Matsuura Y. Efficient gene transfer into various mammalian cells, including non‐hepatic cells, by baculovirus vectors. J. Gen. Virol. 1997;78:2657–2664. doi: 10.1099/0022-1317-78-10-2657. [DOI] [PubMed] [Google Scholar]
- Smith G.E., Summers M.D., Fraser M.J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol. Cell. Biol. 1983;3:2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollerbrant K., Elmen J., Wahlestedt C., Acker J., Leblois‐Prehaud H., Latta‐Mahieu M., Yeh P., Perricaudet M. A novel method using baculovirus‐mediated gene transfer for production of recombinant adeno‐associated virus vectors. J. Gen. Virol. 2001;82:2051–2060. doi: 10.1099/0022-1317-82-9-2051. [DOI] [PubMed] [Google Scholar]
- Song S.U., Boyce F.M. Combination treatment for osteosarcoma with baculoviral vector mediated gene therapy (p53) and chemotherapy (adriamycin) Exp. Mol. Med. 2001;33:46–53. doi: 10.1038/emm.2001.9. [DOI] [PubMed] [Google Scholar]
- Song S.U., Shin S.H., Kim S.K., Choi G.S., Kim W.C., Lee M.H., Kim S.J., Kim I.H., Choi M.S., Hong Y.J., Lee K.H. Effective transduction of osteogenic sarcoma cells by a baculovirus vector. J. Gen. Virol. 2003;84:697–703. doi: 10.1099/vir.0.18772-0. [DOI] [PubMed] [Google Scholar]
- Spenger A., Ernst W., Condreay J.P., Kost T.A., Grabherr R. Influence of promoter choice and trichostatin A treatment on expression of baculovirus delivered genes in mammalian cells. Protein. Expr. Purif. 2004;38:17–23. doi: 10.1016/j.pep.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Stanbridge L.J., Dussupt V., Maitland N.J. Baculoviruses as vectors for gene therapy against human prostate cancer. J. Biomed. Biotechnol. 2003;2003:79–91. doi: 10.1155/S1110724303209049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Porter C.D., Strahan K.M., Preece A.F., Gustafsson K., Cosset F.L., Weiss R.A., Collins M.K.L. Sensitization of cells and retroviruses to human serum by (alpha 1–3) galactosyltransferase. Nature. 1996;379:85–88. doi: 10.1038/379085a0. [DOI] [PubMed] [Google Scholar]
- Tami C., Farber M., Palma E.L., Taboga O. Presentation of antigenic sites from foot‐and‐mouth disease virus on the surface of baculovirus and in the membrane of infected cells. Arch. Virol. 2000;145:1815–1828. doi: 10.1007/s007050070058. [DOI] [PubMed] [Google Scholar]
- Tani H., Nishijima M., Ushijima H., Miyamura T., Matsuura Y. Characterization of cell‐surface determinants important for baculovirus infection. Virology. 2001;279:343–353. doi: 10.1006/viro.2000.0699. [DOI] [PubMed] [Google Scholar]
- Tani H., Limn C.K., Yap C.C., Onishi M., Nozaki M., Nishimune Y., Okahashi N., Kitagawa Y., Watanabe Y., Mochizuki R., Moriishi K., Matsuura Y. In vitro and in vivo gene delivery by recombinant baculoviruses. J. Virol. 2003;77:9799–9808. doi: 10.1128/JVI.77.18.9799-9808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.E., Ehrhardt A., Kay M.A. Progress and problems with the use of viral vectors for gene therapy. Nature Rev. Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Tjia S.T., Altenschildesche G.M.Z., Doerfler W. Autographa californica nuclear polyhedrosis virus (AcNPV) DNA does not persist in mass cultures of mammalian cells. Virology. 1983;125:107–117. doi: 10.1016/0042-6822(83)90067-3. [DOI] [PubMed] [Google Scholar]
- van Loo N.D., Fortunati E., Ehlert E., Rabelink M., Grosveld F., Scholte B.J. Baculovirus infection of nondividing mammalian cells: Mechanisms of entry and nuclear transport of capsids. J. Virol. 2001;75:961–970. doi: 10.1128/JVI.75.2.961-970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkaiah B., Viswanathan P., Habib S., Hasnain S.E. An additional copy of the homologous region (hr1) sequence in the Autographa californica multinucleocapsid polyhedrosis virus genome promotes hyperexpression of foreign genes. Biochemistry. 2004;43:8143–8151. doi: 10.1021/bi049953q. [DOI] [PubMed] [Google Scholar]
- Verma I.M., Somia N. Gene therapy‐promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- Viswanathan P., Venkaiah B., Kumar M.S., Rasheedi S., Vrati S., Bashyam M.D., Hasnain S.E. The homologous region sequence (hr1) of Autographa californica multinucleocapsid polyhedrosis virus can enhance transcription from non‐baculoviral promoters in mammalian cells. J. Biol. Chem. 2003;278:52564–52571. doi: 10.1074/jbc.M309351200. [DOI] [PubMed] [Google Scholar]
- Volkman L.E., Goldsmith P.A. In vitro study of Autographa californica nuclear polyhedrosis virus interaction with nontarget vertebrate host cells. Appl. Environ. Microbiol. 1983;45:1085–1093. doi: 10.1128/aem.45.3.1085-1093.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle M., Jesuthasan S. Baculovirus‐mediated gene expression in zebrafish. Marine Biotechnol. 2003;5:58–63. doi: 10.1007/s10126-002-0050-9. [DOI] [PubMed] [Google Scholar]
- Wang C.‐Y., Wang S. Adeno‐associated virus inverted terminal repeats improve neuronal transgene expression mediated by baculoviral vectors in rat brain. Hum. Gene. Ther. 2005;16:1219–1226. doi: 10.1089/hum.2005.16.1219. [DOI] [PubMed] [Google Scholar]
- Wang K.‐C., Wu J.‐C., Chung Y.‐C., Ho Y.‐C., Chang M.D., Hu Y.‐C. Baculovirus as a highly efficient gene delivery vector for the expression of hepatitis delta virus antigens in mammalian cells. Biotechnol. Bioeng. 2005;89:464–473. doi: 10.1002/bit.20385. [DOI] [PubMed] [Google Scholar]
- Wickham T.J., Granados R.R., Wood H.A., Hammer D.A., Shuler M.L. General analysis of receptor‐mediated viral attachment to cell surfaces. Biophys. J. 1990;58:1501–1516. doi: 10.1016/S0006-3495(90)82495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.Y., Liono L., Chen S.L., Chen C.Y., Chao Y.C. Expression of highly controllable genes in insect cells using a modified tetracycline‐regulated gene expression system. J. Biotechnol. 2000;80:75–83. doi: 10.1016/s0168-1656(00)00247-9. [DOI] [PubMed] [Google Scholar]
- Yap C.C., Ishii K., Aizaki H., Tani H., Aoki Y., Ueda Y., Matsuura Y., Miyamura T. Expression of target genes by coinfection with replication‐deficient viral vectors. J. Gen. Virol. 1998;79:1879–1888. doi: 10.1099/0022-1317-79-8-1879. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Kondoh D., Arai E., Matsuoka H., Seki C., Tanaka T., Okada M., Ishii A. Baculovirus virions displaying Plasmodium berghei circumsporozoite protein protect mice against malaria sporozoite infection. Virology. 2003;316:161–170. doi: 10.1016/j.virol.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Zhou G.Y., Galvan V., Campadelli‐Fiume G., Roizman B. Glycoprotein D or J delivered in trans blocks apoptosis in SK‐N‐SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 2000;74:11782–11791. doi: 10.1128/jvi.74.24.11782-11791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


