Abstract
Recent advances in our understanding of stem/progenitor cells and their potential to repair damaged organs offer the possibility of cell-based treatments for neonatal lung injury. This review summarizes basic concepts of stem/progenitor cell biology and discusses the recent advances and challenges of cell-based therapies for lung diseases, with a particular focus on bronchopulmonary dysplasia (BPD), a form of chronic lung disease that primarily affects very preterm infants. Despite advances in perinatal care, BPD still remains the most common complication of extreme prematurity, and there is no specific treatment.
Keywords: Premature birth, Bronchopulmonary dysplasia, Oxygen, Lung injury, Stem cells, Regeneration, Cell therapy
Key Points.
-
•
Various types of stem/progenitor cells have shown potential promise in preventing and/or repairing neonatal lung injury.
-
•
Mesenchymal stem cells derived from both bone marrow and umbilical cord blood are being popularly studied and appear to function in a paracrine manner rather than through cell engraftment.
-
•
Further knowledge and understanding in this novel and exciting area of research is necessary before safe clinical translation of cell-based therapies is warranted.
-
•
Strong emphasis must be placed on developing and standardizing techniques for stem/progenitor cell definition, isolation, expansion, and therapeutic administration.
-
•
Experimental studies also need to focus on the long-term outcomes of such therapies.
-
•
By identifying the most appropriate “reparative cell(s)” and its source, combined with understanding alternative mechanisms of action beyond cell replacement, we can advance in the quest of providing therapeutic strategies to prevent/repair neonatal lung injury.
Introduction
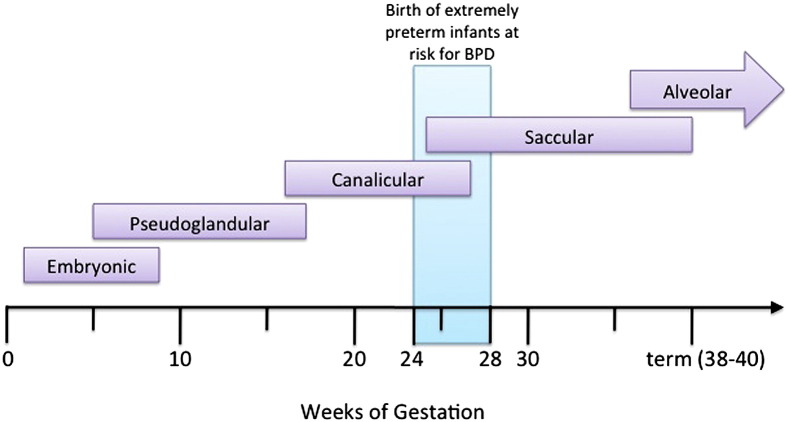
Advances in perinatal care have led to improved survival following very preterm birth, with infants born as early as 23 to 24 weeks of gestation now being capable of survival. However, with this shift in the limit of viability toward a lower gestational age, the task of protecting the more immature lung from injury becomes increasingly challenging. Extreme prematurity is one of the major risk factors for the development of chronic lung disease of prematurity or bronchopulmonary dysplasia (BPD).1 Preterm infants born between 24 and 28 weeks of gestation (ie, extremely preterm) have an immature pulmonary surfactant system, immature airway and vascular architecture, and an underdeveloped surface area for gas exchange (Fig. 1 ).2 Many very preterm infants require prolonged respiratory support to ensure survival, which further increases their risk of developing BPD.
Fig. 1.
Stages and gestational ages of normal lung development and preterm infants at risk of BPD. Schematic depicting stages of lung development in the human: embryonic (1–7 weeks), pseudoglandular (∼5–16/17 weeks), canalicular (∼16/17–24/26 weeks), saccular (∼24–38 weeks), and alveolar (∼36 weeks to postnatal) stages. Preterm infants at risk of developing BPD are born during the late canalicular to early saccular phase of lung development.
Recent evidence suggests that BPD may have long-term respiratory complications that reach beyond childhood. Numerous follow-up studies indicate that children and young adults who were born very preterm are at an increased risk of respiratory symptoms, poor lung function, and lower exercise capacity3, 4, 5, 6, 7 this is especially apparent in infants who have developed BPD. More alarmingly, isolated case studies are surfacing of irreversible arrested alveolar development at adult age in former premature infants with BPD,8, 9 mirroring results from experimental models of BPD.10
Progress toward decreasing the incidence/severity of BPD over the next few years using currently available techniques and strategies is likely (ie, optimization of antenatal management combined with surfactant and early noninvasive ventilatory support targeting lower oxygen saturations).11 However, further understanding of the mechanisms involved in lung development, injury, and repair are necessary to advance toward preventing lung injury and/or promoting lung development/regeneration in prematurely born infants. Exciting discoveries in stem cell biology in recent years may offer new insight into the pathogenesis of BPD and, more importantly, open new therapeutic avenues.
Basic concepts of stem cell biology
Stem cells are primitive cells capable of extensive self-renewal with the potential to give rise to multiple differentiated cellular phenotypes.12 These cells are not only critical for organogenesis and growth during the early stages of development but also contribute to organ repair and regeneration throughout life.
Developmental Potency of Stem Cells
The concept of developmental potency refers to the range of possible fates open to cells during differentiation. Stem cells exhibit varying differentiation potencies, and are typically categorized into embryonic and somatic stem cells. Embryonic stem cells (ESCs) are derived from the early blastocyst and represent the most potent of stem cells owing to their pluripotency (ie, ability to differentiate into cell types derived from all 3 germinal lineages: endoderm, mesoderm, or ectoderm) and their ability for indefinite self-renewal. By contrast, somatic stem cells (also termed adult stem cells [ASCs]) are cells that have assumed increasing degrees of fate restriction and are either multipotent (ie, can differentiate into a limited range of cell types) or unipotent (ie, can generate only one cell type).13 Residual pools of such multipotent or unipotent stem cells are hypothesized to reside in almost all adult organs, and have the ability to contribute to tissue repair and regeneration via repopulation during growth, injury, or disease.
Classical Versus Nonclassical Stem Cell Hierarchies
While stem cells are essential for growth and development, residual pools of ASCs are considered important for tissue repair and maintenance through adulthood. Highly proliferative tissues, such as the intestinal epithelium or the hematopoietic compartment of the bone marrow, depend on a pool of ASCs that are organized in a “classical hierarchy” to maintain homeostasis.14 By contrast, anatomically complex tissues that turn over more slowly (ie, brain, heart, lung, and kidney) do not appear to support a classical stem cell hierarchy. Such tissues are thought to be maintained by stem/progenitor cell populations that are organized in a nonclassical hierarchy and are recruited in a facultative manner for regeneration following injury.
In the lung, several local epithelial cell types function both as differentiated functional cells and as transit-amplifying progenitors that proliferate in response to airway or alveolar injuries.15 Recent research suggests that the adult lung harbors rare populations of multipotent epithelial stem cells that are regulated by specific microenvironmental cellular niches, and are putatively recruited to repopulate the damaged epithelium.16, 17, 18, 19 With new insights into stem cell biology, other types of resident lung stem/progenitor cells have also been described over the past 5 years.
Resident lung stem/progenitor cells
Lungs are complex organs constituted by more than 40 cell types derived from all 3 germ layers.20 Normal lung morphogenesis involves spatiotemporally coordinated interactions between the stem/progenitor cells of different cellular compartments, which are later recapitulated during lung regeneration and repair following injury.21 At present, the localization and properties of lung stem/progenitor cell niches and the type of cells within each niche are of major interest, yet also present controversy. Complexity of the lung architecture combined with an extensive diversity of cell types and niches has hindered the identification of true lung stem/progenitor cells. Because of the exceptionally low rate of epithelial cell turnover in the steady-state adult lung, the use of injury models has become necessary in unveiling the identity, fate, and specificity of resident lung stem/progenitor cells that contribute to homeostatic maintenance in the lung.22 In doing so, it has been observed that relatively differentiated airway and alveolar epithelial cell types are capable of proliferating in response to epithelial injury.15 This observation has drawn the focus of lung stem/progenitor cell research into identifying and defining those epithelial cell subpopulations that appear to contribute to postinjury regeneration.15 Indeed, putative populations of such endogenous progenitor cells within the adult lung have been located in the basal layer of the proximal conducting airways (trachea and major bronchi) and more distally (bronchioles and alveolar ducts) within or near neuroendocrine bodies (NEBs), in the bronchoalveolar duct junction (BADJ), and the alveolar surface.23 A representative list of putative endogenous lung epithelial stem/progenitor cells is summarized in Table 1 . While stem/progenitor cells in the proximal airways have been explored more extensively, the study of distal stem/progenitor cells remains more controversial.
Table 1.
A selective list of candidate endogenous lung stem/progenitor cells in the rodent lung
| Anatomic Location | Candidate Stem/Progenitor Cell | Attributed Differentiated Phenotype | Niche | Defining Characteristics | References |
|---|---|---|---|---|---|
| Proximal trachea | SMG duct cells | Tracheal epithelial cells, SMGs, SMG ducts | SMGs | Express cytokeratin-14 and -5; survives and repopulates tracheal epithelium following hypoxic-ischemic injury; BrdU labeling–retained cells following i.t. detergent or SO2-mediated epithelial injury | 25, 26 |
| Distal trachea and bronchi | Basal cells | Tracheobronchial epithelial cells | Intercartilaginous zone | Cytokeratin-14–expressing multipotent progenitor cells capable of restoring differentiated tracheal epithelium following naphthalene injury; associated with innervated NEBs | 27 |
| Bronchioles | ClaraV cells | Distal airway epithelium | BADJs, NEBs, and PSMCs | Express CCSP; survives and repopulates distal airway epithelium following naphthalene injury; dependent on paracrine signaling of Fgf10 | 29, 30 |
| Bronchioles and alveoli | BASCs | Bronchoalveolar epithelial cells | BADJs | Resistant to naphthalene injury and proliferate in response; coexpress CCSP and SP-C | 17 |
| Pulmonary Oct-4+ stem/progenitor cells | Alveolar type-I and -II pneumocytes | BADJs | Oct4+, SSEA-1+, Sca-1+, cytokeratin-7+ cells; serially passaged, differentiate terminally into type-II and -I pneumocytes; susceptible to SARS-CoV infection | 78 | |
| Multipotent lung epithelial progenitors | Airway and alveolar epithelium | Intrapulmonary airways and alveoli (not localized) | EpCAMhi, CD49f+, CD104+, CD24lo, Sca-1−, CD45−, CD31− lung epithelial colony-forming units, form colonies in Matrigel, serially passaged and retain multipotent potential | 18 | |
| Alveoli | Alveolar type-II pneumocytes | Alveolar type-I pneumocytes | Alveolar surface | All alveolar type-II pneumocytes | 34 |
| A subset of alveolar type-II pneumocytes | Alveolar type-I and mature type-II pneumocytes | Alveolar surface | E-cadherin negative subset of alveolar type-II cells, proliferative, high telomerase activity, resistant to oxygen-induced injury | 37 |
Abbreviations: BADJ, bronchoalveolar duct junction; BASC, bronchoalveolar stem cell; BrdU, 5-bromo-2′-deoxyuridine; CCSP, Clara cell secretory protein; ClaraV, variant Clara; EpCAMhi, epithelial cell adhesion molecule Fgf10, fibroblast growth factor 10; NEBs, neuroendocrine bodies; Oct-4, octamer-binding transcription factor 4; PSMC, parabronchial smooth muscle cell; Sca-1, stem cell antigen 1; SMG, submucosal gland; SP-C, surfactant protein C; SSEA-1, stage-specific embryonic antigen-1; SARS-CoV, severe acute respiratory syndrome coronavirus.
Proximal Airway Stem/Progenitor Cells
Airway submucosal glands (SMGs) and SMG ducts are major secretory structures situated below the epithelium of the proximal trachea.24 Studies have indicated that SMGs may serve as a protective niche for adult epithelial stem/progenitor cells of the proximal airways.25, 26 Using a model of epithelial damage induced by intratracheal detergent or SO2 inhalation, Borthwick and colleagues25 identified a population of 5-bromo-2′-deoxyuridine label–retaining cells that were localized to the gland ducts of the upper trachea and were able to reconstitute a surface-like tracheal epithelium. In support of these findings, SMG duct cells (K5+, K14+) survive severe hypoxic-ischemic injury and contain stem/progenitor cell populations for regenerating the pseudostratified surface epithelium, SMGs, and SMG ducts.26
An additional niche of stem/progenitor cells in the proximal airway is the K14-expressing tracheobronchial basal cells, which have been shown to repopulate the denuded airway epithelium, including columnar secretory and ciliated cells, following naphthalene-induced epithelial ablation.27 This finding indicates that the K14-expressing basal cells are implicated as a stem/progenitor cell for this airway location. The potential contribution of these cells to the repair of the distal lung remains unknown.
Distal Lung Epithelial Stem/Progenitor Cells
With transition from the proximal to distal airways, it can be seen that the notion of multiple niches supporting different populations and their progenitors within the lung is evident. This idea is supported by studies using naphthalene to deplete the airways of Clara cells, revealing a subset of Clara cells that are CCSP+, yet naphthalene resistant. These cells, termed variant Clara (ClaraV) cells, exhibit stem cell characteristics, including ability to efflux Hoechst dye and Sca-1 expression, and have been located either within NEBs or at the bronchoalveolar duct junctions (BADJs).28, 29 More recently, Volckaert and colleagues30 also proposed that parabronchial smooth muscle cells (PSMCs) constitute a stem/progenitor cell niche for the ClaraV cells. Activation of the ClaraV cell for epithelial repair following naphthalene injury was shown to be dependent on the paracrine signaling of fibroblast growth factor 10 from the PSMC niche.30
Recently, multipotent stem cells in the distal lung capable of differentiating into epithelial cells specific to the bronchioles and the alveoli have been identified. Kim and colleagues17 demonstrated the existence of dual-lineage bronchoalveolar stem cells (BASCs) at the BADJ that express both bronchiolar (CCSP+) and alveolar (SP-C+) markers, which proliferate in response to airway and alveolar injury. However, based on the techniques used, there has been some ambiguity regarding the lineage potential23, 31 and contribution of these cells to alveolar repair.32 McQualter and colleagues18 used a multiparameter cell separation strategy and an organotypic in vitro clonogenic assay to detect and characterize a rare population of multipotent adult lung epithelial stem cells that give rise to airway and alveolar epithelial lineages in vitro. More recently, p63-expressing cells in the bronchiolar epithelium have been shown to undergo rapid proliferation after H1N1 influenza virus infection and to radiate to interbronchiolar regions of alveolar ablation. These cells assemble into Krt5+ pods and initiate expression of markers typical of alveoli. Gene-expression profiles of these pods suggest that they are intermediates in the reconstitution of the alveolar-capillary network.33 The presence of such putative endogenous alveolar stem cell populations provides fresh hope of target-directed, regenerative therapies for alveolar diseases.
Cuboidal type-II pneumocytes have long been considered as progenitors of the alveolar epithelium, based on their capacity to replenish themselves and generate terminally differentiated type I pneumocytes.34, 35 Since then, type-II pneumocytes have been speculated to contain a subpopulation of progenitors cells that can undergo reactivation into a progenitor-like state in response to injury cues. Using an acute model of oxygen-induced injury, Driscoll and colleagues36 demonstrated the existence of a telomerase-positive subpopulation within the general type-II cell population during the recovery phase. These findings were further strengthened by a later study in which Reddy and colleagues37 classified type-II cells into E-cadherin–positive and -negative fractions, and showed heightened telomerase activity and injury resistance in the latter subset.
Lung Mesenchymal Stem Cells
Additional lung cell types, including airway smooth muscle, fibroblasts, and the vasculature, are derived from the mesoderm. Interactions between the epithelial cells, mesenchymal microenvironment (including extracellular matrix proteins and growth factors), and the adjacent pulmonary vasculature regulate the structural and functional maturation of the developing lung.21 Current knowledge of lung mesenchymal precursors is limited; however, there is evidence that small populations of resident lung cells expressing certain phenotypic characteristics of mesenchymal cells with progenitor capacity exist within the lung.
Resident lung “side population” (SP) cells, which appear to have both mesenchymal and epithelial potential, have been isolated based on their capacity to efflux Hoechst dye.38, 39, 40 These SP cells have been shown to be present at all levels of the airway tree, and regardless of which lung compartment they were derived from, exhibited a relatively uniform phenotype.40 Although it has been demonstrated that these SP cells are a source of adult lung mesenchymal stem cells (MSCs),39 the role of SP cells in endogenous lung repair is not completely understood.
Furthermore, McQualter and colleagues41 described a population of endogenous fibroblastic progenitor cells with clonogenic potential in the adult lung, which are predominantly representative of mesenchymal cell lineages. The cell fraction defined by McQualter and colleagues18 was of similar cell phenotype (CD45−, CD31−, Sca-1+, CD43+) to the cell fraction defined as BASCs; however, they coexpressed immunophenotypic markers definitive of lung fibroblastic rather than epithelial cells.41 These findings highlight the need for alternative, specific markers to enable precise identification of endogenous stem/progenitor cell subpopulations within the lung.
Following the discovery of the plasticity characteristics of ASCs that allow them to cross lineage barriers and adopt functional phenotypes of other tissues, much interest has been diverted to understanding their role in repair and maintenance of the lungs.42 Experimental evidence indicates that the injured lung stimulates the release and preferential homing of MSCs, a population of ASCs derived from the bone marrow.43, 44 However, the mechanism by which exogenous progenitors, such as bone marrow MSCs, assume lung phenotype remains unclear, as does its clinical significance.45, 46
Lung Endothelial Progenitor Cells
Endothelial progenitor cells (EPCs), a population of vascular precursor cells, have also recently received attention in the context of lung development and regeneration. Indeed, given the importance of lung angiogenesis and vascular growth factors during lung growth and repair, vascular progenitor cells are appealing candidate cells likely to be involved in the same mechanisms.47 However, assessment of the contribution of endogenous lung EPCs in lung vascular repair and lung regeneration and remodeling is impeded by their rarity, lack of distinguishing markers, and the inability to discriminate circulating EPCs and tissue EPCs.22 Alvarez and colleagues48 demonstrated that the lung microvasculature is enriched with a population of EPCs, termed resident microvascular endothelial progenitor cells, which were shown to be highly proliferative and capable of renewing the entire hierarchy of endothelial cell growth potentials. It has been demonstrated that both circulating and resident lung EPCs are likely to contribute to endothelial cell regeneration and repair in the lung.49, 50
The recent surge in our knowledge of stem cell biology and the availability of advanced research tools in this field has motivated researchers in exploring the role of lung stem cells in the pathogenesis of lung diseases. Indeed, several major lung diseases likely involve dysregulation in the numbers and/or the function of resident lung stem/progenitor cells.46 For instance, depletion or functional impairment of alveolar epithelial and/or EPCs could putatively underlie the pathogenesis of alveolar growth arrest or destruction observed in BPD and emphysema, respectively. In such a scenario, augmentation of stem cells is an appealing strategy to minimize lung injury, promote repair, or possibly regenerate lost tissue.
Lung stem/progenitor cells: implications for the pathogenesis of BPD
Recent animal and human studies suggest that damage or depletion of epithelial and/or vascular stem/progenitor cells in the developing lung likely contributes to the pathogenesis of BPD.
Perturbation of Distal Lung Epithelial and Mesenchymal Stem Cells
Exposure of neonatal rodents to high levels of oxygen is extensively used as an injury model to investigate experimental BPD. Irwin and colleagues51 showed a reduction in the number and endothelial differentiation potential of multipotent lung SP cells. Observations from the authors’ own laboratory in an oxygen-challenged neonatal rat model of BPD have also shown decreased numbers of circulating and resident MSCs in the lungs.52 This finding highlights the potential of stem cell supplementation for the prevention or repair of neonatal lung injury. Accordingly, systemic treatment of neonatal hyperoxia–exposed mice with MSCs significantly increases the number of BASCs compared with untreated controls. In addition, treatment of BASCs with MSC-derived conditioned media (CdM) in culture stimulated BASC growth efficiency, indicating a direct effect of MSCs on BASCs.53
In contrast to the aforementioned reports of depleted numbers of stem/progenitor cells, Popova and colleagues54 demonstrated that the presence of MSCs in tracheal aspirates of preterm infants indicated an increased risk for developing BPD. Those cells isolated from the tracheal aspirates expressed the markers STRO-1, CD73, CD90, CD105, CD166, CCR2b, CD13, propyl-4-hydroxylase, and α-smooth muscle actin, and were negative for CD11b, CD31, CD34, and CD45.55 Furthermore, these cells were shown to acquire a myofibroblast phenotype, which suggest that they could contribute to the profibrotic changes and arrested alveolarization in BPD.56 However, in contrast to tracheal aspirate MSCs, human bone marrow–derived MSCs did not undergo myofibroblastic differentiation in response to transforming growth factor β1, suggesting distinct properties between these 2 populations of MSCs.56 Indeed, it is possible that these reported resident lung MSCs are perturbed in BPD, as their cell phenotype is not analogous to the endogenous MSCs described by McQualter and colleagues41 in the absence of lung injury. Therefore, with the growing interest in harnessing the therapeutic effects of stem progenitor cells for neonatal lung injury, it is necessary to perform further thorough investigations to understand the behavior of MSCs from different populations (ie, lung, umbilical cord blood [UCB], bone marrow) in the presence and absence of lung injury, and how this could affect potential cell-based therapies for BPD.57
Perturbation of Lung and Circulating EPCs
Neonatal mice with oxygen-induced chronic lung injury have depleted numbers of putative lung-resident EPCs (CD45−/Sca-1+/CD133+/VEGFR-2+).50 Baker and colleagues58 demonstrated that UCB of preterm infants yielded a higher amount of endothelial colony-forming cells (ECFCs; a specific subset of EPCs) than from UCB of term infants. Preterm ECFCs had an increased susceptibility to in vitro oxygen exposure than term ECFCs.58 Borghesi and colleagues59 reported that the number of ECFCs was lower in UCB of preterm infants who subsequently developed BPD, compared with preterm infants who did not develop BPD. In contrast to the findings of Borghesi and colleagues,59 Paviotti and colleagues60 recently reported no association between the number of EPCs at birth and the subsequent development of BPD. The apparent discordance between studies reporting EPCs in preterm infants highlights the importance of appropriately defining an EPC and establishing criteria similar to the “minimal criteria” for characterizing MSCs.61 Furthermore, assessing EPC function may be more revealing than assessing EPC number.
These observations suggest that the capacity of resident stem cell populations to undergo self-renewal and regeneration can be limited, because of the natural effect of increasing age and/or the presence of disease. This situation forms the rationale for the therapeutic potential of stem cell–based therapies, either through stimulation of endogenous stem cell pools or their therapeutic replacement with exogenous-derived stem cells. Such cell-replacement therapies already show promise in debilitating childhood and adult disorders.62, 63, 64 In the laboratory, stem cell–based strategies have shown therapeutic benefit in experimental models of lung disease.
Therapeutic potential of stem cells to prevent or repair the damaged lung
Numerous studies in experimental animal models provide compelling evidence for the beneficial effects of stem cell therapy approaches for a wide variety of adult lung diseases (Table 2 ), including acute lung injury/acute respiratory distress syndrome, pulmonary hypertension, asthma, and chronic obstructive pulmonary disease (including emphysema).65, 66, 67
Table 2.
Studies testing the therapeutic effect of stem/progenitor cells in experimental adult lung disease models
| Experimental Model | Therapeutic Cell of Product | Outcomes | Suggested Mechanisms | References |
|---|---|---|---|---|
| Bleomycin Lung Injury/Acute Respiratory Distress Syndrome | ||||
| Bleomycin-induced (i.t.) | Human ESC-derived cells with AT2 epithelial phenotype (i.t.) | Improved body weight and survival Improved arterial oxygen saturation Decreased collagen deposition |
Engraftment and AT1 differentiation Paracrine mechanisms |
79 |
| Bone marrow–derived MSCs (i.v.) | Reduced fibrosis and inflammation | IL-1 receptor antagonism Decrease in NO metabolites, proinflammatory, and angiogenic cytokines |
44, 80, 81 | |
| hUC Wharton jelly–derived MSCs (i.v.) | Reduced fibrosis | Decreased TGF-β and TIMP activity Increased MMP-2 activity |
82 | |
| Bone marrow–derived HSCs ± KGF overexpression (i.v.) | Reduced fibrosis | KGF-induced endogenous AT2 cell proliferation | 83 | |
| Bleomycin-induced (i.n.) | hAECs (i.p.) | Reduced fibrosis and collagen deposition Improved lung function Modulated inflammatory response |
Anti-inflammatory effects | 84 |
| Escherichia coli endotoxin-induced (i.p.) | Bone marrow–derived MSCs (i.v.; i.t.) | Improved survival Decreased systemic and local inflammation |
Cell-cell interactions Paracrine mechanisms Decreased proinflammatory and increased anti-inflammatory cytokines Antioxidant mechanisms |
85, 86, 87 |
| E coli endotoxin-induced (i.t.) | iPS cells and CdM (i.v.) | Attenuated lung injury Reduced inflammation Reduced MPO and NF-κB activity Improved Pao2 and lung function |
Paracrine mechanisms Regulation of neutrophil activity Attenuating inflammatory cascade Immunomodulatory effects |
88 |
| Bone marrow–derived MSCs overexpressing Ang-1 (i.v.; i.t.) | Decreased inflammation Decreased alveolar permeability |
Decreased inflammatory cytokines Ang-1–mediated effects |
89, 90 | |
| hUCB-derived MSCs (i.t.) | Increased survival Attenuated lung injury Reduced inflammation Increased MPO activity Inhibited bacterial growth |
Down-modulating inflammatory process Enhancing bacterial clearance |
91 | |
| LPS-induced (i.t.) | Human orbital fat–derived stem/stromal cells (i.v.) | Decreased systemic and local inflammation Decreased alveolar and endothelial permeability |
Inhibition of macrophage and neutrophil-associated inflammatory responses | 92 |
| EPCs (i.v.) | Improved Pao2 and SaO2 Preservation of alveolocapillary permeability Reduced interstitial edema, hyaline membrane formation, hemorrhage |
Paracrine mechanisms Anti-inflammatory effects |
93 | |
| hUCB-derived MSCs (i.t.) | Increased survival Reduced edema, hemorrhage, alveolar and endothelial permeability Reduced inflammation |
Paracrine mechanisms Anti-inflammatory effects |
94 | |
| LPS-induced (i.v.) | EPCs (i.v.) | Reduced pulmonary edema, inflammation, hemorrhage, and hyaline membrane formation Decreased adhesion molecule expression Reduced endothelial and epithelial cell apoptosis |
Engraftment of EPCs Re-endothelialization Downregulation of adhesion molecules Alleviation of inflammatory response Apoptosis prevention |
95 |
| Ventilator-induced | Bone marrow–derived MSCs and CdM (i.v.) | Improved lung function Modulated inflammation Restored lung structure |
Paracrine mechanisms | 96 |
| Pulmonary Hypertension | ||||
| Monocrotaline-induced | Bone marrow–derived MSCs ± eNOS overexpression (i.v.; i.t.) | Improved survival Improved RV pressure overload and function Improved lung structure |
eNOS-mediated vasodilation VEGF-mediated enhanced microvasculature Paracrine effects |
97, 98, 99, 100 |
| Bone marrow–derived EPCs (i.v.) | Restored pulmonary hemodynamics Increased microvascular perfusion |
eNOS-mediated vascular growth | 101 | |
| Peripheral blood-derived EPCs (i.t.) | Improved cardiac function Improved vasculature thickness and lung neovascularization |
102 | ||
| Asthma/Allergic Airway Inflammation | ||||
| Ovalbumin-induced (i.p. and i.t.; nebulized) | Adipose tissue–derived MSCs (i.v.) | Decreased local and systemic allergic response | Decreased Th2 activity | 103, 104 |
| Bone marrow–derived MSCs (i.v.) | Reduced airway hyperresponsiveness and remodeling Reduced serum NO levels Reduced inflammatory cell infiltration and mast cell degranulation |
Immunomodulatory effects Anti-inflammatory effects |
105, 106 | |
| BMC-CdM | Prevented airway inflammation Reduced airway remodeling Prevented airway hyperresponsiveness |
Paracrine mechanisms Anti-inflammatory effects of adipokine, APN |
107 | |
| Ragweed-induced (i.p.) | Bone marrow–derived MSCs (i.v.) | Decreased asthma-specific allergic response | TGF-β production Regulatory T-cell recruitment |
108 |
| Chronic Obstructive Pulmonary Disease/Emphysema | ||||
| Cigarette smoke–induced | Bone marrow–derived MSCs, CdM, and BMCs (i.v.) | Restoration of alveolar structure Increased pulmonary vascularity Alleviation of pulmonary hypertension (by BMCs) |
Paracrine mechanisms Recruitment of BMCs by donor cells |
109 |
| Papain-induced | Bone marrow–derived MSCs (i.v.) | Improved alveolar structure | Engraftment and AT2 differentiation Reduced alveolar epithelial apoptosis |
110 |
| Elastase-induced (i.t.) | Adipose tissue–derived MSCs (i.v. or cultured on PGA and transplanted after LVRS) | Restored gas exchange Improved exercise tolerance |
Growth factor release (HGF, VEGF) | 111, 112 |
| Bone marrow–derived MSCs (i.t.) | Preservation of alveolar structure Reduced inflammation Upregulated growth factors |
Paracrine mechanisms HGF, EGF, and secretory leukocyte protease inhibitor secretion |
113 | |
| Lung resident multilineage progenitors Sca1+CD45−CD31− (i.t.) | Improved survival Attenuated alveolar damage |
Immunomodulatory effects Paracrine mechanisms |
114 | |
Abbreviations: Ang-1, angiopoietin-1; APN, adiponectin; AT1, alveolar epithelial type 1; AT2, alveolar epithelial type 2; BMC, bone marrow–derived cells; CdM, conditioned media; EGF, epidermal growth factor; eNOS, endothelial nitric oxide synthase; EPC, endothelial progenitor cell; HGF, hepatocyte growth factor; HSC, hematopoietic stem cell; hAEC, human amnion epithelial cell; hUC, human umbilical cord; hUCB, human umbilical cord blood; IL, interleukin; i.n., intranasal; i.p., intraperitoneal; iPS, induced pluripotent stem; i.t., intratracheal; i.v., intravenous; KGF, keratinocyte growth factor; LPS, lipopolysaccharide; LVRS, lung volume reduction surgery; MMP-2, matrix metalloproteinase 2; MPO, myeloperoxidase; MSC, mesenchymal stem cell; NF-κB, nuclear factor kappa light-chain enhancer of activated B cells; NO, nitric oxide; Pao2, partial pressure of oxygen in arterial blood; PGA, polyglycolic acid; RV, right ventricle; Sao2, oxygen saturation; TGF-β, transforming growth factor β; Th2, helper T cell type 2; TIMP, tissue inhibitor of metalloproteinase; VEGF, vascular endothelial growth factor.
Of the many different stem/progenitor cell therapies that have been used in experimental models, MSCs appear to be the most extensively examined cell type. MSCs can be sourced from the bone marrow, UCB, Wharton jelly, the placenta, and adipose tissue.68 As outlined in Table 2 benefits of MSC therapy in experimental adult lung diseases include, but are not limited to, improvements in alveolar, airway, and vascular structure; attenuation of lung inflammation; decreased pulmonary fibrosis; reduced pulmonary edema, hemorrhage, and alveolar and endothelial permeability; and restoration of lung function and exercise capacity. Of importance, the beneficial therapeutic actions of MSCs appear to be mediated through paracrine mechanisms and immunomodulatory effects, rather than cell engraftment and direct actions in the lungs.69
The use of MSCs and other types of stem/progenitor cells are also being increasingly examined in experimental models of neonatal lung disease, in particular BPD. Given the perturbations of resident lung stem cells in BPD, the ideal therapeutic approach would involve replenishing the lung with healthy multipotent stem/progenitor cells that repopulate, repair, and regenerate the injured, developing lung. Indeed, several recent studies have demonstrated promising outcomes using different stem/progenitor cell types in animal models of BPD (Fig. 2 , Table 3 ).
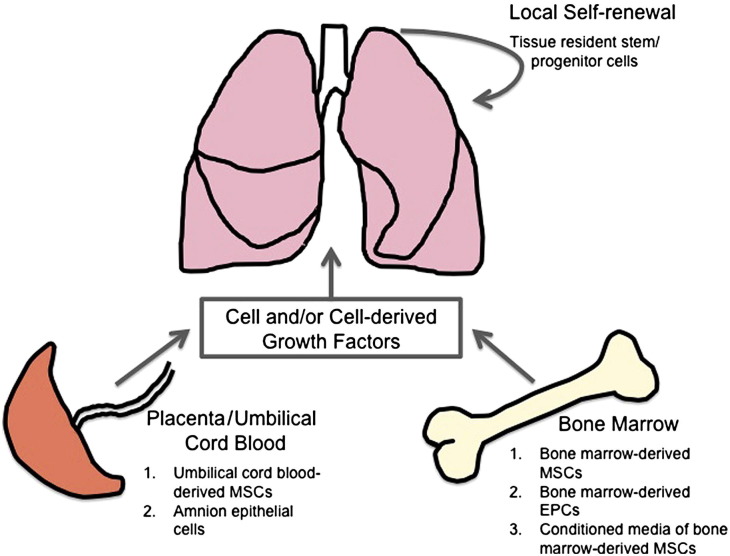
Fig. 2.
Current sources of stem/progenitor cells for lung regeneration in experimental models of neonatal lung injury. Several studies have demonstrated the effects of stem/progenitor cells and stem/progenitor cell-derived growth factors (ie, conditioned media) to promote lung regeneration following neonatal lung injury in animal models of BPD. These cells were sourced from the bone marrow, umbilical cord blood, and placenta amnion.
Table 3.
Studies testing the therapeutic effect of stem/progenitor cells in experimental models of neonatal chronic lung disease
| Experimental Model | Therapeutic Cell or Product | Outcomes | Suggested Mechanism | References |
|---|---|---|---|---|
| Hyperoxia-induced lung injury (mice, rats) | Bone marrow–derived MSCs (i.t.) | Improved survival Improved alveolar structure/prevented alveolar arrest Prevented vascular growth arrest Improved exercise capacity Reduced pulmonary hypertension |
Engraftment as AT2 Paracrine mechanisms |
52 |
| Bone marrow–derived MSCs or CdM (i.v.) | Improved alveolar structure/prevented alveolar arrest Attenuated inflammation Prevented vascular growth arrest Prevented pulmonary hypertension |
Paracrine mechanisms Immunomodulatory effects |
70 | |
| Bone marrow–derived MSCs or CdM (i.v.) | Increased number of BASCs Improved alveolar structure/prevented alveolar arrest |
Stimulation of BASCs Paracrine mechanisms |
53 | |
| Bone marrow–derived MSCs (i.p.) | Improved survival Improved alveolar structure/prevented alveolar arrest Attenuated inflammation Inhibited lung fibrosis |
Engraftment as AT2 Reduction in ECM remodeling and fibrosis gene expression (TGF-β1, collagen 1α, TIMP-1) Anti-inflammatory effects |
71 | |
| hUCB-derived MSCs (i.t.) | Improved survival and growth restriction Improved alveolar structure Attenuated lung fibrosis, inflammation, and ROS activity |
Paracrine anti-inflammatory, antifibrotic, and antioxidative effects | 72, 73 | |
| BMDACs (i.v.) | Improved alveolar structure Improved vascular growth |
Paracrine mechanisms | 74 | |
| LPS-induced (i.a.) lung injury (sheep) | hAECs (i.t.; i.v.) | Improved alveolar structure Increased surfactant protein expression Attenuated inflammation |
Immunomodulatory effects | 75 |
Abbreviations: AT2, alveolar epithelial type 2; BASC, bronchoalveolar stem cell; BMDAC, bone marrow–derived angiogenic cell; CdM, conditioned media; ECM, extracellular matrix; hAEC, human amnion epithelial cell; hUCB, human umbilical cord blood; i.a., intra-amniotic, i.p., intraperitoneal; i.t., intratracheal; i.v., intravenous; LPS, lipopolysaccharide; MSC, mesenchymal stem cell; ROS, reactive oxygen species; TGF-β1, transforming growth factor β1; TIMP-1, tissue inhibitor of metalloproteinase 1.
Mesenchymal Stem Cell Therapy in Experimental BPD
Administration of bone marrow–derived MSCs, either intratracheally, intravenously, or intraperitoneally, have ameliorated numerous aspects of neonatal lung injury, as evident by mitigation of lung inflammation, prevention of lung vascular damage and alveolar growth arrest, inhibition of lung fibrosis, and improvement in exercise tolerance (Fig. 3 ).52, 53, 70, 71 Low engraftment and differentiation of these MSCs into the injured neonatal lung suggest that the potential mechanisms through which MSCs exert their actions are paracrine mediated. These speculations are supported by in vitro and in vivo studies demonstrating that administration of CdM from MSCs has the ability to protect alveolar epithelial and lung microvascular endothelial cells from oxidative stress, prevent oxygen-induced alveolar growth arrest, and stimulate a subset of stem/progenitor cells, namely BASCs, to aid in lung repair.52, 53, 70 Furthermore, the therapeutic benefits of MSC-CdM may surpass those of MSCs, with in vivo findings indicating a more profound therapeutic effect of MSC-CdM in preventing and repairing lung injury than that of MSCs.70
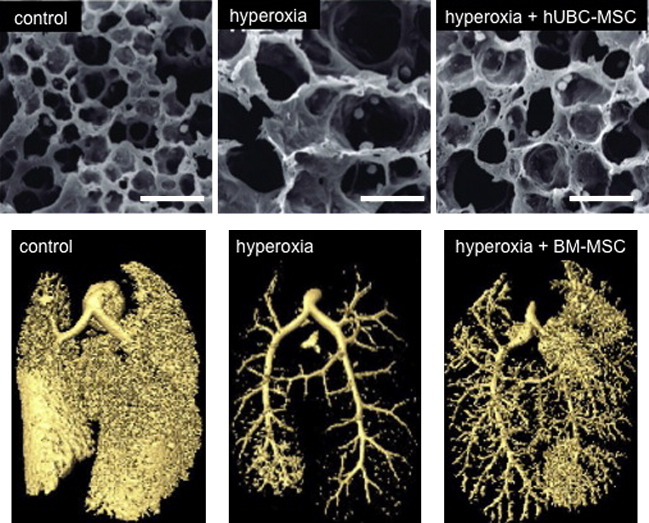
Fig. 3.
Therapeutic effects of bone marrow–derived MSCs and human umbilical cord (hUBC) blood-derived MSCs in experimental oxygen-induced BPD. Intratracheal delivery of MSCs derived from hUBC and from bone marrow (BM) improves hyperoxia-induced alveolar and lung vascular growth in neonatal rats, as demonstrated by electron microscopy (top panels) and micro–computed tomography (bottom panels) of the alveolar structure and pulmonary vasculature, respectively.
(Adapted from Chang YS, Oh W, Choi SJ, et al. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant 2009;18:869–86; with permission; and van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med 2009;180:1131–42; with permission.)
UCB also represents an appealing source of MSCs for therapeutic use in the newborn because of its clinically relevant, easily accessible, ethically viable, and readily available source of stem/progenitor cells. Chang and colleagues72, 73 demonstrated that MSCs obtained from human UCB prevent hyperoxia-induced alveolar growth arrest and alleviate fibrotic changes in the neonatal rat lung (see Fig. 3). Chang and colleagues73 also show that the route of administration may alter the outcome, with intratracheal transplantation resulting in a more prominent attenuation of hyperoxia-induced lung injury than intraperitoneal transplantation. Furthermore, Chang and colleagues72 recently demonstrated the dose-dependent effects of human UCB-derived MSCs in the oxygen-challenged neonatal rat lung. This study indicated that intratracheal delivery of a minimum of 5 × 104 cells is required to exhibit efficient anti-inflammatory, antifibrotic, and antioxidative effects following hyperoxia-induced lung injury in neonatal rats.72 In light of these findings, further studies determining the optimal dose of MSCs for potential clinical benefit in human neonates are anticipated.
Endothelial Progenitor Cell Therapy in Experimental BPD
The therapeutic potential of EPCs in neonatal lung injury has been effectively demonstrated in an oxygen-induced BPD mouse model.74 Treatment of neonatal mice exposed to hyperoxia with intravenously administered bone marrow–derived angiogenic cells (a population of bone marrow myeloid-like precursor cells) showed restoration of the alveolar structure and vessel density to that of control (room air–exposed) levels.74
Amnion Epithelial Cell Therapy in Experimental BPD
The therapeutic potential of human amnion epithelial cells (hAECs) has recently been investigated in a sheep model of neonatal lung injury, induced by lipopolysaccharide (LPS) administration in fetal sheep.75 Because hAECs are sourced from placentae, which are normally discarded after birth, they present an easily accessible and ethically viable candidate for cell therapy. Administration of hAECs to fetal sheep exposed to LPS attenuated inflammation-induced changes in lung function and structure, and reduced pulmonary inflammation.75 Of particular interest is the ability of hAECs to significantly increase the expression levels of SP-A and SP-C. The low engraftment into the lungs indicates that these hAECs act via immune modulation rather than cell engraftment and differentiation. More detailed assessment of the therapeutic potential of these cells in other models of neonatal lung injury will be of interest.
In summary, findings from several exciting studies indicate that a variety of stem/progenitor cells can prevent and/or regenerate neonatal lung injury in experimental models. Additional studies in different animal models of BPD are necessary to broaden the current knowledge and understanding of the therapeutic potential of stem/progenitor cells. In doing so, further evidence for creating a strong rationale for transitioning this potential breakthrough into clinic can be generated.
Remaining challenges
Although stem/progenitor cell therapies present potential promise in preventing and/or repairing lung injury, many gaps in our knowledge and understanding of stem cell biology in health and disease are yet to be filled (Box 1 ). In part, it is important to more precisely define putative reparative cells. One of the obstacles is the lack of biomarkers available for the characterization of candidate stem/progenitor cells in different species22 and the inability to easily study these cells in vivo.
Box 1. Future directions and questions for the use of stem/progenitor cells in neonatal lung injury.
Determine Optimal Stem Cell-Based Strategy for Lung Diseases
-
•What is the best reparative cell to treat a given lung disease?
-
○ESCs, MSCs, EPCs, amniotic fluid stem cells, amnion epithelial cells
-
○
-
•What is the appropriate strategy for cell-based therapies?
-
○Administration of exogenous stem cells: stem cells, stem cell–derived CdM, CdM-derived factors
-
○Protection of endogenous lung progenitor cells
-
○
-
•What is the best mode of delivery of stem cells for lung diseases?
-
○Intravenous
-
○Intratracheal
-
○
Long-Term Outcomes of Cell Therapy in BPD Models
-
•
Can stem/progenitor cell therapies permanently prevent/repair neonatal lung injury, or will continuous treatment be required for life?
-
•Would additional later in life “insults” reduce the protective effects of stem/progenitor cell therapies?
-
○Lung infection
-
○Smoking
-
○
-
•What are the effects of stem/progenitor cell therapies on short-term and long-term lung function outcomes?
-
○Baseline lung function
-
○Challenged lung function: exercise-induced, asthma/allergy-induced
-
○Age-induced decline in lung function
-
○
-
•What are the potential adverse effects of stem cell–based therapies?
-
○Tumor formation
-
○Ectopic tissue formation
-
○Immune rejection of the transplanted stem cells
-
○
Cell Therapy in Other Experimental Models of BPD
-
•What are the effects of stem/progenitor cell therapies in other relevant animal models of neonatal lung injury?
-
○Type of lung injury: ventilator-induced, inflammation/infection-induced
-
○Type of animal model: rodent, sheep, baboon
-
○
Current studies elegantly detail the short-term effects of stem/progenitor cell therapies in animal models of neonatal lung injury.52, 53, 70, 71, 72, 73, 74, 75 However, few of these studies have reported the long-term outcomes (ie, in mid-adult or aged lung) of such stem/progenitor cell therapies,52, 71 which is a vital and clinically important area of research that needs to be understood to warrant safe clinical translation.
In addition, it would be valuable to understand the effects of such stem/progenitor cell therapies in other animal models of neonatal lung injury closely mimicking the clinical setting (ie, ventilator-induced, fetal/neonatal inflammation-induced), rather than the frequently used hyperoxia-induced model; indeed, this is already being used by some.75
Current studies highlight the beneficial effects of stem/progenitor cell therapy on attenuating structural and/or molecular alterations to the injured developing lung, yet the effects on lung function are infrequently reported.52 This aspect of experimental studies requires further investigation and thorough documentation, because the overall aim of treating neonatal lung injury with stem/progenitor cells is to reduce and/or prevent lung dysfunction.
Summary
Half a century since the landmark discovery of stem cells by the Canadian researchers Till and McCulloch in 1961,76 their therapeutic potential in regenerative medicine is now being harnessed for treatment of neonatal lung injury, almost half a century since Northway and colleagues77 described BPD. Various types of stem/progenitor cells have shown benefit in experimental models of neonatal lung injury, with MSCs derived from both bone marrow and UCB being popularly studied, as well as CdM from these cells. However, before safe clinical translation of cell-based therapies is warranted, we must broaden our knowledge and understanding in this novel and exciting area of research. Strong emphasis must be placed on developing and standardizing techniques for stem/progenitor cell definition, isolation, expansion, and therapeutic administration. Experimental studies also need to focus on the long-term outcomes of such therapies. By identifying the most appropriate reparative cell(s) and its source, combined with understanding alternative mechanisms of action beyond cell replacement and assessing the short-term and long-term efficacy and safety, we can advance in the quest of providing therapeutic strategies to prevent and repair neonatal lung injury.
References
- 1.Henderson-Smart D.J., Hutchinson J.L., Donoghue D.A. Prenatal predictors of chronic lung disease in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 2006;91:F40–F45. doi: 10.1136/adc.2005.072264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinsella J.P., Greenough A., Abman S.H. Bronchopulmonary dysplasia. Lancet. 2006;367:1421–1431. doi: 10.1016/S0140-6736(06)68615-7. [DOI] [PubMed] [Google Scholar]
- 3.Brostrom E.B., Thunqvist P., Adenfelt G. Obstructive lung disease in children with mild to severe BPD. Respir Med. 2010;104:362–370. doi: 10.1016/j.rmed.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Doyle L.W., Faber B., Callanan C. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics. 2006;118:108–113. doi: 10.1542/peds.2005-2522. [DOI] [PubMed] [Google Scholar]
- 5.Filippone M., Bonetto G., Corradi M. Evidence of unexpected oxidative stress in airways of adolescents born very preterm. Eur Respir J. 2012 doi: 10.1183/09031936.00185511. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Narang I., Rosenthal M., Cremonesini D. Longitudinal evaluation of airway function 21 years after preterm birth. Am J Respir Crit Care Med. 2008;178:74–80. doi: 10.1164/rccm.200705-701OC. [DOI] [PubMed] [Google Scholar]
- 7.Smith L.J., van Asperen P.P., McKay K.O. Reduced exercise capacity in children born very preterm. Pediatrics. 2008;122:e287–e293. doi: 10.1542/peds.2007-3657. [DOI] [PubMed] [Google Scholar]
- 8.Cutz E., Chiasson D. Chronic lung disease after premature birth. N Engl J Med. 2008;358:743–745. doi: 10.1056/NEJMc073362. [author reply: 745–6] [DOI] [PubMed] [Google Scholar]
- 9.Wong P.M., Lees A.N., Louw J. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008;32:321–328. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 10.Yee M., Chess P.R., McGrath-Morrow S.A. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol. 2009;297:L641–L649. doi: 10.1152/ajplung.00023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jobe A.H. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–172. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blau H.M., Brazelton T.R., Weimann J.M. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson K., McGlynn L., Shiels P.G. Stem cells: outstanding potential and outstanding questions. Scott Med J. 2009;54:35–37. doi: 10.1258/rsmsmj.54.4.35. [DOI] [PubMed] [Google Scholar]
- 14.Stripp B.R. Hierarchical organization of lung progenitor cells: is there an adult lung tissue stem cell? Proc Am Thorac Soc. 2008;5:695–698. doi: 10.1513/pats.200801-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rawlins E.L., Hogan B.L. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 16.Giangreco A., Reynolds S.D., Stripp B.R. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim C.F., Jackson E.L., Woolfenden A.E. Identification of bronchoalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 18.McQualter J.L., Yuen K., Williams B. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawlins E.L., Clark C.P., Xue Y. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim C.F. Paving the road for lung stem cell biology: bronchoalveolar stem cells and other putative distal lung stem cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1092–L1098. doi: 10.1152/ajplung.00015.2007. [DOI] [PubMed] [Google Scholar]
- 21.Shi W., Xu J., Warburton D. Development, repair and fibrosis: what is common and why it matters. Respirology. 2009;14:656–665. doi: 10.1111/j.1440-1843.2009.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQualter J.L., Bertoncello I. Concise review: deconstructing the lung to reveal its regenerative potential. Stem Cells. 2012;30(5):811–816. doi: 10.1002/stem.1055. [DOI] [PubMed] [Google Scholar]
- 23.Bertoncello I., McQualter J.L. Endogenous lung stem cells: what is their potential for use in regenerative medicine? Expert Rev Respir Med. 2010;4:349–362. doi: 10.1586/ers.10.21. [DOI] [PubMed] [Google Scholar]
- 24.Liu X., Engelhardt J.F. The glandular stem/progenitor cell niche in airway development and repair. Proc Am Thorac Soc. 2008;5:682–688. doi: 10.1513/pats.200801-003AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borthwick D.W., Shahbazian M., Krantz Q.T. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 26.Hegab A.E., Ha V.L., Gilbert J.L. Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells. 2011;29:1283–1293. doi: 10.1002/stem.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong K.U., Reynolds S.D., Watkins S. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giangreco A., Shen H., Reynolds S.D. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L624–L630. doi: 10.1152/ajplung.00149.2003. [DOI] [PubMed] [Google Scholar]
- 29.Hong K.U., Reynolds S.D., Giangreco A. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 30.Volckaert T., Dill E., Campbell A. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest. 2011;121:4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder J.C., Teisanu R.M., Stripp B.R. Endogenous lung stem cells and contribution to disease. J Pathol. 2009;217:254–264. doi: 10.1002/path.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawlins E.L., Okubo T., Xue Y. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar P.A., Hu Y., Yamamoto Y. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adamson I.Y., Bowden D.H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974;30:35–42. [PubMed] [Google Scholar]
- 35.Brody J.S., Williams M.C. Pulmonary alveolar epithelial cell differentiation. Annu Rev Physiol. 1992;54:351–371. doi: 10.1146/annurev.ph.54.030192.002031. [DOI] [PubMed] [Google Scholar]
- 36.Driscoll B., Buckley S., Bui K.C. Telomerase in alveolar epithelial development and repair. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1191–L1198. doi: 10.1152/ajplung.2000.279.6.L1191. [DOI] [PubMed] [Google Scholar]
- 37.Reddy R., Buckley S., Doerken M. Isolation of a putative progenitor subpopulation of alveolar epithelial type 2 cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L658–L667. doi: 10.1152/ajplung.00159.2003. [DOI] [PubMed] [Google Scholar]
- 38.Majka S.M., Beutz M.A., Hagen M. Identification of novel resident pulmonary stem cells: form and function of the lung side population. Stem Cells. 2005;23:1073–1081. doi: 10.1634/stemcells.2005-0039. [DOI] [PubMed] [Google Scholar]
- 39.Martin J., Helm K., Ruegg P. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy. 2008;10:140–151. doi: 10.1080/14653240801895296. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds S.D., Shen H., Reynolds P.R. Molecular and functional properties of lung SP cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L972–L983. doi: 10.1152/ajplung.00090.2006. [DOI] [PubMed] [Google Scholar]
- 41.McQualter J.L., Brouard N., Williams B. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells. 2009;27:623–633. doi: 10.1634/stemcells.2008-0866. [DOI] [PubMed] [Google Scholar]
- 42.Herzog E.L., Chai L., Krause D.S. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 43.Liebler J.M., Lutzko C., Banfalvi A. Retention of human bone marrow-derived cells in murine lungs following bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L285–L292. doi: 10.1152/ajplung.00222.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rojas M., Xu J., Woods C.R. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotton D.N., Fine A. Lung stem cells. Cell Tissue Res. 2008;331:145–156. doi: 10.1007/s00441-007-0479-2. [DOI] [PubMed] [Google Scholar]
- 46.Neuringer I.P., Randell S.H. Stem cells and repair of lung injuries. Respir Res. 2004;5:6. doi: 10.1186/1465-9921-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thebaud B., Abman S.H. Bronchopulmonary dysplasia—where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarez D.F., Huang L., King J.A. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol. 2008;294:L419–L430. doi: 10.1152/ajplung.00314.2007. [DOI] [PubMed] [Google Scholar]
- 49.Alphonse R.S., Vadivel A., Waszak P. Existence, functional impairment and therapeutic potential of endothelial colony forming cells (ECFCS) in oxygen-induced arrested alveolar growth. Am J Respir Crit Care Med. 2011;183:A1237. [Google Scholar]
- 50.Balasubramaniam V., Mervis C.F., Maxey A.M. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–L1084. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- 51.Irwin D., Helm K., Campbell N. Neonatal lung side population cells demonstrate endothelial potential and are altered in response to hyperoxia-induced lung simplification. Am J Physiol Lung Cell Mol Physiol. 2007;293:L941–L951. doi: 10.1152/ajplung.00054.2007. [DOI] [PubMed] [Google Scholar]
- 52.van Haaften T., Byrne R., Bonnet S. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tropea K.A., Leder E., Aslam M. Bronchoalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302(9):L829–L837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popova A.P., Bozyk P.D., Bentley J.K. Isolation of tracheal aspirate mesenchymal stromal cells predicts bronchopulmonary dysplasia. Pediatrics. 2010;126:e1127–e1133. doi: 10.1542/peds.2009-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hennrick K.T., Keeton A.G., Nanua S. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med. 2007;175:1158–1164. doi: 10.1164/rccm.200607-941OC. [DOI] [PubMed] [Google Scholar]
- 56.Popova A.P., Bozyk P.D., Goldsmith A.M. Autocrine production of TGF-beta1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am J Physiol Lung Cell Mol Physiol. 2010;298:L735–L743. doi: 10.1152/ajplung.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pierro M., Thebaud B. Mesenchymal stem cells in chronic lung disease: culprit or savior? Am J Physiol Lung Cell Mol Physiol. 2010;298:L732–L734. doi: 10.1152/ajplung.00099.2010. [DOI] [PubMed] [Google Scholar]
- 58.Baker C.D., Ryan S.L., Ingram D.A. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med. 2009;180:454–461. doi: 10.1164/rccm.200901-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borghesi A., Massa M., Campanelli R. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2009;180:540–546. doi: 10.1164/rccm.200812-1949OC. [DOI] [PubMed] [Google Scholar]
- 60.Paviotti G., Fadini G.P., Boscaro E. Endothelial progenitor cells, bronchopulmonary dysplasia and other short-term outcomes of extremely preterm birth. Early Hum Dev. 2011;87:461–465. doi: 10.1016/j.earlhumdev.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 61.Dominici M., Le Blanc K., Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 62.Helmy K.Y., Patel S.A., Silverio K. Stem cells and regenerative medicine: accomplishments to date and future promise. Ther Deliv. 2010;1:693–705. doi: 10.4155/tde.10.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horwitz E.M., Prockop D.J., Fitzpatrick L.A. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 64.Vats A., Bielby R.C., Tolley N.S. Stem cells. Lancet. 2005;366:592–602. doi: 10.1016/S0140-6736(05)66879-1. [DOI] [PubMed] [Google Scholar]
- 65.Agostini C. Stem cell therapy for chronic lung diseases: hope and reality. Respir Med. 2010;104(Suppl 1):S86–S91. doi: 10.1016/j.rmed.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 66.Blaisdell C.J., Gail D.B., Nabel E.G. National Heart, Lung, and Blood Institute perspective: lung progenitor and stem cells—gaps in knowledge and future opportunities. Stem Cells. 2009;27:2263–2270. doi: 10.1002/stem.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sueblinvong V., Weiss D.J. Stem cells and cell therapy approaches in lung biology and diseases. Transl Res. 2010;156:188–205. doi: 10.1016/j.trsl.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee J.W., Gupta N., Serikov V. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther. 2009;9:1259–1270. doi: 10.1517/14712590903213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiss D.J., Bertoncello I., Borok Z. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2011;8:223–272. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aslam M., Baveja R., Liang O.D. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X., Wang H., Shi Y. The role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol Int. 2012;36(6):589–594. doi: 10.1042/CBI20110447. [DOI] [PubMed] [Google Scholar]
- 72.Chang Y.S., Choi S.J., Sung D.K. Intratracheal transplantation of human umbilical cord blood derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011 doi: 10.3727/096368911X565038. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 73.Chang Y.S., Oh W., Choi S.J. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18:869–886. doi: 10.3727/096368909X471189. [DOI] [PubMed] [Google Scholar]
- 74.Balasubramaniam V., Ryan S.L., Seedorf G.J. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L315–L323. doi: 10.1152/ajplung.00089.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vosdoganes P., Hodges R.J., Lim R. Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep. Am J Obstet Gynecol. 2011;205:156.e26–156.e33. doi: 10.1016/j.ajog.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 76.Till J.E., McCulloch E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 77.Northway W.H., Jr., Rosan R.C., Porter D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 78.Ling T.Y., Kuo M.D., Li C.L. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc Natl Acad Sci U S A. 2006;103:9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang D., Morales J.E., Calame D.G. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010;18:625–634. doi: 10.1038/mt.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumamoto M., Nishiwaki T., Matsuo N. Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur Respir J. 2009;34:740–748. doi: 10.1183/09031936.00128508. [DOI] [PubMed] [Google Scholar]
- 81.Ortiz L.A., Dutreil M., Fattman C. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moodley Y., Atienza D., Manuelpillai U. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175:303–313. doi: 10.2353/ajpath.2009.080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aguilar S., Scotton C.J., McNulty K. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS One. 2009;4:e8013. doi: 10.1371/journal.pone.0008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy S., Lim R., Dickinson H. Human amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung function. Cell Transplant. 2011;20:909–923. doi: 10.3727/096368910X543385. [DOI] [PubMed] [Google Scholar]
- 85.Gupta N., Su X., Popov B. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 86.Iyer S.S., Torres-Gonzalez E., Neujahr D.C. Effect of bone marrow-derived mesenchymal stem cells on endotoxin-induced oxidation of plasma cysteine and glutathione in mice. Stem Cells Int. 2010;2010:868076. doi: 10.4061/2010/868076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu J., Woods C.R., Mora A.L. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 88.Yang K.Y., Shih H.C., How C.K. IV delivery of induced pluripotent stem cells attenuates endotoxin-induced acute lung injury in mice. Chest. 2011;140:1243–1253. doi: 10.1378/chest.11-0539. [DOI] [PubMed] [Google Scholar]
- 89.Mei S.H., McCarter S.D., Deng Y. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu J., Qu J., Cao L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214:472–481. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 91.Kim E.S., Chang Y.S., Choi S.J. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir Res. 2011;12:108. doi: 10.1186/1465-9921-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chien M.H., Bien M.Y., Ku C.C. Systemic human orbital fat-derived stem/stromal cell transplantation ameliorates acute inflammation in lipopolysaccharide-induced acute lung injury. Crit Care Med. 2012;40:1245–1253. doi: 10.1097/CCM.0b013e31823bc89a. [DOI] [PubMed] [Google Scholar]
- 93.Lam C.F., Roan J.N., Lee C.H. Transplantation of endothelial progenitor cells improves pulmonary endothelial function and gas exchange in rabbits with endotoxin-induced acute lung injury. Anesth Analg. 2011;112:620–627. doi: 10.1213/ANE.0b013e3182075da4. [DOI] [PubMed] [Google Scholar]
- 94.Sun J., Han Z.B., Liao W. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti- and pro-inflammatory factors. Cell Physiol Biochem. 2011;27:587–596. doi: 10.1159/000329980. [DOI] [PubMed] [Google Scholar]
- 95.Gao X., Chen W., Liang Z. Autotransplantation of circulating endothelial progenitor cells protects against lipopolysaccharide-induced acute lung injury in rabbit. Int Immunopharmacol. 2011;11:1584–1590. doi: 10.1016/j.intimp.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 96.Curley G.F., Hayes M., Ansari B. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67(6):496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 97.Baber S.R., Deng W., Master R.G. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–H1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 98.Kanki-Horimoto S., Horimoto H., Mieno S. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114:I181–I185. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- 99.Raoul W., Wagner-Ballon O., Saber G. Effects of bone marrow-derived cells on monocrotaline- and hypoxia-induced pulmonary hypertension in mice. Respir Res. 2007;8:8. doi: 10.1186/1465-9921-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Umar S., de Visser Y.P., Steendijk P. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H1606–H1616. doi: 10.1152/ajpheart.00590.2009. [DOI] [PubMed] [Google Scholar]
- 101.Zhao Y.D., Courtman D.W., Deng Y. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 102.Takahashi M., Nakamura T., Toba T. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng. 2004;10:771–779. doi: 10.1089/1076327041348563. [DOI] [PubMed] [Google Scholar]
- 103.Cho K.S., Park H.K., Park H.Y. IFATS collection: immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells. 2009;27:259–265. doi: 10.1634/stemcells.2008-0283. [DOI] [PubMed] [Google Scholar]
- 104.Park H.K., Cho K.S., Park H.Y. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev. 2010;19:1811–1818. doi: 10.1089/scd.2009.0513. [DOI] [PubMed] [Google Scholar]
- 105.Firinci F., Karaman M., Baran Y. Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. Int Immunopharmacol. 2011;11:1120–1126. doi: 10.1016/j.intimp.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 106.Ou-Yang H.F., Huang Y., Hu X.B. Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med (Maywood) 2011;236:1461–1467. doi: 10.1258/ebm.2011.011221. [DOI] [PubMed] [Google Scholar]
- 107.Ionescu L.I., Alphonse R.S., Arizmendi N. Airway delivery of soluble factors from plastic-adherent bone marrow cells prevents murine asthma. Am J Respir Cell Mol Biol. 2012;46:207–216. doi: 10.1165/rcmb.2010-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nemeth K., Keane-Myers A., Brown J.M. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huh J.W., Kim S.Y., Lee J.H. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol. 2011;301:L255–L266. doi: 10.1152/ajplung.00253.2010. [DOI] [PubMed] [Google Scholar]
- 110.Zhen G., Liu H., Gu N. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci. 2008;13:3415–3422. doi: 10.2741/2936. [DOI] [PubMed] [Google Scholar]
- 111.Shigemura N., Okumura M., Mizuno S. Lung tissue engineering technique with adipose stromal cells improves surgical outcome for pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:1199–1205. doi: 10.1164/rccm.200603-406OC. [DOI] [PubMed] [Google Scholar]
- 112.Shigemura N., Okumura M., Mizuno S. Autologous transplantation of adipose tissue-derived stromal cells ameliorates pulmonary emphysema. Am J Transplant. 2006;6:2592–2600. doi: 10.1111/j.1600-6143.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 113.Katsha A.M., Ohkouchi S., Xin H. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2011;19:196–203. doi: 10.1038/mt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hegab A.E., Kubo H., Fujino N. Isolation and characterization of murine multipotent lung stem cells. Stem Cells Dev. 2010;19:523–536. doi: 10.1089/scd.2009.0287. [DOI] [PubMed] [Google Scholar]