Abstract
Lower respiratory tract infections by respiratory syncytial virus (RSV) are the foremost cause of infant hospitalization and are implicated in lasting pulmonary impairment and the development of asthma. Neutrophils infiltrate the airways of pediatric patients with RSV-induced bronchiolitis in vast numbers: approximately 80% of infiltrated cells are neutrophils. However, why neutrophils are recruited to the site of viral respiratory tract infection is not clear. In this review we discuss the beneficial and pathologic contributions of neutrophils to the immune response against RSV infection. Neutrophils can limit viral replication and spread, as well as stimulate an effective antiviral adaptive immune response. However, low specificity of neutrophil antimicrobial armaments allows for collateral tissue damage. Neutrophil-induced injury to the airways during the delicate period of infant lung development has lasting adverse consequences for pulmonary architecture and might promote the onset of asthma in susceptible subjects. We suggest that pharmacologic modulation of neutrophils should be explored as a viable future therapy for severe RSV-induced bronchiolitis and thereby prevent the inception of subsequent asthma. The antiviral functions of neutrophils suggest that targeting of neutrophils in patients with RSV-induced bronchiolitis is best performed under the umbrella of antiviral treatment.
Key words: Neutrophil, respiratory syncytial virus, asthma, respiratory syncytial virus bronchiolitis, lower respiratory tract infection, neutrophil extracellular traps, immunopathology, asthma prevention, immune regulation, treatment
Abbreviations used: DC, Dendritic cell; HNP, Human neutrophil peptide; LRTI, Lower respiratory tract infection; MMP, Matrix metalloproteinase; MPO, Myeloperoxidase; NET, Neutrophil extracellular trap; NK, Natural killer; PTX, Pentraxin; ROS, Reactive oxygen species; RSV, Respiratory syncytial virus; TLR, Toll-like receptor
Respiratory syncytial virus (RSV) is a ubiquitous seasonal human pathogen that, on infection of the upper respiratory tract, causes cold-like symptoms in most healthy adults and children. At 2 years of age, nearly all children will have been infected with RSV at least once. Lower respiratory tract infections (LRTIs) by RSV are a major cause of morbidity and mortality among infants. Annual RSV-associated mortality is estimated at a quarter million deaths per year, of which 99% occur in developing countries, and it accounts for approximately 7% of deaths among infants younger than 1 year of age.1 In the developed world 1% to 2% of infants are admitted to the hospital with RSV-induced bronchiolitis; therefore it is the foremost cause of infant hospitalization during the winter season.2, 3 Severe RSV-induced bronchiolitis in infancy is linked to impaired lung function in adulthood and causally implicated in the onset of recurrent wheezing and asthma.4, 5, 6, 7
Massive pulmonary neutrophil infiltration is observed in pediatric patients with RSV-induced bronchiolitis.8, 9, 10 In the lower airways neutrophils account for a median of 76% of infiltrated cells and a median of 93% in the upper airways, as measured in bronchoalveolar lavage fluid and nasopharyngeal aspirates, respectively.9 Similarly, postmortem histopathologic analyses of fatal RSV-related LRTI cases reveal extensive neutrophil infiltration of the airway wall and lumen, as well as the alveoli.11 This begs the question of why neutrophils are recruited en masse to the site of viral respiratory tract infection. A wide variety of inflammatory stimuli purposefully attract neutrophils to the lungs, such as to clear invading bacteria, apoptotic debris, or foreign substances. Could neutrophils also function protectively during RSV infection?
Neutrophils and RSV-induced bronchiolitis disease severity
Neutrophils, the most abundant leukocytes in human circulation, are classically portrayed as unsophisticated, first-line foot soldiers with a role limited to the engulfment and subsequent elimination of invading extracellular bacteria and fungi. In this view lung-infiltrated neutrophils seem out of place during RSV infection. The promiscuous cytotoxicity of neutrophil antimicrobial armaments might even potentiate virus-induced lung injury.12 Indeed, the degree of neutrophilic inflammation correlates with disease severity in patients with RSV-induced bronchiolitis.13 Moreover, a common single nucleotide polymorphism just upstream of the IL-8–encoding gene that is tentatively associated with increased production of IL-8, a potent neutrophil chemoattractant, is more frequent among infants with severe RSV-induced bronchiolitis, in particular among infants who lack other known risk factors.14 Increased IL-8 levels in the airways of patients with bronchiolitis are linked to increased disease severity, as measured based on oxygen saturation, Silverman score, and respiratory rate.15, 16 Genes related to neutrophil function, such as those encoding α-defensin-1 and elastase, are overexpressed in the blood of patients with RSV-induced bronchiolitis, and expression levels positively correlate with disease severity.17 Thus neutrophils are thought to contribute to lung injury in patients with severe RSV-induced bronchiolitis, but their exact role in pathogenesis is still unclear.
Clinical link between RSV-induced bronchiolitis and asthma inception
A large body of epidemiologic evidence, including prospective case-control and cohort studies, implicates RSV-induced bronchiolitis during infancy in the inception of recurrent wheeze and lasting lung function impairment.5, 6, 7, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 However, the association between wheeze and a history of RSV-induced bronchiolitis during infancy partially subsides with age.7, 32 It is not clear to what extent RSV-induced bronchiolitis is related to increased risk of asthma at school age or beyond. Nonetheless, nearly half of children with severe RSV-induced bronchiolitis present with asthma at 6 years of age.5 Estimates indicate that early-life RSV-induced bronchiolitis is responsible for up to 13% of childhood asthma cases.24 Moreover, smoking adults with RSV-induced bronchiolitis during infancy, but not those without LRTIs in early life, have an increased risk of persistent asthma.26 However, association does not necessarily imply causation. Severe RSV-induced bronchiolitis could occur mainly in infants with pre-existing susceptibility to asthma development. Common genetic and/or environmental factors appear to underlie predisposition to both diseases.21
We recently addressed the issue of causality in a randomized, double-blind, placebo-controlled clinical trial with RSV immunoprophylaxis of healthy preterm infants, in which we demonstrated a causal relationship between RSV-induced bronchiolitis and the development of recurrent wheeze.4 Although wheezing in early life is a strong risk factor for asthma in (early) adulthood,33 asthma trials are needed to provide conclusive evidence that RSV disease is causally related to persistent asthma.
Neutrophilic lung inflammation and asthma inception
Neutrophils play an important role in asthma exacerbations by inducing mucus hypersecretion and airway remodeling, which result in acute reversible and progressive irreversible airway obstruction, respectively.34, 35 Although neutrophils are thought to affect lung function decrease, their role in asthma inception during viral bronchiolitis is understudied. Nonetheless, the severity of infant bronchiolitis shows a dose-response relationship with the risk and morbidity of childhood asthma,22 and as discussed above, the degree of neutrophilic inflammation correlates with the severity of RSV-induced bronchiolitis.13, 15, 16 Therefore severe neutrophilic lung inflammation during infancy could tenably predispose children to subsequent wheezing and onset of asthma. Indeed, a functional IL-8 polymorphism more frequent among infants with severe RSV-induced bronchiolitis is also overrepresented in infants who had postbronchiolitis wheezing compared with patients with bronchiolitis who did not have wheezing afterward.14, 36 Neutrophilic inflammation–induced collateral tissue damage and airway sensitization could mediate the development of asthma susceptibility.
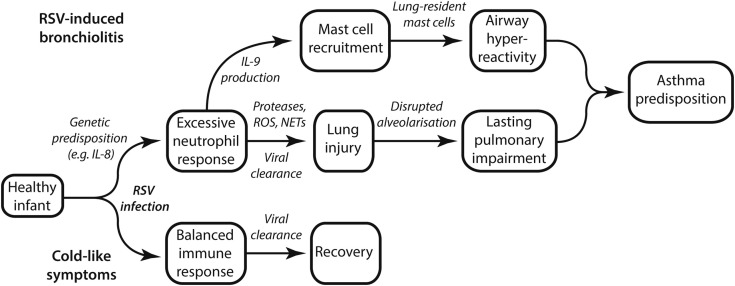
Transient damage to development of infant lungs can have far-reaching and lasting adverse consequences for pulmonary anatomy and function; this is particularly true if the critical process of alveolar multiplication by septation is disrupted.37 Elastin fibers, which are present in alveolar duct and septal walls, provide elasticity to the lungs and transmit cues of mechanical stress to stimulate alveolarization. Neutrophil elastase, which is released on neutrophil recruitment to the lungs, degrades elastin fibers and thereby demolishes lung tissue structure.38 Through elastase release and other lung-damaging mechanisms discussed below, the massive pulmonary neutrophil infiltrate of pediatric patients with RSV-induced bronchiolitis might thus cause irreparable disruption of lung development that predisposes these infants to subsequent asthma inception (Fig 1 ). In addition to inducing airway remodeling through collateral tissue damage, neutrophils might sensitize the airways to asthma through mast cell recruitment. IL-9 is detected in the bronchoalveolar lavage fluid of patients with RSV-induced bronchiolitis, and airway-infiltrated neutrophils are the principal producers.39 In response to pulmonary expression of IL-9, long-lived mast cells migrate to and accumulate in lung tissue.40, 41 Lung-resident mast cells, specifically those located in the bronchial smooth muscle bundles, contribute to airway hyperresponsiveness and are considered crucial to the pathogenesis of (allergic) asthma (Fig 1).42
Fig 1.
Schematic representation of the tentative relationship between neutrophil-induced lung damage and airway sensitization during RSV-induced bronchiolitis and subsequent susceptibility to asthma.
Dampening excessive neutrophilic inflammation during RSV-induced bronchiolitis might protect lung function, curtail airway sensitization, and reduce the risk of recurrent wheezing and asthma. A proof-of-concept, randomized, double-blind, placebo-controlled trial of macrolide treatment in patients with RSV-induced bronchiolitis provides tentative clinical support in favor of dampening neutrophilic inflammation.43 Macrolides have antineutrophilic activities in vitro and attenuate neutrophilic airway inflammation in patients with refractory asthma and during RSV infection in mice.44, 45, 46 In comparison with placebo-treated patients, macrolide-treated patients with RSV-induced bronchiolitis showed reduced nasal lavage IL-8 levels, experienced fewer days after bronchiolitis with respiratory symptoms, tended toward fewer subsequent wheezing episodes, and exhibited a delayed third wheezing episode.43
RSV immunoprophylaxis is costly and available only to high-risk infants, for whom it reduces RSV-related hospitalization by approximately 50%.47 For patients, supportive care is the sole treatment regimen available. Clearly, new treatment options for severe RSV-induced bronchiolitis are required to ameliorate disease and reduce sequelae, asthma in particular. The prominent neutrophilic inflammation in patients with RSV-induced bronchiolitis represents a promising target.
Below, we examine the role of neutrophils in patients with RSV infection and discuss the relevance thereof for therapy and asthma prevention. Because studies performed with RSV in this regard are scarce, we will also draw information from the literature on other viruses, in particular influenza virus, infections with which also exhibit severe pulmonary neutrophil infiltration.
Neutrophil-induced lung injury
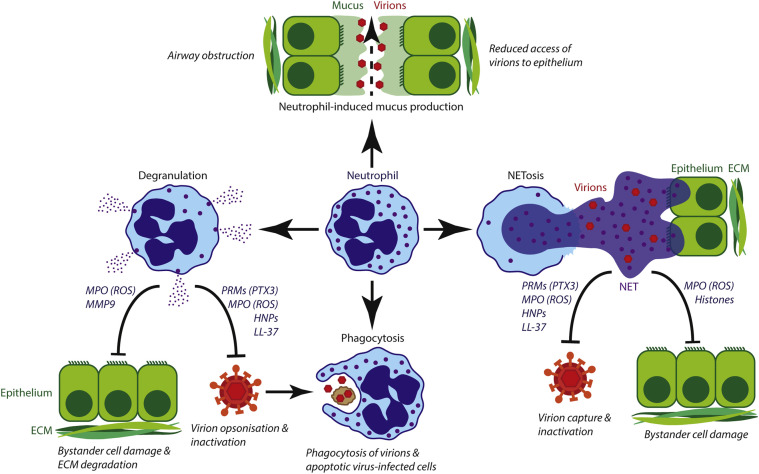
Neutrophils can injure the lungs in multiple ways: (1) release of proteolytic enzymes, including elastase, into the microenvironment through degranulation; (2) production of reactive oxygen species (ROS); (3) formation of neutrophil extracellular traps (NETs) by the cell death program known as NETosis; and (4) stimulation of mucus production (Fig 2 ).
Fig 2.
Protective and lung-injurious roles of neutrophils during respiratory tract viral infection. Neutrophil-induced mucus production limits access of viral particles to the epithelium but obstructs airflow. Degranulation releases antimicrobial mediators that are also cytotoxic to host cells. Phagocytosis of (opsonized) viral particles and virus-infected cells limits viral spread. NETs capture and deactivate viral particles but also damage healthy bystander cells. ECM, Extracellular matrix; PRMs, pattern recognition molecules.
Neutrophil migration into the airways during influenza infection in mice depends on the neutrophil tertiary granule-expressed matrix metalloproteinase (MMP) 9, which digests extracellular matrix. MMP9-mediated neutrophil influx into the airways is required for the control of influenza virus replication in mouse lungs.48 However, excessive MMP9 proteolytic activity in response to high viral loads exacerbates pathology.48 Pulmonary inflammation caused by noninfectious agents, such as cigarette smoke, implicate other neutrophil proteolytic enzymes, including cathepsin G, neutrophil elastase, and proteinase 3, in lung tissue destruction as well.49
The inflammatory milieu in the lungs during RSV infection induces the neutrophil oxidative burst.50 The abundantly produced ROS indiscriminately oxidize biomolecules, which compromises their function. Therefore ROS are potently antimicrobial but also damage host cellular structures. Oxidative stress during RSV infection promotes lung injury.51, 52 Thus neutrophil-derived ROS are likely to contribute to lung damage in patients with RSV infection.
NETs are DNA-based meshes decorated with histones and granular antimicrobial proteins and peptides, including elastase, myeloperoxidase (MPO), and α-defensins. By trapping microbes in NETs, microbial spread can be minimized and microbe killing optimized by high local concentrations of antimicrobial proteins and peptides.53 Exposure of neutrophils to RSV particles in vitro induces NET formation through specific interaction of RSV fusion (F) protein with neutrophil-expressed Toll-like receptor (TLR) 4.54 Data on the in vivo antiviral activity of NETs are conflicting.55 Genetic ablation of peptidylarginine deiminase 4, which is required for NET formation, did not affect viral replication or mortality in mice infected with influenza virus.56 Instead, NETs are located in areas of alveolar damage in the lungs of influenza virus–infected mice.57 In vitro NETs directly damage alveolar epithelial and endothelial cells. The histone component of NETs is chiefly responsible for this cytotoxic effect.58 This suggests that NETs induce lung injury rather than suppressing viral replication. However, the absence of NETs in peptidylarginine deiminase 4–deficient mice does not reduce lung pathology during influenza infection either.56 In this mouse model of human influenza virus infection, NETs appear to play neither a beneficial nor a pathologic role. The contribution of NETs during viral infection might be virus specific and depend on the degree of NET induction.
In addition to directly damaging the respiratory tract, neutrophils can contribute to airway obstruction by inducing mucus production. Mucus forms a protective barrier to viral infection by limiting access of viral particles to the pulmonary epithelium. However, excessive mucus production might cause airway obstruction. Mouse neutrophils promote the expression of mucin, the chief component of mucus, during RSV infection.59 Thus the extensive neutrophil infiltration in the lungs might contribute to the airway obstruction and resultant wheezing observed in patients with severe RSV-induced bronchiolitis.
Pulmonary IL-17 enhances neutrophil recruitment to the lungs early in RSV infection by inducing IL-8 production in airway epithelial cells.60 The amount of IL-17 in the lungs of pediatric patients with RSV-induced bronchiolitis correlates to the degree of subsequent pulmonary neutrophil influx.60 Additionally, IL-17 induces the production of obstructive mucus in the airways.61 This suggests that IL-17 plays a pathogenic role during LRTIs by RSV. The association of a polymorphism in the gene encoding IL-17 with severe RSV-induced bronchiolitis supports this notion.62 Moreover, the response of TH17 cells, noted producers of IL-17, is enhanced in infants with RSV-induced bronchiolitis.63 An RSV mouse model also links IL-17 and TH17 responses to RSV disease severity: pulmonary neutrophil recruitment and lung pathology are exacerbated in RSV-infected mice, which have increased TH17 responses caused by genetic ablation of the IL-27 receptor.64 Moreover, genetic ablation or antibody-mediated blocking of IL-17 in RSV-infected mice reduces neutrophilic inflammation, mucus production, and viral loads and improves the cytotoxic T-cell response against RSV.65 Nonetheless, it is not clear whether local IL-17 levels are protective or harmful. IL-17 reduces airway reactivity of RSV-infected mice presensitized with ovalbumin,66 and mice that received a novel attenuated Bordetella pertussis vaccine were protected from RSV infection in an IL-17–dependent fashion.67 It is possible that a limited IL-17 response is protective, whereas excessive IL-17 production induces immune pathology through unrestrained neutrophil recruitment. However, the early recruitment of neutrophils to the lungs of patients with RSV-induced bronchiolitis is unlikely to be mediated by an adaptive TH17 response because RSV antigen–driven clonal expansion and differentiation of TH17 cells require several days. Instead, there might be a role for natural TH17 cells that acquire effector functions during thymic development and regulate early-phase airway neutrophil responses.68, 69 Thus (natural) TH17 cells and IL-17 might promote a pathogenic neutrophil-dominated response to RSV infection.
Control of viral replication by neutrophils
The neutrophil antimicrobial arsenal, although damaging to the lungs if deployed excessively, has also shown efficacy against viruses in addition to its traditional bacterial and fungal targets. Based on this, a contribution of neutrophils to antiviral defense is suggested.55, 70, 71 Indeed, a beneficial role for neutrophils in patients with viral respiratory tract infections has been reported for influenza virus.72, 73, 74, 75 Neutrophil depletion in mice by neutrophil-specific anti-Ly6G antibody treatment results in enhanced viral replication and lung damage.72 Therefore neutrophil recruitment to the airways during RSV infection could also be a protective response. Neutrophils mediate direct antimicrobial effects through, in essence, 3 known effector mechanisms, namely (1) phagocytosis, (2) degranulation, and (3) NET formation (Fig 2 and Table I ).76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86
Table I.
Neutrophils possess direct antiviral activity
| Mechanism or molecular mediator | Effect and function | Virus/model | References |
|---|---|---|---|
| Phagocytosis | Eliminates virus-infected cells and virions | Influenza virus/in vivo | 76, 77 |
| HNP-1 | Inhibits viral entry into cell | HIV/in vitro | 78 |
| HNP-1 and HNP-2 | Promotes virion aggregation and phagocytosis | Influenza/in vitro | 79 |
| LL-37 | Inactivates virions, protects epithelial cells from infection and cell death, inhibits virion production | RSV/in vitro and clinical association | 80, 81 |
| PTX3 | Reduces virion infectivity | Influenza virus and MHV-1/in vitro and in vivo | 82, 83 |
| MPO | Inactivates virions through HClO production | HIV-1/in vitro | 84 |
| NETosis | Reduces viral infectivity and spread, inactivates virions | Influenza virus and myxoma poxvirus/in vitro and in vivo | 85, 86 |
Neutrophil effector mechanisms, neutrophil-derived molecules, and their respective antiviral activities are listed. The supporting lines of evidence and related references are included.
HClO, Hypochlorous acid; MHV-1, mouse hepatitis virus strain 1.
By means of phagocytosis, neutrophils can eliminate RSV-infected cells, thereby preventing further viral replication, and engulf virions to limit infection of new cells. Blocking the recognition and phagocytosis of apoptotic cells by local Annexin V administration 1 day after influenza virus inoculation increases mortality among mice.76 Both mouse macrophages and neutrophils phagocytose influenza virus–infected apoptotic cells in vivo.77 This process is amplified by TLR4,77 which recognizes RSV F protein expressed on the surfaces of virions and infected cells.87 Moreover, as shown in vitro for herpes simplex virus, antibody- and complement-mediated opsonization facilitates the phagocytosis of viral particles and virus-infected cells by neutrophils.88
Neutrophil granules contain antimicrobial proteins and peptides, including α-defensins 1 to 4 (or human neutrophil peptides [HNPs] 1-4), cathelicidins (ie, LL-37), pentraxin (PTX) 3, and MPO, which are released into the microenvironment on degranulation.89 Neutrophils degranulate in response to in vitro stimulation with RSV particles.90 HNPs are small, cationic amphipathic peptides that demonstrate antiviral activity. For instance, multiple steps of cellular entry by HIV-1 are inhibited by HNP-1,78 and HNPs promote influenza virus aggregation and uptake by neutrophils.79 Whether the antiviral activity of HNPs extends to RSV is not currently known, but the broad antiviral activities of HPNs against multiple enveloped and nonenveloped viruses hints at the possibility.91
Recent in vitro work demonstrates that the cationic antimicrobial peptide LL-37 possesses direct antiviral activity against RSV virions, protects infected epithelial cells against RSV-induced cell death, inhibits production of new viral particles, and reduces the susceptibly of epithelial cells to RSV infection.80 Low serum levels of LL-37 precursor (ie, hCAP-18) are associated with severe infantile RSV-induced bronchiolitis.81
PTXs are soluble pattern recognition molecules that recognize pathogen-associated molecular patterns and mediate innate humoral immunity, functionally resembling antibodies of the adaptive immune system. PTX3 can activate compliment through recruitment of C1q (ie, the classical pathway) and act as opsonins by interacting with the FcγRIII (CD16) and FcγRII (CD32) receptors, which are expressed by phagocytes, including neutrophils.92 Mature neutrophils represent a major reservoir of preformed ready-to-use PTX3, as well as other pattern recognition molecules.93 Direct evidence of PTX3-mediated antiviral activity against RSV is lacking but has been demonstrated for influenza virus and mouse coronavirus. PTX3 reduces the infectivity of both viruses. Moreover, PTX3-deficient mice inoculated with either virus fare worse than their wild-type counterparts but can be rescued by administration of exogenous PTX3.82, 83
MPO catalyzes the production of hypochlorous acid, which is a potent antimicrobial ROS, because of its ability to chlorinate and oxidize a great variety of biomolecules. Secreted and NET-bound MPO deactivates HIV-1 particles in vitro.84, 85 The RSV F protein induces formation of MPO-coated NETs through TLR4,54 and HIV-1 triggers NETosis through a TLR7/TLR8- and ROS-dependent pathway. NET-bound MPO and HNPs abolish the infectivity of HIV-1 particles. In coculture neutrophils reduce the infection of CD4+ T cells by HIV-1 in a NET-dependent manner.85 Thus NETs are elicited by stimulation with virus and possess direct antiviral activity in vitro.
Furthermore, the release of NETs by neutrophils during intravenous myxoma poxvirus challenge in mice protects against viral infection of liver cells.86 Therefore NETs can benefit antiviral defense in vivo. As discussed above, NETosis deficiency does not affect the course of disease in human influenza virus-inoculated mice.56 A contribution, protective or damaging, of NETs to antiviral defense might be virus and organ (eg, liver vs lung) specific.
Neutrophil-mediated immune modulation
Recent studies demonstrate multiple immune regulatory functions for neutrophils. In addition to their role as effector cells in patients with RSV infection described above, neutrophils can also act as immune regulatory cells (Fig 3 ). Although neutrophil-mediated immune regulation in the context of RSV infection has yet to be examined directly, general concepts can be derived from studies with other viruses and guide further research into the severely understudied role of neutrophils in patients with RSV infection. Here we focus on regulatory roles potentially involved in RSV infection. For a more complete overview, the reader is referred to a number of recent reviews.89, 93, 94, 95
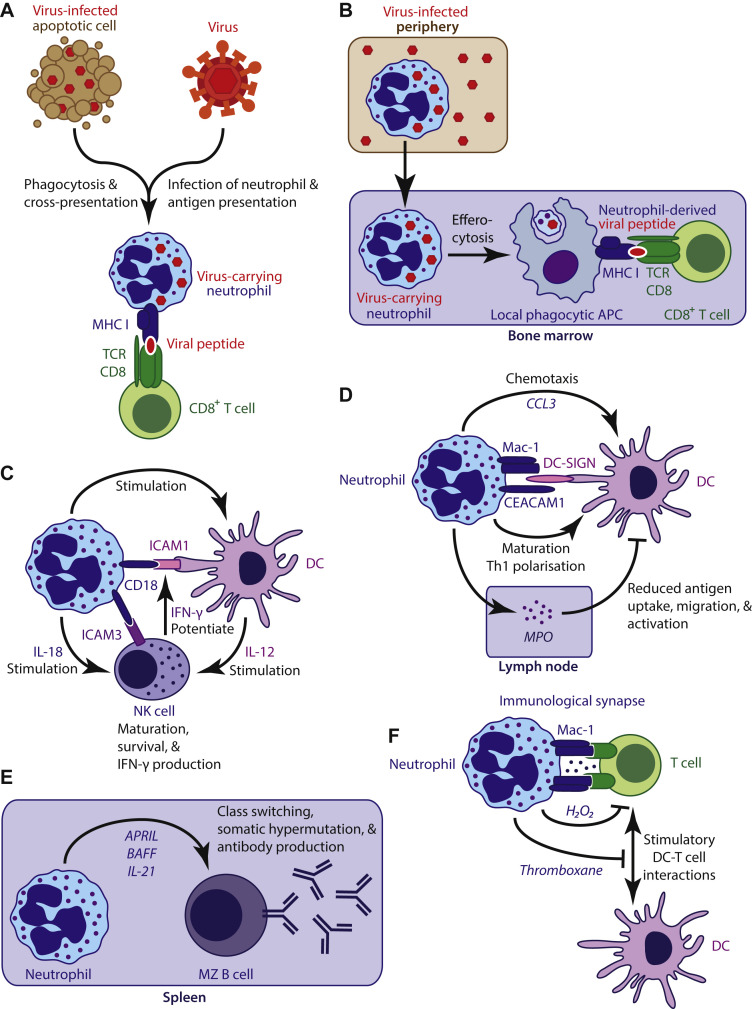
Fig 3.
Neutrophils regulate immune responses. A and B, Neutrophils directly and indirectly present viral antigen to elicit CD8+ T-cell responses. C, In collaboration with DCs, neutrophils maintain NK cell homeostasis. D, Neutrophils recruit, activate, and polarize DCs but can also suppress DC activity. E, Splenic neutrophils produce cytokines to induce immunoglobulin class-switch recombination, somatic hypermutation, and antibody production in marginal zone B cells. F, T-cell activity can be suppressed by neutrophils through direct interactions with T cells and by limiting stimulatory interactions between T cells and DCs. APC, Antigen-presenting cell; APRIL, a proliferation-inducing ligand; BAFF, B cell–activating factor of the TNF family; CEACAM1, carcinoembryonic antigen-related cell adhesion molecule 1; DC-SIGN, dendritic cell–specific intercellular adhesion molecule 3–grabbing nonintegrin; MZ, marginal zone; TCR, T-cell receptor.
Neutrophils can promote antiviral CD8+ T-cell responses. Influenza virus antigen–displaying neutrophils stimulate lung-infiltrated CD8+ T cells to produce IFN-γ, whereas infected epithelial cells only elicit their own cytolysis.96, 97 Stimulation of IFN-γ production by neutrophils can enhance antiviral defense against RSV infection.98 The CD8+ T-cell response to influenza in the airways of mice is maintained by infiltrated neutrophils.75 Neutrophils thus regulate CD8+ T-cell effector function at the site of viral infection. Neutrophils efficiently cross-present phagocytosed antigen to naive CD8+ T cells in vivo to stimulate proliferation, IFN-γ production, and cytolytic activity.99 By acting as viral antigen shuttles, neutrophils can indirectly induce an antiviral CD8+ T-cell response. Mouse neutrophils take up modified vaccinia Ankara virus in skin, transport viral antigen to the bone marrow, and there, through local phagocytic antigen-presenting cells, trigger virus-specific CD8+ T-cell proliferation and establishment of memory.100 Moreover, neutrophils can indirectly stimulate T-cell responses by recruiting immature dendritic cells (DCs) to a site of infection through CCL3 secretion101 and by inducing DC maturation through direct cell-cell contact, which depends on altered glycosylation patterns of Mac-1 (CD11b/CD18) and carcinoembryonic antigen-related cell adhesion molecule 1 (CAECAM1) that are specific to activated neutrophils.102, 103
The homeostasis of innate cytotoxic lymphocytes (ie, natural killer [NK] cells) is neutrophil dependent.104, 105, 106 Neutropenic patients and neutrophil-depleted mice present with immature hyporesponsive NK cells.104 Neutrophils stimulate NK cells through IL-18 production and the interaction of CD18 with NK cell–expressed intercellular adhesion molecule 3. Moreover, neutrophils stimulate DCs to produce NK cell–stimulatory IL-12 through CD18/intercellular adhesion molecule (ICAM) 1 interaction.105, 106 IFN-γ produced by activated NK cells potentiates the interaction between neutrophils and DCs, thus creating a neutrophil-dependent positive feedback loop that supports NK cell maturation, survival, and IFN-γ production.105
The adaptive humoral response is stimulated by neutrophils as well. Through cytokine secretion (ie, B cell–activating factor of the TNF family [BAFF], a proliferation inducing ligand [APRIL], and IL-21), splenic neutrophils induce immunoglobulin class-switch recombination, somatic hypermutation, and antibody production by marginal zone B cells.107
Neutrophils can also suppress adaptive immune responses. Systemic inflammation in human subjects induces a neutrophil subset with T cell–suppressive capabilities. These suppressive neutrophils form Mac-1–dependent immunologic synapses with T cells and locally produce bursts of membrane-associated hydrogen peroxide (H2O2) that suppress T-cell proliferation.108 Furthermore, neutrophil-derived thromboxane limits T-cell cytokine production in response to protein-in-adjuvant vaccination of mice and suppresses the spread of the T-cell response from the draining lymph nodes of the injection site to distal lymph nodes.109 Neutrophils can indirectly suppress T-cell responses through deposition of MPO in lymph nodes, which impairs DC function.110
In a more passive manner neutrophils might contribute to the resolution of inflammation. Depending on environmental stimuli, macrophages can acquire a proinflammatory or proresolution phenotype. The phagocytosis of apoptotic neutrophils by macrophages induces a regulatory phenotype in macrophages, which might contribute to the resolution of inflammation.111 In RSV-infected cotton rats, proresolution macrophages limit lung pathology.112
Neutrophils, RSV-induced bronchiolitis, and asthma: Remaining caveats
Is it conceivable that neutrophils are abundantly present in the airways during severe RSV infection without playing a major role in either the pathogenesis of bronchiolitis or the subsequent inception of asthma? Although indicators of neutrophilic inflammation, including airway IL-8 levels and neutrophil elastase activity, correlate with the severity of bronchiolitis, cell counts of airway-infiltrated neutrophils, which are invariably high, are not associated with RSV disease severity.10, 13, 15, 16, 113 Similar to RSV-induced bronchiolitis, extensive neutrophil infiltration of the airways and alveoli is observed during bacterial pneumonia, usually in the absence of wheezing. If neutrophils contribute to the inception of asthma after bronchiolitis, an increased risk of asthma should also be observed after bacterial respiratory tract infections in early childhood. Evidence in support of this is limited to observations in a high-risk birth cohort.31 Future studies should attempt to validate the relationship between bacterial respiratory tract infection during infancy and the subsequent development of reactive airway disease.
Concluding remarks and implications for therapy
The vast number of lung-infiltrating neutrophils in patients with RSV-induced bronchiolitis appears to be the principal instigator of pulmonary immunopathology. The secretion of proteolytic enzymes, such as elastase, the production of ROS, and NET release can clearly injure the lungs. This might disrupt the delicate process of lung development in infancy and have lasting adverse consequences for lung anatomy, function, and susceptibility to chronic disease, including asthma. However, through direct antiviral activity of these and other neutrophil mechanisms, as well as through regulation of adaptive immune responses, neutrophils can also contribute to a protective antiviral response. In patients with severe RSV-induced bronchiolitis, the balance between neutrophil antimicrobial activity and collateral tissue damage appears to have shifted toward a pathologic response. Hence pharmacologic suppression of neutrophil activity could curtail lung injury. Possible approaches include (1) neutrophil-inhibitory macrolides43, 46; (2) inhibiting damaging neutrophil-derived products, such as elastase114; (3) antagonizing neutrophil-modulatory adenosine115; (4) neutrophil chemokine receptor blockade116, 117; (5) and targeting neutrophil-expressed inhibitory receptors.118, 119 We eagerly await the results of future clinical trials for the treatment of RSV infection with pharmacologic agents that can effectively dampen neutrophilic inflammation, such as the selective small-molecule CXCR2 (IL-8 receptor) antagonist danirixin (ClinicalTrials.gov identifier: NCT02201303).117
The relationship between extensive neutrophil infiltration of the airways in early life and later asthma development might not be limited to RSV infection. Lower respiratory tract illnesses in infants induced by other viruses are also associated with subsequent wheeze and asthma.7, 31, 120 Limited evidence also hints at a causal association between bacterial invasion of the respiratory tract and subsequent asthma development.121, 122, 123 Colonization of neonatal airways by pathogenic bacteria induces neutrophil response–associated cytokines and is associated with development of persistent wheezing and childhood asthma in a prospective birth cohort.124, 125 Moreover, among prospectively followed high-risk children, respiratory tract infections by pathogenic bacteria in early life were associated with an increased risk of school-age asthma.31 In fact, rather than a specific viral or bacterial pathogen, the total number of early respiratory tract infections correlated with the risk of childhood asthma in this study.31
Although injurious when present in excess, neutrophils are essential to antimicrobial defense. The safety of treating RSV-induced bronchiolitis with neutrophil suppression could improve with the administration of RSV antivirals and prophylactic antibiotics. Recently, a newly developed RSV antiviral was successfully tested in a clinical trial wherein healthy adult subjects were challenged with RSV.126 By itself, however, an RSV antiviral might confer limited benefit when administered several days after onset of symptoms. For influenza, oseltamivir is only effective when administered within 36 to 48 hours after symptom onset.127 Similarly, antiviral treatments against RSV should ideally be administered as close to initial infection as possible to be at their most effective, yet RSV loads are already on the decrease when patients are admitted to the intensive care unit.128 Nonetheless, neutrophils are still abundantly present in the lungs,9, 10, 11 and thus neutrophil-mediated damage to the airways persists (Fig 2). Therefore we propose that suppressing neutrophil activity under the umbrella of RSV antivirals and prophylactic antibiotics offers a promising treatment strategy to improve the outcomes of infants with RSV-induced bronchiolitis and reduce the risk of subsequent asthma.
Footnotes
R.J.G. and J.P. are supported by the Netherlands Scientific Organization (NWO Graduate Programme 2012, 022.004.018) and Lung Foundation Netherlands (grant 5.2.14.058JO), respectively.
Disclosure of potential conflict of interest: R.J. Geerdink has received research support from the Netherlands Organisation for Scientific Research (NWO; grant 022.004.018). J. Pillay has received research support from Lung Foundation Netherlands (grant 5.2.14.058JO). L. Meyaard has received research support from the Netherlands Organisation for Scientific Research (NWO), the NWO/ALW Open Programme, the Dutch Arthritis Foundation, and NWO Vici and has consultant arrangements with Novo Nordisk. L. Bont has consultant arrangements with AbbVie, MedImmune, Johnson & Johnson, Ablynx, AIT, and Alios; has received research support from AbbVie, MedImmune, and Johnson & Johnson; and has received payment for lectures from AbbVie.
References
- 1.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth R.L., Openshaw P.J. Bronchiolitis. Lancet. 2006;368:312–322. doi: 10.1016/S0140-6736(06)69077-6. [DOI] [PubMed] [Google Scholar]
- 3.Hervás D., Reina J., Yañez A., del Valle J.M., Figuerola J., Hervás J.A. Epidemiology of hospitalization for acute bronchiolitis in children: differences between RSV and non-RSV bronchiolitis. Eur J Clin Microbiol Infect Dis. 2012;31:1975–1981. doi: 10.1007/s10096-011-1529-y. [DOI] [PubMed] [Google Scholar]
- 4.Blanken M.O., Rovers M.M., Bont L. Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze. N Engl J Med. 2013;369:782–783. doi: 10.1056/NEJMc1307429. [DOI] [PubMed] [Google Scholar]
- 5.Bacharier L.B., Cohen R., Schweiger T., Yin-Declue H., Christie C., Zheng J. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2012;130:91–93. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backman K., Piippo-Savolainen E., Ollikainen H., Koskela H., Korppi M. Adults face increased asthma risk after infant RSV bronchiolitis and reduced respiratory health-related quality of life after RSV pneumonia. Acta Paediatr. 2014;103:850–855. doi: 10.1111/apa.12662. [DOI] [PubMed] [Google Scholar]
- 7.Stein R.T., Sherrill D., Morgan W.J., Holberg C.J., Halonen M., Taussig L.M. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 8.Everard M.L., Milner A.D. The respiratory syncytial virus and its role in acute bronchiolitis. Eur J Pediatr. 1992;151:638–651. doi: 10.1007/BF01957564. [DOI] [PubMed] [Google Scholar]
- 9.Everard M.L., Swarbrick A., Wrightham M., McIntyre J., Dunkley C., James P.D. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994;71:428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNamara P.S., Ritson P., Selby A., Hart C.A., Smyth R.L. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child. 2003;88:922–926. doi: 10.1136/adc.88.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welliver T.P., Garofalo R.P., Hosakote Y., Hintz K.H., Avendano L., Sanchez K. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardoel B.W., Kenny E.F., Sollberger G., Zychlinsky A. The balancing act of neutrophils. Cell Host Microbe. 2014;15:526–536. doi: 10.1016/j.chom.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Yasui K., Baba A., Iwasaki Y., Kubo T., Aoyama K., Mori T. Neutrophil-mediated inflammation in respiratory syncytial viral bronchiolitis. Pediatr Int. 2005;47:190–195. doi: 10.1111/j.1442-200x.2005.02039.x. [DOI] [PubMed] [Google Scholar]
- 14.Hull J., Thomson A., Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55:1023–1027. doi: 10.1136/thorax.55.12.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marguet C., Bocquel N., Benichou J., Basuyau J.P., Hellot M.F., Couderc L. Neutrophil but not eosinophil inflammation is related to the severity of a first acute epidemic bronchiolitis in young infants. Pediatr Allergy Immunol. 2008;19:157–165. doi: 10.1111/j.1399-3038.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 16.Smyth R.L., Mobbs K.J., O'Hea U., Ashby D., Hart C.A. Respiratory syncytial virus bronchiolitis: Disease severity, interleukin-8, and virus genotype. Pediatr Pulmonol. 2002;33:339–346. doi: 10.1002/ppul.10080. [DOI] [PubMed] [Google Scholar]
- 17.Mejias A., Dimo B., Suarez N.M., Garcia C., Suarez-Arrabal M.C., Jartti T. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 2013;10:e1001549. doi: 10.1371/journal.pmed.1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigurs N., Aljassim F., Kjellman B., Robinson P.D., Sigurbergsson F., Bjarnason R. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 19.Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 20.Gern J.E., Rosenthal L.A., Sorkness R.L., Lemanske R.F. Effects of viral respiratory infections on lung development and childhood asthma. J Allergy Clin Immunol. 2005;115:668–675. doi: 10.1016/j.jaci.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stensballe L.G., Simonsen J.B., Thomsen S.F., Larsen A.M., Lysdal S.H., Aaby P. The causal direction in the association between respiratory syncytial virus hospitalization and asthma. J Allergy Clin Immunol. 2009;123:131–137. doi: 10.1016/j.jaci.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 22.Carroll K.N., Wu P., Gebretsadik T., Griffin M.R., Dupont W.D., Mitchel E.F. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–1061.e1. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholas C., Wegienka G., Havstad S., Zoratti E., Ownby D., Johnson C.C. Are severe lung infections in the first 2 years of life related to lung function at 18 years. J Allergy Clin Immunol. 2011;127:AB136. [Google Scholar]
- 24.James K.M., Gebretsadik T., Escobar G.J., Wu P., Carroll K.N., Li S.X. Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J Allergy Clin Immunol. 2013;132:227–229. doi: 10.1016/j.jaci.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zomer-Kooijker K., van der Ent C.K., Ermers M. Increased risk of wheeze and decreased lung function after respiratory syncytial virus infection. PLoS One. 2014;9:e87162. doi: 10.1371/journal.pone.0087162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voraphani N., Stern D.A., Wright A.L., Guerra S., Morgan W.J., Martinez F.D. Risk of current asthma among adult smokers with respiratory syncytial virus illnesses in early life. Am J Respir Crit Care Med. 2014;190:392–398. doi: 10.1164/rccm.201311-2095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman A.S., He Y., Moore M.L., Hershenson M.B., Hartert T.V. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med. 2015;191:34–44. doi: 10.1164/rccm.201405-0901PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu P., Hartert T.V. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9:731–745. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B., Björkstén B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 30.Henderson J., Hilliard T.N., Sherriff A., Stalker D. Shammari Al N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16:386–392. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 31.Bønnelykke K., Vissing N.H., Sevelsted A., Johnston S.L., Bisgaard H. Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol. 2015;136:81–86.e4. doi: 10.1016/j.jaci.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Régnier S.A., Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32:820–826. doi: 10.1097/INF.0b013e31829061e8. [DOI] [PubMed] [Google Scholar]
- 33.Stern D.A., Morgan W.J., Halonen M., Wright A.L., Martinez F.D. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louis R., Djukanovic R. Is the neutrophil a worthy target in severe asthma and chronic obstructive pulmonary disease? Clin Exp Allergy. 2006;36:563–567. doi: 10.1111/j.1365-2222.2006.02493.x. [DOI] [PubMed] [Google Scholar]
- 35.Beeh K.M., Beier J. Handle with care: targeting neutrophils in chronic obstructive pulmonary disease and severe asthma? Clin Exp Allergy. 2006;36:142–157. doi: 10.1111/j.1365-2222.2006.02418.x. [DOI] [PubMed] [Google Scholar]
- 36.Goetghebuer T., Isles K., Moore C., Thomson A., Kwiatkowski D., Hull J. Genetic predisposition to wheeze following respiratory syncytial virus bronchiolitis. Clin Exp Allergy. 2004;34:801–803. doi: 10.1111/j.1365-2222.2004.1947.x. [DOI] [PubMed] [Google Scholar]
- 37.Le Cras T.D., Hardie W.D., Deutsch G.H., Albertine K.H., Ikegami M., Whitsett J.A. Transient induction of TGF-alpha disrupts lung morphogenesis, causing pulmonary disease in adulthood. Am J Physiol Lung Cell Mol Physiol. 2004;287:L718–L729. doi: 10.1152/ajplung.00084.2004. [DOI] [PubMed] [Google Scholar]
- 38.Cavarra E., Martorana P.A., Gambelli F., de Santi M., van Even P., Lungarella G. Neutrophil recruitment into the lungs is associated with increased lung elastase burden, decreased lung elastin, and emphysema in alpha 1 proteinase inhibitor-deficient mice. Lab Invest. 1996;75:273–280. [PubMed] [Google Scholar]
- 39.McNamara P.S., Flanagan B.F., Baldwin L.M., Newland P., Hart C.A., Smyth R.L. Interleukin 9 production in the lungs of infants with severe respiratory syncytial virus bronchiolitis. Lancet. 2004;363:1031–1037. doi: 10.1016/S0140-6736(04)15838-8. [DOI] [PubMed] [Google Scholar]
- 40.Kearley J., Erjefalt J.S., Andersson C., Benjamin E., Jones C.P., Robichaud A. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011;183:865–875. doi: 10.1164/rccm.200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sehra S., Yao W., Nguyen E.T., Glosson-Byers N.L., Akhtar N., Zhou B. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 2015;136:433–440. doi: 10.1016/j.jaci.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradding P., Walls A.F., Holgate S.T. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 43.Beigelman A., Isaacson-Schmid M., Sajol G., Baty J., Rodriguez O.M., Leege E. Randomized trial to evaluate azithromycin's effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135:1171–1178.e1. doi: 10.1016/j.jaci.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedlander A.L., Albert R.K. Chronic macrolide therapy in inflammatory airways diseases. Chest. 2010;138:1202–1212. doi: 10.1378/chest.10-0196. [DOI] [PubMed] [Google Scholar]
- 45.Beigelman A., Mikols C.L., Gunsten S.P., Cannon C.L., Brody S.L., Walter M.J. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir Res. 2010;11:90. doi: 10.1186/1465-9921-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson J.L., Powell H., Boyle M.J., Scott R.J., Gibson P.G. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177:148–155. doi: 10.1164/rccm.200707-1134OC. [DOI] [PubMed] [Google Scholar]
- 47.Mejias A., Ramilo O. Review of palivizumab in the prophylaxis of respiratory syncytial virus (RSV) in high-risk infants. Biologics. 2008;2:433–439. doi: 10.2147/btt.s3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradley L.M., Douglass M.F., Chatterjee D., Akira S., Baaten B.J. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathog. 2012;8:e1002641. doi: 10.1371/journal.ppat.1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guyot N., Wartelle J., Malleret L., Todorov A.A., Devouassoux G., Pacheco Y. Unopposed cathepsin G, neutrophil elastase, and proteinase 3 cause severe lung damage and emphysema. Am J Pathol. 2014;184:2197–2210. doi: 10.1016/j.ajpath.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 50.Bataki E.L., Evans G.S., Everard M.L. Respiratory syncytial virus and neutrophil activation. Clin Exp Immunol. 2005;140:470–477. doi: 10.1111/j.1365-2249.2005.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hosakote Y.M., Liu T., Castro S.M., Garofalo R.P., Casola A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am J Respir Cell Mol Biol. 2009;41:348–357. doi: 10.1165/rcmb.2008-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hosakote Y.M., Jantzi P.D., Esham D.L., Spratt H., Kurosky A., Casola A. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2011;183:1550–1560. doi: 10.1164/rccm.201010-1755OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 54.Funchal G.A., Jaeger N., Czepielewski R.S., Machado M.S., Muraro S.P., Stein R.T. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS One. 2015;10:e0124082. doi: 10.1371/journal.pone.0124082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jenne C.N., Kubes P. Virus-induced NETs—critical component of host defense or pathogenic mediator? PLoS Pathog. 2015;11:e1004546. doi: 10.1371/journal.ppat.1004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hemmers S., Teijaro J.R., Arandjelovic S., Mowen K.A. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011;6:e22043. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.A. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saffarzadeh M., Juenemann C., Queisser M.A., Lochnit G., Barreto G., Galuska S.P. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stokes K.L., Currier M.G., Sakamoto K., Lee S., Collins P.L., Plemper R.K. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. J Virol. 2013;87:10070–10082. doi: 10.1128/JVI.01347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stoppelenburg A.J., Salimi V., Hennus M., Plantinga M., Huis In 't Veld R., Walk J. Local IL-17A potentiates early neutrophil recruitment to the respiratory tract during severe RSV infection. PLoS One. 2013;8:e78461. doi: 10.1371/journal.pone.0078461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Newcomb D.C., Boswell M.G., Sherrill T.P., Polosukhin V.V., Boyd K.L., Goleniewska K. IL-17A induces signal transducers and activators of transcription-6-independent airway mucous cell metaplasia. Am J Respir Cell Mol Biol. 2013;48:711–716. doi: 10.1165/rcmb.2013-0017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janssen R., Bont L., Siezen C.L., Hodemaekers H.M., Ermers M.J., Doornbos G. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis. 2007;196:826–834. doi: 10.1086/520886. [DOI] [PubMed] [Google Scholar]
- 63.Stoppelenburg A.J., de Roock S., Hennus M.P., Bont L., Boes M. Elevated Th17 response in infants undergoing respiratory viral infection. Am J Pathol. 2014;184:1274–1279. doi: 10.1016/j.ajpath.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 64.De Almeida Nagata D.E., Demoor T., Ptaschinski C., Ting H.A., Jang S., Reed M. IL-27R-mediated regulation of IL-17 controls the development of respiratory syncytial virus-associated pathogenesis. Am J Pathol. 2014;184:1807–1818. doi: 10.1016/j.ajpath.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukherjee S., Lindell D.M., Berlin A.A., Morris S.B., Shanley T.P., Hershenson M.B. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol. 2011;179:248–258. doi: 10.1016/j.ajpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Newcomb D.C., Boswell M.G., Reiss S., Zhou W., Goleniewska K., Toki S. IL-17A inhibits airway reactivity induced by respiratory syncytial virus infection during allergic airway inflammation. Thorax. 2013;68:717–723. doi: 10.1136/thoraxjnl-2012-202404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schnoeller C., Roux X., Sawant D., Raze D., Olszewska W., Locht C. Attenuated Bordetella pertussis vaccine protects against respiratory syncytial virus disease via an IL-17-dependent mechanism. Am J Respir Crit Care Med. 2014;189:194–202. doi: 10.1164/rccm.201307-1227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanaka S., Yoshimoto T., Naka T., Nakae S., Iwakura Y., Cua D. Natural occurring IL-17 producing T cells regulate the initial phase of neutrophil mediated airway responses. J Immunol. 2009;183:7523–7530. doi: 10.4049/jimmunol.0803828. [DOI] [PubMed] [Google Scholar]
- 69.Kim J.S., Sklarz T., Banks L.B., Gohil M., Waickman A.T., Skuli N. Natural and inducible TH17 cells are regulated differently by Akt and mTOR pathways. Nat Immunol. 2013;14:611–618. doi: 10.1038/ni.2607. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Gabriel C., Her Z., Ng L.F. Neutrophils: neglected players in viral diseases. DNA Cell Biol. 2013;32:665–675. doi: 10.1089/dna.2013.2211. [DOI] [PubMed] [Google Scholar]
- 71.Drescher B., Bai F. Neutrophil in viral infections, friend or foe? Virus Res. 2013;171:1–7. doi: 10.1016/j.virusres.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tate M.D., Deng Y.M., Jones J.E., Anderson G.P., Brooks A.G., Reading P.C. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol. 2009;183:7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- 73.Dienz O., Rud J.G., Eaton S.M., Lanthier P.A., Burg E., Drew A. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5:258–266. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tate M.D., Ioannidis L.J., Croker B., Brown L.E., Brooks A.G., Reading P.C. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One. 2011;6:e17618. doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tate M.D., Brooks A.G., Reading P.C., Mintern J.D. Neutrophils sustain effective CD8(+) T-cell responses in the respiratory tract following influenza infection. Immunol Cell Biol. 2012;90:197–205. doi: 10.1038/icb.2011.26. [DOI] [PubMed] [Google Scholar]
- 76.Watanabe Y., Hashimoto Y., Shiratsuchi A., Takizawa T., Nakanishi Y. Augmentation of fatality of influenza in mice by inhibition of phagocytosis. Biochem Biophys Res Commun. 2005;337:881–886. doi: 10.1016/j.bbrc.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 77.Hashimoto Y., Moki T., Takizawa T., Shiratsuchi A., Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- 78.Demirkhanyan L.H., Marin M., Padilla-Parra S., Zhan C., Miyauchi K., Jean-Baptiste M. Multifaceted mechanisms of HIV-1 entry inhibition by human α-defensin. J Biol Chem. 2012;287:28821–28838. doi: 10.1074/jbc.M112.375949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tecle T., White M.R., Gantz D., Crouch E.C., Hartshorn K.L. Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J Immunol. 2007;178:8046–8052. doi: 10.4049/jimmunol.178.12.8046. [DOI] [PubMed] [Google Scholar]
- 80.Currie S.M., Findlay E.G., McHugh B.J., Mackellar A., Man T., Macmillan D. The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS One. 2013;8:e73659. doi: 10.1371/journal.pone.0073659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mansbach J.M., Piedra P.A., Borregaard N., Martineau A.R., Neuman M.I., Espinola J.A. Serum cathelicidin level is associated with viral etiology and severity of bronchiolitis. J Allergy Clin Immunol. 2012;130:1007–1008.e1. doi: 10.1016/j.jaci.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reading P.C., Bozza S., Gilbertson B., Tate M., Moretti S., Job E.R. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J Immunol. 2008;180:3391–3398. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 83.Han B., Ma X., Zhang J., Zhang Y., Bai X., Hwang D.M. Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab Invest. 2012;92:1285–1296. doi: 10.1038/labinvest.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klebanoff S.J., Coombs R.W. Viricidal effect of polymorphonuclear leukocytes on human immunodeficiency virus-1. Role of the myeloperoxidase system. J Clin Invest. 1992;89:2014–2017. doi: 10.1172/JCI115810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 86.Jenne C.N., Wong C.H., Zemp F.J., McDonald B., Rahman M.M., Forsyth P.A. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13:169–180. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 87.Kurt-Jones E.A., Popova L., Kwinn L., Haynes L.M., Jones L.P., Tripp R.A. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 88.Van Strijp J.A., Van Kessel K.P., van der Tol M.E., Fluit A.C., Snippe H., Verhoef J. Phagocytosis of herpes simplex virus by human granulocytes and monocytes. Arch Virol. 1989;104:287–298. doi: 10.1007/BF01315550. [DOI] [PubMed] [Google Scholar]
- 89.Amulic B., Cazalet C., Hayes G.L., Metzler K.D., Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 90.Jaovisidha P., Peeples M.E., Brees A.A., Carpenter L.R., Moy J.N. Respiratory syncytial virus stimulates neutrophil degranulation and chemokine release. J Immunol. 1999;163:2816–2820. [PubMed] [Google Scholar]
- 91.Wilson S.S., Wiens M.E., Smith J.G. Antiviral mechanisms of human defensins. J Mol Biol. 2013;425:4965–4980. doi: 10.1016/j.jmb.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bottazzi B., Doni A., Garlanda C., Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 93.Mantovani A., Cassatella M.A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 94.Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scapini P., Cassatella M.A. Social networking of human neutrophils within the immune system. Blood. 2014;124:710–719. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 96.Hufford M.M., Kim T.S., Sun J., Braciale T.J. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J Exp Med. 2011;208:167–180. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hufford M.M., Richardson G., Zhou H., Manicassamy B., García-Sastre A., Enelow R.I. Influenza-infected neutrophils within the infected lungs act as antigen presenting cells for anti-viral CD8(+) T cells. PLoS One. 2012;7:e46581. doi: 10.1371/journal.pone.0046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boelen A., Kwakkel J., Barends M., de Rond L., Dormans J., Kimman T. Effect of lack of Interleukin-4, Interleukin-12, Interleukin-18, or the Interferon-gamma receptor on virus replication, cytokine response, and lung pathology during respiratory syncytial virus infection in mice. J Med Virol. 2002;66:552–560. doi: 10.1002/jmv.2180. [DOI] [PubMed] [Google Scholar]
- 99.Beauvillain C., Delneste Y., Scotet M., Peres A., Gascan H., Guermonprez P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110:2965–2973. doi: 10.1182/blood-2006-12-063826. [DOI] [PubMed] [Google Scholar]
- 100.Duffy D., Perrin H., Abadie V., Benhabiles N., Boissonnas A., Liard C. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity. 2012;37:917–929. doi: 10.1016/j.immuni.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 101.Charmoy M., Brunner-Agten S., Aebischer D., Auderset F., Launois P., Milon G. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 2010;6:e1000755. doi: 10.1371/journal.ppat.1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Gisbergen K.P., Sanchez-Hernandez M., Geijtenbeek T.B., van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J Exp Med. 2005;201:1281–1292. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Gisbergen K.P., Ludwig I.S., Geijtenbeek T.B., van Kooyk Y. Interactions of DC-SIGN with Mac-1 and CEACAM1 regulate contact between dendritic cells and neutrophils. FEBS Lett. 2005;579:6159–6168. doi: 10.1016/j.febslet.2005.09.089. [DOI] [PubMed] [Google Scholar]
- 104.Jaeger B.N., Donadieu J., Cognet C., Bernat C., Ordoñez-Rueda D., Barlogis V. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J Exp Med. 2012;209:565–580. doi: 10.1084/jem.20111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Costantini C., Calzetti F., Perbellini O., Micheletti A., Scarponi C., Lonardi S. Human neutrophils interact with both 6-sulfo LacNAc+ DC and NK cells to amplify NK-derived IFN{gamma}: role of CD18, ICAM-1, and ICAM-3. Blood. 2011;117:1677–1686. doi: 10.1182/blood-2010-06-287243. [DOI] [PubMed] [Google Scholar]
- 106.Spörri R., Joller N., Hilbi H., Oxenius A. A novel role for neutrophils as critical activators of NK cells. J Immunol. 2008;181:7121–7130. doi: 10.4049/jimmunol.181.10.7121. [DOI] [PubMed] [Google Scholar]
- 107.Puga I., Cols M., Barra C.M., He B., Cassis L., Gentile M. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pillay J., Kamp V.M., van Hoffen E., Visser T., Tak T., Lammers J.W. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang C.W., Unanue E.R. Neutrophils control the magnitude and spread of the immune response in a thromboxane A2-mediated process. J Exp Med. 2013;210:375–387. doi: 10.1084/jem.20122183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Odobasic D., Kitching A.R., Yang Y., O'Sullivan K.M., Muljadi R.C., Edgtton K.L. Neutrophil myeloperoxidase regulates T-cell-driven tissue inflammation in mice by inhibiting dendritic cell function. Blood. 2013;121:4195–4204. doi: 10.1182/blood-2012-09-456483. [DOI] [PubMed] [Google Scholar]
- 111.Filardy A.A., Pires D.R., Nunes M.P., Takiya C.M., Freire-de-Lima C.G., Ribeiro-Gomes F.L. Proinflammatory clearance of apoptotic neutrophils induces an IL-12(low)IL-10(high) regulatory phenotype in macrophages. J Immunol. 2010;185:2044–2050. doi: 10.4049/jimmunol.1000017. [DOI] [PubMed] [Google Scholar]
- 112.Shirey K.A., Pletneva L.M., Puche A.C., Keegan A.D., Prince G.A., Blanco J.C. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal Immunol. 2010;3:291–300. doi: 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sheeran P., Jafri H., Carubelli C., Saavedra J., Johnson C., Krisher K. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999;18:115–122. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 114.Henriksen P.A. The potential of neutrophil elastase inhibitors as anti-inflammatory therapies. Curr Opin Hematol. 2014;21:23–28. doi: 10.1097/MOH.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 115.Barletta K.E., Ley K., Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32:856–864. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hay D.W., Sarau H.M. Interleukin-8 receptor antagonists in pulmonary diseases. Curr Opin Pharmacol. 2001;1:242–247. doi: 10.1016/s1471-4892(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 117.Miller B.E., Smart K., Mistry S., Ambery C.L., Bloomer J.C., Connolly P. The pharmacokinetics of conventional and bioenhanced tablet formulations of danirixin (GSK1325756) following oral administration in healthy, elderly, human volunteers. Eur J Drug Metab Pharmacokinet. 2014;39:173–181. doi: 10.1007/s13318-014-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Steevels T.A., Van Avondt K., Westerlaken G.H., Stalpers F., Walk J., Bont L. Signal inhibitory receptor on leukocytes-1 (SIRL-1) negatively regulates the oxidative burst in human phagocytes. Eur J Immunol. 2013;43:1297–1308. doi: 10.1002/eji.201242916. [DOI] [PubMed] [Google Scholar]
- 119.Van Avondt K., Fritsch-Stork R., Derksen R.H., Meyaard L. Ligation of signal inhibitory receptor on leukocytes-1 suppresses the release of neutrophil extracellular traps in systemic lupus erythematosus. PLoS One. 2013;8:e78459. doi: 10.1371/journal.pone.0078459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Calışkan M., Bochkov Y.A., Kreiner-Møller E., Bønnelykke K., Stein M.M., Du G. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beigelman A., Weinstock G.M., Bacharier L.B. The relationships between environmental bacterial exposure, airway bacterial colonization, and asthma. Curr Opin Allergy Clin Immunol. 2014;14:137–142. doi: 10.1097/ACI.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomas A.O., Lemanske R.F., Jackson D.J. Infections and their role in childhood asthma inception. Pediatr Allergy Immunol. 2014;25:122–128. doi: 10.1111/pai.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kloepfer K.M., Lee W.M., Pappas T.E., Kang T.J., Vrtis R.F., Evans M.D. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–1307. doi: 10.1016/j.jaci.2014.02.030. e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bisgaard H., Hermansen M.N., Buchvald F., Loland L., Halkjaer L.B., Bønnelykke K. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 125.Følsgaard N.V., Schjørring S., Chawes B.L., Rasmussen M.A., Krogfelt K.A., Brix S. Pathogenic bacteria colonizing the airways in asymptomatic neonates stimulates topical inflammatory mediator release. Am J Respir Crit Care Med. 2013;187:589–595. doi: 10.1164/rccm.201207-1297OC. [DOI] [PubMed] [Google Scholar]
- 126.Devincenzo J.P., Whitley R.J., Mackman R.L., Scaglioni-Weinlich C., Harrison L., Farrell E. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371:711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 127.Nguyen-Van-Tam J.S., Venkatesan S., Muthuri S.G., Myles P.R. Neuraminidase inhibitors: who, when, where? Clin Microbiol Infect. 2015;21:222–225. doi: 10.1016/j.cmi.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 128.Lukens M.V., van de Pol A.C., Coenjaerts F.E., Jansen N.J., Kamp V.M., Kimpen J.L. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J Virol. 2010;84:2374–2383. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]