Abstract
Background
Allergic reactions to Hymenoptera insect stings remain a major global clinical problem. Although effective, parenteral desensitization regimens require use of costly venom extracts and require frequent visits over extended periods of time.
Objective
Adjuvants are commonly used to enhance the efficacy of infectious disease vaccines, and this study asked whether Advax (Vaxine Pty Ltd, Adelaide, Australia), a novel noninflammatory polysaccharide adjuvant, might provide similar benefits for allergy desensitization.
Methods
A randomized, controlled phase 1/2 trial was undertaken in 27 adults with a history of rapid-onset systemic allergic reactions to honeybee stings and positive specific IgE levels to evaluate the safety and efficacy of honeybee venom immunotherapy (HBVIT) combined with Advax adjuvant. Venom immunotherapy (VIT) was administered monthly for 30 months after achievement of maintenance doses.
Results
Advax-adjuvanted HBVIT was well tolerated. Around week 14 of VIT, specific IgG4 responses peaked in both groups but increased earlier, peaked higher, and were better maintained through the end of the study in the Advax-adjuvanted arm. Several different patterns of serologic response to VIT were seen; some subjects had a dominant IgG4 response, some had a combined IgG4 and IgG1 response, and some had an exclusively IgG1 response. In some subjects specific IgE levels increased during the induction phase and then decreased, whereas in others specific IgE levels progressively decreased from the start of VIT.
Conclusion
Advax adjuvant favorably enhanced the immunogenicity of HBVIT, with an early and prolonged switch to specific IgG4 production. The ability of Advax adjuvant to enhance VIT efficacy warrants further study.
Key words: Hymenoptera, anaphylaxis, immunotherapy, IgG4, inulin, adjuvant, allergy
Abbreviations used: AHBVIT, Honeybee venom immunotherapy combined with Advax adjuvant; CRP, C-reactive protein; HBVIT, Honeybee venom immunotherapy; MCT, Mast cell tryptase; SARS, Severe acute respiratory syndrome; SHBVIT, Standard honeybee venom immunotherapy; VIT, Venom immunotherapy; WCC, White cell count
Graphical abstract
Anaphylactic reactions to Hymenoptera insect stings are a major clinical problem in many parts of the world. Although venom immunotherapy (VIT) can be effective at preventing life-threatening reactions to future stings,1, 2, 3 it can itself cause severe and even (although rare) life-threatening reactions. It is also less effective for honeybee than Vespula species wasp stings4 or Myrmecia pilosula (jack jumper ant) stings, the second most common cause of sting anaphylaxis in South Australia.3 Furthermore, during the course of honeybee venom immunotherapy (HBVIT), immediate systemic reactions, including mild anaphylactic reactions, are common. In large US4 and European5 studies, the rate of systemic reactions was ~40% for subjects undergoing HBVIT. This is similar to the systemic reactions we saw to VIT with jumper ant venom, where the rate was 34%3 by comparison to the lower rate of systemic reaction rates to VIT with vespid venoms in Northern Hemisphere studies.4, 5
The median time taken to achieve a maintenance dose of HBVIT was 14 weeks in a US study of outpatient VIT,6 which is comparable with our experience of about 10 weeks with cluster regimens. Attempts to accelerate the achievement of maintenance doses using “ultrarush” inpatient regimens have had variable effects on reaction rates, interpretation of which is confounded by comparisons being nonrandomized and generally noncontemporaneous, but with some studies suggesting that reaction rates to VIT are even greater on such regimens.7, 8 Reactions to HBVIT are generally more severe and frequent than for vespid VIT.9 A minority of subjects receiving HBVIT do not achieve a maintenance dose of HBVIT, this proportion being quoted as 9% in the retrospective study of Lockey et al6 but in our experience more typically in the range of 5%, leaving this subgroup more susceptible to recurrent sting-induced anaphylaxis.10 Even when subjects have been established on dose regimens for which there is literature evidence of high probability of protection from sting-induced anaphylaxis, questions remain regarding the optimal duration of VIT. There is a propensity, once VIT has been ceased, for the susceptibility to sting-induced anaphylaxis to recur with each further sting.11, 12 The 2016 update of the US Practice Parameters suggests that VIT can be discontinued after 5 years in “low-risk” patients (2/3 of patients on VIT) who will have a less than 3% chance of systemic reaction to subsequent stings, whereas extended or indefinite treatment is suggested for “high-risk” patients (about 1/3 of patients) who have a greater than 40% chance of relapse.13 There is limited evidence that abolition of detectable specific IgE responses to the venom is a marker for protection from sting-induced anaphylaxis. In the studies by Golden's group,14, 15 only a minority of subjects lost skin test reactivity to venom after 5 to 8 years of VIT. After approximately 6 years of VIT, only 25% had negative skin test responses, whereas 70% of responses became negative after 9.6 years of follow-up.15 Thus up to one third of subjects might either need to indefinitely continue VIT, to continue to carry automated adrenaline syringes, or both.
In Australia the commercially available honeybee venom in general use is Albey Bee Venom (Apis mellifera; Hollister-Stier, Spokane, Wash), which is a soluble extract. Several European studies have administered Hymenoptera venoms with alum adjuvant.16, 17, 18 Although alum reduced local adverse reactions to VIT, presumably by binding and delaying allergen release, it has not been shown to accelerate desensitization or reduce the required venom dose. Alum also has potential disadvantages for allergy therapy given its propensity to enhance IgE production and eosinophilia, the opposite of what is desired from an ideal allergy immunotherapy adjuvant.19
Advax (Vaxine Pty Ltd, Adelaide, Australia) is a new adjuvant derived from the natural plant-based polysaccharide delta inulin, which was developed through the National Institutes of Health's Adjuvant Development Program.19 Advax has been shown to provide enhanced vaccine immunogenicity and antigen sparing while having low reactogenicity.20 It drives a strong TH1 response that might help suppress any excess TH2 bias in allergic patients. Notably, when formulated with Advax, vaccines against severe acute respiratory syndrome (SARS) were able to suppress eosinophilic lung pathology after SARS coronavirus infection, whereas this problem was aggravated by formulation of SARS vaccine with the TH2-biasing alum adjuvant.20 Advax adjuvant has been shown to be safe and effective in human vaccine trials,21, 22, 23, 24 and we sought, therefore, to test the hypothesis that Advax adjuvant would enhance the effectiveness of HBVIT.
Methods
Study design
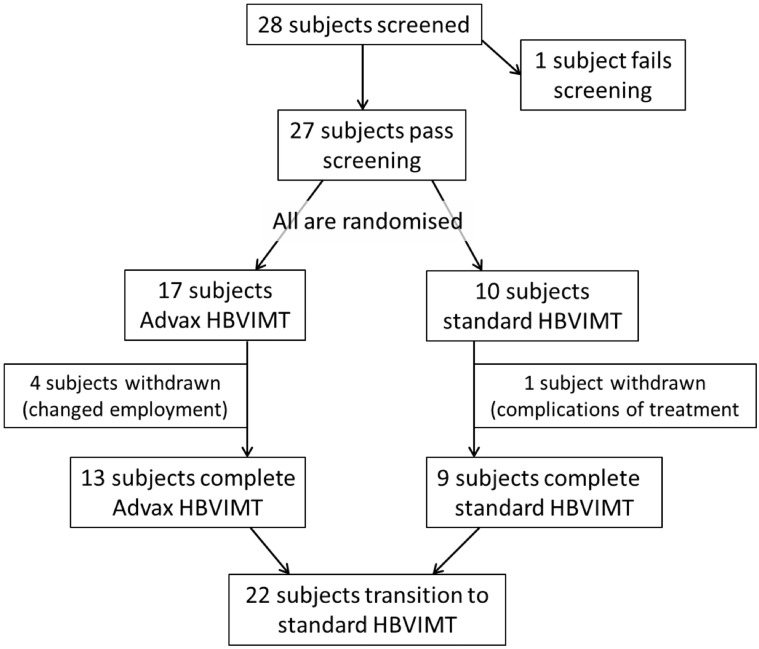
Healthy adults with a history of rapid-onset systemic allergic reactions to honeybee venom and a positive test result for serum specific IgE (>0.35 KU/L Thermo Fisher CAP) were recruited. They were randomized in a 1:2 ratio to cluster (semirush) HBVIT administered by means of subcutaneous injection as either standard honeybee venom immunotherapy (SHBVIT) or combined with Advax adjuvant (AHBVIT; Fig 1 ). The study was approved by the Human Ethics Committees of Flinders Medical Centre and the Royal Adelaide Hospital. It started in May 2008, with the last participant completed in March 2015. In 2018, subsequently, ethics approval was obtained to recall participants to obtain an additional blood sample for measurement of specific IgE and IgG4 levels, 3 to 7 years after participants completed active trial therapy.
Fig 1.
CONSORT diagram of study participants and procedures.
Study participants
Twenty-seven adult subjects aged between 18 and 65 years with a history of a rapid-onset systemic allergic reactions to a bee sting and positive specific IgE levels (>0.35 KU/L Thermo Fisher CAP test) were enrolled. Subjects were excluded if they had previously undergone HBVIT, had baseline mast cell tryptase (MCT) levels of greater than 20 μg/L, were pregnant or lactating, were women of childbearing potential unless using a reliable and appropriate contraceptive method, were receiving concurrent systemic immunosuppressive therapy, had a history of intravenous drug or alcohol abuse, were participating in another clinical trial with an investigational agent within 30 days preceding initiation of treatment, or had any other serious medical, social, or mental conditions that, in the opinion of the investigator, would be detrimental to the subjects or the study. None of the subjects were on treatment with a β-blocker or angiotensin-converting enzyme inhibitor.
VIT administration
Albey Bee Venom was reconstituted with diluent (albumin saline with phenol preservative), as instructed by the manufacturer, and then stored and used within expiration dates according to the manufacturer's recommendations. Advax adjuvant (delta inulin, 50 mg/mL) without preservative in single-dose vials was mixed with honeybee venom under aseptic conditions within 6 hours of use. The scheduled injection was administered as a deep subcutaneous injection into the upper outer arm or abdominal skin (depending on patient preference), according to the schedule in Table E1 in this article's Online Repository at www.jacionline.org. Because Advax–honeybee venom mixtures are opaque and standard honeybee venom is clear, administrator blinding was impractical, and therefore injections were administered by nursing staff aware of the group allocation, whereas both patients and medical investigators remained blind to study group.
Pulse, blood pressure, respiratory rate, oxygen saturation, and skin status (general and at the injection site) were recorded before and 20 minutes after each injection before leaving the clinic (45 minutes after the last injection of attendance) and immediately if symptomatic.
Clinically significant reactions were defined as follows:
-
1.
Immediate systemic hypersensitivity reactions were classified (grade 1, 2, or 3, specifically mild, moderate, or severe) according to the criteria of Brown,25 which are based on evidence of a relationship between particular manifestations and progression to hypotension, hypoxia, or both.
-
2.
Excessive local reactions at injection sites were classified as mild (local swelling, 40-60 mm), moderate (local swelling, 61-100 mm), or severe (local swelling, >100 mm and/or extending across the joint and/or causing significant disability).
-
3.
Systemic reactions and other than immediate hypersensitivity reactions were classified as mild (not interfering significantly with usual activities or requiring treatment other than with oral antihistamines, simple mild analgesics, or both), moderate (fever >38.0°C and short-term [1- to 2-day] interference with usual activities but not meeting criteria for severe), or severe (fever >39.0°C and reaction requiring hospital treatment or admission).
Consistent with usual clinical practice, the schedule was varied as follows:
-
1.
For excessive local reactions, the last dose was repeated, or if the reaction had extended beyond a joint (elbow), the last tolerated dose was given.
-
2.
For mild generalized allergic reactions, at the discretion of the investigator, the dose was repeated or reduced to the last tolerated dose.
-
3.
For more significant generalized reactions, the dose was reduced to the last tolerated dose. At the discretion of the investigator, further increments were reduced.
Laboratory tests
Safety blood tests, including full blood estimation, liver function tests, and measurement of serum MCT, urea and electrolyte, C-reactive protein (CRP), complement C3 and C4, antinuclear antibody, and rheumatoid factor values were assayed by a National Association of Testing Authorities Australia-certified commercial pathology service (SA Pathology, Adelaide, Australia) and collected before commencement of the study, after 4 weeks of VIT (full blood estimation, liver function tests, and urea and electrolyte measurement only), when maintenance was reached (around week 14) and after 30 months of maintenance therapy. Blood collection to assess specific IgE and IgG4 levels was performed immediately before VIT commencement and then at 2, 4, and 8 weeks after starting VIT, on reaching maintenance, and thereafter every third month until the end of the 30-month study follow-up period. Specific IgE and IgG4 levels were measured by using the ImmunoCAP Phadia assay (Thermo Scientific, Uppsala, Sweden) by SA Pathology, according to the manufacturer's instructions. Additional details on the Methods used is included in this article's Online Repository at www.jacionline.org.
In addition, specific total IgG, IgG1, and IgG4 levels were measured by means of ELISA in which 96-well ELISA plates were coated with 100 μL of Albey Bee Venom at 4 μg/mL overnight at 4°C, washed, and then blocked for 1 hour with 1% BSA in PBS. Serum samples diluted 1:100 in blocking buffer were then added to duplicate wells and incubated at room temperature for 2 hours and then washed. Next, biotinylated anti-human total IgG (1:30,000) or anti-human IgG1 or IgG4 (1:5000) was added to the wells and incubated for 1 hour, followed by washing and addition of horseradish peroxidase–conjugated streptavidin (1:1000) for 1 hour, after which plates were thoroughly washed and then tetramethylbenzidine substrate was added for 10 minutes before the reaction was stopped with 50 μL of 1 mol/L phosphoric acid. The OD of each well was read at 450 nm (OD450nm) with a spectrophotometer plate reader (VersaMax; Molecular Devices, Sunnyvale, Calif) and analyzed with SoftMax Pro Software.
Venom skin testing
Venom skin testing was performed at baseline; at weeks 0, 12, and 52; and then at 30 months. Skin tests were performed off antihistamine medication and included a negative control (normal PBS). Dilutions of venom (Albey Bee Venom extract) at 0.001, 0.01, 0.1, and 1.0 mg/mL were injected into the dermis of the underside of the upper forearm. The positive control was histamine acid phosphate at 10 mg/mL through skin prick tests, but otherwise, skin tests were performed through the intradermal route. Skin tests were read at 20 minutes, and a positive test result was recorded if wheals were 3 mm (0.12 in) in diameter or greater than those elicited by the negative control.26
End points
The primary prespecified end points were as follows:
-
1.
number of subjects in each group reaching maintenance;
-
2.
number and grade of objective systemic immediate hypersensitivity reactions in each group; and
-
3.
changes in venom skin test response by group.
Secondary end points included the following:
-
1.
changes from baseline to end point for the in vitro measures of specific IgE and specific IgG4 and
-
2.
number and size of local adverse reactions per subject.
Care of subjects at the end of the study
Based on published studies (reviewed by Golden27), the policy of our unit is to continue maintenance VIT for at least 5 years in subjects who have had immediate systemic reactions to bee stings and on an indefinite basis for those who have had extreme life-threatening reactions (most typically major hypotensive reactions). Because the combination with Advax adjuvant was not a licensed product, after 30 months of maintenance therapy, subjects were exited from their blinded randomized therapy and offered conventional VIT immunotherapy at 100 μg/mo for 5 years or indefinitely, depending on the severity of past sting reactions. Patients who withdrew because of changed work circumstances and had reached maintenance doses were offered opportunities to complete conventional VIT treatment in community-based settings. Routine sting challenges at the end of the trial were not performed because of a lack of resources.
Statistical analysis
Safety and efficacy analyses were performed for all subjects who commenced VIT. Data were analyzed by using STATA (version 14.2; StataCorp, College Station, Tex) and GraphPad Prism (version 5; GraphPad Software, La Jolla, Calif) software. Means and 95% CIs for specific IgE levels, specific IgG4 levels, and specific IgE/specific IgG4 ratios across time are shown. Each outcome was analyzed by using a mixed-effects model that included a random intercept for each subject to account for the within-subject correlation across time. The models included fixed effects for intervention group, time (as a categorical variables from 0-15 for the 16 visits), age category (greater than or less than the median age), and sex. We also included an additional term for baseline values to increase precision and avoid regression to the mean effects.
To assess whether changes in means across time varied differently between groups, each model included a group × time interaction term, and we performed a global test for significance based on 15 df (for the 16 time points). In sensitivity analyses we also assessed whether the effect of sex varied across time by including a sex and time interaction term. Assumptions of the mixed effects models were confirmed by assessing both level 1 (error) and level 2 (individual level) residuals. Differences in means at each time point were considered significant if the global tests were significant (P < .05), and the means at each individual time point were significant at a P value of less than .05 after adjustment for 16 comparisons (Bonferroni adjustment). Data for clinical and demographic characteristics were assessed for normality and are presented as means ± SDs for normally distributed variables and medians (ranges) for nonnormally distributed variables.
Results
Participants' characteristics
Twenty-seven subjects met the selection criteria (1 subject was excluded) and consented to participate in the trial, with 10 randomized to receive SHBVIT and 17 randomized to receive AHBVIT (Fig 1). The low enrollment rate was due to many eligible subjects instead opting for therapy using an ultrarush regimen offered at our center. None of the patients was receiving treatment with a β-blocker or angiotensin-converting enzyme inhibitor. One subject allocated to the AHBVIT group withdrew because of altered work commitments before starting treatment and was not included in further analyses. Baseline characteristics of the remaining 26 subjects are shown in Table I . There was a slightly greater proportion of subjects with a history of hypotensive anaphylaxis to bee sting randomized to the SHBVIT group (5/10), compared to the AHBVIT group (5/16). Three subjects in the SHBVIT group had baseline serum MCT levels ranging from 13 to 19 μg/L, whereas only 1 subject in the AHBVIT group had a marginally increased baseline MCT level of 12 μg/L. None of these subjects had any clinical features of a mast cell disorder. All 4 subjects with increased MCT levels had a history before the trial of anaphylaxis with hypotension in response to a bee sting. One subject with increased MCT levels in the SHBVIT group was stung by 8 bees on separate occasions during the trial with only local reactions. Another subject with increased MCT levels in the SHBVIT group tolerated 2 wasp stings during the trial. The third subject in the SHBVIT group and the 1 subject in the AHBVIT group who had increased MCT levels did not experience any stings during the trial.
Table I.
Subjects' demographics and baseline characteristics
| AHBVIT group | SHBVIT group | P value | |
|---|---|---|---|
| Demographics | |||
| No. of subjects | 16 | 10 | |
| Mean age (y) | 40 | 46 | .14 |
| Male sex (no.) | 11 | 9 | |
| Hypertension | 2 | 0 | |
| Past medical history of malignancy | 1 | 0 | |
| Current smoker | 6 | 1 | |
| General allergy history | |||
| Rhinitis | 1 | 1 | |
| Asthma | 4 | 2 | |
| Non–sting-induced anaphylaxis | 3 | 1 | |
| Bee sting history (baseline) | |||
| Grade of worst honeybee reaction | |||
| 1 | 1 | 0 | |
| 2 | 10 | 5 | .29 |
| 3 | 5 | 5 | |
| Baseline MCT (μg/L) | |||
| Mean | 5.8 | 7.9 | .70 |
| Range | 2-12 | 4-19 | |
| No. >12 μg/L | 0 | 3 | |
| Positive honeybee venom skin test responses per concentration tested | |||
| 0.001 | 2 | 0 | |
| 0.01 | 3 | 3 | |
| 0.1 | 4 | 3 | |
| 1 | 4 | 3 | |
| Total IgE (kU/mL) | |||
| Mean | 275 | 161 | .82 |
| Range | 18-1804 | 7-299 | |
| Honey bee–specific IgE (kU/mL) | |||
| Mean | 22.5 | 10.2 | .20 |
| Range | 0.74-87.9 | 2.6-38 |
Mean total and specific IgE levels, smoking history, comorbidities, and history of other allergic disorders were all greater and/or more frequent at baseline in the AHBVIT group, but there was no statistically significant difference between the groups for age, grade of worst prior bee sting reaction, MCT level, total IgE level, specific IgE level, or specific IgG4 level at baseline.
Reported non–sting-induced anaphylaxis represented reactions to penicillin (2 participants) or sulfite (1 participant) in the AHBVIT group and seafood (1 participant) in the SHBVIT group.
Safety data and adverse reactions
Blood tests taken at 4 weeks, on reaching the maintenance phase, and at study completion revealed no clinically significant laboratory abnormality classed as likely to be related to VIT. In particular, there was no significant difference in inflammatory markers, including CRP (see Fig E1 in this article's Online Repository at www.jacionline.org), and total white cell counts (WCCs; see Fig E2 in this article's Online Repository at www.jacionline.org) over time or between groups, which is consistent with previous reports that Advax is essentially noninflammatory.28
Fig E1.
CRP levels. Data are shown as means and SEs for each group and all study subjects combined for each study time point. Minimums and maximums of normal ranges are shown as horizontal black lines.
Fig E2.
Total WCCs. Data are shown as means and SEs.
Inulin particles are activators of the alternative complement pathway.29 Because subjects in the AHBVIT group received approximately 50 doses or a total of approximately 0.5 g of delta inulin over the study, serum levels of complement factors C3 and C4 were assessed to detect any complement changes. There was no significant difference in serum C3 (see Fig E3 in this article's Online Repository at www.jacionline.org) or C4 (Fig E4 in this article's Online Repository at www.jacionline.org) over time or between groups, suggesting Advax adjuvant administration does not cause complement disturbances.
Fig E3.
Complement C3 levels. Data are shown as means and SEs.
Fig E4.
Complement C4 levels. Data are shown as means and SEs.
MCT levels were measured as a marker of mast cell activation, with no significant differences in MCT levels between study groups, with an overall trend for MCT levels to decrease over time in both groups (see Fig E5 in this article's Online Repository at www.jacionline.org). This is similar to other reports of a decrease in baseline serum MCT levels by 2.5% per year after a mean of 4 years of VIT.30
Fig E5.
Serum MCT levels. Data are shown as means and SEs.
Reported adverse reactions were common in both groups and were predominantly made up of local (see “Secondary end points”) and generalized allergic reactions occurring during the 45-minute observation period after injection (see “Primary end points”). The only adverse events that were classified by the investigators as likely to be related to the Advax adjuvant were small, nontender subcutaneous nodules at the VIT injection sites of some subjects, which regressed over weeks to months. Overall, AHBVIT and SHBVIT were both assessed by the study investigators to be safe and tolerable.
Primary end points
Number of subjects reaching maintenance
Two subjects in the AHBVIT group did not stay in the trial long enough to reach monthly maintenance therapy, with changed work commitments leading to their withdrawal at weeks 7 and 11. All other subjects in the AHBVIT group reached monthly maintenance, but a further 2 subjects later withdrew after varying periods of treatment because of changed employment circumstances. In the SHBVIT group, a single subject was withdrawn after repeated immediate systemic reactions on 4 occasions after reaching the maintenance dose despite intervening reduced doses in an unsuccessful attempt to get around this issue. The other 9 subjects in the SHBVIT group reached monthly maintenance.
Number of systemic immediate hypersensitivity reactions in each group
In the AHBVIT group, 6 (37.5%) of 16 subjects experienced a total of 12 grade 1 reactions, 1 a grade 2 reaction, and none a grade 3 reaction. In the SHBVIT group, 3 (30%) of 10 subjects experienced a total of 10 grade 1 reactions, 2 a grade 2 reaction, and 1 subject, who was withdrawn after a second hypotensive anaphylaxis, 2 grade 3 reactions (see Table E2 in this article's Online Repository at www.jacionline.org). All subjects recovered promptly and fully from these reactions with standard treatment, and no biphasic reactions were observed. None of the 4 subjects with increased baseline MCT levels had an objective systemic reaction to HBVIT.
Venom skin tests by group
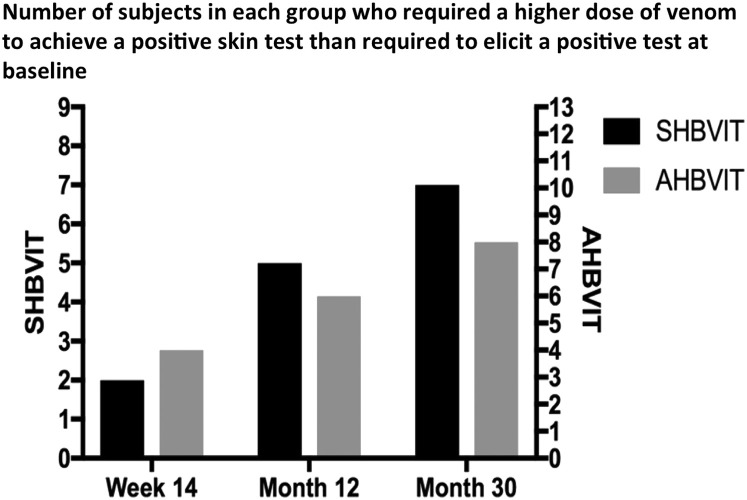
In both groups, venom skin test sensitivity decreased throughout the course of VIT, as evidenced by the increasing concentrations of honeybee venom required to elicit a positive response at week 14 and after 12 and 30 months of maintenance therapy (see Fig E6 and Table E3 in this article's Online Repository at www.jacionline.org). There was no statistical difference in venom skin test sensitivity scores between the 2 groups at any time point.
Fig E6.
Responders with positive VIT skin test responses. The number of responders in each group for each assessed time point (week 14, month 12, and month 30) who tolerated a greater dose of venom than was needed to elicit a positive venom skin test response in that subject at baseline is shown.
Secondary end points
Honey bee venom–specific IgE responses
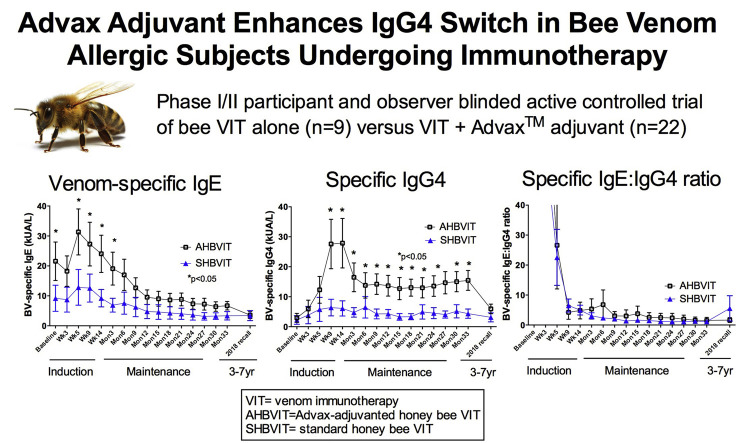
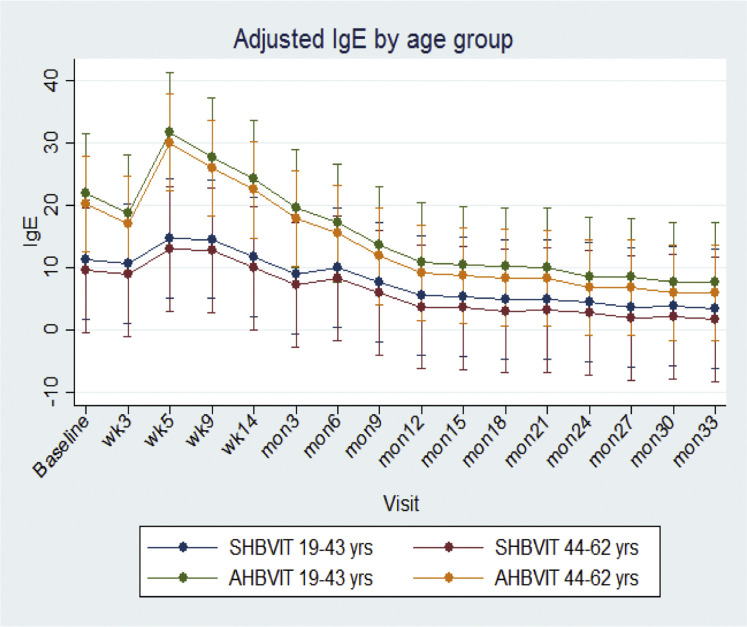
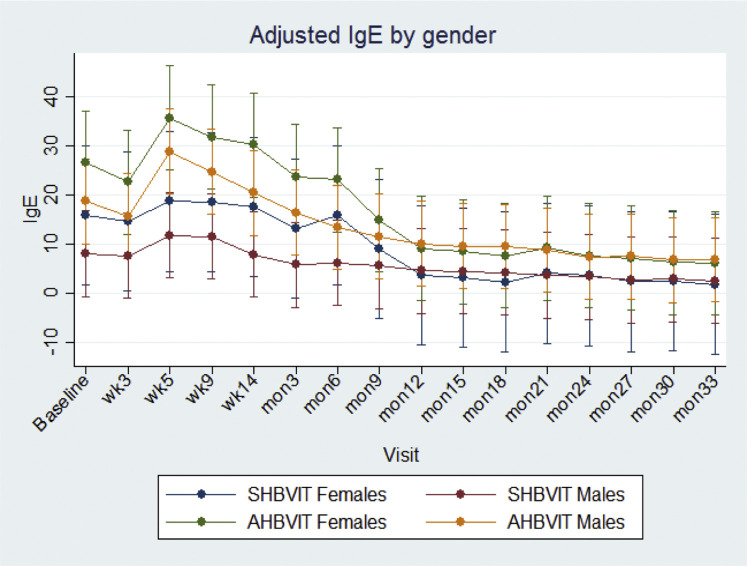
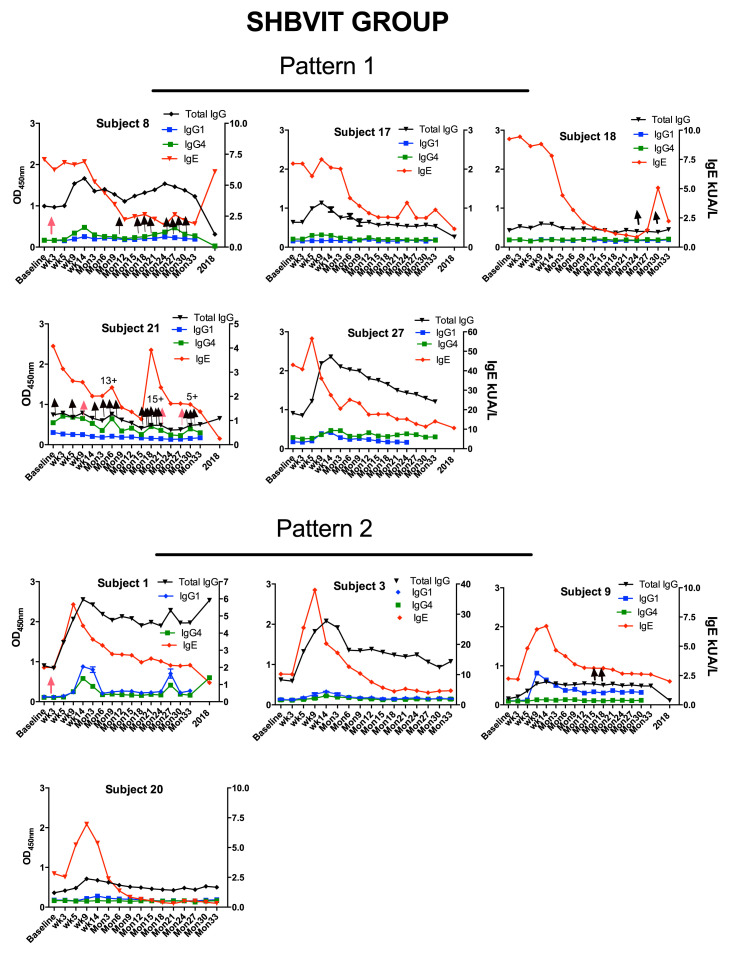
At study entry, there was a nonsignificant trend toward greater specific IgE levels in the AHBVIT group but there was substantial overlap of levels between groups. In both groups there was an overall increase in specific IgE levels on commencement of therapy, reaching a peak around week 9 and then progressively decreasing thereafter (Fig 2 ). Specific IgE levels got back to baseline values by around weeks 14 to 26 in both groups and then progressively decreased through to the end of the study. When analyzed by ANOVA for differences by group, time, sex, and age, the reduction in specific IgE levels was significant for both groups over time (P = .001) but was not significantly different between groups (Fig 2). There was no effect on specific IgE responses of sex (see Fig E7 in this article's Online Repository at www.jacionline.org) or subject's age (see Fig E8 in this article's Online Repository at www.jacionline.org). Examination of the specific IgE responses in individual subjects (see Figs E9 and E10 in this article's Online Repository at www.jacionline.org), revealed 2 different patterns; 1 pattern showed immediate decreases in specific IgE after commencement of VIT, whereas the another pattern showed a large (>2-fold) increase in specific IgE levels between weeks 5 and 9 after VIT initiation with specific IgE only decreasing below baseline levels after 6 to 12 months of maintenance VIT. Approximately half the subjects in both groups fell into each pattern. Subjects who had multiple stings in a short space of time (eg, subjects 8, 14, and 21) had a major increase in their specific IgE levels immediately after this, suggesting the stings had caused either priming or boosting of an underlying IgE memory B-cell response (see Figs E9 and E10).
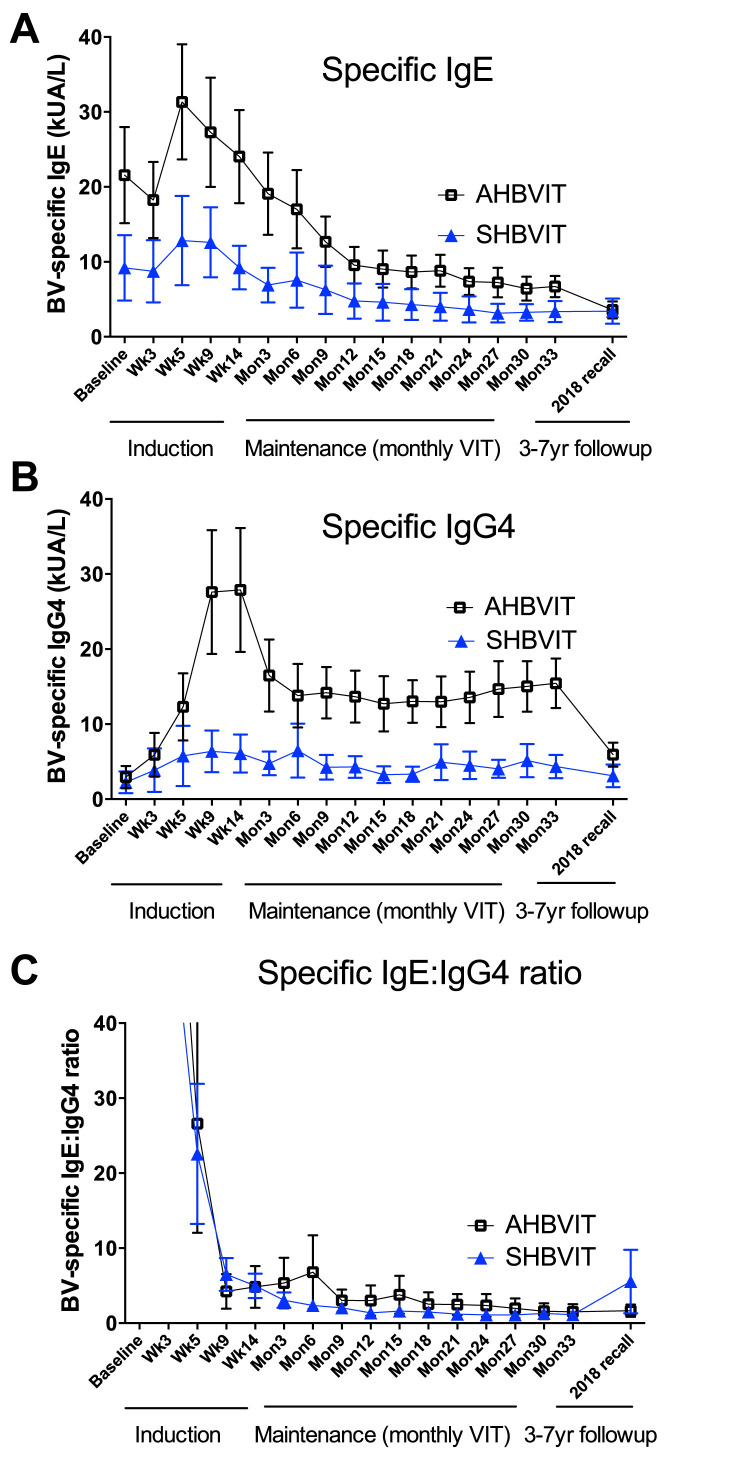
Fig 2.
Specific IgE and IgG4 responses to VIT. Shown are the means of specific IgE levels (A), specific IgG4 levels (B), and specific IgE/IgG4 ratios (C) at baseline and throughout the induction and maintenance phases of VIT, as measured by using the ImmunoCAP assay (mean ± SEM). Shown at the far right of each figure are the mean specific IgE and IgG4 levels in participants able to be recalled in mid-2018, 3 to 7 years after their completion of active study treatment.
Fig E7.
Adjusted marginal means across time for specific IgE by age. Shown are means and 95% CIs.
Fig E8.
Adjusted marginal means across time for specific IgE by sex. Shown are means and 95% CIs.
Fig E9.
Standard HBVIT individual subject serologic response data. Shown are specific total IgG, IgG1, and IgG4 levels, as measured by means of ELISA, and specific IgE levels, as measured by using an ImmunoCAP assay.
Fig E10.
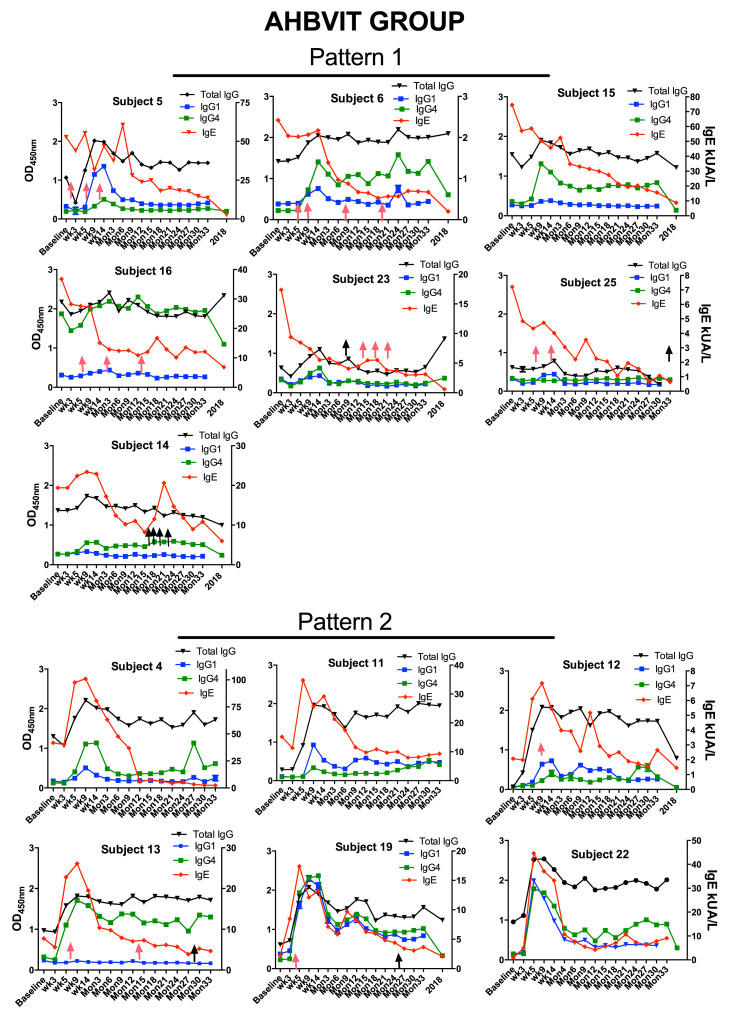
Advax-adjuvanted HBVIT individual subject serologic response data. Shown are specific total IgG, IgG1, and IgG4 levels, as measured by means of ELISA, and specific IgE levels, as measured by using an ImmunoCAP assay.
Specific IgG4 responses
IgG blocking antibodies have been suggested to play a major role in preventing reactions early in VIT when specific IgE levels are still high whereas blocking antibodies may be progressively less important as specific IgE levels decrease over time.31 Hence one measure of early efficacy of VIT might be the induction of specific IgG4, thought to be the dominant blocking subclass. At study entry, mean levels of specific IgG4 were not significantly different between groups (Fig 2), and were low in all subjects bar 1 subject in the AHBVIT group (subject 16, see Fig E10). To exclude the possibility that the high baseline IgG4 result in this subject was due to interfering anti-mouse antibodies, we tested whether their specific IgG4 activity could be adsorbed out by adding mouse sera or bee venom to the soluble phase during the assay. The specific IgG4 activity was adsorbed out by bee venom but not normal mouse sera (data not shown), confirming that this subject had a high specific IgG4 level before starting VIT.
After VIT commencement, specific IgG4 levels rapidly diverged between the SHBVIT and AHBVIT groups, increasing more rapidly and reaching a higher peak in the AHBVIT group, with higher specific IgG4 levels in the AHBVIT group already seen as early as week 5, and with the extent of the difference increasing as VIT progressed (Fig 2). The greater specific IgG4 levels in the AHBVIT group continued through 33 months, 3 months after cessation of study intervention. By contrast, specific IgG4 levels returned back toward baseline levels in many subjects in the SHBVIT group after year 1, despite ongoing VIT.
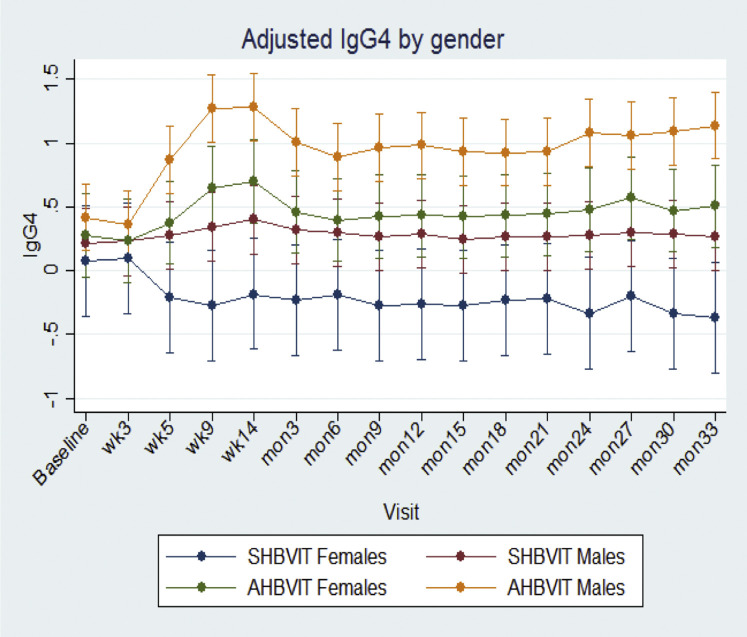
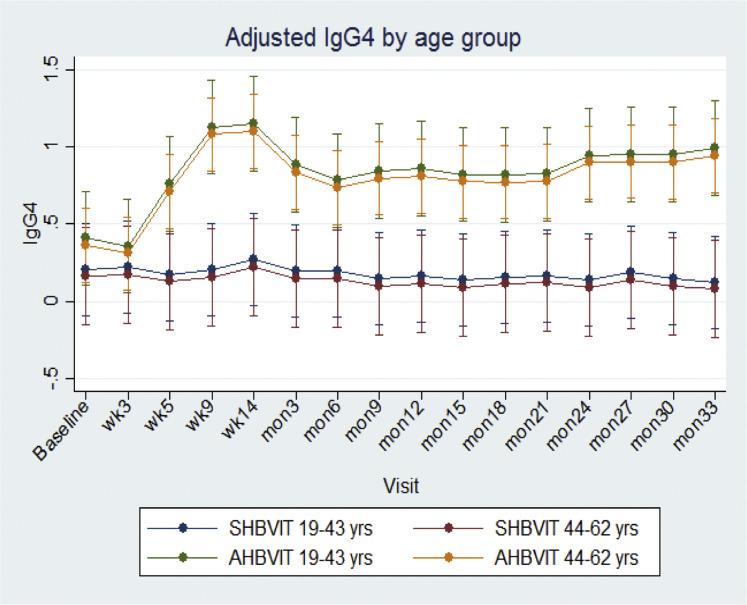
Overall, male subjects had significantly greater specific IgG4 responses than female subjects (male subjects: 0.65 ± 0.06 vs female subjects: 0.26 ± 0.10, P < .002), and in sensitivity analyses there was a significant difference in changes in mean specific IgG4 levels across time according to sex (P = .001; see Fig E11 in this article's Online Repository at www.jacionline.org). No effect of age was seen on IgG4 responses (see Fig E12 in this article's Online Repository at www.jacionline.org). The Advax adjuvant worked well in both male and female subjects and more than compensated for the lower responses of female subjects to VIT. By the end of the study, specific IgG4 levels for female subjects in the AHBVIT group were greater than for male subjects in the SHBVIT group (see Fig E10).
Fig E11.
Adjusted marginal means across time for specific IgG4 levels by sex. Shown are means and 95% CIs.
Fig E12.
Adjusted marginal means across time for specific IgG4 levels by age. Shown are means and 95% CIs.
Specific IgG subclass responses
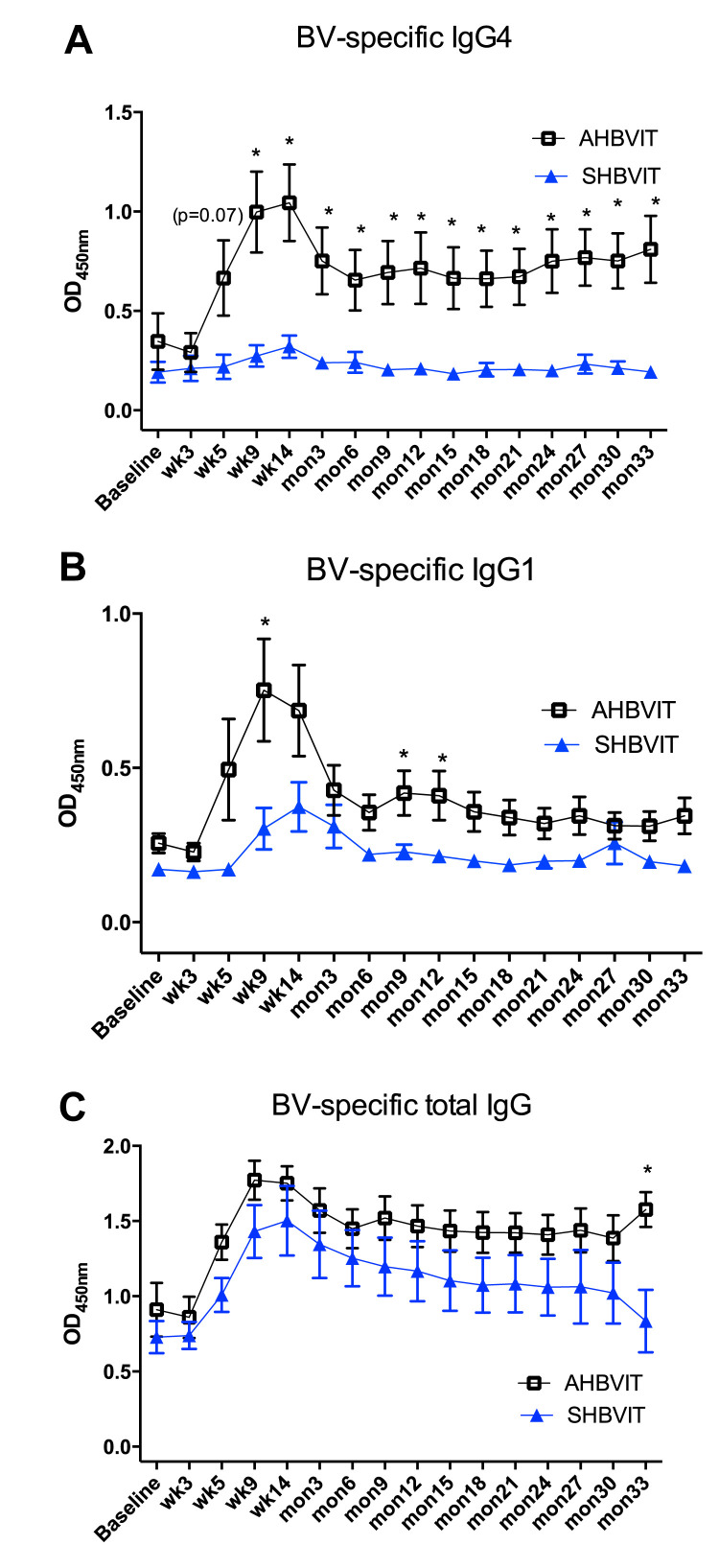
Other IgG subtypes, including IgG1, can also play a role as blocking antibodies.32 Levels of specific total IgG and 2 of its individual subtypes, IgG1 and IgG4, were measured using ELISA. Many subjects showed an increase in specific IgG1 levels that paralleled the specific IgG4 response, peaking between weeks 9 and 14 of the induction phase, followed by a plateau throughout the maintenance phase (Fig 3 ). Specific IgG1 responses, as for specific IgG4 responses, were higher in the AHBVIT group. The majority of subjects in the SHBVIT group had only small increases in specific IgG4 levels, with the majority (5/9) having higher specific IgG1 than IgG4 responses and 1 subject (subject 9, see Fig E9) having an exclusively IgG1 response to HBVIT. By contrast, the majority of subjects in the AHBVIT group (9/13) had higher specific IgG4 than IgG1 responses (see Fig E10). In 4 subjects in the AHBVIT group the specific IgG1 response was initially greater than the specific IgG4 response but by month 30, only 1 of 4 of these subjects still had a marginally higher specific IgG1 than IgG4 level. Hence at month 30, 12 of the 13 subjects in the AHBVIT group had higher specific IgG4 than IgG1 levels.
Fig 3.
Bee venom–specific IgG subclass responses determined by means of ELISA. Shown are means of specific IgG4(A), specific IgG1(B), and specific total IgG (C) levels, as measured by means of ELISA. Means ± SEs are shown. *Time points in which differences between groups were statistically significant (P < .05).
Levels of total specific IgG, the sum of all the IgG subclasses, showed a similar pattern to specific IgG1 and IgG4 levels (Fig 3). The total specific IgG ELISA was noted to have a high background even at baseline relative to the specific IgG1 and IgG4 ELISAs. Nevertheless, specific total IgG levels increased in most subjects after commencement of VIT and then slowly waned over the 3 years of VIT administration. Interestingly, specific total IgG levels in some subjects remained increased over baseline values, even when specific IgG1 and IgG4 levels were relatively low or undetectable, suggesting that the specific total IgG ELISA might have been more sensitive than the IgG1 or IgG4 assays (see Figs E9 and E10).
Long-term follow-up of specific IgE and IgG4 measurement after study completion
In 2018, 3 to 7 years after all participants had completed active study treatment and reverted to ongoing standard HBVIT or had ceased HBVIT altogether, ethics permission was obtained to invite study participants to provide an additional blood sample to assess long-term specific IgE and IgG4 responses. Given the long duration since the end of the study, blood samples were only able to be obtained from 13 of the original participants, 5 from the SHBVIT group and 7 from the AHBVIT group. Both groups showed further reduction of their specific IgE levels from levels measured at the end of the study, with the increased specific IgG4 seen during active VIT in the AHBVIT group having largely attenuated by this time (Fig 2), suggesting that ongoing inclusion of Advax in maintenance VIT may be necessary to maintain high specific IgG4 levels longterm.
Local adverse reactions
Local reactions, as expected, were common and not statistically significant between groups, being reported in 10 (62.5%) of 16 subjects in the AHBVIT group and 4 (40%) of 10 subjects in the SHBVIT group (P = .4, Fisher exact test). Seven (43.8%) of 16 subjects in the AHBVIT group and 2 (22.2%) of 9 subjects in the SHBVIT group reported at least 1 episode where local swelling exceeded 10 cm (P = .4, Fisher exact test). Pain after injection was reported on at least 1 occasion by 6 (37.5%) of 16 subjects in the AHBVIT group and 3 (30%) of 10 subjects in the SHBVIT group. Only 2 local reactions persisted beyond 48 hours. None of these reactions required further measures beyond simple analgesic and/or antihistamine medications.
Delayed systemic reactions
A single subject in the AHBVIT group experienced swelling and pain in the forearm discontinuous from the site of the subcutaneous injection in the upper arm, starting 5 hours after injection and persisting for 40 hours. At 24 hours after injection, he had urticaria, lip angioedema, and some wheezing, but when assessed by his local medical practitioner, he showed no desaturation or hypotension and recovered promptly after intramuscular adrenaline, except for local swelling in the forearm, which persisted for another 24 hours. The subject went on to receive further doses of VIT without major incident.
Incidental honeybee sting reactions
Subjects were encouraged to minimize their sting risk during the study. A total of 65 bee stings were reported during the study, involving 4 subjects in AHBVIT group (see Fig E10) and 3 subjects in the SHBVIT group (see Fig E9). One subject, a beekeeper in the SHBVIT group, experienced early subjective manifestations consistent with a grade 2 reaction to bee stings on 5 of 20 occasions involving a total of 47 stings. Stings in the other subjects were all tolerated, and medical attention was not sought for any sting by any participant. Although all subjects were equipped with adrenaline autoinjectors and instructed in their use for any generalized reaction, no subject used their devices. The subject with the highest baseline MCT level of 19 μg/L reported 8 bee stings with only local reactions, and another subject with increased baseline MCT levels tolerated 2 presumed wasp stings with no adverse reactions.
Discussion
Hymenoptera VIT is of proven efficacy for the stinging insects dominant in Europe and North America and for honeybee and jumper jack ant allergy in Australia. However, problems with HBVIT remain, including significant adverse reaction rates, need for frequent injection visits, failure of some subjects to achieve maintenance, and the possibility of recurrent sensitization after cessation of VIT. Given these shortcomings, an adjuvant able to accelerate desensitization, reduce VIT dose and frequency, and/or prolong duration of protection could have broad utility for allergen immunotherapy. Ideally, such an adjuvant would help drive the immune response to the allergen away from a TH2 bias, thereby helping to reduce eosinophilia and IgE production.
Inulin is a plant-based fructan polymer comprised of a linear chain of fructose units connected by β-(2-1)-glycosidic bonds and capped at the reducing end by an α-D-(1-2)-glucopyranoside ring. It can be crystallized into specific semicrystalline forms of delta inulin in which the inulin chains associate through hydrogen bonding to create ordered antiparallel helices in the form of lamellar sheets.33, 34, 35, 36 Only when in this semicrystalline state, referred to as delta inulin or Advax adjuvant, does inulin acquire unique immunologic properties, including the ability to act as a potent vaccine adjuvant.37
Advax adjuvant has been shown to boost both humoral and cellular immunity and enhance vaccine-mediated protection across a wide variety of viral,20, 38, 39, 40, 41 bacterial,42, 43, 44, 45 and protozoan46 antigens in multiple species, including human subjects. This was the first study to assess the human safety and efficacy of Advax when combined with an allergen used for immunotherapy. The study confirmed that Advax adjuvant was safe and well tolerated, even after participants had received approximately 50 consecutive doses over 3 years. Local reactions were common in both groups throughout the study, as expected, with a nonsignificant trend toward more local reactions, predominantly concentrated in the mild category, in the adjuvant group. There was a significant reduction in venom skin test reactivity in both groups after VIT, although the proviso is that skin tests might be only a weak correlate of clinical protection.12 Sting challenges are considered the gold standard outcome measure for VIT but were not performed in this study because of resource limitations. However, a study is currently underway of Advax adjuvant in ant sting allergy VIT in which ant sting challenges are being performed to assess efficacy that should help address this issue.
Immunotherapy for stinging insect anaphylaxis is thought to work through several mechanisms, including production of IgG blocking antibodies that bind the allergen and reduce effector cell function (mast cells, basophils, and eosinophils), leading to reduced TH2 activation and attenuation of IgE production.47 In keeping with this concept, tolerant beekeepers were shown to have increased levels of specific IgG4 together with no specific IgE.48 Hence induction of specific IgG4 is considered an important surrogate marker of effective allergen immunotherapy.47 Notably, Advax adjuvant markedly increased specific IgG4 responses when compared with the SHBVIT group, with specific IgG4 levels staying high through to the end of the treatment period. By contrast, in the SHBVIT group, peak specific IgG4 levels were lower and rapidly waned back toward baseline levels even with ongoing maintenance therapy. This is consistent with a study that showed only modest ~3-fold increases from baseline in specific IgG4 levels after 2 years of standard VIT with ~2-fold reductions in specific IgE levels.49 It remains unclear whether the increase in specific IgG4 levels or the decrease in specific IgE levels is more relevant to VIT clinical efficacy. This might reflect the previous difficulty in inducing sufficiently high blocking IgG4, a problem that our study suggests can be solved by incorporating Advax adjuvant into VIT. In the clinical response to grass pollen immunotherapy, functional assays of IgG4-associated inhibitory activity, such as inhibition of IgE-allergen interactions and inhibition of CD23-dependent IgE-facilitated allergen binding, correlated better with clinical outcome than immunoreactive IgG4 levels.50 Hence it will be interesting in future studies of Advax-adjuvanted VIT to also include functional assays of IgG4-associated inhibitory activity.
Interestingly, the high specific IgG4 levels had attenuated significantly in AHBVIT participants surveyed 3 to 7 years after completion of study treatment, suggesting that inclusion of Advax adjuvant in ongoing VIT might be necessary to sustain high specific IgG4 levels in the long term. All recalled AHBVIT participants had evidence of even further attenuation of their specific IgE levels from the time of study completion (Fig 2). This continued long-term decrease in specific IgE levels might reflect natural death over time of IgE-producing plasmablasts together with failure to replace these because of depletion of the memory B-cell compartment required to replenish IgE+ plasmablasts and/or the effect of regulatory T cells preventing the generation of IgE-producing B cells. In the future, it would be interesting to test whether functional assays of serum inhibitory activity are greater in subjects receiving Advax and also to directly measure the frequency of specific IgE- and IgG4-positive memory B cells throughout the course of VIT.
A difficulty in designing clinical VIT studies is in selecting which assays to use as potential AIT response biomarkers, a topic recently reviewed by a working party of the European Academy of Allergy and Clinical Immunology.51 Allergen-specific regulatory T cells characterized by IL-10 secretion can play a role in VIT,52 driving production of IL-10– and IgG4-positive regulatory B cells. For example, a previous study in nonallergic beekeepers showed the presence of bee venom–specific, IgG4-positive, IL-10–expressing regulatory B cells, with these regulatory B cells also being found to be increased in allergic subjects after VIT.53 Hence the higher specific IgG4 levels in the AHBVIT group might reflect an effect of Advax adjuvant on expansion of IL-10+ regulatory T cells and regulatory B cells making IL-10 and IgG4. This will be an important question to address in future studies.
Interestingly, male subjects had significantly greater specific IgG4 responses and a nonsignificant trend toward lower specific IgE levels after VIT than female subjects. This is consistent with female subjects, postmenarche, having a more TH2-biased immune system, making them prone to allergy.54 However, after 3 years of VIT, the female subjects in both groups had reduced specific IgE levels, indicating that VIT is effective irrespective of sex.
The role of non-IgG4 subclasses as blocking antibodies in patients with venom allergy remains unclear. Antigen specificity rather than IgG subclass was shown to be the dominant factor determining the ability to block allergen-dependent IgE activity when different IgG subclasses of the same allergen specificity were produced and tested.55 Specific IgG1 levels were also greater in the AHBVIT group, with specific IgG1 behaving similarly to specific IgG4, peaking during the induction phase and then plateauing during the maintenance phase in the AHBVIT group or slowly decreasing in the SHBVIT group. However, examination of the time trends of specific IgG1 and IgG4 in individual subjects revealed a more complex pattern, with some subjects making a dominant specific IgG4 response, some making a combined specific IgG4 and IgG1 response, and a few making exclusively a specific IgG1 response. Notably, 2 subjects who had a good specific IgG1 response but no specific IgG4 response (subjects 9 and 25) had a single bee sting at around months 15 and 32, respectively, with neither having a systemic reaction to this. Just how these IgG subclass differences might influence VIT clinical outcomes will be an important area for future research.
Although specific IgE levels did not differ significantly between the 2 groups, different patterns of IgE response were seen. In one pattern of IgE response, the specific IgE level started to decrease directly after commencement of VIT, with no initial increase in IgE level during the induction phase of VIT. In the other pattern of response, there was a relatively large increase in specific IgE levels between weeks 5 and 9 of VIT initiation, with the IgE levels not decreasing to pre-VIT levels until after at least 6 to 12 months of maintenance VIT but thereafter rapidly decreasing. Approximately half the subjects in the SHBVIT and AHBVIT groups fell into each of these patterns.
Interestingly, the majority of subjects experiencing systemic immediate hypersensitivity reactions to VIT were in the group that did not have an initial increase in IgE, a surprising result because it might have been expected that systemic immediate hypersensitivity reactions would have been exacerbated by the sharp increase in specific IgE levels in some VIT subjects. We speculate that the lack of reactions in this group despite the increase in IgE levels might have been because there was a parallel increase in these subjects of specific IgG1 and IgG4, that might have more than compensated for the increase in IgE.
Only a minority of subjects reported stings after entering the study, with no serious systemic reactions and none requiring adrenaline or other therapy, suggesting the VIT had been effective in these subjects. Interestingly, in 2 subjects, 1 in the SHBVIT group (subject 21) and 1 in the AHBVIT group (subject 14), there was a sharp increase in specific IgE levels in response to a series of bee stings, suggesting the possibility of bee stings restimulating IgE production, despite ongoing VIT. How fresh stings might induce different immune responses to the bee venom used for VIT is not clear but might reflect additional labile vasoactive substances and enzymes contained in fresh venom that are no longer present in preserved bee venom.
In summary, the results support potential benefits of Advax adjuvant in VIT, which is consistent with its benefits previously seen with Advax adjuvant for infectious disease vaccines. Although not directly assessed by this study, the increase in specific IgG4 levels in the Advax adjuvant group during the early induction phase, when only very low doses of allergen were being administered, suggests Advax adjuvant might have useful venom dose-sparing ability, particularly during the early stages of VIT. Importantly, for the first time in human subjects, this study confirmed the safety and tolerability of VIT combined with Advax adjuvant. Hence Advax adjuvant is a promising candidate for VIT, with an ongoing study assessing its ability to provide antigen-sparing effects for ant venom allergy therapy.
Acknowledgments
We thank Aida Ahmadie, Sharen Pringle, Bridgit McAteer, Lynda Borg, Kylie Bragg, Chamindi Abeyratne, Susanne Heinzel, Pam Hudson, Susan Virgin, and Dianne Edwards. We thank Tahir Chaudhry, Rory Hannah, Jason Fok, and Paul Russo for organization and assistance in conduct of the clinical trial. We also thank Dorothee Girard, Rebecca Saunders, Connie Li, Tony Ferrante, Blandine Jouvenaux, and Rodolpho Loubaton for assistance with assays and data analysis.
Footnotes
The study was supported by a grant from the Australian Respiratory and Sleep Medicine Institute. Development of Advax adjuvant was supported by funding from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, under contracts HHSN272200800039C and U01 AI061142, and N.P. is currently supported by contract HHSN272201400053C.
Disclosure of potential conflict of interest: N. Petrovsky is associated with Vaxine Pty Ltd, which holds intellectual property over Advax adjuvant. The rest of the authors declare that they have no relevant conflicts of interest.
Australian New Zealand Clinical Trials Registry: ACTRN12608000379314.
Methods
Treatment protocol
The HBVIT protocol for the AHBVIT group is shown in Table E1. The SHBVIT group received the same protocol but without the addition of the Advax adjuvant.
Laboratory assessments of treatment safety
CRP levels and total WCCs were assessed as markers of inflammation. There were no significant changes in CRP levels (Fig E1) or WCCs (Fig E2) across time or between groups. Data are shown as means ± SEs for each group. Minimums and maximums of normal ranges are shown as horizontal black lines.
Similarly, complement factors C3 and C4 were measured as markers of complement pathway dysregulation. There was no significant change in C3 (Fig E3) or C4 (Fig E4) levels across time or between study groups. Marginally greater C4 levels seen in both groups at the month 30 time point is likely caused by assay calibration drift, rather than a true change.
Serum MCT levels were measured as markers of mast cell activation. There was no significant change in MCT levels across time or between study groups (Fig E5) with, if anything, a trend for MCT levels to reduce over time in both study groups as VIT progressed.
Venom skin tests
There was a steady increase in both groups in the number of responders who at each time point during VIT (week 14, month 12, and month 30) tolerated a greater dose of venom than that required to elicit a positive venom skin test response in that subject at baseline (Fig E6). Although there was a trend toward an increased frequency of responders in the AHBVIT group at the 12- and 30-month time points, the differences between groups were not statistically significant (P = .7, 2-way ANOVA). Overall, in both groups a many-fold greater dose of venom from baseline was required for positive skin reactions by the end of the study (in micrograms per milliliter as log10, Table E3).
Specific IgE responses according to sex and age
Adjusted marginal means across time for specific IgE by age category (Fig E7) and sex (Fig E8), respectively, showed a significant overall difference in the variation in mean specific IgE levels across time between intervention groups (χ2 = 26.9, 15 df, P = .03), with a peak at visit 2 for the AHBVIT group, followed by a rapid decrease compared with a gradual decrease across time in the SHBVIT group. The difference in means at visit 2 was borderline significant (P = .057), but there was no significant difference at the other time points after adjustment for multiple comparisons. There was no significant difference in adjusted mean specific IgE levels between those aged less than 43 years and those aged 43 years or more (9.92 ± 1.31 vs 12.28 ± 1.08, P = .17) or between male and female subjects (11.52 ± 0.97 vs 10.76, P = .69). In sensitivity analyses there were also no differences in the changes in mean specific IgE levels across time by sex (P = .35). Error bars represent 95% CIs.
Specific IgG4 responses according to sex and age
Adjusted marginal means for specific IgG4 levels across time by age were assessed by using a mixed-effects model, with adjustment for age group, sex, time, and baseline values. Means were significantly different overall across time, but there was no effect seen for age (Fig E12). There was a significant effect of sex, with women having lower specific IgG4 responses than men (Fig E11).
Relationship between specific total IgG, IgG1, IgG4, and IgE levels over time in subjects receiving standard and adjuvanted VIT
Specific total IgG, IgG1, and IgG4 levels were measured by means of ELISA and specific IgE levels by using an ImmunoCAP assay. Based on the IgE response, 2 different patterns emerged between subject receiving VIT, with 1 group (pattern 1) showing almost immediate decreases in specific IgE levels after commencement of VIT with absence of an initial increase in IgE level (Figs E9 and E10). The other group (pattern 2) showed a large (>2-fold) increase in specific IgE levels between weeks 5 and 9 of the initiation of VIT, and IgE did not decrease to pre-VIT levels until after 6 to 12 months of maintenance VIT. Approximately half the subjects in the SHBVIT and AHBVIT groups fell into each category. In Figs E9 and E10, pink arrows represent systemic immediate hypersensitivity reactions to VIT, and black arrows indicate incidental bee stings, none of which resulted in systemic reactions. Surprisingly, the majority of subjects experiencing systemic immediate hypersensitivity reactions to VIT were concentrated in the pattern 1 groups. Although analysis of baseline data showed that mean specific IgG4 levels at baseline were more than 4-fold greater in subjects in pattern 1 (mean specific IgG4, 4.13; SD, 6.13) versus pattern 2 (mean specific IgG4, 0.915; SD, 1.6), this difference did not reach statistical significance (P = .088, Mann-Whitney test). It was noticeable that subjects having multiple stings in a short space of time (eg, subjects 8, 14, and 21) had a major increase in their specific IgE levels immediately after this, suggesting repeat priming or boosting of IgE+ B-cell memory responses. The specific total IgG ELISA assay was noted to have a high background relative to specific IgG1 and IgG4 ELISAs for reasons that were not clear. Nevertheless, specific total IgG levels increased in most subjects after commencement of VIT and then stayed high or slowly waned over the 3 years of VIT. Interestingly, specific total IgG levels in some subjects remained increased above baseline values, even when specific IgG1 and IgG4 levels were low or undetectable, suggesting that the specific total IgG assay might be much more sensitive than either the IgG1 or IgG4 assays alone.
Interestingly, those subjects with the greatest specific total IgG levels appeared to have the greatest suppression of IgE levels over time with, for example, waning of specific total IgG levels in subject 8 at their most recent follow-up being associated with an upward spike in specific IgE levels. Similarly, subjects 14, 18, 21, both of whom had minimal increases in specific total IgG levels after VIT, both exhibited major spikes in IgE levels.
Table E1.
Study immunization schedule (AHBVIT group)
| Week | Day | Dose | Venom amount (μg) | Advax (mg) | Venom concentration (μg/mL) | Total volume (μL) |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 0.001 | 5 | 0.02 | 200 |
| 2 | 0.01 | 5 | 0.2 | 200 | ||
| 3 | 0.1 | 5 | 2 | 200 | ||
| 2 | 8 | 4 | 0.3 | 5 | 20 | 200 |
| 5 | 1 | 5 | 20 | 200 | ||
| 6 | 3 | 5 | 200 | 200 | ||
| 3 | 15 | 7 | 6 | 5 | 200 | 200 |
| 8 | 10 | 5 | 200 | 200 | ||
| 9 | 15 | 5 | 200 | 200 | ||
| 4 | 22 | 10 | 20 | 7.5 | 200 | 500 |
| 11 | 25 | 7.5 | 200 | 500 | ||
| 5 | 29 | 12 | 30 | 7.5 | 200 | 500 |
| 13 | 35 | 7.5 | 200 | 500 | ||
| 6 | 36 | 14 | 40 | 7.5 | 200 | 500 |
| 15 | 45 | 7.5 | 200 | 500 | ||
| 7 | 43 | 16 | 50 | 7.5 | 200 | 500 |
| 17 | 50 | 7.5 | 200 | 500 | ||
| 8 | 50 | 18 | 70 | 7.5 | 200 | 500 |
| 19 | 30 | 7.5 | 200 | 500 | ||
| 9 | 57 | 20 | 100 | 15 | 200 | 1000 |
| 11 | 71 | 21 | 100 | 15 | 200 | 1000 |
| 14 | 92 | 22 | 100 | 15 | 200 | 1000 |
| Monthly | 23 | 100 | 15 | 200 | 1000 | |
| 24 | 100 | 15 | 200 | 1000 | ||
| 25 | 100 | 15 | 200 | 1000 | ||
| 26 | 100 | 15 | 200 | 1000 | ||
| 27 | 100 | 15 | 200 | 1000 | ||
Table E2.
Systemic immediate hypersensitivity reactions by grade and study group
| Group size | Reaction grade |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| SHBVIT | 10 | 3 (10) | 1 (2) | 1 (2) |
| AHBVIT | 16 | 5 (12) | 1 (1) | 0 (0) |
Table E3.
Increase in venom dose from baseline required for a positive skin test result (log10 scale)
| Group | SHBVIT mean (range) | AHBVIT mean (range) |
|---|---|---|
| After 14-wk induction phase | 0.22 (0 to 1) | 0.38 (−2 to 2) |
| After 12 mo of maintenance | 0.78 (−1 to 3) | 0.77 (−1 to 3) |
| After 30 mo of maintenance | 1.22 (0 to 3) | 1.00 (−1 to 3) |
References
- 1.Hunt K.J., Valentine M.D., Sobotka A.K., Benton A.W., Amodio F.J., Lichtenstein L.M. A controlled trial of immunotherapy in insect hypersensitivity. N Engl J Med. 1978;299:157–161. doi: 10.1056/NEJM197807272990401. [DOI] [PubMed] [Google Scholar]
- 2.Muller U., Thurnheer U., Patrizzi R., Spiess J., Hoigne R. Immunotherapy in bee sting hypersensitivity. Bee venom versus wholebody extract. Allergy. 1979;34:369–378. doi: 10.1111/j.1398-9995.1979.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 3.Brown S.G., Wiese M.D., Blackman K.E., Heddle R.J. Ant venom immunotherapy: a double-blind, placebo-controlled, crossover trial. Lancet. 2003;361:1001–1006. doi: 10.1016/S0140-6736(03)12827-9. [DOI] [PubMed] [Google Scholar]
- 4.Lockey R.F., Turkeltaub P.C., Olive E.S., Hubbard J.M., Baird-Warren I.A., Bukantz S.C. The Hymenoptera venom study. III: Safety of venom immunotherapy. J Allergy Clin Immunol. 1990;86:775–780. doi: 10.1016/s0091-6749(05)80182-4. [DOI] [PubMed] [Google Scholar]
- 5.Muller U., Helbling A., Berchtold E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. J Allergy Clin Immunol. 1992;89:529–535. doi: 10.1016/0091-6749(92)90319-w. [DOI] [PubMed] [Google Scholar]
- 6.Lockey R.F., Turkeltaub P.C., Olive C.A., Baird-Warren I.A., Olive E.S., Bukantz S.C. The Hymenoptera venom study. II: Skin test results and safety of venom skin testing. J Allergy Clin Immunol. 1989;84:967–974. doi: 10.1016/0091-6749(89)90396-5. [DOI] [PubMed] [Google Scholar]
- 7.Sturm G., Kranke B., Rudolph C., Aberer W. Rush Hymenoptera venom immunotherapy: a safe and practical protocol for high-risk patients. J Allergy Clin Immunol. 2002;110:928–933. doi: 10.1067/mai.2002.129124. [DOI] [PubMed] [Google Scholar]
- 8.Rueff F., Przybilla B., Bilo M.B., Muller U., Scheipl F., Aberer W. Predictors of side effects during the buildup phase of venom immunotherapy for Hymenoptera venom allergy: the importance of baseline serum tryptase. J Allergy Clin Immunol. 2010;126:105–111.e5. doi: 10.1016/j.jaci.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Incorvaia C., Frati F., Dell'Albani I., Robino A., Cattaneo E., Mauro M. Safety of hymenoptera venom immunotherapy: a systematic review. Expert Opin Pharmacother. 2011;12:2527–2532. doi: 10.1517/14656566.2011.616494. [DOI] [PubMed] [Google Scholar]
- 10.Golden D.B. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439–448. doi: 10.1016/j.jaci.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Lerch E., Muller U.R. Long-term protection after stopping venom immunotherapy: results of re-stings in 200 patients. J Allergy Clin Immunol. 1998;101:606–612. doi: 10.1016/S0091-6749(98)70167-8. [DOI] [PubMed] [Google Scholar]
- 12.Golden D.B., Kagey-Sobotka A., Lichtenstein L.M. Survey of patients after discontinuing venom immunotherapy. J Allergy Clin Immunol. 2000;105:385–390. doi: 10.1016/s0091-6749(00)90092-7. [DOI] [PubMed] [Google Scholar]
- 13.Golden D.B., Demain J., Freeman T., Graft D., Tankersley M., Tracy J. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28–54. doi: 10.1016/j.anai.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Urbanek R., Krauss U., Ziupa J., Smedegard G. Venom-specific IgE and IgG antibodies as a measure of the degree of protection in insect-sting-sensitive patients. Clin Allergy. 1983;13:229–234. doi: 10.1111/j.1365-2222.1983.tb02592.x. [DOI] [PubMed] [Google Scholar]
- 15.Golden D.B., Kwiterovich K.A., Kagey-Sobotka A., Lichtenstein L.M. Discontinuing venom immunotherapy: extended observations. J Allergy Clin Immunol. 1998;101:298–305. doi: 10.1016/S0091-6749(98)70239-8. [DOI] [PubMed] [Google Scholar]
- 16.Rueff F., Wolf H., Schnitker J., Ring J., Przybilla B. Specific immunotherapy in honeybee venom allergy: a comparative study using aqueous and aluminium hydroxide adsorbed preparations. Allergy. 2004;59:589–595. doi: 10.1111/j.1398-9995.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 17.Cadario G., Marengo F., Ranghino E., Rossi R., Gatti B., Cantone R. Higher frequency of early local side effects with aqueous versus depot immunotherapy for hymenoptera venom allergy. J Investig Allergol Clin Immunol. 2004;14:127–133. [PubMed] [Google Scholar]
- 18.Quercia O., Rafanelli S., Puccinelli P., Stefanini G.F. The safety of cluster immunotherapy with aluminium hydroxide-adsorbed honey bee venom extract. J Investig Allergol Clin Immunol. 2001;11:27–33. [PubMed] [Google Scholar]
- 19.Petrovsky N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf. 2015;38:1059–1074. doi: 10.1007/s40264-015-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honda-Okubo Y., Barnard D., Ong C.H., Peng B.H., Tseng C.T., Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015;89:2995–3007. doi: 10.1128/JVI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honda-Okubo Y., Rajapaksha H., Sajkov D., Gordon D., Cox M.M.J., Petrovsky N. Panblok-H1+advax H1N1/2009pdm vaccine: insights into rapid development of a delta inulin adjuvanted recombinant pandemic influenza vaccine. Hum Vaccin Immunother. 2017;13:1–11. doi: 10.1080/21645515.2017.1279765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon D.L., Sajkov D., Honda-Okubo Y., Wilks S.H., Aban M., Barr I.G. Human phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax delta inulin adjuvant. Vaccine. 2016;34:3780–3786. doi: 10.1016/j.vaccine.2016.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon D., Kelley P., Heinzel S., Cooper P., Petrovsky N. Immunogenicity and safety of Advax, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled phase 1 study. Vaccine. 2014;32:6469–6477. doi: 10.1016/j.vaccine.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon D.L., Sajkov D., Woodman R.J., Honda-Okubo Y., Cox M.M., Heinzel S. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine. 2012;30:5407–5416. doi: 10.1016/j.vaccine.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown S.G. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371–376. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Seibert S.M., King T.S., Kline D., Mende C., Craig T. Reliability of skin test results when read at different time points. Allergy Asthma Proc. 2011;32:203–205. doi: 10.2500/aap.2011.32.3436. [DOI] [PubMed] [Google Scholar]
- 27.Golden D.B. Long-term outcome after venom immunotherapy. Curr Opin Allergy Clin Immunol. 2010;10:337–341. doi: 10.1097/ACI.0b013e32833bc0ba. [DOI] [PubMed] [Google Scholar]
- 28.Petrovsky N., Cooper P.D. Advax, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine. 2015;33:5920–5926. doi: 10.1016/j.vaccine.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper P.D., Carter M. Anti-complementary action of polymorphic “solubility forms” of particulate inulin. Mol Immunol. 1986;23:895–901. doi: 10.1016/0161-5890(86)90075-1. [DOI] [PubMed] [Google Scholar]
- 30.Dugas-Breit S., Przybilla B., Dugas M., Arnold A., Pfundstein G., Kuchenhoff H. Serum concentration of baseline mast cell tryptase: evidence for a decline during long-term immunotherapy for Hymenoptera venom allergy. Clin Exp Allergy. 2010;40:643–649. doi: 10.1111/j.1365-2222.2009.03436.x. [DOI] [PubMed] [Google Scholar]
- 31.Ferrante A., Mocatta F., Goh D.H. Changes in IgG and IgE antibody levels to bee venom during immunotherapy. Int Arch Allergy Appl Immunol. 1986;81:284–287. doi: 10.1159/000234148. [DOI] [PubMed] [Google Scholar]
- 32.Denepoux S., Eibensteiner P.B., Steinberger P., Vrtala S., Visco V., Weyer A. Molecular characterization of human IgG monoclonal antibodies specific for the major birch pollen allergen Bet v 1. Anti-allergen IgG can enhance the anaphylactic reaction. FEBS Lett. 2000;465:39–46. doi: 10.1016/s0014-5793(99)01703-2. [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Barclay T., Song Y., Joyce P., Sakala I.G., Petrovsky N. Investigation of the biodistribution, breakdown and excretion of delta inulin adjuvant. Vaccine. 2017;35:4382–4388. doi: 10.1016/j.vaccine.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 34.Barclay T.G., Rajapaksha H., Thilagam A., Qian G., Ginic-Markovic M., Cooper P.D. Physical characterization and in silico modeling of inulin polymer conformation during vaccine adjuvant particle formation. Carbohydr Polym. 2016;143:108–115. doi: 10.1016/j.carbpol.2016.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper P.D., Rajapaksha K.H., Barclay T.G., Ginic-Markovic M., Gerson A.R., Petrovsky N. Inulin crystal initiation via a glucose-fructose cross-link of adjacent polymer chains: atomic force microscopy and static molecular modelling. Carbohydr Polym. 2015;117:964–972. doi: 10.1016/j.carbpol.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper P.D., Barclay T.G., Ginic-Markovic M., Petrovsky N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology. 2013;23:1164–1174. doi: 10.1093/glycob/cwt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper P.D., Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising beta-D-[2 -> 1] poly(fructo-furanosyl) alpha-D-glucose polymers. Glycobiology. 2011;21:595–606. doi: 10.1093/glycob/cwq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menon V., Ayala V.I., Rangaswamy S.P., Kalisz I., Whitney S., Galmin L. DNA prime/protein boost vaccination elicits robust humoral response in rhesus macaques using oligomeric simian immunodeficiency virus envelope and Advax delta inulin adjuvant. J Gen Virol. 2017;98:2143–2155. doi: 10.1099/jgv.0.000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honda-Okubo Y., Kolpe A., Li L., Petrovsky N. A single immunization with inactivated H1N1 influenza vaccine formulated with delta inulin adjuvant (Advax) overcomes pregnancy-associated immune suppression and enhances passive neonatal protection. Vaccine. 2014;32:4651–4659. doi: 10.1016/j.vaccine.2014.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrovsky N., Larena M., Siddharthan V., Prow N.A., Hall R.A., Lobigs M. An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody. J Virol. 2013;87:10324–10333. doi: 10.1128/JVI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Layton R.C., Petrovsky N., Gigliotti A.P., Pollock Z., Knight J., Donart N. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine. 2011;29:6242–6251. doi: 10.1016/j.vaccine.2011.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Paula Oliveira Santos B., Trentini M.M., Machado R.B., Rubia Nunes Celes M., Kipnis A., Petrovsky N. Advax4 delta inulin combination adjuvant together with ECMX, a fusion construct of four protective mTB antigens, induces a potent Th1 immune response and protects mice against Mycobacterium tuberculosis infection. Hum Vaccin Immunother. 2017;13:2967–2976. doi: 10.1080/21645515.2017.1368598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Counoupas C., Pinto R., Nagalingam G., Britton W.J., Petrovsky N., Triccas J.A. Delta inulin-based adjuvants promote the generation of polyfunctional CD4(+) T cell responses and protection against Mycobacterium tuberculosis infection. Sci Rep. 2017;7:8582. doi: 10.1038/s41598-017-09119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Del Rio E., Marradi M., Calderon-Gonzalez R., Frande-Cabanes E., Penades S., Petrovsky N. A gold glyco-nanoparticle carrying a Listeriolysin O peptide and formulated with Advax delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine. 2015;33:1465–1473. doi: 10.1016/j.vaccine.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 45.Feinen B., Petrovsky N., Verma A., Merkel T.J. Advax-adjuvanted recombinant protective antigen provides protection against inhalational anthrax that is further enhanced by addition of murabutide adjuvant. Clin Vaccine Immunol. 2014;21:580–586. doi: 10.1128/CVI.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hess J.A., Zhan B., Torigian A.R., Patton J.B., Petrovsky N., Zhan T. The immunomodulatory role of adjuvants in vaccines formulated with the recombinant antigens Ov-103 and Ov-RAL-2 against Onchocerca volvulus in mice. PLoS Negl Trop Dis. 2016;10:e0004797. doi: 10.1371/journal.pntd.0004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozdemir C., Kucuksezer U.C., Akdis M., Akdis C.A. Mechanisms of immunotherapy to wasp and bee venom. Clin Exp Allergy. 2011;41:1226–1234. doi: 10.1111/j.1365-2222.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- 48.Varga E.M., Kausar F., Aberer W., Zach M., Eber E., Durham S.R. Tolerant beekeepers display venom-specific functional IgG4 antibodies in the absence of specific IgE. J Allergy Clin Immunol. 2013;131:1419–1421. doi: 10.1016/j.jaci.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 49.Varga E.M., Francis J.N., Zach M.S., Klunker S., Aberer W., Durham S.R. Time course of serum inhibitory activity for facilitated allergen-IgE binding during bee venom immunotherapy in children. Clin Exp Allergy. 2009;39:1353–1357. doi: 10.1111/j.1365-2222.2009.03303.x. [DOI] [PubMed] [Google Scholar]
- 50.Shamji M.H., Ljorring C., Francis J.N., Calderon M.A., Larche M., Kimber I. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67:217–226. doi: 10.1111/j.1398-9995.2011.02745.x. [DOI] [PubMed] [Google Scholar]
- 51.Shamji M.H., Kappen J.H., Akdis M., Jensen-Jarolim E., Knol E.F., Kleine-Tebbe J. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI position paper. Allergy. 2017;72:1156–1173. doi: 10.1111/all.13138. [DOI] [PubMed] [Google Scholar]
- 52.Akdis C.A., Blesken T., Akdis M., Wuthrich B., Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van de Veen W., Stanic B., Yaman G., Wawrzyniak M., Sollner S., Akdis D.G. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204–1212. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Keselman A., Fang X., White P.B., Heller N.M. Estrogen signaling contributes to sex differences in macrophage polarization during asthma. J Immunol. 2017;199:1573–1583. doi: 10.4049/jimmunol.1601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dodev T.S., Bowen H., Shamji M.H., Bax H.J., Beavil A.J., McDonnell J.M. Inhibition of allergen-dependent IgE activity by antibodies of the same specificity but different class. Allergy. 2015;70:720–724. doi: 10.1111/all.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]