Summary
Background
An outbreak of severe acute respiratory syndrome (SARS) has been reported in Hong Kong. We investigated the viral cause and clinical presentation among 50 patients.
Methods
We analysed case notes and microbiological findings for 50 patients with SARS, representing more than five separate epidemiologically linked transmission clusters. We defined the clinical presentation and risk factors associated with severe disease and investigated the causal agents by chest radiography and laboratory testing of nasopharyngeal aspirates and sera samples. We compared the laboratory findings with those submitted for microbiological investigation of other diseases from patients whose identity was masked.
Findings
Patients' age ranged from 23 to 74 years. Fever, chills, myalgia, and cough were the most frequent complaints. When compared with chest radiographic changes, respiratory symptoms and auscultatory findings were disproportionally mild. Patients who were household contacts of other infected people and had older age, lymphopenia, and liver dysfunction were associated with severe disease. A virus belonging to the family Coronaviridae was isolated from two patients. By use of serological and reverse-transcriptase PCR specific for this virus, 45 of 50 patients with SARS, but no controls, had evidence of infection with this virus.
Interpretation
A coronavirus was isolated from patients with SARS that might be the primary agent associated with this disease. Serological and molecular tests specific for the virus permitted a definitive laboratory diagnosis to be made and allowed further investigation to define whether other cofactors play a part in disease progression.
Published online April 8, 2003 http://image.thelancet.com/extras/03art3477web.pdf
Introduction
An outbreak of atypical pneumonia in Guangdong Province, People's Republic of China, that has continued since November, 2002, is reported to have affected 792 people and caused 31 deaths.1 In adjacent Hong Kong, surveillance of severe atypical pneumonia was heightened in the public hospital network under the Hospital Authority of Hong Kong. By the end of February, 2003, clusters of patients with pneumonia were noted in Hong Kong, along with affected close contacts and health-care workers. The disease did not respond to empirical antimicrobial treatment for acute community-acquired typical or atypical pneumonia. Bacteriological and virological pathogens known to cause pneumonia were not identified. Thus, the new disorder was called severe acute respiratory syndrome (SARS). Subsequently, SARS has spread worldwide to involve patients in North America, Europe, and other Asian countries.1 We investigated patients in Hong Kong to try to identify the causal agent.
Methods
We included in the study 50 patients fitting a modified WHO definition of SARS admitted to three acute regional hospitals in Hong Kong between Feb 26 and March 26, 2003.2 Briefly, the case definition was fever of 38°C or more, cough or shortness of breath, new pulmonary infiltrates on chest radiography, and a history of exposure to a patient with SARS or absence of response to empirical antimicrobial coverage for typical and atypical pneumonia (β lactams and macrolides, fluoroquinolones, or tetracyclines).
We collected nasopharyngeal aspirates and serum samples from all patients. Paired acute and convalescent sera and faeces were available from some patients. A lung-biopsy tissue sample from one patient was processed for viral culture and reverse-transcriptase PCR (RT-PCR) and for routine histopathological examination and electron microscopy. We used as controls nasopharyngeal aspirates, and faeces and sera submitted for microbiological investigation of other diseases from patients whose identities were masked.
The SARS patients' medical records were reviewed retrospectively by the attending physicians and clinical microbiologists. Routine haematological, biochemical, and microbiological work-up was done, including bacterial culture of blood and sputum, serology, and nasopharyngeal aspirates for virology. The nasopharyngeal aspirate was assessed by rapid immunoflourescent antigen detection for influenza A and B, parainfluenza types 1, 2, and 3, respiratory syncytial virus and adenovirus,3 and was cultured for conventional respiratory pathogens on Mardin Darby Canine Kidney, LLC-Mk2, RDE, Hep-2 and MRC-5 cells.4 Subsequently, fetal rhesus kidney (FRhK-4) and A-549 cells were added to the panel of cell lines used. RT-PCR for influenza A5 and human metapneumovirus was done directly on the clinical samples. The degenerate primers used for human metapneumovirus were: first round 5'-AARGTSAATGCATCAGC-3′ and 5′-CAKATTYTG CTTATGCTTTC-3′; nested primers: 5′-ACACCTGT TACAATACCAGC-3′ and 5′-GACTTGAGTCCCA GCTCCA-3′ (sequences using the International Union of Pure Chemistry one-letter code). The size of the nested PCR product was 201 bp. We used an ELISA for mycoplasma to screen cell cultures (Roche Diagnostics, Indianapolis, IN, USA).
Serology and detection of coronavirus
After culture and genetic sequencing of a coronavirus from two patients, we developed an RT-PCR to detect the coronavirus sequence from nasopharyngeal aspiration samples. Total RNA from clinical samples was reverse transcribed with random hexamers and cDNA was amplified with primers 5'-TACACACCT CAGCGTTG-3' and 5'-CACGAACGTGACGAAT-3' in the presence of 2·5 mmol/L magnesium chloride (94°C for 8 min followed by 40 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min).
Coronavirus-infected fetal rhesus kidney cells were fixed in acetone and used in an indirect immunofluorescence assay to detect a serological response to the virus.
Random RT-PCR assay
To find out the genetic sequence information of an unknown RNA virus, we did a random RT-PCR assay. Total RNA from virus-infected and virus-uninfected fetal rhesus kidney cells were isolated. The RNA samples were reverse transcribed with primer 5′-GCCGGAGC TCTGCAGAATTCNNNNNN-3′, where N=A, T, C, or G, and cDNA was amplified by a primer 5′-GCCGGAGCTCTGCAGAATTC-3′. Unique PCR products (in size) in the infected cell preparation were cloned and sequenced, and the genetic homology compared with those in GenBank.
The routine receipt and inoculation of samples was done in a biosafety level-2 laboratory. Laboratory procedures involving culture of the virus was done in biosafety level-3 containment.
Statistical analysis
We compared risk factors associated with complicated and uncomplicated disease with the χ2 test for categorical variables. Continuous variables were tested by Student's t test. A p value of less than 0·05 was taken to be significant. We used SPSS (version 10.0) for all analyses. We did not do multivariate analysis since the number of cases was too small for meaningful results to be obtained.
Role of the funding source
The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or in the writing of the report.
Results
All 50 patients with SARS were ethnic Chinese. They represented five different epidemiologically linked clusters and sporadic cases fitting the case definition. They were admitted to hospital at a mean of 5 days (SD 2·3) after the onset of symptoms. The median age was 42 years (range 23–74) and the female-to-male ratio was 1 to 1·3. Among the patients, 14 (28%) were health-care workers and five (10%) had a history of a visit to a hospital in which there was a major outbreak of SARS, 13 (26%) were household contacts and 12 (24%) had social contacts with patients who had SARS; four (8%) had recently travelled to mainland China.
The presenting complaint in most patients was feverishness or shortness of breath. Cough and myalgia were present in more than half the patients (table 1 ). Upper-respiratory-tract symptoms such as rhinorrhoea (n=12, 24%) and sore throat (n=10, 20%) were present in a few patients. Watery diarrhoea (n=5, 10%) and anorexia (n=5, 10%) were also reported. At initial examination, auscultatory findings such as crepitations and decreased air entry were present in only 19 (38%) patients. Dry cough was reported by 31 (62%) patients. All had radiological evidence of consolidation at the time of admission involving one zone in 36, two zones in 13, and three zones in one.
Table 1.
Symptoms of 50 patients with SARS at presentation
| Clinical symptoms* | Number (%) |
|---|---|
| Fever | 50 (100) |
| Chill or rigors | 37 (74) |
| Cough | 31 (62) |
| Myalgia | 27 (54) |
| Malaise | 25 (50) |
| Running nose | 12 (24) |
| Sore throat | 10 (20) |
| Shortness of breath | 10 (20) |
| Anorexia | 10 (20) |
| Diarrhoea | 5 (10) |
| Headache | 10 (20) |
| Dizziness | 6 (12) |
Truncal maculopapular rash was noted in one patient.
Despite high fever, 49 (98%) patients had no evidence of a leucocytosis. In peripheral blood tests lymphopenia was present in 68%, leucopenia in 26%, thrombocytopenia in 40%, and anaemia in 18% (table 2 ). Alanine aminotransferase (45–350 U/L) and creatinine kinase (141–1379 U/L) were raised in 34% and 26%, respectively.
Table 2.
Initial laboratory findings of 50 patients with SARS
| Laboratory variables | Mean (range) | Number (%) of abnormal | Normal range | |
|---|---|---|---|---|
| Haemoglobin | 12·9 (8·9–15·9) | ‥ | 11·5–16·5 g/dL | |
| Anaemia | ‥ | 9 (18%) | ‥ | |
| White-cell count | 5·17 (1·1–11·4) | ‥ | 4–11×109/L | |
| Leucopenia | ‥ | 13 (26%) | ‥ | |
| Lymphocyte count | 0·78 (0·3–1·5) | ‥ | 1·5–4·0×109/L | |
| Severe lymphopenia (<1·0×109 /L) | ‥ | 34 (68%) | ‥ | |
| Platelet count | 174 (88–351) | ‥ | 150–400×109/L | |
| Thrombocytopenia | ‥ | 20 (40%) | ‥ | |
| Alanine aminotransferase | 63 (11–350) | ‥ | 6–53 U/L | |
| Raised alanine aminotransferase | ‥ | 17 (34%) | ‥ | |
| Albumin | 37 (26–50) | ‥ | 42–54 g/L | |
| Low albumin | ‥ | 34 (68%) | ‥ | |
| Globulin | 33 (21–42) | ‥ | 24–36 g/L | |
| Raised globulin | ‥ | 10 (20%) | ‥ | |
| Creatinine kinase | 244 (31–1379) | ‥ | 34–138 U/L | |
| Raised creatinine kinase | ‥ | 13 (26%) | ‥ | |
Routine microbiological investigation for known viruses and bacteria by culture, antigen detection, and PCR was negative in most cases. Blood culture was positive for Escherichia coli in one man aged 74 years admitted to intensive care. The finding was attributed to a hospital-acquired urinary-tract infection. Klebsiella pneumoniae and Haemophilus influenzae were isolated from the sputum samples of two other patients on admission.
Oral levofloxacin 500 mg every 24 h was given to nine patients, and amoxicillin-clavulanate given intravenously 1·2 g at 8 h intervals or orally 375 mg three times daily, and intravenous or oral clarithromycin 500 mg every 12 h were given to another 40 patients. Four patients received oral oseltamivir 75 mg twice daily. In one patient, intravenous ceftriaxone 2 g every 24 h, oral azithromycin 500 mg every 24 h, and oral amantadine 100 mg twice daily were given for empirical coverage of typical and atypical pneumonia.
At the time of writing, 19 patients had progressed to severe disease with oxygen desaturation requiring intensive care and ventilatory support for a mean of 6·4 days. The mean time between onset of symptoms and worsening was 8·3 days. Intravenous ribavirin 8 mg/kg every 8 h for 7–10 days and steroid (intravenous hydrocortisone 100 mg every 6 h, or hydrocortisone 200 mg every 8 h, or methylprednisolone 1–3 mg/kg every 24 h for two to three doses and tailed off over 2–3 weeks) was given in 49 patients at a mean of 6·7 days after onset of symptoms. Of the six patients given ribavirin and steroids before intubation and ventilation in intensive care, two had a consistent response in terms of resolution of fever, decreased respiratory support, and later radiological resolution, whereas the other four had fluctuating fever and static requirement in respiratory support.
The risk factors associated with severe complicated disease requiring intensive care and ventilatory support were older age, severe lymphopenia, impaired alanine aminotransferase, and delayed starting of ribavirin and steroid (table 3 ). All the complicated cases were treated with ribavirin and steroids after admission to the intensive-care unit, whereas all the uncomplicated cases were started on ribavirin and steroids in the general ward. As expected, 31 uncomplicated cases recovered or improved, whereas eight patients with complicated disease worsened, with one death at the time of writing. All 50 patients had been monitored for a mean of 12 days (SD 6·1) at the time of writing.
Table 3.
Risk factors associated with severe disease requiring intensive care and ventilatory support
| Complicated case (n=19) | Uncomplicated case (n=31) | p | ||
|---|---|---|---|---|
| Mean (SD) age | 49·5 (12·7) | 39·0 (10·7) | 0·005 | |
| Male/female ratio | 8/11 | 14/17 | ||
| Underlying illness | 5* | 1† | 0·05 | |
| Method of contact | ||||
| Travel to China | 1 | 3 | ||
| Health-care worker | 5 | 9 | ||
| Hospital visit | 1 | 4 | ||
| Household contact | 8 | 5 | 0·09 | |
| Social contact | 4 | 10 | ||
| Mean (SD) duration of symptoms to admission (days) | 5·2 (2·0) | 4·7 (2·5) | ||
| Mean (SD) admission temperature (°C) | 38·8 (0·9) | 38·7 (0·8) | ||
| Mean (SD) initial total peripheral WBC count (× 109/L) | 5·1 (2·4) | 5·2 (1·8) | ||
| Mean (SD) initial lymphocyte count (× 109/L) | 0·66 (0·3) | 0·85 (0·3) | 0·04 | |
| Presence of thrombocytopenia (<150× 109/L) | 8 | 12 | ||
| Impaired liver-function test | 11 | 6 | 0·01 | |
| Chest radiographic changes (number of zones affected) | 1·4 | 1·2 | ||
| Mean (SD) day of worsening from onset of symptoms‡ | 8·3 (2·6) | Not applicable | ||
| Number of patients who received ribavirin and steroids | 18 | 31 | ||
| Mean (SD) day of start of ribavirin and steroids from onset of symptoms | 7·7 (2·9) | 5·7 (2·6) | 0·03 | |
| Start of ribavirin and steroids after worsening | 12 | 0 | 0·0001 | |
| Response to ribavirin and steroids§ | 11 | 28 | 0·02 | |
| Outcome | ||||
| Improved or recovered | 10 | 31 | 0·0001 | |
| Not improving | 8 | 0 | 0·0004 | |
| Died | 1 | 0 | ||
WBC=white-blood cell.
Two patients had diabetes mellitus, one had hypertrophic obstructive cardiomyopathy, one had chronic active hepatitis B, and one had brain tumour.
One patient had essential hypertension.
Desaturation requiring intensive-care support.
Response defined as resolution of fever within 48 h, decreased ventilatory support, or radiological improvement.
Two virus isolates, identified as a coronavirus, were isolated from two patients. One was from an open lung biopsy sample from a male Hong Kong Chinese resident aged 53 years and the other from a nasopharyngeal aspirate of a woman aged 42 years with good previous health. The man had a history of 10 h social contact with a Chinese visitor coming from Guangzhou, mainland China, who later died from SARS. 2 days after exposure, this patient presented with fever, malaise, myalgia, and headache. Crepitations were present over the right lower zone and there was a corresponding alevolar shadow on the chest radiograph. Haematological investigation revealed lymphopenia of 0·7×109/L with normal total white-cell and platelet count. Alanine aminotransferase (41 U/L) and creatinine phosphokinase (405 U/L) were impaired. Despite a combination of oral azithromycin, amantadine, and intravenous ceftriaxone, there was increasing bilateral pulmonary infiltrates and progressive oxygen desaturation. Therefore, an open lung biopsy was done 9 days after admission. Histopathological examination showed a mild interstitial inflammation with scattered alveolar pneumocytes showing cytomegaly, granular amphophilic cytoplasm, and enlarged nuclei with prominent nucleoli. No cells showed inclusions typical of herpes virus or adenovirus infection. He required ventilation and intensive care after the surgical procedure. Empirical intravenous ribavirin and hydrocortisone were given. He died 20 days after admission. Coronavirus RNA was detected in his nasopharyngeal aspirate, lung biopsy samples, and postmortem lung samples. He had a significant rise in antibody titre (from 1/200 to 1/1600) to his own coronavirus isolate.
The female patient from whom a coronavirus was isolated had a history of good health. She had recently travelled to Guangzhou for 2 days. She presented with fever and diarrhoea 5 days after return to Hong Kong. Physical examination showed crepitation over the right lower zone, which had a corresponding alveolar shadow on chest radiograph. Investigation revealed leucopenia (2·7×109/L), lymphopenia (0·6×109/L), and thrombocytopenia (104×109/L). Despite empirical antimicrobial coverage with amoxicillin-clavulanate, clarithromycin, and oseltamivir, she worsened 5 days after admission and required mechanical ventilation and intensive care for 5 days. She gradually improved without treatment by ribavirin or steroids. Her nasopharyngeal aspirate was positive on RT-PCR for coronavirus and she seroconverted from titre less than one per 50 to one per 1600 to the coronavirus isolate.
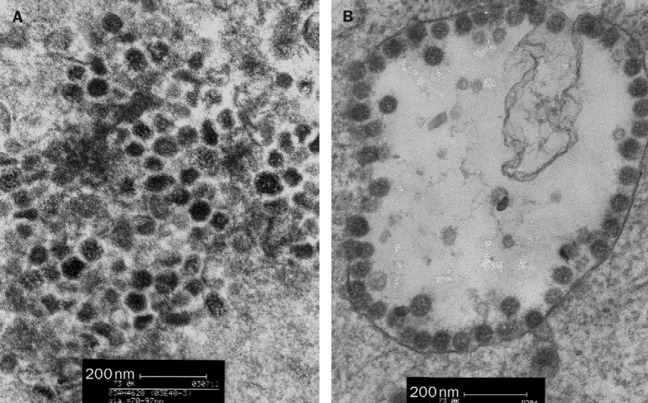
Viruses were isolated on fetal rhesus kidney cells from the lung biopsy and nasopharyngeal aspirate, respectively, of these two patients. The initial cytopathic effect noted was the appearance of rounded refractile cells appearing 2–4 days after inoculation. The cytopathic effect did not progress in the initial culture tubes but on subsequent passage, and appeared in 24 h. The two virus isolates did not react with the routine panel of reagents used to identify virus isolates, including those to influenza A, B, parainfluenza types 1, 2, and 3, adenovirus, and respiratory syncytial virus (DAKO, Glostrup, Denmark). They also did not react in RT-PCR assays for influenza A and human metapneumovirus, or in PCR assays for mycoplasma. The virus was ether sensitive, which shows that it was an enveloped virus. Electron microscopy of negative stained (3% potassium phospho-tungstate, pH 7·0) ultracentrifuged cell-culture extracts showed the presence of pleomorphic enveloped virus particles of around 80–90 nm (range 70–130 nm) in diameter with surface morphology compatible with a coronavirus (figure 1 ). Thin-section electron microscopy of infected cells revealed virus particles of 55–90 nm diameter within smooth walled vesicles in the cytoplasm (figure 2, B ). Virus particles were also seen at the cell surface. The overall findings were compatible with coronavirus infection in the cells.
Figure 1.
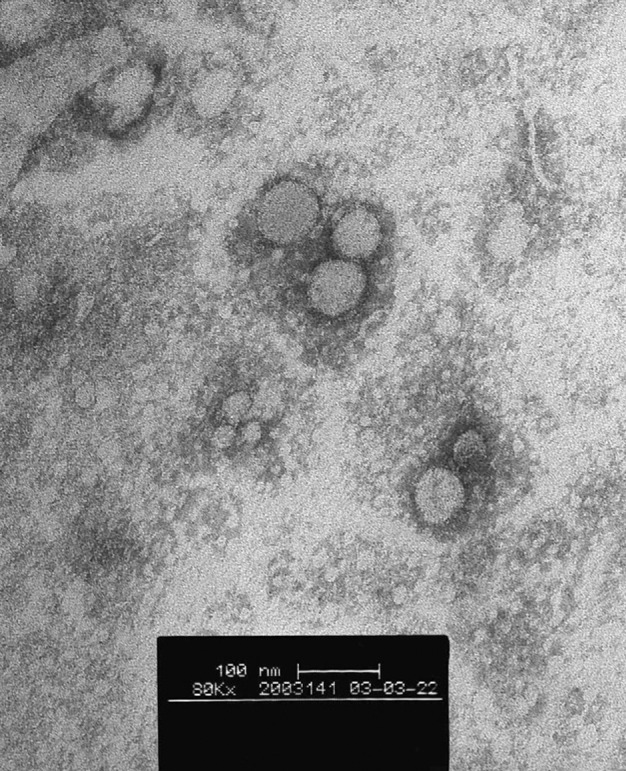
Electron microscopy of ultracentrifuged deposit of cell-culture-grown human pneumonia-associated coronavirus
Negatively stained with 3% potassium phospho-tungstate, pH 7·0.
Figure 2.
Thin-section electron micrograph of lung biopsy sample from patient with SARS (A) and of human pneumonia-associated coronavirus infected cells (B)
A thin-section electron micrograph of the lung biopsy sample from the 53-year-old male contained 60–90 nm viral particles in the cytoplasm of desquamated cells. These viral particles were similar in size and morphology to those observed in the cell cultured virus isolate from both patients (figure 2, A).
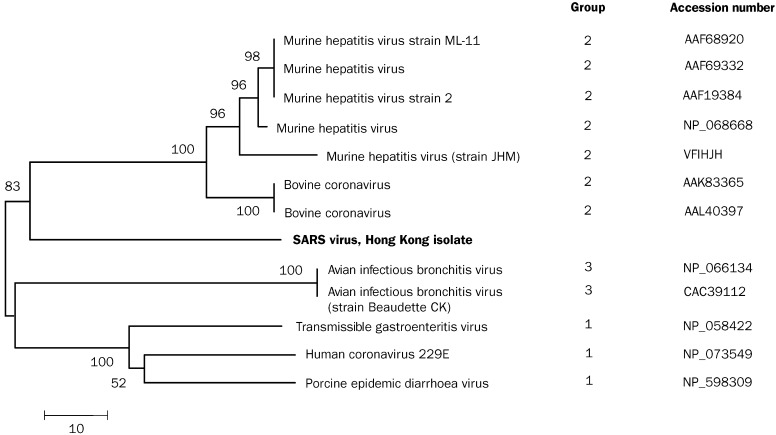
The RT-PCR products generated in a random primer RT-PCR assay were analysed, and unique bands found in the virus-infected samples were cloned and sequenced. Of 30 clones examined, one containing 646 bp of unknown origin was identified. Sequence analysis of this DNA fragment suggested this sequence had a weak homology to viruses of the family of Coronaviridae. Deducted aminoacid sequence (215 aminoacids) from this unknown sequence, however, had the highest homology (57%) to the RNA polymerase of bovine coronavirus and murine hepatitis virus, confirming that this virus belongs to the family of Coronaviridae. Phylogenetic analysis of the protein sequences showed that this virus, although most closely related to the group II coronaviruses, was a distinct virus (figure 3 ).
Figure 3.
Phylogenetic analysis of the partial protein sequence (215 aminoacids) of the coronavirus (SARS)
GenBank accession number AY268070. Tree is constructed by neighbour-jointing method. Horizontal-line distance represents number of sites at which the two sequences compared are different. Bootstrap values deducted from 500 replicates.
Based on the 646 bp sequence of the isolate, specific primers for detecting the new virus were designed for RT-PCR detection of this human pneumonia-associated coronavirus genome in clinical samples. Of the 44 nasopharyngeal samples available from the 50 SARS patients, 22 had evidence of human pneumonia-associated coronavirus RNA. Viral RNA was detectable in ten of 18 faecal samples tested. The specificity of the RT-PCR reaction was confirmed by sequencing selected positive RT-PCR-amplified products. None of 40 nasophararyngeal and faecal samples from patients with unrelated diseases were reactive on RT-PCR.
In 35 of the 50 most recent serum samples from patients with SARS there was evidence of antibody to the coronavirus. Of 32 patients from whom paired acute and convalescent sera were available, all had seroconverted or had more than a four-fold increase in antibody titre to the virus. Five other pairs of sera from additional SARS patients from clusters outside this study group were also tested to provide a wider sampling of SARS patients in the community and all of them seroconverted. None of 80 sera from patients with respiratory or other diseases and none of 200 blood donors had detectable antibody.
If seropositivity to human pneumonia-associated coronavirus in one serum sample or viral RNA detection in the nasopharyngeal aspirates or stools is deemed evidence of infection with the coronavirus, 45 of the 50 patients have evidence of infection. Of the five patients with no virological evidence of coronavirus infection, only one had a serum sample tested more than 14 days after onset of clinical disease.
Discussion
The outbreak of SARS is unusual in several ways, especially in the appearance of clusters of patients with pneumonia in health-care workers and family contacts. In this series of patients, investigations for conventional pathogens of atypical pneumonia proved negative. However, a virus belonging to the family Coronaviridae was isolated from the lung biopsy and nasopharyngeal aspirate of two SARS patients and other patients with SARS had a serological response to this virus.
The family Coronaviridae includes the genus Coronavirus and Torovirus. They are enveloped RNA viruses that cause disease in human beings and animals. The previously known human coronaviruses, types 229E and OC43 are a major cause of the common cold.6 They can occasionally cause pneumonia in older adults, neonates, or immunocompromised patients.7, 8 Coronaviruses have been reported to be an important cause of pneumonia in military recruits, accounting for up to 30% of cases in some studies.9 Human coronaviruses can infect neurons, and viral RNA has been detected in the brain of patients with multiple sclerosis.10 On the other hand, several animal coronaviruses (eg, porcine transmissible gastroenteritis virus, murine hepatitis virus, and avian infectious bronchititis virus) cause respiratory, gastrointestinal, neurological, or hepatic disease in their respective hosts.11
Phylogenetically, human pneumonia-associated coronavirus was not closely related to any known human or animal coronavirus or torovirus. We based our analysis on a 646 bp fragment of the polymerase gene which showed that the virus belongs to antigenic group 2 of the coronaviruses, along with murine hepatitis virus and bovine coronavirus. However, viruses of the Coronaviridae can undergo heterologous recombination within the virus family and genetic analysis of other parts of the genome needs to be done before the nature of this new virus is more conclusively defined.6 The biological, genetic, and clinical data taken together show that the new virus is not one of the two known human coronaviruses. Antibody to the previously recognised human 229E and OC43-like coronaviruses is widespread in the human population.12 The lack of serological reactivity against the novel pneumonia-associated coronavirus among our patients implies that there is little antigenic cross reactivity between it and the 229E or OC43 viruses.
Most patients who had clinically defined SARS had either serological or RT-PCR evidence of infection by this virus. By contrast, neither antibody nor viral RNA was detectable in healthy controls. All 32 patients from whom acute and convalescent sera were available had rising antibody titres to human pneumonia-associated coronavirus, which strengthens the contention that a recent infection with this virus is a necessary factor in the evolution of SARS. In addition, all five pairs of acute and convalescent sera tested from patients from other hospitals in Hong Kong also showed seroconversion to the virus. Five patients who had SARS had no serological or virological evidence of coronavirus infection. They need to have later convalescent sera tested to define whether they seroconvert subsequently. However, the concordance of human pneumonia-associated coronavirus with the clinical definition of SARS seems remarkable, given that clinical case definitions are never perfect.
No evidence of human-metapneumovirus infection, by RT-PCR or rising antibody titre, was detected in any of our patients and no other pathogen was consistently detected. It is therefore highly likely that that this coronavirus is either the cause of SARS or a necessary prerequisite for disease progression. Whether other microbial or non-microbial cofactors play a part in progression of the disease remains to be investigated.
We describe the clinical presentation and complications of SARS. Less than 25% of patients with coronaviral pneumonia had upper-respiratory-tract symptoms. As expected in atypical pneumonia, both respiratory symptoms and positive auscultatory findings were disproportionally mild compared with the chest radiographic findings. Gastrointestinal symptoms were present in 10% of patients. These symptoms are relevant since the viral RNA is detectable in faeces of some patients and coronaviruses have been associated with diarrhoea in animals and human beings.13 The high incidence of altered liver function, leucopenia, severe lymphopenia, thrombocytopenia, and subsequent evolution into adult respiratory distress syndrome suggests a severe systemic inflammatory damage induced by this human pneumonia-associated coronavirus. Thus immunomodulation by steroid treatment may be important to complement the empirical antiviral treatment with ribavirin. It is pertinent that severe human disease associated with the avian influenza subtype H5N1, another virus that has crossed from animals to human beings, has also been postulated to have an immunopathological component.14 In common with H5N1 disease, patients with severe SARS are adults, have lymphopenia, and have variables of organ dysfunction beyond the respiratory tract.15 A window of opportunity of around 8 days exists from the onset of symptoms to respiratory failure. Severe complicated cases are strongly associated with underlying disease and delayed use of ribavirin and steroid treatment. After our clinical experience in the first cases, we started this combination treatment very early in subsequent cases, which were generally uncomplicated at the time of admission. The overall mortality at the time of writing was only 2% with use of this treatment regimen. Eight of 19 complicated cases still had shown no notable response. A detailed analysis of the therapeutic response to this combination regimen is impossible, given the heterogeneous dosing and time of starting treatment. The choice of ribavirin was made on empirical grounds, before the cause was identified. It might have to be reviewed once the in-vitro susceptibility of human pneumonia-associated coronavirus to antivirals is better understood.
Another factor associated with severe disease is acquisition of the disease through household contact. People infected in this way may have a higher dose or duration of viral exposure and the presence of underlying diseases than people exposed, for example, through social contact.
Our clinical description pertains largely to the more severe cases admitted to hospital. We presently have no data on the full clinical spectrum of the emerging coronavirus infection in the community or among outpatients. The availability of diagnostic tests we describe will help address these questions. In addition, it will allow questions to be addressed about the period of virus shedding (and communicability) during convalescence, the presence of virus in other body fluids and excreta, and the presence of virus shedding during the incubation period.
The epidemiological data at present seem to suggest that the virus is spread by droplets or by direct and indirect contact, although airborne spread cannot be ruled out. The finding of infectious virus in the respiratory tract supports this contention. Preliminary evidence also suggests that the virus may be shed in the faeces. However, detection of viral RNA does not prove that the virus is viable or transmissible. If viable virus is detectable in the faeces, this is potentially an additional route of transmission. Several animal coronaviruses are spread via the faecal-oral route.11 Samples from patients with SARS were not readily distinguishable from samples from other patients on receipt in the laboratory. Thus, initial processing of these samples was done under biohazard level-2 containment. However, culture of the virus was done in biosafety level-3 containment. These containment measures have proved successful so far, in that no laboratory infections have been documented.
We have provided evidence that a virus in the coronavirus family is the causal agent of SARS. However it remains possible that other viruses act as opportunistic secondary invaders to increase the disease progression, a hypothesis that needs to be investigated further.
Acknowledgments
Acknowledgments
We thank E K Yeoh of the Hong Kong Government for facilitating this study. This work was greatly facilitated by the WHO Network of laboratories investigating the causes of SARS. The laboratories are Centers for Disease Control and Prevention, Atlanta, GA, USA; The Chinese University of Hong Kong; Erasmus Universiteit, Rotterdam, Netherlands; The Government Virus Unit, Hong Kong SAR; Institut für Medizinische Virologie im Klinikum der Johann Wolfgang Goethe-Universität Frankfurt am Main, Frankfurt, Germany; Institut Pasteur, Paris, France; National Institute of Infectious Diseases, Tokyo, Japan; National Microbiology Laboratory, Population Public Health Branch, Health Canada; Public Health Laboratory Service, Colindale, London, UK; The University of Hong Kong, Virology Unit; Singapore General Hospital, Singapore. We thank the staff of the Department of Microbiology, Queen Mary Hospital, Hong Kong, and the Government Virus Unit of the Department of Health for their technical assistance. We received research funding from Public Health Research (grant A195357), the National Institute of Allergy and Infectious Diseases, USA, the University of Hong Kong, and the Hospital Authority of Hong Kong SAR.
Contributors
J S M Peiris and K Y Yuen are co-principal investigators, jointly wrote the report, and supervised the virological and clinical components of the study. L L M Poon obtained viral sequence data and developed the RT-PCR assay. Y Guan, K H Chan, W Lim, and J M Nicholls did the phylogenetic analysis, clinical virology, and electron microscopy to identify the novel virus. T K Ng, D N C Tsang, R Yung, and W Lim coordinated the microbiological investigations and analysed the overall results. All other researchers were involved in collection and analysis of the clinical data.
Members of the HKU SARS study group
I F N Hung, Department of Medicine, S W Kwan, K F Lo, W H Seto, Department of Microbiology, Queen Mary Hospital, Hong Kong; O T Y Tsang, E Y K Tso, Department of Medicine, Princess Margaret Hospital; Queen Mary Hospital, Hong Kong; W Luk, H Y Ng, L J Zhang, C Y Cheung, O K Wong, W Cheung, Department of Microbiology, University of Hong Kong.
Conflict of interest statement
None declared.
Footnotes
Members listed at the end of paper
References
- 1.WHO Severe acute respiratory syndrome (SARS) Wkly Epidemiol Rec. 2003;78:86. [PubMed] [Google Scholar]
- 2.WHO Severe acute respiratory syndrome (SARS) Wkly Epidemiol Rec. 2003;78:81–83. [PubMed] [Google Scholar]
- 3.Chan KH, Maldeis N, Pope W. Evaluation of directigen flu A+B test for rapid diagnosis of influenza A and B virus infections. J Clin Microbiol. 2002;40:1675–1680. doi: 10.1128/JCM.40.5.1675-1680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiedbrauk DL, Johnston SLG. Manual of clinical virology. Raven Press; New York: 1993. [Google Scholar]
- 5.Fouchier RA, Bestebroer TM, Herfst S, Van Der Kemp L, Rimmelzwaan GF, Osterhaus AD. Detection of influenza A virus from different species by PCR amplification of conserved sequences in the matrix gene. J Clin Microbiol. 2000;38:4096–4101. doi: 10.1128/jcm.38.11.4096-4101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes KV. Coronaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4th edn. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 1187–1203. [Google Scholar]
- 7.El-Sahly HM, Atmar RL, Glezen WP, Greenberg SB. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fotz RJ, Elkordy MA. Coronavirus pneumonia following autologous bone marrow transplantation for breast cancer. Chest. 1999;115:901–905. doi: 10.1378/chest.115.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenzel RP, Hendley JO, Davies JA, Gwaltney JM., Jr Coronavirus infections in military recruits: three-year study with coronavirus strains OC43 and 229E. Am Rev Respir Dis. 1974;109:621–624. doi: 10.1164/arrd.1974.109.6.621. [DOI] [PubMed] [Google Scholar]
- 10.Talbot PJ, Cote G, Arbour N. Human coronavirus OC43 and 229E persistence in neural cell cultures and human brains. Adv Exp Med Biol (in press).
- 11.McIntosh K. Coronaviruses: a comparative review. Curr Top Microbiol Immunol. 1974;63:85–129. [Google Scholar]
- 12.McIntosh K, Kapikian AZ, Turner HC, Hartley JW, Parrott RH, Chanock RM. Seroepidemiologic studies of coronavirus infection in adults and children. Am J Epidemiol. 1970;91:585–592. doi: 10.1093/oxfordjournals.aje.a121171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caul EO, Egglestone SI. Further studies on human enteric coronaviruses. Arch Virol. 1977;54:107–117. doi: 10.1007/BF01314383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung CY, Poon LLM, Lau ASY. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease. Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 15.Yuen KY, Chan PKS, Peiris M. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]