Common problems in ill returned travelers to Brazil are dermatologic conditions, diarrhea, and febrile illnesses, especially dengue. Knowledge of potential risks can help clinicians to advise travelers attending large events in Brazil and to care for those who become ill.
Keywords: Brazil, travelers, mass gatherings, dengue, dermatologic
Abstract
Background. Brazil will host the 2014 FIFA World Cup and the 2016 Olympic and Paralympic Games, events that are expected to attract hundreds of thousands of international travelers. Travelers to Brazil will encounter locally endemic infections as well as mass event–specific risks.
Methods. We describe 1586 ill returned travelers who had visited Brazil and were seen at a GeoSentinel Clinic from July 1997 through May 2013.
Results. The most common travel-related illnesses were dermatologic conditions (40%), diarrheal syndromes (25%), and febrile systemic illness (19%). The most common specific dermatologic diagnoses were cutaneous larva migrans, myiasis, and tungiasis. Dengue and malaria, predominantly Plasmodium vivax, were the most frequently identified specific causes of fever and the most common reasons for hospitalization after travel. Dengue fever diagnoses displayed marked seasonality, although cases were seen throughout the year. Among the 28 ill returned travelers with human immunodeficiency virus (HIV) infection, 11 had newly diagnosed asymptomatic infection and 9 had acute symptomatic HIV.
Conclusions. Our analysis primarily identified infectious diseases among travelers to Brazil. Knowledge of illness in travelers returning from Brazil can assist clinicians to advise prospective travelers and guide pretravel preparation, including itinerary-tailored advice, vaccines, and chemoprophylaxis; it can also help to focus posttravel evaluation of ill returned travelers. Travelers planning to attend mass events will encounter other risks that are not captured in our surveillance network.
Brazil will host the 2014 FIFA (Fédération Internationale de Football Association) World Cup and the 2016 Olympic and Paralympic Games. These events will attract participants and spectators from around the globe. The FIFA World Cup, to be held from 12 June through 14 July 2014 in 12 cities (Figure 1), is expected to draw an estimated 600 000 international tourists and 3 million domestic travelers [1]. The Brazil Olympic Games, which will take place mainly in Rio de Janeiro, will be held 5–21 August 2016, followed by the summer Paralympics, 7–18 September [2]. Based on data from the 2012 Olympics in London, the 2016 Olympic Games and Paralympics are expected to attract 600 000 international visitors, including 15 000–17 000 athletes [3].
Figure 1.
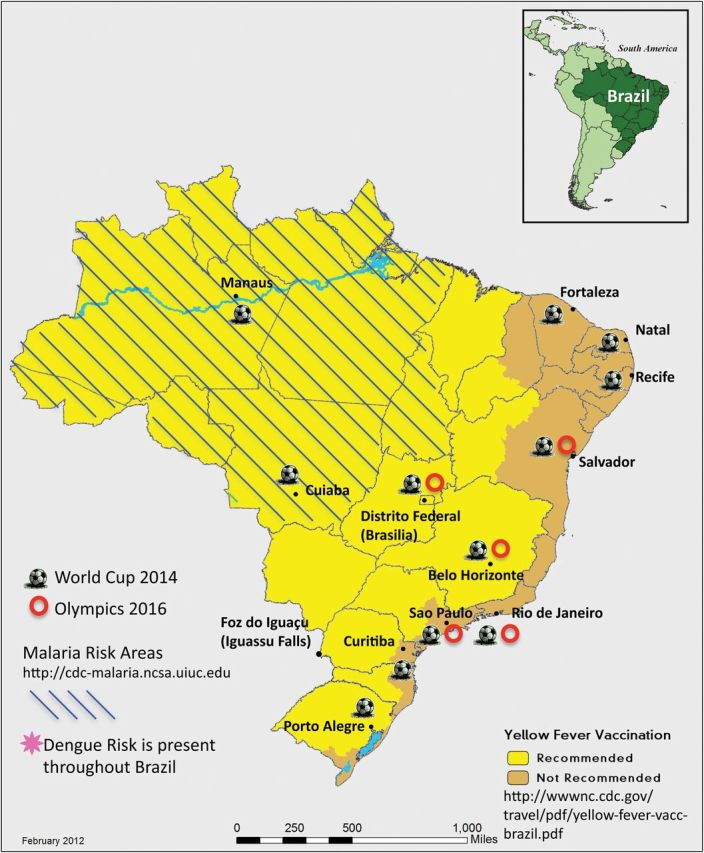
Map of Brazil (includes shading for yellow fever and malaria and all major cities where World Cup and Olympic and Paralympic Games will be held). Sources: http://cdc-malaria.ncsa.uiuc.edu, http://wwwnc.cdc.gov/travel/pdf/yellow-fever-vacc-brazil.pdf, http://www.rio2016.org/en/the-games/maps/venues-map, http://www.fifa.com/worldcup/destination/cities/index.html.
Brazil is a large country with abundant natural resources and varied terrain, including the Amazon basin and tropical rain forests. In Rio de Janeiro alone, one can find beaches, mountains, a rain forest, and a lagoon. Although the sports events will be held at specific venues, travelers to these events may also visit other parts of Brazil.
Brazil has made great progress over the last 3 decades in providing safe water, improved sanitary facilities, and access to healthcare, including vaccination and human immunodeficiency virus (HIV) treatment [4]. However, attendees of these sports events will potentially be at risk for health problems, both infectious and noncommunicable, and exacerbation of chronic diseases. Noncommunicable health risks include intentional and unintentional injuries that can be caused by violence and mass gatherings, and environmental stressors. Infectious diseases include locally endemic infections as well as widely distributed infections, such as influenza and sexually transmitted infections (STIs). Risk for the latter may be heightened during mass gatherings [5].
Deaths from infectious diseases in Brazil have dropped dramatically since 1980 [4], yet malaria and yellow fever (YF) remain endemic in parts of the country (Figure 1). Dengue has caused recent massive urban outbreaks; HIV/AIDS is widespread; leishmaniasis and schistosomiasis are present in focal areas [6, 7].
In anticipation of increased travel to Brazil, we have analyzed data collected by the GeoSentinel Surveillance Network about illness in travelers returned from Brazil. Knowledge of these illnesses can inform health providers about specific risks and guide pretravel preparation; it can also help to focus posttravel evaluation of ill returned travelers. Our data describe primarily infectious diseases and understate the frequency of injury, which may be a greater risk to those attending mass events [8].
METHODS
The GeoSentinel Surveillance Network (www.istm.org/geosentinel) [9] is an international network of specialized travel and tropical medicine clinics located on 6 continents. All sites collect data by using a standard reporting form on ill travelers seen during or after international travel. Anonymized data on demographics, travel history, reason for travel, pretravel advice, hospitalization, major clinical symptoms, and final diagnoses assigned by the GeoSentinel site clinician are electronically entered into a central database. Diagnoses are selected from a standard list of >500 diagnostic codes, and involve syndromic groupings alone if no etiology is defined or syndromic groupings plus specific etiologies where possible. Individual patients can have >1 final diagnosis. All sites use the best reference diagnostic tests available in their own country. Country of exposure is identified by the clinician based on the travelers' itinerary, known endemicity patterns of the destinations visited, and incubation period of the illness.
This evaluation includes ill travelers who were seen after travel at a GeoSentinel site from July 1997 through May 2013, with Brazil declared as the single country of exposure; travelers with another country of exposure stated along with Brazil were excluded. Those traveling for immigration were also excluded. Final diagnoses were included if they had been classified as confirmed or probable. Demographic and travel characteristics of ill travelers returning from Brazil were described by using frequencies and proportions for categorical variables and median and range for continuous variables. Analysis of illness trends over time was based on monthly counts of ill returned travelers aggregated over the entire study period for dengue. Because the World Cup and Olympic Games will take place during the cooler months in Brazil, we specifically examined and aggregated top diagnoses reported during June through September. Numbers of dengue cases per month in Brazil during 2008–2012 are from the Brazilian Ministry of Health (link: http://dtr2004.saude.gov.br/sinanweb/tabnet/dh?sinannet/dengue/bases/denguebrnet.def). All data analysis was performed using SAS software, version 9.2 (Cary, North Carolina).
RESULTS
Table 1 shows the demographic and trip characteristics of 1586 ill returned travelers to Brazil. A majority of these travelers were male (55%), and they were often traveling for tourism (65%). Only 50% sought pretravel advice. Trip duration was <30 days for 59%. Of those who traveled to Brazil, 233 (15%) traveled to other countries within the 6 months before their clinic visit date. Top syndromic diagnoses in ill returned travelers (Table 2) were dermatologic (40%), diarrheal syndromes (25%), and febrile systemic illness (19%). The most frequent specific dermatologic diagnoses were cutaneous parasitic infections including cutaneous larva migrans (CLM), myiasis, and tungiasis; skin and soft tissue infections were also observed (Table 2). CLM was the most common diagnosis during June, July, and September (Table 3).
Table 1.
Demographic and Trip Characteristics of Ill Returned Travelers Exposed in Brazil Seen at GeoSentinel Clinics, July 1997–May 2013 (N = 1586)
| Characteristic | |
|---|---|
| No. (%) | |
| Male sex | 875 (55) |
| Age, y | |
| Median, range | 33 (0–78) |
| <15 | 83 (5) |
| 15–54 | 1306 (83) |
| ≥55 | 193 (12) |
| Sought pretravel advice | |
| Yes | 755 (50) |
| No | 519 (35) |
| Don't know | 226 (15) |
| Expatriate | 134 (9) |
| Travel reason | |
| Tourism | 1030 (65) |
| VFR | 137 (9) |
| Business | 190 (12) |
| Volunteera | 201 (13) |
| Student | 24 (2) |
| Medical tourismb | 1 (<1) |
| Hospitalization | 116 (7) |
| Trip durationc | |
| <30 d | 842 (59) |
| ≥30 d | 579 (41) |
Missing values: age (4), pretravel advice (86), travel reason (3), hospitalization (6), trip duration (165).
Abbreviation: VFR, visiting friends and family.
a Category includes missionary/volunteer/researcher/aid worker.
b Medical tourism: the primary purpose of the travel was to seek medical care and the person developed a health problem as a consequence of this particular travel.
c Trip duration is calculated based on total trip, including travel to countries other than Brazil.
Table 2.
Top Syndrome Groups and Top Diagnosesa of Ill Returned Travelers Exposed in Brazil Seen at GeoSentinel Clinics, July 1997–May 2013 (N = 1586)
| Diagnosis | No. | % |
|---|---|---|
| 1. Dermatologic syndromes | 630 | 40 |
| Cutaneous larva migrans, hookworm-related | 167 | 27 |
| Bite, insectb | 99 | 16 |
| Skin and soft tissue infectionc | 92 | 15 |
| Rash, unknown etiology (nonfebrile) | 43 | 7 |
| Myiasis | 37 | 6 |
| Tungiasis | 35 | 6 |
| Fungal infection (superficial/cutaneous mycosis) | 26 | 4 |
| Rabies, postexposure prophylaxis | 17 | 3 |
| Leishmaniasis, cutaneous | 13 | 2 |
| 2. Diarrheal syndromes | 395 | 25 |
| Acute diarrhea, etiology unknownd | 146 | 37 |
| Diarrhea, chronic unknown | 70 | 18 |
| Giardiasis | 37 | 9 |
| Campylobacter infection | 14 | 4 |
| 3. Febrile syndromes | 297 | 19 |
| Unspecified febrile illnesse | 109 | 37 |
| Dengue | 92 | 31 |
| Malariaf | 25 | 8 |
| Epstein-Barr virus infection/mononucleosis | 17 | 6 |
| Influenza-like illness | 17 | 6 |
a One or more diagnoses are possible for each ill returned traveler.
b Includes bite, insect (including sting), suprainfected.
c Includes skin and soft tissue infection; skin and soft tissue infection, secondary bacterial of existing lesion; skin and soft tissue infection, superficial skin abscess.
d Includes diarrhea, acute bacterial; diarrhea, acute unspecified; gastroenteritis.
e Includes febrile illness unspecified (<3 weeks); viral syndrome (no rash).
f Includes Plasmodium falciparum; Plasmodium vivax (n = 20); species unknown.
Table 3.
Frequency of Top 5 Specific Diagnoses of Ill Returned Travelers Exposed in Brazil Seen at GeoSentinel Clinics, by Month, June– September
| Month and No. of Diagnoses | |||||||
|---|---|---|---|---|---|---|---|
| June | July | August | September | ||||
| CLM | 15 | CLM | 20 | Acute diarrhea, etiology unknowna | 15 | CLM | 11 |
| Acute diarrhea, etiology unknowna | 10 | PI-IBS | 8 | Febrile unspecified <3 wk | 7 | Viral syndrome (no rash) | 10 |
| Dengue | 6 | Acute diarrhea, etiology unknowna | 8 | PI-IBS | 7 | Diarrhea, chronic unknown | 9 |
| Strongyloides | 5 | Giardiasis | 6 | Insect bites and stings | 6 | Acute diarrhea, etiology unknowna | 7 |
| URTI | 5 | Unknown nonfebrile rash | 5 | CLM | 6 | Tungiasis, dengueb | 5 |
Abbreviations: CLM, cutaneous larva migrans, hookworm-related; PI-IBS, irritable bowel syndrome, postinfectious; URTI, upper respiratory tract infection.
a Acute diarrhea, etiology unknown: diarrhea, acute bacterial; diarrhea, acute unspecified; gastroenteritis.
b There were 5 cases for each diagnosis.
Among those with both acute and chronic diarrheal syndromes, no specific pathogen was identified for >50%. Campylobacter, the bacterial gastrointestinal pathogen most often identified, was found in 4%; giardiasis was diagnosed in 9%. Intestinal strongyloidiasis was diagnosed in 15 and hyperinfection syndrome in 1; schistosomiasis was diagnosed in 11.
Among 297 diagnoses of systemic febrile illness were 92 (31%) diagnoses of dengue fever and 25 (8%) diagnoses of malaria, 20 of them Plasmodium vivax (2 each Plasmodium falciparum and malaria species unknown, and 1 Plasmodium ovale). There were 17 diagnoses of Epstein-Barr virus/mononucleosis, 7 of cytomegalovirus, and 17 of influenza-like illness (2 confirmed).
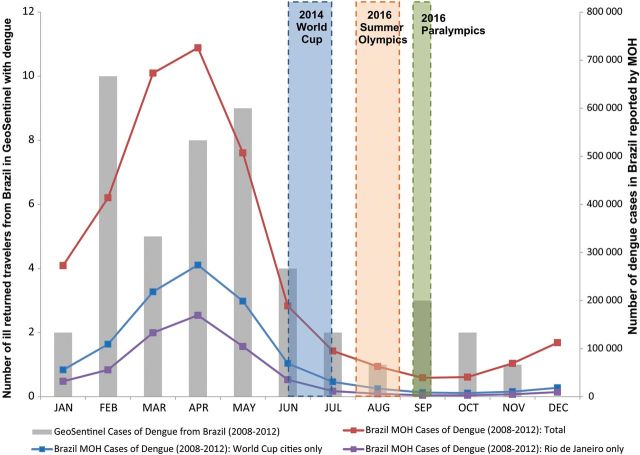
Figure 2 shows the number of dengue fever diagnoses according to month for 2008 through 2012. Table 3 shows top diagnoses during the cooler months in Brazil, when fewer dengue infections were seen.
Figure 2.
Dengue fever diagnoses of ill returned travelers exposed in Brazil seen at GeoSentinel clinics, by month, 2008–2012 (n = 48).
Source: Ministry of Health (MOH), Brazil (http://dtr2004.saude.gov.br/sinanweb/tabnet/dh?sinannet/dengue/bases/denguebrnet.def).
Dengue and malaria were the most common diagnoses leading to hospitalization, recorded for 20% of dengue and 64% of malaria patients. Infrequently diagnosed infections in ill returned travelers to Brazil included rickettsial infections (n = 3), histoplasmosis (n = 3), visceral leishmaniasis (n = 1), and leptospirosis (n = 1). No deaths were recorded.
Although it was not one of the top diagnoses, HIV infection was found in 28 ill returned travelers, including 3 with AIDS. Eleven had newly diagnosed asymptomatic infection and 9 had acute symptomatic HIV. Among other STIs, syphilis was reported in 3, urethritis 3, lymphogranuloma venereum 1, and STI (undefined) 1.
No cases of measles, mumps, rubella, pertussis, acute hepatitis B, or yellow fever were reported, and most vaccine-preventable infections, except for influenza, were infrequent or not observed. Four cases of enteric fever (2 typhoid, 2 paratyphoid fever) and 4 hepatitis A cases were reported. Seventeen returned travelers were seen for rabies postexposure prophylaxis; specifically reported exposures were dogs (n = 7), monkeys (n = 3), and a cat (n = 1).
DISCUSSION
Mass gatherings entail a series of event-specific, individual health risks. In the past, many injuries and casualties have been attributable to stampedes and crush injuries, violence, crime, and even terrorist attacks, traffic accidents, and trauma associated with drug and alcohol intoxication [8]. Emotional stress, aggression, and cardiovascular events may be a heightened risk, in particular during sports events, in addition to outdoor activity–specific health risks such as sunburn, heatstroke, or dehydration [8]. Although weather and environmental factors cannot be altered and many preventive and preemptive measures have to be organized by local authorities, many of the above-listed health risks can be, at least partly, controlled by the individual (eg, using sun protection, continuous rehydration, and avoiding dangerous areas and excess alcohol consumption). Pretravel preparation should include education about these risks.
Travelers to Brazil may encounter Brazil-endemic infections as well as mass event–related problems. Based on GeoSentinel data, we have identified dermatologic problems, diarrhea, and febrile systemic infections (especially dengue) as the most common diagnoses in returned travelers from Brazil. Dengue fever and malaria were the most common reasons for hospitalization.
Skin problems accounted for 40% of diagnoses in ill returned travelers. The most common diagnosis, CLM, was found in 11%. The etiologic agents of CLM are common in Brazil [10]. Almost 90% of dogs and 94.2% of cats in Adrandina Municipality, Sao Paulo, were infected with Ancylostoma caninum and Ancylostoma brasiliense [11]. Thirty percent of beach sand samples from Alto Beach, Pernambuco (near Recife) contained larvae of these organisms [12]. Most travelers acquire infection during beach exposures. Many popular tourist destinations and sites for the sports events, such as Rio de Janeiro, Salvador, Recife, and Fortaleza have coastal locations. Hookworm larvae in sand and soil penetrate the dermis and migrate superficially, causing migrating linear, serpiginous tracks accompanied by severe pruritus [13] (Figure 3). The feet are most often affected (39%), followed by the buttocks and abdomen. Infection may persist weeks to months, rarely up to a year. Oral albendazole or ivermectin provides safe, effective treatment [14, 15]. Prevention is by avoidance of skin contact with soil/sand.
Figure 3.
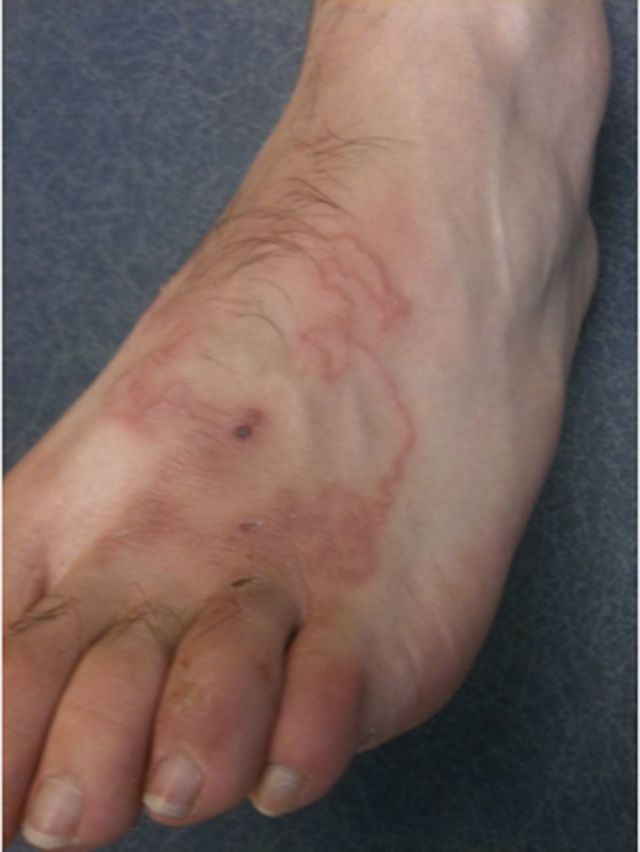
Cutaneous larva migrans: a 26-year-old man returned from Jamaica with a severely pruritic serpiginous rash.
Myiasis and tungiasis each accounted for >2% of diagnoses in ill returned travelers. Furuncular myiasis is a skin infection with the larva of a Diptera fly, most frequently Dermatobium hominis [16]. The adult fly lays its eggs on the underbelly of a mosquito that subsequently feeds on humans. The eggs lodge in the dermis and develop to third-stage larvae over 1–5 months, each manifesting as a small papule that enlarges to a tender nodule with a central punctum through which serosanguinous fluid drains (Figure 4). Treatment is by suffocation and removal of the larva [17]; prevention is by use of insect repellents and/or wearing long sleeves and trousers during the day.
Figure 4.
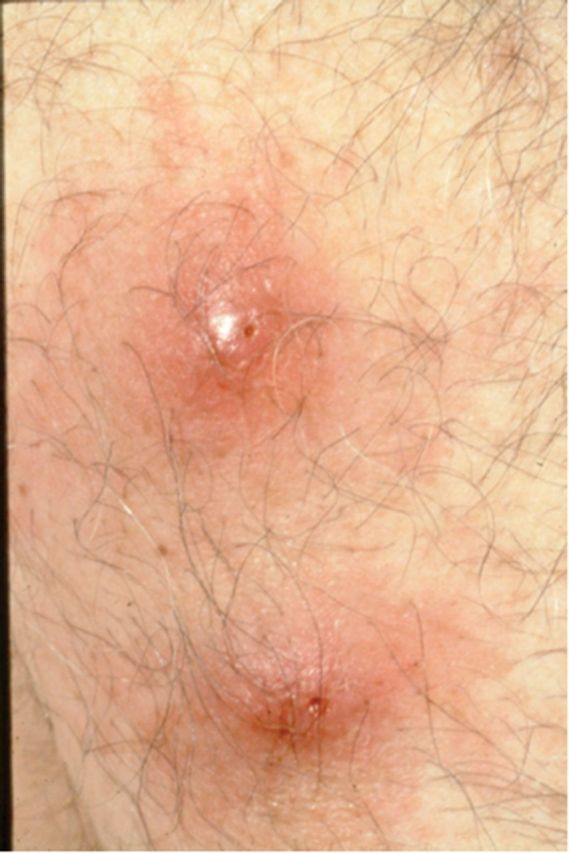
Myiasis: a 50-year-old man returned from Belize feeling intermittent stabbing pain and movement within his arm lesions.
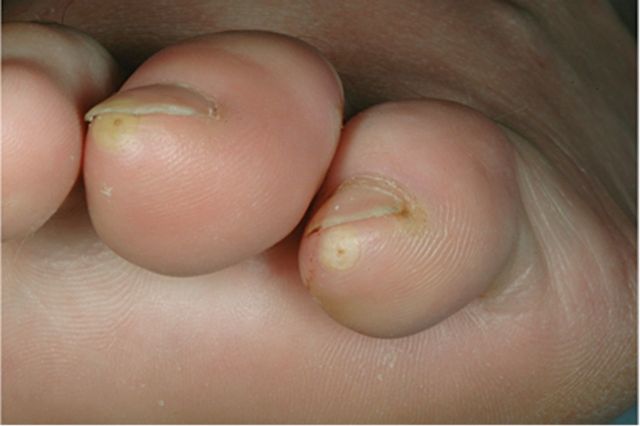
Tungiasis, caused by penetration of the epidermis by the female sand fly Tunga penetrans and related species, can occur virtually anywhere on the skin, although >97% are found on the feet [18, 19]. Black papules at the site of penetration develop into nodules with a pale halo. Treatment is removal of the flea [20] (Figure 5); prevention is by the use of closed-toed footwear and avoidance of skin contact with soil.
Figure 5.
Tungiasis: a 21-year-old woman returned from Peru with painful nodules on her toes.
Cutaneous leishmaniasis was seen infrequently in travelers returned from Brazil, although it remains a public health problem in focal areas (>20 000 new cases reported annually in Brazil) [21]; visceral leishmaniasis is a growing problem [4]. Travelers should be advised to reduce bites from sandflies, whose bite transmits infection, by using insect repellent and/or wearing long-sleeved shirts and trousers during evening hours in endemic regions.
Given the frequency of both acute and chronic diarrheal syndromes among ill travelers returning from Brazil, food and water precautions are recommended. Clinicians may provide symptomatic antidiarrheal agents and antibiotics for presumptive self-treatment should diarrhea develop (eg, loperamide plus quinolone or azithromycin for bacterial pathogens).
Dengue, the most frequent specified cause of systemic febrile illness, was reported in almost 6% of ill returned travelers; 20% were hospitalized. Cases occurred throughout the year (Figure 2). Since 1986, dengue in Brazil has increased in incidence and severity [22]. Massive epidemics have affected urban centers, including Rio de Janeiro and Sao Paulo [22, 23]; 3.5 million cases were reported during 2000–2009. Three dengue serotypes, DENV-1, DENV-2, and DENV-3, circulate widely in Brazil; DENV-4 has caused recent outbreaks [24]. Transmission is most intense during February through June. The day-biting main mosquito vector, Aedes aegypti, is widespread in urban areas. The primary way to avoid infection is to prevent mosquito bites with repellent on exposed skin. Only travelers to certain areas of Brazil will be at risk for malaria, but travelers to all areas will be at risk for dengue.
Malaria transmission persists in the Brazilian Amazon region (Figure 1); about 300 000 cases are reported annually, with P. vivax accounting for >80% and P. falciparum <20% of cases. GeoSentinel cases reflect these proportions. Travelers who will visit malaria-endemic areas can be protected with chemoprophylaxis [25]. Some non-US guidelines recommend standby emergency treatment as an alternative.
No cases of yellow fever were recorded among 1586 ill returned travelers to Brazil; however, cases still occur in Brazil despite vaccination programs. From 1973 through 2008, among the 831 notified cases of YF in Brazil, mortality was 51%. Groups most affected were migrant laborers, farm workers, and tourists [26]. In recent years, the Brazil Ministry of Health has expanded the areas for which YF vaccination is recommended. Vaccination is not recommended for travel to the large eastern cities of Rio de Janeiro, Sao Paulo, Salvador, Recife, and Fortaleza, but is recommended for several cities hosting World Cup events, including Belo Horizonte, Brasilia, and Manaus (Figure 1). Urban areas in Brazil are infested with mosquitoes competent to transmit YF virus [27]. Brazil has reported to the World Health Organization that no YF vaccine is required for entry, but conflicting information has been provided by some Brazilian authorities. Travelers should check their entry requirement with Brazilian authorities in their own countries as well as the Brazil Ministry of Health.
Travel is a risk factor for STIs. An estimated 20%–50% of travelers have casual sex [28, 29]. Approximately half of travel-related sex is unsafe sex [28]. Isolation from family and removal of inhibitions because of anonymity and/or alcohol may contribute to sexual behavior during travel. Among ill travelers who consulted a GeoSentinel site, 0.9% were diagnosed with a travel-related STI [30]. HIV infections, including acute HIV, were reported in 28 returned travelers, including 3 with AIDS, and possibly others infected before their recent trip. In Brazil, an estimated 600 000 people are HIV-infected (mean national seroprevalence <0.6%); about 33 000 new infections are reported yearly [4]. Travelers should be reminded to avoid unsafe sexual practices and exposure to potentially contaminated needles and blood.
The absence of the vaccine-preventable infections measles, rubella, mumps, or pertussis may reflect the high rates of vaccine coverage in Brazil or high coverage in travelers to Brazil. Measles was eliminated from Brazil in 1999. However, travelers should be up to date with these vaccinations; during mass events they will have contact with visitors from measles-endemic countries. Airborne transmission of measles occurred in a domed stadium and other venues during the International Special Olympic Games in 1991 [31]. Superspreading events can rapidly lead to large epidemics [32]. Measles and mumps outbreaks have also followed other mass gatherings [33, 34]. After the Winter Olympic Games in Vancouver (February 2010), a measles outbreak occurred with 82 confirmed cases [35]. Other vaccine-preventable infections, including typhoid fever, hepatitis A, and acute hepatitis B, were infrequent or absent in ill returned travelers, possibly reflecting improved sanitation and access to healthcare in Brazil [4] and pretravel immunization. Hepatitis A vaccine is still recommended for most travelers to Brazil.
The close interaction of large numbers of people from all over the world can facilitate transmission of infections spread from person to person, such as norovirus (outbreaks during 2006 World Cup) and influenza [5, 36, 37]. Although few cases of influenza were reported in our data, during mass gatherings influenza may have greater prominence. During the Salt Lake City Winter Olympic Games, 316 of the 2635 (12%) clinic visits were for respiratory illness, and 188 (59%) travelers were diagnosed with influenza-like illness. Thirty-six had confirmed influenza; 36% of these were in athletes [37]. Surveillance of illnesses at 6 Beijing clinics during the 2008 Olympic and Paralympic Games found that the most common diagnoses for foreign visitors were respiratory, injury/musculoskeletal, and gastrointestinal illnesses [38].
The World Cup and Olympic Games will take place during Brazil's winter season. An analysis of influenza-associated excess mortality data from Brazil for the period 1980–2008 showed no clear seasonality in northern, tropical Brazil. In southern Brazil (location of most sports events; Figure 1), 73.5% of 627 influenza viruses identified through routine surveillance (2000–2008) were detected during April to August, and influenza showed clear seasonality, peaking in June and July [39].
Influenza vaccine is recommended with the vaccine produced for the Southern Hemisphere, but if that is not available, then pretravel vaccination with the Northern Hemisphere strains should be considered. Influenza outbreaks can spread rapidly during mass events, and returning travelers aid global virus dispersal [40].
Limitations
These results reflect sentinel surveillance data among ill returned travelers visiting specialist (GeoSentinel) clinics and are not representative of all ill returned travelers. Individuals traveling to the World Cup or Olympic events may have a different spectrum of illnesses. Those with mild or self-limited illnesses may not seek care or may see their primary care provider. People do not seek care for injuries or STIs at a GeoSentinel clinic. Hospitalized patients may not be captured in the database. Denominators including travelers who remained healthy are lacking, so the incidence of illness in travelers cannot be calculated. Additionally, immunization status and use of malaria chemoprophylaxis are unknown. In only 10% of diagnoses was the probable location of illness acquisition within Brazil recorded, too small to derive meaningful conclusions about regional differences. Finally, this analysis is based on past events. Future risks could be different, including potential disrupters, such as chikungunya virus, a novel coronavirus, and new influenza viruses. Although local transmission of chikungunya virus has not yet been reported in Brazil, competent vectors are present [40]. Since December 2013, local transmission has been reported in the Americas [42]. Introduction into Brazil is a serious threat, given large numbers of visitors from areas with ongoing chikungunya virus transmission [43].
Although this paper highlights risks for travelers to mass sports events, the findings are also relevant to those traveling to Brazil for other reasons and at other times. Preparation for Brazil travel (Table 4) involves reviewing the itinerary for potential need for YF vaccination and malaria chemoprophylaxis. Influenza vaccination, if available, would be prudent for those visiting during May–August, especially for those attending mass events. Travelers should be reminded about avoiding blood-borne infections and STIs, including HIV. Education about avoiding food and water-borne infections, vector-borne infections, and CLM and other common skin infections should be part of the preparation for all travelers (Table 4). The findings in this analysis also will be helpful to clinicians caring for ill returned travelers from Brazil by providing data about more and less common diagnoses and directing them to useful resources.
Table 4.
Recommended Preparations for Travelers Planning to Attend 2014 FIFA World Cup or 2016 Olympics in Brazil
| All travelers should be up-to-date on their routine vaccines. In particular, document receipt of vaccination for (or immunity to): |
|
| Advise travelers on specific risks: |
|
| Review needs based on specific travel destination: |
|
Notes
Acknowledgments. We thank Adam Plier, Kathy Smith, and the GeoSentinel Surveillance Network staff, special advisors, and members of the data use and publication committee for helpful comments. We thank Dr Jay Keystone who supplied the photographs. Additional members of the GeoSentinel Surveillance Network who contributed data (in descending order) are: Gerd-Dieter Burchard, Bernhard-Nocht-Institute for Tropical Medicine, Hamburg, Germany; Rahul Anand and Stephanie S. Gelman, University of Utah, Salt Lake City, Utah; Kevin Kain and Andrea Boggild, University of Toronto, Toronto, Ontario, Canada; Cecilia Perret and Francisca Valdivieso, School of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile; Louis Loutan and François Chappuis, University of Geneva, Geneva, Switzerland; Patricia Schlagenhauf, Rainer Weber, and Robert Steffen, University of Zürich, Zürich, Switzerland; Eric Caumes and Alice Pérignon, Hôpital Pitié-Salpêtrière, Paris, France; Michael D. Libman, Brian Ward, and J. Dick Maclean, McGill University, Montreal, Canada; Martin C. Grobusch, Abram Goorhuis, Peter de Vries, and Kartini Gadroen, University of Amsterdam, Amsterdam, Netherlands; Frank Mockenhaupt and Gunder Harms, Berlin, Germany; Philippe Parola, Fabrice Simon, and Jean Delmont, Hôpital Nord and Hôpital Laveran, Marseille, France; Giampiero Carosi and Francesco Castelli, University of Brescia, Brescia, Italy; Bradley A. Connor, Cornell University, New York, New York; Phyllis E. Kozarsky, Henry Wu, Jessica Fairley, and Carlos Franco-Paredes, Emory University, Atlanta, Georgia; Johan Using, Gabrielle Fröberg, Helena Hervius Askling, and Ulf Bronner, Karolinska University Hospital, Stockholm, Sweden; N. Jean Haulman, David Roesel, and Elaine C. Jong, University of Washington and Harborview Medical Center, Seattle, Washington; Rogelio López-Vélez and Jose Antonio Perez Molina, Hospital Ramon y Cajal, Madrid, Spain; Joseph Torresi and Graham Brown, Royal Melbourne Hospital, Melbourne, Australia; Carmelo Licitra and Antonio Crespo, Orlando Regional Health Center, Orlando, Florida; Anne McCarthy, University of Ottawa, Ottawa, Canada; Vanessa Field, InterHealth, London, UK; John D. Cahill and George McKinley, St Luke's-Roosevelt Hospital Center, New York, New York; Perry J. van Genderen, Havenziekenhuis en Instituut voor Tropische Ziekten, Rotterdam, the Netherlands; Effrossyni Gkrania-Klotsas, Addenbrooke's Hospital, Cambridge, UK; William M. Stauffer and Patricia F. Walker, University of Minnesota, Minneapolis, Minnesota; Shuzo Kanagawa, Yasuyuki Kato, and Yasutaka Mizunno, International Medical Center of Japan, Tokyo, Japan; Marc Shaw and Annemarie Hern, Worldwise Travellers Health and Vaccination Centre, Auckland, New Zealand; Jean Vincelette, Centre Hospitalier de l'Université de Montréal, Montréal, Québec, Canada; David O. Freedman, University of Alabama at Birmingham, Birmingham, Alabama; Susan Anderson, Palo Alto Medical Foundation, Palo Alto, California; Noreen Hynes, R. Bradley Sack, and Robin McKenzie, Johns Hopkins University, Baltimore, Maryland; Thomas B. Nutman and Amy D. Klion, National Institutes of Health, Bethesda, Maryland; Christophe Rapp and Olivier Aoun, Hôpital d'instruction des armées Bégin, Saint Mandé, France; Patrick Doyle and Wayne Ghesquiere, Vancouver General Hospital and Vancouver Island Health Authority, Vancouver and Victoria, British Columbia, Canada; Luis M. Valdez and Hugo Siu, Clínica Anglo Americana, Lima, Peru; Natsuo Tachikawa, Hanako Kurai, and Hiroko Sagara, Yokohama Municipal Citizen's Hospital, Yokohama, Japan; David G. Lalloo and Nicholas J. Beeching, Liverpool School of Tropical Medicine, Liverpool, UK; Alejandra Gurtman, Mount Sinai Medical Center, New York City, New York (October 2002–August 2005 only); Susan McLellan; Tulane University, New Orleans, Louisiana, USA (December 1999–August 2005 only); Elizabeth D. Barnett, Boston University, Boston, Massachusetts; Stefan Hagmann, Michael Henry, and Andy O. Miller, Bronx-Lebanon Hospital Center, Bronx, New York; Marc Mendelson and Peter Vincent, University of Cape Town and Tokai Medicross Travel Clinic, Cape Town, South Africa; Michael W. Lynch, Fresno International Travel Medical Center, Fresno, California (August 2003–February 2010 only); Phi Truong Hoang Phu, Nicole Anderson, Trish Batchelor, and Dominique Meisch, International SOS Clinic, Ho Chi Minh City, Vietnam; Johnnie Yates and Vernon Ansdell, Kaiser Permanente, Honolulu, Hawaii (October 1997–January 2003 only); Prativa Pandey, Rashila Pradhan, and Holly Murphy, CIWEC Clinic Travel Medicine Center, Kathmandu, Nepal; Filipe Basto and Candida Abreu, Hospital de São João, Porto, Portugal.
Financial support. This work was supported by the US Centers for Disease Control and Prevention (cooperative agreement U50 CK000189).
Potential conflicts of interest. L. H. C. has received honoraria from Elsevier, Springer, and Wiley-Blackwell Publishers; consultant fees from Shoreland Inc; and research funding from Xcellerex Inc. K. L. has received travel support from GlaxoSmithKline and Sanofi Pasteur, and research funding from Sanofi Pasteur. J. S. K. has received support for educational presentations from Merck Frosst and Pfizer. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: Adam Plier, Kathy Smith, Gerd-Dieter Burchard, Rahul Anand, Stephanie S. Gelman, Kevin Kain, Andrea Boggild, Cecilia Perret, Francisca Valdivieso, Louis Loutan, François Chappuis, Patricia Schlagenhauf, Rainer Weber, Robert Steffen, Eric Caumes, Alice Pérignon, Michael D. Libman, Brian Ward, J. Dick Maclean, Martin C. Grobusch, Abram Goorhuis, Peter de Vries, Kartini Gadroen, Frank Mockenhaupt, Gunder Harms, Philippe Parola, Fabrice Simon, Jean Delmont, Hôpital Nord, Hôpital Laveran, Giampiero Carosi, Francesco Castelli, Bradley A. Connor, Phyllis E. Kozarsky, Henry Wu, Jessica Fairley, Carlos Franco-Paredes, Johan Using, Gabrielle Fröberg, Helena Hervius Askling, Ulf Bronner, N. Jean Haulman, David Roesel, Elaine C. Jong, Rogelio López-Vélez, Jose Antonio Perez Molina, Joseph Torresi, Graham Brown, Carmelo Licitra, Antonio Crespo, Anne McCarthy, Vanessa Field, John D. Cahill, George McKinley, Perry J. van Genderen, Effrossyni Gkrania-Klotsas, William M. Stauffer, Patricia F. Walker, Shuzo Kanagawa, Yasuyuki Kato, Yasutaka Mizunno, Marc Shaw, Annemarie Hern, Jean Vincelette, David O. Freedman, Susan Anderson, Noreen Hynes, R. Bradley Sack, Robin McKenzie, Thomas B. Nutman, Amy D. Klion, Christophe Rapp, Olivier Aoun, Patrick Doyle, Wayne Ghesquiere, Luis M. Valdez, Hugo Siu, Natsuo Tachikawa, Hanako Kurai, Hiroko Sagara, David G. Lalloo, Nicholas J. Beeching, Alejandra Gurtman, Susan McLellan, Elizabeth D. Barnett, Stefan Hagmann, Michael Henry, Andy O. Miller, Marc Mendelson, Peter Vincent, Michael W. Lynch, Phi Truong Hoang Phu, Nicole Anderson, Trish Batchelor, Dominique Meisch, Johnnie Yates, Vernon Ansdell, Kaiser Permanente, Prativa Pandey, Rashila Pradhan, Holly Murphy, Filipe Basto, and Candida Abreu
References
- 1.Fédération Internationale de Football Association (FIFA. ). Brazil 2014 promotional tour gets underway in Chile . Available at: http://www.fifa.com/worldcup/news/newsid=1663917/index.html. Accessed 16 March 2013.
- 2.Rio 2016 Olympic Games. Available at: http://www.rio2016.com/en. Accessed 16 March 2013. [Google Scholar]
- 3.UK Office for National Statistics. Statistical bulletin: overseas travel and tourism, August 2012. Available at: http://www.ons.gov.uk/ons/dcp171778_282767.pdf . Accessed 7 March 2014.
- 4.Barreto ML, Teixeira MG, Bastos FI, Ximens RAA, Barata RB, Rodrigues LC. Successes and failures in the control of infectious diseases in Brazil: social and environmental context, policies, interventions, and research needs. Lancet. 2011;377:1877–89. doi: 10.1016/S0140-6736(11)60202-X. [DOI] [PubMed] [Google Scholar]
- 5.Abubakar I, Gautret P, Brunette GW, et al. Global perspectives for prevention of infectious diseases associated with mass gatherings. Lancet Infect Dis. 2012;12:66–74. doi: 10.1016/S1473-3099(11)70246-8. [DOI] [PubMed] [Google Scholar]
- 6.Enk MJ, Amorim A, Schall VT. Acute schistosomiasis outbreak in the metropolitan area of Belo Horizonte, Minas Gerais: alert about the risk of unnoticed transmission increased by growing rural tourism. Mem Inst Oswaldo Cruz. 2003;98:745–50. doi: 10.1590/s0074-02762003000600006. [DOI] [PubMed] [Google Scholar]
- 7.Lambertucci JR, Drummond SC, Voieta I, et al. An outbreak of acute Schistosoma mansoni schistosomiasis in a nonendemic area of Brazil: a report on 50 cases, including 5 with severe clinical manifestations. Clin Infect Dis. 2013;57:e1–6. doi: 10.1093/cid/cit157. [DOI] [PubMed] [Google Scholar]
- 8.Steffen R, Bouchama A, Johansson A, et al. Non-communicable health risks during mass gatherings. Lancet Infect Dis. 2012;12:142–9. doi: 10.1016/S1473-3099(11)70293-6. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Surveillance for travel-related diseases-GeoSentinel surveillance system, United States, 1997–2011. MMWR Surveill Summ. 2013;62:1–28. [PubMed] [Google Scholar]
- 10.Jackson A, Heukelbach J, Calheiros CM, Soares Vde L, Harms G, Feldmeier H. A study in a community in Brazil in which cutaneous larva migrans is endemic. Clin Infect Dis. 2006;43:e13–8. doi: 10.1086/505221. [DOI] [PubMed] [Google Scholar]
- 11.Coelho WM, Amarante AF, Apolinário Jde C, Coelho NM, Bresciani KD. Occurrence of Ancylostoma in dogs, cats and public places from Andradina City, São Paulo State, Brazil. Rev Inst Med Trop Sao Paulo. 2011;53:181–4. doi: 10.1590/s0036-46652011000400001. [DOI] [PubMed] [Google Scholar]
- 12.Silva PF, Cavalcanti IM, Irmão JI, Rocha FJ. Common beach sand contamination due to enteroparasites on the southern coast of Pernambuco State, Brazil. Rev Inst Med Trop Sao Paulo. 2009;51:217–8. doi: 10.1590/s0036-46652009000400007. [DOI] [PubMed] [Google Scholar]
- 13.Jelinek T, Maiwald H, Nothdurft HD, Loscher T. Cutaneous larva migrans in travelers: synopsis of histories, symptoms, and treatment of 98 patients. Clin Infect Dis. 1994;19:1062–66. doi: 10.1093/clinids/19.6.1062. [DOI] [PubMed] [Google Scholar]
- 14.Bouchaud O, Houze S, Schiemann R, et al. Cutaneous larva migrans in travelers: a prospective study, with assessment of therapy with ivermectin. Clin Infect Dis. 2000;31:493–98. doi: 10.1086/313942. [DOI] [PubMed] [Google Scholar]
- 15.Vanhaecke C, Perignon A, Monsel G, Regnier S, Bricaire F, Caumes E. The efficacy of single dose ivermectin in the treatment of hookworm related cutaneous larva migrans varies depending on the clinical presentation [epub ahead of print] J Eur Acad Dermatol Venereol. 2013 doi: 10.1111/jdv.12097. [DOI] [PubMed] [Google Scholar]
- 16.Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79–105. doi: 10.1128/CMR.00010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boggild AK, Keystone JS, Kain KC. Furuncular myiasis: a simple and rapid method for extraction of intact Dermatobia hominis larvae. Clin Infect Dis. 2002;35:363–68. doi: 10.1086/341493. [DOI] [PubMed] [Google Scholar]
- 18.Buckendahl J, Heukelbach J, Witt L, Schwalfenberg S, Calheiros CML, Feldmeier H. Topical distribution of the sand flea tunga penetrans in Wistar rats and humans in two endemic areas in Brazil. Am J Trop Med Hyg. 2010;87:125–27. doi: 10.4269/ajtmh.2012.11-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heukelbach J, Wilcke T, Eisele M, Feldmeier H. Ectopic localization of tungiasis. Am J Trop Med Hyg. 2002;67:214–6. doi: 10.4269/ajtmh.2002.67.214. [DOI] [PubMed] [Google Scholar]
- 20.Veraldi S, Valsecchi M. Imported tungiasis: a report of 19 cases and review of the literature. Int J Dermatol. 2007;46:1061–6. doi: 10.1111/j.1365-4632.2007.03280.x. [DOI] [PubMed] [Google Scholar]
- 21.Romero GAS, Vinitius de Farias Guerra M, Gomes Paes M, de Oliveira Macedo V. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: clinical findings and diagnostic approach. Clin Infect Dis. 2001;32:1304–12. doi: 10.1086/319990. [DOI] [PubMed] [Google Scholar]
- 22.Teixeira MG, Costa Mda C, Barreto F, Barreto ML. Dengue: twenty-five years since reemergence in Brazil. Cad Saude Publica. 2009;25(suppl 1):S7–18. doi: 10.1590/s0102-311x2009001300002. [DOI] [PubMed] [Google Scholar]
- 23.Martin JL, Brathwaite O, Zambrano B, et al. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg. 2010;82:128–35. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Souza RP, Rocco IM, Maeda AY, et al. Dengue virus type 4 phylogenetics in Brazil 2011: looking beyond the veil. PLoS Negl Trop Dis. 2011;5:1439. doi: 10.1371/journal.pntd.0001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Health information for international travel. Available at: http://wwwnc.cdc.gov/travel/destinations/brazil.htm. Accessed 17 March 2013.
- 26.Camara FP, de Carvalho LM, Gomes ALB. Demographic profile of sylvatic yellow fever in Brazil from 1973 to 2008. Trans R Soc Trop Med Hyg. 2013;107:324–7. doi: 10.1093/trstmh/trt014. [DOI] [PubMed] [Google Scholar]
- 27.Monath TP. Dengue and yellow fever—challenges for the development and use of vaccines. N Engl J Med. 2007;357:222–25. doi: 10.1056/NEJMp0707161. [DOI] [PubMed] [Google Scholar]
- 28.Vivancos R, Abubakar I, Hunter PR. Foreign travel, casual sex, and sexually transmitted infections: systematic review and meta-analysis. Int J Infect Dis. 2010;14:842–51. doi: 10.1016/j.ijid.2010.02.2251. [DOI] [PubMed] [Google Scholar]
- 29.Gagneux OP, Blochliger CU, Tanner M, Hatz CF. Malaria and casual sex: what travelers know and how they behave. J Travel Med. 1996;3:14–21. doi: 10.1111/j.1708-8305.1996.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 30.Matteelli A, Schlagenhauf P, Carvalho AC, et al. Travel-associated sexually transmitted infections: an observational cross-sectional study of the GeoSentinel surveillance database. Lancet Infect Dis. 2013;13:205–13. doi: 10.1016/S1473-3099(12)70291-8. [DOI] [PubMed] [Google Scholar]
- 31.Ehresmann KR, Kedberg CW, Grimm MB, Norton CA, MacDonald KL, Osterholm MT. An outbreak of measles at in international sporting event with airborne transmission at a domed stadium. J Infect Dis. 1995;171:679–83. doi: 10.1093/infdis/171.3.679. [DOI] [PubMed] [Google Scholar]
- 32.De Serres G, Markowski F, Toth E, et al. Largest measles epidemic in North America in a decade—Quebec, Canada, 2011: contribution of susceptibility, serendipity, and superspreading events. J Infect Dis. 2013;207:990–8. doi: 10.1093/infdis/jis923. [DOI] [PubMed] [Google Scholar]
- 33.Pfaff G, Lohn D, Santibanez S, et al. Spotlight on measles 2010: measles outbreak among travellers returning from a mass gathering, Germany, September to October 2010. Euro Surveill. 2010;15:19750. [PubMed] [Google Scholar]
- 34.Schmid D, Holzmann H, Alfery C, Wallenko H, Popow-Kraupp TH, Allerberger F. Mumps outbreak in young adults following a festival in Austria, 2006. Euro Surveill. 2008;13:8042. doi: 10.2807/ese.13.07.08042-en. [DOI] [PubMed] [Google Scholar]
- 35.British Columbia Centre for Disease Control. Available at: http://www.bccdc.ca/dis-cond/a-z/_m/Measles/default.htm. Accessed 17 March 2013.
- 36.Blyth CC, Foo H, van Hal SJ, et al. Influenza outbreaks during World Youth Day 2008 mass gathering. Emerg Infect Dis. 2010;16:809–15. doi: 10.3201/eid1605.091136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gundlapalli AV, Rubin MA, Samore MH, et al. Influenza, Winter Olympiad, 2002. Emerg Infect Dis. 2006;12:144–6. doi: 10.3201/eid1201.050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jentes ES, Davis XM, MacDonald S, et al. Health risks and travel preparation among foreign visitors and expatriates during the 2008 Beijing Olympic and Paralympic Games. Am J Trop Med Hyg. 2010;82:466–72. doi: 10.4269/ajtmh.2010.09-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freitas FT, Souza LR, Azziz-Baumgartner E, et al. Influenza-associated mortality in southern Brazil, 1980–2008. Epidemiol Infect. 2012;141:1731–40. doi: 10.1017/S0950268812002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan K, Arino J, Hu W, et al. Spread of a novel influenza A (H1N1) virus via global airline transportation. N Engl J Med. 2009;361:212–4. doi: 10.1056/NEJMc0904559. [DOI] [PubMed] [Google Scholar]
- 41.Chaves Tdo S, Pellini AC, Mascheretti M, et al. Travelers as sentinels for chikungunya fever, Brazil. Emerg Infect Dis. 2012;18:529–30. doi: 10.3201/eid1803.110838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. Chikungunya in the Caribbean. Available at: http://wwwnc.cdc.gov/travel/notices/watch/chikungunya-saint-martin. Accessed 20 February 2014.
- 43.Pan American Health Organization. Washington, DC: PAHO; 2011. Preparedness and response for chikungunya virus: introduction in the Americas. Available at: http://www.paho.org/fep/index.php?option=com_content&view=article&id=344&Itemid=1. (also available at: http://www.cdc.gov/chikungunya/ ) Accessed 12 August 2013. [Google Scholar]