Summary
Context
Live-animal markets (wet markets) provide a source of vertebrate and invertebrate animals for customers in tropical and subtropical regions of the world. Wet markets sell live poultry, fish, reptiles, and mammals of every kind. Live-poultry markets (mostly chicken, pigeon, quail, ducks, geese, and a wide range of exotic wild-caught and farm-raised fowl) are usually separated from markets selling fish or red-meat animals, but the stalls can be near each other with no physical separation. Despite the widespread availability of affordable refrigeration, many Asian people prefer live animals for fresh produce. Wet markets are widespread in Asian countries and in countries where Asian people have migrated. Live-poultry markets were the source of the H5N1 bird-influenza virus that transmitted to and killed six of 18people in Hong Kong.
Starting point
Yi Guan and colleagues (Science 2003; 302: 276–78) recently reported the isolation of severe acute respiratory syndrome (SARS) coronavirus (CoV) from Himalayan palm civets (Paguna larvata) in wet markets in Shenzen, southern China. These researchers also found serological evidence of infection in raccoon dogs (Nyctereutes pro-cuyoinboides). Serological evidence for SARS CoV in human beings working in these markets, taken together with the earliest cases of SARS in restaurant workers, supports the contention of a potential zoonotic origin for SARS.
Where next?
Will SARS reappear? This question confronts public-health officials worldwide, particularly infectious disease personnel in those regions of the world most affected by the disease and the economic burden of SARS, including China, Taiwan, and Canada. Will the virus re-emerge from wet markets or from laboratories working with SARS CoV, or are asymptomatic infections ongoing in human beings? Similar questions can be asked about a pandemic of influenza that is probably imminent. Knowledge of the ecology of influenza in wet markets can be used as an early-warning system to detect the reappearance of SARS or pandemic influenza.
The establishment of permanent live-animal markets (wet markets) in many countries means that there is usually carry over of animals from one day to the next, and more expensive animals (eg, pheasants in poultry markets, civet cats in red-meat markets) can stay from days to weeks. Daily introduction of new animals provides optimum conditions for amplification and perpetration of disease agents such as influenza. The influenza virus has a segmented negatively stranded RNA genome with a propensity for reassortment and generation of novel agents. Add the daily human contacts (including children) with the live animals, and conditions are at an optimum for zoonotic transfer and the evolution of infectious disease agents.
Since the late 1970s, live-poultry markets have been known to be a source of influenza viruses,1 and by the early 1990s in the USA live-poultry markets were recognised as the missing link in the epidemiology of influenza.2 More recently live-poultry markets were recognised as the source of the H5N1 bird-influenza virus that transmitted to and killed six of 18 human beings in Hong Kong.3, 4 Both the Asian/57 (H2N2) and Hong Kong/68 (H3N2) influenza pandemics were caused by reassortants containing gene segments from the avian and human influenza viruses,5 although whether wet markets were the source is unknown.
Lessons from US live-poultry markets
Live-poultry markets in the USA have been associated with the emergence of H5 and H7 influenza viruses, which are a threat to commercial poultry. To date, neither the highly pathogenic H5N2 nor the emerging H7N2 viruses in the USA have been shown to be pathogenic for human beings. Notwithstanding, ongoing surveillance provides information on the role of these markets in the continuing evolution of H7N2 viruses with pathogenic potential for poultry,6 and the difficulty or seemingly impossible task of eliminating H7N2 influenza viruses from markets, despite many attempts. Additionally in New York the number of live-poultry markets nearly doubled from 44 in 1994 to over 80 in 2002.7 Surveillance in live-poultry markets in the USA serves as an early-warning system of emerging influenza viruses that are a threat to the poultry industry and potentially to human beings.
Live-poultry markets in Hong Kong
The H5N1 bird-influenza incident in Hong Kong in 1997 left no doubt about the role of live-poultry markets as a source of novel infectious disease agents for human beings. Live-poultry markets were identified as a risk factor for H5N1 influenza,8 and slaughter of all poultry in Hong Kong brought the outbreak in human beings to a stop. To reduce the possibility of re-emergence of H5N1, changes were made in poultry markets in Hong Kong: after 1997 all aquatic birds, including ducks and geese (the original source of influenza viruses), were eliminated from the markets and sold chilled, and a representative sample of each truckload of poultry entering Hong Kong is screened to assess the level of H5N1 immunity. Despite these changes highly pathogenic H5N1 of multiple new genotypes reappeared in retail poultry markets in 2001, and all poultry were again destroyed to prevent transmission to human beings.9 Marketing practices were also changed to reduce the risk from influenza virus, including removal of quail (a newly recognised host susceptible to all subtypes of influenza and a potential intermediate host10, 11) and the introduction of a clean day every month when all markets are emptied simultaneously and cleaned. Despite these additional changes H5N1 viruses reappeared in late 2002 and in early 2003 in the live-bird markets, in wild migrating birds, and on farms, and killed many aquatic birds in Kowloon and Penfold parks in Hong Kong. In February, 2003, H5N1 virus was again transmitted to human beings and killed two infected persons in a family of four, and WHO issued a pandemic alert.12 The reappearance of H5N1 in human beings coincided with the spread of severe acute respiratory syndrome (SARS) and caused initial confusion in the diagnosis of the causative agent of SARS.
Introduction of a second clean day every month in live-poultry markets and the use of inactivated H5N1 vaccine on poultry farms in Hong Kong are further measures being taken to keep H5N1 out of live-poultry markets. What we now know about H5N1 in live-poultry markets illustrates the difficulty in attempting to control infectious disease agents in such markets.
Introduction of these measures in live-poultry markets in Hong Kong reduced the spectrum of influenza viruses compared with such markets in mainland China (figure 1 ). In Hong Kong, since 2000, the influenza subtypes have been limited to H6N1 and H9N2, with intermittent introductions of H5N1. By contrast, multiple different subtypes (H1N1, H2N9, H3N2, H3N3, H3N6, and H4N6) continue to circulate in live-poultry markets in mainland China.13 The use of inactivated H5N1 vaccines and improved sanitation on farms in Hong Kong hold promise for reducing the introduction of H5N1 from the farms. A similar policy of vaccination is being used on farms in the Mainland that provide live poultry to Hong Kong, again holding the promise of reducing the possibility of introduction of H5N1 virus. The transmission of highly pathogenic H5N1 to wild migrating birds in Hong Kong in 2003 and the unknown distribution of these H5N1 viruses in mainland China is a worry. Introduction of a standardised H5N1 influenza vaccine for use on poultry farms in China could further improve vaccine strategy.
Figure 1.

Poultry wet-market in mainland China
Removal of ducks and geese (original source of influenza viruses) from live-poultry markets in Hong Kong reduced number of subtypes of influenza viruses found there. Similar changes have not been made on the mainland.
SARS and influenza: the wet-market dilemma
The host range of SARS coronaviruses in wild or farm-raised animals is not resolved. Yi Guan and colleagues14 recently established a potential zoonotic origin of SARS coronavirus (CoV) and wet markets as a possible source of the original outbreak. These researchers isolated a novel coronavirus from Himalayan palm civets (Paguna larvata) found in live-animal markets in Guangdong and provided serological evidence of infection of other animals (raccoon dogs, Nyctereutes procuyoinboides) and human beings in the markets. The animal isolates were different from the human SARS CoV in that they had an additional 29-nucleotide sequence not found in the human isolates (figure 2 ). WHO has had a task force working with Chinese authorities to develop strategies to define the ecology of SARS CoV. At the October, 2003, meeting Options to Control Influenza, in Okinawa, Japan, reports of replication of SARS CoV with disease signs in primates,15 ferrets and cats,16 and mice17 provide animal models for vaccine and antiviral development, but suggest a wider host range in nature. After the identification of SARS-like CoV in civet cats,14 civets were banned from wet markets in China but are again now available. Customer demand has driven the price for civet cats up to US$200, making it likely that such animals could be obtained whether or not they were banned. We lack information on the transmissibility of the SARS CoV-like virus in animals, the mode of transmission (oral, faecal), the role of intermediate hosts (eg, pigs and quail for influenza), environmental stability, and serological screening at the human-animal interface.
Figure 2.
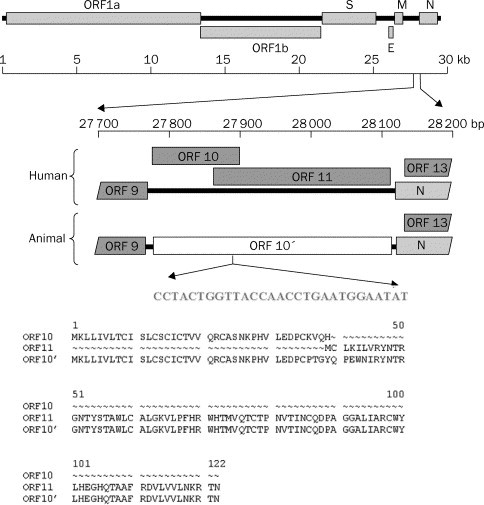
Genomic organisation of human and animal SARS CoV
Human SARS CoV has 29-nucleotide deletion compared with virus isolated from Himalayan palm civet. Reprinted with permission from reference 14.
There are many parallels with pandemic preparedness for influenza at the interface between lower animals and human beings. Thus H5N1 influenza continues to circulate in Asia and periodically spills over to human beings through live-poultry markets. To date, H5N1 viruses have not acquired the ability to transmit efficiently from human being to human being. However, we must be aware of the facts about both influenza and coronaviruses: they are both RNA viruses with known ability to vary by mutation, deletion, reassortment, and recombination.18, 19, 20 The next emergence of H5N1 or SARS CoV might acquire real transmissibility in human beings.
Solution—close the wet markets
Although it may seem a simple matter to close wet markets for the general good of society, it would be no simple matter. Such closure would put thousands of legitimate owners and workers in wet markets out of business, with all the legal issues to be resolved. Continuing demand and high prices would drive the wet-market system underground where monitoring would be impossible. Currently, wet markets serve as an early-warning system for H5N1 influenza in Asia and H7N2 in the USA, and will likely serve the same role for SARS CoV. The immediate goal must be to reduce the virus burden in wet markets, and perhaps the first use of pandemic vaccines should be on farms raising animals for wet markets. Additionally, hygiene and sanitation can be improved immediately, and modified as we understand the ecology of these viruses. Selling of wild-caught animals should be discouraged by education of customers and stall owners about the health risks.
Centralised slaughter of poultry and closure of wet markets in Hong Kong is an ongoing debate—a real dilemma with no simple answers. From a public-health perspective closure of the wet markets would reduce the risk of influenza emergence in Hong Kong. However, from a global-health perspective, unilateral closure of live-poultry markets without closure of the wet markets in the wider region would probably have little impact. The case in point is SARS—there are no red-meat wet markets in Hong Kong but that did not prevent SARS appearing in Hong Kong. Additionally the local early-warning system for influenza would potentially be compromised. The situation with H5N1 influenza is complex, because declaration of its presence would negatively affect poultry exports. With acceptance of the need for openness after the emergence of SARS and the rapid resolution of H7N7 bird-influenza in Holland,21 a precedent has been established for the need for complete openness when dealing with the control of emerging infectious disease agents.
In the longer term, wet markets will be phased out. The younger generation of customers in Asia buy their produce frozen or chilled. Public education about the risks associated with wet markets, especially during the current awareness about SARS, could foster this trend.
Wet markets are not the only risk for re-emergence of SARS. At the time of writing this review there have been two re-emergent cases of SARS, both from laboratory infections. One case in Singapore22 and the other in Taiwan.23 WHO must continue its efforts to promote scientific responsibility for both SARS and influenza viruses. Laboratory regulations globally are inconsistent. We now live in a global village, so universal guidelines need to be adopted. The situation with H2N2 influenza is a case in point. Although H2N2 influenza has not circulated in human beings since 1968 and everyone under the age of 36years is susceptible, the H2N2 virus is widely distributed in laboratories and is still used in some laboratories. The re-emergence of H1N1 influenza, in 1977, that continues to circulate in human beings is another unresolved case. This H1N1 virus remained genetically conserved for 27 years.24 The most likely explanation is that the virus came from a frozen source and a laboratory seems the most probable culprit. Thus SARS CoV and many influenza viruses (eg, H2N2, H5N1, and H7N7 from human beings) must be restricted to Biosafety level 3+ laboratories.
Live-poultry markets are not the only risk for influenza transmission. The increasing numbers of pigs and poultry—the recognised intermediate hosts for influenza—required to feed an expanding human population increases the opportunity for zoonotic transmission.
Acknowledgments
Influenza research at St Jude Children's Research Hospital is supported by Public Health Service grant AI95357 and Cancer Center Support (CORE) grant CA-21765 from the National Institutes of Health, and by the American Lebanese Syrian Associated Charities (ALSAC). I thank Sharon Naron for editorial assistance, and Carol Walsh and Frances Wong for manuscript preparation.
References
- 1.Shortridge KF. Pandemic influenza—a zoonosis? Semin Respir Infect. 1992;7:11–25. [PubMed] [Google Scholar]
- 2.Senne DA, Pearson JE, Panigrahy B. Live poultry markets: a missing link in the epidemiology of avian influenza. Proceedings of Third International Symposium on Avian Influenza, Richmond, Virginia, USA, Animal Health Association, 1992
- 3.DeJong JC, Class ECJ, Osterhaus ADME, Webster RG, Lim WL. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Class ECJ, Osterhaus ADME, van Beek R. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 5.Kawaoka Y, Krauss S, Webster RG. Avian-to-human transmission of the PB1 gene of influenza A virus in the 1957 and 1968 pandemics. J Virol. 1989;63:4603–4608. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senne DA, Suarez DL, Pederson JC, Panigrahy B. Molecular and biological characteristics of H5 and H7 avian influenza viruses in live bird markets of the northeastern United States. Avian Dis. 2003;47:898–904. doi: 10.1637/0005-2086-47.s3.898. [DOI] [PubMed] [Google Scholar]
- 7.Trock SC, Senne DA, Gaeta M, Gonzalez A, Lucio B. Low-pathogenicity avian influenza viruses in live bird markets—what about the livestock area. Avian Dis. 2003;47:1111–1113. doi: 10.1637/0005-2086-47.s3.1111. [DOI] [PubMed] [Google Scholar]
- 8.Mounts AW, Kwong H, Izurieta HS. Case-control study of risk factors for avian influenza A (H5N1) disease, Hong Kong. J Infect Dis. 1999;180:505–508. doi: 10.1086/314903. [DOI] [PubMed] [Google Scholar]
- 9.Sims LD, Ellis TM, Liu KK. Avian influenza in Hong Kong 1997–2002. Avian Dis. 2003;47:832–838. doi: 10.1637/0005-2086-47.s3.832. [DOI] [PubMed] [Google Scholar]
- 10.Perez DR, Lim W, Seiler JP. Role of quail in the interspecies transmission of H9 influenza A viruses. J Virol. 2003;77:3148–3156. doi: 10.1128/JVI.77.5.3148-3156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makarova NV, Hiroishi O, Hiroshi K, Webster RG, Perez DR. Replication and transmission of influenza viruses in Japanese quail. Virology. 2003;310:8–15. doi: 10.1016/s0042-6822(03)00094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Influenza A (H5N1) in Hong Kong special administrative region of China. WHO Disease Alert; Feb 19, 2003. [Google Scholar]
- 13.Liu M, He S, Walker D. The influenza virus gene pool in a poultry market in south central China. Virology. 2003;305:267–275. doi: 10.1006/viro.2002.1762. [DOI] [PubMed] [Google Scholar]
- 14.Guan Y, Zheng BJ, He YQ. Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science. 2003;302:226–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 15.Fouchier RA, Kuiken T, Schutten M. Koch's postulates fulfilled for SARS virus. Nature. 2003;423:240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martina BEE, Haagmans BL, Kuiken T. SARS virus infection of cats and ferrets. Nature. 2003;425:915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enserink M. SARS researchers report new animal models. Science. 2003;302:213. doi: 10.1126/science.302.5643.213a. [DOI] [PubMed] [Google Scholar]
- 18.Ismail MM, Cho KO, Ward LA, Saif LJ, Saif YM. Experimental bovine coronavirus infections in turkey poults and young chickens. Avian Dis. 2001;45:157–253. [PubMed] [Google Scholar]
- 19.Lai MCC, Holmes KV. Coronaviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Field's virology. 4th edn. Lippincott; Philadelphia: 2001. pp. 1163–1186. [Google Scholar]
- 20.Holmes KV. Coronaviridae. In: Knipe DM, Howley PM, editors. Field's virology. 4th edn. Lippincott; Philadelphia: 2001. pp. 1187–1203. [Google Scholar]
- 21.van Kolfschooten F. Dutch veterinarian becomes first victim of avian influenza. Lancet. 2003;361:1444. doi: 10.1016/S0140-6736(03)13156-X. [DOI] [PubMed] [Google Scholar]
- 22.Senior K. Recent Singapore SARS case a laboratory accident. Lancet Infect Dis. 2003;3:679. doi: 10.1016/S1473-3099(03)00815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Society for Infectious Diseases SARS—worldwide (185): Taiwan, probable lab exposure. Promed Mail, Dec 13, 2003: 20031217. 3081. http://www.promedmail.org/pls/askus/f?p=2400:1001:354734443665146201::NO::F2400_P1001_BACK_PAGE,F2400_P1001_PUB_MAIL_ID:1000,23751 (accessed Dec 30, 2003)
- 24.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


