Highlights
-
•
Corn byproduct polysaccharides (CBP) were isolated from corn steep liquor (CSL).
-
•
CBP was further fractionated into polysaccharide fractions (CBP1P and CBP1S).
-
•
CBP1S showed the most potent innate immune system-stimulatory activities.
-
•
Oral administration of CBP1S could facilitate the anti-metastatic effect.
-
•
CBP1S primarily comprises polysaccharide rich in arabinoxylan and glucan.
Keywords: Corn steep liquor, Polysaccharide, Arabinoxylan, Immunostimulating activity, Anti-metastatic activity
Abstract
Corn steep liquor (CSL) is a major by-product of the corn steeping process that is utilized in the wet milling industry. To develop new physiologically active polysaccharides from CSL, polysaccharides were isolated and their innate immunostimulatory and anti-metastatic activities were investigated. Corn byproduct polysaccharides (CBP) were preferentially isolated from CSL and further separated into supernatant (CBP1S) and precipitate (CBP1P) fractions. The anti-complementary activity of CBP1S was more potent than CBP1P and CBP. In addition, CBP1S enhanced production of macrophage-stimulating cytokines (e.g., IL-6 and IL-12) and natural killer (NK) cell-activating substances (e.g., granzyme and interferon-γ). Further, CBP1S significantly inhibited lung metastasis at a dose of 1000 μg per mouse in an experimental lung metastasis model. These results suggest that CBP1S seems to promote the inhibition of lung metastasis through a mechanism leading to stimulation of the innate immune system, and CBP1S could be used as immunostimulating agents and for industrial applications.
1. Introduction
Industrial starch is produced primarily from grains such as corn, wheat, potato, casaba, sweet potato, rice, and barley. Among them, the production of starch from corn has great advantages, attributable to its cultivation superiority, relatively large grains, and ability to be stored for long periods (Serna-Saldivar, 2016). For these reasons, corn starch accounts for fifty percent of the total starch production. Industrial starch is used in 53.1% of the food industry (e.g., foods and beverages) and 37.8% of non-food industries (e.g., plastic or detergent industries 10.4%, paper industry 16.3%, and fermentation products 11.1%). Industrial applications that use starch are expected to continue to increase, and as a result, worldwide starch consumption is expected to increase from approximately 68 million-ton in 2010 to 89 million-ton in 2018 (31.1% increase) (Global Strategic Business Report, 2012).
In addition to starch, a large amount of corn steep liquor (CSL) is generated, attributable to the extraction and isolation processes of starch from corn grain using precipitation with aqueous sulfurous acid method (Liggett & Koffler, 1948). Until recently, CSL was primarily discarded in the ocean as a major byproduct of corn starch production process. However, like other countries, as pf 2016, Korean laws prohibit the disposal of CSL in the water; consequently, CSL disposal is becoming a significant problem in Korea. It is well known that CSL contains various organic compounds, including polysaccharides, organic acids and vitamins as carbon sources, and amino acids and proteins as nitrogen sources) (Hull, Yang, Venzke, Kulhavy, & Montgomery, 1996; Liggett & Koffler, 1948). Although various studies of the industrial applications of the organic compounds have been conducted, most of these studies have examined these compounds for use in growth or synthesis media for microorganisms (Maddipati, Atiyeh, Bellmer, & Huhnke, 2011; Utrilla et al., 2012, Wang et al., 2014; Xi et al., 2013). Moreover, there have been few studies involving the application of CSL as a functional food or therapeutic agent for the promotion of health.
Recently, interest in health-promoting foods and ingredients has been gradually increasing, attributable to the incidence of new infectious diseases, such as H1N1 influenza, Middle East Respiratory Syndrome (MERS), and Severe Acute Respiratory Syndrome (SARS). Particularly, immunostimulatory polysaccharides isolated from natural sources have been recognized as a key class of functional compounds (Lee et al., 2014, Park et al., 2013). In addition, they have been attracting a great deal of interest from the food and biopharmaceutical industries. It is well known that herbal and mushroom extracts include immunostimulatory polysaccharides that promote immune functions by activating the innate and adaptive immune systems (Park et al., 2009, Volman et al., 2010, Wasser, 2002). Indeed, a primary mechanism of immune system potentiation by natural polysaccharides likely involves their ability to activate macrophage and NK cells, as well as the complement system
In this study, we aimed to investigate the potential for using agricultural by-products as health-promoting ingredients. For this, polysaccharides were isolated from CSL and their effects on innate immunostimulatory and anti-metastasis activities were evaluated in several in vitro and in vivo experiments.
2. Materials and methods
2.1. Material and animals
CSL was obtained from Sandolfood (Gangwon, Korea). Specific pathogen-free (SPF), female BALB/c mice (6–8 weeks old) were purchased from Orient Bio Inc. (Seongnam, Korea). The mice were maintained in a clean rack in a 12/12 h light/dark cycle at Kyonggi University. Water and a pellet diet were supplied ad libitum. All animal experiments were carried out according to the instructions of the Ethics Committee for Use of Experimental Animals at Kyonggi University (2016-001).
2.2. Isolation of polysaccharide from CSL
Previously, we developed a rapid isolation method for fractionation of polysaccharides with different characteristics (Lee, Hong, & Shin, 2016; Lee, Hong, & Shin, 2015). Here, two polysaccharide fractions were prepared from crude CSL polysaccharides. Briefly, a concentration of 60 brix CSL was diluted with three volumes distilled water and then four volumes of 95% cold ethanol were added to obtain crude polysaccharide from CSL. After centrifugation at 6000 rpm for 30 min, the precipitates were rapidly fractionated with a 50% ethanol solution. The samples were again centrifuged at 6000 rpm for 30 min, and the resulting supernatant and precipitate were separately divided, concentrated, and lyophilized to obtain the polysaccharide fractions (CBP1S and CBP1P, respectively).
2.3. General analysis
Total carbohydrate contents were determined using the phenol-sulfuric acid method (Dubois, Gilles, Hamilton, Rebers, & Smith, 1956), with galactose as a reference. Uronic acid was measured by the m-hydroxybiphenyl method (Blumenkrantz & Asboe-Hansen, 1973), using galacturonic acid as a reference. The 2-keto-3-deoxy-d-manno-octulosonic acid (KDO) content was determined via a modified thiobarbituric acid (TBA) method (Karkhanis, Zeltner, Jackson, & Carlo, 1978), using KDO as a reference. Protein content was determined by the Bradford method (Bradford, 1976), using bovine serum albumin as a reference. The monosaccharide composition of the polysaccharides was analyzed via the partially modified alditol acetate method of Jones and Albersheim (Jones & Albersheim, 1972) using GC equipment (6000 series, Young-Lin Co., Anyang, Korea) equipped with a SP-2380 capillary (0.2 μm × 0.25 mm × 30 m; Supelco, Bellefonte, PA, USA) and flame ionization detector (FID). The temperature program of the GC was analyzed at 60° C for 1 min, 60 → 220° C (30° C/min), 220° C for 12 min, 220 → 250° C (8° C/min), and 250° C for 15 min, the molar ratio of monosaccharides was calculated from the peak areas and response factors. The molecular weight of polysaccharide was confirmed by high performance size-exclusion chromatography (HPSEC) using an Agilent 1260 Infinity LC system (Agilent Technologies Co., Ltd., Palo Alto, CA, USA) equipped with an Asahipak GS-520, GS-320, and GS-220 linked column (Showa Denko, Co. Ltd., Tokyo, Japan) and refractive index detector (Agilent 1200 series). A total of 20 μL of polysaccharide solution was analyzed using an isocratic mobile phase (50 mM ammoniumformate buffer, pH 5.5) at a flow rate of 0.5 mL/min at room temperature. The molecular weight of the polysaccharide was calculated from the calibration curve generated for standard pullulans (P-800, 400, 200, 100, 50, 20, 10, and 5; Showa Denko Co. Ltd.).
2.4. Anti-complementary activity
Anti-complementary activity was measured by the complement fixation test based on complement consumption and the degree of erythrocyte lysis by residual complement (Mayer, 1961). Normal human serum (NHS) obtained from a healthy adult was used as a complement source. IgM from hemolysin-sensitized sheep erythrocytes (EA cells, 1 × 108 cells/mL) was used as the complementary target. For the first order reaction, several concentrations of CBP1S, gelatin veronal-buffered saline (GVB++, pH 7.4) containing 150 mM Ca2+ and 500 mM Mg2+ (50 μL) were mixed and pre-incubated at 37 °C for 30 min. For the second order reaction, EA cells were added to the first order reaction solution and incubated at 37 °C for 1 h. The reaction was stopped with 2.5 mL PBS and centrifuged at 2500 rpm for 10 min. Absorbance of the supernatant was measured at 412 nm. The anti-complementary activity of the isolated polysaccharides is expressed as percent inhibition of the control total complement hemolysis (TCH)50 for polysaccharide K (PSK) from Coriolus versicolor (Tsukagoshi et al., 1984) per the following equation.
| TCH50 (%) = TCH50 (control) − TCH50 (treated with sample)/TCH50 (control). |
2.5. Measurements of peritoneal macrophage-stimulating cytokines
In order to measure the immunostimulating activity of isolated CBP1S, peritoneal macrophages were harvested from 5% thioglycollate medium (TG)-injected BALB/c mice as previously described (Salki et al., 1988). The collected macrophages were suspended in complete Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and plated at 2.5 × 105 cells/well onto 96-well culture plates. After 2 h, various concentrations of CBP1S were added to the cells and incubated at 37 °C for 24 h. The levels of interleukin (IL)-6, IL-12, and tumor necrosis factor (TNF)-α were measured in the culture supernatant using the respective sandwich enzyme-linked immunosorbent assay (ELISA) set (BD bioscience, San Diego, CA, USA).
2.6. NK cell cytotoxicity against tumor cells
Yac-1 is a Moloney murine leukemia virus-induced lymphoma that does not express major histocompatibility complex (MHC)-I and is sensitive to lysis by NK cells (Kiessling, Klein, & Wigzell, 1975). Therefore, NK-mediated cytotoxicity was determined in Yac-1 and primary cultured splenocytes from sample-treated animals (Yoon et al., 1998). Briefly, three BALB/c mice per group were administered CBP1S intravenously (1000 μg/mouse), and their splenocytes were harvested three days after treatment. Single cell suspensions of splenocytes were added to the Yac-1 cells (1 × 104 cells/well) to obtain effector cell to target cell (E/T) ratios of 100:1, 50:1 and 25:1 in round bottomed 96 well plates, and co-cultured for 24 h. We then measured the levels of interferon-gamma (IFN-γ) and granzyme released from activated NK cells using the respective ELISA set (BD bioscience, San Diego, CA, USA).
2.7. Inhibitory activity of lung metastasis using colon26-M3.1 carcinoma
To measure the anti-metastatic potential of CBP1S, various doses of CBP1S were orally administered twice per day (12 h intervals) for 15 days. After oral administration of the CBP1S, Colon26-M3.1 carcinoma cells (3 × 104 cells/mouse) were intravenously injected into BALB/c mice (Yoo, Saiki, Sato, & Azuma, 1994). The mice were euthanized at 14 daysafter the tumor inoculation, and their lungs were fixed in Bouin's solution (Sigma). Tumor colonies that spread to the lung were counted under a dissecting microscope.
2.8. Methylation analysis
Methylation analysis was performed according to the methods of Hakomori (1964) and Kim, Hong, & Shin (2017) with slight modifications. In summary, 20 μL of glycerol (Sigma) was added to 1 mL of polysaccharide sample (1 mg/mL), and the mixture was dried under a nitrogen-flushing with heating block (Eyela MG-2200, Tokyo, Japan). After the sample was methylated by addition of sodium methylsulfinyl carbanion with DMSO and iodomethane (sigma), the methylated sample was collected using an Sep-pak C18 cartridge (Waters, Dublin, Ireland) eluted with EtOH. The methylated product was hydrolyzed with 1 M TFA for 90 min at 121 °C, reduced with NaBD4 for 150 min, and finally acetylated with acetic anhydride for 150 min at 100 °C. The resulting methylated alditol acetates (PMAAs) were dissolved with 50 μL of acetone (Sigma) and analyzed using a GC–MS (6890 GC/5975 MSD; Agilent, Santa Clara, CA, USA) equipped with a SP-2380 capillary column. The analysis was performed with the following temperature program: 60 °C (1 min) → 150 °C (30∘C/min) → 180 °C (1 °C/min) → 231 °C (1.5 °C/min) → 250 °C (30 °C/min), 250 °C (10 min). PMAAs were identified by their fragment ions and relative retention times, and their mole percentage was estimated from the peak areas and response factors on a flame ionization detector.
2.9. Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 12.0 (SPSS Inc., Chicago, IL, USA). Differences among groups were evaluated by a one-way Analysis of variance (ANOVA) and Duncan's multiple range test. All data are presented as the means ± standard deviation (SD).
3. Results and discussion
3.1. Preparation of polysaccharide fractions from CSL
CBP was preferentially isolated from CSL via 80% EtOH precipitation and successively divided into two polysaccharide fractions using a 50% EtOH rapid separation as previously described (Kim, Kwak, Hong, Suh, & Shin, 2016; Lee et al., 2015). The fractions, termed CBP1S and CBP1P, yielded 4.0% and 6.8%, and estimated as 80 kDa and 4 kD, indicated respectively (Fig. 1 ). In relation to the extraction yield of carbohydrate from CSL, Hull, Yang, Venzke, Kulhavy, & Montgomery (1996) reported that total carbohydrate contents was varied (5.6-17.2 g/L) according to the steaming condition of corn steep, and Prasanth, Hasan, Danielle, & Raymond (2011) reported that CSL included 7.5% total carbohydrate. Both polysaccharide fractions consisted primarily of neutral sugars (81–82%), as determined by chemical composition analysis (Table 1 ). However, the characteristics of CBP1S and CBP1P were distinguished by the concentration of uronic acid and protein. That is, CBP1P contained 17.7% protein and minimal uronic acid. In contrast, CBP1S1 contained 9.5% uronic acid and an equivalent concentration of protein. Ethanol fractionation of crude polysaccharides results in separation of the polysaccharides by molecular size (Lee et al., 2015), therefore, our results indicate that CBP1S, the polysaccharide fraction isolated from CSL that contains molecules of relatively small molecular, seems to be an uronic acid-involving polysaccharide.
Fig. 1.
Scheme for isolation and fractionation of polysaccharide fractions from CSL. Corn byproduct polysaccharide (CBP) was isolated from CSL using 80% ethanol, and further fractionated into CBP1S and CBP1P with 50% ethanol fractionation.
Table 1.
Chemical composition of CBP1P and CBP1S isolated from corn steep liquor.
| CBP1P | CBP1S | |
|---|---|---|
| Molecular weight | 4 kDa | 80 kDa |
| General composition (%) | ||
| Neutral sugar | 82.0 ± 1.0 | 81.0 ± 0.1 |
| Uronic acid | 0.2 ± 0.2 | 9.5 ± 0.1 |
| Protein | 17.7 ± 0.2 | 9.5 ± 0.8 |
| Kdo-like materialsa | – | – |
| Monosaccharide composition (mole%)b | ||
| Rhamnose | – | – |
| Fucose | – | – |
| Arabinose | 0.5 ± 0.0 | 22.3 ± 0.2 |
| Xylose | 0.6 ± 0.0 | 14.3 ± 0.0 |
| Mannose | 0.6 ± 0.0 | 1.4 ± 0.0 |
| Galactose | 3.3 ± 0.0 | 9.5 ± 0.0 |
| Glucose | 77.0 ± 0.0 | 33.5 ± 0.2 |
| Galacturonic acid + Glucuronic acid | 0.2 ± 0.2 | 9.5 ± 0.1 |
Kdo means 2-keto-3-deoxy-d-manno-octulosonic acid.
Mole% of monosaccharide composition was calculated from the detected total carbohydrate.
3.2. Structural characteristics of polysaccharide fractions isolated from CSL
The GC chromatograms of alditol acetate derivative prepared from CBP1S and CBP1P was presented in Supplementary Fig. S1, and the results were summarized in Table 1. Monosaccharide composition analysis revealed that CBP1P consists primarily of glucose units that are most likely glucan-like polysaccharides such as α-glucan (starch) or β-glucan. In contrast, CBP1S comprised glucose, arabinose, and xylose residues (33.5%, 22.3%, and 14.3%, respectively). The high percentage of arabinose and xylose in CBP1S suggests that it is rich in arabinoxylan, which is a major hemicellulosic polysaccharide present in grass plants, including corn (Ebringerová, Hromádková, & Heinze, 2005). Hull et al. (1996) reported changes in the composition of corn steep water during steeping, with arabinose, xylose, and/or glucose as the major polysaccharide units in the corn steep water; their results are similar to the monosaccharide composition of CBP1S reported here. Excluding above reports, to our knowledge, few studies on the sugar composition analysis of CSL have reported so far.
In addition, CBP1S fraction was analyzed using GC–MS following a methylation procedure to deduce the glycosidic linkage conformation of immunostimulating polysaccharide in CSL (Table 2 ). CBP1S contains over than twenty types of glycosyl linkages, consisting primarily of xylopyranosyl (Xylp), arabinofuranosyl (Araf), glucopyranosyl (Glcp) and galactopyranosyl (Galp) linkages, in addition to a small amount of rhamnopyranosyl (Rhap) linkages. Large amount of Xylp linkages (26.2 mol%) and Araf linkages (20.8 mol%) indicated that hemicelluosic polysaccharide arabinoxylan accounts for about half proportion of CBP1S. In addition, large proportion of branched Xylp (2,4-linked, 3,4-linked, and 2,3,4-linked; total of 16.8 mol%) in addition to reducing terminal Araf (15.8 mol%) assumed that (1 → 4)-xylan backbone present in CBP1S arabinoxylan was highly substituted with short Araf residues. The 4-linked Glcp (11.4 mol%) and terminal Glcp (3.6 mol%) accounted for a major portion of Glcp linkages, suggesting that starch and cellulose might be contained in CBP1S with a small amount (about 15% of CBP1S). Meanwhile, various types of Galp linkages in addition to small amount Rhap linkage suggested that CBP1S is likely to contain several side chains of pectic rhamnogalacturonan I (RG-I). That is, Rhap residue of RG-I is usaually substituted with single neutral glycosyl residues and with polymeric side chains consisting predominantly of α-l-(1 → 5)-arabinans, β-(1 → 4)-d-galactans, type I arabinogalactans, type II arabinogalactans, and possibly galacto-arabinans. Consequently, sugar composition and glycosidic linkage analyses indicated that three kinds of polysaccharide coexist in CBP1S, including a large proportion of arabinoxylan (about 50%), linear (1 → 4)-glucan such as starch and cellulose, and side chains branched with Rhap residue of RG-I backbone.
Table 2.
Methylation analysis of glycosidic linkages of CBP1S purified from corn steep liquor.
| Glycosyl residuea | Position of methyl group | Deduced glycosidic linkage | CBP1S (Mole%)b |
|---|---|---|---|
| Rhap | 2- | 3,4- | 3.1 |
| Arap | 2,3,5- | Terminal (f) | 15.8 |
| 3,5- | 2- | 2.0 | |
| 2,3- | 5- | 3.0 | |
| Xylp | 2,3,4- | Terminal (p) | 1.6 |
| 2,3- | 4- | 7.8 | |
| 3- | 2,4- | 5.3 | |
| 2- | 3,4- | 5.3 | |
| – | 2,3,4- | 6.2 | |
| Glcp | 2,3,4,6- | Terminal (p) | 3.6 |
| 2,4,6- | 3- | 0.6 | |
| 3,4,6- | 2- | 0.8 | |
| 2,3,4- | 6- | 0.8 | |
| 2,3,6- | 4- | 11.4 | |
| Galp | 2,3,4,6- | Terminal (p) | 3.1 |
| 2,4,6- | 3- | 1.2 | |
| 2,3,6- | 4- | 0.6 | |
| 2,3,4- | 6- | 1.4 | |
| 2,6- | 3,4- | 1.5 | |
| 2,3- | 4,6- | 2.0 | |
| 2,4- | 3,6- | 3.2 | |
| 2- | 3,4,6- | 0.9 |
p and f mean pyranoside and furanoside, respectively.
Mole% was calculated from the detected neutral sugar (81.0%).
3.3. Anti-complementary activity of CBP1S isolated from CSL
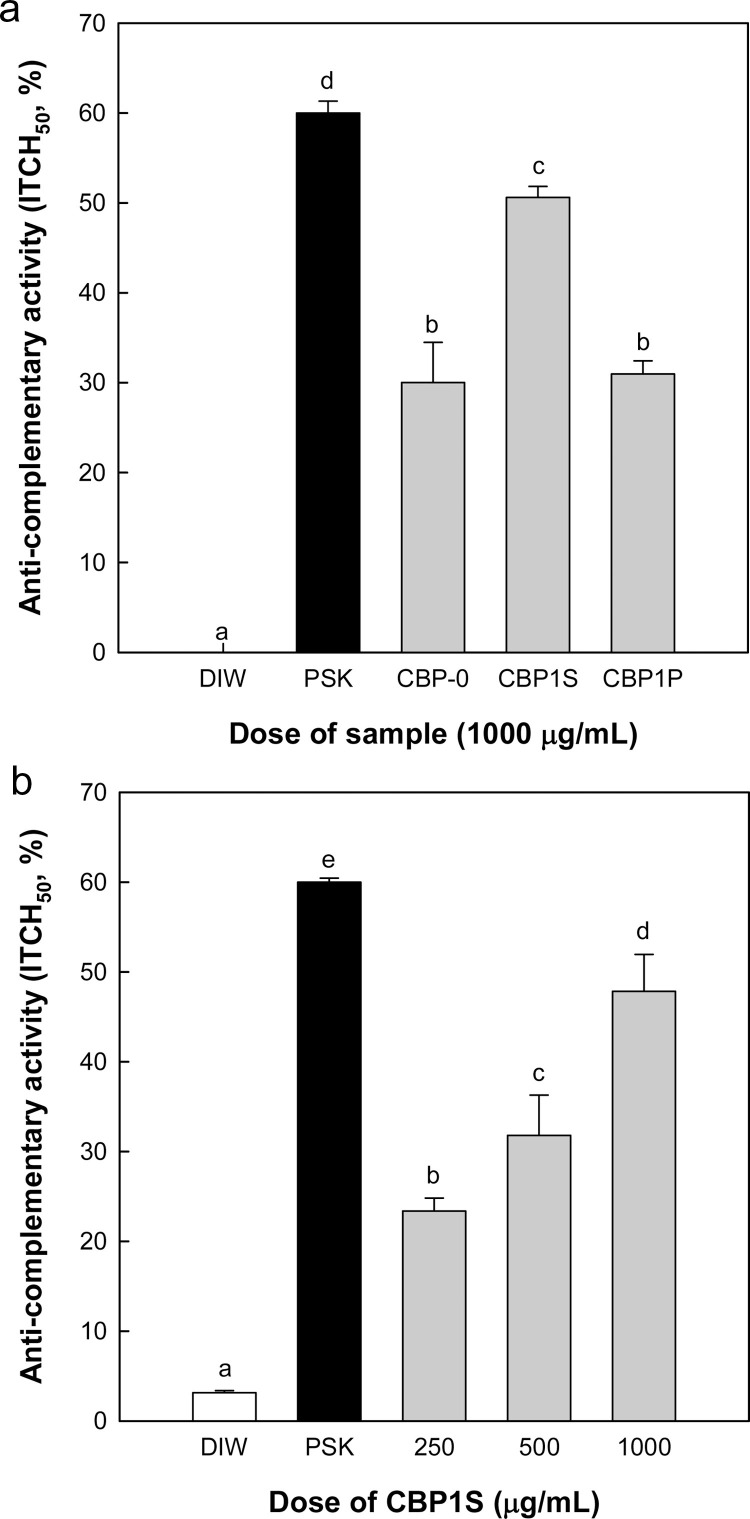
The complement system is a component of innate immunity that enhances the effectiveness of antibodies and phagocytic cells such as macrophages and neutrophils (Dunkelberger & Song, 2010). The measurement of complementary activity was based on the theory that depletion of complement components in human blood can be detected by measuring the overall hemolytic activity (Strong, Lea, & Ward, 1978). Therefore, NHS was cultured with samples (complement activator), and the remaining complement titer was measured by hemolysis of sensitized sheep erythrocytes as an antigen-antibody complex. The regulating samples for the hemolytic activity of complement are referred to as ‘anti-complementary substances” (Strong et al., 1978). In this study, deionized water and PSK were used as negative and positive controls, respectively, and complementary activity was calculated as inhibition of 50% TCH (ITCH50). PSK is a protein-bound polysaccharide isolated from the fruit body of mushrooms, such as Trametes versicol and Coriolus versicolor, which has been the subject of investigation as an experimental adjunctive therapy for various types of cancer (Koji et al., 2007). It is well known a proteoglycan consisting mainly of common fungal β-glucan being comprised of β-1,4 backbone with β-1,3 and β-1,6 side chains. The approximate molecular weight of PSK is 100 kDa, and the protein component is reported at the side chain of β-1,6 glycosidic bond (Hiroshi, Kenichi, & Yoshiharu, 1995). When we estimated the complementary activity of CBP isolated from CSL and its two fractions (CBP1P and CBP1S), CBP1S showed the highest anti-complementary activity among the three polysaccharides (Fig. 2 A). In the results of the relationship between dose and activity of the most active polysaccharide fraction, the significant anti-complementary activity of CBP1S was concentration-dependent (Fig. 2B). Therefore, we used CBP1S as the immunostimulating polysaccharide fraction in all subsequent experiments.
Fig. 2.
Anti-complementary activity of polysaccharide fractions isolated from CSL. Anticomplementary activity was represented as the inhibition of 50% total complementary hemolysis. Distilled water (DIW) was used as a negative reference, and Polysaccharide K (PSK) is an immunoactive polysaccharide isolated from Coriolus versicolor that was used as a positive reference. Means with different superscript letters indicate significant differences at p < 0.05 by Duncan's multiple range test.
3.4. Effect of CBP1S on cytokine production in murine peritoneal macrophages
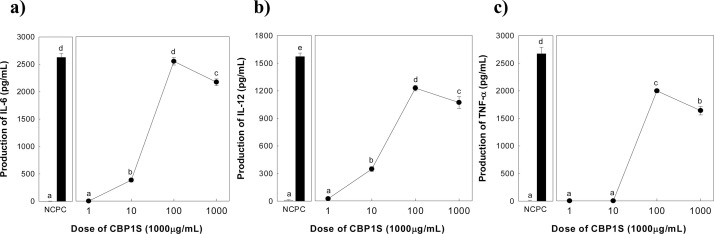
It is well known that one of the primary mechanisms of immune system potentiation by plant polysaccharides appears to involve their ability to influence complement activation in addition to macrophage stimulation (Kim et al., 2016, Kraus and Franz, 1992). Macrophages belonging to the innate immune system can work as antigen-presenting cells following capturing bacteria and virus, etc, by phagolytic activity. Simultaneously, macrophage secretes various cytokines, such as IL-6, IL-12, IL-1β, and TNF-α to activate subsequent immune system functions (Beutler, 2004). Ultimately, these macrophage-produced cytokines play a pivotal role in the communication between the innate and adaptive immune systems as part of the host defense mechanisms (Aderem and Underhill, 1999). CBP1S increased the production of all cytokines tested (IL-6, IL-12, and TNF-α) in murine peritoneal macrophage in a dose-dependent manner, ranging from 1 to 100 μg/mL (Fig. 3 ). However, at a dose of 1000 μg/mL CBP 1S, cytokine production was significantly lower than that observed at a dose of 100 μg/mL.
Fig. 3.
Effect of CBP1S on the production of IL-6 (a), IL-12 (b), and TNF-α (c) in murine peritoneal macrophage. NC and PC mean negative control treated with medium and positive control treated with LPS (5 μg/mL), respectively. Means with different superscript letters indicate significant differences at p < 0.05 by Duncan's multiple range test.
3.5. Effect of CBP1S on NK cell activation in the tumor cells
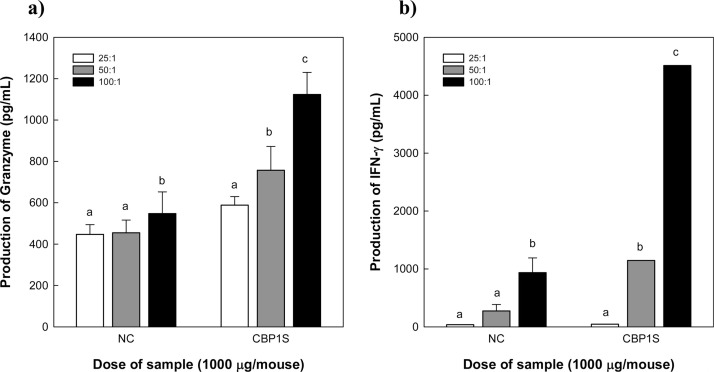
CBP1S-treated peritoneal macrophages produced remarkable levels of IL-12, which is well known to stimulate NK cells and primarily kill viral-infected cells and tumor cells (Vivier et al., 2011). Therefore, we aimed to determine whether CBP1S facilitates NK cell activation. NK cells were isolated from the spleen of CBP1S-injected mice and co-cultured with MHC class I-deficient Yac-1 lymphoma cells. NK cells recognize MHC class I bound in the cellular membrane of target cells to distinguish between self and non-self. If MHC class I is not recognized, perforins and granzymes are secreted to pierce the cell membrane and to degrade the MHC class I-deficient tumor or infected cells (Terunuma, Deng, Dewan, Fujimoto, & Yamamoto, 2008; Vivier et al., 2011; Voskoboinik, Whisstock, & Trapani, 2015). Therefore, we evaluated NK cell activity by measuring NK cell-secreting molecules, macrophage-stimulating IFN-γ and serine protease granzyme in the CBP1S-sensitized mouse spleen cells (effector cells) co-cultured with Yac-1 lymphoma cells (target cells). As shown in Fig. 4 , NK cell activity increased in splenocytes isolated from saline-treated mice (NC) in an effector/target (E/T) ratio-dependent manner. Further, the levels of both granzyme and IFN-γ were dramatically higher in an E/T ratio-dependent manner in the group treated with CBP1S than in the NC group. These results suggest that intravenous administration of CBP1S might be effective for NK cell activation.
Fig. 4.
Granzyme (a) and IFN-γ (b) production activity in NK cells isolated from CBP1S-stimulated Balb/c splenocytes. NK cells (effector cells) collected from CBP1S-injected Balb/c mice were co-cultured with Yac-1 lymphoma cells (target cells) with different effector to target ratios (25:1, 50:1, and 100:1). The production of NK cell-activating substances (granzyme and IFN-γ) was determined in the culture supernatant by sandwich ELISA. The negative control (NC) was NK cells isolated from saline-injected Balb/c mice. Means with different superscript letters indicate significant differences at p < 0.05 by Duncan's multiple range test.
3.6. Anti-metastatic effects promoted by oral administration of CBP1S
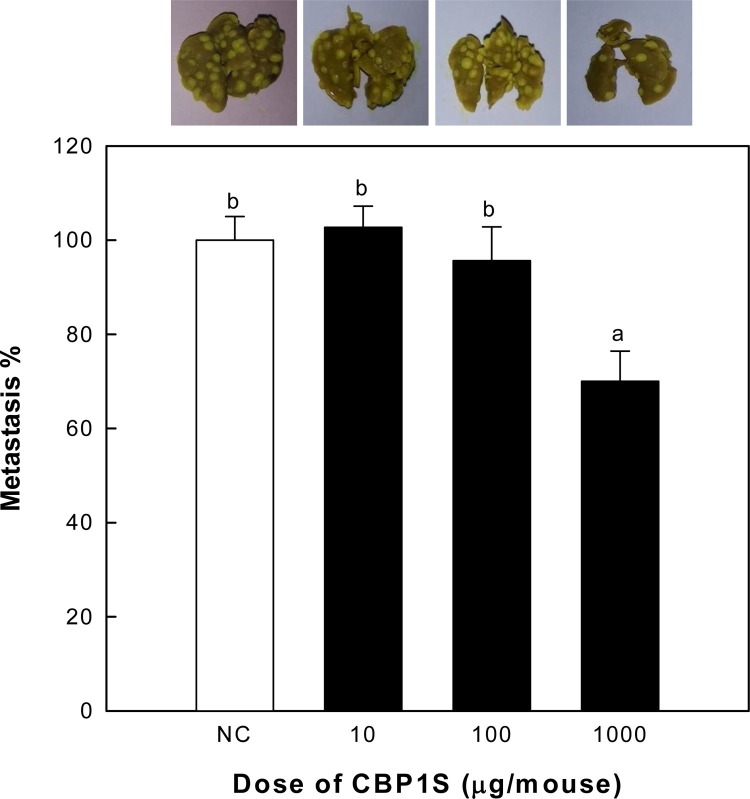
NK cells are well known to play a key role in tumor cell removal and resistance to metastasis in the lung (Yang, Goding, Hokland, & Basse, 2006). According to the above results, CBP1S might be beneficial for facilitating the innate immune system via activation of the complementary system, macrophages and NK cells. In particular, our results reveal that intravenous administration of CBP1S is useful for activating NK cells. Many of the previously reported studies of CSL were in vitro or used injections of the active polysaccharides, with no further oral administration (Pang, Xie, Chen, & Hu, 2012). In addition, it is not clear whether polysaccharides that elicit biological activities in vitro or upon injection are effective when administered orally (Boh, Berovic, Zhang, & Zhi-Bin, 2007). Therefore, we aimed to evaluate the anti-metastatic effects of CBP1S after oral administration. As such, CBP1S was constantly taken orally to mice for 15 days (twice per day). The effects of orally administered CBP1S on tumor metastasis were evaluated based on the number of tumor colonies after inoculating Colon26-M3.1 carcinoma cells, which can metastasize to the lungs in Balb/c mice (Sakamoto et al., 2003). The results indicate that oral administration of CBP1S with a high dose (1000 μg per mouse) inhibits tumor metastasis significantly more than CBP1S administered at lower doses (Fig. 5 ). Lung tumor generation was 37% lower in the group administered 1000 μg CBP1S than that of the NC group administered saline only. Consequently, our results suggest that oral administration of CBP1S could facilitates the anticancer effects, as shown by suppression of tumor migration to the target organ; these effects are likely related to stimulation of the innate immune system, such as the complement system, macrophages and NK cells.
Fig. 5.
Effect of oral administration of CBP1S on lung metastasis produced by inoculating Colon26-M3.1 carcinoma cells. CBP1S was orally administered at various doses to the mice (four mice per group, n = 16) for 10 days (twice per day), and metastatic tumor cells (Colon26-M3.1 cells, 3 × 104) were then intravenously injected into the mice. Mice were euthanized 14 days after tumor inoculation, and the prophylactic effect of CBP1S was estimated as metastatic rate (%) calculated from the munber of colonies in the lung tissue. Saline was administered to the group of negative control (NC) instead of samples. Means with different superscript letters indicate significant differences at p < 0.05 by Duncan's multiple range test.
4. Conclusion
Currently, most CSL is disposed of because of its huge output; therefore, we aimed to investigate whether polysaccharides isolated from CSL can serve as functional food material for humans. Therefore, CBP1S and CBP1P were isolated from CSL, and various immunological activities were evaluated in vitro and in vivo. Consequently, CBP1S primarily comprises highly branched-arabinoxylan-rich polysaccharides with potent innate immune system-stimulatory activities. In addition, our results suggest that CBP1S seems to promote inhibition of lung metastasis through a mechanism leading to stimulation of the innate immune system. Overall, CBP1S shows potential for use in industrial application. On the other hand, the elucidation of molecular target of CBP1S is very important aspect of understanding the molecular mechanisms involved in immune responses (Kim, Hong, & Shin, 2017). In the present study, potent anti-metastatic effect induced by stimulation of CBP1S was closely associated with the activation of immune cells. In fact, it is well known that activation of immune cells such as macrophage and NK cell are closely involved in the intracellular mechanisms, such as mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) pathways. Therefore, we would like to elucidate the molecular mechanism occurred inside the immune cells responsible for the immunological action, when the cells were stimulated with the CBP1S, in near future works.
Conflict of interest
The authors claim no conflicts of interest.
Acknowledgements
This research was financially supported by the Ministry of Trade, Industry and Energy (MOTIE) and Korea Institute for Advancement of Technology (KIAT) through the Research and Promoting Regional specialized Industry. Also, this work was supported by Kyonggi University’s Graduate Research Assistantship 2017.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.carbpol.2017.11.060.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aderem A., Underhill D.M. Mechanisms of phagocytosis in macrophages. Annual Review of Immunology. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Beutler B. Innate immunity: An overview. Molecular Immunology. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Analytical Biochemistry. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Boh B., Berovic M., Zhang J., Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnology Annual Review. 2007;13:265–301. doi: 10.1016/S1387-2656(07)13010-6. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers P., Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28:350–356. [Google Scholar]
- Dunkelberger J.R., Song W.C. Complement and its role in innate and adaptive immune responses. Cell Research. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- Ebringerová A., Hromádková Z., Heinze T. Hemicellulose. In: Heinze T., editor. Polysaccharides I. Springer; Berlin Heidelberg: 2005. pp. 1–67. [Google Scholar]
- Global strategic business report. 2012. Biodegradable Polymers; pp. 1–66. [Google Scholar]
- Hakomori S. A rapid permethylation of glycolipid, and polysaccharidecatalyzed by methylsulfinyl carbanion in mimethyl sulfoxide. Journal of Biochemistry. 1964;55:205–208. [PubMed] [Google Scholar]
- Hiroshi K., Kenichi M., Yoshiharu O. Antimetastatic effects of PSK (Krestin), a protein-bound polysccharide obtained from Basidiomycetes: an overview Cancer Epidemiology. Biomarkers & Prevention. 1995;4:275–281. [PubMed] [Google Scholar]
- Hull S.R., Yang B.Y., Venzke D., Kulhavy K., Montgomery R. Composition of corn steep water during steeping. Journal of Agricultural and Food Chemistry. 1996;44:1857–1863. [Google Scholar]
- Jones T.M., Albersheim P. A gas chromatographic method for the determination of aldose and uronic acid constituents of plant cell wall polysaccharides. Plant Physiology. 1972;49:926–936. doi: 10.1104/pp.49.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y.D., Zeltner J.Y., Jackson J.J., Carlo D.J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of gram-negative bacteria. Analytical Biochemistry. 1978;85:595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. Natural killer cells in the mouse. I: Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. European Journal of Immunology. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kim H., Kwak B.S., Hong H.D., Suh H.J., Shin K.S. Structural features of immunostimulatory polysaccharide purified from pectinase hydrolysate of barley leaf. International Journal of Biological Macromolecules. 2016;87:308–316. doi: 10.1016/j.ijbiomac.2016.02.072. [DOI] [PubMed] [Google Scholar]
- Kim H., Hong H.D., Shin K.S. Structure elucidation of an immunostimulatory arabinoxylan-type polysaccharide prepared from young barley leaves (Hordeum vulgare L.) Carbohydrate Polymers. 2017;157:282–293. doi: 10.1016/j.carbpol.2016.09.056. [DOI] [PubMed] [Google Scholar]
- Koji O., Satoshi T., Michiya K., Takanori M., Yasuhiro K., Junichi S. Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curative resections of gastric cancer. Cancer Immunology Immunotherapy. 2007;56:905–911. doi: 10.1007/s00262-006-0248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J., Franz G. Immunomodulating effects of polysaccharides from medicinal plants. Advances in Experimental Medicine and Biology. 1992;319:299–308. doi: 10.1007/978-1-4615-3434-1_30. [DOI] [PubMed] [Google Scholar]
- Lee E.H. Antitumor metastasis activity of pectic polysaccharide purified from the peels of Korean Citrus Hallabong. Carbohydrate Polymers. 2014;111:72–79. doi: 10.1016/j.carbpol.2014.04.073. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Hong H.D., Shin K.S. Rapid isolation method for preparation of immuno-stimulating rhamnogalacturonans in citrus peels. Korean Journal of Food Science and Technology. 2015;47:286–292. [Google Scholar]
- Lee S.J., Hong H.-D., Shin K.-S. Convenient method for selective isolation of immuno-stimulating polysaccharides from persimmon leaves. Journal of the Korean Society of Food Science and Nutrition. 2016;45:52–60. [Google Scholar]
- Liggett R.W., Koffler H. Corn steep liquor in microbiology. Bacteriological Reviews. 1948;12:297–311. doi: 10.1128/br.12.4.297-311.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddipati P., Atiyeh H.K., Bellmer D.D., Huhnke R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresource Technology. 2011;102:6494–6501. doi: 10.1016/j.biortech.2011.03.047. [DOI] [PubMed] [Google Scholar]
- Mayer M. Complement and complement fixation. In: Kabat E.A., Mayer M.M., editors. Experimental immunochemistry. 2nd ed. CC Thomas Publisher; 1961. pp. 133–240. [Google Scholar]
- Pang G., Xie J., Chen Q., Hu Z. How functional foods play critical roles in human health. Food Science and Human Wellness. 2012;1:26–60. [Google Scholar]
- Park H.R. Enhanced antitumor efficacy of cisplatin in combination with HemoHIM in tumor-bearing mice. BMC Cancer. 2009;9:85. doi: 10.1186/1471-2407-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.R., Lee H.S., Cho S.Y., Kim Y.S., Shin K.S. Anti-metastatic effect of polysaccharide isolated from Colocasia esculenta is exerted through immunostimulation. International Journal of Molecular Medicine. 2013;31:361–368. doi: 10.3892/ijmm.2012.1224. [DOI] [PubMed] [Google Scholar]
- Sakamoto N. Interleukin-10 gene therapy enhances antitumor effect of CPT-11 for lung metastasis of colon26 adenocarcinoma in mice. Gastroenterology. 2003;124:A456–A457. [Google Scholar]
- Salki I. Induction of tumoricidal macrophages and production of cytokines by synthetic muramyl dipeptide analogues. Vaccine. 1988;6:238–244. doi: 10.1016/0264-410x(88)90218-6. [DOI] [PubMed] [Google Scholar]
- Serna-Saldivar S.O. CRC Press; 2016. Cereal grains: properties, processing, and nutritional attributes. [Google Scholar]
- Strong W.M., Lea D.J., Ward D.J. Measurement of total haemolytic complement activity in body fluids. Journal of Clinical Pathology. 1978;31:527–530. doi: 10.1136/jcp.31.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma H., Deng X., Dewan Z., Fujimoto S., Yamamoto N. Potential role of NK cells in the induction of immune responses: Implications for NK cell-based immunotherapy for cancers and viral infections. International Reviews of Immunology. 2008;27:93–110. doi: 10.1080/08830180801911743. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi S. Krestin (PSK) Cancer Treatment Reviews. 1984;11:131–155. doi: 10.1016/0305-7372(84)90005-7. [DOI] [PubMed] [Google Scholar]
- Utrilla J. Engineering and adaptive evolution of Escherichia coli for D-lactate fermentation reveals GatC as a xylose transporter. Metabolic Engineering. 2012;14:469–476. doi: 10.1016/j.ymben.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Vivier E. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman J.J. Effects of mushroom-derived beta-glucan-rich polysaccharide extracts on nitric oxide production by bone marrow-derived macrophages and nuclear factor-kappaB transactivation in Caco-2 reporter cells: Can effects be explained by structure? Molecular Nutrition and Food Research. 2010;54:268–276. doi: 10.1002/mnfr.200900009. [DOI] [PubMed] [Google Scholar]
- Voskoboinik I., Whisstock J.C., Trapani J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nature Reviews Immunology. 2015;15:388–400. doi: 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- Wang F., Hu J.H., Guo C., Liu C.Z. Enhanced laccase production by Trametes versicolor using corn steep liquor as both nitrogen source and inducer. Bioresource Technology. 2014;166:602–605. doi: 10.1016/j.biortech.2014.05.068. [DOI] [PubMed] [Google Scholar]
- Wasser S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- Xi Y.L. Succinic acid production by Actinobacillus succinogenes NJ113 using corn steep liquor powder as nitrogen source. Bioresource Technology. 2013;136:775–779. doi: 10.1016/j.biortech.2013.03.107. [DOI] [PubMed] [Google Scholar]
- Yang Q., Goding S.R., Hokland M.E., Basse P.H. Antitumor activity of NK cells. Immunologic Research. 2006;36:13–25. doi: 10.1385/IR:36:1:13. [DOI] [PubMed] [Google Scholar]
- Yoo Y.C., Saiki I., Sato K., Azuma I. MDP-Lys(L18), a lipophilic derivative of muramyl dipeptide: inhibits the metastasis of haematogenous and non-haematogenous tumours in mice. Vaccine. 1994;12:160–175. doi: 10.1016/0264-410x(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Yoon T.J. Prophylactic effect of Korean mistletoe (Viscum album coloratum) extract on tumor metastasis is mediated by enhancement of NK cell activity. International Journal of Immunopharmacology. 1998;20:163–172. doi: 10.1016/s0192-0561(98)00024-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.