Abstract
In the bovine synepitheliochorial placenta key sites of fetal–maternal interaction are placentomes consisting of maternal caruncles interdigitating with fetal cotyledons. The aim of this study was to establish an epithelial cell line from caruncles of pregnant cows and to develop a model to study restricted trophoblast invasion, pathogenesis of pregnancy associated diseases and pathways of infection and transport. Primary epithelial cells were isolated, successfully subcultured for 32 passages and cryopreserved at various stages. The cultures were termed bovine caruncular epithelial cell line-1 (BCEC-1). Cytokeratin, zonula occludens-1 protein and vimentin but neither α-smooth muscle actin nor desmin were detected by immunofluorescence performed every 5 (±1) passages. These results were confirmed by Western blotting. BCEC-1 were then cultured either without matrix or on fibronectin or collagen coated Transwell® polyester membrane inserts, respectively, enabling separate access to the basal or apical epithelial compartments. Transmission and scanning electron microscopy of BCEC-1 revealed ultrastructural features also observed in vivo, such as apical microvilli and junctional complexes. Transepithelial electrical resistance (TEER) was measured regularly and revealed an increase with advancing confluence in all cultures. Cultures on coated inserts reached confluence and corresponding TEER-levels at an earlier stage. In addition, the cells were tested negative for bovine virus diarrhoea (BVD) virus, but were permissive for the virus. In conclusion, the BCEC-1 cell line retained characteristics of maternal caruncular epithelial cells as observed in vivo and in primary cell cultures and thus will be a highly useful tool for future studies of pathways of invasion, fetal–maternal communication, transport and infection.
Keywords: Bovine placenta, Cell culture, Polarised epithelial cell line, Transepithelial electrical resistance, Bovine virus diarrhoea virus
1. Introduction
In contrast to the haemochorial placenta of humans and rodents, the synepitheliochorial placenta of cows resembles a polarised uterine epithelial barrier facing the fetal chorionic (trophoblast) epithelium. This barrier protects the fetus from a variety of infectious and/or toxic agents. Even maternal immunoglobulins cannot pass this barrier and therefore newborn calves rely on receiving colostrum within the first hours after birth.
Key sites of fetal–maternal interaction are placentomes consisting of maternal caruncles interdigitating with fetal cotyledons. A special feature is the occurrence of “restricted” trophoblast invasion performed by trophoblast giant cells migrating towards and fusing with maternal epithelial cells [1], [2]. Trophoblast invasion may be compared with tumour invasion but in contrast to tumour cells, the trophoblast can regulate its tumour-like attributes and the depth of invasion is limited in a species-specific manner [3]. In the bovine, this migration takes place between only two cell layers suggesting the bovine placenta to be an ideal model to study invasive processes. So far, the expression of potential regulating factors has been studied on in vivo material only [2], [4], [5].
From a clinical point of view, one of the major disorders in bovine reproduction is the retention of the fetal membranes post partum due to an incomplete loosening of the feto-maternal attachment [6]. If not treated in time, it can result in severe reproductive disorders and subsequent economic loss. Surprisingly and different from other ruminants this disease frequently occurs in the cow and the water buffalo. Although many studies have been conducted there is no solution to this problem yet, because the underlying mechanisms are far too complex to be elucidated by in vivo studies.
Taken together, the establishment of an in vitro model to study bovine placental mechanisms is overdue. However, when looking for suitable cell cultures it appears that the majority of studies dealt with primary epithelial cells isolated from the endometrial tissue of non-pregnant cows and seldom differentiated between caruncular and intercaruncular tissues [7], [8]. Such a differentiation, however, is essential because caruncular and intercaruncular (interplacentomal) epithelia differ from each other functionally, e.g. according to the expression pattern of mRNA and proteins [9], [10], [11].
Cell cultures derived from the caruncle of pregnant animals have rarely been established. Furthermore, the few studies undertaken failed to differentiate between the cell types, epithelial cells in particular [12]. So far only one bovine endometrial (epithelial) cell line (BEND) originating from a non-pregnant animal has been characterised and deposited at the American Type Culture Collection (ATCC# CRL-2398) [13]. The BEND cell line has been used successfully in many studies; the majority of which have dealt with prostaglandin synthesis and regulation pathways [14], [15]. However, since it was derived from a non-pregnant animal without differentiating between caruncular and intercaruncular regions, it raises the question as whether it is suitable to study processes occurring during pregnancy. Moreover, the ATCC tested the cells positive for the virus of bovine viral diarrhoea (BVD), a disease of great importance in bovine medicine and management, since it can lead to a reduced milk yield, decreased fertility and abortion. If transmitted intrauterinely to the fetus within the first 120 days of pregnancy, the calf develops an immune tolerance resulting in persistent infection. Superinfection or mutations within the virus in the first few months after birth result in the development of the lethal mucosal disease [16].
In the attempt to create a cell culture model to study fetal–maternal interactions, our group recently established and validated a method to isolate and cultivate primary epithelial cells from the caruncle of pregnant cows from day 100 of pregnancy onwards [17], [18]. The cells were proven to be of maternal origin by fluorescence in situ hybridisation (FISH). It was demonstrated that these cells expressed the epithelial specific proteins cytokeratin and tight junctional zonula occludens-1 as well as integrin subunits and proteins of the extracellular matrix. Endothelial origin could be excluded by the absence of low density lipoprotein (LDL) labelling. Furthermore, it was shown that cryopreservation of the cells is possible. To standardise future experimental conditions, the aim of this study was to create a cell line, representing a functional, differentiated caruncular epithelium from pregnant cows including the assessment of the BVD virus status. We thereby provide the basis for future research on trophoblast invasion, feto-maternal communication, transport and infection in the bovine placenta.
2. Materials and methods
2.1. Tissue collection and primary caruncular epithelial cell culture
A uterus from a pregnant cow (Bos taurus) with a gestational age of 4 months estimated by measurement of the crown-crump length of the fetus (formula by Keller [19]) was retrieved from the local abattoir 20–30 min after slaughter. A primary cell culture of caruncular epithelial cells was created as described by Zeiler et al. [18]. Briefly, a placentome was excised and manually separated into fetal cotyledon and maternal caruncle. The maternal tissue was disaggregated in 200 U/ml collagenase I (Biochrom AG seromed, Berlin, Germany) and the cells were cultured on plastic in flasks supplied with Dulbecco's Modified Eagle Medium (DMEM)/Ham's F12 (PAA Laboratories GmbH, Coelbe, Germany) containing 10% fetal calf serum (FCS, Biochrom) and 1% penicillin/streptomycin (10,000 IU/10,000 μg/ml; Biochrom) in an incubator (5% CO2/95% air at 37 °C). Morphologically, two cell populations could be differentiated: fibroblastoid cells and epithelioid cells previously identified as caruncular epithelial cells [17].
2.2. BCEC-1 cell line
Contaminating fibroblastoid cells were removed during the first 3–4 passages by a two step trypsin incubation protocol (0.05% trypsin/0.02% EDTA solution; Biochrom). In the first step, fibroblastoid cells detaching prior to the epithelial cells were discarded. Then, the remaining epithelial cells were retrieved and seeded in two new cell culture flasks. Subculturing was performed as soon as confluence was reached or after being 14 days in culture. Confluent cultures were either continuously subcultured until passage 32 or cryopreserved in a solution containing 10% dimethylsulphoxide (DMSO, Sigma–Aldrich GmbH, Taufkirchen, Germany), 30% FCS and 60% DMEM/Ham's F12. For immunofluorescence and BVD virus experiments confluent and/or thawed cultures were seeded in 6-well cell culture plates with cover slips. For transmission (TEM) and scanning (SEM) electron microscopy and measurements of the transepithelial electrical resistance (TEER) the cells were seeded in uncoated and coated (fibronectin or collagen A) polyester membrane Transwell®-Clear inserts (12 μm diameter, 0.4 μm pore size, Corning Inc., Acton, USA). Coating with 10 μg/ml fibronectin from the bovine placenta (Sigma–Aldrich) and 0.5 mg/ml collagen A (Biochrom) was performed according to the manufacturer's instructions. A preliminary experiment also included inserts with biopore and polycarbonate membranes (Millicell, Millipore, Billerica, USA) with a pore size of 0.4 μm.
2.3. Immunofluorescence
Immunofluorescence was performed with cultures from passages 4, 15, 18, 21, 25 and 31 using primary antibodies detecting cytokeratin, zonula occludens-1, vimentin, desmin and α-smooth muscle (sm) actin ( Table 1) as previously described [17]. In this study, all antibodies were applied to sections of placentomal tissue (in vivo control) and primary cultures demonstrating cell type specific binding. Briefly, cultures were washed in 0.02 M phosphate buffered saline (PBS, pH 7.3) containing 0.3% Tween-20 (Sigma–Aldrich) three times between each incubation step. Sera and antibodies were diluted in a PBS/0.3% Tween containing 0.1 g/ml bovine serum albumin and glycerol at a ratio of 2:1 (pH 8.0). The cells were fixed in 100% methanol for 10 min and then blocked for 60 min in a 10% normal donkey serum solution (Chemicon Int., Temecula, USA). Incubation with primary antibodies (Table 1) was done overnight at 4 °C. After being rinsed in PBS/Tween, the cultures were incubated for another 60 min with fluorescence conjugated secondary antibodies (Table 1). Finally, the cover slips were removed from the wells and placed face down on slides in Vectashield Hard+Set mounting medium with DAPI (Vector Lab., Burlingame, USA) and viewed under an Olympus BX50 fluorescence microscope (Hamburg, Germany). Incubation in buffer without primary antibodies was used as negative control for unspecific binding of secondary antibodies. If the primary antibodies were derived from different hosts, double labelling was performed.
Table 1.
Antibodies used for immunofluorescence and Western blot analysis
| Dilution IF (μg/ml) | Dilution Western blot (μg/ml) | Manufacturer | ||
|---|---|---|---|---|
| Primary antibodies | ||||
| Rabbit anti-cow cytokeratin (wide spectrum screening) | Polyclonal | 35.7a | 5.4a | DakoCytomation, Carpinteria, USA, Cat. No.: Z0622 |
| Rat anti-Zo-1 tight junction associated polypeptide | Monoclonal | 20 μl/ml | – | Chemicon Int., Temecula, USA, Cat. No.: MAB1420 |
| Mouse anti-vimentin | Monoclonal | 0.69b | 0.14b | DakoCytomation, Carpinteria, USA, Cat. No.: M7020 |
| Mouse anti-human α-smooth muscle actin | Monoclonal | 1.4b | 0.14b | DakoCytomation, Carpinteria, USA, Cat. No.: M0851 |
| Mouse anti-human desmin | Monoclonal | 2.4b | – | DakoCytomation, Carpinteria, USA, Cat. No.: M0760 |
| Secondary antibodies | ||||
| Donkey anti-rabbit IgG, Fluorescein conjugated | 5.0a | – | Chemicon Int., Temecula, USA, Cat. No.: AP182F | |
| Donkey anti-rat IgG, Cy3 conjugated | 3.33a | – | Chemicon Int., Temecula, USA, Cat. No.: AP189C | |
| Donkey anti-mouse IgG, Cy3 conjugated | 3.33a | – | Chemicon Int., Temecula, USA, Cat. No.: AP192C | |
| Biotinylated anti-mouse/anti-rabbit IgG | – | 1.05b | Vector Lab., Burlingame, USA, Cat. No.: BA-1400 | |
Total protein concentration.
Total IgG concentration.
2.4. Western blot analysis
BCEC-1 cultures (passages 18 and 28) were chosen for Western blot analysis. Protein samples from a shock frozen placentome and a primary culture containing a pure epithelioid cell population isolated from a caruncle served as controls. Protein extraction, electrophoresis, Western blot and immunodetection with the antibodies listed in Table 1 was performed as previously described [17]. In this study protein derived from primary fibroblasts isolated from the caruncle (negative for cytokeratin) was included as well as the detection of a desmin protein specific band in placentome derived and fibroblast derived protein only. Briefly, protein was extracted with TRIzol-Reagent (Invitrogen, Karlsruhe, Germany), resuspended in a 1% sodium dodecyl sulphate (SDS) solution, diluted to a final concentration of 1000 μg/ml protein (±10%) and stored at −20 °C until further processing. Gel electrophoresis and Western blotting were performed with the NuPAGE Gel System (Invitrogen) according to the manufacturer's protocol using 10% and 12% NuPAGE Novex Bis-Tris gels. Blotting on a nitrocellulose membrane (0.2 μm pore size) was followed by immunodetection (Vectastain ABC Kit, Vector Labs). The colorimetric reaction was induced using TrueBlue peroxidase substrate (KPL, Gaithersburg, USA).
2.5. Transmission- and scanning electron microscopy (TEM and SEM)
TEM was performed on sections of confluent cultures (passages 11, 18 and 28 as well as a primary cell culture). The monolayer was fixed during a first step in 0.1 M cacodylate buffer (pH 7.2) containing 2% paraformaldehyde, 2% glutaraldehyde and 0.02% picric acid and in a second step in 1% osmium tetroxide in 0.1 M cacodylate buffer. After dehydration in ethanol followed by xylene, the membrane was embedded in Epon (Serva Electrophoresis GmbH, Heidelberg, Germany). Prior to the preparation of ultra thin sections for TEM, semithin sections were cut with a Reichert Ultracut S (Leica GmbH, Wetzlar, Germany), stained with Richardson's stain and assessed via light microscopy to obtain an overview of the sample. Finally, ultra thin sections (70–100 nm) were cut, counterstained with uranyl acetate and lead citrate with a Reichert Ultrastainer (Leica) and viewed under a transmission electron microscope (LEO 912AB, Zeiss, Oberkochen, Germany). Sections were assessed for the presence of epithelium specific structures, i.e. tight junctions, adherent junctions and apical microvilli.
SEM was performed with confluent cultures (passages 12 and 28). The monolayer was fixed in Soerensen buffer containing 1% glutaraldehyde. After dehydration in ethanol followed by isoamylacetate, the cells were dried in a Critical Point Dryer CPD 030 (Bal-Tec GmbH, Schalksmuehle, Germany) and sputter-coated with gold in a Sputtering Device (SCD 020, Balzer Union). The membrane was mounted to the sample holder and viewed using a scanning electron microscope (LEO 1430 Gemini, Zeiss). Samples were assessed for the presence of epithelial specific structures, i.e. the presence of a regular monolayer and apical microvilli.
2.6. Transepithelial electrical resistance (TEER) measurements
TEER measurements were performed on cultures (passages 6, 12 and 19) grown in duplicates on uncoated or fibronectin or collagen A coated polyester membrane Transwell-Clear inserts as described above. TEER measurements with the Epithelial Voltohmmeter (EVOM) and a STX2 Electrode (WPI Inc., Berlin, Germany) were performed regularly until confluence was reached according to the manufacturer's instructions. The cultures were considered confluent as soon as an estimated >95% of the insert membrane was covered with a monolayer observed via light microscopy. TEER levels of cultures from the same origin grown on coated and uncoated inserts were compared. Primary cultures of fibroblastoid cells isolated from the caruncle and seeded in duplicates on coated and uncoated inserts served as a negative control. As soon as TEER values exceeded 200 Ω/cm2 the monolayer was considered to be intact and polarised.
2.7. Infection with the bovine virus diarrhoea (BVD) virus
The BVD virus experiments were performed on cultures of passages 6, 12, 17 and 28 as well as Madin Darby bovine kidney (MDBK) cells (positive control, ATCC Number CCL-22, kindly provided by N. Tautz, Department of Virology, Justus-Liebig-University, Giessen, Germany) which were seeded in triplicate in 6-well cell culture plates. Prior to confluence one well of each cell culture was infected with a non-cytopathogenic BVD virus (NCP8-strain, kindly provided by E.J. Dubovi, New York State College of Veterinary Medicine, Cornell University Ithaca, NY, USA [20]) with a multiplicity of infection (MOI) of 10 and incubated for 48 h. Finally, virus protein was detected via immunofluorescence as described by Lackner et al. [21]. Briefly, the cells were fixed in 2% paraformaldehyde and pestivirus specific NS2/3 protein was detected by the primary antibody code 4 (mAk 8.12.7, kindly provided by N. Tautz [22]) followed by a fluorescence cy3-conjugated secondary antibody (Jackson ImmunoResearch, Suffolk, UK). The cultures were viewed under an inverse fluorescence microscope (Axiovert 35, Zeiss) and the presence of virus was assessed in the non-infected wells and in the infected wells to confirm that the cultures were not contaminated with virus prior to infection and to determine whether infection was possible, respectively.
3. Results
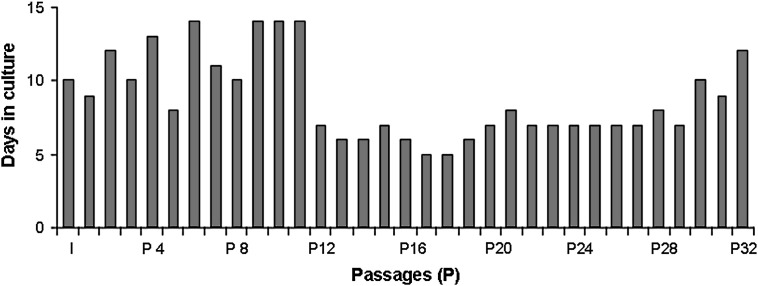
By continuously subculturing, a primary cell culture of bovine caruncular epithelial cells the removal of contaminating fibroblastoid cells was possible within the first four passages, and resulted in a population of cells displaying epithelioid morphology only. During the first 8 passages the cells reached confluence after 11 days (±2). The following three passages were characterised by a low viability of the cells ( Fig. 1) and confluence was hardly reached. By day 14 in culture no further proliferation was observed. Subculturing at this time point stimulated cell proliferation and from passage 12 onwards the proliferation rate accelerated markedly resulting in confluence within 7 days (±1) in culture and remained constant for the subsequent 18 passages. From passage 30 onwards the proliferation rate decreased and because the amount of cryopreserved cultures was deemed sufficient, it was decided to stop further subculturing after the 32nd passage. During the process of subculturing cultures of various passages were successfully cryopreserved for later experiments. The cell line was termed BCEC-1 (Bovine Caruncular Epithelial Cell line 1).
Fig. 1.
Proliferation characteristics of BCEC-1 during establishment of the cell line. The number of days in culture until confluence was reached (y-axis) is shown for primary cells (I) and each subsequent passage (P). During the first 8 passages confluence was reached after 11 days (±2) followed by a period of crisis (low viability and proliferation) until passage 11. From passage 12 onwards viability and proliferation increased markedly resulting in constant time periods (7 days ± 1) in which confluence was reached.
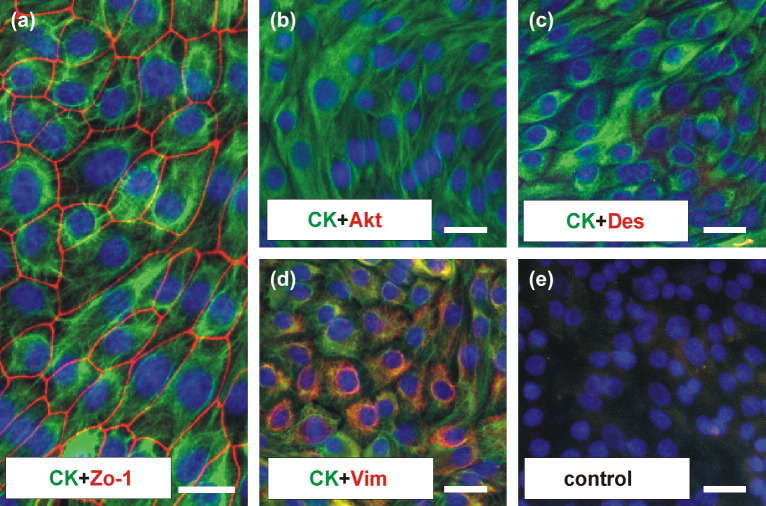
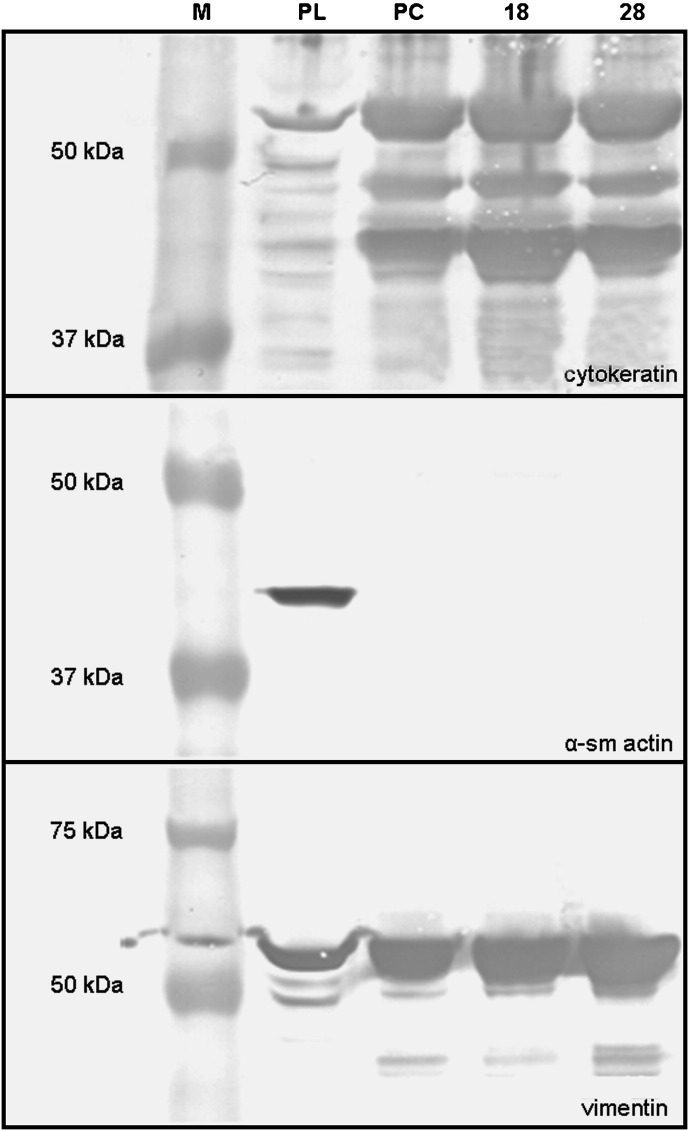
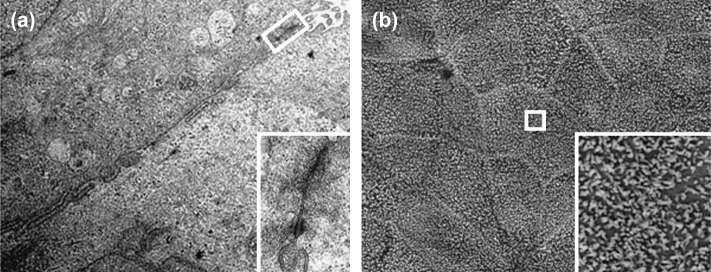
Characterisation of BCEC-1 cells at various time points during subculturing revealed the presence of cytokeratin, zonula occludens-1 and vimentin while the cells remained negative for both α-smooth muscle actin and desmin ( Fig. 2). This result was confirmed by Western blot analysis ( Fig. 3). In addition, TEM and SEM analysis confirmed the presence of epithelium specific characteristics as they show a polarised morphology, the presence of apical microvilli and junctional complexes (zonula occludens, zonula adherens and desmosomes) as well as the growth pattern as a homogenous monolayer of cells ( Fig. 4).
Fig. 2.
Representative pictures demonstrating the characterisation of BCEC-1 via immunofluorescence at regular intervals in culture. The cells of epithelial origin constantly expressed epithelial cytokeratin (a–d, green) and tight junctional zonula occludens-1 (Zo-1) protein (a, red; passage (P) 25). Neither α-smooth muscle actin (Akt) protein (b; no red colour: P15) nor desmin (Des) protein (c; no red colour: P21) was detected. Only vimentin (d, red; P11) a protein of mesenchymal origin was present, a well-known phenomenon in epithelial cell cultures. Nuclei were counterstained with DAPI. Representative negative control (e; P18). Bar: 25 μm.
Fig. 3.
Western blot analysis of protein samples derived from the placentome (PL; positive control), primary epithelial cells (PC) and BCEC-1 cells from passage (P)18 and 28 confirming the specificity of the antibodies and purity of the cultures. Specific bands corresponding to epithelial cytokeratin (wide spectrum screening, 40–54 kDa) and vimentin (57 kDa), but not α-smooth muscle (sm) actin (42 kDa: negative control for cell culture derived protein) were detected in all epithelial cell cultures. M, Molecular weight marker.
Fig. 4.
Transmission (TEM) and scanning (SEM) electron microscopy of BCEC-1 cultures of passage 28 (a) and 11 (b) grown on Transwell inserts. The TEM image (a) shows the lateral contact area of two neighbouring epithelial cells. Higher magnification of the apicolateral region (inset) demonstrates the presence of an epithelial specific junctional complex (zonula occludens, zonula adherens and desmosome). The SEM image (b) shows the apical side of the epithelial monolayer. Higher magnification (inset) demonstrates the presence of caruncular epithelium specific apical microvilli as shown in vivo [33].
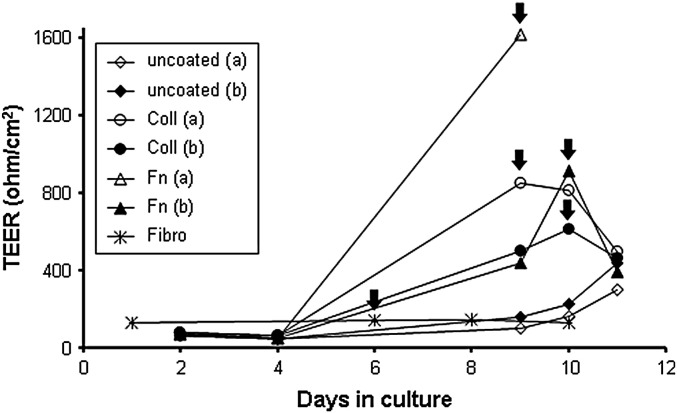
All BCEC-1 cultures used for TEER experiments displayed marked increases in TEER values with progressing confluence independent of culture conditions in comparison to confluent fibroblasts which never developed TEER > 200 Ω/cm2. Culturing cells on coated membranes resulted in an earlier attainment of confluence and subsequent earlier increase of TEER values ( Fig. 5).
Fig. 5.
Representative graph of one out of three independently and in duplicates performed transepithelial electrical resistance (TEER) experiments showing the TEER development of a BCEC-1 passage 12 culture seeded in duplicates (a + b) on uncoated as well as collagen A (Coll) and fibronectin (Fn) coated Transwell insert membranes. Culture on coated inserts lead to an increase in proliferation and an earlier attainment of confluence as demonstrated by an increase of TEER values at an earlier stage when compared to uncoated inserts. This was the case in all experiments. In the experiment shown confluence (arrows) was reached in fibronectin and collagen A coated inserts within day 9 and 10, respectively, whereas the cultures grown on uncoated inserts were close to confluence by the end of the measurements at day 11. Cultures of primary caruncular fibroblasts (Fibro) reaching confluence by day 6 served as negative controls.
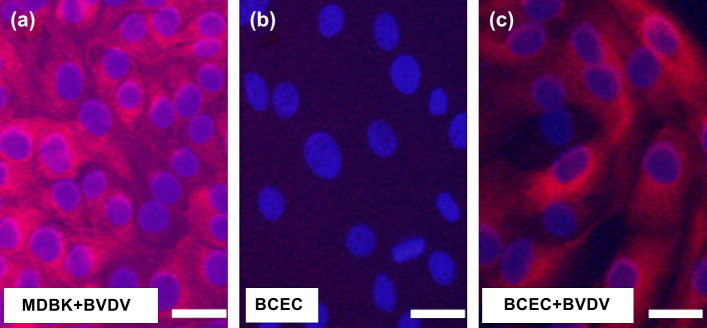
All cultures tested negative for the BVD virus ( Fig. 6). After 48 h of incubation with non-cytopathogenic BVD virus, virus protein could be detected in the cytoplasm of the BCEC-1 cells. Corresponding to the controls (MDBK cells) no cytopathogenic effect was observed.
Fig. 6.
Detection of bovine viral diarrhoea (BVD) virus antigen. Cultures of Madin Darby bovine kidney (MDBK) cells (a, positive control) and BCEC-1 cells (b, c) were incubated with (a, c) and without (b) BVD virus for 48 h and virus antigen was detected in the cytoplasm of infected cells demonstrating that BCEC-1 cells are negative for BVD virus and that an infection is possible. The nuclei were counterstained with DAPI. Bar: 20 μm.
4. Discussion
This is the first study to successfully establish a cell line of bovine caruncular epithelial cells (BCEC-1) from pregnant animals with a morphologically and functionally intact epithelial barrier.
The first issue was to create a population of epithelial cells. The primary culture consisted of several cell types and though the majority of cells was epithelioid, fibroblastoid cells originating from the stroma were also present. Different adherent properties of the cells allowed purification of the culture using a two step trypsination procedure which was carried out on every passage even though no fibroblastoid cells were identified beyond the fourth passage. As has been previously shown in primary cultures of caruncular epithelial cells [17], the BCEC-1 cell line also consistently expressed epithelial cytokeratin and tight junctional zonula occludens-1 as well as vimentin, an intermediate filament protein of mesenchymal origin [23]. The expression of vimentin in primary epithelial cell cultures and epithelial cell lines is a well known phenomenon irrespective of which tissue or organ they were isolated from. It has been described in endometrial cell cultures derived from sheep, pigs, dogs, rats and humans [24], [25], [26], [27], [28]. Furthermore, primary epithelial cells isolated from the endometrium of non-pregnant cows as well as the BEND cell line were shown to be positive for vimentin [29], [30], [31]. It was reported that the cells preserved their epithelial specific properties resulting in successful experiments.
In BCEC-1 cultures the cell morphology, protein expression and the ability to form an intact epithelial barrier was maintained for at least 32 passages. In comparison, the BEND cell line was successfully subcultured for at least 25 passages [14]. In BCEC-1 the variable and prolonged cultivation periods until confluence during the initial passages are partly referable to the process of spontaneous immortalisation. A similar growth pattern was observed during the establishment of the human breast epithelial cell line MCF-10 [32]. The number of viable and proliferating cells decreased within the first 7 passages and spontaneous immortalisation occurred beyond passage 12. Nonetheless, it should be taken into account that the BCEC-1 cells were not counted prior to each seeding and therefore the number of cells transferred to the new flasks was not identical on each occasion. Thus, the differing periods until confluence was reached could possibly be related to different numbers of cells per passage. Nevertheless, the number of days between two passages remained relatively constant between passages 12 and 30. Later experiments with cryopreserved BCEC-1 cells enabled the defining of an optimal seeding density of 7500 cells/cm2, resulting in a subcultivation ratio of 1:4 and a cultivation period of 6 days until confluence on fibronectin coated flasks. These values corresponded to those suggested by the ATCC for the BEND cell line.
The BCEC-1 cells grew successfully on Transwell polyester (PET) membranes allowing further assessment of morphological and functional characteristics. A preliminary experiment revealed that culture was not successful on Millicell inserts with biopore or polycarbonate membranes. Ultrastructural analysis via electron microscopy demonstrated growth of polarised epithelial monolayers with apical microvilli and junctional complexes as described in vivo [33]. The localisation of the tight junctional protein zonula occludens-1 supported the hypothesis that this cell culture model forms an intact epithelial barrier. This assumption was unequivocally confirmed by the detection of a significant transepithelial electrical resistance a soon as confluence was reached.
Coating the membrane with extracellular matrix proteins enhanced cell growth and optimised culture conditions. In most cases fibronectin resulted in higher TEER values than coating with collagen A (personal observations). The positive effect of fibronectin on growth properties of the monolayer was confirmed by the results obtained from cell counting of primary caruncular epithelial cells cultured on different extracellular matrices [34]. Fibronectin is a soluble multiadhesive matrix protein and a major component of the extracellular matrix. Amongst other locations in the bovine placenta, fibronectin is expressed in the basement membrane of the caruncular epithelium [2]. In addition, in primary cultures of caruncular epithelial cells fibronectin secretion was shown to be associated with the expression of specific integrin receptors [18]. Fibronectin secretion is a frequently observed feature of epithelial cell cultures and occurs particularly at the basolateral surface [35], [36]. Taken together these results provide evidence that fibronectin may have a positive effect on the culture conditions of BCEC-1, a procedure which is now used routinely when working with this cell line.
In contrast to the BEND cell line, the BCEC-1 cultures were negative for the BVD-virus antigen but were found to be permissive for the virus suggesting a possible application of the BCEC-1 cell line in future studies on the pathogenesis of pregnancy-related diseases in cattle. Bovine virus diarrhoea (BVD) is a disease of great importance in bovine medicine and has a worldwide distribution [16]. If transmitted transplacentally to the fetus within the first 120 days of pregnancy, the calf develops an immune tolerance resulting in persistent infection with a non-cytopathogenic (ncp) BVD virus. In the first few months after birth superinfection with ncp-virus or mutations within the virus leading to a transformation into a cytopathogenic (cp) virus, result in the development of the lethal mucosal disease. Immunohistological studies demonstrated strong staining of BVD virus antigen in the caruncular epithelial cells of pregnant persistently infected animals [37]. To date, the pathways of infection in a polarised cell culture model derived from the tissue actually participating in vertical transmission have not yet been studied. In a comparable polarised cell culture model of human bronchial epithelial cells (Calu-3) the entry and release of the severe acute respiratory syndrome corona virus (SARS-CoV) almost exclusively from the apical border was demonstrated [38].
In conclusion, BCEC-1 is the first cell line derived from caruncular epithelial cells of a pregnant cow displaying characteristic epithelial features on a morphological and functional basis for at least 32 passages. Culture of BCEC-1 cells in an insert culture system makes a variety of future applications possible, i.e. co-culture invasion assays with trophoblast or tumour cells [39]; studies on pathways of transport, communication and modification of metabolic substances (e.g. hormones, pharmaceuticals) [40], [41], [42] and pathways of infection of pregnancy associated diseases caused by parasites (e.g. Neospora caninum), bacteria (e.g. Bacillus spp.) and viruses (e.g. BDV virus); as well as studies on environmental toxins (e.g. plants, mycotoxins) [[38], [43], [44], [45]].
Acknowledgements
The authors are grateful to M. Fink, A. Hild, S. Kettner, S. Schubert-Porth and K. Wolf (Department of Veterinary Anatomy, Justus-Liebig-University (JLU), Giessen, Germany) for their expert technical assistance. Furthermore, the authors would like to thank Dr. N. Tautz (Department of Veterinary Virology, JLU), Dr. M. Hardt (Centre of Biotechnology, JLU) and Prof. Dr. R. Gerstberger (Department of Veterinary Physiology) for the donation of material as well as the usage of their facilities as well as M. Mohr (Institute for Hygiene and Infectious Diseases of Animals, JLU) and E. Lattwein (Department of Veterinary Virology, JLU) for introduction in TEER measurements and BVD virus detection. This study was supported by a grant from the German Research Foundation (DFG).
References
- 1.Wooding F.B. Current topic: the synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta. 1992;13:101–113. doi: 10.1016/0143-4004(92)90025-o. [DOI] [PubMed] [Google Scholar]
- 2.Pfarrer C.D., Hirsch P., Guillomot M., Leiser R. Interaction of integrin receptors with extracellular matrix is involved in trophoblast giant cell migration in bovine placentomes. Placenta. 2003;24:588–597. doi: 10.1016/s0143-4004(03)00059-6. [DOI] [PubMed] [Google Scholar]
- 3.Soundararajan R., Rao A.J. Trophoblast ‘pseudo-tumorigenesis’: significance and contributory factors. Reprod Biol Endocrinol. 2004;2:15. doi: 10.1186/1477-7827-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfarrer C.D., Heeb C., Leiser R. Expression of gap junctional connexins 26, 32 and 43 in bovine placentomes during pregnancy. Placenta. 2006;27:79–86. doi: 10.1016/j.placenta.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Bücher K., Leiser R., Tiemann U., Pfarrer C.D. Platelet-activating factor receptor (PAF-R) and acetylhydrolase (PAF-AH) are co-expressed in immature bovine trophoblast giant cells throughout gestation, but not at parturition. Prostaglandins Other Lipid Mediat. 2006;79:74–83. doi: 10.1016/j.prostaglandins.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Laven R.A., Peters A.R. Bovine retained placenta: aetiology, pathogenesis and economic loss. Vet Rec. 1996;139:465–471. doi: 10.1136/vr.139.19.465. [DOI] [PubMed] [Google Scholar]
- 7.Fortier M.A., Guilbault L.A., Grasso F. Specific properties of epithelial and stromal cells from the endometrium of cows. J Reprod Fertil. 1988;83:239–248. doi: 10.1530/jrf.0.0830239. [DOI] [PubMed] [Google Scholar]
- 8.Horn S., Bathgate R., Lioutas C., Bracken K., Ivell R. Bovine endometrial epithelial cells as a model system to study oxytocin receptor regulation. Hum Reprod Update. 1998;4:605–614. doi: 10.1093/humupd/4.5.605. [DOI] [PubMed] [Google Scholar]
- 9.Banu S.K., Arosh J.A., Chapdelaine P., Fortier M.A. Expression of prostaglandin transporter in the bovine uterus and fetal membranes during pregnancy. Biol Reprod. 2005;73:230–236. doi: 10.1095/biolreprod.105.039925. [DOI] [PubMed] [Google Scholar]
- 10.Asselin E., Drolet P., Fortier M.A. In vitro response to oxytocin and interferon-τ in bovine endometrial cells from caruncular and inter-caruncular areas. Biol Reprod. 1998;59:241–247. doi: 10.1095/biolreprod59.2.241. [DOI] [PubMed] [Google Scholar]
- 11.Pfarrer C.D., Ruziwa S.D., Winther H., Callesen H., Leiser R., Schams D. Localization of vascular endothelial growth factor (VEGF) and its receptors VEGFR-1 and VEGFR-2 in bovine placentomes from implantation until term. Placenta. 2006;27:889–898. doi: 10.1016/j.placenta.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Shemesh M., Harel-Markowitz E., Gurevich M., Shore L.S. Staurosporine stimulates progesterone production by bovine placental cells. Biol Reprod. 1994;51:146–151. doi: 10.1095/biolreprod51.1.146. [DOI] [PubMed] [Google Scholar]
- 13.Johnson G.A., Austin K.J., Collins A.M., Murdoch W.J., Hansen T.R. Endometrial ISG17 mRNA and a related mRNA are induced by interferon-tau and localized to glandular epithelial and stromal cells from pregnant cows. Endocrine. 1999;10:243–252. doi: 10.1007/BF02738623. [DOI] [PubMed] [Google Scholar]
- 14.Binelli M., Guzeloglu A., Badinga L., Arnold D.R., Sirois J., Hansen T.R. Interferon-τ modulates phorbol ester-induced production of prostaglandin and expression of cyclooxygenase-2 and phospholipase-A2 from bovine endometrial cells. Biol Reprod. 2000;63:417–424. doi: 10.1095/biolreprod63.2.417. [DOI] [PubMed] [Google Scholar]
- 15.Parent J., Fortier M.A. Expression and contribution of three different isoforms of prostaglandin E synthase in the bovine endometrium. Biol Reprod. 2005;73:36–44. doi: 10.1095/biolreprod.104.037036. [DOI] [PubMed] [Google Scholar]
- 16.Thiel H.J., Plagemann P.G.W., Moennig V. Pestiviruses. In: Knipe D.M., Howley P.M., editors. Fields virology. Lippincott-Raven; Philadelphia: 1996. pp. 1059–1073. [Google Scholar]
- 17.Bridger PS, Haupt S, Klisch K, Leiser R, Tinneberg HR, Pfarrer CD. Validation of epitheloid cell cultures isolated from the caruncle and the cotyledon of cattle throughout pregnancy. Theriogenology 2007; in press. [DOI] [PubMed]
- 18.Zeiler M, Leiser R, Johnson GA, Tinneberg HR, Pfarrer CD. Development of an in vitro model for bovine placentation: A comparison of the in vivo and in vitro expression of integrins and components of extracellular matrix in bovine placental cells. Cells Tissues Organs 2007; in press. [DOI] [PubMed]
- 19.Wille K.H. Ueber die pränatale Entwicklung der Dickdarm-Mukosa unter besonderer Berücksichtigung des Epithels. Morphologische sowie histo- und zytochemische Untersuchungen am Blinddarm des Rindes. Habilitation Thesis, Justus-Liebig-University Giessen. Germany. 1984:3–4. [Google Scholar]
- 20.Corapi W.V., Donis R.O., Dubovi E.J. Monoclonal antibody analyses of cytopathic and noncytopathic viruses from fatal bovine viral diarrhea virus infections. J Virol. 1988;62:2823–2827. doi: 10.1128/jvi.62.8.2823-2827.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lackner T., ller A., Pankraz A., Becher P., Thiel H.J., Gorbalenya A.E. Temporal modulation of an autoprotease is crucial for replication and pathogenicity of an RNA virus. J Virol. 2004;78:10765–10775. doi: 10.1128/JVI.78.19.10765-10775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corapi W.V., Donis R.O., Dubovi E.J. Characterization of a panel of monoclonal antibodies and their use in the study of the antigenic diversity of bovine viral diarrhea virus. Am J Vet Res. 1990;51:1388–1394. [PubMed] [Google Scholar]
- 23.Evans R.M. Vimentin: The conundrum of the intermediate filament gene family. BioEssays. 1998;20:79–86. doi: 10.1002/(SICI)1521-1878(199801)20:1<79::AID-BIES11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Johnson G.A., Burghardt R.C., Bazer F.W., Spencer T.E., Newton G.R. Development and characterization of immortalized ovine endometrial cell lines. Biol Reprod. 1999;61:1324–1330. doi: 10.1095/biolreprod61.5.1324. [DOI] [PubMed] [Google Scholar]
- 25.Galabova-Kovacs G., Walter I., Aurich C., Aurich J.E. Steroid receptors in canine endometrial cells can be regulated by estrogen and progesterone under in vitro conditions. Theriogenology. 2004;61:963–976. doi: 10.1016/j.theriogenology.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Classen-Linke I., Kusche M., Knauthe R., Beier H.M. Establishment of a human endometrial cell culture system and characterization of its polarized hormone responsive epithelial cells. Cell Tissue Res. 1997;287:171–185. doi: 10.1007/s004410050743. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z., Paria B.C., Davis D.L. Pig endometrial cells in primary culture: morphology, secretion of prostaglandins and proteins, and effects of pregnancy. J Anim Sci. 1991;69:3005–3015. doi: 10.2527/1991.6973005x. [DOI] [PubMed] [Google Scholar]
- 28.Arslan A., Almazan G., Zingg H.H. Characterization and co-culture of novel nontransformed cell lines derived from rat endometrial epithelium and stroma. In Vitro Cell Dev Biol Anim. 1995;31:140–148. doi: 10.1007/BF02633974. [DOI] [PubMed] [Google Scholar]
- 29.Munson L., Chandler S.K., Schlafer D.H. Cultivation of bovine fetal and adult endometrial epithelial cells. J Tissue Cult Methods. 1988;11:129–133. [Google Scholar]
- 30.Yamauchi N., Yamada O., Takahashi T., Imai K., Hashizume K., Sato T. A three-dimensional cell culture model for bovine endometrium: Regeneration of a multicellular spheroid using ascorbate. Placenta. 2003;24:258–269. doi: 10.1053/plac.2002.0901. [DOI] [PubMed] [Google Scholar]
- 31.Binelli M., Subramaniam P., Diaz T., Johnson G.A., Hansen T.R., Badinga L. Bovine interferon-τ stimulates the Janus kinase-signal transducer and activator of transcription pathway in bovine endometrial epithelial cells. Biol Reprod. 2001;64:654–665. doi: 10.1095/biolreprod64.2.654. [DOI] [PubMed] [Google Scholar]
- 32.Soule H.D., Maloney T.M., Wolman S.R., Peterson W.D., Jr., Brenz R., McGrath C.M. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 33.Leiser R. Development of contact between trophoblast and uterine epithelium during the early stages of implantation in the cow. Zentralbl Veterinarmed [C] 1975;4:63–86. [PubMed] [Google Scholar]
- 34.Pfarrer C.D., Goebel S., Zeiler M., Leiser R., Tinneberg H.R. Binding to a specific matrix enhances proliferation and expression of signalling molecules in bovine caruncular epithelial cells. Placenta. 2006:27. [Google Scholar]
- 35.Walia B., Castaneda F.E., Wang L., Kolachala V.L., Bajaj R., Roman J. Polarized fibronectin secretion induced by adenosine regulates bacterial-epithelial interaction in human intestinal epithelial cells. Biochem J. 2004;382:589–596. doi: 10.1042/BJ20040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hynes R.O., Yamada K.M. Fibronectins: Multifunctional modular glycoproteins. J Cell Biol. 1982;95:369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fredriksen B., Press C.M., Loken T., Ïdegaard S.A. Distribution of viral antigen in uterus, placenta and foetus of cattle persistently infected with bovine virus diarrhoea virus. Vet Microbiol. 1999;64:109–122. doi: 10.1016/s0378-1135(98)00263-6. [DOI] [PubMed] [Google Scholar]
- 38.Tseng C.T.K., Tseng J., Perrone L., Worthy M., Popov V., Peters C.J. Apical entry and release of severe acute respiratory syndrome-associated coronavirus in polarized Calu-3 lung epithelial cells. J Virol. 2005;79:9470–9479. doi: 10.1128/JVI.79.15.9470-9479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khodarev N.N., Yu J., Labay E., Darga T., Brown C.K., Mauceri H.J. Tumour-endothelium interactions in co-culture: Coordinated changes of gene expression profiles and phenotypic properties of endothelial cells. J Cell Sci. 2003;116:1013–1022. doi: 10.1242/jcs.00281. [DOI] [PubMed] [Google Scholar]
- 40.Kitchens K.M., El Sayed M.E.H., Ghandehari H. Transepithelial and endothelial transport of poly (amidoamine) dendrimers. Adv Drug Deliv Rev. 2005;57:2163–2176. doi: 10.1016/j.addr.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Bur M., Huwer H., Lehr C.M., Hagen N., Guldbrandt M., Kim K.J. Assessment of transport rates of proteins and peptides across primary human alveolar epithelial cell monolayers. Eur J Pharm Sci. 2006;28:196–203. doi: 10.1016/j.ejps.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Liu D.Z., Lecluyse E.L., Thakker D.R. Dodecylphosphocholine-mediated enhancement of paracellular permeability and cytotoxicity in Caco-2 cell monolayers. J Pharm Sci. 1999;88:1161–1168. doi: 10.1021/js990094e. [DOI] [PubMed] [Google Scholar]
- 43.Woclawek-Potocka I., Korzekwa A., Bah M.M., Skarzynski D.J., Acosta T.J., Shibaya M. Phytoestrogens modulate prostaglandin production in bovine endometrium: Cell type specificity and intracellular mechanisms. Exp Biol Med (Maywood) 2005;230:326–333. doi: 10.1177/153537020523000506. [DOI] [PubMed] [Google Scholar]
- 44.Agerholm J.S., Jensen N.E., Dantzer V., Jensen H.E., Aarestrup F.M. Experimental infection of pregnant cows with Bacillus licheniformis bacteria. Vet Pathol. 1999;36:191–201. doi: 10.1354/vp.36-3-191. [DOI] [PubMed] [Google Scholar]
- 45.Dubey J.P., Buxton D., Wouda W. Pathogenesis of bovine neosporosis. J Comp Pathol. 2006;134:267–289. doi: 10.1016/j.jcpa.2005.11.004. [DOI] [PubMed] [Google Scholar]