Abstract
Fucoidans are sulfated polysaccharides from brown algae, known to have immunomodulatory activity. Their effects on the response of airway epithelial cells to Toll-like receptor 3 (TLR3) stimulation have not been characterized. Our objective was to evaluate the effects of a marine-sourced fucoidan solution (MFS) on the TLR3-induced expression and/or production of cytokines and prostaglandin by human primary bronchial epithelial cells as a model of the airway epithelium. The cells were incubated with MFS in the presence or absence of Poly(I:C) (a TLR3 agonist that mimics viral RNA). Cytokine expression and production were assessed using RT-qPCR and ELISA. The expression of cyclooxygenase-2 and the production of prostaglandin E2 were also measured. Relative to control, exposure to MFS was associated with lower Poly(I:C)-induced mRNA expression of various cytokines and chemokines, and lower COX-2 production. The MFS inhibited the production of some cytokines (IL-1α, IL-1β, TNFα, and IL-6), chemokines (CCL5, CCL22, CXCL1, CXCL5 and CXCL8) and prostaglandin E2 but did not alter the production of IL-12/25, CCL2 and CCL20. At clinically relevant concentrations, the MFS inhibited the TLR3-mediated production of inflammatory mediators by human primary bronchial epithelial cells - suggesting that locally applied MFS might help to reduce airway inflammation in viral infections.
Keywords: Airway epithelial cell, fucoidan, Cytokine
1. Introduction
Acute viral respiratory tract infection (also known as the common cold) is the most prevalent disease in humans, and is frequently associated with acute exacerbation of asthma. Most common colds are caused by respiratory viruses such as rhinovirus, influenza virus, parainfluenza virus, coronavirus, and respiratory syncytial virus [1].
Epithelial cells constitute the upper and lower airways' front-line defenses against inhaled microbial pathogens. The efficient physical barrier formed by these cells is complemented by the mucociliary escalator, which also contributes significantly to defense of the airways against potentially harmful inhaled physical, chemical and biological agents [2]. After infecting the airway epithelium, human rhinovirus and influenza A virus trigger both innate and adaptive immune responses that are crucial for efficient antiviral control [[3], [4], [5]]. Airway epithelial cells also have an important role in the local regulation of the inflammatory response to viruses by releasing a variety of pleiotropic cytokines and chemokines. The cytokines (such as IL-1 and IL-6) exert a number of actions in the airways, whereas the chemokines (such as CCL5, CCL20, CXCL1, CXCL8, and CXCL10) contribute to the recruitment and activation of selected inflammatory cell populations (including neutrophils, eosinophils, dendritic cells, lymphocytes and natural killer cells) [6,7].
Airway epithelial cells sense potentially dangerous inhaled materials through the latter's interactions with pattern recognition receptors, such as Toll-like receptors (TLRs) [8]. Toll-like receptor 3 (TLR3) recognizes double-stranded RNA (dsRNA), and thus is especially relevant to the airway epithelial cells' response to rhinovirus and influenza A virus [[9], [10], [11], [12]]. This receptor is expressed in the endosomal compartments of dendritic cells and macrophages [13], and is also detected on the surface and in the cytoplasm of airway epithelial cells; as such TLR3 probably contributes to regulation of the cells' innate response [14].
Seaweed products have been used in traditional Chinese herbal medicine for at least the last 500 years. Fucoidans constitute the main class of polysaccharide found in brown algae. They contain high levels of l-fucose and sulfate ester groups, and exert a variety of antiviral [15,16], anti-inflammatory [17] and immunomodulatory effects [18,19]. In particular, fucoidans are known to be ligands for class A scavenger receptors (SR-A), which also belong to the pattern recognition receptor family. It is known that SR-A is a cell surface receptor for dsRNA, and so may also be involved in antiviral immune responses [20]. To the best of our knowledge, the fucoidans' effects on the release of inflammatory mediators induced by TLR3 activation have not previously been characterized. The objective of the present study was to assess the effects of a marine-sourced fucoidan solution (MFS) from Ascophyllum nodosum on the mRNA expression and protein production of cytokines, chemokines and prostaglandin E2 (PGE2) by human primary bronchial epithelial cells following TLR3 activation by Poly(I:C) (a synthetic analog of dsRNA) [21,22].
2. Materials and methods
2.1. Materials
Antibiotics (penicillin, streptomycin, gentamycin, vancomycin, amphotericin, and ceftazidim), DMSO, l-glutamine, heat-inactivated fetal calf serum, bovine pancreas protease, and HEPES were purchased from Sigma-Aldrich (St-Quentin Fallavier, France). Roswell Park Memorial Institute (RPMI) 1640 medium, Dulbecco's Modified Eagle's medium (DMEM) high-glucose DMEM, and bovine serum albumin were bought from Eurobio Biotechnology (Les Ulis, France). High-molecular-weight Poly(I:C) was obtained from InvivoGen (Toulouse, France). Bronchial epithelial cell growth medium (BEGM, supplemented with growth factors and antibiotics), and MycoZap™ were purchased from Lonza (Walkersville, MD, USA). Trypsin 0.25% EDTA was obtained from Gibco® (Thermo Fisher Scientific, Villebon sur Yvette, France). Culture flasks for epithelial cell culture were from TPP® (Trasadingen, Swiss) and Biocoat collagen-coated culture flasks were purchased from Corning® (Sigma-Aldrich, St-Quentin Fallavier, France). All the other cell culture plastics were from CML (Nemours, France).
Untreated sea water drawn from the Atlantic Ocean (off the coast of Brittany, France, by Yslab, Quimper, France) was prepared as follows: sand and carbon filtering, UV treatment, and sterilizing filtration (pore size: 1-0.45-0.2 μm). To lower the solution's tonicity, the seawater's salinity was reduced to 15 g·kg−1 by dilution with purified water.
Ascophyscient® fucoidan-rich Ascophyllum nodosum extract was purchased from Algues&Mer (Ouessant, France). The Ascophyllum nodosum seaweed had grown in a zone covered by the UNESCO World Network of Biosphere Reserves and that had achieved a “good ecological status” according to the European Union's Water Framework Directive 2000/60/EC. The seaweed's thallus was used for fucoidan extraction. The main extraction steps were polyphenol trapping, aqueous extraction, solid/liquid separation, microparticle removal (by filtration), alginate precipitation and removal, further filtration, demineralization, depolymerization, and atomization. The extract's total sugar content was 40% by weight (according to Dubois' method [23], using fucose as the sugar standard), and the total sulfate level was 17% (as determined by liquid chromatography, as described in the International Standard ISO 10304). The remaining components were uronic acid and ash. The l-fucopyranose structure of the backbone of the fucoidan extracted from Ascophyllum nodosum has been previously described, and features alternating α(1 → 3) and α(1 → 4) linkages [24,25]. The mean molecular weight was 18,940 g/mol as determined with high performance size exclusion chromatography coupled to a refractive index detector.
To prepare the MFS, Ascophyllum nodosum extract was dissolved to a final concentration of 0.1% (m/v) in diluted sea water. This concentration was chosen on the basis of previous work [[26], [27], [28], [29]].
2.2. Isolation and culture of human primary bronchial epithelial cells
The use of resected lung tissue for in vitro experiments was approved by the local institutional review board (Comité de Protection des Personnes Ile-de-France VIII, Boulogne-Billancourt, France; reference: DC-2010-1221), and the patients gave their informed consent before the surgical procedure. Lung tissue samples were obtained from 17 patients (10 males and 7 females; 6 smokers, 10 former smokers, 1 non-smoker; mean ± standard error (SD) age: 65.5 ± 7.2 years; FEV1: 74.4 ± 23.7%; pack-years: 36 ± 13; 7 had COPD) undergoing surgical resection for lung carcinoma and who had not received prior chemotherapy. None of the subjects had been treated with immunosuppressants or corticosteroids before surgery. Proximal segmental bronchi collected from sites distant from the tumor were placed in high-glucose DMEM supplemented with l-glutamine, 10% fetal calf serum, HEPES, antibiotics, and antifungal drugs (penicillin, streptomycin, gentamycin, vancomycin, amphotericin, ceftazidim and MycoZap™), and were stored at +4 °C (for bacterial decontamination) for at least 3 days. The bronchial mucus was removed, and the bronchus was cut lengthwise before the addition of protease (10 mg·mL−1) overnight at +4 °C. On the following day, the luminal surface of each bronchus sample was gently scratched in order to detach epithelial cells into the culture medium. After centrifugation of the cell suspension, the cell pellet was resuspended in 30 mL of supplemented high-glucose DMEM and placed in a culture flask for 2 h at 37 °C in a 5% CO2 atmosphere. The supernatant was recovered and placed in a BioCoat collagen-coated flask. On the following day and then every other day, the culture medium was replaced with 15 mL of antibiotic-free high-glucose DMEM and 15 mL of BEGM (referred to hereafter as BEGM-DMEM). When the cells reached confluence (usually after 7 days of culture), they were detached from the plate with trypsin, centrifuged, and resuspended in BEGM-DMEM in a 24-well plate (1 mL per well) for 24 h prior to exposure to pharmacological agents.
2.3. Treatment of bronchial epithelial cells
The 24-well plates were washed, and 1 mL of fresh BEGM-DMEM was added per well. Cells were incubated with either sodium chloride 0.9% (control) or MFS for 1 h (200 μL/well). The solutions were then removed, and the cells were incubated in 2 mL of culture medium in the absence or presence of 10 μg·mL−1 high-molecular-weight Poly(I:C) cells for 24 h. The cell culture supernatants were then collected and stored at −80 °C for later analysis.
2.4. Cytokine and chemokine assays
The production of selected chemokines and cytokines was assessed by measuring their concentrations in the bronchial epithelial cells' supernatants using commercially available ELISAs (Duoset Development System, R&D Systems Europe, Lille, France). The limit of detection was 1 pg.mL−1 for IL-1β, 4 pg·mL−1 for IL-1α and CCL22, 8 pg·mL−1 for TNF-α, CCL2 and CXCL5, 9 pg·mL−1 for IL-6, 16 pg·mL−1 for CCL5, CCL20, CXCL1, and CXCL8, and 32 pg·mL−1 for IL-12/IL-23. Levels of PGE2 were determined using a commercially available ELISA (Cayman Chemical Europe, Tallinn, Estonia).
2.5. Cytotoxicity assays
Cell viability was assessed by measuring LDH release (CytoTox96® Non-Radioactive Cytotoxicity Assay from Promega, Madison, USA).
2.6. Reverse transcriptase - quantitative polymerase chain reaction
Total RNA was prepared using TRIzol® reagent (Life Technologies, Saint Aubin, France), and RNA was extracted according to the manufacturer's instructions. The RNA's intactness was determined by running an aliquot of each sample on an Experion automated electrophoresis station (Bio-Rad, Marnes-la-Coquette, France). The RNA concentration was determined using a Biowave DNA spectrophotometer (Biochrom, Cambridge, UK). Next, 1 μg of total RNA was reverse-transcribed using a SuperScript® III First-strand SuperMix kit (Life Technologies).
Specific TaqMan® arrays based on predesigned reagents (Life Technologies) were used to assay cytokine and chemokine transcripts (customized TaqMan® array 96-well FAST Plate, Applied Biosystems, Foster City, USA). Reverse transcriptase-quantitative PCR was performed using Gene Expression Master Mix (Life Technologies) with 20 ng of cDNA in a StepOnePlus thermocycler (Life Technologies). The thermal cycling conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The housekeeping gene hypoxanthine phosphoribosyltransferase (HPRT1) was used for signal normalization.
2.7. Statistical analysis
Statistical analysis was performed using Prism software (version 6, GraphPad Software, La Jolla, CA, USA). A one-way analysis of variance for repeated measures (or a Friedman test, when appropriate) followed by post-tests or a paired Student's t-test (or a Wilcoxon test, when appropriate) were used to compare treatments (MFS and Poly(I:C)) with controls.
3. Results
3.1. Cytotoxicity
A one-hour incubation with MFS and then 24 h in culture medium alone was not associated with elevated LDH release by the epithelial cells (relative to controls), thus demonstrating the absence of cytotoxicity (Table 1 ).
Table 1.
Cytotoxicity after incubation with marine-sourced fucoidan solution (MFS) for 1 h, followed by 24 h in culture medium. Cytotoxicity was measured using an LDH release assay. The data correspond to the mean ± SEM of 7 to 10 independent experiments.
| Treatment | % of LDH release |
|---|---|
| Sodium chloride 0.9% | 0.36 ± 0.09 |
| MFS | 0.41 ± 0.12 |
| Poly(I:C) | 0.93 ± 0.23 |
| Poly(I:C) + MFS | 0.53 ± 0.20 |
3.2. Effects of the MFS on cytokine expression by bronchial epithelial cells
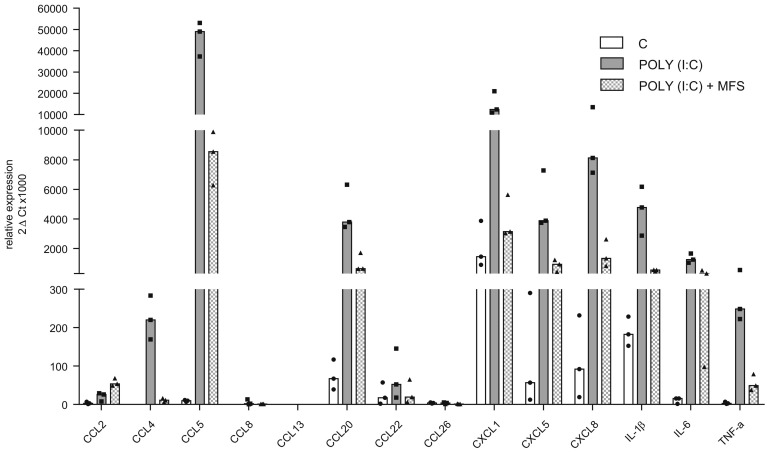
Exposure to Poly(I:C) was associated with the elevated expression (relative to control experiments) of genes coding for the cytokines CCL5, CCL20, CCL22, CXCL1, CXCL5, CXCL8, IL-1β, IL-6, and TNF-α (Fig. 1 ). However, pre-incubation of epithelial cells with MFS inhibited the Poly(I:C)-induced elevation in the expression of these genes. The elevated gene expression of CCL22 was completely blocked by MFS.
Fig. 1.
Effects of the marine-sourced fucoidan solution (MFS) on the Poly(I:C)-induced expression of cytokines by human bronchial epithelial cells. Bronchial epithelial cells were incubated with the MFS or NaCl 0.9% (control) for 1 h, followed by 24 h in culture medium in the absence or presence of Poly(I:C) 10 μg·mL−1. The data are expressed as the median (range) of three independent paired experiments.
3.3. Effects of MFS on cytokine and chemokine production by bronchial epithelial cells
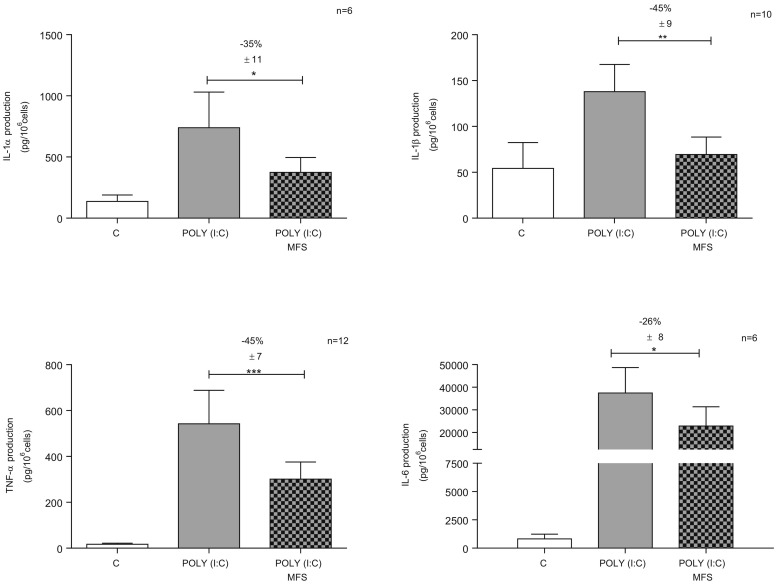
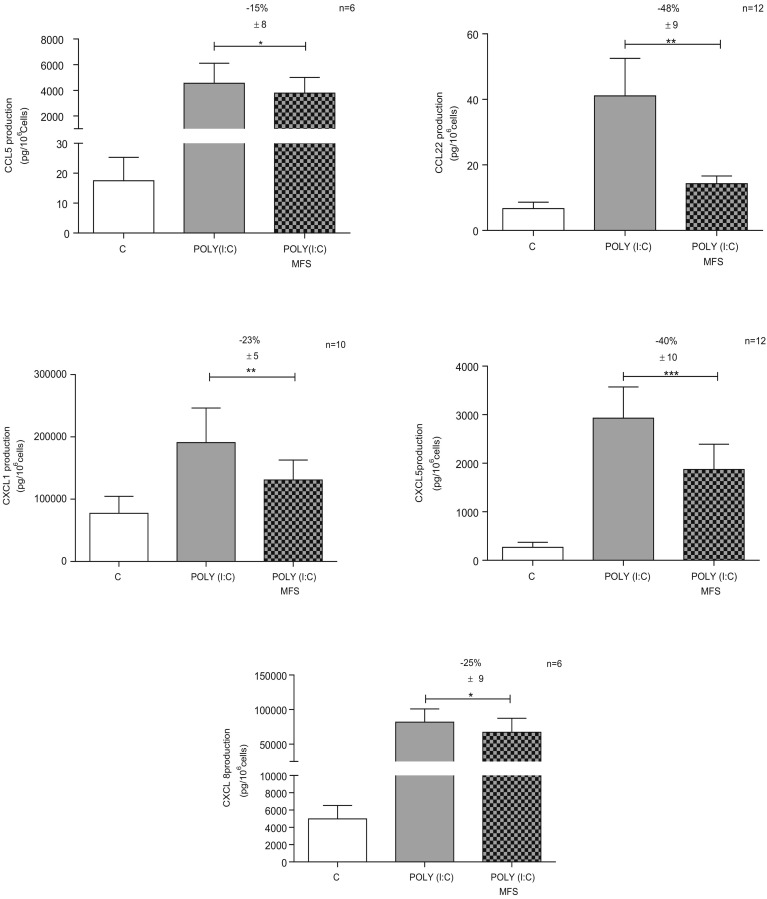
Incubation with MFS did not generally alter the baseline production of cytokines and chemokines by non-stimulated cells (Table 2 ). However, incubation with MFS was associated with a slight relative increase in the baseline production of IL-6, CXCL8 and CCL20 (Table 2). Poly(I:C) induced a marked elevation of the production of all the measured cytokines/chemokines (except for IL-12/IL-23) to a varying extent (from 2.5-fold for IL-1β and CXCL1 to 250-fold for CCL5) (Table 2). Pre-incubation with MFS significantly inhibited the production of IL-1α, IL-1β, TNF-α and IL-6 but not that of IL-12/IL-23 (Table 2, Fig. 2 ). Similarly, pre-incubation with MFS inhibited the Poly(I:C)-induced production of some chemokines (CCL5, CCL22, CXCL1, CXCL5 and CXCL8) but not others (CCL2 and CCL20) (Table 2, Fig. 3 ).
Table 2.
Effects of a marine-sourced fucoidan solution (MFS) on the production of cytokines by human bronchial epithelial cells.
Human bronchial epithelial cells were incubated for 1 h in the presence of a Marine-sourced fucoidan solution (MFS) or sodium chloride 0.9% (control) before being stimulated with Poly (I:C) (10 μg·mL−1) for 24 h. The concentrations of the cytokines are expressed in pg/106 cells. The data correspond to the mean ± SEM for the number (n) of independent experiments indicated. nd: not determined.
| Cytokine | n | Control | MFS | POLY (I:C) | MFS + POLY (I:C) |
|---|---|---|---|---|---|
| IL-1α | 6 | 137 ± 53 | 116 ± 47 | 740 ± 290⁎ | 374 ± 121⁎ |
| IL-1β | 10 | 54 ± 28 | nd | 138 ± 30⁎ | 69 ± 19⁎⁎ |
| TNF-α | 12 | 17 ± 5 | nd | 542 ± 146⁎⁎ | 301 ± 74⁎⁎⁎ |
| IL-6 | 6 | 824 ± 410 | 1138 ± 454⁎ | 37,436 ± 11274⁎ | 22,876 ± 8503⁎ |
| IL-12/IL-23 | 3 | 604 ± 247 | 602 ± 277 | 501 ± 208 | 654 ± 355 |
| CCL2 | 6 | 182 ± 90 | 394 ± 209 | 2112 ± 541⁎⁎ | 1950 ± 579 |
| CCL5 | 6 | 18 ± 8 | 11 ± 5 | 4556 ± 1552⁎ | 3781 ± 1219⁎ |
| CCL20 | 6 | 546 ± 249 | 1036 ± 223⁎ | 4722 ± 891⁎⁎ | 4840 ± 756 |
| CCL22 | 12 | 7 ± 2 | nd | 41 ± 11⁎ | 14 ± 2⁎⁎ |
| CXCL1 | 6/10 | 49,377 ± 11,668 | 55,568 ± 10,827 | 142,614 ± 35149⁎ | 109,981 ± 23871⁎⁎ |
| CXCL5 | 12 | 267 ± 103 | nd | 2927 ± 643⁎⁎ | 1871 ± 522⁎⁎⁎ |
| CXCL8 | 6 | 4985 ± 1554 | 6819 ± 1860⁎ | 81,956 ± 19093⁎⁎ | 67,256 ± 20169⁎ |
p < 0.05 (significant treshold for MFS vs. Control).
p < 0.01 (significant treshold for Poly(I:C) vs Control)
p < 0.001 (significant treshold for MFS + Poly(I:C) vs Poly(I:C)).
Fig. 2.
Effect of the marine-sourced fucoidan solution (MFS) on Poly(I:C)-induced cytokine release (IL-1α, IL-1β, TNF-α, and IL-6) by human bronchial epithelial cells. Bronchial epithelial cells were incubated with the MFS or NaCl 0.9% (control) for 1 h, followed by 24 h in culture medium in the absence or presence of Poly(I:C) 10 μg·mL−1. The data are expressed as the mean ± SEM of 6 to 12 independent, paired experiments. The significance thresholds were *p < 0.05, **p < 0.01, and ***p < 0.001.
Fig. 3.
Effect of the marine-sourced fucoidan solution (MFS) on chemokine release (CCL5, CCL22, CXCL1, CXCL5, and CXCL8) by human bronchial epithelial cells. Bronchial epithelial cells were incubated with the MFS or NaCl 0.9% (control) for 1 h, followed by 24 h in culture medium in the absence or presence of Poly(I:C) 10 μg·mL−1. Data correspond to the mean ± SEM of 6 to 12 independent paired experiments. The significance thresholds were *p < 0.05, **p < 0.01, and ***p < 0.001.
3.4. Effects of MFS on the production of PGE2 by bronchial epithelial cells
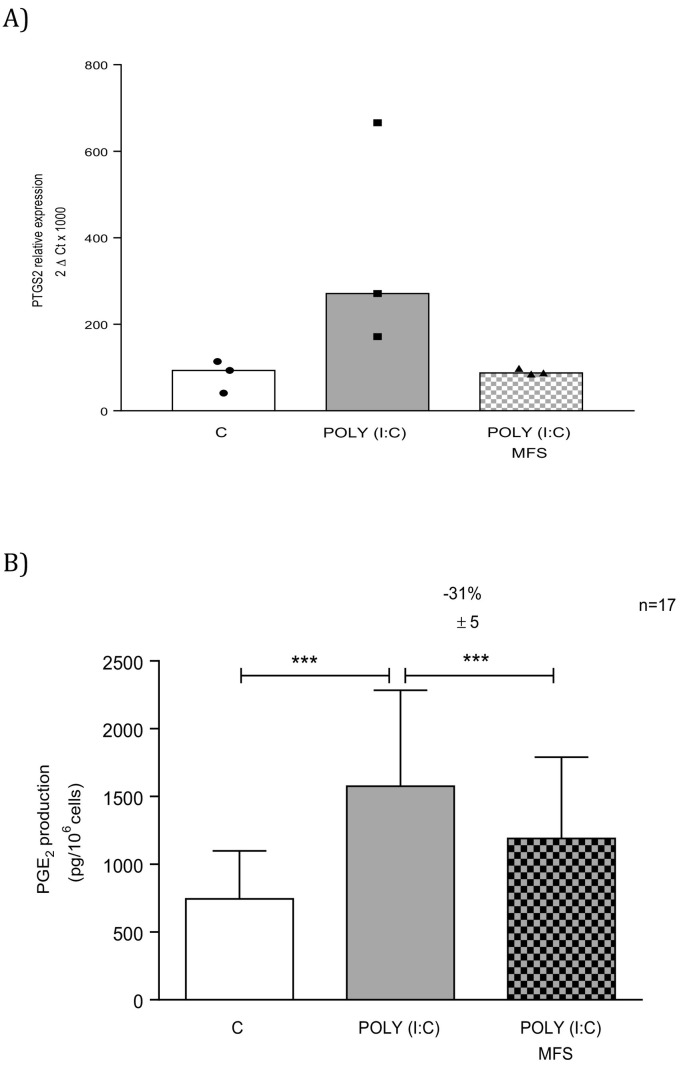
Exposure to MFS did not alter the low baseline level of PGE2 production by non-stimulated bronchial epithelial cells (data not shown) but did markedly inhibit the Poly(I:C)-induced production of PGE2 (Fig. 4 ). This observation was in line with MFS's inhibition of the Poly(I:C)-associated elevation in COX-2 expression (Fig. 4).
Fig. 4.
Effect of the marine-sourced fucoidan solution (MFS) on the mRNA expression of COX-2 and the production of PGE2 by human bronchial epithelial cells. Bronchial epithelial cells were incubated with the MFS or NaCl 0.9% (control) for 1 h, followed by 24 h in culture medium in the absence or presence of Poly(I:C) 10 μg·mL−1. Data correspond as the median (range) of three independent paired experiments (A) or the mean ± SEM of 17 (B) independent paired experiments. The significance threshold was ***p < 0.001.
4. Discussion
The objective of the present study was to explore the effects of a seawater solution of depolymerized fucoidans from Ascophyllum nodosum on human bronchial epithelial cells. An hour of exposure to MFS was enough to inhibit the Poly(I:C)-induced elevation in cytokine/chemokine mRNA expression and protein production, mRNA expression of COX-2, and the production of PGE2 by human bronchial epithelial cells for 24 h.
Fucoidans are abundant, sulfated polysaccharides found only in brown algae. Over the last few decades, fucoidans purified from different species of brown alga have been extensively studied because of their broad spectrum of desirable biological activities, including anticoagulant, antithrombotic, antitumor, anti-inflammatory, and immunomodulatory effects [30]. In a recent in vitro study of RAW 264.7 macrophages, fucoidans purified from the marine brown alga Chnoospora minima were found to inhibit (i) the lipopolysaccharide (LPS)-induced production of nitrous oxide and PGE2 via the downregulation of inducible nitric oxide synthase (iNOS) and COX-2 expression, and (ii) the production of TNF-α, IL1-β, and IL-6 [31]. Furthermore, sulfated polysaccharides extracted from other brown seaweeds (Sargassum hemiphyllum and Sargassum horneri) were shown to reduce pro-inflammatory cytokine production (IL-1β, IL-6 and TNF-α), NO production, and the expression of IL-1β, iNOS, and COX-2 and NF-κB (p65) in LPS-stimulated RAW 246.7 macrophages [32,33]. In macrophages, the mechanisms underlying the fucoidans' anti-inflammatory actions have yet to be elucidated. However, it has been reported that fucoidans regulated signaling pathways involving MAP kinases and NF-κB [34]. Since the MFS did not influence epithelial cell viability, its inhibitory effect was not due to non-specific toxicity.
The present study is the first to have demonstrated that fucoidans (extracted from Ascophyllum nodosum and dissolved in diluted seawater) also have anti-inflammatory effects on human primary bronchial epithelial cells. In particular, the MFS's inhibition of the effects on bronchial epithelial cells of a synthetic viral-like dsRNA (Poly(I:C)) suggests that fucoidans might dampen the epithelium's inflammatory response to viral infections. In addition to the inhibition of the production of various pro-inflammatory cytokines (IL-1α, IL-1β, TNFα, and IL-6) and chemokines (CCL5, CCL22, CXCL1, CXCL5 and CXCL8), the inhibition of PGE2 may have beneficial effect by reducing mucus secretion [35,36]. Further studies are needed to determine whether or not exposure to MFS lowers mucus secretion in the nasal epithelium.
In addition to fucoidans, other algal polysaccharides reportedly exert antiviral activities through various mechanisms of action, such the inhibition of virus binding or internalization and the suppression of DNA replication and/or protein synthesis [37,38]. Scavenger receptors have been described as functional co-receptors of both TLR3 and TLR4 [[39], [40], [41]], and the process of endocytosis might lie at the interface between several underlying mechanisms. Indeed, activation of the SR-A-fucoidan complex is dependent on endocytosis [42], and endosomal internalization of cell-surface TLR3 is critical for the epithelial cells' response to Poly(I:C) [14]. In the present study, the SR-A-fucoidan complex may have been endocytosed (along with TLR3) during the one-hour exposure to MFS, and thus would no longer have been present on the cell surface when Poly(I:C) was added - thus blocking the response to this TLR agonist. However, further studies are required to determine the exact mechanism of action.
The airway epithelium is continuously exposed to a multitude of noxious challenges in inhaled air, and has an important role in regulating the local inflammatory response to viral infections of the upper and lower airways. Earlier research has shown that human bronchial epithelial cell cultures accurately recapitulate the functional characteristics of human nasal epithelial cells, and so could therefore serve as a surrogate for the latter. Indeed, the two cell types have morphologic, histologic and functional similarities. Their respective ciliary activities react to various agonists and antagonists in a similar manner [43]. In experiments on nasal and bronchial epithelial cells harvested from children with cystic fibrosis (with a wide variety of CFTR mutations), the cell types' stimulated and inhibited levels of CFTR were similar [44]. In other studies of human bronchial and nasal epithelial cells, levels of cytokine and chemokine release in vitro were similar for both non-stimulated cells and cells stimulated with TNF-α, IL-1β, IL-13 or even rhinovirus infection [[45], [46], [47]]. Indeed, nasal epithelial cells showed much the same cytokine responses as bronchial epithelial cells when exposed to rhinovirus under air-liquid interface culture or submerged culture conditions; this observation is of particularly relevance to the present study [47]. Taken as a whole, these findings strongly suggest that (i) nasal and bronchial airway epithelial cells can be used interchangeably, and thus (ii) our observation of MFS's anti-inflammatory effects on bronchial epithelial cells can be legitimately transposed to nasal epithelial cells.
Most common colds are caused by respiratory viruses whose number, diversity and antigenic variability limit the development of an effective vaccine. Although the infection is usually self-limiting, it affects people of all age groups and still causes significant morbidity. At present, the standard therapy for the common cold is general care and the treatment of symptoms. Over-the-counter antihistamines are commonly used to relieve symptoms but have a short-term beneficial effect and do not appear to be effective in children [48]. Over-the-counter nasal decongestants are also used, although their effectiveness and safety in children and the clinical relevance of their small symptomatic effect in adults have yet to be demonstrated [49]. Furthermore, the available evidence does not support the use of intranasal corticosteroids for symptomatic relief from the common cold [50]. It was recently shown that iota-carrageenan (a sulfated polysaccharide found in some species of red seaweed) exerts potent activity against respiratory viruses by directly binding to the infectious particles; however, carrageenan does not enter the host cell and is not expected to exert pharmacologic or immune activities [51]. Although iota-carrageenan was found to be effective as nasal spray in three clinical trials [1,51,52], it failed to achieve statistically significant outcomes for the primary and secondary endpoints in the most recent study [53]. Nasal irrigation with saline is often employed as an adjunct treatment for acute upper respiratory tract infections - particularly in children - and might relieve the symptoms to some extent [54]. The MFS assessed here is a ready-to-use, topically applied solution that combines nasal saline irrigation and the topical delivery of fucoidans.
In conclusion, our study results suggest that fucoidans could be used to prevent or treat viral-induced airway inflammation. The MFS may therefore combine the symptomatic benefits of local irrigation with anti-inflammatory activity in the treatment of the common cold.
This research did not receive any specific grant from funding agencies in the public or not-for-profit sectors. The study was partly funded by Yslab and Adebiopharm ER67.
Declarations of interest
MD and RF are employees of Yslab; PD has received funding by Yslab for the present study and consulting fees from Yslab, Astra Zeneca, Sanofi, GlaxoSmithKline, Menarini, ALK-Abello and Stallergenes; SGD, HS, MB, PR and EN have no conflict of interest.
References
- 1.Eccles R., Meier C., Jawad M., Weinmullner R., Grassauer A., Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir. Res. 2010;11:108. doi: 10.1186/1465-9921-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holgate S.T. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol. Rev. 2011;242(1):205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 3.Vareille M., Kieninger E., Edwards M.R., Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011;24(1):210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herold S., Becker C., Ridge K.M., Budinger G.R. Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur. Respir. J. 2015;45(5):1463–1478. doi: 10.1183/09031936.00186214. [DOI] [PubMed] [Google Scholar]
- 5.Veerapandian R., Snyder J.D., Samarasinghe A.E. Influenza in asthmatics: for better or for worse? Front. Immunol. 2018;9:1843. doi: 10.3389/fimmu.2018.01843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spurrell J.C., Wiehler S., Zaheer R.S., Sanders S.P., Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am. J. Phys. Lung Cell. Mol. Phys. 2005;289(1):L85–L95. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- 7.Maciejewski B.A., Jamieson K.C., Arnason J.W., Kooi C., Wiehler S., Traves S.L., Leigh R., Proud D. Rhinovirus-bacteria coexposure synergistically induces CCL20 production from human bronchial epithelial cells. Am. J. Phys. Lung Cell. Mol. Phys. 2017;312(5):L731–L740. doi: 10.1152/ajplung.00362.2016. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R.K., Foster P.S., Rosenberg H.F. Respiratory viral infection, epithelial cytokines, and innate lymphoid cells in asthma exacerbations. J. Leukoc. Biol. 2014;96(3):391–396. doi: 10.1189/jlb.3RI0314-129R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vercammen E., Staal J., Beyaert R. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin. Microbiol. Rev. 2008;21(1):13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillot L., Le Goffic R., Bloch S., Escriou N., Akira S., Chignard M., Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 2005;280(7):5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q., Nagarkar D.R., Bowman E.R., Schneider D., Gosangi B., Lei J., Zhao Y., McHenry C.L., Burgens R.V., Miller D.J., Sajjan U., Hershenson M.B. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J. Immunol. 2009;183(11):6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons K.S., Hsu A.C., Wark P.A. TLR3 and MDA5 signalling, although not expression, is impaired in asthmatic epithelial cells in response to rhinovirus infection. Clin. Exp. Allergy. 2014;44(1):91–101. doi: 10.1111/cea.12218. [DOI] [PubMed] [Google Scholar]
- 13.Blasius A.L., Beutler B. Intracellular toll-like receptors. Immunity. 2010;32(3):305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Ioannidis I., Ye F., McNally B., Willette M., Flano E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J. Virol. 2013;87(6):3261–3270. doi: 10.1128/JVI.01956-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Queiroz K.C., Medeiros V.P., Queiroz L.S., Abreu L.R., Rocha H.A., Ferreira C.V., Juca M.B., Aoyama H., Leite E.L. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed. Pharmacother. 2008;62(5):303–307. doi: 10.1016/j.biopha.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Hidari K.I., Takahashi N., Arihara M., Nagaoka M., Morita K., Suzuki T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008;376(1):91–95. doi: 10.1016/j.bbrc.2008.08.100. [DOI] [PubMed] [Google Scholar]
- 17.Li C., Gao Y., Xing Y., Zhu H., Shen J., Tian J. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia-reperfusion injury in rats via regulating the inflammation response. Food Chem. Toxicol. 2011;49(9):2090–2095. doi: 10.1016/j.fct.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Maruyama H., Tamauchi H., Hashimoto M., Nakano T. Suppression of Th2 immune responses by mekabu fucoidan from Undaria pinnatifida sporophylls. Int. Arch. Allergy Immunol. 2005;137(4):289–294. doi: 10.1159/000086422. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama H., Tamauchi H., Hashimoto M., Nakano T. Vivo 17(3) 2003. Antitumor activity and immune response of Mekabu fucoidan extracted from Sporophyll of Undaria pinnatifida; pp. 245–249. [PubMed] [Google Scholar]
- 20.Limmon G.V., Arredouani M., McCann K.L., Corn Minor R.A., Kobzik L., Imani F. Scavenger receptor class-a is a novel cell surface receptor for double-stranded RNA. FASEB J. 2008;22(1):159–167. doi: 10.1096/fj.07-8348com. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y., Guo M., Wang X., Li J., Wang Y., Ye L., Dai M., Zhou L., Persidsky Y., Ho W. TLR3 activation efficiency by high or low molecular mass poly I:C. Innate Immun. 2013;19(2):184–192. doi: 10.1177/1753425912459975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortier M.E., Kent S., Ashdown H., Poole S., Boksa P., Luheshi G.N. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am. J. Phys. Regul. Integr. Comp. Phys. 2004;287(4):R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 23.Dubois M., Gilles K., Hamilton J.K., Rebers P.A., Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168(4265):167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 24.Ale M.T., Mikkelsen J.D., Meyer A.S. Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs. 2011;9(10):2106–2130. doi: 10.3390/md9102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chevolot L., Foucault A., Chaubet F., Kervarec N., Sinquin C., Fisher A.M., Boisson-Vidal C. Further data on the structure of brown seaweed fucans: relationships with anticoagulant activity. Carbohydr. Res. 1999;319(1–4):154–165. doi: 10.1016/s0008-6215(99)00127-5. [DOI] [PubMed] [Google Scholar]
- 26.Fagon R., Dutot-Wolf M., Tanter C., Hemon M. In: Aqueous Composition Derived From Seawater and Seaweed. W.I.P. Organization, editor. 2018. France. [Google Scholar]
- 27.Hayashi S., Itoh A., Isoda K., Kondoh M., Kawase M., Yagi K. Fucoidan partly prevents CCl4-induced liver fibrosis. Eur. J. Pharmacol. 2008;580(3):380–384. doi: 10.1016/j.ejphar.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang W.N., Chen P.W., Huang C.Y. Compositional characteristics and in vitro evaluations of antioxidant and neuroprotective properties of crude extracts of Fucoidan prepared from compressional puffing-pretreated Sargassum crassifolium. Mar. Drugs. 2017;15(6) doi: 10.3390/md15060183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamasaki-Miyamoto Y., Yamasaki M., Tachibana H., Yamada K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009;57(18):8677–8682. doi: 10.1021/jf9010406. [DOI] [PubMed] [Google Scholar]
- 30.Florez N., Gonzalez-Munoz M.J., Ribeiro D., Fernandes E., Dominguez H., Freitas M. Algae Polysaccharides' chemical characterization and their role in the inflammatory process. Curr. Med. Chem. 2017;24(2):149–175. doi: 10.2174/0929867323666161028160416. [DOI] [PubMed] [Google Scholar]
- 31.Fernando I.P.S., Sanjeewa K.K.A., Samarakoon K.W., Lee W.W., Kim H.S., Kang N., Ranasinghe P., Lee H.S., Jeon Y.J., fucoidan fraction purified from Chnoospora minima A. A potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017;104(Pt A):1185–1193. doi: 10.1016/j.ijbiomac.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 32.Hwang P.A., Chien S.Y., Chan Y.L., Lu M.K., Wu C.H., Kong Z.L., Wu C.J. Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J. Agric. Food Chem. 2011;59(5):2062–2068. doi: 10.1021/jf1043647. [DOI] [PubMed] [Google Scholar]
- 33.Wen Z.S., Xiang X.W., Jin H.X., Guo X.Y., Liu L.J., Huang Y.N., OuYang X.K., Qu Y.L. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int. J. Biol. Macromol. 2016;88:403–413. doi: 10.1016/j.ijbiomac.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Park J., Cha J.D., Choi K.M., Lee K.Y., Han K.M., Jang Y.S. Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo. Int. Immunopharmacol. 2017;43:91–98. doi: 10.1016/j.intimp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Akaba T., Komiya K., Suzaki I., Kozaki Y., Tamaoki J., Rubin B.K. Activating prostaglandin E2 receptor subtype EP4 increases secreted mucin from airway goblet cells. Pulm. Pharmacol. Ther. 2018;48:117–123. doi: 10.1016/j.pupt.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Kook Kim J., Hoon Kim C., Kim K., Jong Jang H., Jik Kim H., Yoon J.H. Effects of prostagladin E(2) on gel-forming mucin secretion in normal human nasal epithelial cells. Acta Otolaryngol. 2006;126(2):174–179. doi: 10.1080/00016480500280033. [DOI] [PubMed] [Google Scholar]
- 37.Wang W., Wang S.X., Guan H.S. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar. Drugs. 2012;10(12):2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmadi A., Zorofchian Moghadamtousi S., Abubakar S., Zandi K. Antiviral potential of algae polysaccharides isolated from marine sources: a review. Biomed. Res. Int. 2015;2015:825203. doi: 10.1155/2015/825203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanai H., Ban T., Wang Z., Choi M.K., Kawamura T., Negishi H., Nakasato M., Lu Y., Hangai S., Koshiba R., Savitsky D., Ronfani L., Akira S., Bianchi M.E., Honda K., Tamura T., Kodama T., Taniguchi T. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462(7269):99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 40.Mukhopadhyay S., Varin A., Chen Y., Liu B., Tryggvason K., Gordon S. SR-A/MARCO-mediated ligand delivery enhances intracellular TLR and NLR function, but ligand scavenging from cell surface limits TLR4 response to pathogens. Blood. 2011;117(4):1319–1328. doi: 10.1182/blood-2010-03-276733. [DOI] [PubMed] [Google Scholar]
- 41.Komai K., Shichita T., Ito M., Kanamori M., Chikuma S., Yoshimura A. Role of scavenger receptors as damage-associated molecular pattern receptors in toll-like receptor activation. Int. Immunol. 2017;29(2):59–70. doi: 10.1093/intimm/dxx010. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X.D., Zhuang Y., Ben J.J., Qian L.L., Huang H.P., Bai H., Sha J.H., He Z.G., Chen Q. Caveolae-dependent endocytosis is required for class a macrophage scavenger receptor-mediated apoptosis in macrophages. J. Biol. Chem. 2011;286(10):8231–8239. doi: 10.1074/jbc.M110.145888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devalia J.L., Davies R.J. Human nasal and bronchial epithelial cells in culture: an overview of their characteristics and function. Allergy Proc. 1991;12(2):71–79. doi: 10.2500/108854191779011783. [DOI] [PubMed] [Google Scholar]
- 44.Brewington J.J., Filbrandt E.T., LaRosa F.J., 3rd, Moncivaiz J.D., Ostmann A.J., Strecker L.M., Clancy J.P. Brushed nasal epithelial cells are a surrogate for bronchial epithelial CFTR studies. JCI Insight. 2018;3(13) doi: 10.1172/jci.insight.99385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDougall C.M., Blaylock M.G., Douglas J.G., Brooker R.J., Helms P.J., Walsh G.M. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am. J. Respir. Cell Mol. Biol. 2008;39(5):560–568. doi: 10.1165/rcmb.2007-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pringle E.J., Richardson H.B., Miller D., Cornish D.S., Devereux G.S., Walsh G.M., Turner S.W. Nasal and bronchial airway epithelial cell mediator release in children. Pediatr. Pulmonol. 2012;47(12):1215–1225. doi: 10.1002/ppul.22672. [DOI] [PubMed] [Google Scholar]
- 47.Roberts N., Al Mubarak R., Francisco D., Kraft M., Chu H.W. Comparison of paired human nasal and bronchial airway epithelial cell responses to rhinovirus infection and IL-13 treatment. Clin. Transl. Med. 2018;7(1):13. doi: 10.1186/s40169-018-0189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Sutter A.I., Saraswat A., van Driel M.L. Antihistamines for the common cold. Cochrane Database Syst. Rev. 2015;11 doi: 10.1002/14651858.CD009345.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deckx L., De Sutter A.I., Guo L., Mir N.A., van Driel M.L. Nasal decongestants in monotherapy for the common cold. Cochrane Database Syst. Rev. 2016;10 doi: 10.1002/14651858.CD009612.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayward G., Thompson M.J., Perera R., Del Mar C.B., Glasziou P.P., Heneghan C.J. Corticosteroids for the common cold. Cochrane Database Syst. Rev. 2015;10 doi: 10.1002/14651858.CD008116.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludwig M., Enzenhofer E., Schneider S., Rauch M., Bodenteich A., Neumann K., Prieschl-Grassauer E., Grassauer A., Lion T., Mueller C.A. Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial. Respir. Res. 2013;14:124. doi: 10.1186/1465-9921-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fazekas T., Eickhoff P., Pruckner N., Vollnhofer G., Fischmeister G., Diakos C., Rauch M., Verdianz M., Zoubek A., Gadner H., Lion T. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complement. Altern. Med. 2012;12:147. doi: 10.1186/1472-6882-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eccles R., Winther B., Johnston S.L., Robinson P., Trampisch M., Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respir. Res. 2015;16:121. doi: 10.1186/s12931-015-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King D., Mitchell B., Williams C.P., Spurling G.K. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015;4 doi: 10.1002/14651858.CD006821.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]