Summary
Background
Severe acute respiratory syndrome (SARS) is a novel infectious disease with global impact. A virus from the family Coronaviridae has been identified as the cause, but the pathogenesis is still unclear.
Methods
Post-mortem tissue samples from six patients who died from SARS in February and March, 2003, and an open lung biopsy from one of these patients were studied by histology and virology. Only one full autopsy was done. Evidence of infection with the SARS-associated coronavirus (SARS-CoV) and human metapneumovirus was sought by reverse-transcriptase PCR and serology. Pathological samples were examined by light and electron microscopy and immunohistochemistry.
Findings
All six patients had serological evidence of recent infection with SARS-CoV. Diffuse alveolar damage was common but not universal. Morphological changes identified were bronchial epithelial denudation, loss of cilia, and squamous metaplasia. Secondary bacterial pneumonia was present in one case. A giant-cell infiltrate was seen in four patients, with a pronounced increase in macrophages in the alveoli and the interstitium of the lung. Haemophagocytosis was present in two patients. The alveolar pneumocytes also showed cytomegaly with granular amphophilic cytoplasm. The patient for whom full autopsy was done had atrophy of the white pulp of the spleen. Electron microscopy revealed viral particles in the cytoplasm of epithelial cells corresponding to coronavirus.
Interpretation
SARS is associated with epithelial-cell proliferation and an increase in macrophages in the lung. The presence of haemophagocytosis supports the contention that cytokine dysregulation may account, at least partly, for the severity of the clinical disease. The case definition of SARS should acknowledge the range of lung pathology associated with this disease.
Published online May 16, 2003 http://image.thelancet.com/extras/03art4347web.pdf
Introduction
Since Nov 1, 2002, an outbreak of severe acute respiratory syndrome (SARS) has affected 33 countries in five continents, with 7053 reported cases and 506 deaths at the time of writing.1 Local transmission has occurred in at least six countries. The first cases of SARS in Hong Kong Special Administrative Region were recognised in February, 2003. As of May 8, 2003, there had been 1661 cases and 208 deaths in the region.
Clinically, the disease is characterised by fever, dyspnoea, lymphopenia, and rapidly progressing changes on radiography.23 Upper-respiratory-tract symptoms are not prominent, but diarrhoea has been reported by some patients. There is no response to conventional antibiotics used to treat atypical pneumonia. The SARS-associated coronavirus (SARS-CoV) has been consistently associated with this disease.3 Although this virus seems to be necessary for the development of SARS, it has not been localised at the site of lung pathology.3, 4 Poutanen and colleagues5 found human metapneumovirus (HMPV) as a second pathogen in patients with SARS and postulated that HMPV potentiates the progression or severity of coronavirus infection.
The use of steroids together with ribavirin has been reported to confer clinical benefit, although randomised clinical trials to support its clinical efficacy are not available. To aid understanding of the pathogenesis of the disease and the relation to current therapy, we report the pathological and virological findings in six patients with SARS who died.
Methods
Selection of patients and autopsy material
All patients who met a modified WHO case definition of SARS6 and who underwent lung biopsy or post-mortem examination during March, 2003, at four major hospitals in the Kowloon hospital cluster were eligible for inclusion. The case definition was fever (temperature 38°C or higher), cough or shortness of breath, new pulmonary infiltrates on chest radiograph, and either a history of exposure to a patient with SARS or a lack of response to empirical antimicrobial coverage for typical and atypical pneumonia (beta-lactams and macrolides, fluoroquinolones or tetracyclines).3 Owing to the potentially infectious nature of the disorder, full autopsy was done in only one case. In the other cases, the post-mortem examination was limited to the lungs. We included only those patients for whom paired serum samples were submitted for virological studies during the course of the illness. Patients with only needle-biopsy samples of organs available were excluded. During the period under study, there were six patients with pathological and virological investigation; one of these also underwent lung biopsy earlier in the course of the illness.
In all cases the autopsy material was fixed in 10% neutral buffered formalin and later processed for electron microscopy. Separate pieces of fresh tissue were sent for virological study; if sufficient quantity was available, they were stored at –70°C for immunofluorescence studies.
Patients
Patient 1, a 37-year-old woman, had good health before the illness. She was admitted on March 13, 2003, with a history of fever, cough, and general muscle pain for 10 days. Oxygen saturation on admission was 89% on 50% oxygen. The chest radiograph showed patchy haziness. The patient soon developed respiratory failure necessitating mechanical ventilation; ribavirin and antibiotic treatment was then started. The ratio of partial arterial pressure of oxygen (PaO2) to fraction of inspired oxygen (FiO2) was 24·2 kPa on the day before death. She died on March 23, after 10 days of assisted ventilation.
Patient 2, a 39-year-old woman who was previously well, had indirect contact with a person who developed fever after return from Guangzhou, China. She subsequently developed fever with shortness of breath and diarrhoea. She had lymphopenia at admission, and the chest radiograph showed infiltrates in the left upper zone. Treatment with ribavirin and steroids was started on March 21. She developed respiratory failure the next day and was intubated. She had repeated cardiac arrests and died on March 24.
Patient 3, a 64-year-old man, developed influenza-like symptoms and left pleuritic chest pain on Feb 15. A chest radiograph taken a day later showed haziness in the left lower zone. He subjectively improved after self-medication with levofloxacin and penicillin. He visited Hong Kong on Feb 21 as a tourist, but his condition then deteriorated. He was admitted directly to the intensive-care unit and intubated on Feb 22. Investigation showed diffuse bilateral ground-glass changes on chest radiograph and severe oxygen desaturation. Despite empirical antimicrobial coverage for typical, atypical, and hospital-acquired pneumonia, his condition continued to deteriorate. He was empirically treated with oseltamivir, foscarnet, intravenous immunoglobulin and, at a later stage, ribavirin and steroids. After ventilation for 10 days with a peak airway pressure of 36 cm water, tidal volume of 730 mL, and PaO2/FiO2 9·8 kPa, he died on March 4 with acute respiratory distress syndrome and multiorgan failure.
Patient 4 was a 53-year-old man with a history of hypertension. He had developed influenza-like symptoms a few days before admission on Feb 28 with chest radiographic changes of diffuse bilateral pneumonia and respiratory failure. He did not respond to doxycycline, amantadine, and cefotaxime. An open lung biopsy was done on March 4. Routine microbiological and virological investigations were negative. He was empirically treated with oseltamivir, steroids, and ribavirin. Non-oliguric renal failure developed and was later complicated by nosocomial pneumonia due to Pseudomonas aeruginosa. He died on March 19, after ventilation for 16 days with a peak airway pressure of 34 cm water, tidal volume of 640 mL, and PaO2/FiO2 17·0 kPa
Patient 5 was a 49-year-old businessman who had a history of hepatitis B liver cirrhosis and portal hypertension. He developed influenza-like symptoms while in Hong Kong but still travelled overseas on Feb 26. Investigation at his destination showed lymphopenia and thrombocytopenia in the peripheral blood examination, and bilateral pulmonary infiltrates on chest radiograph. He went into respiratory failure on March 2, and on March 5 was transferred back to Hong Kong, where he was ventilated. The ventilation pressure was 20/10 cm water most of the time. Positive end-expiratory pressure was 15–17 cm water. The tidal volume was generally kept at 450–650 mL, and the PaO2 was between 70% and 90% and FiO2 between 80% and 100%. His renal function deteriorated progressively from March 9 to March 12, and at the time of death the urea concentration was 10·6 mmol/L and creatinine 218 μmol/L. Total bilirubin varied between 18 μmol/L and 27 μmol/L. Despite intensive support and antimicrobial coverage with piperacillin-tazobactam, azithromycin, and oseltamivir, he died on March 13.
Patient 6 was a 77-year-old man with good health previously. On March 2 he was admitted to hospital for increasing shortness of breath, fever, and cough for 2 days. Investigations showed bilateral patchy consolidations of lower zones on chest radiograph and oxygen desaturation. He was intubated and ventilated by positive end-expiratory pressure mode in the intensive-care unit 2 days after admission. His clinical course was complicated by pneumonia due to meticillin-resistant Staphylococcus aureus. He was ventilated for 12 days with a peak airway pressure of 30 cm water, tidal volume of 540 mL, and PaO2/FiO2 9·4 kPa, but he died on March 15, despite extensive antimicrobial coverage for typical, atypical, and hospital-acquired pneumonia.
The clinical presentations of two of these patients (patients 3 and 4) have been reported previously.2
Investigations
Only one patient (patient 4) had an open lung biopsy done before death. The material was divided into four pieces; one was sent for viral studies, one frozen at –70°C, one fixed in formalin, and the one fixed in 2.5% glutaraldehyde. The latter was processed for transmission electron microscopy.
For immunohistochemistry, paraffin-embedded blocks of the lung tissue were sectioned at 5 μm and dewaxed by standard procedures. Monoclonal antibodies (Dako, Carpintaria, CA, USA) to keratin AE1/3 (1:50), CD3 T-cell (1:5), and CD68 macrophage marker (1:50) were applied on each section, and staining was done with the Ventana Nexes automated immunohistochemical stainer.
Frozen post-mortem lung tissue from one patient (patient 5) was available for study. Cryostat sections were stained with directly conjugated monoclonal antibodies to influenza viruses A and B, adenovirus, and parainfluenza virus (Dako, USA) and examined under a Nikon Eclipse E800M fluorescent microscope.
Routine microbiological investigations included culture of blood and sputum for bacterial pathogens.3 Serology was done with complement-fixation tests for respiratory viruses, chlamydiae, and Mycoplasma pneumoniae.
Total RNA and DNA were extracted from nasopharyngeal aspirates with the Viral RNA minikit and QIAmp DNA minikit (QIAGEN, Hilden, Germany). Reverse-transcriptase (RT) PCR was done for influenza A,7 adenovirus,8 HMPV, and a newly recognised coronavirus subsequently reported to be associated with SARS.3 The RT-PCR protocol has been described previously.3
The biopsy sample or post-mortem lung tissue was homogenised in a tissue grinder, and the supernatant was inoculated onto cell cultures. Viral RNA was extracted from the tissues with the RNeasy minikit (QIAGEN) according to the manufacturer's tissue protocol instructions. RT-PCR for the novel coronavirus and for HMPV was carried out as described above.
Serial dilutions of serum from acute and convalescent patients were tested in parallel for antibodies to the coronavirus and HMPV by an indirect immunofluorescence test on FRhK-4 cells infected with the respective virus.3
Role of the funding source
The sponsors of the study had no role in the study design, data collection, analysis, or interpretation, or in the writing of the report.
Results
Ante-mortem investigations for routine bacterial and viral respiratory pathogens proved unremarkable. However, all six patients had four-fold or greater rises in antibody titre to the SARS-CoV, indicating recent infection with this virus (table 1 ). In addition, several patients had evidence of coronaviral RNA in the nasopharyngeal aspirate, and in one case (patient 4) also in the plasma. Viral RNA was found in the lung biopsy sample of patient 3. No evidence of HMPV infection (rise in antibody titre or viral RNA) was found in any of the patients studied (table 1). Evidence of SARS-CoV RNA was detected in the postmortem lung samples of four patients by RT-PCR. None of these samples had evidence of HMPV RNA.
Table 1.
Virological investigations of patients with fatal SARS
| Patient |
Coronavirus serology |
RT-PCR on nasopharyngeal aspirate before death |
RT-PCR on post-mortem lung |
|||
|---|---|---|---|---|---|---|
| Date | Titre | Coronavirus | HMPV | Coronavirus | HMPV | |
| 1 | March 14 | 1/40 | NA | NA | - | - |
| March 23 | 1/640 | |||||
| 2 | March 19 | 1/10 | NA | NA | + | - |
| March 24 | 1/640 | |||||
| 3 | Feb 26 | 1/50 | + | - | + | - |
| March 3 | 1/200 | |||||
| 4 | March 3 | 1/200 | + | - | + | - |
| March 11 | 1/1600 | |||||
| 5 | March 6 | <1/50 | + | - | - | - |
| March 11 | 1/800 | |||||
| 6 | March 4 | 1/50 | + | - | + | - |
| March 11 | 1/1600 | |||||
NA=not available
In the biopsy sample from patient 4, most of the material was pleural tissue with a small amount of lung parenchyma. The architecture was preserved with a mild increase in interstitial lymphocytes. Cytomegaly was present in a very few pneumocytes; it was characterised by nuclear enlargement, prominent nucleolus, and amphophilic granular cytoplasm (figure 1 ). No typical viral inclusions were identified. There was a mild to moderate increase in alveolar macrophages and early hyaline-membrane formation.
Figure 1.
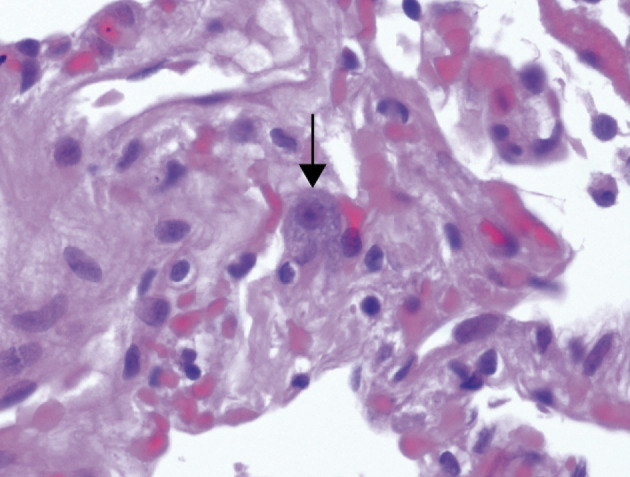
Open lung biopsy from patient 4
Arrow shows an enlarged pneumocyte with amphophilic granular cytoplasm and prominent nucleolus. Haematoxylin and eosin; ×400.
Gross findings in the post-mortem lung tissue were similar among patients (table 2 ). Where the lungs were removed en bloc, they weighed 1000–2100 g and were oedematous, with a greyish brown consolidated cut surface. The consolidation was irregular and patchy, with foci of pale tissue measuring up to several millimetres in diameter. More diffuse involvement was seen in one case (patient 4); mucopurulent material was seen in the tracheobronchial tree.
Table 2.
Summary of histological findings
| Patient | Sex | Age (years) | Date of onset | hospital admission | Date of death | Diffuse alveolar damage | Glant cells | Cytomegaly with granular eoslnophillc cytoplasm | Other pathological findings | Other medical disorders |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 37 | March 3 | March 13 | March 23 | + | - | + | Epithelial denudation | None |
| 2 | Female | 39 | March 16 | March 19 | March 24 | + | - | - | Squamous metaplasia with loss of cilia, haemophagocytosis | None |
| 3 | Male | 64 | Feb 15 | Feb 22 | March 4 | + | ++ | + | Epithelial denudation with fibrin thrombi present in pulmonary vessels | None |
| 4 | Male | 53 | Feb 23 | Feb 28 | March 19 | + | ++ | + | Bronchiolitis obliterans | Hypertension |
| 5 | Male | 49 | Feb 24 | March 5 | March 13 | + | ++ | + | Epithelial denudation, white-pulp atrophy in spleen | Cirrhosis with oesophageal varices |
| 6 | Male | 77 | Feb 28 | March 2 | March 15 | + | ++ | + | Haemophagocytosis | None |
In cases of disease duration of less than 10 days, the histological involvement of the lung varied, with a mixed inflammatory infiltrate, oedema, and hyaline-membrane formation seen (figure 2 ; table 2). The intra-alveolar oedema was granular or vacuolated. Desquamation of pneumocytes was a prominent and consistent feature (figure 3 ).
Figure 2.
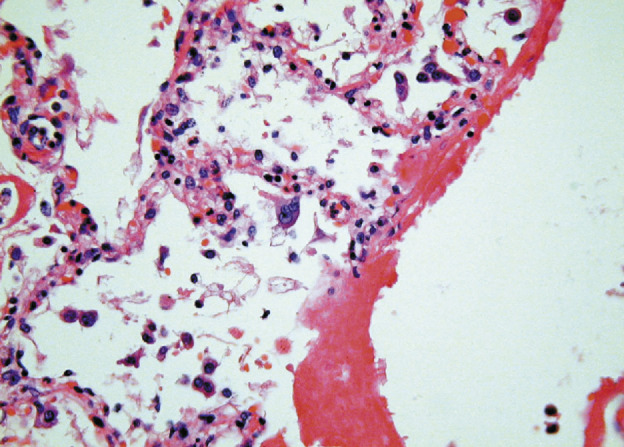
SARS showing hyaline-membrane formation and pneumocyte desquamation with focal giant-cell formation
Haematoxylin and eosin; ×400.
Figure 3.
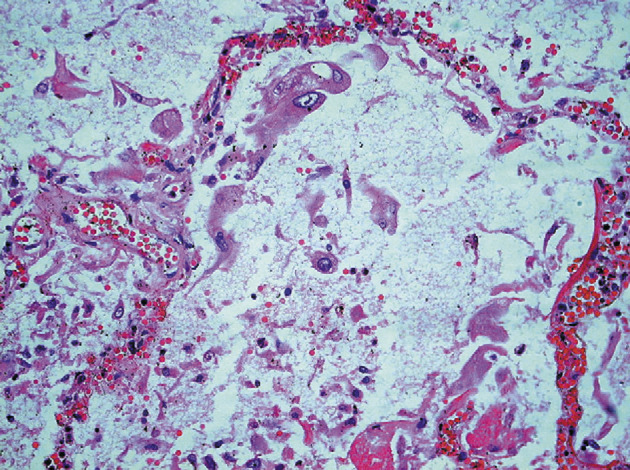
SARS-CoV pneumonia with cytomegaly of pneumocytes
Haematoxylin and eosin; ×400.
All cases showed scattered single enlarged cells that, in most, were associated with large nuclei and prominent nucleoli, similar to the findings in the open lung biopsy sample. Clearing of the chromatin was also seen. Giantcell formation was seen within the alveolar lumen in four cases, and there were a few cells that contained amphophilic to basophilic cytoplasmic granules within enlarged pneumocytes. One patient (patient 3) showed fibrin thrombi within pulmonary vessels, and another (patient 1) showed intimal swelling of pulmonary vessels. In three of four cases, the giant cells were positive for the macrophage marker CD68 (figure 4 ); in one case, they were positive for the epithelial marker AE1/3. In the patient who had frozen tissue available, no staining was identified to adenovirus, parainfluenza virus, or influenza viruses A or B on frozen-section immunofluoresence.
Figure 4.
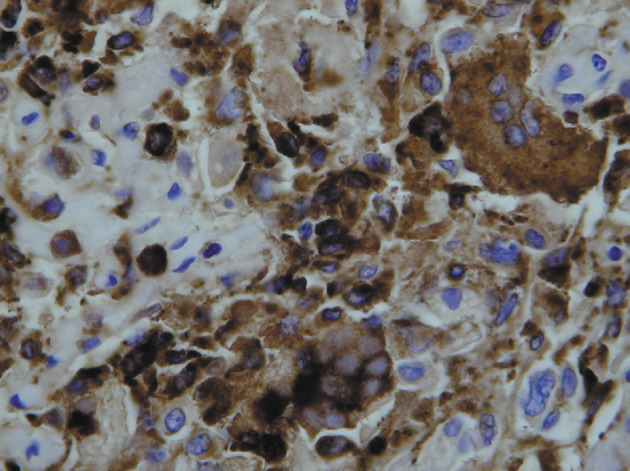
CD68 macrophage antibody staining of giant cells in patient 5
Diaminobenzidine with haematoxylin counterstain; ×400.
One patient, in whom the time from onset of symptoms to death was 8 days, showed squamous metaplasia of the bronchi with loss of cilia (figure 5 ). In addition, within the parenchyma and also within the interstitium there were increased numbers of CD68-positive mononuclear cells and focal haemophagocytosis (figure 6 ). Because of the risk of infection, most post-mortem examinations were limited to the chest, but in patient 5, for whom a full autopsy was done, pronounced white-pulp atrophy of the spleen was seen. In this case, electron microscopy showed dilated Golgi apparatus in the cytoplasm of pneumocytes, and within these organelles, 90 nm viral particles with small external spikes corresponding to coronavirus were identified (figure 7 ). These particles were of similar morphology to those reported previously.3
Figure 5.
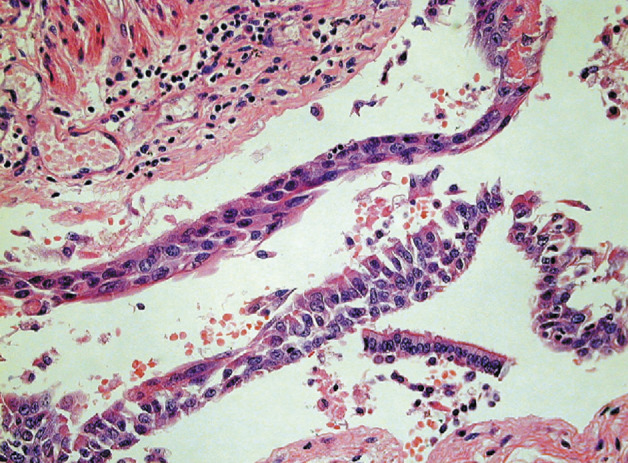
Squamous metaplasia of bronchial mucosa in SARS-CoV pneumonia
Haematoxylin and eosin; ×400.
Figure 6.
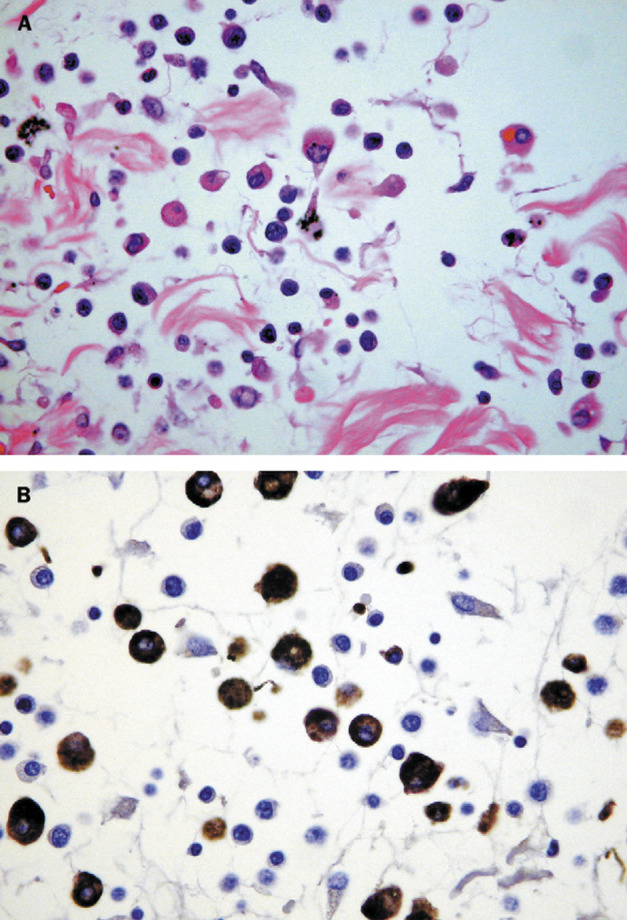
Septal inflammation of patient 2
A: Increased numbers of mononuclear cells with haemophagocytosis. Haematoxylin and eosin; ×400. B: The mononuclear cells are CD68 positive. Diaminobenzidine with haematoxylin counterstain.
Figure 7.
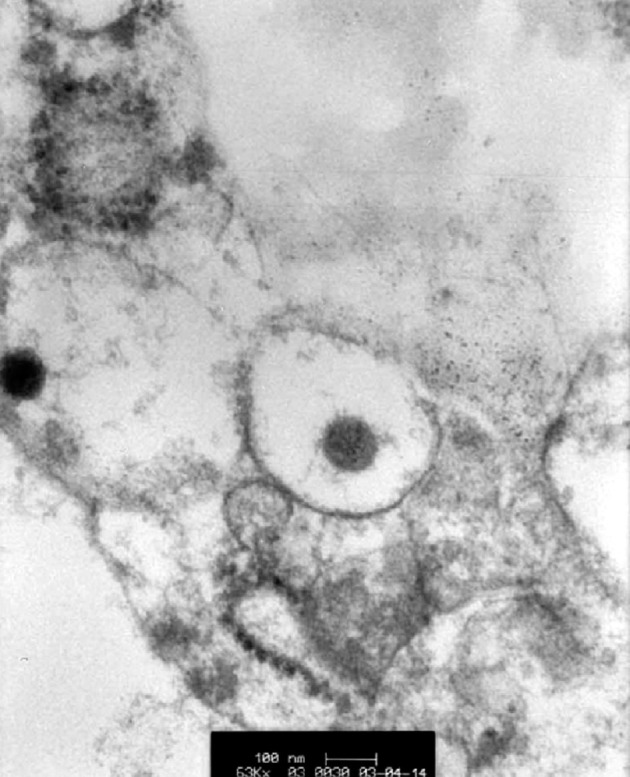
Thin-section electron micrograph showing a dilated secretory vesicle containing a 90 nm coronavirus particle
×63 000.
Discussion
All of the six patients described had serological evidence of recent infection with the SARS-CoV. Five had evidence of viral RNA detectable in samples taken before or after death. In previous studies, healthy controls and patients with unrelated disease had no evidence of SARS-CoV antibody or RNA in the serum or the respiratory tract, respectively.3
In patients with disease of duration less than 10 days there was hyaline-membrane formation, pneumocyte proliferation, and oedema. Diffuse alveolar damage was seen in cases of longer duration. This diffuse alveolar damage typically goes through an exudative phase followed by a proliferative phase,9 although others have described a three-stage process: an inflammatory or exudative phase, a proliferative phase, and a final fibrotic phase.10 Viral infections resulting in diffuse alveolar damage typically fall into two categories; influenza viruses A and B and adenovirus cause most cases of viral pneumonia in immunocompetent adults. Immunocompromised hosts are susceptible to pneumonias caused by cytomegalovirus and other herpesviruses, measles virus, and adenovirus.11 In addition to the diffuse alveolar damage here, morphological features of giant cells and pneumocyte hyperplasia, which appear to be prominent in this viral infection, were seen in these patients.4, 12 Multinucleate giant cells within the alveoli have previously been reported in patients with SARS,4, 12 but their derivation has remained unclear. We have documented that in three of the patients these giant cells are of macrophage origin and in one other patient they are epithelial in origin. These giant cells were not present in the ante-mortem lung biopsy sample and were present only in patients with disease progression for longer than 8 days from admission. Some animal coronaviruses induce syncytium formation in cell culture,13 and SARS-CoV also induces focal syncitium formation4 in Vero cells.
Bronchial epithelial denudation, loss of cilia, and squamous metaplasia were early features. Another notable feature was the presence of large pneumocytes showing an enlarged nucleus and granular amphophilic cytoplasm. These changes were also seen in the open lung biopsy sample from patient 4. Coronavirus assembly takes place in the Golgi apparatus of the cytoplasm;14 in the sections of the coronavirus-infected culture this process was characterised by swelling and increased granularity of the cytoplasm. We therefore hypothesise that this cytoplasmic amphophilia may be due to viral assembly within the Golgi apparatus—a feature seen on electron microscopy of the open lung biopsy sample from patient 4.3
The histological changes of uncomplicated viral pneumonias are rarely described, and reports tend to be derived from post-mortem examination of patients who succumb to the pneumonia; thus, they may not be representative of the majority of pneumonia patients, who survive.3, 15 The same is true for SARS, because the patients admitted to hospital have not been in a suitable medical condition to warrant biopsy. However, several features of fatal coronaviral pneumonia can be identified. Coronavirus infection in the early stages seems to stimulate epithelial cells and results in cellular proliferation and squamous metaplasia in the lung. This type-2 pneumocyte hyperplasia and hypertrophy has also been identified with porcine reproductive and respiratory syndrome virus (an arterivirus tentatively classified under the Coronaviridae 16) as well as porcine respiratory coronavirus (previously known as transmissible gastroenteritis virus, respiratory variant). In those infections mild type-2 pneumocyte proliferation is associated with squamous metaplasia.17 In SARS, macrophage proliferation is more prominent in the consolidated areas of the lung; this distribution is also seen with porcine respiratory coronavirus.18
In contrast to typical diffuse alveolar damage in which neutrophils and fibroblasts are the main cellular agents and macrophages have a lesser role,9 in patients with fatal SARS macrophages are the prominent leucocyte in the alveoli, even in the early stages of the disease. The finding of haemophagocytosis in the lung and white-pulp atrophy of the spleen identified in SARS is reminiscent of that reported in fatal influenza subtype H5N1 disease in 1997.19 Haemophagocytosis has been attributed to cytokine dysregulation.20 Lymphopenia is another feature common to both SARS-CoV and H5N1 influenza pneumonia.21 Both viruses have crossed to human beings from animals or birds.3, 21 Experimental studies in which macrophages are infected in vitro suggest that, compared with conventional human influenza viruses, the subtype H5N1 influenza A viruses isolated in 1997 are hyperinducers of proinflammatory cytokines.22 Human coronavirus OC43 can replicate in human macrophages in vitro.23 Taken together, the similarity of clinical and pathological changes in SARS-CoV pneumonia and H5N1 pneumonia suggest that proinflammatory cytokines released by stimulated macrophages in the alveoli have a prominent role in pathogenesis of SARS, resulting in cytokine dysregulation. This idea has implications for the management of coronaviral pneumonia. Intervention with steroids might modulate this cytokine response and prevent a fatal outcome, as has been proposed for nonviral acute respiratory distress syndrome.24
Although we did not find PCR evidence of a secondary viral infection, such as HMPV, in pigs infected with some isolates of porcine respiratory coronavirus, the respiratory lesions were severe enough to predispose the pigs to secondary bacterial or mycoplasmal infections or to lead to combined viral infections.25 As we have already mentioned, the histological features of porcine reproductive and respiratory syndrome virus infection are similar to those of SARS-CoV infection; it is possible that SARS-CoV infection in human beings may “open the door” to secondary infections, as previously reported.5 The patients who received ribavirin therapy still had PCR and electron-microscopic evidence of SARS-CoV in the lung. This finding supports clinical evidence that a search for a more promising antiviral agent is of high priority in the treatment of this disease.
The recent WHO case definition of SARS when an autopsy is done states that autopsy features showing changes of respiratory distress syndrome without an identifiable cause can be included, whereas those without respiratory distress syndrome should be excluded.26 Previous reports have featured late cases, in which diffuse alveolar damage is pronounced.4, 12 Our series of serologically confirmed cases shows there is a range of morphological changes in SARS, and that in disease of less than 10 days’duration the changes of respiratory distress syndrome may be focal and dissimilar to those previously published cases. Pathologists undertaking these autopsies should be aware of the varied range of these morphological changes.
Acknowledgments
Acknowledgments
We thank Klaus Stöhr and WHO for initiating and coordinating the information exchange between members of the WHO SARS Laboratories network, which has allowed rapid understanding of the aetiology of this disease; S Y Lam, S W Kwan, K F Lo, H Y Ng, C Y Cheung, K Fung, and O K Wong for their assistance; and Trevor Ellis of the Department of Agriculture and Fisheries, Government of the Hong Kong Special Administrative Region for advice. We acknowledge research funding from Public Health Research Grant A195357 from the National Institute of Allergy and Infectious Diseases, USA, the Wellcome Trust grant GR067072/D/02/Z, the University of Hong Kong, and the Hospital Authority of Hong Kong Special Administrative Region.
Contributors
J M Nicholls and J S M Peiris are coprincipal investigators who planned, coordinated, and jointly wrote the report. J M Nicholls supervised and interpreted the immunofluorescence, electron microscopy, and immunohistochemistry. L L M Poon, K H Chan, W Lim, Y Guan, and K Y Yuen supervised the microbiological and virological studies. K C Lee, W F Ng, S T Lai, C Y Leung, C M Chu, P K Hui, K L Mak, K W Yan, and N C Tsang were pathologists or clinicians involved in the management or post-mortem examination of the six patients
Conflict of interest statement
None declared.
References
- 1.WHO Cumulative number of reported cases of severe acute respiratory syndrome. (SARS) http://www.who.int/csr/sarscountry/2003_04_05/en (accessed May 6, 2003).
- 2.Tsang KW, Ho PL, Ooi GC, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med (in press). [DOI] [PubMed]
- 3.Peiris JSM, Lai ST, Poon LLM. Severe acute respiratory syndrome (SARS) is associated with a coronavirus. Lances. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ksiazek TG, Erdman D, Goldsmith C, et-al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med (in press). [DOI] [PubMed]
- 5.Poutanen SM, Low DE, Henry B, et-al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med (in press). [DOI] [PubMed]
- 6.WHO Severe acute respiratory syndrome (SARS) Wkly Epidemiol Rec. 2003;78:81–83. [PubMed] [Google Scholar]
- 7.Fouchier RA, Bestebroer TM, Herfst S, Van Der Kemp L, Rimmelzwaan GF, Osterhaus AD. Detection of influenza A virus from different species by PCR amplification of conserved sequences in the matrix gene. J Clin Microbiol. 2000;38:4096–4101. doi: 10.1128/jcm.38.11.4096-4101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell S, O'Neill HJ, Ong GM. Clinical assessment of a generic DNA amplification assay for the identification of respiratory adenovirus infections. J Clin Virol. 2003;26:331–338. doi: 10.1016/s1386-6532(02)00082-3. [DOI] [PubMed] [Google Scholar]
- 9.Myers JC, Colby TV, Yousem SA. Common pathways and patterns of injury. In: Dail DH, Hammar SP, editors. Pulmonary pathology. 2nd edn. Springer-Verlag; New York: 1994. pp. 57–77. [Google Scholar]
- 10.Bellingan GJ. The pulmonary physician in critical care: 6, the pathogenesis of ALI/ARDS. Thorax. 2002;57:540–546. doi: 10.1136/thorax.57.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim EA, Lee KS, Primack SL. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics. 2002;22:S137–S149. doi: 10.1148/radiographics.22.suppl_1.g02oc15s137. [DOI] [PubMed] [Google Scholar]
- 12.Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med (in press). [DOI] [PubMed]
- 13.World Organization for Animal Health Transmissible gastroenteritis. http://www.oie.int/eng/normes/mmanual/A_00085.htm (accessed May 6, 2003).
- 14.Corse E, Machamer CE. The cytoplasmic tail of infectious bronchitis virus E protein directs Golgi targeting. J Viral. 2002;76:1273–1284. doi: 10.1128/JVI.76.3.1273-1284.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garantziotis S, Howell DN, McAdams HP, Davis RD, Henshaw NG, Palmer SM. Influenza pneumonia in lung transplant recipients: clinical features and association with bronchiolitis obliterans syndrome. Chest. 2001;119:1277–1280. doi: 10.1378/chest.119.4.1277. [DOI] [PubMed] [Google Scholar]
- 16.Halbur PG, Paul PS, Frey ML. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 17.Halbur PG, Paul PS, Vaughn EM, Andrews JJ. Experimental reproduction of pneumonia in gnotobiotic pigs with porcine respiratory coronavirus isolate AR310. J Vet Diagn Invest. 1993;5:184–188. doi: 10.1177/104063879300500207. [DOI] [PubMed] [Google Scholar]
- 18.Pensaert MB. Transmissible gastroentertitis virus (respiritory variant) In: Pensaert MB, editor. Virus infection of porcines. Elsevier Science Publishers; Amsterdam: 1989. pp. 154–165. [Google Scholar]
- 19.To KF, Chan PK, Chan KF. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. 2000;6:60–68. doi: 10.3201/eid0606.000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuen KY, Chan PKS, Peiris M. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancer. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 22.Cheung CY, Poon LLM, Lau ASY. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease. Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 23.Collins AR. Human macrophages are susceptible to coronavirus OC43. Adv Exp Med Biol. 1998;440:635–639. doi: 10.1007/978-1-4615-5331-1_82. [DOI] [PubMed] [Google Scholar]
- 24.Lai KN, Leung JC, Metz CN, Lai FM, Bucala R, Lan HY. Role for macrophage migration inhibitory factor in acute respiratory distress syndrome. J Pathol. 2003;199:496–508. doi: 10.1002/path.1291. [DOI] [PubMed] [Google Scholar]
- 25.Halbur PG, Pallarés FJ, Opriessnig T, Vaughn EM, Paul PS. Pathogenicity of three isolates of porcine respiratory coronavirus in the USA. Vet Rec. 2003;152:358–361. doi: 10.1136/vr.152.12.358. [DOI] [PubMed] [Google Scholar]
- 26.WHO Case definitions for surveillance of severe acute respiratory syndrome (SARS) http://www.who.int/csr/sars/casedefinition/en/ (accessed May 6, 2003).


