Abstract
Objective
Severe acute respiratory syndrome (SARS) was the first massive infectious disease outbreak of the 21st century. However, it is unlikely that this outbreak will be the last. This study aimed to evaluate the long-term psychiatric morbidities in survivors of SARS.
Method
This is a cohort study designed to investigate psychiatric complications among SARS survivors treated in the United Christian Hospital 30 months after the SARS outbreak. Psychiatric morbidities were assessed by the Structured Clinical Interview for DSM-IV, the Impact of Events Scale–Revised and the Hospital Anxiety and Depression Scale. Functional outcomes were assessed by the Medical Outcomes Study 36-Item Short-Form Health Survey.
Results
Ninety subjects were recruited, yielding a response rate of 96.8%. Post-SARS cumulative incidence of DSM-IV psychiatric disorders was 58.9%. Current prevalence for any psychiatric disorder at 30 months post-SARS was 33.3%. One-fourth of the patients had post-traumatic stress disorder (PTSD), and 15.6% had depressive disorders.
Conclusion
The outbreak of SARS can be regarded as a mental health catastrophe. PTSD was the most prevalent long-term psychiatric condition, followed by depressive disorders. Our results highlight the need to enhance preparedness and competence of health care professionals in detecting and managing the psychological sequelae of future comparable infectious disease outbreaks.
Keywords: Severe acute respiratory syndrome, SARS survivors, Cohort, Psychiatric, Post-traumatic stress disorder
1. Introduction
Throughout history, mankind has been affected by various infectious disease epidemics. Traditional medical care tends to focus on the biological impact of such diseases, as well as on infection control. As attention has shifted from infectious diseases to other chronic medical illnesses over the past few decades, our knowledge of the psychological impact of disease outbreaks has become underdeveloped. The occurrence of severe acute respiratory syndrome (SARS) in the 21st century has rekindled concern for this neglected area.
SARS is a highly contagious disease caused by a novel coronavirus [1]. The disease spread rapidly to over 30 countries, with more than 8000 reported cases, and resulted in 774 deaths worldwide [2]. The places that were most affected included Mainland China, Hong Kong, Singapore, Taiwan and Toronto. In Hong Kong, the SARS epidemic began in March 2003. It affected up to 1755 individuals and caused 299 deaths. Understandably, SARS survivors, being the direct victims of this deadly infectious disease, were most affected. Confronted with this novel deadly infectious disease, the experience of witnessing adverse events during hospitalisation, uncertainty regarding one's prognosis and the need for ICU care all constituted a terrifying experience for SARS victims [3].
In the immediate aftermath of the SARS epidemic, psychiatric morbidity of individual patients began to emerge. The main problems involved adjustment reactions with increased anxiety levels [4], [5], [6], [7]. Studies showed that 10–35% of SARS survivors reported having features of anxiety, depression or both during the early recovery phase [3], [8], [9]. Other studies examined the features of post-traumatic stress reactions in SARS survivors in the early postdischarge period [3], [10]. A series of studies using the Chinese version of the Structured Clinical Interview for DSM-IV (SCID) revealed that 45% of the respondents had at least one active diagnosable psychiatric disorder at 2 to 4 weeks after discharge [11]. Persistent psychopathologies such as major depression (23.6%), adjustment disorder (8.1%) and posttraumatic stress disorder (PTSD) (7.3%) were also noted at 6 months postdischarge [12]. However, the reliability, validity and generalisation of the above studies are limited by low response rates of 28% to 65% [3], [8], [9], [10], [11], [12], the sole reliance on self-administered instruments [3], [5], [6], [8], [9], [10] and the use of convenience sampling methods.
In order to better define the long-term psychiatric complications among SARS survivors, we conducted the present study at 30 months after the SARS outbreak, using a more reliable and valid instrument. The results of this study may contribute to a better understanding of the pattern and course of the long-term psychiatric outcomes of SARS. It may also raise awareness of the possible psychiatric impacts that a future reemergence of SARS or other similar infectious disease outbreak could have.
2. Methods
2.1. Study design
A retrospective cohort design was implemented, and SARS-related exposure was ascertained from past medical records. Between September 2005 and March 2006, psychiatric outcome was assessed approximately 30 months post-SARS. The 30-month window began at the time when Hong Kong was removed from the SARS list by the WHO (June 2003). The study was reviewed and approved by the Hong Kong Hospital Authority Research Ethics Committee.
2.2. Participants
The study was conducted in the United Christian Hospital (UCH), a general hospital in a densely populated district in Hong Kong that serves a population of 0.6 million people from diverse socioeconomic backgrounds. Under the follow-up policy of the hospital, the medical department continues to follow up all the SARS patients on a regular basis, rendering it a suitable place to identify eligible study subjects.
Criteria for inclusion were as follows: a history of SARS infection according to WHO criteria [13], [14], hospitalisation at UCH for the index SARS infection, Chinese race and age ≥18 years at the time of the SARS infection. Excluded were those patients with severe communication problems (e.g., deafness, dementia, mental retardation). Patients who received treatment for SARS infection in other hospitals and were transferred back to UCH for follow-up were also excluded because the management involved might vary and because they were likely to be a biased group of patients with few complications.
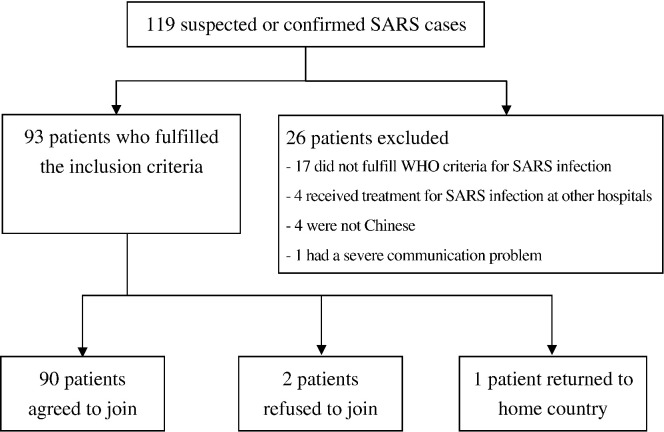
Since April 2003, 119 adult patients had been followed up at UCH after being treated for suspected or confirmed SARS infection. Of these patients, 17 failed to fulfill WHO criteria for SARS infection, four had received SARS infection treatment in other hospitals, four were of Filipino ethnicity and one had a history of a cerebrovascular accident with severe dysphasia and communication problems.
Of the remaining 93 eligible subjects, two refused to participate and one returned to his home country. Thus, the final cohort consisted of 90 subjects, yielding a response rate of 96.8% (Fig. 1 ). The subjects were relatively young (mean age=41.1 years, S.D.=12.1), with a high percentage of females (62.2%). Subjects were also relatively well educated, with 80% having completed secondary school or higher. Twenty-seven (30%) of the subjects were health care workers (HCWs). HCWs in the sample consisted of nursing staff (n=15, 55.6%), health care assistants (n=6, 22.2%) and doctors (n=4, 14.8%). More than two-thirds of all subjects (68.9%) were either cohabiting or married at the time of the SARS outbreak; five subjects' spouses died of SARS, and one subject divorced after the SARS outbreak. Throughout the SARS convalescence period, the rate of unemployment increased markedly from 3.3% to 14.4%, and the rate of retirement from 0 to 4.4%. Among HCWs, 22% were still on intermittent or continuous sick leave at the time of this study, and 7.4% had applied for early retirement.
Fig. 1.
Sample recruitment.
2.3. Procedures and measures
The Chinese version of the Structured Clinical Interview for DSM-IV (SCID) was first administered by our research psychiatrist (IM), who had completed standard training in the use of this instrument. The SCID [15] is a clinician-administered, semistructured interview used for diagnosing major Axis I DSM-IV diagnoses [16]. The output of the SCID is a record of the presence or absence of each of the disorders being considered, for both the current episode (past month) and lifetime occurrence. Because of the difficulty in making a reliable diagnosis for general anxiety disorder and adjustment disorders, the SCID allows only the current diagnoses of these two disorders to be made. So et al. [17], [18], [19] translated the patient edition of the SCID into a bilingual Chinese/English version for use in Chinese (Cantonese)-speaking subjects. Interrater reliability among SCID raters as well as concurrent validity as measured via the relationship between rater and clinician diagnoses is high (kappa for inter-rater reliability=0.84, rater-clinician scores=0.77) [17], [18], [19].
Self-rated scales, including the Chinese version of the Impact of Event Scale-Revised (IES-R) and the Hospital Anxiety and Depression Scale (HADS), were then administered. The Chinese IES-R [20], [21] involves the intrusion, avoidance and hyper-arousal domains to parallel the DSM-IV criteria for PTSD [16]. Concurrent validity has been demonstrated by the moderate to strong relationship between the IES-R and the subscales of the PTSD Checklist (Pearson's correlation between 0.62 and 0.83, with P<.001) [21]. A mean subscale score of 2, representing a moderate level of distress, was chosen as the cut-off point [20], [22]. The Chinese HADS [23], [24] consists of two independent scores that are calculated for anxiety and depression. Concurrent validity has been demonstrated by the close correlation with both the Hamilton Rating Scale of Depression and Hamilton Rating Scale of Anxiety (Pearson's coefficient=0.67 and 0.63, respectively; P<.001) [25]. A score of 11 was used as the cut-off point for each subscale to reflect a moderate level of distress [24]. The scale was previously shown to be a useful screening instrument in SARS patients during both the acute and recovery stages [11].
The MOS 36-Item Short Form (SF-36) [26] was chosen as the functional outcome assessment in this study. It is a self-administered, generic-multidimensional measure of health-related quality of life [27]. The Chinese version has been validated, and norms have been established for the Hong Kong Chinese population [28], [29].
3. Results
Based on the data provided from the Hospital Authority of Hong Kong, there were 1394 SARS survivors in Hong Kong at the time of our study. No statistically significant differences concerning the mean age, gender distribution and proportion of HCWs were found between our sample and the SARS survivor population in Hong Kong (Table 1 ).
Table 1.
Background characteristics of the UCH study sample and the population of Hong Kong SARS survivors
| HK SARS survivor population (N=1394) | UCH Sample (n=90) | P | |
|---|---|---|---|
| Mean age | 40.3±15.9 | 41.1±12.1 | .554a |
| Sex (female) | 829 (59.5%) | 56 (62.2%) | .596b |
| Health care workers | 377 (27%) | 27 (30%) | .242b |
Values are mean±S.D or n (%).
Two-sided independent sample t test.
Chi-square test.
3.1. Medical and psychiatric background
Nine subjects (10%) had one or more chronic medical illnesses before being under active follow-up for SARS, and three subjects (3.3%) had distressing pain conditions before the SARS infection (Table 2 ). Only one subject was known to the psychiatric service before the SARS outbreak. However, according to the SCID criteria, six subjects had a history of psychiatric disorder before the SARS infection: five had a history of a depressive disorder and one had a history of alcoholic dependence. No subjects in our sample received a prior diagnosis of substance use disorder, anxiety disorder or PTSD. Only one subject had experienced trauma that met DSM-IV A1 and A2 criteria for PTSD [16] before SARS infection.
Table 2.
Medical and psychiatric background of subjects (n=90)
| n | % | |
|---|---|---|
| Medical background | ||
| Presence of chronic physical illness before SARS | 9 | 10 |
| Pre-SARS distressing pain condition | 3 | 3.3 |
| Family history of mental illness | 6 | 6.7 |
| Psychiatric background according to DSM-IV criteria (using SCID) | ||
| Pre-SARS depression disorder | 5 | 5.6 |
| Pre-SARS alcohol use disorder | 1 | 1.1 |
| Total pre-SARS psychiatric disorder | 6 | 6.7 |
3.2. Overall medical parameters and effects of SARS on others
The SARS-related medical parameters and sequelae are summarised in Table 3 . The duration of hospitalisation ranged from 19 to 112 days, with a median stay of 27 days.
Table 3.
Overall medical parameters and sequelae of SARS
| Overall medical parameters | |
|---|---|
| Days of hospitalisation, median (minimum–maximum) | 27 (19–112) |
| Cumulative steroid dosage (methylprednisolone equivalent in grams), mean±S.D. | 4.36±0.21 |
| Quarantine camp experience | 0 (0) |
| Desaturation during hospital stay | 29 (32.2) |
| ICU Admission | 9 (10) |
| Intubation | 5 (5.6) |
| Avascular necrosis | 24 (26.7) |
| Permanent lung fibrosis and damage | 0 (0) |
| Presence of family members infected with SARS | 31 (34.4) |
| Presence of family members who died due to SARS | 9 (10) |
| Presence of an acquaintance infected with SARS | 40 (44.4) |
| Presence of an acquaintance who died due to SARS | 26 (28.9) |
Values are shown as n (%), unless otherwise indicated.
During hospitalisation, up to one-third of patients experienced oxygen desaturation. Patients were considered to have experienced desaturation if their SaO2 was less than 90% or if they required oxygen supplementation to maintain optimal oxygen saturation. Nine subjects (10%) were admitted to the intensive care unit; of these subjects, five (5.6%) required intubation.
All SARS patient, except one, were given high doses of steroids with a mean total equivalent dose of 4.36 g of methylprednisolone. There was no permanent lung damage within this cohort. One-fourth of our subjects had varying degrees of avascular necrosis (AVN), as detected by magnetic resonance imaging. Because SARS was highly contagious, more than one-third of our subjects had one or more family members infected with SARS. Nine subjects (10%) had one or more of their family members died of SARS infection.
3.3. Pattern of psychiatric morbidity
Table 4 depicts the rate of psychiatric disorders after the SARS outbreak: (1) post-SARS cumulative incidence, (2) current prevalence 30 months post-SARS and (3) recovery rate, which is defined as having fulfilled the DSM-IV criteria for the psychiatric disorder in the post-SARS period and no longer fulfilling those diagnostic criteria in the last month.
Table 4.
Pattern of psychiatric morbidity (n=90)
| Diagnosis | Subjects with current DSM-IV psychiatric diagnoses |
Recovered subjects |
Subjects with post-SARS DSM-IV psychiatric diagnoses |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Subjects having Axis I disorder | 30 | 33.3 | 23 | 25.6 | 53 | 58.9 |
| Depressive disorder | ||||||
| Major depression | 12 | 13.3 | 28 | 31.1 | 40 | 44.4 |
| Dysthymia | 2 | 2.2 | 0 | 0 | 2 | 2.2 |
| PTSD | 23 | 25.6 | 20 | 22.2 | 43 | 47.8 |
| Anxiety disorders | ||||||
| Panic disorder | 7 | 7.8 | 5 | 5.6 | 12 | 13.3 |
| Agoraphobia | 3 | 3.3 | 3 | 3.3 | 6 | 6.6 |
| Social phobia | 1 | 1.1 | 0 | 0 | 1 | 1.1 |
| Generalised anxiety disorder | 3 | 3.3 | – | – | – | – |
| Other psychiatric disorders | ||||||
| Post-SARS psychotic symptoms | 0 | 0 | 4 | 4.4 | 4 | 4.4 |
| Alcohol- or substance-related disorders | 0 | 0 | 0 | 0 | 0 | 0 |
3.4. Current prevalence
At 30 months post-SARS, the current prevalence of psychiatric disorders over the past month was 33.3% (n=30). The most prevalent current psychiatric diagnoses were PTSD (25.6%), followed by depressive disorders (15.6%).
3.5. Current psychiatric comorbidity
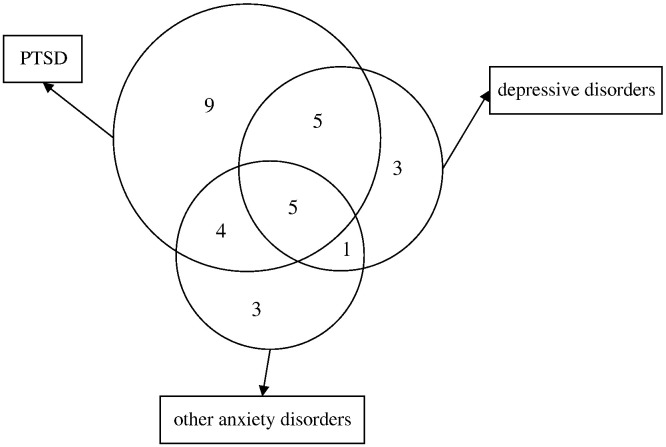
At 30 months post-SARS, 15 subjects (16.7%) had one current psychiatric diagnosis; 10 subjects (11.1%) and five subjects (5.6%) had two and three diagnoses, respectively (Fig. 2 ).
Fig. 2.
Venn diagram showing psychiatric comorbidities at 30 months post-SARS (the numbers indicate the number of subjects suffering from current psychiatric diagnoses at 30 months post-SARS; area does not indicate real proportion).
Sixty-one percent of SARS survivors who had current PTSD also suffered from other current psychiatric disorders. Ten (71%) of 14 patients currently suffering from depressive disorder and six (85.7%) of seven patients currently suffering from panic disorder also had comorbid PTSD.
3.6. Cumulative incidence
Since the SARS outbreak, the incidence of psychiatric disorders among this clinical cohort was 58.9% (n=53/90) (Table 4). Among these 53 patients, 42 (44%) patients suffered from depressive disorders (including major depression and dysthymia) and 43 (47.8%) patients suffered from PTSD at some time point after the SARS outbreak. Other disorders mainly involved the anxiety spectrum disorders, with incidences of 13.3% for panic disorder, 6.6% for agoraphobia and 1.1% for social phobia. New obsessive-compulsive disorder and substance use disorder cases were not diagnosed.
Three subjects reported transient auditory and visual hallucinations during admission for SARS treatment but did not satisfy any psychotic disorder diagnoses.
3.7. Recovery pattern
Overall, 23 (43.4%) of 53 subjects achieved recovery from post-SARS psychiatric disorders. Twenty-eight (66.7%) of the 42 subjects who suffered from depressive disorders also recovered. However, only 20 (46.5%) of the 43 PTSD patients recovered.
Table 5 illustrates the association between the longitudinal course of PTSD and coexisting disorders. For recovery from PTSD, no subjects with only post-SARS PTSD fulfilled the diagnostic criteria of PTSD at 30 months post-SARS. However, 63.9% (n=23/36) of subjects with post-SARS PTSD and other comorbid post-SARS psychiatric disorders still fulfilled the diagnostic criteria of PTSD at 30 months post-SARS.
Table 5.
Changes in diagnostic status from post-SARS to current period (n=90)
| Current psychiatric diagnoses at 30 months post-SARS |
||||||
|---|---|---|---|---|---|---|
| No current psychiatric diagnosis | PTSD Alone | PTSD and other disorders | Other disorders without PTSD | Total | ||
| Post-SARS psychiatric diagnoses | No post-SARS psychiatric disorder | 37 | 0 | 0 | 0 | 37 |
| PTSD alone | 7 | 0 | 0 | 0 | 7 | |
| PTSD and other psychiatric disorders | 8 | 9 | 14 | 5 | 36 | |
| Other disorders without PTSD | 8 | 0 | 0 | 2 | 10 | |
| Total | 60 | 9 | 14 | 7 | 90 | |
3.8. Subjective rating scales
At 30 months post-SARS, the number of participants who surpassed the IES-R subscales cut-off was 19 (21.1%) for intrusion, 18 (20%) for avoidance and 18 (20%) for the hyperarousal subscale. With the use of the HADS instrument, 14 (15.6%) subjects were classified as moderately to severely anxious, while 17 (18.9%) subjects were classified as moderately to severely depressed.
3.9. Quality of life
Table 6 compares the SF-36 score of our study population with the norm value of the Hong Kong general population [29]. Our SARS survivors showed generally poorer performance in all eight domains of the SF-36 at 30 months post-SARS.
Table 6.
Eight domains of SF-36 at 30 months post-SARS (compared to the norm of the general population)
| Quality of life | SARS Subjects (n=90) | HK Population normative values | Pa |
|---|---|---|---|
| Physical functioning | 75.17±22.77 | 91.83±12.89 | <.001⁎⁎ |
| Role limitations due to physical health | 43.54±46.39 | 82.43±30.97 | <.001⁎⁎ |
| Bodily pain | 58.74±29.98 | 83.98±21.89 | <.001⁎⁎ |
| General health | 40.18±26.58 | 55.98±20.18 | <.001⁎⁎ |
| Vitality | 48.82±22.32 | 60.27±18.65 | <.001⁎⁎ |
| Social functioning | 67.07±27.81 | 91.19±16.57 | <.001⁎⁎ |
| Role limitations due to emotional health | 51.70±46.35 | 71.66±38.36 | <.001⁎⁎ |
| Mental health | 61.62±21.57 | 72.79±16.57 | <.001⁎⁎ |
Values are mean±S.D.
Two-sided independent sample t test.
P<.001.
3.10. Subgroup analysis of HCWs
Because HCWs comprised 30% of our study sample, subgroup analysis of the pattern of psychopathology in HCWs was done. Much higher percentages of chronic PTSD were found among HCWs when compared with non-HCWs (40.7% vs. 19%; P=.031).
4. Discussion
4.1. Range of psychiatric disorders and functional outcome
In this cohort study, the post-SARS cumulative incidence of any DSM-IV diagnosable psychiatric disorder was 58.9%. The current prevalence of psychiatric disorders 30 months after SARS remained high, with up to one-third of subjects still suffering from various psychiatric diagnoses. Despite the fact that our cumulative incidence estimation was not drawn from the typical prospective longitudinal design, our figure was fairly compatible with the finding of the Prince of Wales Hospital (PWH) prospective cohort study, which found that 45% of their subjects had at least one active diagnosable psychiatric disorder at 2–4 weeks after discharge [11]. Our incidence figure was, as expected, higher than the PWH figure because the full biopsychosocial impact of SARS had not fully surfaced in the acute stage.
The cumulative incidence rate of PTSD 30 months after SARS (47.8%) can be regarded as a crude approximation of the lifetime prevalence, although underestimation may be possible. The SARS outbreak has created a range of psychiatric conditions similar to that created by other traumatic events [30]. PTSD, depressive disorders and other anxiety spectrum disorders (i.e., panic disorder, agoraphobia, generalised anxiety disorder and social phobia) were detected. The high rates of psychiatric morbidities can also be shown using subjective ratings, such as the HADS and the IES-R. Among the subjects who suffered from any psychiatric conditions at 30 months post-SARS, one-half still had comorbid psychiatric diagnoses. This high level of psychiatric comorbidity was comparable to that obtained in the SARS outcome studies in the acute stage [12], as well as that reported in other trauma studies [31], [32]. Similar to evidence from the acute phase studies [33], [34], [35], this study showed that the lower quality of life seen among SARS survivors, as measured by the SF-36, persisted. Hence, the SARS outbreak in 2003 should not simply be regarded as a medical event but also as a mental health catastrophe with a response compatible to that of other major disasters [36], [37], [38]. There are several factors that account for the high rate of psychiatric morbidities. Patients were confronted with a highly contagious novel virus that required compulsory isolation treatment and administration of experimental drugs [39]. There were imminent threats to their life and physical health as well as fears of cross-infection to family and friends. Patients may have experienced loss if they had family members or friends killed by SARS or the threat of loss even if family members or friends were infected and then recovered [9]. As compared with other disasters (natural or man-made), the nature of this threat was extremely unpredictable because SARS was a novel virus. The duration of threat exposure was prolonged as evidenced by lengthy hospitalisations and the 3-month period that was required for our community to adequately manage the outbreak threat. Furthermore, the psychological damage may have been intensified by social support systems being immobilised by isolation and stigmatisation. The ongoing subsequent biopsychosocial challenges (i.e., functional impairment, pain, fatigue, unemployment and bereavement) may have complicated the situation further. Although the lungs are the key organ involved in SARS [40], the majority of patients have recovered lung function parameters. Though lung function was recovered, many patients were given high-dose steroid therapy, which may have lead to the subsequent complication of AVN [41]. Finally, there is emerging evidence concerning the role of neuroendocrine, neurostructural and neuroimmunological disturbances in psychiatric disorders such as depression and PTSD [32], [42], [43]. Hence, we cannot exclude the possibility that either neurobiological disturbances brought about by high-dose steroid treatment, inflammation secondary to SARS infection or the direct effect of the virus on the central nervous system caused various neuropsychological consequences [44], [45].
Contrary to findings from Western studies of traumatic events [46], [47], [48], new alcohol or substance use disorders were not observed. The use of alcohol as a means of coping may be inhibited by the biologically determined lack of tolerance to alcohol in Asian and Oriental cultures [49]. Alternatively, this maladaptive coping mechanism may be less prominent in a disaster involving a medical disease with possible long-term physical complications. The fact that alcohol consumption is one of the contributing factors to AVN may deter subjects from using alcohol for self-medication of posttraumatic stress symptoms [50]. Furthermore, our subjects could have been more health conscious given that 30% of our sample was composed of HCWs. This percentage of HCWs may have resulted in a lower risk of subsequent substance- or alcohol-related problems. Hence, there may be dynamic interactions among characteristics of the survivors, the nature of the traumatic event and the psycho-behavioural responses to a stressor.
4.2. Comparison with the acute stage psychiatric outcome studies
In contrast to the SARS cohort study in the acute stage [12], this study found that PTSD was the most prevalent condition, followed by depressive disorders. There are several possible explanations for this observation. First, PTSD may have a delayed onset, which may be missed in the acute stage [32], [47]. Second, consistent with other trauma studies [31], [51], [52], this study reported lower PTSD recovery rates compared with that of major depressive disorder. The constellation of PTSD symptoms may be more persistent than the symptoms of depressive disorders [32], [51]. Third, it is possible that depressive disorders represent a more severe form of adjustment problems that may improve gradually with time. Furthermore, the significant nonresponse rate of the acute stage studies may cause underestimation of PTSD, as nonresponse may be associated with a higher rate of PTSD [53].
4.3. Impact for HCW
In this study, a statistically higher rate of PTSD was noted among HCW subjects. This observation is consistent with the finding of higher psychiatric morbidity both in the acute and in the convalescent phase among HCWs [7], [10], [54]. HCWs were required to face various overwhelming threats, including risk of catching a disease with a potentially lethal outcome as well as being indirectly traumatised by close contact with SARS victims [12]. When the HCWs became SARS patient themselves, the sudden role reversal from a care provider to a patient may have created great adjustment challenges, frustration and feeling of helplessness [12]. Fear of stigmatisation and discrimination may have hindered HCWs from seeking proper psychiatric or psychological interventions. Consistent with another study by Maunder et al. [55], long-term occupational effects, including high rate of sick leave and early retirement, were noted among HCWs.
4.4. Clinical implications
In view of the high prevalence of psychiatric disorders, management of SARS survivors should not solely focus on infectious disease treatments. Clinicians who are responsible for following up with SARS patients should be alerted to the possible long-term psychiatric sequelae, specifically PTSD and depression. This study highlights the importance of detecting and treating psychiatric comorbidities, which may exert a great influence on recovery from psychiatric diseases. Despite the extensive long-term psychiatric morbidities, more than one-third of the subjects with persistent psychiatric problems had never received proper specialist help before. Evaluation of psychiatric problems required tactful enquiry of symptoms due to subjects' fear of stigmatisation.
The close similarity of the clinical pattern of psychiatric morbidity between SARS and other disasters suggests that knowledge of disaster response management can be applied while awaiting further research evidence on the effectiveness of psychosocial interventions.
This study illustrates that a novel deadly infectious disease like SARS can cause significant prolonged psychiatric problems. However, SARS will not be the last new infectious disease to take advantage of modern globalisation [56]. This study has highlighted that the psychological impact on survivors of any future comparable infectious disease outbreaks should not be overlooked.
In order to protect HCWs from possible long-term psychiatric impact, the health authority should enhance preparedness with constant risk communication to HCWs. Psychological support of HCWs with self-coping strategies should be provided in a timely manner in order to strengthen resilience and capacity to mitigate fear, anxiety and stress. Mental health professionals should be more proactive and be considered part of the multidisciplinary team that manages SARS or other infectious disease outbreaks [12]. Finally, for pandemic planning, the likelihood of prolonged subjective distress and occupational difficulties in a substantial percentage of HCWs should be factored into surge capacity modelling during and after the pandemic [55]. Further longitudinal follow-up studies of SARS survivors should be conducted to evaluate the course of psychiatric consequences. Multicentre or even cross-country research should be actively encouraged so as to increase the power of research studies and explore cultural differences. The psychological effects of the disease outbreak on other at-risk populations (e.g., victims' families) and their correlation with the patient's psychological health should also be determined. The impact of the threat or actual loss of family members and friends due to the disease outbreak should be explored. In case of an unfortunate future massive outbreak, a prospective study with an early baseline and longitudinal assessment should also be adopted. Research networks with protocols and instruments that have been preselected and propositioned should be established well before the occurrence of a massive outbreak in order to avoid chaotic conditions that may pose various constraints to further studies.
4.5. Strengths and limitations
This study has played an important exploratory role in revealing the long-term psychiatric outcomes of SARS survivors. The excellent response rate has minimised sampling bias, and the use of a structured interview has enhanced diagnostic reliability.
The findings of this study, however, should be interpreted with consideration for the following methodological limitations. First, the pre-SARS data, e.g., subjects' baseline psychopathological profiles, was dependent on the subjects' own recollections. Second, recall bias might have resulted from the estimation of the cumulative incidence of psychiatric conditions in this study.
Additionally, discrimination and stigmatisation were important phenomena in the SARS population. As a result of these phenomena, patients may have been more likely to underreport their psychopathology to avoid the phenomenon of “double stigmatisation.” Conversely, litigation and compensation processes might have caused an overexaggeration of reported psychiatric symptoms. In order to minimise these potential confounding effects, the principal investigator was not involved in the clinical care or medical board assessment of the subjects before the completion of this research. We also stressed that the investigation was used solely for research and that it would not be used for any other purposes. The variables of litigation and compensation were recorded and taken into consideration in the data analysis. No statistically significant association between unresolved litigation or compensation and psychiatric morbidities was found.
In addition, the generalisation of our findings to all survivors of SARS in Hong Kong is limited. However, analysis of this study population and the population of Hong Kong SARS survivors did not reveal significant differences in their basic demographic variables. Finally, no general population or other chronic illness control groups were included for comparison.
In conclusion, this is the first study to report the longer-term psychiatric morbidity in a relatively stable SARS survivor cohort. Despite the above limitations, the results clearly showed that the SARS outbreak should be regarded as a mental health catastrophe. The pattern of longer-term psychiatric morbidity in SARS was comparable to that of other disasters. We should not forget the lessons learned through SARS, as they have taught us much concerning the management of future comparable infectious disease epidemics (e.g., avian influenza). This study highlights the need to enhance preparedness and competence of health care professionals in detecting and managing the psychological sequelae of infectious disease outbreaks. This study may also help make decisions concerning manpower forecasting, an important component of infectious disease outbreak contingency planning.
Acknowledgment
The authors would like to thank Dr. Sammy K.W. Cheng, Professor Y.K. Wing and Dr. Kitty Wu for sharing their experience in performing studies on SARS survivors; Professor William Goggins and Mr. Edward Choi for statistical advice; Dr. S.F. Hung, Dr. Alfred H.T. Pang and Dr. Y.K. Ng for useful comments on earlier drafts; and the nursing staff of the post-SARS integrated and psychiatric clinic for their assistance and support.
Footnotes
The Psychiatric–Medical Comorbidity section will focus on the prevalence and impact of psychiatric disorders in patients with chronic medical illness as well as the prevalence and impact of medical disorders in patients with chronic psychiatric illness.
References
- 1.Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. 2003. http://www.who.int/csr/sars/country/table2004_04_21/en/ Retrieved May 20, 2005 from.
- 3.Wu K.K., Chan S.K., Ma T.M. Posttraumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS) J Trauma Stress. 2005;18(1):39–42. doi: 10.1002/jts.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng S.K.W., Tsang J.S.K., Ku K.H., Wong C.W., Ng Y.K. Psychiatric complications in patients with severe acute respiratory syndrome (SARS) during the acute treatment phase: a series of 10 cases. Br J Psychiatry. 2004;184:359–360. doi: 10.1192/bjp.184.4.359. [DOI] [PubMed] [Google Scholar]
- 5.Chua S.E., Cheung V., McAlonan G.M. Stress and psychological impact on SARS patients during the outbreak. Can J Psychiatry. 2004;49:385–390. doi: 10.1177/070674370404900607. [DOI] [PubMed] [Google Scholar]
- 6.Chua S.E., Cheung V., Cheung C., McAlonan G.M., Wong J.W.S., Cheung E.P.T. Psychological effects of the SARS outbreak in Hong Kong on high-risk health care workers. Can J Psychiatry - Revue Canadienne de Psychiatrie. 2004;49(6):391–393. doi: 10.1177/070674370404900609. [DOI] [PubMed] [Google Scholar]
- 7.Cheng S.K.W., Wong C.W. Psychological intervention with sufferers from severe acute respiratory syndrome (SARS): lessons learnt from empirical findings. Clin Psychol Psychother. 2005;12(1):80–86. [Google Scholar]
- 8.Au A., Chan I., Li P., Chan J., Chan Y.H., Ng F. Correlates of psychological distress in discharged patients recovering from severe acute respiratory syndrome in Hong Kong. Int J Psychosoc Rehabil. 2004;8:41–51. [Google Scholar]
- 9.Cheng S.K.W., Wong C.W., Tsong J., Wong K.C. Psychological distress and negative appraisals in survivors of severe acute respiratory syndrome (SARS) Psychol Med. 2004;34(7):1187–1195. doi: 10.1017/s0033291704002272. [DOI] [PubMed] [Google Scholar]
- 10.Wu K.K., Chan S.K., Ma T.M. Posttraumatic stress after SARS. Emerg Infect Dis. 2005;11(8):1297–1300. doi: 10.3201/eid1108.041083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing YK, Leung CM, Kam IWK. Psychiatric morbidity in severe acute respiratory syndrome (SARS) patient during acute and early recovery stage. Submitted for publication 2004.
- 12.Wing Y.K., Ho S.M.Y. Mental health of patients infected with SARS. In: Chan J.C.K., Wong V.C.W.T., editors. Challenges of Severe Acute Respiratory Syndrome. Elsevier (Singapore) Pte Ltd; Hong Kong: 2006. p. 590. [Google Scholar]
- 13.WHO Case definition for surveillance of severe acute respiratory syndrome (SARS) 2003. http://www.who.int/csr/sars/casedefinition/en/ Retrieved July 25, 2005 from.
- 14.WHO Use of laboratory methods for SARS diagnosis. 2003. http://www.who.int/csr/sars/labmethods/en/ Retrieved July 25, 2005 from.
- 15.First M.B., Spitzer R.L., Gibbon M., William J.B.W. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. [Google Scholar]
- 16.APA . American Psychiatric Association; Washington, DC: 1994. Diagnostic and Statistical Manual of Mental Disorders, 4th edition. [Google Scholar]
- 17.So E., Kam I., Leung C.M., Chung D., Liu Z., Fong S. The Chinese bilingual SCID-I/P project: stage 1 — reliability for mood disorders and schizophrenia. Hong Kong J Psychiatry. 2003;13(1):7–18. [Google Scholar]
- 18.So E., Kam I., Leung C.M., Pang A., Lam L. The Chinese bilingual SCID-I/P project: stage 2 — reliability for anxiety disorders, adjustment disorders, and 'no diagnosis'. Hong Kong J Psychiatry. 2003;13(1):19–25. [Google Scholar]
- 19.So E., Kam I., Lam L. The Chinese bilingual SCID-I/P project: stage 3 — multi-site inter-rater reliability. Hong Kong J Psychiatry. 2005;14(3):19–26. [Google Scholar]
- 20.Weiss D., Marmar C. The Impact of Event Scale–Revised. In: Wilson J.P., Tang C.S., editors. Assessing psychological trauma and PTSD. Guildford Press; New York: 1997. pp. 399–411. [Google Scholar]
- 21.Wu K.K., Chan S.K. Psychometric properties of the Chinese version of the Impact of Event Scale–Revised. Hong Kong J Psychiatry. 2004;14(4):2–8. [Google Scholar]
- 22.Wu K.K., Chan K.S. The development of Chinese version of Impact of Event Scale–Revised (CIES-R) Soc Psychiatry Psychiatr Epidemiol. 2003;38:94–98. doi: 10.1007/s00127-003-0611-x. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond A.S., Snaith R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Leung C.M., Ho S., Kan C.S., Hung C.H., Chen C.N. Evaluation of the Chinese version of the Hospital Anxiety and Depression Scale. Int J Psychosom. 1993;40:29–34. [PubMed] [Google Scholar]
- 25.Leung C.M., Wing Y.K., Kwong P.K., Shum A.L.o.K. Validation of the Chinese-Cantonese version of the Hospital Anxiety and Depression Scale and comparison with the Hamilton Rating Scale of Depression. Acta Psychiatr Scand. 1999;100:456–461. doi: 10.1111/j.1600-0447.1999.tb10897.x. [DOI] [PubMed] [Google Scholar]
- 26.Ware J.E., Sherbourne C.D. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 27.Ware J.E., Grandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51:903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 28.Lam C.L.K., Grandek B., Ren X.S., Chan M.S. Tests of scaling assumptions and construct validity of the Chinese (HK) version of the SF-36 Health Survey. J Clin Epidemiol. 1998;51:1139–1147. doi: 10.1016/s0895-4356(98)00105-x. [DOI] [PubMed] [Google Scholar]
- 29.Lam C.L.K., Lauder I.J., Lam T.P., Gandek B. Population based norming of the Chinese (HK) version of the SF-36 health survey. Hong Kong Pract. 1999;21:460–470. [Google Scholar]
- 30.Moreau C., Zisook S. Rationale for a posttraumatic stress spectrum disorder. Psychiatr Clin North Am. 2002;25(4):775–790. doi: 10.1016/s0193-953x(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 31.Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 32.Foa E.B., Stein D.J., McFarlane A.C. Symptomatology and psychopathology of mental health problems after disaster. J Clin Psychiatry. 2006;67(Suppl 2):15–25. [PubMed] [Google Scholar]
- 33.Hui D.S., Wong K.T., Ko F.W., Tam L.S., Chan D.P., Woo J. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arroliga A.C., Diaz-Guzman E., Wiedemann H.P. Severe acute respiratory syndrome, pulmonary function tests, and quality of life: lessons learned [comment] Chest. 2005;128(3):1088–1089. doi: 10.1378/chest.128.3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau H.M.C., Lee E.W.C., Wong C.N.C., Ng G.Y.F., Jones A.Y.M., Hui D.S.C. The impact of severe acute respiratory syndrome on the physical profile and quality of life. Arch Phys Med Rehabil. 2005;86(6):1134–1140. doi: 10.1016/j.apmr.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.North C.S., Kawasaki A., Spitznagel E.L., Hong B.A. The course of PTSD, major depression, substance abuse and somatization after a natural disaster. J Nerv Ment Dis. 2004;192:823–829. doi: 10.1097/01.nmd.0000146911.52616.22. [DOI] [PubMed] [Google Scholar]
- 37.Wickrama K.A.S., Kaspar V. Family context of mental health risk in Tsunami-exposed adolescents: findings from a pilot study in Sri Lanka. Soc Sci Med. 2007;64(3):713–723. doi: 10.1016/j.socscimed.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 38.Hollifield M., Hewage C., Gunawardena C.N., Kodituwakku P., Bopagoda K., Weerarathnege K. Symptoms and coping in Sri Lanka 20-21 months after the 2004 tsunami. Br J Psychiatry. 2008;192(1):39–44. doi: 10.1192/bjp.bp.107.038422. [DOI] [PubMed] [Google Scholar]
- 39.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu C.M., Leung Y.Y., Hui J.Y.H., Hung I.F.N., Chan V.L., Leung W.S. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J. 2004;23(6):802–804. doi: 10.1183/09031936.04.00096404. [DOI] [PubMed] [Google Scholar]
- 41.Griffith J.F., Antonio G.E., Kumta S.M. Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology. 2005;235(1):168–175. doi: 10.1148/radiol.2351040100. [DOI] [PubMed] [Google Scholar]
- 42.Lau W.M., Qiu G., Helmeste D.M., Lee T.M.C., Tang S.W., So K.F. Corticosteroid decreases subventricular zone cell proliferation, which could be reversed by paroxetine. Restor Neurol Neurosci. 2007;25(1):17–23. [PubMed] [Google Scholar]
- 43.Leonard B.E. Inflammation, depression and dementia: Are they connected? Neurochem Res. 2007;32(10):1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 44.Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10(2):342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheng B., Cheng S.K.W., Lau K.K., Li H.L., Chan E.L.Y. The effects of disease severity, use of corticosteroids and social factors on neuropsychiatric complaints in severe acute respiratory syndrome (SARS) patients at acute and convalescent phases. Eur Psychiatry: J Assoc Eur Psychiatrists. 2005;20(3):236–242. doi: 10.1016/j.eurpsy.2004.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chilcoat H.D., Breslau N. Posttraumatic stress disorder and drug disorders: testing causal pathways. Arch Gen Psychiatry. 1998;55(10):913–917. doi: 10.1001/archpsyc.55.10.913. [DOI] [PubMed] [Google Scholar]
- 47.McFarlane A.C. Posttraumatic stress disorder: a model of the longitudinal course and the role of the risk factors. J Clin Psychiatry. 2000;61:15–20. [PubMed] [Google Scholar]
- 48.Finch E. Social and transcultural aspects of substance misuse. Curr Opin Psychiatry. 2001;14(3):173–177. [Google Scholar]
- 49.Harada S., Argarwal D.P. Distribution of ALDH I deficiency and alcohol consumption. Japanese J Alcohol Stud Drug Depend. 1985;20:234–235. [Google Scholar]
- 50.Chiu P.K.Y., Chiu K.H., Griffith J.F., Leong L.L.Y. Post-SARS avascular necrosis. In: Chan J.C.K., Wong V.C.W.T., editors. Challenges of Severe Acute Respiratory Syndrome. Elsevier(Singapore) Pte Ltd; Hong Kong: 2006. p. 590. [Google Scholar]
- 51.O'Donnell M.L., Creamer M., Pattison P. Posttraumatic stress disorder and depression following trauma: understanding comorbidity. Am J Psychiatry. 2004;161:1390–1396. doi: 10.1176/appi.ajp.161.8.1390. [DOI] [PubMed] [Google Scholar]
- 52.McFarlane A.C., Papay P. Multiple diagnoses in posttraumatic stress disorder in the victims of a natural disaster. J Nerv Ment Dis. 1992;180(8):498–504. doi: 10.1097/00005053-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Weisaeth L. Importance of high response rates in traumatic stress research. Acta Psychiatr Scand, Suppl. 1989;355:131–137. [PubMed] [Google Scholar]
- 54.Tam W.C., Pang P.F., Lam C.W., Chiu F.K. Severe acute respiratory syndrome (SARS) in Hong Kong in 2003: stress and psychological impact among frontline healthcare workers. Psychol Med. 2004;34:1197–1204. doi: 10.1017/s0033291704002247. [DOI] [PubMed] [Google Scholar]
- 55.Maunder R.G., Lancee W.J., Balderson K.E., Bennett J.P., Borgundvaag B., Evans S. Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerg Infect Dis. 2006;12(12):1924–1932. doi: 10.3201/eid1212.060584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong S.S.Y., Yuen K.Y. Avian influenza virus infections in humans. Chest. 2006;129(1):156–168. doi: 10.1378/chest.129.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]