Abstract
The development of potent cholesterol-reducing medications in the last decade of the twentieth century has altered the approach to prevention and treatment of cardiovascular disease (CVD). Initial experience with statins, and more recently with the addition of PCSK9 inhibitors, has proven that human CVD, like that in animal models, can be halted and regressed. Available clinical data show that the lower the achieved level of low-density lipoprotein cholesterol, the greater the regression of disease. Investigative studies are now aimed to understand those factors that both accelerate and impede this healing process. Some of these are likely to be modifiable, and the future of atherosclerotic CVD treatment is likely to be early screening, use of measures to repair atherosclerotic arteries, and prevention of most CVD events.
Keywords: cholesterol, coronary artery disease, myocardial infarction, statin
INTRODUCTION
Although the description of atherosclerosis as a disease associated with excess lipid, primarily cholesterol accumulation within the artery, traces back to the nineteenth century, our understanding that this disease can be cured dates to the mid-twentieth century. Studies in animals and in occasional patients described the reduction of atherosclerosis and the opening of partially occluded arteries with manipulations that markedly reduced circulating levels of cholesterol-containing lipoproteins (1).
More recently, potent cholesterol-reducing medications and the development of improved noninvasive methods to assess vascular disease have confirmed that it is possible to cure, or at least reduce, atherosclerosis. To determine the mechanisms for this, investigative studies first required an animal model that would develop high circulating levels of cholesterol and atherosclerotic lesions. Rats do not develop high levels of cholesterol when their dietary cholesterol is markedly increased; this is because the rat liver reduces its cholesterol biosynthesis (2). In contrast, cholesterolfed rabbits develop atherosclerosis, in part due to a relative deficiency of hepatic lipase (3), the final enzyme in chylomicron and VLDL (very-low-density lipoprotein) metabolism. Regression was first illustrated in this model when investigators showed that a change back to a standard rabbit diet reduced cholesterol-rich arterial plaques (4). Subsequently, studies in monkeys and pigs (1) confirmed the bidirectional changes in atherosclerotic plaque size associated with changes in blood cholesterol (Figure 1). Studies in rabbits also illustrated that the size and/or the composition of lipoproteins was critical for atherosclerosis development. This was accidentally discovered in an investigation of the relationship between atherosclerosis and diabetes; diabetic rabbits have reduced disease despite increased circulating cholesterol and triglyceride levels (5). The reason for this is that the circulating lipoproteins, primarily chylomicrons, are too large to enter the arterial wall (6).
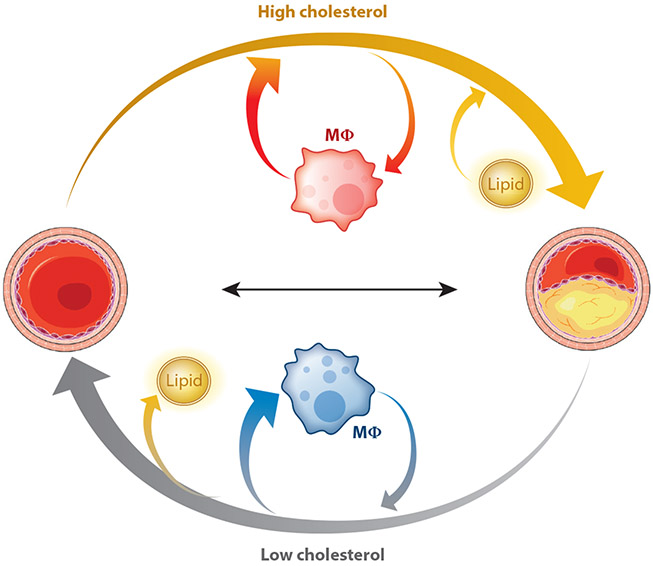
Figure 1.
Cholesterol effects on atherosclerotic lesion biology. Hypercholesterolemia, found in the circulation of most adults in the western world, leads to lipid collection within the arterial wall (yellow arrow). This promotes or is accompanied by the influx of inflammatory macrophages (indicated in red). But atherosclerosis is reversible (gray arrow). Marked reductions in cholesterol reduce the lipid content of the atherosclerotic plaque. Repair also requires the influx of alternatively activated or reparative macrophages (shown in blue) and an increase in arterial collagen. A more stable lesion results, which in humans translates to a reduction in acute clinical events.
Mice can be genetically altered to lack apolipoprotein (Apo)E, which is required for clearance of partially metabolized (remnant) lipoproteins; to lack the low-density lipoprotein receptor (LDLr); or to overexpress ApoB. Such mice become hypercholesterolemic and develop atherosclerosis, especially when fed a diet that contains large amounts of cholesterol and saturated fat. These single genetic variations are sufficient to create atherosclerosis in animals that are otherwise atherosclerosis resistant. Thus, the only ingredient required to produce atherosclerotic lesions is an elevated level of ApoB lipoproteins.
Within the past decade, a number of methods have been developed to explore the biology of atherosclerosis regression in mice (7). Switching from a high-cholesterol to a chow diet allows regression in some models, and usually requires blood cholesterol reductions to less than 200 mg/dl. Transplant of aortic segments with lesions that have developed in hypercholesterolemic mice into mice with low (i.e., normal) cholesterol levels leads to regression. Other regression methods entail genetically reversing hypercholesterolemia (8, 9). As noted below, these experiments have defined many of the biological processes involved in normal and defective regression.
EVIDENCE FOR REGRESSION IN HUMANS
That atheroma can regress in humans has been suggested by autopsy studies after famine and in the setting of chronic wasting disease, including cancer (10-13). Regression has been subsequently confirmed by coronary angiography. As early as the mid-1960s, the first prospective, interventional study of niacin therapy demonstrated improved femoral angiograms (14). Since then, lipid-lowering therapy and intensive lifestyle changes have shown significant angiographic regression of coronary atherosclerosis. The reductions in clinical events are greater than might be predicted from the relatively small changes in lesion size (15-22), with >50% reduction in events in subjects with metabolic syndrome and >80% reduction in others (23). This surprise may be explained by the stabilization of high-risk, lipid-rich, thin-cap atheroma (vulnerable plaques), rather than significant reduction in overall plaque area. This stabilization and reversal have been demonstrated by several invasive and noninvasive imaging modalities (below), highlighting that compositional changes in plaque independent of size changes may be worthwhile to achieve.
Some studies have evaluated only the most severe proximal lesions of the major vessels (24-27), whereas others have included all lesions (28-31), comparing each lesion and/or a global change score of all lesions. As can be expected, more dramatic regression has been noted when fewer, more severe lesions were followed over time (32), which overall has made comparison of these studies challenging. Regardless, regression has been visualized by coronary angiography and has since been confirmed by other invasive and noninvasive imaging modalities as well.
Beyond angiography, intravascular ultrasound (IVUS) offers direct imaging of the artery wall, including the intima, media, and external elastic lamina, with some ability to characterize plaque composition and volume. In several large prospective randomized clinical trials of lipid-lowering therapy in patients with stable coronary artery disease, IVUS has demonstrated plaque regression as measured by percent atheroma volume (PAV) and total atheroma volume (TAV). Higher-intensity statin therapy is associated with small but significant improvements in PAV and TAV paralleling significant reductions in LDL cholesterol, particularly in the lowest LDL cholesterol levels (below 88 mg/dl) (33-36). Addition of ezetimibe to statin therapy results in significantly more regression than high-intensity statin therapy alone (37). More recently, a large randomized trial of the PCSK9 inhibitor evolocumab showed significant reductions in PAV and TAV with mean LDL cholesterol below 40 mg/dl, as compared with progression on statin therapy with mean LDL cholesterol of approximately 90 mg/dl (38). A meta-regression of 11 randomized trials including >7,000 patients showed that at a median of 18 months of follow-up, the rates of plaque volume regression were significantly associated with the incidence of myocardial infarction or revascularization but not with major adverse cardiovascular events (39). Moreover, these IVUS studies have shown evidence of greater regression as LDL cholesterol is lowered well below 70 mg/dl (40).
More limited outcomes benefit with plaque regression has also been demonstrated by carotid ultrasonography. Carotid intima media thickness is a validated measure of carotid atherosclerosis that predicts future cardiovascular events (41). Several randomized trials of lipid-lowering therapy (particularly niacin) have shown significant regression of carotid intima media thickness (41-45) with some associated reduction in clinical cardiovascular events (46); however, larger-scale studies demonstrating reduction in events are lacking.
Other imaging techniques have shown regression as well but lack outcomes data. Optical coherence tomography is a newer intravascular imaging technique that provides higher resolution than IVUS, especially in its ability to image the intima, and thus better visualizes high-risk, thin-cap fibroatheroma, at the expense of poorer definition of the external elastic lamina. To date, one prospective randomized trial has evaluated patients with angina and intermediate, lipid-rich plaque by optical coherence tomography before and after therapy with ezetimibe and fluvastatin versus fluvastatin alone. After nine months, fibrous cap thickness significantly increased in both groups, but the change in cap thickness was significantly greater in the ezetimibe–fluvastatin group, suggesting that greater LDL cholesterol reduction is associated with more favorable plaque morphology (47). Near-infrared spectroscopy is another intravascular imaging technique that can evaluate the extent of plaque lipid content, reported as the lipid-core burden index. In a small prospective randomized trial of short-term high-intensity statin therapy versus standard-of-care statin therapy of patients with multivessel coronary artery disease referred for percutaneous coronary intervention with at least one other severely obstructive lesion, median reduction in lipid-core burden index (reduction in lipid content) was greater in the high-intensity statin group (89).
Noninvasive imaging techniques have similarly demonstrated atheroma regression and favorable changes in plaque composition. Coronary commuted tomography angiography (CCTA) can visualize luminal stenosis as well as plaque composition (calcification versus noncalcification) and arterial remodeling. Noncalcified plaque with spotty calcification, a surrounding ring of high attenuation, positive remodeling, and low Hounsfield units are associated with high risk (48). In one large retrospective study of patients being evaluated for coronary artery disease, those on statin therapy had features of plaque regression with reduction in low-attenuation plaque, reduction in noncalcified plaque, and increase in calcified plaque (49). A randomized trial of lipid-lowering therapy versus placebo in HIV patients showed a significant reduction in noncalcified plaque volume and favorable remodeling of high-risk plaque features with significantly fewer low-attenuation plaques and significantly less positive remodeling in the statin group, as measured by CCTA at baseline and at one year of follow-up (50).
Other noninvasive imaging modalities have highlighted the ability to regress plaque as well. Unlike CCTA, magnetic resonance imagining (MRI) and magnetic resonance angiography (MRA) have trouble imaging coronary anatomy and plaque characteristics in detail, as motion artifact and technical difficulties reduce contrast-to-noise ratio, spatial resolution, and volumetric coverage (38). Therefore, the use of coronary MRA remains largely limited to the proximal portion of all major coronary arteries (38, 51). Imaging of plaque characteristics by MRI has been more successful than lumen analysis (51). No clinical trials to date have been completed to demonstrate coronary artery plaque regression with this technique. However, the use of MRI and MRA to visualize carotid atherosclerosis is more feasible, given that the carotid arteries are of larger caliber and stationary (38,48). Randomized trials of lipid-lowering therapy have demonstrated significant regression of carotid plaque as assessed by MRI after 18 months of therapy in terms of reduction of vessel wall area and volume (52, 53), and after two years and three years of therapy in terms of reduction in lipid-rich necrotic core (54, 55).
Both perfusion and molecular imaging with cardiac positron emission tomography (PET) have also demonstrated plaque regression. Prospective trials of intensive lifestyle intervention with and without pharmacologic therapy demonstrated reduction in size and severity of perfusion abnormalities as compared to antianginal therapy alone. Progressively more aggressive lifestyle intervention approaches were associated with fewer coronary events on follow-up (56, 57). Molecular imaging of plaque inflammation has been validated by PET with the radiotracer 18F-FDG (fluorodeoxyglucose) and has shown reduced inflammation associated with reduced clinical cardiovascular events with use of statin therapy (38).
An important clinical goal should be to use clinical imaging, reductions in plaque size, and indices of instability to predict individual response to therapy. Most vulnerable plaques likely rupture without any clinical significance; therefore, focusing on isolated lesions, e.g., the most advanced plaques, might not be predictive. In contrast, risk of cardiovascular events associates with cardiovascular risk factors, exclusive of imaging (58). Probably because of the multiplicity of lesions, most of which are nonobstructive, medical therapy would be predicted to have advantages over lesion-based therapy in patients with stable coronary artery disease. Thus, while patients with angina have more rapid symptomatic improvement with surgery or stent placement, landmark studies show that medical treatments offer similar long-term benefit (31, 59). This contrasts with the great benefit of interventional approaches during an acute event.
While marked cholesterol reduction will reduce and remodel atherosclerotic lesions in the majority of patients, it is important to identify patients who require additional approaches. Subgroup analysis of the IVUS studies has assessed a number of markers associated with increased CVD and greater inflammation. However, neither higher C-reactive protein (60) nor lipoprotein (a) (61) levels negate the benefits of reducing LDL cholesterol to induce regression. Although patients with diabetes also benefit from marked LDL reduction (62), higher on-treatment LDL levels, hypertension, hyperglycemia, and elevated triglycerides continue to contribute to atherosclerosis progression (33, 35, 62).
MOUSE MODELS AND THE BIOLOGY LEARNED FROM THEM
Animal models allow for elucidation of the pathophysiology underlying the benefits of cholesterol reduction. IVUS studies in humans suggest that cholesterol reduction leads to a rapid reduction in necrotic core and lipid infiltration of the lesions (60), and multiple methods have been developed in mouse models to reproduce this biology, obtain more detailed molecular dissection of the pathology, and identify interventions that improve or inhibit regression. These methods include the transplantation of atherosclerotic aortic segments from a hypercholesterolemic to a low-cholesterol recipient mouse (63), genetic conditional induction of defective liver production of ApoB lipoproteins (64), and transient inhibition of LDL receptors using antisense oligonucleotides (9). These studies have shown that marked cholesterol reduction leads to a rapid decrease in vascular lipid and initiates multiple inflammation-resolving programs in the immune system. For example, the inflammatory (M1) and often cholesterol-loaded macrophages can exit the lesions, and most of the remaining macrophages, which continue to be recruited from monocytes in the circulation, are required for tissue remodeling and creation of a vascular scar (65, 66). As in humans, the regressed lesion may not be altered in size, but its pathology, as reflected by cellular composition and extracellular matrix content, is markedly altered.
From these models, we can learn of many metabolic and cellular factors that prevent normal regression. Increased atherogenesis, however, due to diabetes has been difficult to reproduce in mice. One reason for this is the exacerbation of the hypercholesterolemia in ApoE−/− and LDLr−/− mouse models, which likely swamps out effects of diabetes per se. Experiments in which early lesions are assessed in non-hypercholesterolemic mice have illustrated a role of the receptor for advanced glycation endproducts (RAGE) (67) and the fatty acid metabolizing enzyme acyl CoA synthetase 1 (ACSL1) (68) in enhancing lesion formation in diabetic mice.
In contrast to the confounding effects of extreme hypercholesterolemia in diabetic mouse models of atherosclerosis progression, in regression studies a simpler setting is possible, because to initiate plaque resolution, plasma lipids are returned to normal in both the normoglycemic and hyperglycemic mice (e.g., 69). The defect in atherosclerosis regression due to diabetes is very robust, ~50% impairment (69, 70), and has been tied to hyperglycemic activation of the bone marrow (71). Defective regression is exacerbated by overexpression of the human form of the aberrant glucose metabolizing enzyme aldose reductase (72) and is ameliorated by increasing the production of ApoA-I (73), the major protein of high-density lipoprotein (HDL), which in certain contexts suppresses the proliferation of bone marrow precursors of neutrophils and monocytes (74).
ApoA-I also promotes the regression of atherosclerosis in nondiabetic mice (75). Presumably this is related to its ability to form functional HDL particles, as the lowering of ApoB-containing lipoproteins was ineffective in promoting regression when ApoA-I (and HDL) was deficient (75). Consistent with this was the finding that infusions of functional, but not dysfunctional, HDL promoted plaque regression in ApoE−/− mice (76). These types of studies emphasize the difference between HDL particle functionality and HDL cholesterol. The former is still considered clinically important as an atheroprotective factor by many, and the latter is now considered to be an inadequate marker in intervention trials, despite its inverse association with CVD in observational studies. Besides promoting cholesterol efflux from plaque macrophages, other protective properties of HDL may be promoting these cells to adopt an M2-like state, which is inflammation resolving (65), and reorganizing lipid rafts to make macrophages less responsive to inflammatory stimuli (77-79).
The effect of HDL on macrophage phenotype is a reminder of the broad role of macrophages in atherosclerosis. Historically, they have been shown to be the central cell in the formation and progression of atherosclerotic plaques, and we refer the reader to other reviews for more comprehensive information (e.g., 80-82). One highlight for the purposes of the present review is that the recruitment of CCR2+ circulating monocytes (termed LY6Chi in mice or CD14+CD16− in humans) to the plaque is a major step in atherogenesis because these cells become plaque macrophages. This explains why CCR2 inhibitors have attracted attention as anti-inflammatory agents of potential value in atherosclerosis and other inflammatory diseases (e.g., 83, 84).
What is less well appreciated is that to resolve inflammation in atherosclerotic plaques, the recruitment of CCR2+ cells is also required—even with a dramatic lowering of plasma levels of ApoB-containing lipoproteins (65). In contrast to the fate of the cells recruited in a progression environment, in regression, the LY6Chi monocytes become macrophages with M2-like properties, which then exert processes that serve to dampen inflammation (e.g., by secretion of IL-10) and to clean up dead and dying cells (by efferocytosis). There have been other inflammatory diseases (e.g., 85) in which newly recruited CCR2+ (LY6Chi) monocytes become inflammation resolving. Although these monocytes have traditionally been thought to become only M1 cells, atherosclerosis regression may be an example of the hypothesis (86) that a “burst” of inflammatory cells is required to jump start the inflammation resolution process.
A series of elegant studies (reviewed in 87) has shown the importance of efferocytosis in plaque pathology. It has been known for some time that as plaques advance, macrophage apoptosis is part of a homeostatic mechanism to regulate the population size of these cells. In early stages of atherosclerosis, apparently there are enough healthy macrophages to efferocytose the dying ones, which protects the plaque microenvironment from the release of inflammation-inciting damage-associated molecular patterns (DAMPS), prothrombotic tissue factor, and a variety of chemo and cytokines. As plaques advance, however, efferocytosis efficiency wanes, which promotes the formation and expansion of the necrotic core. Thus, by the time a regression stimulus is applied in the typical mouse models, this process of failed efferocytosis and necrotic core expansion is well entrenched. The favorable change in the plaque microenvironment from the rapid reversal of hyperlipidemia apparently allows the newly recruited LY6Chi monocytes to polarize toward the M2 state and exert robust efferocytosis activity. In the diabetic setting, both in vitro and in vivo, assumption of the M2 state by macrophages is inhibited despite lipid lowering (69, 71). This defect in macrophage biology is a likely contributor to the impaired regression in diabetic mouse models of atherosclerosis.
CONCLUSIONS
Information obtained from animal experiments and the effects of marked cholesterol reduction in humans have changed our view of the natural history of atherosclerosis. A reversal of the hypercholesterolemia that commonly develops in humans in the United States and Western Europe initiates the repair of the vessel. This process, termed regression, is responsible at least in part for the marked reduction in angina and acute events in patients with coronary artery disease. Regression involves loss of lesional lipids, but for success, there also has to be infiltration and conversion of macrophages from an inflammatory to a reparative phenotype, and increased collagen accumulation. That these processes are integral to the maximal response to the regression stimulus of lipid lowering is clear from mouse studies, in which reversal of hypercholesterolemia alone did not fully accomplish favorable plaque remodeling, especially in diabetic mice. A similar phenomenon may be reflected in the CANTOS study, which revealed that in some subjects, CVD risk reduction was observed with aggressive lipid lowering and an anti-inflammatory therapy (88). Thus, augmentation of each of these processes should reduce plaque vulnerability and reduce CVD events.
ACKNOWLEDGMENTS
Work on atherosclerosis and regression was supported by the following grants: HL092969, HL131481, HL135987, HL129433, and HL084312.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Williams KJ, Feig JE, Fisher EA. 2008. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat. Clin. Pract. Cardiovasc. Med 5:91–102 [DOI] [PubMed] [Google Scholar]
- 2.Schoenheimer R 1931. New contributions in sterol metabolism. Science 74:579–84 [DOI] [PubMed] [Google Scholar]
- 3.Clay MA, Hopkins GJ, Ehnholm CP, Barter PJ. 1989. The rabbit as an animal model of hepatic lipase deficiency. Biochim. Biophys. Acta 1002:173–81 [DOI] [PubMed] [Google Scholar]
- 4.McMillan GC, Horlick L, Duff GL. 1955. Cholesterol content of aorta in relation to severity of atherosclerosis; studies during progression and retrogression of experimental lesions. AMA Arch. Pathol 59:285–90 [PubMed] [Google Scholar]
- 5.Duff GL, McMillan GC. 1949. The effect of alloxan diabetes on experimental cholesterol atherosclerosis in the rabbit. J. Exp. Med 89:611–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordestgaard BG, Stender S, Kjeldsen K. 1988. Reduced atherogenesis in cholesterol-fed diabetic rabbits. Giant lipoproteins do not enter the arterial wall. Arteriosclerosis 8:421–28 [DOI] [PubMed] [Google Scholar]
- 7.Fisher EA. 2016. Regression of atherosclerosis: the journey from the liver to the plaque and back. Arterioscler. Thromb. Vasc. Biol 36:226–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, et al. 2011. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123:989–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu D, Hu Y, Huggins LA, Mullick AE, Graham MJ, et al. 2018. Novel reversible model of atherosclerosis and regression using oligonucleotide regulation of the LDL receptor. Circ. Res 122:560–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vartiainen I, Kanerva K. 1947. Arteriosclerosis and war-time. Ann. Med. Intern. Fenn 36:748–58 [PubMed] [Google Scholar]
- 11.Wanscher O, Clemmesen J, Nielsen A. 1951. Negative correlation between atherosclerosis and carcinoma. Br. J. Cancer 5:172–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilens SL. 1947. The resorption of arterial atheromatous deposits in wasting disease. Am. J. Pathol 23:793–804 [PMC free article] [PubMed] [Google Scholar]
- 13.Wissler RW, Vesselinovitch D. 1976. Studies of regression of advanced atherosclerosis in experimental animals and man. Ann. N. Y. Acad. Sci 275:363–78 [DOI] [PubMed] [Google Scholar]
- 14.Ost CR, Stenson S. 1967. Regression of peripheral atherosclerosis during therapy with high doses of nicotinic acid. Scand. J. Clin. Lab. Investig. Suppl 99:241–45 [PubMed] [Google Scholar]
- 15.Ornish D, Brown SE, Scherwitz LW, et al. 1990. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet 336:129–33 [DOI] [PubMed] [Google Scholar]
- 16.Ornish D, Scherwitz LW, Billings JH, et al. 1998. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 280:2001–7 [DOI] [PubMed] [Google Scholar]
- 17.Esselstyn CB Jr., Ellis SG, Medendorp SV, Crowe TD. 1995. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J. Fam. Pract 41:560–68 [PubMed] [Google Scholar]
- 18.Blankenhorn DH, Hodis HN. 1994. George Lyman Duff Memorial Lecture. Arterial imaging and atherosclerosis reversal. Arterioscler. Thromb 14:177–92 [DOI] [PubMed] [Google Scholar]
- 19.Brown BG, Zhao XQ, Sacco DE, Albers JJ. 1993. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation 87:1781–91 [DOI] [PubMed] [Google Scholar]
- 20.Stein Y, Stein O. 2001. Does therapeutic intervention achieve slowing of progression or bona fide regression of atherosclerotic lesions? Arterioscler. Thromb. Vasc. Biol 21:183–88 [DOI] [PubMed] [Google Scholar]
- 21.Kuo PT, Hayase K, Kostis JB, Moreyra AE. 1979. Use of combined diet and colestipol in long-term (7–7 1/2 years) treatment of patients with type II hyperlipoproteinemia. Circulation 59:199–211 [DOI] [PubMed] [Google Scholar]
- 22.Kuo PT. 1982. Regression, retardation of atherosclerosis progression, and collateral circulation. Their functional importance. Chest 81:3–5 [DOI] [PubMed] [Google Scholar]
- 23.Zhao XQ, Krasuski RA, Baer J, et al. 2009. Effects of combination lipid therapy on coronary stenosis progression and clinical cardiovascular events in coronary disease patients with metabolic syndrome: a combined analysis of the Familial Atherosclerosis Treatment Study (FATS), the HDL-Atherosclerosis Treatment Study (HATS), and the Armed Forces Regression Study (AFREGS). Am. J. Cardiol 104:1457–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown G, Albers JJ, Fisher LD, et al. 1990. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N. Engl. J. Med 323:1289–98 [DOI] [PubMed] [Google Scholar]
- 25.Kane JP, Malloy MJ, Ports TA, et al. 1990. Regression of coronary atherosclerosis during treatment of familial hypercholesterolemia with combined drug regimens. JAMA 264:3007–12 [PubMed] [Google Scholar]
- 26.Brensike JF, Levy RI, Kelsey SF, et al. 1984. Effects of therapy with cholestyramine on progression of coronary arteriosclerosis: results of the NHLBI Type II Coronary Intervention Study. Circulation 69:313–24 [DOI] [PubMed] [Google Scholar]
- 27.Blankenhorn DH, Nessim SA, Johnson RL, et al. 1987. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA 257:3233–40 [PubMed] [Google Scholar]
- 28.Cashin-Hemphill L, Mack WJ, Pogoda JM, et al. 1990. Beneficial effects of colestipol-niacin on coronary atherosclerosis. A 4-year follow-up. JAMA 264:3013–17 [PubMed] [Google Scholar]
- 29.Schuler G, Hambrecht R, Schlierf G, et al. 1992. Regular physical exercise and low-fat diet. Effects on progression of coronary artery disease. Circulation 86:1–11 [DOI] [PubMed] [Google Scholar]
- 30.Blankenhorn DH, Azen SP, Kramsch DM, et al. 1993. Coronary angiographic changes with lovastatin therapy. The Monitored Atherosclerosis Regression Study (MARS). Ann. Intern. Med 119:969–76 [DOI] [PubMed] [Google Scholar]
- 31.Buchwald H, Varco RL, Matts JP, et al. 1990. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N. Engl. J. Med 323:946–55 [DOI] [PubMed] [Google Scholar]
- 32.Zhao XQ, Brown BG, Hillger L, et al. 1993. Effects of intensive lipid-lowering therapy on the coronary arteries of asymptomatic subjects with elevated apolipoprotein B. Circulation 88:2744–53 [DOI] [PubMed] [Google Scholar]
- 33.Nissen SE, Tuzcu EM, Schoenhagen P, et al. 2004. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 291:1071–80 [DOI] [PubMed] [Google Scholar]
- 34.Nissen SE, Nicholls SJ, Sipahi I, et al. 2006. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 295:1556–65 [DOI] [PubMed] [Google Scholar]
- 35.Nicholls SJ, Tuzcu EM, Sipahi I, et al. 2007. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 297:499–508 [DOI] [PubMed] [Google Scholar]
- 36.Nicholls SJ, Ballantyne CM, Barter PJ, et al. 2011. Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med 365:2078–87 [DOI] [PubMed] [Google Scholar]
- 37.Tsujita K, Sugiyama S, Sumida H, et al. 2015. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J. Am. Coll. Cardiol 66:495–507 [DOI] [PubMed] [Google Scholar]
- 38.Tarkin JM, Dweck MR, Evans NR, et al. 2016. Imaging atherosclerosis. Circ. Res 118:750–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Ascenzo F, Agostoni P, Abbate A, et al. 2013. Atherosclerotic coronary plaque regression and the risk of adverse cardiovascular events: a meta-regression of randomized clinical trials. Atherosclerosis 226:178–85 [DOI] [PubMed] [Google Scholar]
- 40.Nicholls SJ, Puri R, Anderson T, et al. 2016. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 316:2373–84 [DOI] [PubMed] [Google Scholar]
- 41.Azen SP, Mack WJ, Cashin-Hemphill L, et al. 1996. Progression of coronary artery disease predicts clinical coronary events. Long-term follow-up from the Cholesterol Lowering Atherosclerosis Study. Circulation 93:34–41 [DOI] [PubMed] [Google Scholar]
- 42.Taylor AJ, Kent SM, Flaherty PJ, et al. 2002. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation 106:2055–60 [DOI] [PubMed] [Google Scholar]
- 43.Taylor AJ, Sullenberger LE, Lee HJ, et al. 2004. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 110:3512–17 [DOI] [PubMed] [Google Scholar]
- 44.Taylor AJ, Villines TC, Stanek EJ, et al. 2009. Extended-release niacin or ezetimibe and carotid intima-media thickness. N. Engl. J. Med 361:2113–22 [DOI] [PubMed] [Google Scholar]
- 45.Villines TC, Stanek EJ, Devine PJ, et al. 2010. The ARBITER 6-HALTS trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis): final results and the impact of medication adherence, dose, and treatment duration. J. Am. Coll. Cardiol 55:2721–26 [DOI] [PubMed] [Google Scholar]
- 46.Mulder HJ, Bal ET, Jukema JW, et al. 2000. Pravastatin reduces restenosis two years after percutaneous transluminal coronary angioplasty (REGRESS trial). Am. J. Cardiol 86:742–46 [DOI] [PubMed] [Google Scholar]
- 47.Habara M, Nasu K, Terashima M, et al. 2014. Impact on optical coherence tomographic coronary findings of fluvastatin alone versus fluvastatin + ezetimibe. Am. J. Cardiol 113:580–87 [DOI] [PubMed] [Google Scholar]
- 48.Sandfort V, Lima JA, Bluemke DA. 2015. Noninvasive imaging of atherosclerotic plaque progression: status of coronary computed tomography angiography. Circ. Cardiovasc. Imaging 8:e003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeb I, Li D, Nasir K, et al. 2013. Effect of statin treatment on coronary plaque progression—a serial coronary CT angiography study. Atherosclerosis 231:198–204 [DOI] [PubMed] [Google Scholar]
- 50.Lo J, Lu MT, Ihenachor EJ, et al. 2015. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2:e52–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shishikura D 2016. Noninvasive imaging modalities to visualize atherosclerotic plaques. Cardiovasc. Diagn. Ther 6:340–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corti R, Fuster V, Fayad ZA, et al. 2005. Effects of aggressive versus conventional lipid-lowering therapy by simvastatin on human atherosclerotic lesions: a prospective, randomized, double-blind trial with high-resolution magnetic resonance imaging. J. Am. Coll. Cardiol 46:106–12 [DOI] [PubMed] [Google Scholar]
- 53.Sibley CT, Vavere AL, Gottlieb I, et al. 2013. MRI-measured regression of carotid atherosclerosis induced by statins with and without niacin in a randomised controlled trial: the NIA plaque study. Heart 99:1675–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Underhill HR, Yuan C, Zhao XQ, et al. 2008. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial. Am. Heart J 155:584. [DOI] [PubMed] [Google Scholar]
- 55.Zhao XQ, Dong L, Hatsukami T, et al. 2011. MR imaging of carotid plaque composition during lipid-lowering therapy: a prospective assessment of effect and time course. JACC Cardiovasc. Imaging 4:977–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gould KL, Ornish D, Scherwitz L, et al. 1995. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA 274:894–901 [DOI] [PubMed] [Google Scholar]
- 57.Sdringola S, Nakagawa K, Nakagawa Y, et al. 2003. Combined intense lifestyle and pharmacologic lipid treatment further reduce coronary events and myocardial perfusion abnormalities compared with usualcare cholesterol-lowering drugs in coronary artery disease. J. Am. Coll. Cardiol 41:263–72 [DOI] [PubMed] [Google Scholar]
- 58.Arbab-Zadeh A, Fuster V 2015. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J. Am. Coll. Cardiol 65:846–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pitt B, Waters D, Brown WV, et al. 1999. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment investigators. N. Engl. J. Med 341:70–76 [DOI] [PubMed] [Google Scholar]
- 60.Puri R, Libby P, Nissen SE, et al. 2014. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur. Heart J. Cardiovasc. Imaging 15:380–88 [DOI] [PubMed] [Google Scholar]
- 61.Puri R, Ballantyne CM, Hoogeveen RC, et al. 2017. Lipoprotein(a) and coronary atheroma progression rates during long-term high-intensity statin therapy: insights from SATURN. Atherosclerosis 263:137–44 [DOI] [PubMed] [Google Scholar]
- 62.Stegman B, Puri R, Cho L, et al. 2014. High-intensity statin therapy alters the natural history of diabetic coronary atherosclerosis: insights from SATURN. Diabetes Care 37:3114–20 [DOI] [PubMed] [Google Scholar]
- 63.Reis ED, Li J, Fayad ZA, et al. 2001. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein E-deficient mouse in a novel transplantation model. J. Vascular Surg 34:541–47 [DOI] [PubMed] [Google Scholar]
- 64.Lieu HD, Withycombe SK, Walker Q, et al. 2003. Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation 107:1315–21 [DOI] [PubMed] [Google Scholar]
- 65.Rahman K, Vengrenyuk Y, Ramsey SA, et al. 2017. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J. Clin. Investig 127:2904–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin JD, Nishi H, Poles J, et al. 2019. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight 4:e124574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park L, Raman KG, Lee KJ, et al. 1998. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med 4:1025–31 [DOI] [PubMed] [Google Scholar]
- 68.Kanter JE, Kramer F, Barnhart S, et al. 2012. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. PNAS 109:E715–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parathath S, Grauer L, Huang LS, et al. 2011. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes 60:1759–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gaudreault N, Kumar N, Olivas VR, et al. 2013. Hyperglycemia impairs atherosclerosis regression in mice. Am. J. Pathol 183:1981–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagareddy PR, Murphy AJ, Stirzaker RA, et al. 2013. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 17:695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan C, Hu J, Parathath S, et al. 2018. Human aldose reductase expression prevents atherosclerosis regression in diabetic mice. Diabetes 67:1880–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Distel E, Barrett TJ, Chung K, et al. 2014. miR33 inhibition overcomes deleterious effects of diabetes mellitus on atherosclerosis plaque regression in mice. Circ. Res 115:759–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yvan-Charvet L, Pagler T, Gautier EL, et al. 2010. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328:1689–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feig JE, Rong JX, Shamir R, et al. 2011. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. PNAS 108:7166–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hewing B, Parathath S, Barrett T, et al. 2014. Effects of native and myeloperoxidase-modified apolipoprotein A-I on reverse cholesterol transport and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol 34:779–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy AJ, Woollard KJ, Hoang A, et al. 2008. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb. Vasc. Biol 28:2071–77 [DOI] [PubMed] [Google Scholar]
- 78.Iqbal AJ, Barrett TJ, Taylor L, et al. 2016. Acute exposure to apolipoprotein A1 inhibits macrophage chemotaxis in vitro and monocyte recruitment in vivo. eLife 5:e15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sorci-Thomas MG, Owen JS, Fulp B, et al. 2012. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers. J. Lipid Res 53:1890–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glass CK, Witztum JL. 2001. Atherosclerosis: the road ahead. Cell 104:503–16 [DOI] [PubMed] [Google Scholar]
- 81.Libby P, Ridker PM, Hansson GK. 2011. Progress and challenges in translating the biology of atherosclerosis. Nature 473:317–25 [DOI] [PubMed] [Google Scholar]
- 82.Ruparelia N, Chai JT, Fisher EA, Choudhury RP. 2017. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat. Rev. Cardiol 14:133–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perez-Gomez MV, Sanchez-Nino MD, Sanz AB, et al. 2016. Targeting inflammation in diabetic kidney disease: early clinical trials. Expert Opin. Investig. Drugs 25:1045–58 [DOI] [PubMed] [Google Scholar]
- 84.Bertrand MJ, Tardif JC. 2017. Inflammation and beyond: new directions and emerging drugs for treating atherosclerosis. Expert Opin. Emerg. Drugs 22:1–26 [DOI] [PubMed] [Google Scholar]
- 85.Egawa M, Mukai K, Yoshikawa S, et al. 2013. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity 38:570–80 [DOI] [PubMed] [Google Scholar]
- 86.Nathan C, Ding A. 2010. Nonresolving inflammation. Cell 140:871–82 [DOI] [PubMed] [Google Scholar]
- 87.Tabas I 2017. 2016 Russell Ross Memorial Lecture in Vascular Biology: molecular-cellular mechanisms in the progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol 37:183–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ridker PM, Everett BM, Thuren T, et al. 2017. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med 377:1119–31 [DOI] [PubMed] [Google Scholar]
- 89.Kini AS, Baber U, Kovacic JC, et al. 2013. Changes in plaque lipid content after short-term intensive versus standard statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy). J. Am. Coll. Cardiol 62:21–29 [DOI] [PubMed] [Google Scholar]