Abstract
The complex interplay between cytokines and chemokines regulates innate and adaptive immune responses against pathogens; specifically, cytokine and chemokine expression drives activation of immune effector cells and their recruitment to tissue infection sites. Herein, we inoculated dogs with Leishmania braziliensis antigens plus saponin (the LBSap vaccine), as well as with the vaccine components, and then used real-time PCR to evaluate the kinetics of dermal expression of mRNAs of cytokines (IL-12, IFN-γ, TNF-α, IL-4, IL-13, TGF-β and IL-10) and chemokines (CCL2, CCL4, CCL5, CCL21 and CXCL8) 1, 12, 24 and 48 h after inoculation. We also evaluated the correlation between cytokine and chemokine expression and dermal cellularity. The LBSap vaccine induced high levels of IL-12 and IL-10 expression at 12 and 24 h, respectively. Furthermore, we observed positive correlations between IL-12 and IL-13 expression, IFN-γ and IL-13 expression, and IL-13 and TGF-β expression, suggesting that a mixed cytokine microenvironment developed after immunization with the vaccine. Inoculation with the saponin adjuvant alone induced a chemokine and cytokine expression profile similar to that observed in the LBSap group. CCL4 and CXCL8 chemokine expression was up regulated by the LBSap vaccine. CCL5 expression was initially highest in the LBSap group, but at 48 h, expression was highest in the LB group. Information about the kinetics of the immune response to this vaccine gained using this dog model will help to elucidate the mechanisms of and factors involved in a protective response against Leishmania infection and will aid in establishing rational approaches for the development of vaccines against canine visceral leishmaniasis.
Keywords: Canine visceral leishmaniasis, Cytokines, Chemokines, Vaccine, Saponin, Adjuvants, Real-time PCR
1. Introduction
Human visceral leishmaniasis are heterogeneous diseases caused by obligate intracellular protozoan parasites of the genus Leishmania and are transmitted by the bite of the female phlebotomine sand fly (Desjeux, 2004). Dogs infected with canine visceral leishmaniasis (CVL) constitute the main domestic reservoir and play a central role in the transmission cycle of the parasite to humans (Deane, 1995). Therefore, a canine vaccine may be the most practical and effective method for reducing the incidence of human visceral leishmaniasis, and may also provide a basis for the development of a similar vaccine for humans (Gradoni, 2001, Hommel et al., 1995, Mauel, 2002).
In the search for a potential vaccine against CVL, various approaches involving the dog model have been employed, and the use of purified fractions from parasite extracts (e.g., fucose mannose ligand antigen) (Borja-Cabrera et al., 2004, da Silva et al., 2000) and from parasite cultures (excreted/secreted antigens) (Lemesre et al., 2005, Lemesre et al., 2007) have shown particular promise. Additionally, remarkable results have been obtained following vaccination of dogs with killed parasite vaccines (Giunchetti et al., 2007, Giunchetti et al., 2008a, Lasri et al., 1999, Mayrink et al., 1996, Moreira et al., 2009, Panaro et al., 2001, Vitoriano-Souza et al., 2008, Roatt et al., 2012). These vaccines represent an excellent tool for immunoprophylaxis and control of CVL, considering their wide spectrum of antigenicity, as well as their cost and safety (Giunchetti et al., 2007, Giunchetti et al., 2008a, Lasri et al., 1999, Mayrink et al., 1996, Moreira et al., 2009, Panaro et al., 2001, Vitoriano-Souza et al., 2008). Moreover, in animals that receive saponin as an adjuvant, the major adverse reaction is minor local swelling, indicating that in dogs, overall tolerance to the candidate vaccines appears to be adequate (Giunchetti et al., 2007, Giunchetti et al., 2008a, Giunchetti et al., 2008b, Moreira et al., 2009, Vitoriano-Souza et al., 2008).
Understanding the cellular immune responses of dogs to infection with Leishmania parasites is crucial for vaccine design (Strauss-Ayali et al., 2007). Involvement of IL-10 in the immune response of Leishmania-infected dogs has not yet been established in some organs, because expression of IL-10 mRNA is not elevated in antigen-stimulated peripheral blood mononuclear cells during the course of an experimental infection or in the bone marrow of naturally infected dogs (Quinnell et al., 2001, Santos-Gomes et al., 2002). Lage et al. (2007) suggested that severity CVL also is marked by the balanced splenic production of type 1 and 2 cytokines with the predominant accumulation of IL-10 and IFN-γ as a consequence of increased parasitic load and progression of the symptoms. Furthermore, Menezes-Souza et al. (2011) showed that dogs with high skin parasitism exhibited predominantly immunoregulatory pattern of immune response characterized by increased expression of IL-10 and TGF-β.
IL-4 mRNA is expressed in the bone marrow of some symptomatic dogs (Quinnell et al., 2001), and high IL-4 expression was recently detected locally in skin lesions of L. infantum – infected dogs (Brachelente et al., 2005). Thus, these contradictory findings have been proven that the role of IL-4 in the CVL progression still unclear needing more studies to clarify their real function in the natural history of the canine disease (Menezes-Souza et al., 2011). Interferon-γ expression in antigen-stimulated peripheral blood mononuclear cells, skin lesions and bone marrow of infected dogs has been shown to be elevated (Quinnell et al., 2001, Brachelente et al., 2005, Chamizo et al., 2005, Strauss-Ayali et al., 2005). Thus, this IFN-γ increased is responsible for the main mechanism involved in protective immune response of dogs infected with L. (L.) infantum, through activation of macrophages to kill intracellular amastigotes (Vouldoukis et al., 1996).
The potential roles of chemokines in Leishmania infection include host defense functions such as leucocyte recruitment, participation in cell-mediated immunity, cell activation and anti-Leishmania activity (Rot and von Andrian, 2004). Chemokines play a vital role in the regulation of immunity to various diseases affecting the skin, including human cutaneous leishmaniasis (Gupta et al., 2011). However, the immunomodulatory processes triggered by chemokines in assessments of the immunogenicity of vaccines against CVL have not been well studied, and therefore we addressed this issue in the present study.
Successful immunization against infection by Leishmania spp. requires the participation of both innate and adaptive immune responses. The cytokine and chemokine microenvironment promotes immediate recruitment of cells that drive the immune response. In this prospective study, we investigated the kinetics of the expression of cytokine and chemokine mRNAs in the skin of dogs after immunization with Leishmania braziliensis antigens plus saponin as an adjuvant (the LBSap vaccine), as well as inoculation with the separate components of the vaccine.
2. Materials and methods
2.1. Animals
The details of the proposed study were presented to, and approved by, the Ethical Committee for the Use of Experimental Animals of the Universidade Federal de Ouro Preto, Ouro Preto, MG, Brazil. As subjects, we used 12 mongrel dogs (males and females), 8–12 months old, which were born and raised in the kennels of the Center of Animal Science, Universidade Federal de Ouro Preto. The dogs were treated with an anthelmintic and vaccinated against rabies (Tecpar, Curitiba, PR, Brazil), canine distemper, type 2 adenovirus, coronavirus, parainfluenza, parvovirus and leptospira (Vanguard HTLP 5/CV-L; Pfizer Animal Health, New York, NY, USA). Two days prior to the experiments, the dorsal area of all animals was shaved, and no local reactions were observed.
2.2. Immunization protocol
The dogs were subdivided into four groups of three dogs per group. The Sap group was inoculated with 1 mg of saponin (Sigma Chemical Co., St. Louis, MO, USA), the LB group was inoculated with 600 μg of L. braziliensis total proteins, the LBSap group was inoculated with 600 μg of L. braziliensis total proteins and 1 mg of saponin, and the S group (control) was inoculated with an equivalent volume of sterile 0.9% saline solution. Adjuvants and antigens were diluted with sterile 0.9% saline solution to a total volume of 250 μL. Following application of a general anaesthetic (Thiopentax; Cristália, Itapira, SP, Brazil; 7 mg/kg of body weight), the inoculants were intradermally injected into the shaved dorsal area of the animals. At intervals, skin biopsies were collected from the inoculation areas and stored at −80 °C until required for RNA analysis. Descriptive and kinect studies of the biopsied tissue were performed at 1, 12, 24 and 48 h. The histological analysis was performed according to Vitoriano-Souza et al. (2008). Briefly, a histological examination (morphometric analysis and leucocyte differential counting) of sections stained with haematoxylin and eosin (HE). The kinetics of cell migration was evaluated within three skin layers (outer dermis, inner dermis and hypodermis) and the predominance of cells in the inflammatory infiltrate and their distribution within the skin layers were assessed. A quantitative (morphometric) analysis of the inflammatory cells present in the skin layers was performed by acquiring digital images of pre-marked areas. Images were captured at 40 and 100× magnification using a Leica DM5000B micro-camera (Leica Microsystems-Switzerland Ltd., Heerbrugg, Switzerland) and Leica Application Suite software (version 2.4.0 R1), and analyzed with the aid of Leica QWin (V3) software. In order to identify which types of cells were recruited to the sites of different inoculations sites, the inflammatory cells (neutrophils, eosinophils, macrophages and lymphocytes) were counted and the results were expressed in percentages. Herein, correlation analysis between the cellularity and the cytokines and chemokines mRNA expression in the skin of these same animals was performed.
2.3. Extraction of total RNA and synthesis of first-strand cDNAs
Total RNA was extracted by homogenizing approximately 20 mg of skin tissue with 1 mL of TRIzol reagent (Invitrogen Brasil, São Paulo, SP, Brazil) in a rotor stator. The lysate was incubated at room temperature for 10 min, mixed with chloroform (200 μL) by tube inversion, and centrifuged at 12,000 × g for 10 min at 4 °C. The aqueous phase was collected, and RNA was extracted by means of the SV Total RNA Isolation System (Promega, Madison, WI, USA) according to the recommendations of the manufacturer. After elution, the concentration of purified total RNA was determined by measuring the OD ratio at 260/280 nm using Nanovue® (GE Healthcare, USA). To confirm the integrity of extracted RNA, electrophoresis of a fraction of each RNA sample was performed on a denaturing agarose gel and verified both the 18S and 28S ribosomal RNA (rRNA) bands. The procedure included a DNAse treatment step, and the efficiency of this step was evaluated by PCR amplification of the cDNA reaction mixture without the addition of the ThermoScript enzyme. Each quantitative PCR run was performed with two internal controls assessing both potential contamination by genomic DNA (no reverse transcriptase added) and reagent purity (no cDNA added). First-strand cDNAs were synthesized from 1.0 μg of total RNA using the ThermoScript RT-PCR System (Invitrogen Brasil, São Paulo, SP, Brasil) with oligo-dT primers according to the manufacturer's instructions.
2.4. Design of primers for gene evaluation
Primers were designed with the aid of Gene Runner (ver. 3.05, Hasting Software Inc.) using specific canine sequences obtained from GenBank (http://www.genbank.nlm.nih.gov). The sequences of the primers employed are listed in Table 1 . The primers were synthesized by Eurogentec (Southampton, UK) and reconstituted in nuclease-free water.
Table 1.
Sequences of forward (F) and reverse (R) primers used for real-time PCR quantification of mRNA expression in skin biopsies of immunized dogs.
| Gene | Primer sequence (5′–3′) | Product length (bp) | Reaction efficiency (%) | R2 | GenBank accession no. |
|---|---|---|---|---|---|
| GAPDH | |||||
| F | TTCCACGGCACAGTCAAG | 115 | 99.1 | 0.996 | AB038240 |
| R | ACTCAGCACCAGCATCAC | ||||
| IL-12p40 | |||||
| F | CAGCAGAGAGGGTCAGAGTGG | 109 | 96.5 | 0.989 | U49100 |
| R | ACGACCTCGATGGGTAGGC | ||||
| IFN-γ | |||||
| F | TCAACCCCTTCTCGCCACT | 113 | 95.4 | 0.967 | AF126247 |
| R | GCTGCCTACTTGGTCCCTGA | ||||
| TNF-α | |||||
| F | CGTCCATTCTTGCCCAAAC | 94 | 97.2 | 0.983 | DQ923808 |
| R | AGCCCTGAGCCCTTAATTC | ||||
| IL-4 | |||||
| F | CACCTCCCAACTGATTCCAA | 123 | 96.9 | 0.991 | AF239917 |
| R | CTCGCTGTGAGGATGTTCAA | ||||
| IL-13 | |||||
| F | CCTCCTCAGAGCAAAGTG | 148 | 96.7 | 0.973 | AF244915 |
| R | CCCAGCACAAACAAAGAC | ||||
| IL-10 | |||||
| F | AGAACCACGACCCAGACATC | 129 | 97.1 | 0.993 | U33843 |
| R | CCACCGCCTTGCTCTTATTC | ||||
| TGF-β1 | |||||
| F | AGGATCTGGGCTGGAAGTG | 134 | 95.1 | 0.981 | L34956 |
| R | CGGGTTGTGCTGGTTGTA | ||||
| CCL2 | |||||
| F | CCTGCTGCTATACACTCA | 91 | 96.3 | 0.979 | U29653 |
| R | GCTTCTTTGGGACACTTG | ||||
| CCL4 | |||||
| F | TCCTACTGCCTGCTGCTT | 76 | 95.9 | 0.981 | AB183194 |
| R | GCTGGTCTCAAAGTAATCTGC | ||||
| CCL5 | |||||
| F | TTCTACACCAGCAGCAAG | 136 | 98.7 | 0.991 | AB098562 |
| R | TTCTACACCAGCAGCAAG | ||||
| CCL21 | |||||
| F | AGTCTGGCAAGAAGGGAAAG | 60 | 96.7 | 0.972 | AB164433 |
| R | GGGTCTGTGGCTGTTCAGT | ||||
| CXCL8 | |||||
| F | ACACTCCACACCTTTCCAT | 116 | 98.7 | 0.977 | AF048717 |
| R | GGCACACCTCATTTCCATTG | ||||
2.5. Real-time PCR
Real-time PCR was performed on an ABI Prism 7000 DNA Sequence Detection System using 10 μL of SYBR Green PCR Master Mix (PE Applied Biosystems, Foster City, CA, USA), 4 μL of 100 mM primers and 8 μL of cDNA diluted at 1:5 carried out in a final volume of 20 μL. The samples were incubated at 95 °C for 10 min and then submitted to 40 cycles of 95 °C for 15 s and 60 °C for 1 min, during which time fluorescence data were collected. The efficiency of each pair of primers was evaluated by serial dilution of cDNA according to the protocol developed by PE Applied Biosystems. To evaluate gene expression, we performed three replicate analyses, and the amount of target RNA was normalized with respect to the endogenous control (reference genes) gene GAPDH. Data were generated by means of the 2−ΔΔCt method using the mean value of the ΔCt of the control group as the calibrator (Livak and Schmittgen, 2001). PCR products were cloned with pGEM-T Easy Vector (Promega) and sequenced to check specificity using an ABI 3100 Automated Sequencer (PE Applied Biosystems) and a Dye Terminator Kit.
2.6. Statistical analysis
Statistical analyses were performed with the aid of GraphPad Prism software package (ver. 5.0, GraphPad Software, San Diego, CA, USA). The normality of the data was established using the Kolmogorov–Smirnov test. One-way analysis of variance and Tukey post tests were used to investigate differences between groups. Associations between cytokine expression and leukocytes (%) and between chemokine expression and leukocytes in the dermis of dogs were investigated using Pearson's rank correlation. In all cases, differences were considered significant when P values were ≤0.05.
3. Results
3.1. Cytokine mRNA expression profiles in the skin of dogs after immunization
Evaluation of cytokine mRNA expression is essential for improving our understanding of the protective cellular immune response induced by vaccines against canine leishmaniasis. Therefore, we evaluated the expression of IL-12, IFN-γ, TNF-α, IL-4, IL-13, TGF-β and IL-10 mRNAs in the dermis of dogs 1, 12, 24 and 48 h after inoculation with the LBSap vaccine or its components (Fig. 1 ). At 12 h, expression of IL-12 in the LBSap group was higher than that in the Sap group, and, interestingly, the expression of IL-10 was also higher in the LBSap group than in the Sap group. No significant differences in the expression of IFN-γ, TNF-α, IL-4, IL-13 or TGF-β were observed (Fig. 1).
Fig. 1.

Relative expression of mRNAs of type I (IL-12, IFN-γ and TNF-α), type II (IL-4 and IL-13) and immunoregulatory (IL-10 and TGF-β) cytokines in the dermis of dogs 1, 12, 24 and 48 h after inoculation with L. braziliensis antigens (LB group;  ), saponin (Sap group;
), saponin (Sap group;  ) or L. braziliensis antigens plus saponin (LBSap group; ■). Significant difference (p < 0.05) compared with Sap and LBSap are indicated by the letter “b”.
) or L. braziliensis antigens plus saponin (LBSap group; ■). Significant difference (p < 0.05) compared with Sap and LBSap are indicated by the letter “b”.
We carried out a detailed analysis of the correlations between type 1, type 2 and immunoregulatory cytokines (Fig. 2 ) and found that in the LB group, there was a positive correlation between IL-13 and TNF-α (p < 0.0001, r = 0.8927). In the Sap group, there were positive correlations between IL-13 and TNF-α (p = 0.0002, r = 0.8749), between IL-13 and IL-12 (p = 0.0040, r = 0.7622) and between IL-13 and IFN-γ (p = 0.0170, r = 0.6708). In the LBSap group, positive correlations were observed between IL-13 and IL-12 (p < 0.0001, r = 0.9455) and between IL-13 and IFN-γ (p = 0.0194, r = 0.6606). In the LB group, a positive correlation was observed between IL-10 and IL-4 (p = 0.0014, r = 0.8344), and in the LBSap group a positive correlation was observed between TGF-β and IL-13 (p = 0.0020, r = 0.7948).
Fig. 2.

Correlations between relative expression of (A) type I and II cytokine mRNAs and (B) type II and immunoregulatory cytokine mRNAs in the dermis of dogs inoculated with L. braziliensis antigens (LB group;  ), saponin (Sap group;
), saponin (Sap group;  ) or L. braziliensis antigens plus saponin (LBSap group; ●). Pearson's correlation indexes (r) and p-values are shown on the graphs.
) or L. braziliensis antigens plus saponin (LBSap group; ●). Pearson's correlation indexes (r) and p-values are shown on the graphs.
3.2. Chemokine mRNA expression profiles in the skin of dogs after immunization
We evaluated the expression of the mRNAs of CCL2, CCL4, CCL5, CCL21 and CXCL8 (IL-8) chemokines 1, 12, 24, and 48 h after inoculation the LBSap vaccine or its individual components (Fig. 3 ). At 24 h after inoculation, expression of CCL4 mRNA in the LBSap group was 11.6 times that in the LB group (p < 0.05). At 1 h after inoculation, CCL5 expression was significantly upregulated in the LBSap group in comparison with expression in the LB and Sap groups (4.0-fold and 6.8-fold, respectively; p < 0.05). At 12 and 24 h after inoculation, CCL5 expression in the LB group was higher than that in the Sap group (28.2-fold and 72.1-fold, respectively; p < 0.05). Also, 48 h after inoculation, we observed increases in expression of these chemokine (CCL5) in the LB group relative to expression in the Sap and LBSap groups (6.9-fold and 5.6-fold, respectively; p < 0.05). In addition, CXCL8 expression was significantly higher in the LBSap group than in the LB and Sap groups 24 h (13.9-fold and 6.4-fold, respectively; p < 0.05) and 48 h after inoculation (13.6-fold and 4.7-fold, respectively; p < 0.05). There were no significant differences in expression of CCL2 and CCL21 between the groups.
Fig. 3.
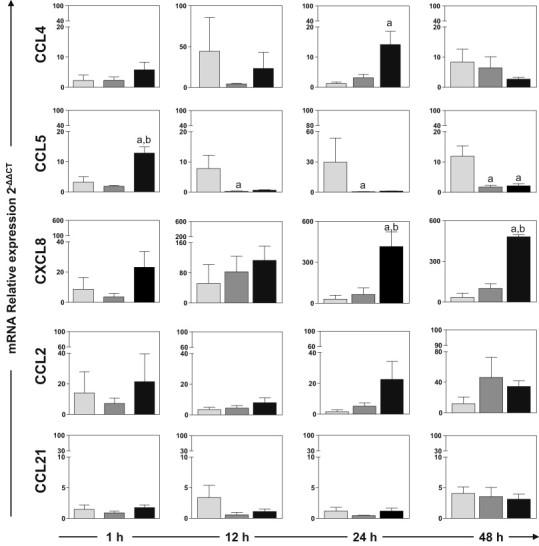
Relative expression of mRNA of chemokines CCL2, CCL4, CCL5, CCL21 and CXCL8 in the dermis of dogs 1, 12, 24 and 48 h after inoculation with L. braziliensis antigens (LB group;  ), saponin (Sap group;
), saponin (Sap group;  ) or L. braziliensis antigens plus saponin (LBSap group; ■). Significant differences (p < 0.05) compared with LB, Sap and LBSap are indicated by the letters “a,” “b” and “c,” respectively.
) or L. braziliensis antigens plus saponin (LBSap group; ■). Significant differences (p < 0.05) compared with LB, Sap and LBSap are indicated by the letters “a,” “b” and “c,” respectively.
3.3. Correlation of inflammatory cells in the outer and inner dermis with cytokine and chemokine expression
We analyzed the correlation between cytokine and chemokine expression and the cellularity of the outer and inner dermis (Fig. 4 ). We observed important correlations between chemokine and cytokine expression and cellularity in the outer dermis. In the Sap group, macrophages and CCL5 expression were positively correlated (p = 0.0005, r = 0.8917), and neutrophils and CCL5 expression were negatively correlated (p = 0.0083, r = −0.7464). In the LBSap group, neutrophils were positively correlated with IFN-γ (p = 0.0015, r = 0.8085), IL-12 (p = 0.0351, r = 0.6103) and IL-4 (p = 0.0240, r = 0.6434) expression.
Fig. 4.

Correlations between relative expression of cytokine and chemokine mRNAs and percentages of macrophages and neutrophils in the outer and inner dermis of dogs after inoculation with L. braziliensis antigens (LB group;  ), saponin (Sap group;
), saponin (Sap group;  ) or L. braziliensis antigens plus saponin (LBSap group; ●). Pearson's correlation indexes (r) and p-values are shown on the graphs.
) or L. braziliensis antigens plus saponin (LBSap group; ●). Pearson's correlation indexes (r) and p-values are shown on the graphs.
In the inner dermis, we observed a positive correlation between neutrophils and IL-13 (p = 0.0479, r = 0.5803) in the LB group. As in the outer dermis, there was a negative correlation between CCL5 and neutrophils (p = 0.0030, r = −0.8020) in the Sap group. In the LBSap group, we observed positive correlations between neutrophils and IL-12 (p = 0.0138, r = 0.6859) and between TNF-α (p = 0.0040, r = 0.7615) and IL-13 (p = 0.0125, r = 0.6930), as well as a negative correlation between lymphocytes and CCL5 (p = 0.0345, r = −0.6117).
4. Discussion
The search for new immunoprophylactics against CVL should focus on prospective antigens capable of intervening in the immune system in such a way as to induce a specific immunological events related to parasite elimination. The development of immunity against infectious agents such as Leishmania spp. requires the concerted action of components of both the innate and the adaptive immune systems (Teixeira et al., 2005). The differential regulation of type 1 and type 2 responses and the association of Th cytokines and immune function are important to study the adaptive immune response to Leishmania infections (Menezes-Souza et al., 2011).
In a previously published study, we demonstrated that both the LBSap vaccine and the saponin adjuvant alone induce intense cell migration in the skin of inoculated dogs, thus triggering the initial immunogenic events (Vitoriano-Souza et al., 2008). The intense expression of inducible NO synthase and selective recruitment of neutrophils to the skin of animals immunized with Sap and LBSap are similar to the reactions observed in animals that exhibit resistance to Leishmania infection (Vitoriano-Souza et al., 2008). Based on these results it is possible to hypothesize that neutrophils function at the front line of the immune system, responding immediately upon request, and direct the migration of other cells to the inoculation area some hours afterwards (macrophages and lymphocytes). It appears, therefore, that neutrophils participate effectively in the adaptive immune response from the very beginning of the process (Vitoriano-Souza et al., 2008). In this sense, the investigation of the kinetics of cell migration to the inoculation area is extremely relevant since the number and types of cells recruited immediately after inoculation will stimulate the innate immune system and will influence the development of acquired immunity.
To expand our investigation of the immune response triggered by the components of the LBSap vaccine, we evaluated expression of cytokine and chemokine mRNAs in the skin of dogs, seeking biomarkers of immunogenicity in the context of the innate immune response. We carried out a kinetics study of the relative expression of the mRNAs of type I, II and immunoregulatory cytokines and chemokines induced by the vaccine and its separate components at the site of inoculation after 1, 12, 24 and 48 h. Additionally, we evaluated the correlations between cytokine and chemokine mRNA expression and cellularity in the outer and inner dermis.
We analyzed expression of IL-12, IFN-γ, TNF-α, IL-4, IL-13, TGF-β and IL-10 mRNAs in the dermis of dogs immunized with the LBSap vaccine and found that expression of IL-12 and IL-10 mRNAs at 12 and 24 h, respectively, was higher in the LBSap group than in the Sap group. Moreover, we observed positive correlations between expression of the mRNAs of type I (IL-12, IFN-γ, TNF-α) and type II cytokines (especially IL-13) in the groups inoculated with the separate vaccine components and with the LBSap vaccine itself. These results suggest that after administration of each inocula, a microenvironment of mixed type I and II cytokines develops in the skin of animals. Cytokines play a decisive role in the regulation of the immune response directed against L. infantum, determining the prevention or progression of the infection (Carrillo and Moreno, 2009). Several studies have demonstrated that a mixed cytokine pattern can be associated with resistance or susceptibility in both vaccine and Leishmania-infection models (Raziuddin and Abdalla, 1994, Peruhype-Magalhaes et al., 2006). In murine leishmaniasis, several researchers have observed that IL-13 synthesis promotes initial IFN-γ production and influences the assembly and maturation of tissue granuloma. However, such experiments have not addressed the mechanism(s) by which IL-13 regulates the expression of anti-leishmanial type 1 response (Murray et al., 2006). Menezes-Souza et al. (Menezes-Souza et al., 2011) demonstrated that asymptomatic Leishmania-infected animals present high expression of IL-13 with concomitant IFN-γ production. The authors suggest that inflammatory cytokine profiles, particularly those driven by IFN-γ, TNF- α and IL-13, could be used as biomarkers for asymptomatic clinical forms in CVL.
In studies of HIV infections was demonstrated effects of both IL-13 and IFN-γ on HIV-1 macrophage infections (Meylan et al., 1993, Kornbluth et al., 1989), to enhance Ag presenting function (de Waal Malefyt et al., 1993, Marshall et al., 1997), and to be associated with improved disease status (increased CD4 or decreased HIV viral load) suggest a contribution by these cytokines in maintaining immune function following HIV-1 infection (Robert et al., 1999).
In this study, we observed increased expression of some chemokines in the skin of dogs inoculated with the various vaccine components. Is important to note that chemokines produced at the site of infection are critical in determining the cells that infiltrate the site and thus in defining the eventual outcome of the disease (Teixeira et al., 2006). In addition, Leishmania parasites also possess a chemotactic factor that selectively attracts neutrophils which can serve as host cells in the early stages of infection (van Zandbergen et al., 2002).
Our data showed increased expression of CCL4 mRNA in the LBSap group 24 h after inoculation. In addition, significant upregulation of CCL5 mRNA expression was observed 1 h after inoculation in the LBSap group. At the other biopsy times, the levels of CCL4 and CCL5 mRNAs were higher in the LB group than in the other groups. These data demonstrate the ability of the LBSap vaccine to induce the production of chemokines (CCL4 and CCL5), which are important in the recruitment of immune cells to the inoculation site. These chemokines preferentially recruit monocytes to the skin, suggesting a host strategy for controlling parasitism during ongoing CVL infection (Menezes-Souza et al., 2011). In addition, the expression of CCL5 may be related to resistance to infection by Leishmania following the cascade of events leading to parasite control during L. major infection (Santiago et al., 2004).
In this study, we observed higher levels of CXCL8 expression in the skin of dogs vaccinated with the LBSap vaccine than in the dogs in the other groups. In the first few hours of Leishmania infection, CXCL8 production amplifies the recruitment of neutrophils to the infection site (van Zandbergen et al., 2002, Badolato et al., 1996, Venuprasad et al., 2002). Neutrophils represent the first group of leukocytes to arrive at the site of infection, and they are thought to serve as Trojan horses for eventual entry of into macrophages, the ultimate cellular host for Leishmania. However, the initial influx of neutrophils seems to be beneficial for Leishmania survival in the infected tissue (van Zandbergen et al., 2004).
The interactions of cytokines and chemokines with various cells in the skin layers after immunization are important for understanding the mechanisms by which adaptive immunity against Leishmania infection is activated. Our correlation analysis between cell types and chemokine expression in the outer dermis showed a positive correlation between macrophages and CCL5 and a negative correlation between neutrophils and CCL5 in the Sap group. These data confirm the role of CCL5 in the recruitment of macrophages and other monocytes. In the LBSap group, neutrophils were positively correlated with increased expression of IFN-γ, IL-12 and IL-4 mRNAs, and a mixed profile of cytokines was observed in the skin. In the inner dermis, neutrophils and IL-13 expression were positively correlated in the LB group. As was the case in the outer dermis, CCL5 expression and neutrophils were negatively correlated in the Sap group. In the LBSap group, neutrophils were positively correlated with expression of IL-12, TNF-α and IL-13 mRNAs.
The development of immunity depends on the migration of appropriate cell populations to infected sites, which in turn depends on the expression of cytokines and chemokines. Additionally, the knowledge of the cell profile of the local inflammatory infiltrate can provide supplementary information concerning the immune response in the microenvironment of the inoculation (Stewart et al., 1984). Taken together, our current results allow us to conclude that in the skin microenvironment of dogs immunized with LBSap vaccine, there was a mixed cytokine profile and a distinctive expression profile for CCL4, CCL5 and CXCL8 mRNAs. Our data can be expected to contribute to the establishment of a rational strategy for the development of vaccines and immunological therapies against visceral leishmaniasis.
Acknowledgements
The study was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil (PRONEX 2007, PPSUS/MS/CNPq/FAPEMIG/SES-MG CBB-APQ-00356-10). The authors wish to extend their gratitude to the staff of the kennels at the Universidade Federal de Ouro Preto for their help and dedication throughout the execution of this project. The authors are also grateful for the use of facilities at CEBIO, Universidade Federal de Minas Gerais, and Rede Mineira de Bioterismo (FAPEMIG), and for support with the provision of experimental animals.
References
- Badolato R., Sacks D.L., Savoia D., Musso T. Leishmania major: infection of human monocytes induces expression of IL-8 and MCAF. Experimental Parasitology. 1996;82:21–26. doi: 10.1006/expr.1996.0003. [DOI] [PubMed] [Google Scholar]
- Borja-Cabrera G.P., Cruz Mendes A., Paraguai de Souza E., Hashimoto Okada L.Y., de A.T.F.A., Kawasaki J.K. Effective immunotherapy against canine visceral leishmaniasis with the FML-vaccine. Vaccine. 2004;22:2234–2243. doi: 10.1016/j.vaccine.2003.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachelente C., Muller N., Doherr M.G., Sattler U., Welle M. Cutaneous leishmaniasis in naturally infected dogs is associated with a T helper-2-biased immune response. Veterinary Pathology. 2005;42:166–175. doi: 10.1354/vp.42-2-166. [DOI] [PubMed] [Google Scholar]
- Carrillo E., Moreno J. Cytokine profiles in canine visceral leishmaniasis. Veterinary Immunology and Immunopathology. 2009;128:67–70. doi: 10.1016/j.vetimm.2008.10.310. [DOI] [PubMed] [Google Scholar]
- Chamizo C., Moreno J., Alvar J. Semi-quantitative analysis of cytokine expression in asymptomatic canine leishmaniasis. Veterinary Immunology and Immunopathology. 2005;103:67–75. doi: 10.1016/j.vetimm.2004.08.010. [DOI] [PubMed] [Google Scholar]
- da Silva V.O., Borja-Cabrera G.P., Correia Pontes N.N., de Souza E.P., Luz K.G., Palatnik M. A phase III trial of efficacy of the FML-vaccine against canine kala-azar in an endemic area of Brazil (Sao Goncalo do Amaranto, RN) Vaccine. 2000;19:1082–1092. doi: 10.1016/s0264-410x(00)00339-x. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Figdor C.G., Huijbens R., Mohan-Peterson S., Bennett B., Culpepper J., Dang W., Zurawski G., de Vries J.E. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes: comparison with IL-4 and modulation by IFN-g or IL-10. Journal of Immunology. 1993;151:6370. [PubMed] [Google Scholar]
- Deane L.M., Deane M.P. Leishmaniose visceral urbana (no cão e no homem) em Sobral, Ceará. Hospital. 1995:75–87. [Google Scholar]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comparative Immunology, Microbiology and Infectious Diseases. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Giunchetti R.C., Correa-Oliveira R., Martins-Filho O.A., Teixeira-Carvalho A., Roatt B.M., de Oliveira Aguiar-Soares R.D. Immunogenicity of a killed Leishmania vaccine with saponin adjuvant in dogs. Vaccine. 2007;25:7674–7686. doi: 10.1016/j.vaccine.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunchetti R.C., Reis A.B., da Silveira-Lemos D., Martins-Filho O.A., Correa-Oliveira R., Bethony J. Antigenicity of a whole parasite vaccine as promising candidate against canine leishmaniasis. Research in Veterinary Science. 2008;85:106–112. doi: 10.1016/j.rvsc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Giunchetti R.C., Correa-Oliveira R., Martins-Filho O.A., Teixeira-Carvalho A., Roatt B.M., de Oliveira Aguiar-Soares R.D. A killed Leishmania vaccine with sand fly saliva extract and saponin adjuvant displays immunogenicity in dogs. Vaccine. 2008;26:623–638. doi: 10.1016/j.vaccine.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradoni L. An update on antileishmanial vaccine candidates and prospects for a canine Leishmania vaccine. Veterinary Parasitology. 2001;100:87–103. doi: 10.1016/s0304-4017(01)00486-1. [DOI] [PubMed] [Google Scholar]
- Gupta G., Dey R., Bhattacharyya S., Majumdar S. Role of exogenous chemokines as immunotherapeutic tool against visceral leishmaniasis. Science Against Microbial Pathogens: Communicating Current Research and Technological Advances. 2011:605–612. [Google Scholar]
- Hommel M., Jaffe C.L., Travi B., Milon G. Experimental models for leishmaniasis and for testing anti-leishmanial vaccines. Annals of Tropical Medicine and Parasitology. 1995;89(Suppl. 1):55–73. doi: 10.1080/00034983.1995.11813015. [DOI] [PubMed] [Google Scholar]
- Kornbluth R.S., Oh P.S., Munis J.R., Cleveland P.H., Richman D.D. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. Journal of Experimental Medicine. 1989;169:1137. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage R.S., Oliveira G.C., Busek S.U., Guerra L.L., Giunchetti R.C., Correa-Oliveira R. Analysis of the cytokine profile in spleen cells from dogs naturally infected by Leishmania chagasi. Veterinary Immunology and Immunopathology. 2007;115:135–145. doi: 10.1016/j.vetimm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Lasri S., Sahibi H., Sadak A., Jaffe C.L., Rhalem A. Immune responses in vaccinated dogs with autoclaved Leishmania major promastigotes. Veterinary Research. 1999;30:441–449. [PubMed] [Google Scholar]
- Lemesre J.L., Holzmuller P., Cavaleyra M., Goncalves R.B., Hottin G., Papierok G. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine. 2005;23:2825–2840. doi: 10.1016/j.vaccine.2004.11.061. [DOI] [PubMed] [Google Scholar]
- Lemesre J.L., Holzmuller P., Goncalves R.B., Bourdoiseau G., Hugnet C., Cavaleyra M. Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: double-blind randomised efficacy field trial. Vaccine. 2007;25:4223–4234. doi: 10.1016/j.vaccine.2007.02.083. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marshall J.D., Robertson S.E., Trinchieri G., Chehimi J. Priming with IL-4 and IL-13 during HIV-1 infection restores in vitro IL-12 production by mononuclear cells of HIV-infected patients. Journal of Immunology. 1997;159:5705. [PubMed] [Google Scholar]
- Mauel J. Vaccination against Leishmania infections. Current Drug Targets: Immune, Endocrine and Metabolic Disorders. 2002;2:201–226. doi: 10.2174/1568008023340631. [DOI] [PubMed] [Google Scholar]
- Mayrink W., Genaro O., Silva J.C., da Costa R.T., Tafuri W.L., Toledo V.P. Phase I and II open clinical trials of a vaccine against Leishmania chagasi infections in dogs. Memorias do Instituto Oswaldo Cruz. 1996;91:695–697. doi: 10.1590/s0074-02761996000600006. [DOI] [PubMed] [Google Scholar]
- Menezes-Souza D., Correa-Oliveira R., Guerra-Sa R., Giunchetti R.C., Teixeira-Carvalho A., Martins-Filho O.A. Cytokine and transcription factor profiles in the skin of dogs naturally infected by Leishmania (Leishmania) chagasi presenting distinct cutaneous parasite density and clinical status. Veterinary Parasitology. 2011;177:39–49. doi: 10.1016/j.vetpar.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Meylan P.R., Spina C.A., Richman D.D., Kornbluth R.S. In vitro differentiation of monocytoid THP-1 cells affects their permissiveness for HIV strains: a model system for studying the cellular basis of HIV differential tropism. Virology. 1993;193:256–267. doi: 10.1006/viro.1993.1121. [DOI] [PubMed] [Google Scholar]
- Moreira N., Giunchetti R.C., Carneiro C.M., Vitoriano-Souza J., Roatt B.M., Malaquias L.C. Histological study of cell migration in the dermis of hamsters after immunisation with two different vaccines against visceral leishmaniasis. Veterinary Immunology and Immunopathology. 2009;128:418–424. doi: 10.1016/j.vetimm.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Murray H.W., Tsai C.W., Liu J., Ma X. Visceral Leishmania donovani infection in interleukin-13−/− mice. Infection and Immunity. 2006;74:2487–2490. doi: 10.1128/IAI.74.4.2487-2490.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaro M.A., Acquafredda A., Lisi S., Lofrumento D.D., Mitolo V., Sisto M. Nitric oxide production by macrophages of dogs vaccinated with killed Leishmania infantum promastigotes. Comparative Immunology, Microbiology and Infectious Diseases. 2001;24:187–195. doi: 10.1016/s0147-9571(00)00026-6. [DOI] [PubMed] [Google Scholar]
- Peruhype-Magalhaes V., Martins-Filho O.A., Prata A., Silva Lde A., Rabello A., Teixeira-Carvalho A. Mixed inflammatory/regulatory cytokine profile marked by simultaneous raise of interferon-gamma and interleukin-10 and low frequency of tumour necrosis factor-alpha(+) monocytes are hallmarks of active human visceral Leishmaniasis due to Leishmania chagasi infection. Clinical and Experimental Immunology. 2006;146:124–132. doi: 10.1111/j.1365-2249.2006.03171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnell R.J., Courtenay O., Shaw M.A., Day M.J., Garcez L.M., Dye C. Tissue cytokine responses in canine visceral leishmaniasis. Journal of Infectious Diseases. 2001;183:1421–1424. doi: 10.1086/319869. [DOI] [PubMed] [Google Scholar]
- Raziuddin S., Abdalla R.E., el-Awad E.H., al-Janadi M. Immunoregulatory and proinflammatory cytokine production in visceral and cutaneous leishmaniasis. Journal of Infectious Diseases. 1994;170:1037–1040. doi: 10.1093/infdis/170.4.1037. [DOI] [PubMed] [Google Scholar]
- Roatt B.M., Aguiar-Soares R.D., Vitoriano-Souza J., Coura-Vital W., Braga S.L., Correa-Oliveira R. Performance of LBSap vaccine after intradermal challenge with L. infantum and saliva of Lu. longipalpis: immunogenicity and parasitological evaluation. PLoS ONE. 2012;7:e49780. doi: 10.1371/journal.pone.0049780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert T., Bailer A.H., Sun Junwei, Joseph B. Margolick, Martin Melissa, Kostman Jay, Montaner Luis J. IL-13 and IFN-g secretion by activated T cells in HIV-1 infection associated with viral suppression and a lack of disease progression. The Journal of Immunology. 1999:7534–7542. [PubMed] [Google Scholar]
- Rot A., von Andrian U.H. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annual Review of Immunology. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- Santiago H.C., Oliveira C.F., Santiago L., Ferraz F.O., de Souza D.G., de-Freitas L.A. Involvement of the chemokine RANTES (CCL5) in resistance to experimental infection with Leishmania major. Infection and Immunity. 2004;72:4918–4923. doi: 10.1128/IAI.72.8.4918-4923.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Gomes G.M., Rosa R., Leandro C., Cortes S., Romao P., Silveira H. Cytokine expression during the outcome of canine experimental infection by Leishmania infantum. Veterinary Immunology and Immunopathology. 2002;88:21–30. doi: 10.1016/s0165-2427(02)00134-4. [DOI] [PubMed] [Google Scholar]
- Stewart R.J., Holloway L.J., Isbister W.H. Peritoneal neutrophilia: a potential indicator of the surgical acute abdomen. Australian and New Zealand Journal of Surgery. 1984;54:565–568. doi: 10.1111/j.1445-2197.1984.tb05447.x. [DOI] [PubMed] [Google Scholar]
- Strauss-Ayali D., Baneth G., Shor S., Okano F., Jaffe C.L. Interleukin-12 augments a Th1-type immune response manifested as lymphocyte proliferation and interferon gamma production in Leishmania infantum-infected dogs. International Journal for Parasitology. 2005;35:63–73. doi: 10.1016/j.ijpara.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Strauss-Ayali D., Baneth G., Jaffe C.L. Splenic immune responses during canine visceral leishmaniasis. Veterinary Research. 2007;38:547–564. doi: 10.1051/vetres:2007015. [DOI] [PubMed] [Google Scholar]
- Teixeira C.R., Teixeira M.J., Gomes R.B., Santos C.S., Andrade B.B., Raffaele-Netto I. Saliva from Lutzomyia longipalpis induces CC chemokine ligand 2/monocyte chemoattractant protein-1 expression and macrophage recruitment. Journal of Immunology. 2005;175:8346–8353. doi: 10.4049/jimmunol.175.12.8346. [DOI] [PubMed] [Google Scholar]
- Teixeira M.J., Teixeira C.R., Andrade B.B., Barral-Netto M., Barral A. Chemokines in host–parasite interactions in leishmaniasis. Trends in Parasitology. 2006;22:32–40. doi: 10.1016/j.pt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- van Zandbergen G., Hermann N., Laufs H., Solbach W., Laskay T. Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infection and Immunity. 2002;70:4177–4184. doi: 10.1128/IAI.70.8.4177-4184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zandbergen G., Klinger M., Mueller A., Dannenberg S., Gebert A., Solbach W. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. Journal of Immunology. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- Venuprasad K., Banerjee P.P., Chattopadhyay S., Sharma S., Pal S., Parab P.B. Human neutrophil-expressed CD28 interacts with macrophage B7 to induce phosphatidylinositol 3-kinase-dependent IFN-gamma secretion and restriction of Leishmania growth. Journal of Immunology. 2002;169:920–928. doi: 10.4049/jimmunol.169.2.920. [DOI] [PubMed] [Google Scholar]
- Vitoriano-Souza J., Reis A.B., Moreira N.D., Giunchetti R.C., Correa-Oliveira R., Carneiro C.M. Kinetics of cell migration to the dermis and hypodermis in dogs vaccinated with antigenic compounds of Leishmania braziliensis plus saponin. Vaccine. 2008;26:3922–3931. doi: 10.1016/j.vaccine.2008.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouldoukis I., Drapier J.C., Nussler A.K., Tselentis Y., Da Silva O.A., Gentilini M. Canine visceral leishmaniasis: successful chemotherapy induces macrophage antileishmanial activity via the l-arginine nitric oxide pathway. Antimicrobial Agents and Chemotherapy. 1996;40:253–256. doi: 10.1128/aac.40.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]


