Abstract
In this study an in vitro assay was optimized to detect feline proliferating lymphocytes as an assessment for the cell-mediated immune response. For this purpose, 5-bromo-2′-deoxyuridine (BrdU) labeling was chosen because of its sensitivity and the possibility of further characterization of proliferating cells. The assay was optimized by selecting the best batch and concentration of fetal bovine serum, β-mercaptoethanol concentration, cell density, BrdU incubation time and antigen presenting cell type. Cats were vaccinated with the attenuated Nobivac vaccine Tricat and the peripheral blood lymphocyte proliferation responses were quantified upon in vitro restimulation with inactivated and infectious feline panleukopenia virus (FPV), feline calicivirus (FCV) and felid herpesvirus 1 (FeHV-1). Proliferation signals were detected with inactivated FeHV-1 in the CD8+ but not in the CD8− T lymphocyte population, with inactivated FCV and FPV in both CD8− and CD8+ T lymphocyte populations. Restimulation with infectious FCV caused significant proliferation in the CD8− T lymphocyte population only while infectious FPV and FeHV-1 seemed to suppress lymphocyte proliferation in both T cell populations. Additional IFN-γ quantification in the culture supernatant revealed a large correlation between the proliferation signals and IFN-γ production, indicating that BrdU labeling is a very reliable technique to assess and characterize feline lymphoproliferative responses to viral antigens in vitro.
Abbreviations: [3H] TdR, tritiated thymidine; BrdU, 5-bromo-2′-deoxyuridine; CMI, cell-mediated immune response; FCV, feline calicivirus; FeHV-1, felid herpesvirus 1; FPV, feline panleukopenia virus; FSC-A, area of forward scatter; GM-CSF, granulocyte macrophage colony-stimulating factor; mDC, monocyte-derived dendritic cells; ME, 2-mercaptoethanol; SSC-A, area of sideward scatter
Keywords: BrdU, Proliferation, Antigen-presenting cells, T lymphocytes, Cat, Immunity
1. Introduction
The most reliable and accurate way to evaluate the proliferation of T cells in response to either mitogens or antigens is to quantify DNA-synthesis (Hughes and Mehmet, 2003). Currently two methods are generally used, the DNA incorporation of either radioactive tritiated thymidine ([3H] TdR) or the fluorescently labeled thymidine analogue 5-bromo-2′-deoxyuridine (BrdU). BrdU labeling has been proven to be advantageous over [3H] thymidine labeling in many aspects. While equally sensitive, there is no need for specialized equipment and special care during handling with the fluorescent compounds. The fluorescent cells are not destroyed during the process allowing additional staining for membrane molecules (e.g. CD3 and CD8) and final analysis is done by flow cytometry (Huong et al., 1991, Motobu et al., 2002). At present, mainly [3H] TdR labeling is used for in vitro restimulation assays in cats. Therefore, FITC BrdU labeling would be an interesting alternative.
A notable problem with in vitro proliferation assays in general is the need for repeated blood collection for the generation of APC and effector cells (lymphocytes). This can be a serious drawback, especially if severely weakened infected animals are involved or if only a single blood sample can be taken. Working with frozen PBMC would present a solution to these problems.
In the present study, the proliferative responses of feline T lymphocytes, originating from vaccinated cats were determined by FITC BrdU labeling upon restimulation with both infectious and inactivated feline panleukopenia virus, feline calicivirus, and felid herpesvirus 1. A double staining for feline CD3 and CD8 was performed, to allow identification of proliferating CTL (CD8+) or CD8− T lymphocytes. All cells, both APC and lymphocytes, originated from PBMC stored in liquid nitrogen, thus bypassing the problem of repeated blood sampling. Subsequent flow cytometrical analysis allowed the specific identification of the subset of proliferating T cells. Both monocytes and monocyte-derived dendritic cells (mDC) were used as APC. Additionally, the production of IFN-γ, the signature molecule of Th1, was evaluated in the cell culture supernatant and compared with the degree of proliferation.
To the author's knowledge, this is the first time that FITC BrdU labeling was used to evaluate in vitro proliferative responses of feline T lymphocytes to viral antigens.
2. Materials and methods
2.1. Viruses and antibodies
Third passages of the belgian feline panleukopenia virus (FPV) isolate 09K444, the feline calicivirus (FCV) reference strain F9 and the belgian felid herpesvirus 1 (FeHV-1) isolate 95K270 in Crandell feline Kidney cells were used. A monoclonal antibody NZM1 against the epsilon chain of feline CD3 was kindly provided by Dr. Yorihiro Nishimura (Tokyo University, Japan) (Nishimura et al., 2004). Monoclonal antibodies recognizing feline CD1a (FE1.5F4) and CD1c (FE5.5C1) were kindly provided by Dr. Peter F. Moore (University of California, USA). Monoclonal antibody DH59B, recognizing CD172a and monoclonal antibody CAT82A, reacting with feline MHC class II, were purchased from Veterinary Medical Research and Development (VMRD, Pullman, USA). Monoclonal antibody FE5.4D2, recognizing feline CD8β and monoclonal antibody TÜK4 against CD14 were purchased from AbD serotec (Dusseldorf, Germany). Conjugated secondary antibodies [Molecular Probes (Invitrogen, Carlsbad, USA)] were goat anti-mouse IgG-R-Phycoerythrin and goat anti-mouse IgG1-Alexa Fluor 647.
2.2. Immunization of cats
Three feline coronavirus-, feline leukemia virus- and feline immunodeficiency virus-negative cats were used as blood donors. All cats were vaccinated with the attenuated Nobivac Tricat vaccine (FPV, FCV and FeHV-1) according to the manufacturer's manual (Intervet Ltd, Boxmeer, The Netherlands). Briefly, the vaccine was resuspended in 1 ml of the accompanying diluens at room temperature and administered subcutaneously. Cats were vaccinated three times, twice at arrival with a 3-week interval and a third time, 4 weeks before the first blood sampling.
2.3. Isolating, freezing and storing PBMC
Blood (10 ml) was collected from the vena jugularis in heparin (15 U ml−1) (Leo, Zaventem, Belgium) and mononuclear cells were separated on Ficoll-Paque. The blood was first diluted with 10 ml of cold phosphorus buffered salt solution (PBS), the 20 ml suspension was then brought onto 20 ml of Ficoll-Paque solution (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) at room temperature. After centrifugation at room temperature, the buffy coat layer, containing the PBMC, was extracted and a lysis step was implemented to remove any unwanted red blood cells. Cell counts typically showed 2–4 × 106 monocytes and 6–14 × 106 lymphocytes. Immediately after purification, cells were frozen and stored in liquid nitrogen (N2). Briefly, maximum 2 × 107 cells ml−1 were resuspended in RPMI supplemented with 30% FBS, 100 U ml−1 penicillin, 0.1 mg ml−1 streptomycin, and 10% DMSO. The cells were then frozen by lowering the temperature slowly with 1 °C min−1 untill −30 °C, followed by a 15 min incubation period at −30 °C and finally lowering the temperature to −150 °C at a rate of 1 °C s−1 (PTLPD81, Orthodyne, Alleur, Belgium). After freezing, cells were stored at −196 °C in liquid nitrogen.
2.4. Generation of monocytes, monocyte-derived dendritic cell and macrophages
In order to generate monocytes, stored cells were thawed and seeded at a concentration of 2 × 105 ml−1 monocytes in a 24-well dish or 96-well dish (Thermo Fisher Scientific, Waltham, MA, USA), in complete RPMI 1640 medium (Invitrogen) containing 10% FBS (Greiner Bio-one, Kremsmuenster, Germany), 100 U ml−1 penicillin, 0.1 mg ml−1streptomycin, 0.1 mg ml−1 gentamycin, 1 mM sodium pyruvate and 1% non-essential amino-acids (100×) (Invitrogen). After overnight incubation, non-adherent cells (typically 0.6–1.4 × 106 cells) were removed by washing the dishes with RPMI 1640. The non-adherent cells consisted of 59 ± 15% of T lymphocytes (as assessed by fluorescent staining with the lymphocyte marker NZM1). The adherent cells consisted of 86 ± 7% of monocytes (as assessed by fluorescent staining with the monocyte marker DH59B).
For the generation of monocyte-derived dendritic cells (mDCs), 2 × 105 ml−1 monocytes were incubated during 7 days in complete medium supplemented with 10 ng ml−1 recombinant feline IL-4 and 50 ng ml−1 recombinant feline granulocyte macrophage colony-stimulating factor (GM-CSF) (R&D systems, Minneaoplis, MN, USA). Cells were refed on days 3 and 5 before application in experiments.
For the generation of monocyte-derived MΦ, 2 × 105 ml−1 monocytes were incubated in complete medium without IL-4 and GM-CSF. Final flow cytometrical characterization occurred on day 7. Briefly, cells were detached by incubation with accutase (Sigma–Aldrich, St. Louis, MO, USA), for 10 minutes at 37 °C after which they were washed with icecold medium. Staining was performed on living cells at 4 °C using monoclonal antibodies targeting CD14, MHC II, CD1a and CD1c, followed by either R-Phycoerythrin (CD14) or Alexa Fluor 647 (MHC II, CD1a and CD1c). Isotype-matched irrelevant monoclonal antibodies were included as controls in each experiment
2.5. In vitro restimulation assay
Monocytes and generated mDC served as APC and were incubated for 1 h with several infectious viruses at a multiplicity of infection (m.o.i.) of 1: FPV, FCV, and FeHV-1. Simultaneously, the APC were incubated for 3 h with equivalent UV-inactivated amounts of each virus (180 mJ cm−2). After antigen pulsing, cells were washed and further incubated with 5 × 105 ml−1 homologous non-adherent cells stored in liquid nitrogen (N2) for 4 days at 37 °C, 5% CO2 in complete RPMI 1640 medium containing 5% FBS (Invitrogen 16000) instead of 10% and 0.05 mM 2-mercaptoethanol (ME) (Invitrogen). Cells were then stained for proliferation with the FITC BrdU flow kit according to manufacturer's instructions (Becton, Dickinson and Company, NJ, USA). Additionally, cells were stained with surface markers against CD3 and CD8 to identify the proliferating cell population. Analysis was done by flow cytometry (FACSCanto, Becton, Dickinson and Company). Data were analyzed using FACSDiva software.
2.6. Analysis of culture supernatant
IFN-γ concentrations in cell culture supernatant were determined using an ELISA specific for feline IFN-γ according to manufacturer's instructions (R&D Systems).
2.7. Statistical analysis
Statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA). For all data, differences between medians were assessed by the Mann–Whitney U test, while correlation was assessed by the Spearman correlation test. Significant differences were considered when P ≤ 0.05.
3. Results
3.1. Optimization of assay parameters
The restimulation assay setup was optimized to obtain maximal proliferation responses while minimizing the background proliferation. In all tests, monocytes were used as APC. To assess maximum responses, the mitogen ConA (Sigma–Aldrich), which induces aspecific lymphocyte proliferation was added after which cells were incubated for 3 days. To evaluate background proliferation mock cultures were incubated during 5 days. Tested parameters were: responder cell concentration [0.5–1–5 (×106 cells ml−1)], FBS concentration [0–1–2.5–5–10–20(%)], 2-mercaptoethanol concentration [0–0.05–0.25 (mM)], and BrdU incubation time [1–2–4–8 (h)]. The tested FBS batches can be found in Table 1 . Additionally, the serum free medium X-vivo 15 was also included (Lonza, Basel, Switzerland). The best results were obtained when 0.5 × 106 cells ml−1 were incubated in RPMI medium with 5% of FBS batch 16000 of Invitrogen and 0.05 mM 2-mercaptoethanol and when cells were incubated with BrdU for 4 h before staining and flowcytometric analysis. Because the need of repeated blood collection for the generation of APC and effector cells (lymphocytes) forms a well-known problem with in vitro proliferation assays, all cells used in the experiments originated from PBMC stored in liquid nitrogen.
Table 1.
Overview of different FBS batches that were tested for the in vitro restimulation assays.
| Fetal bovine serum | ||
|---|---|---|
| Manufacturer | Catalog number | Batch |
| Greiner Bio-one | 758093 | 6845093 |
| Greiner Bio-one | 758093 (antibody depleted) | 6845093 |
| Sigma | F7524 | 030M3396 |
| Sigma | F6178 | 129K0361 |
| Sigma | F2442 | 128K8423 |
| Invitrogen | 16000 | 941273 |
| Gentaur | FB-1001/50 | Batch .8460 |
| Gentaur | FB-1090/50 | Batch .8026 |
| Gentaur | FB-1090/50 | Batch .8033 |
3.2. Generation of monocytes, monocyte-derived dendritic cells and macrophages
In order to generate stronger responses, dendritic cells were generated from monocytes by culturing them with 10 ng ml−1 recombinant feline IL-4 and 50 ng ml−1 recombinant feline GM-CSF. Characterization of mDC was done by using several available monoclonal antibodies to assess four phenotypic markers on feline monocytes, MΦ and mDC: TÜK4 (CD14), CAT82A (MHCII), FE1.5F4 (CD1a), and FE5.5C1 (CD1c). Fig. 1 shows the comparison of mean fluorescence intensities of several stained cell surface antigens on feline monocytes, mDCs and MΦ. There were marked differences between monocytes, mDCs and MΦ concerning the expression of all markers. mDC stained consistently stronger than monocytes and MΦ for all markers [MFI: 16,070 ± 1717 (CD14), 4384 ± 202 (MHCII), 2427 ± 1070 (CD1a) and 3134 ± 1131 (CD1c)], while monocytes did not stain [MFI: 34 ± 6 (CD1a) and 114 ± 30 (CD1c)] or were stained 8 to 14 times less bright [MFI: 929 ± 178 (CD14) and 303 ± 13 (MHCII)] than MΦ [MFI: 12,791 ± 1013 (CD14), 2550 ± 300 (MHCII), 766 ± 707 (CD1a) and 7987 ± 515 (CD1c)]. Finally, Fig. 2 shows that the size and granularity, assessed by forward and side scatter respectively (FSC-A and SSC-A), were found to be significantly higher for mDC [linear value parameter: 115 ± 2 (FSC-A) and 37 ± 1 (SSC-A)] when compared with MФ [linear value parameter: 103 ± 3 (FSC-A) and 23 ± 3 (SSC-A)]. Fig. 3 shows the morphological analysis of MΦ (a) and mDC (b) on a phase contrast microscope. MΦ were generally tightly adherent cells characterized by a highly variable morphology and size and the presence of pseudopodia. mDC were generally non-adherent or loosely adherent cells with a round shape and had numerous fine cytoplasmic protrusions (dendrites) that are typical for dendritic cells.
Fig. 1.
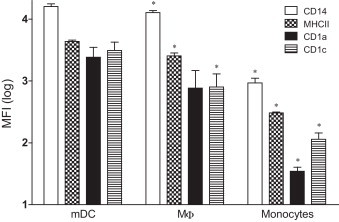
Mean fluorescence intensities (MFI) of different phenotypic markers on monocyte derived dendritic cells (mDC), MФ and monocytes. The MFI of different phenotypic markers on MФ and monocytes were compared with the markers on mDC. Results are shown as mean ± SD (n = 3). Significant differences (p ≤ 0.05) are indicated with an asterisk (*).
Fig. 2.
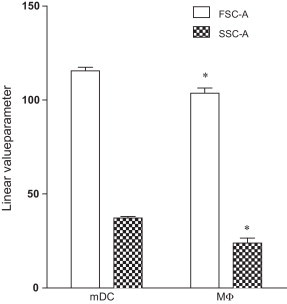
Linear values of the area of forward scatter (FSC-A) and side scatter (SSC-A) from monocyte derived dendritic cells (mDC) and MФ populations. Results are shown as mean ± SD (n = 6). Significant differences (p ≤ 0.05) between mDC and MФ, with regard to FSC-A and SSC-A are indicated with an asterisk (*).
Fig. 3.
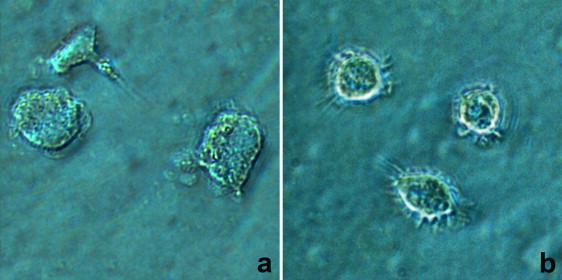
Phase contrast pictures of 7-day-old macrophages (a) and monocyte-derived dendritic cells (b). Magnification was 400×.
3.3. Proliferation response upon in vitro restimulation with monocytes as APC
First, the proliferation response was evaluated using monocytes as APC. This method is the least labor-intensive method. Fig. 4 A shows the detection of proliferating CD3+/CD8+ and CD3+/CD8− lymphocytes. In the CD8+ population, only when inactivated FeHV-1 was presented to monocytes, marginally significant proliferation compared to mock infection (P < 0.05) was observed. In the CD8− T lymphocyte population, a proliferative response could be detected only when inactivated FCV was presented. Additional IFN-γ levels were determined in cell culture supernatant; all samples fell under the detection limit (Fig. 5 ). These results indicate that even weak responses induced by monocytes, known to be poor antigen presenters, can be detected in cats using the BrdU assay.
Fig. 4.
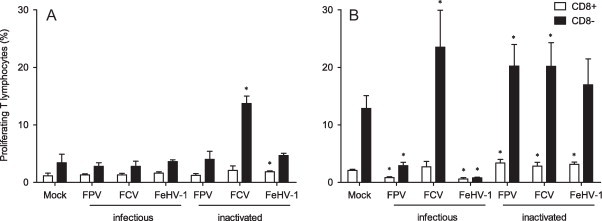
Percentage proliferating T lymphocytes in response to both infectious and inactivated feline panleukopenia virus (FPV), feline calicivirus (FCV), and felid herpes virus 1 (FeHV-1), using monocytes (A) and monocyte derived dendritic cells (B) as APC. Results are shown as mean ± SD (n = 3). Significant differences between mock-treated and virus-treated conditions (p ≤ 0.05) are indicated with an asterisk (*).
Fig. 5.
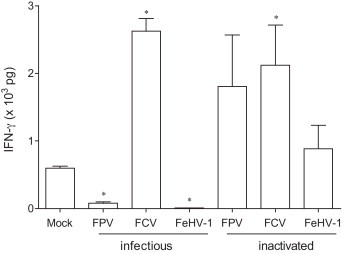
Interferon-γ (IFN-γ) concentration in culture supernatant of the in vitro restimulation assay that analyses proliferation response to infectious and inactivated and feline panleukopenia virus (FPV), feline calicivirus (FCV), and felid herpes virus 1 (FeHV-1) when monocyte derived dendritic cells (mDC) were used as APC. Results are shown as mean ± SEM (n = 3). Significant differences, when comparing each condition with the mock, (p ≤ 0.05) are indicated with an asterisk (*).
3.4. Proliferation response upon in vitro restimulation with mDC as APC
Using the mDC as more potent APC, the restimulation experiment was repeated. Fig. 4B shows the detection of proliferating CD3+/CD8+ and CD3+/CD8− lymphocytes that were in co-culture with antigen-pulsed mDC. When inactivated viruses were used as antigen stimuli, all viruses induced significant proliferation (P ≤ 0.05) in the CD8+ T lymphocyte population while only inactivated FPV and FCV induced proliferation (P ≤ 0.05) in the CD8− T lymphocyte population. Although not significant, inactivated FeHV-1 did induce some proliferation in the CD8− T lymphocyte population. When infectious virus was used, a totally different pattern was detected. Infectious FPV and FeHV-1 caused severe suppression of proliferation (P ≤ 0.05) in both the CD8+ and CD8− population while infectious FCV strongly induced proliferation in the CD8− T lymphocyte proliferation. Additionally, IFN-γ levels were determined in the cell culture supernatant (Fig. 5). Significant production was seen in the presence of both infectious and inactivated FCV while significantly reduced production was seen with infectious FPV and FeHV-1. Although the production was not significant, some IFN-γ was detected in the presence of inactivated FPV and FHC. The concentration of IFN-γ was positively correlated with the proliferation response for all the tested viruses in the CD8− (r = 0.964; p = 0.003) as well as in the CD8+ population (r = 0.679; p = 0.11).
4. Discussion
Currently, four immune parameters can be monitored by flow cytometry in order to evaluate the cell-mediated immune response (CMI): activation-proliferation, cytokine-production, cytotoxicity and receptor-detection (Sandbulte and Roth, 2004, Thiel et al., 2004). Although all parameters play a crucial role in the development of an immune response, the proliferation of antigen-specific T lymphocytes is one of the first things to occur during a CMI (Berke and Clark, 2005). It is therefore not surprising that many studies to this day use the proliferative response to assess the T cell immunological function in response to virus infections.
To our knowledge, this is the first time the BrdU staining method in combination with membrane antigen labeling has been used to evaluate the feline lymphoproliferative response to viral antigens. The first aspect in the development of the assay was to minimize the background proliferation. This phenomenon is also known in other species but felids seem to be especially prone to this (Brenchley and Douek, 2004, Raulf-Heimsoth, 2008) (personal communication, Rochelle Mikkelsen). Optimal results were obtained when 0.5 × 106 cells ml−1 were incubated in medium containing 5% FBS (Invitrogen 16000) and 0.05 mM ME and when cells were incubated with BrdU for 4 h before flowcytometric analysis. The assay was further simplified for practical use by freezing the cells from 1 blood sample in liquid nitrogen. This allowed the generation of APC and effector cells from the same sample, reducing the stress that accompanies repeated blood sampling or allowing analysis when only one blood sample is available. Like in human immunology, several precautions were taken into account in order to preserve the immunophenotypic characteristics (such as proliferation and IFN-γ production) (Bull et al., 2002, Disis et al., 2006). Cells were frozen immediately after isolation, they were kept at −196 °C for the entire storage period and viability of thawed cells was routinely 80–90%.
Proliferation assays using both infectious and inactivated virus as antigen stimuli were set up in order to evaluate if BrdU staining is applicable in cats. Additionally, CD3 and CD8 molecules, both co-receptors for the T cell receptor (TCR), were stained in the membrane of responding lymphocytes in order to characterize the proliferative response more in detail. Some viruses were selected for restimulation: FCV, FPV and FeHV-1. Infectious panleukopenia virus, typically causes severe enteritis, diarrhea and vomiting and is characterized by high mortality rates (Parrish, 1995, Lamm and Rezabek, 2008). Feline calicivirus induces mainly moderate and self-limiting acute oral and upper respiratory tract disease, is rarely fatal and resolves quickly after infection (Radford et al., 2007). Finally, felid herpesvirus 1 causes moderate to severe respiratory disease (Gaskell et al., 2007). These viruses were chosen because well-performing combined vaccines exist for these viruses and are recommended to be administered to cats (Poulet et al., 2009).
BrdU analysis of lymphocyte proliferation, using monocytes as APC revealed overall little response to any virus, with the exception of a strong reaction to inactivated FCV in the CD8− T lymphocyte population. This correlates well with earlier research where proliferation signals were detected in whole and washed feline blood cultures that were stimulated with inactivated FCV (Tham and Studdert, 1987a). However, the same research group also found proliferation signals when stimulation occurred with inactivated FPV and FeHV-1, which could not be detected in our study (Tham and Studdert, 1987b, Tham and Studdert, 1987c). This might be due to the different virus inactivation methods that were used to produce virus antigens for restimulation. Tham and Studdert, 1987a, Tham and Studdert, 1987b, Tham and Studdert, 1987c used β-propiolactone, a chemical disinfectant that preserves most of the immunogenic properties of viral proteins but is very hazardous to work with. It is nowadays mostly replaced by other chemical compounds such as binary ethyleneimine (Larghi and Nebel, 1980). In our study, UV irradiation was applied to inactivate the viruses. Although this is a promising inactivation method that has been used frequently because it mainly targets the genome, there have been reports of structural modifications of viral proteins which can have an influence on the immunogenic capacities of the inactivated virus (Miller and Plageman, 1974). Another group found significant proliferation signals in response to FeHV-1 antigens, however, their method of vaccination and restimulation, using immune stimulating complexes and purified virus protein differed significantly from our method (Martina et al., 2001). In addition, their in vitro restimulation assay involved adding exogenous IL-2 to the incubation medium which could modify the response, especially since, in a normal response, activated lymphocytes produce their own IL-2 and thus stimulate their own proliferation. Furthermore, IL-2 addition has been shown to increase non-stimulated background proliferation (Chain et al., 1987, von Baehr et al., 2001). Overall, there is little data concerning in vitro restimulation techniques in cats and there is a large diversity in used protocols. This makes a comparison of results difficult.
There are a couple of possible explanations for the lack of proliferation when adding inactivated FPV and FeHV-1. There is an inherent difference in antigenic capacity depending on the virus inactivation method, as has been found for feline parvoviruses (Churchill, 1982). Inactivated herpes viruses (e.g. Epstein–Barr virus and bovine herpes virus 1) have been found to be able to induce apoptosis in monocytes and regulate DC development (Li et al., 2002). Furthermore, when comparing proliferation responses to infectious and inactivated bovine herpesvirus 1, using DC and monocytes, the same absence of proliferation was noted when monocytes were used as APC (Renjifo et al., 1999). The authors reported that this was mainly due to a lower viability of monocytes and due to the inhibitory effects of the virion glycoprotein gD, which is also present in FeHV-1. All these facts might contribute to the absence of proliferation when adding inactivated FPV and FeHV-1. Earlier research in humans has shown that dendritic cells are much more capable of stimulating T lymphocytes in comparison to monocytes or macrophages (Steinman, 1991, Sallusto and Lanzavecchia, 1994). In order to better detect proliferation signals to virus antigens, monocyte-derived dendritic cells (mDC) were generated by adding IL-4 and GM-CSF to the culture medium and used as APC in further experiments. Surface phenotyping of feline mDC was performed through labeling, quantifying and comparing surface molecules on monocytes, macrophages and mDCs. Generally, mDC stained brightest for CD14, MHCII, CD1a, and CD1b, followed by MФ while monocytes were not or weakly stained for these surface markers. The same pattern was detected by other authors using feline mDC (Bienzle et al., 2003, Freer et al., 2005, Sprague et al., 2005) and is characteristic for the high antigen presenting capacity of mDC (Zhou and Tedder, 1996). In human immunology, mDC generally lose CD14 expression after differentiation in vitro (Sallusto and Lanzavecchia, 1994). This seems to differ from feline mDC since they stained very brightly for this glycosylphosphatidylinositol receptor. This had already been noted in earlier studies using feline mDC (Bienzle et al., 2003, Freer et al., 2005, Sprague et al., 2005) as well as porcine mDC (Carrasco et al., 2001) and mDC obtained from rhesus macaques (O’Doherty et al., 1997). These differences in CD14 expression might be attributed to the use of certain monoclonal antibodies recognizing CD14 on monocytes but not on DC or MФ, a fact that has also been found for porcine cells. In addition, even when CD14 expression is detected, there could still be a cell type-dependent functional difference of the CD14 receptor (e.g. on porcine monocytes CD14 is a functional LPS receptor while this is not the case for CD14 on porcine mDC) (Carrasco et al., 2001). Similar to the work from Freer et al. (2005), size and granularity, assessed by forward and side scatter respectively (FSC-A and SSC-A), were found to be significantly higher for mDC when compared with MФ. Finally, the morphological analysis of both mDC (non-adherent, round shaped and presence of dendrites) and MФ (tightly adherent, irregular shape and presence of pseudopodia) revealed characteristics similar to those reported in other studies on feline mDC generation (Bienzle et al., 2003, Freer et al., 2005, Sprague et al., 2005). BrdU analysis of lymphocyte proliferation, using mDCs as APC revealed proliferation signals for inactivated FeHV-1 in the CD8+ T lymphocyte population and for inactivated FCV and FPV in both the CD8− and CD8+ T lymphocytes population while infectious FCV caused significant proliferation only in the CD8− T lymphocyte population and infectious FPV and FeHV-1 seemed to suppress lymphocyte proliferation in both T cell populations. The highly pathogenic panleukopenia virus and the immune evasive felid herpesvirus both caused a severe suppression of proliferation during in vitro restimulation. This suppression of proliferation might be caused by certain immunosuppressive mechanisms of the viruses (e.g. down regulation of MHC-I expression with FeHV-1 (Montagnaro et al., 2009), suppression of proliferation after in vivo infection with FPV (Schultz et al., 1976)) or the death of infected cells (80% cell death after FeHV-1 infection in monocytes, results not shown). The fact that inactivated FeHV-1 and FPV do not seem to suppress proliferation indicates that none of the structural proteins are involved but that active infection needs to be present to elicit the immunosuppressive mechanisms.
Another aspect frequently investigated in veterinary immunology is the cytokine production in antigen-stimulated cell cultures (Sandbulte and Roth, 2004). In the complex realm of cytokines, interferons and especially IFN-γ are key molecules in the protection against viruses. IFN-γ is the signature molecule of the Th1 response, has antiviral capacities and is in the adaptive CMI response mainly produced by CD4+ T helper cell (Th1) and CD8+ CTL (Sen, 2001, Katze et al., 2002, Schroder et al., 2004). In order to verify the signals detected by the BrdU labeling method, IFN-γ was detected in the supernatant of the proliferation assay using a sandwich ELISA specific for the detection of feline IFN-γ. IFN-γ-levels were below the detection limit when monocytes were used as APC (results not shown). This absence of production together with the proliferation signal detected with infectious FCV might indicate that a Th2 response rather than a Th1 response is mounted. This hypothesis however, would need to be verified through the detection of typical Th2 cytokines such as IL-4 and IL-13. Another interpretation might be the higher sensitivity of the BrdU labeling in comparison with the IFN-γ ELISA for the detection of Th1 biased CMI. If mDC were used however, there was a large similarity between detected proliferation signals and detected IFN-γ levels, indicating that, when using mDC as APC, the BrdU method is suitable for the in vitro evaluation of CMI responses to viruses.
5. Conclusion
In conclusion, this study demonstrates the suitability of the BrdU labeling technique to detect and characterize specific T lymphocyte proliferative responses to both infectious and inactivated viruses using N2-stored cells from vaccinated cats for the first time. The use of mDC as APC is recommended since only low responses were detected when monocytes were used as APC. The obtained results were verified via the detection of IFN-γ in culture supernatant. In this study, the BrdU labeling method was used to evaluate and describe proliferative responses to FPV, FCV and FeHV-1, some of the most common viral infections in cats. In the future this will be extended to CMI-studies infected cats and other major feline pathogens such as feline infectious peritonitis virus (FIPV) and feline immunodeficiency virus (FIV). Further, this method could be implemented in a bioassay-tool to evaluate CMI responses to other vaccines or to analyze the effect of drugs and other health-promoting products on CMI responses.
Acknowledgments
This work was funded by a Ph.D. grant of the Agency for Innovation by Science and Technology (IWT). The authors would like to express their gratitude to Ytse Noppe for excellent technical assistance and to Sarah Costers and Leslie Bosseler for scientific input and critical reading of the manuscript.
Contributor Information
Ben L. Vermeulen, Email: Ben.Vermeulen@ugent.be.
Hans J. Nauwynck, Email: Hans.Nauwynck@ugent.be.
References
- Berke G., Clark W.R. Springer; Dordrecht: 2005. Killer Lymphocytes. 361 pp. [Google Scholar]
- Bienzle D., Reggeti F., Clark M.E., Chow C. Immunophenotype and functional properties of feline dendritic cells derived from blood and bone marrow. Vet. Immunol. Immunopathol. 2003;96:19–30. doi: 10.1016/s0165-2427(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Brenchley J.M., Douek D.C. Flow cytometric analysis of human antigen-specific T-cell proliferation. Methods Cell Biol. 2004;75:481–496. doi: 10.1016/s0091-679x(04)75019-0. [DOI] [PubMed] [Google Scholar]
- Bull M.E., Gebhard D.G., Tompkins W.A.F., Kennedy-Stoskopf S. Polymorphic expression in the CD8 alpha chain surface receptor of African lions (Panthera leo) Vet. Immunol. Immunopathol. 2002;84:181–189. doi: 10.1016/s0165-2427(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Carrasco C.P., Rigden R.C., Schaffner R., Gerber H., Neuhaus V., Inumaru S., Takamatsu H., Bertoni G., McCullough K.C., Summerfield A. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology. 2001;104:175–184. doi: 10.1046/j.0019-2805.2001.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chain B., Mccafferty I., Wallace G., Askenase P.W. Improvement of the invitro T-cell proliferation assay by a modified method that separates the antigen recognition and Il-2-dependent steps. J. Immunol. Methods. 1987;99:221–228. doi: 10.1016/0022-1759(87)90131-1. [DOI] [PubMed] [Google Scholar]
- Churchill A.E. A potency test for inactivated small animal parvovirus vaccines using chicks. J. Biol. Stand. 1982;10:1–8. doi: 10.1016/s0092-1157(82)80042-5. [DOI] [PubMed] [Google Scholar]
- Disis M.L., dela Rosa C., Goodell V., Kuan L.Y., Chang J.C.C., Kuus-Reichel K., Clay T.M., Lyerly H.K., Bhatia S., Ghanekar S.A., Maino V.C., Maecker H.T. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J. Immunol. Methods. 2006;308:13–18. doi: 10.1016/j.jim.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Freer G., Matteucci D., Mazzetti P., Lozzacco L., Bendinelli M. Generation of feline dendritic cells derived from peripheral blood monocytes for in vivo use. Clin. Diagn. Lab. Immunol. 2005;12:1202–1208. doi: 10.1128/CDLI.12.10.1202-1208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell R., Dawson S., Radford A., Thiry E. Feline herpesvirus. Vet. Res. 2007;38:337–354. doi: 10.1051/vetres:2006063. [DOI] [PubMed] [Google Scholar]
- Hughes D., Mehmet H. BIOS Scientific Publishers; Oxford: 2003. Cell Proliferation & Apoptosis. xvii, 373 pp. [Google Scholar]
- Huong P.L.T., Kolk A.H.J., Eggelte T.A., Verstijnen C.P.H.J., Gilis H., Hendriks J.T. Measurement of antigen specific lymphocyte-proliferation using 5-bromo-deoxyuridine incorporation. J. Immunol. Methods. 1991;140:243–248. doi: 10.1016/0022-1759(91)90377-r. [DOI] [PubMed] [Google Scholar]
- Katze M.G., He Y., Gale M., Jr. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- Lamm C.G., Rezabek G.B. Parvovirus infection in domestic companion animals. Vet. Clin. N. Am. Small Anim. Pract. 2008;38:837–850. doi: 10.1016/j.cvsm.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Larghi O.P., Nebel A.E. Rabies virus inactivation by binary ethylenimine – new method for inactivated vaccine production. J. Clin. Microbiol. 1980;11:120–122. doi: 10.1128/jcm.11.2.120-122.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.Q., Liu D.R., Hutt-Fletcher L., Morgan A., Masucci M.G., Levitsky V. Epstein–Barr virus inhibits the development of dendritic cells by promoting apoptosis of their monocyte precursors in the presence of granulocyte macrophage-colony-stimulating factor and interleukin-4. Blood. 2002;99:3725–3734. doi: 10.1182/blood.v99.10.3725. [DOI] [PubMed] [Google Scholar]
- Martina B.E.E., Airikkala M.I., Harder T.C., van Amerongen G., Osterhaus A.D.M.E. A candidate phocid herpesvirus vaccine that provides protection against feline herpesvirus infection. Vaccine. 2001;20:943–948. doi: 10.1016/s0264-410x(01)00378-4. [DOI] [PubMed] [Google Scholar]
- Miller R.L., Plageman P.G. Effect of ultraviolet-light on mengovirus – formation of uracil dimers, instability and degradation of capsid, and covalent linkage of protein to viral-RNA. J. Virol. 1974;13:729–739. doi: 10.1128/jvi.13.3.729-739.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnaro S., Longo M., Pacilio M., Indovina P., Roberti A., De Martino L., Iovane G., Pagnini U. Feline herpesvirus-1 down-regulates MHC class I expression in an homologous cell system. J. Cell. Biochem. 2009;106:179–185. doi: 10.1002/jcb.21986. [DOI] [PubMed] [Google Scholar]
- Motobu M., El-Abasy M., Na K.J., Hirota Y. Detection of mitogen-induced lymphocyte proliferation by bromodeoxyuridine (BrdU) incorporation in the chicken. J. Vet. Med. Sci. 2002;64:377–379. doi: 10.1292/jvms.64.377. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Shimojima M., Sato E., Izumiya Y., Tohya Y., Mikami T., Miyazawa T. Downmodulation of CD3 epsilon expression CD8 alpha(+) beta(-) T cells of feline immunodeficiency virus-infected cats. J. Gen. Virol. 2004;85:2585–2589. doi: 10.1099/vir.0.80102-0. [DOI] [PubMed] [Google Scholar]
- O’Doherty U., Ignatius R., Bhardwaj N., Pope M. Generation of monocyte-derived dendritic cells from precursors in rhesus macaque blood. J. Immunol. Methods. 1997;207:185–194. doi: 10.1016/s0022-1759(97)00119-1. [DOI] [PubMed] [Google Scholar]
- Parrish C.R. Pathogenesis of feline panleukopenia virus and canine parvovirus. Baillieres Clin. Haematol. 1995;8:57–71. doi: 10.1016/S0950-3536(05)80232-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet H., Jas D., Aeberle C., Lacombe V., Guiot A.L. Onset of immunity in kittens after vaccination with a non-adjuvanted vaccine against feline panleucopenia, feline calicivirus and feline herpesvirus. Vet. J. 2009;182:86–93. doi: 10.1016/j.tvjl.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Radford A.D., Coyne K.P., Dawson S., Porter C.J., Gaskell R.M. Feline calicivirus. Vet. Res. 2007;38:319–335. doi: 10.1051/vetres:2006056. [DOI] [PubMed] [Google Scholar]
- Raulf-Heimsoth M. T cell – primary culture from peripheral blood. Methods Mol. Med. 2008;138:17–30. doi: 10.1007/978-1-59745-366-0_2. [DOI] [PubMed] [Google Scholar]
- Renjifo X., Letellier C., Keil G.M., Ismaili J., Vanderplasschen A., Michel P., Godfroid J., Walravens K., Charlier G., Pastoret P.P., Urbain J., Denis M., Moser M., Kerkhofs P. Susceptibility of bovine antigen-presenting cells to infection by bovine herpesvirus 1 and in vitro presentation to T cells: two independent events. J. Virol. 1999;73:4840–4846. doi: 10.1128/jvi.73.6.4840-4846.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble-antigen by cultured human dendritic cells is maintained by granulocyte-macrophage colony-stimulating factor plus interleukin-4 and down-regulated by tumor-necrosis-factor-alpha. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbulte M.R., Roth J.A. Methods for analysis of cell-mediated immunity in domestic animal species. J. Am. Vet. Med. Assoc. 2004;225:522–530. doi: 10.2460/javma.2004.225.522. [DOI] [PubMed] [Google Scholar]
- Schroder K., Hertzog P.J., Ravasi T., Hume D.A. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Schultz R.D., Mendel H., Scott F.W. Effect of feline panleukopenia virus-infection on development of humoral and cellular immunity. Cornell Vet. 1976;66:324–332. [PubMed] [Google Scholar]
- Sen G.C. Viruses and interferons. Annu. Rev. Microbiol. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- Sprague W.S., Pope M., Hoover E.A. Culture and comparison of feline myeloid dendritic cells vs macrophages. J. Comp. Pathol. 2005;133:136–145. doi: 10.1016/j.jcpa.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Steinman R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Tham K.M., Studdert M.J. Antibody and cell-mediated immune-responses to feline calicivirus following inactivated vaccine and challenge. J. Vet. Med. B. 1987;34:640–654. doi: 10.1111/j.1439-0450.1987.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Tham K.M., Studdert M.J. Antibody and cell-mediated immune-responses to feline herpesvirus-1 following inactivated vaccine and challenge. J. Vet. Med. B. 1987;34:585–597. doi: 10.1111/j.1439-0450.1987.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Tham K.M., Studdert M.J. Antibody and cell mediated immune responses to an inactivated feline panleukopenia virus vaccine. Zentralbl. Veterinarmed. [B] 1987;34:701–712. doi: 10.1111/j.1439-0450.1987.tb00451.x. [DOI] [PubMed] [Google Scholar]
- Thiel A., Scheffold A., Radbruch A. Antigen-specific cytometry – new tools arrived! Clin. Immunol. 2004;111:155–161. doi: 10.1016/j.clim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- von Baehr V., Mayer W., Liebenthal C., von Baehr R., Bieger W., Volk H.D. Improving the in vitro antigen specific T cell proliferation assay: the use of interferon-alpha to elicit antigen specific stimulation and decrease bystander proliferation. J. Immunol. Methods. 2001;251:63–71. doi: 10.1016/s0022-1759(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Zhou L.J., Tedder T.F. CD14(+) blood monocytes can differentiate into functionally mature CD83(+) dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]


