Abstract
Peroxiredoxins (PRDXs) are antioxidant enzymes, known to catalyze peroxide reduction to balance cellular hydrogen peroxide (H2O2) levels, which are essential for cell signaling and metabolism and act as a regulator of redox signaling. Redox signaling is a critical component of cell signaling pathways that are involved in the regulation of cell growth, metabolism, hormone signaling, immune regulation and variety of other physiological functions. Early studies demonstrated that PRDXs regulates cell growth, metabolism and immune regulation and therefore involved in the pathologic regulator or protectant of several cancers, neurodegenerative diseases and inflammatory diseases. Oxidative stress and antioxidant systems are important regulators of redox signaling regulated diseases. In addition, thiol-based redox systems through peroxiredoxins have been demonstrated to regulate several redox-dependent process related diseases. In this review article, we will discuss recent findings regarding PRDXs in the development of diseases and further discuss therapeutic approaches targeting PRDXs. Moreover, we will suggest that PRDXs could be targets of several diseases and the therapeutic agents for targeting PRDXs may have potential beneficial effects for the treatment of cancers, neurodegenerative diseases and inflammatory diseases. Future research should open new avenues for the design of novel therapeutic approaches targeting PRDXs.
Abbreviations: AD, Alzheimer’s disease; AML, Acute myeloid leukemia; ApoE, Apolipoprotein; ARE/EpRE, Antioxidant/electrophile responsive element; COX-2, Cyclooxygenase-2; cdk5, Cyclin-dependent kinase 5; EAE, Experimental autoimmune encephalomyelitis; ER, Endoplasmic reticulum; FOXO, Forkhead box O3; GST, Glutathione S-transferase; HIF-1, Hypoxia inducible factor-1; H2O2, Hydrogen peroxide; ICAM-1, Intercellular adhesion molecule-1; iPLA2, Calcium-independent Phospholipase A2; JNK, c-Jun N-terminal kinase; LPS, Lipopolysaccharide; MCAO, Middle cerebral artery occlusion; MPP(+), 1-methyl-4-phenylpyridinium; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NF-κB, Nuclear factor kappa B; PD, Parkinson’s disease; PDGF, Platelet-derived growth factor; PI3K, phosphoinositide 3-kinase; PRDX, Peroxiredoxin; PTEN, Phosphatase and tensin homolog; RA, Rheumatoid arthritis; RCMD, Refractory cytopenia with multilineage dysplasia;; ROS, Reactive oxygen species; SMCs, Smooth muscle cells; SOD, Superoxide Dismutase; Srx, Sulfiredoxin; TCA, Tricarboxylic acid; TLR, Toll-like receptor; TNF-α, tumor necrosis factor (TNF)-α; TRAIL, TNF-related apoptosis-inducing ligand; Trx, Thioredoxin; TrxR, Thioredoxin reductase; VCAM-1, Vascular cell adhesion molecule-1; VEGF, Vascular endothelial growth factor; 4-HNE, 4-hydroxynonenal
Keywords: Peroxiredoxins, Cancer, Neurodegenerative diseases, Inflammatory diseases, Therapeutic approaches
1. Introduction
Peroxiredoxin (PRDX)s are thiol-specific antioxidant enzymes that reduce various cellular peroxide substrates using cysteine-containing active sites (Fatma et al., 2005, Wood et al., 2003b, Wood et al., 2003a). PRDXs refer to a family of small (22–27 kDa) non-seleno peroxidases currently known to possess six isozymes, namely, PRDX1–6 in mammalian systems (Chae et al., 2012, Wood et al., 2003a). These proteins contain either one (1-Cys PRDX) or two (2-Cys PRDX) redox-active cysteine residues (Schröder et al., 2000, Wood et al., 2003a). The 2-Cys group includes PRDX1 to PRDX5, whereas PRDX6 is the sole member of the 1-Cys group (Immenschuh and Baumgart-Vogt, 2005, Rhee et al., 2005). Despite this difference, peroxidase activities of both 1-Cys and 2-Cys groups commonly contribute to cellular protection against oxidative stress (Dayer et al., 2008, Monteiro et al., 2007). During reaction with oxidant substrates, the cys-residues of PRDXs are oxidized (Chevallet et al., 2003, Schröder et al., 2008). Thioredoxin (Trx) provides the electron for reducing the oxidized PRDX1-5, whereas glutathione is likely to be employed to reduce the oxidized PRDX6 (Poole et al., 2001, Turner-Ivey et al., 2013). Peroxiredoxins (PRDXs) are a ubiquitous family of antioxidant enzymes, known to catalyze peroxide reduction to balance cellular hydrogen peroxide (H2O2) levels, which is essential for cell signaling and metabolism and act as a regulator of redox signaling (Chen et al., 2014, Perkins et al., 2015). Redox signaling is a critical component of cell signaling pathways that are involved in the regulation of cell growth, metabolism, hormone signaling, immune regulation and a variety of other physiological functions (Mishra et al., 2015, Shadel and Horvath, 2015, Trachootham et al., 2008). Therefore, PRDXs are involved in the regulation of cell proliferation, apoptosis, embryonic development, lipid metabolism and immune responses.
PRDXs are involved in particular pathological condition. PRDX isoforms can be considered good therapeutic targets in several cancers such as lung cancer (Jo et al., 2013, Kim et al., 2007, Soini and Kinnula, 2012, Wei et al., 2011), glioblastoma (Deighton et al., 2014, Khalil, 2007), colorectal cancer (Lu et al., 2014a, Lu et al., 2014b, Song et al., 2015), prostate cancer (Riddell et al., 2011, Ummanni et al., 2012) and ovarian cancer (Chung et al., 2010, Wang et al., 2013) where they protect tumor cells, PRDXs are involved in a variety of signaling pathways such as NF-κB signaling pathway, STAT3 pathway, wnt/β-catenin pathway and MAPK kinase pathways (Brigelius-Flohé and Flohé, 2011, Jo et al., 2013, Lu et al., 2014b, Riddell et al., 2010, Yun et al., 2015c, Yun et al., 2015b, Yun et al., 2015a) that are involved in cancer development. So, PRDXs can be explored as therapeutic targets as well as therapeutic tools depending on their role in particular pathological conditions. Several studies have suggested that PRDXs are related with the development of neurodegenerative diseases. PRDXs are involved in neurodegenerative diseases such as AD and PD that are regulated by oxidative stress and redox signaling (Cimini et al., 2013, Yun et al., 2013). PRDXs have distinct functional roles in the brain and provide differential contributions to neuropathologic conditions. The expression patterns of PRDXs are in fact highly variable in different regions of the brain during neurodegenerative disease processes (Hattori & Oikawa, 2007). Given that oxidative damage is involved in the pathogenesis of neurodegenerative diseases, the alteration in PRDX expression would appear to be primarily a consequence of cellular resistance to the oxidative damage. PRDXs are also important regulators of inflammatory diseases such as obesity (Huh et al., 2012, Murri et al., 2013), atherosclerosis (Guo et al., 2012, Park et al., 2011) and rheumatoid arthritis (RA) (Kim et al., 2015, Szabó-Taylor et al., 2012). Inflammation is a complex biological self-defense reaction triggered by tissue damage or infection by pathogens. Redox-regulated transcription factors and protein kinases play important roles in the regulation of inflammation. NF-κB is a well-characterized redox-sensitive transcription factor important in the initiation and progression of inflammation through the production of various cytokines and prostaglandins. PRDXs induce NF-κB activation that is important for the expression of inflammatory proteins (Kim et al., 2013a, Kim et al., 2013b, Riddell et al., 2010). Also, PRDXs are secreted from cells under mild oxidative stress binds Toll-like receptor 4, suggesting that PRDXs are involved in inflammation related disease (Kuang et al., 2014, Riddell et al., 2012, Shichita et al., 2012a).
Although several studies of PRDXs have been published, a comprehensive review of the various features and functions of PRDXs is lacking. In this review article, we will discuss recent findings regarding PRDXs in the development of several diseases and therapeutic approaches.
2. PRDXs
2.1. Structure of PRDXs
Based on its conserved cysteine residues and their catalytic mechanisms, mammalian PRDXs can be divided into three subgroup, namely typical 2-Cys, atypical 2-Cys, and 1-Cys PRDXs. PRDX1-4 belong to typical 2-Cys and PRDX5 belongs to atypical 2-Cys group, within which all members contain two conserved cysteine residues. PRDX6 belongs to 1-Cys PRDXs subgroup in which one cysteine residue is conserved. Moreover, another subgroup named PRDX-Q was extensively found in plants, which also contains two cysteine residues. These structures reveal PRDXs to be very similar, each containing a thioredoxin fold with a few additional secondary-structure elements present as insertions. The most striking differences involve their oligomeric states. The atypical 2-Cys PRDXs are monomeric enzymes, whereas both the typical 2-Cys and the 1-Cys PRDXs are domain-swapped homodimers in which the C terminus of one subunit reaches across the dimer interface to interact with the other subunit (Hirotsu et al., 1999, Schröder et al., 2000, Wood et al., 2003a). In the typical 2-Cys PRDXs, the resolving cysteine is located in the C-terminal arm. Three of the typical 2-Cys PRDXs crystallized as toroid-shaped complexes consisting of a pentameric arrangement of dimers ((a2)5 decamer), consistent with observations that 2-Cys PRDX dimers can form discrete higher-order oligomers (Kitano, Niimura, Nishiyama, & Miki, 1999). AhpC from Amphibacillus xylanus, another 2-Cys PRDX, also crystallizes as an (a2)5 decamer, although no crystal structure for this enzyme has been reported. The structure and sequence of the peroxidatic active site is highly conserved among the PRDX classes (Nelson et al., 2011).
2.2. General functions of PRDXs
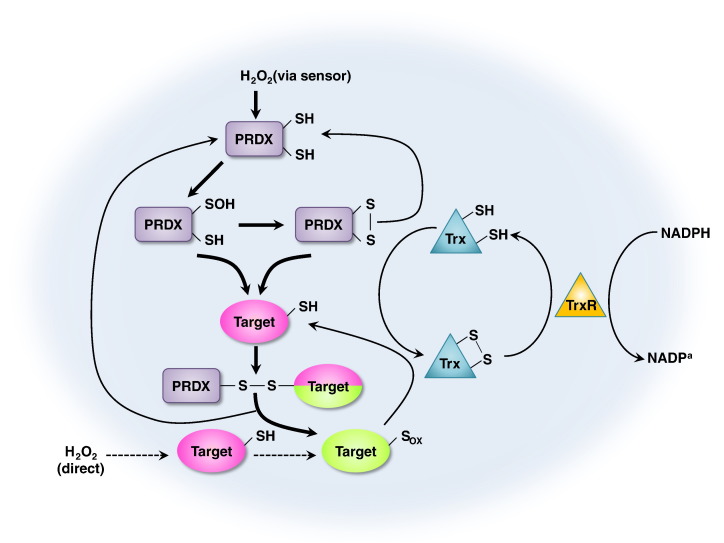
PRDXs contain an active site cysteine that is sensitive to oxidation by H2O2. Mammalian cells express six PRDX isoforms that are localized to various cellular compartments. The oxidized active site cysteine of PRDX can be reduced by a cellular thiol, thus enabling PRDX to function as a locally constrained peroxidase (Rhee, Woo, Kil, & Bae, 2012). Regulation of PRDX via phosphorylation in response to extracellular signals allows the local accumulation of H2O2 and thereby enables its messenger function (Rhee et al., 2012, Woo et al., 2010). The fact that the oxidation state of the active site cysteine of PRDX can be transferred to other proteins that are less intrinsically susceptible to H2O2 also allows PRDX to function as an H2O2 sensor. PRDXs are a ubiquitous family of antioxidant enzymes, known to catalyze peroxide reduction to balance cellular H2O2 levels, which is essential for cell signaling and metabolism. The general function and mechanism of action of the PRDX redox system is in Fig. 1 .
Fig. 1.
General functions of PRDXs. Mechanism of action of the PRDX redox system. PRDXs scavenge H2O2 and become oxidized and inactive. PRDXs use the SH groups as reducing equivalents. This inactivation is reversed by the thioredoxin (Trx)-Trx reductase (TrxR) system that uses NADPH as reducing equivalents. Reduced Trx catalyzes the reduction of disulphides (s-s) within oxidized PRDXs. The oxidized form of PRDXs can then be recycled back to its reduced form by Trx. Further thiol oxidation of PRDXs by higher levels of H2O2 radically inactivates the target proteins allowing H2O2 levels to increase in the cell and to trigger the H2O2-dependent signaling. This inactivation is transient to avoid toxic effects by high levels of H2O2.
PRDX1 is a multifunctional protein originally identified as an intracellular scavenger of H2O2 (Yang et al., 2002). It has been also shown to act as a molecular chaperone with the ability to modulate the actions of numerous molecules, a regulator of transcription, or as an immunomodulator (Jang et al., 2004, O’Leary et al., 2014, Park et al., 2007, Wang et al., 2010). PRDX1 is involved in redox regulation of the cells and reduces peroxides with reducing equivalents provided through the Trx system but not from glutaredoxin (Lehtonen et al., 2004, Neumann et al., 2003). The Trx system is comprised of NADPH, Trx and TrxR, and forms the Trx peroxidase system with PRDXs, and reduces peroxides by NADPH (Watanabe, Nakamura, Masutani, & Yodoi, 2010). PRDX1 is involved in this system. PRDX1 gene is mapped on chromosome 1p34.1 and is a member of the peroxiredoxin family that contains a consensus site (Thr(90)-Pro-Lys-Lys) for phosphorylation by cyclin dependent kinases (Chang et al., 2002, Gisin et al., 2005). PRDX1 is phosphorylated on Thr-90 during the M-phase, which leads to a more than 80% decrease in enzymatic activity through the Trx system (Jang et al., 2006, Neumann et al., 2009). PRDX1 is implicated in redox regulation of the cell and plays an important role in eliminating peroxides produced during metabolism that participates in the signaling of growth factors and tumor necrosis factor-alpha (TNF-α) through regulating the intracellular concentrations of H2O2 (Jamaluddin et al., 2010, Liou and Storz, 2010). PRDX1 is overexpressed in several cancer cells including esophageal, pancreatic, lung, thyroid and oral cancer (Gong et al., 2015, Jiang et al., 2014b, Jiang et al., 2014a, Taniuchi et al., 2015, Yanagawa et al., 1999, Zhang et al., 2012b, Zhang et al., 2012a). Concentrated PRDX1 can be secreted by non-small cell lung cancer cells via unusual pathway (Chang et al., 2005).
PRDX2 has a high reactivity for H2O2. An active Cys on one PRDX2 subunit forms a disulphide bond with the other subunit in the presence of H2O2 (König et al., 2013, Peskin et al., 2007). PRDX2 appears to modulate growth factors and the tumor necrosis factor-alpha-mediated signaling pathway via modulating endogenous H2O2 signaling (Zhao & Wang, 2012). When PRDX2 reacts with peroxide, the peroxidatic cysteine at the active site on one subunit is oxidized to a sulfenic acid (Low, Hampton, Peskin, & Winterbourn, 2007). Through the reduction process of disulphide by Trx, PRDX2 is regenerated and completes the cycle and Trx is in turn regenerated by thioredoxin reductase (TrxR), with reducing equivalents derived from NADPH (Low et al., 2007). PRDX2 reduces hydrogen peroxide and alkyl hydroperoxides involved in cell protection and antiviral activity through the Trx system (Pillay et al., 2011, Sobotta et al., 2015, Watanabe et al., 2010). PRDX2 acts as a H2O2 signal receptor and transmitter in transcription factor redox regulation. PRDX2 forms a redox relay with the transcription factor STAT3 in which oxidative equivalents flow from PRDX2 to STAT3, and the redox relay generates disulphide-linked STAT3 oligomers with attenuated transcriptional activity. Cytokine-induced STAT3 signaling is accompanied by PRDX2 and STAT3 oxidation and is modulated by PRDX2 expression levels (Sobotta et al., 2015). Moon et al demonstrated that PRDX2 inhibits general immune cell responsiveness, which may be regulated by scavenging low levels of reactive oxygen species (ROS) (Moon et al., 2006). PRDX2 also enhances the activation of platelet-derived growth factor (PDGF) receptor and phospholipase Cγ1 in PDGF signaling via the modulation of H2O2 (Choi et al., 2005).
PRDX3 (MER-5, SP22, AOP-1) is known as c-myc, miR-383 and miR-23b target gene and is required for mitochondrial homoeostasis and neoplastic transformation (Pablo et al., 2009; Wonsey, Zeller, & Dang, 2002). PRDX3 contains a mitochondrial localization sequence, which is found exclusively in the mitochondrion, comprises 5% of the total mitochondrial matrix, and uses mitochondrial Trx2 as the electron donor for its peroxidase activity (Gourlay, Bhella, Kelly, Price, & Lindsay, 2003). PRDX3 has been known to eliminate approximately 90% of mitochondrial H2O2 and plays a key role in mitochondrial redox regulation (Chang et al., 2004). The overexpression of PRDX3 has been shown to protect cells from oxidative stress and apoptosis, and knockout studies show an increase in intracellular ROS levels and oxidative stress (Mukhopadhyay et al., 2006, Nonn et al., 2003). PRDX3 protects radical-sensitive enzymes from oxidative damage by a radical-generating system, and acts synergistically with MAP3K13 to regulate the activation of NF-kappa-B in the cytosol (Lin et al., 2007). PRDX3 is involved in the regulation of cellular proliferation, differentiation and antioxidant functions that has been localized in the mitochondria (Huh et al., 2012, Song et al., 2011). Increased expression of PRDX3 was found to be associated with hormonal receptors (estrogen receptor and progesterone receptor) (Karihtala, Mäntyniemi, Kang, Kinnula, & Soini, 2003).
PRDX4 (AOE372, TRANK) is a ubiquitously expressed member of the peroxiredoxin family that is mainly localized in the endoplasmic reticulum (ER), but is also present in the cytosol, lysosome, nucleus, or secreted (Leyens, Donnay, & Knoops, 2003). PRDX4 plays an essential role in the redox balance in the ER. The Cysteine residue of PRDX4 is first oxidized to sulfenic acid form and then forms intermolecular disulphide bond with another PRDX molecule, which can be reversed by the reducing activity of the Trx-Trx reductase system (Mehmeti et al., 2014, Tavender and Bulleid, 2010). Under oxidative stress conditions, however, the Cysteine of PRDX4 undergoes further oxidation to sulfinic/sulfonic acid forms which can only be reduced by sulfiredoxin (Srx) (Jeong, Bae, Toledano, & Rhee, 2012). The hyperoxidized form of PRDX4 loses its antioxidant property but may function as molecular chaperone to facilitate protein folding (Rhee and Woo, 2010, Zito et al., 2010). PRDX4 diminishes oxidative stress by reducing hydrogen peroxide to water in a thiol-dependent catalytic cycle (Schulte, 2011) and has been linked to the regulation of the key pro-inflammatory transcription factor, NF-κB in the cytosol by a modulation of IκB-α phosphorylation (Haridas et al., 1998, Jin et al., 1997). PRDX4 is abundantly expressed in the pancreas, liver and heart, while the lowest expression in blood leukocytes and the brain (Schulte, 2011).
PRDX5 (ACR1, PMP20 or AOEB166) is an antioxidant enzyme implicated as a protective role against oxidative stress through reduction of hydrogen peroxide and alkyl hydroperoxides with reducing equivalents provided through the Trx system and is involved in intracellular redox signaling (Knoops, Goemaere, Van der Eecken, & Declercq, 2010). PRDX5 is a thiol peroxidase that reduces H2O2 105 times faster than free cysteine that shows hydrogen bonds in the active sites (Stephanie et al., 2014). The PRDX5 gene is mapped on chromosome on 11q13.1 that displays mitochondrial presequence and 3 cysteines involved in antioxidant activity with C-terminal peroxisomal sequence (Knoops et al., 2010, Nguyên-nhu et al., 2007). PRDX5 interacts with peroxisome receptor 1 which uses translation initiation sites to produce mitochondrial forms. PRDX5 acts as an antioxidant on different tissues under normal conditions and inflammatory processes through Trx system. The analysis of the 5’-flanking region of human PRDX5 revealed the presence of several putative response elements for transcription factors involved in the response of mammalian cells to oxidative stress such as Nrf2 or NF-κB (Kropotov et al., 2007, Nguyên-nhu et al., 2007). PRDX5 expression is highly increased in vitro in primary macrophages exposed to lipopolysaccharide (LPS) and IFN-γ (Abbas et al., 2009, Diet et al., 2007). Downstream of the LPS/TLR pathway, p38 and c-Jun N-terminal kinase (JNK) were shown also to be major contributors to PRDX5 up-regulation (Diet et al., 2007).
PRDX6 belongs to the ahpC/TSA family and contains 1 Trx domain (Poole, Hall, & Nelson, 2011). Among the six mammalian members of PRDX, PRDX6 has a unique property as a bifunctional enzyme that has glutathione peroxidase and iPLA2 activities (Fisher, 2011). PRDX6 involved in redox regulation of the cell can reduce H2O2 and short chain organic, fatty acid, and phospholipid hydroperoxides (Fisher, 2011). PRDX6 plays a role in the regulation of phospholipid turnover as well as in protection against oxidative injury (Wang, Feinstein, Manevich, Ho, & Fisher, 2006). Site directed mutation analysis has clearly shown that activities of PRDX6 require two distinct active sites, namely, a Cys 47-dependent peroxidase activity and a Ser 32-dependent iPLA2 activity (Chen, Dodia, Feinstein, Jain, & Fisher, 2000). iPLA2 catalyzes the hydrolysis of the sn-2 fatty acyl ester bond of glycerophospholipides to produce free fatty acids and lysophospholipids (Markova et al., 2005, Shalini et al., 2014). PRDX6 prefers phosphatidylcholines as substrates, particularly those with arachidonic acid (AA) or palmitic acid at the sn-2 position (Chen et al., 2000, Manevich and Fisher, 2005). The ability of PRDX6 to release AA seems important, given the crucial role of AA in phospholipid metabolism and cell signaling (Hooks & Cummings, 2008). PRDX6 also protects cells via reducing peroxidazed membrane phospholipids and return to the cytosolic compartment (Wang, Feinstein, & Fisher, 2008). General properties of peroxiredoxin isoforms are briefly summarized in Table 1 , and the cellular localization and the roles of PRDXs are in Fig. 2 .
Table 1.
Properties of peroxiredoxin isoforms.
| PRDX1-4 | PRDX5 | PRDX6 | |
|---|---|---|---|
| Class | 2-cys | Atypical 2-cys | 1-cys |
| Thioredoxin fold superfamily | Yes | Yes | Yes |
| Structure of disulphide bond | Homodimer | Monomer | Heterodimer |
| Reductant | Trx | Trx | GSH |
| PLOOH as substrate | No | No | Yes |
| PLA2 activity | No | No | Yes |
PLOOH, phospholipid hydroperoxide.
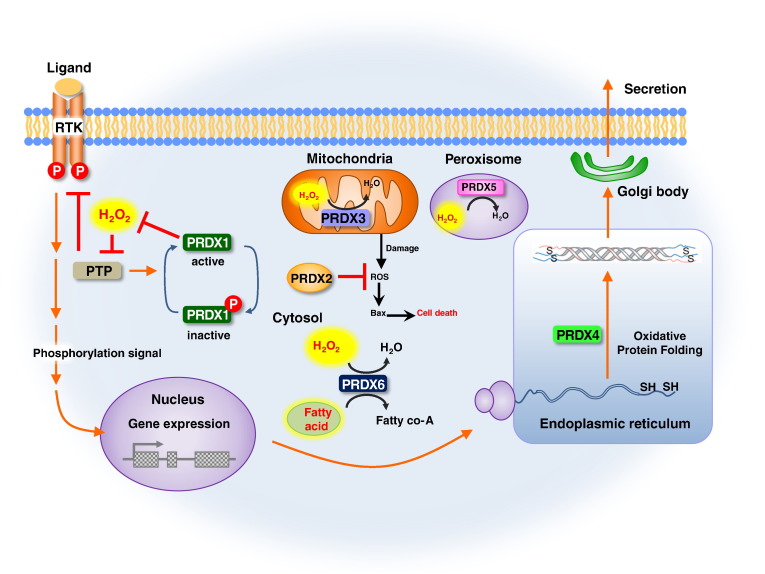
Fig. 2.
Cellular localization and roles of PRDXs. Tyrosine residue of PRDX1 is phosphorylated, which causes the transient inactivation of peroxidase activity. As a result, a local accumulation of H2O2 occurs and sustains phosphorylation signaling from tyrosine kinase for the growth factor receptor via the oxidative inactivation of phosphotyrosine phosphatases. Consequently the cell growth proceeds for a prolonged period as the result of the elevated H2O2 during the inactivation of PRDX1. PRDX2 is localized in the cytosol and scavenges ROS in the cytosol and blocks Bax mediated cell death by inhibition of cellular apoptosis. PRDX3 is localized in the mitochondria. PRDX3 reduces H2O2 level and protects cells from oxidative damages. PRDX4 possesses a hydrophobic N-terminal signal peptide that leads to its secretion from cells and predominant localization in the ER. PRDX5 reduces H2O2 and alkyl hydroperoxides that acts as antioxidant on different tissues under normal conditions and inflammatory processes through Trx system in peroxisomes. PRDX6 involved in redox regulation of the cell and can reduce H2O2 and short chain organic, fatty acid, and phospholipid hydroperoxides. PRDX6 plays a role in the regulation of phospholipid turnover as well as in the protection against oxidative injury.
2.3. Regulation of PRDXs expression
General regulatory pathway of PRDX expression is in Fig. 3 . PRDXs are regulated at multiple levels, including both transcriptional regulation and post-translational modifications (Klotz et al., 2015, Riquier et al., 2014). PRDX1 peroxidase activity is regulated by Pin1. Pin1 facilitates the protein phosphatase 2A-mediated dephosphorylation of PRDX1, which helps to explain the accumulation of the inactive phosphorylated form of PRDX1 in Pin1-/- MEFs (Chu et al., 2013). PRDX2 is silenced by a decreased histone H3 acetylation and DNA hypermethylation in AML (Agrawal Singh et al., 2012). A recent study also demonstrated a critical role for Foxo3a in regulating the gene expression of PRDX3 in human cardiac fibroblasts (Chiribau et al., 2008). In the promoter of human PRDX5 gene, the binding sites for both nuclear respiratory factor 1 and nuclear respiratory factor 2 have been predicted (Kropotov et al., 2007). Increased transcription of PRDX6 has been reported to be mediated by Nrf2 via an antioxidant response element-driven mechanism (Chowdhury et al., 2009). The MAPKs can mediate phosphorylation of PRDX6 at Thr-177 with a consequent marked increase in its aiPLA2 activity (Wu et al., 2009). SP-A and PRDX6 directly interact, which provides a mechanism for regulation of the aiPLA2 activity of PRDX6 by SP-A (Wu et al., 2006). Recent findings suggest that Fkbp52 deficiency diminishes the threshold against OS by reducing PRDX6 levels (Hirota et al., 2010). A recent study also suggested that 67 (phox) binds to phospho-PRDX6 and inhibits its PLA2 activity, an interaction that could function to terminate the PLA2-mediated NOX2 activation signal (Krishnaiah, Dodia, Feinstein, & Fisher, 2013). Moreover, the expression of PRDXs is induced by several factors. PRDX1 is also detected in mouse body fluids, and the secretion of PRDX1 from cultured cells was enhanced following the treatment with cytokines such as TGF-β, oncostatin M and IL1-β (Ishii, Warabi, & Yanagawa, 2012). Another recent study showed PRDX1 and PRDX2 can be secreted from cells as disulphide homodimers in association with exosomes following stimulation by LPS or TNF-α (Mullen, Hanschmann, Lillig, Herzenberg, & Ghezzi, 2015). PRDX1 is up-regulated in cells under oxidative stress conditions. The transcription factor Nrf2 and its inhibitor Keap1 play an essential role in the regulation of the stress-induced PRDX1 gene activation through the antioxidant/electrophile response element (ARE/EpRE) (Takeuchi et al., 2013). The expression levels of PRDX2 and PRDX3 are also up-regulated under oxidative stress conditions (Stresing et al., 2013). PRDX6 is an oxidative stress inducible protein regulated by Nrf2 and activation of the gene expression was observed in mouse lungs by hyperoxia and in cultured lung epithelial cells following treatment with H2O2 or paraquat (Chowdhury et al., 2009). In addition to oxidative stress, keratinocyte growth factor also regulates PRDX6 gene expression (Chowdhury et al., 2014). PRDX6 expression is down regulated upon serum deprivation and subsequently induced in a time-dependent manner in response to KGF, dexamethasone and H2O2 (Gallagher & Phelan, 2007). TNF-α regulates the expression of PRDX6 to influence neuroplasticity (Zhang et al., 2015).
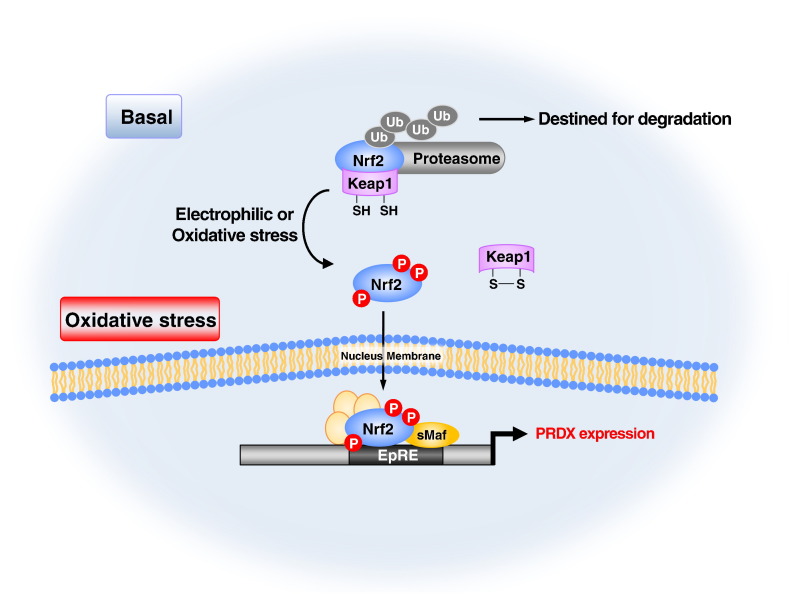
Fig. 3.
General regulatory pathway of PRDX expression. Nrf2 induces the expression of PRDXs in response to oxidative and electrophilic stresses. Under unstressed conditions, Nrf2 is degraded via the ubiquitin (Ub)–proteasome pathway in a Keap1-dependent manner. The Keap1 homodimer binds a single Nrf2 molecule through two-site binding utilizing the DLG and ETGE motifs. When Nrf2 inducers inactivate Keap1 via the modification of cysteine residues (Cys) under oxidative stress and electrophiles modified condition, Nrf2 is stabilized, and de novo synthesized Nrf2 translocates into the nucleus. Nrf2 heterodimerizes with small Maf proteins (sMaf) and activates the expression of target genes including PRDXs through antioxidant response elements (AREs), exerting cytoprotective effects against various diseases and toxic insults. Phosphorylation of Nrf2 by various kinases has also been implicated in the liberation, stability, and trans-activation of Nrf2.
3. PRDXs and cancer
Diseases involved in abnormal inflammatory and metabolic processes, such as cardiovascular dysfunction, cancer, diabetes mellitus and neurodegeneration, often involve abnormal control of ROS (Rahman, 2007). The hyperproliferative property of cancer cells is known to be associated with increased production of intracellular ROS (Cerutti, 1994). Also, cancer cells contain large numbers of abnormal mitochondria and produce large amounts of ROS (Song et al., 2011). In cancer cells, key mitochondrial regulators of cell death and other processes are often altered (Gogvadze, Orrenius, & Zhivotovsky, 2008). Mitochondria of cancer cells differ structurally and functionally from their normal-cell counterparts (Gogvadze et al., 2008, Modica-Napolitano and Singh, 2004). Increased mitochondrial ROS generation and the disturbance of PRDX production in cancer cells may lead to oxidative stress and the induction of apoptosis (Song et al., 2011). The PRDX system is a cellular defense system against oxidative stress (Powers & Jackson, 2008). As PRDXs are antioxidants, they scavenge the H2O2 in cancer cells and they support survival and tumor maintenance by protecting cells from oxidative stress-induced apoptosis (Chatterjee et al., 2011).
3.1. Roles of PRDXs in the cancer development
Several studies have shown that overexpression of PRDXs inhibits the development of cancer or promotes growth of cancers. In this review, we divided the role of PRDXs by benefit and non-benefit roles in several cancers. The relationship between cancer development and PRDXs is summarized in Table 2 .
Table 2.
Summary of function and phylogenetic distribution of PRDX subfamily in different cancer types
| PRDX type | Cancer type | Effects | Results | Mechanisms | Reference |
|---|---|---|---|---|---|
| PRDX1 | Breast cancer | Anti-tumor effect | Suppress the tumor | -PRDX1 interacts with the c-Myc oncogene and suppresses its transcriptional activity -PRDX1 protects the tumor suppressive function of PTEN phosphatase |
Egler et al., 2005; Cao et al., 2009 |
| Tumor promoting effect | Enhance the risk of cancer | -PRDX1 increases risk of local recurrence after radiotherapy -PRDX1 enhances the transactivation potential of NF-κB in ER-deficient breast cancer cells |
O’Leary et al., 2014 | ||
| Oral squamous cell carcinoma | Tumor promoting effect | Promotes cancer | -PRDX1 induces hypoxia of oral cancer -PRDX1 upregulates hemeoxygenase 1 and activates NF-κB pathway |
Yanagawa et al., 2000, Yanagawa et al., 2005 | |
| Bladder cancer | Tumor promoting effect | Increase the proliferation of cancer cells | -PRDX1 regulates the cell cycle by upregulation of phosphorylation of NF-κB subunit | Quan et al., 2006 | |
| Lung cancer | Tumor promoting effect | Promotes tumorigenesis and enhance the drug resistance | -PRDX1 promotes Lung cancer progression - PRDX1 enhances radiotherapy resistance and drug resistance through suppression of FOXO1 induced apoptosis |
Hwang et al., 2013, Jiang et al., 2014a, Jiang et al., 2014b | |
| Esophageal squamous cell carcinoma | Tumor promoting effect | Promotes tumorigenesis | PRDX1 activates mTOR/p70S6K pathway | Gong et al., 2015 | |
| Prostate cancer | Tumor promoting effect | Promotes cancer | PRDX1 interacts with androgen receptor and enhances its transactivation | Chhipa et al., 2009, Park et al., 2007 | |
| Hepatocellular carcinoma | Tumor promoting effect | Promotes the angiogenesis | PRDX1 expression is correlated with angiogenesis factors such as VEGF expression and microvesel density | Sun et al., 2013 | |
| Pancreatic cancer | Tumor promoting effect | Promotes the angiogenesis | PRDX1 upregulates pancreatic cancer invasion by modulating p38 MAPK signaling | Taniuchi et al., 2015 | |
| PRDX2 | Colorectal carcinoma | Tumor promoting effect | Promotes tumorigenesis | -PRDX2 promotes cell growth and inhibit apoptosis -PRDX2 upregulates Wnt/β-catenin pathway |
Lu et al., 2014a, Lu et al., 2014b |
| Prostate cancer | Tumor promoting effect | Promotes cancer | PRDX2 regulates the AR activity and involved in the proliferation of AR-expressing prostate cancer cells | Shiota et al., 2011 | |
| Breast cancer | Tumor promoting effect | Enhance the drug resistance | PRDX2 increases the drug resistance | Wang et al., 2014a, Wang et al., 2014b | |
| PRDX3 | Breast cancer | Tumor promoting effect | Increase the proliferation of cancer cells | PRDX3 regulates cell cycle and cell proliferation | Chua et al., 2010 |
| Prostate cancer | Tumor promoting effect | Enhance the risk of cancer | PRDX3 is upregulated in antiandrogen-resistant tumours and increases resistance to oxidative stress and pro-apoptotic pathway | Whitaker et al., 2013 | |
| Cervical carcinoma | Tumor promoting effect | Increase the proliferation of cancer cells | PRDX3 is increased in cervical carcinoma consistent with cell proliferation marker, ki-67 | Hu et al., 2013 | |
| Thymoma | Tumor promoting effect | Enhance the drug resistance | PRDX3 enhances drug resistant role against drug induced oxidative stress | Nonn et al., 2003 | |
| Hepatocellular carcinoma | Tumor promoting effect | Enhance the risk and promotes tumorigenesis | -PRDX3 increases the cell proliferation and inhibits apoptosis -PRDX3 is involved in chemotherapeutic resistance |
Wang et al., 2014a, Wang et al., 2014b | |
| Ovarian cancer | Tumor promoting effect | Enhance the drug resistance | -PRDX3 inhibits cisplatin induced apoptosis by down regulation of proapoptotic proteins and upregulation of NF-κB pathway | Wang et al., 2013 | |
| PRDX4 | Lung cancer | Tumor promoting effect | Promotes tumorigenesis | PRDX4 binds with Srx and the Srx-PRDX4 axis contributes the maintenance of lung tumor phenotype by AP-1 and MAPK signaling pathway | Wei et al., 2011 |
| Leukemia | Tumor promoting effect | Enhance the risk of cancer | PRDX4 promotes signal transduction from a myeloid growth factor receptor | Zhang et al., 2004 | |
| glioblastoma | Tumor promoting effect | Enhance the risk and promotes tumorigenesis | PRDX4 increases the cell growth and radiation or drug-resistance | Kim et al., 2012 | |
| Oral cavity squamous cell carcinoma | Tumor promoting effect | Promotes the metastasis | PRDX4 increases cell migration and/or metastasis | Chang et al., 2011a, Chang et al., 2011b | |
| Colorectal cancer | Tumor promoting effect | Promotes the metastasis | PRDX4 expression is correlated with depth of invasion and lymph node metastasis | Yi et al., 2014 | |
| PRDX5 | Breast cancer | Anti-tumor effect | Protect from breast cancer | -PRDX5 expression is downregulated in tumor samples obtained from Sudanese breast cancer patients -PRDX5 expression via DNA binding factor (GATA1) repression increases protective function in breast cancer cells |
Elamin et al., 2013 Seo et al., 2012 |
| Grave’s disease | Tumor promoting effect | Promotes the disease | PRDX5 expression is directly correlated with the functional status of epithelial cells | Gérard et al., 2005 | |
| PRDX6 | Ovarian cancer | Tumor promoting effect | Enhance the drug resistance | PRDX5 attenuates cisplatin induced apoptosis | Pak et al., 2011 |
| Liver cancer | Tumor promoting effect | Promotes tumorigenesis | PRDX6 reduces peroxide-induced cell death | Eismann et al., 2009 | |
| Lung cancer | Tumor promoting effect | Promotes lung tumor development | -PRDX6 promotes lung tumor development via its mediated and CCL5-associated activation of the JAK2/STAT3 pathway. -PRDX6 promotes lung tumor progression via its GPx and iPLA2 activities. -PRDX6 promotes invasion and metastasis of lung cancer cells. -PRDX6 promotes lung cancer cell invasion by inducing urokinase-type plasminogen activator via p38 kinase, phosphoinositide 3-kinase, and Akt |
Ho et al., 2010, Jo et al., 2013, Yun et al., 2014a, Yun et al., 2014b, Yun et al., 2015c, Yun et al., 2015b, Yun et al., 2015a | |
| Gastric cancer | Tumor promoting effect | Promotes the metastasis and enhance the drug resistance | -PRDX6 is highly upregulated in metastatic gastric cancer cells, which are relatively resistant to TRAIL as compared with primary cancer cells. | Choi et al., 2011 | |
| Breast cancer | Tumor promoting effect | Promotes the metastasis | -PRDX6 leads to a more invasive phenotype and metastatic potential in human breast cancer through regulation of the level of uPAR, Est-1, MMP-9, RhoC and TIMP-2 expression. | Chang et al., 2007 |
3.1.1. Dual function of PRDX1 in cancer
First, the anti-tumor effect of PRDX1 is well established in breast cancers. Several evidences suggest that PRDX1 may act as a tumor suppressor in breast cancer. PRDX1 interacts with the c-Myc oncogene and suppresses its transcriptional activity (Egler et al., 2005). Cao et al. demonstrated that PRDX1 protects the tumor suppressive function of PTEN phosphatase, likely due to the presence of a ROS sensitive cysteine in the catalytic domain, and reduces predisposition of genetically modified mice to develop Ras-induced mammary tumors (Cao et al., 2009). PRDX1-deficient mice suffer from shortened survival due to the development of hemolytic anemia and multiple tumors, including mammary carcinomas (Neumann et al., 2003). These studies suggest that the breast tumor suppressive role of PRDX1 is mediated via c-Myc or PTEN pathways.
Next, the tumor promoting effect of PRDX1 is well established in breast cancer, oral squamous cell carcinoma, bladder cancer, lung cancer, prostate cancer, hepatocellular carcinoma, esophageal squamous cell carcinoma and pancreatic cancer. The role of PRDX1 in breast cancer has been controversial. Recent studies have shown that overexpression of PRDX1 mRNA in human breast carcinoma is associated with higher tumor grade (Cha, Suh, & Kim, 2009), and high expression of cytoplasmic PRDX1 protein correlated with an increased risk of local recurrence after radiotherapy (Woolston, Storr, Ellis, Morgan, & Martin, 2011). PRDX1 enhances p65-mediated cyclooxygenase (COX)-2 gene expression in estrogen receptor (ER) deficient human breast cancer cells (MDA-MB-231), and knockdown of PRDX1 can attenuate COX-2 expression by reducing the occupancy of NF-κB transactivation potential of NF-κB in ER-deficient-breast cancer cells (Wang et al., 2010). PRDX1 is also overexpressed in oral squamous cell carcinoma (Yanagawa et al., 2005). A recent study provides experimental evidence that overexpression of PRDX1 is involved in oral carcinogenesis by upregulation of hemeoxygenase 1 and activation of NF-κB pathway (Zhang et al., 2014). PRDX1 is also related with bladder cancer. PRDX1 is highly expressed in bladder cancer tissues than in the bladder mucosa of normal controls or in normal mucosa surrounding cancer tissues (Quan et al., 2006). Jiang et al showed that PRDX1 silencing by transfection of PRDX1 shRNA significantly suppressed growth, promoted apoptosis and regulated the cell cycle in bladder cancer cells and reduced the phospho-NF-κB p50 and p65 protein expression suggesting that PRDX1 is related with bladder cancers by regulation of phosphorylation of NF-κB subunit (Jiang et al., 2014a, b). PRDX1 is also related with lung cancer. Abnormal up-regulation of PRDX1 by environmental redox change leads to lung cancer progression and radiotherapy resistance by controlling critical molecules (Kim et al., 2007). Another recent study suggested that down-regulation of PRDX1 significantly inhibited tumor growth and reduced the incidence of spontaneous pulmonary metastasis (Chen et al., 2006). PRDX1 provides resistance to docetaxel treatment through the suppression of FOXO1-induced apoptosis in A549 xenograft tumors, suggesting PRDX1 may be an attractive target in the development of more effective docetaxel-based therapies in lung cancer treatment (Hwang et al., 2013). PRDX1 is also related with prostate cancer. PRDX1 interacts with the androgen receptor and enhances its transactivation that plays a critical role in prostate cancer (Park et al., 2007). One previous study reported that hypoxia increases AR activity in prostate cancer cells, and that PRDX1, which is up-regulated by hypoxia, interacts with AR to enhance the expression of androgen-regulated genes (Chhipa et al., 2009, Park et al., 2006). PRDX1 is highly expressed in hepatocellular carcinoma. PRDX1-positive rate was significantly higher in hepatocellular carcinoma than in adjacent non-tumorous liver tissues (Sun et al., 2013). Actually, PRDX1 immunoreactivity was positively correlated with tumor vascular endothelial growth factor (VEGF) expression and microvessel density (Sun et al., 2013). PRDX1 expression was significantly associated with tumor size, microvascular invasion, Edmondson grade, tumor capsula status, serum AFP, and tumor-node-metastasis stage (Sun et al., 2013). PRDX1 is involved in esophageal squamous cell carcinoma tumorigenesis through regulation of the mTOR/p70S6K pathway (Gong et al., 2015). PRDX1 is also significantly increased in pancreatic cancer by upregulation of angiogenesis (Cai et al., 2015). These studies indicate that PRDX1 has a tumor promoting effect through NF-κB pathway, FOXO1 mediated pathway and mTOR/p70S6K pathway in breast cancer, oral squamous cell carcinoma, bladder cancer, lung cancer, prostate cancer, hepatocellular carcinoma, esophageal squamous cell carcinoma and pancreatic cancer, which suggest that PRDX1 is a potential therapeutic target in these cancer types.
3.1.2. Tumor promoting effect of PRDX2
The tumor promoting effect of PRDX2 is well established in colorectal carcinoma, prostate cancer, breast cancer and cervical cancer. PRDX2 is overexpressed in colorectal carcinoma tissues compared with the matched non-cancer colorectal mucosa tissues and that expression is positively associated with tumor metastasis and the TNM stage by regulation of oxidation induced apoptosis (Lu et al., 2014a). Other researchers also demonstrated that PRDX2 knockdown using a lentiviral vector-mediated specific shRNA inhibited cell growth, stimulated apoptosis, and augmented the production of endogenous ROS that led to an altered expression of proteins associated with the Wnt signaling pathway (Lu et al., 2014b). PRDX2 is also important in prostate cancer. Shiota et al demonstrated that the expression of PRDX2 is most significantly elevated among the PRDX family in prostate cancer (Shiota et al., 2011). They showed that PRDX2 in the nucleus and cytoplasm distinctively regulated AR activity and is involved in the proliferation of AR-expressing prostate cancer cells and in the progression to castration-resistant prostate cancer, suggesting PRDX2 to be a key factor in prostate carcinogenesis and in the progression to castration-resistant prostate cancer (Shiota et al., 2011). PRDX2 is highly inducible by therapeutic radiation and drugs in breast cancer cells (Wang et al., 2014b, Wang et al., 2014a). Several resistant tumor cells have shown upregulated PRDX2 levels in breast cancer. PRDX2 plays a role in protecting the cell membrane from IR-induced oxidative radioresistant breast cancer cells (Diaz, Tamae, Yen, Li, & Wang, 2013). PRDX2 is also significantly upregulated in the early diagnosis of Hepatitis B Virus (HBV) related fibrosis (Lu et al., 2010). PRDX2 is markedly upregulated in neutrophils of refractory cytopenia with multilineage dysplasia (RCMD) patients compared to healthy donors. In addition, white blood cell and neutrophil counts in RCMD patients correlated inversely with PRDX2 expression (Kazama et al., 2014). Among PRDXs, PRDX2 specifically regulates breast cancer cell colonization in lungs by acting on the oxidative and metabolic stress responses of metastatic cells (Stresing et al., 2013). PRDX2 has a strong pattern of increased expression with increasing severity of the lesion, thus PRDX2 is up-regulated in response to cervical cancer development (Kim et al., 2009). These data indicate that PRDX2 is involved in the proliferation or metastasis of cancer, involved in radio- and drug-resistance, and suggest that designing therapeutics targeting PRDX2 may offer a novel strategy for developing treatment for cancer.
3.1.3. Promoting effect of PRDX3 in several cancer types
The tumor promoting effect of PRDX3 is well established in breast cancer, prostate cancer, cervical cancer, hepatocellular carcinoma and ovarian cancer. In a gene silencing study in breast cancer cells, silencing the PRDX3 gene inhibits cell proliferation and induces cell cycle arrest in breast cancers (Chua et al., 2010). PRDX3 is also involved in prostate cancer. PRDX3 is upregulated in endocrine-regulated tumors and in particular prostatic intraepithelial neoplasia and prostate cancer (Whitaker et al., 2013). Also, the PRDX3 protein is significantly upregulated in antiandrogen-resistant prostate cancer cell lines, resulting in an increased resistance to oxidative stress and failure to activate pro-apoptotic pathways (Whitaker et al., 2013). Antiandrogen-resistant prostate cancer cells also possess an upregulation of the tricarboxylic acid (TCA) pathway and resistance to H2O2-induced apoptosis through a failure to activate pro-apoptotic pathways, but knockdown of PRDX3 restored H2O2 sensitivity (Whitaker et al., 2013). PRDX3 is upregulated in response to the development of cervical cancer. The expression of PRDX3 is increased in cervical carcinoma and the pattern of PRDX3 expression in cervical carcinoma was consistent with that of cell proliferation marker, Ki67 (Hu, Gao, & Li, 2013). Nonn et al reported that PRDX3 played a drug-resistant role against drug-induced oxidative stress and subsequent apoptosis of thymoma cells (Nonn et al., 2003). PRDX3 is also involved in hepatocellular carcinoma development. The overexpression of PRDX3 is associated with 94.9% hepatocellular carcinoma, and correlated with poor differentiation (Qiao et al., 2012). Also, PRDX3 is resistant to oxidation-induced apoptosis of Hep-3b cells (Wang et al., 2014a, b). After a low-dose of H2O2 treatment, the ROS level was significantly higher in PRDX3-knockdown Hep-3b cells than in controls. In addition, PRDX3 down-regulation resulted in decreased proliferation, increased apoptosis, and increased caspase 3 activity of Hep-3b cells (Wang et al., 2014a, b). The PRDX3 is also overexpressed in ovarian cancer. Downregulation of PRDX3 upregulated pro-apoptotic proteins Bax, Caspase‑3 and Caspase‑9, and the silencing of PRDX3 triggered cisplatin‑mediated apoptosis in the ovarian cancer cells through suppression of the NF-κB signaling pathway (Duan et al., 2013). These data indicate that PRDX3 is accordingly up-regulated in cancer cells to remove cellular ROS and inhibit apoptosis, which provides a favorable microenvironment for cell proliferation. Therefore, PRDX3 can be a therapeutic target for several cancer treatments.
3.1.4. Tumor promoting effect of PRDX4
The tumor promoting effect of PRDX4 is well established in lung cancer, leukemia, glioblastoma, oral squamous cell carcinoma, cardiovascular disease, hepatic diseases and colorectal carcinoma. PRDX4 is associated with lung cancer. Wei et al demonstrated that PRDX4 along with Srx plays a very important role in tumor progression and metastasis in lung cancer (Wei et al., 2011). Wei et al. showed that Srx preferentially binds to PRDX4 and the Srx-PRDX4 axis contributes significantly to the maintenance of lung tumor phenotype in vitro and the formation of metastases in vivo and also they suggested the Srx-PRDX4 axis in activation of intracellular phosphokinase signaling including AP-1/MMP-9 axis and MAPK signaling (Wei et al., 2011). The expression of PRDX4 is at least 1.5 fold higher in tumor cells compared to control and this finding applies most frequently to adenocarcinoma and modestly to squamous cell carcinoma (Lehtonen et al., 2004). Alteration in expression of PRDX4 results in an alteration to the rate of tumor progression and metastasis which is indicated by anchorage independent colony formation, cell migration and invasion of human lung cancer cells (Park et al., 2008). The ability of PRDX4 to promote tumor progression and metastasis is supposed to be due to its antioxidant properties (Dando et al., 2015). Alteration of PRDX4 expression is proposed to play a role in the development of different types of leukemia (Palande et al., 2011). Palande et al. found that the alteration in genomic sequence and expression level of PRDX4 is rare in acute myeloid leukemia but have found strong reduction in PRDX4 expression in acute promyelocytic leukemia (Palande et al., 2011). Also, it was suggested that due to the alteration in PRDX4 expression, the signal transduction from a myeloid growth factor receptor i.e. the granulocyte colony stimulating factor receptor is affected (Palande et al., 2011). In acute myeloid leukemia (AML) patients, the PRDX4 gene is fused with the AML1 gene between exon 5 and 6 of AML1 and exon 2 of PRDX4 (Zhang et al., 2004). This fusion of AML1 gene with the PRDX4 gene is supposed to play a role in the altered expression of PRDX4 in acute myeloid leukemia (Zhang et al., 2004). PRDX4 is supposed to play a role in the most aggressive primary brain malignancy (Kim et al., 2012). They found that the knockdown of PRDX4 results in reduced Glioblastoma multiform cell growth and radiation resistance along with increased ROS level, DNA damage and apoptosis in in-vitro model, suggesting the importance of PRDX4 in radiation resistance and tumor maintenance of GBM (Kim et al., 2012). PRDX4 is also studied for its role in tumor progression, cell migration and invasiveness in oral cavity squamous cell carcinoma (Chang et al., 2011a, b). Along with the prognostic value of PRDX4, PRDX4 can also be a good therapeutic target in OSCC by virtue of its ability to mediate cell migration and/or metastasis (Liao et al., 2011). Also, PRDX4 is secreted into an extracellular environment, therefore, its plasma concentration may be used as a molecular indicator of various cardiovascular disease and other disorders involving oxidative stress (Schulte, 2011). The increased serum PRDX4 concentration is considered as a good indicator of risk to cardiovascular disease because cardiovascular disease have higher level of oxidative stress and PRDX4 is over-expressed in these conditions (Abbasi et al., 2012). A study in a rat model of Wilson's disease has demonstrated that this disease has a lower level of PRDX4 expression as compared to normal (Ito et al., 2012). PRDX4 can protect the hepatic tissue against the Hydrogen peroxide as well as other ROS causing oxidative stress. The same study has proposed that PRDX4 can be used as a potential biomarker of hepatic diseases as the PRDX4 serum concentration in this model was found to be quite low. Genomic loss of PRDX4 in mice results in testicular atrophy due to elevated spermatogenic cell death (Iuchi et al., 2009). PRDX4 is also related with colorectal carcinoma. The expressions of the PRDX4 gene and protein were higher in colorectal carcinoma compared to those in the adjacent normal tissues and the expression intensity of the PRDX4 protein was correlated with depth of invasion and lymph node metastasis in CRC, and high PRDX4 expression was correlated with short survival time (Yi et al., 2014). These studies suggest that PRDX4 is involved in the tumor promoting effect through formation of Srx-PRDX4 via AP-1/MMP-9 and MAPK signaling, and remove cellular ROS which provides a favorable microenvironment for cell proliferation. Hence, PRDX4 can be a therapeutic target for several cancer treatments.
3.1.5. Beneficial or non-beneficial role of PRDX5 in cancer
The anti-tumor effect of PRDX5 is well established in breast cancer. Recent data showed that of the various PRDX family members, PRDX5 was the only one that was significantly downregulated in tumor samples obtained from Sudanese breast cancer patients (Elamin, Zhu, Hassan, Xu, & Ibrahim, 2013).
The tumor promoting effect of PRDX5 is established in Graves’ disease. PRDX5 expression is higher in the thyroid gland of patients with Graves' disease compared to multinodular goiters, and the level of expression is directly correlated with the functional status of epithelial cells, being higher in multinodular goiters, and even more pronounced in hyperthyroid tissues, such as Graves’ disease (Gérard, Many, Daumerie, Knoops, & Colin, 2005). Wang et al showed that PRDX5 expression is increased in a degenerative tendon compared with normal tendon (Wang et al., 2001). They showed that while PRDX5 was localized to fibroblasts in a normal tendon, it was localized to fibroblasts and endothelial cells in a degenerative tendon (Wang et al., 2001).
These data indicate that PRDX5 may inhibit breast cancers, but promotes Graves’ disease.
3.1.6. Tumor promoting effect of PRDX6
The tumor promoting effect of PRDX6 is well established in lung cancer, ovarian cancer, liver cancer and gastric cancer. PRDX6 has been found at higher levels in lung squamous cell carcinoma patients compared with healthy controls (Zhang et al., 2009). PRDX6 is elevated in several lung diseases including lung cancer, mesothelioma and sarcoidosis (Yun et al., 2014a, b). Invasion- and metastasis-promoting actions of PRDX6 have been found in lung cancer cells (Abbas et al., 2009). The evidence indicates that the activity of PRDX6 contributes to the invasion, promotion, and metastatic ability of lung cancer cells (Ho et al., 2010). We previously found that iPLA2 activity of PRDX6 is critical for lung tumor development in in vivo allograft (Yun et al., 2014a, Yun et al., 2014b). Our recent study demonstrated that PRDX6 promotes tumor development via the JAK2/STAT3 pathway in a urethane-induced lung tumor model (Yun et al., 2015a, Yun et al., 2015b, Yun et al., 2015c). Also, PRDX6 promotes lung tumor progression via its GPx and iPLA2 activities (Yun et al., 2014a, Yun et al., 2014b). In a recent study, the overexpression of PRDX6 attenuated cisplatin-induced apoptosis in human ovarian cancer cells, whereas the reduction of PRDX6 expression increased cell death in liver cancer cells (Pak et al., 2011). PRDX6 is highly upregulated in metastatic gastric cancer cells, which are relatively resistant to TRAIL as compared with primary cancer cells (Choi, Chang, & Jung, 2011). Overexpression of PRDX6 leads to a more invasive phenotype and metastatic potential in human breast cancer through regulation of the levels of Upar, Est-1, MMP-9, RhoC and TIMP-2 expression (Chang et al., 2007). PRDX6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages (Chatterjee et al., 2011). These studies, as well as our own, suggest that PRDX6 promotes lung tumor development via JAK2/STAT3 pathway, its GPx and iPLA2 activities, and is involved in drug resistance of several cancer types, suggesting that PRDX6 can be a therapeutic target for cancer treatment
3.2. Expression of PRDXs in the human cancer patients
PRDX1 is overexpressed or downregulated in the tissue of various cancer patients. Cases exhibiting a low PRDX1 expression level included significantly larger tumor mass cases (P < 0.004), positive lymph node metastasis (P < 0.015), advanced stage (P < 0.002), and poorly differentiated cells (P < 0.020) in oral squamous cell carcinoma (Yanagawa et al., 2000). PRDX1 is highly expressed in human bladder cancer tissues than in the bladder mucosa of normal controls or in normal mucosa surrounding cancer tissues which correlates with development, recurrence and progression of bladder cancer (Quan et al., 2006). Sun et al reported that PRDX1 positive rate was significantly higher (77.1 %) in human hepatocellular carcinoma than in adjacent non-tumorous liver tissues (18.4 %) (Sun et al., 2013). PRDX1 immunoreactivity was positively correlated with VEGF expression and microvessel density and PRDX1 expression was significantly associated with tumor size, microvascular invasion, Edmondson grade, tumor capsula status, serum AFP, and tumor-node-metastasis stage (Sun et al., 2013).
PRDX2 is upregulated in human colorectal carcinoma tissues compared with the matched non cancer colorectal mucosa tissues (Lu et al., 2014a). PRDX2 is markedly upregulated in neutrophils of refractory cytopenia with RCMD patients compared to healthy donors. In addition, white blood cell and neutrophil counts in RCMD patients correlated inversely with the PRDX2 expression (Kazama et al., 2014).
IHC revealed significant overexpression of PRDX3 in prostate cancer, associated with age, increased prostate specific antigen (PSA), tumor stage, or Gleason score, and high PRDX3 staining was associated with early age and elevated Gleason score at time of radical prostatectomy in African-American patients but not in Caucasian patients with prostate cancer (Basu et al., 2011). PRDX3 is upregulated in human endocrine-regulated tumours and in particular prostatic intraepithelial neoplasia and prostate cancer (Whitaker et al., 2013). The overexpression of PRDX3 is associated with 94.9% hepatocellular carcinoma, and correlated with poor differentiation (Qiao et al., 2012). The platinum-resistant ovarian cancer patient group had significantly higher PRDX3 protein compared to the platinum-sensitive ovarian cancer patient group, suggesting PRDX3 may be associated with drug resistance in ovarian cancer (Wang et al., 2013).
The expression of PRDX4 is at least 1.5 fold higher in human tumor cells compared to control and this finding applies most frequently to adenocarcinoma and modestly to squamous cell carcinoma (Lehtonen et al., 2004). PRDX4 expression is elevated in human lung carcinomas compared to nonmalignant tissue, and is mainly associated with adenocarcinoma (Hwang et al., 2015). PRDX4 is also related with colorectal carcinoma. The expressions of the PRDX4 gene and protein were higher in CRC compared to those in the adjacent normal tissues (Yi et al., 2014). The expression intensity of the PRDX4 protein was correlated with depth of invasion, lymph node metastasis and Dukes’ classification in CRC (Yi et al., 2014).
PRDX5 expression in bladder cancer was significantly higher than in normal tissue, as well as corresponding normal bladder mucosa surrounding the cancer (Quan et al., 2006). A recent study demonstrated that PRDX5 expression was higher in the thyroid gland of patients with Graves' disease compared to multinodular goiters (Gérard et al., 2005).
PRDX6 has also been found at higher levels in lung squamous cell carcinoma patients compared with healthy controls (Zhang et al., 2009). Our recent study also showed that the PRDX6 is overexpressed in lung cancer patients (Yun et al., 2015a, Yun et al., 2015b, Yun et al., 2015c). Overexpression of PRDX6 leads to a more invasive phenotype and metastatic potential in human breast cancer (Chang et al., 2007).
3.3. Signaling pathway of PRDXs in the development of cancer
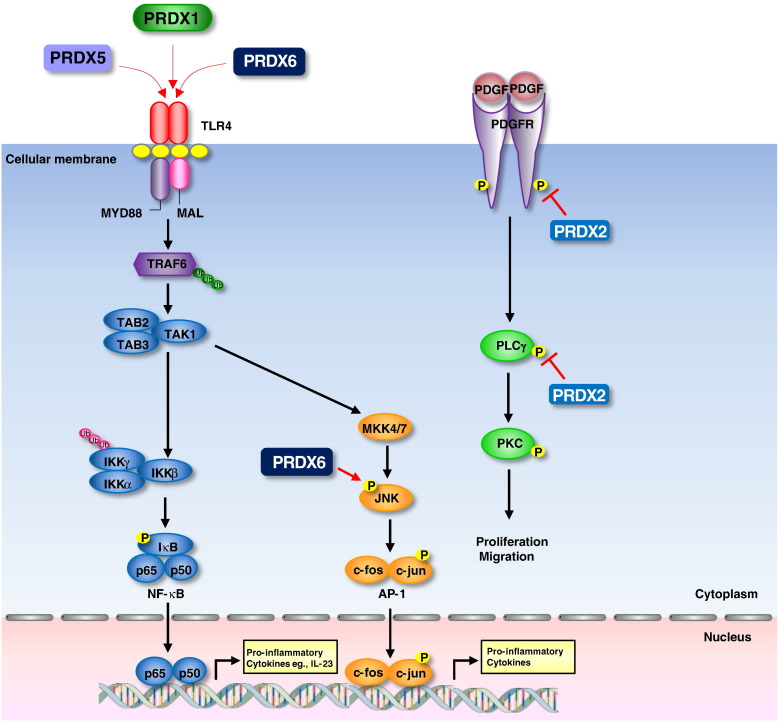
PRDXs are involved in several cancer related signal pathways. Several studies suggested that NF-κB is related with redox signaling pathway. During redox signaling, some PRDXs have been implicated in the regulation of NF-κB through the initial activation in the cytoplasm by controlling the components affecting IκB phosphorylation and subsequent dissociation. In principle, PRDXs could have a different function in the nucleus because NF-κB interactions with DNA are governed by a redox-sensitive cysteine (Cys62) on the p50 subunit of the NF-κB dimer. Oxidation of Cys62 inhibits NF-κB binding and decreases the effectiveness of NF-κB signaling. Also, some PRDXs have also been implicated in the regulation of MAPK pathway during redox signaling. Although inhibiting PRDX appears to have different effects on MAPK activation depending on the cell or stimuli, in some cases the use of different stimuli, or time-points at which MAPK activation is assessed, make comparisons between studies difficult. In any case, the consequence of inhibiting PRDX in any given cell is likely to be determined by the levels of intracellular ROS and intrinsic features of the cell/compartment, such as the capacity to regenerate reduced PRDX, using Srx, or Trx using TrxR and NADPH. Besides these pathways, PRDXs also are involved in several cancer related pathways. Fig. 4 illustrates the roles of PRDXs in the signaling pathways for the development of cancers.
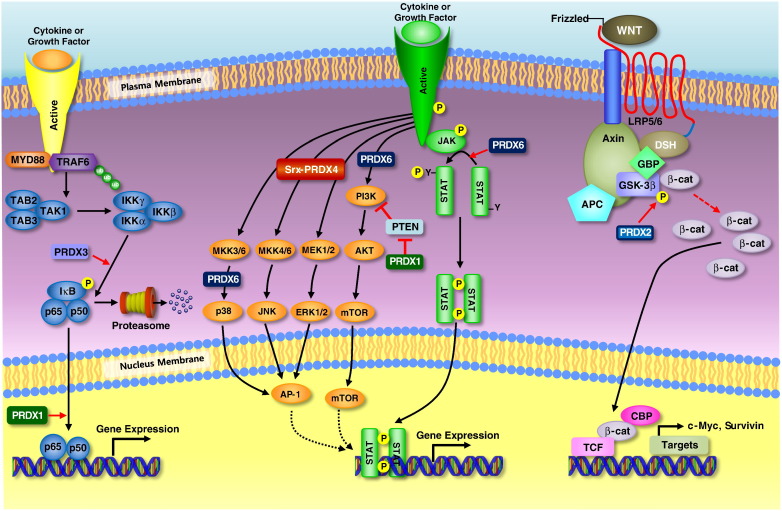
Fig. 4.
Signaling pathway related with cancer development. PRDX1 enhances the transactivation potential of NF-κB in ER-deficient breast cancer cells and upregulates phosphorylation of NF-κB subunit in bladder cancers. PRDX1 promotes tumorigenesis of esophageal squamous cell carcinoma through regulating the activity of mTOR/p70S6K pathway. PRDX2 is involved in the regulation of Wnt/β-catenin signaling in colorectal cancer cells. Specifically, PRDX2 inhibits GSK-3β activity, enhances β-catenin translocation into the nucleus, and inhibits the levels of β-catenin phosphorylation, thus resulting in significant up-regulation of transcription of the LEF/TCF target genes c-Myc and Survivin. PRDX3 is associated with NF-κB signaling pathway. PRDX3 enhances I-κB phosphorylation and induces NF-κB signaling pathway. PRDX3 also acts synergistically with MAP3K13 to regulate the activation of NF-κB in the cytosol. PRDX4 binds with Srx and the Srx-PRDX4 axis contributes to the maintenance of lung tumor phenotype by AP-1 and MAPK signaling pathway. PRDX6 promotes tumor development via the JAK2/STAT3 pathway in a urethane-induced lung tumor model. Upregulation of PRDX6 results in the activation of Akt via PI3K and p38 kinase.
It was shown that overexpression of PRDX1 and PRDX2 suppresses PDGF- and EGF-dependent H2O2 production as well as TNF-α-induced NF-κB transcriptional activity (Kang et al., 1998). Similar studies have since followed using other ligands, such as thyrotropin (TSH), TNF-α, and TNF-related apoptosis-inducing ligand (TRAIL), as well as further work on EGF dependent H2O2 production, in various cell types (Benvenga & Koch, 2014). Collectively, these studies show that 2-Cys PRDXs not only eliminate the levels of intracellular H2O2 that were increased upon receptor stimulation, but also suppress the downstream signaling responses, including NF-κB transcriptional activity, JNK activity and apoptosis (Yang et al., 2007).
PRDX2 is involved in differential regulation of different MAP kinases. Under conditions of TNF-α stimulation in which PRDX2 activity was either partially blocked using a dominant negative mutant version or was completely abolished by gene knockout, JNK and p38 MAP kinase activation were enhanced whereas the activation of extracellular signal related kinase (ERK) was suppressed (Hanschmann, Godoy, Berndt, Hudemann, & Lillig, 2013). It was asserted that the endogenous H2O2 regulated by PRDX2 targets the phosphorylation of the tyrosines at sites 579/581 and 857 in the PDGF receptor, but has no effect on any of the other tyrosine sites that are known to be phosphorylated, whereas exogenously added H2O2 was shown to induce the phosphorylation of all possible tyrosine sites (Choi et al., 2005). It was also shown that the site selective response is controlled solely by PRDX2 and not by other peroxidases, and is achieved through the inactivation of the membrane-associated protein tyrosine phosphatases (PTPs) (Rhee et al., 2012).
Xi et al reported that the protein levels of PRDX3 are significantly reduced in VHL-deficient clear cell renal cell carcinoma (CCRCC) (Xi et al., 2014). Furthermore, stabilization of HIF-1α protein, caused either by VHL deficiency under normoxia, or by hypoxia, significantly reduced PRDX3 expression. Luciferase-reporter and chromatin-immunoprecipitation assays indicated that HIF-1α binds to the hypoxia-responsive elements of PRDX3 promoter and represses its transcription. Finally, shRNA-based assays suggested that PRDX3 downregulation is required for the HIF-1α-dependent proliferation of CCRCC cells (Xi et al., 2014). Ovarian cancer cells transfected with PRDX3/small interfering (Si)‑1 efficiently downregulated the expression of PRDX3 and thus decreased the growth of the ovarian cancer cells in vitro and in vivo. Furthermore, the silencing of PRDX3 triggered cisplatin‑mediated apoptosis in the ovarian cancer cells, which may act through suppression of the NF‑κB signaling pathway (Song et al., 2011). In antiandrogen-resistant LNCaP cell lines, PRDX3 is upregulated and the resistant cells also possess an upregulation of the TCA pathway and resistance to H₂O₂-induced apoptosis through a failure to activate pro-apoptotic pathways. Also, knockdown of PRDX3 restored H₂O₂ sensitivity, and PRDX3 has been identified as a gene induced by oncogenic c-Myc (Whitaker et al., 2013). Its specific localization to mitochondria suggests that PRDX3, together with its mitochondrion-specific electron suppliers Trx2 and Trx reductase (TrxR) 2, might provide a primary line of defense against H2O2 produced by the mitochondrial respiratory chain, as SOD2 does against the superoxide radical. In addition, Srx plays a crucial role by reducing hyperoxidized PRDX3 via translocation into mitochondria. Noh et al. reported that the overexpression of mitochondrion-targeted Srx efficiently promotes the restoration of PRDX3 and results in cellular resistance to apoptosis, with enhanced elimination of mitochondrial H2O2 and decreased rates of ΔΨm collapse (Noh, Baek, Jeong, Rhee, & Chang, 2009). Thus, a Trx-related antioxidant system composed of Trx2, TrxR2, and PRDX3 has been closely associated with the regulation of apoptosis and the redox control of MPT pores for the release of cytochrome c (Noh et al., 2009).
PRDX4 is associated with lung cancer. Srx preferentially binds to PRDX4 and the Srx–PRDX4 axis promotes human lung cancer progression through modulation of receptor tyrosine kinase related signaling (Wei et al., 2011). The same study has also shown the role of Srx-PRDX4 axis in activation of intracellular phosphokinase signaling including AP-1/MMP-9 axis and MAPK signaling (Wei et al., 2011). Also, the Srx-PRDX4 plays a very important role in tumor progression and metastasis in lung cancer. Wei et al demonstrated that an alteration in the expression of PRDX4 results in an alteration in the rate of tumor progression and metastasis which is indicated by anchorage independent colony formation, cell migration and invasion of human lung cancer cells (Wei et al., 2011). This ability of PRDX4 to promote tumor progression and metastasis is supposedly due to its antioxidant properties.
Analysis of the promoter region of the PRDX5 gene and reporter gene assays revealed the promoter region critical for PRDX5 gene regulation to which the novel negative transcription regulator, GATA1 binds in human breast cancer cell lines (Seo, Liu, Chang, & Park, 2012). They showed that knockdown of GATA1 led to an increased expression of PRDX5 and inhibition of apoptosis, suggesting that PRDX5 may protect cells from oxidative stress-mediated apoptosis in a GATA1-regulated manner.
In a recent study, overexpression of PRDX6 attenuates cisplatin-induced apoptosis in human ovarian cancer cells (Pak et al., 2011). Pak et al showed that cisplatin-induced cytotoxicity in ovarian cancer cells cytotoxicity was associated with increased accumulation of intracellular (ROS) and apoptosis mediated by proteolytically activated caspase 3 and 9. Moreover, overexpression of PRDX 6 protein or exposure to N-acetylcysteine (NAC) reversed the apoptotic effect of cisplatin by reducing ROS levels and suppressing the caspase signaling pathway. In contrast, reduction of PRDX6 expression increased peroxide-induced cell death in liver cancer cells (Walsh et al., 2009). They showed that the cancerous hepatoma cell line is significantly more resistant to peroxide-induced cytotoxicity than the non-cancerous cell line, and hepatoma cell line expresses approximately 3-fold PRDX6 than non-cancerous cell line. Also, they showed that transient transfection of cancerous hepatoma cell line with PRDX6 siRNA led to an increase in peroxide-induced cytotoxicity by apoptosis. The invasion-promoting action of PRDX6 has also been confirmed using lung cancer cells, in which upregulation of PRDX6 results in the activation of Akt via phosphoinositide 3-kinase (PI3K) and p38 kinase, which promotes cell invasion by inducing urokinase-type plasminogen activator (uPA) (Ho et al., 2010; Seung et al., 2009). Our study demonstrated that PRDX6 promoted lung tumor growth in an in vivo allograft model. Histopathological and Western blotting examination demonstrated that expression of proliferating cell nuclear antigen, VEGF, MMP-2 and -9, and cyclin-dependent kinases accompanied by increased iPLA2 and GPx activities were increased in the tumor tissues of PRDX6-overexpressing nude mice (Jo et al., 2013). In tumor tissues of PRDX6-overexpressing mice, the activation of mitogen-activated protein kinases and AP-1 DNA binding were also increased (Yun et al., 2014a, Yun et al., 2014b). And the growth of lung cancer cell lines (A549 and NCI-H460) was enhanced by the increase in iPLA2 and GPx activities of PRDX6. In addition, mutant PRDX6 (C47S) attenuated PRDX6-mediated p38, ERK1/2, and AP-1 activities as well as its enzyme activities in the A549 and NCI-H460 lines. Furthermore, tumor growth and p38, ERK1/2, and AP-1 activities were also inhibited in nude mice bearing mutant PRDX6 (C47S) compared to PRDX6. Previously, we showed that urethane (1g/kg)-induced tumor incidence in PRDX6-Tg mice was significantly higher compared to non-Tg mice (Yun et al., 2015a, Yun et al., 2015b, Yun et al., 2015c). In the tumors of PRDX6-Tg mice, the activation of JAK2/STAT3 and STAT3 DNA binding were also increased, accompanied by increased GPx and iPLA2 activities. PRDX6 was colocalized with JAK2 in tumor tissues and lung cancer cells and also showed physical interaction with JAK2, and increased levels of PRDX6 increase the activation of the JAK2/STAT3 pathway. Furthermore, PRDX6-Tg mice showed altered cytokine levels in the tumors, especially leading to increased CCL5 levels. We validated that the activation of JAK2 was also decreased in lung tumors of CCR5-/- mice, and CCL5 increased the JAK2/STAT3 pathway in the lung cancer cells, suggesting that PRDX6 promotes lung tumor development via its mediated and CCL5-associated activation of the JAK2/STAT3 pathway.
4. PRDXs and neurodegenerative diseases
PRDXs are differentially expressed in the brain regions depending on the subfamily. PRDX1 and 6 are expressed in glial cells, whereas PRDX2, 3, 4 and 5 are expressed in neurons (Hanschmann et al., 2013). These data indicate that the two PRDXs have distinct functional roles in the brain and provide differential contributions to neuropathologic conditions. The expression patterns of PRDXs are in fact highly variable in different regions of the brain during neurodegenerative disease processes (Hanschmann et al., 2013). Given that oxidative damage is involved in the pathogenesis of neurodegenerative diseases, the alteration in PRDX expression would appear to be primarily a consequence of cellular resistance to the oxidative damage (Perez-Pinzon, Stetler, & Fiskum, 2012). Several studies suggested that the PRDXs are related with the development of neurodegenerative diseases or neuroprotective effects (Perez-Pinzon et al., 2012, Soriano et al., 2008). In this regard, we reviewed the roles of PRDXs in the development of neurodegenerative diseases and neuroprotective effects. The relationship between neurodegenerative diseases and PRDXs is summarized in Table 3 .
Table 3.
Summary of function of PRDX subfamily in different neurodenerative disease types
| PRDX type | Disease type | Effects | Results | Mechanisms | Reference |
|---|---|---|---|---|---|
| PRDX1 PRDX2 |
Sporadic Creutzfeldt-Jacob disease | Neuroprotective effect | Decrease the disease | PRDX1 is decreased in the frontal cortex of patients with Sporadic Creutzfeldt-Jacob disease | Krapfenbauer, Yoo, Fountoulakis, Mitrova, & Lubec, 2002 |
| Neuroblastoma | Neurodegenerative effect | Enhance the neuroblastoma | PRDX1 is induced by the accumulation of Aβ1-42 peptides in neuroblastoma cells | Lee et al., 2011 | |
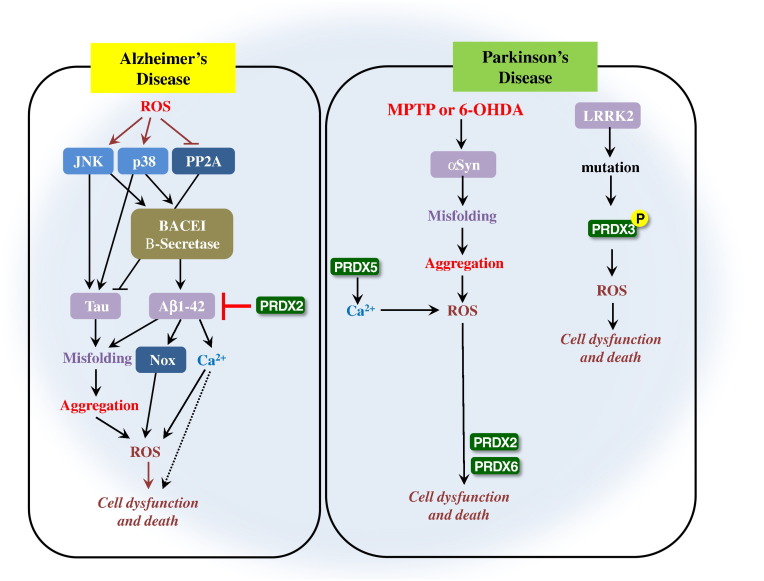
| AD | Neuroprotective effect | Protects the brain | PRDX2 is overexpressed in the brain of AD patients indicating that PRDX2 protects the brain from Aβ1-42 induced neurotoxicity | Cimini et al., 2013 | |
| PD | Neurodegenerative effect | Increase the risk of PD | -PRDX2 is involved in the regulation of 6-OHDA-induced apoptosis signaling in DA induced neuronal cell death -PRDX2 phosphorylation is involved in PD -PRDX2 promotes oxidative stress-induced neuronal cell death in PD |
Hu et al., 2011, Qu et al., 2007, Fang et al., 2007 | |
| PRDX3 | Excitotoxic injury | Neuroprotective effect | Protects the neuron | PRDX3 is involved in the protection of hippocampal neurons from excitotoxic injury | Hattori et al., 2003 |
| Down syndrome | Neuroprotective effect | Protects the brain from Down syndrome | PRDX3 is decreased in frontal cortex of Down syndrome | Krapfenbauer et al., 2003 | |
| Pick’s disease | Neuroprotective effect | Protects the brain from Pick’s disease | PRDX3 is decreased in frontal cortex of Pick’s disease | Krapfenbauer et al., 2003 | |
| PD | Neurodegenerative effect | Increase the risk of PD | PRDX3 phosphorylation is increased in autosomal dominant mutation of LRRK2 that is important for PD and results in the oxidative stress induced neuronal death | Angeles et al., 2011 | |
| PRDX4 | Pick’s disease | Neurodegenerative effect | Increase the risk of Pick’s disease | PRDX4 is significantly increased only in Pick’s disease frontal cortex | Krapfenbauer et al., 2003 |
| PRDX5 | PD | Neurodegenerative effect | Increase the risk of PD | PRDX5 is involved in PD through mitochondrial redox signaling with Ca2 + influx resulting in the development of PD | Davey & Bolaños, 2013 |
| PRDX6 | AD | Neurodegenerative effect | Increase the risk of AD | -PRDX6 accelerates the development of AD through increased amyloidogenesis through independent PLA2 activation and Nrf2 transcription. - PRDX6 activates astrocyte and microglia cells, lipid peroxidation and protein carbonyl levels in PRDX6 overexpressed transgenic mice. |
Shalini et al., 2014, Yun et al., 2013 |
| PD | Neurodegenerative effect | Increase the risk of PD | PRDX6 induces astrocytic activation followed by increased proinflammatory cytokines (TNF-α and IL1-β), 4-HNE, and PRDX6 hyperoxidation in primary cultured astrocytes. | Yun et al., 2015c, Yun et al., 2015b, Yun et al., 2015a |
4.1. Roles of PRDXs in the development of neurodegenerative diseases
4.1.1. Dual function of PRDX1 in neurodegenerative diseases
The neuroprotective role of PRDX1 is well established. PRDX1 has a protective role in counteracting Aβ injury by increasing cell viability, preserving neurites, and decreasing cell death (Cimini et al., 2013). The neurodegenerative role of PRDX1 is also established in neuroblastoma cells (Lee et al., 2011).
4.1.2. Neuroprotective or neurodegenerative effect of PRDX2
The neuroprotective role of PRDX2 is well established in AD and PD. Among the antioxidant PRDX enzymes, PRDX2 is the most abundant in mammalian neurons, making it a prime candidate to defend against oxidative stress (Fang, Nakamura, Cho, Gu, & Lipton, 2007). PRDX2 is overexpressed in the brain of Alzheimer’s disease (AD) indicating that PRDX2 protects the brain from Aβ1-42 induced neurotoxicity (Yao et al., 2007). Another study also suggested that PRDX2 exhibited anti apoptotic effects in DA neurons via suppression of ASK1 dependent activation of JNK and p38 pathways which are activated in PA neurons of PD brains (Hu et al., 2011). The neurodegenerative role of PRDX2 is also established in AD and PD. The role of PRDX2 in PD is controversial. Garcia-Garcia et al suggested that the oxidative modification of PRDX2 is associated with drug induced apoptosis signaling in the model of Parkinson disease (Garcia-Garcia, Zavala-Flores, Rodriguez-Rocha, & Franco, 2012). They suggested that the oxidative modification of PRDX2 is involved in the regulation of 6-OHDA-induced apoptotic signaling in DA induced neuronal cell death. Qu et al defined the role of PRDX2 in PD and suggested that phosphorylation of PRDX2 isoform is involved in PD (Qu et al., 2007). In a histochemical study of brains from AD patients, nitrated PRDX2 was identified. Randall et al. investigated the functional consequences of PRDX2 tyrosine nitration, and demonstrated that nitration was on a non-catalytic residue that resulted in increases in peroxidase activity and resistance to over-oxidation (Randall et al., 2014). Fang et al observed increased S-nitrosylation of PRDX2 in human Parkinson's disease (PD) brains, and S-nitrosylation of PRDX2 inhibited both its enzymatic activity and protective function from oxidative stress (Fang et al., 2007). Dopaminergic neurons, which are lost in PD, become particularly vulnerable, provided a direct link between nitrosative/oxidative stress and neurodegenerative disorders such as PD (Fang et al., 2007). These data suggest that not only is PRDX2 involved in neuroprotection via inhibition of Aβ1-42 induced apoptosis and suppression of JNK and p38 pathways, but also involved in neurodegeneration via oxidative modification, phosphorylation and s-nitrosylation of PRDX2.
4.1.3. Dual function of PRDX3 in neurodegenerative diseases
The neuroprotective role of PRDX3 is well established. PRDX3 is involved in the protection of hippocampal neurons from excitotoxic injury. Hattori et al showed that PRDX3 is up-regulated in the mitochondria of damaged nerve cells (Hattori, Murayama, Noshita, & Oikawa, 2003). Because mitochondria is involved in excitotoxic damage of nerve cells, mitochondrial PRDX3 seems to be neuroprotective against oxidative insults (Hattori et al., 2003). Also, PRDX3 is decreased in the frontal cortex of patients with Down syndrome (P < 0.01) and Pick’s disease (P < 0.001) (Krapfenbauer, Engidawork, Cairns, Fountoulakis, & Lubec, 2003). The neurodegenerative role of PRDX3 is established in PD. A recent study also suggested that the autosomal dominant mutation of LRRK2 that is important for PD, increased the phosphorylation of PRDX3 and resulted in the oxidative stress induced neuronal death (Angeles et al., 2011). These data indicate that PRDX3 is involved in the neuroprotection against oxidative insults in the mitochondria, and involved in neurodegeneration through increased phosphorylation by LRRK2 mutation.
4.1.4. Role of PRDX4 and PRDX5 in neurodegenerative diseases
There are not many research reports in PRDX4 and PRDX5. PRDX4 is significantly increased only in Pick’s disease frontal cortex (Krapfenbauer et al., 2003). PRDX5 is involved in PD through mitochondrial redox signaling with Ca2 + influx resulting in the development of PD (Davey & Bolaños, 2013).
4.1.5. Role of PRDX6 in neuroprotection and neurodegeneration
The neuroprotective role of PRDX6 is established in an experimental autoimmune encephalomyelitis (EAE) model. In the recent study, we also showed that the expression of an PRDX6 is markedly increased in spinal cords of mice with EAE compared to other PRDXs. PRDX6 transgenic mice displayed a significant decrease in clinical severity and attenuated demyelination in EAE compared to wild type mice, suggesting that PRDX6 expression may represent a therapeutic way to restrict inflammation in the central nervous system and potentiate oligodendrocyte survival, and suggest a new molecule for neuroprotective therapies in MS (Yun et al., 2015a, Yun et al., 2015b, Yun et al., 2015c). The neurodegenerative role of PRDX6 is established in AD and PD. Our recent study suggested that overexpression of PRDX6 could accelerate the development of AD through increased amyloidogenesis through independent PLA2 activation and Nrf2 transcription (Yun et al., 2013). In a mouse model after amyloid beta infusion, memory impairment in PRDX6 transgenic mice was worse than C57BL/6 mice. In addition, the astrocytes and microglia cells of amyloid-infused PRDX6 transgenic mice were more activated, lipid peroxidation and protein carbonyl levels were increased and glutathione levels were lower, suggesting that PRDX6 is promoting rather than preventing oxidative stress (Yun et al., 2013). Our recent study also suggested that PRDX6 exacerbates dopaminergic neurodegeneration in a MPTP mouse model of PD (Yun et al., 2015a, Yun et al., 2015b, Yun et al., 2015c). These data indicate that PRDX6 induces neurodegeneration through iPLA2 and nrf2 activation in AD, and induction of MPTP-induced dopaminergic neurodegeneration of PD, but inhibits multiple sclerosis by suppressing inflammation and blood brain barrier disruption in EAE model.
4.2. Signaling pathway of PRDXs in neurodegenerative diseases
The Trx system protects against H2O2-induced apoptosis, and the Trx-PRDX system detoxifies peroxides by transferring reducing equivalents from NADPH to peroxides with cytoprotective and antioxidative effect (Soriano et al., 2008). Several studies reported that PRDX1-4 are implicated in protecting neuronal cells from Aβ toxicity (Yao et al., 2007), peroxide and 1-methyl-4-phenylpyridinium (MPP(+)) toxicity (Qu et al., 2007), oxygen-glucose deprivation and excitotoxicity (Hattori et al., 2003). Also, other PRDXs are also involved in neurodegenerative diseases. Fig. 5 illustrates the roles of PRDXs in the signaling pathways for the neurodegenerative diseases.
Fig. 5.
Possible mechanisms of PRDXs related with neurodegenerative disease. ROS mediate neurotoxicity of AD through modifying the hallmark protein by oxidation. In AD, ROS could also activate c-Jun N-terminal kinases (JNK) and p38, and deactivate protein phosphatase 2A (PP2A). JNK and p38 promote the expression of Tau, which is inhibited by PP2A. The activation of JNK and p38 further stimulate APP cleaving enzyme 1 (BACE1), causing Aβ1-42 accumulation, which leads to activation of NADPH oxidase (Nox) to produce additional O2, and results in Ca2 + influx to elicit excitatory neurotoxicity. PRDX2 inhibits the generation and accumulation of Aβ1-42, and protects the brain from AD neurotoxicity. PRDX6 accelerates cell dysfunction and cell death proceeded from ROS generation. In PD, the α-Syn is aggregated and generates ROS that also generated from Ca2 + accumulation. PRDX5 induces the Ca2 + accumulation that can cause ROS generation. PRDX2 and PRDX5 mediate the neurotoxicity through induction of cell dysfunction and cell death from ROS accumulation. PD is also generated from LRRK2 mutation. This mutation causes PRDX3 phosphorylation, after that ROS accumulates, resulting in cellular dysfunction and cellular death.
Overexpression of PRDX1 protects against 6 hydroxydopamine toxicity, prevents p38 MAPK activation and subsequent activation of caspase-3 in MN9D cells, but apoptotic death signals were enhanced by RNA interference-targeted reduction of PRDX1 (Lee et al., 2008). PRDX2 is involved in neurodegeneration through phosphorylation by cdk2, a serine/threonine kinase, and contributes to PD.
In a recent study, Qu et al. showed that cyclin-dependent kinase 5 (cdk5) inhibits PRDX2 activity, leading to increased levels of ROS in PD models (Qu et al., 2007). They also demonstrated that PRDX2 is a bona fide substrate for the cdk5-p35 complex, which phosphorylated PRDX2 at the threonine residue at position 89. Cyclin-dependent kinase 5, a serine/threonine kinase, contributes to neurodegeneration in PD. This kinase is shown to phosphorylate PRDX2, inactivating it, and thereby sensitizing neurons to the deleterious effects of parkinsonian toxins. Hu et al. explored the role of PRDX2 and showed it inhibited 6 hydroxydopamine-induced ASK1 activation by modulating the redox state of Trx1 preventing its dissociation from ASK1 (Hu et al., 2011). They showed that Lentivirus-mediated PRDX2 overexpression conferred marked in vitro and in vivo neuroprotection against 6-OHDA toxicity in DA neurons, and preserved motor functions involving the dopamine system in mouse. In addition to its role as an antioxidant enzyme, PRDX2 exhibited anti-apoptotic effects in DA neurons via suppression of apoptosis signal-regulating kinase (ASK1)-dependent activation of the c-Jun N-terminal kinase/c-Jun and p38 pro-death pathways, which are also activated in DA neurons of postmortem PD brains. PRDX2 inhibited 6-OHDA-induced ASK1 activation by modulating the redox status of the endogenous ASK1 inhibitor Trx. PRDX2 overexpression maintained Trx in a reduced state by inhibiting the cysteine thiol-disulphide exchange, thereby preventing its dissociation from ASK1.
In cells expressing the LRRK2 mutant gene that is responsible for up to 30–40% of PD cases in some ethnic populations, the phosphorylation of PRDX3 is increased but decreased peroxidase activity and increased death in neuronal cells (Angeles et al., 2011).
Kim et al suggested that apoptosis was inhibited after amyloid beta treatment in PC12 cells overexpressing wild-type PRDX6, but not in cells that overexpressed the C47S catalytic mutant. This indicates that the peroxidase activity of PRDX6 protects PC12 cells from amyloid-induced neurotoxicity (Kim et al., 2013a, b). Our recent study also demonstrated that Aβ1-42-induced memory impairment in PRDX6 transgenic mice was worse than C57BL/6 mice, and the expression of amyloid precursor protein cleavage, C99, β-site APP-cleaving enzyme 1, inducible nitric oxide synthase, and cyclooxygenase-2 was greatly increased (Yun et al., 2013). In addition, the astrocytes and microglia cells of Aβ-infused PRDX6 transgenic mice were more activated, and Aβ also significantly increased lipid peroxidation and protein carbonyl levels, but decreased glutathione levels. Furthermore, we found that translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) to the nucleus was increased in Aβ-infused PRDX6 transgenic mice. These results suggest that the overexpression of PRDX6 could accelerate the development of AD through increased amyloidogenesis through independent PLA2 activation and Nrf2 transcription. We also recently investigated the effects of PRDX6 on MPTP-induced dopaminergic neurodegeneration using PRDX6 Tg mice (Yun et al., 2015a, Yun et al., 2015b, Yun et al., 2015c). PRDX6 Tg mice had a much higher loss of dopaminergic neurons by MPTP administration compared to non-Tg mice. There was also a much higher behavioral impairment and astrocyte activation in PRDX6 Tg mice. MPTP-induced GPx activity was not different between PRDX6 Tg mice and non-Tg mice, which is accompanied by hyperoxidation of PRDX6. Intriguingly, the expression pattern of PRDX6 showed similar distribution and co-localization with astrocytes, but not neurons in the mouse and human brain. Furthermore, we demonstrated that iPLA2 activity of PRDX6 induced astrocytic activation followed by increased proinflammatory cytokines (TNF- α and IL1-β), 4-HNE, and PRDX6 hyperoxidation in primary cultured astrocytes. A recent study suggested the translational potential of blocking TLR4/PRDX6 signaling for the treatment of ischemic stroke (Kuang et al., 2014). They showed that ligustilide (LIG) exerted antineuroinflammatory and neuroprotective effects against an ischemic insult, and showed that TLR4/PRDX6 pathway is involved in the protective effect of LIG against postischemic neuroinflammation and brain injury induced by transient middle cerebral artery occlusion (MCAO) in rats. Also, LIG-induced neuroprotection was accompanied by the improvement of neuropathological alterations, including neuron loss, astrocyte and microglia/macrophage activation, neutrophil and T-lymphocyte invasion, and regulation of inflammatory mediator expression. Moreover, LIG significantly inhibited the expression and extracellular release of PRDX6 and activation of TLR4 signaling, reflected by decreased TLR4 expression, extracellular signal-regulated kinase 1/2 phosphorylation, and transcriptional activity of NF-κB and signal transducer and activator of transcription 3 in the ischemic brain.
5. PRDXs and inflammatory disease
Redox-regulated transcription factors and protein kinases play important roles in the regulation of inflammation (Adler, Yin, Tew, & Ronai, 1999). NF-κB is a well-characterized redox-sensitive transcription factor important in the initiation and progression of inflammation through the production of various cytokines and prostaglandins (Kunsch & Medford, 1999). Nrf2 is another redox-regulated transcription factor important for cellular defense against oxidative and electrophilic stress (Trachootham et al., 2008). Nrf2 activates the expression of stress-dependent cytoprotective genes such as NAD(P)H-quinone oxidoreductase 1 and glutathione S-transferase (GST) through a cis-acting element called the ARE/EpRE (Kobayashi et al., 2006). PRDXs secreted from cells under mild oxidative stress binds TLR4 and PRDXs induces Nrf2 or NF-κB activation that is important for the expression of inflammatory proteins, suggesting that PRDXs are involved in the inflammation related disease (Madrigal-Matute et al., 2012, Westermann et al., 1996).
5.1. Roles of PRDXs in the development of inflammatory disease
PRDXs are related with several inflammatory diseases such as atherosclerosis, RA and obesity. The relationship between inflammatory diseases and PRDXs is summarized in Table 4 .
Table 4.
Summary of function of PRDX subfamily in different inflammatory disease types
| PRDX type | Disease type | Effects | Results | Mechanisms | Reference |
|---|---|---|---|---|---|
| PRDX1 | Atherosclerosis | Benefit effect | Protects against atherosclerosis | PRDX1 is induced by exposing macrophages to oxidized LDL5 and by laminar shear stress in endothelial cells PRDX1 protects against excessive endothelial activation and atherosclerosis |
Kisucka et al., 2008; Mowbray et al., 2008 |
| Rheumatoid arthritis | Non-benefit effect | Enhance the risk of RA | PRDX1 is overexpressed in RA patient tissue by more than 3 fold compared with normal tissues. | Li et al., 2013 | |
| PRDX2 | Atherosclerosis | Benefit effect | Suppresses Atherosclerosis | PRDX2 suppresses the proliferation and migration of SMCs through the site-selective phosphorylation of the PDGF receptor and, furthermore, is involved in the neointimal thickening of SMCs in balloon-injured carotidartery | Kang et al., 2013 |
| Rheumatoid arthritis | Non-benefit effect | Enhance the risk of RA | PRDX2 is involved in the persistence of pro-inflammatory cells in chronic inflammation because PRDX2 protein content of lymphocytes from RA patient was more than 2-fold higher compared with healthy lymphocytes | Szabó-Taylor et al., 2012 | |
| PRDX3 | Obesity | Benefit effect | Decrease the risk of obesity | PRDX3 deficiency increased abnormal lipid accumulation in adipose tissue, due to increased ROS | Huh et al., 2012 |
| PRDX4 | Atherosclerosis | Benefit effect | Protects against atherosclerosis | PRDX4 protects the vascular tissue against ROS and hence, it has ability to inhibit the oxidative stress induced inflammation in various tissues | Guo et al., 2012 |
| Non-benefit effect | Enhance the risk of RA | PRDX4 is increased in synovial tissues of RA patients | Chang et al., 2009 | ||
| Rheumatoid arthritis | Benefit effect | Decrease the risk of obesity | PRDX4 prevents HFD-induced hepatic steatosis and insulin resistance by amelioration of oxidative stress in PRDX4 overexpressed transgenic mice | Ding et al., 2010 | |
| Obesity | Benefit effect | Protects against atherosclerosis | PRDX6-/- macrophages oxidized LDL significantly more than did controls and plasma lipid hydroperoxide levels were higher in atherogenic diet-fed Prdx6-/- mice than in controls. | Wang et al., 2004 | |
| PRDX6 | Rheumatoid arthritis | Non-benefit effect | Enhance the risk of RA | PRDX6 promotes development of RA through activation of NF-κB/AP-1 coupled JNK pathway in the CAIA and AIA-induced arthritis development model | Kim et al., 2015 |
5.1.1. Dual function of PRDX1 in inflammatory diseases
The beneficial role of PRDX1 in inflammatory diseases is well established. In addition, PRDX1 deficiency enhanced the regulated secretion pathway in endothelial cells by the promotion of excessive release of several proinflammatory components. Kisucka et al examined the role of PRDX1 in the apolipoprotein E-deficient (apoE-/-) murine spontaneous model of atherosclerosis (Kisucka et al., 2008). They showed that PRDX1-/-/apoE-/- mice fed normal chow developed larger, more macrophage-rich aortic sinus lesions than PRDX1+/+/apoE-/- mice, despite similar amounts and size distributions of cholesterol in their plasma lipoproteins, suggesting that PRDX1 protects against excessive endothelial activation and atherosclerosis. The role of PRDX1 as non-beneficial in inflammatory diseases is also established. PRDX1 is induced by exposing macrophages to oxidized LDL5 and by laminar shear stress in endothelial cells (Conway and Kinter, 2006, Mowbray et al., 2008). Also, transcription factor Nrf2, one of the erythroid transcription factor NFE2 subunit factors responsible for the induction of defense proteins including PRDX1, is activated in oxidized LDL-treated macrophage cells, thus these data suggested that PRDX1 is involved in macrophage activation (Kisucka et al., 2008). In the RA patient tissue, the PRDX1 is overexpressed by more than 3 fold compared with normal tissues (Li et al., 2013). Demasi et al suggested that PRDX1 induction could be considered a crucial part of the cellular adaptive response to the B-cell differentiation process to cope with the additional H2O2 associated with massive disulphide bond formation during immunoglobulin folding in the ER of plasma cells (Demasi et al., 2007). These data indicate that PRDX1 inhibits inflammatory disease by protection of excessive endothelial activation and atherosclerosis, but induces inflammatory disease via activation of Nrf2.
5.1.2. Dual function of PRDX2 in inflammatory diseases
The beneficial role of PRDX2 in inflammatory diseases is well established. PRDX2, a thiol-specific peroxidase, has been reported to regulate proinflammatory responses, vascular remodeling, and global oxidative stress (Park et al., 2011). PRDX2 removes transiently produced H2O2 in response to activation of various cell surface receptors. PRDX2 regulates platelet-derived growth factor (PDGF) signaling, including enhanced activation of the PDGF receptor and phospholipase Cγ1, and vascular remodeling, including PDGF-dependent neointimal thickening of vascular smooth muscle cells (Choi et al., 2005). Yang et al showed that PRDX2 inhibits general immune cell responsiveness by scavenging low levels of ROS, modulating LPS-induced proinflammatory responses, and protecting against endotoxin-induced lethal shock (Yang et al., 2007). A recent report showed that PRDX2 is more susceptible than PRDX1 to hyperoxidation in cells subjected to sustained global oxidative stress, which suggests that PRDX2 deficiency may lead to accelerated atherosclerosis due to failure to eliminate ROS (Woo et al., 2010). It has recently been shown that PRDX2 suppresses the proliferation and migration of smooth muscle cells (SMCs) through the site-selective phosphorylation of the PDGF receptor and, furthermore, is involved in the neointimal thickening of SMCs in balloon-injured carotidartery (Kang et al., 2013). The role of PRDX2 in inflammatory diseases as non-beneficial in inflammatory diseases is also established. In a recent study by Bayer et al, PRDX2 is increased in the presence of the neutrophils and under acidotic conditions and also oxidation of erythrocyte PRDX2 is increased in a mouse model of endotoxemia (Bayer, Maghzal, Stocker, Hampton, & Winterbourn, 2013). Another recent study also showed that the level of intracellular PRDX2 protein content of lymphocytes from RA patients was more than 2-fold higher compared with healthy lymphocytes suggesting that PRDX2 is involved in the persistence of pro-inflammatory cells in chronic inflammation (Szabó-Taylor et al., 2012). Also, PRDX2 is readily detected in the blood of severe acute respiratory syndrome patients, whereas it is undetectable in normal control blood (Chen et al., 2004). Recombinant PRDX2 induces macrophages to produce TNF-α in the absence of any other stimuli, demonstrating that the Trx peroxidase PRDX2 is appropriately classified as a danger signal, meaning a molecule that is released by cells undergoing stress and acts as an immune mediator (Salzano et al., 2014). These data suggest that PRDX2 suppresses inflammatory diseases through inhibition of PDGF signaling, inhibition of immune cell responsiveness and elimination of ROS, but induces inflammatory diseases through persistence of pro-inflammatory cells and mediation of TNF-α production.
5.1.3. Anti-inflammatory effect of PRDX3
The beneficial role of PRDX3 in inflammatory diseases is well established. PRDX3 deficiency increased abnormal lipid accumulation in adipose tissue, due to increased ROS (Huh et al., 2012). They suggested that endogenous PRDX3 may play an essential role in maintaining normal characteristics of adipocytes and that a defect in PRDX3 alters mitochondrial redox state and function, and adipokine expression in adipocytes leading to metabolic alteration (Huh et al., 2012). These data indicate that PRDX3 protects the inflammatory diseases through elimination of lipid accumulation.
5.1.4. Beneficial or non-beneficial role of PRDX4 in inflammatory diseases
The beneficial role of PRDX4 in inflammatory diseases is well established. It was reported that HFD-induced hepatic steatosis and insulin resistance were prevented in PRDX4 transgenic mice by amelioration of oxidative stress (Nabeshima et al., 2013). It has been suggested that streptozotocin-treated Tg mice, which overexpress PRDX4 in pancreatic islets, can protect pancreatic beta-cells against streptozotocin-induced injury (insulitis) by suppressing increased oxidative stress and inflammatory signaling activation (Ding et al., 2010). The role of PRDX4 in inflammatory diseases as non-beneficial is also established. Chang et al demonstrated that the expression of PRDX4 is increased in synovial tissues of RA patients (Chang et al., 2009). These data indicate that PRDX4 inhibits inflammatory diseases by suppressing oxidative stress and inflammatory signaling.
5.1.5. Pro-or anti-inflammatory effect of PRDX6 in inflammatory diseases
The beneficial role of PRDX6 in inflammatory diseases is well established. Wang et al showed that PRDX6-/- macrophages oxidized LDL significantly more than it did in controls and plasma lipid hydroperoxide levels were higher in atherogenic diet-fed PRDX6-/- mice than in controls (Wang et al., 2004). A recent study showed that increased PRDX6 expression suppresses liver injury induced by free radical-mediated damage via inflammation (Khoontawad et al., 2010). Likewise, an increased liver injury in PRDX6-knockout mice occurred via increased mitochondrial generation of H2O2 (Eismann et al., 2009). The role of PRDX6 in inflammatory diseases as non-beneficial is also established. PRDX6 is also involved in the several inflammation related diseases. Our recent study suggested that overexpression of PRDX6 promotes development of RA through activation of coupling NF-κB/AP-1 with the JNK pathway or NF-κB/AP-1 coupled with the JNK pathway in the CAIA and AIA-induced arthritis development model (Kim et al., 2015). Our data and other data suggest that PRDX6 inhibits inflammatory diseases by suppression of free radical-induced damage and mitochondrial generation of H2O2, and induces inflammatory diseases through activation of NF-κB/AP-1 coupled with the JNK pathway.
5.2. Signaling pathway of PRDXs in the development of inflammatory diseases
PRDXs secreted from cells under mild oxidative stress binds TLR4 and PRDXs induces Nrf2 or NF-κB activation that is important for the expression of inflammatory proteins, suggesting that PRDXs are involved in inflammation related disease (Madrigal-Matute et al., 2012, Westermann et al., 1996). Fig. 6 illustrates the roles of PRDXs in the signaling pathways for the development of inflammatory diseases.
Fig. 6.
Possible mechanisms of PRDXs related with inflammatory disease. PRDX1 can induce activation of NF-κB through TLR4. PRDX1 is secreted from cells under mild oxidative stress and binds with TLR4 which results in induced NF-κB activation that is important for the expression of inflammatory proteins. Recombinant PRDX1 supplemented to the culture medium induced secretion of TNF-α and IL-6 from mouse thioglycolate-elicited peritoneal macrophages and BMDM. This effect of PRDX1 is quite similar to that of LPS as it required membrane proteins CD14 and MD2 and was mediated by the TLR4-MyD88 signaling leading to the activation of NF-κB. It was speculated that PRDXs released from necrotic cells induce NF-κB activation and the production of IL-23. PRDX2 is a negative regulator of PDGF signalling. PRDX2 suppresses activation of PDGF receptor (PDGFR) and phospholipase Cγ1, and subsequently decreases cell proliferation and migration in response to PDGF. Also, PRDX2 is recruited to PDGFR upon PDGF stimulation, and suppresses protein tyrosine phosphatase inactivation. PRDX5 and PRDX6 are TLR4-dependent inducers of infiltrating macrophage activation and the subsequent production of inflammatory mediators from invading T cells in the ischemic brain. Overexpression of PRDX6 promotes development of RA through activation of NF-κB/AP-1 coupled with JNK pathway in the CAIA and AIA-induced arthritis development model.
Riddell et al. found that extracellular PRDX1 can induce activation of NF-κB through TLR4 (Riddell et al., 2012). They showed that incubation of mouse vascular endothelial cells with recombinant PRDX1 caused an increase of VEGF expression that was dependent upon TLR4 and required hypoxia inducible factor-1 (HIF-1) interaction with the VEGF promoter. The induction of VEGF was also dependent upon NF-κB; however, NF-κB interaction with the VEGF promoter was not required for PRDX1 induction of VEGF suggesting that NF-κB was acting indirectly to induce VEGF expression. Riddell et al. also showed that recombinant PRDX1 supplemented to the culture medium induced secretion of TNF-α and IL-6 from mouse thioglycolate-elicited peritoneal macrophages (Riddell et al., 2010). They showed that secretion of cytokines in response to PRDX1 was dependent upon serum and required CD14 and MD2. Binding of PRDX1 to TG-macrophages occurred within minutes and resulted in TLR4 endocytosis. PRDX1 interaction with TLR4 was independent of its peroxidase activity and appeared to be dependent upon its chaperone activity and ability to form decamers. Cytokine expression occurred via the TLR-MyD88 signaling pathway, which resulted in nuclear translocation and activation of NF-κB. These findings suggest that PRDX1 may act as a danger signal similar to other TLR4 binding chaperone molecules such as HSP72. Shichita et al. showed that PRDX1 to 6 all bind to TLR4 and induce IL-23 (Shichita et al., 2012a, Shichita et al., 2012b). The authors identified PRDXs as key initiators of post-ischemic inflammation in the brain. They showed that IL-23 production from infiltrated macrophages induces IL-17-producing T cells, which play a role in the promotion of delayed ischemic brain damages. It was speculated that PRDXs released from necrotic cells induce NF-κB activation and the production of IL-23 (Salzano et al., 2014). The authors identified a domain on PRDXs required for the TLR4-mediated cytokine production. This domain is amino acid residues between 70 and 90 of PRDX5 and homologous regions in other PRDXs that commonly contain β4 sheet and α3 helix regions. It is noted, however, that the activity of domain containing GST-PRDX fusion proteins induced the IL-23 mRNA to a much lesser extent compared to whole PRDXs. The interaction of PRDXs with TLR4 should be strictly regulated by a manner different from that of LPS. Kisucka et al examined the role of PRDX1 in the apoE-/- murine spontaneous model of atherosclerosis (Kisucka et al., 2008). They showed that PRDX1-/-/apoE-/- mice fed normal chow developed larger, more macrophage-rich aortic sinus lesions than PRDX1+/+/apoE-/- mice, despite similar amounts and size distributions of cholesterol in their plasma lipoproteins, suggesting that PRDX1 protects against excessive endothelial activation and atherosclerosis. PRDX1 deficiency enhanced the regulated secretion pathway in endothelial cells by promotion of excessive release of several proinflammatory components of Weibel-Palade bodies, such as P-selectin and von Willebrand factor (Kisucka et al., 2008). However, PRDX1 deficiency did not affect transcriptional regulation of receptors such as intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule-1 (VCAM-1).
One study showed that PRDX2 is highly expressed in endothelial and immune cells in atherosclerotic lesions and blocked the increase of endogenous H2O2 by atherogenic stimulation (Park et al., 2011). Deficiency of PRDX2 in apoE-/- mice accelerated plaque formation with enhanced activation of p65, c-Jun, JNKs, and p38 mitogen-activated protein kinase; and these proatherogenic effects of PRDX2 deficiency were rescued by the administration of the antioxidant ebselen. Also, they showed that PRDX2 deficiency resulted in increased expression of vascular adhesion molecule-1, intercellular adhesion molecule-1, and monocyte chemotactic protein-1, which led to increased immune cell adhesion and infiltration into the aortic intima. Moreover, compared with deficiency of glutathione peroxidase 1 or catalase, PRDX2 deficiency showed a severe predisposition to develop atherosclerosis. Chen et al showed that inflammatory stimuli induce the release of oxidized PRDX2, a ubiquitous redox-active intracellular enzyme (Chen et al., 2004). Once released, the extracellular PRDX2 acts as a redox-dependent inflammatory mediator, triggering macrophages to produce and release TNF-α. The oxidative coupling of glutathione (GSH) to PRDX2 cysteine residues occurs before or during PRDX2 release, a process central to the regulation of immunity (Salzano et al., 2014). Salzano et al identified PRDX2 among the glutathionylated proteins released in vitro by LPS-stimulated macrophages using mass spectrometry proteomic methods. Consistent with being part of an inflammatory cascade, it was found that PRDX2 then induces TNF-α release (Salzano et al., 2014). Importantly, the PRDX2 substrate TRX is also released along with PRDX2, enabling an oxidative cascade that can alter the –SH status of surface proteins and thereby facilitate activation via cytokine and Toll-like receptors (Salzano et al., 2014).
PRDX3 deficiency increased abnormal lipid accumulation in adipose tissue, due to increased ROS. PRDX4 is a secretory antioxidant protein which can be detected in plasma. By virtue of its antioxidant activity, the extracellular PRDX4 can protect the vascular tissue against ROS and hence, it has ability to inhibit the oxidative stress induced inflammation in various tissues and it can also reduce the chances of oxidative stress induced diabetes mellitus in animal models (Ding et al., 2010). Yamada et al demonstrated that streptozotocin-treated Tg mice, which overexpress hPRDX4 in pancreatic islets, can protect pancreatic beta-cells against streptozotocin-induced injury (insulitis) by suppressing increased oxidative stress and inflammatory signaling activation (Yamada, Ding, & Sasaguri, 2012). These observations indicate that Tg mice could become a useful animal model to study the relevance of oxidative stress to inflammation, and that a specific accelerator of PRDX4 might prove to be a potential therapeutic agent for ameliorating various chronic inflammatory diseases. It was also reported that HFD-induced hepatic steatosis and insulin resistance were prevented in PRDX4 transgenic mice by amelioration of oxidative stress (Ding et al., 2010). They showed that PRDX4 protects against the progression of nonalcoholic steatohepatitis and insulin resistance, particularly in the liver, in a nongenetic mouse model of nonalcoholic fatty liver disease /or type 2 diabetes mellitus. These effects of PRDX4 were achieved by reducing oxidative stress and ameliorating hepatic steatosis, inflammatory reaction, apoptotic activity, and fibrogenesis at systemic as well as local levels.
PRDX6 is also involved in the several inflammatory related diseases. Wang et al showed that PRDX6-/- macrophages oxidized LDL significantly more than did controls and plasma lipid hydroperoxide levels were higher in atherogenic diet-fed PRDX6-/- mice than in controls (Wang et al., 2004). Recent study showed that increased PRDX6 expression suppresses liver injury induced by free radical-mediated damage via inflammation (Khoontawad et al., 2010). Likewise, an increased liver injury in PRDX6-knockout mice occurred via increased mitochondrial generation of H2O2 (Eismann et al., 2009). Our recent study suggested that overexpression of PRDX6 promotes development of RA through activation of NF-κB/AP-1 coupled JNK pathway in the CAIA and AIA-induced arthritis development model (Kim et al., 2015).
6. Therapeutic approaches of PRDXs for treatment of disease
PRDXs involved in the several disease related signal pathways. Also, PRDXs are involved in inhibiting the cancer development or promoting growth of cancers. So, they can be a potential therapeutic target through targeting these signal pathways.
PRDX1 has breast tumor suppressive role mediated via c-Myc or PTEN pathways. This study provides that tumour suppressive function of PRDX1 and PTEN through understanding the mechanistic details of PRDX1 peroxidase activity and PTEN oxidation. On the other hand, PRDX1 has a tumor promoting effect through NF-kB pathway in Breast cancer, oral cancer and bladder cancer. PRDX1 suppressed FOXO1 induced apoptosis in lung cancer, activates mTOR/p70S6K pathway in esophageal squamous cell carcinoma and upregulates pancreatic cancer invasion by modulating p38 MAPK signaling. So, enhanced expression of PRDX1 through cancer related pathways serves as a useful marker for the prognosis of patients with this disease and PRDX1 may be a promising molecular target for the therapeutic intervention of these cancer types.
PRDX2 promotes colon cancer through upregulation of Wnt/β-catenin pathway, promotes prostate cancer through upregulation of AR activity. So, designing therapeutics targeting PRDX2 may offer a novel strategy for developing treatment for cancer.
Moreover, several studies demonstrated that overexpression of PRDX1 and PRDX2 has an important role in several drug resistances, and several therapeutic agents targeting PRDX1 and PRDX2 are studied for treatment of cancer. He et al showed that PRDX1 knockdown enhances sensitization to β-lap that is an anticancer agent through modulating ROS accumulation and MAPK activation (He et al., 2013). Also, downregulation of PRDX1 by a novel fully human phage display recombinant antibody induces apoptosis and enhances radiation sensitization in lung carcinoma cells (Guo et al., 2011), indicating that PRDX1 could be targets competing against cancer. The therapeutic agents targeting PRDX1 are studied for treatment of cancer. A recent study demonstrated that theonellasterone, a steroidal metabolite isolated from a Theonellasponge targets the PRDX1 and protects its cysteine over-oxidation induced by H2O2 both in vitro and in vivo. This suggested that THS can be used as an anticancer agent for several cancers (Margarucci et al., 2015). Liu et al also demonstrated that adenanthin, a diterpenoid isolated from the leaves of Rabdosia adenantha inhibits the tumor growth in the mouse acute promyelocytic leukemia mouse model by directly targeting the conserved resolving cysteines of PRDX1 as well as PRDX2 and inhibits their peroxidase activities (Liu et al., 2012). Hou et al also investigated the effect of adenanthin on HCC cells, and found that the natural agent can kill malignant liver cells in vitro and xenografts through targeting PRDX1 and PRDX2, thus increasing ROS level (Hou et al., 2014).
Overexpression of PRDX3 has an important role in several drug resistances. The silencing of PRDX3 triggered cisplatin‑mediated apoptosis in ovarian cancer cells through suppression of the NF‑κB signaling pathway (Duan et al., 2013), suggesting that PRDX3 is involved in drug resistance. PRDX3 also promotes breast cancer through upregulation of cell cycle and cell proliferation, enhances the risk of prostate cancer, thymoma and hepatocellular carcinoma through increased resistance to oxidative stress. Prolonged drug exposure may result in cumulative toxicity. The clinical efficacy of chemotherapy must be enhanced, its attendant toxicity reduced, and resistance overcome. To overcome multidrug resistance in cancer cells, recent chemotherapeutics could be used in combination with other molecules. So, PRDX3 may be a therapeutic target for the combination treatment of several drug resistant cancers.
PRDX4 promotes lung cancer, leukemia, glioblastoma, oral squamous cell carcinoma, cardiovascular disease, hepatic diseases and colorectal carcinoma. PRDX4 is involved in the tumor promoting effect through formation of Srx-PRDX4 via AP-1/MMP-9 and MAPK signaling, and remove cellular ROS which provides a favorable microenvironment for cell proliferation. Prx-4 can be used as an important therapeutic target in this disorder.
PRDX6 promotes lung tumor development via JAK2/STAT3 pathway, its GPx and iPLA2 activities, and is involved in drug resistance of several cancer types, suggesting that PRDX6 can be a therapeutic target for cancer treatment. Our recent study suggested that thiacremonone inhibits tumor growth via inhibition of glutathione peroxidase activity of PRDX6 through interaction, suggesting that thiacremonone may have potential beneficial effects in lung cancer (Jo et al., 2014). These data indicate that PRDX6 could be targets of several cancers and the therapeutic agents for targeting PRDX6 may have potentially beneficial effects in cancer treatment.
PRDXs could be targets for the treatment of neurodegenerative diseases. PRDX1 has a protective role in counteracting Aβ injury by increasing cell viability, preserving neurites, and decreasing cell death (Cimini et al., 2013). These researches provide the neuroprotective function of PRDX1 and understanding the mechanistic details of PRDX1 peroxidase activity in the neurodegenerative diseases.
PRDX2 is involved in neuroprotection via inhibition of Aβ1-42 induced apoptosis and suppression of JNK and p38 pathways, providing the neuroprotective function of PRDX2 and understanding the mechanistic details of PRDX1 peroxidase activity in the neurodegenerative diseases. Also, because cdk5 that inhibits PRDX2 activity leads to increased levels of ROS in PD, cdk5 is used as therapeutic target for treatment of PD. Using novel Cdk5 modulators, Sun et al further showed the mechanism by which Cdk5 can induce oxidative stress in the disease's early stage and cell death in the late stage (Sun et al., 2008). Cdk5 inhibition rescues mitochondrial damage upon neurotoxic insults, thereby revealing Cdk5 as an upstream regulator of mitochondrial dysfunction. As oxidative stress and mitochondrial dysfunction play pivotal roles in promoting neurodegeneration, Cdk5 could be a viable therapeutic target for AD. Zhang et al found that neurotoxin, MPP(+) induces CdK5/p25-dependent phosphorylation of PRDX2, resulting in inhibition of its peroxireductase activity and accumulation of ROS (Zhang et al., 2012a, b). On the other hand, PRDX2 is also involved in neurodegeneration through phosphorylation by cdk2, a serine/threonine kinase, and contributes to PD, providing that PRDX2 can be used for therapeutic target for treatment of PD through targeting this pathway.
PRDX3 is involved in the neuroprotection against oxidative insults in the mitochondria, suggesting that PRDX3 up-regulation might be a useful novel approach for the management of neurodegenerative diseases. On the other hand, PRDX3 is involved in neurodegeneration through increased phosphorylation by LRRK2 mutation, providing therapeutic target in PD patients carrying LRRK2 mutations. PRDX5 is involved in PD through mitochondrial redox signaling with Ca2 + influx resulting in the development of PD (Davey & Bolaños, 2013), also can be used for therapeutic target.
PRDX6 induces neurodegeneration through aiPLA2 and nrf2 activation in AD, and induces neurodegeneration through induction of MPTP-induced dopaminergic neurodegeneration of PD, providing that PRDX6 might be used a useful novel approach for the management of neurodegenerative diseases. On the other hand, PRDX6 inhibits multiple sclerosis by suppressing inflammation and blood brain barrier disruption in EAE model, suggesting a new molecule for neuroprotective therapies in multiple sclerosis.
PRDXs could be targets for the treatment of inflammatory diseases. PRDX2 suppresses atherosclerosis through the site-selective phosphorylation of the PDGF receptor pathways, suggesting upregulation of PRDX2 can be used for treatment of atherosclerosis. Du et al examined the expressions of PRDX2 and Foxo3a that were down-regulated in ischemic brain compared with the normal-control group (Du et al., 2012). They showed that the combined treatment of probucol or atorvastatin dramatically reduced the brain water content and the infarct volume, along with the decrease of PRDX2, Foxo3a and Nrf2 was significantly alleviated in combined treatment group. They suggested that probucol combined with atorvastatin effect in the neuroprotection from the damage caused by MCAO through up-regulation of PRDX2, Foxo3a and Nrf2. On the other hand, PRDX2 enhances the risk of RA because PRDX2 is involved in the persistence of pro-inflammatory cells in chronic inflammation, providing PRDX2 can be used as therapeutic target of RA.
PRDX3 inhibits the risk of obesity through decreased abnormal lipid accumulation in adipose tissue, due to increased ROS, suggesting PRDX3 can be used for the treatment of obesity. PRDX4 protects against atherosclerosis and obesity through inhibiting the oxidative stress induced inflammation in various tissues, suggesting up-regulation might be a useful novel approach for the management of atherosclerosis and obesity. But PRDX4 enhances the risk of RA, providing therapeutic target for treatment of RA.
PRDX6 enhances the risk of RA through activation of NF-κB/AP-1 coupled JNK pathway in the CAIA and AIA-induced arthritis development model, suggesting PRDX6 can be used as therapeutic target for treatment of RA. Moreover, several therapeutic agents targeting PRDX6 are studied for treatment of inflammatory diseases. Among them, Epigallocatechin-3-gallate modulates the levels of the antioxidant enzymes PRDX6, catalase and superoxide dismutase (SOD), as well as PCNA, in NOD (Ohno et al., 2012). They showed that the early PCNA elevation was followed by a decline of PRDX6 protein, in contrast EGCG-fed mice exhibited normal levels of PCNA and PRDX6, comparable to healthy untreated BALB/c mice.
In conclusion, we discussed the roles and mechanisms of PRDXs on cellular homeostasis, diseases development and drug resistance, and propose potential therapeutic approaches for targeting PRDXs. We hope this review provides a foundation for further studies to design novel therapeutic approaches targeting PRDXs.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by National Research Foundation of Korea [NRF] grant funded by the Korea government (MSIP) (No. MRC.2008-0062275).
References
- Abbas K., Breton J., Picot C.R., Quesniaux V., Bouton C., Drapier J.C. Signaling events leading to peroxiredoxin 5 up-regulation in immunostimulated macrophages. Free Radic Biol Med. 2009;47:794–802. doi: 10.1016/j.freeradbiomed.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Abbasi A., Corpeleijn E., Postmus D., Gansevoort R.T., de Jong P.E., Gans R.O. Peroxiredoxin 4, A Novel Circulating Biomarker for Oxidative Stress and the Risk of Incident Cardiovascular Disease and All-Cause Mortality. J Am Heart Assoc. 2012;1:e002956. doi: 10.1161/JAHA.112.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler V., Yin Z., Tew K.D., Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- Agrawal Singh S., Isken F., Agelopoulos K., Klein H.U., Thoennissen N.H., Koehler G. Genome-wide analysis of histone H3 acetylation patterns in AML identifies PRDX2 as an epigenetically silenced tumor suppressor gene. Blood. 2012;119:2346–2357. doi: 10.1182/blood-2011-06-358705. [DOI] [PubMed] [Google Scholar]
- Angeles D.C., Gan B.H., Onstead L., Zhao Y., Lim K.L., Dachsel J. Mutations in LRRK2 increase phosphorylation of peroxiredoxin 3 exacerbating oxidative stress-induced neuronal death. Hum Mutat. 2011;32:1390–1397. doi: 10.1002/humu.21582. [DOI] [PubMed] [Google Scholar]
- Basu A., Banerjee H., Rojas H., Martinez S.R., Roy S., Jia Z. Differential Expression of Peroxiredoxins in Prostate Cancer: Consistent Upregulation of PRDX3 and PRDX4. Prostate. 2011;71:755–765. doi: 10.1002/pros.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer S.B., Maghzal G., Stocker R., Hampton M.B., Winterbourn C.C. Neutrophil-mediated oxidation of erythrocyte peroxiredoxin 2 as a potential marker of oxidative stress in inflammation. FASEB J. 2013;27:3315–3322. doi: 10.1096/fj.13-227298. [DOI] [PubMed] [Google Scholar]
- Benvenga S., Koch C.A. Molecular Pathways Associated with Aggressiveness of Papillary Thyroid Cancer. Curr Genomics. 2014;15:162–170. doi: 10.2174/1389202915999140404100958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohé R., Flohé L. Basic Principles and Emerging Concepts in the Redox Control of Transcription Factors. Antioxid Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C.Y., Zhai L.L., Wu Y., Tang Z.G. Expression and clinical value of peroxiredoxin-1 in patients with pancreatic cancer. Eur J Surg Oncol. 2015;41:228–235. doi: 10.1016/j.ejso.2014.11.037. [DOI] [PubMed] [Google Scholar]
- Cao J., Schulte J., Knight A., Leslie N.R., Zagozdzon A., Bronson R. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti P.A. Oxy-radicals and cancer. Lancet. 1994;344:862–863. doi: 10.1016/s0140-6736(94)92832-0. [DOI] [PubMed] [Google Scholar]
- Cha M.K., Suh K.H., Kim I.H. Overexpression of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J Exp Clin Cancer Res. 2009;28:93. doi: 10.1186/1756-9966-28-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae H.Z., Oubrahim H., Park J.W., Rhee S.G., Chock P.B. Protein Glutathionylation in the Regulation of Peroxiredoxins: A Family of Thiol-Specific Peroxidases That Function As Antioxidants, Molecular Chaperones, and Signal Modulators. Antioxid Redox Signal. 2012;16:506–523. doi: 10.1089/ars.2011.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.H., Multani P.S., Sun K.H., Vincent F., de Pablo Y., Ghosh S. Nuclear envelope dispersion triggered by deregulated Cdk5 precedes neuronal death. Mol Biol Cell. 2011;22:1452–1462. doi: 10.1091/mbc.E10-07-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.P., Yu J.S., Chien K.Y., Lee C.W., Liang Y., Liao C.T. Identification of PRDX4 and P4HA2 as Metastasis-Associated Proteins in Oral Cavity Squamous Cell Carcinoma by Comparative Tissue Proteomics of Microdissected Specimens Using iTRAQ Technology. J Proteome Res. 2011;10:4935–4947. doi: 10.1021/pr200311p. [DOI] [PubMed] [Google Scholar]
- Chang T.S., Cho C.S., Park S., Yu S., Kang S.W., Rhee S.G. Peroxiredoxin III, a Mitochondrion-specific Peroxidase, Regulates Apoptotic Signaling by Mitochondria. J Biol Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- Chang X., Cui Y., Zong M., Zhao Y., Yan X., Chen Y. Identification of Proteins with Increased Expression in Rheumatoid Arthritis Synovial Tissues. J Rheumatol. 2009;36:872–880. doi: 10.3899/jrheum.080939. [DOI] [PubMed] [Google Scholar]
- Chang T.S., Jeong W., Choi S.Y., Yu S., Kang S.W., Rhee S.G. Regulation of Peroxiredoxin I Activity by Cdc2-mediated Phosphorylation. J Biol Chem. 2002;277:25370–25376. doi: 10.1074/jbc.M110432200. [DOI] [PubMed] [Google Scholar]
- Chang J.W., Lee S.H., Jeong J.Y., Chae H.Z., Kim Y.C., Park Z.Y. Peroxiredoxin-I is an autoimmunogenic tumor antigen in non-small cell lung cancer. FEBS Lett. 2005;579:2873–2877. doi: 10.1016/j.febslet.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Chang X.Z., Li D.Q., Hou Y.F., Wu J., Lu J.S., Di G.H. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007;9:R76-R76. doi: 10.1186/bcr1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Feinstein S.I., Dodia C., Sorokina E., Lien Y.C., Nguyen S. Peroxiredoxin 6 Phosphorylation and Subsequent Phospholipase A(2) Activity Are Required for Agonist-mediated Activation of NADPH Oxidase in Mouse Pulmonary Microvascular Endothelium and Alveolar Macrophages. J Biol Chem. 2011;286:11696–11706. doi: 10.1074/jbc.M110.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.H., Chang Y.W., Yao C.W., Chiueh T.S., Huang S.C., Chien K.Y. Plasma proteome of severe acute respiratory syndrome analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proc Natl Acad Sci U S A. 2004;101:17039–17044. doi: 10.1073/pnas.0407992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.W., Dodia C., Feinstein S.I., Jain M.K., Fisher A.B. 1-Cys Peroxiredoxin, a Bifunctional Enzyme with Glutathione Peroxidase and Phospholipase A2 Activities. J Biol Chem. 2000;275:28421–28427. doi: 10.1074/jbc.M005073200. [DOI] [PubMed] [Google Scholar]
- Chen M.F., Keng P.C., Shau H., Wu C.T., Hu Y.C., Liao S.K. Inhibition of lung tumor growth and augmentation of radiosensitivity by decreasing peroxiredoxin I expression. Int J Radiat Oncol Biol Phys. 2006;64:581–591. doi: 10.1016/j.ijrobp.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Chen L., Na R., Ran Q. Enhanced defense against mitochondrial hydrogen peroxide attenuates age-associated cognition decline. Neurobiol Aging. 2014;35:2552–2561. doi: 10.1016/j.neurobiolaging.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Chevallet M., Wagner E., Luche S., van Dorsselaer A., Leize-Wagner E., Rabilloud T. Regeneration of Peroxiredoxins during Recovery after Oxidative Stress: ONLY SOME OVEROXIDIZED PEROXIREDOXINS CAN BE REDUCED DURING RECOVERY AFTER OXIDATIVE STRESS. J Biol Chem. 2003;278:37146–37153. doi: 10.1074/jbc.M305161200. [DOI] [PubMed] [Google Scholar]
- Chhipa R.R., Lee K.S., Onate S., Wu Y., Ip C. Prx1 Enhances Androgen Receptor Function in Prostate Cancer Cells by Increasing Receptor Affinity to Dihydrotestosterone. Mol Cancer Res. 2009;7:1543–1552. doi: 10.1158/1541-7786.MCR-08-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiribau C.B., Cheng L., Cucoranu I.C., Yu Y.S., Clempus R.E., Sorescu D. FOXO3A Regulates Peroxiredoxin III Expression in Human Cardiac Fibroblasts. J Biol Chem. 2008;283:8211–8217. doi: 10.1074/jbc.M710610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H., Chang J.W., Jung Y.K. Peroxiredoxin 6 interferes with TRAIL-induced death-inducing signaling complex formation by binding to death effector domain caspase. Cell Death Differ. 2011;18:405–414. doi: 10.1038/cdd.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M.H., Lee I.K., Kim G.W., Kim B.U., Han Y.H., Yu D.Y. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- Chowdhury I., Fisher A.B., Christofidou-Solomidou M., Gao L., Tao J.Q., Sorokina E.M. Keratinocyte Growth Factor and Glucocorticoid Induction of Human Peroxiredoxin 6 Gene Expression Occur by Independent Mechanisms That Are Synergistic. Antioxid Redox Signal. 2014;20:391–402. doi: 10.1089/ars.2012.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I., Mo Y., Gao L., Kazi A., Fisher A.B., Feinstein S.I. Oxidant Stress Stimulates Expression of the Human Peroxiredoxin 6 (Prdx6) Gene by a Transcriptional Mechanism Involving an Antioxidant Response Element. Free Radic Biol Med. 2009;46:146–153. doi: 10.1016/reeradbiomed.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K.L., Lew Q.J., Rajasegaran V., Kung J.T., Zheng L., Yang Q. Regulation of PRDX1 peroxidase activity by Pin1. Cell Cycle. 2013;12:944–952. doi: 10.4161/cc.23916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua P.J., Lee E.H., Yu Y., Yip G.W.C., Tan P.H., Bay B.H. Silencing the Peroxiredoxin III gene inhibits cell proliferation in breast cancer. Int J Oncol. 2010;36:359–364. [PubMed] [Google Scholar]
- Chung K.H., Lee D.H., Kim Y., Kim T.H., Huh J.H., Chung S.G. Proteomic Identification of Overexpressed PRDX 1 and Its Clinical Implications in Ovarian Carcinoma. J Proteome Res. 2010;9:451–457. doi: 10.1021/pr900811x. [DOI] [PubMed] [Google Scholar]
- Cimini A., Gentile R., Angelucci F., Benedetti E., Pitari G., Giordano A. Neuroprotective effects of PrxI over-expression in an in vitro human Alzheimer's disease model. J Cell Biochem. 2013;114:708–715. doi: 10.1002/jcb.24412. [DOI] [PubMed] [Google Scholar]
- Conway J.P., Kinter M. Dual Role of Peroxiredoxin I in Macrophage-derived Foam Cells. J Biol Chem. 2006;281:27991–28001. doi: 10.1074/jbc.M605026200. [DOI] [PubMed] [Google Scholar]
- Dando I., Cordani M., Dalla Pozza E., Biondani G., Donadelli M., Palmieri M. Antioxidant Mechanisms and ROS-Related MicroRNAs in Cancer Stem Cells. Oxid Med Cell Longev. 2015;2015:425708. doi: 10.1155/2015/425708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey G.P., Bolaños J.P. Peroxiredoxin 5 links mitochondrial redox signalling with calcium dynamics: impact on Parkinson's disease. J Neurochem. 2013;125:332–333. doi: 10.1111/jnc.12171. [DOI] [PubMed] [Google Scholar]
- Dayer R., Fischer B.B., Eggen R.I.L., Lemaire S.D. The Peroxiredoxin and Glutathione Peroxidase Families in Chlamydomonas reinhardtii. Genetics. 2008;179:41–57. doi: 10.1534/genetics.107.086041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighton R.F., Le Bihan T., Martin S.F., Gerth A.M., McCulloch M., Edgar J.M. Interactions among mitochondrial proteins altered in glioblastoma. J Neurooncol. 2014;118:247–256. doi: 10.1007/s11060-014-1430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demasi A.P., Ceratti D., Furuse C., Cury P., Junqueira J.L., Araújo V.C. Expression of peroxiredoxin I in plasma cells of oral inflammatory diseases. Eur J Oral Sci. 2007;115:334–337. doi: 10.1111/j.1600-0722.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- Diaz A.J.G., Tamae D., Yen Y., Li J., Wang T. Enhanced radiation response in radioresistant MCF-7 cells by targeting peroxiredoxin II. Breast Cancer (Dove Med Press) 2013;5:87–101. doi: 10.2147/BCTT.S51378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diet A., Abbas K., Bouton C., Guillon B., Tomasello F., Fourquet S. Regulation of Peroxiredoxins by Nitric Oxide in Immunostimulated Macrophages. J Biol Chem. 2007;282:36199–36205. doi: 10.1074/jbc.M706420200. [DOI] [PubMed] [Google Scholar]
- Ding Y., Yamada S., Wang K.Y., Shimajiri S., Guo X., Tanimoto A. Overexpression of Peroxiredoxin 4 Protects Against High-Dose Streptozotocin-Induced Diabetes by Suppressing Oxidative Stress and Cytokines in Transgenic Mice. Antioxid Redox Signal. 2010;13:1477–1490. doi: 10.1089/ars.2010.3137. [DOI] [PubMed] [Google Scholar]
- Du Y., Zhang X., Ji H., Liu H., Li S., Li L. Probucol and atorvastatin in combination protect rat brains in MCAO model: Upregulating Peroxiredoxin2, Foxo3a and Nrf2 expression. Neurosci Lett. 2012;509:110–115. doi: 10.1016/j.neulet.2011.12.054. [DOI] [PubMed] [Google Scholar]
- Duan J., Lang Y., Song C., Xiong J., Wang Y., Yan Y. siRNA targeting of PRDX3 enhances cisplatin-induced apoptosis in ovarian cancer cells through the suppression of the NF-κB signaling pathway. Mol Med Rep. 2013;7:1688–1694. doi: 10.3892/mmr.2013.1370. [DOI] [PubMed] [Google Scholar]
- Egler R.A., Fernandes E., Rothermund K., Sereika S., de Souza-Pinto N., Jaruga P. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24:8038–8050. doi: 10.1038/sj.onc.1208821. [DOI] [PubMed] [Google Scholar]
- Eismann T., Huber N., Shin T., Kuboki S., Galloway E., Wyder M. Peroxiredoxin-6 protects against mitochondrial dysfunction and liver injury during ischemia-reperfusion in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G266–G274. doi: 10.1152/ajpgi.90583.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamin A., Zhu H., Hassan A.M., Xu N., Ibrahim M.E. Peroxiredoxin V: A candidate breast tumor marker of population specificity. Mol Clin Oncol. 2013;1:541–549. doi: 10.3892/mco.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J., Nakamura T., Cho D.H., Gu Z., Lipton S.A. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104:18742–18747. doi: 10.1073/pnas.0705904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatma N., Kubo E., Sharma P., Beier D.R., Singh D.P. Impaired homeostasis and phenotypic abnormalities in Prdx6-//-mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGF[beta] Cell Death Differ. 2005;12:734–750. doi: 10.1038/sj.cdd.4401597. [DOI] [PubMed] [Google Scholar]
- Fisher A.B. Peroxiredoxin 6: A Bifunctional Enzyme with Glutathione Peroxidase and Phospholipase A(2) Activities. Antioxid Redox Signal. 2011;15:831–844. doi: 10.1089/ars.2010.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher B.M., Phelan S.A. Investigating transcriptional regulation of Prdx6 in mouse liver cells. Free Radic Biol Med. 2007;42:1270–1277. doi: 10.1016/j.freeradbiomed.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia A., Zavala-Flores L., Rodriguez-Rocha H., Franco R. Thiol-Redox Signaling, Dopaminergic Cell Death, and Parkinson's Disease. Antioxid Redox Signal. 2012;17:1764–1784. doi: 10.1089/ars.2011.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard A.C., Many M.C., Daumerie C., Knoops B., Colin I.M. Peroxiredoxin 5 Expression in the Human Thyroid Gland. Thyroid. 2005;15:205–209. doi: 10.1089/thy.2005.15.205. [DOI] [PubMed] [Google Scholar]
- Gisin J., Perren A., Bawohl M., Jochum W. Rare allelic imbalances, but no mutations of the PRDX1 gene in human hepatocellular carcinomas. J Clin Pathol. 2005;58:1229–1231. doi: 10.1136/jcp.2004.024679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogvadze V., Orrenius S., Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Gong F., Hou G., Liu H., Zhang M. Peroxiredoxin 1 promotes tumorigenesis through regulating the activity of mTOR/p70S6K pathway in esophageal squamous cell carcinoma. Med Oncol. 2015;32:1–9. doi: 10.1007/s12032-014-0455-0. [DOI] [PubMed] [Google Scholar]
- Gourlay L.J., Bhella D., Kelly S.M., Price N.C., Lindsay J.G. Structure-Function Analysis of Recombinant Substrate Protein 22 kDa (SP-22): A MITOCHONDRIAL 2-CYS PEROXIREDOXIN ORGANIZED AS A DECAMERIC TOROID. J Biol Chem. 2003;278:32631–32637. doi: 10.1074/jbc.M303862200. [DOI] [PubMed] [Google Scholar]
- Guo Q., Huang X., Zhang J., Luo Y., Peng Z., Li S. Downregulation of Peroxiredoxin I by a Novel Fully Human Phage Display Recombinant Antibody Induces Apoptosis and Enhances Radiation Sensitization in A549 Lung Carcinoma Cells. Cancer Biother Radiopharm. 2011;27:307–316. doi: 10.1089/cbr.2011.0989. [DOI] [PubMed] [Google Scholar]
- Guo X., Yamada S., Tanimoto A., Ding Y., Wang K.Y., Shimajiri S. Overexpression of Peroxiredoxin 4 Attenuates Atherosclerosis in Apolipoprotein E Knockout Mice. Antioxid Redox Signal. 2012;17:1362–1375. doi: 10.1089/ars.2012.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanschmann E.M., Godoy J.R., Berndt C., Hudemann C., Lillig C.H. Thioredoxins, Glutaredoxins, and Peroxiredoxins—Molecular Mechanisms and Health Significance: from Cofactors to Antioxidants to Redox Signaling. Antioxid Redox Signal. 2013;19:1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haridas V., Ni J., Meager A., Su J., Yu G.L., Zhai Y. Cutting Edge: TRANK, a Novel Cytokine That Activates NF-κB and c-Jun N-Terminal Kinase. J Immunol. 1998;161:1–6. [PubMed] [Google Scholar]
- Hattori F., Oikawa S. Peroxiredoxins in the Central Nervous System. (2007) Subcell Biochem. 2007;44:357–374. doi: 10.1007/978-1-4020-6051-9_17. [DOI] [PubMed] [Google Scholar]
- Hattori F., Murayama N., Noshita T., Oikawa S. Mitochondrial peroxiredoxin-3 protects hippocampal neurons from excitotoxic injury in vivo. J Neurochem. 2003;86:860–868. doi: 10.1046/j.1471-4159.2003.01918.x. [DOI] [PubMed] [Google Scholar]
- He T., Banach-Latapy A., Vernis L., Dardalhon M., Chanet R., Huang M.-E. Peroxiredoxin 1 knockdown potentiates β-lapachone cytotoxicity through modulation of reactive oxygen species and mitogen-activated protein kinase signals. Carcinogenesis. 2013;34:760–769. doi: 10.1093/carcin/bgs389. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Acar N., Tranguch S., Burnum K.E., Xie H., Kodama A. Uterine FK506-binding protein 52 (FKBP52)–peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress. Proc Natl Acad Sci U S A. 2010;107:15577–15582. doi: 10.1073/pnas.1009324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu S., Abe Y., Okada K., Nagahara N., Hori H., Nishino T. Crystal structure of a multifunctional 2-Cys peroxiredoxin heme-binding protein 23 kDa/proliferation-associated gene product. Proc Natl Acad Sci U S A. 1999;96:12333–12338. doi: 10.1073/pnas.96.22.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J.N., Lee S.B., Lee S.S., Yoon S.H., Kang G.Y., Hwang S.G. Phospholipase A2 Activity of Peroxiredoxin 6 Promotes Invasion and Metastasis of Lung Cancer Cells. Mol Cancer Ther. 2010;9:825–832. doi: 10.1158/1535-7163.MCT-09-0904. [DOI] [PubMed] [Google Scholar]
- Hooks S.B., Cummings B.S. Role of Ca2 +-independent phospholipase A2 in cell growth and signaling. Biochem Pharmacol. 2008;76:1059–1067. doi: 10.1016/j.bcp.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J.K., Huang Y., He W., Yan Z.W., Fan L., Liu M.H. Adenanthin targets peroxiredoxin I/II to kill hepatocellular carcinoma cells. Cell Death Dis. 2014;5:e1400. doi: 10.1038/cddis.2014.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.X., Gao Q.U.N., Li L. Peroxiredoxin 3 is a novel marker for cell proliferation in cervical cancer. Biomed Rep. 2013;1:228–230. doi: 10.3892/br.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Weng Z., Chu C.T., Zhang L., Cao G., Gao Y. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the ASK1 signaling cascade. J Neurosci. 2011;31:247–261. doi: 10.1523/JNEUROSCI.4589-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J.Y., Kim Y., Jeong J., Park J., Kim I., Huh K.H. Peroxiredoxin 3 Is a Key Molecule Regulating Adipocyte Oxidative Stress, Mitochondrial Biogenesis, and Adipokine Expression. Antioxid Redox Signal. 2012;16:229–243. doi: 10.1089/ars.2010.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K.E., Park C., Seol C.H., Hwang Y.R., Hwang J.S., Jung J.W. Elevated Prx1 Provides Resistance to Docetaxel, But Is Not Associated with Predictive Significance in Lung Cancer. Tuberc Respir Dis (Seoul) 2013;75:59–66. doi: 10.4046/trd.2013.75.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.A., Song J.S., Yu D.Y., Kim H.R., Park H.J., Park Y.S. Peroxiredoxin 4 as an independent prognostic marker for survival in patients with early-stage lung squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8:6627–6635. [PMC free article] [PubMed] [Google Scholar]
- Immenschuh S., Baumgart-Vogt E. Peroxiredoxins, Oxidative Stress, and Cell Proliferation. Antioxid Redox Signal. 2005;7:768–777. doi: 10.1089/ars.2005.7.768. [DOI] [PubMed] [Google Scholar]
- Ishii T., Warabi E., Yanagawa T. Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J Clin Biochem Nutr. 2012;50:91–105. doi: 10.3164/jcbn.11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R., Takahashi M., Ihara H., Tsukamoto H., Fujii J., Ikeda Y. Measurement of peroxiredoxin-4 serum levels in rat tissue and its use as a potential marker for hepatic disease. Mol Med Rep. 2012;6:379–384. doi: 10.3892/mmr.2012.935. [DOI] [PubMed] [Google Scholar]
- Iuchi Y., Okada F., Tsunoda S., Kibe N., Shirasawa N., Ikawa M. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem J. 2009;419:149–158. doi: 10.1042/BJ20081526. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M., Wiktorowicz J.E., Soman K.V., Boldogh I., Forbus J.D., Spratt H. Role of Peroxiredoxin 1 and Peroxiredoxin 4 in Protection of Respiratory Syncytial Virus-Induced Cysteinyl Oxidation of Nuclear Cytoskeletal Proteins. J Virol. 2010;84:9533–9545. doi: 10.1128/JVI.01005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H.H., Kim S.Y., Park S.K., Jeon H.S., Lee Y.M., Jung J.H. Phosphorylation and concomitant structural changes in human 2-Cys peroxiredoxin isotype I differentially regulate its peroxidase and molecular chaperone functions. FEBS Lett. 2006;580:351–355. doi: 10.1016/j.febslet.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Jang H.H., Lee K.O., Chi Y.H., Jung B.G., Park S.K., Park J.H. Two Enzymes in One: Two Yeast Peroxiredoxins Display Oxidative Stress-Dependent Switching from a Peroxidase to a Molecular Chaperone Function. Cell. 2004;117:625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Jeong W., Bae S.H., Toledano M.B., Rhee S.G. Role of sulfiredoxin as a regulator of peroxiredoxin function and regulation of its expression. Free Radic Biol Med. 2012;53:447–456. doi: 10.1016/j.freeradbiomed.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Jiang H., Wu L., Mishra M., Chawsheen H.A., Wei Q. Expression of peroxiredoxin 1 and 4 promotes human lung cancer malignancy. Am J Cancer Res. 2014;4:445–460. [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Xiao X., Ren J., Tang Y., Weng H., Yang Q. Proteomic analysis of bladder cancer indicates Prx-I as a key molecule in BI-TK/GCV treatment system. PLoS One. 2014;9:e98764. doi: 10.1371/journal.pone.0098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D.Y., Chae H.Z., Rhee S.G., Jeang K.T. Regulatory Role for a Novel Human Thioredoxin Peroxidase in NF-κB Activation. J Biol Chem. 1997;272:30952–30961. doi: 10.1074/jbc.272.49.30952. [DOI] [PubMed] [Google Scholar]
- Jo M., Yun H.M., Park K.R., Park M.H., Kim T.M., Pak J.H. Lung tumor growth-promoting function of peroxiredoxin 6. Free Radic Biol Med. 2013;61:453–463. doi: 10.1016/j.freeradbiomed.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Jo M., Yun H.M., Park K.R., Park M.H., Lee D.H., Cho S.H. Anti-Cancer Effect of Thiacremonone through Down Regulation of Peroxiredoxin 6. PLoS One. 2014;9:e91508. doi: 10.1371/journal.pone.0091508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.W., Chae H.Z., Seo M.S., Kim K., Baines I.C., Rhee S.G. Mammalian Peroxiredoxin Isoforms Can Reduce Hydrogen Peroxide Generated in Response to Growth Factors and Tumor Necrosis Factor-α. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- Kang D.H., Lee D.J., Kim J., Lee J.Y., Kim H.W., Kwon K. Vascular Injury Involves the Overoxidation of Peroxiredoxin Type II and Is Recovered by the Peroxiredoxin Activity Mimetic That Induces Reendothelialization. Circulation. 2013;128:834–844. doi: 10.1161/CIRCULATIONAHA.113.001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karihtala P., Mäntyniemi A., Kang S.W., Kinnula V.L., Soini Y. Peroxiredoxins in Breast Carcinoma. Clin Cancer Res. 2003;9:3418–3424. [PubMed] [Google Scholar]
- Kazama H., Teramura M., Kurihara S., Yoshinaga K., Kato T., Motoji T. Peroxiredoxin 2 expression is increased in neutrophils of patients with refractory cytopenia with multilineage dysplasia. Br J Haematol. 2014;166:720–728. doi: 10.1111/bjh.12954. [DOI] [PubMed] [Google Scholar]
- Khalil A.A. Biomarker discovery: A proteomic approach for brain cancer profiling. Cancer Sci. 2007;98:201–213. doi: 10.1111/j.1349-7006.2007.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoontawad J., Wongkham C., Hiraku Y., Yongvanit P., Prakobwong S., Boonmars T. Proteomic identification of peroxiredoxin 6 for host defence against Opisthorchis viverrini infection. Parasite Immunol. 2010;32:314–323. doi: 10.1111/j.1365-3024.2009.01189.x. [DOI] [PubMed] [Google Scholar]
- Kim I.K., Lee K.J., Rhee S., Seo S.B., Pak J.H. Protective effects of Peroxiredoxin 6 overexpression on amyloid β-induced apoptosis in PC12 cells. Free Radic Res. 2013;47:836–846. doi: 10.3109/10715762.2013.833330. [DOI] [PubMed] [Google Scholar]
- Kim S.U., Park Y.H., Min J.S., Sun H.N., Han Y.H., Hua J.M. Peroxiredoxin I is a ROS/p38 MAPK-dependent inducible antioxidant that regulates NF-κB-mediated iNOS induction and microglial activation. J Neuroimmunol. 2013;259:26–36. doi: 10.1016/j.jneuroim.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Bogner P.N., Ramnath N., Park Y., Yu J., Park Y.M. Elevated Peroxiredoxin 1, but not NF-E2–Related Factor 2, Is an Independent Prognostic Factor for Disease Recurrence and Reduced Survival in Stage I Non–Small Cell Lung Cancer. Clin Cancer Res. 2007;13:3875–3882. doi: 10.1158/1078-0432.CCR-06-2893. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Lee D.H., Jo M.R., Son D.J., Park M.H., Hwang C.J. Exacerbation of Collagen Antibody–Induced Arthritis in Transgenic Mice Overexpressing Peroxiredoxin 6. Arthritis Rheum. 2015;67:3058–3069. doi: 10.1002/art.39284. [DOI] [PubMed] [Google Scholar]
- Kim T.H., Song J., Alcantara Llaguno S.R., Murnan E., Liyanarachchi S., Palanichamy K. Suppression of Peroxiredoxin 4 in Glioblastoma Cells Increases Apoptosis and Reduces Tumor Growth. PLoS One. 2012;7:e42818. doi: 10.1371/journal.pone.0042818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Yu M., Han S., Oh I., Choi Y.J., Kim S. Expression of human peroxiredoxin isoforms in response to cervical carcinogenesis. Oncol Rep. 2009;21:1391–1396. [PubMed] [Google Scholar]
- Kisucka J., Chauhan A.K., Patten I.S., Yesilaltay A., Neumann C., Van Etten R.A. Peroxiredoxin1 Prevents Excessive Endothelial Activation and Early Atherosclerosis. Circ Res. 2008;103:598–605. doi: 10.1161/CIRCRESAHA.108.174870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano K., Niimura Y., Nishiyama Y., Miki K. Stimulation of Peroxidase Activity by Decamerization Related to Ionic Strength: AhpC Protein from Amphibacillus xylanus. J Biochem. 1999;126:313–319. doi: 10.1093/oxfordjournals.jbchem.a022451. [DOI] [PubMed] [Google Scholar]
- Klotz L.O., Sánchez-Ramos C., Prieto-Arroyo I., Urbánek P., Steinbrenner H., Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops B., Goemaere J., Van der Eecken V., Declercq J.P. Peroxiredoxin 5: Structure, Mechanism, and Function of the Mammalian Atypical 2-Cys Peroxiredoxin. Antioxid Redox Signal. 2010;15:817–829. doi: 10.1089/ars.2010.3584. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Kang M.I., Watai Y., Tong K.I., Shibata T., Uchida K. Oxidative and Electrophilic Stresses Activate Nrf2 through Inhibition of Ubiquitination Activity of Keap1. Mol Cell Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J., Galliardt H., Jütte P., Schäper S., Dittmann L., Dietz K.J. The conformational bases for the two functionalities of 2-cysteine peroxiredoxins as peroxidase and chaperone. J Exp Bot. 2013;64:3483–3497. doi: 10.1093/jxb/ert184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapfenbauer K., Engidawork E., Cairns N., Fountoulakis M., Lubec G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003;967:152–160. doi: 10.1016/s0006-8993(02)04243-9. [DOI] [PubMed] [Google Scholar]
- Krapfenbauer K., Yoo B.C., Fountoulakis M., Mitrova E., Lubec G. Expression patterns of antioxidant proteins in brains of patients with sporadic Creutzfeldt-Jacob disease. Electrophoresis. 2002;23:2541–2547. doi: 10.1002/1522-2683(200208)23:15<2541::AID-ELPS2541>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Krishnaiah S.Y., Dodia C., Feinstein S.I., Fisher A.B. p67(phox) terminates the phospholipase A(2)-derived signal for activation of NADPH oxidase (NOX2) FASEB J. 2013;27:2066–2073. doi: 10.1096/fj.12-222133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropotov A., Usmanova N., Serikov V., Zhivotovsky B., Tomilin N. Mitochondrial targeting of human peroxiredoxin V protein and regulation of PRDX5 gene expression by nuclear transcription factors controlling biogenesis of mitochondria. FASEB J. 2007;274:5804–5814. doi: 10.1111/j.1742-4658.2007.06103.x. [DOI] [PubMed] [Google Scholar]
- Kuang X., Wang L.F., Yu L., Li Y.J., Wang Y.N., He Q. Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic Biol Med. 2014;71:165–175. doi: 10.1016/j.freeradbiomed.2014.03.028. [DOI] [PubMed] [Google Scholar]
- Kunsch C., Medford R.M. Oxidative Stress as a Regulator of Gene Expression in the Vasculature. Circ Res. 1999;85:753–766. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Goo J.S., Kim J.E., Nam S.H., Hwang I.S., Choi S.I. Peroxiredoxin I regulates the component expression of γ-secretase complex causing the Alzheimer's disease. Lab Anim Res. 2011;27:293–299. doi: 10.5625/lar.2011.27.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.M., Park S.H., Shin D.I., Hwang J.Y., Park B., Park Y.J. Oxidative Modification of Peroxiredoxin Is Associated with Drug-induced Apoptotic Signaling in Experimental Models of Parkinson Disease. J Biol Chem. 2008;283:9986–9998. doi: 10.1074/jbc.M800426200. [DOI] [PubMed] [Google Scholar]
- Lehtonen S.T., Svensk A.M., Soini Y., Pääkkö P., Hirvikoski P., Kang S.W. Peroxiredoxins, a novel protein family in lung cancer. Int J Cancer. 2004;111:514–521. doi: 10.1002/ijc.20294. [DOI] [PubMed] [Google Scholar]
- Leyens G., Donnay I., Knoops B. Cloning of bovine peroxiredoxins—gene expression in bovine tissues and amino acid sequence comparison with rat, mouse and primate peroxiredoxins. Comp Biochem Physiol B Biochem Mol Biol. 2003;136:943–955. doi: 10.1016/s1096-4959(03)00290-2. [DOI] [PubMed] [Google Scholar]
- Li X.J., Xu M., Zhao X.Q., Zhao J.N., Chen F.F., Yu W. Proteomic analysis of synovial fibroblast-like synoviocytes from rheumatoid arthritis. Clin Exp Rheumatol. 2013;31:552–558. [PubMed] [Google Scholar]
- Liao K.A., Tsay Y.G., Huang L.C., Huang H.Y., Li C.F., Wu T.F. Search for the Tumor-Associated Proteins of Oral Squamous Cell Carcinoma Collected in Taiwan using Proteomics Strategy. J Proteome Res. 2011;10:2347–2358. doi: 10.1021/pr101146w. [DOI] [PubMed] [Google Scholar]
- Lin J.F., Xu J., Tian H.Y., Gao X., Chen Q.X., Gu Q. Identification of candidate prostate cancer biomarkers in prostate needle biopsy specimens using proteomic analysis. Int J Cancer. 2007;121:2596–2605. doi: 10.1002/ijc.23016. [DOI] [PubMed] [Google Scholar]
- Liou G.Y., Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:10. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.X., Yin Q.Q., Zhou H.C., Wu Y.L., Pu J.X., Xia L. Adenanthin targets peroxiredoxin I and II to induce differentiation of leukemic cells. Nat Chem Biol. 2012;8:486–493. doi: 10.1038/nchembio.935. [DOI] [PubMed] [Google Scholar]
- Low F.M., Hampton M.B., Peskin A.V., Winterbourn C.C. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood. 2007;109:2611–2617. doi: 10.1182/blood-2006-09-048728. [DOI] [PubMed] [Google Scholar]
- Lu W., Fu Z., Wang H., Feng J., Wei J., Guo J. Peroxiredoxin 2 is upregulated in colorectal cancer and contributes to colorectal cancer cells’ survival by protecting cells from oxidative stress. Mol Cell Biochem. 2014;387:261–270. doi: 10.1007/s11010-013-1891-4. [DOI] [PubMed] [Google Scholar]
- Lu W., Fu Z., Wang H., Feng J., Wei J., Guo J. Peroxiredoxin 2 knockdown by RNA interference inhibits the growth of colorectal cancer cells by downregulating Wnt/β-catenin signaling. Cancer Lett. 2014;343:190–199. doi: 10.1016/j.canlet.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Lu Y., Liu J., Lin C., Wang H., Jiang Y., Wang J. Peroxiredoxin 2: a potential biomarker for early diagnosis of Hepatitis B Virus related liver fibrosis identified by proteomic analysis of the plasma. BMC Gastroenterol. 2010;10:115-115. doi: 10.1186/1471-230X-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal-Matute J., Martinez-Pinna R., Fernandez-Garcia C.E., Ramos-Mozo P., Burillo E., Egido J. Cell Stress Proteins in Atherothrombosis. Oxid Med Cell Longev. 2012;2012:232464. doi: 10.1155/2012/232464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manevich Y., Fisher A.B. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med. 2005;38:1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Margarucci L., Monti M.C., Tosco A., Esposito R., Zampella A., Sepe V. Theonellasterone, a steroidal metabolite isolated from a Theonella sponge, protects peroxiredoxin-1 from oxidative stress reactions. Chem Commun (Camb) 2015;51:1591–1593. doi: 10.1039/c4cc09205h. [DOI] [PubMed] [Google Scholar]
- Markova M., Koratkar R.A., Silverman K.A., Sollars V.E., MacPhee-Pellini M., Walters R. Diversity in secreted PLA2-IIA activity among inbred mouse strains that are resistant or susceptible to ApcMin//+ tumorigenesis. Oncogene. 2005;24:6450–6458. doi: 10.1038/sj.onc.1208791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmeti I., Lortz S., Elsner M., Lenzen S. Peroxiredoxin 4 Improves Insulin Biosynthesis and Glucose-induced Insulin Secretion in Insulin-secreting INS-1E Cells. J Biol Chem. 2014;289:26904–26913. doi: 10.1074/jbc.M114.568329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M., Jiang H., Wu L., Chawsheen H.A., Wei Q. The sulfiredoxin–peroxiredoxin (Srx–Prx) axis in cell signal transduction and cancer development. Cancer Lett. 2015;366:150–159. doi: 10.1016/j.canlet.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica-Napolitano J.S., Singh K.K. Mitochondrial dysfunction in cancer. Mitochondrion. 2004;4:755–762. doi: 10.1016/j.mito.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Monteiro G., Horta B.B., Pimenta D.C., Augusto O., Netto L.E. Reduction of 1-Cys peroxiredoxins by ascorbate changes the thiol-specific antioxidant paradigm, revealing another function of vitamin C. Proc Natl Acad Sci U S A. 2007;104:4886–4891. doi: 10.1073/pnas.0700481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon E.Y., Noh Y.W., Han Y.H., Kim S.U., Kim J.M., Yu D.Y. T lymphocytes and dendritic cells are activated by the deletion of peroxiredoxin II (Prx II) gene. I Immunol Lett. 2006;102:184–190. doi: 10.1016/j.imlet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Mowbray A.L., Kang D.H., Rhee S.G., Kang S.W., Jo H. L Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. J Biol Chem. 2008;283:1622–1627. doi: 10.1074/jbc.M707985200. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S.S., Leung K.S., Hicks M.J., Hastings P.J., Youssoufian H., Plon S.E. Defective mitochondrial peroxiredoxin-3 results in sensitivity to oxidative stress in Fanconi anemia. J Cell Biol. 2006;175:225–235. doi: 10.1083/jcb.200607061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen L., Hanschmann E.M., Lillig C.H., Herzenberg L.A., Ghezzi P. Cysteine Oxidation Targets Peroxiredoxins 1 and 2 for Exosomal Release through a Novel Mechanism of Redox-Dependent Secretion. Mol Med. 2015;21:98–108. doi: 10.2119/molmed.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murri M., Insenser M., Bernal-Lopez M.R., Perez-Martinez P., Escobar-Morreale H.F., Tinahones F.J. Proteomic analysis of visceral adipose tissue in pre-obese patients with type 2 diabetes. Mol Cell Endocrinol. 2013;376:99–106. doi: 10.1016/j.mce.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Nabeshima A., Yamada S., Guo X., Tanimoto A., Wang K.Y., Shimajiri S. Peroxiredoxin 4 Protects Against Nonalcoholic Steatohepatitis and Type 2 Diabetes in a Nongenetic Mouse Model. Antioxid Redox Signal. 2013;19:1983–1998. doi: 10.1089/ars.2012.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K.J., Knutson S.T., Soito L., Klomsiri C., Poole L.B., Fetrow J.S. Analysis of the peroxiredoxin family: using active site structure and sequence information for global classification and residue analysis. Proteins. 2011;79:947–964. doi: 10.1002/prot.22936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C.A., Cao J., Manevich Y. Peroxiredoxin 1 and its role in cell signaling. Cell Cycle. 2009;8:4072–4078. doi: 10.4161/cc.8.24.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C.A., Krause D.S., Carman C.V., Das S., Dubey D.P., Abraham J.L. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- Nguyên-nhu N.T., Berck J., Clippe A., Duconseille E., Cherif H., Boone C. Human peroxiredoxin 5 gene organization, initial characterization of its promoter and identification of alternative forms of mRNA. Biochim Biophys Acta. 2007;1769:472–483. doi: 10.1016/j.bbaexp.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Noh Y.H., Baek J.Y., Jeong W., Rhee S.G., Chang T.S. Sulfiredoxin Translocation into Mitochondria Plays a Crucial Role in Reducing Hyperoxidized Peroxiredoxin III. J Biol Chem. 2009;284:8470–8477. doi: 10.1074/jbc.M808981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonn L., Berggren M., Powis G. Increased Expression of Mitochondrial Peroxiredoxin-3 (Thioredoxin Peroxidase-2) Protects Cancer Cells Against Hypoxia and Drug-Induced Hydrogen Peroxide-Dependent Apoptosis11CA52995 and CA772049. Mol Cancer Res. 2003;1:682–689. [PubMed] [Google Scholar]
- O’Leary P.C., Terrile M., Bajor M., Gaj P., Hennessy B.T., Mills G.B. Peroxiredoxin-1 protects estrogen receptor α from oxidative stress-induced suppression and is a protein biomarker of favorable prognosis in breast cancer. Breast Cancer Res. 2014;16:R79-R79. doi: 10.1186/bcr3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Yu H., Dickinson D., Chu T.C., Ogbureke K., Derossi S. Epigallocatechin-3-gallate modulates antioxidant and DNA repair-related proteins in exocrine glands of a primary Sjogren's syndrome mouse model prior to disease onset. Autoimmunity. 2012;45:540–546. doi: 10.3109/08916934.2012.710860. [DOI] [PubMed] [Google Scholar]
- Pak J.H., Choi W.H., Lee H.M., Joo W.D., Kim J.H., Kim Y.T. Peroxiredoxin 6 Overexpression Attenuates Cisplatin-Induced Apoptosis in Human Ovarian Cancer Cells. Cancer Invest. 2011;29:21–28. doi: 10.3109/07357907.2010.535056. [DOI] [PubMed] [Google Scholar]
- Palande K.K., Beekman R., van der Meeren L.E., Beverloo H.B., Valk P.J.M., Touw I.P. The Antioxidant Protein Peroxiredoxin 4 Is Epigenetically Down Regulated in Acute Promyelocytic Leukemia. PLoS One. 2011;6:e16340. doi: 10.1371/journal.pone.0016340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Kim Y.J., Gao A.C., Mohler J.L., Onate S.A., Hidalgo A.A. Hypoxia Increases Androgen Receptor Activity in Prostate Cancer Cells. Cancer Res. 2006;66:5121–5129. doi: 10.1158/0008-5472.CAN-05-1341. [DOI] [PubMed] [Google Scholar]
- Park H.J., Kim B.G., Lee S.J., Heo S.H., Kim J.Y., Kwon T.H. Proteomic Profiling of Endothelial Cells in Human Lung Cancer. J Proteome Res. 2008;7:1138–1150. doi: 10.1021/pr7007237. [DOI] [PubMed] [Google Scholar]
- Park J.G., Yoo J.Y., Jeong S.J., Choi J.H., Lee M.R., Lee M.N. Peroxiredoxin 2 Deficiency Exacerbates Atherosclerosis in Apolipoprotein E–Deficient Mice. Circ Res. 2011;109:739–749. doi: 10.1161/CIRCRESAHA.111.245530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Yu X., Ip C., Mohler J.L., Bogner P.N., Park Y.M. Peroxiredoxin 1 Interacts with Androgen Receptor and Enhances Its Transactivation. Cancer Res. 2007;67:9294–9303. doi: 10.1158/0008-5472.CAN-07-0651. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon M.A., Stetler R.A., Fiskum G. Novel mitochondrial targets for neuroprotection. J Cereb Blood Flow Metab. 2012;32:1362–1376. doi: 10.1038/jcbfm.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A., Nelson K.J., Parsonage D., Poole L.B., Karplus P.A. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem Sci. 2015;40:435–445. doi: 10.1016/j.tibs.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin A.V., Low F.M., Paton L.N., Maghzal G.J., Hampton M.B., Winterbourn C.C. The High Reactivity of Peroxiredoxin 2 with H2O2 Is Not Reflected in Its Reaction with Other Oxidants and Thiol Reagents. J Biol Chem. 2007;282:11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- Pillay C., Hofmeyr J.H., Rohwer J. The logic of kinetic regulation in the thioredoxin system. BMC Syst Biol. 2011;5:15. doi: 10.1186/1752-0509-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole L.B., Hall A., Nelson K.J. Current Protocols in Toxicology. John Wiley & Sons, Inc.; 2001. Overview of Peroxiredoxins in Oxidant Defense and Redox Regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole, L. B., Hall, A., & Nelson, K. J. (2011). Overview of Peroxiredoxins in oxidant defense and redox regulation. Curr Protoc Toxicol (Chapter 7:Unit7.9). [DOI] [PMC free article] [PubMed]
- Powers S.K., Jackson M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao B., Wang J., Xie J., Niu Y., Ye S., Wan Q. Detection and identification of peroxiredoxin 3 as a biomarker in hepatocellular carcinoma by a proteomic approach. Int J Mol Med. 2012;29:832–840. doi: 10.3892/ijmm.2012.916. [DOI] [PubMed] [Google Scholar]
- Qu D., Rashidian J., Mount M.P., Aleyasin H., Parsanejad M., Lira A. Role of Cdk5-Mediated Phosphorylation of Prx2 in MPTP Toxicity and Parkinson's Disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Quan C., Cha E.J., Lee H.L., Han K.H., Lee K.M., Kim W.J. Enhanced Expression of Peroxiredoxin I and VI Correlates with Development, Recurrence and Progression of Human Bladder Cancer. J Urol. 2006;175:1512–1516. doi: 10.1016/S0022-5347(05)00659-2. [DOI] [PubMed] [Google Scholar]
- Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- Randall L.M., Manta B., Hugo M., Gil M., Batthyàny C., Trujillo M. Nitration Transforms a Sensitive Peroxiredoxin 2 into a More Active and Robust Peroxidase. J Biol Chem. 2014;289:15536–15543. doi: 10.1074/jbc.M113.539213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S.G., Woo H.A. Multiple Functions of Peroxiredoxins: Peroxidases, Sensors and Regulators of the Intracellular Messenger H2O2, and Protein Chaperones. Antioxid Redox Signal. 2010;15:781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- Rhee S.G., Chae H.Z., Kim K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Rhee S.G., Woo H.A., Kil I.S., Bae S.H. Peroxiredoxin Functions as a Peroxidase and a Regulator and Sensor of Local Peroxides. J Biol Chem. 2012;287:4403–4410. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell J.R., Bshara W., Moser M.T., Spernyak J.A., Foster B.A., Gollnick S.O. Peroxiredoxin 1 Controls Prostate Cancer Growth through Toll-Like Receptor 4 Dependent Regulation of Tumor Vasculature. Cancer Res. 2011;71:1637–1646. doi: 10.1158/0008-5472.CAN-10-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell J.R., Maier P., Sass S.N., Moser M.T., Foster B.A., Gollnick S.O. Peroxiredoxin 1 Stimulates Endothelial Cell Expression of VEGF via TLR4 Dependent Activation of HIF-1α. PLoS One. 2012;7:e50394. doi: 10.1371/journal.pone.0050394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell J.R., Wang X.-Y., Minderman H., Gollnick S.O. Peroxiredoxin 1 Stimulates Secretion of Pro-Inflammatory Cytokines by Binding to Toll-like Receptor 4. J J Immunol. 2010;184:1022–1030. doi: 10.4049/jimmunol.0901945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquier S., Breton J., Abbas K., Cornu D., Bouton C., Drapier J.C. Peroxiredoxin post-translational modifications by redox messengers. Redox Biol. 2014;2:777–785. doi: 10.1016/j.redox.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzano S., Checconi P., Hanschmann E.M., Lillig C.H., Bowler L.D., Chan P. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci U S A. 2014;111:12157–12162. doi: 10.1073/pnas.1401712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder E., Brennan J.P., Eaton P. Cardiac peroxiredoxins undergo complex modifications during cardiac oxidant stress. Am J Physiol Heart Circ Physiol. 2008;295:H425–H433. doi: 10.1152/ajpheart.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder E., Littlechild J.A., Lebedev A.A., Errington N., Vagin A.A., Isupov M.N. Crystal structure of decameric 2-Cys peroxiredoxin from human erythrocytes at 1.7Å resolution. Structure. 2000;8:605–615. doi: 10.1016/s0969-2126(00)00147-7. [DOI] [PubMed] [Google Scholar]
- Schulte J. Peroxiredoxin 4: a multifunctional biomarker worthy of further exploration. BMC Med. 2011;9:137-137. doi: 10.1186/1741-7015-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M.J., Liu X., Chang M., Park J.H. GATA-binding protein 1 is a novel transcription regulator of peroxiredoxin 5 in human breast cancer cells. Int J Oncol. 2012;40:655–664. doi: 10.3892/ijo.2011.1236. [DOI] [PubMed] [Google Scholar]
- Shadel G.S., Horvath T.L. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalini S.M., Chew W.S., Rajkumar R., Dawe G.S., Ong W.Y. Role of constitutive calcium-independent phospholipase A2 beta in hippocampo-prefrontal cortical long term potentiation and spatial working memory. Neurochem Int. 2014;78:96–104. doi: 10.1016/j.neuint.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Shichita T., Ago T., Kamouchi M., Kitazono T., Yoshimura A., Ooboshi H. Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J Neurochem. 2012;123:29–38. doi: 10.1111/j.1471-4159.2012.07941.x. [DOI] [PubMed] [Google Scholar]
- Shichita T., Hasegawa E., Kimura A., Morita R., Sakaguchi R., Takada I. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med. 2012;18:911–917. doi: 10.1038/nm.2749. [DOI] [PubMed] [Google Scholar]
- Shiota M., Yokomizo A., Kashiwagi E., Takeuchi A., Fujimoto N., Uchiumi T. Peroxiredoxin 2 in the nucleus and cytoplasm distinctly regulates androgen receptor activity in prostate cancer cells. Free Radic Biol Med. 2011;51:78–87. doi: 10.1016/j.freeradbiomed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Sobotta M.C., Liou W., Stöcker S., Talwar D., Oehler M., Ruppert T. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol. 2015;11:64–70. doi: 10.1038/nchembio.1695. [DOI] [PubMed] [Google Scholar]
- Soini Y., Kinnula V.L. High association of peroxiredoxins with lung cancer. Lung Cancer. 2012;78:167. doi: 10.1016/j.lungcan.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Song I.S., Jeong Y.J., Jeong S.H., Heo H.J., Kim H.K., Bae K.B. FOXM1-Induced PRX3 Regulates Stemness and Survival of Colon Cancer Cells via Maintenance of Mitochondrial Function. Gastroenterology. 2015;149 doi: 10.1053/j.gastro.2015.06.007. (1006-1016.e1009) [DOI] [PubMed] [Google Scholar]
- Song I.S., Kim H.K., Jeong S.H., Lee S.R., Kim N., Rhee B.D. Mitochondrial Peroxiredoxin III is a Potential Target for Cancer Therapy. Int J Mol Sci. 2011;12:7163–7185. doi: 10.3390/ijms12107163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano F.X., Léveillé F., Papadia S., Higgins L.G., Varley J., Baxter P. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem. 2008;107:533–543. doi: 10.1111/j.1471-4159.2008.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stresing V., Baltziskueta E., Rubio N., Blanco J., Arriba M., Valls J. Peroxiredoxin 2 specifically regulates the oxidative and metabolic stress response of human metastatic breast cancer cells in lungs. Oncogene. 2013;32:724–735. doi: 10.1038/onc.2012.93. [DOI] [PubMed] [Google Scholar]
- Sun K.H., de Pablo Y., Vincent F., Johnson E.O., Chavers A.K., Shah K. Novel Genetic Tools Reveal Cdk5's Major Role in Golgi Fragmentation in Alzheimer's Disease. Mol Biol Cell. 2008;19:3052–3069. doi: 10.1091/mbc.E07-11-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q.K., Zhu J.Y., Wang W., Lv Y., Zhou H.C., Yu J.H. Diagnostic and prognostic significance of peroxiredoxin 1 expression in human hepatocellular carcinoma. Med Oncol. 2013;31:1–9. doi: 10.1007/s12032-013-0786-2. [DOI] [PubMed] [Google Scholar]
- Szabó-Taylor K.É., Eggleton P., Turner C.A., Faro M.L., Tarr J.M., Tóth S. Lymphocytes from rheumatoid arthritis patients have elevated levels of intracellular peroxiredoxin 2, and a greater frequency of cells with exofacial peroxiredoxin 2, compared with healthy human lymphocytes. Int J Biochem Cell Biol. 2012;44:1223–1231. doi: 10.1016/j.biocel.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Morizane Y., Kamami-Levy C., Suzuki J., Kayama M., Cai W. AMP-dependent Kinase Inhibits Oxidative Stress-induced Caveolin-1 Phosphorylation and Endocytosis by Suppressing the Dissociation between c-Abl and Prdx1 Proteins in Endothelial Cells. J Biol Chem. 2013;288:20581–20591. doi: 10.1074/jbc.M113.460832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi K., Furihata M., Hanazaki K., Iwasaki S., Tanaka K., Shimizu T. Peroxiredoxin 1 Promotes Pancreatic Cancer Cell Invasion by Modulating p38 MAPK Activity. Pancreas. 2015;44:331–340. doi: 10.1097/MPA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavender T.J., Bulleid N.J. Peroxiredoxin IV protects cells from oxidative stress by removing H(2)O(2) produced during disulphide formation. J Cell Sci. 2010;123:2672–2679. doi: 10.1242/jcs.067843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D., Lu W., Ogasawara M.A., Valle N.R., Huang P. Redox Regulation of Cell Survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Ivey B., Manevich Y., Schulte J., Kistner-Griffin E., Jezierska-Drutel A., Liu Y. Role for Prdx1 as a specific sensor in redox-regulated senescence in breast cancer. Oncogene. 2013;32:5302–5314. doi: 10.1038/onc.2012.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ummanni R., Barreto F., Venz S., Scharf C., Barett C., Mannsperger H.A. Peroxiredoxins 3 and 4 Are Overexpressed in Prostate Cancer Tissue and Affect the Proliferation of Prostate Cancer Cells in Vitro. J Proteome Res. 2012;11:2452–2466. doi: 10.1021/pr201172n. [DOI] [PubMed] [Google Scholar]
- Walsh B., Pearl A., Suchy S., Tartaglio J., Visco K., Phelan S.A. Overexpression of Prdx6 and resistance to peroxide-induced death in Hepa1-6 cells: Prdx suppression increases apoptosis. Redox Rep. 2009;14:275–284. doi: 10.1179/135100009X12525712409652. [DOI] [PubMed] [Google Scholar]
- Wang T., Diaz A.J.G., Yen Y. The role of peroxiredoxin II in chemoresistance of breast cancer cells. Breast Cancer (Dove Med Press) 2014;6:73–80. doi: 10.2147/BCTT.S61281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Feinstein S.I., Fisher A.B. Peroxiredoxin 6 as an antioxidant enzyme: Protection of lung alveolar epithelial type II cells from H2O2-induced oxidative stress. J Cell Biochem. 2008;104:1274–1285. doi: 10.1002/jcb.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Feinstein S.I., Manevich Y., Ho Y.S., Fisher A.B. Peroxiredoxin 6 Gene-Targeted Mice Show Increased Lung Injury with Paraquat-Induced Oxidative Stress. Antioxid Redox Signal. 2006;8:229–237. doi: 10.1089/ars.2006.8.229. [DOI] [PubMed] [Google Scholar]
- Wang X., He S., Sun J.M., Delcuve G.P., Davie J.R. Selective Association of Peroxiredoxin 1 with Genomic DNA and COX-2 Upstream Promoter Elements in Estrogen Receptor Negative Breast Cancer Cells. Mol Biol Cell. 2010;21:2987–2995. doi: 10.1091/mbc.E10-02-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.G., Li L., Liu C.H., Hong S., Zhang M.J. Peroxiredoxin 3 is resistant to oxidation-induced apoptosis of Hep-3b cells. Clin Transl Oncol. 2014;16:561–566. doi: 10.1007/s12094-013-1117-y. [DOI] [PubMed] [Google Scholar]
- Wang X., Phelan S.A., Petros C., Taylor E.F., Ledinski G., Jürgens G. Peroxiredoxin 6 deficiency and atherosclerosis susceptibility in mice: significance of genetic background for assessing atherosclerosis. Atherosclerosis. 2004;177:61–70. doi: 10.1016/j.atherosclerosis.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Wang X.Y., Wang H.J., Li X.Q. Peroxiredoxin III protein expression is associated with platinum resistance in epithelial ovarian cancer. Tumour Biol. 2013;34:2275–2281. doi: 10.1007/s13277-013-0769-0. [DOI] [PubMed] [Google Scholar]
- Wang M.X., Wei A., Yuan J., Clippe A., Bernard A., Knoops B. Antioxidant Enzyme Peroxiredoxin 5 Is Upregulated in Degenerative Human Tendon. Biochem Biophys Res Commun. 2001;284:667–673. doi: 10.1006/bbrc.2001.4991. [DOI] [PubMed] [Google Scholar]
- Watanabe R., Nakamura H., Masutani H., Yodoi J. Anti-oxidative, anti-cancer and anti-inflammatory actions by thioredoxin 1 and thioredoxin-binding protein-2. Pharmacol Ther. 2010;127:261–270. doi: 10.1016/j.pharmthera.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Wei Q., Jiang H., Xiao Z., Baker A., Young M.R., Veenstra T.D. Sulfiredoxin–Peroxiredoxin IV axis promotes human lung cancer progression through modulation of specific phosphokinase signaling. Proc Natl Acad Sci U S A. 2011;108:7004–7009. doi: 10.1073/pnas.1013012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann P., Knoblich M., Maier O., Lindschau C., Haller H. Protein kinase C bound to the Golgi apparatus supports the formation of constitutive transport vesicles. Biochem J. 1996;320:651–658. doi: 10.1042/bj3200651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker H.C., Patel D., Howat W.J., Warren A.Y., Kay J.D., Sangan T. Peroxiredoxin-3 is overexpressed in prostate cancer and promotes cancer cell survival by protecting cells from oxidative stress. Br J Cancer. 2013;109:983–993. doi: 10.1038/bjc.2013.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonsey D.R., Zeller K.I., Dang C.V. The c-Myc target gene PRDX3 is required for mitochondrial homeostasis and neoplastic transformation. Proc Natl Acad Sci U S A. 2002;99:6649–6654. doi: 10.1073/pnas.102523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo H.A., Yim S.H., Shin D.H., Kang D., Yu D.Y., Rhee S.G. Inactivation of Peroxiredoxin I by Phosphorylation Allows Localized H2O2 Accumulation for Cell Signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Wood Z.A., Poole L.B., Karplus P.A. Peroxiredoxin Evolution and the Regulation of Hydrogen Peroxide Signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- Wood Z.A., Schröder E., Robin Harris J., Poole L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- Woolston C.M., Storr S.J., Ellis I.O., Morgan D.A., Martin S.G. Expression of thioredoxin system and related peroxiredoxin proteins is associated with clinical outcome in radiotherapy treated early stage breast cancer. Radiother Oncol. 2011;100:308–313. doi: 10.1016/j.radonc.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Wu Y., Feinstein S.I., Manevich Y., Chowdhury I., Pak J.H., Kazi A. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A(2) activity. Biochem J. 2009;419:669–679. doi: 10.1042/BJ20082061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.Z., Manevich Y., Baldwin J.L., Dodia C., Yu K., Feinstein S.I. Interaction of Surfactant Protein A with Peroxiredoxin 6 Regulates Phospholipase A2 Activity. J Biol Chem. 2006;281:7515–7525. doi: 10.1074/jbc.M504525200. [DOI] [PubMed] [Google Scholar]
- Xi H., Gao Y.H., Han D.Y., Li Q.Y., Feng L.J., Zhang W. Hypoxia inducible factor-1α suppresses Peroxiredoxin 3 expression to promote proliferation of CCRCC cells. FEBS Lett. 2014;588:3390–3394. doi: 10.1016/j.febslet.2014.07.030. [DOI] [PubMed] [Google Scholar]
- Yamada S., Ding Y., Sasaguri Y. Peroxiredoxin 4: critical roles in inflammatory diseases. J UOEH. 2012;34:27–39. doi: 10.7888/juoeh.34.27. [DOI] [PubMed] [Google Scholar]
- Yanagawa T., Ishikawa T., Ishii T., Tabuchi K., Iwasa S., Bannai S. Peroxiredoxin I expression in human thyroid tumors. Cancer Lett. 1999;145:127–132. doi: 10.1016/s0304-3835(99)00243-8. [DOI] [PubMed] [Google Scholar]
- Yanagawa T., Iwasa S., Ishii T., Tabuchi K., Yusa H., Onizawa K. Peroxiredoxin I expression in oral cancer: a potential new tumor marker. Cancer Lett. 2000;156:27–35. doi: 10.1016/s0304-3835(00)00434-1. [DOI] [PubMed] [Google Scholar]
- Yanagawa T., Omura K., Harada H., Ishii T., Uwayama J., Nakaso K. Peroxiredoxin I expression in tongue squamous cell carcinomas as involved in tumor recurrence. Int J Oral Maxillofac Surg. 2005;34:915–920. doi: 10.1016/j.ijom.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Yang K.S., Kang S.W., Woo H.A., Hwang S.C., Chae H.Z., Kim K. Inactivation of Human Peroxiredoxin I during Catalysis as the Result of the Oxidation of the Catalytic Site Cysteine to Cysteine-sulfinic Acid. J Biol Chem. 2002;277:38029–38036. doi: 10.1074/jbc.M206626200. [DOI] [PubMed] [Google Scholar]
- Yang C.S., Lee D.S., Song C.H., An S.J., Li S., Kim J.M. Roles of peroxiredoxin II in the regulation of proinflammatory responses to LPS and protection against endotoxin-induced lethal shock. J Exp Med. 2007;204:583–594. doi: 10.1084/jem.20061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Taylor M., Davey F., Ren Y., Aiton J., Coote P. Interaction of amyloid binding alcohol dehydrogenase/Aβ mediates up-regulation of peroxiredoxin II in the brains of Alzheimer’s disease patients and a transgenic Alzheimer’s disease mouse model. Mol Cell Neurosci. 2007;35:377–382. doi: 10.1016/j.mcn.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Yi N.A., Xiao M.B., Ni W.K., Jiang F., Lu C.H., Ni R.Z. High expression of peroxiredoxin 4 affects the survival time of colorectal cancer patients, but is not an independent unfavorable prognostic factor. Mol Clin Oncol. 2014;2:767–772. doi: 10.3892/mco.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H.M., Choi D.Y., Oh K.W., Hong J.T. PRDX6 Exacerbates Dopaminergic Neurodegeneration in a MPTP Mouse Model of Parkinson’s Disease. Mol Neurobiol. 2015;52:422–431. doi: 10.1007/s12035-014-8885-4. [DOI] [PubMed] [Google Scholar]
- Yun H.M., Jin P., Han J.Y., Lee M.S., Han S.B., Oh K.W. Acceleration of the Development of Alzheimer’s Disease in Amyloid Beta-Infused Peroxiredoxin 6 Overexpression Transgenic Mice. Mol Neurobiol. 2013;48:941–951. doi: 10.1007/s12035-013-8479-6. [DOI] [PubMed] [Google Scholar]
- Yun H.M., Park M.H., Kim D.H., Ahn Y.J., Park K.R., Kim T.M. Loss of presenilin 2 is associated with increased iPLA2 activity and lung tumor development. Oncogene. 2014;33:5193–5200. doi: 10.1038/onc.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H.M., Park K.R., Kim E.C., Hong J.T. PRDX6 controls multiple sclerosis by suppressing inflammation and blood brain barrier disruption. Oncotarget. 2015;6:20875–20884. doi: 10.18632/oncotarget.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H.M., Park K.R., Lee H.P., Lee D.H., Jo M., Shin D.H. PRDX6 promotes lung tumor progression via its GPx and iPLA2 activities. Free Radic Biol Med. 2014;69:367–376. doi: 10.1016/j.freeradbiomed.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Yun H.M., Park K.R., Park M.H., Kim D.H., Jo M.R., Kim J.Y. PRDX6 promotes tumor development via the JAK2/STAT3 pathway in a urethane-induced lung tumor model. Free Radic Biol Med. 2015;80:136–144. doi: 10.1016/j.freeradbiomed.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Emmanuel N., Kamboj G., Chen J., Shurafa M., Van Dyke D.L. PRDX4, a member of the peroxiredoxin family, is fused to AML1 (RUNX1) in an acute myeloid leukemia patient with a t(X;21)(p22;q22) Genes Chromosomes Cancer. 2004;40:365–370. doi: 10.1002/gcc.20050. [DOI] [PubMed] [Google Scholar]
- Zhang M., Hou M., Ge L., Miao C., Zhang J., Jing X. Induction of Peroxiredoxin 1 by Hypoxia Regulates Heme Oxygenase-1 via NF-κB in Oral Cancer. PLoS One. 2014;9:e105994. doi: 10.1371/journal.pone.0105994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Liu W., Szumlinski K.K., Lew J. p10, the N-terminal domain of p35, protects against CDK5/p25-induced neurotoxicity. Proc Natl Acad Sci U S A. 2012;109:20041–20046. doi: 10.1073/pnas.1212914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Shi L.L., Gao X., Jiang D., Zhong Z.Q., Zeng X. Lentivirus-mediated Inhibition of Tumour Necrosis Factor-α improves motor function associated with PRDX6 in spinal cord contusion rats. Sci Rep. 2015;5:8486. doi: 10.1038/srep08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Wang S., Zhang M., Weng Z., Li P., Gan Y. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke. 2012;43:1390–1397. doi: 10.1161/STROKEAHA.111.647420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.Z., Xiao Z.F., Li C., Xiao Z.Q., Yang F., Li D.J. Triosephosphate isomerase and peroxiredoxin 6, two novel serum markers for human lung squamous cell carcinoma. Cancer Sci. 2009;100:2396–2401. doi: 10.1111/j.1349-7006.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Wang Q. The protective effect of peroxiredoxin II on oxidative stress induced apoptosis in pancreatic β-cells. Cell Biosci. 2012;2:22. doi: 10.1186/2045-3701-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito E., Melo E.P., Yang Y., Wahlander Å., Neubert T.A., Ron D. Oxidative protein folding by an endoplasmic reticulum localized peroxiredoxin. Mol Cell. 2010;40:787–797. doi: 10.1016/j.molcel.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]