Summary
Objectives
To determine the aetiology, clinical features and prognosis of CAP during the first post-pandemic influenza season. We also assessed the factors associated with severe disease and tested the ability of a scoring system for identifying influenza A (H1N1)pdm09-related pneumonia.
Methods
Prospective cohort study carried out at 10 tertiary hospitals of Spain. All adults hospitalised with CAP from December 01, 2010 to March 31, 2011 were analysed.
Results
A total of 747 adults with CAP required hospitalisation. The aetiology was determined in 315 (42.2%) patients, in whom 154 (21.9%) were due to bacteria, 125 (16.7%) were due to viruses and 36 (4.8%) were mixed (due to viruses and bacteria). The most frequently isolated bacteria were Streptococccus pneumoniae. Among patients with viral pneumonia, the most common organism identified were influenza A (H1N1)pdm09. Independent factors associated with severe disease were impaired consciousness, septic shock, tachypnea, hyponatremia, hypoxemia, influenza B, and influenza A (H1N1)pdm09. The scoring system evaluated did not differentiate reliably between patients with influenza A (H1N1)pdm09-related pneumonia and those with other aetiologies.
Conclusions
The frequency of bacterial and viral pneumonia during the first post-pandemic influenza season was similar. The main identified virus was influenza A (H1N1)pdm09, which was associated with severe disease. Although certain presenting clinical features may allow recognition of influenza A (H1N1)pdm09-related pneumonia, it is difficult to express them in a reliable scoring system.
Keywords: Clinical features, Community-acquired pneumonia, Influenza A (H1N1)pdm09, Mortality, Post-pandemic period
Introduction
Community-acquired pneumonia (CAP) is one of the world's major public health problems. The aetiology of CAP has been under constant study in different local settings. The most frequently documented causative pathogens of CAP are Streptococcus pneumoniae, Haemophilus influenzae and Legionella pneumophila.1, 2, 3 Evidence of viral infection has been detected in 15%–56% of cases, mainly by influenza viruses, respiratory syncytial virus, parainfluenza viruses and adenovirus.4
Notably, CAP was one of the most frequent complications of influenza A (H1N1)pdm09 infection during the pandemic period. In addition, it was associated with high morbidity and mortality.5, 6 The reported incidence of pneumonia in hospitalised patients with influenza A (H1N1)pdm09 infection was 23%–66%,7, 8, 9 with primary viral pneumonia being the main cause of admission to intensive care units.10, 11 Similarly, bacterial co-infection was associated with worse prognosis.7, 12
Knowledge of the predominant microbial patterns in CAP is the basis for initial decisions about its empirical antimicrobial treatment.3 Importantly, influenza A (H1N1)pdm09 continues to circulate as a seasonal virus after the pandemic period. It is therefore crucial to determine the microbial patterns and outcomes in CAP in the post-pandemic influenza seasons.13 In addition, although Bewick et al.14 developed a scoring system for identifying patients with influenza A (H1N1)pdm09-related pneumonia, this has not been validated to date.
In this multicentre, prospective cohort study conducted in Spain, we aimed to determine the aetiology, clinical features and prognosis of hospitalised adults with CAP during the first post-pandemic influenza season (2010–2011). We also assessed the factors associated with severe disease and tested the reliability of a scoring system for identifying patients with influenza A (H1N1)pdm09-related pneumonia.
Patients and methods
Setting, patients and study design
This prospective cohort study was carried out at 10 tertiary hospitals in different areas of Spain. All adults admitted to hospital for at least 24 h with CAP from December 01, 2010 to March 31, 2011 were prospectively recruited and followed up. Cases were identified at the emergency department by attending physicians or investigators. The study was approved by the Ethics Committee of the coordinating centre, Hospital Universitari de Bellvitge, and informed consent was obtained from patients.
Clinical assessment and follow-up
Patients were seen during their hospital stay by one or more of the investigators at each participating hospital, who recorded clinical data in a standardized, computer-assisted protocol. Data were collected on demographic features, comorbidities, clinical features, biochemical analyses, chest X-ray findings, therapy, complications and in-hospital mortality. To stratify patients according to risk, we used pneumonia severity index (PSI) and CURB-65.15, 16 Completed protocols were carefully revised by two clinical investigators prior to the final validation.
Definitions
CAP was defined by the presence of a new infiltrate on chest X-rays, together with at least two symptoms of a lower respiratory tract infection (fever or hypothermia, new cough with or without sputum production, pleuritic chest pain, dyspnoea or altered breath sounds on auscultation) and no alternative diagnosis during follow-up. Viral CAP was diagnosed if a virus was detected by multiplex PCR, and the respiratory and blood bacterial cultures and urine antigen tests were negative. Bacterial CAP was diagnosed in patients with one or more positive cultures obtained from blood, normally sterile fluids or sputum and/or a positive urinary antigen test, and with no viral pathogens detected. A mixed infection was defined as the presence of both respiratory virus and bacteria, as defined above.
Comorbidities were assessed by the Charlson Comorbidity Index.17 Complications were defined as any untoward circumstances occurring during hospitalisation. Multilobar pneumonia was defined as chests X-ray infiltrate involving two or more lobes. The diagnosis of septic shock was based on systolic blood pressure of less than 90 mmHg and requirement of vasopressors after adequate volume reposition. Severe disease was defined as the composite outcome of intensive care unit admission or in-hospital mortality. In-hospital mortality was defined as death from any cause during hospitalisation.
Microbiological studies
Two specific one-step multiplex RT-PCR on nasopharyngeal swab or bronchoalveolar lavage were performed in each participating centre and were used for typing (A/B) and subtyping of the hemagglutinin (H1/novel H1/H3/H5) the influenza virus as described elsewhere.5 The Real Time RT-PCR Protocol for Detection and Characterization of Influenza A (H1N1)v supplied by Centers for Disease Control (CDC, Atlanta, US) was used to confirm our positive results.
In addition, nasopharyngeal swab or bronchoalveolar lavage samples were used to obtain ADN and ARN using an extraction protocol with magnetic particles (Sample Preparation Systems RNA and DNA, Promega, Abbott, USA) at the coordinating centre. RT-PCR was used for the detection of influenza A and B, rhinovirus, respiratory syncytial virus A and B, parainfluenza virus types 1, 2, 3, and 4, coronavirus types OC43 and 229E, adenovirus and human metapneumovirus as described elsewhere.18, 19 Gene amplification products were identified by electrophoresis in 3% agarose gels and afterwards ethidium bromide staining.
Bacterial pathogens in blood, normally sterile fluids, sputum and other samples were investigated by standard microbiological procedures within the first 48 h after admission. Sputum samples were considered of good quality if they had <10 squamous cells and >25 leukocytes per low-power field. The finding of the S. pneumoniae antigen in urine was detected by a rapid immunochromatographic assay (NOW Assay; Binax Inc, Portland, Maine) or enzyme-linked immunosorbent assay (ELISA-Bartels, Bartels, Trinity Biotech, Wicklow, Ireland). L. pneumophila serogroup 1 antigen in urine was detected by an immunochromatographic method (NOW Legionella Urinary Antigen Test; Binax Inc).
Statistical analysis
All proportions were calculated as percentages of the patients with available data. We compared patients with influenza A (H1N1)pdm09-related CAP and patients with bacterial CAP. To detect significant differences between groups, we used the chi-square test or Fisher exact test for categorical variables and the t test or Mann–Whitney test for continuous variables, as appropriate. A multivariate logistic analysis, including significant variables in univariate analysis and clinically important variables, was performed to detect factors associated with severe disease. The relative risks were expressed as odds ratios (OR) and 95% CI. The possibility of multicollinearity among variables was measured by means of correlation coefficient matrix. A value of 0.75 or higher among variables were considered as multicollinearity. Calibration of the model was evaluated by the goodness-of-fit according to the Hosmer–Lemeshow test. We also tested the ability of the Bewick et al.14 scoring system for identifying patients with influenza A (H1N1)pdm09-related pneumonia by means of the area under receiver operating characteristic curve (AUROC) and sensitivity, specificity and likelihood ratios (LR). The Bewick 5-point scoring system is a model based in five clinical criteria: age ≤ 65 years, mental orientation, temperature ≥ 38 °C, leucocyte count ≤12 × 10(9)/l and bilateral radiographic consolidation. Statistical significance was established at P < 0.05. All P values reported are 2-tailed. The results were analyzed using SPSS version 15.0 (SPSS Inc, Chicago, Illinois).
Results
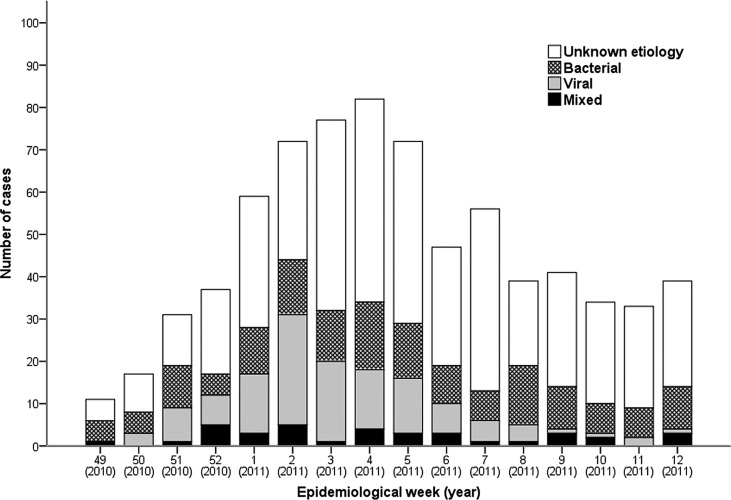
All 747 adult patients admitted with CAP to the participating centres were included in the analysis. The distribution of cases during the study period is shown in Fig. 1 . Most patients were hospitalised between epidemiological weeks 2 and 5, 2011 (January 09 – February 05).
Figure 1.
Distribution of cases and causative agentsa by week during the study period (2010–2011). aMost cases of community-acquired pneumonia caused by influenza A (H1N1)pdm09 (76.7%) and influenza B (62.5%) occurred during epidemiological weeks 1–6 and 6–9, respectively. According to data provided by the Spanish Influenza Surveillance System (http://vgripe.isciii.es/gripe/inicio.do), the highest incidence of influenza cases during the first post-pandemic influenza season period were in weeks 2–3 (236 cases/100 000 inhabitants).
Aetiology, clinical features and outcomes of CAP
Table 1 shows the distribution of causative organisms in hospitalised patients with CAP. An etiological diagnosis could be established in 315 (42.2%) patients. According to the etiological diagnosis, 154 (21.9%) were due to bacteria, 125 (16.7%) to viruses, and 36 (4.8%) to viruses and bacteria. The most frequently documented bacteria was S. pneumoniae. Among patients with viral pneumonia, the most common organism were influenza A (H1N1)pdm09.
Table 1.
Causative organisms in hospitalized patients with CAP during the first post-pandemic influenza season (2010–2011).a
| N = 747 n (%) | |
|---|---|
| Bacterial | 154 (21.9)b |
| Streptococcus pneumoniae | 98 (13.1) |
| Haemophilus influenzae | 13 (1.7) |
| Staphylococcus aureusc | 11 (1.5) |
| Pseudomonas aeruginosa | 9 (1.2) |
| Legionella pneumophila | 6 (0.8) |
| Others | 17 (2.2) |
| Viral | 125 (16.7) |
| Influenza A (H1N1)pdm09 | 96 (12.8) |
| Rhinovirus | 16 (2.1) |
| Influenza B | 5 (0.6) |
| Parainfluenza | 4 (0.5) |
| Others | 4 (0.5) |
| Mixed | 36 (4.8) |
| Influenza A (H1N1)pdm09 + S. Pneumoniae | 11 (1.5) |
| Rhinovirus + S. pneumoniae | 3 (0.4) |
| Influenza B + S. pneumoniae | 3 (0.4) |
| RSV + S. pneumoniae | 3 (0.4) |
| Others | 16 (2.1) |
| Unknown aetiology | 432 (57.2) |
CAP, community-acquired pneumonia; RSV, respiratory syncytial virus.
The number of diagnostic test performed were: RT-PCR 667, sputum culture 382, blood culture 445, pleural effusion culture 40, bronchoalveolar lavage culture (BAL) 49, pneumococcal urinary antigen test (PUAT) 617, and Legionella pneumophila urinary antigen test 570.
S. pneumoniae was identified by means of sputum culture (26 cases), blood culture (23), BAL (3), and PUAT (96); Haemophilus influenzae by sputum culture (15), blood culture (2), and BAL (1); Staphylococcus aureus by sputum culture (8), blood culture (6), and BAL (3); Pseudomonas aeruginosa sputum culture (13), blood culture (2), and BAL (1); and Legionella pneumophila by urinary antigen test (6).
Six cases were methicillin-resistant S. aureus.
Compared with patients who did not require ICU admission, patients who required ICU admission had more frequently influenza A (H1N1)pdm09 (47.9% vs 10.3%; P < .001), influenza B (4.3% vs 0.6%; P = .001), and Streptococcus spp. (3% vs 0%; P = .02). The frequency of bacteraemia was not different between these two groups (8.7% vs 11.8%; P = .36). Likewise, the proportion of patients in whom microbiological tests were performed were similar in both groups (97.9% vs 99.2%; P = .33).
The epidemiology, clinical features and laboratory findings of hospitalised patients with CAP are detailed in Table 2 . Their median age was 65 and over half were male. Patients with viral pneumonia were younger than patients with other aetiologies. Five hundred and fifty-three (74%) patients had comorbidities, mainly chronic pulmonary disease, chronic heart disease and diabetes mellitus. Patients with viral pneumonia had comorbid conditions less frequently than the remaining patients. The most frequent clinical features were cough, dyspnoea, pleuritic chest pain and arthralgia. In chest X-rays, unilobar alveolar infiltrates were the most frequent finding (59.7%). Empyema was documented in a low proportion of cases (1.6%).
Table 2.
Epidemiology, clinical features and laboratory findings at admission of hospitalized patients with CAP during the first post-pandemic influenza season (2010–2011).
| Characteristic | All cases N = 747 | Bacterial n = 154 | Viral n = 125 | Mixed n = 36 | Unknown n = 432 |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age, median (IQR), years | 65 (49–78) | 67 (53–78) | 55 (43–65) | 60 (50.5–77.5) | 68 (51–79) |
| Male sex | 423 (56.8) | 84 (54.5) | 70 (56) | 21 (60) | 248 (57.5) |
| Current smoker | 211 (28.3) | 49 (31.8) | 37 (29.6) | 11 (30.6) | 114 (26.5) |
| Alcohol abuse | 73 (9.8) | 12 (7.8) | 14 (11.2) | 6 (16.7) | 41 (9.5) |
| Comorbidities | 553 (74) | 122 (79.2) | 75 (60) | 29 (80.6) | 327 (75.7) |
| Chronic pulmonary disease | 269 (36) | 73 (47.4) | 36 (28.8) | 14 (38.9) | 146 (33.8) |
| Chronic heart disease | 197 (26.4) | 33 (21.4) | 20 (16) | 9 (25) | 135 (31.3) |
| Diabetes mellitus | 171 (22.9) | 36 (23.4) | 14 (11.2) | 6 (16.7) | 115 (26.6) |
| Immunosuppression | 99 (13.3) | 23 (14.9) | 22 (17.6) | 9 (25) | 45 (10.4) |
| Clinical features | |||||
| Cough | 650 (87.2) | 132 (85.7) | 115 (92) | 33 (91.7) | 372 (86.2) |
| Rhinorrea | 74 (10.8) | 11 (8.3) | 27 (22.1) | 3 (8.6) | 34 (8.6) |
| Dyspnoea | 563 (75.6) | 117 (76) | 97 (77.6) | 29 (80.6) | 320 (74.4) |
| Pleuritic chest pain | 240 (32.3) | 58 (37.7) | 28 (22.6) | 8 (22.2) | 146 (34) |
| Arthralgia | 208 (27.9) | 40 (26) | 50 (40) | 13 (36.1) | 105 (24.4) |
| Fever (>38.0 C) | 215 (29.3) | 49 (32.2) | 40 (33.3) | 13 (36.1) | 113 (26.6) |
| Tachycardia (>100 beats-min-1) | 309 (41.7) | 84 (55.3) | 48 (38.7) | 16 (45.7) | 161 (37.4) |
| Tachypnea (>30 breaths-min-1) | 161 (25.8) | 44 (34.4) | 38 (34.9) | 10 (33.3) | 69 (19.4) |
| Impaired consciousness | 87 (11.7) | 21 (13.6) | 16 (12.8) | 8 (22.2) | 42 (9.8) |
| Septic shock | 35 (4.7) | 10 (6.5) | 10 (8) | 2 (5.6) | 13 (3) |
| Laboratory and radiographic findings | |||||
| Hypoxemia (sat02 < 90%) | 240 (35.7) | 57 (41.3) | 50 (44.6) | 19 (55.4) | 114 (29.3) |
| Leukocytosis (leukocytes >12 109/L) | 370 (49.9) | 96 (62.7) | 36 (29.3) | 15 (42.9) | 223 (51.7) |
| Hyponatremia (sodium <130 mEq/L) | 70 (9.5) | 21 (13.8) | 22 (17.6) | 4 (11.1) | 23 (5.4) |
| Multilobar pneumonia | 301 (40.3) | 51 (33.1) | 77 (61.6) | 17 (47.2) | 156 (36.1) |
| Pleural effusion | 100 (13.5) | 25 (16.4) | 15 (12.1) | 4 (11.1) | 56 (13) |
| CAP-specific scores | |||||
| High-risk PSI classesa | 348 (46.6) | 89 (57.8) | 50 (40) | 21 (58.3) | 188 (43.5) |
| High-risk CURB-65 groupsb | 283 (37.9) | 74 (48.1) | 34 (27.2) | 23 (63.9) | 152 (32.5) |
CAP, community-acquired pneumonia; IQR, interquartile range; PSI, pneumonia severity index.
Patients were stratified into the following risk classes according to their PSI score: low risk (≤90 points, classes I, II and III) and high risk (>90 points, classes IV and V).
Patients were stratified into the following risk classes according to their CURB-65 score: low risk (0–1 point) and high risk (>1 point).
The clinical outcomes of patients are given in Table 3 . Respiratory complications (need for mechanical ventilation, need for non-invasive mechanical ventilation, ARDS and/or empyema) were the most frequent ones documented during hospitalisation, followed by acute cardiac events and nosocomial infections. Ninety-four (12.6%) patients required ICU admission and 61 (8.2%) died. Patients with viral and mixed pneumonia required ICU admission more frequently and had higher in-hospital mortality. The main causes of mortality were respiratory failure/acute respiratory distress syndrome, multi-organ failure, septic shock and acute decompensated underlying disease.
Table 3.
Clinical outcomes of hospitalized patients with CAP during the first post-pandemic influenza season (2010–2011).
| Characteristic | All cases N = 747 | Bacterial n = 154 | Viral n = 125 | Mixed n = 36 | Unknown n = 432 |
|---|---|---|---|---|---|
| In-hospital complications | |||||
| Acute cardiac eventsa | 79 (10.6) | 14 (9.1) | 18 (14.4) | 1 (2.8) | 46 (10.6) |
| Nosocomial infections | 31 (4.1) | 5 (3.2) | 13 (10.4) | 1 (2.8) | 12 (2.8) |
| ICU admissionb | 94 (12.6) | 18 (11.7) | 41 (32.8) | 11 (30.6) | 24 (5.6) |
| Need for mechanical ventilation (intubation) | 59 (7.9) | 9 (5.8) | 30 (24.4) | 8 (22.2) | 12 (2.8) |
| ARDS | 58 (7.8) | 11 (7.1) | 27 (21.6) | 9 (25) | 11 (2.5) |
| Time to clinical stability, median (IQR), days | 2 (1–4) | 2 (1–5) | 3 (1–7) | 4 (2–7) | 2 (1–3) |
| Length of hospital stay, median (IQR), days | 8 (5–13) | 9 (6–14) | 9 (6–15) | 8 (6.5–16.5) | 8 (5–11) |
| In-hospital mortality | 61 (8.2) | 11 (7.1) | 22 (17.6) | 6 (16.7) | 22 (5.1) |
ARDS, acute respiratory distress syndrome; CAP, community-acquired pneumonia; ICU, intensive care unit; IQR, interquartile range.
New-onset or worsening cardiac arrhythmias, new-onset or worsening congestive heart failure and/or myocardial infarction.
79 (84.9%) patients required intubation and/or non-invasive mechanical ventilation.
Predictors of severe disease
Severe disease (ICU admission or in-hospital mortality) occurred in 124 (16.6%) patients. In the univariate analysis, factors associated with severe disease were age (<65 years), current smoker, alcohol abuse, influenza vaccine (2010–2011), impaired consciousness, septic shock, influenza A (H1N1)pdm09, influenza B, Streptococcus spp., hyponatremia, multilobar pneumonia, hypoxemia, tachypnea, high-risk PSI and CURB65 classes. Although comorbidities were not related with severe disease, chronic pulmonary disease was a protective condition and immunosuppression was a risk condition for this complication (data not shown). The results of multivariate logistic regression analysis for factors possibly associated with severe disease are shown in Table 4 . Multicollinearity among variables was not documented. Independent factors related with severe disease were impaired consciousness, septic shock, tachypnea, hyponatremia, hypoxemia, influenza B, and influenza A (H1N1)pdm09. The value of Hosmer–Lemeshow test for the model was 0.86.
Table 4.
Multivariate analysis for factors associated with severe disease in hospitalized patients with CAP during the first post-pandemic influenza season (2010–2011).
| OR | 95% CI | P value | |
|---|---|---|---|
| Age (<65 years) | 1.22 | (0.54–2.73) | 0.62 |
| Female sex | 0.51 | (0.25–1.06) | 0.07 |
| Comorbiditiesa | 0.69 | (0.31–1.64) | 0.37 |
| Current smoker | 1.04 | (0.50–2.15) | 0.90 |
| Alcohol abuse | 2.15 | (0.81–5.69) | 0.12 |
| Influenza vaccine (2010–2011) | 0.81 | (0.38–1.71) | 0.58 |
| Impaired consciousness | 4.14 | (1.73–9.94) | 0.001 |
| Septic shock | 21.26 | (5.53–81.66) | <0.001 |
| Tachypnea (>30 breaths-min-1) | 3.89 | (2.01–7.53) | <0.001 |
| Hyponatremia (sodium <130 mEq/L) | 3.16 | (1.19–8.38) | 0.02 |
| Hypoxemia (sat02 < 90%) | 2.04 | (1.07–3.90) | 0.03 |
| Multilobar pneumonia | 1.69 | (0.87–3.26) | 0.11 |
| Influenza A (H1N1)pdm09 | 5.47 | (2.58–11.57) | <0.001 |
| Influenza B | 11.67 | (1.42–95.99) | 0.02 |
| S. aureus | 2.35 | (0.27–20.25) | 0.43 |
| Pneumococcal pneumonia | 1.82 | (0.82–3.99) | 0.14 |
CAP, community-acquired pneumonia; CI, confidence interval; OR, odds ratio.
If chronic pulmonary disease, diabetes mellitus and immunosuppression were included in the model, none of these comorbidities were significantly associated with severe disease. However, chronic heart disease was independently related with higher risk of severe disease (OR 2.41 95% CI 1.11–5.28; P = .02).
Because ICU admission was not standardised and therapeutic limitation orders were not recorded, a post hoc multivariate analysis was performed considering ICU admission and intubation as dependent variable. Factors independently associated with ICU admission and intubation were septic shock, influenza A (H1N1)pdm09, influenza B, hyponatremia, and hypoxemia. The value of Hosmer–Lemeshow test for the model was 0.86.
Validation of a clinical model for identifying patients with influenza A (H1N1)pdm09-related pneumonia
The results of applying the scoring system described by Bewick et al.14 are summarized in Table 5 . Twenty-one patients were excluded from this analysis because of missing data. The incidences of influenza A (H1N1)pdm09-related pneumonia ranged from 1.6% for patients with less than one clinical criterion to 27% for subjects with 4 or more clinical criteria. The AUROC of the model to predict influenza A (H1N1)pdm09-related pneumonia was 0.77 (95% CI, 0.71–0.82). Using the optimal cut-off point (highest Youden index) of >3, the model had 56.6% sensitivity (95% CI, 45.8–67.1) and 86.1% specificity (95% CI, 83.2–88.8) for predicting influenza A (H1N1)pdm09-related pneumonia (LR+ 4.1 and LR- 0.5).
Table 5.
Results of application of the Bewick scoring system for identifying influenza A (H1N1)pdm09-related pneumonia.a, b
| Points | Bacterial n = 151 | (H1N1)pdm09 n = 90 | Mixed ((H1N1)pdm09) n = 16 | Other viruses n = 28 | Unknown etiology n = 422 |
|---|---|---|---|---|---|
| 0 | 1 (0.7) | 1 (1.1) | 0 (0) | 1 (3.6) | 10 (2.4) |
| 1 | 35 (23.2) | 2 (2.2) | 1 (6.3) | 4 (14.3) | 98 (23.2) |
| 2 | 56 (37.1) | 15 (16.7) | 2 (12.5) | 10 (35.7) | 129 (30.6) |
| 3 | 48 (31.8) | 21 (23.3) | 6 (37.5) | 9 (32.1) | 120 (28.4) |
| 4 | 9 (6) | 32 (35.6) | 5 (31.3) | 4 (14.3) | 54 (12.8) |
| 5 | 2 (1.3) | 16 (21.1) | 2 (12.5) | 0 (0) | 11 (2.6) |
| AUROC (95% CI) | NA | 0.77 (0.71–0.82) | 0.70 (0.58–0.82) | NA | NA |
AUROC, Area under receiver operating characteristic curve; CI, confidence interval; NA, not applicable.
Twenty-one patients were excluded from this analysis because of missing data.
Data from mixed (viral (no H1N1) and bacterial) were not shown.
Discussion
This multicentre, prospective cohort with 747 consecutive hospitalised adults, the largest study to date, provides a comprehensive insight into the aetiology, clinical features and outcomes of CAP during the first post-pandemic influenza season (2010–2011). The main results were that: (a) the frequency of bacterial pneumonia and viral pneumonia was similar, (b) independent factors associated with severe disease (ICU admission or mortality) were altered mental state at presentation, septic shock, tachypnea, hyponatremia, multilobar infiltrates in chest X-ray and influenza A (H1N1)pdm09-related pneumonia, and (c) the scoring system evaluated did not distinguish reliably between influenza A (H1N1)pdm09-related pneumonia and other aetiologies of CAP.
In the present study, the percentage of patients with aetiologies (42.2%) and the main etiological agents causing bacterial CAP were comparable to those in several previous series.1, 2, 20, 21 S. pneumoniae was found to be the leading pathogen in bacterial CAP. We also found a relatively high percentage of Gram-negative bacilli (2.2%) and Staphylococcus aureus (1.5%) among the pathogens involved. However, it should be noted that 13.3% of our cases were immunosuppressed. Mixed infections were relatively frequent (most commonly involving S. pneumoniae together with a respiratory virus). Moreover, we also documented a high percentage of viral CAP, which may be mainly attributable to the high number of influenza A (H1N1)pdm09-related pneumonia cases. In this regard, according to surveillance reports, influenza A (H1N1)pdm09 was the predominant virus causing illness in Europe and the Middle East during the 2010–2011 winter season.22, 23 In Spain, there were 4747 detections of influenza viruses during the 2010–11 winter season. Of the total virus detections, 71.9% were influenza A viruses, mainly influenza A (H1N1)pdm09 (97%), 27.8% influenza B and 0.3% influenza C.24
Our study identified factors associated with severe disease in hospitalised patients with CAP during the first post-pandemic influenza season. These factors are markers of more severe pneumonia at hospital admission (impaired consciousness, septic shock, tachypnea, hyponatremia, and hypoxemia) and have been described as prognostic factors in previous CAP studies.3 Interestingly, we also documented that influenza A (H1N1)pdm09-related pneumonia was independently associated with higher risk of severe disease. Compared with other aetiologies, influenza A (H1N1)pdm09-related pneumonia had a three times greater risk of ICU admission and in-hospital mortality. This explains the higher morbidity and mortality found in patients with viral pneumonia in the present study. Recently, it was documented in England, Greece, Spain and Taiwan22, 23, 25, 26 that, in December 2010 and January 2011, there was a sharp rise in influenza-related hospitalisations, ICU admissions and fatalities than in the 2009-2010 season. Increasing age and certain comorbidities, as well as a delay in the administration of antiviral treatment, have been reported as causes of higher morbidity and mortality from influenza A (H1N1)pdm09 infection during this period.26, 27
Moreover, as recently reported by Gutiérrez-Pizarraya et al.,28 we also documented an unexpected severity of cases of influenza B infection in patients that required hospitalisation during the first post-pandemic influenza season. Interestingly, we did not find comorbidities as independent risk factor for severe disease. However, it is extensively known that influenza A (H1N1)pdm09 affected mainly younger patients without comorbid conditions.5, 29 In addition, we documented that the frequency of comorbid conditions was higher in patients with bacterial CAP.
A previous study reported that there is a clinical profile that increases the likelihood of the diagnosis of influenza A (H1N1)pdm09-related pneumonia rather than interpandemic CAP.14 In the present study, patients with influenza A (H1N1)pdm09-related pneumonia were significantly younger and were less likely to have comorbid conditions. Similarly, patients with influenza A (H1N1)pdm09-related pneumonia presented more frequently with rhinorrhoea, sore throat, arthralgia and headache, but less commonly tachycardia and pleuritic chest pain. Laboratory findings showed that these patients also had high alanine aminotransferase more often, but leucocytosis less frequently. Alveolar unilobar infiltrates in chest X-rays were found more commonly in patients with bacterial pneumonia (data not shown).
The accuracy of the scoring system described by Bewick et al.14 for identifying influenza A (H1N1)pdm09-related pneumonia was moderate in the present external validation, as the statistical analysis demonstrates (AUROC 0.77). However, it should be noted that the model was less useful for identifying patients with influenza A (H1N1)pdm09-related pneumonia and bacterial co-infection (AUROC 0.70). In addition, sensitivity and (to a lesser extent) specificity were low in diagnosis of influenza A (H1N1)pdm09-related pneumonia in this clinical context. Thus, a course of antiviral therapy guided solely by the application of the model would have left 2.5% (including those with bacterial co-infection) of the patients with influenza A (H1N1)pdm09-related pneumonia without specific coverage (score of 0 or 1), and would have led to administration of an unnecessary antiviral regimen to 58.2% of patients classified with scores of 4 or 5. In addition, the model was unable to distinguish between influenza A (H1N1)pdm09-related pneumonia and other aetiologies in most cases, because 41% of influenza A (H1N1)pdm09-related pneumonia cases and 51.6% of cases with other aetiologies fell into the values of 3 and 4. Thus, rather than identifying patients who benefit from treatment, the most useful finding of the scoring system proposed by Bewick et al.14 is that antiviral treatment might be avoided in some patients with a score of 0 or 1.
The strengths of the present study are its prospective and multicentre design, the large number of consecutive hospitalised patients with CAP included across Spain and its comprehensive collection of clinical data. Thus, this cohort is representative of patients hospitalised with a pneumonic illness during the first post-pandemic period. In addition, to the best of our knowledge, no prior study has evaluated microbial patterns and outcomes in CAP during this period. Moreover, nearly 90% of patients underwent viral PCR and 95% underwent one or more bacterial identification tests. However, our study has several limitations that should be acknowledged. Firstly, we did not compare our cohort of patients with those of other epidemiological periods to explore for differences in outcomes. Secondly, PCR testing was mainly performed on samples obtained via a nasopharyngeal swab. Evidence suggests that the yield of positive PCR results from nasopharyngeal swabs in patients with confirmed positive lower respiratory tract samples is nearly 80%. However, sampling was repeated when clinical suspicion was high in some cases in the present study. Thirdly, as serologic methods were not used to determine antibodies against atypical agents, these microorganisms are under-represented. Lastly, hospital and ICU admission criteria were not standardized. However, to detect factors associated with severe disease, we performed a multivariate analysis to control for confounding factors. In addition, a post hoc analysis was performed considering ICU admission and intubation as dependent variable. Notably, the usefulness of biomarkers to predict prognosis was not evaluated in the present study.
In conclusion, the frequency of bacterial pneumonia and viral pneumonia in hospitalised adults during the first post-pandemic influenza season was similar. The main virus isolated was influenza A (H1N1)pdm09, which was independently associated with severe disease. Although certain clinical features on first examination of the patient may allow recognition of influenza A (H1N1)pdm09-related pneumonia, it is difficult to express them in a reliable scoring system.
Role of the funding sources
The funding sources had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Competing interests
All authors declare that they have no conflicting interests that are relevant to this article.
Acknowledgements
This research was supported by the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, Programa de Investigación sobre gripe A/H1N1 (GR09/0014). It was co-financed by Ministerio de Economía y Competitividad, Instituto de Salud Carlos III – co-financed by European Development Regional Fund "A way to achieve Europe" ERDF, Spanish Network for the Research in Infectious Diseases (REIPI RD12/0015). DV is the recipient of a research grant from the REIPI.
Appendix.
Other members of the Novel Influenza A (H1N1) Study Group are: Melchor Riera (Hospital Universitario Son Dureta – Son Espases, Palma de Mallorca); Antoni Payeras and Victoria Férnandez-Baca (Hospital Son Llàtzer, Palma de Mallorca); Antonio Gutiérrez, Teresa Aydillo, Magdalena Sánchez, Giovana Osorio, Caridad Milara, Pilar Perez-Romero and Jerónimo Pachón (Hospital Universitario Virgen del Rocío, Sevilla); Felipe Fernández-Cuenca and Jesús Rodríguez-Baño (Hospital Universitario Virgen Macarena, Sevilla); Carmen Casillas Ruiz, Celia García de la Fuente, María Victoria Sanjuán, and José Antonio Parra (Hospital Universitario Marqués de Valdecilla, Santander); Julián Torre-Cisneros, Manuel Casal, Manuel Causse, Juan Gutiérrez-Aroca, Rosario Lara, Antonio Rivero, Fernando Rodríguez, and Rocio Tejero (Hospital Universitario Reina Sofía – IMIBIC, University of Córdoba, Córdoba); Joaquín Martínez-Montauti (SCIAS – Hospital de Barcelona, Barcelona); Francisca Portero Azorín, Antonio Ramos Martínez and Teresa Álvarez-Espejo Montiel (Hospital Universitario Puerta de Hierro, Madrid); Sarah Caro-Bragado and José Ramón Paño-Pardo (Hospital Universitario La Paz – IDIPAZ, Madrid), Spain.
References
- 1.Ruiz M., Ewig S., Marcos M.A., Martinez J.A., Arancibia F., Mensa J. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160:397–405. doi: 10.1164/ajrccm.160.2.9808045. [DOI] [PubMed] [Google Scholar]
- 2.Rosón B., Carratalà J., Dorca J., Casanova A., Manresa F., Gudiol F. Etiology, reasons for hospitalization, risk classes, and outcomes of community-acquired pneumonia in patients hospitalized on the basis of conventional admission criteria. Clin Infect Dis. 2001;33:158–165. doi: 10.1086/321808. [DOI] [PubMed] [Google Scholar]
- 3.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C. Infectious Disease Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl. 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viasus D., Paño-Pardo J.R., Pachón J., Riera M., López-Medrano F., Payeras A. Pneumonia complicating pandemic (H1N1) 2009: risk factors, clinical features, and outcomes. Medicine (Baltimore) 2011;90:328–336. doi: 10.1097/MD.0b013e31822e67a7. [DOI] [PubMed] [Google Scholar]
- 6.Jain S., Benoit S.R., Skarbinski J., Bramley A.M., Finelli L., 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team Influenza-associated pneumonia among hospitalized patients with 2009 pandemic influenza A (H1N1) virus–United States, 2009. Clin Infect Dis. 2012;54:1221–1229. doi: 10.1093/cid/cis197. [DOI] [PubMed] [Google Scholar]
- 7.Viasus D., Paño-Pardo J.R., Pachón J., Campins A., López-Medrano F., Villoslada A. Factors associated with severe disease in hospitalized adults with pandemic (H1N1) 2009 in Spain. Clin Microbiol Infect. 2011;17:738–746. doi: 10.1111/j.1469-0691.2010.03362.x. [DOI] [PubMed] [Google Scholar]
- 8.Jain S., Kamimoto L., Bramley A.M., Schmitz A.M., Benoit S.R., Louie J. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 9.Louie J.K., Acosta M., Winter K., Jean C., Gavali S., Schechter R. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A., Zarychanski R., Pinto R., Cook D.J., Marshall J., Lacroix J. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 11.Rello J., Rodríguez A., Ibañez P., Socias L., Cebrian J., Marques A. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice T.W., Rubinson L., Uyeki T.M., Vaughn F.L., John B.B., Miller R.R., 3rd Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med. 2012;40:1487–1498. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haessler S., Schimmel J.J. Managing community-acquired pneumonia during flu season. Cleve Clin J Med. 2012;79:67–78. doi: 10.3949/ccjm.79a.11108. [DOI] [PubMed] [Google Scholar]
- 14.Bewick T., Myles P., Greenwood S., Nguyen-Van-Tam J.S., Brett S.J., Semple M.G. Clinical and laboratory features distinguishing pandemic H1N1 influenza-related pneumonia from interpandemic community-acquired pneumonia in adults. Thorax. 2011;66:247–252. doi: 10.1136/thx.2010.151522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine M.J., Auble T.E., Yealy D.M., Hanusa B.H., Weissfeld L.A., Singer D.E. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 16.Lim W.S., van der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2009;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Coiras M.T., Aguilar J.C., García M.L., Casas I., Pérez-Breña P. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested-PCR assays. J Med Virol. 2004;72:484–495. doi: 10.1002/jmv.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schildgen O., Geikowski T., Glatzel T., Simon A., Wilkesmann A., Roggendorf M. New variant of the human metapneumovirus (HMPV) associated with an acute and severe exacerbation of asthma bronchiale. J Clin Virol. 2004;31:283–288. doi: 10.1016/j.jcv.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Johansson N., Kalin M., Tiveljung-Lindell A., Giske C.G., Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50(2):202–209. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Baum H., Welte T., Marre R., Suttorp N., Ewig S. CAPNETZ study group. Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J. 2010;35:598–605. doi: 10.1183/09031936.00091809. [DOI] [PubMed] [Google Scholar]
- 22.Bolotin S., Pebody R., White P.J., McMenamin J., Perera L., Nguyen-Van-Tam J.S., UK Severe Influenza Surveillance System (USISS) Steering Group A new sentinel surveillance system for severe influenza in England shows a shift in age distribution of hospitalised cases in the post-pandemic period. PLoS One. 2012;7:e30279. doi: 10.1371/journal.pone.0030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Athanasiou M., Baka A., Andreopoulou A., Spala G., Karageorgou K., Kostopoulos L. Influenza surveillance during the post-pandemic influenza 2010/11 season in Greece, 04 October 2010 to 22 May 2011. Euro Surveill. 2011;16 pii: 20004. [PubMed] [Google Scholar]
- 24.Vigilancia de la gripe en España. Área de Vigilancia de la Salud Pública. Centro Nacional de Epidemiología. Instituto de Salud Carlos III; Temporada 2010–2011. http://vgripe.isciii.es/gripe/documentos/20102011/InformesAnuales/Informe_GRIPE_Temporada_2010-11_07092011.pdf Available at: [Google Scholar]
- 25.Chuang J.H., Huang A.S., Huang W.T., Liu M.T., Chou J.H., Chang F.Y. Nationwide surveillance of influenza during the pandemic (2009–10) and post-pandemic (2010–11) periods in Taiwan. PLoS One. 2012;7:e36120. doi: 10.1371/journal.pone.0036120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viasus D., Cordero E., Rodríguez-Baño J., Oteo J.A., Fernández-Navarro A., Ortega L. Changes in epidemiology, clinical features and severity of influenza A (H1N1) 2009 pneumonia in the first post-pandemic influenza season. Clin Microbiol Infect. 2012;18:E55–E62. doi: 10.1111/j.1469-0691.2011.03753.x. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Loeches I., Díaz E., Vidaur L., Torres A., Laborda C., Granada R. Pandemic and post-pandemic influenza A (H1N1) infection in critically ill patients. Crit Care. 2011;15:R286. doi: 10.1186/cc10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutiérrez-Pizarraya A., Pérez-Romero P., Alvarez R., Aydillo T.A., Osorio-Gómez G., Milara-Ibáñez C. Unexpected severity of cases of influenza B infection in patients that required hospitalization during the first postpandemic wave. J Infect. 2012;65:423–430. doi: 10.1016/j.jinf.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Lee N., Chan P.K., Lui G.C., Wong B.C., Sin W.W., Choi K.W. Complications and outcomes of pandemic 2009 Influenza A (H1N1) virus infection in hospitalized adults: how do they differ from those in seasonal influenza? J Infect Dis. 2011;203(12):1739–1747. doi: 10.1093/infdis/jir187. [DOI] [PubMed] [Google Scholar]