Abstract
Bactericidal action of Al2O3, Ag/Al2O3 and AgCl/Al2O3 on pure culture of Escherichia coli K 12 was studied. Ag/Al2O3 and AgCl/Al2O3 demonstrated a stronger bactericidal activity than Al2O3. The colony-forming ability of E. coli was completely lost in 0.5 min on both of Ag/Al2O3 and AgCl/Al2O3 at room temperature in air. The configuration of the bacteria on the catalyst surface was observed using scanning electron microscopy (SEM). Reactive oxygen species (ROS) play an important role in the expression of the bactericidal activity on the surface of catalysts by assay with O2/N2 bubbling and scavenger for ROS. Furthermore, the formation of CO2 as an oxidation product could be detected by diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and be deduced by total carbon analysis. These results strongly support that the bactericidal process on the surface of Ag/Al2O3 and AgCl/Al2O3 was caused by the catalytic oxidation.
Keywords: Silver, Alumina, Bactericidal activity, Catalytic oxidation, Reactive oxygen species, E. coli
1. Introduction
Sterilization is an important procedure to maintain a sanitary environment. For this purpose, many chemical germicides have been used. With these applications, the potential dangers of the compounds to human health and environment are concerned. TiO2 photocatalysts applied in water disinfection process were noticeable, which provided an alternative to conventional chemical germicides [1], [2], [3], [4], [5]. However, this technology requires a relatively complex device and photo energy. Recently, we have reported the inactivation efficiency of Ag/Al2O3 and Cu/Al2O3 to SARS coronavirus, bacteria and yeast [6]. The microbes are completely inactivated in 5 min on Ag/Al2O3 surface at room temperature in air, and we suggest a catalytic oxidation mechanism in this disinfection process.
Even though the bactericidal effect of compounds containing silver has been investigated for a long time, its mechanism is still controversial. Some reports insisted that the antibacterial activity of silver-loaded compounds is depended on the cytotoxicity of Ag+ eluted from the compounds into system containing the microorganisms [7], [8]. At the same time, it has been suggested that the reactive oxygen species play an important role in the expression of the bactericidal activity in water [9], [10].
To identify the inactivation mechanism of bactericidal process on the surface of Ag/Al2O3 in air, e.g. by catalytic oxidation or by toxicity of heavy metals, Ag/Al2O3 and AgCl/Al2O3 were used in this study since they have different amount of Ag+ which may be eluted from the catalysts. In this study, we also report that reactive oxygen species (ROS) play an important role in the expression of the bactericidal activity on the surface of catalysts.
2. Experimental
2.1. Catalysts
Ag/Al2O3 and AgCl/Al2O3 (Ag 5 wt%) were prepared by impregnation and precipitation methods respectively. After Al2O3 powder was introduced into an appropriate amount of silver nitrate aqueous solution, the mixture was stirred at room temperature. As for AgCl/Al2O3, an appropriate amount of ammonium chloride aqueous solution was added into the mixture at a rate of 0.5 ml/min, while the Ag/Al2O3 need not this step. Both of the wet samples were dried at 120 °C for 12 h, and then calcined in air at 600 °C for 3 h [11]. Before using, Al2O3, Ag/Al2O3 and AgCl/Al2O3 powders were pressed into wafers of ca. 20 mg/cm2. XRD is used to investigate the silver phase on γ-Al2O3. In the case of Ag/Al2O3, only the γ-Al2O3 phase was detected when the silver loading was 5 wt%, and the absence of diffraction lines of silver phase on 5 wt% Ag/Al2O3 catalyst indicates that silver is at a very high dispersion degree. To 5 wt% AgCl/Al2O3, however, the peaks attributable to AgCl phase was observed at 2θ of 27.76°, 32.24°, 55.86°, 57.34° and 76.54° (PDF-ICDD 85-1355, PDF-ICDD 31-1238). The absence of diffraction lines of silver phase on 5 wt% Ag/Al2O3 catalyst indicates that silver is at a very high dispersion degree. TEM was also used to investigate the silver phase on γ-Al2O3. As for 5 wt% Ag/Al2O3, the silver particle (ranging from 15 to 20 nm) at a very high dispersion degree was observed. In the case of 5 wt% AgCl/Al2O3, the silver particle was greater than that of 5 wt% Ag/Al2O3 (ranging from 20 to 50 nm).
2.2. Culture of micro-organisms
E. coli K 12 strain ATCC23716 was inoculated into LB broth (Fluka Co. 61748) and grew aerobically for 24 h at 37 °C with constant agitation. Aliquots of the culture were inoculated into fresh medium and incubated at 37 °C for 4–5 h until reaching an exponential growth phase. Bacterial cells were collected using centrifugation at 10,000 rpm for 10 min, and the bacterial pellet was washed with sterile water. Finally, bacterial cells were suspended in the water then diluted to the required cell density corresponding to 108 colony forming units per milliliter (CFU/ml).
2.3. Bactericidal experiment and analysis method
A 20 μl aliquot of the E. coli suspension was applied onto the wafer surface of catalyst, and the contacting times (refers to the bacteria contacting time with the wafers of catalyst before sampling) were for 0.5, 2, 5, 10 and 20 min at room temperature. The survival cells were washed off from the catalyst wafers with sterile water. 0.5 ml eluate was immediately injected into 4.5 ml 0.9% NaCl aqueous solution to eliminate the effect of Ag+ [12], and then plated on LB agar (Fluka Co.61746) plates. The plates were incubated at 37 °C for 24 h before counting. All experiments were repeated three times. The wafers of catalysts were investigated using SEM during the bactericidal process. Before SEM measurement, the E. coli on the wafers were fixed with glutaraldehyde and osmium tetroxide, drained with ethanol/water with the concentrations of ethanol increasing. The absolute ethanol was replaced by dimethoxymethane, and the samples underwent critical point drying with CO2. The wafers were glued onto stages with conductive silver and metallized with gold. The samples were microscoped and photographed with a scanning electron microscope (Fei QUANTA 200).
The bactericidal activity was measured under either aerobic or anaerobic conditions, which was achieved by oxygen or nitrogen bubbling to investigate the effect of dissolved oxygen. Superoxide dismutase (SOD, Sigma) was used to investigate the effect of ROS on bactericidal activity. Fifty unit per ml and 100 unit/ml of SOD solution was made, respectively. The solution was added to the E. coli K12 suspension and mixed sufficiently. A 20 μl of the mixture of E. coli suspension and SOD solution was applied onto the wafer surface of catalyst, and the sampling times were for 0.5, 2, 5, 10 and 20 min at room temperature.
In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) method was used to investigate the dynamic changes of IR spectra on Ag/Al2O3 surface during the bactericidal process, and DRIFTS spectra were recorded on a Nexus 670 (Thermo Nicolet) FT-IR, equipped with an in situ diffuse reflection chamber and a high sensitivity MCT detector. After E. coli suspension and catalyst powder were mixed completely, the mixed sample was placed in a ceramic crucible in the in situ chamber. Total carbon (TC) content of mixture of E. coli and catalysts is determined using Apollo 9000 Analyzer (Terkmar Dohmann) during the bactericidal process. It was observed that TC decreased during the catalytic oxidation process, which implied that TC of E. coli had converted to CO2 and released from the catalysts. The formation of CO2 during this process can be calculated using the following equation:
where TC0 signifies initial TC and TCi is TC at different times during this process.
3. Results and discussion
3.1. Bactericidal effects of the catalysts
The bactericidal activity of Al2O3, Ag/Al2O3 and AgCl/Al2O3 in air was investigated. Fig. 1 shows the time course changes with the viable cell count of E. coli. The process where Al2O3 used as host compounds (Fig. 1a) shows that the cell concentration decreased from 2.8 × 108 CFU/ml to 1 × 106 CFU/ml within 20 min after addition. Obviously, the processes with Ag/Al2O3 (Fig. 1b) and AgCl/Al2O3 (Fig. 1c) show a higher bactericidal effect on E. coli than Al2O3. The bacteria lost their colony forming ability in 0.5 min after applied onto the wafer of Ag/Al2O3 and AgCl/Al2O3. The spoiling of bacteria was also observed by SEM photographs as shown in Fig. 2 .
Fig. 1.
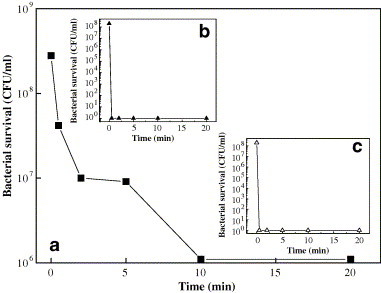
E. coli K 12 inactivation on (a) Al2O3, (b) AgCl/Al2O3 and (c) Ag/Al2O3 surface. Initial concentration of bacteria: 2.8 × 108 CFU/ml.
Fig. 2.
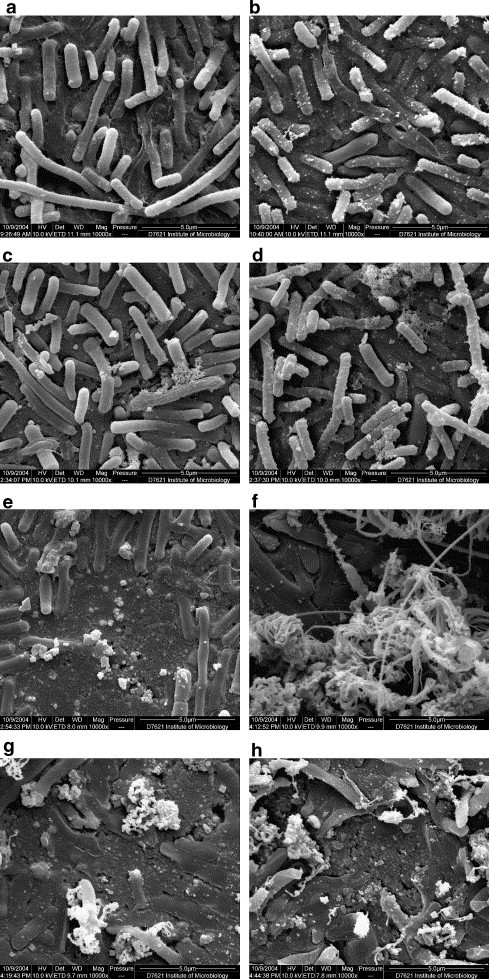
SEM images of E. coli adsorbed on (a) Al2O3 at room temperature for 0.5 min and (b) 24 h, on (c) AgCl/Al2O3 for 0.5 min, (d) for 0.5 h, (e) for 1 h and on (f) Ag/Al2O3 for 0.5 min, (g) for 0.5 h and (h) for 1 h.
Fig. 2a and b show the SEM images of E. coli adhered on Al2O3 at room temperature for 0.5 min and 24 h, respectively. Although some cells were damaged after E. coli exposured to the wafer of Al2O3 for 0.5 min, the viable cells were still detectable using plate counting method. Some cells collapsed and led to release of intracellular constituents, however, their membrane and intracellular constituents covered the surface even after 24 h. Fig. 2c–e and f–h show the SEM images of E. coli adhered on AgCl/Al2O3 and Ag/Al2O3 at room temperature for 0.5 min, 0.5 and 1 h, respectively. As shown in Fig. 1, it is obvious that Ag/Al2O3 and AgCl/Al2O3 gave a higher bactericidal effect on E. coli than Al2O3. Although AgCl/Al2O3 exhibited a similar bactericidal ability comparing with Ag/Al2O3, the SEM images indicate that different mechanisms are involved in the bactericidal action of AgCl/Al2O3 and Ag/Al2O3. The cell membrane damage mainly started at one end of E. coli on the surface of AgCl/Al2O3 (Fig. 2c), and intracellular constituents were released from the damaged site. After exposure time of 0.5 h, the cell was further spoiled (Fig. 2d). After 1 h, the clean catalyst surface reappeared (Fig. 2e). The damage of bacteria on the surface of Ag/Al2O3 was more serious than that of AgCl/Al2O3 (Fig. 2f–h). Even though after 30 s on AgCl/Al2O3, a great deal of intracellular constituents spouted from damaged sites on the cell membrane (Fig. 2f). After exposure time of 0.5 h, E. coli cells shrank and the catalyst surface covered by the cells reappeared (Fig. 2g, h).
As mentioned above, the bactericidal mechanism of Ag containing compounds is still controversial [8], [9], [10], [11]. During the process of the E. coli suspension contacted with Ag/Al2O3 and AgCl/Al2O3, Ag+ released unavoidablely. In order to confirm the bactericidal effect of Ag+, the catalyst wafers were dip in sterile water for 10 min and the quantitative analysis of Ag+ in the eluate was carried out with an atomic adsorption spectrophotometer. The bactericidal effect of a series of AgNO3 (10−9, 10−8, 10−5, 10−4, 10−3 M) contacting with E. coli suspension for 10 min were determined. The correlation between the concentration of Ag+ and the quantity of survival cells was reflected in a curve, so the effect of Ag+ released from the catalyst can be confirmed. As shown in Fig. 3 , the colony-forming ability of E. coli lost in Ag+ containing solution when the concentration of Ag+ is more than 10−4 M. The concentrations of Ag+ eluted from Ag/Al2O3 and AgCl/Al2O3 were less than 2.9 × 10−4 M and 4.7 × 10−7 M under such experimental conditions, respectively. According to this result, Ag+ was released from the catalysts, and the bactericidal ability of the Ag/Al2O3 would be related to the amount of Ag+ dissolved in a certain extent. However the amount of Ag+ eluted from AgCl/Al2O3 is too little to cause any bactericidal effect against E. coli.
Fig. 3.
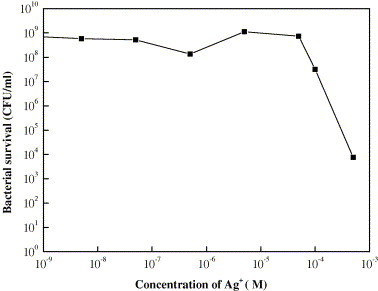
Effect of the concentration of Ag+ on the colony-forming ability of E. coli.
3.2. Effect of oxygen and nitrogen gas bubbling
The effect of oxygen and nitrogen gas bubbling on the bactericidal activity was also investigated. This investigation was performed to obtain information about the role of oxygen in the bactericidal activity of Ag/Al2O3 and AgCl/Al2O3. 0.5 g Al2O3, Ag/Al2O3 and AgCl/Al2O3 powder was added into 40 ml sterilized water respectively, and then the aerobic and anaerobic condition was achieved by oxygen and nitrogen gas bubbling for 40 min before piping the bacteria into the system. The bubbling was continued throughout the treatment period. The bactericidal activity of Al2O3 under the same condition was studied as the control experiment. The results are shown in Fig. 4 . Under the anaerobic condition, the bactericidal activity of Ag/Al2O3 sharply decreased compared to that in the aerobic condition. Though the bactericidal activity of AgCl/Al2O3 was lower than Ag/Al2O3, it can also be obviously observed that the bactericidal activity in aerobic condition is better than that in anaerobic condition. These results suggest that nitrogen gas bubbling drastically suppresses the bactericidal activity of Ag/Al2O3, and indicate that the presence of oxygen is necessary for the bactericidal activity by Ag/Al2O3 and AgCl/Al2O3.
Fig. 4.
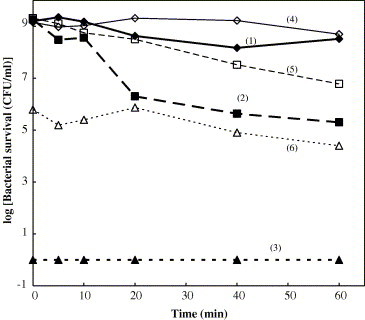
Effect of O2 and N2 bubbling on the bactericidal activity of Ag/Al2O3 and AgCl/Al2O3: (1) Al2O3, (2) AgCl/Al2O3, (3) Ag/Al2O3 in aerated condition; (4) Al2O3, (5) AgCl/Al2O3, (6) Ag/Al2O3 in nonaerated condition.
3.3. Effect of ROS on the surface of catalysts in the bactericidal process
From above experimental results, we consider that oxygen can be activated to ROS by the catalyst, which play an important role in the bactericidal activity. The ROS might contain superoxide anions, hydrogen peroxide and hydroxyl radicals. SOD is a scavenger for superoxide anions and hydroxyl radicals. The effect of addition of SOD on the bactericidal activity was investigated (Fig. 5 ). Compared with the situation without SOD, in the presence of SOD, the bacterial survival apparently increased on the surface of Ag/Al2O3 and AgCl/Al2O3, respectively. The addition of 100 unit/ml SOD drastically suppressed the bactericidal activity. The survival bacterial was kept more than 1 × 106 CFU/ml when 100 unit/ml of SOD added (The survival bacteria were less than 5 × 105 CFU/ml when 50 unit/ml of SOD added). This result indicates that the formation of superoxide anions and hydroxyl radicals contributes to the bactericidal activity.
Fig. 5.
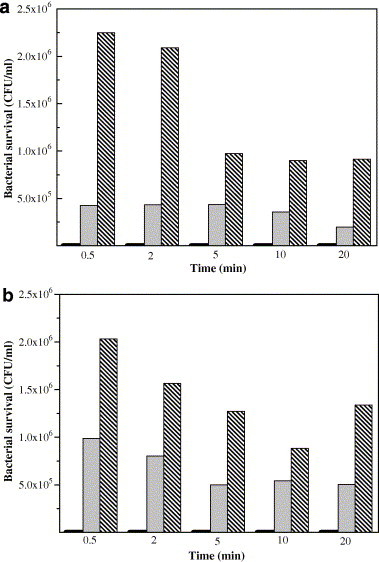
Effect of SOD against ROS on the bactericidal activity of (a) Ag/Al2O3 and (b) AgCl/Al2O3, respectively.  0 unit/ml of SOD (The bactericidal survival in the case of 0 unit/ml of SOD reached 0.0E + 00 CFU/ml);
0 unit/ml of SOD (The bactericidal survival in the case of 0 unit/ml of SOD reached 0.0E + 00 CFU/ml);  50 unit/ml of SOD;
50 unit/ml of SOD;  100 unit/ml of SOD.
100 unit/ml of SOD.
3.4. Formation of CO2 during bactericidal process
As shown in Fig. 6 , Ag/Al2O3 added with E. coli was investigated using DRIFTS during exposure of the sample to O2 for 42 h at 44 °C. After exposure of the sample to pure O2 for 18 h, peaks at 2339 and 2362 cm−1 assigned to gas phase CO2 were observed, and the intensity of CO2 peaks increased with time increasing. The appearance of gas phase CO2 strongly supports that the bactericidal process on Ag/Al2O3 not only leads to the cell death, but also causes the completely catalytic oxidation of bacteria and other intermediates.
Fig. 6.
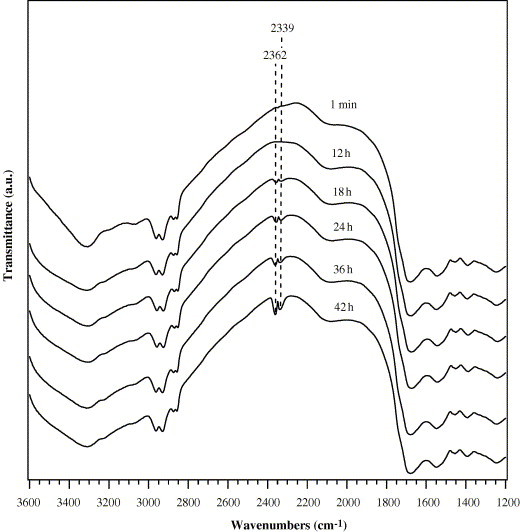
Dynamic changes of in situ DRIFTS spectra of Ag/Al2O3 as a function of time during exposure of the mixed sample to pure O2 at 44 °C. Before measurement, the catalyst powder was mixed with E. coli suspension.
Furthermore, the formation of CO2 was investigated by TC analysis as shown in Fig. 7 . About 49% and 28% of TC of E. coli were converted to CO2 in air at 44 °C after the E. coli cells were added to the surfaces of Ag/Al2O3 and AgCl/Al2O3 for 125 h, respectively.
Fig. 7.
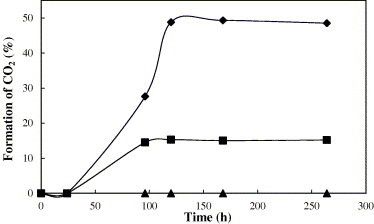
Detection of CO2 produced during the bactericidal process at 44 °C by TC analysis: (♦) Ag/Al2O3; (■) AgCl/Al2O3; (▴) Al2O3.
It should be noticed that the time of surface reappearance is different from the time producing CO2. In the SEM images, we can only see the surface uncovered with bacteria reappeared, but the surface might not as clean as the fresh surface, where small molecules might adsorb. The small molecules might have not been oxygenated completely into CO2, but they already could not be seen using SEM. In addition, the catalyst could adsorb a mass of CO2, because of its large specific surface area. Therefore the detection of CO2 desorbed from the catalyst will be delayed.
4. Conclusion
Ag/Al2O3 and AgCl/Al2O3 demonstrated a stronger bactericidal activity than Al2O3. The colony-forming ability of E. coli was completely lost in 0.5 min on both of Ag/Al2O3 and AgCl/Al2O3 at room temperature in air. Relying on SEM investigation, the different bactericidal mechanisms were identified on the surfaces of Ag/Al2O3 and AgCl/Al2O3. The role of Ag+ eluted from AgCl/Al2O3 can be ignored in our experiments. Oxygen, which can be activated to ROS by catalyst, is essential for the expression of bactericidal activity. The strong catalytic oxidation ability was expressed on the Ag/Al2O3 surface, and CO2 as the ultimate product was observed during the bactericidal process. All of these results indicate that the catalytic oxidation is the essential mechanism in bactericidal process. Further work is needed to clarify the details of these actions.
5. Abbreviations
- ROS
reactive oxygen species
- SEM
scanning electron microscopy
- SARS
severe acute respiratory syndrome
- XRD
X-ray polycrystalline diffraction
- TEM
transmission electron microscopy
- CFU/ml
colony forming units per milliliter
- SOD
superoxide dismutase
- DRIFTS
diffuse reflectance infrared Fourier transform spectroscopy
- TC
total carbon
Acknowledgement
This work was financially supported by the National Natural Science Foundation of China (50621804, 50538090).
References
- 1.Yu J.C., Ho W., Lin J., Yip H., Wong P. Environ. Sci. Technol. 2003;37:2296–2301. doi: 10.1021/es0259483. [DOI] [PubMed] [Google Scholar]
- 2.Rincón A., Pulgarin C. Appl. Catal. B. 2004;51:283–302. [Google Scholar]
- 3.Rincón A., Pulgarin C. Appl. Catal. B. 2004;49:99–112. [Google Scholar]
- 4.Huang Z., Maness P., Blake D.M., Wolfrum E.J., Smolinski S.L., Jacoby W.A. J. Photochem. Photobio. A. 2000;130:163–170. [Google Scholar]
- 5.Tatsuma T., Takeda S., Saitoh S., Ohko Y., Fujishima A. Electrochem. Commun. 2003;5:793–796. [Google Scholar]
- 6.He H., Dong X., Yang M., Yang Q., Duan S., Yu Y., Han J., Zhang C., Chen L., Yang X. Catal. Commun. 2004;5:170–172. doi: 10.1016/j.catcom.2003.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowling P., Betts A.J., Pope C., McConnell M.L., Eloy R., Arnaud M.N. Surf. Coat. Tech. 2003;163–164:637–640. [Google Scholar]
- 8.Sökmen M., Candan F., Sümer Z. J. Photoch. Photobio A: Chemistry. 2001;143:241–244. [Google Scholar]
- 9.Inoue Y., Hoshino M., Takahashi H., Noguchi T., Murata T., Kanzaki Y., Hamashima H. M. Sasatsu J. Inorg. Biochem. 2002;92:37–42. doi: 10.1016/s0162-0134(02)00489-0. [DOI] [PubMed] [Google Scholar]
- 10.Le Pape H., Solano-Serena F., Contini P., Devillers C., Maftah A., Leprat P. J. Inorg. Biochem. 2004;98:1054–1060. doi: 10.1016/j.jinorgbio.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 11.He H., Zhang C.B., Yu Y.B. Catal. Today. 2004;90:191–197. [Google Scholar]
- 12.Matsumura Yoshinobu. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 2003;69:4278–4281. doi: 10.1128/AEM.69.7.4278-4281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


