Highlights
-
•
Multiple intestinal pathogen species were present in both healthy and diarrheic calves.
-
•
Decreased haptoglobin concentrations were associated with weak signs of eimeriosis.
-
•
Increased haptoglobin and fibrinogen concentrations were associated with respiratory tract infection and umbilical infection.
Keywords: Eimeriosis, Eimeria spp., Acute phase response, Haptoglobin, Calf, Diarrhoea, Intestinal pathogens
Abstract
In this study, the association between Eimeria spp. related signs and innate immune response in dairy calves was examined. Calves (n = 100) aged 15–60 days were clinically examined and faecal samples, blood samples and deep nasopharyngeal swabs obtained. The samples were analysed for intestinal pathogens, acute phase proteins and WBC count, and respiratory tract pathogens, respectively.
Diarrhoea was diagnosed in 32.6% (23.3–43.0%, 95% CI) of calves. An association between the pathogenic Eimeria spp. and diarrhoea was detected by multiple correspondence analysis. Eimeria related signs (diarrhoea, presence of pathogenic species and total oocyst count) were combined resulting a four level variable. Calves with weak signs of eimeriosis had decreased haptoglobin concentrations (p = 0.02) and increased fibrinogen concentrations (p = 0.048) compared to no signs. Increased haptoglobin and fibrinogen concentrations were associated with respiratory tract infection and umbilical infection. Serum amyloid A and WBC counts showed no association with signs of eimeriosis or clinical diagnoses.
1. Introduction
Eimeriosis is caused by pathogenic Eimeria spp. and is considered a universal problem of dairy and beef calves. Clinical eimeriosis (coccidiosis) manifests itself as moderate to severe, and sometimes bloody, diarrhoea, inappetence and lethargy [1]. Chronic or subclinical eimeriosis reduces growth rate and can result in considerable economic losses [2], [3]. As the mere presence of Eimeria spp. is not conclusive for them being the etiologic agents of diarrhoea, an oocyst count of pathogenic species of 500 oocyst per gram of faeces (opg) in calves with simultaneous signs of diarrhoea has been used to indicate eimeriosis for research purposes [4], [5]. The oocysts are formed during gamogony, which with second merogony is the most disruptive stage for the intestine in the Eimeria life cycle [1]. The histopathological lesions during gamogony in the ileum include oedema, congestion, haemorrhage, mucosal ulceration, blunting of mucosal ridges and ulceration of the lamina propria [6].
An effective response of the local immune system against the Eimeria sporozoites has been recorded for in vitro models, where macrophages reduce the numbers of sporozoites [7]. One indicator of the innate immune response is the synthesis of the acute phase proteins (APPs) that are synthesised in the liver at the beginning of the acute phase of the inflammatory response initiated by infection, neoplasia or tissue destruction [8]. The initial location or cause of the inflammation cannot be determined with APP measurements, but the magnitude of the increase correlates with the extent and acuteness of the inflammation [9]. Most studies concentrate on the changes in haptoglobin (Hp), serum amyloid A (SAA) and fibrinogen (Fb), the major acute phase proteins in cattle [10]. In calves, research has concentrated on experimental and natural respiratory infections, where the association with the disease and the APPs has been confirmed [11], [12]. Association between diarrhoea and increased APP concentrations or intestinal pathogens and increased APP concentrations has also been studied, but the results are inconclusive [13], [14]. As severe tissue destruction occurs during eimeriosis, the acute phase response would be expected to follow [7].
The aim of the study was to explore possible associations between the various severities of eimeriosis and APPs under natural conditions while controlling the effects of inflammatory diseases and other intestinal pathogens on the acute phase response.
2. Material and methods
2.1. Animals and clinical examination
The study included 100 calves of 15–60 days of age from 20 free or tie-stall farms visited between April and June 2010. Inclusion criteria were: farms with more than 40 milking cows located near the Production Animal Hospital University of the Helsinki in southern Finland volunteering to participate in the study. Exclusion criteria were: farms that used prophylactic rotavirus vaccination or metaphylactic coccidiosis treatment (toltrazuril). Farmers reported no use of antimicrobials prior to the visit.
Two veterinarians with similar instructions performed the sample collections and clinical examinations. All the calves aged 15–60 days of age during the sampling visit were included in the study, and examined once. The calves were clinically examined for respiratory inflammation, assessed for diarrhoea, palpated for umbilical defects and examined for joint lesions in carpal, hock and fetlock joints. Two faecal samples were collected from the rectum of each calf into plastic bags, blood samples were collected from the jugular vein by vacuum venipuncture into EDTA and plain serum tubes, and a deep nasopharyngeal swab was also obtained.
All examinations and sample collections were performed on commercial family-owned farms with minimal stress to the calves, according to guidelines of the Council for International Organizations of Medical Sciences (International Guiding Principles for Biomedical Research involving Animals).
2.2. Faecal samples: handling and examination
After the collection the faecal samples were sent with cooling element packs to either the Finnish Food Safety Authority Evira on the day of the farm visit, or stored refrigerated and sent in weekly batches to the Estonian University of Life Sciences.
Eimeria oocysts were concentrated, floated, and counted in a reading chamber constructed from glass slides as previously described [15], [16], [17] and the species were identified according to Levine [18]. The presence of Cryptosporidium spp. and Giardia spp. was examined using fluorescein staining (fluorescein isothiocyanate antibody, Crypto/Giardia Cel, Cellabs, Pty Ltd, Australia) at the Estonian University of Life Sciences. Cryptosporidium species were identified at the Finnish Food Safety Authority Evira using a restriction fragment length polymorphism PCR [19] after a positive finding by modified Ziehl Neelsen staining. All samples underwent bacteriological culture; salmonella was detected with ISO 6579:2002 [20]. Rotavirus and bovine coronavirus (BCV) were detected from faecal samples using ELISA (Duo Digestive Kit, Bio-X, Jemelle, Belgium).
For nine calves not all the pathogens were analysed; from five calves no faecal sample was obtained, for three calves only presence of rotavirus, coronavirus and salmonella was analysed and for one calf only presence of Eimeria spp., Cryptosporidium spp. and Giardia spp. was analysed. The observations of these calves were excluded in the analysis concerning all the detected intestinal pathogens.
2.3. Acute phase proteins and haematology
Blood samples were analysed for white blood cell (WBC) count, fibrinogen (Fb), Hp and SAA. EDTA samples were stored in refrigeration until the WBC count was performed using an automatic counter (scil Vet ABC, scil animal care company GmbH, Viernheim, Germany) and Fb was determined using the heat precipitation method [21] within 28 h after the sampling. Serum was separated by centrifugation and stored in a freezer at −20 °C until Hp and SAA analyses were performed. Hp analysis was performed with the haemoglobin-binding assay [22], modified by Alsemgeest et al. [23] by using tetramethylbenzidine (TMB) (0.06 mg/ml) as a chromogen instead of o-dianisidine. The analysis for SAA was performed using a commercial sandwich ELISA kit (Phase SAA assay, Tridelta Development Ltd., Maynooth, Co. Kildare, Ireland) according to the manufacturer's instructions.
2.4. Deep nasopharyngeal swabs: handling and examination
The deep nasopharyngeal swabs were collected using a sterile 27 cm cotton swab, guarded with a silicon tube (Medical Wire Equipment Ltd., Corsham, England; [24]). The swabs were rinsed in NaCl and in mycoplasma F-medium [25] and the media were cold transported to the laboratory within 24 h. Samples were examined for aerobic and anaerobic respiratory tract bacteria, bovine respiratory syncytial virus, and bovine corona virus as described in Autio [26].
2.5. Statistical methods
To account for multicollinearity associated with the correlation of diarrhoea caused by different pathogens, a multiple correspondence analysis (MCA) was performed to obtain an overall view of the associations between the findings for pathogens in faecal samples and the presence of diarrhoea. The idea of the multiple correspondence analysis is to carry out cross-tabulations of multiple categorical variables simultaneously and explore relationships within a set of categorical variables, resulting in two-dimensional scatterplot summary. In the summary, clusters of points are associated with each other, and clusters furthest from the inter-section of the axes have the strongest internal association [27]. Test values were computed for evaluating variables’ correlation with model dimensions (axes). The test values were considered to be the standardised coordinates and were interpreted as the number of standard deviations from the centre of gravity of the analysis. Absolute test values higher than the threshold value of 1.96 were considered to be statistically significant. For the MCA, the Cryptosporidium findings from immunofluorescence staining were used. The MCA was conducted with XLSTAT (Version 2010.4.01, Addinsoft) software.
The associations between the eimeriosis related signs, the acute phase protein concentrations and clinical signs were examined using a linear mixed model. Based on clinical examination, calves were categorised having the signs of: diarrhoea (pasty-like or watery faeces), respiratory infection (temperature >39.5 °C, respiratory rate >40/min, and increased respiratory sounds), umbilical infection (enlarged, warm or tender umbilicus), joint lesion (swelling, abrasions or heat in the joint or on the skin close to the joint) or healthy (none of the previous conditions applicable). Calves having simultaneously signs of diarrhoea and any other signs were placed into the category of the other sign, considered the primary sign, so each calf had only one condition or was categorised as healthy. The final variable “Signs of diseases” was generated by combining all the individual signs. For forming eimeriosis severity categories three criteria related with Eimeria infection were assessed: presence of diarrhoea, total oocyst count >500 opg in faecal sample and presence of pathogenic Eimeria species (E. bovis or E. zuernii). If no criteria was met no evidence of eimeriosis was recorded (category 1), if one of the criteria was met the evidence of eimeriosis was considered weak (category 2), if two criteria were met the evidence was considered moderate (category 3), and if all three criteria were met the evidence were considered strong (category 4).
Associations between severity of eimeriosis and APP concentrations were examined by linear mixed model; separate models were built Hp, SAA, Fb, or WBC as an outcome. Age, gender and “Signs of disease” were added into the models to control their possible effect on acute phase proteins or WBC. “Gender” was removed, if no effect was detected. Each intestinal pathogen (rotavirus, Giardia spp., or Cryptosporidium spp.) was also inserted to the model, but none showed significant results. Herd was included as a random effect in all models. For the model using SAA as an outcome, the variable “positive finding from respiratory tract samples” (yes/no) was added, as the examination of the data showed a possible connection of positive respiratory tract sample and SAA concentration. Inverse transformation for Hp, Fb and WBC and logarithmic transformation for SAA were used in the linear mixed model to normalise the data. The difference of intestinal pathogen frequency over age groups (age in weeks) was tested by Fisher's exact test. The Fisher's exact test, linear modelling and the diagnostics were done using Stata/IC 11.2 for Windows (StataCorp LP, College Station, TX, USA). p values <0.05 were considered indicating statistically significant difference.
3. Results
The final sampling consisted of 79 heifer calves and 21 bull calves, mainly of Ayrshire breed (79%; Holstein Friesian 19%, mixed breeds 2%). The calves were equally sampled according to their age (age in weeks shown in Fig. 1 ). Diarrhoea was most frequently diagnosed in calves, but umbilical infections (9.6%; 95% CI 5.5–16.0%) and respiratory tract infections (6.3%, 2.9–11.7%) were also common. Joint lesions were observed in 2.8% (0.8–7.1%) of the calves. No signs of disease were observed in 63.3% (54.9–71.3%) of the calves. No difference in observed pathogens with respect to age of calves was observed (shown in Fig. 1).
Fig. 1.
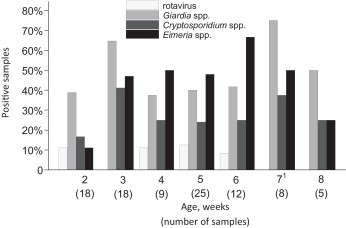
The proportion of positive samples for the examined pathogens in calves aged 2–8 weeks. No statistically significant differences between pathogen occurrence at different ages was observed. 1The only calf positive with bovine coronavirus was seven weeks old, the bar is left out for clarity.
3.1. Intestinal pathogens and diarrhoea
Faecal samples were obtained from 95 calves, of which almost a third (32.6%; 23.3–43.0%, 95% CI) suffered from diarrhoea. Giardia spp., Eimeria spp., Cryptosporidium spp., rotavirus and bovine coronavirus were present in 47.8% (37.3–58.5%), 42.4% (32.1–53.1%), 27.2% (18.4–37.4%), 7.4% (3.0–14.7%) and 1.1% (0.002–5.8%) of samples, respectively. The frequency of intestinal pathogens showed no difference between the age groups (Fig. 1). No salmonella was detected from the samples. The samples positive for Cryptosporidium spp. in Ziehl Neelsen staining were found to be either C. ryanae or C. bovis; no C. parvum or C. andersoni were detected. More than one pathogen was detected in 36.2% (26.5–46.7%) of samples, while 44.7% (34.4–55.3%) of the samples were positive for only one pathogen species. Both diarrheic and non-diarrheic calves provided samples with one or several intestinal pathogens (Fig. 2 ), showing that intestinal pathogens were common also in the non-diarrheic samples.
Fig. 2.
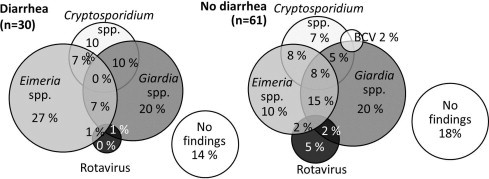
Euler diagrams for intestinal pathogens in diarrheic and non-diarrheic Finnish dairy calves less than 2 months of age. BCV = bovine coronavirus.
The multiple correspondence analysis (MCA) (Fig. 3 ) showed that the presence of the different Eimeria species correlated with each other, and the presence of two pathogenic Eimeria species, E. bovis and E. zuernii, were correlated with each other and with the presence of diarrhoea. Of the samples positive for Eimeria spp., 92.3% (79.1–98.4%) were positive for either E. bovis or E. zuernii and more than one Eimeria species was present in 74.4% (57.9–87.0%) of the samples. The third pathogenic species, E. alabamensis was detected in 6.5% (2.4–13.7%) of samples.
Fig. 3.
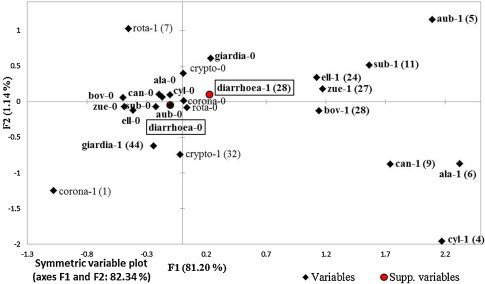
A graphical display of the multiple correspondence analysis, with respect to axes 1 (F1) and 2 (F2) for presence of diarrhoea and pathogens in faecal samples from 91 calves. Bolded pathogens are significantly related with F1. Number 1 after pathogen means presence of particular pathogen in faecal samples or diarrhoea and 0 no presence or no diarrhoea. Number of positive samples is in the parenthesis. Abbreviations: ala = E. alabamensis, aub = E. auburnensis, bov = E. bovis, can = E. canadensis, cyl = E. cylindrica, ell = E. ellipsoidalis, sub = E. subspherica, zue = E. zuernii, corona = bovine coronavirus, crypto = Cryptosporidium spp., giardia = Giardia spp., rota = rotavirus.
The MCA showed that presence of Giardia spp., Cryptosporidium spp. or rotavirus was negatively correlated with the presence of diarrhoea. The samples negative for Eimeria spp. were also more related with non-diarrheic samples.
3.2. Eimeriosis and acute phase response
Median and standard deviation of Eimeria oocyst count in positive samples was 2180 opg ± 17,290. Calves were quite evenly distributed to the two lowest categories of eimeriosis severity (the number of calves in each category can be found in Table 1 ), but fewer calves suffered from strong signs of eimeriosis (Table 1).
Table 1.
Associations of 1/haptoglobina concentration with eimeriosis related signs, diagnosis, gender and age in Finnish dairy calves.
| Variable | n | Estimate | 95% CI | p value | Wald test p value |
|---|---|---|---|---|---|
| Eimeriosis signs | 0.15 | ||||
| No signs | 36 | 0 | |||
| Weak signs | 22 | 2.21 | 0.055; 4.36 | 0.04 | |
| Moderate signs | 25 | 1.64 | −0.32; 3.54 | 0.10 | |
| Strong signs | 9 | 0.54 | −2.65; 3.74 | 0.74 | |
| Signs of disease | 0.01 | ||||
| Healthy | 52 | 0 | |||
| Diarrhoea | 20 | −1.19 | −3.44; 1.06 | 0.30 | |
| Respiratory infection | 9 | −3.83 | −6.76; −0.91 | 0.01 | |
| Umbilical inflammation | 9 | −3.33 | −5.99; −0.67 | 0.01 | |
| Joint problem | 2 | −4.26 | −9.68; 1.17 | 0.12 | |
| Gender | 0.005 | ||||
| Female | 74 | 0 | |||
| Male | 18 | 2.75 | 0.79; 4.72 | 0.01 | |
| Age (days) | 92 | 0.06 | −0.001; 0.13 | 0.052 |
p values showing significance difference (p = 0.05) are bolded.
To normalise the data Hp values were divided from 1, so a negative estimate means a positive effect.
In the linear mixed models decreased Hp concentrations (p = 0.02; Table 1; Fig. 4 ) and increased Fb concentrations (p = 0.048, Table 2 ) were associated with weak signs of eimeriosis. There were no statistically significant associations between eimeriosis and SAA concentration or WBC count (results not shown). Also no association was detected between APPs and intestinal pathogens other than Eimeria spp.
Fig. 4.
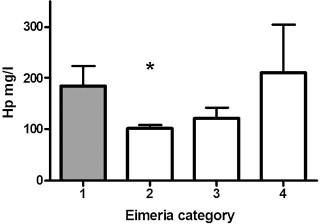
The mean haptoglobin (Hp) concentrations (+standard deviation) for Finnish dairy calves aged 15–60 days with different Eimeria infection status. Eimeria category: 1 = no Eimeria or diarrhoea, 2 = weak signs of eimeriosis, 3 = moderate signs of eimeriosis, 4 = strong signs of eimeriosis. Statistical difference as compared to category 1; *p = 0.04.
Table 2.
Associations of 1/fibrinogena concentration with eimeriosis related signs, diagnosis, gender and age in Finnish dairy calves.
| Variable | n | Estimate | 95% CI | p value | Wald test p value |
|---|---|---|---|---|---|
| Eimeriosis signs | 0.13 | ||||
| No signs | 35 | 0 | |||
| Weak signs | 22 | −0.033 | −0.066; −0.0003 | 0.048 | |
| Moderate signs | 25 | −0.007 | −0.025; 0.039 | 0.67 | |
| Strong signs | 9 | −0.010 | −0.061; 0.042 | 0.72 | |
| Signs of disease | 91 | 0.03 | |||
| Healthy | 52 | 0 | |||
| Diarrhoea | 20 | −0.002 | −0.037; 0.033 | 0.91 | |
| Respiratory infection | 9 | −0.047 | −0.091; −0.002 | 0.04 | |
| Umbilical inflammation | 8 | −0.052 | −0.093; −0.012 | 0.01 | |
| Joint problem | 2 | −0.031 | −0.110; 0.050 | 0.45 | |
| Age (days) | 91 | 0.001 | −0.001;0.002 | 0.32 |
p values showing significance difference (p = 0.05) are bolded.
To normalise the data Fb values were divided from 1, so a negative estimate means a positive effect.
3.3. Acute phase proteins and diseases
The means and variation of Hp, Fb and SAA concentrations and WBC in different conditions can be seen in Fig. 5 . Elevated Hp and Fb concentrations were associated with respiratory infections or umbilical infections (Table 1, Table 2). Age was negatively associated with SAA concentration (p = 0.002), but Hp, Fb or WBC showed no associations with age. Elevated SAA concentration was associated positively with positive culture from deep nasopharyngeal swab samples (p = 0.02; detailed results of the model not shown). Positive culture was defined as a positive finding for Pasteurella multocida, Histophilus somni, Trueperella pyogenes, Mannheimia haemolytica, Mannheimia varigena, Mycoplasma dispar or Ureaplasma diversum. Half of the clinically healthy calves with positive respiratory tract culture (50%; 95% CI 27.2–72.8%) had SAA concentrations over the reference value of 178 mg/l (mean 198 mg/l ± 148 mg/l). No viruses were detected from the respiratory tract samples.
Fig. 5.
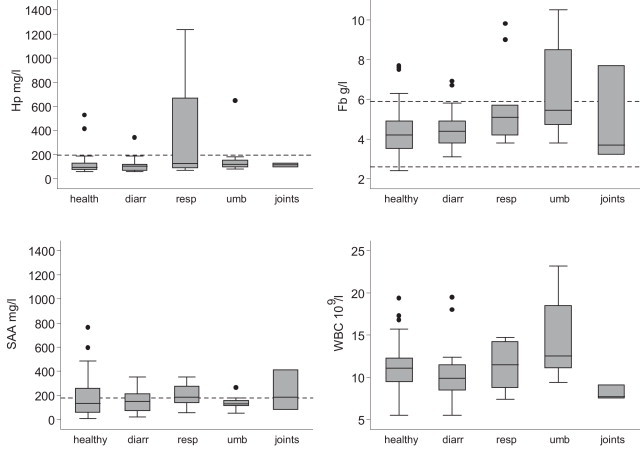
A box plot of haptoglobin (Hp), fibrinogen (Fb) and serum amyloid A (SAA) concentrations and white blood cell (WBC) count for different diseases. Broken lines indicate reference values (Seppä-Lassila et al.[48]). See text for statistically significant differences in APP or WBC count between diseases. Abbreviations: diarr = diarrhoea, resp = respiratory tract infection, umb = umbilical disease, joints = joint lesion.
4. Discussion
In the study associations between intestinal pathogens, diarrhoea and acute phase response were explored in natural conditions. The results showed pathogenic Eimeria spp. to be associated with diarrhoea and mild signs of Eimeria infection associated with decreased Hp concentrations and increased Fb concentrations.
4.1. Intestinal pathogens and diarrhoea
Almost third of the examined calves showed signs of diarrhoea, which is more frequent than observed in Swedish [28] or Norwegian studies [29]. In the current study, setting diagnosis of diarrhoea based on one evaluation only can be questioned because changes in diet or poor feeding practices can cause momentary changes in faecal consistency. The assessment of faecal consistency is also subjective, although many of the visits were performed by both of the veterinarians conducting the study, thus reducing the inter-observer variation.
Various intestinal pathogens are frequently detected both in diarrheic [29], [30], [31] and healthy calves [29], [32], but for choosing the right treatment, prevention measures and possible prophylaxis, detecting the etiological agent of diarrhoea is necessary. Amongst all intestinal pathogens detected E. bovis and E. zuernii showed association with diarrhoea in this study. The study also showed a higher prevalence of samples positive for both Cryptosporidium spp. and Giardia spp. in diarrheic calves than in healthy calves, but the MCA indicated no association between diarrhoea and Giardia spp. or diarrhoea and Cryptosporidium spp. Only non-pathogenic species C. ryanae and C. bovis were detected in this study. The non-pathogenicity of C. bovis is being questioned [33], but the evidence is not strong. Giardia spp. have also been considered to be non-pathogenic, but there is evidence that Giardia is an important intestinal pathogen of calves [34]. In the current study Giardia spp. was not determined to genus or assemblage level, and thus pathogenicity of Giardia cannot be reliably assessed.
Rotavirus is a well-established intestinal pathogen, but the current study provided no evidence of association between rotavirus and diarrhoea. The calves in the study were slightly older than calves typically suffering from rotavirus, and there were only few positive samples for rotavirus. Calves usually continue to shed rotavirus for a week, even if the clinical signs have ceased [35], which could explain the lack of association between rotavirus and diarrhoea as the few rotavirus-positive calves might have been sampled soon after the diarrhoea has ceased.
Many common intestinal pathogens in central Europe and North America are rare in Finland; BVDV is nearly eradicated, salmonellosis is very rare in cattle and C. parvum is met only occasionally, although the occurrence of C. parvum is increasing [36], [37]. This parasite is already frequently found in young calves in Sweden [38]. In Finland, Eimeria spp. and rotavirus are the most common pathogens detected in diarrheic samples of calves [37].
4.2. Eimeriosis and acute phase response
Confirming Eimeria species as the cause of eimeriosis can be challenging because several intestinal pathogens cause similar symptoms [39], [40], not all species of Eimeria are equally pathogenic, the number of oocysts shed in the patent period is not solely a good measure of the severity of the infection, and symptoms often precede shedding of oocysts. The presence of pathogenic Eimeria spp. oocysts and diarrhoea, however, showed a relation in the MCA analysis.
In the present study, a negative association between eimeriosis severity and Hp or Fb was established, but SAA and WBC counts were not associated with Eimeria related signs. When building the variable for Eimeria related signs, different cut-off limits for coccidia oocyst counts were investigated. In a cross-sectional study we can expect to find animals harbouring different stages of Eimeria in the intestine, resulting in varying and possibly low numbers of excreted oocysts in the faeces. A relatively low cut-off point at 500 opg of total oocyst count (modified from Mundt et al. [4]) provided the most reasonable categorisation, when combined with the information on the presence of pathogenic or non-pathogenic Eimeria spp. and the presence of diarrhoea.
In the current study, a decrease in Hp and an increase in Fb concentrations were associated with weak signs of Eimeria. Although the Eimeria sporozoites and merozoites exist only briefly in the lumen of the intestine or are intracellular, thus attempting to evade the immune system, they are capable of inducing an innate immune response [7] and also haematological changes [41], revealing a systemic response. Studies exploring the acute phase response and Eimeria are few. A positive acute phase response and a pro-inflammatory cytokine response have been reported in experimentally induced caprine coccidiosis [42], supporting the existence of systemic response at least in experimental studies. In the current study with natural infection in calves, farms with known coccidia problems might have been more likely to participate in the study. However, this possible bias should not affect the relationship between Hp and eimeriosis.
As the decreased Hp concentration was associated only with milder signs of eimeriosis, the association is not probably directly related to the inflammation process, but is more a reflection of Hp's action as a haemoglobin scavenger [43]. Hp exists in blood at very low concentrations, when no inflammation process is in progress [8]. Eimeria merozoites induce haemorrhaging injuries on the wall of the intestine [6], resulting in haemolysis and excessive amounts of haemoglobin in the tissues. Hp binds to haemoglobin, thus decreasing the amount of measurable Hp determined by the haemoglobin binding method used in this study. Arthington et al. [44] discovered an unexpected and unexplained decrease in Hp concentrations when exploring how transporting and commingling calves affects the acute phase response, and initial decrease in Hp concentration was also observed in the early phase of experimental E. coli mastitis [45]. Hp binding to haemoglobin could explain these observations, as minor, haemorrhaging injuries or haemolysis can occur in these stressful situations or in the early phase of inflammation, where vascular permeability increases.
4.3. Acute phase proteins and the diagnoses
Elevated Hp and Fb concentrations were associated with respiratory infections and umbilical infections. No association between diarrhoea and APPs or WBC count was observed in the study, although diarrhoea was one of the components in Eimeria related signs variable. Diarrhoea can be viewed rather as a disorder than a definite diagnosis, and common mechanisms of diarrhoea (hypersecretory or malabsorption) do not involve the initiation of the inflammatory pathway. Increased concentrations of Hp and SAA in naturally infected diarrheic calves with or without intestinal pathogens (Eimeria spp. not examined), have been reported [13], although the concentrations on the whole still remained fairly low.
The SAA concentrations have previously shown association with age [46] and diseases [14], but in this study there were no associations with any of the diseases and SAA concentrations. SAA is considered to be the most sensitive bovine APP [47], but in this study the results conflict. The calves classified “healthy” had increased SAA concentration, the mean close to the upper reference value of healthy calves [48]. The difference between APP concentrations of healthy and diseased calves is hard to detect when it is small. Our group of “healthy” includes some calves with some of the respiratory infection signs (temperature >39.5 °C, respiratory rate >40/min, or increased respiratory sounds). The concentrations of SAA, sensitive to inflammation, might be affected by these minor signs of illness, as the models with Hp and Fb functioned despite the slightly indistinct definition of healthy. Absence of apparent disease is not equal to health, as reviewed by Gunnarsson [49], but for simplicity the term was used in this study. Using the current study design the onset, duration and acuteness of the inflammation were unclear, which increased the dispersion of the APP measurements.
5. Conclusions
The relevance of various pathogens in diarrhoea is difficult to assess. The presence of diarrhoea appears to associate with pathogenic Eimeria species, in contrast to other intestinal pathogens examined and observed. A decrease in haptoglobin may imply of mild eimeriosis, although inflammatory disease concurrently appearing elsewhere in the host might increase the APPs, making conclusions of the situation challenging. However, this study showed an association between an intestinal pathogen and innate immunology.
Conflict of interest statement
None of the authors has any financial or personal relationship with organisations or people that could influence the content or conclusions of the study.
Acknowledgements
Warmest thanks to veterinary students participating the farm visits and especially to Marjo Kuosa for assisting in the data collection. This study was partly funded by grants from Finnish Foundation of Veterinary Research, Finnish Veterinary Foundation, and by institutional research funding (IUT number 8-1) of the Estonian Ministry of Education and Research.
References
- 1.Daugschies A., Najdrowski M. Eimeriosis in cattle: current understanding. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2005;52:417–427. doi: 10.1111/j.1439-0450.2005.00894.x. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald P.R. The economic impact of coccidiosis in domestic animals. Adv. Vet. Sci. Comp. Med. 1980;24:121–143. [PubMed] [Google Scholar]
- 3.Lassen B., Østergaard S. Estimation of the economical effects of Eimeria infections in Estonian dairy herds using a stochastic model. Prev. Vet. Med. 2012;106:258–265. doi: 10.1016/j.prevetmed.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Mundt H.-C., Bangoura B., Mengel H., Keidel J., Daugschies A. Control of clinical coccidiosis of calves due to Eimeria bovis and Eimeria zuernii with toltrazuril under field conditions. Parasitol. Res. 2005;97(Suppl. 1):S134–S142. doi: 10.1007/s00436-005-1457-9. [DOI] [PubMed] [Google Scholar]
- 5.Bangoura B., Mundt H.-C., Schmäschke R., Westphal B., Daugschies A. Prevalence of Eimeria bovis and Eimeria zuernii in German cattle herds and factors influencing oocyst excretion. Parasitol. Res. 2012;110:875–881. doi: 10.1007/s00436-011-2569-z. [DOI] [PubMed] [Google Scholar]
- 6.Friend S.C., Stockdale P.H.G. Experimental Eimeria bovis infection in calves: a histopathological study. Can. J. Comp. Med. 1980;44:129–140. [PMC free article] [PubMed] [Google Scholar]
- 7.Taubert A., Behrendt J.H., Suhwold A., Zahner H., Hermosilla C. Monocyte- and macrophage-mediated immune reactions against Eimeria bovis. Vet. Parasitol. 2009;164:141–153. doi: 10.1016/j.vetpar.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Petersen H.H., Nielsen J.P., Heegaard P.M.H. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- 9.Horadagoda N.U., Knox K.M., Gibbs H.A., Reid S.W., Horadagoda A., Edwards S.E. Acute phase proteins in cattle: discrimination between acute and chronic inflammation. Vet. Rec. 1999;144:437–441. doi: 10.1136/vr.144.16.437. [DOI] [PubMed] [Google Scholar]
- 10.Ceciliani F., Ceron J.J., Eckersall P.D., Sauerwein H. Acute phase proteins in ruminants. J. Proteomics. 2012;75:4207–4231. doi: 10.1016/j.jprot.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Gånheim C., Hultén C., Carlsson U., Kindahl H., Niskanen R., Persson Waller K. The acute phase response in calves experimentally infected with bovine viral diarrhoea virus and/or Mannheimia haemolytica. J. Vet. Med. Ser. B. 2003;50:183–190. doi: 10.1046/j.1439-0450.2003.00658.x. [DOI] [PubMed] [Google Scholar]
- 12.Orro T., Pohjanvirta T., Rikula U., Huovilainen A., Alasuutari S., Sihvonen L. Acute phase protein changes in calves during an outbreak of respiratory disease caused by bovine respiratory syncytial virus. Comp. Immunol. Microbiol. Infect. Dis. 2011;34:23–29. doi: 10.1016/j.cimid.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pourjafar M., Badiei K., Nazifi S., Naghib S.M. Acute phase response in Holstein dairy calves affected with diarrhoea. Bulg. J. Vet. Med. 2011;14:142–149. [Google Scholar]
- 14.Tóthová C., Nagy O., Seidel H., Kováč G. Acute phase proteins in relation to various inflammatory diseases of calves. Comp. Clin. Pathol. 2012;21:1037–1042. [Google Scholar]
- 15.Henriksen S., Korsholm H. Parasitologisk undersøgelse af fæcesprøver. Konstruktion og anvendelse af et enkelt opbygget tællekammar. Dansk Vet. 1984;67:1193–1196. [Google Scholar]
- 16.Roepstroff A., Nansen P. FAO; Rome, Italy: 1998. Epidemiology, Diagnosis, and Control of Helminth Parasites of Swine, FAO Animal Health Manual; pp. 51–56. [Google Scholar]
- 17.Lassen B., Viltrop A., Raaperi K., Järvis T. Eimeria and Cryptosporidium in Estonian dairy farms in regard to age, species, and diarrhoea. Vet. Parasitol. 2009;166:212–219. doi: 10.1016/j.vetpar.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Levine N. Apicomplexa: the Coccidia proper. In: Levine N., editor. Veterinary Protozoology. The Iowa University State Press; Iowa, USA: 1985. pp. 130–232. [Google Scholar]
- 19.Feng Y., Ortega Y., He G., Das P., Xu M., Zhang X. Wide geographic distribution of Cryptosporidium bovis and the deer-like genotype in bovines. Vet. Parasitol. 2007;144:1–9. doi: 10.1016/j.vetpar.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 20.ISO . 2007. Horizontal Method for the Detection of Salmonella spp. 6579:2002/amd 2007. [Google Scholar]
- 21.Millar H.R., Simpson J.G., Stalker A.L. An evaluation of the heat precipitation method for plasma fibrinogen estimation. J. Clin. Pathol. 1971;24:827–830. doi: 10.1136/jcp.24.9.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makimura S., Suzuki N. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Jpn. J. Vet. Sci.Nippon Juigaku Zasshi. 1982;44:15–21. doi: 10.1292/jvms1939.44.15. [DOI] [PubMed] [Google Scholar]
- 23.Alsemgeest S.P., Kalsbeek H.C., Wensing T., Koeman J.P., van Ederen A.M., Gruys E. Concentrations of serum amyloid-A (SAA) and haptoglobin (HP) as parameters of inflammatory diseases in cattle. Vet. Q. 1994;16:21–23. doi: 10.1080/01652176.1994.9694410. [DOI] [PubMed] [Google Scholar]
- 24.DeRosa D.C., Mechor G.D., Staats J.J., Chengappa M.M., Shryock T.R. Comparison of Pasteurella spp. simultaneously isolated from nasal and transtracheal swabs from cattle with clinical signs of bovine respiratory disease. J. Clin. Microbiol. 2000;38:327–332. doi: 10.1128/jcm.38.1.327-332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friis N.F., Krogh H.V. Isolation of mycoplasmas from Danish cattle. Nord. Vet. Med. 1983;35:74–81. [PubMed] [Google Scholar]
- 26.Autio T., Pohjanvirta T., Holopainen R., Rikula U., Pentikainen J., Huovilainen A. Etiology of respiratory disease in non-vaccinated, non-medicated calves in rearing herds. Vet. Microbiol. 2007;119:256–265. doi: 10.1016/j.vetmic.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dohoo I., Martin W., Stryhn H. Model-building strategies. In: Dohoo I., Martin W., Stryhn H., editors. Veterinary Epidemiologic Research. AVC Inc.; Prince Edward Island, Canada: 2003. pp. 317–334. [Google Scholar]
- 28.Lundborg G.K., Scensson E.C., Oltenacu P.A. Herd-level risk factors for infectious diseases in Swedish dairy calves aged 0–90 days. Prev. Vet. Med. 2005;68:123–143. doi: 10.1016/j.prevetmed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Gulliksen S.M., Jor E., Lie K.I., Hamnes I.S., Loken T., Akerstedt J. Enteropathogens and risk factors for diarrhea in Norwegian dairy calves. J. Dairy Sci. 2009;92:5057–5066. doi: 10.3168/jds.2009-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoet A.E., Smiley J., Thomas C., Nielsen P.R., Wittum T.E., Saif L.J. Association of enteric shedding of bovine torovirus (Breda virus) and other enteropathogens with diarrhea in veal calves. Am. J. Vet. Res. 2003;64:485–490. doi: 10.2460/ajvr.2003.64.485. [DOI] [PubMed] [Google Scholar]
- 31.Lanz Uhde F., Kaufmann T., Sager H., Albini S., Zanoni R., Schelling E. Prevalence of four enteropathogens in the faeces of young diarrhoeic dairy calves in Switzerland. Vet. Rec. 2008;163:362–366. doi: 10.1136/vr.163.12.362. [DOI] [PubMed] [Google Scholar]
- 32.Busato A., Lentze T., Hofer D., Burnens A., Hentrich B., Gaillard C. A case control study of potential enteric pathogens for calves raised in cow-calf herds. Zentralbl Vet. B. 1998;45:519–528. doi: 10.1111/j.1439-0450.1998.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 33.Silverlås C., Bosaeus-Reineck H., Näslund K., Björkman C. Is there a need for improved Cryptosporidium diagnostics in Swedish calves? Int. J. Parasitol. 2013;43:155–161. doi: 10.1016/j.ijpara.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geurden T., Vercruysse J., Claerebout E. Is Giardia a significant pathogen in production animals? A review. Exp. Parasitol. 2010;124:98–106. doi: 10.1016/j.exppara.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Archaumbalt D., Morin G., Elazhary Y., Hoet R.S. Study of virus excretion in feces of diarrheic and asymptomatic calves infected with rotavirus. J. Vet. Med. 1990;37:73–76. doi: 10.1111/j.1439-0450.1990.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 36.Autio T., Syrjälä P., Pohjanvirta T., Karhukorpi J., Pelkonen S. Book of Abstracts. XXVI World Buiatrics Congress; Santiago, Chile: 2010. Prevalence and molecular characterization of Cryptosporidium in calves and a case of human cryptosporidiosis associated with calves in Finland. [Google Scholar]
- 37.Finnish Food Safety Authority Evira . vol. 7. Eviran julkaisuja; 2014. (Animal Diseases in Finland 2013). (Evira publications; only in Finnish) [Google Scholar]
- 38.Silverlas C., Näslund K., Björkman C., Mattsson J.G. Molecular characterisation of Cryptosporidium isolates from Swedish dairy cattle in relation to age, diarrhoea and region. Vet. Parasitol. 2010;169:289–295. doi: 10.1016/j.vetpar.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Cornelissen A.W., Verstegen R., van den Brand H., Perie N.M., Eysker M., Lam T.J. An observational study of Eimeria species in housed cattle on Dutch dairy farms. Vet. Parasitol. 1995;56:7–16. doi: 10.1016/0304-4017(94)00671-x. [DOI] [PubMed] [Google Scholar]
- 40.Björkman C., Svensson C., Christensson B., de Verdier K. Cryptosporidium parvum and Giardia intestinalis in calf diarrhoea in Sweden. Acta Vet. Scand. 2003;44:145–152. doi: 10.1186/1751-0147-44-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bangoura B., Daugschies A., Fuerll M. Influence of experimental Eimeria zuernii infection on clinical blood chemistry in calves. Vet. Parasitol. 2007;150:46–53. doi: 10.1016/j.vetpar.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Hashemnia M., Khodakaram-Tafti A., Razavi S.M., Nazifi S. Changing patterns of acute phase proteins and inflammatory mediators in experimental caprine coccidiosis. Korean J. Parasitol. 2011;49:213–219. doi: 10.3347/kjp.2011.49.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eaton J.W., Brandt P., Mahoney J.R., Lee J.T., Jr. Haptoglobin: a natural bacteriostat. Science. 1982;215:691–693. doi: 10.1126/science.7036344. [DOI] [PubMed] [Google Scholar]
- 44.Arthington J.D., Eichert S.D., Kunkle W.E., Martin F.G. Effect of transportation and commingling on the acute-phase protein response, growth, and feed intake of newly weaned beef calves. J. Anim. Sci. 2003;81:1120–1125. doi: 10.2527/2003.8151120x. [DOI] [PubMed] [Google Scholar]
- 45.Suojala L., Orro T., Järvinen H., Saatsi J., Pyörälä S. Acute phase response in two consecutive experimentally induced E. coli intramammary infections in dairy cows. Acta Vet. Scand. 2008;50:18. doi: 10.1186/1751-0147-50-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orro T., Jacobsen S., LePage J., Niewold T., Alasuutari S., Soveri T. Temporal changes in serum concentrations of acute phase proteins in newborn dairy calves. Vet. J. 2008;176:182–187. doi: 10.1016/j.tvjl.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Werling D., Arnold M., Kun G., Tooten P.C.J., Gruys E., Kreuzer M., Langhans W. Characterisation of the acute phase response of heifers to a prolonged low dose infusion of lipopolysaccharide. Res. Vet. Sci. 1996;61:252–257. doi: 10.1016/s0034-5288(96)90073-9. [DOI] [PubMed] [Google Scholar]
- 48.Seppä-Lassila L., Orro T., LePage J.P., Soveri T. Reference values for acute phase proteins in calves and its clinical application. Vet. Rec. 2013;173:319. doi: 10.1136/vr.101233. [DOI] [PubMed] [Google Scholar]
- 49.Gunnarsson S. The conceptualisation of health and disease in veterinary medicine. A review. Acta Vet. Scand. 2006;48:20. doi: 10.1186/1751-0147-48-20. [DOI] [PMC free article] [PubMed] [Google Scholar]


