Summary
Background
Respiratory viruses are detectable in a large proportion of adults hospitalised with acute respiratory illness. For influenza and other viruses there is evidence that viral load and persistence are associated with certain clinical outcomes but it is not known if there is an association between viral load and hospital length of stay.
Methods
306 adults hospitalised with viral acute respiratory illness were studied. Associations between viral load and length of stay were examined. Multiple linear regression analysis was performed to control for age, comorbidity, influenza vaccine status, duration of illness prior to hospitalisation, bacterial co-infection, clinical group and virus subtype.
Results
High viral load was associated with a longer duration of hospitalisation for all patients (p < 0.0001). This remained significant across all virus types and clinical groups and when adjusted for age, comorbidity, duration of illness prior to hospitalisation, bacterial co-infection and other factors.
Conclusions
High viral loads are associated with prolonged hospital length of stay in adults with viral acute respiratory illness. This further supports existing evidence demonstrating that viral acute respiratory illness is a viral load driven process and suggests that viral load could be used in clinical practise to predict prolonged hospitalisation and prioritise antivirals.
International Standard Randomised Controlled Trial Number (ISRCTN): 21521552
Keywords: Respiratory viruses, Viral load, Acute respiratory illness, Adults, Length of stay, Hospitalisation
Highlights
-
•
It is not known if viral load is associated with length of stay in viral acute respiratory illness.
-
•
We examined 306 adult patients hospitalised with confirmed viral acute respiratory illness.
-
•
High viral load was associated with longer length of stay for all virus subtypes and all clinical groups.
-
•
This could be used in clinical practise to identify high risk patients and prioritise antivirals.
Introduction
Acute respiratory tract infections are responsible for a huge burden of disease and are the third most common cause of death worldwide.1 Although bacteria have previously been considered to be the principal aetiological agents of severe respiratory infection, the global importance of respiratory viruses in this group has been increasingly recognised in recent years.2, 3, 4, 5, 6, 7, 8 There were around 400,000 hospital admissions with acute respiratory infection in England in 2014,9 excluding exacerbations of chronic lung disease, and recent studies have shown that respiratory viruses are detectable in around 40–50% of hospitalised adults with acute respiratory illness.4, 10
For influenza virus, human challenge models have demonstrated that viral load, as measured by viral culture or polymerase chain reaction (PCR) on upper respiratory tract samples, is correlated with symptom severity and the magnitude of the host inflammatory response.11, 12, 13 For hospitalised adults with severe and complicated seasonal influenza viral load is highest at the point of hospitalisation14 and is higher, with more protracted shedding, compared with outpatients.14 In addition, the time to viral clearance is related to certain clinical outcomes in this group, including length of hospital stay.14, 15 In hospitalised patients with the virulent avian H5N1 influenza A, who generally have life threatening disease, the pharyngeal viral load on admission to hospital is higher than in patients with seasonal influenza16 and is higher in non-survivors versus those who survive.17
For the other respiratory viruses there are also data in adults to suggest a relationship between viral load and clinical outcomes. In a human challenge model using healthy adult volunteers, RSV nasal viral load is closely correlated with clinical symptoms and recovery.18 Observational studies have shown that RSV viral load is higher and viral shedding more protracted in hospitalised adults compared to outpatients,19 and that a high viral load in hospitalised adults is a risk factor for the development of complications including respiratory failure and the need for mechanical ventilation.20, 21 In human challenge models of asthma and COPD exacerbation, using challenge with rhinovirus, viral load measured in sputum correlates with the degree of host inflammatory response.22, 23 In addition, a study involving healthy university students with naturally acquired rhinovirus infection demonstrated lower viral loads in individuals with asymptomatic rhinovirus detection compared to individuals with symptomatic colds.24
The most appropriate end-points for clinical trials of respiratory virus antivirals in hospitalised adults have not been agreed. As antiviral drugs work by inhibiting viral replication and available evidence suggests that virological and clinical outcomes are closely correlated, virological end-points have been suggested and used as primary outcome measures for antiviral trials.17 Additional studies to further characterise the details of the relationship between clinical and virological outcomes in hospitalised adults are therefore required.
For clinical outcome measures, admission to intensive care units (ICU) or death are relatively rare outcomes in adults hospitalised with acute respiratory illness and severity scoring tools to predict ICU admission and death have not proven to be useful in hospitalised adults with influenza.25 Other potential outcome measures such as time to clinical stability and symptom scoring for influenza illness are unreliable and potentially flawed in this population, especially in the elderly who may not display typical clinical features and who may also have cognitive impairment. Length of hospital stay is the key determinant of cost in patients hospitalised with acute respiratory illness26 and so represents an outcome measure which is both clinically and economically important. For this reason it is often employed as a primary or secondary outcome measure in clinical trials in hospitalised patients. Although affected by factors other than acute illness such as patient age and comorbidity, and variations in practice between different centres, providing these can be adequately controlled for hospital length of stay represents a useful and robust clinical outcome measure. It has been suggested that prolonged hospitalisation (e.g. >5–7 days) could be used as a clinical end-point for future trials of influenza antivirals in addition to other rarer clinical events such as ICU admission and death.17 Defining the relationship between viral load and length of hospital stay is therefore of significant interest and importance.
The aim of this study was to examine the associations between viral load, as measured by real-time PCR cycle threshold (Ct) value on upper respiratory tract samples at the point of admission, and hospital length of stay for a large cohort of adults hospitalised with confirmed viral acute respiratory illness. An association between high viral load and prolonged length of stay would support existing evidence suggesting that viral acute respiratory illness is a viral load driven process and could be used clinically to identify patients at risk of prolonged hospitalisation and for stratification of participants in clinical trials of candidate antiviral agents.
Methods
Study design
This was a cohort study using patients recruited as part of a prospective trial of rapid diagnostic tests for influenza and Streptococcus pneumoniae.26 The study was approved by the Leicestershire, Northamptonshire and Rutland Ethics Committee prior to commencement of the study and all patients gave written informed consent.
Patients
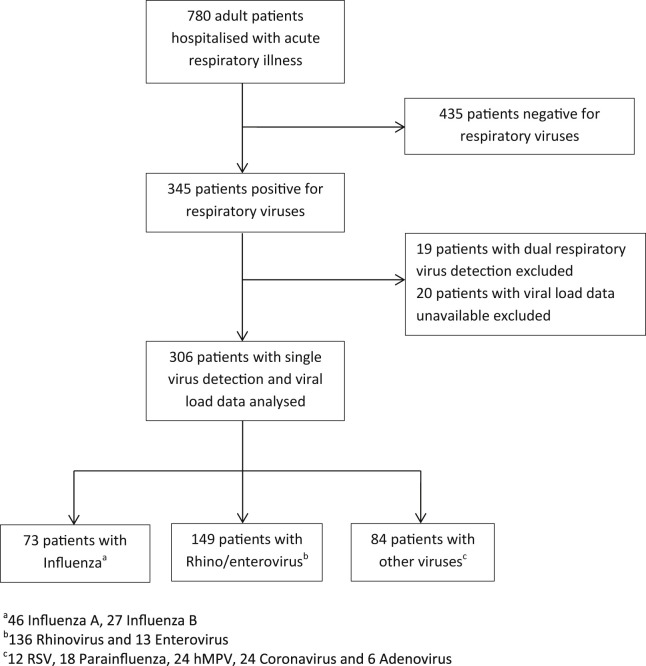
Patients were adults hospitalised with acute respiratory illness as identified by discharge ICD-10 (International classification of disease, tenth edition) code classification27 and confirmed by case note review. Subjects were participants in a large prospective study of rapid near patient testing for S. pneumoniae and influenza and met the following inclusion criteria; adult aged over 18 years of age, able and willing to give written informed consent (or a relative or carer is able and willing to give informed assent), acute exacerbation of a chronic respiratory disease or new onset of acute respiratory illness, of less than 7 days duration, able to be recruited within 16 h of hospital admission. Patients were recruited during the winter months of 2005–2008, across two hospital sites with acute medical admission units, within the University Hospitals of Leicester NHS Trust, UK. Patients were tested for respiratory virus infection as detailed below and those testing positive for respiratory viruses and with viral load data available were included in this study. Patients with dual viral detections were excluded. Fig. 1 shows the trial profile for this study.
Figure 1.
Flow chart of patients enrolled and included in the analysis.
Clinical data
Demographic and clinical data (presenting symptoms and signs, comorbidity, medications use, prior antibiotic use, smoking history, and vaccination status) were collected at enrolment and outcome data (duration of hospitalisation measured up to 30 days following admission, admission to high dependency and intensive care units, in-hospital mortality and 30 day mortality) were collected retrospectively and recorded on a standardised case report form. Radiological and laboratory data were collated retrospectively using computerised imaging and laboratory systems. All patients had a chest radiograph performed within 24 h of admission and all radiographs were reported by a consultant radiologist independent from the study team. Full details of clinical groups and their definitions have been described previously.4
Samples
Blood, urine, sputum and nasopharyngeal swab samples were collected at enrolment. Nasopharyngeal swab samples were collected by trained research staff according to a standard protocol. Swabs were stored in viral transport medium and frozen at −80 °C prior to testing. Definitions of viral and bacterial infection and the diagnostic methods for bacteria have been described previously.4
PCR methods
Briefly, viral nucleic acid was extracted using the XtractorGene nucleic acid extraction procedure (Corbett Life Sciences, Sydney, Australia) according to the manufacturer's instructions. A starting volume of 200 μL was used to elute 60 μL of viral RNA/DNA and 5 μL of this eluate was used for each PCR reaction, with a 25 μL final reaction volume.
Four quadriplex real-time (TaqMan®) PCR assays were employed to detect the following respiratory viruses; influenza A and B, respiratory syncytial virus (RSV) A and B, parainfluenza (PIV) types 1–4, adenovirus, enterovirus, rhinovirus, human metapneumovirus (hMPV), group 1 coronaviruses (HCoV-229E and HCoV-NL63), group 2 coronaviruses (HCoV-OC43 and HCoV-HKU1), and SARS associated coronavirus. Full details of the PCR methods including primer and probe sequences, controls and cycling conditions have been previously described.4 All respiratory virus testing was performed by the same staff using the Rotor-Gene 3000 PCR machine (Corbett Life Sciences, Sydney, Australia) with standard laboratory protocols. Laboratory staff were blinded to clinical data.
Comparison of Ct values
The real-time PCR Cycle threshold (Ct) value represents the first PCR cycle in which the fluorescent signal for the target (i.e. viral RNA) is greater than the minimal detection level. Ct values are inversely proportional to the quantity of target, offering a semi-quantitative assessment of viral load,28 so that a low Ct value represents a high viral load and vice versa.
Statistical analysis
Statistical analysis was performed by an independent statistician using PRISM Version 6 (Graphpad Software, La Jolla, CA) and Stata v13.1 (StataCorp, 2013). Baseline characteristics are presented using appropriate summary measures. Initially, Ct value (viral load) was categorised into 4 groups (≤20, 21–25, 26–30 and >30) and the Kruskal–Wallis test was used to explore differences between baseline characteristics across these groups. The primary analysis was a zero-truncated negative binomial regression used to assess the association between the outcome variable duration of hospitalisation (measured in days) and Ct value (viral load). The multivariable model controlled for age, sex, comorbidity, influenza vaccine status, duration of illness prior to hospitalisation, presence of bacteria, clinical group (exacerbation of asthma, exacerbation of COPD, community acquired pneumonia and influenza-like illness/bronchitis) and virus subtype (Influenza A and B, Rhino/enterovirus and other viruses); these covariates were identified a priori. A zero-truncated model was chosen due to the fact that duration of hospitalisation could not be less than one day; negative binomial regression was chosen due to over-dispersion of the outcome, suggesting Poisson regression was not appropriate.
Results
A total of 306 patients were studied; 73 with influenza (46 influenza A and 27 influenza B), 149 with rhino/enterovirus (136 rhinovirus and 13 enterovirus) and 84 with other viruses (12 RSV, 18 parainfluenza virus 1–4, 24 human metapneumovirus, 24 coronavirus and 6 adenovirus). No patients were treated with neuraminidase inhibitors or any other antiviral agents prior to or during their inpatient stay. 56 patients had pneumonia, 108 had exacerbation of COPD, 95 had exacerbation of asthma and 47 had Influenza-like illness/bronchitis combined. Baseline characteristics including demographic, clinical, laboratory and outcome data for all patients and by viral load (Ct value) category are shown in Table 1 .
Table 1.
Baseline characteristics, laboratory and outcome data for all patients combined (n = 306) and by Ct value (viral load).
| All patients n = 306 |
Ct value (viral load) |
p Valuea | p Valueb | ||||
|---|---|---|---|---|---|---|---|
| ≤20 n = 42 |
21–25 n = 87 |
26–30 n = 94 |
>30 n = 83 |
||||
| Demographics | |||||||
| Age, years | 61 [41–74] | 73 [36–85] | 61 [41–71] | 63 [43–73] | 59 [40–73] | 0.146 | 0.031 |
| Female sex (%) | 174 (57) | 28 (67) | 43 (49) | 61 (65) | 42 (51) | 0.060 | 0.183 |
| White ethnicity (%) | 271 (89) | 37 (88) | 74 (85) | 82 (87) | 78 (94) | 0.304 | 1.000 |
| Current smoker (%) | 92 (30) | 9 (21) | 28 (32) | 30 (34) | 25 (30) | 0.608 | 0.209 |
| Influenza vaccinec | 171 (56) | 24 (57) | 43 (49) | 60 (67) | 44 (53) | 0.242 | 1.000 |
| Comorbidity | |||||||
| Cardiovascular (%) | 128 (43) | 22 (54) | 39 (45) | 39 (42) | 32 (40) | 0.486 | 0.180 |
| Respiratory (%) | 232 (77) | 30 (73) | 65 (76) | 76 (82) | 57 (70) | 0.357 | 0.701 |
| Renal (%) | 7 (2) | 1 (2) | 0 (0) | 3 (3) | 3 (4) | 0.382 | 1.000 |
| Liver (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA |
| Diabetes mellitus (%) | 35 (12) | 6 (15) | 9 (10) | 8 (8) | 12 (15) | 0.551 | 0.598 |
| Cancer (%) | 6 (2) | 1 (2) | 1 (1) | 3 (3) | 0 (0) | 0.385 | 0.522 |
| Clinical group | |||||||
| Asthma (%) | 95 (31) | 10 (24) | 33 (38) | 34 (36) | 18 (22) | 0.058 | 0.369 |
| IECOPD (%) | 108 (35) | 13 (31) | 37 (43) | 29 (31) | 29 (35) | 0.368 | 0.604 |
| CAP (%) | 56 (18) | 8 (19) | 5 (6) | 15 (16) | 28 (34) | <0.0001 | 0.833 |
| ILI/bronchitis (%) | 47 (15) | 11 (26) | 12 (14) | 16 (17) | 8 (10) | 0.100 | 0.062 |
| Virus type | |||||||
| Influenza (%) | 73 (24) | 13 (31) | 20 (23) | 20 (21) | 20 (24) | 0.671 | 0.247 |
| Rhino/enterovirus (%) | 149 (49) | 16 (38) | 42 (48) | 46 (49) | 45 (54) | 0.303 | 0.183 |
| Other virusesd (%) | 84 (27) | 13 (31) | 25 (29) | 28 (30) | 18 (22) | 0.578 | 0.580 |
| Clinical features | |||||||
| Duration of illness priore, days | 4 [2–7] | 4 [2–7] | 4 [2–7] | 4 [2–6.4] | 4 [2–7] | 0.879 | 0.643 |
| Antibiotics priore (%) | 73 (24) | 9 (21) | 21 (24) | 26 (28) | 17 (20) | 0.723 | 0.846 |
| Antibiotics during (%) | 243 (82) | 37 (88) | 67 (81) | 76 (82) | 63 (79) | 0.655 | 0.386 |
| IV Antibiotics during (%) | 88 (30) | 16 (38) | 26 (31) | 19 (20) | 27 (34) | 0.112 | 0.204 |
| Pulse rate, bpm | 98.5 [85–112] | 95 [84–111] | 100 [90–115] | 98 [84–114] | 96 [84–110] | 0.347 | 0.514 |
| Respiratory rate, bpm | 22 [20–26] | 23 [19–24] | 23 [20–26] | 22 [20–25] | 23 [20–26] | 0.567 | 0.988 |
| Systolic blood pressure, mmHg | 130 [118–145] | 134 [121–157] | 130 [115–145] | 134 [120–147] | 125 [115–140] | 0.165 | 0.361 |
| Temperature, °C | 37 [36.5–37.5] | 37 [36.6–37.5] | 37 [36.5–37.7] | 37 [36.5–37.4] | 37 [36.4–37.5] | 0.728 | 0.821 |
| O2 Saturations, % | 96 [93–98] | 96 [93–98] | 96 [94–98] | 96 [93–98] | 95 [92–97] | 0.437 | 0.801 |
| Laboratory | |||||||
| WCC ×109/L | 10.5 [7.8–13.8] | 9.9 [7.1–12.8] | 10.2 [7.7–13] | 10.8 [7.9–14] | 11.2 [8.3–14.5] | 0.133 | 0.134 |
| CRP, mg/L | 28 [13–71] | 31 [14–77] | 26 [10–56] | 27 [8–63] | 36 [14–127] | 0.205 | 0.523 |
| Ct value | 27 [23–31] | 18 [17–20] | 23 [22–24] | 28 [27–29] | 33 [32–34] | NA | NA |
| Pneumococcal detection (%) | 37 (12) | 5 (12) | 9 (10) | 10 (11) | 13 (16) | 0.691 | 1.000 |
| Any bacteria detected (%) | 57 (19) | 7 (17) | 12 (14) | 15 (16) | 23 (28) | 0.093 | 0.833 |
| Outcome | |||||||
| Length of stay, days | 2 [1–6] | 6 [2–10.3] | 2 [1–5] | 2 [1–4] | 2 [1–5] | 0.0002 | <0.0001 |
| ICU admission (%) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0.441 | 1.000 |
| Death (%) | 9 (3) | 1 (2) | 0 (0) | 6 (7) | 2 (2) | 0.083 | 1.000 |
Data are presented as number (%) and median [Inter-quartile range].
Abbreviations are: Ct, real-time PCR cycle threshold (a low Ct value represents a high viral load and vice versa); IECOPD, infective exacerbation of COPD; CAP, community acquired pneumonia; ILI, Influenza-like illness; IV, intravenous; WCC, white cell count; CRP, C reactive protein; ICU, intensive care unit; NA, not applicable.
p values highlighted in bold are statistically significant (i.e., p <0.05).
Across all Ct value groups (Kruskal–Wallis test).
Between high viral load group (Ct ≤ 20) and other groups combined (Chi squared test).
Receipt this influenza season.
Includes; Respiratory Syncytial Virus, parainfluenza 1–4, human metapneumovirus, coronavirus and adenovirus.
Prior to hospitalisation.
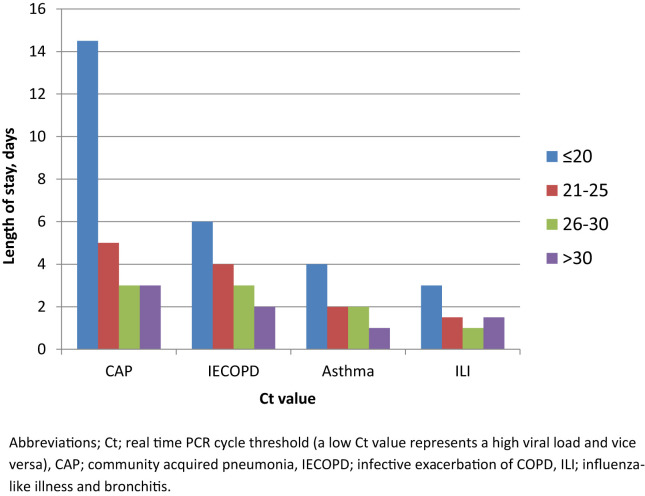
Age and hospital length of stay were associated with a Ct values of ≤20 (i.e. a high viral load), p = 0.031 and p < 0.0001 respectively. For all patients combined, Ct value was inversely correlated with hospital length of stay, i.e. length of stay increased as Ct value decreased (viral load increased), r = −0.17 [95% CI −0.28 to −0.06], p = 0.002 (Spearman's rank correlation). Length of stay was longer for patients with Ct ≤ 20 (high viral load) for all patients combined (p < 0.0001) and for patients with influenza (p = 0.005), rhino/enterovirus (p = 0.004) and other viruses (p = 0.044), and also by clinical groups; for pneumonia (p = 0.012), exacerbation of asthma (p = 0.016), exacerbation of COPD (p = 0.02) and ILI/bronchitis (p = 0.025). Examining individual clinical groups by virus subtype, the association between hospital length of stay and Ct value (viral load) was strongest for influenza in patients with exacerbation of COPD (p = 0.007) and pneumonia (p = 0.028), and for rhino/enterovirus in patients with asthma exacerbation (p = 0.006) although the small numbers of patients infected with certain viral subtypes in some clinical groups meant that meaningful comparisons were not possible for all subgroups (Table 2 and Fig. 2 ).
Table 2.
Duration of hospitalisation (in days) by Ct value (viral load) for all patient and individual clinical groups, by virus subtype.
| Ct value (viral load) |
p Valuea | p Valueb | ||||
|---|---|---|---|---|---|---|
| ≤20 | 21–25 | 26–30 | >30 | |||
| All patients (n) | ||||||
| All viruses (306) | 6 [2–10] | 2 [1–5] | 2 [1–4] | 2 [1–5] | 0.0002 | <0.0001 |
| Influenza (73) | 7 [2.5–13.5] | 2 [1–3.8] | 2 [1–4] | 2.5 [1–6] | 0.019 | 0.005 |
| Rhino/entero (149) | 4 [2–7.8] | 2 [1–4.3] | 2 [1–4] | 2 [1–4] | 0.052 | 0.004 |
| Other virusesc (84) | 6 [2.5–13] | 4 [1.5–8] | 3 [1–6] | 3.5 [1–5.5] | 0.236 | 0.044 |
| Asthma | ||||||
| All viruses (95) | 4 [1.8–7] | 2 [1–3] | 2 [1–4] | 1 [1–2.3] | 0.086 | 0.016 |
| Influenza (19) | 2 [2–2] | 2 [1–3] | 3 [1–4.3] | 2 [1–3.5] | NA | NA |
| Rhino/entero (58) | 4 [1.3–7] | 1 [1–2] | 1 [1–3.3] | 1 [1–2] | 0.071 | 0.006 |
| Other virusesc (18) | 7 [7–7] | 3 [1.5–7.5] | 2 [1–6.3] | 3 [2–4] | NA | NA |
| IECOPD | ||||||
| All viruses (108) | 6 [3–13.5] | 4 [1–7] | 3 [1–6] | 2 [1–5.5] | 0.089 | 0.020 |
| Influenza (20) | 8.5 [6–17.5] | 2 [1–5.5] | 2 [1–6] | 3 [1.5–6.5] | 0.078 | 0.007 |
| Rhino/entero (47) | 4.5 [3–6] | 4 [1–8] | 2.5 [1–6] | 2 [1–6] | 0.386 | 0.574 |
| Other virusesc (41) | 4 [2–17] | 4 [1.5–8] | 4.5 [1.5–5.8] | 4 [1–6] | 0.919 | 0.542 |
| CAP | ||||||
| All viruses (56) | 14.5 [3.8–20] | 5 [2.5–11] | 3 [2–11] | 3 [1–6] | 0.077 | 0.012 |
| Influenza (23) | 17 [3–21] | 6.5 [1–12] | 1.5 [1–2] | 7.5 [3–9] | 0.073 | 0.028 |
| Rhino/entero (30) | 14 [2–30] | 4.5 [4–5] | 3.5 [2.3–14] | 3 [1–5.5] | 0.415 | 0.249 |
| Other virusesc (13) | 10.5 [6–15] | 10 [10–10] | 11 [3–23] | 2 [1–12] | NA | 0.423 |
| ILI/bronchitis | ||||||
| All viruses (47) | 3 [2–8] | 1.5 [1–5.5] | 1 [1–3] | 1.5 [1–5.5 | 0.098 | 0.025 |
| Influenza (21) | 3 [1–8] | 1 [1–8.5] | 1 [1–3.5] | 2.5 [1–6.3] | 0.807 | 0.493 |
| Rhino/entero (14) | 3 [2–8] | 1 [1–1] | 1 [1–2.8] | 1.5 [1–2] | NA | NA |
| Other virusesc (12) | 8 [2–11] | 3 [1–7] | 1 [1–4] | NA | NA | 0.155 |
Data are presented as median [Inter-quartile range].
Abbreviations: Ct; real-time PCR cycle threshold (a low Ct value represents a high viral load and vice versa); CAP, community acquired pneumonia; IECOPD, infective exacerbation of COPD; ILI, influenza-like illness; NA, not applicable.
p values highlighted in bold are statistically significant (i.e., p.<0.05).
Across all groups (Kruskal–Wallis test).
Between high viral load group (Ct ≤ 20) and other groups combined (Chi squared).
Includes; Respiratory Syncytial Virus, parainfluenza 1–4, human metapneumovirus, coronavirus and adenovirus.
Figure 2.
Median hospital length of stay by Ct value (viral load) for all viruses combined (n = 306), by individual clinical groups.
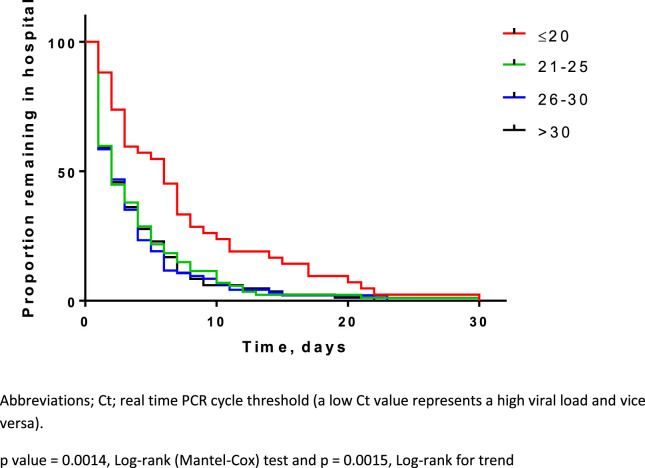
Examining other markers of severity, in patients with pneumonia, CURB65 score was higher in patients with influenza and Ct ≤ 20 (high viral load), p = 0.038, but not in patients with other viruses detected (shown in Supplementary Appendix, Table S1). Fig. 3 demonstrates Kaplan–Meier survival curves for hospital length of stay by Ct value (viral load) for all patients combined. Multiple logistic regression analysis demonstrated that Ct value (viral load) was associated with hospital length of stay independently of age, sex, comorbidity, influenza vaccine status, duration of illness prior to hospitalisation, bacterial co-infection, clinical group and virus subtype (p < 0.001). For every unit decrease in Ct value (i.e. increase in viral load) there was a 6% increase in hospital length of stay. Age, comorbidity (other than respiratory disease), bacterial infection and clinical group were also independently associated with duration of hospitalisation (Table 3 ).
Figure 3.
Kaplan–Meier survival curve showing duration of hospitalisation by Ct value (viral load) for all patients (n = 306).
Table 3.
Multiple logistic regression analysis showing associations between duration of hospitalisation (in days) and other variables, for all patients (n = 306).
| Variable | Ratio | 95% CI | p Value | |
|---|---|---|---|---|
| Ct valuea | 0.94 | 0.92 | 0.97 | <0.001 |
| Agea | 1.02 | 1.01 | 1.04 | <0.001 |
| Duration of illness prior to admission, days | 0.99 | 0.93 | 1.07 | 0.965 |
| Sex (reference group: female) | 0.80 | 0.58 | 1.11 | 0.181 |
| Influenza vaccine (reference group: no) | 0.94 | 0.67 | 1.31 | 0.700 |
| Comorbidity (reference group: none) | 1.47 | 1.05 | 2.07 | 0.025 |
| Antibiotics prior to admission (reference group: none) | 1.12 | 0.78 | 1.60 | 0.544 |
| Current smoker (reference group: no) | 1.11 | 0.78 | 1.58 | 0.563 |
| Bacterial detection (reference group: no) | 1.78 | 1.18 | 2.67 | 0.006 |
| Clinical group | ||||
| Asthma (reference group) | – | – | – | – |
| IECOPD | 1.16 | 0.72 | 1.85 | 0.538 |
| Pneumonia | 1.44 | 0.79 | 2.63 | 0.236 |
| ILI/bronchitis | 0.46 | 0.21 | 0.98 | 0.044 |
| Virus type | ||||
| Influenza (reference group) | – | – | – | – |
| Rhino/enterovirus | 0.92 | 0.62 | 1.38 | 0.692 |
| Other virusesb | 1.12 | 0.74 | 1.70 | 0.588 |
Abbreviations: Ct, real-time PCR Cycle threshold (a low Ct value represents a high viral load and vice versa); IECOPD, infective exacerbation of COPD; CAP, community acquired pneumonia; ILI, Influenza-like illness; CI, confidence interval.
Note: For each unit decrease in Ct value (i.e. increase in viral load), there is a 6% increase in expected days in hospital. Likewise, for each additional year of age, length of stay increases by 2%. The presence of at least one of any recorded comorbidity (cardiovascular, renal or liver disease, malignancy or diabetes) increases length of stay by 47% relative to people without any of these comorbidities. The detection of bacteria was associated with a 78% increase in length of stay. Patients with ILI/bronchitis had a length of stay 46% shorter than those with asthma.
p values highlighted in bold are statistically significant (i.e., p <0.05).
Variables are mean-centred.
Includes; Respiratory Syncytial Virus, parainfluenza 1–4, human metapneumovirus, human coronavirus and adenovirus.
Discussion
This large cohort study of adults hospitalised with viral acute respiratory illness demonstrates that viral load, as measured on nasopharyngeal samples at the point of admission to hospital, is associated with hospital length of stay and that a high viral load is strongly associated with a longer duration of hospitalisation. This was a consistent finding across all clinical groups and all virus subtypes and was maintained when adjusting for age, comorbidity, duration of illness, bacterial co-infection and other potentially confounding factors. Our results support the existing body of evidence suggesting that respiratory viruses may cause severe acute respiratory illness and that the resulting clinical illness is a viral load driven process with clinical outcomes predicted by viral load magnitude, kinetics and persistence. Whilst there is already a substantial body of evidence suggesting a relationship between certain clinical outcomes and viral load for influenza and some other respiratory viruses, this is the first study to demonstrate an association between viral load and hospital length of stay and to show a relationship for multiple clinical groups and across multiple viral subtypes within the same study. Our findings suggest that the detection of a high nasopharyngeal viral load in an adult patient hospitalised with acute respiratory illness could be used clinically to identify patients at high risk of prolonged hospitalisation. Although currently most laboratories test for influenza using real-time PCR and so generate Ct values routinely, these are not generally available to clinicians or used for clinical decision making. This study suggest that these results could be passed on to clinicians and used to inform clinical decision making including the priority use of neuraminidase inhibitors for influenza and antivirals for other viruses when they become available.
The median duration of illness prior to hospitalisation was 4 days (inter-quartile range 2–7 days) in our cohort and there was no detectable association between the duration of illnesses prior to hospitalisation and viral load at presentation. This suggests that in contrast to healthy adults with uncomplicated viral infection (where viral load peaks quickly and declines rapidly) for hospitalised adults with complicated respiratory viral illness, the viral load remains an important determinant of clinical outcome at this later time point and is therefore potentially modifiable with effective antiviral therapy. This is consistent with a number of observational studies of hospitalised adults with influenza showing reduction in viral loads and clinical benefits from neuraminidase inhibitors even when started beyond 48 h of symptom duration.14, 29, 30 Although not explored in this study a high viral load is also suggestive of increased infectiousness and an increased risk of nosocomial spread and so patients with a high viral load detected on admission to hospital would represent a priority group for side room isolation and enhanced infection control practices.
Respiratory virus nasopharyngeal viral load has been used as a primary endpoint in clinical trials of novel antivirals on the basis of the association between viral load, clinical symptoms and outcome. This study strengthens this position and extends it to patients with other respiratory viruses such as rhinovirus and to patients in specific, less well studied clinical groups such as exacerbation of asthma and COPD. Furthermore this study suggests that high viral load could be used as a method to stratify patients in future trials of novel candidate antivirals agents where duration of hospitalisation is used as an outcome measure.
The strengths of our study include the large numbers of patients with confirmed viral acute respiratory illness, the consistency of the PCR methods for all patients and the use of multiple logistic regression analysis to adjust for confounding variables such as age, comorbidity, duration of illness prior to hospitalisation and bacterial co-infection. In addition no patients with influenza were treated with neuraminidase inhibitors in our cohort which is consistent with UK prescribing practice at the time of the study and prior to the H1N1 influenza pandemic of 2009, and allows a unique opportunity to examine the associations between viral load and clinical outcomes in untreated patients with influenza without confounding from antiviral treatment.
Although this is a large ‘real world’ clinical study, and as such is generalizable to typical adult patients hospitalised with acute respiratory illness, it does have several limitations. Retrospective observational studies are prone to bias and although this has been well controlled for using our multivariate model, including variables known to potentially influence hospital length of stay, we cannot exclude the possibility of other unpredicted and unmeasured variables influencing the results. The findings of this study are however biologically plausible and are consistent with previously published studies demonstrating a relationship between viral load and clinical outcomes. Furthermore the association seen in our study between high viral load and increased severity of influenza pneumonia using the CURB65 score suggests that high viral load is indeed a marker of severity in patients hospitalised with acute viral respiratory illness.
We did not use a formal quantitative PCR (qPCR) assay in this study and instead relied on the semi-quantitative Ct value from our real-time PCR assays. Real-time PCR Ct values are now considered by many to be a robust surrogate for quantified viral load and are increasingly reported in published studies of acute viral illness including those examining respiratory viruses6, 31 and also in the recent Ebola virus pandemic.32, 33 Furthermore, the use of protocolised sampling techniques, processing by dedicated research staff and the removal of inter-operator variability of results interpretation (as all samples were analysed by the same scientists in the same laboratory) gives reassurance as to the robustness of the Ct result in this study. Although all patients had respiratory swabs taken from the nasopharynx, as is standard clinical practise when testing for respiratory viruses in the UK, this site may be insensitive for the detection of respiratory viruses in some patients with lower respiratory tract infection.34 Some studies have found increased rates of respiratory virus detection from sputum compared to upper respiratory tract samples34, 35 and so it is possible that some of our patients that were excluded due to negative PCR on nasopharyngeal samples may have actually had detectable viruses in their lower respiratory tract. It is also possible that when viruses are detected from both upper and lower respiratory samples that the viral load might be different between the two. We would suggest that the findings of this study are validated in a large prospective cohort incorporating sampling from the upper and lower respiratory tract and examining viral kinetics and persistence over the course of hospitalisation.
In summary this study demonstrates that a high viral load measured on nasopharyngeal samples at the point of hospitalisation is associated with longer length of stay in adult patients with viral acute respiratory illness, for all virus subtypes and across all clinical groups. If confirmed in further studies this could be used in clinical practice to identify patients at risk of prolonged hospital stay and to prioritise for antiviral treatment and enhanced infection control practises.
Notes
Funding
This work was supported by the National Institute of Health Research though the Health Technology Assessment programme (HTA), grant number [03/39/18]. The views and opinions expressed in this manuscript are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS or the Department of Health.
Competing interests
None declared for SE, MM, SB, MC and SP. KGN has been an ad hoc consultant to GSK, MSD, and Novartis. He has received funding to speak at meetings organised by Novartis, Baxter, Berna Biotech, Esteves, Roche and the European Scientific Working Group on Influenza, and has received H5 vaccines from Novartis to support an MRC-funded research project, and H1N1 vaccines from Baxter AG and GSK. TWC has been an investigator on clinical trials sponsored by Novartis, Baxter AG and GSK.
Author contributions
Conception and design: TWC, KGN, MM, MC and SP. Patient enrolment and data collection: TWC, SB, MM and KGN. Analysis and interpretation of the data: TWC, SE, KGN. Drafting of the article: TWC, SE, MM, SB, MC, SP and KGN.
Ethical approval
The study was approved by the Leicestershire, Northamptonshire and Rutland Ethics Committee and all patients gave informed written consent.
Acknowledgements
We would like to thank all the hospital staff at UHL involved in the care of the patients in this study. The assays used were developed as part of an ongoing collaboration between HPA East of England and the Respiratory Virus reference laboratory at the Centre for Infections, London. We thank Alison Bermingham for her assistance in the validation of some of the PCR assays used in this study.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jinf.2016.09.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.World Health Organisation fact sheet 310; Top 10 causes of death 2012. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/. [accessed 24.02.15].
- 2.Jain S., Williams D.J., Arnold S.R., Ampofo K., Bramley A.M., Reed C. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Acremont V., Kilowoko M., Kyungu E., Philipina S., Sangu W., Kahama-Maro J. Beyond malaria – causes of fever in outpatient Tanzanian children. N Engl J Med. 2014;370:809–817. doi: 10.1056/NEJMoa1214482. [DOI] [PubMed] [Google Scholar]
- 4.Clark T.W., Medina M.J., Batham S., Curran M.D., Parmar S., Nicholson K.G. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J Infect. 2014;69:507–515. doi: 10.1016/j.jinf.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M. Community-acquired pneumonia requiring hospitalization among U.S. Adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Self W.H., Williams D.J., Zhu Y., Ampofo K., Pavia A.T., Chappell J.D. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis. 2015;213:584–591. doi: 10.1093/infdis/jiv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byington C.L., Ampofo K., Stockmann C., Adler F.R., Herbener A., Miller T. Community surveillance of respiratory viruses among families in the Utah better identification of germs-longitudinal viral epidemiology (BIG-LoVE) study. Clin Infect Dis. 2015;61:1217–1224. doi: 10.1093/cid/civ486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi T., McLean K., Campbell H., Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta-analysis. J Glob Health. 2015;5:010408. doi: 10.7189/jogh.05.010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hospital Episode Statistics, Admitted Patient Care – England, 2014–15 [NS], Health and Social Care Information Centre. Available at: http://www.hscic.gov.uk/catalogue/PUB20104. [accessed 24.02.16].
- 10.Falsey A.R., Becker K.L., Swinburne A.J., Nylen E.S., Formica M.A., Hennessey P.A. Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J Infect Dis. 2013;208:432–441. doi: 10.1093/infdis/jit190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz R.S., Hayden F.G., Calfee D.P., Cass L.M., Peng A.W., Alvord W.G. Nasal cytokine and chemokine responses in experimental influenza A virus infection: results of a placebo-controlled trial of intravenous zanamivir treatment. J Infect Dis. 1999;180:586–593. doi: 10.1086/314938. [DOI] [PubMed] [Google Scholar]
- 12.Hayden F.G., Fritz R., Lobo M.C., Alvord W., Strober W., Straus S.E. Local and systemic cytokine responses during experimental human influenza A virus infection: relation to symptom formation and host defense. J Clin Invest. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrat F., Vergu E., Ferguson N.M., Lemaitre M., Cauchemez S., Leach S. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167:775–785. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 14.Lee N., Chan P.K., Hui D.S., Rainer T.H., Wong E., Choi K.W. Viral loads and duration of shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200:492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee N., Chan P.K., Choi K.W., Lui G., Wong B., Cockram C.S. Factors associated with early hospital discharge of adult influenza patients. Antivir Ther. 2007;12:501–508. [PubMed] [Google Scholar]
- 16.de Jong M.D., Simmons C.P., Thanh T.T., Hien V.M., Smith G.J., Chau T.N. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ison M.G., de Jong M.D., Gilligan K.J., Higgs E.S., Pavia A.T., Pierson J. End points for testing influenza antiviral treatments for patients at high risk of severe and life-threatening disease. End points for testing influenza antiviral treatments for patients at high risk of severe and life-threatening disease. J Infect Dis. 2010;201:1654–1662. doi: 10.1086/652498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeVincenzo J.P., Wilkinson T., Vaishnaw A., Cehelsky J., Meyers R., Nochur S. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;182:1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh E.E., Peterson D.R., Kalkanoglu A.E., Lee F.E., Falsey A.R. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis. 2013;207:1424–1432. doi: 10.1093/infdis/jit038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan C.B., Walsh E.E., Peterson D.R., Lee F.E., Falsey A.R. Risk factors for respiratory failure associated with respiratory syncytial virus infection in adults. J Infect Dis. 2009;200(8):1242–1246. doi: 10.1086/605948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee N., Chan M.C., Lui G.C., Li R., Wong R.Y., Yung I.M. High viral load and respiratory failure in adults hospitalized for respiratory syncytial virus infections. J Infect Dis. 2015;212:1237–1240. doi: 10.1093/infdis/jiv248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallia P., Message S.D., Gielen V., Contoli M., Gray K., Kebadze T. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J., Message S.D., Qiu Y., Mallia P., Kebadze T., Contoli M. Airway inflammation and illness severity in response to experimental rhinovirus infection in asthma. Chest. 2014;145:1219–1229. doi: 10.1378/chest.13-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granados A., Goodall E.C., Luinstra K., Smieja M., Mahony J. Comparison of asymptomatic and symptomatic rhinovirus infections in university students: incidence, species diversity, and viral load. Diagn Microbiol Infect Dis. 2015;82:292–296. doi: 10.1016/j.diagmicrobio.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller M.P., McGeer A.J., Hassan K., Marshall J., Christian M., Toronto Invasive Bacterial Disease Network Evaluation of pneumonia severity and acute physiology scores to predict ICU admission and mortality in patients hospitalized for influenza. PLoS One. 2010;5:e9563. doi: 10.1371/journal.pone.0009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson K.G., Abrams K.R., Batham S., Medina M.J., Warren F.C., Barer M. Randomised controlled trial and health economic evaluation of the impact of diagnostic testing for influenza, respiratory syncytial virus and Streptococcus pneumoniae infection on the management of acute admissions in the elderly and high-risk 18- to 64-year-olds. Health Technol Assess. 2014;18 doi: 10.3310/hta18360. 1–274, vii–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bramer G.R. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat Q. 1988;41:32–36. [PubMed] [Google Scholar]
- 28.Ginzinger D.G. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002;30:503–512. doi: 10.1016/s0301-472x(02)00806-8. [DOI] [PubMed] [Google Scholar]
- 29.Lee N., Choi K.W., Chan P.K., Hui D.S., Lui G.C., Wong B.C. Outcomes of adults hospitalised with severe influenza. Thorax. 2010;65(6):510–515. doi: 10.1136/thx.2009.130799. [DOI] [PubMed] [Google Scholar]
- 30.Muthuri S.G., Venkatesan S., Myles P.R., Leonardi-Bee J., Al Khuwaitir T.S., Al Mamun A. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2:395–404. doi: 10.1016/S2213-2600(14)70041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu L., Wang Z., Chen Y., Ding W., Jia H., Chan J.F. Clinical, virological, and histopathological manifestations of fatal human infections by avian influenza A(H7N9) virus. Clin Infect Dis. 2013;57:1449–1457. doi: 10.1093/cid/cit541. [DOI] [PubMed] [Google Scholar]
- 32.Spengler J.R., McElroy A.K., Harmon J.R., Ströher U., Nichol S.T., Spiropoulou C.F. Relationship between Ebola virus real-time quantitative polymerase chain reaction-based threshold cycle value and virus isolation from human plasma. J Infect Dis. 2015;212(Suppl. 2):S346–S349. doi: 10.1093/infdis/jiv187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan T., Mu J., Qin E., Wang Y., Liu L., Wu D. Clinical characteristics of 154 patients suspected of having Ebola virus disease in the Ebola holding center of Jui Government Hospital in Sierra Leone during the 2014 Ebola outbreak. Eur J Clin Microbiol Infect Dis. 2015;34:2089–2095. doi: 10.1007/s10096-015-2457-z. [DOI] [PubMed] [Google Scholar]
- 34.Jeong J.H., Kim K.H., Jeong S.H., Park J.W., Lee S.M., Seo Y.H. Comparison of sputum and nasopharyngeal swabs for detection of respiratory viruses. J Med Virol. 2014;86:2122–2127. doi: 10.1002/jmv.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falsey A.R., Formica M.A., Walsh E.E. Yield of sputum for viral detection by reverse transcriptase PCR in adults hospitalized with respiratory illness. J Clin Microbiol. 2012;50:21–24. doi: 10.1128/JCM.05841-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.