Abstract
Objective
Neopterin is generated and released in increased amounts by macrophages upon activation by interferon-gamma during cellular immune response. In this study, we aimed to investigate serum neopterin levels in patients with Crimean–Congo hemorrhagic fever (CCHF) and its clinical significance as a predictor factor of mortality.
Methods
Neopterin concentrations on the first day of hospitalization were measured in serum samples from 51 CCHF patients. Serum neopterin levels and other clinical–laboratory parameters for fatal and nonfatal CCHF patients were compared.
Results
Serum neopterin levels (73.22 ± 54.30 nmol/L) were highly elevated in all CCHF patients (p < 0.0001) with higher levels in fatal group (153.66 ± 81.34 nmol/L, p = 0.0001) compared to nonfatal disease (55.99 ± 24.09 nmol/L). In univariate analysis, the level of neopterin on the first day of hospitalization, bleeding, platelet count, aspartate transferase and lactate dehydrogenase were associated with mortality. In multivariate analysis, only the serum level of neopterin was associated with mortality. As a mortality risk factor, area under the curve was 0.939 (p = 0.0001, 95% confidence interval: 0.85–1.00).
Conclusions
In this first study of serum neopterin levels for CCHF, elevated serum neopterin level showing strong activation of monocytes/macrophages was a risk factor for CCHF.
Keywords: Neopterin, Crimean–Congo hemorrhagic fever, Mortality, Risk factors
Introduction
Crimean–Congo hemorrhagic fever (CCHF) is an acute tick-borne viral illness with the potential of human-to-human transmission affecting multiple organ systems and characterized by ecchymosis, visceral bleeding and hepatic dysfunction.1, 2 The virus belongs to the genus Nairovirus in the Bunyaviridea family.3, 4 The reported fatality rate ranges 5–50%.2, 3 Increased white blood cell count (WBC), decreased platelet count, elevated alanine transferase (ALT), aspartate transferase (AST), lactate dehydrogenase (LDH) and creatine kinase (CK) levels, prolonged activated partial thromboplastin time (aPTT) and prothrombin time (PT), decreased fibrinogen, echhymosis, melena, hematemesis, somnolence, and absence or low detection of specific IgM and IgG antibodies against CCHF have been described as risk factors for fatal CCHF in different studies.2, 5, 6
The pathogenesis of CCHF is not well described. Their ability to disable the host immune response by attacking and manipulating the cells that initiate the antiviral response is a common pathogenic feature of hemorrhagic fever viruses.7 The vascular endothelium is both direct and indirect target of all viral hemorrhagic viruses. Mononuclear phagocytes are also target cells for viral hemorrhagic viruses. They are activated upon infection and release certain cytokines and chemokines. These mediators indirectly target the endothelium.8, 9 Serum levels of the proinflammatory cytokines IL-6 and TNF-α were higher in fatal CCHF patients.10, 11
Neopterin derivatives are produced by human monocyte-derived macrophages and dendritic cells upon stimulation by interferons. Plasma or urine neopterin concentrations reflect the activation of the macrophage/monocyte line cells.12 Neopterin has become one of the useful tools to assess the intensity of the cell-mediated immune response. It appears to be an indicator of severity, progression, and outcome of infectious and inflammatory diseases.13
In this study, we aimed to investigate serum neopterin levels in patients with CCHF and its clinical significance as a predictor factor of mortality.
Patients and methods
We studied 51 CCHF patients who were admitted to Ankara Numune Education and Research Hospital (ANERH) during the spring and summer of 2006. Suspected patients for CCHF were defined as the cases with clinical symptoms and signs of CCHF such as fever, myalgia, malaise, and bleeding, and also the history of tick bite or inhabit (or travel to) endemic region. Diagnosis of all patients in the study group was confirmed with elevated IgM antibodies and/or viral RNA by RT-PCR. Blood samples of suspected cases were collected on admission to hospital for IgM antibodies and RT-PCR test of CCHF virus. The IgM antibodies were detected by using ELISA at Refik Saydam Hygiene Center of Ankara, Turkey. TaqMan-based one-step RT-PCR assay was used for detection of CCHF virus RNA.14 The assay was performed in a Perkin–Elmer 7700 Sequence Detection System by using the combination of reverse-transcriptase (MBI Fermentas, Vilnius, Lithuania) and hot start Taq DNA polymerase (Birion GmBH, Munchen, Germany) enzymes. All of the manipulations were performed in a biosafety class II cabinet.
Age, sex, duration of symptoms, presence of fever, myalgia, bleeding, tick bite, platelet count, WBC, ALT, AST, LDH, CK levels, aPTT and international normalized ratio (INR) on the first day of hospitalization were recorded for all patients.
Sera collected on the first day and last day (exitus day for fatal CCHF or discharge day for nonfatal CCHF) of hospitalization for each patient were analyzed in the study. All sera were collected in tubes covered with tin foil. Then they were stored at −80 °C until analysis. Serum neopterin concentration was measured with a high-performance liquid chromatography device (Hewlett–Packard 1050, USA) using a fluorometry detector, as defined by Alrashed et al.15 The results were calculated as nmol/L.
The serum levels of neopterin on the first day of hospitalization in CCHF patients were compared with the serum levels of neopterin of healthy controls (n = 30). We also compared serum neopterin levels and other clinical–laboratory parameters for two groups (patients with fatal and nonfatal CCHF).
Statistical analysis
Groups were compared by Chi-square tests and Mann–Whitney U test. Univariate and multivariate analysis were used to determine variables associated with mortality. In order to determine the cut-off point and to quantify the accuracy of serum neopterin, we used the Receiver Operating Characteristics (ROC) analysis.16 Statistical analysis was performed by using the SPSS 11.5 Statistical Package Program for Windows (SPSS Inc., Chicago, Illinois, USA). Differences were considered significant at p < 0.05.
Results
Clinical and laboratory features for 51 CCHF patients in this study are summarized in Table 1. Nine of the cases died because of CCHF (fatal CCHF group). Forty-two cases were survived (nonfatal CCHF group). The overall fatality rate was 17.6%.
Table 1.
Clinical and laboratory features for 51 CCHF patients
| Patients with nonfatal CCHF (n:42) | Patients with fatal CCHF (n:9) | p | Univariate analysis of risk factors for mortality |
|||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | ||||
| Mean age | 49.6 ± 16.1 | 55 ± 17.1 | NS | 1.022 | 0.975–1.070 | 0.367 |
| Female (n) | 17 (40.5%) | 6 (66.7%) | NS | 0.340c | 0.075–1.549c | 0.163c |
| Mean duration of symptoms (days) | 5.8 ± 3.0 | 6.0 ± 5.8 | NS | 1.017 | 0.799–1.293 | 0.894 |
| Mean temperature (°C) | 37.6 ± 1.0 | 37.8 ± 0.9 | NS | – | – | – |
| Serum neopterin levela (nmol/L) | 55.9 ± 24.0 | 153.6 ± 81.3 | 0.0001 | 1.074 | 1.024–1.127 | 0.004 |
| Serum neopterin levelb (nmol/L) | 28.5 ± 17.0 | 173.1 ± 152.1 | 0.002 | |||
| Fever (n) | 38 (90.5%) | 9 (100%) | NS | – | 1.005–1.108 | 0.999 |
| Myalgia (n) | 40 (95.2%) | 9 (100%) | NS | – | – | 0.999 |
| Bleeding (n) | 14 (33.3%) | 8 (88.9%) | 0.003 | 16 | 1.817–140.919 | 0.012 |
| Tick bite (n) | 27 (64.3%) | 6 (66.7%) | NS | 0.984 | 0.880–1.101 | 0.779 |
| WBCa (ml) | 2723 ± 1418 | 3411 ± 2870 | NS | 1.00 | 1.000–1.001 | 0.287 |
| Platelet counta (ml) | 71,404 ± 43,712 | 22,111 ± 14,895 | 0.001 | 0.955 | 0.921–0.990 | 0.012 |
| ASTa (IU/L) | 357 ± 543 | 1207 ± 1265 | <0.0001 | 1.001 | 1.000–1.002 | 0.033 |
| ALTa (IU/L) | 210 ± 288 | 429 ± 609 | 0.029 | 1.001 | 1.000–1.003 | 0.139 |
| LDHa (IU/L) | 623 ± 383 | 2380 ± 2491 | 0.0001 | 1.004 | 1.001–1.006 | 0.005 |
| CKa (IU/L) | 666 ± 881 | 933 ± 672 | NS | 1.000 | 1.000–1.001 | 0.400 |
| aPTTa | 36.8 ± 15.2 | 44.1 ± 20.9 | NS | 1.002 | 0.998–1.006 | 0.248 |
| INRa | 95.3 ± 29.5 | 104.5 ± 33.0 | NS | 1.003 | 0.994–1.019 | 0.439 |
NS: not significant, CCHF: Crimean–Congo hemorrhagic fever, WBC: white blood cell count, ALT: alanine transferase, AST: aspartate transferase, LDH: lactate dehydrogenase, CK: creatine kinase, aPTT: activated partial thromboplastin time, INR: international normalized ratio, and OR: odds ratio.
On the first day of admission to the hospital.
On the last day of admission to the hospital.
Risk of male patients according to females.
Serum neopterin levels raised in all CCHF patients on the first day of hospitalization (>10 nmol/L), reaching a mean concentration of 73.22 ± 54.30 nmol/L. The mean neopterin concentration of healthy blood donors was 4.78 ± 1.26 nmol/L. Thus, the mean neopterin concentration in CCHF patients exceeded that of the controls by approximately 15-fold (p < 0.0001).
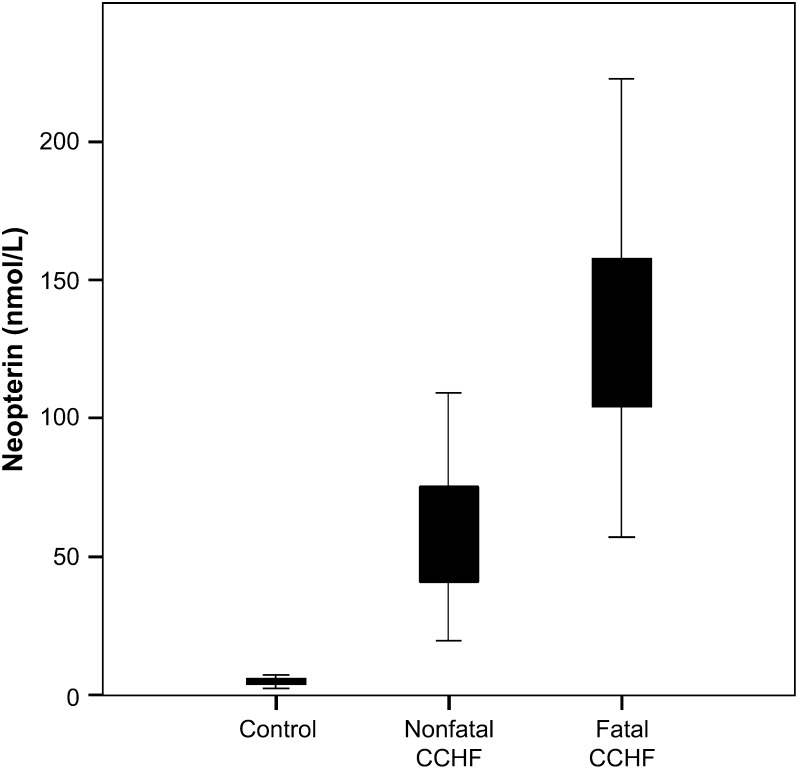
The mean concentration of neopterin in fatal group (153.66 ± 81.34 nmol/L) was nearly threefold higher than that of nonfatal group (55.99 ± 24.09 nmol/L) ( Fig. 1). The difference between fatal and nonfatal groups was statistically significant (p = 0.0001). The levels of neopterin at the last day of hospitalization in fatal group (173.14 ± 152.16 nmol/L) were also significantly higher than that of nonfatal group (28.53 ± 17.01 nmol/L) (p = 0.002).
Figure 1.
Serum neopterin levels in fatal and nonfatal CCHF patients compared with control cases.
The other parameters analyzed for mortality are also presented in Table 1. In univariate analysis, the serum level of neopterin on the first day of hospitalization, bleeding, platelet count, AST and LDH were associated with mortality (Table 1). Multivariate analysis was done excluding parameters with very low odds ratios (AST and LDH). Only, the serum level of neopterin on the first day of hospitalization was associated with mortality ( Table 2).
Table 2.
Multivariate analysis of risk factors for mortality of CCHF patients
| Factors | OR | 95% CI interval | p |
|---|---|---|---|
| Serum neopterin levela (nmol/L) | 1.088 | 1.012–1.170 | 0.022 |
| Bleedinga (n) | 23.028 | 0.265–2003.313 | 0.169 |
| Platelet counta (/ml) | 0.966 | 0.904–1.031 | 0.296 |
OR: odds ratio.
On the first day of admission to the hospital.
ROC curves were generated to determine the value of neopterin on the first day of hospitalization as a mortality risk factor. AUC was 0.939 (p = 0.0001, 95% confidence interval (CI): 0.85–1.00). According to a cut-off value of 105 nmol/L, the negative and positive predictive values were 95.2% and 77.8%, respectively, with 77.8% sensitivity and 95.2% specificity.
Discussion
Although serum neopterin has been investigated in some studies with hemorrhagic fevers including Dengue fever17, 18 and Ebola hemorrhagic fever,19 it has not been studied in CCHF disease previously. Recently, Chan et al. studied neopterin concentrations in 110 patients with acute Dengue Fever (DF).17 They found higher neopterin concentrations in acute DF sera compared with those in measles and influenza and in healthy controls. Baize et al. studied inflammatory mediators in patients with Ebola hemorrhagic fever during two recent outbreaks in Gabon.19 They also found higher levels of serum neopterin concentrations compared to controls. In the current study, we found increased serum neopterin levels in CCHF patients after admission to hospital. Since the mean time for hospital admission was approximately same for both fatal (6 ± 5.8 days) and nonfatal (5.8 ± 3.0 days) groups in the current study, measurements ‘on the first day of admission of patients to the hospital’ were practical and appropriate to compare both groups. The mean serum neopterin concentration in CCHF patients exceeded that of the controls by approximately 15-fold. According to our knowledge, this is the first study in the literature to evaluate neopterin levels in CCHF patients. The very high serum neopterin levels may suggest that monocytes/macrophages are strongly activated during CCHF infection. Neopterin is mainly secreted by activated monocytes/macrophages in response to IFNγ.20
Serum neopterin levels have been studied in other viral diseases including severe acute respiratory syndrome (SARS),21 cytomegalovirus (CMV),22 rubella,23 measles and influenza infection.17 Our finding of increased serum level of neopterin in CCHF agrees with results from other viral infections such as Dengue fever, rubella, Ebola, measles and influenza.17, 18, 19, 23
The clinical significance of neopterin was also determined in hemorrhagic fevers including Dengue fever and Ebola virus infection.17, 18, 19 Higher increase of neopterin level in DF patients was associated with longer duration of fever and thus predicted the clinical course of the disease.17, 18 In Ebola infection, Baize et al. found low but significant levels of neopterin in survivors during the symptomatic phase and significantly high neopterin concentrations in fatalities.19 In the current study, we also analyzed the clinical significance of increased neopterin in early stage of hospitalization of CCHF patients. The mean concentration of neopterin in the patients with fatal CCHF disease was nearly threefold higher than that of nonfatal CCHF disease. The mean concentrations of neopterin before death in patients with fatal CCHF disease were even higher than the mean serum neopterin of the first day of hospitalization. However, the levels of neopterin decreased to lower levels in patients with nonfatal CCHF. This shows that those CCHF patients with lower neopterin concentrations are more likely to recover than others. High serum neopterin levels in fatal patients may reflect severe inflammation and intense activation of cell-mediated immune response in severe CCHF disease. Recently, in another study we found that viral load ≥1 × 109 RNA copies/ml could be considered to predict a fatal outcome in CCHF disease.24 Together with viral load, neopterin could be a measure of the severity of CCHF disease.
The overall fatality rate in CCHF disease was 17.6% in the current study. Our fatality rate is higher than the average for Turkey (5.4%). As ANERH is a referral and tertiary-care hospital, more complicated cases are transferred here from other hospitals.
In the studies analyzing the risk factors in CCHF, Swanepoel et al. studied the parameters during the first five days of illness. However, Ergonul et al. and Cevik et al. used highest and lowest levels of parameters in their studies.5, 6 In the current study, the parameters on the first day of admission were used to predict mortality. Different predictors of fatality were reported as risk factors for CCHF2, 5, 6 and those previously defined severity criteria were not completely consistent with each other. Among these parameters decreased platelet count, prolonged aPTT, elevated AST and ALT levels were common in the univariate analysis of three different studies. In multivariate analysis only ALT,6 and existence of somnolence, melena, prolonged aPTT and decreased platelet count5 were predictors of fatality. In the current study, bleeding, decreased platelet count, elevated neopterin, AST and LDH were identified as predictors of fatality in univariate analysis. In our multivariate analysis, only serum neopterin level was associated with fatal outcome. Further studies including larger number of patients are necessary to clarify these inconsistencies.
Conclusion
This is the first study of serum neopterin levels for CCHF disease. In the current study, we report that elevated serum neopterin levels can be detected in CCHF with higher levels in fatal cases. This finding suggests that monocytes/macrophages are strongly activated during CCHF disease.
References
- 1.Whitehouse C.A. Crimean–Congo hemorrhagic fever. Antiviral Res. 2004;64:145–160. doi: 10.1016/j.antiviral.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Swanepoel R., Gill D.E., Shepherd A.J., Leman P.A., Mynhardt J.H., Harvey S. The clinical pathology of Crimean–Congo hemorrhagic fever. Rev Infect Dis. 1989;11(Suppl. 4):S794–S800. doi: 10.1093/clinids/11.supplement_4.s794. [DOI] [PubMed] [Google Scholar]
- 3.Vorou R., Pierroutsakos I.N., Maltezou H.C. Crimean–Congo hemorrhagic fever. Curr Opin Infect Dis. 2007;20:495–500. doi: 10.1097/QCO.0b013e3282a56a0a. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Update: management of patients with suspected viral hemorrhagic fever – United States. MMWR Morb Mortal Wkly Rep. 1995 Jun 30;44(25):475–479. [PubMed] [Google Scholar]
- 5.Cevik MA, Erbay A, Bodur H, Gülderen E, Baştuğ A, Kubar A, et al. Clinical and laboratory features of Crimean–Congo haemorrhagic fever: predictors of fatality. Int J Infect Dis, in press, doi:10.1016/j.ijid.2007.09.010. [DOI] [PubMed]
- 6.Ergonul O., Celikbas A., Baykam N., Eren S., Dokuzoguz B. Analysis of risk-factors among patients with Crimean–Congo haemorrhagic fever virus infection: severity criteria revisited. Clin Microbiol Infect. 2006;12:551–554. doi: 10.1111/j.1469-0691.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- 7.Geisbert T.W., Jahrling P.B. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10:S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- 8.Schnittler H.J., Feldmann H. Viral hemorrhagic fever – a vascular disease? Thromb Haemost. 2003;89:967–972. [PubMed] [Google Scholar]
- 9.Papa A., Bino S., Velo E., Harxhi A., Kota M., Antoniadis A. Cytokine levels in Crimean–Congo hemorrhagic fever. J Clin Virol. 2006 Aug;36(4):272–276. doi: 10.1016/j.jcv.2006.04.007. [Epub 2006 Jun 9] [DOI] [PubMed] [Google Scholar]
- 10.Ergonul O., Tuncbilek S., Baykam N., Celikbas A., Dokuzoguz B. Evaluation of serum levels of interleukin (IL)-6, IL-10, and tumor necrosis factor-alpha in patients with Crimean–Congo hemorrhagic fever. J Infect Dis. 2006;1(193):941–944. doi: 10.1086/500836. [DOI] [PubMed] [Google Scholar]
- 11.Bray M. Comparative pathogenesis of Crimean–Congo hemorrhagic fever and Ebola hemorrhagic fever. In: Ergonul O., Whitehouse C.A., editors. Crimean–Congo hemorrhagic fever: a global perspective. Springer; Dordrecht (NL): 2007. pp. 221–231. [Google Scholar]
- 12.Ruokonen E., Ilkka L., Niskanen M., Takala J. Procalcitonin and neopterin as indicators of infection in critically ill patients. Acta Anaesthesiol Scand. 2002;46:398–404. doi: 10.1034/j.1399-6576.2002.460412.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann G., Wirleitner B., Fuchs D. Potential role of immune system activation-associated production of neopterin derivatives in humans. Inflamm Res. 2003;52:313–321. doi: 10.1007/s00011-003-1181-9. [DOI] [PubMed] [Google Scholar]
- 14.Yapar M., Aydogan H., Pahsa A., Besirbellioglu B.A., Bodur H., Basustaoglu A.C. Rapid and quantitative detection of Crimean–Congo hemorrhagic fever virus by one-step real-time reverse-transcriptase PCR. Jpn J Infect Dis. 2005;58:358–362. [PubMed] [Google Scholar]
- 15.Alrashed M., Abougoush M., Akgul E.O., Erbil M.K. Detection method of serum and urine neopterin levels by high performance liquid chromatography. Gulhane MJ. 2002;44:273–277. [in Turkish] [Google Scholar]
- 16.Swets J.A. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 17.Chan C.P., Choi J.W., Cao K.Y., Wang M., Gao Y., Zhou D.H. Detection of serum neopterin for early assessment of dengue virus infection. J Infect. 2006;53:152–158. doi: 10.1016/j.jinf.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babb K., Carrington C.V., Monteil M.A. A preliminary study of neopterin as a potential marker for severe dengue virus infection. Trans R Soc Trop Med Hyg. 1999;93:447–448. doi: 10.1016/s0035-9203(99)90155-4. [DOI] [PubMed] [Google Scholar]
- 19.Baize S., Leroy E.M., Georges A.J., Georges-Courbot M.C., Capron M., Bedjabaga I. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol. 2002;128:163–168. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs D., Hausen A., Reibnegger G., Werner E.R., Dierich M.P., Wachter H. Neopterin as a marker for activated cell-mediated immunity: application in HIV infection. Immunol Today. 1988;9:150–155. doi: 10.1016/0167-5699(88)91203-0. [DOI] [PubMed] [Google Scholar]
- 21.Zheng B.J., Cao K.Y., Chan C.P.Y., Choi J.W., Leung W., Leung M. Serum neopterin for early assessment of severity of severe acute respiratory syndrome. Clin Immunol. 2005;116:18. doi: 10.1016/j.clim.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jungraithmayr T.C., Reschke M., Grebe S.O., Lange H., Radsak K., Mueller T.F. Assessment of cytomegalovirus infections using neopterin and a new immunoblot. Clin Chim Acta. 2001;310:63–69. doi: 10.1016/s0009-8981(01)00528-9. [DOI] [PubMed] [Google Scholar]
- 23.Zaknun D., Weiss G., Glatzl J., Wachter H., Fuchs D. Neopterin levels during acute rubella in children. Clin Infect Dis. 1993;17:521–522. doi: 10.1093/clinids/17.3.521. [DOI] [PubMed] [Google Scholar]
- 24.Cevik M.A., Erbay A., Bodur H., Eren S.S., Akinci E., Sener K. Viral load as a predictor of outcome in Crimean–Congo hemorrhagic fever. Clin Infect Dis. 2007;45:96–100. doi: 10.1086/521244. [DOI] [PubMed] [Google Scholar]