Abstract
The giant panda (Ailuropoda melanoleuca) is an endangered species and indigenous to China. In mammals, multiple subtypes of interferon-α (IFN-α) exist, most of which possess antiviral activity. Little is known about giant panda IFN-α genes and the role they may play in giant panda immunological responses to viruses. We have cloned genes encoding 12 giant panda IFN-α (AmIFN-α or AmIFNA) subtypes that share from 90 to 99% amino acid sequence identity. AmIFN-α12 has one additional amino acid at position 57, which is not present in other subtypes. Sequence identity of the AmIFN-α proteins encoded by the 12 genes compared to human IFN-α2 is approximately 58%. Unlike most of the human subtypes, each of the 12 giant panda IFN sequences has an N-glycosylation recognition site. Expression of all 12 AmIFN-α subtypes in 293 cells was confirmed by SDS-PAGE and Western blotting analysis. The antiviral activity and antiproliferative activity of each AmIFN-α subtype produced in transiently transfected 293 cell cultures were tested in vitro. All AmIFN-α subtypes were found to be stable at pH 2 or 65 °C and to exhibit antiviral activity. Some IFN subtypes (AmIFN-α8 and AmIFN-α4) showed higher biological activity levels than others, whereas AmIFN-α11 exhibited lower activity. AmIFN-α had various antiproliferative activities to different target cells. To B16 cells, AmIFN-α3, AmIFN-α4, AmIFN-α8 had the highest activities, while to K562 cells, AmIFN-α3, AmIFN-α7, AmIFN-α10 had the highest activities. The various IFN-α subtypes displayed a good correlation between their antiviral and antiproliferative potencies.
Keywords: Giant panda (Ailuropoda melanoleuca), Interferon-α, Gene cloning, Expression, HEK293 cell, Antiviral activity, Antiproliferative activity
1. Introduction
Alpha interferons (IFN-α) are the first cytokines discovered. They are produced as a first line of defense against viral infection by inducing genes, including 2′,5′-oligoadenylate synthetase (2-5-OAS), RNase L, guanylate binding protein, MxA protein and the dsRNA-dependent protein kinase (PKR) (Samuel, 2001), which interfere with viral replication and activate natural killer cells. IFNs play an important role in the host antiviral response but are also recognized for their antiproliferative and immunomodulatory activities (Issacs and Lindenman, 1957, Fleischmann et al., 1998, Biron, 1998, Biron, 2001). They are encoded by an intronless multigene family, and widely distributed among the vertebrates, and share a same receptor. Multiple genes encoding IFN-α subtypes have been identified in several mammalian species, including human, equine, bovine, canine and murine species (Roberts et al., 1998). More than 10 different subtypes of IFN-α have been reported in mice and 15 or more subtypes in humans (Roberts et al., 1997, Roberts et al., 1998, Diaz et al., 1994).
The giant panda (Ailuropoda melanoleuca) is indigenous to China (Chen, 1997). Except for man's encroachment on the territories of giant panda, diseases are probably the greatest threats. Although effective measures have been carried out to protect giant panda in breeding and reproduction, prevention and control of infectious diseases, especially virus diseases, it is still an arduous and pressing task. Recently, canine distemper virus, canine coronavirus, canine adenovirus, minute virus, parainfluenza virus have been detected in giant panda (Tennant et al., 1993, He et al., 2000, Gao et al., 2003, Qiao et al., 2004). In China, Xia and He had separated canine distemper virus and coronavirus from liver of giant panda, respectively. Therefore, it is very important to study the immune system of giant panda, especially immune function genes. So we have cloned the genes coding for the IFN-α and expressed them in eukaryotic cells and tested their biological activities which could be attributable to enhance the survival ability of giant panda.
2. Materials and methods
2.1. Strains and vectors
Escherichia coli JM109 used in this study was kept in our lab. The pMDT-18 and pcDNA3.1 (+) vector were purchased from TaKaRa and Clontech, respectively.
2.2. Cell culture
Human embryonic kidney cells (293 cell), Hela cells, B16 melanoma cells, MDBK cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, and 100 μg/ml of streptomycin. K562 cells were cultured in RPMI 1640 (Gibco., USA) with 10% FBS.
2.3. Genomic DNA isolation and PCR cloning of giant panda IFN-α subtypes
Genomic DNA was extracted from liver sample of giant panda (natural death, Sichuan, Baoxing, China) by using DNA isolation kit (Takara) and used as template to clone giant panda IFN-α genes following polymerase chain reaction (PCR) strategy. The primers for giant panda (A. melanoleuca) IFN-α (AmIFN-α) were designed corresponding to conserved regions of carnivora. The sequences of forward and reverse primers were 5′-ATGGC (CG) CTGCCCT (CG) (TC) TCCTTCTTGGTG-3′ and 5′-TCATTTCT (CT) (GC) CTCCTA (ACG) TCTTTCTTGC-3′, respectively. All primers used for PCR and sequencing were made by Sangon Inc., China.
Amplification of giant panda genomic IFN-α was carried out by PCR in a final volume of 50 μl containing 1× buffer, 0.2 μM of each dNTPs, 500 nM primers, 200 ng of DNA, and 1 U/ml Pfu high fidelity DNA polymerase (Takara). The amplification profile included one initial denaturation step at 94 °C for 5 min, then 30 cycles using the following conditions: 94 °C for 45 s, 58 °C for 45 s, and 72 °C for 1 min, and a final extension at 72 °C for 10 min.
2.4. Cloning and sequencing of AmIFN-α genes
PCR products from 1% agarose gel were purified with a recover kit (Omega, China) and ligated into pMDT-18 vector. The ligated products were proliferated in Escherichia coli JM109 cells and the resulted plasmids were followed PCR verification and enzymatic digestion with EcoRI and HindIII (TaKaRa, China).
2.5. Expression of AmIFN-α genes in mammalian cells
Coding fragments of the AmIFN-α subtype clones were sub-cloned into eukaryotic expression vector pcDNA3.1 (+) as followings. The plasmid of each IFN-α subtype was used as template for PCR with the primers containing BamHI and XhoI restriction sites at the 5′ and 3′ ends, respectively. The sequences of the forward and reverse primers were 5′-CGGGATCC AACATGGCGCTGCCCT (GC) (TC) TCCTT-3′ and 5′-CCGCTCGAGTCATTTCT (TC) (GC) CTCCTTAGTCTT-3′, respectively. AACATG is a bona fide Kozak consensus sequence for translation. The PCR conditions included a 5 min denaturation step at 94 °C, followed by 30 cycles of 45 s at 94 °C, 45 s at 58 °C, and 1 min at 72 °C. A final extension for 10 min at 72 °C was then performed. The resulted fragments were purified, digested with BamHI and XhoI and ligated into the corresponding cloning sites of pcDNA3.1 (+) vector. Every recombinant plasmid was confirmed by sequencing and named as pAmIFN-α1, pAmIFN-α2, pAmIFN-α3, pAmIFN-α4, pAmIFN-α5, pAmIFN-α6, pAmIFN-α7, pAmIFN-α8, pAmIFN-α9, pAmIFN-α10, pAmIFN-α11, and pAmIFN-α12. Minipreparations of the plasmids were obtained using the endo-free plasmid mini kit (Omega).
Human embryonic kidney cells (HEK293 cell) were seeded at 2 × 105 cells/100 mm dish in DMEM, and incubated at 37 °C in a CO2 incubator until the cells reached approximately 50–80% confluence. AmIFN-α plasmid DNA (2 μg) was diluted in 100 μl serum-free and antibiotic-free DMEM while mixed gently. Six microliters of liposome sofast (Xiame, China) were mixed gently with 94 μl serum-free and antibiotic-free DMEM and then the mixture was added to 100 μl DNA solutions drop wise with vortex. The mixtures were incubated at room temperature (RT) for 20 min. The DNA–liposome complexes were overlaid on top of the cells and incubated at 37 °C for additional 72 h.
2.6. SDS-PAGE and Western blotting analysis
SDS-PAGE was performed with 5% stacking gel and 12% (v/v) resolving gel and the proteins were visualized by silver staining. After SDS-PAGE, the proteins were transferred onto the nitrocellulose membrane from gel without staining. The membrane was blocked with 3% BAS in TBS (2 M Tris–HCl, pH 7.5, 10 mM NaCl) at room temperature for 2 h. The blot was washed 3× 10 min with TBS, followed by an overnight incubation with a mouse anti-human IFN-α monoclonal (Abcam) that was at diluted 1:100 in blocking solution (TBS with 0.5% BSA). After washing 3× 10 min with TBS, the blot was incubated with a goat anti-mouse IgG conjugated to horseradish peroxidase in TBS with 0.5% BSA (diluted 1:5000). Finally, nitrocellulose sheets were washed (3× 10 min) with TBS (Briones and Maria, 2003). Blots were incubated with this solution for 1–3 min. The reaction was stopped with tap water, and the blots were dried with paper towels and stored away from light at room temperature.
2.7. pH and thermo stability assay
Supernatants collected from transfected 293 cells were treated at pH 2 for 24 h at 4 °C, or for 1 h at 65 °C, respectively. Antiviral activities were compared between treated and untreated samples.
2.8. Cytopathic effect (CPE) reduction assay
The antiviral activity of AmIFN-α was determined in vitro by utilizing a standard VSV assay as previously described with minor changes (Vogel et al., 1991, Wonderling et al., 2002). The antiviral activity of AmIFN-α was assayed in a cytopathic effect reduction assay using MDBK cells challenged with vesicular stomatitis virus (VSV) in 96-well microtiter plates. Briefly, MDBK cells were seeded into 96-well plate in a concentration of 5 × 104 cells/ml. After 24 h, serial dilutions containing IFN-α supernatants were added to 96-well plates, and each sample had three replicates. After cells were incubated for 24 h, the supernatants were removed and the cells were infected with VSV at 4 × 104 plaque forming unit (pfu/ml). After 24 h, cells were washed three times with PBS and stained with methyl violet solution. The cells were then washed with deionized water and allowed to dry. The methyl violet was eluted with 100 ml of methanol and absorbance was measured at 570 nm on a Microplate Reader 3550 (Bio-Rad, USA). The activity of giant panda interferon-α was shown as the reciprocal of the dilution leading to 50% cytopathic effect. The supernatants of pcDNA3.1 (+) and purified human IFN-α2b (Zhongshan, China) were as negative control and positive control, respectively. These activities were normalized to the activity calculated for AmIFN-α6 in the same experiment and corrected for the quantification of the amount of IFN measured in the supernatants.
2.9. Antiproliferative activity assay
The antiproliferative activity of AmIFN-α was determined as previously described (Van Pesch et al., 2004) with minor changes. B16 melanoma and K562 cells were seeded in 96-well plates at a density of 1000 cells/well, respectively. After 24 h, serial dilutions of the IFN-containing supernatants (100 μl) were added to the cells. After incubation for 72 h, the cell density were quantified by using the WST-1 cell proliferation reagent (Biorot, Nanjing, China). Briefly, 10 μl of WST-1 (10 mg/ml) were added per well and incubated for 2 h at 37 °C. Absorbance was read at 450 nm. The activity of AmIFN-α was shown as the reciprocal of the dilution leading to 50% inhibition of cell proliferation. The supernatants of pcDNA3.1 (+) and purified human IFN-α2b (Zhongshan, China) were as negative control and positive control, respectively. For each independent experiment, the assay was performed three times. Activity is expressed relative to that of IFN-α6 and corrected for the quantification of the amount of IFN measured in the supernatants.
2.10. Nucleotide sequence accession numbers
The GenBank accession numbers for AmIFN-α nucleotide sequences are as follows: DQ392967 (AmIFN-α1), DQ392968 (AmIFN-α2), DQ392970 (AmIFN-α3), DQ392969 (AmIFN-α4), DQ392972 (AmIFN-α5), DQ392971 (AmIFN-α6), DQ392974 (AmIFN-α7), DQ392973 (AmIFN-α8), DQ392975 (AmIFN-α9), DQ392976 (AmIFN-α10), DQ392977 (AmIFN-α11), DQ392978 (AmIFN-α12) and DQ392978 (AmIFN-α pseudogene).
3. Results
3.1. Cloning and sequence analysis of giant panda IFN-α genes
The genes encoding AmIFN-α subtypes were cloned by PCR from giant panda DNA. The primers for AmIFN-α were designed based on the conserved regions of carnivora IFN-α genes. PCR products were cloned into a TA cloning vector and the cloned fragments were sequenced. DNA sequence analyses indicated that there were 12 subtypes of AmIFN-α genes and one IFN-α pseudogene, termed AmIFN-α1–AmIFN-α12 and AmIFN-α pseudogene, respectively. The AmIFN-α pseudogene, had changes in nucleotides at 23 and 74 which leads to a stop codon at amino acid position 24.
Comparison of the AmFN-α subtype sequences demonstrated that homologies of nucleotides and amino acids approximate were 94–99 and 90–99%, respectively (Fig. 1 and Table 1 ). All deduced AmIFN-α sequences contained an N-terminal secretory signal sequence from residue 1–23 and a putative N-glycosylation site at position 102–104. The predicted length of the mature polypeptides contained either 165 (AmIFN-α1–AmIFN-α11) or 166 (AmIFN-α12) amino acid residues. AmIFN-α12 contained one more amino acid residue at position 54 (Lys). The predicted molecular mass of the protein with signal peptide was 20 kDa. Each of the deduced mature sequences contained six cysteine residues. The cysteine residues at positions 1, 29, 99 and 137 were very similar to the highly conserved cysteines in other mammalian IFN-α proteins at positions 1, 29, 99, and 139 (Roberts et al., 1997), suggesting a common tertiary structure. Five proline residues were conserved among all of the giant panda IFN-α sequences at the positions of 4, 25, 39, 111, and 142, which correspond to the conserved positions of 4, 26, 39, 110, and 139 in other mammalian IFN-α protein (Roberts et al., 1997).
Fig. 1.

Amino acid sequence homology among giant panda IFN-α subtypes. Consensus sequences are shown as AmIFN-α1. Identical amino acids are indicated by dots. Residues 1–23 are signal sequence.
Table 1.
Homology (%) of amino acids and nucleotides among AmIFN-α subtypes
| IFN-α1 | IFN-α2 | IFN-α3 | IFN-α4 | IFN-α5 | IFN-α6 | IFN-α7 | IFN-α8 | IFN-α9 | IFN-α10 | IFN-α11 | IFN-α12 | IFN pseudogene | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-α1 | – | 99.3 | 99.5 | 99.1 | 97.7 | 99.5 | 98.8 | 99.1 | 99.5 | 99.5 | 95.9 | 95.8 | 98.9 |
| IFN-α2 | 99.7 | – | 99.5 | 99.1 | 97.7 | 99.5 | 98.8 | 98.9 | 99.1 | 99.6 | 95.6 | 95.6 | 98.9 |
| IFN-α3 | 98.9 | 99.5 | – | 99.6 | 97.9 | 99.6 | 98.9 | 99.1 | 98.9 | 99.6 | 95.7 | 95.6 | 99.1 |
| IFN-α4 | 98.0 | 98.3 | 99.5 | – | 97.9 | 99.3 | 99.3 | 99.1 | 99.3 | 99.3 | 96.1 | 95.6 | 98.8 |
| IFN-α5 | 95.9 | 95.2 | 95.2 | 95.2 | – | 98.2 | 97.9 | 98.1 | 97.5 | 97.9 | 94.7 | 97.0 | 97.3 |
| IFN-α6 | 98.9 | 99.7 | 98.9 | 98.0 | 96.6 | – | 98.9 | 99.1 | 98.9 | 99.6 | 95.7 | 95.9 | 99.1 |
| IFN-α7 | 97.9 | 98.0 | 97.9 | 98.0 | 96.6 | 97.9 | – | 98.8 | 99.3 | 98.9 | 95.7 | 95.9 | 98.8 |
| IFN-α8 | 98.9 | 98.0 | 97.9 | 98.0 | 96.3 | 97.6 | 97.6 | – | 99.1 | 99.1 | 96.3 | 95.8 | 98.6 |
| IFN-α9 | 98.3 | 98.0 | 97.9 | 98.4 | 95.9 | 97.6 | 98.3 | 98.3 | – | 99.1 | 96.1 | 95.8 | 98.8 |
| IFN-α10 | 98.3 | 99.7 | 98.9 | 98.0 | 95.9 | 98.3 | 97.6 | 97.6 | 97.6 | – | 95.8 | 95.9 | 99.1 |
| IFN-α11 | 93.8 | 93.5 | 92.5 | 93.5 | 90.1 | 92.1 | 94.5 | 94.5 | 93.6 | 92.1 | – | 94.4 | 95.2 |
| IFN-α12 | 90.4 | 91.0 | 90.4 | 91.0 | 91.0 | 91.5 | 92.3 | 90.4 | 91.5 | 91.5 | 88.3 | – | 95.4 |
“–” same sequence. The upper line shows identities at nucleotide level; the lower line shows identities at the amino acid level. The sequences for alignment were from the following GenBank accession numbers: IFN-α1 (AmIFN-α1, DQ392967), IFN-α2 (AmIFN-α2, DQ392968), IFN-α3 (AmIFN-α3, DQ392970), IFN-α4 (AmIFN-α4, DQ392969), IFN-α5 (AmIFN-α5, DQ392972), IFN-α6 (AmIFN-α6, DQ392971), IFN-α7 (AmIFN-α7, DQ392974), IFN-α8 (AmIFN-α8, DQ392973), IFN-α9 (AmIFN-α9, DQ392975), IFN-α10 (AmIFN-α10, DQ392976), IFN-α11 (AmIFN-α11, DQ392977), IFN-α12 (AmIFN-α12, DQ392978) and IFN-α pseudogene (AmIFN-α pseudogene, DQ392978).
Comparison of the AmIFN-α gene sequences to feline IFN-α sequences demonstrated homologies of approximately 72%, respectively. Comparison of the amino acid sequences of all giant panda subtypes with huIFN-α2 (J00207) and huIFN-α1 (M11003) showed approximate homologies of 56–59 and 58–60%, respectively (Fig. 2 ). Homologies of the dog IFN-α6 (NP_001007129) and mouse IFN-α7 (NP_032360) amino acid sequences compared with the giant panda subtypes were approximately 67–71 and 49–53%, respectively.
Fig. 2.
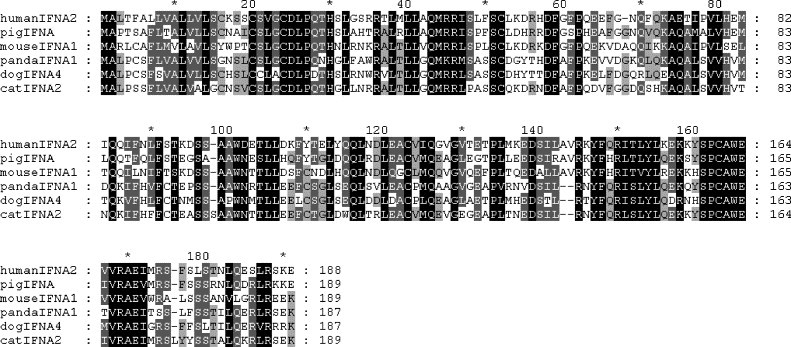
Alignment of the deduced amino acid sequences of IFNA (IFN-α) among giant panda and other animals. The sequences for alignment were from the following GenBank accession numbers: BC074937 (humanIFNA2), AY526089 (pigIFNA), AY225950 (mouseIFNA1), DQ392967 (AmIFNA1), XP_537941 (dogIFNA4) and AY117394 (catIFNA2). Identical residues were in dark grey and similar residues were in light grey. Residues 1–23 were signal sequence.
To better determine the evolutionary position of AmIFN-α, a phylogenetic tree was constructed. All AmIFN-α genes clustered in the same group (Fig. 3 ). AmIFN-α clustered with interferon from other mammalian, such as cat and dog interferon, originating an in-group with respect to other mammalian species.
Fig. 3.
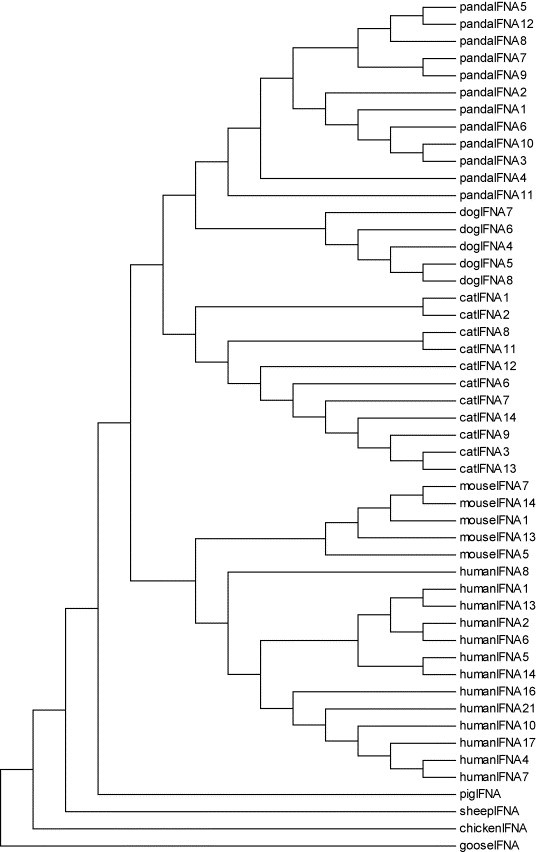
Phylogenetic tree based on nucleotide sequences of giant panda interferon-α and type I IFN from different species by the maximum parsimony method. GenBank accession numbers of sequences used in the tree: XP_537941 (dogIFNA4), AB125934 (dogIFNA5), NM_001007128 (dogIFNA6), NM_001006654 (dogIFNA7) and NM_001007130 (dogIFNA8); AY117395 (catIFNA1), AY117394 (catIFNA2), AY117394 (catIFNA3), AY117391 (catIFNA6), AB094996 (catIFNA7), AB094997 (catIFNA8), AB095000 (catIFNA11), AB095001 (catIFNA12) and AB095002 (catIFNA13); AY225950 (mouseIFNA1), NM_010505 (mouseIFNA5), NM_008334 (mouseIFNA7), AY190047 (mouseIFNA13) and NM_206975 (mouseIFNA14); BC074928 (humanIFNA1), BC074937 (humanIFNA2), BC074965 (humanIFNA4), NM_002169 (humanIFNA5), NM_021002 (humanIFNA6), BC074992 (humanIFNA7), NM_002170 (humanIFNA8), NM_002171 (humanIFNA10), NM_006900 (humanIFNA13), NM_002172 (humanIFNA14), NM_002173 (humanIFNA16), NM_021268 (humanIFNA17) and NM_002175 (humanIFNA21); AY526089 (pigIFNA); AY802984 (sheepIFNA); AB0021154 (chickenIFNA); AY524422 (gooseIFNA).
3.2. Expression of the AmIFN-α genes in 293 cells
The coding sequence of each AmIFN-α was sub-cloned into a eukaryotic expression vector pcDNA3.1 (+), the recombinant plasmids and pcDNA3.1 vector were used to transfect into HEK293 cells. Supernatants obtained from AmIFN-α transiently transfected 293 cells were concentrated 10-fold using acetone. Recombinant protein was visualized on SDS-PAGE by silver staining. Western blotting analysis of the supernatants using monoclonal antibody against huIFN-α indicated that all 12 subtypes of AmIFN-α genes were expressed and secreted (Fig. 4 ).
Fig. 4.
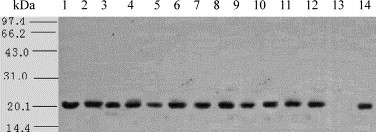
Western blotting of AmIFN-α subtypes in 293 cells. Lanes 1–12: AmIFN-α1–AmIFN-α12; lane 13: pcDNA3.1 (+) vector; lane 14: human IFN-α2b.
3.3. Antiviral activities of the IFN subtypes
The relative amounts of the different IFN subtypes in the supernatants were quantified by PhosphorImager after SDS-PAGE. The antiviral activities of these supernatants were determined against VSV. The measured antiviral activities were then corrected for the amount of IFN to calculate the relative antiviral activities of the various IFN subtypes. No measurable activity could be detected in the supernatants from cells transfected with the vector alone. All IFN-α subtypes displayed antiviral activities, but different ones had various antiviral activities (Fig. 5 ). For example, AmIFN-α3, AmIFN-α4 and AmIFN-α8 showed the highest antiviral potencies, four-fold activity levels compared to AmIFN-α6, whereas AmIFN-α2, AmIFN-α5, AmIFN-α7 and AmIFN-α11 displayed lower activity than AmIFN-α6.
Fig. 5.
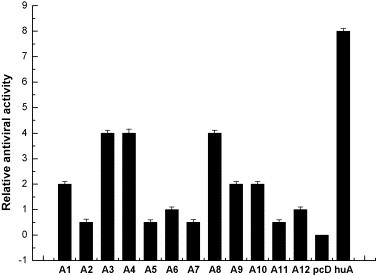
Relative antiviral activity of AmIFN-α subtypes. The activities were normalized to the activity calculated for AmIFN-α6 (that is set as 1 to represent the reciprocal of the dilution leading to 50% cytopathic effect) in the same experiment. The relative antiviral activity of each AmIFN-α subtype was the mean value of three replicates. Standard deviation was the statistics value of three replicates. A1: pAmIFN-α1; A2: pAmIFN-α2; A3: pAmIFN-α3; A4: pAmIFN-α4; A5: pAmIFN-α5; A6: pAmIFN-α6; A7: pAmIFN-α7; A8: pAmIFN-α8; A9: pAmIFN-α9; A10: pAmIFN-α10; A11: pAmIFN-α11; A12: pAmIFN-α12; pcD: pcDNA3.1 (+) vector; huA: human IFN-α2b.
We tested the stability of 12 subtypes of AmIFN-α at pH 2 and 65 °C, respectively. Therefore, the supernatants containing IFN-α were treated at pH 2 or 65 °C, and then used in the antiviral assay in parallel with untreated supernatants. The results showed that all AmIFN-α were stable at pH 2 for 24 h or 65 °C for 1 h because no loss in antiviral activity was detected in treated and untreated samples (data not shown).
3.4. Antiproliferative activity
To determine whether some of the AmIFN-α subtypes evolved to acquire distinct biological functions, the relative antiproliferative activities of the different AmIFN-α subtypes were also determined by assaying the growth inhibition of B16 murine melanoma cells and K562 cells. Results presented in Fig. 6, Fig. 7 show the antiproliferative potencies of the different subtypes.
Fig. 6.
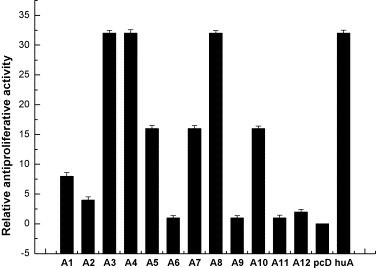
Relative antiproliferative activity of AmIFN-α subtypes to B16 cells. The activities were normalized to the activity calculated for AmIFN-α6 (that is set as 1 to represent the reciprocal of the dilution leading to 50% inhibition of cell proliferation) in the same experiment. The relative antiviral activity of each AmIFN-α subtype was the mean value of three replicates. Standard deviation was the statistics value of three replicates. A1: pAmIFN-α1; A2: pAmIFN-α2; A3: pAmIFN-α3; A4: pAmIFN-α4; A5: pAmIFN-α5; A6: pAmIFN-α6; A7: pAmIFN-α7; A8: pAmIFN-α8; A9: pAmIFN-α9; A10: pAmIFN-α10; A11: pAmIFN-α11; A12: pAmIFN-α12; pcD: pcDNA3.1 (+) vector; huA: human IFN-α2b.
Fig. 7.
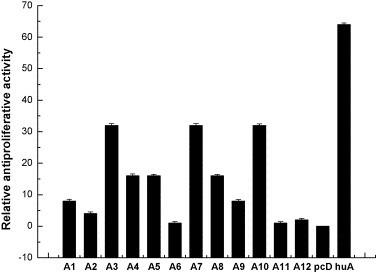
Relative antiproliferative activity of AmIFN-α subtypes to K562 cells. The activities were normalized to the activity calculated for AmIFN-α6 (that is set as 1 to represent the reciprocal of the dilution leading to 50% inhibition of cell proliferation) in the same experiment. The relative antiviral activity of each AmIFN-α subtype was the mean value of three replicates. Standard deviation was the statistics value of three replicates. A1: pAmIFN-α1; A2: pAmIFN-α2; A3: pAmIFN-α3; A4: pAmIFN-α4; A5: pAmIFN-α5; A6: pAmIFN-α6; A7: pAmIFN-α7; A8: pAmIFN-α8; A9: pAmIFN-α9; A10: pAmIFN-α10; A11: pAmIFN-α11; A12: pAmIFN-α12; pcD: pcDNA3.1 (+) vector; huA: human IFN-α2b.
To B16 murine melanoma cells (Fig. 6), AmIFN-α3, AmIFN-α4 and AmIFN-α8 showed the highest antiproliferative potencies, 32-fold activity higher than AmIFN-α6, whereas AmIFN-α5, AmIFN-α7 and AmIFN-α10 showed 16-fold activity higher than AmIFN-α6. AmIFN-α9 and AmIFN-α11 displayed the same activity as AmIFN-α6.
To K562 cells (Fig. 7), AmIFN-α3, AmIFN-α7 and AmIFN-α10 showed the highest antiproliferative potencies, 32-fold activity higher than AmIFN-α6, whereas AmIFN-α4, AmIFN-α5 and AmIFN-α8 showed 16-fold activity higher than AmIFN-α6. AmIFN-α11 displayed the same activity as AmIFN-α6.
4. Discussion
In this report, we describe the cloning of genes encoding 12 giant panda type I IFN proteins. To confirm that sequences existed in the giant panda were not generated during either the PCR amplification or sequencing process, or both, two independent PCRs and sequencing were conducted. These clones were sequenced bidirectionally at least twice, and the consistent results from more than two positive clones were regarded as one subtype.
All AmIFN-α genes are intronless. Similar to other mammalian type I IFN subtypes (Wonderling et al., 2002, Nagai et al., 2004, Nakamura et al., 1992), the differences among 12 giant panda IFN-α protein sequences are based on a small number of amino acid changes. The differences observed among the AmIFN-α products are the length of the proteins, which has been reported for other species, such as the mouse, pig, and horse, but not for human IFN-α, which has the same size in different subtypes. This size variation, where described, has been related to deletions or insertions in the nucleotide sequence.
Multiple IFN-α genes have been detected in many species (Velan et al., 1985, Harrison et al., 2004, Henco et al., 1985, Goeddel et al., 1981, Chaplin et al., 1996). It was found that giant panda IFN-α has 12 subtypes. The woodchuck IFN-α proteins are encoded by a multigenic family composed of at least 10 subtypes (Berraondo et al., 2002). The reason for this redundancy remains unknown. Type I IFNs compete for common binding sites on human cells (Meager, 1998) and are believed to share a common receptor (Kunzi and Rowe, 2001). However, it seems that the different IFN-α subtypes, although sharing some activities, have different functional profiles. Within the IFN-α subfamily, genes are clustered by species, suggesting the possibility that IFN-α genes have been duplicated independently. In the case of IFN-α, there is evidence that the IFN subfamily may have diversified adaptively in mammals. Existence of so many IFN-α subtypes raises the question regarding whether there are biological differences among these proteins. Although IFN-α subtype proteins from the same species are extremely homologous, differences in biological potency are often observed (Ortaldo et al., 1984, Mouchel-Vielh et al., 1992, Foster et al., 1996, Yeow et al., 1998, Baldwin et al., 2004). Different subtypes of huIFN-α reveal various antiviral activities. HuIFN-α8 was reportedly the most potent while huIFN-α1 provided the least amount of antiviral activity (Foster et al., 1996). Several authors have reported that muIFN-α4 was 5–10 times more active than muIFN-α1 (Van Heuvel et al., 1986, Van Heuvel et al., 1988).
To compare the properties of the different AmIFN-α subtypes, the recombinant plasmids expressing the various IFN-α subtypes were transiently transfected in 293 cells in parallel with the vector pcDNA3.1. Supernatants from transfected 293 cells were collected and analyzed. We demonstrated antiviral and antiproliferative activities of all AmIFN-α subtypes, and observed that different subtypes had different activities. As reported for human IFN-α subtypes, the differences in activities might be concerned with differences in receptor binding affinities (Aguet et al., 1984, Meister et al., 1986, Piehler and Schreiber, 1999, Yamaoka et al., 1999). Maybe subtle modification in the protein structure accounts for the variation in biological activities. As the activity of mouse IFN-α (Van Pesch et al., 2004), our results showed a good correlation between the antiviral and antiproliferative potencies of the IFN subtypes.
In conclusion, this is the first report of the cloning and characterization of the giant panda IFN-α family with facilitates the way for systematic studies of the biological and therapeutic effects of this cytokine. AmIFN-α is likely to be efficient against both chronic viral infections and neoplastic diseases that affect the giant panda population. It will be significant importance for further studies to protect this endangered species.
Acknowledgment
The authors would like to thank Dr. Feng for his kind help with checking the language in the paper.
References
- Aguet M., Grobke M., Dreiding P. Various human interferon-alpha subclasses cross-react with common receptors: their binding affinities correlate with their specific biological activities. Virology. 1984;132:211–216. doi: 10.1016/0042-6822(84)90105-3. [DOI] [PubMed] [Google Scholar]
- Baldwin S.L., Powell T.D., Sellins K.S., Radecki S.V., Cohen J.J., Milhausen M.J. The biological effects of five feline IFN-α subtypes. Vet. Immunol. Immunopathol. 2004;99:153–167. doi: 10.1016/j.vetimm.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Berraondo P., Garcia-Navarro R., Gonzalez-Aseguinolaza G., Vales A., Blanco-Urgoiti B., Larrea E., Riezu-Boj J.I., Prieto J., Ruiz J. The woodchuck interferon-alpha system: cloning, family description, and biologic activity. J. Med. Virol. 2002;68(3):424–432. doi: 10.1002/jmv.10221. [DOI] [PubMed] [Google Scholar]
- Biron C.A. Role of early cytokines, including α and β interferons (IFN-α/β), in innate and adaptive immune respones to viral infections. Semin. Immunol. 1998;5:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- Biron C.A. Interferons alpha and beta as immune regulators—a new look. Immunity. 2001;14:661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- Briones P., Maria A.V. Western blotting with diaminobenzidine detection for the diagnosis of congenital disorders of glycosylation. J. Neurosci. Methods. 2003;125:167–171. doi: 10.1016/s0165-0270(03)00051-7. [DOI] [PubMed] [Google Scholar]
- Chaplin P.J., Entrican G., Gelder K.I., Collins R.A. Cloning and biologic activities of a bovine interferon-alpha isolated from the epithelium of a rotavirus-infected calf. J. Interferon Cytokine Res. 1996;16(1):25–30. doi: 10.1089/jir.1996.16.25. [DOI] [PubMed] [Google Scholar]
- Chen D.Y. Active salvage of the giant panda won studying its reproductive biology in China. Life Sci. 1997;9:31–33. (in Chinese) [Google Scholar]
- Diaz M.O., Pomykala H.M., Bohlander S.K., Maltepe E., Malik K., Brownstein B., Olopade O.I. Structure of the human type-I interferon gene cluster determined from YAC clonecontig. Genomics. 1994;22:540–552. doi: 10.1006/geno.1994.1427. [DOI] [PubMed] [Google Scholar]
- Fleischmann W.R., Masoor J., Wu T.Y., Fleischmann C.M. Orally administered IFN-alpha acts alone and in synergistic combination with intraperitoneally administered IFN-gamma to exert an antitumor effect against B16 melanoma in mice. J. Interferon Cytokine Res. 1998;18:17–20. doi: 10.1089/jir.1998.18.17. [DOI] [PubMed] [Google Scholar]
- Foster G.R., Rodrigues O., Ghouze F., Schulte-Frohlinde E., Testa D., Liao M.J., Stark G.R., Leadbeater L., Thomas H.C. Different relative activities of human cell-derived interferon-α subtypes: IFN-α8 has very high antiviral potency. J. Interferon Cytokine Res. 1996;16:1027–1033. doi: 10.1089/jir.1996.16.1027. [DOI] [PubMed] [Google Scholar]
- Gao F.S., Hu G.X., Xia X.Z., Po Y.T., Yu C., Qiao J. Diagnosed the combined infection of canine distemper and canine coronavirus of giant panda by united PCR. Acta Jilin Agric. Univ. 2003;25(1):91–93. (in Chinese) [Google Scholar]
- Goeddel D.V., Leung D.W., Dull T.J., Gross M., Lawn R.M., McCandliss R., Seeburg P.H., Ullrich A., Yelverton E., Gray P.W. The structure of eight distinct cloned human leukocyte interferon cDNAs. Nature. 1981;290(5801):20–26. doi: 10.1038/290020a0. [DOI] [PubMed] [Google Scholar]
- Harrison G.A., McNicol K.A., Deane E.M. Interferon alpha/beta genes from a marsupial, Macropus eugenii. Dev. Comp. Immunol. 2004;28(9):927–940. doi: 10.1016/j.dci.2004.02.002. [DOI] [PubMed] [Google Scholar]
- He H.B., Li J.Z., Xia X.Z., Yu C., Fan Q.S., Huang G., Qiu W., Yen Z. Nucleotide seqnecnce analysis of the haemagglutinin protein gene of giant panda strain of canine distemper virus. Virol. Sin. 2000;15(3):291–296. (in Chinese) [Google Scholar]
- Henco K., Brosius J., Fujisawa A., Fujisawa J.I., Haynes J.R., Hochstadt J., Kovacic T., Pasek M., Schambock A., Schmid J. Structural relationship of human interferon alpha genes and pseudogenes. J. Mol. Biol. 1985;185(2):227–260. doi: 10.1016/0022-2836(85)90401-2. [DOI] [PubMed] [Google Scholar]
- Issacs D., Lindenman J. Virus interference. 1. The interferon. Proc. R. Soc. Lond. B. 1957;147:258–273. [Google Scholar]
- Kunzi M.S., Rowe P.P. IFNα, IFNβ, IFNγ ligands. In: Oppenheim J.J., Feldman M., Duram S.K., Hirano T., Vilcek J., Nicola N.A., editors. Cytokine Reference, vol. 1, Ligands. Academic Press; California: 2001. pp. 627–639. [Google Scholar]
- Meager A. Interferons alpha, beta, and omega. In: Mire-Sluis A., Thorpe R., editors. Cytokines. Academic Press; California: 1998. pp. 361–389. [Google Scholar]
- Meister A., Uze G., Mogensen K.E., Gresser I., Tovey M.G., Grutter M., Meyer F. Biological activities and receptor binding of two human recombinant interferons and their hybrids. J. Gen. Virol. 1986;67:1633–1643. doi: 10.1099/0022-1317-67-8-1633. [DOI] [PubMed] [Google Scholar]
- Mouchel-Vielh E., Lutfalla G., Mogensen K.E., Uze G. Specific antiviral activities of the human alpha interferons are determined at the level of receptor (IFNAR) structure. FEBS Lett. 1992;313:255–259. doi: 10.1016/0014-5793(92)81204-y. [DOI] [PubMed] [Google Scholar]
- Nagai A., Taira O., Ishikawam M., Hiramatsum K., Hohdatsum T., Koyamam H., Araim S., Satom H., Nakanom K., Maehara N. Cloning of cDNAs encoding multiple subtypes of feline interferon-alpha from the feline epitherial cell line. J. Vet. Med. Sci. 2004;66(6):725–728. doi: 10.1292/jvms.66.725. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Sudo T., Matsuda S., Yanai A. Molecular cloning of feline interferon cDNA by direct expression. Biosci. Biotechnol. Biochem. 1992;56:211–214. doi: 10.1271/bbb.56.211. [DOI] [PubMed] [Google Scholar]
- Ortaldo J.R., Herbermann R.B., Harvey C., Osheroff P., Pan Y.C.E., Kelder B., Pestka S. A species of human α interferon that lacks the ability to boost human natural killer activity. Proc. Natl. Acad. Sci. U.S.A. 1984;81:4926–4929. doi: 10.1073/pnas.81.15.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehler J., Schreiber G. Mutational and structural analysis of the binding interface between types I interferons and their receptor IFNAR2. J. Mol. Biol. 1999;294:223–237. doi: 10.1006/jmbi.1999.3230. [DOI] [PubMed] [Google Scholar]
- Qiao J., Xia X.Z., Hu G.X., Qi R.L., Xie Z.J., Rui F., Yang S.T., Huang G. Cloning, sequence analysis and expression in Escherichia coli of nucleoprotein gene of canine coronavirus giant panda isolate. Acta Theriol. Sin. 2004;24(3):248–253. (in Chinese) [Google Scholar]
- Roberts R.M., Liu L., Alexenko A. New and atypical families of type 1 interferons in mammals: comparative functions, structures, and evolutionary relationships. Prog. Nucl. Acid Res. Mol. Biol. 1997;56:287–325. doi: 10.1016/s0079-6603(08)61008-9. [DOI] [PubMed] [Google Scholar]
- Roberts R.M., Liu L., Guo Q., Leaman D., Bixby J. The evolution of the type I interferons. J. Interferon Cytokine Res. 1998;18:805–816. doi: 10.1089/jir.1998.18.805. (published erratum appears in J. Interferon Cytokine Res 19 (4) (1999) 427) [DOI] [PubMed] [Google Scholar]
- Samuel C.E. Antiviral actions of interferons. Clinc. Microbial. Rev. 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant B.J., Gskell R.M., Jones R.C., Gaskell C.J. Studies on the epizootiology of canine coxonavixus. Vet. Rec. 1993;132(2):7–11. doi: 10.1136/vr.132.1.7. [DOI] [PubMed] [Google Scholar]
- Van Heuvel M., Bosveld I.J., Mooren A.A., Trapman J., Zwarthoff E.C. Properties of natural and hybrid murine alpha interferons. J. Gen. Virol. 1986;67:2215–2222. doi: 10.1099/0022-1317-67-10-2215. [DOI] [PubMed] [Google Scholar]
- Van Heuvel M., Bosveld I.J., Klaassen P., Zwarthoff E.C., Trapman J. Structure–function analysis of murine interferon-alpha: antiviral properties of novel hybrid interferons. J. Interferon Res. 1988;8:5–14. doi: 10.1089/jir.1988.8.5. [DOI] [PubMed] [Google Scholar]
- Van Pesch V., Lanaya H., Renauld J.C., Michiels T. Characterization of the murine alpha interferon gene family. J. Virol. 2004;78(15):8219–8228. doi: 10.1128/JVI.78.15.8219-8228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velan B., Cohen S., Grosfeld H., Leitner M., Shafferman A. Bovine interferon alpha genes, structure and expression. J. Biol. Chem. 1985;260:5498–5504. [PubMed] [Google Scholar]
- Vogel S.N., Friedman R.M., Hogan M.M. Measurement of antiviral activity induced by interferons α, β and γ. In: Coligan J.E., Kruisbeek A.M., Margulies D.H., Shevach E.M., Strober W., editors. Current Protocols in Immunology. Wiley; New York: 1991. pp. 691–694. [DOI] [PubMed] [Google Scholar]
- Wonderling R., Powell T., Baldwin S., Morales T., Snyder S., Keiser K., Hunter S., Best E., McDermott M., Milhausen M. Cloning, expression, purification, and biological activity of five feline type I interferons. Vet. Immunol. Immunopathol. 2002;89(1/2):13–27. doi: 10.1016/s0165-2427(02)00188-5. [DOI] [PubMed] [Google Scholar]
- Yamaoka T., Kojima S., Ichi S., Kashiwazaki Y., Koide T., Sokawa Y. Biologic and binding activities of IFN-alpha subtypes in ACHN human renal cell carcinoma cells and Daudi Burkitt's lymphoma cells. J. Interferon Cytokine Res. 1999;19:1343–1349. doi: 10.1089/107999099312803. [DOI] [PubMed] [Google Scholar]
- Yeow W.S., Lawson C.M., Beilharz M.W. Antiviral activities of individual murine IFN-α subtypes in vivo: intramuscular injection of IFN expression constructs reduces cytomegalovirus replication. J. Immunol. 1998;160:2932–2939. [PubMed] [Google Scholar]


