Highlights
-
•
Information about vector-borne bacteria of camels is scarce.
-
•
Anaplasmataceae, SFG rickettsiae, Bartonella and Borrelia were investigated in blood of 200 dromedary camels of Iran.
-
•
PCR results revealed 30 animals (15%) to be infected with Anaplasmataceae bacteria.
-
•
BlastN® analysis of positive samples revealed identity with “Candidatus Anaplasma camelii” isolates.
-
•
This paper reviews the current knowledge on camels’ tick-borne bacteria including microscopy, serology and molecular studies.
Keywords: Camelus dromedarius, Dromedary, Anaplasma, Tick-borne, Vector-borne, Review
Abstract
Despite close association between camels and humans, molecular based studies on vector-borne pathogens infecting camels are scarce compared to other animals in Iran. The current study was carried out to investigate the occurrence of vector-borne bacteria in the blood of dromedaries by molecular tools. A total of 200 peripheral blood samples were collected from apparently healthy animals. Microscopic examination was performed on Giemsa-stained blood smears, and drops of blood were spotted on Whatman FTA® cards for molecular analyses. Genomic DNA was extracted from the cards, and PCR amplification followed by sequencing of positive samples was carried out for the detection of Anaplasmataceae, spotted fever group (SFG) rickettsiae, Bartonella spp. and Borrelia spp. Intra-cytic forms of any blood pathogens could not be detected by light microscopy. PCR results revealed 30 animals (15%) to be infected with Anaplasmataceae bacteria. Analyses of sequences revealed a strain of Anaplasma sp. identical to Candidatus Anaplasma camelii isolated from camels, cattle and deer in Asia and Africa. Neither SFG rickettsiae, nor Borrelia or Bartonella species were found. Further studies for determining epidemiological role of camels and its zoonotic potential are recommended. This paper reviews the current knowledge on camels’ tickborne bacteria including microscopy, serology and molecular studies.
1. Introduction
Camels are susceptible to a wide range of pathogenic microorganisms and they may act as carriers or reservoirs for several animal and zoonotic diseases [1]. However, apart from camel brucellosis and the Middle East respiratory syndrome (MERS), reports of camel-to-human transmission of zoonotic agents are either anecdotal or unsubstantiated.
Human and animal infections with members of the Anaplasmataceae family are increasingly recognized as important, in part emerging and potentially fatal arthropod-transmitted diseases for humans and animals. From the genus Anaplasma to date Anaplasma phagocytophilum, A. platys, A. ovis and A. capra have been recognized to infect humans [[2], [3], [4], [5]]. In camels Anaplasma organisms have been reported in blood smears by light microscopy [6,7] and antibodies against them in blood serum [8,9]. However, so far the only Anaplasma species confirmed by DNA sequencing in camels are Candidatus Anaplasma camelii (genetically close to A. platys), A. phagocytophilum and A. ovis [[10], [11], [12], [13], [14], [15], [16], [17], [18]]. Clinical signs of natural infections are described as fever, pale and icteric conjunctiva suggestive of anaemia, dullness, anorexia, diarrhoea, loss of appetite, emaciation, coughing, lacrimation, rough hair coat, abortion, and/or infertility [7,19]. Intramuscular administration of oxytetracycline at 20 mg/kg as specific therapy and injectable B-complex, iron, folic acid and hydroxycobalamin as supportive therapy is the recommended therapeutic regimen [7,19]. Infection with and antibodies against several species of Rickettsia [20,21], as well as infection with Bartonella [22] and Borrelia [23,24] have been also documented.
According to official estimates, around 183,900 camels live in Iran [25]. Given the growing scientific and public health interest in camels, we investigated the occurrence of selected vector-borne bacteria; Anaplasmataceae, spotted fever group (SFG) rickettsiae, Bartonella spp. and Borrelia spp. in domestic dromedary camels from Iran to get a deeper insight into the spectrum of pathogens circulating in this host population. We also review the current knowledge on cameline tick-borne bacteria including microscopical, serological and molecular studies.
2. Materials and methods
2.1. Sampling and microscopy examinations
Totally 200 clinically healthy one-humped dromedaries (Camelus dromedarius) of both sexes (36 females and 164 males), aged between one and nine years were sampled from June to July 2014 in central and south-eastern Iran from six different locations. In previous studies, blood of these animals was examined for the presence of filarioid helminths, piroplasms and trypanosomes. Deraiophoronema evansi was detected in 16 out of 200 samples and one positive sample each with Theileria annulata and Trypanosoma evansi using PCR and sequencing were found. For details on the study population and method of sampling see Sazmand et al. [26,27]. Thin blood smears were prepared from each sample, and stained with Giemsa for light microscopic examination.
2.2. DNA extraction, PCRs and DNA sequencing
Genomic DNA was extracted from blood spots on FTA® cards (5 mm2) with the QIAamp® DNA Blood Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s recommended protocol.
We performed PCRs targeting a 345 bp fragment of the 16S rDNA using the primers EHR16SD (5’-GGTACCYACAGAAGAAGTCC-3’) and EHR16SR (5’-TAGCACTCATCGTTTACAGC-3’) [28]. For the phylogenetic network analyses, we sequenced a 1012 bp fragment of the 16S rDNA using the newly designed primers Ana16SF (5’-GCAGACGGGTGAGTAATGCATAG-3’) and Ana16SR (5’-CTTGACATCATCCCCACCTTCCT-3’). Annealing temperatures were 53 °C and 56 °C in the latter two PCRs, respectively. Screening for Rickettsia spp. was carried out with the primers ITS-F (5′-GATAGGTCGGGTGTGGAAG-3′) and ITS-R (5′-TCGGGATGG GATCGTGTG-3′), amplifying the ca. 360 bp long intergenic spacer region between the 23S and 5S rDNAs, at 52 °C annealing temperature [29]. The samples were also screened for Borrelia burgdorferi s.l. using the primers P1 (5′-ACGCTGGCAGTGCGTCTTAA-3′) and P2 (5′-CTGATATCAACAGATTCCACCC-3′) targeting ab 650 bp section of the 16S rDNA, at 64 °C annealing temperature [30]. For the detection of Bartonella spp. a hypervariable intergenic transcribed spacer 16S-23S rRNA (ITS) was targeted using the primers Bart/16-23 F (5’-TTGATAAGCGTGAGGTCGGAGG-3’) and Bart/16-23R (5’-CAAAGCAGGTGCTCTCCCAG-3’), at 64 °C annealing temperature [31].
All PCRs were performed with the GoTaq® G2 Polymerase (Promega, Madison, Wisconsin, USA) and started with an initial denaturation for 2 min at 94 °C, followed by 35 cycles with 30 s at 94 °C, 30 s at the respective annealing temperatures (see above), 1 min at 72 °C, and a final extension for 10 min at 72 °C. PCR-products were sent to Microsynth Austria GmbH (Vienna, Austria) for purification and sequencing in both directions using the PCR primers.
2.3. Sequence analyses
The raw forward and reverse sequences (and electropherograms) were carefully analyzed in Bioedit 7.0.8.0 [32]. In order to visualize the relation between Anaplasma lineages, we calculated a Median Joining network with the 16S rDNA sequences (1012 bp) of Anaplasma-positive samples and data published on NCBI GenBank®. Calculations were performed with Network v.4.6.0.0 (Fluxus Technology Ltd., Suffolk, UK) applying the default settings. The network was post-processed with the MP (Maximum parsimony) option in order to reduce unnecessary median vectors.
2.4. Statistical analyses
Pearsons’ X2 and Kruskal–Wallis tests were used for the determination of relations between infections and sex or age of the camels with IBM SPSS Statistics 20.0 software. P values < 0.05 were considered significant.
2.5. Ethical considerations
Samples of Kerman province were obtained from slaughtered camels, and samples of Sistan-va-Baloochestan province were taken from live animals with official permission and under supervision of Provincial Veterinary Organization in accordance with the veterinary laws of I. R. Iran.
3. Results
Intracellular forms of blood pathogens could not be detected by light microscopy. However, 30 samples (15%; CI95 = 10–20%) were positive in the PCR assays specifically targeting the 16S rDNAs of Anaplasmataceae. The longer 16S fragment (1012 bp) was sequenced from 21 of the Anaplasma-positive samples, whereby all featured identical sequences. By performing a BLAST search in NCBI GenBank® database, we retrieved another 68 Anaplasma sequences, which covered the complete 16S rDNA sequence and showed more than 99.5% sequence similarity. Median Joining networks were calculated based on the alignment containing all these 68 sequences and the 21 sequences of the present study (Fig. 1 ).
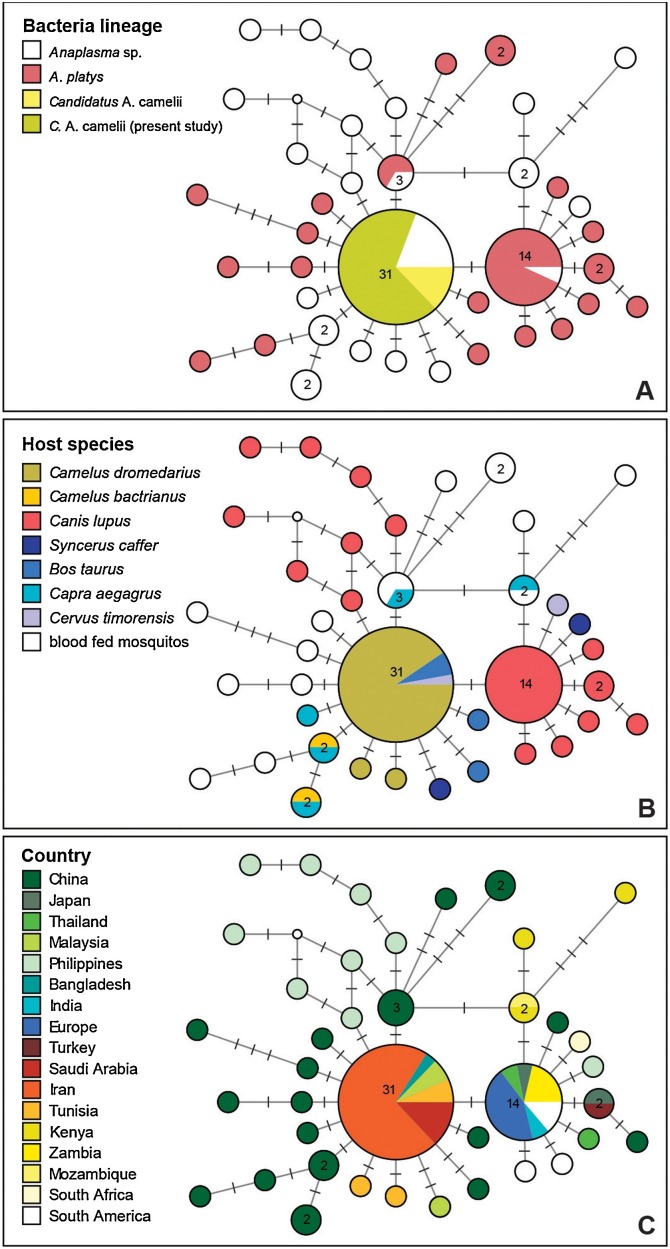
Fig. 1.
Median Joining network with 16S sequences (1012 bp) of Candidatus Anaplasma camelii, Anaplasma platys, and related lineages. The size of the circles corresponds to the number of sequences featuring the same genotype. Bars on branches indicate the number of substitutions between genotypes. In the three figures we indicate (A) the parasite species identified in the original publication, (B) the host species, and (C) the geographic origin of samples.
The lineage detected in the present study is identical to Candidatus Anaplasma camelii isolated from blood and spleen, respectively, of dromedary camels in Saudi Arabia (KF843823–28, [11]), Iran (KX765882 [15]), and Tunisia (KM401906–07 [12]). Moreover, the same lineage was found also in blood of Javanese rusa which is a deer native to the islands of Indonesia and East Timor (Rusa timorensis) (MG910989) and cattle (MG910990) in Malaysia [33], and in cattle in Bangladesh (MF576175 [34]). The 1012 bp section of the 16S differs only in one position from sequences of Anaplasma platys isolated from dogs worldwide. Moreover, several other genotypes, differing in one or a few position only, were isolated from dromedaries in Tunisia, and in Bactrian camels, Mongolian gazelles, goats, cattle, and blood fed mosquitoes in China. Several related genotypes, differing in one or several positions, were isolated from blood of goats, Mongolian gazelle, cattle, and blood fed mosquitoes in China, and dogs from the Philippines.
Statistically, there were no significant correlations between PCR positive results and age or sex of the animals. Candidatus Anaplasma camelii was present in five out of six study sites with prevalence ranging 5–43.3% in different regions (Table 1 ). One camel positive for Anaplasma spp. in Shahr-e-Babak was co-infected with filaroid Deraiophoronema evansi detected in a previous study [27]. The PCR screenings for Borrelia burgdorferi, Bartonella spp. and Rickettsia spp. were all negative. The 16S sequences of Candidatus Anaplasma camelii obtained from 21 camels in the present study were deposited in Genbank® (www.ncbi.nlm.nih.gov) under the accession numbers MK726038–MK726058.
Table 1.
Distribution of Anaplasma infection in camels according to sampling sites.
| Province | Sampling site | Number of collected samples | Number of infected camels (%) |
|---|---|---|---|
| Kerman | Shahr-e-Bababk | 20 | 3 (15) |
| Kerman | 60 | 5 (8.3) | |
| Kahnooj | 20 | 1 (5) | |
| Sistan-va-Baloochestan | Zabol | 30 | 13 (43.3) |
| Zahedan | 10 | 0 (0) | |
| Mirjaveh | 60 | 8 (13.3) | |
| Total | 200 | 30 (15) |
4. Discussion
In the present study, microscopy and molecular techniques were employed for examination of clinically healthy Iranian dromedaries’ blood for Anaplasmataceae, SFG rickettsiae, Bartonella spp. and Borrelia spp. We detected a strain of Anaplasma sp. identical to Candidatus Anaplasma camelii isolated from camels, cattle and deer in Asia and Africa [11,12,15,33,34].
In this study, Anaplasma bacteria were not detected in Giemsa-stained blood smears. As presented in Table 2 , Anaplasma spp. have been detected in erythrocytes of camels by light microscopy examination in Iran, Iraq, Saudi Arabia, India, Nigeria and Egypt with prevalence rates of up to 83.9%. One report also described Anaplasma organisms in cytoplasm of monocytes in Omani dromedaries [35]. It is worth noting that haemotropic Mycoplasma spp. can also infect dromedaries [36,37], so due to a high possibility of misdiagnosis of Anaplasma spp. morulae with other pathogens, application of PCR is highly recommended, as confirmed in the current study.
Table 2.
Prevalence rate of Anaplasma infection in dromedary camels by light microscopical examination.
| Country | Region | No. tested | No. positive | % Positive | Reference |
|---|---|---|---|---|---|
| Egypt | Kom Hamada | 135 | 15 | 11.1 | [38] |
| Matruh | 53 | 8 | 15.1 | [39] | |
| Matrouh | 331 | 157 | 47.4 | [40] | |
| Iran | Golestan | 100 | 2 | 2 | [17] |
| Kerman, Sistan-va-Baloochestan | 200 | 0 | 0 | This study | |
| Khorasan Razavi | 35 | 6 | 17.1 | [41] | |
| Mashhad | 262 | 0 | 0 | [42] | |
| Yazd | 114 | 0 | 0 | [43] | |
| Iraq | Najaf | 160 | 44 | 27.5 | [44] |
| Ninava | 62 | 52 | 83.9 | [6] | |
| Nigeria | Maiduguri | 105 | 4 | 3.8 | [45] |
| Maiduguri | 202 | 41 | 20.3 | [46] | |
| Zaria | 85 | 2 | 2.3 | [47] | |
| Saudi Arabia | Riyadh | 138 | 32 | 23.2 | [48] |
| Riyadh, Makkah | 237 | 72 | 30.4 | [19] |
Camel anaplasmosis with species that have tropism to erythrocytes, monocytes or granulocytes have been studied serologically in the past decades. As summarized in Table 3 seroprevalences of 0–53.85% have been reported. However, since correct serological testing for Anaplasma/Ehrlichia infections is hampered by cross-reactivity [49] and the phase of bacteriaemia may be shorter than the period of seropositivity, no DNA of Anaplasma was detected in serum-positive dromedaries [21].
Table 3.
Seroprevalence of Anaplasma in camels (Camelus dromedarius and Camelus bactrianus).
| Host | Country | Region | No. tested | No. positive | % Positive | Assay | Target organism | Reference |
|---|---|---|---|---|---|---|---|---|
| Camelus dromedarius | Iraq | Najaf, Wasit | 120 | 13 | 10.8 | ELISAd | A. marginale | [50] |
| Kenya | Ol Jogi | NSe | 0 | 0 | CFf | Anaplasma spp. | [51] | |
| Nigeria | Kano, Maiduguri, Jos | 28 | 3 | 10.7 | IFATa, CTATb, RCATc | A. marginale | [39] | |
| Spain | Canary Islands | 100 | 3 | 3 | ELISAd | A. marginale, A. ovis, A. centrale | [21] | |
| Tunisia | Sidi Bouzid, Bouficha, Douz | 226 | 66 | 29.2 | IFATa | A. phagocytophilum | [9] | |
| United Arab Emirates | Dubai | 1119 | 5 | 0.4 | ELISAd | A. marginale, A. ovis, A. centrale | [8] | |
| Camelus bactrianus | Mongolia | Terelj, Dalanjargalan, Sainshand | 104 | 56 | 53.8 | IFATa | A. phagocytophilum | [52] |
IFAT: indirect flourescent antibody test.
CTAT: capillary-tube agglutination test.
RCAT: rapid card agglutination test.
ELISA: enzyme-linked immunosorbent assay.
NS: not stated.
CFT: complement fixation test.
We detected a strain of Anaplasma sp. in 15% of the tested camels which featured 16S sequences identical to Candidatus Anaplasma camelii isolated from camels in Saudi Arabia, Tunisia and Iran [11,12,15]. Phylogenetic analyses based on DNA sequencing in our study supports the assumption that the Candidatus Anaplasma camelii lineage is genetically divergent from A. platys and may present a novel species.
With recent advances in diagnosis using molecular methods, reports of infection with Anaplasma spp. with tropism for platelets are increasing in one- and two-humped camels (Table 4 ). In six out of eight studies Anaplasma genotypes confirmed by nucleotide sequencing were genetically related to A. platys and Bastos et al. [11] proposed to name this genotype “Candidatus Anaplasma camelii”. Phylogenetic analyses in our study supports that Candidatus Anaplasma camelii lineage is genetically divergent from A. platys and may present a novel species.
Table 4.
Overview on molecular detection of Anaplasma in dromedary and Bactrian camels.
| Host | Country | Region | Type of sample | No. tested | No. positive | % Positive | Sequencing of PCR Products | Accession No. | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Camelus dromedarius | Spain | Canary Island | Serum | 3 | 0 | 0 | – | – | [21] |
| Egypt | Matrouh | Blood | 331 | 223 | 67.4 | – | – | [40] | |
| Iran | Golestan | Blood | 100 | 2 | 2 | + | KT601343, KU321250 | [17] | |
| Fars | Blood | 100 | 6 | 6 | + | KX765882 | [15] | ||
| Yazd, Zabol, Kerman, Bandar-Abbas, Hoveyze | Blood | 207 | 71 | 34.3 | Five positive samples | – | [18] | ||
| Kerman, Sistan-va-Baloochestan | Blood | 200 | 30 | 15 | + | MH794244, MH794245 | This study | ||
| Morocco | Tiznit, Guelmim, Laâyoune, Es-Semara, Boujdour, Oued Ed Dahab | Blood | 106 | 42 | 39.6 | + | KX074079 | [14] | |
| Nigeria | Sokoto | Blood | 36 | 22 | 61.1 | + | KJ832066, KJ832067 | [13] | |
| Pakistan | Mianwali | Blood | 150 | 20 | 13.3 | + | NSa | [16] | |
| Saudi Arabia | Riyadh | Blood | 77 | 0 | 0 | – | – | [55] | |
| Unizah | Spleen | 100 | 26 | 26 | + | KF843823–KF843828, KJ814955–KJ814959 | [11] | ||
| Tunisia | Sidi Bouzid, Bouficha, Douz | Blood | 226 | 40 | 17.7 | + | KM401905–KM401908 | [50] | |
| United Arab Emirates | Dubai | Blood | 55 | 0 | 0 | – | – | [56] | |
| Camelus bactrianus | China | Xinjiang | Blood | 279 | 20 | 7.2 | + | KP939254, KP939255, KP939257, KP939258, KP939260, KP939261, KR011925, KR011927 | [10] |
NS: not stated.
In two studies in Iran in 2018, A. ovis was confirmed in two dromedaries, which were positive in microscopy [17], and A. phagocytophilum was detected in the blood of 34.2% of 207 tested dromedaries in five areas of the country [18]. In a previous study, Anaplasma strains from dromedary camels in Iran and Tunisia were consistently placed on a divergent cluster from those found in Bacterian camel in China suggesting that (i) the causative agents of anaplasmosis in two species of Camelus dromedarius and Camelus bactrianus are independent from each other, (ii) genetic diversity of Candidatus Anaplasma camelii is not dependent on the geographical area, and (iii) it is related to host species [15]. Our finding of identical lineages in deer and cattle in Malaysia [33] and cattle in Bangladesh [34], suggest that Candidatus Anaplasma camelii is not geographically restricted to the Middle East and North Africa as well as lack of host specificity.
Interestingly most genotypes differing in just one nucleotide were found in cattle, goat or blood-fed mosquitoes in China, but the Candidatus Anaplasma camelii genotype was not found in China. However, the largest diversity of similar genotypes was found in Asia (China, Philippines, Malaysia), and the same is the case with A. platys which differs only by one nucleotide [10,33,34,53,54]. The occurrence of identical genotypes in Iran, Bangladesh and Malaysia might suggest that Candidatus Anaplasma camelii did not originate in camels in the Middle East but in Eastern Asia.
All of the Bactrian and dromedary camels tested for Anaplasma spp. by means of PCR were apparently healthy with no obvious signs of anaplasmosis, except for camels investigated by Ait Lbacha et al. in Morroco [14]. However, in an outbreak of disease in dromedaries in Morocco clinical signs of oedema, anorexia, respiratory distress, and sudden death similar to the clinical signs observed in cattle acutely infected with A. phagocytophilum were observed [14]. As Anaplasma infection might not be the sole cause of the observed clinical signs in camels of the latter study, experimental infections of will clarify the outcome of infection in this species.
The vectors of Candidatus Anaplasma camelii are still bot known. Camels of Iran are mainly infested with hard ticks of the genus Hyalomma [1]. Similarly in Saudi Arabia, Tunisia and Morocco, infection of dromedaries with Anaplasma platys-like organisms occurs in dromedaries primarily infested with Hyalomma spp. ticks [11,12,14], suggesting their potential role as vectors of Candidatus Anaplasma camelii. Furthermore, genotypes similar to Candidatus Anaplasma camelii were found in blood-fed mosquitoes in China indicating that mosquitoes might play a role in the transmission and evolution of Anaplasma species [53]. Researchers have identified Anaplasma, Ehrlichia, Candidatus Neoehrlichia, and Rickettsia bacteria in multiple mosquitoes and different life stages (egg, larvae, pupae, and adult) showing that mosquitoes may have played an important role in the transmission and evolution of Rickettsiales [53]. Hence, further studies are needed to investigate the actual vector competences for Candidatus Anaplasma camelii.
No Rickettsia spp. were detected in the present study that could be due to very short bacteremic period. Similarly, Erbaş et al. could not detect this bacteria in blood samples from 50 camels in Turkey [57]. However, there are two reports of R. aeschlimannii and Rickettsia sp. DNA in serum and blood samples from dromedary camels [20,21]. Antibodies against R. prowazekii, R. mooseri, R. rickettsia, R. conorii and Rickettsia sp. have been detected in camel blood serum with prevalences of up to 83% [21,58] and R. aeschlimannii, R. africae, R. sibirica mongolitimonae and Rickettsia sp. have been identified in several tick species collected from camels [20,[59], [60], [61], [62], [63], [64], [65], [66]]. Despite this, there are no reports on diseases in camels caused by these organisms.
Also, Bartonella DNA was not detected in the blood of camels in the present study, although in 2014 a novel species, B. dromedarii, was isolated from 18% of apparently healthy domesticated dromedaries in Israel [22]. The potential role of B. dromedarii as a zoonotic agent is still unknown. Recently, DNA from B. bovis and B. rochalimae was confirmed in Hyalomma dromedarii ticks collected from a single camel in Palestine. None of the 19 blood samples from camels in the latter study area were positive for Bartonella spp. [67].
Borrelia spirochetes DNA could not be found in our study which could be due to the fact that the bacteria do not prevail in the blood for longer time periods after infection. In principle, camels are susceptible to infections with Borrelia and in a seroprevalence survey on Borrelia in humans man and domestic animals from Egypt, 47.8% of the camels had serum antibodies against Borrelia sp., which was much higher than in buffaloes, cattle, goats or sheep (10.9–23.8%) [68]. In recent years DNA of Borrelia burgdorferi sensu lato has been detected in blood of 1.3% (3/232) of tested dromedary camels in Tunisia [23] and 3.6% (5/138) Bactrian camels in China [24]. These findings suggest that camels could play a role in Lyme disease and/or relapsing fever.
5. Conclusion
In the present article we reviewed literature about infection of camels with vector-borne bacteria, in particular Anaplasmataceae, SFG rickettsiae, Bartonella spp. and Borrelia spp. We also confirmed infection of Iranian one-humped camels with Candidatus Anaplasma camelii by molecular analysis. However, further investigations on vectors, hosts, reservoirs, pathogenicity in camels and zoonotic potential of this pathogen are required. Control of tick infestation is highly recommended to reduce infection pressure with tick-borne pathogens in camels. The risk of transmission of infections of camels with Rickettsiales by mosquitoes must be evaluated further.
Conflict of interests
No conflict of interests is declared.
Acknowledgments
We thank Dr. Mehdi Hosseini (Sistan-va-Baloochestan Provincial Veterinary Organization), Dr. Georg Gerhard Duscher (Institute of Parasitology, Department of Pathobiology, University of Veterinary Medicine Vienna, Austria), Mr. Mansour Aminzadeh (Laboratory of Parasitology, Faculty of Veterinary Medicine, Shahid Bahonar University of Kerman) for their help. The study was conducted within the framework of EurNegVec COST Action TD1303.
References
- 1.Sazmand A., Joachim A. Parasitic diseases of camels in Iran (1931–2017)—a literature review. Parasite. 2017;24:21. doi: 10.1051/parasite/2017024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breitschwerdt E.B., Hegarty B.C., Qurollo B.A., Saito T.B., Maggi R.G., Blanton L.S., Bouyer D.H. Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasit. Vectors. 2014;7(1):298. doi: 10.1186/1756-3305-7-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rikihisa Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat. Rev. Microbiol. 2010;8(5):328–339. doi: 10.1038/nrmicro2318. [DOI] [PubMed] [Google Scholar]
- 4.Hosseini-Vasoukolaei N., Oshaghi M.A., Shayan P., Vatandoost H., Babamahmoudi F., Yaghoobi-Ershadi M.R., Telmadarraiy Z., Mohtarami F. Anaplasma infection in ticks, livestock and human in Ghaemshahr, Mazandaran Province, Iran. J. Arthropod. Borne Dis. 2014;8(2):204–211. [PMC free article] [PubMed] [Google Scholar]
- 5.Li H., Zheng Y.-C., Ma L., Jia N., Jiang B.-G., Jiang R.-R., Huo Q.-B., Wang Y.-W., Liu H.-B., Chu Y.-L. Human infection with a novel tick-borne Anaplasma species in China: a surveillance study. Lancet Infect. Dis. 2015;15(6):663–670. doi: 10.1016/S1473-3099(15)70051-4. [DOI] [PubMed] [Google Scholar]
- 6.Alsaad K.M. Clinical, hematological and biochemical studies of anaplasmosis in Arabian one-humped camels (Camelus dromedaries) J. Anim. Vet. Adv. 2009;8(11):2106–2109. [Google Scholar]
- 7.Sudan V., Sharma R.L., Borah M.K. Subclinical anaplasmosis in camel (Camelus dromedarius) and its successful therapeutic management. J. Parasit. Dis. 2012;38(2):163–165. doi: 10.1007/s12639-012-0206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wernery U., Thomas R., Syriac G., Raghavan R., Kletzka S. Seroepidemiological studies for the detection of antibodies against nine infectious diseases in dairy dromedaries (part-i) J. Camel Pract. Res. 2007;14(2):85–90. [Google Scholar]
- 9.Ben Said M., Belkahia H., Sayahi L., Aloui M., Jemli M.H., Hadj Mohamed B., Sassi L., Darghouth M.A., Djaïem A.A., Bayoudh M., Messadi L. First serological study of the prevalence of Anaplasma phagocytophilum in dromedary (Camelus dromedarius) in Tunisia. Bull. Soc. Pathol. Exot. 2014;107(1):1–6. doi: 10.1007/s13149-013-0323-8. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Yang J., Chen Z., Qin G., Li Y., Li Q., Liu J., Liu Z., Guan G., Yin H., Luo J., Zhang L. Anaplasma infection of Bactrian camels (Camelus bactrianus) and ticks in Xinjiang, China. Parasit. Vectors. 2015;8(1):313. doi: 10.1186/s13071-015-0931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastos A.D.S., Mohammed O.B., Bennett N.C., Petevinos C., Alagaili A.N. Molecular detection of novel Anaplasmataceae closely related to Anaplasma platys and Ehrlichia canis in the dromedary camel (Camelus dromedarius) Vet. Microbiol. 2015;179(3–4):310–314. doi: 10.1016/j.vetmic.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Belkahia H., Ben Said M., Sayahi L., Alberti A., Messadi L. Detection of novel strains genetically related to Anaplasma platys in Tunisian one-humped camels (Camelus dromedarius) J. Infect. Dev.ctries. 2015;9(10):1117–1125. doi: 10.3855/jidc.6950. [DOI] [PubMed] [Google Scholar]
- 13.Lorusso V., Wijnveld M., Latrofa M.S., Fajinmi A., Majekodunmi A.O., Dogo A.G., Igweh A.C., Otranto D., Jongejan F., Welburn S.C., Picozzi K. Canine and ovine tick-borne pathogens in camels, Nigeria. Vet. Parasitol. 2016;228:90–92. doi: 10.1016/j.vetpar.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ait Lbacha H., Zouagui Z., Alali S., Rhalem A., Petit E., Ducrotoy M.J., Boulouis H.-J., Maillard R. “Candidatus anaplasma camelii” in one-humped camels (Camelus dromedarius) in Morocco: a novel and emerging anaplasma species? Infect. Dis. Poverty. 2017;6(1):1. doi: 10.1186/s40249-016-0216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharifiyazdi H., Jafari S., Ghane M., Nazifi S., Sanati A. Molecular investigation of Anaplasma and Ehrlichia natural infections in the dromedary camel (Camelus dromedarius) in Iran. Comp. Clin. Pathol. 2017;26(1):99–103. [Google Scholar]
- 16.Azmat M., Ijaz M., Farooqi S., Ghaffar A., Ali A., Masud A., Saleem S., Rehman A., Ali M., Mehmood K. Molecular epidemiology, associated risk factors, and phylogenetic analysis of anaplasmosis in camel. Microb. Pathog. 2018;123:377–384. doi: 10.1016/j.micpath.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 17.Noaman V. Molecular detection of novel genetic variants associated to Anaplasma ovis among dromedary camels in Iran. Arch. Razi Inst. 2018;73(1):11–18. doi: 10.22092/ARI.2018.114055. [DOI] [PubMed] [Google Scholar]
- 18.Bahrami S., Hamidinejat H., Ganjali-Tafreshi A.-R. First molecular detection of Anaplasma phagocytophilum in dromedaries (Camelus dromedarius) J. Zoo Wildl. Med. 2018;49(4):844–848. doi: 10.1638/2017-0165.1. [DOI] [PubMed] [Google Scholar]
- 19.Ismael A.B., Swelum A.A., Khalaf A.F., Alowaimer A.N. First evidence of natural anaplasmosis in Camelus dromedarius in Saudi Arabia. J. Camel Pract. Res. 2016;23(1):95–100. [Google Scholar]
- 20.Kleinerman G., Baneth G., Mumcuoglu K.Y., van Straten M., Berlin D., Apanaskevich D.A., Abdeen Z., Nasereddin A., Harrus S. Molecular detection of Rickettsia africae, Rickettsia aeschlimannii, and Rickettsia sibirica mongolitimonae in camels and Hyalomma spp. ticks from Israel. Vector Borne Zoonotic Dis. 2013;13(12):851–856. doi: 10.1089/vbz.2013.1330. [DOI] [PubMed] [Google Scholar]
- 21.Mentaberre G., Gutiérrez C., Rodríguez N.F., Joseph S., González-Barrio D., Cabezón O., de la Fuente J., Gortazar C., Boadella M. A transversal study on antibodies against selected pathogens in dromedary camels in the Canary Islands, Spain. Vet. Microbiol. 2013;167(3–4):468–473. doi: 10.1016/j.vetmic.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Rasis M., Rudoler N., Schwartz D., Giladi M. Bartonella dromedarii sp. nov. isolated from domesticated camels (Camelus dromedarius) in Israel. Vector Borne Zoonotic Dis. 2014;14(11):775–782. doi: 10.1089/vbz.2014.1663. [DOI] [PubMed] [Google Scholar]
- 23.Said M.B., Belkahia H., Alberti A., Abdi K., Zhioua M., Daaloul-Jedidi M., Messadi L. First molecular evidence of Borrelia burgdorferi sensu lato in goats, sheep, cattle and camels in Tunisia. Ann. Agric. Environ. Med. 2016;23(3):442–447. doi: 10.5604/12321966.1219184. [DOI] [PubMed] [Google Scholar]
- 24.Zhai B., Niu Q., Liu Z., Yang J., Pan Y., Li Y., Zhao H., Luo J., Yin H. First detection and molecular identification of Borrelia species in Bactrian camel (Camelus bactrianus) from Northwest China. Infect. Genet. Evol. 2018;64:149–155. doi: 10.1016/j.meegid.2018.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry of Agriculture Jihad . 2018. Annual Production Report. Tehran, Iran [in Persian] [Google Scholar]
- 26.Sazmand A., Eigner B., Mirzaei M., Hekmatimoghaddam S., Harl J., Duscher G.G., Fuehrer H.-P., Joachim A. Molecular identification of hemoprotozoan parasites in camels (Camelus dromedarius) of Iran. Iran. J. Parasitol. 2016;11(4):568–573. [PMC free article] [PubMed] [Google Scholar]
- 27.Sazmand A., Eigner B., Mirzaei M., Hekmatimoghaddam S., Harl J., Duscher G.G., Fuehrer H.-P., Joachim A. Molecular identification and phylogenetic analysis of Dipetalonema evansi (LEWIS, 1882) in camels (Camelus dromedarius) of Iran. Parasitol. Res. 2016;115(4):1605–1610. doi: 10.1007/s00436-015-4896-y. [DOI] [PubMed] [Google Scholar]
- 28.Parola P., Roux V., Camicas J.-L., Baradji I., Brouqui P., Raoult D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 2000;94(6):707–708. doi: 10.1016/s0035-9203(00)90243-8. [DOI] [PubMed] [Google Scholar]
- 29.Vitorino L., Zé-Zé L., Sousa A., Bacellar F., Tenreiro R. rRNA intergenic spacer regions for phylogenetic analysis of Rickettsia species. Ann. N. Y. Acad. Sci. 2003;990:726–733. doi: 10.1111/j.1749-6632.2003.tb07451.x. [DOI] [PubMed] [Google Scholar]
- 30.Liebisch G., Sohns B., Bautsch W. Detection and typing of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks attached to human skin by PCR. J. Clin. Microbiol. 1998;36(11):3355–3358. doi: 10.1128/jcm.36.11.3355-3358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García-Esteban C., Gil H., Rodríguez-Vargas M., Gerrikagoitia X., Barandika J., Escudero R., Jado I., García-Amil C., Barral M., García-Pérez A.L., Bhide M., Anda P. Molecular method for Bartonella species identification in clinical and environmental samples. J. Clin. Microbiol. 2008;46(2):776–779. doi: 10.1128/JCM.01720-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 33.Koh F.X., Panchadcharam C., Sitam F.T., Tay S.T. Molecular investigation of Anaplasma spp. in domestic and wildlife animals in Peninsular Malaysia. Vet. Parasitol. Reg. Stud. Rep. 2018;13:141–147. doi: 10.1016/j.vprsr.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Roy B., Krücken J., Ahmed J., Majumder S., Baumann M., Clausen P.H., Nijhof A. Molecular identification of tick‐borne pathogens infecting cattle in Mymensingh district of Bangladesh reveals emerging species of Anaplasma and Babesia. Transbound. Emerg. Dis. 2018;65(2):e231–e242. doi: 10.1111/tbed.12745. [DOI] [PubMed] [Google Scholar]
- 35.Wernery U., Musa B., Kinne J. Rickettsia-like disease in dromedaries. J. Camel Pract. Res. 2001;8(1):7–9. [Google Scholar]
- 36.Nazifi S., Oryan A., Bahrami S., Razavi S.M. Evaluation of haematological and serum biochemical parameters in Iranian camels (Camelus dromedarius) infected with haemotrophic Mycoplasma (Eperythrozoon) spp. Comp. Clin. Pathol. 2009;18(3):329–332. [Google Scholar]
- 37.Wernery U. Mycoplasmosis in camelids with own investigations. J. Camel Pract. Res. 2012;19(2):135–142. [Google Scholar]
- 38.Hegazy E., Mahmoud A., Khadr A., Elshemey T., El-Rahman A. Evaluation of molecular technique and microscopical examination for diagnosis of blood parasites in equine and camels. Alexandria J. Vet. Sci. 2017;55(1):217–224. [Google Scholar]
- 39.Osman A.O., El-Metwaly H.A., Wahba A.A., Hefny S.F. Studies on causes of abortion in Maghrabian camels. Egypt. J. Agr. Res. 2016;94(4):955–967. [Google Scholar]
- 40.El-Naga T., Barghash S. Blood parasites in camels (Camelus dromedarius) in Northern West Coast of Egypt. J. Bacteriol. Parasitol. 2016;7:1. [Google Scholar]
- 41.Ghazvinian K., Khodaiean T. Anaplasmosis among camels in Iran and observation of abnormalities in infected blood films. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2017;10(7):475–478. [Google Scholar]
- 42.Borji H., Razmi G.R., Parandeh S. Epidemiological study on haemoparasites of dromedary (Camelus dromedarius) in Iran. J. Camel Pract. Res. 2009;16(2):217–219. [Google Scholar]
- 43.Hekmatimoghaddam S., Sazmand A., Rasooli A., Hamidinejat H., Jafari H. Laboratory tests in dromedary camels naturally infected with piroplasms in Iran: study and review of literature. J. Camel Pract. Res. 2012;19(2):217–221. [Google Scholar]
- 44.Al-Amery A.M., Faraj A.A., Majeed S.A. Detection of haemorotozoa in camel in Al-Najaf Province, Iraq. Int. J. Adv. Biol. Res. 2017;7(2):238–241. [Google Scholar]
- 45.Bamaiyi P.H., Kalu A.U., Ali M. Haemoparasites of the trade camel (Camelus dromedarius) arriving for slaughter at Maiduguri, Borno State, Nigeria. Cont. J. Vet. Sci. 2011;5(1):18–21. [Google Scholar]
- 46.Wakil Y., Lawal J.R., Gazali Y.A., Mustpha F.B., Bello A.M., Mshelia E.S., Ayomikun A.M. Survey on prevalence of haemoparasites of trade camels (Camelus dromedarius) in Maiduguri; Nigeria. Pyrex J. Vet. Med. Anim. Sci. 2016;1(2):7–10. [Google Scholar]
- 47.Mohammed A.K., Sackey A.K.B., Tekdek L.B., Gefu J.O. Common health problems of the one humped camel (Camelus dromedarius) introduced into sub-humid climate in Zaria, Nigeria. Res. J. Anim. Sci. 2007;1(1):1–5. [Google Scholar]
- 48.Al-Khatib R.M., Mazloum K.S., Al Nakhli H.M. Incidence of anaplasmosis and FMD in camel (Camelus dromedaries) Assiut Vet. Med. J. 2012;58(133):41–48. [Google Scholar]
- 49.Al-Adhami B., Scandrett W.B., Lobanov V.A., Gajadhar A.A. Serological cross-reactivity between Anaplasma marginale and an Ehrlichia species in naturally and experimentally infected cattle. J. Vet. Diagn. Invest. 2011;23(6):1181–1188. doi: 10.1177/1040638711425593. [DOI] [PubMed] [Google Scholar]
- 50.Al-Gharban H.A.J., Sr A.L.T. Seroclinical diagnosis of Anaplasma marginale bacteria in carrier arabian one-humped camels. Basrah J. Vet. Res. 2016;15(2):346–359. [Google Scholar]
- 51.Kimber K.R., Lubroth J., Dubovi E.J., Berninger M.L., Demaar T.W. Serologic survey of selected viral, bacterial, and protozoal agents in captive and free‐ranging ungulates from central Kenya. Ann. N. Y. Acad. Sci. 2002;969(1):217–223. doi: 10.1111/j.1749-6632.2002.tb04382.x. [DOI] [PubMed] [Google Scholar]
- 52.von Fricken M.E., Lkhagvatseren S., Boldbaatar B., Nymadawa P., Weppelmann T.A., Baigalmaa B.-O., Anderson B.D., Reller M.E., Lantos P.M., Gray G.C. Estimated seroprevalence of Anaplasma spp. and spotted fever group Rickettsia exposure among herders and livestock in Mongolia. Acta Trop. 2018;177:179–185. doi: 10.1016/j.actatropica.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo W.-P., Tian J.-H., Lin X.-D., Ni X.-B., Chen X.-P., Liao Y., Yang S.-Y., Dumler J.S., Holmes E.C., Zhang Y.-Z. Extensive genetic diversity of Rickettsiales bacteria in multiple mosquito species. Sci. Rep. 2016;6:38770. doi: 10.1038/srep38770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ybañez A.P., Ybañez R.H.D., Yokoyama N., Inokuma H. Multiple infections of Anaplasma platys variants in Philippine dogs. Vet. World. 2016;9(12):1456. doi: 10.14202/vetworld.2016.1456-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohammed A., Sharma A., Saied M., Osman O., Al-Balowi M., Salih D., Singla L. Lack of evidence for infection of camels with tick-borne diseases in Riyadh region, Saudi Arabia. Sudan J. Vet. Res. 2017;32:39–40. [Google Scholar]
- 56.Wernery U., Pfister K., Marina R., Hakimudin F., Silaghi C. No evidence of Mycoplasma haemolamae and Anaplasma marginale in anaemic dromedaries in the United Arab Emirates. J. Camel Pract. Res. 2014;21(1):5–8. [Google Scholar]
- 57.Erbas G., Parin U., Kirkan S., Savasan S., Yüksel H., Balat G. Molecular identification of tick-borne zoonotic bacteria in one humped camel (Camelus dromedarius) J. Camel Pract. Res. 2018;25(1):89–92. [Google Scholar]
- 58.Wernery U., Kinne J., Schuster R.K. OIE (World Organisation for Animal Health); Paris, France: 2014. Camelid Infectious Disorders. [Google Scholar]
- 59.Al-Deeb M.A., Muzaffar S.B., Abu-Zeid Y.A., Enan M.R., Karim S. First record of a spotted fever group Rickettsia sp. and Theileria annulata in Hyalomma dromedarii (Acari: Ixodidae) ticks in the United Arab Emirates. Florida Entomol. 2015;98(1):135–139. [Google Scholar]
- 60.Abdel-Shafy S., Allam N.A.T., Mediannikov O., Parola P., Raoult D. Molecular detection of spotted fever group rickettsiae associated with ixodid ticks in Egypt. Vector Borne Zoonotic Dis. 2012;12(5):346–359. doi: 10.1089/vbz.2010.0241. [DOI] [PubMed] [Google Scholar]
- 61.Loftis A.D., Reeves W.K., Szumlas D.E., Abbassy M.M., Helmy I.M., Moriarity J.R., Dasch G.A. Rickettsial agents in Egyptian ticks collected from domestic animals. Exp. Appl. Acarol. 2006;40(1):67–81. doi: 10.1007/s10493-006-9025-2. [DOI] [PubMed] [Google Scholar]
- 62.Djerbouh A., Kernif T., Beneldjouzi A., Socolovschi C., Kechemir N., Parola P., Raoult D., Bitam I. The first molecular detection of Rickettsia aeschlimannii in the ticks of camels from southern Algeria. Ticks Tick Borne Dis. 2012;3(5–6):374–376. doi: 10.1016/j.ttbdis.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 63.Kernif T., Djerbouh A., Mediannikov O., Ayach B., Rolain J.-M., Raoult D., Parola P., Bitam I. Rickettsia africae in Hyalomma dromedarii ticks from sub-Saharan Algeria. Ticks Tick Borne Dis. 2012;3(5–6):377–379. doi: 10.1016/j.ttbdis.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Kamani J., Baneth G., Apanaskevich D.A., Mumcuoglu K.Y., Harrus S. Molecular detection of Rickettsia aeschlimannii in Hyalomma spp. ticks from camels (Camelus dromedarius) in Nigeria, West Africa. Med. Vet. Entomol. 2015;29(2):205–209. doi: 10.1111/mve.12094. [DOI] [PubMed] [Google Scholar]
- 65.Demoncheaux J.-P., Socolovschi C., Davoust B., Haddad S., Raoult D., Parola P. First detection of Rickettsia aeschlimannii in Hyalomma dromedarii ticks from Tunisia. Ticks Tick Borne Dis. 2012;3(5–6):398–402. doi: 10.1016/j.ttbdis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 66.Koka H., Sang R., Kutima H.L., Musila L. The detection of spotted fever group Rickettsia DNA in tick samples from pastoral communities in Kenya. J. Med. Entomol. 2017;54(3):774–780. doi: 10.1093/jme/tjw238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ereqat S., Nasereddin A., Vayssier-Taussat M., Abdelkader A., Al-Jawabreh A., Zaid T., Azmi K., Abdeen Z. Molecular evidence of Bartonella species in ixodid ticks and domestic animals in Palestine. Front. Microbiol. 2016;7:1217. doi: 10.3389/fmicb.2016.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helmy N. Seasonal abundance of Ornithodoros (O.) savignyi and prevalence of infection with Borrelia spirochetes in Egypt. J. Egypt. Soc. Parasitol. 2000;30(2):607–619. [PubMed] [Google Scholar]