Abstract
β-Defensins are cationic peptides which form part of the innate immune response of the respiratory epithelium. Due to their antimicrobial properties and immunostimulatory activity, β-defensins are potential tools for the treatment and prevention of respiratory disease. In dogs, infectious respiratory disease is a common problem, particularly in housed animals. This study aimed to assess the presence of four β-defensins in the canine respiratory tract and to use quantitative real-time PCR to determine mRNA levels following microbial challenge. Three β-defensins, CBD1, CBD103 and CBD108, were detected in respiratory cells. All three defensins were also readily expressed in skin samples, while their expression in lymphoid tissues and the kidney was low and inconsistent. Treatment of primary tracheal epithelial cells with lipopolysaccharide (LPS) or infection with canine respiratory coronavirus led to decreased expression of CBD103 and CBD108, while cells infected with canine parainfluenza virus had lower levels of CBD1 and CBD108. Furthermore CBD103 was demonstrated to have antimicrobial activity against the respiratory pathogen Bordetella bronchiseptica.
Keywords: Defensin, Dog, Respiratory, Bordetella bronchiseptica
1. Introduction
The respiratory tract is the site of entry for many pathogens. The first line of defence to prevent colonisation of the respiratory epithelium consists of physical measures, such as the ciliary clearance, as well as antimicrobial substances in the airway secretions. Respiratory epithelial cells produce antimicrobial peptides such as defensins, which form an important part of the innate immune response of the respiratory tract (Diamond et al., 2000, Singh et al., 1998). Defensins are a family of cationic peptides, which are produced by neutrophils, macrophages or epithelial cells and which are divided into α, β and θ (theta) defensins according to their structural properties (Ganz, 2003). Human β-defensins show direct inhibitory activities against viruses such as HIV and influenza virus (Leikina et al., 2005, Quinones-Mateu et al., 2003) as well as bacteria such as E. coli and Staphylococcus aureus (Krishnakumari et al., 2006). In addition, β-defensins have immunostimulatory functions including chemotaxis and activation of antigen-presenting cells (Ganz, 2003). In the canine genome 43 putative β-defensins have been identified by similarity search (14). Of these, canine β-defensin 122 (CBD122) has been detected in a range of tissues including the lung and was shown to inhibit the growth of gram-positive and gram-negative bacteria (Sang et al., 2005). Two further defensins (CBD1 and CBD103) have been found in canine skin, however their antimicrobial properties or their potential presence in the canine respiratory tract have not been investigated. Infectious respiratory disease is the most common health problem of housed dogs. Vaccination frequently does not provide a sufficiently rapid immune response to prevent infection of dogs entering a kennel. Traditionally canine parainfluenza virus (CPIV), canine adenovirus type 2 and the bacterium Bordetella bronchiseptica have been associated with canine infectious respiratory disease (CIRD) and are included in vaccines against the disease complex (Buonavoglia and Martella, 2007, Jacobs et al., 2005). However an ever-increasing number of pathogens including viruses and bacteria has been shown to be present in this disease complex (Chalker et al., 2003a, Chalker et al., 2004, Crawford et al., 2005, Erles et al., 2003). β-Defensins may offer novel routes for the prevention and treatment of diseases, including CIRD, by acting as antimicrobials, either after topical application or by up-regulation of defensin production in the host cell, and by acting as mediators to stimulate the innate immune response. In addition naturally occurring and artificial cationic peptides may be used as vaccine adjuvants (Charoenvit et al., 2004, Fritz et al., 2004). This study therefore investigated the presence of β-defensins in the canine respiratory tract and their regulation following microbial challenge and determined the antimicrobial action of CBD103 against the respiratory pathogen B. bronchiseptica.
2. Materials and methods
2.1. RNA extraction and cDNA synthesis
RNA was extracted from cells using the RNeasy kit (Qiagen, Crawley) following the manufacturer's protocol for cultured cells. The RNA was eluted into 30 μl of RNase-free water. Genomic DNA was removed by digestion with RQDNase (Promega, Southampton) for 30 min at 37 °C. Reverse transcription was performed using 7 μl of RNA, 0.5 μg random hexamers (GE Healthcare, Little Chalfont), 1 μl of ImPromII reverse transcriptase (Promega) and a MgCl2 concentration of 3 mM in a total reaction volume of 20 μl.
Total cDNA was quantified using the Quant-iT™ PicoGreen Assay Kit (Invitrogen, Paisley) which selectively detects dsDNA and DNA–RNA hybrids (cDNA) (Seville et al., 1996). The assay was performed using a modification of a previously published protocol (Whelan et al., 2003). Briefly the λ DNA standard was diluted in a 5-fold dilution series from 1 μg/ml to 1.6 ng/ml. cDNA samples were tested in duplicate at a final dilution of 1:100. The fluorescence was measured using the SPECTRAmax M2 Plate reader (Molecular devices, Wokingham) with an excitation at 480 nm and emission at 520 nm. The linear standard curve was constructed for the λ DNA standard concentration vs. fluorescence units. The concentration of the cDNA was calculated from the linear equation after adjusting for the dilution.
2.2. Conventional PCR
The sequences for the intron spanning primers for CBD1, CBD102, CBD103 and CBD108 cDNAs are given in Table 1 . PCR was carried out using Taq polymerase (Promega) and a final concentration of 2.5 mM MgCl2. The conditions for the PCR were as follows: initial denaturation at 95 °C for 5 min, 35 cycles of 95 °C for 1 min, 55 °C for 40 s and 72 °C for 40 s and a final extension at 72 °C for 10 min. PCR products were analysed on 1% agarose gels and visualised by ethidium bromide. The expected product sizes were 256 bp for CBD1, 177 bp for CBD102, 143 bp for CBD103 and 195 bp for CBD108. PCR products were cloned into the pGEM-T Easy vector (Promega) and submitted to The Sequencing Service (University of Dundee, UK) to confirm the integrity of the clones.
Table 1.
Primer sequences.
| Primer | Sequence | Position | Genbank accession number |
|---|---|---|---|
| CBD1-1 | 5′-TCT-ACT-TGC-TGC-TGC-TGC-TTC-TGT-3′ | 163–186 | NM_001113713 |
| CBD1-2 | 5′-ACT-TCA-CTG-GGC-TCA-ATG-GAC-TTC-3′ | 418–395 | NM_001113713 |
| CBD103-1 | 5′-CCT-GTC-GCG-AAG-CCT-GTT-GG-3′ | 92–111 | NM_001129980 |
| CBD103-2 | 5′-CGC-ACC-GAC-CGC-TCC-TTA-TTC-T-3′ | 234–213 | NM_001129980 |
| CBD108-1 | 5′-CTG-CTC-CTC-GCC-CTG-CTC-TTC-TTT-3′ | 16–39 | DQ011977 |
| CBD108-2 | 5′-GGG-TGT-AGT-AGG-CTC-GAT-GAT-GGA-3′ | 210–187 | DQ011977 |
| CBD102-1 | 5′-GTC-TGT-ATC-GTA-ATT-GGT-GGT-GAA-3′ | 39–62 | DQ011971 |
| CBD102-2 | 5′-CTG-GAG-TAG-GTG-ACG-AGA-ATA-AAA-3′ | 215–192 | DQ011971 |
2.3. Quantitative PCR
The β-defensin PCR products cloned into pGEM-T Easy were used to generate a standard curve. A 10-fold dilution series of the plasmid DNA (108 to 101 copies/μl) was prepared and used to produce the standard curve.
Real-time PCR was performed using SYBR Green Jumpstart Taq Ready Mix (Sigma, Gillingham) with final concentrations of 0.5 μM of each primer and 3 mM MgCl2 for CBD1, 3.5 mM MgCl2 for CBD103 or 2.5 mM MgCl2 for CBD108, respectively. For each reaction 1 μl of cDNA template or standard was added to a total reaction volume of 25 μl. Samples were analysed in duplicate. In each run, the standard dilution series was included in triplicate. The cycling conditions were 95 °C for 10 min, followed by 40 cycles of 94 °C for 10 s, 55 °C for 20 s and 72 °C for 30 s. Following the extension, a melting step of 80 °C for CBD103 and of 84 °C for CBD1 and CBD108 was performed after which the plate read was taken. This was followed by melting curve analysis from 55 to 95 °C over 20 min and a re-annealing step of 72 °C for 5 min. All reactions were performed on a DNA Engine Opticon 2 (Bio-Rad, Hemel Hempstead).
Analyses of copy number, linear regression and melting curve analysis were performed for each experiment using the Opticon Monitor 2 Software version 3.1 (Bio-Rad). Quantitative data was normalized by cDNA concentration.
2.4. Culture of canine tracheal epithelial cells (CTEC)
Dogs were received from UK re-homing centres having been euthanised due to behavioural concerns and unsuitability to re-home. The whole trachea, from larynx to carina, was aseptically removed at necropsy within 2–3 h of death. The trachea was washed briefly in sterile PBS, then washed twice for 1 h at 37 °C in Dulbecco's modified Eagle's medium (DMEM) containing 50 μg/ml gentamycin, 100 U/ml penicillin, 100 μg/ml streptomycin and 2.5 μg/ml amphotericin B (Sigma).
Tracheal tissue was screened for contamination with respiratory viruses or mycoplasmas by PCR and for other bacterial contaminants by culture on blood agar plates (Becton-Dickinson, Oxford). PCR assays for the detection of canine adenovirus type 2, canine herpesvirus, canine parainfluenza virus (CPIV), canine respiratory coronavirus (CRCoV), canine distemper virus and Mycoplasma spp. were performed as previously described (Erles et al., 2004, Erles et al., 2003, Frisk et al., 1999, Kobayashi et al., 1995). DNA was extracted using the DNeasy Tissue Kit (Qiagen) according to the manufacturer's instructions; RNA extraction and cDNA synthesis were performed as described above.
Epithelial cells were obtained by the incubation of the complete trachea over night at 4 °C with Proteinase type XIV (Sigma) at a concentration of 0.5 mg/ml in PBS (PAA, Yeovil). Following proteinase treatment foetal calf serum (FCS) was added to a final concentration of 2.5%. Cells were harvested by centrifugation at 200 × g for 10 min at 15 °C. Cell pellets from one trachea were resuspended in complete DMEM/Ham's F12 (containing 20% FCS (PAA), 50 μg/ml gentamycin, 100 U/ml penicillin, 100 μg/ml streptomycin and 2.5 μg/ml amphotericin B). Cells were incubated in a tissue culture flask for 2 h at 37 °C and 5% CO2 to allow fibroblasts to settle. The supernatant containing the epithelial cells was then seeded onto four 12-well plates coated with collagen type I (Becton-Dickinson). Cells were maintained and all experiments were carried out at 37 °C and 5% CO2.
2.5. Treatment with lipopolysaccharide (LPS)
Confluent cultures of CTEC derived from one dog (CTEC-7) were treated with E. coli O55:B5 LPS (Sigma), 10 μg/ml in complete DMEM/Ham's F12 for 8 h and 24 h. Control cultures were incubated with medium only.
2.6. Virus infection
Confluent cultures were infected with CRCoV or CPIV at a multiplicity of infection of 0.1 for 1 h. Preliminary experiments using CRCoV had shown no changes to β-defensin mRNA levels at 24 h, 48 h and 72 h whereas changes were observed at 96 h and 120 h. For the analysis of CRCoV infection cells derived from two different dogs were used and analysed after 96 h (CTEC-10) or 120 h (CTEC-8); CPIV-infected cultures were analysed after 72 h (CTEC-11) to prevent cell loss due to cytopathic effect.
2.7. Tissue samples
Tissue samples from two dogs were used to assess mRNA levels of canine β-defensins. The samples included trachea, skin, lung, palatine tonsil, bronchial lymph node, spleen, liver, kidney, duodenum, jejunum, ileum, colon and mesenteric lymph node. RNA extraction and cDNA synthesis were performed as described above.
2.8. Bacterial culture
B. bronchiseptica isolate Bb49 was obtained from a dog with respiratory disease and grown on Bordet-Gengou agar plates (Becton-Dickinson) (Chalker et al., 2003b). Bacteria were quantified by plating serial 10-fold dilutions on Bordet-Gengou agar.
2.9. Antimicrobial activity of CBD103
A 45-amino-acid CBD103 peptide (aa 23–67) was synthesised by PPR Ltd (Wickham). The peptide was lyophilised and converted to the HCl salt. The peptide was dissolved in DMSO to a concentration of 30 μg/μl.
CBD103 peptide was diluted in sodium phosphate buffer (SPB), pH 7.4, to working concentrations of 1 mg/ml, 100 μg/ml and 10 μg/ml. As the peptide stock was dissolved in DMSO the concentration of DMSO was 3% in the 1 mg/ml dilution. Therefore 3% DMSO in SPB was used as the negative control.
100 μl of CBD103 dilutions or 3% DMSO were added in duplicate to the wells of a 96-well microtitre plate. 100 μl B. bronchiseptica (105 CFU in SPB) was added and incubated with the peptide or control for 1 h at 37 °C. 10-fold dilutions were prepared in SPB, plated onto Bordet-Gengou agar plates in quadruplicate and incubated for 48 h at 37 °C after which the number of colonies was determined.
Inhibition of the activity of CBD103 peptide by NaCl was determined at 1 mg/ml and 100 μg/ml peptide concentrations and 150 mM and 300 mM NaCl concentrations. Assays were carried out as above with NaCl being added to the peptide dilutions to obtain final concentrations of 150 mm and 300 mM after the addition of bacterial suspension.
2.10. Statistical analysis
Means and standard deviations were calculated and the differences between experimental groups were assessed by Mann–Whitney test using SPSS (version 17) software. Differences were considered statistically significant if p ≤ 0.05.
3. Results
3.1. Defensin levels in canine respiratory cells
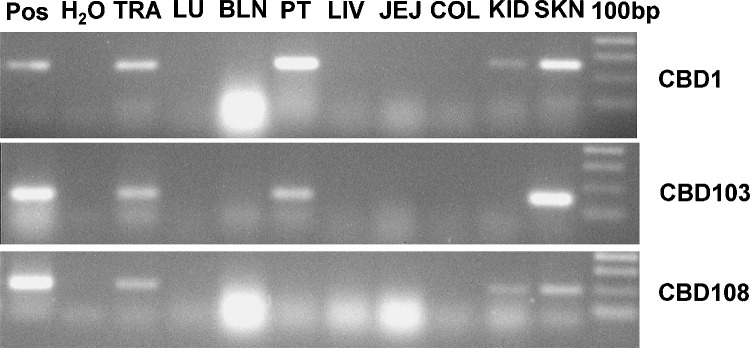
Conventional PCR was used to determine the presence of four β-defensins in the canine trachea. CBD1, CBD103 and CBD108 were found to be present whereas all tracheal samples as well as CTEC were negative for CBD102. The expression of CBD102 was therefore not further analysed.
The quantities of mRNA of CBD1, CBD103 and CBD108 were determined by quantitative PCR in untreated CTEC. The mRNA levels for CBD103 were found to be the highest with an average level of 4684 copies per ng of cDNA over all time points assessed. The average copy number for CBD1 was 336 copies per ng of cDNA and 18 copies per ng of cDNA for CBD108. When comparing different CTEC preparations, the mean copy numbers ranged from 191 to 9361 copies per ng of cDNA for CBD103, 13 to 890 copies per ng for CBD1 and 1.4 to 44 copies per ng for CBD108. The mRNA levels were also analysed within the same preparation of untreated primary cells over time. Levels of CBD1 increased over time whereas levels of CBD103 decreased and levels of CBD108 remained relatively stable (Table 2 ).
Table 2.
Levels of β-defensin mRNA over time in untreated primary tracheal epithelial cells.
| Time point | CBD1 | CBD103 | CBD108 |
|---|---|---|---|
| 24 h | 12.3 (±6.11) | 8088.3 (±2857.89) | 3.9 (±2.89) |
| 48 h | 20.1 (±3.49) | 5485.9 (±1749.12) | 5.7 (±1.68) |
| 72 h | 71.3 (±33.56) | 2818.7 (±1025.32) | 4.5 (±1.05) |
| 96 h | 74.6 (±37.39) | 1792.2 (±654.58) | 2.7 (±0.96) |
Values denote copies per ng of cDNA; values in brackets show the standard deviation. Four wells were analysed per time point.
3.2. Defensin production following stimulation with LPS
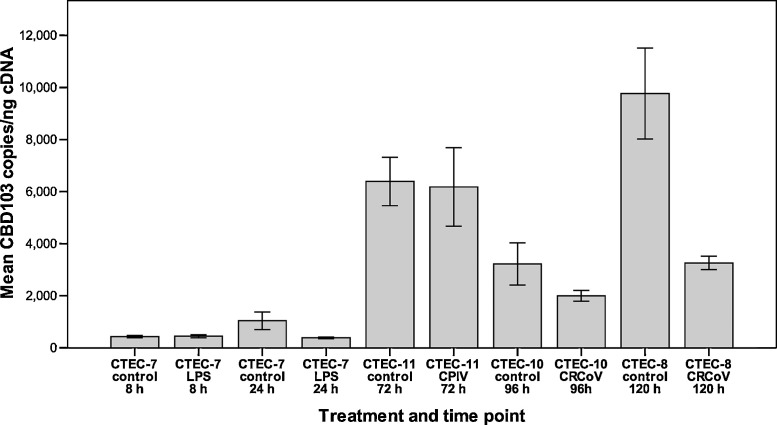
CBD103 mRNA levels remained unchanged at 8 h following LPS treatment (p = 0.481). At 24 h the levels of CBD103 in LPS-treated cells were reduced 1.9-fold compared to the control cells (Fig. 1 ). The reduction was statistically significant (p = 0.043).
Fig. 1.
Mean levels of CBD103 mRNA in canine tracheal epithelial cells (CTEC). The numbers (CTEC-7 to CTEC-11) refer to cells derived from different animals. Cells were treated with LPS (10 μg/ml) or infected with CRCoV or CPIV and analysed by quantitative real-time PCR. Ten wells were analysed per time point and per treatment for CTEC-7 and 24 wells were analysed per treatment for CTEC-8, CTEC-10 and CTEC-11. Error bars show standard error of the mean.
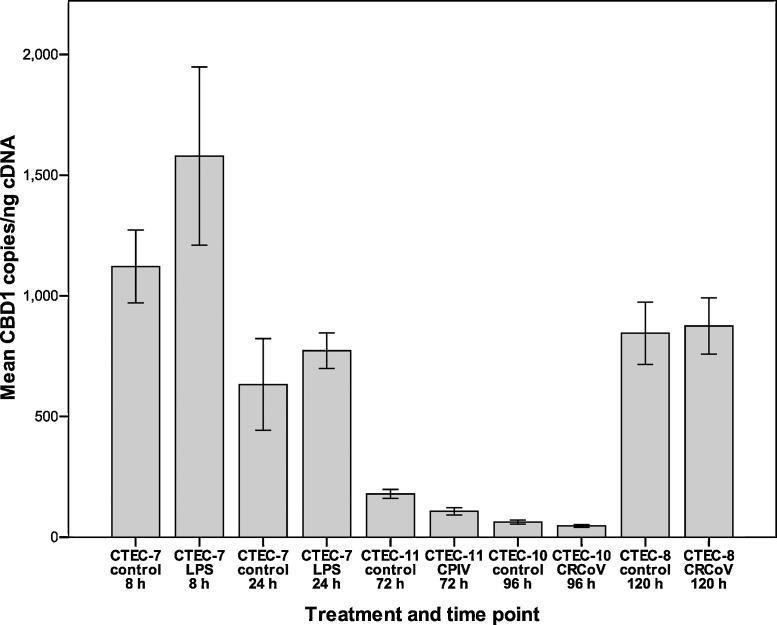
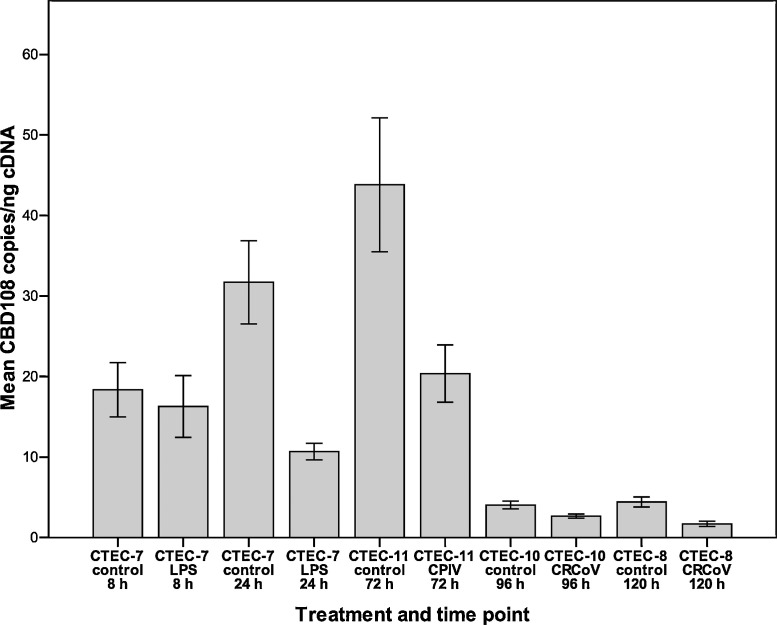
For CBD1 a small increase (1.4-fold) was detected after 8 h in LPS-treated cells and after 24 h (1.2-fold) (Fig. 2 ). The changes in mRNA levels were not statistically significant (p = 0.684 and 0.356, respectively). The levels of CBD108 mRNA were found to be unchanged in LPS-treated cells at 8 h (p = 0.684). At 24 h CBD108 levels in LPS-treated cells were 3-fold lower compared to control cells (p < 0.001, Fig. 3 ).
Fig. 2.
Mean levels of CBD1 mRNA in canine tracheal epithelial cells (CTEC). The numbers (CTEC-7 to CTEC-11) refer to cells derived from different animals. Cells were treated with LPS (10 μg/ml) or infected with CRCoV or CPIV and analysed by quantitative real-time PCR. Ten wells were analysed per time point and per treatment for CTEC-7 and 24 wells were analysed per treatment for CTEC-8, CTEC-10 and CTEC-11. Error bars show standard error of the mean.
Fig. 3.
Mean levels of CBD108 mRNA in canine tracheal epithelial cells (CTEC). The numbers (CTEC-7 to CTEC-11) refer to cells derived from different animals. Cells were treated with LPS (10 μg/ml) or infected with CRCoV or CPIV and analysed by quantitative real-time PCR. Ten wells were analysed per time point and per treatment for CTEC-7 and 24 wells were analysed per treatment for CTEC-8, CTEC-10 and CTEC-11. Error bars show standard error of the mean.
3.3. TNF-α production following LPS treatment
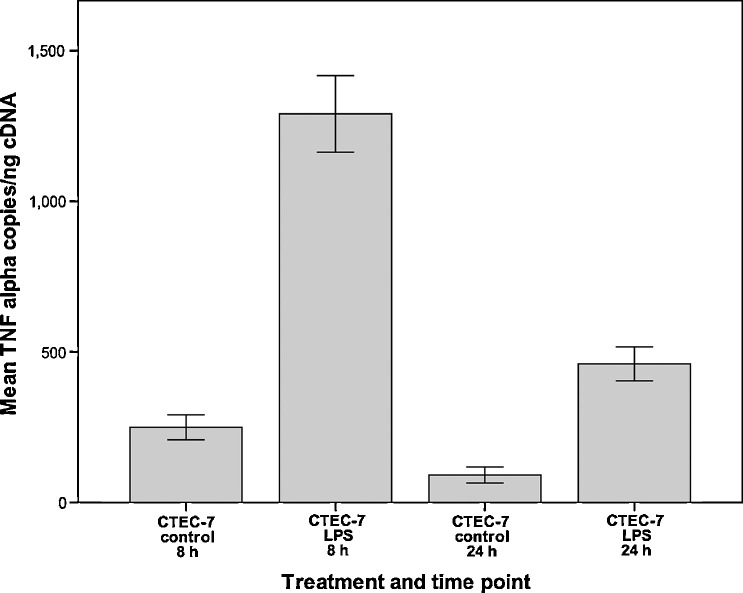
The effect of LPS treatment on the production of TNF-α was assessed at 8 h and 24 h (Fig. 4 ). At both time points LPS-treated cells had 5-fold increased levels of TNF-α compared to untreated control cells (p = 0.008 for both time points).
Fig. 4.
Mean levels of TNF-α mRNA in canine tracheal epithelial cells (CTEC-7). Cells were treated with LPS (10 μg/ml) and analysed by quantitative real-time PCR. Ten wells were analysed per time point and per treatment. Error bars show standard error of the mean.
3.4. Defensin production following viral infection
Following infection with CRCoV the levels of CBD103 were decreased 1.6-fold at 96 h (p = 0.214) and 2.7-fold at 120 h (p < 0.001, Fig. 1). For CBD1 the mRNA levels were 1.3-fold reduced at 96 h (p = 0.12) and unchanged at 120 h (p = 0.454, Fig. 2). The levels of CBD108 mRNA were 1.5-fold decreased at 96 h (p = 0.014) and 2.5-fold decreased at 120 h (p = 0.002, Fig. 3). Analysis of earlier time points (24 h, 48 h, and 72 h post-infection) showed no change in mRNA levels for β-defensins following CRCoV-infection (data not shown).
Following CPIV infection no change was detected in CBD103 levels (p = 0.232, Fig. 1). The levels of CBD1 (Fig. 2) and CBD108 (Fig. 3) were reduced 1.7-fold and 2.2-fold, respectively (p = 0.056 for both).
3.5. Expression of β-defensins in other canine tissues
Lung, spleen, liver, mesenteric lymph node and the small and large intestines were negative by conventional as well as qPCR. All three β-defensins were detected in the trachea and the skin using conventional PCR (Fig. 5 ) as well as qPCR. In addition CBD103-specific peaks were detected by qPCR in the palatine tonsil and the bronchial lymph node of one dog. By conventional PCR the bronchial lymph node sample was negative. CBD1 was detected in the palatine tonsil and kidney of one dog and CBD108 was detected in the kidney of one dog. Levels of β-defensins in lymphoid tissues and kidney were low and mRNA was not detected in tissues from both dogs.
Fig. 5.
Presence of β-defensins in canine tissues analysed by PCR. Positive control (Pos), trachea (TRA), lung (LU), bronchial lymph node (BLN), palatine tonsil (PT), liver (LIV), jejunum (JEJ), colon (COL), kidney (KID), skin (SKN), 100 bp molecular weight marker.
All tissue samples were negative for CBD102 when assessed using conventional PCR.
3.6. Effect of CBD103 on B. bronchiseptica
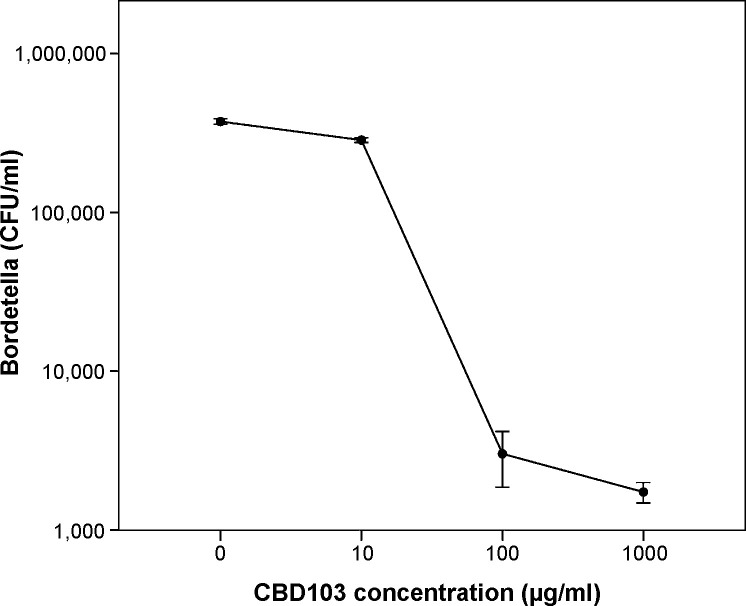
The number of B. bronchiseptica was on average reduced 200-fold following incubation with 1 mg/ml CBD103 and 124-fold following incubation with 100 μg/ml (Fig. 6 ). Treatment with 10 μg/ml CBD103 did not affect bacterial viability.
Fig. 6.
Effect of CBD103 on the viability of Bordetella bronchiseptica. Viability of bacteria was determined after 1 h incubation with CBD103 peptide by colony counting assay (colony forming units per ml). The data are based on four independent experiments. Error bars show standard error of the mean.
Addition of NaCl to a total concentration of 150 mM and 300 mM prevented the antibacterial effect of CBD103 on B. bronchiseptica at peptide concentrations of 1 mg/ml and 100 μg/ml.
4. Discussion
β-Defensins are components of the innate immune response which are important in protecting mucosal surfaces such as the respiratory tract from microbial invasion. In humans the presence and regulation of β-defensins in airway epithelial cells has been studied extensively (Schutte and McCray, 2002, Singh et al., 1998, Tsutsumi-Ishii and Nagaoka, 2003) however only one canine β-defensin, CBD122, has been shown to be present in the lung (Sang et al., 2005). The present study therefore assessed the presence of four β-defensins in the canine respiratory tract. Three of these (CBD1, CBD103 and CBD108) were found to be present in canine tracheal samples and in primary tracheal epithelial cells. Treatment of these cells with LPS did not lead to increased β-defensin production. This is in contrast to studies in other species where β-defensins have been found to be up-regulated in epithelial cells following incubation with LPS. Levels of the human β-defensin 2 (hBD2) were increased in vaginal epithelial cells after LPS treatment (Pivarcsi et al., 2005) and production of the bovine tracheal antimicrobial peptide (TAP) was induced by LPS in tracheal epithelial cells (Diamond et al., 2000). The up-regulation is thought to be mediated through toll-like receptor 4 (TLR-4) via the NF-κB pathway (Froy, 2005). In addition treatment of epithelial cells with inflammatory mediators such as IL-1β and TNF-α has been shown to induce β-defensin production (Diamond et al., 2000, Harder et al., 2001, Tsutsumi-Ishii and Nagaoka, 2003). The concentration of LPS (10 μg/ml) used in the present study was based on previous experiments using tracheal organ cultures in which this dose had led to an up-regulation of pro-inflammatory mediators (Priestnall et al., 2009). TNF-α was indeed up-regulated in CTEC following LPS treatment at both time points tested. Our results therefore indicate that the canine β-defensins CBD1, CBD103 and CBD108 may not be regulated via TLR-4 in tracheal epithelial cells. It is possible that different signalling pathways are responsible for the activation of these defensins. Additional cell types such as lymphocytes and macrophages that are present in the respiratory tract but absent from cultures of epithelial cells may be necessary to induce defensin production. Studies assessing the effect of supernatants from LPS-stimulated canine macrophages or lymphocytes on canine tracheal cells may allow identifying the pathways involved in regulation of β-defensins in the respiratory tract of the dog.
Several studies have analysed the effect of viral infection on the production of defensins. Human rhinovirus-16 has been shown to induce hBD2 and hBD3 production in primary bronchial epithelial cells (Proud et al., 2004). As viral replication was essential for this effect it was concluded that the up-regulation was mediated through intracellular double-stranded viral RNA. Expression of human β-defensins was also increased in respiratory papillomas compared to control tissues (Chong et al., 2006). Sheep β-defensin 1 was up-regulated in the lung of neonatal lambs following experimental infection with ovine parainfluenza virus type 1 (Grubor et al., 2004) whereas infection with bovine respiratory syncytial virus did not significantly increase expression of this defensin (Kawashima et al., 2006). In mice infection with influenza virus led to increased expression of murine β-defensins in the respiratory tract (Chong et al., 2008). In our study both CRCoV and CPIV failed to induce the expression of canine β-defensins despite both viruses producing double-stranded RNA intermediates during replication. β-Defensin levels were instead reduced at some time points. Down-regulation of β-defensins in the lungs of 2-week-old pigs has recently been reported following experimental infection with porcine reproductive and respiratory syndrome virus (Sang et al., 2009). It has moreover been shown that CRCoV-infected tracheal organ cultures exhibit decreased levels of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-8 compared to control cultures (Priestnall et al., 2009). The mechanism for this apparent down-regulation is not known, however it may involve disruption of signalling through TLR-3. It will be necessary to determine if CRCoV and CPIV infections are able to prevent defensin up-regulation by other mediators. However these mediators remain to be determined for canine defensins.
It is not clear if the observed down-regulation of β-defensins following challenge with LPS or viruses is of biological significance. CBD108 showed significant reductions in copy numbers, however the mRNA levels in the untreated epithelial cells were low and it is uncertain if a further reduction would affect the ability of the cells to prevent microbial infection.
In addition to tracheal samples β-defensins were also detected in other tissues including skin, lymphoid tissues and kidney. The tissue distribution of CBD103 appears to be similar to that of its ortholog hBD3 (Harder et al., 2001). Candille et al. (2007) have reported the expression of CBD103 and CBD1 in canine skin but were not able to detect CBD102 or CBD108. In our study CBD108 levels in the skin were low and CBD102 was not detected in any tissue samples tested. CBD102 has previously been reported to be present in the skin although expression was variable (Wingate et al., 2009). The levels of mRNA in some of the tissue samples tested in the present study may have been elevated due to inflammatory processes. The samples were from dogs euthanised due to behavioural problems and the only clinical data available was the absence of clinical respiratory disease. Moreover variable expression may be due to sampling of different areas within one tissue.
The antimicrobial activity of CBD103 was assessed against the respiratory pathogen B. bronchiseptica. The peptide was shown to significantly reduce the number of viable bacteria after incubation for one hour. The inhibitory concentrations determined for CBD103 in this study were about 10-fold higher than those determined for hBD3 for a range of bacteria (Harder et al., 2001), however B. bronchiseptica has been shown to be more resistant to treatment with antimicrobial peptides than other bacteria (Banemann et al., 1998). While no data were available for the effect of other canine β-defensins on B. bronchiseptica, a similar study using CBD122 obtained minimum inhibitory concentrations of 20–50 μg/ml for gram-negative pathogens of the canine urogenital tract and 10–100 μg/ml for gram-positive pathogens (Sang et al., 2005).
This study has shown that CBD103 is present at high levels in the canine upper respiratory tract and that it has antibacterial activity towards an important cause of respiratory disease in dogs. Complex respiratory diseases such as CIRD require novel approaches for prevention and treatment. CBD103 may be a candidate for the development of topical treatments of the upper respiratory tract. A possible drawback however is its salt sensitivity at physiological NaCl concentrations of 150 mM similar to other β-defensins (Maisetta et al., 2008, Sang et al., 2005). The NaCl concentration in human airway surface fluid has been shown to be below that of serum (Knowles et al., 1997, Smith et al., 1996); however other factors such as presence of proteins may also interfere with the activity of defensins (Maisetta et al., 2008). It therefore remains to be determined if canine β-defensins are active in conditions found in the upper respiratory tract in vivo. CBD1 and CBD108 were present at lower levels compared to CBD103 and it is not clear if they play a role in the defence of the canine tracheal epithelium. However, CBD1 as well as CBD103 may be important for the innate immune response of the skin.
Acknowledgements
This work was supported by the RCVS Trust. The authors wish to thank Sandra Greaves for technical assistance.
References
- Banemann A., Deppisch H., Gross R. The lipopolysaccharide of Bordetella bronchiseptica acts as a protective shield against antimicrobial peptides. Infect. Immun. 1998;66:5607–5612. doi: 10.1128/iai.66.12.5607-5612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonavoglia C., Martella V. Canine respiratory viruses. Vet. Res. 2007;38:355–373. doi: 10.1051/vetres:2006058. [DOI] [PubMed] [Google Scholar]
- Candille S.I., Kaelin C.B., Cattanach B.M., Yu B., Thompson D.A., Nix M.A., Kerns J.A., Schmutz S.M., Millhauser G.L., Barsh G.S. A β-defensin mutation causes black coat color in domestic dogs. Science. 2007;318:1418–1423. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker V.J., Brooks H.W., Brownlie J. The association of Streptococcus equi subsp. zooepidemicus with canine infectious respiratory disease. Vet. Microbiol. 2003;95:149–156. doi: 10.1016/S0378-1135(03)00155-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker V.J., Owen W.M., Paterson C., Barker E., Brooks H., Rycroft A.N., Brownlie J. Mycoplasmas associated with canine infectious respiratory disease. Microbiology. 2004;150:3491–3497. doi: 10.1099/mic.0.26848-0. [DOI] [PubMed] [Google Scholar]
- Chalker V.J., Toomey C., Opperman S., Brooks H.W., Ibuoye M.A., Brownlie J., Rycroft A.N. Respiratory disease in kennelled dogs: serological responses to Bordetella bronchiseptica lipopolysaccharide do not correlate with bacterial isolation or clinical respiratory symptoms. Clin. Diagn. Lab. Immunol. 2003;10:352–356. doi: 10.1128/CDLI.10.3.352-356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenvit Y., Brice G.T., Bacon D., Majam V., Williams J., Abot E., Ganeshan H., Sedegah M., Doolan D.L., Carucci D.J., Zimmerman D.H. A small peptide (CEL-1000) derived from the beta-chain of the human major histocompatibility complex class II molecule induces complete protection against malaria in an antigen-independent manner. Antimicrob. Agents Chemother. 2004;48:2455–2463. doi: 10.1128/AAC.48.7.2455-2463.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong K.T., Thangavel R.R., Tang X. Enhanced expression of murine [beta]-defensins (MBD-1, -2,- 3, and -4) in upper and lower airway mucosa of influenza virus infected mice. Virology. 2008;380:136–143. doi: 10.1016/j.virol.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Chong K.T., Xiang L., Wang X., Jun E.L., Xi L.F., Schweinfurth J.M. High level expression of human epithelial beta-defensins (hBD-1, 2 and 3) in papillomavirus induced lesions. Virol. J. 2006;3:75. doi: 10.1186/1743-422X-3-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford P.C., Dubovi E.J., Castleman W.L., Stephenson I., Gibbs E.P.J., Chen L., Smith C., Hill R.C., Ferro P., Pompey J., Bright R.A., Medina M.-J., Johnson C.M., Olsen C.W., Cox N.J., Klimov A.I., Katz J.M., Donis R.O. Transmission of equine influenza virus to dogs. Science. 2005;310:482–485. doi: 10.1126/science.1117950. 10.1126/science.1117950. [DOI] [PubMed] [Google Scholar]
- Diamond G., Kaiser V., Rhodes J., Russell J.P., Bevins C.L. Transcriptional regulation of beta-defensin gene expression in tracheal epithelial cells. Infect. Immun. 2000;68:113–119. doi: 10.1128/iai.68.1.113-119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Dubovi E.J., Brooks H.W., Brownlie J. Longitudinal study of viruses associated with canine infectious respiratory disease. J. Clin. Microbiol. 2004;42:4524–4529. doi: 10.1128/JCM.42.10.4524-4529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisk A.L., Konig M., Moritz A., Baumgartner W. Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. J. Clin. Microbiol. 1999;37:3634–3643. doi: 10.1128/jcm.37.11.3634-3643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J.H., Brunner S., Birnstiel M.L., Buschle M., Gabain A., Mattner F., Zauner W. The artificial antimicrobial peptide KLKLLLLLKLK induces predominantly a TH2-type immune response to co-injected antigens. Vaccine. 2004;22:3274–3284. doi: 10.1016/j.vaccine.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Froy O. Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell Microbiol. 2005;7:1387–1397. doi: 10.1111/j.1462-5822.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Grubor B., Gallup J.M., Meyerholz D.K., Crouch E.C., Evans R.B., Brogden K.A., Lehmkuhl H.D., Ackermann M.R. Enhanced surfactant protein and defensin mRNA levels and reduced viral replication during parainfluenza virus type 3 pneumonia in neonatal lambs. Clin. Diagn. Lab. Immunol. 2004;11:599–607. doi: 10.1128/CDLI.11.3.599-607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder J., Bartels J., Christophers E., Schroder J.-M. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- Jacobs A.A., Theelen R.P., Jaspers R., Horspool L.J., Sutton D., Bergman J.G., Paul G. Protection of dogs for 13 months against Bordetella bronchiseptica and canine parainfluenza virus with a modified live vaccine. Vet. Rec. 2005;157:19–23. doi: 10.1136/vr.157.1.19. [DOI] [PubMed] [Google Scholar]
- Kawashima K., Meyerholz D.K., Gallup J.M., Grubor B., Lazic T., Lehmkuhl H.D., Ackermann M.R. Differential expression of ovine innate immune genes by preterm and neonatal lung epithelia infected with respiratory syncytial virus. Viral Immunol. 2006;19:316–323. doi: 10.1089/vim.2006.19.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M.R., Robinson J.M., Wood R.E., Pue C.A., Mentz W.M., Wager G.C., Gatzy J.T., Boucher R.C. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J. Clin. Invest. 1997;100:2588–2595. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Yamamoto K., Eguchi M., Kubo M., Nakagami S., Wakisaka S., Kaizuka M., Ishii H. Rapid detection of mycoplasma contamination in cell cultures by enzymatic detection of polymerase chain reaction (PCR) products. J. Vet. Med. Sci. 1995;57:769–771. doi: 10.1292/jvms.57.769. [DOI] [PubMed] [Google Scholar]
- Krishnakumari V., Singh S., Nagaraj R. Antibacterial activities of synthetic peptides corresponding to the carboxy-terminal region of human [beta]-defensins 1–3. Peptides. 2006;27:2607–2613. doi: 10.1016/j.peptides.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Leikina E., Delanoe-Ayari H., Melikov K., Cho M.S., Chen A., Waring A.J., Wang W., Xie Y., Loo J.A., Lehrer R.I., Chernomordik L.V. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat. Immunol. 2005;6:995–1001. doi: 10.1038/ni1248. [DOI] [PubMed] [Google Scholar]
- Maisetta G., Di Luca M., Esin S., Florio W., Brancatisano F.L., Bottai D., Campa M., Batoni G. Evaluation of the inhibitory effects of human serum components on bactericidal activity of human beta defensin 3. Peptides. 2008;29:1–6. doi: 10.1016/j.peptides.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Pivarcsi A., Nagy I., Koreck A., Kis K., Kenderessy-Szabo A., Szell M., Dobozy A., Kemeny L. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human [beta]-defensin-2 in vaginal epithelial cells. Microb. Infect. 2005;7:1117–1127. doi: 10.1016/j.micinf.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Priestnall S.L., Mitchell J.A., Brooks H.W., Brownlie J., Erles K. Quantification of mRNA encoding cytokines and chemokines and assessment of ciliary function in canine tracheal epithelium during infection with canine respiratory coronavirus (CRCoV) Vet. Immunol. Immunopathol. 2009;127:38–46. doi: 10.1016/j.vetimm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud D., Sanders S.P., Wiehler S. Human rhinovirus infection induces airway epithelial cell production of human beta-defensin 2 both in vitro and in vivo. J. Immunol. 2004;172:4637–4645. doi: 10.4049/jimmunol.172.7.4637. [DOI] [PubMed] [Google Scholar]
- Quinones-Mateu M.E., Lederman M.M., Feng Z., Chakraborty B., Weber J., Rangel H.R., Marotta M.L., Mirza M., Jiang B., Kiser P., Medvik K., Sieg S.F., Weinberg A. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. Aids. 2003;17:F39–48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- Sang Y., Ortega M.T., Blecha F., Prakash O., Melgarejo T. Molecular cloning and characterization of three beta-defensins from canine testes. Infect. Immun. 2005;73:2611–2620. doi: 10.1128/IAI.73.5.2611-2620.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y., Ruchala P., Lehrer R.I., Ross C.R., Rowland R.R., Blecha F. Antimicrobial host defense peptides in an arteriviral infection: differential peptide expression and virus inactivation. Viral Immunol. 2009;22:235–242. doi: 10.1089/vim.2009.0005. [DOI] [PubMed] [Google Scholar]
- Schutte B.C., McCray P.B., Jr. [beta]-defensins in lung host defense. Annu. Rev. Physiol. 2002;64:709–748. doi: 10.1146/annurev.physiol.64.081501.134340. [DOI] [PubMed] [Google Scholar]
- Seville M., West A.B., Cull M.G., McHenry C.S. Fluorometric assay for DNA polymerases and reverse transcriptase. Biotechniques. 1996;21:664–672. doi: 10.2144/96214st04. [DOI] [PubMed] [Google Scholar]
- Singh P.K., Jia H.P., Wiles K., Hesselberth J., Liu L., Conway B.A., Greenberg E.P., Valore E.V., Welsh M.J., Ganz T., Tack B.F., McCray P.B., Jr. Production of beta-defensins by human airway epithelia. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.J., Travis S.M., Greenberg E.P., Welsh M.J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Tsutsumi-Ishii Y., Nagaoka I. Modulation of human {beta}-defensin-2 transcription in pulmonary epithelial cells by lipopolysaccharide-stimulated mononuclear phagocytes via proinflammatory cytokine production. J. Immunol. 2003;170:4226–4236. doi: 10.4049/jimmunol.170.8.4226. [DOI] [PubMed] [Google Scholar]
- Whelan J.A., Russell N.B., Whelan M.A. A method for the absolute quantification of cDNA using real-time PCR. J. Immunol. Methods. 2003;278:261–269. doi: 10.1016/s0022-1759(03)00223-0. [DOI] [PubMed] [Google Scholar]
- Wingate K.V., Torres S.M., Silverstein K.A., Hendrickson J.A., Rutherford M.S. Expression of endogenous antimicrobial peptides in normal canine skin. Vet. Dermatol. 2009;20:19–26. doi: 10.1111/j.1365-3164.2008.00707.x. [DOI] [PubMed] [Google Scholar]