Abstract
Human cytomegalovirus (HCMV) infection is associated with severe morbidity and mortality in immunocompromised individuals, mainly transplant recipients and AIDS patients, and is the most frequent cause of congenital malformations in newborn children. To date, few drugs are licensed for the treatment of HCMV infections, most of which target the viral DNA polymerase and suffer from many drawbacks, including long-term toxicity, low potency, and poor bioavailability. In addition, the emergence of drug-resistant viral strains is becoming an increasing problem for disease management. Finally, none of the current anti-HCMV drugs have been approved for the treatment of congenital infections. For all these reasons, there is still a strong need for new anti-HCMV drugs with novel mechanisms of action. The first events of the virus replication cycle, including attachment, entry, immediate-early gene expression, and immediate-early functions—in particular that of Immediate-Early 2 protein—represent attractive targets for the development of novel antiviral compounds. Such inhibitors would block not only the expression of viral immediate-early proteins, which play a key role in the pathogenesis of HCMV infection, but also the host immunomodulation and the changes to cell physiology induced by the first events of virus infection. This review describes the current knowledge on the initial phases of HCMV replication, their validation as potential novel antiviral targets, and the development of compounds that block such processes.
Abbreviations: ACV, acyclovir; AIDS, acquired immunodeficiency syndrome; CDV, cidofovir; CMV, cytomegalovirus; CpG, Cytosine-guanosine; crs, cis-repression sequence; E, Early; EC50 or EC90, Effective Concentration that results in 50% or 90% inhibition, respectively; EGFR, epidermal growth factor receptor; FOS, foscarnet; GCV, ganciclovir; GPI, glycosyl-phosphatidylinositol; HCMV, human cytomegalovirus; HDAC, histone deacetylase; HELF, human embryonic lung fibroblast; HIV, human immunodeficiency virus; HS, heparan sulfate; HSPGs, heparan sulfate proteoglycans; HSV, herpes simplex virus; IE, Immediate-Early; L, Late; LF, lactoferrin; mAb, monoclonal antibody; MIE, Major Immediate-Early; MIEP, Major Immediate-Early Promoter; MOI, multiplicity of infection; NF-kB, Nuclear Factor-kB; ODNs, oligodeoxynucleotides; PI3K, phosphatydilinositol 3-kinase; siRNA, small-interfering RNA; TBP, TATA box-binding protein; TFs, transcription factors; VACV, valaciclovir; VGCV, valganciclovir
Keywords: Human cytomegalovirus, Antiviral therapy, Virus attachment, Virus entry, Gene expression, Novel antiviral strategies
1. Introduction
Human cytomegalovirus (HCMV) is an enveloped, double-stranded DNA virus that has been included in the Herpesviridae family on the basis of its virion structure, kinetics of viral gene expression, and life-long persistence in the host (Landolfo et al., 2003, Mocarski et al., 2007, Britt, 2008). The strict species specificity for humans, the salivary gland tropism, and the slow growth in cell cultures make HCMV the prototype member of the beta-herpesvirus subfamily.
The HCMV genome consists of a linear, double-stranded 230-kbp DNA, the largest among herpesviruses. It is contained by an icosahedral protein capsid that is surrounded by a proteinaceous layer termed tegument. In turn, these structures are enclosed in a lipid bilayer called envelope. The mature virion particle is 150–200 nm in diameter.
A large number of virally encoded envelope glycoproteins are exposed on the virion surface whose primary function is to mediate virus attachment and entry into the host cells. After penetration into the cytoplasm, the genome migrates to the nucleus where it undergoes the processes of gene expression and replication. In productive infection, HCMV gene expression is a temporally coordinated and regulated cascade of transcriptional events that lead to the synthesis of three classes of viral proteins designated as immediate-early (IE), early (E), and late (L). Transcription of the L genes occurs after genome replication. HCMV gene transcription and genome replication are catalyzed by the cellular RNA polymerase II and by the viral DNA polymerase, respectively. The latter enzyme is the target of most licensed anti-HCMV drugs.
HCMV is an important opportunistic pathogen responsible of significant morbidity and mortality in susceptible individuals like those with immature or immunocompromised immune system. To date, no vaccine is available to prevent HCMV infection and few drugs are licensed to manage HCMV diseases. In recent years, many research groups focused their efforts in exploiting alternative targets for the prophylaxis and therapy of HCMV infections and new HCMV inhibitors have been identified. This review will examine the early events of HCMV replication as targets for the development of novel anti-HCMV therapies. The processes of HCMV attachment, entry, and IE genes expression will be described. A special emphasis will be placed on molecules that inhibit these processes, discussing their mechanism of action, their therapeutical potential, and their drawbacks.
2. Epidemiology and clinical features of HCMV infections
HCMV infections occur in all geographic locations and socioeconomic groups, although high population density and low sanitary conditions increase the risk of being infected (Cannon et al., 2010). HCMV can be transmitted via saliva, sexual activity, placental transfer, breastfeeding, blood transfusion, and solid organ or hematopoietic stem cell transplantation. After primary infection, HCMV establishes a lifelong latent infection that can periodically reactivate with shedding of infectious virus in body fluids (i.e., urine, saliva, tears, milk, semen, and cervical secretions) for months to years. In healthy individuals, HCMV infections are efficiently controlled by host immune responses and usually run asymptomatically, except for some cases of mononucleosis-like syndrome. Other rare complications of primary HCMV infections in immunocompetent individuals include arthralgia and arthritis, ulcerative colitis, pneumonitis, hepatitis, aseptic meningitis, and myocarditis (Gandhi & Khanna, 2004). By contrast, HCMV is responsible of severe morbidity and mortality in immunocompromised individuals like those with untreated acquired immunodeficiency syndrome (AIDS) and transplant recipients receiving immunosuppressive agents. Retinitis is the primary manifestation of HCMV infection in AIDS patients with low CD4+ T-cell counts, while transplant recipients are at great risk of developing pneumonia, gastrointestinal disease or to suffer an acute graft rejection (Gandhi and Khanna, 2004, Steininger et al., 2006, Buyck et al., 2010). In addition, HCMV is the most common cause of congenital infections. In HCMV-seronegative pregnant women, HCMV can be transmitted from mother to fetus in approximately 32% of cases. About 12.7% of infected newborns manifest symptoms at birth or during the first year of life. These include birth defects and developmental disabilities, such as microcephaly, hearing loss, vision loss, and mental retardation (Dollard et al., 2007, Kenneson and Cannon, 2007).
Finally, a large body of evidences link HCMV infections with the acceleration of a number of vascular diseases such as atherosclerosis, restenosis, and transplant vascular sclerosis that promotes chronic allograft rejection (Streblow et al., 2008).
3. Current anti-human cytomegalovirus strategies and available drugs
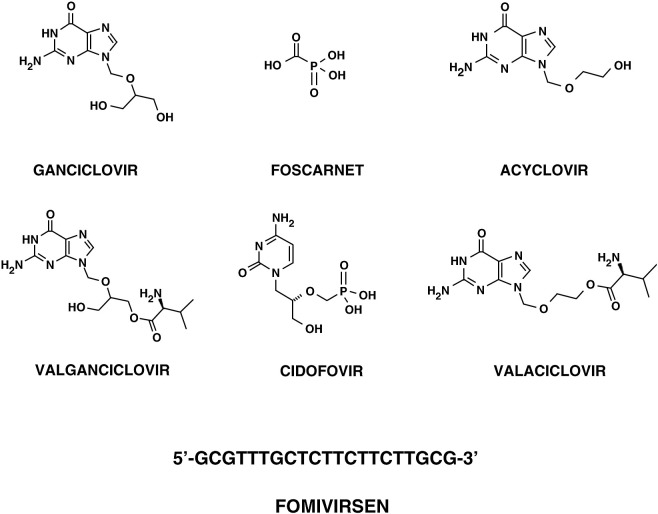
Three major therapeutic approaches are currently employed to manage HCMV infections and diseases: prophylactic therapy without evidence for virus replication, pre-emptive therapeutic suppression of virus replication in the absence of disease, and treatment of an established disease (Singh, 2006, Snydman, 2006). To date, a very few antiviral drugs have been developed and employed for either prophylactic or pre-emptive therapies and for direct treatment of HCMV disease. Five compounds are currently licensed to treat established HCMV infections: ganciclovir (GCV), its oral prodrug valganciclovir (VGCV), foscarnet (FOS), cidofovir (CDV), and fomivirsen. High-dose acyclovir (ACV), and more recently valaciclovir (VACV), have also been used for prophylaxis against HCMV in transplant recipients, while they are not recommended for treatment of active HCMV disease. No antiviral agent has been approved as yet for the treatment of congenital HCMV infections. With the exception of fomivirsen, an antisense oligonucleotide that is directed against the HCMV Major Immediate-Early (MIE) gene locus (see Section 4.4.2 for a description of fomivirsen), all the other compounds target, either directly or indirectly, the viral DNA polymerase (Yeh & Coen, 2004).
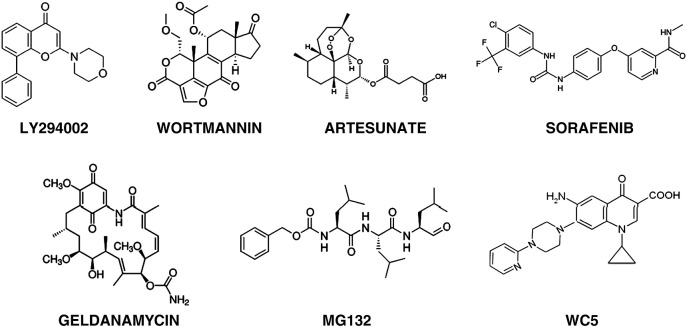
Ganciclovir [9-(1,3-dihydroxy-2-propoxymethyl)guanine; Fig. 1 ] was the first antiviral agent approved for treatment of HCMV infections, and remains the first-line choice for the treatment of HCMV diseases (Markham and Faulds, 1994, Razonable and Emery, 2004). GCV is an acyclic nucleoside analog of 2′-deoxyguanosine and is a prodrug. In HCMV-infected cells, GCV undergoes a first step of phosphorylation by the viral UL97 protein kinase and is then converted by cellular kinases to the active triphosphate form (GCV-TP) (Littler et al., 1992, Sullivan et al., 1992). GCV-TP interacts with the catalytic subunit (UL54) of the viral DNA polymerase and inhibits DNA synthesis by competing with the natural nucleoside triphosphate dGTP and acting as a chain terminator. GCV inhibits the viral polymerase more potently than the cellular DNA polymerase α. Mutations conferring resistance to GCV have been mapped to both UL54 (DNA polymerase) and UL97 (protein kinase) genes. The mutations associated with resistance to GCV generally map in the conserved subdomains of UL54, and can confer cross-resistance to CDV, or less commonly to FOS (Gilbert & Boivin, 2005). Before the introduction of the highly active antiretroviral therapy, the emergence of resistant strains was a major concern in AIDS patients who received a prolonged maintenance therapy (Erice et al., 1989, Lurain et al., 1994, Baldanti et al., 1995, Baldanti et al., 1998). Currently, drug-resistance still represents an important problem in transplant recipients (Lurain & Chou, 2010). The side effects of GCV include hematologic abnormalities (primarily neutropenia, anemia, and thrombocytopenia) and, based on preclinical toxicologic studies, probable long-term reproductive toxicity (Faulds and Heel, 1990, Markham and Faulds, 1994). GCV is currently available as an intravenous formulation (Cytovene IV®, Roche) and as a sustained-release intraocular implant (Vitrasert®, Baush & Lomb) approved for the treatment of HCMV retinitis. A GCV oral formulation (250 and 500 mg capsules; Cytovene®, Roche) received approval in 1994 for treatment of HCMV retinitis, but only as maintenance therapy. Although the oral formulation represented an important advance in treatment options for maintenance and prophylactic therapies, the low bioavailability (approximately 5%) and the high pill burden from the t.i.d. (three times a day) regimen posed severe limitations. In fact, oral GCV is no longer marketed.
Fig. 1.
Licensed anti-HCMV drugs.
To circumvent the low oral bioavailability of GCV, a prodrug—valganciclovir (Valacyte®, Roche; Fig. 1)—was developed. VGCV is the l-valyl ester of GCV and has an oral bioavailability of around 60%. It is rapidly metabolized to the active form (i.e., GCV) in the intestinal wall and in liver. Valganciclovir was approved in 2000 for treatment of HCMV retinitis in AIDS patients (Cvetkovic & Wellington, 2005) and later for prophylactic treatment of HCMV in solid organ transplant recipients. VGCV has now replaced oral GCV in clinical practice.
Foscarnet (Fig. 1) was the second drug approved for treatment of HCMV retinitis in AIDS patients since 1991. FOS is a pyrophosphate analog that inhibits the viral DNA polymerase (Chrisp and Clissold, 1991, Wagstaff and Bryson, 1994). Differently from GCV and CDV, it does not require prior activation by virus-encoded or cellular enzymes. It binds to the pyrophosphate binding site and blocks the cleavage of the pyrophosphate group from the terminal nucleoside triphosphate added to the growing DNA chain. Point mutations conferring resistance to FOS have been mapped in UL54 gene. Moreover, cross-resistance has been observed between GCV and FOS in several laboratory and clinical isolates. FOS is available as Foscavir® (AstraZeneca), the trisodium salt of phosphonoformic acid, which is administered intravenously. FOS is considered a second-line therapy; however, it is the drug of choice for patients failing GCV therapy due to viral resistance, or for those who cannot be treated with GCV due to dose-limiting neutropenia or leucopenia (Razonable & Emery, 2004). Due to its potential nephrotoxicity, its administration requires slow infusion, extensive prehydration, and frequent monitoring of serum creatinine levels (Naesens & De Clercq, 2001).
Cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine]; Fig. 1 is an acyclic nucleoside phosphonate analog of dCMP. It has a mechanism of action similar to that of GCV, but, being an acyclic nucleoside phosphonate, is not dependent on activation by a virus-encoded enzyme for activity. It is converted by cellular kinases to the diphosphoryl form, which then acts as an inhibitor of the viral DNA polymerase, causing premature chain termination in viral DNA synthesis (De Clercq & Holy, 2005). CDV was approved in 1996 for treatment of HCMV retinitis in AIDS patients. CDV is available only as an intravenous formulation (Vistide®, Gilead), because its oral bioavailability is very low (less than 5%). One of its advantages compared with GCV and FOS is its long intracellular half-life (Aduma et al., 1995); as such, good efficacy can be achieved with infrequent dosing. The major limitation of CDV is its severe renal toxicity, which can lead to electrolyte imbalance (Lea & Bryson, 1996); in fact, it requires coadministration of probenecid and prehydratation to reduce the nephrotoxic effects. Neutropenia is another side-effect that can be associated with CDV, and it was also shown to be both carcinogenic and teratogenic in preclinical toxicological studies (Lea & Bryson, 1996). For all these reasons, CDV remains a second-line therapy. The potential of CDV prodrugs to avoid renal tubular uptake and concentration is under evaluation.
Finally, acyclovir [9-(2-hydroxyethoxymethyl)guanine]; Fig. 1 is an analo of 2′-deoxyguanosine (Wagstaff et al., 1994). Like GCV, ACV is a prodrug that is phosphorylated by UL97 and subsequently by cellular kinases to the triphosphate form, the active form that promotes chain termination of the growing viral DNA. ACV is a less efficient substrate than GCV; in addition, ACV-TP displays a shorter half-life compared to GCV-TP in infected cells, resulting in lower intracellular levels of the active drug. Probably for these reasons, ACV exhibits a lower in vitro potency compared to GCV in HCMV-infected cells. Like for GCV, drug resistance arises from mutations in UL54 or UL97 genes. Valaciclovir (Valtrex®, GlaxoSmithKline; Fig. 1) is the l-valyl ester of ACV (Lowance et al., 1999) and presents higher bioavailability (55% compared to 6–10% of oral ACV) (Perry & Faulds, 1996). Both ACV and VACV lack sufficient potency to be used for treatment of active HCMV disease. Their primary use in transplant patients has been for suppression of HSV reactivation, but prophylactic treatment with these drugs can also reduce the occurrence of HCMV infection and diseases in solid organ transplant recipients. In particular, VACV has been approved in several countries for prophylaxis of HCMV infections in renal or heart transplant recipients.
The currently available anti-HCMV drugs have several drawbacks that limit their clinical utility (Villarreal, 2003). Most of the compounds have limited oral bioavailability, and thus must be administered intravenously. In addition, the currently approved systemic drugs have an unfavorable safety profile, with severe acute and long-term toxicities. Of special concern for a pediatric population is long-term reproductive toxicity and carcinogenicity. The emergence of drug-resistant viral strains also poses an increasing problem for disease management. Since most of the approved anti-HCMV compounds share a similar mechanism of action, targeting the viral DNA polymerase, mutant viruses resistant to one drug can sometime be resistant to others, although this is not a rule. Finally, the safety and efficacy of the currently available drugs in the treatment of congenital HCMV infection are still the subject of some debate despite the results from randomized controlled trials (see for example Kimberlin et al., 2003). Thus, there is still a strong need to identify new targets for anti-HCMV chemotherapy and to develop novel antiviral compounds and treatment strategies.
4. Early events in human cytomegalovirus replication: antiviral targets and inhibitors
The initial phases of HCMV replication cycle might provide useful targets for new antiviral drugs. Indeed, a number of reports on antiviral compounds that inhibit HCMV replication at an early stage have been recently published. Such a great interest has been raised by the compelling notion that the inhibition of HCMV replication at an initial stage, e.g., by inhibiting virus attachment and entry or by interfering with the expression and/or activity of IE gene products, could be beneficial at more than one level. In particular, drugs that target attachment and entry would be especially attractive, because such drugs would not need to enter cells to exert activity and would inhibit the synthesis of downstream, potentially toxic viral proteins. In addition, although cell-free virus transmission is believed to be unlikely because HCMV replication is highly cell-associated, cell-free virus is commonly found in body fluids such as urine, saliva and breast milk, and often at high titers (Britt, 2008). Thus, the block of virus attachment might prevent HCMV transmission via these excretions and reduce disseminated infections in severely immunocompromised patients, in which large amounts of cell-free infectious virus have been documented in the peripheral blood (Britt, 2008). Several compounds that inhibit attachment and/or entry of HCMV have been identified (see 4.1.1, 4.2.1). Most of these are relatively large, charged molecules exemplified by heparin, which binds to certain viral glycoproteins that mediate the initial attachment of HCMV to heparan sulfate moieties on the cell surface (Kari & Gehrz, 1992).
Two other particularly desirable stages at which to inhibit viral replication are transcription and translation events that lead to expression of IE proteins. Importantly, there is increasing evidence that IE proteins, in particular IE2-86 in concert with IE1-72, play crucial roles in the pathogenesis of HCMV infection by inducing a broad dysregulation of host gene expression. This leads to changes in host cell physiology and contributes to HCMV-induced cell cycle alterations, immunomodulation, and pro-inflammatory response (Stinski & Petrik, 2008). The currently available anti-HCMV drugs targeting the viral DNA polymerase have no impact on IE1-72- and IE2-86-induced effects that are produced before the early phase. Thus, when HCMV DNA replication is inhibited by one of such drugs, IE gene products accumulate in infected cells causing disturbances of host cell functions. The importance of IE functions has led to the suggestion that the prevention of their expression and/or functions may provide an alternative strategy for the inhibition of HCMV reactivation, replication, and immunopathogenesis. Fomivirsen is the only marketed anti-HCMV drug that is not targeted at the DNA polymerase; in fact, it potently inhibits the expression of the IE2-86 protein, thus preventing both HCMV replication and IE2-86-mediated effects such as pro-inflammatory immune mechanisms (Azad et al., 1993, Anderson et al., 1996, Perry and Balfour, 1999, Grillone and Lanz, 2001). Although fomivirsen has very limited therapeutic applications, the introduction of this drug into clinical use has provided a proof-of-concept that the inhibition of HCMV replication at an early stage is a feasible antiviral strategy. Novel anti-HCMV agents that block IE protein expression or activity, without causing major adverse effects, may be exploited for the treatment of patients that do not respond to the currently used inhibitors of viral DNA replication.
4.1. Virus attachment
The wide spectrum of diseases caused by HCMV correlates with its remarkable broad organ and cell tropism (Landolfo et al., 2003, Gerna et al., 2004). Epithelial cells lining the rhinopharinx and the genital tract represent two major portals of HCMV entry into the body during primary infection if the route of transmission is oral or sexual, respectively. In addition, endothelial cells of the vascular tree are infected through blood trasfusions or during a disseminated infection (Revello & Gerna, 2010). However, in HCMV-infected individuals many other cell types have been found infected, beside epithelial and endothelial cells. These include fibroblasts, smooth muscle cells, stromal cells, monocytes/macrophages, neutrophils, neuronal cells, and hepatocytes (Dankner et al., 1990, Cinque et al., 1997, van Den Pol et al., 1999, Sinzger et al., 2008). In vitro, several cell types are permissive for HCMV replication, although high cell-free virus yields can be obtained only in human skin, human embryonic lung fibroblasts (HELF), or in human umbilical vein endothelial cells (Revello et al., 1998, Hahn et al., 2004, Gerna et al., 2005). Of note, however, HCMV is able to bind, fuse, and initiate replication in almost all vertebrate cell types tested (Nowlin et al., 1991). The ubiquitous distribution of HCMV infection and the wide range of susceptible cells depend, at least in part, on the presence of broadly expressed receptors and cell-specific receptors that may act in combination to mediate the processes of virus attachment and entry.
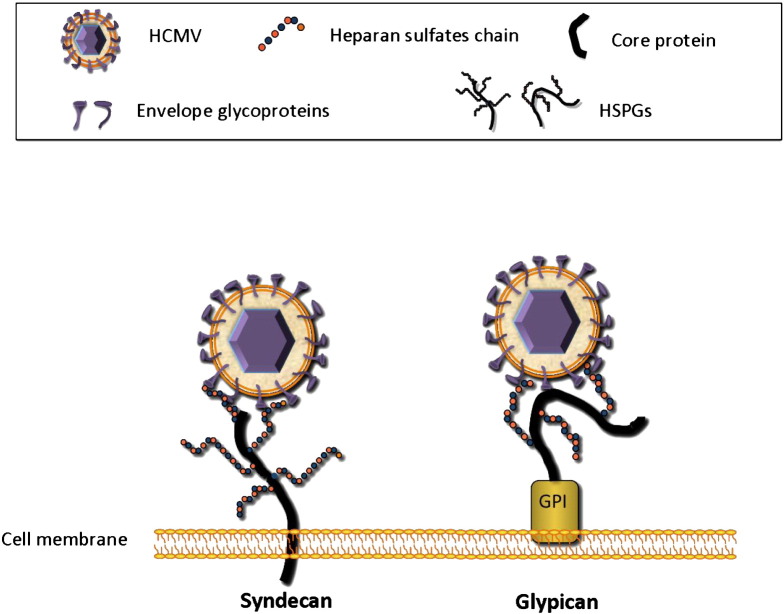
HCMV penetrates into host cells through a complex series of events that begins with adsorption onto heparan sulfate proteoglycans (HSPGs), a process that concentrates the virus on the cell surface (Compton et al., 1993). HSPGs are widely expressed on the surface of almost all eukaryotic cell types and typically consist of a core protein linked to heparan sulfates (HS) chains. HS are made up of unbranched anionic polysaccharides composed of variably sulfated repeating disaccharides units. The disaccharides unit that occurs most frequently within HS is composed of a glucuronic acid linked to N-acetylglucosamine and undergoes several modifications in the Golgi apparatus, including N-deacetylation and N-sulfation of glucosamine, C5 epimerization of glucuronic acid to form iduronic acid residues, 2-O-sulfation of iduronic and glucuronic acid residues, as well as 6-O-sulfation and 3-O-sulfation of glucosamine residue (Lindahl et al., 1994, Lindahl et al., 1998, Esko and Lindahl, 2001, Turnbull et al., 2001, Gorsi and Stringer, 2007). Syndecans and glypicans are two types of cell-surface HSPGs. The syndecans family comprises transmembrane HSPGs, while glypicans are typically glycosyl-phosphatidylinositol (GPI)-anchored HSPGs (Fig. 2 ). To date, it is not known whether HCMV binds to syndecans or glypicans. HCMV attachment to HSPGs seems to be primarly mediated by the glycoprotein complex gM/gN (Kari and Gehrz, 1992, Kari and Gehrz, 1993). The HCMV glycoprotein gB is not absolutely required for virus attachment (Isaacson & Compton, 2009) despite its ability to bind heparin, a soluble mimic of HSPGs (Compton et al., 1993).
Fig. 2.
HCMV attachment to cell surface HSPGs. Positively charged regions of the viral glycoprotein complex gM/gN interact with negatively charged heparan sulfate chains linked to a core protein. This process concentrates the virus on the cell surface. Syndecans and glypicans are the major cell-surface HSPGs. The syndecans family comprises transmembrane HSPGs, while glypicans are typically glycosyl-phosphatidylinositol (GPI)-anchored HSPGs. Since it has not yet been elucidated whether HCMV adsorbs to syndecans or glypican, both molecules are shown.
4.1.1. Attachment inhibitors
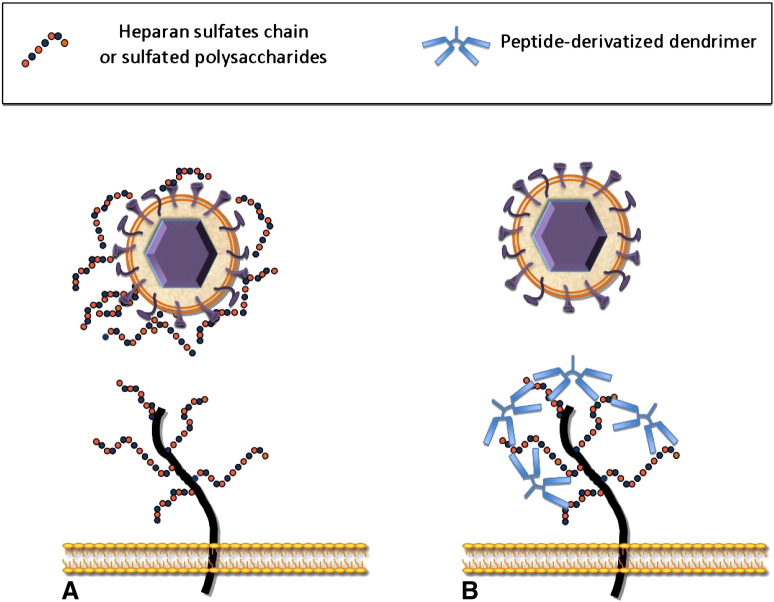
Compounds belonging to this class exert their antiviral activity by preventing HCMV interaction to HSPGs, although additional mechanisms might be involved in some cases. One type of inhibitors consists of sulfated polysaccharides, a large group of charged polymers that mimic HS chains. These compounds interact with viral glycoproteins thus blocking virus attachment to HSPGs by competitive inhibition (Fig. 3 A). A second type of inhibitors, namely peptide-derivatized dendrimers, acts through an opposite mechanism. They bind to HSPGs and are thus capable of competing with HCMV attachment to cell surface (Fig. 3B>). Lactoferrin, a component of the innate immune response, antagonizes HCMV infection at least in part by binding HSPGs, and therefore is included in this section. The clinical potential of these inhibitors is yet to be determined.
Fig. 3.
Mechanism of action of HCMV attachment inhibitors. (A) Sulfated polysaccharides are negatively charged molecules that mimic heparan sulfate chains. They interact with viral glycoproteins and block virus attachment to HSPGs by competitive inhibition. (B) Peptide-derivatized dendrimers contain clusters of basic amino acids that bind to the negatively charged sulfate and carboxyl groups of HS, thus inhibiting HCMV attachment to cell surface. To simplify this figure, only syndecans are shown.
4.1.1.1. Sulfated polysaccharides
These polyanionic compounds interfere with electrostatic interactions between the negatively charged sulfated/carboxyl groups of the HS chains exposed on the cell surface and the positively charged regions of HCMV glycoproteins (Ghosh et al., 2009).
As a general rule, antiviral potency can be increased by augmenting the degree of sulfation (i.e., the number of sulfate groups per monosaccharide residue), or the length of the oligosaccharide chain, or both (Witvrouw & De Clercq, 1997). The former feature raises the likelihood that the oligosaccharide will successfully interact with the HS-binding domain of a virus attachment protein, whereas the latter enables a single oligosaccharide to simultaneously make contacts with multiple copies of the virus attachment protein, as well as to induce cross-linking of the virions.
Over the last three decades, many sulfated polysaccharides from marine algae, cyanobacteria, and animal sources have shown potent anti-HCMV activity in preclinical studies. In addition, some semi-synthetic compounds also inhibit HCMV attachment. Table 1 summarizes the sulfated polysaccharides endowed with anti-HCMV activity and their potency.
Table 1.
Sulfated polysaccharides that inhibit HCMV attachment.
| Compound | EC50 (μg/ml)a | References |
|---|---|---|
| Sulfated polysaccharides from marine algae | ||
| Fucoidan | 2 | Baba et al., 1988 |
| κ-Carrageenan | 2.8 | Baba et al., 1988 |
| λ-Carrageenan | 0.3 | Baba et al., 1988 |
| Calcium spirulan | 8.3 | Hayashi et al., 1996b |
| A sulfate polysaccaride from Sargassum horneri | 3.3 | Hoshino et al., 1998 |
| Rhamnan sulfate | 1.7 | Lee et al., 1999 |
| Sulfated polysaccharides from cyanobacteria | ||
| Nostoflan | 0.47 | Kanekiyo et al., 2005 |
| Spirulan-like substance (TK-V2b) | 31 | Rechter et al., 2006 |
| Spirulan-like substance (TK-V3a) | 1.4 | Rechter et al., 2006 |
| Spirulan-like substance (TK-V3b) | 2.2 | Rechter et al., 2006 |
| Spirulan-like substance (TK-V4a) | 2.3 | Rechter et al., 2006 |
| Sulfated polysaccharides from animal source | ||
| Heparin | 0.75–2.60 | Andrei et al., 1991 |
| Heparin | 0.5 | Neyts et al., 1992 |
| Semi-synthetic compounds | ||
| Dextran sulfate | 0.5 | Baba et al., 1988 |
| Dextran sulfate | 0.25–0.77 | Andrei et al., 1991 |
| Dextran sulfate (Mw 1000) | 3.0 | Neyts et al., 1992 |
| Dextran sulfate (Mw 5000) | 0.7 | Neyts et al., 1992 |
| Dextran sulfate (Mw 40,000) | 0.3 | Neyts et al., 1992 |
| Dextran sulfate (Mw 70,000) | 0.6 | Neyts et al., 1992 |
| Pentosan polysulfate | 1.7 | Baba et al., 1988 |
| Pentosan polysulfate | 0.65 - 1.70 | Andrei et al., 1991 |
| Pentosan polysulfate | 1.7 | Neyts et al., 1992 |
| Sulfated bacterial glycosaminoglycan (Org 31581) | 0.3 | Baba et al., 1990 |
| Chemically degraded heparin (Org 31733) | 1.0 | Baba et al., 1990 |
| Sulfated derivatives of the E. coli K5 capsular polysaccharide | ||
| K5–N,OS(H) | 0.03 | Mercorelli et al., 2010 |
| K5-N,OS(L) | 0.29 | Mercorelli et al., 2010 |
This table reports only the EC50 obtained in attachment/binding assays carried out on fibroblasts infected with HCMV laboratory strains (e.g., AD169, Towne) to make the values as much comparable as possible among the different studies.
According to Table 1, the λ-Carrageenan and Nostoflan appear to be the most active in vitro among the sulfated polysaccharides of natural origin. However, it must be noted that a direct comparison of the antiviral potencies is of limited value since most of the compounds were tested with different antiviral assays and experimental settings. The latter include the cell type, the virus strain, and the time of compound addition. Interestingly, an additional mechanism of antiviral action was reported for spirulan-like polysaccharides. Besides inhibition of virus attachment and penetration, these compounds seem to induce intracellular antiviral mechanisms probably by altering membrane properties or cellular signaling processes (Rechter et al., 2006). This additional effect should be taken into account when assessing the antiviral activity of sulfated polysaccharides, as they may interact with the cell surface inducing intracellular signaling pathways.
Dextran sulfate, pentosan polysulfate, bacterial sulfated glycosaminoglycan, and chemically degraded heparin are semi-synthetic compounds that have been reported as HCMV inhibitors since the early 90s. More recently, a new class of semi-synthetic polysaccharides emerged as potent inhibitors of viral attachment, i.e., the sulfated derivatives of E. coli K5 capsular polysaccharide. As they are active against three sexually transmitted viruses, namely human immunodeficiency virus (HIV), human papillomavirus, and herpes simplex virus types-1 and -2 (HSV-1 and -2), they are promising candidates as ingredients in topical microbicides (Vicenzi et al., 2003, Lembo et al., 2008, Pinna et al., 2008, Rusnati et al., 2009). The capsular K5 polysaccharide from E. coli has the same structure as the heparin/HS biosynthetic precursor, the N-acetyl-heparosan. This observation represented the starting point for the synthesis of K5 derivatives by chemical or enzymatic methods. Indeed, the addition of sulfate groups at the N- or O-position of the sugars led to the synthesis of chemically defined compounds with different degrees of sulfation and charge distribution (Rusnati et al., 2005). Unlike heparin and other sulfated polysaccharides, they are devoid of anticoagulant activity and can be tailored in structure and molecular weight to closely mimic cell surface HS and to obtain a good toxicological profile. Very recently, a panel of K5 derivatives was tested against HCMV and some of them proved active (Mercorelli et al., 2010). The most active compound, K5-N,OS(H), higly sulfated at both the N- and O-positions, was able to potently inhibit the replication of laboratory HCMV strains, of clinical viral isolates, and of virus strains resistant to the anti-HCMV drugs currently used for therapy. Moreover, K5-N,OS(H) inhibited the cell-to-cell spread of the virus.
Taken as a group, sulfated polysaccharides have emerged as broad spectrum antiviral compounds and some of them entered in clinical trials as active ingredients of topical microbicides for the prevention of the sexual transmission of HIV (Rusnati et al., 2009). However, they appear less attractive to treat systemic infections like those sustained by HCMV. A major undesired effect associated with the use of many sulfated polysaccharides in vivo is their anticoagulant activity. However, a number of compounds that have minimal or no effect on plasma coagulation could be selected, including fucoidan, galactan, spirulan, xylomannan, heteroglycan, and K5 derivatives (Hayashi et al., 1996a, Ghosh et al., 2004, Rusnati et al., 2005, Adhikari et al., 2006, Chattopadhyay et al., 2007 Mandal et al., 2008, Chattopadhyay et al., 2008). Among these, fucoidan, spirulan, and K5 derivatives are active against HCMV (Table 1). Another factor that limits the use of sulfated polysaccharides in vivo is poor bioavailability (Flexner et al., 1991), although some of them can be adsorbed after oral administration (Ghosh et al., 2009). The only sulfated polysaccharide that showed anti-HCMV activity in humans is the semi-synthetic compound curdlan sulfate, a potent anti-HIV inhibitor in vitro (Gordon et al., 1994). In a 21-day intravenous tolerance study in HIV- and HCMV-infected individuals, 12 out of 21 patients tested were HCMV-negative at the end of 21 days and curdlan sulfate was well tolerated (Gordon et al., 1997).
4.1.1.2. Peptide-derivatized dendrimers
Two peptide-derivatized dendrimers, named SB105 and SB105_A10, were recently reported as potent inhibitors of HCMV attachment (Luganini et al., 2010). They consist of a lysine peptidyl branching core and four covalently attached surface peptide functional units, containing clusters of basic amino acids that bind to the negatively charged sulfate and carboxyl groups of HS. Indeed, SB105 and its derivative SB105_A10, in which the C-terminal glutamine residue of the functional ASLRVRIKKQ group was removed, could bind heparin immobilized on a BIAcore sensor chip, a “cell-free” model that resembles interaction with cell surface HS (Donalisio et al., 2010). Moreover, binding of SB105 and SB105_A10 to cell surface was prevented by soluble heparin as well as by pretreatment with heparinase I or heparitinase I (Luganini et al., 2010), indicating the ability of these dendrimers to bind cellular HS. This mode of action allows SB105 and SB105_A10 to prevent attachment by several HCMV strains (including GCV-resistant clinical isolates) to both primary fibroblasts and endothelial cells. Indeed, pretreatment of HELF cells with SB105 and SB105_A10 produced a significant inhibition of HCMV AD169 replication (EC50: 0.22 and 0.29 μM, respectively). This effect is neither virus strain specific nor cell type specific since it was also observed in HELFs infected with the clinical isolate AL-1 (EC50: 0.23 μM for SB105 and 0.25 μM for SB105_A10) and in human umbilical vein endothelial cells infected with the endotheliotropic VR1814 strain (EC50: 0.24 and 0.34 μM, respectively).
The use of peptide-derivatized dendrimers against HCMV is still at the preclinical stage of development. SB105 and SB105_A10 are attractive molecules due to their small size (nanometric), their ability to display multiple copies of active surface groups (multivalency), and their high anti -HCMV potency. Moreover, as they target a cellular component, the emergence of HCMV resistant strains could be less likely. However, a possible drawback of their mode of action is the induction of undesired cellular signaling cascades or the alteration of cellular responses. As HS interact with a variety of extracellular ligands and participate in many events during cell adhesion, migration, proliferation, and differentiation (Perrimon and Bernfield, 2000, Tumova et al., 2000), this issue should be throughly addressed in preclinical studies before candidating SB105 and SB105_A10 as antiviral molecules for the prevention and/or control of HCMV infections.
4.1.1.3. Lactoferrin
Lactoferrin (LF) is an iron-binding globular glycoprotein with a molecular mass of about 80 kDa. In vivo, it is produced by neutrophils, by the mammary glands during lactation, and by mucosal epithelial cells (Iyer and Lonnerdal, 1993, Baynes and Bezwoda, 1994, Lonnerdal and Iyer, 1995). Therefore, LF is present at high levels in milk and colostrum, and is also found in most mucosal secretions and body fluids including uterine fluid, vaginal secretion, seminal fluid, saliva, bile, pancreatic juice, small intestine secretions, nasal secretion, and tears (Levay and Viljoen, 1995, Lonnerdal and Iyer, 1995). LF exerts a broad antimicrobial (Iyer and Lonnerdal, 1993, Levay and Viljoen, 1995, Lonnerdal and Iyer, 1995, Kuipers et al., 1999) and antiviral activity (Harmsen et al., 1995, Marchetti et al., 1996, Shimizu et al., 1996, Swart et al., 1996, Grover et al., 1997, van der Strate et al., 2001a) and thus is considered to be a part of the innate defense, mainly at mucoses.
Early studies reported the anti-HCMV activity of intact LF from bovine or human milk, colostrum, or serum, with EC50 values of 36 μg/ml for bovine LF and 90 μg/ml for human LF against the viral strain AD169 (Harmsen et al., 1995, Swart et al., 1999). Subsequent investigations confirmed these findings with the viral strain RC256 obtaining EC50 values somewhat lower than those determined with AD169 (15 μg/ml for bovine LF and 60 μg/ml for human LF) (Beljaars et al., 2004). In all studies, bovine LF was more potent than the human isoform.
It was proposed that the activity of LF occurs at the level of virus attachment and entry, since preincubation of target cells with LF is essential, while the protective effect decreases if LF is added after infection (Harmsen et al., 1995, Beljaars et al., 2004). The N-terminal portion of the protein, called lactoferricin, is required for antiviral activity and contains a stretch of positively charged amino acids that bind to HSPGs (van Berkel et al., 1997, Swart et al., 1999, Andersen et al., 2001). Moreover, addition of positive charges to LF through amination increases its anti-HCMV effects, whereas negatively charged acylated derivatives of LF are devoid of this activity (Swart et al., 1999, Beljaars et al., 2004). Finally, preincubation of LF with heparin mutually blocks each other's antiviral activity (Andersen et al., 2001). Taken together, these studies demonstrate that the anti-HCMV activity of LF requires a specific distribution of positive and negative charges within the molecule, and suggest that at least part of the antiviral activity may be accomplished by preventing the virus from docking onto the cell surface HSPGs and entry into the cell. However, the mechanism of action of LF against HCMV remains to be fully elucidated.
The fact that LF is one of the antimicrobial component of breast milk and that breast-feeding is a high-risk factor for postnatal transmission of HCMV led some researchers to hypothesize that HCMV could be inhibited by secretions containing high levels of LF, thereby preventing transmission to the newborn. This hypothesis was disproved by a study that demonstrated that LF concentrations in breast milk do not correlate with HCMV transmission to preterm neonates (van der Strate et al., 2001b).
In 2003, van der Strate and colleagues demonstrated a synergistic effect between bovine LF and CDV in vitro that could be explained by inhibition of two different steps in HCMV replication (van der Strate et al., 2003). By contrast, combination with ACV or FOS resulted in antagonism, whereas neither synergy nor antagonism was observed when LF was combined with GCV. Although the mechanism of these interactions remains to be elucidated, a combination therapy consisting of LF with CDV deserves further investigation in vivo. The anti-HCMV activity of LF was studied in vivo in rat cytomegalovirus models with and without immune suppression (Beljaars et al., 2004). A moderate antiviral effect was observed that might be explained by a suboptimal dosage regimen of LF or by the presence of antibodies against LF. The latter factor is unlikely to play a role in humans treated with human LF. An earlier study demonstrated the ability of LF to inhibit murine cytomegalovirus replication in vivo by stimulating the activity of natural killer cells (Shimizu et al., 1996). By contrast, the study by Beljaars and colleagues indicated that the contribution of the immunomodulatory activity of LF to the overall antiviral activity is less important as compared to the direct inhibitory effects on HCMV attachment and entry (Beljaars et al., 2004).
4.2. Virus entry
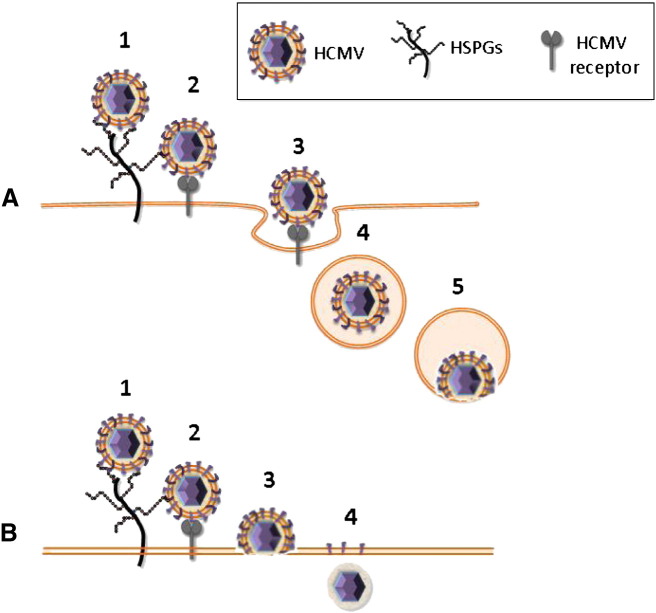
The HCMV entry process is yet not fully understood. Virus attachment to HSPGs is followed by a more stable interaction with post-attachment entry receptor(s) which triggers signal transduction cascades. Eventually, virus entry results in the release of the virion or virion components directly into the cell (Fig. 4 ). The epidermal growth factor receptor (EGFR) and integrins have been proposed as HCMV receptors but their role is still controversial. The findings that EGFR expression on HELF cells and breast carcinoma cells correlates with the susceptibility of these cells to HCMV infection and that anti-EGFR antibodies blocked HCMV entry into HELFs supported a role of EGFR as a HCMV entry receptor (Wang et al., 2003). Moreover, it has been recently demonstrated that inhibition of EGFR signaling abrogates HCMV entry into monocytes, indicating that EGFR can serve as a cellular tropism receptor (Chan et al., 2009). However, EGFR is not expressed on several HCMV permissive cell types and other studies demonstrated that it is not required for HCMV entry and signaling (Cobbs et al., 2007, Isaacson et al., 2007) and that the platelet-derived growth factor receptor-α rather than EGFR is necessary for efficient viral entry (Soroceanu et al., 2008). Integrins α2β1, α6β1, and αVβ3 were also identified as cellular receptors for HCMV entry (Feire et al., 2004). Evidences supporting a role for integrins as entry receptors include the identification of an integrin binding disintegrin-like domain within HCMV gB protein, a physical interaction of this domain with β1 integrin, and the ability of a soluble form of the domain or derived peptides to block HCMV infection at a post-attachment step (Feire et al., 2004, Feire et al., 2010). Most of the studies that support a role for EGFR and integrins as entry receptors were carried out by infecting fibroblasts with the HCMV laboratory strain AD169. This strain harbors deletions, mutations, and rearrangements within a genomic area that comprises the UL128, UL130, and UL131 genes which are important determinants of HCMV tropism for endothelial and epithelial cells (Cha et al., 1996, Murphy et al., 2003, Dolan et al., 2004, Hahn et al., 2004, Wang and Shenk, 2005a, Wang and Shenk, 2005b). The role of EGFR and integrins as entry receptors has not been confirmed for epithelial and endothelial cells infected with viral strains endowed with a broader cell tropism. It has been shown that HCMV entry into epithelial and endothelial cells depends on a complex of viral proteins made up of gH/gL/pUL128/pUL130/pUL131 and requires endocytosis and low pH-dependent fusion (Fig. 4A) (Wang and Shenk, 2005a, Ryckman et al., 2006, Ryckman et al., 2008). By contrast, HCMV entry into fibroblasts is mediated by the gH/gL/gO complex and involves a pH-independent fusion of the viral envelope with the cell plasma membrane (Fig. 4B) (Compton et al., 1992, Huber and Compton, 1998). At the end of the replicative cycle, viral progeny can spread directly to adjacent cells through cellular contacts or across cellular junctions, by a mechanism called cell-to-cell spread (Johnson & Huber, 2002). A number of HCMV proteins have been proposed to contribute to cell-to-cell spread. These include pUL128–pUL131 both in endothelial cells and in fibroblasts and gO or gB in fibroblasts (Jiang et al., 2008, Isaacson and Compton, 2009).
Fig. 4.
HCMV entry into epithelial and endothelial cells (A) or into fibroblasts (B). Virus attachment to HSPGs (1) is followed by a more stable interaction with a post-attachment entry receptor (2) which triggers intracellular signaling cascades. HCMV entry into epithelial and endothelial cells depends on the viral proteins gH/gL/pUL128/pUL130/pUL131 and requires endocytosis (3, 4) and low pH-dependent fusion (5). HCMV entry into fibroblasts is mediated by the gH/gL/gO complex and involves a pH-independent fusion of the viral envelope with the plasma membrane (3) resulting in release of capsid and tegument proteins directly into the cytoplasm (4).
4.2.1. Entry inhibitors
The efforts to identify pharmacological agents that can interfere with HCMV entry have resulted in a heterogeneous group of compounds that act at multiple stages of the entry process and have distinct mechanisms of action. These include natural compounds like dietary polyphenols, small molecules, synthetic peptides and oligonucleotides, and neutralizing antibodies (Table 2 ). Generally, entry inhibitors exert their biological properties by inhibiting protein–protein interactions or by preventing membrane fusion and receptor activation. Most of them are still at an early stage of development. Currently, only anti-HCMV hyperimmune globulin preparations are licensed for clinical use.
Table 2.
Anti-HCMV compounds that inhibit virus entry.
| Compound | Chemical backbone | Target (HCMV function affected) | Status | References |
|---|---|---|---|---|
| Baicalein | Flavonoid (5,6,7-trihydroxyflavone) | EGFR tyrosine kinase activity (Block of virus entry) | Pre-clinical | Evers et al., 2005 |
| Resveratrol | Polyphenol (3,5,4′-trihydroxy-trans-stilbene) | EGFR tyrosine kinase activity (Block of virus entry) | Pre-clinical | Atreides et al., 2002, Evers et al., 2004 |
| CFI02 | Thiourea small molecule | gB (Block of virion fusion) | Pre-clinical | Jones et al., 2004 |
| Peptides containing heptad repeat motif | α-amino acid oligomers | Heptad repeat motif in gB (Block of virus entry) | Pre-clinical | Lopper & Compton, 2004 |
| β-peptides | β-amino acid oligomers | Heptad repeat motif in gB (Block of virus entry) | Pre-clinical | English et al., 2005 |
| CpG 2006 | Phosphorothioate-modified oligonucleotides | Unknown (Block of virus entry) | Pre-clinical | Luganini et al., 2008a |
| Cytogam (CSL Behring), Cytotect (Biotests AG), and others | HCMV hyperimmune globulin preparations | Envelope glycoproteins (Block of virus entry) | Licensed for the prophylaxis of HCMV diseases in solid organ transplant recipients, either alone or in combination with GCV | Cui et al., 2008 |
| Human monoclonal antibodies | Human monoclonal immunoglobulin | Envelope glycoproteins (Block of virus entry) | Pre-clinical | Funaro et al., 2008, Macagno et al., 2010 |
4.2.1.1. Polyphenols
Polyphenols are a large group of antioxidant compounds, many of which are contained in fruits, vegetables, and beverages (tea, coffee, beer, wine, and fruit drinks). Among these, flavonoids and resveratrol have been shown to inhibit HCMV replication.
4.2.1.2. Flavonoids
Flavonoids are a class of secondary plant metabolites containing 15 carbon atoms. They are widespread in nature, being fruits, tea, and soybean the most important dietary source for humans. All these compounds possess a marked antioxidant effect (Heim et al., 2002). Beside this property, many reports proposed additional biological activities including anti-inflammatory, anti-proliferative, vasculoprotective, antimicrobial, and antiviral effects (Formica and Regelson, 1995, Cushnie and Lamb, 2005, Friedman, 2007, Rathee et al., 2009, Androutsopoulos et al., 2010). Indeed, different flavonoids inhibit replication of a wide array of viruses in vitro, including HSV, poliovirus, adenovirus, respiratory syncytial virus, Sindbis virus, parainfluenza virus, pseudorabies virus, Rous sarcoma virus, SARS coronavirus, influenza virus, and HIV (Amoros et al., 1992, Hayashi et al., 1997, Lin et al., 1999, Mitrocotsa et al., 2000, Robin et al., 2001, Akula et al., 2002, Chiang et al., 2003, Wei et al., 2004, Yi et al., 2004, Cos et al., 2008). These observations, along with the fact that various flavonoids affect cellular signaling pathways that are required for HCMV replication, e.g. those in which phosphatidyl inositol 3-kinase (PI3K), Akt, and mitogen-activated protein kinases are involved, stimulated the search of flavonoids endowed with anti-HCMV activity. A study by Evers et al. (2005) identified nine flavonoids that block the replication of the HCMV strain Towne. Among these, baicalein (5,6,7-trihydroxyflavone) was the most potent inhibitor (EC50: 1.2 μM). This compound was originally isolated from the roots of Scutellaria baicalensis, an herb widely used in Chinese traditional medicine for treatment of chronic hepatitis, allergy, thrombotic stroke, and inflammatory diseases (Shang et al., 2010). Several studies confirmed a potent anti-inflammatory effect of baicalein (Sekiya and Okuda, 1982, Huang et al., 1994, Chen et al., 2004, Huang et al., 2005). As far as the anti-HCMV activity, it was demonstrated that baicalein acts prior to IE protein expression. Its primary mechanism of action is to inhibit HCMV entry by blocking EGFR tyrosine kinase activity, a cellular function required for HCMV penetration (Wang et al., 2003, Chan et al., 2009). Safety and efficacy issues remain to be addressed in vivo (see also next section).
4.2.1.3. Resveratrol
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a natural compound produced by several plants in response to bacterial or fungal infections or other stresses. It is found in the skin of red grapes, in red wine, in plums, and in peanuts, which represent primary dietary sources of resveratrol for humans. Moreover, it can be produced by chemical synthesis (Farina et al., 2006) or by an engineered Saccharomyces cerevisiae (Trantas et al., 2009). Many studies reported a beneficial effect of resveratrol in the prevention or in slowing the progression of inflammation, cardiovascular diseases, and cancer (Baur & Sinclair, 2006). It was also shown that resveratrol inhibits infection by several viruses in vitro including HSV-1 and -2, varicella-zoster virus, SARS coronavirus, and influenza virus (Docherty et al., 1999, Palamara et al., 2005, Docherty et al., 2006, Li et al., 2006). Moreover, it was active in combination therapy against HIV (Heredia et al., 2000). In vivo studies in mice showed that topical administration of resveratrol inhibits HSV replication in the vagina and limits extra-vaginal disease (Docherty et al., 2004, Docherty et al., 2005). The anti-HCMV activity of resveratrol was initially documented in 2002 and then confirmed in 2004 during a screening of a panel of stilbenes and related compounds (Atreides et al., 2002, Evers et al., 2004). The latter study reported an EC50 of 1.7 μM for resveratrol against the HCMV strain Towne, with a selectivity index of at least 50. The other stilbenes or stilbene-like compounds examinated lacked of antiviral activity suggesting that the phenolic hydroxyl groups of resveratrol are required for such activity. Investigation of its mode of action revealed that resveratrol acts within the first 4 h of infection and prevents IE genes expression. It was found that resveratrol blocks a number of cellular events triggered by HCMV early after infection (Evers et al., 2004). These include EGFR phosphorylation, PI3K signaling and the activation of the transcription factors Sp1 and Nuclear Factor-κB (NF-κB) (Johnson et al., 2001, Wang et al., 2003). In light of these findings, the authors proposed that the primary mechanism of action of resveratrol against HCMV is to block EGFR activation, thus preventing virus entry. The efficacy of resveratrol as an anti-HCMV agent remains to be investigated in vivo. Its pharmacokinetics and short half-life and the fact that it is extensively metabolized in the body may require a high intake of resveratrol to achieve antiviral activity (Baur & Sinclair, 2006). Like some anticancer drugs (e.g., etoposide and doxorubicin), resveratrol and flavonoids (including baicalein) inhibit the activity of cellular DNA topoisomerase, raising concerns about their carcinogenic potential for the fetus, which has insufficient detoxification systems (Jo et al., 2006, Leone et al., 2010). Indeed, high intake of polyphenol/flavonoid compounds during pregnancy is suspected to increase the risk of infant and early childhood leukemia by causing site-specific DNA cleavage in the MLL gene (Ross, 2000, Strick et al., 2000, Paolini et al., 2003, Barjesteh van Waalwijk van Doorn-Khosrovani et al., 2007, Azarova et al., 2010). These safety issues discourage the development of polyphenols as drugs for the treatment of HCMV infection in pregnant women.
4.2.1.4. CFI02
This compound is a thiourea small molecule identified during a high-throughput screening of a proprietary chemical library at Wyeth Research (Pearl River, New York, USA) (Jones et al., 2004). CFI02 emerged as a potent and selective inhibitor of HCMV. At a multiplicity of infection (MOI) of 0.3, its EC50 against the HCMV AD169 strain was 0.04 μM (determined with an automated assay), while its EC90 was 0.12 μM in yield reduction assays. At MOI of 1 or 3, the EC90 increased only 1.5- and 2.5-fold, respectively. In addition, CFI02 prevented cell-to-cell spread of HCMV. In a cytotoxicity assay, the 50% cytotoxic concentration of CFI02 was >50 μM. Thus, its selectivity index is >2000. Of interest, the antiviral activity of CFI02 is very specific: neither HSV, varicella-zoster virus, respiratory syncytial virus, nor the closely related beta-herpesviruses murine CMV, simian CMV, and rat CMV are inhibited by CFI02. Mechanism-of-action studies revealed that CFI02 acts within the first 2 h of the HCMV replication cycle and remains effective when added after attachment. CFI02 prevents cytoplasm-to-nucleus transport of virions and viral IE/E gene expression. Its inhibitory effect can be reversed with polyethylene glycol, a chemical mediator of membrane fusion. Moreover, mutant viruses resistant to CFI02 bear mutations in gB that are sufficient to confer resistance. Taken together, these findings indicated that CFI02 prevents HCMV entry by inhibiting gB-mediated virion envelope fusion with the cell plasma membrane.
Although CFI02 has not been developed clinically due to in vivo stability and metabolic issues, its discovery validated gB-mediated virion fusion as a target for the development of new anti-HCMV strategies.
4.2.1.5. Peptides
The successful development of the HIV entry inhibitor Enfuvirtide for clinical use encouraged the rational design of peptides as inhibitors of HCMV entry. Enfuvirtide is a synthetic peptide of 36-amino acids corresponding to the C-terminal heptad repeat segment of the HIV fusion protein gp41 (Kilby et al., 1998). The heptad repeat motif consists of a seven-amino-acid pattern in which hydrophobic side chains occur at the first and fourth position. This structural motif is the basis of a common mode of helix association in proteins, namely the α-helical coiled-coil (Lupas, 1996). A heptad repeat motif was also identified in a region of HCMV gB and gH glycoproteins and it was proposed to play a role in HCMV fusion mechanisms (Lopper & Compton, 2004). In support to this hypothesis, it was demonstrated that peptides corresponding to these regions prevent entry of a variety of clinical and laboratory HCMV strains in a specific manner (Lopper & Compton, 2004). These findings validated the heptad repeat motif as a target for the development of HCMV entry inhibitors, although the peptides displayed a low potency compared to that of Enfuvirtide. Thus, alternative strategies were explored to disrupt coiled-coil interactions. A promising line of research was opened by the rational development of β-amino-acid oligomers (β-peptides) designed to inhibit gB-mediated HCMV entry (English et al., 2005). These peptides display a secondary structure called 12-helix that mimicks putative α-helical segments within the gB glycoprotein. Some β-peptides were identified as highly specific inhibitors of HCMV entry that probably act by altering the viral fusion machinery (English et al., 2005). The intrinsic advantages of β-peptides with respect to the gB-derived α-peptides are a higher antiviral potency and resistance to proteolytic degradation. Further studies are however required to determine their therapeutical potential.
4.2.1.6. Phosphorothioate-modified oligonucleotides
CpG oligodeoxynucleotides (ODNs) are synthetic, single-stranded DNA fragments that contain unmethylated cytosine–guanosine (CpG) dinucleotides (Krieg, 2006). CpG dinucleotides are relatively common in viral and bacterial genomes and are recognized as a pathogen-associated molecular pattern by Toll-like receptor 9. Therefore, either genomes containing unmethylated CpG dinucleotides or CpG ODNs trigger an immunomodulatory cascade that results in the secretion of a variety of proinflammatory and antiviral cytokines and in the activation of innate and adaptative immune responses. These properties stimulated the development of CpG ODNs as vaccine adjuvants and suggested their potential for protecting against viral infections in a non-specific manner (Ashkar et al., 2003, Dong et al., 2003, Schlaepfer et al., 2004, Wong et al., 2005, Kamstrup et al., 2006, Krieg, 2006, Jiang et al., 2011). CpG ODNs belong to three different classes, i.e., A, B, and C, on the basis of distinct structural and biological features. Very recently, Luganini and colleagues reported that the prototype B class CpG 2006 and other related ODNs display a potent and selective anti-HCMV activity (EC50: 0.02 μM against HCMV AD169) (Luganini et al., 2008a). Studies with mutant versions of CpG 2006 revealed that its activity is independent from the presence of CpG dinucleotides but requires a phosphorothioate backbone. Interestingly, CpG 2006 exerts its antiviral activity by blocking virus entry with a mechanism that does not involve the activation of Toll-like Receptor 9 pathway (Luganini et al., 2008a). This novel mode of action of CpG 2006 may represent a starting point for the development of anti-HCMV strategies based on phosphorothioate-modified ODNs. Importantly, a number of studies carried out on rodents and humans revealed a good safety profile of CpG ODNs (Krieg, 2006).
4.2.1.7. HCMV hyperimmune globulin and human monoclonal antibodies
Passive immunization with HCMV hyperimmune globulin is a feasible strategy for the prophylaxis of HCMV infections and to prevent symptomatic HCMV diseases. Currently, three preparations of intravenous immunoglobulin enriched for antibodies against HCMV are available on the market: Cytogam (CSL Behring), Cytotect (Biotests AG), and a preparation produced by the Australian Red Cross blood service. These products contain high titers of neutralizing antibodies that prevent HCMV entry into endothelial and epithelial cells (Cui et al., 2008). A number of studies and clinical trials support the use of HCMV hyperimmune globulin for the prophylaxis of HCMV diseases in solid organ transplant recipients, either alone or in combination with GCV (Snydman, 2001, Valantine et al., 2001, Bonaros et al., 2008). By contrast, their use in hematological malignancies and hematopoietic stem cell transplantation is still controversial (Raanani et al., 2008). Passive immunization with CMV hyperimmune globulin has also been studied for the in utero treatment and prevention of congenital HCMV infections. Preclinical studies were based mostly on the guinea pig model of cytomegalovirus infection because the guinea pig CMV crosses the placenta causing infection to the fetus like HCMV in humans. Moreover, the guinea pig and human placenta are similar (Schleiss, 2008). Improved perinatal outcomes after treatment of congenital guinea pig CMV infection were observed, paving the way to clinical trials in humans (Griffith et al., 1985, Bratcher et al., 1995). When administered to pregnant women with a primary CMV infection, HCMV hyperimmune globulin has shown promising results for both the treatment and the prevention of fetal infection (Adler & Nigro, 2009). However, randomized, blinded, controlled trials are needed to assess the benefits of passive immunization with HCMV hyperimmune globulin in HCMV congenital infections.
A major drawback of an immunotherapy based on hyperimmune globulin is the need to administer very high doses intravenously in order to reach therapeutic concentrations of neutralizing antibodies. At least in part, this is a consequence of their low potency observed in vitro (Roy & Grundy, 1992). As for as safety, intravenous immunoglobulins are the most purified blood derivatives and have been administered safely to humans for decades. Nevertheless, the risk of transmission of infectious agents, including viruses and prions, cannot be completely eliminated.
To overcome these limitations and to improve the therapeutic potential of passive immunization against HCMV, two groups have recently generated human monoclonal antibodies (mAbs) that potently neutralize HCMV infection in vitro (Funaro et al., 2008, Macagno et al., 2010). Both groups utilized immortalized B cells from HCMV-immune donors as a source of antibodies. Immunoglobulins produced by these B cells are expected to be highly relevant for the body's defense against HCMV, and therefore provide a favorable starting point for generation of highly protective mAbs. Funaro and colleagues developed an innovative technology and exploited it to generate a panel of mAbs that neutralize HCMV infection of fibroblasts or endothelial cells with a potency about 20-fold higher than that of commercially available HCMV hyperimmune globulins (Funaro et al., 2008). The most active mAbs showed a strong binding to the gB or the gH viral glycoproteins. The VH and VL variable regions of the most promising candidate mAb were cloned, sequenced, and used to generate a recombinant IgG that displayed a neutralizing activity similar to that of the parental mAb. Macagno and colleagues were able to generate extremely potent mAbs that neutralize HCMV infection in endothelial, epithelial, and myeloid cells with EC90 values in the low pM range (Macagno et al., 2010). Most of them bind to conformational epitopes made up by the gH/gL/UL128-131A complex. In conclusion, human natural or recombinant mAbs are promising candidates for passive immunotherapy endowed with higher potency than that of hyperimmune immunoglobulins and devoid of the risk of unwanted transmission of infectious pathogens associated with the use of plasma derivatives. Moreover, they are expected to avoid some of the side effects of humanized and mouse mAbs, including immunogenicity, a rapid removal from the blood, and systemic inflammatory effects.
4.3. Post-entry events prior to viral gene expression
In this section, the post-entry events that occur during HCMV replication before DNA synthesis, which are summarized Fig. 5 , will be described.
Fig. 5.
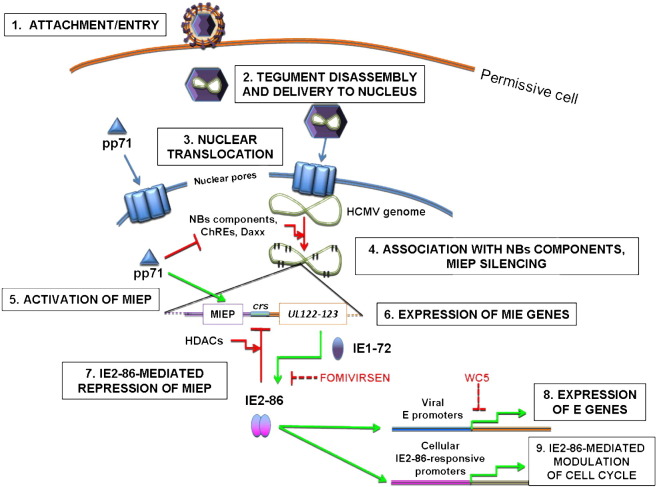
Schematic representation of post-entry events occurring prior to viral genome replication in HCMV cycle. HCMV entry in permissive host cell (1) occurs either via fusion with the plasma membrane (in fibroblasts) or via receptor-mediated endocytosis (in epithelial and endothelial cells). Once in the cytoplasm, viral tegument disassembly occurs (2). Some viral tegument proteins, like pUL47/pUL48 and maybe ppUL32, are engaged in the transport of virus capsids through the cytoplasm and in intranuclear translocation of viral genome via nuclear pores (3); other tegument proteins, such as pp71, are able to localize autonomously into the nucleus. After intranuclear delivery, HCMV genome quickly becomes associated with Nuclear Bodies (NBs) components, in particular Daxx, and with chromatin remodeling enzymes (ChREs) (4) in a process that would lead to the Major Immediate-Early Promoter (MIEP) silencing without the counteracting effect of pp71. The pp71 protein, in fact, causes the degradation of Daxx and blocks MIEP silencing, thus activating MIE gene expression (5). After expression of MIE genes (6), IE2-86 acts as a repressor of its own promoter in concert with histone deacetylases (HDACs) by binding to crs (7). Furthermore, it acts as a transactivator of viral and cellular promoters to induce viral early (E) gene expression (8) and modulate the cell-cycle progression of infected cell (9). Depicted are also viral targets affected by fomivirsen and WC5.
4.3.1. Transport of viral genome into the nucleus
The events that follow HCMV entry are still poorly characterized. Despite the fact that it is not known yet how the tegument disassembles, there is evidence that at this stage some proteins of the viral tegument play a major role in guiding the transport of viral capsids from the cytoplasm to the cell nucleus and the subsequent intranuclear translocation of viral genome. The tegument proteins that most likely are involved in genome-containing capsid transport to the nucleus are pUL48, pUL47, and perhaps pp150/UL32. The current knowledge on the activities of these proteins as well as recent experimental data obtained from studies on other herpesviruses support a model for HCMV post-fusion events clearly illustrated in (Kalejta, 2008).
After entry, HCMV exploits the cellular microtubule network to travel from the cell membrane (in the case of entry via fusion in fibroblasts) or endocytic vesicles (in the case of entry via receptor-mediated endocytosis in epithelial/endothelial cells) to the nucleus. The viral tegument protein pp150 and the complex formed by pUL47/pUL48 proteins are involved in the transport of viral capsid at the nuclear membrane (Bechtel & Shenk, 2002); however, their definite cellular binding partner(s) has not been identified yet. A recent study on HSV-1 reported that plus/minus directed microtubule motors bind to viral capsids using different tegument proteins, including also the HCMV pUL48 ortholog pUL36 (Radtke et al., 2010). Furthermore, the disruption of cellular microtubule network caused by the drug nocodazole prevents viral IE gene expression (Ogawa-Goto et al., 2003), thus highlighting the potential of this process as a target of novel antiviral drugs. Once the viral capsids reach the nuclear membrane, they are supposed to dock at the nuclear pore complex and then to release the viral genome into the nucleus. Again, the events and the players involved in these processes are still poorly characterized. For HSV-1, two capsid-associated tegument proteins, i.e., pUL25 and pUL36, were recently shown to play a role in facilitating the nuclear translocation of viral genome. In fact, pUL25 was found to interact with two cellular nucleoporins (Pasdeloup et al., 2009); in addition, proteolytic cleavage of pUL36 at the nuclear membrane was shown to be required for efficient release of viral DNA into the nucleus (Jovasevic et al., 2008).
Despite the importance of the post-fusion processes in virus replication, no anti-HCMV drug that inhibits either the transport of capsids or the nuclear translocation of viral genome has been developed so far. The identification of the viral proteins involved in these processes and the understanding of the protein–protein interactions necessary for post-fusion events of HCMV cycle are strongly required. Indeed, the disruption of such protein–protein interactions could lead to inhibition of virus replication and thus may represent a novel strategy for the development of new anti-HCMV drugs, an approach that has already been successfully applied for interactions between other herpesvirus proteins (Liuzzi et al., 1994, Marcello et al., 1994, Loregian et al., 1999, Loregian et al., 2002, Loregian et al., 2003, Pilger et al., 2004, Loregian and Palù, 2005a, Loregian and Palù, 2005b, Loregian and Coen, 2006).
4.3.2. Activation of Major-Immediate Early gene expression
The nuclear translocation of HCMV genome is not sufficient to induce the onset of lytic replication. In fact, there is compelling experimental evidence that the tegument protein pp71/UL82 has to localize into the nucleus of HCMV-infected cells to efficiently promote viral IE gene expression (Penkert & Kalejta, 2010). After entry into the nucleus, HCMV genome is “attacked” by components of an intrinsic cellular antiviral defense in order to reach viral genome silencing (Tavalai & Stamminger, 2008). The effectors of this antiviral defense are primarily chromatin remodeling enzymes (Woodhall et al., 2006, Cuevas-Bennett and Shenk, 2008, Nitzsche et al., 2008) and proteins generally found as constituents of Nuclear Bodies. Thanks to the activities of these proteins, and in particular to the activity of the transcriptional corepressor Daxx, immediately after entry the incoming HCMV genome could be assimilated to transcriptionally inactive heterochromatin. However, the pp71 protein counteracts Daxx-mediated Major Immediate-Early Promoter (MIEP) repression first by displacing Daxx from its corepressor ATRX (Lukashchuk et al., 2008), by inducing its SUMOylation (Hwang & Kalejta, 2009), and eventually by targeting it to proteasomal degradation via an ubiquitin-independent pathway (Hwang & Kalejta, 2007). By these mechanisms, pp71 is therefore able to efficiently contrast the inhibition of IE gene expression mediated by Daxx. The pp71 protein also targets Rb family members to degradation, thus contributing to G0/G1 progression of virus-infected cells (Kalejta & Shenk, 2003).
4.4. Major-Immediate Early gene expression
4.4.1. Major-Immediate Early enhancer-containing promoter
The MIE region of HCMV genome represents one of the most complex DNA sequences ever studied in biology. It consists of the MIE locus UL122–123, which encodes the IE1-72 and IE2-86 proteins. Their expression is regulated by an upstream rightward located, enhancer-containing promoter, i.e., the MIEP. Relative to the transcription start site at nucleotide +1, the MIEP spans bases up to −39, while the enhancer extends up to base −750. The enhancer is in turn divided into two portions: the proximal enhancer (from base −39 to base −300) and the distal enhancer (from base −300 to base −550). In addition, a unique region is located between bases −350 and −750. The two components of MIE enhancer are not equivalent; in fact, the deletion of the distal portion of the enhancer is tolerated at high MOI and recombinant viruses lacking of this region still replicate, albeit slowly, showing a small-plaque phenotype (Meier and Pruessner, 2000, Meier et al., 2002). In contrast, the deletion of the proximal enhancer results in non-viable viruses, indicating that this region is absolutely required for virus replication (Isomura et al., 2004).
Like eukaryotic enhancers, the MIE enhancer of HCMV contains a high number of repeated, cis-acting binding sites for cellular transcription factors (TFs) that, once bound, attract RNA polymerase II and other components of the basal transcription machinery to permit MIE gene expression. The number and the distribution of these binding sites vary in the CMVs, thus reflecting a possible example of adaptation throughout co-evolution with different hosts to reach the most efficient viral replication (for a review see Stinski & Isomura, 2008). In keeping with this hypothesis, the enhancers of murine CMV and HCMV are not interchangeable (Isomura & Stinski, 2003). The HCMV MIE enhancer contains TF binding sites in common with other CMVs, e.g., sites for NF-кB, CREB/ATF, AP-1, and Sp1 (Hunninghake et al., 1989, Liu and Stinski, 1992, Keller et al., 2003), as well binding sites unique for HCMV, e.g., sites for retinoic acid receptor, Elk-1, Serum Response Factor, CRE-Binding Protein, and γ-interferon-activated sites (Angulo and Ghazal, 1995, Netterwald et al., 2005). However, the minimal enhancer sequence for efficient MIE gene expression has been identified in two Sp1/Sp3 binding sites (GC boxes) located at bases −55 and −75, based on the observation that mutations of these sites impair MIE gene transcription (Isomura et al., 2005). The MIE enhancer of HCMV is flanked by two divergent viral promoters, i.e., the MIEP (discussed below) and the UL127 gene promoter. The unique region of MIEP acts like an insulator element since it contains binding sites for cellular repressors (Chao et al., 2004, Lashmit et al., 2004) and provides negative regulation of UL127 promoter, thus blocking the influence of MIE enhancer on transcription from UL127. Also the MIE enhancer is subjected to negative regulation leading to repression of MIE gene expression, which could be an option from a therapeutical point of view. In fact, the MIE region of HCMV has a key role in virus reactivation from latency; of note is the fact that IE proteins are not expressed in non-permissive cells and during latency, reflecting their role in acute infection as well as in reactivation.
The MIEP region spans bases from +1 to −39 and contains a TATA box, a cis-repression sequence (crs) located between the TATA box and the transcription start site, and an initiator sequence (Macias et al., 1996, Isomura et al., 2008). The TATA box serves as a recognition site for the basal transcription machinery assembly, since basal TFs are recruited at the promoter by TATA box-binding protein (TBP), which interacts with the other TFs and ultimately promotes the binding of RNA polymerase II to MIEP. HCMV requires both promoter and enhancer to efficiently induce MIE gene transcription; an enhancerless recombinant HCMV is in fact not able to replicate (Isomura et al., 2004). Also the crs is essential for virus replication, since the introduction of single point mutations in the crs abrogates the ability of a recombinant virus to replicate (Isomura et al., 2008).
4.4.2. Inhibitors of Major-Immediate Early gene expression
The pivotal role played by MIEP functions and MIE gene expression for the progression of productive infection makes them attractive targets for novel antiviral strategies. A proof-of-principle for the feasibility of this approach is provided by the sole approved anti-HCMV drug that blocks MIE gene expression, i.e., fomivirsen. Fomivirsen (ISIS 2922; Fig. 1), marketed as Vitravene® (Novartis Ophtalmics), is an antisense ODN composed of 21 phosphorothioate-linked nucleotides (5′-GCGTTTGCTCTTCTTCTTGCG-3′), which was identified in a screening of oligonucleotides complementary to the mRNA of HCMV MIE transcriptional unit (Azad et al., 1993). This ODN is able to hybridize with IE2 mRNA, thus blocking the expression of the IE2-86 protein. This was initially supposed to be the unique mechanism of antiviral action of fomivirsen, however other (sequence-unrelated) effects on the expression of other viral genes, e.g., UL123, have been reported (Azad et al., 1993). Furthermore, in a fomivirsen-resistant virus no mutation in the IE2 gene region complementary to fomivirsen was detected (Mulamba et al., 1998). Therefore, beside IE2-86 expression, other viral functions might be affected by this antiviral compound, such as adsorption and entry, targets of other antiviral ODNs (Shogan et al., 2006, Luganini et al., 2008a). Fomivirsen/Vitravene® has been approved by Food and Drug Administration only for ophtalmic use and in U.S. is recommended for the treatment of HCMV-induced retinitis in AIDS patients. It has been withdrawn from the European market for commercial reasons; however, it remains still available for European patients when needed. Fomivirsen/Vitravene® is typically administered as an intravitreal injection of 330 μg/0.5 ml in two doses every two weeks, but it is not recommended for patients who have been treated with CDV, because this may increase one of fomivirsen typical side effects, i.e., eye inflammation. In addition, other ocular or systemic side-effects have been reported for fomivirsen, which restrict its use only to topical administration. In general, antisense ODNs and small-interfering RNAs (siRNAs) represent a promising class of antiviral agents; however, they suffer from several drawbacks, such as need of repeated administration, poor bioavailability, low stability in fluids, and inefficient uptake, thus limiting their clinical applications (for a review see Fattal & Bochot, 2008). For these reasons, in the past years many efforts have been spent to extend the use of antisense ODNs and siRNAs as systemic drugs and to improve their pharmacokinetics and cell uptake by exploiting nanoparticulate delivery systems. These approaches include association with cationic polymers and polymeric nanocapsules (Fattal and Bochot, 2008, de Martimprey et al., 2009). As an example of successful application of these strategies, it was reported that the use of albumin nanoparticles for the combined delivery of GCV and a phosphodiester analog of fomivirsen significatly improved the effectiveness of these drugs (Irache et al., 2005).
In addition to fomivirsen, a series of other compounds have been reported to exert a repressive effect on HCMV MIEP activation or to inhibit MIE gene expression (Table 3 ); however, for many of these the exact mechanism of action remains yet to be elucidated. For example, it is known that trichostatin A, an inhibitor of histone deacetylases (HDACs), triggers MIEP reactivation (Ferguson et al., 2011); therefore, one may speculate that inhibitors of histone acetylases or histone methyltransferases could exert anti-HCMV activity by silencing the MIEP. Furthermore, the pharmacological inhibition of cellular signaling pathways important for MIEP activation results in impairment of virus replication (Johnson et al., 2001). In this context, there is compelling evidence that drugs able to perturb the cellular PI3K/Akt signaling pathway or NF-κB activation have also a significant impact on MIEP activation. The PI3K protein belongs to the class of cellular tyrosine kinases and is activated by different mitogenic stimuli. Its activation triggers a phosphorylation cascade together with other cellular kinases, including Akt. Infection with HCMV strongly up-regulates the PI3K/Akt signaling pathway (Johnson et al., 2001); PI3K-dependent activation of Akt and p70S6K leads eventually to NF-κB activation, a transcription factor important for inducing MIE gene expression. In line with this, the PI3K inhibitors LY294002 and wortmannin (Fig. 6 ) were reported to inhibit both MIE gene expression and viral DNA synthesis by preventing NF-κB activation and, possibly, the phosphorylation of other cellular proteins required for efficient virus replication. Also other inhibitors of kinases, acting both upstream and downstream PI3K in cell signaling, were reported to have anti-HCMV activity. For example, worthy of note is artesunate (Fig. 6). This compound was initially identified as an anti-malaria agent and is currently used for treatment of Plasmodium spp. infections (Rosenthal, 2008). Later, artesunate was discovered to inhibit HCMV-induced transactivation activities of both NF-κB and Sp1, necessary for the activation of MIEP (Efferth et al., 2002). Studies to elucidate the mechanism of the anti-HCMV activity of artesunate indicated that it blocks the activation of two effectors of PI3K pathway, i.e., Akt and p70S6K. Further work is needed to fully understand the antiviral mechanism of this drug, which could represent an alternative option for treatment of HCMV infections. In fact, artesunate has shown a good bioavailability profile and lack of significant toxicity in clinical anti-malaria studies (Hien et al., 1992a, Hien et al., 1992b, Nosten, 1994).
Table 3.
Anti-HCMV compounds that inhibit MIE gene expression or IE2-86 activities.
| Compound | Chemical Backbone | Target (HCMV Function Affected) | Status | References |
|---|---|---|---|---|
| LY294002 | 2-morpholin-4-yl-8-phenylchromen-4-one | PI3K inhibitor (Block of MIE gene expression) | Clinical trials for cancer treatment | Johnson et al., 2001 |
| Wortmannin | Furanosteroid (from P. funiculosum, P. wortmannii) | PI3K inhibitor (Block of MIE gene expression) | Derivatives in clinical trials for cancer treatment | Johnson et al., 2001 |
| Artesunate | Semisynthetic derivative of artemisinin (from A. annua) | Akt/p70S6K inhibitor (Block of MIE gene expression) | Approved as an anti-malaria drug | Efferth et al., 2002 |
| Sorafenib/Nevaxar® | 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino] phenoxy]-N-methyl-pyridine-2-carboxamide | Multi-targeted Tyr-kinase inhibitor (Block of MIE gene expression) | Approved as an anti-cancer drug | Michaelis et al., 2011 |
| Geldanamycin | Benzoquinone ansamycin (from S. hygroscopicus) | Hsp90 inhibitor (Block of MIE gene expression) | Pre-clinical | Basha et al., 2005 |
| MG132 | N-(benzyloxycarbonyl)leucinylleucinyl-leucinal | Proteasome inhibitor (Block of MIE gene expression) | Pre-clinical | Prosch et al., 2003 |
| CMV423 | 2-Chloro-3-pyridin-3-yl-5,6,7,8-tetrahydroindolizine-1-carboxamide | Unknown (Block of MIE gene expression) | Pre-clinical | Snoeck et al., 2002 |
| Pristimerim | Triterpenoid quinone methide (from M. heterophylla) | Unknown (Block of MIE gene expression) | Pre-clinical | Murayama et al., 2007 |
| DPPC | 1-(3,5-dichloro-4-pyridyl) piperidine-4-carboxamide | Unknown (Block of MIE gene expression) | Pre-clinical | Fukui et al., 2008 |
| Berberine | Ammonium salt of isoquinoline alkaloid (from R. coptidis) | Unknown (Block of a viral function prior to DNA synthesis) | Pre-clinical | Hayashi et al., 2007 |
| Genistein | 5,7-Dihydroxy-3-(4-hydroxyphenyl)chromen-4-one | Unknown (Block of IE proteins functioning) | Pre-clinical | Evers et al., 2005 |
| Fomivirsen/Vitravene® | Phosphorothioate oligonucleotide | IE2-86 mRNA and ? (Block of IE2-86 expression) | Approved as an anti-HCMV drug | Azad et al., 1993 |
| WC5 | 6-Aminoquinolone | IE2-86 transactivation activity and ? (Block of E/L proteins expression) | Pre-clinical | Loregian et al., 2010 |
Fig. 6.
Chemical structure of some unlicensed inhibitors of MIEP functions, MIE gene expression, and IE2-86 activities.
Most recently, it was reported that Sorafenib/Nexavar® (Fig. 6), a multi-targeted tyrosine kinase inhibitor used for anti-cancer treatment, also blocks HCMV replication at non-toxic, clinically relevant concentrations (Michaelis et al., 2011). It inhibits MIEP activity and MIE gene expression in a panel of different cell lines, but the exact mechanism of the anti-HCMV activity of Sorafenib has not yet been established. Nevertheless, there are indications suggesting that the inhibition of the cellular kinase Raf could be involved, although a role for Raf in MIE gene expression has not been described previously (Michaelis et al., 2011). Raf is a cell signaling effector acting before the mitogen-activated kinases MEK and ERK/MAPK, but it has also been involved in the NF-κB activation pathway (Wellbrock et al., 2004). Further studies will be necessary to establish whether Sorafenib might represent a novel anti-HCMV drug, in particular for cancer patients who are at risk of HCMV reactivation as prospected in (Michaelis et al., 2011).
Finally, also inhibitors of cellular processes that are not directly involved in virus replication have been shown to exert anti-HCMV activity by perturbing cellular functions necessary for triggering MIE gene expression. This is the case of geldanamycin (Fig. 6), which is an Hsp90 inhibitor that interferes with HCMV replication (Basha et al., 2005). The inhibition of molecular chaperone Hsp90 leads to the inactivation of its client proteins, including Akt and other kinases involved in PI3K signaling. Another example is represented by proteasome inhibitors, in particular MG132 (Fig. 6), which have shown the ability to block HCMV replication (Prosch et al., 2003). The transcription factor NF-κB is one of the most important activators of MIEP and it is found in an inactive cytoplasmatic complex with its repressor IκB. The activation of diverse kinases in response to extracellular stimuli leads to phosphorylation of the latter and to its proteasomal degradation via an ubiquitin-dependent pathway, resulting in the activation of NF-κB. Therefore, the anti-HCMV activity reported for MG132 might be mainly due to the cytosolic accumulation of IκB caused by lack of proteasomal degradation. The failed release of NF-κB blocks its nuclear translocation and abrogates IE gene expression. Proteasome inhibitors, however, have demonstrated also other inhibitory effects on HCMV processes (Kaspari et al., 2008).
In summary, a variety of compounds have been reported to have anti-HCMV activity at this stage, but they act by exerting their primary inhibitory activity on cellular targets. In fact, it should be kept in mind that none of these compounds was first discovered as an anti-HCMV agent neither was specifically designed to inhibit a particular viral function, as in the case of fomivirsen. Furthermore, although most of the compounds reported in Table 3 can inhibit HCMV replication at concentrations that are clinically relevant, none of them has a selectivity index comparable to that of GCV. Perturbing cellular pathways and/or functions might be not a feasible strategy, since nonspecific adverse effects cannot be excluded. Therefore, accurate studies are needed to establish whether these inhibitors could be useful in clinical anti-HCMV settings.
4.5. Biological functions of IE2-86 protein
The most abundant HCMV IE proteins originate from the UL122–123 loci under control of the MIE enhancer-promoter. The MIE proteins can be detected from 1.5 h p.i. (Ishov et al., 1997) and are called IE1-72 and IE2-86. These two proteins are the product of alternative splicing events that lead to different mRNAs. As a consequence, IE1-72 and IE2-86 have the first two exons in common, while are divergent at the C-terminus, with IE1-72 ending with exon 4-derived aa sequence and IE2-86 with exon 5. Other gene products originate from the UL122 locus, i.e. IE2-60 and IE2-40 proteins, shorter IE2 forms which are dispensable for HCMV replication, but have been reported to play a role in L genes expression and in MIEP repression (White et al., 2007).
IE1-72 is not essential for virus replication, although it greatly augments the efficiency of HCMV infection by acting as a transactivator as well as by interacting with cellular proteins, thus cooperating with IE2-86 in creating the most favorable environment for virus replication. At low MOI, the lack of IE1-72 affects viral replication; however at high MOI its absence appears to be compensated by the activity of other viral and/or cellular proteins (Gawn & Greaves, 2002).
IE2-86 is a multi-tasking protein that is essential for virus replication; in fact, recombinant viruses carrying deletions in exon 5 are not able to complete the replication cycle, because of lack of E genes expression (Marchini et al., 2001). The transactivation properties of IE2-86 on E genes cannot be complemented by the activity of other either viral or cellular proteins. The fact that IE2-86 functions are indispensable for virus growth makes it an appealing target for novel antiviral strategies; however, to date no drug that affects IE2-86 functions has been licensed. Below are discussed the main roles that IE2-86 fulfills to render the infected host cell the most suitable environment for HCMV replication and that could be targeted by antiviral compounds.
In addition to IE1-72 and IE2-86, several ancillary IE proteins exist (Colberg-Poley, 1996, Castillo and Kowalik, 2002, Mercorelli et al., 2008), which are encoded by the HCMV UL36-38, TRS1/IRS1 and US3 loci. However, although most of these proteins are known to play an important role in HCMV replication and thus could be potential antiviral targets, the only antiviral agent specifically directed against one of these proteins, i.e. a phosphorothioate oligonucleotide—GEM 132 (UL36ANTI)—complementary to the intron–exon boundary of the IE genes UL36–UL37 has not been further developed since the initial reports (Pari et al., 1995, Smith and Pari, 1995, Dvorchik and Marquis, 2000) and no other compound specifically directed against these viral targets has been described in the last decade.
4.5.1. Autoregulation of Major-Immediate Early Promoter
The ability of IE2-86 to repress its own promoter is essential for virus replication. IE2-86 forms homodimers and binds directly to a DNA sequence in the MIEP, i.e. crs, located between the TATA box and the transcription start site. This regulatory element consists of a 14-bp sequence of a GC dinucleotide flanked by two A/T rich regions (Waheed et al., 1998). The binding of IE2-86 to crs physically blocks the recruitment of RNA polymerase II and thus prevents the assembly of the pre-initiation complex and subsequent MIE gene expression. Mutations in both crs and IE2-86 able to impair the DNA–protein interaction have a lethal effect in virus replication (Petrik et al., 2007, Isomura et al., 2008). However, it seems that the crs might have a dual role during HCMV replication. In fact, it has been reported that in the first phases of HCMV replication an unknown cellular protein of about 150 kDa interacts with crs, and that this interaction results in increase of MIE gene expression (Isomura et al., 2008). Then, with the progression of virus replication, IE2-86 is abundantly expressed and becomes able to compete with this cellular protein for crs binding, thus exerting a repressive effect on its own promoter. Furthermore, IE2-86 is able to interact with repressive chromatin remodelling enzymes such as HDAC-1 and HDAC-2 (Reeves et al., 2006, Park et al., 2007). The recruitment of these proteins by IE2-86 causes the deacetylation of histones at MIEP and modifications in the chromatin status of the promoter leading to silencing of MIE genes.
4.5.2. Regulation of viral and cellular gene expression
One of the most striking features of IE2-86 is its ability to function both as a transactivator of gene expression and as a repressor. IE2-86 is the major transactivator of viral E genes and the IE1-72 protein enhances this activity (Nevels et al., 2004). The transactivation properties of IE2-86 mainly rely on protein–protein interactions. In fact, IE2-86 interacts with several components of the basal transcription machinery, including TBP, TFIIB, and TAFII130-TAF4 (Caswell et al., 1993), showing by itself TAF-like functions (Lukac et al., 1994, Lukac et al., 1997). Furthermore, both IE2-86 and IE1-72 attract histone acetylases to permit the activation of viral E promoters (Bryant et al., 2000, Nevels et al., 2004). However, there are also evidences of a direct binding of IE2-86 to DNA sequences in E promoters required for efficient promoter activation (Schwartz et al., 1994, Scully et al., 1995). The transactivating functions of IE2-86 are essential for the progression of HCMV lytic replication cycle, since point mutations that affect the ability of IE2-86 either to bind E promoters (Petrik et al., 2007) or to interact with basal transcription factors (Harris et al., 2010) abrogate HCMV replication. Furthermore, although RNA polymerase II and TBP are recruited at viral E promoters in the absence of IE2-86, the latter remains essential to achieve E genes expression (Petrik et al., 2007). IE2-86 also acts as a repressor of key cellular regulators, e.g., the tumor suppressor Rb (Hagemeier et al., 1994). Through the interaction with Rb, IE2-86 induces the activation of E2F-responsive genes whose products are responsible of G1/S progression in HCMV-infected cells, thus providing a way to bypass the cellular quiescence.
IE2-86 is also an immunomodulator, responsible for the block of pro-inflammatory signaling necessary for the survival of HCMV-infected cells by a mechanism that involves NF-kB inhibition (Taylor & Bresnahan, 2005). In fact, IE2-86 acts as a NF-kB antagonist by blocking its DNA-binding activity and hence attenuating the expression of NF-kB-dependent pro-inflammatory chemokines (Taylor and Bresnahan, 2006a, Taylor and Bresnahan, 2006b, Poole et al., 2008).
During the past years, many efforts have been aimed at identifying whether a transactivation domain in IE2-86 could be separated from a domain responsible of autoregulation. A definitive answer has to be found yet. A core domain was initially identified in IE2-86C-terminus from amino acid 450 to 452 that was apparently required for all functions (Asmar et al., 2004). A more recent study, which characterized the effects of two double mutations in IE2-86, i.e., P535A/Y537A and H446A/H452A, shed further light on this aspect (Petrik et al., 2007). In fact, the authors showed that a P535A/Y537A mutant is still able to autoregulate the MIEP even though it is not competent for transactivation, suggesting that IE2-86 likely uses two independent mechanisms for crs binding and for activation of viral E promoters. Very recently, an analysis of the effects of point mutations in IE2-86 between amino acids 435 and 545 was performed to assess the impact of single aa changes on IE2-86 transactivating activity both in vitro and in the context of viral replication (Harris et al., 2010). This study evidenced that residue Y544 is essential for UL54 promoter activation while P535 is not; in contrast, mutation of either P535 or Y544 inhibits UL112–113 promoter transactivation. Thus, the possibility that IE2-86 might use distinct mechanisms to activate different responsive promoters was suggested. Nevertheless, the identification of IE2-86 residues essential for autoregulatory/transactivating functions merits further investigation, because the comprehension at a molecular level of the mechanism(s) of action of this protein could provide a route for developing IE2-86 inhibitors.
4.5.3. Inhibitors of IE2-86 functions
A number of compounds have been reported to block viral E and L gene expression but not IE gene expression (Table 3), and for these a mechanism involving inhibition of IE2-86 functions has been suggested. Examples are represented by genistein (Evers et al., 2005) or berberine (Hayashi et al., 2007). In addition, inhibitors of cellular kinases, such as MEK and MAPK, have been shown to suppress IE1-72 and IE2-86 phosphorylation, which results in inhibition of HCMV delayed E gene expression and viral DNA replication (Johnson et al., 2001, Chen and Stinski, 2002). Since phosphorylation levels of IE2-86 have been correlated to its transactivation activity (Barrasa et al., 2005), the inhibition of this IE2-86 post-translational modification should be further investigated as a potential antiviral target.
Recently, a class of compounds, namely 6-aminoquinolones, has emerged as novel antiviral compounds that act by blocking viral transactivating functions (Parolin et al., 2003). More recently, a 6-aminoquinolone—termed WC5 (Fig. 6)—that potently and selectively inhibits HCMV replication (EC50: 0.9 μM) was identified (Mercorelli et al., 2009). A remarkable feature of WC5 is that it specifically inhibits HCMV replication, exhibiting a selectivity index (~500) more favorable than that of GCV, while it has no significant activity against other human herpesviruses (Mercorelli et al., 2009). Importantly, WC5 was also potently active against clinical strains of HCMV recovered from infected patients, as well as against virus strains resistant to drugs currently used for anti-HCMV therapy, e.g., GCV, FOS, and CDV. For these reasons, WC5 was considered as a promising lead for the development of novel anti-HCMV drugs. Further studies performed to elucidate the mode of action of WC5 demonstrated that it acts by a mechanism different from all of the licensed anti-HCMV drugs (Loregian et al., 2010). In fact, WC5 does not inhibit either HCMV DNA polymerase activity or IE gene expression, but interferes with the transactivation activity of IE2-86 protein. By exploiting a cell-based assay developed to identify IE2-86 inhibitors (Luganini et al., 2008b), it was demonstrated that WC5 is able to inhibit the IE2-86-mediated transactivation of viral E genes, thus blocking E and L gene expression (Loregian et al., 2010). Importantly, WC5 acts synergistically with GCV against HCMV replication, while the cytotoxic effect of the two compounds in combination is additive (Loregian et al., 2010). These findings open the possibility of a combination therapy with similar synergistic effects in vivo that could provide a means to treat HCMV infection while reducing drug toxicity and the outcome of drug-resistant mutants.
WC5 is the first antiviral compound reported to directly block an activity of the essential IE2-86 protein; however, taking advantage of the cell-based assay developed by Luganini and colleagues (Luganini et al., 2008b), other IE2-86 inhibitors could be identified. Deeper investigation is needed to elucidate the mechanism of inhibition of IE2-86 transactivating activity at molecular level and to verify whether in the antiviral activity of WC5 other functions/activities of IE2-86 might be involved, whose inhibition could contribute to the block of virus replication in WC5-treated cells.
5. Concluding remarks
HCMV infections are still associated with severe morbidity and mortality, especially in the vulnerable immunocompromised patient, despite the availability of five drugs that are currently licensed for antiviral therapy. Thus, new anti-HCMV agents are urgently needed. Inhibition of HCMV entry and expression of IE genes represents an emerging field of study and could provide efficacious strategies for the reduction or ablation of productive infection. Indeed, the successful development of HIV entry inhibitors (Kuritzkes, 2009, Joly et al., 2010) has provided the impetus for designing antiviral compounds targeting one or more essential steps occurring early in virus replicative cycle.
To date, there are a number of promising novel compounds which target the initial phases of HCMV replication and are at different stages of development. Thus, there is strong hope that at least a few of the compounds described here, or their derivatives, will reach the marketplace and help to improve the treatment options for this area of high unmet medical need. To do this, they should circumvent the toxicity, bioavailability, resistance, and efficacy issues associated with the current anti-HCMV drugs. Moreover, the availability of compounds targeting other viral events beside genome replication could allow the design of more active therapeutic protocols based on the combined use of multiple anti-HCMV drugs, like the highly active antiretroviral therapy that has changed the lives of HIV-infected patients. Research efforts to dissect further the molecular interactions and mechanisms involved in the first events of HCMV life cycle are clearly necessary to identify other novel drug targets and to develop new inhibitors against these targets.
Acknowledgments
Work in the authors' laboratories was supported by MURST EX60%, Progetto di Ricerca di Ateneo 2007 (grant no. CPDA074945), and PRIN 2008 (grant no. 20085FF4J4) to A.L, by Regione Veneto to G.P., and by Regione Piemonte (Ricerca Finalizzata 2008-bis) and PRIN 2008 (grant no. 20085FF4J4) to D.L.
Contributor Information
Giorgio Palù, Email: giorgio.palu@unipd.it.
Arianna Loregian, Email: arianna.loregian@unipd.it.
References
- Adhikari U., Mateu C.G., Chattopadhyay K., Pujol C.A., Damonte E.B., Ray B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry. 2006;67:2474–2482. doi: 10.1016/j.phytochem.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Adler S.P., Nigro G. Findings and conclusions from CMV hyperimmune globulin treatment trials. J Clin Virol. 2009;46(Suppl 4):S54–S57. doi: 10.1016/j.jcv.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Aduma P., Connelly M.C., Srinivas R.V., Fridland A. Metabolic diversity and antiviral activities of acyclic nucleoside phosphonates. Mol Pharmacol. 1995;47:816–822. [PubMed] [Google Scholar]
- Akula S.M., Hurley D.J., Wixon R.L., Wang C., Chase C.C. Effect of genistein on replication of bovine herpesvirus type 1. Am J Vet Res. 2002;63:1124–1128. doi: 10.2460/ajvr.2002.63.1124. [DOI] [PubMed] [Google Scholar]
- Amoros M., Simoes C.M., Girre L., Sauvager F., Cormier M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J Nat Prod. 1992;55:1732–1740. doi: 10.1021/np50090a003. [DOI] [PubMed] [Google Scholar]
- Andersen J.H., Osbakk S.A., Vorland L.H., Traavik T., Gutteberg T.J. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antiviral Res. 2001;51:141–149. doi: 10.1016/s0166-3542(01)00146-2. [DOI] [PubMed] [Google Scholar]
- Anderson K.P., Fox M.C., Brown-Driver V., Martin M.J., Azad R.F. Inhibition of human cytomegalovirus immediate-early gene expression by an antisense oligonucleotide complementary to immediate-early RNA. Antimicrob Agents Chemother. 1996;40:2004–2011. doi: 10.1128/aac.40.9.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei G., Snoeck R., Schols D., Goubau P., Desmyter J., De Clercq E. Comparative activity of selected antiviral compounds against clinical isolates of human cytomegalovirus. Eur J Clin Microbiol Infect Dis. 1991;10:1026–1033. doi: 10.1007/BF01984924. [DOI] [PubMed] [Google Scholar]
- Androutsopoulos V.P., Papakyriakou A., Vourloumis D., Tsatsakis A.M., Spandidos D.A. Dietary flavonoids in cancer therapy and prevention: substrates and inhibitors of cytochrome P450 CYP1 enzymes. Pharmacol Ther. 2010;126:9–20. doi: 10.1016/j.pharmthera.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Angulo A., Ghazal P. Regulation of human cytomegalovirus by retinoic acid. Scand J Infect Dis Suppl. 1995;99:113–115. [PubMed] [Google Scholar]
- Ashkar A.A., Bauer S., Mitchell W.J., Vieira J., Rosenthal K.L. Local delivery of CpG oligodeoxynucleotides induces rapid changes in the genital mucosa and inhibits replication, but not entry, of herpes simplex virus type 2. J Virol. 2003;77:8948–8956. doi: 10.1128/JVI.77.16.8948-8956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmar J., Wiebusch L., Truss M., Hagemeier C. The putative zinc finger of the human cytomegalovirus IE2 86-kilodalton protein is dispensable for DNA binding and autorepression, thereby demarcating a concise core domain in the C terminus of the protein. J Virol. 2004;78:11853–11864. doi: 10.1128/JVI.78.21.11853-11864.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreides S.-P.A., Wilkins C., Ekworomadu C.O., Docherty J.J., Dix R.D. Does resveratrol exhibit antiviral properties against human cytomegalovirus replication? Invest Ophthalmol Vis Sci. 2002;43 E-Abstract 4325. [Google Scholar]
- Azad R.F., Driver V.B., Tanaka K., Crooke R.M., Anderson K.P. Antiviral activity of a phosphorothioate oligonucleotide complementary to RNA of the human cytomegalovirus major immediate-early region. Antimicrob Agents Chemother. 1993;37:1945–1954. doi: 10.1128/aac.37.9.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarova A.M., Lin R.K., Tsai Y.C., Liu L.F., Lin C.P., Lyu Y.L. Genistein induces topoisomerase IIbeta- and proteasome-mediated DNA sequence rearrangements: implications in infant leukemia. Biochem Biophys Res Commun. 2010;399:66–71. doi: 10.1016/j.bbrc.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., Snoeck R., Pauwels R., de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988;32:1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., De Clercq E., Schols D., Pauwels R., Snoeck R., Van Boeckel C. Novel sulfated polysaccharides: dissociation of anti-human immunodeficiency virus activity from antithrombin activity. J Infect Dis. 1990;161:208–213. doi: 10.1093/infdis/161.2.208. [DOI] [PubMed] [Google Scholar]
- Baldanti F., Silini E., Sarasini A., Talarico C.L., Stanat S.C., Biron K.K. A three-nucleotide deletion in the UL97 open reading frame is responsible for the ganciclovir resistance of a human cytomegalovirus clinical isolate. J Virol. 1995;69:796–800. doi: 10.1128/jvi.69.2.796-800.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldanti F., Simoncini L., Talarico C.L., Sarasini A., Biron K.K., Gerna G. Emergence of a ganciclovir-resistant human cytomegalovirus strain with a new UL97 mutation in an AIDS patient. AIDS. 1998;12:816–818. [PubMed] [Google Scholar]
- Barjesteh van Waalwijk van Doorn-Khosrovani S., Janssen J., Maas L.M., Godschalk R.W., Nijhuis J.G., van Schooten F.J. Dietary flavonoids induce MLL translocations in primary human CD34+ cells. Carcinogenesis. 2007;28:1703–1709. doi: 10.1093/carcin/bgm102. [DOI] [PubMed] [Google Scholar]
- Barrasa M.I., Harel N.Y., Alwine J.C. The phosphorylation status of the serine-rich region of the human cytomegalovirus 86-kilodalton major immediate-early protein IE2/IEP86 affects temporal viral gene expression. J Virol. 2005;79:1428–1437. doi: 10.1128/JVI.79.3.1428-1437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha W., Kitagawa R., Uhara M., Imazu H., Uechi K., Tanaka J. Geldanamycin, a potent and specific inhibitor of Hsp90, inhibits gene expression and replication of human cytomegalovirus. Antivir Chem Chemother. 2005;16:135–146. doi: 10.1177/095632020501600206. [DOI] [PubMed] [Google Scholar]
- Baur J.A., Sinclair D. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Baynes R.D., Bezwoda W.R. Lactoferrin and the inflammatory response. Adv Exp Med Biol. 1994;357:133–141. doi: 10.1007/978-1-4615-2548-6_13. [DOI] [PubMed] [Google Scholar]
- Bechtel J.T., Shenk T. Human cytomegalovirus UL47 tegument protein functions after entry and before immediate-early gene expression. J Virol. 2002;76:1043–1050. doi: 10.1128/JVI.76.3.1043-1050.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljaars L., van der Strate B.W., Bakker H.I., Reker-Smit C., van Loenen-Weemaes A.M., Wiegmans F.C. Inhibition of citomegalovirus infection by lactoferrin in vitro and in vivo. Antiviral Res. 2004;63:197–208. doi: 10.1016/j.antiviral.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Bonaros N., Mayer B., Schachner T., Laufer G., Kocher A. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: a meta-analysis. Clin Transplant. 2008;22:89–97. doi: 10.1111/j.1399-0012.2007.00750.x. [DOI] [PubMed] [Google Scholar]
- Bratcher D.F., Bourne N., Bravo F.J., Schleiss M.R., Slaoui M., Myers M.G. Effect of passive antibody on congenital cytomegalovirus infection in guinea pigs. J Infect Dis. 1995;172:944–950. doi: 10.1093/infdis/172.4.944. [DOI] [PubMed] [Google Scholar]
- Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol. 2008;325:417–470. doi: 10.1007/978-3-540-77349-8_23. [DOI] [PubMed] [Google Scholar]
- Bryant L.A., Mixon P., Davidson M., Bannister A.J., Kouzarides T., Sinclair J.H. The human cytomegalovirus 86-kilodalton major immediate-early protein interacts physically and functionally with histone acetyltransferase P/CAF. J Virol. 2000;74:7230–7237. doi: 10.1128/jvi.74.16.7230-7237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyck H.C., Griffiths P.D., Emery V.C. Human cytomegalovirus (HCMV). Replication kinetics in stem cell transplant recipients following anti-HCMV therapy. J Clin Virol. 2010;49:32–36. doi: 10.1016/j.jcv.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Cannon M.J., Schmid D.S., Hyde T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- Castillo J.P., Kowalik T.F. Human cytomegalovirus immediate early proteins and cell growth control. Gene. 2002;290:19–34. doi: 10.1016/s0378-1119(02)00566-8. [DOI] [PubMed] [Google Scholar]
- Caswell R., Hagemeier C., Chiou C.J., Hayward G., Kouzarides T., Sinclair J. The human cytomegalovirus 86 K immediate early (IE) 2 protein requires the basic region of the TATA-box binding protein (TBP) for binding, and interacts with TBP and transcription factor TFIIB via regions of IE2 required for transcriptional regulation. J Gen Virol. 1993;74:2691–2698. doi: 10.1099/0022-1317-74-12-2691. [DOI] [PubMed] [Google Scholar]
- Cha T., Tom E., Kemble G., Duke G., Mocarski E., Spaete R. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol. 1996;70:78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G., Nogalski M.T., Yurochko A.D. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc Natl Acad Sci U S A. 2009;106:22369–22374. doi: 10.1073/pnas.0908787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao S.H., Harada J.N., Hyndman F., Gao X., Nelson C.G., Chanda S.K. PDX1, a cellular homeoprotein, binds to and regulates the activity of human cytomegalovirus immediate early promoter. J Biol Chem. 2004;279:16111–16120. doi: 10.1074/jbc.M312304200. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay K., Mateu C.G., Mandal P., Pujol C.A., Damonte E.B., Ray B. Galactan sulfate of Grateloupia indica: isolation, structural features and antiviral activity. Phytochemistry. 2007;68:2428–2435. doi: 10.1016/j.phytochem.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay K., Ghosh T., Pujol C.A., Carlucci M.J., Damonte E.B., Ray B. Polysaccharides from Gracilaria corticata: sulfation, chemical characterization and anti-HSV activities. Int J Biol Macromol. 2008;43:346–351. doi: 10.1016/j.ijbiomac.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Chen J., Stinski M.F. Role of regulatory elements and the MAPK/ERK or p38 MAPK pathways for activation of human cytomegalovirus gene expression. J Virol. 2002;76:4873–4885. doi: 10.1128/JVI.76.10.4873-4885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.J., Raung S.L., Liao S.L., Chen S.Y. Inhibition of inducible nitric oxide synthase expression by baicalein in endotoxin/cytokine-stimulated microglia. Biochem Pharmacol. 2004;67:957–965. doi: 10.1016/j.bcp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Chiang L.C., Chiang W., Liu M.C., Lin C.C. In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids. J Antimicrob Chemother. 2003;52:194–198. doi: 10.1093/jac/dkg291. [DOI] [PubMed] [Google Scholar]
- Chrisp P., Clissold S.P. Foscarnet. A review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs. 1991;41:104–129. doi: 10.2165/00003495-199141010-00009. [DOI] [PubMed] [Google Scholar]
- Cinque P., Marenzi R., Ceresa D. Cytomegalovirus infections of the nervous system. Intervirology. 1997;40:85–97. doi: 10.1159/000150536. [DOI] [PubMed] [Google Scholar]
- Cobbs C.S., Soroceanu L., Denham S., Zhang W., Britt W.J., Pieper R. Human cytomegalovirus induces cellular tyrosine kinase signaling and promotes glioma cell invasiveness. J Neurooncol. 2007;85:271–280. doi: 10.1007/s11060-007-9423-2. [DOI] [PubMed] [Google Scholar]
- Colberg-Poley A.M. Functional roles of immediate early proteins encoded by the human cytomegalovirus UL36–38, UL115–119, TRS1/IRS1 and US3 loci. Intervirology. 1996;39:350–360. doi: 10.1159/000150506. [DOI] [PubMed] [Google Scholar]
- Compton T., Nepomuceno R.R., Nowlin D.M. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology. 1992;191:387–395. doi: 10.1016/0042-6822(92)90200-9. [DOI] [PubMed] [Google Scholar]
- Compton T., Nowlin D.M., Cooper N.R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- Cos P., Maes L., Vlietinck A., Pieters L. Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection—an update (1998–2007) Planta Med. 2008;74:1323–1337. doi: 10.1055/s-2008-1081314. [DOI] [PubMed] [Google Scholar]
- Cuevas-Bennett C., Shenk T. Dynamic histone H3 acetylation and methylation at human cytomegalovirus promoters during replication in fibroblasts. J Virol. 2008;82:9525–9536. doi: 10.1128/JVI.00946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Meza M.P., Adler S.P., McVoy M.A. Human immune sera contain high titers of antibodies that block cytomegalovirus entry into epithelial cells but are induced at low titers by CMV vaccines. Vaccine. 2008;26:5760–5766. doi: 10.1016/j.vaccine.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushnie T.P., Lamb A.J. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetkovic R.S., Wellington K. Valganciclovir: a review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs. 2005;65:859–878. doi: 10.2165/00003495-200565060-00012. [DOI] [PubMed] [Google Scholar]
- Dankner W.M., McCutchan J.A., Richman D.D., Hirata K., Spector S.A. Localization of human cytomegalovirus in peripheral blood leukocytes by in situ hybridization. J Infect Dis. 1990;161:31–36. doi: 10.1093/infdis/161.1.31. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Holy A. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat Rev Drug Discov. 2005;4:928–940. doi: 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]
- de Martimprey H., Vauthier C., Malvy C., Couvreur P. Polymer nanocarriers for the delivery of small fragments of nucleic acids: oligonucleotides and siRNA. Eur J Pharm Biopharm. 2009;71:490–504. doi: 10.1016/j.ejpb.2008.09.024. [DOI] [PubMed] [Google Scholar]
- Docherty J.J., Fu M.M., Stiffler B.S., Limperos R.J., Pokabla C.M., DeLucia A.L. Resveratrol inhibition of herpes simplex virus replication. Antiviral Res. 1999;43:145–155. doi: 10.1016/s0166-3542(99)00042-x. [DOI] [PubMed] [Google Scholar]
- Docherty J.J., Smith J.S., Fu M.M., Stoner T., Booth T. Effect of topically applied resveratrol on cutaneous herpes simplex virus infections in hairless mice. Antiviral Res. 2004;61:19–26. doi: 10.1016/j.antiviral.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Docherty J.J., Fu M.M., Hah J.M., Sweet T.J., Faith S.A., Booth T. Effect of resveratrol on herpes simplex virus vaginal infection in the mouse. Antiviral Res. 2005;67:155–162. doi: 10.1016/j.antiviral.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Docherty J.J., Sweet T.J., Bailey E., Faith S.A., Booth T. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antiviral Res. 2006;72:171–177. doi: 10.1016/j.antiviral.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Dolan A., Cunningham C., Hector R.D., Hassan-Walker A.F., Lee L., Addison C. Genetic content of wild-type human cytomegalovirus. J Gen Virol. 2004;85:1301–1312. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- Dollard S.C., Grosse S.D., Ross D.S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- Donalisio M., Rusnati M., Civra A., Bugatti A., Allemand D., Pirri G. Identification of a dendrimeric heparan sulfate-binding peptide that inhibits infectivity of genital types of human papillomaviruses. Antimicrob Agents Chemother. 2010;54:4290–4299. doi: 10.1128/AAC.00471-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Mori I., Hossain M.J., Liu B., Kimura Y. An immunostimulatory oligodeoxynucleotide containing a cytidine–guanosine motif protects senescence-accelerated mice from lethal influenza virus by augmenting the T helper type 1 response. J Gen Virol. 2003;84:1623–1628. doi: 10.1099/vir.0.19029-0. [DOI] [PubMed] [Google Scholar]
- Dvorchik B.H., Marquis J.K. Disposition and toxicity of a mixed backbone antisense oligonucleotide, targeted against human cytomegalovirus, after intravitreal injection of escalating single doses in the rabbit. Drug Metab Dispos. 2000;28:1255–1261. [PubMed] [Google Scholar]
- Efferth T., Marschall M., Wang X., Huong S.M., Hauber I., Olbrich A. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J Mol Med. 2002;80:233–242. doi: 10.1007/s00109-001-0300-8. [DOI] [PubMed] [Google Scholar]
- English E.P., Chumanov R.S., Gellman S.H., Compton T. Rational development of beta-peptide inhibitors of human cytomegalovirus entry. J Biol Chem. 2005;281:2661–2667. doi: 10.1074/jbc.M508485200. [DOI] [PubMed] [Google Scholar]
- Erice A., Chou S., Biron K.K., Stanat S.C., Balfour H.H., Jr., Jordan M.C. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N Engl J Med. 1989;320:289–293. doi: 10.1056/NEJM198902023200505. [DOI] [PubMed] [Google Scholar]
- Esko J.D., Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest. 2001;108:169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers D.L., Wang X., Huong S.M., Huang D.Y., Huang E.S. 3,4′,5-Trihydroxy-trans-stilbene (resveratrol) inhibits human cytomegalovirus replication and virus-induced cellular signaling. Antiviral Res. 2004;63:85–95. doi: 10.1016/j.antiviral.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Evers D.L., Chao C.F., Wang X., Zhang Z., Huong S.M., Huang E.S. Human cytomegalovirus-inhibitory flavonoids: studies on antiviral activity and mechanism of action. Antiviral Res. 2005;68:124–134. doi: 10.1016/j.antiviral.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina A., Ferranti C., Marra C. An improved synthesis of resveratrol. Nat Prod Res. 2006;20:247–252. doi: 10.1080/14786410500059532. [DOI] [PubMed] [Google Scholar]
- Fattal E., Bochot A. State of the art and perspectives for the delivery of antisense oligonucleotides and siRNA by polymeric nanocarriers. Int J Pharm. 2008;364:237–248. doi: 10.1016/j.ijpharm.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Faulds D., Heel R.C. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs. 1990;39:597–638. doi: 10.2165/00003495-199039040-00008. [DOI] [PubMed] [Google Scholar]
- Feire A.L., Koss H., Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci U S A. 2004;101:15470–15475. doi: 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feire A.L., Roy R.M., Manley K., Compton T. The glycoprotein B disintegrin-like domain binds beta 1 integrin to mediate cytomegalovirus entry. J Virol. 2010;84:10026–10037. doi: 10.1128/JVI.00710-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson L.R., Tatham A.L., Lin Z., Denny W.A. Epigenetic regulation of gene expression as an anticancer drug target. Curr Cancer Drug Targets. 2011;11:199–212. doi: 10.2174/156800911794328510. [DOI] [PubMed] [Google Scholar]
- Flexner C., Barditch-Crovo P.A., Kornhauser D.M., Farzadegan H., Nerhood L.J., Chaisson R.E. Pharmacokinetics, toxicity, and activity of intravenous dextran sulfate. Antimicrob Agents Chemother. 1991;35:2544–2550. doi: 10.1128/aac.35.12.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formica J.V., Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol Nutr Food Res. 2007;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Shindoh K., Yamamoto Y., Koyano S., Kosugi I., Yamaguchi T. Establishment of a cell-based assay for screening of compounds inhibiting very early events in the cytomegalovirus replication cycle and characterization of a compound identified using the assay. Antimicrob Agents Chemother. 2008;52:2420–2427. doi: 10.1128/AAC.00134-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funaro A., Gribaudo G., Luganini A., Ortolan E., Lo Buono N., Licenzi E. Generation of potent neutralizing human monoclonal antibodies against cytomegalovirus infection from immune B cells. BMC Biotechnol. 2008;8:85. doi: 10.1186/1472-6750-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M.K., Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- Gawn J.M., Greaves R.F. Absence of IE1 p72 protein function during low-multiplicity infection by human cytomegalovirus results in a broad block to viral delayed-early gene expression. J Virol. 2002;76:4441–4455. doi: 10.1128/JVI.76.9.4441-4455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Baldanti F., Revello M.G. Pathogenesis of human cytomegalovirus infection and cellular targets. Hum Immunol. 2004;65:381–386. doi: 10.1016/j.humimm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Gerna G., Percivalle E., Lilleri D., Lozza L., Fornaia C., Hahn G. Dendritic cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8þ T cells. J Gen Virol. 2005;86:275–284. doi: 10.1099/vir.0.80474-0. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Adhikari U., Ghosal P., Pujol C.A., Carlucci M.J., Damonte E.B. In vitro anti-herpetic activity of sulfated polysaccharide fractions from Caulerpa racemosa. Phytochemistry. 2004;65:3151–3157. doi: 10.1016/j.phytochem.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Ghosh T., Chattopadhyay K., Marschall M., Karmakar P., Mandal P., Ray B. Focus on antivirally active sulfated polysaccharides: from structure–activity analysis to clinical evaluation. Glycobiology. 2009;19:2–15. doi: 10.1093/glycob/cwn092. [DOI] [PubMed] [Google Scholar]
- Gilbert C., Boivin G. Human cytomegalovirus resistance to antiviral drugs. Antimicrob Agents Chemother. 2005;49:873–883. doi: 10.1128/AAC.49.3.873-883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M., Guralnik M., Kaneko Y., Mimura T., Baker M., Lang W. A phase I study of curdlan sulfate—an HIV inhibitor. Tolerance, pharmacokinetics and effects on coagulation and on CD4 lymphocytes. J Med. 1994;25:163–180. [PubMed] [Google Scholar]
- Gordon M., Deeks S., De Marzo C., Goodgame J., Guralnik M., Lang W. Curdlan sulfate (CRDS) in a 21-day intravenous tolerance study in human immunodeficiency virus (HIV) and cytomegalovirus (CMV) infected patients: indication of anti-CMV activity with low toxicity. J Med. 1997;28:108–128. [PubMed] [Google Scholar]
- Gorsi B., Stringer S.E. Tinkering with heparan sulfate sulfation to steer development. Trends Cell Biol. 2007;4:173–177. doi: 10.1016/j.tcb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Griffith B.P., Lavallee J.T., Jennings T.A., Hsiung G.D. Transmission of maternal cytomegalovirus-specific immunity in the guinea pig. Clin Immunol Immunopathol. 1985;35:169–181. doi: 10.1016/0090-1229(85)90063-7. [DOI] [PubMed] [Google Scholar]
- Grillone L.R., Lanz R. Fomivirsen. Drugs Today. 2001;37:245–255. doi: 10.1358/dot.2001.37.4.620590. [DOI] [PubMed] [Google Scholar]
- Grover M., Giouzeppos O., Schnagl R.D., May J.T. Effect of human milk prostaglandins and lactoferrin on respiratory syncytial virus and rotavirus. Acta Paediatr. 1997;86:315–316. doi: 10.1111/j.1651-2227.1997.tb08896.x. [DOI] [PubMed] [Google Scholar]
- Hagemeier C., Caswell R., Hayhurst G., Sinclair J., Kouzarides T. Functional interaction between the HCMV IE2 transactivator and the retinoblastoma protein. EMBO J. 1994;13:2897–2903. doi: 10.1002/j.1460-2075.1994.tb06584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn G., Revello M.G., Patrone M., Percivalle E., Campanili G., Saracini A. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol. 2004;78:10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen M.C., Swart P.J., de Béthune M.P., Pauwels R., De Clercq E., The T.H. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J Infect Dis. 1995;172:380–388. doi: 10.1093/infdis/172.2.380. [DOI] [PubMed] [Google Scholar]
- Harris S.M., Bullock B., Westgard E., Zhu H., Stenberg R.M., Kerry J.A. Functional properties of the human cytomegalovirus IE86 protein required for transcriptional regulation and virus replication. J Virol. 2010;84:8839–8848. doi: 10.1128/JVI.00327-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Hayashi T., Kojima I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: in vitro and ex vivo evaluation of anti-herpes simplex virus and anti human immunodeficiency virus activities. AIDS Res Hum Retroviruses. 1996;12:1463–1471. doi: 10.1089/aid.1996.12.1463. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Hayashi K., Maeda M., Kojima I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J Nat Prod. 1996;59:83–87. doi: 10.1021/np960017o. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Hayashi T., Otsuka H., Takeda Y. Antiviral activity of 5,6,7-trimethoxyflavone and its potentiation of the antiherpes activity of acyclovir. J Antimicrob Chemother. 1997;39:821–824. doi: 10.1093/jac/39.6.821. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Minoda K., Nagaoka Y., Hayashi T., Uesato S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg Med Chem Lett. 2007;17:1562–1564. doi: 10.1016/j.bmcl.2006.12.085. [DOI] [PubMed] [Google Scholar]
- Heim K.E., Tagliaferro A.R., Bobilya D.J. Flavonoid antioxidants: chemistry, metabolism and structure–activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Heredia A., Davis C., Redfield R. Synergistic inhibition of HIV-1 in activated and resting peripheral blood mononuclear cells, monocyte-derived macrophages, and selected drug-resistant isolates with nucleoside analogues combined with a natural product, resveratrol. J Acquir Immune Defic Syndr. 2000;25:246–255. doi: 10.1097/00126334-200011010-00006. [DOI] [PubMed] [Google Scholar]
- Hien T.T., Arnold K., Vinh H., Cuong B.M., Phu N.H., Chau T.T. Comparison of artemisinin suppositories with intravenous artesunate and intravenous quinine in the treatment of cerebral malaria. Trans R Soc Trop Med Hyg. 1992;86:582–583. doi: 10.1016/0035-9203(92)90137-2. [DOI] [PubMed] [Google Scholar]
- Hien T.T., Phu N.H., Mai N.T., Chau T.T., Trang T.T., Loc P.P. An open randomized comparison of intravenous and intramuscular artesunate in severe falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:584–585. doi: 10.1016/0035-9203(92)90138-3. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Hayashi T., Hayashi K., Hamada J., Lee J.B., Sankawa U. An antivirally active sulfated polysaccharide from Sargassum horneri (TURNER) C. AGARDH. Biol Pharm Bull. 1998;21:730–734. doi: 10.1248/bpb.21.730. [DOI] [PubMed] [Google Scholar]
- Huang H.C., Hsieh L.M., Chen H.W., Lin Y.S., Chen J.S. Effects of baicalein and esculetin on transduction signals and growth factors expression in T-lymphoid leukemia cells. Eur J Pharmacol. 1994;268:73–78. doi: 10.1016/0922-4106(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Huang Y., Tsang S.Y., Yao X., Chen Z.Y. Biological properties of baicalein in cardiovascular system. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:177–184. doi: 10.2174/1568006043586206. [DOI] [PubMed] [Google Scholar]
- Huber M.T., Compton T. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J Virol. 1998;72:8191–8197. doi: 10.1128/jvi.72.10.8191-8197.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G.W., Monick M.M., Liu B., Stinski M.F. The promoter-regulatory region of the major immediate-early gene of human cytomegalovirus responds to T-lymphocyte stimulation and contains functional cyclic AMP-response elements. J Virol. 1989;63:3026–3033. doi: 10.1128/jvi.63.7.3026-3033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J., Kalejta R.F. Proteasome-dependent, ubiquitin-independent degradation of Daxx by the viral pp 71 protein in human cytomegalovirus-infected cells. Virology. 2007;367:334–338. doi: 10.1016/j.virol.2007.05.037. [DOI] [PubMed] [Google Scholar]
- Hwang J., Kalejta R.F. Human cytomegalovirus protein pp 71 induces Daxx SUMOylation. J Virol. 2009;83:6591–6598. doi: 10.1128/JVI.02639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irache J.M., Merodio M., Arnedo A., Camapanero M.A., Mirshahi M., Espuelas S. Albumin nanoparticles for the intravitreal delivery of anticytomegaloviral drugs. Mini Rev Med Chem. 2005;5:293–305. doi: 10.2174/1389557053175335. [DOI] [PubMed] [Google Scholar]
- Isaacson M.K., Compton T. Human cytomegalovirus glycoprotein B is required for virus entry and cell-to-cell spread but not for virion attachment, assembly, or egress. J Virol. 2009;83:3891–3903. doi: 10.1128/JVI.01251-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson M.K., Feire A.L., Compton T. Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J Virol. 2007;81:6241–6247. doi: 10.1128/JVI.00169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishov A.M., Stenberg R.M., Maul G.G. Human cytomegalovirus immediate early interaction with host nuclear structures: definition of an immediate transcript environment. J Cell Biol. 1997;138:5–16. doi: 10.1083/jcb.138.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura H., Stinski M.F. The human cytomegalovirus major immediate-early enhancer determines the efficiency of immediate-early gene transcription and viral replication in permissive cells at low multiplicity of infection. J Virol. 2003;77:3602–3614. doi: 10.1128/JVI.77.6.3602-3614.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura H., Tsurumi T., Stinski M.F. Role of the proximal enhancer of the major immediate-early promoter in human cytomegalovirus replication. J Virol. 2004;78:12788–12799. doi: 10.1128/JVI.78.23.12788-12799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura H., Stinski M.F., Kudoh A., Daikoku T., Shirata N., Tsurumi T. Two Sp1/Sp3 binding sites in the major immediate-early proximal enhancer of human cytomegalovirus have a significant role in viral replication. J Virol. 2005;79:9597–9607. doi: 10.1128/JVI.79.15.9597-9607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura H., Stinski M.F., Kudoh A., Nakayama S., Murata T., Sato Y. A cis element between the TATA Box and the transcription start site of the major immediate-early promoter of human cytomegalovirus determines efficiency of viral replication. J Virol. 2008;82:849–858. doi: 10.1128/JVI.01593-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S., Lonnerdal B. Lactoferrin, lactoferrin receptors and iron metabolism. Eur J Clin Nutr. 1993;47:232–241. [PubMed] [Google Scholar]
- Jiang X.J., Adler B., Sampaio K.L., Digel M., Jahn G., Ettischer N. UL74 of human cytomegalovirus contributes to virus release by promoting secondary envelopment of virions. J Virol. 2008;82:2802–2812. doi: 10.1128/JVI.01550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Zhao H., Li X.F., Deng Y.Q., Liu J., Xu L.J. CpG oligodeoxynucleotides protect against the 2009 H1N1 pandemic influenza virus infection in a murine model. Antiviral Res. 2011;89:124–126. doi: 10.1016/j.antiviral.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Jo J.Y., Gonzalez de Mejia E., Lila M.A. Catalytic inhibition of human DNA topoisomerase II by interactions of grape cell culture polyphenols. J Agric Food Chem. 2006;54:2083–2087. doi: 10.1021/jf052700z. [DOI] [PubMed] [Google Scholar]
- Johnson D.C., Huber M.T. Directed egress of animal viruses promotes cell-to-cell spread. J Virol. 2002;76:1–8. doi: 10.1128/JVI.76.1.1-8.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.A., Wang X., Ma X.L., Huong S.M., Huang E.S. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J Virol. 2001;75:6022–6032. doi: 10.1128/JVI.75.13.6022-6032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly V., Jidar K., Tatay M., Yeni P. Enfuvirtide: from basic investigations to current clinical use. Expert Opin Pharmacother. 2010;11:2701–2713. doi: 10.1517/14656566.2010.522178. [DOI] [PubMed] [Google Scholar]
- Jones T.R., Lee S.W., Johann S.V., Razinkov V., Visalli R.J., Feld B. Specific inhibition of human cytomegalovirus glycoprotein B-mediated fusion by a novel thiourea small molecule. J Virol. 2004;78:1289–1300. doi: 10.1128/JVI.78.3.1289-1300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovasevic V., Liang L., Roizman B. Proteolytic cleavage of VP1-2 is required for release of herpes simplex virus 1 DNA into the nucleus. J Virol. 2008;82:3311–3319. doi: 10.1128/JVI.01919-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalejta R.F. Functions of human cytomegalovirus tegument proteins prior to immediate early gene expression. Curr Top Microbiol Immunol. 2008;325:101–115. doi: 10.1007/978-3-540-77349-8_6. [DOI] [PubMed] [Google Scholar]
- Kalejta R.F., Shenk T. Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp 71 protein. Proc Natl Acad Sci U S A. 2003;100:3263–3268. doi: 10.1073/pnas.0538058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamstrup S., Frimann T.H., Barfoed A.M. Protection of Balb/c mice against infection with FMDV by immunostimulation with CpG oligonucleotides. Antiviral Res. 2006;72:42–48. doi: 10.1016/j.antiviral.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kanekiyo K., Lee J.B., Hayashi K., Takenaka H., Hayakawa Y., Endo S. Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc flagelliforme. J Nat Prod. 2005;68:1037–1041. doi: 10.1021/np050056c. [DOI] [PubMed] [Google Scholar]
- Kari B., Gehrz R. A human cytomegalovirus glycoprotein complex designated gC-II is a major heparin-binding component of the envelope. J Virol. 1992;66:1761–1764. doi: 10.1128/jvi.66.3.1761-1764.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari B., Gehrz R. Structure, composition and heparin binding properties of a human cytomegalovirus glycoprotein complex designated gC-II. J Gen Virol. 1993;74:255–264. doi: 10.1099/0022-1317-74-2-255. [DOI] [PubMed] [Google Scholar]
- Kaspari M., Tavalai N., Stamminger T., Zimmermann A., Schilf R., Bogner E. Proteasome inhibitor MG132 blocks viral DNA replication and assembly of human cytomegalovirus. FEBS Lett. 2008;582:666–672. doi: 10.1016/j.febslet.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Keller M.J., Wheeler D.G., Cooper E., Meier J.L. Role of the human cytomegalovirus major immediate-early promoter's 19-base-pair-repeat cyclic AMP-response element in acutely infected cells. J Virol. 2003;77:6666–6675. doi: 10.1128/JVI.77.12.6666-6675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneson A., Cannon M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- Kilby J.M., Hopkins S., Venetta T.M., Di Massimo B., Cloud G.A., Lee J.Y. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1232–1233. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- Kimberlin D.W., Lin C.Y., Sanchez P.J., Demmler G.J., Dankner W., Shelton M. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143:16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- Krieg A.M. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;6:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- Kuipers M.E., de Vries-Hospers H.G., Eikelboom M.C., Meijer D.K.F., Swart P.J. Synergistic fungistatic effects of lactoferrin in combination with antifungal drugs against clinical Candida isolates. Antimicrob Agents Chemother. 1999;43:2635–2641. doi: 10.1128/aac.43.11.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuritzkes D.R. HIV-1 entry inhibitors: an overview. Curr Opin HIV AIDS. 2009;4:82–87. doi: 10.1097/COH.0b013e328322402e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfo S., Gariglio M., Gribaudo G., Lembo D. The human cytomegalovirus. Pharmacol Ther. 2003;98:269–297. doi: 10.1016/s0163-7258(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Lashmit P.E., Lundquist C.A., Meier J.L., Stinski M.F. Cellular repressor inhibits human cytomegalovirus transcription from the UL127 promoter. J Virol. 2004;78:5113–5123. doi: 10.1128/JVI.78.10.5113-5123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea A.P., Bryson H.M. Cidofovir. Drugs. 1996;52:225–230. doi: 10.2165/00003495-199652020-00006. [DOI] [PubMed] [Google Scholar]
- Lee J.B., Hayashi K., Hayashi T., Sankawa U., Maeda M. Antiviral activities against HSV-1, HCMV, and HIV-1 of rhamnan sulfate from Monostroma latissimum. Planta Med. 1999;65:439–441. doi: 10.1055/s-2006-960804. [DOI] [PubMed] [Google Scholar]
- Lembo D., Donalisio M., Rusnati M., Bugatti A., Cornaglia M., Cappello P. Sulfated K5 Escherichia coli polysaccharide derivatives as wide-range inhibitors of genital types of human papillomavirus. Antimicrob Agents Chemother. 2008;52:1374–1381. doi: 10.1128/AAC.01467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone S., Cornetta T., Basso E., Cozzi R. Resveratrol induces DNA double-strand breaks through human topoisomerase II interaction. Cancer Lett. 2010;295:167–172. doi: 10.1016/j.canlet.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Levay P.F., Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- Li Y.Q., Li Z.L., Zhao W.J., Wen R.X., Meng Q.W., Zeng Y. Synthesis of stilbene derivatives with inhibition of SARS coronavirus replication. Eur J Med Chem. 2006;41:1084–1089. doi: 10.1016/j.ejmech.2006.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.M., Flavin M.T., Schure R., Chen F.C., Sidwell R., Barnard D.L. Antiviral activities of biflavonoids. Planta Med. 1999;65:120–125. doi: 10.1055/s-1999-13971. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Lidholt K., Spillmann D., Kjellen L. More to “heparin” than anticoagulation. Thromb Res. 1994;75:1–32. doi: 10.1016/0049-3848(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Gullberg M.K., Kjellen L. Regulated diversity of heparan sulfate. J Biol Chem. 1998;273:24979–24982. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- Littler E., Stuart A.D., Chee M.S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992;358:160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- Liu B., Stinski M.F. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J Virol. 1992;66:4434–4444. doi: 10.1128/jvi.66.7.4434-4444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi M., Deziel R., Moss N., Beaulieu P., Bonneau A.M., Bousquet C. A potent peptidomimetic inhibitor of HSV ribonucleotide reductase with antiviral activity in vivo. Nature. 1994;372:695–698. doi: 10.1038/372695a0. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B., Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- Lopper M., Compton T. Coiled-coil domains in glycoproteins B and H are involved in human cytomegalovirus membrane fusion. J Virol. 2004;78:8333–8341. doi: 10.1128/JVI.78.15.8333-8341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loregian A., Coen D.M. Selective anti-cytomegalovirus compounds discovered by screening for inhibitors of subunit interactions of the viral polymerase. Chem Biol. 2006;13:191–200. doi: 10.1016/j.chembiol.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Loregian A., Palù G. Disruption of the interactions between the subunits of herpesvirus DNA polymerases as a novel antiviral strategy. Clin Microbiol Infect. 2005;11:437–446. doi: 10.1111/j.1469-0691.2005.01149.x. [DOI] [PubMed] [Google Scholar]
- Loregian A., Palù G. Disruption of protein–protein interactions: towards new targets for chemotherapy. J Cell Physiol. 2005;204:750–762. doi: 10.1002/jcp.20356. [DOI] [PubMed] [Google Scholar]
- Loregian A., Papini E., Satin B., Marsden H.S., Hirst T.R., Palù G. Intranuclear delivery of an antiviral peptide mediated by the B subunit of Escherichia coli heat-labile enterotoxin. Proc Natl Acad Sci U S A. 1999;96:5221–5226. doi: 10.1073/pnas.96.9.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loregian A., Marsden H.S., Palù G. Protein–protein interactions as targets for antiviral chemotherapy. Rev Med Virol. 2002;12:239–262. doi: 10.1002/rmv.356. [DOI] [PubMed] [Google Scholar]
- Loregian A., Rigatti R., Murphy M., Schievano E., Palù G., Marsden H.S. Inhibition of human cytomegalovirus (HCMV) DNA polymerase by C-terminal peptides from the UL54 subunit. J Virol. 2003;77:8336–8344. doi: 10.1128/JVI.77.15.8336-8344.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loregian A., Mercorelli B., Muratore G., Sinigalia E., Pagni S., Massari S. The 6-aminoquinolone WC5 inhibits human cytomegalovirus replication at an early stage by interfering with the transactivating activity of viral immediate-early 2 protein. Antimicrob Agents Chemother. 2010;54:1930–1940. doi: 10.1128/AAC.01730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowance D., Neumayer H.H., Legendre C.M., Squifflet J.P., Kovarik J., Brennan P.J. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group. N Engl J Med. 1999;340:1462–1470. doi: 10.1056/NEJM199905133401903. [DOI] [PubMed] [Google Scholar]
- Luganini A., Caposio P., Landolfo S., Gribaudo G. Phosphorothioate-modified oligodeoxynucleotides inhibit human cytomegalovirus replication by blocking virus entry. Antimicrob Agents Chemother. 2008;52:1111–1120. doi: 10.1128/AAC.00987-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luganini A., Caposio P., Mondini M., Landolfo S., Gribaudo G. New cell-based indicator assays for the detection of human cytomegalovirus infection and screening of inhibitors of viral immediate-early 2 protein activity. J Appl Microbiol. 2008;105:1791–1801. doi: 10.1111/j.1365-2672.2008.03927.x. [DOI] [PubMed] [Google Scholar]
- Luganini A., Giuliani A., Pirri G., Pizzuto L., Landolfo S., Gribaudo G. Peptide-derivatized dendrimers inhibit human cytomegalovirus infection by blocking virus binding to cell surface heparan sulfate. Antiviral Res. 2010;85:532–540. doi: 10.1016/j.antiviral.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Lukac D.M., Manuppello J.R., Alwine J.C. Transcriptional activation by the human cytomegalovirus immediate-early proteins: requirements for simple promoter structures and interactions with multiple components of the transcription complex. J Virol. 1994;68:5184–5193. doi: 10.1128/jvi.68.8.5184-5193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac D.M., Harel N.Y., Tanese N., Alwine J.C. TAF-like functions of human cytomegalovirus immediate-early proteins. J Virol. 1997;71:7227–7239. doi: 10.1128/jvi.71.10.7227-7239.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashchuk V., McFarlane S., Everett R.D., Preston C.M. Human cytomegalovirus protein pp 71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection. J Virol. 2008;82:12543–12554. doi: 10.1128/JVI.01215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A. Coiled coils: new structures and new functions. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- Lurain N.S., Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010;23:689–712. doi: 10.1128/CMR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurain N.S., Spafford L.E., Thompson K.D. Mutation in the UL97 open reading frame of human cytomegalovirus strains resistant to ganciclovir. J Virol. 1994;68:4427–4431. doi: 10.1128/jvi.68.7.4427-4431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macagno A., Bernasconi N.L., Vanzetta F., Dander E., Sarasini A., Revello M.G. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128–131A complex. J Virol. 2010;84:1005–1013. doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias M.P., Huang L., Lashmit P.E., Stinski M.F. Cellular or viral protein binding to a cytomegalovirus promoter transcription initiation site: effects on transcription. J Virol. 1996;70:3628–3635. doi: 10.1128/jvi.70.6.3628-3635.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P., Pujol C.A., Carlucci M.J., Chattopadhyay K., Damonte E.B., Ray B. Sulfated xylomannan of Scinaia hetai: isolation, structural features and antiviral activity. Phytochemistry. 2008;69:2193–2199. doi: 10.1016/j.phytochem.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Marcello A., Loregian A., Cross A., Marsden H.S., Hirst T.R., Palù G. Specific inhibition of herpes virus replication by receptor-mediated entry of an antiviral peptide linked to Escherichia coli enterotoxin B subunit. Proc Natl Acad Sci U S A. 1994;91:8994–8998. doi: 10.1073/pnas.91.19.8994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti M., Longhi C., Conte M.P., Pisani S., Valenti P., Seganti L. Lactoferrin inhibits herpes simplex virus type 1 adsorption to Vero cells. Antiviral Res. 1996;29:221–231. doi: 10.1016/0166-3542(95)00840-3. [DOI] [PubMed] [Google Scholar]
- Marchini A., Liu H., Zhu H. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J Virol. 2001;75:1870–1878. doi: 10.1128/JVI.75.4.1870-1878.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham A., Faulds D. Ganciclovir. An update of its therapeutic use in cytomegalovirus infection. Drugs. 1994;48:455–484. doi: 10.2165/00003495-199448030-00009. [DOI] [PubMed] [Google Scholar]
- Meier J.L., Pruessner J.A. The human cytomegalovirus major immediate-early distal enhancer region is required for efficient viral replication and immediate-early gene expression. J Virol. 2000;74:1602–1613. doi: 10.1128/jvi.74.4.1602-1613.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J.L., Keller M.J., McCoy J.J. Requirement of multiple cis-acting elements in the human cytomegalovirus major immediate-early distal enhancer for viral gene expression and replication. J Virol. 2002;76:313–326. doi: 10.1128/JVI.76.1.313-326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercorelli B., Sinigalia E., Loregian A., Palu G. Human cytomegalovirus DNA replication: antiviral targets and drugs. Rev Med Virol. 2008;18:177–210. doi: 10.1002/rmv.558. [DOI] [PubMed] [Google Scholar]
- Mercorelli B., Muratore G., Sinigalia E., Tabarrini O., Biasolo M.A., Cecchetti V. A 6-aminoquinolone compound, WC5, with potent and selective anti-human cytomegalovirus activity. Antimicrob Agents Chemother. 2009;53:312–315. doi: 10.1128/AAC.00988-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercorelli B., Oreste P., Sinigalia E., Muratore G., Lembo D., Palù G. Sulfated derivatives of Escherichia coli K5 capsular polysaccharide are potent inhibitors of human cytomegalovirus. Antimicrob Agents Chemother. 2010;54:4561–4567. doi: 10.1128/AAC.00721-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M., Paulus C., Loschmann N., Dauth S., Stange E., Doerr H.W. The multi-targeted kinase inhibitor sorafenib inhibits human cytomegalovirus replication. Cell Mol Life Sci. 2011;68:1079–1090. doi: 10.1007/s00018-010-0510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrocotsa D., Mitaku S., Axarlis S., Harvala C., Malamas M. Evaluation of the antiviral activity of kaempferol and its glycosides against human cytomegalovirus. Planta Med. 2000;66:377–379. doi: 10.1055/s-2000-8550. [DOI] [PubMed] [Google Scholar]
- Mocarski E.S., Shenk T., Pass R.F. Cytomegaloviruses. In: Knipe D.M., Howley P.M., Griffin D.E., Lamb R.A., Martin M.A., Roizman B., Straus S.E., editors. Fields Virology. 5th ed. Lippincott Williams & Wilkins; 2007. pp. 2701–2772. [Google Scholar]
- Mulamba G.B., Hu A., Azad R.F., Anderson K.P., Coen D.M. Human cytomegalovirus mutant with sequence-dependent resistance to the phosphorothioate oligonucleotide fomivirsen (ISIS 2922) Antimicrob Agents Chemother. 1998;42:971–973. doi: 10.1128/aac.42.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T., Eizuru Y., Yamada R., Sadanari H., Matsubara K., Rukung G. Anticytomegalovirus activity of pristimerin, a triterpenoid quinone methide isolated from Maytenus heterophylla (Eckl. & Zeyh.) Antivir Chem Chemother. 2007;18:133–139. doi: 10.1177/095632020701800303. [DOI] [PubMed] [Google Scholar]
- Murphy E., Yu D., Grimwood J., Schmutz J., Dickson M., Jarvis M.A. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc Natl Acad Sci U S A. 2003;100:14976–14981. doi: 10.1073/pnas.2136652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naesens L., De Clercq E. Recent developments in herpesvirus therapy. Herpes. 2001;8:12–16. [PubMed] [Google Scholar]
- Netterwald J., Yang S., Wang W., Ghanny S., Cody M., Soteropoulos P. Two gamma interferon-activated site-like elements in the human cytomegalovirus major immediate-early promoter/enhancer are important for viral replication. J Virol. 2005;79:5035–5046. doi: 10.1128/JVI.79.8.5035-5046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevels M., Paulus C., Shenk T. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc Natl Acad Sci U S A. 2004;101:17234–17239. doi: 10.1073/pnas.0407933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyts J., Snoeck R., Schols D., Balzarini J., Esko J.D., Van Schepdael A. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology. 1992;189:48–58. doi: 10.1016/0042-6822(92)90680-n. [DOI] [PubMed] [Google Scholar]
- Nitzsche A., Paulus C., Nevels M. Temporal dynamics of cytomegalovirus chromatin assembly in productively infected human cells. J Virol. 2008;82:11167–11180. doi: 10.1128/JVI.01218-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosten F. Artemisinin: large community studies. Trans R Soc Trop Med Hyg. 1994;88(Suppl 1):S45–S46. doi: 10.1016/0035-9203(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Nowlin D.M., Cooper N.R., Compton T. Expression of a human cytomegalovirus receptor correlates with infectibility of cells. J Virol. 1991;65:3114–3121. doi: 10.1128/jvi.65.6.3114-3121.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa-Goto K., Tanaka K., Gibson W., Moriishi E., Miura Y., Kurata T. Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid. J Virol. 2003;77:8541–8547. doi: 10.1128/JVI.77.15.8541-8547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamara A.T., Nencioni L., Aquilano K., De Chiara G., Hernandez L., Cozzolino F. Inhibition of influenza A virus replication by resveratrol. J Infect Dis. 2005;191:1719–1729. doi: 10.1086/429694. [DOI] [PubMed] [Google Scholar]
- Paolini M., Sapone A., Valgimigli L. Avoidance of bioflavonoid supplements during pregnancy: a pathway to infant leukemia? Mutat Res. 2003;527:99–101. doi: 10.1016/s0027-5107(03)00057-5. [DOI] [PubMed] [Google Scholar]
- Pari G.S., Field A.K., Smith J.A. Potent antiviral activity of an antisense oligonucleotide complementary to the intron-exon boundary of human cytomegalovirus genes UL36 and UL37. Antimicrob Agents Chemother. 1995;39:1157–1161. doi: 10.1128/aac.39.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.J., Kim Y.E., Pham H.T., Kim E.T., Chung Y.H., Ahn J.H. Functional interaction of the human cytomegalovirus IE2 protein with histone deacetylase 2 in infected human fibroblasts. J Gen Virol. 2007;88:3214–3223. doi: 10.1099/vir.0.83171-0. [DOI] [PubMed] [Google Scholar]
- Parolin C., Gatto B., Del Vecchio C., Pecere T., Tramontano E., Cecchetti V. New anti-human immunodeficiency virus type 1 6-aminoquinolones: mechanism of action. Antimicrob Agents Chemother. 2003;47:889–896. doi: 10.1128/AAC.47.3.889-896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasdeloup D., Blondel D., Isidro A.L., Rixon F.J. Herpesvirus capsid association with the nuclear pore complex and viral DNA release involve the nucleoporin CAN/Nup214 and the capsid protein pUL25. J Virol. 2009;83:6610–6623. doi: 10.1128/JVI.02655-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkert R.R., Kalejta R.F. Nuclear localization of tegument-delivered pp 71 in human cytomegalovirus-infected cells is facilitated by one or more factors present in terminally differentiated fibroblasts. J Virol. 2010;84:9853–9863. doi: 10.1128/JVI.00500-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Bernfield M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 2000;404:725–728. doi: 10.1038/35008000. [DOI] [PubMed] [Google Scholar]
- Perry C.M., Balfour J.A. Fomivirsen. Drugs. 1999;57:375–380. doi: 10.2165/00003495-199957030-00010. [DOI] [PubMed] [Google Scholar]
- Perry C.M., Faulds D. Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs. 1996;52:754–772. doi: 10.2165/00003495-199652050-00009. [DOI] [PubMed] [Google Scholar]
- Petrik D.T., Schmitt K.P., Stinski M.F. The autoregulatory and transactivating functions of the human cytomegalovirus IE86 protein use independent mechanisms for promoter binding. J Virol. 2007;81:5807–5818. doi: 10.1128/JVI.02437-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilger B.D., Cui C., Coen D.M. Identification of a small molecule that inhibits herpes simplex virus DNA polymerase subunit interactions and viral replication. Chem Biol. 2004;11:647–654. doi: 10.1016/j.chembiol.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Pinna D., Oreste P., Coradin T., Kajaste-Rudnitski A., Ghezzi S., Zappetti G. Inhibition of herpes simplex virus types 1 and 2 in vitro infection by sulfated derivatives of Escherichia coli K5 polysaccharide. Antimicrob Agents Chemother. 2008;52:3078–3084. doi: 10.1128/AAC.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole E., Atkins E., Nakayama T., Yoshie O., Groves I., Alcami A. NF-kappaB-mediated activation of the chemokine CCL22 by the product of the human cytomegalovirus gene UL144 escapes regulation by viral IE86. J Virol. 2008;82:4250–4256. doi: 10.1128/JVI.02156-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosch S., Priemer C., Hoflich C., Liebenthaf C., Babel N., Kruger D.H. Proteasome inhibitors: a novel tool to suppress human cytomegalovirus replication and virus-induced immune modulation. Antivir Ther. 2003;8:555–567. [PubMed] [Google Scholar]
- Raanani P., Gafter-Gvili A., Paul M., Ben-Bassat I., Leibovici L., Shpilberg O. Immunoglobulin prophylaxis in hematological malignancies and hematopoietic stem cell transplantation. Cochrane Database Syst Rev. 2008;8:CD006501. doi: 10.1002/14651858.CD006501.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke K., Kieneke D., Wolfstein A., Michael K., Steffen W., Scholz T. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog. 2010;6:e1000991. doi: 10.1371/journal.ppat.1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathee P., Chaudhary H., Rathee S., Rathee D., Kumar V., Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets. 2009;8:229–235. doi: 10.2174/187152809788681029. [DOI] [PubMed] [Google Scholar]
- Razonable R.R., Emery V.C. Management of CMV infection and disease in transplant patients. Herpes. 2004;11:77–86. [PubMed] [Google Scholar]
- Rechter S., Konig T., Auerochs S., Thulke S., Walter H., Dornenburg H., Walter C., Marschall M. Antiviral activity of Arthrospira-derived spirulan-like substances. Antiviral Res. 2006;72:197–206. doi: 10.1016/j.antiviral.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Reeves M., Murphy J., Greaves R., Fairley J., Brehm A., Sinclair J. Autorepression of the human cytomegalovirus major immediate-early promoter/enhancer at late times of infection is mediated by the recruitment of chromatin remodeling enzymes by IE86. J Virol. 2006;80:9998–10009. doi: 10.1128/JVI.01297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revello M.G., Gerna G. Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications. Rev Med Virol. 2010;20:136–155. doi: 10.1002/rmv.645. [DOI] [PubMed] [Google Scholar]
- Revello M.G., Percivalle E., Arbustivi E., Pardi R., Sozzoni S., Gerna G. In vitro generation of human cytomegalovirus pp 65 antigenemia, viremia, and leukoDNAemia. J Clin Invest. 1998;101:2686–2692. doi: 10.1172/JCI1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin V., Irurzun A., Amoros M., Boustie J., Carrasco L. Antipoliovirus flavonoids from Psiadia dentata. Antivir Chem Chemother. 2001;12:283–291. doi: 10.1177/095632020101200503. [DOI] [PubMed] [Google Scholar]
- Rosenthal P.J. Artesunate for the treatment of severe falciparum malaria. N Engl J Med. 2008;358:1829–1836. doi: 10.1056/NEJMct0709050. [DOI] [PubMed] [Google Scholar]
- Ross J.A. Dietary flavonoids and the MLL gene: a pathway to infant leukemia? Proc Natl Acad Sci U S A. 2000;97:4411–4413. doi: 10.1073/pnas.97.9.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D.M., Grundy J.E. Evaluation of neutralizing antibody titers against human cytomegalovirus in intravenous gamma globulin preparations. Transplantation. 1992;54:1109–1110. [PubMed] [Google Scholar]
- Rusnati M., Oreste P., Zappetti G., Presta M. Biotechnological engineering of heparin/heparan sulphate: a novel area of multi-target drug discovery. Curr Pharm Des. 2005;11:2489–2499. doi: 10.2174/1381612054367553. [DOI] [PubMed] [Google Scholar]
- Rusnati M., Vicenzi E., Donalisio M., Oreste P., Landolfo S., Lembo D. Sulfated K5 Escherichia coli polysaccharide derivatives: a novel class of candidate antiviral microbicides. Pharmacol Ther. 2009;123:310–322. doi: 10.1016/j.pharmthera.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Ryckman B.J., Jarvis M.A., Drummond D.D., Nelson J.A., Johnson D.C. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol. 2006;80:710–722. doi: 10.1128/JVI.80.2.710-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckman B.J., Rainish B.L., Chase M.C., Borton J.A., Nelson J.A., Jarvis M.A. Characterization of the human cytomegalovirus gH/gL/UL128–131 complex that mediates entry into epithelial and endothelial cells. J Virol. 2008;82:60–70. doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer E., Audige A., von Beust B., Manolova V., Weber M., Joller H. CpG oligodeoxynucleotides block human immunodeficiency virus type 1 replication in human lymphoid tissue infected ex vivo. J Virol. 2004;78:12344–12354. doi: 10.1128/JVI.78.22.12344-12354.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiss M.R. Comparison of vaccine strategies against congenital CMV infection in the guinea pig model. J Clin Virol. 2008;41:224–230. doi: 10.1016/j.jcv.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Schwartz R., Sommer M.H., Scully A., Spector D.H. Site-specific binding of the human cytomegalovirus IE2 86-kilodalton protein to an early gene promoter. J Virol. 1994;68:5613–5622. doi: 10.1128/jvi.68.9.5613-5622.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully A.L., Sommer M.H., Schwartz R., Spector D.H. The human cytomegalovirus IE2 86-kilodalton protein interacts with an early gene promoter via site-specific DNA binding and protein–protein associations. J Virol. 1995;69:6533–6540. doi: 10.1128/jvi.69.10.6533-6540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya K., Okuda H. Selective inhibition of platelet lipoxygenase by baicalein. Biochem Biophys Res Commun. 1982;105:1090–1095. doi: 10.1016/0006-291x(82)91081-6. [DOI] [PubMed] [Google Scholar]
- Shang X., He X., He X., Li M., Zhang R., Fan P. The genus Scutellaria an ethnopharmacological and phytochemical review. J Ethnopharmacol. 2010;24:279–313. doi: 10.1016/j.jep.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Matsuzawa H., Okada K., Tazume S., Dosako S., Kawasaki Y. Lactoferrin-mediated protection of the host from murine cytomegalovirus infection by a T-cell-dependent augmentation of natural killer cell activity. Arch Virol. 1996;141:1875–1889. doi: 10.1007/BF01718201. [DOI] [PubMed] [Google Scholar]
- Shogan B., Kruse L., Mulamba G.B., Hu A., Coen D.M. Virucidal activity of a GT-rich oligonucleotide against herpes simplex virus mediated by glycoprotein B. J Virol. 2006;80:4740–4747. doi: 10.1128/JVI.80.10.4740-4747.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. Antiviral drugs for cytomegalovirus in transplant recipients: advantages of preemptive therapy. Rev Med Virol. 2006;16:281–287. doi: 10.1002/rmv.513. [DOI] [PubMed] [Google Scholar]
- Sinzger C., Digel M., Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol. 2008;325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- Smith J.A., Pari G.S. Expression of human cytomegalovirus UL36 and UL37 genes is required for viral DNA replication. J Virol. 1995;69:1925–1931. doi: 10.1128/jvi.69.3.1925-1931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeck R., Andrei G., Bodaghi B., Lagneaux L., Daelemans D., de Clercq E. 2-Chloro-3-pyridin-3-yl-5,6,7,8-tetrahydroindolizine-1-carboxamide (CMV423), a new lead compound for the treatment of human cytomegalovirus infections. Antiviral Res. 2002;55:413–424. doi: 10.1016/s0166-3542(02)00074-8. [DOI] [PubMed] [Google Scholar]
- Snydman D.R. Historical overview of the use of cytomegalovirus hyperimmune globulin in organ transplantation. Transpl Infect Dis. 2001;3(Suppl 2):6–13. doi: 10.1034/j.1399-3062.2001.00002.x. [DOI] [PubMed] [Google Scholar]
- Snydman D.R. The case for cytomegalovirus prophylaxis in solid organ transplantation. Rev Med Virol. 2006;16:289–295. doi: 10.1002/rmv.514. [DOI] [PubMed] [Google Scholar]
- Soroceanu L., Akhavan A., Cobbs C.S. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature. 2008;455:391–395. doi: 10.1038/nature07209. [DOI] [PubMed] [Google Scholar]
- Steininger C., Puchhammer-Stockl E., Popow-Kraupp T. Cytomegalovirus disease in the era of highly active antiretroviral therapy (HAART) J Clin Virol. 2006;37:1–9. doi: 10.1016/j.jcv.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Stinski M.F., Isomura H. Role of the cytomegalovirus major immediate early enhancer in acute infection and reactivation from latency. Med Microbiol Immunol. 2008;197:223–231. doi: 10.1007/s00430-007-0069-7. [DOI] [PubMed] [Google Scholar]
- Stinski M.F., Petrik D.T. Functional roles of the human cytomegalovirus essential IE86 protein. Curr Top Microbiol Immunol. 2008;325:133–152. doi: 10.1007/978-3-540-77349-8_8. [DOI] [PubMed] [Google Scholar]
- Streblow D.N., Dumortier J., Moses A.V., Orloff S.L., Nelson J.A. Mechanisms of cytomegalovirus-accelerated vascular disease: induction of paracrine factors that promote angiogenesis and wound healing. Curr Top Microbiol Immunol. 2008;325:397–415. doi: 10.1007/978-3-540-77349-8_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick R., Strissel P.L., Borgers S., Smith S.L., Rowley J.D. Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia. Proc Natl Acad Sci U S A. 2000;97:4790–4795. doi: 10.1073/pnas.070061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan V., Talarico C.L., Stanat S.C., Davis M., Coen D.M., Biron K.K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992;358:162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- Swart P.J., Kuipers M.E., Smit C., Pauwels R., de Béthune M.P., De Clercq E. Antiviral effects of milk proteins: acylation results in polyanionic compounds with potent activity against human immunodeficiency virus types 1 and 2 in vitro. AIDS Res Hum Retroviruses. 1996;12:769–775. doi: 10.1089/aid.1996.12.769. [DOI] [PubMed] [Google Scholar]
- Swart P.J., Harmsen M.C., Kuipers M.E., Van Dijk A.A., van der Strate B.W.A., Van Berkel P.H.C. Charge modification of plasma and milk proteins results in antiviral active compounds. J Pept Sci. 1999;5:563–576. doi: 10.1002/(SICI)1099-1387(199912)5:12<563::AID-PSC226>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Tavalai N., Stamminger T. New insights into the role of the subnuclear structure ND10 for viral infection. Biochim Biophys Acta. 2008;1783:2207–2221. doi: 10.1016/j.bbamcr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Taylor R.T., Bresnahan W.A. Human cytomegalovirus immediate-early 2 gene expression blocks virus-induced beta interferon production. J Virol. 2005;79:3873–3877. doi: 10.1128/JVI.79.6.3873-3877.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.T., Bresnahan W.A. Human cytomegalovirus IE86 attenuates virus- and tumor necrosis factor alpha-induced NFkappaB-dependent gene expression. J Virol. 2006;80:10763–10771. doi: 10.1128/JVI.01195-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R.T., Bresnahan W.A. Human cytomegalovirus immediate-early 2 protein IE86 blocks virus-induced chemokine expression. J Virol. 2006;80:920–928. doi: 10.1128/JVI.80.2.920-928.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantas E., Panopoulos N., Ververidis F. Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metab Eng. 2009;11:355–366. doi: 10.1016/j.ymben.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Tumova S., Woods A., Couchman J.R. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int J Biochem Cell Biol. 2000;32:269–288. doi: 10.1016/s1357-2725(99)00116-8. [DOI] [PubMed] [Google Scholar]
- Turnbull J., Powell A., Guimond S. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 2001;11:75–82. doi: 10.1016/s0962-8924(00)01897-3. [DOI] [PubMed] [Google Scholar]
- Valantine H.A., Luikart H., Doyle R., Theodore J., Hunt S., Oyer P. Impact of cytomegalovirus hyperimmune globulin on outcome after cardiothoracic transplantation: a comparative study of combined prophylaxis with CMV hyperimmune globulin plus ganciclovir versus ganciclovir alone. Transplantation. 2001;72:1647–1652. doi: 10.1097/00007890-200111270-00012. [DOI] [PubMed] [Google Scholar]
- van Berkel P.H.C., Geerts M.E.J., van Veen H.A., Mericskay M., de Boer H.A., Nuijens J.H. N-terminal stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem J. 1997;328:145–151. doi: 10.1042/bj3280145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Den Pol A.N., Mocarski E., Saederup N., Vieira J., Meier T.J. Cytomegalovirus cell tropism, replication, and gene transfer in brain. J Neurosci. 1999;19:10948–10965. doi: 10.1523/JNEUROSCI.19-24-10948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Strate B.W., Beljaars L., Molema G., Harmsen M.C., Meijer D.K.F. Antiviral activities of lactoferrin. Antiviral Res. 2001;52:225–239. doi: 10.1016/s0166-3542(01)00195-4. [DOI] [PubMed] [Google Scholar]
- van der Strate B.W.A., Harmsen M.C., Schafer P., Swart P.J., The T.H., Jahn G. Viral load in breast milk correlates with transmission of human cytomegalovirus to preterm neonates, but lactoferrin concentrations do not. Clin Diagn Lab Immunol. 2001;8:818–821. doi: 10.1128/CDLI.8.4.818-821.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Strate B.W.A., De Boer F.M., Bakker H.I., Meijer D.K.F., Molema G., Harmsen M.C. Synergy of bovine lactoferrin with the anti-cytomegalovirus drug cidofovir in vitro. Antiviral Res. 2003;58:159–165. doi: 10.1016/s0166-3542(02)00211-5. [DOI] [PubMed] [Google Scholar]
- Vicenzi E., Gatti A., Ghezzi S., Oreste P., Zoppetti G., Poli G. Broad spectrum inhibition of HIV-1 infection by sulfated K5 Escherichia coli polysaccharide derivatives. AIDS. 2003;17:177–181. doi: 10.1097/00002030-200301240-00006. [DOI] [PubMed] [Google Scholar]
- Villarreal E.C. Current and potential therapies for the treatment of herpes-virus infections. Prog Drug Res. 2003;60:263–307. doi: 10.1007/978-3-0348-8012-1_8. [DOI] [PubMed] [Google Scholar]
- Wagstaff A.J., Bryson H.M. Foscarnet. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs. 1994;48:199–226. doi: 10.2165/00003495-199448020-00007. [DOI] [PubMed] [Google Scholar]
- Wagstaff A.J., Faulds D., Goa K.L. Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47:153–205. doi: 10.2165/00003495-199447010-00009. [DOI] [PubMed] [Google Scholar]
- Waheed I., Chiou C.J., Ahn J.H., Hayward G.S. Binding of the human cytomegalovirus 80-kDa immediate-early protein (IE2) to minor groove A/T-rich sequences bounded by CG dinucleotides is regulated by protein oligomerization and phosphorylation. Virology. 1998;252:235–257. doi: 10.1006/viro.1998.9448. [DOI] [PubMed] [Google Scholar]
- Wang D., Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A. 2005;102:18153–18158. doi: 10.1073/pnas.0509201102. BIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Shenk T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol. 2005;79:10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Huong S.M., Chiu M.L., Raab-Traub N., Huang E.S. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- Wei F., Ma S.C., Ma L.Y., But P.P., Lin R.C., Khan I.A. Antiviral flavonoids from the seeds of Aesculus chinensis. J Nat Prod. 2004;67:650–653. doi: 10.1021/np030470h. [DOI] [PubMed] [Google Scholar]
- Wellbrock C., Karasarides M., Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- White E.A., Del Rosario C.J., Sanders R.L., Spector D.H. The IE2 60-kilodalton and 40-kilodalton proteins are dispensable for human cytomegalovirus replication but are required for efficient delayed early and late gene expression and production of infectious virus. J Virol. 2007;81:2573–2583. doi: 10.1128/JVI.02454-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witvrouw M., De Clercq E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen Pharmacol. 1997;29:497–511. doi: 10.1016/s0306-3623(96)00563-0. [DOI] [PubMed] [Google Scholar]
- Wong J.P., Nagata L.P., Christopher M.E., Salazar A.M., Dale R.M. Prophylaxis of acute respiratory virus infections using nucleic acid-based drugs. Vaccine. 2005;23:2266–2268. doi: 10.1016/j.vaccine.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Woodhall D.L., Groves I.J., Reeves M.B., Wilkinson G., Sinclair J.H. Human Daxx-mediated repression of human cytomegalovirus gene expression correlates with a repressive chromatin structure around the major immediate early promoter. J Biol Chem. 2006;281:37652–37660. doi: 10.1074/jbc.M604273200. [DOI] [PubMed] [Google Scholar]
- Yeh R., Coen D.M. Pharmacology of viral replication. In: Golan DE T.A.J., Armstrong E.J., Galanter J.M., Armstrong A.W., Arnaout R.A., Rose H.S., editors. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott, Williams & Wilkins; Baltimore: 2004. pp. 545–564. [Google Scholar]
- Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]