Abstract
Objectives
To investigate the genetic character of Human bocavirus (HBoV) among children with severe acute respiratory infection (SARI) in China.
Methods
We screened 993 respiratory samples for HBoV by PCR among hospitalized children with SARI between September 2007 and March 2014. Four of HBoV1 samples were selected for complete genomes analysis by next-generation sequencing.
Results
The results show that 200 (20.1%) out of 993 samples were HBoV-positive, most of these HBoV belong to HBoV1 subtype (n = 197), HBoV2 (n = 1) and HBoV3 (n = 2) were also detected. Fifty (5.04%) of 993 SARI patient were detected as HBoV-positive only. Four HBoV1 genomes in this study were conserved and showed no significant difference among the nucleotide diversity from different regions. Analyses of evolutionary rates showed that NS1 exhibited the highest degree of conservation while the VP1 gene exhibited the fastest rate of evolution at 4.20 × 10−4 substitutions/site/year. The nucleotide deletions and substitutions occurred in NP1 and VP1 represented novel molecular signatures enabling subtype differentiation between HBoVs.
Conclusions
We described some new characteristics in the epidemiology of HBoV among children with SARI, these data will significantly expand the current knowledge of HBoV epidemic and genomic characterization among children with SARI.
Keywords: Human bocavirus, Severe acute respiratory infection, Genome, Phylogenetic analysis, Evolutionary rate
Highlights
-
•
The infection rate of HBoV among inpatient children with SARI in China was about 20.1%.
-
•
A comparative analysis of HBoV genomes identified a total of three deletions within NP1 and VP1 together.
-
•
There was no significant difference among the nucleotide diversity of HBoV1 from different regions.
Introduction
Human bocavirus1–4 (HBoV) represents a novel pathogen associated with gastrointestinal and respiratory tract illnesses.1, 2, 3 According to the latest ICTV classification of parvovirus, HBoV1 and HBoV3 belonged to Primate bocaparvovirus 1 species; HBoV2 and HBoV4 are part of P. bocaparvovirus 2 species.4 The genome of HBoV is ∼5.3 kb in length, and is divided into four partially overlapping genes, namely NS1, NP1, VP1, and VP2, VP2 is totally included within VP1.5, 6 While the prototype HBoV (HBoV1) were first discovered from nasopharyngeal samples,7 three additional viruses (HBoV2–4) have since been discovered in stool specimens, classified based upon their close phylogenetic relationship with HBoV1.8, 9
HBoV1 is the most commonly reported genotype and occurring primarily in pediatric patients with respiratory tract infection, but also gastrointestinal symptoms are often observed.10, 11 In contrast, HBoV2 are preferentially detected in stool samples and appear to be more strongly associated with enteric disease, HBoV3 and HBoV4 are occasionally detected in faeces and too rare for any associations.2 Since the discovery of HBoVs, numerous epidemiological surveillance efforts examining HBoV prevalence in children have been performed across multiple regions, comprising Thailand, United States, France, Jordan and Brazil.10, 11, 12, 13, 14
Severe acute respiratory infection (SARI) is among the leading causes of morbidity and mortality among children globally.15 HBoV infection in children has been reported associated with respiratory tract infection, a few cases reported that HBoV as the cause of SARI among Children.16, 17, 18 However, prolonged shed periods of HBoV and high co-infection detection resulted in the on-going debate of the HBoV as the agent of SARI19; In addition, the comprehensive research of HBoV genome among children with SARI were limited, especially in China. To better understand the molecular epidemiology and characterization of HBoV genome in children with SARI, we investigated the prevalence of HBoV among 993 inpatient children with SARI in China. Analyses of genome characterization were also performed.
Methods
Study subjects and sample collection
From Sep 2007 to Mar 2014, a total of 993 nasopharyngeal aspirates (NPAs) or induced sputum (IS) were randomly collected from hospitalized children with SARI in Beijing (n = 259), Shanghai (n = 441) and Zhejiang (n = 293) area. The case of SARI was defined according to the World Health Organization case definition for all hospitalized children in whom the onset of illness occurred within seven days of admission. Most of the patients had received clinical diagnosis of respiratory tract infection, including pneumonia, acute bronchitis/bronchiolitis, asthma exacerbation and acute pharyngitis. The most common respiratory symptoms included fever (temperature ≥38 °C), cough, sore throat, shortness of breath, vomit, dyspnea and so on.15 In addition, none of the samples come from the patients in Pediatric intensive care unit (PICU) and most of the children have no co-morbidities, such as heart and liver diseases. All the samples were collected by medical professionals and placed in a tube containing of viral transportation medium and stored at −80 °C. This project was approved by the Research Ethics Committee of Beijing Children's Hospital, Children's Hospital of Fudan University, Wenzhou Medical College, and the Institutional Review Board at the China CDC. Written informed consent was obtained on the participants' behalf from their parents or guardians.
Molecular typing of HBoV and co-infection detection
Viral nucleic acids were extracted from virus transport medium by the QIAamp MinElute Virus Spin Kit (QIAGEN, Germany), according to the instructions provided by the manufacturer. As previously described,8 partial VP1/VP2 gene fragment was amplified by nested PCR to screen and type HBoV infection. The first round-PCR primers were F1 (5′-CGCCGTGGCTCCTGCTCT-3′) and R1 (5′-TGTTCGCCATCACAAAAGATGTG-3′) with 609 bp product, the second-round PCR primers were F2 (5′-GGCTCCTGCTCTAGGAAATAAAGAG-3′) and R2 (5′-CCTGCTGTTAGGTCGTTGTTGTATGT-3′) with 576 bp PCR product. Positive products were cloned into pMDT-18 vector and sequenced by ABI 3730xl automated sequencer. HBoV co-infection with other respiratory viruses, including HRSV, HRV/EV, HAdV, HMPV, HPIV1–4, influenza A/B virus and HCoVs (-OC43, -229E, -NL63, -HKU1), was also screened as described previously.20, 21
Sequencing and phylogenetic analysis of HBoV1 genome
Four samples of HBoV1 infection only in this study were used for complete genomes sequencing by next-generation sequencing. Samples were pretreated as previously,22 and the amplified DNA was used as a template for Illumina Hiseq 2500 sequencing, paired-end reads (2 × 125 bp reads) were assembled into contigs by CLC genomic workbench.
To analyze genetic variation of HBoV detected, nucleotide sequences were compared to strains available from GenBank. Nucleotide sequence alignment was conducted through MAFFT version 5.23 Phylogenetic and molecular evolutionary analyses were constructed by Neighbor-Joining Method using MEGA 5.024 with the bootstrap value of 1000.
Evolutionary rate and diversity analysis
Evolutionary rates were calculated using the Bayesian Markov Chain Monte Carlo approach employed by BEAST version 1.7.25 Fragments of the NS1, NP1 and VP1 genes of HBoV were aligned separately and used to calculate the rate of nucleotide substitutions/site/year, under an uncorrelated lognormal-relaxed clock model of rate variation among lineages. The best-fit models were selected by jModelTest, with the following models: HKY + I + G for NP1, GTR + G for NS1 and GTR + I + G for VP1. The evolutionary rates of individual genes were then compared to identify the differences in conservation throughout the genome. DNA SP5 software26 was used to analyze the nucleic acid sequence diversity of the HBoV genomes.
Molecular modeling
The model of HBoV1 VP1 (NC_007455) was based on the crystal structure of a capsid viral protein of Adeno-associated virus (PDB code: 4IOV)27 which shared a close genetic relationship with HBoVs. Molecular modeling was performed manually using the COOT software under the guidance of Fo-Fc and 2Fo-Fc electron density maps. Consequently, we refined the initial rigid body, and performed a series of restrained TLS refinements using Refmac5. Additional rounds of refinements were performed using the phenix refine program implemented in the Phenix package with isotropic ADP refinement and bulk solvent modeling. We assessed the stereochemical quality of the final models with the program PROCHECK. Eventually, the molecular model was generated using PyMOL (http://www.pymol.org/).
Accession numbers and statistical analysis
The nucleotide sequences generated in this study have been deposited in GenBank under the accession numbers KM378039∼KM378094, KM877548∼KM877592, KM8877594∼KM877614 and KM464728∼KM464730. Data were analyzed by the chi-squared test using SAS software version 9.2. P < 0.05 was considered statistically significant.
Results
Epidemiological data
All the respiratory samples were collected from hospitalized children with SARI in Beijing, Shanghai and Zhejiang from 2007 to 2014. The sample information was provided in Table 1 . The median age of the population in each of the three regions studied is 7 months, 7.5 months and 1 year, respectively. No significant differences were observed regarding gender and age distribution (P > 0.05) and the characteristics of three regions' population was matched well. In all, 200 (20.1%) of 993 SARI patient were positive for HBoV and the infection rates of HBoV among inpatient in Beijing, Zhejiang and Shanghai area were 21.6% (56/259), 22.5% (66/293) and 17.7% (78/441), respectively. No significant difference was observed regarding infection rate (P > 0.05).
Table 1.
Demographic and prevalence data in this study.
| Total | Beijing | Shanghai | Zhejiang | P value | |
|---|---|---|---|---|---|
| Samples | |||||
| Number | 993 | 259 | 441 | 293 | |
| Type | NPAs or IS | NPAs | NPAs | IS | |
| Population | |||||
| Male/Female | 631/362 | 155/104 | 281/160 | 195/98 | 0.2618 |
| Age range | 0.3M–14Y | 1M∼6Y2M | 0.3M–14Y | 0.5M–11Y | |
| Median age | 7.5M | 7M | 1Y | 7.5M | 0.5233 |
| HBoV detection | |||||
| Positive No. | 200 | 56 | 78 | 66 | |
| Rate (%) | 20.1% | 21.6% | 17.7% | 22.5% | 0.2187 |
| HBoV1 No. | 197 | 54 | 78 | 65 | |
| HBoV2 No. | 1 | 1 | 0 | 0 | |
| HBoV3 No. | 2 | 1 | 0 | 1 | |
| HBoV4 No. | 0 | 0 | 0 | 0 | |
| HBoV detected only | 50 | 3 | 23 | 24 | |
| Co-infection* No. | 150 | 53 | 55 | 42 | |
| Co-infection rate | 75% | 94.6% | 70.5% | 63.6% | 0.0002 |
M: month, Y: year, No.: number, NPAs: Nasopharyngeal aspirates, IS: induced sputum, co-infection* detection with other viruses including HRSV, HRV/EV, HAdV, HMPV, HPIV, influenza A/B virus and HCoVs (-OC43, -229E, -NL63, -HKU1).
Prevalence and co-infection analysis of HBoV
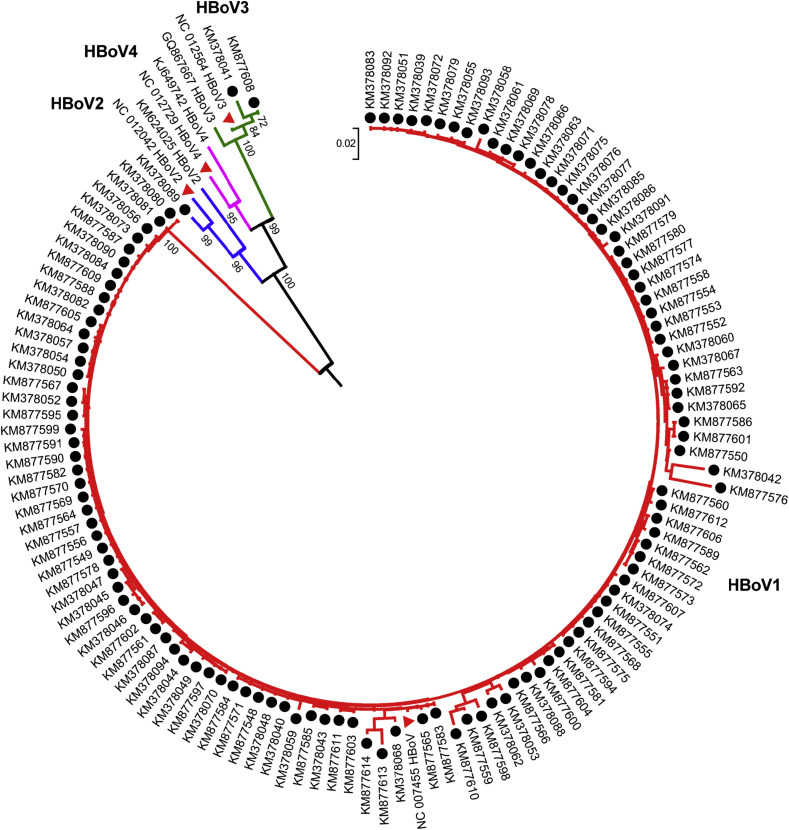
Phylogenetic analysis based on partial VP1/VP2 sequences indicated that HBoV1 was the most prevalent in China. The strains isolated from Beijing were identified as HBoV1 (n = 54), HBoV2 (n = 1) and HBoV3 (n = 1). Similarly, of the 66 HBoV-positive samples collected from Zhejiang Province, 65 were HBoV1, one was HBoV3 (Fig. 1 ). In addition, all the HBoV-positive samples from Shanghai city belonged to HBoV1. Combined with the three cohorts, the most frequently detected strains in China was HBoV1 (98.5%, [197/200]), followed by HBoV3 (1%, 2/200) HBoV2 (0.5%, 1/200) (Fig. 1). Fifty (5.04%) of 993 SARI patient were detected as HBoV positive only, about 25% among all 200 HBoV positive patients with SARI. Co-infection with other respiratory viruses were found in 150 (75%) of 200 HBoV positive patients with SARI, including 53 patients (53/56, 94.6%) from Beijing, 55 (55/78, 70.5%) from Shanghai and 42 (42/66, 63.6%) from Zhejiang Province, respectively (Table 1). The co-infection rate of HBoV among SARI patients from Beijing was significantly higher than that from Shanghai and Zhejiang area. The dominant co-infection with HBoV were human Rhinovirus (HRV), human adenovirus (HAdV), human respiratory syncytial virus (HRSV), human parainfluenza virus (HPIV), influenza virus A and human coronavirus OC43 (Table S1).
Figure 1.
Phylogenetic analysis of HBoV detected among Children with SARI. The phylogenetic tree was constructed based on partial VP1/VP2 gene sequence. All the sequences presented here were indicated in black solid ball and Reference strains of HBoV were indicated in red solid triangle. Sequences were aligned by Neighbor-Joining method with 1000 bootstrap replicates using MEGA 5.0.
Identification of four complete genomes of HBoV1 and novel signatures for distinguishing HBoV1 from HBoV2–4
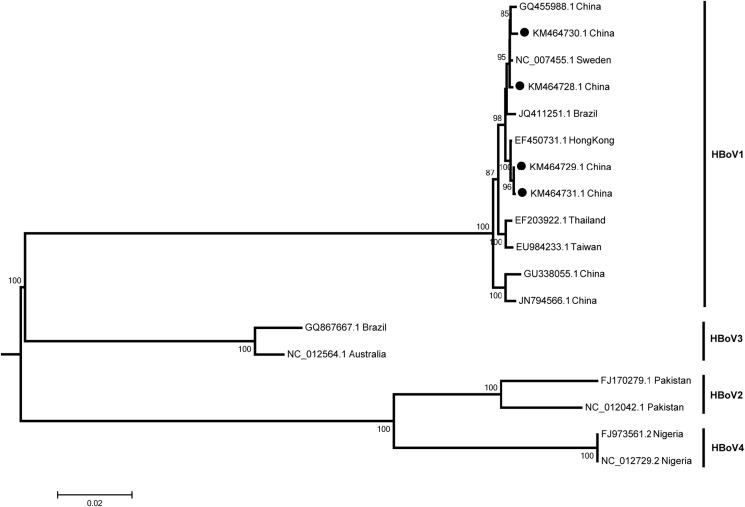
Through high-throughput sequencing, four complete genomes of HBoV were obtained (KM464728, KM464729, KM464730 and KM464731), which all belong to HBoV1 (Fig. 2 ). All four genomes of HBoV1 sequenced in this study were shown to be closely related to each other at the nucleotide (nt) level (99.47%–99.74% nt identity for each genome) and to be closely related (99.60%–99.87%) to prototypes HBoV1 (NC_007455.1).
Figure 2.
Phylogenetic analysis of HBoV based on the complete genome detected in China. Four HBoV1 (indicated in black solid ball) presented in this study and 14 other HBoV representative strains were analyzed using the neighbor joining method with 1000 bootstrap replicates by MEGA 5.0 program, number as the nodes represent bootstrap support.
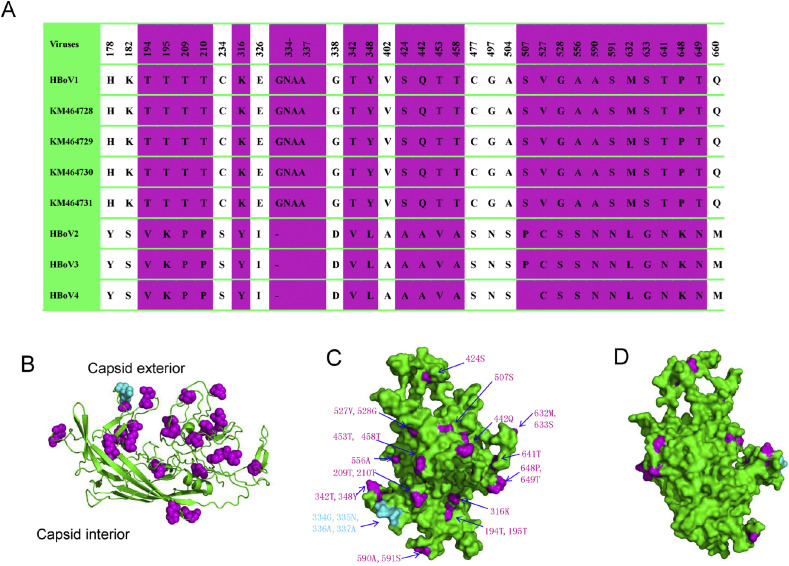
A comparative analysis of the four HBoV1 genomes presented here and all available other HBoV genomes identified a total of three deletions within NP1 and VP1 together, which could be used to distinguishing HBoV1 from HBoV2–4. These differences include a 3-bp deletion in VP1 of HBoV1 at nucleotides 419–421, a 12-bp deletion in VP1 of HBoV2–4 at nucleotides 993–996 and 1004–1011, and a 3-bp deletion in NP1 of HBoV2–4 at nucleotides 88–90 (Fig. 3 ). To further investigate the potential implications of VP1 variations in HBoV1, relative to HBoV2–4, we generated a 3D model of HBoV1 VP1 based on the crystal structure of a capsid viral protein of Adeno-associated virus (PDB code: 4IOV) (Fig. 4 ). According to the previous report,27 the structure of VP1 contains two parts: the capsid exterior and capsid interior. The unique substitutions and insertions (residues 334–337) within HBoV1 compared to HBoV2–4 mapped primarily to the capsid exterior. Interestingly, among the 32 unique substitutions, 19 were related with serine or threonine (substitutions of S/T with other amino acids), which has a dramatic influence on the hydrophobicity of the protein. These consensus nucleotide deletions or substitutions can be used to distinguish HBoV1 from HBoV2–4 and may be associated with pathogenicity of HBoV.
Figure 3.
Molecular characteristics of HBoV. Nucleotide deletions were detected in NP1 and VP1, including deletions at nucleotides 419–421, 993–996, and 1004–1011 in VP1, along with 88–90 in NP1 gene.
Figure 4.
Structural modeling of HBoV1 VP1 and its unique substitutions compared to HBoV2–4. (A) Amino acid substitutions unique to HBoV1 that are otherwise conserved in HBoV2–4; amino acid substitutions with similar properties (such as S with T, D with E, etc.) were not included. Residues presented on the exterior surface of the capsid are shown in purple. HBoV1∼HBoV4 represents their consensus amino acid sequences, KM464728-KM464731 indicated the four strains presented in this study. (B) Structural overview of HBoV1 VP1. Unique substitutions are shown in purple spheres; additional residues are depicted as cyan balls. (C) The capsid exterior and (D) interior of VP1. The majority of VP1 substitutions unique to HBoV1 are shown to localize to the capsid exterior.
Evolutionary and genetic diversity of HBoV1
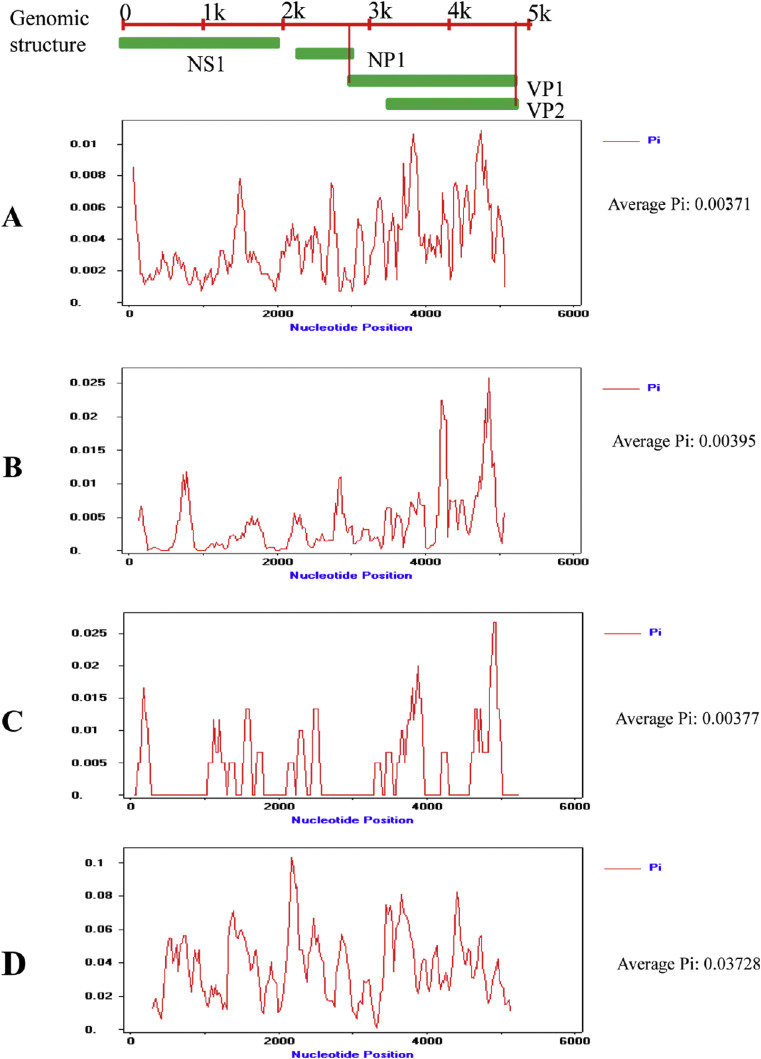
To determine the differences in gene-specific mutation rates, we calculated and compared the mutation rates of NS1, NP1 and VP1 of HBoV1 prevalent in China. The evolutionary rates of NS1, NP1 and VP1 of HBoV1 were 2.840 × 10−5, 3.917 × 10−4 and 4.204 × 10−4 substitutions/site/year with the ESS values >200, respectively (Table 2 ). Comparative analysis showed that the evolutionary rate of VP1 is much faster than that of NS1 for HBoV1, and HBoV evolved relatively slowly in China. No genetic recombination event was found among HBoV1 from China. In addition, analysis of DNA polymorphisms within HBoV1 and HBoV2 was performed using the DNA SP5 software. The overall mean of diversities (Pi) were 0.00371(HBoV1 in China), 0.00395(HBoV1 out of China), 0.00377 (HBoV1 in this study) and 0.03728(HBoV2), respectively. There was no significant difference among the nucleotide diversity of HBoV1 from different regions and the nucleotide sequences diversity of VP1 gene was greater than others. In addition, the high degree of nucleotide sequence diversity in HBoV2 is greater than that of HBoV1 as expected (Fig. 5 ).
Table 2.
Evolutionary rate of main genes of HBoV1 circulating in China.
| Gene | NS1 | NP1 | VP1 |
|---|---|---|---|
| Mean rate/Site year | 2.840 × 10−5 | 3.917 × 10−4 | 4.204 × 10−4 |
| 95% HPD: Lower Upper |
4.249 × 10−6 8.417 × 10−5 |
1.135 × 10−4 6.929 × 10−4 |
1.879 × 10−4 6.489 × 10−4 |
Figure 5.
Nucleic acid diversity analysis of HBoV1. The x-axis represents the genomic position of HBoV without 3′ and 5′ termini; the y-axis shows Pi. Pi represents the average number of nucleotide differences per site between two nucleotide sequences. (A) All available HBoV1 strains circulating in China, (B) all available HBoV1 strains circulating in other countries (C) the four HBoV1 strains detected in this study, (D) all available HBoV2 strains circulating worldwide. HBoV2 group was used as a control.
Discussion
HBoV is a novel parvovirus associated with respiratory tract infections in infants or children, including four genotypes (HBoV1-HBoV4).8, 28 Among the four recognized HBoV genotypes, HBoV1 have more often been associated with SARI. The prevalence of different HBoV genotype varies among the same region. Only limited data is available on the prevalence of HBoV infection in SARI children. Here, we performed a comprehensive molecular epidemiological study of HBoV in China between 2007 and 2014 and made genomic characterization analyses.
Our study indicated that HBoV was frequently detected in SARI children from 2007 to 2014 in China; the frequency of HBoV1 was much higher than that of other HBoV subtypes. However, the infection rates of HBoV among hospitalized children with SARI in three different regions were almost consistent (21.6%, 22.5% and 17.7%). Previous reports demonstrate the prevalence of HBoV varies considerably between 1.5% and 19% in children with acute respiratory tract infections (ARTIs).29, 30 However, the prevalence of HBoV among children with SARI was more common (200/993, 20.1%) in this study. Furthermore, fifty (5.04%) of 993 SARI patient were detected as HBoV positive only, about 25% among all 200 HBoV positive patients with SARI. These data indicated that the HBoV infection may play an important role among children with SARI, although co-infection of HBoV with other viruses was much higher than that of HBoV infection alone. In addition other factors may also affect the infection rate of HBoV, including assay sensitivity, specificity, sampling time and sampling locations, more respiratory samples were needed to provide more detailed information about the prevalence of HBoV among the children with SARI.
We calculated the evolutionary rates of individual HBoV1 genes prevalent in China, identifying strong conservation in NS1 (2.840 × 10−5 substitutions/site/year), compared with VP1, which exhibited substantially higher mutation rates (4.204 × 10−4 substitutions/site/year). Furthermore, the evolutionary rate of HBoV1 VP1 is more than 10 time than that of NS1, indicating the strongest degree of conservation in non-structural protein NS1; while VP1, which encodes the capsid protein, mutated significantly more rapidly than other genes. However, we only calculated the evolutionary rates of HBoV1 genes, rather than HBoV2–4 genes, due to genomic recombinant31, 32 and limited number of genome sequence. More extensive studies will be needed to address the evolution of HBoVs.
Comparative analysis of the HBoV1 genome sequences presented here revealed consistent and reproducible nucleotide deletions and substitution. Three sets of deletions within NP1 and VP1 were shown to be diagnostic for HBoV1, clearly differentiating these from those of HBoV2–4. This difference is consistent with phenotypic analyses, which show HBoV1 to be the most commonly occurring in respiratory tract samples, while HBoV2–4 are detected mainly in gastrointestinal samples and are presumably enteric. Furthermore, the unique substitutions within HBoV1 relatively to HBoV2–4 mapped primarily to the capsid exterior. These otherwise uncommon substitutions and insertions within HBoV1 VP1 are likely to play a major role in the antigenicity of HBoV species and may account for differences in tissue tropism between HBoV1 and HBoV2–4. Further studies are necessary to validate these findings and to confirm the effects of these nucleotide differences on tissue tropism and pathogenicity.
In summary, we first reported the circulating HBoV genotype and their genome character among children with SARI in China, providing more evidence for a causal role of HBoV1 in SARI. Novel molecular signatures were identified for distinguishing HBoV1 from other HBoV subtypes. This study complements and significantly expands upon the current knowledge of HBoV infection and SARI among children in China.
Funding
This work was supported by grants from the State Megaproject for Infectious Disease Research of China (2011ZX10004001, 2013ZX10004601 and 2013ZX10004605).
Conflicts of interest
Nil.
Author contributions
Conceived and designed the experiments: Wj T, Yq W and J S. Performed the experiments: Yq W, Ym L, Yj Z. Analyzed the data: Yq W, Ym L, J L, Zd X and J S. Contributed to the writing of the manuscript: Wj T, Yq W, J S.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jinf.2016.05.014.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Allander T., Jartti T., Gupta S., Niesters H.G., Lehtinen P., Vuorinen T. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44(7):904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur J.L., Higgins G.D., Davidson G.P., Givney R.C., Ratcliff R.M. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog. 2009;5(4):e1000391. doi: 10.1371/journal.ppat.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vicente D., Cilla G., Montes M., Pérez-Yarza E.G., Pérez-Trallero E. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis. 2007;13(4):636. doi: 10.3201/eid1304.061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotmore S.F., Agbandje-McKenna M., Chiorini J.A., Mukha D.V., Pintel D.J., Qiu J. The family Parvoviridae. Arch Virol. 2014;159(5):1239–1247. doi: 10.1007/s00705-013-1914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schildgen O., Qiu J., Söderlund-Venermo M. Genomic features of the human bocaviruses. Future Virol. 2012;7(1):31–39. doi: 10.2217/fvl.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurda B.L., Parent K.N., Bladek H., Sinkovits R.S., DiMattia M.A., Rence C. Human bocavirus capsid structure: insights into the structural repertoire of the parvoviridae. J Virol. 2010;84(12):5880–5889. doi: 10.1128/JVI.02719-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102(36):12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapoor A., Simmonds P., Slikas E., Li L., Bodhidatta L., Sethabutr O. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J Infect Dis. 2010;201(11):1633–1643. doi: 10.1086/652416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapoor A., Slikas E., Simmonds P., Chieochansin T., Naeem A., Shaukat S. A newly identified bocavirus species in human stool. J Infect Dis. 2009;199(2):196–200. doi: 10.1086/595831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khamrin P., Malasao R., Chaimongkol N., Ukarapol N., Kongsricharoern T., Okitsu S. Circulating of human bocavirus 1, 2, 3, and 4 in pediatric patients with acute gastroenteritis in Thailand. Infect Genet Evol. 2012;12(3):565–569. doi: 10.1016/j.meegid.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Kesebir D., Vazquez M., Weibel C., Shapiro E.D., Ferguson D., Landry M.L. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis. 2006;194(9):1276–1282. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foulongne V., Olejnik Y., Perez V., Elaerts S., Rodière M., Segondy M. Human bocavirus in French children. Emerg Infect Dis. 2006;12(8):1251. doi: 10.3201/eid1208.060213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan N.M., Dove W., Abu-Zeid A.F., Shamoon H.E., Abd-Eldayem S.A., Hart C.A. Human bocavirus infection among children, Jordan. Emerg Infect Dis. 2006;12(9):1418. doi: 10.3201/eid1209.060417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos N., Peret T.C., Humphrey C.D., Albuquerque M.C.M., Silva R.C., Benati F.J. Human bocavirus species 2 and 3 in Brazil. J Clin Virol. 2010;48(2):127–130. doi: 10.1016/j.jcv.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Nair H., Brooks W.A., Katz M., Roca A., Berkley J.A., Madhi S.A. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378(9807):1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 16.Chieochansin T., Samransamruajkit R., Chutinimitkul S., Payungporn S., Hiranras T., Theamboonlers A. Human bocavirus (HBoV) in Thailand: clinical manifestations in a hospitalized pediatric patient and molecular virus characterization. J Infect. 2008;56(2):137–142. doi: 10.1016/j.jinf.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moesker F.M., van Kampen J.J., van der Eijk A.A., van Rossum A.M., de Hoog M., Schutten M. Human bocavirus infection as a cause of severe acute respiratory tract infection in children. Clin Microbiol Infect. 2015;21(10) doi: 10.1016/j.cmi.2015.06.014. 964. e1–964. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pogka V., Moutousi A., Kossyvakis A., Kalliaropoulos A., Sgouras D.N., Giannaki M. Genetic variability of human metapneumo-and bocaviruses in children with respiratory tract infections. Influenza Other Resp. 2014;8(1):107–115. doi: 10.1111/irv.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franz A., Adams O., Willems R., Bonzel L., Neuhausen N., Schweizer-Krantz S. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol. 2010;48(4):239–245. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C., Zhu N., Xie Z., Lu R., He B., Liu C. Viral etiology and clinical profiles of children with severe acute respiratory infections in China. PLoS One. 2013;8(8):e72606. doi: 10.1371/journal.pone.0072606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong R., Shen L., Yin W., Zhou W., Lu J., Zheng M. Prevalence of human parvovirus B19, bocavirus, and PARV4 in blood samples from the general population of China and lack of a correlation between parvovirus and hepatitis B Co-Infection. PLos One. 2013;8(5) doi: 10.1371/journal.pone.0064391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He B., Zhang Y., Xu L., Yang W., Yang F., Feng Y. Identification of diverse alphacoronaviruses and genomic characterization of a novel severe acute respiratory syndrome-like coronavirus from bats in china. J Virol. 2014;88(12):7070–7082. doi: 10.1128/JVI.00631-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 25.Drummond A.J., Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7(1):214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Librado P., Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 27.Mikals K., Nam H.-J., Van Vliet K., Vandenberghe L.H., Mays L.E., McKenna R. The structure of AAVrh32. 33, a novel gene delivery vector. J Struct Biol. 2014;186(2):308–317. doi: 10.1016/j.jsb.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin E.T., Kuypers J., McRoberts J.P., Englund J.A., Zerr D.M. Human bocavirus-1 primary infection and shedding in infants. J Infect Dis. 2015:jiv044. doi: 10.1093/infdis/jiv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L., He X., Zhang D., Feng F., Wang Z., Guan L. Surveillance and genome analysis of human bocavirus in patients with respiratory infection in Guangzhou, China. PLoS One. 2012;7(9):e44876. doi: 10.1371/journal.pone.0044876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold J.C., Singh K.K., Spector S.A., Sawyer M.H. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin Infect Dis. 2006;43(3):283–288. doi: 10.1086/505399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu X., Wang X., Ni B., Shen H., Wang H., Zhang X. Recombination analysis based on the complete genome of bocavirus. Virol J. 2011;8:182. doi: 10.1186/1743-422X-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao M., Zhu R., Qian Y., Deng J., Wang F., Sun Y. Prevalence analysis of different human bocavirus genotypes in pediatric patients revealed intra-genotype recombination. Infect Genet Evol. 2014;27:382–388. doi: 10.1016/j.meegid.2014.08.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.