Highlights
-
•
TLR agonists (LPS, Pam3CSK4 and CpG ODN), when administered in ovo in chicken embryo interfered with the replication of IBV.
-
•
Time & optimum dose of TLR agonists can interfere with the replication of IBV.
-
•
All the TLR agonists up-regulated the expression of different pro-inflammatory cytokines.
-
•
The antiviral genes in CAM of ECEs that may play an important role in inhibition of IBV replication.
-
•
LPS appeared to be the better candidate for adjuvant against IBV.
Keywords: Infectious bronchitis virus, TLR ligands, Embryonated chicken eggs, Cytokine expression, IBV replication
Abstract
Avian infectious bronchitis (IB) is an acute, highly infectious and contagious viral disease of chickens caused by avian infectious bronchitis virus (IBV) belonging to the genus Coronavirus and family Coronaviridae. It can affect all age groups of birds. The toll-like receptors (TLRs) are a major class of innate immune pattern recognition receptors that have a key role in immune response and defense against various infections.The TLRs are essential for initiation of innate immune responses and in the development of adaptive immune responses. An in ovo model was employed to study the antiviral activity of TLR ligands (Pam3CSK4, LPS and CpG ODN) on replication of IBV. It was hypothesized that optimum dose and specific timing of TLR ligands may reduce viral load of IBV in specific pathogen free (SPF) embryonated chicken eggs (ECEs). Further, the mechanism involved in the TLR-mediated antiviral response in chorioallantoic membrane (CAM) of ECEs was investigated. The ECEs of 9–11 days old were treated with different doses (high, intermediate and low) of TLR-2 (Pam3CSK4), TLR-4 (LPS) and TLR-21 (CpG ODN) ligands. In addition, to know the timing of TLR ligand treatment, six time intervals were analyzed viz. 36, 24 and 12 h prior to infection, time of infection (co-administration of TLR ligands and avian IBV) and 12 and 24 h post-IBV infection. For studying the relative expression of immuno-stimulatory genes (IFN-α, IFN-β, IFN-γ, IL-1β, iNOS and OAS) in CAM, TLR ligands were administered through intra-allantoicroute and CAM were collected at 4, 8 and 16 h post treatment. The results demonstrated that intermediate dose of all the three TLR ligands significantly reduced virus titers and used in the present study. However, the LPS reduced virus titer pre- and post-IBV infection but Pam3CSK4 and CpG ODN reduced only pre-IBV infection. Further analysis showed that TLR ligands induced IFN-γ, IL-1β and IFN stimulated genes viz. iNOS and OAS genes in CAM. The present study pointed towards the novel opportunities for rational design of LPS as immuno-stimulatory agent in chickens with reference to IBV. It may be speculated that in ovo administration of these TLR ligands may enhance resistance against viral infection in neonatal chicken and may contribute towards the development of more effective and safer vaccines including in ovo vaccines.
1. Introduction
Avian infectious bronchitis (IB) is a highly contagious disease of chickens caused by infectious bronchitis virus (IBV) belonging to genus Coronavirus, sub-genus Gammacoronavirus. The IBV is an enveloped virus having positive-sense and single stranded RNA of 27.6 Kbp (Boursnell et al., 1989; Cavanagh, 2007). IB is a major problem of poultry industry causing huge economic losses to poultry industry through decrease in quality of eggs & production and increase in the susceptibility for secondary bacterial infections in chickens. Emergence of new variant serotypes and genotypes of IBV in the field is frequent because of mutations and/or recombination of the hypervariable region of the S1 gene which do not cross-protect and therefore hinder complete control of the disease by the routinely used vaccination programs (Cavanagh et al., 1992; Chhabra et al., 2015). A large number of IBV serotypes exist worldwide and several serotypes can co-circulate in a region (Capua et al., 1999).
In IBV infections, melanoma differentiation-associated protein 5 (MDA5) is a primary sensor in chicken cells that leads to production of interferon (Kint et al., 2015; Chhabra et al., 2016). The innate immune response activates when IBV binds to the receptors on the mucosal linings of the tracheal tissue (Rahman et al., 2009) and this immune response may be due to pathways in which TLRs are activated (Guo et al., 2008; Wang et al., 2006). TLRs are evolutionarily conserved pattern recognition receptors (PRRs) present across various species including human, mice, fish and chicken and recognize pathogen associated molecule patterns (PAMPs) (Keestra et al., 2013). In chicken, TLR1A and B, TLR2A and B, TLR3, TLR4, TLR5, TLR7, TLR15 and TLR21 have been identified. TLR15 is unique to chickens and TLR21 is a functional homologue of mammalian TLR9 which recognizes CpG ODN in chickens (Paul et al., 2013). These TLR mediated responses interlink innate with adaptive immunity (Akira and Takeda, 2004) and play a critical role in inducing appropriate immune responses against pathogens by influencing the polarization of antigen-specific CD4 + T cell responses.
Several TLR ligands have been used as an prophylactic agents against various diseases and also as an adjuvants in different vaccines like CpG Oligodeoxynucleotides (CpG ODN) with avian influenza virus (AIV) subtype H5N1 inactivated oil emulsion vaccine (Wang et al., 2009). The TLR-2 ligand Pam3CSK4 administered as an adjuvant has been shown to enhance antibody titer against human serum albumin (Erhard et al., 2000). Monophosphoryl lipid A (MPLA), a LPS derivative enhanced antigen specific antibody titer by 10- to 20- fold when compared to vaccine alone. Purified MPLA has been approved as an adjuvant in hepatitis B vaccine, Fendrix™ (Thoelen et al., 2001). When polyinosinic :polycytidylic acid (Poly I:C), CpG ODNand lipopolysaccharide (LPS) were given to chickens 24 h prior to infection with AIV, it significantly reduced the viral shedding (Paul et al., 2012). Further results demonstrated that treatment with these ligands enhanced the protective effect of vaccination against influenza virus in vivo (Paul et al., 2014).
Effective control of IBV involves identification of the virus serotype causing the disease followed by vaccination with an appropriate vaccine against that serotype (Cavanagh, 2007). However, there are only a few different serotypes of IBV vaccines available for use, whereas countless different types and variants of the virus capable of causing disease are found throughout the world. For protection against IBV through a successful vaccination program, it is essential to identify the prevalent genotypes in the region and to determine the role of TLR ligands in enhancing the protective potential of IBV vaccine. The in ovo vaccination has been recognized as an attractive choice for vaccination in poultry. However, there is some problem with in ovo vaccination like low immunogenicity in case of killed vaccine and embryo lethality due to live vaccines (Rautenschlein et al., 1999; Sharma et al., 2002). These challenges may be resolved by use of TLR ligands. Further, the TLR ligands may also act as an immune enhancer with killed vaccine or reduce embryo mortality by enhancement of innate immune responses in live vaccines (Rautenschlein et al., 2002).
The objective of the present study was to examine the effect of different TLR agonists, administered in ovo, on IBV replication at different time points. Further to investigate the possible mechanism associated with antiviral effect, the activation of innate immune response in CAM of ECEs by different TLR agonist was also assessed.
2. Materials and methods
2.1. Infectious bronchitis virus
The IBV used in the present study was isolated and propagated in 9–11 days old SPF embryonated chicken eggs by inoculation through allantoic cavity route. One hundred and five (105) tissue samples comprising trachea, lung, kidneys and caecal tonsilsfrom IBV suspected birds (dead) were processed for RT-PCR. Out of four PCR positive samples, one sample propagated well in ECEs (IBV3Hisar2018) and after third passage demonstrated characteristic IBV lesions viz. curled and stunted/dwarfed embryos (Fig. 1 ). The IBV isolate IBV3Hisar2018 had 99–100 % sequence similarity with S1 (partial) gene of IBV vaccine strain 4/91 (KF377577.1) (China) and IBV isolate CK/CH/GD/XX16-2 S1 gene, partial cds (MF447753.1) (China). The bulk production of IBV was done in 9–11 days old SPF embryonated chicken eggs through intra-allantoic route and virus titration done subsequently. End point titers were expressed as 50 % embryo infective doses (EID50) per ml (Reed and Muench, 1938). Allantoic fluid was checked for contamination of NDV, ILTV, mycoplasma, bacterial or fungal contamination. The presence of IBV in allantoic fluid was re-confirmed by S1 (partial) gene based PCR (Cavanagh et al., 2001) and used for further experiments.
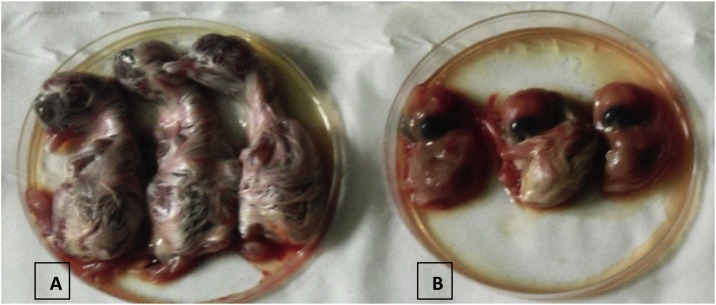
Fig. 1.
Embryos showing curling and dwarfism typical of IBV. A: Control; B: IBV inoculated embryo.
2.2. TLR ligands
The TLR2 ligand (Pam3CSK4, Catalogue no.Tlrl-pms) and TLR21 ligand (CpG ODN 1826, Catalogue no. Tlrl-1826) were procured from InvivoGen, California (USA) and TLR4 ligand (lipopolysaccharide from E. coli O26:B6, Catalogue no. L8274) from Sigma-Aldrich, USA.The ligands were dissolved in sterile phosphate-buffered saline (PBS, pH 7.4) to its working concentrations.
2.3. Optimization of dose and treatment of embryonated chicken eggs with TLR ligands
The SPF embryonated chicken eggs were treated with different doses of TLR ligand as described in Table 1 . The 9–11 day old embryonated chicken eggs were candled, their surface disinfected with 70 % ethanol, and TLR agonists injected through allantoic route. After 24 h of incubation, the eggs were infected with 100 μl of IBV (104.8 EID50) and kept in incubator. The eggs were candled at 24 h intervals for checking the viability. The allantoic fluid was harvested after 72 h of incubation, and virus titer quantified by real time PCR (qPCR).
Table 1.
Different doses of TLR ligands injected into embryonated chicken eggs.
| S.No. | Ligand | TLR | High dose, μg/egg |
Intermediate dose, μg/egg | Low dose, μg/egg |
|---|---|---|---|---|---|
| 1 | LPS | TLR4 | 20 | 2 | 0.2 |
| 2 | Pam3CSK4 | TLR2 | 200 | 10 | 1.0 |
| 3 | CpG ODN | TLR9 | 20 | 4 | 0.5 |
In order to determine the effect of in ovo administration of TLR ligand on IBV replication in embryonated chicken eggs, six ECEs (9–11 days old) in each group were administered with an optimal dose: LPS @ 2 μg/egg, Pam3CSK4 @ 10 μg/egg and CpG ODN @ 4 μg/egg of each TLR ligand through allantoic route at different times: 36, 24 and 12 h prior to infection, at the time of infection (co-administration of TLR ligands and avian IBV) as well as 12 and 24 h post-infection. The control group was treated with DPBS. After 24 h of incubation, ECEs were infected with 100 μl of IBV (104.8 EID50) and kept in incubator and candling done at 24 h intervals. The allantoic fluid was harvested 72 h post-infection. The virus titer was quantified in allantoic fluid by real time PCR (qPCR). The progression of IBV N gene RNA expression in cellular RNA extracted from allantoic fluid was used as an indicator of virus infection progression
2.3.1. RNA isolation from allantoic fluid and cDNA synthesis
The total RNA isolation from allantoic fluid was done using Trizol reagent (Amresco, USA) and the complementary DNA (cDNA) was synthesized from total RNA using Revertaid™First Strand cDNA Synthesis Kit (Thermo Scientific, USA), following manufacturer’s instructions. One μg of RNA was used for cDNA synthesis. One μg of total RNA and one μl of random hexamer primer were made upto 12 μl with nuclease free water, incubated at 65 °C for 5 min and following reaction mixture added (Table 2 )
Table 2.
Reaction mixture for RT-PCR.
| Sr. No. | Reaction Components | Amount |
|---|---|---|
| 1. | Reaction buffer 5 X | 4.0 μl |
| 2. | Ribolock™ RNase inhibitor | 1.0 μl |
| 3. | 10 mM dNTP mix | 2.0 μl |
| 4. | MoMuLV (Moloney murine leukemia virus) Reverse Transcriptase | 1.0 μl |
| Total | 8.0 μl |
The reactants were mixed gently by spinning, incubated at 42 °C for 60 min for cDNA synthesis and the reaction terminated by heating at 70 °C for 5 min. The cDNA product was stored at −20 °C, until further used.
2.3.2. Real-time polymerase chain reaction for amplification of N gene of IBV
The differential mRNA expression was assessed by real-time quantitative reverse transcription (RT-qPCR) by using a PikoReal Real-Time PCR (Thermo Scientific) and QuantiTect SYBR Green PCR Kit (Qiagen, Germany). Real-time PCR (qPCR) was performed in a reaction volume of 20 μl (Table 3 ). All non-treatment controls and treated samples were carried out in triplicate on the same plate.The RT-qPCR cycling conditions were set as initial incubation at 95 °C for 15 min followed by 95 °C for 15 s, 60 °C for 30 s, and 76 °C for 30 s for 45 cycles (Table 4 ). The final step was to obtain a melt curve for the PCR products to determine the specificity of amplification. The relative expression of viral N gene was calculated relative to the expression of the β-actin gene and expressed as n-fold increase or decrease relative to the control samples (Dar et al., 2009). The cycle at which the sample amplicon reporter dye concentration crossed the pre-set threshold was recorded as the cycle threshold (Ct) value. The data of quantitative real time PCR were analyzed by 2−ΔΔCt method (Pfaffl, 2001) to derive the relative fold change in mRNA expression of IBV N gene.The primers used in RT-qPCR are shown in Table 5 .
Table 3.
Reaction mixture for real-time PCR for amplification of N gene of IBV.
| Sr. No. | Reaction components | Volume |
|---|---|---|
| 1 | Diluted cDNA (1:10 in NFW) | 2.0 μl |
| 2 | SYBR® Green master mix | 10.0μl |
| 3 | Forward primers (N gene) | 0.5 μl |
| 4 | Reverse primer (N gene) | 0.5 μl |
| 5 | RNase-free water | 7.0 μl |
| Total Volume | 20.0μl |
Table 4.
Thermal profile for amplification of N gene of IBV by real-time PCR.
| Segment /cycles | Step | Time | Temperature |
|---|---|---|---|
| I/1 | Initial Denaturation | 15:00 min | 95 °C |
| II/45 | Denaturation | 00:15 min | 95 °C |
| Annealing | 00:30 min | 60 °C | |
| Extension | 00:30 min | 76 °C | |
| III/3 | Melting curve | Hold time:00:01 min | 60 °C-95 °C |
Table 5.
Primers used for quantitative real time PCR (qPCR) analysis.
| Gene | Primer Sequence (5′-3′) | References |
|---|---|---|
| IFN-α | F: ATC CTG CTG CTC ACG CTC CTT CT R: GGT GTT GCT GGT GTC CAG GAT G |
Paul et al. (2011) |
| IFN-β | F: ACA CTG ACA AGT CAA AGC CGC ACA R: AGT CGT TCA TCG GGA GCT TGG C |
Villanueva et al. (2011) |
| IFN-γ | F: GCC TCC AGC TCC TTC AGA ATA CG R: CTG GAT CTG GTT GAG GAG GCT GT |
Brisbin et al. (2010) |
| OAS | F: AGA ACT GCA GAA GAA CTT TGT C R: GCT TCA ACA TCT CCT TGT ACC |
Villanueva et al. (2011) |
| IL 1-β | F:GTG AGG CTC AAC ATT GCG CTG TA R: TGT CCA GGC GGT AGA AGA TGA AG |
Paul et al. (2011) |
| iNOS | F: GGC AGC AGC GTC TCT ATG ACT TG R: GAC TTT AGG CTG CCC AGG TTG |
Abdul-Careem et al., 2007 |
| β-Actin | F: CAA CAC AGT GCT GTC TGG TGG TA R: ATC GTA CTC CTG CTT GCT GAT CC |
Paul et al. (2011) |
| N gene | F: GAAGAAAACCAGTCCCAGATGCTTGG R: GTTGGAATAGTGCGCTTGCAATACCG |
Dar et al.(2009) |
2.4. Gene expression in the CAM stimulated with TLR ligands
To understand the TLRs ligand activity, the possible mechanism of antiviral response in CAM was assessed. Six ECEs (9–11 days old) in each group were administered with an optimal dose (as determined earlier; LPS @2 μg/egg, Pam3CSK4 @10 μg/egg and CpG ODN@ 4 μg/egg) of each TLR ligand through allantoic route. The control group was treated with DPBS. At 4, 8 and 16 h post-treatment, the surface of eggs containing viable embryo (determined by candling) was disinfected with 70 % ethanol. The adhering CAM was collected for RNA extraction. The relative expression of various genes (IFN-α, IFN-β, IFN-γ, IL-1β, OAS and iNOS) were assessed relative to β-actin gene using quantitative real-time PCR (Barjesteh et al., 2013).
2.4.1. RNA extraction from CAM and cDNA synthesis
The total RNA isolation from CAM was done using Trizol reagent (Amresco, USA) as per the manufacturer’s protocol and 5 μg of total RNA was used for cDNA synthesis using Revertaid™ First Strand cDNA Synthesis Kit (Thermo Scientific, USA), following manufacturer’s instructions and described in Section 2.3.1
2.4.2. Real-time Polymerase chain reaction relative gene expression in the CAM
The expression level of IFN-α, IFN-β, IFN-γ, IL-1β, OAS and iNOS was analysed by RT-qPCR in PikoReal Real-Time PCR machine (Thermo Scientific) using QuantiTect SYBR Green PCR Kit (Qiagen, Germany) following manufacturer’s instructions and described in section 2.3.2. The RT-qPCR cycling conditions were set as initial incubation at 95 °C for 15 min followed by 95 °C for 10 s, 64 °C for 15 s, and 72 °C for 15 s for 45 cycles (Table 6 ). The data of quantitative real time PCR were analyzed by 2−ΔΔCt method (Pfaffl, 2001) to derive the relative fold change in mRNA expression of different genes studied. The primers used in RT-qPCR are shown in Table 5.
Table 6.
Thermal profile for cytokines expression by real-time PCR.
| Segment /cycle | Step | Time | Temperature |
|---|---|---|---|
| I/1 | Denaturation | 15:00 min | 95 °C |
| II/45 | Denaturation | 00:10 min | 95 °C |
| Annealing | 00:15 min | 64 °C | |
| Extension | 00:15 min | 72 °C | |
| III/3 | Melting curve | Hold time:00:01 min | 60 °C-95 °C |
3. Statistical analysis
The mean relative expression for each group was statistically analyed by one way ANOVA [least significance difference (LSD) and Duncan‘s Test] for ‘T’ distribution (P value) using SPSS (16.0) Software. For statistical analysis of virus replication data in two different groups, independent t-test was performed to check the difference between two groups at different hours significant at P<0.05 (Snedecor and William, 1989).
4. Results
4.1. Optimization of dose of TLR ligands
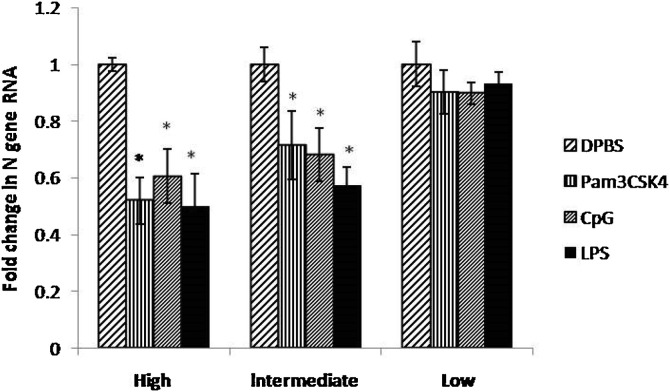
To know the optimum dose of TLR ligands (Pam3CSK4, CpG ODN and LPS), SPF embryonated chicken eggs were treated with three different doses of TLR ligands (high, intermediate and low dose) and virus titer in allantoic fluid was analyzed for N gene of IBV by real time PCR. There were six replicates in each group. The high dose of TLR ligands (Pam3CSK4, LPS and CpG ODN) significantly reduced IBV titers (P < 0.05) in allantoic fluid; but caused high mortality of chicken embryos than that of other two doses. Therefore, the high dose of TLR ligands was not used in further experiments. The intermediate dose of these TLR ligands also significantly (P < 0.05) reduced IBV titers in allantoic fluid than that of control group and caused very low mortality of embryonated chicken eggs. Low doses of TLR ligands did not significantly (P < 0.05) reduced virus titers and did not show any mortality of embryonated chicken eggs. As a result, intermediate dose (P < 0.05) of TLRs was selected for further experiments.
4.2. TLR ligands decrease viral replication in embryonated chicken eggs
The ECEs were treated with three different doses of Pam3CSK4, LPS and CpG ODN (Table 1) 24 h prior to infection. The high dose of TLR ligands (Pam3CSK4, LPS and CpG ODN) significantly reduced IBV titers (P < 0.05) in allantoic fluid; but caused high mortality of chicken embryos than that of other two doses (Fig. 2 ). Therefore, the high dose of TLR ligands was not used in further experiments. The intermediate dose of these TLR ligands also showed significantly (P < 0.05) reduced IBV titers in allantoic fluid than that of control group and caused very low mortality of ECEs. Low doses of TLR ligand did not significantly (P < 0.05) reduced virus titers and did not show any mortality of ECEs. As a result, inter mediate dose (P < 0.05) of TLRs was selected for further experiments.
Fig. 2.
Relative fold change in IBV N gene mRNA transcript expression in allantoic fluid after treatment of ECEs with different doses of TLR ligands (high, intermediate and low dose Significant difference (P < 0.05) between a test group and the PBS group are indicated by *. There were six replicates in each group.
4.3. Effect of TLR ligands treatment on IBV replication at different time intervals
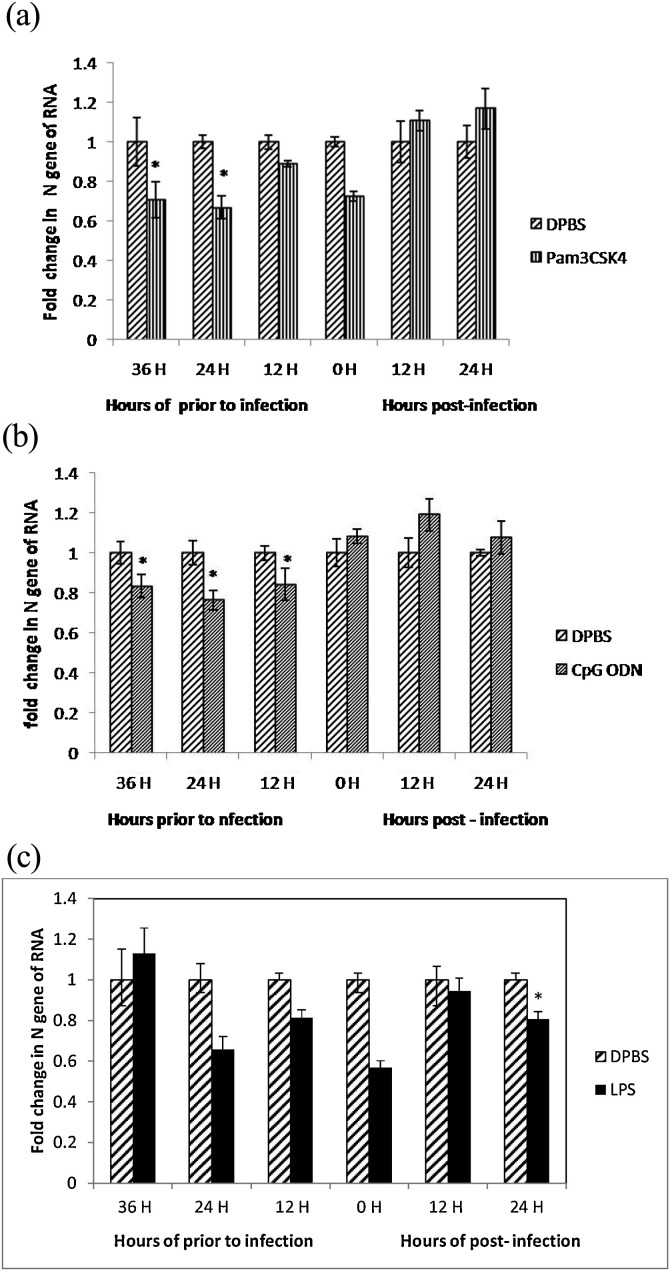
The optimum time for TLR ligands treatment was determined by treating the ECEs at six different time intervals [36, 24 and 12 h prior to infection, time of infection (co-administration of TLR ligands and avian IBV) and 12 and 24 h post-infection]. Subsequently, the ECEs were infected with 100 μl of IBV (104.8 EID50), allantoic fluid collected and IBV titres quantified by relative real time PCR. The treatment of ECEs with LPS @2 μg/egg at 24, 12 h prior to infection and at time of virus infection significantly (P < 0.05) reduced virus titres (0.2 to 0.3 fold) in allantoic fluid. A non-significant increase in virus titre was also observed at 36 h prior to IBV infection. The LPS treatment non-significantly reduced the virus titres at 12 h post-IBV infection. Moreover, the ECEs treated with Pam3CSK4 @ 10 μg/egg at 36 and 24 h prior to IBV infection significantly (P < 0.05) reduced virus titres (0.3 to 0.4 fold) in allantoic fluid while non-significant increase in virus titres were also observed at 12 and 24 h post IBV infection. Treatment of ECEs with CpG ODN @4 μg/egg at 36, 24 and 12 h prior to infection significantly (P < 0.05) reduced virus titer (0.2 to 0.03 fold) in allantoic fluid. The CpG ODN showed non-significant increase in IBV titre at the time of virus infection (0 h), 12 and 24 h post IBV infection (Figs. 3 (a), (b), (c)).
Fig. 3.
(a)Relative fold changes in IBV N gene mRNA transcript expression in allantoic fluid after treatment of ECEs with Pam3CSK4 at different time intervals.
The statistical analysis of each treatment and control group was done using independent sample t-test to compare the mean of two different groups using SPSS (16.0) and significant difference between a test group and the PBS group are indicated by *. There were six replicates in each group. (b) Relative fold changes in IBV N gene mRNA transcript expression in allantoic fluid after treatment of ECEs with CpG ODN at different time intervals.
The statistical analysis of each treatment and control group was done using independent sample t-test to compare the mean of two different groups using SPSS (16.0) and significant difference between a test group and the PBS group are indicated by *. There were six replicates in each group. (c) Relative fold changes in IBV N gene mRNA transcript expression in allantoic fluid after treatment of ECEs with LPS at different time intervals.
The statistical analysis of each treatment and control group was done using independent sample t-test to compare the mean of two different group using SPSS (16.0) and significant difference between a test group and the PBS group are indicated by *. There were six replicate in each group.
4.4. Induction of gene expression in the CAM by TLR ligands
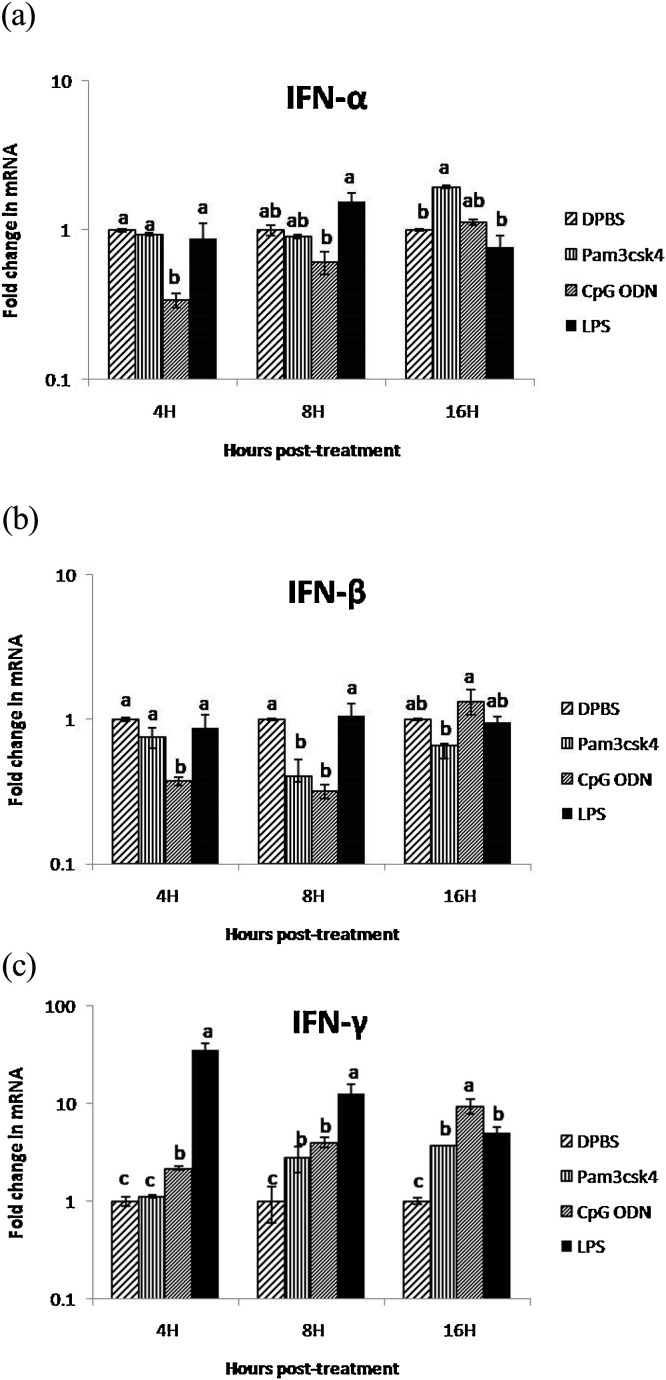
All the three TLR ligands (Pam3CSK4, CpG ODN and LPS) did not show significant (P < 0.05) increase in the expression of IFN-α and IFN-β in CAM of ECEs at any time point. CpG ODN and LPS demonstrated non-significant down regulation of IFN-α at 4 h post treatment and the expression of IFN-β was down regulated at 4 and 8 h post-treatment with CpG ODN while, Pam3CSK4 showed down regulation at 8 and 16 h post-treatment. The LPS induced 35-, 12- and 5-fold increase (significant at P < 0.05) in expression of IFN-γ in CAM at 4, 8 and 16 h post-treatment, respectively (Fig. 3c). The CpG ODN showed significant up-regulation (approximately 10 fold) at 16 h post-treatment and Pam3CSK4 induced four-fold increase in IFN-γexpression at 16 h post-treatment (Figs. 4 (a), (b), (c)).
Fig. 4.
(a) Fold change in IFN-α gene mRNA transcript expression in CAM after treatment with TLR ligands at different time points
(Different superscript represent the significant difference within the group and between the group statistical analysis of IFN− α expression was performed by one way ANOVA followed by Duncan’s post hoc test for multiple comparison to examine the effect of TLR ligands. P value of <0.05 was considered as significant). (b) Fold change in IFN-β gene mRNA transcript expression in CAM after treatment with TLR ligands at different time points.
(Different superscript represent the significant difference within the group and between the group statistical analysis of IFN− β expression was performed by one way ANOVA followed by Duncan’s post hoc test for multiple comparison to examine the effect of TLR ligands. P value of <0.05 was considered as significant). (c) Fold change in IFN-γ gene mRNA transcript expression in CAM after treatment with TLR ligands at different time points
(Different superscript represent the significant difference within the group and between the group statistical analysis of IFN− γ expression was performed by one way ANOVA followed by Duncan’s post hoc test for multiple comparison to examine the effect of TLR ligands. P value of <0.05 was considered as significant).
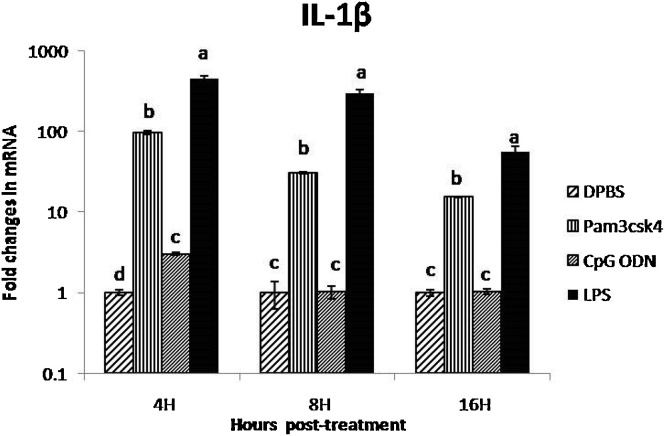
The expression of IL-1βwas significantly up-regulated in CAM treated with LPS at 4, 8 and 16 h post treatment by 452-, 300- and 56-fold, respectively (Fig. 5 ). Treatment of ECEs with Pam3CSK4 significantly up-regulated the expression of IL-1 β in CAM at 4, 8 and 16 h post treatment by 96-, 30- and 15- fold, respectively. The CpG ODN demonstrated very low or little effect on expression of IL-1β in CAM at all the three intervals.
Fig. 5.
Fold change in IL-1β gene mRNA transcript expression in CAM after treatment with TLR ligands at different time points
(Different superscript represent the significant difference within the group and between the group statistical analysis of IL−1β expression was performed by one way ANOVA followed by Duncan’s post hoc test for multiple comparison to examine the effect of TLR ligands. P value of <0.05 was considered as significant).
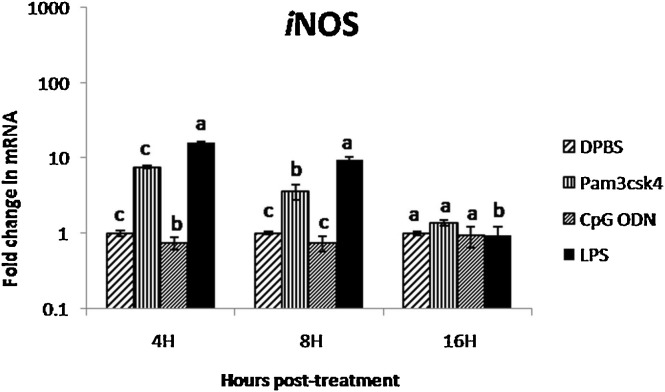
The LPS induced 16- and 10-fold significant (P < 0.05) increase in expression of iNOS in CAM at 4 and 8 h post treatment, respectively (Fig. 6 ). The Pam3CSK4 demonstrated significant (8-fold) expression at 4 h post treatment in CAM. The CpG ODN did not show significant impact on expression of iNOS in CAM of ECEs.
Fig. 6.
Fold change in iNOS gene mRNA transcript expression in CAM after treatment with TLR ligands at different time points
(Different superscript represent the significant difference within the group and between the group statistical analysis of iNOS expression was performed by one way ANOVA followed by Duncan’s post hoc test for multiple comparison to examine the effect of TLR ligands. P value of <0.05 was considered as significant).
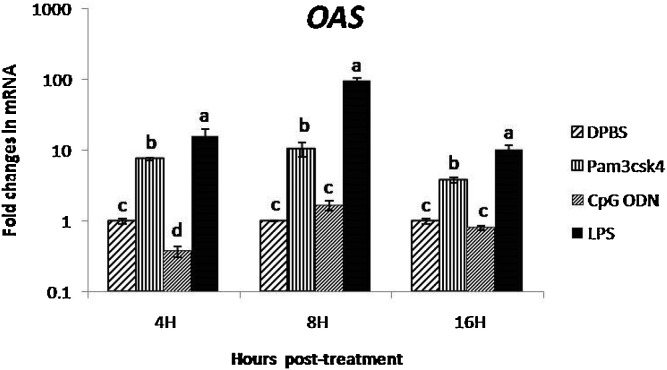
The LPS induced 16-, 96- and 10-fold significant (P < 0.05) increase in expression of OAS in CAM at 4, 8 and 16 h post treatment, respectively (Fig. 7 ). The Pam3CSK4 showed significant up regulation of OAS in CAM at 4 and 8 h post treatment. The CpG ODN induced 1.6-fold expression of OAS in CAM at 8 h post-treatment.
Fig. 7.
Fold change in OAS gene mRNA transcript expression in CAM after treatment with TLR ligands at different time points
(Different superscript represent the significant difference within the group and between the group statistical analysis of OAS expression was performed by one way ANOVA followed by Duncan’s post hoc test for multiple comparison to examine the effect of TLR ligands. P values of <0.05 was consider as significant).
5. Discussion
In the present study, an in vivo model was established to study the effect of TLR ligands on IBV replication in SPF embryonated chicken eggs. The TLRs are preferentially expressed in lymphocyte, dendritic cells (DCs) and macrophages. The engagement of TLRs in DCs links innate with adaptive immunity. The knowledge gained in the TLR research also provides an opportunity to modulate immune responses by targeting innate cells using their cognate ligands. TLR ligands have been tried as adjuvants along with many bacterial and viral vaccines in mammals as well as birds. The present study was conducted with the hypothesis that TLR ligands (Pam3CSK4, LPS and CpG ODN) may reduce IBV replication in allantoic fluid of ECEs and may increase expression of different cytokines in CAM of ECEs.
In the present study, the IBV was selected as a model because of its economic importance and highly infectious nature. IBV infection is worldwide distributed and control of IBV is very difficult because of presence of multiple serotypes and variants of virus are continuously emerging which are not cross protecting each other (Cavanagh and Naqi, 2003). For protection against IBV through a successful vaccination program, it is essential to identify the prevalent genotypes in the region and to determine the role of TLR ligands in enhancing the protective potential of IBV vaccine. As we know, the development of a new vaccine is very difficult task, so it is very important to improve the efficacy and delivery of existing IBV vaccine.
It was observed in the present study that dose of TLR ligands have a significant impact on inhibition of IBV replication in allantoic fluid of ECEs. The use of optimum dose (intermediate dose) of TLR ligands was an important factor in their ability to inhibit IBV replication in the ECEs without causing high mortality. The low dose of TLR ligands did not inhibit IBV replication. Probably, the low dose of TLR ligands might have been degraded early, especially the CpG ODN in allantoic fluid (Zhu et al., 2010). The high doses of TLR ligands caused the death of chicken embryos. Further studies are required to determine the kinetics and metabolism of these ligands in ECEs.
The timings of TLR administration were also a very important part of the present study; the LPS reduced the virus titer prior to infection, at the time of infection and post-infection in ECEs. The previous studies have shown that the pre-treatment of LPS provided protection against the lethal influenza virus challenge in mice (Shinya et al., 2011) and pre-treatment of chicken macrophages with LPS reduced the ability of AIV to infect these cells (Barjesteh et al., 2014). Bandoro and Runstadler (2017) have shown antiviral effects of LPS on influenza virus. The LPS induced NO production can lead to antiviral response against ILTV replication (Haddadi et al., 2013). Further, the LPS also increased the expression of IFN-γ, IL-1β, IL-8, IL-10, iNOS, and MHC-II in the spleen at 2 h post treatment in chicken (Sijben et al., 2003; Paul et al., 2011). These cytokines may play an important role in antiviral response of LPS. In our study, the Pam3SCK4 and CpG ODN significantly reduced virus titer only when administered prior to infection. The antiviral activity of Pam3CSK4 and CpG ODN has been demonstrated by Barjesteh et al. (2014) in which the pre-treatment of chicken macrophages with Pam3CSK4 and CpG ODN reduced the ability of AIV to infect these cells. This transient response induced by Pam3CSK4 and CpG ODN may be due to different profiles of immune responses induced by these TLRs or difference in pharmacokinetics and pharmacodynamics of these TLR ligands. The serum enzymes increased TLR ligands metabolism and decreased their half-life (Engel et al., 2011). For example, the CpG ODN was rapidly degraded when exposed to serum and decreased its half-life. This may explain, to some extent, the duration of response after administration of these TLR ligands. Furthermore, the treatment of TLR ligands prior to viral infection in ECEs may provide sufficient time for expression of antiviral response, including IFN, ISG and non-ISG proteins. These TLR ligands also increased the expression of different antiviral genes. The previous studies have proved that these antiviral factors can block viral replication at different stages of their replication (Goubau et al., 2013). Conversely, treatment of chicken embryos with LPS induced antiviral response post-infection. This may be due to the ISGs that interfere with the translation, assembly and release of virus (Goubau et al., 2013).
The effect of TLR ligands on expression of different cytokine expression (IFN-α, IFN-β, IFN-γ, IL-1β, iNOS and OAS) in CAM was also investigated in the present study. Following treatment with LPS, Pam3CSK4 and CpG ODN, the expression of type-1 interferon (IFN-α and IFN-β) did not increase in the CAM of ECEs. This result is also supported by the findings of Paul et al. (2011) in which expression of IFN-α did not change after treatment of chicken with CpG ODN and LPS in spleen of chicken. Similar results were also observed by Dar et al. (2009) wherein expression of IFN-α was not significantly increased after CpG ODN treatment in spleen of chicken embryos. There is possibility that the expression of type-1 interferon may be increased in later time points (not included in the present study). In IBV infection, the main source of proinflammatory cytokine is macrophages in respiratory tract (Amarasinghe et al., 2018). Induction of type-1 interferon is one of the earliest responses of host immune system to any viral infection (Diebold et al., 2004). Also, they form a bridge between innate and adaptive immune response as dendritic cells produce as well as undergo maturation by IFN-α/ IFN-β (Le Bon and Tough, 2002). They promote antiviral state directly through production of interferon inducible genes which inhibit viral transcription and translation, promote apoptosis of infected cells and activate antigen presenting cells (Samuel, 2001).
Contrary to the type-1 interferon, the expression of IFN-γ in CAM of ECEs was significantly increased by all the three TLR ligands. Dar et al. (2009) have also reported significant up-regulation of IFN-γ by CpG ODN in spleen of chicken embryos. A significant up-regulation of IFN-γ by CpG ODN, LPS and Pam3CSK4 has also been reported in the spleen of chickens (Paul et al., 2011, 2013). Similar to mammals (Huang et al., 1993), chicken IFN-γ has pleiotropic effects on different immune cells viz. antiviral activity against viral infections (Digby and Lowenthal, 1995; Song et al., 1997), stimulation of macrophages and natural killer cells to induce cell mediated immune responses (Lowenthal et al., 1997) and increased expression of MHC antigens (Weining et al., 1996; Song et al., 1997).
The IL-1β is a proinflammatory cytokine produced mainly by monocytes and causes fever, hypotension and production of other cytokines viz. IL-6. In addition, the IL-1β has also been demonstrated to be mainly involved in innate immune responses (Dinarello, 1996). In the present study, following embryonic treatment with LPS and Pam3CSK4, the IL-1β expression was increased in the CAM of ECEs. Conversely, no change in IL-1β expression in CAM of ECEs was noticed after treatment with CpG ODN. Similar results were observed in an in vivo study, in which expression of IL-1β significantly up-regulated in chicken spleen after treatment with Pam3CSK4 (Paul et al., 2013) and LPS (Paul et al., 2011). These results are also supported by Barjesteh et al. (2015) in which Pam3CSK4 and LPS significantly up-regulated the expression of IL-1β in CAM of ECEs. Contrary to our findings the expression of IL-1β was significantly increased in spleen of chicken embryos by treatment of CpG ODN (Dar et al., 2009). This difference may be due to type of cells present in CAM (Parvizi et al., 2012). The expression of IL-1β in CAM may recruit other cells like macrophages to the site of virus replication and these cells may be the source of iNOS. The iNOS catalyzed the production of NO from l-arginine using NADPH and molecular oxygen (Aktan, 2004). Nitric oxide (NO) plays an important role in host defense against infectious agents and tumors (Eisenstein, 2001). Various TLRs upon recognizing their respective ligands can activate macrophages and other immune cells to secrete iNOS and NO. In chickens, the ability of NO synthesis after stimulation with various TLR ligands in monocytes have been evaluated (He et al., 2006). Antiviral activity of NO has been demonstrated against ILTV in chicken macrophage (Haddadiet al., 2013). In the present study, Pam3CSK4 and LPS significantly up-regulated the expression of iNOS in CAM of ECEs. However, no change was observed after CpG ODN treatment. Similar findings were observed by Paul et al., (2011) in which expression of iNOS was significantly increased by LPS in chicken spleen. In another in vitro study, LPS treatment up-regulated the transcription of iNOS and NO production in chicken macrophages (He et al., 2011) and monocytes (He et al., 2006). These results are also supported by Barjesteh et al. (2015) in which Pam3CSK4 and LPS significantly up-regulated the expression of iNOS in CAM of ECEs. The inducible nitric oxide synthase (iNOS or NOS-2), one of the enzymes secreted by macrophages in response to intracellular pathogen, certain tumor cells, microbial products such as lipopolysaccharide and cytokine IFN-γ are of importance in immune response.
The OAS is the critical part of interferon-dependent host defense system against viruses. Further, the OAS activation induced RNAse L and led to the inactivation of viral mRNA (Rogozin et al., 2003; Itsui et al., 2006). The Pam3CSK4 and LPS increased the expression of OAS in CAM of ECEs but CpG ODN did not upregulated the expression of OAS in CAM. Although, CpG ODN significantly increased the expression of OAS in spleen of chicken embryos (Dar et al., 2009) and lung & spleen of chicken (Paul et al., 2012). This difference may be due to the type of cells present in the CAM (Parvizi et al., 2012).
The IFN-γ induced NO production and up-regulated interferon stimulating gene (ISGs) such as OAS, ds RNA protein kinase and RNaseL (Huang et al., 1993; Sedger et al., 1999). As a result, the IFN-γ may inhibit the replication of IBV in ECEs through NO production, up-regulation of ISGs such as OAS, IL-1β, and iNOS and activation of cellular immune response.
In conclusion, the optimum dose(s) of TLR agonists (chosen on the basis of significantly reduced virus titers without causing mortality of embryos), when administered in ovo in chicken embryo can interfere with the replication of IBV. The timings of TLR ligands treatment also had significant impact on the inhibition of virus replication in a way that only LPS reduced virus titer pre- and post-IBV infection but Pam3CSK4 and CpG ODN reduced virus titer only when administered pre-IBV infection. On the basis of these results, the LPS appeared to be the better candidate for adjuvant against IBV. All TLR ligands tested in the present study up-regulated the expression of pro-inflammatory cytokines and antiviral genes in CAM of ECEs that may play an important role in inhibition of IBV replication. It may also be speculated that in ovo administration of these TLR ligands may enhance resistance to viral infection in neonatal chickens and that these TLR ligands may contribute towards the development of more effective and safer vaccines including in ovo vaccines. Further studies are required to confirm the dose and time of treatment with different TLR ligands in SPF eggs as well as commercial birds.
Author statement
B.K.S. developed the hypotheses, designed and performed the experiments, analyzed and interpreted data, and drafted the manuscript as a part of his Ph.D. research work; N.K.K. administrated the overall research project, oversaw the experiments, analysis and interpretation of data and reviewed the manuscript; S.B. assisted with the experiments; R.C. designed and monitored experiments, analyzed and interpreted the data, and critically revised the manuscript. The authors declare no competing financial interests.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
This study was funded by the Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar.
References
- Abdul-Careem M.F., Hunter B.D., Parvizi P., Haghighi H.R., Thanthrige-Don N., Sharif S. Cytokine gene expression patterns associated with immunization against Marek’s disease in chickens. Vaccine. 2007;25(3):424–432. doi: 10.1016/j.vaccine.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4(7):499. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75(6):639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Amarasinghe A., Abdul-Cader M.S., Almatrouk Z., van der Meer F., Cork S.C., Gomis S., Abdul-Careem M.F. Induction of innate host responses characterized by production of interleukin (IL)-1β and recruitment of macrophages to the respiratory tract of chickens following infection with infectious bronchitis virus (IBV) Vet. Microbiol. 2018;215:1–10. doi: 10.1016/j.vetmic.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Bandoro C., Runstadler J.A. Bacterial lipopolysaccharide destabilizes influenza viruses. mSphere. 2017;2(5):e00267–17. doi: 10.1128/mSphere.00267-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barjesteh N., Hodgins D.C., Paul M.S., Quinteiro-Filho W.M., DePass C., Monteiro M.A., Sharif S. Induction of chicken cytokine responses in vivo and in vitro by lipooligosaccharide of Campylobacter jejuni HS: 10. Vet. Microbiol. 2013;164(1-2):122–130. doi: 10.1016/j.vetmic.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Barjesteh N., Behboudi S., Brisbin J.T., Villanueva A.I., Nagy É., Sharif S. TLR ligands induce antiviral responses in chicken macrophages. PLoS One. 2014;9(8):e105713. doi: 10.1371/journal.pone.0105713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barjesteh N., Shojadoost B., Brisbin J.T., Emam M., Hodgins D.C., Nagy É., Sharif S. Reduction of avian influenza virus shedding by administration of Toll-like receptor ligands to chickens. Vaccine. 2015;33(38):4843–4849. doi: 10.1016/j.vaccine.2015.07.070. [DOI] [PubMed] [Google Scholar]
- Boursnell M.E.G., Binns M.M., Brown T.D.K., Cavanagh D., Tomley F.M. Molecular biology of avian infectious bronchitis virus. Prog. Vet. Microbiol. Immunol. 1989;5:65–82. [PubMed] [Google Scholar]
- Brisbin J.T., Gong J., Parvizi P., Sharif S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin.Vaccine Immunol. 2010;17(9):1337–1343. doi: 10.1128/CVI.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capua I., Minta Z., Karpinska E., Mawditt K., Britton P., Cavanagh D., Gough R.E. Co-circulation of four types of infectious bronchitis virus (793/B, 624/I, B1648 and Massachusetts) Avian Pathol. 1999;28(6):587–592. doi: 10.1080/03079459994380. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38(2):281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Naqi S.A. Infectious bronchitis. Avian Dis. 2003;11:101–119. [Google Scholar]
- Cavanagh D., Davis P.J., Cook J.K., Li D., Kant A., Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992;21:33–43. doi: 10.1080/03079459208418816. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Mawditt K., Sharma M., Drury S.E., Ainsworth H.L., Britton P., Gough R.E. Detection of a coronavirus from turkey poults in Europe genetically related to infectious bronchitis virus of chickens. Avian Pathol. 2001;30(4):355–368. doi: 10.1080/03079450120066368. [DOI] [PubMed] [Google Scholar]
- Chhabra R., Forrester A., Lemiere S., Awad F., Chantrey J., Ganapathy K. Mucosal, cellular, and humoral immune responses induced by different live infectious bronchitis virus vaccination regimes and protection conferred against infectious bronchitis virus Q1 strain. Clin. Vaccine Immunol. 2015;22:1050–1059. doi: 10.1128/CVI.00368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra R., Kuchipudi S.V., Chantrey J., Ganapathy K. Pathogenicity and tissue tropism of infectious bronchitis virus is associated with elevated apoptosis and innate immune responses. Virology. 2016;488:232–241. doi: 10.1016/j.virol.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar A., Potter A., Tikoo S., Gerdts V., Lai K., Babiuk L.A., Mutwiri G. CpGoligodeoxynucleotides activate innate immune response that suppresses infectious bronchitis virus replication in chicken embryos. Avian Dis. 2009;53(2):261–267. doi: 10.1637/8560-121808-Reg.1. [DOI] [PubMed] [Google Scholar]
- Diebold S.S., Kaisho T., Hemmi H., Akira S., Sousa C.R. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Digby M.R., Lowenthal J.W. Cloning and expression of the chicken interferon-γ gene. J. Interferon Cytokine Res. 1995;15(11):939–945. doi: 10.1089/jir.1995.15.939. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–2147. [PubMed] [Google Scholar]
- Eisenstein T.K. Implications of Salmonella-induced nitric oxide (NO) for host defense and vaccines: NO, an antimicrobial, antitumor, immunosuppressive and immunoregulatory molecule. Microbes Infect. 2001;3(14-15):1223–1231. doi: 10.1016/s1286-4579(01)01482-4. [DOI] [PubMed] [Google Scholar]
- Engel A.L., Holt G.E., Lu H. The pharmacokinetics of Toll-like receptor agonists and the impact on the immune system. Expert Rev. Clin. Pharmacol. 2011;4(2):275–289. doi: 10.1586/ecp.11.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhard M.H., Schmidt P., Zinsmeister P., Hofmann A., Munster U., Kaspers B., Wiesmuller K.H., Bessler W.G., Stangassinger M. Adjuvant effects of various lipopeptides and interferon-γ on the humoral immune response of chickens. Poult. Sci. 2000;79(9):1264–1270. doi: 10.1093/ps/79.9.1264. [DOI] [PubMed] [Google Scholar]
- Goubau D., Deddouche S., e Sousa C.R. Cytosolic sensing of viruses. Immunity. 2013;38(5):855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Rosa A.J., Chen D.G., Wang X. Molecular mechanisms of primary and secondary mucosal immunity using avian infectious bronchitis virus as a model system. Vet. Immunol.Immunopathol. 2008;121(3-4):332–343. doi: 10.1016/j.vetimm.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadi S., Kim D.S., Jasmine H., van der Meer F., Czub M., Abdul-Careem M.F. Induction of Toll-like receptor 4 signaling in avian macrophages inhibits infectious laryngotracheitis virus replication in a nitric oxide dependent way. Vet. Immunol.Immunopathol. 2013;155(4):270–275. doi: 10.1016/j.vetimm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- He H., Genovese K.J., Nisbet D.J., Kogut M.H. Profile of Toll-like receptor expressions and induction of nitric oxide synthesis by Toll-like receptor agonists in chicken monocytes. Mol. Immunol. 2006;43(7):783–789. doi: 10.1016/j.molimm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- He H., Genovese K.J., Kogut M.H. Modulation of chicken macrophage effector function by TH1/TH2 cytokines. Cytokine. 2011;53(3):363–369. doi: 10.1016/j.cyto.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Huang S.I., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R.M., Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259(5102):1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Itsui Y., Sakamoto N., Kurosaki M., Kanazawa N., Tanabe Y., Koyama T., Takeda Y., Nakagawa M., Kakinuma S., Sekine Y., Maekawa S. Expressional screening of interferon‐stimulated genes for antiviral activity against hepatitis C virus replication. J. Viral Hepat. 2006;13(10):690–700. doi: 10.1111/j.1365-2893.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- Keestra A.M., de Zoete M.R., Bouwman L.I., Vaezirad M.M., Van Putten J.P. Unique features of chicken Toll-like receptors. Dev. Comp. Immunol. 2013;41(3):316–323. doi: 10.1016/j.dci.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Kint J., Fernandez-Gutierrez M., Maier H.J., Britton P., Langereis M.A., Koumans J., Wiegertjes G.F., Forlenza M. Activation of the chicken type I interferon response by infectious bronchitis coronavirus. J. Virol. 2015;89(2):1156–1167. doi: 10.1128/JVI.02671-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A., Tough D.F. Links between innate and adaptive immunity via type I interferon. Curr.Opin.Immunol. 2002;14(4):432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- Lowenthal J.W., York J.J., O’Neil T.E., Rhodes S., Prowse S.J., Strom A.D.G., Digby M.R. In vivo effects of chicken interferon-γ during infection with Eimeria. J. Interferon Cytokine Res. 1997;17(9):551–558. doi: 10.1089/jir.1997.17.551. [DOI] [PubMed] [Google Scholar]
- Parvizi P., Mallick A.I., Haq K., Haghighi H.R., Orouji S., Thanthrige-Don N., St. Paul M., Brisbin J.T., Read L.R., Sharif S. A toll-like receptor 3 ligand enhances protective effects of vaccination against Marek’s disease virus and hinders tumor development in chickens. Viral Immunol. 2012;25(5):394–401. doi: 10.1089/vim.2012.0033. [DOI] [PubMed] [Google Scholar]
- Paul M.S., Mallick A.I., Haq K., Orouji S., Abdul-Careem M.F., Sharif S. In vivo administration of ligands for chicken toll-like receptors 4 and 21 induces the expression of immune system genes in the spleen. Vet. Immunol.Immunopathol. 2011;144(3-4):228–237. doi: 10.1016/j.vetimm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Paul M.S., Paolucci S., Read L.R., Sharif S. Characterization of responses elicited by Toll-like receptor agonists in cells of the bursa of Fabricius in chickens. Vet. Immunol.Immunopathol. 2012;149(3-4):237–244. doi: 10.1016/j.vetimm.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Paul M.S., Brisbin J.T., Abdul-Careem M.F., Sharif S. Immunostimulatory properties of Toll-like receptor ligands in chickens. Vet. Immunol.Immunopathol. 2013;152(3-4):191–199. doi: 10.1016/j.vetimm.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Paul M., Barjesteh N., Brisbin J.T., Villaneueva A.I., Read L.R., Hodgins D., Nagy E., Sharif S. Effects of ligands for Toll-like receptors 3, 4, and 21 as adjuvants on the immunogenicity of an avian influenza vaccine in chickens. Viral Immunol. 2014;27(4):167–173. doi: 10.1089/vim.2013.0124. [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29(9) doi: 10.1093/nar/29.9.e45. e45-e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S.A.E., El-Kenawy A.A., Neumann U., Herrler G., Winter C. Comparative analysis of the sialic acid binding activity and the tropism for the respiratory epithelium of four different strains of avian infectious bronchitis virus. Avian Pathol. 2009;38(1):41–45. doi: 10.1080/03079450802632049. [DOI] [PubMed] [Google Scholar]
- Rautenschlein S., Sharma J.M., Winslow B.J., McMillen J., Junker D., Cochran M. Embryo vaccination of turkeys against Newcastle disease infection with recombinant fowlpox virus constructs containing interferons as adjuvants. Vaccine. 1999;18(5-6):426–433. doi: 10.1016/s0264-410x(99)00254-6. [DOI] [PubMed] [Google Scholar]
- Rautenschlein S., Sheikh A.M., Patnayak D.P., Miller R.L., Sharma J.M., Goyal S.M. Effect of an immunomodulator on the efficacy of an attenuated vaccine against avian pneumovirus in turkeys. Avian Dis. 2002;46(3):555–561. doi: 10.1637/0005-2086(2002)046[0555:EOAIOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27(3):493–497. [Google Scholar]
- Rogozin I.B., Aravind L., Koonin E.V. Differential action of natural selection on the N and C-terminal domains of 2′-5′ oligoadenylatesynthetases and the potential nuclease function of the C-terminal domain. J. Mol. Biol. Mol. 2003;326(5):1449–1461. doi: 10.1016/s0022-2836(03)00055-x. [DOI] [PubMed] [Google Scholar]
- Samuel C.E. Antiviral actions of interferons. Clin.Microbiol. Rev. 2001;14(4):778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedger L.M., Shows D.M., Blanton R.A., Peschon J.J., Goodwin R.G., Cosman D., Wiley S.R. IFN-γ mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J. Immunol. 1999;163(2):920–926. [PubMed] [Google Scholar]
- Sharma J.M., Zhang Y., Jensen D., Rautenschlein S., Yeh H.Y. Field trial in commercial broilers with a multivalent in ovo vaccine comprising a mixture of live viral vaccines against Marek’s disease, infectious bursal disease, Newcastle disease, and fowl pox. Avian Dis. 2002;46(3):613–622. doi: 10.1637/0005-2086(2002)046[0613:FTICBW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Shinya K., Okamura T., Sueta S., Kasai N., Tanaka M., Ginting T.E., Makino A., Eisfeld A.J., Kawaoka Y. Toll-like receptor pre-stimulation protects mice against lethal infection with highly pathogenic influenza viruses. Virol. J. 2011;8(1):97. doi: 10.1186/1743-422X-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijben J.W., Klasing K.C., Schrama J.W., Parmentier H.K., van der Poel J.J., Savelkoul H.F., Kaiser P. Early in vivo cytokine genes expression in chickens after challenge with Salmonella typhimurium lipopolysaccharide and modulation by dietary n− 3 polyunsaturated fatty acids. Dev. Comp. Immunol. 2003;27(6-7):611–619. doi: 10.1016/s0145-305x(03)00031-4. [DOI] [PubMed] [Google Scholar]
- Snedecor G.W.C., William G. 1989. Statistical METHODS/GEORGE W. Snedecor and William G. Cochran (No. QA276. 12. S6313 1989.) [Google Scholar]
- Song K.D., Lillehoj H.S., Choi K.D., Zarlenga D., Han J.Y. Expression and functional characterization of recombinant chicken interferon-gamma. Vet. Immunol.Immunopathol. 1997;58(3-4):321–333. doi: 10.1016/s0165-2427(97)00034-2. [DOI] [PubMed] [Google Scholar]
- Thoelen S., De Clercq N., Tornieporth N. A prophylactic hepatitis B vaccine with a novel adjuvant system. Vaccine. 2001;19(17-19):2400–2403. doi: 10.1016/s0264-410x(00)00462-x. [DOI] [PubMed] [Google Scholar]
- Villanueva A.I., Kulkarni R.R., Sharif S. Synthetic double-stranded RNA oligonucleotides are immunostimulatory for chicken spleen cells. Dev. Comp. Immunol. 2011;35(1):28–34. doi: 10.1016/j.dci.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Rosa A.J., Oliverira H.N., Rosa G.J., Guo X., Travnicek M., Girshick T. Transcriptome of local innate and adaptive immunity during early phase of infectious bronchitis viral infection. Viral Immunol. 2006;19(4):768–774. doi: 10.1089/vim.2006.19.768. [DOI] [PubMed] [Google Scholar]
- Wang Y., Shan C., Ming S., Liu Y., Du Y., Jiang G. Immunoadjuvant effects of bacterial genomic DNA and CpGoligodeoxynucleotides on avian influenza virus subtype H5N1 inactivated oil emulsion vaccine in chicken. Res. Vet. Sci. 2009;86(3):399–405. doi: 10.1016/j.rvsc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Weining K.C., Schultz U., Münster U., Kaspers B., Staeheli P. Biological properties of recombinant chicken interferon‐γ. Eur. J. Immunol. 1996;26(10):2440–2447. doi: 10.1002/eji.1830261026. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Meng W., Li X., Gao H., Hanagata N. Design of mesoporous silica/cytosine− phosphodiester− guanine oligodeoxynucleotide complexes to enhance delivery efficiency. J. Phys. Chem. C. 2010;115(2):447–452. [Google Scholar]