Abstract
In a feeding trial, sows and piglets were fed with the probiotic bacterium Bacillus cereus var. toyoi as a feed additive, and the effects on immune cell populations were examined. The development of the gut immune system was determined for piglets at the ages of 14, 28, 35 and 56 days post partum. Tissue samples of the Jejunum and the continuous Peyer's patch were used for enumeration of intraepithelial lymphocyte populations by fluorescence activated flow cytometry and fluorescence microscopy. Both independent methods of investigation led to similar results: the population of intraepithelial CD8+ T cells was significantly enhanced in the probiotic group piglets (p ≤ 0.05), and the numbers of γδ T cells tended to be higher in the intestinal epithelium (p < 0.1) at the time of weaning (day 28). Lamina propria lymphocytes were also influenced by the treatment. Application of B. cereus var. toyoi resulted in significantly more CD25+ lymphocytes and γδ T cells in the probiotic group post-weaning. The occurrence of pathogenic Escherichia coli serogroups was also less frequent in the feces of piglets from the probiotic group. The finding that the CD8+ T cell population in the intestinal mucosa showed changes on day 28 indicated that the influence of B. cereus var. toyoi supplementation on the intestinal immune system started before weaning, an observation supported by changes in the intestinal microflora observed during the suckling-period. The results suggest that feeding of B. cereus var. toyoi to sows may result in beneficial effects on piglet health status independent of their feed supplementation.
Keywords: Porcine, Pig, Intestinal immunity, Probiotic, Bacillus cereus var. toyoi, Intraepithelial lymphocytes (IEL), CD8, Lamina propria mucosa, E. coli
1. Introduction
The recent ban on antibiotics as antimicrobial growth promoters in the European Union has stimulated the interest in probiotic feed supplements in livestock farming as an alternative means of reducing infectious bacterial loads in animal stock. Many of the bacterial species already used as probiotics are members of the indigenous (commensal) intestinal flora of the host (de Champs et al., 2003, Jin et al., 2000). These commensals naturally colonize the intestinal tract, which is one important quality that makes them candidates for use as probiotics (Massi et al., 2004). In addition, autochthonous bacteria are involved in the development and maintenance of oral tolerance against food antigens (Moreau and Corthier, 1988, Tanaka and Ishikawa, 2004). However, commensal bacterial species would not be expected to stimulate mucosal or systemic immune responses, at least not as long as they do not breach the intestinal barrier or enter the bloodstream (Scharek et al., 2005, Westendorf et al., 2005). Under normal conditions, commensal bacteria should be tolerated by the local immune system of the host, and only a few studies have reported immune stimulatory effects of indigenous bacteria (Talham et al., 1999). Bacterial colonization of the gut and infection by enteropathogens also has an impact on the number of intraepithelial lymphocytes (IELs) associated with the intestinal tract. Consistent with this, as early as 1 month post partum, specific pathogen free pigs show lower amounts of IELs than conventional pigs of the same age (Rothkötter et al., 1999).
Bacillus cereus var. toyoi is not a member of the normal porcine gut flora, but is a common soil inhabitant. A number of studies have assigned probiotic characteristics to this particular Bacillus strain (NCIMB 40112; ToyoCerin®), and it has been authorized in the EU for use as a probiotic feed additive for sows and piglets and several other farm animal species. In animal studies with various strains of B. cereus, positive effects such as increased weight gain, improved feed conversion ratios and lower mortality rates of piglets have been reported (Kirchgessner et al., 1993, Alexopoulos et al., 2001), and B. cereus var. toyoi was correlated with a reduced incidence of post-weaning diarrhoea (Taras et al., 2005a). However, since B. cereus var. toyoi is not a member of the indigenous intestinal flora of pigs and produces a strong hemolysin (P. Schierack, personnel communication), we considered the possibility that it might be more immunogenic compared to other probiotics. Indeed, immunogenic properties of B. cereus spores have been shown previously in mice, resulting in expression of inflammatory cytokines in the gut and modest, spore-specific IgG titers. Furthermore, the in vitro-germination of spores in macrophages initiates the induction of pro-inflammatory cytokine gene expression (Duc le et al., 2004).
In this communication we assessed the effects of B. cereus var. toyoi feed supplementation of sows and piglets on the development of the gut associated immune system by use of fluorescence activated cell scanning (FACS) and immunohistochemistry. The immunological investigations were part of an interdisciplinary research project including histological, physiological, health and performance data assessed by affiliated institutes within a larger study “An Integrative Analysis of Mechanisms of Probiotic Action in Pigs”, funded by the Deutsche Forschungsgemeinschaft (DFG). The immunological results presented here are discussed with regard to the intestinal colonization by B. cereus var. toyoi and health performance data reported previously by Taras et al. (2005a) for the same groups of animals. Our results illustrate additional, specific changes on particular lymphocyte populations attributable to B. cereus var. toyoi.
2. Material and methods
2.1. Animal study
A group of 10 sows (crossbred Landrasse and Duroc) was chosen at random to serve as the probiotic group and were provided with the probiotic B. cereus var. toyoi (ToyoCerin, Lohmann Animal Health, Cuxhaven, Germany) in the feed. To establish the complete, long-term effects on the immune system, both sows as well as the piglets of the probiotic group were treated throughout the course of the study. For sows, feed supplementation started 25 days after insemination. Sows were fed throughout pregnancy, with feed supplementation continuing during lactation. Litter size within each treatment group was adjusted to meet an exclusion criterion of at least nine but not more than 15 living piglets 24 h after birth. Piglets of the probiotic group had free access to supplemented feed beginning on day 15 post partum, and received the same probiotic supplement during and post-weaning (day 28). A group of 10, untreated sows and their piglets served as the control group for the study. Housing facilities for sows and piglets of both treatment groups were of identical construction and environmental conditions and spatially separated from each other. The lighting program was 16 h light and 8 h of darkness. The room temperature and relative humidity were adjusted to 21.5 °C and 65.0%, respectively. The mean concentration (± S.D.) of the supplemented B. cereus var. toyoi in the food for gestating sows, lactating sows, nursed piglets and weaned piglets were determined as 2.6 (± 1.0) × 105, 4.0 (± 1.2) × 105, 1.3 (± 0.5) × 106 and 1.4 (± 0.4) × 106 cfu/g dry weight food, respectively, as previously described (Taras et al., 2005a). From each treatment group a total of five litters were randomly chosen and one piglet per litter was selected at random at the end of days 14, 28, 35 and 56 (i.e., 20 piglets per treatment), to gain tissue samples. Tissue samples obtained from all piglets were submitted for viral diagnostics to exclude the presence of intestinal viruses. No infection with Rota- or Corona-viruses was detected in any of the samples. The viral diagnostics were independently carried out by the Centre of Health Services of Berlin (State Laboratory for examination of Food, Drugs and Epizootics, Berlin, Germany).
2.2. Animal treatment and intestinal sections
Piglets were first anaesthetized with Ketamin and Pentobarbital, and blood was taken from the Vena jugularis and collected in a heparinised tube. While still under anaesthesia, the abdominal cavity was opened and the appropriate sections of the intestine were isolated with clamps. Subsequently, the piglets were euthanized by injection of Pentobarbital, the intestinal tract was removed, and 50 cm of the proximal jejunum beginning with the end of the plica duodenocolica was transferred to PBS. A second 50 cm section was taken from the distal Jejunum (ending at the plica ileocaecalis) and transferred into PBS. Both intestinal samples were excised lengthwise and washed twice with PBS to remove ingesta. Tissue samples were transferred to collection medium (Hanks balanced salts solution without Ca2+ and Mg2+, 0.5 mg/ml gentamicin and penicillin–streptomycin 20 IU/0.02 mg in 1 ml of HBSS) until further processing.
The study was approved by the local animal welfare committee of the Federal Ministry of Consumer Protection, Food and Agriculture (No. G0037/02).
2.3. Tissue samples
The first 1 cm of the proximal jejunum beginning with the end of the plica duodenocolica was snap-frozen in liquid nitrogen. Cryostat sections (6–8 μm in thickness), embedded in OCT Compound (Plano GmbH, Wetzlar), were transferred to poly-l-lysine-coated glass slides (Menzel Gläser GmbH & Co. KG, Braunschweig), air-dried and stored at −80 °C. Sections were warmed to room temperature before fixing in acetone for 10 min at 4 °C. After three, 5 min washes in Tris-buffered saline (TBS), 20% swine serum in 1% BSA–TBS was applied for 30 min at room temperature to block non-specific antibody binding. Incubation with primary antibody (CD45, CD21, CD11R1 and TcR1 dilution 1:10; CD3, CD8 and CD4 dilution 1:5), was carried out in 5% swine serum in 1% BSA/TBS for 1 h at room temperature in a dark, humidified chamber, followed by three 5 min washes in TBS. Sections were then incubated with fluorescence labelled secondary antibodies for 30 min at room temperature in a dark humidified chamber, followed by washing as above. Slides were stained with 4′,6′-diamidino-2-phenylindole (DAPI; Roche Diagnostics GmbH, Mannheim) (1:100 in PBS) for 2 min in the dark and washed as above. The slides were then coated with lycerol and sealed with a coverslip. Negative controls consisted of slides incubated with an equal volume of incubation buffer (5% swine serum–1% BSA/TBS) without the primary monoclonal antibody.
2.4. Antibodies
The antibodies used in this study included: mouse anti-porcine CD45, clone MAC323 and mouse anti-porcine CD11R1, clone MIL4 (Biozol Diagnostica, Eching, Germany); mouse anti-porcine CD21, clone BB6-11C9.6 and mouse anti-porcine CD3, clone PPT3 (Southern Biotechnology associates, Birmingham, USA); mouse anti TcR-1, clone PGBL22-A (Acris, Bad Nauheim, Germany); mouse anti-porcine CD8a, clone PT8 and mouse anti-porcine CD4, clone PT4 (Pharmingen, San Diego, CA, USA). Goat anti-mouse IgG-PE and goat anti-mouse IgG-FITC antibodies were obtained from Caltag (Burlingame, CA, USA).
2.5. Fluorescence microscopy
For quantification of intestinal lymphocyte subsets, a fluorescence microscope with an attached digital camera was used. For morphometric analysis we used the MetaMorph 6.1 software (Visitron Systems, Puchheim, Germany). Villus-area was calculated within the marked regions circumscribing individual villi. Lymphocyte numbers for a given surface area in the Lamina propria were calculated per 105 per μm2. Similarly, the length of epithelium samples was measured by use of a calibrated mouse-controlled cursor, and numbers of intraepithelial lymphocytes (IEL) were calculated per 102 μm of epithelium. For each primary antibody three fields were selected in samples from every animal.
2.6. Cell isolation for flow cytometry
The isolation of intestinal cells was performed as described by Solano-Aguilar et al. (2000) with modifications. In brief, for isolation of intraepithelial lymphocytes (IEL), tissue sections taken from the proximal jejunum were cut into 3 cm sections and incubated in Hanks balanced salt solution with DTT (HBSS-DTT; HBSS without Ca2+ and Mg2+, 2 mM DTT, 0.01 mM Hepes) and gently shaken for 5 min at 37 °C. The medium was discarded and replaced with HBSS–EDTA (HBSS–EDTA; HBSS without Ca2+ and Mg2+, 1 mM EDTA, 1 mM Hepes) and further incubated for 35 min at 37 °C with gentle shaking. After passage through sterile 210 μm Nylon mesh, the cell suspensions were collected and centrifuged at 600 × g for 10 min at 4 °C. Cell pellets were resuspended in RPMI medium and kept on ice. Incubation of the tissue sections was repeated twice. All three cell suspensions attained were combined and centrifuged again. Sediments were resuspended in 25% Percoll in HBSS and centrifuged at 600 × g for 30 min. The cell debris (top layer) was aspirated, the Percoll solution was removed, and the cell sediment was resuspended in RPMI. The resulting whole cell suspensions (containing leukocytes and epithelial cells) were subjected to flow cytometry.
To isolate lymphocytes from the continuous Peyer's patch, tissue sections from the distal jejunum were transferred into HBSS–DTT, and incubated at 37 °C under gentle shaking for 20 min. Medium was discarded and replaced with HBSS–EDTA, and sections were incubated at 37 °C with shaking for 20 min. The medium was discarded and the tissues were minced using scalpel blades. Released cells were collected after passage through a 210 μm nylon mesh and centrifuged at 600 × g for 10 min. Cell pellets were resuspended in 28 ml of a 40% Percoll solution and divided into four fractions. Seven millilitres of aliquots were pipetted into 15 ml conical tubes and underlayed with an equal volume of 70% Percoll (density: 1.13 g/ml) solution. Cell separation was carried out by centrifugation at 1000 × g for 30 min, and lymphoid cells were collected from the 40–70% layer interface and subsequently analysed by flow cytometry.
Peripheral blood mononuclear cells (PBMC) from selected piglets of different ages were separated from heparinised blood by gradient centrifugation (Ficoll-Paque, Amersham Biosciences, Uppsala, Sweden) at 800 × g for 20 min.
2.7. Flow cytometry
CD4 and CD8 surface antigens were stained with labelled primary mouse anti-porcine CD4a, clone 74-12-4, conjugated to FITC or mouse anti-porcine CD8a, clone 76-2-11, conjugated to R-phycoerythrin (Southern Biotechnology Associates, Birmingham, USA) in a one-step incubation. For each reaction, 5 × 105 cells were exposed to saturating concentrations of antibody in a 100 μl volume of PBS with 0.2% BSA for 20 min on ice. Cells were washed with 3 ml of PBS–0.2% BSA (300 × g, 10 min) and resuspended in 1 ml of PBS–0.2% BSA. CD14, CD21, and TcR-1 (γδ T cell receptor) were detected by use of unlabelled primary antibodies followed by washing, and incubation with a fluorescence labelled secondary antibody (goat anti-mouse IgG, Beckman Coulter, Krefeld, Germany) under the same conditions as described above. CD3 surface antigen was detected using a biotinylated antibody (mouse anti-porcine CD3ɛ, Biotin conjugate, Clone BB238E6, Southern Biotechnology Associates, Birmingham, USA) followed by a wash, and incubation with streptavidin conjugated to PC5 (Streptavidin-PC5, Beckman Coulter, Krefeld, Germany).
After a second washing process, cells were resuspended in 1 ml of PBS–0.2% BSA. Propidium iodide (0.5 μg/ml) was added to each sample, and cells were assayed by flow cytometry using an EPICS XL flow cytometer equipped with a 488 nm argon laser (Beckman 193 Coulter, Krefeld, Germany).
2.8. Determination of Escherichia coli serogroups
Fecal samples from individual sows were collected at intervals of ca. 90, 30 and 10 days a.p. Intestinal contents of sacrificed piglets were taken from ileum and/or colon of piglets at 14, 28, 35 and 56 days post partum. Intestinal sections were clamped and sealed off with surgical thread before removal to prevent loss of contents and to maintain the intestinal conditions during anaerobic transport. The samples from sows and piglets were processed as previously described (Scharek et al., 2005). Serotyping of E. coli isolates from blood–agar plates was followed by single colony purification on Gassner and Chromagar Orientation plates (Mast Diagnostica, Reinfeld, Germany) followed by agglutination tests with antisera against O204 specific antigens corresponding to the O108, O138, O139, O141, O147, O149 and O157 serogroups (BfR Dessau, Berlin, Germany).
2.9. Statistical analysis
Statistical evaluation was performed by explorative data analysis and the non-parametric Mann–Whitney test using the software SPSS 12.0, SPSS Inc. p-Values below an α-level of 0.05 were considered as significant.
Methods to assess the health performance and to monitor the colonization of the intestinal tract with the administered B. cereus var. toyoi are described elsewhere (Taras et al., 2005a).
3. Results
3.1. Epithelium of the proximal jejunum
Quantitative assessment of microscopically distinct epithelial cells proved difficult due to the density of the epithelial layer. From flow cytometry determinations, the fraction of total leukocytes (CD45+) in the upper jejunal epithelium developed similarly in both groups of piglets. All animals showed an increase in CD45+ intraepithelial cells between days 14 and 56 (Fig. 1 ). The fraction of γδ T-cells (TcR1+) within the epithelium tended to be higher in the probiotic group (Fig. 2 ). The phenotype of CD8+ cells in the intestinal epithelium differs in some aspects from that commonly found in blood peripheral mononuclear cells (PBMC) preparations of piglets in that a clear distinction between CD8high and CD8low cells was not possible with lymphocytes derived from the intestinal epithelium. Almost all CD8+ cells showed bright fluorescence, indicating high expression levels (Fig. 3a). In the peripheral blood of 56-day-old piglets, a large proportion of the CD8+ cells was CD8low but CD3− (Fig. 3b). In contrast, only a small fraction of the IELs showed the CD8low CD3− phenotype, averaging less than 5% during whole animal study (data not shown).
Fig. 1.
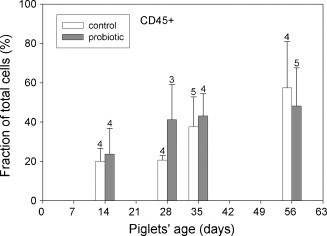
Leukocytes (CD45+) in the proximal jejunal epithelium isolated from control animals (open bars) and probiotic animals (filled bars) were analysed by flow cytometry. Percent values were calculated as a fraction of all living cells isolated from the epithelial layer (= 100%). Number of samples for each determination is shown above the error bars. Data were analysed by Welch's t-statistic.
Fig. 2.
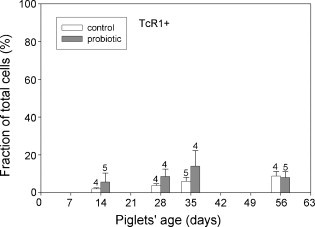
γδ T cells (TcR-1+) in the proximal jejenum of control piglets (open bars) and probiotic piglets (filled bars) were analysed by flow cytometry. Percent values were calculated as a fraction of all epithelial cells (= 100%). Number of samples for each determination is shown above the error bars. Data were analysed by Welch's t-statistic.
Fig. 3.
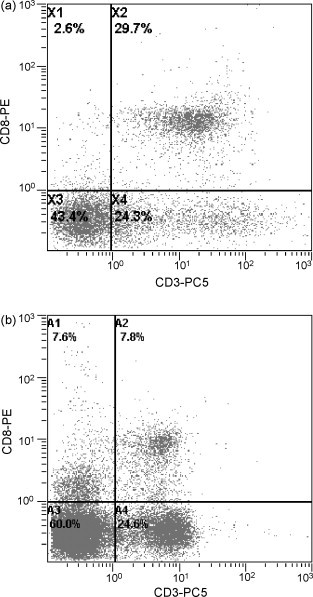
(a) A dot plot of a doubly stained IELs with anti-porcine CD3ɛ (secondary labelled with PC5) and anti-porcine CD8a (primary labelled with PE) is shown. A clear discrimination between CD8high and CD8low cells is not possible with lymphocytes derived from the intestinal epithelium. Most CD8+ cells were also CD3+ (region X2 = 29.7%). The IEL-sample was taken from a 56 days old probiotic piglet. (b) Dot plot of a doubly stained PBMC with anti-porcine CD3ɛ (second. labelled with PC5) and anti-porcine CD8a (prim. labelled with PE) showing a clear difference between CD8high and CD8low cells. A large proportion of the CD8low cells are CD3− NK cells (region A1 = 7.6%). The blood sample was taken from a 56 days old probiotic piglet (same animal as shown in (a)).
The frequency of CD3/CD8 double-positive T cells in the epithelial layer differed between the two experimental groups in that probiotic animals had significantly more CD3/CD8 double-positive T cells in the epithelial layer on day 28 (Fig. 4 ). This result obtained by flow cytometry was confirmed by the immune histochemical investigations of samples from the same animals. In the B. cereus var. toyoi-supplemented group, significantly more CD8+ lymphocytes were present in the tissue sections compared to samples from control animals on day 35 (Fig. 5 ). Furthermore, both methods revealed no CD4+ cells present in the epithelial layer.
Fig. 4.
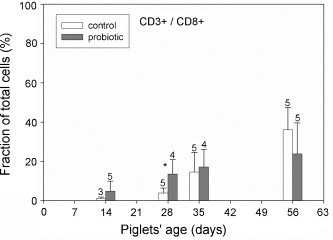
CD8/CD3 double-positive cells in the proximal jejunal epithelium of control animals (open bars) and probiotic animals (filled bars) were analysed by flow cytometry. Percent values were calculated as a fraction of all living cells isolated from the epithelial layer (= 100%). Probiotic animals showed significantly more CD8/CD3 double-positive T cells in the epithelial layer on day 28 (p ≤ 0.05) as indicated by an asterisk. Number of samples for each determination is shown above the error bars. Data were analysed by Welch's t-statistic.
Fig. 5.
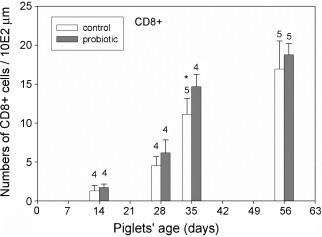
Intraepithelial CD8+ cells counted in tissue sections from control (open bars) and probiotic animals (filled bars) were analysed. The number of positive cells counted microscopically revealed significantly more CD8+ cells in the jejunal epithelium of the probiotic animals at day 35 (p ≤ 0.05) as indicated by an asterisk.
3.2. Lamina propria mucosae
Histological investigations showed that with few exceptions, all animals of both groups developed principally in the same direction. The fraction of CD45+ cells showed an increase between days 14 and 56. Although the number of CD45+ cells counted microscopically in whole tissue sections (epithelium and Lamina propria mucosae) was on average higher in the probiotic group, no significant differences were found between the groups (data not shown). Furthermore, the number of Lamina propria CD4+ cells was similar in both groups (data not shown). However, significant differences between B. cereus var. toyoi-treated piglets and untreated control groups were observed for CD25+ cells and γδ T-lymphocytes in the Lamina propria mucosae. Quantitative analysis revealed significantly more CD25+ lymphocytes (Fig. 6 ) and γδ T cells (Fig. 7 ) in tissue sections of the probiotic piglets on day 35 (p ≤ 0.05). Flow cytometry was not applied for investigation of Lamina propria lymphocytes.
Fig. 6.
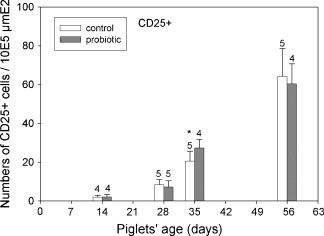
Lamina propria CD25+ cells were counted in tissue sections from the control (open bars) and probiotic animals (filled bars). The number of positive cells counted microscopically revealed significantly more CD25+ cells in the Lamina propria of the probiotic animals at day 35 (p ≤ 0.05) as indicated by an asterisk.
Fig. 7.
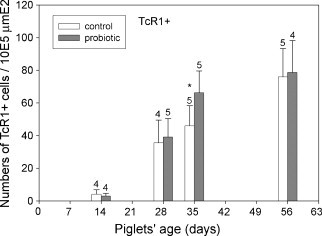
Lamina propria γδ T-cells in tissue sections from the control (open bars) and probiotic animals (filled bars) were counted microscopically. Significantly more γδ T-cells were found in the Lamina propria of the probiotic animals at day 35 (p ≤ 0.05) as indicated by an asterisk.
3.3. Peyer's patches
No differences were observed concerning the populations of CD4, CD8 or double-positive CD4/CD8 cells in the continuous distal Peyer's patches (PPs). Only low numbers of T cells were detected in the continuous ileal PPs of both groups. The average percentages for the various cell populations assessed in this tissue are summarized in Table 1 . The vast majority of cells in PP were CD21+ (complement receptor 2; C3d-receptor), which identifies them as either mature B cells or follicular dendritic cells (Table 1). No differences in the numbers of CD21+ cells were detected between the two experimental groups of piglets.
Table 1.
Mean percentages (arithmetic mean) and standard deviations of CD4+ and CD8+ cells counted in suspension from continuous ileal PPs
| Age (days) | CD21 |
CD4 |
CD8 |
|||
|---|---|---|---|---|---|---|
| Control | Probiotic | Control | Probiotic | Control | Probiotic | |
| 14 | 69.8 ± 11.5 | 45.9 ± 63.7 | 4.6 ± 2.8 | 3.8 ± 5.7 | 6.3 ± 2.5 | 3.5 ± 1.1 |
| 28 | 78.1 ± 21.9 | 42.8 ± 29.6 | 2.9 ± 1.9 | 4.5 ± 3.9 | 5.2. ± 0.6 | 5.5 ± 1.6 |
| 35 | 90.1 ± 3.6 | 82.6 ± 17.6 | 1.7 ± 1.2 | 1.1 ± 0.4 | 2.9 ± 1.2 | 3.1 ± 1.0 |
| 56 | 73.1 ± 15.2 | 84.0 ± 11.8 | 2.7 ± 2.1 | 3.0 ± 1.7 | 10.1 ± 10.9 | 7.0 ± 3.9 |
Cell counts of all animals tested at any time point of the study were combined.
3.4. Frequency of pathogenic E. coli serogroups
Prior to the birth of piglets, the sows chosen at random and assigned to either control or probiotic-supplemented groups were examined for the presence of typical E. coli serotypes associated with diarrhoea in swine herds (Bertschinger and Fairbrother, 1999). As shown in Fig. 8A, similar isolation frequencies of these common, pathogen-associated E. coli serogroups were found in the samples from the sows of both groups. Likewise, piglets of the control group harboured seven of the nine serogroups also found in the sows, consistent with carry-over contamination from sows to piglets. In contrast, despite equal numbers of piglets tested (19 from each group) only two of the nine pathogen-associated serogroups were isolated from the samples of probiotic piglets (Fig. 8B). These results were consistent with the previously reported probiotic effects of other B. cereus strains (Gedek et al., 1993, Alexopoulos et al., 2001).
Fig. 8.
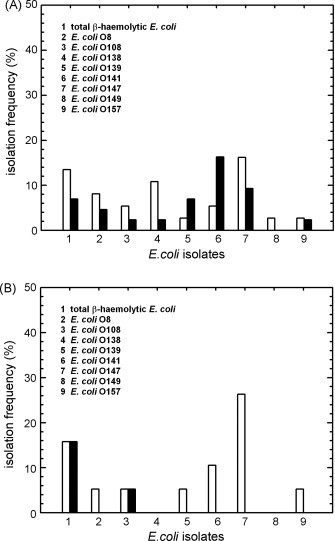
(A) Isolation frequency of total ®-haemolytic E. coli and pathogen-associated E. coli serovars, isolated from feces of control (open bars, n = 37) and probiotic supplemented sows (filled bars, n = 43). (B) Isolation frequency of total ®-haemolytic E. coli and pathogenic E. coli serovars, isolated from the intestinal contents of control (open bars, n = 19) and probiotic supplemented piglets (filled bars, n = 19).
4. Discussion
In this study, we investigated the effects of the probiotic feed supplement, B. cereus var. toyoi (NCIMB 40112; ToyoCerin®), on the immune cell populations associated with the first line of intestinal defence, the epithelial layer. We applied two different methods to determine changes in lymphocyte populations, fluorescence activated cell scanning (FACS), and immunohistochemistry of tissue samples. Both methods yielded consistent results with regard to the age-dependent development of CD45+ cell populations throughout the entire jejunal tissue sections analysed (epithelium and Lamina propria mucosae), and showed an increasing fraction of CD45+ cells.
The protocol used for the purification of intestinal epithelium leukocytes for flow cytometry retains the epithelial cells in the cell suspension. Using this procedure, this allowed us to follow the development of leukocyte numbers relative to epithelial cell numbers rather than only the proportional shifts between different lymphocyte populations. We found between 18% and 24% IELs (CD45+) in the jejunal epithelium of the control group at the age of 28 days. This fraction of leukocytes within the cell suspension is consistent with previous determinations reported by Rothkötter et al. (1994), who measured the portion of CD2+ cells in suspension after EDTA incubation of jejunal tissue samples. In a second study, Rothkötter et al. (1999) reported 15–19% IELs within 1 month old piglet jejunal epithelium in stained tissue sections and found that the number of IELs was lower in specific pathogen free pigs compared to conventional animals. These observations indicate that the proliferation of IELs is influenced by the intestinal flora. The higher portion of CD45+ cells in the epithelial fraction determined by our analysis (up to 60% at the age of 2 months) is probably due to the exclusion of the dead cells from the calculation (stained with propidium iodide). The fraction of dead cells varied between 25% and 35% in the cell preparations (data not shown), with most of them originating from epithelial cells. With both methods of assessing CD45+ cells, no significant differences between control animals and probiotic piglets were observed, although the average number of leukocytes tended to be higher in the probiotic group. There was no or only a limited influence of B. cereus on the morphology of the mucous membrane of the piglets with regard to villus height and crypt depth. Neither the number of goblet cells was significantly affected by the treatment (Reiter et al., 2006), and no symptoms of inflammation were found in the intestinal mucosa (K. Weyrauch, personnel communication).
A differential analysis of lymphocyte populations present in the epithelial layer revealed that certain lymphocyte populations increased relative to epithelial cells when piglets had been fed B. cereus var. toyoi indicating a rise in absolute cell numbers. For example, CD3/CD8 double-positive IELs were significantly higher in the epithelial layer of the probiotic animals at day 28 post partum (Fig. 4). A similar tendency was previously observed for the population of CD8+ T cells in the blood of the probiotic piglets (Altherr, 2005). An increase in mucosal CD8+ cells without signs of intestinal inflammation was also recently reported after the treatment of pigs with E. coli Nissle (Duncker et al., 2006). In addition, the portion of TcR1+ (γδ) T cells tended to be higher in the probiotic group (Fig. 2). These differences were found to be significant in an ANOVA analysis considering the data from the whole sampling period (data not shown). TcR1+ lymphocytes were only rarely detected in the epithelium microscopically, but consistently abundant in the Lamina propria of the jejunal tissue samples. Since the numbers of epithelial γδ T cells determined by flow cytometry amounted to less than 5% of total cells in over a third of all samples, it was not possible to apply statistical data analysis in this instance. However, Lamina propria γδ T-cells were significantly higher in the probiotic animals at the age of 35 days (p ≤ 0.05). The increased numbers of CD3/CD8 double-positive IELs and γδ T cells in the Lamina propria mucosae might be interpreted as an accelerated development of the intestinal immune system. The control animals reached similar numbers of CD8+ IELs and mucosal γδ T cells at the age of 56 days.
In other determinations, we found that a large fraction of the intestinal γδ T-cell population is also CD8+ (40–60% at the age of 1 month, data not shown). This fraction of γδ T-cells merges in the CD8 population, and with the available surface antigen staining we were unable to differentiate between the CD8+ and CD8− γδ T cells in the current animal study. Discrimination between CD8high and CD8low cells is not feasible with IELs because almost all CD8+ cells from the intestinal epithelium show a bright fluorescence. The CD8low population found in blood samples of pigs harbours the CD4/CD8 double-positive subtype of memory helper cells (Yang and Parkhouse, 1996, Zuckermann, 1999). Recently, Denyer et al. (2006) showed that a subset of CD4/CD8 double-positive T-cells also produce perforin and therefore appear to represent cytotoxic T-cells. In our study, CD4/CD8 double-positive T cells were absent in the jejunal epithelium as shown by flow cytometry analysis (data not shown). In blood samples, a considerable fraction of the CD8+ cells is CD3−, and was therefore considered to represent NK cells (Krog et al., 2003, Denyer et al., 2006). The percentage of the latter CD8+ CD3− cells in the IEL fraction of most piglets is below 5%, and no impact of ToyoCerin treatment on this cell population was observed (data not shown). Results obtained in situ with immune histochemical investigations concerning the development of the intraepithelial CD8+ cells were consistent with flow cytometry determinations: CD8+ cells were significantly (p ≤ 0.05) enhanced in the epithelium of probiotic piglets at the age of 35 days (Fig. 5).
Despite the fact that significant differences were only detectable for certain parameters at certain time points we consider the results relevant for two reasons: (1) the p-values were calculated with a conservative statistical test (parameter free Mann–Whitney test), and these results are supported by ANOVA analysis yielding similar results (not shown). (2) As we obtained similar results with both the FACS analysis and immunohistochemistry, a “multiple testing error” would appear highly unlikely. While both methods yielded significant differences, there was a 1 week time-lag between the cytometry and immunohistological data, which we consider to be due to the limitations of the thin sections. However, at the time when the flow data show significant differences (day 28) the tissue data showed tendencies in the same direction. The cell suspensions for flow cytometry originate from a whole cell preparations with full cell surface exposure, whereas the samples for the immunohistology originate from thin sections which do not expose more than one layer of tissue or cells and therefore likely underestimate the presence of the labelled cells.
In humans, CD8+ IELs are regarded as memory cytotoxic T cells or freshly activated cells (Halstensen et al., 1990, Cheroutre, 2005). Bailey et al. (1992) found that 20% of directly isolated porcine IELs expressed CD25, which identifies them as recently activated T cells (Bailey et al., 1992). In earlier studies, CD8+ IELs from pigs were described as cytotoxic T-cells (Wilson et al., 1986). Surprisingly, Denyer et al. (2006) recently reported that 90% of the perforin-producing lymphocytes isolated from the peripheral blood were CD3−. In the present study, only a minor portion of CD8 cells in the jejunal epithelium showed a CD3− phenotype (below 5%). In the absence of further information about the expression status of perforin, we consider the CD8/CD3 double-positive cells in the epithelium to be cytotoxic T-cells. In addition, the CD8/CD3 double-positive cells do not represent a uniform cell population, since the γδ T-cells also partially merge with this cell fraction. It is likely that the CD8/CD3 double-positive cells can be divided into further phenotypically and functionally different populations.
Histological investigations of Lamina propria tissue samples revealed that CD25+ cells and γδ T-cells were significantly enhanced in the probiotic group (compare Fig. 6, Fig. 7). Flow cytometry was not applied to Lamina propria cells in this study because of the necessity for enzymatic digestion of these tissues prior to analysis, a considerable disadvantage compared to the histological method. From the mouse model it is known that CD4+/CD25+ T cells are able to suppress the development of colitis and Th1 cytokine production (Kanai et al., 2006). However, since there were no differences between the groups concerning the number of CD4+ cells, we consider it unlikely that the increased CD25+ cells in the Lamina propria are regulatory T cells (Veltkamp et al., 2006). We therefore suggest the increase in Lamina propria CD25+ cells in the current study is indicative of an immune stimulation (Bailey et al., 1992). Furthermore, the observed increase in CD8+ IELs from day 14 post partum (Fig. 4) is suggestive of a more developed gut-associated immune system of the probiotic group piglets at weaning age or earlier.
Regarding the health performance of the two groups, more piglets were nursed for a longer time in the probiotic group, which resulted in a significantly higher total of nursing days within one litter in the probiotic group (290 day versus 270 day, p = 0.04). Applying the definition of diarrhoea as liquid feces on at least two consecutive days, only 23 piglets (27.4%) from the probiotic group showed such signs of diarrhoea compared to 52 (67.5%) of the control piglets (p < 0.001) (Taras et al., 2005a). These observations might imply that the improved health performance of the treated animals may in part be due to the immune-modulating effect of B. cereus var. toyoi described in this communication. In addition, as noted above, B. cereus var. toyoi affected the intestinal flora of the piglets before weaning, indicating that the administration of the probiotic to the sows is probably an important factor for the subsequent beneficial effects in the piglets. Supportive of this suggestion, it has been shown that piglets take up several grams (about 20 g) of feces per day (Sansom and Gleed, 1982). Indeed, prior to weaning the piglets in this animal trial apparently took up more probiotic bacteria with the feces of the mother sows than with the supplemented feed (Taras et al., 2005a). Consistent with a mean B. cereus var. toyoi concentration of 9.6 (+ 1.9) × 105/g dry matter (DM) in feces of probiotic sows seven days post partum, feces of probiotic piglets collected on day 7 already contained 1.3 (+ 0.3) × 105 probiotic cfu/g DM. Probiotic bacteria were also detected in digesta of piglets on day 14, i.e., before supplemented prestarter feed was offered. The concentration of viable cell counts in the colon ascendens approximately doubled from day 14 (before supplemented feed was offered to the suckling piglets) to day 28 (before piglets were weaned), which means only a slight increase in microbial cell counts. On day 35 (6 days after weaning) the concentration of viable cell counts of B. cereus was already five times higher than that at day 28 (Taras et al., 2005a). Furthermore, the numbers of CD3+ CD8+ cells tended to be higher in the probiotic piglets even before day 28. For these reasons, we suggest that the probiotic feeding of the sows has a greater impact on the immune system of the piglets than the uptake of supplemented feed by the piglets before weaning.
Since the pathogen-associated E. coli serogroups were isolated from the sows at similar frequencies, it might be expected that the pathogen load for both groups of piglets would also be similar (Fig. 8A). However, pathogenic E. coli serogroups were less frequent in the probiotic piglets (Fig. 8B). On the other hand, there was neither a temporal accumulation of these E. coli serovars in the piglets’ feces, nor was the occurrence of pathogenic E. coli serovars clearly correlated with diarrhoea or other symptoms of disease in the piglets. Hence, no direct correlation between the immunological changes and the differences in the E. coli frequency was apparent. Furthermore, high numbers of E. coli harbouring virulence genes and/or with hemolytic activity do not necessarily correlate with disease (Schierack et al., 2006). Nevertheless, we suggest that the occurrence or absence of these pathogen-associated serovars is an indication of the immune status and the health condition of the animals. The E. coli pathovars may be expected to influence the immune system of the animals and vice versa.
A similar observation was made in a prior feeding trial. Piglets fed a probiotic Enterococcus faecium strain also showed a reduction in pathogenic E. coli serogroups (Scharek et al., 2005). Likewise, the rate of carryover infections with Chlamydia was lower in this group of piglets (Pollmann et al., 2005), and the percentages of piglets with post-weaning diarrhoea was also lower in that animal study (Taras et al., 2005a). In the actual animal trial with B. cereus var. toyoi the reduction of post-weaning diarrhoea was even more apparent than in the animal trial with E. faecium (Taras et al., 2005b).
In contrast to a previous animal study with the probiotic Enterococcus faecium SF68 (NCIMB 10415), we found that B. cereus toyoi had a different effect on the development of intraepithelial lymphocytes. Enterococcus faecium is a normal, autochthonous inhabitant of the porcine gut, and therefore may not be expected to stimulate the piglets’ immune system (Scharek et al., 2005). In contrast, B. cereus var. toyoi does not belong to the normal intestinal flora in the pigs, and the intestinal milieu is an unnatural habitat for this spore-forming soil inhabitant. B. cereus apparently sporulates in the intestinal tract after initial germination and shows limited rounds of growth and division cycles in the gut (Taras et al., 2005a, Jadamus et al., 2001). It remains unclear whether spores or metabolically active bacteria are responsible for the observed changes in the intestinal immune system. However, previous reports have indicated that spores can be immunostimulatory and lead to increased expression of certain cell activation markers after oral administration, e.g., CD25 on peripheral-blood lymphocytes (Duc le et al., 2004, Caruso et al., 1993). It therefore seems likely that the observed immune stimulation in the probiotic group of piglets was triggered by microbial antigens. However, as noted above, it has been shown that B. cereus var. toyoi promotes changes in the composition of the gut flora in pigs (Jadamus et al., 2001). In view of the complexity of the microbial flora, it cannot be excluded that B. cereus var. toyoi affects other microbial populations which are ultimately responsible for the observed immune modulation.
Taken together such results indicate that the immune modulating effect of B. cereus var. toyoi correlates with a better health performance of the treated animals. As noted above, all indications suggest that B. cereus var. toyoi started to affect the intestinal flora of the piglets prior to weaning. Therefore, the administration of the probiotic feed to the sows is probably an important factor for the beneficial effects observed in the piglets at later stages.
5. Conclusions
Feed supplementation of B. cereus var. toyoi to sows and piglets was shown to affect the intestinal immune system of the piglets at the time of weaning (age 28 days) and shortly thereafter such that the intestinal epithelial CD8/CD3 double-positive cell populations were enhanced in the probiotic group. In contrast to CD8+ cells in peripheral blood preparations, most CD8+ cells in the jejunal epithelium are T-cells, and CD8+ CD3− Natural Killer cells were rare in the epithelium. Furthermore, CD25+ cell counts were enhanced in the Lamina propria mucosae. This apparent immune modulation was transitory and occurred during the most critical phase of the piglets’ development with respect to intestinal infections. In addition, the frequency of pathogen-associated E. coli serogroups were less frequent in the probiotic-treated piglets. Taking the performance data of Taras et al. (2005a) into consideration, the observed alterations in the immune system shown here indicate an improved health status of the piglets.
Acknowledgements
We would like to thank B. Drewes for help with the immune-histochemistry, and M. Filter and F. Antonelli for statistical analysis. Thanks are also extended to D. Taras for organizing the animal experiments and the personnel of the Institut für Tierernährung who helped to carry out the animal tests. We thank K. Tedin for critical reading and helpful comments on the manuscript. Furthermore we would like to thank A. Lübke for the determination of E. coli serogroup. This study was funded by the Deutsche Forschungsgemeinschaft (DFG), Grant FOR 438.
References
- Alexopoulos C., Karagiannidis A., Kritas S.K., Boscos C., Georgoulakis I.E., Kyriakis S.C. Field evaluation of a bioregulator containing live Bacillus cereus spores on health status and performance of sows and their litters. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2001;48:137–145. doi: 10.1046/j.1439-0442.2001.00342.x. [DOI] [PubMed] [Google Scholar]
- Altherr, B.J.H., 2005. Untersuchungen zum Einfluss von Bacillus cereus var. toyoi auf die zelluläre Immunität des Schweins. Inaugural-Dissertation, Fachbereich Veterinärmedizin, Freie Universität Berlin, Journal-Nr. 2958.
- Bailey M., Stevens K., Bland P.W., Stokes C.R. A monoclonal antibody recognising an epitope associated with pig interleukin-2 receptors. J. Immunol. Methods. 1992;153:85–91. doi: 10.1016/0022-1759(92)90309-h. [DOI] [PubMed] [Google Scholar]
- Bertschinger H.U., Fairbrother J.M. eighth ed. Blackwell Science Ltd.; Oxford: 1999. Diseases of swine. pp. 431–468. [Google Scholar]
- Caruso A., Flamminio G., Folghera S., Peroni L., Foresti I., Balsari A., Turano A. Expression of activation markers on peripheral-blood lymphocytes following oral administration of Bacillus subtilis spores. Int. J. Immunopharmacol. 1993;15:87–92. doi: 10.1016/0192-0561(93)90084-c. [DOI] [PubMed] [Google Scholar]
- Cheroutre H. IELs: Enforcing law and order in the court of the intestinal epithelium. Immunol. Rev. 2005;206:114–131. doi: 10.1111/j.0105-2896.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- de Champs C., Maroncle N., Balestrino D., Rich C., Forestier C. Persistence of colonization of intestinal mucosa by a probiotic strain, Lactobacillus casei subsp. rhamnosus Lcr35, after oral consumption. J. Clin. Microbiol. 2003;41:1270–1273. doi: 10.1128/JCM.41.3.1270-1273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer M.S., Wileman T.E., Stirling C.M.A., Zuber B., Takamatsu H.-H. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of Natural Killer, Cytotoxic T, Natural Killer T and MHC un-retricted cytotoxic T-cells. Vet. Immunol. Immunopathol. 2006;110:279–292. doi: 10.1016/j.vetimm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Duc le H., Hong H.A., Uyen N.Q., Cutting S.M. Intracellular fate and immunogenicity of B. subtilis spores. Vaccine. 2004;22:1873–1885. doi: 10.1016/j.vaccine.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Duncker S.C., Lorentz A., Schroeder B., Breves G., Bischoff S.C. Effect of orally administered probiotic E. coli strain Nissle 1917 on intestinal mucosal immune cells of healthy young pigs. Vet. Immunol. Immunopathol. 2006;111:239–250. doi: 10.1016/j.vetimm.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Gedek B., Kirchgessner M., Wiehler S., Bott A., Eidelsburger U., Roth F.X. Zur nutritiven Wirksamkeit von Bacillus cereus als Probiotikum in der Ferkelaufzucht. Arch. Tierernähr. 1993;44:215–226. doi: 10.1080/17450399309386071. (in German with English abstract) [DOI] [PubMed] [Google Scholar]
- Halstensen T.S., Scott H., Brandtzaeg P. Human CD8+ intraepithelial T lymphocytes are mainly CD45RA-RB+ and show increased co-expression of CD45R0 in celiac disease. Eur. J. Immunol. 1990;20:1825–1830. doi: 10.1002/eji.1830200829. [DOI] [PubMed] [Google Scholar]
- Jadamus A., Vahjen W., Simon O. Growth behaviour of a spore forming probiotic strain in the gastrointestinal tract of broiler chicken and piglets. Arch. Tierernähr. 2001;54:1–17. doi: 10.1080/17450390109381962. [DOI] [PubMed] [Google Scholar]
- Jin L.Z., Marquardt R.R., Zhao X. A strain of Enterococcus faecium (18C23) inhibits adhesion of enterotoxigenic Escherichia coli K88 to porcine small intestine mucus. Appl. Environ. Microbiol. 2000;66:4200–4204. doi: 10.1128/aem.66.10.4200-4204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai T., Tanimoto K., Nemoto Y., Fujii R., Makita S., Totsuka T., Watanabe M. Naturally arising CD4+ CD25+ regulatory T cells suppress the expansion of colitogenic CD4+ CD44highCD62L− effector memory T cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G1051–G1058. doi: 10.1152/ajpgi.00429.2005. [DOI] [PubMed] [Google Scholar]
- Kirchgessner M., Roth F.X., Eidelsburger U., Gedek B. Zur nutritiven Wirksamkeit von Bacillus cereus als Probiotikum in der Ferkelaufzucht.1. Mitteilung-Einfluß auf Wachstumsparameter und gastrointestinales Milieu. Arch. Tierernähr. 1993;44:111–121. doi: 10.1080/17450399309386062. (in German with English abstract) [DOI] [PubMed] [Google Scholar]
- Krog J., Hokland M., Andersen S.K., Tonnesen E. Phenotypic characterisation of porcine counterparts of human NK cell populations—implications for pre-clinical studies. APMIS Suppl. 2003;111:133–139. [PubMed] [Google Scholar]
- Massi M., Vitali B., Federici F., Matteuzzi D., Brigidi P. Identification method based on PCR combined with automated ribotyping for tracking probiotic Lactobacillus strains colonizing the human gut and vagina. J. Appl. Microbiol. 2004;96:777–786. doi: 10.1111/j.1365-2672.2004.02228.x. [DOI] [PubMed] [Google Scholar]
- Moreau M.C., Corthier G. Effect of the gastrointestinal microflora on induction and maintenance of oral tolerance to ovalbumin in C3H/HeJ mice. Infect. Immun. 1988;56:2766–2768. doi: 10.1128/iai.56.10.2766-2768.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann M., Nordhoff M., Pospischil A., Tedin K., Wieler L.H. Effects of a probiotic strain of Enterococcus faecium on the rate of natural chlamydia infection in swine. Infect. Immun. 73. 2005:4346–4353. doi: 10.1128/IAI.73.7.4346-4353.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter K., Eggebrecht S., Drewes B., Riess M., Weyrauch K.D. Effect of Enterococcus faecium and Bacillus cereus var. toyoi on the morphology of the intestinal mucous membrane in piglets. Biologia Bratislava. 2006;6:1–7. [Google Scholar]
- Rothkötter H.-J., Möllhoff S., Pabst R. The influence of age and breeding conditions on the number and proliferation of intraepithelial lymphocytes in pigs. Scand. J. Immunol. 1999;50:31–38. doi: 10.1046/j.1365-3083.1999.00557.x. [DOI] [PubMed] [Google Scholar]
- Rothkötter H.-J., Kirchhoff T., Pabst R. Lymphoid and non-lymphoid cells in the epithelium and Lamina propria of intestinal mucosa of pigs. Gut. 1994;35:1582–1589. doi: 10.1136/gut.35.11.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom B.F., Gleed P.T. The ingestion of sow's faeces by suckling piglets. Br. J. Nutr. 1982;46:451–456. doi: 10.1079/bjn19810053. [DOI] [PubMed] [Google Scholar]
- Scharek L., Guth J., Reiter K., Weyrauch K.D., Tara D., Schwerk P., Schierack P., Schmidt M.F., Wieler L.H., Tedin K. Influence of a probiotic Enterococcus faecium strain on development of the immune system of sows and piglets. Vet. Immunol. Immunopathol. 2005;105:151–161. doi: 10.1016/j.vetimm.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Schierack P., Steinruck H., Kleta S., Vahjen W. Virulence factor gene profiles of Escherichia coli isolates from clinically healthy pigs. Appl. Environ. Microbiol. 2006;72:6680–6686. doi: 10.1128/AEM.02952-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano-Aguilar G.I., Vengroski K.G., Beshah E., Lunney J.K. Isolation and purification of lymphocyte subsets from gut-associated lymphoid tissue in neonatal swine. J. Immunol. Methods. 2000;241:185–199. doi: 10.1016/s0022-1759(00)00209-x. [DOI] [PubMed] [Google Scholar]
- Talham G.L., Jiang H.Q., Bos N.A., Cebra J.J. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect. Immun. 1999;67:1992–2000. doi: 10.1128/iai.67.4.1992-2000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Ishikawa H. Role of intestinal bacterial flora in oral tolerance induction. Histol. Histopathol. 2004;19:907–914. doi: 10.14670/HH-19.907. (Review) [DOI] [PubMed] [Google Scholar]
- Taras D., Vahjen W., Macha M., Simon O. Response of performance characteristics and fecal consistency to long-lasting dietary supplementation with the probiotic strain Bacillus cereus var. toyoi to sows and piglets. Arch. Anim. Nutr. 2005;59:405–417. doi: 10.1080/17450390500353168. [DOI] [PubMed] [Google Scholar]
- Taras D., Vahjen W., Macha M., Simon O. Performance, diarrhoea incidence, and occurrence of Escherichia coli virulence genes during long-term administration of a probiotic Enterococcus faecium strain to sows and piglets. J. Anim. Sci. 2005;84:608–617. doi: 10.2527/2006.843608x. [DOI] [PubMed] [Google Scholar]
- Veltkamp C., Ruhwald R., Giesem T., Autschbach F., Kaden I., Veltkamp R., Sartor R.B., Stremmel W. CD4+ CD25+ cell depletion from the normal CD4+ T cell pool prevents tolerance toward the intestinal flora and leads to chronic colitis in immunodeficient mice. Inflamm. Bowel Dis. 2006;12:437–446. doi: 10.1097/00054725-200606000-00002. [DOI] [PubMed] [Google Scholar]
- Westendorf A.M., Gunzer F., Deppenmeier S., Tapadar D., Hunger J.K., Schmidt M.A., Buer J., Bruder D. Intestinal immunity of Escherichia coli NISSLE 1917: a safe carrier for therapeutic molecules. FEMS Immunol. Med. Microbiol. 2005;43:373–384. doi: 10.1016/j.femsim.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Wilson A.D., Stokes C.R., Bourne F.J. Morphology and functional characteristics of isolated porcine intraepithelial lymphocytes. Immunology. 1986;59:109–113. [PMC free article] [PubMed] [Google Scholar]
- Yang H., Parkhouse R.M. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology. 1996;89:76–83. doi: 10.1046/j.1365-2567.1996.d01-705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F.A. Extrathymic CD4/CD8 double positive T cells. Vet. Immunol. Immunopathol. 1999;72:55–66. doi: 10.1016/s0165-2427(99)00118-x. (Review) [DOI] [PubMed] [Google Scholar]


