Abstract
In this study, we infected NOD/Scid/Jak3null mice engrafted human peripheral blood leukocytes (hu-PBL-NOJ) with measles virus Edmonston B strain (MV-Edm) expressing hepatitis C virus (HCV) envelope proteins (rMV-E1E2) to evaluate the immunogenicity as a vaccine candidate. Although human leukocytes could be isolated from the spleen of mock-infected mice during the 2-weeks experiment, the proportion of engrafted human leukocytes in mice infected with MV (103–105 pfu) or rMV-E1E2 (104 pfu) was decreased. Viral infection of the splenocytes was confirmed by the development of cytopathic effects (CPEs) in co-cultures of splenocytes and B95a cells and verified using RT-PCR. Finally, human antibodies against MV were more frequently observed than E2-specific antibodies in serum from mice infected with a low dose of virus (MV, 100–101 pfu, and rMV-E1E2, 101–102 pfu). These results showed the possibility of hu-PBL-NOJ mice for the evaluation of the immunogenicity of viral proteins.
Keywords: MV, HCV, E1, E2, Human PBL, NOD/Scid/Jak3null mouse
1. Introduction
Hepatitis C virus (HCV) is a member of the Flaviviridae family and is the causative agent of both chronic hepatitis and hepatocellular carcinoma (HCC) [1], [2], [3]. 170 million people are infected with HCV worldwide [4], [5]. Despite prevention efforts and advanced treatment strategies, including combined PEGylated alpha interferron (PEGIFN-α) and ribavirin therapy [6], [7], the clinical efficacy of this treatment is limited [8], [9]. Alternative novel antiviral agents that have been shown to elicit effective responses in chronically infected patients, such as inhibitors of viral protease, helicase, and polymerase, are currently being developed but are expensive [10]. Therefore, the development of an effective vaccine that either induces the production of high-titer, long-lasting, and cross-reactive neutralising antibodies or induces a cellular immune response is important.
Immunological approaches to control HCV infection have proven to be ineffective, in part because HCV adapts to escape from the host immune system [11]. Furthermore, a high percentage of immunocompetent individuals are infected by HCV despite their ability to mount an active immune response [12]. A preventive HCV vaccine is required to protect unexposed individuals from HCV infection. This vaccine will most likely need to target the viral envelope glycoprotein, E1 and E2, and must also be bivalent, safe, and provide long-lasting protective immunity. To address this challenge, we evaluated the immunogenicity of a live-attenuated recombinant vector derived from the pediatric measles virus (MV) that expresses HCV antigens. The MV vaccine is a well-known, live-attenuated vaccine and has proven to be one of the safest, most stable, and effective human vaccines [13]. This vaccine is produced on a large scale in many countries and used at low cost through the Extended Program on Immunisation of the WHO [14], [15]. While this vaccine has been shown to induce life-long immunity with a single dose, boosting is effective. Efforts to develop vaccines using recombinant MV expressing different proteins derived from dengue virus [16], [17], human immunodeficiency virus (HIV) [18], [19], [20], [21], Human papilloma virus (HPV) [22], Severe acute respiratory syndrome (SARS) [23], or West Nile virus (WNV) [24] have been described. We constructed a recombinant MV expressing the E1 and E2 envelope glycoproteins of HCV (rMV-E1E2) [25] and demonstrated that this virus could infect B95a cells and express HCV E1.
HCV research has long been hampered by the lack of an animal model that reproduces HCV infection in humans. The model in which severe combined immunodeficient (SCID) mice are transplanted with human peripheral blood leukocyte (PBL) is a well-established system to study human immunity (hu-PBL-SCID). This mouse develops all human lymphoid cell lineages that repopulate the animal's lymphoid organs. Our group previously generated the non-obese diabetic (NOD)/SCID/Janus kinase 3 (Jak3) knockout (NOJ) mouse model and then established a human hemolymphoid system in this mouse [26], [27]. In this study, we infect human PBL-transplanted NOJ mice with MV and rMV-E1E2 and then characterise the humoral immune responses elicited by the transplanted human cells, in order to evaluate rMV-E1E2 as a vaccine candidate.
2. Materials and methods
2.1. Cells
B95a cells, a marmoset B cell line [28], were used for viral titration and rescue, and were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated foetal calf serum (FCS).
2.2. Plasmid construction and viral rescue
The cDNAs encoding HCV E1 and E2 were obtained from the plasmid HCR6CNS2 [29]. We used replication-competent MV-based vectors (pMV; Edmonston B strain of MV) [25]. The E1 and E2 cDNAs were cloned into the Fse I site of pMV and the resulting clone, pMV-E1E2, was used to rescue the infectious recombinant MV expressing the HCV envelope glycoproteins (rMV-E1E2), as reported previously [30].
2.3. Generation of humanised mice
Mice were reconstituted as described previously [26], [27]. The NOD/SCID/JAK3null strain was established by backcrossing JAK3null and the NOD Cg-PrkdcScid strains for ten generations. All animal experiments were performed according to the guidelines of Institutional Animal Committee or Ethics Committee of Kumamoto University.
2.4. Preparation of human blood leukocytes and transplantation
Peripheral blood leukocytes were isolated from blood donors using Ficoll–Hypaque density gradient centrifugation. A total of 5 × 106 cells were transplanted into the spleen of irradiated (2 Gy) 4-week-old mice.
2.5. MV and MV-E1E2 infection
We injected 100–105 pfu of MV or 100–102 or 104 pfu of MV-E1E2 intraperitoneally for MV and MV-E1E2 infection, respectively. As a negative control, a group of mice was injected with RPMI 1640. Mice were monitored for 2 weeks and then euthanised. The spleens and peripheral blood were collected for analysis.
2.6. Flow cytometry
Isolated splenocytes were stained with APC-Cy7-conjugated anti-mouse CD45 (BD Pharmingen) to detect the murine leukocytes and either APC- or pacific blue- conjugated anti-human CD45 (DAKO) to detect human leukocytes. All data were analysed using FlowJo (Tree Star).
2.7. Confirmation of viral infection
The viral infection of the human leukocytes was confirmed using co-culture with B95a cells followed by RT-PCR. Suspensions of isolated splenocytes were co-cultured with B95a cells and the formation of cytopathic effects (CPEs) was monitored for 2 weeks. Additionally, RNA was isolated from the supernatant of the co-cultures using ISOGEN-LS (Nippon gene) according to manufacturer's instructions. MV RNA was detected using reverse transcript-PCR (RT-PCR) with the sense primer, 5′-ACTCGGTATCACTGCCGAGGATGCAAGGC-3′ (1256–1284) and anti-sense primer 5′-CAGCGTCGTCATCGCTCTCTCC-3′ (2077–2056) or 5′-atggcagaagagcaggcacg-3′ (1807–1826). HCV E1 or E2 was amplified using E1-S-1051 5′-ccgttgctgggtggcactta-3′ and E1-AS-1314 5′-atcatcatgtcccaagccat-3′ or E2-S-1600 5′-ctggcacatcaacaggactg-3′ and E2-AS-1960 5′-aaggagcagcacgtctgtct-3′.
2.8. ELISA
Anti-MV antibody titers were determined by using an ELISA assay. 96-well plates were coated with a 25 μg/ml solution of MV-infected B95a lysate or recombinant E2-expressing baculovirus-infected Sf9 lysate as antigen, respectively. The plates were consecutively incubated with sera (1:100) recovered from hu-PBL-NOJ mice, peroxidase-conjugated rabbit-human IgG (DAKO), and TMB Peroxidase EIA Substrate Kit (Bio-Rad) at 37 °C for 1 h. Optimal density values were measured at 450 nm.
An anti-MV-NP antibody (Millipore, MA, USA) and normal mouse serum (NMS) were used as positive control and negative control respectively.
2.9. Western blot analysis
Total protein extracts from E2-expressing baculovirus-infected Sf9 lysate were separated by SDS-PAGE. The primary antibodies used for Western blots were as follows: sera from mice (1:100) and anti-E2 monoclonal antibody (1:5000). Peroxidase-conjugated secondary antibodies were added and incubated with the mixture for 1 h at room temperature.
3. Results
3.1. Construction of recombinant measles virus expressing E1 and E2
The HCV genes corresponding to the envelope proteins E1 and E2 were sub-cloned in between the N and P genes of the MV vector (Fig. 1A). The HCV E1 and E2 genes included the putative signal peptide sequences at the N terminus and the transmembrane domain at the C terminus [31]. The plasmid vector pMV-E1E2 was introduced with supporting plasmids into 293T cells to rescue the recombinant viruses. The expression of the E1 and E2 proteins by rMV-E1E2 was examined by Western blot (Fig. 1B) and immunofluorescence (Fig. 1C).
Fig. 1.
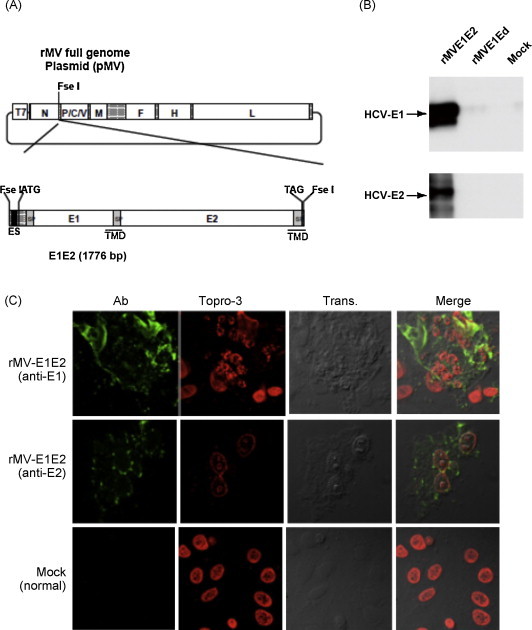
Construction of the recombinant MV vectors. (A) The rMV full genome vector derived from the MV-Ed strain is illustrated in the upper panel and is labelled with letters as follows: N, nucleocapsid; P, phosphoprotein; M, matrix; F, fusion; H, hemagglutinin; and L, large. T7 indicates the T7 RNA polymerase promoter. The cDNA encoding the HCV envelope glycoproteins (E1 and E2) containing the signal peptide sequence (SP) and the transmembrane domain (TMD, underlined) regions, the N gene end signal (E), the P gene start signal (S), and the intercistronic region of the H protein genes at the 5′ end, which was flanked by Fse I sites at both ends, was introduced into the unique Fse I site in between the N and P genes in the pMV vector. The resulting plasmid was designated pMV-E1E2. (B) The HCV E1 and E2 proteins were detected in rMV-E1E2-, rMV-Ed- and mock-infected B95a cells by western blot with MoAb 384 and 544 (arrows). (C) rMV-E1E2-infected B95a cells were stained with MoAb 299 (anti-E1) or MoAb 187 (anti-E2) and analysed by immunofluorescence. Nuclei were stained with Topro-3 and the bright field and merged images are indicated (400×).
3.2. Infection of hu-PBL-NOJ mice with MV and rMV-E1E2
All hu-PBL-NOJ mouse infections were observed for 14 days (Fig. 2A). Infections with MV and rMV-E1E2 were confirmed by first co-culturing the human leukocytes isolated from the spleens of infected mice with B95a cells and then verifying the presence of virus by RT-PCR. In all the MV (103–104 pfu) or rMV-E1E2 (104 pfu)-infected hu-PBL-NOJ mice, CPEs were observed in co-cultures with splenocytes (Table 1 ; Fig. 2B). The results of the co-culture assays are in agreement with results that were obtained by RT-PCR; positive bands were observed in the mice infected with 103–104 pfu of MV and 104 pfu of rMV-E1E2 (Fig. 2C). These results demonstrate that the rescued MV and rMV-E1E2 are able to infect transplanted human PBL.
Fig. 2.
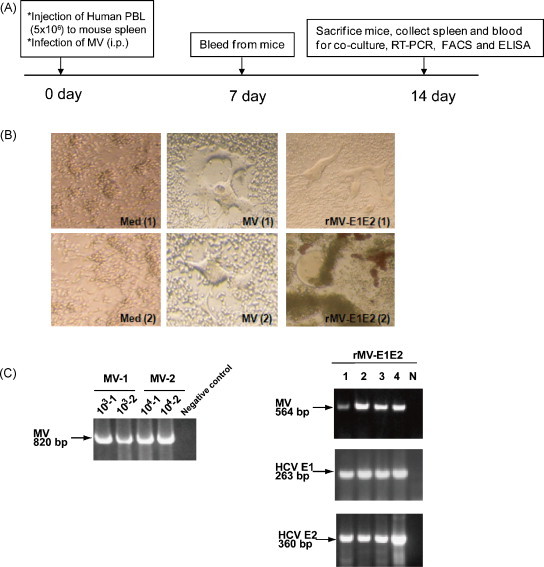
Infection of hu-PBL-NOD/Scid mice with rMV and rMV-E1E2. (A) Course of infection of hu-PBL-NOD/SCID mice with MV and rMV-E1E2. (B) CPE formation in co-cultures of splenocytes isolated from MV- (MV1, 2), rMV-E1E2-, or mock-infected hu-PBL-NOD/SCID and B95a cells (40× magnification). (C) Detection of viral RNA by RT-PCR. Detection of MV in MV-1- or 2-infected mouse splenocyte co-cultures (820 bp) and rMV-E1E2-infected splenocyte co-cultures (564 bp), and HCV E1 (263 bp) and E2 (360 bp) in rMV-E1E2 (104 pfu)-infected splenocyte co-cultures (arrows).
Table 1.
Summary of MV and MV-E1E2 infection of hu-PBL-NOJ mice.
| Virus | Amount of virus (PFU) | No. tested | CPE | RT-PCR |
|---|---|---|---|---|
| Mock | Medium | 7 | 0/7 | 0/7 |
| MV | 100 | 3 | 0/3 | 0/3 |
| 101 | 3 | 0/3 | 0/3 | |
| 102 | 3 | 0/3 | 0/3 | |
| 103 | 2 | 2/2 | 2/2 | |
| 104 | 2 | 2/2 | 2/2 | |
| MV-E1E2 | 100 | 3 | 0/3 | 0/3 |
| 101 | 3 | 0/3 | 0/3 | |
| 102 | 3 | 0/3 | 1/3 | |
| 104 | 4 | 4/4 | 4/4 | |
3.3. Proportion of engrafted human leukocytes in MV- and rMV-E1E2- infected hu-PBL-NOJ mice
We also examined the splenocytes of infected mice simultaneously, using flow cytometry to determine the proportion of human cells in the spleen (Fig. 3 , Table 2 ). In the MV-infected hu-PBL-NOJ mice, a population of human leukocytes was observed in the mice that were infected with 100–101 pfu, whereas few human leukocytes were observed in mice infected with 102–104 pfu. In contrast, in the rMV-E1E2-infected mice, a population of human leukocytes was detected in mice that were inoculated with 100–102 pfu. The ratio of human leukocytes settlement in both groups of mice was inversely correlated with the results from the RT-PCR and co-culture assays (Table 1).
Fig. 3.
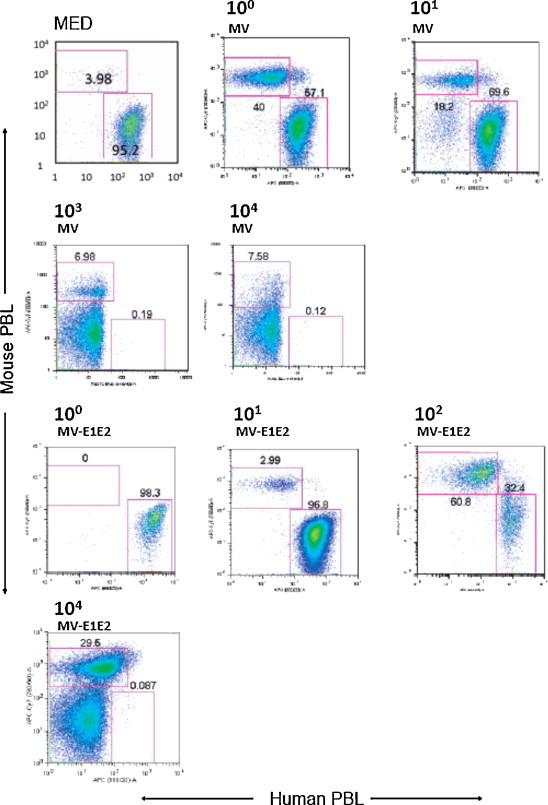
Flow cytometric analysis of splenocytes isolated from hu-PBL-NOJ mice inoculated with medium, MV-Ed (100–104 pfu), or rMV-E1E2 (100–102 or 104 pfu). Splenocytes, consisting of both human and murine cells, were stained with antibodies against human or mouse CD45. Representative flow cytometric profiles of each group of infected mice are shown. The percentages of mouse and human leukocytes are shown.
Table 2.
Proportion of human peripheral leukocytes in the spleen of MV-, rMV-E1E2, or mock-infected hu-PBL-NOJ mice.
| Virus | Amount of virus (PFU) | No. tested | huPBL settlement (average ± S.D.%) |
|---|---|---|---|
| Mock | Medium | 6 | 90.9 ± 13.1 |
| MV | 100 | 3 | 92.7 ± 11.2 |
| 101 | 3 | 58.4 ± 50.6 | |
| 102 | 3 | 55.1 ± 49.9 | |
| 103 | 4 | 4.9 ± 6 | |
| 104 | 2 | 1.7 | |
| MV-E1E2 | 100 | 2 | 79.6 |
| 101 | 2 | 96.0 | |
| 102 | 3 | 56.2 ± 36.2 | |
| 104 | 3 | 0.34 ± 0.4 | |
3.4. Humoral response of MV- and rMV-E1E2-infected hu-PBL-NOJ mice
To examine the immune response against MV and rMV-E1E2 by the transplanted human PBLs, we measured human MV- or HCV-specific antibodies using an ELISA with an MV-infected B95a cell lysate (Fig. 4A) or recombinant HCV E2 protein (Fig. 4B). A significant amount of human antibody against MV antigens was detected in the sera from mice that were infected with MV (100–101 pfu) or rMV-E1E2 (101–102 pfu) (Fig. 4A and B). However, only one mouse, which was infected with 102 pfu of rMV-E1E2, generated human antibodies against HCV E2 (Fig. 5A). The antibody responses in this mouse were confirmed by Western blot analysis (Fig. 5B).
Fig. 4.
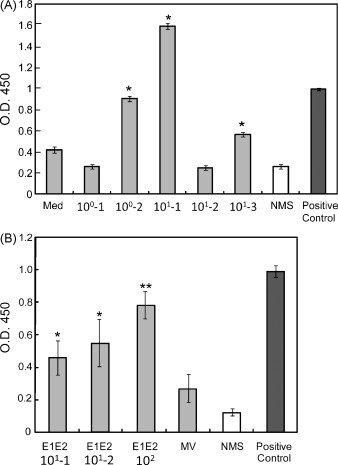
Detection of human MV-specific antibodies in the serum of rMV- or rMV-E1E2-infected mice. (A) Serum (1:100) from MV-infected mice (100–101 pfu) was analysed by ELISA using an MV-infected B95a cell lysate as the target. An anti-MV-NP antibody was used as a positive control and NMS indicates normal mouse serum. The asterisk (*) indicates a significant reaction (p < 0.01) compared to the medium alone control. (B) Serum (1:100) from rMV-E1E2-infected mice (101–102 pfu) was analysed by ELISA. An anti-MV-NP antibody was used as a positive control and NMS indicates normal mouse serum. The double asterisk (**) indicates a highly significant reaction (p < 0.001) compared to NMS and a single asterisk (*) indicates a significant reaction (p < 0.05) compared to NMS.
Fig. 5.
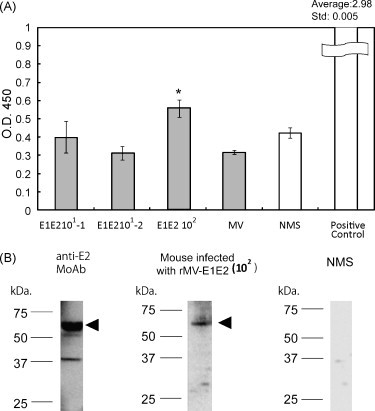
Detection of E2-specific antibodies using ELISA and western blot. (A) Baculovirus-expressed E2 protein was used as the ELISA antigen and serum (diluted 1:100) from rMV-E1E2-infected mice (101–102 pfu) was analysed. Anti-E2 monoclonal antibody (MoAb 544) was used as positive control. The asterisk (*) indicates a significant reaction (p < 0.05) compared to NMS. (B) An anti-E2 monoclonal antibody (MoAb 544), serum from rMV-E1E2 infected mice (1:100), or normal mouse serum (1:100) was used as primary antibodies in a western blot to detect baculovirus-expressed E2 protein. The triangles indicate bands that correspond to HCV E2.
4. Discussion
The development of a vaccine against HCV has relied on several tools, including recombinant proteins and peptides that are derived from HCV antigens [12], [32], [33], [34]. HCV E1 E2 proteins play essential roles in the entry of HCV into host cells. Therefore, these proteins represent ideal targets for neutralising antibodies to block viral entry.
Several studies have used the measles virus as a vector for expression of other viral proteins [16], [17]. In this study, we examined the infectivity of a rescued Edmonston B strain of MV and a recombinant rMV-E1E2 that was constructed using reverse genetics [25], [30]. We demonstrate that these viruses can infect hu-PBL-NOJ mice. This is the first report demonstrating that rescued virus, including a recombinant virus, can infect hu-PBL-NOJ mice. Furthermore, an adequate viral titer could control the generation of antibodies in these mice. Based on the flow cytometry data, most of the human leukocytes disappeared following infection with high virus titer (103–104 pfu) and human antibody was not detected in these mice (data not shown). In contrast, a population of human leukocytes was detected in the mice that were inoculated with a lower dose of virus (100–102 pfu). In addition, we could detect human antibodies in the serum of mice that were infected with a low dose of virus, suggesting that this viral titer range is suitable for the induction of an antibody response that targets rMVs in the hu-PBL-NOJ mouse system. This range of virus concentration is adequate for antibody production and the resulting antibody response might suppress the viral growth of 100–101 pfu MVs in hu-PBL-NOJ mice.
The humanised mouse is a promising model for studying the transmission of the live, attenuated Edmonston B strain of the measles virus. There have been several reports detailing the infection of experimental transgenic mice that expresses human CD46 and CD150 with some strains of measles virus [35], [36]. However, unlike in other animal models, a population of human cells is the target of the virus in this study. Furthermore, the use of hu-PBL-NOJ mice allows one to monitor the immune response that is generated by human leukocytes against the immunogen. Based on our results, hu-PBL-NOJ mice should be a useful tool for studying the immune response during early MV or rMV-E1E2 infection. Since there is no animal model of HCV infection, monitoring the immune response of human leukocytes permits the accurate evaluation of potential vaccine candidates.
We detected a significant amount of MV-specific antibodies in the rMV-E1E2-infected mice (n = 3). However, only one mouse produced E2-specific antibodies and no mice produced E1-specific antibodies. This result could be explained by the hypothesis that the immunogenicity of the E1 and E2, especially E1 protein might be lower than the immunogenicity of the MV proteins, an observation that is consistent with previous studies [37], [38], [39], [40].
Further development of the hu-PBL-NOJ mouse model system will allow us to characterise not only the immediate immune response, but also the long-term evolution of the human immune response against measles virus and recombinant measles viruses. This system will make it possible to evaluate the immunogenicity of potential vaccine targets using human PBLs, which is indispensable for the development of an effective vaccine of HCV.
Acknowledgements
We would like to thank Drs. M.A. Billeter and K. Takeuchi for providing the MV Edmonston B strain rescue system, F. Ikeda and M. Yoneda for their technical support. This work was supported by grants from the Ministry of Health and Welfare of Japan, the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, and the Cooperative Research Project on Clinical and Epidemiological Studies of Emerging and Re-emerging Infectious Diseases.
References
- 1.Di Bisceglie A.M., Carithers R.L., Jr., Gores G.J. Hepatocellular carcinoma. Hepatology. 1998;28(4):1161–1165. doi: 10.1002/hep.510280436. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi J. Hepatitis C virus and hepatocarcinogenesis. Intervirology. 1999;42(2–3):205–210. doi: 10.1159/000024962. [DOI] [PubMed] [Google Scholar]
- 3.Michielsen P.P., Francque S.M., van Dongen J.L. Viral hepatitis and hepatocellular carcinoma. World J Surg Oncol. 2005;3:27. doi: 10.1186/1477-7819-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat, 1999;6(1):35–47. [PubMed]
- 5.Zoulim F. Clinical consequences of hepatitis C virus infection. Rev Med Virol. 2003;13(1):57–68. doi: 10.1002/rmv.371. [DOI] [PubMed] [Google Scholar]
- 6.Bruchfeld A. Ribavirin treatment in dialysis patients with chronic hepatitis C virus infection—a pilot study. J Viral Hepat. 2001;8(4):287–292. doi: 10.1046/j.1365-2893.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 7.Mazzella G. Alpha interferon treatment may prevent hepatocellular carcinoma in HCV-related liver cirrhosis. J Hepatol. 1996;24(2):141–147. doi: 10.1016/s0168-8278(96)80022-5. [DOI] [PubMed] [Google Scholar]
- 8.Kohara M. Hepatitis C virus genotypes 1 and 2 respond to interferon-alpha with different virologic kinetics. J Infect Dis. 1995;172(4):934–938. doi: 10.1093/infdis/172.4.934. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura H. Interferon treatment for patients with chronic hepatitis C infected with high viral load of genotype 2 virus. Hepatogastroenterology. 2002;49(47):1373–1376. [PubMed] [Google Scholar]
- 10.Bukh J. Studies of hepatitis C virus in chimpanzees and their importance for vaccine development. Intervirology. 2001;44(2–3):132–142. doi: 10.1159/000050040. [DOI] [PubMed] [Google Scholar]
- 11.Bowen D.G., Walker C.M. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J Exp Med. 2005;201(11):1709–1714. doi: 10.1084/jem.20050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lechmann M., Liang T.J. Vaccine development for hepatitis C. Semin Liver Dis. 2000;20(2):211–226. doi: 10.1055/s-2000-9947. [DOI] [PubMed] [Google Scholar]
- 13.Combredet C. A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J Virol. 2003;77(21):11546–11554. doi: 10.1128/JVI.77.21.11546-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naniche D. Decrease in measles virus-specific CD4 T cell memory in vaccinated subjects. J Infect Dis. 2004;190(8):1387–1395. doi: 10.1086/424571. [DOI] [PubMed] [Google Scholar]
- 15.Ovsyannikova I.G. Frequency of measles virus-specific CD4+ and CD8+ T cells in subjects seronegative or highly seropositive for measles vaccine. Clin Diagn Lab Immunol. 2003;10(3):411–416. doi: 10.1128/CDLI.10.3.411-416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandler S. Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus. PLoS Negl Trop Dis. 2007;1(3):e96. doi: 10.1371/journal.pntd.0000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandler S., Tangy F. Recombinant vector derived from live attenuated measles virus: potential for flavivirus vaccines. Comp Immunol Microbiol Infect Dis. 2008;31(2–3):271–291. doi: 10.1016/j.cimid.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Guerbois M. Live attenuated measles vaccine expressing HIV-1 Gag virus like particles covered with gp160DeltaV1V2 is strongly immunogenic. Virology. 2009;388(1):191–203. doi: 10.1016/j.virol.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 19.Liniger M. Recombinant measles viruses expressing single or multiple antigens of human immunodeficiency virus (HIV-1) induce cellular and humoral immune responses. Vaccine. 2009;27(25–26):3299–3305. doi: 10.1016/j.vaccine.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorin C. A recombinant live attenuated measles vaccine vector primes effective HLA-A0201-restricted cytotoxic T lymphocytes and broadly neutralizing antibodies against HIV-1 conserved epitopes. Vaccine. 2005;23(36):4463–4472. doi: 10.1016/j.vaccine.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 21.Lorin C. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J Virol. 2004;78(1):146–157. doi: 10.1128/JVI.78.1.146-157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cantarella G. Recombinant measles virus-HPV vaccine candidates for prevention of cervical carcinoma. Vaccine. 2009;27(25–26):3385–3390. doi: 10.1016/j.vaccine.2009.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liniger M. Induction of neutralising antibodies and cellular immune responses against SARS coronavirus by recombinant measles viruses. Vaccine. 2008;26(17):2164–2174. doi: 10.1016/j.vaccine.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Despres P. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J Infect Dis. 2005;191(2):207–214. doi: 10.1086/426824. [DOI] [PubMed] [Google Scholar]
- 25.Radecke F. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14(23):5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada S. Early development of human hematopoietic and acquired immune systems in new born NOD/Scid/Jak3null mice intrahepatic engrafted with cord blood-derived CD34+ cells. Int J Hematol. 2008;88(5):476–482. doi: 10.1007/s12185-008-0215-z. [DOI] [PubMed] [Google Scholar]
- 27.Hattori S. Potent activity of a nucleoside reverse transcriptase inhibitor, 4’-ethynyl-2-fluoro-2’-deoxyadenosine, against human immunodeficiency virus type 1 infection in a model using human peripheral blood mononuclear cell-transplanted NOD/SCID Janus kinase 3 knockout mice. Antimicrob Agents Chemother. 2009;53(9):3887–3893. doi: 10.1128/AAC.00270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobune F., Sakata H., Sugiura A. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J Virol. 1990;64(2):700–705. doi: 10.1128/jvi.64.2.700-705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsukiyama-Kohara K. Activation of the CKI-CDK-Rb-E2F pathway in full genome hepatitis C virus-expressing cells. J Biol Chem. 2004;279(15):14531–14541. doi: 10.1074/jbc.M312822200. [DOI] [PubMed] [Google Scholar]
- 30.Yoneda M. Rinderpest virus phosphoprotein gene is a major determinant of species-specific pathogenicity. J Virol. 2004;78(12):6676–6681. doi: 10.1128/JVI.78.12.6676-6681.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Op De Beeck A., Cocquerel L., Dubuisson J. Biogenesis of hepatitis C virus envelope glycoproteins. J Gen Virol. 2001;82(Pt 11):2589–2595. doi: 10.1099/0022-1317-82-11-2589. [DOI] [PubMed] [Google Scholar]
- 32.Beyene A. Hepatitis C virus envelope glycoproteins and potential for vaccine development. Vox Sang. 2002;83(Suppl 1):27–32. doi: 10.1111/j.1423-0410.2002.tb05262.x. [DOI] [PubMed] [Google Scholar]
- 33.Seong Y.R. Immunogenicity of the E1E2 proteins of hepatitis C virus expressed by recombinant adenoviruses. Vaccine. 2001;19(20–22):2955–2964. doi: 10.1016/s0264-410x(00)00534-x. [DOI] [PubMed] [Google Scholar]
- 34.Stamataki Z. Hepatitis C virus envelope glycoprotein immunization of rodents elicits cross-reactive neutralizing antibodies. Vaccine. 2007;25(45):7773–7784. doi: 10.1016/j.vaccine.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 35.Ohno S. Measles virus infection of SLAM (CD150) knockin mice reproduces tropism and immunosuppression in human infection. J Virol. 2007;81(4):1650–1659. doi: 10.1128/JVI.02134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sellin C.I. High pathogenicity of wild-type measles virus infection in CD150 (SLAM) transgenic mice. J Virol. 2006;80(13):6420–6429. doi: 10.1128/JVI.00209-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falkowska E. Hepatitis C virus envelope glycoprotein E2 glycans modulate entry, CD81 binding, and neutralization. J Virol. 2007;81(15):8072–8079. doi: 10.1128/JVI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helle F. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J Virol. 2007;81(15):8101–8111. doi: 10.1128/JVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson P. Reactivity of synthetic peptides representing selected sections of hepatitis C virus core and envelope proteins with a panel of hepatitis C virus-seropositive human plasma. J Med Virol. 1997;51(1):67–79. doi: 10.1002/(sici)1096-9071(199701)51:1<67::aid-jmv11>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 40.Hoofnagle J.H. Course and outcome of hepatitis C. Hepatology. 2002;36(5 Suppl 1):S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]


