Abstract
Canine non-rabies combined vaccines are widely used to protect animals from infectious agents, and also play an important role in public health. We performed a large-scale survey to investigate vaccine-associated adverse events (VAAEs), including anaphylaxis, in Japan by distributing questionnaires on VAAEs to veterinary hospitals from April 1, 2006 through May 31, 2007. Valid responses were obtained for 57,300 vaccinated dogs at 573 animal hospitals; we obtained VAAEs information for last 100 vaccinated dogs in each veterinary hospital. We found that of the 57,300, 359 dogs showed VAAEs. Of the 359 dogs, death was observed in 1, anaphylaxis in 41, dermatological signs in 244, gastrointestinal signs in 160, and other signs in 106. Onset of VAAEs was mostly observed within 12 h after vaccination (n = 299, 83.3%). In this study, anaphylaxis events occurred within 60 min after vaccination, and about half of these events occurred within 5 min (n = 19, 46.3%). Furthermore, where anaphylaxis was reported, additional information to support the diagnosis was obtained by reinvestigation. Our resurvey of dogs with anaphylaxis yielded responses on 31 dogs; 27 of these demonstrated collapse (87.1%), 24 demonstrated cyanosis (77.4%), and both signs occurred in 22 (71.0%). Higher rates of animal VAAEs, anaphylaxis, and death were found in Japan than in other countries. Further investigations, including survey studies, will be necessary to elucidate the interaction between death and vaccination and the risk factors for VAAEs, and thus develop safer vaccines. Moreover, it may also be necessary to continually update the data of VAAEs.
Keywords: Canine non-rabies combined vaccine, Adverse event, Anaphylaxis
1. Introduction
Canine non-rabies combined vaccines, containing zoonotic pathogen leptospira, are widely used in small animal veterinary medicine. Vaccination is aimed at protecting animals from infectious agents and plays an important role in public health. No vaccine, however, is completely effective or without adverse reactions, and vaccine-associated adverse events (VAAEs) do occur, albeit infrequently, after vaccinations. Epidemiological surveys in the United Kingdom (Gaskell et al., 2002) and the United States (Moore et al., 2005) identified the rates for VAAEs as 0.093 and 38.2/10,000 vaccinated dogs, respectively.
VAAEs can include anaphylaxis, which is an acute multi-system, severe type I hypersensitivity reaction (Sakaguchi et al., 1995, Roth, 1999) occasionally observed in humans and dogs after vaccination, and which sometimes causes death (Brooks, 1991, Sakaguchi et al., 2000). According to large epidemiological studies on canine VAAEs, the incidences of anaphylaxis were 0.018/10,000 vaccinated dogs in the United Kingdom (Gaskell et al., 2002) and 0.65/10,000 vaccinated dogs in the United States (Moore et al., 2005). Based on a survey of a small number (35) of Japanese veterinary hospitals, the anaphylaxis rate in Japan appeared to be somewhat higher, at 0.17% (6/3477 vaccinated dogs) (Fujimura, 2006). However, to date, no large-scale investigation had been carried out on VAAEs associated with canine non-rabies combined vaccine in Japan. Moreover, although incidences of anaphylaxis were provided, none of the previous studies (Gaskell et al., 2002, Moore et al., 2005, Fujimura, 2006) considered important details, such as concurrent symptomatic states.
Here, we report the results of a large-scale survey of VAAEs in Japan, based on diagnosis made by experienced veterinarians in their practices. Our results reveal useful information on VAAEs, including critical detail on anaphylaxis and death caused by canine non-rabies combined vaccines. It is useful to record vaccine reactions routinely, and report VAAEs to specific agents such as local governments in veterinary medicine as well as human medicine.
2. Materials and methods
2.1. Experimental design and questionnaire for study of canine vaccine associated adverse events
The questionnaires about adverse reactions to canine non-rabies combined vaccines were distributed by the Japan Small Animal Veterinary Association to veterinary hospitals in Japan from April 1, 2006 through May 31, 2007. The questionnaires not only recorded standard information: date of birth, breed, sex and neuter status, weight, and date of vaccination, but also included important factors associated with adverse reactions, such as type of vaccination, signs, and time of their onset since vaccination (Fig. 1 ). Practicing veterinarians diagnosed adverse reactions and classified them into 5 groups according to clinical signs: death, anaphylaxis, dermatological signs (swelling of face, pruritus, urticaria, flush, and erythema), gastro-intestinal signs (vomiting and diarrhea), and other signs (including hypodynamia and anorexia). In cases of anaphylaxis, additional information was obtained by reinvestigation to support the diagnoses. To define the population, each responding veterinarian also stated the number of affected cases per last 100 vaccinated dogs.
Fig. 1.
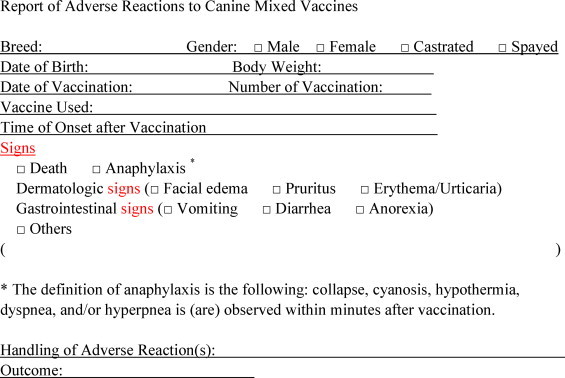
The questionnaire form used for the investigation of vaccine-associated adverse events (VAAEs). The questionnaire was distributed by the Japan Small Animal Veterinary Association to veterinary hospitals in Japan from April 1, 2006 through May 31, 2007.
2.2. Vaccines
The following non-rabies combined vaccines for dogs are commonly used in Japan: combined live vaccines composed of canine parvovirus, canine distemper virus, canine adenovirus type 2, and canine parainfluenza virus (group 1); adding live or inactivated coronavirus to group 1 (group 2); adding inactivated leptospira to group 1 (group 3); adding inactivated coronavirus and leptospira to group 1 (group 4); adding live coronavirus and inactivated leptospira to group 1 (group 5). Duramune MX5 (Kyoritsu Seiyaku Corporation, Tokyo, Japan; Fort Dodge Animal Health, Iowa, USA), Eurican 5 (Merial Animal Health, Lyon, France), and Nobivac DHPPi (Intervet, Boxmeer, Netherlands) were classified as group 1; Canine-6II (Kyoto Biken Laboratories, Kyoto, Japan), Duramune DX6, and Vanguard Plus 5/CV (Pfizer Animal Health, New York, USA) were classified as group 2; Canine-8, Eurican 7, and Nobivac DHPPi+L as group 3; Duramune DX8 and Vanguard Plus 5/CV-L as group 4; and Canine-9 (II) as group 5.
2.3. Statistical analyses
Sex and neuter status were analyzed as categorical data. Continuous variables of age and weight were converted to categorical variables because nonlinear trends were detected in the model-fitting process. Dogs were grouped on the basis of age at date of vaccination as follows: 2–9 months, 9 months to 1.5 years, 1.5–2.5 years, 2.5–3.5 years, 3.5–5.5 years, 5.5–8.5 years, and >8.5 years. Weight was converted from continuous to categorical data of 0–5 kg, 5–10 kg, and > 10 kg. The P values for anaphylaxis in total VAAEs were evaluated using a multivariate unconditional logistic regression model. The variants included sex and neuter status, weight, age, and vaccine group. The model was assessed for significance by use of the le Cessie–van Houwelingen test. A value of P < 0.05 was considered significant. Statistical analysis was performed with R version 2.11.1 (www.r-project.org/).
3. Results
3.1. Total adverse reactions
Valid responses were obtained for 57,300 vaccinated dogs, from 573 animal hospitals; of these, 359 dogs were diagnosed with VAAE (62.7/10,000 vaccinated dogs). Unfortunately, the detailed information regarding dogs with no adverse events could not be obtained in the present study. Of the 359 dogs, anaphylaxis was observed in 41, dermatological signs in 244, gastrointestinal signs in 160, and other signs in 106 (Table 1 ). A single death (0.2/10,000 vaccinated dogs) was reported within a few days after vaccination.
Table 1.
Number and incidence of clinical signs of vaccine-associated adverse events (VAAEs) in 57,300 vaccinated dogs.
| Clinical signs | Numbers of VAAEsa (per 10,000 dogs) |
|---|---|
| Anaphylaxis | 41 (7.2) |
| Death | 1 (0.2) |
| Dermatological signs | 244 (42.6) |
| Gastrointestinal signs | 160 (27.9) |
| Others | 106 (18.5) |
Total number was over 359 because multiple signs were present in some dogs.
In decreasing order of frequency, 181 (50.4%) Miniature Dachshunds, 37 (10.3%) Chihuahuas, 18 (5.0%) Mixed-breeds, and 17 (4.7%) Toy Poodles were reported to have VAAEs in the present survey; other breeds were involved less frequently. The population with VAAEs included 145 (40.4%) sexually intact males, 156 (43.5%) sexually intact females, 24 (6.7%) castrated males, 24 (6.7%) spayed females, and 10 (2.8%) unknown. Almost half these dogs (n = 164, 45.7%) were between the ages of 2 and 9 months. Dogs in the weight category of 0–5 kg had the highest frequency of adverse reactions (n = 249, 69.4%). Most adverse events (n = 299, 83.3%) were observed within 12 h after vaccination (Fig. 2 ).
Fig. 2.
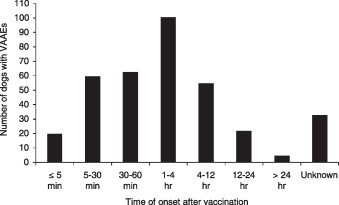
Numbers of dogs with vaccine-associated adverse events (VAAEs), grouped by time of onset of signs. A total of 359 dogs exhibited VAAEs, including one death. Signs manifested within 12 h of vaccination in 299 cases.
3.2. Anaphylaxis and death
More detailed information about vaccine-associated anaphylaxis is provided in Table 2, Table 3 , as well as Fig. 3 . Miniature Dachshunds (n = 13) accounted for approximately 30% of the anaphylaxis cases. Anaphylaxis occurred in four Miniature Schnauzers out of a population of the eight with VAAEs. Of 41 dogs diagnosed with anaphylaxis, 17 (41.5%) were sexually intact males, 18 (43.9%) were intact females, 3 (7.3%) were castrated males, and 3 (7.3%) were spayed females. According to age and weight, the greatest frequency of anaphylaxis was recorded among dogs aged 2–9 months (n = 20, 48.8%) and among dogs weighting less than 5 kg (n = 27, 65.9%), respectively. A multivariate logistic regression model, including sex and neuter status, weight, and age satisfied requirements for goodness of fit (P = 0.12). In the final model, there was no significant relationship between anaphylaxis and the factors of sex and neuter status (P = 0.58), weight (P = 0.15), age (P = 0.24), or vaccine group (P = 0.96). All cases of anaphylaxis occurred within 60 min after vaccinations, and about half occurred within 5 min (n = 19, 46.3%) (Fig. 3).
Table 2.
Data obtained for 41 cases, where anaphylaxis followed vaccination.
| Breeda | Sexb | Age (years) | Weight (kg) | Onset time (min) | Diagnostic reasonc |
|---|---|---|---|---|---|
| Beagle | M | 3.4 | 8.0 | 10 | ND |
| C.K.C. Spaniel | F | 0.2 | 2.2 | 1 | Collapse, Cyanosis, Hyperpnea |
| Chihuahua | F | 0.2 | 0.8 | 3 | ND |
| J.R. Terrier | M | 1.8 | 4.4 | 2 | Collapse, Cyanosis |
| M. Dachshund | F | 0.2 | 1.7 | 5 | Collapse, Cyanosis, Hyperpnea |
| F | 0.2 | 1.7 | 10 | Collapse, Cyanosis | |
| F | 0.2 | 2.4 | 5 | Collapse, Cyanosis | |
| F | 0.2 | 2.6 | 10 | Collapse, Cyanosis | |
| F | 0.3 | 1.5 | 5 | ND | |
| M | 0.4 | 1.7 | 30–40 | Collapse, Cyanosis | |
| S | 1.4 | 4.9 | 15 | ND | |
| M | 2.1 | 4.4 | 30 | Cyanosis | |
| C | 3.3 | 5.6 | 30 | Collapse, Cyanosis, Hypothermia | |
| S | 3.5 | 6.8 | 40 | Collapse, Cyanosis | |
| M | 4.7 | 5.6 | <5 | Collapse, Cyanosis | |
| C | 5.3 | 4.4 | 30 | Collapse, Cyanosis | |
| M | 6.8 | 4.6 | 15 | ND | |
| M. Schnauzer | M | 0.2 | 0.6 | 1 | Collapse, Hyperpnea |
| M | 0.4 | 3.7 | 15 | Collapse, Cyanosis | |
| F | 2.2 | 6.2 | 10 | ND | |
| F | 2.4 | 9.5 | 10 | Collapse, Cyanosis, Hypothermia, Dyspnea | |
| Mix | M | 0.2 | 2.0 | 10 | Collapse, Cyanosis |
| M | 4.2 | 11.0 | <10 | ND | |
| F | 7.1 | 3.7 | 15 | ND | |
| Newfoundland | M | 0.3 | 16.1 | 11 | Collapse, Cyanosis |
| Pekingese | M | 2.6 | 5.9 | 1 | ND |
| Pomeranian | M | 0.3 | 1.0 | 15 | Collapse |
| Pug | F | 3.0 | 7.2 | 5 | Collapse, Cyanosis |
| Shiba | M | 0.2 | 10.5 | 10 | Collapse, Cyanosis |
| F | NA | 9.0 | 5 | Collapse, Cyanosis | |
| S. Sheepdog | F | 9.6 | 11.5 | 5 | Collapse, Cyanosis |
| Shih Tzu | F | 0.2 | 1.6 | <5 | ND |
| M | 0.3 | 1.9 | 1 | Collapse, Hyperpnea | |
| F | 0.3 | 2.2 | 60 | Cyanosis, Hyperpnea | |
| Toy Poodle | F | 0.2 | 1.3 | <5 | Collapse |
| F | 0.2 | 2.4 | <5 | Collapse, Cyanosis | |
| S | 1.4 | 2.5 | 5–10 | Collapse, Cyanosis, Hyperpnea | |
| M | 1.4 | 2.6 | 5 | Collapse, Cyanosis | |
| M | 13.3 | 3.8 | 5 | ND | |
| Welsh Corgi | F | 0.2 | 1.7 | 1 | Collapse |
| C | 1.3 | 14.8 | 60 | ND |
ND: no data.
C.K.C. Spaniel: Cavalier King Charles Spaniel, J.R. Terrier: Jack Russell Terrier, M. Dachshund; Miniature Dachshund, M. Schnauzer: Miniature Schnauzer, S. Sheepdog: Shetland Sheepdog.
M: sexually intact male, F: sexually intact female, C: castrated male, S: spayed female.
Diagnostic reasons were obtained from reinvestigation.
Table 3.
Number of signs exhibited by anaphylactic dogs (n = 31).
| Anaphylactic signs | na | % |
|---|---|---|
| Collapse | 27 | 87.1 |
| Cyanosis | 24 | 77.4 |
| Hyperpnea | 6 | 19.4 |
| Hypothermia | 2 | 6.5 |
| Dyspnea | 1 | 3.2 |
Total number was over 31 because multiple signs were present in some dogs.
Fig. 3.
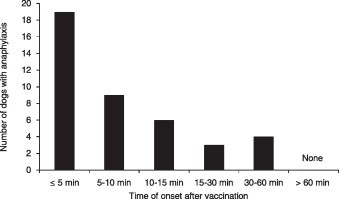
Numbers of dogs with anaphylaxis, grouped by time of onset. A total of 41 dogs exhibited anaphylaxis signs; anaphylaxis set in within 60 min of vaccination, and within 5 min for 19 cases.
When we attempted to resurvey the 41 dogs that experienced anaphylaxis, responses were received for 31 of these dogs (recovery ratio = 75.6%). As shown in Table 3, collapse and cyanosis were observed in almost all 31 of the dogs, with 27 (87.1%) demonstrating collapse, 24 (77.4%) demonstrating cyanosis, and 22 (71.0%) showing both signs. A single death (Chihuahua, intact female, 2 months, 0.9 kg), occurring 38 h after vaccination, was reported in our questionnaire.
4. Discussion
The risks involved in vaccination of dogs were highlighted by previous large epidemiological studies (Gaskell et al., 2002, Moore et al., 2005); however, surveys on VAAEs had only been performed on a small scale in Japan (Ohmori et al., 2002, Ohmori et al., 2005a, Fujimura, 2006). Here, we present the results of a large-scale investigation of VAAEs at Japanese veterinary hospitals. We found a VAAE rate of 62.7/10,000 vaccinated dogs, which is much higher than the rates reported in the United Kingdom (0.093/10,000 vaccinated dogs) (Gaskell et al., 2002) and the United States (38.2/10,000 vaccinated dogs) (Moore et al., 2005). Previous reports suggested that small breed dogs (<10 kg), especially Dachshunds, were more prone to VAAEs than larger dogs (Gaskell et al., 2002, Moore et al., 2005, Moore and Hogenesch, 2010). According to the Japan Kennel Club (http://www.jkc.or.jp), Miniature Dachshund, Chihuahua, and Toy Poodle are the most popular breeds in this country, and account for just over half of the total registrations in the Club (50.3%). This popular breed bias and the proposed breed susceptibility may contribute to higher VAAEs rate in Japan than in other countries.
Factors known to cause vaccine reactions include the primary vaccine agent or antigen, adjuvants, preservatives, stabilizers, and residues from tissue culture used in vaccine production (Hogenesch et al., 1999, Roth, 1999, Georgitis and Fasano, 2001). Our previous study reported large amounts of bovine serum albumin (BSA) and bovine IgG contents in canine vaccines (Ohmori et al., 2005b). Furthermore, we found IgE reactivity against fetal calf serum (FCS) components in dogs with allergic reactions after vaccination, suggesting that most of these reactions might be caused by FCS components derived from the culture media used to produce vaccines (Ohmori et al., 2005b, Ohmori et al., 2007). Many vaccines that are commonly used in Japan are imported from abroad, although a few are made in Japan. Multilateral studies are necessary to clarify the relationship between VAAEs and vaccine by-products, and to ascertain whether certain vaccines are more likely to give rise to VAAEs.
Among the many clinical signs of adverse reactions to vaccines, anaphylaxis is the most dramatic (Roth, 1999). In the present investigation, the incidence of anaphylaxis (7.2/10,000 vaccinated dogs) in dogs was remarkably higher than those reported in previous studies (Gaskell et al., 2002, Moore et al., 2005, Moore and Hogenesch, 2010). The dogs showing anaphylaxis in the present study included many small breeds such as Miniature Dachshund and Miniature Schnauzer. This implies that a breed predisposition may play a key role in anaphylaxis after vaccination of dogs, just as in total VAAEs, and that genetic factors may be involved in these clinical signs.
Moore et al. (2005) have indicated that the risk of VAAEs was significantly increased for small and neutered dogs, highest for dogs approximately 1–3 years old, and least for dogs ≥6 years of age. Unfortunately, as the information regarding dogs with no adverse events could not be obtained in the present study, the odds ratios of VAAEs could not be estimated; however, we did estimate the P values for anaphylaxis according to adverse reactions after vaccine administrations using a logistic regression model. Additionally, we were not able to analyze the relationship between breed and anaphylaxis due to small sample numbers for many breeds. However, no significant differences were observed in the anaphylaxis risk by sex and neuter status, weight, and age; thus, our data seem to show the same tendency of potential risk factors for VAAEs in the results of Moore et al. (2005). The results of the present study suggest that various factors such as weight and age could predict the potential risk of adverse reactions, including anaphylaxis after vaccination, which may allow practicing veterinarians to allay the anxiety of at least a few owners regarding vaccination.
As only a single death was reported in this investigation, a direct association between death and vaccine administration was not clear; however, as an incidence of 0.02/10,000 vaccinated dogs was reported in the United State (Moore et al., 2005), the mortality rate also appeared to be increased in Japan. This case did not show anaphylactic signs, although other clinical sign was observed. Further extensive surveys are needed to uncover the predictive factors and clinical signs that precede such outcomes, in order to prevent such unfortunate events. Moreover, to clarify the interaction between death and vaccination, comparison with randomized unvaccinated dogs may be effective.
5. Conclusions
We present data regarding VAAEs, and in particular, on anaphylaxis, based on a large-scale study in Japanese veterinary hospitals. Higher rates of VAAEs, anaphylaxis, and death were observed in Japan compared to other countries. Further international studies aimed at obtaining similar information will be necessary to elucidate the risk factors, including breed predisposition, the quality of vaccine products, and prior sensitization with variable allergens, for VAAEs, with a view to developing safer vaccines. In addition, as our study was not current, it may be also be necessary to continually update the data of VAAEs.
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgments
The authors would like to thank all staff of participating veterinary hospitals for their kind assistance in responding to the questionnaires. We also thank the Japan Small Animal Veterinary Association for its support. We thank Dr. Sakae Inouye in Otsuma Women's University for critical review of this manuscript. This research was partially supported by the Promotion and Mutual Aid Corporation for Private Schools of Japan, Grant-in-Aid for Matching Fund Subsidy for Private University, and by a Project Grant awarded by the Azabu University Research Services Division.
References
- Brooks R. Adverse reactions to canine and feline vaccines. Aust. Vet. J. 1991;68:342–344. doi: 10.1111/j.1751-0813.1991.tb03096.x. [DOI] [PubMed] [Google Scholar]
- Fujimura M. A survey on the adverse reactions to combined vaccines in a small group of veterinary hospitals (35 hospitals) J. Jap. Vet. Med. Assoc. 2006;46:17–21. (in Japanese) [Google Scholar]
- Gaskell R.M., Gettinby G., Graham S.J., Skilton D. Veterinary Products Committee working group report on feline and canine vaccination. Vet. Rec. 2002;150:126–134. [PubMed] [Google Scholar]
- Georgitis J.W., Fasano M.B. Allergenic components of vaccines and avoidance of vaccination-related adverse events. Curr. Allergy Asthma Rep. 2001;1:11–17. doi: 10.1007/s11882-001-0091-6. [DOI] [PubMed] [Google Scholar]
- Hogenesch H., Azcona-Olivera J., Scott-Moncrieff C., Snyder P.W., Glickman L.T. Vaccine-induced autoimmunity in the dog. Adv. Vet. Med. 1999;41:733–747. doi: 10.1016/s0065-3519(99)80056-1. [DOI] [PubMed] [Google Scholar]
- Moore G.E., Guptill L.F., Ward M.P., Glickman N.W., Faunt K.K., Lewis H.B., Glickman L.T. Adverse events diagnosed within three days of vaccine administration in dogs. J. Am. Vet. Med. Assoc. 2005;227:1102–1108. doi: 10.2460/javma.2005.227.1102. [DOI] [PubMed] [Google Scholar]
- Moore G.E., Hogenesch H. Adverse vaccinal events in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 2010;40:393–407. doi: 10.1016/j.cvsm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Ohmori K., Masuda K., Sakaguchi M., Kaburagi Y., Ohno K., Tsujimoto H. A retrospective study on adverse reactions to canine vaccines in Japan. J. Vet. Med. Sci. 2002;64:851–853. doi: 10.1292/jvms.64.851. [DOI] [PubMed] [Google Scholar]
- Ohmori K., Sakaguchi M., Kaburagi Y., Maeda S., Masuda K., Ohno K., Tsujimoto H. Suspected allergic reactions after vaccination in 85 dogs in Japan. Vet. Rec. 2005;156:87–88. doi: 10.1136/vr.156.3.87. [DOI] [PubMed] [Google Scholar]
- Ohmori K., Masuda K., Maeda S., Kaburagi Y., Kurata K., Ohno K., Deboer D.J., Tsujimoto H., Sakaguchi M. IgE reactivity to vaccine components in dogs that developed immediate-type allergic reactions after vaccination. Vet. Immunol. Immunopathol. 2005;104:249–256. doi: 10.1016/j.vetimm.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori K., Masuda K., DeBoer D.J., Sakaguchi M., Tsujimoto H. Immunoblot analysis for IgE-reactive components of fetal calf serum in dogs that developed allergic reactions after non-rabies vaccination. Vet. Immunol. Immunopathol. 2007;115:166–171. doi: 10.1016/j.vetimm.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J.A. Mechanistic bases for adverse vaccine reactions and vaccine failures. Adv. Vet. Med. 1999;41:681–700. doi: 10.1016/S0065-3519(99)80053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi M., Ogura H., Inouye S. IgE antibody to gelatin in children with immediate-type reactions to measles and mumps vaccines. J. Allergy Clin. Immunol. 1995;96:563–565. doi: 10.1016/s0091-6749(95)70304-7. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Nakayama T., Fujita H., Toda M., Inouye S. Minimum estimated incidence in Japan of anaphylaxis to live virus vaccines including gelatin. Vaccine. 2000;19:431–436. doi: 10.1016/s0264-410x(00)00206-1. [DOI] [PubMed] [Google Scholar]


