Abstract
The Iberian lynx is the most endangered felid species in the world, confined nowadays to two isolated metapopulations in the southwest of Spain, where less than 200 individuals survive. Little is known about the diseases that affect these animals in the wild or in captivity. Kidney samples from necropsies of 27 Iberian lynxes, wild and captive, were examined by histopathology, immunohistochemistry (IgG, IgM, IgA, laminin, type IV collagen, and fibronectin), electron microscopy (n = 8) and immunogold labelling for IgM, IgG and IgA in one case, in order to characterize the glomerulopathy prevalent in this species. Urinalyses from records were available for 9 of the necropsied animals and blood and urine samples from 23 free ranging and captive Iberian lynxes were prospectively obtained in order to evaluate the renal function of the living population. A focal, diffuse membranous glomerulonephritis (MGN) that progressed with age was diagnosed in all but one of the animals in different stages not associated to concurrently known infectious diseases. Positive immunoexpression of IgM and IgG was observed in the glomerular capillary basement membranes and intramembranous electron-dense deposits, compatible with immune complexes (ICs) were seen with electron microscopy.
The immunogold labelling was also positive for IgM and IgG in the electron-dense areas. The serum biochemistry and urinalyses also revealed signs of mild chronic kidney disease in 16 of the 23 animals evaluated. In conclusion, the membranous glomerulopathy affecting the Iberian lynx is a progressive disease of immune origin. We postulate a possible genetic predisposition towards the disease, enhanced by inbreeding and a possible connection to an immune-mediated systemic disease.
Abbreviations: BUN, blood urea nitrogen; CKD, chronic kidney disease; FCV, feline calicivirus; FCoV, feline coronavirus; FeLV, feline leukaemia virus; FIV, feline immunodeficiency virus; ICs, immune complexes; IRIS, International Renal Interest Society; MALT, mucosa-associated lymphoid tissue; MGN, membranous glomerulonephritis; PAS, periodic acid schiff reagent; PBS, phosphate buffer solution; RT, room temperature; TBS, Tris buffer solution; USG, urine specific gravity
Keywords: Membranous glomerulonephritis, Iberian lynx, Immune complexes
1. Introduction
The Iberian lynx is considered to be the most endangered felid species in the world (Nowell and Jackson, 1996). Its situation is critical due to habitat loss and fragmentation, being confined nowadays to two isolated metapopulations in the southwest of Spain (Gaona et al., 1998, Ferreras, 2001, Rodriguez and Delibes, 2002). Today, less than 200 animals are estimated to be left (Palomares et al., 2000, Guzmán et al., 2002). The remaining population presents signs of inbreeding and allele dropout (Johnson et al., 2004, Godoy, 2006).
Very little is known about the diseases that affect these animals in the wild or in captivity. Bovine tuberculosis has been reported to be the cause of death of some Iberian lynxes during the years 1998 and 2003 that probably acquired the disease by eating on infected fallow deer (Dama dama) or wild boars (Sus scrofa) of the area (Briones et al., 2000, Aranaz et al., 2004, Peña et al., 2006). A recent histopathological study of our group, evaluating the status of peripheral lymph nodes, spleen and MALT (mucosa-associated lymphoid tissue) in 17 Iberian lynxes necropsied between the years 1998 and 2003, has reported a generalized immune depletion in these animals, apparently not related to infectious agents or malnutrition (Peña et al., 2006).
Routine histological examination of kidneys obtained from the necropsy of 24 animals between the years 1998 and 2005, including the 17 in the above-cited study, revealed a high prevalence of glomerulonephritis (Peña, unpublished data). This disease is common in the domestic cat (Felis cati), especially the membranous type, and is usually of immune origin (Grant Maxie, 1993, Glick et al., 1978). Glomerulonephritis can be associated to viral infections caused by feline coronavirus (FCoV), feline leukaemia virus (FeLV) and the feline immunodeficiency virus (FIV) (Glick et al., 1978, Slauson and Lewis, 1979, Grant Maxie, 1993, Newman et al., 2007). These pathogens are known to affect a wide variety of wild felids such as the cheetah (Acinonyx jubatus), the African lion (Pantera leo), the puma (Puma concolor), the Canada lynx (Lynx canadensis), etc. (Roelke et al., 2006, Kennedy et al., 2003, Biek et al., 2002).
Also, many non-specific parasitic infections that can affect domestic animals are associated with immune-mediated glomerulonephritis (Newman et al., 2007). A case of glomerulonephritis associated to heartworm infection has been described in the black-footed cat (Felis nigripes) (Deem et al., 1998). Furthermore, a non-immunogenic glomerulosclerosis has been reported in captive cheetahs (Acinonyx jubatus) (Munson, 1993, Bolton and Munson, 1999, Munson et al., 1999).
Rare diseases of uncertain origin are frequently described in endangered animals, usually related to poor genetic diversity or to captive conditions. The study of these diseases is important because of their relevance in endangered species conservation programs. A good example of this is the cheetah, considered a paradigm for disease vulnerability (Munson et al., 2005).
This is the first report of glomerulonephritis in the Iberian lynx. The main objectives of this study were to characterize by means of histology, immunohistochemistry, and electron microscopy, the type of glomerulopathy detected in the Iberian lynx, to determine its pathogenesis, and to evaluate serum biochemical and urinary parameters related to renal function in wild and captive Iberian lynx surviving in the southwest of Spain. The membranous glomerulopathy affecting the Iberian lynx is a progressive disease of immune origin. We postulate that the Iberian lynx has a likely genetic predisposition towards the disease, enhanced by inbreeding, and is potentially connected to a possible immune-mediated systemic disease.
2. Materials and methods
2.1. Animals
Twenty-seven necropsies were performed on Iberian lynxes between the years 1998 and 2005. Twenty-one animals came from the wild and six from captivity with ages that ranged from 44 days to 17 years. There were 15 females and 12 males. The main cause of death were car accidents (n = 17), followed by tuberculosis (n = 3), traumatisms (other than car accidents, n = 2), fights (n = 2), squamous cell carcinomas (n = 1), and other diseases (n = 2). Serology and PCR results for various infectious agents that included those related to glomerulonephritis (FCoV, feline calicivirus (FCV), FIV, and FeLV,) were provided. With the exception of one animal that was positive for FeLV in serology, and two that were PCR positive for FCoV (without histological signs of feline infectious peritonitis), the remaining animals were negative for these agents.
2.2. Histopathology and immunohistochemistry
Right and left kidney samples from 27 necropsies were fixed in formalin, paraffin embedded and sectioned 4 μm wide for hematoxilin-eosin, PAS (Periodic Acid Schiff reagent), Masson's trichromic, Congo red and methenamine silver stains.
In order to quantify the lesions in the kidneys, each glomerulus was classified according to the severity of lesions in three categories: (1) healthy or mild, (2) moderate and (3) severe glomerulonephritis 148 or glomerulosclerosis. The percentages of each category of glomeruli were established in all samples.
Kidneys were then classified as stage 1 or apparently healthy when over 50% of the glomeruli belonged to category 1 (unaltered or presenting slight changes). The remaining kidneys were classified considering the most prevalent category of glomerular lesion (stage 2, moderate kidney affection; stage 3, severe kidney affection).
The streptavidin–biotin peroxidase method was used on dewaxed kidney sections in order to detect the expression of fibronectin, laminin, and type IV collagen, components of the glomerular basement membrane. This technique was also used for IgA, IgM, and IgG, markers of the immunoglobulin fraction of immune complexes.
Antigens were unmasked with trypsin (Sigma® 0.01%, 37 °C, 10 min) for the detection of laminin, fibronectin, IgA, IgM, and IgG while a buffer citrate (tri-sodium citrate Panreac®, 0.3%; pH 6) heated in a microwave oven for 15 min was used to unmask type IV collagen antigen. Endogenous peroxidase was deactivated in all cases (H2O2 1.5% in methanol) at room temperature (RT), for 15 min. Unspecific staining of laminin and fibronectin was blocked incubating the slides with normal swine serum (Dako®, X0901, dilution 1/30, 30 min, RT) and with normal rabbit serum (Dako®, X0902, 1/30) for IgA, IgM, and IgG). The primary antibodies used (all incubated at 4 °C overnight) were polyclonal rabbit anti-laminin (Dako®, Z0097, 1/100), polyclonal rabbit anti-fibronectin (Dako®, A0245, 1/500), monoclonal mouse anti-type IV collagen (Dako®, clone CIV 22, 1/30), polyclonal goat anti-feline IgA (Serotec®, AA125, 1/1000), polyclonal goat anti-feline IgM (Serotec®, AA124, 1/100) and polyclonal goat anti-feline IgG (Serotec®, 173 AA126, 1/1000). Biotinylated secondary antibodies swine anti-rabbit (for laminin and fibronectin, Dako® E0353, 1/200,), rabbit anti-goat (for IgA, IgG, and IgM, Pierce®, 31732, 1/200) and rabbit anti-mouse (for type IV collagen, Dako® E0465, 1/200) were used (incubated 30 min at RT) after washing. Slides were then washed and incubated with a streptavidin peroxidase conjugate (Zymed® 43-4323, 1/400, 30 min RT). A chromogen solution containing 3-3′ diaminobenzidine tetrachloride (Sigma Chemical Co. D5059) and H2O2 in distilled water was used for developing, and finally, slides were counterstained with hematoxylin (Sigma® GH5-2-16). All washes were done with TBS (Tris buffer solution pH 7.4). The glomerular capillary basement membranes served as internal controls for type IV collagen, laminin and fibronectin, while lymphoid tissues with plasmatic cells from Iberian lynx were used as positive controls for IgM, IgG and IgA. Negative controls were used substituting the primary antibodies with TBS.
2.3. Electron microscopy and immunogold labelling
Selected kidney samples fixed in formalin (n = 4) and in 2.5% glutaraldehyde (n = 4) from animals without concurrent diseases or detected viruses were processed for electron microscopy. After fixation, the samples were washed in a phosphate buffer (PBS, pH 7.2) and included in 1% osmium (diluted in PBS) for 1 h at room temperature. They were then washed in distilled water and dehydrated in acetones of increasing percentage (30, 50, 70, 80, and 100%). The samples were gradually infiltrated in a Müllenhauer mixture resin and solidified at 60 °C for 24 h. The resin blocks were then processed and studied in the Electron Microscopy Center of the Complutense 197 University of Madrid, Spain.
One additional kidney sample fixed in formalin was selected for immunogold labelling of IgM, IgG and IgA. The sample was thoroughly washed in PBS at 4 °C until clear of formalin. It was then dehydrated in alcohols of increasing percentage (30, 50, 75, 95, and 100%) while the temperature was lowered gradually (from 0 °C to −20 °C for alcohols at 30 and 50%, respectively) until reaching −30 °C. Resin blocks were obtained by gradually infiltrating the sample with Lowicryl® resin at −30 °C. Tissue samples were obtained from the resin blocks and placed in grids for immunogold labelling. Polyclonal goat anti-feline IgM, IgA and IgG (Serotec®, AA124, AA125, AA126; 1/25) were used as primary antibodies. The grids were incubated at 4 °C overnight and later washed with 0.001 M glycine in TBS. Then they were incubated for 1 h with 12 nm colloidal gold-AffiniPure donkey anti-goat IgG (Jackson ImmunoResearch®, 705-205-147, 1/200). The grids were washed again with the glycine solution.
They were stained with 2% uranyl acetate and Reynolds lead citrate, washing with distilled water after both and finally dried and observed by electron microscopy.
2.4. Blood and urine analyses
Urinalyses from records were available for nine of the necropsied animals. These were retrieved and evaluated.
A prospective study of 23 free ranging and captive Iberian lynxes was carried out (Table 1 ). Blood samples (n = 23) and urine samples (n = 17) were collected from anaesthetized Iberian lynxes (Table 1). Serum biochemistry parameters related to renal function (total proteins, albumin, blood urea nitrogen (BUN), creatinine, calcium, and phosphorus) were determined. The analytical results have been evaluated based on published values for the Canada lynx (Felis lynx canadensis) (Weaber and Johnson, 1995), the European lynx (Lynx lynx) (Ryser-Degiorgis, personal communication), and the domestic cat (DiBartola and Rutgers, 1994).
Table 1.
Prospective study of analytical parameters related to renal function in the Iberian lynx
| Case no. | Name | Sex | Origin | Free/captive | Blood samples | Urine samples | CKDa | Proteinuriab |
|---|---|---|---|---|---|---|---|---|
| 1 | Adelfa | F | Sierra Morena | Captive | Yes | Yes | 1 | NP |
| 2 | Aliaga | F | Sierra Morena | Captive | Yes | No | 0 | |
| 3 | Arcex | M | Sierra Morena | Captive | Yes | No | 2 | |
| 4 | Arrayan | M | Doñana | Free | Yes | Yes | 1 | NP |
| 5 | Almoradux | M | Doñana | Captive | Yes | No | 0 | |
| 6 | Boj | F | Doñana | Captive | Yes | Yes | 1 | BP |
| 7 | Brezo | M | Captive breed | Captive | Yes | Yes | 0 | NP |
| 8 | Brisa | F | Captive breed | Captive | Yes | Yes | 0 | NP |
| 9 | Cantareras | F | Sierra Morena | Captive | Yes | No | 0 | |
| 10 | Cromo | M | Sierra Morena | Captive | Yes | Yes | 2 | NP |
| 11 | Esperanza | F | Doñana | Captive | Yes | No | 3 | |
| 12 | Fran | M | Sierra Morena | Captive | Yes | Yes | 2 | NP |
| 13 | Garfio | M | Sierra Morena | Captive | Yes | No | 3 | |
| 14 | Jabata II | F | Doñana | Free | Yes | Yes | 1 | NP |
| 15 | Artemisa | F | Sierra Morena | Captive | Yes | Yes | 0 | NP |
| 16 | JUB | M | Sierra Morena | Captive | Yes | Yes | 2 | NP |
| 17 | Morena | F | Sierra Morena | Captive | Yes | Yes | 3 | BP |
| 18 | Nati II | M | Doñana | Free | Yes | Yes | 2 | P |
| 19 | Pavón | M | Doñana | Free | Yes | Yes | 1 | NP |
| 20 | Rayuela | F | Doñana | Free | Yes | Yes | 1 | NP |
| 21 | Román | M | Doñana | Free | Yes | Yes | 2 | BP |
| 22 | Viciosa | F | Doñana | Free | Yes | Yes | 0 | NP |
| 23 | Wari | F | Doñana | Free | Yes | Yes | 2 | NP |
Stage of chronic kidney disease following the IRIS classification system.
NP, nonproteinuric; P, proteinuric; BP, borderline proteinuric.
The urinalysis included urine specific gravity (USG), albumin, glucose, leukocytes, blood, and examination of the urine sediment. The protein-to creatinine ratio was also measured in order to quantify proteinuria.
Chronic kidney disease (CKD) was categorized in four stages using serum chemistry concentrations and considering the presence of markers of kidney damage according to the recommendations of the International Renal Interest Society (IRIS) (Polzin, 2004). The markers of kidney damage used for the Iberian lynx were: elevated BUN, hypoalbuminemia, elevated serum creatinine concentration, hyperphosphatemia, hypocalcemia, impaired urine concentrating ability (USG was considered normal when ≥1030), proteinuria, and renal hematuria. These must be confirmed to be of kidney origin and at least one must be present to identify the existence of renal disease. Stage 1 (non-azotemic) was considered when markers of kidney damage were present and serum creatinine concentration was <1.6, stage 2 (mild renal azotemia) presented markers and creatinine values between 1.6 and 2.8 mg/dl. In stage 3 (moderate renal azotemia) creatinine values must be 2.8–3.3 mg/dl, and in stage 4 (severe renal azotemia), creatinine values exceed 5 mg/dl. The stages were further subcategorized using the protein-to-creatinine ratio as proteinuric (ratio >1.0), non-proteinuric (ratio <0.5) or borderline proteinuric (ratio 0.5–1.0).
2.5. Statistical analyses
The associations of epidemiological, pathological and immunohistochemical data were studied using computer software SPSS. Categorical variables were analyzed by Pearson and Yates Chi-square tests. Pearson correlation coefficients were used to study continuous variables. Levene F-tests were used to analyze the homogeneity of variances. If variances were equal, F-tests or pooled t-tests were chosen to evaluate the variables. If variances were not equal, then Welch tests or separate variance t-tests were selected. Values p < 0.05 were considered significant. Non-parametric statistical tests (Kruskal–Wallis and Median Test) have been performed when necessary.
Epidemiological variables included: age as a numerical variable in months and as a categorical variable: (young, 0–12 months; young adult, 13–24 months; and adult/senile, >24 months), gender, origin (Sierra Morena or Doñana), and free or captive. In order to evaluate the kidney lesion, four histopathological variables were used: (a) percentage of glomeruli in category 1, (b) percentage of glomeruli in category 2, (c) percentage glomeruli in category 3 and (d) stage of kidney affection (stage 1, none or minimal lesion; stages 2 and 3, moderate and severe) (categorical). Other variables were considered as present or absent such as interstitial nephritis, peritubular fibrosis, and hyaline cylinders. Immunohistochemical variables concerning normal or increased expression of laminin, IV collagen, and fibronectin and the presence or absence of IgG, IgM, and IgA were also included.
3. Results
3.1. Histopathology
A focal, diffuse membranous glomerulonephritis (MGN) was diagnosed in all of the animals, regardless of their age, sex, and whether they came from captivity or from the wild, except a 44-day-old cub that did not present any changes in the glomeruli. Sclerotic glomeruli were focally scattered in the cortex, and stained blue in the mesangium with Masson's trichromic (Fig. 1 ). Bowman's capsules appeared focally thickened.
Fig. 1.
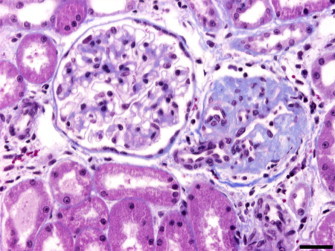
Iberian lynx. Kidney. Masson's trichromic. Membranous glomerulonephritis and glomerulosclerosis. Bar = 34 μm.
The kidneys were classified according to the percentage of altered glomeruli. Ten animals were found to have unaltered or slight changes in over 50% of the glomeruli (stage 1), 16 presented moderate MGN (stage 2) and only one animal had a severe MGN (stage 3).
Interstitial fibrosis was seen in 15 animals and chronic (lympho-plasmacytic) interstitial nephritis in 11 (not including the granulomas found in the kidneys of the three animals with tuberculosis). Other renal lesions such as calcifications and hyaline cylinders in tubules were seen in seven animals. The Congo red stain was negative in all of the animals.
Severity of MGN was found to increase significantly with age: high percentages of normal and minimally altered glomeruli were significantly associated to young animals (mean 66.0% ± S.D. 21.5, median 61.0%) while lower percentages were associated to young adults (mean 40.44% ± SD 19.3, median 35.0% of unaltered glomeruli) and adult/senile animals (mean 28.40% ± SD 28.40, 21.0%) (p = 0.016 ANOVA test). The non-parametric Kruskal–Wallis (p = 0.023) and the Median tests (p = 0.012) were also significant for this association. There was also a positive correlation between the age (in months) 295 and the percentage of severely affected glomeruli (p = 0.001).
The presence of interstitial lympho-plasmacytic infiltrates (chronic interstitial nephritis) was significantly associated to the advanced grade of MGN (p = 0.025). Interstitial fibrosis was evident in advanced MGN (p = 0.056).
3.2. Immunohistochemistry
A slight increase of the three components of the glomerular basement membrane, laminin, fibronectin and type IV collagen was observed in glomeruli at beginning or middle stages of glomerulonephritis, while in glomeruli presenting glomerulosclerosis, laminin and fibronectin would always appear intensely expressed, in a focal or diffuse matter, and type IV collagen could either be intensely expressed (Fig. 2 ) or negative. Laminin expression was significantly associated with the advanced grade of MGN (p = 0.013).
Fig. 2.
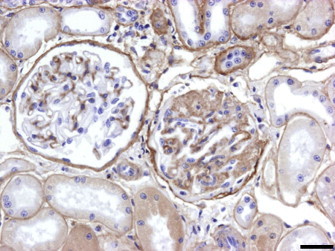
Iberian lynx. Kidney. Streptavidin–biotin peroxidase anti-type IV collagen. Same field as Fig. 1. Intense staining of thickened basement membranes and glomerulosclerosis. Bar = 34 μm.
A positive immunoexpression of IgM and IgG was observed in the thickened glomerular capillary basement membranes in early stages of glomerulonephritis (Fig. 3, Fig. 4 ), while in glomerulosclerosis, the expression of these two immunoglobulins was negative. IgA expression was negative in all cases.
Fig. 3.
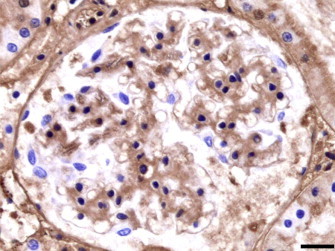
Iberian lynx. Kidney. Streptavidin–biotin peroxidase anti-IgG. IgG deposit along the thickened glomerular basement membranes. Bar = 29 μm.
Fig. 4.
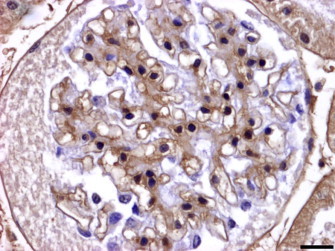
Iberian lynx. Kidney. Streptavidin–biotin peroxidase anti-IgM. IgM deposit along the thickened glomerular basement membranes. Bar = 29 μm.
3.3. Electron microscopy and immunogold labelling
Electron microscopy revealed irregularly thickened glomerular capillary basement membranes (n = 6/8) and effacement of foot processes (n = 4/8) (Fig. 5 ). Electron-dense deposits, compatible with immune complexes (ICs), were located intramembranously (n = 6/8) (Fig. 6 ). Some electron-lucent areas also were seen in the basement membranes, compatible with areas where immune complexes were being reabsorbed (n = 5/8). In glomerulosclerosis, the capillary basement membranes were thickened and wrinkled; foot processes were effaced and sometimes lost. Electron-dense deposits were not found in this stage.
Fig. 5.
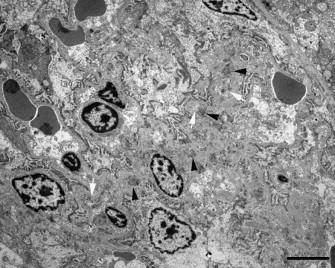
Iberian lynx. Glomerulus. Electron microscopy. Wrinkled and thickened basement membranes with rests of immune complexes (ICs) (black arrowheads) and areas of reabsorption (white arrows). Bar = 5 μm.
Fig. 6.
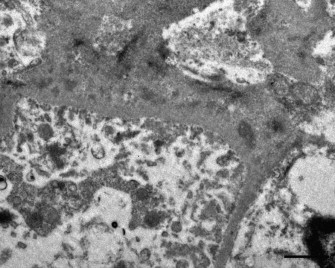
Iberian lynx. Glomerulus. Electron microscopy. Detail of basement membrane with ICs and reabsorption areas. Bar = 1 μm.
The immunogold labelling for IgM and IgG stained positive the electron-dense structures that were compatible with (ICs) (Fig. 7 ), while IgA was not detected.
Fig. 7.
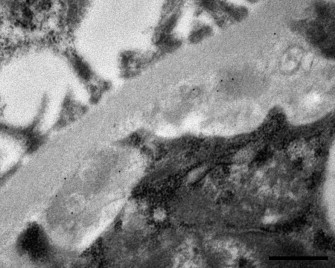
Iberian lynx. Glomerular basement membrane. Electron microscopy. Immunogold labelling for IgM (12 nm colloidal gold particles) detecting IgM in the ICs. Bar = 500 nm.
3.4. Blood and urine analyses
The urinalyses available from nine of the necropsied animals presented low urine specific gravity (USG) in five cases (USG < 1030), three of which presented proteinuria qualitatively measured (albumin ≥30 mg/dl). Two other animals presented proteinuria (albumin ≥300 mg/dl) with normal specific gravity.
Haematuria was seen in six animals and glycosuria was present in one. Serum biochemistry parameters of the 23 live Iberian lynxes were: total proteins (range 5.3–12.3 g/dl; mean 8.6 ± S.D. 1.6; median 8.7), albumin (range 3.27–5,29 g/dl; mean 4.4 ± S.D. 0.6; median 4.47), BUN (range 10–151 mg/dl; mean 58 ± S.D. 31; median 59.0), creatinine (range 0.6–3.3 mg/dl; mean 1.7 ± S.D. 0.8; median 1.6), calcium (range 5.1–11.1 mg/dl; mean 8.2 ± S.D. 2; median 8.9), and phosphorus (range 5.3–13.2 mg/dl; mean 7.4 ± S.D. 2.1; median 6.8). Twelve animals had hyperphosphatemia (phosphorus > 6.5 mg/dl), 9 presented hypocalcemia (calcium < 8.2 mg/dl), 11 animals had uremia (BUN > 60 mg/dl), 10 presented elevated creatinine (creatinine > 1.6 mg/dl). Nine lynxes presented decreased USG (<1030) and proteinuria was detected qualitatively in 10 animals.
According to the IRIS CKD classification system, five animals did not present any signs of kidney damage, two presented only slight hyperphosphatemia and uremia that could not be attributed to the kidney so were considered healthy also, six animals were classified in stage 1 (non-azotemic), seven in stage 2 (mild-azotemia), and three animals in stage 3 (moderate renal azotemia). One animal in stage 2 was subclassified as proteinuric and three animals were subclassified as borderline proteinuric in stages 1, 2 and 3 (Table 1).
4. Discussion
Membranous glomerulonephritis (MGN) in different stages was the main lesion described in the kidneys of the Iberian lynxes. This disease is characterized by an irregular increase of the glomerular capillary basement membranes due to the deposit of circulating immune complexes, or specific antibody attachment to components of the glomerulus (Franklin et al., 1973, Valaitis, 2002, Newman et al., 2007).
The immunohistochemistry revealed basement membranes to be thickened by specific components such as laminin, fibronectin and type IV collagen. The detection of immunoglobulins G and M in the capillary basement with immunohistochemistry along with the intramembranous electron-dense deposits seen with the electron microscopy, confirm the presence of ICs in our samples and an immune origin of the GN. The immunogold labelling results matched the immunohistochemistry since both IgM and IgG were detected over the electron-dense deposits and IgA was absent. Signs of complete and partial reabsortion of ICs were also detected with electron microscopy, but not when there were histological features of sclerosis.
Immune complexes are usually present during initial stages of the disease and are progressively reabsorbed by the basement membrane, which thickens after the process. Repeated injury on the basement membranes can impair their capacity to reabsorb immune complexes and regenerate, and therefore sclerosis of the membranes occurs as it loses its structural detail and capacity to eliminate ICs (Valaitis, 2002).
The most severe lesions, including the glomerulosclerosis, were significantly related to the age of the animals, which corresponds with a progressive degenerative disease.
The other lesions found in many of the kidneys such as chronic interstitial nephritis and tubular fibrosis were less frequent and related to the severity of the MGN, and therefore were considered secondary to the disease. The presence of hyaline cylinders indicates some degree of filtration impairment of the tubuli, as consequence of the glomerular injury (Newman et al., 2007).
The study of the urinalysis available from some of the necropsied animals was important to determine if there were analytical signs of renal dysfunction that corresponded with the histological findings. Unfortunately, only nine urinalyses of the necropsied animals were available. Nevertheless, the presence of proteinuria and low urine specific gravity in five animals, suggests that filtration might have been impaired to some degree. Glycosuria and hematuria were also present in some cases. These are unspecific signs of renal damage and there was no information available about existing extrarenal infections at the time of sampling, therefore their interpretation should be taken with caution.
Blood and urine sampling of the living population was important to evaluate if there was a correspondence between the prevalent histopathological findings and the existence of chronic kidney disease (CKD) in the live animals. There is one study published on serum biochemical characteristics of the Iberian lynx done in 16 wild Iberian lynxes (Beltrán and Delibes, 1991), and recently another study is being done with a larger number of animals by members of this team, describing the parameters observed in the population regardless of the presence of infection or other diseases. Due to this situation the IRIS staging system is useful since it permits the diagnoses of CKD even when reference ranges are not very accurate (Polzin, 2004).
According to the results obtained with the IRIS classification system, 16 out of 23 animals presented some signs of chronic kidney disease, though only three presented moderate azotemia and none of them were found in the most severe stage. The presence of renal disease seems to exist among the living population, both captive and wild, and this would correlate with the histological changes detected in the necropsied animals.
Another common feature of renal failure is hyperphosphatemia and therefore was included as a marker of kidney disease. It appears when the glomerular filtration rate declines around 20% below normal (Grauer and DiBartola, 2000) and it was detected in 12 of the animals. In two of them it was not considered since the urinary analyses were not available and it could have been due to recent ingestion. Hypocalcemia was present in four of the animals with hyperphosphatemia.
The main consequence of hyperphosphatemia is the development of secondary renal hyperparathyroidism (Nagode and Chew, 1992, Kates et al., 1997, Barber and Elliot, 1998). It is usually associated to hypocalcemia, though hypercalcemia or normal serum calcium concentrations accompanying hyperphosphatemia can also be found due to the compensation of the parathyroid hormone (PTH) (Barber and Elliot, 1998). The consequences of a secondary hyperparathyroidism are completely unknown in the Iberian lynx, but it may be interesting to explore animals during sampling or in future necropsies for signs of pain or osteodistrophia seen in other species (Barber and Elliot, 1998).
Membranous glomerulonephritis is a common glomerular affection in cats that can be associated to viral infections (FeLV, FIV, FCoV, etc.), neoplasms, intoxications (methyl mercury), familial diseases, autoimmune pathologies, or in many cases it is considered idiopathic (Glick et al., 1978, Enestrom and Hultman, 1984, Newman et al., 2007). Membranous glomerulonephritis can also be secondary to other systemic immunological diseases such as systemic lupus erythematosus or immune complex polyarteritis in dogs and cats (Newman et al., 2007).
The only Iberian lynx without signs of membranous glomerulonephritis was the 44-day-old female cub that had died in a sibling quarrel. With the exception of the few animals with neoplasias or tuberculosis, or the three lynxes that were PCR positive for viral detection (seven animals altogether), features that could explain the presence of MGN were not found in the rest of the animals.
Other vascular and metabolic diseases like hypertension and diabetes mellitus are non-immunogenic causes of glomerular disease (Alpers, 2004) and the glomerular capillary basement membranes can also appear thickened due to hypertension or sclerosis (Buckalew, 1994, Newman et al., 2007).
Hypertension, probably associated to stress, genetic and dietary factors (a high protein diet), was postulated to be the cause of the glomerulosclerosis described in a high percentage of captive cheetahs (Bolton and Munson, 1999). The pathogenesis of the glomerular injury caused by these diseases lacks an immune component and therefore is different to the one seen in the Iberian lynx.
However, the Iberian lynx is also a carnivore, and high protein ingestion can cause hypertension and accelerate progressive renal failure, which leads to glomerulosclerosis (Buckalew, 1994). Therefore, this could be contributing to the progression of the disease, as occurs in the cheetah, even though the main cause of the glomerulopathy is immunogenic.
Inherited glomerulopathies have been described in many breeds of dogs and there are reports in cats, these are primary glomerular diseases (Grauer and DiBartola, 2000, Casal et al., 2004). It may be interesting to take these into consideration since the Iberian lynx displays very low genetic diversity for a felid, with two remnant populations that have undergone a recent founder effect or population bottleneck (Godoy, 2006). Recent studies have found that it possesses a deficiency of heterozygosity possibly due to inbreeding and some degree of allele loss (Johnson et al., 2004). This could encourage the inheritance of a certain glomerular disease among the population. Animals as young as 5 or 11 months presented glomerulonephritis suggesting there may be a predisposition for the disease.
Also, the cause of the generalized immune depletion described in 17 of the animals (Peña et al., 2006) included in this study remains undetermined and a possible relationship with the membranous glomerulonephritis should be taken into consideration.
As with many species in danger of extinction, with the handicap of low genetic diversity that seem to lead to uncommon diseases, the importance of a detailed study of the diseases that may affect the Iberian lynx is evident.
In conclusion, the membranous glomerulopathy that affects the Iberian lynx is a progressive disease of immune origin. We postulate that the Iberian lynx may be genetically prone to the disease, which could be enhanced by inbreeding and also may have a connection with an immune-mediated systemic disease. Future studies are necessary. Nevertheless, none of the animals are known to have died from chronic renal failure yet, though the results indicate that it may be widely extended, considering that the remaining population is of less than 200 individuals. Because of the critical state of this endangered species, signs of progression of this disease should be watched for, especially in the wild population.
Acknowledgements
This research was supported by the Spanish Ministry of Environment, Research Project 90/2002 in agreement with the Environmental Council of the “Junta de Andalucía”, “Dirección General de Investigación” Project CGL2004-00346/BOS and “Red Nacional de Parques Nacionales” Project 17/2006. We thank all the veterinarians and biologists involved with the field work, management and sampling of the Iberian lynxes at Doñana National Park and Sierra Morena. We also thank Dr. Marie-Pierre Ryser-Degiorgis for the serum biochemical reference values of the European lynx. We are grateful to Ana Vicente, Agustín Fernández and Dr. María Luisa García for their assistance processing the electron microscopy samples and to Pedro Aranda for his histotechnology assistance, and to Pedro Cuesta for his assistance in the statistical analysis.
References
- Alpers C.E. The Kidney. In: Kumas V., Abbas A.K., Fausto N., editors. Robbins and Cotran Pathologic Basis of Disease. 7th Ed. Elsevier Saunders; Philadelphia: 2004. pp. 979–993. [Google Scholar]
- Aranaz A., De Juan I., Montero N., Sánchez C., Galka M., Delso C., Alvarez J., Romero B., Bezos J., Vela A.I., Briones V., Mateos A., Domínguez L. Bovine tuberculosis (Mycobacterium bovis) in wildlife in Spain. J. Clin. Microbiol. 2004;42(6):2602–2608. doi: 10.1128/JCM.42.6.2602-2608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber P.J., Elliot J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J. Small. Anim. Pract. 1998;39:108–116. doi: 10.1111/j.1748-5827.1998.tb03613.x. [DOI] [PubMed] [Google Scholar]
- Beltrán J.F., Delibes M. Hematological and serum chemical characteristics of the Iberian lynx (Lynx pardina) in southwestern Spain. Can. J. Zool. 1991;69:840–846. [Google Scholar]
- Biek R., Zarnke R.L., Gillin C., Wild M., Squires J.R., Poss M. Serologic survey for viral and bacterial infections in western populations of Canada lynx (Lynx Canadensis) J. Wildl. Dis. 2002;38(4):840–845. doi: 10.7589/0090-3558-38.4.840. [DOI] [PubMed] [Google Scholar]
- Bolton L.A., Munson L. Glomerulosclerosis in captive cheetahs (Acinonyx jubatus) Vet. Pathol. 1999;36:14–22. doi: 10.1354/vp.36-1-14. [DOI] [PubMed] [Google Scholar]
- Briones V., de Juan l., Sánchez C., Vela A.I., Galka M., Montero N., Goyache J., Aranaz A., Mateos A., Domínguez L. Bovine tuberculosis and the endangered Iberian lynx. Emerg. Infect. Dis. 2000;6(2):189–191. doi: 10.3201/eid0602.000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckalew V.M., Jr. Pathophysiology of progressive renal failure. South. Med. J. 1994;87(10):517. doi: 10.1097/00007611-199410000-00014. [DOI] [PubMed] [Google Scholar]
- Casal M.L., Dambach D.M., Meister T., Jezyk P.F., Patterson D.F., Hemthorn P.S. Familial glomerulopathy in the Bullmastiff. Vet. Pathol. 2004;41:319–325. doi: 10.1354/vp.41-4-319. [DOI] [PubMed] [Google Scholar]
- Deem S.L., Heard D.J., LaRock R. Heartworm (Dirofilaria immitis) disease and glomerulonephritis in a black-footed cat (Felis nigripes) J. Zoo Wildl. Med. 1998;29(2):199–202. [PubMed] [Google Scholar]
- DiBartola S.P., Rutgers H.C. Diseases of the kidney. In: Sherding R.G., editor. The cat, diseases and clinical management. 2nd Ed. Saunders; Philadelphia: 1994. pp. 1711–1717. [Google Scholar]
- Enestrom S., Hultman P. Immunemediated glomerulonephritis induced by mercury chloride in mice. Experimentia. 1984;40(11):1234–1240. doi: 10.1007/BF01946653. [DOI] [PubMed] [Google Scholar]
- Ferreras P. Landscape structure and asymmetrical inter-patch connectivity in a metapopulation of the endangered Iberian lynx. Biol. Conserv. 2001;100(1):125–136. [Google Scholar]
- Franklin W.A., Jennings R.B., Earle D.P. Membranous glomerulonephritis: long-term serial observations on clinical course and morphology. Kidney Int. 1973;4:36. doi: 10.1038/ki.1973.78. [DOI] [PubMed] [Google Scholar]
- Gaona P., Ferreras P., Delibes M. Dynamics and viability of a metapopulation of the endangered Iberian lynx (Lynx pardinus) Ecol. Monogr. 1998;68(3):349–370. [Google Scholar]
- Glick A.D., Horn R.G., Holscher M. Characterization of feline glomerulonephritis associated with viral-induced hematopoietic neoplasms. Am. J. Pathol. 1978;92:321–332. [PMC free article] [PubMed] [Google Scholar]
- Godoy, J.A., 2006. Iberian lynx population genetics: Doñana, Sierra Morena and ex-situ program. www.lynxexsitu.es/comunicaion/noticias/noticias.php.
- Grant Maxie M. The urinary system. In: Jubb K.V.F., Kennedy P.C., Palmer N., editors. Pathology of Domestic Animals. 4th Ed. Academic Press; San Diego: 1993. pp. 475–487. [Google Scholar]
- Grauer G.F., DiBartola S.P. Glomerular disease. In: Ettinger S.J., Feldman E.C., editors. Textbook of Internal Medicine, Diseases of the dog and cat. 5th Ed. Saunders; Philadelphia: 2000. pp. 1662–1678. [Google Scholar]
- Guzmán J.N., García F.J., Garrote G., Perez de Ayala R., Iglesias M.C. Iberian lynx (Lynx pardinus) distribution and current conservation status in Spain. Proceedings of the International Seminar on the Iberian lynx; Andújar, Spain, October; 2002. pp. 18–20. [Google Scholar]
- Johnson W.E., Godoy A., Palomares F., Delibes M., Fernández M., Revilla E., O’Brien S.j. Phylogenetic and phylogeographic analysis of Iberian lynx populations. J. Hered. 2004;95(1):19–28. doi: 10.1093/jhered/esh006. [DOI] [PubMed] [Google Scholar]
- Kates D.M., Sherrard D.J., Andress D.L. Evidence that serum phosphate is independently associated with serum PTH in patients with chronic renal failure. Am. J. Kidney Dis. 1997;30:809–813. doi: 10.1016/s0272-6386(97)90086-x. [DOI] [PubMed] [Google Scholar]
- Kennedy M., Kania S., Stylianides E., Bertschinger H., Keet D., Van Vuuren M. Detection of feline coronavirus infection in southern African nondomestic felids. J. Wildl. Dis. 2003;39(3):529–535. doi: 10.7589/0090-3558-39.3.529. [DOI] [PubMed] [Google Scholar]
- Munson L. Diseases of captive cheetahs (Acinonyx jubatus): results of the Cheetah Research Council pathology survey 1989–1992. Zoo Biol. 1993;12:105–124. [Google Scholar]
- Munson L., Nesbit J.W., Meltzer D.G., Colly L.P., Bolton L., Kriek N.P. Diseases of captive cheetahs (Acinonyx jubatus) in South Africa: a 20-year retrospective survey. J. Zoo Wildl. Med. 1999;30(3):342–347. [PubMed] [Google Scholar]
- Munson L., Terio K.A., Worley M., Jago M., Bagot-Smith A., Marker L. Extrinsic factors significantly affect patterns of disease in free ranging and captive cheetah (Acinonyx jubatus) populations. J. Wildl. Dis. 2005;45(3):542–548. doi: 10.7589/0090-3558-41.3.542. [DOI] [PubMed] [Google Scholar]
- Nagode L.A., Chew D.J. Nephrocalcinosis caused by hyperparathyroidism in progression of renal failure: treatment with calcitriol. Semin. Vet. Med. Surg. (Small Anim.) 1992;7:202–220. [PubMed] [Google Scholar]
- Newman S.J., Confer A.W., Panciera R.J. Urinary system. In: McGavin M.D., Zachary J.F., editors. Pathologic Basis of Veterinary Disease. 4th Ed. Mosby Elsevier; St. Louis: 2007. pp. 613–692. [Google Scholar]
- Nowell and Jackson, 1996. Wild Cats—Status Survey and Conservation Action Plan. IUCN/SSC, Gland, Switzerland.
- Palomares F., Delibes M., Revilla E. Iberian Lynx in a fragmented landscape: predispersal, dispersal, and postdispersal habitats. Conserv. Biol. 2000;14(3):809–818. [Google Scholar]
- Peña L., García P., Jiménez M.A., Benito A., Pérez Alenza M.D., Sánchez B. Histopathological and immunohistochemical findings in lymphoid tissues of the endangered Iberian lynx (Lynx pardinus) Comp. Immunol. Microbial. Infect. Dis. 2006;29(2–3):114–126. doi: 10.1016/j.cimid.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzin, D.J., 2004. Evaluating patients with chronic kidney disease (vet-383). Western Veterinary Conference 2004. www.vin.com.
- Rodriguez A., Delibes M. Internal structure and patterns of contraction in the geographic range of the Iberian lynx. Ecography. 2002;25(3):314–328. [Google Scholar]
- Roelke M.E., Pecon-Slattery J., Taylor S., Citino S., Brown E., Packer C., Vandewoude S., O’Brien S.J. T-lymphocyte profiles in fiv-infected wild lions and pumas reveal CD4 depletion. J. Wildl. Dis. 2006;42(2):234–248. doi: 10.7589/0090-3558-42.2.234. [DOI] [PubMed] [Google Scholar]
- Slauson D.O., Lewis R.M. Comparative pathology of glomerulonephritis in animals. Vet. Pathol. 1979;16:135–164. doi: 10.1177/030098587901600201. [DOI] [PubMed] [Google Scholar]
- Valaitis J. Atlas of Electrón Microscopy with Histopathological Bases and Immunofluoresence Findings. ASCP Press; Chicago: 2002. Renal glomerular disease. p. 99. [Google Scholar]
- Weaber J.L., Johnson M.R. Hematologic and serum chemistry values of captive Canadian lynx. J. Wildl. Dis. 1995;31:212–215. doi: 10.7589/0090-3558-31.2.212. [DOI] [PubMed] [Google Scholar]


